User login
Patterns and Appropriateness of Thrombophilia Testing in an Academic Medical Center
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
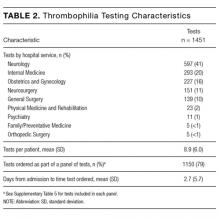
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
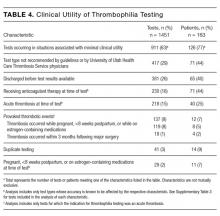
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
© 2017 Society of Hospital Medicine
A Randomized Controlled Trial of a CPR Decision Support Video for Patients Admitted to the General Medicine Service
Discussions about cardiopulmonary resuscitation (CPR) can be difficult due to their association with end of life. The Patient Self Determination Act (H.R.4449 — 101st Congress [1989-1990]) and institutional standards mandate collaboration between care providers and patients regarding goals of care in emergency situations such as cardiopulmonary arrest. The default option is to provide CPR, which may involve chest compressions, intubation, and/or defibrillation. Yet numerous studies show that a significant number of patients have no code preference documented in their medical chart, and even fewer report a conversation with their care provider about their wishes regarding CPR.1-3 CPR is an invasive and potentially painful procedure with a higher chance of failure than success4, and yet many patients report that their provider did not discuss with them the risks and benefits of resuscitation.5,6 Further highlighting the importance of individual discussions about CPR preferences is the reality that factors such as age and disease burden further skew the likelihood of survival after cardiopulmonary arrest.7
Complicating the lack of appropriate provider and patient discussion of the risks and benefits of resuscitation are significant misunderstandings about CPR in the lay population. Patients routinely overestimate the likelihood of survival following CPR.8,9 This may be partially due to the portrayal of CPR in the lay media as highly efficacious.10 Other factors known to prevent effective provider-and-patient discussions about CPR preferences are providers’ discomfort with the subject11 and perceived time constraints.12
Informational videos have been developed to assist patients with decision making about CPR and have been shown to impact patients’ choices in the setting of life-limiting diseases such as advanced cancer,13-14 serious illness with a prognosis of less than 1 year,15 and dementia.16 While discussion of code status is vitally important in end-of-life planning for seriously ill individuals, delayed discussion of CPR preferences is associated with a significant increase in the number of invasive procedures performed at the end of life, increased length of stay in the hospital, and increased medical cost.17 Despite clear evidence that earlier discussion of resuscitation options are valuable, no studies have examined the impact of a video about code status options in the general patient population.
Here we present our findings of a randomized trial in patients hospitalized on the general medicine wards who were 65 years of age or older, regardless of illness severity or diagnosis. The video tool was a supplement for, rather than a replacement of, standard provider and patient communication about code preferences, and we compared patients who watched the video against controls who had standard discussions with their providers. Our video detailed the process of chest compressions and intubation during CPR and explained the differences between the code statuses: full code, do not resuscitate (DNR), and do not resuscitate/do not intubate (DNR/DNI). We found a significant difference between the 2 groups, with significantly more individuals in the video group choosing DNR/DNI. These findings suggest that video support tools may be a useful supplement to traditional provider discussions about code preferences in the general patient population.
METHODS
We enrolled patients from the general medicine wards at the Minneapolis VA Hospital from September 28, 2015 to October 23, 2015. Eligibility criteria included age 65 years or older, ability to provide informed consent, and ability to communicate in English. Study recruitment and data collection were performed by a study coordinator who was a house staff physician and had no role in the care of the participants. The medical charts of all general medicine patients were reviewed to determine if they met the age criteria. The physician of record for potential participants was contacted to assess if the patient was able to provide informed consent and communicate in English. Eligible patients were approached and informed consent was obtained from those who chose to participate in the study. After obtaining informed consent, patients were randomized using a random number generator to the intervention or usual-care arm of the study.
Those who were assigned to the intervention arm watched a 6-minute long video explaining the code-preference choices of full code, DNR, or DNR/DNI. Full code was described as possibly including CPR, intubation, and/or defibrillation depending on the clinical situation. Do not resuscitate was described as meaning no CPR or defibrillation but possible intubation in the case of respiratory failure. Do not resuscitate/do not intubate was explained as meaning no CPR, no defibrillation, and no intubation but rather permitting “natural death” to occur. The video showed a mock code with chest compressions, defibrillation, and intubation on a mannequin as well as palliative care specialists who discussed potential complications and survival rates of inhospital resuscitation.
The video was created at the University of Minnesota with the departments of palliative care and internal medicine (www.mmcgmeservices.org/codestat.html). After viewing the video, participants in the intervention arm filled out a questionnaire designed to assess their knowledge and beliefs about CPR and trust in their medical care providers. They were asked to circle their code preference. The participants’ medical teams were made aware of the code preferences and were counseled to discuss code preferences further if it was different from their previously documented code preference.
Participants in the control arm were assigned to usual care. At the institution where this study occurred, a discussion about code preferences between the patient and their medical team is considered the standard of care. After informed consent was obtained, participants filled out the same questionnaire as the participants in the intervention arm. They were asked to circle their code status preference. If they chose to ask questions about resuscitation, these were answered, but the study coordinator did not volunteer information about resuscitation or intervene in the medical care of the participants in any way.
All participants’ demographic characteristics and outcomes were described using proportions for categorical variables and means ± standard deviation for continuous variables. The primary outcome was participants’ stated code preference (full code, DNR, or DNR/DNI). Secondary outcomes included comparison of trust in medical providers, resuscitation beliefs, and desire for life-prolonging interventions as obtained from the questionnaire.
We analyzed code preferences between the intervention and control groups using Fisher exact test. We used analysis of variance (ANOVA) to compare questionnaire responses between the 2 groups. All reported P values are 2-sided with P < 0.05 considered significant. The project originally targeted a sample size of 194 participants for 80% power to detect a 20% difference in the code preference choices between intervention and control groups. Given the short time frame available to enroll participants, the target sample size was not reached. Propitiously, the effect size was greater than originally expected.
RESULTS
Study Participants
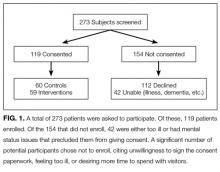
A total of 273 potentially eligible patients were approached to participate and 119 (44%) enrolled. (Figure 1). Of the 154 patients that were deemed eligible after initial screening, 42 patients were unable to give consent due to the severity of their illness or because of their mental status. Another 112 patients declined participation in the study, citing reasons such as disinterest in the consent paperwork, desire to spend time with visitors, and unease with the subject matter. Patients who declined participation did not differ significantly by age, sex, or race from those enrolled in the study.
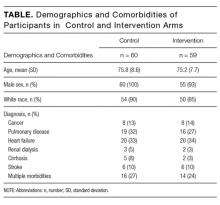
Among the 119 participants, 60 were randomized to the control arm, and 59 were randomized to the intervention arm. Participants in the 2 arms did not differ significantly in age, sex, or race (P > 0.05), although all 4 women in the study were randomized to the intervention arm. Eighty-seven percent of the study population identified as white with the remainder including black, Asian, Pacific Islander, Native American, or declining to answer. The mean age was 75.8 years in the control arm vs. 75.2 years in the intervention arm.
Primary diagnoses in the study group ranged widely from relatively minor skin infections to acute pancreatitis. The control arm and the intervention arm did not differ significantly in the incidence of heart failure, pulmonary disease, renal dialysis, cirrhosis, stroke, or active cancer (P > 0.05). Patients were considered as having a stroke if they had suffered a stroke during their hospital admission or if they had long-term sequelae of prior stroke. Patients were considered as having active cancer if they were currently undergoing treatment or had metastases. Participants were considered as having multiple morbidities if they possessed 2 or more of the listed conditions. Between the control arm and the intervention arm, there was no significant difference in the number of participants with multiple morbidities (27% in the control group and 24% in the video group).
Code Status Preference
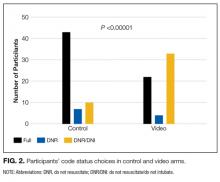
There was a significant difference in the code status preferences of the intervention arm and the control arm (P < 0.00001; Figure 2). In the control arm, 71% of participants chose full code, 12% chose DNR, and 17% chose DNR/DNI. In the intervention arm, only 37% chose full code, 7% chose DNR, and 56% chose DNR/DNI.
Secondary outcomes
Participants in the control and intervention arms were asked about their trust in their medical team (Question 1, Figure 3). There was no significant difference, but a trend towards less trust in the intervention group (P = 0.083) was seen with 93% of the control arm and 76% of the intervention arm agreeing with the statement “My doctors and healthcare team want what is best for me.”
Question 2, “If I choose to avoid resuscitation efforts, I will not receive care,” was designed to assess participants’ knowledge and perception about the care they would receive if they chose DNR/DNI as their code status. No significant difference was seen between the control and the interventions arms, with 28% of the control group agreeing with the statement, compared to 22% of the video group.
For question 3, participants were asked to respond to the statement “I would like to live as long as possible, even if I never leave the hospital.” No significant differences were seen between the control and the intervention arms, with 22% of both groups agreeing with the statement.
When we examined participant responses by the code status chosen, a significantly higher percentage of participants who chose full code agreed with the statement in question 3 (P = 0.0133). Of participants who chose full code, 27% agreed with the statement, compared to 18% of participants who chose DNR and 12% of participants who chose DNR/DNI. There was no significant difference (P > 0.05) between participant code status choice and either Question 1 or 2.
DISCUSSION
This study examined the effect of watching a video about CPR and intubation on the code status preferences of hospitalized patients. Participants who viewed a video about CPR and intubation were more likely to choose to forgo these treatments. Participants who chose CPR and intubation were more likely to agree that they would want to live as long as possible even if that time were spent in a medical setting.
To our knowledge, this is the first study to examine the role of a video decision support tool about code choices in the general hospital population, regardless of prognosis. Previous work has trialed the use of video support tools in hospitalized patients with a prognosis of less than 1 year,15 patients admitted to the ICU,18 and outpatients with cancer18 and those with dementia.16 Unlike previous studies, our study included a variety of illness severity.
Discussions about resuscitation are important for all adults admitted to the hospital because of the unpredictable nature of illness and the importance of providing high-quality care at the end of life. A recent study indicates that in-hospital cardiopulmonary arrest occurs in almost 1 per 1000 hospital days.19 These discussions are particularly salient for patients 65 years and older because of the higher incidence of death in this group. Inpatient admission is often a result of a change in health status, making it an important time for patients to reassess their resuscitation preferences based on their physical state and known comorbidities.
Video tools supplement the traditional code status discussion in several key ways. They provide a visual simulation of the procedures that occur during a typical resuscitation. These tools can help patients understand what CPR and intubation entail and transmit information that might be missed in verbal discussions. Visual media is now a common way for patients to obtain medical information20-22 and may be particularly helpful to patients who have low health literacy.23Video tools also help ensure that patients receive all the facts about resuscitation irrespective of how busy their provider may be or how comfortable the provider is with the topic. Lastly, video tools can reinforce information that is shared in the initial code status discussion. Given the significant differences in code status preference between our control and video arms, it is clear that the video tool has a significant impact on patient choices.
While we feel that our study clearly indicates the utility of video tools in code status discussion in hospitalized patients, there are some limitations. The current study enrolled participants who were predominantly white and male. All participants were recruited from the Minneapolis Veterans Affairs Health Care System, Minnesota. The relatively homogenous study population may impact the study’s generalizability. Another potential limitation of our study was the large number of eligible participants who declined to participate (41%), with many citing that they did not want to sign the consent paperwork. Additionally, the study coordinator was not blinded to the randomization of the participants, which could result in ascertainment bias. Also of concern was a trend, albeit nonsignificant, towards less trust in the healthcare team in the video group. Because the study was not designed to assess trust in the healthcare team both before and after the intervention, it is unclear if this difference was a result of the video.
Another area of potential concern is that visual images can be edited to sway viewers’ opinions based on the way content is presented. In our video, we included input from palliative care and internal medicine specialists. Cardiopulmonary resuscitation and intubation were performed on a CPR mannequin. The risks and benefits of CPR and intubation were discussed, as were the implications of choosing DNR or DNR/DNI code statuses.
The questionnaire that we used to assess participants’ knowledge and beliefs about resuscitation showed no differences between the control and the intervention arms of the study. We were surprised that a significant number of participants in the intervention group agreed with the statement, “If I choose to avoid resuscitation efforts, I will not receive care.” Our video specifically addressed the common belief that choosing DNR/DNI or DNR code statuses means that a patient will not continue to receive medical care. It is possible that participants were confused by the way the question was worded or that they understood the question to apply only to care received after a cardiopulmonary arrest had occurred.
This study and several others14-16 show that the use of video tools impacts participants’ code status preferences. There is clinical and humanistic importance in helping patients make informed decisions regarding whether or not they would want CPR and/or intubation if their heart were to stop or if they were to stop breathing. The data suggest that video tools are an efficient way to improve patient care and should be made widely available.
Disclosures: The authors report no conflicts of interest.
