User login
Webcast: How to use the CDC's online tools to manage complex cases in contraception
| Access the online tools referenced in this Webcast: |
| Access the online tools referenced in this Webcast: |
| Access the online tools referenced in this Webcast: |
Staphylococcal Scalded Skin Syndrome in Pregnancy
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
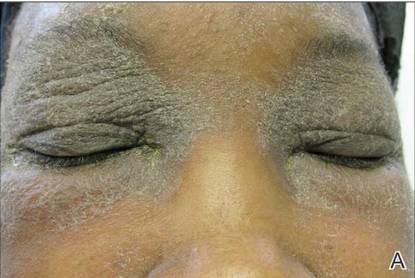
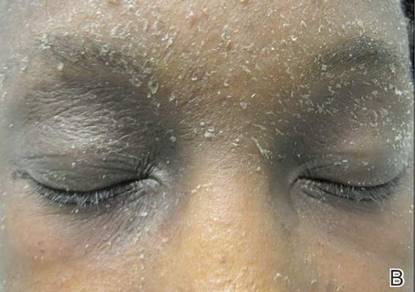
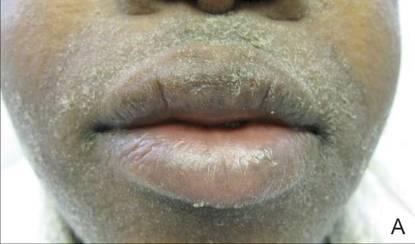
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
IgA increase linked to fewer infections in CLL patients on ibrutinib
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
Increases in IgA levels were associated with a reduced risk of infections in 84 chronic lymphocytic leukemia (CLL) patients participating in a trial of ibrutinib 420 mg once daily.
After 28 months of ibrutinib treatment, 69 (82%) patients had developed 177 infections. Lower rates of infections were found in those who experienced an IgA increase of at least 50% from their baseline values (P = .03), reported Dr. Clare Sun of the hematology branch of the National Heart, Lung and Blood Institute in Bethesda, Md., and her associates.
At baseline, the patients’ median IgA value was 0.47 g/L; after 6 months of treatment with ibrutinib, the median IgA value was 0.74 g/L. The levels of IgA continued to rise in the next 12 months (n = 43, median increase of 45%, P less than 0001), and patients’ IgA levels at 24 months also were greater than their baseline levels (n = 28, median increase of 64%, P less than .0001).
Using serum-free light chain measures to distinguish clonal and normal B cells, researchers also found recovery of normal B cells and increases in B-cell precursors in bone marrow and in normal B cells in the peripheral blood. This growth, however, was not large enough to raise the majority of patients’ normal B cells to normal levels.
The findings suggest “ibrutinib allows for a clinically meaningful recovery of humoral immune function in patients with CLL,” Dr. Sun and her associates wrote. “The rapidity of increase in IgA suggests that pre-existing antibody-producing cells may be secreting more immunoglobulins, whilst CLL cells, which impair immunoglobulin production, are removed by ibrutinib.”
The patients also had a decline in IgG levels, however, which did not appear to have an adverse impact. The patients’ IgG levels remained stable during the first 6 months of treatment, but by 12 months they had decreased (n = 35, median reduction of 4%, P < .0006), falling further at 24 months (n = 21, median reduction of 23%, P < .0001).
Because ibrutinib may be given indefinitely, extended follow-up is needed to determine the immunologic consequences of prolonged Bruton’s tyrosine kinase inhibition, the researchers wrote.
Read the full study in Blood (2015. doi: 10.1182/blood-2015-04-639203).
FROM BLOOD
JAK inhibitors could treat Sézary syndrome, team says

Photo courtesy of NIGMS
Patients with Sézary syndrome (SS) may have a more complex array of genetic mutations than we thought, according to a paper published in Nature Communications.
Investigators uncovered a genomic landscape that, they believe, can be used to design personalized treatment regimens for SS patients.
In particular, the team found mutations in the JAK/STAT pathway and discovered that JAK-mutated SS cells are sensitive to JAK inhibitors.
To conduct this research, the investigators sequenced SS patient samples using 3 different approaches. They performed whole-genome sequencing (n=6), whole-exome sequencing (n=66), and array comparative genomic hybridization-based copy-number analysis (n=80).
“We basically found chromosomal chaos in all of our samples,” said study author Kojo Elenitoba-Johnson, MD, of the University of Pennsylvania in Philadelphia.
“We did not expect the degree of genetic complexity that we found in our study.”
The investigators identified previously unknown, recurrent, loss-of-function mutations that target genes regulating epigenetic pathways.
One of these targets is ARID1A, and the team found that loss-of-function mutations and/or deletions in ARID1A occurred in more than 40% of the SS genome studied.
The investigators also identified gain-of-function mutations in PLCG1, JAK1, JAK3, STAT3, and STAT5B.
And in preliminary drug-mutation matching studies, JAK1-mutated SS cells were sensitive to JAK inhibitors.
“With knowledge like this, we can design clinical trials using JAK inhibitors for SS patients based on their JAK mutations,” Dr Elenitoba-Johnson said. “But this is just the start. These results highlight the genetic vulnerabilities that we can use in designing precision medicine therapies.”
Now, the investigators want to develop a molecular taxonomy for mutations in SS patients. Using the sequencing technology they used in this study, the team hopes to pinpoint the exact mistakes in each patient’s SS-related genes.
From this, the investigators hope to identify distinct subsets of the disease and stratify patients for precision therapy based on their mutations and the inhibitors available for those mutations. ![]()

