User login
Physician liability in opioid deaths
Question: Regarding opioid deaths, which of the following is incorrect?
A. The term refers to accidental or intentional deaths caused mostly by heroin.
B. They are reaching epidemic proportions.
C. May form the basis for a wrongful death lawsuit.
D. May lead to loss of medical license.
E. The physician may face prosecution for homicide.
Answer: A. Opioids are a class of drugs that include the illegal drug heroin, as well as prescription drugs such as fentanyl, oxycodone, hydrocodone, codeine, and morphine. To be sure, opioid deaths occur in addicts from the deliberate or accidental use of heroin; but other opioids, especially painkillers, are also widely implicated. In addition, deaths have resulted from the careless, negligent, reckless, or wanton conduct of doctors who prescribe them without the proper indications or in inappropriate amounts, and then fail to provide careful follow-up.
Physicians may face both civil and criminal liabilities in such a situation. One remedy sought in wrongful death is a civil action, i.e., a malpractice lawsuit against the negligent doctor. The plaintiff is asserting that by violating community professional standards, the physician’s substandard conduct breached his duty of due care and was a proximate cause of the patient’s death. The evidentiary proof that is required to sustain such an allegation is “more probable than not” or “preponderance of evidence,” and expert medical testimony is typically necessary to establish the requisite standard of care and causation. Where there is gross negligence, i.e., egregious conduct that was reckless, the jury may award punitive damages.
Not infrequently, the wayward doctor faces triple liability: a civil lawsuit, state medical board action, and criminal prosecution for homicide. Given the publicity over soaring opioid death rates, one can expect aggressive prosecution of dealers and doctors alike.
This was not the first such case in Oklahoma. In 2014, a 71-year-old pain management doctor pleaded guilty to eight counts of second-degree murder in connection with several drug overdose deaths and will serve 8 years in prison. The doctor had reportedly prescribed more controlled drugs than any other physician in the state of Oklahoma. These drugs included hydrocodone, oxycodone, alprazolam, diazepam (Valium), and carisoprodol (Soma) – as many as 600 pills at a time. He allegedly accepted only cash payment for the office visits, and a review of his patient files revealed inadequate assessment of patient complaints or physical findings to justify the prescriptions.
Other states have been equally aggressive in prosecuting doctors over opioid deaths from reckless prescribing habits.
For the first time, New York in 2014 convicted a doctor of manslaughter in the overdose deaths of patients from oxycodone and alprazolam (Xanax). Some of the patients were prescribed as many as 500-800 pills over a 5- to 6-week period. The defendant, an anesthesiologist and pain management specialist, allegedly saw upward of 90 patients a day in his Queens weekend storefront clinic, charging them on a per-prescription basis. In his defense, he claimed that he was simply trying to help suffering people who misused medications and who misled him (“tough patients and good liars”).
Likewise, a Los Angeles–area doctor was recently convicted of second-degree murder for prescribing painkillers that killed three patients, and he was sentenced to 30 years to life in prison.
According to the Centers for Disease Control and Prevention, both drug overdose and opioid-involved deaths continue to increase in the United States.2 The majority of drug overdose deaths (more than 6 out of 10) involve an opioid, and the number has quadrupled since 1999.2 It has been estimated that more than 18,000 overdose deaths in 2014 involved prescription painkillers, while an additional 10,000 fatalities were attributed to heroin and 5,000 to fentanyl and other synthetic opioids. Overdose deaths exceed motor vehicle accidents as the leading cause of injury-related deaths. About 90 Americans die every day from an opioid overdose, and opioids have been forecast to kill 500,000 Americans over the next decade.
The CDC acknowledges that prescription opioids are a driving factor, noting that since 1999, the amount sold in the United States has nearly quadrupled, yet there has not been an overall change in the amount of pain that Americans report.
States such as Missouri, faced with the skyrocketing cost of treating the opioid epidemic, have sued the drug manufacturers, blaming them for their “campaign of fraud and deception.” At the same time, doctors have been deemed the “biggest culprit” for the opioid addiction epidemic, and one author has pointedly asserted that “by refusing to accept their inability to separate pain relief from addiction, physicians have long suffered the sin of hubris – and their patients have paid the price.”3
The U.S. Surgeon General recently took the historic step of writing to all American doctors asking for their help. And the American Medical Association has developed an educational module explaining the epidemic and how opioid misuse is linked to heroin addiction. The module also outlines risk-reducing steps when using opioids for pain relief.4
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. Some of the materials here have appeared in previous columns by the author. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. Available at www.foxnews.om/health/2017/06/26/oklahoma-doctor-charged-in-opioid-deaths-5-patients.html. Accessed June 28, 2017.
2. Available at www.cdc.gov/drugoverdose/epidemic/index.html. Accessed June 28, 2017.
3. Available at www.thedailybeast.com/the-doctors-who-started-the-opioid-epidemic. Accessed June 27, 2017.
4. https://www.end-opioid-epidemic.org. Accessed July 5, 2017.
Question: Regarding opioid deaths, which of the following is incorrect?
A. The term refers to accidental or intentional deaths caused mostly by heroin.
B. They are reaching epidemic proportions.
C. May form the basis for a wrongful death lawsuit.
D. May lead to loss of medical license.
E. The physician may face prosecution for homicide.
Answer: A. Opioids are a class of drugs that include the illegal drug heroin, as well as prescription drugs such as fentanyl, oxycodone, hydrocodone, codeine, and morphine. To be sure, opioid deaths occur in addicts from the deliberate or accidental use of heroin; but other opioids, especially painkillers, are also widely implicated. In addition, deaths have resulted from the careless, negligent, reckless, or wanton conduct of doctors who prescribe them without the proper indications or in inappropriate amounts, and then fail to provide careful follow-up.
Physicians may face both civil and criminal liabilities in such a situation. One remedy sought in wrongful death is a civil action, i.e., a malpractice lawsuit against the negligent doctor. The plaintiff is asserting that by violating community professional standards, the physician’s substandard conduct breached his duty of due care and was a proximate cause of the patient’s death. The evidentiary proof that is required to sustain such an allegation is “more probable than not” or “preponderance of evidence,” and expert medical testimony is typically necessary to establish the requisite standard of care and causation. Where there is gross negligence, i.e., egregious conduct that was reckless, the jury may award punitive damages.
Not infrequently, the wayward doctor faces triple liability: a civil lawsuit, state medical board action, and criminal prosecution for homicide. Given the publicity over soaring opioid death rates, one can expect aggressive prosecution of dealers and doctors alike.
This was not the first such case in Oklahoma. In 2014, a 71-year-old pain management doctor pleaded guilty to eight counts of second-degree murder in connection with several drug overdose deaths and will serve 8 years in prison. The doctor had reportedly prescribed more controlled drugs than any other physician in the state of Oklahoma. These drugs included hydrocodone, oxycodone, alprazolam, diazepam (Valium), and carisoprodol (Soma) – as many as 600 pills at a time. He allegedly accepted only cash payment for the office visits, and a review of his patient files revealed inadequate assessment of patient complaints or physical findings to justify the prescriptions.
Other states have been equally aggressive in prosecuting doctors over opioid deaths from reckless prescribing habits.
For the first time, New York in 2014 convicted a doctor of manslaughter in the overdose deaths of patients from oxycodone and alprazolam (Xanax). Some of the patients were prescribed as many as 500-800 pills over a 5- to 6-week period. The defendant, an anesthesiologist and pain management specialist, allegedly saw upward of 90 patients a day in his Queens weekend storefront clinic, charging them on a per-prescription basis. In his defense, he claimed that he was simply trying to help suffering people who misused medications and who misled him (“tough patients and good liars”).
Likewise, a Los Angeles–area doctor was recently convicted of second-degree murder for prescribing painkillers that killed three patients, and he was sentenced to 30 years to life in prison.
According to the Centers for Disease Control and Prevention, both drug overdose and opioid-involved deaths continue to increase in the United States.2 The majority of drug overdose deaths (more than 6 out of 10) involve an opioid, and the number has quadrupled since 1999.2 It has been estimated that more than 18,000 overdose deaths in 2014 involved prescription painkillers, while an additional 10,000 fatalities were attributed to heroin and 5,000 to fentanyl and other synthetic opioids. Overdose deaths exceed motor vehicle accidents as the leading cause of injury-related deaths. About 90 Americans die every day from an opioid overdose, and opioids have been forecast to kill 500,000 Americans over the next decade.
The CDC acknowledges that prescription opioids are a driving factor, noting that since 1999, the amount sold in the United States has nearly quadrupled, yet there has not been an overall change in the amount of pain that Americans report.
States such as Missouri, faced with the skyrocketing cost of treating the opioid epidemic, have sued the drug manufacturers, blaming them for their “campaign of fraud and deception.” At the same time, doctors have been deemed the “biggest culprit” for the opioid addiction epidemic, and one author has pointedly asserted that “by refusing to accept their inability to separate pain relief from addiction, physicians have long suffered the sin of hubris – and their patients have paid the price.”3
The U.S. Surgeon General recently took the historic step of writing to all American doctors asking for their help. And the American Medical Association has developed an educational module explaining the epidemic and how opioid misuse is linked to heroin addiction. The module also outlines risk-reducing steps when using opioids for pain relief.4
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. Some of the materials here have appeared in previous columns by the author. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. Available at www.foxnews.om/health/2017/06/26/oklahoma-doctor-charged-in-opioid-deaths-5-patients.html. Accessed June 28, 2017.
2. Available at www.cdc.gov/drugoverdose/epidemic/index.html. Accessed June 28, 2017.
3. Available at www.thedailybeast.com/the-doctors-who-started-the-opioid-epidemic. Accessed June 27, 2017.
4. https://www.end-opioid-epidemic.org. Accessed July 5, 2017.
