User login
COMBI-AD: Adjuvant combo halves relapses in BRAF V600-mutated melanoma
MADRID – A combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) delivered in the adjuvant setting was associated with a halving of the risk for relapse compared with placebo among patients with advanced melanoma with BRAF V600 mutations, late-breaking results from a phase 3 trial show.
Among 438 patients with stage III BRAF V600-mutated melanoma randomly assigned after complete surgical resection to dabrafenib/trametinib in the COMBI-AD trial, the estimated rate of 3-year relapse-free survival (RFS) was 58%, compared with 39% for 432 patients assigned to double placebos. This difference translated into a hazard ratio for relapse with the dabrafenib/trametinib combination of 0.47 (P less than .001).
“The relapse-free survival benefits were observed across all 12 subgroups which have been evaluated, so there’s not a single subgroup that is an outlier,” he said in a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
Results of the study were published online concurrently in the New England Journal of Medicine.
In previous phase 3 trials in patients with BRAF V600 mutated metastatic or unresectable melanoma, the combination of dabrafenib and trametinib improved survival. Because treatment options for patients with resectable stage III melanomas are limited and less than optimal, the COMBI-AD investigators sought to explore whether the combination could improve outcomes when used in the adjuvant setting.
In the study reported by Dr. Hauschild, patients with completely resected, high-risk stage IIIA, IIIB, or IIIC cutaneous melanoma with the BRAF V600EK mutation who were surgically free of disease within 12 weeks of randomization were stratified by BRAF mutation status and disease stage, and then randomly assigned to receive either dabrafenib 150 mg twice daily plus trametinib 2 mg once daily, or two matched placebos.
The RFS curves separated early in the study, and at 1 year the rate of RFS was 88% among patients treated with the combinations, compared with 56% for patients who got placebo. The respective rates at 2 and 3 years of follow-up were 67% vs. 44%, and, as noted before, 58% vs. 39%.
At this first interim analysis, the 1-year OS rate with dabrafenib/trametinib was 97% compared with 94% for placebo. Respective rates at 2 and 3 years of follow-up were 91% vs. 83%, and 86% vs. 77%, but as noted, the Kaplan-Meier survival curves appear to separate, but have yet to reach the prespecified boundary for significance.
As might be expected, the incidence of any grade 3 or 4 adverse events was higher in the combination group than in the placebo group, but there were no fatal adverse events related to assigned treatment. In all, 26% of patients assigned to dabrafenib/trametinib had to discontinue treatment due to adverse events, compared with 3% of patients assigned to placebo.
Dr. Hauschild said that the results of the COMBI-AD study and the Checkmate 238 study presented on the same day “will make a change in our textbooks and our current guidelines, because we have at least two new treatment options, and I think this is a new treatment option and a good day for our melanoma patients.”
His remarks were echoed by Olivier Michielin, MD, PhD, of the Swiss Institute of Bioinformatics in Lausanne. He said that “we now have, with the data, two fantastic new options. We couldn’t dream those studies to be so positive. This is really something that will open new features for our patients.”
Dr. Michielin was invited by ESMO to comment on the study.
COMBI-AD was sponsored by GlaxoSmithKline. Dabrafenib and trametinib have been owned by Novartis AG since March, 2015. Dr. Hauschild disclosed trial support, honoraria, and/or consultancy fees from Novartis and others. Dr. Michielin disclosed consulting and/or honoraria from Amgen, BMS, Roche, MSD, Novartis, and GSK.
MADRID – A combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) delivered in the adjuvant setting was associated with a halving of the risk for relapse compared with placebo among patients with advanced melanoma with BRAF V600 mutations, late-breaking results from a phase 3 trial show.
Among 438 patients with stage III BRAF V600-mutated melanoma randomly assigned after complete surgical resection to dabrafenib/trametinib in the COMBI-AD trial, the estimated rate of 3-year relapse-free survival (RFS) was 58%, compared with 39% for 432 patients assigned to double placebos. This difference translated into a hazard ratio for relapse with the dabrafenib/trametinib combination of 0.47 (P less than .001).
“The relapse-free survival benefits were observed across all 12 subgroups which have been evaluated, so there’s not a single subgroup that is an outlier,” he said in a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
Results of the study were published online concurrently in the New England Journal of Medicine.
In previous phase 3 trials in patients with BRAF V600 mutated metastatic or unresectable melanoma, the combination of dabrafenib and trametinib improved survival. Because treatment options for patients with resectable stage III melanomas are limited and less than optimal, the COMBI-AD investigators sought to explore whether the combination could improve outcomes when used in the adjuvant setting.
In the study reported by Dr. Hauschild, patients with completely resected, high-risk stage IIIA, IIIB, or IIIC cutaneous melanoma with the BRAF V600EK mutation who were surgically free of disease within 12 weeks of randomization were stratified by BRAF mutation status and disease stage, and then randomly assigned to receive either dabrafenib 150 mg twice daily plus trametinib 2 mg once daily, or two matched placebos.
The RFS curves separated early in the study, and at 1 year the rate of RFS was 88% among patients treated with the combinations, compared with 56% for patients who got placebo. The respective rates at 2 and 3 years of follow-up were 67% vs. 44%, and, as noted before, 58% vs. 39%.
At this first interim analysis, the 1-year OS rate with dabrafenib/trametinib was 97% compared with 94% for placebo. Respective rates at 2 and 3 years of follow-up were 91% vs. 83%, and 86% vs. 77%, but as noted, the Kaplan-Meier survival curves appear to separate, but have yet to reach the prespecified boundary for significance.
As might be expected, the incidence of any grade 3 or 4 adverse events was higher in the combination group than in the placebo group, but there were no fatal adverse events related to assigned treatment. In all, 26% of patients assigned to dabrafenib/trametinib had to discontinue treatment due to adverse events, compared with 3% of patients assigned to placebo.
Dr. Hauschild said that the results of the COMBI-AD study and the Checkmate 238 study presented on the same day “will make a change in our textbooks and our current guidelines, because we have at least two new treatment options, and I think this is a new treatment option and a good day for our melanoma patients.”
His remarks were echoed by Olivier Michielin, MD, PhD, of the Swiss Institute of Bioinformatics in Lausanne. He said that “we now have, with the data, two fantastic new options. We couldn’t dream those studies to be so positive. This is really something that will open new features for our patients.”
Dr. Michielin was invited by ESMO to comment on the study.
COMBI-AD was sponsored by GlaxoSmithKline. Dabrafenib and trametinib have been owned by Novartis AG since March, 2015. Dr. Hauschild disclosed trial support, honoraria, and/or consultancy fees from Novartis and others. Dr. Michielin disclosed consulting and/or honoraria from Amgen, BMS, Roche, MSD, Novartis, and GSK.
MADRID – A combination of the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist) delivered in the adjuvant setting was associated with a halving of the risk for relapse compared with placebo among patients with advanced melanoma with BRAF V600 mutations, late-breaking results from a phase 3 trial show.
Among 438 patients with stage III BRAF V600-mutated melanoma randomly assigned after complete surgical resection to dabrafenib/trametinib in the COMBI-AD trial, the estimated rate of 3-year relapse-free survival (RFS) was 58%, compared with 39% for 432 patients assigned to double placebos. This difference translated into a hazard ratio for relapse with the dabrafenib/trametinib combination of 0.47 (P less than .001).
“The relapse-free survival benefits were observed across all 12 subgroups which have been evaluated, so there’s not a single subgroup that is an outlier,” he said in a briefing prior to his presentation of the data in a presidential symposium at the European Society for Medical Oncology Congress.
