User login
Alzheimer’s anti-tau drug fails phase III, but posts some benefit in monotherapy subanalysis
TORONTO – A highly anticipated phase III trial of an anti-tau drug has posted negative topline results, conferring no cognitive or functional benefits when given in conjunction with standard-of-care Alzheimer’s disease medications.
The drug, LTMX (TauRx, Singapore), also did not slow the progression of brain atrophy on imaging in either of two doses tested, according to a company press release.
Although the study didn’t meet its clinical endpoints in the overall cohort of 891 patients with mild-moderate disease, TauRx promoted it as “promising,” based on a subgroup analysis of the 15% of patients who took the drug as monotherapy.
Among these patients, LMTX was associated with dose-dependent, statistically significant improvements in the Alzheimer’s Disease Assessment Scale measures of cognition (ADAS-cog) and Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL). The drug was also associated with a slowing of brain ventricular expansion, compared with controls, suggesting that it could be preserving brain mass.
Nevertheless, the trial must be read as another negative one, said David S. Knopman, MD, who moderated a press briefing where the data were presented.
“I must say I am disappointed by the results because in my view of clinical trials forged from 30 years of experience, the only thing that really counts is the prespecified primary outcome,” said Dr. Knopman of the Mayo Clinic, Rochester, Minn. “I think the secondary results are interesting, especially imaging findings. But our experience of secondary analyses in this field is that they are fraught with hidden biases. And because this is a small subset of just 15%, it’s very difficult to interpret.”
Details of the study
The 15-month study comprised 891 patients with mild-moderate Alzheimer’s disease. Most of these (85%) were taking standard-of-care symptomatic Alzheimer’s disease medications. Patients were randomized to 75 mg twice daily, 125 mg twice daily, or placebo, which necessarily consisted of a small amount of the medication. LMTX is a derivative of the dye methylene blue and colors urine when excreted. The inactive dose is enough to provide that color so that blinding can be maintained.
Patients were grouped according to whether they took the study drug as add-on therapy (85%) or as monotherapy (15%). However, the results were presented in a somewhat unusual way, with the placebo patients in each therapeutic regimen grouped together. Thus, there was no way to compare the placebo-treated patients who did not receive standard-of-care medications against those who received LMTX monotherapy without standard-of-care medications; instead, the benefits reported in the active monotherapy group were compared with the results seen in placebo patients in both the mono- and add-on groups.
The reason for this was that the numbers in each group were small, said Serge Gauthier, MD, who presented the LMTX data at the Alzheimer’s Association International Conference 2016. Among the monotherapy group, 42 took 75 mg twice daily, 40 took 125 mg twice daily, and 54 took placebo. He suggested that these numbers could be pooled with those in a similar phase III trial of LMTX in about 800 patients with mild disease, which will be completed this fall.
“My proposal would be to combine these groups and then we would really be able to understand what we’re seeing in the control monotherapy versus the study drug monotherapy groups,” said Dr. Gauthier of McGill University, Montreal.
The study was conducted at 115 sites across 16 countries in Europe, North America, and Asia. All of the patients had a clinical diagnosis of Alzheimer’s disease; no one underwent amyloid PET imaging. The patients’ mean age was 70.6 years, and their baseline Mini Mental State Exam score was 18.7.
At the study’s end, patients in the monotherapy group taking 75 mg twice daily had declined 6.3 points less on the ADAS-cog than did the grouped placebo patients, indicating preserved cognition. Those taking 125 mg twice daily declined 5.8 points less than did the grouped placebo patients. On the ADCS-ADL, patients taking 75 mg twice daily scored 6.5 points higher than did the placebo group, indicating better function, and those taking 125 mg twice daily scored 6.9 points higher than did the placebo group.
Lateral ventricular volume expansion on MRI was significantly less than that seen in placebo-treated patients. For those taking 75 mg twice a day, ventricular expansion was reduced by 38%; for those taking 125 mg twice a day, expansion was reduced by 33%. This was accompanied by significant slowing of whole brain atrophy, Dr. Gauthier said, adding that this finding has never been reported in an Alzheimer’s drug trial.
Speculation on lack of effect with standard-of-care medications
Confoundingly, however, LMTX showed no benefit at all in the patients who were taking the usual Alzheimer’s medications. Nor were there similar changes in brain volume.
“We are struggling with this information,” Dr. Gauthier said. “Why this difference in the 15%? They were not older, they did not have milder disease, and there were no obvious differences. The only thing we saw was that they were more likely to have come from Eastern Europe, where access to these drugs is reduced.”
That, however, could play a key role in the findings, Dr. Knopman said in an interview.
“To be honest, I think people who entered the study not on standard of care were in regions where they were not getting any good medical care, and when they became part of this trial they began to get better medical care and experienced a pronounced placebo effect.”
He couldn’t explain how a placebo effect could be related to the MRI findings, although he did say that other medical conditions can be related to changes in brain volume. Quitting alcohol is a big one – alcoholics who stop drinking do experience increases in whole brain volume. And, Dr. Knopman pointed out, alcoholism is rampant in Eastern Europe, where most of these patients lived.
The finding is more problematic because there’s no way to compare the active monotherapy group with the placebo monotherapy group, he said.
“My suspicion is that if they had shown the differences between the monotherapy placebo and the monotherapy active groups, the curves would have looked a lot like what we saw in the add-on therapy groups.”
In an interview, Claude Wischik, MD, PhD, cofounder and executive chair of TauRx and primary investigator on all of the LMTX studies, dismissed Dr. Knopman’s suggestion.
“There’s no geography in the world that can change brain volume,” he said. “You can’t shift the brain simply by wanting it.” And while he fell short of suggesting that LMTX is affecting neurogenesis, he did say that the drug is directly responsible for modifying brain physiology.
Dr. Knopman also pointed out that the lack of baseline amyloid PET imaging almost certainly means that there were patients with other, non-Alzheimer’s dementias in the trial. Baseline amyloid PET imaging is now standard because up to 30% of patients in older antiamyloid studies have now been shown to have not even had the disease. Without baseline amyloid PET imaging to confirm diagnosis, “there’s no telling what they were treating” with LMTX, he said.
The drug’s failure as an add-on therapy is problematic, Dr. Knopman said. The symptomatic Alzheimer’s medications are generally considered to have a very low interaction profile with any other drug. This lack of efficacy, he suggested, is another hint that the benefit in the monotherapy group could be a fluke.
Dr. Wischik said this is not due to pharmacokinetics, but rather to the induction of a cellular clearance pathway called the P-glycoprotein 1 transport pathway.
“The most plausible explanation is this transporter hypothesis. If you’re taking a drug chronically – like an Alzheimer’s medication – this extrusion pathway is turned on. Its net effect is to excrete the drugs from the brain and enhance kidney excretion.” This would accelerate LMTX clearance to the point of inactivity, he said.
When asked if this would be problematic for other drugs taken chronically – statins, for example – Dr. Wischik said the cholinesterase inhibitors were responsible for activating the P-glycoprotein 1 transport pathway. He said there were no other drug interactions observed to inhibit the effect of LMTX.
Dr. Gauthier said research will proceed on LMTX, probably targeting patients with mild Alzheimer’s – or even prodromal disease – who are not yet taking an Alzheimer’s medication. In fact, he suggested that any future it might have would most likely be as part of a staged treatment. LMTX could be given early with the aim of delaying symptom onset, at which time treatment could accelerate to a symptomatic medication, and then, perhaps, to more aggressive measures like an antiamyloid, should one ever come to market.
Dr. Gauthier is on the TauRx advisory board. Dr. Knopman is an investigator on a trial of LMTX in frontotemporal dementia, but has no financial ties with the company.
On Twitter @alz_gal
TORONTO – A highly anticipated phase III trial of an anti-tau drug has posted negative topline results, conferring no cognitive or functional benefits when given in conjunction with standard-of-care Alzheimer’s disease medications.
The drug, LTMX (TauRx, Singapore), also did not slow the progression of brain atrophy on imaging in either of two doses tested, according to a company press release.
Although the study didn’t meet its clinical endpoints in the overall cohort of 891 patients with mild-moderate disease, TauRx promoted it as “promising,” based on a subgroup analysis of the 15% of patients who took the drug as monotherapy.
Among these patients, LMTX was associated with dose-dependent, statistically significant improvements in the Alzheimer’s Disease Assessment Scale measures of cognition (ADAS-cog) and Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL). The drug was also associated with a slowing of brain ventricular expansion, compared with controls, suggesting that it could be preserving brain mass.
Nevertheless, the trial must be read as another negative one, said David S. Knopman, MD, who moderated a press briefing where the data were presented.
“I must say I am disappointed by the results because in my view of clinical trials forged from 30 years of experience, the only thing that really counts is the prespecified primary outcome,” said Dr. Knopman of the Mayo Clinic, Rochester, Minn. “I think the secondary results are interesting, especially imaging findings. But our experience of secondary analyses in this field is that they are fraught with hidden biases. And because this is a small subset of just 15%, it’s very difficult to interpret.”
Details of the study
The 15-month study comprised 891 patients with mild-moderate Alzheimer’s disease. Most of these (85%) were taking standard-of-care symptomatic Alzheimer’s disease medications. Patients were randomized to 75 mg twice daily, 125 mg twice daily, or placebo, which necessarily consisted of a small amount of the medication. LMTX is a derivative of the dye methylene blue and colors urine when excreted. The inactive dose is enough to provide that color so that blinding can be maintained.
Patients were grouped according to whether they took the study drug as add-on therapy (85%) or as monotherapy (15%). However, the results were presented in a somewhat unusual way, with the placebo patients in each therapeutic regimen grouped together. Thus, there was no way to compare the placebo-treated patients who did not receive standard-of-care medications against those who received LMTX monotherapy without standard-of-care medications; instead, the benefits reported in the active monotherapy group were compared with the results seen in placebo patients in both the mono- and add-on groups.
The reason for this was that the numbers in each group were small, said Serge Gauthier, MD, who presented the LMTX data at the Alzheimer’s Association International Conference 2016. Among the monotherapy group, 42 took 75 mg twice daily, 40 took 125 mg twice daily, and 54 took placebo. He suggested that these numbers could be pooled with those in a similar phase III trial of LMTX in about 800 patients with mild disease, which will be completed this fall.
“My proposal would be to combine these groups and then we would really be able to understand what we’re seeing in the control monotherapy versus the study drug monotherapy groups,” said Dr. Gauthier of McGill University, Montreal.
The study was conducted at 115 sites across 16 countries in Europe, North America, and Asia. All of the patients had a clinical diagnosis of Alzheimer’s disease; no one underwent amyloid PET imaging. The patients’ mean age was 70.6 years, and their baseline Mini Mental State Exam score was 18.7.
At the study’s end, patients in the monotherapy group taking 75 mg twice daily had declined 6.3 points less on the ADAS-cog than did the grouped placebo patients, indicating preserved cognition. Those taking 125 mg twice daily declined 5.8 points less than did the grouped placebo patients. On the ADCS-ADL, patients taking 75 mg twice daily scored 6.5 points higher than did the placebo group, indicating better function, and those taking 125 mg twice daily scored 6.9 points higher than did the placebo group.
Lateral ventricular volume expansion on MRI was significantly less than that seen in placebo-treated patients. For those taking 75 mg twice a day, ventricular expansion was reduced by 38%; for those taking 125 mg twice a day, expansion was reduced by 33%. This was accompanied by significant slowing of whole brain atrophy, Dr. Gauthier said, adding that this finding has never been reported in an Alzheimer’s drug trial.
Speculation on lack of effect with standard-of-care medications
Confoundingly, however, LMTX showed no benefit at all in the patients who were taking the usual Alzheimer’s medications. Nor were there similar changes in brain volume.
“We are struggling with this information,” Dr. Gauthier said. “Why this difference in the 15%? They were not older, they did not have milder disease, and there were no obvious differences. The only thing we saw was that they were more likely to have come from Eastern Europe, where access to these drugs is reduced.”
That, however, could play a key role in the findings, Dr. Knopman said in an interview.
“To be honest, I think people who entered the study not on standard of care were in regions where they were not getting any good medical care, and when they became part of this trial they began to get better medical care and experienced a pronounced placebo effect.”
He couldn’t explain how a placebo effect could be related to the MRI findings, although he did say that other medical conditions can be related to changes in brain volume. Quitting alcohol is a big one – alcoholics who stop drinking do experience increases in whole brain volume. And, Dr. Knopman pointed out, alcoholism is rampant in Eastern Europe, where most of these patients lived.
The finding is more problematic because there’s no way to compare the active monotherapy group with the placebo monotherapy group, he said.
“My suspicion is that if they had shown the differences between the monotherapy placebo and the monotherapy active groups, the curves would have looked a lot like what we saw in the add-on therapy groups.”
In an interview, Claude Wischik, MD, PhD, cofounder and executive chair of TauRx and primary investigator on all of the LMTX studies, dismissed Dr. Knopman’s suggestion.
“There’s no geography in the world that can change brain volume,” he said. “You can’t shift the brain simply by wanting it.” And while he fell short of suggesting that LMTX is affecting neurogenesis, he did say that the drug is directly responsible for modifying brain physiology.
Dr. Knopman also pointed out that the lack of baseline amyloid PET imaging almost certainly means that there were patients with other, non-Alzheimer’s dementias in the trial. Baseline amyloid PET imaging is now standard because up to 30% of patients in older antiamyloid studies have now been shown to have not even had the disease. Without baseline amyloid PET imaging to confirm diagnosis, “there’s no telling what they were treating” with LMTX, he said.
The drug’s failure as an add-on therapy is problematic, Dr. Knopman said. The symptomatic Alzheimer’s medications are generally considered to have a very low interaction profile with any other drug. This lack of efficacy, he suggested, is another hint that the benefit in the monotherapy group could be a fluke.
Dr. Wischik said this is not due to pharmacokinetics, but rather to the induction of a cellular clearance pathway called the P-glycoprotein 1 transport pathway.
“The most plausible explanation is this transporter hypothesis. If you’re taking a drug chronically – like an Alzheimer’s medication – this extrusion pathway is turned on. Its net effect is to excrete the drugs from the brain and enhance kidney excretion.” This would accelerate LMTX clearance to the point of inactivity, he said.
When asked if this would be problematic for other drugs taken chronically – statins, for example – Dr. Wischik said the cholinesterase inhibitors were responsible for activating the P-glycoprotein 1 transport pathway. He said there were no other drug interactions observed to inhibit the effect of LMTX.
Dr. Gauthier said research will proceed on LMTX, probably targeting patients with mild Alzheimer’s – or even prodromal disease – who are not yet taking an Alzheimer’s medication. In fact, he suggested that any future it might have would most likely be as part of a staged treatment. LMTX could be given early with the aim of delaying symptom onset, at which time treatment could accelerate to a symptomatic medication, and then, perhaps, to more aggressive measures like an antiamyloid, should one ever come to market.
Dr. Gauthier is on the TauRx advisory board. Dr. Knopman is an investigator on a trial of LMTX in frontotemporal dementia, but has no financial ties with the company.
On Twitter @alz_gal
TORONTO – A highly anticipated phase III trial of an anti-tau drug has posted negative topline results, conferring no cognitive or functional benefits when given in conjunction with standard-of-care Alzheimer’s disease medications.
The drug, LTMX (TauRx, Singapore), also did not slow the progression of brain atrophy on imaging in either of two doses tested, according to a company press release.
Although the study didn’t meet its clinical endpoints in the overall cohort of 891 patients with mild-moderate disease, TauRx promoted it as “promising,” based on a subgroup analysis of the 15% of patients who took the drug as monotherapy.
Among these patients, LMTX was associated with dose-dependent, statistically significant improvements in the Alzheimer’s Disease Assessment Scale measures of cognition (ADAS-cog) and Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL). The drug was also associated with a slowing of brain ventricular expansion, compared with controls, suggesting that it could be preserving brain mass.
Nevertheless, the trial must be read as another negative one, said David S. Knopman, MD, who moderated a press briefing where the data were presented.
“I must say I am disappointed by the results because in my view of clinical trials forged from 30 years of experience, the only thing that really counts is the prespecified primary outcome,” said Dr. Knopman of the Mayo Clinic, Rochester, Minn. “I think the secondary results are interesting, especially imaging findings. But our experience of secondary analyses in this field is that they are fraught with hidden biases. And because this is a small subset of just 15%, it’s very difficult to interpret.”
