User login
Chemical could aid malaria control

Photo courtesy of the CDC
A chemical that disrupts hormone signaling in mosquitoes may reduce their ability to transmit malaria, according to a study published in PLOS Pathogens.
The findings suggest a potential new approach to combat spread of the disease.
“As insecticide resistance is spreading, new intervention methods to control mosquitoes are urgently needed,” said study author Flaminia Catteruccia, PhD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Our study provides a new strategy based on the use of a non-toxic compound that prevents transmission of malaria parasites without killing the mosquito.”
Dr Catteruccia and her colleagues treated adult female Anopheles gambiae mosquitoes with a chemical known as dibenzoylhydrazine (DBH) to see how it would impact their biological processes.
DBH mimics the action of the steroid hormone 20-hydroxyecdysone, which plays a key role in the reproductive cycle of the female mosquito.
The researchers found various aspects of the mosquitoes’ life cycle to be disrupted after treatment with DBH.
DBH-treated mosquitoes produced and laid fewer eggs, didn’t mate successfully, and died more rapidly than non-treated mosquitoes. The effects were greater the higher the DBH dose.
And DBH-treated mosquitoes were less likely to be infected by malaria parasites.
To further explore the potential of hormone targeting as a malaria control tactic, the researchers fed their experimental results into a mathematical model of the mosquito life cycle.
The results suggest that applying DBH to bed nets or spraying it indoors could potentially reduce malaria transmission as effectively as insecticides.
The researchers noted that DBH compounds are not toxic to mammals, which would make them ideally suited for use in bed nets, where low toxicity is essential. ![]()

Photo courtesy of the CDC
A chemical that disrupts hormone signaling in mosquitoes may reduce their ability to transmit malaria, according to a study published in PLOS Pathogens.
The findings suggest a potential new approach to combat spread of the disease.
“As insecticide resistance is spreading, new intervention methods to control mosquitoes are urgently needed,” said study author Flaminia Catteruccia, PhD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Our study provides a new strategy based on the use of a non-toxic compound that prevents transmission of malaria parasites without killing the mosquito.”
Dr Catteruccia and her colleagues treated adult female Anopheles gambiae mosquitoes with a chemical known as dibenzoylhydrazine (DBH) to see how it would impact their biological processes.
DBH mimics the action of the steroid hormone 20-hydroxyecdysone, which plays a key role in the reproductive cycle of the female mosquito.
The researchers found various aspects of the mosquitoes’ life cycle to be disrupted after treatment with DBH.
DBH-treated mosquitoes produced and laid fewer eggs, didn’t mate successfully, and died more rapidly than non-treated mosquitoes. The effects were greater the higher the DBH dose.
And DBH-treated mosquitoes were less likely to be infected by malaria parasites.
To further explore the potential of hormone targeting as a malaria control tactic, the researchers fed their experimental results into a mathematical model of the mosquito life cycle.
The results suggest that applying DBH to bed nets or spraying it indoors could potentially reduce malaria transmission as effectively as insecticides.
The researchers noted that DBH compounds are not toxic to mammals, which would make them ideally suited for use in bed nets, where low toxicity is essential. ![]()

Photo courtesy of the CDC
A chemical that disrupts hormone signaling in mosquitoes may reduce their ability to transmit malaria, according to a study published in PLOS Pathogens.
The findings suggest a potential new approach to combat spread of the disease.
“As insecticide resistance is spreading, new intervention methods to control mosquitoes are urgently needed,” said study author Flaminia Catteruccia, PhD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Our study provides a new strategy based on the use of a non-toxic compound that prevents transmission of malaria parasites without killing the mosquito.”
Dr Catteruccia and her colleagues treated adult female Anopheles gambiae mosquitoes with a chemical known as dibenzoylhydrazine (DBH) to see how it would impact their biological processes.
DBH mimics the action of the steroid hormone 20-hydroxyecdysone, which plays a key role in the reproductive cycle of the female mosquito.
The researchers found various aspects of the mosquitoes’ life cycle to be disrupted after treatment with DBH.
DBH-treated mosquitoes produced and laid fewer eggs, didn’t mate successfully, and died more rapidly than non-treated mosquitoes. The effects were greater the higher the DBH dose.
And DBH-treated mosquitoes were less likely to be infected by malaria parasites.
To further explore the potential of hormone targeting as a malaria control tactic, the researchers fed their experimental results into a mathematical model of the mosquito life cycle.
The results suggest that applying DBH to bed nets or spraying it indoors could potentially reduce malaria transmission as effectively as insecticides.
The researchers noted that DBH compounds are not toxic to mammals, which would make them ideally suited for use in bed nets, where low toxicity is essential. ![]()
Congenital CMV linked to increased risk of ALL

Photo by Vera Kratochvil
Newborns with congenital cytomegalovirus (CMV) infection may have an increased risk of developing acute lymphoblastic leukemia (ALL), according to a study published in Blood.
The data also suggest the risk may be particularly high among Hispanic children.
Researchers said this is the first study to suggest that congenital CMV infection is a risk factor for childhood ALL and is more prominent in Hispanic children.
To conduct this study, the researchers first identified all known infections present in the bone marrow of 127 children diagnosed with ALL and 38 children diagnosed with acute myeloid leukemia (AML).
The team found CMV infection was prevalent in children with ALL but rare in those with AML.
Next, the researchers looked for CMV in newborn blood samples from 268 children who went on to develop ALL. The team compared the samples with samples from 270 healthy children.
“Our goal in tracking CMV back from the time of diagnosis to the womb was to establish that this infection occurred well before initiation of disease,” said lead study author Stephen Francis, PhD, of the University of Nevada and University of California, San Francisco.
He and his colleagues found that children who went on to develop ALL were nearly 4 times more likely than control subjects to be CMV-positive at birth. The odds ratio was 3.71 (P=0.0016).
The odds ratio was 5.9 in Hispanic children and 2.1 in non-Hispanic whites. The researchers said this finding is particularly interesting because of the high rate of ALL observed in Hispanics.
“If it’s true that in utero CMV is one of the initiating events in the development of childhood leukemia, then control of the virus has the potential to be a prevention target,” Dr Francis said. “That’s the real take-home message.”
While this research is in the early stages, the researchers hope these results will inspire more studies that will validate these findings and lead to the development of a CMV vaccine.
“This is the first step, but if we do end up finding a causal link to the most common childhood cancer, we hope that will light a fire in terms of stopping mother-to-child transmission of CMV,” Dr Francis said. ![]()

Photo by Vera Kratochvil
Newborns with congenital cytomegalovirus (CMV) infection may have an increased risk of developing acute lymphoblastic leukemia (ALL), according to a study published in Blood.
The data also suggest the risk may be particularly high among Hispanic children.
Researchers said this is the first study to suggest that congenital CMV infection is a risk factor for childhood ALL and is more prominent in Hispanic children.
To conduct this study, the researchers first identified all known infections present in the bone marrow of 127 children diagnosed with ALL and 38 children diagnosed with acute myeloid leukemia (AML).
The team found CMV infection was prevalent in children with ALL but rare in those with AML.
Next, the researchers looked for CMV in newborn blood samples from 268 children who went on to develop ALL. The team compared the samples with samples from 270 healthy children.
“Our goal in tracking CMV back from the time of diagnosis to the womb was to establish that this infection occurred well before initiation of disease,” said lead study author Stephen Francis, PhD, of the University of Nevada and University of California, San Francisco.
He and his colleagues found that children who went on to develop ALL were nearly 4 times more likely than control subjects to be CMV-positive at birth. The odds ratio was 3.71 (P=0.0016).
The odds ratio was 5.9 in Hispanic children and 2.1 in non-Hispanic whites. The researchers said this finding is particularly interesting because of the high rate of ALL observed in Hispanics.
