User login
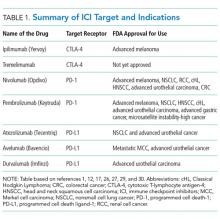
Things We Do for No Reason: Hospitalization for the Evaluation of Patients with Low-Risk Chest Pain
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Chest pain is one of the most common complaints among patients presenting to the emergency department. Moreover, at least 30% of patients who present with chest pain are admitted for observation, and >70% of those admitted with chest pain undergo cardiac stress testing (CST) during hospitalization. Several clinical risk prediction models have validated evaluation processes for managing patients with chest pain, helping to identify those at a low risk of major adverse cardiac events. Among these, the Thrombolysis in Myocardial Infarction or HEART score can identify patients safe to be discharged with outpatient CST within 72 h. It is unnecessary to hospitalize all low-risk patients for cardiac testing because it may expose them to needless risk and avoidable care costs, with little additional benefit.
CLINICAL SCENARIO
A 60-year-old man with a history of osteoarthritis and depression presented to our emergency department (ED) with a 1-month history of left-sided chest pain that was present both at rest and exertion. There were no aggravating or relieving factors for the pain and no associated shortness of breath, diaphoresis, nausea, or lightheadedness. He smoked a half pack of cigarettes daily for 5 years in his twenties. The patient was taking aspirin 81 mg daily and paroxetine 40 mg daily, which he had been taking for 10 years. There was a family history of coronary artery disease in his mother, father, and sister. On examination, he was afebrile, with a blood pressure of 138/78 mm Hg and a heart rate of 62 beats/min; he appeared well, with no abnormal cardiopulmonary findings. Investigation revealed a normal initial troponin I level (<0.034 mg/mL) and normal electrocardiogram (ECG) with normal sinus rhythm (75 beats/min), normal axis, no ST changes, and no Q waves. He was therefore admitted to the hospital for further evaluation.
BACKGROUND
Each year, >7 million patients visit ED for chest pain in the United States,1 with approximately 13% diagnosed with acute coronary syndromes (ACSs).2 Over 30% of patients who present to ED with chest pain are hospitalized for observation, symptom evaluation, and risk stratification.3 In 2012, the mean Medicare reimbursement cost was $1,741 for in-hospital observation,4 with up to 70% of admitted patients undergoing cardiac stress testing (CST) before discharge.5
WHY YOU MIGHT THINK HOSPITALIZATION IS HELPFUL FOR THE EVALUATION OF LOW-RISK CHEST PAIN
A scientific statement by the American Heart Association in 2010 recommended that patients considered to be at low risk for ACS after initial evaluation (based on presenting symptoms, past history, ECG findings, and initial cardiac biomarkers) should undergo CST within 72 h (preferably within 24 h) of presentation to provoke ischemia or detect anatomic coronary artery disease.6 Early exercise treadmill testing as part of an accelerated diagnostic pathway can also reduce the length of stays (LOS) in hospital and lower the medical costs.7 Moreover, when there is noncompliance or poor accessibility, failure to pursue early exercise testing in a hospital could result in a loss of patients to follow-up. Hospitalization for testing through accelerated diagnostic pathways may improve access to care and reduce clinical and legal risks associated with a major adverse cardiac event (MACE).
WHY HOSPITALIZATION FOR THE EVALUATION OF LOW-RISK CHEST PAIN IS UNNECESSARY FOR MANY PATIENTS
Clinical Risk Prediction Models
When a patient initially presents with chest pain, it should be determined if the symptoms are related to ACS or some other diagnosis. Hospitalization is required for patients with ACS but may not be for those without ACS and those with a low risk of inducible ischemia. Clinical risk scores and risk prediction models, such as the Thrombolysis in Myocardial Infarction (TIMI) and HEART scores, have been used in accelerated diagnostic protocols to determine a patient’s likelihood of having ACS. Several large trials of these clinical risk prediction models have validated the processes for evaluating patients with chest pain.
The TIMI risk score, the most well-known model, assesses risk based on the presence or absence of 7 characteristics (Appendix 1). It should be noted that the patient population studied for initial validation of this model comprised high-risk patients with unstable angina or non-ST elevation myocardial infarction who would benefit from early or urgent invasive therapy.8 In this population, TIMI scores of 0-1 are associated with low risk, with a 4.7% risk of ACS at 14 days.8 In another study of patients presenting to ED with undifferentiated chest pain and a TIMI score of zero, the risk of MACE at 30 days was approximately 2%.9
The HEART score is also used for patients presenting to ED with undifferentiated chest pain and assesses 5 separate variables scored 0–2 (Appendix 2). The original research gave a score of 2 to a troponin I level greater than twice the upper limit of the normal level,10 whereas a subsequent validation study gave a score of 2 to a troponin I or T level greater than or equal to 3 times the upper limit of the normal level.11 Patients are considered at low, intermediate, and high risk based on scores of 0–3, 4–6, and 7–10, respectively.10,11 Backus et al. performed a prospective randomized trial of 2388 patients who presented to ED with chest pain to validate the HEART score and compare it to the TIMI risk score. The HEART score performed better than the TIMI risk score in low-risk patients, with TIMI scores of 0-1 and HEART scores of 0–3 having a 6-week MACE risk of 2.8% and 1.7%, respectively.11
A HEART pathway was developed that combines the HEART score with serial troponin I assays assessed at the time of initial presentation and approximately 3 h later.12 Mahler et al. randomized 282 patients presenting to ED with chest pain to either the HEART pathway or conventional care. Patients with low-risk HEART scores and an abnormal troponin I level were admitted for cardiology consultation, whereas discharge was recommended for those with low scores and a normal troponin I level. Despite nearly 20% of the study cohort having a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting, approximately 40% of patients in the HEART pathway were identified as low risk, increasing early discharge rates by 21.3% and decreasing the average LOS by 12 h. No low-risk patient suffered a MACE within 30 days, and the HEART pathway had a sensitivity and a negative predictive value of approximately 99%.
Costs and Harms of Hospitalization for Cardiac Testing
Hospitalization carries measurable risks.13,14 Between 2008 and 2013, Weinstock et al. evaluated the outcom
Outpatient CST can be reliably and safely performed for patients with chest pain.16-18 There is no clear evidence that earlier CST leads to improved patient outcomes, and CST in the absence of acute ischemia (or ACS) increases the rates of angiography and revascularization without improvements in the rate of myocardial infarction.19-21 Given the costs of in-hospital observation4 and the dubious benefits of providing CST for patients with low-risk chest pain, admitting all patients with low-risk chest pain exposes them to costs and harms with little potential benefit.
WHEN HOSPITALIZATION MAY BE REASONABLE TO EVALUATE LOW-RISK CHEST PAIN
Patients presenting with chest pain with either dynamic ECG changes or an elevated troponin level require hospitalization for further ACS diagnosis and treatment. When ACS cannot be clearly diagnosed at the initial evaluation, healthcare providers should use clinical risk prediction models to stratify patients. Those deemed to be at an intermediate or high risk by these models should be hospitalized for further evaluation, as should those at low risk but for whom access to outpatient follow-up is difficult (eg, those without health insurance).
WHAT YOU SHOULD DO INSTEAD OF HOSPITALIZATION FOR LOW-RISK CHEST PAIN
A complete history and physical examination, along with ECG and cardiac biomarker testing, are required for all patients presenting with chest pain. Validated clinical risk prediction models should then be used to determine the likelihood of a cardiac event. Fanaroff et al. reported that low-risk HEART scores of 0–3 and TIMI scores of 0-1 gave positive likelihood ratios of 0.2 and 0.31, respectively.22 Using a pre-test probability of 13%, as reported by Bhuiya et al.,2 the likelihood of ACS or MACE within 6 weeks is 2.9% for patients with low-risk HEART scores and 4.4% for those with low-risk TIMI scores.22 These risk prediction models allow clinicians to provide a shared decision-making plan with the patient and discuss the risks and benefits of in-hospital versus outpatient cardiac testing, especially among patients with access to appropriate outpatient follow-up.23 Low-risk patients can be referred for outpatient testing within 72 h, reducing hospitalization-associated costs and harms.
RECOMMENDATIONS
- Patients presenting with chest pain should undergo a complete history taking and physical examination, as well as ECG and cardiac biomarker testing (eg, troponin I level at presentation and approximately 3 h later).
- Clinical risk prediction models, such as TIMI or HEART scores, should then be used to determine the risk of MACE.
- Patients at a low risk may be safely discharged with outpatient CST performed within 72 h.
- Patients at an intermediate or high risk of MACE should be hospitalized for further evaluation, as should those with low-risk chest pain who are unable to attend follow-up for outpatient CST within 72 h.
- Clinicians should provide a shared decision-making plan with each patient, taking care to discuss the risks and benefits of in-hospital versus outpatient CST.
CONCLUSION
The risk of MACE should be assessed in all patients presenting to ED with low-risk chest pain to avoid unnecessary hospitalization that exposes them to potential costs and harms with few additional benefits. If the risk scoring system was applied to the patient described in our original clinical scenario, he would have had a HEART score of 3 (ie, 1 point for a moderately suspicious history, 1 point for the age of 60 years, and 1 point for a positive family history) and a TIMI score of 1 (ie, 1 point for aspirin use within past 7 days). Therefore, he could be stratified as having a low-risk presentation. With a second negative troponin I test at 3 h, discharge from ED with timely outpatient CST within 72 h would be an appropriate management strategy.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
1. Centers for Disease Control. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. 2011. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed October 7, 2015.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;(43):1-8. PubMed
3. Cotterill PG, Deb P, Shrank WH, Pines JM. Variation in chest pain emergency department admission rates and acute myocardial infarction and death within 30 days in the Medicare population. Acad Emerg Med. 2015;22(8):955-964. PubMed
4. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries, OEI-02-12-00040. 2013. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed May 15, 2017.
5. Penumetsa SC, Mallidi J, Friderici JL, Hiser W, Rothberg MB. Outcomes of patients admitted for observation of chest pain. Arch Inter Med. 2012;172(11):873-877. PubMed
6. Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756-1776. PubMed
7. Hutter AM, Jr., Amsterdam EA, Jaffe AS. 31st Bethesda Conference. Emergency Cardiac Care. Task force 2: Acute coronary syndromes: Section 2B--Chest discomfort evaluation in the hospital. J Am Coll Cardiol. 2000;35(4):853-862. PubMed
8. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835-842. PubMed
9. Pollack CV, Jr., Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13(1):13-18. PubMed
10. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008; 16(6):191-196. PubMed
11. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. PubMed
12. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203. PubMed
13. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Inter Med. 2003;138(3):161-167. PubMed
14. James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122-128. PubMed
15. Weinstock MB, Weingart S, Orth F, et al. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med. 2015;175(7):1207-1212. PubMed
16. Meyer MC, Mooney RP, Sekera AK. A critical pathway for patients with acute chest pain and low risk for short-term adverse cardiac events: role of outpatient stress testing. Ann Emerg Med. 2006;47(5):427-435. PubMed
17. Lai C, Noeller TP, Schmidt K, King P, Emerman CL. Short-term risk after initial observation for chest pain. J Emerg Med. 2003;25(4):357-362. PubMed
18. Scheuermeyer FX, Innes G, Grafstein E, et al. Safety and efficiency of a chest pain diagnostic algorithm with selective outpatient stress testing for emergency department patients with potential ischemic chest pain. Ann Emerg Med. 2012;59(4):256-264 e253. PubMed
19. Safavi KC, Li SX, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. PubMed
20. Foy AJ, Liu G, Davidson WR, Jr., Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015; 175(3):428-436. PubMed
21. Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular testing and clinical outcomes in emergency department patients with chest pain. JAMA Intern Med. 2017;177(8):1175-1182. PubMed
22. Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does this patient with chest pain have acute coronary syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2015;314(18):1955-1965. PubMed
23. Hess EP, Hollander JE, Schaffer JT, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ. 2016;355:i6165. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Chest pain is one of the most common complaints among patients presenting to the emergency department. Moreover, at least 30% of patients who present with chest pain are admitted for observation, and >70% of those admitted with chest pain undergo cardiac stress testing (CST) during hospitalization. Several clinical risk prediction models have validated evaluation processes for managing patients with chest pain, helping to identify those at a low risk of major adverse cardiac events. Among these, the Thrombolysis in Myocardial Infarction or HEART score can identify patients safe to be discharged with outpatient CST within 72 h. It is unnecessary to hospitalize all low-risk patients for cardiac testing because it may expose them to needless risk and avoidable care costs, with little additional benefit.
CLINICAL SCENARIO
A 60-year-old man with a history of osteoarthritis and depression presented to our emergency department (ED) with a 1-month history of left-sided chest pain that was present both at rest and exertion. There were no aggravating or relieving factors for the pain and no associated shortness of breath, diaphoresis, nausea, or lightheadedness. He smoked a half pack of cigarettes daily for 5 years in his twenties. The patient was taking aspirin 81 mg daily and paroxetine 40 mg daily, which he had been taking for 10 years. There was a family history of coronary artery disease in his mother, father, and sister. On examination, he was afebrile, with a blood pressure of 138/78 mm Hg and a heart rate of 62 beats/min; he appeared well, with no abnormal cardiopulmonary findings. Investigation revealed a normal initial troponin I level (<0.034 mg/mL) and normal electrocardiogram (ECG) with normal sinus rhythm (75 beats/min), normal axis, no ST changes, and no Q waves. He was therefore admitted to the hospital for further evaluation.
BACKGROUND
Each year, >7 million patients visit ED for chest pain in the United States,1 with approximately 13% diagnosed with acute coronary syndromes (ACSs).2 Over 30% of patients who present to ED with chest pain are hospitalized for observation, symptom evaluation, and risk stratification.3 In 2012, the mean Medicare reimbursement cost was $1,741 for in-hospital observation,4 with up to 70% of admitted patients undergoing cardiac stress testing (CST) before discharge.5
WHY YOU MIGHT THINK HOSPITALIZATION IS HELPFUL FOR THE EVALUATION OF LOW-RISK CHEST PAIN
A scientific statement by the American Heart Association in 2010 recommended that patients considered to be at low risk for ACS after initial evaluation (based on presenting symptoms, past history, ECG findings, and initial cardiac biomarkers) should undergo CST within 72 h (preferably within 24 h) of presentation to provoke ischemia or detect anatomic coronary artery disease.6 Early exercise treadmill testing as part of an accelerated diagnostic pathway can also reduce the length of stays (LOS) in hospital and lower the medical costs.7 Moreover, when there is noncompliance or poor accessibility, failure to pursue early exercise testing in a hospital could result in a loss of patients to follow-up. Hospitalization for testing through accelerated diagnostic pathways may improve access to care and reduce clinical and legal risks associated with a major adverse cardiac event (MACE).
WHY HOSPITALIZATION FOR THE EVALUATION OF LOW-RISK CHEST PAIN IS UNNECESSARY FOR MANY PATIENTS
Clinical Risk Prediction Models
When a patient initially presents with chest pain, it should be determined if the symptoms are related to ACS or some other diagnosis. Hospitalization is required for patients with ACS but may not be for those without ACS and those with a low risk of inducible ischemia. Clinical risk scores and risk prediction models, such as the Thrombolysis in Myocardial Infarction (TIMI) and HEART scores, have been used in accelerated diagnostic protocols to determine a patient’s likelihood of having ACS. Several large trials of these clinical risk prediction models have validated the processes for evaluating patients with chest pain.