Results of the study were published online concurrently in the New England Journal of Medicine.
In previous phase 3 trials in patients with BRAF V600 mutated metastatic or unresectable melanoma, the combination of dabrafenib and trametinib improved survival. Because treatment options for patients with resectable stage III melanomas are limited and less than optimal, the COMBI-AD investigators sought to explore whether the combination could improve outcomes when used in the adjuvant setting.
In the study reported by Dr. Hauschild, patients with completely resected, high-risk stage IIIA, IIIB, or IIIC cutaneous melanoma with the BRAF V600EK mutation who were surgically free of disease within 12 weeks of randomization were stratified by BRAF mutation status and disease stage, and then randomly assigned to receive either dabrafenib 150 mg twice daily plus trametinib 2 mg once daily, or two matched placebos.
The RFS curves separated early in the study, and at 1 year the rate of RFS was 88% among patients treated with the combinations, compared with 56% for patients who got placebo. The respective rates at 2 and 3 years of follow-up were 67% vs. 44%, and, as noted before, 58% vs. 39%.
At this first interim analysis, the 1-year OS rate with dabrafenib/trametinib was 97% compared with 94% for placebo. Respective rates at 2 and 3 years of follow-up were 91% vs. 83%, and 86% vs. 77%, but as noted, the Kaplan-Meier survival curves appear to separate, but have yet to reach the prespecified boundary for significance.
As might be expected, the incidence of any grade 3 or 4 adverse events was higher in the combination group than in the placebo group, but there were no fatal adverse events related to assigned treatment. In all, 26% of patients assigned to dabrafenib/trametinib had to discontinue treatment due to adverse events, compared with 3% of patients assigned to placebo.
Dr. Hauschild said that the results of the COMBI-AD study and the Checkmate 238 study presented on the same day “will make a change in our textbooks and our current guidelines, because we have at least two new treatment options, and I think this is a new treatment option and a good day for our melanoma patients.”
His remarks were echoed by Olivier Michielin, MD, PhD, of the Swiss Institute of Bioinformatics in Lausanne. He said that “we now have, with the data, two fantastic new options. We couldn’t dream those studies to be so positive. This is really something that will open new features for our patients.”
Dr. Michielin was invited by ESMO to comment on the study.
COMBI-AD was sponsored by GlaxoSmithKline. Dabrafenib and trametinib have been owned by Novartis AG since March, 2015. Dr. Hauschild disclosed trial support, honoraria, and/or consultancy fees from Novartis and others. Dr. Michielin disclosed consulting and/or honoraria from Amgen, BMS, Roche, MSD, Novartis, and GSK.
AT ESMO 2017
Key clinical point: Adjuvant therapy with a BRAF/MEK inhibitor combination significantly improved outcomes for patients with stage III completely resectable melanoma.
Major finding: The hazard ratio for relapse with the dabrafenib/trametinib combination vs. placebo was 0.47 (P less than .001).
Data source: Randomized, placebo-controlled phase 3 trial of 870 patients with stage III, completely resectable BRAF-mutated melanoma.
Disclosures: COMBI-AD was sponsored by GlaxoSmithKline. Dabrafenib and trametinib have been owned by Novartis AG since March, 2015. Dr. Hauschild disclosed trial support, honoraria, and/or consultancy fees from Novartis and others. Dr. Michielin disclosed consulting and/or honoraria from Amgen, BMS, Roche, MSD, Novartis, and GSK.
Using Multi-Condition Assessment to Optimize Management of Mental Health in Primary Care
Click Here to Read Supplement.
Topics include:
- Detecting Mental Health Conditions
- What Is the Multi-Condition M3 Checklist?
- Office Procedure for Use of Checklist
Faculty/Faculty Disclosure:
Steven Daviss, MD
Chief Medical Informatics Officer,
M3 Information,
Rockville, Maryland
Gerald Hurowitz, MD
Chief Medical Officer to M3 Information,
Rockville, Maryland
Assistant Professor of Clinical Psychiatry,
Columbia University
Larry Culpepper, MD
Professor of Family Medicine
Boston University School of Medicine
Massachusetts
Drs. Daviss, Hurowitz, and Culpepper are members in M-3 Information, LLC.
Click Here to Read Supplement.
Topics include:
- Detecting Mental Health Conditions
- What Is the Multi-Condition M3 Checklist?
- Office Procedure for Use of Checklist
Faculty/Faculty Disclosure:
Steven Daviss, MD
Chief Medical Informatics Officer,
M3 Information,
Rockville, Maryland
Gerald Hurowitz, MD
Chief Medical Officer to M3 Information,
Rockville, Maryland
Assistant Professor of Clinical Psychiatry,
Columbia University
Larry Culpepper, MD
Professor of Family Medicine
Boston University School of Medicine
Massachusetts
Drs. Daviss, Hurowitz, and Culpepper are members in M-3 Information, LLC.
Click Here to Read Supplement.
Topics include:
- Detecting Mental Health Conditions
- What Is the Multi-Condition M3 Checklist?
- Office Procedure for Use of Checklist
Faculty/Faculty Disclosure:
Steven Daviss, MD
Chief Medical Informatics Officer,
M3 Information,
Rockville, Maryland
Gerald Hurowitz, MD
Chief Medical Officer to M3 Information,
Rockville, Maryland
Assistant Professor of Clinical Psychiatry,
Columbia University
Larry Culpepper, MD
Professor of Family Medicine
Boston University School of Medicine
Massachusetts
Drs. Daviss, Hurowitz, and Culpepper are members in M-3 Information, LLC.
Clinical trial: Complex VHR using biologic or synthetic mesh
The Study Comparing the Efficacy, Safety, and Cost of a Permanent, Synthetic Prosthetic Versus a Biologic Prosthetic in the One-Stage Repair of Ventral Hernias in Clean and Contaminated Wounds is an interventional trial currently recruiting patients with ventral hernias.
The trial will compare results of ventral hernia repair in patients who have received a biologic mesh made from pig skin with those who received a synthetic mesh made in a laboratory. Both study groups will include patients with and without infection near the hernia. Synthetic mesh is hypothesized to have a higher rate of early postoperative infection, while biologic mesh is hypothesized to have a higher rate of recurrence.
Patients will be included in the trial if they have a ventral hernia; are older than 21 years; are not pregnant; and have no allergic, religious, or ethical objections to polypropylene or porcine prosthetics. Reasons for exclusion include severe malnutrition, pre-existing parenchymal liver disease, immunocompromisation, and refractory ascites.
The primary outcome measure is recurrence within 24 months of surgery, and the secondary outcome measure is wound events within 24 months of surgery. Other outcome measures include early postoperative complications within 1 month of surgery and quality of life, pain, activity level, and overall cost with 24 months of surgery.
The study will end in June 2019. About 330 people are expected to be included in the final analysis.
Find more information at the study page on Clinicaltrials.gov.
The Study Comparing the Efficacy, Safety, and Cost of a Permanent, Synthetic Prosthetic Versus a Biologic Prosthetic in the One-Stage Repair of Ventral Hernias in Clean and Contaminated Wounds is an interventional trial currently recruiting patients with ventral hernias.
The trial will compare results of ventral hernia repair in patients who have received a biologic mesh made from pig skin with those who received a synthetic mesh made in a laboratory. Both study groups will include patients with and without infection near the hernia. Synthetic mesh is hypothesized to have a higher rate of early postoperative infection, while biologic mesh is hypothesized to have a higher rate of recurrence.
Patients will be included in the trial if they have a ventral hernia; are older than 21 years; are not pregnant; and have no allergic, religious, or ethical objections to polypropylene or porcine prosthetics. Reasons for exclusion include severe malnutrition, pre-existing parenchymal liver disease, immunocompromisation, and refractory ascites.