“If it’s true that in utero CMV is one of the initiating events in the development of childhood leukemia, then control of the virus has the potential to be a prevention target,” Dr Francis said. “That’s the real take-home message.”
While this research is in the early stages, the researchers hope these results will inspire more studies that will validate these findings and lead to the development of a CMV vaccine.
“This is the first step, but if we do end up finding a causal link to the most common childhood cancer, we hope that will light a fire in terms of stopping mother-to-child transmission of CMV,” Dr Francis said. ![]()

Photo by Vera Kratochvil
Newborns with congenital cytomegalovirus (CMV) infection may have an increased risk of developing acute lymphoblastic leukemia (ALL), according to a study published in Blood.
The data also suggest the risk may be particularly high among Hispanic children.
Researchers said this is the first study to suggest that congenital CMV infection is a risk factor for childhood ALL and is more prominent in Hispanic children.
To conduct this study, the researchers first identified all known infections present in the bone marrow of 127 children diagnosed with ALL and 38 children diagnosed with acute myeloid leukemia (AML).
The team found CMV infection was prevalent in children with ALL but rare in those with AML.
Next, the researchers looked for CMV in newborn blood samples from 268 children who went on to develop ALL. The team compared the samples with samples from 270 healthy children.
“Our goal in tracking CMV back from the time of diagnosis to the womb was to establish that this infection occurred well before initiation of disease,” said lead study author Stephen Francis, PhD, of the University of Nevada and University of California, San Francisco.
He and his colleagues found that children who went on to develop ALL were nearly 4 times more likely than control subjects to be CMV-positive at birth. The odds ratio was 3.71 (P=0.0016).
The odds ratio was 5.9 in Hispanic children and 2.1 in non-Hispanic whites. The researchers said this finding is particularly interesting because of the high rate of ALL observed in Hispanics.
“If it’s true that in utero CMV is one of the initiating events in the development of childhood leukemia, then control of the virus has the potential to be a prevention target,” Dr Francis said. “That’s the real take-home message.”
While this research is in the early stages, the researchers hope these results will inspire more studies that will validate these findings and lead to the development of a CMV vaccine.
“This is the first step, but if we do end up finding a causal link to the most common childhood cancer, we hope that will light a fire in terms of stopping mother-to-child transmission of CMV,” Dr Francis said. ![]()
How to Categorize Pediatric Asthma
Does Chronic Complaining Mask Acute Problem?
ANSWER
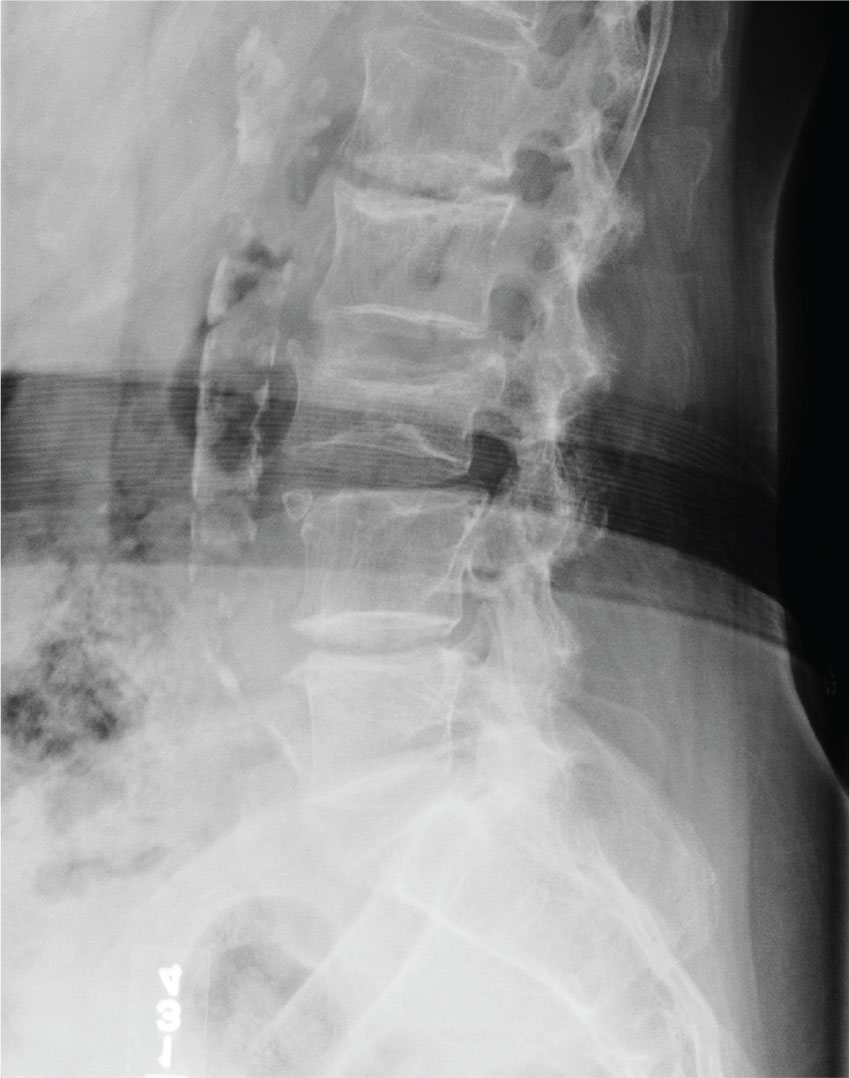
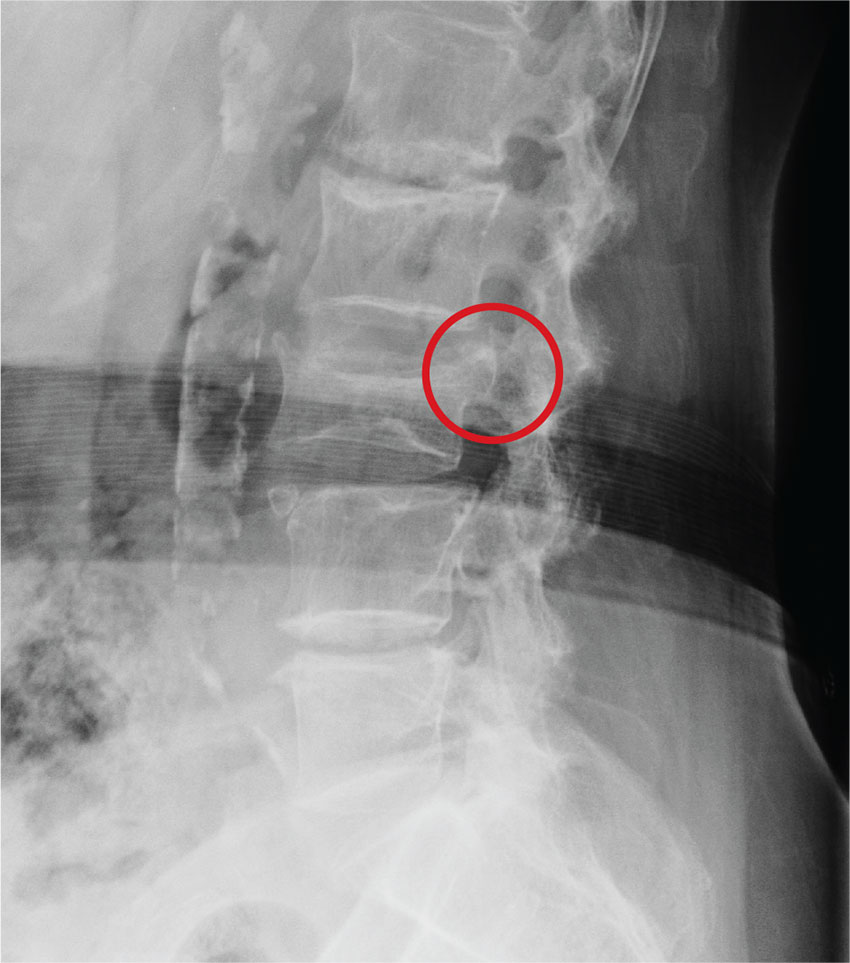
The radiograph demonstrates age-related degenerative changes. Of note is a moderate compression fracture of L3 with close to 50% loss of height. It is difficult to say for sure the fracture is acute; by plain film alone, it would be deemed age indeterminate.
