User login
What’s Eating You? Ant-Induced Alopecia (Pheidole)
Case Report
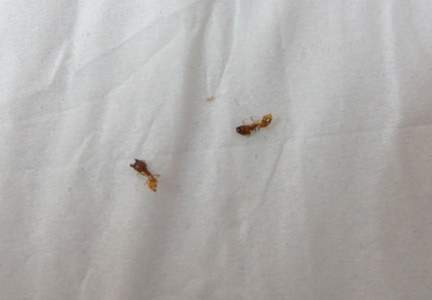
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Practice Points
- Ant-induced alopecia should be considered in the differential diagnosis for patients from endemic regions (eg, Iran, Turkey) with new-onset localized hair loss or in patients recently visiting those areas with a concordant history.
- Ant-induced alopecia is thought to result from mechanical and/or chemical breakage, most commonly caused by Pheidole ants, leaving follicles intact and allowing for hair regrowth without treatment through the normal hair cycle.
Complex picture emerges of prescription opioid abuse
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
FROM JAMA
Key clinical point: The percentage of nonmedical use of prescription opioids declined during the last decade, but the prevalence of use disorders, the frequency of abuse, and related mortality all increased.
Major finding: The 1-year prevalence of opioid use disorders rose from 0.6% to 0.9%, that of high-frequency use increased from 0.3% to 0.4%, and that of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000.
Data source: An analysis of time trends in prescription opioid use, based on two nationally representative data sets involving 472,200 adults.
Disclosures: The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
Spironolactone for Adult Female Acne
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
Cancer survivors have poor diets, study suggests

New research suggests that cancer survivors in the US may need dietary interventions to improve their health.
The study showed that, overall, cancer survivors did not adhere to federal dietary guidelines as well as a matched control population.
Cancer survivors tended to consume more empty calories and less fiber than controls.
Fang Fang Zhang, MD, PhD, of Tufts University in Boston, Massachusetts, and his colleagues reported these findings in Cancer.
The team evaluated the diets of 1533 adult cancer survivors who participated in the National Health and Nutrition Examination Survey from 1999 to 2010. The investigators also assessed the diets of 3075 individuals who had no history of cancer and were matched to the cancer survivors by age, sex, and race/ethnicity.
The goal was to determine how subjects’ diets aligned with advice from the 2010 Dietary Guidelines for Americans, which was jointly issued by the Department of Agriculture and the Department of Health and Human Services.
Cancer survivors had poor adherence to the guidelines, with a total Healthy Eating Index score of 47.2 out of 100, compared with a score of 48.3 in the adults without a history of cancer (P=0.03).
Cancer survivors had a significantly lower mean score for empty calories compared to the noncancer group—13.6 and 14.4, respectively (P=0.001). This suggested the cancer group had a higher consumption of calories from solid fats, alcohol, and added sugars.
Cancer survivors also had significantly lower dietary intake of fiber than the noncancer group—15.0 and 15.9 g per day, respectively (P=0.02).
Compared to recommended values, cancer survivors had low dietary intakes of vitamin D (31% of the recommended intake), vitamin E (47%), potassium (55%), and calcium (73%) but high intakes of saturated fat (112%) and sodium (133%).
The investigators noted that diet quality in cancer survivors increased linearly with age. The older the age, the better the diet quality.
Survivors with lower education (high school or less) had significantly worse diet quality than those with higher education. And survivors who were current smokers had significantly worse diet quality than non-smokers or former smokers.
For the 4 major cancer types in the US (breast, prostate, lung, and colorectal), breast cancer survivors had the best diet quality, and lung cancer survivors had the worst diet quality.
The investigators said that knowing how well cancer survivors adhere to federal dietary guidelines can help inform evidence-based priorities for improving their nutritional intake.
“Dietary changes that include more fiber, fruit, and vegetables in the diet and less fat, sodium, and added sugar would be important for cancer survivors,” Dr Zhang said.
“Oncology care providers can play critical roles in reinforcing the importance of a healthful diet and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors’ overall health.” ![]()

New research suggests that cancer survivors in the US may need dietary interventions to improve their health.
The study showed that, overall, cancer survivors did not adhere to federal dietary guidelines as well as a matched control population.
Cancer survivors tended to consume more empty calories and less fiber than controls.
Fang Fang Zhang, MD, PhD, of Tufts University in Boston, Massachusetts, and his colleagues reported these findings in Cancer.
The team evaluated the diets of 1533 adult cancer survivors who participated in the National Health and Nutrition Examination Survey from 1999 to 2010. The investigators also assessed the diets of 3075 individuals who had no history of cancer and were matched to the cancer survivors by age, sex, and race/ethnicity.
The goal was to determine how subjects’ diets aligned with advice from the 2010 Dietary Guidelines for Americans, which was jointly issued by the Department of Agriculture and the Department of Health and Human Services.
Cancer survivors had poor adherence to the guidelines, with a total Healthy Eating Index score of 47.2 out of 100, compared with a score of 48.3 in the adults without a history of cancer (P=0.03).
Cancer survivors had a significantly lower mean score for empty calories compared to the noncancer group—13.6 and 14.4, respectively (P=0.001). This suggested the cancer group had a higher consumption of calories from solid fats, alcohol, and added sugars.
Cancer survivors also had significantly lower dietary intake of fiber than the noncancer group—15.0 and 15.9 g per day, respectively (P=0.02).
