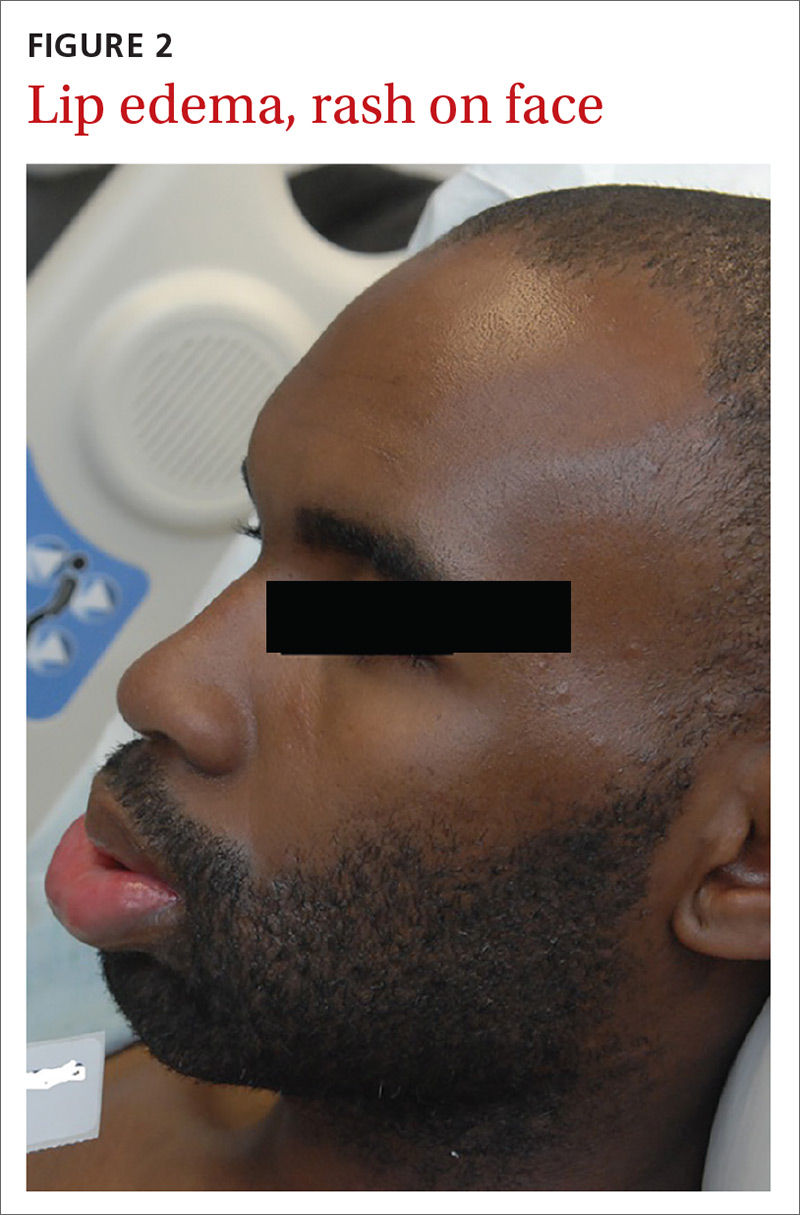
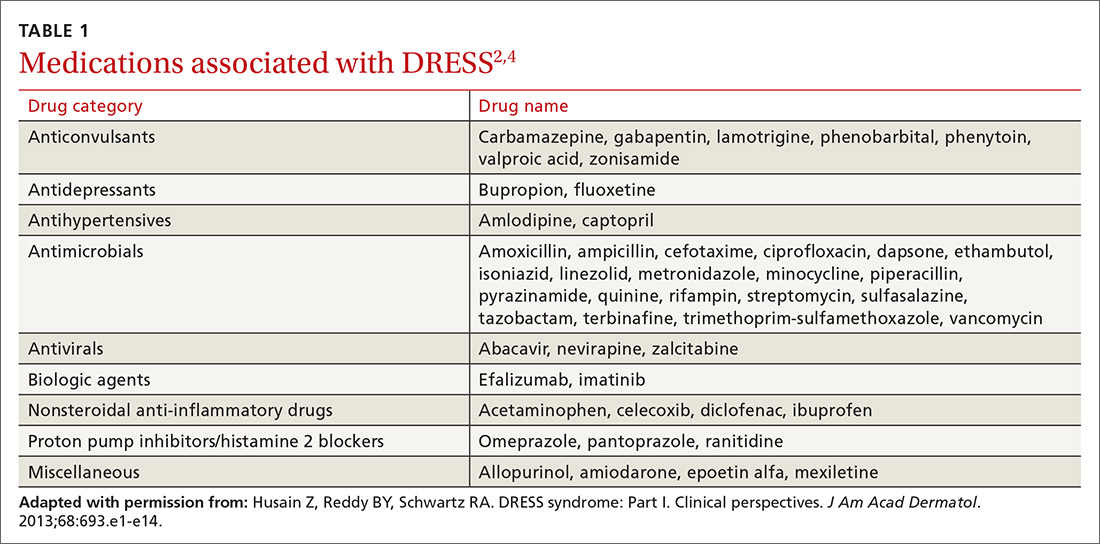
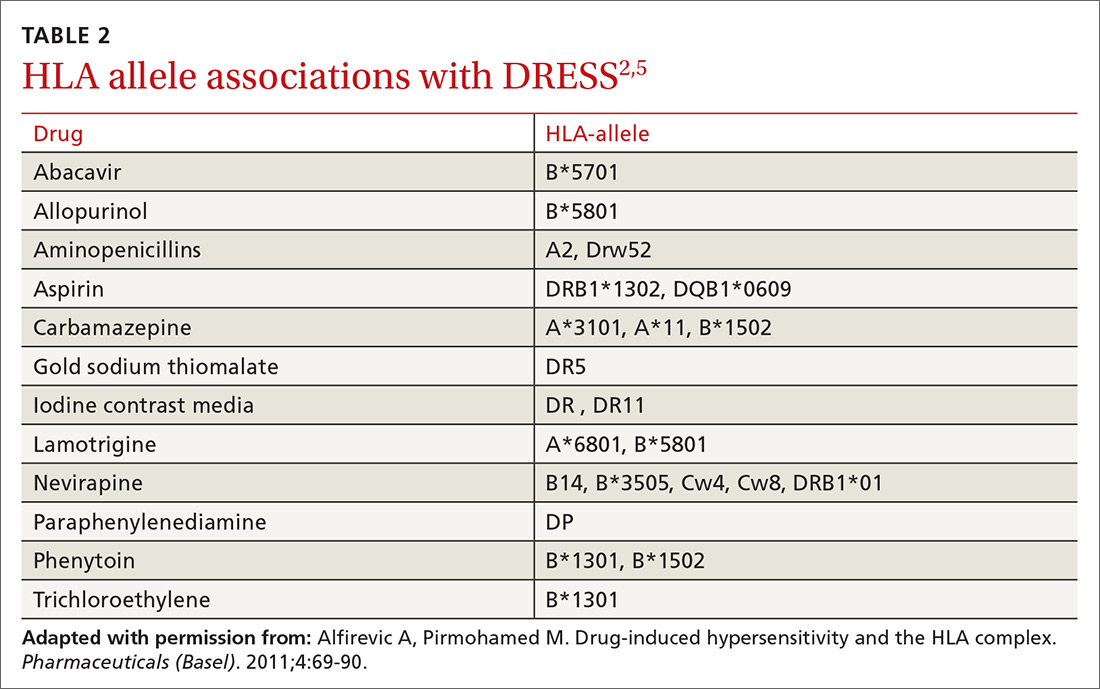
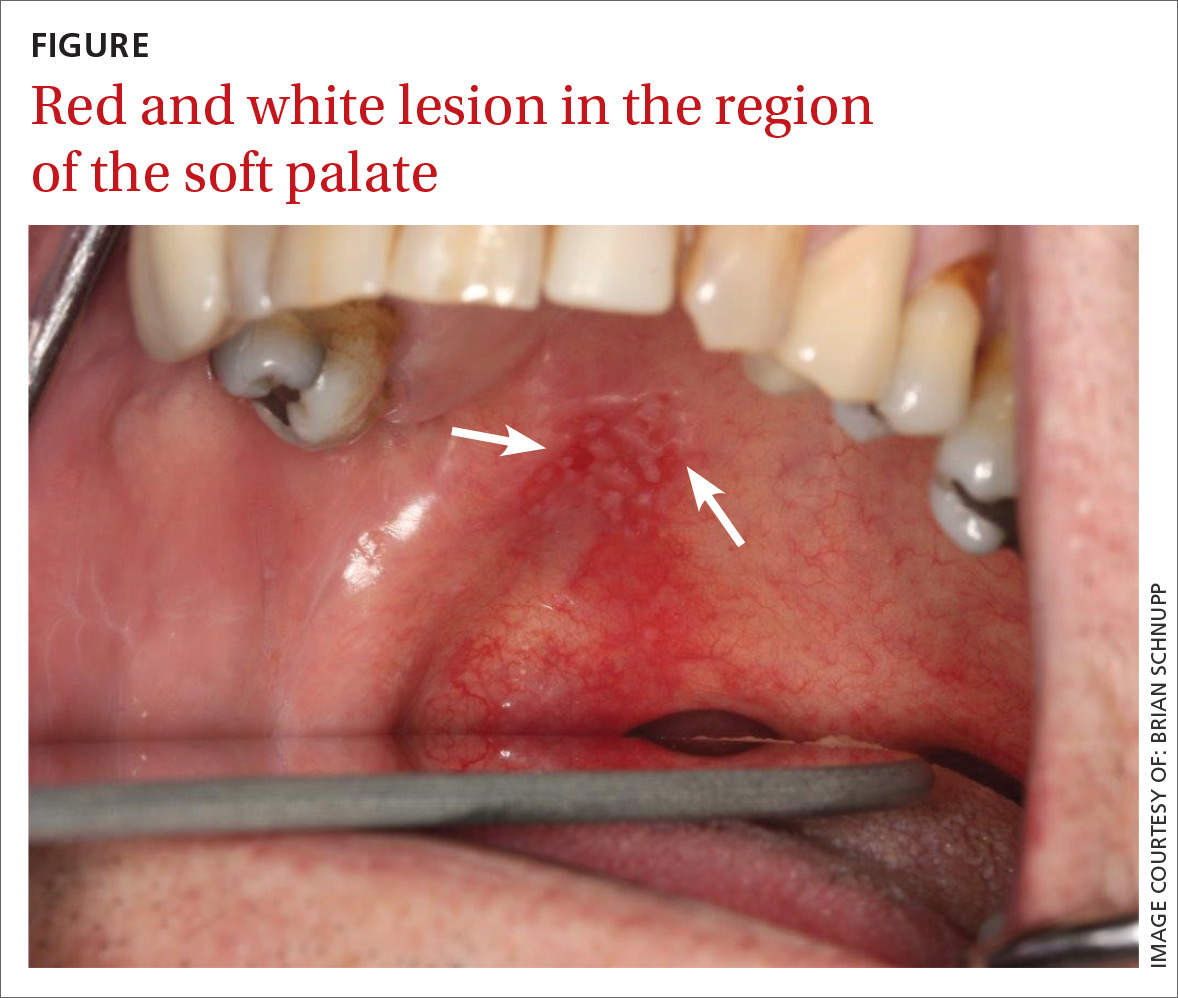
User login
When cold-induced vasospasm is the tip of the iceberg
For many patients, Raynaud symptoms are mild enough to not even mention to their primary care provider, and conversely, there is little reason for most clinicians to routinely inquire about such symptoms. So it may surprise some readers to read about the nuances of diagnosis and treatment discussed by Shapiro and Wigley in this issue of the Journal.
To a rheumatologist, Raynaud phenomenon, particularly of recent onset in an adult, raises the specter of an underlying systemic inflammatory disease. The phenomenon is not linked to a specific diagnosis; it is associated with lupus, rheumatoid arthritis, cryoglobulinemia, inflammatory myopathy, Sjögren syndrome, and, in its severe form, with the scleroderma syndromes. We focus on differentiating between these rheumatic disorders once we have discarded nonrheumatic causes such as atherosclerotic arterial disease, carcinoma, embolism, Buerger disease, medications, smoking, or thrombosis.
But rheumatologists are toward the bottom of the diagnostic funnel—we see these patients when an underlying disease is already suspected. The real challenge is for the primary care providers who first recognize the digital vasospasm on examination or are told of the symptoms by their patient. These clinicians need to know which initial reflexive actions are warranted and which can wait, for, as noted by Shapiro and Wigley, there are several options.
The first action is to try to determine the timeline, although Raynaud disease often has an insidious onset or the patient doesn’t recall the onset. New and sudden onset likely has a stronger association with an underlying disease. A focused physical examination should look for digital stigmata of ischemic damage; the presence of digital ulcers or healed digital pits indicates a possible vascular occlusive component in addition to the vascular spasm. This strongly suggests scleroderma or Buerger disease, as tissue damage doesn’t occur in (primary) Raynaud disease or generally even with Raynaud phenomenon associated with lupus or other rheumatic disorders. Sclerodactyly should be looked for: diffuse finger puffiness, skin-tightening, or early signs such as loss of the usual finger skin creases. Telangiectasia (not vascular spiders or cherry angiomata) should be searched for, particularly on the palms, face, and inner lips, as these vascular lesions are common in patients with limited scleroderma. Careful auscultation for basilar lung crackles should be done. Distal pulses should all be assessed, and bruits in the neck, abdomen and inguinal areas should be carefully sought.
Patients should be questioned about any symptom-associated reduction in exercise tolerance and particularly about trouble swallowing, “heartburn,” and symptoms of reflux. Although patients with Raynaud disease may have demonstrable esophageal dysmotility, the presence of significant, new, or worsened symptoms raises the concern of scleroderma. Patients should be asked about symptoms of malabsorption. Specific questioning should be directed at eliciting a history of joint stiffness and especially muscle weakness. The latter can be approached by inquiring about new or progressive difficulty in specific tasks such as walking up steps, brushing hair, and arising from low chairs or the toilet. Distinguishing muscle weakness from general fatigue is not always easy, but it is important.
Shapiro and Wigley discuss the extremely useful evaluation of nailfold capillaries, which can be done with a standard magnifier or ophthalmoscope. This is very valuable to help predict the development or current presence of a systemic rheumatic disease. But this is not a technique that most clinicians are familiar with. A potentially useful surrogate or adjunctive test, especially in the setting of new-onset Raynaud, is the antinuclear antibody (ANA) test; I prefer the immunofluorescent assay. While a positive test alone (with Raynaud) does not define the presence of any rheumatic disease, several older studies suggest that patients with a new onset of Raynaud phenomenon and a positive ANA test are more likely to develop a systemic autoimmune disorder than if the test is negative. Those who do so (and this is far from all) are most likely to have the disease manifest within a few years. Hence, if the ANA test is positive but the history, physical examination, and limited laboratory testing (complete blood cell count with differential, complete metabolic panel, creatine kinase, and urinalysis) are normal, it is reasonable to reexamine the patient in 3 months and then every 6 months for 2 to 3 years, repeating the focused history and physical examination. It is also reasonable at some point to refer these patients to a rheumatologist.
Since Raynaud phenomenon is common, and the associated severe rheumatic disorders associated with it are rare, it is easy to not recognize Raynaud phenomenon as a clue to the onset of a potentially severe systemic disease. Yet with a few simple questions, a focused examination, and minimal laboratory testing, patients who are more likely to harbor a systemic disease can usually be treated symptomatically if necessary, and appropriately triaged to observation or for subspecialty referral.
For many patients, Raynaud symptoms are mild enough to not even mention to their primary care provider, and conversely, there is little reason for most clinicians to routinely inquire about such symptoms. So it may surprise some readers to read about the nuances of diagnosis and treatment discussed by Shapiro and Wigley in this issue of the Journal.
To a rheumatologist, Raynaud phenomenon, particularly of recent onset in an adult, raises the specter of an underlying systemic inflammatory disease. The phenomenon is not linked to a specific diagnosis; it is associated with lupus, rheumatoid arthritis, cryoglobulinemia, inflammatory myopathy, Sjögren syndrome, and, in its severe form, with the scleroderma syndromes. We focus on differentiating between these rheumatic disorders once we have discarded nonrheumatic causes such as atherosclerotic arterial disease, carcinoma, embolism, Buerger disease, medications, smoking, or thrombosis.
But rheumatologists are toward the bottom of the diagnostic funnel—we see these patients when an underlying disease is already suspected. The real challenge is for the primary care providers who first recognize the digital vasospasm on examination or are told of the symptoms by their patient. These clinicians need to know which initial reflexive actions are warranted and which can wait, for, as noted by Shapiro and Wigley, there are several options.
The first action is to try to determine the timeline, although Raynaud disease often has an insidious onset or the patient doesn’t recall the onset. New and sudden onset likely has a stronger association with an underlying disease. A focused physical examination should look for digital stigmata of ischemic damage; the presence of digital ulcers or healed digital pits indicates a possible vascular occlusive component in addition to the vascular spasm. This strongly suggests scleroderma or Buerger disease, as tissue damage doesn’t occur in (primary) Raynaud disease or generally even with Raynaud phenomenon associated with lupus or other rheumatic disorders. Sclerodactyly should be looked for: diffuse finger puffiness, skin-tightening, or early signs such as loss of the usual finger skin creases. Telangiectasia (not vascular spiders or cherry angiomata) should be searched for, particularly on the palms, face, and inner lips, as these vascular lesions are common in patients with limited scleroderma. Careful auscultation for basilar lung crackles should be done. Distal pulses should all be assessed, and bruits in the neck, abdomen and inguinal areas should be carefully sought.
Patients should be questioned about any symptom-associated reduction in exercise tolerance and particularly about trouble swallowing, “heartburn,” and symptoms of reflux. Although patients with Raynaud disease may have demonstrable esophageal dysmotility, the presence of significant, new, or worsened symptoms raises the concern of scleroderma. Patients should be asked about symptoms of malabsorption. Specific questioning should be directed at eliciting a history of joint stiffness and especially muscle weakness. The latter can be approached by inquiring about new or progressive difficulty in specific tasks such as walking up steps, brushing hair, and arising from low chairs or the toilet. Distinguishing muscle weakness from general fatigue is not always easy, but it is important.
Shapiro and Wigley discuss the extremely useful evaluation of nailfold capillaries, which can be done with a standard magnifier or ophthalmoscope. This is very valuable to help predict the development or current presence of a systemic rheumatic disease. But this is not a technique that most clinicians are familiar with. A potentially useful surrogate or adjunctive test, especially in the setting of new-onset Raynaud, is the antinuclear antibody (ANA) test; I prefer the immunofluorescent assay. While a positive test alone (with Raynaud) does not define the presence of any rheumatic disease, several older studies suggest that patients with a new onset of Raynaud phenomenon and a positive ANA test are more likely to develop a systemic autoimmune disorder than if the test is negative. Those who do so (and this is far from all) are most likely to have the disease manifest within a few years. Hence, if the ANA test is positive but the history, physical examination, and limited laboratory testing (complete blood cell count with differential, complete metabolic panel, creatine kinase, and urinalysis) are normal, it is reasonable to reexamine the patient in 3 months and then every 6 months for 2 to 3 years, repeating the focused history and physical examination. It is also reasonable at some point to refer these patients to a rheumatologist.
