User login
Study reveals patterns of concurrent MRI lesions in knee OA
Groupings of coexisting MRI lesions of the tibiofemoral and patellofemoral joints were linked to the risk of subsequent radiographic osteoarthritis, investigators reported. Their analysis of data from the prospective, observational MOST study was published online Sept. 28 in Arthritis and Rheumatism.
“The magnitude of lesions such as cartilage damage and coexisting meniscal damage appear to be the main distinction between the subgroups,” said Dr. Jingbo Niu of Boston University and her associates. Several studies have linked individual MRI lesions with incident knee OA, but patterns of coexisting lesions more accurately reflect real-world injuries, such as anterior cruciate ligament tears, which tend to affect more than one knee structure, the investigators noted.
Because directly comparing lesions in a multivariate model does not account for chronology, the investigators used latent class analysis to identify subgroups of coexisting MRI lesions of the tibiofemoral and patellofemoral joints, such as cartilage damage, meniscal tear, meniscal extrusion, synovitis, and effusion. Then they modeled associations between these subgroups and incident OA of the knee (Arthritis Rheum. 2015 Sep 28. doi: 10.1002/art.3943).
Among 885 knees from the MOST study, 203 developed radiographic tibiofemoral OA and 64 developed patellofemoral OA after up to 84 months of follow-up, the researchers reported. Latent class analysis identified four groups of MRI lesions for each knee joint, which exhibited sequentially increasing baseline severity for all MRI features except meniscal damage, the investigators added.
For the patellofemoral joint, the odds of incident knee OA rose sequentially with increasing MRI severity, ranging from 1.0 for minimal lesions to 13.7 for severe lesions (95% confidence interval, 5.0-37.0), according to the study. In contrast, the odds of incident knee radiographic OA (ROA) of the tibiofemoral joint were highest for the “mild” and “severe” groups, which had the most meniscal damage and the most extensive history of knee injury and surgery. Odds ratios for these two groups were 5.6 (95% CI, 3.4-9.4) and 5.0 (95% CI, 2.8-9.0), respectively, said the researchers. “Meniscal damage might play a prominent role in the development of incident ROA in the tibiofemoral joint but not the patellofemoral joint,” they added.
Patients in the MOST study had a high risk of knee OA at baseline, which could limit the generalizability of the findings, Dr. Niu and her associates noted.
The National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Disease, both a part of the National Institutes of Health, supported the study. The investigators did not report conflicts of interest.
It was not until recently that the osteoarthritis community directed a shift toward new ways of halting joint damage rather than the palliative approach of analgesics followed by joint replacement. However, the sequential failures of novel disease-modifying therapies attempting to target diverse pathogenic mechanisms have highlighted the need to target early disease and to better identify and target distinct disease phenotypes. In this light of recent efforts to uncover the various mechanisms leading to joint deterioration, Niu et al. have used a novel approach to detect distinct clusters of multiple joint abnormalities on MRI at the preradiographic stage. They also examined the association between each cluster and the risk of radiographic OA during follow-up.
Probably the most important [conclusion from this study] is that it is possible to identify distinct phenotypes of joint damage as early as at the preradiographic phase. Consequently, it is intuitive that alternative pathways caused by various risk factors exist and play diverse roles in the process of joint destruction. Phenotyping patients in regards to their genetic profile, serologic, and MRI markers, demographic features, metabolic status, etc., is promising and probably the best solution to achieve improvements in the way we are treating OA patients.
The use of these results to assess prognosis seems premature in clinical research and inappropriate in the clinical setting. Nevertheless, the latent class analysis is an attractive approach in the field of OA and can aid in unraveling the pathogenesis of this enigmatic disease.
Dr. Leticia A. Deveza and Dr. David J. Hunter are with the department of rheumatology at the University of Sydney. They declared no conflicts of interest. These comments are from their accompanying editorial (Arthritis Rheum. 2015 Sep 28 doi: 10.1002/art.39439).
It was not until recently that the osteoarthritis community directed a shift toward new ways of halting joint damage rather than the palliative approach of analgesics followed by joint replacement. However, the sequential failures of novel disease-modifying therapies attempting to target diverse pathogenic mechanisms have highlighted the need to target early disease and to better identify and target distinct disease phenotypes. In this light of recent efforts to uncover the various mechanisms leading to joint deterioration, Niu et al. have used a novel approach to detect distinct clusters of multiple joint abnormalities on MRI at the preradiographic stage. They also examined the association between each cluster and the risk of radiographic OA during follow-up.
Probably the most important [conclusion from this study] is that it is possible to identify distinct phenotypes of joint damage as early as at the preradiographic phase. Consequently, it is intuitive that alternative pathways caused by various risk factors exist and play diverse roles in the process of joint destruction. Phenotyping patients in regards to their genetic profile, serologic, and MRI markers, demographic features, metabolic status, etc., is promising and probably the best solution to achieve improvements in the way we are treating OA patients.
The use of these results to assess prognosis seems premature in clinical research and inappropriate in the clinical setting. Nevertheless, the latent class analysis is an attractive approach in the field of OA and can aid in unraveling the pathogenesis of this enigmatic disease.
Dr. Leticia A. Deveza and Dr. David J. Hunter are with the department of rheumatology at the University of Sydney. They declared no conflicts of interest. These comments are from their accompanying editorial (Arthritis Rheum. 2015 Sep 28 doi: 10.1002/art.39439).
It was not until recently that the osteoarthritis community directed a shift toward new ways of halting joint damage rather than the palliative approach of analgesics followed by joint replacement. However, the sequential failures of novel disease-modifying therapies attempting to target diverse pathogenic mechanisms have highlighted the need to target early disease and to better identify and target distinct disease phenotypes. In this light of recent efforts to uncover the various mechanisms leading to joint deterioration, Niu et al. have used a novel approach to detect distinct clusters of multiple joint abnormalities on MRI at the preradiographic stage. They also examined the association between each cluster and the risk of radiographic OA during follow-up.
Probably the most important [conclusion from this study] is that it is possible to identify distinct phenotypes of joint damage as early as at the preradiographic phase. Consequently, it is intuitive that alternative pathways caused by various risk factors exist and play diverse roles in the process of joint destruction. Phenotyping patients in regards to their genetic profile, serologic, and MRI markers, demographic features, metabolic status, etc., is promising and probably the best solution to achieve improvements in the way we are treating OA patients.
The use of these results to assess prognosis seems premature in clinical research and inappropriate in the clinical setting. Nevertheless, the latent class analysis is an attractive approach in the field of OA and can aid in unraveling the pathogenesis of this enigmatic disease.
Dr. Leticia A. Deveza and Dr. David J. Hunter are with the department of rheumatology at the University of Sydney. They declared no conflicts of interest. These comments are from their accompanying editorial (Arthritis Rheum. 2015 Sep 28 doi: 10.1002/art.39439).
Groupings of coexisting MRI lesions of the tibiofemoral and patellofemoral joints were linked to the risk of subsequent radiographic osteoarthritis, investigators reported. Their analysis of data from the prospective, observational MOST study was published online Sept. 28 in Arthritis and Rheumatism.
“The magnitude of lesions such as cartilage damage and coexisting meniscal damage appear to be the main distinction between the subgroups,” said Dr. Jingbo Niu of Boston University and her associates. Several studies have linked individual MRI lesions with incident knee OA, but patterns of coexisting lesions more accurately reflect real-world injuries, such as anterior cruciate ligament tears, which tend to affect more than one knee structure, the investigators noted.
Because directly comparing lesions in a multivariate model does not account for chronology, the investigators used latent class analysis to identify subgroups of coexisting MRI lesions of the tibiofemoral and patellofemoral joints, such as cartilage damage, meniscal tear, meniscal extrusion, synovitis, and effusion. Then they modeled associations between these subgroups and incident OA of the knee (Arthritis Rheum. 2015 Sep 28. doi: 10.1002/art.3943).
Among 885 knees from the MOST study, 203 developed radiographic tibiofemoral OA and 64 developed patellofemoral OA after up to 84 months of follow-up, the researchers reported. Latent class analysis identified four groups of MRI lesions for each knee joint, which exhibited sequentially increasing baseline severity for all MRI features except meniscal damage, the investigators added.
For the patellofemoral joint, the odds of incident knee OA rose sequentially with increasing MRI severity, ranging from 1.0 for minimal lesions to 13.7 for severe lesions (95% confidence interval, 5.0-37.0), according to the study. In contrast, the odds of incident knee radiographic OA (ROA) of the tibiofemoral joint were highest for the “mild” and “severe” groups, which had the most meniscal damage and the most extensive history of knee injury and surgery. Odds ratios for these two groups were 5.6 (95% CI, 3.4-9.4) and 5.0 (95% CI, 2.8-9.0), respectively, said the researchers. “Meniscal damage might play a prominent role in the development of incident ROA in the tibiofemoral joint but not the patellofemoral joint,” they added.
Patients in the MOST study had a high risk of knee OA at baseline, which could limit the generalizability of the findings, Dr. Niu and her associates noted.
The National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Disease, both a part of the National Institutes of Health, supported the study. The investigators did not report conflicts of interest.
Groupings of coexisting MRI lesions of the tibiofemoral and patellofemoral joints were linked to the risk of subsequent radiographic osteoarthritis, investigators reported. Their analysis of data from the prospective, observational MOST study was published online Sept. 28 in Arthritis and Rheumatism.
