User login
Ciprofloxacin cured gyrA wild-type Neisseria gonorrhoeae infections
SAN DIEGO – Ciprofloxacin cured 100% of gyrase A wild-type Neisseria gonorrhoeae infections, and physicians prescribed it significantly more frequently when they received electronic reminders of test results and recommendations, in a single-center study.
“Recent reports of untreatable gonorrhea have caused great concern. Treatment with ceftriaxone may be a major driver of resistance, and reducing its use may curb the emergence of resistant infections,” Lao-Tzu Allan-Blitz, a medical student at the David Geffen School of Medicine at the University of California, Los Angeles, said at an annual scientific meeting on infectious diseases.
The Centers for Disease Control and Prevention ranks multidrug-resistant N. gonorrhoeae third among all drug-resistant threats in the United States, Mr. Allan-Blitz noted during an oral presentation at the meeting. Beginning in the late 1990s, strains of N. gonorrhoeae developed resistance to sulfanilamides, penicillin, tetracycline, and fluoroquinolones, leaving only the extended-spectrum cephalosporins for empiric treatment. Recent reports of cephalosporin-resistant N. gonorrhoeae in other countries have raised the specter of untreatable gonorrhea.
Because antimicrobial resistance can shift in response to selective pressure, experts are exploring the use of antibiotics once considered ineffective for treating N. gonorrhoeae infections. At UCLA, researchers developed a real-time reverse transcription polymerase chain reaction test for a mutation of codon 91 in the gyrase A (gyrA) gene in N. gonorrhoeae that reliably predicts resistance to ciprofloxacin.
Test results take 24-48 hours. The test is not Food and Drug Administration approved but has been validated in accordance with Clinical Laboratory Improvement Amendments, Mr. Allan-Blitz said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In November 2015, UCLA Health began gyrA genotyping all N. gonorrhoeae positive specimens, and in May 2016, it began sending providers electronic reminders of genotype results and treatment recommendations. For gyrA wild-type infections, UCLA Health recommends 500 mg oral ciprofloxacin, Mr. Allan-Blitz said.
Initial test-of-cure data are promising. All 25 patients with wild-type infections who received ciprofloxacin and returned 7-90 days later tested negative for N. gonorrhoeae. Culture sites included the urethra (seven cases), pharynx (seven cases), rectum (seven cases), and genitals (four cases), Mr. Allan-Blitz said. “Prior studies have demonstrated that reminder notifications improve uptake of antimicrobial stewardship,” he said. “Other health centers should consider implementing the gyrA assay, and using reminder notifications may improve uptake by providers.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SAN DIEGO – Ciprofloxacin cured 100% of gyrase A wild-type Neisseria gonorrhoeae infections, and physicians prescribed it significantly more frequently when they received electronic reminders of test results and recommendations, in a single-center study.
“Recent reports of untreatable gonorrhea have caused great concern. Treatment with ceftriaxone may be a major driver of resistance, and reducing its use may curb the emergence of resistant infections,” Lao-Tzu Allan-Blitz, a medical student at the David Geffen School of Medicine at the University of California, Los Angeles, said at an annual scientific meeting on infectious diseases.
The Centers for Disease Control and Prevention ranks multidrug-resistant N. gonorrhoeae third among all drug-resistant threats in the United States, Mr. Allan-Blitz noted during an oral presentation at the meeting. Beginning in the late 1990s, strains of N. gonorrhoeae developed resistance to sulfanilamides, penicillin, tetracycline, and fluoroquinolones, leaving only the extended-spectrum cephalosporins for empiric treatment. Recent reports of cephalosporin-resistant N. gonorrhoeae in other countries have raised the specter of untreatable gonorrhea.
Because antimicrobial resistance can shift in response to selective pressure, experts are exploring the use of antibiotics once considered ineffective for treating N. gonorrhoeae infections. At UCLA, researchers developed a real-time reverse transcription polymerase chain reaction test for a mutation of codon 91 in the gyrase A (gyrA) gene in N. gonorrhoeae that reliably predicts resistance to ciprofloxacin.
Test results take 24-48 hours. The test is not Food and Drug Administration approved but has been validated in accordance with Clinical Laboratory Improvement Amendments, Mr. Allan-Blitz said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In November 2015, UCLA Health began gyrA genotyping all N. gonorrhoeae positive specimens, and in May 2016, it began sending providers electronic reminders of genotype results and treatment recommendations. For gyrA wild-type infections, UCLA Health recommends 500 mg oral ciprofloxacin, Mr. Allan-Blitz said.
Initial test-of-cure data are promising. All 25 patients with wild-type infections who received ciprofloxacin and returned 7-90 days later tested negative for N. gonorrhoeae. Culture sites included the urethra (seven cases), pharynx (seven cases), rectum (seven cases), and genitals (four cases), Mr. Allan-Blitz said. “Prior studies have demonstrated that reminder notifications improve uptake of antimicrobial stewardship,” he said. “Other health centers should consider implementing the gyrA assay, and using reminder notifications may improve uptake by providers.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
SAN DIEGO – Ciprofloxacin cured 100% of gyrase A wild-type Neisseria gonorrhoeae infections, and physicians prescribed it significantly more frequently when they received electronic reminders of test results and recommendations, in a single-center study.
“Recent reports of untreatable gonorrhea have caused great concern. Treatment with ceftriaxone may be a major driver of resistance, and reducing its use may curb the emergence of resistant infections,” Lao-Tzu Allan-Blitz, a medical student at the David Geffen School of Medicine at the University of California, Los Angeles, said at an annual scientific meeting on infectious diseases.
The Centers for Disease Control and Prevention ranks multidrug-resistant N. gonorrhoeae third among all drug-resistant threats in the United States, Mr. Allan-Blitz noted during an oral presentation at the meeting. Beginning in the late 1990s, strains of N. gonorrhoeae developed resistance to sulfanilamides, penicillin, tetracycline, and fluoroquinolones, leaving only the extended-spectrum cephalosporins for empiric treatment. Recent reports of cephalosporin-resistant N. gonorrhoeae in other countries have raised the specter of untreatable gonorrhea.
Because antimicrobial resistance can shift in response to selective pressure, experts are exploring the use of antibiotics once considered ineffective for treating N. gonorrhoeae infections. At UCLA, researchers developed a real-time reverse transcription polymerase chain reaction test for a mutation of codon 91 in the gyrase A (gyrA) gene in N. gonorrhoeae that reliably predicts resistance to ciprofloxacin.
Test results take 24-48 hours. The test is not Food and Drug Administration approved but has been validated in accordance with Clinical Laboratory Improvement Amendments, Mr. Allan-Blitz said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In November 2015, UCLA Health began gyrA genotyping all N. gonorrhoeae positive specimens, and in May 2016, it began sending providers electronic reminders of genotype results and treatment recommendations. For gyrA wild-type infections, UCLA Health recommends 500 mg oral ciprofloxacin, Mr. Allan-Blitz said.
Initial test-of-cure data are promising. All 25 patients with wild-type infections who received ciprofloxacin and returned 7-90 days later tested negative for N. gonorrhoeae. Culture sites included the urethra (seven cases), pharynx (seven cases), rectum (seven cases), and genitals (four cases), Mr. Allan-Blitz said. “Prior studies have demonstrated that reminder notifications improve uptake of antimicrobial stewardship,” he said. “Other health centers should consider implementing the gyrA assay, and using reminder notifications may improve uptake by providers.”
The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
AT IDWEEK 2017
Key clinical point:
Major finding: The cure rate was 100% among 25 patients who received ciprofloxacin for wild-type gyrA gonorrhea.
Data source: A single-center study of 582 patients with gonorrhea.
Disclosures: The National Institutes of Health provided funding. The investigators reported having no conflicts of interest.
Rectal swabs concurred with stool tests in children with GI illness
SAN DIEGO – Clinicians who treat children with acute gastrointestinal illness should consider testing rectal swabs when they need to rapidly identify enteropathogens and cannot immediately obtain a bulk stool sample, Stephen Freedman, MD, said at an annual scientific meeting on infectious diseases.
Among 1,519 children and adolescents with diarrhea, vomiting, or both symptoms, diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively, Dr. Freedman reported on behalf of the Alberta Provincial Pediatric Enteric Infection Team.
Kappa values for concordance were 0.76 overall (95% confidence interval [CI], 0.71-0.80), .82 for viruses (0.79-0.86), and .74 for bacteria (0.68-0.80). A kappa value between 0.61 and 0.80 indicates “substantial” concordance between two results, while a value between 0.81 and 1.0 suggests “near perfect” concordance, explained Dr. Freedman of the University of Calgary (Alta.). In addition, 95% of health care providers and 82% of home caregivers considered rectal swabs easy to use, while 10% considered them unacceptable. “Recommendations against rectal swab use should be reconsidered,” he said.
Traditional testing of diarrheal bulk stool is highly specific, but burdensome and subject to various handling issues that can substantially delay diagnosis and outbreak detection, Dr. Freedman said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“Flocked rectal swabs are used at the point of care and are quick and acceptable, but there are few precedents in the literature for their use in children or in patients with vomiting without diarrhea,” he said.
To help fill that gap, the researchers collected 1,147 stool specimens and 1,468 rectal swabs from patients under age 18 years who were seen at emergency departments in Calgary and Edmonton for diarrhea, vomiting, or both, with at least three episodes in the previous 24 hours. All of the patients had been ill for less than 7 days and had no detected psychiatric illness or neutropenia. Stool and rectal samples were evaluated three ways – by routine enteric bacterial culture, an in-house gastroenteric viral panel, and with the polymerase chain reaction-based Luminex xTAG Gastrointestinal Pathogen Panel. Swabs were taken by rotating them 360 degrees one time within the anus. Stool and swab specimens collected at home were stored at room temperature for less than 12 hours.
Among all paired specimens, 76% of stool samples and 68% of rectal swabs tested positive for at least one pathogen (P less than .0001). Thus, stool testing had about a 30% higher odds of detection than did swab testing in the same patient (OR, 1.3; 95% CI, 1.3-1.5). Odds ratios also favored stool testing in subgroups of patients with diarrhea (OR, 1.2; 95% CI, 1.1-1.4) or isolated vomiting (OR, 1.8; 95% CI, 1.5-2.1).