1. Dunn RH, Ahn J, Bernstein J. End-of-life care planning and fragility fractures of the hip: are we missing a valuable opportunity? Clin Orthop Relat Res 2016;474(7):1736-1739. PubMed
2. Warren MB, Lapid MI, McKean AJ, Cha SS, Stevens MA, Brekke FM, et al. Code status discussions in psychiatric and medical inpatients. J Clin Psychiatry. 2015;76(1):49-53. PubMed
3. Bhatia HL, Patel NR, Choma NN, Grande J, Giuse DA, Lehmann CU. Code status and resuscitation options in the electronic health record. Resuscitation. 2015;87:14-20. PubMed
4. Singh S, Namrata, Grewal A, Gautam PL, Luthra N, Kaur A. Evaluation of cardiopulmonary resuscitation (CPR) for patient outcomes and their predictors. J Clin Diagn Res. 2016;10(1):UC01-UC04. PubMed
5. Anderson WG, Chase R, Pantilat SZ, Tulsky JA, Auerbach AD. Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359-366. PubMed
6. Einstein DJ, Einstein KL, Mathew P. Dying for advice: code status discussions between resident physicians and patients with advanced cancer--a national survey. J Palliat Med. 2015;18(6):535-541. PubMed
7. Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the Age-combined Charlson Co-morbidity Index in a cohort study from a two-site Swedish University hospital. Resuscitation. 2016;99:79-83. PubMed
8. Zijlstra TJ, Leenman-Dekker SJ, Oldenhuis HK, Bosveld HE, Berendsen AJ. Knowledge and preferences regarding cardiopulmonary resuscitation: A survey among older patients. Patient Educ Couns. 2016;99(1):160-163. PubMed
9. Wilson ME, Akhoundi A, Krupa AK, Hinds RF, Litell JM, Gajic O, Kashani K. Development, validation, and results of a survey to measure understanding of cardiopulmonary resuscitation choices among ICU patients and their surrogate decision makers. BMC Anesthesiol. 2014;14:15. PubMed
10. Harris D, Willoughby H. Resuscitation on television: realistic or ridiculous? A quantitative observational analysis of the portrayal of cardiopulmonary resuscitation in television medical drama. Resuscitation. 2009;80(11):1275-1279. PubMed
11. Mills LM, Rhoads C, Curtis JR. Medical student training on code status discussions: how far have we come? J Palliat Med. 2016;19(3):323-325. PubMed
12. Binder AF, Huang GC, Buss MK. Uninformed consent: do medicine residents lack the proper framework for code status discussions? J Hosp Med. 2016;11(2):111-116. PubMed
13. Volandes AE, Levin TT, Slovin S, Carvajal RD, O’Reilly EM, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention-postintervention study. Cancer. 2012;118(17):4331-4338. PubMed
14. El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305-310. PubMed
15. El-Jawahri A, Mitchell SL, Paasche-Orlow MK, Temel JS, Jackson VA, Rutledge RR, et al. A randomized controlled trial of a CPR and intubation video decision support tool for hospitalized patients. J Gen Intern Med. 2015;30(8):1071-1080. PubMed
16. Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. PubMed
17. Celso BG, Meenrajan S. The triad that matters: palliative medicine, code status, and health care costs. Am J Hosp Palliat Care. 2010;27(6):398-401. PubMed
18. Wilson ME, Krupa A, Hinds RF, Litell JM, Swetz KM, Akhoundi A, et al. A video to improve patient and surrogate understanding of cardiopulmonary resuscitation choices in the ICU: a randomized controlled trial. Crit Care Med. 2015;43(3):621-629. PubMed
19. Overdyk FJ, Dowling O, Marino J, Qiu J, Chien HL, Erslon M, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. PubMed
20. Stacey D, Samant R, Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58(5)293-304. PubMed
21. Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59(6):379-390. PubMed
22. O’Brien MA, Whelan TJ, Villasis-Keever M, Gafni A, Charles C, Roberts R, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27(6):974-985. PubMed
23. Sudore RL, Landefeld CS, Pérez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns. 2009;75(3):398-402. PubMed
Discussions about cardiopulmonary resuscitation (CPR) can be difficult due to their association with end of life. The Patient Self Determination Act (H.R.4449 — 101st Congress [1989-1990]) and institutional standards mandate collaboration between care providers and patients regarding goals of care in emergency situations such as cardiopulmonary arrest. The default option is to provide CPR, which may involve chest compressions, intubation, and/or defibrillation. Yet numerous studies show that a significant number of patients have no code preference documented in their medical chart, and even fewer report a conversation with their care provider about their wishes regarding CPR.1-3 CPR is an invasive and potentially painful procedure with a higher chance of failure than success4, and yet many patients report that their provider did not discuss with them the risks and benefits of resuscitation.5,6 Further highlighting the importance of individual discussions about CPR preferences is the reality that factors such as age and disease burden further skew the likelihood of survival after cardiopulmonary arrest.7
Complicating the lack of appropriate provider and patient discussion of the risks and benefits of resuscitation are significant misunderstandings about CPR in the lay population. Patients routinely overestimate the likelihood of survival following CPR.8,9 This may be partially due to the portrayal of CPR in the lay media as highly efficacious.10 Other factors known to prevent effective provider-and-patient discussions about CPR preferences are providers’ discomfort with the subject11 and perceived time constraints.12
Informational videos have been developed to assist patients with decision making about CPR and have been shown to impact patients’ choices in the setting of life-limiting diseases such as advanced cancer,13-14 serious illness with a prognosis of less than 1 year,15 and dementia.16 While discussion of code status is vitally important in end-of-life planning for seriously ill individuals, delayed discussion of CPR preferences is associated with a significant increase in the number of invasive procedures performed at the end of life, increased length of stay in the hospital, and increased medical cost.17 Despite clear evidence that earlier discussion of resuscitation options are valuable, no studies have examined the impact of a video about code status options in the general patient population.
Here we present our findings of a randomized trial in patients hospitalized on the general medicine wards who were 65 years of age or older, regardless of illness severity or diagnosis. The video tool was a supplement for, rather than a replacement of, standard provider and patient communication about code preferences, and we compared patients who watched the video against controls who had standard discussions with their providers. Our video detailed the process of chest compressions and intubation during CPR and explained the differences between the code statuses: full code, do not resuscitate (DNR), and do not resuscitate/do not intubate (DNR/DNI). We found a significant difference between the 2 groups, with significantly more individuals in the video group choosing DNR/DNI. These findings suggest that video support tools may be a useful supplement to traditional provider discussions about code preferences in the general patient population.
METHODS
We enrolled patients from the general medicine wards at the Minneapolis VA Hospital from September 28, 2015 to October 23, 2015. Eligibility criteria included age 65 years or older, ability to provide informed consent, and ability to communicate in English. Study recruitment and data collection were performed by a study coordinator who was a house staff physician and had no role in the care of the participants. The medical charts of all general medicine patients were reviewed to determine if they met the age criteria. The physician of record for potential participants was contacted to assess if the patient was able to provide informed consent and communicate in English. Eligible patients were approached and informed consent was obtained from those who chose to participate in the study. After obtaining informed consent, patients were randomized using a random number generator to the intervention or usual-care arm of the study.
Those who were assigned to the intervention arm watched a 6-minute long video explaining the code-preference choices of full code, DNR, or DNR/DNI. Full code was described as possibly including CPR, intubation, and/or defibrillation depending on the clinical situation. Do not resuscitate was described as meaning no CPR or defibrillation but possible intubation in the case of respiratory failure. Do not resuscitate/do not intubate was explained as meaning no CPR, no defibrillation, and no intubation but rather permitting “natural death” to occur. The video showed a mock code with chest compressions, defibrillation, and intubation on a mannequin as well as palliative care specialists who discussed potential complications and survival rates of inhospital resuscitation.
The video was created at the University of Minnesota with the departments of palliative care and internal medicine (www.mmcgmeservices.org/codestat.html). After viewing the video, participants in the intervention arm filled out a questionnaire designed to assess their knowledge and beliefs about CPR and trust in their medical care providers. They were asked to circle their code preference. The participants’ medical teams were made aware of the code preferences and were counseled to discuss code preferences further if it was different from their previously documented code preference.
Participants in the control arm were assigned to usual care. At the institution where this study occurred, a discussion about code preferences between the patient and their medical team is considered the standard of care. After informed consent was obtained, participants filled out the same questionnaire as the participants in the intervention arm. They were asked to circle their code status preference. If they chose to ask questions about resuscitation, these were answered, but the study coordinator did not volunteer information about resuscitation or intervene in the medical care of the participants in any way.
All participants’ demographic characteristics and outcomes were described using proportions for categorical variables and means ± standard deviation for continuous variables. The primary outcome was participants’ stated code preference (full code, DNR, or DNR/DNI). Secondary outcomes included comparison of trust in medical providers, resuscitation beliefs, and desire for life-prolonging interventions as obtained from the questionnaire.
We analyzed code preferences between the intervention and control groups using Fisher exact test. We used analysis of variance (ANOVA) to compare questionnaire responses between the 2 groups. All reported P values are 2-sided with P < 0.05 considered significant. The project originally targeted a sample size of 194 participants for 80% power to detect a 20% difference in the code preference choices between intervention and control groups. Given the short time frame available to enroll participants, the target sample size was not reached. Propitiously, the effect size was greater than originally expected.
RESULTS
Study Participants
A total of 273 potentially eligible patients were approached to participate and 119 (44%) enrolled. (Figure 1). Of the 154 patients that were deemed eligible after initial screening, 42 patients were unable to give consent due to the severity of their illness or because of their mental status. Another 112 patients declined participation in the study, citing reasons such as disinterest in the consent paperwork, desire to spend time with visitors, and unease with the subject matter. Patients who declined participation did not differ significantly by age, sex, or race from those enrolled in the study.
Among the 119 participants, 60 were randomized to the control arm, and 59 were randomized to the intervention arm. Participants in the 2 arms did not differ significantly in age, sex, or race (P > 0.05), although all 4 women in the study were randomized to the intervention arm. Eighty-seven percent of the study population identified as white with the remainder including black, Asian, Pacific Islander, Native American, or declining to answer. The mean age was 75.8 years in the control arm vs. 75.2 years in the intervention arm.
Primary diagnoses in the study group ranged widely from relatively minor skin infections to acute pancreatitis. The control arm and the intervention arm did not differ significantly in the incidence of heart failure, pulmonary disease, renal dialysis, cirrhosis, stroke, or active cancer (P > 0.05). Patients were considered as having a stroke if they had suffered a stroke during their hospital admission or if they had long-term sequelae of prior stroke. Patients were considered as having active cancer if they were currently undergoing treatment or had metastases. Participants were considered as having multiple morbidities if they possessed 2 or more of the listed conditions. Between the control arm and the intervention arm, there was no significant difference in the number of participants with multiple morbidities (27% in the control group and 24% in the video group).
Code Status Preference
There was a significant difference in the code status preferences of the intervention arm and the control arm (P < 0.00001; Figure 2). In the control arm, 71% of participants chose full code, 12% chose DNR, and 17% chose DNR/DNI. In the intervention arm, only 37% chose full code, 7% chose DNR, and 56% chose DNR/DNI.
Secondary outcomes
Participants in the control and intervention arms were asked about their trust in their medical team (Question 1, Figure 3). There was no significant difference, but a trend towards less trust in the intervention group (P = 0.083) was seen with 93% of the control arm and 76% of the intervention arm agreeing with the statement “My doctors and healthcare team want what is best for me.”
Question 2, “If I choose to avoid resuscitation efforts, I will not receive care,” was designed to assess participants’ knowledge and perception about the care they would receive if they chose DNR/DNI as their code status. No significant difference was seen between the control and the interventions arms, with 28% of the control group agreeing with the statement, compared to 22% of the video group.
For question 3, participants were asked to respond to the statement “I would like to live as long as possible, even if I never leave the hospital.” No significant differences were seen between the control and the intervention arms, with 22% of both groups agreeing with the statement.
When we examined participant responses by the code status chosen, a significantly higher percentage of participants who chose full code agreed with the statement in question 3 (P = 0.0133). Of participants who chose full code, 27% agreed with the statement, compared to 18% of participants who chose DNR and 12% of participants who chose DNR/DNI. There was no significant difference (P > 0.05) between participant code status choice and either Question 1 or 2.
DISCUSSION
This study examined the effect of watching a video about CPR and intubation on the code status preferences of hospitalized patients. Participants who viewed a video about CPR and intubation were more likely to choose to forgo these treatments. Participants who chose CPR and intubation were more likely to agree that they would want to live as long as possible even if that time were spent in a medical setting.
To our knowledge, this is the first study to examine the role of a video decision support tool about code choices in the general hospital population, regardless of prognosis. Previous work has trialed the use of video support tools in hospitalized patients with a prognosis of less than 1 year,15 patients admitted to the ICU,18 and outpatients with cancer18 and those with dementia.16 Unlike previous studies, our study included a variety of illness severity.
Discussions about resuscitation are important for all adults admitted to the hospital because of the unpredictable nature of illness and the importance of providing high-quality care at the end of life. A recent study indicates that in-hospital cardiopulmonary arrest occurs in almost 1 per 1000 hospital days.19 These discussions are particularly salient for patients 65 years and older because of the higher incidence of death in this group. Inpatient admission is often a result of a change in health status, making it an important time for patients to reassess their resuscitation preferences based on their physical state and known comorbidities.
Video tools supplement the traditional code status discussion in several key ways. They provide a visual simulation of the procedures that occur during a typical resuscitation. These tools can help patients understand what CPR and intubation entail and transmit information that might be missed in verbal discussions. Visual media is now a common way for patients to obtain medical information20-22 and may be particularly helpful to patients who have low health literacy.23Video tools also help ensure that patients receive all the facts about resuscitation irrespective of how busy their provider may be or how comfortable the provider is with the topic. Lastly, video tools can reinforce information that is shared in the initial code status discussion. Given the significant differences in code status preference between our control and video arms, it is clear that the video tool has a significant impact on patient choices.
While we feel that our study clearly indicates the utility of video tools in code status discussion in hospitalized patients, there are some limitations. The current study enrolled participants who were predominantly white and male. All participants were recruited from the Minneapolis Veterans Affairs Health Care System, Minnesota. The relatively homogenous study population may impact the study’s generalizability. Another potential limitation of our study was the large number of eligible participants who declined to participate (41%), with many citing that they did not want to sign the consent paperwork. Additionally, the study coordinator was not blinded to the randomization of the participants, which could result in ascertainment bias. Also of concern was a trend, albeit nonsignificant, towards less trust in the healthcare team in the video group. Because the study was not designed to assess trust in the healthcare team both before and after the intervention, it is unclear if this difference was a result of the video.
Another area of potential concern is that visual images can be edited to sway viewers’ opinions based on the way content is presented. In our video, we included input from palliative care and internal medicine specialists. Cardiopulmonary resuscitation and intubation were performed on a CPR mannequin. The risks and benefits of CPR and intubation were discussed, as were the implications of choosing DNR or DNR/DNI code statuses.
The questionnaire that we used to assess participants’ knowledge and beliefs about resuscitation showed no differences between the control and the intervention arms of the study. We were surprised that a significant number of participants in the intervention group agreed with the statement, “If I choose to avoid resuscitation efforts, I will not receive care.” Our video specifically addressed the common belief that choosing DNR/DNI or DNR code statuses means that a patient will not continue to receive medical care. It is possible that participants were confused by the way the question was worded or that they understood the question to apply only to care received after a cardiopulmonary arrest had occurred.