“The magnitude of lesions such as cartilage damage and coexisting meniscal damage appear to be the main distinction between the subgroups,” said Dr. Jingbo Niu of Boston University and her associates. Several studies have linked individual MRI lesions with incident knee OA, but patterns of coexisting lesions more accurately reflect real-world injuries, such as anterior cruciate ligament tears, which tend to affect more than one knee structure, the investigators noted.
Because directly comparing lesions in a multivariate model does not account for chronology, the investigators used latent class analysis to identify subgroups of coexisting MRI lesions of the tibiofemoral and patellofemoral joints, such as cartilage damage, meniscal tear, meniscal extrusion, synovitis, and effusion. Then they modeled associations between these subgroups and incident OA of the knee (Arthritis Rheum. 2015 Sep 28. doi: 10.1002/art.3943).
Among 885 knees from the MOST study, 203 developed radiographic tibiofemoral OA and 64 developed patellofemoral OA after up to 84 months of follow-up, the researchers reported. Latent class analysis identified four groups of MRI lesions for each knee joint, which exhibited sequentially increasing baseline severity for all MRI features except meniscal damage, the investigators added.
For the patellofemoral joint, the odds of incident knee OA rose sequentially with increasing MRI severity, ranging from 1.0 for minimal lesions to 13.7 for severe lesions (95% confidence interval, 5.0-37.0), according to the study. In contrast, the odds of incident knee radiographic OA (ROA) of the tibiofemoral joint were highest for the “mild” and “severe” groups, which had the most meniscal damage and the most extensive history of knee injury and surgery. Odds ratios for these two groups were 5.6 (95% CI, 3.4-9.4) and 5.0 (95% CI, 2.8-9.0), respectively, said the researchers. “Meniscal damage might play a prominent role in the development of incident ROA in the tibiofemoral joint but not the patellofemoral joint,” they added.
Patients in the MOST study had a high risk of knee OA at baseline, which could limit the generalizability of the findings, Dr. Niu and her associates noted.
The National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Disease, both a part of the National Institutes of Health, supported the study. The investigators did not report conflicts of interest.
FROM ARTHRITIS & RHEUMATISM
Key clinical point: Phenotypes of MRI lesions of the tibiofemoral and patellofemoral joints were differentially associated with risk of incident radiographic osteoarthritis.
Major finding: An MRI of the tibiofemoral and patellofemoral joints revealed minimal, mild, moderate, and severe lesions, with corresponding changes in the odds of incident OA.
Data source: Analysis of cohort data for 885 knees from the multicenter, prospective, observational MOST study.
Disclosures: The National Institute on Aging and National Institute of Arthritis and Musculoskeletal and Skin Disease, both a part of the National Institutes of Health, supported the study. The investigators did not report conflicts of interest.
BCVI: Screen with CT angiography, confirm with DSA
LAS VEGAS – Management of blunt cerebrovascular injuries using 64-channel computed tomographic angiography screening coupled with digital subtraction angiography for a definitive diagnosis is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate, according to a review of 228 cases.
The computed tomographic angiography (CTA) screening was positive in 189 patients (83%), and digital subtraction angiography (DSA) confirmed injury in 104 (55%) of those. The remaining 39 patients were found to have no injury on DSA, Dr. Charles P. Shahan of the University of Tennessee, Memphis reported at the annual meeting of the American Association for the Surgery of Trauma (AAST).
Stroke related to blunt cerebrovascular injury (BCVI) occurred in five patients (4.8%); three of those patients were symptomatic at the time of presentation, and two became symptomatic while on therapy for a known lesion. None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke, Dr. Shahan said.
The current study follows a prior study reported at the 2013 AAST annual meeting that suggested that 64-channel multidetector CTA could be the primary screening tool for BCVI. The previously used 32-channel multidetector CTA was found to be inadequate, with a sensitivity of only 52%. Sensitivity increased to 68% with the 64-channel CTA, but the positive predictive value remained remarkably low at 36%, he said.
That study led to a change in the screening algorithm, replacing DSA with CTA for screening, and reserving DSA for definitive BCVI diagnosis following a positive CTA or unexplained neurologic findings, he explained, noting that the rationale was that most injuries missed were low-grade injuries less likely to result in further injury, and that with CTA alone, about two-thirds of patients would be treated unnecessarily because of the false-positive rate.
The purpose of the current study was to evaluate outcomes in the wake of the algorithm change and to assess the potential for missed, clinically significant BCVI.
Study subjects were patients who underwent DSA over an 18-month period after implementation of the algorithm change. Most (64%) were men with a mean age of 43 years and a mean injury severity score of 22 out of 75, indicating moderate or severe injury.
The stroke rate was statistically unchanged in the second study, compared with the first. The findings demonstrate the safety and efficacy of the current management algorithm for BCVI, as well as the value of using DSA to identify false-positive CTA findings. In fact, definitive diagnosis by DSA led to avoidance of potentially harmful anticoagulation in 45% of CTA-positive patients, with no increase in the incidence of strokes resulting from injuries missed by CTA, Dr. Shahan said.
“Considering there were 85 false-positive CTAs, and also considering that our average length of heparin time is approximately 7 days prior to reevaluation, we’ve extrapolated this to nearly 600 heparin infusion days that were avoided by confirmatory DSA testing,” he said, concluding that “CTA with 64-channel multidetector technology with experienced radiology staff in a high-volume center can be safe and effective for BCVI screening.”
He added, however, that false-positive rates with CTA continue to necessitate DSA confirmation to avoid overtreatment.
“We feel that CTA screening with DSA confirmation has allowed us to maintain an acceptably low stroke rate and prevented a tremendous amount of unnecessary anticoagulation in these patients,” he said.
Dr. Clay Cothren Burlew, who was an invited discussant for Dr. Shahan’s paper, applauded Dr. Shahan and his colleagues for “continuing to question the validity of CTA as our primary diagnostic modality for BCVI,” and said the findings made her “stop and think, should we all be doing confirmatory angiography? … Are CTAs actually overcalling 45% of the injuries that we identify?”
Dr. Burlew of the University of Colorado, Denver, questioned whether the high rate of false positives is a result of radiologists who “overcall” questionable findings knowing that a confirmatory angiogram will quickly follow.
“I think this evaluation should be a model for others. Each institution should critically review their individual rates and methods of BCVI diagnosis,” she said, adding that “for centers with a marked increase in the identification of BCVI following institution of CTA as their screening tool, consideration of confirmatory angiography is recommended due to the potential false-positive rate of up to 45%.”
However, confirmatory angiography may not be warranted at centers whose screen yields remain the same with no missed injuries, she said.
“All programs should evaluate their injuries, appropriateness of diagnosis, and impact of subsequent treatment. Only then will we have optimal outcomes,” she concluded.
Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
LAS VEGAS – Management of blunt cerebrovascular injuries using 64-channel computed tomographic angiography screening coupled with digital subtraction angiography for a definitive diagnosis is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate, according to a review of 228 cases.
The computed tomographic angiography (CTA) screening was positive in 189 patients (83%), and digital subtraction angiography (DSA) confirmed injury in 104 (55%) of those. The remaining 39 patients were found to have no injury on DSA, Dr. Charles P. Shahan of the University of Tennessee, Memphis reported at the annual meeting of the American Association for the Surgery of Trauma (AAST).
Stroke related to blunt cerebrovascular injury (BCVI) occurred in five patients (4.8%); three of those patients were symptomatic at the time of presentation, and two became symptomatic while on therapy for a known lesion. None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke, Dr. Shahan said.
The current study follows a prior study reported at the 2013 AAST annual meeting that suggested that 64-channel multidetector CTA could be the primary screening tool for BCVI. The previously used 32-channel multidetector CTA was found to be inadequate, with a sensitivity of only 52%. Sensitivity increased to 68% with the 64-channel CTA, but the positive predictive value remained remarkably low at 36%, he said.
That study led to a change in the screening algorithm, replacing DSA with CTA for screening, and reserving DSA for definitive BCVI diagnosis following a positive CTA or unexplained neurologic findings, he explained, noting that the rationale was that most injuries missed were low-grade injuries less likely to result in further injury, and that with CTA alone, about two-thirds of patients would be treated unnecessarily because of the false-positive rate.
The purpose of the current study was to evaluate outcomes in the wake of the algorithm change and to assess the potential for missed, clinically significant BCVI.
Study subjects were patients who underwent DSA over an 18-month period after implementation of the algorithm change. Most (64%) were men with a mean age of 43 years and a mean injury severity score of 22 out of 75, indicating moderate or severe injury.
The stroke rate was statistically unchanged in the second study, compared with the first. The findings demonstrate the safety and efficacy of the current management algorithm for BCVI, as well as the value of using DSA to identify false-positive CTA findings. In fact, definitive diagnosis by DSA led to avoidance of potentially harmful anticoagulation in 45% of CTA-positive patients, with no increase in the incidence of strokes resulting from injuries missed by CTA, Dr. Shahan said.
“Considering there were 85 false-positive CTAs, and also considering that our average length of heparin time is approximately 7 days prior to reevaluation, we’ve extrapolated this to nearly 600 heparin infusion days that were avoided by confirmatory DSA testing,” he said, concluding that “CTA with 64-channel multidetector technology with experienced radiology staff in a high-volume center can be safe and effective for BCVI screening.”
He added, however, that false-positive rates with CTA continue to necessitate DSA confirmation to avoid overtreatment.
“We feel that CTA screening with DSA confirmation has allowed us to maintain an acceptably low stroke rate and prevented a tremendous amount of unnecessary anticoagulation in these patients,” he said.