However, many stool specimens were never submitted, Dr. Freedman said. When the researchers assumed that these unsubmitted samples all tested negative, the diagnostic yield of stool samples fell to 57% and several odds ratios inverted in favor of rectal swabs. The study findings did not change when the researchers excluded positive results for Clostridium difficile in children younger than 2 years or when they restricted the analysis to paired specimens obtained within 24 hours.
The researchers are continuing to explore the diagnostic yield of rectal swab tests for multidrug-resistant pathogens, including those that are notifiable to public health departments, Dr. Freedman said.
Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
SAN DIEGO – Clinicians who treat children with acute gastrointestinal illness should consider testing rectal swabs when they need to rapidly identify enteropathogens and cannot immediately obtain a bulk stool sample, Stephen Freedman, MD, said at an annual scientific meeting on infectious diseases.
Among 1,519 children and adolescents with diarrhea, vomiting, or both symptoms, diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively, Dr. Freedman reported on behalf of the Alberta Provincial Pediatric Enteric Infection Team.
Kappa values for concordance were 0.76 overall (95% confidence interval [CI], 0.71-0.80), .82 for viruses (0.79-0.86), and .74 for bacteria (0.68-0.80). A kappa value between 0.61 and 0.80 indicates “substantial” concordance between two results, while a value between 0.81 and 1.0 suggests “near perfect” concordance, explained Dr. Freedman of the University of Calgary (Alta.). In addition, 95% of health care providers and 82% of home caregivers considered rectal swabs easy to use, while 10% considered them unacceptable. “Recommendations against rectal swab use should be reconsidered,” he said.
Traditional testing of diarrheal bulk stool is highly specific, but burdensome and subject to various handling issues that can substantially delay diagnosis and outbreak detection, Dr. Freedman said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“Flocked rectal swabs are used at the point of care and are quick and acceptable, but there are few precedents in the literature for their use in children or in patients with vomiting without diarrhea,” he said.
To help fill that gap, the researchers collected 1,147 stool specimens and 1,468 rectal swabs from patients under age 18 years who were seen at emergency departments in Calgary and Edmonton for diarrhea, vomiting, or both, with at least three episodes in the previous 24 hours. All of the patients had been ill for less than 7 days and had no detected psychiatric illness or neutropenia. Stool and rectal samples were evaluated three ways – by routine enteric bacterial culture, an in-house gastroenteric viral panel, and with the polymerase chain reaction-based Luminex xTAG Gastrointestinal Pathogen Panel. Swabs were taken by rotating them 360 degrees one time within the anus. Stool and swab specimens collected at home were stored at room temperature for less than 12 hours.
Among all paired specimens, 76% of stool samples and 68% of rectal swabs tested positive for at least one pathogen (P less than .0001). Thus, stool testing had about a 30% higher odds of detection than did swab testing in the same patient (OR, 1.3; 95% CI, 1.3-1.5). Odds ratios also favored stool testing in subgroups of patients with diarrhea (OR, 1.2; 95% CI, 1.1-1.4) or isolated vomiting (OR, 1.8; 95% CI, 1.5-2.1).
However, many stool specimens were never submitted, Dr. Freedman said. When the researchers assumed that these unsubmitted samples all tested negative, the diagnostic yield of stool samples fell to 57% and several odds ratios inverted in favor of rectal swabs. The study findings did not change when the researchers excluded positive results for Clostridium difficile in children younger than 2 years or when they restricted the analysis to paired specimens obtained within 24 hours.
The researchers are continuing to explore the diagnostic yield of rectal swab tests for multidrug-resistant pathogens, including those that are notifiable to public health departments, Dr. Freedman said.
Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
SAN DIEGO – Clinicians who treat children with acute gastrointestinal illness should consider testing rectal swabs when they need to rapidly identify enteropathogens and cannot immediately obtain a bulk stool sample, Stephen Freedman, MD, said at an annual scientific meeting on infectious diseases.
Among 1,519 children and adolescents with diarrhea, vomiting, or both symptoms, diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively, Dr. Freedman reported on behalf of the Alberta Provincial Pediatric Enteric Infection Team.
Kappa values for concordance were 0.76 overall (95% confidence interval [CI], 0.71-0.80), .82 for viruses (0.79-0.86), and .74 for bacteria (0.68-0.80). A kappa value between 0.61 and 0.80 indicates “substantial” concordance between two results, while a value between 0.81 and 1.0 suggests “near perfect” concordance, explained Dr. Freedman of the University of Calgary (Alta.). In addition, 95% of health care providers and 82% of home caregivers considered rectal swabs easy to use, while 10% considered them unacceptable. “Recommendations against rectal swab use should be reconsidered,” he said.
Traditional testing of diarrheal bulk stool is highly specific, but burdensome and subject to various handling issues that can substantially delay diagnosis and outbreak detection, Dr. Freedman said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
“Flocked rectal swabs are used at the point of care and are quick and acceptable, but there are few precedents in the literature for their use in children or in patients with vomiting without diarrhea,” he said.
To help fill that gap, the researchers collected 1,147 stool specimens and 1,468 rectal swabs from patients under age 18 years who were seen at emergency departments in Calgary and Edmonton for diarrhea, vomiting, or both, with at least three episodes in the previous 24 hours. All of the patients had been ill for less than 7 days and had no detected psychiatric illness or neutropenia. Stool and rectal samples were evaluated three ways – by routine enteric bacterial culture, an in-house gastroenteric viral panel, and with the polymerase chain reaction-based Luminex xTAG Gastrointestinal Pathogen Panel. Swabs were taken by rotating them 360 degrees one time within the anus. Stool and swab specimens collected at home were stored at room temperature for less than 12 hours.
Among all paired specimens, 76% of stool samples and 68% of rectal swabs tested positive for at least one pathogen (P less than .0001). Thus, stool testing had about a 30% higher odds of detection than did swab testing in the same patient (OR, 1.3; 95% CI, 1.3-1.5). Odds ratios also favored stool testing in subgroups of patients with diarrhea (OR, 1.2; 95% CI, 1.1-1.4) or isolated vomiting (OR, 1.8; 95% CI, 1.5-2.1).
However, many stool specimens were never submitted, Dr. Freedman said. When the researchers assumed that these unsubmitted samples all tested negative, the diagnostic yield of stool samples fell to 57% and several odds ratios inverted in favor of rectal swabs. The study findings did not change when the researchers excluded positive results for Clostridium difficile in children younger than 2 years or when they restricted the analysis to paired specimens obtained within 24 hours.
The researchers are continuing to explore the diagnostic yield of rectal swab tests for multidrug-resistant pathogens, including those that are notifiable to public health departments, Dr. Freedman said.
Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
AT IDWEEK 2017
Key clinical point: and cannot immediately obtain a bulk stool sample.
Major finding: Diagnostic yields of paired stool and rectal swab specimens were 76% and 68%, respectively.
Data source: A multicenter retrospective cohort study of 1,519 children and adolescents with acute-onset diarrhea, vomiting, or both.
Disclosures: Dr. Freedman disclosed ties to Copan Diagnostics, Luminex, and Alere.
Steroids underused in bacterial meningitis despite low risk
SAN DIEGO – Physicians often skipped out on using steroids when treating bacterial meningitis even though the benefits clearly outweigh the risks, Cinthia Gallegos, MD, reported during an oral presentation at an annual meeting on infectious diseases.
In a recent multicenter retrospective cohort study, only 40% of adults with bacterial meningitis received steroids within 4 hours of hospital admission, as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and only 14% received steroids concomitantly or 10-20 minutes prior to antibiotic initiation, as recommended by the Infectious Diseases Society of America (IDSA), said Dr. Gallegos, an infectious disease fellow at University of Texas, Houston.
“Steroids are being underutilized in our patient population,” she said. “And when steroids are used, they are being used later than is recommended.”
To evaluate the prevalence of guideline-concordant steroid use, Dr. Gallegos and her associates analyzed the medical records of 120 adults with culture-confirmed, community-acquired bacterial meningitis treated at 10 Houston-area hospitals between 2008 and 2016.
Median duration of steroid therapy was 4 hours, which is consistent with IDSA guidelines, she noted.
Among the five patients (4%) who developed delayed cerebral thrombosis, three had Streptococcus pneumoniae meningitis, one had methicillin-resistant Staphylococcus aureus meningitis, and one had Listeria meningitis. All had received either dexamethasone monotherapy or dexamethasone and methylprednisolone within 4 hours of antibiotic initiation. They showed an initial improvement in clinical course, including normal CT and MRI, but their clinical condition deteriorated between 5 and 12 days later. “Repeat imaging showed thrombosis of different areas of the brain,” Dr. Gallegos said. Two patients died, two developed moderate or severe disability, and one fully recovered. The patients ranged in age from 26 to 69; three were male, and two were female.
The 4% rate closely resembles what is seen in the Netherlands, said Diederik van de Beek, MD, PhD, of the Academic Medical Center in Amsterdam, who comoderated the session at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “We have some recent data where we did autopsies of cases and we saw a huge amount of bacterial fragments around the blood vessels,” he said. “We have seen this in previous autopsy studies, but here it was a massive amount of bacterial fragments.”
Researchers have suggested that delayed cerebral thrombosis in bacterial meningitis results from increases in C5a and C5b-9 levels in the cerebrospinal fluid and from an increase in the tissue factor VII pathway, Dr. Gallegos said.
Researchers think that these patients historically developed vasculitis, but that this complication “has disappeared somewhat in the dexamethasone era,” said Dr. van de Beek, lead author of the 2016 ESCMID guidelines on bacterial meningitis. “It appears that some patients are ‘pro-inflammatory’ and still react 7-9 days after treatment,” he said. “The difficult question is whether we give 4 days of steroids or longer. A clinical trial is not feasible, so we [recommend] 4 days.”
