User login
Lessons emerge from Europe’s first enterovirus-related brain stem encephalitis outbreak
MADRID – Ninety-two percent of Spanish children sickened during the first-ever outbreak of enterovirus-associated brain stem encephalitis in western Europe survived with no long-term sequelae, Nuria Worner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We think that aggressive treatments should be restricted to those patients with important neurologic involvement,” declared Dr. Worner of Vall d’Hebron University Hospital in Barcelona. “We can say that no patients with milder involvement and without warning signs during the first 24 hours after onset of neurologic involvement went on to develop fulminant symptoms.”
Notable outbreaks of enterovirus A71 (EV-A71)-associated brain stem encephalitis occurred in Southeast Asia, Australia, and China in the late 1990s.
Dr. Worner reported on 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016. Their median age was 25 months, 57% were male, and a median of 2 days of symptoms of mild viral illness transpired before neurologic symptoms arose. Prior to presenting to a hospital, 21% of the children had been diagnosed with hand-foot-and-mouth disease, and 13% with herpangina.
Initial preadmission symptoms included fever in 94% of cases, sleepiness in 86%, ataxia in 75%, tremor in 47%, myoclonus in 40%, and a rash in 26%.
Fifty-five percent of the children had EV RNA isolated from both throat and feces, 26% from the throat only, and 19% only from their feces. Eighty-seven percent of serotyped EV were EV-A71.
Ninety percent of children underwent lumbar puncture. Particularly noteworthy was the finding that EV was detected in the cerebrospinal fluid of a mere 3% of patients, although pleocytosis was present in 84%.
Brain MRI showed brain stem encephalitis along with myelitis in 50% of patients, brain stem myelitis without encephalitis in 29%, myelitis elsewhere in 2%, and normal findings in 19%.
Ground zero for the outbreak was Barcelona and the surrounding region of Catalonia; indeed, 130 of the 196 (66%) affected children came from there. The Catalan health department and pediatric infectious disease specialists quickly created standardized case severity definitions and treatment recommendations; they distributed them nationally.
Mild EV-A71–associated brain stem disease was defined as two or more of the following: tremor, myoclonus, mild ataxia, and/or significant drowsiness. The recommendation in these mild cases was for no treatment other than supportive care and careful in-hospital monitoring.
Patients with moderate involvement had to meet the definition for mild disease plus more pronounced ataxia or bulbar motor neuron involvement marked by slurred speech, drooling, dysphagia, apnea, abolition of the gag reflex, and/or an abnormal respiratory pattern. Moderately affected patients received two doses of intravenous immunoglobulin (IVIG), each dosed at 1 g/kg per 24 hours. Admission to the pediatric ICU was individualized for patients with moderate EV-A71–associated brain stem encephalitis.
Severe disease was categorized as bulbar motor neuron involvement plus neurogenic cardiorespiratory failure. Those patients were uniformly admitted to a pediatric ICU and given the two doses of IVIG. The need for systemic steroids was determined on an individual basis.
Forty percent of patients received IVIG and systemic steroids, 24% received IVIG only, 2% systemic steroids only, and 34% received no treatment other than supportive care.
Twenty-six percent of children were admitted to a pediatric ICU for a median stay of 3.5 days. Nine percent of children were placed on mechanical ventilation.
As the disease evolved, the most frequent neurologic complications included slurred speech in 15% of children, abnormal breathing pattern in 11%, seizures in 10%, acute flaccid paralysis in 9%, and cardiorespiratory failure with pulmonary edema in 9%, all occurring within the first hours after hospital admission.
The median hospital length of stay for the full study population was 6 days. The survival rate was 99.5%, with the sole death being due to cardiorespiratory failure.
With 1-6 months of follow-up since the acute episode of EV-A71–associated brain stem encephalitis, the long-term sequelae included two cases of limb paresis and two cases of paresis of a cranial nerve, one child with residual seizures, and one with hypoxic-ischemic encephalopathy.
Asked why the fatality rate in the Spanish outbreak was so much lower than in the earlier Australasian outbreaks, Dr. Worner cited Catalan physicians’ quick recognition of what was underway – and, more importantly, a difference in the EV-A71 viral subgenotype. Most of the most severe cases in Asia and Australia involved the C-4 subgenotype, while in Spain, the predominant subgenotype involved in the outbreak was C-1.