This study and several others14-16 show that the use of video tools impacts participants’ code status preferences. There is clinical and humanistic importance in helping patients make informed decisions regarding whether or not they would want CPR and/or intubation if their heart were to stop or if they were to stop breathing. The data suggest that video tools are an efficient way to improve patient care and should be made widely available.
Disclosures: The authors report no conflicts of interest.
Discussions about cardiopulmonary resuscitation (CPR) can be difficult due to their association with end of life. The Patient Self Determination Act (H.R.4449 — 101st Congress [1989-1990]) and institutional standards mandate collaboration between care providers and patients regarding goals of care in emergency situations such as cardiopulmonary arrest. The default option is to provide CPR, which may involve chest compressions, intubation, and/or defibrillation. Yet numerous studies show that a significant number of patients have no code preference documented in their medical chart, and even fewer report a conversation with their care provider about their wishes regarding CPR.1-3 CPR is an invasive and potentially painful procedure with a higher chance of failure than success4, and yet many patients report that their provider did not discuss with them the risks and benefits of resuscitation.5,6 Further highlighting the importance of individual discussions about CPR preferences is the reality that factors such as age and disease burden further skew the likelihood of survival after cardiopulmonary arrest.7
Complicating the lack of appropriate provider and patient discussion of the risks and benefits of resuscitation are significant misunderstandings about CPR in the lay population. Patients routinely overestimate the likelihood of survival following CPR.8,9 This may be partially due to the portrayal of CPR in the lay media as highly efficacious.10 Other factors known to prevent effective provider-and-patient discussions about CPR preferences are providers’ discomfort with the subject11 and perceived time constraints.12
Informational videos have been developed to assist patients with decision making about CPR and have been shown to impact patients’ choices in the setting of life-limiting diseases such as advanced cancer,13-14 serious illness with a prognosis of less than 1 year,15 and dementia.16 While discussion of code status is vitally important in end-of-life planning for seriously ill individuals, delayed discussion of CPR preferences is associated with a significant increase in the number of invasive procedures performed at the end of life, increased length of stay in the hospital, and increased medical cost.17 Despite clear evidence that earlier discussion of resuscitation options are valuable, no studies have examined the impact of a video about code status options in the general patient population.
Here we present our findings of a randomized trial in patients hospitalized on the general medicine wards who were 65 years of age or older, regardless of illness severity or diagnosis. The video tool was a supplement for, rather than a replacement of, standard provider and patient communication about code preferences, and we compared patients who watched the video against controls who had standard discussions with their providers. Our video detailed the process of chest compressions and intubation during CPR and explained the differences between the code statuses: full code, do not resuscitate (DNR), and do not resuscitate/do not intubate (DNR/DNI). We found a significant difference between the 2 groups, with significantly more individuals in the video group choosing DNR/DNI. These findings suggest that video support tools may be a useful supplement to traditional provider discussions about code preferences in the general patient population.
METHODS
We enrolled patients from the general medicine wards at the Minneapolis VA Hospital from September 28, 2015 to October 23, 2015. Eligibility criteria included age 65 years or older, ability to provide informed consent, and ability to communicate in English. Study recruitment and data collection were performed by a study coordinator who was a house staff physician and had no role in the care of the participants. The medical charts of all general medicine patients were reviewed to determine if they met the age criteria. The physician of record for potential participants was contacted to assess if the patient was able to provide informed consent and communicate in English. Eligible patients were approached and informed consent was obtained from those who chose to participate in the study. After obtaining informed consent, patients were randomized using a random number generator to the intervention or usual-care arm of the study.
Those who were assigned to the intervention arm watched a 6-minute long video explaining the code-preference choices of full code, DNR, or DNR/DNI. Full code was described as possibly including CPR, intubation, and/or defibrillation depending on the clinical situation. Do not resuscitate was described as meaning no CPR or defibrillation but possible intubation in the case of respiratory failure. Do not resuscitate/do not intubate was explained as meaning no CPR, no defibrillation, and no intubation but rather permitting “natural death” to occur. The video showed a mock code with chest compressions, defibrillation, and intubation on a mannequin as well as palliative care specialists who discussed potential complications and survival rates of inhospital resuscitation.
The video was created at the University of Minnesota with the departments of palliative care and internal medicine (www.mmcgmeservices.org/codestat.html). After viewing the video, participants in the intervention arm filled out a questionnaire designed to assess their knowledge and beliefs about CPR and trust in their medical care providers. They were asked to circle their code preference. The participants’ medical teams were made aware of the code preferences and were counseled to discuss code preferences further if it was different from their previously documented code preference.
Participants in the control arm were assigned to usual care. At the institution where this study occurred, a discussion about code preferences between the patient and their medical team is considered the standard of care. After informed consent was obtained, participants filled out the same questionnaire as the participants in the intervention arm. They were asked to circle their code status preference. If they chose to ask questions about resuscitation, these were answered, but the study coordinator did not volunteer information about resuscitation or intervene in the medical care of the participants in any way.
All participants’ demographic characteristics and outcomes were described using proportions for categorical variables and means ± standard deviation for continuous variables. The primary outcome was participants’ stated code preference (full code, DNR, or DNR/DNI). Secondary outcomes included comparison of trust in medical providers, resuscitation beliefs, and desire for life-prolonging interventions as obtained from the questionnaire.
We analyzed code preferences between the intervention and control groups using Fisher exact test. We used analysis of variance (ANOVA) to compare questionnaire responses between the 2 groups. All reported P values are 2-sided with P < 0.05 considered significant. The project originally targeted a sample size of 194 participants for 80% power to detect a 20% difference in the code preference choices between intervention and control groups. Given the short time frame available to enroll participants, the target sample size was not reached. Propitiously, the effect size was greater than originally expected.
RESULTS
Study Participants
A total of 273 potentially eligible patients were approached to participate and 119 (44%) enrolled. (Figure 1). Of the 154 patients that were deemed eligible after initial screening, 42 patients were unable to give consent due to the severity of their illness or because of their mental status. Another 112 patients declined participation in the study, citing reasons such as disinterest in the consent paperwork, desire to spend time with visitors, and unease with the subject matter. Patients who declined participation did not differ significantly by age, sex, or race from those enrolled in the study.
Among the 119 participants, 60 were randomized to the control arm, and 59 were randomized to the intervention arm. Participants in the 2 arms did not differ significantly in age, sex, or race (P > 0.05), although all 4 women in the study were randomized to the intervention arm. Eighty-seven percent of the study population identified as white with the remainder including black, Asian, Pacific Islander, Native American, or declining to answer. The mean age was 75.8 years in the control arm vs. 75.2 years in the intervention arm.
Primary diagnoses in the study group ranged widely from relatively minor skin infections to acute pancreatitis. The control arm and the intervention arm did not differ significantly in the incidence of heart failure, pulmonary disease, renal dialysis, cirrhosis, stroke, or active cancer (P > 0.05). Patients were considered as having a stroke if they had suffered a stroke during their hospital admission or if they had long-term sequelae of prior stroke. Patients were considered as having active cancer if they were currently undergoing treatment or had metastases. Participants were considered as having multiple morbidities if they possessed 2 or more of the listed conditions. Between the control arm and the intervention arm, there was no significant difference in the number of participants with multiple morbidities (27% in the control group and 24% in the video group).
Code Status Preference
There was a significant difference in the code status preferences of the intervention arm and the control arm (P < 0.00001; Figure 2). In the control arm, 71% of participants chose full code, 12% chose DNR, and 17% chose DNR/DNI. In the intervention arm, only 37% chose full code, 7% chose DNR, and 56% chose DNR/DNI.
Secondary outcomes
Participants in the control and intervention arms were asked about their trust in their medical team (Question 1, Figure 3). There was no significant difference, but a trend towards less trust in the intervention group (P = 0.083) was seen with 93% of the control arm and 76% of the intervention arm agreeing with the statement “My doctors and healthcare team want what is best for me.”
Question 2, “If I choose to avoid resuscitation efforts, I will not receive care,” was designed to assess participants’ knowledge and perception about the care they would receive if they chose DNR/DNI as their code status. No significant difference was seen between the control and the interventions arms, with 28% of the control group agreeing with the statement, compared to 22% of the video group.
For question 3, participants were asked to respond to the statement “I would like to live as long as possible, even if I never leave the hospital.” No significant differences were seen between the control and the intervention arms, with 22% of both groups agreeing with the statement.
When we examined participant responses by the code status chosen, a significantly higher percentage of participants who chose full code agreed with the statement in question 3 (P = 0.0133). Of participants who chose full code, 27% agreed with the statement, compared to 18% of participants who chose DNR and 12% of participants who chose DNR/DNI. There was no significant difference (P > 0.05) between participant code status choice and either Question 1 or 2.
DISCUSSION
This study examined the effect of watching a video about CPR and intubation on the code status preferences of hospitalized patients. Participants who viewed a video about CPR and intubation were more likely to choose to forgo these treatments. Participants who chose CPR and intubation were more likely to agree that they would want to live as long as possible even if that time were spent in a medical setting.
To our knowledge, this is the first study to examine the role of a video decision support tool about code choices in the general hospital population, regardless of prognosis. Previous work has trialed the use of video support tools in hospitalized patients with a prognosis of less than 1 year,15 patients admitted to the ICU,18 and outpatients with cancer18 and those with dementia.16 Unlike previous studies, our study included a variety of illness severity.
Discussions about resuscitation are important for all adults admitted to the hospital because of the unpredictable nature of illness and the importance of providing high-quality care at the end of life. A recent study indicates that in-hospital cardiopulmonary arrest occurs in almost 1 per 1000 hospital days.19 These discussions are particularly salient for patients 65 years and older because of the higher incidence of death in this group. Inpatient admission is often a result of a change in health status, making it an important time for patients to reassess their resuscitation preferences based on their physical state and known comorbidities.
Video tools supplement the traditional code status discussion in several key ways. They provide a visual simulation of the procedures that occur during a typical resuscitation. These tools can help patients understand what CPR and intubation entail and transmit information that might be missed in verbal discussions. Visual media is now a common way for patients to obtain medical information20-22 and may be particularly helpful to patients who have low health literacy.23Video tools also help ensure that patients receive all the facts about resuscitation irrespective of how busy their provider may be or how comfortable the provider is with the topic. Lastly, video tools can reinforce information that is shared in the initial code status discussion. Given the significant differences in code status preference between our control and video arms, it is clear that the video tool has a significant impact on patient choices.
While we feel that our study clearly indicates the utility of video tools in code status discussion in hospitalized patients, there are some limitations. The current study enrolled participants who were predominantly white and male. All participants were recruited from the Minneapolis Veterans Affairs Health Care System, Minnesota. The relatively homogenous study population may impact the study’s generalizability. Another potential limitation of our study was the large number of eligible participants who declined to participate (41%), with many citing that they did not want to sign the consent paperwork. Additionally, the study coordinator was not blinded to the randomization of the participants, which could result in ascertainment bias. Also of concern was a trend, albeit nonsignificant, towards less trust in the healthcare team in the video group. Because the study was not designed to assess trust in the healthcare team both before and after the intervention, it is unclear if this difference was a result of the video.
Another area of potential concern is that visual images can be edited to sway viewers’ opinions based on the way content is presented. In our video, we included input from palliative care and internal medicine specialists. Cardiopulmonary resuscitation and intubation were performed on a CPR mannequin. The risks and benefits of CPR and intubation were discussed, as were the implications of choosing DNR or DNR/DNI code statuses.
The questionnaire that we used to assess participants’ knowledge and beliefs about resuscitation showed no differences between the control and the intervention arms of the study. We were surprised that a significant number of participants in the intervention group agreed with the statement, “If I choose to avoid resuscitation efforts, I will not receive care.” Our video specifically addressed the common belief that choosing DNR/DNI or DNR code statuses means that a patient will not continue to receive medical care. It is possible that participants were confused by the way the question was worded or that they understood the question to apply only to care received after a cardiopulmonary arrest had occurred.
This study and several others14-16 show that the use of video tools impacts participants’ code status preferences. There is clinical and humanistic importance in helping patients make informed decisions regarding whether or not they would want CPR and/or intubation if their heart were to stop or if they were to stop breathing. The data suggest that video tools are an efficient way to improve patient care and should be made widely available.
Disclosures: The authors report no conflicts of interest.