The TIMI risk score, the most well-known model, assesses risk based on the presence or absence of 7 characteristics (Appendix 1). It should be noted that the patient population studied for initial validation of this model comprised high-risk patients with unstable angina or non-ST elevation myocardial infarction who would benefit from early or urgent invasive therapy.8 In this population, TIMI scores of 0-1 are associated with low risk, with a 4.7% risk of ACS at 14 days.8 In another study of patients presenting to ED with undifferentiated chest pain and a TIMI score of zero, the risk of MACE at 30 days was approximately 2%.9
The HEART score is also used for patients presenting to ED with undifferentiated chest pain and assesses 5 separate variables scored 0–2 (Appendix 2). The original research gave a score of 2 to a troponin I level greater than twice the upper limit of the normal level,10 whereas a subsequent validation study gave a score of 2 to a troponin I or T level greater than or equal to 3 times the upper limit of the normal level.11 Patients are considered at low, intermediate, and high risk based on scores of 0–3, 4–6, and 7–10, respectively.10,11 Backus et al. performed a prospective randomized trial of 2388 patients who presented to ED with chest pain to validate the HEART score and compare it to the TIMI risk score. The HEART score performed better than the TIMI risk score in low-risk patients, with TIMI scores of 0-1 and HEART scores of 0–3 having a 6-week MACE risk of 2.8% and 1.7%, respectively.11
A HEART pathway was developed that combines the HEART score with serial troponin I assays assessed at the time of initial presentation and approximately 3 h later.12 Mahler et al. randomized 282 patients presenting to ED with chest pain to either the HEART pathway or conventional care. Patients with low-risk HEART scores and an abnormal troponin I level were admitted for cardiology consultation, whereas discharge was recommended for those with low scores and a normal troponin I level. Despite nearly 20% of the study cohort having a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting, approximately 40% of patients in the HEART pathway were identified as low risk, increasing early discharge rates by 21.3% and decreasing the average LOS by 12 h. No low-risk patient suffered a MACE within 30 days, and the HEART pathway had a sensitivity and a negative predictive value of approximately 99%.
Costs and Harms of Hospitalization for Cardiac Testing
Hospitalization carries measurable risks.13,14 Between 2008 and 2013, Weinstock et al. evaluated the outcom
Outpatient CST can be reliably and safely performed for patients with chest pain.16-18 There is no clear evidence that earlier CST leads to improved patient outcomes, and CST in the absence of acute ischemia (or ACS) increases the rates of angiography and revascularization without improvements in the rate of myocardial infarction.19-21 Given the costs of in-hospital observation4 and the dubious benefits of providing CST for patients with low-risk chest pain, admitting all patients with low-risk chest pain exposes them to costs and harms with little potential benefit.
WHEN HOSPITALIZATION MAY BE REASONABLE TO EVALUATE LOW-RISK CHEST PAIN
Patients presenting with chest pain with either dynamic ECG changes or an elevated troponin level require hospitalization for further ACS diagnosis and treatment. When ACS cannot be clearly diagnosed at the initial evaluation, healthcare providers should use clinical risk prediction models to stratify patients. Those deemed to be at an intermediate or high risk by these models should be hospitalized for further evaluation, as should those at low risk but for whom access to outpatient follow-up is difficult (eg, those without health insurance).
WHAT YOU SHOULD DO INSTEAD OF HOSPITALIZATION FOR LOW-RISK CHEST PAIN
A complete history and physical examination, along with ECG and cardiac biomarker testing, are required for all patients presenting with chest pain. Validated clinical risk prediction models should then be used to determine the likelihood of a cardiac event. Fanaroff et al. reported that low-risk HEART scores of 0–3 and TIMI scores of 0-1 gave positive likelihood ratios of 0.2 and 0.31, respectively.22 Using a pre-test probability of 13%, as reported by Bhuiya et al.,2 the likelihood of ACS or MACE within 6 weeks is 2.9% for patients with low-risk HEART scores and 4.4% for those with low-risk TIMI scores.22 These risk prediction models allow clinicians to provide a shared decision-making plan with the patient and discuss the risks and benefits of in-hospital versus outpatient cardiac testing, especially among patients with access to appropriate outpatient follow-up.23 Low-risk patients can be referred for outpatient testing within 72 h, reducing hospitalization-associated costs and harms.
RECOMMENDATIONS
- Patients presenting with chest pain should undergo a complete history taking and physical examination, as well as ECG and cardiac biomarker testing (eg, troponin I level at presentation and approximately 3 h later).
- Clinical risk prediction models, such as TIMI or HEART scores, should then be used to determine the risk of MACE.
- Patients at a low risk may be safely discharged with outpatient CST performed within 72 h.
- Patients at an intermediate or high risk of MACE should be hospitalized for further evaluation, as should those with low-risk chest pain who are unable to attend follow-up for outpatient CST within 72 h.
- Clinicians should provide a shared decision-making plan with each patient, taking care to discuss the risks and benefits of in-hospital versus outpatient CST.
CONCLUSION
The risk of MACE should be assessed in all patients presenting to ED with low-risk chest pain to avoid unnecessary hospitalization that exposes them to potential costs and harms with few additional benefits. If the risk scoring system was applied to the patient described in our original clinical scenario, he would have had a HEART score of 3 (ie, 1 point for a moderately suspicious history, 1 point for the age of 60 years, and 1 point for a positive family history) and a TIMI score of 1 (ie, 1 point for aspirin use within past 7 days). Therefore, he could be stratified as having a low-risk presentation. With a second negative troponin I test at 3 h, discharge from ED with timely outpatient CST within 72 h would be an appropriate management strategy.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Chest pain is one of the most common complaints among patients presenting to the emergency department. Moreover, at least 30% of patients who present with chest pain are admitted for observation, and >70% of those admitted with chest pain undergo cardiac stress testing (CST) during hospitalization. Several clinical risk prediction models have validated evaluation processes for managing patients with chest pain, helping to identify those at a low risk of major adverse cardiac events. Among these, the Thrombolysis in Myocardial Infarction or HEART score can identify patients safe to be discharged with outpatient CST within 72 h. It is unnecessary to hospitalize all low-risk patients for cardiac testing because it may expose them to needless risk and avoidable care costs, with little additional benefit.
CLINICAL SCENARIO
A 60-year-old man with a history of osteoarthritis and depression presented to our emergency department (ED) with a 1-month history of left-sided chest pain that was present both at rest and exertion. There were no aggravating or relieving factors for the pain and no associated shortness of breath, diaphoresis, nausea, or lightheadedness. He smoked a half pack of cigarettes daily for 5 years in his twenties. The patient was taking aspirin 81 mg daily and paroxetine 40 mg daily, which he had been taking for 10 years. There was a family history of coronary artery disease in his mother, father, and sister. On examination, he was afebrile, with a blood pressure of 138/78 mm Hg and a heart rate of 62 beats/min; he appeared well, with no abnormal cardiopulmonary findings. Investigation revealed a normal initial troponin I level (<0.034 mg/mL) and normal electrocardiogram (ECG) with normal sinus rhythm (75 beats/min), normal axis, no ST changes, and no Q waves. He was therefore admitted to the hospital for further evaluation.
BACKGROUND
Each year, >7 million patients visit ED for chest pain in the United States,1 with approximately 13% diagnosed with acute coronary syndromes (ACSs).2 Over 30% of patients who present to ED with chest pain are hospitalized for observation, symptom evaluation, and risk stratification.3 In 2012, the mean Medicare reimbursement cost was $1,741 for in-hospital observation,4 with up to 70% of admitted patients undergoing cardiac stress testing (CST) before discharge.5
WHY YOU MIGHT THINK HOSPITALIZATION IS HELPFUL FOR THE EVALUATION OF LOW-RISK CHEST PAIN
A scientific statement by the American Heart Association in 2010 recommended that patients considered to be at low risk for ACS after initial evaluation (based on presenting symptoms, past history, ECG findings, and initial cardiac biomarkers) should undergo CST within 72 h (preferably within 24 h) of presentation to provoke ischemia or detect anatomic coronary artery disease.6 Early exercise treadmill testing as part of an accelerated diagnostic pathway can also reduce the length of stays (LOS) in hospital and lower the medical costs.7 Moreover, when there is noncompliance or poor accessibility, failure to pursue early exercise testing in a hospital could result in a loss of patients to follow-up. Hospitalization for testing through accelerated diagnostic pathways may improve access to care and reduce clinical and legal risks associated with a major adverse cardiac event (MACE).
WHY HOSPITALIZATION FOR THE EVALUATION OF LOW-RISK CHEST PAIN IS UNNECESSARY FOR MANY PATIENTS
Clinical Risk Prediction Models
When a patient initially presents with chest pain, it should be determined if the symptoms are related to ACS or some other diagnosis. Hospitalization is required for patients with ACS but may not be for those without ACS and those with a low risk of inducible ischemia. Clinical risk scores and risk prediction models, such as the Thrombolysis in Myocardial Infarction (TIMI) and HEART scores, have been used in accelerated diagnostic protocols to determine a patient’s likelihood of having ACS. Several large trials of these clinical risk prediction models have validated the processes for evaluating patients with chest pain.
The TIMI risk score, the most well-known model, assesses risk based on the presence or absence of 7 characteristics (Appendix 1). It should be noted that the patient population studied for initial validation of this model comprised high-risk patients with unstable angina or non-ST elevation myocardial infarction who would benefit from early or urgent invasive therapy.8 In this population, TIMI scores of 0-1 are associated with low risk, with a 4.7% risk of ACS at 14 days.8 In another study of patients presenting to ED with undifferentiated chest pain and a TIMI score of zero, the risk of MACE at 30 days was approximately 2%.9
The HEART score is also used for patients presenting to ED with undifferentiated chest pain and assesses 5 separate variables scored 0–2 (Appendix 2). The original research gave a score of 2 to a troponin I level greater than twice the upper limit of the normal level,10 whereas a subsequent validation study gave a score of 2 to a troponin I or T level greater than or equal to 3 times the upper limit of the normal level.11 Patients are considered at low, intermediate, and high risk based on scores of 0–3, 4–6, and 7–10, respectively.10,11 Backus et al. performed a prospective randomized trial of 2388 patients who presented to ED with chest pain to validate the HEART score and compare it to the TIMI risk score. The HEART score performed better than the TIMI risk score in low-risk patients, with TIMI scores of 0-1 and HEART scores of 0–3 having a 6-week MACE risk of 2.8% and 1.7%, respectively.11
A HEART pathway was developed that combines the HEART score with serial troponin I assays assessed at the time of initial presentation and approximately 3 h later.12 Mahler et al. randomized 282 patients presenting to ED with chest pain to either the HEART pathway or conventional care. Patients with low-risk HEART scores and an abnormal troponin I level were admitted for cardiology consultation, whereas discharge was recommended for those with low scores and a normal troponin I level. Despite nearly 20% of the study cohort having a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting, approximately 40% of patients in the HEART pathway were identified as low risk, increasing early discharge rates by 21.3% and decreasing the average LOS by 12 h. No low-risk patient suffered a MACE within 30 days, and the HEART pathway had a sensitivity and a negative predictive value of approximately 99%.
Costs and Harms of Hospitalization for Cardiac Testing
Hospitalization carries measurable risks.13,14 Between 2008 and 2013, Weinstock et al. evaluated the outcom
Outpatient CST can be reliably and safely performed for patients with chest pain.16-18 There is no clear evidence that earlier CST leads to improved patient outcomes, and CST in the absence of acute ischemia (or ACS) increases the rates of angiography and revascularization without improvements in the rate of myocardial infarction.19-21 Given the costs of in-hospital observation4 and the dubious benefits of providing CST for patients with low-risk chest pain, admitting all patients with low-risk chest pain exposes them to costs and harms with little potential benefit.
WHEN HOSPITALIZATION MAY BE REASONABLE TO EVALUATE LOW-RISK CHEST PAIN
Patients presenting with chest pain with either dynamic ECG changes or an elevated troponin level require hospitalization for further ACS diagnosis and treatment. When ACS cannot be clearly diagnosed at the initial evaluation, healthcare providers should use clinical risk prediction models to stratify patients. Those deemed to be at an intermediate or high risk by these models should be hospitalized for further evaluation, as should those at low risk but for whom access to outpatient follow-up is difficult (eg, those without health insurance).
WHAT YOU SHOULD DO INSTEAD OF HOSPITALIZATION FOR LOW-RISK CHEST PAIN
A complete history and physical examination, along with ECG and cardiac biomarker testing, are required for all patients presenting with chest pain. Validated clinical risk prediction models should then be used to determine the likelihood of a cardiac event. Fanaroff et al. reported that low-risk HEART scores of 0–3 and TIMI scores of 0-1 gave positive likelihood ratios of 0.2 and 0.31, respectively.22 Using a pre-test probability of 13%, as reported by Bhuiya et al.,2 the likelihood of ACS or MACE within 6 weeks is 2.9% for patients with low-risk HEART scores and 4.4% for those with low-risk TIMI scores.22 These risk prediction models allow clinicians to provide a shared decision-making plan with the patient and discuss the risks and benefits of in-hospital versus outpatient cardiac testing, especially among patients with access to appropriate outpatient follow-up.23 Low-risk patients can be referred for outpatient testing within 72 h, reducing hospitalization-associated costs and harms.
RECOMMENDATIONS
- Patients presenting with chest pain should undergo a complete history taking and physical examination, as well as ECG and cardiac biomarker testing (eg, troponin I level at presentation and approximately 3 h later).
- Clinical risk prediction models, such as TIMI or HEART scores, should then be used to determine the risk of MACE.
- Patients at a low risk may be safely discharged with outpatient CST performed within 72 h.
- Patients at an intermediate or high risk of MACE should be hospitalized for further evaluation, as should those with low-risk chest pain who are unable to attend follow-up for outpatient CST within 72 h.
- Clinicians should provide a shared decision-making plan with each patient, taking care to discuss the risks and benefits of in-hospital versus outpatient CST.
CONCLUSION
The risk of MACE should be assessed in all patients presenting to ED with low-risk chest pain to avoid unnecessary hospitalization that exposes them to potential costs and harms with few additional benefits. If the risk scoring system was applied to the patient described in our original clinical scenario, he would have had a HEART score of 3 (ie, 1 point for a moderately suspicious history, 1 point for the age of 60 years, and 1 point for a positive family history) and a TIMI score of 1 (ie, 1 point for aspirin use within past 7 days). Therefore, he could be stratified as having a low-risk presentation. With a second negative troponin I test at 3 h, discharge from ED with timely outpatient CST within 72 h would be an appropriate management strategy.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
1. Centers for Disease Control. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. 2011. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed October 7, 2015.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;(43):1-8. PubMed
3. Cotterill PG, Deb P, Shrank WH, Pines JM. Variation in chest pain emergency department admission rates and acute myocardial infarction and death within 30 days in the Medicare population. Acad Emerg Med. 2015;22(8):955-964. PubMed
4. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries, OEI-02-12-00040. 2013. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed May 15, 2017.