Question: Regarding opioid deaths, which of the following is incorrect?
A. The term refers to accidental or intentional deaths caused mostly by heroin.
B. They are reaching epidemic proportions.
C. May form the basis for a wrongful death lawsuit.
D. May lead to loss of medical license.
E. The physician may face prosecution for homicide.
Answer: A. Opioids are a class of drugs that include the illegal drug heroin, as well as prescription drugs such as fentanyl, oxycodone, hydrocodone, codeine, and morphine. To be sure, opioid deaths occur in addicts from the deliberate or accidental use of heroin; but other opioids, especially painkillers, are also widely implicated. In addition, deaths have resulted from the careless, negligent, reckless, or wanton conduct of doctors who prescribe them without the proper indications or in inappropriate amounts, and then fail to provide careful follow-up.
Physicians may face both civil and criminal liabilities in such a situation. One remedy sought in wrongful death is a civil action, i.e., a malpractice lawsuit against the negligent doctor. The plaintiff is asserting that by violating community professional standards, the physician’s substandard conduct breached his duty of due care and was a proximate cause of the patient’s death. The evidentiary proof that is required to sustain such an allegation is “more probable than not” or “preponderance of evidence,” and expert medical testimony is typically necessary to establish the requisite standard of care and causation. Where there is gross negligence, i.e., egregious conduct that was reckless, the jury may award punitive damages.
Not infrequently, the wayward doctor faces triple liability: a civil lawsuit, state medical board action, and criminal prosecution for homicide. Given the publicity over soaring opioid death rates, one can expect aggressive prosecution of dealers and doctors alike.
This was not the first such case in Oklahoma. In 2014, a 71-year-old pain management doctor pleaded guilty to eight counts of second-degree murder in connection with several drug overdose deaths and will serve 8 years in prison. The doctor had reportedly prescribed more controlled drugs than any other physician in the state of Oklahoma. These drugs included hydrocodone, oxycodone, alprazolam, diazepam (Valium), and carisoprodol (Soma) – as many as 600 pills at a time. He allegedly accepted only cash payment for the office visits, and a review of his patient files revealed inadequate assessment of patient complaints or physical findings to justify the prescriptions.
Other states have been equally aggressive in prosecuting doctors over opioid deaths from reckless prescribing habits.
For the first time, New York in 2014 convicted a doctor of manslaughter in the overdose deaths of patients from oxycodone and alprazolam (Xanax). Some of the patients were prescribed as many as 500-800 pills over a 5- to 6-week period. The defendant, an anesthesiologist and pain management specialist, allegedly saw upward of 90 patients a day in his Queens weekend storefront clinic, charging them on a per-prescription basis. In his defense, he claimed that he was simply trying to help suffering people who misused medications and who misled him (“tough patients and good liars”).
Likewise, a Los Angeles–area doctor was recently convicted of second-degree murder for prescribing painkillers that killed three patients, and he was sentenced to 30 years to life in prison.
According to the Centers for Disease Control and Prevention, both drug overdose and opioid-involved deaths continue to increase in the United States.2 The majority of drug overdose deaths (more than 6 out of 10) involve an opioid, and the number has quadrupled since 1999.2 It has been estimated that more than 18,000 overdose deaths in 2014 involved prescription painkillers, while an additional 10,000 fatalities were attributed to heroin and 5,000 to fentanyl and other synthetic opioids. Overdose deaths exceed motor vehicle accidents as the leading cause of injury-related deaths. About 90 Americans die every day from an opioid overdose, and opioids have been forecast to kill 500,000 Americans over the next decade.
The CDC acknowledges that prescription opioids are a driving factor, noting that since 1999, the amount sold in the United States has nearly quadrupled, yet there has not been an overall change in the amount of pain that Americans report.
States such as Missouri, faced with the skyrocketing cost of treating the opioid epidemic, have sued the drug manufacturers, blaming them for their “campaign of fraud and deception.” At the same time, doctors have been deemed the “biggest culprit” for the opioid addiction epidemic, and one author has pointedly asserted that “by refusing to accept their inability to separate pain relief from addiction, physicians have long suffered the sin of hubris – and their patients have paid the price.”3
The U.S. Surgeon General recently took the historic step of writing to all American doctors asking for their help. And the American Medical Association has developed an educational module explaining the epidemic and how opioid misuse is linked to heroin addiction. The module also outlines risk-reducing steps when using opioids for pain relief.4
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. Some of the materials here have appeared in previous columns by the author. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. Available at www.foxnews.om/health/2017/06/26/oklahoma-doctor-charged-in-opioid-deaths-5-patients.html. Accessed June 28, 2017.
2. Available at www.cdc.gov/drugoverdose/epidemic/index.html. Accessed June 28, 2017.
3. Available at www.thedailybeast.com/the-doctors-who-started-the-opioid-epidemic. Accessed June 27, 2017.
4. https://www.end-opioid-epidemic.org. Accessed July 5, 2017.
Adverse childhood experiences link to worse SLE
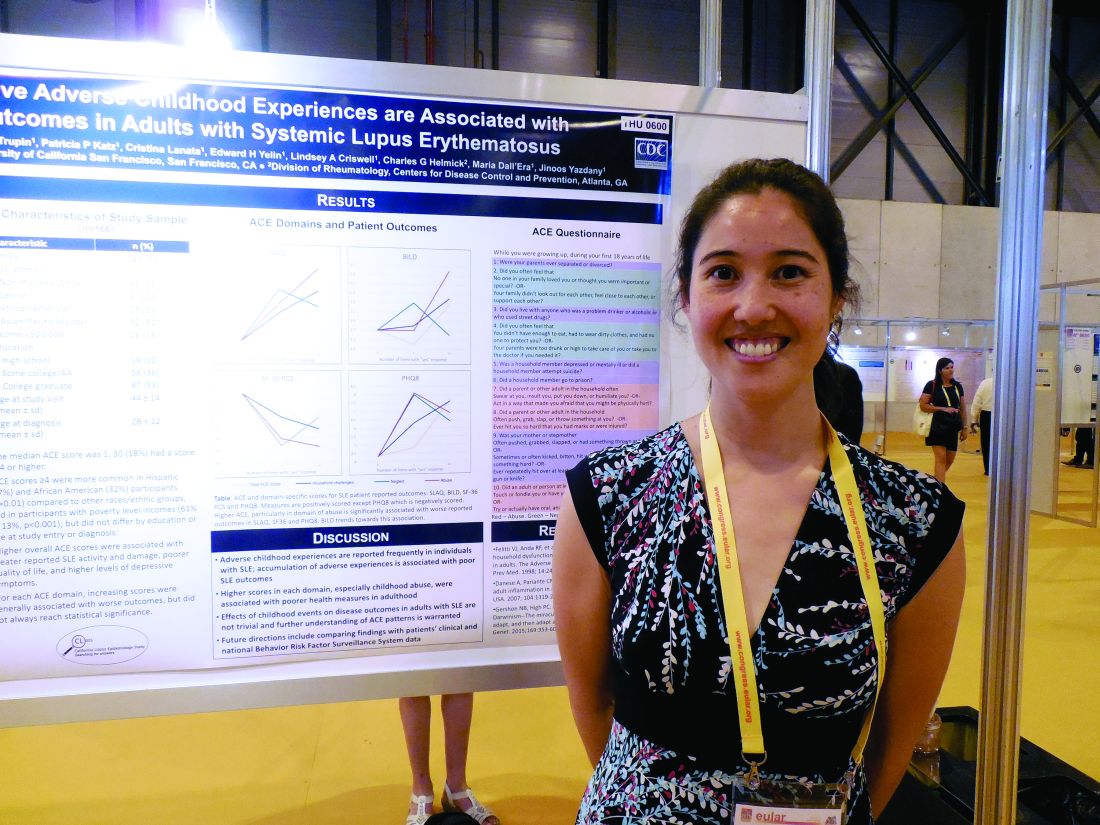
MADRID – Adult patients with systemic lupus erythematosus (SLE) who had several adverse experiences as children generally had a worse disease state than did similar adult lupus patients with no adverse childhood experiences in a study of 166 California lupus patients.
Higher numbers of self-reported episodes of household challenges, neglect, and especially childhood abuse were associated with worse SLE outcomes, Kimberly DeQuattro, MD, said while presenting a poster at the European Congress of Rheumatology.
It remains unclear “why there is an association between childhood trauma and outcomes of chronic disease, especially autoimmune disease,” she said in an interview. “We want to know, What does it mean [clinically] to have been abused or neglected, and is it irreversible?” She called for further understanding of childhood adverse experiences and their consequences. These patients might benefit from referrals to psychologists or social workers, Dr. DeQuattro suggested.
The data for her analysis came from 166 adult SLE patients who participated in the California Lupus Epidemiology Study and completed several disease activity surveys, as well as the Adverse Childhood Experience survey, which measures episodes of abuse, neglect, and household challenges. The patients averaged 44 years old and had been diagnosed with SLE for an average of about 16 years. More than 60% of the 166 participants reported at least one episode on this survey. “One event does not make a big difference,” Dr. DeQuattro said, but that wasn’t the case for the SLE patients who reported having four of more of these childhood events.
Patients with a higher number of adverse childhood experiences had worse disease states, as measured by the Systemic Lupus Activity Questionnaire; the Brief Index of Lupus Damage; the 36-Item Short Form Health Survey, a measure of quality of life; and the Patient Health Questionnaire 8, a measure of depression, the researchers said in their report. For example, the average score on the Systemic Lupus Activity Questionnaire was 6.0 in 66 patients with no adverse childhood experiences and 11.8 in the 30 patients who reported having four or more adverse childhood experiences.
