User login
Chemo quadruples risk for myeloid cancers
ORLANDO – Patients who undergo cytotoxic chemotherapy, even in the modern era, are at increased risk for developing myeloid neoplasms, based on data from a cohort of nearly 750,000 adults who were initially treated with chemotherapy and survived at least 1 year after diagnosis.
In the cohort, the standardized incidence ratio (SIR) for treatment-related acute myeloid leukemia (tAML) or myelodysplastic syndrome (MDS) was four times greater than would be expected in the general population, reported Lindsay M. Morton, Ph.D., of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“We now demonstrate that there are elevated risks for treatment-related leukemia after treatment for a broad spectrum of first primary malignancies that are generally consistent with what we know about changing treatment practices,” she said at the American Society of Hematology annual meeting.
“The number of cancer survivors in the United States has increased dramatically, to reach nearly 14 million individuals today, and in just the next few years the number is expected to reach more than 18 million people, which means that the long-term health of this population is of great clinical importance as well as public health importance,” Dr. Morton emphasized.
The link between cytotoxic chemotherapy and leukemia risk has been known since the 1960s, with certain classes of agents carrying higher risks than others, including platinum-containing compounds, alkylating agents (for example, cyclophosphamide, melphalan, chlorambucil), topoisomerase II inhibitors (doxorubicin, daunorubicin, epirubicin, etc.), and antimetabolites (5-fluorauracil, capecitabine, gemcitabine, et al.).
Treatment-related leukemias are associated with higher doses of these agents, and the trend in contemporary medicine is to use more of these agents upfront for the treatment of primary malignancies. Yet estimates of the risk of tAML, MDS, or other malignancies have been hard to come by because of the relative rarity of cases and the sporadic reports in the literature, Dr. Morton said.
The investigators previously reported that risk for tAML and other myeloid neoplasms changed over time, and showed that since the 1990s there was an uptick in risk for patients treated with chemotherapy for cancers of bone, joints, and endometrium, and since 2000 for patients treated with chemotherapy for cancers of the esophagus, cervix and prostate.
For example, risks for tAML were higher in the 1970s for patients with ovarian cancer treated with melphalan, a highly leukemogenic agent, but dropped somewhat with the switch to platinum-based agents. Similarly, women with breast cancer had a comparatively high risk with the use of melphalan, a decline in risk with the introduction of cyclophosphamide, and then an increase with the addition of anthracyclines and dose-dense regimens.
Risk update
To get a better idea of the magnitude of risk in the modern era, Dr. Morton and colleagues sifted through Surveillance, Epidemiology, and End Results (SEER) data to identify a cohort of 746,007 adults who were initially treated with chemotherapy and survived for at least 1 year following a diagnosis with a first primary malignancy from 2000 through 2012. They calculated SIRs based on variables that included age, race, sex, malignancy type and treatment period.
They looked at four categories of myeloid neoplasms as defined by World Health Organization criteria: AML/MDS, chronic myeloid leukemia, myeloproliferative neoplasms (MPN) negative for BCR-ABL (Philadelphia-negative), and chronic myelomonocytic leukemia (CMML).
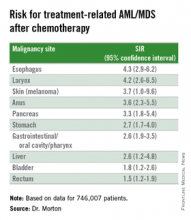
They found that 2,071 patients developed treatment-related AML/MDS, translating into a fourfold incidence compared with the general population (SIR 4.1, 95% confidence interval [CI] 3.9-4.2), 106 were diagnosed with CMML
They also identified novel risk for tAML/MDS after chemotherapy by malignancy (see table).
The investigators found that breast cancer, non-Hodgkin lymphoma, and lung cancer were most commonly associated with tAML/MDS (SIRs 4.1, 7.3, and 4.1, respectively, all significant).
In addition, although the overall numbers of cases were small, the investigators noted “strikingly elevated” risks for cancers of bone (SIR 35.1, CI. 16.9-64.6). testes (15.6, CI, 9.2-24.6), and soft tissue (12.6, CI=7.7-19.4),
Risk for tAML/MD was more modestly elevated for cancers of the brain, ovaries, endometrium, cervix, and prostate, and for Hodgkin lymphoma, chronic lymphocytic leukemia, and multiple myeloma.
Adding radiotherapy to chemotherapy for cancers of the breast, lung, and stomach cancers showed a trend toward heightened tAML/MDS risk, but this was not significant.
An elevated risk for CMML was also seen after chemotherapy for lung cancer (SIR 2.5, CI, 1.3-4.4), breast cancer (1.8, CI, 1.3-2.5), and non-Hodgkin lymphoma (2.1, CI, 1.2-3.4). There was elevated risk for CMML following chemotherapy for breast cancer (3.0, CI. 1.7-5.0) and non-Hodgkin lymphoma (4.2, CI, 2.4-6.9).
There were no increased risks for other myeloproliferative neoplasms after chemotherapy for any first primary cancer, however.
“This reminds us that with new uses of standard agents and introduction of new agents, it’s critical to carefully weigh the risks and benefits of systemic therapy,” Dr. Morton said.
The investigators plan to quantify risks associated with specific drugs and doses, she added.
The study was supported by the National Cancer Institute. Dr. Morton reported no relevant conflicts of interest to disclose.
ORLANDO – Patients who undergo cytotoxic chemotherapy, even in the modern era, are at increased risk for developing myeloid neoplasms, based on data from a cohort of nearly 750,000 adults who were initially treated with chemotherapy and survived at least 1 year after diagnosis.
In the cohort, the standardized incidence ratio (SIR) for treatment-related acute myeloid leukemia (tAML) or myelodysplastic syndrome (MDS) was four times greater than would be expected in the general population, reported Lindsay M. Morton, Ph.D., of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“We now demonstrate that there are elevated risks for treatment-related leukemia after treatment for a broad spectrum of first primary malignancies that are generally consistent with what we know about changing treatment practices,” she said at the American Society of Hematology annual meeting.
“The number of cancer survivors in the United States has increased dramatically, to reach nearly 14 million individuals today, and in just the next few years the number is expected to reach more than 18 million people, which means that the long-term health of this population is of great clinical importance as well as public health importance,” Dr. Morton emphasized.
The link between cytotoxic chemotherapy and leukemia risk has been known since the 1960s, with certain classes of agents carrying higher risks than others, including platinum-containing compounds, alkylating agents (for example, cyclophosphamide, melphalan, chlorambucil), topoisomerase II inhibitors (doxorubicin, daunorubicin, epirubicin, etc.), and antimetabolites (5-fluorauracil, capecitabine, gemcitabine, et al.).
Treatment-related leukemias are associated with higher doses of these agents, and the trend in contemporary medicine is to use more of these agents upfront for the treatment of primary malignancies. Yet estimates of the risk of tAML, MDS, or other malignancies have been hard to come by because of the relative rarity of cases and the sporadic reports in the literature, Dr. Morton said.
The investigators previously reported that risk for tAML and other myeloid neoplasms changed over time, and showed that since the 1990s there was an uptick in risk for patients treated with chemotherapy for cancers of bone, joints, and endometrium, and since 2000 for patients treated with chemotherapy for cancers of the esophagus, cervix and prostate.
For example, risks for tAML were higher in the 1970s for patients with ovarian cancer treated with melphalan, a highly leukemogenic agent, but dropped somewhat with the switch to platinum-based agents. Similarly, women with breast cancer had a comparatively high risk with the use of melphalan, a decline in risk with the introduction of cyclophosphamide, and then an increase with the addition of anthracyclines and dose-dense regimens.
Risk update
To get a better idea of the magnitude of risk in the modern era, Dr. Morton and colleagues sifted through Surveillance, Epidemiology, and End Results (SEER) data to identify a cohort of 746,007 adults who were initially treated with chemotherapy and survived for at least 1 year following a diagnosis with a first primary malignancy from 2000 through 2012. They calculated SIRs based on variables that included age, race, sex, malignancy type and treatment period.
They looked at four categories of myeloid neoplasms as defined by World Health Organization criteria: AML/MDS, chronic myeloid leukemia, myeloproliferative neoplasms (MPN) negative for BCR-ABL (Philadelphia-negative), and chronic myelomonocytic leukemia (CMML).
They found that 2,071 patients developed treatment-related AML/MDS, translating into a fourfold incidence compared with the general population (SIR 4.1, 95% confidence interval [CI] 3.9-4.2), 106 were diagnosed with CMML
They also identified novel risk for tAML/MDS after chemotherapy by malignancy (see table).
The investigators found that breast cancer, non-Hodgkin lymphoma, and lung cancer were most commonly associated with tAML/MDS (SIRs 4.1, 7.3, and 4.1, respectively, all significant).
In addition, although the overall numbers of cases were small, the investigators noted “strikingly elevated” risks for cancers of bone (SIR 35.1, CI. 16.9-64.6). testes (15.6, CI, 9.2-24.6), and soft tissue (12.6, CI=7.7-19.4),
Risk for tAML/MD was more modestly elevated for cancers of the brain, ovaries, endometrium, cervix, and prostate, and for Hodgkin lymphoma, chronic lymphocytic leukemia, and multiple myeloma.
Adding radiotherapy to chemotherapy for cancers of the breast, lung, and stomach cancers showed a trend toward heightened tAML/MDS risk, but this was not significant.
An elevated risk for CMML was also seen after chemotherapy for lung cancer (SIR 2.5, CI, 1.3-4.4), breast cancer (1.8, CI, 1.3-2.5), and non-Hodgkin lymphoma (2.1, CI, 1.2-3.4). There was elevated risk for CMML following chemotherapy for breast cancer (3.0, CI. 1.7-5.0) and non-Hodgkin lymphoma (4.2, CI, 2.4-6.9).
There were no increased risks for other myeloproliferative neoplasms after chemotherapy for any first primary cancer, however.
“This reminds us that with new uses of standard agents and introduction of new agents, it’s critical to carefully weigh the risks and benefits of systemic therapy,” Dr. Morton said.
The investigators plan to quantify risks associated with specific drugs and doses, she added.
The study was supported by the National Cancer Institute. Dr. Morton reported no relevant conflicts of interest to disclose.
ORLANDO – Patients who undergo cytotoxic chemotherapy, even in the modern era, are at increased risk for developing myeloid neoplasms, based on data from a cohort of nearly 750,000 adults who were initially treated with chemotherapy and survived at least 1 year after diagnosis.
In the cohort, the standardized incidence ratio (SIR) for treatment-related acute myeloid leukemia (tAML) or myelodysplastic syndrome (MDS) was four times greater than would be expected in the general population, reported Lindsay M. Morton, Ph.D., of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“We now demonstrate that there are elevated risks for treatment-related leukemia after treatment for a broad spectrum of first primary malignancies that are generally consistent with what we know about changing treatment practices,” she said at the American Society of Hematology annual meeting.
“The number of cancer survivors in the United States has increased dramatically, to reach nearly 14 million individuals today, and in just the next few years the number is expected to reach more than 18 million people, which means that the long-term health of this population is of great clinical importance as well as public health importance,” Dr. Morton emphasized.
The link between cytotoxic chemotherapy and leukemia risk has been known since the 1960s, with certain classes of agents carrying higher risks than others, including platinum-containing compounds, alkylating agents (for example, cyclophosphamide, melphalan, chlorambucil), topoisomerase II inhibitors (doxorubicin, daunorubicin, epirubicin, etc.), and antimetabolites (5-fluorauracil, capecitabine, gemcitabine, et al.).
Treatment-related leukemias are associated with higher doses of these agents, and the trend in contemporary medicine is to use more of these agents upfront for the treatment of primary malignancies. Yet estimates of the risk of tAML, MDS, or other malignancies have been hard to come by because of the relative rarity of cases and the sporadic reports in the literature, Dr. Morton said.
