User login
Recommend high CBD, low THC products to marijuana-using patients with psychosis
NEW ORLEANS – Marijuana and psychosis don’t mix, according to Erica Rapp, MD, an assistant professor of psychiatry at the University of Colorado Anschutz Medical Campus, Aurora.
Regular use is associated with increased positive psychotic symptoms, a wide-range of poor psycho-social outcomes, reduced medication adherence, and a higher rate of relapse that’s not entirely explained by reduced adherence. One study even found an increased risk of suicide, she said at the American Psychiatric Association’s Institute on Psychiatric Services meeting.
Epidemiological studies, meanwhile, have found a “robust” association between regular marijuana use and an increased risk of schizophrenia, about two-times higher in the general population and about four-times higher in people who are predisposed to psychosis. It’s possible that marijuana doesn’t actually increase the rate of psychotic disorders, but just makes them come on sooner. “While that is almost certainly true, it doesn’t exclude the overall increased risk,” Dr. Rapp said.
Researchers are working to unravel the cross-talk between marijuana and psychosis. It seems that with heavy, regular use, you “are basically loading your brain with dopamine; [perhaps] people become more sensitized to dopamine-induced perceptual and cognitive problems,” she said.
“Luckily, if we can get people to stop use early in the course of their psychotic illness, they do much better. There are improvements in mood, anxiety, positive psychotic symptoms, medication adherence, and global functioning,” she said.
It’s not easy to get people to stop, however.
The notion of “marijuana as a potent cause of schizophrenia ... is something that users do not like to hear.” Many consider marijuana “a medicine, and that if they smoke it, they are going to be healthier. If you suggest that” marijuana might do “some bad things in addition to all the great things they think it’s doing, they immediately think you are a narc, and shut down. It’s hard to have an open conversion,” Dr. Rapp said.
At this point, it seems that it’s the tetrahydrocannabinol (THC) in marijuana that causes problems for people who have or who are prone to psychosis, and that it’s the cannabidiol (CBD) component that’s responsible for the therapeutic effects. CBD appears to be a dopamine D2 receptor antagonist, and some small pilot studies have found anti-psychotic effects. “CBD actually has some potential as a treatment” for psychosis, especially for negative symptoms, she said.
So, when abstinence isn’t an option, “I try to steer people towards CBD, rather than THC. Labels in dispensaries tell you what the THC and CBD content are. Those can be really unreliable, but it’s something.” Staff can point out high CBD products. “I also usually caution against smoking. I tell people that smoking anything has bad effects on your health,” she said. Edibles are among the many alternative formulations.
Patients who are truly interested in therapeutic benefits appreciate the message. “If they say, ‘oh, that doesn’t sound like fun,’ it tells me they are really looking for the psychoactive THC high,” she said.
Dr. Rapp did not have any industry disclosures.
NEW ORLEANS – Marijuana and psychosis don’t mix, according to Erica Rapp, MD, an assistant professor of psychiatry at the University of Colorado Anschutz Medical Campus, Aurora.
Regular use is associated with increased positive psychotic symptoms, a wide-range of poor psycho-social outcomes, reduced medication adherence, and a higher rate of relapse that’s not entirely explained by reduced adherence. One study even found an increased risk of suicide, she said at the American Psychiatric Association’s Institute on Psychiatric Services meeting.
Epidemiological studies, meanwhile, have found a “robust” association between regular marijuana use and an increased risk of schizophrenia, about two-times higher in the general population and about four-times higher in people who are predisposed to psychosis. It’s possible that marijuana doesn’t actually increase the rate of psychotic disorders, but just makes them come on sooner. “While that is almost certainly true, it doesn’t exclude the overall increased risk,” Dr. Rapp said.
Researchers are working to unravel the cross-talk between marijuana and psychosis. It seems that with heavy, regular use, you “are basically loading your brain with dopamine; [perhaps] people become more sensitized to dopamine-induced perceptual and cognitive problems,” she said.
“Luckily, if we can get people to stop use early in the course of their psychotic illness, they do much better. There are improvements in mood, anxiety, positive psychotic symptoms, medication adherence, and global functioning,” she said.
It’s not easy to get people to stop, however.
The notion of “marijuana as a potent cause of schizophrenia ... is something that users do not like to hear.” Many consider marijuana “a medicine, and that if they smoke it, they are going to be healthier. If you suggest that” marijuana might do “some bad things in addition to all the great things they think it’s doing, they immediately think you are a narc, and shut down. It’s hard to have an open conversion,” Dr. Rapp said.
At this point, it seems that it’s the tetrahydrocannabinol (THC) in marijuana that causes problems for people who have or who are prone to psychosis, and that it’s the cannabidiol (CBD) component that’s responsible for the therapeutic effects. CBD appears to be a dopamine D2 receptor antagonist, and some small pilot studies have found anti-psychotic effects. “CBD actually has some potential as a treatment” for psychosis, especially for negative symptoms, she said.
So, when abstinence isn’t an option, “I try to steer people towards CBD, rather than THC. Labels in dispensaries tell you what the THC and CBD content are. Those can be really unreliable, but it’s something.” Staff can point out high CBD products. “I also usually caution against smoking. I tell people that smoking anything has bad effects on your health,” she said. Edibles are among the many alternative formulations.
Patients who are truly interested in therapeutic benefits appreciate the message. “If they say, ‘oh, that doesn’t sound like fun,’ it tells me they are really looking for the psychoactive THC high,” she said.
Dr. Rapp did not have any industry disclosures.
NEW ORLEANS – Marijuana and psychosis don’t mix, according to Erica Rapp, MD, an assistant professor of psychiatry at the University of Colorado Anschutz Medical Campus, Aurora.
Regular use is associated with increased positive psychotic symptoms, a wide-range of poor psycho-social outcomes, reduced medication adherence, and a higher rate of relapse that’s not entirely explained by reduced adherence. One study even found an increased risk of suicide, she said at the American Psychiatric Association’s Institute on Psychiatric Services meeting.
Epidemiological studies, meanwhile, have found a “robust” association between regular marijuana use and an increased risk of schizophrenia, about two-times higher in the general population and about four-times higher in people who are predisposed to psychosis. It’s possible that marijuana doesn’t actually increase the rate of psychotic disorders, but just makes them come on sooner. “While that is almost certainly true, it doesn’t exclude the overall increased risk,” Dr. Rapp said.
Researchers are working to unravel the cross-talk between marijuana and psychosis. It seems that with heavy, regular use, you “are basically loading your brain with dopamine; [perhaps] people become more sensitized to dopamine-induced perceptual and cognitive problems,” she said.
“Luckily, if we can get people to stop use early in the course of their psychotic illness, they do much better. There are improvements in mood, anxiety, positive psychotic symptoms, medication adherence, and global functioning,” she said.
It’s not easy to get people to stop, however.
The notion of “marijuana as a potent cause of schizophrenia ... is something that users do not like to hear.” Many consider marijuana “a medicine, and that if they smoke it, they are going to be healthier. If you suggest that” marijuana might do “some bad things in addition to all the great things they think it’s doing, they immediately think you are a narc, and shut down. It’s hard to have an open conversion,” Dr. Rapp said.
At this point, it seems that it’s the tetrahydrocannabinol (THC) in marijuana that causes problems for people who have or who are prone to psychosis, and that it’s the cannabidiol (CBD) component that’s responsible for the therapeutic effects. CBD appears to be a dopamine D2 receptor antagonist, and some small pilot studies have found anti-psychotic effects. “CBD actually has some potential as a treatment” for psychosis, especially for negative symptoms, she said.
So, when abstinence isn’t an option, “I try to steer people towards CBD, rather than THC. Labels in dispensaries tell you what the THC and CBD content are. Those can be really unreliable, but it’s something.” Staff can point out high CBD products. “I also usually caution against smoking. I tell people that smoking anything has bad effects on your health,” she said. Edibles are among the many alternative formulations.
Patients who are truly interested in therapeutic benefits appreciate the message. “If they say, ‘oh, that doesn’t sound like fun,’ it tells me they are really looking for the psychoactive THC high,” she said.
Dr. Rapp did not have any industry disclosures.
AT IPS 2017
No benefit found in pre-bariatric surgery weight loss programs
NATIONAL HARBOR, MD – Many third-party payers require candidates for bariatric surgery to complete weight loss programs in order to qualify for reimbursement, but two new studies presented at Obesity Week 2017 have found no identifiable justification for the delay in treatment.
“When comparing those who did or did not participate in a weight management program, there was no significant benefit in regard to surgery complications, patient rate of followup, or percent excess weight loss at 12 months,” reported Andrew Schneider, MD, who is completing his residency in general surgery in the Greenville Health Systems, Greenville, South Carolina.
No significant differences were observed in a long list of procedural and outcome variables including operating time, length of hospital stay, and excess weight loss (EWL) at 3, 6, and 12 months, according to Dr. Schneider, who emphasized that no differences even approached significance.
A second study, evaluating the effect of presurgical weight management programs from a different perspective, drew the same conclusion. In this study, the goal was to correlate the number of preoperative weight loss sessions with change in multiple outcomes including EWL, according Genna Hymowitz, PhD, a psychologist at the Stony Brook Medicine Bariatric and Metabolic Weight Loss Center, Stony Brook, New York.
No correlation was observed between number of presurgical weight management program visits and any outcome evaluated in followup out to 12 months, according to Dr. Hymowitz. There was one exception.
“The number of visits attended and weight loss 3 weeks after surgery was a negative correlation, suggesting that the number of sessions attended was associated with lower excess weight loss,” Dr. Hymowitz reported.
Insurance company requirements for presurgical weight management programs vary widely, but the American Society for Metabolic and Bariatric Surgery (ASMBS) concluded in a position statement issued in 2011 that they are unsupported by controlled evidence. According to this statement, which referenced several clinical studies, “there is no evidence of any kind that insurance mandated preoperative weight loss…has any clear impact on postoperative outcomes or weight loss.”
In the ASBMS statement, the objection is directed at specific requirements for medically supervised weight loss program. These can demand six or more months of participation before reimbursement for surgery will be granted. In the ASBMS statement, mandated treatment required by insurance companies is distinguished from Medicare policy. Medicare reimbursement requires patients to fail medical treatment prior to bariatric surgery but providers are allowed to define failure. In contrast, specified periods of medical management required by insurance companies can have the effect of delaying treatment with proven efficacy in appropriate candidates.
Asked to speculate why insurance companies mandate supervised weight loss program for bariatric surgery eligibility, Dr. Schneider suggested that it might be considered a method to evaluate patient motivation and compliance. However, he also acknowledged that the requirement is likely to provide a barrier for some individuals thereby reducing surgical costs for the third-party payers.
While there are now several studies, including those cited in the ASBMS position statement, arguing that these mandates should be eliminated, longer followup is needed, according to Maher El Chaar, MD, Co-Medical Director, Bariatric surgery, St. Luke’s University Hospital, Allentown, Pennsylvania. One of the moderators for the Obesity Week session in which the two latest studies were presented, Dr. El Chaar said that insurance company representatives with whom he has spoken insist that longer-term studies are needed.
“When I point out that there is no data supporting mandated weight management programs, they tell me that there is very little data beyond 12 months,” Dr. El Chaar explained. He suggested data beyond 12 months could be helpful in the effort to get these requirements waived.
Dr. Schneider and Dr. Hymowitz reported no relevant financial relationships.
NATIONAL HARBOR, MD – Many third-party payers require candidates for bariatric surgery to complete weight loss programs in order to qualify for reimbursement, but two new studies presented at Obesity Week 2017 have found no identifiable justification for the delay in treatment.
“When comparing those who did or did not participate in a weight management program, there was no significant benefit in regard to surgery complications, patient rate of followup, or percent excess weight loss at 12 months,” reported Andrew Schneider, MD, who is completing his residency in general surgery in the Greenville Health Systems, Greenville, South Carolina.
No significant differences were observed in a long list of procedural and outcome variables including operating time, length of hospital stay, and excess weight loss (EWL) at 3, 6, and 12 months, according to Dr. Schneider, who emphasized that no differences even approached significance.
A second study, evaluating the effect of presurgical weight management programs from a different perspective, drew the same conclusion. In this study, the goal was to correlate the number of preoperative weight loss sessions with change in multiple outcomes including EWL, according Genna Hymowitz, PhD, a psychologist at the Stony Brook Medicine Bariatric and Metabolic Weight Loss Center, Stony Brook, New York.
No correlation was observed between number of presurgical weight management program visits and any outcome evaluated in followup out to 12 months, according to Dr. Hymowitz. There was one exception.
“The number of visits attended and weight loss 3 weeks after surgery was a negative correlation, suggesting that the number of sessions attended was associated with lower excess weight loss,” Dr. Hymowitz reported.
Insurance company requirements for presurgical weight management programs vary widely, but the American Society for Metabolic and Bariatric Surgery (ASMBS) concluded in a position statement issued in 2011 that they are unsupported by controlled evidence. According to this statement, which referenced several clinical studies, “there is no evidence of any kind that insurance mandated preoperative weight loss…has any clear impact on postoperative outcomes or weight loss.”