5. Penumetsa SC, Mallidi J, Friderici JL, Hiser W, Rothberg MB. Outcomes of patients admitted for observation of chest pain. Arch Inter Med. 2012;172(11):873-877. PubMed
6. Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756-1776. PubMed
7. Hutter AM, Jr., Amsterdam EA, Jaffe AS. 31st Bethesda Conference. Emergency Cardiac Care. Task force 2: Acute coronary syndromes: Section 2B--Chest discomfort evaluation in the hospital. J Am Coll Cardiol. 2000;35(4):853-862. PubMed
8. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835-842. PubMed
9. Pollack CV, Jr., Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13(1):13-18. PubMed
10. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008; 16(6):191-196. PubMed
11. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. PubMed
12. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203. PubMed
13. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Inter Med. 2003;138(3):161-167. PubMed
14. James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122-128. PubMed
15. Weinstock MB, Weingart S, Orth F, et al. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med. 2015;175(7):1207-1212. PubMed
16. Meyer MC, Mooney RP, Sekera AK. A critical pathway for patients with acute chest pain and low risk for short-term adverse cardiac events: role of outpatient stress testing. Ann Emerg Med. 2006;47(5):427-435. PubMed
17. Lai C, Noeller TP, Schmidt K, King P, Emerman CL. Short-term risk after initial observation for chest pain. J Emerg Med. 2003;25(4):357-362. PubMed
18. Scheuermeyer FX, Innes G, Grafstein E, et al. Safety and efficiency of a chest pain diagnostic algorithm with selective outpatient stress testing for emergency department patients with potential ischemic chest pain. Ann Emerg Med. 2012;59(4):256-264 e253. PubMed
19. Safavi KC, Li SX, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. PubMed
20. Foy AJ, Liu G, Davidson WR, Jr., Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015; 175(3):428-436. PubMed
21. Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular testing and clinical outcomes in emergency department patients with chest pain. JAMA Intern Med. 2017;177(8):1175-1182. PubMed
22. Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does this patient with chest pain have acute coronary syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2015;314(18):1955-1965. PubMed
23. Hess EP, Hollander JE, Schaffer JT, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ. 2016;355:i6165. PubMed
1. Centers for Disease Control. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. 2011. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed October 7, 2015.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;(43):1-8. PubMed
3. Cotterill PG, Deb P, Shrank WH, Pines JM. Variation in chest pain emergency department admission rates and acute myocardial infarction and death within 30 days in the Medicare population. Acad Emerg Med. 2015;22(8):955-964. PubMed
4. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries, OEI-02-12-00040. 2013. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed May 15, 2017.
5. Penumetsa SC, Mallidi J, Friderici JL, Hiser W, Rothberg MB. Outcomes of patients admitted for observation of chest pain. Arch Inter Med. 2012;172(11):873-877. PubMed
6. Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122(17):1756-1776. PubMed
7. Hutter AM, Jr., Amsterdam EA, Jaffe AS. 31st Bethesda Conference. Emergency Cardiac Care. Task force 2: Acute coronary syndromes: Section 2B--Chest discomfort evaluation in the hospital. J Am Coll Cardiol. 2000;35(4):853-862. PubMed
8. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835-842. PubMed
9. Pollack CV, Jr., Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13(1):13-18. PubMed
10. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008; 16(6):191-196. PubMed
11. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. PubMed
12. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203. PubMed
13. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Inter Med. 2003;138(3):161-167. PubMed
14. James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122-128. PubMed
15. Weinstock MB, Weingart S, Orth F, et al. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med. 2015;175(7):1207-1212. PubMed
16. Meyer MC, Mooney RP, Sekera AK. A critical pathway for patients with acute chest pain and low risk for short-term adverse cardiac events: role of outpatient stress testing. Ann Emerg Med. 2006;47(5):427-435. PubMed
17. Lai C, Noeller TP, Schmidt K, King P, Emerman CL. Short-term risk after initial observation for chest pain. J Emerg Med. 2003;25(4):357-362. PubMed
18. Scheuermeyer FX, Innes G, Grafstein E, et al. Safety and efficiency of a chest pain diagnostic algorithm with selective outpatient stress testing for emergency department patients with potential ischemic chest pain. Ann Emerg Med. 2012;59(4):256-264 e253. PubMed
19. Safavi KC, Li SX, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546-553. PubMed
20. Foy AJ, Liu G, Davidson WR, Jr., Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015; 175(3):428-436. PubMed
21. Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular testing and clinical outcomes in emergency department patients with chest pain. JAMA Intern Med. 2017;177(8):1175-1182. PubMed
22. Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does this patient with chest pain have acute coronary syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2015;314(18):1955-1965. PubMed
23. Hess EP, Hollander JE, Schaffer JT, et al. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ. 2016;355:i6165. PubMed
© 2018 Society of Hospital Medicine
Numeracy, Health Literacy, Cognition, and 30-Day Readmissions among Patients with Heart Failure
Most studies to identify risk factors for readmission among patients with heart failure (HF) have focused on demographic and clinical characteristics.1,2 Although easy to extract from administrative databases, this approach fails to capture the complex psychosocial and cognitive factors that influence the ability of HF patients to manage their disease in the postdischarge period, as depicted in the framework by Meyers et al.3 (2014). To date, studies have found low health literacy, decreased social support, and cognitive impairment to be associated with health behaviors and outcomes among HF patients, including decreased self-care,4 low HF-specific knowledge,5 medication nonadherence,6 hospitalizations,7 and mortality.8-10 Less, however, is known about the effect of numeracy on HF outcomes, such as 30-day readmission.
Numeracy, or quantitative literacy, refers to the ability to access, understand, and apply numerical data to health-related decisions.11 It is estimated that 110 million people in the United States have limited numeracy skills.12 Low numeracy is a risk factor for poor glycemic control among patients with diabetes,13 medication adherence in HIV/AIDS,14 and worse blood pressure control in hypertensives.15 Much like these conditions, HF requires that patients understand, use, and act on numerical information. Maintaining a low-salt diet, monitoring weight, adjusting diuretic doses, and measuring blood pressure are tasks that HF patients are asked to perform on a daily or near-daily basis. These tasks are particularly important in the posthospitalization period and could be complicated by medication changes, which might create additional challenges for patients with inadequate numeracy. Additionally, cognitive impairment, which is a highly prevalent comorbid condition among adults with HF,16,17 might impose additional barriers for those with inadequate numeracy who do not have adequate social support. However, to date, numeracy in the context of HF has not been well described.
Herein, we examined the effects of numeracy, alongside health literacy and cognition, on 30-day readmission risk among patients hospitalized for acute decompensated HF (ADHF).
METHODS
Study Design
The Vanderbilt Inpatient Cohort Study (VICS) is a prospective observational study of patients admitted with cardiovascular disease to Vanderbilt University Medical Center (VUMC), an academic tertiary care hospital. VICS was designed to investigate the impact of social determinants of health on postdischarge health outcomes. A detailed description of the study rationale, design, and methods is described elsewhere.3
Briefly, participants completed a baseline interview while hospitalized, and follow-up phone calls were conducted within 1 week of discharge, at 30 days, and at 90 days. At 30 and 90 days postdischarge, healthcare utilization was ascertained by review of medical records and patient report. Clinical data about the index hospitalization were also abstracted. The Vanderbilt University Institutional Review Board approved the study.
Study Population
Patients hospitalized from 2011 to 2015 with a likely diagnosis of acute coronary syndrome and/or ADHF, as determined by a physician’s review of the medical record, were identified as potentially eligible. Research assistants assessed these patients for the presence of the following exclusion criteria: less than 18 years of age, non-English speaking, unstable psychiatric illness, a low likelihood of follow-up (eg, no reliable telephone number), on hospice, or otherwise too ill to complete an interview. Additionally, those with severe cognitive impairment, as assessed from the medical record (such as seeing a note describing dementia), and those with delirium, as assessed by the brief confusion assessment method, were excluded from enrollment in the study.18,19 Those who died before discharge or during the 30-day follow-up period were excluded. For this analysis, we restricted our sample to only include participants who were hospitalized for ADHF.
Outcome Measure: 30-Day Readmission
The main outcome was all-cause readmission to any hospital within 30 days of discharge, as determined by patient interview, review of electronic medical records from VUMC, and review of outside hospital records.
Main Exposures: Numeracy, Health Literacy, and Cognitive Impairment
Numeracy was assessed with a 3-item version of the Subjective Numeracy Scale (SNS-3), which quantifies the patients perceived quantitative abilities.20 Other authors have shown that the SNS-3 has a correlation coefficient of 0.88 with the full-length SNS-8 and a Cronbach’s alpha of 0.78.20-22 The SNS-3 is reported as the mean on a scale from 1 to 6, with higher scores reflecting higher numeracy.
Subjective health literacy was assessed by using the 3-item Brief Health Literacy Screen (BHLS).23 Scores range from 3 to 15, with higher scores reflecting higher literacy. Objective health literacy was assessed with the short form of the Test of Functional Health Literacy in Adults (sTOFHLA).24,25 Scores may be categorized as inadequate (0-16), marginal (17-22), or adequate (23-36).
We assessed cognition by using the 10-item Short Portable Mental Status Questionnaire (SPMSQ).26 The SPMSQ, which describes a person’s capacity for memory, structured thought, and orientation, has been validated and has demonstrated good reliability and validity.27 Scores of 0 were considered to reflect intact cognition, and scores of 1 or more were considered to reflect any cognitive impairment, a scoring approach employed by other authors.28 We used this approach, rather than the traditional scoring system developed by Pfeiffer et al.26 (1975), because it would be the most sensitive to detect any cognitive impairment in the VICS cohort, which excluded those with severe cognition impairment, dementia, and delirium.
Covariates
During the hospitalization, participants completed an in-person interviewer-administered baseline assessment composed of demographic information, including age, self-reported race (white and nonwhite), educational attainment, home status (married, not married and living with someone, not married and living alone), and household income.
Clinical and diagnostic characteristics abstracted from the medical record included a medical history of HF, HF subtype (classified by left ventricular ejection fraction [LVEF]), coronary artery disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), and comorbidity burden as summarized by the van Walraven-Elixhauser score.29,30 Depressive symptoms were assessed during the 2 weeks prior to the hospitalization by using the first 8 items of the Patient Health Questionnaire.31 Scores ranged from 0 to 24, with higher scores reflecting more severe depressive symptoms. Laboratory values included estimated glomerular filtration rate (eGFR), hemoglobin (g/dl), sodium (mg/L), and brain natriuretic peptide (BNP) (pg/ml) from the last laboratory draw before discharge. Smoking status was also assessed (current and former/nonsmokers).
Hospitalization characteristics included length of stay in days, number of prior admissions in the last year, and transfer to the intensive care unit during the index admission.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics. The Kruskal-Wallis test and the Pearson χ2 test were used to determine the association between patient characteristics and levels of numeracy, literacy, and cognition separately. The unadjusted relationship between patient characteristics and 30-day readmission was assessed by using Wilcoxon rank sums tests for continuous variables and Pearson χ2 tests for categorical variables. In addition, a correlation matrix was performed to assess the correlations between numeracy, health literacy, and cognition (supplementary Figure 1).
To examine the association between numeracy, health literacy, and cognition and 30-day readmissions, a series of multivariable Poisson (log-linear) regression models were fit.32 Like other studies, numeracy, health literacy, and cognition were examined as categorical and continuous measures in models.33 Each model was modified with a sandwich estimator for robust standard errors. Log-linear models were chosen over logistic regression models for ease of interpretation because (exponentiated) parameters correspond to risk ratios (RRs) as opposed to odds ratios. Furthermore, the fitting challenges associated with log-linear models when predicted probabilities are near 0 or 1 were not present in these analyses. Redundancy analyses were conducted to ensure that independent variables were not highly correlated with a linear combination of the other independent variables. To avoid case-wise deletion of records with missing covariates, we employed multiple imputation with 10 imputation samples by using predictive mean matching.34,35 All analyses were conducted in R version 3.1.2 (The R Foundation, Vienna, Austria).36
RESULTS
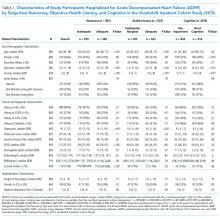
Overall, 883 patients were included in this analysis (supplementary Figure 2). Of the 883 participants, 46% were female and 76% were white (Table 1). Their median age was 60 years (interdecile range [IDR] 39-78) and the median educational attainment was 13.5 years (IDR 11-18).
DISCUSSION
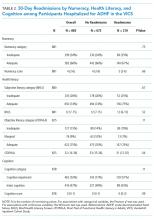
This is the first study to examine the effect of numeracy alongside literacy and cognition on 30-day readmission risk among patients hospitalized with ADHF. Overall, we found that 33.9% of participants had inadequate numeracy skills, and 24.6% had inadequate or marginal health literacy. In unadjusted and adjusted models, numeracy was not associated with 30-day readmission. Although (objective) low health literacy was associated with 30-day readmission in unadjusted models, it was not in adjusted models. Additionally, though 53% of participants had any cognitive impairment, readmission did not differ significantly by this factor. Taken together, these findings suggest that other factors may be greater determinants of 30-day readmissions among patients hospitalized for ADHF.
Only 1 other study has examined the effect of numeracy on readmission risk among patients hospitalized for HF. In this multicenter prospective study, McNaughton et al.37 found low numeracy to be associated with higher odds of recidivism to the emergency department (ED) or hospital within 30 days. Our findings may differ from theirs for a few reasons. First, their study had a significantly higher percentage of individuals with low numeracy (55%) compared with ours (33.9%). This may be because they did not exclude individuals with severe cognitive impairment, and their patient population was of lower socioeconomic status (SES) than ours. Low SES is associated with higher 30-day readmissions among HF patients1,10 throughout the literature, and low numeracy is associated with low SES in other diseases.13,38,39 Finally, they studied recidivism, which was defined as any unplanned return to the ED or hospital within 30 days of the index ED visit for acute HF. We only focused on 30-day readmissions, which also may explain why our results differed.
We found that health literacy was not associated with 30-day readmissions, which is consistent with the literature. Although an association between health literacy and mortality exists among adults with HF, several studies have not found an association between health literacy and 30- and 90-day readmission among adults hospitalized for HF.8,9,40 Although we found an association between objective health literacy and 30-day readmission in unadjusted analyses, we did not find one in the multivariable model. This, along with our numeracy finding, suggests that numeracy and literacy may not be driving the 30-day readmission risk among patients hospitalized with ADHF.
We examined cognition alongside numeracy and literacy because it is a prevalent condition among HF patients and because it is associated with adverse outcomes among patients with HF, including readmission.41,42 Studies have shown that HF preferentially affects certain cognitive domains,43 some of which are vital to HF self-care activities. We found that 53% of patients had any cognitive impairment, which is consistent with the literature of adults hospitalized for ADHF.44,45 Cognitive impairment was not, however, associated with 30-day readmissions. There may be a couple reasons for this. First, we measured cognitive impairment with the SPMSQ, which, although widely used and well-validated, does not assess executive function, the domain most commonly affected in HF patients with cognitive impairment.46 Second, patients with severe cognitive impairment and those with delirium were excluded from this study, which may have limited our ability to detect differences in readmission by this factor.