The primary outcome measure is recurrence within 24 months of surgery, and the secondary outcome measure is wound events within 24 months of surgery. Other outcome measures include early postoperative complications within 1 month of surgery and quality of life, pain, activity level, and overall cost with 24 months of surgery.
The study will end in June 2019. About 330 people are expected to be included in the final analysis.
Find more information at the study page on Clinicaltrials.gov.
The Study Comparing the Efficacy, Safety, and Cost of a Permanent, Synthetic Prosthetic Versus a Biologic Prosthetic in the One-Stage Repair of Ventral Hernias in Clean and Contaminated Wounds is an interventional trial currently recruiting patients with ventral hernias.
The trial will compare results of ventral hernia repair in patients who have received a biologic mesh made from pig skin with those who received a synthetic mesh made in a laboratory. Both study groups will include patients with and without infection near the hernia. Synthetic mesh is hypothesized to have a higher rate of early postoperative infection, while biologic mesh is hypothesized to have a higher rate of recurrence.
Patients will be included in the trial if they have a ventral hernia; are older than 21 years; are not pregnant; and have no allergic, religious, or ethical objections to polypropylene or porcine prosthetics. Reasons for exclusion include severe malnutrition, pre-existing parenchymal liver disease, immunocompromisation, and refractory ascites.
The primary outcome measure is recurrence within 24 months of surgery, and the secondary outcome measure is wound events within 24 months of surgery. Other outcome measures include early postoperative complications within 1 month of surgery and quality of life, pain, activity level, and overall cost with 24 months of surgery.
The study will end in June 2019. About 330 people are expected to be included in the final analysis.
Find more information at the study page on Clinicaltrials.gov.
SUMMARY FROM CLINICALTRIALS.GOV
Sleep issues vary by menopausal status
Perimenopausal women aged 40-59 years were less likely than were others in the same age group to average at least 7 hours’ sleep each night in 2015, according to the National Center for Health Statistics.
Among the perimenopausal women in that age group, 56% said that they slept less than 7 hours, on average, in a 24-hour period, compared with 40.5% of postmenopausal women and 32.5% of those who were premenopausal. Overall, 35.1% of women aged 40-59 did not average at least 7 hours of sleep per night, the NCHS reported in a data brief released Sept. 7.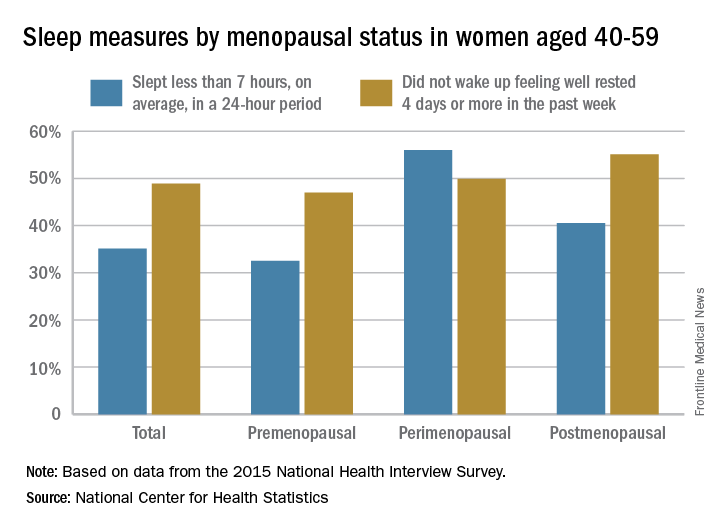
For this analysis, about 74% of the women included were premenopausal (still had a menstrual cycle), 4% were perimenopausal (last menstrual cycle was 1 year before or less), and 22% were postmenopausal (no menstrual cycle for more than 1 year or surgical menopause after removal of their ovaries).
Perimenopausal women aged 40-59 years were less likely than were others in the same age group to average at least 7 hours’ sleep each night in 2015, according to the National Center for Health Statistics.
Among the perimenopausal women in that age group, 56% said that they slept less than 7 hours, on average, in a 24-hour period, compared with 40.5% of postmenopausal women and 32.5% of those who were premenopausal. Overall, 35.1% of women aged 40-59 did not average at least 7 hours of sleep per night, the NCHS reported in a data brief released Sept. 7.
For this analysis, about 74% of the women included were premenopausal (still had a menstrual cycle), 4% were perimenopausal (last menstrual cycle was 1 year before or less), and 22% were postmenopausal (no menstrual cycle for more than 1 year or surgical menopause after removal of their ovaries).
Perimenopausal women aged 40-59 years were less likely than were others in the same age group to average at least 7 hours’ sleep each night in 2015, according to the National Center for Health Statistics.
Among the perimenopausal women in that age group, 56% said that they slept less than 7 hours, on average, in a 24-hour period, compared with 40.5% of postmenopausal women and 32.5% of those who were premenopausal. Overall, 35.1% of women aged 40-59 did not average at least 7 hours of sleep per night, the NCHS reported in a data brief released Sept. 7.
For this analysis, about 74% of the women included were premenopausal (still had a menstrual cycle), 4% were perimenopausal (last menstrual cycle was 1 year before or less), and 22% were postmenopausal (no menstrual cycle for more than 1 year or surgical menopause after removal of their ovaries).
Your job: Provide oral health promotion, fluoride varnish
Learn the “Nuts and Bolts of Office-Based Oral Health Promotion and Fluoride Varnish” from Melinda Clark, MD, and Rocio Quiñonez, DMD.
Caries affect 50% of 5- to 9-year-olds and 78% of 17-year-olds, yet 25% of poor children don’t see a dentist by age 5. You are in an excellent position to provide “timely preventive oral health interventions” in your office, the United States Preventive Services Task Force recommends that “primary care clinicians apply fluoride varnish to the primary teeth of all infants and children starting at the age of primary tooth eruption,” and fluoride varnish was added to the Bright Futures Periodicity Schedule in 2015.
At the American Academy of Pediatrics’ annual meeting in Chicago, Dr. Clark and Dr. Quiñonez will address the importance of dealing with early childhood caries (ECC), defined as one or more decayed, missing from dental caries, or filled tooth surfaces in any primary tooth in a preschool-age child between birth and younger than 6 years of age. ECC can result in missed school, inappropriate use of over-the-counter pain medication, disturbed sleep, eating dysfunction, infection, and even death.
Dr. Clark is an associate professor of pediatrics at Albany (N.Y.) Medical College, and Dr. Quiñonez is an associate professor of pediatric dentistry and pediatrics at the University of North Carolina at Chapel Hill.
Dr. Clark and Dr. Quiñonez will be presenting Sunday, Sept. 17, from 4 p.m. to 5:30 p.m., and Monday, Sept. 18, from 8:30 a.m. to 10 a.m. You don’t want to miss it!
Learn the “Nuts and Bolts of Office-Based Oral Health Promotion and Fluoride Varnish” from Melinda Clark, MD, and Rocio Quiñonez, DMD.
Caries affect 50% of 5- to 9-year-olds and 78% of 17-year-olds, yet 25% of poor children don’t see a dentist by age 5. You are in an excellent position to provide “timely preventive oral health interventions” in your office, the United States Preventive Services Task Force recommends that “primary care clinicians apply fluoride varnish to the primary teeth of all infants and children starting at the age of primary tooth eruption,” and fluoride varnish was added to the Bright Futures Periodicity Schedule in 2015.