The investigators previously reported that risk for tAML and other myeloid neoplasms changed over time, and showed that since the 1990s there was an uptick in risk for patients treated with chemotherapy for cancers of bone, joints, and endometrium, and since 2000 for patients treated with chemotherapy for cancers of the esophagus, cervix and prostate.
For example, risks for tAML were higher in the 1970s for patients with ovarian cancer treated with melphalan, a highly leukemogenic agent, but dropped somewhat with the switch to platinum-based agents. Similarly, women with breast cancer had a comparatively high risk with the use of melphalan, a decline in risk with the introduction of cyclophosphamide, and then an increase with the addition of anthracyclines and dose-dense regimens.
Risk update
To get a better idea of the magnitude of risk in the modern era, Dr. Morton and colleagues sifted through Surveillance, Epidemiology, and End Results (SEER) data to identify a cohort of 746,007 adults who were initially treated with chemotherapy and survived for at least 1 year following a diagnosis with a first primary malignancy from 2000 through 2012. They calculated SIRs based on variables that included age, race, sex, malignancy type and treatment period.
They looked at four categories of myeloid neoplasms as defined by World Health Organization criteria: AML/MDS, chronic myeloid leukemia, myeloproliferative neoplasms (MPN) negative for BCR-ABL (Philadelphia-negative), and chronic myelomonocytic leukemia (CMML).
They found that 2,071 patients developed treatment-related AML/MDS, translating into a fourfold incidence compared with the general population (SIR 4.1, 95% confidence interval [CI] 3.9-4.2), 106 were diagnosed with CMML
They also identified novel risk for tAML/MDS after chemotherapy by malignancy (see table).
The investigators found that breast cancer, non-Hodgkin lymphoma, and lung cancer were most commonly associated with tAML/MDS (SIRs 4.1, 7.3, and 4.1, respectively, all significant).
In addition, although the overall numbers of cases were small, the investigators noted “strikingly elevated” risks for cancers of bone (SIR 35.1, CI. 16.9-64.6). testes (15.6, CI, 9.2-24.6), and soft tissue (12.6, CI=7.7-19.4),
Risk for tAML/MD was more modestly elevated for cancers of the brain, ovaries, endometrium, cervix, and prostate, and for Hodgkin lymphoma, chronic lymphocytic leukemia, and multiple myeloma.
Adding radiotherapy to chemotherapy for cancers of the breast, lung, and stomach cancers showed a trend toward heightened tAML/MDS risk, but this was not significant.
An elevated risk for CMML was also seen after chemotherapy for lung cancer (SIR 2.5, CI, 1.3-4.4), breast cancer (1.8, CI, 1.3-2.5), and non-Hodgkin lymphoma (2.1, CI, 1.2-3.4). There was elevated risk for CMML following chemotherapy for breast cancer (3.0, CI. 1.7-5.0) and non-Hodgkin lymphoma (4.2, CI, 2.4-6.9).
There were no increased risks for other myeloproliferative neoplasms after chemotherapy for any first primary cancer, however.
“This reminds us that with new uses of standard agents and introduction of new agents, it’s critical to carefully weigh the risks and benefits of systemic therapy,” Dr. Morton said.
The investigators plan to quantify risks associated with specific drugs and doses, she added.
The study was supported by the National Cancer Institute. Dr. Morton reported no relevant conflicts of interest to disclose.
AT ASH 2015
Key clinical point: This study quantifies the risks for treatment-related myeloid cancers after chemotherapy.
Major finding: Chemotherapy is associated with a fourfold risk for treatment-related AML/MDS, compared with the general population.
Data source: Retrospective review of data on 746,007 adults treated with chemotherapy for a first primary malignancy.
Disclosures: The National Cancer Institute supported the study. Dr. Morton reported having no conflicts of interest to disclose.
More complete cytogenetic responses at 12 months with radotinib than imatinib
Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than imatinib was at a minimum 12 months of follow-up in a randomized, open-label, phase III clinical trialof patients with newly diagnosed chronic myeloid leukemia-chronic phase (CML-CP).
Radotinib, an investigational BCR-ABL1 tyrosine kinase inhibitor developed by IL-YANG Pharmaceuticals, is approved in Korea for the treatment of CML-CP in patients who have failed prior TKIs.
Dr. Jae-Yong Kwak of Chonbuk National University Medical School and Hospital, Jeonju, South Korea, and his colleagues randomized 241 patients to either radotinib 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81), or imatinib 400 mg once daily (n = 81). All three study groups were balanced in regard to baseline age, gender, race, and Sokal risk score.
At a minimum follow-up of 12 months, the proportions of patients receiving a study drug were 86% (69/79) in the radotinib 300 mg twice-daily group, 72% (58/81) in the radotinib 400 mg twice-daily group, and 82% (66/81) in the imatinib 400 mg once-daily group.
The rates of major molecular response at 12 months were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib (30%), Dr. Kwak reported at the annual meeting of the American Society of Hematology in Orlando.
Among responders, the median times to major molecular response were shorter on radotinib 300 mg b.i.d. (5.7 months) and radotinib 400 mg b.i.d. (5.6 months) than on imatinib (8.2 months). The MR4.5 rates by 12 months were also higher for both radotinib 300 mg b.i.d. (15%) and 400 mg b.i.d. (14%), compared with imatinib (9%). The complete cytogenetic response rates by 12 months were 91% for radotinib 300 mg b.i.d. (P = .0120), compared with imatinib (77%). None of the patients in the study had progressed to accelerated phase or blast crisis at 12 months.
Drug discontinuation due to adverse events (AEs) or laboratory abnormalities occurred in 9% of patients on radotinib 300 mg b.i.d., 20% on radotinib 400 mg b.i.d., and 6% on imatinib.
The major side effects included grade 3/4 thrombocytopenia in 16% of patients receiving radotinib 300 mg b.i.d., 14% on radotinib 400 mg b.i.d., and 20% receiving imatinib. Grade 3/4 neutropenia occurred in 19%, 24%, and 30% for radotinib 300 mg b.i.d., 400 mg b.i.d., and imatinib, respectively.
Overall, grade 3/4 nonlaboratory AEs were uncommon in all groups. The most common nonlaboratory AEs in the radotinib groups were skin rash (about 33% in both), nausea/vomiting (about 23% in both), headache (19% and 31%), and pruritus (19% and 30%). In the imatinib group, the most common adverse events were edema (35%), myalgia (28%), nausea/vomiting (27%), and skin rash (22%).
Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
On Twitter @maryjodales
Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than imatinib was at a minimum 12 months of follow-up in a randomized, open-label, phase III clinical trialof patients with newly diagnosed chronic myeloid leukemia-chronic phase (CML-CP).
Radotinib, an investigational BCR-ABL1 tyrosine kinase inhibitor developed by IL-YANG Pharmaceuticals, is approved in Korea for the treatment of CML-CP in patients who have failed prior TKIs.
Dr. Jae-Yong Kwak of Chonbuk National University Medical School and Hospital, Jeonju, South Korea, and his colleagues randomized 241 patients to either radotinib 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81), or imatinib 400 mg once daily (n = 81). All three study groups were balanced in regard to baseline age, gender, race, and Sokal risk score.
At a minimum follow-up of 12 months, the proportions of patients receiving a study drug were 86% (69/79) in the radotinib 300 mg twice-daily group, 72% (58/81) in the radotinib 400 mg twice-daily group, and 82% (66/81) in the imatinib 400 mg once-daily group.
The rates of major molecular response at 12 months were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib (30%), Dr. Kwak reported at the annual meeting of the American Society of Hematology in Orlando.
Among responders, the median times to major molecular response were shorter on radotinib 300 mg b.i.d. (5.7 months) and radotinib 400 mg b.i.d. (5.6 months) than on imatinib (8.2 months). The MR4.5 rates by 12 months were also higher for both radotinib 300 mg b.i.d. (15%) and 400 mg b.i.d. (14%), compared with imatinib (9%). The complete cytogenetic response rates by 12 months were 91% for radotinib 300 mg b.i.d. (P = .0120), compared with imatinib (77%). None of the patients in the study had progressed to accelerated phase or blast crisis at 12 months.
Drug discontinuation due to adverse events (AEs) or laboratory abnormalities occurred in 9% of patients on radotinib 300 mg b.i.d., 20% on radotinib 400 mg b.i.d., and 6% on imatinib.
The major side effects included grade 3/4 thrombocytopenia in 16% of patients receiving radotinib 300 mg b.i.d., 14% on radotinib 400 mg b.i.d., and 20% receiving imatinib. Grade 3/4 neutropenia occurred in 19%, 24%, and 30% for radotinib 300 mg b.i.d., 400 mg b.i.d., and imatinib, respectively.
Overall, grade 3/4 nonlaboratory AEs were uncommon in all groups. The most common nonlaboratory AEs in the radotinib groups were skin rash (about 33% in both), nausea/vomiting (about 23% in both), headache (19% and 31%), and pruritus (19% and 30%). In the imatinib group, the most common adverse events were edema (35%), myalgia (28%), nausea/vomiting (27%), and skin rash (22%).
Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
On Twitter @maryjodales
Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than imatinib was at a minimum 12 months of follow-up in a randomized, open-label, phase III clinical trialof patients with newly diagnosed chronic myeloid leukemia-chronic phase (CML-CP).
Radotinib, an investigational BCR-ABL1 tyrosine kinase inhibitor developed by IL-YANG Pharmaceuticals, is approved in Korea for the treatment of CML-CP in patients who have failed prior TKIs.
Dr. Jae-Yong Kwak of Chonbuk National University Medical School and Hospital, Jeonju, South Korea, and his colleagues randomized 241 patients to either radotinib 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81), or imatinib 400 mg once daily (n = 81). All three study groups were balanced in regard to baseline age, gender, race, and Sokal risk score.
At a minimum follow-up of 12 months, the proportions of patients receiving a study drug were 86% (69/79) in the radotinib 300 mg twice-daily group, 72% (58/81) in the radotinib 400 mg twice-daily group, and 82% (66/81) in the imatinib 400 mg once-daily group.
The rates of major molecular response at 12 months were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib (30%), Dr. Kwak reported at the annual meeting of the American Society of Hematology in Orlando.
Among responders, the median times to major molecular response were shorter on radotinib 300 mg b.i.d. (5.7 months) and radotinib 400 mg b.i.d. (5.6 months) than on imatinib (8.2 months). The MR4.5 rates by 12 months were also higher for both radotinib 300 mg b.i.d. (15%) and 400 mg b.i.d. (14%), compared with imatinib (9%). The complete cytogenetic response rates by 12 months were 91% for radotinib 300 mg b.i.d. (P = .0120), compared with imatinib (77%). None of the patients in the study had progressed to accelerated phase or blast crisis at 12 months.
Drug discontinuation due to adverse events (AEs) or laboratory abnormalities occurred in 9% of patients on radotinib 300 mg b.i.d., 20% on radotinib 400 mg b.i.d., and 6% on imatinib.
The major side effects included grade 3/4 thrombocytopenia in 16% of patients receiving radotinib 300 mg b.i.d., 14% on radotinib 400 mg b.i.d., and 20% receiving imatinib. Grade 3/4 neutropenia occurred in 19%, 24%, and 30% for radotinib 300 mg b.i.d., 400 mg b.i.d., and imatinib, respectively.
Overall, grade 3/4 nonlaboratory AEs were uncommon in all groups. The most common nonlaboratory AEs in the radotinib groups were skin rash (about 33% in both), nausea/vomiting (about 23% in both), headache (19% and 31%), and pruritus (19% and 30%). In the imatinib group, the most common adverse events were edema (35%), myalgia (28%), nausea/vomiting (27%), and skin rash (22%).
Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
On Twitter @maryjodales
FROM ASH 2015
Key clinical point: Radotinib was associated with significantly higher complete cytogenetic responses and major molecular responses than was imatinib at a minimum 12 months of follow-up.
Major finding: By 12 months, the rates of major molecular response were significantly higher in patients receiving radotinib 300 mg b.i.d. (52%, P = .0044) and radotinib 400 mg b.i.d. (46%, P = .0342), compared with imatinib 400 mg/day (30%).
Data source: Randomized, open-label, phase III clinical trial involving 241 patients.
Disclosures: Dr. Kwak had no relevant disclosures. Some of his colleagues received research funding from IL-YANG Pharmaceutical Co. and Alexion Pharmaceuticals.
Nilotinib safe, effective as first-line therapy for CML-CP patients age 65 and older
ORLANDO – Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with chronic myeloid leukemia in chronic phase (CML-CP), based on results from a subanalysis of the ENEST1st study.
The analysis of the ENEST1st study, reported by Dr. Francis J. Giles, compared outcomes for 1,089 newly diagnosed CML-CP patients, 19% were aged 65 years or older and 81% were younger than age 65 years. All patients had typical transcripts and were treated for 3 months or less with nilotinib 300 mg twice daily in the open-label study.
For those 65 years and older, Sokal risk scores were low in 4.5%, intermediate in 61.2%, and high in 23.4%, with missing data for 10.9%. For younger patients, Sokal risk scores were low in 42.1%, intermediate in 32%, and high in 16.9%, with missing data for 9.
At 18 months, there was an overall 38.4% rate (95% CI, 35.5%-41.3%) of MR4 grade molecular response, which was defined as BCR-ABL level of 0.01% or less on the International Scale or undetectable BCR-ABL in cDNA with at least 10,000 ABL transcripts.
The MR4 rate at 18 months did not significantly vary by age. For patients under age 65, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%); among patients aged 65 and older, the incidence of MR4 was 48.3% (95% CI, 41.4% - 55.2%). The MR4.5 rate by 18 months was 32.5% in younger patients and 28.4% in older patients, reported Dr. Giles of the Institute for Drug Development, Cancer Therapy and Research Center, at the University of Texas Health Science Center at San Antonio, and his colleagues.
Based on Sokal score, the MR4 rate by 18 months in younger patients was 53.6% (low), 45.2% (intermediate), and 35.4% (high), respectively. For older patients, the MR4 rate by 18 months based on Sokal score was 44.4% (low), 49.6% (intermediate), and 44.7% (high).
Six patients (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 patients (1.2%) died by 24 months. The most common adverse events were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
On Twitter @maryjodales
ORLANDO – Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with chronic myeloid leukemia in chronic phase (CML-CP), based on results from a subanalysis of the ENEST1st study.
The analysis of the ENEST1st study, reported by Dr. Francis J. Giles, compared outcomes for 1,089 newly diagnosed CML-CP patients, 19% were aged 65 years or older and 81% were younger than age 65 years. All patients had typical transcripts and were treated for 3 months or less with nilotinib 300 mg twice daily in the open-label study.
For those 65 years and older, Sokal risk scores were low in 4.5%, intermediate in 61.2%, and high in 23.4%, with missing data for 10.9%. For younger patients, Sokal risk scores were low in 42.1%, intermediate in 32%, and high in 16.9%, with missing data for 9.
At 18 months, there was an overall 38.4% rate (95% CI, 35.5%-41.3%) of MR4 grade molecular response, which was defined as BCR-ABL level of 0.01% or less on the International Scale or undetectable BCR-ABL in cDNA with at least 10,000 ABL transcripts.
The MR4 rate at 18 months did not significantly vary by age. For patients under age 65, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%); among patients aged 65 and older, the incidence of MR4 was 48.3% (95% CI, 41.4% - 55.2%). The MR4.5 rate by 18 months was 32.5% in younger patients and 28.4% in older patients, reported Dr. Giles of the Institute for Drug Development, Cancer Therapy and Research Center, at the University of Texas Health Science Center at San Antonio, and his colleagues.
Based on Sokal score, the MR4 rate by 18 months in younger patients was 53.6% (low), 45.2% (intermediate), and 35.4% (high), respectively. For older patients, the MR4 rate by 18 months based on Sokal score was 44.4% (low), 49.6% (intermediate), and 44.7% (high).
Six patients (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 patients (1.2%) died by 24 months. The most common adverse events were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
On Twitter @maryjodales
ORLANDO – Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with chronic myeloid leukemia in chronic phase (CML-CP), based on results from a subanalysis of the ENEST1st study.
The analysis of the ENEST1st study, reported by Dr. Francis J. Giles, compared outcomes for 1,089 newly diagnosed CML-CP patients, 19% were aged 65 years or older and 81% were younger than age 65 years. All patients had typical transcripts and were treated for 3 months or less with nilotinib 300 mg twice daily in the open-label study.
For those 65 years and older, Sokal risk scores were low in 4.5%, intermediate in 61.2%, and high in 23.4%, with missing data for 10.9%. For younger patients, Sokal risk scores were low in 42.1%, intermediate in 32%, and high in 16.9%, with missing data for 9.
At 18 months, there was an overall 38.4% rate (95% CI, 35.5%-41.3%) of MR4 grade molecular response, which was defined as BCR-ABL level of 0.01% or less on the International Scale or undetectable BCR-ABL in cDNA with at least 10,000 ABL transcripts.
The MR4 rate at 18 months did not significantly vary by age. For patients under age 65, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%); among patients aged 65 and older, the incidence of MR4 was 48.3% (95% CI, 41.4% - 55.2%). The MR4.5 rate by 18 months was 32.5% in younger patients and 28.4% in older patients, reported Dr. Giles of the Institute for Drug Development, Cancer Therapy and Research Center, at the University of Texas Health Science Center at San Antonio, and his colleagues.
Based on Sokal score, the MR4 rate by 18 months in younger patients was 53.6% (low), 45.2% (intermediate), and 35.4% (high), respectively. For older patients, the MR4 rate by 18 months based on Sokal score was 44.4% (low), 49.6% (intermediate), and 44.7% (high).
Six patients (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 patients (1.2%) died by 24 months. The most common adverse events were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
On Twitter @maryjodales
FROM ASH 2015
Key clinical point: Age did not affect molecular response or the incidence of adverse reactions to nilotinib among patients with CML-CP.
Major finding: For patients younger than age 65 years, the cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4%-52.1%); among patients aged 65 years and older, the incidence of MR4 was 48.3% (95% CI, 41.4%-55.2%).
Data source: The analysis of the ENEST1st study compared outcomes for 1,089 newly diagnosed CML-CP patients.
Disclosures: Novartis is the sponsor of the ENEST1st study. Dr. Giles consults for and receives honoraria and research funding from Novartis.
Chagas disease: Neither foreign nor untreatable
Chagas disease is a vector-borne parasitic disease, endemic to the Americas, that remains as little recognized by U.S. patients and practitioners as the obscure winged insects that transmit it.
Transmission occurs when triatomine bugs, commonly called “kissing bugs,” pierce the skin to feed and leave behind parasite-infected feces that can enter the bloodstream; pregnant women can also transmit Chagas to their newborns.
About a third of patients infected with Trypanosoma cruzi, the protozoan parasite that causes Chagas, will develop cardiac abnormalities such as cardiomyopathy, arrhythmias, and heart failure – often decades after becoming infected. In the United States, where blood banks began screening for Chagas in 2007, patients without symptoms are likely to learn they are positive only after donating blood.
Conventional wisdom has long maintained that Chagas is limited to Central and South America. But immigration from Chagas-endemic countries, such as El Salvador, Mexico, and Bolivia, means more people are living with the disease in the United States.
“One percent of the Latin American immigrant population we screen [in Los Angeles] has Chagas,” said Dr. Sheba K. Meymandi, cardiologist and director of Center of Excellence for Chagas Disease at Olive View–UCLA Medical Center in Los Angeles, who also works with the city’s health department to detect Chagas. “That’s huge.”
Meanwhile, blood banks are discovering more cases among people without ties to Latin America, and species of kissing bugs native to the southern United States are increasingly recognized as a non-negligible source of Chagas transmission. Of 39 Chagas cases reported to Texas health authorities in 2013 and 2014, 12 were thought to be locally acquired.
Dr. Heather Yun, an infectious disease specialist at the San Antonio Military Medical Center, said risk factors for local transmission are not well established, but “we think people who are living in poverty in substandard housing, people who spend a lot of time outdoors, especially at night, and people involved with direct blood contact with wild game in Southern parts of the United States” may be at higher risk.
A U.S. disease
Evidence is amassing quickly that Chagas is a U.S. disease. But U.S. clinicians still lag in their knowledge of it, say physicians treating Chagas cases. “In medical school we get a 2-hour lecture on it, and it’s always been presented as an exotic disease and one you don’t treat,” Dr. Meymandi said.
The persistent perception of Chagas as a foreign disease means clinicians are inclined to dismiss positive results from a blood screening, particularly from someone who is not from Latin America. Yet cardiologists, ID practitioners, obstetricians, and primary care physicians all need to be aware that cases do occur in the United States and are potentially treatable.
Dr. Laila Woc-Colburn, an infectious disease and tropical medicine specialist at Baylor College of Medicine in Houston, said many people with Chagas never make it to an infectious disease specialist or cardiologist for a work-up. “When you test positive on serology [after a blood donation], you get a letter recommending you consult your physician. Most will go to their primary care doctors, who might say ‘this isn’t a disease in the United States.’ In Houston, that is often the case.”
Dr. Meymandi, who has treated hundreds of patients with Chagas with and without cardiac involvement, said any physician with a potential Chagas case must act. “If you get someone that’s positive, it’s your duty as a physician to confirm the positivity with CDC,” she said.
Dr. Yun concurred. “The most important message is, do something,” she said. “Don’t just assume it’s a false positive.”
Diagnosis is not simple and requires testing beyond the initial ELISA assay used in blood-bank screening. Confirmatory tests must be carried out in coordination with the Centers for Disease Control and Prevention. Also, with no agents approved by the Food and Drug Administration to treat Chagas, treatment is available only through the CDC’s investigational drugs protocol. Both drugs used in Chagas, benznidazole and nifurtimox, come with serious adverse effects that must be closely monitored.
“It’s time consuming, filling out the forms, getting the consent, tracking and sending back lab results to CDC in order to get drugs – it’s not like you can just write a prescription,” Dr. Meymandi said. But, “if you don’t know how to treat the patient or don’t have time, find someone like me,” she noted, adding that she is available to counsel any physician daunted by a potential Chagas case.
Treatment options
No formal clinical algorithm exists for Chagas, but Dr. Meymandi, Dr. Yun, and Dr. Woc-Colburn all pointed to a 2007 JAMA article, which describes diagnosis and treatment protocols, as an important reference for clinicians to start with. It’s “the best approximation of a clinical guideline we have,” Dr. Yun said (JAMA. 2007;298[18]:2171-81. doi:10.1001/jama.298.18.2171).
Dr. Meymandi, who has treated more Chagas patients than has any other U.S. clinician, said that treatment has changed somewhat since the JAMA article was published. In 2007, she said, nifurtimox was the main drug available through CDC, while benznidazole, which is somewhat better tolerated and has shorter treatment duration, has since become the first-line agent.
“We’ve lowered the dose of benznidazole, maxing out at 400 mg/day to decrease the toxicity,” she said. Also, treatment is now being extended to some patients aged 60 years and older.
The decision to treat or not treat, clinicians say, depends on the patient’s age, disease progression, comorbidities and potential serious drug interactions, and willingness to tolerate side effects that, with nifurtimox especially, can include skin sloughing, rash, and psychological and neurologic symptoms including depression and peripheral neuropathy.