Dr. DeQuattro had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Adult patients with systemic lupus erythematosus (SLE) who had several adverse experiences as children generally had a worse disease state than did similar adult lupus patients with no adverse childhood experiences in a study of 166 California lupus patients.
Higher numbers of self-reported episodes of household challenges, neglect, and especially childhood abuse were associated with worse SLE outcomes, Kimberly DeQuattro, MD, said while presenting a poster at the European Congress of Rheumatology.
It remains unclear “why there is an association between childhood trauma and outcomes of chronic disease, especially autoimmune disease,” she said in an interview. “We want to know, What does it mean [clinically] to have been abused or neglected, and is it irreversible?” She called for further understanding of childhood adverse experiences and their consequences. These patients might benefit from referrals to psychologists or social workers, Dr. DeQuattro suggested.
The data for her analysis came from 166 adult SLE patients who participated in the California Lupus Epidemiology Study and completed several disease activity surveys, as well as the Adverse Childhood Experience survey, which measures episodes of abuse, neglect, and household challenges. The patients averaged 44 years old and had been diagnosed with SLE for an average of about 16 years. More than 60% of the 166 participants reported at least one episode on this survey. “One event does not make a big difference,” Dr. DeQuattro said, but that wasn’t the case for the SLE patients who reported having four of more of these childhood events.
Patients with a higher number of adverse childhood experiences had worse disease states, as measured by the Systemic Lupus Activity Questionnaire; the Brief Index of Lupus Damage; the 36-Item Short Form Health Survey, a measure of quality of life; and the Patient Health Questionnaire 8, a measure of depression, the researchers said in their report. For example, the average score on the Systemic Lupus Activity Questionnaire was 6.0 in 66 patients with no adverse childhood experiences and 11.8 in the 30 patients who reported having four or more adverse childhood experiences.
Dr. DeQuattro had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Adult patients with systemic lupus erythematosus (SLE) who had several adverse experiences as children generally had a worse disease state than did similar adult lupus patients with no adverse childhood experiences in a study of 166 California lupus patients.
Higher numbers of self-reported episodes of household challenges, neglect, and especially childhood abuse were associated with worse SLE outcomes, Kimberly DeQuattro, MD, said while presenting a poster at the European Congress of Rheumatology.
It remains unclear “why there is an association between childhood trauma and outcomes of chronic disease, especially autoimmune disease,” she said in an interview. “We want to know, What does it mean [clinically] to have been abused or neglected, and is it irreversible?” She called for further understanding of childhood adverse experiences and their consequences. These patients might benefit from referrals to psychologists or social workers, Dr. DeQuattro suggested.
The data for her analysis came from 166 adult SLE patients who participated in the California Lupus Epidemiology Study and completed several disease activity surveys, as well as the Adverse Childhood Experience survey, which measures episodes of abuse, neglect, and household challenges. The patients averaged 44 years old and had been diagnosed with SLE for an average of about 16 years. More than 60% of the 166 participants reported at least one episode on this survey. “One event does not make a big difference,” Dr. DeQuattro said, but that wasn’t the case for the SLE patients who reported having four of more of these childhood events.
Patients with a higher number of adverse childhood experiences had worse disease states, as measured by the Systemic Lupus Activity Questionnaire; the Brief Index of Lupus Damage; the 36-Item Short Form Health Survey, a measure of quality of life; and the Patient Health Questionnaire 8, a measure of depression, the researchers said in their report. For example, the average score on the Systemic Lupus Activity Questionnaire was 6.0 in 66 patients with no adverse childhood experiences and 11.8 in the 30 patients who reported having four or more adverse childhood experiences.
Dr. DeQuattro had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: The Systemic Lupus Activity Questionnaire score averaged 6.0 with no adverse childhood experiences and 11.8 with four or more experiences.
Data source: Survey results from 166 adult lupus patients enrolled in the California Lupus Epidemiology Study.
Disclosures: Dr. DeQuattro had no relevant financial disclosures.
First-line obinutuzumab monotherapy in CLL linked to good response, reduced toxicity
NEW YORK – Obinutuzumab monotherapy was effective for the first-line treatment of chronic lymphocytic leukemia in a small study of patients with a high rate of unmutated immunoglobulin heavy-chain variable region (IGHV) genes.
The overall response rate to obinutuzumab, a type 2 anti-CD20 humanized monoclonal antibody, was 100% in 20 previously untreated patients. Median progression-free survival was 30 months, and no deaths occurred at a median of 23 months follow-up, Nathan D. Gay, MD, reported in a poster at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia (iwCLL).
The good results imply that initial monotherapy may be an alternative approach that limits the toxicity associated with the recommended combination of chlorambucil and obinutuzumab.
At the time of analysis, 3 of the 20 patients (15%) had relapsed, and the mean time to next line therapy was 29 months,” Dr. Gay of Oregon Health & Science University, Portland and his colleagues wrote. Minimal residual disease (MRD) analysis completed in 16 patients showed that 5 (31%) were MRD-negative 6 months after the completion of therapy.
Study participants were adults with a median age of 62.5 years and a median cumulative illness rating scale score of 6.5 on the 0-56 scale. Most (80%) had unmutated IGHV and none harbored 17p deletion. All met iwCLL diagnostic criteria for CLL based on peripheral blood counts and flow cytometry,
All but one patient received 6 cycles of intravenous obinutuzumab given at 100 mg on day 1, 900 mg on day 2, 1000 mg on days 8 and 15 of the first cycle, and 1000 mg on day 1 for cycles 2-6.
The remaining patient discontinued treatment after two cycles because of grade 4 neutropenia.
Obinutuzumab is approved for use in combination with oral chlorambucil in patients with previously untreated CLL. The approval was based on the CLL11 study, which demonstrated improved overall response, complete response rate, and peripheral blood MRD negativity rates with obinutuzumab plus chlorambucil, vs. rituximab plus chlorambucil, the authors said. Based on those findings, obinutuzumab plus chlorambucil is considered a standard of care option in treatment-naive CLL lacking del(17p)/TP53 mutation in patients who are not candidates for first-line therapy with fludarabine, cyclophosphamide, and rituximab (FCR).
However, while chlorambucil is generally well tolerated, it has limited efficacy and is associated with overall grade 3-4 toxicity of about 44%. Obinutuzumab has significant single-agent activity in previously untreated CLL and was shown in a recent phase 2 dose-response study to be associated with an overall response rate of 49%-67% and complete response rates of 5%-20%, but data on the efficacy of first line obinutuzumab monotherapy using standard dosing outside of a clinical trial are lacking, they said.
The current study represents an analysis of all patients treated with first line obinutuzumab monotherapy at Oregon Health & Science University.
In the current study, the most common side effects were infusion reactions and cytopenias. Grade 3 or higher neutropenia, anemia, and thrombocytopenia occurred in 32%, 11%, and 32% of patients, respectively, and one patient developed a grade 3 infection.
“In our cohort of patients with untreated CLL, we found first line obinutuzumab monotherapy to be very effective and well tolerated,” they wrote, noting that this was true despite a high rate of unmutated IGHV. “These data, using first-line obinutuzumab monotherapy, compare favorably with combination therapy with chlorambucil.
“Omitting chlorambucil from this combination in favor of initial obinutuzumab monotherapy may eliminate the short- and long-term toxicity associated with the use of chemotherapy,” they concluded.
The authors reported having no disclosures.
NEW YORK – Obinutuzumab monotherapy was effective for the first-line treatment of chronic lymphocytic leukemia in a small study of patients with a high rate of unmutated immunoglobulin heavy-chain variable region (IGHV) genes.
The overall response rate to obinutuzumab, a type 2 anti-CD20 humanized monoclonal antibody, was 100% in 20 previously untreated patients. Median progression-free survival was 30 months, and no deaths occurred at a median of 23 months follow-up, Nathan D. Gay, MD, reported in a poster at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia (iwCLL).
The good results imply that initial monotherapy may be an alternative approach that limits the toxicity associated with the recommended combination of chlorambucil and obinutuzumab.
At the time of analysis, 3 of the 20 patients (15%) had relapsed, and the mean time to next line therapy was 29 months,” Dr. Gay of Oregon Health & Science University, Portland and his colleagues wrote. Minimal residual disease (MRD) analysis completed in 16 patients showed that 5 (31%) were MRD-negative 6 months after the completion of therapy.
Study participants were adults with a median age of 62.5 years and a median cumulative illness rating scale score of 6.5 on the 0-56 scale. Most (80%) had unmutated IGHV and none harbored 17p deletion. All met iwCLL diagnostic criteria for CLL based on peripheral blood counts and flow cytometry,
All but one patient received 6 cycles of intravenous obinutuzumab given at 100 mg on day 1, 900 mg on day 2, 1000 mg on days 8 and 15 of the first cycle, and 1000 mg on day 1 for cycles 2-6.
The remaining patient discontinued treatment after two cycles because of grade 4 neutropenia.
Obinutuzumab is approved for use in combination with oral chlorambucil in patients with previously untreated CLL. The approval was based on the CLL11 study, which demonstrated improved overall response, complete response rate, and peripheral blood MRD negativity rates with obinutuzumab plus chlorambucil, vs. rituximab plus chlorambucil, the authors said. Based on those findings, obinutuzumab plus chlorambucil is considered a standard of care option in treatment-naive CLL lacking del(17p)/TP53 mutation in patients who are not candidates for first-line therapy with fludarabine, cyclophosphamide, and rituximab (FCR).