Details of the study
The 15-month study comprised 891 patients with mild-moderate Alzheimer’s disease. Most of these (85%) were taking standard-of-care symptomatic Alzheimer’s disease medications. Patients were randomized to 75 mg twice daily, 125 mg twice daily, or placebo, which necessarily consisted of a small amount of the medication. LMTX is a derivative of the dye methylene blue and colors urine when excreted. The inactive dose is enough to provide that color so that blinding can be maintained.
Patients were grouped according to whether they took the study drug as add-on therapy (85%) or as monotherapy (15%). However, the results were presented in a somewhat unusual way, with the placebo patients in each therapeutic regimen grouped together. Thus, there was no way to compare the placebo-treated patients who did not receive standard-of-care medications against those who received LMTX monotherapy without standard-of-care medications; instead, the benefits reported in the active monotherapy group were compared with the results seen in placebo patients in both the mono- and add-on groups.
The reason for this was that the numbers in each group were small, said Serge Gauthier, MD, who presented the LMTX data at the Alzheimer’s Association International Conference 2016. Among the monotherapy group, 42 took 75 mg twice daily, 40 took 125 mg twice daily, and 54 took placebo. He suggested that these numbers could be pooled with those in a similar phase III trial of LMTX in about 800 patients with mild disease, which will be completed this fall.
“My proposal would be to combine these groups and then we would really be able to understand what we’re seeing in the control monotherapy versus the study drug monotherapy groups,” said Dr. Gauthier of McGill University, Montreal.
The study was conducted at 115 sites across 16 countries in Europe, North America, and Asia. All of the patients had a clinical diagnosis of Alzheimer’s disease; no one underwent amyloid PET imaging. The patients’ mean age was 70.6 years, and their baseline Mini Mental State Exam score was 18.7.
At the study’s end, patients in the monotherapy group taking 75 mg twice daily had declined 6.3 points less on the ADAS-cog than did the grouped placebo patients, indicating preserved cognition. Those taking 125 mg twice daily declined 5.8 points less than did the grouped placebo patients. On the ADCS-ADL, patients taking 75 mg twice daily scored 6.5 points higher than did the placebo group, indicating better function, and those taking 125 mg twice daily scored 6.9 points higher than did the placebo group.
Lateral ventricular volume expansion on MRI was significantly less than that seen in placebo-treated patients. For those taking 75 mg twice a day, ventricular expansion was reduced by 38%; for those taking 125 mg twice a day, expansion was reduced by 33%. This was accompanied by significant slowing of whole brain atrophy, Dr. Gauthier said, adding that this finding has never been reported in an Alzheimer’s drug trial.
Speculation on lack of effect with standard-of-care medications
Confoundingly, however, LMTX showed no benefit at all in the patients who were taking the usual Alzheimer’s medications. Nor were there similar changes in brain volume.
“We are struggling with this information,” Dr. Gauthier said. “Why this difference in the 15%? They were not older, they did not have milder disease, and there were no obvious differences. The only thing we saw was that they were more likely to have come from Eastern Europe, where access to these drugs is reduced.”
That, however, could play a key role in the findings, Dr. Knopman said in an interview.
“To be honest, I think people who entered the study not on standard of care were in regions where they were not getting any good medical care, and when they became part of this trial they began to get better medical care and experienced a pronounced placebo effect.”
He couldn’t explain how a placebo effect could be related to the MRI findings, although he did say that other medical conditions can be related to changes in brain volume. Quitting alcohol is a big one – alcoholics who stop drinking do experience increases in whole brain volume. And, Dr. Knopman pointed out, alcoholism is rampant in Eastern Europe, where most of these patients lived.
The finding is more problematic because there’s no way to compare the active monotherapy group with the placebo monotherapy group, he said.
“My suspicion is that if they had shown the differences between the monotherapy placebo and the monotherapy active groups, the curves would have looked a lot like what we saw in the add-on therapy groups.”
In an interview, Claude Wischik, MD, PhD, cofounder and executive chair of TauRx and primary investigator on all of the LMTX studies, dismissed Dr. Knopman’s suggestion.
“There’s no geography in the world that can change brain volume,” he said. “You can’t shift the brain simply by wanting it.” And while he fell short of suggesting that LMTX is affecting neurogenesis, he did say that the drug is directly responsible for modifying brain physiology.
Dr. Knopman also pointed out that the lack of baseline amyloid PET imaging almost certainly means that there were patients with other, non-Alzheimer’s dementias in the trial. Baseline amyloid PET imaging is now standard because up to 30% of patients in older antiamyloid studies have now been shown to have not even had the disease. Without baseline amyloid PET imaging to confirm diagnosis, “there’s no telling what they were treating” with LMTX, he said.
The drug’s failure as an add-on therapy is problematic, Dr. Knopman said. The symptomatic Alzheimer’s medications are generally considered to have a very low interaction profile with any other drug. This lack of efficacy, he suggested, is another hint that the benefit in the monotherapy group could be a fluke.
Dr. Wischik said this is not due to pharmacokinetics, but rather to the induction of a cellular clearance pathway called the P-glycoprotein 1 transport pathway.
“The most plausible explanation is this transporter hypothesis. If you’re taking a drug chronically – like an Alzheimer’s medication – this extrusion pathway is turned on. Its net effect is to excrete the drugs from the brain and enhance kidney excretion.” This would accelerate LMTX clearance to the point of inactivity, he said.
When asked if this would be problematic for other drugs taken chronically – statins, for example – Dr. Wischik said the cholinesterase inhibitors were responsible for activating the P-glycoprotein 1 transport pathway. He said there were no other drug interactions observed to inhibit the effect of LMTX.
Dr. Gauthier said research will proceed on LMTX, probably targeting patients with mild Alzheimer’s – or even prodromal disease – who are not yet taking an Alzheimer’s medication. In fact, he suggested that any future it might have would most likely be as part of a staged treatment. LMTX could be given early with the aim of delaying symptom onset, at which time treatment could accelerate to a symptomatic medication, and then, perhaps, to more aggressive measures like an antiamyloid, should one ever come to market.
Dr. Gauthier is on the TauRx advisory board. Dr. Knopman is an investigator on a trial of LMTX in frontotemporal dementia, but has no financial ties with the company.
On Twitter @alz_gal
AT AAIC 2016
Key clinical point: The anti-tau drug LMTX didn’t improve cognition or function as add-on therapy for Alzheimer’s disease, but did offer hints of benefit as a monotherapy.
Major finding: Patients who took LMTX as monotherapy declined 6 points less on the ADAS-cog scale over 15 months, compared with those who took placebo.
Data source: The trial randomized 891 patients to placebo or to LMTX 75 mg twice daily or LMTX 125 mg twice daily .
Disclosures: The study was sponsored by TauRx. Dr. Serge Gauthier is on the company’s advisory board. Dr. David Knopman is an investigator on a LMTX study for frontotemporal dementia, but has no financial ties with the company.
The benefits of doing ultrasound exams in your office
Point-of-care ultrasound is increasingly being integrated into clinical practice, as an adjunct to the physical examination and patient history,1 and into medical school curricula across North America.2,3 Research confirms that this technology improves patient survival in emergency medicine settings;4 however, the benefits of point-of-care ultrasound administered by family physicians (FPs) in the office setting are less well documented.
Here we provide a comprehensive review of the indications for ultrasound in the office setting, which range from diagnosing musculoskeletal injuries and guiding injections to screening for abdominal aortic aneurysm (AAA). We also address the accuracy and cost-effectiveness of ultrasound use and the training needed to make family medicine ultrasound (FAMUS) successful.
Ultrasound: A useful screening tool for abdominal aortic aneurysm
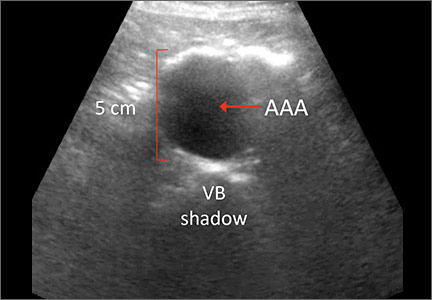
The US Preventive Services Task Force (USPSTF) recommends one-time screening for abdominal aortic aneurysm (AAA) in men ages 65 to 75 years who have ever smoked (See: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.) Ultrasound is a reliable tool for identifying AAA5 (FIGURE 1); its sensitivity and specificity range from 94% to 98.9% and 98% to 100%, respectively.6-9 It is also superior to physical examination for AAAs,10 which has a sensitivity of 29% for small AAAs (30-39 mm) and 76% for larger AAAs (>50 mm).11
Most importantly, research has demonstrated that long-term mortality benefits are associated with ultrasound screening of asymptomatic patients for AAA. For example, one study found that screening asymptomatic men ages 65 to 74 (a population-based sample, with no particular risk factors) for AAA resulted in a reduction in all-cause mortality and that the benefit of AAA-related mortality continued to accumulate throughout follow-up.12
In fact, nationwide programs to screen for AAA using ultrasound have been established in England, Northern Ireland, Scotland, Sweden, the United States, and Wales to help prevent deaths associated with AAA rupture.13 Despite the documented benefits of ultrasound screening for AAA, a large retrospective cohort study conducted in an American integrated health care system found that only about 9% of patients eligible for screening according to USPSTF guidelines were screened for AAA with ultrasound in primary care practices in 2012.14
While most AAA screening occurs in the hospital, screening for the condition can be just as easily and effectively performed in an FP’s office or outpatient clinic. A Canadian prospective observational study demonstrated that aortic diameter measurements were comparable whether they were obtained by ultrasound performed by an office-based physician (who had completed an emergency ultrasonography course and performed at least 50 ultrasonographer-supervised ultrasound scans of the aorta), or by a hospital-based technologist whose scans were then reviewed by a radiologist.15 (See the TABLE for an overview of the research involving family medicine ultrasound.)
The office-based scans had a high degree of correlation (0.81) with the hospital-based ones, a sensitivity and specificity of 100%, and lasted a mean of 3.5 minutes. The researchers concluded that ultrasound screening for AAA can be safely performed in the office setting by FPs who are trained to use point-of-care ultrasound technology, and that the screening can be completed within the time constraints of a typical family practice office visit.15
In a separate study, cardiologists compared hand-held ultrasound screening for AAA to standard 2-dimensional echocardiography. This study found that screening for AAA in an outpatient clinic with a hand-held ultrasound device is feasible and accurate with a sensitivity of 88% and a specificity of 98%.16
Ultrasound in the obstetrician’s office—and the FP’s office, too
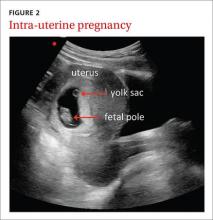
The use of ultrasound in obstetrics (FIGURE 2) is particularly well documented, with evidence supporting the use of FAMUS for various obstetrical indications dating back 30 years.17 The American Academy of Family Physicians has a position paper endorsing diagnostic ultrasound for women’s health care and has offered obstetric ultrasound courses organized by, and for, FPs since 1989.18
In a prospective observational study conducted in the United Kingdom, an FP and a nurse midwife used ultrasound to assess 240 pregnant women presenting with vaginal bleeding in early pregnancy.19 Fetal heartbeat detection by an office ultrasound scan predicted fetal progression to 20 weeks with a sensitivity of 97% and a specificity of 98%. The clinicians also detected anomalies such as molar pregnancy, blighted ovum, and ectopic pregnancy.
FAMUS and its ability to accurately estimate delivery date was examined in another prospective study involving 186 patients at a community health center.20 Accuracy for the estimated date of delivery was 96% using stratified confidence intervals for first-, second-, and third-trimester examinations. The office-based ultrasound scans also detected one case of placenta previa, one fetal death, and 2 unsuspected twin pregnancies. Another study showed no difference in estimations of gestational age provided by ultrasound performed by supervised FP residents with 3 years’ ultrasound training (including 3 lectures per year and an annual 4-hour workshop), and radiologists.21
Further evidence that FAMUS can confirm fetal death and multiple gestations was provided by a retrospective review of almost 498 obstetric ultrasound examinations.22 FPs accurately predicted the presence or absence of fetal death, multiple gestations, and the estimated date of confinement. Another study demonstrated that 86% of 248 FP obstetrical scans were judged acceptable by a radiologist, 10% were repeated due to technical errors and subsequently found to be acceptable, and 3% were unacceptable and referred for formal ultrasound.23 These scans were performed by FPs who completed 5 days of theory and hands-on training and 3 half-days of apprenticeship in an ultrasound laboratory.
In a study conducted in Tanzania, bedside ultrasound scans performed by nurse midwives had 100% agreement with scans performed by a sonographer when evaluating for twins, the presence of fetal heartbeat, or fetal positioning. Overall, bedside ultrasound aided in the diagnosis (39%) and management plan (22%) of 542 patients.24 It is important to note, as highlighted in a multisite study, that consultation with specialists when appropriate is paramount to the successful use of ultrasound by the FP for prenatal care.25
Guiding joint injections, assessing LV function
Sports/exercise medicine. FPs with expertise in sports and exercise medicine commonly use office ultrasound to diagnose musculoskeletal (MSK) injuries, including rotator cuff tears, muscle ruptures, tendinitis, and bursitis.26 It is superior to magnetic resonance imaging (MRI) in terms of cost-to-benefit ratio, precision, and sensitivity (due, in part, to the fact that clinicians can obtain patient feedback during the examination).26 In addition, a review of office-based procedures for MSK indications demonstrated the usefulness of ultrasound for the guidance of joint aspirations and joint and tendon injections.27 Ultrasound guidance is commonly used to ensure procedural accuracy during aspirations and injections of the shoulder (glenohumeral joint; subacromial bursa), elbow, wrist (carpal tunnel tendons), hip, knee, and ankle.27-29
Cardiology (FIGURE 3). General practitioners in Norway found that 8 hours of training on a hand-held ultrasound device was sufficient to assess left ventricular function with a sensitivity and specificity of 78% and 83%, respectively.30 Their measurements of septal mitral annular excursion (a surrogate measurement of left ventricular function) were similar to those of a cardiologist using the same device and added no more than 5 minutes to the examination.
Other uses. In a separate study, military FPs with 16 hours of training found that FAMUS was easy to learn and effective in the outpatient and inpatient setting for the detection of AAA, trauma, musculoskeletal injuries, and certain obstetric, echocardiographic, and biliary indications.31 They reported that the average time spent per ultrasound examination was one to 5 minutes for the majority of the indications.
The authors of a retrospective study involving a suburban family practice reported that FAMUS was successfully used to identify the causes of epigastric and right upper quadrant pain, and to check post-void residual urinary bladder volume.32
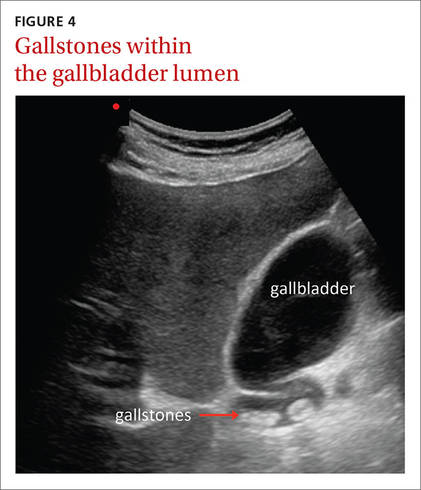
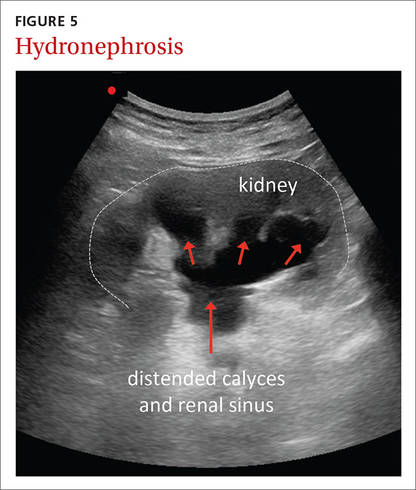
The ultrasound-assisted physical examination can detect pathologies not apparent on history and physical examination alone (FIGURES 4 and 5). In one study, an FP used ultrasound in the office to identify pathologies in 31% of patients that were not detected on physical examination alone. The pathologies included AAAs, a thyroid cyst, mitral stenosis, gallstones, renal cysts, urinary retention, hydronephrosis, ectopic kidney, and an endometrial tumor.33
 |  |
In another study, an FP performed ultrasound examinations on 189 patients during their annual exams.34 The technology identified pathologies that were not suspected after clinical assessment in 22% of these patients. With the emphasis in the current clinical landscape on choosing diagnostic tests wisely, it will be important to determine if findings like these positively impact patient care.35,36
Portable ultrasound machines are affordable
The relative affordability of portable ultrasound contributes to the cost-effectiveness of FAMUS. For FPs seeking to initiate an office-based ultrasound program, expenses to consider include the price of the machine itself, which ranges from $7500 to $50,000, depending on the technology included. Other expenses include the cost of disposables (eg, ultrasound gel and disinfectant wipes or spray), which may total about $400 per year.