As for the curious finding that EV was detectable in the cerebrospinal fluid of a mere 3% of the Spanish children, she said the explanation is unknown. The two main possibilities are that the CNS symptoms were due to a parenchymal brain infection rather than to EV-A71 infection of meningeal tissue. Alternatively, the CNS involvement may have been a manifestation of an immunologic response to the infection, rather than being due to the virus itself.
Dr. Worner reported having no financial conflicts of interest.
MADRID – Ninety-two percent of Spanish children sickened during the first-ever outbreak of enterovirus-associated brain stem encephalitis in western Europe survived with no long-term sequelae, Nuria Worner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We think that aggressive treatments should be restricted to those patients with important neurologic involvement,” declared Dr. Worner of Vall d’Hebron University Hospital in Barcelona. “We can say that no patients with milder involvement and without warning signs during the first 24 hours after onset of neurologic involvement went on to develop fulminant symptoms.”
Notable outbreaks of enterovirus A71 (EV-A71)-associated brain stem encephalitis occurred in Southeast Asia, Australia, and China in the late 1990s.
Dr. Worner reported on 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016. Their median age was 25 months, 57% were male, and a median of 2 days of symptoms of mild viral illness transpired before neurologic symptoms arose. Prior to presenting to a hospital, 21% of the children had been diagnosed with hand-foot-and-mouth disease, and 13% with herpangina.
Initial preadmission symptoms included fever in 94% of cases, sleepiness in 86%, ataxia in 75%, tremor in 47%, myoclonus in 40%, and a rash in 26%.
Fifty-five percent of the children had EV RNA isolated from both throat and feces, 26% from the throat only, and 19% only from their feces. Eighty-seven percent of serotyped EV were EV-A71.
Ninety percent of children underwent lumbar puncture. Particularly noteworthy was the finding that EV was detected in the cerebrospinal fluid of a mere 3% of patients, although pleocytosis was present in 84%.
Brain MRI showed brain stem encephalitis along with myelitis in 50% of patients, brain stem myelitis without encephalitis in 29%, myelitis elsewhere in 2%, and normal findings in 19%.
Ground zero for the outbreak was Barcelona and the surrounding region of Catalonia; indeed, 130 of the 196 (66%) affected children came from there. The Catalan health department and pediatric infectious disease specialists quickly created standardized case severity definitions and treatment recommendations; they distributed them nationally.
Mild EV-A71–associated brain stem disease was defined as two or more of the following: tremor, myoclonus, mild ataxia, and/or significant drowsiness. The recommendation in these mild cases was for no treatment other than supportive care and careful in-hospital monitoring.
Patients with moderate involvement had to meet the definition for mild disease plus more pronounced ataxia or bulbar motor neuron involvement marked by slurred speech, drooling, dysphagia, apnea, abolition of the gag reflex, and/or an abnormal respiratory pattern. Moderately affected patients received two doses of intravenous immunoglobulin (IVIG), each dosed at 1 g/kg per 24 hours. Admission to the pediatric ICU was individualized for patients with moderate EV-A71–associated brain stem encephalitis.
Severe disease was categorized as bulbar motor neuron involvement plus neurogenic cardiorespiratory failure. Those patients were uniformly admitted to a pediatric ICU and given the two doses of IVIG. The need for systemic steroids was determined on an individual basis.
Forty percent of patients received IVIG and systemic steroids, 24% received IVIG only, 2% systemic steroids only, and 34% received no treatment other than supportive care.
Twenty-six percent of children were admitted to a pediatric ICU for a median stay of 3.5 days. Nine percent of children were placed on mechanical ventilation.
As the disease evolved, the most frequent neurologic complications included slurred speech in 15% of children, abnormal breathing pattern in 11%, seizures in 10%, acute flaccid paralysis in 9%, and cardiorespiratory failure with pulmonary edema in 9%, all occurring within the first hours after hospital admission.
The median hospital length of stay for the full study population was 6 days. The survival rate was 99.5%, with the sole death being due to cardiorespiratory failure.