Dr. Clay Cothren Burlew, who was an invited discussant for Dr. Shahan’s paper, applauded Dr. Shahan and his colleagues for “continuing to question the validity of CTA as our primary diagnostic modality for BCVI,” and said the findings made her “stop and think, should we all be doing confirmatory angiography? … Are CTAs actually overcalling 45% of the injuries that we identify?”
Dr. Burlew of the University of Colorado, Denver, questioned whether the high rate of false positives is a result of radiologists who “overcall” questionable findings knowing that a confirmatory angiogram will quickly follow.
“I think this evaluation should be a model for others. Each institution should critically review their individual rates and methods of BCVI diagnosis,” she said, adding that “for centers with a marked increase in the identification of BCVI following institution of CTA as their screening tool, consideration of confirmatory angiography is recommended due to the potential false-positive rate of up to 45%.”
However, confirmatory angiography may not be warranted at centers whose screen yields remain the same with no missed injuries, she said.
“All programs should evaluate their injuries, appropriateness of diagnosis, and impact of subsequent treatment. Only then will we have optimal outcomes,” she concluded.
Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
LAS VEGAS – Management of blunt cerebrovascular injuries using 64-channel computed tomographic angiography screening coupled with digital subtraction angiography for a definitive diagnosis is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate, according to a review of 228 cases.
The computed tomographic angiography (CTA) screening was positive in 189 patients (83%), and digital subtraction angiography (DSA) confirmed injury in 104 (55%) of those. The remaining 39 patients were found to have no injury on DSA, Dr. Charles P. Shahan of the University of Tennessee, Memphis reported at the annual meeting of the American Association for the Surgery of Trauma (AAST).
Stroke related to blunt cerebrovascular injury (BCVI) occurred in five patients (4.8%); three of those patients were symptomatic at the time of presentation, and two became symptomatic while on therapy for a known lesion. None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke, Dr. Shahan said.
The current study follows a prior study reported at the 2013 AAST annual meeting that suggested that 64-channel multidetector CTA could be the primary screening tool for BCVI. The previously used 32-channel multidetector CTA was found to be inadequate, with a sensitivity of only 52%. Sensitivity increased to 68% with the 64-channel CTA, but the positive predictive value remained remarkably low at 36%, he said.
That study led to a change in the screening algorithm, replacing DSA with CTA for screening, and reserving DSA for definitive BCVI diagnosis following a positive CTA or unexplained neurologic findings, he explained, noting that the rationale was that most injuries missed were low-grade injuries less likely to result in further injury, and that with CTA alone, about two-thirds of patients would be treated unnecessarily because of the false-positive rate.
The purpose of the current study was to evaluate outcomes in the wake of the algorithm change and to assess the potential for missed, clinically significant BCVI.
Study subjects were patients who underwent DSA over an 18-month period after implementation of the algorithm change. Most (64%) were men with a mean age of 43 years and a mean injury severity score of 22 out of 75, indicating moderate or severe injury.
The stroke rate was statistically unchanged in the second study, compared with the first. The findings demonstrate the safety and efficacy of the current management algorithm for BCVI, as well as the value of using DSA to identify false-positive CTA findings. In fact, definitive diagnosis by DSA led to avoidance of potentially harmful anticoagulation in 45% of CTA-positive patients, with no increase in the incidence of strokes resulting from injuries missed by CTA, Dr. Shahan said.
“Considering there were 85 false-positive CTAs, and also considering that our average length of heparin time is approximately 7 days prior to reevaluation, we’ve extrapolated this to nearly 600 heparin infusion days that were avoided by confirmatory DSA testing,” he said, concluding that “CTA with 64-channel multidetector technology with experienced radiology staff in a high-volume center can be safe and effective for BCVI screening.”
He added, however, that false-positive rates with CTA continue to necessitate DSA confirmation to avoid overtreatment.
“We feel that CTA screening with DSA confirmation has allowed us to maintain an acceptably low stroke rate and prevented a tremendous amount of unnecessary anticoagulation in these patients,” he said.
Dr. Clay Cothren Burlew, who was an invited discussant for Dr. Shahan’s paper, applauded Dr. Shahan and his colleagues for “continuing to question the validity of CTA as our primary diagnostic modality for BCVI,” and said the findings made her “stop and think, should we all be doing confirmatory angiography? … Are CTAs actually overcalling 45% of the injuries that we identify?”
Dr. Burlew of the University of Colorado, Denver, questioned whether the high rate of false positives is a result of radiologists who “overcall” questionable findings knowing that a confirmatory angiogram will quickly follow.
“I think this evaluation should be a model for others. Each institution should critically review their individual rates and methods of BCVI diagnosis,” she said, adding that “for centers with a marked increase in the identification of BCVI following institution of CTA as their screening tool, consideration of confirmatory angiography is recommended due to the potential false-positive rate of up to 45%.”
However, confirmatory angiography may not be warranted at centers whose screen yields remain the same with no missed injuries, she said.
“All programs should evaluate their injuries, appropriateness of diagnosis, and impact of subsequent treatment. Only then will we have optimal outcomes,” she concluded.
Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Use of 64-channel CT angiography screening coupled with digital subtraction angiography for a definitive diagnosis of blunt cerebrovascular injury is safe and effective for identifying clinically significant injury and for maintaining a low stroke rate.
Major finding: None of the patients who had a negative screening CTA, including three with injuries missed on CTA, had a stroke.
Data source: A review of 228 patients with possible BCVI.
Disclosures: Dr. Shahan and Dr. Burlew reported having no relevant financial disclosures.
AGA: Biopsy normal gastric mucosa for H. pylori during esophagogastroduodenoscopy
Obtain gastric biopsies to check for Helicobacter pylori infection in patients undergoing routine esophagogastroduodenoscopy for dyspepsia, even if their mucosa appears normal and they’re immunocompetent, the American Gastroenterological Association advises in a new guideline for upper gastrointestinal biopsy to evaluate dyspepsia in the adult patient in the absence of visible mucosal lesions, which was published in the October issue of Gastroenterology.
The group suggests taking five biopsy specimens from the gastric body and antrum using the updated Sydney System; placing the samples in the same jar; and relying on routine staining to make the call. “Experienced GI pathologists can determine the anatomic location of biopsy specimens sent from the stomach, which obviates the need for” – and cost of – “separating specimens into multiple jars.” They can also identify “virtually all cases of H. pylori .... Therefore, routine use of ancillary special staining” needlessly adds cost, said the guideline authors, led by Dr. Yu-Xiao Yang of the University of Pennsylvania in Philadelphia (Gastroenterology. 2015 Aug 14. doi: 10.1053/j.gastro.2015.07.039).
The group also counsels against routine biopsies of normal-appearing esophagus and gastroesophageal junctions in patients undergoing esophagogastroduodenoscopy (EGD) for dyspepsia, regardless of their immune status.
It does recommend biopsies of normal-appearing duodenum in immunocompromised patients to check for opportunistic infections and graft-versus-host disease (GVHD) after bone marrow transplant, but counsels against routine duodenum biopsies in immunocompetent patients undergoing EGD solely for dyspepsia if signs and symptoms of celiac disease are absent. Again, routine use of special staining isn’t necessary. “Studies using [hematoxylin and eosin] counting methods show similar results as those obtained using CD3 [T-cell marker] stains,” the authors wrote.
The purpose of the guideline is to establish evidence-based standards for biopsies of normal-appearing mucosa in the upper gastrointestinal tract. No such standards existed until now, so “there [is] likely wide practice variation in whether or not such biopsies of normal-appearing mucosa are obtained,” they said.
The advice is based on a thorough review of the medical literature, and a grading of its strength. The guideline is meant for adult patients undergoing EGD solely for dyspepsia, and assumes that endoscopic biopsy has negligible complications.
The esophageal biopsy recommendations are strong, meaning that “most individuals should receive the recommended course of action.” The gastric biopsy advice is a mix of both strong and conditional recommendations, while the duodenal biopsy recommendations are conditional. Evidence for each recommendation ranged from very low to moderate.
Regarding gastric biopsy, H. pylori can lurk in normal-looking mucosa, and evidence supports detection and treatment for both symptom relief and cancer risk reduction. In the immunocompromised, gastric biopsies can also help detect cytomegalovirus infection.
The five-biopsy Sydney System gathers specimens from the lesser and greater curve of the gastric antrum, lesser curvature of the corpus, middle portion of the greater curvature of the corpus, and the incisura angularis. The thorough approach probably detects H. pylori missed by a three-biopsy method, with little added cost.
Esophageal biopsies of normal mucosa aren’t necessary, the authors said, because “although a number of microscopic changes in the esophageal mucosa can be seen in patients with” gastroesophageal reflux disease, they aren’t much help in distinguishing it from heartburn or even healthy controls. For immune compromised patients, GVHD is more likely to show up at other sites, such as the duodenum.
Although the group counsels against routine duodenum biopsies in immunocompetent patients, it did note that “if the suspicion of celiac disease is high, biopsies of the normal-appearing duodenum may be of value even if serologies ... are negative.”
Funding source and disclosures for the work are available from AGA.
Obtain gastric biopsies to check for Helicobacter pylori infection in patients undergoing routine esophagogastroduodenoscopy for dyspepsia, even if their mucosa appears normal and they’re immunocompetent, the American Gastroenterological Association advises in a new guideline for upper gastrointestinal biopsy to evaluate dyspepsia in the adult patient in the absence of visible mucosal lesions, which was published in the October issue of Gastroenterology.