In-office exams facilitate savings elsewhere. Other factors that contribute to the cost-effectiveness of FAMUS include reduced radiologist expenses and hospital visits. The cost savings of in-office ultrasound was highlighted almost 30 years ago when the cost of a FAMUS obstetrical scan was reported to be half that of a radiologist scan.23 This same study reported that increased costs for additional investigations caused by incidental findings using FAMUS could be offset by the decreased costs associated with an earlier diagnosis of serious conditions.23
A 2002 study demonstrated that office-based FAMUS scans (N=131) reduced the number of hospital scans, emergency admissions, and outpatient and inpatient hospital visits.37 Although the unit cost of a FAMUS scan was higher than an inpatient one, the total cost of the FAMUS scan was lower due to decreased hospital visits. In addition, research has shown that patients are more satisfied with office-based ultrasound examinations and prefer ultrasound performed by their FP to hospital-based ultrasound scans.31,37
Training: Cost and availability
Training in office-based ultrasound is available at the undergraduate, postgraduate, and continuing medical education levels. Undergraduate bedside ultrasound education is evident in medical schools around the globe including in Australia, Austria, Canada, China, Germany, France, the United States, and the United Kingdom.3 In an American survey of family medicine residency programs published in 2015, only 2.2% reported an established ultrasound curriculum; however, 29% had started a program within the past year.38 In Canada, one- and 2-day bedside ultrasound courses are offered to family medicine residents at a number of universities. And continuing medical education (CME) courses in bedside ultrasound are available to physicians on a regular basis internationally.39 In North America, CME courses exist specifically for urban and rural family medicine clinicians,40-43 and offer training for a wide range of applications.
Courses are often available for $1000 to $2000. Many of these courses run over a one- to 3-day period. Some provide a general overview of ultrasound for the primary care physician while others specialize in topics such as musculoskeletal uses, obstetric uses, or emergency department echocardiography.40-44
Challenges remain
More research is necessary to demonstrate that office-based ultrasound produces patient outcomes that are comparable to those resulting from hospital-based ultrasound. Also, bedside ultrasound is only as good as the operator who performs the examination,45 which highlights the importance of developing bedside ultrasound training programs tailored for FPs. National policies are essential for standardizing indications, training, and credentialing so that this effective tool can be used in a safe and effective manner.
CORRESPONDENCE
Peter Steinmetz, MD, CCFP, St. Mary’s Hospital, 3830 Ave Lacombe, Montreal, Quebec, Canada H3T1M5; peter.steinmetz@mcgill.ca.
ACKNOWLEDGEMENTS
We thank Assistant Professor Marion Dove, MD, CCFP, Department of Family Medicine, McGill University, for her suggestions and critical review of an earlier version of the manuscript. The term FAMUS is pending registration and is advertised with the Canadian Intellectual Property Office (Steinmetz, Volume 63, Issue 3217).
1. Solomon SD, Saldana F. Point-of-care ultrasound in medical education - stop listening and look. N Engl J Med. 2014;370:1083-1085.
2. Bahner DP, Goldman E, Way D, et al. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686.
3. Steinmetz P, Dobrescu O, Oleskevich S, et al. Bedside ultrasound education in Canadian medical schools: a national survey. Can Med Educ J. 2016;7:e78-e86.
4. Deshpande R, Akhtar S, Haddadin AS. Utility of ultrasound in the ICU. Curr Opin Anaesthesiol. 2014;27:123-132.
5. Wilmink ABM, Hubbard CSFF, Quick CRG. Quality of the measurement of the infrarenal aortic diameter by ultrasound. J Med Screen. 1997;4:49-53.
6. Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455-460.
7. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
8. Nusbaum JW, Freimanis AK, Thomford NR. Echography in the diagnosis of abdominal aortic aneurysm. Arch Surg. 1971;102:385-388.
9. Wilmink ABM, Forshaw M, Quick CRG, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125-127.
10. Lynch RM. Accuracy of abdominal examination in the diagnosis of non-ruptured abdominal aortic aneurysm. Accid Emerg Nurs. 2004;12:99-107.
11. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77-82.
12. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
13. Stather PW, Dattani N, Bown MJ, et al. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45:231-234.
14. Ruff AL, Teng K, Hu B, et al. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283-288.
15. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-178.
16. Vourvouri EC, Poldermans D, Schinkel AF, et al. Abdominal aortic aneurysm screening using a hand-held ultrasound device. “A pilot study”. Eur J Vasc Endovasc Surg. 2001;22:352-354.
17. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
18. American Academy of Family Physicians. Position Paper: Diagnostic ultrasonography in women’s health care. 2013. Available at http://www.aafp.org/about/policies/all/ultrasonography-diagnostic.html. Accessed 2013.
19. Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract. 1996;46:7-9.
20. Rodney WM, Prislin MD, Orientale E, et al. Family practice obstetric ultrasound in an urban community health center. Birth outcomes and examination accuracy of the initial 227 cases. J Fam Pract. 1990;30:163-168.
21. Keith R, Frisch L. Fetal biometry: a comparison of family physicians and radiologists. Fam Med. 2001;33:111-114.
22. Ornstein SM, Smith MA, Peggs J, et al. Obstetric ultrasound by family physicians. Adequacy as assessed by pregnancy outcome. J Fam Pract. 1990;30:403-408.
23. Hahn RG, Ho S, Roi LD, et al. Cost-effectiveness of office obstetrical ultrasound in family practice: preliminary considerations. J Am Board Fam Pract. 1988;1:33-38.
24. Stein W, Katunda I, Butoto C. A two-level ultrasonographic service in a maternity care unit of a rural district hospital in Tanzania. Trop Doct. 2008;38:125-126.
25. Morgan WC, Rodney WM, Hahn R, et al. Ultrasound for the primary care physician. Applications in family-centered obstetrics. Postgrad Med. 1988;83:103-107.
26. Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21:57-61.
27. Royall NA, Farrin E, Bahner DP, et al. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2:57-66.
28. Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin; New York: Springer; 2007.
29. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer; 2011.
30. Mjolstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
31. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-477.
32. Chan VSP, Piterman L, McCall L. Use of clinical ultrasonography in an Australian suburban family practice: its indications and findings. Hong Kong Practitioner. 1999;21:405-415.
33. Siepel T, Clifford DS, James PA, et al. The ultrasound-assisted physical examination in the periodic health evaluation of the elderly. J Fam Pract. 2000;49:628-632.
34. Rosenthal TC, Siepel T, Zubler J, et al. The use of ultrasonography to scan the abdomen of patients presenting for routine physical examinations. J Fam Pract. 1994;38:380-385.
35. Choosing Wisely Canada. Available at: http://www.choosingwiselycanada.org. Accessed 2016.
36. Hale I. Add to cart? Can Fam Physician. 2015;61:937-939.
37. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med. 2002;24:88-94.
38. Hall JW, Holman H, Bornemann P, et al. Point of Care Ultrasound in Family Medicine Residency Programs: A CERA Study. Fam Med. 2015;47:706-711.
39. WINFOCUS-World Interactive Network Focused on Critical UltraSound. Available at: http://www.winfocus.it/#winfocus. Accessed 2016.
40. McGill University. McGill Ultrasound Evaluation Program (MUSE). Bedside ultrasound course for primary care clinicians (MUSE 1.0). Available at: www.mcgill.ca/medsimcentre/muse. Accessed 2016.
41. The University of British Columbia. Faculty of Medicine UBC CPD (Continuing Professional Development). CPD/CME courses. Available at: http://ubccpd.ca/courses?combine=ultrasound&field_target_audience_tid=3&field_learning_type_tid=All&field_location_tid=All&field_cost_tid=All&
field_credit_type_tid=All&field_number_of_credits_tid=All&field_event_date_value_1%5Bvalue%5D%5Bdate%5D=. Accessed 2016.
42. Emergency Department Echo (EDE). Available at: www.edecourse.com. Accessed 2016.
43. McGill University. MUSE 2.0 Advanced Bedside Ultrasound Course. Available at: http://www.mcgill.ca/medsimcentre/channels/event/muse-20-advanced-bedside-ultrasound-course-256963. Accessed 2016.
44. Gulfcoast Ultrasound Institute. Available at: https://www.gcus.com. Accessed 2016.
45. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79-85.
Point-of-care ultrasound is increasingly being integrated into clinical practice, as an adjunct to the physical examination and patient history,1 and into medical school curricula across North America.2,3 Research confirms that this technology improves patient survival in emergency medicine settings;4 however, the benefits of point-of-care ultrasound administered by family physicians (FPs) in the office setting are less well documented.
Here we provide a comprehensive review of the indications for ultrasound in the office setting, which range from diagnosing musculoskeletal injuries and guiding injections to screening for abdominal aortic aneurysm (AAA). We also address the accuracy and cost-effectiveness of ultrasound use and the training needed to make family medicine ultrasound (FAMUS) successful.
Ultrasound: A useful screening tool for abdominal aortic aneurysm
The US Preventive Services Task Force (USPSTF) recommends one-time screening for abdominal aortic aneurysm (AAA) in men ages 65 to 75 years who have ever smoked (See: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.) Ultrasound is a reliable tool for identifying AAA5 (FIGURE 1); its sensitivity and specificity range from 94% to 98.9% and 98% to 100%, respectively.6-9 It is also superior to physical examination for AAAs,10 which has a sensitivity of 29% for small AAAs (30-39 mm) and 76% for larger AAAs (>50 mm).11
Most importantly, research has demonstrated that long-term mortality benefits are associated with ultrasound screening of asymptomatic patients for AAA. For example, one study found that screening asymptomatic men ages 65 to 74 (a population-based sample, with no particular risk factors) for AAA resulted in a reduction in all-cause mortality and that the benefit of AAA-related mortality continued to accumulate throughout follow-up.12
In fact, nationwide programs to screen for AAA using ultrasound have been established in England, Northern Ireland, Scotland, Sweden, the United States, and Wales to help prevent deaths associated with AAA rupture.13 Despite the documented benefits of ultrasound screening for AAA, a large retrospective cohort study conducted in an American integrated health care system found that only about 9% of patients eligible for screening according to USPSTF guidelines were screened for AAA with ultrasound in primary care practices in 2012.14
While most AAA screening occurs in the hospital, screening for the condition can be just as easily and effectively performed in an FP’s office or outpatient clinic. A Canadian prospective observational study demonstrated that aortic diameter measurements were comparable whether they were obtained by ultrasound performed by an office-based physician (who had completed an emergency ultrasonography course and performed at least 50 ultrasonographer-supervised ultrasound scans of the aorta), or by a hospital-based technologist whose scans were then reviewed by a radiologist.15 (See the TABLE for an overview of the research involving family medicine ultrasound.)
The office-based scans had a high degree of correlation (0.81) with the hospital-based ones, a sensitivity and specificity of 100%, and lasted a mean of 3.5 minutes. The researchers concluded that ultrasound screening for AAA can be safely performed in the office setting by FPs who are trained to use point-of-care ultrasound technology, and that the screening can be completed within the time constraints of a typical family practice office visit.15
In a separate study, cardiologists compared hand-held ultrasound screening for AAA to standard 2-dimensional echocardiography. This study found that screening for AAA in an outpatient clinic with a hand-held ultrasound device is feasible and accurate with a sensitivity of 88% and a specificity of 98%.16
Ultrasound in the obstetrician’s office—and the FP’s office, too
The use of ultrasound in obstetrics (FIGURE 2) is particularly well documented, with evidence supporting the use of FAMUS for various obstetrical indications dating back 30 years.17 The American Academy of Family Physicians has a position paper endorsing diagnostic ultrasound for women’s health care and has offered obstetric ultrasound courses organized by, and for, FPs since 1989.18
In a prospective observational study conducted in the United Kingdom, an FP and a nurse midwife used ultrasound to assess 240 pregnant women presenting with vaginal bleeding in early pregnancy.19 Fetal heartbeat detection by an office ultrasound scan predicted fetal progression to 20 weeks with a sensitivity of 97% and a specificity of 98%. The clinicians also detected anomalies such as molar pregnancy, blighted ovum, and ectopic pregnancy.
FAMUS and its ability to accurately estimate delivery date was examined in another prospective study involving 186 patients at a community health center.20 Accuracy for the estimated date of delivery was 96% using stratified confidence intervals for first-, second-, and third-trimester examinations. The office-based ultrasound scans also detected one case of placenta previa, one fetal death, and 2 unsuspected twin pregnancies. Another study showed no difference in estimations of gestational age provided by ultrasound performed by supervised FP residents with 3 years’ ultrasound training (including 3 lectures per year and an annual 4-hour workshop), and radiologists.21
Further evidence that FAMUS can confirm fetal death and multiple gestations was provided by a retrospective review of almost 498 obstetric ultrasound examinations.22 FPs accurately predicted the presence or absence of fetal death, multiple gestations, and the estimated date of confinement. Another study demonstrated that 86% of 248 FP obstetrical scans were judged acceptable by a radiologist, 10% were repeated due to technical errors and subsequently found to be acceptable, and 3% were unacceptable and referred for formal ultrasound.23 These scans were performed by FPs who completed 5 days of theory and hands-on training and 3 half-days of apprenticeship in an ultrasound laboratory.
In a study conducted in Tanzania, bedside ultrasound scans performed by nurse midwives had 100% agreement with scans performed by a sonographer when evaluating for twins, the presence of fetal heartbeat, or fetal positioning. Overall, bedside ultrasound aided in the diagnosis (39%) and management plan (22%) of 542 patients.24 It is important to note, as highlighted in a multisite study, that consultation with specialists when appropriate is paramount to the successful use of ultrasound by the FP for prenatal care.25
Guiding joint injections, assessing LV function
Sports/exercise medicine. FPs with expertise in sports and exercise medicine commonly use office ultrasound to diagnose musculoskeletal (MSK) injuries, including rotator cuff tears, muscle ruptures, tendinitis, and bursitis.26 It is superior to magnetic resonance imaging (MRI) in terms of cost-to-benefit ratio, precision, and sensitivity (due, in part, to the fact that clinicians can obtain patient feedback during the examination).26 In addition, a review of office-based procedures for MSK indications demonstrated the usefulness of ultrasound for the guidance of joint aspirations and joint and tendon injections.27 Ultrasound guidance is commonly used to ensure procedural accuracy during aspirations and injections of the shoulder (glenohumeral joint; subacromial bursa), elbow, wrist (carpal tunnel tendons), hip, knee, and ankle.27-29
Cardiology (FIGURE 3). General practitioners in Norway found that 8 hours of training on a hand-held ultrasound device was sufficient to assess left ventricular function with a sensitivity and specificity of 78% and 83%, respectively.30 Their measurements of septal mitral annular excursion (a surrogate measurement of left ventricular function) were similar to those of a cardiologist using the same device and added no more than 5 minutes to the examination.
Other uses. In a separate study, military FPs with 16 hours of training found that FAMUS was easy to learn and effective in the outpatient and inpatient setting for the detection of AAA, trauma, musculoskeletal injuries, and certain obstetric, echocardiographic, and biliary indications.31 They reported that the average time spent per ultrasound examination was one to 5 minutes for the majority of the indications.