Compared to recommended values, cancer survivors had low dietary intakes of vitamin D (31% of the recommended intake), vitamin E (47%), potassium (55%), and calcium (73%) but high intakes of saturated fat (112%) and sodium (133%).
The investigators noted that diet quality in cancer survivors increased linearly with age. The older the age, the better the diet quality.
Survivors with lower education (high school or less) had significantly worse diet quality than those with higher education. And survivors who were current smokers had significantly worse diet quality than non-smokers or former smokers.
For the 4 major cancer types in the US (breast, prostate, lung, and colorectal), breast cancer survivors had the best diet quality, and lung cancer survivors had the worst diet quality.
The investigators said that knowing how well cancer survivors adhere to federal dietary guidelines can help inform evidence-based priorities for improving their nutritional intake.
“Dietary changes that include more fiber, fruit, and vegetables in the diet and less fat, sodium, and added sugar would be important for cancer survivors,” Dr Zhang said.
“Oncology care providers can play critical roles in reinforcing the importance of a healthful diet and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors’ overall health.” ![]()

New research suggests that cancer survivors in the US may need dietary interventions to improve their health.
The study showed that, overall, cancer survivors did not adhere to federal dietary guidelines as well as a matched control population.
Cancer survivors tended to consume more empty calories and less fiber than controls.
Fang Fang Zhang, MD, PhD, of Tufts University in Boston, Massachusetts, and his colleagues reported these findings in Cancer.
The team evaluated the diets of 1533 adult cancer survivors who participated in the National Health and Nutrition Examination Survey from 1999 to 2010. The investigators also assessed the diets of 3075 individuals who had no history of cancer and were matched to the cancer survivors by age, sex, and race/ethnicity.
The goal was to determine how subjects’ diets aligned with advice from the 2010 Dietary Guidelines for Americans, which was jointly issued by the Department of Agriculture and the Department of Health and Human Services.
Cancer survivors had poor adherence to the guidelines, with a total Healthy Eating Index score of 47.2 out of 100, compared with a score of 48.3 in the adults without a history of cancer (P=0.03).
Cancer survivors had a significantly lower mean score for empty calories compared to the noncancer group—13.6 and 14.4, respectively (P=0.001). This suggested the cancer group had a higher consumption of calories from solid fats, alcohol, and added sugars.
Cancer survivors also had significantly lower dietary intake of fiber than the noncancer group—15.0 and 15.9 g per day, respectively (P=0.02).
Compared to recommended values, cancer survivors had low dietary intakes of vitamin D (31% of the recommended intake), vitamin E (47%), potassium (55%), and calcium (73%) but high intakes of saturated fat (112%) and sodium (133%).
The investigators noted that diet quality in cancer survivors increased linearly with age. The older the age, the better the diet quality.
Survivors with lower education (high school or less) had significantly worse diet quality than those with higher education. And survivors who were current smokers had significantly worse diet quality than non-smokers or former smokers.
For the 4 major cancer types in the US (breast, prostate, lung, and colorectal), breast cancer survivors had the best diet quality, and lung cancer survivors had the worst diet quality.
The investigators said that knowing how well cancer survivors adhere to federal dietary guidelines can help inform evidence-based priorities for improving their nutritional intake.
“Dietary changes that include more fiber, fruit, and vegetables in the diet and less fat, sodium, and added sugar would be important for cancer survivors,” Dr Zhang said.
“Oncology care providers can play critical roles in reinforcing the importance of a healthful diet and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors’ overall health.” ![]()
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 1: Overview, Clinical Characteristics, and Laboratory Evaluation
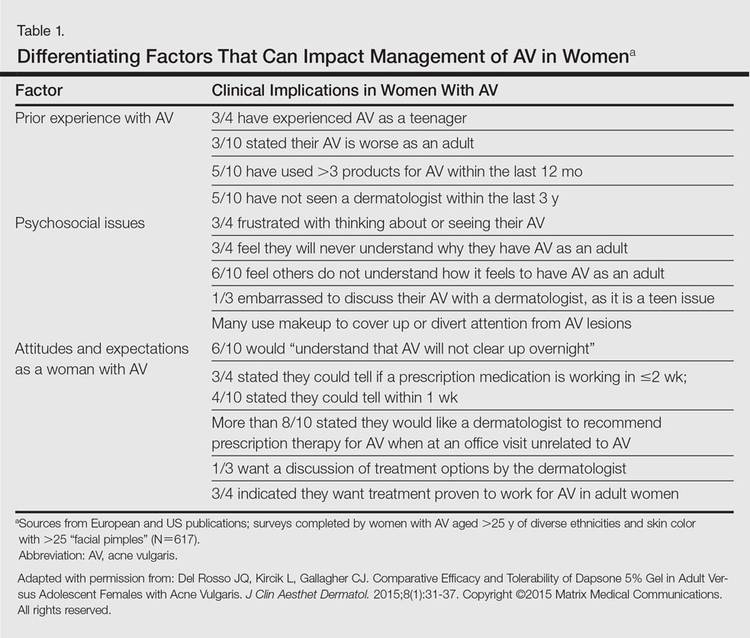
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
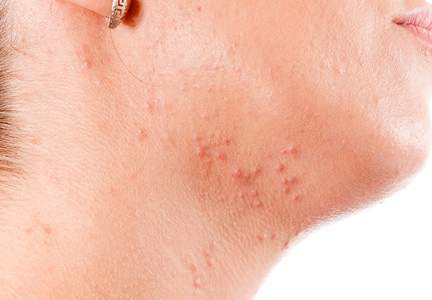
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
Practice Points
- Acne in adult women is common and may persist beyond the adolescent years or may be late in onset with emergence usually during the early to mid-20s.