With 1-6 months of follow-up since the acute episode of EV-A71–associated brain stem encephalitis, the long-term sequelae included two cases of limb paresis and two cases of paresis of a cranial nerve, one child with residual seizures, and one with hypoxic-ischemic encephalopathy.
Asked why the fatality rate in the Spanish outbreak was so much lower than in the earlier Australasian outbreaks, Dr. Worner cited Catalan physicians’ quick recognition of what was underway – and, more importantly, a difference in the EV-A71 viral subgenotype. Most of the most severe cases in Asia and Australia involved the C-4 subgenotype, while in Spain, the predominant subgenotype involved in the outbreak was C-1.
As for the curious finding that EV was detectable in the cerebrospinal fluid of a mere 3% of the Spanish children, she said the explanation is unknown. The two main possibilities are that the CNS symptoms were due to a parenchymal brain infection rather than to EV-A71 infection of meningeal tissue. Alternatively, the CNS involvement may have been a manifestation of an immunologic response to the infection, rather than being due to the virus itself.
Dr. Worner reported having no financial conflicts of interest.
MADRID – Ninety-two percent of Spanish children sickened during the first-ever outbreak of enterovirus-associated brain stem encephalitis in western Europe survived with no long-term sequelae, Nuria Worner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We think that aggressive treatments should be restricted to those patients with important neurologic involvement,” declared Dr. Worner of Vall d’Hebron University Hospital in Barcelona. “We can say that no patients with milder involvement and without warning signs during the first 24 hours after onset of neurologic involvement went on to develop fulminant symptoms.”
Notable outbreaks of enterovirus A71 (EV-A71)-associated brain stem encephalitis occurred in Southeast Asia, Australia, and China in the late 1990s.
Dr. Worner reported on 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016. Their median age was 25 months, 57% were male, and a median of 2 days of symptoms of mild viral illness transpired before neurologic symptoms arose. Prior to presenting to a hospital, 21% of the children had been diagnosed with hand-foot-and-mouth disease, and 13% with herpangina.
Initial preadmission symptoms included fever in 94% of cases, sleepiness in 86%, ataxia in 75%, tremor in 47%, myoclonus in 40%, and a rash in 26%.
Fifty-five percent of the children had EV RNA isolated from both throat and feces, 26% from the throat only, and 19% only from their feces. Eighty-seven percent of serotyped EV were EV-A71.
Ninety percent of children underwent lumbar puncture. Particularly noteworthy was the finding that EV was detected in the cerebrospinal fluid of a mere 3% of patients, although pleocytosis was present in 84%.
Brain MRI showed brain stem encephalitis along with myelitis in 50% of patients, brain stem myelitis without encephalitis in 29%, myelitis elsewhere in 2%, and normal findings in 19%.
Ground zero for the outbreak was Barcelona and the surrounding region of Catalonia; indeed, 130 of the 196 (66%) affected children came from there. The Catalan health department and pediatric infectious disease specialists quickly created standardized case severity definitions and treatment recommendations; they distributed them nationally.
Mild EV-A71–associated brain stem disease was defined as two or more of the following: tremor, myoclonus, mild ataxia, and/or significant drowsiness. The recommendation in these mild cases was for no treatment other than supportive care and careful in-hospital monitoring.
Patients with moderate involvement had to meet the definition for mild disease plus more pronounced ataxia or bulbar motor neuron involvement marked by slurred speech, drooling, dysphagia, apnea, abolition of the gag reflex, and/or an abnormal respiratory pattern. Moderately affected patients received two doses of intravenous immunoglobulin (IVIG), each dosed at 1 g/kg per 24 hours. Admission to the pediatric ICU was individualized for patients with moderate EV-A71–associated brain stem encephalitis.
Severe disease was categorized as bulbar motor neuron involvement plus neurogenic cardiorespiratory failure. Those patients were uniformly admitted to a pediatric ICU and given the two doses of IVIG. The need for systemic steroids was determined on an individual basis.
Forty percent of patients received IVIG and systemic steroids, 24% received IVIG only, 2% systemic steroids only, and 34% received no treatment other than supportive care.
Twenty-six percent of children were admitted to a pediatric ICU for a median stay of 3.5 days. Nine percent of children were placed on mechanical ventilation.
