User login
Sneak Peek: The Hospital Leader blog – Nov. 2017
What we expect and what we get from work
Are American workers becoming happier with less? An interesting article in the Wall Street Journal reported on the findings of a recent survey of U.S. workers by the Conference Board, a research organization. Although the survey wasn’t specific to health care, much less to hospitalists, I see some parallels that might cause many of us to stop and think more carefully about what we expect from our work.
American workers today tend to have less job security and fewer employer-paid benefits than they did in previous generations. A companion graphic in the WSJ reported that, while in 1973 only 6% of Americans said they worked too many hours and 7% said they had trouble completing their work in the time allotted, by 2016 26% said they often worked more than 48 hours a week and half said they work during their free time at least periodically. Two-thirds of Americans now say they need to spend at least half of their day working at high speeds or meeting tight deadlines.
Yet, despite these trends, the Conference Board found that overall, U.S. workers are more satisfied with their jobs than they have been in the past. The WSJ article posits that workers are happier at work because they have adjusted to lower expectations of the employer-employee relationship. In addition, workers have more flexibility today to change jobs or companies to find the right fit or pursue advancement, and often have more influence over when, where, and how they do their jobs than ever before. Many are working as temps or independent contractors, or in similar “contingent” arrangements. Finally, more employers are offering a wider array of tools to aid with work-life balance, such as paid medical and family leave.
So what does all this have to do with hospitalists?
Read the full post at hospitalleader.org.
Also on The Hospital Leader…
- 95% of inpatient providers would get an F on this exam by Brad Flansbaum, DO, MPH, MHM
- When it comes to health care violence, silence isn’t an option by Danielle Scheurer, MD, MSCR, SFHM,
- How do we keep our providers safe? by Tracy Cardin, ACNP-BC, SFHM
- We have a voice. It’s time we use it #DoctorsSpeakOut by Vineet Arora, MD, MAPP, MHM
What we expect and what we get from work
Are American workers becoming happier with less? An interesting article in the Wall Street Journal reported on the findings of a recent survey of U.S. workers by the Conference Board, a research organization. Although the survey wasn’t specific to health care, much less to hospitalists, I see some parallels that might cause many of us to stop and think more carefully about what we expect from our work.
American workers today tend to have less job security and fewer employer-paid benefits than they did in previous generations. A companion graphic in the WSJ reported that, while in 1973 only 6% of Americans said they worked too many hours and 7% said they had trouble completing their work in the time allotted, by 2016 26% said they often worked more than 48 hours a week and half said they work during their free time at least periodically. Two-thirds of Americans now say they need to spend at least half of their day working at high speeds or meeting tight deadlines.
Yet, despite these trends, the Conference Board found that overall, U.S. workers are more satisfied with their jobs than they have been in the past. The WSJ article posits that workers are happier at work because they have adjusted to lower expectations of the employer-employee relationship. In addition, workers have more flexibility today to change jobs or companies to find the right fit or pursue advancement, and often have more influence over when, where, and how they do their jobs than ever before. Many are working as temps or independent contractors, or in similar “contingent” arrangements. Finally, more employers are offering a wider array of tools to aid with work-life balance, such as paid medical and family leave.
So what does all this have to do with hospitalists?
Read the full post at hospitalleader.org.
Also on The Hospital Leader…
- 95% of inpatient providers would get an F on this exam by Brad Flansbaum, DO, MPH, MHM
- When it comes to health care violence, silence isn’t an option by Danielle Scheurer, MD, MSCR, SFHM,
- How do we keep our providers safe? by Tracy Cardin, ACNP-BC, SFHM
- We have a voice. It’s time we use it #DoctorsSpeakOut by Vineet Arora, MD, MAPP, MHM
What we expect and what we get from work
Are American workers becoming happier with less? An interesting article in the Wall Street Journal reported on the findings of a recent survey of U.S. workers by the Conference Board, a research organization. Although the survey wasn’t specific to health care, much less to hospitalists, I see some parallels that might cause many of us to stop and think more carefully about what we expect from our work.
American workers today tend to have less job security and fewer employer-paid benefits than they did in previous generations. A companion graphic in the WSJ reported that, while in 1973 only 6% of Americans said they worked too many hours and 7% said they had trouble completing their work in the time allotted, by 2016 26% said they often worked more than 48 hours a week and half said they work during their free time at least periodically. Two-thirds of Americans now say they need to spend at least half of their day working at high speeds or meeting tight deadlines.
Yet, despite these trends, the Conference Board found that overall, U.S. workers are more satisfied with their jobs than they have been in the past. The WSJ article posits that workers are happier at work because they have adjusted to lower expectations of the employer-employee relationship. In addition, workers have more flexibility today to change jobs or companies to find the right fit or pursue advancement, and often have more influence over when, where, and how they do their jobs than ever before. Many are working as temps or independent contractors, or in similar “contingent” arrangements. Finally, more employers are offering a wider array of tools to aid with work-life balance, such as paid medical and family leave.
So what does all this have to do with hospitalists?
Read the full post at hospitalleader.org.
Also on The Hospital Leader…
- 95% of inpatient providers would get an F on this exam by Brad Flansbaum, DO, MPH, MHM
- When it comes to health care violence, silence isn’t an option by Danielle Scheurer, MD, MSCR, SFHM,
- How do we keep our providers safe? by Tracy Cardin, ACNP-BC, SFHM
- We have a voice. It’s time we use it #DoctorsSpeakOut by Vineet Arora, MD, MAPP, MHM
VIDEO: Study supports close follow-up of patients with high-risk adenomas plus serrated polyps
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
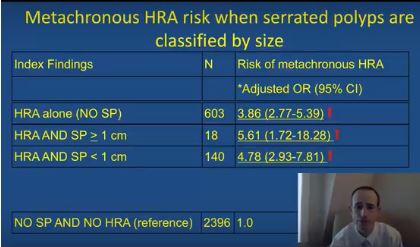
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: High-risk adenomas and the synchronous presence of serrated polyps significantly increased the risk of metachronous high-risk adenomas.
Major finding: Compared with individuals with unremarkable colonoscopies, the odds ratio was 5.6 after adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies.
Data source: Analyses of index and follow-up colonoscopies of 5,433 individuals from a population-based surveillance registry.
Disclosures: The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
Upfront chemotherapy yields excellent survival in patients with MZLs
For patients with advanced or recurrent marginal zone lymphomas (MZL) typically treated with radiotherapy, antibiotics, single-agent therapy, or observation, upfront chemotherapy was associated with high rates of both failure-free and overall survival at 10 years.
A retrospective analysis of data on 44 patients with either extranodal MZL (MALT), splenic MZL (SMZL), or nodal MZL (NMZL) treated with either the standard of care (for early-stage MALT) or with chemotherapy plus rituximab (for patients with advanced MALT, SMZL, or NMZL) showed a projected 10-year failure-free survival rate of 80%, and an overall survival rate of 100%, reported José L. Ortega, MD, of the University of Puerto Rico in San Juan, and his colleagues (Clin Lymphoma Myeloma Leuk. 2017 Sep 23. pii: S2152-2650[17]30632-8. doi: 10.1016/j.clml.2017.09.014).
“Although the watch and wait modality is still the most-used strategy for patients with advanced MZLs, with chemotherapy traditionally reserved for relapsed or advanced symptomatic disease, our data suggest that upfront chemotherapy is very effective for patients with advanced MALT, SMZL, and NMZL. However, it was not possible to definitely exclude that a less-aggressive approach such as single-agent rituximab could yield similar results,” the investigators wrote.
The standard of care for patients with localized MALT has traditionally been either antibiotic therapy for gastric MALT, or radiotherapy when antibiotics are not feasible. There is no standard of care, however, for either SMZL or NMZL, which are typically managed with either observation or single-agent rituximab, the investigators stated.
To see whether upfront chemotherapy with either FND-R (fludarabine, mitoxantrone, dexamethasone, and rituximab) or CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab) could improve outcomes in patients with advanced or recurrent MZLs, the investigators conducted a retrospective study of outcomes for 44 patients treated at their institution.
Group 1 comprised 22 patients with early-stage MALT treated with either radiotherapy or antibiotics, with or without surgery. Group 2 comprised 9 patients with advanced MALT, 9 with SMZL, and 4 with NMZL. Patients in this group underwent upfront chemotherapy with either FND-R (14 patients) or CHOP-R (8 patients). In addition, 16 patients in group 2 received maintenance rituximab.
All patients in each group had complete remissions. Two patients in group 1 had relapses (one at 70 months and one at 75 months) of stage I MALT that had previously been treated with radiotherapy. Both patients underwent salvage FND-R, and remained disease free at 27 and 39 months after relapse. There were no relapses in group 2.
The investigators deemed long-term toxicities to be “acceptable,” with most adverse effects in group 2 being hematologic in origin, including grade 3 or 4 neutropenia in 70%, thrombocytopenia in 22%, and anemia in 17%. Nonhematologic adverse events were mostly grade 1 or 2. There were no second malignancies reported at the most recent follow-up.
The investigators noted that the high complete remission rate and durable remissions with FND-R suggest that it has excellent activity against MZL, and that the long failure-free survival suggests the possibility of cure. They acknowledged, however, that their impressions were based on retrospective data and a small sample size, and that larger clinical trials are needed to confirm their results.
The investigators reported having no conflicts of interest.
For patients with advanced or recurrent marginal zone lymphomas (MZL) typically treated with radiotherapy, antibiotics, single-agent therapy, or observation, upfront chemotherapy was associated with high rates of both failure-free and overall survival at 10 years.
A retrospective analysis of data on 44 patients with either extranodal MZL (MALT), splenic MZL (SMZL), or nodal MZL (NMZL) treated with either the standard of care (for early-stage MALT) or with chemotherapy plus rituximab (for patients with advanced MALT, SMZL, or NMZL) showed a projected 10-year failure-free survival rate of 80%, and an overall survival rate of 100%, reported José L. Ortega, MD, of the University of Puerto Rico in San Juan, and his colleagues (Clin Lymphoma Myeloma Leuk. 2017 Sep 23. pii: S2152-2650[17]30632-8. doi: 10.1016/j.clml.2017.09.014).
“Although the watch and wait modality is still the most-used strategy for patients with advanced MZLs, with chemotherapy traditionally reserved for relapsed or advanced symptomatic disease, our data suggest that upfront chemotherapy is very effective for patients with advanced MALT, SMZL, and NMZL. However, it was not possible to definitely exclude that a less-aggressive approach such as single-agent rituximab could yield similar results,” the investigators wrote.
The standard of care for patients with localized MALT has traditionally been either antibiotic therapy for gastric MALT, or radiotherapy when antibiotics are not feasible. There is no standard of care, however, for either SMZL or NMZL, which are typically managed with either observation or single-agent rituximab, the investigators stated.
To see whether upfront chemotherapy with either FND-R (fludarabine, mitoxantrone, dexamethasone, and rituximab) or CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab) could improve outcomes in patients with advanced or recurrent MZLs, the investigators conducted a retrospective study of outcomes for 44 patients treated at their institution.
Group 1 comprised 22 patients with early-stage MALT treated with either radiotherapy or antibiotics, with or without surgery. Group 2 comprised 9 patients with advanced MALT, 9 with SMZL, and 4 with NMZL. Patients in this group underwent upfront chemotherapy with either FND-R (14 patients) or CHOP-R (8 patients). In addition, 16 patients in group 2 received maintenance rituximab.