At the American Academy of Pediatrics’ annual meeting in Chicago, Dr. Clark and Dr. Quiñonez will address the importance of dealing with early childhood caries (ECC), defined as one or more decayed, missing from dental caries, or filled tooth surfaces in any primary tooth in a preschool-age child between birth and younger than 6 years of age. ECC can result in missed school, inappropriate use of over-the-counter pain medication, disturbed sleep, eating dysfunction, infection, and even death.
Dr. Clark is an associate professor of pediatrics at Albany (N.Y.) Medical College, and Dr. Quiñonez is an associate professor of pediatric dentistry and pediatrics at the University of North Carolina at Chapel Hill.
Dr. Clark and Dr. Quiñonez will be presenting Sunday, Sept. 17, from 4 p.m. to 5:30 p.m., and Monday, Sept. 18, from 8:30 a.m. to 10 a.m. You don’t want to miss it!
Learn the “Nuts and Bolts of Office-Based Oral Health Promotion and Fluoride Varnish” from Melinda Clark, MD, and Rocio Quiñonez, DMD.
Caries affect 50% of 5- to 9-year-olds and 78% of 17-year-olds, yet 25% of poor children don’t see a dentist by age 5. You are in an excellent position to provide “timely preventive oral health interventions” in your office, the United States Preventive Services Task Force recommends that “primary care clinicians apply fluoride varnish to the primary teeth of all infants and children starting at the age of primary tooth eruption,” and fluoride varnish was added to the Bright Futures Periodicity Schedule in 2015.
At the American Academy of Pediatrics’ annual meeting in Chicago, Dr. Clark and Dr. Quiñonez will address the importance of dealing with early childhood caries (ECC), defined as one or more decayed, missing from dental caries, or filled tooth surfaces in any primary tooth in a preschool-age child between birth and younger than 6 years of age. ECC can result in missed school, inappropriate use of over-the-counter pain medication, disturbed sleep, eating dysfunction, infection, and even death.
Dr. Clark is an associate professor of pediatrics at Albany (N.Y.) Medical College, and Dr. Quiñonez is an associate professor of pediatric dentistry and pediatrics at the University of North Carolina at Chapel Hill.
Dr. Clark and Dr. Quiñonez will be presenting Sunday, Sept. 17, from 4 p.m. to 5:30 p.m., and Monday, Sept. 18, from 8:30 a.m. to 10 a.m. You don’t want to miss it!
New Data Show Rise in Epilepsy
Between 2010 and 2015, the number of adults with active epilepsy rose from 2.3 million to 3 million, according to the CDC. The number of children with epilepsy rose from 450,000 to 470,000.
The increases are likely due to population growth, the CDC says, or other unknown factors, such as an increased willingness to disclose. However, most states do not have data on epilepsy prevalence. This is the first time estimates have been modeled for every state. Moreover, epilepsy has been assessed only intermittently in population surveys. Before 2010, the last national estimates were based on 1986-1990 data.
Obviously, epilepsy is not rare. It also is a serious public health issue. People with epilepsy often have other conditions, such as stroke, heart disease, depression, or developmental delay, which complicate epilepsy management, impair quality of life, and contribute to early mortality, the CDC says. Epilepsy also is the costliest and second most common of 5 chronic conditions that have adverse impact on academic and health outcomes in children and adolescents. For instance, children with seizures are more likely to live in poverty, and their parents more frequently report food insecurity.
The CDC suggests that health care providers and others can use the findings to ensure that evidence-based programs meet the complex needs of adults and children living with epilepsy and reduce the disparities resulting from it.
Between 2010 and 2015, the number of adults with active epilepsy rose from 2.3 million to 3 million, according to the CDC. The number of children with epilepsy rose from 450,000 to 470,000.
The increases are likely due to population growth, the CDC says, or other unknown factors, such as an increased willingness to disclose. However, most states do not have data on epilepsy prevalence. This is the first time estimates have been modeled for every state. Moreover, epilepsy has been assessed only intermittently in population surveys. Before 2010, the last national estimates were based on 1986-1990 data.
Obviously, epilepsy is not rare. It also is a serious public health issue. People with epilepsy often have other conditions, such as stroke, heart disease, depression, or developmental delay, which complicate epilepsy management, impair quality of life, and contribute to early mortality, the CDC says. Epilepsy also is the costliest and second most common of 5 chronic conditions that have adverse impact on academic and health outcomes in children and adolescents. For instance, children with seizures are more likely to live in poverty, and their parents more frequently report food insecurity.
The CDC suggests that health care providers and others can use the findings to ensure that evidence-based programs meet the complex needs of adults and children living with epilepsy and reduce the disparities resulting from it.
Between 2010 and 2015, the number of adults with active epilepsy rose from 2.3 million to 3 million, according to the CDC. The number of children with epilepsy rose from 450,000 to 470,000.
The increases are likely due to population growth, the CDC says, or other unknown factors, such as an increased willingness to disclose. However, most states do not have data on epilepsy prevalence. This is the first time estimates have been modeled for every state. Moreover, epilepsy has been assessed only intermittently in population surveys. Before 2010, the last national estimates were based on 1986-1990 data.
Obviously, epilepsy is not rare. It also is a serious public health issue. People with epilepsy often have other conditions, such as stroke, heart disease, depression, or developmental delay, which complicate epilepsy management, impair quality of life, and contribute to early mortality, the CDC says. Epilepsy also is the costliest and second most common of 5 chronic conditions that have adverse impact on academic and health outcomes in children and adolescents. For instance, children with seizures are more likely to live in poverty, and their parents more frequently report food insecurity.
The CDC suggests that health care providers and others can use the findings to ensure that evidence-based programs meet the complex needs of adults and children living with epilepsy and reduce the disparities resulting from it.
Biosimilar deemed equivalent to reference drug in FL
MADRID—The biosimilar GP2013 has demonstrated equivalence to its reference drug rituximab in patients with previously untreated, advanced-stage follicular lymphoma (FL), according to researchers.
Treatment with GP2013 plus cyclophosphamide, vincristine, and prednisone (CVP) produced a similar overall response rate (ORR) as rituximab plus CVP in the phase 3 ASSIST-FL trial.
Survival rates were also similar between the treatment arms, as were adverse events (AEs).
Results from this study were published in The Lancet Haematology and presented at ESMO 2017 Congress (abstract 994O).
The study was funded by Hexal AG, a Sandoz company (part of the Novartis group), which is marketing GP2013 as Rixathon in Europe.
Patients and treatment
The trial included 629 patients with previously untreated, advanced-stage FL. They were randomized to receive 8 cycles of GP2013-CVP (n=314) or rituximab-CVP (n=315). Responders in either arm could receive monotherapy maintenance for up to 2 years.
The mean age was 57.5 in the GP2013 arm and 56.4 in the rituximab arm. Fifty-eight percent and 54% of patients, respectively, were female.
Fifty-seven percent of patients in the GP2013 arm and 56% in the rituximab arm had an ECOG performance status of 0. Forty percent and 39%, respectively, had a status of 1. Two percent and 4%, respectively, had a status of 2. (For the remaining 1% of patients in each arm, data on performance status were missing.)
Patients had an Ann Arbor stage of III—46% in the GP2013 arm and 43% in the GP2013 arm—or IV—54% in the GP2013 arm and 57% in the rituximab arm.
Fifty-six percent of patients in each arm were high-risk according to FLIPI. Thirty-four percent in the GP2013 arm and 33% in the rituximab arm were intermediate-risk. Ten percent and 11%, respectively, were low-risk.
Fourteen percent of patients in the GP2013 arm and 18% in the rituximab arm had bulky disease. Fifteen percent and 13%, respectively, had splenic involvement.
