User login
Standing Linked to Reduced Obesity
Study Overview
Objective. To examine the cross-sectional relationships between standing time, obesity, and metabolic syndrome.
Design. Cross-sectional study.
Setting and participants. Participants were patients aged 20–79 years old attending Cooper Clinic in Dallas for a preventive medicine visit who enrolled in the Cooper Center Longitudinal Study, an ongoing prospective investigation established in 1970 to explore the effects of physical activity on morbidity and mortality [1]. Included in the analysis were those enrolled starting in 2010, when questions pertaining to standing patterns began to be included in the medical history. Patients who did not have complete information or who had a history of myocardial infarction, stroke, or cancer were excluded.
Measures. Obesity was directly measured using body mass index (≥ 30), waist circumference (men: ≥ 102 cm; women: ≥ 88 cm), and body fat percentage (men: ≥ 25%; women ≥ 30%) and was adjusted for history of diabetes and hypertension. Metabolic syndrome, a clustering of risk factors that increase the risk for heart disease, stroke, and diabetes, was assessed. Participants’ standing patterns were ascertained from responses to survey questions derived from the Canada Fitness Survey Questionnaire (“For those activities that you do most days of the week, such as work, school, and housework, how much time do you spend standing: Almost all of the time, ¾ of the time, ½ of the time, ¼ of the time, almost none of the time?”). Leisure-time physical activity was determined based on responses to survey questions, and answers were used to categorize participants as either meeting or not meeting the Physical Activity Guidelines for Americans.
Results. The study sample consisted of 7075 participants, who were primarily white and college educated. Over two-thirds were men and the mean age was 50.0 ± 10.1 years. Multivariable analysis showed that in men, increased standing was significantly associated with a lower likelihood of elevated body fat percentage. Specifically, standing a quarter of the time was linked to a 32% reduced likelihood of obesity (body fat percentage), standing half the time was associated with a 59% reduced likelihood of obesity, but standing more than three-quarters of the time was not associated with a lower risk of obesity. In women, standing a quarter, half, and three-quarters of the time was associated with 35%, 47%, and 57% respective reductions in the likelihood of abdominal obesity (waist circumference). No relationship between standing and metabolic syndrome was found among women or men.
The study also examined whether physical activity in conjunction with standing provided additional reduction risk for obesity. The study showed that 150 minutes of moderate activity and/or 75 minutes of vigorous activity per week added to standing time was associated with significant reduction in the probability of obesity and metabolic syndrome in both women and men.
Conclusion. Standing a quarter of the time per day or more is associated with reduced odds of obesity. The inverse relationship of standing to obesity and metabolic syndrome is more robust when combined with health-promoting leisure-time physical activity.
Commentary
Obesity is considered one of the main risk factors for cardiovascular diseases worldwide. Obesity-related conditions include heart disease, stroke, type 2 diabetes, and certain types of cancer, some of the leading causes of preventable death. The effects of obesity among Americans add more than $147 billion in medical costs to the U.S. economy annually [2].
Obesity is a national epidemic, with more than 78.9 million obese adults in the United States [2]. Studies have shown that Americans are currently less active as compared to past decades [3]. This decline in physical activity combined with other factors, such as the ubiquity of low-cost high-energy foods and beverages, has likely contributed to the high rate of obesity.
This cross-sectional study aimed to assess the relationship between standing time, obesity, and metabolic syndrome alongside and independent of leisure-time physical activity. The researchers found that standing for at least one quarter of the day is linked to lower odds of obesity, which was directly assessed through 3 measures: BMI, body fat percentage, and waist circumference. The apparent benefit of standing is an important finding in light of obesity being such an important public health concern.
The large sample size is a strength of this study in terms of statistical power; however, there are important limitations that must be acknowledged. First, given the cross-sectional design, no causal inferences can be made. Moreover, while obesity and metabolic syndrome were objectively measured, standing and physical activity were based on self-report, which may lead to over- or underestimation of these behaviors. In addition, due to the survey measure used in the study, it is unclear whether study participants were standing still or standing and moving. More information in this regard would be helpful. Longitudinal research is encouraged in order to provide better evidence of these relationships and their effects.
In addition, cultural aspects were not assessed in this study. Racial and ethnic differences may influence the relationship between the variables of physical activity and obesity reduction.
Applications for Clinical Practice
Obesity is a complex but preventable health problem commonly associated with sedentary lifestyle. Physical activity is recommended as a component of weight management for prevention of weight gain and for weight loss [4]. Whether standing more often will aid in reducing obesity cannot be determined from this study.
—Paloma Cesar de Sales, BS, RN, MS
1. Shuval K, Finley CE, Barlow CE, et al. Sedentary behavior, cardiorespiratory fitness, physical activity, and cardiometabolic risk in men: the cooper center longitudinal study. Mayo Clin Proc 2014;89:1052–62.
2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14.
3. Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012;13:659–80.
4. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(25 Suppl 2):S102–38.
Study Overview
Objective. To examine the cross-sectional relationships between standing time, obesity, and metabolic syndrome.
Design. Cross-sectional study.
Setting and participants. Participants were patients aged 20–79 years old attending Cooper Clinic in Dallas for a preventive medicine visit who enrolled in the Cooper Center Longitudinal Study, an ongoing prospective investigation established in 1970 to explore the effects of physical activity on morbidity and mortality [1]. Included in the analysis were those enrolled starting in 2010, when questions pertaining to standing patterns began to be included in the medical history. Patients who did not have complete information or who had a history of myocardial infarction, stroke, or cancer were excluded.
Measures. Obesity was directly measured using body mass index (≥ 30), waist circumference (men: ≥ 102 cm; women: ≥ 88 cm), and body fat percentage (men: ≥ 25%; women ≥ 30%) and was adjusted for history of diabetes and hypertension. Metabolic syndrome, a clustering of risk factors that increase the risk for heart disease, stroke, and diabetes, was assessed. Participants’ standing patterns were ascertained from responses to survey questions derived from the Canada Fitness Survey Questionnaire (“For those activities that you do most days of the week, such as work, school, and housework, how much time do you spend standing: Almost all of the time, ¾ of the time, ½ of the time, ¼ of the time, almost none of the time?”). Leisure-time physical activity was determined based on responses to survey questions, and answers were used to categorize participants as either meeting or not meeting the Physical Activity Guidelines for Americans.
Results. The study sample consisted of 7075 participants, who were primarily white and college educated. Over two-thirds were men and the mean age was 50.0 ± 10.1 years. Multivariable analysis showed that in men, increased standing was significantly associated with a lower likelihood of elevated body fat percentage. Specifically, standing a quarter of the time was linked to a 32% reduced likelihood of obesity (body fat percentage), standing half the time was associated with a 59% reduced likelihood of obesity, but standing more than three-quarters of the time was not associated with a lower risk of obesity. In women, standing a quarter, half, and three-quarters of the time was associated with 35%, 47%, and 57% respective reductions in the likelihood of abdominal obesity (waist circumference). No relationship between standing and metabolic syndrome was found among women or men.
The study also examined whether physical activity in conjunction with standing provided additional reduction risk for obesity. The study showed that 150 minutes of moderate activity and/or 75 minutes of vigorous activity per week added to standing time was associated with significant reduction in the probability of obesity and metabolic syndrome in both women and men.
Conclusion. Standing a quarter of the time per day or more is associated with reduced odds of obesity. The inverse relationship of standing to obesity and metabolic syndrome is more robust when combined with health-promoting leisure-time physical activity.
Commentary
Obesity is considered one of the main risk factors for cardiovascular diseases worldwide. Obesity-related conditions include heart disease, stroke, type 2 diabetes, and certain types of cancer, some of the leading causes of preventable death. The effects of obesity among Americans add more than $147 billion in medical costs to the U.S. economy annually [2].
Obesity is a national epidemic, with more than 78.9 million obese adults in the United States [2]. Studies have shown that Americans are currently less active as compared to past decades [3]. This decline in physical activity combined with other factors, such as the ubiquity of low-cost high-energy foods and beverages, has likely contributed to the high rate of obesity.
This cross-sectional study aimed to assess the relationship between standing time, obesity, and metabolic syndrome alongside and independent of leisure-time physical activity. The researchers found that standing for at least one quarter of the day is linked to lower odds of obesity, which was directly assessed through 3 measures: BMI, body fat percentage, and waist circumference. The apparent benefit of standing is an important finding in light of obesity being such an important public health concern.
The large sample size is a strength of this study in terms of statistical power; however, there are important limitations that must be acknowledged. First, given the cross-sectional design, no causal inferences can be made. Moreover, while obesity and metabolic syndrome were objectively measured, standing and physical activity were based on self-report, which may lead to over- or underestimation of these behaviors. In addition, due to the survey measure used in the study, it is unclear whether study participants were standing still or standing and moving. More information in this regard would be helpful. Longitudinal research is encouraged in order to provide better evidence of these relationships and their effects.
In addition, cultural aspects were not assessed in this study. Racial and ethnic differences may influence the relationship between the variables of physical activity and obesity reduction.
Applications for Clinical Practice
Obesity is a complex but preventable health problem commonly associated with sedentary lifestyle. Physical activity is recommended as a component of weight management for prevention of weight gain and for weight loss [4]. Whether standing more often will aid in reducing obesity cannot be determined from this study.
—Paloma Cesar de Sales, BS, RN, MS
Study Overview
Objective. To examine the cross-sectional relationships between standing time, obesity, and metabolic syndrome.
Design. Cross-sectional study.
Setting and participants. Participants were patients aged 20–79 years old attending Cooper Clinic in Dallas for a preventive medicine visit who enrolled in the Cooper Center Longitudinal Study, an ongoing prospective investigation established in 1970 to explore the effects of physical activity on morbidity and mortality [1]. Included in the analysis were those enrolled starting in 2010, when questions pertaining to standing patterns began to be included in the medical history. Patients who did not have complete information or who had a history of myocardial infarction, stroke, or cancer were excluded.
Measures. Obesity was directly measured using body mass index (≥ 30), waist circumference (men: ≥ 102 cm; women: ≥ 88 cm), and body fat percentage (men: ≥ 25%; women ≥ 30%) and was adjusted for history of diabetes and hypertension. Metabolic syndrome, a clustering of risk factors that increase the risk for heart disease, stroke, and diabetes, was assessed. Participants’ standing patterns were ascertained from responses to survey questions derived from the Canada Fitness Survey Questionnaire (“For those activities that you do most days of the week, such as work, school, and housework, how much time do you spend standing: Almost all of the time, ¾ of the time, ½ of the time, ¼ of the time, almost none of the time?”). Leisure-time physical activity was determined based on responses to survey questions, and answers were used to categorize participants as either meeting or not meeting the Physical Activity Guidelines for Americans.
Results. The study sample consisted of 7075 participants, who were primarily white and college educated. Over two-thirds were men and the mean age was 50.0 ± 10.1 years. Multivariable analysis showed that in men, increased standing was significantly associated with a lower likelihood of elevated body fat percentage. Specifically, standing a quarter of the time was linked to a 32% reduced likelihood of obesity (body fat percentage), standing half the time was associated with a 59% reduced likelihood of obesity, but standing more than three-quarters of the time was not associated with a lower risk of obesity. In women, standing a quarter, half, and three-quarters of the time was associated with 35%, 47%, and 57% respective reductions in the likelihood of abdominal obesity (waist circumference). No relationship between standing and metabolic syndrome was found among women or men.
The study also examined whether physical activity in conjunction with standing provided additional reduction risk for obesity. The study showed that 150 minutes of moderate activity and/or 75 minutes of vigorous activity per week added to standing time was associated with significant reduction in the probability of obesity and metabolic syndrome in both women and men.
Conclusion. Standing a quarter of the time per day or more is associated with reduced odds of obesity. The inverse relationship of standing to obesity and metabolic syndrome is more robust when combined with health-promoting leisure-time physical activity.
Commentary
Obesity is considered one of the main risk factors for cardiovascular diseases worldwide. Obesity-related conditions include heart disease, stroke, type 2 diabetes, and certain types of cancer, some of the leading causes of preventable death. The effects of obesity among Americans add more than $147 billion in medical costs to the U.S. economy annually [2].
Obesity is a national epidemic, with more than 78.9 million obese adults in the United States [2]. Studies have shown that Americans are currently less active as compared to past decades [3]. This decline in physical activity combined with other factors, such as the ubiquity of low-cost high-energy foods and beverages, has likely contributed to the high rate of obesity.
This cross-sectional study aimed to assess the relationship between standing time, obesity, and metabolic syndrome alongside and independent of leisure-time physical activity. The researchers found that standing for at least one quarter of the day is linked to lower odds of obesity, which was directly assessed through 3 measures: BMI, body fat percentage, and waist circumference. The apparent benefit of standing is an important finding in light of obesity being such an important public health concern.
The large sample size is a strength of this study in terms of statistical power; however, there are important limitations that must be acknowledged. First, given the cross-sectional design, no causal inferences can be made. Moreover, while obesity and metabolic syndrome were objectively measured, standing and physical activity were based on self-report, which may lead to over- or underestimation of these behaviors. In addition, due to the survey measure used in the study, it is unclear whether study participants were standing still or standing and moving. More information in this regard would be helpful. Longitudinal research is encouraged in order to provide better evidence of these relationships and their effects.
In addition, cultural aspects were not assessed in this study. Racial and ethnic differences may influence the relationship between the variables of physical activity and obesity reduction.
Applications for Clinical Practice
Obesity is a complex but preventable health problem commonly associated with sedentary lifestyle. Physical activity is recommended as a component of weight management for prevention of weight gain and for weight loss [4]. Whether standing more often will aid in reducing obesity cannot be determined from this study.
—Paloma Cesar de Sales, BS, RN, MS
1. Shuval K, Finley CE, Barlow CE, et al. Sedentary behavior, cardiorespiratory fitness, physical activity, and cardiometabolic risk in men: the cooper center longitudinal study. Mayo Clin Proc 2014;89:1052–62.
2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14.
3. Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012;13:659–80.
4. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(25 Suppl 2):S102–38.
1. Shuval K, Finley CE, Barlow CE, et al. Sedentary behavior, cardiorespiratory fitness, physical activity, and cardiometabolic risk in men: the cooper center longitudinal study. Mayo Clin Proc 2014;89:1052–62.
2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14.
3. Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012;13:659–80.
4. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(25 Suppl 2):S102–38.
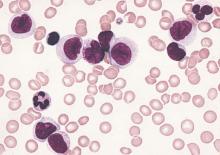
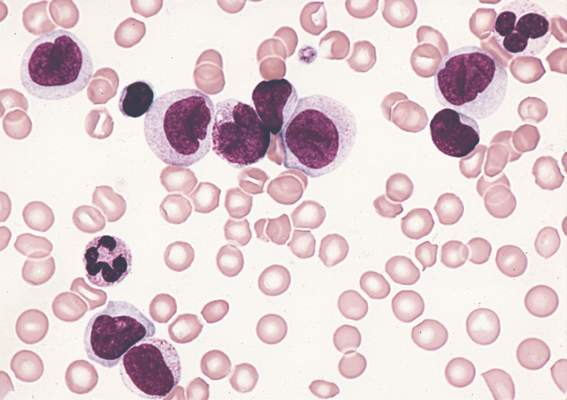
Hodgkin lymphoma going untreated in patients with HIV

