User login
Stem cell therapy significantly improves ulcer healing
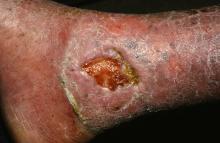
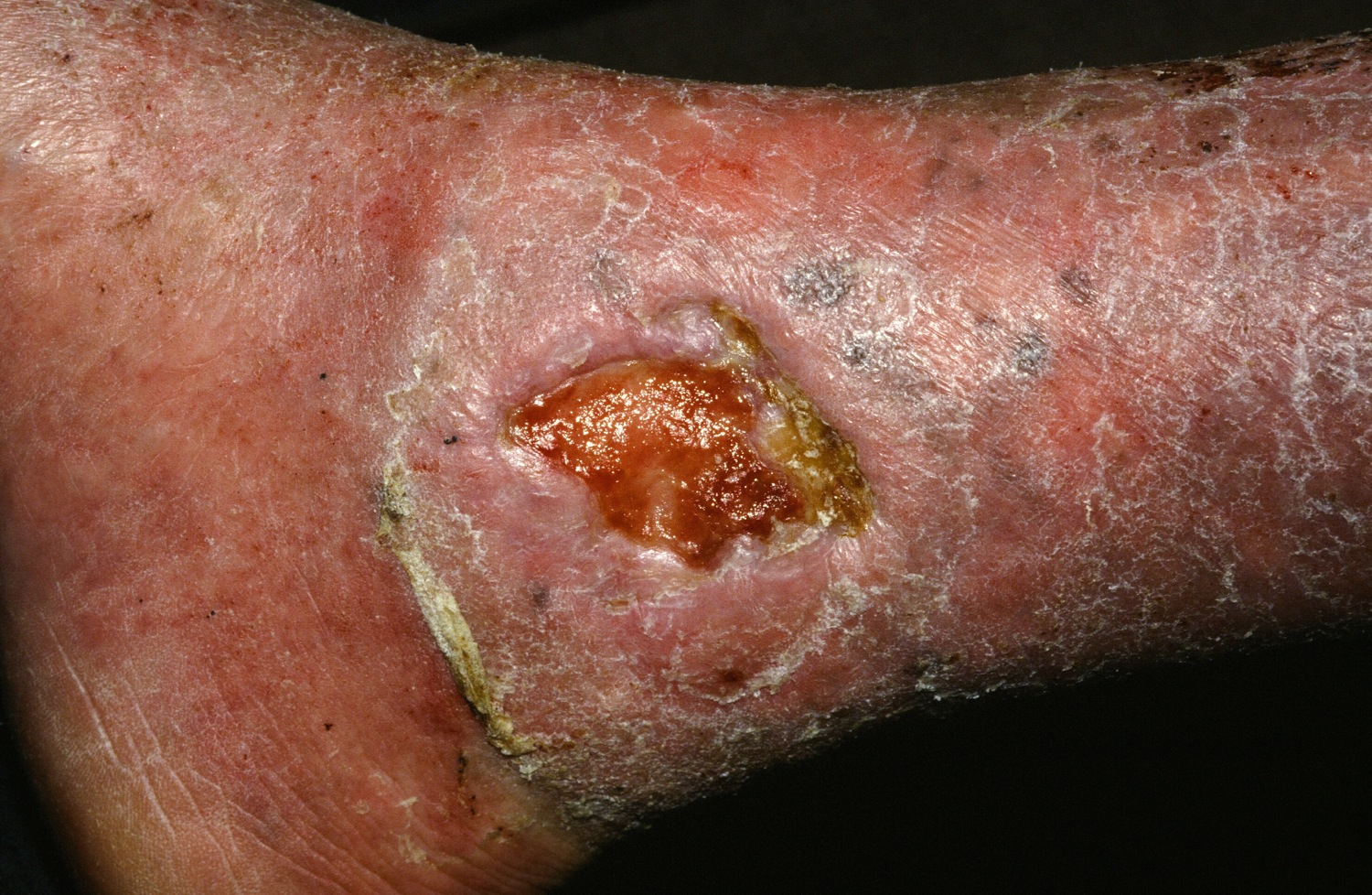
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
PORTLAND, ORE. – Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy, according to the results of a small randomized, controlled, double-blind pilot trial.
“Topical application of autologous, bone-marrow–derived mesenchymal stem cells may be an effective way to promote healing in patients with difficult-to-heal wounds,” said Ayman Grada, MD, of the department of dermatology at Boston University. “However, larger studies are needed to confirm this finding.”
“Various treatment modalities have been used, but treatment outcomes are not always satisfactory,” said Dr. Grada. “In about 60% of cases, wounds fail to close, and there is also a high rate of recurrence.”
Preclinical work in several animal models indicated that applying mesenchymal stem cells to wounds accelerated healing through a variety of mechanisms, Dr. Grada noted. Based on that premise, he and his associates hypothesized that autologous cultured mesenchymal stem cells could accelerate wound healing in humans.
To test that idea, they randomly assigned the 11 trial participants to one of two control treatments or to the stem cell intervention. Four patients received normal saline with conventional standard care, three patients received fibrin spray plus conventional therapy, and four patients received conventional therapy plus autologous mesenchymal stem cells delivered in fibrin spray at a dose of 1 x 106 cells per square centimeter of wound surface. Patients were treated every 3 weeks, up to three times or until complete wound healing, and were followed for up to 24 weeks.
To acquire the stem cells, the researchers obtained 30- to 50-mL samples of bone marrow aspirate from the iliac crest, then separated and cultured the cells in-house. The controls underwent sham aspiration with needles that did not penetrate the bone, Dr. Grada said. At each 4-week follow-up visit, the investigators measured the perimeter and area of each wound and analyzed the results with public domain software called ImageJ. They calculated the linear advance of the wound margin by dividing change in area by average perimeter.
The healing rate of the intervention group outpaced that of either control group at each time point measured, Dr. Grada said. Average weekly healing rates by time point ranged between –0.002 cm and 0.006 cm for the saline group and between –0.05 cm and 0.01 cm for the fibrin spray group. Neither of these control groups achieved meaningful wound closure by week 24.
In contrast, stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week. The study was too small for conventional statistical analysis, but a Bayesian time aggregated one-way analysis of variance yielded a statistically significant difference in healing rates among groups (P less than .0005).
Dr. Grada also discussed several case studies. An 82-year-old white woman with a decades-long history of venous ulcers experienced complete wound healing with mesenchymal stem cell therapy, which enabled her to become more independent within her long-term care facility. A 75-year-old African American woman achieved 80% wound healing with stem cell therapy after previously having failed to benefit from two applications of bioengineered skin.
Finally, a 39-year-old man with chronic, treatment-resistant venous ulcers achieved partial wound healing. “He has almost healed, with very thin epidermal coverage, but never to the point of no exudate and complete closure,” Dr. Grada said. “Therefore, we could not declare him healed, even though the ulcer was smaller at the end of the study.”
No patient in the study experienced adverse events from treatment. However, recruiting for the trial was difficult, because patients were reluctant to undergo bone marrow aspiration, Dr. Grada said.
Previous work indicates that the initial rate at which the wound heals dictates its final rate (J Am Acad Dermatol. 1993 Mar;28[3]:418-21), and that 4 weeks is enough to establish a healing trend, he noted. Dr. Grada concluded by quoting Hippocrates: “Natural forces within us are the true healers of disease.”
The National Institutes of Health supported the trial. Dr. Grada had no conflicts of interest.
At SID 2017
Key clinical point: Treating chronic venous leg ulcers with mesenchymal stem cells and fibrin spray significantly improved wound healing, compared with vehicle control or saline plus conventional therapy.
Major finding: Neither control group achieved meaningful wound closure by week 24, while stem cell recipients experienced consistent wound closure at rates of 0.11-0.13 cm per week (P less than .0005 for difference in healing rates among groups).
Data source: A randomized, controlled, double-blind pilot trial of 11 patients.
Disclosures: The National Institutes of Health supported the study. Dr. Grada had no conflicts of interest.