All patients in each group had complete remissions. Two patients in group 1 had relapses (one at 70 months and one at 75 months) of stage I MALT that had previously been treated with radiotherapy. Both patients underwent salvage FND-R, and remained disease free at 27 and 39 months after relapse. There were no relapses in group 2.
The investigators deemed long-term toxicities to be “acceptable,” with most adverse effects in group 2 being hematologic in origin, including grade 3 or 4 neutropenia in 70%, thrombocytopenia in 22%, and anemia in 17%. Nonhematologic adverse events were mostly grade 1 or 2. There were no second malignancies reported at the most recent follow-up.
The investigators noted that the high complete remission rate and durable remissions with FND-R suggest that it has excellent activity against MZL, and that the long failure-free survival suggests the possibility of cure. They acknowledged, however, that their impressions were based on retrospective data and a small sample size, and that larger clinical trials are needed to confirm their results.
The investigators reported having no conflicts of interest.
For patients with advanced or recurrent marginal zone lymphomas (MZL) typically treated with radiotherapy, antibiotics, single-agent therapy, or observation, upfront chemotherapy was associated with high rates of both failure-free and overall survival at 10 years.
A retrospective analysis of data on 44 patients with either extranodal MZL (MALT), splenic MZL (SMZL), or nodal MZL (NMZL) treated with either the standard of care (for early-stage MALT) or with chemotherapy plus rituximab (for patients with advanced MALT, SMZL, or NMZL) showed a projected 10-year failure-free survival rate of 80%, and an overall survival rate of 100%, reported José L. Ortega, MD, of the University of Puerto Rico in San Juan, and his colleagues (Clin Lymphoma Myeloma Leuk. 2017 Sep 23. pii: S2152-2650[17]30632-8. doi: 10.1016/j.clml.2017.09.014).
“Although the watch and wait modality is still the most-used strategy for patients with advanced MZLs, with chemotherapy traditionally reserved for relapsed or advanced symptomatic disease, our data suggest that upfront chemotherapy is very effective for patients with advanced MALT, SMZL, and NMZL. However, it was not possible to definitely exclude that a less-aggressive approach such as single-agent rituximab could yield similar results,” the investigators wrote.
The standard of care for patients with localized MALT has traditionally been either antibiotic therapy for gastric MALT, or radiotherapy when antibiotics are not feasible. There is no standard of care, however, for either SMZL or NMZL, which are typically managed with either observation or single-agent rituximab, the investigators stated.
To see whether upfront chemotherapy with either FND-R (fludarabine, mitoxantrone, dexamethasone, and rituximab) or CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab) could improve outcomes in patients with advanced or recurrent MZLs, the investigators conducted a retrospective study of outcomes for 44 patients treated at their institution.
Group 1 comprised 22 patients with early-stage MALT treated with either radiotherapy or antibiotics, with or without surgery. Group 2 comprised 9 patients with advanced MALT, 9 with SMZL, and 4 with NMZL. Patients in this group underwent upfront chemotherapy with either FND-R (14 patients) or CHOP-R (8 patients). In addition, 16 patients in group 2 received maintenance rituximab.
All patients in each group had complete remissions. Two patients in group 1 had relapses (one at 70 months and one at 75 months) of stage I MALT that had previously been treated with radiotherapy. Both patients underwent salvage FND-R, and remained disease free at 27 and 39 months after relapse. There were no relapses in group 2.
The investigators deemed long-term toxicities to be “acceptable,” with most adverse effects in group 2 being hematologic in origin, including grade 3 or 4 neutropenia in 70%, thrombocytopenia in 22%, and anemia in 17%. Nonhematologic adverse events were mostly grade 1 or 2. There were no second malignancies reported at the most recent follow-up.
The investigators noted that the high complete remission rate and durable remissions with FND-R suggest that it has excellent activity against MZL, and that the long failure-free survival suggests the possibility of cure. They acknowledged, however, that their impressions were based on retrospective data and a small sample size, and that larger clinical trials are needed to confirm their results.
The investigators reported having no conflicts of interest.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The 10-year projected failure-free survival rate was 80%, and the overall survival rate was 100%.
Data source: Retrospective single-center study of 44 patients with marginal zone lymphomas.
Disclosures: The investigators reported having no conflicts of interest.
Marginal zone lymphoma treatment studies to be presented at ASH
Findings from several studies on marginal zone lymphoma (MZL) will be presented during oral and poster sessions at the annual meeting of the American Society of Hematology, with a focus on evaluating combination treatment approaches.
Some of the MZL treatment–related studies include the assessment of chlorambucil plus rituximab in patients with extranodal marginal zone B-cell lymphoma of mucosa associated lymphoid tissue, the combination of bendamustine with rituximab for first-line treatment of splenic MZL, and the safety and progression-free survival associated with lenalidomide and rituximab in previously untreated patients with MZL.
Other studies consider how to approach refractory disease. One study looks at the use of ibrutinib in patients with relapsed/refractory MZL, and researchers will also present findings from a systematic literature review of the efficacy and safety of various treatments among patients with relapsed/refractory MZL.
Abstract 1506: IELSG-38: A Phase II Study of Chlorambucil in Combination with Rituximab Followed by Maintenance Therapy with Subcutaneous Rituximab in Patients with Extranodal Marginal Zone B-Cell Lymphoma of Mucosa Associated Lymphoid Tissue (MALT) .
Abstract 4062: Bendamustine in Combination with Rituximab as First-Line Treatment of Splenic Marginal Zone Lymphoma (BRISMA). Results of the IELSG-36 Phase II Study.
Abstract 3026: Ibrutinib Therapy in Patients with Relapsed/Refractory Marginal Zone Lymphoma: Analysis by Prior Rituximab Treatment and Baseline Mutations.
Abstract 4040: Safety and Activity of Lenalidomide and Rituximab in Previously Untreated Marginal Zone Lymphoma: Subgroup Analysis and Long-Term Follow-Up of an Open-Label Phase II Trial.
Abstract 2783: Systematic Literature Review of the Clinical Efficacy and Safety of Treatments in the Relapsed/Refractory Setting for Patients with Follicular Lymphoma or Marginal Zone Lymphoma.
Findings from several studies on marginal zone lymphoma (MZL) will be presented during oral and poster sessions at the annual meeting of the American Society of Hematology, with a focus on evaluating combination treatment approaches.
Some of the MZL treatment–related studies include the assessment of chlorambucil plus rituximab in patients with extranodal marginal zone B-cell lymphoma of mucosa associated lymphoid tissue, the combination of bendamustine with rituximab for first-line treatment of splenic MZL, and the safety and progression-free survival associated with lenalidomide and rituximab in previously untreated patients with MZL.
Other studies consider how to approach refractory disease. One study looks at the use of ibrutinib in patients with relapsed/refractory MZL, and researchers will also present findings from a systematic literature review of the efficacy and safety of various treatments among patients with relapsed/refractory MZL.
Abstract 1506: IELSG-38: A Phase II Study of Chlorambucil in Combination with Rituximab Followed by Maintenance Therapy with Subcutaneous Rituximab in Patients with Extranodal Marginal Zone B-Cell Lymphoma of Mucosa Associated Lymphoid Tissue (MALT) .
Abstract 4062: Bendamustine in Combination with Rituximab as First-Line Treatment of Splenic Marginal Zone Lymphoma (BRISMA). Results of the IELSG-36 Phase II Study.
Abstract 3026: Ibrutinib Therapy in Patients with Relapsed/Refractory Marginal Zone Lymphoma: Analysis by Prior Rituximab Treatment and Baseline Mutations.
Abstract 4040: Safety and Activity of Lenalidomide and Rituximab in Previously Untreated Marginal Zone Lymphoma: Subgroup Analysis and Long-Term Follow-Up of an Open-Label Phase II Trial.
Abstract 2783: Systematic Literature Review of the Clinical Efficacy and Safety of Treatments in the Relapsed/Refractory Setting for Patients with Follicular Lymphoma or Marginal Zone Lymphoma.
Findings from several studies on marginal zone lymphoma (MZL) will be presented during oral and poster sessions at the annual meeting of the American Society of Hematology, with a focus on evaluating combination treatment approaches.
Some of the MZL treatment–related studies include the assessment of chlorambucil plus rituximab in patients with extranodal marginal zone B-cell lymphoma of mucosa associated lymphoid tissue, the combination of bendamustine with rituximab for first-line treatment of splenic MZL, and the safety and progression-free survival associated with lenalidomide and rituximab in previously untreated patients with MZL.
Other studies consider how to approach refractory disease. One study looks at the use of ibrutinib in patients with relapsed/refractory MZL, and researchers will also present findings from a systematic literature review of the efficacy and safety of various treatments among patients with relapsed/refractory MZL.
Abstract 1506: IELSG-38: A Phase II Study of Chlorambucil in Combination with Rituximab Followed by Maintenance Therapy with Subcutaneous Rituximab in Patients with Extranodal Marginal Zone B-Cell Lymphoma of Mucosa Associated Lymphoid Tissue (MALT) .
Abstract 4062: Bendamustine in Combination with Rituximab as First-Line Treatment of Splenic Marginal Zone Lymphoma (BRISMA). Results of the IELSG-36 Phase II Study.
Abstract 3026: Ibrutinib Therapy in Patients with Relapsed/Refractory Marginal Zone Lymphoma: Analysis by Prior Rituximab Treatment and Baseline Mutations.
Abstract 4040: Safety and Activity of Lenalidomide and Rituximab in Previously Untreated Marginal Zone Lymphoma: Subgroup Analysis and Long-Term Follow-Up of an Open-Label Phase II Trial.
Abstract 2783: Systematic Literature Review of the Clinical Efficacy and Safety of Treatments in the Relapsed/Refractory Setting for Patients with Follicular Lymphoma or Marginal Zone Lymphoma.
FROM ASH 2017
What Are the Long-Term Neurologic Complications of Childhood Cancer?
KANSAS CITY, MO—As cancer treatment advances, the prevalence of childhood cancer survivors increases; as a result, physicians see more neurologic complications of surgery, chemotherapy, and radiation, according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“There is an increasing impact of neurologic and neuropsychological toxicity that underscores the need for intervention and follow-up over the lifespan,” said Nicole Ullrich, MD, PhD, Director of Neurologic Neuro-Oncology at the Dana-Farber/Boston Children's Cancer and Blood Disorders Center.
Lifelong Effects
The prevalence of adult survivors of childhood cancer is one in 250 people, and there are more than 270,000 childhood cancer survivors in the United States. These cancer survivors “experience long-term toxicities affecting their respiratory system, cardiovascular system, cerebrovascular system, reproductive system, and a gamut of other late effects of treatment that we need to think about as they continue to advance into adulthood,” said Dr. Ullrich.
“Ninety-five percent of all cancer survivors will have a chronic health problem by the age of 45, and 80% will have a severe or life-threatening condition,” she said. The risk is greater for patients with CNS malignancies.
Childhood cancer survivors may develop physical, cognitive, and psychological issues later in life. These late effects have a significant impact on their quality and quantity of life. Neurologic issues can include headache, seizures, and stroke.
Headache and Seizures
Patients with elevated intracranial pressure or large tumors have an increased risk of headache. Nontumor causes of headache may include medications, such as antiemetic drugs and chemotherapy. Radiation therapy may cause acute radiation necrosis and long-term vascular issues. Supportive therapies, including steroids, antacids, and antinausea regimens, can cause headaches as well as sleep disruption, said Dr. Ullrich.