“If you don’t have side effects, you’re not taking the drugs,” Dr. Meymandi said. Dr. Woc-Colburn noted that polypharmacy was a major consideration when treating older adults for Chagas. “If I have a patient who has diabetes, obesity, [and] end-stage renal disease, it’s not going to be ideal to give [benznidazole].”
Recent, highly anticipated results from BENEFIT, a large randomized trial (n = 2,854) showed that benznidazole reduced parasite load but was not helpful in halting cardiac damage at 5 years’ follow-up in patients with established Chagas cardiomyopathy (N Engl J Med. 2015 Oct;373:1295-306. doi:10.1056/NEJMoa1507574).
Dr. Meymandi, whose earlier research established that Chagas cardiomyopathy carries significantly higher morbidity and mortality than does non–Chagas cardiomyopathy (Circulation. 2012;126:A18171), said that the BENEFIT results underscore the need for physicians to be bullish in their approach to treating Chagas soon after diagnosis.
“It doesn’t matter if they’re symptomatic or asymptomatic. You can’t wait till they progress to treat. If you wait for the progression of disease you’ve lost the battle. You can’t wait and follow conservatively until you see the complications, because once those complications have started the parasitic load is too high for you to have an impact,” she said.
Dr. Yun said that given the toxicity of current treatment, she hoped to see more studies show clearer evidence of clinical benefit, “either reductions in mortality or reductions in end organ disease.” Most studies “have focused on clearance of parasite, which is important, but it’s not as important decreasing the risk of death or cardiomyopathy or heart failure.”
Rick Tarleton, Ph.D., a biologist the University of Georgia, in Athens, who has worked on Chagas for more than 30 years, said that because Chagas pathology is directly tied to parasite load – and not, as people have suggested in the past, an autoimmune reaction resulting from parasite exposure – drug treatment may prove to be worthwhile even in patients with significant cardiac involvement.
“You get rid of the parasite, you get rid of the progression of the disease,” Dr. Tarleton said. Even the findings from the BENEFIT trial, he said, did not lead him to conclude that treatment in people with established cardiac disease was futile.
“If you’re treating people who are already chronically infected and showing symptoms, the question is not have you reversed the damage, it’s have you stopped accumulating damage,” he noted. “And a 5-year follow-up is probably not long enough to know whether you’ve stopped accumulating.”
“We have drugs, they’re not great, they do have side effects, they don’t always work,” Dr. Tarleton said. “But they’re better than nothing. And they ought to be more widely used.”
Dr. Meymandi said that current supplies of benznidazole at CDC are low and that a dozen patients at her clinic are awaiting treatment. Meanwhile, access may soon be complicated further by the announcement, this month, that KaloBios Pharmaceuticals had bought the rights to seek FDA approval of benznidazole and market it in the United States.
The same company’s CEO came under fire in recent months for acquiring rights to an inexpensive drug to treat toxoplasmosis in AIDS patients, then announcing a price increase from $13.50 to $750 a pill.
“Everyone’s really concerned,” Dr. Meymandi said, “because Chagas is a disease of the poor.”
Chagas disease is a vector-borne parasitic disease, endemic to the Americas, that remains as little recognized by U.S. patients and practitioners as the obscure winged insects that transmit it.
Transmission occurs when triatomine bugs, commonly called “kissing bugs,” pierce the skin to feed and leave behind parasite-infected feces that can enter the bloodstream; pregnant women can also transmit Chagas to their newborns.
About a third of patients infected with Trypanosoma cruzi, the protozoan parasite that causes Chagas, will develop cardiac abnormalities such as cardiomyopathy, arrhythmias, and heart failure – often decades after becoming infected. In the United States, where blood banks began screening for Chagas in 2007, patients without symptoms are likely to learn they are positive only after donating blood.
Conventional wisdom has long maintained that Chagas is limited to Central and South America. But immigration from Chagas-endemic countries, such as El Salvador, Mexico, and Bolivia, means more people are living with the disease in the United States.
“One percent of the Latin American immigrant population we screen [in Los Angeles] has Chagas,” said Dr. Sheba K. Meymandi, cardiologist and director of Center of Excellence for Chagas Disease at Olive View–UCLA Medical Center in Los Angeles, who also works with the city’s health department to detect Chagas. “That’s huge.”
Meanwhile, blood banks are discovering more cases among people without ties to Latin America, and species of kissing bugs native to the southern United States are increasingly recognized as a non-negligible source of Chagas transmission. Of 39 Chagas cases reported to Texas health authorities in 2013 and 2014, 12 were thought to be locally acquired.
Dr. Heather Yun, an infectious disease specialist at the San Antonio Military Medical Center, said risk factors for local transmission are not well established, but “we think people who are living in poverty in substandard housing, people who spend a lot of time outdoors, especially at night, and people involved with direct blood contact with wild game in Southern parts of the United States” may be at higher risk.
A U.S. disease
Evidence is amassing quickly that Chagas is a U.S. disease. But U.S. clinicians still lag in their knowledge of it, say physicians treating Chagas cases. “In medical school we get a 2-hour lecture on it, and it’s always been presented as an exotic disease and one you don’t treat,” Dr. Meymandi said.
The persistent perception of Chagas as a foreign disease means clinicians are inclined to dismiss positive results from a blood screening, particularly from someone who is not from Latin America. Yet cardiologists, ID practitioners, obstetricians, and primary care physicians all need to be aware that cases do occur in the United States and are potentially treatable.
Dr. Laila Woc-Colburn, an infectious disease and tropical medicine specialist at Baylor College of Medicine in Houston, said many people with Chagas never make it to an infectious disease specialist or cardiologist for a work-up. “When you test positive on serology [after a blood donation], you get a letter recommending you consult your physician. Most will go to their primary care doctors, who might say ‘this isn’t a disease in the United States.’ In Houston, that is often the case.”
Dr. Meymandi, who has treated hundreds of patients with Chagas with and without cardiac involvement, said any physician with a potential Chagas case must act. “If you get someone that’s positive, it’s your duty as a physician to confirm the positivity with CDC,” she said.
Dr. Yun concurred. “The most important message is, do something,” she said. “Don’t just assume it’s a false positive.”
Diagnosis is not simple and requires testing beyond the initial ELISA assay used in blood-bank screening. Confirmatory tests must be carried out in coordination with the Centers for Disease Control and Prevention. Also, with no agents approved by the Food and Drug Administration to treat Chagas, treatment is available only through the CDC’s investigational drugs protocol. Both drugs used in Chagas, benznidazole and nifurtimox, come with serious adverse effects that must be closely monitored.
“It’s time consuming, filling out the forms, getting the consent, tracking and sending back lab results to CDC in order to get drugs – it’s not like you can just write a prescription,” Dr. Meymandi said. But, “if you don’t know how to treat the patient or don’t have time, find someone like me,” she noted, adding that she is available to counsel any physician daunted by a potential Chagas case.
Treatment options
No formal clinical algorithm exists for Chagas, but Dr. Meymandi, Dr. Yun, and Dr. Woc-Colburn all pointed to a 2007 JAMA article, which describes diagnosis and treatment protocols, as an important reference for clinicians to start with. It’s “the best approximation of a clinical guideline we have,” Dr. Yun said (JAMA. 2007;298[18]:2171-81. doi:10.1001/jama.298.18.2171).
Dr. Meymandi, who has treated more Chagas patients than has any other U.S. clinician, said that treatment has changed somewhat since the JAMA article was published. In 2007, she said, nifurtimox was the main drug available through CDC, while benznidazole, which is somewhat better tolerated and has shorter treatment duration, has since become the first-line agent.
“We’ve lowered the dose of benznidazole, maxing out at 400 mg/day to decrease the toxicity,” she said. Also, treatment is now being extended to some patients aged 60 years and older.
The decision to treat or not treat, clinicians say, depends on the patient’s age, disease progression, comorbidities and potential serious drug interactions, and willingness to tolerate side effects that, with nifurtimox especially, can include skin sloughing, rash, and psychological and neurologic symptoms including depression and peripheral neuropathy.
“If you don’t have side effects, you’re not taking the drugs,” Dr. Meymandi said. Dr. Woc-Colburn noted that polypharmacy was a major consideration when treating older adults for Chagas. “If I have a patient who has diabetes, obesity, [and] end-stage renal disease, it’s not going to be ideal to give [benznidazole].”
Recent, highly anticipated results from BENEFIT, a large randomized trial (n = 2,854) showed that benznidazole reduced parasite load but was not helpful in halting cardiac damage at 5 years’ follow-up in patients with established Chagas cardiomyopathy (N Engl J Med. 2015 Oct;373:1295-306. doi:10.1056/NEJMoa1507574).
Dr. Meymandi, whose earlier research established that Chagas cardiomyopathy carries significantly higher morbidity and mortality than does non–Chagas cardiomyopathy (Circulation. 2012;126:A18171), said that the BENEFIT results underscore the need for physicians to be bullish in their approach to treating Chagas soon after diagnosis.
“It doesn’t matter if they’re symptomatic or asymptomatic. You can’t wait till they progress to treat. If you wait for the progression of disease you’ve lost the battle. You can’t wait and follow conservatively until you see the complications, because once those complications have started the parasitic load is too high for you to have an impact,” she said.
Dr. Yun said that given the toxicity of current treatment, she hoped to see more studies show clearer evidence of clinical benefit, “either reductions in mortality or reductions in end organ disease.” Most studies “have focused on clearance of parasite, which is important, but it’s not as important decreasing the risk of death or cardiomyopathy or heart failure.”
Rick Tarleton, Ph.D., a biologist the University of Georgia, in Athens, who has worked on Chagas for more than 30 years, said that because Chagas pathology is directly tied to parasite load – and not, as people have suggested in the past, an autoimmune reaction resulting from parasite exposure – drug treatment may prove to be worthwhile even in patients with significant cardiac involvement.
“You get rid of the parasite, you get rid of the progression of the disease,” Dr. Tarleton said. Even the findings from the BENEFIT trial, he said, did not lead him to conclude that treatment in people with established cardiac disease was futile.
“If you’re treating people who are already chronically infected and showing symptoms, the question is not have you reversed the damage, it’s have you stopped accumulating damage,” he noted. “And a 5-year follow-up is probably not long enough to know whether you’ve stopped accumulating.”
“We have drugs, they’re not great, they do have side effects, they don’t always work,” Dr. Tarleton said. “But they’re better than nothing. And they ought to be more widely used.”
Dr. Meymandi said that current supplies of benznidazole at CDC are low and that a dozen patients at her clinic are awaiting treatment. Meanwhile, access may soon be complicated further by the announcement, this month, that KaloBios Pharmaceuticals had bought the rights to seek FDA approval of benznidazole and market it in the United States.
The same company’s CEO came under fire in recent months for acquiring rights to an inexpensive drug to treat toxoplasmosis in AIDS patients, then announcing a price increase from $13.50 to $750 a pill.
“Everyone’s really concerned,” Dr. Meymandi said, “because Chagas is a disease of the poor.”
Chagas disease is a vector-borne parasitic disease, endemic to the Americas, that remains as little recognized by U.S. patients and practitioners as the obscure winged insects that transmit it.
Transmission occurs when triatomine bugs, commonly called “kissing bugs,” pierce the skin to feed and leave behind parasite-infected feces that can enter the bloodstream; pregnant women can also transmit Chagas to their newborns.
About a third of patients infected with Trypanosoma cruzi, the protozoan parasite that causes Chagas, will develop cardiac abnormalities such as cardiomyopathy, arrhythmias, and heart failure – often decades after becoming infected. In the United States, where blood banks began screening for Chagas in 2007, patients without symptoms are likely to learn they are positive only after donating blood.