- Adult women with acne often are frustrated, as they perceive it as a disorder of teenagers and are perplexed by its presence later in life. They often are distressed by unpredictable flares as well as difficulty with covering lesions and associated dyschromia and scarring.
- Clinical patterns of acne in adult women are mixed inflammatory and comedonal facial acne or a U-shaped pattern of inflammatory lesions involving the lower face and neck.
- Laboratory testing is not considered mandatory in all cases. The clinician is encouraged to carefully evaluate each case and determine if further evaluation to detect a cause of androgen excess is warranted.
Tracking system helps reduce blood use

Photo by Juan D. Alfonso
CHICAGO—Researchers say an electronic tracking system has enabled a group of hospitals to significantly reduce the amount of blood transfused after operations.
This system also cut costs by an estimated $2.5 million over 2 years and contributed to lower infection rates without harming patients.
These results were presented at the 2015 Clinical Congress of the American College of Surgeons and published in the Journal of the American College of Surgeons.
In 2012, Intermountain Healthcare implemented the blood ordering and tracking system, along with a program to educate hospital staff, in 22 hospitals across Utah. This includes trauma centers, small rural hospitals, and large community medical centers.
Intermountain employs approximately 1200 physicians and 550 advanced practice clinicians, and another 3000 to 4000 independent physicians have privileges at Intermountain hospitals.
Before Intermountain implemented its blood tracking system, general surgeons, orthopedic surgeons, and urologists each used different hematocrit levels to order blood.
Now, Intermountain uses a consistent threshold across all disciplines—less than 23%. However, physicians can still order blood for patients with hematocrit above that threshold when they feel it is medically necessary.
Results
In 2011, 6% of all patients at Intermountain facilities received blood. Today, only 4% do, according to study author Mark J. Ott, MD, chief medical director of Intermountain Healthcare’s central region.
“So a third of our patients didn’t get blood who used to,” Dr Ott said. “That’s a giant change. That’s tens of thousands of units of blood a year that didn’t get used.”
Before the program started (January 1, 2012), Intermountain facilities transfused almost 50 units of packed red blood cells per 1000 patient days. By January 31, 2015, that rate had declined to about 35.5 units, a reduction of around 30%.
Over the same time period, the percentage of patients transfused with a hematocrit of 23% or greater decreased from 60% to 34%.
The researchers said these reductions in blood use reduced costs by about $2.5 million over the 2-year period, assuming each unit of packed red blood cells costs $300.
In addition, the rate of hospital-acquired infections for both the general hospital population and patients who received blood declined significantly over the 2-year period.
The overall infection rate fell from 1.66 to 0.81 per 1000 patient days. Among patients who received blood, infection rates declined around 33%.
Dr Ott noted that the reduction in infections was also impacted by other initiatives within the health system aimed at reducing surgical site infections and ambulating patients earlier after operations.
“So I cannot tell you that those decreases in hospital-acquired infections are solely due to patients receiving less blood, but it’s part of the picture,” he said. “And we did not see worse outcomes in patients.” ![]()

Photo by Juan D. Alfonso
CHICAGO—Researchers say an electronic tracking system has enabled a group of hospitals to significantly reduce the amount of blood transfused after operations.
This system also cut costs by an estimated $2.5 million over 2 years and contributed to lower infection rates without harming patients.
These results were presented at the 2015 Clinical Congress of the American College of Surgeons and published in the Journal of the American College of Surgeons.
In 2012, Intermountain Healthcare implemented the blood ordering and tracking system, along with a program to educate hospital staff, in 22 hospitals across Utah. This includes trauma centers, small rural hospitals, and large community medical centers.
Intermountain employs approximately 1200 physicians and 550 advanced practice clinicians, and another 3000 to 4000 independent physicians have privileges at Intermountain hospitals.
Before Intermountain implemented its blood tracking system, general surgeons, orthopedic surgeons, and urologists each used different hematocrit levels to order blood.
Now, Intermountain uses a consistent threshold across all disciplines—less than 23%. However, physicians can still order blood for patients with hematocrit above that threshold when they feel it is medically necessary.
Results
In 2011, 6% of all patients at Intermountain facilities received blood. Today, only 4% do, according to study author Mark J. Ott, MD, chief medical director of Intermountain Healthcare’s central region.
“So a third of our patients didn’t get blood who used to,” Dr Ott said. “That’s a giant change. That’s tens of thousands of units of blood a year that didn’t get used.”
Before the program started (January 1, 2012), Intermountain facilities transfused almost 50 units of packed red blood cells per 1000 patient days. By January 31, 2015, that rate had declined to about 35.5 units, a reduction of around 30%.
Over the same time period, the percentage of patients transfused with a hematocrit of 23% or greater decreased from 60% to 34%.
The researchers said these reductions in blood use reduced costs by about $2.5 million over the 2-year period, assuming each unit of packed red blood cells costs $300.
In addition, the rate of hospital-acquired infections for both the general hospital population and patients who received blood declined significantly over the 2-year period.