cultured lymphocyte
Image courtesy of the CDC
Patients with HIV-associated Hodgkin lymphoma may not be getting potentially curative treatment, according to a study published in the journal AIDS.
The study showed that 16% of HIV-positive patients did not receive treatment for their lymphoma, compared to 9% of Hodgkin lymphoma patients who were HIV-negative.
“Hodgkin lymphoma is generally believed to be highly curable,” said study author Adam Olszewski, MD, of Brown University in Providence, Rhode Island.
“We have an expectation to cure over 90% of early stage patients and even 70% to 80% of quite advanced cases.”
It hasn’t been clear whether HIV-positive patients with Hodgkin lymphoma survive the cancer as well as people who are HIV-negative. While some small studies, particularly in Europe, have shown that HIV status makes no difference to survival, observations in the US population suggest that being HIV-positive makes survival less likely.
The new study, which is the largest of its kind to date, may reconcile that conflict. It suggests that, in the US, the reason people with HIV seem to fare worse with the cancer is because they are less likely to be treated for it.
The study included 2090 cases of HIV-associated Hodgkin lymphoma recorded in the National Cancer Data Base between 2004 and 2012, as well as 41,846 cases of Hodgkin lymphoma in patients who were HIV-negative.
The unadjusted 5-year overall survival was 66% for HIV-positive patients and 80% for the HIV-negative population.
Among the HIV-positive patients, 81% received chemotherapy (12% in combination with radiation), 13% received any radiation therapy, and 16% received no treatment for their lymphoma. The corresponding numbers for HIV-negative patients were 87%, 31%, and 9%, respectively (P<0.00001 for all comparisons).
The researchers assessed patient- and disease-related factors associated with the risk of not receiving chemotherapy in the HIV-positive population.
And they found the risk was significantly higher for patients who were older than 40, male, “nonwhite” (black, Hispanic, or Asian/”other”), did not have health insurance, lived in areas with the lowest median income, and had early stage Hodgkin lymphoma or an undetermined histology.
Dr Olszewski said the lack of treatment among HIV-positive patients could be due to a lingering assumption that they won’t tolerate the treatment well. Or some patients may be declining treatment, either for HIV (thereby making them seem more vulnerable) or for the lymphoma itself.
He noted, however, that lymphoma treatment can be effective for and tolerated by HIV-positive patients, especially when the lymphoma subtype is known.
Among the patients who received chemotherapy in this study, there was no significant difference in the hazard of death between HIV-positive and HIV-negative patients who had one of the defined classical histologic subtypes: nodular sclerosis, mixed cellularity, lymphocyte-rich, or lymphocyte-depleted Hodgkin lymphoma. However, mortality was significantly higher for HIV-positive patients with an undetermined histologic subtype. ![]()

cultured lymphocyte
Image courtesy of the CDC
Patients with HIV-associated Hodgkin lymphoma may not be getting potentially curative treatment, according to a study published in the journal AIDS.
The study showed that 16% of HIV-positive patients did not receive treatment for their lymphoma, compared to 9% of Hodgkin lymphoma patients who were HIV-negative.
“Hodgkin lymphoma is generally believed to be highly curable,” said study author Adam Olszewski, MD, of Brown University in Providence, Rhode Island.
“We have an expectation to cure over 90% of early stage patients and even 70% to 80% of quite advanced cases.”
It hasn’t been clear whether HIV-positive patients with Hodgkin lymphoma survive the cancer as well as people who are HIV-negative. While some small studies, particularly in Europe, have shown that HIV status makes no difference to survival, observations in the US population suggest that being HIV-positive makes survival less likely.
The new study, which is the largest of its kind to date, may reconcile that conflict. It suggests that, in the US, the reason people with HIV seem to fare worse with the cancer is because they are less likely to be treated for it.
The study included 2090 cases of HIV-associated Hodgkin lymphoma recorded in the National Cancer Data Base between 2004 and 2012, as well as 41,846 cases of Hodgkin lymphoma in patients who were HIV-negative.
The unadjusted 5-year overall survival was 66% for HIV-positive patients and 80% for the HIV-negative population.
Among the HIV-positive patients, 81% received chemotherapy (12% in combination with radiation), 13% received any radiation therapy, and 16% received no treatment for their lymphoma. The corresponding numbers for HIV-negative patients were 87%, 31%, and 9%, respectively (P<0.00001 for all comparisons).
The researchers assessed patient- and disease-related factors associated with the risk of not receiving chemotherapy in the HIV-positive population.
And they found the risk was significantly higher for patients who were older than 40, male, “nonwhite” (black, Hispanic, or Asian/”other”), did not have health insurance, lived in areas with the lowest median income, and had early stage Hodgkin lymphoma or an undetermined histology.
Dr Olszewski said the lack of treatment among HIV-positive patients could be due to a lingering assumption that they won’t tolerate the treatment well. Or some patients may be declining treatment, either for HIV (thereby making them seem more vulnerable) or for the lymphoma itself.
He noted, however, that lymphoma treatment can be effective for and tolerated by HIV-positive patients, especially when the lymphoma subtype is known.
Among the patients who received chemotherapy in this study, there was no significant difference in the hazard of death between HIV-positive and HIV-negative patients who had one of the defined classical histologic subtypes: nodular sclerosis, mixed cellularity, lymphocyte-rich, or lymphocyte-depleted Hodgkin lymphoma. However, mortality was significantly higher for HIV-positive patients with an undetermined histologic subtype. ![]()

