User login
EADV: Vismodegib treatment breaks don’t hurt efficacy
COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
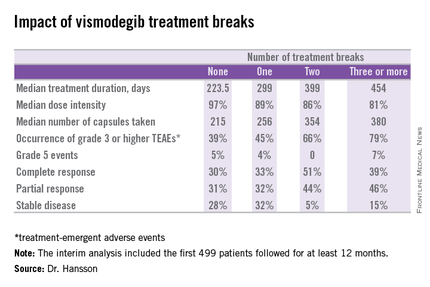
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.
COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.

COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.

AT THE EADV CONGRESS
Key clinical point: Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t compromise efficacy.
Major finding: The complete response rate to vismodegib in patients with advanced BCC was intriguingly higher in those with more treatment breaks due to adverse events.
Data source: A prespecified interim analysis of the first 499 patients with advanced BCC enrolled in STEVIE, a large ongoing phase II, long-term, open-label international safety study of vismodegib.
Disclosures: The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. The presenter reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.
Making sense of the expanded myeloma treatment landscape
ORLANDO – The moment the Food and Drug Administration approved daratumumab, ixazomib, and elotuzumab in rapid-fire succession over 15 days in November 2015, Dr. S. Vincent Rajkumar’s phone started ringing.
As with other multiple myeloma experts, three common questions kept cropping up:
• For previously untreated patients, should we add bortezomib to lenalidomide plus dexamethasone (Rd) based on the S0777 results?
• For previously treated patients, should we add ixazomib or elotuzumab to Rd?
• Should we add daratumumab to frontline therapy right out of the box?
Daratumumab (Darzalex), ixazomib (Ninlaro), and elotuzumab (Empliciti) are welcome additions to the armamentarium, but the problem with this plethora of riches is that numerous treatments already exist for frontline multiple myeloma, observed Dr. Rajkumar, professor of medicine at the Mayo Clinic in Rochester, Minn.
In fact, the National Comprehensive Cancer Network guidelines list 22 possible newly diagnosed myeloma regimens that can be potentially recommended for patients.
“This definitely leads to confusion in the community. And this was the result of the fact that we didn’t have a single, good randomized trial with a survival benefit of a modern therapy against another modern therapy,” Dr. Rajkumar said at the annual meeting of the American Society of Hematology during a joint FDA/ASH symposium on the three newly approved agents.
This quandary was solved at ASH with phase III randomized data from the Southwest Oncology Group S0777 study showing a significant overall survival advantage with a triplet of bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) followed by continuous Rd maintenance compared with Rd alone and ongoing maintenance in untreated patients who did not intend to receive stem cell transplant, he said.
Median overall survival was 75 months for the triplet vs. 64 months for the Rd doublet (hazard ratio, 0.709; two-sided log-rank P = .0250), and median PFS 43 months vs. 30 months (HR, 0.712; one-sided P = .0018), study author Dr. Brian Durie, of Cedars-Sinai Comprehensive Cancer Center in Los Angeles, reported (Abstract 25).
The VRd triplet is already in use in the United States, but based on the S0777 results, many groups, including the Mayo Clinic, have changed treatment guidelines and now “prefer bortezomib, len-dex for frontline therapy, not just in transplant candidates, but also in non-transplant candidates,” Dr. Rajkumar said.
In countries where VRd is not possible, bortezomib, thalidomide, and dexamethasone (VTd) is a second option.
Rd is an appropriate therapy for non-transplant candidates who are frail or aged 75 years or older, he said, adding that there is no need to add bortezomib for patients already on Rd and doing well.
“If your patient is doing well on a doublet, leave them alone,” Dr. Rajkumar advised.
Similarly, for patients with relapsed myeloma who are doing well on Rd, there isn’t “an urgent need” to add ixazomib or elotuzumab, but rather, he said, “We can reserve those for when the patient progresses.”
Ixazomib is approved in combination with Rd after at least one prior therapy, but the oral proteasome inhibitor may have a role in the frontline treatment of standard-risk patients. It is a very simple regimen, just three pills a month, and “the side effect profile is outstanding; virtually difficult to tell who’s taking placebo and who’s taking drug,” Dr. Rajkumar observed.
In addition, some patients may not have access to bortezomib because of insurance reasons or can’t drive to the clinic once a week to get the shot, while others may be too frail to get an intravenous or subcutaneous shot or may have neuropathy.
“For whatever reason, I think it is reasonable to keep in mind that we may have a situation where we can use ixazomib/len-dex in clinical practice if the patient’s best interests so dictate,” he said.
For high-risk patients (deletion 17p or translocations t(4;14), t(14;16), t(14;20), VRd or VTd are obvious upfront choices. Based on four phase II trials and the ASPIRE results in the relapsed and refractory setting, however, the Mayo Clinic has already decided that the recently approved second-generation proteasome inhibitor carfilzomib (Kyprolis) plus Rd is also worth considering.
Adding a monoclonal antibody such as elotuzumab or daratumumab to a VRd triplet or ixazomib, lenalidomide, and dexamethasone (IRd) triplet may be another way to improve outcomes in high-risk patients, who still die with a median overall survival of 3 years, Dr. Rajkumar said. This strategy is already being used in the ongoing SWOG S1211 study.
For maintenance therapy after VRd or VTd and autologous stem cell transplant, he recommended lenalidomide for standard-risk patients and bortezomib-based maintenance for high-risk patients, but said ixazomib-based maintenance with the addition of monoclonal antibodies may also have a role in high-risk patients.
What may be more important going forward is how these three drugs will be used in clinical trials, Dr. Rajkumar observed.
“We’d rather put all patients on clinical trials than any of the recommendations I made,” he said. “The problem is that clinical trials have to be appropriately designed.”
Several phase III trials are already ongoing comparing a doublet versus a triplet (IRd vs. Rd, elotuzumab-Rd vs. Rd, and daratumumab-Rd vs. Rd) in the frontline setting, so the key question for future trials is which triplet: VRd, KRd, elotuzumab-Rd, or daratumumab-Rd, and to what endpoint.
Progression-free survival can remain a primary endpoint for comparing two triplets in the frontline, but PFS alone is not enough in the maintenance setting and investigators should look to other primary endpoints such as PFS2, PFS1 vs. PFS2, overall survival with a higher type 1 error than currently used, or PFS plus validated patient-reported or quality of life outcomes, Dr. Rajkumar said.
Relapsed/refractory disease
Speaking on how the three new agents fit into the relapsed or refractory space,Dr. Paul Richardson, of Dana-Farber Cancer Institute, Boston, said three-drug platforms are emerging as a standard of care for relapsed or refractory disease after studies have shown time and time again they are better than doublets.
He highlighted phase III data reported at ASH by Dr. Philippe Moreau from TOURMALINE-MM1 (Abstract 727) showing a 35% improvement in PFS with weekly oral ixazomib plus lenalidomide-dexamethasone vs. Rd alone in relapsed and/or refractory multiple myeloma.
This translated into a median 6-month gain in PFS compared with an almost 9-month PFS benefit seen in ASPIRE with carfilzomib plus Rd, but cross-trial comparisons should be approached with some caution and both hazard ratios were very robust, he said. In addition, as previously observed, ixazomib is remarkably well tolerated.
“I think ixazomib, particularly in older patients and particularly in patients with high-risk disease, will be very useful in the context of the three-drug or even greater combinations. So there’s a strong rationale for its use,” Dr. Richardson said.
He went on to say that elotuzumab has shown remarkable anti-myeloma activity in the relapsed and refractory setting, improving both the overall response rate and PFS when used in combination with Ld vs. Ld alone in the ELOQUENT-2 trial. Updated results from ELOQUENT-2 were presented at the ASH meeting (Abstract 28).
A PFS benefit was also seen when elotuzumab was added to bortezomib and dexamethasone, with a 24% reduction in the risk of disease progression or death reported in a study presented at ASH by myeloma expert Dr. Antonio Palumbo (Abstract 510).
“My point in showing this is that when you think of elotuzumab being used with lenalidomide and dexamethasone in relapse, many of our patients are actually on them as maintenance when it occurs, therefore elotuzumab may have a role in combination, for example, with proteasome inhibitors in this same setting,” Dr. Richardson said.
Several pomalidomide-based triple therapy combinations have been evaluated in advanced relapsed or refractory myeloma, with a phase II study (Abstract 506) reported that morning at ASH showing the third-generation immunomodulatory drug (IMiD) pomalidomide induced responses in 60% of heavily pretreated patients when partnered with pembrolizumab and dexamethasone.
Combination strategies with daratumumab are also very provocative, particularly in the context of IMiDs, he noted. A phase Ib study reported in the same early morning session by Dr. Ajai Chari (Abstract 508) had a “very encouraging” overall response rate of 71% with daratumumab plus pomalidomide and dexamethasone in heavily pretreated patients, including 43% very good partial responses or better, and an overall response rate of 67% among double-refractory patients.
“Daratumumab and elotuzumab, in my view, as first-in-class monoclonal antibodies, are paradigm-changing agents,” Dr. Richardson concluded. “They provide us with this mutation-driven ability to overdrive the impact of those mutations and the important point is that they prescribe an entirely non-crossresistant strategy that can be easily added to existing platforms of drugs.”
Dr. Rajkumar reported discussion of off-label drug use for elotuzumab, daratumumab, ixazomib, and carfilzomib in untreated myeloma, maintenance, and early relapse. Dr. Richardson reported membership on a board of directors or advisory committee for Millennium Takeda, Celgene, Janssen, Bristol-Myers Squibb, and Novartis, and research funding from Millennium Takeda and Celgene.
ORLANDO – The moment the Food and Drug Administration approved daratumumab, ixazomib, and elotuzumab in rapid-fire succession over 15 days in November 2015, Dr. S. Vincent Rajkumar’s phone started ringing.
As with other multiple myeloma experts, three common questions kept cropping up:
• For previously untreated patients, should we add bortezomib to lenalidomide plus dexamethasone (Rd) based on the S0777 results?
• For previously treated patients, should we add ixazomib or elotuzumab to Rd?
• Should we add daratumumab to frontline therapy right out of the box?
Daratumumab (Darzalex), ixazomib (Ninlaro), and elotuzumab (Empliciti) are welcome additions to the armamentarium, but the problem with this plethora of riches is that numerous treatments already exist for frontline multiple myeloma, observed Dr. Rajkumar, professor of medicine at the Mayo Clinic in Rochester, Minn.
In fact, the National Comprehensive Cancer Network guidelines list 22 possible newly diagnosed myeloma regimens that can be potentially recommended for patients.
“This definitely leads to confusion in the community. And this was the result of the fact that we didn’t have a single, good randomized trial with a survival benefit of a modern therapy against another modern therapy,” Dr. Rajkumar said at the annual meeting of the American Society of Hematology during a joint FDA/ASH symposium on the three newly approved agents.
This quandary was solved at ASH with phase III randomized data from the Southwest Oncology Group S0777 study showing a significant overall survival advantage with a triplet of bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) followed by continuous Rd maintenance compared with Rd alone and ongoing maintenance in untreated patients who did not intend to receive stem cell transplant, he said.
Median overall survival was 75 months for the triplet vs. 64 months for the Rd doublet (hazard ratio, 0.709; two-sided log-rank P = .0250), and median PFS 43 months vs. 30 months (HR, 0.712; one-sided P = .0018), study author Dr. Brian Durie, of Cedars-Sinai Comprehensive Cancer Center in Los Angeles, reported (Abstract 25).
The VRd triplet is already in use in the United States, but based on the S0777 results, many groups, including the Mayo Clinic, have changed treatment guidelines and now “prefer bortezomib, len-dex for frontline therapy, not just in transplant candidates, but also in non-transplant candidates,” Dr. Rajkumar said.
In countries where VRd is not possible, bortezomib, thalidomide, and dexamethasone (VTd) is a second option.
Rd is an appropriate therapy for non-transplant candidates who are frail or aged 75 years or older, he said, adding that there is no need to add bortezomib for patients already on Rd and doing well.
“If your patient is doing well on a doublet, leave them alone,” Dr. Rajkumar advised.
Similarly, for patients with relapsed myeloma who are doing well on Rd, there isn’t “an urgent need” to add ixazomib or elotuzumab, but rather, he said, “We can reserve those for when the patient progresses.”
Ixazomib is approved in combination with Rd after at least one prior therapy, but the oral proteasome inhibitor may have a role in the frontline treatment of standard-risk patients. It is a very simple regimen, just three pills a month, and “the side effect profile is outstanding; virtually difficult to tell who’s taking placebo and who’s taking drug,” Dr. Rajkumar observed.
In addition, some patients may not have access to bortezomib because of insurance reasons or can’t drive to the clinic once a week to get the shot, while others may be too frail to get an intravenous or subcutaneous shot or may have neuropathy.
“For whatever reason, I think it is reasonable to keep in mind that we may have a situation where we can use ixazomib/len-dex in clinical practice if the patient’s best interests so dictate,” he said.
For high-risk patients (deletion 17p or translocations t(4;14), t(14;16), t(14;20), VRd or VTd are obvious upfront choices. Based on four phase II trials and the ASPIRE results in the relapsed and refractory setting, however, the Mayo Clinic has already decided that the recently approved second-generation proteasome inhibitor carfilzomib (Kyprolis) plus Rd is also worth considering.
Adding a monoclonal antibody such as elotuzumab or daratumumab to a VRd triplet or ixazomib, lenalidomide, and dexamethasone (IRd) triplet may be another way to improve outcomes in high-risk patients, who still die with a median overall survival of 3 years, Dr. Rajkumar said. This strategy is already being used in the ongoing SWOG S1211 study.
For maintenance therapy after VRd or VTd and autologous stem cell transplant, he recommended lenalidomide for standard-risk patients and bortezomib-based maintenance for high-risk patients, but said ixazomib-based maintenance with the addition of monoclonal antibodies may also have a role in high-risk patients.
What may be more important going forward is how these three drugs will be used in clinical trials, Dr. Rajkumar observed.
“We’d rather put all patients on clinical trials than any of the recommendations I made,” he said. “The problem is that clinical trials have to be appropriately designed.”
Several phase III trials are already ongoing comparing a doublet versus a triplet (IRd vs. Rd, elotuzumab-Rd vs. Rd, and daratumumab-Rd vs. Rd) in the frontline setting, so the key question for future trials is which triplet: VRd, KRd, elotuzumab-Rd, or daratumumab-Rd, and to what endpoint.
Progression-free survival can remain a primary endpoint for comparing two triplets in the frontline, but PFS alone is not enough in the maintenance setting and investigators should look to other primary endpoints such as PFS2, PFS1 vs. PFS2, overall survival with a higher type 1 error than currently used, or PFS plus validated patient-reported or quality of life outcomes, Dr. Rajkumar said.
Relapsed/refractory disease
Speaking on how the three new agents fit into the relapsed or refractory space,Dr. Paul Richardson, of Dana-Farber Cancer Institute, Boston, said three-drug platforms are emerging as a standard of care for relapsed or refractory disease after studies have shown time and time again they are better than doublets.
He highlighted phase III data reported at ASH by Dr. Philippe Moreau from TOURMALINE-MM1 (Abstract 727) showing a 35% improvement in PFS with weekly oral ixazomib plus lenalidomide-dexamethasone vs. Rd alone in relapsed and/or refractory multiple myeloma.
This translated into a median 6-month gain in PFS compared with an almost 9-month PFS benefit seen in ASPIRE with carfilzomib plus Rd, but cross-trial comparisons should be approached with some caution and both hazard ratios were very robust, he said. In addition, as previously observed, ixazomib is remarkably well tolerated.
“I think ixazomib, particularly in older patients and particularly in patients with high-risk disease, will be very useful in the context of the three-drug or even greater combinations. So there’s a strong rationale for its use,” Dr. Richardson said.
He went on to say that elotuzumab has shown remarkable anti-myeloma activity in the relapsed and refractory setting, improving both the overall response rate and PFS when used in combination with Ld vs. Ld alone in the ELOQUENT-2 trial. Updated results from ELOQUENT-2 were presented at the ASH meeting (Abstract 28).
A PFS benefit was also seen when elotuzumab was added to bortezomib and dexamethasone, with a 24% reduction in the risk of disease progression or death reported in a study presented at ASH by myeloma expert Dr. Antonio Palumbo (Abstract 510).
“My point in showing this is that when you think of elotuzumab being used with lenalidomide and dexamethasone in relapse, many of our patients are actually on them as maintenance when it occurs, therefore elotuzumab may have a role in combination, for example, with proteasome inhibitors in this same setting,” Dr. Richardson said.
Several pomalidomide-based triple therapy combinations have been evaluated in advanced relapsed or refractory myeloma, with a phase II study (Abstract 506) reported that morning at ASH showing the third-generation immunomodulatory drug (IMiD) pomalidomide induced responses in 60% of heavily pretreated patients when partnered with pembrolizumab and dexamethasone.
Combination strategies with daratumumab are also very provocative, particularly in the context of IMiDs, he noted. A phase Ib study reported in the same early morning session by Dr. Ajai Chari (Abstract 508) had a “very encouraging” overall response rate of 71% with daratumumab plus pomalidomide and dexamethasone in heavily pretreated patients, including 43% very good partial responses or better, and an overall response rate of 67% among double-refractory patients.
“Daratumumab and elotuzumab, in my view, as first-in-class monoclonal antibodies, are paradigm-changing agents,” Dr. Richardson concluded. “They provide us with this mutation-driven ability to overdrive the impact of those mutations and the important point is that they prescribe an entirely non-crossresistant strategy that can be easily added to existing platforms of drugs.”
Dr. Rajkumar reported discussion of off-label drug use for elotuzumab, daratumumab, ixazomib, and carfilzomib in untreated myeloma, maintenance, and early relapse. Dr. Richardson reported membership on a board of directors or advisory committee for Millennium Takeda, Celgene, Janssen, Bristol-Myers Squibb, and Novartis, and research funding from Millennium Takeda and Celgene.
ORLANDO – The moment the Food and Drug Administration approved daratumumab, ixazomib, and elotuzumab in rapid-fire succession over 15 days in November 2015, Dr. S. Vincent Rajkumar’s phone started ringing.
As with other multiple myeloma experts, three common questions kept cropping up:
• For previously untreated patients, should we add bortezomib to lenalidomide plus dexamethasone (Rd) based on the S0777 results?
• For previously treated patients, should we add ixazomib or elotuzumab to Rd?
• Should we add daratumumab to frontline therapy right out of the box?
Daratumumab (Darzalex), ixazomib (Ninlaro), and elotuzumab (Empliciti) are welcome additions to the armamentarium, but the problem with this plethora of riches is that numerous treatments already exist for frontline multiple myeloma, observed Dr. Rajkumar, professor of medicine at the Mayo Clinic in Rochester, Minn.
In fact, the National Comprehensive Cancer Network guidelines list 22 possible newly diagnosed myeloma regimens that can be potentially recommended for patients.
“This definitely leads to confusion in the community. And this was the result of the fact that we didn’t have a single, good randomized trial with a survival benefit of a modern therapy against another modern therapy,” Dr. Rajkumar said at the annual meeting of the American Society of Hematology during a joint FDA/ASH symposium on the three newly approved agents.
This quandary was solved at ASH with phase III randomized data from the Southwest Oncology Group S0777 study showing a significant overall survival advantage with a triplet of bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) followed by continuous Rd maintenance compared with Rd alone and ongoing maintenance in untreated patients who did not intend to receive stem cell transplant, he said.
Median overall survival was 75 months for the triplet vs. 64 months for the Rd doublet (hazard ratio, 0.709; two-sided log-rank P = .0250), and median PFS 43 months vs. 30 months (HR, 0.712; one-sided P = .0018), study author Dr. Brian Durie, of Cedars-Sinai Comprehensive Cancer Center in Los Angeles, reported (Abstract 25).
The VRd triplet is already in use in the United States, but based on the S0777 results, many groups, including the Mayo Clinic, have changed treatment guidelines and now “prefer bortezomib, len-dex for frontline therapy, not just in transplant candidates, but also in non-transplant candidates,” Dr. Rajkumar said.
In countries where VRd is not possible, bortezomib, thalidomide, and dexamethasone (VTd) is a second option.
Rd is an appropriate therapy for non-transplant candidates who are frail or aged 75 years or older, he said, adding that there is no need to add bortezomib for patients already on Rd and doing well.
“If your patient is doing well on a doublet, leave them alone,” Dr. Rajkumar advised.
Similarly, for patients with relapsed myeloma who are doing well on Rd, there isn’t “an urgent need” to add ixazomib or elotuzumab, but rather, he said, “We can reserve those for when the patient progresses.”
Ixazomib is approved in combination with Rd after at least one prior therapy, but the oral proteasome inhibitor may have a role in the frontline treatment of standard-risk patients. It is a very simple regimen, just three pills a month, and “the side effect profile is outstanding; virtually difficult to tell who’s taking placebo and who’s taking drug,” Dr. Rajkumar observed.
In addition, some patients may not have access to bortezomib because of insurance reasons or can’t drive to the clinic once a week to get the shot, while others may be too frail to get an intravenous or subcutaneous shot or may have neuropathy.
“For whatever reason, I think it is reasonable to keep in mind that we may have a situation where we can use ixazomib/len-dex in clinical practice if the patient’s best interests so dictate,” he said.
For high-risk patients (deletion 17p or translocations t(4;14), t(14;16), t(14;20), VRd or VTd are obvious upfront choices. Based on four phase II trials and the ASPIRE results in the relapsed and refractory setting, however, the Mayo Clinic has already decided that the recently approved second-generation proteasome inhibitor carfilzomib (Kyprolis) plus Rd is also worth considering.
Adding a monoclonal antibody such as elotuzumab or daratumumab to a VRd triplet or ixazomib, lenalidomide, and dexamethasone (IRd) triplet may be another way to improve outcomes in high-risk patients, who still die with a median overall survival of 3 years, Dr. Rajkumar said. This strategy is already being used in the ongoing SWOG S1211 study.
For maintenance therapy after VRd or VTd and autologous stem cell transplant, he recommended lenalidomide for standard-risk patients and bortezomib-based maintenance for high-risk patients, but said ixazomib-based maintenance with the addition of monoclonal antibodies may also have a role in high-risk patients.
What may be more important going forward is how these three drugs will be used in clinical trials, Dr. Rajkumar observed.
“We’d rather put all patients on clinical trials than any of the recommendations I made,” he said. “The problem is that clinical trials have to be appropriately designed.”
Several phase III trials are already ongoing comparing a doublet versus a triplet (IRd vs. Rd, elotuzumab-Rd vs. Rd, and daratumumab-Rd vs. Rd) in the frontline setting, so the key question for future trials is which triplet: VRd, KRd, elotuzumab-Rd, or daratumumab-Rd, and to what endpoint.
Progression-free survival can remain a primary endpoint for comparing two triplets in the frontline, but PFS alone is not enough in the maintenance setting and investigators should look to other primary endpoints such as PFS2, PFS1 vs. PFS2, overall survival with a higher type 1 error than currently used, or PFS plus validated patient-reported or quality of life outcomes, Dr. Rajkumar said.
Relapsed/refractory disease
Speaking on how the three new agents fit into the relapsed or refractory space,Dr. Paul Richardson, of Dana-Farber Cancer Institute, Boston, said three-drug platforms are emerging as a standard of care for relapsed or refractory disease after studies have shown time and time again they are better than doublets.
He highlighted phase III data reported at ASH by Dr. Philippe Moreau from TOURMALINE-MM1 (Abstract 727) showing a 35% improvement in PFS with weekly oral ixazomib plus lenalidomide-dexamethasone vs. Rd alone in relapsed and/or refractory multiple myeloma.
This translated into a median 6-month gain in PFS compared with an almost 9-month PFS benefit seen in ASPIRE with carfilzomib plus Rd, but cross-trial comparisons should be approached with some caution and both hazard ratios were very robust, he said. In addition, as previously observed, ixazomib is remarkably well tolerated.