The overall infection rate fell from 1.66 to 0.81 per 1000 patient days. Among patients who received blood, infection rates declined around 33%.
Dr Ott noted that the reduction in infections was also impacted by other initiatives within the health system aimed at reducing surgical site infections and ambulating patients earlier after operations.
“So I cannot tell you that those decreases in hospital-acquired infections are solely due to patients receiving less blood, but it’s part of the picture,” he said. “And we did not see worse outcomes in patients.” ![]()

Photo by Juan D. Alfonso
CHICAGO—Researchers say an electronic tracking system has enabled a group of hospitals to significantly reduce the amount of blood transfused after operations.
This system also cut costs by an estimated $2.5 million over 2 years and contributed to lower infection rates without harming patients.
These results were presented at the 2015 Clinical Congress of the American College of Surgeons and published in the Journal of the American College of Surgeons.
In 2012, Intermountain Healthcare implemented the blood ordering and tracking system, along with a program to educate hospital staff, in 22 hospitals across Utah. This includes trauma centers, small rural hospitals, and large community medical centers.
Intermountain employs approximately 1200 physicians and 550 advanced practice clinicians, and another 3000 to 4000 independent physicians have privileges at Intermountain hospitals.
Before Intermountain implemented its blood tracking system, general surgeons, orthopedic surgeons, and urologists each used different hematocrit levels to order blood.
Now, Intermountain uses a consistent threshold across all disciplines—less than 23%. However, physicians can still order blood for patients with hematocrit above that threshold when they feel it is medically necessary.
Results
In 2011, 6% of all patients at Intermountain facilities received blood. Today, only 4% do, according to study author Mark J. Ott, MD, chief medical director of Intermountain Healthcare’s central region.
“So a third of our patients didn’t get blood who used to,” Dr Ott said. “That’s a giant change. That’s tens of thousands of units of blood a year that didn’t get used.”
Before the program started (January 1, 2012), Intermountain facilities transfused almost 50 units of packed red blood cells per 1000 patient days. By January 31, 2015, that rate had declined to about 35.5 units, a reduction of around 30%.
Over the same time period, the percentage of patients transfused with a hematocrit of 23% or greater decreased from 60% to 34%.
The researchers said these reductions in blood use reduced costs by about $2.5 million over the 2-year period, assuming each unit of packed red blood cells costs $300.
In addition, the rate of hospital-acquired infections for both the general hospital population and patients who received blood declined significantly over the 2-year period.
The overall infection rate fell from 1.66 to 0.81 per 1000 patient days. Among patients who received blood, infection rates declined around 33%.
Dr Ott noted that the reduction in infections was also impacted by other initiatives within the health system aimed at reducing surgical site infections and ambulating patients earlier after operations.
“So I cannot tell you that those decreases in hospital-acquired infections are solely due to patients receiving less blood, but it’s part of the picture,” he said. “And we did not see worse outcomes in patients.” ![]()
Eltrombopag can benefit kids with chronic ITP

Photo by Logan Tuttle
Results of 2 studies suggest eltrombopag can be safe and effective in children of all ages affected by chronic immune thrombocytopenia (ITP).
In both trials, patients who received eltrombopag were significantly more likely to achieve stable platelet counts than patients who received placebo.
And eltrombopag did not increase the rate of serious adverse events (AEs).
These studies are the phase 2 PETIT trial, which was published in The Lancet Haematology, and the phase 3 PETIT2 trial, which was published in The Lancet.
“The studies, funded by GlaxoSmithKline, provide clinicians with much-needed evidence to help decide when eltrombopag would benefit pediatric patients and provide dosage regimens suitable for pediatric patients,” said investigator John Grainger, PhD, of The University of Manchester in the UK.
Phase 2 trial
The PETIT trial included 67 ITP patients who were stratified by age cohort (12-17 years, 6-11 years, and 1-5 years) and randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The most common AEs (in the eltrombopag and placebo groups, respectively) were headache (30% vs 43%), upper respiratory tract infection (25% vs 10%), and diarrhea (16% vs 5%).
Grade 3/4 AEs occurred in 11% of patients receiving eltrombopag and 19% of patients receiving placebo. Serious AEs occurred in 9% and 10%, respectively. There were no thrombotic events or malignancies in either group.
Phase 3 trial
The PETIT2 trial included 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%, compared to 3.4% of patients in the placebo arm (P<0.001).
AEs that occurred more frequently with eltrombopag than with placebo included nasopharyngitis (17%), rhinitis (16%), upper respiratory tract infection (11%), and cough (11%).
Serious AEs occurred in 8% of patients who received eltrombopag and 14% who received placebo. There were no deaths, malignancies, or thromboses during this trial.
It was based on these studies that eltrombopag was approved for use in US children older than 1 year of age. The drug is currently under review for this indication in the European Union. ![]()

Photo by Logan Tuttle
Results of 2 studies suggest eltrombopag can be safe and effective in children of all ages affected by chronic immune thrombocytopenia (ITP).
In both trials, patients who received eltrombopag were significantly more likely to achieve stable platelet counts than patients who received placebo.
And eltrombopag did not increase the rate of serious adverse events (AEs).
These studies are the phase 2 PETIT trial, which was published in The Lancet Haematology, and the phase 3 PETIT2 trial, which was published in The Lancet.