ORR and survival
The patients had a median follow-up of 23.8 months. The primary efficacy endpoint was equivalence in ORR, defined by a 95% confidence interval (CI) with a margin of ± 12% standard deviation.
The primary endpoint was met, as the ORR was 87% in the GP2013 arm and 88% in the rituximab arm, with a difference of –0.40% (95% CI –5.94%, 5.14%).
The complete response rate was 15% in the GP2013 arm and 13% in the rituximab arm. The partial response rates were 72% and 74%, respectively.
The median progression-free survival and overall survival have not been reached. However, the progression-free survival rate was 70% in the GP2013 arm and 76% in the rituximab arm (hazard ratio [HR]=1.31; 95% CI 1.02, 1.69).
The overall survival rate was 93% in the GP2013 arm and 91% in the rituximab arm (HR=0.77; 95% CI 0.49, 1.22).
Safety
During the combination phase, the incidence of AEs was 93% in the GP2013 arm and 91% in the rituximab arm. The incidence of serious AEs was 23% and 20%, respectively.
The most frequent AEs (in the GP2013 and rituximab arms, respectively) were neutropenia (26% and 30%), constipation (22% and 20%), and nausea (16% and 13%). The most common grade 3/4 AE was neutropenia (18% and 21%).
There were 11 deaths reported during the combination phase—4 in the GP2013 arm and 7 in the rituximab arm.
Three deaths in the GP2013 arm (sudden death, septic shock, and respiratory failure) and 2 deaths in the rituximab arm (multiple organ dysfunction syndrome and sepsis) were suspected to be related to study treatment.
During the maintenance phase, the incidence of AEs was 63% in the GP2013 arm and 57% in the rituximab arm. The incidence of serious AEs was 6% and 4%, respectively.
The most frequent AEs (in the GP2013 and rituximab arms, respectively) were infections and infestations (20% and 27%), neutropenia (10% and 6%), cough (9% and 6%), and upper respiratory tract infection (3% and 6%). The most common grade 3/4 AE was neutropenia (7% and 4%).
There were 4 deaths reported during the maintenance phase, 2 in each treatment arm. ![]()
MADRID—The biosimilar GP2013 has demonstrated equivalence to its reference drug rituximab in patients with previously untreated, advanced-stage follicular lymphoma (FL), according to researchers.
Treatment with GP2013 plus cyclophosphamide, vincristine, and prednisone (CVP) produced a similar overall response rate (ORR) as rituximab plus CVP in the phase 3 ASSIST-FL trial.
Survival rates were also similar between the treatment arms, as were adverse events (AEs).
Results from this study were published in The Lancet Haematology and presented at ESMO 2017 Congress (abstract 994O).
The study was funded by Hexal AG, a Sandoz company (part of the Novartis group), which is marketing GP2013 as Rixathon in Europe.
Patients and treatment
The trial included 629 patients with previously untreated, advanced-stage FL. They were randomized to receive 8 cycles of GP2013-CVP (n=314) or rituximab-CVP (n=315). Responders in either arm could receive monotherapy maintenance for up to 2 years.
The mean age was 57.5 in the GP2013 arm and 56.4 in the rituximab arm. Fifty-eight percent and 54% of patients, respectively, were female.
Fifty-seven percent of patients in the GP2013 arm and 56% in the rituximab arm had an ECOG performance status of 0. Forty percent and 39%, respectively, had a status of 1. Two percent and 4%, respectively, had a status of 2. (For the remaining 1% of patients in each arm, data on performance status were missing.)
Patients had an Ann Arbor stage of III—46% in the GP2013 arm and 43% in the GP2013 arm—or IV—54% in the GP2013 arm and 57% in the rituximab arm.
Fifty-six percent of patients in each arm were high-risk according to FLIPI. Thirty-four percent in the GP2013 arm and 33% in the rituximab arm were intermediate-risk. Ten percent and 11%, respectively, were low-risk.
Fourteen percent of patients in the GP2013 arm and 18% in the rituximab arm had bulky disease. Fifteen percent and 13%, respectively, had splenic involvement.
ORR and survival
The patients had a median follow-up of 23.8 months. The primary efficacy endpoint was equivalence in ORR, defined by a 95% confidence interval (CI) with a margin of ± 12% standard deviation.
The primary endpoint was met, as the ORR was 87% in the GP2013 arm and 88% in the rituximab arm, with a difference of –0.40% (95% CI –5.94%, 5.14%).
The complete response rate was 15% in the GP2013 arm and 13% in the rituximab arm. The partial response rates were 72% and 74%, respectively.
The median progression-free survival and overall survival have not been reached. However, the progression-free survival rate was 70% in the GP2013 arm and 76% in the rituximab arm (hazard ratio [HR]=1.31; 95% CI 1.02, 1.69).
The overall survival rate was 93% in the GP2013 arm and 91% in the rituximab arm (HR=0.77; 95% CI 0.49, 1.22).
Safety
During the combination phase, the incidence of AEs was 93% in the GP2013 arm and 91% in the rituximab arm. The incidence of serious AEs was 23% and 20%, respectively.
The most frequent AEs (in the GP2013 and rituximab arms, respectively) were neutropenia (26% and 30%), constipation (22% and 20%), and nausea (16% and 13%). The most common grade 3/4 AE was neutropenia (18% and 21%).
There were 11 deaths reported during the combination phase—4 in the GP2013 arm and 7 in the rituximab arm.
Three deaths in the GP2013 arm (sudden death, septic shock, and respiratory failure) and 2 deaths in the rituximab arm (multiple organ dysfunction syndrome and sepsis) were suspected to be related to study treatment.
During the maintenance phase, the incidence of AEs was 63% in the GP2013 arm and 57% in the rituximab arm. The incidence of serious AEs was 6% and 4%, respectively.
The most frequent AEs (in the GP2013 and rituximab arms, respectively) were infections and infestations (20% and 27%), neutropenia (10% and 6%), cough (9% and 6%), and upper respiratory tract infection (3% and 6%). The most common grade 3/4 AE was neutropenia (7% and 4%).
There were 4 deaths reported during the maintenance phase, 2 in each treatment arm. ![]()
MADRID—The biosimilar GP2013 has demonstrated equivalence to its reference drug rituximab in patients with previously untreated, advanced-stage follicular lymphoma (FL), according to researchers.
Treatment with GP2013 plus cyclophosphamide, vincristine, and prednisone (CVP) produced a similar overall response rate (ORR) as rituximab plus CVP in the phase 3 ASSIST-FL trial.
Survival rates were also similar between the treatment arms, as were adverse events (AEs).
Results from this study were published in The Lancet Haematology and presented at ESMO 2017 Congress (abstract 994O).
The study was funded by Hexal AG, a Sandoz company (part of the Novartis group), which is marketing GP2013 as Rixathon in Europe.
Patients and treatment
The trial included 629 patients with previously untreated, advanced-stage FL. They were randomized to receive 8 cycles of GP2013-CVP (n=314) or rituximab-CVP (n=315). Responders in either arm could receive monotherapy maintenance for up to 2 years.
The mean age was 57.5 in the GP2013 arm and 56.4 in the rituximab arm. Fifty-eight percent and 54% of patients, respectively, were female.
Fifty-seven percent of patients in the GP2013 arm and 56% in the rituximab arm had an ECOG performance status of 0. Forty percent and 39%, respectively, had a status of 1. Two percent and 4%, respectively, had a status of 2. (For the remaining 1% of patients in each arm, data on performance status were missing.)