Since Raynaud phenomenon is common, and the associated severe rheumatic disorders associated with it are rare, it is easy to not recognize Raynaud phenomenon as a clue to the onset of a potentially severe systemic disease. Yet with a few simple questions, a focused examination, and minimal laboratory testing, patients who are more likely to harbor a systemic disease can usually be treated symptomatically if necessary, and appropriately triaged to observation or for subspecialty referral.
For many patients, Raynaud symptoms are mild enough to not even mention to their primary care provider, and conversely, there is little reason for most clinicians to routinely inquire about such symptoms. So it may surprise some readers to read about the nuances of diagnosis and treatment discussed by Shapiro and Wigley in this issue of the Journal.
To a rheumatologist, Raynaud phenomenon, particularly of recent onset in an adult, raises the specter of an underlying systemic inflammatory disease. The phenomenon is not linked to a specific diagnosis; it is associated with lupus, rheumatoid arthritis, cryoglobulinemia, inflammatory myopathy, Sjögren syndrome, and, in its severe form, with the scleroderma syndromes. We focus on differentiating between these rheumatic disorders once we have discarded nonrheumatic causes such as atherosclerotic arterial disease, carcinoma, embolism, Buerger disease, medications, smoking, or thrombosis.
But rheumatologists are toward the bottom of the diagnostic funnel—we see these patients when an underlying disease is already suspected. The real challenge is for the primary care providers who first recognize the digital vasospasm on examination or are told of the symptoms by their patient. These clinicians need to know which initial reflexive actions are warranted and which can wait, for, as noted by Shapiro and Wigley, there are several options.
The first action is to try to determine the timeline, although Raynaud disease often has an insidious onset or the patient doesn’t recall the onset. New and sudden onset likely has a stronger association with an underlying disease. A focused physical examination should look for digital stigmata of ischemic damage; the presence of digital ulcers or healed digital pits indicates a possible vascular occlusive component in addition to the vascular spasm. This strongly suggests scleroderma or Buerger disease, as tissue damage doesn’t occur in (primary) Raynaud disease or generally even with Raynaud phenomenon associated with lupus or other rheumatic disorders. Sclerodactyly should be looked for: diffuse finger puffiness, skin-tightening, or early signs such as loss of the usual finger skin creases. Telangiectasia (not vascular spiders or cherry angiomata) should be searched for, particularly on the palms, face, and inner lips, as these vascular lesions are common in patients with limited scleroderma. Careful auscultation for basilar lung crackles should be done. Distal pulses should all be assessed, and bruits in the neck, abdomen and inguinal areas should be carefully sought.
Patients should be questioned about any symptom-associated reduction in exercise tolerance and particularly about trouble swallowing, “heartburn,” and symptoms of reflux. Although patients with Raynaud disease may have demonstrable esophageal dysmotility, the presence of significant, new, or worsened symptoms raises the concern of scleroderma. Patients should be asked about symptoms of malabsorption. Specific questioning should be directed at eliciting a history of joint stiffness and especially muscle weakness. The latter can be approached by inquiring about new or progressive difficulty in specific tasks such as walking up steps, brushing hair, and arising from low chairs or the toilet. Distinguishing muscle weakness from general fatigue is not always easy, but it is important.
Shapiro and Wigley discuss the extremely useful evaluation of nailfold capillaries, which can be done with a standard magnifier or ophthalmoscope. This is very valuable to help predict the development or current presence of a systemic rheumatic disease. But this is not a technique that most clinicians are familiar with. A potentially useful surrogate or adjunctive test, especially in the setting of new-onset Raynaud, is the antinuclear antibody (ANA) test; I prefer the immunofluorescent assay. While a positive test alone (with Raynaud) does not define the presence of any rheumatic disease, several older studies suggest that patients with a new onset of Raynaud phenomenon and a positive ANA test are more likely to develop a systemic autoimmune disorder than if the test is negative. Those who do so (and this is far from all) are most likely to have the disease manifest within a few years. Hence, if the ANA test is positive but the history, physical examination, and limited laboratory testing (complete blood cell count with differential, complete metabolic panel, creatine kinase, and urinalysis) are normal, it is reasonable to reexamine the patient in 3 months and then every 6 months for 2 to 3 years, repeating the focused history and physical examination. It is also reasonable at some point to refer these patients to a rheumatologist.
Since Raynaud phenomenon is common, and the associated severe rheumatic disorders associated with it are rare, it is easy to not recognize Raynaud phenomenon as a clue to the onset of a potentially severe systemic disease. Yet with a few simple questions, a focused examination, and minimal laboratory testing, patients who are more likely to harbor a systemic disease can usually be treated symptomatically if necessary, and appropriately triaged to observation or for subspecialty referral.
Liquid biopsy predicts checkpoint inhibitor response
The overall response rate to immune checkpoint inhibitors was 45% among cancer patients who had more than three variants of unknown significance in their circulating tumor DNA; among those with three or fewer, the response rate was 15%, according to a University of California, San Diego, investigation with 69 subjects.
Higher mutation burdens in circulating tumor DNA (ctDNA) also correlated with improved progression-free and overall survival across 20 cancer types, the investigators reported (Clin Cancer Res. 2017 Oct. 1. doi: 10.1158/1078-0432.CCR-17-1439).
Tumor mutation burdens can predict response to checkpoint inhibitors, but they are usually assessed by tissue biopsy, which is costly and invasive. The findings suggest that blood tests could replace tissue biopsies to green-light immune checkpoint inhibitor treatment.
“Our current results may be clinically exploitable. ... Liquid biopsies that assess blood-derived ctDNA are noninvasive, easily acquired, and inexpensive. The ctDNA derived from blood may also represent shed DNA from multiple metastatic sites, whereas tissue genomics reflects only the piece of tissue removed,” said investigators led by Yulian Khagi, MD, a hematology-oncology fellow at the university.
In a press statement, Dr. Khagi said “If verified by further studies, clinicians will be able to utilize the ... results of this simple blood test to make determinations about whether to use checkpoint inhibitor–based immune therapy in a variety of tumor types.”
The 69 patients were a median of 56 years old, and 43 (62.3%) were men. Melanoma, lung cancer, and head and neck cancer were the most common malignancies. The majority of patients had anti–PD-1 or PD-L1 monotherapy.
For most patients, blood samples were drawn a month or 2 before treatment. Next-generation sequencing (Guardant360) was done on ctDNA to detect alterations in cancer genes. Of the 69 patients, 20 (29%) had more than three variants of unknown significance (VUS); the rest had three or fewer.
The median overall survival was 15.3 months from the start of immunotherapy. For patients with three or fewer VUS, median overall survival was 10.72 months; for patients with more, median overall survival could not be calculated because more than half were alive at the study’s conclusion.
Median progression-fee survival was 2.07 months with three or fewer VUS, versus 3.84 months with more. The findings were statistically significant.
Similar results were found when all genomic alterations, not just VUS, were examined and dichotomized as six or more versus fewer than six.
“The number of genes assayed in our ctDNA analysis was only between 54 and 70. Unlike targeted NGS [next-generation sequencing] of tumor tissue, which often tests for hundreds of genes and allows a relatively accurate estimate of total mutational burden, targeted NGS of plasma ctDNA provides only a limited snapshot of the cancer genome. More extensive ctDNA gene panels merit investigation to determine if they increase the correlative value of our findings,” the investigators said.
The work was funded by the Joan and Irwin Jacobs Fund and the National Cancer Institute. Dr. Khagi had no industry disclosures. Three authors reported financial ties to a number of companies, including Boehringer, Merck, Guardant, and Pfizer. The senior author has ownership interests in CureMatch.
The overall response rate to immune checkpoint inhibitors was 45% among cancer patients who had more than three variants of unknown significance in their circulating tumor DNA; among those with three or fewer, the response rate was 15%, according to a University of California, San Diego, investigation with 69 subjects.
Higher mutation burdens in circulating tumor DNA (ctDNA) also correlated with improved progression-free and overall survival across 20 cancer types, the investigators reported (Clin Cancer Res. 2017 Oct. 1. doi: 10.1158/1078-0432.CCR-17-1439).
Tumor mutation burdens can predict response to checkpoint inhibitors, but they are usually assessed by tissue biopsy, which is costly and invasive. The findings suggest that blood tests could replace tissue biopsies to green-light immune checkpoint inhibitor treatment.
“Our current results may be clinically exploitable. ... Liquid biopsies that assess blood-derived ctDNA are noninvasive, easily acquired, and inexpensive. The ctDNA derived from blood may also represent shed DNA from multiple metastatic sites, whereas tissue genomics reflects only the piece of tissue removed,” said investigators led by Yulian Khagi, MD, a hematology-oncology fellow at the university.
In a press statement, Dr. Khagi said “If verified by further studies, clinicians will be able to utilize the ... results of this simple blood test to make determinations about whether to use checkpoint inhibitor–based immune therapy in a variety of tumor types.”
The 69 patients were a median of 56 years old, and 43 (62.3%) were men. Melanoma, lung cancer, and head and neck cancer were the most common malignancies. The majority of patients had anti–PD-1 or PD-L1 monotherapy.
For most patients, blood samples were drawn a month or 2 before treatment. Next-generation sequencing (Guardant360) was done on ctDNA to detect alterations in cancer genes. Of the 69 patients, 20 (29%) had more than three variants of unknown significance (VUS); the rest had three or fewer.
The median overall survival was 15.3 months from the start of immunotherapy. For patients with three or fewer VUS, median overall survival was 10.72 months; for patients with more, median overall survival could not be calculated because more than half were alive at the study’s conclusion.
Median progression-fee survival was 2.07 months with three or fewer VUS, versus 3.84 months with more. The findings were statistically significant.
Similar results were found when all genomic alterations, not just VUS, were examined and dichotomized as six or more versus fewer than six.
“The number of genes assayed in our ctDNA analysis was only between 54 and 70. Unlike targeted NGS [next-generation sequencing] of tumor tissue, which often tests for hundreds of genes and allows a relatively accurate estimate of total mutational burden, targeted NGS of plasma ctDNA provides only a limited snapshot of the cancer genome. More extensive ctDNA gene panels merit investigation to determine if they increase the correlative value of our findings,” the investigators said.
The work was funded by the Joan and Irwin Jacobs Fund and the National Cancer Institute. Dr. Khagi had no industry disclosures. Three authors reported financial ties to a number of companies, including Boehringer, Merck, Guardant, and Pfizer. The senior author has ownership interests in CureMatch.
The overall response rate to immune checkpoint inhibitors was 45% among cancer patients who had more than three variants of unknown significance in their circulating tumor DNA; among those with three or fewer, the response rate was 15%, according to a University of California, San Diego, investigation with 69 subjects.
Higher mutation burdens in circulating tumor DNA (ctDNA) also correlated with improved progression-free and overall survival across 20 cancer types, the investigators reported (Clin Cancer Res. 2017 Oct. 1. doi: 10.1158/1078-0432.CCR-17-1439).
Tumor mutation burdens can predict response to checkpoint inhibitors, but they are usually assessed by tissue biopsy, which is costly and invasive. The findings suggest that blood tests could replace tissue biopsies to green-light immune checkpoint inhibitor treatment.
“Our current results may be clinically exploitable. ... Liquid biopsies that assess blood-derived ctDNA are noninvasive, easily acquired, and inexpensive. The ctDNA derived from blood may also represent shed DNA from multiple metastatic sites, whereas tissue genomics reflects only the piece of tissue removed,” said investigators led by Yulian Khagi, MD, a hematology-oncology fellow at the university.
In a press statement, Dr. Khagi said “If verified by further studies, clinicians will be able to utilize the ... results of this simple blood test to make determinations about whether to use checkpoint inhibitor–based immune therapy in a variety of tumor types.”
The 69 patients were a median of 56 years old, and 43 (62.3%) were men. Melanoma, lung cancer, and head and neck cancer were the most common malignancies. The majority of patients had anti–PD-1 or PD-L1 monotherapy.
For most patients, blood samples were drawn a month or 2 before treatment. Next-generation sequencing (Guardant360) was done on ctDNA to detect alterations in cancer genes. Of the 69 patients, 20 (29%) had more than three variants of unknown significance (VUS); the rest had three or fewer.
The median overall survival was 15.3 months from the start of immunotherapy. For patients with three or fewer VUS, median overall survival was 10.72 months; for patients with more, median overall survival could not be calculated because more than half were alive at the study’s conclusion.
Median progression-fee survival was 2.07 months with three or fewer VUS, versus 3.84 months with more. The findings were statistically significant.
Similar results were found when all genomic alterations, not just VUS, were examined and dichotomized as six or more versus fewer than six.
“The number of genes assayed in our ctDNA analysis was only between 54 and 70. Unlike targeted NGS [next-generation sequencing] of tumor tissue, which often tests for hundreds of genes and allows a relatively accurate estimate of total mutational burden, targeted NGS of plasma ctDNA provides only a limited snapshot of the cancer genome. More extensive ctDNA gene panels merit investigation to determine if they increase the correlative value of our findings,” the investigators said.
The work was funded by the Joan and Irwin Jacobs Fund and the National Cancer Institute. Dr. Khagi had no industry disclosures. Three authors reported financial ties to a number of companies, including Boehringer, Merck, Guardant, and Pfizer. The senior author has ownership interests in CureMatch.
FROM CLINICAL CANCER RESEARCH
Key clinical point:
Major finding: The overall response rate to immune checkpoint inhibitors was 45% among cancer patients who had more than three variants of unknown significance in their circulating tumor DNA; among those with three or fewer, the response rate was 15%.
Data source: Review of 69 cancer patients.
Disclosures: The work was funded by the Joan and Irwin Jacobs Fund and the National Cancer Institute. Three investigators reported financial ties to a number of companies, including Boehringer, Merck, Guardant, and Pfizer. The senior author has ownership interests in CureMatch.
Obesity: When to consider medication
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4-6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
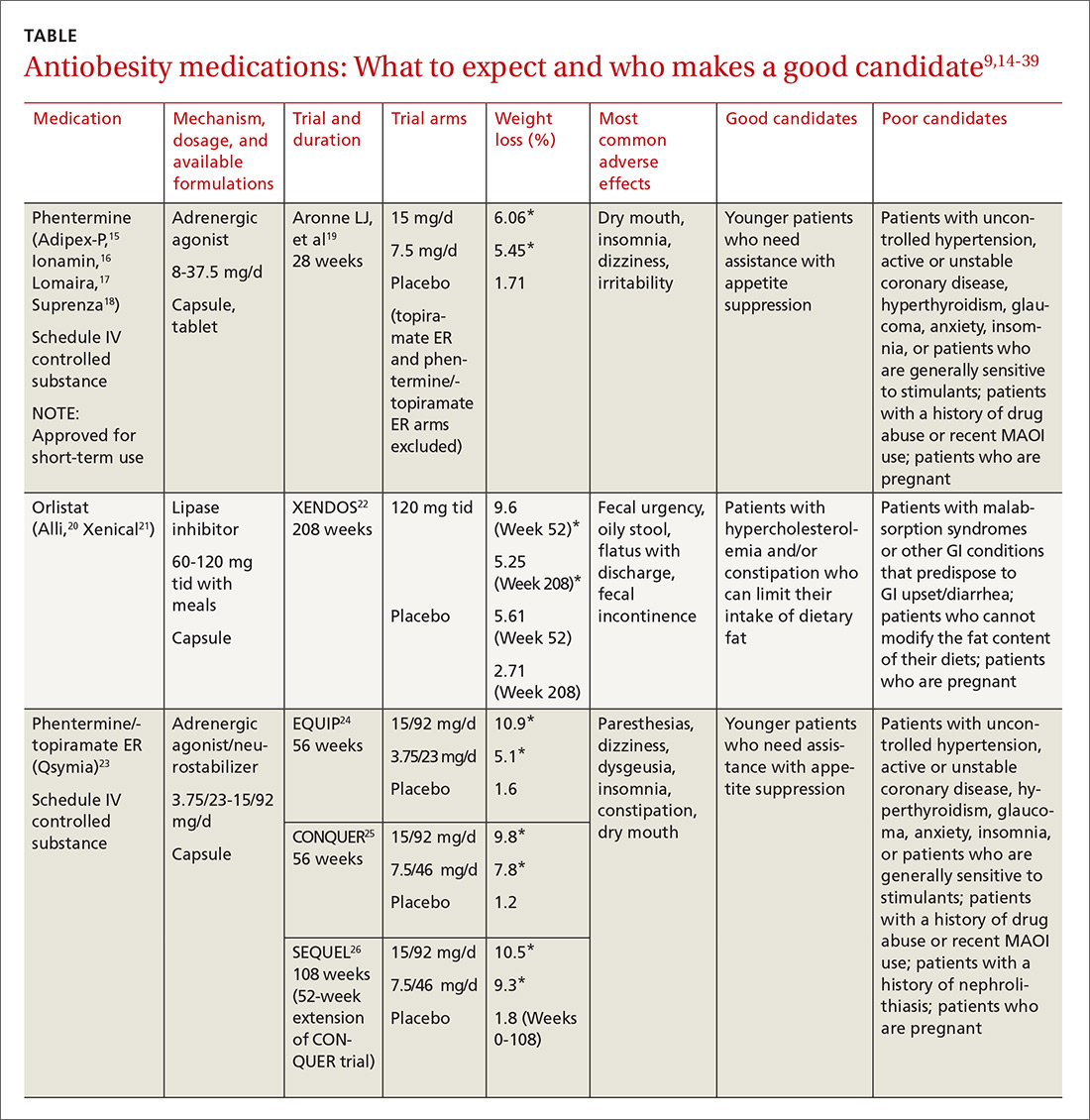
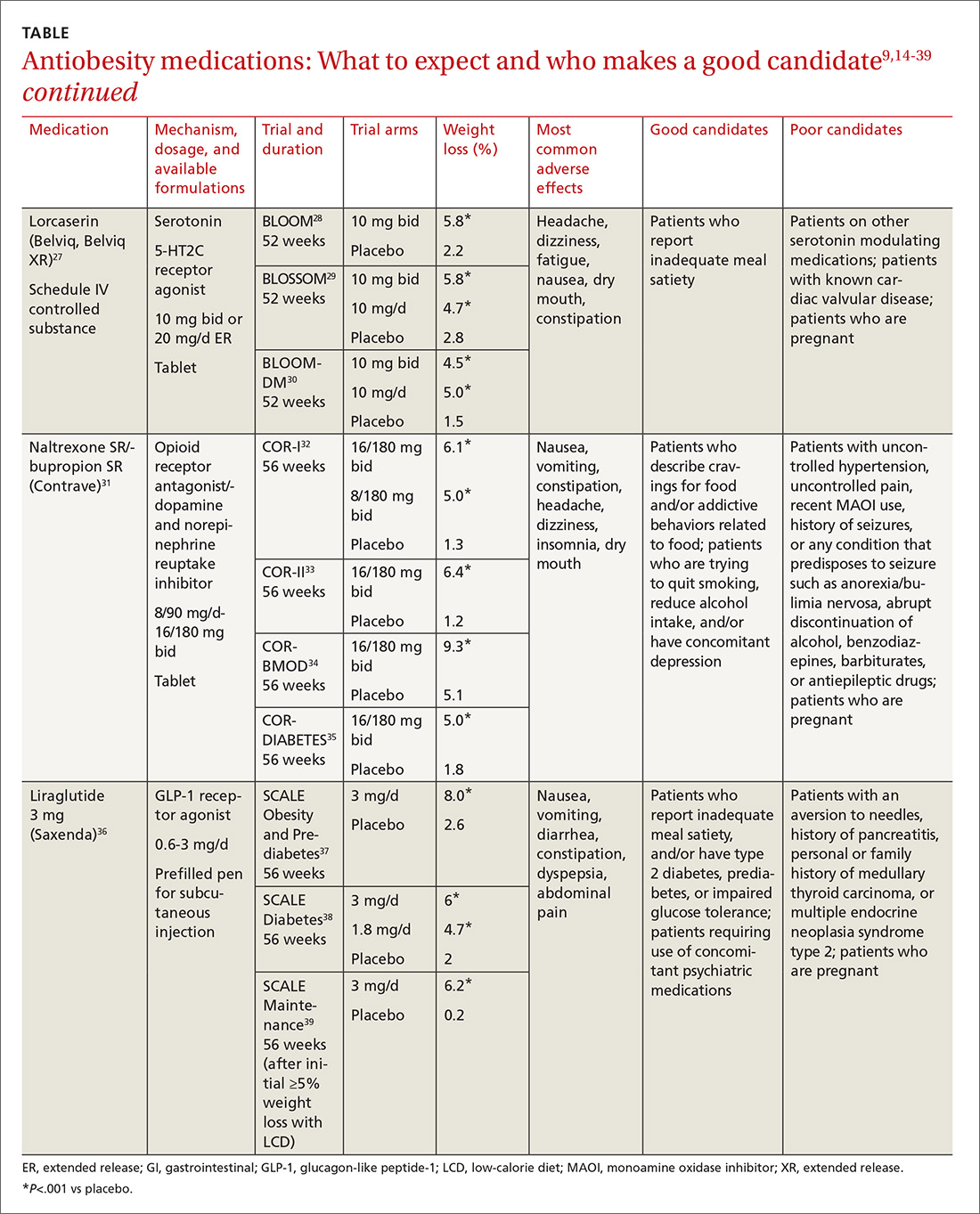
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14-39). These medications have the potential to not only limit weight gain, but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—namely family practitioners—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehensive treatment plan that includes diet, physical activity, and behavioral modification.