As the disease evolved, the most frequent neurologic complications included slurred speech in 15% of children, abnormal breathing pattern in 11%, seizures in 10%, acute flaccid paralysis in 9%, and cardiorespiratory failure with pulmonary edema in 9%, all occurring within the first hours after hospital admission.
The median hospital length of stay for the full study population was 6 days. The survival rate was 99.5%, with the sole death being due to cardiorespiratory failure.
With 1-6 months of follow-up since the acute episode of EV-A71–associated brain stem encephalitis, the long-term sequelae included two cases of limb paresis and two cases of paresis of a cranial nerve, one child with residual seizures, and one with hypoxic-ischemic encephalopathy.
Asked why the fatality rate in the Spanish outbreak was so much lower than in the earlier Australasian outbreaks, Dr. Worner cited Catalan physicians’ quick recognition of what was underway – and, more importantly, a difference in the EV-A71 viral subgenotype. Most of the most severe cases in Asia and Australia involved the C-4 subgenotype, while in Spain, the predominant subgenotype involved in the outbreak was C-1.
As for the curious finding that EV was detectable in the cerebrospinal fluid of a mere 3% of the Spanish children, she said the explanation is unknown. The two main possibilities are that the CNS symptoms were due to a parenchymal brain infection rather than to EV-A71 infection of meningeal tissue. Alternatively, the CNS involvement may have been a manifestation of an immunologic response to the infection, rather than being due to the virus itself.
Dr. Worner reported having no financial conflicts of interest.
AT ESPID 2017
Key clinical point:
Major finding: Ninety-two percent of Spanish children involved in an outbreak of enterovirus-associated brain stem encephalitis survived with no long-term sequelae.
Data source: A retrospective review of 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016 during the first-ever outbreak of this condition in western Europe.
Disclosures: Dr. Worner reported having no financial conflicts of interest.
Exploring Noninvasive Presurgical Brain Mapping
Although invasive procedures like cortical stimulation mapping and the Wada test have traditionally been employed to determine the location of motor and language-related areas of the brain prior to ablative epilepsy surgery, a recent review of noninvasive procedures suggests several may have merit. Papanicolaou et al outline the value of magnetoencephalography, functional magnetic resonance imaging, and transcranial magnetic stimulation to accomplish the same purpose and explain the rationale and conditions in which these approaches may be worth considering.
Papanicolaou AC, Rezaie R, Narayana S et al. On the relative merits of invasive and non-invasive pre-surgical brain mapping: New tools in ablative epilepsy surgery [published online ahead of print July 3, 2017]. Epilepsy Res. 2017; doi: 10.1016/j.eplepsyres.2017.07.002.
Although invasive procedures like cortical stimulation mapping and the Wada test have traditionally been employed to determine the location of motor and language-related areas of the brain prior to ablative epilepsy surgery, a recent review of noninvasive procedures suggests several may have merit. Papanicolaou et al outline the value of magnetoencephalography, functional magnetic resonance imaging, and transcranial magnetic stimulation to accomplish the same purpose and explain the rationale and conditions in which these approaches may be worth considering.
Papanicolaou AC, Rezaie R, Narayana S et al. On the relative merits of invasive and non-invasive pre-surgical brain mapping: New tools in ablative epilepsy surgery [published online ahead of print July 3, 2017]. Epilepsy Res. 2017; doi: 10.1016/j.eplepsyres.2017.07.002.
Although invasive procedures like cortical stimulation mapping and the Wada test have traditionally been employed to determine the location of motor and language-related areas of the brain prior to ablative epilepsy surgery, a recent review of noninvasive procedures suggests several may have merit. Papanicolaou et al outline the value of magnetoencephalography, functional magnetic resonance imaging, and transcranial magnetic stimulation to accomplish the same purpose and explain the rationale and conditions in which these approaches may be worth considering.
Papanicolaou AC, Rezaie R, Narayana S et al. On the relative merits of invasive and non-invasive pre-surgical brain mapping: New tools in ablative epilepsy surgery [published online ahead of print July 3, 2017]. Epilepsy Res. 2017; doi: 10.1016/j.eplepsyres.2017.07.002.