The authors of a retrospective study involving a suburban family practice reported that FAMUS was successfully used to identify the causes of epigastric and right upper quadrant pain, and to check post-void residual urinary bladder volume.32
The ultrasound-assisted physical examination can detect pathologies not apparent on history and physical examination alone (FIGURES 4 and 5). In one study, an FP used ultrasound in the office to identify pathologies in 31% of patients that were not detected on physical examination alone. The pathologies included AAAs, a thyroid cyst, mitral stenosis, gallstones, renal cysts, urinary retention, hydronephrosis, ectopic kidney, and an endometrial tumor.33
 |  |
In another study, an FP performed ultrasound examinations on 189 patients during their annual exams.34 The technology identified pathologies that were not suspected after clinical assessment in 22% of these patients. With the emphasis in the current clinical landscape on choosing diagnostic tests wisely, it will be important to determine if findings like these positively impact patient care.35,36
Portable ultrasound machines are affordable
The relative affordability of portable ultrasound contributes to the cost-effectiveness of FAMUS. For FPs seeking to initiate an office-based ultrasound program, expenses to consider include the price of the machine itself, which ranges from $7500 to $50,000, depending on the technology included. Other expenses include the cost of disposables (eg, ultrasound gel and disinfectant wipes or spray), which may total about $400 per year.
In-office exams facilitate savings elsewhere. Other factors that contribute to the cost-effectiveness of FAMUS include reduced radiologist expenses and hospital visits. The cost savings of in-office ultrasound was highlighted almost 30 years ago when the cost of a FAMUS obstetrical scan was reported to be half that of a radiologist scan.23 This same study reported that increased costs for additional investigations caused by incidental findings using FAMUS could be offset by the decreased costs associated with an earlier diagnosis of serious conditions.23
A 2002 study demonstrated that office-based FAMUS scans (N=131) reduced the number of hospital scans, emergency admissions, and outpatient and inpatient hospital visits.37 Although the unit cost of a FAMUS scan was higher than an inpatient one, the total cost of the FAMUS scan was lower due to decreased hospital visits. In addition, research has shown that patients are more satisfied with office-based ultrasound examinations and prefer ultrasound performed by their FP to hospital-based ultrasound scans.31,37
Training: Cost and availability
Training in office-based ultrasound is available at the undergraduate, postgraduate, and continuing medical education levels. Undergraduate bedside ultrasound education is evident in medical schools around the globe including in Australia, Austria, Canada, China, Germany, France, the United States, and the United Kingdom.3 In an American survey of family medicine residency programs published in 2015, only 2.2% reported an established ultrasound curriculum; however, 29% had started a program within the past year.38 In Canada, one- and 2-day bedside ultrasound courses are offered to family medicine residents at a number of universities. And continuing medical education (CME) courses in bedside ultrasound are available to physicians on a regular basis internationally.39 In North America, CME courses exist specifically for urban and rural family medicine clinicians,40-43 and offer training for a wide range of applications.
Courses are often available for $1000 to $2000. Many of these courses run over a one- to 3-day period. Some provide a general overview of ultrasound for the primary care physician while others specialize in topics such as musculoskeletal uses, obstetric uses, or emergency department echocardiography.40-44
Challenges remain
More research is necessary to demonstrate that office-based ultrasound produces patient outcomes that are comparable to those resulting from hospital-based ultrasound. Also, bedside ultrasound is only as good as the operator who performs the examination,45 which highlights the importance of developing bedside ultrasound training programs tailored for FPs. National policies are essential for standardizing indications, training, and credentialing so that this effective tool can be used in a safe and effective manner.
CORRESPONDENCE
Peter Steinmetz, MD, CCFP, St. Mary’s Hospital, 3830 Ave Lacombe, Montreal, Quebec, Canada H3T1M5; peter.steinmetz@mcgill.ca.
ACKNOWLEDGEMENTS
We thank Assistant Professor Marion Dove, MD, CCFP, Department of Family Medicine, McGill University, for her suggestions and critical review of an earlier version of the manuscript. The term FAMUS is pending registration and is advertised with the Canadian Intellectual Property Office (Steinmetz, Volume 63, Issue 3217).
Point-of-care ultrasound is increasingly being integrated into clinical practice, as an adjunct to the physical examination and patient history,1 and into medical school curricula across North America.2,3 Research confirms that this technology improves patient survival in emergency medicine settings;4 however, the benefits of point-of-care ultrasound administered by family physicians (FPs) in the office setting are less well documented.
Here we provide a comprehensive review of the indications for ultrasound in the office setting, which range from diagnosing musculoskeletal injuries and guiding injections to screening for abdominal aortic aneurysm (AAA). We also address the accuracy and cost-effectiveness of ultrasound use and the training needed to make family medicine ultrasound (FAMUS) successful.
Ultrasound: A useful screening tool for abdominal aortic aneurysm
The US Preventive Services Task Force (USPSTF) recommends one-time screening for abdominal aortic aneurysm (AAA) in men ages 65 to 75 years who have ever smoked (See: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening.) Ultrasound is a reliable tool for identifying AAA5 (FIGURE 1); its sensitivity and specificity range from 94% to 98.9% and 98% to 100%, respectively.6-9 It is also superior to physical examination for AAAs,10 which has a sensitivity of 29% for small AAAs (30-39 mm) and 76% for larger AAAs (>50 mm).11
Most importantly, research has demonstrated that long-term mortality benefits are associated with ultrasound screening of asymptomatic patients for AAA. For example, one study found that screening asymptomatic men ages 65 to 74 (a population-based sample, with no particular risk factors) for AAA resulted in a reduction in all-cause mortality and that the benefit of AAA-related mortality continued to accumulate throughout follow-up.12
In fact, nationwide programs to screen for AAA using ultrasound have been established in England, Northern Ireland, Scotland, Sweden, the United States, and Wales to help prevent deaths associated with AAA rupture.13 Despite the documented benefits of ultrasound screening for AAA, a large retrospective cohort study conducted in an American integrated health care system found that only about 9% of patients eligible for screening according to USPSTF guidelines were screened for AAA with ultrasound in primary care practices in 2012.14
While most AAA screening occurs in the hospital, screening for the condition can be just as easily and effectively performed in an FP’s office or outpatient clinic. A Canadian prospective observational study demonstrated that aortic diameter measurements were comparable whether they were obtained by ultrasound performed by an office-based physician (who had completed an emergency ultrasonography course and performed at least 50 ultrasonographer-supervised ultrasound scans of the aorta), or by a hospital-based technologist whose scans were then reviewed by a radiologist.15 (See the TABLE for an overview of the research involving family medicine ultrasound.)
The office-based scans had a high degree of correlation (0.81) with the hospital-based ones, a sensitivity and specificity of 100%, and lasted a mean of 3.5 minutes. The researchers concluded that ultrasound screening for AAA can be safely performed in the office setting by FPs who are trained to use point-of-care ultrasound technology, and that the screening can be completed within the time constraints of a typical family practice office visit.15
In a separate study, cardiologists compared hand-held ultrasound screening for AAA to standard 2-dimensional echocardiography. This study found that screening for AAA in an outpatient clinic with a hand-held ultrasound device is feasible and accurate with a sensitivity of 88% and a specificity of 98%.16
Ultrasound in the obstetrician’s office—and the FP’s office, too
The use of ultrasound in obstetrics (FIGURE 2) is particularly well documented, with evidence supporting the use of FAMUS for various obstetrical indications dating back 30 years.17 The American Academy of Family Physicians has a position paper endorsing diagnostic ultrasound for women’s health care and has offered obstetric ultrasound courses organized by, and for, FPs since 1989.18
In a prospective observational study conducted in the United Kingdom, an FP and a nurse midwife used ultrasound to assess 240 pregnant women presenting with vaginal bleeding in early pregnancy.19 Fetal heartbeat detection by an office ultrasound scan predicted fetal progression to 20 weeks with a sensitivity of 97% and a specificity of 98%. The clinicians also detected anomalies such as molar pregnancy, blighted ovum, and ectopic pregnancy.
FAMUS and its ability to accurately estimate delivery date was examined in another prospective study involving 186 patients at a community health center.20 Accuracy for the estimated date of delivery was 96% using stratified confidence intervals for first-, second-, and third-trimester examinations. The office-based ultrasound scans also detected one case of placenta previa, one fetal death, and 2 unsuspected twin pregnancies. Another study showed no difference in estimations of gestational age provided by ultrasound performed by supervised FP residents with 3 years’ ultrasound training (including 3 lectures per year and an annual 4-hour workshop), and radiologists.21
Further evidence that FAMUS can confirm fetal death and multiple gestations was provided by a retrospective review of almost 498 obstetric ultrasound examinations.22 FPs accurately predicted the presence or absence of fetal death, multiple gestations, and the estimated date of confinement. Another study demonstrated that 86% of 248 FP obstetrical scans were judged acceptable by a radiologist, 10% were repeated due to technical errors and subsequently found to be acceptable, and 3% were unacceptable and referred for formal ultrasound.23 These scans were performed by FPs who completed 5 days of theory and hands-on training and 3 half-days of apprenticeship in an ultrasound laboratory.
In a study conducted in Tanzania, bedside ultrasound scans performed by nurse midwives had 100% agreement with scans performed by a sonographer when evaluating for twins, the presence of fetal heartbeat, or fetal positioning. Overall, bedside ultrasound aided in the diagnosis (39%) and management plan (22%) of 542 patients.24 It is important to note, as highlighted in a multisite study, that consultation with specialists when appropriate is paramount to the successful use of ultrasound by the FP for prenatal care.25
Guiding joint injections, assessing LV function
Sports/exercise medicine. FPs with expertise in sports and exercise medicine commonly use office ultrasound to diagnose musculoskeletal (MSK) injuries, including rotator cuff tears, muscle ruptures, tendinitis, and bursitis.26 It is superior to magnetic resonance imaging (MRI) in terms of cost-to-benefit ratio, precision, and sensitivity (due, in part, to the fact that clinicians can obtain patient feedback during the examination).26 In addition, a review of office-based procedures for MSK indications demonstrated the usefulness of ultrasound for the guidance of joint aspirations and joint and tendon injections.27 Ultrasound guidance is commonly used to ensure procedural accuracy during aspirations and injections of the shoulder (glenohumeral joint; subacromial bursa), elbow, wrist (carpal tunnel tendons), hip, knee, and ankle.27-29
Cardiology (FIGURE 3). General practitioners in Norway found that 8 hours of training on a hand-held ultrasound device was sufficient to assess left ventricular function with a sensitivity and specificity of 78% and 83%, respectively.30 Their measurements of septal mitral annular excursion (a surrogate measurement of left ventricular function) were similar to those of a cardiologist using the same device and added no more than 5 minutes to the examination.
Other uses. In a separate study, military FPs with 16 hours of training found that FAMUS was easy to learn and effective in the outpatient and inpatient setting for the detection of AAA, trauma, musculoskeletal injuries, and certain obstetric, echocardiographic, and biliary indications.31 They reported that the average time spent per ultrasound examination was one to 5 minutes for the majority of the indications.
The authors of a retrospective study involving a suburban family practice reported that FAMUS was successfully used to identify the causes of epigastric and right upper quadrant pain, and to check post-void residual urinary bladder volume.32
The ultrasound-assisted physical examination can detect pathologies not apparent on history and physical examination alone (FIGURES 4 and 5). In one study, an FP used ultrasound in the office to identify pathologies in 31% of patients that were not detected on physical examination alone. The pathologies included AAAs, a thyroid cyst, mitral stenosis, gallstones, renal cysts, urinary retention, hydronephrosis, ectopic kidney, and an endometrial tumor.33
 |  |
In another study, an FP performed ultrasound examinations on 189 patients during their annual exams.34 The technology identified pathologies that were not suspected after clinical assessment in 22% of these patients. With the emphasis in the current clinical landscape on choosing diagnostic tests wisely, it will be important to determine if findings like these positively impact patient care.35,36
Portable ultrasound machines are affordable
The relative affordability of portable ultrasound contributes to the cost-effectiveness of FAMUS. For FPs seeking to initiate an office-based ultrasound program, expenses to consider include the price of the machine itself, which ranges from $7500 to $50,000, depending on the technology included. Other expenses include the cost of disposables (eg, ultrasound gel and disinfectant wipes or spray), which may total about $400 per year.
In-office exams facilitate savings elsewhere. Other factors that contribute to the cost-effectiveness of FAMUS include reduced radiologist expenses and hospital visits. The cost savings of in-office ultrasound was highlighted almost 30 years ago when the cost of a FAMUS obstetrical scan was reported to be half that of a radiologist scan.23 This same study reported that increased costs for additional investigations caused by incidental findings using FAMUS could be offset by the decreased costs associated with an earlier diagnosis of serious conditions.23
A 2002 study demonstrated that office-based FAMUS scans (N=131) reduced the number of hospital scans, emergency admissions, and outpatient and inpatient hospital visits.37 Although the unit cost of a FAMUS scan was higher than an inpatient one, the total cost of the FAMUS scan was lower due to decreased hospital visits. In addition, research has shown that patients are more satisfied with office-based ultrasound examinations and prefer ultrasound performed by their FP to hospital-based ultrasound scans.31,37
Training: Cost and availability
Training in office-based ultrasound is available at the undergraduate, postgraduate, and continuing medical education levels. Undergraduate bedside ultrasound education is evident in medical schools around the globe including in Australia, Austria, Canada, China, Germany, France, the United States, and the United Kingdom.3 In an American survey of family medicine residency programs published in 2015, only 2.2% reported an established ultrasound curriculum; however, 29% had started a program within the past year.38 In Canada, one- and 2-day bedside ultrasound courses are offered to family medicine residents at a number of universities. And continuing medical education (CME) courses in bedside ultrasound are available to physicians on a regular basis internationally.39 In North America, CME courses exist specifically for urban and rural family medicine clinicians,40-43 and offer training for a wide range of applications.
Courses are often available for $1000 to $2000. Many of these courses run over a one- to 3-day period. Some provide a general overview of ultrasound for the primary care physician while others specialize in topics such as musculoskeletal uses, obstetric uses, or emergency department echocardiography.40-44
Challenges remain
More research is necessary to demonstrate that office-based ultrasound produces patient outcomes that are comparable to those resulting from hospital-based ultrasound. Also, bedside ultrasound is only as good as the operator who performs the examination,45 which highlights the importance of developing bedside ultrasound training programs tailored for FPs. National policies are essential for standardizing indications, training, and credentialing so that this effective tool can be used in a safe and effective manner.
CORRESPONDENCE
Peter Steinmetz, MD, CCFP, St. Mary’s Hospital, 3830 Ave Lacombe, Montreal, Quebec, Canada H3T1M5; peter.steinmetz@mcgill.ca.
ACKNOWLEDGEMENTS
We thank Assistant Professor Marion Dove, MD, CCFP, Department of Family Medicine, McGill University, for her suggestions and critical review of an earlier version of the manuscript. The term FAMUS is pending registration and is advertised with the Canadian Intellectual Property Office (Steinmetz, Volume 63, Issue 3217).
1. Solomon SD, Saldana F. Point-of-care ultrasound in medical education - stop listening and look. N Engl J Med. 2014;370:1083-1085.
2. Bahner DP, Goldman E, Way D, et al. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686.
3. Steinmetz P, Dobrescu O, Oleskevich S, et al. Bedside ultrasound education in Canadian medical schools: a national survey. Can Med Educ J. 2016;7:e78-e86.
4. Deshpande R, Akhtar S, Haddadin AS. Utility of ultrasound in the ICU. Curr Opin Anaesthesiol. 2014;27:123-132.
5. Wilmink ABM, Hubbard CSFF, Quick CRG. Quality of the measurement of the infrarenal aortic diameter by ultrasound. J Med Screen. 1997;4:49-53.
6. Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455-460.
7. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
8. Nusbaum JW, Freimanis AK, Thomford NR. Echography in the diagnosis of abdominal aortic aneurysm. Arch Surg. 1971;102:385-388.
9. Wilmink ABM, Forshaw M, Quick CRG, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125-127.
10. Lynch RM. Accuracy of abdominal examination in the diagnosis of non-ruptured abdominal aortic aneurysm. Accid Emerg Nurs. 2004;12:99-107.
11. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77-82.
12. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
13. Stather PW, Dattani N, Bown MJ, et al. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45:231-234.
14. Ruff AL, Teng K, Hu B, et al. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283-288.
15. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-178.
16. Vourvouri EC, Poldermans D, Schinkel AF, et al. Abdominal aortic aneurysm screening using a hand-held ultrasound device. “A pilot study”. Eur J Vasc Endovasc Surg. 2001;22:352-354.
17. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
18. American Academy of Family Physicians. Position Paper: Diagnostic ultrasonography in women’s health care. 2013. Available at http://www.aafp.org/about/policies/all/ultrasonography-diagnostic.html. Accessed 2013.
19. Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract. 1996;46:7-9.
20. Rodney WM, Prislin MD, Orientale E, et al. Family practice obstetric ultrasound in an urban community health center. Birth outcomes and examination accuracy of the initial 227 cases. J Fam Pract. 1990;30:163-168.
21. Keith R, Frisch L. Fetal biometry: a comparison of family physicians and radiologists. Fam Med. 2001;33:111-114.
22. Ornstein SM, Smith MA, Peggs J, et al. Obstetric ultrasound by family physicians. Adequacy as assessed by pregnancy outcome. J Fam Pract. 1990;30:403-408.
23. Hahn RG, Ho S, Roi LD, et al. Cost-effectiveness of office obstetrical ultrasound in family practice: preliminary considerations. J Am Board Fam Pract. 1988;1:33-38.
24. Stein W, Katunda I, Butoto C. A two-level ultrasonographic service in a maternity care unit of a rural district hospital in Tanzania. Trop Doct. 2008;38:125-126.
25. Morgan WC, Rodney WM, Hahn R, et al. Ultrasound for the primary care physician. Applications in family-centered obstetrics. Postgrad Med. 1988;83:103-107.
26. Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21:57-61.
27. Royall NA, Farrin E, Bahner DP, et al. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2:57-66.
28. Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin; New York: Springer; 2007.
29. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer; 2011.
30. Mjolstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
31. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-477.
32. Chan VSP, Piterman L, McCall L. Use of clinical ultrasonography in an Australian suburban family practice: its indications and findings. Hong Kong Practitioner. 1999;21:405-415.
33. Siepel T, Clifford DS, James PA, et al. The ultrasound-assisted physical examination in the periodic health evaluation of the elderly. J Fam Pract. 2000;49:628-632.
34. Rosenthal TC, Siepel T, Zubler J, et al. The use of ultrasonography to scan the abdomen of patients presenting for routine physical examinations. J Fam Pract. 1994;38:380-385.
35. Choosing Wisely Canada. Available at: http://www.choosingwiselycanada.org. Accessed 2016.
36. Hale I. Add to cart? Can Fam Physician. 2015;61:937-939.
37. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med. 2002;24:88-94.
38. Hall JW, Holman H, Bornemann P, et al. Point of Care Ultrasound in Family Medicine Residency Programs: A CERA Study. Fam Med. 2015;47:706-711.
39. WINFOCUS-World Interactive Network Focused on Critical UltraSound. Available at: http://www.winfocus.it/#winfocus. Accessed 2016.
40. McGill University. McGill Ultrasound Evaluation Program (MUSE). Bedside ultrasound course for primary care clinicians (MUSE 1.0). Available at: www.mcgill.ca/medsimcentre/muse. Accessed 2016.
41. The University of British Columbia. Faculty of Medicine UBC CPD (Continuing Professional Development). CPD/CME courses. Available at: http://ubccpd.ca/courses?combine=ultrasound&field_target_audience_tid=3&field_learning_type_tid=All&field_location_tid=All&field_cost_tid=All&
field_credit_type_tid=All&field_number_of_credits_tid=All&field_event_date_value_1%5Bvalue%5D%5Bdate%5D=. Accessed 2016.
42. Emergency Department Echo (EDE). Available at: www.edecourse.com. Accessed 2016.
43. McGill University. MUSE 2.0 Advanced Bedside Ultrasound Course. Available at: http://www.mcgill.ca/medsimcentre/channels/event/muse-20-advanced-bedside-ultrasound-course-256963. Accessed 2016.
44. Gulfcoast Ultrasound Institute. Available at: https://www.gcus.com. Accessed 2016.
45. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79-85.
1. Solomon SD, Saldana F. Point-of-care ultrasound in medical education - stop listening and look. N Engl J Med. 2014;370:1083-1085.
2. Bahner DP, Goldman E, Way D, et al. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686.
3. Steinmetz P, Dobrescu O, Oleskevich S, et al. Bedside ultrasound education in Canadian medical schools: a national survey. Can Med Educ J. 2016;7:e78-e86.
4. Deshpande R, Akhtar S, Haddadin AS. Utility of ultrasound in the ICU. Curr Opin Anaesthesiol. 2014;27:123-132.
5. Wilmink ABM, Hubbard CSFF, Quick CRG. Quality of the measurement of the infrarenal aortic diameter by ultrasound. J Med Screen. 1997;4:49-53.
6. Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med. 2005;29:455-460.
7. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
8. Nusbaum JW, Freimanis AK, Thomford NR. Echography in the diagnosis of abdominal aortic aneurysm. Arch Surg. 1971;102:385-388.
9. Wilmink ABM, Forshaw M, Quick CRG, et al. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125-127.
10. Lynch RM. Accuracy of abdominal examination in the diagnosis of non-ruptured abdominal aortic aneurysm. Accid Emerg Nurs. 2004;12:99-107.
11. Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77-82.
12. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
13. Stather PW, Dattani N, Bown MJ, et al. International variations in AAA screening. Eur J Vasc Endovasc Surg. 2013;45:231-234.
14. Ruff AL, Teng K, Hu B, et al. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283-288.
15. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-178.
16. Vourvouri EC, Poldermans D, Schinkel AF, et al. Abdominal aortic aneurysm screening using a hand-held ultrasound device. “A pilot study”. Eur J Vasc Endovasc Surg. 2001;22:352-354.
17. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
18. American Academy of Family Physicians. Position Paper: Diagnostic ultrasonography in women’s health care. 2013. Available at http://www.aafp.org/about/policies/all/ultrasonography-diagnostic.html. Accessed 2013.
19. Everett CB, Preece E. Women with bleeding in the first 20 weeks of pregnancy: value of general practice ultrasound in detecting fetal heart movement. Br J Gen Pract. 1996;46:7-9.
20. Rodney WM, Prislin MD, Orientale E, et al. Family practice obstetric ultrasound in an urban community health center. Birth outcomes and examination accuracy of the initial 227 cases. J Fam Pract. 1990;30:163-168.
21. Keith R, Frisch L. Fetal biometry: a comparison of family physicians and radiologists. Fam Med. 2001;33:111-114.
22. Ornstein SM, Smith MA, Peggs J, et al. Obstetric ultrasound by family physicians. Adequacy as assessed by pregnancy outcome. J Fam Pract. 1990;30:403-408.
23. Hahn RG, Ho S, Roi LD, et al. Cost-effectiveness of office obstetrical ultrasound in family practice: preliminary considerations. J Am Board Fam Pract. 1988;1:33-38.
24. Stein W, Katunda I, Butoto C. A two-level ultrasonographic service in a maternity care unit of a rural district hospital in Tanzania. Trop Doct. 2008;38:125-126.
25. Morgan WC, Rodney WM, Hahn R, et al. Ultrasound for the primary care physician. Applications in family-centered obstetrics. Postgrad Med. 1988;83:103-107.
26. Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21:57-61.
27. Royall NA, Farrin E, Bahner DP, et al. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2:57-66.
28. Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin; New York: Springer; 2007.
29. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. New York: Springer; 2011.
30. Mjolstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
31. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-477.
32. Chan VSP, Piterman L, McCall L. Use of clinical ultrasonography in an Australian suburban family practice: its indications and findings. Hong Kong Practitioner. 1999;21:405-415.
33. Siepel T, Clifford DS, James PA, et al. The ultrasound-assisted physical examination in the periodic health evaluation of the elderly. J Fam Pract. 2000;49:628-632.
34. Rosenthal TC, Siepel T, Zubler J, et al. The use of ultrasonography to scan the abdomen of patients presenting for routine physical examinations. J Fam Pract. 1994;38:380-385.
35. Choosing Wisely Canada. Available at: http://www.choosingwiselycanada.org. Accessed 2016.
36. Hale I. Add to cart? Can Fam Physician. 2015;61:937-939.
37. Wordsworth S, Scott A. Ultrasound scanning by general practitioners: is it worthwhile? J Public Health Med. 2002;24:88-94.
38. Hall JW, Holman H, Bornemann P, et al. Point of Care Ultrasound in Family Medicine Residency Programs: A CERA Study. Fam Med. 2015;47:706-711.
39. WINFOCUS-World Interactive Network Focused on Critical UltraSound. Available at: http://www.winfocus.it/#winfocus. Accessed 2016.
40. McGill University. McGill Ultrasound Evaluation Program (MUSE). Bedside ultrasound course for primary care clinicians (MUSE 1.0). Available at: www.mcgill.ca/medsimcentre/muse. Accessed 2016.
41. The University of British Columbia. Faculty of Medicine UBC CPD (Continuing Professional Development). CPD/CME courses. Available at: http://ubccpd.ca/courses?combine=ultrasound&field_target_audience_tid=3&field_learning_type_tid=All&field_location_tid=All&field_cost_tid=All&
field_credit_type_tid=All&field_number_of_credits_tid=All&field_event_date_value_1%5Bvalue%5D%5Bdate%5D=. Accessed 2016.
42. Emergency Department Echo (EDE). Available at: www.edecourse.com. Accessed 2016.
43. McGill University. MUSE 2.0 Advanced Bedside Ultrasound Course. Available at: http://www.mcgill.ca/medsimcentre/channels/event/muse-20-advanced-bedside-ultrasound-course-256963. Accessed 2016.
44. Gulfcoast Ultrasound Institute. Available at: https://www.gcus.com. Accessed 2016.
45. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79-85.
Influenza: A vaccine we love to hate
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.
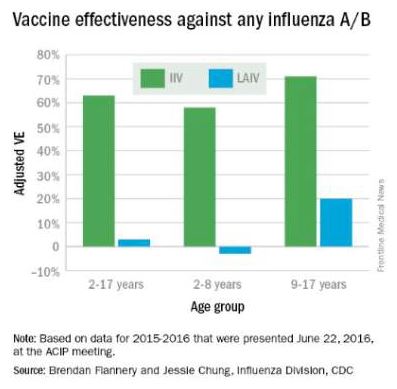
ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at pdnews@frontlinemedcom.com.
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at pdnews@frontlinemedcom.com.
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at pdnews@frontlinemedcom.com.
Sleep Apnea in Later Life More Than Doubles Subsequent Alzheimer’s Disease Risk
DENVER—Obstructive sleep apnea (OSA) diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Omonigho Bubu, MD, MPH, said at the 2016 Annual Meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-response relationship was apparent. The more severe an individual’s OSA, as reflected in a higher apnea–hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
Researchers identified several possible contributing factors for the observed relationship between OSA and Alzheimer’s disease. Patients with OSA and more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were patients with OSA and less severely disrupted sleep measures, added Dr. Bubu, a PhD candidate at the University of South Florida in Tampa.
The study included 756 patients age 65 and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001–2005. They were matched by age, race, sex, BMI, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had various medical problems, but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, BMI, and education level, OSA was independently associated with a 2.2-fold increased risk of Alzheimer’s disease. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for APOE ε4 allele status, which is a known risk factor for OSA and Alzheimer’s disease, so it remains unclear whether the association is “all related to APOE,” said Richard J. Caselli, MD, Professor of Neurology at the Mayo Clinic in Scottsdale, Arizona.
Time to onset of Alzheimer’s disease was shorter in patients with OSA. The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5–14 apnea–hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, which had 15–29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.
Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk, and non-Hispanic whites were at 1.87-fold increased risk. Patients with OSA and a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls; those with at least some college or technical school were at 1.82-fold risk, and patients with OSA who had attended graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease, as opposed to an early expression of the dementing disease process, whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Short sleep duration of less than six hours as well as a mean total sleep time greater than nine hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of six to nine hours. Patients with high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
—Bruce Jancin
Suggested Reading
Kheirandish-Gozal L, Philby ME, Alonso-Álvarez ML, et al. Biomarkers of Alzheimer disease in children with obstructive sleep apnea: effect of adenotonsillectomty. Sleep. 2016;39(6):1225-1232.
DENVER—Obstructive sleep apnea (OSA) diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Omonigho Bubu, MD, MPH, said at the 2016 Annual Meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-response relationship was apparent. The more severe an individual’s OSA, as reflected in a higher apnea–hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
Researchers identified several possible contributing factors for the observed relationship between OSA and Alzheimer’s disease. Patients with OSA and more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were patients with OSA and less severely disrupted sleep measures, added Dr. Bubu, a PhD candidate at the University of South Florida in Tampa.
The study included 756 patients age 65 and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001–2005. They were matched by age, race, sex, BMI, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had various medical problems, but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, BMI, and education level, OSA was independently associated with a 2.2-fold increased risk of Alzheimer’s disease. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for APOE ε4 allele status, which is a known risk factor for OSA and Alzheimer’s disease, so it remains unclear whether the association is “all related to APOE,” said Richard J. Caselli, MD, Professor of Neurology at the Mayo Clinic in Scottsdale, Arizona.
Time to onset of Alzheimer’s disease was shorter in patients with OSA. The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5–14 apnea–hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, which had 15–29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.
Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk, and non-Hispanic whites were at 1.87-fold increased risk. Patients with OSA and a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls; those with at least some college or technical school were at 1.82-fold risk, and patients with OSA who had attended graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease, as opposed to an early expression of the dementing disease process, whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Short sleep duration of less than six hours as well as a mean total sleep time greater than nine hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of six to nine hours. Patients with high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
—Bruce Jancin
DENVER—Obstructive sleep apnea (OSA) diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Omonigho Bubu, MD, MPH, said at the 2016 Annual Meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-response relationship was apparent. The more severe an individual’s OSA, as reflected in a higher apnea–hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
Researchers identified several possible contributing factors for the observed relationship between OSA and Alzheimer’s disease. Patients with OSA and more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were patients with OSA and less severely disrupted sleep measures, added Dr. Bubu, a PhD candidate at the University of South Florida in Tampa.
The study included 756 patients age 65 and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001–2005. They were matched by age, race, sex, BMI, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had various medical problems, but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, BMI, and education level, OSA was independently associated with a 2.2-fold increased risk of Alzheimer’s disease. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for APOE ε4 allele status, which is a known risk factor for OSA and Alzheimer’s disease, so it remains unclear whether the association is “all related to APOE,” said Richard J. Caselli, MD, Professor of Neurology at the Mayo Clinic in Scottsdale, Arizona.
Time to onset of Alzheimer’s disease was shorter in patients with OSA. The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5–14 apnea–hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, which had 15–29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.
Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk, and non-Hispanic whites were at 1.87-fold increased risk. Patients with OSA and a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls; those with at least some college or technical school were at 1.82-fold risk, and patients with OSA who had attended graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease, as opposed to an early expression of the dementing disease process, whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Short sleep duration of less than six hours as well as a mean total sleep time greater than nine hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of six to nine hours. Patients with high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
—Bruce Jancin
Suggested Reading
Kheirandish-Gozal L, Philby ME, Alonso-Álvarez ML, et al. Biomarkers of Alzheimer disease in children with obstructive sleep apnea: effect of adenotonsillectomty. Sleep. 2016;39(6):1225-1232.
Suggested Reading
Kheirandish-Gozal L, Philby ME, Alonso-Álvarez ML, et al. Biomarkers of Alzheimer disease in children with obstructive sleep apnea: effect of adenotonsillectomty. Sleep. 2016;39(6):1225-1232.
Disordered sleep: Ask the right questions to reveal this hidden confounder
It seems like common sense: Sleeping poorly results in not feeling good. The truth is that many of our patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. The busy life that many patients have does not allow adequate time for sleep. In fact, I have encountered patients who think of sleep as an inconvenience that takes away time from other pursuits.
Sleep deprivation in psychiatric disorders
Sleep deprivation occurs when the duration or quality of sleep is inadequate. Inadequate sleep duration can be caused by insomnia or simply not allowing enough time for sleep (1 aspect of poor sleep hygiene). Poor sleep quality often is caused by sleep-disordered breathing.