Photo courtesy of NIGMS
Patients with Sézary syndrome (SS) may have a more complex array of genetic mutations than we thought, according to a paper published in Nature Communications.
Investigators uncovered a genomic landscape that, they believe, can be used to design personalized treatment regimens for SS patients.
In particular, the team found mutations in the JAK/STAT pathway and discovered that JAK-mutated SS cells are sensitive to JAK inhibitors.
To conduct this research, the investigators sequenced SS patient samples using 3 different approaches. They performed whole-genome sequencing (n=6), whole-exome sequencing (n=66), and array comparative genomic hybridization-based copy-number analysis (n=80).
“We basically found chromosomal chaos in all of our samples,” said study author Kojo Elenitoba-Johnson, MD, of the University of Pennsylvania in Philadelphia.
“We did not expect the degree of genetic complexity that we found in our study.”
The investigators identified previously unknown, recurrent, loss-of-function mutations that target genes regulating epigenetic pathways.
One of these targets is ARID1A, and the team found that loss-of-function mutations and/or deletions in ARID1A occurred in more than 40% of the SS genome studied.
The investigators also identified gain-of-function mutations in PLCG1, JAK1, JAK3, STAT3, and STAT5B.
And in preliminary drug-mutation matching studies, JAK1-mutated SS cells were sensitive to JAK inhibitors.
“With knowledge like this, we can design clinical trials using JAK inhibitors for SS patients based on their JAK mutations,” Dr Elenitoba-Johnson said. “But this is just the start. These results highlight the genetic vulnerabilities that we can use in designing precision medicine therapies.”
Now, the investigators want to develop a molecular taxonomy for mutations in SS patients. Using the sequencing technology they used in this study, the team hopes to pinpoint the exact mistakes in each patient’s SS-related genes.
From this, the investigators hope to identify distinct subsets of the disease and stratify patients for precision therapy based on their mutations and the inhibitors available for those mutations. ![]()

Photo courtesy of NIGMS
Patients with Sézary syndrome (SS) may have a more complex array of genetic mutations than we thought, according to a paper published in Nature Communications.
Investigators uncovered a genomic landscape that, they believe, can be used to design personalized treatment regimens for SS patients.
In particular, the team found mutations in the JAK/STAT pathway and discovered that JAK-mutated SS cells are sensitive to JAK inhibitors.
To conduct this research, the investigators sequenced SS patient samples using 3 different approaches. They performed whole-genome sequencing (n=6), whole-exome sequencing (n=66), and array comparative genomic hybridization-based copy-number analysis (n=80).
“We basically found chromosomal chaos in all of our samples,” said study author Kojo Elenitoba-Johnson, MD, of the University of Pennsylvania in Philadelphia.
“We did not expect the degree of genetic complexity that we found in our study.”
The investigators identified previously unknown, recurrent, loss-of-function mutations that target genes regulating epigenetic pathways.
One of these targets is ARID1A, and the team found that loss-of-function mutations and/or deletions in ARID1A occurred in more than 40% of the SS genome studied.
The investigators also identified gain-of-function mutations in PLCG1, JAK1, JAK3, STAT3, and STAT5B.
And in preliminary drug-mutation matching studies, JAK1-mutated SS cells were sensitive to JAK inhibitors.
“With knowledge like this, we can design clinical trials using JAK inhibitors for SS patients based on their JAK mutations,” Dr Elenitoba-Johnson said. “But this is just the start. These results highlight the genetic vulnerabilities that we can use in designing precision medicine therapies.”
Now, the investigators want to develop a molecular taxonomy for mutations in SS patients. Using the sequencing technology they used in this study, the team hopes to pinpoint the exact mistakes in each patient’s SS-related genes.
From this, the investigators hope to identify distinct subsets of the disease and stratify patients for precision therapy based on their mutations and the inhibitors available for those mutations. ![]()
BMI linked to need for blood transfusion

Photo courtesy of UAB Hospital
VIENNA—New research suggests that having a higher body mass index (BMI) is associated with a decreased need for blood transfusion among patients undergoing hip or knee replacement surgery.
In this retrospective, single-center study, overweight and obese patients were less likely than patients with a normal BMI to require blood transfusions.
The investigators said these results add to the conflicting body of research examining the association between BMI and blood transfusions in this patient population.
The results were presented at the International Society for Technology in Arthroplasty Annual Congress.
“The results were surprising to us,” said investigator Craig Silverton, DO, of Henry Ford Health System in Detroit, Michigan.
“It goes against the normal thought process. It’s hard to explain, but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
For this study, Dr Silverton and his colleagues evaluated 2399 patients, 1503 of whom underwent knee replacement and 896 of whom underwent hip surgery.
The investigators divided patients into 3 groups according to BMI: normal (BMI less than 25), overweight (BMI of 25 to 29.9), and obese (BMI more than 30).
As BMI increased, there was a significant increase in the estimated blood loss for both types of surgery.
Among hip surgery patients, the estimated blood loss was 268.2± 313.9 mL in patients with a normal BMI, 282.0 ± 208.7 mL in overweight patients, and 330.5 ± 302.4 mL in obese patients.
Among knee surgery patients, the estimated blood loss was 85.7 ± 153.8 mL in patients with a normal BMI, 90.5 ± 164.6 mL in overweight patients, and 89.4 ± 72.4 mL in obese patients.
However, with increasing BMI, there was a significant decrease in the estimated blood volume lost.
Among hip surgery patients, the estimated blood volume lost was 6.12% ± 8.12 in patients with a normal BMI, 4.92% ± 3.05 in overweight patients, and 4.50% ± 3.25 in obese patients.
Among knee surgery patients, the estimated blood volume lost was 2.05% ± 4.00 in patients with a normal BMI, 1.55% ± 2.73 in overweight patients, and 1.26% ± 1.01 in obese patients.
Likewise, there was a significant reduction in transfusion rates as BMI increased.
Among hip surgery patients, the transfusion rate was 34.8% for those with a normal BMI, 27.6% for those who were overweight, and 21.9% for obese patients.
Among knee surgery patients, the transfusion rate was 17.3% for those with a normal BMI, 11.4% for those who were overweight, and 8.3% for obese patients.
The investigators noted that there was no identifiable relationship between BMI and deep vein thrombosis, pulmonary embolism, myocardial infarction, length of hospital stay, 30-day readmission rate, or preoperative hemoglobin level.
There was a trend toward increased deep surgical site infections with increased BMI, but only among patients who underwent hip surgery. ![]()