“The studies, funded by GlaxoSmithKline, provide clinicians with much-needed evidence to help decide when eltrombopag would benefit pediatric patients and provide dosage regimens suitable for pediatric patients,” said investigator John Grainger, PhD, of The University of Manchester in the UK.
Phase 2 trial
The PETIT trial included 67 ITP patients who were stratified by age cohort (12-17 years, 6-11 years, and 1-5 years) and randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The most common AEs (in the eltrombopag and placebo groups, respectively) were headache (30% vs 43%), upper respiratory tract infection (25% vs 10%), and diarrhea (16% vs 5%).
Grade 3/4 AEs occurred in 11% of patients receiving eltrombopag and 19% of patients receiving placebo. Serious AEs occurred in 9% and 10%, respectively. There were no thrombotic events or malignancies in either group.
Phase 3 trial
The PETIT2 trial included 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%, compared to 3.4% of patients in the placebo arm (P<0.001).
AEs that occurred more frequently with eltrombopag than with placebo included nasopharyngitis (17%), rhinitis (16%), upper respiratory tract infection (11%), and cough (11%).
Serious AEs occurred in 8% of patients who received eltrombopag and 14% who received placebo. There were no deaths, malignancies, or thromboses during this trial.
It was based on these studies that eltrombopag was approved for use in US children older than 1 year of age. The drug is currently under review for this indication in the European Union. ![]()

Photo by Logan Tuttle
Results of 2 studies suggest eltrombopag can be safe and effective in children of all ages affected by chronic immune thrombocytopenia (ITP).
In both trials, patients who received eltrombopag were significantly more likely to achieve stable platelet counts than patients who received placebo.
And eltrombopag did not increase the rate of serious adverse events (AEs).
These studies are the phase 2 PETIT trial, which was published in The Lancet Haematology, and the phase 3 PETIT2 trial, which was published in The Lancet.
“The studies, funded by GlaxoSmithKline, provide clinicians with much-needed evidence to help decide when eltrombopag would benefit pediatric patients and provide dosage regimens suitable for pediatric patients,” said investigator John Grainger, PhD, of The University of Manchester in the UK.
Phase 2 trial
The PETIT trial included 67 ITP patients who were stratified by age cohort (12-17 years, 6-11 years, and 1-5 years) and randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The most common AEs (in the eltrombopag and placebo groups, respectively) were headache (30% vs 43%), upper respiratory tract infection (25% vs 10%), and diarrhea (16% vs 5%).
Grade 3/4 AEs occurred in 11% of patients receiving eltrombopag and 19% of patients receiving placebo. Serious AEs occurred in 9% and 10%, respectively. There were no thrombotic events or malignancies in either group.
Phase 3 trial
The PETIT2 trial included 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%, compared to 3.4% of patients in the placebo arm (P<0.001).
AEs that occurred more frequently with eltrombopag than with placebo included nasopharyngitis (17%), rhinitis (16%), upper respiratory tract infection (11%), and cough (11%).
Serious AEs occurred in 8% of patients who received eltrombopag and 14% who received placebo. There were no deaths, malignancies, or thromboses during this trial.
It was based on these studies that eltrombopag was approved for use in US children older than 1 year of age. The drug is currently under review for this indication in the European Union. ![]()
Regimen may lengthen survival in AL amyloidosis

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()
A primer on sexuality and gender identity
I am a relatively young physician. When I started medical school 10 years ago, I thought that most medical school campuses would be fairly progressive. This was not the case for me.
My school did not have a nondiscrimination policy on sexual orientation or gender identity at the time, nor do I recall any lectures about this patient population. So during my first year of medical school, I embarked on a mission to educate both my classmates and the faculty about sexual orientation, gender identity, and related health disparities. My fellow classmates and the administration received my efforts warmly; nevertheless, this effort to educate was an incredible challenge for me. Surely other medical school campuses were already discussing the importance of sexuality and gender identity, I thought.
Fast forward to the year 2011. A study in JAMA found that many medical schools fall short in teaching the next generation of physicians about lesbian, gay, bisexual, and transgender (LGBT) health (JAMA. 2011;306[9]:971-7).
Things may have improved for LGBT people, but the world of medicine has yet to catch up. If LGBT medical education is lacking today, imagine how lacking it was for those who went to medical school decades ago. It is my hope that with this new column, we as a medical community can make up for lost time.
Why should physicians, especially pediatricians, care about LGBT health? Although LGBT youth comprise less than 10% of the adolescent population, they have a disproportionate share of health problems compared with their heterosexual peers. LGBT youth are three times as likely to attempt suicide and almost two times as likely to abuse alcohol and drugs compared with heterosexual youth. Among homeless teens in the United States, a whopping 40% are LGBT. HIV still plagues young gay males – especially those of color – and young gay and bisexual women experience an inordinate amount of dating violence from both men and women. Most appalling of all, every 3 days, a transgender person is murdered. These sobering statistics highlight the impact sexual orientation and gender identity have on health.
Why do LGBT youth experience such enormous health problems? A rich body of evidence points to stigma and discrimination as a likely cause. We are familiar with stories of how LGBT youth are kicked out of their homes after coming out to their parents or how male teens suffered bullying for being perceived as “too feminine.” Nonetheless, we tend to ignore the more subtle ways LGBT youth experience stigma and discrimination through our heterosexist language and behavior. Although we could dismiss the phrase “that’s so gay” as just another variation of “that’s so dumb,” an LGBT teen might think “if something is that dumb, then so am I.”