Left untreated, bacterial meningitis is fatal in up to 70% of cases, and about one in five survivors faces limb loss or neurologic disability, according to the Centers for Disease Control and Prevention. The advent of penicillin and other antibiotics dramatically improved survival, but death rates remained around 10% for meningitis associated with Neisseria meningitides and Haemophilus influenza infection, and often exceeded 30% for S. pneumoniae meningitis. “That’s important because besides antibiotics, the only treatment that decreases mortality has been shown to be steroids,” Dr. Gallegos said.
High-quality evidence supports their use. In a double-blind, randomized, multicenter trial of 301 adults with bacterial meningitis, adjunctive dexamethasone was associated with a 50% improvement in mortality, compared with adjunctive placebo (N Engl J Med. 2002 Nov 14;347[20]:1549-56). Other data confirm that steroids do not prevent vancomycin from concentrating in CSF or increase the risk of hippocampal apoptosis. But although both IDSA and ESCMID endorse steroids as adjunctive therapy to help control intracranial pressure in patients with bacterial meningitis, studies have shown much higher rates of steroid use in the Netherlands, Sweden, and Denmark than in the United States.
The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
SAN DIEGO – Physicians often skipped out on using steroids when treating bacterial meningitis even though the benefits clearly outweigh the risks, Cinthia Gallegos, MD, reported during an oral presentation at an annual meeting on infectious diseases.
In a recent multicenter retrospective cohort study, only 40% of adults with bacterial meningitis received steroids within 4 hours of hospital admission, as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and only 14% received steroids concomitantly or 10-20 minutes prior to antibiotic initiation, as recommended by the Infectious Diseases Society of America (IDSA), said Dr. Gallegos, an infectious disease fellow at University of Texas, Houston.
“Steroids are being underutilized in our patient population,” she said. “And when steroids are used, they are being used later than is recommended.”
To evaluate the prevalence of guideline-concordant steroid use, Dr. Gallegos and her associates analyzed the medical records of 120 adults with culture-confirmed, community-acquired bacterial meningitis treated at 10 Houston-area hospitals between 2008 and 2016.
Median duration of steroid therapy was 4 hours, which is consistent with IDSA guidelines, she noted.
Among the five patients (4%) who developed delayed cerebral thrombosis, three had Streptococcus pneumoniae meningitis, one had methicillin-resistant Staphylococcus aureus meningitis, and one had Listeria meningitis. All had received either dexamethasone monotherapy or dexamethasone and methylprednisolone within 4 hours of antibiotic initiation. They showed an initial improvement in clinical course, including normal CT and MRI, but their clinical condition deteriorated between 5 and 12 days later. “Repeat imaging showed thrombosis of different areas of the brain,” Dr. Gallegos said. Two patients died, two developed moderate or severe disability, and one fully recovered. The patients ranged in age from 26 to 69; three were male, and two were female.
The 4% rate closely resembles what is seen in the Netherlands, said Diederik van de Beek, MD, PhD, of the Academic Medical Center in Amsterdam, who comoderated the session at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “We have some recent data where we did autopsies of cases and we saw a huge amount of bacterial fragments around the blood vessels,” he said. “We have seen this in previous autopsy studies, but here it was a massive amount of bacterial fragments.”
Researchers have suggested that delayed cerebral thrombosis in bacterial meningitis results from increases in C5a and C5b-9 levels in the cerebrospinal fluid and from an increase in the tissue factor VII pathway, Dr. Gallegos said.
Researchers think that these patients historically developed vasculitis, but that this complication “has disappeared somewhat in the dexamethasone era,” said Dr. van de Beek, lead author of the 2016 ESCMID guidelines on bacterial meningitis. “It appears that some patients are ‘pro-inflammatory’ and still react 7-9 days after treatment,” he said. “The difficult question is whether we give 4 days of steroids or longer. A clinical trial is not feasible, so we [recommend] 4 days.”
Left untreated, bacterial meningitis is fatal in up to 70% of cases, and about one in five survivors faces limb loss or neurologic disability, according to the Centers for Disease Control and Prevention. The advent of penicillin and other antibiotics dramatically improved survival, but death rates remained around 10% for meningitis associated with Neisseria meningitides and Haemophilus influenza infection, and often exceeded 30% for S. pneumoniae meningitis. “That’s important because besides antibiotics, the only treatment that decreases mortality has been shown to be steroids,” Dr. Gallegos said.
High-quality evidence supports their use. In a double-blind, randomized, multicenter trial of 301 adults with bacterial meningitis, adjunctive dexamethasone was associated with a 50% improvement in mortality, compared with adjunctive placebo (N Engl J Med. 2002 Nov 14;347[20]:1549-56). Other data confirm that steroids do not prevent vancomycin from concentrating in CSF or increase the risk of hippocampal apoptosis. But although both IDSA and ESCMID endorse steroids as adjunctive therapy to help control intracranial pressure in patients with bacterial meningitis, studies have shown much higher rates of steroid use in the Netherlands, Sweden, and Denmark than in the United States.
The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
SAN DIEGO – Physicians often skipped out on using steroids when treating bacterial meningitis even though the benefits clearly outweigh the risks, Cinthia Gallegos, MD, reported during an oral presentation at an annual meeting on infectious diseases.
In a recent multicenter retrospective cohort study, only 40% of adults with bacterial meningitis received steroids within 4 hours of hospital admission, as recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and only 14% received steroids concomitantly or 10-20 minutes prior to antibiotic initiation, as recommended by the Infectious Diseases Society of America (IDSA), said Dr. Gallegos, an infectious disease fellow at University of Texas, Houston.
“Steroids are being underutilized in our patient population,” she said. “And when steroids are used, they are being used later than is recommended.”
To evaluate the prevalence of guideline-concordant steroid use, Dr. Gallegos and her associates analyzed the medical records of 120 adults with culture-confirmed, community-acquired bacterial meningitis treated at 10 Houston-area hospitals between 2008 and 2016.
Median duration of steroid therapy was 4 hours, which is consistent with IDSA guidelines, she noted.
Among the five patients (4%) who developed delayed cerebral thrombosis, three had Streptococcus pneumoniae meningitis, one had methicillin-resistant Staphylococcus aureus meningitis, and one had Listeria meningitis. All had received either dexamethasone monotherapy or dexamethasone and methylprednisolone within 4 hours of antibiotic initiation. They showed an initial improvement in clinical course, including normal CT and MRI, but their clinical condition deteriorated between 5 and 12 days later. “Repeat imaging showed thrombosis of different areas of the brain,” Dr. Gallegos said. Two patients died, two developed moderate or severe disability, and one fully recovered. The patients ranged in age from 26 to 69; three were male, and two were female.
The 4% rate closely resembles what is seen in the Netherlands, said Diederik van de Beek, MD, PhD, of the Academic Medical Center in Amsterdam, who comoderated the session at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “We have some recent data where we did autopsies of cases and we saw a huge amount of bacterial fragments around the blood vessels,” he said. “We have seen this in previous autopsy studies, but here it was a massive amount of bacterial fragments.”
Researchers have suggested that delayed cerebral thrombosis in bacterial meningitis results from increases in C5a and C5b-9 levels in the cerebrospinal fluid and from an increase in the tissue factor VII pathway, Dr. Gallegos said.
Researchers think that these patients historically developed vasculitis, but that this complication “has disappeared somewhat in the dexamethasone era,” said Dr. van de Beek, lead author of the 2016 ESCMID guidelines on bacterial meningitis. “It appears that some patients are ‘pro-inflammatory’ and still react 7-9 days after treatment,” he said. “The difficult question is whether we give 4 days of steroids or longer. A clinical trial is not feasible, so we [recommend] 4 days.”
Left untreated, bacterial meningitis is fatal in up to 70% of cases, and about one in five survivors faces limb loss or neurologic disability, according to the Centers for Disease Control and Prevention. The advent of penicillin and other antibiotics dramatically improved survival, but death rates remained around 10% for meningitis associated with Neisseria meningitides and Haemophilus influenza infection, and often exceeded 30% for S. pneumoniae meningitis. “That’s important because besides antibiotics, the only treatment that decreases mortality has been shown to be steroids,” Dr. Gallegos said.
High-quality evidence supports their use. In a double-blind, randomized, multicenter trial of 301 adults with bacterial meningitis, adjunctive dexamethasone was associated with a 50% improvement in mortality, compared with adjunctive placebo (N Engl J Med. 2002 Nov 14;347[20]:1549-56). Other data confirm that steroids do not prevent vancomycin from concentrating in CSF or increase the risk of hippocampal apoptosis. But although both IDSA and ESCMID endorse steroids as adjunctive therapy to help control intracranial pressure in patients with bacterial meningitis, studies have shown much higher rates of steroid use in the Netherlands, Sweden, and Denmark than in the United States.
The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
AT IDWEEK 2017
Key clinical point:
Major finding: Five of 120 (4%) of patients developed delayed cerebral thrombosis. Only 40% received steroids within the maximum recommended time frame.
Data source: A retrospective multicenter study of 120 adults with culture-confirmed bacterial meningitis.
Disclosures: The Grant A. Starr Foundation provided funding. The investigators had no conflicts of interest.
C. auris: ‘A yeast that acts like a bacteria’
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at candidaauris@cdc.gov.
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at candidaauris@cdc.gov.
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
SAN DIEGO – The rise of Candida auris as a superbug represents a paradigm shift, because, in the words of Dr. Tom M. Chiller, it’s a yeast that acts like a bacteria.
“Treatment resistance is now the norm,” Dr. Chiller, chief of the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta, said an annual scientific meeting on infectious diseases. “It thrives on skin, it contaminates patient rooms, and it spreads readily in health care settings.”
Since it was first described in Japan in 2009, C. auris has been identified in multiple countries in four continents, including the United States, prompting the CDC to issue a clinical alert to health care facilities in June of 2016. To date, more than 130 cases have been reported in 10 states, mostly in New York and New Jersey. At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, Dr. Chiller said that C. auris is a challenging superbug for four main reasons:
It’s not easily identified
Matrix assisted laser desorption ionization–time of flight (MALDI-TOF) or DNA sequencing are required to make the diagnosis. “It turns out that only about 25% of clinical labs have MALDI-TOF available, so we’re still lacking in our ability to identify it,” he said.