[polldaddy:9840472]
CASE 1 Melissa C, a 27-year-old woman with obesity (BMI 33 kg/m2), hyperlipidemia, and migraine headaches, presents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she’s taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for Ms. C?
Discussion
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .”
SIDEBAR
Before prescribing antiobesity medication . . .Have a frank discussion with the patient and be sure to cover the following points:
- The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
- Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
- Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
- Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30-34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24-26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Norman S, a 52-year-old overweight man (BMI 29 kg/m2) with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma, has recently hit a plateau with his weight loss. He lost 45 pounds secondary to diet and exercise, but hasn’t been able to lose any more. He also struggles with constant hunger. His medications include metformin 1000 mg bid, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until he undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
Mr. S is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
Discussion
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. He has worked hard to lose a significant number of pounds, and is now at high risk of regaining them. That’s because his appetite has increased with his new exercise regimen, but his energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of his opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
He could try orlistat, especially if he struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for his diabetes and may also promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOM-DM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of his initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomic testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 Kathryn M, a 38-year-old woman with obesity (BMI 42 kg/m2), obstructive sleep apnea, gastroesophageal reflux disease, and depression, is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
Ms. M smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discussion
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like Ms. M, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to Ms. M that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for Ms. M because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion could also help Ms. M quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses Ms. M’s problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg could also be used and should certainly be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 William P, a 65-year-old man with obesity (BMI 39 kg/m2) who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about his weight. He lost 100 lbs following surgery and maintained his weight for 3 years, but then regained 30 lbs. He comes in for an office visit because he’s concerned about his increasing blood sugar and wants to prevent further weight gain. His medications include metformin 1000 mg bid, lisinopril 5 mg/d, carvedilol 12.5 mg bid, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. He denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for Mr. P?
Discussion
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of his heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given his need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide;48 however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37-39 Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
CORRESPONDENCE
Katherine H. Saunders, MD, DABOM, Comprehensive Weight Control Center, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; kph2001@med.cornell.edu.
1. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
2. Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.
4. Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
5. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
6. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612-1619.
7. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
8. Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
9. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
10. US Food and Drug Administration. Drug approval package. Qsymia. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
11. Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. Available at: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
12. Drugs.com. Contrave approval history. Available at: https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
13. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
14. Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
15. Adipex-P package insert. Available at: http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
16. Ionamin package insert. Available at: http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
17. Lomaira package insert. Available at: https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
18. Suprenza package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
19. Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
20. Alli package labeling. Available at: http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
21. Xenical package insert. Available at: https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28, 2017.
22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
23. Qsymia package insert. Available at: https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
24. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
27. Belviq package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
28. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
29. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
30. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
31. Contrave package insert. Available at: https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
32. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
33. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
34. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
35. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
36. Saxenda package insert. Available at: http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
37. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
38. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
39. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
40. Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. [Epub ahead of print]
41. Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
42. Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. Available at: http://www.qsymiarems.com. Accessed January 16, 2017.
43. Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
44. US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
45. Belviq XR package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
46. Smith SR, O’Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
48. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
49. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4-6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14-39). These medications have the potential to not only limit weight gain, but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—namely family practitioners—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehensive treatment plan that includes diet, physical activity, and behavioral modification.
[polldaddy:9840472]
CASE 1 Melissa C, a 27-year-old woman with obesity (BMI 33 kg/m2), hyperlipidemia, and migraine headaches, presents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she’s taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for Ms. C?
Discussion
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .”
SIDEBAR
Before prescribing antiobesity medication . . .Have a frank discussion with the patient and be sure to cover the following points:
- The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
- Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
- Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
- Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30-34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24-26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Norman S, a 52-year-old overweight man (BMI 29 kg/m2) with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma, has recently hit a plateau with his weight loss. He lost 45 pounds secondary to diet and exercise, but hasn’t been able to lose any more. He also struggles with constant hunger. His medications include metformin 1000 mg bid, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until he undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
Mr. S is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
Discussion
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. He has worked hard to lose a significant number of pounds, and is now at high risk of regaining them. That’s because his appetite has increased with his new exercise regimen, but his energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of his opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
He could try orlistat, especially if he struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for his diabetes and may also promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOM-DM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of his initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomic testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 Kathryn M, a 38-year-old woman with obesity (BMI 42 kg/m2), obstructive sleep apnea, gastroesophageal reflux disease, and depression, is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
Ms. M smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discussion
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like Ms. M, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to Ms. M that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for Ms. M because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion could also help Ms. M quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses Ms. M’s problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg could also be used and should certainly be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 William P, a 65-year-old man with obesity (BMI 39 kg/m2) who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about his weight. He lost 100 lbs following surgery and maintained his weight for 3 years, but then regained 30 lbs. He comes in for an office visit because he’s concerned about his increasing blood sugar and wants to prevent further weight gain. His medications include metformin 1000 mg bid, lisinopril 5 mg/d, carvedilol 12.5 mg bid, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. He denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for Mr. P?
Discussion
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of his heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given his need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide;48 however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37-39 Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
CORRESPONDENCE
Katherine H. Saunders, MD, DABOM, Comprehensive Weight Control Center, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; kph2001@med.cornell.edu.
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4-6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14-39). These medications have the potential to not only limit weight gain, but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—namely family practitioners—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehensive treatment plan that includes diet, physical activity, and behavioral modification.
[polldaddy:9840472]
CASE 1 Melissa C, a 27-year-old woman with obesity (BMI 33 kg/m2), hyperlipidemia, and migraine headaches, presents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she’s taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for Ms. C?
Discussion
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .”
SIDEBAR
Before prescribing antiobesity medication . . .Have a frank discussion with the patient and be sure to cover the following points:
- The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
- Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
- Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
- Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30-34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24-26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Norman S, a 52-year-old overweight man (BMI 29 kg/m2) with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma, has recently hit a plateau with his weight loss. He lost 45 pounds secondary to diet and exercise, but hasn’t been able to lose any more. He also struggles with constant hunger. His medications include metformin 1000 mg bid, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until he undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
Mr. S is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
Discussion
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. He has worked hard to lose a significant number of pounds, and is now at high risk of regaining them. That’s because his appetite has increased with his new exercise regimen, but his energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of his opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
He could try orlistat, especially if he struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for his diabetes and may also promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOM-DM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of his initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomic testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 Kathryn M, a 38-year-old woman with obesity (BMI 42 kg/m2), obstructive sleep apnea, gastroesophageal reflux disease, and depression, is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
Ms. M smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discussion
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like Ms. M, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to Ms. M that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for Ms. M because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion could also help Ms. M quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses Ms. M’s problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg could also be used and should certainly be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 William P, a 65-year-old man with obesity (BMI 39 kg/m2) who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about his weight. He lost 100 lbs following surgery and maintained his weight for 3 years, but then regained 30 lbs. He comes in for an office visit because he’s concerned about his increasing blood sugar and wants to prevent further weight gain. His medications include metformin 1000 mg bid, lisinopril 5 mg/d, carvedilol 12.5 mg bid, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. He denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for Mr. P?
Discussion
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of his heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given his need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide;48 however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37-39 Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
CORRESPONDENCE
Katherine H. Saunders, MD, DABOM, Comprehensive Weight Control Center, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medicine, 1165 York Avenue, New York, NY 10065; kph2001@med.cornell.edu.
1. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
2. Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.
4. Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
5. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
6. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612-1619.
7. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
8. Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
9. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
10. US Food and Drug Administration. Drug approval package. Qsymia. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
11. Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. Available at: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
12. Drugs.com. Contrave approval history. Available at: https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
13. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
14. Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
15. Adipex-P package insert. Available at: http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
16. Ionamin package insert. Available at: http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
17. Lomaira package insert. Available at: https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
18. Suprenza package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
19. Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
20. Alli package labeling. Available at: http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
21. Xenical package insert. Available at: https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28, 2017.
22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
23. Qsymia package insert. Available at: https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
24. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
27. Belviq package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
28. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
29. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
30. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
31. Contrave package insert. Available at: https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
32. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
33. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
34. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
35. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
36. Saxenda package insert. Available at: http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
37. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
38. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
39. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
40. Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. [Epub ahead of print]
41. Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
42. Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. Available at: http://www.qsymiarems.com. Accessed January 16, 2017.
43. Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
44. US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
45. Belviq XR package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
46. Smith SR, O’Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
48. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
49. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
1. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
2. Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023.
4. Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
5. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
6. Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24:1612-1619.
7. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
8. Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
9. Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
10. US Food and Drug Administration. Drug approval package. Qsymia. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
11. Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQ® (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. Available at: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
12. Drugs.com. Contrave approval history. Available at: https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
13. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
14. Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
15. Adipex-P package insert. Available at: http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
16. Ionamin package insert. Available at: http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
17. Lomaira package insert. Available at: https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
18. Suprenza package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
19. Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
20. Alli package labeling. Available at: http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
21. Xenical package insert. Available at: https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28, 2017.
22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
23. Qsymia package insert. Available at: https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
24. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
25. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
26. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
27. Belviq package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
28. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
29. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
30. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
31. Contrave package insert. Available at: https://contrave.com/wp-content/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
32. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
33. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
34. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
35. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
36. Saxenda package insert. Available at: http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
37. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
38. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
39. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
40. Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. [Epub ahead of print]
41. Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
42. Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. Available at: http://www.qsymiarems.com. Accessed January 16, 2017.
43. Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
44. US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
45. Belviq XR package insert. Available at: https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
46. Smith SR, O’Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
47. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
48. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
49. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
From The Journal of Family Practice | 2017;66(10):608-616.
PRACTICE RECOMMENDATIONS
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
› Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
› Continue an antiobesity medication if it is deemed effective and well tolerated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
As cord blood inventory increases, use declines
Recently, the US has seen a decline in the use of cord blood in transplants, according to a study by the RAND Corporation.
The research revealed a significant increase in the national cord blood inventory but also suggested many cord blood units may go unused because they have a total nucleated cell (TNC) count of less than 1.25 billion cells per unit.
RAND researchers conducted this study by examining past research about cord blood banks, analyzing data on public cord blood bank collection and use, and interviewing stakeholders such as blood bank leaders and transplant center physicians.
The data showed the number of hematopoietic stem cell transplants (HSCTs) performed in the US has increased in recent years.
However, the percentage of HSCTs in which cord blood is used has declined, from about 12% (n=822) of all HSCTs in 2010 to about 8% (n=718) of all HSCTs in 2015.
At the same time, public cord blood banks in the US have greatly increased their inventory. This is, at least in part, because the federal government began offering subsidies to public contractor banks with the goal of helping them obtain 150,000 new units of high-quality cord blood.
The numeric goal has been met, as the inventory currently exceeds 200,000 units. However, many of the units cannot be considered high-quality because they have a TNC count of less than 1.25 billion cells.
The researchers noted that the government subsidies are not structured to discourage the processing and banking of cord blood units with lower TNC counts.
In fact, the team found that about half of the national inventory is made up of cord blood units with TNC counts of less than 1.25 billion cells. They said the probability that such a unit will be used in a given year is about 0.1%, and there’s an 11% chance it will ever be used.
On the other hand, there is a 1% to 3% chance a cord blood unit with a TNC count of more than 1.5 billion will be used in a given year, and there’s a 61% chance it will ever be used.
Therefore, the researchers recommend the federal government find ways to encourage public cord blood banks to collect samples with higher TNC counts, as they will help expand the utility of the national inventory.
Support for this research was provided by the US Department of Health and Human Services, Office of the Assistant Secretary of Health. ![]()
Recently, the US has seen a decline in the use of cord blood in transplants, according to a study by the RAND Corporation.
The research revealed a significant increase in the national cord blood inventory but also suggested many cord blood units may go unused because they have a total nucleated cell (TNC) count of less than 1.25 billion cells per unit.
RAND researchers conducted this study by examining past research about cord blood banks, analyzing data on public cord blood bank collection and use, and interviewing stakeholders such as blood bank leaders and transplant center physicians.
The data showed the number of hematopoietic stem cell transplants (HSCTs) performed in the US has increased in recent years.
However, the percentage of HSCTs in which cord blood is used has declined, from about 12% (n=822) of all HSCTs in 2010 to about 8% (n=718) of all HSCTs in 2015.
At the same time, public cord blood banks in the US have greatly increased their inventory. This is, at least in part, because the federal government began offering subsidies to public contractor banks with the goal of helping them obtain 150,000 new units of high-quality cord blood.
The numeric goal has been met, as the inventory currently exceeds 200,000 units. However, many of the units cannot be considered high-quality because they have a TNC count of less than 1.25 billion cells.
The researchers noted that the government subsidies are not structured to discourage the processing and banking of cord blood units with lower TNC counts.
In fact, the team found that about half of the national inventory is made up of cord blood units with TNC counts of less than 1.25 billion cells. They said the probability that such a unit will be used in a given year is about 0.1%, and there’s an 11% chance it will ever be used.
On the other hand, there is a 1% to 3% chance a cord blood unit with a TNC count of more than 1.5 billion will be used in a given year, and there’s a 61% chance it will ever be used.
Therefore, the researchers recommend the federal government find ways to encourage public cord blood banks to collect samples with higher TNC counts, as they will help expand the utility of the national inventory.
Support for this research was provided by the US Department of Health and Human Services, Office of the Assistant Secretary of Health. ![]()
Recently, the US has seen a decline in the use of cord blood in transplants, according to a study by the RAND Corporation.
The research revealed a significant increase in the national cord blood inventory but also suggested many cord blood units may go unused because they have a total nucleated cell (TNC) count of less than 1.25 billion cells per unit.
RAND researchers conducted this study by examining past research about cord blood banks, analyzing data on public cord blood bank collection and use, and interviewing stakeholders such as blood bank leaders and transplant center physicians.
The data showed the number of hematopoietic stem cell transplants (HSCTs) performed in the US has increased in recent years.
However, the percentage of HSCTs in which cord blood is used has declined, from about 12% (n=822) of all HSCTs in 2010 to about 8% (n=718) of all HSCTs in 2015.
At the same time, public cord blood banks in the US have greatly increased their inventory. This is, at least in part, because the federal government began offering subsidies to public contractor banks with the goal of helping them obtain 150,000 new units of high-quality cord blood.
The numeric goal has been met, as the inventory currently exceeds 200,000 units. However, many of the units cannot be considered high-quality because they have a TNC count of less than 1.25 billion cells.
The researchers noted that the government subsidies are not structured to discourage the processing and banking of cord blood units with lower TNC counts.
In fact, the team found that about half of the national inventory is made up of cord blood units with TNC counts of less than 1.25 billion cells. They said the probability that such a unit will be used in a given year is about 0.1%, and there’s an 11% chance it will ever be used.
On the other hand, there is a 1% to 3% chance a cord blood unit with a TNC count of more than 1.5 billion will be used in a given year, and there’s a 61% chance it will ever be used.
Therefore, the researchers recommend the federal government find ways to encourage public cord blood banks to collect samples with higher TNC counts, as they will help expand the utility of the national inventory.
Support for this research was provided by the US Department of Health and Human Services, Office of the Assistant Secretary of Health. ![]()
Confusion recurs 2 weeks after fall
A 77-year-old woman presented to the emergency department complaining of a headache following a syncopal episode (while standing) earlier that day. She said that she’d lost consciousness for several minutes, and then experienced several minutes of mild confusion that resolved spontaneously.
On physical exam, she was oriented to person and place, but not time. She had a contusion in her left occipitoparietal region without extensive bruising or deformity. The patient had normal cardiopulmonary, abdominal, and neurologic exams. Her past medical history included hypertension and normal pressure hydrocephalus, and her vital signs were within normal limits. She was taking aspirin once daily.The patient’s initial head and neck computerized tomography (CT) scans were normal (FIGURE 1), but she was hospitalized because of her confusion. During her hospitalization, the patient had mild episodic headaches that resolved with acetaminophen. The next day, her confusion resolved, and repeat CT scans were unchanged. She was discharged within 24 hours.
Two weeks later, the patient returned to the hospital after her daughter found her on the toilet, unable to stand up from the sitting position. She was confused and experienced a worsening of headache during transport to the hospital. No recurrent falls or additional episodes of trauma were reported. A CT scan was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Delayed acute subdural hematoma
The CT scan (FIGURE 2) revealed an acute on chronic large left frontotemporoparietal and a right frontoparietal subdural hematoma (SDH) with resultant left to right subfalcine herniation. The patient was given a diagnosis of a delayed acute subdural hematoma (DASH)—an acute subdural hematoma that is not apparent on an initial CT scan, but is detected on follow-up CT imaging days or weeks after the injury.1 The incidence of DASH is approximately 0.5% among acute SDH patients who require operative treatment.1
Because DASH is rare, there is a lot of uncertainty surrounding its presentation, pathophysiology, and outcomes. In the few cases that have been described, patients have varied from those who were healthy, and had no coagulation abnormalities, to those who were elderly and taking anticoagulants.2,3 In addition, the period between the head injury and the development of SDH is variable.3
While not much is known about DASH, the mechanism of acute SDH has been widely studied and researched. Acute SDH, which typically follows a head trauma, results from the tearing of bridging veins that lack supporting structures and are most vulnerable to injury when crossing the subdural space.4 The potential pathophysiology for DASH is not completely understood, but is likely to involve subtle damage to the bridging veins of the brain that continue to leak over a matter of hours and days.1,5
Two risk factors to consider. Increasing age and use of oral anticoagulants can increase the risk of developing an intracranial lesion after head injury.3 Due to the infrequency of DASH, the same risk factors for SDH should be considered for DASH. These factors make it increasingly important to establish guidelines on how to approach mild traumatic brain injury (TBI) in both DASH and SDH, especially for those who are elderly or have been on anticoagulation therapy.