A woman with heavy noncyclical bleeding 6 weeks after abortion
A) Retained products of conception (RPOC) INCORRECT
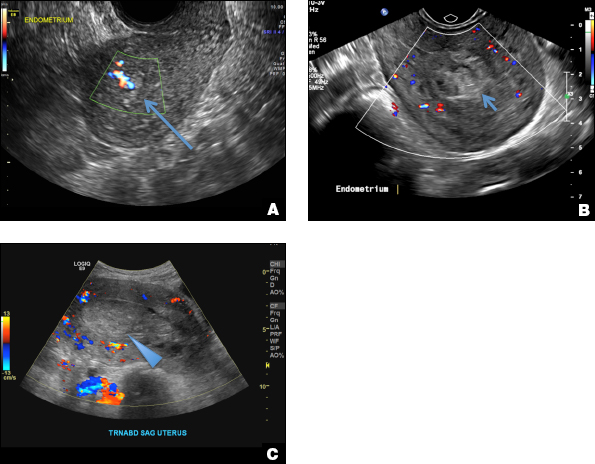
RPOC is a common complication arising from the presence of retained placental or fetal tissue after delivery, spontaneous, or elective abortion and is diagnosed by the presence of chorionic villi suggesting trophoblastic or placental tissue.1,2 Interpreting imaging findings is often a challenge secondary to the nonspecific findings of RPOC and often overlapping imaging features with blood products and enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). Management usually is based on clinical findings in collaboration with supportive imaging features. On ultrasound, RPOC is suspected when there is a thickened endometrial echo complex (>8 mm to 13 mm) and/or the presence of an endometrial mass. Additionally, increased vascularity on color Doppler significantly increases the likelihood of RPOC; the absence of vascularity can be seen with both blood products and avascular RPOC.1 Vascularity in RPOC can be differentiated from EMV by its extension into the endometrium.
B) Complete hydatidiform mole INCORRECT
Complete hydatidiform mole (CHM) usually presents early in gestation with markedly elevated β-hCG. On ultrasound, it appears classically as an echogenic mass with innumerable small cystic spaces creating a “snowstorm or cluster of grape appearance” from hydropic chorionic villi along with larger irregular fluid collections and the absence of fetal parts.3 Ovarian hyperstimulation from elevated β-hCG can result in large bilateral ovarian cysts called theca lutein cysts.3
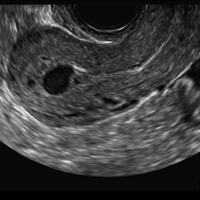
C) Enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). CORRECT
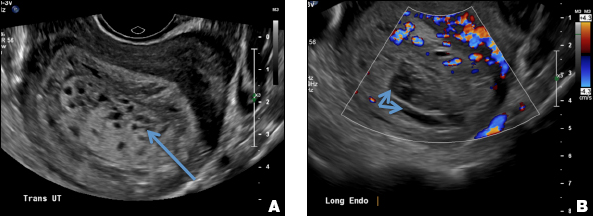
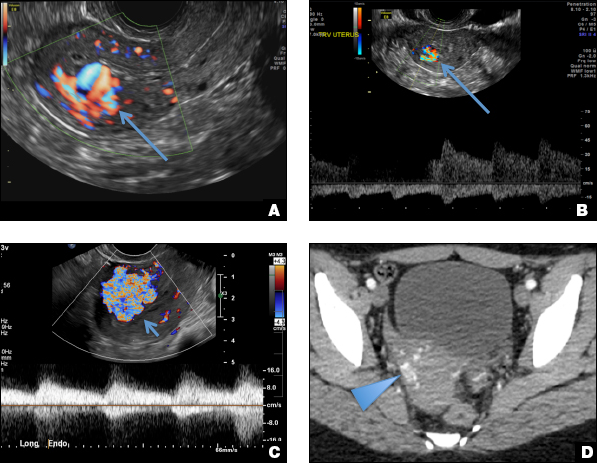
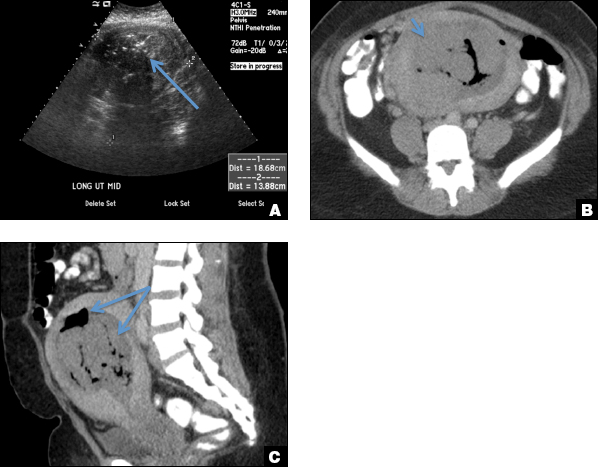
EMV is an extremely rare cause of postpregnancy hemorrhage most often seen secondary to iatrogenic causes but can also be congenital or acquired from excessive hormone stimulation.2 On ultrasound, EMV appears as a hypoechoic vascular lesion or serpiginous network of vessels located in the myometrium with increased velocity and low resistance waveform on spectral Doppler.4 Subinvolution of placental site implantation where there is failure of the vessels to involute can sometimes be indistinguishable from acquired EMV and occasionally difficult to differentiate from RPOC.1 In stable patients with equivocal ultrasound findings, magnetic resonance imaging (MRI) with its superior contrast resolution can help delineate the endometrium from myometrium and clearly identify EMV as serpiginous signal voids in the myometrium with avid enhancement following contrast.5 Computed tomography (CT) angiogram is also of value in both the diagnosis and pretreatment planning of EMV prior to transcatheter uterine artery embolization.
D) Endometritis INCORRECT
Endometritis is a common cause of fever and sepsis in the postpartum state, but it can also occur after procedures such as uterine fibroid embolization (UFE). On ultrasound, the endometrium usually is distended with avascular echogenic fluid. The presence of shadowing gas in the appropriate clinical setting is concerning for endometritis. CT scan can confirm the presence of gas and evaluate for septic thrombophlebitis, an uncommon, life-threatening complication of endometritis.
- Sellmyer MA, Desser TS, Maturen KE, Jeffrey RB Jr, Kamaya A. Physiologic, histologic, and imaging features of retained products of conception. RadioGraphics. 2013;33(3):781–796.
- Plunk M, Lee JH, Kani K, Dighe M. Imaging of postpartum complications: a multimodality review. AJR. 2013;200(2):W143–W154.
- Shaaban AM, Rezvani M, Haroun RR, et al. Gestational trophoblastic disease: clinical and imaging features. RadioGraphics. 2017;37(2):681–700.
- Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–e10.
- Yoon DJ, Jones M, Taani JA, Buhimschi C, Dowell JD. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. Am J Perinatol Rep. 2016;6(1):e6–e14.
A) Retained products of conception (RPOC) INCORRECT
RPOC is a common complication arising from the presence of retained placental or fetal tissue after delivery, spontaneous, or elective abortion and is diagnosed by the presence of chorionic villi suggesting trophoblastic or placental tissue.1,2 Interpreting imaging findings is often a challenge secondary to the nonspecific findings of RPOC and often overlapping imaging features with blood products and enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). Management usually is based on clinical findings in collaboration with supportive imaging features. On ultrasound, RPOC is suspected when there is a thickened endometrial echo complex (>8 mm to 13 mm) and/or the presence of an endometrial mass. Additionally, increased vascularity on color Doppler significantly increases the likelihood of RPOC; the absence of vascularity can be seen with both blood products and avascular RPOC.1 Vascularity in RPOC can be differentiated from EMV by its extension into the endometrium.

B) Complete hydatidiform mole INCORRECT
Complete hydatidiform mole (CHM) usually presents early in gestation with markedly elevated β-hCG. On ultrasound, it appears classically as an echogenic mass with innumerable small cystic spaces creating a “snowstorm or cluster of grape appearance” from hydropic chorionic villi along with larger irregular fluid collections and the absence of fetal parts.3 Ovarian hyperstimulation from elevated β-hCG can result in large bilateral ovarian cysts called theca lutein cysts.3

C) Enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). CORRECT
EMV is an extremely rare cause of postpregnancy hemorrhage most often seen secondary to iatrogenic causes but can also be congenital or acquired from excessive hormone stimulation.2 On ultrasound, EMV appears as a hypoechoic vascular lesion or serpiginous network of vessels located in the myometrium with increased velocity and low resistance waveform on spectral Doppler.4 Subinvolution of placental site implantation where there is failure of the vessels to involute can sometimes be indistinguishable from acquired EMV and occasionally difficult to differentiate from RPOC.1 In stable patients with equivocal ultrasound findings, magnetic resonance imaging (MRI) with its superior contrast resolution can help delineate the endometrium from myometrium and clearly identify EMV as serpiginous signal voids in the myometrium with avid enhancement following contrast.5 Computed tomography (CT) angiogram is also of value in both the diagnosis and pretreatment planning of EMV prior to transcatheter uterine artery embolization.