However, while chlorambucil is generally well tolerated, it has limited efficacy and is associated with overall grade 3-4 toxicity of about 44%. Obinutuzumab has significant single-agent activity in previously untreated CLL and was shown in a recent phase 2 dose-response study to be associated with an overall response rate of 49%-67% and complete response rates of 5%-20%, but data on the efficacy of first line obinutuzumab monotherapy using standard dosing outside of a clinical trial are lacking, they said.
The current study represents an analysis of all patients treated with first line obinutuzumab monotherapy at Oregon Health & Science University.
In the current study, the most common side effects were infusion reactions and cytopenias. Grade 3 or higher neutropenia, anemia, and thrombocytopenia occurred in 32%, 11%, and 32% of patients, respectively, and one patient developed a grade 3 infection.
“In our cohort of patients with untreated CLL, we found first line obinutuzumab monotherapy to be very effective and well tolerated,” they wrote, noting that this was true despite a high rate of unmutated IGHV. “These data, using first-line obinutuzumab monotherapy, compare favorably with combination therapy with chlorambucil.
“Omitting chlorambucil from this combination in favor of initial obinutuzumab monotherapy may eliminate the short- and long-term toxicity associated with the use of chemotherapy,” they concluded.
The authors reported having no disclosures.
NEW YORK – Obinutuzumab monotherapy was effective for the first-line treatment of chronic lymphocytic leukemia in a small study of patients with a high rate of unmutated immunoglobulin heavy-chain variable region (IGHV) genes.
The overall response rate to obinutuzumab, a type 2 anti-CD20 humanized monoclonal antibody, was 100% in 20 previously untreated patients. Median progression-free survival was 30 months, and no deaths occurred at a median of 23 months follow-up, Nathan D. Gay, MD, reported in a poster at the annual meeting of the International Workshop on Chronic Lymphocytic Leukemia (iwCLL).
The good results imply that initial monotherapy may be an alternative approach that limits the toxicity associated with the recommended combination of chlorambucil and obinutuzumab.
At the time of analysis, 3 of the 20 patients (15%) had relapsed, and the mean time to next line therapy was 29 months,” Dr. Gay of Oregon Health & Science University, Portland and his colleagues wrote. Minimal residual disease (MRD) analysis completed in 16 patients showed that 5 (31%) were MRD-negative 6 months after the completion of therapy.
Study participants were adults with a median age of 62.5 years and a median cumulative illness rating scale score of 6.5 on the 0-56 scale. Most (80%) had unmutated IGHV and none harbored 17p deletion. All met iwCLL diagnostic criteria for CLL based on peripheral blood counts and flow cytometry,
All but one patient received 6 cycles of intravenous obinutuzumab given at 100 mg on day 1, 900 mg on day 2, 1000 mg on days 8 and 15 of the first cycle, and 1000 mg on day 1 for cycles 2-6.
The remaining patient discontinued treatment after two cycles because of grade 4 neutropenia.
Obinutuzumab is approved for use in combination with oral chlorambucil in patients with previously untreated CLL. The approval was based on the CLL11 study, which demonstrated improved overall response, complete response rate, and peripheral blood MRD negativity rates with obinutuzumab plus chlorambucil, vs. rituximab plus chlorambucil, the authors said. Based on those findings, obinutuzumab plus chlorambucil is considered a standard of care option in treatment-naive CLL lacking del(17p)/TP53 mutation in patients who are not candidates for first-line therapy with fludarabine, cyclophosphamide, and rituximab (FCR).
However, while chlorambucil is generally well tolerated, it has limited efficacy and is associated with overall grade 3-4 toxicity of about 44%. Obinutuzumab has significant single-agent activity in previously untreated CLL and was shown in a recent phase 2 dose-response study to be associated with an overall response rate of 49%-67% and complete response rates of 5%-20%, but data on the efficacy of first line obinutuzumab monotherapy using standard dosing outside of a clinical trial are lacking, they said.
The current study represents an analysis of all patients treated with first line obinutuzumab monotherapy at Oregon Health & Science University.
In the current study, the most common side effects were infusion reactions and cytopenias. Grade 3 or higher neutropenia, anemia, and thrombocytopenia occurred in 32%, 11%, and 32% of patients, respectively, and one patient developed a grade 3 infection.
“In our cohort of patients with untreated CLL, we found first line obinutuzumab monotherapy to be very effective and well tolerated,” they wrote, noting that this was true despite a high rate of unmutated IGHV. “These data, using first-line obinutuzumab monotherapy, compare favorably with combination therapy with chlorambucil.
“Omitting chlorambucil from this combination in favor of initial obinutuzumab monotherapy may eliminate the short- and long-term toxicity associated with the use of chemotherapy,” they concluded.
The authors reported having no disclosures.
AT THE iwCLL MEETING
Key clinical point:
Major finding: Overall response rate, 100%; median progression-free survival, 30 months.
Data source: A study of 20 patients treated with obinutuzumab monotherapy.
Disclosures: The authors reported having no disclosures.
Course and outcome of Guillain-Barré syndrome measured in ongoing study
The International Guillain-Barré Syndrome Outcome Study (IGOS) is actively recruiting patients with Guillain-Barré Syndrome (GBS) to examine disease course and outcome, according to ClinicalTrials.gov.
IGOS is conducted by the members of the Inflammatory Neuropathy Consortium and Peripheral Nerve Society, and the researchers plan to identify clinical and biological determinants and predictors of disease course and outcome in individual patients with GBS as early as possible after onset of disease. It is a prospective study with standardized collection of clinical data and biomaterials from a large group of well-defined GBS patients during a long follow-up period. Patients will be divided into four cohorts: GBS patients with a follow-up of 1-3 years, normal controls, infectious controls, and other neurological diseases.
The primary outcome is to receive a disability score and Medical Research Council sum score within a 1-year time frame. Secondary outcomes include Overall Neuropathy Limitations Scale, Fatigue Severity Scale, EurQol EQ-5D health questionnaire, and Rasch-built Overall Disability Scale, all in a 1-year time frame. The information will be used to understand the diversity in clinical presentation and response to treatment of GBS and will also be used to develop new prognostic models to predict the clinical course and outcome accurately in individual patients with GBS.
Enrollment for the study started May 2012 and the researchers aim to enroll an estimated 4,000 participants. As of April 2017, the IGOS has enrolled more than 1,500 patients, according to the study’s website. The study is expected to be completed by January 2019. All patients with GBS or variants of GBS, including the Miller Fisher syndrome and overlap syndromes, are eligible for the study.
Currently, patients with GBS have not shown improvement over the last 20 years. It is estimated that 10%-20% of patients remain severely disabled and about 5% die from GBS.
Find the full summary here.
The International Guillain-Barré Syndrome Outcome Study (IGOS) is actively recruiting patients with Guillain-Barré Syndrome (GBS) to examine disease course and outcome, according to ClinicalTrials.gov.
IGOS is conducted by the members of the Inflammatory Neuropathy Consortium and Peripheral Nerve Society, and the researchers plan to identify clinical and biological determinants and predictors of disease course and outcome in individual patients with GBS as early as possible after onset of disease. It is a prospective study with standardized collection of clinical data and biomaterials from a large group of well-defined GBS patients during a long follow-up period. Patients will be divided into four cohorts: GBS patients with a follow-up of 1-3 years, normal controls, infectious controls, and other neurological diseases.
The primary outcome is to receive a disability score and Medical Research Council sum score within a 1-year time frame. Secondary outcomes include Overall Neuropathy Limitations Scale, Fatigue Severity Scale, EurQol EQ-5D health questionnaire, and Rasch-built Overall Disability Scale, all in a 1-year time frame. The information will be used to understand the diversity in clinical presentation and response to treatment of GBS and will also be used to develop new prognostic models to predict the clinical course and outcome accurately in individual patients with GBS.
Enrollment for the study started May 2012 and the researchers aim to enroll an estimated 4,000 participants. As of April 2017, the IGOS has enrolled more than 1,500 patients, according to the study’s website. The study is expected to be completed by January 2019. All patients with GBS or variants of GBS, including the Miller Fisher syndrome and overlap syndromes, are eligible for the study.
Currently, patients with GBS have not shown improvement over the last 20 years. It is estimated that 10%-20% of patients remain severely disabled and about 5% die from GBS.
Find the full summary here.
The International Guillain-Barré Syndrome Outcome Study (IGOS) is actively recruiting patients with Guillain-Barré Syndrome (GBS) to examine disease course and outcome, according to ClinicalTrials.gov.
IGOS is conducted by the members of the Inflammatory Neuropathy Consortium and Peripheral Nerve Society, and the researchers plan to identify clinical and biological determinants and predictors of disease course and outcome in individual patients with GBS as early as possible after onset of disease. It is a prospective study with standardized collection of clinical data and biomaterials from a large group of well-defined GBS patients during a long follow-up period. Patients will be divided into four cohorts: GBS patients with a follow-up of 1-3 years, normal controls, infectious controls, and other neurological diseases.
The primary outcome is to receive a disability score and Medical Research Council sum score within a 1-year time frame. Secondary outcomes include Overall Neuropathy Limitations Scale, Fatigue Severity Scale, EurQol EQ-5D health questionnaire, and Rasch-built Overall Disability Scale, all in a 1-year time frame. The information will be used to understand the diversity in clinical presentation and response to treatment of GBS and will also be used to develop new prognostic models to predict the clinical course and outcome accurately in individual patients with GBS.
Enrollment for the study started May 2012 and the researchers aim to enroll an estimated 4,000 participants. As of April 2017, the IGOS has enrolled more than 1,500 patients, according to the study’s website. The study is expected to be completed by January 2019. All patients with GBS or variants of GBS, including the Miller Fisher syndrome and overlap syndromes, are eligible for the study.