It’s easily transmitted
C. auris “is really happy in a hospital room,” Dr. Chiller said. “You can grow it from the floor, on the bottom of shoes, and on hand alcohol dispensers. It also likes the skin, and it also likes to grow in slightly higher temperatures. You find it readily in the axilla and groin. Those are the main locations we’re using for developing screening culture techniques.”
It’s difficult to treat
Treatment, if clinically indicated, includes an echinocandin such as micafungin, anidulafungin, and caspofungin at standard dosing. However, there have been cases of development of resistance to echinocandins while on therapy. “That bothers me,” Dr. Chiller said. “We don’t like to see that happen, and I am concerned. These bugs are really happy to be resistant, but based on the epidemiology, we remain convinced that it’s important to treat with an echinocandin.”
It can cause severe invasive disease and death
Global epidemiologic evaluation of the first 50 or so cases found that some patients were on antifungal treatment when C. auris was isolated. The mortality was greater than 60%, and there was a clustering in some hospitals. “Some hospitals reported that up to 40% of candidemia cases were from C. auris,” he said.
Among cases in the United States to date, the median age of affected patients is 70 years and patients’ 30-day mortality is about 30%. “They were quite ill, with multiple underlying conditions and indwelling devices,” Dr. Chiller said. They had “extensive health care exposure” with stays in acute care hospitals and nursing homes with ventilator units, and several recent cases with travel and health care exposures abroad, mainly to India, Pakistan, Venezuela, and South Africa.
Clinicians should report suspected cases to their local health department or to the CDC at candidaauris@cdc.gov.
“We also want them to implement and reinforce infection control measures,” Dr. Chiller advised. “Get the lab to review other potential Candida cases or Candida species you might have. Conduct contact tracing to identify other colonized patients, and consider point-prevalence surveys.”
He reported having no financial disclosures.
REPORTING FROM ID WEEK 2017
Pediatric psoriasis carries sharply increased risk of selected autoimmune comorbidities
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.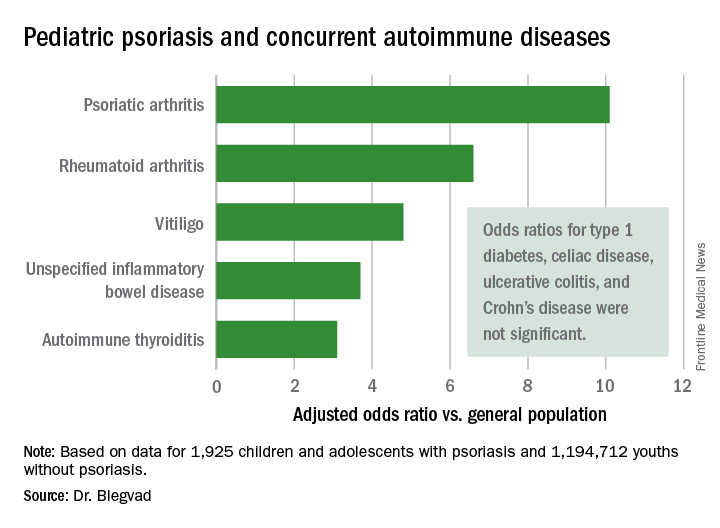
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Pediatric psoriasis patients were at an adjusted 6.6-fold increased risk of comorbid rheumatoid arthritis, 4.8-fold risk of vitiligo, and significantly increased risks of several other autoimmune diseases, compared with matched youths without psoriasis.
Data source: A cross-sectional study of all children and adolescents living in Denmark at the end of 2012.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
Metals may surprise as sources of contact dermatitis
, according to Jennifer H. Perryman, MD, of the Greeley Skin Clinic in Fort Collins, Colo.
For example, metal from orthopedic implants can cause contact dermatitis, Dr. Perryman said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The cutaneous complications of metal implants generally are eczematous, but they can be urticarial and vasculitic as well, with symptoms either generalized or localized. Dr. Perryman explained. Noncutaneous complications from contact dermatitis associated with the metal include chronic joint pain, and a loosening and dysfunction of the device.
It is a case of “chicken or the egg: Metal allergy causes device failure, or device failure causes metal allergy,” Dr. Perryman said.
Dental implants also can be unforeseen causes of contact dermatitis, she noted. The bone cement used in some implants may contain a variety of potential irritants such as methyl methacrylate, N,N-dimethyl-p-toluidine (DPT), benzoyl peroxide, gentamicin, and hydroquinone.
Metal allergy in the mouth most often presents as a reaction resembling oral lichen planus, with lesions that are reticular, atrophic, erosive, or plaque-like. These lesions usually erupt next to the implant, she said. Some patients also experience burning mouth syndrome from amalgam tattoos. However, some patients who test positive for metal allergies in general have developed a tolerance for dental implants as a result of having worn braces in the past.
Metal eyelid weights implanted to treat lagophthalmos are another rare, but potential allergen to consider, said Dr. Perryman. These weights often are made of gold, and Dr. Perryman cited a study in which four patients with gold eyelid weights experienced inflammatory reactions. Patch testing revealed gold sodium thiosulfate as the cause of their allergic contact dermatitis (Dermatitis. 2008 May-Jun;19[3]:148-53). Other options for these patients include platinum weights, hyaluronic acid, ointment, and taping, she said.
Dr. Perryman had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
, according to Jennifer H. Perryman, MD, of the Greeley Skin Clinic in Fort Collins, Colo.
For example, metal from orthopedic implants can cause contact dermatitis, Dr. Perryman said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The cutaneous complications of metal implants generally are eczematous, but they can be urticarial and vasculitic as well, with symptoms either generalized or localized. Dr. Perryman explained. Noncutaneous complications from contact dermatitis associated with the metal include chronic joint pain, and a loosening and dysfunction of the device.
It is a case of “chicken or the egg: Metal allergy causes device failure, or device failure causes metal allergy,” Dr. Perryman said.
Dental implants also can be unforeseen causes of contact dermatitis, she noted. The bone cement used in some implants may contain a variety of potential irritants such as methyl methacrylate, N,N-dimethyl-p-toluidine (DPT), benzoyl peroxide, gentamicin, and hydroquinone.
Metal allergy in the mouth most often presents as a reaction resembling oral lichen planus, with lesions that are reticular, atrophic, erosive, or plaque-like. These lesions usually erupt next to the implant, she said. Some patients also experience burning mouth syndrome from amalgam tattoos. However, some patients who test positive for metal allergies in general have developed a tolerance for dental implants as a result of having worn braces in the past.
Metal eyelid weights implanted to treat lagophthalmos are another rare, but potential allergen to consider, said Dr. Perryman. These weights often are made of gold, and Dr. Perryman cited a study in which four patients with gold eyelid weights experienced inflammatory reactions. Patch testing revealed gold sodium thiosulfate as the cause of their allergic contact dermatitis (Dermatitis. 2008 May-Jun;19[3]:148-53). Other options for these patients include platinum weights, hyaluronic acid, ointment, and taping, she said.
Dr. Perryman had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
, according to Jennifer H. Perryman, MD, of the Greeley Skin Clinic in Fort Collins, Colo.
For example, metal from orthopedic implants can cause contact dermatitis, Dr. Perryman said at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
The cutaneous complications of metal implants generally are eczematous, but they can be urticarial and vasculitic as well, with symptoms either generalized or localized. Dr. Perryman explained. Noncutaneous complications from contact dermatitis associated with the metal include chronic joint pain, and a loosening and dysfunction of the device.
It is a case of “chicken or the egg: Metal allergy causes device failure, or device failure causes metal allergy,” Dr. Perryman said.
Dental implants also can be unforeseen causes of contact dermatitis, she noted. The bone cement used in some implants may contain a variety of potential irritants such as methyl methacrylate, N,N-dimethyl-p-toluidine (DPT), benzoyl peroxide, gentamicin, and hydroquinone.
Metal allergy in the mouth most often presents as a reaction resembling oral lichen planus, with lesions that are reticular, atrophic, erosive, or plaque-like. These lesions usually erupt next to the implant, she said. Some patients also experience burning mouth syndrome from amalgam tattoos. However, some patients who test positive for metal allergies in general have developed a tolerance for dental implants as a result of having worn braces in the past.
Metal eyelid weights implanted to treat lagophthalmos are another rare, but potential allergen to consider, said Dr. Perryman. These weights often are made of gold, and Dr. Perryman cited a study in which four patients with gold eyelid weights experienced inflammatory reactions. Patch testing revealed gold sodium thiosulfate as the cause of their allergic contact dermatitis (Dermatitis. 2008 May-Jun;19[3]:148-53). Other options for these patients include platinum weights, hyaluronic acid, ointment, and taping, she said.
Dr. Perryman had no financial conflicts to disclose. SDEF and this news organization are owned by Frontline Medical Communications.
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Delayed appropriate therapy affects outcomes in patients at risk for CRE infections
SAN DIEGO – Among patients with serious infections due to Enterobacteriaceae, delayed appropriate therapy has a stronger association with outcomes, relative to presence of carbapenem-resistant Enterobacteriaceae, according to an analysis of national hospital data.
“We need to reconsider how we approach patients with serious Gram-negative infections,” lead study author Thomas Lodise, PharmD, PhD, said at an annual scientific meeting on infectious diseases. “We kind of take this wait-and-see approach in infectious diseases; we wait a couple of days, then we get aggressive. You would never do this in oncology. I don’t know how many more studies we need to show that early therapy matters. We talk about antibiotic stewardship. One of the fundamental pillars of stewardship is getting it right the first time, and we fail to do this in the majority of patients with serious Gram-negative infections.”
At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, he noted that delayed appropriate therapy is associated with increased rates of clinical failure and mortality, longer lengths of stay, longer durations of antibiotic treatment, and greater in-hospital costs. “Similarly, patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have poorer outcomes, such as increased risk of mortality or of being discharged to a long-term care facility, compared with patients with infections caused by carbapenem-susceptible Enterobacteriaceae isolates,” said Dr. Lodise of the Albany (N.Y.) College of Pharmacy and Health Sciences. “Although CRE and delayed appropriate therapy have both been associated with worse outcomes, the impact of each of these factors on clinical and economic outcomes is not well understood.”