Implementation of a Communication Training Program Is Associated with Reduction of Antipsychotic Medication Use in Nursing Homes
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
Nocturnal Seizures Linked to Severe Hypoxemia
Patients who have seizures while asleep are more likely to experience severe and longer episodes of hypoxemia compared with seizures while awake according to an examination of 48 recorded seizures from 20 adults with epilepsy. The analysis also suggested that an increased risk of sudden death may be caused by hypoxemia from nocturnal seizures.
Latreille V, Abdennadher M, Dworetzky BA et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression [published online ahead of print July 17, 2017]. Epilepsia. 2017;doi: 10.1111/epi.13841.
Patients who have seizures while asleep are more likely to experience severe and longer episodes of hypoxemia compared with seizures while awake according to an examination of 48 recorded seizures from 20 adults with epilepsy. The analysis also suggested that an increased risk of sudden death may be caused by hypoxemia from nocturnal seizures.
Latreille V, Abdennadher M, Dworetzky BA et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression [published online ahead of print July 17, 2017]. Epilepsia. 2017;doi: 10.1111/epi.13841.
Patients who have seizures while asleep are more likely to experience severe and longer episodes of hypoxemia compared with seizures while awake according to an examination of 48 recorded seizures from 20 adults with epilepsy. The analysis also suggested that an increased risk of sudden death may be caused by hypoxemia from nocturnal seizures.
Latreille V, Abdennadher M, Dworetzky BA et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression [published online ahead of print July 17, 2017]. Epilepsia. 2017;doi: 10.1111/epi.13841.
Finding a Noninvasive Approach to Epilepsy Diagnosis
A combination of metabolites and genomic indicators hold promise as a noninvasive biomarker for epilepsy suggests a recent study published in Epilepsia. Wu et al found that a metabolomic-genomic signature that included reduced lactate and increased creatine plus phosphocreatine and choline was able to predict the existence of an altered metabolic state in epileptic brain regions, suggesting it may eventually be an alternative to surgically invasive approaches to diagnosis.
Wu HC, Dachet F, Ghoddoussi F, et al. Altered metabolomic-genomic signature: a potential noninvasive biomarker of epilepsy [published online ahead of print July 17, 2017]. Epilepsia. 2017; doi: 10.1111/epi.13848.
A combination of metabolites and genomic indicators hold promise as a noninvasive biomarker for epilepsy suggests a recent study published in Epilepsia. Wu et al found that a metabolomic-genomic signature that included reduced lactate and increased creatine plus phosphocreatine and choline was able to predict the existence of an altered metabolic state in epileptic brain regions, suggesting it may eventually be an alternative to surgically invasive approaches to diagnosis.
Wu HC, Dachet F, Ghoddoussi F, et al. Altered metabolomic-genomic signature: a potential noninvasive biomarker of epilepsy [published online ahead of print July 17, 2017]. Epilepsia. 2017; doi: 10.1111/epi.13848.
A combination of metabolites and genomic indicators hold promise as a noninvasive biomarker for epilepsy suggests a recent study published in Epilepsia. Wu et al found that a metabolomic-genomic signature that included reduced lactate and increased creatine plus phosphocreatine and choline was able to predict the existence of an altered metabolic state in epileptic brain regions, suggesting it may eventually be an alternative to surgically invasive approaches to diagnosis.
Wu HC, Dachet F, Ghoddoussi F, et al. Altered metabolomic-genomic signature: a potential noninvasive biomarker of epilepsy [published online ahead of print July 17, 2017]. Epilepsia. 2017; doi: 10.1111/epi.13848.
Deep Brain Stimulation Offers Benefit for Intractable Epilepsy
Intracranial deep brain stimulation (DBS) may offer some benefit to patients with epilepsy who do not respond to more conservative therapy, but most of the evidence comes from short-term randomized controlled trials, according to a recent review from the Cochrane Library. Experts concluded that anterior thalamic DBS, responsive ictal onset zone stimulation, and hippocampal DBS can reduce the frequency of seizures in patients with refractory epilepsy.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017 Jul 18;7:CD008497.