Patients had an Ann Arbor stage of III—46% in the GP2013 arm and 43% in the GP2013 arm—or IV—54% in the GP2013 arm and 57% in the rituximab arm.
Fifty-six percent of patients in each arm were high-risk according to FLIPI. Thirty-four percent in the GP2013 arm and 33% in the rituximab arm were intermediate-risk. Ten percent and 11%, respectively, were low-risk.
Fourteen percent of patients in the GP2013 arm and 18% in the rituximab arm had bulky disease. Fifteen percent and 13%, respectively, had splenic involvement.
ORR and survival
The patients had a median follow-up of 23.8 months. The primary efficacy endpoint was equivalence in ORR, defined by a 95% confidence interval (CI) with a margin of ± 12% standard deviation.
The primary endpoint was met, as the ORR was 87% in the GP2013 arm and 88% in the rituximab arm, with a difference of –0.40% (95% CI –5.94%, 5.14%).
The complete response rate was 15% in the GP2013 arm and 13% in the rituximab arm. The partial response rates were 72% and 74%, respectively.
The median progression-free survival and overall survival have not been reached. However, the progression-free survival rate was 70% in the GP2013 arm and 76% in the rituximab arm (hazard ratio [HR]=1.31; 95% CI 1.02, 1.69).
The overall survival rate was 93% in the GP2013 arm and 91% in the rituximab arm (HR=0.77; 95% CI 0.49, 1.22).
Safety
During the combination phase, the incidence of AEs was 93% in the GP2013 arm and 91% in the rituximab arm. The incidence of serious AEs was 23% and 20%, respectively.
The most frequent AEs (in the GP2013 and rituximab arms, respectively) were neutropenia (26% and 30%), constipation (22% and 20%), and nausea (16% and 13%). The most common grade 3/4 AE was neutropenia (18% and 21%).
There were 11 deaths reported during the combination phase—4 in the GP2013 arm and 7 in the rituximab arm.
Three deaths in the GP2013 arm (sudden death, septic shock, and respiratory failure) and 2 deaths in the rituximab arm (multiple organ dysfunction syndrome and sepsis) were suspected to be related to study treatment.
During the maintenance phase, the incidence of AEs was 63% in the GP2013 arm and 57% in the rituximab arm. The incidence of serious AEs was 6% and 4%, respectively.
The most frequent AEs (in the GP2013 and rituximab arms, respectively) were infections and infestations (20% and 27%), neutropenia (10% and 6%), cough (9% and 6%), and upper respiratory tract infection (3% and 6%). The most common grade 3/4 AE was neutropenia (7% and 4%).
There were 4 deaths reported during the maintenance phase, 2 in each treatment arm. ![]()
Treatment for WDTC tied to increased risk of AML
MADRID—New research suggests patients with well-differentiated thyroid cancer (WDTC) are more likely to develop acute myeloid leukemia (AML) if they receive radioactive iodine (RAI) after surgery.
Of the WDTC patients studied, those who only underwent surgery were less likely to develop AML.
WDTC patients who did develop AML had a far worse prognosis than WDTC patients without AML and patients with de novo AML.
These results were presented at the ESMO 2017 Congress (abstract 996O).
Researchers already knew that the risk of developing leukemia is increased after radiation and RAI treatment for WDTC.
However, the risk of AML following RAI treatment in WDTC survivors had not been fully characterized, according to Remco J. Molenaar, an MD/PhD candidate at Academic Medical Centre in Amsterdam, Netherlands.
Therefore, Molenaar and colleagues reviewed data from all 18 registries in the Surveillance Epidemiology and End Results database for WDTC cases treated solely with surgery or by surgery and RAI.
The researchers identified 148,215 patients who were diagnosed with WDTC from 1973 to 2014. Fifty-five percent were treated with surgery alone, and 45% were treated with surgery and RAI.
The median follow-up was 4.3 person-years. AML occurred in 44 patients in the surgery-only arm and 56 patients in the RAI arm.
A comparison to the background rates in the general population showed that patients receiving surgery plus RAI had an increased risk of developing AML (relative risk=5.6; 95% confidence interval [CI] 3.8, 8.1; P<0.0001), after correcting for age, sex, and year of WDTC diagnosis.
This risk peaked within the first 3 years following RAI and subsequently regressed to baseline rates.
In multivariate analysis that corrected for sex and WDTC tumor size, 3 variables emerged as independent predictors of AML development. There was an increased risk of AML associated with:
- Patient age (hazard ratio [HR]=1.03; 95% CI 1.02, 1.05; P<0.001)
- WDTC tumor stage (HR=1.36; 95% CI 1.04, 1.79; P=0.03)
- Receiving RAI after thyroidectomy vs thyroidectomy alone (HR=1.38; 95% CI 1.09, 1.75; P=0.007).
The researchers also found prognosis is significantly poorer in WDTC patients who develop AML following RAI and in patients with spontaneous AML development.
Case-control analyses revealed that WDTC patients who developed AML after surgery and RAI survived one-third as long as matched control patients who were successfully treated for WDTC but did not develop AML. The median overall survival was 7.4 years and 24.4 years, respectively (P<0.0001).
Patients who were diagnosed with AML after RAI treatment also had a significantly worse prognosis than patients with de novo AML. The median overall survival was 1.2 years and 3.5 years, respectively (P=0.004).
The researchers noted that rates of AML in WDTC survivors will likely continue to rise due to the increasing incidence of WDTC, the young ages at which most WDTC diagnoses are made, and the otherwise high survival rates of patients with WDTC. ![]()
MADRID—New research suggests patients with well-differentiated thyroid cancer (WDTC) are more likely to develop acute myeloid leukemia (AML) if they receive radioactive iodine (RAI) after surgery.
Of the WDTC patients studied, those who only underwent surgery were less likely to develop AML.
WDTC patients who did develop AML had a far worse prognosis than WDTC patients without AML and patients with de novo AML.
These results were presented at the ESMO 2017 Congress (abstract 996O).
Researchers already knew that the risk of developing leukemia is increased after radiation and RAI treatment for WDTC.
However, the risk of AML following RAI treatment in WDTC survivors had not been fully characterized, according to Remco J. Molenaar, an MD/PhD candidate at Academic Medical Centre in Amsterdam, Netherlands.
Therefore, Molenaar and colleagues reviewed data from all 18 registries in the Surveillance Epidemiology and End Results database for WDTC cases treated solely with surgery or by surgery and RAI.
The researchers identified 148,215 patients who were diagnosed with WDTC from 1973 to 2014. Fifty-five percent were treated with surgery alone, and 45% were treated with surgery and RAI.
The median follow-up was 4.3 person-years. AML occurred in 44 patients in the surgery-only arm and 56 patients in the RAI arm.
A comparison to the background rates in the general population showed that patients receiving surgery plus RAI had an increased risk of developing AML (relative risk=5.6; 95% confidence interval [CI] 3.8, 8.1; P<0.0001), after correcting for age, sex, and year of WDTC diagnosis.
This risk peaked within the first 3 years following RAI and subsequently regressed to baseline rates.
In multivariate analysis that corrected for sex and WDTC tumor size, 3 variables emerged as independent predictors of AML development. There was an increased risk of AML associated with:
- Patient age (hazard ratio [HR]=1.03; 95% CI 1.02, 1.05; P<0.001)
- WDTC tumor stage (HR=1.36; 95% CI 1.04, 1.79; P=0.03)
- Receiving RAI after thyroidectomy vs thyroidectomy alone (HR=1.38; 95% CI 1.09, 1.75; P=0.007).
The researchers also found prognosis is significantly poorer in WDTC patients who develop AML following RAI and in patients with spontaneous AML development.