Photo courtesy of UAB Hospital
VIENNA—New research suggests that having a higher body mass index (BMI) is associated with a decreased need for blood transfusion among patients undergoing hip or knee replacement surgery.
In this retrospective, single-center study, overweight and obese patients were less likely than patients with a normal BMI to require blood transfusions.
The investigators said these results add to the conflicting body of research examining the association between BMI and blood transfusions in this patient population.
The results were presented at the International Society for Technology in Arthroplasty Annual Congress.
“The results were surprising to us,” said investigator Craig Silverton, DO, of Henry Ford Health System in Detroit, Michigan.
“It goes against the normal thought process. It’s hard to explain, but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
For this study, Dr Silverton and his colleagues evaluated 2399 patients, 1503 of whom underwent knee replacement and 896 of whom underwent hip surgery.
The investigators divided patients into 3 groups according to BMI: normal (BMI less than 25), overweight (BMI of 25 to 29.9), and obese (BMI more than 30).
As BMI increased, there was a significant increase in the estimated blood loss for both types of surgery.
Among hip surgery patients, the estimated blood loss was 268.2± 313.9 mL in patients with a normal BMI, 282.0 ± 208.7 mL in overweight patients, and 330.5 ± 302.4 mL in obese patients.
Among knee surgery patients, the estimated blood loss was 85.7 ± 153.8 mL in patients with a normal BMI, 90.5 ± 164.6 mL in overweight patients, and 89.4 ± 72.4 mL in obese patients.
However, with increasing BMI, there was a significant decrease in the estimated blood volume lost.
Among hip surgery patients, the estimated blood volume lost was 6.12% ± 8.12 in patients with a normal BMI, 4.92% ± 3.05 in overweight patients, and 4.50% ± 3.25 in obese patients.
Among knee surgery patients, the estimated blood volume lost was 2.05% ± 4.00 in patients with a normal BMI, 1.55% ± 2.73 in overweight patients, and 1.26% ± 1.01 in obese patients.
Likewise, there was a significant reduction in transfusion rates as BMI increased.
Among hip surgery patients, the transfusion rate was 34.8% for those with a normal BMI, 27.6% for those who were overweight, and 21.9% for obese patients.
Among knee surgery patients, the transfusion rate was 17.3% for those with a normal BMI, 11.4% for those who were overweight, and 8.3% for obese patients.
The investigators noted that there was no identifiable relationship between BMI and deep vein thrombosis, pulmonary embolism, myocardial infarction, length of hospital stay, 30-day readmission rate, or preoperative hemoglobin level.
There was a trend toward increased deep surgical site infections with increased BMI, but only among patients who underwent hip surgery. ![]()

Photo courtesy of UAB Hospital
VIENNA—New research suggests that having a higher body mass index (BMI) is associated with a decreased need for blood transfusion among patients undergoing hip or knee replacement surgery.
In this retrospective, single-center study, overweight and obese patients were less likely than patients with a normal BMI to require blood transfusions.
The investigators said these results add to the conflicting body of research examining the association between BMI and blood transfusions in this patient population.
The results were presented at the International Society for Technology in Arthroplasty Annual Congress.
“The results were surprising to us,” said investigator Craig Silverton, DO, of Henry Ford Health System in Detroit, Michigan.
“It goes against the normal thought process. It’s hard to explain, but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
For this study, Dr Silverton and his colleagues evaluated 2399 patients, 1503 of whom underwent knee replacement and 896 of whom underwent hip surgery.
The investigators divided patients into 3 groups according to BMI: normal (BMI less than 25), overweight (BMI of 25 to 29.9), and obese (BMI more than 30).
As BMI increased, there was a significant increase in the estimated blood loss for both types of surgery.
Among hip surgery patients, the estimated blood loss was 268.2± 313.9 mL in patients with a normal BMI, 282.0 ± 208.7 mL in overweight patients, and 330.5 ± 302.4 mL in obese patients.
Among knee surgery patients, the estimated blood loss was 85.7 ± 153.8 mL in patients with a normal BMI, 90.5 ± 164.6 mL in overweight patients, and 89.4 ± 72.4 mL in obese patients.
However, with increasing BMI, there was a significant decrease in the estimated blood volume lost.
Among hip surgery patients, the estimated blood volume lost was 6.12% ± 8.12 in patients with a normal BMI, 4.92% ± 3.05 in overweight patients, and 4.50% ± 3.25 in obese patients.
Among knee surgery patients, the estimated blood volume lost was 2.05% ± 4.00 in patients with a normal BMI, 1.55% ± 2.73 in overweight patients, and 1.26% ± 1.01 in obese patients.
Likewise, there was a significant reduction in transfusion rates as BMI increased.
Among hip surgery patients, the transfusion rate was 34.8% for those with a normal BMI, 27.6% for those who were overweight, and 21.9% for obese patients.
Among knee surgery patients, the transfusion rate was 17.3% for those with a normal BMI, 11.4% for those who were overweight, and 8.3% for obese patients.
The investigators noted that there was no identifiable relationship between BMI and deep vein thrombosis, pulmonary embolism, myocardial infarction, length of hospital stay, 30-day readmission rate, or preoperative hemoglobin level.
There was a trend toward increased deep surgical site infections with increased BMI, but only among patients who underwent hip surgery. ![]()
Trio wins Nobel Prize for DNA repair discoveries