D) Endometritis INCORRECT
Endometritis is a common cause of fever and sepsis in the postpartum state, but it can also occur after procedures such as uterine fibroid embolization (UFE). On ultrasound, the endometrium usually is distended with avascular echogenic fluid. The presence of shadowing gas in the appropriate clinical setting is concerning for endometritis. CT scan can confirm the presence of gas and evaluate for septic thrombophlebitis, an uncommon, life-threatening complication of endometritis.

A) Retained products of conception (RPOC) INCORRECT
RPOC is a common complication arising from the presence of retained placental or fetal tissue after delivery, spontaneous, or elective abortion and is diagnosed by the presence of chorionic villi suggesting trophoblastic or placental tissue.1,2 Interpreting imaging findings is often a challenge secondary to the nonspecific findings of RPOC and often overlapping imaging features with blood products and enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). Management usually is based on clinical findings in collaboration with supportive imaging features. On ultrasound, RPOC is suspected when there is a thickened endometrial echo complex (>8 mm to 13 mm) and/or the presence of an endometrial mass. Additionally, increased vascularity on color Doppler significantly increases the likelihood of RPOC; the absence of vascularity can be seen with both blood products and avascular RPOC.1 Vascularity in RPOC can be differentiated from EMV by its extension into the endometrium.

B) Complete hydatidiform mole INCORRECT
Complete hydatidiform mole (CHM) usually presents early in gestation with markedly elevated β-hCG. On ultrasound, it appears classically as an echogenic mass with innumerable small cystic spaces creating a “snowstorm or cluster of grape appearance” from hydropic chorionic villi along with larger irregular fluid collections and the absence of fetal parts.3 Ovarian hyperstimulation from elevated β-hCG can result in large bilateral ovarian cysts called theca lutein cysts.3

C) Enhanced myometrial vascularity (EMV) also known as arteriovenous malformation (AVM). CORRECT
EMV is an extremely rare cause of postpregnancy hemorrhage most often seen secondary to iatrogenic causes but can also be congenital or acquired from excessive hormone stimulation.2 On ultrasound, EMV appears as a hypoechoic vascular lesion or serpiginous network of vessels located in the myometrium with increased velocity and low resistance waveform on spectral Doppler.4 Subinvolution of placental site implantation where there is failure of the vessels to involute can sometimes be indistinguishable from acquired EMV and occasionally difficult to differentiate from RPOC.1 In stable patients with equivocal ultrasound findings, magnetic resonance imaging (MRI) with its superior contrast resolution can help delineate the endometrium from myometrium and clearly identify EMV as serpiginous signal voids in the myometrium with avid enhancement following contrast.5 Computed tomography (CT) angiogram is also of value in both the diagnosis and pretreatment planning of EMV prior to transcatheter uterine artery embolization.

D) Endometritis INCORRECT
Endometritis is a common cause of fever and sepsis in the postpartum state, but it can also occur after procedures such as uterine fibroid embolization (UFE). On ultrasound, the endometrium usually is distended with avascular echogenic fluid. The presence of shadowing gas in the appropriate clinical setting is concerning for endometritis. CT scan can confirm the presence of gas and evaluate for septic thrombophlebitis, an uncommon, life-threatening complication of endometritis.

- Sellmyer MA, Desser TS, Maturen KE, Jeffrey RB Jr, Kamaya A. Physiologic, histologic, and imaging features of retained products of conception. RadioGraphics. 2013;33(3):781–796.
- Plunk M, Lee JH, Kani K, Dighe M. Imaging of postpartum complications: a multimodality review. AJR. 2013;200(2):W143–W154.
- Shaaban AM, Rezvani M, Haroun RR, et al. Gestational trophoblastic disease: clinical and imaging features. RadioGraphics. 2017;37(2):681–700.
- Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–e10.
- Yoon DJ, Jones M, Taani JA, Buhimschi C, Dowell JD. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. Am J Perinatol Rep. 2016;6(1):e6–e14.
- Sellmyer MA, Desser TS, Maturen KE, Jeffrey RB Jr, Kamaya A. Physiologic, histologic, and imaging features of retained products of conception. RadioGraphics. 2013;33(3):781–796.
- Plunk M, Lee JH, Kani K, Dighe M. Imaging of postpartum complications: a multimodality review. AJR. 2013;200(2):W143–W154.
- Shaaban AM, Rezvani M, Haroun RR, et al. Gestational trophoblastic disease: clinical and imaging features. RadioGraphics. 2017;37(2):681–700.
- Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovacs S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731.e1–e10.
- Yoon DJ, Jones M, Taani JA, Buhimschi C, Dowell JD. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. Am J Perinatol Rep. 2016;6(1):e6–e14.
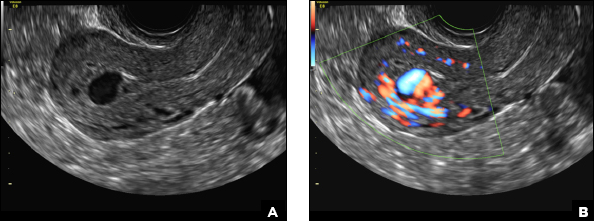
A 25-year-old woman presents to her ObGyn’s office with heavy noncyclical bleeding 6 weeks after a first-trimester suction curettage abortion. Transvaginal pelvic ultrasonography of the uterus with grayscale (A) and color Doppler (B) are performed.
Difficult-to-Detect Low-Grade Infections Responsible for Poor Outcomes in Total Knee Arthroplasty
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
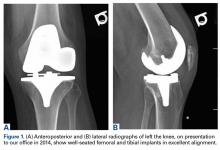
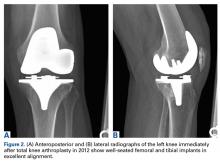
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
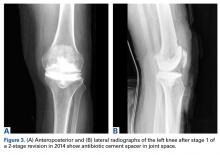
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
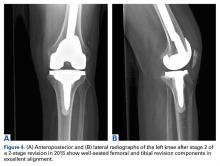
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Mild Persistent Asthma: Characteristics, Treatment, and Unmet Needs
This newsletter, Mild Persistent Asthma: Characteristics, Treatment, and Unmet Needs, discusses the characteristics and burden of mild persistent asthma and provides practical information on its diagnosis and appropriate management.
Click here to read the supplement
PA-C
Former American Academy of Physician Assistants (AAPA) Liaison to the American Academy of Allergy Asthma and Immunology (AAAAI) and former Liaison from AAAAI to the AAPA.
This newsletter, Mild Persistent Asthma: Characteristics, Treatment, and Unmet Needs, discusses the characteristics and burden of mild persistent asthma and provides practical information on its diagnosis and appropriate management.
Click here to read the supplement
PA-C
Former American Academy of Physician Assistants (AAPA) Liaison to the American Academy of Allergy Asthma and Immunology (AAAAI) and former Liaison from AAAAI to the AAPA.
This newsletter, Mild Persistent Asthma: Characteristics, Treatment, and Unmet Needs, discusses the characteristics and burden of mild persistent asthma and provides practical information on its diagnosis and appropriate management.
Click here to read the supplement
PA-C
Former American Academy of Physician Assistants (AAPA) Liaison to the American Academy of Allergy Asthma and Immunology (AAAAI) and former Liaison from AAAAI to the AAPA.
Anxiety and Depressive Disorders May Be Equally Prevalent in Patients With Epilepsy
Contrary to the widespread belief that depressive disorders are more common than anxiety disorders among people with epilepsy, these psychiatric comorbidities appear to have equivalent prevalence, according to research published online ahead of print May 3 in Epilepsia. In addition, variability in the observed prevalence of anxiety disorders in previous studies partly results from the method of diagnosis.
“These findings also challenge widely held assumptions that psychiatric comorbidity is more common in people with drug-resistant epilepsy,” said Amelia J. Scott, a PhD candidate at the University of Sydney.
Comorbid anxiety and depressive disorders are highly prevalent in patients with epilepsy, compared with the general population. The prevalence of anxiety disorders reported in previous studies of people with epilepsy has been highly variable, however. Ms. Scott and colleagues conducted a study to clarify the prevalence of anxiety and depressive disorders in patients with epilepsy and to determine which factors account for the variability in estimates of these disorders’ prevalence.