Differential Dx
The differential diagnosis for our patient’s decline and altered mentation weeks after the initial event included worsening normal pressure hydrocephalus, cerebrovascular accident, and seizure.
Normal pressure hydrocephalus typically has a more chronic onset than DASH. It manifests with the classic triad of dementia, incontinence, and magnetic or festinating gait (“wild, wet, and wobbly”).
Cerebrovascular accidents are most often associated with focal neurologic deficits, which can be ischemic or hemorrhagic. If hemorrhagic, the hemorrhage is typically parenchymal and not subdural.
Seizure, especially partial complex seizure, can arise after trauma and may not involve obvious motor movements. Symptoms generally abate over a few minutes to hours with treatment. Electroencephalogram and CT scan can differentiate seizure from a subdural hematoma.
Keep DASH on your radar screen
The American College of Emergency Physicians states that a non-contrast head CT scan is indicated in head trauma patients with loss of consciousness if one or more of the following is present: age >60 years, vomiting, headache, drug or alcohol intoxication, short-term memory deficits, posttraumatic seizure, Glasgow Coma Scale score of <15, focal neurologic deficits, and coagulopathy.6
Some have suggested that the initial head CT scan be delayed by up to 8 hours to prevent missing a slowly developing intracranial hemorrhage. Others suggest that the CT scan be repeated at 24 hours. Still others have suggested that patients with even mild TBI be admitted for a period of observation if any risk factors, such as age or history of anticoagulation therapy, are noted.
Because there is no evidence to support delaying the initial head CT scan, physicians should be thorough in their evaluation of head trauma patients with loss of consciousness and consider a repeat CT scan of the head if worsening of any symptoms occurs. Physicians should also consider a repeat CT scan of the head for patients at high risk, including the elderly and those who have taken anticoagulants.
In addition, patients with traumatic head injuries must be properly counseled to return if they experience repeated vomiting, worsening headache, memory loss, confusion, focal neurologic deficit, abnormal behavior, increased sleepiness, or seizures.6 An extra precaution for high-risk patients includes suggesting adequate follow-up with a primary care physician to help monitor recovery and prevent any occurrences of DASH from going unnoticed.
Our patient underwent a mini-craniotomy. Postoperatively, she was discharged to a skilled nursing facility and ultimately made a complete recovery.
CORRESPONDENCE
Andrew Muck, MD, Department of Emergency Medicine, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MSC 7736, San Antonio, TX 78229; muck@uthscsa.edu.
1. Cohen T, Gudeman S. In: Narayan RK, ed. Delayed Traumatic Intracranial Hematoma. Neurotrauma. New York, NY: McGraw-Hill;1995:689-701.
2. Matsuda W, Sugimoto K, Sato N, et al. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: a case report and brief review. J Trauma. 2008;65:461-463.
3. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851-E856.
4. Culotta VP, Sermentilli ME, Gerold K, et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245-250.
5. Shabani S, Nguyen HS, Doan N, et al. Case Report and Review of Literature of Delayed Acute Subdural Hematoma. World Neurosurg. 2016;96:66-71.
6. Jagoda AS, Bazarian JJ, Bruns Jr JJ, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714-748.
A 77-year-old woman presented to the emergency department complaining of a headache following a syncopal episode (while standing) earlier that day. She said that she’d lost consciousness for several minutes, and then experienced several minutes of mild confusion that resolved spontaneously.
On physical exam, she was oriented to person and place, but not time. She had a contusion in her left occipitoparietal region without extensive bruising or deformity. The patient had normal cardiopulmonary, abdominal, and neurologic exams. Her past medical history included hypertension and normal pressure hydrocephalus, and her vital signs were within normal limits. She was taking aspirin once daily.The patient’s initial head and neck computerized tomography (CT) scans were normal (FIGURE 1), but she was hospitalized because of her confusion. During her hospitalization, the patient had mild episodic headaches that resolved with acetaminophen. The next day, her confusion resolved, and repeat CT scans were unchanged. She was discharged within 24 hours.
Two weeks later, the patient returned to the hospital after her daughter found her on the toilet, unable to stand up from the sitting position. She was confused and experienced a worsening of headache during transport to the hospital. No recurrent falls or additional episodes of trauma were reported. A CT scan was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Delayed acute subdural hematoma
The CT scan (FIGURE 2) revealed an acute on chronic large left frontotemporoparietal and a right frontoparietal subdural hematoma (SDH) with resultant left to right subfalcine herniation. The patient was given a diagnosis of a delayed acute subdural hematoma (DASH)—an acute subdural hematoma that is not apparent on an initial CT scan, but is detected on follow-up CT imaging days or weeks after the injury.1 The incidence of DASH is approximately 0.5% among acute SDH patients who require operative treatment.1
Because DASH is rare, there is a lot of uncertainty surrounding its presentation, pathophysiology, and outcomes. In the few cases that have been described, patients have varied from those who were healthy, and had no coagulation abnormalities, to those who were elderly and taking anticoagulants.2,3 In addition, the period between the head injury and the development of SDH is variable.3
While not much is known about DASH, the mechanism of acute SDH has been widely studied and researched. Acute SDH, which typically follows a head trauma, results from the tearing of bridging veins that lack supporting structures and are most vulnerable to injury when crossing the subdural space.4 The potential pathophysiology for DASH is not completely understood, but is likely to involve subtle damage to the bridging veins of the brain that continue to leak over a matter of hours and days.1,5
Two risk factors to consider. Increasing age and use of oral anticoagulants can increase the risk of developing an intracranial lesion after head injury.3 Due to the infrequency of DASH, the same risk factors for SDH should be considered for DASH. These factors make it increasingly important to establish guidelines on how to approach mild traumatic brain injury (TBI) in both DASH and SDH, especially for those who are elderly or have been on anticoagulation therapy.
Differential Dx
The differential diagnosis for our patient’s decline and altered mentation weeks after the initial event included worsening normal pressure hydrocephalus, cerebrovascular accident, and seizure.
Normal pressure hydrocephalus typically has a more chronic onset than DASH. It manifests with the classic triad of dementia, incontinence, and magnetic or festinating gait (“wild, wet, and wobbly”).
Cerebrovascular accidents are most often associated with focal neurologic deficits, which can be ischemic or hemorrhagic. If hemorrhagic, the hemorrhage is typically parenchymal and not subdural.
Seizure, especially partial complex seizure, can arise after trauma and may not involve obvious motor movements. Symptoms generally abate over a few minutes to hours with treatment. Electroencephalogram and CT scan can differentiate seizure from a subdural hematoma.
Keep DASH on your radar screen
The American College of Emergency Physicians states that a non-contrast head CT scan is indicated in head trauma patients with loss of consciousness if one or more of the following is present: age >60 years, vomiting, headache, drug or alcohol intoxication, short-term memory deficits, posttraumatic seizure, Glasgow Coma Scale score of <15, focal neurologic deficits, and coagulopathy.6
Some have suggested that the initial head CT scan be delayed by up to 8 hours to prevent missing a slowly developing intracranial hemorrhage. Others suggest that the CT scan be repeated at 24 hours. Still others have suggested that patients with even mild TBI be admitted for a period of observation if any risk factors, such as age or history of anticoagulation therapy, are noted.
Because there is no evidence to support delaying the initial head CT scan, physicians should be thorough in their evaluation of head trauma patients with loss of consciousness and consider a repeat CT scan of the head if worsening of any symptoms occurs. Physicians should also consider a repeat CT scan of the head for patients at high risk, including the elderly and those who have taken anticoagulants.
In addition, patients with traumatic head injuries must be properly counseled to return if they experience repeated vomiting, worsening headache, memory loss, confusion, focal neurologic deficit, abnormal behavior, increased sleepiness, or seizures.6 An extra precaution for high-risk patients includes suggesting adequate follow-up with a primary care physician to help monitor recovery and prevent any occurrences of DASH from going unnoticed.
Our patient underwent a mini-craniotomy. Postoperatively, she was discharged to a skilled nursing facility and ultimately made a complete recovery.
CORRESPONDENCE
Andrew Muck, MD, Department of Emergency Medicine, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MSC 7736, San Antonio, TX 78229; muck@uthscsa.edu.
A 77-year-old woman presented to the emergency department complaining of a headache following a syncopal episode (while standing) earlier that day. She said that she’d lost consciousness for several minutes, and then experienced several minutes of mild confusion that resolved spontaneously.
On physical exam, she was oriented to person and place, but not time. She had a contusion in her left occipitoparietal region without extensive bruising or deformity. The patient had normal cardiopulmonary, abdominal, and neurologic exams. Her past medical history included hypertension and normal pressure hydrocephalus, and her vital signs were within normal limits. She was taking aspirin once daily.The patient’s initial head and neck computerized tomography (CT) scans were normal (FIGURE 1), but she was hospitalized because of her confusion. During her hospitalization, the patient had mild episodic headaches that resolved with acetaminophen. The next day, her confusion resolved, and repeat CT scans were unchanged. She was discharged within 24 hours.
Two weeks later, the patient returned to the hospital after her daughter found her on the toilet, unable to stand up from the sitting position. She was confused and experienced a worsening of headache during transport to the hospital. No recurrent falls or additional episodes of trauma were reported. A CT scan was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Delayed acute subdural hematoma
The CT scan (FIGURE 2) revealed an acute on chronic large left frontotemporoparietal and a right frontoparietal subdural hematoma (SDH) with resultant left to right subfalcine herniation. The patient was given a diagnosis of a delayed acute subdural hematoma (DASH)—an acute subdural hematoma that is not apparent on an initial CT scan, but is detected on follow-up CT imaging days or weeks after the injury.1 The incidence of DASH is approximately 0.5% among acute SDH patients who require operative treatment.1
Because DASH is rare, there is a lot of uncertainty surrounding its presentation, pathophysiology, and outcomes. In the few cases that have been described, patients have varied from those who were healthy, and had no coagulation abnormalities, to those who were elderly and taking anticoagulants.2,3 In addition, the period between the head injury and the development of SDH is variable.3
While not much is known about DASH, the mechanism of acute SDH has been widely studied and researched. Acute SDH, which typically follows a head trauma, results from the tearing of bridging veins that lack supporting structures and are most vulnerable to injury when crossing the subdural space.4 The potential pathophysiology for DASH is not completely understood, but is likely to involve subtle damage to the bridging veins of the brain that continue to leak over a matter of hours and days.1,5
Two risk factors to consider. Increasing age and use of oral anticoagulants can increase the risk of developing an intracranial lesion after head injury.3 Due to the infrequency of DASH, the same risk factors for SDH should be considered for DASH. These factors make it increasingly important to establish guidelines on how to approach mild traumatic brain injury (TBI) in both DASH and SDH, especially for those who are elderly or have been on anticoagulation therapy.
Differential Dx
The differential diagnosis for our patient’s decline and altered mentation weeks after the initial event included worsening normal pressure hydrocephalus, cerebrovascular accident, and seizure.
Normal pressure hydrocephalus typically has a more chronic onset than DASH. It manifests with the classic triad of dementia, incontinence, and magnetic or festinating gait (“wild, wet, and wobbly”).
Cerebrovascular accidents are most often associated with focal neurologic deficits, which can be ischemic or hemorrhagic. If hemorrhagic, the hemorrhage is typically parenchymal and not subdural.
Seizure, especially partial complex seizure, can arise after trauma and may not involve obvious motor movements. Symptoms generally abate over a few minutes to hours with treatment. Electroencephalogram and CT scan can differentiate seizure from a subdural hematoma.
Keep DASH on your radar screen
The American College of Emergency Physicians states that a non-contrast head CT scan is indicated in head trauma patients with loss of consciousness if one or more of the following is present: age >60 years, vomiting, headache, drug or alcohol intoxication, short-term memory deficits, posttraumatic seizure, Glasgow Coma Scale score of <15, focal neurologic deficits, and coagulopathy.6
Some have suggested that the initial head CT scan be delayed by up to 8 hours to prevent missing a slowly developing intracranial hemorrhage. Others suggest that the CT scan be repeated at 24 hours. Still others have suggested that patients with even mild TBI be admitted for a period of observation if any risk factors, such as age or history of anticoagulation therapy, are noted.
Because there is no evidence to support delaying the initial head CT scan, physicians should be thorough in their evaluation of head trauma patients with loss of consciousness and consider a repeat CT scan of the head if worsening of any symptoms occurs. Physicians should also consider a repeat CT scan of the head for patients at high risk, including the elderly and those who have taken anticoagulants.
In addition, patients with traumatic head injuries must be properly counseled to return if they experience repeated vomiting, worsening headache, memory loss, confusion, focal neurologic deficit, abnormal behavior, increased sleepiness, or seizures.6 An extra precaution for high-risk patients includes suggesting adequate follow-up with a primary care physician to help monitor recovery and prevent any occurrences of DASH from going unnoticed.
Our patient underwent a mini-craniotomy. Postoperatively, she was discharged to a skilled nursing facility and ultimately made a complete recovery.
CORRESPONDENCE
Andrew Muck, MD, Department of Emergency Medicine, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MSC 7736, San Antonio, TX 78229; muck@uthscsa.edu.
1. Cohen T, Gudeman S. In: Narayan RK, ed. Delayed Traumatic Intracranial Hematoma. Neurotrauma. New York, NY: McGraw-Hill;1995:689-701.
2. Matsuda W, Sugimoto K, Sato N, et al. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: a case report and brief review. J Trauma. 2008;65:461-463.
3. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851-E856.
4. Culotta VP, Sermentilli ME, Gerold K, et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245-250.
5. Shabani S, Nguyen HS, Doan N, et al. Case Report and Review of Literature of Delayed Acute Subdural Hematoma. World Neurosurg. 2016;96:66-71.
6. Jagoda AS, Bazarian JJ, Bruns Jr JJ, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714-748.
1. Cohen T, Gudeman S. In: Narayan RK, ed. Delayed Traumatic Intracranial Hematoma. Neurotrauma. New York, NY: McGraw-Hill;1995:689-701.
2. Matsuda W, Sugimoto K, Sato N, et al. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: a case report and brief review. J Trauma. 2008;65:461-463.
3. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851-E856.
4. Culotta VP, Sermentilli ME, Gerold K, et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245-250.
5. Shabani S, Nguyen HS, Doan N, et al. Case Report and Review of Literature of Delayed Acute Subdural Hematoma. World Neurosurg. 2016;96:66-71.
6. Jagoda AS, Bazarian JJ, Bruns Jr JJ, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714-748.
Fever, rash, and leukopenia in a 32-year-old man • Dx?
THE CASE
A 32-year-old man was admitted to our hospital with fever, chills, malaise, leukopenia, and a rash. About 3 weeks earlier, he’d had oral maxillofacial surgery and started a 10-day course of prophylactic amoxicillin/clavulanic acid. Fifteen days after the surgery, he developed a fever (temperature, 103˚ F), chills, arthralgia, myalgia, cough, diarrhea, and malaise. He was seen by his physician, who obtained a chest x-ray showing a lingular infiltrate. The physician diagnosed influenza and pneumonia in this patient, and prescribed oseltamivir, azithromycin, and an additional course of amoxicillin/clavulanic acid.
Upon admission to the hospital, laboratory tests revealed a white blood cell count (WBC) of 3.1 k/mcL (normal: 3.2-10.8 k/mcL). The patient’s physical examination was notable for lip edema, white mucous membrane plaques, submandibular and inguinal lymphadenopathy, and a morbilliform rash across his chest (FIGURE 1). Broad-spectrum antibiotics were initiated for presumed sepsis.
On hospital day (HD) 1, tests revealed a WBC count of 1.8 k/mcL, an erythrocyte sedimentation rate of 53 mm/hr (normal: 20-30 mm/hr for women, 15-20 mm/hr for men), and a C-reactive protein level of 6.7 mg/dL (normal: <0.5 mg/dL). A repeat chest x-ray and orofacial computerized tomography scan were normal.
By HD 3, all bacterial cultures were negative, but the patient was positive for human herpesvirus (HHV)-6 on viral cultures. His leukopenia persisted and he had elevated levels of alanine transaminase ranging from 40 to 73 U/L (normal: 6-43 U/L) and aspartate aminotransferase ranging from 66 to 108 U/L (normal range: 10-40 U/L), both downtrending during his hospitalization. He also had elevated levels of antinuclear antibodies (ANAs) and anti-Smith (Sm) antibody titers.
A posterior-auricular biopsy was consistent with lymphocytic perivasculitis. The rash continued to progress, involving his chest, abdomen, and face (FIGURE 2). Bacterial and viral cultures remained negative and on HD 4, broad-spectrum antibiotics were discontinued.
THE DIAGNOSIS
We diagnosed the patient with DRESS (drug reaction with eosinophilia and systemic symptoms) based on persistent fever, onset of cutaneous manifestations (facial edema and morbilliform eruption), lymphadenopathy, increased liver function tests, and recent exposure to an offending drug. The patient did not have eosinophilia; however, atypical lymphocytes were present on his peripheral smear.
DISCUSSION
DRESS is typically characterized by fever, rash, eosinophilia, atypical lymphocytes, lymphadenopathy, and organ involvement (primarily liver, but multiple organ systems can be affected).1 Patients with severe symptoms have renal involvement, anemia, respiratory and cardiac symptoms (chest pain, tachycardia, and myocarditis), and transaminase levels up to 5 times greater than normal.1-3 Anticonvulsants and antibiotics are the most common offending classes among the medications that are associated with DRESS (TABLE 1).2,4
The reported incidence of DRESS is between one in 1000 and one in 10,000 drug exposures.1 Due to the broad presentation and a lack of established diagnostic criteria associated with DRESS, this number may be even higher. DRESS has a 10% mortality rate,1 and hepatic necrosis is the most common cause of death.2
Certain people may be more prone to DRESS. People with certain gene mutations that code for drug detoxification enzymes have shown a greater incidence of DRESS.5 Viral reactivation, commonly of HHV-6, has also been shown to have an effect on the pathogenesis of DRESS. Additionally, genetic predisposition involving specific human leukocyte antigens (HLAs) makes certain people more prone to the development of DRESS (TABLE 2).2,5
Case reports have demonstrated a link between certain autoimmune syndromes and DRESS, specifically Grave’s disease and type 1 diabetes mellitus.2
A unique finding of this case was the presence of elevated ANA and anti-Sm antibody titers at initial presentation, with spontaneous negative seroconversion 2 months later. Because of these 2 findings, as well as the patient’s leukopenia and rash, he briefly met 4 of the 11 criteria set forth by the American College of Rheumatology for a diagnosis of systemic lupus erythematosus (SLE).6 It is unclear whether the transiently elevated anti-Sm antibody titers were an acute phase reactant due to DRESS, a viral illness, or an evolving autoimmune process.
The false-positive rate for anti-Sm antibodies in association with DRESS has not been previously reported.
MAKING THE DIAGNOSIS
Distinguishing DRESS from other life-threatening cutaneous drug reactions, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, can be difficult. Likewise, acute bacterial/viral infections, autoimmune syndromes, vasculitis, and hematologic diseases can mimic DRESS.7 Exposure to an offending drug 2 to 6 weeks prior to the onset of symptoms is supportive of DRESS.