In the ASBMS statement, the objection is directed at specific requirements for medically supervised weight loss program. These can demand six or more months of participation before reimbursement for surgery will be granted. In the ASBMS statement, mandated treatment required by insurance companies is distinguished from Medicare policy. Medicare reimbursement requires patients to fail medical treatment prior to bariatric surgery but providers are allowed to define failure. In contrast, specified periods of medical management required by insurance companies can have the effect of delaying treatment with proven efficacy in appropriate candidates.
Asked to speculate why insurance companies mandate supervised weight loss program for bariatric surgery eligibility, Dr. Schneider suggested that it might be considered a method to evaluate patient motivation and compliance. However, he also acknowledged that the requirement is likely to provide a barrier for some individuals thereby reducing surgical costs for the third-party payers.
While there are now several studies, including those cited in the ASBMS position statement, arguing that these mandates should be eliminated, longer followup is needed, according to Maher El Chaar, MD, Co-Medical Director, Bariatric surgery, St. Luke’s University Hospital, Allentown, Pennsylvania. One of the moderators for the Obesity Week session in which the two latest studies were presented, Dr. El Chaar said that insurance company representatives with whom he has spoken insist that longer-term studies are needed.
“When I point out that there is no data supporting mandated weight management programs, they tell me that there is very little data beyond 12 months,” Dr. El Chaar explained. He suggested data beyond 12 months could be helpful in the effort to get these requirements waived.
Dr. Schneider and Dr. Hymowitz reported no relevant financial relationships.
NATIONAL HARBOR, MD – Many third-party payers require candidates for bariatric surgery to complete weight loss programs in order to qualify for reimbursement, but two new studies presented at Obesity Week 2017 have found no identifiable justification for the delay in treatment.
“When comparing those who did or did not participate in a weight management program, there was no significant benefit in regard to surgery complications, patient rate of followup, or percent excess weight loss at 12 months,” reported Andrew Schneider, MD, who is completing his residency in general surgery in the Greenville Health Systems, Greenville, South Carolina.
No significant differences were observed in a long list of procedural and outcome variables including operating time, length of hospital stay, and excess weight loss (EWL) at 3, 6, and 12 months, according to Dr. Schneider, who emphasized that no differences even approached significance.
A second study, evaluating the effect of presurgical weight management programs from a different perspective, drew the same conclusion. In this study, the goal was to correlate the number of preoperative weight loss sessions with change in multiple outcomes including EWL, according Genna Hymowitz, PhD, a psychologist at the Stony Brook Medicine Bariatric and Metabolic Weight Loss Center, Stony Brook, New York.
No correlation was observed between number of presurgical weight management program visits and any outcome evaluated in followup out to 12 months, according to Dr. Hymowitz. There was one exception.
“The number of visits attended and weight loss 3 weeks after surgery was a negative correlation, suggesting that the number of sessions attended was associated with lower excess weight loss,” Dr. Hymowitz reported.
Insurance company requirements for presurgical weight management programs vary widely, but the American Society for Metabolic and Bariatric Surgery (ASMBS) concluded in a position statement issued in 2011 that they are unsupported by controlled evidence. According to this statement, which referenced several clinical studies, “there is no evidence of any kind that insurance mandated preoperative weight loss…has any clear impact on postoperative outcomes or weight loss.”
In the ASBMS statement, the objection is directed at specific requirements for medically supervised weight loss program. These can demand six or more months of participation before reimbursement for surgery will be granted. In the ASBMS statement, mandated treatment required by insurance companies is distinguished from Medicare policy. Medicare reimbursement requires patients to fail medical treatment prior to bariatric surgery but providers are allowed to define failure. In contrast, specified periods of medical management required by insurance companies can have the effect of delaying treatment with proven efficacy in appropriate candidates.
Asked to speculate why insurance companies mandate supervised weight loss program for bariatric surgery eligibility, Dr. Schneider suggested that it might be considered a method to evaluate patient motivation and compliance. However, he also acknowledged that the requirement is likely to provide a barrier for some individuals thereby reducing surgical costs for the third-party payers.
While there are now several studies, including those cited in the ASBMS position statement, arguing that these mandates should be eliminated, longer followup is needed, according to Maher El Chaar, MD, Co-Medical Director, Bariatric surgery, St. Luke’s University Hospital, Allentown, Pennsylvania. One of the moderators for the Obesity Week session in which the two latest studies were presented, Dr. El Chaar said that insurance company representatives with whom he has spoken insist that longer-term studies are needed.
“When I point out that there is no data supporting mandated weight management programs, they tell me that there is very little data beyond 12 months,” Dr. El Chaar explained. He suggested data beyond 12 months could be helpful in the effort to get these requirements waived.
Dr. Schneider and Dr. Hymowitz reported no relevant financial relationships.
AT OBESITY WEEK 2017
Key clinical point: Two studies concluded mandated weight loss programs prior to bariatric surgery offer no clinical value.
Major finding: When compared for weight loss at 3, 6, or 12 months after surgery, there was no difference in weight change for participants versus non-participants.
Data source: Retrospective and prospective analyses.
Disclosures: Dr. Schneider and Dr. Hymowitz reported no relevant financial relationships.
New BACE1 study launches in the shadow of verubecestat’s demise
BOSTON – Undeterred by a failed BACE inhibitor study with worrisome adverse events, an ambitious new clinical trial will investigate a different BACE-inhibiting molecule as an Alzheimer’s preventive in people at high genetic risk of Alzheimer’s disease.
The global Generation 2 trial intends to recruit about 3,300 cognitively normal subjects aged 60-75 years, who have either one or two copies of the apolipoprotein e4 (ApoE4) allele, said Pierre Tariot, MD, who announced the new study during the Clinical Trials on Alzheimer’s Disease 2017 meeting.
The Alzheimer Preventive Initiative and industry partners Novartis and Amgen are sponsoring the trial, which is a sister study to Generation 1. Generation 1, now ongoing, targets cognitively normal ApoE4 subjects only. It is a four-armed trial, comparing placebo to both CNP520 and an active immunotherapy called CAD106. Together, the studies comprise the Generation Program.
Both trials are event-driven, and will run 5-8 years. Generation 2 has a dual primary endpoint – a successful trial will find either a delay in progression to mild cognitive impairment or dementia relative to placebo, or significant differences from baseline in cognitive change as measured by the Alzheimer’s Preclinical Composite Cognitive test, or both.
The study partners are enthusiastic about CNP520, which performed well in its safety and tolerability studies. In an interview, Dr. Tariot called the CNP520 “likely a best-in-class molecule.”
“The only adverse events we saw in the entire safety program were a few cases of itchy skin,” said Dr. Tariot, director of the Banner Alzheimer Institute in Phoenix. “One of the reasons we chose Novartis as a study partner is that this drug of theirs looked remarkably clean – so clean that when we were deciding what doses to look at, the decision related only to what degree of BACE inhibition we wanted, not safety.”
The drug seemed very well-tolerated at every dose tested (1 mg, 10 mg, 25 mg, and 75 mg). Generation 1 employs a 50-mg oral dose per day. Generation 2 will investigate both 50 mg and 15 mg.
Site investigators are solidly behind the choice, said Anton Porsteinsson, MD, a Generation investigator at the University of Rochester (N.Y.) Medical Center, despite the unveiling at CTAD of worrisome adverse events seen in Merck’s failed EPOCH trial.
“I am even more convinced now that BACE inhibitors are drugs that need to be used early and that are probably highly indicated for this population,” he said in an interview. Dr. Porsteinsson wasn’t overly disturbed by data that Merck released during the meeting on its BACE inhibitor, verubecestat. In February, the company pulled the plug on its EPOCH trial investigating verubecestat in patients with mild-moderate AD. An interim analysis determined that there was no chance of success with the molecule.
Despite the general disappointment of yet another rainy-day parade in late-stage AD drug trials, researchers who heard the EPOCH post-mortem during CTAD expressed considerable concern over the unexpected, wide-ranging, and serious adverse events the trial accumulated.
Although there were no cases of Amyloid-Related Imaging Abnormalities (ARIA), a number of adverse events occurred significantly more often in the active groups than the placebo group. These included rash, falls and injuries, insomnia, headache, anxiety, suicidal ideation, diarrhea, dizziness, and weight loss.
At least some of these were hinted at in the bench science that brought verubecestat to late-stage clinical development. BACE is important for proper muscle function, and some BACE-knockout mice displayed a decrease in muscle spindles, receptors that sense changes in the length of muscle fibers. Other peculiarities in the mice have included axon targeting errors, reduced myelination, memory impairment, neurochemical abnormalities, alterations in neurogenesis and astrogenesis, increased age-related neurodegeneration, reduced spine density, retinal pathology, endophenotypes of schizophrenia, and seizures.
But the verubecestat findings aren’t a show-stopper for the more target-specific CNP520, Dr. Porsteinsson said. Verubecestat inhibited both BACE1 and BACE2; CNP520, only BACE1. And Dr. Porsteinsson, like most researchers, believes that preventing the accumulation of neurotoxic AB species will probably be much more effective clinically than trying to dissolve large stubborn brain plaques, which have already wreaked cognitive havoc.
In EPOCH, “even in people with advanced disease and a high load of insoluble plaques; they saw an 80% reduction in the production of AB and a 4% decrease in plaque burden. To me that signals this is not the class to use in moderate patients, or even mild, but in these very early stages where you don’t have full saturation, it might just be perfect,” he said.
“Do the side effects give me pause? Obviously and maybe mostly because we don’t know exactly what the driver was. But what we do is keep a close eye on everyone, maybe do extra safety monitoring, Most importantly, we picked a drug that shows less issues with any of these problems.”
Cognitively normal ApoE4 carriers – especially homozygotes – are an extremely important population to study, both in terms of clinical and scientific need. The gene is the single largest genetic risk factor for Alzheimer’s; those who carry two copies are 60% more likely than the general population to develop Alzheimer’s.
“These people truly need an effective intervention,” said Dr. Porsteinsson. “At the preclinical stage, they don’t truly have a disease yet; they are living their lives unimpaired. But something is percolating, and the results won’t be good. Even early on, there are fairly significant changes going on in the brain: a buildup of amyloid and tau, excess oxidative damage, inflammation. And if you don’t deal with it early on, it’s like trying to cure cancer once it’s metastasized.”
In the larger scientific picture, success in a primary prevention trial would finally put to rest questions about the amyloid cascade hypothesis and explain the long string of anti-amyloid drug trial failures, all of which were targeted at people with more advanced disease.
To enroll the entire cohort, Generation 2 will need to screen about 30,000 people. This sounds like a daunting task, but a new digital platform makes it eminently do-able, Dr. Tariot said. Both Generation studies are enrolling online. The consumer-friendly website offers detailed information about Alzheimer’s disease in general, ApoE4 risk, the studies’ structure, and the investigational medications. Most importantly, Dr. Tariot said, the website has a direct link to GeneMatch. Hosted by Banner Alzheimer Institute, GeneMatch prescreens potential trial participants (U.S. residents aged 55-75 years) by taking baseline demographic information, and mailing out free, simple-to-use cheek swab kits for genetic analysis. The program then stores and sorts the information, matching volunteers with appropriate trials according to location, age, interest, and genetic status. People don’t necessarily learn their ApoE4 status, unless they are recruited into a genetics-driven trial.
Launched 2 years ago, GeneMatch has already accrued more than 280,000 volunteers – a pretty remarkable achievement in itself, Dr. Tariot said. “People are very motivated to help find a cure for Alzheimer’s. Many of them have a family member who has the disease. Others are concerned about themselves, and many people just want to be part of something important.”
Dr. Porsteinsson agreed, relaying a startling interaction he had with a patient, who found out he was not qualified for a Generation study, meaning, of course, that he was ApoE4 negative.
“He told me he was actually kind of disappointed, because he believes so much in this study, and wanted to be part of doing something important for humanity,” Dr. Porsteinsson said. “Of course, I didn’t let him off the hook. I directed him to a bunch of other studies he was qualified for. We need everyone’s help to solve this.”
Both Dr. Tariot and Dr. Porsteinsson have reported financial relationships with numerous pharmaceutical companies.
* This story was updated 11/7/17.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – Undeterred by a failed BACE inhibitor study with worrisome adverse events, an ambitious new clinical trial will investigate a different BACE-inhibiting molecule as an Alzheimer’s preventive in people at high genetic risk of Alzheimer’s disease.
The global Generation 2 trial intends to recruit about 3,300 cognitively normal subjects aged 60-75 years, who have either one or two copies of the apolipoprotein e4 (ApoE4) allele, said Pierre Tariot, MD, who announced the new study during the Clinical Trials on Alzheimer’s Disease 2017 meeting.
The Alzheimer Preventive Initiative and industry partners Novartis and Amgen are sponsoring the trial, which is a sister study to Generation 1. Generation 1, now ongoing, targets cognitively normal ApoE4 subjects only. It is a four-armed trial, comparing placebo to both CNP520 and an active immunotherapy called CAD106. Together, the studies comprise the Generation Program.