“I think ixazomib, particularly in older patients and particularly in patients with high-risk disease, will be very useful in the context of the three-drug or even greater combinations. So there’s a strong rationale for its use,” Dr. Richardson said.
He went on to say that elotuzumab has shown remarkable anti-myeloma activity in the relapsed and refractory setting, improving both the overall response rate and PFS when used in combination with Ld vs. Ld alone in the ELOQUENT-2 trial. Updated results from ELOQUENT-2 were presented at the ASH meeting (Abstract 28).
A PFS benefit was also seen when elotuzumab was added to bortezomib and dexamethasone, with a 24% reduction in the risk of disease progression or death reported in a study presented at ASH by myeloma expert Dr. Antonio Palumbo (Abstract 510).
“My point in showing this is that when you think of elotuzumab being used with lenalidomide and dexamethasone in relapse, many of our patients are actually on them as maintenance when it occurs, therefore elotuzumab may have a role in combination, for example, with proteasome inhibitors in this same setting,” Dr. Richardson said.
Several pomalidomide-based triple therapy combinations have been evaluated in advanced relapsed or refractory myeloma, with a phase II study (Abstract 506) reported that morning at ASH showing the third-generation immunomodulatory drug (IMiD) pomalidomide induced responses in 60% of heavily pretreated patients when partnered with pembrolizumab and dexamethasone.
Combination strategies with daratumumab are also very provocative, particularly in the context of IMiDs, he noted. A phase Ib study reported in the same early morning session by Dr. Ajai Chari (Abstract 508) had a “very encouraging” overall response rate of 71% with daratumumab plus pomalidomide and dexamethasone in heavily pretreated patients, including 43% very good partial responses or better, and an overall response rate of 67% among double-refractory patients.
“Daratumumab and elotuzumab, in my view, as first-in-class monoclonal antibodies, are paradigm-changing agents,” Dr. Richardson concluded. “They provide us with this mutation-driven ability to overdrive the impact of those mutations and the important point is that they prescribe an entirely non-crossresistant strategy that can be easily added to existing platforms of drugs.”
Dr. Rajkumar reported discussion of off-label drug use for elotuzumab, daratumumab, ixazomib, and carfilzomib in untreated myeloma, maintenance, and early relapse. Dr. Richardson reported membership on a board of directors or advisory committee for Millennium Takeda, Celgene, Janssen, Bristol-Myers Squibb, and Novartis, and research funding from Millennium Takeda and Celgene.
EXPERT ANALYSIS FROM ASH 2015
Smartphones feasible modality for collecting data in bipolar disorders
Smartphone surveys of mood and social stress might be useful in identifying mental changes in bipolar disorder patients, according to a pilot feasibility study by Stefani Schwartz of the department of psychiatry at Pennsylvania State University, Hershey, and her associates.
Ten bipolar disorder patients and 10 healthy controls recruited for the study were given smartphones and asked to complete surveys of mood and social stress twice a day at random for 14 days. The surveys included a visual analog scale to record ratings of mood, energy, speed of thoughts, and impulsivity, in which participants could choose any point along a scale of 0-100 by moving a sliding marker; and a Likert scale to measure social stress. For this part, participants revealed whether they were with others and whether they would rather be alone.
Completion rates were similar among the groups: a median of 95% in the bipolar disorder group and 88% in the healthy control group (P = 0.68). Median scores of the 14-day mean mood and energy in the bipolar disorder group were significantly lower in the bipolar disorder group, while speed of thoughts, impulsivity, and social stress were not significantly different between the groups. Median scores of the 14-day range for mood, speed of thoughts, and impulsivity did differ from the healthy controls, while energy and social stress did not differ significantly.
Prolonged monitoring might be required to detect prodromal symptoms of an impending major episode among patients with bipolar disorder, the authors wrote. Also, the findings are preliminary in light of many factors, including the small sample. Nevertheless, the techniques used in this study “could be investigated in subjects in different treatment settings to explore the sensitivity of detection of changes in symptoms,” the investigators wrote.
Read the article in the Journal of Affective Disorders (http://dx.doi.org/10.1016/j.jad.2015.11.013).
Smartphone surveys of mood and social stress might be useful in identifying mental changes in bipolar disorder patients, according to a pilot feasibility study by Stefani Schwartz of the department of psychiatry at Pennsylvania State University, Hershey, and her associates.
Ten bipolar disorder patients and 10 healthy controls recruited for the study were given smartphones and asked to complete surveys of mood and social stress twice a day at random for 14 days. The surveys included a visual analog scale to record ratings of mood, energy, speed of thoughts, and impulsivity, in which participants could choose any point along a scale of 0-100 by moving a sliding marker; and a Likert scale to measure social stress. For this part, participants revealed whether they were with others and whether they would rather be alone.
Completion rates were similar among the groups: a median of 95% in the bipolar disorder group and 88% in the healthy control group (P = 0.68). Median scores of the 14-day mean mood and energy in the bipolar disorder group were significantly lower in the bipolar disorder group, while speed of thoughts, impulsivity, and social stress were not significantly different between the groups. Median scores of the 14-day range for mood, speed of thoughts, and impulsivity did differ from the healthy controls, while energy and social stress did not differ significantly.
Prolonged monitoring might be required to detect prodromal symptoms of an impending major episode among patients with bipolar disorder, the authors wrote. Also, the findings are preliminary in light of many factors, including the small sample. Nevertheless, the techniques used in this study “could be investigated in subjects in different treatment settings to explore the sensitivity of detection of changes in symptoms,” the investigators wrote.
Read the article in the Journal of Affective Disorders (http://dx.doi.org/10.1016/j.jad.2015.11.013).
Smartphone surveys of mood and social stress might be useful in identifying mental changes in bipolar disorder patients, according to a pilot feasibility study by Stefani Schwartz of the department of psychiatry at Pennsylvania State University, Hershey, and her associates.
Ten bipolar disorder patients and 10 healthy controls recruited for the study were given smartphones and asked to complete surveys of mood and social stress twice a day at random for 14 days. The surveys included a visual analog scale to record ratings of mood, energy, speed of thoughts, and impulsivity, in which participants could choose any point along a scale of 0-100 by moving a sliding marker; and a Likert scale to measure social stress. For this part, participants revealed whether they were with others and whether they would rather be alone.
Completion rates were similar among the groups: a median of 95% in the bipolar disorder group and 88% in the healthy control group (P = 0.68). Median scores of the 14-day mean mood and energy in the bipolar disorder group were significantly lower in the bipolar disorder group, while speed of thoughts, impulsivity, and social stress were not significantly different between the groups. Median scores of the 14-day range for mood, speed of thoughts, and impulsivity did differ from the healthy controls, while energy and social stress did not differ significantly.
Prolonged monitoring might be required to detect prodromal symptoms of an impending major episode among patients with bipolar disorder, the authors wrote. Also, the findings are preliminary in light of many factors, including the small sample. Nevertheless, the techniques used in this study “could be investigated in subjects in different treatment settings to explore the sensitivity of detection of changes in symptoms,” the investigators wrote.
Read the article in the Journal of Affective Disorders (http://dx.doi.org/10.1016/j.jad.2015.11.013).
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Great day, new tanning bed restrictions proposed
It’s a great day for our patients! The Food and Drug Administration has proposed new tanning bed restrictions that would not allow those under 18 years old to use tanning beds and would require adults to sign a written acknowledgment that certifies that they have been warned of the risks of tanning beds. These are proposed rules, so it is important for you to write and support them (or ask for even more). The rule is available for review and comments are accepted through March 21, 2016, at www.regulations.gov.
The American Academy of Dermatology and numerous state dermatology societies have been advocating for such restrictions for many years.
The real question is why has it taken so long. At a meeting almost 6 years ago, an FDA advisory committee agreed that these devices were hazardous and recommended greater restrictions. The answer is multifactorial, long, but an important one for dermatologists who have been patient advocates on this issue for many long years.
First, we had to establish a scientific basis for our efforts. Many contributed to this, from establishing basic science of ultraviolet injury, to demonstration of ultraviolet carcinogenesis in an animal model (a fish no less!), to publishing compelling numbers confirming the epidemic of skin cancer (for which I will take some credit). Once the literature was in place, we had the good fortune to have a dermatologist (a former resident of mine and good friend) who was acting surgeon general (Dr. Boris Lushniak) and brought the Centers for Disease Control and Prevention on board and declared ultraviolet radiation exposure a national health crisis.
During this time, the tanning industry was not idle. They were not highly organized and were caught off guard by the imposition of a federal tanning tax several years ago. Since then, they have become more organized and have hired lobbyists (reportedly the same ones who represented big tobacco) and petitioned congress for relief as small businesses. They claim they provide something that is healthful (vitamin D is good, right?), are small businesses, and sell something that people can get by walking outside, but in a timelier manner. Never mind that they are clustered around high school and college campuses and sell their carcinogenic wares to unsuspecting teenage girls. I remember arguing with a tanning lobbyist at a state hearing who claimed that dermatologists were trying to line their pockets because we sell ultraviolet radiation in our offices.
There are powerful social drivers behind tanning bed use. A tan in our office cubicle-based work force implies wealth and success since no one really has time to sit around a pool and cultivate one. The young are healthy and indestructible and haven’t seen or won’t believe the carnage wrought by skin cancer. Some do buy the pictures of resultant wrinkling. The good news is that tanning is nowhere as nearly addictive as tobacco and should be easier to vanquish. As the data continue to roll in, and as more movie stars go under the knife for skin cancer, the momentum builds. We are making progress and we will continue the campaign because prevention efforts will save more anguish and lives than an army of dermatologists.
It has taken 50 years to turn the tide on cigarette smoking and with a similarly long cancer latency, tanning will take persistent effort. The problem, however, has been identified, the solution obvious, and an ultimate victory for our patients inevitable. Dermatologists everywhere should take great pride in this victory at the FDA. Remember, tanning is the new tobacco!
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics.
It’s a great day for our patients! The Food and Drug Administration has proposed new tanning bed restrictions that would not allow those under 18 years old to use tanning beds and would require adults to sign a written acknowledgment that certifies that they have been warned of the risks of tanning beds. These are proposed rules, so it is important for you to write and support them (or ask for even more). The rule is available for review and comments are accepted through March 21, 2016, at www.regulations.gov.
The American Academy of Dermatology and numerous state dermatology societies have been advocating for such restrictions for many years.
The real question is why has it taken so long. At a meeting almost 6 years ago, an FDA advisory committee agreed that these devices were hazardous and recommended greater restrictions. The answer is multifactorial, long, but an important one for dermatologists who have been patient advocates on this issue for many long years.
First, we had to establish a scientific basis for our efforts. Many contributed to this, from establishing basic science of ultraviolet injury, to demonstration of ultraviolet carcinogenesis in an animal model (a fish no less!), to publishing compelling numbers confirming the epidemic of skin cancer (for which I will take some credit). Once the literature was in place, we had the good fortune to have a dermatologist (a former resident of mine and good friend) who was acting surgeon general (Dr. Boris Lushniak) and brought the Centers for Disease Control and Prevention on board and declared ultraviolet radiation exposure a national health crisis.
During this time, the tanning industry was not idle. They were not highly organized and were caught off guard by the imposition of a federal tanning tax several years ago. Since then, they have become more organized and have hired lobbyists (reportedly the same ones who represented big tobacco) and petitioned congress for relief as small businesses. They claim they provide something that is healthful (vitamin D is good, right?), are small businesses, and sell something that people can get by walking outside, but in a timelier manner. Never mind that they are clustered around high school and college campuses and sell their carcinogenic wares to unsuspecting teenage girls. I remember arguing with a tanning lobbyist at a state hearing who claimed that dermatologists were trying to line their pockets because we sell ultraviolet radiation in our offices.
There are powerful social drivers behind tanning bed use. A tan in our office cubicle-based work force implies wealth and success since no one really has time to sit around a pool and cultivate one. The young are healthy and indestructible and haven’t seen or won’t believe the carnage wrought by skin cancer. Some do buy the pictures of resultant wrinkling. The good news is that tanning is nowhere as nearly addictive as tobacco and should be easier to vanquish. As the data continue to roll in, and as more movie stars go under the knife for skin cancer, the momentum builds. We are making progress and we will continue the campaign because prevention efforts will save more anguish and lives than an army of dermatologists.
It has taken 50 years to turn the tide on cigarette smoking and with a similarly long cancer latency, tanning will take persistent effort. The problem, however, has been identified, the solution obvious, and an ultimate victory for our patients inevitable. Dermatologists everywhere should take great pride in this victory at the FDA. Remember, tanning is the new tobacco!
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics.
It’s a great day for our patients! The Food and Drug Administration has proposed new tanning bed restrictions that would not allow those under 18 years old to use tanning beds and would require adults to sign a written acknowledgment that certifies that they have been warned of the risks of tanning beds. These are proposed rules, so it is important for you to write and support them (or ask for even more). The rule is available for review and comments are accepted through March 21, 2016, at www.regulations.gov.
The American Academy of Dermatology and numerous state dermatology societies have been advocating for such restrictions for many years.
The real question is why has it taken so long. At a meeting almost 6 years ago, an FDA advisory committee agreed that these devices were hazardous and recommended greater restrictions. The answer is multifactorial, long, but an important one for dermatologists who have been patient advocates on this issue for many long years.
First, we had to establish a scientific basis for our efforts. Many contributed to this, from establishing basic science of ultraviolet injury, to demonstration of ultraviolet carcinogenesis in an animal model (a fish no less!), to publishing compelling numbers confirming the epidemic of skin cancer (for which I will take some credit). Once the literature was in place, we had the good fortune to have a dermatologist (a former resident of mine and good friend) who was acting surgeon general (Dr. Boris Lushniak) and brought the Centers for Disease Control and Prevention on board and declared ultraviolet radiation exposure a national health crisis.
During this time, the tanning industry was not idle. They were not highly organized and were caught off guard by the imposition of a federal tanning tax several years ago. Since then, they have become more organized and have hired lobbyists (reportedly the same ones who represented big tobacco) and petitioned congress for relief as small businesses. They claim they provide something that is healthful (vitamin D is good, right?), are small businesses, and sell something that people can get by walking outside, but in a timelier manner. Never mind that they are clustered around high school and college campuses and sell their carcinogenic wares to unsuspecting teenage girls. I remember arguing with a tanning lobbyist at a state hearing who claimed that dermatologists were trying to line their pockets because we sell ultraviolet radiation in our offices.
There are powerful social drivers behind tanning bed use. A tan in our office cubicle-based work force implies wealth and success since no one really has time to sit around a pool and cultivate one. The young are healthy and indestructible and haven’t seen or won’t believe the carnage wrought by skin cancer. Some do buy the pictures of resultant wrinkling. The good news is that tanning is nowhere as nearly addictive as tobacco and should be easier to vanquish. As the data continue to roll in, and as more movie stars go under the knife for skin cancer, the momentum builds. We are making progress and we will continue the campaign because prevention efforts will save more anguish and lives than an army of dermatologists.
It has taken 50 years to turn the tide on cigarette smoking and with a similarly long cancer latency, tanning will take persistent effort. The problem, however, has been identified, the solution obvious, and an ultimate victory for our patients inevitable. Dermatologists everywhere should take great pride in this victory at the FDA. Remember, tanning is the new tobacco!
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics.
Hospital Medicine 2016 Returns to San Diego
A great thing about San Diego is that the weather there is to a meteorologist as the common cold is to a doctor: not too challenging. If you guess mild and sunny, you won’t be far off.
Read more about the new tracks, speakers at HM16.
The HM16 program, on the other hand, might be a challenge. There’s a lot to choose from. The latest in clinical care, technology, practice management, building better relationships with patients—it all will be covered and then some. TH
A great thing about San Diego is that the weather there is to a meteorologist as the common cold is to a doctor: not too challenging. If you guess mild and sunny, you won’t be far off.
Read more about the new tracks, speakers at HM16.
The HM16 program, on the other hand, might be a challenge. There’s a lot to choose from. The latest in clinical care, technology, practice management, building better relationships with patients—it all will be covered and then some. TH
A great thing about San Diego is that the weather there is to a meteorologist as the common cold is to a doctor: not too challenging. If you guess mild and sunny, you won’t be far off.
Read more about the new tracks, speakers at HM16.
The HM16 program, on the other hand, might be a challenge. There’s a lot to choose from. The latest in clinical care, technology, practice management, building better relationships with patients—it all will be covered and then some. TH
A New Schedule Could Be Better for Your Hospitalist Group
Present “hospitalist” in a word association exercise to a wide range of healthcare personnel in clinical and administrative roles, and many would instantly respond with “seven-on/seven-off schedule.”
Some numbers from SHM’s 2014 State of Hospital Medicine report:
- 53.8%: Portion of hospitalist groups using a seven-on/seven-off schedule.
- 182: Median number of shifts worked annually by a full-time hospitalist (standard contract hours, does not include “extra” shifts).
- 65%: Portion of groups having day shifts that are 12.0–13.9 hours in length.
These numbers suggest to me that, at least outside of academia, the standard hospitalist is working 12-hour shifts on a seven-on/seven-off schedule. And that mirrors my experience working on-site with hundreds of hospitalist groups across the country.
In other words, the hospitalist marketplace has spoken unambiguously regarding the favored work schedule. In some ways, it is a defining feature of hospitalist practice. In the same way that a defining characteristic of Millennials is devotion to social media and that air travel is associated with cramped seats, this work schedule is a defining characteristic for hospitalists.
Schedule Benefits? Many …
There is a reason for its popularity: It is simple to understand and operationalize, it provides for good hospitalist-patient continuity, and having every other week off is often cited as a principle reason for becoming a hospitalist (in many cases, it might only take a clerk or administrator a few hours to create a group’s work schedule for a whole year). Many hospitalist groups have followed this schedule for a decade or longer, and while they might have periodically discussed moving to an entirely different model, most have stuck with what they know.
I’m convinced this schedule will be around for many years to come.
Not Ideal in All Respects
Despite this schedule’s popularity, I regularly talk with hospitalists who say it has become very stressful and monotonous. They say they would really like to change to something else but feel stuck by the complexity of alternative models and the difficulty achieving consensus within the group regarding what model offers enough advantages—and acceptable costs—to be worth it.
They cite as shortcomings of the seven-on/seven-off schedule:
- It can be a Herculean task to alter the schedule to arrange a day or two off during the regularly scheduled week. They often give up on the effort, and over time, this can lead to some resentment toward their work.
- There is a tendency to adopt a systole-diastole lifestyle, with no activities other than work during the week on (e.g., no trips to the gym, dinners out with family, etc.) and an effort to move all of these into the week off. They’ll say, “What other profession requires one to shut down their personal life for seven days every other week?”
- It can be difficult to reliably use the seven days off productively. Sometimes it might be better to return to work after only two to four days off if at other times it were easy to arrange more than seven consecutive days off.
- The “switch day” can be difficult for the hospital. Such schedules nearly always are arranged so that all the doctors conclude seven days of work on the same day and are replaced by others the following day. Every hospitalist patient (typically more than half of all patients in the hospital) gets a new doctor on the same day, and the whole hospital runs less efficiently as a result.
Change Your Schedule?
Who am I kidding? Few groups, probably none to be precise, are likely to change their schedule as a result of reading this column. But I’m among what seems to be a small contingent who believe alternative schedules can work. Whether your group decides to pursue a different model should be entirely up to its members, but it is worthwhile to periodically discuss the costs and benefits of your current schedule as well as what other options might be practical. In most cases the discussion will conclude without any significant change, but discussing it periodically might turn up worthwhile small adjustments.
But if your group is ready to make a meaningful change away from a rigid seven-on/seven-off schedule, the first step could be to vary the number of days off. No longer would all in the group switch on the same day; only one doctor would switch at a time (unless there are more than seven day shifts), and that could occur on any day of the week.
To illustrate, let’s say you’re in a group with four day shifts. For this week, Dr. Plant might start Monday after four days off, Dr. Bonham has had 11 days off and starts Tuesday, Dr. Page starts Friday after nine days off, and Dr. Jones starts Saturday after six days off. Each will work seven consecutive day shifts, and the number of off days will vary depending on their own wishes and the needs of the group. This is much more complicated to schedule, but varying the switch day and number of days off between weeks can be good for work-life balance.
Some will quickly identify difficulties, such as how to get the kids’ nanny to match a varying work schedule like this. I know many hospitalists who have done this successfully and are glad they did, but I’m sure there are also many for whom changing to a schedule like this might require moving from their current terrific childcare arrangements to a new one, something that they (justifiably) are unwilling to do.
And if your group successfully moves to a seven-on/X-off schedule (i.e., varied number of days off), you could next think about varying the number of consecutive days worked. Maybe it could range from no fewer than five or six (to preserve reasonable continuity) to as many as 10 or 11 as long as you have the stamina.
I don’t have research proving this would be a better schedule. But my own career, and the experiences of a number of others I’ve spoken with, is enough to convince me it’s worth considering. TH