Conventional wisdom has long maintained that Chagas is limited to Central and South America. But immigration from Chagas-endemic countries, such as El Salvador, Mexico, and Bolivia, means more people are living with the disease in the United States.
“One percent of the Latin American immigrant population we screen [in Los Angeles] has Chagas,” said Dr. Sheba K. Meymandi, cardiologist and director of Center of Excellence for Chagas Disease at Olive View–UCLA Medical Center in Los Angeles, who also works with the city’s health department to detect Chagas. “That’s huge.”
Meanwhile, blood banks are discovering more cases among people without ties to Latin America, and species of kissing bugs native to the southern United States are increasingly recognized as a non-negligible source of Chagas transmission. Of 39 Chagas cases reported to Texas health authorities in 2013 and 2014, 12 were thought to be locally acquired.
Dr. Heather Yun, an infectious disease specialist at the San Antonio Military Medical Center, said risk factors for local transmission are not well established, but “we think people who are living in poverty in substandard housing, people who spend a lot of time outdoors, especially at night, and people involved with direct blood contact with wild game in Southern parts of the United States” may be at higher risk.
A U.S. disease
Evidence is amassing quickly that Chagas is a U.S. disease. But U.S. clinicians still lag in their knowledge of it, say physicians treating Chagas cases. “In medical school we get a 2-hour lecture on it, and it’s always been presented as an exotic disease and one you don’t treat,” Dr. Meymandi said.
The persistent perception of Chagas as a foreign disease means clinicians are inclined to dismiss positive results from a blood screening, particularly from someone who is not from Latin America. Yet cardiologists, ID practitioners, obstetricians, and primary care physicians all need to be aware that cases do occur in the United States and are potentially treatable.
Dr. Laila Woc-Colburn, an infectious disease and tropical medicine specialist at Baylor College of Medicine in Houston, said many people with Chagas never make it to an infectious disease specialist or cardiologist for a work-up. “When you test positive on serology [after a blood donation], you get a letter recommending you consult your physician. Most will go to their primary care doctors, who might say ‘this isn’t a disease in the United States.’ In Houston, that is often the case.”
Dr. Meymandi, who has treated hundreds of patients with Chagas with and without cardiac involvement, said any physician with a potential Chagas case must act. “If you get someone that’s positive, it’s your duty as a physician to confirm the positivity with CDC,” she said.
Dr. Yun concurred. “The most important message is, do something,” she said. “Don’t just assume it’s a false positive.”
Diagnosis is not simple and requires testing beyond the initial ELISA assay used in blood-bank screening. Confirmatory tests must be carried out in coordination with the Centers for Disease Control and Prevention. Also, with no agents approved by the Food and Drug Administration to treat Chagas, treatment is available only through the CDC’s investigational drugs protocol. Both drugs used in Chagas, benznidazole and nifurtimox, come with serious adverse effects that must be closely monitored.
“It’s time consuming, filling out the forms, getting the consent, tracking and sending back lab results to CDC in order to get drugs – it’s not like you can just write a prescription,” Dr. Meymandi said. But, “if you don’t know how to treat the patient or don’t have time, find someone like me,” she noted, adding that she is available to counsel any physician daunted by a potential Chagas case.
Treatment options
No formal clinical algorithm exists for Chagas, but Dr. Meymandi, Dr. Yun, and Dr. Woc-Colburn all pointed to a 2007 JAMA article, which describes diagnosis and treatment protocols, as an important reference for clinicians to start with. It’s “the best approximation of a clinical guideline we have,” Dr. Yun said (JAMA. 2007;298[18]:2171-81. doi:10.1001/jama.298.18.2171).
Dr. Meymandi, who has treated more Chagas patients than has any other U.S. clinician, said that treatment has changed somewhat since the JAMA article was published. In 2007, she said, nifurtimox was the main drug available through CDC, while benznidazole, which is somewhat better tolerated and has shorter treatment duration, has since become the first-line agent.
“We’ve lowered the dose of benznidazole, maxing out at 400 mg/day to decrease the toxicity,” she said. Also, treatment is now being extended to some patients aged 60 years and older.
The decision to treat or not treat, clinicians say, depends on the patient’s age, disease progression, comorbidities and potential serious drug interactions, and willingness to tolerate side effects that, with nifurtimox especially, can include skin sloughing, rash, and psychological and neurologic symptoms including depression and peripheral neuropathy.
“If you don’t have side effects, you’re not taking the drugs,” Dr. Meymandi said. Dr. Woc-Colburn noted that polypharmacy was a major consideration when treating older adults for Chagas. “If I have a patient who has diabetes, obesity, [and] end-stage renal disease, it’s not going to be ideal to give [benznidazole].”
Recent, highly anticipated results from BENEFIT, a large randomized trial (n = 2,854) showed that benznidazole reduced parasite load but was not helpful in halting cardiac damage at 5 years’ follow-up in patients with established Chagas cardiomyopathy (N Engl J Med. 2015 Oct;373:1295-306. doi:10.1056/NEJMoa1507574).
Dr. Meymandi, whose earlier research established that Chagas cardiomyopathy carries significantly higher morbidity and mortality than does non–Chagas cardiomyopathy (Circulation. 2012;126:A18171), said that the BENEFIT results underscore the need for physicians to be bullish in their approach to treating Chagas soon after diagnosis.
“It doesn’t matter if they’re symptomatic or asymptomatic. You can’t wait till they progress to treat. If you wait for the progression of disease you’ve lost the battle. You can’t wait and follow conservatively until you see the complications, because once those complications have started the parasitic load is too high for you to have an impact,” she said.
Dr. Yun said that given the toxicity of current treatment, she hoped to see more studies show clearer evidence of clinical benefit, “either reductions in mortality or reductions in end organ disease.” Most studies “have focused on clearance of parasite, which is important, but it’s not as important decreasing the risk of death or cardiomyopathy or heart failure.”
Rick Tarleton, Ph.D., a biologist the University of Georgia, in Athens, who has worked on Chagas for more than 30 years, said that because Chagas pathology is directly tied to parasite load – and not, as people have suggested in the past, an autoimmune reaction resulting from parasite exposure – drug treatment may prove to be worthwhile even in patients with significant cardiac involvement.
“You get rid of the parasite, you get rid of the progression of the disease,” Dr. Tarleton said. Even the findings from the BENEFIT trial, he said, did not lead him to conclude that treatment in people with established cardiac disease was futile.
“If you’re treating people who are already chronically infected and showing symptoms, the question is not have you reversed the damage, it’s have you stopped accumulating damage,” he noted. “And a 5-year follow-up is probably not long enough to know whether you’ve stopped accumulating.”
“We have drugs, they’re not great, they do have side effects, they don’t always work,” Dr. Tarleton said. “But they’re better than nothing. And they ought to be more widely used.”
Dr. Meymandi said that current supplies of benznidazole at CDC are low and that a dozen patients at her clinic are awaiting treatment. Meanwhile, access may soon be complicated further by the announcement, this month, that KaloBios Pharmaceuticals had bought the rights to seek FDA approval of benznidazole and market it in the United States.
The same company’s CEO came under fire in recent months for acquiring rights to an inexpensive drug to treat toxoplasmosis in AIDS patients, then announcing a price increase from $13.50 to $750 a pill.
“Everyone’s really concerned,” Dr. Meymandi said, “because Chagas is a disease of the poor.”
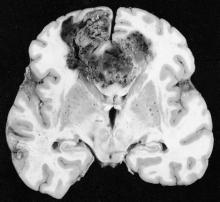
Tumor-treating fields may improve outcomes in glioblastoma
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
FROM JAMA
Key clinical point: Maintenance therapy with tumor-treating fields (TTFields) plus temozolomide resulted in longer survival, compared with temozolomide alone, for patients with glioblastoma.
Major finding: Median progression-free survival for TTFields plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (HR, 0.62; 98.7% CI, 0.43-0.89; P = .001).
Data sources: Interim analysis of the randomized trial included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group.
Disclosures: The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck, and Novartis. Several of his coauthors reported ties to industry.
Predictors for Surgical Management of Small Bowel Obstruction
Clinical question: Are there clinical or computerized tomography (CT) findings that identify which patients will need early surgical management in adhesive small bowel obstruction (ASBO)?
Background: Previous studies determined adverse outcomes resulting from delayed surgery in patients with ASBO: increased length of stay (LOS), complications, and mortality. Most patients respond to nonoperative management, however.
Study design: Prospective observational study.
Setting: Three academic and tertiary referral medical centers.
Synopsis: Using multivariate analysis of 202 patients admitted with presumed adhesive ASBO without immediate surgical need, of whom 52 required eventual surgical intervention, this study found three predictors for needing operative care: no flatus (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.51-7.12; P=0.003), as well as the CT findings of a high-grade obstruction, defined as only minimal passage of air and fluid into the distal small bowel or colon (OR, 2.44; 95% CI, 1.10-5.43; P=0.028) or the presence of free fluid (OR, 2.59; 95% CI, 1.13-5.90; P=0.023).
Despite these associations, clinicians should not view these findings as indications for surgery. Of the patients who responded to nonoperative management, one-third had no flatus, and on CT one-third had high-grade obstruction and half had free fluid. Instead, because patients with these findings are at an increased risk of failing nonoperative management, they should be observed more closely and reassessed more frequently.
Bottom line: Patients without flatus or with the presence of free fluid or high-grade obstruction on CT are at an increased risk of requiring surgical management for ASBO.
Citation: Kulvatunyou N, Pandit V, Moutamn S, et al. A multi-institution prospective observational study of small bowel obstruction: clinical and computerized tomography predictors of which patients may require early surgery. J Trauma Acute Care Surg. 2015;79(3):393-398.
Clinical question: Are there clinical or computerized tomography (CT) findings that identify which patients will need early surgical management in adhesive small bowel obstruction (ASBO)?
Background: Previous studies determined adverse outcomes resulting from delayed surgery in patients with ASBO: increased length of stay (LOS), complications, and mortality. Most patients respond to nonoperative management, however.
Study design: Prospective observational study.
Setting: Three academic and tertiary referral medical centers.
Synopsis: Using multivariate analysis of 202 patients admitted with presumed adhesive ASBO without immediate surgical need, of whom 52 required eventual surgical intervention, this study found three predictors for needing operative care: no flatus (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.51-7.12; P=0.003), as well as the CT findings of a high-grade obstruction, defined as only minimal passage of air and fluid into the distal small bowel or colon (OR, 2.44; 95% CI, 1.10-5.43; P=0.028) or the presence of free fluid (OR, 2.59; 95% CI, 1.13-5.90; P=0.023).
Despite these associations, clinicians should not view these findings as indications for surgery. Of the patients who responded to nonoperative management, one-third had no flatus, and on CT one-third had high-grade obstruction and half had free fluid. Instead, because patients with these findings are at an increased risk of failing nonoperative management, they should be observed more closely and reassessed more frequently.
Bottom line: Patients without flatus or with the presence of free fluid or high-grade obstruction on CT are at an increased risk of requiring surgical management for ASBO.
Citation: Kulvatunyou N, Pandit V, Moutamn S, et al. A multi-institution prospective observational study of small bowel obstruction: clinical and computerized tomography predictors of which patients may require early surgery. J Trauma Acute Care Surg. 2015;79(3):393-398.
Clinical question: Are there clinical or computerized tomography (CT) findings that identify which patients will need early surgical management in adhesive small bowel obstruction (ASBO)?
Background: Previous studies determined adverse outcomes resulting from delayed surgery in patients with ASBO: increased length of stay (LOS), complications, and mortality. Most patients respond to nonoperative management, however.
Study design: Prospective observational study.
Setting: Three academic and tertiary referral medical centers.