Both trials are event-driven, and will run 5-8 years. Generation 2 has a dual primary endpoint – a successful trial will find either a delay in progression to mild cognitive impairment or dementia relative to placebo, or significant differences from baseline in cognitive change as measured by the Alzheimer’s Preclinical Composite Cognitive test, or both.
The study partners are enthusiastic about CNP520, which performed well in its safety and tolerability studies. In an interview, Dr. Tariot called the CNP520 “likely a best-in-class molecule.”
“The only adverse events we saw in the entire safety program were a few cases of itchy skin,” said Dr. Tariot, director of the Banner Alzheimer Institute in Phoenix. “One of the reasons we chose Novartis as a study partner is that this drug of theirs looked remarkably clean – so clean that when we were deciding what doses to look at, the decision related only to what degree of BACE inhibition we wanted, not safety.”
The drug seemed very well-tolerated at every dose tested (1 mg, 10 mg, 25 mg, and 75 mg). Generation 1 employs a 50-mg oral dose per day. Generation 2 will investigate both 50 mg and 15 mg.
Site investigators are solidly behind the choice, said Anton Porsteinsson, MD, a Generation investigator at the University of Rochester (N.Y.) Medical Center, despite the unveiling at CTAD of worrisome adverse events seen in Merck’s failed EPOCH trial.
“I am even more convinced now that BACE inhibitors are drugs that need to be used early and that are probably highly indicated for this population,” he said in an interview. Dr. Porsteinsson wasn’t overly disturbed by data that Merck released during the meeting on its BACE inhibitor, verubecestat. In February, the company pulled the plug on its EPOCH trial investigating verubecestat in patients with mild-moderate AD. An interim analysis determined that there was no chance of success with the molecule.
Despite the general disappointment of yet another rainy-day parade in late-stage AD drug trials, researchers who heard the EPOCH post-mortem during CTAD expressed considerable concern over the unexpected, wide-ranging, and serious adverse events the trial accumulated.
Although there were no cases of Amyloid-Related Imaging Abnormalities (ARIA), a number of adverse events occurred significantly more often in the active groups than the placebo group. These included rash, falls and injuries, insomnia, headache, anxiety, suicidal ideation, diarrhea, dizziness, and weight loss.
At least some of these were hinted at in the bench science that brought verubecestat to late-stage clinical development. BACE is important for proper muscle function, and some BACE-knockout mice displayed a decrease in muscle spindles, receptors that sense changes in the length of muscle fibers. Other peculiarities in the mice have included axon targeting errors, reduced myelination, memory impairment, neurochemical abnormalities, alterations in neurogenesis and astrogenesis, increased age-related neurodegeneration, reduced spine density, retinal pathology, endophenotypes of schizophrenia, and seizures.
But the verubecestat findings aren’t a show-stopper for the more target-specific CNP520, Dr. Porsteinsson said. Verubecestat inhibited both BACE1 and BACE2; CNP520, only BACE1. And Dr. Porsteinsson, like most researchers, believes that preventing the accumulation of neurotoxic AB species will probably be much more effective clinically than trying to dissolve large stubborn brain plaques, which have already wreaked cognitive havoc.
In EPOCH, “even in people with advanced disease and a high load of insoluble plaques; they saw an 80% reduction in the production of AB and a 4% decrease in plaque burden. To me that signals this is not the class to use in moderate patients, or even mild, but in these very early stages where you don’t have full saturation, it might just be perfect,” he said.
“Do the side effects give me pause? Obviously and maybe mostly because we don’t know exactly what the driver was. But what we do is keep a close eye on everyone, maybe do extra safety monitoring, Most importantly, we picked a drug that shows less issues with any of these problems.”
Cognitively normal ApoE4 carriers – especially homozygotes – are an extremely important population to study, both in terms of clinical and scientific need. The gene is the single largest genetic risk factor for Alzheimer’s; those who carry two copies are 60% more likely than the general population to develop Alzheimer’s.
“These people truly need an effective intervention,” said Dr. Porsteinsson. “At the preclinical stage, they don’t truly have a disease yet; they are living their lives unimpaired. But something is percolating, and the results won’t be good. Even early on, there are fairly significant changes going on in the brain: a buildup of amyloid and tau, excess oxidative damage, inflammation. And if you don’t deal with it early on, it’s like trying to cure cancer once it’s metastasized.”
In the larger scientific picture, success in a primary prevention trial would finally put to rest questions about the amyloid cascade hypothesis and explain the long string of anti-amyloid drug trial failures, all of which were targeted at people with more advanced disease.
To enroll the entire cohort, Generation 2 will need to screen about 30,000 people. This sounds like a daunting task, but a new digital platform makes it eminently do-able, Dr. Tariot said. Both Generation studies are enrolling online. The consumer-friendly website offers detailed information about Alzheimer’s disease in general, ApoE4 risk, the studies’ structure, and the investigational medications. Most importantly, Dr. Tariot said, the website has a direct link to GeneMatch. Hosted by Banner Alzheimer Institute, GeneMatch prescreens potential trial participants (U.S. residents aged 55-75 years) by taking baseline demographic information, and mailing out free, simple-to-use cheek swab kits for genetic analysis. The program then stores and sorts the information, matching volunteers with appropriate trials according to location, age, interest, and genetic status. People don’t necessarily learn their ApoE4 status, unless they are recruited into a genetics-driven trial.
Launched 2 years ago, GeneMatch has already accrued more than 280,000 volunteers – a pretty remarkable achievement in itself, Dr. Tariot said. “People are very motivated to help find a cure for Alzheimer’s. Many of them have a family member who has the disease. Others are concerned about themselves, and many people just want to be part of something important.”
Dr. Porsteinsson agreed, relaying a startling interaction he had with a patient, who found out he was not qualified for a Generation study, meaning, of course, that he was ApoE4 negative.
“He told me he was actually kind of disappointed, because he believes so much in this study, and wanted to be part of doing something important for humanity,” Dr. Porsteinsson said. “Of course, I didn’t let him off the hook. I directed him to a bunch of other studies he was qualified for. We need everyone’s help to solve this.”
Both Dr. Tariot and Dr. Porsteinsson have reported financial relationships with numerous pharmaceutical companies.
* This story was updated 11/7/17.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – Undeterred by a failed BACE inhibitor study with worrisome adverse events, an ambitious new clinical trial will investigate a different BACE-inhibiting molecule as an Alzheimer’s preventive in people at high genetic risk of Alzheimer’s disease.
The global Generation 2 trial intends to recruit about 3,300 cognitively normal subjects aged 60-75 years, who have either one or two copies of the apolipoprotein e4 (ApoE4) allele, said Pierre Tariot, MD, who announced the new study during the Clinical Trials on Alzheimer’s Disease 2017 meeting.
The Alzheimer Preventive Initiative and industry partners Novartis and Amgen are sponsoring the trial, which is a sister study to Generation 1. Generation 1, now ongoing, targets cognitively normal ApoE4 subjects only. It is a four-armed trial, comparing placebo to both CNP520 and an active immunotherapy called CAD106. Together, the studies comprise the Generation Program.
Both trials are event-driven, and will run 5-8 years. Generation 2 has a dual primary endpoint – a successful trial will find either a delay in progression to mild cognitive impairment or dementia relative to placebo, or significant differences from baseline in cognitive change as measured by the Alzheimer’s Preclinical Composite Cognitive test, or both.
The study partners are enthusiastic about CNP520, which performed well in its safety and tolerability studies. In an interview, Dr. Tariot called the CNP520 “likely a best-in-class molecule.”
“The only adverse events we saw in the entire safety program were a few cases of itchy skin,” said Dr. Tariot, director of the Banner Alzheimer Institute in Phoenix. “One of the reasons we chose Novartis as a study partner is that this drug of theirs looked remarkably clean – so clean that when we were deciding what doses to look at, the decision related only to what degree of BACE inhibition we wanted, not safety.”
The drug seemed very well-tolerated at every dose tested (1 mg, 10 mg, 25 mg, and 75 mg). Generation 1 employs a 50-mg oral dose per day. Generation 2 will investigate both 50 mg and 15 mg.
Site investigators are solidly behind the choice, said Anton Porsteinsson, MD, a Generation investigator at the University of Rochester (N.Y.) Medical Center, despite the unveiling at CTAD of worrisome adverse events seen in Merck’s failed EPOCH trial.
“I am even more convinced now that BACE inhibitors are drugs that need to be used early and that are probably highly indicated for this population,” he said in an interview. Dr. Porsteinsson wasn’t overly disturbed by data that Merck released during the meeting on its BACE inhibitor, verubecestat. In February, the company pulled the plug on its EPOCH trial investigating verubecestat in patients with mild-moderate AD. An interim analysis determined that there was no chance of success with the molecule.
Despite the general disappointment of yet another rainy-day parade in late-stage AD drug trials, researchers who heard the EPOCH post-mortem during CTAD expressed considerable concern over the unexpected, wide-ranging, and serious adverse events the trial accumulated.
Although there were no cases of Amyloid-Related Imaging Abnormalities (ARIA), a number of adverse events occurred significantly more often in the active groups than the placebo group. These included rash, falls and injuries, insomnia, headache, anxiety, suicidal ideation, diarrhea, dizziness, and weight loss.
At least some of these were hinted at in the bench science that brought verubecestat to late-stage clinical development. BACE is important for proper muscle function, and some BACE-knockout mice displayed a decrease in muscle spindles, receptors that sense changes in the length of muscle fibers. Other peculiarities in the mice have included axon targeting errors, reduced myelination, memory impairment, neurochemical abnormalities, alterations in neurogenesis and astrogenesis, increased age-related neurodegeneration, reduced spine density, retinal pathology, endophenotypes of schizophrenia, and seizures.
But the verubecestat findings aren’t a show-stopper for the more target-specific CNP520, Dr. Porsteinsson said. Verubecestat inhibited both BACE1 and BACE2; CNP520, only BACE1. And Dr. Porsteinsson, like most researchers, believes that preventing the accumulation of neurotoxic AB species will probably be much more effective clinically than trying to dissolve large stubborn brain plaques, which have already wreaked cognitive havoc.
In EPOCH, “even in people with advanced disease and a high load of insoluble plaques; they saw an 80% reduction in the production of AB and a 4% decrease in plaque burden. To me that signals this is not the class to use in moderate patients, or even mild, but in these very early stages where you don’t have full saturation, it might just be perfect,” he said.
“Do the side effects give me pause? Obviously and maybe mostly because we don’t know exactly what the driver was. But what we do is keep a close eye on everyone, maybe do extra safety monitoring, Most importantly, we picked a drug that shows less issues with any of these problems.”
Cognitively normal ApoE4 carriers – especially homozygotes – are an extremely important population to study, both in terms of clinical and scientific need. The gene is the single largest genetic risk factor for Alzheimer’s; those who carry two copies are 60% more likely than the general population to develop Alzheimer’s.
“These people truly need an effective intervention,” said Dr. Porsteinsson. “At the preclinical stage, they don’t truly have a disease yet; they are living their lives unimpaired. But something is percolating, and the results won’t be good. Even early on, there are fairly significant changes going on in the brain: a buildup of amyloid and tau, excess oxidative damage, inflammation. And if you don’t deal with it early on, it’s like trying to cure cancer once it’s metastasized.”
In the larger scientific picture, success in a primary prevention trial would finally put to rest questions about the amyloid cascade hypothesis and explain the long string of anti-amyloid drug trial failures, all of which were targeted at people with more advanced disease.
To enroll the entire cohort, Generation 2 will need to screen about 30,000 people. This sounds like a daunting task, but a new digital platform makes it eminently do-able, Dr. Tariot said. Both Generation studies are enrolling online. The consumer-friendly website offers detailed information about Alzheimer’s disease in general, ApoE4 risk, the studies’ structure, and the investigational medications. Most importantly, Dr. Tariot said, the website has a direct link to GeneMatch. Hosted by Banner Alzheimer Institute, GeneMatch prescreens potential trial participants (U.S. residents aged 55-75 years) by taking baseline demographic information, and mailing out free, simple-to-use cheek swab kits for genetic analysis. The program then stores and sorts the information, matching volunteers with appropriate trials according to location, age, interest, and genetic status. People don’t necessarily learn their ApoE4 status, unless they are recruited into a genetics-driven trial.
Launched 2 years ago, GeneMatch has already accrued more than 280,000 volunteers – a pretty remarkable achievement in itself, Dr. Tariot said. “People are very motivated to help find a cure for Alzheimer’s. Many of them have a family member who has the disease. Others are concerned about themselves, and many people just want to be part of something important.”
Dr. Porsteinsson agreed, relaying a startling interaction he had with a patient, who found out he was not qualified for a Generation study, meaning, of course, that he was ApoE4 negative.
“He told me he was actually kind of disappointed, because he believes so much in this study, and wanted to be part of doing something important for humanity,” Dr. Porsteinsson said. “Of course, I didn’t let him off the hook. I directed him to a bunch of other studies he was qualified for. We need everyone’s help to solve this.”
Both Dr. Tariot and Dr. Porsteinsson have reported financial relationships with numerous pharmaceutical companies.