This scoring system can help. The RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) has developed a scoring system to aid in the accurate diagnosis of DRESS.1,8 The scoring consists of 8 categories: fever, eosinophilia, enlarged lymph nodes, atypical lymphocytes, skin involvement, organ involvement, time of resolution, and the evaluation of other potential causes.1 Each category is graded a number from -1 (not supportive of DRESS) to 2 (highly supportive of DRESS) based on the patient’s presentation. The total score grades potential cases as “no,” “possible,” “probable,” or “definite.”1,8 In one review, cases classified as “probable” or “definite” by the RegiSCAR scoring system constituted 88% of the cases reported in the literature.1
Two tests that can also aid in the diagnosis of DRESS include patch testing (exposing the skin to a diluted version of the suspected offending drug and observing for a local reaction) and lymphocyte transformation tests. The latter are a better method of diagnosing drug-induced DRESS, with a sensitivity of 60% to 70%, and a specificity of 85%.9 However, this testing is not readily available.
Once DRESS is diagnosed, the offending drug should be immediately discontinued. For mild cases, supportive treatment is recommended. For more severe cases, the use of corticosteroids tapered over several months is the treatment of choice.10 Further studies are needed to determine the optimal type of corticosteroids, as well as the dose, route, and duration of therapy. Immunotherapy, plasmapheresis, and antivirals have been used with mixed results.10,11
Our patient was started on topical and systemic oral corticosteroids. Within 24 hours, his fever resolved and his rash improved. By HD 7, his laboratory values were normal and he was discharged.
The patient was advised that in the future, he should avoid exposure to the penicillin class of medication.
THE TAKEAWAY
The presence of rash, fever, lymphadenopathy, eosinophilia, atypical lymphocytes, liver involvement, and HHV-6 reactivation in the absence of sepsis should raise suspicion for DRESS. Early diagnosis, discontinuation of the culprit drug, and timely treatment are imperative in the management of the condition. Due to a potential genetic predisposition to DRESS, clinicians should use caution when treating first-degree family members with the same class of medication that was problematic for their relative. Long-term sequelae, such as Grave’s disease and diabetes mellitus, have been reported following DRESS. Therefore, long-term monitoring with appropriate testing is recommended.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
3. Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequelae of DRESS-2 cases. J Am Acad Dermatol. 2011;65:889-890.
4. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1-21.
5. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity and the HLA complex. Pharmaceuticals (Basel). 2011;4:69-90.
6. American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at: https://www.rheumatology.org/Portals/0/Files/1982%20SLE%20Classification_Excerpt.pdf. Accessed August 30, 2017.
7. Descamps V, Ben Saïd B, Sassolas B, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2010;137:703-708.
8. Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
9. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820.
10. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome part II: management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
11. Funck-Brentano E, Duong TA, Bouvresses S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252.
THE CASE
A 32-year-old man was admitted to our hospital with fever, chills, malaise, leukopenia, and a rash. About 3 weeks earlier, he’d had oral maxillofacial surgery and started a 10-day course of prophylactic amoxicillin/clavulanic acid. Fifteen days after the surgery, he developed a fever (temperature, 103˚ F), chills, arthralgia, myalgia, cough, diarrhea, and malaise. He was seen by his physician, who obtained a chest x-ray showing a lingular infiltrate. The physician diagnosed influenza and pneumonia in this patient, and prescribed oseltamivir, azithromycin, and an additional course of amoxicillin/clavulanic acid.
Upon admission to the hospital, laboratory tests revealed a white blood cell count (WBC) of 3.1 k/mcL (normal: 3.2-10.8 k/mcL). The patient’s physical examination was notable for lip edema, white mucous membrane plaques, submandibular and inguinal lymphadenopathy, and a morbilliform rash across his chest (FIGURE 1). Broad-spectrum antibiotics were initiated for presumed sepsis.
On hospital day (HD) 1, tests revealed a WBC count of 1.8 k/mcL, an erythrocyte sedimentation rate of 53 mm/hr (normal: 20-30 mm/hr for women, 15-20 mm/hr for men), and a C-reactive protein level of 6.7 mg/dL (normal: <0.5 mg/dL). A repeat chest x-ray and orofacial computerized tomography scan were normal.
By HD 3, all bacterial cultures were negative, but the patient was positive for human herpesvirus (HHV)-6 on viral cultures. His leukopenia persisted and he had elevated levels of alanine transaminase ranging from 40 to 73 U/L (normal: 6-43 U/L) and aspartate aminotransferase ranging from 66 to 108 U/L (normal range: 10-40 U/L), both downtrending during his hospitalization. He also had elevated levels of antinuclear antibodies (ANAs) and anti-Smith (Sm) antibody titers.
A posterior-auricular biopsy was consistent with lymphocytic perivasculitis. The rash continued to progress, involving his chest, abdomen, and face (FIGURE 2). Bacterial and viral cultures remained negative and on HD 4, broad-spectrum antibiotics were discontinued.
THE DIAGNOSIS
We diagnosed the patient with DRESS (drug reaction with eosinophilia and systemic symptoms) based on persistent fever, onset of cutaneous manifestations (facial edema and morbilliform eruption), lymphadenopathy, increased liver function tests, and recent exposure to an offending drug. The patient did not have eosinophilia; however, atypical lymphocytes were present on his peripheral smear.
DISCUSSION
DRESS is typically characterized by fever, rash, eosinophilia, atypical lymphocytes, lymphadenopathy, and organ involvement (primarily liver, but multiple organ systems can be affected).1 Patients with severe symptoms have renal involvement, anemia, respiratory and cardiac symptoms (chest pain, tachycardia, and myocarditis), and transaminase levels up to 5 times greater than normal.1-3 Anticonvulsants and antibiotics are the most common offending classes among the medications that are associated with DRESS (TABLE 1).2,4
The reported incidence of DRESS is between one in 1000 and one in 10,000 drug exposures.1 Due to the broad presentation and a lack of established diagnostic criteria associated with DRESS, this number may be even higher. DRESS has a 10% mortality rate,1 and hepatic necrosis is the most common cause of death.2
Certain people may be more prone to DRESS. People with certain gene mutations that code for drug detoxification enzymes have shown a greater incidence of DRESS.5 Viral reactivation, commonly of HHV-6, has also been shown to have an effect on the pathogenesis of DRESS. Additionally, genetic predisposition involving specific human leukocyte antigens (HLAs) makes certain people more prone to the development of DRESS (TABLE 2).2,5
Case reports have demonstrated a link between certain autoimmune syndromes and DRESS, specifically Grave’s disease and type 1 diabetes mellitus.2
A unique finding of this case was the presence of elevated ANA and anti-Sm antibody titers at initial presentation, with spontaneous negative seroconversion 2 months later. Because of these 2 findings, as well as the patient’s leukopenia and rash, he briefly met 4 of the 11 criteria set forth by the American College of Rheumatology for a diagnosis of systemic lupus erythematosus (SLE).6 It is unclear whether the transiently elevated anti-Sm antibody titers were an acute phase reactant due to DRESS, a viral illness, or an evolving autoimmune process.
The false-positive rate for anti-Sm antibodies in association with DRESS has not been previously reported.
MAKING THE DIAGNOSIS
Distinguishing DRESS from other life-threatening cutaneous drug reactions, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, can be difficult. Likewise, acute bacterial/viral infections, autoimmune syndromes, vasculitis, and hematologic diseases can mimic DRESS.7 Exposure to an offending drug 2 to 6 weeks prior to the onset of symptoms is supportive of DRESS.
This scoring system can help. The RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) has developed a scoring system to aid in the accurate diagnosis of DRESS.1,8 The scoring consists of 8 categories: fever, eosinophilia, enlarged lymph nodes, atypical lymphocytes, skin involvement, organ involvement, time of resolution, and the evaluation of other potential causes.1 Each category is graded a number from -1 (not supportive of DRESS) to 2 (highly supportive of DRESS) based on the patient’s presentation. The total score grades potential cases as “no,” “possible,” “probable,” or “definite.”1,8 In one review, cases classified as “probable” or “definite” by the RegiSCAR scoring system constituted 88% of the cases reported in the literature.1
Two tests that can also aid in the diagnosis of DRESS include patch testing (exposing the skin to a diluted version of the suspected offending drug and observing for a local reaction) and lymphocyte transformation tests. The latter are a better method of diagnosing drug-induced DRESS, with a sensitivity of 60% to 70%, and a specificity of 85%.9 However, this testing is not readily available.
Once DRESS is diagnosed, the offending drug should be immediately discontinued. For mild cases, supportive treatment is recommended. For more severe cases, the use of corticosteroids tapered over several months is the treatment of choice.10 Further studies are needed to determine the optimal type of corticosteroids, as well as the dose, route, and duration of therapy. Immunotherapy, plasmapheresis, and antivirals have been used with mixed results.10,11
Our patient was started on topical and systemic oral corticosteroids. Within 24 hours, his fever resolved and his rash improved. By HD 7, his laboratory values were normal and he was discharged.
The patient was advised that in the future, he should avoid exposure to the penicillin class of medication.
THE TAKEAWAY
The presence of rash, fever, lymphadenopathy, eosinophilia, atypical lymphocytes, liver involvement, and HHV-6 reactivation in the absence of sepsis should raise suspicion for DRESS. Early diagnosis, discontinuation of the culprit drug, and timely treatment are imperative in the management of the condition. Due to a potential genetic predisposition to DRESS, clinicians should use caution when treating first-degree family members with the same class of medication that was problematic for their relative. Long-term sequelae, such as Grave’s disease and diabetes mellitus, have been reported following DRESS. Therefore, long-term monitoring with appropriate testing is recommended.
THE CASE
A 32-year-old man was admitted to our hospital with fever, chills, malaise, leukopenia, and a rash. About 3 weeks earlier, he’d had oral maxillofacial surgery and started a 10-day course of prophylactic amoxicillin/clavulanic acid. Fifteen days after the surgery, he developed a fever (temperature, 103˚ F), chills, arthralgia, myalgia, cough, diarrhea, and malaise. He was seen by his physician, who obtained a chest x-ray showing a lingular infiltrate. The physician diagnosed influenza and pneumonia in this patient, and prescribed oseltamivir, azithromycin, and an additional course of amoxicillin/clavulanic acid.
Upon admission to the hospital, laboratory tests revealed a white blood cell count (WBC) of 3.1 k/mcL (normal: 3.2-10.8 k/mcL). The patient’s physical examination was notable for lip edema, white mucous membrane plaques, submandibular and inguinal lymphadenopathy, and a morbilliform rash across his chest (FIGURE 1). Broad-spectrum antibiotics were initiated for presumed sepsis.
On hospital day (HD) 1, tests revealed a WBC count of 1.8 k/mcL, an erythrocyte sedimentation rate of 53 mm/hr (normal: 20-30 mm/hr for women, 15-20 mm/hr for men), and a C-reactive protein level of 6.7 mg/dL (normal: <0.5 mg/dL). A repeat chest x-ray and orofacial computerized tomography scan were normal.
By HD 3, all bacterial cultures were negative, but the patient was positive for human herpesvirus (HHV)-6 on viral cultures. His leukopenia persisted and he had elevated levels of alanine transaminase ranging from 40 to 73 U/L (normal: 6-43 U/L) and aspartate aminotransferase ranging from 66 to 108 U/L (normal range: 10-40 U/L), both downtrending during his hospitalization. He also had elevated levels of antinuclear antibodies (ANAs) and anti-Smith (Sm) antibody titers.
A posterior-auricular biopsy was consistent with lymphocytic perivasculitis. The rash continued to progress, involving his chest, abdomen, and face (FIGURE 2). Bacterial and viral cultures remained negative and on HD 4, broad-spectrum antibiotics were discontinued.
THE DIAGNOSIS
We diagnosed the patient with DRESS (drug reaction with eosinophilia and systemic symptoms) based on persistent fever, onset of cutaneous manifestations (facial edema and morbilliform eruption), lymphadenopathy, increased liver function tests, and recent exposure to an offending drug. The patient did not have eosinophilia; however, atypical lymphocytes were present on his peripheral smear.
DISCUSSION
DRESS is typically characterized by fever, rash, eosinophilia, atypical lymphocytes, lymphadenopathy, and organ involvement (primarily liver, but multiple organ systems can be affected).1 Patients with severe symptoms have renal involvement, anemia, respiratory and cardiac symptoms (chest pain, tachycardia, and myocarditis), and transaminase levels up to 5 times greater than normal.1-3 Anticonvulsants and antibiotics are the most common offending classes among the medications that are associated with DRESS (TABLE 1).2,4
The reported incidence of DRESS is between one in 1000 and one in 10,000 drug exposures.1 Due to the broad presentation and a lack of established diagnostic criteria associated with DRESS, this number may be even higher. DRESS has a 10% mortality rate,1 and hepatic necrosis is the most common cause of death.2
Certain people may be more prone to DRESS. People with certain gene mutations that code for drug detoxification enzymes have shown a greater incidence of DRESS.5 Viral reactivation, commonly of HHV-6, has also been shown to have an effect on the pathogenesis of DRESS. Additionally, genetic predisposition involving specific human leukocyte antigens (HLAs) makes certain people more prone to the development of DRESS (TABLE 2).2,5
Case reports have demonstrated a link between certain autoimmune syndromes and DRESS, specifically Grave’s disease and type 1 diabetes mellitus.2
A unique finding of this case was the presence of elevated ANA and anti-Sm antibody titers at initial presentation, with spontaneous negative seroconversion 2 months later. Because of these 2 findings, as well as the patient’s leukopenia and rash, he briefly met 4 of the 11 criteria set forth by the American College of Rheumatology for a diagnosis of systemic lupus erythematosus (SLE).6 It is unclear whether the transiently elevated anti-Sm antibody titers were an acute phase reactant due to DRESS, a viral illness, or an evolving autoimmune process.
The false-positive rate for anti-Sm antibodies in association with DRESS has not been previously reported.
MAKING THE DIAGNOSIS
Distinguishing DRESS from other life-threatening cutaneous drug reactions, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, can be difficult. Likewise, acute bacterial/viral infections, autoimmune syndromes, vasculitis, and hematologic diseases can mimic DRESS.7 Exposure to an offending drug 2 to 6 weeks prior to the onset of symptoms is supportive of DRESS.
This scoring system can help. The RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) has developed a scoring system to aid in the accurate diagnosis of DRESS.1,8 The scoring consists of 8 categories: fever, eosinophilia, enlarged lymph nodes, atypical lymphocytes, skin involvement, organ involvement, time of resolution, and the evaluation of other potential causes.1 Each category is graded a number from -1 (not supportive of DRESS) to 2 (highly supportive of DRESS) based on the patient’s presentation. The total score grades potential cases as “no,” “possible,” “probable,” or “definite.”1,8 In one review, cases classified as “probable” or “definite” by the RegiSCAR scoring system constituted 88% of the cases reported in the literature.1
Two tests that can also aid in the diagnosis of DRESS include patch testing (exposing the skin to a diluted version of the suspected offending drug and observing for a local reaction) and lymphocyte transformation tests. The latter are a better method of diagnosing drug-induced DRESS, with a sensitivity of 60% to 70%, and a specificity of 85%.9 However, this testing is not readily available.
Once DRESS is diagnosed, the offending drug should be immediately discontinued. For mild cases, supportive treatment is recommended. For more severe cases, the use of corticosteroids tapered over several months is the treatment of choice.10 Further studies are needed to determine the optimal type of corticosteroids, as well as the dose, route, and duration of therapy. Immunotherapy, plasmapheresis, and antivirals have been used with mixed results.10,11
Our patient was started on topical and systemic oral corticosteroids. Within 24 hours, his fever resolved and his rash improved. By HD 7, his laboratory values were normal and he was discharged.
The patient was advised that in the future, he should avoid exposure to the penicillin class of medication.
THE TAKEAWAY
The presence of rash, fever, lymphadenopathy, eosinophilia, atypical lymphocytes, liver involvement, and HHV-6 reactivation in the absence of sepsis should raise suspicion for DRESS. Early diagnosis, discontinuation of the culprit drug, and timely treatment are imperative in the management of the condition. Due to a potential genetic predisposition to DRESS, clinicians should use caution when treating first-degree family members with the same class of medication that was problematic for their relative. Long-term sequelae, such as Grave’s disease and diabetes mellitus, have been reported following DRESS. Therefore, long-term monitoring with appropriate testing is recommended.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
3. Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequelae of DRESS-2 cases. J Am Acad Dermatol. 2011;65:889-890.
4. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1-21.
5. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity and the HLA complex. Pharmaceuticals (Basel). 2011;4:69-90.
6. American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at: https://www.rheumatology.org/Portals/0/Files/1982%20SLE%20Classification_Excerpt.pdf. Accessed August 30, 2017.
7. Descamps V, Ben Saïd B, Sassolas B, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2010;137:703-708.
8. Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
9. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820.
10. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome part II: management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
11. Funck-Brentano E, Duong TA, Bouvresses S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
3. Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequelae of DRESS-2 cases. J Am Acad Dermatol. 2011;65:889-890.
4. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1-21.
5. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity and the HLA complex. Pharmaceuticals (Basel). 2011;4:69-90.
6. American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at: https://www.rheumatology.org/Portals/0/Files/1982%20SLE%20Classification_Excerpt.pdf. Accessed August 30, 2017.
7. Descamps V, Ben Saïd B, Sassolas B, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2010;137:703-708.
8. Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
9. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820.
10. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome part II: management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
11. Funck-Brentano E, Duong TA, Bouvresses S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252.
Are oral emergency contraceptives a safe & effective form of long-term birth control?
EVIDENCE SUMMARY
A systematic review of 22 trials (13 case series, 8 prospective, nonrandomized studies, and one randomized controlled trial; 12,407 patients) conducted in Europe, Asia, and the Americas evaluated the likelihood of pregnancy with repeated use of precoital and postcoital hormonal contraception.1 Some trials used more than one dose or medication. Many had inadequate reporting of research methods. Results were reported using the Pearl Index (PI)—the number of pregnancies per 100 woman-years.
In 11 studies (2700 patients), women took 750 mcg of levonorgestrel from 24 hours before to 24 hours after intercourse for an average duration of 5 cycles or months. Coital frequency varied from 1 to 15 times per month. The PI ranged from 0 to 18.6, with a pooled PI of 5.4 (95% confidence interval [CI], 4.1-7.0). Three of the trials (915 patients), with research methods reported as good, had a pooled PI of 8.9 (95% CI, 5.1-14.4). No serious adverse effects were reported in 10 of the 11 studies, but menstrual irregularity was commonly observed. In one of the largest studies (1315 patients), only 3% of women discontinued treatment because of adverse effects.
Six other trials (5785 patients) of levonorgestrel taken at doses ranging from 150 mcg to 1 mg for a mean duration of 9.2 cycles reported PIs of 0 to 9. Breakthrough bleeding was the most common adverse event. When all 17 studies of levonorgestrel were combined, the PI was 4.9 (95% CI, 4.3-5.5). The remaining studies in the systematic review described medicines not commonly used for emergency contraception or not available in the United States.
Other reported adverse effects: Headache, nausea, abdominal pain
A prospective, open-label study enrolled 321 women 18 to 45 years of age from Asia, Europe, and South America to evaluate the safety and efficacy of levonorgestrel 1.5 mg taken before or within 24 hours of intercourse as the exclusive means of contraception.2 Women who were lactating or recently postpartum were excluded; condoms were permitted for women who had concerns about risk of sexually transmitted illness. Data analysis included estimates of perfect use (consistent and correct use of levonorgestrel only) and typical use (use of other contraceptive methods in addition to levonorgestrel).