Intracranial deep brain stimulation (DBS) may offer some benefit to patients with epilepsy who do not respond to more conservative therapy, but most of the evidence comes from short-term randomized controlled trials, according to a recent review from the Cochrane Library. Experts concluded that anterior thalamic DBS, responsive ictal onset zone stimulation, and hippocampal DBS can reduce the frequency of seizures in patients with refractory epilepsy.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017 Jul 18;7:CD008497.
Intracranial deep brain stimulation (DBS) may offer some benefit to patients with epilepsy who do not respond to more conservative therapy, but most of the evidence comes from short-term randomized controlled trials, according to a recent review from the Cochrane Library. Experts concluded that anterior thalamic DBS, responsive ictal onset zone stimulation, and hippocampal DBS can reduce the frequency of seizures in patients with refractory epilepsy.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017 Jul 18;7:CD008497.
Managing psychiatric illness during pregnancy and breastfeeding: Tools for decision making
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.
Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
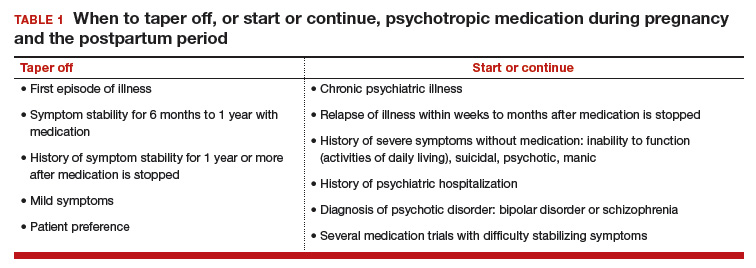
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).
In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
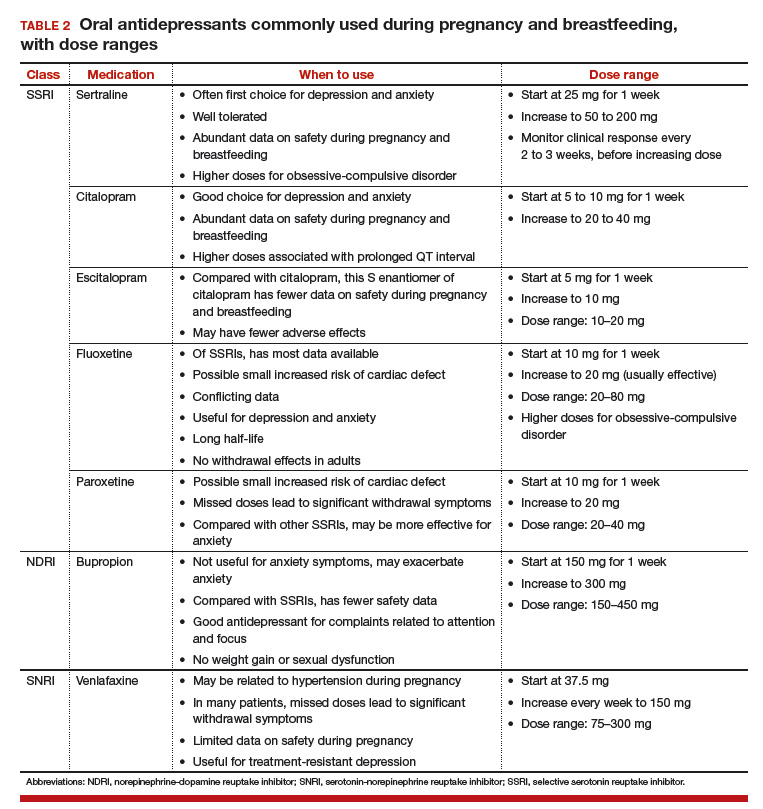
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29
One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.
Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).
In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29
One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.
Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).
In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29
One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
Association between concurrent use of prescription opiates and benzos
Title: Short periods of concurrent benzodiazepine and opioid use increase overdose risk
Clinical Question: What is the impact of concurrent benzodiazepine use in chronic versus intermittent opioid use on risk for opioid overdose?
Study Design: Retrospective study.
Setting: Private insurance administrative claims in the United States.