Of concern, though, is the posterior portion of the superior endplate of L3, which appears to be posteriorly displaced into the spinal canal. This finding suggests possible retropulsion and, if this fracture was in fact acute, would suggest a possible unstable fracture. Further imaging is warranted.
The patient underwent noncontrast CT of the lumbar spine, which demonstrated that the fracture was acute. It also indicated that there was dorsal retropulsion causing close to 50% canal compromise. The patient was subsequently admitted for further workup and evaluation.

ANSWER
The radiograph demonstrates age-related degenerative changes. Of note is a moderate compression fracture of L3 with close to 50% loss of height. It is difficult to say for sure the fracture is acute; by plain film alone, it would be deemed age indeterminate.
Of concern, though, is the posterior portion of the superior endplate of L3, which appears to be posteriorly displaced into the spinal canal. This finding suggests possible retropulsion and, if this fracture was in fact acute, would suggest a possible unstable fracture. Further imaging is warranted.
The patient underwent noncontrast CT of the lumbar spine, which demonstrated that the fracture was acute. It also indicated that there was dorsal retropulsion causing close to 50% canal compromise. The patient was subsequently admitted for further workup and evaluation.

ANSWER
The radiograph demonstrates age-related degenerative changes. Of note is a moderate compression fracture of L3 with close to 50% loss of height. It is difficult to say for sure the fracture is acute; by plain film alone, it would be deemed age indeterminate.
Of concern, though, is the posterior portion of the superior endplate of L3, which appears to be posteriorly displaced into the spinal canal. This finding suggests possible retropulsion and, if this fracture was in fact acute, would suggest a possible unstable fracture. Further imaging is warranted.
The patient underwent noncontrast CT of the lumbar spine, which demonstrated that the fracture was acute. It also indicated that there was dorsal retropulsion causing close to 50% canal compromise. The patient was subsequently admitted for further workup and evaluation.
An 80-year-old woman who resides in a nursing home is sent to urgent care for evaluation of back pain. There have been no witnessed injuries or falls. The notes from the nursing home staff indicate that the patient, who has severe dementia, tends to chronically complain about one ailment or another. However, the patient’s family seems to feel she is complaining more than usual.
Her only other medical history is controlled hypertension. Her vital signs are stable. On physical exam, you note an elderly female who is pleasant but very confused. She is able to follow simple commands and appears to be able to move all her extremities well, with no obvious neurologic compromise. Inspection of her back does not demonstrate any obvious wounds, bruising, or step-offs. She does have generalized tenderness as you palpate her lumbar region.
The triage nurse already ordered lumbar spine radiographs, which have been completed. The lateral view is shown. What is your impression?
PIK3 inhibitor gives slight PFS edge at high cost for HR+/HER2– advanced breast cancer
SAN ANTONIO – A combination of a PI3K inhibitor and selective estrogen receptor down-regulator (SERD) met its primary endpoint of 2.1 months better progression-free survival (PFS) in postmenopausal women with locally advanced or metastatic breast cancer who were quickly running out of other treatment options.
Yet the small gain in PFS came at a very high price in terms of toxicities, including mood disorders that may have led to patient suicide attempts, according to investigators.
The BELLE-3 trial looked at the combination of the SERD fulvestrant (Faslodex) and an experimental inhibitor of the PI3 kinase, buparlisib, in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor–2 (HER2)-negative breast cancer treated with an aromatase inhibitor (AI) who experienced disease progression either on or after receiving therapy with an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1).
The combination of fulvestrant and buparlisib was associated with a median PFS of 3.9 months, compared with 1.8 months for fulvestrant and placebo (P less than .001), Angelo Di Leo, MD, of Ospedale Misericordia e Dolce in Prato, Italy, reported at the San Antonio Breast Cancer Symposium,
Objective response rates (ORR) were low, at 7.8% in the combination arm, and 2.1% in the fulvestrant-plus-placebo arm.
Although the PFS difference was statistically significant, “the higher rate of toxicity in patients receiving buparlisib and fulvestrant, including transaminase elevations and mood disorders, may represent a clinically relevant challenge for future development of this compound in this particular group of patients,” Dr. Di Leo said.
Blocks AKT pathway
The preclinical rationale for the use of a P13K inhibitor after disease progression on mTORC1 inhibitor is that current mTOR inhibitors such as everolimus have a feedback mechanism that activates the AKT pathway, and that the use of P13K inhibitors can “abrogate or attenuate this activation, potentially blocking that pathway,” explained coinvestigator Ruth O’Regan, MD, head of the division of hematology and oncology at the University of Wisconsin–Madison School of Medicine and Public Health. Dr. O’Regan discussed the BELLE-3 findings in a briefing prior to Dr. Di Leo’s presentation of the data in general session.
In BELLE-3, 432 postmenopausal women with HR+/HER2-, AI-pretreated, locally advanced or metastatic breast cancer that had progressed on or after treatment with an mTOR inhibitor as the last line of therapy were enrolled. The patients were stratified by the presence or absence of visceral disease and then randomized on a 2:1 basis to fulvestrant 500 mg daily plus either buparlisib 100 mg/day (289 patients), or placebo (143).
As noted, the primary endpoint of investigator-assessed PFS favored the addition of buparlisib, with a hazard ratio for progression of 0.67 (P less than .001). PFS results by independent central review were similar (HR 0.57, P less than .001).
The ORR for the buparlisib/fulvestrant combination, 7.6%, consisted of 0.3% complete responses, and 7.3% partial responses. The ORR for placebo/fulvestrant, 2.1%, was composed entirely of partial responses. The respective clinical benefit rates, defined as a combination of complete and partial responses and stable disease, were 24.6% and 15.4.
The benefit of buparlisib was evidently entirely among patients with visceral disease, with a PFS of 3.1 vs. 1.5 months. In contrast, PFS among patients with no visceral disease was 4.2 vs. 4.1 months, respectively, and was not significant.
In addition, the P13K inhibitor seemed to benefit patients with PIK3CA mutations detected in either the primary tumor or in circulating DNA samples, but not patients with wild-type PIK3CA.
Depression, anxiety with combination
Patients assigned to buparlisib/fulvestrant had substantially higher proportions of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations compared with patients on placebo fulvestrant, as well as more reported depression and anxiety. Three patients in the buparlisib arm attempted suicide. There were no reported suicide attempts in the placebo arm.
Dr. O’Regan said at the briefing that mood disorders are known adverse events associated with buparlisib, and that patients with psychiatric disorders were excluded from the trial.
Carlos Arteaga, MD, co-leader of the Breast Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., who moderated the briefing, said in an interview that PI kinase mutations do not appear to be a good target, and that a pan-PIK3 inhibitor such as buparlisib may be hitting too many targets at once.
Inhibiting all of the PIK3 isoforms – alpha, beta, gamma, and delta – “may be a little too tough,” he said.
The trial should serve as impetus for developing agents that inhibit only the alpha isoform of PIK3 which mutates and appears to be the driver of some types of breast cancer, he said.
Following Dr. Di Leo’s presentation, perennial SABCS gadfly Steven Vogl, MD, New York, told the speaker that “you presented this very nicely, it’s very interesting biology. I don’t think you’d want to do this again to a human, right? Three month prolongation in PFS, miserable, with diarrhea, depression – you don’t want to do this again, is that correct?”