Approximately 15% to 25% of children with a brain tumor present with a seizure. Seizures are more often associated with low-grade tumors. Children with solid tumors or leukemia often have seizures without clinical or radiologic signs of a structural lesion. When a surgeon removes the tumor, there may be associated areas of dysplasia that surround the tumor, said Dr. Ullrich. Strategies such as electrocorticography or intraoperative monitoring can help identify epileptic zones for removal during surgery.
Potential causes of seizures include the tumor itself, surrounding edema, areas of cortical dysplasia, hyperexcitability related to neurotransmitters and glutamate levels, and scar formation that occurs after tumor resection. Individuals who have had cortically based or temporal lobe tumors or who have had incomplete resection or preexisting seizures before diagnosis have the highest risk of developing seizures, even years after completion of therapy.
EEG can help confirm seizures and distinguish between seizure types. It also can aid in the choice of an anticonvulsant. “We tend to lean more towards non-enzyme-inducing anticonvulsants in order to not interfere with concurrent chemotherapy,” said Dr. Ullrich. If seizures are acutely related to a drug or infection, Dr. Ullrich aims to withdraw anticonvulsants as soon as possible.
Surgery and Chemotherapy
Children who have had a gross total resection of the primary tumor may still be at risk for acute neurologic, neurosensory, and neuromotor issues, endocrine dysfunction, cerebellar mutism, and other neuropsychological deficits, said Dr. Ullrich.
Deficits and long-term effects of brain tumor treatment mainly depend on tumor location. Maximal tumor resection may cause hypopituitarism, vascular issues, and vision issues. Image-guided therapy can help surgeons remove most, if not all, of the tumor during the initial resection.
Chemotherapy-related neurologic effects are common, said Dr. Ullrich. One of the main side effects is chemotherapy-induced peripheral neuropathy (ie, any injury or inflammation to the peripheral nerve due to administration of a chemotherapeutic drug). Patients with peripheral neuropathy may exhibit changes in their gait, loss of reflexes, and sensory changes. Pediatric patients with Charcot-Marie-Tooth disease or other hereditary neuropathies have a greater risk of chemotherapy-induced peripheral neuropathy.
Radiation
Age at the time of radiation, radiation field, genetic predisposition, and total dose are all risk factors for radiation-induced cognitive injury. One study found that children younger than 7 had the most significant decline in overall IQ after radiation. This research led to a shift in the development of treatment protocols and inspired physicians to strive to decrease and eliminate the use of radiation in the youngest patients, said Dr. Ullrich.
Another consequence of radiation may be the development of secondary neoplasms. The mean interval between the time of radiation and the development of secondary tumors is around eight years. These secondary neoplasms resulting from radiation often have anaplastic features. Childhood cancer survivors should see a dermatologist to monitor radiated areas for skin cancer.
Stroke and SMART Syndrome
Stroke is increasingly recognized as a late consequence of cancer treatment, especially in patients who have been treated for leukemia and brain tumors. Studies have found that prior radiation is an independent predictor of stroke. Mueller et al found that pediatric cancer survivors with hypertension had a fourfold increased risk of stroke, compared with sibling controls.
“Screen for correctable risk factors such as hypertension, hypercholesterolemia, hyperlipidemia, obesity, and sedentary lifestyle,” said Dr. Ullrich.
SMART syndrome (stroke-like migraine attacks after radiation therapy) is another potential complication that can occur years after radiation therapy. The syndrome can present like a transient ischemic attack, and symptoms can last hours to days. This syndrome can be treated with aggressive preventive headache care.
Mitigation and Prevention
Neurologists can take steps to help prevent or ameliorate some of these late effects. The Children's Oncology Group has created Passport for Care, a tool that allows patients to share a summary of their cancer treatments and follow-up recommendations with their primary care providers. The Children's Oncology Group also has created long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.
In addition, new and refined surgical techniques help detect and remove residual tumor after surgery. Proton beam radiation, intensity-modulated radiation therapy, and other approaches can help reduce doses of radiation, potentially reducing cognitive risks. “The goal is to shift the paradigm from just categorizing the late effects to mitigating them and actually preventing them in the first place,” said Dr. Ullrich.
—Erica Tricarico
Suggested Reading
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649-655.
KANSAS CITY, MO—As cancer treatment advances, the prevalence of childhood cancer survivors increases; as a result, physicians see more neurologic complications of surgery, chemotherapy, and radiation, according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“There is an increasing impact of neurologic and neuropsychological toxicity that underscores the need for intervention and follow-up over the lifespan,” said Nicole Ullrich, MD, PhD, Director of Neurologic Neuro-Oncology at the Dana-Farber/Boston Children's Cancer and Blood Disorders Center.
Lifelong Effects
The prevalence of adult survivors of childhood cancer is one in 250 people, and there are more than 270,000 childhood cancer survivors in the United States. These cancer survivors “experience long-term toxicities affecting their respiratory system, cardiovascular system, cerebrovascular system, reproductive system, and a gamut of other late effects of treatment that we need to think about as they continue to advance into adulthood,” said Dr. Ullrich.
“Ninety-five percent of all cancer survivors will have a chronic health problem by the age of 45, and 80% will have a severe or life-threatening condition,” she said. The risk is greater for patients with CNS malignancies.
Childhood cancer survivors may develop physical, cognitive, and psychological issues later in life. These late effects have a significant impact on their quality and quantity of life. Neurologic issues can include headache, seizures, and stroke.
Headache and Seizures
Patients with elevated intracranial pressure or large tumors have an increased risk of headache. Nontumor causes of headache may include medications, such as antiemetic drugs and chemotherapy. Radiation therapy may cause acute radiation necrosis and long-term vascular issues. Supportive therapies, including steroids, antacids, and antinausea regimens, can cause headaches as well as sleep disruption, said Dr. Ullrich.
Approximately 15% to 25% of children with a brain tumor present with a seizure. Seizures are more often associated with low-grade tumors. Children with solid tumors or leukemia often have seizures without clinical or radiologic signs of a structural lesion. When a surgeon removes the tumor, there may be associated areas of dysplasia that surround the tumor, said Dr. Ullrich. Strategies such as electrocorticography or intraoperative monitoring can help identify epileptic zones for removal during surgery.
Potential causes of seizures include the tumor itself, surrounding edema, areas of cortical dysplasia, hyperexcitability related to neurotransmitters and glutamate levels, and scar formation that occurs after tumor resection. Individuals who have had cortically based or temporal lobe tumors or who have had incomplete resection or preexisting seizures before diagnosis have the highest risk of developing seizures, even years after completion of therapy.
EEG can help confirm seizures and distinguish between seizure types. It also can aid in the choice of an anticonvulsant. “We tend to lean more towards non-enzyme-inducing anticonvulsants in order to not interfere with concurrent chemotherapy,” said Dr. Ullrich. If seizures are acutely related to a drug or infection, Dr. Ullrich aims to withdraw anticonvulsants as soon as possible.
Surgery and Chemotherapy
Children who have had a gross total resection of the primary tumor may still be at risk for acute neurologic, neurosensory, and neuromotor issues, endocrine dysfunction, cerebellar mutism, and other neuropsychological deficits, said Dr. Ullrich.
Deficits and long-term effects of brain tumor treatment mainly depend on tumor location. Maximal tumor resection may cause hypopituitarism, vascular issues, and vision issues. Image-guided therapy can help surgeons remove most, if not all, of the tumor during the initial resection.
Chemotherapy-related neurologic effects are common, said Dr. Ullrich. One of the main side effects is chemotherapy-induced peripheral neuropathy (ie, any injury or inflammation to the peripheral nerve due to administration of a chemotherapeutic drug). Patients with peripheral neuropathy may exhibit changes in their gait, loss of reflexes, and sensory changes. Pediatric patients with Charcot-Marie-Tooth disease or other hereditary neuropathies have a greater risk of chemotherapy-induced peripheral neuropathy.
Radiation
Age at the time of radiation, radiation field, genetic predisposition, and total dose are all risk factors for radiation-induced cognitive injury. One study found that children younger than 7 had the most significant decline in overall IQ after radiation. This research led to a shift in the development of treatment protocols and inspired physicians to strive to decrease and eliminate the use of radiation in the youngest patients, said Dr. Ullrich.
Another consequence of radiation may be the development of secondary neoplasms. The mean interval between the time of radiation and the development of secondary tumors is around eight years. These secondary neoplasms resulting from radiation often have anaplastic features. Childhood cancer survivors should see a dermatologist to monitor radiated areas for skin cancer.
Stroke and SMART Syndrome
Stroke is increasingly recognized as a late consequence of cancer treatment, especially in patients who have been treated for leukemia and brain tumors. Studies have found that prior radiation is an independent predictor of stroke. Mueller et al found that pediatric cancer survivors with hypertension had a fourfold increased risk of stroke, compared with sibling controls.
“Screen for correctable risk factors such as hypertension, hypercholesterolemia, hyperlipidemia, obesity, and sedentary lifestyle,” said Dr. Ullrich.
SMART syndrome (stroke-like migraine attacks after radiation therapy) is another potential complication that can occur years after radiation therapy. The syndrome can present like a transient ischemic attack, and symptoms can last hours to days. This syndrome can be treated with aggressive preventive headache care.
Mitigation and Prevention
Neurologists can take steps to help prevent or ameliorate some of these late effects. The Children's Oncology Group has created Passport for Care, a tool that allows patients to share a summary of their cancer treatments and follow-up recommendations with their primary care providers. The Children's Oncology Group also has created long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.
In addition, new and refined surgical techniques help detect and remove residual tumor after surgery. Proton beam radiation, intensity-modulated radiation therapy, and other approaches can help reduce doses of radiation, potentially reducing cognitive risks. “The goal is to shift the paradigm from just categorizing the late effects to mitigating them and actually preventing them in the first place,” said Dr. Ullrich.
—Erica Tricarico
Suggested Reading
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649-655.
KANSAS CITY, MO—As cancer treatment advances, the prevalence of childhood cancer survivors increases; as a result, physicians see more neurologic complications of surgery, chemotherapy, and radiation, according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“There is an increasing impact of neurologic and neuropsychological toxicity that underscores the need for intervention and follow-up over the lifespan,” said Nicole Ullrich, MD, PhD, Director of Neurologic Neuro-Oncology at the Dana-Farber/Boston Children's Cancer and Blood Disorders Center.
Lifelong Effects
The prevalence of adult survivors of childhood cancer is one in 250 people, and there are more than 270,000 childhood cancer survivors in the United States. These cancer survivors “experience long-term toxicities affecting their respiratory system, cardiovascular system, cerebrovascular system, reproductive system, and a gamut of other late effects of treatment that we need to think about as they continue to advance into adulthood,” said Dr. Ullrich.
“Ninety-five percent of all cancer survivors will have a chronic health problem by the age of 45, and 80% will have a severe or life-threatening condition,” she said. The risk is greater for patients with CNS malignancies.
Childhood cancer survivors may develop physical, cognitive, and psychological issues later in life. These late effects have a significant impact on their quality and quantity of life. Neurologic issues can include headache, seizures, and stroke.
Headache and Seizures
Patients with elevated intracranial pressure or large tumors have an increased risk of headache. Nontumor causes of headache may include medications, such as antiemetic drugs and chemotherapy. Radiation therapy may cause acute radiation necrosis and long-term vascular issues. Supportive therapies, including steroids, antacids, and antinausea regimens, can cause headaches as well as sleep disruption, said Dr. Ullrich.