* This story was updated 11/7/17.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT CTAD
Seven years after bariatric surgery, more than 40% still off insulin
NATIONAL HARBOR, MD – Forty-four percent of insulin-dependent patients with type 2 diabetes mellitus (DM2) were at their glycemic target without insulin a median of seven years after surgery. The data from the largest study to evaluate long-term outcomes in this population were presented at Obesity Week 2017.
“These data confirm that the impressive metabolic effects of bariatric surgery in patients with type 2 diabetes are sustained beyond five years,” reported Ali Aminian, MD, a surgeon who specializes in bariatric procedures at the Cleveland Clinic, Cleveland, Ohio. He said that long-term efficacy has not been well characterized previously.
Reaching the glycemic target, defined as less than 7% HbA1c, without insulin was only one of the primary endpoints. The other was diabetes remission, which was defined as HbA1c less than 6.5%, fasting blood glucose less than 126 mg/dL, and being off all diabetes medications. This was observed in 15% of the patients after a median of 7 years followup.
Contrasting short-term results, defined as outcomes one to two years after bariatric surgery with the long-term followup, Dr. Aminian was able to show that declines were relatively modest over time. For example, 51% were at the glycemic target off insulin at the short-term mark, which translates into an absolute decline of only 7% relative to the 44% observed at the long-term followup assessment.
Similarly, 70% had achieved the American Diabetes Association (ADA) goal of less than 7% within the first two years of surgery, while 59% remained at this goal at the most recent followup. The proportion taking insulin at the short-term mark was 36% rising only to 40% long-term.
When data were stratified by procedure, results favored RYGB over sleeve gastrectomy. For example, 47% of the RYGB patients versus 33% of the sleeve gastrectomy patients were able to reach the ADA goal without insulin at the end of the study. The proportions in diabetes remission were 17% and 10%, respectively. RYGB was also associated with greater improvement in BMI (median -12 vs. - 8 kg/m2) and reduced late weight gain (median 20% vs. 31%).
However, Dr. Aminian, who did not provide statistical calculations for these differences, cautioned that higher risk patients might have been preferentially selected for sleeve gastrectomy. He noted that difference in median HbA1c levels was significantly lower in the RYGB group two years after surgery (P less than .001) but the numerical advantage had lost significance at the last followup (P = .32).
In an evaluation of predictors for glycemic control, a shorter duration of diabetes (less than 10 years) and good glycemic control prior to surgery were both predictors of achieving the primary outcomes on the basis of a multivariate analysis, according to Dr. Aminian. Younger age was a marginal predictor, but Dr. Aminian said that neither type of procedure nor presurgical BMI predicted outcomes from the multivariate analysis.
Relative to baseline, there were significant improvements in median LDL (P = .001). In addition, HDL, triglyceride levels, systolic, and diastolic blood pressure measurements were all significantly improved, both short-term and long-term after bariatric surgery (all P values less than .001), according to Dr. Aminian. When expressed as ADA goals, 82% of participants had blood pressure less than 140/90 mm Hg 7 years after surgery relative to 44% at baseline (P less than .001). The proportion with LDL less 100 mg/dL approached, but did not reach clinical significance (61% vs. 70%; P=0.06).
“When you consider all three parameters [ADA targets for glycemic control, blood pressure control, and lipid control], only 3% of patients met all three targets at baseline but 32% [P< less than .001] were at these targets at long-term followup,” Dr. Aminian reported.
Dr. Aminian reported having no relevant financial relationships.
As the invited discussant on these data, Raul Rosenthal, MD, Director, Bariatric and Metabolic Institute, Cleveland Clinic Florida, Weston, Florida, reiterated that time with diabetes prior to bariatric surgery may be an important predictor of postsurgical control of metabolic parameters.
“I published a paper about 10 years ago on outcomes in patients with diabetes, and in our experience 5 years was the limit. If you have a history of 5 years or less with diabetes, the chance of going into remission were 80%, and if it was more than 5 years, the likelihood dropped dramatically,” Dr. Rosenthal noted. He indicated duration of diabetes deserves further evaluation for its potential relevance to the optimal timing of bariatric surgery.
NATIONAL HARBOR, MD – Forty-four percent of insulin-dependent patients with type 2 diabetes mellitus (DM2) were at their glycemic target without insulin a median of seven years after surgery. The data from the largest study to evaluate long-term outcomes in this population were presented at Obesity Week 2017.
“These data confirm that the impressive metabolic effects of bariatric surgery in patients with type 2 diabetes are sustained beyond five years,” reported Ali Aminian, MD, a surgeon who specializes in bariatric procedures at the Cleveland Clinic, Cleveland, Ohio. He said that long-term efficacy has not been well characterized previously.
Reaching the glycemic target, defined as less than 7% HbA1c, without insulin was only one of the primary endpoints. The other was diabetes remission, which was defined as HbA1c less than 6.5%, fasting blood glucose less than 126 mg/dL, and being off all diabetes medications. This was observed in 15% of the patients after a median of 7 years followup.
Contrasting short-term results, defined as outcomes one to two years after bariatric surgery with the long-term followup, Dr. Aminian was able to show that declines were relatively modest over time. For example, 51% were at the glycemic target off insulin at the short-term mark, which translates into an absolute decline of only 7% relative to the 44% observed at the long-term followup assessment.
Similarly, 70% had achieved the American Diabetes Association (ADA) goal of less than 7% within the first two years of surgery, while 59% remained at this goal at the most recent followup. The proportion taking insulin at the short-term mark was 36% rising only to 40% long-term.
When data were stratified by procedure, results favored RYGB over sleeve gastrectomy. For example, 47% of the RYGB patients versus 33% of the sleeve gastrectomy patients were able to reach the ADA goal without insulin at the end of the study. The proportions in diabetes remission were 17% and 10%, respectively. RYGB was also associated with greater improvement in BMI (median -12 vs. - 8 kg/m2) and reduced late weight gain (median 20% vs. 31%).
However, Dr. Aminian, who did not provide statistical calculations for these differences, cautioned that higher risk patients might have been preferentially selected for sleeve gastrectomy. He noted that difference in median HbA1c levels was significantly lower in the RYGB group two years after surgery (P less than .001) but the numerical advantage had lost significance at the last followup (P = .32).
In an evaluation of predictors for glycemic control, a shorter duration of diabetes (less than 10 years) and good glycemic control prior to surgery were both predictors of achieving the primary outcomes on the basis of a multivariate analysis, according to Dr. Aminian. Younger age was a marginal predictor, but Dr. Aminian said that neither type of procedure nor presurgical BMI predicted outcomes from the multivariate analysis.
Relative to baseline, there were significant improvements in median LDL (P = .001). In addition, HDL, triglyceride levels, systolic, and diastolic blood pressure measurements were all significantly improved, both short-term and long-term after bariatric surgery (all P values less than .001), according to Dr. Aminian. When expressed as ADA goals, 82% of participants had blood pressure less than 140/90 mm Hg 7 years after surgery relative to 44% at baseline (P less than .001). The proportion with LDL less 100 mg/dL approached, but did not reach clinical significance (61% vs. 70%; P=0.06).
“When you consider all three parameters [ADA targets for glycemic control, blood pressure control, and lipid control], only 3% of patients met all three targets at baseline but 32% [P< less than .001] were at these targets at long-term followup,” Dr. Aminian reported.
Dr. Aminian reported having no relevant financial relationships.
As the invited discussant on these data, Raul Rosenthal, MD, Director, Bariatric and Metabolic Institute, Cleveland Clinic Florida, Weston, Florida, reiterated that time with diabetes prior to bariatric surgery may be an important predictor of postsurgical control of metabolic parameters.
“I published a paper about 10 years ago on outcomes in patients with diabetes, and in our experience 5 years was the limit. If you have a history of 5 years or less with diabetes, the chance of going into remission were 80%, and if it was more than 5 years, the likelihood dropped dramatically,” Dr. Rosenthal noted. He indicated duration of diabetes deserves further evaluation for its potential relevance to the optimal timing of bariatric surgery.
NATIONAL HARBOR, MD – Forty-four percent of insulin-dependent patients with type 2 diabetes mellitus (DM2) were at their glycemic target without insulin a median of seven years after surgery. The data from the largest study to evaluate long-term outcomes in this population were presented at Obesity Week 2017.
“These data confirm that the impressive metabolic effects of bariatric surgery in patients with type 2 diabetes are sustained beyond five years,” reported Ali Aminian, MD, a surgeon who specializes in bariatric procedures at the Cleveland Clinic, Cleveland, Ohio. He said that long-term efficacy has not been well characterized previously.
Reaching the glycemic target, defined as less than 7% HbA1c, without insulin was only one of the primary endpoints. The other was diabetes remission, which was defined as HbA1c less than 6.5%, fasting blood glucose less than 126 mg/dL, and being off all diabetes medications. This was observed in 15% of the patients after a median of 7 years followup.
Contrasting short-term results, defined as outcomes one to two years after bariatric surgery with the long-term followup, Dr. Aminian was able to show that declines were relatively modest over time. For example, 51% were at the glycemic target off insulin at the short-term mark, which translates into an absolute decline of only 7% relative to the 44% observed at the long-term followup assessment.
Similarly, 70% had achieved the American Diabetes Association (ADA) goal of less than 7% within the first two years of surgery, while 59% remained at this goal at the most recent followup. The proportion taking insulin at the short-term mark was 36% rising only to 40% long-term.
When data were stratified by procedure, results favored RYGB over sleeve gastrectomy. For example, 47% of the RYGB patients versus 33% of the sleeve gastrectomy patients were able to reach the ADA goal without insulin at the end of the study. The proportions in diabetes remission were 17% and 10%, respectively. RYGB was also associated with greater improvement in BMI (median -12 vs. - 8 kg/m2) and reduced late weight gain (median 20% vs. 31%).
However, Dr. Aminian, who did not provide statistical calculations for these differences, cautioned that higher risk patients might have been preferentially selected for sleeve gastrectomy. He noted that difference in median HbA1c levels was significantly lower in the RYGB group two years after surgery (P less than .001) but the numerical advantage had lost significance at the last followup (P = .32).
In an evaluation of predictors for glycemic control, a shorter duration of diabetes (less than 10 years) and good glycemic control prior to surgery were both predictors of achieving the primary outcomes on the basis of a multivariate analysis, according to Dr. Aminian. Younger age was a marginal predictor, but Dr. Aminian said that neither type of procedure nor presurgical BMI predicted outcomes from the multivariate analysis.
Relative to baseline, there were significant improvements in median LDL (P = .001). In addition, HDL, triglyceride levels, systolic, and diastolic blood pressure measurements were all significantly improved, both short-term and long-term after bariatric surgery (all P values less than .001), according to Dr. Aminian. When expressed as ADA goals, 82% of participants had blood pressure less than 140/90 mm Hg 7 years after surgery relative to 44% at baseline (P less than .001). The proportion with LDL less 100 mg/dL approached, but did not reach clinical significance (61% vs. 70%; P=0.06).
“When you consider all three parameters [ADA targets for glycemic control, blood pressure control, and lipid control], only 3% of patients met all three targets at baseline but 32% [P< less than .001] were at these targets at long-term followup,” Dr. Aminian reported.
Dr. Aminian reported having no relevant financial relationships.
As the invited discussant on these data, Raul Rosenthal, MD, Director, Bariatric and Metabolic Institute, Cleveland Clinic Florida, Weston, Florida, reiterated that time with diabetes prior to bariatric surgery may be an important predictor of postsurgical control of metabolic parameters.
“I published a paper about 10 years ago on outcomes in patients with diabetes, and in our experience 5 years was the limit. If you have a history of 5 years or less with diabetes, the chance of going into remission were 80%, and if it was more than 5 years, the likelihood dropped dramatically,” Dr. Rosenthal noted. He indicated duration of diabetes deserves further evaluation for its potential relevance to the optimal timing of bariatric surgery.
AT OBESITY WEEK 2017
Key clinical point: In the largest study to follow insulin-dependent patients after bariatric surgery, substantial benefits persist after median 7 years of followup.
Major finding: Among 252 insulin-dependent patients followed for a minimum of 5 years, 44% remain off insulin and 15% are off all anti-diabetic medications.
Data source: Retrospective single-center analysis.
Disclosures: Dr. Aminian reported having no relevant financial relationships.
Nebulized glycopyrrolate improves lung function in COPD
TORONTO – Glycopyrrolate, a novel nebulized long-acting muscarinic antagonist (LAMA) in development, was well-tolerated and significantly improved lung function and health status in COPD patients regardless of baseline lung function or age, according to a subgroup analysis of pooled results from two randomized trials.*
There are currently no nebulized LAMAs approved for use in the U.S.
Jill Ohar, MD, from Wake Forest University School of Medicine (Winston-Salem, N.C.), presented this secondary analysis of the GOLDEN-3 and GOLDEN-4 trials at the CHEST annual meeting. She and her colleagues evaluated the efficacy and safety of glycopyrrolate in patients with a forced expiratory volume 1(FEV1) % predicted of less than 50 and an FEV1 % predicted of greater than or equal to 50, in age ranges of less than 65 years, greater than or equal to 65 years and at least 75 years, as measured by trough FEV1.