The investigators searched electronic databases to find studies that reported the prevalence of anxiety and depressive disorders in people with epilepsy until July 2016. Journal articles or dissertations that reported on current diagnoses of anxiety and depressive disorders based on a structured diagnostic interview or a clinician evaluation were included. Clinician evaluations followed the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition or the International Classification of Diseases, Tenth Revision or more recent.
The investigators excluded studies of participants younger than 16. In addition, studies that reported diagnoses of anxiety and depressive disorders using self-report measures and studies that only reported a depression diagnosis or only an anxiety diagnosis were also excluded. Finally, researchers excluded studies if recruitment was based on additional medical comorbidity or on results of prescreening measures of distress.
Extracted data included the prevalence of anxiety and depressive disorders and moderators of interest (eg, method of diagnosis and prevalence of drug-resistant epilepsy). Using these data, Ms. Scott and colleagues conducted a meta-analysis of the overall pooled prevalence of anxiety and depressive disorders.
In all, 27 studies met the inclusion criteria. The pooled prevalence of anxiety disorders was 20.2%, and the pooled prevalence of depressive disorders was 22.9%. Ms. Scott and colleagues also observed that the method of diagnosis significantly affected the observed prevalence of anxiety disorders. The prevalence of anxiety disorders based on unstructured clinician assessment was 8.1%, compared with a prevalence of 27.3% based on a structured clinical interview. No significant moderators of depressive disorder diagnosis were reported, however.
“Future research should aim to improve the detection and management of comorbidities in people with epilepsy, particularly anxiety disorders, which have remained relatively neglected,” said Ms. Scott. “An improvement in our understanding, detection, and management of both anxiety and depressive disorders in people with epilepsy is crucial to improve the quality of life of people with epilepsy.”
—Erica Tricarico
Suggested Reading
Scott AJ, Sharpe L, Hunt C, Gandy M. Anxiety and depressive disorders in people with epilepsy: A meta-analysis. Epilepsia. 2017 May 3 [Epub ahead of print].
Contrary to the widespread belief that depressive disorders are more common than anxiety disorders among people with epilepsy, these psychiatric comorbidities appear to have equivalent prevalence, according to research published online ahead of print May 3 in Epilepsia. In addition, variability in the observed prevalence of anxiety disorders in previous studies partly results from the method of diagnosis.
“These findings also challenge widely held assumptions that psychiatric comorbidity is more common in people with drug-resistant epilepsy,” said Amelia J. Scott, a PhD candidate at the University of Sydney.
Comorbid anxiety and depressive disorders are highly prevalent in patients with epilepsy, compared with the general population. The prevalence of anxiety disorders reported in previous studies of people with epilepsy has been highly variable, however. Ms. Scott and colleagues conducted a study to clarify the prevalence of anxiety and depressive disorders in patients with epilepsy and to determine which factors account for the variability in estimates of these disorders’ prevalence.
The investigators searched electronic databases to find studies that reported the prevalence of anxiety and depressive disorders in people with epilepsy until July 2016. Journal articles or dissertations that reported on current diagnoses of anxiety and depressive disorders based on a structured diagnostic interview or a clinician evaluation were included. Clinician evaluations followed the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition or the International Classification of Diseases, Tenth Revision or more recent.
The investigators excluded studies of participants younger than 16. In addition, studies that reported diagnoses of anxiety and depressive disorders using self-report measures and studies that only reported a depression diagnosis or only an anxiety diagnosis were also excluded. Finally, researchers excluded studies if recruitment was based on additional medical comorbidity or on results of prescreening measures of distress.
Extracted data included the prevalence of anxiety and depressive disorders and moderators of interest (eg, method of diagnosis and prevalence of drug-resistant epilepsy). Using these data, Ms. Scott and colleagues conducted a meta-analysis of the overall pooled prevalence of anxiety and depressive disorders.
In all, 27 studies met the inclusion criteria. The pooled prevalence of anxiety disorders was 20.2%, and the pooled prevalence of depressive disorders was 22.9%. Ms. Scott and colleagues also observed that the method of diagnosis significantly affected the observed prevalence of anxiety disorders. The prevalence of anxiety disorders based on unstructured clinician assessment was 8.1%, compared with a prevalence of 27.3% based on a structured clinical interview. No significant moderators of depressive disorder diagnosis were reported, however.
“Future research should aim to improve the detection and management of comorbidities in people with epilepsy, particularly anxiety disorders, which have remained relatively neglected,” said Ms. Scott. “An improvement in our understanding, detection, and management of both anxiety and depressive disorders in people with epilepsy is crucial to improve the quality of life of people with epilepsy.”
—Erica Tricarico
Suggested Reading
Scott AJ, Sharpe L, Hunt C, Gandy M. Anxiety and depressive disorders in people with epilepsy: A meta-analysis. Epilepsia. 2017 May 3 [Epub ahead of print].
Contrary to the widespread belief that depressive disorders are more common than anxiety disorders among people with epilepsy, these psychiatric comorbidities appear to have equivalent prevalence, according to research published online ahead of print May 3 in Epilepsia. In addition, variability in the observed prevalence of anxiety disorders in previous studies partly results from the method of diagnosis.
“These findings also challenge widely held assumptions that psychiatric comorbidity is more common in people with drug-resistant epilepsy,” said Amelia J. Scott, a PhD candidate at the University of Sydney.
Comorbid anxiety and depressive disorders are highly prevalent in patients with epilepsy, compared with the general population. The prevalence of anxiety disorders reported in previous studies of people with epilepsy has been highly variable, however. Ms. Scott and colleagues conducted a study to clarify the prevalence of anxiety and depressive disorders in patients with epilepsy and to determine which factors account for the variability in estimates of these disorders’ prevalence.
The investigators searched electronic databases to find studies that reported the prevalence of anxiety and depressive disorders in people with epilepsy until July 2016. Journal articles or dissertations that reported on current diagnoses of anxiety and depressive disorders based on a structured diagnostic interview or a clinician evaluation were included. Clinician evaluations followed the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition or the International Classification of Diseases, Tenth Revision or more recent.
The investigators excluded studies of participants younger than 16. In addition, studies that reported diagnoses of anxiety and depressive disorders using self-report measures and studies that only reported a depression diagnosis or only an anxiety diagnosis were also excluded. Finally, researchers excluded studies if recruitment was based on additional medical comorbidity or on results of prescreening measures of distress.
Extracted data included the prevalence of anxiety and depressive disorders and moderators of interest (eg, method of diagnosis and prevalence of drug-resistant epilepsy). Using these data, Ms. Scott and colleagues conducted a meta-analysis of the overall pooled prevalence of anxiety and depressive disorders.
In all, 27 studies met the inclusion criteria. The pooled prevalence of anxiety disorders was 20.2%, and the pooled prevalence of depressive disorders was 22.9%. Ms. Scott and colleagues also observed that the method of diagnosis significantly affected the observed prevalence of anxiety disorders. The prevalence of anxiety disorders based on unstructured clinician assessment was 8.1%, compared with a prevalence of 27.3% based on a structured clinical interview. No significant moderators of depressive disorder diagnosis were reported, however.
“Future research should aim to improve the detection and management of comorbidities in people with epilepsy, particularly anxiety disorders, which have remained relatively neglected,” said Ms. Scott. “An improvement in our understanding, detection, and management of both anxiety and depressive disorders in people with epilepsy is crucial to improve the quality of life of people with epilepsy.”
—Erica Tricarico
Suggested Reading
Scott AJ, Sharpe L, Hunt C, Gandy M. Anxiety and depressive disorders in people with epilepsy: A meta-analysis. Epilepsia. 2017 May 3 [Epub ahead of print].
Acne improved in most women treated with spironolactone
Most of the postadolescent women with acne in a retrospective review of 400 patients treated over a 4-year period improved with spironolactone treatment, with a low rate of side effects, according to the authors.
“The vast majority of our patients improved on spironolactone, despite many previously failing other acne treatments,” wrote Radhika Grandhi, MD, and Ali Alikhan, MD, of the department of dermatology, University of Cincinnati. They conducted a review of 400 postadolescent female patients treated with spironolactone from July 2012 through February 2016, representing 292 patient years of experience.
Of the 400 patients, 345 improved, 27 were unchanged, 11 became worse, and 17 had an indeterminate response. Most (220 of 253 or 87%) of previously treated patients improved with spironolactone as did most (137 of 147 or 93%) of previously untreated patients.
About half of patients were on a combination of spironolactone, at least one topical agent, and an oral treatment; almost 90% of these patients improved with spironolactone. Of the remainder, 81% of those on spironolactone plus an oral medication improved, and 95% of those on spironolactone plus a topical agent improved. Of the 41 patients on spironolactone monotherapy, 35 (85%) improved.