Currently, patients with GBS have not shown improvement over the last 20 years. It is estimated that 10%-20% of patients remain severely disabled and about 5% die from GBS.
Find the full summary here.
SUMMARY FROM CLINICALTRIALS.GOV
Dermatofibrosarcoma Protuberans
To the Editor:
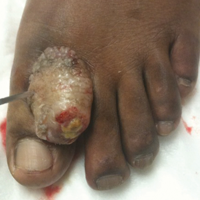
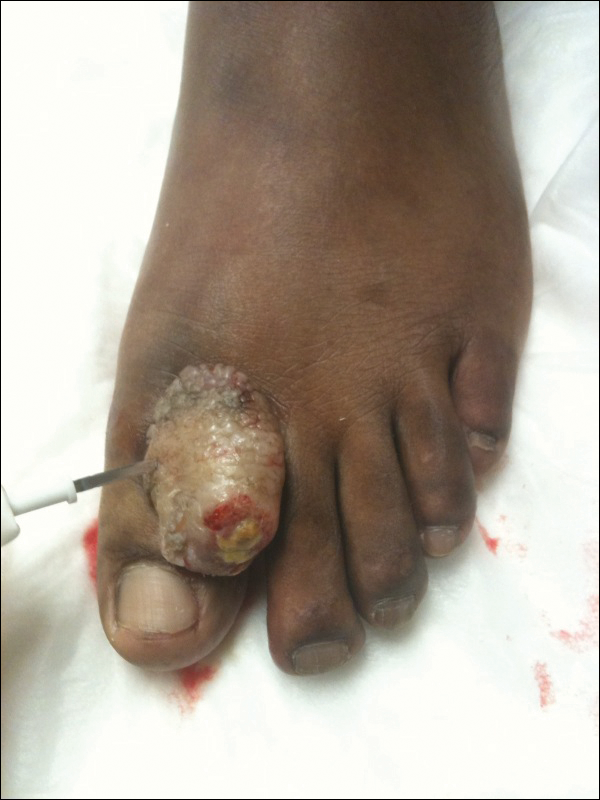
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.
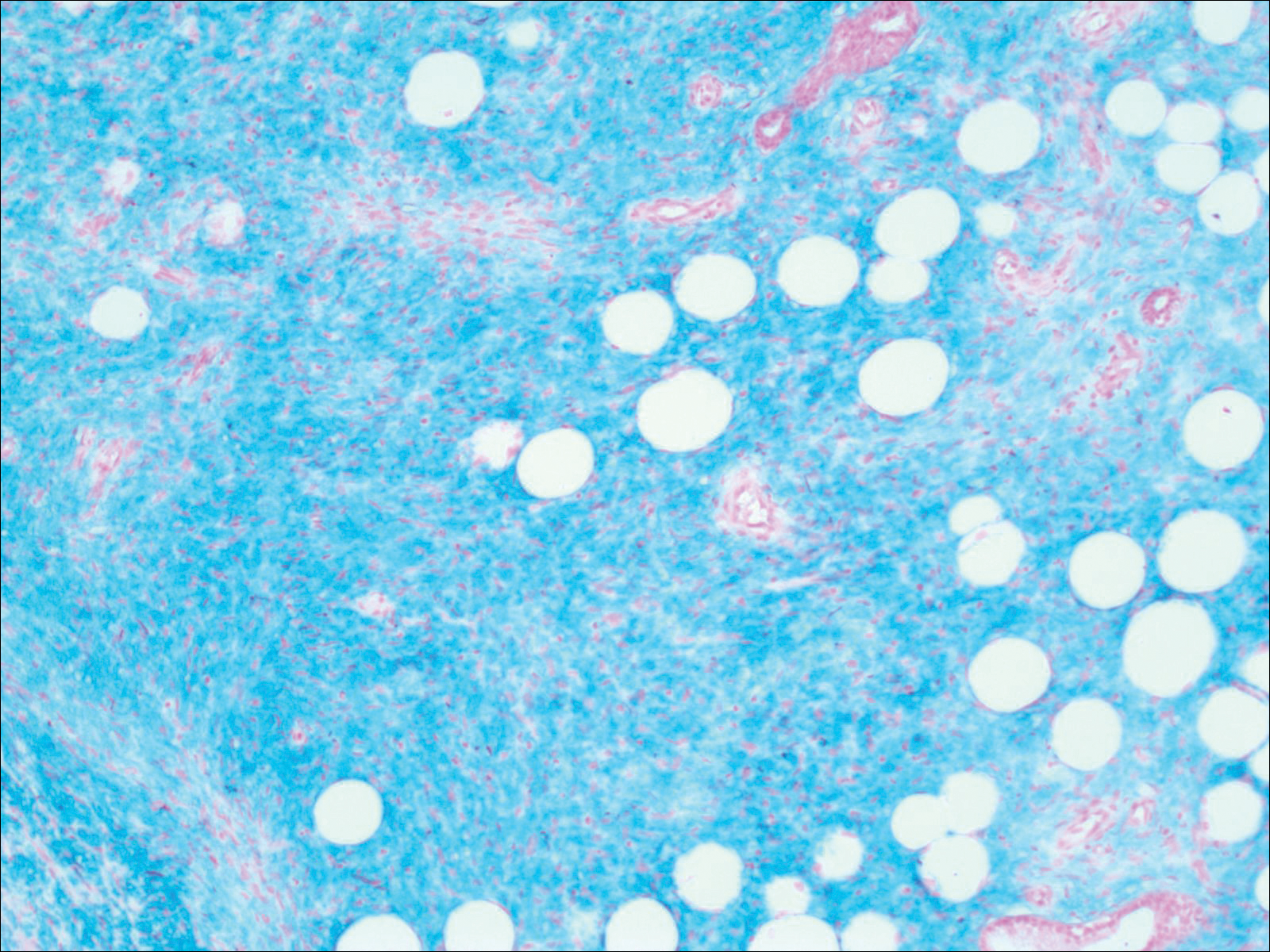
Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.
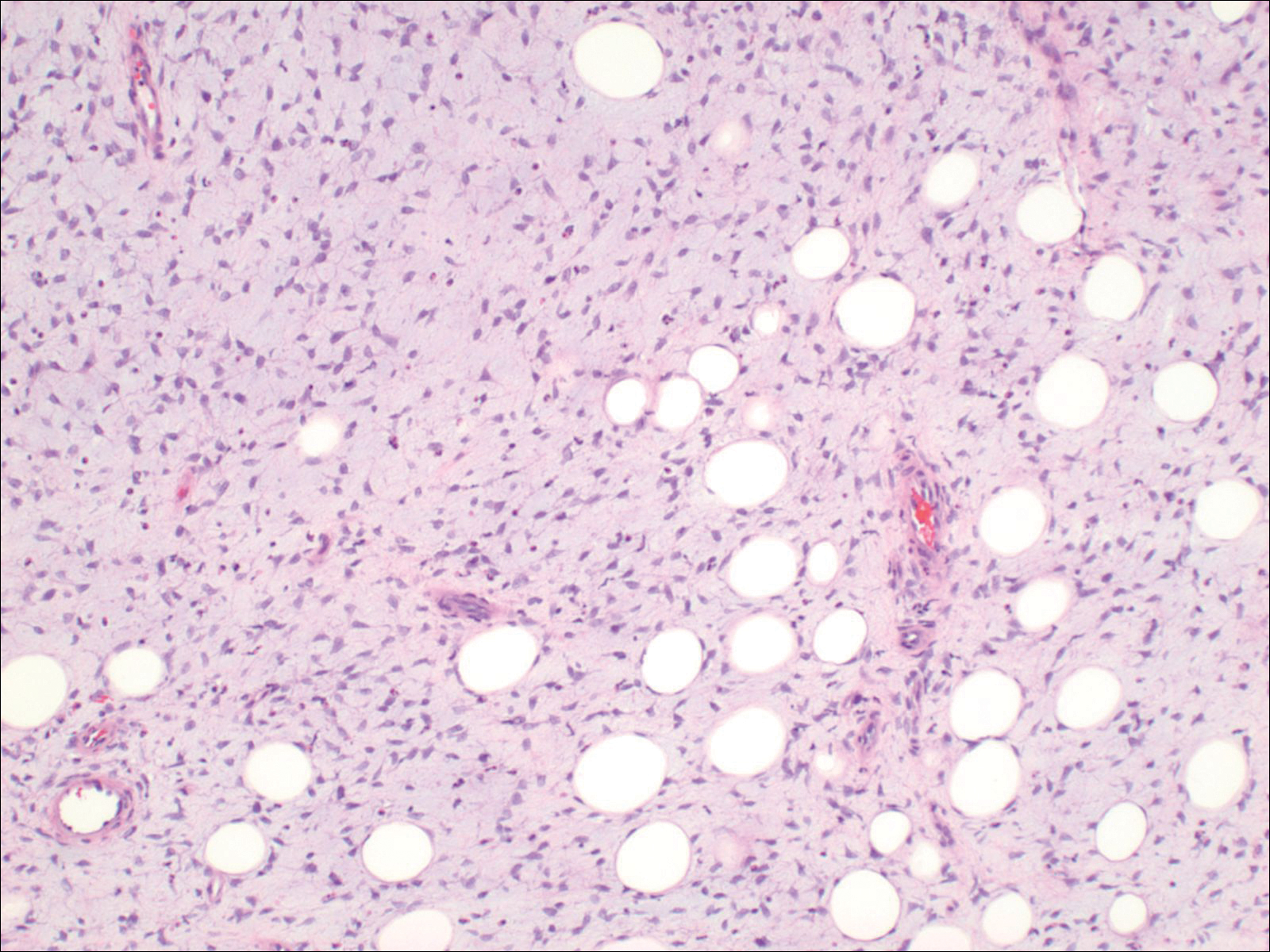
Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
Practice Points
- Consider dermatofibrosarcoma protuberans for a keloidlike enlarging lesion when there is no history of trauma or prior keloid formation.