Image by Tom Ellenberger
Three researchers have won this year’s Nobel Prize in Chemistry for mechanistic studies of DNA repair.
Tomas Lindahl, MD, PhD, Paul Modrich, PhD, and Aziz Sancar, MD, PhD, each mapped how DNA repair systems function at a detailed molecular level.
Their work has provided insight into how cells function, knowledge that can be used in the development of new cancer treatments, among other applications.
In the early 1970s, scientists believed that DNA was an extremely stable molecule, but Dr Lindahl demonstrated that DNA decays at a rate that ought to have made life on Earth impossible.
This insight led to the discovery of molecular machinery known as base excision repair, which constantly counteracts the collapse of our DNA.
For his part, Dr Sancar mapped nucleotide excision repair, the mechanism that cells use to repair UV damage to DNA.
People born with defects in this repair system will develop skin cancer if they are exposed to sunlight. The cell also utilizes nucleotide excision repair to correct defects caused by mutagenic substances, among other things.
Dr Modrich demonstrated how the cell corrects errors that occur when DNA is replicated during cell division.
This mechanism, mismatch repair, reduces the error frequency during DNA replication by about a thousand-fold. Congenital defects in mismatch repair are known, for example, to cause a hereditary variant of colon cancer.
About the winners
Tomas Lindahl was born in 1938 in Stockholm, Sweden. He earned his PhD in 1967 and his MD in 1970, both from Karolinska Institutet in Sweden. He is currently emeritus group leader at the Francis Crick Institute in London, UK.
Paul Modrich was born in 1946. In 1973, he earned his PhD from Stanford University in California. He is currently an investigator at Howard Hughes Medical Institute in Chevy Chase, Maryland, and a professor at Duke University School of Medicine in Durham, North Carolina.
Aziz Sancar was born in 1946 in Savur, Turkey. He earned his MD in 1969 from Istanbul University in Turkey and his PhD in 1977 from the University of Texas in Dallas. He is currently a professor at the University of North Carolina School of Medicine in Chapel Hill. ![]()

Image by Tom Ellenberger
Three researchers have won this year’s Nobel Prize in Chemistry for mechanistic studies of DNA repair.
Tomas Lindahl, MD, PhD, Paul Modrich, PhD, and Aziz Sancar, MD, PhD, each mapped how DNA repair systems function at a detailed molecular level.
Their work has provided insight into how cells function, knowledge that can be used in the development of new cancer treatments, among other applications.
In the early 1970s, scientists believed that DNA was an extremely stable molecule, but Dr Lindahl demonstrated that DNA decays at a rate that ought to have made life on Earth impossible.
This insight led to the discovery of molecular machinery known as base excision repair, which constantly counteracts the collapse of our DNA.
For his part, Dr Sancar mapped nucleotide excision repair, the mechanism that cells use to repair UV damage to DNA.
People born with defects in this repair system will develop skin cancer if they are exposed to sunlight. The cell also utilizes nucleotide excision repair to correct defects caused by mutagenic substances, among other things.
Dr Modrich demonstrated how the cell corrects errors that occur when DNA is replicated during cell division.
This mechanism, mismatch repair, reduces the error frequency during DNA replication by about a thousand-fold. Congenital defects in mismatch repair are known, for example, to cause a hereditary variant of colon cancer.
About the winners
Tomas Lindahl was born in 1938 in Stockholm, Sweden. He earned his PhD in 1967 and his MD in 1970, both from Karolinska Institutet in Sweden. He is currently emeritus group leader at the Francis Crick Institute in London, UK.
Paul Modrich was born in 1946. In 1973, he earned his PhD from Stanford University in California. He is currently an investigator at Howard Hughes Medical Institute in Chevy Chase, Maryland, and a professor at Duke University School of Medicine in Durham, North Carolina.
Aziz Sancar was born in 1946 in Savur, Turkey. He earned his MD in 1969 from Istanbul University in Turkey and his PhD in 1977 from the University of Texas in Dallas. He is currently a professor at the University of North Carolina School of Medicine in Chapel Hill. ![]()

Image by Tom Ellenberger
Three researchers have won this year’s Nobel Prize in Chemistry for mechanistic studies of DNA repair.
Tomas Lindahl, MD, PhD, Paul Modrich, PhD, and Aziz Sancar, MD, PhD, each mapped how DNA repair systems function at a detailed molecular level.
Their work has provided insight into how cells function, knowledge that can be used in the development of new cancer treatments, among other applications.
In the early 1970s, scientists believed that DNA was an extremely stable molecule, but Dr Lindahl demonstrated that DNA decays at a rate that ought to have made life on Earth impossible.
This insight led to the discovery of molecular machinery known as base excision repair, which constantly counteracts the collapse of our DNA.
For his part, Dr Sancar mapped nucleotide excision repair, the mechanism that cells use to repair UV damage to DNA.
People born with defects in this repair system will develop skin cancer if they are exposed to sunlight. The cell also utilizes nucleotide excision repair to correct defects caused by mutagenic substances, among other things.
Dr Modrich demonstrated how the cell corrects errors that occur when DNA is replicated during cell division.
This mechanism, mismatch repair, reduces the error frequency during DNA replication by about a thousand-fold. Congenital defects in mismatch repair are known, for example, to cause a hereditary variant of colon cancer.
About the winners
Tomas Lindahl was born in 1938 in Stockholm, Sweden. He earned his PhD in 1967 and his MD in 1970, both from Karolinska Institutet in Sweden. He is currently emeritus group leader at the Francis Crick Institute in London, UK.
Paul Modrich was born in 1946. In 1973, he earned his PhD from Stanford University in California. He is currently an investigator at Howard Hughes Medical Institute in Chevy Chase, Maryland, and a professor at Duke University School of Medicine in Durham, North Carolina.
Aziz Sancar was born in 1946 in Savur, Turkey. He earned his MD in 1969 from Istanbul University in Turkey and his PhD in 1977 from the University of Texas in Dallas. He is currently a professor at the University of North Carolina School of Medicine in Chapel Hill. ![]()
Case suggests GSIs could treat Notch-mutated ALL
before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors. ![]()