The group suggests taking five biopsy specimens from the gastric body and antrum using the updated Sydney System; placing the samples in the same jar; and relying on routine staining to make the call. “Experienced GI pathologists can determine the anatomic location of biopsy specimens sent from the stomach, which obviates the need for” – and cost of – “separating specimens into multiple jars.” They can also identify “virtually all cases of H. pylori .... Therefore, routine use of ancillary special staining” needlessly adds cost, said the guideline authors, led by Dr. Yu-Xiao Yang of the University of Pennsylvania in Philadelphia (Gastroenterology. 2015 Aug 14. doi: 10.1053/j.gastro.2015.07.039).
The group also counsels against routine biopsies of normal-appearing esophagus and gastroesophageal junctions in patients undergoing esophagogastroduodenoscopy (EGD) for dyspepsia, regardless of their immune status.
It does recommend biopsies of normal-appearing duodenum in immunocompromised patients to check for opportunistic infections and graft-versus-host disease (GVHD) after bone marrow transplant, but counsels against routine duodenum biopsies in immunocompetent patients undergoing EGD solely for dyspepsia if signs and symptoms of celiac disease are absent. Again, routine use of special staining isn’t necessary. “Studies using [hematoxylin and eosin] counting methods show similar results as those obtained using CD3 [T-cell marker] stains,” the authors wrote.
The purpose of the guideline is to establish evidence-based standards for biopsies of normal-appearing mucosa in the upper gastrointestinal tract. No such standards existed until now, so “there [is] likely wide practice variation in whether or not such biopsies of normal-appearing mucosa are obtained,” they said.
The advice is based on a thorough review of the medical literature, and a grading of its strength. The guideline is meant for adult patients undergoing EGD solely for dyspepsia, and assumes that endoscopic biopsy has negligible complications.
The esophageal biopsy recommendations are strong, meaning that “most individuals should receive the recommended course of action.” The gastric biopsy advice is a mix of both strong and conditional recommendations, while the duodenal biopsy recommendations are conditional. Evidence for each recommendation ranged from very low to moderate.
Regarding gastric biopsy, H. pylori can lurk in normal-looking mucosa, and evidence supports detection and treatment for both symptom relief and cancer risk reduction. In the immunocompromised, gastric biopsies can also help detect cytomegalovirus infection.
The five-biopsy Sydney System gathers specimens from the lesser and greater curve of the gastric antrum, lesser curvature of the corpus, middle portion of the greater curvature of the corpus, and the incisura angularis. The thorough approach probably detects H. pylori missed by a three-biopsy method, with little added cost.
Esophageal biopsies of normal mucosa aren’t necessary, the authors said, because “although a number of microscopic changes in the esophageal mucosa can be seen in patients with” gastroesophageal reflux disease, they aren’t much help in distinguishing it from heartburn or even healthy controls. For immune compromised patients, GVHD is more likely to show up at other sites, such as the duodenum.
Although the group counsels against routine duodenum biopsies in immunocompetent patients, it did note that “if the suspicion of celiac disease is high, biopsies of the normal-appearing duodenum may be of value even if serologies ... are negative.”
Funding source and disclosures for the work are available from AGA.
Obtain gastric biopsies to check for Helicobacter pylori infection in patients undergoing routine esophagogastroduodenoscopy for dyspepsia, even if their mucosa appears normal and they’re immunocompetent, the American Gastroenterological Association advises in a new guideline for upper gastrointestinal biopsy to evaluate dyspepsia in the adult patient in the absence of visible mucosal lesions, which was published in the October issue of Gastroenterology.
The group suggests taking five biopsy specimens from the gastric body and antrum using the updated Sydney System; placing the samples in the same jar; and relying on routine staining to make the call. “Experienced GI pathologists can determine the anatomic location of biopsy specimens sent from the stomach, which obviates the need for” – and cost of – “separating specimens into multiple jars.” They can also identify “virtually all cases of H. pylori .... Therefore, routine use of ancillary special staining” needlessly adds cost, said the guideline authors, led by Dr. Yu-Xiao Yang of the University of Pennsylvania in Philadelphia (Gastroenterology. 2015 Aug 14. doi: 10.1053/j.gastro.2015.07.039).
The group also counsels against routine biopsies of normal-appearing esophagus and gastroesophageal junctions in patients undergoing esophagogastroduodenoscopy (EGD) for dyspepsia, regardless of their immune status.
It does recommend biopsies of normal-appearing duodenum in immunocompromised patients to check for opportunistic infections and graft-versus-host disease (GVHD) after bone marrow transplant, but counsels against routine duodenum biopsies in immunocompetent patients undergoing EGD solely for dyspepsia if signs and symptoms of celiac disease are absent. Again, routine use of special staining isn’t necessary. “Studies using [hematoxylin and eosin] counting methods show similar results as those obtained using CD3 [T-cell marker] stains,” the authors wrote.
The purpose of the guideline is to establish evidence-based standards for biopsies of normal-appearing mucosa in the upper gastrointestinal tract. No such standards existed until now, so “there [is] likely wide practice variation in whether or not such biopsies of normal-appearing mucosa are obtained,” they said.
The advice is based on a thorough review of the medical literature, and a grading of its strength. The guideline is meant for adult patients undergoing EGD solely for dyspepsia, and assumes that endoscopic biopsy has negligible complications.
The esophageal biopsy recommendations are strong, meaning that “most individuals should receive the recommended course of action.” The gastric biopsy advice is a mix of both strong and conditional recommendations, while the duodenal biopsy recommendations are conditional. Evidence for each recommendation ranged from very low to moderate.
Regarding gastric biopsy, H. pylori can lurk in normal-looking mucosa, and evidence supports detection and treatment for both symptom relief and cancer risk reduction. In the immunocompromised, gastric biopsies can also help detect cytomegalovirus infection.
The five-biopsy Sydney System gathers specimens from the lesser and greater curve of the gastric antrum, lesser curvature of the corpus, middle portion of the greater curvature of the corpus, and the incisura angularis. The thorough approach probably detects H. pylori missed by a three-biopsy method, with little added cost.
Esophageal biopsies of normal mucosa aren’t necessary, the authors said, because “although a number of microscopic changes in the esophageal mucosa can be seen in patients with” gastroesophageal reflux disease, they aren’t much help in distinguishing it from heartburn or even healthy controls. For immune compromised patients, GVHD is more likely to show up at other sites, such as the duodenum.
Although the group counsels against routine duodenum biopsies in immunocompetent patients, it did note that “if the suspicion of celiac disease is high, biopsies of the normal-appearing duodenum may be of value even if serologies ... are negative.”
Funding source and disclosures for the work are available from AGA.
FROM GASTROENTEROLOGY
Ponatinib’s toxicity limits use for upfront chronic phase CML
The tyrosine kinase inhibitor ponatinib produced a high degree of complete cytogenetic responses when used in first-line treatment of patients with chronic myeloid leukemia in chronic phase. But the drug’s toxicities, especially its propensity for causing thromboembolic events, mitigate against its upfront use, investigators say.
In a small phase II study, 90% of evaluable patients with chronic-phase chronic myeloid leukemia (CML) treated with the tyrosine kinase inhibitor (TKI) had a complete cytogenetic response (CCyR) after 3 months, and 94% had a CCyR after 6 months, but that efficacy came at the cost of significant adverse events requiring dose reductions, treatment interruptions, and early termination of the trial for safety reasons at the recommendation of the Food and Drug Administration, reported Dr. Preetesh Jain of MD Anderson Cancer Center in Houston, Tex., and colleagues.
“[O]ur study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy,” the investigators wrote (Lancet Haematol. 2015;2[9]:e376-e383).
Ponatinib (Iclusig) is a third-generation TKI with action against malignancies with mutations in the ABL kinase domain that make the cancers resistant to first- and second-generation TKIs such as imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna).
Ponatinib is approved in the United States for treatment of adult patients with CML positive for the T3151 mutation in chronic phase, accelerated phase, or blast phase, or T3151-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). It is also approved for treatment of adults with chronic phase, accelerated phase, or blast phase CML or Ph+ALL for whom no other TKI is indicated.
The drug labeling carries a boxed warning about increased risk for vascular occlusion, heart failure, and hepatotoxicity. The warning notes that “[a]rterial and venous thrombosis and occlusions have occurred in at least 27% of Iclusig-treated patients, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures.”
In a single-arm, open label trial, the investigators enrolled 51 patients with recently diagnosed CML in chronic phase, starting them on doses of oral ponatinib 45 mg daily. The protocol was later amended to a starting dose of 30 mg daily because of the high frequency of dose reductions required among patients started at 45 mg. Following a warning from the FDA about vascular complications with the drug, patients were started on daily low-dose aspirin (81 mg), and all drug doses were reduced to either 30 mg or 15 mg daily.
One of the patients discontinued therapy prior to assessment because of the FDA warning, leaving 50 for assessment at 6 months.
Of 46 patients evaluable for the primary outcome of CCyR by 6 months, 43 (94%) achieved it. Major molecular responses, defined as a BCR-ABL to ABL transcript ratio of 0.1% or lower, occurred in 40 of 50 patients (80%) evaluable for this secondary endpoint, and deep molecular response (MR4.5), defined as a ratio less than 0.0032% or lower, occurred in 28 of 50 (55%).
Cardiovascular events, primarily hypertension, occurred in 25 patients (49%), 15 (29%) had grade 3-4 myelosuppression, and 5 patients (10%) developed cerebrovascular or vaso-occlusive disease.
In all, 43 patients (85%) needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated in June 2014, a little more than a year after recruitment began.