1. Dunn RH, Ahn J, Bernstein J. End-of-life care planning and fragility fractures of the hip: are we missing a valuable opportunity? Clin Orthop Relat Res 2016;474(7):1736-1739. PubMed
2. Warren MB, Lapid MI, McKean AJ, Cha SS, Stevens MA, Brekke FM, et al. Code status discussions in psychiatric and medical inpatients. J Clin Psychiatry. 2015;76(1):49-53. PubMed
3. Bhatia HL, Patel NR, Choma NN, Grande J, Giuse DA, Lehmann CU. Code status and resuscitation options in the electronic health record. Resuscitation. 2015;87:14-20. PubMed
4. Singh S, Namrata, Grewal A, Gautam PL, Luthra N, Kaur A. Evaluation of cardiopulmonary resuscitation (CPR) for patient outcomes and their predictors. J Clin Diagn Res. 2016;10(1):UC01-UC04. PubMed
5. Anderson WG, Chase R, Pantilat SZ, Tulsky JA, Auerbach AD. Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359-366. PubMed
6. Einstein DJ, Einstein KL, Mathew P. Dying for advice: code status discussions between resident physicians and patients with advanced cancer--a national survey. J Palliat Med. 2015;18(6):535-541. PubMed
7. Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the Age-combined Charlson Co-morbidity Index in a cohort study from a two-site Swedish University hospital. Resuscitation. 2016;99:79-83. PubMed
8. Zijlstra TJ, Leenman-Dekker SJ, Oldenhuis HK, Bosveld HE, Berendsen AJ. Knowledge and preferences regarding cardiopulmonary resuscitation: A survey among older patients. Patient Educ Couns. 2016;99(1):160-163. PubMed
9. Wilson ME, Akhoundi A, Krupa AK, Hinds RF, Litell JM, Gajic O, Kashani K. Development, validation, and results of a survey to measure understanding of cardiopulmonary resuscitation choices among ICU patients and their surrogate decision makers. BMC Anesthesiol. 2014;14:15. PubMed
10. Harris D, Willoughby H. Resuscitation on television: realistic or ridiculous? A quantitative observational analysis of the portrayal of cardiopulmonary resuscitation in television medical drama. Resuscitation. 2009;80(11):1275-1279. PubMed
11. Mills LM, Rhoads C, Curtis JR. Medical student training on code status discussions: how far have we come? J Palliat Med. 2016;19(3):323-325. PubMed
12. Binder AF, Huang GC, Buss MK. Uninformed consent: do medicine residents lack the proper framework for code status discussions? J Hosp Med. 2016;11(2):111-116. PubMed
13. Volandes AE, Levin TT, Slovin S, Carvajal RD, O’Reilly EM, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention-postintervention study. Cancer. 2012;118(17):4331-4338. PubMed
14. El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305-310. PubMed
15. El-Jawahri A, Mitchell SL, Paasche-Orlow MK, Temel JS, Jackson VA, Rutledge RR, et al. A randomized controlled trial of a CPR and intubation video decision support tool for hospitalized patients. J Gen Intern Med. 2015;30(8):1071-1080. PubMed
16. Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. PubMed
17. Celso BG, Meenrajan S. The triad that matters: palliative medicine, code status, and health care costs. Am J Hosp Palliat Care. 2010;27(6):398-401. PubMed
18. Wilson ME, Krupa A, Hinds RF, Litell JM, Swetz KM, Akhoundi A, et al. A video to improve patient and surrogate understanding of cardiopulmonary resuscitation choices in the ICU: a randomized controlled trial. Crit Care Med. 2015;43(3):621-629. PubMed
19. Overdyk FJ, Dowling O, Marino J, Qiu J, Chien HL, Erslon M, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. PubMed
20. Stacey D, Samant R, Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58(5)293-304. PubMed
21. Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59(6):379-390. PubMed
22. O’Brien MA, Whelan TJ, Villasis-Keever M, Gafni A, Charles C, Roberts R, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27(6):974-985. PubMed
23. Sudore RL, Landefeld CS, Pérez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns. 2009;75(3):398-402. PubMed
1. Dunn RH, Ahn J, Bernstein J. End-of-life care planning and fragility fractures of the hip: are we missing a valuable opportunity? Clin Orthop Relat Res 2016;474(7):1736-1739. PubMed
2. Warren MB, Lapid MI, McKean AJ, Cha SS, Stevens MA, Brekke FM, et al. Code status discussions in psychiatric and medical inpatients. J Clin Psychiatry. 2015;76(1):49-53. PubMed
3. Bhatia HL, Patel NR, Choma NN, Grande J, Giuse DA, Lehmann CU. Code status and resuscitation options in the electronic health record. Resuscitation. 2015;87:14-20. PubMed
4. Singh S, Namrata, Grewal A, Gautam PL, Luthra N, Kaur A. Evaluation of cardiopulmonary resuscitation (CPR) for patient outcomes and their predictors. J Clin Diagn Res. 2016;10(1):UC01-UC04. PubMed
5. Anderson WG, Chase R, Pantilat SZ, Tulsky JA, Auerbach AD. Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359-366. PubMed
6. Einstein DJ, Einstein KL, Mathew P. Dying for advice: code status discussions between resident physicians and patients with advanced cancer--a national survey. J Palliat Med. 2015;18(6):535-541. PubMed
7. Piscator E, Hedberg P, Göransson K, Djärv T. Survival after in-hospital cardiac arrest is highly associated with the Age-combined Charlson Co-morbidity Index in a cohort study from a two-site Swedish University hospital. Resuscitation. 2016;99:79-83. PubMed
8. Zijlstra TJ, Leenman-Dekker SJ, Oldenhuis HK, Bosveld HE, Berendsen AJ. Knowledge and preferences regarding cardiopulmonary resuscitation: A survey among older patients. Patient Educ Couns. 2016;99(1):160-163. PubMed
9. Wilson ME, Akhoundi A, Krupa AK, Hinds RF, Litell JM, Gajic O, Kashani K. Development, validation, and results of a survey to measure understanding of cardiopulmonary resuscitation choices among ICU patients and their surrogate decision makers. BMC Anesthesiol. 2014;14:15. PubMed
10. Harris D, Willoughby H. Resuscitation on television: realistic or ridiculous? A quantitative observational analysis of the portrayal of cardiopulmonary resuscitation in television medical drama. Resuscitation. 2009;80(11):1275-1279. PubMed
11. Mills LM, Rhoads C, Curtis JR. Medical student training on code status discussions: how far have we come? J Palliat Med. 2016;19(3):323-325. PubMed
12. Binder AF, Huang GC, Buss MK. Uninformed consent: do medicine residents lack the proper framework for code status discussions? J Hosp Med. 2016;11(2):111-116. PubMed
13. Volandes AE, Levin TT, Slovin S, Carvajal RD, O’Reilly EM, et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention-postintervention study. Cancer. 2012;118(17):4331-4338. PubMed
14. El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305-310. PubMed
15. El-Jawahri A, Mitchell SL, Paasche-Orlow MK, Temel JS, Jackson VA, Rutledge RR, et al. A randomized controlled trial of a CPR and intubation video decision support tool for hospitalized patients. J Gen Intern Med. 2015;30(8):1071-1080. PubMed
16. Volandes AE, Paasche-Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. PubMed
17. Celso BG, Meenrajan S. The triad that matters: palliative medicine, code status, and health care costs. Am J Hosp Palliat Care. 2010;27(6):398-401. PubMed
18. Wilson ME, Krupa A, Hinds RF, Litell JM, Swetz KM, Akhoundi A, et al. A video to improve patient and surrogate understanding of cardiopulmonary resuscitation choices in the ICU: a randomized controlled trial. Crit Care Med. 2015;43(3):621-629. PubMed
19. Overdyk FJ, Dowling O, Marino J, Qiu J, Chien HL, Erslon M, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. PubMed
20. Stacey D, Samant R, Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58(5)293-304. PubMed
21. Lin GA, Aaronson DS, Knight SJ, Carroll PR, Dudley RA. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59(6):379-390. PubMed
22. O’Brien MA, Whelan TJ, Villasis-Keever M, Gafni A, Charles C, Roberts R, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27(6):974-985. PubMed
23. Sudore RL, Landefeld CS, Pérez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns. 2009;75(3):398-402. PubMed
© 2017 Society of Hospital Medicine
Sneak Peek: Journal of Hospital Medicine – August 2017
BACKGROUND: There is increasing recognition that patients have critical insights into care experiences, including about breakdowns in care. Harnessing patient perspectives for hospital improvement requires an in-depth understanding of the types of breakdowns patients identify and the impact of these events.
RESULTS: Of 979 interviewees, 386 (39.4%) believed they had experienced at least one breakdown in care. The most common reported breakdowns involved information exchange (n = 158; 16.1%), medications (n = 120; 12.3%), delays in admission (n = 90; 9.2%), team communication (n = 65; 6.6%), providers’ manner (n = 62; 6.3%), and discharge (n = 56; 5.7%). Of the 386 interviewees who reported a breakdown, 140 (36.3%) perceived associated harm. Patient-perceived harms included physical (e.g., pain), emotional (e.g., distress, worry), damage to relationship with providers, need for additional care or prolonged hospital stay, and life disruption. We found higher rates of reporting breakdowns among younger (less than 60 years old) patients (45.4% vs. 34.5%; P less than .001), those with at least some college education (46.8% vs 32.7%; P less than .001), and those with another person (family or friend) present during the interview or interviewed in lieu of the patient (53.4% vs 37.8%; P = .002).
CONCLUSIONS: When asked directly, almost 4 out of 10 hospitalized patients reported a breakdown in their care. Patient-perceived breakdowns in care are frequently associated with perceived harm, illustrating the importance of detecting and addressing these events.
Also in JHM
Excess readmission vs. excess penalties: Maximum readmission penalties as a function of socioeconomics and geography
AUTHORS: Chris M. Caracciolo, MPH, Devin M. Parker, MS, Emily Marshall, MS, Jeremiah R. Brown, PhD, MS
Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patientsAUTHORS: Maya Dewan, MD, MPH, Heather Wolfe, MD, MSHP, Richard Lin, MD, Eileen Ware, RN, Michelle Weiss, RN, Lihai Song, MS, Matthew MacMurchy, BA, Daniela Davis, MD, MSCE, Christopher P. Bonafide MD MSCE
Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: A qualitative studyAUTHORS: Bennett W. Clark, MD, Katelyn Baron, MSIOP, Kathleen Tynan-McKiernan, RN, MSN, Meredith Campbell Britton, LMSW, Karl E. Minges, PhD, Sarwat I. Chaudhry, MD
Use of postacute facility care in children hospitalized with acute respiratory illnessAUTHORS: Jay G. Berry, MD, MPH, Karen M. Wilson, MD, MPH, FAAP, Helene Dumas, PT, MS, Edwin Simpser, MD, Jane O’Brien, MD, Kathleen Whitford, PNP, Rachna May, MD, Vineeta Mittal, MD, Nancy Murphy, MD, David Steinhorn, MD, Rishi Agrawal, MD, MPH, Kris Rehm, MD, Michelle Marks, DO, FAAP, SFHM, Christine Traul, MD, Michael Dribbon, PhD, Christopher J. Haines, DO, MBA, FAAP, FACEP, Matt Hall, PhD
A contemporary assessment of mechanical complication rates and trainee perceptions of central venous catheter insertionAUTHORS: Lauren Heidemann, MD, Niket Nathani, MD, Rommel Sagana, MD, Vineet Chopra, MD, MSc, Michael Heung, MD, MS
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
BACKGROUND: There is increasing recognition that patients have critical insights into care experiences, including about breakdowns in care. Harnessing patient perspectives for hospital improvement requires an in-depth understanding of the types of breakdowns patients identify and the impact of these events.
RESULTS: Of 979 interviewees, 386 (39.4%) believed they had experienced at least one breakdown in care. The most common reported breakdowns involved information exchange (n = 158; 16.1%), medications (n = 120; 12.3%), delays in admission (n = 90; 9.2%), team communication (n = 65; 6.6%), providers’ manner (n = 62; 6.3%), and discharge (n = 56; 5.7%). Of the 386 interviewees who reported a breakdown, 140 (36.3%) perceived associated harm. Patient-perceived harms included physical (e.g., pain), emotional (e.g., distress, worry), damage to relationship with providers, need for additional care or prolonged hospital stay, and life disruption. We found higher rates of reporting breakdowns among younger (less than 60 years old) patients (45.4% vs. 34.5%; P less than .001), those with at least some college education (46.8% vs 32.7%; P less than .001), and those with another person (family or friend) present during the interview or interviewed in lieu of the patient (53.4% vs 37.8%; P = .002).
CONCLUSIONS: When asked directly, almost 4 out of 10 hospitalized patients reported a breakdown in their care. Patient-perceived breakdowns in care are frequently associated with perceived harm, illustrating the importance of detecting and addressing these events.
Also in JHM
Excess readmission vs. excess penalties: Maximum readmission penalties as a function of socioeconomics and geography
AUTHORS: Chris M. Caracciolo, MPH, Devin M. Parker, MS, Emily Marshall, MS, Jeremiah R. Brown, PhD, MS
Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patientsAUTHORS: Maya Dewan, MD, MPH, Heather Wolfe, MD, MSHP, Richard Lin, MD, Eileen Ware, RN, Michelle Weiss, RN, Lihai Song, MS, Matthew MacMurchy, BA, Daniela Davis, MD, MSCE, Christopher P. Bonafide MD MSCE
Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: A qualitative studyAUTHORS: Bennett W. Clark, MD, Katelyn Baron, MSIOP, Kathleen Tynan-McKiernan, RN, MSN, Meredith Campbell Britton, LMSW, Karl E. Minges, PhD, Sarwat I. Chaudhry, MD
Use of postacute facility care in children hospitalized with acute respiratory illnessAUTHORS: Jay G. Berry, MD, MPH, Karen M. Wilson, MD, MPH, FAAP, Helene Dumas, PT, MS, Edwin Simpser, MD, Jane O’Brien, MD, Kathleen Whitford, PNP, Rachna May, MD, Vineeta Mittal, MD, Nancy Murphy, MD, David Steinhorn, MD, Rishi Agrawal, MD, MPH, Kris Rehm, MD, Michelle Marks, DO, FAAP, SFHM, Christine Traul, MD, Michael Dribbon, PhD, Christopher J. Haines, DO, MBA, FAAP, FACEP, Matt Hall, PhD
A contemporary assessment of mechanical complication rates and trainee perceptions of central venous catheter insertionAUTHORS: Lauren Heidemann, MD, Niket Nathani, MD, Rommel Sagana, MD, Vineet Chopra, MD, MSc, Michael Heung, MD, MS
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
BACKGROUND: There is increasing recognition that patients have critical insights into care experiences, including about breakdowns in care. Harnessing patient perspectives for hospital improvement requires an in-depth understanding of the types of breakdowns patients identify and the impact of these events.
RESULTS: Of 979 interviewees, 386 (39.4%) believed they had experienced at least one breakdown in care. The most common reported breakdowns involved information exchange (n = 158; 16.1%), medications (n = 120; 12.3%), delays in admission (n = 90; 9.2%), team communication (n = 65; 6.6%), providers’ manner (n = 62; 6.3%), and discharge (n = 56; 5.7%). Of the 386 interviewees who reported a breakdown, 140 (36.3%) perceived associated harm. Patient-perceived harms included physical (e.g., pain), emotional (e.g., distress, worry), damage to relationship with providers, need for additional care or prolonged hospital stay, and life disruption. We found higher rates of reporting breakdowns among younger (less than 60 years old) patients (45.4% vs. 34.5%; P less than .001), those with at least some college education (46.8% vs 32.7%; P less than .001), and those with another person (family or friend) present during the interview or interviewed in lieu of the patient (53.4% vs 37.8%; P = .002).