cultured lymphocyte
Image courtesy of the CDC
Patients with HIV-associated Hodgkin lymphoma may not be getting potentially curative treatment, according to a study published in the journal AIDS.
The study showed that 16% of HIV-positive patients did not receive treatment for their lymphoma, compared to 9% of Hodgkin lymphoma patients who were HIV-negative.
“Hodgkin lymphoma is generally believed to be highly curable,” said study author Adam Olszewski, MD, of Brown University in Providence, Rhode Island.
“We have an expectation to cure over 90% of early stage patients and even 70% to 80% of quite advanced cases.”
It hasn’t been clear whether HIV-positive patients with Hodgkin lymphoma survive the cancer as well as people who are HIV-negative. While some small studies, particularly in Europe, have shown that HIV status makes no difference to survival, observations in the US population suggest that being HIV-positive makes survival less likely.
The new study, which is the largest of its kind to date, may reconcile that conflict. It suggests that, in the US, the reason people with HIV seem to fare worse with the cancer is because they are less likely to be treated for it.
The study included 2090 cases of HIV-associated Hodgkin lymphoma recorded in the National Cancer Data Base between 2004 and 2012, as well as 41,846 cases of Hodgkin lymphoma in patients who were HIV-negative.
The unadjusted 5-year overall survival was 66% for HIV-positive patients and 80% for the HIV-negative population.
Among the HIV-positive patients, 81% received chemotherapy (12% in combination with radiation), 13% received any radiation therapy, and 16% received no treatment for their lymphoma. The corresponding numbers for HIV-negative patients were 87%, 31%, and 9%, respectively (P<0.00001 for all comparisons).
The researchers assessed patient- and disease-related factors associated with the risk of not receiving chemotherapy in the HIV-positive population.
And they found the risk was significantly higher for patients who were older than 40, male, “nonwhite” (black, Hispanic, or Asian/”other”), did not have health insurance, lived in areas with the lowest median income, and had early stage Hodgkin lymphoma or an undetermined histology.
Dr Olszewski said the lack of treatment among HIV-positive patients could be due to a lingering assumption that they won’t tolerate the treatment well. Or some patients may be declining treatment, either for HIV (thereby making them seem more vulnerable) or for the lymphoma itself.
He noted, however, that lymphoma treatment can be effective for and tolerated by HIV-positive patients, especially when the lymphoma subtype is known.
Among the patients who received chemotherapy in this study, there was no significant difference in the hazard of death between HIV-positive and HIV-negative patients who had one of the defined classical histologic subtypes: nodular sclerosis, mixed cellularity, lymphocyte-rich, or lymphocyte-depleted Hodgkin lymphoma. However, mortality was significantly higher for HIV-positive patients with an undetermined histologic subtype. ![]()
Watchdog says trial was unethical
Photo courtesy of
The Medicines Company
The watchdog group Public Citizen is alleging that ethics violations were made during the CHAMPION PHOENIX trial, in which researchers compared cangrelor and clopidogrel as thromboprophylaxis for patients undergoing coronary stent procedures.
Public Citizen said the trial “needlessly threatened” the lives of subjects assigned to the control group, and trial participants may not have been informed about the potential risks of enrollment.
Public Citizen sent a letter to the Office of Research Oversight (ORO) at the US Department of Veterans Affairs (VA) asserting that the CHAMPION PHOENIX trial unnecessarily increased the risk of death, heart attack, and other adverse cardiac events for subjects placed in the control group.
The trial was conducted at 153 institutions around the world, including 3 VA facilities: the Dallas VA Medical Center, the Jesse Brown VA Medical Center in Chicago, and the VA Boston Healthcare System. The study involved more than 11,000 subjects, 84 of whom were patients at the 3 VA medical centers.
The primary goal of the trial was to determine whether cangrelor is more effective than clopidogrel at preventing death, heart attacks, and other serious cardiac complications in patients undergoing coronary artery stent procedures.
Public Citizen said the increased risk to subjects in the control group resulted from failure to ensure they were treated with clopidogrel prior to their coronary stent procedures.
An analysis by a senior medical reviewer at the US Food and Drug Administration revealed that failure to administer the necessary antiplatelet treatment occurred in 89% of subjects enrolled at the 3 VA facilities, compared with 30% of subjects enrolled at non-VA facilities.
“The seriously flawed trial protocol paved the way for inappropriate and shocking delays in antiplatelet therapy for subjects enrolled in the control group at all trial institutions,” said Michael Carome, MD, director of Public Citizen’s Health Research Group.
“Inexplicably, the rate of such delays was extraordinarily high at the VA trial sites, making participation in the trial even more hazardous for subjects randomized to the control group at those sites compared with other sites.”
Public Citizen’s complaint contends that the trial’s research protocol was unethical because it failed to require that control group subjects receive lifesaving antiplatelet medications as soon as possible prior to undergoing coronary artery stent procedures.
Prior research conducted by the same research team, expert clinical practice guidelines, and other data available to the researchers established that withholding clopidogrel until after the coronary stent procedures constitutes substandard care.
Indeed, one of the two lead researchers for the trial stated publicly before the trial’s initiation, “If you ask the experts, they will all tell you to give antiplatelet therapy upfront before the PCI [coronary stent] procedure.”
Public Citizen is calling on the ORO to investigate why the institutional review boards responsible for reviewing human research at the 3 VA medical facilities approved the trial given a design that was unethical and failed to minimize the risks to the control group subjects.
The complaint also urges the ORO to investigate whether proper consent of the subjects was obtained in light of the risks, as required by the VA’s human subjects protection rules.
Public Citizen said another issue that requires further inquiry—but falls outside the scope of the ORO’s jurisdiction—is whether inappropriate delays in antiplatelet therapy were more widespread at VA healthcare facilities for patients who did not participate in the trial.
In a separate letter, Public Citizen urged the VA’s Office of Inspector General to launch an investigation to find out. ![]()

Photo courtesy of
The Medicines Company
The watchdog group Public Citizen is alleging that ethics violations were made during the CHAMPION PHOENIX trial, in which researchers compared cangrelor and clopidogrel as thromboprophylaxis for patients undergoing coronary stent procedures.
Public Citizen said the trial “needlessly threatened” the lives of subjects assigned to the control group, and trial participants may not have been informed about the potential risks of enrollment.
Public Citizen sent a letter to the Office of Research Oversight (ORO) at the US Department of Veterans Affairs (VA) asserting that the CHAMPION PHOENIX trial unnecessarily increased the risk of death, heart attack, and other adverse cardiac events for subjects placed in the control group.
The trial was conducted at 153 institutions around the world, including 3 VA facilities: the Dallas VA Medical Center, the Jesse Brown VA Medical Center in Chicago, and the VA Boston Healthcare System. The study involved more than 11,000 subjects, 84 of whom were patients at the 3 VA medical centers.
The primary goal of the trial was to determine whether cangrelor is more effective than clopidogrel at preventing death, heart attacks, and other serious cardiac complications in patients undergoing coronary artery stent procedures.
Public Citizen said the increased risk to subjects in the control group resulted from failure to ensure they were treated with clopidogrel prior to their coronary stent procedures.
An analysis by a senior medical reviewer at the US Food and Drug Administration revealed that failure to administer the necessary antiplatelet treatment occurred in 89% of subjects enrolled at the 3 VA facilities, compared with 30% of subjects enrolled at non-VA facilities.
“The seriously flawed trial protocol paved the way for inappropriate and shocking delays in antiplatelet therapy for subjects enrolled in the control group at all trial institutions,” said Michael Carome, MD, director of Public Citizen’s Health Research Group.
“Inexplicably, the rate of such delays was extraordinarily high at the VA trial sites, making participation in the trial even more hazardous for subjects randomized to the control group at those sites compared with other sites.”
Public Citizen’s complaint contends that the trial’s research protocol was unethical because it failed to require that control group subjects receive lifesaving antiplatelet medications as soon as possible prior to undergoing coronary artery stent procedures.
Prior research conducted by the same research team, expert clinical practice guidelines, and other data available to the researchers established that withholding clopidogrel until after the coronary stent procedures constitutes substandard care.
Indeed, one of the two lead researchers for the trial stated publicly before the trial’s initiation, “If you ask the experts, they will all tell you to give antiplatelet therapy upfront before the PCI [coronary stent] procedure.”
Public Citizen is calling on the ORO to investigate why the institutional review boards responsible for reviewing human research at the 3 VA medical facilities approved the trial given a design that was unethical and failed to minimize the risks to the control group subjects.
The complaint also urges the ORO to investigate whether proper consent of the subjects was obtained in light of the risks, as required by the VA’s human subjects protection rules.
Public Citizen said another issue that requires further inquiry—but falls outside the scope of the ORO’s jurisdiction—is whether inappropriate delays in antiplatelet therapy were more widespread at VA healthcare facilities for patients who did not participate in the trial.
In a separate letter, Public Citizen urged the VA’s Office of Inspector General to launch an investigation to find out. ![]()

Photo courtesy of
The Medicines Company
The watchdog group Public Citizen is alleging that ethics violations were made during the CHAMPION PHOENIX trial, in which researchers compared cangrelor and clopidogrel as thromboprophylaxis for patients undergoing coronary stent procedures.
Public Citizen said the trial “needlessly threatened” the lives of subjects assigned to the control group, and trial participants may not have been informed about the potential risks of enrollment.
Public Citizen sent a letter to the Office of Research Oversight (ORO) at the US Department of Veterans Affairs (VA) asserting that the CHAMPION PHOENIX trial unnecessarily increased the risk of death, heart attack, and other adverse cardiac events for subjects placed in the control group.
The trial was conducted at 153 institutions around the world, including 3 VA facilities: the Dallas VA Medical Center, the Jesse Brown VA Medical Center in Chicago, and the VA Boston Healthcare System. The study involved more than 11,000 subjects, 84 of whom were patients at the 3 VA medical centers.
The primary goal of the trial was to determine whether cangrelor is more effective than clopidogrel at preventing death, heart attacks, and other serious cardiac complications in patients undergoing coronary artery stent procedures.
Public Citizen said the increased risk to subjects in the control group resulted from failure to ensure they were treated with clopidogrel prior to their coronary stent procedures.
An analysis by a senior medical reviewer at the US Food and Drug Administration revealed that failure to administer the necessary antiplatelet treatment occurred in 89% of subjects enrolled at the 3 VA facilities, compared with 30% of subjects enrolled at non-VA facilities.
“The seriously flawed trial protocol paved the way for inappropriate and shocking delays in antiplatelet therapy for subjects enrolled in the control group at all trial institutions,” said Michael Carome, MD, director of Public Citizen’s Health Research Group.
“Inexplicably, the rate of such delays was extraordinarily high at the VA trial sites, making participation in the trial even more hazardous for subjects randomized to the control group at those sites compared with other sites.”
Public Citizen’s complaint contends that the trial’s research protocol was unethical because it failed to require that control group subjects receive lifesaving antiplatelet medications as soon as possible prior to undergoing coronary artery stent procedures.
Prior research conducted by the same research team, expert clinical practice guidelines, and other data available to the researchers established that withholding clopidogrel until after the coronary stent procedures constitutes substandard care.
Indeed, one of the two lead researchers for the trial stated publicly before the trial’s initiation, “If you ask the experts, they will all tell you to give antiplatelet therapy upfront before the PCI [coronary stent] procedure.”
Public Citizen is calling on the ORO to investigate why the institutional review boards responsible for reviewing human research at the 3 VA medical facilities approved the trial given a design that was unethical and failed to minimize the risks to the control group subjects.
The complaint also urges the ORO to investigate whether proper consent of the subjects was obtained in light of the risks, as required by the VA’s human subjects protection rules.
Public Citizen said another issue that requires further inquiry—but falls outside the scope of the ORO’s jurisdiction—is whether inappropriate delays in antiplatelet therapy were more widespread at VA healthcare facilities for patients who did not participate in the trial.
In a separate letter, Public Citizen urged the VA’s Office of Inspector General to launch an investigation to find out. ![]()
Concomitant Sensitization to Inhaled Budesonide and Oral Nystatin Presenting as Allergic Contact Stomatitis and Systemic Allergic Contact Dermatitis
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.
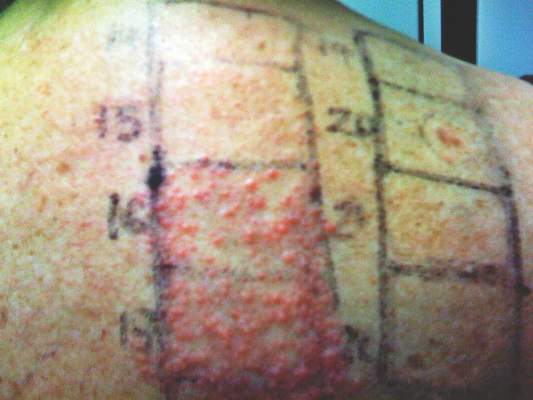
Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
Practice Points
- When lesions develop in the oral cavity during treatment with inhaled corticosteroids, delayed contact allergy should be considered in the differential diagnosis along with fungal infection.
- Although it generally is not considered to be allergenic due to its poor intestinal absorption, oral nystatin may induce systemic allergic disorders.
- All drugs involved in a presumed allergic reaction must be evaluated since concomitant sensitization to multiple drugs could be present. Patch and challenge testing should be conducted to diagnose allergic contact dermatitis and assess drug cross-reactivity.
Recalcitrant Hailey-Hailey Disease Responds to Oral Tacrolimus and Botulinum Toxin Type A
To the Editor:
Hailey-Hailey disease, also known as familial benign pemphigus, is a chronic blistering skin disorder that typically presents as a recurrent vesicular or bullous dermatitis found predominantly in the intertriginous regions of the body. Because current treatment regimens for Hailey-Hailey disease are fairly limited, novel treatments may be explored in intractable cases.
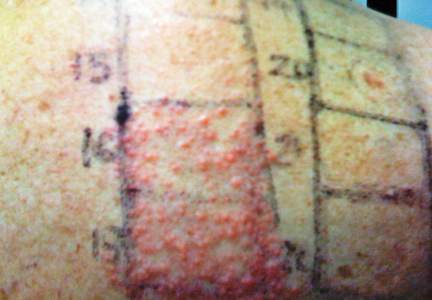
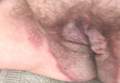
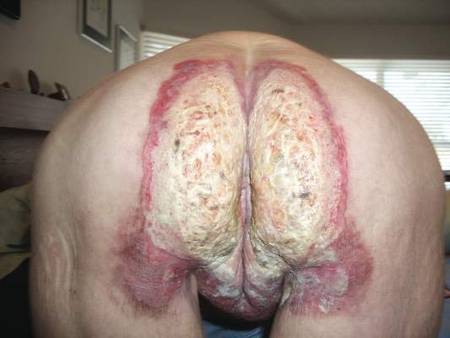
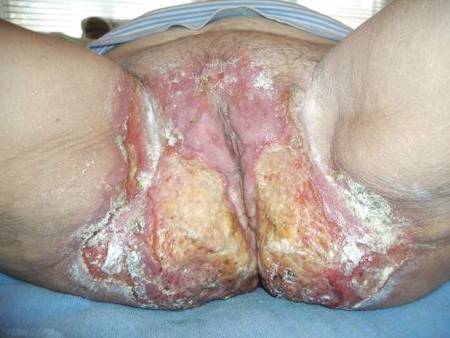
A 71-year-old woman presented with well-demarcated erythematous plaques with erosions and white verrucous regions in the perivulvar, vaginal, and perianal areas of 1 month’s duration (Figure 1). The lesions were excruciatingly pruritic and excoriated. The patient reported no personal or family history of similar lesions.