Approximately 15% to 25% of children with a brain tumor present with a seizure. Seizures are more often associated with low-grade tumors. Children with solid tumors or leukemia often have seizures without clinical or radiologic signs of a structural lesion. When a surgeon removes the tumor, there may be associated areas of dysplasia that surround the tumor, said Dr. Ullrich. Strategies such as electrocorticography or intraoperative monitoring can help identify epileptic zones for removal during surgery.
Potential causes of seizures include the tumor itself, surrounding edema, areas of cortical dysplasia, hyperexcitability related to neurotransmitters and glutamate levels, and scar formation that occurs after tumor resection. Individuals who have had cortically based or temporal lobe tumors or who have had incomplete resection or preexisting seizures before diagnosis have the highest risk of developing seizures, even years after completion of therapy.
EEG can help confirm seizures and distinguish between seizure types. It also can aid in the choice of an anticonvulsant. “We tend to lean more towards non-enzyme-inducing anticonvulsants in order to not interfere with concurrent chemotherapy,” said Dr. Ullrich. If seizures are acutely related to a drug or infection, Dr. Ullrich aims to withdraw anticonvulsants as soon as possible.
Surgery and Chemotherapy
Children who have had a gross total resection of the primary tumor may still be at risk for acute neurologic, neurosensory, and neuromotor issues, endocrine dysfunction, cerebellar mutism, and other neuropsychological deficits, said Dr. Ullrich.
Deficits and long-term effects of brain tumor treatment mainly depend on tumor location. Maximal tumor resection may cause hypopituitarism, vascular issues, and vision issues. Image-guided therapy can help surgeons remove most, if not all, of the tumor during the initial resection.
Chemotherapy-related neurologic effects are common, said Dr. Ullrich. One of the main side effects is chemotherapy-induced peripheral neuropathy (ie, any injury or inflammation to the peripheral nerve due to administration of a chemotherapeutic drug). Patients with peripheral neuropathy may exhibit changes in their gait, loss of reflexes, and sensory changes. Pediatric patients with Charcot-Marie-Tooth disease or other hereditary neuropathies have a greater risk of chemotherapy-induced peripheral neuropathy.
Radiation
Age at the time of radiation, radiation field, genetic predisposition, and total dose are all risk factors for radiation-induced cognitive injury. One study found that children younger than 7 had the most significant decline in overall IQ after radiation. This research led to a shift in the development of treatment protocols and inspired physicians to strive to decrease and eliminate the use of radiation in the youngest patients, said Dr. Ullrich.
Another consequence of radiation may be the development of secondary neoplasms. The mean interval between the time of radiation and the development of secondary tumors is around eight years. These secondary neoplasms resulting from radiation often have anaplastic features. Childhood cancer survivors should see a dermatologist to monitor radiated areas for skin cancer.
Stroke and SMART Syndrome
Stroke is increasingly recognized as a late consequence of cancer treatment, especially in patients who have been treated for leukemia and brain tumors. Studies have found that prior radiation is an independent predictor of stroke. Mueller et al found that pediatric cancer survivors with hypertension had a fourfold increased risk of stroke, compared with sibling controls.
“Screen for correctable risk factors such as hypertension, hypercholesterolemia, hyperlipidemia, obesity, and sedentary lifestyle,” said Dr. Ullrich.
SMART syndrome (stroke-like migraine attacks after radiation therapy) is another potential complication that can occur years after radiation therapy. The syndrome can present like a transient ischemic attack, and symptoms can last hours to days. This syndrome can be treated with aggressive preventive headache care.
Mitigation and Prevention
Neurologists can take steps to help prevent or ameliorate some of these late effects. The Children's Oncology Group has created Passport for Care, a tool that allows patients to share a summary of their cancer treatments and follow-up recommendations with their primary care providers. The Children's Oncology Group also has created long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers.
In addition, new and refined surgical techniques help detect and remove residual tumor after surgery. Proton beam radiation, intensity-modulated radiation therapy, and other approaches can help reduce doses of radiation, potentially reducing cognitive risks. “The goal is to shift the paradigm from just categorizing the late effects to mitigating them and actually preventing them in the first place,” said Dr. Ullrich.
—Erica Tricarico
Suggested Reading
Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649-655.
Adjusting fecal immunochemical test thresholds improved their performance
Physicians can minimize the heterogeneity of fecal immunochemical colorectal cancer screening tests by adjusting thresholds for positivity, according to researchers. The report is in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2017.09.018).
“Rather than simply using thresholds recommended by the manufacturer, screening programs should choose thresholds based on intended levels of specificity and manageable positivity rates,” wrote PhD student Anton Gies of the German Cancer Research Center and the National Center for Tumor Diseases in Heidelberg, Germany, with his associates.
The investigators directly compared nine different fecal immunochemical assays using stool samples from 516 individuals, of whom 216 had advanced adenoma or colorectal cancer. Using thresholds recommended by manufacturers (2-17 mcg Hb/g feces) produced widely ranging sensitivities (22%-46%) and specificities (86%-98%). Using a uniform threshold of 15 mcg Hb/g feces narrowed the range of specificity (94%-98%), but sensitivities remained quite variable (16%-34%). Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively), the researchers reported.
Increasingly, physicians are using fecal immunochemical testing to screen for colorectal neoplasia. In a prior study (Ann Intern Med. 2009 Feb 3;150[3]:162-90) investigators evaluated the diagnostic performance of six qualitative point-of-care fecal immunochemical tests among screening colonoscopy patients in Germany, and found that the tests had highly variable sensitivities and specificities for the detection of colorectal neoplasia. Not surprisingly, the most sensitive tests were the least specific, and vice versa, which is the problem with using fixed thresholds in qualitative fecal immunochemical tests, the researchers asserted.
Quantitative fecal immunochemical tests are more flexible than qualitative assays because users can adjust thresholds based on fecal hemoglobin concentrations. However, very few studies had directly compared sensitivities and specificities among quantitative fecal immunochemical tests, and “it is unclear to what extent differences ... reflect true heterogeneity in test performance or differences in study populations or varying pre-analytical conditions,” the investigators wrote. Patients in their study underwent colonoscopies in Germany between 2005 and 2010, and fecal samples were stored at –80 °C until analysis. The researchers calculated test sensitivities and specificities by using colonoscopy and histology reports evaluated by blinded, trained research assistants.
“Apparent heterogeneity in diagnostic performance of quantitative fecal immunochemical tests can be overcome to a large extent by adjusting thresholds to yield defined levels of specificity or positivity rates,” the investigators concluded. Only 16 patients in this study had colorectal cancer, which made it difficult to pinpoint test sensitivity for this finding, they noted. However, they found similar sensitivity estimates for colorectal cancer in an ancillary clinical study.
Manufacturers provided test kits free of charge. There were no external funding sources, and the researchers reported having no conflicts of interest.
The fecal immunochemical test (FIT) is an important option for colorectal cancer screening, endorsed by guidelines and effective for mass screening using mailed outreach. Patients offered FIT or a choice between FIT and colonoscopy are more likely to be screened.
In the United States, FIT is a qualitative test (reported as positive or negative), based on Food and Drug Administration regulations, in an attempt to simplify clinical decision making. In Europe, FIT has been used quantitatively, with adjustable positivity rate and sensitivity pegged to available colonoscopy resources. Adding complexity, there are multiple FIT brands, each with varying performance, even at similar hemoglobin concentrations. Each brand has a different sensitivity, specificity, and positivity rate, because reagents, buffers, and collection devices vary. Ambient temperature during mailing and transport time to processing labs can also affect test performance.
Theodore R. Levin, MD, is chief of gastroenterology, Kaiser Permanente Medical Center, Walnut Creek, Calif. He has no conflicts of interest.
The fecal immunochemical test (FIT) is an important option for colorectal cancer screening, endorsed by guidelines and effective for mass screening using mailed outreach. Patients offered FIT or a choice between FIT and colonoscopy are more likely to be screened.
In the United States, FIT is a qualitative test (reported as positive or negative), based on Food and Drug Administration regulations, in an attempt to simplify clinical decision making. In Europe, FIT has been used quantitatively, with adjustable positivity rate and sensitivity pegged to available colonoscopy resources. Adding complexity, there are multiple FIT brands, each with varying performance, even at similar hemoglobin concentrations. Each brand has a different sensitivity, specificity, and positivity rate, because reagents, buffers, and collection devices vary. Ambient temperature during mailing and transport time to processing labs can also affect test performance.
Theodore R. Levin, MD, is chief of gastroenterology, Kaiser Permanente Medical Center, Walnut Creek, Calif. He has no conflicts of interest.
The fecal immunochemical test (FIT) is an important option for colorectal cancer screening, endorsed by guidelines and effective for mass screening using mailed outreach. Patients offered FIT or a choice between FIT and colonoscopy are more likely to be screened.
In the United States, FIT is a qualitative test (reported as positive or negative), based on Food and Drug Administration regulations, in an attempt to simplify clinical decision making. In Europe, FIT has been used quantitatively, with adjustable positivity rate and sensitivity pegged to available colonoscopy resources. Adding complexity, there are multiple FIT brands, each with varying performance, even at similar hemoglobin concentrations. Each brand has a different sensitivity, specificity, and positivity rate, because reagents, buffers, and collection devices vary. Ambient temperature during mailing and transport time to processing labs can also affect test performance.
Theodore R. Levin, MD, is chief of gastroenterology, Kaiser Permanente Medical Center, Walnut Creek, Calif. He has no conflicts of interest.
Physicians can minimize the heterogeneity of fecal immunochemical colorectal cancer screening tests by adjusting thresholds for positivity, according to researchers. The report is in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2017.09.018).
“Rather than simply using thresholds recommended by the manufacturer, screening programs should choose thresholds based on intended levels of specificity and manageable positivity rates,” wrote PhD student Anton Gies of the German Cancer Research Center and the National Center for Tumor Diseases in Heidelberg, Germany, with his associates.
The investigators directly compared nine different fecal immunochemical assays using stool samples from 516 individuals, of whom 216 had advanced adenoma or colorectal cancer. Using thresholds recommended by manufacturers (2-17 mcg Hb/g feces) produced widely ranging sensitivities (22%-46%) and specificities (86%-98%). Using a uniform threshold of 15 mcg Hb/g feces narrowed the range of specificity (94%-98%), but sensitivities remained quite variable (16%-34%). Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively), the researchers reported.
Increasingly, physicians are using fecal immunochemical testing to screen for colorectal neoplasia. In a prior study (Ann Intern Med. 2009 Feb 3;150[3]:162-90) investigators evaluated the diagnostic performance of six qualitative point-of-care fecal immunochemical tests among screening colonoscopy patients in Germany, and found that the tests had highly variable sensitivities and specificities for the detection of colorectal neoplasia. Not surprisingly, the most sensitive tests were the least specific, and vice versa, which is the problem with using fixed thresholds in qualitative fecal immunochemical tests, the researchers asserted.
Quantitative fecal immunochemical tests are more flexible than qualitative assays because users can adjust thresholds based on fecal hemoglobin concentrations. However, very few studies had directly compared sensitivities and specificities among quantitative fecal immunochemical tests, and “it is unclear to what extent differences ... reflect true heterogeneity in test performance or differences in study populations or varying pre-analytical conditions,” the investigators wrote. Patients in their study underwent colonoscopies in Germany between 2005 and 2010, and fecal samples were stored at –80 °C until analysis. The researchers calculated test sensitivities and specificities by using colonoscopy and histology reports evaluated by blinded, trained research assistants.