CONCLUSIONS: When asked directly, almost 4 out of 10 hospitalized patients reported a breakdown in their care. Patient-perceived breakdowns in care are frequently associated with perceived harm, illustrating the importance of detecting and addressing these events.
Also in JHM
Excess readmission vs. excess penalties: Maximum readmission penalties as a function of socioeconomics and geography
AUTHORS: Chris M. Caracciolo, MPH, Devin M. Parker, MS, Emily Marshall, MS, Jeremiah R. Brown, PhD, MS
Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patientsAUTHORS: Maya Dewan, MD, MPH, Heather Wolfe, MD, MSHP, Richard Lin, MD, Eileen Ware, RN, Michelle Weiss, RN, Lihai Song, MS, Matthew MacMurchy, BA, Daniela Davis, MD, MSCE, Christopher P. Bonafide MD MSCE
Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: A qualitative studyAUTHORS: Bennett W. Clark, MD, Katelyn Baron, MSIOP, Kathleen Tynan-McKiernan, RN, MSN, Meredith Campbell Britton, LMSW, Karl E. Minges, PhD, Sarwat I. Chaudhry, MD
Use of postacute facility care in children hospitalized with acute respiratory illnessAUTHORS: Jay G. Berry, MD, MPH, Karen M. Wilson, MD, MPH, FAAP, Helene Dumas, PT, MS, Edwin Simpser, MD, Jane O’Brien, MD, Kathleen Whitford, PNP, Rachna May, MD, Vineeta Mittal, MD, Nancy Murphy, MD, David Steinhorn, MD, Rishi Agrawal, MD, MPH, Kris Rehm, MD, Michelle Marks, DO, FAAP, SFHM, Christine Traul, MD, Michael Dribbon, PhD, Christopher J. Haines, DO, MBA, FAAP, FACEP, Matt Hall, PhD
A contemporary assessment of mechanical complication rates and trainee perceptions of central venous catheter insertionAUTHORS: Lauren Heidemann, MD, Niket Nathani, MD, Rommel Sagana, MD, Vineet Chopra, MD, MSc, Michael Heung, MD, MS
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
VA Health Equity Report Details Disparities in Care
“A good understanding of the diverse veteran populations is imperative if the VA is to genuinely resolve the inequities for those at high risk and with the most need,” says the introduction to the first-ever National Veteran Health Equity Report. The report, which contains demographic information on veterans who received VHA care in Fiscal Year (FY) 2013, is designed to provide that comparative information on minorities, women, and other veteran groups. For example:
- Although women represented only about 7% of patients in FY2013, their numbers in VHA have more than doubled since 2000—140% growth, far outstripping the 63% growth among men over the same period;
- In FY2013, 46% of veterans were aged ≥ 65 years;
- More than one-third of veterans lived in rural areas;
- Almost one-half of VHA patients had a service-connected disability. All racial/ethnic minority patient groups, compared with whites, were more likely to have a service-connected disability;
- A higher proportion of women had a service-connected disability, and women are twice as likely to be diagnosed with depression;
- Overall, 33% of VHA patients had ≥ 1 mental health diagnoses. Women and blacks/African Americans were “over-represented” among patients diagnosed with serious mental illness;
- Veterans with serious mental illness also had higher diagnosis rates for musculoskeletal disorders (60% vs 43%) and gastrointestinal conditions (48% vs 30%). In fact, among the top 20 diagnosed conditions, rates for the SMI group exceeded that for the veterans with no mental health diagnosis for 17 conditions by a margin of > 10% for 7. The largest disparities were in tobacco use disorder, psychosocial factors, spine disorders, and housing insufficiency; and
- The only condition in which the diagnosed rate in a racial/ethnic group exceeded that for whites by a margin of 10% was PTSD, diagnosed in 21% of American Indian/Alaska Natives, versus 11% of whites.
Although the report targeted 6 million veterans accessing VA care in FY13, the estimated number of living veterans is about 22 million. It is a “starting place,” the developers promise. Next iterations will continue to evolve to meet the unique needs of diverse veterans.
“A good understanding of the diverse veteran populations is imperative if the VA is to genuinely resolve the inequities for those at high risk and with the most need,” says the introduction to the first-ever National Veteran Health Equity Report. The report, which contains demographic information on veterans who received VHA care in Fiscal Year (FY) 2013, is designed to provide that comparative information on minorities, women, and other veteran groups. For example:
- Although women represented only about 7% of patients in FY2013, their numbers in VHA have more than doubled since 2000—140% growth, far outstripping the 63% growth among men over the same period;
- In FY2013, 46% of veterans were aged ≥ 65 years;
- More than one-third of veterans lived in rural areas;
- Almost one-half of VHA patients had a service-connected disability. All racial/ethnic minority patient groups, compared with whites, were more likely to have a service-connected disability;
- A higher proportion of women had a service-connected disability, and women are twice as likely to be diagnosed with depression;
- Overall, 33% of VHA patients had ≥ 1 mental health diagnoses. Women and blacks/African Americans were “over-represented” among patients diagnosed with serious mental illness;
- Veterans with serious mental illness also had higher diagnosis rates for musculoskeletal disorders (60% vs 43%) and gastrointestinal conditions (48% vs 30%). In fact, among the top 20 diagnosed conditions, rates for the SMI group exceeded that for the veterans with no mental health diagnosis for 17 conditions by a margin of > 10% for 7. The largest disparities were in tobacco use disorder, psychosocial factors, spine disorders, and housing insufficiency; and
- The only condition in which the diagnosed rate in a racial/ethnic group exceeded that for whites by a margin of 10% was PTSD, diagnosed in 21% of American Indian/Alaska Natives, versus 11% of whites.
Although the report targeted 6 million veterans accessing VA care in FY13, the estimated number of living veterans is about 22 million. It is a “starting place,” the developers promise. Next iterations will continue to evolve to meet the unique needs of diverse veterans.
“A good understanding of the diverse veteran populations is imperative if the VA is to genuinely resolve the inequities for those at high risk and with the most need,” says the introduction to the first-ever National Veteran Health Equity Report. The report, which contains demographic information on veterans who received VHA care in Fiscal Year (FY) 2013, is designed to provide that comparative information on minorities, women, and other veteran groups. For example:
- Although women represented only about 7% of patients in FY2013, their numbers in VHA have more than doubled since 2000—140% growth, far outstripping the 63% growth among men over the same period;
- In FY2013, 46% of veterans were aged ≥ 65 years;
- More than one-third of veterans lived in rural areas;
- Almost one-half of VHA patients had a service-connected disability. All racial/ethnic minority patient groups, compared with whites, were more likely to have a service-connected disability;
- A higher proportion of women had a service-connected disability, and women are twice as likely to be diagnosed with depression;
- Overall, 33% of VHA patients had ≥ 1 mental health diagnoses. Women and blacks/African Americans were “over-represented” among patients diagnosed with serious mental illness;
- Veterans with serious mental illness also had higher diagnosis rates for musculoskeletal disorders (60% vs 43%) and gastrointestinal conditions (48% vs 30%). In fact, among the top 20 diagnosed conditions, rates for the SMI group exceeded that for the veterans with no mental health diagnosis for 17 conditions by a margin of > 10% for 7. The largest disparities were in tobacco use disorder, psychosocial factors, spine disorders, and housing insufficiency; and
- The only condition in which the diagnosed rate in a racial/ethnic group exceeded that for whites by a margin of 10% was PTSD, diagnosed in 21% of American Indian/Alaska Natives, versus 11% of whites.
Although the report targeted 6 million veterans accessing VA care in FY13, the estimated number of living veterans is about 22 million. It is a “starting place,” the developers promise. Next iterations will continue to evolve to meet the unique needs of diverse veterans.
Study: Reference pricing does reduce prescription costs
Reference pricing effectively encourages patients to spend significantly less on prescription drugs, according to research published in NEJM.
Under the reference pricing strategy, the insurer or employer establishes its maximum contribution toward the price of therapeutically similar drugs, and the patient must pay the remainder out of pocket.
The insurer/employer contribution is based on the price of the lowest-priced drug in the therapeutic class, called the reference drug.
“If the patient chooses a cheap or moderately priced option, the employer’s contribution will cover most of the cost,” said study author James C. Robinson, PhD, of the University of California at Berkeley.
“However, if the patient insists on a particularly high-priced option, he or she will need to make a meaningful payment from personal resources.”
It has been theorized that this policy would encourage patients to save money by selecting cheaper drugs. However, little is known about how the policy has actually influenced patient spending.
The new study showed that reference pricing was associated with significant changes in drug selection and spending for patients covered by employment-based insurance in the US.
Researchers analyzed changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the US, before and after an alliance of private employers began using reference pricing.
The trends were compared to a cohort without reference pricing. The study’s dataset included 1.1 million prescriptions reimbursed from 2010 to 2014.
Implementation of reference pricing was associated with a 7% increase in prescriptions filled for the low-price reference drug within its therapeutic class, a 14% decrease in average price paid, and a 5% increase in consumer cost-sharing.
In the first 18 months after implementation, employers’ spending dropped $1.34 million, and employees’ cost-sharing increased $120,000.
Based on these findings, Dr Robinson and his colleagues concluded that reference pricing may be one instrument for influencing patients’ drug choices and drug prices paid by employers and insurers. The team believes that, in the future, pharmaceutical companies charging “premium prices” may need to demonstrate that their drugs provide “premium performance.”
“There is huge and unjustified variation within and across geographic areas in the prices charged for almost every test and treatment, drug and device, office visit and hospitalization,” Dr Robinson said.
“It’s not a surprise when one considers that most patients are covered by health insurance and, hence, do not shop among competing providers on the basis of price. Some providers look at price-unconscious consumer demand and ask themselves, ‘Why don’t we raise our prices?’”
This research was funded by the US Agency for Healthcare Research and Quality and the Genentech Foundation. ![]()
Reference pricing effectively encourages patients to spend significantly less on prescription drugs, according to research published in NEJM.
Under the reference pricing strategy, the insurer or employer establishes its maximum contribution toward the price of therapeutically similar drugs, and the patient must pay the remainder out of pocket.
The insurer/employer contribution is based on the price of the lowest-priced drug in the therapeutic class, called the reference drug.
“If the patient chooses a cheap or moderately priced option, the employer’s contribution will cover most of the cost,” said study author James C. Robinson, PhD, of the University of California at Berkeley.
“However, if the patient insists on a particularly high-priced option, he or she will need to make a meaningful payment from personal resources.”
It has been theorized that this policy would encourage patients to save money by selecting cheaper drugs. However, little is known about how the policy has actually influenced patient spending.
The new study showed that reference pricing was associated with significant changes in drug selection and spending for patients covered by employment-based insurance in the US.
Researchers analyzed changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the US, before and after an alliance of private employers began using reference pricing.
The trends were compared to a cohort without reference pricing. The study’s dataset included 1.1 million prescriptions reimbursed from 2010 to 2014.
Implementation of reference pricing was associated with a 7% increase in prescriptions filled for the low-price reference drug within its therapeutic class, a 14% decrease in average price paid, and a 5% increase in consumer cost-sharing.
In the first 18 months after implementation, employers’ spending dropped $1.34 million, and employees’ cost-sharing increased $120,000.
Based on these findings, Dr Robinson and his colleagues concluded that reference pricing may be one instrument for influencing patients’ drug choices and drug prices paid by employers and insurers. The team believes that, in the future, pharmaceutical companies charging “premium prices” may need to demonstrate that their drugs provide “premium performance.”
“There is huge and unjustified variation within and across geographic areas in the prices charged for almost every test and treatment, drug and device, office visit and hospitalization,” Dr Robinson said.
“It’s not a surprise when one considers that most patients are covered by health insurance and, hence, do not shop among competing providers on the basis of price. Some providers look at price-unconscious consumer demand and ask themselves, ‘Why don’t we raise our prices?’”
This research was funded by the US Agency for Healthcare Research and Quality and the Genentech Foundation. ![]()
Reference pricing effectively encourages patients to spend significantly less on prescription drugs, according to research published in NEJM.
Under the reference pricing strategy, the insurer or employer establishes its maximum contribution toward the price of therapeutically similar drugs, and the patient must pay the remainder out of pocket.
The insurer/employer contribution is based on the price of the lowest-priced drug in the therapeutic class, called the reference drug.
“If the patient chooses a cheap or moderately priced option, the employer’s contribution will cover most of the cost,” said study author James C. Robinson, PhD, of the University of California at Berkeley.
“However, if the patient insists on a particularly high-priced option, he or she will need to make a meaningful payment from personal resources.”
It has been theorized that this policy would encourage patients to save money by selecting cheaper drugs. However, little is known about how the policy has actually influenced patient spending.
The new study showed that reference pricing was associated with significant changes in drug selection and spending for patients covered by employment-based insurance in the US.
Researchers analyzed changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the US, before and after an alliance of private employers began using reference pricing.
The trends were compared to a cohort without reference pricing. The study’s dataset included 1.1 million prescriptions reimbursed from 2010 to 2014.
Implementation of reference pricing was associated with a 7% increase in prescriptions filled for the low-price reference drug within its therapeutic class, a 14% decrease in average price paid, and a 5% increase in consumer cost-sharing.
In the first 18 months after implementation, employers’ spending dropped $1.34 million, and employees’ cost-sharing increased $120,000.
Based on these findings, Dr Robinson and his colleagues concluded that reference pricing may be one instrument for influencing patients’ drug choices and drug prices paid by employers and insurers. The team believes that, in the future, pharmaceutical companies charging “premium prices” may need to demonstrate that their drugs provide “premium performance.”
“There is huge and unjustified variation within and across geographic areas in the prices charged for almost every test and treatment, drug and device, office visit and hospitalization,” Dr Robinson said.
“It’s not a surprise when one considers that most patients are covered by health insurance and, hence, do not shop among competing providers on the basis of price. Some providers look at price-unconscious consumer demand and ask themselves, ‘Why don’t we raise our prices?’”