Present “hospitalist” in a word association exercise to a wide range of healthcare personnel in clinical and administrative roles, and many would instantly respond with “seven-on/seven-off schedule.”
Some numbers from SHM’s 2014 State of Hospital Medicine report:
- 53.8%: Portion of hospitalist groups using a seven-on/seven-off schedule.
- 182: Median number of shifts worked annually by a full-time hospitalist (standard contract hours, does not include “extra” shifts).
- 65%: Portion of groups having day shifts that are 12.0–13.9 hours in length.
These numbers suggest to me that, at least outside of academia, the standard hospitalist is working 12-hour shifts on a seven-on/seven-off schedule. And that mirrors my experience working on-site with hundreds of hospitalist groups across the country.
In other words, the hospitalist marketplace has spoken unambiguously regarding the favored work schedule. In some ways, it is a defining feature of hospitalist practice. In the same way that a defining characteristic of Millennials is devotion to social media and that air travel is associated with cramped seats, this work schedule is a defining characteristic for hospitalists.
Schedule Benefits? Many …
There is a reason for its popularity: It is simple to understand and operationalize, it provides for good hospitalist-patient continuity, and having every other week off is often cited as a principle reason for becoming a hospitalist (in many cases, it might only take a clerk or administrator a few hours to create a group’s work schedule for a whole year). Many hospitalist groups have followed this schedule for a decade or longer, and while they might have periodically discussed moving to an entirely different model, most have stuck with what they know.
I’m convinced this schedule will be around for many years to come.
Not Ideal in All Respects
Despite this schedule’s popularity, I regularly talk with hospitalists who say it has become very stressful and monotonous. They say they would really like to change to something else but feel stuck by the complexity of alternative models and the difficulty achieving consensus within the group regarding what model offers enough advantages—and acceptable costs—to be worth it.
They cite as shortcomings of the seven-on/seven-off schedule:
- It can be a Herculean task to alter the schedule to arrange a day or two off during the regularly scheduled week. They often give up on the effort, and over time, this can lead to some resentment toward their work.
- There is a tendency to adopt a systole-diastole lifestyle, with no activities other than work during the week on (e.g., no trips to the gym, dinners out with family, etc.) and an effort to move all of these into the week off. They’ll say, “What other profession requires one to shut down their personal life for seven days every other week?”
- It can be difficult to reliably use the seven days off productively. Sometimes it might be better to return to work after only two to four days off if at other times it were easy to arrange more than seven consecutive days off.
- The “switch day” can be difficult for the hospital. Such schedules nearly always are arranged so that all the doctors conclude seven days of work on the same day and are replaced by others the following day. Every hospitalist patient (typically more than half of all patients in the hospital) gets a new doctor on the same day, and the whole hospital runs less efficiently as a result.
Change Your Schedule?
Who am I kidding? Few groups, probably none to be precise, are likely to change their schedule as a result of reading this column. But I’m among what seems to be a small contingent who believe alternative schedules can work. Whether your group decides to pursue a different model should be entirely up to its members, but it is worthwhile to periodically discuss the costs and benefits of your current schedule as well as what other options might be practical. In most cases the discussion will conclude without any significant change, but discussing it periodically might turn up worthwhile small adjustments.
But if your group is ready to make a meaningful change away from a rigid seven-on/seven-off schedule, the first step could be to vary the number of days off. No longer would all in the group switch on the same day; only one doctor would switch at a time (unless there are more than seven day shifts), and that could occur on any day of the week.
To illustrate, let’s say you’re in a group with four day shifts. For this week, Dr. Plant might start Monday after four days off, Dr. Bonham has had 11 days off and starts Tuesday, Dr. Page starts Friday after nine days off, and Dr. Jones starts Saturday after six days off. Each will work seven consecutive day shifts, and the number of off days will vary depending on their own wishes and the needs of the group. This is much more complicated to schedule, but varying the switch day and number of days off between weeks can be good for work-life balance.
Some will quickly identify difficulties, such as how to get the kids’ nanny to match a varying work schedule like this. I know many hospitalists who have done this successfully and are glad they did, but I’m sure there are also many for whom changing to a schedule like this might require moving from their current terrific childcare arrangements to a new one, something that they (justifiably) are unwilling to do.
And if your group successfully moves to a seven-on/X-off schedule (i.e., varied number of days off), you could next think about varying the number of consecutive days worked. Maybe it could range from no fewer than five or six (to preserve reasonable continuity) to as many as 10 or 11 as long as you have the stamina.
I don’t have research proving this would be a better schedule. But my own career, and the experiences of a number of others I’ve spoken with, is enough to convince me it’s worth considering. TH