“Apparent heterogeneity in diagnostic performance of quantitative fecal immunochemical tests can be overcome to a large extent by adjusting thresholds to yield defined levels of specificity or positivity rates,” the investigators concluded. Only 16 patients in this study had colorectal cancer, which made it difficult to pinpoint test sensitivity for this finding, they noted. However, they found similar sensitivity estimates for colorectal cancer in an ancillary clinical study.
Manufacturers provided test kits free of charge. There were no external funding sources, and the researchers reported having no conflicts of interest.
Physicians can minimize the heterogeneity of fecal immunochemical colorectal cancer screening tests by adjusting thresholds for positivity, according to researchers. The report is in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2017.09.018).
“Rather than simply using thresholds recommended by the manufacturer, screening programs should choose thresholds based on intended levels of specificity and manageable positivity rates,” wrote PhD student Anton Gies of the German Cancer Research Center and the National Center for Tumor Diseases in Heidelberg, Germany, with his associates.
The investigators directly compared nine different fecal immunochemical assays using stool samples from 516 individuals, of whom 216 had advanced adenoma or colorectal cancer. Using thresholds recommended by manufacturers (2-17 mcg Hb/g feces) produced widely ranging sensitivities (22%-46%) and specificities (86%-98%). Using a uniform threshold of 15 mcg Hb/g feces narrowed the range of specificity (94%-98%), but sensitivities remained quite variable (16%-34%). Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively), the researchers reported.
Increasingly, physicians are using fecal immunochemical testing to screen for colorectal neoplasia. In a prior study (Ann Intern Med. 2009 Feb 3;150[3]:162-90) investigators evaluated the diagnostic performance of six qualitative point-of-care fecal immunochemical tests among screening colonoscopy patients in Germany, and found that the tests had highly variable sensitivities and specificities for the detection of colorectal neoplasia. Not surprisingly, the most sensitive tests were the least specific, and vice versa, which is the problem with using fixed thresholds in qualitative fecal immunochemical tests, the researchers asserted.
Quantitative fecal immunochemical tests are more flexible than qualitative assays because users can adjust thresholds based on fecal hemoglobin concentrations. However, very few studies had directly compared sensitivities and specificities among quantitative fecal immunochemical tests, and “it is unclear to what extent differences ... reflect true heterogeneity in test performance or differences in study populations or varying pre-analytical conditions,” the investigators wrote. Patients in their study underwent colonoscopies in Germany between 2005 and 2010, and fecal samples were stored at –80 °C until analysis. The researchers calculated test sensitivities and specificities by using colonoscopy and histology reports evaluated by blinded, trained research assistants.
“Apparent heterogeneity in diagnostic performance of quantitative fecal immunochemical tests can be overcome to a large extent by adjusting thresholds to yield defined levels of specificity or positivity rates,” the investigators concluded. Only 16 patients in this study had colorectal cancer, which made it difficult to pinpoint test sensitivity for this finding, they noted. However, they found similar sensitivity estimates for colorectal cancer in an ancillary clinical study.
Manufacturers provided test kits free of charge. There were no external funding sources, and the researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: To minimize the heterogeneity of fecal immunochemical screening tests, adjust thresholds to produce a predetermined specificity or a manageable rate of positivity.
Major finding: Adjusting detection thresholds to obtain preset specificities (99%, 97%, or 93%) greatly narrowed both sensitivity (14%-18%, 21%-24%, and 30%-35%, respectively) and rates of positivity (2.8%-3.4%, 5.8%-6.1%, and 10%-11%, respectively).
Data source: A comparison of nine different fecal immunochemical assays in 516 patients, of whom 216 had colorectal neoplasias.
Disclosures: Manufacturers provided test kits free of charge. There were no other external sources of support, and the researchers reported having no conflicts of interest.
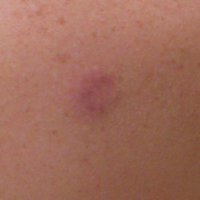
Confluent and reticulated papillomatosis
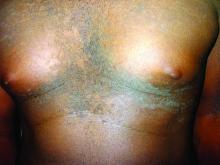
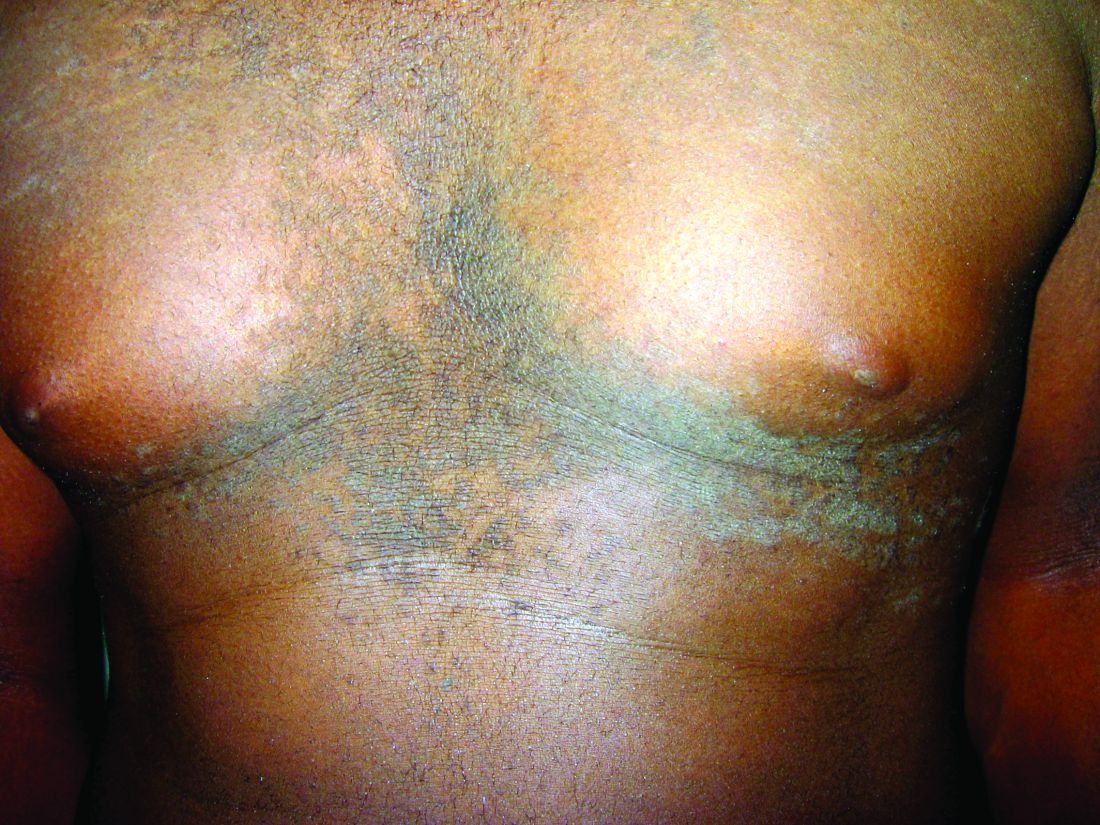
Confluent and reticulated papillomatosis of Gougerot and Carteaud, also known as Gougerot-Carteaud syndrome, is an uncommon skin disorder of young individuals characterized by hyperkeratotic or verrucous brown papules or plaques that coalesce centrally and by a reticulated pattern peripherally. It was first described by two French dermatologists, Gougerot and Carteaud, in 1927.1 Initially, the distinct entity of CARP was contested, with some dermatologists believing it to be a variant of acanthosis nigricans. However, CARP is now recognized as a distinct, though rare, dermatosis.
Histopathology reveals findings similar to those that may be found in acanthosis nigricans and epidermal nevi. Classic characteristics of CARP include hyperkeratosis, papillomatosis, increased basal melanin pigmentation, and mild acanthosis. Occasionally, there may be perivascular lymphocytic infiltrates in the superficial dermis.3,4
The etiology of CARP is unknown. CARP’s resolution in response to antibiotics and the isolation of two bacterial actinomycetes, Rhodococcus and Dietzia papillomatosis, from skin scrapings of CARP patients have led some to believe that its etiology is bacterial. However, no bacterial species have been consistently isolated from CARP patients. The prevailing theory of the past was that CARP was an abnormal host response to the fungus Malassezia furfur. Inconsistent detection of the fungus in skin scrapings, as well as persistence of the skin lesions after fungal clearance with antifungal therapy, has debunked this theory. An underlying disorder of keratinization resulting in hyperproliferation also has been suggested given reports of familial CARP and electron microscopy studies demonstrating focal-enhanced expression of keratin-16 in the stratum granulosom.5 Other theories include a cutaneous response to underlying endocrinopathies, ultraviolet light, and localized amyloidosis.1
Diagnosis and differential
CARP is poorly recognized by clinicians and frequently initially misdiagnosed due to its similar appearance to other disorders, most commonly tinea versicolor and acanthosis nigricans. Davis et al. proposed criteria for diagnosis of CARP requiring 1) presence of scaly, reticulated and papillomatous brown macules and patches; 2) distribution over the upper trunk and neck; 3) negative fungal staining of scales; 4) no improvement following antifungal treatment; and 5) improvement following minocycline.2
Tinea versicolor may appear similar to CARP, but unlike CARP, will respond to antifungal treatment and may demonstrate hyphae and yeast on KOH preparation. Acanthosis nigricans and CARP both may present with velvety, hyperpigmented plaques in individuals of obese habitus or with insulin resistance, but peripheral reticulation will be absent in acanthosis nigricans. However, acanthosis nigricans and CARP may coexist, and this coexistence is not uncommonly seen in individuals with obesity and/or insulin resistance. Darier’s disease may look similar to cases of CARP without pigmentary change, but it often will have accompanying nail changes. Macular or lichen amyloidosis may present with pruritic brown macules or papules, but skin biopsy will have positive amyloid staining. The use of 70% alcohol swabbing to diagnose terra firma-forme dermatosis, with lesions disappearing with swabbing, is classic and used to differentiate it from CARP. Other conditions to consider include seborrheic dermatitis, epidermal nevi, verruca plana, epidermodysplasia verruciformis, and acne vulgaris.1,2,4
Treatment
Minocycline is the first-line treatment for CARP: 80% of patients may have complete resolution with minocycline, while the remainder experience at least 50% clearance of skin lesions.2 However, recurrence after stopping minocycline treatment is not uncommon. The mechanism by which minocycline works is unknown. Second-line treatment for those who cannot tolerate minocycline are macrolide antibiotics.6 Other treatment options with reported success include oral isotretinoin and topical retinoids, including tretinoin gel and tazarotene cream.3,7 Appropriate strength topical corticosteroids may be used for pruritus.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, as well as the vice chair of the department of dermatology and a professor of dermatology and pediatrics at UC San Diego. They report having no conflicts of interest or financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. Clin Cosmet Investig Dermatol. 2016 Aug 25;9:217-23.
2. Br J Dermatol. 2006 Feb;154(2):287-93.
3. Arch Dermatol. 2012 Apr;148(4):505-8.
4. J Am Acad Dermatol. 2003 Dec;49(6):1182-4.
5. Arch Dermatol. 2002 Feb;138(2):276-7.
6. J Am Acad Dermatol. 2001;44(4):652-5.
7. Am J Clin Dermatol. 2006;7(5):305-13.
Confluent and reticulated papillomatosis of Gougerot and Carteaud, also known as Gougerot-Carteaud syndrome, is an uncommon skin disorder of young individuals characterized by hyperkeratotic or verrucous brown papules or plaques that coalesce centrally and by a reticulated pattern peripherally. It was first described by two French dermatologists, Gougerot and Carteaud, in 1927.1 Initially, the distinct entity of CARP was contested, with some dermatologists believing it to be a variant of acanthosis nigricans. However, CARP is now recognized as a distinct, though rare, dermatosis.