In an effort to assess the independent and combined impact of CRE and delayed appropriate therapy on clinical and economic outcomes among hospitalized U.S. patients with serious infections due to Enterobacteriaceae, Dr. Lodise and his associates drew from the Premier Hospital Database, which includes information for about 500 acute-care hospitals in the United States, including the 150 hospitals that provided admission records and microbiological data assessed in the current analysis.
The researchers evaluated adults hospitalized between July 2011 and September 2014. The index date was defined as the earliest culture positive for at least one Gram-negative bacteria of interest, and patients were stratified based on whether the pathogen was CRE or non-CRE. Appropriate therapy was defined as receipt of an antibiotic regimen with microbiological activity against all pathogens identified within the index culture on the index date or within the subsequent 2-day period. All subsequent receipt of such therapy was defined as delayed appropriate therapy.
In all, 50,069 patients with a mean age of 66 years were included in the study. Of these, 514 (1%) harbored infections caused by CRE, and 49,555 (99%) had infections caused by a pathogen other than CRE. Multivariate adjusted analysis revealed significant differences between the CRE group and the non-CRE group in duration of antibiotic therapy (a mean of 8.5 days vs. 7.5 days, respectively); length of stay (a mean of 8.4 days vs. 7.6 days), and in-hospital cost (a mean of $19,816 vs. a mean of $15,165; P less than .01 for all associations). In addition, CRE patients were less likely to be discharged home (odds ratio [OR], .3) and more likely to die in the hospital or be discharged to hospice (OR, 2.2).
When outcomes of patients infections due to Enterobacteriaceae species were stratified by timing of appropriate therapy (timely vs. delayed) and CRE status (CRE vs. non-CRE), without exception the burden of serious infections was least among patients with infections due to non-CRE who received timely appropriate therapy, and greatest among patients with infections due to CRE in whom appropriate therapy was delayed. A gradient effect was observed across strata, and weighted towards timing of receipt of initial therapy. For example, the mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days). Similarly, mean in-hospital costs post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of $9,875) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of $25,506).
“This study demonstrates the importance of early identification of patients at risk for delayed appropriate therapy, through the use of clinical criteria for risk stratification or rapid diagnostic tools,” Dr. Lodise concluded. “The findings also highlight the need to shift current treatment practices away from antibiotic escalation strategies that contribute to delayed appropriate therapy and toward early aggressive, appropriate therapy in patients at risk for CRE infection.”
Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He has also been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
SAN DIEGO – Among patients with serious infections due to Enterobacteriaceae, delayed appropriate therapy has a stronger association with outcomes, relative to presence of carbapenem-resistant Enterobacteriaceae, according to an analysis of national hospital data.
“We need to reconsider how we approach patients with serious Gram-negative infections,” lead study author Thomas Lodise, PharmD, PhD, said at an annual scientific meeting on infectious diseases. “We kind of take this wait-and-see approach in infectious diseases; we wait a couple of days, then we get aggressive. You would never do this in oncology. I don’t know how many more studies we need to show that early therapy matters. We talk about antibiotic stewardship. One of the fundamental pillars of stewardship is getting it right the first time, and we fail to do this in the majority of patients with serious Gram-negative infections.”
At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, he noted that delayed appropriate therapy is associated with increased rates of clinical failure and mortality, longer lengths of stay, longer durations of antibiotic treatment, and greater in-hospital costs. “Similarly, patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have poorer outcomes, such as increased risk of mortality or of being discharged to a long-term care facility, compared with patients with infections caused by carbapenem-susceptible Enterobacteriaceae isolates,” said Dr. Lodise of the Albany (N.Y.) College of Pharmacy and Health Sciences. “Although CRE and delayed appropriate therapy have both been associated with worse outcomes, the impact of each of these factors on clinical and economic outcomes is not well understood.”
In an effort to assess the independent and combined impact of CRE and delayed appropriate therapy on clinical and economic outcomes among hospitalized U.S. patients with serious infections due to Enterobacteriaceae, Dr. Lodise and his associates drew from the Premier Hospital Database, which includes information for about 500 acute-care hospitals in the United States, including the 150 hospitals that provided admission records and microbiological data assessed in the current analysis.
The researchers evaluated adults hospitalized between July 2011 and September 2014. The index date was defined as the earliest culture positive for at least one Gram-negative bacteria of interest, and patients were stratified based on whether the pathogen was CRE or non-CRE. Appropriate therapy was defined as receipt of an antibiotic regimen with microbiological activity against all pathogens identified within the index culture on the index date or within the subsequent 2-day period. All subsequent receipt of such therapy was defined as delayed appropriate therapy.
In all, 50,069 patients with a mean age of 66 years were included in the study. Of these, 514 (1%) harbored infections caused by CRE, and 49,555 (99%) had infections caused by a pathogen other than CRE. Multivariate adjusted analysis revealed significant differences between the CRE group and the non-CRE group in duration of antibiotic therapy (a mean of 8.5 days vs. 7.5 days, respectively); length of stay (a mean of 8.4 days vs. 7.6 days), and in-hospital cost (a mean of $19,816 vs. a mean of $15,165; P less than .01 for all associations). In addition, CRE patients were less likely to be discharged home (odds ratio [OR], .3) and more likely to die in the hospital or be discharged to hospice (OR, 2.2).
When outcomes of patients infections due to Enterobacteriaceae species were stratified by timing of appropriate therapy (timely vs. delayed) and CRE status (CRE vs. non-CRE), without exception the burden of serious infections was least among patients with infections due to non-CRE who received timely appropriate therapy, and greatest among patients with infections due to CRE in whom appropriate therapy was delayed. A gradient effect was observed across strata, and weighted towards timing of receipt of initial therapy. For example, the mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days). Similarly, mean in-hospital costs post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of $9,875) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of $25,506).
“This study demonstrates the importance of early identification of patients at risk for delayed appropriate therapy, through the use of clinical criteria for risk stratification or rapid diagnostic tools,” Dr. Lodise concluded. “The findings also highlight the need to shift current treatment practices away from antibiotic escalation strategies that contribute to delayed appropriate therapy and toward early aggressive, appropriate therapy in patients at risk for CRE infection.”
Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He has also been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
SAN DIEGO – Among patients with serious infections due to Enterobacteriaceae, delayed appropriate therapy has a stronger association with outcomes, relative to presence of carbapenem-resistant Enterobacteriaceae, according to an analysis of national hospital data.
“We need to reconsider how we approach patients with serious Gram-negative infections,” lead study author Thomas Lodise, PharmD, PhD, said at an annual scientific meeting on infectious diseases. “We kind of take this wait-and-see approach in infectious diseases; we wait a couple of days, then we get aggressive. You would never do this in oncology. I don’t know how many more studies we need to show that early therapy matters. We talk about antibiotic stewardship. One of the fundamental pillars of stewardship is getting it right the first time, and we fail to do this in the majority of patients with serious Gram-negative infections.”
At the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, he noted that delayed appropriate therapy is associated with increased rates of clinical failure and mortality, longer lengths of stay, longer durations of antibiotic treatment, and greater in-hospital costs. “Similarly, patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have poorer outcomes, such as increased risk of mortality or of being discharged to a long-term care facility, compared with patients with infections caused by carbapenem-susceptible Enterobacteriaceae isolates,” said Dr. Lodise of the Albany (N.Y.) College of Pharmacy and Health Sciences. “Although CRE and delayed appropriate therapy have both been associated with worse outcomes, the impact of each of these factors on clinical and economic outcomes is not well understood.”
In an effort to assess the independent and combined impact of CRE and delayed appropriate therapy on clinical and economic outcomes among hospitalized U.S. patients with serious infections due to Enterobacteriaceae, Dr. Lodise and his associates drew from the Premier Hospital Database, which includes information for about 500 acute-care hospitals in the United States, including the 150 hospitals that provided admission records and microbiological data assessed in the current analysis.
The researchers evaluated adults hospitalized between July 2011 and September 2014. The index date was defined as the earliest culture positive for at least one Gram-negative bacteria of interest, and patients were stratified based on whether the pathogen was CRE or non-CRE. Appropriate therapy was defined as receipt of an antibiotic regimen with microbiological activity against all pathogens identified within the index culture on the index date or within the subsequent 2-day period. All subsequent receipt of such therapy was defined as delayed appropriate therapy.
In all, 50,069 patients with a mean age of 66 years were included in the study. Of these, 514 (1%) harbored infections caused by CRE, and 49,555 (99%) had infections caused by a pathogen other than CRE. Multivariate adjusted analysis revealed significant differences between the CRE group and the non-CRE group in duration of antibiotic therapy (a mean of 8.5 days vs. 7.5 days, respectively); length of stay (a mean of 8.4 days vs. 7.6 days), and in-hospital cost (a mean of $19,816 vs. a mean of $15,165; P less than .01 for all associations). In addition, CRE patients were less likely to be discharged home (odds ratio [OR], .3) and more likely to die in the hospital or be discharged to hospice (OR, 2.2).
When outcomes of patients infections due to Enterobacteriaceae species were stratified by timing of appropriate therapy (timely vs. delayed) and CRE status (CRE vs. non-CRE), without exception the burden of serious infections was least among patients with infections due to non-CRE who received timely appropriate therapy, and greatest among patients with infections due to CRE in whom appropriate therapy was delayed. A gradient effect was observed across strata, and weighted towards timing of receipt of initial therapy. For example, the mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days). Similarly, mean in-hospital costs post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of $9,875) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of $25,506).
“This study demonstrates the importance of early identification of patients at risk for delayed appropriate therapy, through the use of clinical criteria for risk stratification or rapid diagnostic tools,” Dr. Lodise concluded. “The findings also highlight the need to shift current treatment practices away from antibiotic escalation strategies that contribute to delayed appropriate therapy and toward early aggressive, appropriate therapy in patients at risk for CRE infection.”
Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He has also been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
REPORTING FROM ID WEEK 2017
Key clinical point:
Major finding: The mean LOS post index culture date rank was lowest among non-CRE patients who received timely appropriate therapy (a mean of 5 days) and greatest among patients infected with CRE who received delayed appropriate therapy (a mean of 8.8 days).