Synopsis: Using multivariate analysis of 202 patients admitted with presumed adhesive ASBO without immediate surgical need, of whom 52 required eventual surgical intervention, this study found three predictors for needing operative care: no flatus (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.51-7.12; P=0.003), as well as the CT findings of a high-grade obstruction, defined as only minimal passage of air and fluid into the distal small bowel or colon (OR, 2.44; 95% CI, 1.10-5.43; P=0.028) or the presence of free fluid (OR, 2.59; 95% CI, 1.13-5.90; P=0.023).
Despite these associations, clinicians should not view these findings as indications for surgery. Of the patients who responded to nonoperative management, one-third had no flatus, and on CT one-third had high-grade obstruction and half had free fluid. Instead, because patients with these findings are at an increased risk of failing nonoperative management, they should be observed more closely and reassessed more frequently.
Bottom line: Patients without flatus or with the presence of free fluid or high-grade obstruction on CT are at an increased risk of requiring surgical management for ASBO.
Citation: Kulvatunyou N, Pandit V, Moutamn S, et al. A multi-institution prospective observational study of small bowel obstruction: clinical and computerized tomography predictors of which patients may require early surgery. J Trauma Acute Care Surg. 2015;79(3):393-398.
Adding Advanced Molecular Techniques to Standard Blood Cultures May Improve Patient Outcomes
Clinical question: Does the addition of rapid multiplex polymerase chain reaction molecular techniques to standard blood culture bottle (BCB) processing, with or without antimicrobial stewardship recommendations, affect antimicrobial utilization and patient outcomes?
Background: Standard BCB processing typically requires two days to provide identification and susceptibility testing results. PCR-based molecular testing is available to test positive BCB and deliver specific susceptibility results more rapidly, typically within one hour. Earlier results could improve antimicrobial utilization, limit antimicrobial resistance, decrease the risk of Clostridium difficile colitis, improve patient outcomes, and decrease healthcare costs. The impact of these techniques on outcomes is uncertain.
Study design: Prospective, randomized controlled trial (RCT).
Setting: Single large tertiary academic medical center.
Synopsis: Nearly 750 patients were randomized to conventional BCB processing (control), BCB with rapid multiplex PCR and templated recommendations (rmPCR), or BCB with rapid multiplex PCR and real-time antimicrobial stewardship provided by an infectious disease physician or specially trained pharmacist (rmPCR/AS). Time to microorganism identification was reduced from 22.3 hours in the control arm to 1.3 hours in the intervention arms. Both intervention groups had decreased use of broad spectrum piperacillin-tazobactam, increased use of narrow spectrum β-lactam, and decreased treatment of contaminants. Time to appropriate empiric treatment modification was shortest in the rmPCR/AS group.
Groups did not differ in mortality, length of stay, or cost, although an adequately powered study may show beneficial effects in these outcomes.
Bottom line: The addition of rapid multiplex PCR, ideally combined with antimicrobial stewardship, improves antimicrobial utilization in patients with positive blood cultures.
Citation: Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080.
Clinical question: Does the addition of rapid multiplex polymerase chain reaction molecular techniques to standard blood culture bottle (BCB) processing, with or without antimicrobial stewardship recommendations, affect antimicrobial utilization and patient outcomes?
Background: Standard BCB processing typically requires two days to provide identification and susceptibility testing results. PCR-based molecular testing is available to test positive BCB and deliver specific susceptibility results more rapidly, typically within one hour. Earlier results could improve antimicrobial utilization, limit antimicrobial resistance, decrease the risk of Clostridium difficile colitis, improve patient outcomes, and decrease healthcare costs. The impact of these techniques on outcomes is uncertain.
Study design: Prospective, randomized controlled trial (RCT).
Setting: Single large tertiary academic medical center.
Synopsis: Nearly 750 patients were randomized to conventional BCB processing (control), BCB with rapid multiplex PCR and templated recommendations (rmPCR), or BCB with rapid multiplex PCR and real-time antimicrobial stewardship provided by an infectious disease physician or specially trained pharmacist (rmPCR/AS). Time to microorganism identification was reduced from 22.3 hours in the control arm to 1.3 hours in the intervention arms. Both intervention groups had decreased use of broad spectrum piperacillin-tazobactam, increased use of narrow spectrum β-lactam, and decreased treatment of contaminants. Time to appropriate empiric treatment modification was shortest in the rmPCR/AS group.
Groups did not differ in mortality, length of stay, or cost, although an adequately powered study may show beneficial effects in these outcomes.
Bottom line: The addition of rapid multiplex PCR, ideally combined with antimicrobial stewardship, improves antimicrobial utilization in patients with positive blood cultures.
Citation: Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080.
Clinical question: Does the addition of rapid multiplex polymerase chain reaction molecular techniques to standard blood culture bottle (BCB) processing, with or without antimicrobial stewardship recommendations, affect antimicrobial utilization and patient outcomes?
Background: Standard BCB processing typically requires two days to provide identification and susceptibility testing results. PCR-based molecular testing is available to test positive BCB and deliver specific susceptibility results more rapidly, typically within one hour. Earlier results could improve antimicrobial utilization, limit antimicrobial resistance, decrease the risk of Clostridium difficile colitis, improve patient outcomes, and decrease healthcare costs. The impact of these techniques on outcomes is uncertain.
Study design: Prospective, randomized controlled trial (RCT).
Setting: Single large tertiary academic medical center.
Synopsis: Nearly 750 patients were randomized to conventional BCB processing (control), BCB with rapid multiplex PCR and templated recommendations (rmPCR), or BCB with rapid multiplex PCR and real-time antimicrobial stewardship provided by an infectious disease physician or specially trained pharmacist (rmPCR/AS). Time to microorganism identification was reduced from 22.3 hours in the control arm to 1.3 hours in the intervention arms. Both intervention groups had decreased use of broad spectrum piperacillin-tazobactam, increased use of narrow spectrum β-lactam, and decreased treatment of contaminants. Time to appropriate empiric treatment modification was shortest in the rmPCR/AS group.
Groups did not differ in mortality, length of stay, or cost, although an adequately powered study may show beneficial effects in these outcomes.
Bottom line: The addition of rapid multiplex PCR, ideally combined with antimicrobial stewardship, improves antimicrobial utilization in patients with positive blood cultures.
Citation: Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080.
Osteoarticular pain affects CML patients stopping TKI

Photo courtesy of ASH
ORLANDO, FL—Cases of musculoskeletal pain have been reported after patients stop taking tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML).
TKI discontinuation trials—notably, the STOP imatinib (STIM) trials and EURO-SKI trial—have been conducted to assess the feasibility of maintaining molecular remission once patients discontinue a TKI.
However, none of the studies collected low-grade events before or after patients discontinued TKI therapy.
So investigators collected data from the STIM2 study and EUROSKI trial and recorded all events from the time of TKI discontinuation.
They discovered that about 23% of patients who stopped TKI therapy experienced a withdrawal syndrome (WS) consisting largely of musculoskeletal pain, regardless of the TKI they were taking.
Philippe Rousselot, MD, PhD, of University of Versailles St-Quentin-en-Yvelines, Versailles, France, discussed this finding at the 2015 ASH Annual Meeting as abstract 137.*
Dr Rousselot noted that investigators first reported the TKI WS in 2014 in CML patients enrolled on the EURO-SKI trial who were discontinuing imatinib (Richter et al, JCO 2014).
A team of French investigators undertook the current observational study to estimate the prevalence of the WS and to identify clinical factors associated with it.
They collected, prospectively, the adverse events from all 428 French patients who were enrolled in the STIM2 (n=204) and EURO-SKI (n=224) trials. And they compared patients who stopped taking TKIs and had a painful WS to those who stopped TKIs and did not have a painful syndrome.
Patient characteristics
Patient characteristics were well balanced between the STIM2 and EURO-SKI groups, with the exception of the median time on TKI before discontinuation. In the STIM2 group, patients were a median of 77.4 months on TKI therapy. In the EURO-SKI group, the median time on a TKI was 100.4 months (P<0.001).
In all, there were 208 male and 220 female patients included. They were a median age of 64 (range, 53–73) and 63 (range, 53–70) years in the STIM2 and EURO-SKI groups, respectively.
Sokal scores were also comparable between the cohorts, with most patients falling in the low and intermediate ranges.
Prevalence and characteristics of WS
Overall, 326 patients (76.2%) were without WS and 102 (23.8%) had WS. In the STIM2 cohort, 193 patients (86.2%) were without WS and 31 (13.8%) had WS. In the EURO-SKI cohort, 133 patients (65.2%) were without WS and 71 (34.8%) had WS.
“And these differences [between cohorts] are significant,” Dr Rousselot pointed out.
Investigators analyzed clinical characteristics of WS in 40 patients and determined that the median time from TKI discontinuation to WS was 21 days, and the median duration of WS was 7 months (range, 3–30).
Pain was located in the shoulder and spine for 67% of the patients and elsewhere in 33%. About two-thirds of patients (62.5%) experienced grade 1–2 pain, and 37.5% experienced grade 3–4 pain.
Nineteen patients resumed TKI therapy, “because of loss of MMR [major molecular response] or loss of clinical response,” Dr Rousselot said.
And the pain disappeared in 52.6% of them when they resumed TKI therapy. The median duration of TKI therapy before WS pain disappeared was 3 weeks.
Risk factors for WS
Investigators determined that CML duration, time on a TKI, and previous history of osteoarticular symptoms were risk factors for WS.
Patients without WS had CML for a shorter time—a mean of 8.7 ± 3.1 months, compared to 9.7 ± 3.8 for those with WS (P=0.02).
Patients without WS were also on a TKI for a shorter time—a median of 81.2 months (range, 61.2–108.0), compared to 97.3 months (range, 73.7–122.9) for those with WS (P<0.001).
Patients with a previous history of osteoarticular symptoms were more likely to experience WS—22.9%, compared to 9.8% without a previous history (P=0.002).
Most patients were receiving imatinib—323 without WS and 100 with WS. The 1 patient receiving dasatinib had no WS. And of the 4 patients receiving nilotinib, 2 had WS and 2 didn’t.
And so the type of TKI therapy—dasatinib, imatinib, or nilotinib—was not significant (P=0.42).
Investigators performed a multivariate analysis adjusted for gender, CML duration, and Sokal score, and 2 risk factors emerged: previous history of osteoarticular symptoms (relative risk: 2.08) and time on TKI (relative risk: 2.23).
Discussion
Dr Rousselot compared the Richter trial (Richter et al, JCO 2014) to the current study and noted that the Richter trial, with an enrollment of 50 patients, had a WS prevalence of 30%. But the current trial had a prevalence of 24%.
The difference in WS may be due to time on TKI, Dr Rousselot said, as patients in the Richter trial were on TKI treatment for a longer period of time.
“The time of onset is the same [in both trials],” Dr Rousselot said, as are the TKI used, location of pain, and duration of pain.
“So what we can say is [with] shorter TKI treatment . . . , we have a higher risk of molecular relapse but a lower risk of withdrawal syndrome.”
And with longer TKI treatment, the converse appears to be true. It reduces the risk of molecular relapse but raises the risk of withdrawal syndrome. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of ASH
ORLANDO, FL—Cases of musculoskeletal pain have been reported after patients stop taking tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML).
TKI discontinuation trials—notably, the STOP imatinib (STIM) trials and EURO-SKI trial—have been conducted to assess the feasibility of maintaining molecular remission once patients discontinue a TKI.
However, none of the studies collected low-grade events before or after patients discontinued TKI therapy.
So investigators collected data from the STIM2 study and EUROSKI trial and recorded all events from the time of TKI discontinuation.