Histopathologic examination of multiple biopsies from the periphery of the plaques showed acantholysis of the epidermis and surface necrosis with negative direct immunofluorescence. A diagnosis of Hailey-Hailey disease was made. Over several months following the initial presentation, the patient was treated with regimens of corticosteroids, antibiotics, antifungals, acitretin, and topical tacrolimus (which showed minimal response), but the condition continued to progress and thus warranted a more aggressive approach. After a 4-week course of oral cyclosporine 1.25 mg/kg twice daily, some healthy granulation tissue had formed, but new erosions continued to develop on the vulva, labia, and intergluteal cleft (Figure 2). Subsequently, a 2-month course of methotrexate yielded similar results with minimal healing and new necrotic areas (Figure 3).
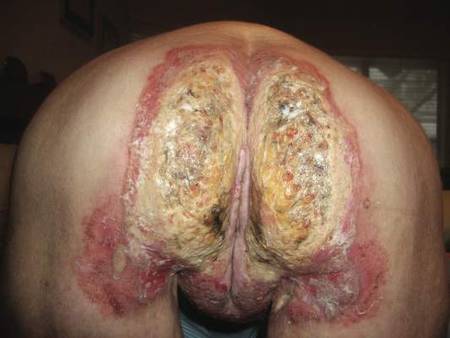
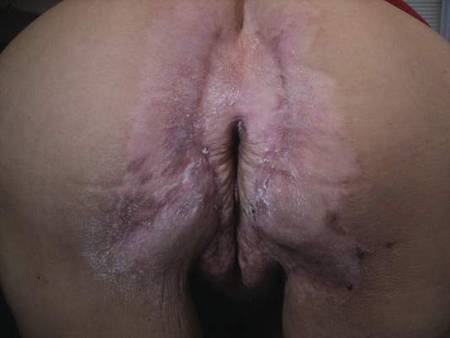
Following methotrexate therapy, treatment with several other oral agents was considered such as azathioprine, mycophenolate mofetil, and oral tacrolimus. Since the patient had previously shown minimal response to topical tacrolimus, a course of oral treatment (0.05 mg/kg twice daily) was initiated. At 4 weeks’ follow-up, extensive healing of the lesions was noted (Figure 4) and the patient reported that the painful pruritus had improved.
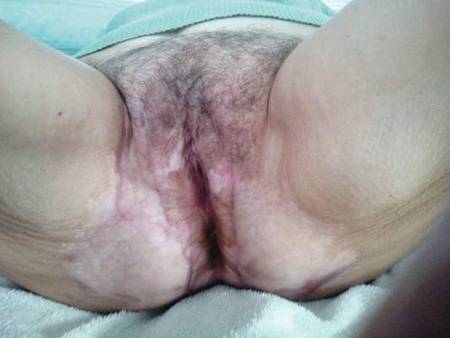
After 6 weeks of treatment, there still were a few small lesions in the intergluteal cleft, which were treated with botulinum toxin type A (100 U diluted with 5 cc of bacteriostatic saline per cm2). After 3 separate injections with botulinum toxin type A and oral tacrolimus for 9 months, complete resolution of the lesions was obtained (Figure 5). After more than 6 months of remission, oral tacrolimus slowly was decreased by 1 mg every 6 weeks until treatment was stopped completely. She has remained in remission without oral treatment. After tapering the tacrolimus, botulinum toxin A injections were continued every 6 months for maintenance with the use of topical tacrolimus 3 to 4 times weekly (Figure 6).
The inheritance of Hailey-Hailey disease is autosomal dominant with incomplete penetrance, although spontaneous mutations are implicated in up to 30% of patients.1,2 The pathogenesis of Hailey-Hailey disease involves a mutation in the ATPase, Ca++ transporting, type 2C, member 1 gene (ATP2C1), which encodes for the hSPCA1 protein.1-2 The malfunction of this ATPase leads to inadequacy of the keratinocyte adhesive barrier resulting in acantholysis and intraepidermal vesicle formation. Macerated plaques also may be found instead of intact vesicles.3
Initial presentation of lesions in Hailey-Hailey disease typically occurs in the third or fourth decades of life but may present at any age.4 Areas exposed to increased amounts of friction (eg, axillae, groin, neck, perineum) commonly are involved.5 Lesions may occur in a relapsing and remitting course and usually are more prominent in summer months because they are exacerbated by sunburn and frictional trauma. Secondary bacterial or fungal infections are common and antibiotics and antifungals often are necessary to prevent progression of the lesions.5,6
Various treatment regimens for Hailey-Hailey disease include topical and oral corticosteroids and antibiotics.5 Topical and oral retinoids, calcitriol, topical tacrolimus, cyclosporin, methotrexate, and even botulinum toxin A have been reported to be effective for refractory cases.7-12 Our case describes a novel regimen of oral tacrolimus in conjunction with botulinum toxin A used in the successful treatment of recalcitrant Hailey-Hailey disease.
Tacrolimus binds to the immunophilin FK506 binding protein, which inhibits calcineurin. Calcineurin, a protein phosphatase, is necessary for T-cell activation through the nuclear factor of activated T cells.This inhibition of calcineurin blocks the expression of several cytokines.13 The efficacy of oral tacrolimus demonstrates that cellular immunity could play a role in the pathogenic mechanism of Hailey-Hailey disease.
Contraindications to oral tacrolimus therapy include renal or hepatic impairment, breast-feeding, pregnancy, and certain neoplastic diseases. There also is an increased risk of patients developing malignancies such as lymphoma or skin cancer due to immunosuppression. Use of oral tacrolimus also requires routine laboratory monitoring of renal and hepatic function, potassium, and blood glucose levels.13
Botulinum toxin A injections augmented the therapeutic approach in our patient possibly by controlling secretions of sweat and mucous, which may cause maceration and lead to exacerbation of Hailey-Hailey disease. Control of secretions may help in creating an environment that is less prone to exacerbation of lesions and secondary infection.14 The combination of oral tacrolimus and botulinum toxin A injections provided a safe therapeutic option for recalcitrant Hailey-Hailey disease in our patient.
- Fairclough RJ, Dode L, Vanoevelen J, et al. Effect of Hailey-Hailey Disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J Biol Chem. 2003; 278:24721-24730.
- Dobson-Stone C, Fairclough R, Dunne E, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338-343.
- Warycha M, Patel R, Meehan S, et al. Familial benign chronic pemphigus (Hailey-Hailey disease). Dermatol Online J. 2009;15:15.
- Tchernev GJ, Cardosa C. Familial benign chronic pemphigus (Hailey-Hailey Disease): use of topical immunomodulators as a modern treatment option. Rev Med Chil. 2011;139:633-637.
- Hunt R, O’Reilly K, Ralston J, et al. Familial benign chronic pemphigus (Hailey-Hailey disease). Dermatol Online J. 2010;16:14.
- Berger EM, Galadari HI, Gottlieb AB. Successful treatment of Hailey-Hailey with acitretin. J Drugs Dermatol. 2007;6:734-736.
- Rabeni EJ, Cunningham NM. Effective treatment of Hailey-Hailey disease with topical tacrolimus. J Am Acad Dermatol. 2002;47:797-798.
- Sand C, Thomsen HK. Topical tacrolimus ointment is an effective therapy for Hailey-Hailey disease. Arch Dermatol. 2003;139:1401-1402.
- Bianchi L, Chimenti MS, Giunta A. Treatment of Hailey-Hailey with topical calcitriol. J Am Acad of Dermatol. 2004;51:475-476.
- Berth-Jones J, Smith SG, Graham-Brown RA. Benign familial chronic pemphigus (Hailey-Hailey disease) responds to cyclosporin. Clin Exp Dermatol. 1995;20:70-72.
- Vilarinho CF, Ventura F, Brito C. Methotrexate for refractory Hailey-Hailey disease. J Eur Acad Dermatol Venereol. 2010;24:106.
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treatment. 2008;19:251-254.
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill; 2009.14. Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374.
To the Editor:
Hailey-Hailey disease, also known as familial benign pemphigus, is a chronic blistering skin disorder that typically presents as a recurrent vesicular or bullous dermatitis found predominantly in the intertriginous regions of the body. Because current treatment regimens for Hailey-Hailey disease are fairly limited, novel treatments may be explored in intractable cases.
A 71-year-old woman presented with well-demarcated erythematous plaques with erosions and white verrucous regions in the perivulvar, vaginal, and perianal areas of 1 month’s duration (Figure 1). The lesions were excruciatingly pruritic and excoriated. The patient reported no personal or family history of similar lesions.

Histopathologic examination of multiple biopsies from the periphery of the plaques showed acantholysis of the epidermis and surface necrosis with negative direct immunofluorescence. A diagnosis of Hailey-Hailey disease was made. Over several months following the initial presentation, the patient was treated with regimens of corticosteroids, antibiotics, antifungals, acitretin, and topical tacrolimus (which showed minimal response), but the condition continued to progress and thus warranted a more aggressive approach. After a 4-week course of oral cyclosporine 1.25 mg/kg twice daily, some healthy granulation tissue had formed, but new erosions continued to develop on the vulva, labia, and intergluteal cleft (Figure 2). Subsequently, a 2-month course of methotrexate yielded similar results with minimal healing and new necrotic areas (Figure 3).


Following methotrexate therapy, treatment with several other oral agents was considered such as azathioprine, mycophenolate mofetil, and oral tacrolimus. Since the patient had previously shown minimal response to topical tacrolimus, a course of oral treatment (0.05 mg/kg twice daily) was initiated. At 4 weeks’ follow-up, extensive healing of the lesions was noted (Figure 4) and the patient reported that the painful pruritus had improved.