- Treatments such as Mohs micrographic surgery or oral imatinib mesylate can provide lower recurrence rates in appropriate patients as stand-alone or adjuvant therapy.
Use of Pap smears, mammography on the decline
Use of Pap smears and mammography has slowly but steadily declined since both peaked in the year 2000, according to the National Center for Health Statistics.
The age-adjusted rate of women aged 18 years and over who reported having had a Pap smear in the past 3 years dropped from 81.3% in 2000 to 70.2% in 2015. Over that same time period, the age-adjusted rate of women aged 40 years and over who had a mammogram over the previous 2 years declined from 70.4% in 2000 to 64% in 2015, the NCHS reported in “Health, United States, 2016.”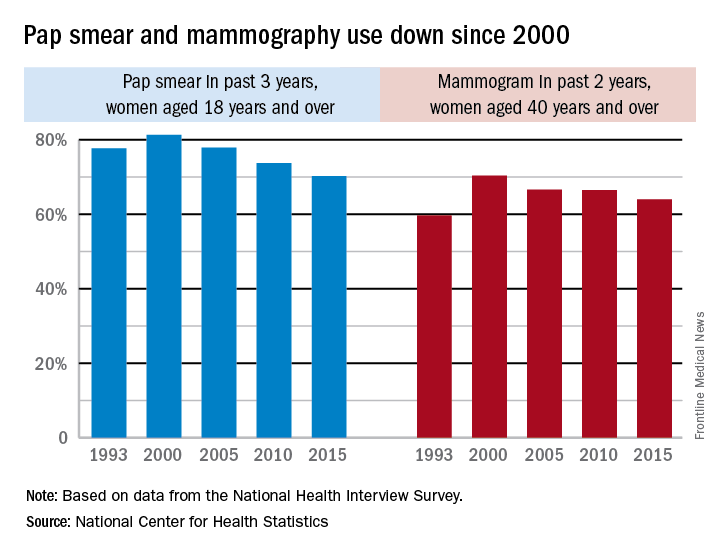
The pattern for mammography use, however, was somewhat different. Declines in use were consistent by age group, but not by race. White women were less likely to get a mammogram in 2015 (65.3%), compared with 2000 (71.4%), while Hispanic women experienced a small drop from 61.2% to 60.9%, but black, American Indian/Alaska Native, and Asian women all increased their use of mammography over that time period, the NCHS reported.
Use of Pap smears and mammography has slowly but steadily declined since both peaked in the year 2000, according to the National Center for Health Statistics.
The age-adjusted rate of women aged 18 years and over who reported having had a Pap smear in the past 3 years dropped from 81.3% in 2000 to 70.2% in 2015. Over that same time period, the age-adjusted rate of women aged 40 years and over who had a mammogram over the previous 2 years declined from 70.4% in 2000 to 64% in 2015, the NCHS reported in “Health, United States, 2016.”
The pattern for mammography use, however, was somewhat different. Declines in use were consistent by age group, but not by race. White women were less likely to get a mammogram in 2015 (65.3%), compared with 2000 (71.4%), while Hispanic women experienced a small drop from 61.2% to 60.9%, but black, American Indian/Alaska Native, and Asian women all increased their use of mammography over that time period, the NCHS reported.
Use of Pap smears and mammography has slowly but steadily declined since both peaked in the year 2000, according to the National Center for Health Statistics.
The age-adjusted rate of women aged 18 years and over who reported having had a Pap smear in the past 3 years dropped from 81.3% in 2000 to 70.2% in 2015. Over that same time period, the age-adjusted rate of women aged 40 years and over who had a mammogram over the previous 2 years declined from 70.4% in 2000 to 64% in 2015, the NCHS reported in “Health, United States, 2016.”
The pattern for mammography use, however, was somewhat different. Declines in use were consistent by age group, but not by race. White women were less likely to get a mammogram in 2015 (65.3%), compared with 2000 (71.4%), while Hispanic women experienced a small drop from 61.2% to 60.9%, but black, American Indian/Alaska Native, and Asian women all increased their use of mammography over that time period, the NCHS reported.
The Drive to Reduce HCV Among Native Americans
American Indians/Alaska Natives have the highest rate of new hepatitis C virus (HCV) infections of all ethnic groups, according to recent surveillance data from CDC.
The IHS has distributed updated guidelines on HCV prevention, testing, and treatment to IHS facilities. The guidelines are based on those of the National Viral Hepatitis Action Plan for 2017-2020, the U.S. Preventive Services Task Force, the CDC, and the American Association for the Study of Liver Diseases.
Tribal programs are an important element of the outreach. In 2015, for instance, the Cherokee Nation became the first tribe in the U.S. to launch an HCV Elimination Project, with the goal of screening 80,000 patients over the next 3 years. Last year, 23,000 patients were screened.
Other programs provide telehealth and teleconsultation services to treat patients on-site, rather than referring them to facilities far from their communities. A pharmacist-led treatment program, for example, in conjunction with a local physician and Project ECHO (Extension for Community Health Outcomes) and telehealth programs, has successfully cured > 10 patients on the Fort Peck reservation.
An estimated 20,000 to 40,000 IHS patients need HCV treatment. The IHS encourages anyone with HCV—even those who had unsuccessful treatment in the past—to seek care as soon as possible.
American Indians/Alaska Natives have the highest rate of new hepatitis C virus (HCV) infections of all ethnic groups, according to recent surveillance data from CDC.
The IHS has distributed updated guidelines on HCV prevention, testing, and treatment to IHS facilities. The guidelines are based on those of the National Viral Hepatitis Action Plan for 2017-2020, the U.S. Preventive Services Task Force, the CDC, and the American Association for the Study of Liver Diseases.
Tribal programs are an important element of the outreach. In 2015, for instance, the Cherokee Nation became the first tribe in the U.S. to launch an HCV Elimination Project, with the goal of screening 80,000 patients over the next 3 years. Last year, 23,000 patients were screened.
Other programs provide telehealth and teleconsultation services to treat patients on-site, rather than referring them to facilities far from their communities. A pharmacist-led treatment program, for example, in conjunction with a local physician and Project ECHO (Extension for Community Health Outcomes) and telehealth programs, has successfully cured > 10 patients on the Fort Peck reservation.
An estimated 20,000 to 40,000 IHS patients need HCV treatment. The IHS encourages anyone with HCV—even those who had unsuccessful treatment in the past—to seek care as soon as possible.
American Indians/Alaska Natives have the highest rate of new hepatitis C virus (HCV) infections of all ethnic groups, according to recent surveillance data from CDC.
The IHS has distributed updated guidelines on HCV prevention, testing, and treatment to IHS facilities. The guidelines are based on those of the National Viral Hepatitis Action Plan for 2017-2020, the U.S. Preventive Services Task Force, the CDC, and the American Association for the Study of Liver Diseases.
Tribal programs are an important element of the outreach. In 2015, for instance, the Cherokee Nation became the first tribe in the U.S. to launch an HCV Elimination Project, with the goal of screening 80,000 patients over the next 3 years. Last year, 23,000 patients were screened.
Other programs provide telehealth and teleconsultation services to treat patients on-site, rather than referring them to facilities far from their communities. A pharmacist-led treatment program, for example, in conjunction with a local physician and Project ECHO (Extension for Community Health Outcomes) and telehealth programs, has successfully cured > 10 patients on the Fort Peck reservation.
An estimated 20,000 to 40,000 IHS patients need HCV treatment. The IHS encourages anyone with HCV—even those who had unsuccessful treatment in the past—to seek care as soon as possible.
Friends Don't Let Friends Ignore Skin Problems
This 58-year-old woman was unaware there was a problem with her neck skin until friends took a picture and showed it to her. She was surprised and distressed, thinking the changes were new and therefore representative of serious disease.
She denies having any associated symptoms but does admit to a great deal of sun exposure over the years. Her history is significant for a basal cell carcinoma, removed from her chest many years ago. She also has a history of smoking and early COPD.
EXAMINATION
A solid sheet of fine, blanchable telangiectasias spreads across the patient’s upper anterior neck, extending down onto her chest. It spares the skin directly under her chin, leaving an unaffected white oval area.
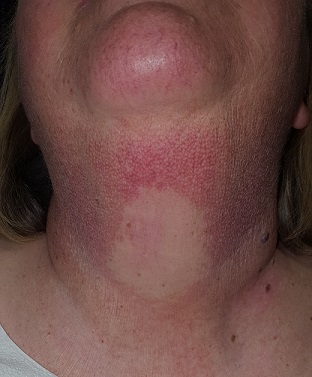
Elsewhere, the patient has a great deal of dermatoheliosis superimposed on her type II skin, including solar lentigines, weathering, and focal solar elastosis.
What is the diagnosis?
This particular pattern of mottled hyper- and hypopigmented skin is a result of overexposure to UV light. The name for this common problem—seen far more commonly in women than in men—is poikiloderma of Civatte (PC). This case is typical in that the changes manifested and progressed so slowly that the patient didn’t notice.
PC can manifest with combinations of red, brown, and yellow discoloration around the neck. In this case, the dominant color was red. The oval area of spared skin was created by the shade of the patient’s chin.
Similar changes can be seen with other conditions, such as poikiloderma vasculare atrophicans, a manifestation of small plaque parapsoriasis. However, this typically affects areas below the waist and does not have areas of sparing.
Treatment has been attempted with lasers and peels, yielding mixed success. Because of her condition’s benignancy, this patient did not opt for treatment.