At baseline, weight, blood pressure, and hemoglobin were documented, and follow-up visits occurred at 2.5, 4.5, and 6.5 months. Pregnancy tests, blood pressure, and adverse effects were assessed at each visit; weight and hemoglobin were evaluated at the final visit. The primary outcome measure was the PI in women younger than 35 years who used only levonorgestrel for contraception.
In women younger than 35 years (208 patients), the PI was 11 (95% CI, 5.7-13.1) with perfect use and 10.3 (95% CI, 5.4-19.9) with typical use. In all ages 18 to 45 years, the PI was 7.1 (95% CI, 3.8-13.1) for typical use and 7.5 (95% CI, 4-13.9) for perfect use. Most women took 4 to 6 doses per month.
The most commonly reported adverse effects were headache (29%), nausea or abdominal pain (16%), influenza (11%), and acne or candidiasis (8%). Bleeding patterns varied with a tendency toward longer bleeding initially and lighter menstrual periods and less anemia in some patients at the end of the study.
RECOMMENDATIONS
The Office of Population Research at Princeton University suggests that moderate repeat use of emergency contraceptives is unlikely to cause serious harm, but estimates that women using progestin-only emergency contraception on a regular basis would have a 20% chance of pregnancy in a year.3
The American College of Obstetricians and Gynecologists states that long-term use of emergency contraception is less effective than other methods and may result in higher hormone levels and more adverse effects than other established means.4
The International Consortium for Emergency Contraception concluded that there is no basis for limiting the number of times that emergency contraceptives may be used in a menstrual cycle, that emergency contraceptives are safe, and that, although they are less effective than other forms of long-term contraception, using them repeatedly is more effective than using no method.5
The Society of Obstetricians and Gynecologists of Canada states that emergency contraception is intended for occasional use as a backup method.6 The Society also notes that repeat use isn’t as effective as regular use of other forms of contraception.
The Faculty of Sexual & Reproductive Healthcare of the (British) Royal College of Obstetricians and Gynaecologists says that use of levonorgestrel can be considered even if previously used one or more times in a menstrual cycle (SOR: D, based on non-analytical studies and expert opinion).7 The organization also recommends that emergency contraceptive providers share with patients that oral emergency contraceptive methods should not be used for long-term contraception (SOR: Good Practice Point, based on clinical experience of the guideline development group).
The Guttmacher Institute reports that without contraception, approximately 85% of sexually active women become pregnant each year.8 Long-acting reversible methods, such as implants and intrauterine devices, have annual pregnancy rates of 0.05% to 0.8%. With perfect (consistent and correct) use, combined oral contraceptives have a 0.3% annual pregnancy rate, but the rate rises to 9% with typical use. Condoms, when used perfectly, are associated with a 2% annual rate of pregnancy compared with an 18% rate with typical use.
1. Halpern V, Raymond EG, Lopez LM. Repeated use of pre-and postcoital hormonal contraception for the prevention of pregnancy. Cochrane Database Syst Rev. 2014 Sep 26;(9):CD007595.
2. Festin MPR, Bahamondes L, Nguyen TMH, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5mg. Hum Reprod. 2016;31:530-540.
3. Trussell J, Raymond EG, Cleland K. Emergency Contraception: A Last Chance to Prevent Unintended Pregnancy. Princeton, NJ: Office of Population Research & Association of Reproductive Health Professionals, June 2017. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 28, 2017.
4. American College of Obstetricians and Gynecologists. Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
5. International Consortium for Emergency Contraception. Repeated Use of Emergency Contraceptive Pills: The Facts. New York, NY: ICEC, October 2015. Available at: www.cecinfo.org/custom-content/uploads/2015/10/ICEC_Repeat-Use_Oct-2015.pdf. Accessed June 28, 2017.
6. Dunn S, Guilbert E, Burnett M, et al. Emergency contraception. J Obstet Can. 2012;34:870–878.
7. Faculty of Sexual & Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists. FSRH Guideline: Emergency Contraception. March 2017 (Updated May 29, 2017). Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed June 28, 2017.
8. Guttmacher Institute. Contraceptive Use in the United States. New York, NY: Guttmacher Institute, September 2016. Available at: www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Accessed June 28, 2017.
EVIDENCE SUMMARY
A systematic review of 22 trials (13 case series, 8 prospective, nonrandomized studies, and one randomized controlled trial; 12,407 patients) conducted in Europe, Asia, and the Americas evaluated the likelihood of pregnancy with repeated use of precoital and postcoital hormonal contraception.1 Some trials used more than one dose or medication. Many had inadequate reporting of research methods. Results were reported using the Pearl Index (PI)—the number of pregnancies per 100 woman-years.
In 11 studies (2700 patients), women took 750 mcg of levonorgestrel from 24 hours before to 24 hours after intercourse for an average duration of 5 cycles or months. Coital frequency varied from 1 to 15 times per month. The PI ranged from 0 to 18.6, with a pooled PI of 5.4 (95% confidence interval [CI], 4.1-7.0). Three of the trials (915 patients), with research methods reported as good, had a pooled PI of 8.9 (95% CI, 5.1-14.4). No serious adverse effects were reported in 10 of the 11 studies, but menstrual irregularity was commonly observed. In one of the largest studies (1315 patients), only 3% of women discontinued treatment because of adverse effects.
Six other trials (5785 patients) of levonorgestrel taken at doses ranging from 150 mcg to 1 mg for a mean duration of 9.2 cycles reported PIs of 0 to 9. Breakthrough bleeding was the most common adverse event. When all 17 studies of levonorgestrel were combined, the PI was 4.9 (95% CI, 4.3-5.5). The remaining studies in the systematic review described medicines not commonly used for emergency contraception or not available in the United States.
Other reported adverse effects: Headache, nausea, abdominal pain
A prospective, open-label study enrolled 321 women 18 to 45 years of age from Asia, Europe, and South America to evaluate the safety and efficacy of levonorgestrel 1.5 mg taken before or within 24 hours of intercourse as the exclusive means of contraception.2 Women who were lactating or recently postpartum were excluded; condoms were permitted for women who had concerns about risk of sexually transmitted illness. Data analysis included estimates of perfect use (consistent and correct use of levonorgestrel only) and typical use (use of other contraceptive methods in addition to levonorgestrel).
At baseline, weight, blood pressure, and hemoglobin were documented, and follow-up visits occurred at 2.5, 4.5, and 6.5 months. Pregnancy tests, blood pressure, and adverse effects were assessed at each visit; weight and hemoglobin were evaluated at the final visit. The primary outcome measure was the PI in women younger than 35 years who used only levonorgestrel for contraception.
In women younger than 35 years (208 patients), the PI was 11 (95% CI, 5.7-13.1) with perfect use and 10.3 (95% CI, 5.4-19.9) with typical use. In all ages 18 to 45 years, the PI was 7.1 (95% CI, 3.8-13.1) for typical use and 7.5 (95% CI, 4-13.9) for perfect use. Most women took 4 to 6 doses per month.
The most commonly reported adverse effects were headache (29%), nausea or abdominal pain (16%), influenza (11%), and acne or candidiasis (8%). Bleeding patterns varied with a tendency toward longer bleeding initially and lighter menstrual periods and less anemia in some patients at the end of the study.
RECOMMENDATIONS
The Office of Population Research at Princeton University suggests that moderate repeat use of emergency contraceptives is unlikely to cause serious harm, but estimates that women using progestin-only emergency contraception on a regular basis would have a 20% chance of pregnancy in a year.3
The American College of Obstetricians and Gynecologists states that long-term use of emergency contraception is less effective than other methods and may result in higher hormone levels and more adverse effects than other established means.4
The International Consortium for Emergency Contraception concluded that there is no basis for limiting the number of times that emergency contraceptives may be used in a menstrual cycle, that emergency contraceptives are safe, and that, although they are less effective than other forms of long-term contraception, using them repeatedly is more effective than using no method.5
The Society of Obstetricians and Gynecologists of Canada states that emergency contraception is intended for occasional use as a backup method.6 The Society also notes that repeat use isn’t as effective as regular use of other forms of contraception.
The Faculty of Sexual & Reproductive Healthcare of the (British) Royal College of Obstetricians and Gynaecologists says that use of levonorgestrel can be considered even if previously used one or more times in a menstrual cycle (SOR: D, based on non-analytical studies and expert opinion).7 The organization also recommends that emergency contraceptive providers share with patients that oral emergency contraceptive methods should not be used for long-term contraception (SOR: Good Practice Point, based on clinical experience of the guideline development group).
The Guttmacher Institute reports that without contraception, approximately 85% of sexually active women become pregnant each year.8 Long-acting reversible methods, such as implants and intrauterine devices, have annual pregnancy rates of 0.05% to 0.8%. With perfect (consistent and correct) use, combined oral contraceptives have a 0.3% annual pregnancy rate, but the rate rises to 9% with typical use. Condoms, when used perfectly, are associated with a 2% annual rate of pregnancy compared with an 18% rate with typical use.
EVIDENCE SUMMARY
A systematic review of 22 trials (13 case series, 8 prospective, nonrandomized studies, and one randomized controlled trial; 12,407 patients) conducted in Europe, Asia, and the Americas evaluated the likelihood of pregnancy with repeated use of precoital and postcoital hormonal contraception.1 Some trials used more than one dose or medication. Many had inadequate reporting of research methods. Results were reported using the Pearl Index (PI)—the number of pregnancies per 100 woman-years.
In 11 studies (2700 patients), women took 750 mcg of levonorgestrel from 24 hours before to 24 hours after intercourse for an average duration of 5 cycles or months. Coital frequency varied from 1 to 15 times per month. The PI ranged from 0 to 18.6, with a pooled PI of 5.4 (95% confidence interval [CI], 4.1-7.0). Three of the trials (915 patients), with research methods reported as good, had a pooled PI of 8.9 (95% CI, 5.1-14.4). No serious adverse effects were reported in 10 of the 11 studies, but menstrual irregularity was commonly observed. In one of the largest studies (1315 patients), only 3% of women discontinued treatment because of adverse effects.
Six other trials (5785 patients) of levonorgestrel taken at doses ranging from 150 mcg to 1 mg for a mean duration of 9.2 cycles reported PIs of 0 to 9. Breakthrough bleeding was the most common adverse event. When all 17 studies of levonorgestrel were combined, the PI was 4.9 (95% CI, 4.3-5.5). The remaining studies in the systematic review described medicines not commonly used for emergency contraception or not available in the United States.
Other reported adverse effects: Headache, nausea, abdominal pain
A prospective, open-label study enrolled 321 women 18 to 45 years of age from Asia, Europe, and South America to evaluate the safety and efficacy of levonorgestrel 1.5 mg taken before or within 24 hours of intercourse as the exclusive means of contraception.2 Women who were lactating or recently postpartum were excluded; condoms were permitted for women who had concerns about risk of sexually transmitted illness. Data analysis included estimates of perfect use (consistent and correct use of levonorgestrel only) and typical use (use of other contraceptive methods in addition to levonorgestrel).
At baseline, weight, blood pressure, and hemoglobin were documented, and follow-up visits occurred at 2.5, 4.5, and 6.5 months. Pregnancy tests, blood pressure, and adverse effects were assessed at each visit; weight and hemoglobin were evaluated at the final visit. The primary outcome measure was the PI in women younger than 35 years who used only levonorgestrel for contraception.
In women younger than 35 years (208 patients), the PI was 11 (95% CI, 5.7-13.1) with perfect use and 10.3 (95% CI, 5.4-19.9) with typical use. In all ages 18 to 45 years, the PI was 7.1 (95% CI, 3.8-13.1) for typical use and 7.5 (95% CI, 4-13.9) for perfect use. Most women took 4 to 6 doses per month.
The most commonly reported adverse effects were headache (29%), nausea or abdominal pain (16%), influenza (11%), and acne or candidiasis (8%). Bleeding patterns varied with a tendency toward longer bleeding initially and lighter menstrual periods and less anemia in some patients at the end of the study.
RECOMMENDATIONS
The Office of Population Research at Princeton University suggests that moderate repeat use of emergency contraceptives is unlikely to cause serious harm, but estimates that women using progestin-only emergency contraception on a regular basis would have a 20% chance of pregnancy in a year.3
The American College of Obstetricians and Gynecologists states that long-term use of emergency contraception is less effective than other methods and may result in higher hormone levels and more adverse effects than other established means.4
The International Consortium for Emergency Contraception concluded that there is no basis for limiting the number of times that emergency contraceptives may be used in a menstrual cycle, that emergency contraceptives are safe, and that, although they are less effective than other forms of long-term contraception, using them repeatedly is more effective than using no method.5
The Society of Obstetricians and Gynecologists of Canada states that emergency contraception is intended for occasional use as a backup method.6 The Society also notes that repeat use isn’t as effective as regular use of other forms of contraception.
The Faculty of Sexual & Reproductive Healthcare of the (British) Royal College of Obstetricians and Gynaecologists says that use of levonorgestrel can be considered even if previously used one or more times in a menstrual cycle (SOR: D, based on non-analytical studies and expert opinion).7 The organization also recommends that emergency contraceptive providers share with patients that oral emergency contraceptive methods should not be used for long-term contraception (SOR: Good Practice Point, based on clinical experience of the guideline development group).
The Guttmacher Institute reports that without contraception, approximately 85% of sexually active women become pregnant each year.8 Long-acting reversible methods, such as implants and intrauterine devices, have annual pregnancy rates of 0.05% to 0.8%. With perfect (consistent and correct) use, combined oral contraceptives have a 0.3% annual pregnancy rate, but the rate rises to 9% with typical use. Condoms, when used perfectly, are associated with a 2% annual rate of pregnancy compared with an 18% rate with typical use.
1. Halpern V, Raymond EG, Lopez LM. Repeated use of pre-and postcoital hormonal contraception for the prevention of pregnancy. Cochrane Database Syst Rev. 2014 Sep 26;(9):CD007595.
2. Festin MPR, Bahamondes L, Nguyen TMH, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5mg. Hum Reprod. 2016;31:530-540.
3. Trussell J, Raymond EG, Cleland K. Emergency Contraception: A Last Chance to Prevent Unintended Pregnancy. Princeton, NJ: Office of Population Research & Association of Reproductive Health Professionals, June 2017. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 28, 2017.
4. American College of Obstetricians and Gynecologists. Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
5. International Consortium for Emergency Contraception. Repeated Use of Emergency Contraceptive Pills: The Facts. New York, NY: ICEC, October 2015. Available at: www.cecinfo.org/custom-content/uploads/2015/10/ICEC_Repeat-Use_Oct-2015.pdf. Accessed June 28, 2017.
6. Dunn S, Guilbert E, Burnett M, et al. Emergency contraception. J Obstet Can. 2012;34:870–878.
7. Faculty of Sexual & Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists. FSRH Guideline: Emergency Contraception. March 2017 (Updated May 29, 2017). Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed June 28, 2017.
8. Guttmacher Institute. Contraceptive Use in the United States. New York, NY: Guttmacher Institute, September 2016. Available at: www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Accessed June 28, 2017.
1. Halpern V, Raymond EG, Lopez LM. Repeated use of pre-and postcoital hormonal contraception for the prevention of pregnancy. Cochrane Database Syst Rev. 2014 Sep 26;(9):CD007595.
2. Festin MPR, Bahamondes L, Nguyen TMH, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5mg. Hum Reprod. 2016;31:530-540.
3. Trussell J, Raymond EG, Cleland K. Emergency Contraception: A Last Chance to Prevent Unintended Pregnancy. Princeton, NJ: Office of Population Research & Association of Reproductive Health Professionals, June 2017. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 28, 2017.
4. American College of Obstetricians and Gynecologists. Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
5. International Consortium for Emergency Contraception. Repeated Use of Emergency Contraceptive Pills: The Facts. New York, NY: ICEC, October 2015. Available at: www.cecinfo.org/custom-content/uploads/2015/10/ICEC_Repeat-Use_Oct-2015.pdf. Accessed June 28, 2017.
6. Dunn S, Guilbert E, Burnett M, et al. Emergency contraception. J Obstet Can. 2012;34:870–878.
7. Faculty of Sexual & Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists. FSRH Guideline: Emergency Contraception. March 2017 (Updated May 29, 2017). Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-emergency-contraception-march-2017/. Accessed June 28, 2017.
8. Guttmacher Institute. Contraceptive Use in the United States. New York, NY: Guttmacher Institute, September 2016. Available at: www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Accessed June 28, 2017.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes, but not as effective as some other methods. Annual pregnancy rates in women using pericoital levonorgestrel 150 mcg to 1 mg range from 4.9% to 8.9%; menstrual irregularity is the most common adverse effect (strength of recommendation [SOR]: B, Cochrane review of lower-quality trials).
In women younger than 35 years who have sexual intercourse 6 or fewer times per month, correct and consistent use of pericoital levonorgestrel 1.5 mg results in an annual pregnancy rate of 11% (SOR: B, one large prospective, open-label trial).
Pericoital contraception is less effective than long-acting reversible contraceptives (annual pregnancy rates of 0.05%-0.8%) or perfect use of combined oral contraceptives (0.3% annual pregnancy rate), but similar to, or better than, typical use of combined oral contraception (9%) and condoms (18%).
When to “CAP” off treatment for pneumonia
ILLUSTRATIVE CASE
A 65-year-old woman is admitted to your inpatient service from your family health center. She is diagnosed with community-acquired pneumonia (CAP) based on a 5-day history of cough and fever and a positive chest x-ray. She now requires oxygen at rest. She has a past medical history of hypertension and diabetes, both of which have been controlled on oral medications. Antibiotic therapy is initiated for the treatment of the pneumonia, but what treatment duration is ideal?
The World Health Organization estimates that pneumonia is the third most common cause of mortality worldwide, causing 3.2 million deaths per year.2 Appropriate prescribing of antibiotics is critical for the successful treatment of CAP.
The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) created consensus guidelines, published in 2007, for the treatment of CAP.3 These guidelines recommend a minimum 5-day course of antibiotics if the patient is clinically stable, which is defined as: afebrile for 48 hours, heart rate ≤100 beats/minute, respiratory rate ≤24 respirations/minute, systolic blood pressure ≥90 mm Hg, oxygen saturation ≥90%, normal mental status, and able to tolerate oral intake. Longer antibiotic treatment durations are recommended on an individualized basis, if, for example, the isolated pathogen is not susceptible to the initial antibiotic or if the infection was caused by an extrapulmonary source.