Histopathology reveals findings similar to those that may be found in acanthosis nigricans and epidermal nevi. Classic characteristics of CARP include hyperkeratosis, papillomatosis, increased basal melanin pigmentation, and mild acanthosis. Occasionally, there may be perivascular lymphocytic infiltrates in the superficial dermis.3,4
The etiology of CARP is unknown. CARP’s resolution in response to antibiotics and the isolation of two bacterial actinomycetes, Rhodococcus and Dietzia papillomatosis, from skin scrapings of CARP patients have led some to believe that its etiology is bacterial. However, no bacterial species have been consistently isolated from CARP patients. The prevailing theory of the past was that CARP was an abnormal host response to the fungus Malassezia furfur. Inconsistent detection of the fungus in skin scrapings, as well as persistence of the skin lesions after fungal clearance with antifungal therapy, has debunked this theory. An underlying disorder of keratinization resulting in hyperproliferation also has been suggested given reports of familial CARP and electron microscopy studies demonstrating focal-enhanced expression of keratin-16 in the stratum granulosom.5 Other theories include a cutaneous response to underlying endocrinopathies, ultraviolet light, and localized amyloidosis.1
Diagnosis and differential
CARP is poorly recognized by clinicians and frequently initially misdiagnosed due to its similar appearance to other disorders, most commonly tinea versicolor and acanthosis nigricans. Davis et al. proposed criteria for diagnosis of CARP requiring 1) presence of scaly, reticulated and papillomatous brown macules and patches; 2) distribution over the upper trunk and neck; 3) negative fungal staining of scales; 4) no improvement following antifungal treatment; and 5) improvement following minocycline.2
Tinea versicolor may appear similar to CARP, but unlike CARP, will respond to antifungal treatment and may demonstrate hyphae and yeast on KOH preparation. Acanthosis nigricans and CARP both may present with velvety, hyperpigmented plaques in individuals of obese habitus or with insulin resistance, but peripheral reticulation will be absent in acanthosis nigricans. However, acanthosis nigricans and CARP may coexist, and this coexistence is not uncommonly seen in individuals with obesity and/or insulin resistance. Darier’s disease may look similar to cases of CARP without pigmentary change, but it often will have accompanying nail changes. Macular or lichen amyloidosis may present with pruritic brown macules or papules, but skin biopsy will have positive amyloid staining. The use of 70% alcohol swabbing to diagnose terra firma-forme dermatosis, with lesions disappearing with swabbing, is classic and used to differentiate it from CARP. Other conditions to consider include seborrheic dermatitis, epidermal nevi, verruca plana, epidermodysplasia verruciformis, and acne vulgaris.1,2,4
Treatment
Minocycline is the first-line treatment for CARP: 80% of patients may have complete resolution with minocycline, while the remainder experience at least 50% clearance of skin lesions.2 However, recurrence after stopping minocycline treatment is not uncommon. The mechanism by which minocycline works is unknown. Second-line treatment for those who cannot tolerate minocycline are macrolide antibiotics.6 Other treatment options with reported success include oral isotretinoin and topical retinoids, including tretinoin gel and tazarotene cream.3,7 Appropriate strength topical corticosteroids may be used for pruritus.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, as well as the vice chair of the department of dermatology and a professor of dermatology and pediatrics at UC San Diego. They report having no conflicts of interest or financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. Clin Cosmet Investig Dermatol. 2016 Aug 25;9:217-23.
2. Br J Dermatol. 2006 Feb;154(2):287-93.
3. Arch Dermatol. 2012 Apr;148(4):505-8.
4. J Am Acad Dermatol. 2003 Dec;49(6):1182-4.
5. Arch Dermatol. 2002 Feb;138(2):276-7.
6. J Am Acad Dermatol. 2001;44(4):652-5.
7. Am J Clin Dermatol. 2006;7(5):305-13.
Confluent and reticulated papillomatosis of Gougerot and Carteaud, also known as Gougerot-Carteaud syndrome, is an uncommon skin disorder of young individuals characterized by hyperkeratotic or verrucous brown papules or plaques that coalesce centrally and by a reticulated pattern peripherally. It was first described by two French dermatologists, Gougerot and Carteaud, in 1927.1 Initially, the distinct entity of CARP was contested, with some dermatologists believing it to be a variant of acanthosis nigricans. However, CARP is now recognized as a distinct, though rare, dermatosis.
Histopathology reveals findings similar to those that may be found in acanthosis nigricans and epidermal nevi. Classic characteristics of CARP include hyperkeratosis, papillomatosis, increased basal melanin pigmentation, and mild acanthosis. Occasionally, there may be perivascular lymphocytic infiltrates in the superficial dermis.3,4
The etiology of CARP is unknown. CARP’s resolution in response to antibiotics and the isolation of two bacterial actinomycetes, Rhodococcus and Dietzia papillomatosis, from skin scrapings of CARP patients have led some to believe that its etiology is bacterial. However, no bacterial species have been consistently isolated from CARP patients. The prevailing theory of the past was that CARP was an abnormal host response to the fungus Malassezia furfur. Inconsistent detection of the fungus in skin scrapings, as well as persistence of the skin lesions after fungal clearance with antifungal therapy, has debunked this theory. An underlying disorder of keratinization resulting in hyperproliferation also has been suggested given reports of familial CARP and electron microscopy studies demonstrating focal-enhanced expression of keratin-16 in the stratum granulosom.5 Other theories include a cutaneous response to underlying endocrinopathies, ultraviolet light, and localized amyloidosis.1
Diagnosis and differential
CARP is poorly recognized by clinicians and frequently initially misdiagnosed due to its similar appearance to other disorders, most commonly tinea versicolor and acanthosis nigricans. Davis et al. proposed criteria for diagnosis of CARP requiring 1) presence of scaly, reticulated and papillomatous brown macules and patches; 2) distribution over the upper trunk and neck; 3) negative fungal staining of scales; 4) no improvement following antifungal treatment; and 5) improvement following minocycline.2
Tinea versicolor may appear similar to CARP, but unlike CARP, will respond to antifungal treatment and may demonstrate hyphae and yeast on KOH preparation. Acanthosis nigricans and CARP both may present with velvety, hyperpigmented plaques in individuals of obese habitus or with insulin resistance, but peripheral reticulation will be absent in acanthosis nigricans. However, acanthosis nigricans and CARP may coexist, and this coexistence is not uncommonly seen in individuals with obesity and/or insulin resistance. Darier’s disease may look similar to cases of CARP without pigmentary change, but it often will have accompanying nail changes. Macular or lichen amyloidosis may present with pruritic brown macules or papules, but skin biopsy will have positive amyloid staining. The use of 70% alcohol swabbing to diagnose terra firma-forme dermatosis, with lesions disappearing with swabbing, is classic and used to differentiate it from CARP. Other conditions to consider include seborrheic dermatitis, epidermal nevi, verruca plana, epidermodysplasia verruciformis, and acne vulgaris.1,2,4
Treatment
Minocycline is the first-line treatment for CARP: 80% of patients may have complete resolution with minocycline, while the remainder experience at least 50% clearance of skin lesions.2 However, recurrence after stopping minocycline treatment is not uncommon. The mechanism by which minocycline works is unknown. Second-line treatment for those who cannot tolerate minocycline are macrolide antibiotics.6 Other treatment options with reported success include oral isotretinoin and topical retinoids, including tretinoin gel and tazarotene cream.3,7 Appropriate strength topical corticosteroids may be used for pruritus.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, as well as the vice chair of the department of dermatology and a professor of dermatology and pediatrics at UC San Diego. They report having no conflicts of interest or financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. Clin Cosmet Investig Dermatol. 2016 Aug 25;9:217-23.
2. Br J Dermatol. 2006 Feb;154(2):287-93.
3. Arch Dermatol. 2012 Apr;148(4):505-8.
4. J Am Acad Dermatol. 2003 Dec;49(6):1182-4.
5. Arch Dermatol. 2002 Feb;138(2):276-7.
6. J Am Acad Dermatol. 2001;44(4):652-5.
7. Am J Clin Dermatol. 2006;7(5):305-13.
A 17-year-old male presents to the dermatology clinic for brown lesions on his central chest and back that have been present for about a year. The brown areas gradually have become scaly over time. They are asymptomatic. His pediatrician had given him hydrocortisone ointment to apply to the lesions, but there was no improvement. Review of systems was otherwise negative.
Higher water intake linked to less hyperuricemia in gout
SAN DIEGO – While more hydration seems to improve gout, there’s little research into the connection between the two. Now, a new study suggests a strong link between low water consumption and hyperuricemia, a possible sign that boosting water intake will help some gout patients.
While it’s too early to confirm a clinically relevant connection, “there is a statistically significant inverse association between water consumption and high uric acid levels,” said Patricia Kachur, MD, a third-year internal medicine resident at the University of Central Florida, Ocala (Fla.) Regional Medical Center. Dr. Kachur, who spoke about the findings in an interview, is lead author of a study presented at the annual meeting of the American College of Rheumatology.
An abstract presented at the 2009 ACR annual meeting reported fewer gout attacks (adjusted odds ratio, 0.54; 95% confidence interval, 0.32-0.90) in 535 gout patients who reported drinking more than eight glasses of water over a 24-hour period, compared with those who drank one or fewer.
For the new study, Dr. Kachur and her colleagues examined findings from 539 participants with gout (but not chronic kidney disease) out of 17,321 individuals who took part in the National Health and Nutrition Examination Survey from 2009 to 2014.
Of the 539 participants, 39% were defined as having hyperuricemia (6.0 mg/dL or greater), with the rest having a low or normal level. Those with hyperuricemia were significantly more likely to be male and have obesity and hypertension.
The investigators defined high water intake as three or more liters of water per day for men and 2.2 or more liters for women. Of the 539 participants, 116 (22%) had high water intake.
The researchers found a lower risk of developing hyperuricemia in those with higher water intake, compared with those with lower intake (adjusted OR, 0.421; 95% CI, 0.262-0.679; P = .0007).
“These findings do not say anything about water and gout – not yet anyway,” Dr. Kachur said. “Rather there is a possibility that outpatient water intake has an association with lower uric acid levels in people afflicted by gout even after considering multiple other factors such as gender, race, BMI, age, hypertension, and diabetes mellitus.”
Indeed, she and her colleagues decided against publishing the results of their 2009 study “because there is a major challenge in interpreting these data.”
“Given that people only consume a finite amount of liquids each day, is it that consuming more water is beneficial or that drinking less of ‘bad’ fluids (for example, sodas, sugar-sweetened juices) is beneficial? We were not able to disentangle this issue,” she explained.
Still, she said, there are explanations about why water intake could be beneficial for gout. “Intravascular volume depletion increases the concentration of serum urate, and increased serum urate beyond the saturation threshold can result in crystallization,” she said. “With heat-related dehydration, there may also be metabolic acidosis and/or electrolyte abnormalities that can lead to decreased urate secretion in renal tubules, and an acidic pH can decrease solubility of serum urate.”
Dr. Neogi does encourage appropriate gout patients to make sure they drink enough water, especially if it is hot. She cowrote a 2014 study that linked gout flares to high temperatures and extremes of humidity, which can lead to dehydration (Am J Epidemiol. 2014 Aug 15;180[4]:372-7).