Study details: An analysis of 50,069 adults hospitalized with serious infections due to Enterobacteriaceae between July 2011 and September 2014.
Disclosures: Allergan funded the study. Dr. Lodise disclosed that he has received consulting fees or honoraria from Allergan. He also has been a consultant for Merck, Achaogen, Zavante, and The Medicines Company.
Oral bioidentical combo improves quality of life, vasomotor symptoms
PHILADELPHIA – An oral estradiol/progesterone formulation significantly improved menopause-related quality of life, compared with placebo, for up to 1 year after beginning treatment, according to a new study.
If approved, the new formulation “may be an option for the estimated millions of women currently using less-regulated and unapproved compounded bioidentical hormone therapy,” said James Simon, MD, the study’s senior author.
Patients receiving the combination therapy, termed TX-001HR, experienced a significant improvement in quality of life, compared with placebo and compared with baseline, at all study points, said Dr. Simon of George Washington University, Washington.
Using the Menopause-Specific Quality of Life questionnaire (MENQOL), Dr. Simon and his coinvestigators found that women taking the combination therapy saw reductions in the vasomotor domain of MENQOL within 12 weeks of beginning the study. The significant symptomatic improvement persisted for the full year that patients were followed.
For patients with particularly bothersome vasomotor symptoms, vasomotor domain scores ranged from 6.9 to 7.2 at baseline and were 2.8-3.6 with TX-001HR and 4.4 with placebo at month 12, according to Dr. Simon.
Speaking during a top abstracts session at the annual meeting of the North American Menopause Society, Dr. Simon said that TX-001HR combines the physiologic sex hormones 17-beta estradiol and progesterone (E2/P4) into a single oral soft-gel.
The phase 3 randomized, double blind, placebo-controlled REPLENISH trial explored the safety and efficacy of one of four dose combinations of E2/P4. A total of 1,833 patients were randomized to receive E2/P4 in doses of 1.0/100 mg, 0.5/100 mg, 0.5/50 mg, or 0.25/50 mg, or to receive placebo. An approximately equal number of patients were allocated to each study arm, except that 151 patients were allocated to receive placebo.
The MENQOL is structured so that the 29 items in the symptom inventory are grouped into four domains: vasomotor, psychosocial, physical, and sexual. Significant reductions were seen at 12 weeks for all patients in overall MENQOL scores and for the four domains.
The REPLENISH investigators also performed a separate analysis of data from the subset of patients who had moderate to severe vasomotor symptoms (VMS). At the 6- and 12-month assessment points, the VMS patients on all but the lowest dose of TX-001HR had significant improvement over placebo.
“Independent of treatment, the largest correlation observed was between changes in moderate to severe VMS frequency and changes in the MENQOL vasomotor symptom domain score at 12 weeks,” said Dr. Simon. The quality of life and moderate to severe VMS frequency were highly correlated, he added (rho = 0.561, P less than .0001). Improvements in the other MENQOL domains were also highly correlated with reduction in moderate to severe frequency (P less than .0001 for all).
Among patients who reported significant improvement on the MENQOL, said Dr. Simon, more of the TX-001HR patients had improvements that were judged to be clinically significant compared to those taking placebo. Women who experienced a minimal clinically important difference in their symptoms had a weekly improvement of 34 fewer VMS events. Those who had a stronger response, which was judged to be clinically important, had a weekly improvement of 44 fewer VMS events.
Dr. Simon reported financial relationships with several pharmaceutical companies, including TherapeuticsMD, the sponsor of the THX-001HR clinical trials.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – An oral estradiol/progesterone formulation significantly improved menopause-related quality of life, compared with placebo, for up to 1 year after beginning treatment, according to a new study.
If approved, the new formulation “may be an option for the estimated millions of women currently using less-regulated and unapproved compounded bioidentical hormone therapy,” said James Simon, MD, the study’s senior author.
Patients receiving the combination therapy, termed TX-001HR, experienced a significant improvement in quality of life, compared with placebo and compared with baseline, at all study points, said Dr. Simon of George Washington University, Washington.
Using the Menopause-Specific Quality of Life questionnaire (MENQOL), Dr. Simon and his coinvestigators found that women taking the combination therapy saw reductions in the vasomotor domain of MENQOL within 12 weeks of beginning the study. The significant symptomatic improvement persisted for the full year that patients were followed.
For patients with particularly bothersome vasomotor symptoms, vasomotor domain scores ranged from 6.9 to 7.2 at baseline and were 2.8-3.6 with TX-001HR and 4.4 with placebo at month 12, according to Dr. Simon.
Speaking during a top abstracts session at the annual meeting of the North American Menopause Society, Dr. Simon said that TX-001HR combines the physiologic sex hormones 17-beta estradiol and progesterone (E2/P4) into a single oral soft-gel.
The phase 3 randomized, double blind, placebo-controlled REPLENISH trial explored the safety and efficacy of one of four dose combinations of E2/P4. A total of 1,833 patients were randomized to receive E2/P4 in doses of 1.0/100 mg, 0.5/100 mg, 0.5/50 mg, or 0.25/50 mg, or to receive placebo. An approximately equal number of patients were allocated to each study arm, except that 151 patients were allocated to receive placebo.
The MENQOL is structured so that the 29 items in the symptom inventory are grouped into four domains: vasomotor, psychosocial, physical, and sexual. Significant reductions were seen at 12 weeks for all patients in overall MENQOL scores and for the four domains.
The REPLENISH investigators also performed a separate analysis of data from the subset of patients who had moderate to severe vasomotor symptoms (VMS). At the 6- and 12-month assessment points, the VMS patients on all but the lowest dose of TX-001HR had significant improvement over placebo.
“Independent of treatment, the largest correlation observed was between changes in moderate to severe VMS frequency and changes in the MENQOL vasomotor symptom domain score at 12 weeks,” said Dr. Simon. The quality of life and moderate to severe VMS frequency were highly correlated, he added (rho = 0.561, P less than .0001). Improvements in the other MENQOL domains were also highly correlated with reduction in moderate to severe frequency (P less than .0001 for all).
Among patients who reported significant improvement on the MENQOL, said Dr. Simon, more of the TX-001HR patients had improvements that were judged to be clinically significant compared to those taking placebo. Women who experienced a minimal clinically important difference in their symptoms had a weekly improvement of 34 fewer VMS events. Those who had a stronger response, which was judged to be clinically important, had a weekly improvement of 44 fewer VMS events.
Dr. Simon reported financial relationships with several pharmaceutical companies, including TherapeuticsMD, the sponsor of the THX-001HR clinical trials.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – An oral estradiol/progesterone formulation significantly improved menopause-related quality of life, compared with placebo, for up to 1 year after beginning treatment, according to a new study.
If approved, the new formulation “may be an option for the estimated millions of women currently using less-regulated and unapproved compounded bioidentical hormone therapy,” said James Simon, MD, the study’s senior author.
Patients receiving the combination therapy, termed TX-001HR, experienced a significant improvement in quality of life, compared with placebo and compared with baseline, at all study points, said Dr. Simon of George Washington University, Washington.
Using the Menopause-Specific Quality of Life questionnaire (MENQOL), Dr. Simon and his coinvestigators found that women taking the combination therapy saw reductions in the vasomotor domain of MENQOL within 12 weeks of beginning the study. The significant symptomatic improvement persisted for the full year that patients were followed.
For patients with particularly bothersome vasomotor symptoms, vasomotor domain scores ranged from 6.9 to 7.2 at baseline and were 2.8-3.6 with TX-001HR and 4.4 with placebo at month 12, according to Dr. Simon.
Speaking during a top abstracts session at the annual meeting of the North American Menopause Society, Dr. Simon said that TX-001HR combines the physiologic sex hormones 17-beta estradiol and progesterone (E2/P4) into a single oral soft-gel.
The phase 3 randomized, double blind, placebo-controlled REPLENISH trial explored the safety and efficacy of one of four dose combinations of E2/P4. A total of 1,833 patients were randomized to receive E2/P4 in doses of 1.0/100 mg, 0.5/100 mg, 0.5/50 mg, or 0.25/50 mg, or to receive placebo. An approximately equal number of patients were allocated to each study arm, except that 151 patients were allocated to receive placebo.
The MENQOL is structured so that the 29 items in the symptom inventory are grouped into four domains: vasomotor, psychosocial, physical, and sexual. Significant reductions were seen at 12 weeks for all patients in overall MENQOL scores and for the four domains.
The REPLENISH investigators also performed a separate analysis of data from the subset of patients who had moderate to severe vasomotor symptoms (VMS). At the 6- and 12-month assessment points, the VMS patients on all but the lowest dose of TX-001HR had significant improvement over placebo.
“Independent of treatment, the largest correlation observed was between changes in moderate to severe VMS frequency and changes in the MENQOL vasomotor symptom domain score at 12 weeks,” said Dr. Simon. The quality of life and moderate to severe VMS frequency were highly correlated, he added (rho = 0.561, P less than .0001). Improvements in the other MENQOL domains were also highly correlated with reduction in moderate to severe frequency (P less than .0001 for all).
Among patients who reported significant improvement on the MENQOL, said Dr. Simon, more of the TX-001HR patients had improvements that were judged to be clinically significant compared to those taking placebo. Women who experienced a minimal clinically important difference in their symptoms had a weekly improvement of 34 fewer VMS events. Those who had a stronger response, which was judged to be clinically important, had a weekly improvement of 44 fewer VMS events.
Dr. Simon reported financial relationships with several pharmaceutical companies, including TherapeuticsMD, the sponsor of the THX-001HR clinical trials.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT NAMS 2017
Key clinical point:
Major finding: Patients taking TX-100HR had significant improvements in a menopause-related quality of life scale at all study time points.
Data source: REPLENISH, a phase 3, randomized, double blind, placebo-controlled study of 1,833 postmenopausal women.
Disclosures: Dr. Simon reported financial relationships with multiple pharmaceutical companies, including TherapeuticsMD, sponsor of the REPLENISH trial.
Time to take the fear out of the hormone therapy conversation
PHILADELPHIA – It’s time to be clear about the benefits of hormone therapy for many women in midlife, JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said during the keynote address at the group’s annual meeting.