As in prior studies, we found that a history of DM and more hospitalizations in the prior year were independently associated with 30-day readmissions in fully adjusted models. Like other studies, in adjusted models, we found that LVEF and a history of HF were not independently associated with 30-day readmission.47-49 This, however, is not surprising because recent studies have shown that, although HF patients are at risk for multiple hospitalizations, early readmission after a hospitalization for ADHF specifically is often because of reasons unrelated to HF or a non-cardiovascular cause in general.50,51
Although a negative study, several important themes emerged. First, while we were able to assess numeracy, health literacy, and cognition, none of these measures were HF-specific. It is possible that we did not see an effect on readmission because our instruments failed to assess domains specific to HF, such as monitoring weight changes, following a low-salt diet, and interpreting blood pressure. Currently, however, no HF-specific objective numeracy measure exists. With respect to health literacy, only 1 HF-specific measure exists,52 although it was only recently developed and validated. Second, while numeracy may not be a driving influence of all-cause 30-day readmissions, it may be associated with other health behaviors and quality metrics that we did not examine here, such as self-care, medication adherence, and HF-specific readmissions. Third, it is likely that the progression of HF itself, as well as the clinical management of patients following discharge, contribute significantly to 30-day readmissions. Increased attention to predischarge processes for HF patients occurred at VUMC during the study period; close follow-up and evidence-directed therapies may have mitigated some of the expected associations. Finally, we were not able to assess numeracy of participants’ primary caregivers who may help patients at home, especially postdischarge. Though a number of studies have examined the role of family caregivers in the management of HF,53,54 none have examined numeracy levels of caregivers in the context of HF, and this may be worth doing in future studies.
Overall, our study has several strengths. The size of the cohort is large and there were high response rates during the follow-up period. Unlike other HF readmission studies, VICS accounts for readmissions to outside hospitals. Approximately 35% of all hospitalizations in VICS are to outside facilities. Thus, the ascertainment of readmissions to hospitals other than Vanderbilt is more comprehensive than if readmissions to VUMC were only considered. We were able to include a number of clinical comorbidities, laboratory and diagnostic tests from the index admission, and hospitalization characteristics in our analyses. Finally, we performed additional analyses to investigate the correlation between numeracy, literacy, and cognition; ultimately, we found that the majority of these correlations were weak, which supports our ability to study them simultaneously among VICS participants.
Nonetheless, we note some limitations. Although we captured readmissions to outside hospitals, the study took place at a single referral center in Tennessee. Though patients were diverse in age and comorbidities, they were mostly white and of higher SES. Finally, we used home status as a proxy for social support, which may underestimate the support that home care workers provide.
In conclusion, in this prospective longitudinal study of adults hospitalized with ADHF, inadequate numeracy was present in more than a third of patients, and low health literacy was present in roughly a quarter of patients. Neither numeracy nor health literacy, however, were associated with 30-day readmissions in adjusted analyses. Any cognitive impairment, although present in roughly one-half of patients, was not associated with 30-day readmission either. Our findings suggest that other influences may play a more dominant role in determining 30-day readmission rates in patients hospitalized for ADHF than inadequate numeracy, low health literacy, or cognitive impairment as assessed here.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute (R01 HL109388) and in part by the National Center for Advancing Translational Sciences (UL1 TR000445-06). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health. The authors’ funding sources did not participate in the planning, collection, analysis, or interpretation of data or in the decision to submit for publication. Dr. Sterling is supported by T32HS000066 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Mixon has a VA Health Services Research and Development Service Career Development Award at the Tennessee Valley Healthcare System, Department of Veterans Affairs (CDA 12-168). This material was presented at the Society of General Internal Medicine Annual Meeting on April 20, 2017, in Washington, DC.
Disclosure
Dr. Kripalani reports personal fees from Verustat, personal fees from SAI Interactive, and equity from Bioscape Digital, all outside of the submitted work. Dr. Rothman and Dr. Wallston report personal fees from EdLogics outside of the submitted work. All of the other authors have nothing to disclose
1. Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch of Intern Med. 2008;168(13):1371-1386. PubMed
33. Bohannon AD, Fillenbaum GG, Pieper CF, Hanlon JT, Blazer DG. Relationship of race/ethnicity and blood pressure to change in cognitive function. J Am Geriatr Soc. 2002;50(3):424-429. PubMed
38. Abdel-Kader K, Dew MA, Bhatnagar M, et al. Numeracy Skills in CKD: Correlates and Outcomes. Clin J Am Soc Nephrol. 2010;5(9):1566-1573. PubMed
Most studies to identify risk factors for readmission among patients with heart failure (HF) have focused on demographic and clinical characteristics.1,2 Although easy to extract from administrative databases, this approach fails to capture the complex psychosocial and cognitive factors that influence the ability of HF patients to manage their disease in the postdischarge period, as depicted in the framework by Meyers et al.3 (2014). To date, studies have found low health literacy, decreased social support, and cognitive impairment to be associated with health behaviors and outcomes among HF patients, including decreased self-care,4 low HF-specific knowledge,5 medication nonadherence,6 hospitalizations,7 and mortality.8-10 Less, however, is known about the effect of numeracy on HF outcomes, such as 30-day readmission.
Numeracy, or quantitative literacy, refers to the ability to access, understand, and apply numerical data to health-related decisions.11 It is estimated that 110 million people in the United States have limited numeracy skills.12 Low numeracy is a risk factor for poor glycemic control among patients with diabetes,13 medication adherence in HIV/AIDS,14 and worse blood pressure control in hypertensives.15 Much like these conditions, HF requires that patients understand, use, and act on numerical information. Maintaining a low-salt diet, monitoring weight, adjusting diuretic doses, and measuring blood pressure are tasks that HF patients are asked to perform on a daily or near-daily basis. These tasks are particularly important in the posthospitalization period and could be complicated by medication changes, which might create additional challenges for patients with inadequate numeracy. Additionally, cognitive impairment, which is a highly prevalent comorbid condition among adults with HF,16,17 might impose additional barriers for those with inadequate numeracy who do not have adequate social support. However, to date, numeracy in the context of HF has not been well described.
Herein, we examined the effects of numeracy, alongside health literacy and cognition, on 30-day readmission risk among patients hospitalized for acute decompensated HF (ADHF).
METHODS
Study Design
The Vanderbilt Inpatient Cohort Study (VICS) is a prospective observational study of patients admitted with cardiovascular disease to Vanderbilt University Medical Center (VUMC), an academic tertiary care hospital. VICS was designed to investigate the impact of social determinants of health on postdischarge health outcomes. A detailed description of the study rationale, design, and methods is described elsewhere.3
Briefly, participants completed a baseline interview while hospitalized, and follow-up phone calls were conducted within 1 week of discharge, at 30 days, and at 90 days. At 30 and 90 days postdischarge, healthcare utilization was ascertained by review of medical records and patient report. Clinical data about the index hospitalization were also abstracted. The Vanderbilt University Institutional Review Board approved the study.
Study Population
Patients hospitalized from 2011 to 2015 with a likely diagnosis of acute coronary syndrome and/or ADHF, as determined by a physician’s review of the medical record, were identified as potentially eligible. Research assistants assessed these patients for the presence of the following exclusion criteria: less than 18 years of age, non-English speaking, unstable psychiatric illness, a low likelihood of follow-up (eg, no reliable telephone number), on hospice, or otherwise too ill to complete an interview. Additionally, those with severe cognitive impairment, as assessed from the medical record (such as seeing a note describing dementia), and those with delirium, as assessed by the brief confusion assessment method, were excluded from enrollment in the study.18,19 Those who died before discharge or during the 30-day follow-up period were excluded. For this analysis, we restricted our sample to only include participants who were hospitalized for ADHF.
Outcome Measure: 30-Day Readmission
The main outcome was all-cause readmission to any hospital within 30 days of discharge, as determined by patient interview, review of electronic medical records from VUMC, and review of outside hospital records.
Main Exposures: Numeracy, Health Literacy, and Cognitive Impairment
Numeracy was assessed with a 3-item version of the Subjective Numeracy Scale (SNS-3), which quantifies the patients perceived quantitative abilities.20 Other authors have shown that the SNS-3 has a correlation coefficient of 0.88 with the full-length SNS-8 and a Cronbach’s alpha of 0.78.20-22 The SNS-3 is reported as the mean on a scale from 1 to 6, with higher scores reflecting higher numeracy.
Subjective health literacy was assessed by using the 3-item Brief Health Literacy Screen (BHLS).23 Scores range from 3 to 15, with higher scores reflecting higher literacy. Objective health literacy was assessed with the short form of the Test of Functional Health Literacy in Adults (sTOFHLA).24,25 Scores may be categorized as inadequate (0-16), marginal (17-22), or adequate (23-36).
We assessed cognition by using the 10-item Short Portable Mental Status Questionnaire (SPMSQ).26 The SPMSQ, which describes a person’s capacity for memory, structured thought, and orientation, has been validated and has demonstrated good reliability and validity.27 Scores of 0 were considered to reflect intact cognition, and scores of 1 or more were considered to reflect any cognitive impairment, a scoring approach employed by other authors.28 We used this approach, rather than the traditional scoring system developed by Pfeiffer et al.26 (1975), because it would be the most sensitive to detect any cognitive impairment in the VICS cohort, which excluded those with severe cognition impairment, dementia, and delirium.
Covariates
During the hospitalization, participants completed an in-person interviewer-administered baseline assessment composed of demographic information, including age, self-reported race (white and nonwhite), educational attainment, home status (married, not married and living with someone, not married and living alone), and household income.
Clinical and diagnostic characteristics abstracted from the medical record included a medical history of HF, HF subtype (classified by left ventricular ejection fraction [LVEF]), coronary artery disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), and comorbidity burden as summarized by the van Walraven-Elixhauser score.29,30 Depressive symptoms were assessed during the 2 weeks prior to the hospitalization by using the first 8 items of the Patient Health Questionnaire.31 Scores ranged from 0 to 24, with higher scores reflecting more severe depressive symptoms. Laboratory values included estimated glomerular filtration rate (eGFR), hemoglobin (g/dl), sodium (mg/L), and brain natriuretic peptide (BNP) (pg/ml) from the last laboratory draw before discharge. Smoking status was also assessed (current and former/nonsmokers).
Hospitalization characteristics included length of stay in days, number of prior admissions in the last year, and transfer to the intensive care unit during the index admission.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics. The Kruskal-Wallis test and the Pearson χ2 test were used to determine the association between patient characteristics and levels of numeracy, literacy, and cognition separately. The unadjusted relationship between patient characteristics and 30-day readmission was assessed by using Wilcoxon rank sums tests for continuous variables and Pearson χ2 tests for categorical variables. In addition, a correlation matrix was performed to assess the correlations between numeracy, health literacy, and cognition (supplementary Figure 1).
To examine the association between numeracy, health literacy, and cognition and 30-day readmissions, a series of multivariable Poisson (log-linear) regression models were fit.32 Like other studies, numeracy, health literacy, and cognition were examined as categorical and continuous measures in models.33 Each model was modified with a sandwich estimator for robust standard errors. Log-linear models were chosen over logistic regression models for ease of interpretation because (exponentiated) parameters correspond to risk ratios (RRs) as opposed to odds ratios. Furthermore, the fitting challenges associated with log-linear models when predicted probabilities are near 0 or 1 were not present in these analyses. Redundancy analyses were conducted to ensure that independent variables were not highly correlated with a linear combination of the other independent variables. To avoid case-wise deletion of records with missing covariates, we employed multiple imputation with 10 imputation samples by using predictive mean matching.34,35 All analyses were conducted in R version 3.1.2 (The R Foundation, Vienna, Austria).36
RESULTS
Overall, 883 patients were included in this analysis (supplementary Figure 2). Of the 883 participants, 46% were female and 76% were white (Table 1). Their median age was 60 years (interdecile range [IDR] 39-78) and the median educational attainment was 13.5 years (IDR 11-18).
DISCUSSION
This is the first study to examine the effect of numeracy alongside literacy and cognition on 30-day readmission risk among patients hospitalized with ADHF. Overall, we found that 33.9% of participants had inadequate numeracy skills, and 24.6% had inadequate or marginal health literacy. In unadjusted and adjusted models, numeracy was not associated with 30-day readmission. Although (objective) low health literacy was associated with 30-day readmission in unadjusted models, it was not in adjusted models. Additionally, though 53% of participants had any cognitive impairment, readmission did not differ significantly by this factor. Taken together, these findings suggest that other factors may be greater determinants of 30-day readmissions among patients hospitalized for ADHF.
Only 1 other study has examined the effect of numeracy on readmission risk among patients hospitalized for HF. In this multicenter prospective study, McNaughton et al.37 found low numeracy to be associated with higher odds of recidivism to the emergency department (ED) or hospital within 30 days. Our findings may differ from theirs for a few reasons. First, their study had a significantly higher percentage of individuals with low numeracy (55%) compared with ours (33.9%). This may be because they did not exclude individuals with severe cognitive impairment, and their patient population was of lower socioeconomic status (SES) than ours. Low SES is associated with higher 30-day readmissions among HF patients1,10 throughout the literature, and low numeracy is associated with low SES in other diseases.13,38,39 Finally, they studied recidivism, which was defined as any unplanned return to the ED or hospital within 30 days of the index ED visit for acute HF. We only focused on 30-day readmissions, which also may explain why our results differed.
We found that health literacy was not associated with 30-day readmissions, which is consistent with the literature. Although an association between health literacy and mortality exists among adults with HF, several studies have not found an association between health literacy and 30- and 90-day readmission among adults hospitalized for HF.8,9,40 Although we found an association between objective health literacy and 30-day readmission in unadjusted analyses, we did not find one in the multivariable model. This, along with our numeracy finding, suggests that numeracy and literacy may not be driving the 30-day readmission risk among patients hospitalized with ADHF.
We examined cognition alongside numeracy and literacy because it is a prevalent condition among HF patients and because it is associated with adverse outcomes among patients with HF, including readmission.41,42 Studies have shown that HF preferentially affects certain cognitive domains,43 some of which are vital to HF self-care activities. We found that 53% of patients had any cognitive impairment, which is consistent with the literature of adults hospitalized for ADHF.44,45 Cognitive impairment was not, however, associated with 30-day readmissions. There may be a couple reasons for this. First, we measured cognitive impairment with the SPMSQ, which, although widely used and well-validated, does not assess executive function, the domain most commonly affected in HF patients with cognitive impairment.46 Second, patients with severe cognitive impairment and those with delirium were excluded from this study, which may have limited our ability to detect differences in readmission by this factor.
As in prior studies, we found that a history of DM and more hospitalizations in the prior year were independently associated with 30-day readmissions in fully adjusted models. Like other studies, in adjusted models, we found that LVEF and a history of HF were not independently associated with 30-day readmission.47-49 This, however, is not surprising because recent studies have shown that, although HF patients are at risk for multiple hospitalizations, early readmission after a hospitalization for ADHF specifically is often because of reasons unrelated to HF or a non-cardiovascular cause in general.50,51
Although a negative study, several important themes emerged. First, while we were able to assess numeracy, health literacy, and cognition, none of these measures were HF-specific. It is possible that we did not see an effect on readmission because our instruments failed to assess domains specific to HF, such as monitoring weight changes, following a low-salt diet, and interpreting blood pressure. Currently, however, no HF-specific objective numeracy measure exists. With respect to health literacy, only 1 HF-specific measure exists,52 although it was only recently developed and validated. Second, while numeracy may not be a driving influence of all-cause 30-day readmissions, it may be associated with other health behaviors and quality metrics that we did not examine here, such as self-care, medication adherence, and HF-specific readmissions. Third, it is likely that the progression of HF itself, as well as the clinical management of patients following discharge, contribute significantly to 30-day readmissions. Increased attention to predischarge processes for HF patients occurred at VUMC during the study period; close follow-up and evidence-directed therapies may have mitigated some of the expected associations. Finally, we were not able to assess numeracy of participants’ primary caregivers who may help patients at home, especially postdischarge. Though a number of studies have examined the role of family caregivers in the management of HF,53,54 none have examined numeracy levels of caregivers in the context of HF, and this may be worth doing in future studies.