My fellow columnists and I hope that this column will help you get to know a very vulnerable, yet special, population. We will ask you to rethink what you have learned about sexuality and gender. Here, we will start with the basics.
What is the difference between sex and gender?
Sex is the biological distinction between male and female that is determined chromosomally (XX versus XY, although there are variations) and phenotypically, such as organs like the penis or vagina. Gender is a range of characteristics that a culture assigns as typically male and female, which encompasses both anatomy and behaviors. For example, an individual assigned as male because he was born with a penis is also expected to be proactive, a problem solver, stoic, and the breadwinner of the family. Although we’d like to believe that there are clear distinctions between the two solely on the basis of anatomy, we often see many people diverge from behaviors that are typically assigned to a gender. In modern day U.S. society, there are an increasing number of men who stay home to take care of their children – a typically female role. In other words, gender is a spectrum ranging from the very masculine to the very feminine and everything else in between.
What is gender identity?
Gender identity is our own sense of maleness or femaleness. This identity can be based on a variety of factors, including the sex organ one is born with and the culture one is raised in. It also is possible for some people to feel that they do not fit neatly into male or female categories. At the end of the day, only you can determine your gender identity, despite beliefs and attitudes in society about which appearances and behaviors are stereotypically male or female.
Transgender people are individuals who experience a mismatch between their gender identity and their assigned sex at birth. The word “trans” is Latin for “the other side,” highlighting the discrepancy between one’s gender identity and assigned sex. In contrast, people who identify as their assigned sex would be called cisgender. The word “cis” is Latin for “the same side.” A transgender male is someone who was assigned female at birth, but identifies as a male, whereas a transgender female is someone who was assigned male at birth, but identifies as a female. You also may also hear the terms “FTM” (female to male) and “MTF” (male to female) to describe transgender males and females, respectively.
What is sexual orientation?
Sexual orientation refers to our pattern of emotional and/or physical attraction to people who are the same or the opposite gender. The most common in this society is heterosexual, where one finds the opposite gender attractive. Those who identify as gay or lesbian find the same gender attractive. A person who identifies as bisexual finds both genders attractive. There are other sexual orientations that are not as commonly known. Someone who is pansexual is attracted to any sex or gender identity. Asexuals are individuals who don’t find anyone sexually attractive, but could be attracted to someone romantically or emotionally irrespective of sex or gender.
Just as gender is fluid, so is sexuality. Alfred Kinsey, a well-known sexologist, introduced the concept of sexual fluidity with the Kinsey Scale. With this scale, people rate themselves on how attracted they are to each sex, ranging from 0 – meaning exclusively attracted to the opposite sex – to 3 – equally attracted to both sexes – to 6 – exclusively attracted to the same sex. It is possible to move along the spectrum in either direction over time or stay in one place. It is also possible for our sexual identity (i.e. lesbian, gay, bisexual) and sexual behavior (i.e. whom we are having sex with) to not perfectly overlap; attraction is complex. Finally, people often confuse gender identity and sexual orientation. These are two separate concepts and not dependent on each other. For example, someone who was assigned female at birth but now identifies as male can still be attracted to men.
This primer is by no means complete or comprehensive and runs the risk of being oversimplistic. Nevertheless, I hope it will get you thinking about the nature of sexuality and gender identity and how they affect health. In the next couple of months, you will read more on the complexities of sexuality and gender identity, advice on how to talk to your patients about these topics, how to make your clinic a safe place for LGBT youth, the transition process for transgender youth, and much more. I encourage you stick around to learn how you can help this vulnerable, but amazing, group of young people. Until next time …
Dr. Montano is an adolescent medicine fellow at Children’s Hospital of Pittsburgh of UPMC and a postdoctoral fellow in the department of pediatrics the University of Pittsburgh.
I am a relatively young physician. When I started medical school 10 years ago, I thought that most medical school campuses would be fairly progressive. This was not the case for me.
My school did not have a nondiscrimination policy on sexual orientation or gender identity at the time, nor do I recall any lectures about this patient population. So during my first year of medical school, I embarked on a mission to educate both my classmates and the faculty about sexual orientation, gender identity, and related health disparities. My fellow classmates and the administration received my efforts warmly; nevertheless, this effort to educate was an incredible challenge for me. Surely other medical school campuses were already discussing the importance of sexuality and gender identity, I thought.
Fast forward to the year 2011. A study in JAMA found that many medical schools fall short in teaching the next generation of physicians about lesbian, gay, bisexual, and transgender (LGBT) health (JAMA. 2011;306[9]:971-7).
Things may have improved for LGBT people, but the world of medicine has yet to catch up. If LGBT medical education is lacking today, imagine how lacking it was for those who went to medical school decades ago. It is my hope that with this new column, we as a medical community can make up for lost time.
Why should physicians, especially pediatricians, care about LGBT health? Although LGBT youth comprise less than 10% of the adolescent population, they have a disproportionate share of health problems compared with their heterosexual peers. LGBT youth are three times as likely to attempt suicide and almost two times as likely to abuse alcohol and drugs compared with heterosexual youth. Among homeless teens in the United States, a whopping 40% are LGBT. HIV still plagues young gay males – especially those of color – and young gay and bisexual women experience an inordinate amount of dating violence from both men and women. Most appalling of all, every 3 days, a transgender person is murdered. These sobering statistics highlight the impact sexual orientation and gender identity have on health.