Case-control analyses revealed that WDTC patients who developed AML after surgery and RAI survived one-third as long as matched control patients who were successfully treated for WDTC but did not develop AML. The median overall survival was 7.4 years and 24.4 years, respectively (P<0.0001).
Patients who were diagnosed with AML after RAI treatment also had a significantly worse prognosis than patients with de novo AML. The median overall survival was 1.2 years and 3.5 years, respectively (P=0.004).
The researchers noted that rates of AML in WDTC survivors will likely continue to rise due to the increasing incidence of WDTC, the young ages at which most WDTC diagnoses are made, and the otherwise high survival rates of patients with WDTC. ![]()
MADRID—New research suggests patients with well-differentiated thyroid cancer (WDTC) are more likely to develop acute myeloid leukemia (AML) if they receive radioactive iodine (RAI) after surgery.
Of the WDTC patients studied, those who only underwent surgery were less likely to develop AML.
WDTC patients who did develop AML had a far worse prognosis than WDTC patients without AML and patients with de novo AML.
These results were presented at the ESMO 2017 Congress (abstract 996O).
Researchers already knew that the risk of developing leukemia is increased after radiation and RAI treatment for WDTC.
However, the risk of AML following RAI treatment in WDTC survivors had not been fully characterized, according to Remco J. Molenaar, an MD/PhD candidate at Academic Medical Centre in Amsterdam, Netherlands.
Therefore, Molenaar and colleagues reviewed data from all 18 registries in the Surveillance Epidemiology and End Results database for WDTC cases treated solely with surgery or by surgery and RAI.
The researchers identified 148,215 patients who were diagnosed with WDTC from 1973 to 2014. Fifty-five percent were treated with surgery alone, and 45% were treated with surgery and RAI.
The median follow-up was 4.3 person-years. AML occurred in 44 patients in the surgery-only arm and 56 patients in the RAI arm.
A comparison to the background rates in the general population showed that patients receiving surgery plus RAI had an increased risk of developing AML (relative risk=5.6; 95% confidence interval [CI] 3.8, 8.1; P<0.0001), after correcting for age, sex, and year of WDTC diagnosis.
This risk peaked within the first 3 years following RAI and subsequently regressed to baseline rates.
In multivariate analysis that corrected for sex and WDTC tumor size, 3 variables emerged as independent predictors of AML development. There was an increased risk of AML associated with:
- Patient age (hazard ratio [HR]=1.03; 95% CI 1.02, 1.05; P<0.001)
- WDTC tumor stage (HR=1.36; 95% CI 1.04, 1.79; P=0.03)
- Receiving RAI after thyroidectomy vs thyroidectomy alone (HR=1.38; 95% CI 1.09, 1.75; P=0.007).
The researchers also found prognosis is significantly poorer in WDTC patients who develop AML following RAI and in patients with spontaneous AML development.
Case-control analyses revealed that WDTC patients who developed AML after surgery and RAI survived one-third as long as matched control patients who were successfully treated for WDTC but did not develop AML. The median overall survival was 7.4 years and 24.4 years, respectively (P<0.0001).
Patients who were diagnosed with AML after RAI treatment also had a significantly worse prognosis than patients with de novo AML. The median overall survival was 1.2 years and 3.5 years, respectively (P=0.004).
The researchers noted that rates of AML in WDTC survivors will likely continue to rise due to the increasing incidence of WDTC, the young ages at which most WDTC diagnoses are made, and the otherwise high survival rates of patients with WDTC. ![]()
Synthetic heparin poised for clinical trials, team says
Researchers say they have synthesized low molecular weight heparin (LMWH) that may someday replace animal-sourced heparin.
The team created heparin dodecasaccharides (12-mers) using a manufacturing method that yielded gram quantities—roughly 1000-fold more than previous approaches used to synthesize LMWHs.
One of these dodecasaccharides, called 12-mer-1, demonstrated safety and efficacy in animals models.
Robert Linhardt, PhD, of Rensselaer Polytechnic Institute in Troy, New York, and his colleagues detailed this research in Science Translational Medicine.
The researchers tested 12-mer-1 in a mouse model of venous thrombosis induced by stenosis of the inferior vena cava. Twenty-four hours after stenosis, 12-mer-1 had reduced clot weight by about 60% (P<0.05), when compared to phosphate-buffered saline.
The effects of 12-mer-1 were comparable to those achieved with enoxaparin. However, the dose of 12-mer-1 (1.5 mg/kg) was one-fifth the dose of enoxaparin (7.5 mg/kg). This suggests 12-mer-1 has “considerably higher antithrombotic potency” than enoxaparin, according to the researchers.
Dr Linhardt and his colleagues also tested 12-mer-1 in a mouse model of sickle cell disease. The team said the anticoagulant (given at 2.0 mg/kg every 8 hours for 7 days) “significantly attenuated the activation of coagulation” when compared to saline (P<0.05).
The researchers then tested the clearance of 12-mer-1 in mice with severe kidney failure.
The team observed significant impairment of clearance for both high-dose (1.5 mg/kg) and low-dose (0.3 mg/kg) 12-mer-1 (P<0.05). However, the impairment of 12-mer-1 clearance was dependent upon the severity of the kidney injury.
The researchers also performed toxicology studies of 12-mer-1 in rats. The animals received a single dose of 12-mer-1 at 3600 mg/kg per day for 7 consecutive days.
The rats experienced a decrease in white blood cell counts, but this was within the historical control data range. Two additional doses of 12-mer-1 (400 and 1200 mg/kg per day) produced similar results.
Therefore, Dr Linhardt and his colleagues concluded that 12-mer-1 was well-tolerated.
The researchers also noted that anticoagulation with 12-mer-1 was completely reversible via treatment with protamine, which could potentially reduce bleeding risk.
The team believes that, with substantial optimization, 12-mer-1 could be suitable for industrial-scale synthesis.
“This is at the cusp of clinical trials and commercial use,” Dr Linhardt said. “There is no question about the science. We have proven that this is a safer, more effective alternative to its natural counterpart, and what now determines its success or failure is the marketplace.” ![]()
Researchers say they have synthesized low molecular weight heparin (LMWH) that may someday replace animal-sourced heparin.
The team created heparin dodecasaccharides (12-mers) using a manufacturing method that yielded gram quantities—roughly 1000-fold more than previous approaches used to synthesize LMWHs.
One of these dodecasaccharides, called 12-mer-1, demonstrated safety and efficacy in animals models.
Robert Linhardt, PhD, of Rensselaer Polytechnic Institute in Troy, New York, and his colleagues detailed this research in Science Translational Medicine.
The researchers tested 12-mer-1 in a mouse model of venous thrombosis induced by stenosis of the inferior vena cava. Twenty-four hours after stenosis, 12-mer-1 had reduced clot weight by about 60% (P<0.05), when compared to phosphate-buffered saline.
The effects of 12-mer-1 were comparable to those achieved with enoxaparin. However, the dose of 12-mer-1 (1.5 mg/kg) was one-fifth the dose of enoxaparin (7.5 mg/kg). This suggests 12-mer-1 has “considerably higher antithrombotic potency” than enoxaparin, according to the researchers.
Dr Linhardt and his colleagues also tested 12-mer-1 in a mouse model of sickle cell disease. The team said the anticoagulant (given at 2.0 mg/kg every 8 hours for 7 days) “significantly attenuated the activation of coagulation” when compared to saline (P<0.05).
The researchers then tested the clearance of 12-mer-1 in mice with severe kidney failure.