before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors. ![]()

before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors. ![]()
Nevus of Ota/Oculodermal Melancytosis: A Rare Report of an Oral Mucosal Lesion Involving the Hard Palate
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
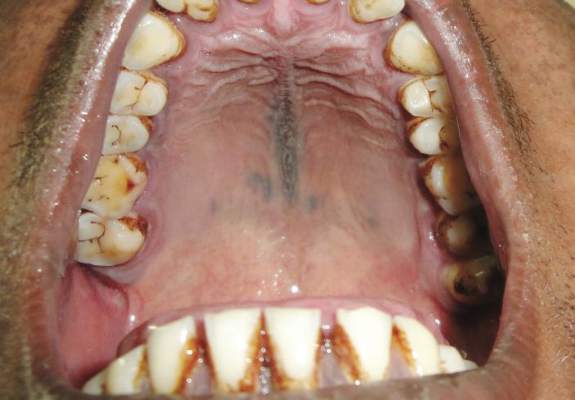
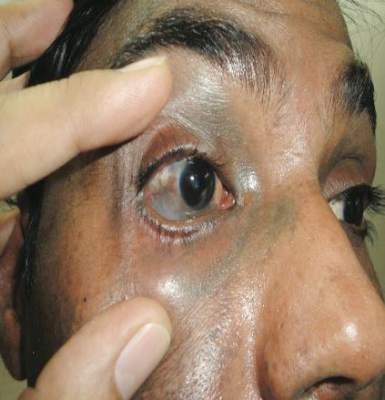
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).
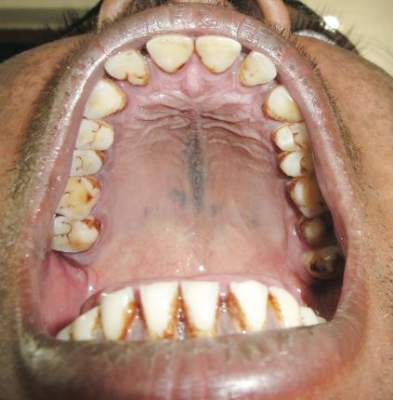
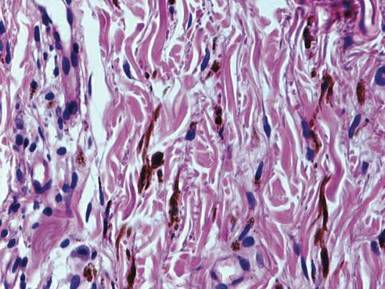
Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.
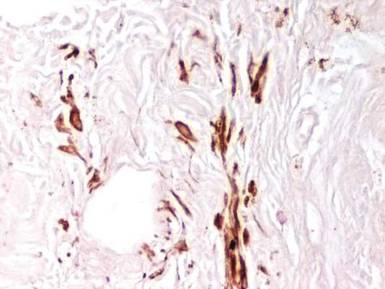
Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).

Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.



Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).

Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.



Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
New guideline allows use of estrogen for hot flashes – with caveats
Clinicians treating vasomotor and genitourinary symptoms of menopause should consider estrogen therapy for healthy women with moderate to severe symptoms and no contraindications to hormone therapy, according to a new clinical practice guideline issued by the Endocrine Society.
The guideline, developed by an international panel, is designed to be a comprehensive document that emphasizes individualized clinical recommendations and generally takes a conservative approach to balancing risks and benefits, said Dr. Cynthia Stuenkel, chair of the task force that developed the guideline and professor of medicine at the University of California, San Diego.
“We need to be very mindful of the individual health concerns of our patient – is the individual therapy that we choose safe for her?” noted Dr. Stuenkel during a web-hosted press conference announcing the publication of the guideline.
For women under 60 years of age or fewer than 10 years past menopause who have bothersome vasomotor symptoms (VMS) and are without contraindications, the guideline suggests initiating estrogen therapy, supplemented by a progestogen for those women who have a uterus (J Clin Endocrinol Metab. 2015. doi: 10.1210/jc.2015-2236).
The discussion regarding treatment options should be grounded by a obtaining a baseline history of and assessing risk for cardiovascular disease (CVD) and breast cancer. “Menopause is a portal to the second half of life,” so clinicians should address bone health, smoking cessation, alcohol use, and cardiovascular and cancer risks and screening in their discussion, said Dr. Stuenkel.
The panel makes specific recommendations to tailor treatment depending on risk. For example, women at intermediate to high risk of breast cancer should be steered toward nonhormonal therapies to relieve VMS. Those at moderate risk of CVD can consider transdermal estradiol, while nonhormonal therapies are recommended for the high–CVD risk group.
The genitourinary symptoms of menopause (GSM) can include not just vulvovaginal atrophy but also urinary frequency and recurrent urinary tract infections, said Dr. Stuenkel, so the panel used the broader terminology to address estrogen’s effect on both organ systems.
An initial trial of vaginal moisturizers, used at least twice weekly, supplemented by lubricants as needed before sexual activity, should be the first-line treatment for GSM. For women with persistent symptoms and no history of estrogen-dependent cancers, low-dose vaginal estrogen therapy is a logical next step and does not require accompanying progestogen treatment, according to the guideline.
For women with symptomatic GSM who have had breast or endometrial cancer whose symptoms are not sufficiently treated by nonhormonal methods, low-dose vaginal estrogen is a consideration. This option should be considered with a shared decision-making approach that involves the patient’s oncologist.
Conjugated equine estrogens plus bazedoxefine, a novel selective estrogen receptor modulator (Duavee) can treat VMS and provide protection against bone loss, said the task force. The guideline recommends against the use of custom-compounded hormonal therapy and recommends the use of Food and Drug Administration–approved formulations. Ospemifene can be considered in women with significant dyspareunia and without contraindications, which include a history of breast cancer.
In conclusion, the guideline calls for ongoing rigorous study of the optimal agents and dosing for treatment of symptoms, how best to balance symptom relief with chronic disease prevention, and the merits of long-term hormone therapy beyond the period when symptomatic relief of VMS is needed. “International registries and clinical trials are overdue to address the long-reaching implications of these important issues,” said Dr. Stuenkel and coauthors of the guideline.
Dr. Stuenkel reported no relevant financial disclosures. Three task force members, Dr. Susan Davis, Dr. JoAnn Pinkerton, and Dr. Richard Santen, reported financial ties to pharmaceutical companies. Cosponsoring organizations included the Australasian Menopause Society, the British Menopause Society, European Menopause and Andropause Society, the European Society of Endocrinology, and the International Menopause Society.
On Twitter @karioakes
Clinicians treating vasomotor and genitourinary symptoms of menopause should consider estrogen therapy for healthy women with moderate to severe symptoms and no contraindications to hormone therapy, according to a new clinical practice guideline issued by the Endocrine Society.
The guideline, developed by an international panel, is designed to be a comprehensive document that emphasizes individualized clinical recommendations and generally takes a conservative approach to balancing risks and benefits, said Dr. Cynthia Stuenkel, chair of the task force that developed the guideline and professor of medicine at the University of California, San Diego.
“We need to be very mindful of the individual health concerns of our patient – is the individual therapy that we choose safe for her?” noted Dr. Stuenkel during a web-hosted press conference announcing the publication of the guideline.
For women under 60 years of age or fewer than 10 years past menopause who have bothersome vasomotor symptoms (VMS) and are without contraindications, the guideline suggests initiating estrogen therapy, supplemented by a progestogen for those women who have a uterus (J Clin Endocrinol Metab. 2015. doi: 10.1210/jc.2015-2236).
The discussion regarding treatment options should be grounded by a obtaining a baseline history of and assessing risk for cardiovascular disease (CVD) and breast cancer. “Menopause is a portal to the second half of life,” so clinicians should address bone health, smoking cessation, alcohol use, and cardiovascular and cancer risks and screening in their discussion, said Dr. Stuenkel.
The panel makes specific recommendations to tailor treatment depending on risk. For example, women at intermediate to high risk of breast cancer should be steered toward nonhormonal therapies to relieve VMS. Those at moderate risk of CVD can consider transdermal estradiol, while nonhormonal therapies are recommended for the high–CVD risk group.
The genitourinary symptoms of menopause (GSM) can include not just vulvovaginal atrophy but also urinary frequency and recurrent urinary tract infections, said Dr. Stuenkel, so the panel used the broader terminology to address estrogen’s effect on both organ systems.
An initial trial of vaginal moisturizers, used at least twice weekly, supplemented by lubricants as needed before sexual activity, should be the first-line treatment for GSM. For women with persistent symptoms and no history of estrogen-dependent cancers, low-dose vaginal estrogen therapy is a logical next step and does not require accompanying progestogen treatment, according to the guideline.
For women with symptomatic GSM who have had breast or endometrial cancer whose symptoms are not sufficiently treated by nonhormonal methods, low-dose vaginal estrogen is a consideration. This option should be considered with a shared decision-making approach that involves the patient’s oncologist.
Conjugated equine estrogens plus bazedoxefine, a novel selective estrogen receptor modulator (Duavee) can treat VMS and provide protection against bone loss, said the task force. The guideline recommends against the use of custom-compounded hormonal therapy and recommends the use of Food and Drug Administration–approved formulations. Ospemifene can be considered in women with significant dyspareunia and without contraindications, which include a history of breast cancer.