Similarly, both glycopyrrolate doses produced significant (P less than .05) and clinically meaningful lung function improvements vs. placebo in participants less than 65 years of age, at least 65 years, and greater than or equal to 75 years.
Glycopyrrolate use for 12 weeks led to greater improvements over placebo in St. George’s Respiratory Questionnaire (SGRQ) total score, in patients in both lung function classes. There were a higher percentage of SGRQ responders in the treatment arms compared to placebo arms.
The highest SGRQ improvement in SGRQ (−6.287) was seen in the 47 patients that comprised the at-least-75 years of age subgroup receiving glycopyrrolate 25 mcg BID. “It’s a small number of people, but I think it’s [valuable] to see if the very aged act in any way differently than the entire greater than or equal to 65-year-old group,” said Dr. Ohar.
Adverse event rates were similar for placebo and both glycopyrrolate doses, with no safety signals seen according to baseline lung function or age. Few cardiovascular events of special interest were seen.
“Looking at major adverse cardiovascular events, such as fatal MIs, other cardiovascular deaths, arrhythmias, etc., we see nothing that would suggest that the drug overall is associated with an undue number of these versus placebo,” reported Dr. Ohar.
GOLDEN 3 and 4 were replicate, 12-week, phase 3, randomized, double-blind, placebo-controlled studies that evaluated glycopyrrolate solution administered by an investigational eFlow Close System (eFLOW CS) nebulizer in individuals with moderate-to-very severe COPD, including those with continued background use of a long-acting beta2-agonist (LABA), with or without an inhaled corticosteroid (ICS). In each of the trials, about 30% of patients were on LABA ICS, noted Dr. Ohar in her presentation. A total of 653 subjects were randomized in GOLDEN 3 and 641 in GOLDEN 4.
Its manufacturer, Sunovion Pharmaceuticals, resubmitted the product to the FDA in June 2017 in response to a Complete Response Letter received from the FDA in May 2017. The FDA is expected to act on the new submission on December 15, 2017. The novel agent is being considered for the long-term, maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and/or emphysema.
Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
*This article was updated on Nov. 6, 2017.
Eric Gartman, MD, FCCP, comments: If approved, this would represent the first nebulized LAMA available in the U.S. – so in the small population of patients that is unable to utilize standard delivery devices, this would provide an option. It is unclear if this medication must be administered via the proprietary nebulizer that was used in the study – but if so, this would certainly add to the already extremely high cost of respiratory medications and further limit access for many patients.
Eric Gartman, MD, FCCP, comments: If approved, this would represent the first nebulized LAMA available in the U.S. – so in the small population of patients that is unable to utilize standard delivery devices, this would provide an option. It is unclear if this medication must be administered via the proprietary nebulizer that was used in the study – but if so, this would certainly add to the already extremely high cost of respiratory medications and further limit access for many patients.
Eric Gartman, MD, FCCP, comments: If approved, this would represent the first nebulized LAMA available in the U.S. – so in the small population of patients that is unable to utilize standard delivery devices, this would provide an option. It is unclear if this medication must be administered via the proprietary nebulizer that was used in the study – but if so, this would certainly add to the already extremely high cost of respiratory medications and further limit access for many patients.
TORONTO – Glycopyrrolate, a novel nebulized long-acting muscarinic antagonist (LAMA) in development, was well-tolerated and significantly improved lung function and health status in COPD patients regardless of baseline lung function or age, according to a subgroup analysis of pooled results from two randomized trials.*
There are currently no nebulized LAMAs approved for use in the U.S.
Jill Ohar, MD, from Wake Forest University School of Medicine (Winston-Salem, N.C.), presented this secondary analysis of the GOLDEN-3 and GOLDEN-4 trials at the CHEST annual meeting. She and her colleagues evaluated the efficacy and safety of glycopyrrolate in patients with a forced expiratory volume 1(FEV1) % predicted of less than 50 and an FEV1 % predicted of greater than or equal to 50, in age ranges of less than 65 years, greater than or equal to 65 years and at least 75 years, as measured by trough FEV1.
Similarly, both glycopyrrolate doses produced significant (P less than .05) and clinically meaningful lung function improvements vs. placebo in participants less than 65 years of age, at least 65 years, and greater than or equal to 75 years.
Glycopyrrolate use for 12 weeks led to greater improvements over placebo in St. George’s Respiratory Questionnaire (SGRQ) total score, in patients in both lung function classes. There were a higher percentage of SGRQ responders in the treatment arms compared to placebo arms.
The highest SGRQ improvement in SGRQ (−6.287) was seen in the 47 patients that comprised the at-least-75 years of age subgroup receiving glycopyrrolate 25 mcg BID. “It’s a small number of people, but I think it’s [valuable] to see if the very aged act in any way differently than the entire greater than or equal to 65-year-old group,” said Dr. Ohar.
Adverse event rates were similar for placebo and both glycopyrrolate doses, with no safety signals seen according to baseline lung function or age. Few cardiovascular events of special interest were seen.
“Looking at major adverse cardiovascular events, such as fatal MIs, other cardiovascular deaths, arrhythmias, etc., we see nothing that would suggest that the drug overall is associated with an undue number of these versus placebo,” reported Dr. Ohar.
GOLDEN 3 and 4 were replicate, 12-week, phase 3, randomized, double-blind, placebo-controlled studies that evaluated glycopyrrolate solution administered by an investigational eFlow Close System (eFLOW CS) nebulizer in individuals with moderate-to-very severe COPD, including those with continued background use of a long-acting beta2-agonist (LABA), with or without an inhaled corticosteroid (ICS). In each of the trials, about 30% of patients were on LABA ICS, noted Dr. Ohar in her presentation. A total of 653 subjects were randomized in GOLDEN 3 and 641 in GOLDEN 4.
Its manufacturer, Sunovion Pharmaceuticals, resubmitted the product to the FDA in June 2017 in response to a Complete Response Letter received from the FDA in May 2017. The FDA is expected to act on the new submission on December 15, 2017. The novel agent is being considered for the long-term, maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and/or emphysema.
Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
*This article was updated on Nov. 6, 2017.
TORONTO – Glycopyrrolate, a novel nebulized long-acting muscarinic antagonist (LAMA) in development, was well-tolerated and significantly improved lung function and health status in COPD patients regardless of baseline lung function or age, according to a subgroup analysis of pooled results from two randomized trials.*
There are currently no nebulized LAMAs approved for use in the U.S.
Jill Ohar, MD, from Wake Forest University School of Medicine (Winston-Salem, N.C.), presented this secondary analysis of the GOLDEN-3 and GOLDEN-4 trials at the CHEST annual meeting. She and her colleagues evaluated the efficacy and safety of glycopyrrolate in patients with a forced expiratory volume 1(FEV1) % predicted of less than 50 and an FEV1 % predicted of greater than or equal to 50, in age ranges of less than 65 years, greater than or equal to 65 years and at least 75 years, as measured by trough FEV1.
Similarly, both glycopyrrolate doses produced significant (P less than .05) and clinically meaningful lung function improvements vs. placebo in participants less than 65 years of age, at least 65 years, and greater than or equal to 75 years.
Glycopyrrolate use for 12 weeks led to greater improvements over placebo in St. George’s Respiratory Questionnaire (SGRQ) total score, in patients in both lung function classes. There were a higher percentage of SGRQ responders in the treatment arms compared to placebo arms.
The highest SGRQ improvement in SGRQ (−6.287) was seen in the 47 patients that comprised the at-least-75 years of age subgroup receiving glycopyrrolate 25 mcg BID. “It’s a small number of people, but I think it’s [valuable] to see if the very aged act in any way differently than the entire greater than or equal to 65-year-old group,” said Dr. Ohar.
Adverse event rates were similar for placebo and both glycopyrrolate doses, with no safety signals seen according to baseline lung function or age. Few cardiovascular events of special interest were seen.
“Looking at major adverse cardiovascular events, such as fatal MIs, other cardiovascular deaths, arrhythmias, etc., we see nothing that would suggest that the drug overall is associated with an undue number of these versus placebo,” reported Dr. Ohar.
GOLDEN 3 and 4 were replicate, 12-week, phase 3, randomized, double-blind, placebo-controlled studies that evaluated glycopyrrolate solution administered by an investigational eFlow Close System (eFLOW CS) nebulizer in individuals with moderate-to-very severe COPD, including those with continued background use of a long-acting beta2-agonist (LABA), with or without an inhaled corticosteroid (ICS). In each of the trials, about 30% of patients were on LABA ICS, noted Dr. Ohar in her presentation. A total of 653 subjects were randomized in GOLDEN 3 and 641 in GOLDEN 4.
Its manufacturer, Sunovion Pharmaceuticals, resubmitted the product to the FDA in June 2017 in response to a Complete Response Letter received from the FDA in May 2017. The FDA is expected to act on the new submission on December 15, 2017. The novel agent is being considered for the long-term, maintenance treatment of airflow obstruction in people with COPD, including chronic bronchitis and/or emphysema.
Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
*This article was updated on Nov. 6, 2017.
AT CHEST 2017
Key clinical point: Nebulized glycopyrrolate improved lung function and was well tolerated irrespective of baseline lung function or age.
Major finding: Statistically and clinically meaningful improvements in trough FEV1 at 12 weeks were seen in individuals, regardless of their baseline FEV1 % predicted.
Data source: Pooled findings from 2 RCTs, GOLDEN 3 and GOLDEN 4, that together included 1,294 moderate-to-very severe COPD patients.
Disclosures: Dr. Ohar reported that she serves on the advisory boards of several pharmaceutical companies. The other three authors are employees of Sunovion Pharmaceuticals Inc.
Remimazolam surpasses midazolam for bronchoscopy sedation
TORONTO – An investigational sedative, remimazolam, that’s similar to midazolam but with faster onset and offset, resulted in significantly better procedural success compared with midazolam in a multicenter, phase III trial with 431 patients.
The results also showed that remimazolam was as safe as midazolam (Versed), with a very similar adverse event profile, said Gerard A. Silvestri, MD, FCCP, at the CHEST annual meeting.
The bronchoscopy trial enrolled patients at any of 15 U.S. centers with an American Society of Anesthesiologists (ASA) physical status classification of I-III and scheduled for diagnostic or therapeutic bronchoscopy. The enrolled patients averaged 62 years of age, and 38% were in ASA class III.
All patients received initial sedation treatment with fentanyl, followed by a three-to-one randomization to blinded remimazolam, blinded placebo that included midazolam rescue, or open-label midazolam. The study’s primary efficacy endpoint was procedural success, defined as patients who underwent the complete procedure without need for an alternative sedative and without need for more than five doses of the patient’s assigned medication within any 15 minute period during the procedure or need for more than three midazolam doses within any 12-minute period in the patients randomized to receive midazolam.
This primary endpoint occurred in 83% of 303 patients in the remimazolam arm, 5% of 59 patients in the placebo arm, and 34% of 69 patients in the midazolam arm, a statistically significant difference between the remimazolam patients and each of the comparator groups, reported Dr. Silvestri, a professor of medicine and a lung cancer pulmonologist at the Medical University of South Carolina in Charleston.
The results also demonstrated the faster inset and offset of remimazolam. Treatment achieved adequate sedation to start the procedure after a median of 5 minutes with remimazolam, a median of 15.5 minutes with midazolam, and a median of 17 minutes among patients in the placebo group. Once sedation finished, patients returned to being fully alert after a median of 6 minutes with remimazolam, a median of 12 minutes with midazolam, and a median of 13.5 minutes for patients in the placebo arm.
“What’s nice about remimazolam is that the adverse event profile is exactly the same as with placebo and midazolam, and you have a reversal agent,” the same as what’s used for midazolam, he said.
Dr. Silvestri suggested several additional studies he would like to see run on remimazolam to better understand its clinical performance and role. These include studying the drug in the elderly, patients with an ASA classification of IV, obese patients, and those on high narcotic doses. He also suggested comparing remimazolam directly with propofol, testing remimazolam as a stand-alone agent without fentanyl co-administration, and trying the drug during other pulmonary procedures such as pleural-catheter placement and other invasive procedures, and in ICU patients.
The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
TORONTO – An investigational sedative, remimazolam, that’s similar to midazolam but with faster onset and offset, resulted in significantly better procedural success compared with midazolam in a multicenter, phase III trial with 431 patients.
The results also showed that remimazolam was as safe as midazolam (Versed), with a very similar adverse event profile, said Gerard A. Silvestri, MD, FCCP, at the CHEST annual meeting.
The bronchoscopy trial enrolled patients at any of 15 U.S. centers with an American Society of Anesthesiologists (ASA) physical status classification of I-III and scheduled for diagnostic or therapeutic bronchoscopy. The enrolled patients averaged 62 years of age, and 38% were in ASA class III.
All patients received initial sedation treatment with fentanyl, followed by a three-to-one randomization to blinded remimazolam, blinded placebo that included midazolam rescue, or open-label midazolam. The study’s primary efficacy endpoint was procedural success, defined as patients who underwent the complete procedure without need for an alternative sedative and without need for more than five doses of the patient’s assigned medication within any 15 minute period during the procedure or need for more than three midazolam doses within any 12-minute period in the patients randomized to receive midazolam.