Present “hospitalist” in a word association exercise to a wide range of healthcare personnel in clinical and administrative roles, and many would instantly respond with “seven-on/seven-off schedule.”
Some numbers from SHM’s 2014 State of Hospital Medicine report:
- 53.8%: Portion of hospitalist groups using a seven-on/seven-off schedule.
- 182: Median number of shifts worked annually by a full-time hospitalist (standard contract hours, does not include “extra” shifts).
- 65%: Portion of groups having day shifts that are 12.0–13.9 hours in length.
These numbers suggest to me that, at least outside of academia, the standard hospitalist is working 12-hour shifts on a seven-on/seven-off schedule. And that mirrors my experience working on-site with hundreds of hospitalist groups across the country.
In other words, the hospitalist marketplace has spoken unambiguously regarding the favored work schedule. In some ways, it is a defining feature of hospitalist practice. In the same way that a defining characteristic of Millennials is devotion to social media and that air travel is associated with cramped seats, this work schedule is a defining characteristic for hospitalists.
Schedule Benefits? Many …
There is a reason for its popularity: It is simple to understand and operationalize, it provides for good hospitalist-patient continuity, and having every other week off is often cited as a principle reason for becoming a hospitalist (in many cases, it might only take a clerk or administrator a few hours to create a group’s work schedule for a whole year). Many hospitalist groups have followed this schedule for a decade or longer, and while they might have periodically discussed moving to an entirely different model, most have stuck with what they know.
I’m convinced this schedule will be around for many years to come.
Not Ideal in All Respects
Despite this schedule’s popularity, I regularly talk with hospitalists who say it has become very stressful and monotonous. They say they would really like to change to something else but feel stuck by the complexity of alternative models and the difficulty achieving consensus within the group regarding what model offers enough advantages—and acceptable costs—to be worth it.
They cite as shortcomings of the seven-on/seven-off schedule:
- It can be a Herculean task to alter the schedule to arrange a day or two off during the regularly scheduled week. They often give up on the effort, and over time, this can lead to some resentment toward their work.
- There is a tendency to adopt a systole-diastole lifestyle, with no activities other than work during the week on (e.g., no trips to the gym, dinners out with family, etc.) and an effort to move all of these into the week off. They’ll say, “What other profession requires one to shut down their personal life for seven days every other week?”
- It can be difficult to reliably use the seven days off productively. Sometimes it might be better to return to work after only two to four days off if at other times it were easy to arrange more than seven consecutive days off.
- The “switch day” can be difficult for the hospital. Such schedules nearly always are arranged so that all the doctors conclude seven days of work on the same day and are replaced by others the following day. Every hospitalist patient (typically more than half of all patients in the hospital) gets a new doctor on the same day, and the whole hospital runs less efficiently as a result.
Change Your Schedule?
Who am I kidding? Few groups, probably none to be precise, are likely to change their schedule as a result of reading this column. But I’m among what seems to be a small contingent who believe alternative schedules can work. Whether your group decides to pursue a different model should be entirely up to its members, but it is worthwhile to periodically discuss the costs and benefits of your current schedule as well as what other options might be practical. In most cases the discussion will conclude without any significant change, but discussing it periodically might turn up worthwhile small adjustments.
But if your group is ready to make a meaningful change away from a rigid seven-on/seven-off schedule, the first step could be to vary the number of days off. No longer would all in the group switch on the same day; only one doctor would switch at a time (unless there are more than seven day shifts), and that could occur on any day of the week.
To illustrate, let’s say you’re in a group with four day shifts. For this week, Dr. Plant might start Monday after four days off, Dr. Bonham has had 11 days off and starts Tuesday, Dr. Page starts Friday after nine days off, and Dr. Jones starts Saturday after six days off. Each will work seven consecutive day shifts, and the number of off days will vary depending on their own wishes and the needs of the group. This is much more complicated to schedule, but varying the switch day and number of days off between weeks can be good for work-life balance.
Some will quickly identify difficulties, such as how to get the kids’ nanny to match a varying work schedule like this. I know many hospitalists who have done this successfully and are glad they did, but I’m sure there are also many for whom changing to a schedule like this might require moving from their current terrific childcare arrangements to a new one, something that they (justifiably) are unwilling to do.
And if your group successfully moves to a seven-on/X-off schedule (i.e., varied number of days off), you could next think about varying the number of consecutive days worked. Maybe it could range from no fewer than five or six (to preserve reasonable continuity) to as many as 10 or 11 as long as you have the stamina.
I don’t have research proving this would be a better schedule. But my own career, and the experiences of a number of others I’ve spoken with, is enough to convince me it’s worth considering. TH