“I think buparlisib is probably not the best compound to be used in this particular setting of patients,” Dr. Di Leo said, but added that a better-tolerated PI3K inhibitor might be more effective.
“Actually, the study is raising an important question: Should we use the PI3K inhibitor in place of the mTOR inhibitor, or perhaps, as the study suggests, should we use the P13K inhibitors sequentially, after the mTOR inhibitor. This is an open question,” he replied.
Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
SAN ANTONIO – A combination of a PI3K inhibitor and selective estrogen receptor down-regulator (SERD) met its primary endpoint of 2.1 months better progression-free survival (PFS) in postmenopausal women with locally advanced or metastatic breast cancer who were quickly running out of other treatment options.
Yet the small gain in PFS came at a very high price in terms of toxicities, including mood disorders that may have led to patient suicide attempts, according to investigators.
The BELLE-3 trial looked at the combination of the SERD fulvestrant (Faslodex) and an experimental inhibitor of the PI3 kinase, buparlisib, in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor–2 (HER2)-negative breast cancer treated with an aromatase inhibitor (AI) who experienced disease progression either on or after receiving therapy with an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1).
The combination of fulvestrant and buparlisib was associated with a median PFS of 3.9 months, compared with 1.8 months for fulvestrant and placebo (P less than .001), Angelo Di Leo, MD, of Ospedale Misericordia e Dolce in Prato, Italy, reported at the San Antonio Breast Cancer Symposium,
Objective response rates (ORR) were low, at 7.8% in the combination arm, and 2.1% in the fulvestrant-plus-placebo arm.
Although the PFS difference was statistically significant, “the higher rate of toxicity in patients receiving buparlisib and fulvestrant, including transaminase elevations and mood disorders, may represent a clinically relevant challenge for future development of this compound in this particular group of patients,” Dr. Di Leo said.
Blocks AKT pathway
The preclinical rationale for the use of a P13K inhibitor after disease progression on mTORC1 inhibitor is that current mTOR inhibitors such as everolimus have a feedback mechanism that activates the AKT pathway, and that the use of P13K inhibitors can “abrogate or attenuate this activation, potentially blocking that pathway,” explained coinvestigator Ruth O’Regan, MD, head of the division of hematology and oncology at the University of Wisconsin–Madison School of Medicine and Public Health. Dr. O’Regan discussed the BELLE-3 findings in a briefing prior to Dr. Di Leo’s presentation of the data in general session.
In BELLE-3, 432 postmenopausal women with HR+/HER2-, AI-pretreated, locally advanced or metastatic breast cancer that had progressed on or after treatment with an mTOR inhibitor as the last line of therapy were enrolled. The patients were stratified by the presence or absence of visceral disease and then randomized on a 2:1 basis to fulvestrant 500 mg daily plus either buparlisib 100 mg/day (289 patients), or placebo (143).
As noted, the primary endpoint of investigator-assessed PFS favored the addition of buparlisib, with a hazard ratio for progression of 0.67 (P less than .001). PFS results by independent central review were similar (HR 0.57, P less than .001).
The ORR for the buparlisib/fulvestrant combination, 7.6%, consisted of 0.3% complete responses, and 7.3% partial responses. The ORR for placebo/fulvestrant, 2.1%, was composed entirely of partial responses. The respective clinical benefit rates, defined as a combination of complete and partial responses and stable disease, were 24.6% and 15.4.
The benefit of buparlisib was evidently entirely among patients with visceral disease, with a PFS of 3.1 vs. 1.5 months. In contrast, PFS among patients with no visceral disease was 4.2 vs. 4.1 months, respectively, and was not significant.
In addition, the P13K inhibitor seemed to benefit patients with PIK3CA mutations detected in either the primary tumor or in circulating DNA samples, but not patients with wild-type PIK3CA.
Depression, anxiety with combination
Patients assigned to buparlisib/fulvestrant had substantially higher proportions of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations compared with patients on placebo fulvestrant, as well as more reported depression and anxiety. Three patients in the buparlisib arm attempted suicide. There were no reported suicide attempts in the placebo arm.
Dr. O’Regan said at the briefing that mood disorders are known adverse events associated with buparlisib, and that patients with psychiatric disorders were excluded from the trial.
Carlos Arteaga, MD, co-leader of the Breast Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., who moderated the briefing, said in an interview that PI kinase mutations do not appear to be a good target, and that a pan-PIK3 inhibitor such as buparlisib may be hitting too many targets at once.
Inhibiting all of the PIK3 isoforms – alpha, beta, gamma, and delta – “may be a little too tough,” he said.
The trial should serve as impetus for developing agents that inhibit only the alpha isoform of PIK3 which mutates and appears to be the driver of some types of breast cancer, he said.
Following Dr. Di Leo’s presentation, perennial SABCS gadfly Steven Vogl, MD, New York, told the speaker that “you presented this very nicely, it’s very interesting biology. I don’t think you’d want to do this again to a human, right? Three month prolongation in PFS, miserable, with diarrhea, depression – you don’t want to do this again, is that correct?”
“I think buparlisib is probably not the best compound to be used in this particular setting of patients,” Dr. Di Leo said, but added that a better-tolerated PI3K inhibitor might be more effective.
“Actually, the study is raising an important question: Should we use the PI3K inhibitor in place of the mTOR inhibitor, or perhaps, as the study suggests, should we use the P13K inhibitors sequentially, after the mTOR inhibitor. This is an open question,” he replied.
Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
SAN ANTONIO – A combination of a PI3K inhibitor and selective estrogen receptor down-regulator (SERD) met its primary endpoint of 2.1 months better progression-free survival (PFS) in postmenopausal women with locally advanced or metastatic breast cancer who were quickly running out of other treatment options.
Yet the small gain in PFS came at a very high price in terms of toxicities, including mood disorders that may have led to patient suicide attempts, according to investigators.
The BELLE-3 trial looked at the combination of the SERD fulvestrant (Faslodex) and an experimental inhibitor of the PI3 kinase, buparlisib, in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor–2 (HER2)-negative breast cancer treated with an aromatase inhibitor (AI) who experienced disease progression either on or after receiving therapy with an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1).
The combination of fulvestrant and buparlisib was associated with a median PFS of 3.9 months, compared with 1.8 months for fulvestrant and placebo (P less than .001), Angelo Di Leo, MD, of Ospedale Misericordia e Dolce in Prato, Italy, reported at the San Antonio Breast Cancer Symposium,
Objective response rates (ORR) were low, at 7.8% in the combination arm, and 2.1% in the fulvestrant-plus-placebo arm.
Although the PFS difference was statistically significant, “the higher rate of toxicity in patients receiving buparlisib and fulvestrant, including transaminase elevations and mood disorders, may represent a clinically relevant challenge for future development of this compound in this particular group of patients,” Dr. Di Leo said.
Blocks AKT pathway
The preclinical rationale for the use of a P13K inhibitor after disease progression on mTORC1 inhibitor is that current mTOR inhibitors such as everolimus have a feedback mechanism that activates the AKT pathway, and that the use of P13K inhibitors can “abrogate or attenuate this activation, potentially blocking that pathway,” explained coinvestigator Ruth O’Regan, MD, head of the division of hematology and oncology at the University of Wisconsin–Madison School of Medicine and Public Health. Dr. O’Regan discussed the BELLE-3 findings in a briefing prior to Dr. Di Leo’s presentation of the data in general session.
In BELLE-3, 432 postmenopausal women with HR+/HER2-, AI-pretreated, locally advanced or metastatic breast cancer that had progressed on or after treatment with an mTOR inhibitor as the last line of therapy were enrolled. The patients were stratified by the presence or absence of visceral disease and then randomized on a 2:1 basis to fulvestrant 500 mg daily plus either buparlisib 100 mg/day (289 patients), or placebo (143).