The most common adverse effect reported was hyperkalemia in six patients, followed by facial/perioral xerosis, erythema, irritation, and pruritus in four, and one report each of severe headaches, feeling sick, heavy menstrual cycles, back pain, abdominal fullness, and breast tenderness.
These data suggest that spironolactone, a second-line acne treatment, “is a useful drug, and the results further support that spironolactone is a safe medication with tolerable side effects,” the authors noted. ‘It may be speculated that an increased use of spironolactone may decrease our dependence on oral antibiotics in acne management, which is paramount in this age of antibiotic stewardship.”
For the full study, go to (Dermatology doi: 10.1159/000471799).
Most of the postadolescent women with acne in a retrospective review of 400 patients treated over a 4-year period improved with spironolactone treatment, with a low rate of side effects, according to the authors.
“The vast majority of our patients improved on spironolactone, despite many previously failing other acne treatments,” wrote Radhika Grandhi, MD, and Ali Alikhan, MD, of the department of dermatology, University of Cincinnati. They conducted a review of 400 postadolescent female patients treated with spironolactone from July 2012 through February 2016, representing 292 patient years of experience.
Of the 400 patients, 345 improved, 27 were unchanged, 11 became worse, and 17 had an indeterminate response. Most (220 of 253 or 87%) of previously treated patients improved with spironolactone as did most (137 of 147 or 93%) of previously untreated patients.
About half of patients were on a combination of spironolactone, at least one topical agent, and an oral treatment; almost 90% of these patients improved with spironolactone. Of the remainder, 81% of those on spironolactone plus an oral medication improved, and 95% of those on spironolactone plus a topical agent improved. Of the 41 patients on spironolactone monotherapy, 35 (85%) improved.
The most common adverse effect reported was hyperkalemia in six patients, followed by facial/perioral xerosis, erythema, irritation, and pruritus in four, and one report each of severe headaches, feeling sick, heavy menstrual cycles, back pain, abdominal fullness, and breast tenderness.
These data suggest that spironolactone, a second-line acne treatment, “is a useful drug, and the results further support that spironolactone is a safe medication with tolerable side effects,” the authors noted. ‘It may be speculated that an increased use of spironolactone may decrease our dependence on oral antibiotics in acne management, which is paramount in this age of antibiotic stewardship.”
For the full study, go to (Dermatology doi: 10.1159/000471799).
Most of the postadolescent women with acne in a retrospective review of 400 patients treated over a 4-year period improved with spironolactone treatment, with a low rate of side effects, according to the authors.
“The vast majority of our patients improved on spironolactone, despite many previously failing other acne treatments,” wrote Radhika Grandhi, MD, and Ali Alikhan, MD, of the department of dermatology, University of Cincinnati. They conducted a review of 400 postadolescent female patients treated with spironolactone from July 2012 through February 2016, representing 292 patient years of experience.
Of the 400 patients, 345 improved, 27 were unchanged, 11 became worse, and 17 had an indeterminate response. Most (220 of 253 or 87%) of previously treated patients improved with spironolactone as did most (137 of 147 or 93%) of previously untreated patients.
About half of patients were on a combination of spironolactone, at least one topical agent, and an oral treatment; almost 90% of these patients improved with spironolactone. Of the remainder, 81% of those on spironolactone plus an oral medication improved, and 95% of those on spironolactone plus a topical agent improved. Of the 41 patients on spironolactone monotherapy, 35 (85%) improved.
The most common adverse effect reported was hyperkalemia in six patients, followed by facial/perioral xerosis, erythema, irritation, and pruritus in four, and one report each of severe headaches, feeling sick, heavy menstrual cycles, back pain, abdominal fullness, and breast tenderness.
These data suggest that spironolactone, a second-line acne treatment, “is a useful drug, and the results further support that spironolactone is a safe medication with tolerable side effects,” the authors noted. ‘It may be speculated that an increased use of spironolactone may decrease our dependence on oral antibiotics in acne management, which is paramount in this age of antibiotic stewardship.”
For the full study, go to (Dermatology doi: 10.1159/000471799).
FROM DERMATOLOGY
Grassroots policymaking demands that hospitalists team up
LAS VEGAS – Alla Zilbering, MD, sat at attention for hours during HM17, jotting notes like a scribe about the myriad of federal rules that are pretty rapidly pushing hospitalists and health care as a whole away from fee-for-service payments to a world where doctors are paid for quality.
So, why did she do it? Why all that time on policy, instead of practice?
Because Dr. Zilbering felt compelled to get more involved. As a lead hospitalist at Cigna-HealthSpring, a Medicare Advantage program in Philadelphia, she’s already part of initiatives to improve transitions of care and reduce readmissions.
However, she said she wants to do more. “I’m feeling like, unless you actually address the policy, you can’t get that far in terms of what you can physically do with a patient.”
HM17 was the meeting for her, then. SHM, this year, unveiled its first Health Policy Mini Track, dedicated to updating attendees on the implementation of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), the Bundled Payments for Care Improvement initiative, and a host of other federal programs. Hospitalists were updated on a litany of advocacy efforts, including observation status, interoperability of electronic health records systems, and the recent launch of the first hospitalist billing code.
Two of the meeting’s three keynote speakers were Washington veterans who confirmed that, while nightly news reports may suggest that health care reforms contained in the Affordable Care Act are constantly in flux, the trajectory toward paying for higher quality care at lower costs shows no signs of abating.
Plenary speaker Patrick Conway, MD, MSc, MHM, deputy administrator for innovation and quality at the Centers for Medicare & Medicaid Services and director of its Center for Medicare and Medicaid Innovation, noted that the proposed American Health Care Act doesn’t have a “single word dealing with the Innovation Center,” which is the government agency tasked with supporting the development and testing of new payment and service delivery models.
He added that the policy’s gravitation away from fee-for-service toward alternative payment models will ideally lead to better patient outcomes, more coordinated care, and financial savings. So, he urged hospitalists to continue to help design those new payment and care-delivery systems.
M.A. Williams, MD, FHM, the medical director of perioperative services at Porter Adventist Hospital in Denver, said that the way to help design those systems is to get involved. Policy may seem like an issue for C-suite denizens and wonks, but individual practitioners can make more impact than they think.
“Learn enough to be dangerous and go to your CMO [or] whoever you can get a meeting with because MACRA is going to effect all physicians in the organization, even if the system is not doing anything active about it,” Dr. Williams said. “If you show interest and show that you have a little bit of knowledge, you’d be surprised with what kind of traction you might be able to get.”
And that traction isn’t just within the walls of a given institution, Dr. Greeno said. He wants more hospitalists involved in the society’s overall advocacy efforts. That includes lobbying Congress both in person and with phone calls, letters, and emails and pressuring people at home via conduits like SHM’s Grassroots Network, which has nearly 1,200 members from 490 states.
Don’t think those things work? Dr. Greeno said, one need look no further than the new C6 Medicare billing code for hospitalists that went live in April. That didn’t come to pass without a concentrated effort.
“That was a ton of work by our staff and several years of lobbying,” he said. “We had to be able to explain to them why our data should be treated differently as a specialty and compared only to other hospitalists as opposed to other internists or family practitioners.”
The code will help differentiate hospitalists at a time when MACRA will force changes in how hospitalists are paid. But, it will also define the specialty in a way that has never before been accomplished.
“It is an identity within Medicare,” said Josh Boswell, SHM’s director of government affairs.
While the ACA and the potential repeal of its insurance reforms have taken center stage in the media, Dr. Greeno urged hospitalists to focus more on the implementation and rule-making via MACRA.
The bill, which eliminated the Sustainable Growth Rate formula, states that, starting in 2019, Medicare payments will be provided through one of two pathways. The first is the Merit-based Incentive Payment System that combines the Physician Quality Reporting System, the Physician Value-Based Modifier, and Meaningful Use into a single performance-based payment system.
The second option is Alternative Payment Models, which is meant to incentivize the adoption of payment models that move physicians away from fee-for-service models more quickly. To qualify in this pathway, the criteria require elements of “upside and downside financial risk,” as well as meeting threshold requirements for either patients or payments. Those physicians that meet the criteria qualify for a 5% incentive payment.
The first payments in 2019 are based on performance data for 2017. As most hospitalists won’t quality for APMs in the first year, they will default to the MIPS pathway, Dr. Greeno said.