In conclusion, the guideline calls for ongoing rigorous study of the optimal agents and dosing for treatment of symptoms, how best to balance symptom relief with chronic disease prevention, and the merits of long-term hormone therapy beyond the period when symptomatic relief of VMS is needed. “International registries and clinical trials are overdue to address the long-reaching implications of these important issues,” said Dr. Stuenkel and coauthors of the guideline.
Dr. Stuenkel reported no relevant financial disclosures. Three task force members, Dr. Susan Davis, Dr. JoAnn Pinkerton, and Dr. Richard Santen, reported financial ties to pharmaceutical companies. Cosponsoring organizations included the Australasian Menopause Society, the British Menopause Society, European Menopause and Andropause Society, the European Society of Endocrinology, and the International Menopause Society.
On Twitter @karioakes
Clinicians treating vasomotor and genitourinary symptoms of menopause should consider estrogen therapy for healthy women with moderate to severe symptoms and no contraindications to hormone therapy, according to a new clinical practice guideline issued by the Endocrine Society.
The guideline, developed by an international panel, is designed to be a comprehensive document that emphasizes individualized clinical recommendations and generally takes a conservative approach to balancing risks and benefits, said Dr. Cynthia Stuenkel, chair of the task force that developed the guideline and professor of medicine at the University of California, San Diego.
“We need to be very mindful of the individual health concerns of our patient – is the individual therapy that we choose safe for her?” noted Dr. Stuenkel during a web-hosted press conference announcing the publication of the guideline.
For women under 60 years of age or fewer than 10 years past menopause who have bothersome vasomotor symptoms (VMS) and are without contraindications, the guideline suggests initiating estrogen therapy, supplemented by a progestogen for those women who have a uterus (J Clin Endocrinol Metab. 2015. doi: 10.1210/jc.2015-2236).
The discussion regarding treatment options should be grounded by a obtaining a baseline history of and assessing risk for cardiovascular disease (CVD) and breast cancer. “Menopause is a portal to the second half of life,” so clinicians should address bone health, smoking cessation, alcohol use, and cardiovascular and cancer risks and screening in their discussion, said Dr. Stuenkel.
The panel makes specific recommendations to tailor treatment depending on risk. For example, women at intermediate to high risk of breast cancer should be steered toward nonhormonal therapies to relieve VMS. Those at moderate risk of CVD can consider transdermal estradiol, while nonhormonal therapies are recommended for the high–CVD risk group.
The genitourinary symptoms of menopause (GSM) can include not just vulvovaginal atrophy but also urinary frequency and recurrent urinary tract infections, said Dr. Stuenkel, so the panel used the broader terminology to address estrogen’s effect on both organ systems.
An initial trial of vaginal moisturizers, used at least twice weekly, supplemented by lubricants as needed before sexual activity, should be the first-line treatment for GSM. For women with persistent symptoms and no history of estrogen-dependent cancers, low-dose vaginal estrogen therapy is a logical next step and does not require accompanying progestogen treatment, according to the guideline.
For women with symptomatic GSM who have had breast or endometrial cancer whose symptoms are not sufficiently treated by nonhormonal methods, low-dose vaginal estrogen is a consideration. This option should be considered with a shared decision-making approach that involves the patient’s oncologist.
Conjugated equine estrogens plus bazedoxefine, a novel selective estrogen receptor modulator (Duavee) can treat VMS and provide protection against bone loss, said the task force. The guideline recommends against the use of custom-compounded hormonal therapy and recommends the use of Food and Drug Administration–approved formulations. Ospemifene can be considered in women with significant dyspareunia and without contraindications, which include a history of breast cancer.
In conclusion, the guideline calls for ongoing rigorous study of the optimal agents and dosing for treatment of symptoms, how best to balance symptom relief with chronic disease prevention, and the merits of long-term hormone therapy beyond the period when symptomatic relief of VMS is needed. “International registries and clinical trials are overdue to address the long-reaching implications of these important issues,” said Dr. Stuenkel and coauthors of the guideline.
Dr. Stuenkel reported no relevant financial disclosures. Three task force members, Dr. Susan Davis, Dr. JoAnn Pinkerton, and Dr. Richard Santen, reported financial ties to pharmaceutical companies. Cosponsoring organizations included the Australasian Menopause Society, the British Menopause Society, European Menopause and Andropause Society, the European Society of Endocrinology, and the International Menopause Society.
On Twitter @karioakes
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Devices can help relieve dyspareunia in midlife
LAS VEGAS – Physicians looking for more options for alleviating dyspareunia in postmenopausal women should consider vaginal dilators, specialized vibrators, and pelvic floor physical therapy, according to Susan Kellogg Spadt, Ph.D.
These approaches can prevent or overcome some of the changes in the pelvic anatomy that can occur with menopause or with prolonged periods without sexual activity, Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, said at the NAMS 2015 Annual Meeting.
Along with the topical atrophy that can occur with the low estrogen state of menopause, hypertonus and foreshortening of the pelvic floor muscles can occur in some postmenopausal women. Multiparous women may experience significant muscle laxity. Some women, especially those who may have gone without intercourse for prolonged periods, may also have vaginal stenosis. These can all present barriers to sexual health for women in midlife, she said.
Pelvic floor physical therapy, said Dr. Kellogg Spadt, is critical to help with all of these physical changes. Clinicians can find physical therapists certified in women’s health through the website of the American Physical Therapy Association (www.apta.org).
With the guidance of a physical therapist, women can learn about home use of a series of graduated vaginal dilators. Beginning with a smaller size, patients typically insert a dilator several times a week (up to daily) for 5-10 minutes, changing size every 2-3 weeks. During the changeover week, patients can start with the smaller size for 5 minutes and then change to the larger dilator for the second half of the session. This consistent, but gradual, approach is well tolerated and produces good results, she said.
Physical therapists may also use a pelvic wand, such as the Therawand, for women who have hypertonus of the pelvic musculature. This S-shaped acrylic wand is inserted into the vagina and provides direct internal pressure on the pubococcygeus and puborectalis muscles, facilitating trigger point release. Pelvic wands can be used in physical therapy sessions, but patients can also learn how to use the devices at home, Dr. Kellogg Spadt said.