As noted, the primary endpoint of investigator-assessed PFS favored the addition of buparlisib, with a hazard ratio for progression of 0.67 (P less than .001). PFS results by independent central review were similar (HR 0.57, P less than .001).
The ORR for the buparlisib/fulvestrant combination, 7.6%, consisted of 0.3% complete responses, and 7.3% partial responses. The ORR for placebo/fulvestrant, 2.1%, was composed entirely of partial responses. The respective clinical benefit rates, defined as a combination of complete and partial responses and stable disease, were 24.6% and 15.4.
The benefit of buparlisib was evidently entirely among patients with visceral disease, with a PFS of 3.1 vs. 1.5 months. In contrast, PFS among patients with no visceral disease was 4.2 vs. 4.1 months, respectively, and was not significant.
In addition, the P13K inhibitor seemed to benefit patients with PIK3CA mutations detected in either the primary tumor or in circulating DNA samples, but not patients with wild-type PIK3CA.
Depression, anxiety with combination
Patients assigned to buparlisib/fulvestrant had substantially higher proportions of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations compared with patients on placebo fulvestrant, as well as more reported depression and anxiety. Three patients in the buparlisib arm attempted suicide. There were no reported suicide attempts in the placebo arm.
Dr. O’Regan said at the briefing that mood disorders are known adverse events associated with buparlisib, and that patients with psychiatric disorders were excluded from the trial.
Carlos Arteaga, MD, co-leader of the Breast Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., who moderated the briefing, said in an interview that PI kinase mutations do not appear to be a good target, and that a pan-PIK3 inhibitor such as buparlisib may be hitting too many targets at once.
Inhibiting all of the PIK3 isoforms – alpha, beta, gamma, and delta – “may be a little too tough,” he said.
The trial should serve as impetus for developing agents that inhibit only the alpha isoform of PIK3 which mutates and appears to be the driver of some types of breast cancer, he said.
Following Dr. Di Leo’s presentation, perennial SABCS gadfly Steven Vogl, MD, New York, told the speaker that “you presented this very nicely, it’s very interesting biology. I don’t think you’d want to do this again to a human, right? Three month prolongation in PFS, miserable, with diarrhea, depression – you don’t want to do this again, is that correct?”
“I think buparlisib is probably not the best compound to be used in this particular setting of patients,” Dr. Di Leo said, but added that a better-tolerated PI3K inhibitor might be more effective.
“Actually, the study is raising an important question: Should we use the PI3K inhibitor in place of the mTOR inhibitor, or perhaps, as the study suggests, should we use the P13K inhibitors sequentially, after the mTOR inhibitor. This is an open question,” he replied.
Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
AT SABCS 2016
Key clinical point: The PI3K inhibitor buparlisib plus fulvestrant slightly prolonged progression-free survival of HR+/HER2– breast cancer pretreated with an aromatase inhibitor and mTOR inhibitor.
Major finding: The combination met its primary endpoint of better PFS than fulvestrant/placebo, but with high liver toxicity and mood disorders.
Data source: Randomized phase III trial of 432 women with hormone receptor–positive, HER2-negative, AI-pretreated breast cancer that progressed on or after mTOR inhibitor therapy.
Disclosures: Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
More tricuspid valve regurgitation should be fixed
CHICAGO – Fixing the tricuspid valve should be part of left-sided heart operations in many cases of functional tricuspid regurgitation, but study data and international guidelines supporting the practice are too frequently ignored, said Steven Bolling, MD.
Speaking during Heart Valve Summit 2016, Dr. Bolling said that of the approximately four million U.S. individuals with mitral regurgitation, about 1.6 million, or 40%, have concomitant tricuspid regurgitation (TR). Yet, he said, only about 7,000 concomitant tricuspid valve (TV) repairs are performed in the 60,000 patients receiving mitral valve (MV) repair surgery annually, for a TV repair rate of less than 12%. “Tricuspid regurgitation is ignored,” said Dr. Bolling, a conference organizer and professor of surgery at the University of Michigan, Ann Arbor.
A 2015 study followed 645 consecutive patients who underwent primary repair of degenerative mitral regurgitation. The patients who had concomitant TVR, he said, “had far less TR, better right ventricle function, and it’s safe. There was lower mortality and morbidity” (J Am Coll Cardiol. 2015 May 12;65[18]:1931-8).
Citing a study of 5,589 patients undergoing surgery for mitral valve regurgitation only, 16% of these had severe – grade 3-4+ – TR preoperatively. However, at discharge, 62% of those had severe residual TR. Despite a “good” mitral result, said Dr. Bolling, multiple studies dating back to the 1980s have demonstrated that surgical repair of just the mitral valve still results in functional tricuspid regurgitation (FTR) rates of up to 67%. “There’s no guarantee of FTR ‘getting better,’” said Dr. Bolling.
The problem lies fundamentally in the annular dilation and change of shape of the tricuspid annulus, and so these issues must be addressed for a good functional result, he said. This dilation and distortion has been shown to occur in up to 75% of all cases of MR (Circulation. 2006;114:1-492).
“Placing an ‘undersized’ tricuspid ring is actually restoring normal sizing to the annulus,” said Dr. Bolling. The normal tricuspid annular dimension is 2.8 cm, plus or minus 0.5 cm, he said. Patients fare better both in the immediate postoperative period and at follow-up with an “undersized” TV repair for FTR, he said.
And surgeons shouldn’t worry about stenosis with an “undersized” TV repair, he said. High school geometry shows that a 26-mm valve diameter yields an area of about 4 square cm, for a 2- to 3-mm gradient, said Dr. Bolling.
Detection of tricuspid regurgitation can itself be a tricky prospect, because tricuspid regurgitation is dynamic. “You should look for functional tricuspid regurgitation preoperatively,” said Dr. Bolling. “Under anesthesia, four-plus TR can become mild.” Accordingly, any significant intraoperative TR or a dilated annulus should be considered indications for tricuspid valve repair, he said.
Though adding TV repair to mitral surgery may add some complexity, it does not necessarily add risk, said Dr. Bolling, citing a study of 110 matched patients with FTR that found a trend toward lower 30-day mortality for combined repair, when compared to mitral repair only (2% versus 8.5%, P = .2).
However, the single-intervention group had a 40% rate of tricuspid progression compared to 5% when both valves were repaired, and the 5-year survival rate was higher for those who had the combined surgery (74% versus 45%; Ann Thorac Surg. 2009 Mar;87[3]:698-703).
According to American College of Cardiology/American Heart Association (ACC/AHA) guidelines for managing valvular heart disease, which were last updated in 2014, patients with severe TR who are undergoing left-sided valve surgery should have concomitant TV repair, a class I recommendation.
The European Society of Cardiology and the European Association for Cardio-Thoracic Surgery (ESC/EACTS) 2012 guidelines are in accord with the ACC/AHA for this population, also issuing a class I recommendation.
For patients with greater than mild TR who have tricuspid annular dilation or right-sided heart failure, TV repair is a class IIa recommendation, according to the ACC/AHA guidelines. For patients with FTR who also have either pulmonary hypertension or right ventricular dilation or dysfunction, TV repair is an ACC/AHA class IIb recommendation.
In the European guidelines, patients with moderate secondary TR with a tricuspid annulus over 40 mm in diameter who are undergoing left-sided valve surgery, or who have right ventricular dilation or dysfunction, should undergo TV repair. This is a class IIa recommendation in the ESC/EACTS schema.
Dr. Bolling reported financial relationships with the Sorin Group, Medtronic, and Edwards Lifesciences.
koakes@frontlinemedcom.com
On Twitter @karioakes
CHICAGO – Fixing the tricuspid valve should be part of left-sided heart operations in many cases of functional tricuspid regurgitation, but study data and international guidelines supporting the practice are too frequently ignored, said Steven Bolling, MD.