“I want to take fear out of the conversation. Hormone therapy remains the most effective treatment for vasomotor symptoms and the genitourinary syndrome of menopause and has been shown to prevent bone loss and fracture,” said Dr. Pinkerton, who also chaired the advisory panel that penned the 2017 NAMS position statement on hormone therapy.
Hormone therapy is currently approved by the Food and Drug Administration as first-line therapy to relieve vasomotor symptoms (VMS). Low-dose vaginal estrogen therapy is also a first-line treatment for the genitourinary syndrome of menopause, since it can directly address vulvovaginal atrophy.
An additional approved indication for systemic hormone therapy (HT) is the prevention of bone loss and fracture reduction in postmenopausal women who have increased risk of osteoporosis or fractures. It’s also FDA approved for women who had hypogonadism, primary ovarian insufficiency, or premature surgical menopause, who may use HT until the average age of menopause – about 52 years.
Unopposed systemic estrogen should not be used as HT in women with an intact uterus because of the elevated risk of endometrial cancer, and all indications assume there are no contraindications to HT use.
The position statement was developed by an expert panel, and has been endorsed by a number of international menopause societies, other American women’s health societies, and the American Association of Clinical Endocrinologists.
Cardiovascular risks
Early analysis of cardiovascular health data from the large, prospective Women’s Health Initiative trial raised significant concerns about increased risk. But further study of data from the Women’s Health Initiative, as well as meta-analyses of randomized controlled trials, have yielded a more nuanced view of the relationship between HT and cardiovascular disease, she said.
“Age matters,” Dr. Pinkerton said. “Data show that there is reduced heart disease in women who start [hormone replacement] early.” There is increasing data, she said, to support the “timing hypothesis.”
“Women who start HT before the age of 60 years, or within 10 years of menopause, may have a reduced risk of coronary heart disease,” Dr. Pinkerton said. “There is concern of increased risk of [coronary heart disease] in women who initiate hormone therapy more than 10 or 20 years from menopause.”
Use of HT is associated with a significantly increased risk of venous thromboembolism, a risk that increases with time, as does the risk of stroke and pulmonary embolism. Using lower doses or transdermal HT may reduce this risk, but “the lack of comparative randomized controlled trial data limit recommendations,” she said.
Transdermal therapy can also be considered for women with metabolic syndrome, hypertriglyceridemia, and fatty liver, since this route avoids first-pass hepatic metabolism.
Breast cancer
“The effect of hormone therapy on breast cancer risk is complex and conflicting,” said Dr Pinkerton, noting that breast cancer risk from HT may depend on many factors, including whether progestins are added to estrogen, the dose and duration of HT use, and how HT is administered.
Regarding the use of vaginal estrogen for women who have had breast cancer, Dr. Pinkerton said, “It’s a data-free zone.” Systemic absorption of vaginally-dosed estrogen is minimal, but the decision to use vaginal estrogen for a breast cancer survivor who is experiencing genitourinary syndrome of menopause symptoms should always be made in consultation with the woman’s oncologist and in shared decision-making with the patient herself, Dr. Pinkerton added.
Bioidentical HT
“Unique concerns about safety surround the use of compounded bioidentical hormone therapy,” Dr. Pinkerton said.
The lack of regulation and monitoring, together with lax labeling requirements, are areas of concern. Accurate dosing may not be occurring, and data are lacking to support safety and efficacy of compounded bioidentical products, she said. Neither is there evidence to support routine testing of serum or salivary hormone levels, she added.
Symptom relief
For isolated symptoms of genitourinary syndrome of menopause, low-dose vaginal preparations are safe and effective, Dr. Pinkerton said. For women who are symptomatic, use of either low-dose vaginal estrogen or systemic HT increases sexual function scores; however, she said, “hormone therapy is not recommended as the sole treatment of other sexual function problems,” such as diminished libido, though it can be a useful adjunct.
“Hormone therapy is the most effective treatment for hot flashes,” said Dr. Pinkerton, and using HT improves sleep quality and duration in women with bothersome nighttime hot flashes.
Fracture prevention
Data from the Women’s Health Initiative showed a highly significant 33% reduction in hip fractures for women using both estrogen alone and estrogen with progestogen. “That seems to get forgotten,” Dr. Pinkerton said. Though HT’s osteoporosis and fracture prevention effects stop when HT is discontinued, there’s no evidence of elevated fracture risk above baseline in women who have used HT and then stopped.
“Younger women may need higher doses to protect bone, but make sure you get adequate endometrial protection if you do that,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia.
Unapproved uses
“Hormone therapy is not recommended at any age to prevent or treat cognition or dementia,” said Dr. Pinkerton, citing a lack of data to support its use for these reasons. Observational data may show some reduction in risk of Alzheimer’s disease in women who use HT at younger ages or soon after menopause, she said.
Though HT users have a reduced risk of developing type 2 diabetes, diabetes prevention is not a Food and Drug Administration–approved indication for HT. Abdominal fat accumulation and weight gain may be reduced by HT as well, Dr. Pinkerton said.
Similarly, there are no data to support the use of HT for the treatment of clinical depression. Perimenopausal – but not postmenopausal – women may see some benefit from estrogen therapy; progestins may actually contribute to mood disturbance, she said.
Special populations
“Systemic hormone therapy is not recommended for survivors of breast cancer,” Dr. Pinkerton said. Any consideration for systemic HT in this population should include the oncologist, and only be entertained after other nonhormonal options have been tried, she said.
Women with a family history of breast or ovarian cancer, or with the BRCA mutation, do not appear to have their risk increased by the use of HT, though the ovarian cancer data are limited and observational, Dr. Pinkerton said.
The NAMS position statement also addresses the use of HT in other special populations, including survivors of other cancers and women who have primary ovarian insufficiency or early menopause, BRCA-positive women who have undergone oophorectomy, and those over age 65 years.
“The recommendation to routinely discontinue systemic hormone therapy after age 65 is not supported by data,” Dr. Pinkerton said. “I would tell you that there’s a lack of good data about prolonged duration. What I tell patients is, we really are in another data-free zone.” She recommends an individualized approach that balances benefits and risks and includes ongoing surveillance.
New message
“So what do I want us to do? I want us to change the message,” she said. Rather than advocating for HT to be used in “the lowest dose, for the shortest period of time,” she said the new message should be for women to use “appropriate hormone therapy to meet their treatment goals.”
The bottom line? After accounting for women who should avoid HT for specific contraindications, “benefits are likely to outweigh risks for symptomatic women who initiate hormone therapy when aged younger than 60 years and within 10 years of menopause,” Dr. Pinkerton said.
Dr. Pinkerton reported that she has received grant or research support from TherapeuticsMD.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – It’s time to be clear about the benefits of hormone therapy for many women in midlife, JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said during the keynote address at the group’s annual meeting.
“I want to take fear out of the conversation. Hormone therapy remains the most effective treatment for vasomotor symptoms and the genitourinary syndrome of menopause and has been shown to prevent bone loss and fracture,” said Dr. Pinkerton, who also chaired the advisory panel that penned the 2017 NAMS position statement on hormone therapy.
Hormone therapy is currently approved by the Food and Drug Administration as first-line therapy to relieve vasomotor symptoms (VMS). Low-dose vaginal estrogen therapy is also a first-line treatment for the genitourinary syndrome of menopause, since it can directly address vulvovaginal atrophy.
An additional approved indication for systemic hormone therapy (HT) is the prevention of bone loss and fracture reduction in postmenopausal women who have increased risk of osteoporosis or fractures. It’s also FDA approved for women who had hypogonadism, primary ovarian insufficiency, or premature surgical menopause, who may use HT until the average age of menopause – about 52 years.
Unopposed systemic estrogen should not be used as HT in women with an intact uterus because of the elevated risk of endometrial cancer, and all indications assume there are no contraindications to HT use.
The position statement was developed by an expert panel, and has been endorsed by a number of international menopause societies, other American women’s health societies, and the American Association of Clinical Endocrinologists.
Cardiovascular risks
Early analysis of cardiovascular health data from the large, prospective Women’s Health Initiative trial raised significant concerns about increased risk. But further study of data from the Women’s Health Initiative, as well as meta-analyses of randomized controlled trials, have yielded a more nuanced view of the relationship between HT and cardiovascular disease, she said.
“Age matters,” Dr. Pinkerton said. “Data show that there is reduced heart disease in women who start [hormone replacement] early.” There is increasing data, she said, to support the “timing hypothesis.”
“Women who start HT before the age of 60 years, or within 10 years of menopause, may have a reduced risk of coronary heart disease,” Dr. Pinkerton said. “There is concern of increased risk of [coronary heart disease] in women who initiate hormone therapy more than 10 or 20 years from menopause.”
Use of HT is associated with a significantly increased risk of venous thromboembolism, a risk that increases with time, as does the risk of stroke and pulmonary embolism. Using lower doses or transdermal HT may reduce this risk, but “the lack of comparative randomized controlled trial data limit recommendations,” she said.
Transdermal therapy can also be considered for women with metabolic syndrome, hypertriglyceridemia, and fatty liver, since this route avoids first-pass hepatic metabolism.
Breast cancer
“The effect of hormone therapy on breast cancer risk is complex and conflicting,” said Dr Pinkerton, noting that breast cancer risk from HT may depend on many factors, including whether progestins are added to estrogen, the dose and duration of HT use, and how HT is administered.
Regarding the use of vaginal estrogen for women who have had breast cancer, Dr. Pinkerton said, “It’s a data-free zone.” Systemic absorption of vaginally-dosed estrogen is minimal, but the decision to use vaginal estrogen for a breast cancer survivor who is experiencing genitourinary syndrome of menopause symptoms should always be made in consultation with the woman’s oncologist and in shared decision-making with the patient herself, Dr. Pinkerton added.
Bioidentical HT
“Unique concerns about safety surround the use of compounded bioidentical hormone therapy,” Dr. Pinkerton said.
The lack of regulation and monitoring, together with lax labeling requirements, are areas of concern. Accurate dosing may not be occurring, and data are lacking to support safety and efficacy of compounded bioidentical products, she said. Neither is there evidence to support routine testing of serum or salivary hormone levels, she added.