After 6 weeks of treatment, there still were a few small lesions in the intergluteal cleft, which were treated with botulinum toxin type A (100 U diluted with 5 cc of bacteriostatic saline per cm2). After 3 separate injections with botulinum toxin type A and oral tacrolimus for 9 months, complete resolution of the lesions was obtained (Figure 5). After more than 6 months of remission, oral tacrolimus slowly was decreased by 1 mg every 6 weeks until treatment was stopped completely. She has remained in remission without oral treatment. After tapering the tacrolimus, botulinum toxin A injections were continued every 6 months for maintenance with the use of topical tacrolimus 3 to 4 times weekly (Figure 6).


The inheritance of Hailey-Hailey disease is autosomal dominant with incomplete penetrance, although spontaneous mutations are implicated in up to 30% of patients.1,2 The pathogenesis of Hailey-Hailey disease involves a mutation in the ATPase, Ca++ transporting, type 2C, member 1 gene (ATP2C1), which encodes for the hSPCA1 protein.1-2 The malfunction of this ATPase leads to inadequacy of the keratinocyte adhesive barrier resulting in acantholysis and intraepidermal vesicle formation. Macerated plaques also may be found instead of intact vesicles.3
Initial presentation of lesions in Hailey-Hailey disease typically occurs in the third or fourth decades of life but may present at any age.4 Areas exposed to increased amounts of friction (eg, axillae, groin, neck, perineum) commonly are involved.5 Lesions may occur in a relapsing and remitting course and usually are more prominent in summer months because they are exacerbated by sunburn and frictional trauma. Secondary bacterial or fungal infections are common and antibiotics and antifungals often are necessary to prevent progression of the lesions.5,6
Various treatment regimens for Hailey-Hailey disease include topical and oral corticosteroids and antibiotics.5 Topical and oral retinoids, calcitriol, topical tacrolimus, cyclosporin, methotrexate, and even botulinum toxin A have been reported to be effective for refractory cases.7-12 Our case describes a novel regimen of oral tacrolimus in conjunction with botulinum toxin A used in the successful treatment of recalcitrant Hailey-Hailey disease.
Tacrolimus binds to the immunophilin FK506 binding protein, which inhibits calcineurin. Calcineurin, a protein phosphatase, is necessary for T-cell activation through the nuclear factor of activated T cells.This inhibition of calcineurin blocks the expression of several cytokines.13 The efficacy of oral tacrolimus demonstrates that cellular immunity could play a role in the pathogenic mechanism of Hailey-Hailey disease.
Contraindications to oral tacrolimus therapy include renal or hepatic impairment, breast-feeding, pregnancy, and certain neoplastic diseases. There also is an increased risk of patients developing malignancies such as lymphoma or skin cancer due to immunosuppression. Use of oral tacrolimus also requires routine laboratory monitoring of renal and hepatic function, potassium, and blood glucose levels.13
Botulinum toxin A injections augmented the therapeutic approach in our patient possibly by controlling secretions of sweat and mucous, which may cause maceration and lead to exacerbation of Hailey-Hailey disease. Control of secretions may help in creating an environment that is less prone to exacerbation of lesions and secondary infection.14 The combination of oral tacrolimus and botulinum toxin A injections provided a safe therapeutic option for recalcitrant Hailey-Hailey disease in our patient.
To the Editor:
Hailey-Hailey disease, also known as familial benign pemphigus, is a chronic blistering skin disorder that typically presents as a recurrent vesicular or bullous dermatitis found predominantly in the intertriginous regions of the body. Because current treatment regimens for Hailey-Hailey disease are fairly limited, novel treatments may be explored in intractable cases.
A 71-year-old woman presented with well-demarcated erythematous plaques with erosions and white verrucous regions in the perivulvar, vaginal, and perianal areas of 1 month’s duration (Figure 1). The lesions were excruciatingly pruritic and excoriated. The patient reported no personal or family history of similar lesions.

Histopathologic examination of multiple biopsies from the periphery of the plaques showed acantholysis of the epidermis and surface necrosis with negative direct immunofluorescence. A diagnosis of Hailey-Hailey disease was made. Over several months following the initial presentation, the patient was treated with regimens of corticosteroids, antibiotics, antifungals, acitretin, and topical tacrolimus (which showed minimal response), but the condition continued to progress and thus warranted a more aggressive approach. After a 4-week course of oral cyclosporine 1.25 mg/kg twice daily, some healthy granulation tissue had formed, but new erosions continued to develop on the vulva, labia, and intergluteal cleft (Figure 2). Subsequently, a 2-month course of methotrexate yielded similar results with minimal healing and new necrotic areas (Figure 3).


Following methotrexate therapy, treatment with several other oral agents was considered such as azathioprine, mycophenolate mofetil, and oral tacrolimus. Since the patient had previously shown minimal response to topical tacrolimus, a course of oral treatment (0.05 mg/kg twice daily) was initiated. At 4 weeks’ follow-up, extensive healing of the lesions was noted (Figure 4) and the patient reported that the painful pruritus had improved.

After 6 weeks of treatment, there still were a few small lesions in the intergluteal cleft, which were treated with botulinum toxin type A (100 U diluted with 5 cc of bacteriostatic saline per cm2). After 3 separate injections with botulinum toxin type A and oral tacrolimus for 9 months, complete resolution of the lesions was obtained (Figure 5). After more than 6 months of remission, oral tacrolimus slowly was decreased by 1 mg every 6 weeks until treatment was stopped completely. She has remained in remission without oral treatment. After tapering the tacrolimus, botulinum toxin A injections were continued every 6 months for maintenance with the use of topical tacrolimus 3 to 4 times weekly (Figure 6).


The inheritance of Hailey-Hailey disease is autosomal dominant with incomplete penetrance, although spontaneous mutations are implicated in up to 30% of patients.1,2 The pathogenesis of Hailey-Hailey disease involves a mutation in the ATPase, Ca++ transporting, type 2C, member 1 gene (ATP2C1), which encodes for the hSPCA1 protein.1-2 The malfunction of this ATPase leads to inadequacy of the keratinocyte adhesive barrier resulting in acantholysis and intraepidermal vesicle formation. Macerated plaques also may be found instead of intact vesicles.3
Initial presentation of lesions in Hailey-Hailey disease typically occurs in the third or fourth decades of life but may present at any age.4 Areas exposed to increased amounts of friction (eg, axillae, groin, neck, perineum) commonly are involved.5 Lesions may occur in a relapsing and remitting course and usually are more prominent in summer months because they are exacerbated by sunburn and frictional trauma. Secondary bacterial or fungal infections are common and antibiotics and antifungals often are necessary to prevent progression of the lesions.5,6
Various treatment regimens for Hailey-Hailey disease include topical and oral corticosteroids and antibiotics.5 Topical and oral retinoids, calcitriol, topical tacrolimus, cyclosporin, methotrexate, and even botulinum toxin A have been reported to be effective for refractory cases.7-12 Our case describes a novel regimen of oral tacrolimus in conjunction with botulinum toxin A used in the successful treatment of recalcitrant Hailey-Hailey disease.
Tacrolimus binds to the immunophilin FK506 binding protein, which inhibits calcineurin. Calcineurin, a protein phosphatase, is necessary for T-cell activation through the nuclear factor of activated T cells.This inhibition of calcineurin blocks the expression of several cytokines.13 The efficacy of oral tacrolimus demonstrates that cellular immunity could play a role in the pathogenic mechanism of Hailey-Hailey disease.
Contraindications to oral tacrolimus therapy include renal or hepatic impairment, breast-feeding, pregnancy, and certain neoplastic diseases. There also is an increased risk of patients developing malignancies such as lymphoma or skin cancer due to immunosuppression. Use of oral tacrolimus also requires routine laboratory monitoring of renal and hepatic function, potassium, and blood glucose levels.13
Botulinum toxin A injections augmented the therapeutic approach in our patient possibly by controlling secretions of sweat and mucous, which may cause maceration and lead to exacerbation of Hailey-Hailey disease. Control of secretions may help in creating an environment that is less prone to exacerbation of lesions and secondary infection.14 The combination of oral tacrolimus and botulinum toxin A injections provided a safe therapeutic option for recalcitrant Hailey-Hailey disease in our patient.
- Fairclough RJ, Dode L, Vanoevelen J, et al. Effect of Hailey-Hailey Disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J Biol Chem. 2003; 278:24721-24730.
- Dobson-Stone C, Fairclough R, Dunne E, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338-343.
- Warycha M, Patel R, Meehan S, et al. Familial benign chronic pemphigus (Hailey-Hailey disease). Dermatol Online J. 2009;15:15.
- Tchernev GJ, Cardosa C. Familial benign chronic pemphigus (Hailey-Hailey Disease): use of topical immunomodulators as a modern treatment option. Rev Med Chil. 2011;139:633-637.
- Hunt R, O’Reilly K, Ralston J, et al. Familial benign chronic pemphigus (Hailey-Hailey disease). Dermatol Online J. 2010;16:14.
- Berger EM, Galadari HI, Gottlieb AB. Successful treatment of Hailey-Hailey with acitretin. J Drugs Dermatol. 2007;6:734-736.
- Rabeni EJ, Cunningham NM. Effective treatment of Hailey-Hailey disease with topical tacrolimus. J Am Acad Dermatol. 2002;47:797-798.
- Sand C, Thomsen HK. Topical tacrolimus ointment is an effective therapy for Hailey-Hailey disease. Arch Dermatol. 2003;139:1401-1402.
- Bianchi L, Chimenti MS, Giunta A. Treatment of Hailey-Hailey with topical calcitriol. J Am Acad of Dermatol. 2004;51:475-476.
- Berth-Jones J, Smith SG, Graham-Brown RA. Benign familial chronic pemphigus (Hailey-Hailey disease) responds to cyclosporin. Clin Exp Dermatol. 1995;20:70-72.
- Vilarinho CF, Ventura F, Brito C. Methotrexate for refractory Hailey-Hailey disease. J Eur Acad Dermatol Venereol. 2010;24:106.
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treatment. 2008;19:251-254.
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill; 2009.14. Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374.
- Fairclough RJ, Dode L, Vanoevelen J, et al. Effect of Hailey-Hailey Disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J Biol Chem. 2003; 278:24721-24730.
- Dobson-Stone C, Fairclough R, Dunne E, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338-343.
- Warycha M, Patel R, Meehan S, et al. Familial benign chronic pemphigus (Hailey-Hailey disease). Dermatol Online J. 2009;15:15.
- Tchernev GJ, Cardosa C. Familial benign chronic pemphigus (Hailey-Hailey Disease): use of topical immunomodulators as a modern treatment option. Rev Med Chil. 2011;139:633-637.
- Hunt R, O’Reilly K, Ralston J, et al. Familial benign chronic pemphigus (Hailey-Hailey disease). Dermatol Online J. 2010;16:14.
- Berger EM, Galadari HI, Gottlieb AB. Successful treatment of Hailey-Hailey with acitretin. J Drugs Dermatol. 2007;6:734-736.
- Rabeni EJ, Cunningham NM. Effective treatment of Hailey-Hailey disease with topical tacrolimus. J Am Acad Dermatol. 2002;47:797-798.
- Sand C, Thomsen HK. Topical tacrolimus ointment is an effective therapy for Hailey-Hailey disease. Arch Dermatol. 2003;139:1401-1402.
- Bianchi L, Chimenti MS, Giunta A. Treatment of Hailey-Hailey with topical calcitriol. J Am Acad of Dermatol. 2004;51:475-476.
- Berth-Jones J, Smith SG, Graham-Brown RA. Benign familial chronic pemphigus (Hailey-Hailey disease) responds to cyclosporin. Clin Exp Dermatol. 1995;20:70-72.
- Vilarinho CF, Ventura F, Brito C. Methotrexate for refractory Hailey-Hailey disease. J Eur Acad Dermatol Venereol. 2010;24:106.
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treatment. 2008;19:251-254.
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill; 2009.14. Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374.
Hong Kong zygomycosis deaths pinned to dirty hospital laundry
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Clinicians need to maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,
Major finding: Of 195 environmental samples at the contaminated laundry, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples.
Data source: Epidemiological study in Hong Kong.
Disclosures: Hong Kong government services helped support the work. The authors do not have any financial conflicts of interest.
Study confirms resistant malaria in Cambodia