They discovered that about 23% of patients who stopped TKI therapy experienced a withdrawal syndrome (WS) consisting largely of musculoskeletal pain, regardless of the TKI they were taking.
Philippe Rousselot, MD, PhD, of University of Versailles St-Quentin-en-Yvelines, Versailles, France, discussed this finding at the 2015 ASH Annual Meeting as abstract 137.*
Dr Rousselot noted that investigators first reported the TKI WS in 2014 in CML patients enrolled on the EURO-SKI trial who were discontinuing imatinib (Richter et al, JCO 2014).
A team of French investigators undertook the current observational study to estimate the prevalence of the WS and to identify clinical factors associated with it.
They collected, prospectively, the adverse events from all 428 French patients who were enrolled in the STIM2 (n=204) and EURO-SKI (n=224) trials. And they compared patients who stopped taking TKIs and had a painful WS to those who stopped TKIs and did not have a painful syndrome.
Patient characteristics
Patient characteristics were well balanced between the STIM2 and EURO-SKI groups, with the exception of the median time on TKI before discontinuation. In the STIM2 group, patients were a median of 77.4 months on TKI therapy. In the EURO-SKI group, the median time on a TKI was 100.4 months (P<0.001).
In all, there were 208 male and 220 female patients included. They were a median age of 64 (range, 53–73) and 63 (range, 53–70) years in the STIM2 and EURO-SKI groups, respectively.
Sokal scores were also comparable between the cohorts, with most patients falling in the low and intermediate ranges.
Prevalence and characteristics of WS
Overall, 326 patients (76.2%) were without WS and 102 (23.8%) had WS. In the STIM2 cohort, 193 patients (86.2%) were without WS and 31 (13.8%) had WS. In the EURO-SKI cohort, 133 patients (65.2%) were without WS and 71 (34.8%) had WS.
“And these differences [between cohorts] are significant,” Dr Rousselot pointed out.
Investigators analyzed clinical characteristics of WS in 40 patients and determined that the median time from TKI discontinuation to WS was 21 days, and the median duration of WS was 7 months (range, 3–30).
Pain was located in the shoulder and spine for 67% of the patients and elsewhere in 33%. About two-thirds of patients (62.5%) experienced grade 1–2 pain, and 37.5% experienced grade 3–4 pain.
Nineteen patients resumed TKI therapy, “because of loss of MMR [major molecular response] or loss of clinical response,” Dr Rousselot said.
And the pain disappeared in 52.6% of them when they resumed TKI therapy. The median duration of TKI therapy before WS pain disappeared was 3 weeks.
Risk factors for WS
Investigators determined that CML duration, time on a TKI, and previous history of osteoarticular symptoms were risk factors for WS.
Patients without WS had CML for a shorter time—a mean of 8.7 ± 3.1 months, compared to 9.7 ± 3.8 for those with WS (P=0.02).
Patients without WS were also on a TKI for a shorter time—a median of 81.2 months (range, 61.2–108.0), compared to 97.3 months (range, 73.7–122.9) for those with WS (P<0.001).
Patients with a previous history of osteoarticular symptoms were more likely to experience WS—22.9%, compared to 9.8% without a previous history (P=0.002).
Most patients were receiving imatinib—323 without WS and 100 with WS. The 1 patient receiving dasatinib had no WS. And of the 4 patients receiving nilotinib, 2 had WS and 2 didn’t.
And so the type of TKI therapy—dasatinib, imatinib, or nilotinib—was not significant (P=0.42).
Investigators performed a multivariate analysis adjusted for gender, CML duration, and Sokal score, and 2 risk factors emerged: previous history of osteoarticular symptoms (relative risk: 2.08) and time on TKI (relative risk: 2.23).
Discussion
Dr Rousselot compared the Richter trial (Richter et al, JCO 2014) to the current study and noted that the Richter trial, with an enrollment of 50 patients, had a WS prevalence of 30%. But the current trial had a prevalence of 24%.
The difference in WS may be due to time on TKI, Dr Rousselot said, as patients in the Richter trial were on TKI treatment for a longer period of time.
“The time of onset is the same [in both trials],” Dr Rousselot said, as are the TKI used, location of pain, and duration of pain.
“So what we can say is [with] shorter TKI treatment . . . , we have a higher risk of molecular relapse but a lower risk of withdrawal syndrome.”
And with longer TKI treatment, the converse appears to be true. It reduces the risk of molecular relapse but raises the risk of withdrawal syndrome. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of ASH
ORLANDO, FL—Cases of musculoskeletal pain have been reported after patients stop taking tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML).
TKI discontinuation trials—notably, the STOP imatinib (STIM) trials and EURO-SKI trial—have been conducted to assess the feasibility of maintaining molecular remission once patients discontinue a TKI.
However, none of the studies collected low-grade events before or after patients discontinued TKI therapy.
So investigators collected data from the STIM2 study and EUROSKI trial and recorded all events from the time of TKI discontinuation.
They discovered that about 23% of patients who stopped TKI therapy experienced a withdrawal syndrome (WS) consisting largely of musculoskeletal pain, regardless of the TKI they were taking.
Philippe Rousselot, MD, PhD, of University of Versailles St-Quentin-en-Yvelines, Versailles, France, discussed this finding at the 2015 ASH Annual Meeting as abstract 137.*
Dr Rousselot noted that investigators first reported the TKI WS in 2014 in CML patients enrolled on the EURO-SKI trial who were discontinuing imatinib (Richter et al, JCO 2014).
A team of French investigators undertook the current observational study to estimate the prevalence of the WS and to identify clinical factors associated with it.
They collected, prospectively, the adverse events from all 428 French patients who were enrolled in the STIM2 (n=204) and EURO-SKI (n=224) trials. And they compared patients who stopped taking TKIs and had a painful WS to those who stopped TKIs and did not have a painful syndrome.
Patient characteristics
Patient characteristics were well balanced between the STIM2 and EURO-SKI groups, with the exception of the median time on TKI before discontinuation. In the STIM2 group, patients were a median of 77.4 months on TKI therapy. In the EURO-SKI group, the median time on a TKI was 100.4 months (P<0.001).
In all, there were 208 male and 220 female patients included. They were a median age of 64 (range, 53–73) and 63 (range, 53–70) years in the STIM2 and EURO-SKI groups, respectively.
Sokal scores were also comparable between the cohorts, with most patients falling in the low and intermediate ranges.
Prevalence and characteristics of WS
Overall, 326 patients (76.2%) were without WS and 102 (23.8%) had WS. In the STIM2 cohort, 193 patients (86.2%) were without WS and 31 (13.8%) had WS. In the EURO-SKI cohort, 133 patients (65.2%) were without WS and 71 (34.8%) had WS.
“And these differences [between cohorts] are significant,” Dr Rousselot pointed out.
Investigators analyzed clinical characteristics of WS in 40 patients and determined that the median time from TKI discontinuation to WS was 21 days, and the median duration of WS was 7 months (range, 3–30).
Pain was located in the shoulder and spine for 67% of the patients and elsewhere in 33%. About two-thirds of patients (62.5%) experienced grade 1–2 pain, and 37.5% experienced grade 3–4 pain.
Nineteen patients resumed TKI therapy, “because of loss of MMR [major molecular response] or loss of clinical response,” Dr Rousselot said.
And the pain disappeared in 52.6% of them when they resumed TKI therapy. The median duration of TKI therapy before WS pain disappeared was 3 weeks.
Risk factors for WS
Investigators determined that CML duration, time on a TKI, and previous history of osteoarticular symptoms were risk factors for WS.
Patients without WS had CML for a shorter time—a mean of 8.7 ± 3.1 months, compared to 9.7 ± 3.8 for those with WS (P=0.02).
Patients without WS were also on a TKI for a shorter time—a median of 81.2 months (range, 61.2–108.0), compared to 97.3 months (range, 73.7–122.9) for those with WS (P<0.001).
Patients with a previous history of osteoarticular symptoms were more likely to experience WS—22.9%, compared to 9.8% without a previous history (P=0.002).
Most patients were receiving imatinib—323 without WS and 100 with WS. The 1 patient receiving dasatinib had no WS. And of the 4 patients receiving nilotinib, 2 had WS and 2 didn’t.
And so the type of TKI therapy—dasatinib, imatinib, or nilotinib—was not significant (P=0.42).
Investigators performed a multivariate analysis adjusted for gender, CML duration, and Sokal score, and 2 risk factors emerged: previous history of osteoarticular symptoms (relative risk: 2.08) and time on TKI (relative risk: 2.23).
Discussion
Dr Rousselot compared the Richter trial (Richter et al, JCO 2014) to the current study and noted that the Richter trial, with an enrollment of 50 patients, had a WS prevalence of 30%. But the current trial had a prevalence of 24%.
The difference in WS may be due to time on TKI, Dr Rousselot said, as patients in the Richter trial were on TKI treatment for a longer period of time.
“The time of onset is the same [in both trials],” Dr Rousselot said, as are the TKI used, location of pain, and duration of pain.
“So what we can say is [with] shorter TKI treatment . . . , we have a higher risk of molecular relapse but a lower risk of withdrawal syndrome.”
And with longer TKI treatment, the converse appears to be true. It reduces the risk of molecular relapse but raises the risk of withdrawal syndrome. ![]()
*Data in the abstract differ from the presentation.
Fear really does curdle blood, study suggests

Photo by Graham Colm
Horror movies may actually be “blood-curdling,” according to a study published in The BMJ.
The research showed that watching a scary movie was associated with an increase in the clotting protein factor VIII (FVIII).
Study subjects were more likely to experience an increase in FVIII while watching a horror film than an educational film.
So using the term “blood-curdling” to describe feeling extreme fear may be justified, researchers said.
The term dates back to medieval times and is based on the concept that fear would “run the blood cold” or “curdle” the blood, but the validity of this theory has never been studied.
So Banne Nemeth, MD, of Leiden University Medical Centre in The Netherlands, and his colleagues set out to assess whether acute fear can curdle blood.
Their study involved 24 healthy volunteers age 30 or younger. Fourteen subjects were assigned to watch a frightening movie followed by a non-threatening educational movie, and 10 were assigned to watch the movies in reverse order.
The movies were viewed more than a week apart at the same time of day and in a comfortable and relaxed environment. Both lasted approximately 90 minutes.
Before and after each movie (within 15 minutes), blood samples were taken and analyzed for markers of clotting activity.
After each movie, participants rated the fear they experienced using a visual analogue fear scale ranging from 0 (no fear at all) to 10 (worst fear imaginable). They also reported whether they had already seen the movie and completed a general questionnaire on lifestyle and favorite movie genre.
The horror movie was perceived to be more frightening than the educational movie, with a 5.4 mean difference in fear rating scores.
The difference in FVIII levels before and after watching the movies was higher for the horror movie than for the educational movie. The mean difference of differences was 11.1 IU/dL (95% confidence interval: 1.2 to 21.0 IU/dL).
FVIII levels increased in 12 (57%) subjects during the horror movie and in 3 (14%) during the educational movie. FVIII levels decreased in 18 (86%) participants during the educational movie and in 9 (43%) during the horror movie.
However, the researchers found no effect of either movie on thrombin-antithrombin complexes, D-dimer, or prothrombin fragments 1+2. They said this suggests that although acute fear may trigger coagulation, it does not lead to actual clot formation.
The team pointed out some study limitations but concluded that, in young and healthy adults, “watching blood-curdling movies is associated with an increase in blood coagulant factor VIII without actual thrombin formation.”
The researchers believe this phenomenon may confer an important evolutionary benefit by preparing the body for blood loss during life-threatening situations. ![]()

Photo by Graham Colm
Horror movies may actually be “blood-curdling,” according to a study published in The BMJ.
The research showed that watching a scary movie was associated with an increase in the clotting protein factor VIII (FVIII).