Overall, our study has several strengths. The size of the cohort is large and there were high response rates during the follow-up period. Unlike other HF readmission studies, VICS accounts for readmissions to outside hospitals. Approximately 35% of all hospitalizations in VICS are to outside facilities. Thus, the ascertainment of readmissions to hospitals other than Vanderbilt is more comprehensive than if readmissions to VUMC were only considered. We were able to include a number of clinical comorbidities, laboratory and diagnostic tests from the index admission, and hospitalization characteristics in our analyses. Finally, we performed additional analyses to investigate the correlation between numeracy, literacy, and cognition; ultimately, we found that the majority of these correlations were weak, which supports our ability to study them simultaneously among VICS participants.
Nonetheless, we note some limitations. Although we captured readmissions to outside hospitals, the study took place at a single referral center in Tennessee. Though patients were diverse in age and comorbidities, they were mostly white and of higher SES. Finally, we used home status as a proxy for social support, which may underestimate the support that home care workers provide.
In conclusion, in this prospective longitudinal study of adults hospitalized with ADHF, inadequate numeracy was present in more than a third of patients, and low health literacy was present in roughly a quarter of patients. Neither numeracy nor health literacy, however, were associated with 30-day readmissions in adjusted analyses. Any cognitive impairment, although present in roughly one-half of patients, was not associated with 30-day readmission either. Our findings suggest that other influences may play a more dominant role in determining 30-day readmission rates in patients hospitalized for ADHF than inadequate numeracy, low health literacy, or cognitive impairment as assessed here.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute (R01 HL109388) and in part by the National Center for Advancing Translational Sciences (UL1 TR000445-06). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health. The authors’ funding sources did not participate in the planning, collection, analysis, or interpretation of data or in the decision to submit for publication. Dr. Sterling is supported by T32HS000066 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Mixon has a VA Health Services Research and Development Service Career Development Award at the Tennessee Valley Healthcare System, Department of Veterans Affairs (CDA 12-168). This material was presented at the Society of General Internal Medicine Annual Meeting on April 20, 2017, in Washington, DC.
Disclosure
Dr. Kripalani reports personal fees from Verustat, personal fees from SAI Interactive, and equity from Bioscape Digital, all outside of the submitted work. Dr. Rothman and Dr. Wallston report personal fees from EdLogics outside of the submitted work. All of the other authors have nothing to disclose
Most studies to identify risk factors for readmission among patients with heart failure (HF) have focused on demographic and clinical characteristics.1,2 Although easy to extract from administrative databases, this approach fails to capture the complex psychosocial and cognitive factors that influence the ability of HF patients to manage their disease in the postdischarge period, as depicted in the framework by Meyers et al.3 (2014). To date, studies have found low health literacy, decreased social support, and cognitive impairment to be associated with health behaviors and outcomes among HF patients, including decreased self-care,4 low HF-specific knowledge,5 medication nonadherence,6 hospitalizations,7 and mortality.8-10 Less, however, is known about the effect of numeracy on HF outcomes, such as 30-day readmission.
Numeracy, or quantitative literacy, refers to the ability to access, understand, and apply numerical data to health-related decisions.11 It is estimated that 110 million people in the United States have limited numeracy skills.12 Low numeracy is a risk factor for poor glycemic control among patients with diabetes,13 medication adherence in HIV/AIDS,14 and worse blood pressure control in hypertensives.15 Much like these conditions, HF requires that patients understand, use, and act on numerical information. Maintaining a low-salt diet, monitoring weight, adjusting diuretic doses, and measuring blood pressure are tasks that HF patients are asked to perform on a daily or near-daily basis. These tasks are particularly important in the posthospitalization period and could be complicated by medication changes, which might create additional challenges for patients with inadequate numeracy. Additionally, cognitive impairment, which is a highly prevalent comorbid condition among adults with HF,16,17 might impose additional barriers for those with inadequate numeracy who do not have adequate social support. However, to date, numeracy in the context of HF has not been well described.
Herein, we examined the effects of numeracy, alongside health literacy and cognition, on 30-day readmission risk among patients hospitalized for acute decompensated HF (ADHF).
METHODS
Study Design
The Vanderbilt Inpatient Cohort Study (VICS) is a prospective observational study of patients admitted with cardiovascular disease to Vanderbilt University Medical Center (VUMC), an academic tertiary care hospital. VICS was designed to investigate the impact of social determinants of health on postdischarge health outcomes. A detailed description of the study rationale, design, and methods is described elsewhere.3
Briefly, participants completed a baseline interview while hospitalized, and follow-up phone calls were conducted within 1 week of discharge, at 30 days, and at 90 days. At 30 and 90 days postdischarge, healthcare utilization was ascertained by review of medical records and patient report. Clinical data about the index hospitalization were also abstracted. The Vanderbilt University Institutional Review Board approved the study.
Study Population
Patients hospitalized from 2011 to 2015 with a likely diagnosis of acute coronary syndrome and/or ADHF, as determined by a physician’s review of the medical record, were identified as potentially eligible. Research assistants assessed these patients for the presence of the following exclusion criteria: less than 18 years of age, non-English speaking, unstable psychiatric illness, a low likelihood of follow-up (eg, no reliable telephone number), on hospice, or otherwise too ill to complete an interview. Additionally, those with severe cognitive impairment, as assessed from the medical record (such as seeing a note describing dementia), and those with delirium, as assessed by the brief confusion assessment method, were excluded from enrollment in the study.18,19 Those who died before discharge or during the 30-day follow-up period were excluded. For this analysis, we restricted our sample to only include participants who were hospitalized for ADHF.
Outcome Measure: 30-Day Readmission
The main outcome was all-cause readmission to any hospital within 30 days of discharge, as determined by patient interview, review of electronic medical records from VUMC, and review of outside hospital records.
Main Exposures: Numeracy, Health Literacy, and Cognitive Impairment
Numeracy was assessed with a 3-item version of the Subjective Numeracy Scale (SNS-3), which quantifies the patients perceived quantitative abilities.20 Other authors have shown that the SNS-3 has a correlation coefficient of 0.88 with the full-length SNS-8 and a Cronbach’s alpha of 0.78.20-22 The SNS-3 is reported as the mean on a scale from 1 to 6, with higher scores reflecting higher numeracy.
Subjective health literacy was assessed by using the 3-item Brief Health Literacy Screen (BHLS).23 Scores range from 3 to 15, with higher scores reflecting higher literacy. Objective health literacy was assessed with the short form of the Test of Functional Health Literacy in Adults (sTOFHLA).24,25 Scores may be categorized as inadequate (0-16), marginal (17-22), or adequate (23-36).
We assessed cognition by using the 10-item Short Portable Mental Status Questionnaire (SPMSQ).26 The SPMSQ, which describes a person’s capacity for memory, structured thought, and orientation, has been validated and has demonstrated good reliability and validity.27 Scores of 0 were considered to reflect intact cognition, and scores of 1 or more were considered to reflect any cognitive impairment, a scoring approach employed by other authors.28 We used this approach, rather than the traditional scoring system developed by Pfeiffer et al.26 (1975), because it would be the most sensitive to detect any cognitive impairment in the VICS cohort, which excluded those with severe cognition impairment, dementia, and delirium.
Covariates
During the hospitalization, participants completed an in-person interviewer-administered baseline assessment composed of demographic information, including age, self-reported race (white and nonwhite), educational attainment, home status (married, not married and living with someone, not married and living alone), and household income.
Clinical and diagnostic characteristics abstracted from the medical record included a medical history of HF, HF subtype (classified by left ventricular ejection fraction [LVEF]), coronary artery disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), and comorbidity burden as summarized by the van Walraven-Elixhauser score.29,30 Depressive symptoms were assessed during the 2 weeks prior to the hospitalization by using the first 8 items of the Patient Health Questionnaire.31 Scores ranged from 0 to 24, with higher scores reflecting more severe depressive symptoms. Laboratory values included estimated glomerular filtration rate (eGFR), hemoglobin (g/dl), sodium (mg/L), and brain natriuretic peptide (BNP) (pg/ml) from the last laboratory draw before discharge. Smoking status was also assessed (current and former/nonsmokers).
Hospitalization characteristics included length of stay in days, number of prior admissions in the last year, and transfer to the intensive care unit during the index admission.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics. The Kruskal-Wallis test and the Pearson χ2 test were used to determine the association between patient characteristics and levels of numeracy, literacy, and cognition separately. The unadjusted relationship between patient characteristics and 30-day readmission was assessed by using Wilcoxon rank sums tests for continuous variables and Pearson χ2 tests for categorical variables. In addition, a correlation matrix was performed to assess the correlations between numeracy, health literacy, and cognition (supplementary Figure 1).
To examine the association between numeracy, health literacy, and cognition and 30-day readmissions, a series of multivariable Poisson (log-linear) regression models were fit.32 Like other studies, numeracy, health literacy, and cognition were examined as categorical and continuous measures in models.33 Each model was modified with a sandwich estimator for robust standard errors. Log-linear models were chosen over logistic regression models for ease of interpretation because (exponentiated) parameters correspond to risk ratios (RRs) as opposed to odds ratios. Furthermore, the fitting challenges associated with log-linear models when predicted probabilities are near 0 or 1 were not present in these analyses. Redundancy analyses were conducted to ensure that independent variables were not highly correlated with a linear combination of the other independent variables. To avoid case-wise deletion of records with missing covariates, we employed multiple imputation with 10 imputation samples by using predictive mean matching.34,35 All analyses were conducted in R version 3.1.2 (The R Foundation, Vienna, Austria).36
RESULTS
Overall, 883 patients were included in this analysis (supplementary Figure 2). Of the 883 participants, 46% were female and 76% were white (Table 1). Their median age was 60 years (interdecile range [IDR] 39-78) and the median educational attainment was 13.5 years (IDR 11-18).
DISCUSSION
This is the first study to examine the effect of numeracy alongside literacy and cognition on 30-day readmission risk among patients hospitalized with ADHF. Overall, we found that 33.9% of participants had inadequate numeracy skills, and 24.6% had inadequate or marginal health literacy. In unadjusted and adjusted models, numeracy was not associated with 30-day readmission. Although (objective) low health literacy was associated with 30-day readmission in unadjusted models, it was not in adjusted models. Additionally, though 53% of participants had any cognitive impairment, readmission did not differ significantly by this factor. Taken together, these findings suggest that other factors may be greater determinants of 30-day readmissions among patients hospitalized for ADHF.
Only 1 other study has examined the effect of numeracy on readmission risk among patients hospitalized for HF. In this multicenter prospective study, McNaughton et al.37 found low numeracy to be associated with higher odds of recidivism to the emergency department (ED) or hospital within 30 days. Our findings may differ from theirs for a few reasons. First, their study had a significantly higher percentage of individuals with low numeracy (55%) compared with ours (33.9%). This may be because they did not exclude individuals with severe cognitive impairment, and their patient population was of lower socioeconomic status (SES) than ours. Low SES is associated with higher 30-day readmissions among HF patients1,10 throughout the literature, and low numeracy is associated with low SES in other diseases.13,38,39 Finally, they studied recidivism, which was defined as any unplanned return to the ED or hospital within 30 days of the index ED visit for acute HF. We only focused on 30-day readmissions, which also may explain why our results differed.
We found that health literacy was not associated with 30-day readmissions, which is consistent with the literature. Although an association between health literacy and mortality exists among adults with HF, several studies have not found an association between health literacy and 30- and 90-day readmission among adults hospitalized for HF.8,9,40 Although we found an association between objective health literacy and 30-day readmission in unadjusted analyses, we did not find one in the multivariable model. This, along with our numeracy finding, suggests that numeracy and literacy may not be driving the 30-day readmission risk among patients hospitalized with ADHF.
We examined cognition alongside numeracy and literacy because it is a prevalent condition among HF patients and because it is associated with adverse outcomes among patients with HF, including readmission.41,42 Studies have shown that HF preferentially affects certain cognitive domains,43 some of which are vital to HF self-care activities. We found that 53% of patients had any cognitive impairment, which is consistent with the literature of adults hospitalized for ADHF.44,45 Cognitive impairment was not, however, associated with 30-day readmissions. There may be a couple reasons for this. First, we measured cognitive impairment with the SPMSQ, which, although widely used and well-validated, does not assess executive function, the domain most commonly affected in HF patients with cognitive impairment.46 Second, patients with severe cognitive impairment and those with delirium were excluded from this study, which may have limited our ability to detect differences in readmission by this factor.
As in prior studies, we found that a history of DM and more hospitalizations in the prior year were independently associated with 30-day readmissions in fully adjusted models. Like other studies, in adjusted models, we found that LVEF and a history of HF were not independently associated with 30-day readmission.47-49 This, however, is not surprising because recent studies have shown that, although HF patients are at risk for multiple hospitalizations, early readmission after a hospitalization for ADHF specifically is often because of reasons unrelated to HF or a non-cardiovascular cause in general.50,51
Although a negative study, several important themes emerged. First, while we were able to assess numeracy, health literacy, and cognition, none of these measures were HF-specific. It is possible that we did not see an effect on readmission because our instruments failed to assess domains specific to HF, such as monitoring weight changes, following a low-salt diet, and interpreting blood pressure. Currently, however, no HF-specific objective numeracy measure exists. With respect to health literacy, only 1 HF-specific measure exists,52 although it was only recently developed and validated. Second, while numeracy may not be a driving influence of all-cause 30-day readmissions, it may be associated with other health behaviors and quality metrics that we did not examine here, such as self-care, medication adherence, and HF-specific readmissions. Third, it is likely that the progression of HF itself, as well as the clinical management of patients following discharge, contribute significantly to 30-day readmissions. Increased attention to predischarge processes for HF patients occurred at VUMC during the study period; close follow-up and evidence-directed therapies may have mitigated some of the expected associations. Finally, we were not able to assess numeracy of participants’ primary caregivers who may help patients at home, especially postdischarge. Though a number of studies have examined the role of family caregivers in the management of HF,53,54 none have examined numeracy levels of caregivers in the context of HF, and this may be worth doing in future studies.
Overall, our study has several strengths. The size of the cohort is large and there were high response rates during the follow-up period. Unlike other HF readmission studies, VICS accounts for readmissions to outside hospitals. Approximately 35% of all hospitalizations in VICS are to outside facilities. Thus, the ascertainment of readmissions to hospitals other than Vanderbilt is more comprehensive than if readmissions to VUMC were only considered. We were able to include a number of clinical comorbidities, laboratory and diagnostic tests from the index admission, and hospitalization characteristics in our analyses. Finally, we performed additional analyses to investigate the correlation between numeracy, literacy, and cognition; ultimately, we found that the majority of these correlations were weak, which supports our ability to study them simultaneously among VICS participants.