Sleep deprivation can result in either sleepiness or fatigue. Sleepiness is a propensity to fall asleep; fatigue is a lack of energy that is not alleviated by additional sleep. Fatigue is more likely to be associated with a psychiatric disorder; sleepiness is more predominant in sleep disorders (although there is significant overlap). For example, patients with a major depressive disorder can experience fatigue as much as patients with sleep deprivation, but the latter also is more likely to result in sleepiness. Trouble concentrating is seen in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and sleep deprivation.1
Insomnia or poor sleep hygiene can be diagnosed with a thorough sleep history. I take special care to consider sleep problems by presenting 5 groups of questions to the patient (Box).
Sleep-disordered breathing
A sleep study is required to accurately diagnose sleep-disordered breathing. Unless this diagnosis is specifically looked for, it remains hidden from both physicians and patients. Clues to the presence of sleep-disordered breathing include snorting, snoring, and gasping for air during sleep; witnessed apnea during sleep; nighttime awakening; daytime fatigue; nocturia; mouth breathing or dry mouth; acid reflux; irritability; morning headache; nighttime sweating; and low libido. Risk factors for sleep-disordered breathing include obesity; smoking; menopause; family history; increasing age; and anatomical factors (eg, deviation of the nasal septum; retrognathia; long face syndrome; high-arched narrow hard palate; large tonsils, uvula, or tongue).2
Measuring sleep quality
Some patients are unaware of the extent to which they are sleepy. The most widely used scale to measure sleepiness is the Epworth Sleepiness Scale.3 Sleep specialists view a score of ≥10 on the Epworth scale as indicative of daytime sleepiness. In addition, a patient’s daily consumption of caffeinated beverages can be a clue to excessive sleepiness or, at least, fatigue. If the degree of sleepiness cannot be determined subjectively, objective measures, such as the Multiple Sleep Latency Test (MSLT), can quantify it. In a randomly selected sample from the general population, 13.4% had excessive daytime sleepiness as measured by the MSLT.4
Adult ADHD and sleep deprivation
In my practice, sleep problems confound both the diagnosis and treatment of psychiatric disorders, especially ADHD. Often, patients who report ADHD symptoms have no clear history of ADHD during childhood. In these cases, I always consider the possibility that their ADHD symptoms are due to sleep deprivation. Sleep deprivation can mimic the poor executive function and difficulty concentrating that is often seen in ADHD, because such deprivation is associated with decreased activity in the prefrontal cortex during wakefulness.5
In patients who provide a clear history of ADHD symptoms during childhood, it is possible that inadequate sleep exacerbates ADHD symptoms as adults. Unless sleep deprivation is diagnosed and treated in these patients, they can end up taking a higher-than-necessary dosage of a stimulant. Also, patients who have ADHD might have a difficult time managing their sleep schedule because of poor executive functioning. This, in turn, can result in additional sleep deprivation, thus worsening their ADHD symptoms, creating a vicious circle.
Psychotropics and sedation
Many psychiatric medications list sedation as a side effect. Patients with untreated sleep problems might be more likely to notice this side effect because sleep problems contribute to their fatigue. I have had patients who were unable to tolerate sedative medications until their sleep apnea was treated.
In conclusion
It is important to consider sleep deprivation in your differential diagnosis of psychiatric patients. This will allow for more accurate diagnosis and treatment and, in some cases, can avoid treatment resistance.
1. Stahl SM. Excessive sleepiness. San Diego, CA: NEI Press; 2005.
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736-747.
3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
4. Drake CL, Roehrs TA, Richardson GS, et al. Epidemiology and morbidity of excessive daytime sleepiness. Sleep. 2002;25:A91-A92.
5. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
It seems like common sense: Sleeping poorly results in not feeling good. The truth is that many of our patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. The busy life that many patients have does not allow adequate time for sleep. In fact, I have encountered patients who think of sleep as an inconvenience that takes away time from other pursuits.
Sleep deprivation in psychiatric disorders
Sleep deprivation occurs when the duration or quality of sleep is inadequate. Inadequate sleep duration can be caused by insomnia or simply not allowing enough time for sleep (1 aspect of poor sleep hygiene). Poor sleep quality often is caused by sleep-disordered breathing.
Sleep deprivation can result in either sleepiness or fatigue. Sleepiness is a propensity to fall asleep; fatigue is a lack of energy that is not alleviated by additional sleep. Fatigue is more likely to be associated with a psychiatric disorder; sleepiness is more predominant in sleep disorders (although there is significant overlap). For example, patients with a major depressive disorder can experience fatigue as much as patients with sleep deprivation, but the latter also is more likely to result in sleepiness. Trouble concentrating is seen in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and sleep deprivation.1
Insomnia or poor sleep hygiene can be diagnosed with a thorough sleep history. I take special care to consider sleep problems by presenting 5 groups of questions to the patient (Box).
Sleep-disordered breathing
A sleep study is required to accurately diagnose sleep-disordered breathing. Unless this diagnosis is specifically looked for, it remains hidden from both physicians and patients. Clues to the presence of sleep-disordered breathing include snorting, snoring, and gasping for air during sleep; witnessed apnea during sleep; nighttime awakening; daytime fatigue; nocturia; mouth breathing or dry mouth; acid reflux; irritability; morning headache; nighttime sweating; and low libido. Risk factors for sleep-disordered breathing include obesity; smoking; menopause; family history; increasing age; and anatomical factors (eg, deviation of the nasal septum; retrognathia; long face syndrome; high-arched narrow hard palate; large tonsils, uvula, or tongue).2
Measuring sleep quality
Some patients are unaware of the extent to which they are sleepy. The most widely used scale to measure sleepiness is the Epworth Sleepiness Scale.3 Sleep specialists view a score of ≥10 on the Epworth scale as indicative of daytime sleepiness. In addition, a patient’s daily consumption of caffeinated beverages can be a clue to excessive sleepiness or, at least, fatigue. If the degree of sleepiness cannot be determined subjectively, objective measures, such as the Multiple Sleep Latency Test (MSLT), can quantify it. In a randomly selected sample from the general population, 13.4% had excessive daytime sleepiness as measured by the MSLT.4
Adult ADHD and sleep deprivation
In my practice, sleep problems confound both the diagnosis and treatment of psychiatric disorders, especially ADHD. Often, patients who report ADHD symptoms have no clear history of ADHD during childhood. In these cases, I always consider the possibility that their ADHD symptoms are due to sleep deprivation. Sleep deprivation can mimic the poor executive function and difficulty concentrating that is often seen in ADHD, because such deprivation is associated with decreased activity in the prefrontal cortex during wakefulness.5
In patients who provide a clear history of ADHD symptoms during childhood, it is possible that inadequate sleep exacerbates ADHD symptoms as adults. Unless sleep deprivation is diagnosed and treated in these patients, they can end up taking a higher-than-necessary dosage of a stimulant. Also, patients who have ADHD might have a difficult time managing their sleep schedule because of poor executive functioning. This, in turn, can result in additional sleep deprivation, thus worsening their ADHD symptoms, creating a vicious circle.
Psychotropics and sedation
Many psychiatric medications list sedation as a side effect. Patients with untreated sleep problems might be more likely to notice this side effect because sleep problems contribute to their fatigue. I have had patients who were unable to tolerate sedative medications until their sleep apnea was treated.
In conclusion
It is important to consider sleep deprivation in your differential diagnosis of psychiatric patients. This will allow for more accurate diagnosis and treatment and, in some cases, can avoid treatment resistance.
It seems like common sense: Sleeping poorly results in not feeling good. The truth is that many of our patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. The busy life that many patients have does not allow adequate time for sleep. In fact, I have encountered patients who think of sleep as an inconvenience that takes away time from other pursuits.
Sleep deprivation in psychiatric disorders
Sleep deprivation occurs when the duration or quality of sleep is inadequate. Inadequate sleep duration can be caused by insomnia or simply not allowing enough time for sleep (1 aspect of poor sleep hygiene). Poor sleep quality often is caused by sleep-disordered breathing.
Sleep deprivation can result in either sleepiness or fatigue. Sleepiness is a propensity to fall asleep; fatigue is a lack of energy that is not alleviated by additional sleep. Fatigue is more likely to be associated with a psychiatric disorder; sleepiness is more predominant in sleep disorders (although there is significant overlap). For example, patients with a major depressive disorder can experience fatigue as much as patients with sleep deprivation, but the latter also is more likely to result in sleepiness. Trouble concentrating is seen in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and sleep deprivation.1
Insomnia or poor sleep hygiene can be diagnosed with a thorough sleep history. I take special care to consider sleep problems by presenting 5 groups of questions to the patient (Box).
Sleep-disordered breathing
A sleep study is required to accurately diagnose sleep-disordered breathing. Unless this diagnosis is specifically looked for, it remains hidden from both physicians and patients. Clues to the presence of sleep-disordered breathing include snorting, snoring, and gasping for air during sleep; witnessed apnea during sleep; nighttime awakening; daytime fatigue; nocturia; mouth breathing or dry mouth; acid reflux; irritability; morning headache; nighttime sweating; and low libido. Risk factors for sleep-disordered breathing include obesity; smoking; menopause; family history; increasing age; and anatomical factors (eg, deviation of the nasal septum; retrognathia; long face syndrome; high-arched narrow hard palate; large tonsils, uvula, or tongue).2
Measuring sleep quality
Some patients are unaware of the extent to which they are sleepy. The most widely used scale to measure sleepiness is the Epworth Sleepiness Scale.3 Sleep specialists view a score of ≥10 on the Epworth scale as indicative of daytime sleepiness. In addition, a patient’s daily consumption of caffeinated beverages can be a clue to excessive sleepiness or, at least, fatigue. If the degree of sleepiness cannot be determined subjectively, objective measures, such as the Multiple Sleep Latency Test (MSLT), can quantify it. In a randomly selected sample from the general population, 13.4% had excessive daytime sleepiness as measured by the MSLT.4
Adult ADHD and sleep deprivation
In my practice, sleep problems confound both the diagnosis and treatment of psychiatric disorders, especially ADHD. Often, patients who report ADHD symptoms have no clear history of ADHD during childhood. In these cases, I always consider the possibility that their ADHD symptoms are due to sleep deprivation. Sleep deprivation can mimic the poor executive function and difficulty concentrating that is often seen in ADHD, because such deprivation is associated with decreased activity in the prefrontal cortex during wakefulness.5
In patients who provide a clear history of ADHD symptoms during childhood, it is possible that inadequate sleep exacerbates ADHD symptoms as adults. Unless sleep deprivation is diagnosed and treated in these patients, they can end up taking a higher-than-necessary dosage of a stimulant. Also, patients who have ADHD might have a difficult time managing their sleep schedule because of poor executive functioning. This, in turn, can result in additional sleep deprivation, thus worsening their ADHD symptoms, creating a vicious circle.
Psychotropics and sedation
Many psychiatric medications list sedation as a side effect. Patients with untreated sleep problems might be more likely to notice this side effect because sleep problems contribute to their fatigue. I have had patients who were unable to tolerate sedative medications until their sleep apnea was treated.
In conclusion
It is important to consider sleep deprivation in your differential diagnosis of psychiatric patients. This will allow for more accurate diagnosis and treatment and, in some cases, can avoid treatment resistance.
1. Stahl SM. Excessive sleepiness. San Diego, CA: NEI Press; 2005.
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736-747.
3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
4. Drake CL, Roehrs TA, Richardson GS, et al. Epidemiology and morbidity of excessive daytime sleepiness. Sleep. 2002;25:A91-A92.
5. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
1. Stahl SM. Excessive sleepiness. San Diego, CA: NEI Press; 2005.
2. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736-747.
3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
4. Drake CL, Roehrs TA, Richardson GS, et al. Epidemiology and morbidity of excessive daytime sleepiness. Sleep. 2002;25:A91-A92.
5. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
Is Chemotherapy a Good Choice for Neuroendocrine Tumors?
How effective is chemotherapy in treating neuroendocrine tumors (NETs)? Unfortunately, there is a lack of strong evidence from randomized trials, according to researchers from Gosford Hospital, University of Sydney; Royal North Shore Hospital, Northern Cancer Institute, and Macquarie University Hospitals, all in Australia; and Memorial Sloan Kettering Cancer Center in New York.
Related: Complex Malignancies: A Diagnostic and Therapeutic Trilemma
Few systematic reviews about chemotherapy for patients with NET have been published, and no meta-analyses have been done, the researchers say. They decided to rectify that deficiency and winnowed several hundred “potentially relevant” studies to 8 after examination.
Six studies compared one chemotherapy regimen against another (usually the standard streptozotocin [STZ] plus 5-fluorouracil [5FU]). Two studies compared chemotherapy with interferon (IFN). The researchers found no studies comparing chemotherapy with targeted therapy or somatostatin analogues and none comparing chemotherapy with best-supportive care.
Related: Harnessing Vaccines to Treat Cancers
Their analysis revealed no difference between STZ/5FU and other chemotherapies in response rate, progression-free survival (PFS), 1-year overall survival (OS), and OS. Response rates seemed to be higher for IFN, but no PFS or OS were demonstrated.
Any recommendations for chemotherapy as an option are “largely based on weak evidence,” the researchers charge. The problem, they say, is that the overall quality of the studies was poor. Only one met all features of a randomized trial with low risk of biases. The researchers cite inadequate randomization, lack of blinding, missing data, and selective reporting, among other flaws. In spite of the drawbacks of the studies they examined, their review remains the largest and most comprehensive review and appraisal of the existing evidence of chemotherapy in NET, the researchers say.
Related: Finding Synchronous Cancers
The past few years have seen the “evolution of an impressive array of systemic options,” they conclude, and “it remains to be seen whether chemotherapy shows enough efficacy compared to these options to warrant its side effect profile.” They point out that their review highlights not only the paucity of strong evidence, but also an urgent need of good randomized data—especially on the new, promising chemotherapeutic agents.
Source:
Wong MH, Chan DL, Lee A, et al. PLoS One. 2016;11(6):e0158140.
doi: 10.1371/journal.pone.0158140
How effective is chemotherapy in treating neuroendocrine tumors (NETs)? Unfortunately, there is a lack of strong evidence from randomized trials, according to researchers from Gosford Hospital, University of Sydney; Royal North Shore Hospital, Northern Cancer Institute, and Macquarie University Hospitals, all in Australia; and Memorial Sloan Kettering Cancer Center in New York.
Related: Complex Malignancies: A Diagnostic and Therapeutic Trilemma
Few systematic reviews about chemotherapy for patients with NET have been published, and no meta-analyses have been done, the researchers say. They decided to rectify that deficiency and winnowed several hundred “potentially relevant” studies to 8 after examination.
Six studies compared one chemotherapy regimen against another (usually the standard streptozotocin [STZ] plus 5-fluorouracil [5FU]). Two studies compared chemotherapy with interferon (IFN). The researchers found no studies comparing chemotherapy with targeted therapy or somatostatin analogues and none comparing chemotherapy with best-supportive care.
Related: Harnessing Vaccines to Treat Cancers
Their analysis revealed no difference between STZ/5FU and other chemotherapies in response rate, progression-free survival (PFS), 1-year overall survival (OS), and OS. Response rates seemed to be higher for IFN, but no PFS or OS were demonstrated.
Any recommendations for chemotherapy as an option are “largely based on weak evidence,” the researchers charge. The problem, they say, is that the overall quality of the studies was poor. Only one met all features of a randomized trial with low risk of biases. The researchers cite inadequate randomization, lack of blinding, missing data, and selective reporting, among other flaws. In spite of the drawbacks of the studies they examined, their review remains the largest and most comprehensive review and appraisal of the existing evidence of chemotherapy in NET, the researchers say.
Related: Finding Synchronous Cancers
The past few years have seen the “evolution of an impressive array of systemic options,” they conclude, and “it remains to be seen whether chemotherapy shows enough efficacy compared to these options to warrant its side effect profile.” They point out that their review highlights not only the paucity of strong evidence, but also an urgent need of good randomized data—especially on the new, promising chemotherapeutic agents.
Source:
Wong MH, Chan DL, Lee A, et al. PLoS One. 2016;11(6):e0158140.
doi: 10.1371/journal.pone.0158140
How effective is chemotherapy in treating neuroendocrine tumors (NETs)? Unfortunately, there is a lack of strong evidence from randomized trials, according to researchers from Gosford Hospital, University of Sydney; Royal North Shore Hospital, Northern Cancer Institute, and Macquarie University Hospitals, all in Australia; and Memorial Sloan Kettering Cancer Center in New York.