Fewer doses of malaria drug just as effective

Photo by Sarah Mattison
A trial of African children suggests that 3 doses of artesunate can be just as effective as 5 doses for treating severe malaria.
A 3-dose intramuscular (IM) artesunate regimen proved noninferior to 5 doses of IM artesunate.
However, a 3-dose intravenous (IV) artesunate regimen was not as effective.
Peter Kremsner, MD, of Eberhard Karls Universität Tübingen in Germany, and his colleagues reported these results in PLOS Medicine.
The World Health Organization recommends that patients with severe malaria be given a 5-dose regimen of IV or IM artesunate at the time of admission (0 hours) and at 12, 24, 48, and 72 hours. However, in resource-limited settings, administering 5 doses on schedule can be challenging.
So Dr Kremsner and his colleagues wanted to determine if 3 doses of artesunate would be just as effective. They conducted an open-label, randomized, controlled trial investigating the efficacy of 3-dose IV or IM artesunate at 0, 24, and 48 hours.
The researchers enrolled 1047 children (0.5 to 10 years of age) who had severe malaria and were treated at 7 different sites in 5 African countries. The children were randomized to receive a total artesunate dose of 12 mg/kg as a control regimen of 5 IM injections (n=348) or 3 injections of 4 mg/kg either IM (n=348) or IV (n=351).
Of these children, 1002 received treatment per-protocol—331 in the 5-dose group, 338 in the 3-dose IM group, and 333 in the 3-dose IV group.
Seventy-eight percent of patients in the 3-dose IM group had about 99% parasite clearance at 24 hours, as did 79% of patients in the 5-dose IM group, a result that met a preset criterion for noninferiority (P=0.02).
However, the 3-dose IV regimen did not meet the noninferiority criterion. Seventy-four percent of these children had about 99% parasite clearance at 24 hours (P=0.24).
Twenty-two percent of the entire study population developed delayed anemia, but there was no difference in the incidence of this adverse event between the treatment arms.
The researchers said further studies are needed to clarify whether treatment with artesunate or the malaria infection itself was responsible for the delayed anemia. And patients receiving the drug should be monitored for this complication.
The team also said their findings suggest a 3-dose IM artesunate regimen can be effective against severe malaria in children, but this study did have limitations. For instance, due to practical constraints, the primary endpoint was parasite clearance at 24 hours rather than survival. ![]()