However, these recommendations are not routinely followed. Practitioners often make it their custom to prescribe longer courses of antibiotics.4 And yet we know that there are several reasons to consider shorter courses of antibiotics, including lower health care costs, fewer adverse effects, and lower rates of bacterial resistance.5-7
Two meta-analyses were performed to compare the safety and efficacy of short- (≤7 days) vs long-course (>7 days) antibiotic therapy in CAP.8,9 Both meta-analyses found no difference in efficacy or safety between shorter and longer courses of antibiotic treatment regimens for CAP. Secondary outcomes noted a trend toward decreased antibiotic-associated adverse events with shorter courses of therapy.8,9
While these meta-analyses supported shorter courses of antibiotics for CAP, there are limitations to the broad implementation of their findings. Studies included in these analyses utilized a variety of antibiotic treatment regimens and longer courses (7 days vs 5 days) that are not recommended by the IDSA/ATS guidelines. Additionally, studies included both inpatient and outpatient treatment groups, so findings may not apply to an exclusively inpatient CAP population.8,9
This study sought to validate the IDSA/ATS guidelines recommending a 5-day course of antibiotics for hospitalized patients with CAP.1
STUDY SUMMARY
No differences in clinical outcomes between 5 days of Tx—and longer
This multicenter, double-blind, noninferiority randomized trial compared short-term antibiotic treatment duration (5 days) to physician-discretion antibiotic treatment duration among 312 patients ≥18 years of age admitted for CAP to one of 4 teaching hospitals in Spain.1 Pneumonia was diagnosed on chest radiograph with at least one symptom: cough, fever, dyspnea, or chest pain. Patients were excluded if, among other things, they had an immunocompromising condition, lived in a nursing home, had a recent hospital stay, used antibiotics within the previous 30 days, or had an uncommon pathogen, such as Pseudomonas aeruginosa or Staphylococcus aureus.1
Patients were randomized after receiving a minimum of 5 days of antibiotics to an intervention group (where, if clinically stable, no further antibiotics were given) or a control group (where physicians determined antibiotic duration).1 Primary outcomes were clinical success rate at Days 10 and 30 from admission, defined as resolution of signs and symptoms of CAP without further antibiotics, and improvement of CAP-related symptoms as determined by an 18-item CAP symptom questionnaire. This questionnaire was scored 0 to 90, where higher scores indicated greater severity. Secondary outcomes included: duration of antibiotic use, time to clinical improvement, mortality, hospital readmission, hospital length of stay, and CAP recurrence.1A total of 312 patients were randomized with 162 patients in the intervention group and 150 patients in the control group. The mean age of patients in the intervention and control groups was 66.2 and 64.7 years, respectively. Other baseline demographics were similar between the groups. Nearly 80% of patients received quinolone treatment; <10% received a beta-lactam plus a macrolide.1
Clinical success rates were similar for the control and intervention groups, respectively, at Day 10 (49% vs 56%; P=.18) and Day 30 (89% vs 92%; P=.33). There was shorter median treatment duration with antibiotics in the intervention group compared with the control group (5 days vs 10 days; P<.001) and fewer 30-day hospital readmissions (1.4% vs 6.6%; P=.02). There were no differences for other secondary outcomes.1
WHAT’S NEW
Clinical support for 2007 guidelines
This is the first study to clinically support the IDSA/ATS guidelines, which state that a 5-day course of antibiotic therapy for hospitalized adults with CAP is effective and without increased risk of adverse events.
CAVEATS
Generaliz
This study focused on antibiotic duration for the treatment of CAP in hospitalized patients and mainly used quinolone antibiotics. It remains unclear if duration of therapy is as effective in the outpatient setting or when using alternative antibiotic regimens.
If patients continued to have symptoms (such as fever or low oxygen saturation on room air) after 5 days of antibiotic treatment, antibiotic treatment was continued in the study. Thus, patients in real life who continue to have symptoms may need individualized therapy and may require more than 5 days of antibiotics.
CHALLENGES TO IMPLEMENTATION
Antibiotics end before clinical improvement occurs
This study noted an average of 12 days in both groups for patients to achieve clinical improvement, with upwards of 15 to 18 days for patients to return to normal activity. Patients and providers may be dissatisfied if the treatment course ends days before clinical improvement of symptoms. This may cause prescribers to lengthen the duration of antibiotic therapy inappropriately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257-1265.
2. World Health Organization. The top 10 causes of death. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index.html. Accessed September 5, 2017.
3. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Aliberti S, Blasi F, Zanaboni AM, et al. Duration of antibiotic therapy in hospitalised patients with community-acquired pneumonia. Eur Respir J. 2010;36:128-134.
5. Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of ß-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365-370.
6. Opmeer BC, el Moussaoui R, Bossuyt PM, et al. Costs associated with shorter duration of antibiotic therapy in hospitalized patients with mild-to-moderate severe community-acquired pneumonia. J Antimicrob Chemother. 2007;60:1131-1136.
7. File TM Jr. Clinical efficacy of newer agents in short-duration therapy for community-acquired pneumonia. Clin Infect Dis. 2004;39(suppl 3):S159-S164.
8. Li JZ, Winston LG, Moore DH, et al. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
9. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
ILLUSTRATIVE CASE
A 65-year-old woman is admitted to your inpatient service from your family health center. She is diagnosed with community-acquired pneumonia (CAP) based on a 5-day history of cough and fever and a positive chest x-ray. She now requires oxygen at rest. She has a past medical history of hypertension and diabetes, both of which have been controlled on oral medications. Antibiotic therapy is initiated for the treatment of the pneumonia, but what treatment duration is ideal?
The World Health Organization estimates that pneumonia is the third most common cause of mortality worldwide, causing 3.2 million deaths per year.2 Appropriate prescribing of antibiotics is critical for the successful treatment of CAP.
The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) created consensus guidelines, published in 2007, for the treatment of CAP.3 These guidelines recommend a minimum 5-day course of antibiotics if the patient is clinically stable, which is defined as: afebrile for 48 hours, heart rate ≤100 beats/minute, respiratory rate ≤24 respirations/minute, systolic blood pressure ≥90 mm Hg, oxygen saturation ≥90%, normal mental status, and able to tolerate oral intake. Longer antibiotic treatment durations are recommended on an individualized basis, if, for example, the isolated pathogen is not susceptible to the initial antibiotic or if the infection was caused by an extrapulmonary source.
However, these recommendations are not routinely followed. Practitioners often make it their custom to prescribe longer courses of antibiotics.4 And yet we know that there are several reasons to consider shorter courses of antibiotics, including lower health care costs, fewer adverse effects, and lower rates of bacterial resistance.5-7
Two meta-analyses were performed to compare the safety and efficacy of short- (≤7 days) vs long-course (>7 days) antibiotic therapy in CAP.8,9 Both meta-analyses found no difference in efficacy or safety between shorter and longer courses of antibiotic treatment regimens for CAP. Secondary outcomes noted a trend toward decreased antibiotic-associated adverse events with shorter courses of therapy.8,9
While these meta-analyses supported shorter courses of antibiotics for CAP, there are limitations to the broad implementation of their findings. Studies included in these analyses utilized a variety of antibiotic treatment regimens and longer courses (7 days vs 5 days) that are not recommended by the IDSA/ATS guidelines. Additionally, studies included both inpatient and outpatient treatment groups, so findings may not apply to an exclusively inpatient CAP population.8,9
This study sought to validate the IDSA/ATS guidelines recommending a 5-day course of antibiotics for hospitalized patients with CAP.1
STUDY SUMMARY
No differences in clinical outcomes between 5 days of Tx—and longer
This multicenter, double-blind, noninferiority randomized trial compared short-term antibiotic treatment duration (5 days) to physician-discretion antibiotic treatment duration among 312 patients ≥18 years of age admitted for CAP to one of 4 teaching hospitals in Spain.1 Pneumonia was diagnosed on chest radiograph with at least one symptom: cough, fever, dyspnea, or chest pain. Patients were excluded if, among other things, they had an immunocompromising condition, lived in a nursing home, had a recent hospital stay, used antibiotics within the previous 30 days, or had an uncommon pathogen, such as Pseudomonas aeruginosa or Staphylococcus aureus.1
Patients were randomized after receiving a minimum of 5 days of antibiotics to an intervention group (where, if clinically stable, no further antibiotics were given) or a control group (where physicians determined antibiotic duration).1 Primary outcomes were clinical success rate at Days 10 and 30 from admission, defined as resolution of signs and symptoms of CAP without further antibiotics, and improvement of CAP-related symptoms as determined by an 18-item CAP symptom questionnaire. This questionnaire was scored 0 to 90, where higher scores indicated greater severity. Secondary outcomes included: duration of antibiotic use, time to clinical improvement, mortality, hospital readmission, hospital length of stay, and CAP recurrence.1A total of 312 patients were randomized with 162 patients in the intervention group and 150 patients in the control group. The mean age of patients in the intervention and control groups was 66.2 and 64.7 years, respectively. Other baseline demographics were similar between the groups. Nearly 80% of patients received quinolone treatment; <10% received a beta-lactam plus a macrolide.1
Clinical success rates were similar for the control and intervention groups, respectively, at Day 10 (49% vs 56%; P=.18) and Day 30 (89% vs 92%; P=.33). There was shorter median treatment duration with antibiotics in the intervention group compared with the control group (5 days vs 10 days; P<.001) and fewer 30-day hospital readmissions (1.4% vs 6.6%; P=.02). There were no differences for other secondary outcomes.1
WHAT’S NEW
Clinical support for 2007 guidelines
This is the first study to clinically support the IDSA/ATS guidelines, which state that a 5-day course of antibiotic therapy for hospitalized adults with CAP is effective and without increased risk of adverse events.
CAVEATS
Generaliz
This study focused on antibiotic duration for the treatment of CAP in hospitalized patients and mainly used quinolone antibiotics. It remains unclear if duration of therapy is as effective in the outpatient setting or when using alternative antibiotic regimens.
If patients continued to have symptoms (such as fever or low oxygen saturation on room air) after 5 days of antibiotic treatment, antibiotic treatment was continued in the study. Thus, patients in real life who continue to have symptoms may need individualized therapy and may require more than 5 days of antibiotics.
CHALLENGES TO IMPLEMENTATION
Antibiotics end before clinical improvement occurs
This study noted an average of 12 days in both groups for patients to achieve clinical improvement, with upwards of 15 to 18 days for patients to return to normal activity. Patients and providers may be dissatisfied if the treatment course ends days before clinical improvement of symptoms. This may cause prescribers to lengthen the duration of antibiotic therapy inappropriately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 65-year-old woman is admitted to your inpatient service from your family health center. She is diagnosed with community-acquired pneumonia (CAP) based on a 5-day history of cough and fever and a positive chest x-ray. She now requires oxygen at rest. She has a past medical history of hypertension and diabetes, both of which have been controlled on oral medications. Antibiotic therapy is initiated for the treatment of the pneumonia, but what treatment duration is ideal?
The World Health Organization estimates that pneumonia is the third most common cause of mortality worldwide, causing 3.2 million deaths per year.2 Appropriate prescribing of antibiotics is critical for the successful treatment of CAP.
The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) created consensus guidelines, published in 2007, for the treatment of CAP.3 These guidelines recommend a minimum 5-day course of antibiotics if the patient is clinically stable, which is defined as: afebrile for 48 hours, heart rate ≤100 beats/minute, respiratory rate ≤24 respirations/minute, systolic blood pressure ≥90 mm Hg, oxygen saturation ≥90%, normal mental status, and able to tolerate oral intake. Longer antibiotic treatment durations are recommended on an individualized basis, if, for example, the isolated pathogen is not susceptible to the initial antibiotic or if the infection was caused by an extrapulmonary source.
However, these recommendations are not routinely followed. Practitioners often make it their custom to prescribe longer courses of antibiotics.4 And yet we know that there are several reasons to consider shorter courses of antibiotics, including lower health care costs, fewer adverse effects, and lower rates of bacterial resistance.5-7
Two meta-analyses were performed to compare the safety and efficacy of short- (≤7 days) vs long-course (>7 days) antibiotic therapy in CAP.8,9 Both meta-analyses found no difference in efficacy or safety between shorter and longer courses of antibiotic treatment regimens for CAP. Secondary outcomes noted a trend toward decreased antibiotic-associated adverse events with shorter courses of therapy.8,9
While these meta-analyses supported shorter courses of antibiotics for CAP, there are limitations to the broad implementation of their findings. Studies included in these analyses utilized a variety of antibiotic treatment regimens and longer courses (7 days vs 5 days) that are not recommended by the IDSA/ATS guidelines. Additionally, studies included both inpatient and outpatient treatment groups, so findings may not apply to an exclusively inpatient CAP population.8,9
This study sought to validate the IDSA/ATS guidelines recommending a 5-day course of antibiotics for hospitalized patients with CAP.1
STUDY SUMMARY
No differences in clinical outcomes between 5 days of Tx—and longer
This multicenter, double-blind, noninferiority randomized trial compared short-term antibiotic treatment duration (5 days) to physician-discretion antibiotic treatment duration among 312 patients ≥18 years of age admitted for CAP to one of 4 teaching hospitals in Spain.1 Pneumonia was diagnosed on chest radiograph with at least one symptom: cough, fever, dyspnea, or chest pain. Patients were excluded if, among other things, they had an immunocompromising condition, lived in a nursing home, had a recent hospital stay, used antibiotics within the previous 30 days, or had an uncommon pathogen, such as Pseudomonas aeruginosa or Staphylococcus aureus.1
Patients were randomized after receiving a minimum of 5 days of antibiotics to an intervention group (where, if clinically stable, no further antibiotics were given) or a control group (where physicians determined antibiotic duration).1 Primary outcomes were clinical success rate at Days 10 and 30 from admission, defined as resolution of signs and symptoms of CAP without further antibiotics, and improvement of CAP-related symptoms as determined by an 18-item CAP symptom questionnaire. This questionnaire was scored 0 to 90, where higher scores indicated greater severity. Secondary outcomes included: duration of antibiotic use, time to clinical improvement, mortality, hospital readmission, hospital length of stay, and CAP recurrence.1A total of 312 patients were randomized with 162 patients in the intervention group and 150 patients in the control group. The mean age of patients in the intervention and control groups was 66.2 and 64.7 years, respectively. Other baseline demographics were similar between the groups. Nearly 80% of patients received quinolone treatment; <10% received a beta-lactam plus a macrolide.1
Clinical success rates were similar for the control and intervention groups, respectively, at Day 10 (49% vs 56%; P=.18) and Day 30 (89% vs 92%; P=.33). There was shorter median treatment duration with antibiotics in the intervention group compared with the control group (5 days vs 10 days; P<.001) and fewer 30-day hospital readmissions (1.4% vs 6.6%; P=.02). There were no differences for other secondary outcomes.1
WHAT’S NEW
Clinical support for 2007 guidelines
This is the first study to clinically support the IDSA/ATS guidelines, which state that a 5-day course of antibiotic therapy for hospitalized adults with CAP is effective and without increased risk of adverse events.
CAVEATS
Generaliz
This study focused on antibiotic duration for the treatment of CAP in hospitalized patients and mainly used quinolone antibiotics. It remains unclear if duration of therapy is as effective in the outpatient setting or when using alternative antibiotic regimens.
If patients continued to have symptoms (such as fever or low oxygen saturation on room air) after 5 days of antibiotic treatment, antibiotic treatment was continued in the study. Thus, patients in real life who continue to have symptoms may need individualized therapy and may require more than 5 days of antibiotics.
CHALLENGES TO IMPLEMENTATION
Antibiotics end before clinical improvement occurs
This study noted an average of 12 days in both groups for patients to achieve clinical improvement, with upwards of 15 to 18 days for patients to return to normal activity. Patients and providers may be dissatisfied if the treatment course ends days before clinical improvement of symptoms. This may cause prescribers to lengthen the duration of antibiotic therapy inappropriately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257-1265.
2. World Health Organization. The top 10 causes of death. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index.html. Accessed September 5, 2017.
3. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Aliberti S, Blasi F, Zanaboni AM, et al. Duration of antibiotic therapy in hospitalised patients with community-acquired pneumonia. Eur Respir J. 2010;36:128-134.
5. Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of ß-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365-370.
6. Opmeer BC, el Moussaoui R, Bossuyt PM, et al. Costs associated with shorter duration of antibiotic therapy in hospitalized patients with mild-to-moderate severe community-acquired pneumonia. J Antimicrob Chemother. 2007;60:1131-1136.
7. File TM Jr. Clinical efficacy of newer agents in short-duration therapy for community-acquired pneumonia. Clin Infect Dis. 2004;39(suppl 3):S159-S164.
8. Li JZ, Winston LG, Moore DH, et al. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
9. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
1. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257-1265.
2. World Health Organization. The top 10 causes of death. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index.html. Accessed September 5, 2017.
3. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Aliberti S, Blasi F, Zanaboni AM, et al. Duration of antibiotic therapy in hospitalised patients with community-acquired pneumonia. Eur Respir J. 2010;36:128-134.
5. Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of ß-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365-370.
6. Opmeer BC, el Moussaoui R, Bossuyt PM, et al. Costs associated with shorter duration of antibiotic therapy in hospitalized patients with mild-to-moderate severe community-acquired pneumonia. J Antimicrob Chemother. 2007;60:1131-1136.
7. File TM Jr. Clinical efficacy of newer agents in short-duration therapy for community-acquired pneumonia. Clin Infect Dis. 2004;39(suppl 3):S159-S164.
8. Li JZ, Winston LG, Moore DH, et al. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783-790.
9. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841-1854.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe 5 days of antibiotic treatment for inpatients with community-acquired pneumonia because it produces the same clinical success rates as longer treatment regimens, but is associated with fewer negative patient outcomes.1
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality randomized control trial.
Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257-1265.1
Intraoral lesion • history of cirrhosis and smoking • Dx?
THE CASE
A 56-year-old white man presented at our dental clinic for routine care. The intraoral examination revealed an asymptomatic red lesion with white vesicle-like areas on the right side of the soft palate (FIGURE). The extraoral examination was normal, and regional lymph nodes were nonpalpable. The patient’s medical history included liver cirrhosis and pancreatitis. He also had a 30-year history of alcohol misuse (1-5 drinks per day) and a 30-pack-year smoking history. (The patient had stopped drinking at the time of presentation, and had quit smoking 2 years earlier.) We instructed him to gargle with warm salt water at home and return in 2 weeks. At follow-up, the lesion was unresolved, so a biopsy was performed.
THE DIAGNOSIS
The clinical diagnosis was erythroplakia. Trauma from food burn and inflammation of the salivary gland were both considered, but ultimately ruled out due to lack of symptoms and persistence of the lesion after 14 days. The pathology report confirmed a diagnosis of squamous cell carcinoma (SCC) in situ. Based on the pathology report, we referred the patient to an oral surgeon for wide surgical excision with evaluation of the margins.
Because of its location and subtle presentation, the lesion could have been easily overlooked, underscoring the importance of routinely going beyond dentition to examine the soft tissues of the mouth.
DISCUSSION
SCC is the most common cancer found in the oral cavity, accounting for 90% of all oral malignancies.1,2 Other malignancies include lymphomas, sarcomas, melanomas, salivary gland neoplasms, and metastasis from other sites.3,4 Predisposing factors include tobacco use (namely inhaled methods and chewing tobacco), alcohol misuse, human papillomavirus infection, and chewing betel nut.1,5 (Betel nuts grow on a species of palm tree mainly found in India, Pakistan, and Bangladesh. They are commonly chewed for their caffeine-like effect and are known to be carcinogenic.)
Presentation. SCC of the oral cavity can have various presentations. The lesion can appear as white, red, a mix of white and red, as a mass, or as a nonhealing ulcer. While some patients may be asymptomatic (as was ours), some may have signs and symptoms such as pain, bleeding, difficulty swallowing, difficulty wearing dentures, or a neck mass.6 A history of smoking and alcohol misuse, which was present in this case, should heighten suspicion and prompt further investigation of oral lesions.
Location. The most common intraoral site for oral cancer is the tongue (on the posterolateral border) followed by the floor of the mouth. Other common sites in descending order are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.1 (Our patient’s lesion was on the border of the hard and soft palate).
Treatment of oral cancer is surgical. In some cases, depending on the stage and size of the tumor, radiation and chemotherapy may be considered.3,5 Approximately two-thirds of oral cancers are detected in the later stages.7 The 5-year survival rate for people with oral SCC found at stages III or IV ranges from 32% to 45%, while the rate for those with SCC detected at stages I or II is 58% to 72%.1 Patients with a history of oral cancer have a 20-fold increased risk of a recurrence in the oral cavity or of developing cancer in the surrounding areas, such as the larynx, esophagus, and lungs, underscoring the necessity of adequate follow-up in these patients.2,3,5
Who is at risk?