This research was funded by the US Agency for Healthcare Research and Quality and the Genentech Foundation. ![]()
Triplet eradicates B-ALL, prolongs survival in mice
A 3-drug combination has demonstrated long-term efficacy against acute lymphoblastic leukemia (ALL) in mice, according to research published in Nature Communications.
Researchers found that when the production of nucleotides is stopped, a DNA replication stress response is activated, and this allows ALL cells to survive.
The team used these findings to devise a 3-drug regimen that blocks both of the nucleotide biosynthetic pathways and inhibits the replication stress response.
One of the drugs is triapine (3-AP), which inhibits ribonucleotide reductase (RNR) and enhances salvage nucleotide biosynthesis.
Another drug is DI-82, which inhibits the nucleoside salvage kinase deoxycytidine kinase (dCK).
And the third drug is VE-822, which inhibits the replication stress-sensing kinase ataxia telangiectasia and Rad3-related protein (ATR).
The researchers tested these 3 drugs in a mouse model of pre-B-ALL. Starting on day 7 after inoculation of pre-B-ALL, the mice received 3-AP and DI-82 twice a day and VE-822 once a day.
The team said mice in the control group succumbed to their disease within 17 days. However, all of the mice that received the 3-drug combination were still disease-free 442 days after they had stopped treatment (on day 42).
The researchers said the combination was well-tolerated, as mice maintained their body weight and enjoyed long-term survival without any detectable pathology.
The team also tested a dosing regimen in which all 3 drugs were given once a day.
Although this was less effective than the first regimen, 4 of 5 mice had no detectable disease 313 days after stopping treatment.
Two of the researchers involved in this study are co-founders and equity holders of Trethera Corp, a company developing a dCK inhibitor known as TRE-515.
The University of California (where most of the study authors work) has patented intellectual property for small-molecule dCK inhibitors, and it was licensed by Trethera. ![]()
A 3-drug combination has demonstrated long-term efficacy against acute lymphoblastic leukemia (ALL) in mice, according to research published in Nature Communications.
Researchers found that when the production of nucleotides is stopped, a DNA replication stress response is activated, and this allows ALL cells to survive.
The team used these findings to devise a 3-drug regimen that blocks both of the nucleotide biosynthetic pathways and inhibits the replication stress response.
One of the drugs is triapine (3-AP), which inhibits ribonucleotide reductase (RNR) and enhances salvage nucleotide biosynthesis.
Another drug is DI-82, which inhibits the nucleoside salvage kinase deoxycytidine kinase (dCK).
And the third drug is VE-822, which inhibits the replication stress-sensing kinase ataxia telangiectasia and Rad3-related protein (ATR).
The researchers tested these 3 drugs in a mouse model of pre-B-ALL. Starting on day 7 after inoculation of pre-B-ALL, the mice received 3-AP and DI-82 twice a day and VE-822 once a day.
The team said mice in the control group succumbed to their disease within 17 days. However, all of the mice that received the 3-drug combination were still disease-free 442 days after they had stopped treatment (on day 42).
The researchers said the combination was well-tolerated, as mice maintained their body weight and enjoyed long-term survival without any detectable pathology.
The team also tested a dosing regimen in which all 3 drugs were given once a day.
Although this was less effective than the first regimen, 4 of 5 mice had no detectable disease 313 days after stopping treatment.
Two of the researchers involved in this study are co-founders and equity holders of Trethera Corp, a company developing a dCK inhibitor known as TRE-515.
The University of California (where most of the study authors work) has patented intellectual property for small-molecule dCK inhibitors, and it was licensed by Trethera. ![]()
A 3-drug combination has demonstrated long-term efficacy against acute lymphoblastic leukemia (ALL) in mice, according to research published in Nature Communications.
Researchers found that when the production of nucleotides is stopped, a DNA replication stress response is activated, and this allows ALL cells to survive.
The team used these findings to devise a 3-drug regimen that blocks both of the nucleotide biosynthetic pathways and inhibits the replication stress response.
One of the drugs is triapine (3-AP), which inhibits ribonucleotide reductase (RNR) and enhances salvage nucleotide biosynthesis.
Another drug is DI-82, which inhibits the nucleoside salvage kinase deoxycytidine kinase (dCK).
And the third drug is VE-822, which inhibits the replication stress-sensing kinase ataxia telangiectasia and Rad3-related protein (ATR).
The researchers tested these 3 drugs in a mouse model of pre-B-ALL. Starting on day 7 after inoculation of pre-B-ALL, the mice received 3-AP and DI-82 twice a day and VE-822 once a day.
The team said mice in the control group succumbed to their disease within 17 days. However, all of the mice that received the 3-drug combination were still disease-free 442 days after they had stopped treatment (on day 42).
The researchers said the combination was well-tolerated, as mice maintained their body weight and enjoyed long-term survival without any detectable pathology.
The team also tested a dosing regimen in which all 3 drugs were given once a day.
Although this was less effective than the first regimen, 4 of 5 mice had no detectable disease 313 days after stopping treatment.
Two of the researchers involved in this study are co-founders and equity holders of Trethera Corp, a company developing a dCK inhibitor known as TRE-515.
The University of California (where most of the study authors work) has patented intellectual property for small-molecule dCK inhibitors, and it was licensed by Trethera. ![]()
Drug granted orphan designation for chemo-induced ototoxicity
The US Food and Drug Administration (FDA) has granted orphan drug designation to SENS-401 to be used for the prevention of platinum-induced ototoxicity in pediatric patients.
Platinum-based chemotherapies, particularly cisplatin, can induce severe hearing loss in cancer patients, but there is no pharmaceutical agent approved to treat this side effect.
“Hearing loss in pediatric oncology patients is one of the most frequent side effects of cisplatin treatment and may disable them for the rest of their lives,” said Nawal Ouzren, CEO of Sensorion, the company developing SENS-401.
“Based on its unique profile and the data generated to date, we believe SENS-401 has the potential to be a safe and effective treatment for this serious medical condition where a significant unmet need exists. As such, we look forward to working with the FDA and EMA [European Medicines Agency] to set up an IND [investigational new drug application] and design a phase 2 clinical trial in order to evaluate SENS-401 in this indication.”
About SENS-401
SENS-401 (R-azasetron besylate) is a small molecule intended to protect and preserve inner ear tissue when lesions cause progressive or sequelar hearing impairments. The drug can be taken orally or via an injection.
SENS-401 is one of the two enantiomer forms of SENS-218 (azasetron), a racemic molecule belonging to the family of setrons marketed in Asia under the name Serotone. Pharmacological and pharmacokinetic tests have shown a superior profile for SENS-401 compared with the other enantiomer or the racemic form.
Healthy subjects demonstrated a “very good clinical tolerance” to SENS-401 in a phase 1 study, according to Sensorion. The company is planning to launch a phase 2 trial of the drug for platinum-induced ototoxicity in 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to SENS-401 to be used for the prevention of platinum-induced ototoxicity in pediatric patients.
Platinum-based chemotherapies, particularly cisplatin, can induce severe hearing loss in cancer patients, but there is no pharmaceutical agent approved to treat this side effect.
“Hearing loss in pediatric oncology patients is one of the most frequent side effects of cisplatin treatment and may disable them for the rest of their lives,” said Nawal Ouzren, CEO of Sensorion, the company developing SENS-401.
“Based on its unique profile and the data generated to date, we believe SENS-401 has the potential to be a safe and effective treatment for this serious medical condition where a significant unmet need exists. As such, we look forward to working with the FDA and EMA [European Medicines Agency] to set up an IND [investigational new drug application] and design a phase 2 clinical trial in order to evaluate SENS-401 in this indication.”
About SENS-401
SENS-401 (R-azasetron besylate) is a small molecule intended to protect and preserve inner ear tissue when lesions cause progressive or sequelar hearing impairments. The drug can be taken orally or via an injection.
SENS-401 is one of the two enantiomer forms of SENS-218 (azasetron), a racemic molecule belonging to the family of setrons marketed in Asia under the name Serotone. Pharmacological and pharmacokinetic tests have shown a superior profile for SENS-401 compared with the other enantiomer or the racemic form.
Healthy subjects demonstrated a “very good clinical tolerance” to SENS-401 in a phase 1 study, according to Sensorion. The company is planning to launch a phase 2 trial of the drug for platinum-induced ototoxicity in 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to SENS-401 to be used for the prevention of platinum-induced ototoxicity in pediatric patients.
Platinum-based chemotherapies, particularly cisplatin, can induce severe hearing loss in cancer patients, but there is no pharmaceutical agent approved to treat this side effect.
“Hearing loss in pediatric oncology patients is one of the most frequent side effects of cisplatin treatment and may disable them for the rest of their lives,” said Nawal Ouzren, CEO of Sensorion, the company developing SENS-401.
“Based on its unique profile and the data generated to date, we believe SENS-401 has the potential to be a safe and effective treatment for this serious medical condition where a significant unmet need exists. As such, we look forward to working with the FDA and EMA [European Medicines Agency] to set up an IND [investigational new drug application] and design a phase 2 clinical trial in order to evaluate SENS-401 in this indication.”
About SENS-401
SENS-401 (R-azasetron besylate) is a small molecule intended to protect and preserve inner ear tissue when lesions cause progressive or sequelar hearing impairments. The drug can be taken orally or via an injection.
SENS-401 is one of the two enantiomer forms of SENS-218 (azasetron), a racemic molecule belonging to the family of setrons marketed in Asia under the name Serotone. Pharmacological and pharmacokinetic tests have shown a superior profile for SENS-401 compared with the other enantiomer or the racemic form.
Healthy subjects demonstrated a “very good clinical tolerance” to SENS-401 in a phase 1 study, according to Sensorion. The company is planning to launch a phase 2 trial of the drug for platinum-induced ototoxicity in 2018.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Study findings cast doubt on community-acquired pneumonia diagnostic practices
New studies raise doubts on the reliability of physical exam findings in suspected pediatric community-acquired pneumonia cases and on the value of blood cultures in hospitalized pediatric CAP cases.
In one study, 128 cases of suspected CAP in children aged 3 months to 18 years presenting to an ED from July 2013 to May 2016 underwent paired assessments within 20 minutes of each other. Only 3 of 19 exam findings used to diagnose CAP – wheezing, retractions, and respiratory rate – had acceptable levels of inter-rater reliability, with the lower end of the 95% confidential interval at a Fleiss’ kappa value of 0.4 or higher.
“The reliability of these findings must be considered in the clinical management and research of children with CAP,” said lead author Todd Florin, MD, associate research director in emergency medicine at Cincinnati Children’s Hospital Medical Center and his associates. (Pediatrics. 2017;140[3]:e20170310)
In a retrospective cohort analysis, researchers found that just 2.5% of 2,568 hospitalized children with CAP who had a blood culture performed actually grew a pathogen. And of the detected pathogens, 82% were susceptible to penicillin. Streptococcus pneumoniae accounted for 78% of all the pathogens that were found; it was detected in only 2% of all children who had blood cultures taken.
Just 11 children – or 0.43% of the children with a blood culture performed – had growth of a pathogen that was not treatable with penicillin, said lead author Mark Neuman, MD, director of research at Boston Children’s Hospital and his associates (Pediatrics. 2017;140[3]:e20171013).
The analysis was drawn from a cohort of 7,509 children hospitalized from 2007 to 2011, with children with complex chronic conditions excluded. Data for the analysis came from the Pediatric Health Information System Plus database, in which administrative, billing, laboratory, and radiographic information is stored from six tertiary children’s hospitals.
The investigators said that one challenge is that when blood cultures are drawn early in the course of evaluation and treatment, the severity of the child’s CAP might not be apparent, which makes it difficult to know which children would benefit from a blood culture.
“The routine performance of blood cultures in these children may not be indicated,” Dr. Neuman and his associates said. “Researchers in future studies should seek to identify the clinical characteristics of children in whom obtaining blood cultures would lead to changes in clinical management, especially when identifying those patients at risk for CAP caused by organisms not susceptible to guideline-recommended, narrow-spectrum antibiotics.”
Both studies were funded by the National Institutes of Health. For the physician exam study, additional funding was provided by individual grants from the Gerber Foundation, the National Center for Research Resources and the National Center for Advancing Translational Sciences, the National Institute for Allergy and Infectious Diseases and the National Institutes of Health, and a Trustee Award from Cincinnati Children’s Hospital Medical Center. For the blood cultures study, individual researchers received funding from the National Institute of Allergy and Infectious Diseases and the Agency for Healthcare Research and Quality. For the physician exam study, no financial disclosures were reported. For the blood cultures study, Anne Blaschke, MD, PhD, reported receiving research funding from and other financial relationships with BioFire Diagnostics.
New studies raise doubts on the reliability of physical exam findings in suspected pediatric community-acquired pneumonia cases and on the value of blood cultures in hospitalized pediatric CAP cases.
In one study, 128 cases of suspected CAP in children aged 3 months to 18 years presenting to an ED from July 2013 to May 2016 underwent paired assessments within 20 minutes of each other. Only 3 of 19 exam findings used to diagnose CAP – wheezing, retractions, and respiratory rate – had acceptable levels of inter-rater reliability, with the lower end of the 95% confidential interval at a Fleiss’ kappa value of 0.4 or higher.
“The reliability of these findings must be considered in the clinical management and research of children with CAP,” said lead author Todd Florin, MD, associate research director in emergency medicine at Cincinnati Children’s Hospital Medical Center and his associates. (Pediatrics. 2017;140[3]:e20170310)
In a retrospective cohort analysis, researchers found that just 2.5% of 2,568 hospitalized children with CAP who had a blood culture performed actually grew a pathogen. And of the detected pathogens, 82% were susceptible to penicillin. Streptococcus pneumoniae accounted for 78% of all the pathogens that were found; it was detected in only 2% of all children who had blood cultures taken.
Just 11 children – or 0.43% of the children with a blood culture performed – had growth of a pathogen that was not treatable with penicillin, said lead author Mark Neuman, MD, director of research at Boston Children’s Hospital and his associates (Pediatrics. 2017;140[3]:e20171013).
The analysis was drawn from a cohort of 7,509 children hospitalized from 2007 to 2011, with children with complex chronic conditions excluded. Data for the analysis came from the Pediatric Health Information System Plus database, in which administrative, billing, laboratory, and radiographic information is stored from six tertiary children’s hospitals.