Photo by Sarah Mattison
A trial of African children suggests that 3 doses of artesunate can be just as effective as 5 doses for treating severe malaria.
A 3-dose intramuscular (IM) artesunate regimen proved noninferior to 5 doses of IM artesunate.
However, a 3-dose intravenous (IV) artesunate regimen was not as effective.
Peter Kremsner, MD, of Eberhard Karls Universität Tübingen in Germany, and his colleagues reported these results in PLOS Medicine.
The World Health Organization recommends that patients with severe malaria be given a 5-dose regimen of IV or IM artesunate at the time of admission (0 hours) and at 12, 24, 48, and 72 hours. However, in resource-limited settings, administering 5 doses on schedule can be challenging.
So Dr Kremsner and his colleagues wanted to determine if 3 doses of artesunate would be just as effective. They conducted an open-label, randomized, controlled trial investigating the efficacy of 3-dose IV or IM artesunate at 0, 24, and 48 hours.
The researchers enrolled 1047 children (0.5 to 10 years of age) who had severe malaria and were treated at 7 different sites in 5 African countries. The children were randomized to receive a total artesunate dose of 12 mg/kg as a control regimen of 5 IM injections (n=348) or 3 injections of 4 mg/kg either IM (n=348) or IV (n=351).
Of these children, 1002 received treatment per-protocol—331 in the 5-dose group, 338 in the 3-dose IM group, and 333 in the 3-dose IV group.
Seventy-eight percent of patients in the 3-dose IM group had about 99% parasite clearance at 24 hours, as did 79% of patients in the 5-dose IM group, a result that met a preset criterion for noninferiority (P=0.02).
However, the 3-dose IV regimen did not meet the noninferiority criterion. Seventy-four percent of these children had about 99% parasite clearance at 24 hours (P=0.24).
Twenty-two percent of the entire study population developed delayed anemia, but there was no difference in the incidence of this adverse event between the treatment arms.
The researchers said further studies are needed to clarify whether treatment with artesunate or the malaria infection itself was responsible for the delayed anemia. And patients receiving the drug should be monitored for this complication.
The team also said their findings suggest a 3-dose IM artesunate regimen can be effective against severe malaria in children, but this study did have limitations. For instance, due to practical constraints, the primary endpoint was parasite clearance at 24 hours rather than survival. ![]()

Photo by Sarah Mattison
A trial of African children suggests that 3 doses of artesunate can be just as effective as 5 doses for treating severe malaria.
A 3-dose intramuscular (IM) artesunate regimen proved noninferior to 5 doses of IM artesunate.
However, a 3-dose intravenous (IV) artesunate regimen was not as effective.
Peter Kremsner, MD, of Eberhard Karls Universität Tübingen in Germany, and his colleagues reported these results in PLOS Medicine.
The World Health Organization recommends that patients with severe malaria be given a 5-dose regimen of IV or IM artesunate at the time of admission (0 hours) and at 12, 24, 48, and 72 hours. However, in resource-limited settings, administering 5 doses on schedule can be challenging.
So Dr Kremsner and his colleagues wanted to determine if 3 doses of artesunate would be just as effective. They conducted an open-label, randomized, controlled trial investigating the efficacy of 3-dose IV or IM artesunate at 0, 24, and 48 hours.
The researchers enrolled 1047 children (0.5 to 10 years of age) who had severe malaria and were treated at 7 different sites in 5 African countries. The children were randomized to receive a total artesunate dose of 12 mg/kg as a control regimen of 5 IM injections (n=348) or 3 injections of 4 mg/kg either IM (n=348) or IV (n=351).
Of these children, 1002 received treatment per-protocol—331 in the 5-dose group, 338 in the 3-dose IM group, and 333 in the 3-dose IV group.
Seventy-eight percent of patients in the 3-dose IM group had about 99% parasite clearance at 24 hours, as did 79% of patients in the 5-dose IM group, a result that met a preset criterion for noninferiority (P=0.02).
However, the 3-dose IV regimen did not meet the noninferiority criterion. Seventy-four percent of these children had about 99% parasite clearance at 24 hours (P=0.24).
Twenty-two percent of the entire study population developed delayed anemia, but there was no difference in the incidence of this adverse event between the treatment arms.
The researchers said further studies are needed to clarify whether treatment with artesunate or the malaria infection itself was responsible for the delayed anemia. And patients receiving the drug should be monitored for this complication.
The team also said their findings suggest a 3-dose IM artesunate regimen can be effective against severe malaria in children, but this study did have limitations. For instance, due to practical constraints, the primary endpoint was parasite clearance at 24 hours rather than survival. ![]()
Drug gets priority review as CLL treatment

Image by Mary Ann Thompson
Despite previous safety concerns, the US Food and Drug Administration (FDA) has granted priority review for the BCL-2 inhibitor venetoclax.
The FDA is reviewing the drug as a potential treatment for patients with chronic lymphocytic leukemia (CLL), including those with 17p deletion, who have received at least 1 prior therapy.
A priority review designation is granted to drugs thought to have the potential to provide significant improvements in the treatment, prevention, or diagnosis of a disease.
The designation means the FDA’s goal is to take action on a drug application within 6 months, compared to 10 months under standard review.
Venetoclax has proven active against CLL and other hematologic malignancies, but it is known to induce tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Venetoclax received breakthrough therapy designation from the FDA last year for the treatment of patients with relapsed or refractory CLL and 17p deletion. This designation is designed to expedite the development and review of medicines intended to treat serious or life-threatening diseases.
The new drug application for venetoclax is based, in part, on data from the phase 2 M13-982 study, which were just presented at the 2015 ASH Annual Meeting.
Phase 2 trial
M13-982 is an open-label, single-arm, multicenter study in which researchers are evaluating the efficacy and safety of venetoclax in patients with relapsed, refractory, or previously untreated CLL with 17p deletion.
The study included 107 patients with relapsed or refractory disease, and all but 1 had 17p deletion. An additional 50 patients with relapsed, refractory, or previously untreated disease have been enrolled in the safety expansion cohort.
The primary endpoint of the study is overall response rate as determined by an independent review committee, and secondary endpoints include complete response, partial response, duration of response, progression-free survival, and overall survival. The level of minimal residual disease (MRD) in peripheral blood and/or bone marrow was assessed in a subset of patients.
The study met its primary endpoint, with an overall response rate of 79.4% among the 107 patients with relapsed or refractory disease. In addition, 7.5% of patients achieved a complete response, with or without complete recovery of blood counts in the bone marrow.
Forty-five patients had an assessment for MRD in the blood. Of these, 18 patients achieved MRD-negativity. Ten of these 18 patients also had bone marrow assessments, and 6 were MRD-negative.
At 1 year, 84.7% of all responses and 94.4% of MRD-negative responses were maintained. The 1-year progression-free survival and overall survival rates were 72% and 86.7%, respectively.
The most common serious adverse events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%). The most common grade 3-4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
Laboratory TLS was reported in 5 patients. None had clinical consequences.
Venetoclax is under development by AbbVie and Genentech/Roche. ![]()

Image by Mary Ann Thompson
Despite previous safety concerns, the US Food and Drug Administration (FDA) has granted priority review for the BCL-2 inhibitor venetoclax.
The FDA is reviewing the drug as a potential treatment for patients with chronic lymphocytic leukemia (CLL), including those with 17p deletion, who have received at least 1 prior therapy.
A priority review designation is granted to drugs thought to have the potential to provide significant improvements in the treatment, prevention, or diagnosis of a disease.
The designation means the FDA’s goal is to take action on a drug application within 6 months, compared to 10 months under standard review.
Venetoclax has proven active against CLL and other hematologic malignancies, but it is known to induce tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Venetoclax received breakthrough therapy designation from the FDA last year for the treatment of patients with relapsed or refractory CLL and 17p deletion. This designation is designed to expedite the development and review of medicines intended to treat serious or life-threatening diseases.
The new drug application for venetoclax is based, in part, on data from the phase 2 M13-982 study, which were just presented at the 2015 ASH Annual Meeting.
Phase 2 trial
M13-982 is an open-label, single-arm, multicenter study in which researchers are evaluating the efficacy and safety of venetoclax in patients with relapsed, refractory, or previously untreated CLL with 17p deletion.
The study included 107 patients with relapsed or refractory disease, and all but 1 had 17p deletion. An additional 50 patients with relapsed, refractory, or previously untreated disease have been enrolled in the safety expansion cohort.
The primary endpoint of the study is overall response rate as determined by an independent review committee, and secondary endpoints include complete response, partial response, duration of response, progression-free survival, and overall survival. The level of minimal residual disease (MRD) in peripheral blood and/or bone marrow was assessed in a subset of patients.
The study met its primary endpoint, with an overall response rate of 79.4% among the 107 patients with relapsed or refractory disease. In addition, 7.5% of patients achieved a complete response, with or without complete recovery of blood counts in the bone marrow.
Forty-five patients had an assessment for MRD in the blood. Of these, 18 patients achieved MRD-negativity. Ten of these 18 patients also had bone marrow assessments, and 6 were MRD-negative.
At 1 year, 84.7% of all responses and 94.4% of MRD-negative responses were maintained. The 1-year progression-free survival and overall survival rates were 72% and 86.7%, respectively.
The most common serious adverse events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%). The most common grade 3-4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
Laboratory TLS was reported in 5 patients. None had clinical consequences.
Venetoclax is under development by AbbVie and Genentech/Roche. ![]()