Symptom relief
For isolated symptoms of genitourinary syndrome of menopause, low-dose vaginal preparations are safe and effective, Dr. Pinkerton said. For women who are symptomatic, use of either low-dose vaginal estrogen or systemic HT increases sexual function scores; however, she said, “hormone therapy is not recommended as the sole treatment of other sexual function problems,” such as diminished libido, though it can be a useful adjunct.
“Hormone therapy is the most effective treatment for hot flashes,” said Dr. Pinkerton, and using HT improves sleep quality and duration in women with bothersome nighttime hot flashes.
Fracture prevention
Data from the Women’s Health Initiative showed a highly significant 33% reduction in hip fractures for women using both estrogen alone and estrogen with progestogen. “That seems to get forgotten,” Dr. Pinkerton said. Though HT’s osteoporosis and fracture prevention effects stop when HT is discontinued, there’s no evidence of elevated fracture risk above baseline in women who have used HT and then stopped.
“Younger women may need higher doses to protect bone, but make sure you get adequate endometrial protection if you do that,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia.
Unapproved uses
“Hormone therapy is not recommended at any age to prevent or treat cognition or dementia,” said Dr. Pinkerton, citing a lack of data to support its use for these reasons. Observational data may show some reduction in risk of Alzheimer’s disease in women who use HT at younger ages or soon after menopause, she said.
Though HT users have a reduced risk of developing type 2 diabetes, diabetes prevention is not a Food and Drug Administration–approved indication for HT. Abdominal fat accumulation and weight gain may be reduced by HT as well, Dr. Pinkerton said.
Similarly, there are no data to support the use of HT for the treatment of clinical depression. Perimenopausal – but not postmenopausal – women may see some benefit from estrogen therapy; progestins may actually contribute to mood disturbance, she said.
Special populations
“Systemic hormone therapy is not recommended for survivors of breast cancer,” Dr. Pinkerton said. Any consideration for systemic HT in this population should include the oncologist, and only be entertained after other nonhormonal options have been tried, she said.
Women with a family history of breast or ovarian cancer, or with the BRCA mutation, do not appear to have their risk increased by the use of HT, though the ovarian cancer data are limited and observational, Dr. Pinkerton said.
The NAMS position statement also addresses the use of HT in other special populations, including survivors of other cancers and women who have primary ovarian insufficiency or early menopause, BRCA-positive women who have undergone oophorectomy, and those over age 65 years.
“The recommendation to routinely discontinue systemic hormone therapy after age 65 is not supported by data,” Dr. Pinkerton said. “I would tell you that there’s a lack of good data about prolonged duration. What I tell patients is, we really are in another data-free zone.” She recommends an individualized approach that balances benefits and risks and includes ongoing surveillance.
New message
“So what do I want us to do? I want us to change the message,” she said. Rather than advocating for HT to be used in “the lowest dose, for the shortest period of time,” she said the new message should be for women to use “appropriate hormone therapy to meet their treatment goals.”
The bottom line? After accounting for women who should avoid HT for specific contraindications, “benefits are likely to outweigh risks for symptomatic women who initiate hormone therapy when aged younger than 60 years and within 10 years of menopause,” Dr. Pinkerton said.
Dr. Pinkerton reported that she has received grant or research support from TherapeuticsMD.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – It’s time to be clear about the benefits of hormone therapy for many women in midlife, JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said during the keynote address at the group’s annual meeting.
“I want to take fear out of the conversation. Hormone therapy remains the most effective treatment for vasomotor symptoms and the genitourinary syndrome of menopause and has been shown to prevent bone loss and fracture,” said Dr. Pinkerton, who also chaired the advisory panel that penned the 2017 NAMS position statement on hormone therapy.
Hormone therapy is currently approved by the Food and Drug Administration as first-line therapy to relieve vasomotor symptoms (VMS). Low-dose vaginal estrogen therapy is also a first-line treatment for the genitourinary syndrome of menopause, since it can directly address vulvovaginal atrophy.
An additional approved indication for systemic hormone therapy (HT) is the prevention of bone loss and fracture reduction in postmenopausal women who have increased risk of osteoporosis or fractures. It’s also FDA approved for women who had hypogonadism, primary ovarian insufficiency, or premature surgical menopause, who may use HT until the average age of menopause – about 52 years.
Unopposed systemic estrogen should not be used as HT in women with an intact uterus because of the elevated risk of endometrial cancer, and all indications assume there are no contraindications to HT use.
The position statement was developed by an expert panel, and has been endorsed by a number of international menopause societies, other American women’s health societies, and the American Association of Clinical Endocrinologists.
Cardiovascular risks
Early analysis of cardiovascular health data from the large, prospective Women’s Health Initiative trial raised significant concerns about increased risk. But further study of data from the Women’s Health Initiative, as well as meta-analyses of randomized controlled trials, have yielded a more nuanced view of the relationship between HT and cardiovascular disease, she said.
“Age matters,” Dr. Pinkerton said. “Data show that there is reduced heart disease in women who start [hormone replacement] early.” There is increasing data, she said, to support the “timing hypothesis.”
“Women who start HT before the age of 60 years, or within 10 years of menopause, may have a reduced risk of coronary heart disease,” Dr. Pinkerton said. “There is concern of increased risk of [coronary heart disease] in women who initiate hormone therapy more than 10 or 20 years from menopause.”
Use of HT is associated with a significantly increased risk of venous thromboembolism, a risk that increases with time, as does the risk of stroke and pulmonary embolism. Using lower doses or transdermal HT may reduce this risk, but “the lack of comparative randomized controlled trial data limit recommendations,” she said.
Transdermal therapy can also be considered for women with metabolic syndrome, hypertriglyceridemia, and fatty liver, since this route avoids first-pass hepatic metabolism.
Breast cancer
“The effect of hormone therapy on breast cancer risk is complex and conflicting,” said Dr Pinkerton, noting that breast cancer risk from HT may depend on many factors, including whether progestins are added to estrogen, the dose and duration of HT use, and how HT is administered.
Regarding the use of vaginal estrogen for women who have had breast cancer, Dr. Pinkerton said, “It’s a data-free zone.” Systemic absorption of vaginally-dosed estrogen is minimal, but the decision to use vaginal estrogen for a breast cancer survivor who is experiencing genitourinary syndrome of menopause symptoms should always be made in consultation with the woman’s oncologist and in shared decision-making with the patient herself, Dr. Pinkerton added.
Bioidentical HT
“Unique concerns about safety surround the use of compounded bioidentical hormone therapy,” Dr. Pinkerton said.
The lack of regulation and monitoring, together with lax labeling requirements, are areas of concern. Accurate dosing may not be occurring, and data are lacking to support safety and efficacy of compounded bioidentical products, she said. Neither is there evidence to support routine testing of serum or salivary hormone levels, she added.
Symptom relief
For isolated symptoms of genitourinary syndrome of menopause, low-dose vaginal preparations are safe and effective, Dr. Pinkerton said. For women who are symptomatic, use of either low-dose vaginal estrogen or systemic HT increases sexual function scores; however, she said, “hormone therapy is not recommended as the sole treatment of other sexual function problems,” such as diminished libido, though it can be a useful adjunct.
“Hormone therapy is the most effective treatment for hot flashes,” said Dr. Pinkerton, and using HT improves sleep quality and duration in women with bothersome nighttime hot flashes.
Fracture prevention
Data from the Women’s Health Initiative showed a highly significant 33% reduction in hip fractures for women using both estrogen alone and estrogen with progestogen. “That seems to get forgotten,” Dr. Pinkerton said. Though HT’s osteoporosis and fracture prevention effects stop when HT is discontinued, there’s no evidence of elevated fracture risk above baseline in women who have used HT and then stopped.
“Younger women may need higher doses to protect bone, but make sure you get adequate endometrial protection if you do that,” said Dr. Pinkerton, professor of obstetrics and gynecology at the University of Virginia.
Unapproved uses
“Hormone therapy is not recommended at any age to prevent or treat cognition or dementia,” said Dr. Pinkerton, citing a lack of data to support its use for these reasons. Observational data may show some reduction in risk of Alzheimer’s disease in women who use HT at younger ages or soon after menopause, she said.
Though HT users have a reduced risk of developing type 2 diabetes, diabetes prevention is not a Food and Drug Administration–approved indication for HT. Abdominal fat accumulation and weight gain may be reduced by HT as well, Dr. Pinkerton said.
Similarly, there are no data to support the use of HT for the treatment of clinical depression. Perimenopausal – but not postmenopausal – women may see some benefit from estrogen therapy; progestins may actually contribute to mood disturbance, she said.
Special populations
“Systemic hormone therapy is not recommended for survivors of breast cancer,” Dr. Pinkerton said. Any consideration for systemic HT in this population should include the oncologist, and only be entertained after other nonhormonal options have been tried, she said.
Women with a family history of breast or ovarian cancer, or with the BRCA mutation, do not appear to have their risk increased by the use of HT, though the ovarian cancer data are limited and observational, Dr. Pinkerton said.
The NAMS position statement also addresses the use of HT in other special populations, including survivors of other cancers and women who have primary ovarian insufficiency or early menopause, BRCA-positive women who have undergone oophorectomy, and those over age 65 years.
“The recommendation to routinely discontinue systemic hormone therapy after age 65 is not supported by data,” Dr. Pinkerton said. “I would tell you that there’s a lack of good data about prolonged duration. What I tell patients is, we really are in another data-free zone.” She recommends an individualized approach that balances benefits and risks and includes ongoing surveillance.
New message
“So what do I want us to do? I want us to change the message,” she said. Rather than advocating for HT to be used in “the lowest dose, for the shortest period of time,” she said the new message should be for women to use “appropriate hormone therapy to meet their treatment goals.”
The bottom line? After accounting for women who should avoid HT for specific contraindications, “benefits are likely to outweigh risks for symptomatic women who initiate hormone therapy when aged younger than 60 years and within 10 years of menopause,” Dr. Pinkerton said.
Dr. Pinkerton reported that she has received grant or research support from TherapeuticsMD.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT NAMS 2017
Biologic approved for moderate to severe psoriasis in adolescents
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.