The investigators said that one challenge is that when blood cultures are drawn early in the course of evaluation and treatment, the severity of the child’s CAP might not be apparent, which makes it difficult to know which children would benefit from a blood culture.
“The routine performance of blood cultures in these children may not be indicated,” Dr. Neuman and his associates said. “Researchers in future studies should seek to identify the clinical characteristics of children in whom obtaining blood cultures would lead to changes in clinical management, especially when identifying those patients at risk for CAP caused by organisms not susceptible to guideline-recommended, narrow-spectrum antibiotics.”
Both studies were funded by the National Institutes of Health. For the physician exam study, additional funding was provided by individual grants from the Gerber Foundation, the National Center for Research Resources and the National Center for Advancing Translational Sciences, the National Institute for Allergy and Infectious Diseases and the National Institutes of Health, and a Trustee Award from Cincinnati Children’s Hospital Medical Center. For the blood cultures study, individual researchers received funding from the National Institute of Allergy and Infectious Diseases and the Agency for Healthcare Research and Quality. For the physician exam study, no financial disclosures were reported. For the blood cultures study, Anne Blaschke, MD, PhD, reported receiving research funding from and other financial relationships with BioFire Diagnostics.
New studies raise doubts on the reliability of physical exam findings in suspected pediatric community-acquired pneumonia cases and on the value of blood cultures in hospitalized pediatric CAP cases.
In one study, 128 cases of suspected CAP in children aged 3 months to 18 years presenting to an ED from July 2013 to May 2016 underwent paired assessments within 20 minutes of each other. Only 3 of 19 exam findings used to diagnose CAP – wheezing, retractions, and respiratory rate – had acceptable levels of inter-rater reliability, with the lower end of the 95% confidential interval at a Fleiss’ kappa value of 0.4 or higher.
“The reliability of these findings must be considered in the clinical management and research of children with CAP,” said lead author Todd Florin, MD, associate research director in emergency medicine at Cincinnati Children’s Hospital Medical Center and his associates. (Pediatrics. 2017;140[3]:e20170310)
In a retrospective cohort analysis, researchers found that just 2.5% of 2,568 hospitalized children with CAP who had a blood culture performed actually grew a pathogen. And of the detected pathogens, 82% were susceptible to penicillin. Streptococcus pneumoniae accounted for 78% of all the pathogens that were found; it was detected in only 2% of all children who had blood cultures taken.
Just 11 children – or 0.43% of the children with a blood culture performed – had growth of a pathogen that was not treatable with penicillin, said lead author Mark Neuman, MD, director of research at Boston Children’s Hospital and his associates (Pediatrics. 2017;140[3]:e20171013).
The analysis was drawn from a cohort of 7,509 children hospitalized from 2007 to 2011, with children with complex chronic conditions excluded. Data for the analysis came from the Pediatric Health Information System Plus database, in which administrative, billing, laboratory, and radiographic information is stored from six tertiary children’s hospitals.
The investigators said that one challenge is that when blood cultures are drawn early in the course of evaluation and treatment, the severity of the child’s CAP might not be apparent, which makes it difficult to know which children would benefit from a blood culture.
“The routine performance of blood cultures in these children may not be indicated,” Dr. Neuman and his associates said. “Researchers in future studies should seek to identify the clinical characteristics of children in whom obtaining blood cultures would lead to changes in clinical management, especially when identifying those patients at risk for CAP caused by organisms not susceptible to guideline-recommended, narrow-spectrum antibiotics.”
Both studies were funded by the National Institutes of Health. For the physician exam study, additional funding was provided by individual grants from the Gerber Foundation, the National Center for Research Resources and the National Center for Advancing Translational Sciences, the National Institute for Allergy and Infectious Diseases and the National Institutes of Health, and a Trustee Award from Cincinnati Children’s Hospital Medical Center. For the blood cultures study, individual researchers received funding from the National Institute of Allergy and Infectious Diseases and the Agency for Healthcare Research and Quality. For the physician exam study, no financial disclosures were reported. For the blood cultures study, Anne Blaschke, MD, PhD, reported receiving research funding from and other financial relationships with BioFire Diagnostics.
FROM PEDIATRICS
Key clinical point:
Major finding: In one study, only 3 of 19 exam findings used to diagnose CAP – wheezing, retractions, and respiratory rate – had acceptable levels of inter-rater reliability in cases in which paired assessments were done 20 minutes apart after patients presented to the ED with suspected CAP. In another study, just 2.5% of hospitalized children with CAP who had a blood culture performed actually grew a pathogen.
Data source: An ongoing prospective cohort study of 128 pediatric patients presenting to an emergency room with suspected CAP, and a retrospective analysis of data collected on hospitalizations from 2007 to 2011 at six tertiary children’s hospitals.
Disclosures: The studies were funded by the National Institutes of Health and by other grants to individual researchers. For the physician exam study, no relevant financial disclosures were reported. For the blood cultures study, Anne Blaschke, MD, PhD, reports receiving research funding from and other financial relationships with BioFire Diagnostics.
Navigating the complex landscape of IBD therapies
I provided an update on existing, new, and upcoming medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC), with a focus on studies presented at Digestive Disease Week® 2017.
In one study of over 13,000 inflammatory bowel disease (IBD) patients in Medicare/Medicaid databases, it was found that among those treated with corticosteroids in the previous year, patients started on a tumor necrosis factor (TNF) inhibitor within the next year had mortality rates that were at least 22% lower than those of patients treated with prolonged corticosteroids over the next 12 months (Gastroenterology. 2017;152[5 Suppl 1]:S65-5). Initial results of the CALM study were presented, comparing a treat-to-target (T2T) algorithmic medical escalation approach in moderate to severe CD to a more conventional approach. Medical therapy was primarily adalimumab based and was escalated based on “success criteria,” which included not only symptomatic remission but also normalization of serum C-reactive protein and fecal calprotectin. At week 48, the rate of endoscopic remission was significantly higher (45.9%) in the T2T group than in conventionally managed patients (30.3%, P = .01), thus demonstrating the superiority of a T2T approach (Gastroenterology 2017;152[5 Suppl 1]:S155).
Ustekinumab is a monoclonal antibody to interleukins 12 and 23, and was approved for moderate to severe CD last year on the basis of the pivotal UNITI-1, UNITI-2, and IM-UNITI trials (N Engl J Med. 2016;375:1946-60). A weight-based intravenous loading dose was shown to be effective at inducing clinical response in both patients who had failed or were intolerant to anti-TNF therapy and those who had not. The responders in both induction trials were randomized to two subcutaneous doses of ustekinumab or placebo, and at the end of the 44-week trial, the drug met multiple efficacy endpoints, including clinical remission, clinical response, steroid-free remission, and sustained clinical remission. In another abstract, the rate of tuberculosis reactivation within the clinical development program of ustekinumab across all indications (6,581 patients, over 12,000 patient-years of follow-up) was significantly lower at 0.02 cases per 100 patient-years compared with the rates seen in the golimumab (0.24 per 100) and infliximab (0.39 per 100) development programs (Gastroenterology 2017;152[5 Suppl 1]:S596), illustrating that the safety profile of ustekinumab may be significantly different from that of anti-TNF agents.
Tofacitinib, which inhibits mainly JAK1 and JAK3 receptors, is an emergent oral small molecule drug for UC. Three phase 3 randomized placebo-controlled trials (OCTAVE-1, OCTAVE-2, and OCTAVE Sustain) of tofacitinib treatment in moderately to severely active UC patients have been recently published (N Engl J Med. 2017;376:1723-36). The rates of clinical remission at week 8 were significantly greater in patients who were treated with 10 mg tofacitinib than placebo in both induction trials, and results were similar regardless of anti-TNF exposure status. Clinical responders in the induction studies were randomized to placebo or two doses of tofacitinib. At week 52, remission rates were significantly higher in the patients treated with 10 mg tofacitinib twice daily and 5 mg tofacitinib twice daily than those receiving placebo. The percentages of tofacitinib-treated patients who achieved mucosal healing were significantly greater than those in the placebo group. Serious infections occurred significantly more frequently in the tofacitinib than placebo group during induction, but not during maintenance. However, rates of herpes zoster were higher with maintenance therapy at 10 mg twice daily (5.1%) than with placebo (0.5%). A recently published phase 2 study of filgotinib, a selective JAK1 inhibitor, reported that the remission rate at week 10 was significantly higher in active CD patients receiving 200 mg of filgotinib daily than in those receiving placebo (Lancet 2017;389:266-75). A phase 2 trial of another selective JAK1 inhibitor, upadacitinib (ABT-494), for induction therapy in CD patients with a history of failure or intolerance to TNF-antagonists, was presented at DDW (Gastroenterology 2017;152[5 Suppl 1]:S1308-9). Higher rates of clinical remission at week 16 were seen in patients on 6 mg upadacitinib twice daily than placebo, and several doses of upadacitinib were significantly better than placebo for inducing endoscopic remission at week 12 or 16. Serious adverse events were seen in 9%-15% of CD patients treated with these two agents (vs. 4%-5% in placebo-treated patients).
Smad7 regulates the signaling of transforming growth factor (TGF)-beta1, an anti-inflammatory cytokine. Mongersen is an orally delivered anti-sense oligonucleotide that inhibits Smad7 and restores TGF-beta1 signaling, and is being developed for CD. The efficacy of induction therapy for active CD patients with limited active disease (terminal ileum or proximal colon) was demonstrated in a phase 2 study (N Engl J Med. 2015;372:1104-13). Interestingly, this study showed significantly higher rates of clinical remission at day 15 with mongersen. However, there were no endoscopic data available in this trial, baseline serum C-reactive protein concentrations were low, and did not decrease significantly. This drug appears to be well tolerated, and serious adverse events were not significantly higher than for placebo. In a phase 1b study, correlations between clinical and endoscopic outcomes were explored, and among 52 CD patients, SES-CD reductions of at least 25% at week 12 were seen in 37% of mongersen-treated patients (Gastroenterology. 2017;152[5 Suppl 1]:S198).
In summary, the future of IBD medical therapy is bright due to the recent introduction of therapies with novel mechanisms of action and favorable safety profiles (e.g., vedolizumab and ustekinumab), potentially lower-cost biosimilars, and multiple compounds in the drug development pipeline.
Dr. Loftus is professor of medicine, Mayo Clinic College of Medicine, director of the Inflammatory Bowel Disease Interest Group, the division of gastroenterology and hepatology, Rochester, Minn. He made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
I provided an update on existing, new, and upcoming medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC), with a focus on studies presented at Digestive Disease Week® 2017.
In one study of over 13,000 inflammatory bowel disease (IBD) patients in Medicare/Medicaid databases, it was found that among those treated with corticosteroids in the previous year, patients started on a tumor necrosis factor (TNF) inhibitor within the next year had mortality rates that were at least 22% lower than those of patients treated with prolonged corticosteroids over the next 12 months (Gastroenterology. 2017;152[5 Suppl 1]:S65-5). Initial results of the CALM study were presented, comparing a treat-to-target (T2T) algorithmic medical escalation approach in moderate to severe CD to a more conventional approach. Medical therapy was primarily adalimumab based and was escalated based on “success criteria,” which included not only symptomatic remission but also normalization of serum C-reactive protein and fecal calprotectin. At week 48, the rate of endoscopic remission was significantly higher (45.9%) in the T2T group than in conventionally managed patients (30.3%, P = .01), thus demonstrating the superiority of a T2T approach (Gastroenterology 2017;152[5 Suppl 1]:S155).
Ustekinumab is a monoclonal antibody to interleukins 12 and 23, and was approved for moderate to severe CD last year on the basis of the pivotal UNITI-1, UNITI-2, and IM-UNITI trials (N Engl J Med. 2016;375:1946-60). A weight-based intravenous loading dose was shown to be effective at inducing clinical response in both patients who had failed or were intolerant to anti-TNF therapy and those who had not. The responders in both induction trials were randomized to two subcutaneous doses of ustekinumab or placebo, and at the end of the 44-week trial, the drug met multiple efficacy endpoints, including clinical remission, clinical response, steroid-free remission, and sustained clinical remission. In another abstract, the rate of tuberculosis reactivation within the clinical development program of ustekinumab across all indications (6,581 patients, over 12,000 patient-years of follow-up) was significantly lower at 0.02 cases per 100 patient-years compared with the rates seen in the golimumab (0.24 per 100) and infliximab (0.39 per 100) development programs (Gastroenterology 2017;152[5 Suppl 1]:S596), illustrating that the safety profile of ustekinumab may be significantly different from that of anti-TNF agents.
Tofacitinib, which inhibits mainly JAK1 and JAK3 receptors, is an emergent oral small molecule drug for UC. Three phase 3 randomized placebo-controlled trials (OCTAVE-1, OCTAVE-2, and OCTAVE Sustain) of tofacitinib treatment in moderately to severely active UC patients have been recently published (N Engl J Med. 2017;376:1723-36). The rates of clinical remission at week 8 were significantly greater in patients who were treated with 10 mg tofacitinib than placebo in both induction trials, and results were similar regardless of anti-TNF exposure status. Clinical responders in the induction studies were randomized to placebo or two doses of tofacitinib. At week 52, remission rates were significantly higher in the patients treated with 10 mg tofacitinib twice daily and 5 mg tofacitinib twice daily than those receiving placebo. The percentages of tofacitinib-treated patients who achieved mucosal healing were significantly greater than those in the placebo group. Serious infections occurred significantly more frequently in the tofacitinib than placebo group during induction, but not during maintenance. However, rates of herpes zoster were higher with maintenance therapy at 10 mg twice daily (5.1%) than with placebo (0.5%). A recently published phase 2 study of filgotinib, a selective JAK1 inhibitor, reported that the remission rate at week 10 was significantly higher in active CD patients receiving 200 mg of filgotinib daily than in those receiving placebo (Lancet 2017;389:266-75). A phase 2 trial of another selective JAK1 inhibitor, upadacitinib (ABT-494), for induction therapy in CD patients with a history of failure or intolerance to TNF-antagonists, was presented at DDW (Gastroenterology 2017;152[5 Suppl 1]:S1308-9). Higher rates of clinical remission at week 16 were seen in patients on 6 mg upadacitinib twice daily than placebo, and several doses of upadacitinib were significantly better than placebo for inducing endoscopic remission at week 12 or 16. Serious adverse events were seen in 9%-15% of CD patients treated with these two agents (vs. 4%-5% in placebo-treated patients).