In 2015, there were an estimated 45,780 new cases of oral cavity and pharyngeal cancer and 8650 deaths from these causes.8 Although oral cancer accounts for only 3% of all cancers in the United States, it is the eighth most common cancer in males and the 15th most common in females.1 Prevalence differs tremendously by location, however. In India, for example, oral cancer accounts for 30% of all cancers.9 Regardless of location, incidence increases with age; 62 is the average age at diagnosis.2 Oral cancers are also more common among African Americans than among Caucasians.1,3,5
Smokers are 2 to 3 times more likely to develop oral cancer than nonsmokers.1 This risk increases with amount and duration of smoking.1 The combination of smoking and alcohol use has a synergistic effect, increasing the likelihood of developing oral cancer 15 fold.1,3
Alcohol use. Among male patients with oral cancer, one-third are heavy alcohol users.1 In fact, one study found that 20% of these patients have cirrhosis of the liver.1 Thus, it makes good clinical sense to routinely examine the soft tissue of the oral cavity for abnormalities in patients with alcohol-induced cirrhosis of the liver.
Our patient. We placed our patient on a 3-month recall and stressed the importance of not smoking. The patient had surgery and a good outcome was documented. The patient indicated at follow-up that he’d started drinking again and was referred for counseling.
TAKEAWAY
It’s important to pay attention to color differences in the oral cavity on routine visits, particularly in patients with known risk factors for SCC. Patients with a lesion in the oral cavity should be seen again within 2 weeks. If the lesion is unresolved, the patient should be referred for further examination and/or biopsy. The possibility of recurrent oral cancer or cancer in the surrounding areas makes these patients good candidates for frequent follow-up examinations.
We strongly suggest that primary care physicians encourage their patients with the known predisposing risk factors of tobacco use and chronic alcohol misuse to quit these habits, visit their dentists for annual oral cancer screenings, and report any oral symptoms promptly to their medical and/or dental care providers. The asymptomatic nature of many of these lesions underscores the importance of following this advice. As is the case with most other cancers, survival rate is dependent on the stage of the disease at diagnosis.
1. Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. 4th ed. Philadelphia, PA: Elsevier, Inc; 2016:374-388.
2. American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? Available at: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed August 28, 2017.
3. The Oral Cancer Foundation. Oral cancer facts. Available at: http://oralcancerfoundation.org/facts/. Accessed August 28, 2017.
4. Zini A, Czerninski R, Sqan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299-305.
5. National Institute of Health. National Cancer Institute. Oral Cavity and Oropharyngeal Cancer Screening (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck/patient/oral-screening-pdq. Accessed August 28, 2017.
6. Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642-647.
7. Dodd VJ, Schenck DP, Chaney EH, et al. Assessing oral cancer awareness among rural Latino migrant workers. J Immigr Minor Health. 2016;18:552-560.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
9. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932.
THE CASE
A 56-year-old white man presented at our dental clinic for routine care. The intraoral examination revealed an asymptomatic red lesion with white vesicle-like areas on the right side of the soft palate (FIGURE). The extraoral examination was normal, and regional lymph nodes were nonpalpable. The patient’s medical history included liver cirrhosis and pancreatitis. He also had a 30-year history of alcohol misuse (1-5 drinks per day) and a 30-pack-year smoking history. (The patient had stopped drinking at the time of presentation, and had quit smoking 2 years earlier.) We instructed him to gargle with warm salt water at home and return in 2 weeks. At follow-up, the lesion was unresolved, so a biopsy was performed.
THE DIAGNOSIS
The clinical diagnosis was erythroplakia. Trauma from food burn and inflammation of the salivary gland were both considered, but ultimately ruled out due to lack of symptoms and persistence of the lesion after 14 days. The pathology report confirmed a diagnosis of squamous cell carcinoma (SCC) in situ. Based on the pathology report, we referred the patient to an oral surgeon for wide surgical excision with evaluation of the margins.
Because of its location and subtle presentation, the lesion could have been easily overlooked, underscoring the importance of routinely going beyond dentition to examine the soft tissues of the mouth.
DISCUSSION
SCC is the most common cancer found in the oral cavity, accounting for 90% of all oral malignancies.1,2 Other malignancies include lymphomas, sarcomas, melanomas, salivary gland neoplasms, and metastasis from other sites.3,4 Predisposing factors include tobacco use (namely inhaled methods and chewing tobacco), alcohol misuse, human papillomavirus infection, and chewing betel nut.1,5 (Betel nuts grow on a species of palm tree mainly found in India, Pakistan, and Bangladesh. They are commonly chewed for their caffeine-like effect and are known to be carcinogenic.)
Presentation. SCC of the oral cavity can have various presentations. The lesion can appear as white, red, a mix of white and red, as a mass, or as a nonhealing ulcer. While some patients may be asymptomatic (as was ours), some may have signs and symptoms such as pain, bleeding, difficulty swallowing, difficulty wearing dentures, or a neck mass.6 A history of smoking and alcohol misuse, which was present in this case, should heighten suspicion and prompt further investigation of oral lesions.
Location. The most common intraoral site for oral cancer is the tongue (on the posterolateral border) followed by the floor of the mouth. Other common sites in descending order are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.1 (Our patient’s lesion was on the border of the hard and soft palate).
Treatment of oral cancer is surgical. In some cases, depending on the stage and size of the tumor, radiation and chemotherapy may be considered.3,5 Approximately two-thirds of oral cancers are detected in the later stages.7 The 5-year survival rate for people with oral SCC found at stages III or IV ranges from 32% to 45%, while the rate for those with SCC detected at stages I or II is 58% to 72%.1 Patients with a history of oral cancer have a 20-fold increased risk of a recurrence in the oral cavity or of developing cancer in the surrounding areas, such as the larynx, esophagus, and lungs, underscoring the necessity of adequate follow-up in these patients.2,3,5
Who is at risk?
In 2015, there were an estimated 45,780 new cases of oral cavity and pharyngeal cancer and 8650 deaths from these causes.8 Although oral cancer accounts for only 3% of all cancers in the United States, it is the eighth most common cancer in males and the 15th most common in females.1 Prevalence differs tremendously by location, however. In India, for example, oral cancer accounts for 30% of all cancers.9 Regardless of location, incidence increases with age; 62 is the average age at diagnosis.2 Oral cancers are also more common among African Americans than among Caucasians.1,3,5
Smokers are 2 to 3 times more likely to develop oral cancer than nonsmokers.1 This risk increases with amount and duration of smoking.1 The combination of smoking and alcohol use has a synergistic effect, increasing the likelihood of developing oral cancer 15 fold.1,3
Alcohol use. Among male patients with oral cancer, one-third are heavy alcohol users.1 In fact, one study found that 20% of these patients have cirrhosis of the liver.1 Thus, it makes good clinical sense to routinely examine the soft tissue of the oral cavity for abnormalities in patients with alcohol-induced cirrhosis of the liver.
Our patient. We placed our patient on a 3-month recall and stressed the importance of not smoking. The patient had surgery and a good outcome was documented. The patient indicated at follow-up that he’d started drinking again and was referred for counseling.
TAKEAWAY
It’s important to pay attention to color differences in the oral cavity on routine visits, particularly in patients with known risk factors for SCC. Patients with a lesion in the oral cavity should be seen again within 2 weeks. If the lesion is unresolved, the patient should be referred for further examination and/or biopsy. The possibility of recurrent oral cancer or cancer in the surrounding areas makes these patients good candidates for frequent follow-up examinations.
We strongly suggest that primary care physicians encourage their patients with the known predisposing risk factors of tobacco use and chronic alcohol misuse to quit these habits, visit their dentists for annual oral cancer screenings, and report any oral symptoms promptly to their medical and/or dental care providers. The asymptomatic nature of many of these lesions underscores the importance of following this advice. As is the case with most other cancers, survival rate is dependent on the stage of the disease at diagnosis.
THE CASE
A 56-year-old white man presented at our dental clinic for routine care. The intraoral examination revealed an asymptomatic red lesion with white vesicle-like areas on the right side of the soft palate (FIGURE). The extraoral examination was normal, and regional lymph nodes were nonpalpable. The patient’s medical history included liver cirrhosis and pancreatitis. He also had a 30-year history of alcohol misuse (1-5 drinks per day) and a 30-pack-year smoking history. (The patient had stopped drinking at the time of presentation, and had quit smoking 2 years earlier.) We instructed him to gargle with warm salt water at home and return in 2 weeks. At follow-up, the lesion was unresolved, so a biopsy was performed.
THE DIAGNOSIS
The clinical diagnosis was erythroplakia. Trauma from food burn and inflammation of the salivary gland were both considered, but ultimately ruled out due to lack of symptoms and persistence of the lesion after 14 days. The pathology report confirmed a diagnosis of squamous cell carcinoma (SCC) in situ. Based on the pathology report, we referred the patient to an oral surgeon for wide surgical excision with evaluation of the margins.
Because of its location and subtle presentation, the lesion could have been easily overlooked, underscoring the importance of routinely going beyond dentition to examine the soft tissues of the mouth.
DISCUSSION
SCC is the most common cancer found in the oral cavity, accounting for 90% of all oral malignancies.1,2 Other malignancies include lymphomas, sarcomas, melanomas, salivary gland neoplasms, and metastasis from other sites.3,4 Predisposing factors include tobacco use (namely inhaled methods and chewing tobacco), alcohol misuse, human papillomavirus infection, and chewing betel nut.1,5 (Betel nuts grow on a species of palm tree mainly found in India, Pakistan, and Bangladesh. They are commonly chewed for their caffeine-like effect and are known to be carcinogenic.)
Presentation. SCC of the oral cavity can have various presentations. The lesion can appear as white, red, a mix of white and red, as a mass, or as a nonhealing ulcer. While some patients may be asymptomatic (as was ours), some may have signs and symptoms such as pain, bleeding, difficulty swallowing, difficulty wearing dentures, or a neck mass.6 A history of smoking and alcohol misuse, which was present in this case, should heighten suspicion and prompt further investigation of oral lesions.
Location. The most common intraoral site for oral cancer is the tongue (on the posterolateral border) followed by the floor of the mouth. Other common sites in descending order are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.1 (Our patient’s lesion was on the border of the hard and soft palate).
Treatment of oral cancer is surgical. In some cases, depending on the stage and size of the tumor, radiation and chemotherapy may be considered.3,5 Approximately two-thirds of oral cancers are detected in the later stages.7 The 5-year survival rate for people with oral SCC found at stages III or IV ranges from 32% to 45%, while the rate for those with SCC detected at stages I or II is 58% to 72%.1 Patients with a history of oral cancer have a 20-fold increased risk of a recurrence in the oral cavity or of developing cancer in the surrounding areas, such as the larynx, esophagus, and lungs, underscoring the necessity of adequate follow-up in these patients.2,3,5
Who is at risk?
In 2015, there were an estimated 45,780 new cases of oral cavity and pharyngeal cancer and 8650 deaths from these causes.8 Although oral cancer accounts for only 3% of all cancers in the United States, it is the eighth most common cancer in males and the 15th most common in females.1 Prevalence differs tremendously by location, however. In India, for example, oral cancer accounts for 30% of all cancers.9 Regardless of location, incidence increases with age; 62 is the average age at diagnosis.2 Oral cancers are also more common among African Americans than among Caucasians.1,3,5
Smokers are 2 to 3 times more likely to develop oral cancer than nonsmokers.1 This risk increases with amount and duration of smoking.1 The combination of smoking and alcohol use has a synergistic effect, increasing the likelihood of developing oral cancer 15 fold.1,3
Alcohol use. Among male patients with oral cancer, one-third are heavy alcohol users.1 In fact, one study found that 20% of these patients have cirrhosis of the liver.1 Thus, it makes good clinical sense to routinely examine the soft tissue of the oral cavity for abnormalities in patients with alcohol-induced cirrhosis of the liver.
Our patient. We placed our patient on a 3-month recall and stressed the importance of not smoking. The patient had surgery and a good outcome was documented. The patient indicated at follow-up that he’d started drinking again and was referred for counseling.
TAKEAWAY
It’s important to pay attention to color differences in the oral cavity on routine visits, particularly in patients with known risk factors for SCC. Patients with a lesion in the oral cavity should be seen again within 2 weeks. If the lesion is unresolved, the patient should be referred for further examination and/or biopsy. The possibility of recurrent oral cancer or cancer in the surrounding areas makes these patients good candidates for frequent follow-up examinations.
We strongly suggest that primary care physicians encourage their patients with the known predisposing risk factors of tobacco use and chronic alcohol misuse to quit these habits, visit their dentists for annual oral cancer screenings, and report any oral symptoms promptly to their medical and/or dental care providers. The asymptomatic nature of many of these lesions underscores the importance of following this advice. As is the case with most other cancers, survival rate is dependent on the stage of the disease at diagnosis.
1. Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. 4th ed. Philadelphia, PA: Elsevier, Inc; 2016:374-388.
2. American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? Available at: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed August 28, 2017.
3. The Oral Cancer Foundation. Oral cancer facts. Available at: http://oralcancerfoundation.org/facts/. Accessed August 28, 2017.
4. Zini A, Czerninski R, Sqan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299-305.
5. National Institute of Health. National Cancer Institute. Oral Cavity and Oropharyngeal Cancer Screening (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck/patient/oral-screening-pdq. Accessed August 28, 2017.
6. Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642-647.
7. Dodd VJ, Schenck DP, Chaney EH, et al. Assessing oral cancer awareness among rural Latino migrant workers. J Immigr Minor Health. 2016;18:552-560.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
9. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932.
1. Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. 4th ed. Philadelphia, PA: Elsevier, Inc; 2016:374-388.
2. American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancer? Available at: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html. Accessed August 28, 2017.
3. The Oral Cancer Foundation. Oral cancer facts. Available at: http://oralcancerfoundation.org/facts/. Accessed August 28, 2017.
4. Zini A, Czerninski R, Sqan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299-305.
5. National Institute of Health. National Cancer Institute. Oral Cavity and Oropharyngeal Cancer Screening (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck/patient/oral-screening-pdq. Accessed August 28, 2017.
6. Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642-647.
7. Dodd VJ, Schenck DP, Chaney EH, et al. Assessing oral cancer awareness among rural Latino migrant workers. J Immigr Minor Health. 2016;18:552-560.
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
9. Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932.
Is obesity a disease?
It depends on whom you ask. But if you ask me, obesity should not be labeled a disease.
I understand the rationale for calling obesity a disease—it helps legitimize the time we spend treating obesity and aids in getting paid for that time. Some people have distinct diseases, such as Prader-Willi syndrome, hypothyroidism, and Cushing’s syndrome that can cause obesity, and perhaps massive obesity is best categorized and treated as a disease. But the “garden variety” obesity that affects nearly 40% of the US adult population1 behaves more like a risk factor than a disease. Think of other continuous variables like blood pressure and cholesterol—the higher the measurement, the higher the risk of a plethora of medical problems.
Obesity is a global public health problem that is due largely—at least in this country—to the widespread availability of inexpensive, calorie-packed foods, as well as a desire by a screen-addicted society to stay home and “play” online rather than outdoors. Obesity is a health risk factor produced by our current social milieu and modified by genetics and personal health habits.
So what can we do? We need to recognize our limited, but important, role and remain nonjudgmental with our overweight and obese patients when they are unsuccessful at losing weight. It is easy to play the blame game, even in subtle ways. Recognizing that obesity is more of a social issue than a personal behavioral issue is a great place to start. Asking patients what they want to do and helping them set goals and find the resources to reach their goals can be helpful. Celebrating even small decreases in weight or increases in physical activity is always good medicine. Remember that a 5% to 10% weight loss has medically beneficial effects, especially for patients with diabetes.2
In addition to recommendations (and referrals) to help patients reduce calories and increase exercise, we have other weight-loss tools to draw upon. Gastric bypass surgery is certainly effective—especially for obese patients with diabetes. And
So whether you consider obesity a disease, or not, we now have even more ways with which to combat it.
1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1-8.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
It depends on whom you ask. But if you ask me, obesity should not be labeled a disease.
I understand the rationale for calling obesity a disease—it helps legitimize the time we spend treating obesity and aids in getting paid for that time. Some people have distinct diseases, such as Prader-Willi syndrome, hypothyroidism, and Cushing’s syndrome that can cause obesity, and perhaps massive obesity is best categorized and treated as a disease. But the “garden variety” obesity that affects nearly 40% of the US adult population1 behaves more like a risk factor than a disease. Think of other continuous variables like blood pressure and cholesterol—the higher the measurement, the higher the risk of a plethora of medical problems.
Obesity is a global public health problem that is due largely—at least in this country—to the widespread availability of inexpensive, calorie-packed foods, as well as a desire by a screen-addicted society to stay home and “play” online rather than outdoors. Obesity is a health risk factor produced by our current social milieu and modified by genetics and personal health habits.
So what can we do? We need to recognize our limited, but important, role and remain nonjudgmental with our overweight and obese patients when they are unsuccessful at losing weight. It is easy to play the blame game, even in subtle ways. Recognizing that obesity is more of a social issue than a personal behavioral issue is a great place to start. Asking patients what they want to do and helping them set goals and find the resources to reach their goals can be helpful. Celebrating even small decreases in weight or increases in physical activity is always good medicine. Remember that a 5% to 10% weight loss has medically beneficial effects, especially for patients with diabetes.2
In addition to recommendations (and referrals) to help patients reduce calories and increase exercise, we have other weight-loss tools to draw upon. Gastric bypass surgery is certainly effective—especially for obese patients with diabetes. And
So whether you consider obesity a disease, or not, we now have even more ways with which to combat it.
It depends on whom you ask. But if you ask me, obesity should not be labeled a disease.
I understand the rationale for calling obesity a disease—it helps legitimize the time we spend treating obesity and aids in getting paid for that time. Some people have distinct diseases, such as Prader-Willi syndrome, hypothyroidism, and Cushing’s syndrome that can cause obesity, and perhaps massive obesity is best categorized and treated as a disease. But the “garden variety” obesity that affects nearly 40% of the US adult population1 behaves more like a risk factor than a disease. Think of other continuous variables like blood pressure and cholesterol—the higher the measurement, the higher the risk of a plethora of medical problems.
Obesity is a global public health problem that is due largely—at least in this country—to the widespread availability of inexpensive, calorie-packed foods, as well as a desire by a screen-addicted society to stay home and “play” online rather than outdoors. Obesity is a health risk factor produced by our current social milieu and modified by genetics and personal health habits.
So what can we do? We need to recognize our limited, but important, role and remain nonjudgmental with our overweight and obese patients when they are unsuccessful at losing weight. It is easy to play the blame game, even in subtle ways. Recognizing that obesity is more of a social issue than a personal behavioral issue is a great place to start. Asking patients what they want to do and helping them set goals and find the resources to reach their goals can be helpful. Celebrating even small decreases in weight or increases in physical activity is always good medicine. Remember that a 5% to 10% weight loss has medically beneficial effects, especially for patients with diabetes.2
In addition to recommendations (and referrals) to help patients reduce calories and increase exercise, we have other weight-loss tools to draw upon. Gastric bypass surgery is certainly effective—especially for obese patients with diabetes. And
So whether you consider obesity a disease, or not, we now have even more ways with which to combat it.
1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1-8.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
1. Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;219:1-8.
2. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.