Synopsis: In 315,428 privately insured adults younger than 65 without malignancy who filled at least one opioid prescription between 2001 and 2013, concurrent benzodiazepine use doubled (increasing from 9% to 17%) and was associated with an increased risk for hospitalization for opioid overdose (1.16% versus 2.42%, odds ratio = 2.14, P less than .001). The risk was increased in both chronic opioid users versus nonusers (5.36% versus 3.13%, odds ratio = 1.8, CI, 1.67-1.96, P less than .001) and in intermittent opioid users as compared to nonusers (1.45% versus 1.02%, odds ratio = 1.42, CI, 1.33-1.51, P less than .001).
These results are similar to prior studies performed in other patient populations, but add to those by including short periods of co-prescription between opioid and benzodiazepine prescriptions (including a single day of overlap). Limitations of this study include that it included only patients who were seen in the ED or hospital.
Bottom Line: There may be no safe duration of opioid use in patients who are also taking benzodiazepines.
Citation: Sun EC, Dixit, A, Humphreys K, et. al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356(760):1-7.
Dr. Barrett is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: Short periods of concurrent benzodiazepine and opioid use increase overdose risk
Clinical Question: What is the impact of concurrent benzodiazepine use in chronic versus intermittent opioid use on risk for opioid overdose?
Study Design: Retrospective study.
Setting: Private insurance administrative claims in the United States.
Synopsis: In 315,428 privately insured adults younger than 65 without malignancy who filled at least one opioid prescription between 2001 and 2013, concurrent benzodiazepine use doubled (increasing from 9% to 17%) and was associated with an increased risk for hospitalization for opioid overdose (1.16% versus 2.42%, odds ratio = 2.14, P less than .001). The risk was increased in both chronic opioid users versus nonusers (5.36% versus 3.13%, odds ratio = 1.8, CI, 1.67-1.96, P less than .001) and in intermittent opioid users as compared to nonusers (1.45% versus 1.02%, odds ratio = 1.42, CI, 1.33-1.51, P less than .001).
These results are similar to prior studies performed in other patient populations, but add to those by including short periods of co-prescription between opioid and benzodiazepine prescriptions (including a single day of overlap). Limitations of this study include that it included only patients who were seen in the ED or hospital.
Bottom Line: There may be no safe duration of opioid use in patients who are also taking benzodiazepines.
Citation: Sun EC, Dixit, A, Humphreys K, et. al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356(760):1-7.
Dr. Barrett is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: Short periods of concurrent benzodiazepine and opioid use increase overdose risk
Clinical Question: What is the impact of concurrent benzodiazepine use in chronic versus intermittent opioid use on risk for opioid overdose?
Study Design: Retrospective study.
Setting: Private insurance administrative claims in the United States.
Synopsis: In 315,428 privately insured adults younger than 65 without malignancy who filled at least one opioid prescription between 2001 and 2013, concurrent benzodiazepine use doubled (increasing from 9% to 17%) and was associated with an increased risk for hospitalization for opioid overdose (1.16% versus 2.42%, odds ratio = 2.14, P less than .001). The risk was increased in both chronic opioid users versus nonusers (5.36% versus 3.13%, odds ratio = 1.8, CI, 1.67-1.96, P less than .001) and in intermittent opioid users as compared to nonusers (1.45% versus 1.02%, odds ratio = 1.42, CI, 1.33-1.51, P less than .001).
These results are similar to prior studies performed in other patient populations, but add to those by including short periods of co-prescription between opioid and benzodiazepine prescriptions (including a single day of overlap). Limitations of this study include that it included only patients who were seen in the ED or hospital.
Bottom Line: There may be no safe duration of opioid use in patients who are also taking benzodiazepines.
Citation: Sun EC, Dixit, A, Humphreys K, et. al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356(760):1-7.
Dr. Barrett is assistant professor in the division of hospital medicine at the University of New Mexico.
First trimester antibiotics may increase birth defect risk
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Key clinical point:
Major finding: Clindamycin exposure during the first trimester is associated with an 81% increased risk of ventricular/septal defect and 67% greater risk of musculoskeletal system malformations.
Data source: Population-based cohort study in 139,938 liveborn singletons.
Disclosures: The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Reducing Readmissions or Length of Stay—Which Is More Important?
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
© 2017 Society of Hospital Medicine