Speaking during Heart Valve Summit 2016, Dr. Bolling said that of the approximately four million U.S. individuals with mitral regurgitation, about 1.6 million, or 40%, have concomitant tricuspid regurgitation (TR). Yet, he said, only about 7,000 concomitant tricuspid valve (TV) repairs are performed in the 60,000 patients receiving mitral valve (MV) repair surgery annually, for a TV repair rate of less than 12%. “Tricuspid regurgitation is ignored,” said Dr. Bolling, a conference organizer and professor of surgery at the University of Michigan, Ann Arbor.
A 2015 study followed 645 consecutive patients who underwent primary repair of degenerative mitral regurgitation. The patients who had concomitant TVR, he said, “had far less TR, better right ventricle function, and it’s safe. There was lower mortality and morbidity” (J Am Coll Cardiol. 2015 May 12;65[18]:1931-8).
Citing a study of 5,589 patients undergoing surgery for mitral valve regurgitation only, 16% of these had severe – grade 3-4+ – TR preoperatively. However, at discharge, 62% of those had severe residual TR. Despite a “good” mitral result, said Dr. Bolling, multiple studies dating back to the 1980s have demonstrated that surgical repair of just the mitral valve still results in functional tricuspid regurgitation (FTR) rates of up to 67%. “There’s no guarantee of FTR ‘getting better,’” said Dr. Bolling.
The problem lies fundamentally in the annular dilation and change of shape of the tricuspid annulus, and so these issues must be addressed for a good functional result, he said. This dilation and distortion has been shown to occur in up to 75% of all cases of MR (Circulation. 2006;114:1-492).
“Placing an ‘undersized’ tricuspid ring is actually restoring normal sizing to the annulus,” said Dr. Bolling. The normal tricuspid annular dimension is 2.8 cm, plus or minus 0.5 cm, he said. Patients fare better both in the immediate postoperative period and at follow-up with an “undersized” TV repair for FTR, he said.
And surgeons shouldn’t worry about stenosis with an “undersized” TV repair, he said. High school geometry shows that a 26-mm valve diameter yields an area of about 4 square cm, for a 2- to 3-mm gradient, said Dr. Bolling.
Detection of tricuspid regurgitation can itself be a tricky prospect, because tricuspid regurgitation is dynamic. “You should look for functional tricuspid regurgitation preoperatively,” said Dr. Bolling. “Under anesthesia, four-plus TR can become mild.” Accordingly, any significant intraoperative TR or a dilated annulus should be considered indications for tricuspid valve repair, he said.
Though adding TV repair to mitral surgery may add some complexity, it does not necessarily add risk, said Dr. Bolling, citing a study of 110 matched patients with FTR that found a trend toward lower 30-day mortality for combined repair, when compared to mitral repair only (2% versus 8.5%, P = .2).
However, the single-intervention group had a 40% rate of tricuspid progression compared to 5% when both valves were repaired, and the 5-year survival rate was higher for those who had the combined surgery (74% versus 45%; Ann Thorac Surg. 2009 Mar;87[3]:698-703).
According to American College of Cardiology/American Heart Association (ACC/AHA) guidelines for managing valvular heart disease, which were last updated in 2014, patients with severe TR who are undergoing left-sided valve surgery should have concomitant TV repair, a class I recommendation.
The European Society of Cardiology and the European Association for Cardio-Thoracic Surgery (ESC/EACTS) 2012 guidelines are in accord with the ACC/AHA for this population, also issuing a class I recommendation.
For patients with greater than mild TR who have tricuspid annular dilation or right-sided heart failure, TV repair is a class IIa recommendation, according to the ACC/AHA guidelines. For patients with FTR who also have either pulmonary hypertension or right ventricular dilation or dysfunction, TV repair is an ACC/AHA class IIb recommendation.
In the European guidelines, patients with moderate secondary TR with a tricuspid annulus over 40 mm in diameter who are undergoing left-sided valve surgery, or who have right ventricular dilation or dysfunction, should undergo TV repair. This is a class IIa recommendation in the ESC/EACTS schema.
Dr. Bolling reported financial relationships with the Sorin Group, Medtronic, and Edwards Lifesciences.
koakes@frontlinemedcom.com
On Twitter @karioakes
CHICAGO – Fixing the tricuspid valve should be part of left-sided heart operations in many cases of functional tricuspid regurgitation, but study data and international guidelines supporting the practice are too frequently ignored, said Steven Bolling, MD.
Speaking during Heart Valve Summit 2016, Dr. Bolling said that of the approximately four million U.S. individuals with mitral regurgitation, about 1.6 million, or 40%, have concomitant tricuspid regurgitation (TR). Yet, he said, only about 7,000 concomitant tricuspid valve (TV) repairs are performed in the 60,000 patients receiving mitral valve (MV) repair surgery annually, for a TV repair rate of less than 12%. “Tricuspid regurgitation is ignored,” said Dr. Bolling, a conference organizer and professor of surgery at the University of Michigan, Ann Arbor.
A 2015 study followed 645 consecutive patients who underwent primary repair of degenerative mitral regurgitation. The patients who had concomitant TVR, he said, “had far less TR, better right ventricle function, and it’s safe. There was lower mortality and morbidity” (J Am Coll Cardiol. 2015 May 12;65[18]:1931-8).
Citing a study of 5,589 patients undergoing surgery for mitral valve regurgitation only, 16% of these had severe – grade 3-4+ – TR preoperatively. However, at discharge, 62% of those had severe residual TR. Despite a “good” mitral result, said Dr. Bolling, multiple studies dating back to the 1980s have demonstrated that surgical repair of just the mitral valve still results in functional tricuspid regurgitation (FTR) rates of up to 67%. “There’s no guarantee of FTR ‘getting better,’” said Dr. Bolling.
The problem lies fundamentally in the annular dilation and change of shape of the tricuspid annulus, and so these issues must be addressed for a good functional result, he said. This dilation and distortion has been shown to occur in up to 75% of all cases of MR (Circulation. 2006;114:1-492).
“Placing an ‘undersized’ tricuspid ring is actually restoring normal sizing to the annulus,” said Dr. Bolling. The normal tricuspid annular dimension is 2.8 cm, plus or minus 0.5 cm, he said. Patients fare better both in the immediate postoperative period and at follow-up with an “undersized” TV repair for FTR, he said.
And surgeons shouldn’t worry about stenosis with an “undersized” TV repair, he said. High school geometry shows that a 26-mm valve diameter yields an area of about 4 square cm, for a 2- to 3-mm gradient, said Dr. Bolling.
Detection of tricuspid regurgitation can itself be a tricky prospect, because tricuspid regurgitation is dynamic. “You should look for functional tricuspid regurgitation preoperatively,” said Dr. Bolling. “Under anesthesia, four-plus TR can become mild.” Accordingly, any significant intraoperative TR or a dilated annulus should be considered indications for tricuspid valve repair, he said.
Though adding TV repair to mitral surgery may add some complexity, it does not necessarily add risk, said Dr. Bolling, citing a study of 110 matched patients with FTR that found a trend toward lower 30-day mortality for combined repair, when compared to mitral repair only (2% versus 8.5%, P = .2).
However, the single-intervention group had a 40% rate of tricuspid progression compared to 5% when both valves were repaired, and the 5-year survival rate was higher for those who had the combined surgery (74% versus 45%; Ann Thorac Surg. 2009 Mar;87[3]:698-703).
According to American College of Cardiology/American Heart Association (ACC/AHA) guidelines for managing valvular heart disease, which were last updated in 2014, patients with severe TR who are undergoing left-sided valve surgery should have concomitant TV repair, a class I recommendation.