This primary endpoint occurred in 83% of 303 patients in the remimazolam arm, 5% of 59 patients in the placebo arm, and 34% of 69 patients in the midazolam arm, a statistically significant difference between the remimazolam patients and each of the comparator groups, reported Dr. Silvestri, a professor of medicine and a lung cancer pulmonologist at the Medical University of South Carolina in Charleston.
The results also demonstrated the faster inset and offset of remimazolam. Treatment achieved adequate sedation to start the procedure after a median of 5 minutes with remimazolam, a median of 15.5 minutes with midazolam, and a median of 17 minutes among patients in the placebo group. Once sedation finished, patients returned to being fully alert after a median of 6 minutes with remimazolam, a median of 12 minutes with midazolam, and a median of 13.5 minutes for patients in the placebo arm.
“What’s nice about remimazolam is that the adverse event profile is exactly the same as with placebo and midazolam, and you have a reversal agent,” the same as what’s used for midazolam, he said.
Dr. Silvestri suggested several additional studies he would like to see run on remimazolam to better understand its clinical performance and role. These include studying the drug in the elderly, patients with an ASA classification of IV, obese patients, and those on high narcotic doses. He also suggested comparing remimazolam directly with propofol, testing remimazolam as a stand-alone agent without fentanyl co-administration, and trying the drug during other pulmonary procedures such as pleural-catheter placement and other invasive procedures, and in ICU patients.
The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
TORONTO – An investigational sedative, remimazolam, that’s similar to midazolam but with faster onset and offset, resulted in significantly better procedural success compared with midazolam in a multicenter, phase III trial with 431 patients.
The results also showed that remimazolam was as safe as midazolam (Versed), with a very similar adverse event profile, said Gerard A. Silvestri, MD, FCCP, at the CHEST annual meeting.
The bronchoscopy trial enrolled patients at any of 15 U.S. centers with an American Society of Anesthesiologists (ASA) physical status classification of I-III and scheduled for diagnostic or therapeutic bronchoscopy. The enrolled patients averaged 62 years of age, and 38% were in ASA class III.
All patients received initial sedation treatment with fentanyl, followed by a three-to-one randomization to blinded remimazolam, blinded placebo that included midazolam rescue, or open-label midazolam. The study’s primary efficacy endpoint was procedural success, defined as patients who underwent the complete procedure without need for an alternative sedative and without need for more than five doses of the patient’s assigned medication within any 15 minute period during the procedure or need for more than three midazolam doses within any 12-minute period in the patients randomized to receive midazolam.
This primary endpoint occurred in 83% of 303 patients in the remimazolam arm, 5% of 59 patients in the placebo arm, and 34% of 69 patients in the midazolam arm, a statistically significant difference between the remimazolam patients and each of the comparator groups, reported Dr. Silvestri, a professor of medicine and a lung cancer pulmonologist at the Medical University of South Carolina in Charleston.
The results also demonstrated the faster inset and offset of remimazolam. Treatment achieved adequate sedation to start the procedure after a median of 5 minutes with remimazolam, a median of 15.5 minutes with midazolam, and a median of 17 minutes among patients in the placebo group. Once sedation finished, patients returned to being fully alert after a median of 6 minutes with remimazolam, a median of 12 minutes with midazolam, and a median of 13.5 minutes for patients in the placebo arm.
“What’s nice about remimazolam is that the adverse event profile is exactly the same as with placebo and midazolam, and you have a reversal agent,” the same as what’s used for midazolam, he said.
Dr. Silvestri suggested several additional studies he would like to see run on remimazolam to better understand its clinical performance and role. These include studying the drug in the elderly, patients with an ASA classification of IV, obese patients, and those on high narcotic doses. He also suggested comparing remimazolam directly with propofol, testing remimazolam as a stand-alone agent without fentanyl co-administration, and trying the drug during other pulmonary procedures such as pleural-catheter placement and other invasive procedures, and in ICU patients.
The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: The rate of successful bronchoscopies was 83% with remimazolam, 34% with midazolam, and 5% with placebo.
Data source: A multicenter, phase III trial with 431 patients.
Disclosures: The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
Hispanics bear brunt of NAFLD disease burden
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.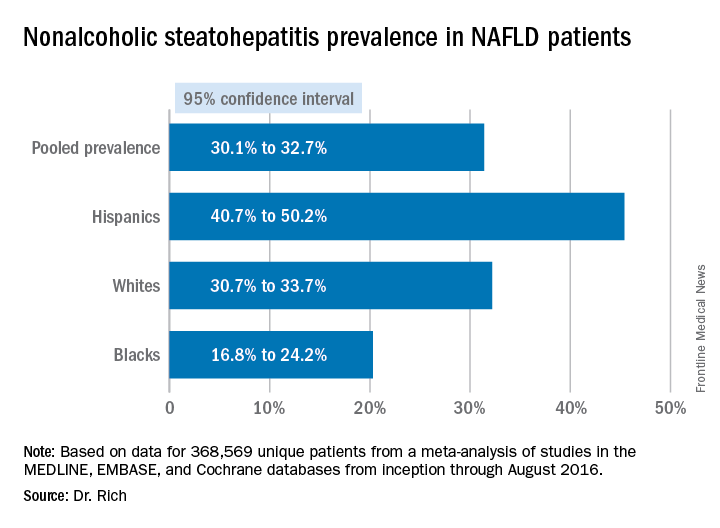
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
AT THE LIVER MEETING 2017
Key clinical point: Hispanics have a much higher incidence of fatty liver disease than other ethnic groups.
Major finding: NAFLD prevalence is highest in Hispanics and lower in blacks, compared with whites, although differences between groups are smaller in high-risk cohorts (range, 47.6%-55.5%) than in population-based cohorts (range, 13.0%-22.9%).
Data source: Meta-analysis of 34 studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that involved 368,569 patients.
Disclosures: Dr. Rich had no financial relationships to disclose.
Failure to diagnose is a continuing challenge
Question: A middle-aged woman developed cellulitis after sustaining multiple mosquito bites in her lower left leg. The area of infection did not appear to reach the knee, which housed a prosthesis implanted there 3 years earlier. Over the next few days, she had significant knee pain, which was attributed to the surrounding cellulitis. Her pulse rate reached 105 beats per min, and her temperature was 101° F, but she was continued on oral antibiotics as an outpatient. Later that evening, she collapsed at home.
Which of the following is best?
A. Fever and tachycardia alone are enough to make the diagnosis of systemic inflammatory response syndrome (SIRS) and should have raised sepsis as a cause.
B. In septic patients, even a short delay in antibiotic administration can significantly affect morbidity and mortality.
C. Failure to diagnose is the most common basis for a medical malpractice claim.
D. The doctor may have anchored his diagnosis on the mosquito-bite incident, and should have considered a septic joint and/or sepsis in the differential.
E. All are correct.
Answer: E. “Failure to diagnose” is a legal term, whereas in medical usage we tend to use terms such “missed diagnosis,” “overlooked condition,” or “diagnostic error.” If such failure is shown to be a breach of the standard of care and is proven to be a proximate cause of the patient’s injury, then a case for medical negligence is made out. Even if the situation is atypical or complex, there still is the duty to refer, if customarily required, to an appropriate specialist, and failure to do so may also constitute negligence.
Diagnostic errors tend to cause the most severe injuries, especially in hospitalized patients. Roughly 5% of autopsies uncover a diagnostic error that was amenable to appropriate treatment, and some 50,000 annual hospital deaths may be the result of a delayed, incorrect, or overlooked diagnosis.
Failure to diagnose occurs in both outpatient and in-hospital settings, recurring examples being myocardial infarction, dissecting aneurysm, pulmonary embolism, appendicitis, ectopic pregnancy, meningitis, cancers, and fractures.
In a records-review study covering a large urban Veterans Affairs facility and an integrated private health care system in a primary care setting, the authors reported that pneumonia, heart failure, acute renal failure, cancer, and urinary tract infections (UTIs) were frequently missed diagnoses.2 They identified 190 diagnostic errors over a 12-month period in 2006-2007, and they attributed them to “process breakdowns” involving the practitioner-patient clinical encounter, referrals, patient factors, follow-up and tracking of diagnostic information, and interpretation of test results. Deficiencies in bedside history taking, physical exam, and test ordering were common; significantly, there was no documentation of an initial differential diagnosis in 80% of misdiagnosed cases.
Malpractice carriers regularly compile data regarding the nature of their covered losses, and their reports on diagnostic errors, although not subject to the usual scientific peer review, have generally corroborated the published literature.
For example, data from 2009 to 2013 collected by MIEC, a large malpractice mutual insurance company on the West Coast, impute almost half of all general medicine claims to diagnostic errors.3 The cases, frequently involving cancer and heart disease, resulted in high-severity injuries and death. Lapses in clinical judgment, communication, and patient-related behavior issues were the primary contributing factors that affected the diagnosis-related claims. Pitfalls included errors in patient assessment, diagnostic processing, provider follow-up, and referral to specialists.
Recent reports have drawn attention to sepsis, an example of SIRS, as an important missed diagnosis, often with deadly consequences. It has been pointed out that if a sepsis case goes to trial, jurors will immediately learn that mortality rates are increased if antibiotics are delayed, even for a short period. In one study, each hour’s delay increased mortality by 7.6%, mortality being 21.1% if antibiotics were given in the first hour, compared with 58% if delayed by more than 6 hours.4
To avoid suits, physicians should be alert to seemingly minor vital sign changes, such as new tachypnea or tachycardia. Notably, patients can have severe sepsis and septic shock without fever or hypothermia. Uncomplicated sepsis is common and can quickly progress to severe sepsis, with organ failure and septic shock. The Surviving Sepsis Campaign has estimated that more than 750,000 individuals develop severe sepsis in North America each year, with mortality around 50%.
The Sullivan Group,which comprises a team of professionals dedicated to perfecting a system solution that reduces medical error and improves patient safety, recently published a wrongful-death narrative from undiagnosed sepsis.5 The decedent gave birth to her first child after 24 hours of labor, sustaining severe vaginal and rectal tearing. Three days later, she began experiencing chills, nausea, worsening vaginal pain, and fever. Her temperature reached 101.9° F (38.8° C). The following day, 4 days after delivery, she was seen by a nurse practitioner in the emergency department with symptoms of nausea, abdominal and back pain, and fever. She was tachycardic at 115 per minute.
The presence of fever plus tachycardia should have raised the diagnosis of SIRS, especially in view of her abdominal pain and a recent complicated delivery. Instead, the practitioner diagnosed a UTI and discharged her on antibiotics. That same afternoon, she collapsed and was admitted for sepsis. Despite an emergency hysterectomy, her condition worsened, she developed multiple organ failure and septic shock, and died the next day. The source of her sepsis was endometritis, and the jury returned a $20 million verdict for the plaintiff.
Observers of medical errors point to our recurring failure to continue to consider alternatives after forming an initial tentative diagnosis, and warn us about the various cognitive biases familiar to behavioral economists but ignored by many doctors.6 These include anchoring bias, in which one is locked into an aspect of the case; framing bias, in which there is misdirection because of the way the problem was posed; availability bias, in which things are judged by what comes readily to mind, such as a recent experience; and confirmation bias, in which one looks for confirmatory evidence of one’s preferred diagnosis while ignoring evidence to the contrary.
In the case outlined earlier, the Sullivan Group noted that the practitioner did not consider sepsis because of cognitive bias, anchoring, and premature closure. The trial documents indicated that the urinalysis did not show bacteria, but the practitioner may have settled – prematurely – on the UTI diagnosis, based on the presence of WBCs in the urine and her obstetrics history. Having anchored on that thought process and prematurely closed her decision making, the practitioner then ignored the elevated white blood cell count with a left shift, and a depressed platelet count of 50,000. Perhaps UTI was a reasonable consideration in the differential, but the working diagnosis of sepsis should have been first and foremost.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials may have been published in earlier columns in Internal Medicine News, and can be accessed at www.mdedge.com/taxonomy/term/83/path_term/21/latest. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. BMJ Qual Saf. 2013 Aug;22(8):672-80.
2. JAMA Intern Med. 2013 Mar 25;173(6):418-25.
3. MIEC, the Exchange, Issue 8, March 2017.
4. Crit Care Med. 2006 Jun;34(6):1589-96.
5. The Sullivan Group. Case: Avoiding cognitive bias in diagnosing sepsis.
6. Acad Med. 2003 Aug;78(8):775-80.
Question: A middle-aged woman developed cellulitis after sustaining multiple mosquito bites in her lower left leg. The area of infection did not appear to reach the knee, which housed a prosthesis implanted there 3 years earlier. Over the next few days, she had significant knee pain, which was attributed to the surrounding cellulitis. Her pulse rate reached 105 beats per min, and her temperature was 101° F, but she was continued on oral antibiotics as an outpatient. Later that evening, she collapsed at home.
Which of the following is best?