Why do LGBT youth experience such enormous health problems? A rich body of evidence points to stigma and discrimination as a likely cause. We are familiar with stories of how LGBT youth are kicked out of their homes after coming out to their parents or how male teens suffered bullying for being perceived as “too feminine.” Nonetheless, we tend to ignore the more subtle ways LGBT youth experience stigma and discrimination through our heterosexist language and behavior. Although we could dismiss the phrase “that’s so gay” as just another variation of “that’s so dumb,” an LGBT teen might think “if something is that dumb, then so am I.”
My fellow columnists and I hope that this column will help you get to know a very vulnerable, yet special, population. We will ask you to rethink what you have learned about sexuality and gender. Here, we will start with the basics.
What is the difference between sex and gender?
Sex is the biological distinction between male and female that is determined chromosomally (XX versus XY, although there are variations) and phenotypically, such as organs like the penis or vagina. Gender is a range of characteristics that a culture assigns as typically male and female, which encompasses both anatomy and behaviors. For example, an individual assigned as male because he was born with a penis is also expected to be proactive, a problem solver, stoic, and the breadwinner of the family. Although we’d like to believe that there are clear distinctions between the two solely on the basis of anatomy, we often see many people diverge from behaviors that are typically assigned to a gender. In modern day U.S. society, there are an increasing number of men who stay home to take care of their children – a typically female role. In other words, gender is a spectrum ranging from the very masculine to the very feminine and everything else in between.
What is gender identity?
Gender identity is our own sense of maleness or femaleness. This identity can be based on a variety of factors, including the sex organ one is born with and the culture one is raised in. It also is possible for some people to feel that they do not fit neatly into male or female categories. At the end of the day, only you can determine your gender identity, despite beliefs and attitudes in society about which appearances and behaviors are stereotypically male or female.
Transgender people are individuals who experience a mismatch between their gender identity and their assigned sex at birth. The word “trans” is Latin for “the other side,” highlighting the discrepancy between one’s gender identity and assigned sex. In contrast, people who identify as their assigned sex would be called cisgender. The word “cis” is Latin for “the same side.” A transgender male is someone who was assigned female at birth, but identifies as a male, whereas a transgender female is someone who was assigned male at birth, but identifies as a female. You also may also hear the terms “FTM” (female to male) and “MTF” (male to female) to describe transgender males and females, respectively.
What is sexual orientation?
Sexual orientation refers to our pattern of emotional and/or physical attraction to people who are the same or the opposite gender. The most common in this society is heterosexual, where one finds the opposite gender attractive. Those who identify as gay or lesbian find the same gender attractive. A person who identifies as bisexual finds both genders attractive. There are other sexual orientations that are not as commonly known. Someone who is pansexual is attracted to any sex or gender identity. Asexuals are individuals who don’t find anyone sexually attractive, but could be attracted to someone romantically or emotionally irrespective of sex or gender.
Just as gender is fluid, so is sexuality. Alfred Kinsey, a well-known sexologist, introduced the concept of sexual fluidity with the Kinsey Scale. With this scale, people rate themselves on how attracted they are to each sex, ranging from 0 – meaning exclusively attracted to the opposite sex – to 3 – equally attracted to both sexes – to 6 – exclusively attracted to the same sex. It is possible to move along the spectrum in either direction over time or stay in one place. It is also possible for our sexual identity (i.e. lesbian, gay, bisexual) and sexual behavior (i.e. whom we are having sex with) to not perfectly overlap; attraction is complex. Finally, people often confuse gender identity and sexual orientation. These are two separate concepts and not dependent on each other. For example, someone who was assigned female at birth but now identifies as male can still be attracted to men.
This primer is by no means complete or comprehensive and runs the risk of being oversimplistic. Nevertheless, I hope it will get you thinking about the nature of sexuality and gender identity and how they affect health. In the next couple of months, you will read more on the complexities of sexuality and gender identity, advice on how to talk to your patients about these topics, how to make your clinic a safe place for LGBT youth, the transition process for transgender youth, and much more. I encourage you stick around to learn how you can help this vulnerable, but amazing, group of young people. Until next time …
Dr. Montano is an adolescent medicine fellow at Children’s Hospital of Pittsburgh of UPMC and a postdoctoral fellow in the department of pediatrics the University of Pittsburgh.
I am a relatively young physician. When I started medical school 10 years ago, I thought that most medical school campuses would be fairly progressive. This was not the case for me.
My school did not have a nondiscrimination policy on sexual orientation or gender identity at the time, nor do I recall any lectures about this patient population. So during my first year of medical school, I embarked on a mission to educate both my classmates and the faculty about sexual orientation, gender identity, and related health disparities. My fellow classmates and the administration received my efforts warmly; nevertheless, this effort to educate was an incredible challenge for me. Surely other medical school campuses were already discussing the importance of sexuality and gender identity, I thought.
Fast forward to the year 2011. A study in JAMA found that many medical schools fall short in teaching the next generation of physicians about lesbian, gay, bisexual, and transgender (LGBT) health (JAMA. 2011;306[9]:971-7).
Things may have improved for LGBT people, but the world of medicine has yet to catch up. If LGBT medical education is lacking today, imagine how lacking it was for those who went to medical school decades ago. It is my hope that with this new column, we as a medical community can make up for lost time.