Smad7 regulates the signaling of transforming growth factor (TGF)-beta1, an anti-inflammatory cytokine. Mongersen is an orally delivered anti-sense oligonucleotide that inhibits Smad7 and restores TGF-beta1 signaling, and is being developed for CD. The efficacy of induction therapy for active CD patients with limited active disease (terminal ileum or proximal colon) was demonstrated in a phase 2 study (N Engl J Med. 2015;372:1104-13). Interestingly, this study showed significantly higher rates of clinical remission at day 15 with mongersen. However, there were no endoscopic data available in this trial, baseline serum C-reactive protein concentrations were low, and did not decrease significantly. This drug appears to be well tolerated, and serious adverse events were not significantly higher than for placebo. In a phase 1b study, correlations between clinical and endoscopic outcomes were explored, and among 52 CD patients, SES-CD reductions of at least 25% at week 12 were seen in 37% of mongersen-treated patients (Gastroenterology. 2017;152[5 Suppl 1]:S198).
In summary, the future of IBD medical therapy is bright due to the recent introduction of therapies with novel mechanisms of action and favorable safety profiles (e.g., vedolizumab and ustekinumab), potentially lower-cost biosimilars, and multiple compounds in the drug development pipeline.
Dr. Loftus is professor of medicine, Mayo Clinic College of Medicine, director of the Inflammatory Bowel Disease Interest Group, the division of gastroenterology and hepatology, Rochester, Minn. He made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
I provided an update on existing, new, and upcoming medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC), with a focus on studies presented at Digestive Disease Week® 2017.
In one study of over 13,000 inflammatory bowel disease (IBD) patients in Medicare/Medicaid databases, it was found that among those treated with corticosteroids in the previous year, patients started on a tumor necrosis factor (TNF) inhibitor within the next year had mortality rates that were at least 22% lower than those of patients treated with prolonged corticosteroids over the next 12 months (Gastroenterology. 2017;152[5 Suppl 1]:S65-5). Initial results of the CALM study were presented, comparing a treat-to-target (T2T) algorithmic medical escalation approach in moderate to severe CD to a more conventional approach. Medical therapy was primarily adalimumab based and was escalated based on “success criteria,” which included not only symptomatic remission but also normalization of serum C-reactive protein and fecal calprotectin. At week 48, the rate of endoscopic remission was significantly higher (45.9%) in the T2T group than in conventionally managed patients (30.3%, P = .01), thus demonstrating the superiority of a T2T approach (Gastroenterology 2017;152[5 Suppl 1]:S155).
Ustekinumab is a monoclonal antibody to interleukins 12 and 23, and was approved for moderate to severe CD last year on the basis of the pivotal UNITI-1, UNITI-2, and IM-UNITI trials (N Engl J Med. 2016;375:1946-60). A weight-based intravenous loading dose was shown to be effective at inducing clinical response in both patients who had failed or were intolerant to anti-TNF therapy and those who had not. The responders in both induction trials were randomized to two subcutaneous doses of ustekinumab or placebo, and at the end of the 44-week trial, the drug met multiple efficacy endpoints, including clinical remission, clinical response, steroid-free remission, and sustained clinical remission. In another abstract, the rate of tuberculosis reactivation within the clinical development program of ustekinumab across all indications (6,581 patients, over 12,000 patient-years of follow-up) was significantly lower at 0.02 cases per 100 patient-years compared with the rates seen in the golimumab (0.24 per 100) and infliximab (0.39 per 100) development programs (Gastroenterology 2017;152[5 Suppl 1]:S596), illustrating that the safety profile of ustekinumab may be significantly different from that of anti-TNF agents.
Tofacitinib, which inhibits mainly JAK1 and JAK3 receptors, is an emergent oral small molecule drug for UC. Three phase 3 randomized placebo-controlled trials (OCTAVE-1, OCTAVE-2, and OCTAVE Sustain) of tofacitinib treatment in moderately to severely active UC patients have been recently published (N Engl J Med. 2017;376:1723-36). The rates of clinical remission at week 8 were significantly greater in patients who were treated with 10 mg tofacitinib than placebo in both induction trials, and results were similar regardless of anti-TNF exposure status. Clinical responders in the induction studies were randomized to placebo or two doses of tofacitinib. At week 52, remission rates were significantly higher in the patients treated with 10 mg tofacitinib twice daily and 5 mg tofacitinib twice daily than those receiving placebo. The percentages of tofacitinib-treated patients who achieved mucosal healing were significantly greater than those in the placebo group. Serious infections occurred significantly more frequently in the tofacitinib than placebo group during induction, but not during maintenance. However, rates of herpes zoster were higher with maintenance therapy at 10 mg twice daily (5.1%) than with placebo (0.5%). A recently published phase 2 study of filgotinib, a selective JAK1 inhibitor, reported that the remission rate at week 10 was significantly higher in active CD patients receiving 200 mg of filgotinib daily than in those receiving placebo (Lancet 2017;389:266-75). A phase 2 trial of another selective JAK1 inhibitor, upadacitinib (ABT-494), for induction therapy in CD patients with a history of failure or intolerance to TNF-antagonists, was presented at DDW (Gastroenterology 2017;152[5 Suppl 1]:S1308-9). Higher rates of clinical remission at week 16 were seen in patients on 6 mg upadacitinib twice daily than placebo, and several doses of upadacitinib were significantly better than placebo for inducing endoscopic remission at week 12 or 16. Serious adverse events were seen in 9%-15% of CD patients treated with these two agents (vs. 4%-5% in placebo-treated patients).
Smad7 regulates the signaling of transforming growth factor (TGF)-beta1, an anti-inflammatory cytokine. Mongersen is an orally delivered anti-sense oligonucleotide that inhibits Smad7 and restores TGF-beta1 signaling, and is being developed for CD. The efficacy of induction therapy for active CD patients with limited active disease (terminal ileum or proximal colon) was demonstrated in a phase 2 study (N Engl J Med. 2015;372:1104-13). Interestingly, this study showed significantly higher rates of clinical remission at day 15 with mongersen. However, there were no endoscopic data available in this trial, baseline serum C-reactive protein concentrations were low, and did not decrease significantly. This drug appears to be well tolerated, and serious adverse events were not significantly higher than for placebo. In a phase 1b study, correlations between clinical and endoscopic outcomes were explored, and among 52 CD patients, SES-CD reductions of at least 25% at week 12 were seen in 37% of mongersen-treated patients (Gastroenterology. 2017;152[5 Suppl 1]:S198).
In summary, the future of IBD medical therapy is bright due to the recent introduction of therapies with novel mechanisms of action and favorable safety profiles (e.g., vedolizumab and ustekinumab), potentially lower-cost biosimilars, and multiple compounds in the drug development pipeline.
Dr. Loftus is professor of medicine, Mayo Clinic College of Medicine, director of the Inflammatory Bowel Disease Interest Group, the division of gastroenterology and hepatology, Rochester, Minn. He made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Copper IUDs increase bacterial vaginosis risk
PARK CITY, UTAH – Copper IUDs really do increase the risk of bacterial vaginosis (BV), according to a longitudinal study of 234 women in Harare, Zimbabwe.
This notion has “always been a little bit controversial; it’s commonly believed by some and refuted by others,” but the findings from the new research “are real and generalizable,” said Sharon Hillier, PhD, the study’s senior investigator and the director of reproductive infectious disease research at Magee-Womens Hospital of the University of Pittsburgh.
The African women in this study were all free of HIV and sexually transmitted infections; on average, they were about 26 years old; and most were married and sexually active. As part of a larger look into the role of vaginal dysbiosis in HIV acquisition, they were given five options for contraception: three kinds of injectables; one implant; and the nonhormonal copper IUD.
The women were divided almost evenly among the five options. The researchers followed them for 6 months with routine vaginal swabs and polymerase chain reaction testing during the follicular phase of menses. Women who opted for the copper IUD were slightly less likely to report being married and sexually active.
Almost a third of the women had BV at baseline, a little higher than the prevalence in American women.
Women who opted for hormonal contraceptives had no change in BV prevalence or vaginal microbiota.
However, BV prevalence in women who opted for the copper IUD increased from 27% at baseline to 34% at 30 days, 39% at 90 days, and 44% at 180 days. There was an increase in concentrations of Gardnerella vaginalis and Atopobium vaginae that was not seen in the hormonal contraception groups. Overall, copper IUDs showed a twofold increase in the relative risk of BV.
“I don’t think there’s anything here that’s particularly alarming. This would not dissuade me from recommending a copper IUD. It’s a very effective and safe nonhormonal way of having long-acting reversible contraception, but if a woman gets a copper IUD and she has recurrent BV, you need to understand that the IUD may be playing a role,” Dr. Hillier said in an interview at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
The increased risk is probably because IUDs cause heavier and longer menstrual bleeding, which is known to disturb the vaginal microbiome. Work is underway to see if removing the IUD reverses the effects, Dr. Hillier said.
Most of the women in the study opted to keep their IUDs in place after 6 months.
The Gates Foundation supported the work. Dr. Hillier is a consultant for Merck and Symbiomix and a researcher for Becton Dickinson and Cepheid.
PARK CITY, UTAH – Copper IUDs really do increase the risk of bacterial vaginosis (BV), according to a longitudinal study of 234 women in Harare, Zimbabwe.
This notion has “always been a little bit controversial; it’s commonly believed by some and refuted by others,” but the findings from the new research “are real and generalizable,” said Sharon Hillier, PhD, the study’s senior investigator and the director of reproductive infectious disease research at Magee-Womens Hospital of the University of Pittsburgh.
The African women in this study were all free of HIV and sexually transmitted infections; on average, they were about 26 years old; and most were married and sexually active. As part of a larger look into the role of vaginal dysbiosis in HIV acquisition, they were given five options for contraception: three kinds of injectables; one implant; and the nonhormonal copper IUD.
The women were divided almost evenly among the five options. The researchers followed them for 6 months with routine vaginal swabs and polymerase chain reaction testing during the follicular phase of menses. Women who opted for the copper IUD were slightly less likely to report being married and sexually active.
Almost a third of the women had BV at baseline, a little higher than the prevalence in American women.
Women who opted for hormonal contraceptives had no change in BV prevalence or vaginal microbiota.
However, BV prevalence in women who opted for the copper IUD increased from 27% at baseline to 34% at 30 days, 39% at 90 days, and 44% at 180 days. There was an increase in concentrations of Gardnerella vaginalis and Atopobium vaginae that was not seen in the hormonal contraception groups. Overall, copper IUDs showed a twofold increase in the relative risk of BV.
“I don’t think there’s anything here that’s particularly alarming. This would not dissuade me from recommending a copper IUD. It’s a very effective and safe nonhormonal way of having long-acting reversible contraception, but if a woman gets a copper IUD and she has recurrent BV, you need to understand that the IUD may be playing a role,” Dr. Hillier said in an interview at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
The increased risk is probably because IUDs cause heavier and longer menstrual bleeding, which is known to disturb the vaginal microbiome. Work is underway to see if removing the IUD reverses the effects, Dr. Hillier said.
Most of the women in the study opted to keep their IUDs in place after 6 months.
The Gates Foundation supported the work. Dr. Hillier is a consultant for Merck and Symbiomix and a researcher for Becton Dickinson and Cepheid.
PARK CITY, UTAH – Copper IUDs really do increase the risk of bacterial vaginosis (BV), according to a longitudinal study of 234 women in Harare, Zimbabwe.
This notion has “always been a little bit controversial; it’s commonly believed by some and refuted by others,” but the findings from the new research “are real and generalizable,” said Sharon Hillier, PhD, the study’s senior investigator and the director of reproductive infectious disease research at Magee-Womens Hospital of the University of Pittsburgh.
The African women in this study were all free of HIV and sexually transmitted infections; on average, they were about 26 years old; and most were married and sexually active. As part of a larger look into the role of vaginal dysbiosis in HIV acquisition, they were given five options for contraception: three kinds of injectables; one implant; and the nonhormonal copper IUD.
The women were divided almost evenly among the five options. The researchers followed them for 6 months with routine vaginal swabs and polymerase chain reaction testing during the follicular phase of menses. Women who opted for the copper IUD were slightly less likely to report being married and sexually active.
Almost a third of the women had BV at baseline, a little higher than the prevalence in American women.
Women who opted for hormonal contraceptives had no change in BV prevalence or vaginal microbiota.
However, BV prevalence in women who opted for the copper IUD increased from 27% at baseline to 34% at 30 days, 39% at 90 days, and 44% at 180 days. There was an increase in concentrations of Gardnerella vaginalis and Atopobium vaginae that was not seen in the hormonal contraception groups. Overall, copper IUDs showed a twofold increase in the relative risk of BV.
“I don’t think there’s anything here that’s particularly alarming. This would not dissuade me from recommending a copper IUD. It’s a very effective and safe nonhormonal way of having long-acting reversible contraception, but if a woman gets a copper IUD and she has recurrent BV, you need to understand that the IUD may be playing a role,” Dr. Hillier said in an interview at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
The increased risk is probably because IUDs cause heavier and longer menstrual bleeding, which is known to disturb the vaginal microbiome. Work is underway to see if removing the IUD reverses the effects, Dr. Hillier said.
Most of the women in the study opted to keep their IUDs in place after 6 months.
The Gates Foundation supported the work. Dr. Hillier is a consultant for Merck and Symbiomix and a researcher for Becton Dickinson and Cepheid.
AT IDSOG
Key clinical point:
Major finding: Baterial vaginosis prevalence in women who opted for the copper IUD increased from 27% at baseline to 34% at 30 days, 39% at 90 days, and 44% at 180 days.
Data source: A longitudinal cohort study of 234 women.
Disclosures: The Gates Foundation supported the work. The senior investigator is a consultant for Merck and Symbiomix and a researcher for Becton Dickinson and Cepheid.