“This bill will have a greater impact on ... providers than any piece of legislation in our lifetime,” he noted. “Now, the ACA had a bigger impact on consumers, but, in terms of us as providers, MACRA is a sea change.”
The topic is so important, SHM has created a website at www.macraforhm.org that is meant to serve as a tutorial to the law’s basics. The guide is intended to educate hospitalists and to motivate them to get involved in the policy work that affects them all, Dr. Greeno said
“If you don’t know how the system works, you can’t influence it,” he added. “My view of the world is, if you’re not at the table, you’re on the menu.”
LAS VEGAS – Alla Zilbering, MD, sat at attention for hours during HM17, jotting notes like a scribe about the myriad of federal rules that are pretty rapidly pushing hospitalists and health care as a whole away from fee-for-service payments to a world where doctors are paid for quality.
So, why did she do it? Why all that time on policy, instead of practice?
Because Dr. Zilbering felt compelled to get more involved. As a lead hospitalist at Cigna-HealthSpring, a Medicare Advantage program in Philadelphia, she’s already part of initiatives to improve transitions of care and reduce readmissions.
However, she said she wants to do more. “I’m feeling like, unless you actually address the policy, you can’t get that far in terms of what you can physically do with a patient.”
HM17 was the meeting for her, then. SHM, this year, unveiled its first Health Policy Mini Track, dedicated to updating attendees on the implementation of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), the Bundled Payments for Care Improvement initiative, and a host of other federal programs. Hospitalists were updated on a litany of advocacy efforts, including observation status, interoperability of electronic health records systems, and the recent launch of the first hospitalist billing code.
Two of the meeting’s three keynote speakers were Washington veterans who confirmed that, while nightly news reports may suggest that health care reforms contained in the Affordable Care Act are constantly in flux, the trajectory toward paying for higher quality care at lower costs shows no signs of abating.
Plenary speaker Patrick Conway, MD, MSc, MHM, deputy administrator for innovation and quality at the Centers for Medicare & Medicaid Services and director of its Center for Medicare and Medicaid Innovation, noted that the proposed American Health Care Act doesn’t have a “single word dealing with the Innovation Center,” which is the government agency tasked with supporting the development and testing of new payment and service delivery models.
He added that the policy’s gravitation away from fee-for-service toward alternative payment models will ideally lead to better patient outcomes, more coordinated care, and financial savings. So, he urged hospitalists to continue to help design those new payment and care-delivery systems.
M.A. Williams, MD, FHM, the medical director of perioperative services at Porter Adventist Hospital in Denver, said that the way to help design those systems is to get involved. Policy may seem like an issue for C-suite denizens and wonks, but individual practitioners can make more impact than they think.
“Learn enough to be dangerous and go to your CMO [or] whoever you can get a meeting with because MACRA is going to effect all physicians in the organization, even if the system is not doing anything active about it,” Dr. Williams said. “If you show interest and show that you have a little bit of knowledge, you’d be surprised with what kind of traction you might be able to get.”
And that traction isn’t just within the walls of a given institution, Dr. Greeno said. He wants more hospitalists involved in the society’s overall advocacy efforts. That includes lobbying Congress both in person and with phone calls, letters, and emails and pressuring people at home via conduits like SHM’s Grassroots Network, which has nearly 1,200 members from 490 states.
Don’t think those things work? Dr. Greeno said, one need look no further than the new C6 Medicare billing code for hospitalists that went live in April. That didn’t come to pass without a concentrated effort.
“That was a ton of work by our staff and several years of lobbying,” he said. “We had to be able to explain to them why our data should be treated differently as a specialty and compared only to other hospitalists as opposed to other internists or family practitioners.”
The code will help differentiate hospitalists at a time when MACRA will force changes in how hospitalists are paid. But, it will also define the specialty in a way that has never before been accomplished.
“It is an identity within Medicare,” said Josh Boswell, SHM’s director of government affairs.
While the ACA and the potential repeal of its insurance reforms have taken center stage in the media, Dr. Greeno urged hospitalists to focus more on the implementation and rule-making via MACRA.
The bill, which eliminated the Sustainable Growth Rate formula, states that, starting in 2019, Medicare payments will be provided through one of two pathways. The first is the Merit-based Incentive Payment System that combines the Physician Quality Reporting System, the Physician Value-Based Modifier, and Meaningful Use into a single performance-based payment system.
The second option is Alternative Payment Models, which is meant to incentivize the adoption of payment models that move physicians away from fee-for-service models more quickly. To qualify in this pathway, the criteria require elements of “upside and downside financial risk,” as well as meeting threshold requirements for either patients or payments. Those physicians that meet the criteria qualify for a 5% incentive payment.
The first payments in 2019 are based on performance data for 2017. As most hospitalists won’t quality for APMs in the first year, they will default to the MIPS pathway, Dr. Greeno said.
“This bill will have a greater impact on ... providers than any piece of legislation in our lifetime,” he noted. “Now, the ACA had a bigger impact on consumers, but, in terms of us as providers, MACRA is a sea change.”
The topic is so important, SHM has created a website at www.macraforhm.org that is meant to serve as a tutorial to the law’s basics. The guide is intended to educate hospitalists and to motivate them to get involved in the policy work that affects them all, Dr. Greeno said
“If you don’t know how the system works, you can’t influence it,” he added. “My view of the world is, if you’re not at the table, you’re on the menu.”
LAS VEGAS – Alla Zilbering, MD, sat at attention for hours during HM17, jotting notes like a scribe about the myriad of federal rules that are pretty rapidly pushing hospitalists and health care as a whole away from fee-for-service payments to a world where doctors are paid for quality.
So, why did she do it? Why all that time on policy, instead of practice?
Because Dr. Zilbering felt compelled to get more involved. As a lead hospitalist at Cigna-HealthSpring, a Medicare Advantage program in Philadelphia, she’s already part of initiatives to improve transitions of care and reduce readmissions.
However, she said she wants to do more. “I’m feeling like, unless you actually address the policy, you can’t get that far in terms of what you can physically do with a patient.”
HM17 was the meeting for her, then. SHM, this year, unveiled its first Health Policy Mini Track, dedicated to updating attendees on the implementation of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), the Bundled Payments for Care Improvement initiative, and a host of other federal programs. Hospitalists were updated on a litany of advocacy efforts, including observation status, interoperability of electronic health records systems, and the recent launch of the first hospitalist billing code.
Two of the meeting’s three keynote speakers were Washington veterans who confirmed that, while nightly news reports may suggest that health care reforms contained in the Affordable Care Act are constantly in flux, the trajectory toward paying for higher quality care at lower costs shows no signs of abating.
Plenary speaker Patrick Conway, MD, MSc, MHM, deputy administrator for innovation and quality at the Centers for Medicare & Medicaid Services and director of its Center for Medicare and Medicaid Innovation, noted that the proposed American Health Care Act doesn’t have a “single word dealing with the Innovation Center,” which is the government agency tasked with supporting the development and testing of new payment and service delivery models.
He added that the policy’s gravitation away from fee-for-service toward alternative payment models will ideally lead to better patient outcomes, more coordinated care, and financial savings. So, he urged hospitalists to continue to help design those new payment and care-delivery systems.
M.A. Williams, MD, FHM, the medical director of perioperative services at Porter Adventist Hospital in Denver, said that the way to help design those systems is to get involved. Policy may seem like an issue for C-suite denizens and wonks, but individual practitioners can make more impact than they think.
“Learn enough to be dangerous and go to your CMO [or] whoever you can get a meeting with because MACRA is going to effect all physicians in the organization, even if the system is not doing anything active about it,” Dr. Williams said. “If you show interest and show that you have a little bit of knowledge, you’d be surprised with what kind of traction you might be able to get.”
And that traction isn’t just within the walls of a given institution, Dr. Greeno said. He wants more hospitalists involved in the society’s overall advocacy efforts. That includes lobbying Congress both in person and with phone calls, letters, and emails and pressuring people at home via conduits like SHM’s Grassroots Network, which has nearly 1,200 members from 490 states.
Don’t think those things work? Dr. Greeno said, one need look no further than the new C6 Medicare billing code for hospitalists that went live in April. That didn’t come to pass without a concentrated effort.
“That was a ton of work by our staff and several years of lobbying,” he said. “We had to be able to explain to them why our data should be treated differently as a specialty and compared only to other hospitalists as opposed to other internists or family practitioners.”
The code will help differentiate hospitalists at a time when MACRA will force changes in how hospitalists are paid. But, it will also define the specialty in a way that has never before been accomplished.