Image by Ute Frevert
and Margaret Shear
Results of a new study confirm that dihydroartemisinin-piperaquine, the first-line treatment for Plasmodium falciparum malaria infection in Cambodia, has failed in certain provinces due to parasite resistance to both artemisinin and piperaquine.
Dihydroartemisinin-piperaquine is an artemisinin combination therapy (ACT) that combines fast-acting artemisinin with a long-acting partner drug, piperaquine.
Resistance to artemisinin in parts of Southeast Asia is well-documented, but, until now, only a few studies have presented clear evidence of piperaquine resistance.
Additional results from this study suggest that artesunate, a form of artemisinin, plus mefloquine, a different long-acting partner drug, should be the first-line ACT in areas where dihydroartemisinin-piperaquine treatment has failed.
Chanaki Amaratunga, PhD, of the National Institute of Allergy and Infectious Diseases in Rockville, Maryland, and colleagues reported these results in The Lancet Infectious Diseases.
The researchers assessed the efficacy of dihydroartemisinin-piperaquine treatment in 241 malaria-afflicted participants ages 2 to 65. The subjects lived in 3 different Cambodian provinces with varying levels of artemisinin resistance.
After monitoring parasite levels in the blood for 63 days, the researchers found that parasites had reemerged despite initial clearance in 45.7% of participants in Pursat, 15.9% of participants in Preah Vihear, and 1.67% of participants in Ratanakiri.
The results indicate the ACT is failing in Pursat and Preah Vihear, where artemisinin resistance is common, but remains highly efficacious in Ratanakiri, where resistance is uncommon.
Laboratory tests showed the parasites from dihydroartemisinin-piperaquine failures contained a genetic marker of artemisinin resistance and had a decreased susceptibility to piperaquine, demonstrating that both artemisinin and piperaquine resistance contributed to treatment failures.
However, the parasites also showed an increased susceptibility to mefloquine and completely lacked the molecular marker for mefloquine resistance.
These findings informed new World Health Organization guidelines reinstating artesunate plus mefloquine as the first-line ACT in Cambodia where dihydroartemisinin-piperaquine treatment has failed.
The researchers said the findings also provide evidence to initiate surveillance programs to track the spread of piperaquine resistance and clinical trials to test alternative combination therapies. ![]()

Image by Ute Frevert
and Margaret Shear
Results of a new study confirm that dihydroartemisinin-piperaquine, the first-line treatment for Plasmodium falciparum malaria infection in Cambodia, has failed in certain provinces due to parasite resistance to both artemisinin and piperaquine.
Dihydroartemisinin-piperaquine is an artemisinin combination therapy (ACT) that combines fast-acting artemisinin with a long-acting partner drug, piperaquine.
Resistance to artemisinin in parts of Southeast Asia is well-documented, but, until now, only a few studies have presented clear evidence of piperaquine resistance.
Additional results from this study suggest that artesunate, a form of artemisinin, plus mefloquine, a different long-acting partner drug, should be the first-line ACT in areas where dihydroartemisinin-piperaquine treatment has failed.
Chanaki Amaratunga, PhD, of the National Institute of Allergy and Infectious Diseases in Rockville, Maryland, and colleagues reported these results in The Lancet Infectious Diseases.
The researchers assessed the efficacy of dihydroartemisinin-piperaquine treatment in 241 malaria-afflicted participants ages 2 to 65. The subjects lived in 3 different Cambodian provinces with varying levels of artemisinin resistance.
After monitoring parasite levels in the blood for 63 days, the researchers found that parasites had reemerged despite initial clearance in 45.7% of participants in Pursat, 15.9% of participants in Preah Vihear, and 1.67% of participants in Ratanakiri.
The results indicate the ACT is failing in Pursat and Preah Vihear, where artemisinin resistance is common, but remains highly efficacious in Ratanakiri, where resistance is uncommon.
Laboratory tests showed the parasites from dihydroartemisinin-piperaquine failures contained a genetic marker of artemisinin resistance and had a decreased susceptibility to piperaquine, demonstrating that both artemisinin and piperaquine resistance contributed to treatment failures.
However, the parasites also showed an increased susceptibility to mefloquine and completely lacked the molecular marker for mefloquine resistance.
These findings informed new World Health Organization guidelines reinstating artesunate plus mefloquine as the first-line ACT in Cambodia where dihydroartemisinin-piperaquine treatment has failed.
The researchers said the findings also provide evidence to initiate surveillance programs to track the spread of piperaquine resistance and clinical trials to test alternative combination therapies. ![]()