“The amount of water intake that is beneficial for gout is not known, so patients should follow general recommendations for water intake. In addition, I strongly encourage patients with gout to avoid or limit the amount of liquid consumed in the form of regular sodas and sweetened drinks or juices, particularly those with high-fructose corn syrup, and alcohol,” she said. “With regards to tea or coffee, if patients drink either tea or coffee, they can continue to do so and to use only low-fat or nonfat milk and little or no sugar.”
Meanwhile, she said, “there are some data to suggest that cherry juice – true natural cherry juice from fruit, not ‘cherry drinks’ – can be beneficial for gout. We are formally testing cherry juice in a trial.”
What’s next for research into water intake and gout? “The clinical correlation is missing in the study,” said Dr. Kachur, lead author of the new study. “Targeted surveys of gout patients, hopefully followed by a randomized controlled trial regulating water intake, can help make those connections.”
Dr. Kachur and other study authors reported having no relevant disclosures. Dr. Neogi reported having no relevant disclosures. No specific study funding was reported.
SAN DIEGO – While more hydration seems to improve gout, there’s little research into the connection between the two. Now, a new study suggests a strong link between low water consumption and hyperuricemia, a possible sign that boosting water intake will help some gout patients.
While it’s too early to confirm a clinically relevant connection, “there is a statistically significant inverse association between water consumption and high uric acid levels,” said Patricia Kachur, MD, a third-year internal medicine resident at the University of Central Florida, Ocala (Fla.) Regional Medical Center. Dr. Kachur, who spoke about the findings in an interview, is lead author of a study presented at the annual meeting of the American College of Rheumatology.
An abstract presented at the 2009 ACR annual meeting reported fewer gout attacks (adjusted odds ratio, 0.54; 95% confidence interval, 0.32-0.90) in 535 gout patients who reported drinking more than eight glasses of water over a 24-hour period, compared with those who drank one or fewer.
For the new study, Dr. Kachur and her colleagues examined findings from 539 participants with gout (but not chronic kidney disease) out of 17,321 individuals who took part in the National Health and Nutrition Examination Survey from 2009 to 2014.
Of the 539 participants, 39% were defined as having hyperuricemia (6.0 mg/dL or greater), with the rest having a low or normal level. Those with hyperuricemia were significantly more likely to be male and have obesity and hypertension.
The investigators defined high water intake as three or more liters of water per day for men and 2.2 or more liters for women. Of the 539 participants, 116 (22%) had high water intake.
The researchers found a lower risk of developing hyperuricemia in those with higher water intake, compared with those with lower intake (adjusted OR, 0.421; 95% CI, 0.262-0.679; P = .0007).
“These findings do not say anything about water and gout – not yet anyway,” Dr. Kachur said. “Rather there is a possibility that outpatient water intake has an association with lower uric acid levels in people afflicted by gout even after considering multiple other factors such as gender, race, BMI, age, hypertension, and diabetes mellitus.”
Indeed, she and her colleagues decided against publishing the results of their 2009 study “because there is a major challenge in interpreting these data.”
“Given that people only consume a finite amount of liquids each day, is it that consuming more water is beneficial or that drinking less of ‘bad’ fluids (for example, sodas, sugar-sweetened juices) is beneficial? We were not able to disentangle this issue,” she explained.
Still, she said, there are explanations about why water intake could be beneficial for gout. “Intravascular volume depletion increases the concentration of serum urate, and increased serum urate beyond the saturation threshold can result in crystallization,” she said. “With heat-related dehydration, there may also be metabolic acidosis and/or electrolyte abnormalities that can lead to decreased urate secretion in renal tubules, and an acidic pH can decrease solubility of serum urate.”
Dr. Neogi does encourage appropriate gout patients to make sure they drink enough water, especially if it is hot. She cowrote a 2014 study that linked gout flares to high temperatures and extremes of humidity, which can lead to dehydration (Am J Epidemiol. 2014 Aug 15;180[4]:372-7).
“The amount of water intake that is beneficial for gout is not known, so patients should follow general recommendations for water intake. In addition, I strongly encourage patients with gout to avoid or limit the amount of liquid consumed in the form of regular sodas and sweetened drinks or juices, particularly those with high-fructose corn syrup, and alcohol,” she said. “With regards to tea or coffee, if patients drink either tea or coffee, they can continue to do so and to use only low-fat or nonfat milk and little or no sugar.”
Meanwhile, she said, “there are some data to suggest that cherry juice – true natural cherry juice from fruit, not ‘cherry drinks’ – can be beneficial for gout. We are formally testing cherry juice in a trial.”
What’s next for research into water intake and gout? “The clinical correlation is missing in the study,” said Dr. Kachur, lead author of the new study. “Targeted surveys of gout patients, hopefully followed by a randomized controlled trial regulating water intake, can help make those connections.”
Dr. Kachur and other study authors reported having no relevant disclosures. Dr. Neogi reported having no relevant disclosures. No specific study funding was reported.
SAN DIEGO – While more hydration seems to improve gout, there’s little research into the connection between the two. Now, a new study suggests a strong link between low water consumption and hyperuricemia, a possible sign that boosting water intake will help some gout patients.
While it’s too early to confirm a clinically relevant connection, “there is a statistically significant inverse association between water consumption and high uric acid levels,” said Patricia Kachur, MD, a third-year internal medicine resident at the University of Central Florida, Ocala (Fla.) Regional Medical Center. Dr. Kachur, who spoke about the findings in an interview, is lead author of a study presented at the annual meeting of the American College of Rheumatology.
An abstract presented at the 2009 ACR annual meeting reported fewer gout attacks (adjusted odds ratio, 0.54; 95% confidence interval, 0.32-0.90) in 535 gout patients who reported drinking more than eight glasses of water over a 24-hour period, compared with those who drank one or fewer.
For the new study, Dr. Kachur and her colleagues examined findings from 539 participants with gout (but not chronic kidney disease) out of 17,321 individuals who took part in the National Health and Nutrition Examination Survey from 2009 to 2014.
Of the 539 participants, 39% were defined as having hyperuricemia (6.0 mg/dL or greater), with the rest having a low or normal level. Those with hyperuricemia were significantly more likely to be male and have obesity and hypertension.
The investigators defined high water intake as three or more liters of water per day for men and 2.2 or more liters for women. Of the 539 participants, 116 (22%) had high water intake.
The researchers found a lower risk of developing hyperuricemia in those with higher water intake, compared with those with lower intake (adjusted OR, 0.421; 95% CI, 0.262-0.679; P = .0007).
“These findings do not say anything about water and gout – not yet anyway,” Dr. Kachur said. “Rather there is a possibility that outpatient water intake has an association with lower uric acid levels in people afflicted by gout even after considering multiple other factors such as gender, race, BMI, age, hypertension, and diabetes mellitus.”
Indeed, she and her colleagues decided against publishing the results of their 2009 study “because there is a major challenge in interpreting these data.”
“Given that people only consume a finite amount of liquids each day, is it that consuming more water is beneficial or that drinking less of ‘bad’ fluids (for example, sodas, sugar-sweetened juices) is beneficial? We were not able to disentangle this issue,” she explained.
Still, she said, there are explanations about why water intake could be beneficial for gout. “Intravascular volume depletion increases the concentration of serum urate, and increased serum urate beyond the saturation threshold can result in crystallization,” she said. “With heat-related dehydration, there may also be metabolic acidosis and/or electrolyte abnormalities that can lead to decreased urate secretion in renal tubules, and an acidic pH can decrease solubility of serum urate.”
Dr. Neogi does encourage appropriate gout patients to make sure they drink enough water, especially if it is hot. She cowrote a 2014 study that linked gout flares to high temperatures and extremes of humidity, which can lead to dehydration (Am J Epidemiol. 2014 Aug 15;180[4]:372-7).
“The amount of water intake that is beneficial for gout is not known, so patients should follow general recommendations for water intake. In addition, I strongly encourage patients with gout to avoid or limit the amount of liquid consumed in the form of regular sodas and sweetened drinks or juices, particularly those with high-fructose corn syrup, and alcohol,” she said. “With regards to tea or coffee, if patients drink either tea or coffee, they can continue to do so and to use only low-fat or nonfat milk and little or no sugar.”
Meanwhile, she said, “there are some data to suggest that cherry juice – true natural cherry juice from fruit, not ‘cherry drinks’ – can be beneficial for gout. We are formally testing cherry juice in a trial.”
What’s next for research into water intake and gout? “The clinical correlation is missing in the study,” said Dr. Kachur, lead author of the new study. “Targeted surveys of gout patients, hopefully followed by a randomized controlled trial regulating water intake, can help make those connections.”
Dr. Kachur and other study authors reported having no relevant disclosures. Dr. Neogi reported having no relevant disclosures. No specific study funding was reported.
AT ACR 2017
Key clinical point:
Major finding: Gout patients with the highest water intake were less likely than others to have hyperuricemia (aOR, 0.421).
Data source: 539 participants with gout (but not chronic kidney disease) out of 17,321 in the National Health and Nutrition Examination Survey, 2009-2014.
Disclosures: The study authors reported having no relevant disclosures. No specific study funding was reported.
Robotic hysterectomy plus mini-lap outperformed open procedure
NATIONAL HARBOR, MD. – Robotic hysterectomy combined with extraction of the uterus via mini-laparotomy led to significantly shorter lengths of stay, lower estimated blood loss, and fewer postoperative complications compared with open hysterectomy when the uterus weighed more than 250 grams.
Gynecologic surgeons are seeking ways to safely perform minimally invasive hysterectomy on patients with larger uteri in light of the 2014 Food and Drug Administration admonition regarding power morcellation. To this end, Natasha Gupta, MD, and her colleagues at the University of Tennessee, Chattanooga, retrospectively reviewed all patients with uterine sizes larger than 250 grams undergoing hysterectomy at their institution between 2012 and 2015.
“For the mini-laparotomy, the technique utilizes a customized incision connecting the two left port sites, followed by the removal of the specimen via this incision,” Dr. Gupta said at the AAGL Global Congress.
Patient factors and outcomes were compared via Student t-tests and Chi-square analysis.
Mean length of stay was significantly shorter for patients who underwent robotic hysterectomy/mini-laparotomy, at 1.4 days vs. 5.4 days for those with open hysterectomy (P = .000) as was mean estimated blood loss – 119.9 mL vs. 547.5 mL, respectively (P = .000). Postoperative complications were seen in fewer patients who underwent robotic hysterectomy/mini-laparotomy, 9 of 82 patients vs. 15 of 58 open hysterectomy patients.
Mean operative time was significantly longer in robotic hysterectomy/mini-laparotomy patients – 191.6 minutes vs. 162.8 minutes (P = .005) – but that was expected, Dr. Gupta noted. Patient factors such as hypertension, diabetes, history of spontaneous vaginal delivery and/or cesarean delivery, and body mass index, as well as uterine pathology, were not significantly different between the groups.
“Mini-laparotomy combined with minimally invasive hysterectomy is a very safe and feasible technique for tissue extraction where contained morcellation is either not preferred or not available,” Dr. Gupta said.
Dr. Gupta reported having no relevant financial conflicts of interest.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
NATIONAL HARBOR, MD. – Robotic hysterectomy combined with extraction of the uterus via mini-laparotomy led to significantly shorter lengths of stay, lower estimated blood loss, and fewer postoperative complications compared with open hysterectomy when the uterus weighed more than 250 grams.