TAKE-HOME LEARNING POINTS
- Poikiloderma of Civatte (PC) is a permanent skin change caused by overexposure to the sun or another UV source; it is more common in women than men.
- PC manifests with mottled hyper- or hypopigmented patches of skin on the anterior neck and upper chest, which develop gradually over the course of decades. Many patients also have sheets of telangiectasias covering the affected area.
- A distinct area of sparing (usually oval) is typically seen on the upper anterior neck, due to the chin’s shading of this area.
- Laser treatment has been somewhat successful in lightening the affected skin.
This 58-year-old woman was unaware there was a problem with her neck skin until friends took a picture and showed it to her. She was surprised and distressed, thinking the changes were new and therefore representative of serious disease.
She denies having any associated symptoms but does admit to a great deal of sun exposure over the years. Her history is significant for a basal cell carcinoma, removed from her chest many years ago. She also has a history of smoking and early COPD.
EXAMINATION
A solid sheet of fine, blanchable telangiectasias spreads across the patient’s upper anterior neck, extending down onto her chest. It spares the skin directly under her chin, leaving an unaffected white oval area.

Elsewhere, the patient has a great deal of dermatoheliosis superimposed on her type II skin, including solar lentigines, weathering, and focal solar elastosis.
What is the diagnosis?
This particular pattern of mottled hyper- and hypopigmented skin is a result of overexposure to UV light. The name for this common problem—seen far more commonly in women than in men—is poikiloderma of Civatte (PC). This case is typical in that the changes manifested and progressed so slowly that the patient didn’t notice.
PC can manifest with combinations of red, brown, and yellow discoloration around the neck. In this case, the dominant color was red. The oval area of spared skin was created by the shade of the patient’s chin.
Similar changes can be seen with other conditions, such as poikiloderma vasculare atrophicans, a manifestation of small plaque parapsoriasis. However, this typically affects areas below the waist and does not have areas of sparing.
Treatment has been attempted with lasers and peels, yielding mixed success. Because of her condition’s benignancy, this patient did not opt for treatment.
TAKE-HOME LEARNING POINTS
- Poikiloderma of Civatte (PC) is a permanent skin change caused by overexposure to the sun or another UV source; it is more common in women than men.
- PC manifests with mottled hyper- or hypopigmented patches of skin on the anterior neck and upper chest, which develop gradually over the course of decades. Many patients also have sheets of telangiectasias covering the affected area.
- A distinct area of sparing (usually oval) is typically seen on the upper anterior neck, due to the chin’s shading of this area.
- Laser treatment has been somewhat successful in lightening the affected skin.
This 58-year-old woman was unaware there was a problem with her neck skin until friends took a picture and showed it to her. She was surprised and distressed, thinking the changes were new and therefore representative of serious disease.
She denies having any associated symptoms but does admit to a great deal of sun exposure over the years. Her history is significant for a basal cell carcinoma, removed from her chest many years ago. She also has a history of smoking and early COPD.
EXAMINATION
A solid sheet of fine, blanchable telangiectasias spreads across the patient’s upper anterior neck, extending down onto her chest. It spares the skin directly under her chin, leaving an unaffected white oval area.

Elsewhere, the patient has a great deal of dermatoheliosis superimposed on her type II skin, including solar lentigines, weathering, and focal solar elastosis.
What is the diagnosis?
This particular pattern of mottled hyper- and hypopigmented skin is a result of overexposure to UV light. The name for this common problem—seen far more commonly in women than in men—is poikiloderma of Civatte (PC). This case is typical in that the changes manifested and progressed so slowly that the patient didn’t notice.
PC can manifest with combinations of red, brown, and yellow discoloration around the neck. In this case, the dominant color was red. The oval area of spared skin was created by the shade of the patient’s chin.
Similar changes can be seen with other conditions, such as poikiloderma vasculare atrophicans, a manifestation of small plaque parapsoriasis. However, this typically affects areas below the waist and does not have areas of sparing.
Treatment has been attempted with lasers and peels, yielding mixed success. Because of her condition’s benignancy, this patient did not opt for treatment.
TAKE-HOME LEARNING POINTS
- Poikiloderma of Civatte (PC) is a permanent skin change caused by overexposure to the sun or another UV source; it is more common in women than men.
- PC manifests with mottled hyper- or hypopigmented patches of skin on the anterior neck and upper chest, which develop gradually over the course of decades. Many patients also have sheets of telangiectasias covering the affected area.
- A distinct area of sparing (usually oval) is typically seen on the upper anterior neck, due to the chin’s shading of this area.
- Laser treatment has been somewhat successful in lightening the affected skin.
Blinatumomab granted full approval to treat rel/ref BCP-ALL
The US Food and Drug Administration (FDA) has approved the supplemental biologics license application (sBLA) for blinatumomab (Blincyto®).
The aim of the sBLA was to expand the indication for blinatumomab to include all patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and to convert blinatumomab’s accelerated approval to a full approval.
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed or refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Now, the FDA has granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA also recently approved the sBLA for blinatumomab to be infused over 7 days with preservative, adding to the previously approved administration options for infusion over 24 and 48 hours (preservative-free).
The blinatumomab intravenous bag for a 7-day infusion contains Bacteriostatic 0.9% Sodium Chloride, USP (containing 0.9% benzyl alcohol), which permits continuous intravenous infusion of blinatumomab at 28 mcg/day or 15 mcg/m²/day for a total of 7 days.
The 7-day infusion is not recommended for patients weighing less than 22 kg due to the risk of serious and sometimes fatal adverse events associated with benzyl alcohol in pediatric patients. See the full prescribing information for details.
The prescribing information for blinatumomab includes a boxed warning detailing the risk of cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a Risk Evaluation and Mitigation Strategy program in the US intended to inform healthcare providers about these risks.
Blinatumomab is marketed by Amgen.
Trial results
With this sBLA, Amgen sought to make blinatumomab available as a treatment for patients with Ph+ relapsed/refractory BCP-ALL (as well as Ph-).
To this end, the application included data from the ALCANTARA study, which were published in the Journal of Clinical Oncology.
In this trial, researchers evaluated blinatumomab in adults with Ph+ relapsed/refractory BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor.
Thirty-six percent of patients achieved a complete response or complete response with partial hematologic recovery within the first 2 cycles of blinatumomab treatment. Of these patients, 88% were minimal residual disease-negative.
The most frequent adverse events (AEs) in this trial were pyrexia (58%), neurologic events (47%), febrile neutropenia (40%), and headache (31%). Three patients had grade 1/2 cytokine release syndrome, and 3 patients had grade 3 neurologic AEs.
The sBLA also included overall survival (OS) data from the phase 3 TOWER trial, which was intended to support the conversion of blinatumomab’s accelerated approval to a full approval.
Results from the TOWER trial were published in NEJM.
In this study, researchers compared blinatumomab to standard of care (SOC) chemotherapy (4 different regimens) in adults with Ph- relapsed/refractory BCP-ALL.
Blinatumomab produced higher response rates and nearly doubled OS compared to SOC. The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm. The hazard ratio for death was 0.71 (P=0.012).
The incidence of grade 3 or higher AEs was higher in the SOC arm, but the incidence of serious AEs was higher in the blinatumomab arm. ![]()
The US Food and Drug Administration (FDA) has approved the supplemental biologics license application (sBLA) for blinatumomab (Blincyto®).
The aim of the sBLA was to expand the indication for blinatumomab to include all patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and to convert blinatumomab’s accelerated approval to a full approval.
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed or refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Now, the FDA has granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA also recently approved the sBLA for blinatumomab to be infused over 7 days with preservative, adding to the previously approved administration options for infusion over 24 and 48 hours (preservative-free).
The blinatumomab intravenous bag for a 7-day infusion contains Bacteriostatic 0.9% Sodium Chloride, USP (containing 0.9% benzyl alcohol), which permits continuous intravenous infusion of blinatumomab at 28 mcg/day or 15 mcg/m²/day for a total of 7 days.
The 7-day infusion is not recommended for patients weighing less than 22 kg due to the risk of serious and sometimes fatal adverse events associated with benzyl alcohol in pediatric patients. See the full prescribing information for details.
The prescribing information for blinatumomab includes a boxed warning detailing the risk of cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a Risk Evaluation and Mitigation Strategy program in the US intended to inform healthcare providers about these risks.
Blinatumomab is marketed by Amgen.
Trial results
With this sBLA, Amgen sought to make blinatumomab available as a treatment for patients with Ph+ relapsed/refractory BCP-ALL (as well as Ph-).
To this end, the application included data from the ALCANTARA study, which were published in the Journal of Clinical Oncology.
In this trial, researchers evaluated blinatumomab in adults with Ph+ relapsed/refractory BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor.
Thirty-six percent of patients achieved a complete response or complete response with partial hematologic recovery within the first 2 cycles of blinatumomab treatment. Of these patients, 88% were minimal residual disease-negative.
The most frequent adverse events (AEs) in this trial were pyrexia (58%), neurologic events (47%), febrile neutropenia (40%), and headache (31%). Three patients had grade 1/2 cytokine release syndrome, and 3 patients had grade 3 neurologic AEs.
The sBLA also included overall survival (OS) data from the phase 3 TOWER trial, which was intended to support the conversion of blinatumomab’s accelerated approval to a full approval.
Results from the TOWER trial were published in NEJM.
In this study, researchers compared blinatumomab to standard of care (SOC) chemotherapy (4 different regimens) in adults with Ph- relapsed/refractory BCP-ALL.
Blinatumomab produced higher response rates and nearly doubled OS compared to SOC. The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm. The hazard ratio for death was 0.71 (P=0.012).