Image by Ute Frevert
and Margaret Shear
Results of a new study confirm that dihydroartemisinin-piperaquine, the first-line treatment for Plasmodium falciparum malaria infection in Cambodia, has failed in certain provinces due to parasite resistance to both artemisinin and piperaquine.
Dihydroartemisinin-piperaquine is an artemisinin combination therapy (ACT) that combines fast-acting artemisinin with a long-acting partner drug, piperaquine.
Resistance to artemisinin in parts of Southeast Asia is well-documented, but, until now, only a few studies have presented clear evidence of piperaquine resistance.
Additional results from this study suggest that artesunate, a form of artemisinin, plus mefloquine, a different long-acting partner drug, should be the first-line ACT in areas where dihydroartemisinin-piperaquine treatment has failed.
Chanaki Amaratunga, PhD, of the National Institute of Allergy and Infectious Diseases in Rockville, Maryland, and colleagues reported these results in The Lancet Infectious Diseases.
The researchers assessed the efficacy of dihydroartemisinin-piperaquine treatment in 241 malaria-afflicted participants ages 2 to 65. The subjects lived in 3 different Cambodian provinces with varying levels of artemisinin resistance.
After monitoring parasite levels in the blood for 63 days, the researchers found that parasites had reemerged despite initial clearance in 45.7% of participants in Pursat, 15.9% of participants in Preah Vihear, and 1.67% of participants in Ratanakiri.
The results indicate the ACT is failing in Pursat and Preah Vihear, where artemisinin resistance is common, but remains highly efficacious in Ratanakiri, where resistance is uncommon.
Laboratory tests showed the parasites from dihydroartemisinin-piperaquine failures contained a genetic marker of artemisinin resistance and had a decreased susceptibility to piperaquine, demonstrating that both artemisinin and piperaquine resistance contributed to treatment failures.
However, the parasites also showed an increased susceptibility to mefloquine and completely lacked the molecular marker for mefloquine resistance.
These findings informed new World Health Organization guidelines reinstating artesunate plus mefloquine as the first-line ACT in Cambodia where dihydroartemisinin-piperaquine treatment has failed.
The researchers said the findings also provide evidence to initiate surveillance programs to track the spread of piperaquine resistance and clinical trials to test alternative combination therapies. ![]()
Antilymphocyte globulin curbs chronic graft-versus-host disease
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
FROM NEJM
Key clinical point: Chronic graft-versus-host disease (GVHD) after allogeneic stem cell transplantation with peripheral blood stem cells was less than half as frequent with antilymphocyte globulin added to the conditioning regimen.
Major finding: Cumulative incidence of chronic GVHD at 2 years was 32.2% with antilymphocyte globulin vs 68.7% without it.
Data source: The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012.
Disclosures: Research was supported in part by Neovii Biotech. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Noncompete clauses: Be wary, negotiate early
Noncompete clauses can severely limit a doctor’s business options and create serious financial challenges, so negotiate with employers early and watch out for tricky contract terms that could stifle future opportunities.
That is the advice from health law experts around the country. They point out that when it comes to noncompete clauses – employment contract language that limits where physicians can practice after employment ends or is terminated – doctors should pay close attention, especially to the following:
Geographical limitations
Distance requirements within noncompete provisions are a top issue that can trip up doctors, Bloomfield Hills, Mich., health law attorney Mark S. Kopson said. The clause typically specifies that a physician cannot practice within a certain radius of the former employer. However, if an employer has three offices for instance, that 10-mile radius can quickly become a 30-mile radius or more depending how the provision is worded. Mr. Kopson recalled a recent client who practiced for 5 years in one office and was transferred to an office in another town for 30 days. He was then terminated, and his employer attempted to enforce contract terms that would prevent him from practicing within a 10-mile radius of both offices. A court determined that the employer was acting in bad faith and sought an unfair competitive advantage.
“But No. 1, you don’t want to have to go to court,” Mr. Kopson said. “And No. 2, you can have the best lawyer, but once it’s in the hands of the judge or the jury, anything can happen. That’s why you really want to do the work on this up front.”
When negotiating noncompete clauses, be cognizant of where distances are being measured from and around, the legal experts stress. Also, be clear with employers about what defines a reasonable distance, based on the geographic spread of their patient base.
“What’s reasonable for a family practice physician is probably not going to be reasonable for a pediatric neurosurgeon, as they draw their patients from varying distances,” he said. “Also, negotiate to ensure that the length of time of the restriction is reasonable. Taking into account, both the distance and time period, the physician must still be able to earn a living.”
Time frame restrictions
Negotiate the shortest duration that you can, advises Greenbelt, Md., labor and employment attorney Jay P. Holland. Noncompete provisions typically limit a doctor from practicing around a certain radius for 1-5 years, but some employers may try to enforce longer time periods.
“Consider your career and lifestyle goals carefully prior to entering into a noncompete,” Mr. Holland said. “The first approach should always be an attempt to exclude the noncompete from your prospective agreement if you are joining a practice. If a noncompete is unavoidable, then strive to make it the least onerous possible. Ask yourself prior to signing an agreement, ‘If I were to leave this practice, what are the restrictions I could live with? Are the restrictions reasonable?’ ”
Knowing your state’s law is key. State regulation of noncompete provisions widely differ. States such as California broadly hold that noncompete contracts are per se invalid – even if narrowly tailored – unless necessary to protect trade secrets. States such as Maryland allow the provisions only if area and duration restrictions are reasonable and do not impose undue hardship on employees. Three states – Colorado, Delaware, and Massachusetts – have laws that strictly prohibit noncompete clauses in physician contracts.
“Most other states will generally enforce noncompete clauses so long as their terms are reasonable in light of the interests of the employer, the employee, and the general public,” Mr. Holland said. “Therefore, noncompete clauses should be no greater in scope than is necessary to protect the business or goodwill of the employer.”
Patient retention problems
Watch out for contract language referring to “trade secrets,” adds Los Angeles health law attorney Andrew H. Selesnick. Trade secret clauses are often lengthy and typically state that physicians cannot use or retain information from the employer that is considered confidential. Because patient lists are usually considered confidential, these terms could potentially prohibit patients from following their doctor.
“If you want to leave and take your patients with you, there may be some trade secret implications associated with that,” Mr. Selesnick said. “The ability to be able to move patients is significant and can have significant financial impacts. Know what you’re getting into.”
If bringing patients with them to a new practice, doctors should make sure the employment agreement excludes these patients from any nonsolicitation provision at the time the doctor leaves, notes Mr. Holland. Include language that states physicians can retain patients they originally brought to the practice when they depart without violating the agreement.
Make sure to review any proposed noncompete clauses in relation to proposed termination provisions, Mr. Kopson said. Doctors should negotiate language that ensures noncompete obligations will be null and void if physicians are terminated without cause (if such terminations are permitted by the contract), or if the employer breaches the contract.
Seeking the advice of an experienced contract attorney before signing a noncompete clause can save doctors significant time, money, and heartache in the long run, Mr. Kopson notes.
“The biggest risk is signing a contract that has such a clause with an expectation that it will not be enforced,” he said. “If [clauses are] properly drafted, they’re going to be binding. If you get the help up front, it’s going to be a lot less expensive than having your life turned upside down because you’re stuck with a noncompete that has bad terms in it.”
Unreasonable terms
Once signed, getting out of non-compete clauses can be tricky, Mr. Selesnick said. However, doctors can usually escape them if they can prove the terms are unreasonable.
“You can get out of them, especially if they’re very restrictive and say you can’t practice within an area that may prevent you from earning your livelihood,” Mr. Selesnick said. “Courts [generally] think that employees should be able to leave and be able to get a job elsewhere, even if it’s across the street.”
Courts are typically more favorable to physician-employees than independent contractors when it comes to noncompete clauses, Mr. Selesnick said. Independent contractors are generally viewed as having more power over their work than physician-employees. They may have a tougher time convincing a court that such provisions will harm their employment options.
When seeking to enforce a disputed noncompete agreement, employers frequently will request a court-ordered temporary restraining order or injunction to enforce the clause, Mr. Holland said. Judges consider general principles of fairness and equity, and balance the relative harm to the employer and the employee, when deciding whether to issue the injunction. The employee-physician can also try to beat the employer to the courthouse steps by filing a “declaratory judgment” lawsuit that seeks guidance from the court on the contract’s enforceability.
“Typically, employers attempt to do that which is in their best economic interest,” Mr. Holland said. “If a proposal can be negotiated where the employer’s economic well-being is not threatened, then the employer should have a strong interest in a compromise.”
On Twitter @legal_med
Noncompete clauses can severely limit a doctor’s business options and create serious financial challenges, so negotiate with employers early and watch out for tricky contract terms that could stifle future opportunities.
That is the advice from health law experts around the country. They point out that when it comes to noncompete clauses – employment contract language that limits where physicians can practice after employment ends or is terminated – doctors should pay close attention, especially to the following:
Geographical limitations
Distance requirements within noncompete provisions are a top issue that can trip up doctors, Bloomfield Hills, Mich., health law attorney Mark S. Kopson said. The clause typically specifies that a physician cannot practice within a certain radius of the former employer. However, if an employer has three offices for instance, that 10-mile radius can quickly become a 30-mile radius or more depending how the provision is worded. Mr. Kopson recalled a recent client who practiced for 5 years in one office and was transferred to an office in another town for 30 days. He was then terminated, and his employer attempted to enforce contract terms that would prevent him from practicing within a 10-mile radius of both offices. A court determined that the employer was acting in bad faith and sought an unfair competitive advantage.
“But No. 1, you don’t want to have to go to court,” Mr. Kopson said. “And No. 2, you can have the best lawyer, but once it’s in the hands of the judge or the jury, anything can happen. That’s why you really want to do the work on this up front.”
When negotiating noncompete clauses, be cognizant of where distances are being measured from and around, the legal experts stress. Also, be clear with employers about what defines a reasonable distance, based on the geographic spread of their patient base.
“What’s reasonable for a family practice physician is probably not going to be reasonable for a pediatric neurosurgeon, as they draw their patients from varying distances,” he said. “Also, negotiate to ensure that the length of time of the restriction is reasonable. Taking into account, both the distance and time period, the physician must still be able to earn a living.”
Time frame restrictions
Negotiate the shortest duration that you can, advises Greenbelt, Md., labor and employment attorney Jay P. Holland. Noncompete provisions typically limit a doctor from practicing around a certain radius for 1-5 years, but some employers may try to enforce longer time periods.
“Consider your career and lifestyle goals carefully prior to entering into a noncompete,” Mr. Holland said. “The first approach should always be an attempt to exclude the noncompete from your prospective agreement if you are joining a practice. If a noncompete is unavoidable, then strive to make it the least onerous possible. Ask yourself prior to signing an agreement, ‘If I were to leave this practice, what are the restrictions I could live with? Are the restrictions reasonable?’ ”
Knowing your state’s law is key. State regulation of noncompete provisions widely differ. States such as California broadly hold that noncompete contracts are per se invalid – even if narrowly tailored – unless necessary to protect trade secrets. States such as Maryland allow the provisions only if area and duration restrictions are reasonable and do not impose undue hardship on employees. Three states – Colorado, Delaware, and Massachusetts – have laws that strictly prohibit noncompete clauses in physician contracts.
“Most other states will generally enforce noncompete clauses so long as their terms are reasonable in light of the interests of the employer, the employee, and the general public,” Mr. Holland said. “Therefore, noncompete clauses should be no greater in scope than is necessary to protect the business or goodwill of the employer.”
Patient retention problems
Watch out for contract language referring to “trade secrets,” adds Los Angeles health law attorney Andrew H. Selesnick. Trade secret clauses are often lengthy and typically state that physicians cannot use or retain information from the employer that is considered confidential. Because patient lists are usually considered confidential, these terms could potentially prohibit patients from following their doctor.
“If you want to leave and take your patients with you, there may be some trade secret implications associated with that,” Mr. Selesnick said. “The ability to be able to move patients is significant and can have significant financial impacts. Know what you’re getting into.”
If bringing patients with them to a new practice, doctors should make sure the employment agreement excludes these patients from any nonsolicitation provision at the time the doctor leaves, notes Mr. Holland. Include language that states physicians can retain patients they originally brought to the practice when they depart without violating the agreement.
Make sure to review any proposed noncompete clauses in relation to proposed termination provisions, Mr. Kopson said. Doctors should negotiate language that ensures noncompete obligations will be null and void if physicians are terminated without cause (if such terminations are permitted by the contract), or if the employer breaches the contract.
Seeking the advice of an experienced contract attorney before signing a noncompete clause can save doctors significant time, money, and heartache in the long run, Mr. Kopson notes.
“The biggest risk is signing a contract that has such a clause with an expectation that it will not be enforced,” he said. “If [clauses are] properly drafted, they’re going to be binding. If you get the help up front, it’s going to be a lot less expensive than having your life turned upside down because you’re stuck with a noncompete that has bad terms in it.”
Unreasonable terms
Once signed, getting out of non-compete clauses can be tricky, Mr. Selesnick said. However, doctors can usually escape them if they can prove the terms are unreasonable.
“You can get out of them, especially if they’re very restrictive and say you can’t practice within an area that may prevent you from earning your livelihood,” Mr. Selesnick said. “Courts [generally] think that employees should be able to leave and be able to get a job elsewhere, even if it’s across the street.”
Courts are typically more favorable to physician-employees than independent contractors when it comes to noncompete clauses, Mr. Selesnick said. Independent contractors are generally viewed as having more power over their work than physician-employees. They may have a tougher time convincing a court that such provisions will harm their employment options.
When seeking to enforce a disputed noncompete agreement, employers frequently will request a court-ordered temporary restraining order or injunction to enforce the clause, Mr. Holland said. Judges consider general principles of fairness and equity, and balance the relative harm to the employer and the employee, when deciding whether to issue the injunction. The employee-physician can also try to beat the employer to the courthouse steps by filing a “declaratory judgment” lawsuit that seeks guidance from the court on the contract’s enforceability.
“Typically, employers attempt to do that which is in their best economic interest,” Mr. Holland said. “If a proposal can be negotiated where the employer’s economic well-being is not threatened, then the employer should have a strong interest in a compromise.”