The European Society of Cardiology and the European Association for Cardio-Thoracic Surgery (ESC/EACTS) 2012 guidelines are in accord with the ACC/AHA for this population, also issuing a class I recommendation.
For patients with greater than mild TR who have tricuspid annular dilation or right-sided heart failure, TV repair is a class IIa recommendation, according to the ACC/AHA guidelines. For patients with FTR who also have either pulmonary hypertension or right ventricular dilation or dysfunction, TV repair is an ACC/AHA class IIb recommendation.
In the European guidelines, patients with moderate secondary TR with a tricuspid annulus over 40 mm in diameter who are undergoing left-sided valve surgery, or who have right ventricular dilation or dysfunction, should undergo TV repair. This is a class IIa recommendation in the ESC/EACTS schema.
Dr. Bolling reported financial relationships with the Sorin Group, Medtronic, and Edwards Lifesciences.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM HEART VALVE SUMMIT 2016
AGA Patient INFO Center now offers gMed instructions
The Patient INFO Center, AGA’s digital library of patient education, continues to grow and expand. As new topics and educational resources are added, there are also opportunities to enhance the experience of using the library to better connect with patients.
In addition to electronic health record systems Athena, Cerner, and EPIC, you can now integrate AGA’s patient education materials into gMed. Based on member feedback and popular demand, AGA created instructions with gMed consultants and tested them with members to ensure accuracy and ease of use.
Check out the newly added gMed instructions and everything else the Patient INFO Center has to offer.
Have thoughts on the Patient INFO Center? Email communications@gastro.org.
The Patient INFO Center, AGA’s digital library of patient education, continues to grow and expand. As new topics and educational resources are added, there are also opportunities to enhance the experience of using the library to better connect with patients.
In addition to electronic health record systems Athena, Cerner, and EPIC, you can now integrate AGA’s patient education materials into gMed. Based on member feedback and popular demand, AGA created instructions with gMed consultants and tested them with members to ensure accuracy and ease of use.
Check out the newly added gMed instructions and everything else the Patient INFO Center has to offer.
Have thoughts on the Patient INFO Center? Email communications@gastro.org.
The Patient INFO Center, AGA’s digital library of patient education, continues to grow and expand. As new topics and educational resources are added, there are also opportunities to enhance the experience of using the library to better connect with patients.
In addition to electronic health record systems Athena, Cerner, and EPIC, you can now integrate AGA’s patient education materials into gMed. Based on member feedback and popular demand, AGA created instructions with gMed consultants and tested them with members to ensure accuracy and ease of use.
Check out the newly added gMed instructions and everything else the Patient INFO Center has to offer.
Have thoughts on the Patient INFO Center? Email communications@gastro.org.
Addressing Patient Concerns About Treatment Safety Data
Mutations missed in early-onset colorectal cancer
As many as one in six patients with early-onset colorectal cancer (CRC) have a pathogenic genetic mutation, but around one-third of these patients may not have met the criteria for genetic testing for at least one of their mutations under current guidelines, researchers say.
Rachel Pearlman, MS, CGC, of The Ohio State University Comprehensive Cancer Center, and her coauthors reported the results of multigene panel testing of 450 patients aged under 50 years, from 51 institutions, who had been diagnosed with CRC (JAMA Oncol 2016 Dec 15. doi: 10.1001/jamaoncol.2016.5194).
Overall, 16% of patients were found to have a pathogenic or likely pathogenic cancer susceptibility gene mutations, with 83.3% having at least one gene mutation.
Thirty-seven patients had Lynch syndrome; 13 were MLH1, 16 were LSH2, 1 patient was MSH2/monoallelic MUTYH; 2 were MSH6, and 5 were PMS2.
“While the prevalence of Lynch syndrome reported herein (8.4%) is consistent with previous publications, this is the first study to our knowledge to determine the prevalence and spectrum of other hereditary cancer syndromes (8%) found in an unselected series of patients with early-onset CRC,” the authors wrote.
Forty-eight patients (10.7%) had mismatch repair–deficient tumors, nine of which were in high-penetrance genes linked to CRC risk.
But for 145 patients, their genetic variants were of uncertain significance. Thirteen patients had mutations in high- or moderate-penetrance genes not traditionally associated with CRC, including ATM, ATM/ CHEK2, BRCA1, BRCA2, CDKN2A, and PALB2.
The authors pointed out that the multigene panel testing approach enables identification of hereditary cancer syndromes in patients who might not have otherwise met the criteria for testing.
“Importantly, 24 of 72 patients (33.3%) with pathogenic mutations did not meet NCCN Guidelines for at least 1 of the gene(s) in which they were found to have a mutation,” the researchers noted. These included three patients with MMR-deficient tumors who had additional mutations in genes that would not have been assessed, one patient with an MMR-proficient tumor who was also found to have Lynch syndrome, and six patients with BRCA1/2 mutations.
“Previous studies have reported early-onset CRC in women with BRCA1 mutations and BRCA2 mutations in families with familial colorectal cancer type X,” they noted.
The Ohio Colorectal Cancer Prevention Initiative (OCCPI) is supported by a grant from Pelotonia, and by the National Cancer Institute. Myriad Genetics Inc. donated next-generation sequencing testing. Nine authors disclosed ties with private industry, including Myriad Genetics.
This study illustrates the shortcomings of current algorithms for diagnosing and managing younger patients with CRC. First, although family history is one of the main components used to stratify an individual’s risk for CRC, it is imperfect because only one in five younger patients with CRC reported having a first-degree relative with CRC. Second, although clinical criteria define the phenotypes typically associated with specific gene mutations, variability in penetrance and expressivity can result in overlap among the different hereditary cancer syndromes (e.g., BRCA germline mutations in younger patients with CRC).
The findings of this large population-based study demonstrate that the incorporation of multigene panel genetic testing in the evaluation of patients with CRC will increase the diagnosis of individuals with genetic predisposition to cancer and will expand current knowledge regarding the associated phenotypes, further supporting the cost-effectiveness of testing that can guide management for patients with cancer and their at-risk relatives. The study found germline mutations in one in six patients with CRC and has argued for comprehensive germline genetic testing of patients diagnosed at younger than 50 years.
Eduardo Vilar-Sanchez, MD, PhD, is in the department of clinical cancer prevention and clinical cancer genetics program at The University of Texas MD Anderson Cancer Center, Houston. Elena M. Stoffel, MD, is in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are adapted from an editorial (JAMA Oncology 2016 Dec 15. doi: 10.1001/jamaoncol.2016.5193). No conflicts of interest were declared.
This study illustrates the shortcomings of current algorithms for diagnosing and managing younger patients with CRC. First, although family history is one of the main components used to stratify an individual’s risk for CRC, it is imperfect because only one in five younger patients with CRC reported having a first-degree relative with CRC. Second, although clinical criteria define the phenotypes typically associated with specific gene mutations, variability in penetrance and expressivity can result in overlap among the different hereditary cancer syndromes (e.g., BRCA germline mutations in younger patients with CRC).
The findings of this large population-based study demonstrate that the incorporation of multigene panel genetic testing in the evaluation of patients with CRC will increase the diagnosis of individuals with genetic predisposition to cancer and will expand current knowledge regarding the associated phenotypes, further supporting the cost-effectiveness of testing that can guide management for patients with cancer and their at-risk relatives. The study found germline mutations in one in six patients with CRC and has argued for comprehensive germline genetic testing of patients diagnosed at younger than 50 years.
Eduardo Vilar-Sanchez, MD, PhD, is in the department of clinical cancer prevention and clinical cancer genetics program at The University of Texas MD Anderson Cancer Center, Houston. Elena M. Stoffel, MD, is in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are adapted from an editorial (JAMA Oncology 2016 Dec 15. doi: 10.1001/jamaoncol.2016.5193). No conflicts of interest were declared.