“It is an identity within Medicare,” said Josh Boswell, SHM’s director of government affairs.
While the ACA and the potential repeal of its insurance reforms have taken center stage in the media, Dr. Greeno urged hospitalists to focus more on the implementation and rule-making via MACRA.
The bill, which eliminated the Sustainable Growth Rate formula, states that, starting in 2019, Medicare payments will be provided through one of two pathways. The first is the Merit-based Incentive Payment System that combines the Physician Quality Reporting System, the Physician Value-Based Modifier, and Meaningful Use into a single performance-based payment system.
The second option is Alternative Payment Models, which is meant to incentivize the adoption of payment models that move physicians away from fee-for-service models more quickly. To qualify in this pathway, the criteria require elements of “upside and downside financial risk,” as well as meeting threshold requirements for either patients or payments. Those physicians that meet the criteria qualify for a 5% incentive payment.
The first payments in 2019 are based on performance data for 2017. As most hospitalists won’t quality for APMs in the first year, they will default to the MIPS pathway, Dr. Greeno said.
“This bill will have a greater impact on ... providers than any piece of legislation in our lifetime,” he noted. “Now, the ACA had a bigger impact on consumers, but, in terms of us as providers, MACRA is a sea change.”
The topic is so important, SHM has created a website at www.macraforhm.org that is meant to serve as a tutorial to the law’s basics. The guide is intended to educate hospitalists and to motivate them to get involved in the policy work that affects them all, Dr. Greeno said
“If you don’t know how the system works, you can’t influence it,” he added. “My view of the world is, if you’re not at the table, you’re on the menu.”
Examining How the Body Responds to Ebola
“Unprecedented detail” was observed about how a patient’s clinical condition changes in response to Ebola virus disease and treatment. That’s what NIH researchers who analyzed daily gene activation found in a 26-day study of 1 patient.
The patient, who was admitted to the NIH Clinical Center on day 7 of illness, received intensive supportive care, including fluids and electrolytes, but did not receive any experimental Ebola drugs. The researchers took blood samples daily to measure the rise and decline of virus replication and to track the timing, intensity, and duration of expression of numerous immune system genes. They correlated changes in gene expression with subsequent alterations in the patient’s clinical condition, such as development and resolution of blood-clotting dysfunction.
The researchers pinpointed “key transition points” in the response to infection, NIH says. For example, they observed a marked decline in antiviral responses that correlated with clearance of virus from white blood cells. The researchers also found that most host responses shifted rapidly from activating genes involved in cell damage and inflammation toward those linked to promotion of cellular and organ repair—a “pivot” that came before the patient began showing signs of clinical improvement.
Although the study centered on only 1 patient, the researchers say it may help inform the development of treatments designed to boost or accelerate host factors that counter the virus and promote healing.
“Unprecedented detail” was observed about how a patient’s clinical condition changes in response to Ebola virus disease and treatment. That’s what NIH researchers who analyzed daily gene activation found in a 26-day study of 1 patient.
The patient, who was admitted to the NIH Clinical Center on day 7 of illness, received intensive supportive care, including fluids and electrolytes, but did not receive any experimental Ebola drugs. The researchers took blood samples daily to measure the rise and decline of virus replication and to track the timing, intensity, and duration of expression of numerous immune system genes. They correlated changes in gene expression with subsequent alterations in the patient’s clinical condition, such as development and resolution of blood-clotting dysfunction.
The researchers pinpointed “key transition points” in the response to infection, NIH says. For example, they observed a marked decline in antiviral responses that correlated with clearance of virus from white blood cells. The researchers also found that most host responses shifted rapidly from activating genes involved in cell damage and inflammation toward those linked to promotion of cellular and organ repair—a “pivot” that came before the patient began showing signs of clinical improvement.
Although the study centered on only 1 patient, the researchers say it may help inform the development of treatments designed to boost or accelerate host factors that counter the virus and promote healing.
“Unprecedented detail” was observed about how a patient’s clinical condition changes in response to Ebola virus disease and treatment. That’s what NIH researchers who analyzed daily gene activation found in a 26-day study of 1 patient.
The patient, who was admitted to the NIH Clinical Center on day 7 of illness, received intensive supportive care, including fluids and electrolytes, but did not receive any experimental Ebola drugs. The researchers took blood samples daily to measure the rise and decline of virus replication and to track the timing, intensity, and duration of expression of numerous immune system genes. They correlated changes in gene expression with subsequent alterations in the patient’s clinical condition, such as development and resolution of blood-clotting dysfunction.
The researchers pinpointed “key transition points” in the response to infection, NIH says. For example, they observed a marked decline in antiviral responses that correlated with clearance of virus from white blood cells. The researchers also found that most host responses shifted rapidly from activating genes involved in cell damage and inflammation toward those linked to promotion of cellular and organ repair—a “pivot” that came before the patient began showing signs of clinical improvement.
Although the study centered on only 1 patient, the researchers say it may help inform the development of treatments designed to boost or accelerate host factors that counter the virus and promote healing.
A Rare Case Among Rare Cancer Cases
Cardiac tumors are rare and usually benign. An exception is the primary cardiac schwannoma. Only 17 cases have been reported, and most of those were malignant, say a team of doctors from McMaster University and Hamilton General Hospital, both in Ontario, Canada. Schwannomas, composed of Schwann cells, are tumors of nerve sheaths commonly found in cranial and peripheral nerves. The clinicians’ account of an “interesting case” of a patient with a benign schwannoma was unusual—and the fact that it was in the heart makes it “exceedingly rare.”
Their patient, a 47-year-old woman who had been treated for ovarian cancer, had no symptoms of a cardiac schwannoma. During the workup for the ovarian cancer, the clinicians discovered a large mass between the right atrium and right ventricle. Because she was asymptomatic, her physicians decided to closely monitor the tumor’s growth.
The chemotherapy for ovarian cancer did not reduce the cardiac schwannoma. As the disease progressed and the tumor grew, the patient developed symptoms, including sharp chest pain, shortness of breath, and palpitations.
Related: Rare Cancer Gets Timely Right Treatment
The prognosis of benign cardiac tumor depends on resectability, the clinicians say. After complete resection the prognosis is excellent, and adjuvant therapy is not needed. If surgery is not indicated, chemotherapy is an option. The patient chose to have surgery to remove the tumor. During the operation, the surgeons found that the blood supply of the tumor came from a branch of the right coronary artery and the posterior descending artery (PDA).
The surgery, which included resection, ligation of the PDA , and a saphenous vein graft for a coronary bypass to the distal PDA, was successful. A definite diagnosis of cardiac tumors can be completed only after histologic examination of samples taken at autopsy or surgical resection, the clinicians note. The gross and microscopic evidence confirmed diagnosis of benign schwannoma of the heart.
Related: In Rare Case Colorectal Cancer Causes Thrombus
Source:
Koujanian S, Pawlowicz B, Landry D, Alexopoulou I, Nair V. Hum Pathol. 2017;(8):24-26
Cardiac tumors are rare and usually benign. An exception is the primary cardiac schwannoma. Only 17 cases have been reported, and most of those were malignant, say a team of doctors from McMaster University and Hamilton General Hospital, both in Ontario, Canada. Schwannomas, composed of Schwann cells, are tumors of nerve sheaths commonly found in cranial and peripheral nerves. The clinicians’ account of an “interesting case” of a patient with a benign schwannoma was unusual—and the fact that it was in the heart makes it “exceedingly rare.”
Their patient, a 47-year-old woman who had been treated for ovarian cancer, had no symptoms of a cardiac schwannoma. During the workup for the ovarian cancer, the clinicians discovered a large mass between the right atrium and right ventricle. Because she was asymptomatic, her physicians decided to closely monitor the tumor’s growth.
The chemotherapy for ovarian cancer did not reduce the cardiac schwannoma. As the disease progressed and the tumor grew, the patient developed symptoms, including sharp chest pain, shortness of breath, and palpitations.
Related: Rare Cancer Gets Timely Right Treatment
The prognosis of benign cardiac tumor depends on resectability, the clinicians say. After complete resection the prognosis is excellent, and adjuvant therapy is not needed. If surgery is not indicated, chemotherapy is an option. The patient chose to have surgery to remove the tumor. During the operation, the surgeons found that the blood supply of the tumor came from a branch of the right coronary artery and the posterior descending artery (PDA).