A. Fever and tachycardia alone are enough to make the diagnosis of systemic inflammatory response syndrome (SIRS) and should have raised sepsis as a cause.
B. In septic patients, even a short delay in antibiotic administration can significantly affect morbidity and mortality.
C. Failure to diagnose is the most common basis for a medical malpractice claim.
D. The doctor may have anchored his diagnosis on the mosquito-bite incident, and should have considered a septic joint and/or sepsis in the differential.
E. All are correct.
Answer: E. “Failure to diagnose” is a legal term, whereas in medical usage we tend to use terms such “missed diagnosis,” “overlooked condition,” or “diagnostic error.” If such failure is shown to be a breach of the standard of care and is proven to be a proximate cause of the patient’s injury, then a case for medical negligence is made out. Even if the situation is atypical or complex, there still is the duty to refer, if customarily required, to an appropriate specialist, and failure to do so may also constitute negligence.
Diagnostic errors tend to cause the most severe injuries, especially in hospitalized patients. Roughly 5% of autopsies uncover a diagnostic error that was amenable to appropriate treatment, and some 50,000 annual hospital deaths may be the result of a delayed, incorrect, or overlooked diagnosis.
Failure to diagnose occurs in both outpatient and in-hospital settings, recurring examples being myocardial infarction, dissecting aneurysm, pulmonary embolism, appendicitis, ectopic pregnancy, meningitis, cancers, and fractures.
In a records-review study covering a large urban Veterans Affairs facility and an integrated private health care system in a primary care setting, the authors reported that pneumonia, heart failure, acute renal failure, cancer, and urinary tract infections (UTIs) were frequently missed diagnoses.2 They identified 190 diagnostic errors over a 12-month period in 2006-2007, and they attributed them to “process breakdowns” involving the practitioner-patient clinical encounter, referrals, patient factors, follow-up and tracking of diagnostic information, and interpretation of test results. Deficiencies in bedside history taking, physical exam, and test ordering were common; significantly, there was no documentation of an initial differential diagnosis in 80% of misdiagnosed cases.
Malpractice carriers regularly compile data regarding the nature of their covered losses, and their reports on diagnostic errors, although not subject to the usual scientific peer review, have generally corroborated the published literature.
For example, data from 2009 to 2013 collected by MIEC, a large malpractice mutual insurance company on the West Coast, impute almost half of all general medicine claims to diagnostic errors.3 The cases, frequently involving cancer and heart disease, resulted in high-severity injuries and death. Lapses in clinical judgment, communication, and patient-related behavior issues were the primary contributing factors that affected the diagnosis-related claims. Pitfalls included errors in patient assessment, diagnostic processing, provider follow-up, and referral to specialists.
Recent reports have drawn attention to sepsis, an example of SIRS, as an important missed diagnosis, often with deadly consequences. It has been pointed out that if a sepsis case goes to trial, jurors will immediately learn that mortality rates are increased if antibiotics are delayed, even for a short period. In one study, each hour’s delay increased mortality by 7.6%, mortality being 21.1% if antibiotics were given in the first hour, compared with 58% if delayed by more than 6 hours.4
To avoid suits, physicians should be alert to seemingly minor vital sign changes, such as new tachypnea or tachycardia. Notably, patients can have severe sepsis and septic shock without fever or hypothermia. Uncomplicated sepsis is common and can quickly progress to severe sepsis, with organ failure and septic shock. The Surviving Sepsis Campaign has estimated that more than 750,000 individuals develop severe sepsis in North America each year, with mortality around 50%.
The Sullivan Group,which comprises a team of professionals dedicated to perfecting a system solution that reduces medical error and improves patient safety, recently published a wrongful-death narrative from undiagnosed sepsis.5 The decedent gave birth to her first child after 24 hours of labor, sustaining severe vaginal and rectal tearing. Three days later, she began experiencing chills, nausea, worsening vaginal pain, and fever. Her temperature reached 101.9° F (38.8° C). The following day, 4 days after delivery, she was seen by a nurse practitioner in the emergency department with symptoms of nausea, abdominal and back pain, and fever. She was tachycardic at 115 per minute.
The presence of fever plus tachycardia should have raised the diagnosis of SIRS, especially in view of her abdominal pain and a recent complicated delivery. Instead, the practitioner diagnosed a UTI and discharged her on antibiotics. That same afternoon, she collapsed and was admitted for sepsis. Despite an emergency hysterectomy, her condition worsened, she developed multiple organ failure and septic shock, and died the next day. The source of her sepsis was endometritis, and the jury returned a $20 million verdict for the plaintiff.
Observers of medical errors point to our recurring failure to continue to consider alternatives after forming an initial tentative diagnosis, and warn us about the various cognitive biases familiar to behavioral economists but ignored by many doctors.6 These include anchoring bias, in which one is locked into an aspect of the case; framing bias, in which there is misdirection because of the way the problem was posed; availability bias, in which things are judged by what comes readily to mind, such as a recent experience; and confirmation bias, in which one looks for confirmatory evidence of one’s preferred diagnosis while ignoring evidence to the contrary.
In the case outlined earlier, the Sullivan Group noted that the practitioner did not consider sepsis because of cognitive bias, anchoring, and premature closure. The trial documents indicated that the urinalysis did not show bacteria, but the practitioner may have settled – prematurely – on the UTI diagnosis, based on the presence of WBCs in the urine and her obstetrics history. Having anchored on that thought process and prematurely closed her decision making, the practitioner then ignored the elevated white blood cell count with a left shift, and a depressed platelet count of 50,000. Perhaps UTI was a reasonable consideration in the differential, but the working diagnosis of sepsis should have been first and foremost.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials may have been published in earlier columns in Internal Medicine News, and can be accessed at www.mdedge.com/taxonomy/term/83/path_term/21/latest. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. BMJ Qual Saf. 2013 Aug;22(8):672-80.
2. JAMA Intern Med. 2013 Mar 25;173(6):418-25.
3. MIEC, the Exchange, Issue 8, March 2017.
4. Crit Care Med. 2006 Jun;34(6):1589-96.
5. The Sullivan Group. Case: Avoiding cognitive bias in diagnosing sepsis.
6. Acad Med. 2003 Aug;78(8):775-80.
Question: A middle-aged woman developed cellulitis after sustaining multiple mosquito bites in her lower left leg. The area of infection did not appear to reach the knee, which housed a prosthesis implanted there 3 years earlier. Over the next few days, she had significant knee pain, which was attributed to the surrounding cellulitis. Her pulse rate reached 105 beats per min, and her temperature was 101° F, but she was continued on oral antibiotics as an outpatient. Later that evening, she collapsed at home.
Which of the following is best?
A. Fever and tachycardia alone are enough to make the diagnosis of systemic inflammatory response syndrome (SIRS) and should have raised sepsis as a cause.
B. In septic patients, even a short delay in antibiotic administration can significantly affect morbidity and mortality.
C. Failure to diagnose is the most common basis for a medical malpractice claim.
D. The doctor may have anchored his diagnosis on the mosquito-bite incident, and should have considered a septic joint and/or sepsis in the differential.
E. All are correct.
Answer: E. “Failure to diagnose” is a legal term, whereas in medical usage we tend to use terms such “missed diagnosis,” “overlooked condition,” or “diagnostic error.” If such failure is shown to be a breach of the standard of care and is proven to be a proximate cause of the patient’s injury, then a case for medical negligence is made out. Even if the situation is atypical or complex, there still is the duty to refer, if customarily required, to an appropriate specialist, and failure to do so may also constitute negligence.
Diagnostic errors tend to cause the most severe injuries, especially in hospitalized patients. Roughly 5% of autopsies uncover a diagnostic error that was amenable to appropriate treatment, and some 50,000 annual hospital deaths may be the result of a delayed, incorrect, or overlooked diagnosis.
Failure to diagnose occurs in both outpatient and in-hospital settings, recurring examples being myocardial infarction, dissecting aneurysm, pulmonary embolism, appendicitis, ectopic pregnancy, meningitis, cancers, and fractures.
In a records-review study covering a large urban Veterans Affairs facility and an integrated private health care system in a primary care setting, the authors reported that pneumonia, heart failure, acute renal failure, cancer, and urinary tract infections (UTIs) were frequently missed diagnoses.2 They identified 190 diagnostic errors over a 12-month period in 2006-2007, and they attributed them to “process breakdowns” involving the practitioner-patient clinical encounter, referrals, patient factors, follow-up and tracking of diagnostic information, and interpretation of test results. Deficiencies in bedside history taking, physical exam, and test ordering were common; significantly, there was no documentation of an initial differential diagnosis in 80% of misdiagnosed cases.
Malpractice carriers regularly compile data regarding the nature of their covered losses, and their reports on diagnostic errors, although not subject to the usual scientific peer review, have generally corroborated the published literature.
For example, data from 2009 to 2013 collected by MIEC, a large malpractice mutual insurance company on the West Coast, impute almost half of all general medicine claims to diagnostic errors.3 The cases, frequently involving cancer and heart disease, resulted in high-severity injuries and death. Lapses in clinical judgment, communication, and patient-related behavior issues were the primary contributing factors that affected the diagnosis-related claims. Pitfalls included errors in patient assessment, diagnostic processing, provider follow-up, and referral to specialists.
Recent reports have drawn attention to sepsis, an example of SIRS, as an important missed diagnosis, often with deadly consequences. It has been pointed out that if a sepsis case goes to trial, jurors will immediately learn that mortality rates are increased if antibiotics are delayed, even for a short period. In one study, each hour’s delay increased mortality by 7.6%, mortality being 21.1% if antibiotics were given in the first hour, compared with 58% if delayed by more than 6 hours.4
To avoid suits, physicians should be alert to seemingly minor vital sign changes, such as new tachypnea or tachycardia. Notably, patients can have severe sepsis and septic shock without fever or hypothermia. Uncomplicated sepsis is common and can quickly progress to severe sepsis, with organ failure and septic shock. The Surviving Sepsis Campaign has estimated that more than 750,000 individuals develop severe sepsis in North America each year, with mortality around 50%.
The Sullivan Group,which comprises a team of professionals dedicated to perfecting a system solution that reduces medical error and improves patient safety, recently published a wrongful-death narrative from undiagnosed sepsis.5 The decedent gave birth to her first child after 24 hours of labor, sustaining severe vaginal and rectal tearing. Three days later, she began experiencing chills, nausea, worsening vaginal pain, and fever. Her temperature reached 101.9° F (38.8° C). The following day, 4 days after delivery, she was seen by a nurse practitioner in the emergency department with symptoms of nausea, abdominal and back pain, and fever. She was tachycardic at 115 per minute.
The presence of fever plus tachycardia should have raised the diagnosis of SIRS, especially in view of her abdominal pain and a recent complicated delivery. Instead, the practitioner diagnosed a UTI and discharged her on antibiotics. That same afternoon, she collapsed and was admitted for sepsis. Despite an emergency hysterectomy, her condition worsened, she developed multiple organ failure and septic shock, and died the next day. The source of her sepsis was endometritis, and the jury returned a $20 million verdict for the plaintiff.
Observers of medical errors point to our recurring failure to continue to consider alternatives after forming an initial tentative diagnosis, and warn us about the various cognitive biases familiar to behavioral economists but ignored by many doctors.6 These include anchoring bias, in which one is locked into an aspect of the case; framing bias, in which there is misdirection because of the way the problem was posed; availability bias, in which things are judged by what comes readily to mind, such as a recent experience; and confirmation bias, in which one looks for confirmatory evidence of one’s preferred diagnosis while ignoring evidence to the contrary.
In the case outlined earlier, the Sullivan Group noted that the practitioner did not consider sepsis because of cognitive bias, anchoring, and premature closure. The trial documents indicated that the urinalysis did not show bacteria, but the practitioner may have settled – prematurely – on the UTI diagnosis, based on the presence of WBCs in the urine and her obstetrics history. Having anchored on that thought process and prematurely closed her decision making, the practitioner then ignored the elevated white blood cell count with a left shift, and a depressed platelet count of 50,000. Perhaps UTI was a reasonable consideration in the differential, but the working diagnosis of sepsis should have been first and foremost.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials may have been published in earlier columns in Internal Medicine News, and can be accessed at www.mdedge.com/taxonomy/term/83/path_term/21/latest. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. BMJ Qual Saf. 2013 Aug;22(8):672-80.
2. JAMA Intern Med. 2013 Mar 25;173(6):418-25.
3. MIEC, the Exchange, Issue 8, March 2017.
4. Crit Care Med. 2006 Jun;34(6):1589-96.
5. The Sullivan Group. Case: Avoiding cognitive bias in diagnosing sepsis.
6. Acad Med. 2003 Aug;78(8):775-80.
ORBITA: PCI no better than meds for stable angina
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
AT TCT 2017
Key clinical point:
Major finding: PCI on top of intensive antianginal medications was not significantly more effective at improving exercise tolerance than sham PCI.
Data source: ORBITA, a randomized, multicenter, blinded, sham-controlled study of 200 patients with mild angina and single-vessel CAD.