Vibrators are another tool in addressing dyspareunia. The Intensity exerciser/vibrator is intended for therapeutic use as well as sexual pleasure. The device is powered by four AA batteries and is known to produce very intense orgasms with powerful pelvic muscle contractions. This might be deleterious and even painful for patients with an already tight pelvic floor, but it can be helpful for women with muscle laxity, Dr. Kellogg Spadt said. It can also be an important part of sex therapy for some women, “bringing orgasms to the orgasmless,” she said.
Another device option is Fiera, a small hands-free device that provides a low level of vibration to the clitoris and anterior vulva. It’s not designed to produce an orgasm, but to assist with arousal, so tissues are lubricated and engorged by the time the woman is ready to engage in partner sex play, she said.
Dr. Kellogg Stadt reported being a consultant to or on the advisory board of Neogyn and Nuelle, which markets Fiera. She is also on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
LAS VEGAS – Physicians looking for more options for alleviating dyspareunia in postmenopausal women should consider vaginal dilators, specialized vibrators, and pelvic floor physical therapy, according to Susan Kellogg Spadt, Ph.D.
These approaches can prevent or overcome some of the changes in the pelvic anatomy that can occur with menopause or with prolonged periods without sexual activity, Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, said at the NAMS 2015 Annual Meeting.
Along with the topical atrophy that can occur with the low estrogen state of menopause, hypertonus and foreshortening of the pelvic floor muscles can occur in some postmenopausal women. Multiparous women may experience significant muscle laxity. Some women, especially those who may have gone without intercourse for prolonged periods, may also have vaginal stenosis. These can all present barriers to sexual health for women in midlife, she said.
Pelvic floor physical therapy, said Dr. Kellogg Spadt, is critical to help with all of these physical changes. Clinicians can find physical therapists certified in women’s health through the website of the American Physical Therapy Association (www.apta.org).
With the guidance of a physical therapist, women can learn about home use of a series of graduated vaginal dilators. Beginning with a smaller size, patients typically insert a dilator several times a week (up to daily) for 5-10 minutes, changing size every 2-3 weeks. During the changeover week, patients can start with the smaller size for 5 minutes and then change to the larger dilator for the second half of the session. This consistent, but gradual, approach is well tolerated and produces good results, she said.
Physical therapists may also use a pelvic wand, such as the Therawand, for women who have hypertonus of the pelvic musculature. This S-shaped acrylic wand is inserted into the vagina and provides direct internal pressure on the pubococcygeus and puborectalis muscles, facilitating trigger point release. Pelvic wands can be used in physical therapy sessions, but patients can also learn how to use the devices at home, Dr. Kellogg Spadt said.
Vibrators are another tool in addressing dyspareunia. The Intensity exerciser/vibrator is intended for therapeutic use as well as sexual pleasure. The device is powered by four AA batteries and is known to produce very intense orgasms with powerful pelvic muscle contractions. This might be deleterious and even painful for patients with an already tight pelvic floor, but it can be helpful for women with muscle laxity, Dr. Kellogg Spadt said. It can also be an important part of sex therapy for some women, “bringing orgasms to the orgasmless,” she said.
Another device option is Fiera, a small hands-free device that provides a low level of vibration to the clitoris and anterior vulva. It’s not designed to produce an orgasm, but to assist with arousal, so tissues are lubricated and engorged by the time the woman is ready to engage in partner sex play, she said.
Dr. Kellogg Stadt reported being a consultant to or on the advisory board of Neogyn and Nuelle, which markets Fiera. She is also on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
LAS VEGAS – Physicians looking for more options for alleviating dyspareunia in postmenopausal women should consider vaginal dilators, specialized vibrators, and pelvic floor physical therapy, according to Susan Kellogg Spadt, Ph.D.
These approaches can prevent or overcome some of the changes in the pelvic anatomy that can occur with menopause or with prolonged periods without sexual activity, Dr. Kellogg Spadt, a certified sexual counselor and professor of obstetrics and gynecology at Drexel University, Philadelphia, said at the NAMS 2015 Annual Meeting.
Along with the topical atrophy that can occur with the low estrogen state of menopause, hypertonus and foreshortening of the pelvic floor muscles can occur in some postmenopausal women. Multiparous women may experience significant muscle laxity. Some women, especially those who may have gone without intercourse for prolonged periods, may also have vaginal stenosis. These can all present barriers to sexual health for women in midlife, she said.
Pelvic floor physical therapy, said Dr. Kellogg Spadt, is critical to help with all of these physical changes. Clinicians can find physical therapists certified in women’s health through the website of the American Physical Therapy Association (www.apta.org).
With the guidance of a physical therapist, women can learn about home use of a series of graduated vaginal dilators. Beginning with a smaller size, patients typically insert a dilator several times a week (up to daily) for 5-10 minutes, changing size every 2-3 weeks. During the changeover week, patients can start with the smaller size for 5 minutes and then change to the larger dilator for the second half of the session. This consistent, but gradual, approach is well tolerated and produces good results, she said.
Physical therapists may also use a pelvic wand, such as the Therawand, for women who have hypertonus of the pelvic musculature. This S-shaped acrylic wand is inserted into the vagina and provides direct internal pressure on the pubococcygeus and puborectalis muscles, facilitating trigger point release. Pelvic wands can be used in physical therapy sessions, but patients can also learn how to use the devices at home, Dr. Kellogg Spadt said.
Vibrators are another tool in addressing dyspareunia. The Intensity exerciser/vibrator is intended for therapeutic use as well as sexual pleasure. The device is powered by four AA batteries and is known to produce very intense orgasms with powerful pelvic muscle contractions. This might be deleterious and even painful for patients with an already tight pelvic floor, but it can be helpful for women with muscle laxity, Dr. Kellogg Spadt said. It can also be an important part of sex therapy for some women, “bringing orgasms to the orgasmless,” she said.
Another device option is Fiera, a small hands-free device that provides a low level of vibration to the clitoris and anterior vulva. It’s not designed to produce an orgasm, but to assist with arousal, so tissues are lubricated and engorged by the time the woman is ready to engage in partner sex play, she said.
Dr. Kellogg Stadt reported being a consultant to or on the advisory board of Neogyn and Nuelle, which markets Fiera. She is also on the speakers bureau of Novo Nordisk and Shionogi.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE NAMS 2015 ANNUAL MEETING