Why should physicians, especially pediatricians, care about LGBT health? Although LGBT youth comprise less than 10% of the adolescent population, they have a disproportionate share of health problems compared with their heterosexual peers. LGBT youth are three times as likely to attempt suicide and almost two times as likely to abuse alcohol and drugs compared with heterosexual youth. Among homeless teens in the United States, a whopping 40% are LGBT. HIV still plagues young gay males – especially those of color – and young gay and bisexual women experience an inordinate amount of dating violence from both men and women. Most appalling of all, every 3 days, a transgender person is murdered. These sobering statistics highlight the impact sexual orientation and gender identity have on health.
Why do LGBT youth experience such enormous health problems? A rich body of evidence points to stigma and discrimination as a likely cause. We are familiar with stories of how LGBT youth are kicked out of their homes after coming out to their parents or how male teens suffered bullying for being perceived as “too feminine.” Nonetheless, we tend to ignore the more subtle ways LGBT youth experience stigma and discrimination through our heterosexist language and behavior. Although we could dismiss the phrase “that’s so gay” as just another variation of “that’s so dumb,” an LGBT teen might think “if something is that dumb, then so am I.”
My fellow columnists and I hope that this column will help you get to know a very vulnerable, yet special, population. We will ask you to rethink what you have learned about sexuality and gender. Here, we will start with the basics.
What is the difference between sex and gender?
Sex is the biological distinction between male and female that is determined chromosomally (XX versus XY, although there are variations) and phenotypically, such as organs like the penis or vagina. Gender is a range of characteristics that a culture assigns as typically male and female, which encompasses both anatomy and behaviors. For example, an individual assigned as male because he was born with a penis is also expected to be proactive, a problem solver, stoic, and the breadwinner of the family. Although we’d like to believe that there are clear distinctions between the two solely on the basis of anatomy, we often see many people diverge from behaviors that are typically assigned to a gender. In modern day U.S. society, there are an increasing number of men who stay home to take care of their children – a typically female role. In other words, gender is a spectrum ranging from the very masculine to the very feminine and everything else in between.
What is gender identity?
Gender identity is our own sense of maleness or femaleness. This identity can be based on a variety of factors, including the sex organ one is born with and the culture one is raised in. It also is possible for some people to feel that they do not fit neatly into male or female categories. At the end of the day, only you can determine your gender identity, despite beliefs and attitudes in society about which appearances and behaviors are stereotypically male or female.
Transgender people are individuals who experience a mismatch between their gender identity and their assigned sex at birth. The word “trans” is Latin for “the other side,” highlighting the discrepancy between one’s gender identity and assigned sex. In contrast, people who identify as their assigned sex would be called cisgender. The word “cis” is Latin for “the same side.” A transgender male is someone who was assigned female at birth, but identifies as a male, whereas a transgender female is someone who was assigned male at birth, but identifies as a female. You also may also hear the terms “FTM” (female to male) and “MTF” (male to female) to describe transgender males and females, respectively.
What is sexual orientation?
Sexual orientation refers to our pattern of emotional and/or physical attraction to people who are the same or the opposite gender. The most common in this society is heterosexual, where one finds the opposite gender attractive. Those who identify as gay or lesbian find the same gender attractive. A person who identifies as bisexual finds both genders attractive. There are other sexual orientations that are not as commonly known. Someone who is pansexual is attracted to any sex or gender identity. Asexuals are individuals who don’t find anyone sexually attractive, but could be attracted to someone romantically or emotionally irrespective of sex or gender.
Just as gender is fluid, so is sexuality. Alfred Kinsey, a well-known sexologist, introduced the concept of sexual fluidity with the Kinsey Scale. With this scale, people rate themselves on how attracted they are to each sex, ranging from 0 – meaning exclusively attracted to the opposite sex – to 3 – equally attracted to both sexes – to 6 – exclusively attracted to the same sex. It is possible to move along the spectrum in either direction over time or stay in one place. It is also possible for our sexual identity (i.e. lesbian, gay, bisexual) and sexual behavior (i.e. whom we are having sex with) to not perfectly overlap; attraction is complex. Finally, people often confuse gender identity and sexual orientation. These are two separate concepts and not dependent on each other. For example, someone who was assigned female at birth but now identifies as male can still be attracted to men.
This primer is by no means complete or comprehensive and runs the risk of being oversimplistic. Nevertheless, I hope it will get you thinking about the nature of sexuality and gender identity and how they affect health. In the next couple of months, you will read more on the complexities of sexuality and gender identity, advice on how to talk to your patients about these topics, how to make your clinic a safe place for LGBT youth, the transition process for transgender youth, and much more. I encourage you stick around to learn how you can help this vulnerable, but amazing, group of young people. Until next time …
Dr. Montano is an adolescent medicine fellow at Children’s Hospital of Pittsburgh of UPMC and a postdoctoral fellow in the department of pediatrics the University of Pittsburgh.
Listen Now: Dr. Michael Murphy Discusses Use of Medical Scribes
Michael Murphy, MD, the co-founder and CEO of ScribeAmerica, talks about the business model that has led to the growing use of medical scribes.
Michael Murphy, MD, the co-founder and CEO of ScribeAmerica, talks about the business model that has led to the growing use of medical scribes.
Michael Murphy, MD, the co-founder and CEO of ScribeAmerica, talks about the business model that has led to the growing use of medical scribes.