Gynecologic surgeons are seeking ways to safely perform minimally invasive hysterectomy on patients with larger uteri in light of the 2014 Food and Drug Administration admonition regarding power morcellation. To this end, Natasha Gupta, MD, and her colleagues at the University of Tennessee, Chattanooga, retrospectively reviewed all patients with uterine sizes larger than 250 grams undergoing hysterectomy at their institution between 2012 and 2015.
“For the mini-laparotomy, the technique utilizes a customized incision connecting the two left port sites, followed by the removal of the specimen via this incision,” Dr. Gupta said at the AAGL Global Congress.
Patient factors and outcomes were compared via Student t-tests and Chi-square analysis.
Mean length of stay was significantly shorter for patients who underwent robotic hysterectomy/mini-laparotomy, at 1.4 days vs. 5.4 days for those with open hysterectomy (P = .000) as was mean estimated blood loss – 119.9 mL vs. 547.5 mL, respectively (P = .000). Postoperative complications were seen in fewer patients who underwent robotic hysterectomy/mini-laparotomy, 9 of 82 patients vs. 15 of 58 open hysterectomy patients.
Mean operative time was significantly longer in robotic hysterectomy/mini-laparotomy patients – 191.6 minutes vs. 162.8 minutes (P = .005) – but that was expected, Dr. Gupta noted. Patient factors such as hypertension, diabetes, history of spontaneous vaginal delivery and/or cesarean delivery, and body mass index, as well as uterine pathology, were not significantly different between the groups.
“Mini-laparotomy combined with minimally invasive hysterectomy is a very safe and feasible technique for tissue extraction where contained morcellation is either not preferred or not available,” Dr. Gupta said.
Dr. Gupta reported having no relevant financial conflicts of interest.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
NATIONAL HARBOR, MD. – Robotic hysterectomy combined with extraction of the uterus via mini-laparotomy led to significantly shorter lengths of stay, lower estimated blood loss, and fewer postoperative complications compared with open hysterectomy when the uterus weighed more than 250 grams.
Gynecologic surgeons are seeking ways to safely perform minimally invasive hysterectomy on patients with larger uteri in light of the 2014 Food and Drug Administration admonition regarding power morcellation. To this end, Natasha Gupta, MD, and her colleagues at the University of Tennessee, Chattanooga, retrospectively reviewed all patients with uterine sizes larger than 250 grams undergoing hysterectomy at their institution between 2012 and 2015.
“For the mini-laparotomy, the technique utilizes a customized incision connecting the two left port sites, followed by the removal of the specimen via this incision,” Dr. Gupta said at the AAGL Global Congress.
Patient factors and outcomes were compared via Student t-tests and Chi-square analysis.
Mean length of stay was significantly shorter for patients who underwent robotic hysterectomy/mini-laparotomy, at 1.4 days vs. 5.4 days for those with open hysterectomy (P = .000) as was mean estimated blood loss – 119.9 mL vs. 547.5 mL, respectively (P = .000). Postoperative complications were seen in fewer patients who underwent robotic hysterectomy/mini-laparotomy, 9 of 82 patients vs. 15 of 58 open hysterectomy patients.
Mean operative time was significantly longer in robotic hysterectomy/mini-laparotomy patients – 191.6 minutes vs. 162.8 minutes (P = .005) – but that was expected, Dr. Gupta noted. Patient factors such as hypertension, diabetes, history of spontaneous vaginal delivery and/or cesarean delivery, and body mass index, as well as uterine pathology, were not significantly different between the groups.
“Mini-laparotomy combined with minimally invasive hysterectomy is a very safe and feasible technique for tissue extraction where contained morcellation is either not preferred or not available,” Dr. Gupta said.
Dr. Gupta reported having no relevant financial conflicts of interest.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
AT AAGL 2017
Key clinical point:
Major finding: Mean length of stay was 1.4 days with robotic hysterectomy/mini-laparotomy vs. 5.4 days for open hysterectomy (P = .000).
Data source: A single-center retrospective review of all hysterectomies with uteri larger than 250 grams from the period of 2012-2015.
Disclosures: The study had no outside funding. Dr. Gupta reported having no relevant conflicts of interest.
Asymptomatic Pink Plaque on the Scapula
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.
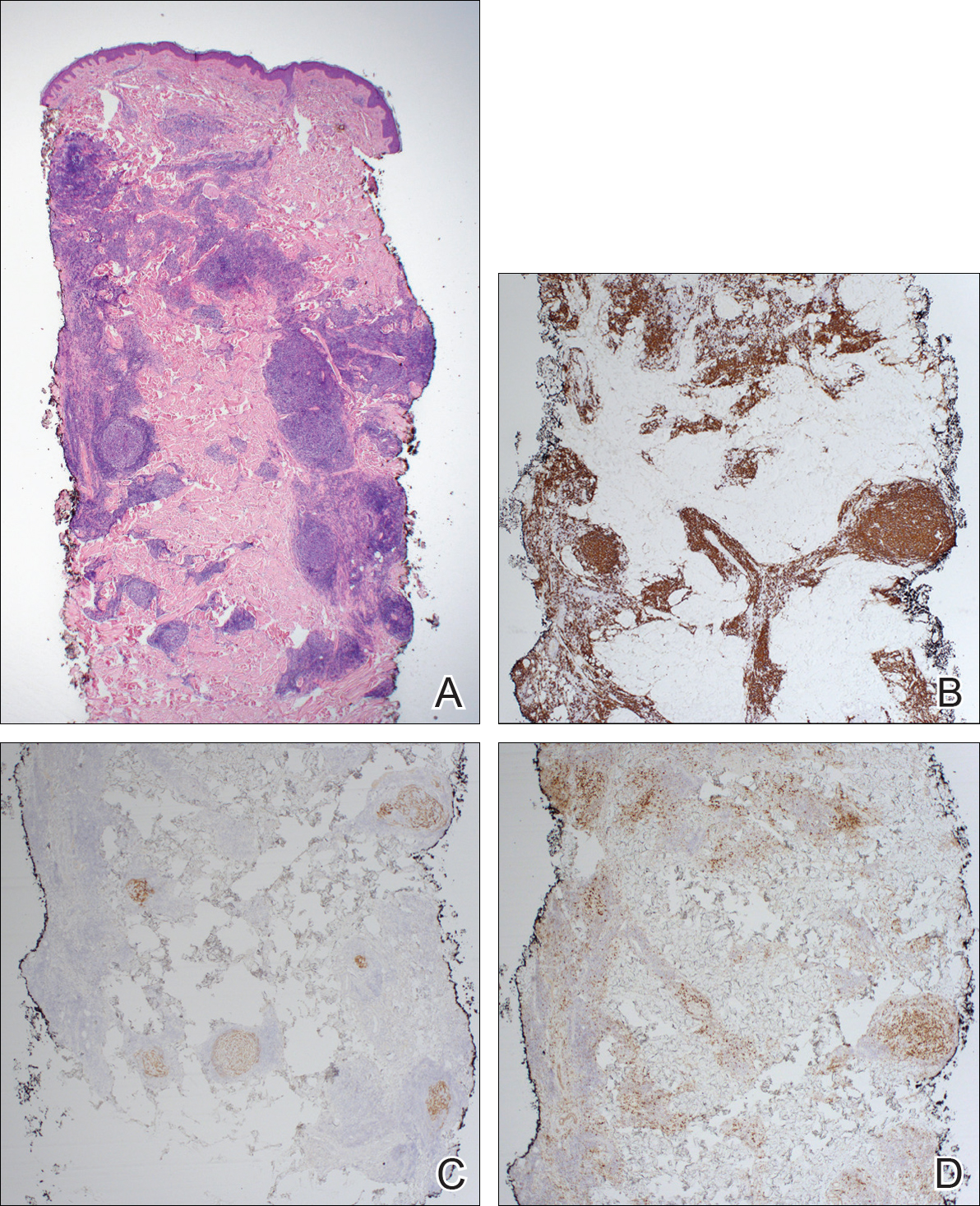
Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.

Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.

Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
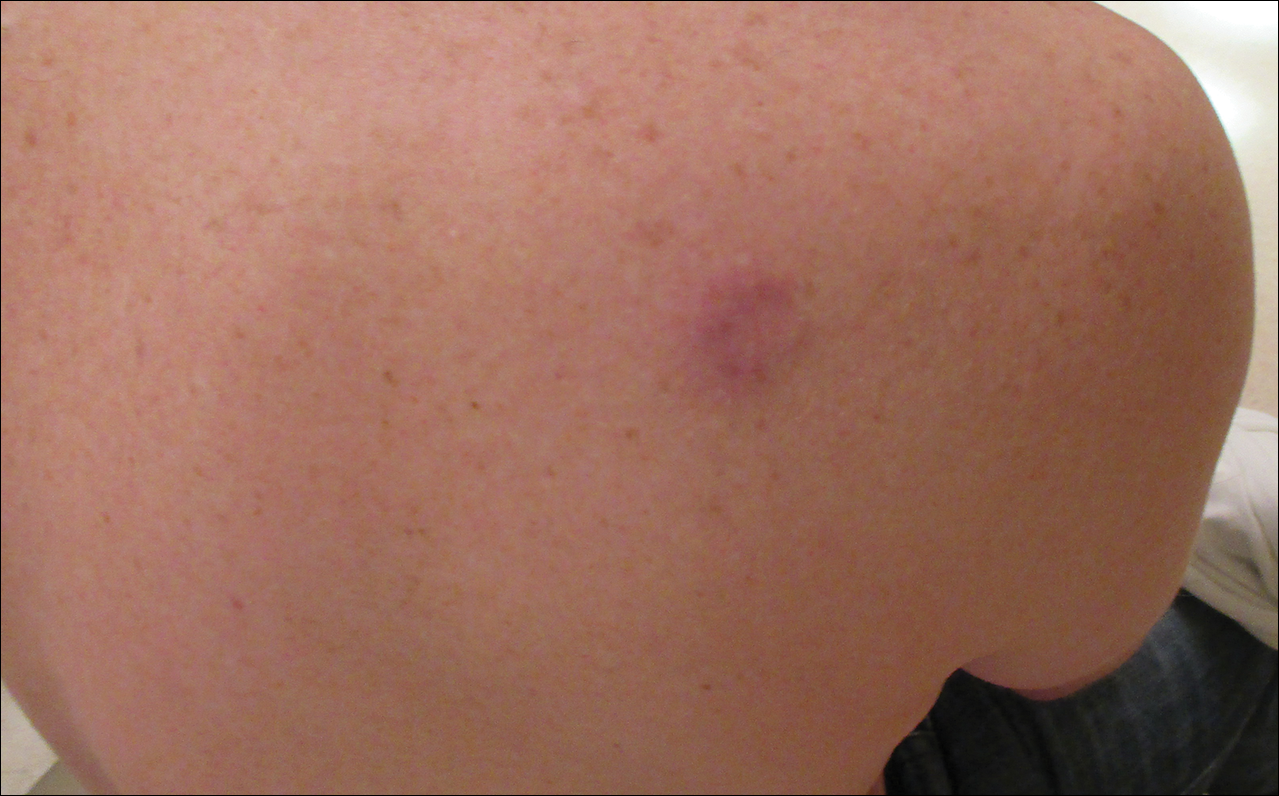
A 36-year-old man presented with a pink plaque on the right side of the scapula of 1 year's duration. The plaque had not grown and was completely asymptomatic. Physical examination revealed a violaceous, pink, 2-cm nodule with overlying telangiectasia. No other concerning lesions were identified on total-body skin examination. A punch biopsy was obtained.