Image by Mary Ann Thompson
Despite previous safety concerns, the US Food and Drug Administration (FDA) has granted priority review for the BCL-2 inhibitor venetoclax.
The FDA is reviewing the drug as a potential treatment for patients with chronic lymphocytic leukemia (CLL), including those with 17p deletion, who have received at least 1 prior therapy.
A priority review designation is granted to drugs thought to have the potential to provide significant improvements in the treatment, prevention, or diagnosis of a disease.
The designation means the FDA’s goal is to take action on a drug application within 6 months, compared to 10 months under standard review.
Venetoclax has proven active against CLL and other hematologic malignancies, but it is known to induce tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Venetoclax received breakthrough therapy designation from the FDA last year for the treatment of patients with relapsed or refractory CLL and 17p deletion. This designation is designed to expedite the development and review of medicines intended to treat serious or life-threatening diseases.
The new drug application for venetoclax is based, in part, on data from the phase 2 M13-982 study, which were just presented at the 2015 ASH Annual Meeting.
Phase 2 trial
M13-982 is an open-label, single-arm, multicenter study in which researchers are evaluating the efficacy and safety of venetoclax in patients with relapsed, refractory, or previously untreated CLL with 17p deletion.
The study included 107 patients with relapsed or refractory disease, and all but 1 had 17p deletion. An additional 50 patients with relapsed, refractory, or previously untreated disease have been enrolled in the safety expansion cohort.
The primary endpoint of the study is overall response rate as determined by an independent review committee, and secondary endpoints include complete response, partial response, duration of response, progression-free survival, and overall survival. The level of minimal residual disease (MRD) in peripheral blood and/or bone marrow was assessed in a subset of patients.
The study met its primary endpoint, with an overall response rate of 79.4% among the 107 patients with relapsed or refractory disease. In addition, 7.5% of patients achieved a complete response, with or without complete recovery of blood counts in the bone marrow.
Forty-five patients had an assessment for MRD in the blood. Of these, 18 patients achieved MRD-negativity. Ten of these 18 patients also had bone marrow assessments, and 6 were MRD-negative.
At 1 year, 84.7% of all responses and 94.4% of MRD-negative responses were maintained. The 1-year progression-free survival and overall survival rates were 72% and 86.7%, respectively.
The most common serious adverse events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%). The most common grade 3-4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
Laboratory TLS was reported in 5 patients. None had clinical consequences.
Venetoclax is under development by AbbVie and Genentech/Roche. ![]()
PBSCs used to treat BMF despite drawbacks

Photo from the Canterbury
District Health Board
Although studies have suggested that peripheral blood stem cells (PBSCs) are not the ideal graft source for patients with bone marrow failure (BMF), new research suggests transplant centers worldwide are still using PBSCs in these patients.
The study included data on more than 3000 hematopoietic stem cell transplants (HSCTs) performed in patients with BMF.
The numbers revealed that PBSCs were most-used in the Asia-Pacific region, Africa, and the Eastern Mediterranean region. But they were also used in Europe and the Americas.
Ayami Yoshimi, MD, PhD, of the University of Freiburg, Germany, and colleagues disclosed these findings in a letter to JAMA.
The researchers noted that bone marrow is the recommended graft source for HSCT in patients with BMF, as studies have shown that PBSCs are associated with higher rates of graft-vs-host disease and lower rates of survival.
With this in mind, the team examined the graft sources used in patients with BMF who underwent HSCTs from 2009 through 2010. The researchers looked at 194 World Health Organization member states and found that 74 had reported at least 1 HSCT during that time period.
Of the 114,217 transplants performed, there were 3282 allogeneic HSCTs in patients with BMF. Overall, the most-used graft source was bone marrow (54%), followed by PBSCs (41%), and then cord blood (5%).
Bone marrow was used most commonly in the Americas (75%) and in Europe (60%) but not in the Eastern Mediterranean region and Africa (46%) or in the Asia-Pacific region (41%; excluding Japan, 19%).
The researchers also looked at graft source according to donor type, both overall and by region, but they excluded the 180 cord blood transplants from this analysis.
The team found that, among patients who had a related donor, 57% received bone marrow and 43% received PBSCs.
For related HSCTs in the Americas, 75% of patients received bone marrow and 25% received PBSCs. In Europe, 63% received bone marrow and 37% received PBSCs. In the Eastern Mediterranean and Africa, 47% received bone marrow and 53% received PBSCs. And in the Asia-Pacific region, 37% received bone marrow and 63% received PBSCs.
Among patients who had unrelated donors, 57% received bone marrow and 43% received PBSCs.
For unrelated HSCTs in the Americas, 74% of patients received bone marrow and 26% received PBSCs. In Europe, 56% received bone marrow and 44% received PBSCs. In the Asia-Pacific region, 47% received bone marrow and 53% received PBSCs. And in the Eastern Mediterranean and Africa, 100% received PBSCs.
The use of bone marrow increased from 20% in countries with low and low-middle incomes to 50% in countries with high-middle incomes and 64% in countries with high incomes (P<0.001). There was a significant association between gross national income per capita and stem cell source (P=0.002).
The researchers speculated that PBSCs are still used in BMF patients, despite the disadvantages, because transplant centers routinely obtain PBSCs for other indications, cell separators are available at any transplant center, and PBSC transplants can be performed at a lower cost than bone marrow transplants.
The team said the association between graft source and income supports the idea that short-term financial considerations are important.
So transplant organizations and authorities should help foster regional-accredited bone marrow harvest centers for patients with nonmalignant disorders. And unrelated donor registries should provide information on the necessity of bone marrow donation for patients with BMF. ![]()

Photo from the Canterbury
District Health Board
Although studies have suggested that peripheral blood stem cells (PBSCs) are not the ideal graft source for patients with bone marrow failure (BMF), new research suggests transplant centers worldwide are still using PBSCs in these patients.
The study included data on more than 3000 hematopoietic stem cell transplants (HSCTs) performed in patients with BMF.
The numbers revealed that PBSCs were most-used in the Asia-Pacific region, Africa, and the Eastern Mediterranean region. But they were also used in Europe and the Americas.
Ayami Yoshimi, MD, PhD, of the University of Freiburg, Germany, and colleagues disclosed these findings in a letter to JAMA.
The researchers noted that bone marrow is the recommended graft source for HSCT in patients with BMF, as studies have shown that PBSCs are associated with higher rates of graft-vs-host disease and lower rates of survival.
With this in mind, the team examined the graft sources used in patients with BMF who underwent HSCTs from 2009 through 2010. The researchers looked at 194 World Health Organization member states and found that 74 had reported at least 1 HSCT during that time period.
Of the 114,217 transplants performed, there were 3282 allogeneic HSCTs in patients with BMF. Overall, the most-used graft source was bone marrow (54%), followed by PBSCs (41%), and then cord blood (5%).
Bone marrow was used most commonly in the Americas (75%) and in Europe (60%) but not in the Eastern Mediterranean region and Africa (46%) or in the Asia-Pacific region (41%; excluding Japan, 19%).
The researchers also looked at graft source according to donor type, both overall and by region, but they excluded the 180 cord blood transplants from this analysis.
The team found that, among patients who had a related donor, 57% received bone marrow and 43% received PBSCs.
For related HSCTs in the Americas, 75% of patients received bone marrow and 25% received PBSCs. In Europe, 63% received bone marrow and 37% received PBSCs. In the Eastern Mediterranean and Africa, 47% received bone marrow and 53% received PBSCs. And in the Asia-Pacific region, 37% received bone marrow and 63% received PBSCs.
Among patients who had unrelated donors, 57% received bone marrow and 43% received PBSCs.
For unrelated HSCTs in the Americas, 74% of patients received bone marrow and 26% received PBSCs. In Europe, 56% received bone marrow and 44% received PBSCs. In the Asia-Pacific region, 47% received bone marrow and 53% received PBSCs. And in the Eastern Mediterranean and Africa, 100% received PBSCs.
The use of bone marrow increased from 20% in countries with low and low-middle incomes to 50% in countries with high-middle incomes and 64% in countries with high incomes (P<0.001). There was a significant association between gross national income per capita and stem cell source (P=0.002).
The researchers speculated that PBSCs are still used in BMF patients, despite the disadvantages, because transplant centers routinely obtain PBSCs for other indications, cell separators are available at any transplant center, and PBSC transplants can be performed at a lower cost than bone marrow transplants.
The team said the association between graft source and income supports the idea that short-term financial considerations are important.
So transplant organizations and authorities should help foster regional-accredited bone marrow harvest centers for patients with nonmalignant disorders. And unrelated donor registries should provide information on the necessity of bone marrow donation for patients with BMF. ![]()

Photo from the Canterbury
District Health Board
Although studies have suggested that peripheral blood stem cells (PBSCs) are not the ideal graft source for patients with bone marrow failure (BMF), new research suggests transplant centers worldwide are still using PBSCs in these patients.
The study included data on more than 3000 hematopoietic stem cell transplants (HSCTs) performed in patients with BMF.
The numbers revealed that PBSCs were most-used in the Asia-Pacific region, Africa, and the Eastern Mediterranean region. But they were also used in Europe and the Americas.
Ayami Yoshimi, MD, PhD, of the University of Freiburg, Germany, and colleagues disclosed these findings in a letter to JAMA.
The researchers noted that bone marrow is the recommended graft source for HSCT in patients with BMF, as studies have shown that PBSCs are associated with higher rates of graft-vs-host disease and lower rates of survival.
With this in mind, the team examined the graft sources used in patients with BMF who underwent HSCTs from 2009 through 2010. The researchers looked at 194 World Health Organization member states and found that 74 had reported at least 1 HSCT during that time period.
Of the 114,217 transplants performed, there were 3282 allogeneic HSCTs in patients with BMF. Overall, the most-used graft source was bone marrow (54%), followed by PBSCs (41%), and then cord blood (5%).
Bone marrow was used most commonly in the Americas (75%) and in Europe (60%) but not in the Eastern Mediterranean region and Africa (46%) or in the Asia-Pacific region (41%; excluding Japan, 19%).
The researchers also looked at graft source according to donor type, both overall and by region, but they excluded the 180 cord blood transplants from this analysis.
The team found that, among patients who had a related donor, 57% received bone marrow and 43% received PBSCs.
For related HSCTs in the Americas, 75% of patients received bone marrow and 25% received PBSCs. In Europe, 63% received bone marrow and 37% received PBSCs. In the Eastern Mediterranean and Africa, 47% received bone marrow and 53% received PBSCs. And in the Asia-Pacific region, 37% received bone marrow and 63% received PBSCs.
Among patients who had unrelated donors, 57% received bone marrow and 43% received PBSCs.
For unrelated HSCTs in the Americas, 74% of patients received bone marrow and 26% received PBSCs. In Europe, 56% received bone marrow and 44% received PBSCs. In the Asia-Pacific region, 47% received bone marrow and 53% received PBSCs. And in the Eastern Mediterranean and Africa, 100% received PBSCs.
The use of bone marrow increased from 20% in countries with low and low-middle incomes to 50% in countries with high-middle incomes and 64% in countries with high incomes (P<0.001). There was a significant association between gross national income per capita and stem cell source (P=0.002).
The researchers speculated that PBSCs are still used in BMF patients, despite the disadvantages, because transplant centers routinely obtain PBSCs for other indications, cell separators are available at any transplant center, and PBSC transplants can be performed at a lower cost than bone marrow transplants.
The team said the association between graft source and income supports the idea that short-term financial considerations are important.
So transplant organizations and authorities should help foster regional-accredited bone marrow harvest centers for patients with nonmalignant disorders. And unrelated donor registries should provide information on the necessity of bone marrow donation for patients with BMF. ![]()
Team identifies new mechanism of megakaryocyte differentiation