The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
The tyrosine kinase inhibitor ponatinib produced a high degree of complete cytogenetic responses when used in first-line treatment of patients with chronic myeloid leukemia in chronic phase. But the drug’s toxicities, especially its propensity for causing thromboembolic events, mitigate against its upfront use, investigators say.
In a small phase II study, 90% of evaluable patients with chronic-phase chronic myeloid leukemia (CML) treated with the tyrosine kinase inhibitor (TKI) had a complete cytogenetic response (CCyR) after 3 months, and 94% had a CCyR after 6 months, but that efficacy came at the cost of significant adverse events requiring dose reductions, treatment interruptions, and early termination of the trial for safety reasons at the recommendation of the Food and Drug Administration, reported Dr. Preetesh Jain of MD Anderson Cancer Center in Houston, Tex., and colleagues.
“[O]ur study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy,” the investigators wrote (Lancet Haematol. 2015;2[9]:e376-e383).
Ponatinib (Iclusig) is a third-generation TKI with action against malignancies with mutations in the ABL kinase domain that make the cancers resistant to first- and second-generation TKIs such as imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna).
Ponatinib is approved in the United States for treatment of adult patients with CML positive for the T3151 mutation in chronic phase, accelerated phase, or blast phase, or T3151-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). It is also approved for treatment of adults with chronic phase, accelerated phase, or blast phase CML or Ph+ALL for whom no other TKI is indicated.
The drug labeling carries a boxed warning about increased risk for vascular occlusion, heart failure, and hepatotoxicity. The warning notes that “[a]rterial and venous thrombosis and occlusions have occurred in at least 27% of Iclusig-treated patients, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures.”
In a single-arm, open label trial, the investigators enrolled 51 patients with recently diagnosed CML in chronic phase, starting them on doses of oral ponatinib 45 mg daily. The protocol was later amended to a starting dose of 30 mg daily because of the high frequency of dose reductions required among patients started at 45 mg. Following a warning from the FDA about vascular complications with the drug, patients were started on daily low-dose aspirin (81 mg), and all drug doses were reduced to either 30 mg or 15 mg daily.
One of the patients discontinued therapy prior to assessment because of the FDA warning, leaving 50 for assessment at 6 months.
Of 46 patients evaluable for the primary outcome of CCyR by 6 months, 43 (94%) achieved it. Major molecular responses, defined as a BCR-ABL to ABL transcript ratio of 0.1% or lower, occurred in 40 of 50 patients (80%) evaluable for this secondary endpoint, and deep molecular response (MR4.5), defined as a ratio less than 0.0032% or lower, occurred in 28 of 50 (55%).
Cardiovascular events, primarily hypertension, occurred in 25 patients (49%), 15 (29%) had grade 3-4 myelosuppression, and 5 patients (10%) developed cerebrovascular or vaso-occlusive disease.
In all, 43 patients (85%) needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated in June 2014, a little more than a year after recruitment began.
The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
The tyrosine kinase inhibitor ponatinib produced a high degree of complete cytogenetic responses when used in first-line treatment of patients with chronic myeloid leukemia in chronic phase. But the drug’s toxicities, especially its propensity for causing thromboembolic events, mitigate against its upfront use, investigators say.
In a small phase II study, 90% of evaluable patients with chronic-phase chronic myeloid leukemia (CML) treated with the tyrosine kinase inhibitor (TKI) had a complete cytogenetic response (CCyR) after 3 months, and 94% had a CCyR after 6 months, but that efficacy came at the cost of significant adverse events requiring dose reductions, treatment interruptions, and early termination of the trial for safety reasons at the recommendation of the Food and Drug Administration, reported Dr. Preetesh Jain of MD Anderson Cancer Center in Houston, Tex., and colleagues.
“[O]ur study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy,” the investigators wrote (Lancet Haematol. 2015;2[9]:e376-e383).
Ponatinib (Iclusig) is a third-generation TKI with action against malignancies with mutations in the ABL kinase domain that make the cancers resistant to first- and second-generation TKIs such as imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna).
Ponatinib is approved in the United States for treatment of adult patients with CML positive for the T3151 mutation in chronic phase, accelerated phase, or blast phase, or T3151-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). It is also approved for treatment of adults with chronic phase, accelerated phase, or blast phase CML or Ph+ALL for whom no other TKI is indicated.
The drug labeling carries a boxed warning about increased risk for vascular occlusion, heart failure, and hepatotoxicity. The warning notes that “[a]rterial and venous thrombosis and occlusions have occurred in at least 27% of Iclusig-treated patients, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures.”
In a single-arm, open label trial, the investigators enrolled 51 patients with recently diagnosed CML in chronic phase, starting them on doses of oral ponatinib 45 mg daily. The protocol was later amended to a starting dose of 30 mg daily because of the high frequency of dose reductions required among patients started at 45 mg. Following a warning from the FDA about vascular complications with the drug, patients were started on daily low-dose aspirin (81 mg), and all drug doses were reduced to either 30 mg or 15 mg daily.
One of the patients discontinued therapy prior to assessment because of the FDA warning, leaving 50 for assessment at 6 months.
Of 46 patients evaluable for the primary outcome of CCyR by 6 months, 43 (94%) achieved it. Major molecular responses, defined as a BCR-ABL to ABL transcript ratio of 0.1% or lower, occurred in 40 of 50 patients (80%) evaluable for this secondary endpoint, and deep molecular response (MR4.5), defined as a ratio less than 0.0032% or lower, occurred in 28 of 50 (55%).
Cardiovascular events, primarily hypertension, occurred in 25 patients (49%), 15 (29%) had grade 3-4 myelosuppression, and 5 patients (10%) developed cerebrovascular or vaso-occlusive disease.
In all, 43 patients (85%) needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated in June 2014, a little more than a year after recruitment began.
The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
FROM LANCET HAEMATOLOGY
Key clinical point: The multi-tyrosine kinase inhibitor ponatinib (Iclusig) may not be suitable as first-line therapy for CML in chronic phase.
Major finding: Among evaluable patients, 94% had a complete cytogenic response by 6 months, but most also required treatment interruptions or dose reductions because of toxicities.
Data source: Single-arm phase II trial of 51 patients with recently diagnosed chronic myeloid leukemia in chronic phase.
Disclosures: The study was sponsored by MD Anderson Cancer Center with partial support from Ariad Pharmaceuticals. Coauthors Hagop Kantarjian, Elias Jabbour, and Jorge Cortes report receiving grants, research support, or serving as consultants to Ariad.
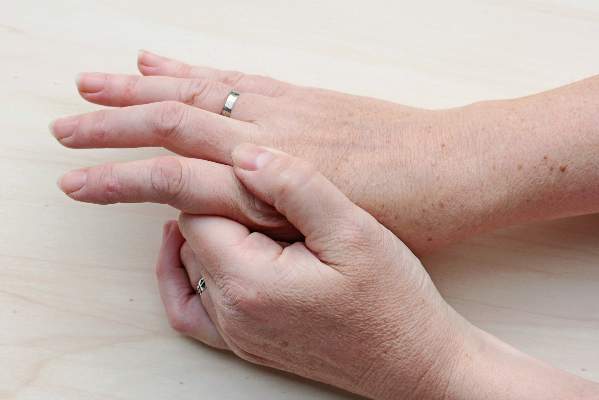
Inflammatory features linked to erosive development in hand OA
Among adults with hand osteoarthritis, ultrasonographic evidence of persistent joint-level inflammation increased the odds of subsequent erosions by as much as 11 times, said authors of a 2-year prospective study.
“These observations implicate a role for inflammation in the pathogenesis of erosive osteoarthritis and might render new therapeutic options that can halt erosive development,” said Dr. Marion C. Kortekaas and her associates at Leiden (the Netherlands) University Medical Center. The findings appeared online in Arthritis & Rheumatology.
The pathogenesis of erosive hand OA remains poorly understood, despite its high clinical burden, the researchers noted. To assess potential risk factors for erosive development, they used standard ultrasonographic methods to examine the interphalangeal joints of 56 consecutive patients who presented to a rheumatology outpatient clinic with hand OA based on American College of Rheumatology criteria. They also scored radiographs for osteophytes or joint-space narrowing with the OARSI method and used the Verbruggen-Veys method to identify and exclude joints that were already eroded (E phase) or remodeled (R phase) at baseline (Arthritis Rheum. 2015 Sep 28. doi: 10.1002/art.39438). At baseline, 18 patients had ultrasonographic evidence of erosions in a total of 51 interphalangeal joints, the investigators said. At the 2.3-year follow-up, a total of 38 interphalangeal joints from 26 patients showed erosive development.
After accounting for age, gender, body mass index, and baseline cartilage and bone abnormalities, all ultrasonographic features of inflammation that were at least grade 1 at baseline and follow-up predicted erosive development. Persistent power Doppler signal was the strongest risk factor, yielding an odds ratio of 11.4 in the adjusted model (95% confidence interval, 2.7-49.1). Other significant risk factors included moderate to severe baseline synovial thickening (OR, 8.8; 95% CI, 2.4-32.3) and power Doppler signal (OR, 7.1; 95% CI, 1.9-26.9).
“The present study confirms that inflammatory US features found at baseline are associated with erosive development on the joint level in hand OA,” the investigators wrote. “These associations are already found after 2 years of follow-up, which is important for future prospective trials.”
The researchers reported having no funding sources or conflicts of interest.
Among adults with hand osteoarthritis, ultrasonographic evidence of persistent joint-level inflammation increased the odds of subsequent erosions by as much as 11 times, said authors of a 2-year prospective study.