The team observed significant impairment of clearance for both high-dose (1.5 mg/kg) and low-dose (0.3 mg/kg) 12-mer-1 (P<0.05). However, the impairment of 12-mer-1 clearance was dependent upon the severity of the kidney injury.
The researchers also performed toxicology studies of 12-mer-1 in rats. The animals received a single dose of 12-mer-1 at 3600 mg/kg per day for 7 consecutive days.
The rats experienced a decrease in white blood cell counts, but this was within the historical control data range. Two additional doses of 12-mer-1 (400 and 1200 mg/kg per day) produced similar results.
Therefore, Dr Linhardt and his colleagues concluded that 12-mer-1 was well-tolerated.
The researchers also noted that anticoagulation with 12-mer-1 was completely reversible via treatment with protamine, which could potentially reduce bleeding risk.
The team believes that, with substantial optimization, 12-mer-1 could be suitable for industrial-scale synthesis.
“This is at the cusp of clinical trials and commercial use,” Dr Linhardt said. “There is no question about the science. We have proven that this is a safer, more effective alternative to its natural counterpart, and what now determines its success or failure is the marketplace.” ![]()
Researchers say they have synthesized low molecular weight heparin (LMWH) that may someday replace animal-sourced heparin.
The team created heparin dodecasaccharides (12-mers) using a manufacturing method that yielded gram quantities—roughly 1000-fold more than previous approaches used to synthesize LMWHs.
One of these dodecasaccharides, called 12-mer-1, demonstrated safety and efficacy in animals models.
Robert Linhardt, PhD, of Rensselaer Polytechnic Institute in Troy, New York, and his colleagues detailed this research in Science Translational Medicine.
The researchers tested 12-mer-1 in a mouse model of venous thrombosis induced by stenosis of the inferior vena cava. Twenty-four hours after stenosis, 12-mer-1 had reduced clot weight by about 60% (P<0.05), when compared to phosphate-buffered saline.
The effects of 12-mer-1 were comparable to those achieved with enoxaparin. However, the dose of 12-mer-1 (1.5 mg/kg) was one-fifth the dose of enoxaparin (7.5 mg/kg). This suggests 12-mer-1 has “considerably higher antithrombotic potency” than enoxaparin, according to the researchers.
Dr Linhardt and his colleagues also tested 12-mer-1 in a mouse model of sickle cell disease. The team said the anticoagulant (given at 2.0 mg/kg every 8 hours for 7 days) “significantly attenuated the activation of coagulation” when compared to saline (P<0.05).
The researchers then tested the clearance of 12-mer-1 in mice with severe kidney failure.
The team observed significant impairment of clearance for both high-dose (1.5 mg/kg) and low-dose (0.3 mg/kg) 12-mer-1 (P<0.05). However, the impairment of 12-mer-1 clearance was dependent upon the severity of the kidney injury.
The researchers also performed toxicology studies of 12-mer-1 in rats. The animals received a single dose of 12-mer-1 at 3600 mg/kg per day for 7 consecutive days.
The rats experienced a decrease in white blood cell counts, but this was within the historical control data range. Two additional doses of 12-mer-1 (400 and 1200 mg/kg per day) produced similar results.
Therefore, Dr Linhardt and his colleagues concluded that 12-mer-1 was well-tolerated.
The researchers also noted that anticoagulation with 12-mer-1 was completely reversible via treatment with protamine, which could potentially reduce bleeding risk.
The team believes that, with substantial optimization, 12-mer-1 could be suitable for industrial-scale synthesis.
“This is at the cusp of clinical trials and commercial use,” Dr Linhardt said. “There is no question about the science. We have proven that this is a safer, more effective alternative to its natural counterpart, and what now determines its success or failure is the marketplace.” ![]()
The Benefits of Exercise for Patients With Multiple Sclerosis
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
Q) Should I recommend exercise to my patients living with MS?
Multiple sclerosis (MS) causes varied symptoms and functional impairment, depending on what part of the central nervous system is involved. Currently, many patients living with MS have sedentary lifestyles, which increases the risk for comorbidities such as cardiovascular disease, type 2 diabetes, and osteoporosis.1-3
Some MS symptoms—ambulatory difficulty, balance impairment, heat intolerance, muscle weakness, spasticity, visual impairment, and fatigue—act as obstacles to routine physical exercise; they also typically worsen over the course of the disease.2-5 In addition, psychosocial factors such as lower levels of education, single status, smoking, and depression or anxiety have been shown to increase the likelihood that a patient will not meet the World Health Organization’s recommendations on physical activity for health.1
For many years, MS patients were advised against physical activity out of concern that it would exacerbate symptoms.6 It is likely still true that patients who fear worsened symptoms or have higher levels of disability avoid physical activity.2-5 Unfortunately, for persons living with MS, this cycle of fear and reduced activity perpetuates itself, resulting in increased disability and decreased quality of life. Thankfully, many of the physical and social factors that prevent patients from exercising are modifiable.1,4
Many types of exercise have been studied in patients living with MS; those shown to be beneficial include regimens focused on cardiovascular fitness, resistance training, balance, and flexibility. Evidence supports the benefits of exercise training for improving overall fitness, muscle strength, ambulation, cognition, spasticity, fatigue, and anxiety and depression in patients with MS.2-4,6-9 Exercise with aerobic, anaerobic, or resistance training has been considered an important nonpharmacologic treatment for MS patients to improve quality of life without worsening disease symptoms.9 There is increasing evidence that engaging in more physical activity and improving physical fitness is an important modality to improve disease course and slow progression over time.
Any increase in symptoms related to exercise is transient, and there is no evidence of lasting harmful effects on overall day-to-day functioning or association with disease progression.6,10 Patient reports of the perceived benefits of exercise include maintenance of physical function, increased social involvement, and feelings of self-management and control.5 Thus, if patients can comply with an exercise regimen, much of the initial disability that limited their activity may be reduced.
More research is needed to fully elucidate what type of exercise is most beneficial for an individual patient.4,5,8,9 However, the benefits of exercise are clear: It can significantly improve quality of life by enhancing psychologic and physical functioning.1,3,5,6,8 Given this information, patients living with MS have incentives to exercise. Health care providers should endorse the benefits of exercise and work to help patients reduce barriers to physical activity.1-5—RR
Rebecca Rahn, MPA-C, MSCS
Augusta MS Center
Neurology Department, Augusta University, Georgia
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.
1. Reider N, Salter AR, Cutter GR, et al. Potentially modifiable factors associated with physical activity in people living with multiple sclerosis. Res Nurs Health. 2017;40(2):143-152.
2. Sebastiao E, Learmonth YC, Motl RM. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross sectional study. Am J Phys Med Rehabil. 2017;96:357-361.
3. Vister E, Tijsma ME, Hoang PD, Lord SR. Fatigue, physical activity, quality of life, and fall risk in people with multiple sclerosis. Int J MS Care. 2017;19:91-98.
4. Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31-39.
5. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. 2016;38(13):1227-1242.
6. Paul L, Coote S, Crosbie J, et al. Core outcome measures for exercise studies in people with multiple sclerosis: recommendations from a multidisciplinary consensus meeting. Mult Scler. 2014;20(12):1641-1650.
7. Sandroff BM, Motl RW, Scuddler MR, Deluca J. Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev. 2016;26(3):271-294.
8. Hugos CL, Bourdette D, Chen YCZ, Cameron M. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: a randomized, controlled pilot trial. Mult Scler J Exp Transl Clin. 2017;3(1):1-11.
9. Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91-99.
10. Smith RM, Adeney-Steel M, Fulcher G, Longley WA. Symptom change with exercise is a temporary phenomenon for people with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(5):723-727.