The surgery, which included resection, ligation of the PDA , and a saphenous vein graft for a coronary bypass to the distal PDA, was successful. A definite diagnosis of cardiac tumors can be completed only after histologic examination of samples taken at autopsy or surgical resection, the clinicians note. The gross and microscopic evidence confirmed diagnosis of benign schwannoma of the heart.
Related: In Rare Case Colorectal Cancer Causes Thrombus
Source:
Koujanian S, Pawlowicz B, Landry D, Alexopoulou I, Nair V. Hum Pathol. 2017;(8):24-26
Cardiac tumors are rare and usually benign. An exception is the primary cardiac schwannoma. Only 17 cases have been reported, and most of those were malignant, say a team of doctors from McMaster University and Hamilton General Hospital, both in Ontario, Canada. Schwannomas, composed of Schwann cells, are tumors of nerve sheaths commonly found in cranial and peripheral nerves. The clinicians’ account of an “interesting case” of a patient with a benign schwannoma was unusual—and the fact that it was in the heart makes it “exceedingly rare.”
Their patient, a 47-year-old woman who had been treated for ovarian cancer, had no symptoms of a cardiac schwannoma. During the workup for the ovarian cancer, the clinicians discovered a large mass between the right atrium and right ventricle. Because she was asymptomatic, her physicians decided to closely monitor the tumor’s growth.
The chemotherapy for ovarian cancer did not reduce the cardiac schwannoma. As the disease progressed and the tumor grew, the patient developed symptoms, including sharp chest pain, shortness of breath, and palpitations.
Related: Rare Cancer Gets Timely Right Treatment
The prognosis of benign cardiac tumor depends on resectability, the clinicians say. After complete resection the prognosis is excellent, and adjuvant therapy is not needed. If surgery is not indicated, chemotherapy is an option. The patient chose to have surgery to remove the tumor. During the operation, the surgeons found that the blood supply of the tumor came from a branch of the right coronary artery and the posterior descending artery (PDA).
The surgery, which included resection, ligation of the PDA , and a saphenous vein graft for a coronary bypass to the distal PDA, was successful. A definite diagnosis of cardiac tumors can be completed only after histologic examination of samples taken at autopsy or surgical resection, the clinicians note. The gross and microscopic evidence confirmed diagnosis of benign schwannoma of the heart.
Related: In Rare Case Colorectal Cancer Causes Thrombus
Source:
Koujanian S, Pawlowicz B, Landry D, Alexopoulou I, Nair V. Hum Pathol. 2017;(8):24-26
Group creates ‘authentic’ HSCs from endothelial cells
Researchers say they have found a way to convert adult mouse endothelial cells into “authentic” hematopoietic stem cells (HSCs).
The team says these HSCs have a transcriptome and long-term self-renewal capacity that are similar to those of adult HSCs that are produced naturally.
In addition, the lab-generated HSCs were capable of engraftment and multi-lineage reconstitution in mice.
“This is a game-changing breakthrough that brings us closer not only to treat blood disorders, but also to deciphering the complex biology of stem-cell self-renewal machinery,” said Shahin Rafii, MD, of Weill Cornell Medicine in New York, New York.
“This is exciting because it provides us with a path towards generating clinically useful quantities of normal stem cells for transplantation that may help us cure patients with genetic and acquired blood diseases,” added Joseph Scandura, MD, PhD, also of Weill Cornell Medicine.
Drs Scandura and Rafii and their colleagues described this research in Nature.
The researchers took vascular endothelial cells from adult mice and induced expression of the transcription-factor-encoding genes Fosb, Gfi1, Runx1, and Spi1.
The cells were grown and multiplied in co-culture with an engineered vascular niche.
This produced HSCs that were transplanted into irradiated mice.
The researchers said these HSCs were capable of long-term engraftment and hematopoietic reconstitution of myelopoiesis and both innate and adaptive immune function.
In addition, the HSCs were endowed with the same genetic attributes as normal adult HSCs.
If this method of generating HSCs in the lab can be scaled up and applied to humans, it could have wide-ranging clinical implications, according to the researchers.
“It might allow us to provide healthy stem cells to patients who need bone marrow donors but have no genetic match,” Dr Scandura said. “It could lead to new ways to cure leukemia and may help us correct genetic defects that cause blood diseases like sickle cell anemia.”
“More importantly, our vascular niche stem cell expansion model may be employed to clone the key unknown growth factors produced by this niche that are essential for self-perpetuation of stem cells,” Dr Rafii said. “Identification of those factors could be important for unraveling the secrets of stem cells’ longevity and translating the potential of stem cell therapy to the clinical setting.” ![]()
Researchers say they have found a way to convert adult mouse endothelial cells into “authentic” hematopoietic stem cells (HSCs).
The team says these HSCs have a transcriptome and long-term self-renewal capacity that are similar to those of adult HSCs that are produced naturally.
In addition, the lab-generated HSCs were capable of engraftment and multi-lineage reconstitution in mice.
“This is a game-changing breakthrough that brings us closer not only to treat blood disorders, but also to deciphering the complex biology of stem-cell self-renewal machinery,” said Shahin Rafii, MD, of Weill Cornell Medicine in New York, New York.
“This is exciting because it provides us with a path towards generating clinically useful quantities of normal stem cells for transplantation that may help us cure patients with genetic and acquired blood diseases,” added Joseph Scandura, MD, PhD, also of Weill Cornell Medicine.
Drs Scandura and Rafii and their colleagues described this research in Nature.
The researchers took vascular endothelial cells from adult mice and induced expression of the transcription-factor-encoding genes Fosb, Gfi1, Runx1, and Spi1.
The cells were grown and multiplied in co-culture with an engineered vascular niche.
This produced HSCs that were transplanted into irradiated mice.
The researchers said these HSCs were capable of long-term engraftment and hematopoietic reconstitution of myelopoiesis and both innate and adaptive immune function.
In addition, the HSCs were endowed with the same genetic attributes as normal adult HSCs.
If this method of generating HSCs in the lab can be scaled up and applied to humans, it could have wide-ranging clinical implications, according to the researchers.
“It might allow us to provide healthy stem cells to patients who need bone marrow donors but have no genetic match,” Dr Scandura said. “It could lead to new ways to cure leukemia and may help us correct genetic defects that cause blood diseases like sickle cell anemia.”
“More importantly, our vascular niche stem cell expansion model may be employed to clone the key unknown growth factors produced by this niche that are essential for self-perpetuation of stem cells,” Dr Rafii said. “Identification of those factors could be important for unraveling the secrets of stem cells’ longevity and translating the potential of stem cell therapy to the clinical setting.” ![]()
Researchers say they have found a way to convert adult mouse endothelial cells into “authentic” hematopoietic stem cells (HSCs).
The team says these HSCs have a transcriptome and long-term self-renewal capacity that are similar to those of adult HSCs that are produced naturally.
In addition, the lab-generated HSCs were capable of engraftment and multi-lineage reconstitution in mice.
“This is a game-changing breakthrough that brings us closer not only to treat blood disorders, but also to deciphering the complex biology of stem-cell self-renewal machinery,” said Shahin Rafii, MD, of Weill Cornell Medicine in New York, New York.
“This is exciting because it provides us with a path towards generating clinically useful quantities of normal stem cells for transplantation that may help us cure patients with genetic and acquired blood diseases,” added Joseph Scandura, MD, PhD, also of Weill Cornell Medicine.
Drs Scandura and Rafii and their colleagues described this research in Nature.
The researchers took vascular endothelial cells from adult mice and induced expression of the transcription-factor-encoding genes Fosb, Gfi1, Runx1, and Spi1.
The cells were grown and multiplied in co-culture with an engineered vascular niche.
This produced HSCs that were transplanted into irradiated mice.
The researchers said these HSCs were capable of long-term engraftment and hematopoietic reconstitution of myelopoiesis and both innate and adaptive immune function.
In addition, the HSCs were endowed with the same genetic attributes as normal adult HSCs.
If this method of generating HSCs in the lab can be scaled up and applied to humans, it could have wide-ranging clinical implications, according to the researchers.
“It might allow us to provide healthy stem cells to patients who need bone marrow donors but have no genetic match,” Dr Scandura said. “It could lead to new ways to cure leukemia and may help us correct genetic defects that cause blood diseases like sickle cell anemia.”
“More importantly, our vascular niche stem cell expansion model may be employed to clone the key unknown growth factors produced by this niche that are essential for self-perpetuation of stem cells,” Dr Rafii said. “Identification of those factors could be important for unraveling the secrets of stem cells’ longevity and translating the potential of stem cell therapy to the clinical setting.” ![]()