Related: Complex Malignancies: A Diagnostic and Therapeutic Trilemma
Few systematic reviews about chemotherapy for patients with NET have been published, and no meta-analyses have been done, the researchers say. They decided to rectify that deficiency and winnowed several hundred “potentially relevant” studies to 8 after examination.
Six studies compared one chemotherapy regimen against another (usually the standard streptozotocin [STZ] plus 5-fluorouracil [5FU]). Two studies compared chemotherapy with interferon (IFN). The researchers found no studies comparing chemotherapy with targeted therapy or somatostatin analogues and none comparing chemotherapy with best-supportive care.
Related: Harnessing Vaccines to Treat Cancers
Their analysis revealed no difference between STZ/5FU and other chemotherapies in response rate, progression-free survival (PFS), 1-year overall survival (OS), and OS. Response rates seemed to be higher for IFN, but no PFS or OS were demonstrated.
Any recommendations for chemotherapy as an option are “largely based on weak evidence,” the researchers charge. The problem, they say, is that the overall quality of the studies was poor. Only one met all features of a randomized trial with low risk of biases. The researchers cite inadequate randomization, lack of blinding, missing data, and selective reporting, among other flaws. In spite of the drawbacks of the studies they examined, their review remains the largest and most comprehensive review and appraisal of the existing evidence of chemotherapy in NET, the researchers say.
Related: Finding Synchronous Cancers
The past few years have seen the “evolution of an impressive array of systemic options,” they conclude, and “it remains to be seen whether chemotherapy shows enough efficacy compared to these options to warrant its side effect profile.” They point out that their review highlights not only the paucity of strong evidence, but also an urgent need of good randomized data—especially on the new, promising chemotherapeutic agents.
Source:
Wong MH, Chan DL, Lee A, et al. PLoS One. 2016;11(6):e0158140.
doi: 10.1371/journal.pone.0158140
Clinical value of costly ER/PR testing of ductal carcinoma biopsies questioned
Limiting hormone receptor testing of diagnostic core biopisies containing ductal carcinoma in situ (DCIS) could save up to $35 million in associated health care costs every year in the United States.
The results of a cost analysis conducted by researchers at Johns Hopkins University were so striking that the hospital has now eliminated reflex testing of core needle biopsies for DCIS, Christopher J. VandenBussche, MD, and his colleagues wrote in the American Journal of Surgical Pathology (2016;40:1090-9).
“We suggest that reflex [hormone receptor] testing of core needle biopsy specimens harboring DCIS should not be performed, as the results do not guide the next step in therapy,” wrote Dr. VandenBussche of Johns Hopkins University, Baltimore. “On the basis of this study, we have convinced our clinicians and no longer reflexively perform estrogen- and progesterone-receptor [ER/PR] testing on core needle biopsy specimens with DCIS at Hopkins and encourage other institutions to follow suit.”
Conducting expensive hormone receptor testing of these biopsy samples before surgery doesn’t make clinical or financial sense, for several reasons, the team said:
• Hormone receptor status at biopsy has no effect on the next treatment step, as DCIS patients always progress first to surgical excision, not neoadjuvant hormone therapy.
• Surgery sometimes reveals extensive breast cancer in patents with pure DCIS, and these cancers will always need ER/PR testing, rendering irrelevant biopsy testing.
• Because ER and PR labeling are often heterogeneous in DCIS, negative results for ER/PR on small core needle biopsy specimens would have to be repeated anyway on surgical excision specimens with larger amounts of DCIS, to be sure that the result is truly negative.
• Hormone therapy for DCIS does decrease recurrence, but it doesn’t impact survival – and it does carry significant adverse effects. Therefore, many women refuse hormone therapy if they do have ER/PR-positive tumors.
• The independent role of PR status in DCIS is unproven, so testing for it is not supported by clinical evidence.
To examine the costs associated with reflexive ER/PR testing, the investigators reviewed 58 core needle biopsies of pure DCIS followed by surgical excision. None of the patients had neoadjuvant hormone therapy. Of the 58 tumors, 76% were pure DCIS, and 16% were DCIS with invasive breast cancer. Most of the DCIS (47) tumors were ER+/PR+; 6 were ER-/PR-; and 5 were ER+/PR-.
The team reviewed the records of 49 patients who underwent surgical excision and ended up with a diagnosis of pure DCIS. These included 46 ER-positive cases, and 3 that were ER-negative in both biopsy and excision.
The findings suggested that ER/PR results from either the biopsy and the surgical excision samples were relevant to therapy in only a portion of these patients.
“We found that the ER/PR results after surgical excision impacted therapy in at most only 16 of 49 cases (33%),” they said. These included the 3 ER-/PR-negative cases, which would, in any case, not have triggered hormone therapy; 10 patients who chose hormone therapy despite a Hopkins oncologist’s neutral recommendation; 1 of 2 who took hormone therapy on a Hopkins oncologist’s recommendation; and 2 of 3 who took it after seeing a non-Hopkins oncologist.
“In contrast,” the authors wrote, “in 33 of the cases (67%), the ER/PR result of the DCIS after surgical excision did not impact therapy.” All of these were ER-positive cases, including 4 patients who opted for bilateral mastectomies (no subsequent role for neoadjuvant therapy), 8 who declined to meet with an oncologist, 1 for whom hormone therapy was contraindicated, 8 (of 8) who declined hormone therapy when their Hopkins oncologist did not recommend it, 10 (of 20) who declined hormone therapy when their Hopkins oncologist was neutral about its risk/benefit ratio, 1 (of 2) who declined hormone therapy when the Hopkins oncologist recommended it, and the 1 patient (of 3) who declined hormone therapy after visiting a non-Hopkins oncologist.
After reviewing the costs associated with these cases, “we found that ER testing … costing $20,685.72 ($357 per patient) had been performed unnecessarily,” the investigators said. In addition, if the PR testing – which has never been proven clinically important in DCIS – had been eliminated, there would have been an additional $86.46 savings per patient, a total savings of $5,014.
“Extrapolating the increased cost of $583 per DCIS diagnosis on core needle biopsy to 60,000 new cases of DCIS in the United States each year, reflex core needle biopsy ER/PR testing unnecessarily increases costs by approximately $35 million,” the authors said. “We recommend that ER/PR not be reflexively ordered on core needle biopsy specimens or surgical excision specimens containing DCIS, but instead that ER alone be performed on surgical excision specimens only when hormone therapy is a serious consideration after medical oncology consultation.”
The group noted that this total reflects the total amount billed, not typical reimbursement. “Regardless,” they said, “the cost of this testing is staggering.”
The authors did not mention the study’s funding source. They had no relevant financial disclosures.
On Twitter @Alz_Gal
It is important to note that the authors are not recommending hormone receptor testing be completely abandoned for ductal carcinoma in situ. They are simply suggesting that the testing should be deferred to being performed on the surgical resection specimen rather than the diagnostic core needle biopsy tissue.
The sample size used (58 cases) is clearly extremely small and cannot be used as the basis for sweeping recommendations for the many thousands of DCIS cases that are diagnosed in the United States annually. This study is from a major academic program; other programs may run the risk of patients not having the ER testing done at all if it is no longer a routine component of pathology testing on DCIS tissue.
The case is probably stronger for abandoning PR testing in DCIS, since this value is very unlikely to influence management decisions on its own.
 |
Dr. Lisa Newman |
Other institutions should be encouraged to perform their own analyses to monitor the cost-effectiveness of ER/PR testing on core needle biopsies revealing DCIS. A study of this type should also be considered for large, multisite data sets, such as the American College of Surgeons’ National Cancer Database.
Lisa Newman, MD, FACS, is director of the breast oncology program for the Henry Ford Health System, and medical director for the Henry Ford International Center for the Study of Breast Cancer Subtypes, both in Detroit. She also serves as adjunct professor of health management and policy at the University of Michigan School of Public Health, Detroit. She has no relevant financial disclosures.
It is important to note that the authors are not recommending hormone receptor testing be completely abandoned for ductal carcinoma in situ. They are simply suggesting that the testing should be deferred to being performed on the surgical resection specimen rather than the diagnostic core needle biopsy tissue.
The sample size used (58 cases) is clearly extremely small and cannot be used as the basis for sweeping recommendations for the many thousands of DCIS cases that are diagnosed in the United States annually. This study is from a major academic program; other programs may run the risk of patients not having the ER testing done at all if it is no longer a routine component of pathology testing on DCIS tissue.
The case is probably stronger for abandoning PR testing in DCIS, since this value is very unlikely to influence management decisions on its own.
 |
Dr. Lisa Newman |
Other institutions should be encouraged to perform their own analyses to monitor the cost-effectiveness of ER/PR testing on core needle biopsies revealing DCIS. A study of this type should also be considered for large, multisite data sets, such as the American College of Surgeons’ National Cancer Database.
Lisa Newman, MD, FACS, is director of the breast oncology program for the Henry Ford Health System, and medical director for the Henry Ford International Center for the Study of Breast Cancer Subtypes, both in Detroit. She also serves as adjunct professor of health management and policy at the University of Michigan School of Public Health, Detroit. She has no relevant financial disclosures.
It is important to note that the authors are not recommending hormone receptor testing be completely abandoned for ductal carcinoma in situ. They are simply suggesting that the testing should be deferred to being performed on the surgical resection specimen rather than the diagnostic core needle biopsy tissue.
The sample size used (58 cases) is clearly extremely small and cannot be used as the basis for sweeping recommendations for the many thousands of DCIS cases that are diagnosed in the United States annually. This study is from a major academic program; other programs may run the risk of patients not having the ER testing done at all if it is no longer a routine component of pathology testing on DCIS tissue.
The case is probably stronger for abandoning PR testing in DCIS, since this value is very unlikely to influence management decisions on its own.
 |
Dr. Lisa Newman |
Other institutions should be encouraged to perform their own analyses to monitor the cost-effectiveness of ER/PR testing on core needle biopsies revealing DCIS. A study of this type should also be considered for large, multisite data sets, such as the American College of Surgeons’ National Cancer Database.
Lisa Newman, MD, FACS, is director of the breast oncology program for the Henry Ford Health System, and medical director for the Henry Ford International Center for the Study of Breast Cancer Subtypes, both in Detroit. She also serves as adjunct professor of health management and policy at the University of Michigan School of Public Health, Detroit. She has no relevant financial disclosures.
Limiting hormone receptor testing of diagnostic core biopisies containing ductal carcinoma in situ (DCIS) could save up to $35 million in associated health care costs every year in the United States.
The results of a cost analysis conducted by researchers at Johns Hopkins University were so striking that the hospital has now eliminated reflex testing of core needle biopsies for DCIS, Christopher J. VandenBussche, MD, and his colleagues wrote in the American Journal of Surgical Pathology (2016;40:1090-9).
“We suggest that reflex [hormone receptor] testing of core needle biopsy specimens harboring DCIS should not be performed, as the results do not guide the next step in therapy,” wrote Dr. VandenBussche of Johns Hopkins University, Baltimore. “On the basis of this study, we have convinced our clinicians and no longer reflexively perform estrogen- and progesterone-receptor [ER/PR] testing on core needle biopsy specimens with DCIS at Hopkins and encourage other institutions to follow suit.”
Conducting expensive hormone receptor testing of these biopsy samples before surgery doesn’t make clinical or financial sense, for several reasons, the team said:
• Hormone receptor status at biopsy has no effect on the next treatment step, as DCIS patients always progress first to surgical excision, not neoadjuvant hormone therapy.
• Surgery sometimes reveals extensive breast cancer in patents with pure DCIS, and these cancers will always need ER/PR testing, rendering irrelevant biopsy testing.
• Because ER and PR labeling are often heterogeneous in DCIS, negative results for ER/PR on small core needle biopsy specimens would have to be repeated anyway on surgical excision specimens with larger amounts of DCIS, to be sure that the result is truly negative.
• Hormone therapy for DCIS does decrease recurrence, but it doesn’t impact survival – and it does carry significant adverse effects. Therefore, many women refuse hormone therapy if they do have ER/PR-positive tumors.
• The independent role of PR status in DCIS is unproven, so testing for it is not supported by clinical evidence.
To examine the costs associated with reflexive ER/PR testing, the investigators reviewed 58 core needle biopsies of pure DCIS followed by surgical excision. None of the patients had neoadjuvant hormone therapy. Of the 58 tumors, 76% were pure DCIS, and 16% were DCIS with invasive breast cancer. Most of the DCIS (47) tumors were ER+/PR+; 6 were ER-/PR-; and 5 were ER+/PR-.
The team reviewed the records of 49 patients who underwent surgical excision and ended up with a diagnosis of pure DCIS. These included 46 ER-positive cases, and 3 that were ER-negative in both biopsy and excision.
The findings suggested that ER/PR results from either the biopsy and the surgical excision samples were relevant to therapy in only a portion of these patients.
“We found that the ER/PR results after surgical excision impacted therapy in at most only 16 of 49 cases (33%),” they said. These included the 3 ER-/PR-negative cases, which would, in any case, not have triggered hormone therapy; 10 patients who chose hormone therapy despite a Hopkins oncologist’s neutral recommendation; 1 of 2 who took hormone therapy on a Hopkins oncologist’s recommendation; and 2 of 3 who took it after seeing a non-Hopkins oncologist.
“In contrast,” the authors wrote, “in 33 of the cases (67%), the ER/PR result of the DCIS after surgical excision did not impact therapy.” All of these were ER-positive cases, including 4 patients who opted for bilateral mastectomies (no subsequent role for neoadjuvant therapy), 8 who declined to meet with an oncologist, 1 for whom hormone therapy was contraindicated, 8 (of 8) who declined hormone therapy when their Hopkins oncologist did not recommend it, 10 (of 20) who declined hormone therapy when their Hopkins oncologist was neutral about its risk/benefit ratio, 1 (of 2) who declined hormone therapy when the Hopkins oncologist recommended it, and the 1 patient (of 3) who declined hormone therapy after visiting a non-Hopkins oncologist.
After reviewing the costs associated with these cases, “we found that ER testing … costing $20,685.72 ($357 per patient) had been performed unnecessarily,” the investigators said. In addition, if the PR testing – which has never been proven clinically important in DCIS – had been eliminated, there would have been an additional $86.46 savings per patient, a total savings of $5,014.
“Extrapolating the increased cost of $583 per DCIS diagnosis on core needle biopsy to 60,000 new cases of DCIS in the United States each year, reflex core needle biopsy ER/PR testing unnecessarily increases costs by approximately $35 million,” the authors said. “We recommend that ER/PR not be reflexively ordered on core needle biopsy specimens or surgical excision specimens containing DCIS, but instead that ER alone be performed on surgical excision specimens only when hormone therapy is a serious consideration after medical oncology consultation.”
The group noted that this total reflects the total amount billed, not typical reimbursement. “Regardless,” they said, “the cost of this testing is staggering.”
The authors did not mention the study’s funding source. They had no relevant financial disclosures.
On Twitter @Alz_Gal
Limiting hormone receptor testing of diagnostic core biopisies containing ductal carcinoma in situ (DCIS) could save up to $35 million in associated health care costs every year in the United States.
The results of a cost analysis conducted by researchers at Johns Hopkins University were so striking that the hospital has now eliminated reflex testing of core needle biopsies for DCIS, Christopher J. VandenBussche, MD, and his colleagues wrote in the American Journal of Surgical Pathology (2016;40:1090-9).
“We suggest that reflex [hormone receptor] testing of core needle biopsy specimens harboring DCIS should not be performed, as the results do not guide the next step in therapy,” wrote Dr. VandenBussche of Johns Hopkins University, Baltimore. “On the basis of this study, we have convinced our clinicians and no longer reflexively perform estrogen- and progesterone-receptor [ER/PR] testing on core needle biopsy specimens with DCIS at Hopkins and encourage other institutions to follow suit.”
Conducting expensive hormone receptor testing of these biopsy samples before surgery doesn’t make clinical or financial sense, for several reasons, the team said:
• Hormone receptor status at biopsy has no effect on the next treatment step, as DCIS patients always progress first to surgical excision, not neoadjuvant hormone therapy.
• Surgery sometimes reveals extensive breast cancer in patents with pure DCIS, and these cancers will always need ER/PR testing, rendering irrelevant biopsy testing.