On Twitter @legal_med
Noncompete clauses can severely limit a doctor’s business options and create serious financial challenges, so negotiate with employers early and watch out for tricky contract terms that could stifle future opportunities.
That is the advice from health law experts around the country. They point out that when it comes to noncompete clauses – employment contract language that limits where physicians can practice after employment ends or is terminated – doctors should pay close attention, especially to the following:
Geographical limitations
Distance requirements within noncompete provisions are a top issue that can trip up doctors, Bloomfield Hills, Mich., health law attorney Mark S. Kopson said. The clause typically specifies that a physician cannot practice within a certain radius of the former employer. However, if an employer has three offices for instance, that 10-mile radius can quickly become a 30-mile radius or more depending how the provision is worded. Mr. Kopson recalled a recent client who practiced for 5 years in one office and was transferred to an office in another town for 30 days. He was then terminated, and his employer attempted to enforce contract terms that would prevent him from practicing within a 10-mile radius of both offices. A court determined that the employer was acting in bad faith and sought an unfair competitive advantage.
“But No. 1, you don’t want to have to go to court,” Mr. Kopson said. “And No. 2, you can have the best lawyer, but once it’s in the hands of the judge or the jury, anything can happen. That’s why you really want to do the work on this up front.”
When negotiating noncompete clauses, be cognizant of where distances are being measured from and around, the legal experts stress. Also, be clear with employers about what defines a reasonable distance, based on the geographic spread of their patient base.
“What’s reasonable for a family practice physician is probably not going to be reasonable for a pediatric neurosurgeon, as they draw their patients from varying distances,” he said. “Also, negotiate to ensure that the length of time of the restriction is reasonable. Taking into account, both the distance and time period, the physician must still be able to earn a living.”
Time frame restrictions
Negotiate the shortest duration that you can, advises Greenbelt, Md., labor and employment attorney Jay P. Holland. Noncompete provisions typically limit a doctor from practicing around a certain radius for 1-5 years, but some employers may try to enforce longer time periods.
“Consider your career and lifestyle goals carefully prior to entering into a noncompete,” Mr. Holland said. “The first approach should always be an attempt to exclude the noncompete from your prospective agreement if you are joining a practice. If a noncompete is unavoidable, then strive to make it the least onerous possible. Ask yourself prior to signing an agreement, ‘If I were to leave this practice, what are the restrictions I could live with? Are the restrictions reasonable?’ ”
Knowing your state’s law is key. State regulation of noncompete provisions widely differ. States such as California broadly hold that noncompete contracts are per se invalid – even if narrowly tailored – unless necessary to protect trade secrets. States such as Maryland allow the provisions only if area and duration restrictions are reasonable and do not impose undue hardship on employees. Three states – Colorado, Delaware, and Massachusetts – have laws that strictly prohibit noncompete clauses in physician contracts.
“Most other states will generally enforce noncompete clauses so long as their terms are reasonable in light of the interests of the employer, the employee, and the general public,” Mr. Holland said. “Therefore, noncompete clauses should be no greater in scope than is necessary to protect the business or goodwill of the employer.”
Patient retention problems
Watch out for contract language referring to “trade secrets,” adds Los Angeles health law attorney Andrew H. Selesnick. Trade secret clauses are often lengthy and typically state that physicians cannot use or retain information from the employer that is considered confidential. Because patient lists are usually considered confidential, these terms could potentially prohibit patients from following their doctor.
“If you want to leave and take your patients with you, there may be some trade secret implications associated with that,” Mr. Selesnick said. “The ability to be able to move patients is significant and can have significant financial impacts. Know what you’re getting into.”
If bringing patients with them to a new practice, doctors should make sure the employment agreement excludes these patients from any nonsolicitation provision at the time the doctor leaves, notes Mr. Holland. Include language that states physicians can retain patients they originally brought to the practice when they depart without violating the agreement.
Make sure to review any proposed noncompete clauses in relation to proposed termination provisions, Mr. Kopson said. Doctors should negotiate language that ensures noncompete obligations will be null and void if physicians are terminated without cause (if such terminations are permitted by the contract), or if the employer breaches the contract.
Seeking the advice of an experienced contract attorney before signing a noncompete clause can save doctors significant time, money, and heartache in the long run, Mr. Kopson notes.
“The biggest risk is signing a contract that has such a clause with an expectation that it will not be enforced,” he said. “If [clauses are] properly drafted, they’re going to be binding. If you get the help up front, it’s going to be a lot less expensive than having your life turned upside down because you’re stuck with a noncompete that has bad terms in it.”
Unreasonable terms
Once signed, getting out of non-compete clauses can be tricky, Mr. Selesnick said. However, doctors can usually escape them if they can prove the terms are unreasonable.
“You can get out of them, especially if they’re very restrictive and say you can’t practice within an area that may prevent you from earning your livelihood,” Mr. Selesnick said. “Courts [generally] think that employees should be able to leave and be able to get a job elsewhere, even if it’s across the street.”
Courts are typically more favorable to physician-employees than independent contractors when it comes to noncompete clauses, Mr. Selesnick said. Independent contractors are generally viewed as having more power over their work than physician-employees. They may have a tougher time convincing a court that such provisions will harm their employment options.
When seeking to enforce a disputed noncompete agreement, employers frequently will request a court-ordered temporary restraining order or injunction to enforce the clause, Mr. Holland said. Judges consider general principles of fairness and equity, and balance the relative harm to the employer and the employee, when deciding whether to issue the injunction. The employee-physician can also try to beat the employer to the courthouse steps by filing a “declaratory judgment” lawsuit that seeks guidance from the court on the contract’s enforceability.
“Typically, employers attempt to do that which is in their best economic interest,” Mr. Holland said. “If a proposal can be negotiated where the employer’s economic well-being is not threatened, then the employer should have a strong interest in a compromise.”
On Twitter @legal_med
ACR: Sulfasalazine reduces TNFi antibodies but more poorly than methotrexate
SAN FRANCISCO – Sulfasalazine prevents formation of antibodies against tumor necrosis factor inhibitors, but probably not as well as methotrexate, according to a European study of 140 axial spondyloarthritis patients.
“The effect of sulfasalazine on the development of antidrug antibodies has not been studied before. Our initial hypothesis was that methotrexate would [reduce] antibody formation” because it’s been shown to do that before, but that “sulfasalazine would not. This was a surprise to us,” said senior investigator Dr. Alejandro Balsa, chief of rheumatology at La Paz University Hospital in Madrid.
The findings suggest that sulfasalazine, like methotrexate, might “prevent immunogenicity and, hence ... secondary failure of” a tumor necrosis factor inhibitor (TNFi), he said at the annual meeting of the American College of Rheumatology.
Thirty-one patients (22%) were on infliximab (Remicade) and 109 (78%) were on adalimumab (Humira) in the year-long study, which was conducted in Madrid and Amsterdam.
Of the 90 patients on TNFi monotherapy, 33 (37%) developed TNFi antibodies, including 3 of 13 (23%) on infliximab monotherapy and 30 of 77 (39%) on adalimumab alone. The difference was not statistically significant.
Of the 50 patients on concomitant therapy, antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including three cases on infliximab and three on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
The trend toward better antibody protection with methotrexate was, again, not significant, probably because of the small numbers in the study.
Methotrexate and sulfasalazine are not routinely prescribed for axial spondyloarthritis; patients were on them in the study to help with peripheral manifestations. The drugs only prevent antibodies if started before a TNFi. “Once patients develop anti-[TNF] antibodies,” it’s too late, Dr. Balsa noted.
Despite the promising results, he said there’s not enough data at this point to recommend routine pretreatment with methotrexate or sulfasalazine to prevent TNFi antibodies.
As expected, antibodies diminished the clinical effect of a TNFi. Less than a quarter of antibody patients, versus more than a half free from antibodies, reached the investigators’ mark for low disease activity at 1 year: clinical improvement plus a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score below 4 and normal C-reactive protein (P = .03).
Also at 1 year, patients on monotherapy had an overall BASDAI improvement of about 1 point on the 10-point scale. Patients on methotrexate gained an additional point or so (P = .04), while those on sulfasalazine gained about a half point extra (P = .16).
“We only saw significant improvements in patients treated with methotrexate, probably because 100% cotreated with methotrexate had free” serum TNFi “at 1 year, as compared with only 82% cotreated with sulfasalazine,” Dr. Balsa said.
Oddly, the investigators detected free serum TNFi at 1 year in 78 (87%) monotherapy patients, which was more than in those cotreated with sulfasalazine; he didn’t address the finding.
The mean BASDAI at baseline was 6. Ankylosing spondylitis was the most common diagnosis in the study. More than half the subjects were men, the majority of patients were HLA-B27 positive, and their mean disease duration was 11 years.
Dr. Balsa’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
SAN FRANCISCO – Sulfasalazine prevents formation of antibodies against tumor necrosis factor inhibitors, but probably not as well as methotrexate, according to a European study of 140 axial spondyloarthritis patients.
“The effect of sulfasalazine on the development of antidrug antibodies has not been studied before. Our initial hypothesis was that methotrexate would [reduce] antibody formation” because it’s been shown to do that before, but that “sulfasalazine would not. This was a surprise to us,” said senior investigator Dr. Alejandro Balsa, chief of rheumatology at La Paz University Hospital in Madrid.
The findings suggest that sulfasalazine, like methotrexate, might “prevent immunogenicity and, hence ... secondary failure of” a tumor necrosis factor inhibitor (TNFi), he said at the annual meeting of the American College of Rheumatology.
Thirty-one patients (22%) were on infliximab (Remicade) and 109 (78%) were on adalimumab (Humira) in the year-long study, which was conducted in Madrid and Amsterdam.
Of the 90 patients on TNFi monotherapy, 33 (37%) developed TNFi antibodies, including 3 of 13 (23%) on infliximab monotherapy and 30 of 77 (39%) on adalimumab alone. The difference was not statistically significant.
Of the 50 patients on concomitant therapy, antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including three cases on infliximab and three on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
The trend toward better antibody protection with methotrexate was, again, not significant, probably because of the small numbers in the study.
Methotrexate and sulfasalazine are not routinely prescribed for axial spondyloarthritis; patients were on them in the study to help with peripheral manifestations. The drugs only prevent antibodies if started before a TNFi. “Once patients develop anti-[TNF] antibodies,” it’s too late, Dr. Balsa noted.
Despite the promising results, he said there’s not enough data at this point to recommend routine pretreatment with methotrexate or sulfasalazine to prevent TNFi antibodies.
As expected, antibodies diminished the clinical effect of a TNFi. Less than a quarter of antibody patients, versus more than a half free from antibodies, reached the investigators’ mark for low disease activity at 1 year: clinical improvement plus a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score below 4 and normal C-reactive protein (P = .03).
Also at 1 year, patients on monotherapy had an overall BASDAI improvement of about 1 point on the 10-point scale. Patients on methotrexate gained an additional point or so (P = .04), while those on sulfasalazine gained about a half point extra (P = .16).
“We only saw significant improvements in patients treated with methotrexate, probably because 100% cotreated with methotrexate had free” serum TNFi “at 1 year, as compared with only 82% cotreated with sulfasalazine,” Dr. Balsa said.
Oddly, the investigators detected free serum TNFi at 1 year in 78 (87%) monotherapy patients, which was more than in those cotreated with sulfasalazine; he didn’t address the finding.
The mean BASDAI at baseline was 6. Ankylosing spondylitis was the most common diagnosis in the study. More than half the subjects were men, the majority of patients were HLA-B27 positive, and their mean disease duration was 11 years.
Dr. Balsa’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
SAN FRANCISCO – Sulfasalazine prevents formation of antibodies against tumor necrosis factor inhibitors, but probably not as well as methotrexate, according to a European study of 140 axial spondyloarthritis patients.
“The effect of sulfasalazine on the development of antidrug antibodies has not been studied before. Our initial hypothesis was that methotrexate would [reduce] antibody formation” because it’s been shown to do that before, but that “sulfasalazine would not. This was a surprise to us,” said senior investigator Dr. Alejandro Balsa, chief of rheumatology at La Paz University Hospital in Madrid.
The findings suggest that sulfasalazine, like methotrexate, might “prevent immunogenicity and, hence ... secondary failure of” a tumor necrosis factor inhibitor (TNFi), he said at the annual meeting of the American College of Rheumatology.
Thirty-one patients (22%) were on infliximab (Remicade) and 109 (78%) were on adalimumab (Humira) in the year-long study, which was conducted in Madrid and Amsterdam.
Of the 90 patients on TNFi monotherapy, 33 (37%) developed TNFi antibodies, including 3 of 13 (23%) on infliximab monotherapy and 30 of 77 (39%) on adalimumab alone. The difference was not statistically significant.
Of the 50 patients on concomitant therapy, antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including three cases on infliximab and three on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
The trend toward better antibody protection with methotrexate was, again, not significant, probably because of the small numbers in the study.
Methotrexate and sulfasalazine are not routinely prescribed for axial spondyloarthritis; patients were on them in the study to help with peripheral manifestations. The drugs only prevent antibodies if started before a TNFi. “Once patients develop anti-[TNF] antibodies,” it’s too late, Dr. Balsa noted.
Despite the promising results, he said there’s not enough data at this point to recommend routine pretreatment with methotrexate or sulfasalazine to prevent TNFi antibodies.
As expected, antibodies diminished the clinical effect of a TNFi. Less than a quarter of antibody patients, versus more than a half free from antibodies, reached the investigators’ mark for low disease activity at 1 year: clinical improvement plus a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score below 4 and normal C-reactive protein (P = .03).
Also at 1 year, patients on monotherapy had an overall BASDAI improvement of about 1 point on the 10-point scale. Patients on methotrexate gained an additional point or so (P = .04), while those on sulfasalazine gained about a half point extra (P = .16).
“We only saw significant improvements in patients treated with methotrexate, probably because 100% cotreated with methotrexate had free” serum TNFi “at 1 year, as compared with only 82% cotreated with sulfasalazine,” Dr. Balsa said.
Oddly, the investigators detected free serum TNFi at 1 year in 78 (87%) monotherapy patients, which was more than in those cotreated with sulfasalazine; he didn’t address the finding.
The mean BASDAI at baseline was 6. Ankylosing spondylitis was the most common diagnosis in the study. More than half the subjects were men, the majority of patients were HLA-B27 positive, and their mean disease duration was 11 years.
Dr. Balsa’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
AT THE ACR ANNUAL MEETING
Key clinical point: Pretreatment with sulfasalazine seems to prevent TNFi antibodies, but not as well as methotrexate.
Major finding: Antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including 3 cases on infliximab and 3 on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
Data source: European study of 140 axial spondyloarthritis patients
Disclosures: The senior investigator’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.