Nonetheless, we note some limitations. Although we captured readmissions to outside hospitals, the study took place at a single referral center in Tennessee. Though patients were diverse in age and comorbidities, they were mostly white and of higher SES. Finally, we used home status as a proxy for social support, which may underestimate the support that home care workers provide.
In conclusion, in this prospective longitudinal study of adults hospitalized with ADHF, inadequate numeracy was present in more than a third of patients, and low health literacy was present in roughly a quarter of patients. Neither numeracy nor health literacy, however, were associated with 30-day readmissions in adjusted analyses. Any cognitive impairment, although present in roughly one-half of patients, was not associated with 30-day readmission either. Our findings suggest that other influences may play a more dominant role in determining 30-day readmission rates in patients hospitalized for ADHF than inadequate numeracy, low health literacy, or cognitive impairment as assessed here.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute (R01 HL109388) and in part by the National Center for Advancing Translational Sciences (UL1 TR000445-06). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health. The authors’ funding sources did not participate in the planning, collection, analysis, or interpretation of data or in the decision to submit for publication. Dr. Sterling is supported by T32HS000066 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr. Mixon has a VA Health Services Research and Development Service Career Development Award at the Tennessee Valley Healthcare System, Department of Veterans Affairs (CDA 12-168). This material was presented at the Society of General Internal Medicine Annual Meeting on April 20, 2017, in Washington, DC.
Disclosure
Dr. Kripalani reports personal fees from Verustat, personal fees from SAI Interactive, and equity from Bioscape Digital, all outside of the submitted work. Dr. Rothman and Dr. Wallston report personal fees from EdLogics outside of the submitted work. All of the other authors have nothing to disclose
1. Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch of Intern Med. 2008;168(13):1371-1386. PubMed
33. Bohannon AD, Fillenbaum GG, Pieper CF, Hanlon JT, Blazer DG. Relationship of race/ethnicity and blood pressure to change in cognitive function. J Am Geriatr Soc. 2002;50(3):424-429. PubMed
38. Abdel-Kader K, Dew MA, Bhatnagar M, et al. Numeracy Skills in CKD: Correlates and Outcomes. Clin J Am Soc Nephrol. 2010;5(9):1566-1573. PubMed
1. Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch of Intern Med. 2008;168(13):1371-1386. PubMed
33. Bohannon AD, Fillenbaum GG, Pieper CF, Hanlon JT, Blazer DG. Relationship of race/ethnicity and blood pressure to change in cognitive function. J Am Geriatr Soc. 2002;50(3):424-429. PubMed
38. Abdel-Kader K, Dew MA, Bhatnagar M, et al. Numeracy Skills in CKD: Correlates and Outcomes. Clin J Am Soc Nephrol. 2010;5(9):1566-1573. PubMed
© 2018 Society of Hospital Medicine
Scratching Beneath the Surface
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
© 2018 Society of Hospital Medicine
DDSEP® 8 Quick Quiz - February 2018 Question 2
Answer: C
Rationale
The patient has ascending cholangitis. After stabilization and initiation of antibiotics, the next most appropriate step is ERCP. The patient is high risk for postsphincterotomy bleeding as he is on three antithrombotic agents. The most prudent course of action is ERCP with stent placement. ERCP and stent placement is not contraindicated in patients on antithrombotic agents. This will allow for confirmation of the diagnosis as well as therapy for the obstruction. Once the patient has recovered, he can return on an elective basis, off antithrombotic agents, for definitive management of the common bile duct stone. MRCP would allow for a diagnosis; however, it is not therapeutic, and in the setting of cholangitis, management of the obstruction is necessary. Continued medical management neither provides information regarding diagnosis nor treats the obstruction. Percutaneous biliary drain would provide appropriate drainage but, as he is at a high risk for bleeding, ERCP with stent placement is a better therapeutic option in this patient.
References
1. Committee, ASGE Standards of Practice, et al. Management of anti-thrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70(6):1060-70.
2. Boustiere C., Veitch A., Vanbiervliet G., et al. Endoscopy and antiplatelet agents. Endoscopy. 2011;43(5):445-61.
Answer: C
Rationale
The patient has ascending cholangitis. After stabilization and initiation of antibiotics, the next most appropriate step is ERCP. The patient is high risk for postsphincterotomy bleeding as he is on three antithrombotic agents. The most prudent course of action is ERCP with stent placement. ERCP and stent placement is not contraindicated in patients on antithrombotic agents. This will allow for confirmation of the diagnosis as well as therapy for the obstruction. Once the patient has recovered, he can return on an elective basis, off antithrombotic agents, for definitive management of the common bile duct stone. MRCP would allow for a diagnosis; however, it is not therapeutic, and in the setting of cholangitis, management of the obstruction is necessary. Continued medical management neither provides information regarding diagnosis nor treats the obstruction. Percutaneous biliary drain would provide appropriate drainage but, as he is at a high risk for bleeding, ERCP with stent placement is a better therapeutic option in this patient.
References
1. Committee, ASGE Standards of Practice, et al. Management of anti-thrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70(6):1060-70.
2. Boustiere C., Veitch A., Vanbiervliet G., et al. Endoscopy and antiplatelet agents. Endoscopy. 2011;43(5):445-61.
Answer: C
Rationale
The patient has ascending cholangitis. After stabilization and initiation of antibiotics, the next most appropriate step is ERCP. The patient is high risk for postsphincterotomy bleeding as he is on three antithrombotic agents. The most prudent course of action is ERCP with stent placement. ERCP and stent placement is not contraindicated in patients on antithrombotic agents. This will allow for confirmation of the diagnosis as well as therapy for the obstruction. Once the patient has recovered, he can return on an elective basis, off antithrombotic agents, for definitive management of the common bile duct stone. MRCP would allow for a diagnosis; however, it is not therapeutic, and in the setting of cholangitis, management of the obstruction is necessary. Continued medical management neither provides information regarding diagnosis nor treats the obstruction. Percutaneous biliary drain would provide appropriate drainage but, as he is at a high risk for bleeding, ERCP with stent placement is a better therapeutic option in this patient.
References
1. Committee, ASGE Standards of Practice, et al. Management of anti-thrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70(6):1060-70.
2. Boustiere C., Veitch A., Vanbiervliet G., et al. Endoscopy and antiplatelet agents. Endoscopy. 2011;43(5):445-61.
A 76-year-old man presents with 2 days of epigastric abdominal pain radiating to the back accompanied by nausea, vomiting, fevers, and chills. His past medical history is notable for diabetes, hypertension, coronary artery disease, on clopidogrel and aspirin, as well as atrial fibrillation, for which he is on warfarin. Vital signs at presentation are temperature of 39.1°C, blood pressure of 88/58 mm Hg, and a heart rate of 110 beats per minute. Labs reveal a WBC count of 15,000/mm3, total bilirubin of 4.0 mg/dL, alkaline phosphatase of 234 IU/L, AST 120 IU/L, ALT 131 IU/L, and an INR of 2.7. An abdominal ultrasound reveals a common bile duct dilated to 1.5 cm.
Following fluid resuscitation and initiation of antibiotics, what is the next most appropriate step?
DDSEP® 8 Quick Quiz - February 2018 Question 1
Answer: B
Rationale
Hypergastrinemia is normally a physiologic response to hypochlorhydria. When hypergastrinemia occurs in the setting of acidic gastric pH, it is considered inappropriate. Gastrinoma is a component of MEN-1 syndrome that results in abnormal gastrin release and acid hypersecretion. Gastric outlet obstruction may lead to stomach distention and persistent stimulation by retained food, causing increased gastrin release and acid secretion. Chronic renal failure leads to a decrease in clearance of gastrin from the circulation, resulting in hypergastrinemia. This increase in circulating gastrin results in stimulation of parietal cells to release acid into the gastric lumen. Therefore, the hypergastrinemia associated with chronic renal failure is inappropriate, given the high serum gastrin level despite low intragastric pH. Retained antrum results when a small portion of antrum is left attached to the duodenal bulb (afferent loop) during a Billroth II surgical procedure. As a result, the G cells from the retained antrum are displaced from the stomach and excluded from the inhibitory effects of gastric acid. The lack of negative feedback leads to persistently high gastrin release and resultant acid production. H. pylori pangastritis results in suppression of acid secretion, leading to a high intragastric pH. Therefore, it represents an appropriate cause for hypergastrinemia.
Reference
1. Murugesan S.V., Varro A., Pritchard D.M. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther. 2009;29:1055-68.
Answer: B
Rationale
Hypergastrinemia is normally a physiologic response to hypochlorhydria. When hypergastrinemia occurs in the setting of acidic gastric pH, it is considered inappropriate. Gastrinoma is a component of MEN-1 syndrome that results in abnormal gastrin release and acid hypersecretion. Gastric outlet obstruction may lead to stomach distention and persistent stimulation by retained food, causing increased gastrin release and acid secretion. Chronic renal failure leads to a decrease in clearance of gastrin from the circulation, resulting in hypergastrinemia. This increase in circulating gastrin results in stimulation of parietal cells to release acid into the gastric lumen. Therefore, the hypergastrinemia associated with chronic renal failure is inappropriate, given the high serum gastrin level despite low intragastric pH. Retained antrum results when a small portion of antrum is left attached to the duodenal bulb (afferent loop) during a Billroth II surgical procedure. As a result, the G cells from the retained antrum are displaced from the stomach and excluded from the inhibitory effects of gastric acid. The lack of negative feedback leads to persistently high gastrin release and resultant acid production. H. pylori pangastritis results in suppression of acid secretion, leading to a high intragastric pH. Therefore, it represents an appropriate cause for hypergastrinemia.
Reference
1. Murugesan S.V., Varro A., Pritchard D.M. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther. 2009;29:1055-68.
Answer: B
Rationale
Hypergastrinemia is normally a physiologic response to hypochlorhydria. When hypergastrinemia occurs in the setting of acidic gastric pH, it is considered inappropriate. Gastrinoma is a component of MEN-1 syndrome that results in abnormal gastrin release and acid hypersecretion. Gastric outlet obstruction may lead to stomach distention and persistent stimulation by retained food, causing increased gastrin release and acid secretion. Chronic renal failure leads to a decrease in clearance of gastrin from the circulation, resulting in hypergastrinemia. This increase in circulating gastrin results in stimulation of parietal cells to release acid into the gastric lumen. Therefore, the hypergastrinemia associated with chronic renal failure is inappropriate, given the high serum gastrin level despite low intragastric pH. Retained antrum results when a small portion of antrum is left attached to the duodenal bulb (afferent loop) during a Billroth II surgical procedure. As a result, the G cells from the retained antrum are displaced from the stomach and excluded from the inhibitory effects of gastric acid. The lack of negative feedback leads to persistently high gastrin release and resultant acid production. H. pylori pangastritis results in suppression of acid secretion, leading to a high intragastric pH. Therefore, it represents an appropriate cause for hypergastrinemia.
Reference
1. Murugesan S.V., Varro A., Pritchard D.M. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther. 2009;29:1055-68.
Which of the following conditions is associated with hypergastrinemia and elevated gastric pH?
Make the Diagnosis - February 2018
Neurofibromatosis (NF) is an autosomal dominant genetic neurocutaneous disorder. There are eight subtypes of NF: NF type 1-7 and NF-NOS, or not otherwise specified. Neurofibromatosis type 1 (NF-1), or von Recklinghausen disease, is the most common and is a result of a genetic mutation on chromosome 17 that is involved in producing a protein called neurofibromin. Neurofibromin is a tumor suppressor that suppresses products of ras proto-oncogenes. When it is absent, tumor progression may occur.
Von Recklinghausen NF-1 appears in childhood, usually by age 10. Diagnosis requires the presence of at least 2 of the following 7 criteria:
•Six or more café au lait macules measuring 5 mm in diameter or greater in prepubertal children and measuring greater than 15 mm in postpubertal children.
•Axillary or inguinal freckling (Crowe’s sign).
•Two or more neurofibromas or one plexiform neurofibroma.
•Optic nerve glioma.
•Two or more iris hamartomas (Lisch nodules).
•Sphenoid dysplasia or long-bone abnormalities, such as pseudoarthrosis.
•First degree relative with NF-1.
The diagnosis is usually made via physical examination. Supportive tests include an ophthalmologic exam to detect Lisch nodules and cataracts. A neurological evaluation is essential. Imaging examinations can identify bony abnormalities and tumor growths. Also, genetic testing to identify genetic mutations can be performed.
Neurofibromatosis type 2 results from a genetic mutation located on chromosome 22 that produces a protein called merlin and occurs in adolescence. Acoustic or vestibular neuromas may occur; these interfere with the transmission of sound and maintaining balance. Symptoms include gradual hearing loss, tinnitus, poor balance, and headaches. Radiosurgery and cochlear implants have shown a role for symptomatic treatment in patients with NF-2.
This case and photo were submitted by Parteek Singla, MD, of the division of dermatology at Washington University and Barnes Jewish Hospital, both in St. Louis, and by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Neurofibromatosis (NF) is an autosomal dominant genetic neurocutaneous disorder. There are eight subtypes of NF: NF type 1-7 and NF-NOS, or not otherwise specified. Neurofibromatosis type 1 (NF-1), or von Recklinghausen disease, is the most common and is a result of a genetic mutation on chromosome 17 that is involved in producing a protein called neurofibromin. Neurofibromin is a tumor suppressor that suppresses products of ras proto-oncogenes. When it is absent, tumor progression may occur.
Von Recklinghausen NF-1 appears in childhood, usually by age 10. Diagnosis requires the presence of at least 2 of the following 7 criteria:
•Six or more café au lait macules measuring 5 mm in diameter or greater in prepubertal children and measuring greater than 15 mm in postpubertal children.
•Axillary or inguinal freckling (Crowe’s sign).
•Two or more neurofibromas or one plexiform neurofibroma.
•Optic nerve glioma.
•Two or more iris hamartomas (Lisch nodules).
•Sphenoid dysplasia or long-bone abnormalities, such as pseudoarthrosis.
•First degree relative with NF-1.
The diagnosis is usually made via physical examination. Supportive tests include an ophthalmologic exam to detect Lisch nodules and cataracts. A neurological evaluation is essential. Imaging examinations can identify bony abnormalities and tumor growths. Also, genetic testing to identify genetic mutations can be performed.
Neurofibromatosis type 2 results from a genetic mutation located on chromosome 22 that produces a protein called merlin and occurs in adolescence. Acoustic or vestibular neuromas may occur; these interfere with the transmission of sound and maintaining balance. Symptoms include gradual hearing loss, tinnitus, poor balance, and headaches. Radiosurgery and cochlear implants have shown a role for symptomatic treatment in patients with NF-2.
This case and photo were submitted by Parteek Singla, MD, of the division of dermatology at Washington University and Barnes Jewish Hospital, both in St. Louis, and by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Neurofibromatosis (NF) is an autosomal dominant genetic neurocutaneous disorder. There are eight subtypes of NF: NF type 1-7 and NF-NOS, or not otherwise specified. Neurofibromatosis type 1 (NF-1), or von Recklinghausen disease, is the most common and is a result of a genetic mutation on chromosome 17 that is involved in producing a protein called neurofibromin. Neurofibromin is a tumor suppressor that suppresses products of ras proto-oncogenes. When it is absent, tumor progression may occur.