• Because ER and PR labeling are often heterogeneous in DCIS, negative results for ER/PR on small core needle biopsy specimens would have to be repeated anyway on surgical excision specimens with larger amounts of DCIS, to be sure that the result is truly negative.
• Hormone therapy for DCIS does decrease recurrence, but it doesn’t impact survival – and it does carry significant adverse effects. Therefore, many women refuse hormone therapy if they do have ER/PR-positive tumors.
• The independent role of PR status in DCIS is unproven, so testing for it is not supported by clinical evidence.
To examine the costs associated with reflexive ER/PR testing, the investigators reviewed 58 core needle biopsies of pure DCIS followed by surgical excision. None of the patients had neoadjuvant hormone therapy. Of the 58 tumors, 76% were pure DCIS, and 16% were DCIS with invasive breast cancer. Most of the DCIS (47) tumors were ER+/PR+; 6 were ER-/PR-; and 5 were ER+/PR-.
The team reviewed the records of 49 patients who underwent surgical excision and ended up with a diagnosis of pure DCIS. These included 46 ER-positive cases, and 3 that were ER-negative in both biopsy and excision.
The findings suggested that ER/PR results from either the biopsy and the surgical excision samples were relevant to therapy in only a portion of these patients.
“We found that the ER/PR results after surgical excision impacted therapy in at most only 16 of 49 cases (33%),” they said. These included the 3 ER-/PR-negative cases, which would, in any case, not have triggered hormone therapy; 10 patients who chose hormone therapy despite a Hopkins oncologist’s neutral recommendation; 1 of 2 who took hormone therapy on a Hopkins oncologist’s recommendation; and 2 of 3 who took it after seeing a non-Hopkins oncologist.
“In contrast,” the authors wrote, “in 33 of the cases (67%), the ER/PR result of the DCIS after surgical excision did not impact therapy.” All of these were ER-positive cases, including 4 patients who opted for bilateral mastectomies (no subsequent role for neoadjuvant therapy), 8 who declined to meet with an oncologist, 1 for whom hormone therapy was contraindicated, 8 (of 8) who declined hormone therapy when their Hopkins oncologist did not recommend it, 10 (of 20) who declined hormone therapy when their Hopkins oncologist was neutral about its risk/benefit ratio, 1 (of 2) who declined hormone therapy when the Hopkins oncologist recommended it, and the 1 patient (of 3) who declined hormone therapy after visiting a non-Hopkins oncologist.
After reviewing the costs associated with these cases, “we found that ER testing … costing $20,685.72 ($357 per patient) had been performed unnecessarily,” the investigators said. In addition, if the PR testing – which has never been proven clinically important in DCIS – had been eliminated, there would have been an additional $86.46 savings per patient, a total savings of $5,014.
“Extrapolating the increased cost of $583 per DCIS diagnosis on core needle biopsy to 60,000 new cases of DCIS in the United States each year, reflex core needle biopsy ER/PR testing unnecessarily increases costs by approximately $35 million,” the authors said. “We recommend that ER/PR not be reflexively ordered on core needle biopsy specimens or surgical excision specimens containing DCIS, but instead that ER alone be performed on surgical excision specimens only when hormone therapy is a serious consideration after medical oncology consultation.”
The group noted that this total reflects the total amount billed, not typical reimbursement. “Regardless,” they said, “the cost of this testing is staggering.”
The authors did not mention the study’s funding source. They had no relevant financial disclosures.
On Twitter @Alz_Gal
FROM THE AMERICAN JOURNAL OF SURGICAL PATHOLOGY
Key clinical point: Hormone receptor testing of diagnostic core needle biopsies should not be automatic.
Major finding: Limiting testing to excised surgical section specimens could save up to $35 million a year in the United States.
Data source: The cost analysis examined testing and treatment of 58 patients with ductal carcinoma in situ.
Disclosures: The authors had no relevant financial disclosures.
First-in-class agent shows early promise in treating clear cell renal cell carcinoma
CHICAGO – Investigational agent PT2385, an inhibitor of hypoxia-inducible factor 2-alpha (HIF-2alpha), appears safe and tolerable and demonstrated early evidence of clinical activity in heavily pretreated patients with advanced clear cell renal cell carcinoma, investigators reported at the annual meeting of the American Society of Clinical Oncology.
In a small, phase I dose-escalation study, 39% of patients in the study achieved complete response, partial response, or stable disease lasting at least 16 weeks.
PT2385 is a first-in-class, orally administered selective small molecule inhibitor of HIF-2alpha, a transcription factor with a role in clear cell renal cell carcinoma.
“Hypoxia-inducible factor 2-alpha is a key oncogenic driver of clear cell renal cell carcinoma,” Kevin Courtney, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, said at the meeting.
A total of 51 patients (n = 26 for dose escalation cohort and n = 25 in dose expansion cohort) met the study’s eligibility requirements of having a diagnosis of clear cell renal cell carcinoma, an Eastern Cooperative Oncology Group score ranging from 0 to 1, and at least one prior anticancer therapy. The median age of the cohort was 65 years with the youngest participant being 29 years and the oldest being 80 years old. The majority of patients were male (71%), had received four or more prior systemic therapies (53%), and had undergone vascular endothelial growth factor therapy (98%). Patients were treated with continuous twice-daily oral dosing of PT2385 until progression or toxicity. A total of three patients were treated at the 100-mg dose, three at the 200-mg dose, four at the 400-mg dose, seven at the 800-mg dose, six at a dose of 1,200 mg, and three at a dose of 1,800 mg.
“No dose-limiting toxicity was observed at any dose level,” Dr. Courtney said.
Based on safety, pharmokinetic and pharmodynamic data, 800 mg twice daily was the selected dose for the expansion cohort, he reported.
Of 51 patients treated, 1 patient had a complete response, 3 achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks. The most common grade one or two adverse events were anemia (n = 18), peripheral edema (n = 18), fatigue (n = 18), nausea (n = 15), and back pain (n = 12). Only two grade four adverse events (low lymphocyte count) were reported.
“Notably, hypertension was not seen,” Dr. Courtney said.
At the time the study concluded, the four patients who achieved complete or partial responses remained on the treatment, and five had received treatment for a year or longer.
The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
On Twitter @jessnicolecraig
CHICAGO – Investigational agent PT2385, an inhibitor of hypoxia-inducible factor 2-alpha (HIF-2alpha), appears safe and tolerable and demonstrated early evidence of clinical activity in heavily pretreated patients with advanced clear cell renal cell carcinoma, investigators reported at the annual meeting of the American Society of Clinical Oncology.
In a small, phase I dose-escalation study, 39% of patients in the study achieved complete response, partial response, or stable disease lasting at least 16 weeks.
PT2385 is a first-in-class, orally administered selective small molecule inhibitor of HIF-2alpha, a transcription factor with a role in clear cell renal cell carcinoma.
“Hypoxia-inducible factor 2-alpha is a key oncogenic driver of clear cell renal cell carcinoma,” Kevin Courtney, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, said at the meeting.
A total of 51 patients (n = 26 for dose escalation cohort and n = 25 in dose expansion cohort) met the study’s eligibility requirements of having a diagnosis of clear cell renal cell carcinoma, an Eastern Cooperative Oncology Group score ranging from 0 to 1, and at least one prior anticancer therapy. The median age of the cohort was 65 years with the youngest participant being 29 years and the oldest being 80 years old. The majority of patients were male (71%), had received four or more prior systemic therapies (53%), and had undergone vascular endothelial growth factor therapy (98%). Patients were treated with continuous twice-daily oral dosing of PT2385 until progression or toxicity. A total of three patients were treated at the 100-mg dose, three at the 200-mg dose, four at the 400-mg dose, seven at the 800-mg dose, six at a dose of 1,200 mg, and three at a dose of 1,800 mg.
“No dose-limiting toxicity was observed at any dose level,” Dr. Courtney said.
Based on safety, pharmokinetic and pharmodynamic data, 800 mg twice daily was the selected dose for the expansion cohort, he reported.
Of 51 patients treated, 1 patient had a complete response, 3 achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks. The most common grade one or two adverse events were anemia (n = 18), peripheral edema (n = 18), fatigue (n = 18), nausea (n = 15), and back pain (n = 12). Only two grade four adverse events (low lymphocyte count) were reported.
“Notably, hypertension was not seen,” Dr. Courtney said.
At the time the study concluded, the four patients who achieved complete or partial responses remained on the treatment, and five had received treatment for a year or longer.
The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
On Twitter @jessnicolecraig
CHICAGO – Investigational agent PT2385, an inhibitor of hypoxia-inducible factor 2-alpha (HIF-2alpha), appears safe and tolerable and demonstrated early evidence of clinical activity in heavily pretreated patients with advanced clear cell renal cell carcinoma, investigators reported at the annual meeting of the American Society of Clinical Oncology.
In a small, phase I dose-escalation study, 39% of patients in the study achieved complete response, partial response, or stable disease lasting at least 16 weeks.
PT2385 is a first-in-class, orally administered selective small molecule inhibitor of HIF-2alpha, a transcription factor with a role in clear cell renal cell carcinoma.
“Hypoxia-inducible factor 2-alpha is a key oncogenic driver of clear cell renal cell carcinoma,” Kevin Courtney, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, said at the meeting.
A total of 51 patients (n = 26 for dose escalation cohort and n = 25 in dose expansion cohort) met the study’s eligibility requirements of having a diagnosis of clear cell renal cell carcinoma, an Eastern Cooperative Oncology Group score ranging from 0 to 1, and at least one prior anticancer therapy. The median age of the cohort was 65 years with the youngest participant being 29 years and the oldest being 80 years old. The majority of patients were male (71%), had received four or more prior systemic therapies (53%), and had undergone vascular endothelial growth factor therapy (98%). Patients were treated with continuous twice-daily oral dosing of PT2385 until progression or toxicity. A total of three patients were treated at the 100-mg dose, three at the 200-mg dose, four at the 400-mg dose, seven at the 800-mg dose, six at a dose of 1,200 mg, and three at a dose of 1,800 mg.
“No dose-limiting toxicity was observed at any dose level,” Dr. Courtney said.
Based on safety, pharmokinetic and pharmodynamic data, 800 mg twice daily was the selected dose for the expansion cohort, he reported.
Of 51 patients treated, 1 patient had a complete response, 3 achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks. The most common grade one or two adverse events were anemia (n = 18), peripheral edema (n = 18), fatigue (n = 18), nausea (n = 15), and back pain (n = 12). Only two grade four adverse events (low lymphocyte count) were reported.
“Notably, hypertension was not seen,” Dr. Courtney said.
At the time the study concluded, the four patients who achieved complete or partial responses remained on the treatment, and five had received treatment for a year or longer.
The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
On Twitter @jessnicolecraig
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: The first-in-class oral HIF-2alpha inhibitor PT2385 demonstrated early evidence of clinical activity in patients with advanced clear cell renal cell carcinoma. The drug also appears safe and tolerable.
Major finding: Overall, one patient had a complete response, three achieved partial responses, and 16 patients achieved stable disease for at least 16 weeks.
Data source: A phase I dose-escalation and expansion study of 51 heavily pretreated patients with advanced clear cell renal cell carcinoma.
Disclosures: The study was funded by Peloton Therapeutics. Dr. Courtney reported having stock and ownership interest in, serving in advisory roles for, or receiving research funding from multiple companies. Several coinvestigators reported receiving research funding from or holding patents with multiple companies including Peloton Therapeutics.
GERD and sleep disorders often go hand in glove
Gastroesophageal reflux disease (GERD) is fertile soil for medical and psychiatric comorbid conditions, with sleep disorders leading the way, according to Maurice M. Ohayon, MD, who presented the findings of a longitudinal, population-based study on GERD and its "fellow travelers" at the annual meeting of the Associated Professional Sleep Societies. Insomnia, obstructive sleep apnea, and restless legs syndrome were among the most prevalent comorbidities of GERD. However, one sleep disorder symptom was reported to be a problem in up to 46% of those with the digestive disease. To learn more, see Family Practice News at: http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/gerd-and-sleep-disorders-often-go-hand-in-glove/1dc81a99b64d296e03b86aa28893fbc7.html.
Gastroesophageal reflux disease (GERD) is fertile soil for medical and psychiatric comorbid conditions, with sleep disorders leading the way, according to Maurice M. Ohayon, MD, who presented the findings of a longitudinal, population-based study on GERD and its "fellow travelers" at the annual meeting of the Associated Professional Sleep Societies. Insomnia, obstructive sleep apnea, and restless legs syndrome were among the most prevalent comorbidities of GERD. However, one sleep disorder symptom was reported to be a problem in up to 46% of those with the digestive disease. To learn more, see Family Practice News at: http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/gerd-and-sleep-disorders-often-go-hand-in-glove/1dc81a99b64d296e03b86aa28893fbc7.html.
Gastroesophageal reflux disease (GERD) is fertile soil for medical and psychiatric comorbid conditions, with sleep disorders leading the way, according to Maurice M. Ohayon, MD, who presented the findings of a longitudinal, population-based study on GERD and its "fellow travelers" at the annual meeting of the Associated Professional Sleep Societies. Insomnia, obstructive sleep apnea, and restless legs syndrome were among the most prevalent comorbidities of GERD. However, one sleep disorder symptom was reported to be a problem in up to 46% of those with the digestive disease. To learn more, see Family Practice News at: http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/gerd-and-sleep-disorders-often-go-hand-in-glove/1dc81a99b64d296e03b86aa28893fbc7.html.
Early sustained viral response linked to better outcomes among HCV patients
Patients with hepatitis C virus (HCV) infections may have the best long-term outcomes if they achieve sustained viral response (SVR) before they develop clinically significant portal hypertension, according to a study reported on in the July issue of Gastroenterology. On the other hand, patients in stage 2 cirrhosis were more likely than stage 1 patients to develop liver decompensation and to die of hepatocellular carcinoma, regardless of SVR. “The available evidence, including our own, suggests that it is opportune to treat HCV as early as possible, in order to reduce progression to stages of cirrhosis in which a viral cure may be less likely lead to ultimate achievement of a major benefit,” said Dr. Vito Di Marco and his associates from the University of Palermo, Italy. More on the study, and its limitations, is available at Family Practice News: http://www.familypracticenews.com/specialty-focus/infectious-diseases/single-article-page/early-sustained-viral-response-linked-to-better-outcomes-among-hcv-patients/5089546903e73ad3c6947ff3bd3f8dbc.html.
Patients with hepatitis C virus (HCV) infections may have the best long-term outcomes if they achieve sustained viral response (SVR) before they develop clinically significant portal hypertension, according to a study reported on in the July issue of Gastroenterology. On the other hand, patients in stage 2 cirrhosis were more likely than stage 1 patients to develop liver decompensation and to die of hepatocellular carcinoma, regardless of SVR. “The available evidence, including our own, suggests that it is opportune to treat HCV as early as possible, in order to reduce progression to stages of cirrhosis in which a viral cure may be less likely lead to ultimate achievement of a major benefit,” said Dr. Vito Di Marco and his associates from the University of Palermo, Italy. More on the study, and its limitations, is available at Family Practice News: http://www.familypracticenews.com/specialty-focus/infectious-diseases/single-article-page/early-sustained-viral-response-linked-to-better-outcomes-among-hcv-patients/5089546903e73ad3c6947ff3bd3f8dbc.html.
Patients with hepatitis C virus (HCV) infections may have the best long-term outcomes if they achieve sustained viral response (SVR) before they develop clinically significant portal hypertension, according to a study reported on in the July issue of Gastroenterology. On the other hand, patients in stage 2 cirrhosis were more likely than stage 1 patients to develop liver decompensation and to die of hepatocellular carcinoma, regardless of SVR. “The available evidence, including our own, suggests that it is opportune to treat HCV as early as possible, in order to reduce progression to stages of cirrhosis in which a viral cure may be less likely lead to ultimate achievement of a major benefit,” said Dr. Vito Di Marco and his associates from the University of Palermo, Italy. More on the study, and its limitations, is available at Family Practice News: http://www.familypracticenews.com/specialty-focus/infectious-diseases/single-article-page/early-sustained-viral-response-linked-to-better-outcomes-among-hcv-patients/5089546903e73ad3c6947ff3bd3f8dbc.html.