“These observations implicate a role for inflammation in the pathogenesis of erosive osteoarthritis and might render new therapeutic options that can halt erosive development,” said Dr. Marion C. Kortekaas and her associates at Leiden (the Netherlands) University Medical Center. The findings appeared online in Arthritis & Rheumatology.
The pathogenesis of erosive hand OA remains poorly understood, despite its high clinical burden, the researchers noted. To assess potential risk factors for erosive development, they used standard ultrasonographic methods to examine the interphalangeal joints of 56 consecutive patients who presented to a rheumatology outpatient clinic with hand OA based on American College of Rheumatology criteria. They also scored radiographs for osteophytes or joint-space narrowing with the OARSI method and used the Verbruggen-Veys method to identify and exclude joints that were already eroded (E phase) or remodeled (R phase) at baseline (Arthritis Rheum. 2015 Sep 28. doi: 10.1002/art.39438). At baseline, 18 patients had ultrasonographic evidence of erosions in a total of 51 interphalangeal joints, the investigators said. At the 2.3-year follow-up, a total of 38 interphalangeal joints from 26 patients showed erosive development.
After accounting for age, gender, body mass index, and baseline cartilage and bone abnormalities, all ultrasonographic features of inflammation that were at least grade 1 at baseline and follow-up predicted erosive development. Persistent power Doppler signal was the strongest risk factor, yielding an odds ratio of 11.4 in the adjusted model (95% confidence interval, 2.7-49.1). Other significant risk factors included moderate to severe baseline synovial thickening (OR, 8.8; 95% CI, 2.4-32.3) and power Doppler signal (OR, 7.1; 95% CI, 1.9-26.9).
“The present study confirms that inflammatory US features found at baseline are associated with erosive development on the joint level in hand OA,” the investigators wrote. “These associations are already found after 2 years of follow-up, which is important for future prospective trials.”
The researchers reported having no funding sources or conflicts of interest.
Among adults with hand osteoarthritis, ultrasonographic evidence of persistent joint-level inflammation increased the odds of subsequent erosions by as much as 11 times, said authors of a 2-year prospective study.
“These observations implicate a role for inflammation in the pathogenesis of erosive osteoarthritis and might render new therapeutic options that can halt erosive development,” said Dr. Marion C. Kortekaas and her associates at Leiden (the Netherlands) University Medical Center. The findings appeared online in Arthritis & Rheumatology.
The pathogenesis of erosive hand OA remains poorly understood, despite its high clinical burden, the researchers noted. To assess potential risk factors for erosive development, they used standard ultrasonographic methods to examine the interphalangeal joints of 56 consecutive patients who presented to a rheumatology outpatient clinic with hand OA based on American College of Rheumatology criteria. They also scored radiographs for osteophytes or joint-space narrowing with the OARSI method and used the Verbruggen-Veys method to identify and exclude joints that were already eroded (E phase) or remodeled (R phase) at baseline (Arthritis Rheum. 2015 Sep 28. doi: 10.1002/art.39438). At baseline, 18 patients had ultrasonographic evidence of erosions in a total of 51 interphalangeal joints, the investigators said. At the 2.3-year follow-up, a total of 38 interphalangeal joints from 26 patients showed erosive development.
After accounting for age, gender, body mass index, and baseline cartilage and bone abnormalities, all ultrasonographic features of inflammation that were at least grade 1 at baseline and follow-up predicted erosive development. Persistent power Doppler signal was the strongest risk factor, yielding an odds ratio of 11.4 in the adjusted model (95% confidence interval, 2.7-49.1). Other significant risk factors included moderate to severe baseline synovial thickening (OR, 8.8; 95% CI, 2.4-32.3) and power Doppler signal (OR, 7.1; 95% CI, 1.9-26.9).
“The present study confirms that inflammatory US features found at baseline are associated with erosive development on the joint level in hand OA,” the investigators wrote. “These associations are already found after 2 years of follow-up, which is important for future prospective trials.”
The researchers reported having no funding sources or conflicts of interest.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Ultrasonographic evidence of inflammation significantly predicted the development of erosions in hand osteoarthritis.
Major finding: All inflammatory ultrasonographic features that were at least grade 1 at baseline and follow-up significantly predicted erosive development.
Data source: Single-center, prospective, ultrasonographic and radiographic study of 56 patients who met ACR criteria for hand OA.
Disclosures: The investigators reported having no funding sources or conflicts of interest.
Word choice affects public perception of drugs

Photo courtesy of the FDA
Using the words “breakthrough” and “promising” to describe new drugs affects the public’s perception of the drugs’ effectiveness, according to a study published in JAMA Internal Medicine.
Investigators noted that, in everyday usage, the term “breakthrough” represents a highly significant or definitive advance.
However, the US Food and Drug Administration’s (FDA’s) “breakthrough therapy designation” has a different meaning.
Since the Safety and Innovation Act became law in 2012, the FDA can assign breakthrough designation to a drug that “treats a serious or life-threatening condition” and “may demonstrate a substantial improvement . . . over available therapies” based on preliminary evidence.
And since the creation of the Safety and Innovation Act, all FDA press releases announcing the approval of breakthrough-designated drugs have used the term “breakthrough,” while about half have used the term “promising.”
“Today, patients and their families can easily find FDA press releases on the Internet, or they often hear about them in the news,” said study author Steven Woloshin, MD, of The Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
“But the reality is that unless patients fully understand how the FDA is using the term ‘breakthrough,’ they may have unwarranted confidence in the evidence supporting drug claims. So we thought it was important to test how these terms affect the judgement of people without medical training.”
Survey details
The investigators conducted an online survey of 597 Americans. Participants were randomly given 1 of 5 short descriptions of a recently approved drug.
The descriptions were based on an FDA press release for a metastatic lung cancer breakthrough-designated drug that was conditionally approved based on the surrogate outcome of tumor shrinkage.
The first, “facts-only” description described the drug as meeting the criteria for breakthrough designation but did not actually use the term “breakthrough.”
A second and a third description included the facts and added the terms “breakthrough” and “promising,” respectively.
The fourth, “tentative” description included the facts, used the word “breakthrough,” and used the following FDA-required language for professional labeling:
The FDA pointed out that the drug was approved based on tumor shrinkage but that an improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The fifth, “definitive” description included the same information as the tentative description, but “may be contingent” was changed to “is contingent.”
Participants were then asked to judge the drug’s benefit, harm, and strength of evidence.
Results
The investigators found that adding either “breakthrough” or “promising” in the description significantly increased the percentage of participants who rated the drug as “very” or “completely” effective compared with the facts-only description—23% and 25% vs 11%.
Adding “breakthrough” or “promising” to the description also significantly increased the number of people who reported believing that evidence supporting the drug is “strong” or “extremely strong”—59% and 63% vs 43%.
At the same time, adding either the tentative or definitive explanations significantly reduced the percentage of participants who believed (incorrectly) that the drug had been “proven to save lives”—16% tentative and 10% definitive vs 31% breakthrough.
Finally, when participants were asked which of 2 drugs—one described as “breakthrough,” the other as meeting the breakthrough criteria—they would take for a potentially deadly condition, 92% chose the “breakthrough” drug.
“Our findings clearly indicate that words like ‘breakthrough’ and ‘promising’ increase people’s beliefs in a drug’s effectiveness,” said Lisa Schwartz, MD, of The Dartmouth Institute for Health Policy and Clinical Practice.
“In light of [the findings], press releases with neutral terms and that clearly explain the limited evidence supporting what breakthrough designation and accelerated approval mean might help consumers make more accurate judgements about these drugs.” ![]()

Photo courtesy of the FDA
Using the words “breakthrough” and “promising” to describe new drugs affects the public’s perception of the drugs’ effectiveness, according to a study published in JAMA Internal Medicine.
Investigators noted that, in everyday usage, the term “breakthrough” represents a highly significant or definitive advance.
However, the US Food and Drug Administration’s (FDA’s) “breakthrough therapy designation” has a different meaning.
Since the Safety and Innovation Act became law in 2012, the FDA can assign breakthrough designation to a drug that “treats a serious or life-threatening condition” and “may demonstrate a substantial improvement . . . over available therapies” based on preliminary evidence.
And since the creation of the Safety and Innovation Act, all FDA press releases announcing the approval of breakthrough-designated drugs have used the term “breakthrough,” while about half have used the term “promising.”
“Today, patients and their families can easily find FDA press releases on the Internet, or they often hear about them in the news,” said study author Steven Woloshin, MD, of The Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
“But the reality is that unless patients fully understand how the FDA is using the term ‘breakthrough,’ they may have unwarranted confidence in the evidence supporting drug claims. So we thought it was important to test how these terms affect the judgement of people without medical training.”
Survey details
The investigators conducted an online survey of 597 Americans. Participants were randomly given 1 of 5 short descriptions of a recently approved drug.
The descriptions were based on an FDA press release for a metastatic lung cancer breakthrough-designated drug that was conditionally approved based on the surrogate outcome of tumor shrinkage.
The first, “facts-only” description described the drug as meeting the criteria for breakthrough designation but did not actually use the term “breakthrough.”
A second and a third description included the facts and added the terms “breakthrough” and “promising,” respectively.
The fourth, “tentative” description included the facts, used the word “breakthrough,” and used the following FDA-required language for professional labeling:
The FDA pointed out that the drug was approved based on tumor shrinkage but that an improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The fifth, “definitive” description included the same information as the tentative description, but “may be contingent” was changed to “is contingent.”
Participants were then asked to judge the drug’s benefit, harm, and strength of evidence.