Von Recklinghausen NF-1 appears in childhood, usually by age 10. Diagnosis requires the presence of at least 2 of the following 7 criteria:
•Six or more café au lait macules measuring 5 mm in diameter or greater in prepubertal children and measuring greater than 15 mm in postpubertal children.
•Axillary or inguinal freckling (Crowe’s sign).
•Two or more neurofibromas or one plexiform neurofibroma.
•Optic nerve glioma.
•Two or more iris hamartomas (Lisch nodules).
•Sphenoid dysplasia or long-bone abnormalities, such as pseudoarthrosis.
•First degree relative with NF-1.
The diagnosis is usually made via physical examination. Supportive tests include an ophthalmologic exam to detect Lisch nodules and cataracts. A neurological evaluation is essential. Imaging examinations can identify bony abnormalities and tumor growths. Also, genetic testing to identify genetic mutations can be performed.
Neurofibromatosis type 2 results from a genetic mutation located on chromosome 22 that produces a protein called merlin and occurs in adolescence. Acoustic or vestibular neuromas may occur; these interfere with the transmission of sound and maintaining balance. Symptoms include gradual hearing loss, tinnitus, poor balance, and headaches. Radiosurgery and cochlear implants have shown a role for symptomatic treatment in patients with NF-2.
This case and photo were submitted by Parteek Singla, MD, of the division of dermatology at Washington University and Barnes Jewish Hospital, both in St. Louis, and by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Baby boomers are the hepatitis C generation
Increases in hepatitis C–related inpatient stays for baby boomers from 2005 to 2014 far outpaced those of older adults, while younger adults saw their admissions drop over that period, according to the Agency for Healthcare Research and Quality.
For the baby boomers (adults aged 52-72 years), the rate of inpatient stays involving hepatitis C with or without hepatitis B, HIV, or alcoholic liver disease rose from 300.7 per 100,000 population in 2005 to 503.1 per 100,000 in 2014 – an increase of over 67%. For patients aged 73 years and older, that rate went from 104.4 in 2005 to 117.1 in 2014, which translates to a 12% increase, and for patients aged 18-51 years, it dropped 15%, from 182.5 to 155.4, the AHRQ said in a statistical brief.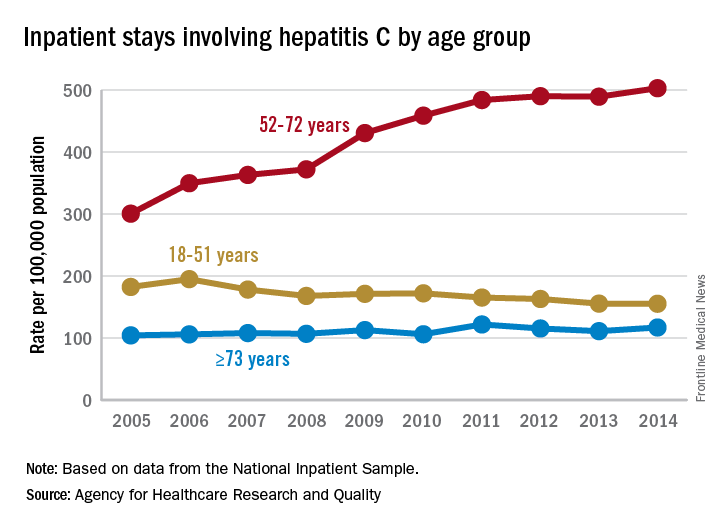
Along with the increased hospitalizations, “acute hepatitis C cases nearly tripled from 2010 through 2015,” the report noted, which was “likely the result of increasing injection drug use due to the growing opioid epidemic.”
Increases in hepatitis C–related inpatient stays for baby boomers from 2005 to 2014 far outpaced those of older adults, while younger adults saw their admissions drop over that period, according to the Agency for Healthcare Research and Quality.
For the baby boomers (adults aged 52-72 years), the rate of inpatient stays involving hepatitis C with or without hepatitis B, HIV, or alcoholic liver disease rose from 300.7 per 100,000 population in 2005 to 503.1 per 100,000 in 2014 – an increase of over 67%. For patients aged 73 years and older, that rate went from 104.4 in 2005 to 117.1 in 2014, which translates to a 12% increase, and for patients aged 18-51 years, it dropped 15%, from 182.5 to 155.4, the AHRQ said in a statistical brief.
Along with the increased hospitalizations, “acute hepatitis C cases nearly tripled from 2010 through 2015,” the report noted, which was “likely the result of increasing injection drug use due to the growing opioid epidemic.”
Increases in hepatitis C–related inpatient stays for baby boomers from 2005 to 2014 far outpaced those of older adults, while younger adults saw their admissions drop over that period, according to the Agency for Healthcare Research and Quality.
For the baby boomers (adults aged 52-72 years), the rate of inpatient stays involving hepatitis C with or without hepatitis B, HIV, or alcoholic liver disease rose from 300.7 per 100,000 population in 2005 to 503.1 per 100,000 in 2014 – an increase of over 67%. For patients aged 73 years and older, that rate went from 104.4 in 2005 to 117.1 in 2014, which translates to a 12% increase, and for patients aged 18-51 years, it dropped 15%, from 182.5 to 155.4, the AHRQ said in a statistical brief.
Along with the increased hospitalizations, “acute hepatitis C cases nearly tripled from 2010 through 2015,” the report noted, which was “likely the result of increasing injection drug use due to the growing opioid epidemic.”
Fingolimod cuts pediatric MS relapse rate more than interferon beta-1a
Pediatric patients with relapsing remitting multiple sclerosis (MS) had fewer relapses after receiving the oral drug fingolimod when compared with patients who received intramuscular interferon beta-1a in the randomized, double-blind PARADIGMS study, suggesting that the sphingosine-1-phosphate receptor modulator could offer a new treatment option to patients younger than 18 years.
A new agent in this patient population is particularly important because most children and adolescents have the relapsing remitting form of the disease, and generally experience a relapse rate that is two to three times higher than that seen in people with adult-onset multiple sclerosis.
The phase 3 PARADIGMS study is the first international controlled trial to evaluate the safety and efficacy of fingolimod in pediatric and adolescent patients. Dr. Chitnis and her colleagues randomized 215 participants aged 10-17 years to up to 0.5 mg/day of fingolimod based on body weight or to a once-a-week intramuscular injection of 30 mcg of interferon beta-1a. The trial lasted 2 years and was followed by an open-label extension for an additional 5 years.
The annualized relapse rate was the primary endpoint. The fingolimod group experienced 25 relapses in 180 patient-years, compared with 120 relapses in 163 patient-years in the interferon beta-1a group.
MRI findings and outcomes associated with relapse were secondary endpoints. The researchers found that, compared with the interferon beta-1a group, patients randomized to fingolimod had fewer lesions identified on MRI: There was a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
“These results indicate that fingolimod is more effective than the current standard of care, beta-interferon, in patients aged 10-17 and is a consideration for treatment in teenagers,” said Dr. Chitnis, director of the Partners Pediatric MS Center at the MassGeneral Hospital for Children, Boston. “The overall safety profile was reasonable, and there were no new major adverse events observed in comparison to adult studies.”
Participants were primarily Caucasian (92%) and female (62%). The mean age of each group at randomization was similar: 15.2 years in the fingolimod group and 15.4 years in the interferon beta-1a group. Disease duration since onset of first symptom was shorter in the fingolimod patients, a mean of 1.9 years, compared with a mean 2.4 years in the interferon beta-1a patients. At baseline, patients in both groups reported a mean 1.5 relapses in the previous year and a median Expanded Disability Status Scale (EDSS) score of 1.5.
To be included in the study, the children and teenagers had to have an EDSS score of 0 to 5.5; one or more relapses in the past year or two relapses in the previous two years; or MRI evidence of one or more gadolinium-enhancing lesions in the 6 months prior to trial randomization.
Fingolimod (Gilenya) is approved for the first-line treatment of relapsing forms of MS in adults in the United States. It is not yet FDA-approved for treatment of pediatric patients.
Dr. Chitnis said she was somewhat surprised by the strength of the findings in the PARADIGMS study. “These results showed very strong efficacy in young patients. However, as this study was being conducted, our group looked in more detail at the young adult subpopulation in the pivotal fingolimod adult studies, there was an improved effect in younger adults [those younger than 20 or younger than 30], compared to the entire group. Thus, one could extrapolate that the effects in adolescents would follow and show even greater efficacy.”
The study was sponsored by Novartis, the maker of fingolimod. Dr. Chitnis and nearly all of her coauthors disclosed financial ties to Novartis. Three authors are employees of Novartis.
SOURCE: Chitnis T et al. ACTRIMS Forum 2018, Abstract P025.
Pediatric patients with relapsing remitting multiple sclerosis (MS) had fewer relapses after receiving the oral drug fingolimod when compared with patients who received intramuscular interferon beta-1a in the randomized, double-blind PARADIGMS study, suggesting that the sphingosine-1-phosphate receptor modulator could offer a new treatment option to patients younger than 18 years.
A new agent in this patient population is particularly important because most children and adolescents have the relapsing remitting form of the disease, and generally experience a relapse rate that is two to three times higher than that seen in people with adult-onset multiple sclerosis.
The phase 3 PARADIGMS study is the first international controlled trial to evaluate the safety and efficacy of fingolimod in pediatric and adolescent patients. Dr. Chitnis and her colleagues randomized 215 participants aged 10-17 years to up to 0.5 mg/day of fingolimod based on body weight or to a once-a-week intramuscular injection of 30 mcg of interferon beta-1a. The trial lasted 2 years and was followed by an open-label extension for an additional 5 years.
The annualized relapse rate was the primary endpoint. The fingolimod group experienced 25 relapses in 180 patient-years, compared with 120 relapses in 163 patient-years in the interferon beta-1a group.
MRI findings and outcomes associated with relapse were secondary endpoints. The researchers found that, compared with the interferon beta-1a group, patients randomized to fingolimod had fewer lesions identified on MRI: There was a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
“These results indicate that fingolimod is more effective than the current standard of care, beta-interferon, in patients aged 10-17 and is a consideration for treatment in teenagers,” said Dr. Chitnis, director of the Partners Pediatric MS Center at the MassGeneral Hospital for Children, Boston. “The overall safety profile was reasonable, and there were no new major adverse events observed in comparison to adult studies.”
Participants were primarily Caucasian (92%) and female (62%). The mean age of each group at randomization was similar: 15.2 years in the fingolimod group and 15.4 years in the interferon beta-1a group. Disease duration since onset of first symptom was shorter in the fingolimod patients, a mean of 1.9 years, compared with a mean 2.4 years in the interferon beta-1a patients. At baseline, patients in both groups reported a mean 1.5 relapses in the previous year and a median Expanded Disability Status Scale (EDSS) score of 1.5.
To be included in the study, the children and teenagers had to have an EDSS score of 0 to 5.5; one or more relapses in the past year or two relapses in the previous two years; or MRI evidence of one or more gadolinium-enhancing lesions in the 6 months prior to trial randomization.
Fingolimod (Gilenya) is approved for the first-line treatment of relapsing forms of MS in adults in the United States. It is not yet FDA-approved for treatment of pediatric patients.
Dr. Chitnis said she was somewhat surprised by the strength of the findings in the PARADIGMS study. “These results showed very strong efficacy in young patients. However, as this study was being conducted, our group looked in more detail at the young adult subpopulation in the pivotal fingolimod adult studies, there was an improved effect in younger adults [those younger than 20 or younger than 30], compared to the entire group. Thus, one could extrapolate that the effects in adolescents would follow and show even greater efficacy.”
The study was sponsored by Novartis, the maker of fingolimod. Dr. Chitnis and nearly all of her coauthors disclosed financial ties to Novartis. Three authors are employees of Novartis.
SOURCE: Chitnis T et al. ACTRIMS Forum 2018, Abstract P025.
Pediatric patients with relapsing remitting multiple sclerosis (MS) had fewer relapses after receiving the oral drug fingolimod when compared with patients who received intramuscular interferon beta-1a in the randomized, double-blind PARADIGMS study, suggesting that the sphingosine-1-phosphate receptor modulator could offer a new treatment option to patients younger than 18 years.
A new agent in this patient population is particularly important because most children and adolescents have the relapsing remitting form of the disease, and generally experience a relapse rate that is two to three times higher than that seen in people with adult-onset multiple sclerosis.
The phase 3 PARADIGMS study is the first international controlled trial to evaluate the safety and efficacy of fingolimod in pediatric and adolescent patients. Dr. Chitnis and her colleagues randomized 215 participants aged 10-17 years to up to 0.5 mg/day of fingolimod based on body weight or to a once-a-week intramuscular injection of 30 mcg of interferon beta-1a. The trial lasted 2 years and was followed by an open-label extension for an additional 5 years.
The annualized relapse rate was the primary endpoint. The fingolimod group experienced 25 relapses in 180 patient-years, compared with 120 relapses in 163 patient-years in the interferon beta-1a group.
MRI findings and outcomes associated with relapse were secondary endpoints. The researchers found that, compared with the interferon beta-1a group, patients randomized to fingolimod had fewer lesions identified on MRI: There was a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
“These results indicate that fingolimod is more effective than the current standard of care, beta-interferon, in patients aged 10-17 and is a consideration for treatment in teenagers,” said Dr. Chitnis, director of the Partners Pediatric MS Center at the MassGeneral Hospital for Children, Boston. “The overall safety profile was reasonable, and there were no new major adverse events observed in comparison to adult studies.”
Participants were primarily Caucasian (92%) and female (62%). The mean age of each group at randomization was similar: 15.2 years in the fingolimod group and 15.4 years in the interferon beta-1a group. Disease duration since onset of first symptom was shorter in the fingolimod patients, a mean of 1.9 years, compared with a mean 2.4 years in the interferon beta-1a patients. At baseline, patients in both groups reported a mean 1.5 relapses in the previous year and a median Expanded Disability Status Scale (EDSS) score of 1.5.
To be included in the study, the children and teenagers had to have an EDSS score of 0 to 5.5; one or more relapses in the past year or two relapses in the previous two years; or MRI evidence of one or more gadolinium-enhancing lesions in the 6 months prior to trial randomization.
Fingolimod (Gilenya) is approved for the first-line treatment of relapsing forms of MS in adults in the United States. It is not yet FDA-approved for treatment of pediatric patients.
Dr. Chitnis said she was somewhat surprised by the strength of the findings in the PARADIGMS study. “These results showed very strong efficacy in young patients. However, as this study was being conducted, our group looked in more detail at the young adult subpopulation in the pivotal fingolimod adult studies, there was an improved effect in younger adults [those younger than 20 or younger than 30], compared to the entire group. Thus, one could extrapolate that the effects in adolescents would follow and show even greater efficacy.”
The study was sponsored by Novartis, the maker of fingolimod. Dr. Chitnis and nearly all of her coauthors disclosed financial ties to Novartis. Three authors are employees of Novartis.
SOURCE: Chitnis T et al. ACTRIMS Forum 2018, Abstract P025.
FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: The fingolimod group experienced 25 relapses in 180 patient-years, compared with 120 relapses in 163 patient-years in the interferon beta-1a group.
Study details: International, randomized, double-blind, parallel-group study of 215 people aged 10-17 years.
Disclosures: The study was sponsored by Novartis, the maker of fingolimod. Dr. Chitnis and nearly all of her coauthors disclosed financial ties to Novartis. Three authors are employees of Novartis.
Source: Chitnis T et al. ACTRIMS Forum 2018, Abstract P025.
Days of Therapy Avoided: A Novel Method for Measuring the Impact of an Antimicrobial Stewardship Program to Stop Antibiotics
A proposed metric to quantify the impact of an antimicrobial stewardship program (ASP) is using changes in the antibiotic days of therapy (DOT) per 1000 patient-days, which is the total number of days any dose of an antibiotic is administered during a specified time period, standardized by the number of patient-days.1 Although DOT is useful for comparing antibiotic use among hospitals or time periods, this metric is a composite result of an ASP’s often multifaceted approach to improving antibiotic use. Thus, DOT provides a loose estimate of the direct impact of specific ASP activities and does not quantify the amount of antibiotics directly avoided or direct cost savings on the patient level. To ameliorate this, we reviewed our institution’s ASP prospective audit and feedback (PAF) and applied a novel metric, days of therapy avoided (DOTA), to calculate the number of antibiotic days avoided that directly result from our ASP’s actions targeting antibiotic stoppage. From DOTA, we also calculate attributable cost savings.
METHODS
To quantify the direct impact of PAF, DOTA (Table) was calculated. Antibiotic costs avoided were calculated by multiplying the average wholesale price (AWP) per day (range: $0.44-$534; mean: $67.85) by DOTA. This calculation was done twice under 2 assumptions: that PAF led to the prevention of (1) 1 more day of antibiotic prescription and (2) the remainder of the documented or assumed LOT.
RESULTS
Over 4 years, the ASP made 1594 interventions to stop antibiotics. Accepted interventions totaled 1151 (72%): 513 (44.5%) for NI and 638 (55.4%) for TC, involving 431 and 575 unique patients, respectively. Nearly half (45.8%) of the NI interventions targeted asymptomatic bacteriuria, whereas respiratory tract infections were the most common (42.2%) indication for the TC intervention.
Under the most conservative assumption that each accepted PAF recommendation avoided 1 day of unnecessary antibiotics, we estimated a total of 1151 DOTA; 690 (59.9%) were intravenous antibiotics. The average DOT on which the PAF note was written was 3.07 ± 1.69 for NI and 6.38 ± 2.73 for TC. A planned LOT was documented for only 36.7% of the courses. On the basis of documented or assumed LOT, we estimate that the NI and TC interventions led to between 1077 and 2826 DOTA and between 397 and 1598 DOTA, respectively. Potential fluoroquinolone DOTA ranged from 300 to 1126; for third- and fourth-generation cephalosporins, there were 314 to 1017 DOTA.
Using the conservative estimate of 1151 DOTA, the costs avoided totaled $16,700, which includes $10,700 for intravenous antibiotics. When the AWP per day of each antibiotic was applied to the remaining LOTs avoided, the maximum potential cost savings was $67,100. Additional cost savings may have been realized if indirect expenses, such as pharmacy preparation and nursing administration time or costs of medical supplies, were evaluated.
CONCLUSION
We investigated DOTA as a measure of the direct patient-level and intervention-specific impact of an ASP’s PAF. DOTA may be useful for ASPs with limited access to an electronic record or electronically generated DOT reports because DOTA and cost savings can be tracked manually and prospectively with each accepted intervention. DOTA can also help ASPs identify which clinical conditions are responsible for the most antibiotic overuse, and thus may benefit from the development of clinical treatment guidelines. We found that the highest yield areas for DOTA were targeting asymptomatic bacteriuria (NI) and respiratory infections (TC). In doing so, these have also succeeded in reducing high-risk, broad-spectrum antimicrobials, such as fluoroquinolones and advanced-generation cephalosporins. Further research is needed to assess if DOTA correlates with other ASP metrics and clinical outcomes; however, current evidence supports that reducing unnecessary antibiotic use is fundamental to reducing antibiotic resistance and adverse events.10
The limitations of measuring DOTA include time consumption, particularly if not collected prospectively. However, we make several conclusions. ASP PAF stopping antibiotics was well accepted and reduced antibiotic use. Second, calculating DOTA requires little technology and only knowledge of the planned LOT and drug costs. DOTA also identifies which infectious indications to focus PAF efforts on and gain the greatest impact. Overall, DOTA is a simple, useful, and promising measurement of the direct antibiotic and economic impacts of specific ASP PAF and warrants further investigation as an ASP metric.
Acknowledgments
The authors thank the patients and RGH staff, particularly the departments of infectious diseases, pharmacy, and internal medicine, for their support.
Disclosure
The authors declare no conflicts of interest. This study was previously presented in poster form at the Society for Healthcare Epidemiology of America Spring Conference in St. Louis, Missouri (March 29-31, 2017).
1. Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds-Ashley ES. Structured Taskforce of Experts Working at Reliable Standards for Stewardship Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis. 2016;64(3):377-383. PubMed
2. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. PubMed
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52. PubMed
4. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. PubMed
5. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intraabdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. PubMed
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Supplement 2):S27-S72. PubMed
7. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. PubMed
8. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. PubMed
9. Cohen SH, Gerding DN, Johnson S, Kelly CP. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. PubMed
10. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ 2017;358:j3418. PubMed
A proposed metric to quantify the impact of an antimicrobial stewardship program (ASP) is using changes in the antibiotic days of therapy (DOT) per 1000 patient-days, which is the total number of days any dose of an antibiotic is administered during a specified time period, standardized by the number of patient-days.1 Although DOT is useful for comparing antibiotic use among hospitals or time periods, this metric is a composite result of an ASP’s often multifaceted approach to improving antibiotic use. Thus, DOT provides a loose estimate of the direct impact of specific ASP activities and does not quantify the amount of antibiotics directly avoided or direct cost savings on the patient level. To ameliorate this, we reviewed our institution’s ASP prospective audit and feedback (PAF) and applied a novel metric, days of therapy avoided (DOTA), to calculate the number of antibiotic days avoided that directly result from our ASP’s actions targeting antibiotic stoppage. From DOTA, we also calculate attributable cost savings.
METHODS
To quantify the direct impact of PAF, DOTA (Table) was calculated. Antibiotic costs avoided were calculated by multiplying the average wholesale price (AWP) per day (range: $0.44-$534; mean: $67.85) by DOTA. This calculation was done twice under 2 assumptions: that PAF led to the prevention of (1) 1 more day of antibiotic prescription and (2) the remainder of the documented or assumed LOT.
RESULTS
Over 4 years, the ASP made 1594 interventions to stop antibiotics. Accepted interventions totaled 1151 (72%): 513 (44.5%) for NI and 638 (55.4%) for TC, involving 431 and 575 unique patients, respectively. Nearly half (45.8%) of the NI interventions targeted asymptomatic bacteriuria, whereas respiratory tract infections were the most common (42.2%) indication for the TC intervention.
Under the most conservative assumption that each accepted PAF recommendation avoided 1 day of unnecessary antibiotics, we estimated a total of 1151 DOTA; 690 (59.9%) were intravenous antibiotics. The average DOT on which the PAF note was written was 3.07 ± 1.69 for NI and 6.38 ± 2.73 for TC. A planned LOT was documented for only 36.7% of the courses. On the basis of documented or assumed LOT, we estimate that the NI and TC interventions led to between 1077 and 2826 DOTA and between 397 and 1598 DOTA, respectively. Potential fluoroquinolone DOTA ranged from 300 to 1126; for third- and fourth-generation cephalosporins, there were 314 to 1017 DOTA.
Using the conservative estimate of 1151 DOTA, the costs avoided totaled $16,700, which includes $10,700 for intravenous antibiotics. When the AWP per day of each antibiotic was applied to the remaining LOTs avoided, the maximum potential cost savings was $67,100. Additional cost savings may have been realized if indirect expenses, such as pharmacy preparation and nursing administration time or costs of medical supplies, were evaluated.
CONCLUSION
We investigated DOTA as a measure of the direct patient-level and intervention-specific impact of an ASP’s PAF. DOTA may be useful for ASPs with limited access to an electronic record or electronically generated DOT reports because DOTA and cost savings can be tracked manually and prospectively with each accepted intervention. DOTA can also help ASPs identify which clinical conditions are responsible for the most antibiotic overuse, and thus may benefit from the development of clinical treatment guidelines. We found that the highest yield areas for DOTA were targeting asymptomatic bacteriuria (NI) and respiratory infections (TC). In doing so, these have also succeeded in reducing high-risk, broad-spectrum antimicrobials, such as fluoroquinolones and advanced-generation cephalosporins. Further research is needed to assess if DOTA correlates with other ASP metrics and clinical outcomes; however, current evidence supports that reducing unnecessary antibiotic use is fundamental to reducing antibiotic resistance and adverse events.10
The limitations of measuring DOTA include time consumption, particularly if not collected prospectively. However, we make several conclusions. ASP PAF stopping antibiotics was well accepted and reduced antibiotic use. Second, calculating DOTA requires little technology and only knowledge of the planned LOT and drug costs. DOTA also identifies which infectious indications to focus PAF efforts on and gain the greatest impact. Overall, DOTA is a simple, useful, and promising measurement of the direct antibiotic and economic impacts of specific ASP PAF and warrants further investigation as an ASP metric.
Acknowledgments
The authors thank the patients and RGH staff, particularly the departments of infectious diseases, pharmacy, and internal medicine, for their support.
Disclosure
The authors declare no conflicts of interest. This study was previously presented in poster form at the Society for Healthcare Epidemiology of America Spring Conference in St. Louis, Missouri (March 29-31, 2017).
A proposed metric to quantify the impact of an antimicrobial stewardship program (ASP) is using changes in the antibiotic days of therapy (DOT) per 1000 patient-days, which is the total number of days any dose of an antibiotic is administered during a specified time period, standardized by the number of patient-days.1 Although DOT is useful for comparing antibiotic use among hospitals or time periods, this metric is a composite result of an ASP’s often multifaceted approach to improving antibiotic use. Thus, DOT provides a loose estimate of the direct impact of specific ASP activities and does not quantify the amount of antibiotics directly avoided or direct cost savings on the patient level. To ameliorate this, we reviewed our institution’s ASP prospective audit and feedback (PAF) and applied a novel metric, days of therapy avoided (DOTA), to calculate the number of antibiotic days avoided that directly result from our ASP’s actions targeting antibiotic stoppage. From DOTA, we also calculate attributable cost savings.
METHODS
To quantify the direct impact of PAF, DOTA (Table) was calculated. Antibiotic costs avoided were calculated by multiplying the average wholesale price (AWP) per day (range: $0.44-$534; mean: $67.85) by DOTA. This calculation was done twice under 2 assumptions: that PAF led to the prevention of (1) 1 more day of antibiotic prescription and (2) the remainder of the documented or assumed LOT.
RESULTS
Over 4 years, the ASP made 1594 interventions to stop antibiotics. Accepted interventions totaled 1151 (72%): 513 (44.5%) for NI and 638 (55.4%) for TC, involving 431 and 575 unique patients, respectively. Nearly half (45.8%) of the NI interventions targeted asymptomatic bacteriuria, whereas respiratory tract infections were the most common (42.2%) indication for the TC intervention.
Under the most conservative assumption that each accepted PAF recommendation avoided 1 day of unnecessary antibiotics, we estimated a total of 1151 DOTA; 690 (59.9%) were intravenous antibiotics. The average DOT on which the PAF note was written was 3.07 ± 1.69 for NI and 6.38 ± 2.73 for TC. A planned LOT was documented for only 36.7% of the courses. On the basis of documented or assumed LOT, we estimate that the NI and TC interventions led to between 1077 and 2826 DOTA and between 397 and 1598 DOTA, respectively. Potential fluoroquinolone DOTA ranged from 300 to 1126; for third- and fourth-generation cephalosporins, there were 314 to 1017 DOTA.
Using the conservative estimate of 1151 DOTA, the costs avoided totaled $16,700, which includes $10,700 for intravenous antibiotics. When the AWP per day of each antibiotic was applied to the remaining LOTs avoided, the maximum potential cost savings was $67,100. Additional cost savings may have been realized if indirect expenses, such as pharmacy preparation and nursing administration time or costs of medical supplies, were evaluated.
CONCLUSION
We investigated DOTA as a measure of the direct patient-level and intervention-specific impact of an ASP’s PAF. DOTA may be useful for ASPs with limited access to an electronic record or electronically generated DOT reports because DOTA and cost savings can be tracked manually and prospectively with each accepted intervention. DOTA can also help ASPs identify which clinical conditions are responsible for the most antibiotic overuse, and thus may benefit from the development of clinical treatment guidelines. We found that the highest yield areas for DOTA were targeting asymptomatic bacteriuria (NI) and respiratory infections (TC). In doing so, these have also succeeded in reducing high-risk, broad-spectrum antimicrobials, such as fluoroquinolones and advanced-generation cephalosporins. Further research is needed to assess if DOTA correlates with other ASP metrics and clinical outcomes; however, current evidence supports that reducing unnecessary antibiotic use is fundamental to reducing antibiotic resistance and adverse events.10
The limitations of measuring DOTA include time consumption, particularly if not collected prospectively. However, we make several conclusions. ASP PAF stopping antibiotics was well accepted and reduced antibiotic use. Second, calculating DOTA requires little technology and only knowledge of the planned LOT and drug costs. DOTA also identifies which infectious indications to focus PAF efforts on and gain the greatest impact. Overall, DOTA is a simple, useful, and promising measurement of the direct antibiotic and economic impacts of specific ASP PAF and warrants further investigation as an ASP metric.
Acknowledgments
The authors thank the patients and RGH staff, particularly the departments of infectious diseases, pharmacy, and internal medicine, for their support.
Disclosure
The authors declare no conflicts of interest. This study was previously presented in poster form at the Society for Healthcare Epidemiology of America Spring Conference in St. Louis, Missouri (March 29-31, 2017).
1. Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds-Ashley ES. Structured Taskforce of Experts Working at Reliable Standards for Stewardship Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis. 2016;64(3):377-383. PubMed
2. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. PubMed
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52. PubMed
4. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. PubMed
5. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intraabdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. PubMed
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Supplement 2):S27-S72. PubMed
7. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. PubMed
8. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. PubMed
9. Cohen SH, Gerding DN, Johnson S, Kelly CP. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. PubMed
10. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ 2017;358:j3418. PubMed
1. Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds-Ashley ES. Structured Taskforce of Experts Working at Reliable Standards for Stewardship Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis. 2016;64(3):377-383. PubMed
2. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. PubMed
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52. PubMed
4. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. PubMed
5. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intraabdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. PubMed
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Supplement 2):S27-S72. PubMed
7. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. PubMed
8. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. PubMed
9. Cohen SH, Gerding DN, Johnson S, Kelly CP. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. PubMed
10. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ 2017;358:j3418. PubMed
© 2018 Society of Hospital Medicine
Immunotherapy-Induced Colitis: An Emerging Problem for the Hospitalist
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
© 2018 Society of Hospital Medicine