The incidence of grade 3 or higher AEs was higher in the SOC arm, but the incidence of serious AEs was higher in the blinatumomab arm. ![]()
The US Food and Drug Administration (FDA) has approved the supplemental biologics license application (sBLA) for blinatumomab (Blincyto®).
The aim of the sBLA was to expand the indication for blinatumomab to include all patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and to convert blinatumomab’s accelerated approval to a full approval.
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed or refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Now, the FDA has granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA also recently approved the sBLA for blinatumomab to be infused over 7 days with preservative, adding to the previously approved administration options for infusion over 24 and 48 hours (preservative-free).
The blinatumomab intravenous bag for a 7-day infusion contains Bacteriostatic 0.9% Sodium Chloride, USP (containing 0.9% benzyl alcohol), which permits continuous intravenous infusion of blinatumomab at 28 mcg/day or 15 mcg/m²/day for a total of 7 days.
The 7-day infusion is not recommended for patients weighing less than 22 kg due to the risk of serious and sometimes fatal adverse events associated with benzyl alcohol in pediatric patients. See the full prescribing information for details.
The prescribing information for blinatumomab includes a boxed warning detailing the risk of cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a Risk Evaluation and Mitigation Strategy program in the US intended to inform healthcare providers about these risks.
Blinatumomab is marketed by Amgen.
Trial results
With this sBLA, Amgen sought to make blinatumomab available as a treatment for patients with Ph+ relapsed/refractory BCP-ALL (as well as Ph-).
To this end, the application included data from the ALCANTARA study, which were published in the Journal of Clinical Oncology.
In this trial, researchers evaluated blinatumomab in adults with Ph+ relapsed/refractory BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor.
Thirty-six percent of patients achieved a complete response or complete response with partial hematologic recovery within the first 2 cycles of blinatumomab treatment. Of these patients, 88% were minimal residual disease-negative.
The most frequent adverse events (AEs) in this trial were pyrexia (58%), neurologic events (47%), febrile neutropenia (40%), and headache (31%). Three patients had grade 1/2 cytokine release syndrome, and 3 patients had grade 3 neurologic AEs.
The sBLA also included overall survival (OS) data from the phase 3 TOWER trial, which was intended to support the conversion of blinatumomab’s accelerated approval to a full approval.
Results from the TOWER trial were published in NEJM.
In this study, researchers compared blinatumomab to standard of care (SOC) chemotherapy (4 different regimens) in adults with Ph- relapsed/refractory BCP-ALL.
Blinatumomab produced higher response rates and nearly doubled OS compared to SOC. The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm. The hazard ratio for death was 0.71 (P=0.012).
The incidence of grade 3 or higher AEs was higher in the SOC arm, but the incidence of serious AEs was higher in the blinatumomab arm. ![]()
ODAC recommends approval of CTL019 in rel/ref ALL
The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval for the chimeric antigen receptor (CAR) T-cell therapy CTL019 (tisagenlecleucel).
The committee voted 10 to 0 in favor of approving CTL019 for the treatment of pediatric and young adult patients (ages 3 to 25) with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA will consider this vote as it reviews the biologics license application (BLA) for CTL019, but the agency is not obligated to follow the ODAC’s recommendation.
The BLA for CTL019 is supported by results from 3 trials.
This includes a pilot study, which was presented at the 2015 ASH Annual Meeting; the phase 2 ENSIGN trial, which was presented at the 2016 ASH Annual Meeting; and the phase 2 ELIANA study, which was recently presented at the 22nd Congress of the European Hematology Association (EHA).
ELIANA enrolled 88 patients with relapsed/refractory B-cell ALL, and 68 of them received CTL019.
Nine patients did not receive CTL019 due to death or adverse events, 7 patients were affected by manufacturing failures, and 4 patients were pending infusion at last follow-up.
Most of the infused patients (n=65) received lymphodepleting chemotherapy prior to CTL019 (single dose). The median dose was 3.0 × 106 (range, 0.2-5.4 × 106) transduced CTL019 cells/kg.
Sixty-three patients were evaluable for efficacy.
The overall response rate—complete response (CR) plus CR with incomplete hematologic recovery (CRi)—was 83% (52/63). All patients with CR/CRis were minimal residual disease-negative in the bone marrow.
Sixty-eight patients were evaluated for safety.
Serious adverse events occurred in 69% of patients. These included life-threatening cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis, neurological events that occurred with CRS or after CRS was resolved, coagulopathies with CRS, and life-threatening infections.
Seventy-eight percent of patients had CRS—21% with grade 3 and 27% with grade 4 CRS. There were no deaths from CRS.
Forty-four percent of patients had neurological toxicities—15% grade 3 or higher. These included encephalopathy, delirium, hallucinations, somnolence, cognitive disorder, seizure, depressed level of consciousness, mental status changes, dysphagia, muscular weakness, and dysarthria.
Severe infectious complications occurred in 26% of patients, and 3 patients died of such complications.
Eleven patients died after receiving CTL019—7 due to ALL, 1 from cerebral hemorrhage, 1 from encephalitis, 1 from a respiratory tract infection, and 1 from systemic mycosis. ![]()
The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval for the chimeric antigen receptor (CAR) T-cell therapy CTL019 (tisagenlecleucel).
The committee voted 10 to 0 in favor of approving CTL019 for the treatment of pediatric and young adult patients (ages 3 to 25) with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA will consider this vote as it reviews the biologics license application (BLA) for CTL019, but the agency is not obligated to follow the ODAC’s recommendation.
The BLA for CTL019 is supported by results from 3 trials.
This includes a pilot study, which was presented at the 2015 ASH Annual Meeting; the phase 2 ENSIGN trial, which was presented at the 2016 ASH Annual Meeting; and the phase 2 ELIANA study, which was recently presented at the 22nd Congress of the European Hematology Association (EHA).
ELIANA enrolled 88 patients with relapsed/refractory B-cell ALL, and 68 of them received CTL019.
Nine patients did not receive CTL019 due to death or adverse events, 7 patients were affected by manufacturing failures, and 4 patients were pending infusion at last follow-up.
Most of the infused patients (n=65) received lymphodepleting chemotherapy prior to CTL019 (single dose). The median dose was 3.0 × 106 (range, 0.2-5.4 × 106) transduced CTL019 cells/kg.
Sixty-three patients were evaluable for efficacy.
The overall response rate—complete response (CR) plus CR with incomplete hematologic recovery (CRi)—was 83% (52/63). All patients with CR/CRis were minimal residual disease-negative in the bone marrow.
Sixty-eight patients were evaluated for safety.
Serious adverse events occurred in 69% of patients. These included life-threatening cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis, neurological events that occurred with CRS or after CRS was resolved, coagulopathies with CRS, and life-threatening infections.
Seventy-eight percent of patients had CRS—21% with grade 3 and 27% with grade 4 CRS. There were no deaths from CRS.
Forty-four percent of patients had neurological toxicities—15% grade 3 or higher. These included encephalopathy, delirium, hallucinations, somnolence, cognitive disorder, seizure, depressed level of consciousness, mental status changes, dysphagia, muscular weakness, and dysarthria.
Severe infectious complications occurred in 26% of patients, and 3 patients died of such complications.
Eleven patients died after receiving CTL019—7 due to ALL, 1 from cerebral hemorrhage, 1 from encephalitis, 1 from a respiratory tract infection, and 1 from systemic mycosis. ![]()
The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval for the chimeric antigen receptor (CAR) T-cell therapy CTL019 (tisagenlecleucel).
The committee voted 10 to 0 in favor of approving CTL019 for the treatment of pediatric and young adult patients (ages 3 to 25) with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA will consider this vote as it reviews the biologics license application (BLA) for CTL019, but the agency is not obligated to follow the ODAC’s recommendation.
The BLA for CTL019 is supported by results from 3 trials.
This includes a pilot study, which was presented at the 2015 ASH Annual Meeting; the phase 2 ENSIGN trial, which was presented at the 2016 ASH Annual Meeting; and the phase 2 ELIANA study, which was recently presented at the 22nd Congress of the European Hematology Association (EHA).
ELIANA enrolled 88 patients with relapsed/refractory B-cell ALL, and 68 of them received CTL019.
Nine patients did not receive CTL019 due to death or adverse events, 7 patients were affected by manufacturing failures, and 4 patients were pending infusion at last follow-up.
Most of the infused patients (n=65) received lymphodepleting chemotherapy prior to CTL019 (single dose). The median dose was 3.0 × 106 (range, 0.2-5.4 × 106) transduced CTL019 cells/kg.
Sixty-three patients were evaluable for efficacy.
The overall response rate—complete response (CR) plus CR with incomplete hematologic recovery (CRi)—was 83% (52/63). All patients with CR/CRis were minimal residual disease-negative in the bone marrow.
Sixty-eight patients were evaluated for safety.
Serious adverse events occurred in 69% of patients. These included life-threatening cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis, neurological events that occurred with CRS or after CRS was resolved, coagulopathies with CRS, and life-threatening infections.
Seventy-eight percent of patients had CRS—21% with grade 3 and 27% with grade 4 CRS. There were no deaths from CRS.
Forty-four percent of patients had neurological toxicities—15% grade 3 or higher. These included encephalopathy, delirium, hallucinations, somnolence, cognitive disorder, seizure, depressed level of consciousness, mental status changes, dysphagia, muscular weakness, and dysarthria.
Severe infectious complications occurred in 26% of patients, and 3 patients died of such complications.
Eleven patients died after receiving CTL019—7 due to ALL, 1 from cerebral hemorrhage, 1 from encephalitis, 1 from a respiratory tract infection, and 1 from systemic mycosis. ![]()