Results
The investigators found that adding either “breakthrough” or “promising” in the description significantly increased the percentage of participants who rated the drug as “very” or “completely” effective compared with the facts-only description—23% and 25% vs 11%.
Adding “breakthrough” or “promising” to the description also significantly increased the number of people who reported believing that evidence supporting the drug is “strong” or “extremely strong”—59% and 63% vs 43%.
At the same time, adding either the tentative or definitive explanations significantly reduced the percentage of participants who believed (incorrectly) that the drug had been “proven to save lives”—16% tentative and 10% definitive vs 31% breakthrough.
Finally, when participants were asked which of 2 drugs—one described as “breakthrough,” the other as meeting the breakthrough criteria—they would take for a potentially deadly condition, 92% chose the “breakthrough” drug.
“Our findings clearly indicate that words like ‘breakthrough’ and ‘promising’ increase people’s beliefs in a drug’s effectiveness,” said Lisa Schwartz, MD, of The Dartmouth Institute for Health Policy and Clinical Practice.
“In light of [the findings], press releases with neutral terms and that clearly explain the limited evidence supporting what breakthrough designation and accelerated approval mean might help consumers make more accurate judgements about these drugs.” ![]()

Photo courtesy of the FDA
Using the words “breakthrough” and “promising” to describe new drugs affects the public’s perception of the drugs’ effectiveness, according to a study published in JAMA Internal Medicine.
Investigators noted that, in everyday usage, the term “breakthrough” represents a highly significant or definitive advance.
However, the US Food and Drug Administration’s (FDA’s) “breakthrough therapy designation” has a different meaning.
Since the Safety and Innovation Act became law in 2012, the FDA can assign breakthrough designation to a drug that “treats a serious or life-threatening condition” and “may demonstrate a substantial improvement . . . over available therapies” based on preliminary evidence.
And since the creation of the Safety and Innovation Act, all FDA press releases announcing the approval of breakthrough-designated drugs have used the term “breakthrough,” while about half have used the term “promising.”
“Today, patients and their families can easily find FDA press releases on the Internet, or they often hear about them in the news,” said study author Steven Woloshin, MD, of The Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire.
“But the reality is that unless patients fully understand how the FDA is using the term ‘breakthrough,’ they may have unwarranted confidence in the evidence supporting drug claims. So we thought it was important to test how these terms affect the judgement of people without medical training.”
Survey details
The investigators conducted an online survey of 597 Americans. Participants were randomly given 1 of 5 short descriptions of a recently approved drug.
The descriptions were based on an FDA press release for a metastatic lung cancer breakthrough-designated drug that was conditionally approved based on the surrogate outcome of tumor shrinkage.
The first, “facts-only” description described the drug as meeting the criteria for breakthrough designation but did not actually use the term “breakthrough.”
A second and a third description included the facts and added the terms “breakthrough” and “promising,” respectively.
The fourth, “tentative” description included the facts, used the word “breakthrough,” and used the following FDA-required language for professional labeling:
The FDA pointed out that the drug was approved based on tumor shrinkage but that an improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
The fifth, “definitive” description included the same information as the tentative description, but “may be contingent” was changed to “is contingent.”
Participants were then asked to judge the drug’s benefit, harm, and strength of evidence.
Results
The investigators found that adding either “breakthrough” or “promising” in the description significantly increased the percentage of participants who rated the drug as “very” or “completely” effective compared with the facts-only description—23% and 25% vs 11%.
Adding “breakthrough” or “promising” to the description also significantly increased the number of people who reported believing that evidence supporting the drug is “strong” or “extremely strong”—59% and 63% vs 43%.
At the same time, adding either the tentative or definitive explanations significantly reduced the percentage of participants who believed (incorrectly) that the drug had been “proven to save lives”—16% tentative and 10% definitive vs 31% breakthrough.
Finally, when participants were asked which of 2 drugs—one described as “breakthrough,” the other as meeting the breakthrough criteria—they would take for a potentially deadly condition, 92% chose the “breakthrough” drug.
“Our findings clearly indicate that words like ‘breakthrough’ and ‘promising’ increase people’s beliefs in a drug’s effectiveness,” said Lisa Schwartz, MD, of The Dartmouth Institute for Health Policy and Clinical Practice.
“In light of [the findings], press releases with neutral terms and that clearly explain the limited evidence supporting what breakthrough designation and accelerated approval mean might help consumers make more accurate judgements about these drugs.” ![]()
Team identifies therapeutic target for HIT
Researchers believe they have identified a therapeutic target for heparin-induced thrombocytopenia (HIT).
The team noted that HIT is caused by antibodies to complexes that form between platelet factor 4 (PF4), which is released from activated platelets, and heparin or cellular glycosaminoglycans.
The researchers elucidated the crystal structure of 3 PF4 complexes and found evidence suggesting that tetramerization of PF4 is targetable.
Zheng Cai, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described this work in Nature Communications.
Previously, the researchers identified KKO, a murine monoclonal antibody to PF4/heparin complexes that causes HIT in a murine model. The team said human HIT antibodies compete with KKO for binding to PF4/heparin, and KKO augments the formation of pathogenic immune complexes.
The researchers also identified RTO, an isotype-matched, anti-PF4 antibody that binds to PF4 but does not generate pathogenic complexes.
For the current study, the team described and compared the crystal structures of PF4 in complex with Fabs derived from KKO and RTO to the structure of PF4 in complex with fondaparinux.
The researchers noted that PF4 molecules can exist singly as monomers, doubly as dimers, and as a 4-part complex called a tetramer, which have an “open” end and a “closed” end.
The crystal structure of PF4 in complex with fondaparinux showed that fondaparinux binds to the “closed” end of the PF4 tetramer, which stabilizes the tetramer.
The crystal structure of PF4 in complex with KKO showed that KKO binds to the “open” end of the stabilized tetramer, making contact with 3 of 4 monomers in the tetramer.
The researchers said this helps explain the requirement for heparin as a backbone for the complex. They also said this finding provides new insight into how a normal host protein such as PF4 can be converted into a target of the host immune system, which leads to an autoimmune disorder.
The crystal structure of PF4 in complex with RTO showed that RTO binds to PF4 monomers rather than tetramers. And RTO binds to the monomers in a way that prevents them from combining into tetramers.
Via cell experiments, the researchers confirmed that RTO prevents the formation of antigenic complexes, as well as the activation of platelets by KKO and human HIT antibodies. RTO also prevented clot formation caused by KKO in a mouse model of HIT.
These results suggest that binding of RTO to PF4 monomers prevents the formation of pathogenic complexes that are central to the pathology of HIT. So the researchers believe RTO can provide the basis for new diagnostics and may pave the way for a therapy to stop HIT early in its progression. ![]()

Researchers believe they have identified a therapeutic target for heparin-induced thrombocytopenia (HIT).
The team noted that HIT is caused by antibodies to complexes that form between platelet factor 4 (PF4), which is released from activated platelets, and heparin or cellular glycosaminoglycans.
The researchers elucidated the crystal structure of 3 PF4 complexes and found evidence suggesting that tetramerization of PF4 is targetable.
Zheng Cai, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described this work in Nature Communications.
Previously, the researchers identified KKO, a murine monoclonal antibody to PF4/heparin complexes that causes HIT in a murine model. The team said human HIT antibodies compete with KKO for binding to PF4/heparin, and KKO augments the formation of pathogenic immune complexes.
The researchers also identified RTO, an isotype-matched, anti-PF4 antibody that binds to PF4 but does not generate pathogenic complexes.
For the current study, the team described and compared the crystal structures of PF4 in complex with Fabs derived from KKO and RTO to the structure of PF4 in complex with fondaparinux.
The researchers noted that PF4 molecules can exist singly as monomers, doubly as dimers, and as a 4-part complex called a tetramer, which have an “open” end and a “closed” end.
The crystal structure of PF4 in complex with fondaparinux showed that fondaparinux binds to the “closed” end of the PF4 tetramer, which stabilizes the tetramer.
The crystal structure of PF4 in complex with KKO showed that KKO binds to the “open” end of the stabilized tetramer, making contact with 3 of 4 monomers in the tetramer.
The researchers said this helps explain the requirement for heparin as a backbone for the complex. They also said this finding provides new insight into how a normal host protein such as PF4 can be converted into a target of the host immune system, which leads to an autoimmune disorder.
The crystal structure of PF4 in complex with RTO showed that RTO binds to PF4 monomers rather than tetramers. And RTO binds to the monomers in a way that prevents them from combining into tetramers.
Via cell experiments, the researchers confirmed that RTO prevents the formation of antigenic complexes, as well as the activation of platelets by KKO and human HIT antibodies. RTO also prevented clot formation caused by KKO in a mouse model of HIT.
These results suggest that binding of RTO to PF4 monomers prevents the formation of pathogenic complexes that are central to the pathology of HIT. So the researchers believe RTO can provide the basis for new diagnostics and may pave the way for a therapy to stop HIT early in its progression. ![]()

Researchers believe they have identified a therapeutic target for heparin-induced thrombocytopenia (HIT).
The team noted that HIT is caused by antibodies to complexes that form between platelet factor 4 (PF4), which is released from activated platelets, and heparin or cellular glycosaminoglycans.
The researchers elucidated the crystal structure of 3 PF4 complexes and found evidence suggesting that tetramerization of PF4 is targetable.
Zheng Cai, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described this work in Nature Communications.
Previously, the researchers identified KKO, a murine monoclonal antibody to PF4/heparin complexes that causes HIT in a murine model. The team said human HIT antibodies compete with KKO for binding to PF4/heparin, and KKO augments the formation of pathogenic immune complexes.