Disclosures: ORBITA was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. The presenter reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
CV outcomes better with SGLT2 inhibitor than DPP4 inhibitor in T2DM study
LISBON – The sodium-glucose co-transporter 2 (SGLT-2) inhibitor dapagliflozin (Farxiga) posed a lower risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors in a large real-world, observational study.
In CVD-REAL NORDIC, treatment with the SGLT-2 inhibitor reduced the incidence of major cardiovascular adverse events by 21% as compared to DDP-4 inhibitors (hazard ratio, 0.79; 95% confidence interval, 0.67-0.94; P = .006).
Anna Norhammar, MD, PhD, of the Institute of Medicine, Cardiology Unit, at Karolinska Institutet in Stockholm, presented the findings at the annual meeting of the European Association for the Study of Diabetes.
She said: “These results are in line with previous clinical trials and meta-analyses, but extend the results to a broader CV risk population and with a commonly used comparator.”
Indeed, the findings build on those from the widely reported EMPA-REG Outcome (New Engl J Med. 2015;373;2117-8) and CANVAS (New Engl J Med. 2017;377:644-7) randomized controlled trials. These trials respectively showed empagliflozin and canagliflozin significantly reduced the risk for MACE and heart failure in patients with T2DM versus placebo. As the majority of patients in these trials had established CV disease, this suggested a class effect for SGLT2 inhibitors, Dr. Norhammar explained.
CVD-REAL NORDIC is part of a larger, multinational, observational, comparative effectiveness study looking at the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM. Altogether around 90,000 patients have been recruited in Sweden, Norway, and Denmark.
Previously, Dr. Norhammar and co-investigators have reported that SGLT2 inhibitors lowered MACE (HR, 0.78; 95% CI 0.69–0.87, P less than .0001) and heart failure hospitalization (HR, 0.70; 95% CI 0.61–0.81, P less than .0001) relative to all glucose-lowering drugs (Lancet Diabetes Endocrinol. 2017;709–17).
“However, the comparator group used in that study, other [glucose-lowering drugs], consisted of almost 50% patients with T2DM treated with insulin or sul[f]onylureas,” they wrote in Diabetes, Obesity and Metabolism (8 Sep., doi: 10.1111/dom.13077). Insulin or sulfonylureas, they add, “have been shown to have increased associated CV risks” compared with DPP4 inhibitors and it is “not fully clear to what extent this could have influenced risk estimates.”
Furthermore, the CVD-NORDIC investigators note that the comparator group reflected real-world practice and it is important to look at treatment strategies more specifically, hence why they decided to do an analysis comparing SGLT2 and DPP4 inhibitors.
The study population for the current analysis consisted of 40,909 patients with T2DM who were newly initiated on either dapagliflozin (n=10,227) or a DPP4 inhibitor (n=30,682) between 2012 and 2015. The mean age was 61 years, and around 23% had prior CV disease and 5% had previous heart failure.
After a mean follow-up of 11.3 months, 177 MACE events had occurred among the 10,227 dapagliflozin-treated patients and 695 among the 30,681 DPP4 inhibitor-treated patients, giving respective event rates of 1.86 and 2.34 per 100 patient years. MACE was defined as a composite of nonfatal myocardial infarction, nonfatal stroke, and CV mortality.
“Dapagliflozin is the most commonly used SGLT2 inhibitor in the Nordic countries,” Dr. Norhammar said, explaining why this particular SGLT2 inhibitor was used in the analysis.
While there was a clear benefit of using dapagliflozin over DPP4 inhibitors in terms of MACE, heart failure hospitalization, and all-cause mortality, there was a “neutral” effect on atrial fibrillation and severe hypoglycaemia, with respective HRs of 0.92 (95% CI 0.76–1.12; P = .41) and 0.94 (95% CI 0.74–1.19; P = .62).
Dr. Norhammar said these “neutral results for atrial fibrillation and severe hypoglycaemia,” were “in line with expectations, and suggest a low likelihood of confounding.”
As this was an observational study, one of the limitations is that could be confounding factors that could not be adequately matched in the analysis. The events were not adjudicated and the study didn’t look at safety.
Dr. Norhammar noted that the results of the DECLARE-TIMI 58 trials were now needed to see if the potential CV benefits of using an SGLT2 inhibitor over other gluc0se-lowering medications might extend into patients at lower CV risk.
AstraZeneca supported the study.
Dr. Norhammar disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
LISBON – The sodium-glucose co-transporter 2 (SGLT-2) inhibitor dapagliflozin (Farxiga) posed a lower risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors in a large real-world, observational study.
In CVD-REAL NORDIC, treatment with the SGLT-2 inhibitor reduced the incidence of major cardiovascular adverse events by 21% as compared to DDP-4 inhibitors (hazard ratio, 0.79; 95% confidence interval, 0.67-0.94; P = .006).
Anna Norhammar, MD, PhD, of the Institute of Medicine, Cardiology Unit, at Karolinska Institutet in Stockholm, presented the findings at the annual meeting of the European Association for the Study of Diabetes.
She said: “These results are in line with previous clinical trials and meta-analyses, but extend the results to a broader CV risk population and with a commonly used comparator.”
Indeed, the findings build on those from the widely reported EMPA-REG Outcome (New Engl J Med. 2015;373;2117-8) and CANVAS (New Engl J Med. 2017;377:644-7) randomized controlled trials. These trials respectively showed empagliflozin and canagliflozin significantly reduced the risk for MACE and heart failure in patients with T2DM versus placebo. As the majority of patients in these trials had established CV disease, this suggested a class effect for SGLT2 inhibitors, Dr. Norhammar explained.
CVD-REAL NORDIC is part of a larger, multinational, observational, comparative effectiveness study looking at the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM. Altogether around 90,000 patients have been recruited in Sweden, Norway, and Denmark.
Previously, Dr. Norhammar and co-investigators have reported that SGLT2 inhibitors lowered MACE (HR, 0.78; 95% CI 0.69–0.87, P less than .0001) and heart failure hospitalization (HR, 0.70; 95% CI 0.61–0.81, P less than .0001) relative to all glucose-lowering drugs (Lancet Diabetes Endocrinol. 2017;709–17).
“However, the comparator group used in that study, other [glucose-lowering drugs], consisted of almost 50% patients with T2DM treated with insulin or sul[f]onylureas,” they wrote in Diabetes, Obesity and Metabolism (8 Sep., doi: 10.1111/dom.13077). Insulin or sulfonylureas, they add, “have been shown to have increased associated CV risks” compared with DPP4 inhibitors and it is “not fully clear to what extent this could have influenced risk estimates.”
Furthermore, the CVD-NORDIC investigators note that the comparator group reflected real-world practice and it is important to look at treatment strategies more specifically, hence why they decided to do an analysis comparing SGLT2 and DPP4 inhibitors.
The study population for the current analysis consisted of 40,909 patients with T2DM who were newly initiated on either dapagliflozin (n=10,227) or a DPP4 inhibitor (n=30,682) between 2012 and 2015. The mean age was 61 years, and around 23% had prior CV disease and 5% had previous heart failure.
After a mean follow-up of 11.3 months, 177 MACE events had occurred among the 10,227 dapagliflozin-treated patients and 695 among the 30,681 DPP4 inhibitor-treated patients, giving respective event rates of 1.86 and 2.34 per 100 patient years. MACE was defined as a composite of nonfatal myocardial infarction, nonfatal stroke, and CV mortality.
“Dapagliflozin is the most commonly used SGLT2 inhibitor in the Nordic countries,” Dr. Norhammar said, explaining why this particular SGLT2 inhibitor was used in the analysis.
While there was a clear benefit of using dapagliflozin over DPP4 inhibitors in terms of MACE, heart failure hospitalization, and all-cause mortality, there was a “neutral” effect on atrial fibrillation and severe hypoglycaemia, with respective HRs of 0.92 (95% CI 0.76–1.12; P = .41) and 0.94 (95% CI 0.74–1.19; P = .62).
Dr. Norhammar said these “neutral results for atrial fibrillation and severe hypoglycaemia,” were “in line with expectations, and suggest a low likelihood of confounding.”
As this was an observational study, one of the limitations is that could be confounding factors that could not be adequately matched in the analysis. The events were not adjudicated and the study didn’t look at safety.
Dr. Norhammar noted that the results of the DECLARE-TIMI 58 trials were now needed to see if the potential CV benefits of using an SGLT2 inhibitor over other gluc0se-lowering medications might extend into patients at lower CV risk.
AstraZeneca supported the study.
Dr. Norhammar disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
LISBON – The sodium-glucose co-transporter 2 (SGLT-2) inhibitor dapagliflozin (Farxiga) posed a lower risk of cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors in a large real-world, observational study.
In CVD-REAL NORDIC, treatment with the SGLT-2 inhibitor reduced the incidence of major cardiovascular adverse events by 21% as compared to DDP-4 inhibitors (hazard ratio, 0.79; 95% confidence interval, 0.67-0.94; P = .006).
Anna Norhammar, MD, PhD, of the Institute of Medicine, Cardiology Unit, at Karolinska Institutet in Stockholm, presented the findings at the annual meeting of the European Association for the Study of Diabetes.
She said: “These results are in line with previous clinical trials and meta-analyses, but extend the results to a broader CV risk population and with a commonly used comparator.”
Indeed, the findings build on those from the widely reported EMPA-REG Outcome (New Engl J Med. 2015;373;2117-8) and CANVAS (New Engl J Med. 2017;377:644-7) randomized controlled trials. These trials respectively showed empagliflozin and canagliflozin significantly reduced the risk for MACE and heart failure in patients with T2DM versus placebo. As the majority of patients in these trials had established CV disease, this suggested a class effect for SGLT2 inhibitors, Dr. Norhammar explained.
CVD-REAL NORDIC is part of a larger, multinational, observational, comparative effectiveness study looking at the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM. Altogether around 90,000 patients have been recruited in Sweden, Norway, and Denmark.
Previously, Dr. Norhammar and co-investigators have reported that SGLT2 inhibitors lowered MACE (HR, 0.78; 95% CI 0.69–0.87, P less than .0001) and heart failure hospitalization (HR, 0.70; 95% CI 0.61–0.81, P less than .0001) relative to all glucose-lowering drugs (Lancet Diabetes Endocrinol. 2017;709–17).
“However, the comparator group used in that study, other [glucose-lowering drugs], consisted of almost 50% patients with T2DM treated with insulin or sul[f]onylureas,” they wrote in Diabetes, Obesity and Metabolism (8 Sep., doi: 10.1111/dom.13077). Insulin or sulfonylureas, they add, “have been shown to have increased associated CV risks” compared with DPP4 inhibitors and it is “not fully clear to what extent this could have influenced risk estimates.”
Furthermore, the CVD-NORDIC investigators note that the comparator group reflected real-world practice and it is important to look at treatment strategies more specifically, hence why they decided to do an analysis comparing SGLT2 and DPP4 inhibitors.
The study population for the current analysis consisted of 40,909 patients with T2DM who were newly initiated on either dapagliflozin (n=10,227) or a DPP4 inhibitor (n=30,682) between 2012 and 2015. The mean age was 61 years, and around 23% had prior CV disease and 5% had previous heart failure.
After a mean follow-up of 11.3 months, 177 MACE events had occurred among the 10,227 dapagliflozin-treated patients and 695 among the 30,681 DPP4 inhibitor-treated patients, giving respective event rates of 1.86 and 2.34 per 100 patient years. MACE was defined as a composite of nonfatal myocardial infarction, nonfatal stroke, and CV mortality.
“Dapagliflozin is the most commonly used SGLT2 inhibitor in the Nordic countries,” Dr. Norhammar said, explaining why this particular SGLT2 inhibitor was used in the analysis.
While there was a clear benefit of using dapagliflozin over DPP4 inhibitors in terms of MACE, heart failure hospitalization, and all-cause mortality, there was a “neutral” effect on atrial fibrillation and severe hypoglycaemia, with respective HRs of 0.92 (95% CI 0.76–1.12; P = .41) and 0.94 (95% CI 0.74–1.19; P = .62).
Dr. Norhammar said these “neutral results for atrial fibrillation and severe hypoglycaemia,” were “in line with expectations, and suggest a low likelihood of confounding.”
As this was an observational study, one of the limitations is that could be confounding factors that could not be adequately matched in the analysis. The events were not adjudicated and the study didn’t look at safety.
Dr. Norhammar noted that the results of the DECLARE-TIMI 58 trials were now needed to see if the potential CV benefits of using an SGLT2 inhibitor over other gluc0se-lowering medications might extend into patients at lower CV risk.
AstraZeneca supported the study.
Dr. Norhammar disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.
AT EASD 2017
Key clinical point:
Major finding: Hazard ratios for MACE, heart failure hospitalization, and all-cause mortality were a respective 0.79, 0.62, and 0.59, comparing SGLT2 with DPP4 inhibitors.
Data source: CVD-NORDIC, part of a multinational, observational study comparing the real-world effect of SGLT2 inhibitors versus other glucose-lowering drugs on CV outcomes in patients with T2DM.
Disclosures: AstraZeneca supported the study. The presenting author disclosed acting as a consultant to and receiving honoraria from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and MSD Sweden.