Study subjects were more likely to experience an increase in FVIII while watching a horror film than an educational film.
So using the term “blood-curdling” to describe feeling extreme fear may be justified, researchers said.
The term dates back to medieval times and is based on the concept that fear would “run the blood cold” or “curdle” the blood, but the validity of this theory has never been studied.
So Banne Nemeth, MD, of Leiden University Medical Centre in The Netherlands, and his colleagues set out to assess whether acute fear can curdle blood.
Their study involved 24 healthy volunteers age 30 or younger. Fourteen subjects were assigned to watch a frightening movie followed by a non-threatening educational movie, and 10 were assigned to watch the movies in reverse order.
The movies were viewed more than a week apart at the same time of day and in a comfortable and relaxed environment. Both lasted approximately 90 minutes.
Before and after each movie (within 15 minutes), blood samples were taken and analyzed for markers of clotting activity.
After each movie, participants rated the fear they experienced using a visual analogue fear scale ranging from 0 (no fear at all) to 10 (worst fear imaginable). They also reported whether they had already seen the movie and completed a general questionnaire on lifestyle and favorite movie genre.
The horror movie was perceived to be more frightening than the educational movie, with a 5.4 mean difference in fear rating scores.
The difference in FVIII levels before and after watching the movies was higher for the horror movie than for the educational movie. The mean difference of differences was 11.1 IU/dL (95% confidence interval: 1.2 to 21.0 IU/dL).
FVIII levels increased in 12 (57%) subjects during the horror movie and in 3 (14%) during the educational movie. FVIII levels decreased in 18 (86%) participants during the educational movie and in 9 (43%) during the horror movie.
However, the researchers found no effect of either movie on thrombin-antithrombin complexes, D-dimer, or prothrombin fragments 1+2. They said this suggests that although acute fear may trigger coagulation, it does not lead to actual clot formation.
The team pointed out some study limitations but concluded that, in young and healthy adults, “watching blood-curdling movies is associated with an increase in blood coagulant factor VIII without actual thrombin formation.”
The researchers believe this phenomenon may confer an important evolutionary benefit by preparing the body for blood loss during life-threatening situations. ![]()

Photo by Graham Colm
Horror movies may actually be “blood-curdling,” according to a study published in The BMJ.
The research showed that watching a scary movie was associated with an increase in the clotting protein factor VIII (FVIII).
Study subjects were more likely to experience an increase in FVIII while watching a horror film than an educational film.
So using the term “blood-curdling” to describe feeling extreme fear may be justified, researchers said.
The term dates back to medieval times and is based on the concept that fear would “run the blood cold” or “curdle” the blood, but the validity of this theory has never been studied.
So Banne Nemeth, MD, of Leiden University Medical Centre in The Netherlands, and his colleagues set out to assess whether acute fear can curdle blood.
Their study involved 24 healthy volunteers age 30 or younger. Fourteen subjects were assigned to watch a frightening movie followed by a non-threatening educational movie, and 10 were assigned to watch the movies in reverse order.
The movies were viewed more than a week apart at the same time of day and in a comfortable and relaxed environment. Both lasted approximately 90 minutes.
Before and after each movie (within 15 minutes), blood samples were taken and analyzed for markers of clotting activity.
After each movie, participants rated the fear they experienced using a visual analogue fear scale ranging from 0 (no fear at all) to 10 (worst fear imaginable). They also reported whether they had already seen the movie and completed a general questionnaire on lifestyle and favorite movie genre.
The horror movie was perceived to be more frightening than the educational movie, with a 5.4 mean difference in fear rating scores.
The difference in FVIII levels before and after watching the movies was higher for the horror movie than for the educational movie. The mean difference of differences was 11.1 IU/dL (95% confidence interval: 1.2 to 21.0 IU/dL).
FVIII levels increased in 12 (57%) subjects during the horror movie and in 3 (14%) during the educational movie. FVIII levels decreased in 18 (86%) participants during the educational movie and in 9 (43%) during the horror movie.
However, the researchers found no effect of either movie on thrombin-antithrombin complexes, D-dimer, or prothrombin fragments 1+2. They said this suggests that although acute fear may trigger coagulation, it does not lead to actual clot formation.
The team pointed out some study limitations but concluded that, in young and healthy adults, “watching blood-curdling movies is associated with an increase in blood coagulant factor VIII without actual thrombin formation.”
The researchers believe this phenomenon may confer an important evolutionary benefit by preparing the body for blood loss during life-threatening situations. ![]()
A potential target for IDH1-mutant cancers

acute myeloid leukemia
Researchers say they have identified a potential therapeutic target for IDH1-mutant malignancies.
Preclinical experiments showed that tumors characterized by IDH1 mutations are “extremely vulnerable” to depletion of NAD+, a metabolic cofactor.
“Our finding . . . supports the proposal that medications that can decrease levels of this metabolite, which are in development, have the potential to specifically treat these cancers,” said Daniel Cahill, MD, PhD, of Massachusetts General Hospital in Boston.
Dr Cahill and his colleagues described their research in Cancer Cell.
IDH1 and IDH2 are known to play essential roles in cellular metabolism, including the processes by which cells convert glucose and other nutrients into ATP, providing the energy needed for cellular survival.
In 2008, researchers discovered that IDH1 mutations are involved in several cancers. The gene is mutated in around 20% of adult gliomas, in more than 10% of acute myeloid leukemias, and in a smaller percentage of other cancers.
One clear result of IDH1 mutation is a 100-fold elevation in levels of the metabolite 2-HG, which is known to mediate several properties that lead to tumor development. Drugs that decrease levels of 2-HG are currently under development.
Dr Cahill and his colleagues wanted to find additional ways of blocking the mutation’s effects, so they inhibited mutant IDH1 in tumor cells. They were surprised to find that this reduced 2-HG levels, but, in many cases, that reduction did not halt cell growth.
Detailed metabolic profiling of IDH1-mutant cells revealed that inhibiting the mutated enzyme greatly increased levels of NAD+, a cofactor that plays a role in several cellular energy processes.
Additional experiments showed that depletion of NAD+ induced the death of IDH1-mutant tumor cells and inhibited tumor growth in an animal model of glioma.
“Accumulation of excess 2-HG is known to drive several changes leading to tumor development, but our results indicate that simply depleting 2-HG levels was not sufficient to halt the growth of several types of later-stage IDH1-mutant tumors,” said Hiroaki Wakimoto, MD, PhD, of Massachusetts General Hospital.
“In addition, we found that mutant IDH1 reduces expression of an enzyme that maintains NAD+ levels, rendering IDH1-mutant tumor cells highly sensitive to direct NAD+ depletion. Several drugs that inhibit the synthesis of NAD+ are already in clinical trials, and these agents may prove useful for patients with IDH-mutant cancers. While we primarily focused on IDH1-mutant gliomas, we also found evidence that NAD+ inhibition could slow the growth of other types of cancer with this mutation.” ![]()

acute myeloid leukemia
Researchers say they have identified a potential therapeutic target for IDH1-mutant malignancies.
Preclinical experiments showed that tumors characterized by IDH1 mutations are “extremely vulnerable” to depletion of NAD+, a metabolic cofactor.
“Our finding . . . supports the proposal that medications that can decrease levels of this metabolite, which are in development, have the potential to specifically treat these cancers,” said Daniel Cahill, MD, PhD, of Massachusetts General Hospital in Boston.
Dr Cahill and his colleagues described their research in Cancer Cell.
IDH1 and IDH2 are known to play essential roles in cellular metabolism, including the processes by which cells convert glucose and other nutrients into ATP, providing the energy needed for cellular survival.
In 2008, researchers discovered that IDH1 mutations are involved in several cancers. The gene is mutated in around 20% of adult gliomas, in more than 10% of acute myeloid leukemias, and in a smaller percentage of other cancers.
One clear result of IDH1 mutation is a 100-fold elevation in levels of the metabolite 2-HG, which is known to mediate several properties that lead to tumor development. Drugs that decrease levels of 2-HG are currently under development.
Dr Cahill and his colleagues wanted to find additional ways of blocking the mutation’s effects, so they inhibited mutant IDH1 in tumor cells. They were surprised to find that this reduced 2-HG levels, but, in many cases, that reduction did not halt cell growth.
Detailed metabolic profiling of IDH1-mutant cells revealed that inhibiting the mutated enzyme greatly increased levels of NAD+, a cofactor that plays a role in several cellular energy processes.
Additional experiments showed that depletion of NAD+ induced the death of IDH1-mutant tumor cells and inhibited tumor growth in an animal model of glioma.
“Accumulation of excess 2-HG is known to drive several changes leading to tumor development, but our results indicate that simply depleting 2-HG levels was not sufficient to halt the growth of several types of later-stage IDH1-mutant tumors,” said Hiroaki Wakimoto, MD, PhD, of Massachusetts General Hospital.
“In addition, we found that mutant IDH1 reduces expression of an enzyme that maintains NAD+ levels, rendering IDH1-mutant tumor cells highly sensitive to direct NAD+ depletion. Several drugs that inhibit the synthesis of NAD+ are already in clinical trials, and these agents may prove useful for patients with IDH-mutant cancers. While we primarily focused on IDH1-mutant gliomas, we also found evidence that NAD+ inhibition could slow the growth of other types of cancer with this mutation.” ![]()

acute myeloid leukemia
Researchers say they have identified a potential therapeutic target for IDH1-mutant malignancies.
Preclinical experiments showed that tumors characterized by IDH1 mutations are “extremely vulnerable” to depletion of NAD+, a metabolic cofactor.
“Our finding . . . supports the proposal that medications that can decrease levels of this metabolite, which are in development, have the potential to specifically treat these cancers,” said Daniel Cahill, MD, PhD, of Massachusetts General Hospital in Boston.
Dr Cahill and his colleagues described their research in Cancer Cell.
IDH1 and IDH2 are known to play essential roles in cellular metabolism, including the processes by which cells convert glucose and other nutrients into ATP, providing the energy needed for cellular survival.
In 2008, researchers discovered that IDH1 mutations are involved in several cancers. The gene is mutated in around 20% of adult gliomas, in more than 10% of acute myeloid leukemias, and in a smaller percentage of other cancers.
One clear result of IDH1 mutation is a 100-fold elevation in levels of the metabolite 2-HG, which is known to mediate several properties that lead to tumor development. Drugs that decrease levels of 2-HG are currently under development.
Dr Cahill and his colleagues wanted to find additional ways of blocking the mutation’s effects, so they inhibited mutant IDH1 in tumor cells. They were surprised to find that this reduced 2-HG levels, but, in many cases, that reduction did not halt cell growth.
Detailed metabolic profiling of IDH1-mutant cells revealed that inhibiting the mutated enzyme greatly increased levels of NAD+, a cofactor that plays a role in several cellular energy processes.
Additional experiments showed that depletion of NAD+ induced the death of IDH1-mutant tumor cells and inhibited tumor growth in an animal model of glioma.
“Accumulation of excess 2-HG is known to drive several changes leading to tumor development, but our results indicate that simply depleting 2-HG levels was not sufficient to halt the growth of several types of later-stage IDH1-mutant tumors,” said Hiroaki Wakimoto, MD, PhD, of Massachusetts General Hospital.
“In addition, we found that mutant IDH1 reduces expression of an enzyme that maintains NAD+ levels, rendering IDH1-mutant tumor cells highly sensitive to direct NAD+ depletion. Several drugs that inhibit the synthesis of NAD+ are already in clinical trials, and these agents may prove useful for patients with IDH-mutant cancers. While we primarily focused on IDH1-mutant gliomas, we also found evidence that NAD+ inhibition could slow the growth of other types of cancer with this mutation.” ![]()