in the bone marrow
Investigators have discovered a new mechanism of megakaryocyte differentiation, according to a paper published in eLife.
They found that overexpression of the methyltransferase enzyme PRMT1 in acute megakaryocytic leukemia blocks megakaryocyte differentiation by downregulating levels of the RNA-binding protein RBM15.
The team therefore believes that targeting PRMT1 could restore megakaryocyte differentiation in this malignancy.
They also think their findings could lead to new approaches for researching and treating other hematologic malignancies and solid tumors.
Xinyang Zhao, PhD, of the University of Alabama at Birmingham, and his colleagues began this study looking at PRMT1, which attaches a methyl group onto specific arginine amino acid residues of target proteins.
The investigators screened for proteins that were tagged with methyl groups by PRMT1 and selected one of them—RBM15—for further study. RBM15 was of interest because a mutant fusion of RBM15 and MKL1 proteins is associated with acute megakaryoblastic leukemia.
The team discovered that when a cell’s PRMT1 levels are high, a greater proportion of RBM15 is tagged with methyl groups on certain arginine residues. This tagging causes a ligase called CNOT4 to mark RBM15 with another tag, ubiquitin, which marks the protein for transport to the cell’s garbage removal machinery.
The methyl-tagged RBM15 proteins rapidly disappear, even though the amount of RBM15 messenger RNA does not change. Thus, the expression levels of PRMT1 inversely affect the amount of RBM15.
When the concentration of RBM15 is low, megakaryocytic progenitor cells cannot move forward to differentiation. But when the concentration of RBM15 is high enough, the progenitor cells differentiate into mature megakaryocytes.
The investigators also found that RBM15 binds to intron regions of the pre-messenger RNA for genes known to be important in megakaryocyte differentiation, including 3 transcription factors—RUNX1, GATA1, and TAL1—that are important for normal and abnormal hematopoiesis.
And RBM15 appears to recruit the splicing factor SF3B1 to correctly splice exons. When RBM15 is low, one or more exons are not correctly spliced.
The team said this is a new mechanism for cell differentiation, initiated by methylation of RNA-binding proteins.
“The regulation of alternative splicing by RBM15 through SF3B1 is an exciting and novel pathway that clearly participates in the decision of a megakaryocyte to grow or differentiate,” said John Crispino, PhD, of the Northwestern University Feinberg School of Medicine in Chicago, Illinois, who was not involved in this study.
“These findings suggest that modulation of RBM15 activity by suppressing PRMT1 activity may change the splicing pattern of megakaryocytic tumor cells and facilitate their differentiation.”
The investigators also believe RBM15 may have broader functions in cells. They found that RBM15 binds directly to the pre-messenger RNA of 1257 genes. Among them are genes involved in metabolic regulation.
In agreement with this finding, the team discovered that overexpression of PRMT1 or reduced expression of RBM15 enhances the creation of more mitochondria.
The investigators have further identified metabolic pathways regulated by PRMT1 in leukemia cells. They said these data, in a manuscript under preparation, will further link tumorigenesis to metabolic pathways.
The team also noted that SF3B1 contains mutations in more than 70% of myelodysplastic syndrome patients and 20% of chronic lymphocytic leukemia patients, and mutated SF3B1 appears in other hematologic malignancies as well.
So the investigators believe that understanding the PRMT1-RBM15 axis can shed new light on SF3B1-mutated hematologic malignancies and may lead to targeting PRMT1 as a novel therapy for myelodysplastic syndromes. The team is already testing PRMT1 inhibitors. ![]()

in the bone marrow
Investigators have discovered a new mechanism of megakaryocyte differentiation, according to a paper published in eLife.
They found that overexpression of the methyltransferase enzyme PRMT1 in acute megakaryocytic leukemia blocks megakaryocyte differentiation by downregulating levels of the RNA-binding protein RBM15.
The team therefore believes that targeting PRMT1 could restore megakaryocyte differentiation in this malignancy.
They also think their findings could lead to new approaches for researching and treating other hematologic malignancies and solid tumors.
Xinyang Zhao, PhD, of the University of Alabama at Birmingham, and his colleagues began this study looking at PRMT1, which attaches a methyl group onto specific arginine amino acid residues of target proteins.
The investigators screened for proteins that were tagged with methyl groups by PRMT1 and selected one of them—RBM15—for further study. RBM15 was of interest because a mutant fusion of RBM15 and MKL1 proteins is associated with acute megakaryoblastic leukemia.
The team discovered that when a cell’s PRMT1 levels are high, a greater proportion of RBM15 is tagged with methyl groups on certain arginine residues. This tagging causes a ligase called CNOT4 to mark RBM15 with another tag, ubiquitin, which marks the protein for transport to the cell’s garbage removal machinery.
The methyl-tagged RBM15 proteins rapidly disappear, even though the amount of RBM15 messenger RNA does not change. Thus, the expression levels of PRMT1 inversely affect the amount of RBM15.
When the concentration of RBM15 is low, megakaryocytic progenitor cells cannot move forward to differentiation. But when the concentration of RBM15 is high enough, the progenitor cells differentiate into mature megakaryocytes.
The investigators also found that RBM15 binds to intron regions of the pre-messenger RNA for genes known to be important in megakaryocyte differentiation, including 3 transcription factors—RUNX1, GATA1, and TAL1—that are important for normal and abnormal hematopoiesis.
And RBM15 appears to recruit the splicing factor SF3B1 to correctly splice exons. When RBM15 is low, one or more exons are not correctly spliced.
The team said this is a new mechanism for cell differentiation, initiated by methylation of RNA-binding proteins.
“The regulation of alternative splicing by RBM15 through SF3B1 is an exciting and novel pathway that clearly participates in the decision of a megakaryocyte to grow or differentiate,” said John Crispino, PhD, of the Northwestern University Feinberg School of Medicine in Chicago, Illinois, who was not involved in this study.
“These findings suggest that modulation of RBM15 activity by suppressing PRMT1 activity may change the splicing pattern of megakaryocytic tumor cells and facilitate their differentiation.”
The investigators also believe RBM15 may have broader functions in cells. They found that RBM15 binds directly to the pre-messenger RNA of 1257 genes. Among them are genes involved in metabolic regulation.
In agreement with this finding, the team discovered that overexpression of PRMT1 or reduced expression of RBM15 enhances the creation of more mitochondria.
The investigators have further identified metabolic pathways regulated by PRMT1 in leukemia cells. They said these data, in a manuscript under preparation, will further link tumorigenesis to metabolic pathways.
The team also noted that SF3B1 contains mutations in more than 70% of myelodysplastic syndrome patients and 20% of chronic lymphocytic leukemia patients, and mutated SF3B1 appears in other hematologic malignancies as well.
So the investigators believe that understanding the PRMT1-RBM15 axis can shed new light on SF3B1-mutated hematologic malignancies and may lead to targeting PRMT1 as a novel therapy for myelodysplastic syndromes. The team is already testing PRMT1 inhibitors. ![]()

in the bone marrow
Investigators have discovered a new mechanism of megakaryocyte differentiation, according to a paper published in eLife.
They found that overexpression of the methyltransferase enzyme PRMT1 in acute megakaryocytic leukemia blocks megakaryocyte differentiation by downregulating levels of the RNA-binding protein RBM15.
The team therefore believes that targeting PRMT1 could restore megakaryocyte differentiation in this malignancy.
They also think their findings could lead to new approaches for researching and treating other hematologic malignancies and solid tumors.
Xinyang Zhao, PhD, of the University of Alabama at Birmingham, and his colleagues began this study looking at PRMT1, which attaches a methyl group onto specific arginine amino acid residues of target proteins.
The investigators screened for proteins that were tagged with methyl groups by PRMT1 and selected one of them—RBM15—for further study. RBM15 was of interest because a mutant fusion of RBM15 and MKL1 proteins is associated with acute megakaryoblastic leukemia.
The team discovered that when a cell’s PRMT1 levels are high, a greater proportion of RBM15 is tagged with methyl groups on certain arginine residues. This tagging causes a ligase called CNOT4 to mark RBM15 with another tag, ubiquitin, which marks the protein for transport to the cell’s garbage removal machinery.
The methyl-tagged RBM15 proteins rapidly disappear, even though the amount of RBM15 messenger RNA does not change. Thus, the expression levels of PRMT1 inversely affect the amount of RBM15.
When the concentration of RBM15 is low, megakaryocytic progenitor cells cannot move forward to differentiation. But when the concentration of RBM15 is high enough, the progenitor cells differentiate into mature megakaryocytes.
The investigators also found that RBM15 binds to intron regions of the pre-messenger RNA for genes known to be important in megakaryocyte differentiation, including 3 transcription factors—RUNX1, GATA1, and TAL1—that are important for normal and abnormal hematopoiesis.
And RBM15 appears to recruit the splicing factor SF3B1 to correctly splice exons. When RBM15 is low, one or more exons are not correctly spliced.
The team said this is a new mechanism for cell differentiation, initiated by methylation of RNA-binding proteins.
“The regulation of alternative splicing by RBM15 through SF3B1 is an exciting and novel pathway that clearly participates in the decision of a megakaryocyte to grow or differentiate,” said John Crispino, PhD, of the Northwestern University Feinberg School of Medicine in Chicago, Illinois, who was not involved in this study.
“These findings suggest that modulation of RBM15 activity by suppressing PRMT1 activity may change the splicing pattern of megakaryocytic tumor cells and facilitate their differentiation.”
The investigators also believe RBM15 may have broader functions in cells. They found that RBM15 binds directly to the pre-messenger RNA of 1257 genes. Among them are genes involved in metabolic regulation.
In agreement with this finding, the team discovered that overexpression of PRMT1 or reduced expression of RBM15 enhances the creation of more mitochondria.
The investigators have further identified metabolic pathways regulated by PRMT1 in leukemia cells. They said these data, in a manuscript under preparation, will further link tumorigenesis to metabolic pathways.
The team also noted that SF3B1 contains mutations in more than 70% of myelodysplastic syndrome patients and 20% of chronic lymphocytic leukemia patients, and mutated SF3B1 appears in other hematologic malignancies as well.
So the investigators believe that understanding the PRMT1-RBM15 axis can shed new light on SF3B1-mutated hematologic malignancies and may lead to targeting PRMT1 as a novel therapy for myelodysplastic syndromes. The team is already testing PRMT1 inhibitors. ![]()