This study illustrates the shortcomings of current algorithms for diagnosing and managing younger patients with CRC. First, although family history is one of the main components used to stratify an individual’s risk for CRC, it is imperfect because only one in five younger patients with CRC reported having a first-degree relative with CRC. Second, although clinical criteria define the phenotypes typically associated with specific gene mutations, variability in penetrance and expressivity can result in overlap among the different hereditary cancer syndromes (e.g., BRCA germline mutations in younger patients with CRC).
The findings of this large population-based study demonstrate that the incorporation of multigene panel genetic testing in the evaluation of patients with CRC will increase the diagnosis of individuals with genetic predisposition to cancer and will expand current knowledge regarding the associated phenotypes, further supporting the cost-effectiveness of testing that can guide management for patients with cancer and their at-risk relatives. The study found germline mutations in one in six patients with CRC and has argued for comprehensive germline genetic testing of patients diagnosed at younger than 50 years.
Eduardo Vilar-Sanchez, MD, PhD, is in the department of clinical cancer prevention and clinical cancer genetics program at The University of Texas MD Anderson Cancer Center, Houston. Elena M. Stoffel, MD, is in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are adapted from an editorial (JAMA Oncology 2016 Dec 15. doi: 10.1001/jamaoncol.2016.5193). No conflicts of interest were declared.
As many as one in six patients with early-onset colorectal cancer (CRC) have a pathogenic genetic mutation, but around one-third of these patients may not have met the criteria for genetic testing for at least one of their mutations under current guidelines, researchers say.
Rachel Pearlman, MS, CGC, of The Ohio State University Comprehensive Cancer Center, and her coauthors reported the results of multigene panel testing of 450 patients aged under 50 years, from 51 institutions, who had been diagnosed with CRC (JAMA Oncol 2016 Dec 15. doi: 10.1001/jamaoncol.2016.5194).
Overall, 16% of patients were found to have a pathogenic or likely pathogenic cancer susceptibility gene mutations, with 83.3% having at least one gene mutation.
Thirty-seven patients had Lynch syndrome; 13 were MLH1, 16 were LSH2, 1 patient was MSH2/monoallelic MUTYH; 2 were MSH6, and 5 were PMS2.
“While the prevalence of Lynch syndrome reported herein (8.4%) is consistent with previous publications, this is the first study to our knowledge to determine the prevalence and spectrum of other hereditary cancer syndromes (8%) found in an unselected series of patients with early-onset CRC,” the authors wrote.
Forty-eight patients (10.7%) had mismatch repair–deficient tumors, nine of which were in high-penetrance genes linked to CRC risk.
But for 145 patients, their genetic variants were of uncertain significance. Thirteen patients had mutations in high- or moderate-penetrance genes not traditionally associated with CRC, including ATM, ATM/ CHEK2, BRCA1, BRCA2, CDKN2A, and PALB2.
The authors pointed out that the multigene panel testing approach enables identification of hereditary cancer syndromes in patients who might not have otherwise met the criteria for testing.
“Importantly, 24 of 72 patients (33.3%) with pathogenic mutations did not meet NCCN Guidelines for at least 1 of the gene(s) in which they were found to have a mutation,” the researchers noted. These included three patients with MMR-deficient tumors who had additional mutations in genes that would not have been assessed, one patient with an MMR-proficient tumor who was also found to have Lynch syndrome, and six patients with BRCA1/2 mutations.
“Previous studies have reported early-onset CRC in women with BRCA1 mutations and BRCA2 mutations in families with familial colorectal cancer type X,” they noted.
The Ohio Colorectal Cancer Prevention Initiative (OCCPI) is supported by a grant from Pelotonia, and by the National Cancer Institute. Myriad Genetics Inc. donated next-generation sequencing testing. Nine authors disclosed ties with private industry, including Myriad Genetics.
As many as one in six patients with early-onset colorectal cancer (CRC) have a pathogenic genetic mutation, but around one-third of these patients may not have met the criteria for genetic testing for at least one of their mutations under current guidelines, researchers say.
Rachel Pearlman, MS, CGC, of The Ohio State University Comprehensive Cancer Center, and her coauthors reported the results of multigene panel testing of 450 patients aged under 50 years, from 51 institutions, who had been diagnosed with CRC (JAMA Oncol 2016 Dec 15. doi: 10.1001/jamaoncol.2016.5194).
Overall, 16% of patients were found to have a pathogenic or likely pathogenic cancer susceptibility gene mutations, with 83.3% having at least one gene mutation.
Thirty-seven patients had Lynch syndrome; 13 were MLH1, 16 were LSH2, 1 patient was MSH2/monoallelic MUTYH; 2 were MSH6, and 5 were PMS2.
“While the prevalence of Lynch syndrome reported herein (8.4%) is consistent with previous publications, this is the first study to our knowledge to determine the prevalence and spectrum of other hereditary cancer syndromes (8%) found in an unselected series of patients with early-onset CRC,” the authors wrote.
Forty-eight patients (10.7%) had mismatch repair–deficient tumors, nine of which were in high-penetrance genes linked to CRC risk.
But for 145 patients, their genetic variants were of uncertain significance. Thirteen patients had mutations in high- or moderate-penetrance genes not traditionally associated with CRC, including ATM, ATM/ CHEK2, BRCA1, BRCA2, CDKN2A, and PALB2.
The authors pointed out that the multigene panel testing approach enables identification of hereditary cancer syndromes in patients who might not have otherwise met the criteria for testing.
“Importantly, 24 of 72 patients (33.3%) with pathogenic mutations did not meet NCCN Guidelines for at least 1 of the gene(s) in which they were found to have a mutation,” the researchers noted. These included three patients with MMR-deficient tumors who had additional mutations in genes that would not have been assessed, one patient with an MMR-proficient tumor who was also found to have Lynch syndrome, and six patients with BRCA1/2 mutations.
“Previous studies have reported early-onset CRC in women with BRCA1 mutations and BRCA2 mutations in families with familial colorectal cancer type X,” they noted.
The Ohio Colorectal Cancer Prevention Initiative (OCCPI) is supported by a grant from Pelotonia, and by the National Cancer Institute. Myriad Genetics Inc. donated next-generation sequencing testing. Nine authors disclosed ties with private industry, including Myriad Genetics.
FROM JAMA ONCOLOGY
Key clinical point: As many as one in six patients with early-onset colorectal cancer have a pathogenic genetic mutation but around one-third of these patients may not have met the criteria for genetic testing for at least one of their mutations, under current guidelines.
Major finding: 16% of patients with early-onset colorectal cancer were found to have a pathogenic or likely pathogenic cancer susceptibility gene mutation.
Data source: Cohort study of 450 patients aged under 50 years diagnosed with colorectal cancer.
Disclosures: The Ohio Colorectal Cancer Prevention Initiative (OCCPI) is supported by a grant from Pelotonia, and by the National Cancer Institute. Myriad Genetics Inc. donated next-generation sequencing testing. Nine authors disclosed ties with private industry, including Myriad Genetics.
Role of the Kidney in Type 2 Diabetes and Mechanism of Action of Sodium Glucose Cotransporter-2 Inhibitors
While type 2 diabetes (T2D) is commonly seen in primary care, it is difficult to manage successfully over time. This series offers brief eNewsletters written by clinical experts that are designed to assist in the clinical management of patients with T2D.
While type 2 diabetes (T2D) is commonly seen in primary care, it is difficult to manage successfully over time. This series offers brief eNewsletters written by clinical experts that are designed to assist in the clinical management of patients with T2D.
While type 2 diabetes (T2D) is commonly seen in primary care, it is difficult to manage successfully over time. This series offers brief eNewsletters written by clinical experts that are designed to assist in the clinical management of patients with T2D.