The researchers also identified RTO, an isotype-matched, anti-PF4 antibody that binds to PF4 but does not generate pathogenic complexes.
For the current study, the team described and compared the crystal structures of PF4 in complex with Fabs derived from KKO and RTO to the structure of PF4 in complex with fondaparinux.
The researchers noted that PF4 molecules can exist singly as monomers, doubly as dimers, and as a 4-part complex called a tetramer, which have an “open” end and a “closed” end.
The crystal structure of PF4 in complex with fondaparinux showed that fondaparinux binds to the “closed” end of the PF4 tetramer, which stabilizes the tetramer.
The crystal structure of PF4 in complex with KKO showed that KKO binds to the “open” end of the stabilized tetramer, making contact with 3 of 4 monomers in the tetramer.
The researchers said this helps explain the requirement for heparin as a backbone for the complex. They also said this finding provides new insight into how a normal host protein such as PF4 can be converted into a target of the host immune system, which leads to an autoimmune disorder.
The crystal structure of PF4 in complex with RTO showed that RTO binds to PF4 monomers rather than tetramers. And RTO binds to the monomers in a way that prevents them from combining into tetramers.
Via cell experiments, the researchers confirmed that RTO prevents the formation of antigenic complexes, as well as the activation of platelets by KKO and human HIT antibodies. RTO also prevented clot formation caused by KKO in a mouse model of HIT.
These results suggest that binding of RTO to PF4 monomers prevents the formation of pathogenic complexes that are central to the pathology of HIT. So the researchers believe RTO can provide the basis for new diagnostics and may pave the way for a therapy to stop HIT early in its progression. ![]()
Hematocrit level may predict need for transfusion

Photo by Elise Amendola
A young trauma patient’s hematocrit level at hospital admission may predict the need for transfusion, new research suggests.
Results of this retrospective, single-center study indicate that children and adolescents with a hematocrit level of 35% or less at admission are more likely than their peers with higher hematocrit levels to require a transfusion after trauma.
The study was published in the Journal of Trauma and Acute Care Surgery.
“A quick and cost-effective measure, such as admission hematocrit, to identify pediatric patients who are at a high risk for bleeding could provide a critical improvement in optimizing care for children, while reducing costs,” said study author Christopher P. Gayer, MD, PhD, of Children's Hospital Los Angeles (CHLA) in California.
For this research, Dr Gayer and his colleagues examined the medical records of all patients, ages 0 to 17, who presented to the level 1 pediatric trauma center at CHLA between 2005 and 2013.
Of all the patients, 1341 had hematocrit measured at admission. The researchers divided this group into patients who required an intervention—transfusion or operation—for their bleeding (n=93) and those who did not (n=1248).
The mean hematocrit was 38.0 for patients who did not require an intervention and 34.3 for those who did (P<0.01). The mean hematocrit was 26.9 in patients who required a transfusion secondary to bleeding and 36.0 in those who required an operative intervention for nonhemostatic indications (P<0.01).
The researchers then analyzed a subset of patients who had an abdominal CT scan, as these patients had a definitive presence or absence of intra-abdominal injury. There was a significant decrease in admission hematocrit between patients who required a transfusion for an intra-abdominal injury and those who did not—29% and 37%, respectively (P<0.01).
Serial hematocrit values remained significantly lower in the patients who required a transfusion up to 67 hours after admission (P=0.04). The researchers said this suggests that hematocrit is a valuable predictor for requiring a transfusion at least 2 days after an injury, and it may be useful for patients presenting well after their initial injury.
The team then evaluated whether an admission hematocrit cutoff of 35% or less could predict the need for transfusion. For all patients, a cutoff of 35% or less had a sensitivity of 94%, specificity of 77%, positive-predictive value of 5%, and negative predictive value of 99.9%.
In patients who had an abdominal CT, a cutoff hematocrit of 35% or less had a sensitivity of 90%, specificity of 76%, positive predictive value of 21%, and negative predictive value of 99%.
These results led the researchers to conclude that this hematocrit cutoff may be a reliable screening tool.
“Admission hematocrit can be done rapidly in the trauma bay, is relatively inexpensive, causes minimal harm, and can aid in critical decision-making and rapid identification of occult bleeding,” said study author Jamie Golden, MD, of CHLA.
“Our results show that a hematocrit level of less than 35% on admission predicts a greater likelihood for the need of transfusion in pediatric blunt trauma patients.”
The researchers stressed that a doctor's concern in the face of clinical signs of hemorrhagic shock should always take priority over lab data. However, a repeat hematocrit can be quickly and easily performed if clinically indicated.
They added that the results of their study require validation in a prospective, multicenter study. ![]()

Photo by Elise Amendola
A young trauma patient’s hematocrit level at hospital admission may predict the need for transfusion, new research suggests.
Results of this retrospective, single-center study indicate that children and adolescents with a hematocrit level of 35% or less at admission are more likely than their peers with higher hematocrit levels to require a transfusion after trauma.
The study was published in the Journal of Trauma and Acute Care Surgery.
“A quick and cost-effective measure, such as admission hematocrit, to identify pediatric patients who are at a high risk for bleeding could provide a critical improvement in optimizing care for children, while reducing costs,” said study author Christopher P. Gayer, MD, PhD, of Children's Hospital Los Angeles (CHLA) in California.
For this research, Dr Gayer and his colleagues examined the medical records of all patients, ages 0 to 17, who presented to the level 1 pediatric trauma center at CHLA between 2005 and 2013.
Of all the patients, 1341 had hematocrit measured at admission. The researchers divided this group into patients who required an intervention—transfusion or operation—for their bleeding (n=93) and those who did not (n=1248).
The mean hematocrit was 38.0 for patients who did not require an intervention and 34.3 for those who did (P<0.01). The mean hematocrit was 26.9 in patients who required a transfusion secondary to bleeding and 36.0 in those who required an operative intervention for nonhemostatic indications (P<0.01).
The researchers then analyzed a subset of patients who had an abdominal CT scan, as these patients had a definitive presence or absence of intra-abdominal injury. There was a significant decrease in admission hematocrit between patients who required a transfusion for an intra-abdominal injury and those who did not—29% and 37%, respectively (P<0.01).
Serial hematocrit values remained significantly lower in the patients who required a transfusion up to 67 hours after admission (P=0.04). The researchers said this suggests that hematocrit is a valuable predictor for requiring a transfusion at least 2 days after an injury, and it may be useful for patients presenting well after their initial injury.
The team then evaluated whether an admission hematocrit cutoff of 35% or less could predict the need for transfusion. For all patients, a cutoff of 35% or less had a sensitivity of 94%, specificity of 77%, positive-predictive value of 5%, and negative predictive value of 99.9%.
In patients who had an abdominal CT, a cutoff hematocrit of 35% or less had a sensitivity of 90%, specificity of 76%, positive predictive value of 21%, and negative predictive value of 99%.
These results led the researchers to conclude that this hematocrit cutoff may be a reliable screening tool.
“Admission hematocrit can be done rapidly in the trauma bay, is relatively inexpensive, causes minimal harm, and can aid in critical decision-making and rapid identification of occult bleeding,” said study author Jamie Golden, MD, of CHLA.
“Our results show that a hematocrit level of less than 35% on admission predicts a greater likelihood for the need of transfusion in pediatric blunt trauma patients.”
The researchers stressed that a doctor's concern in the face of clinical signs of hemorrhagic shock should always take priority over lab data. However, a repeat hematocrit can be quickly and easily performed if clinically indicated.
They added that the results of their study require validation in a prospective, multicenter study. ![]()

Photo by Elise Amendola
A young trauma patient’s hematocrit level at hospital admission may predict the need for transfusion, new research suggests.
Results of this retrospective, single-center study indicate that children and adolescents with a hematocrit level of 35% or less at admission are more likely than their peers with higher hematocrit levels to require a transfusion after trauma.
The study was published in the Journal of Trauma and Acute Care Surgery.
“A quick and cost-effective measure, such as admission hematocrit, to identify pediatric patients who are at a high risk for bleeding could provide a critical improvement in optimizing care for children, while reducing costs,” said study author Christopher P. Gayer, MD, PhD, of Children's Hospital Los Angeles (CHLA) in California.
For this research, Dr Gayer and his colleagues examined the medical records of all patients, ages 0 to 17, who presented to the level 1 pediatric trauma center at CHLA between 2005 and 2013.
Of all the patients, 1341 had hematocrit measured at admission. The researchers divided this group into patients who required an intervention—transfusion or operation—for their bleeding (n=93) and those who did not (n=1248).
The mean hematocrit was 38.0 for patients who did not require an intervention and 34.3 for those who did (P<0.01). The mean hematocrit was 26.9 in patients who required a transfusion secondary to bleeding and 36.0 in those who required an operative intervention for nonhemostatic indications (P<0.01).
The researchers then analyzed a subset of patients who had an abdominal CT scan, as these patients had a definitive presence or absence of intra-abdominal injury. There was a significant decrease in admission hematocrit between patients who required a transfusion for an intra-abdominal injury and those who did not—29% and 37%, respectively (P<0.01).
Serial hematocrit values remained significantly lower in the patients who required a transfusion up to 67 hours after admission (P=0.04). The researchers said this suggests that hematocrit is a valuable predictor for requiring a transfusion at least 2 days after an injury, and it may be useful for patients presenting well after their initial injury.
The team then evaluated whether an admission hematocrit cutoff of 35% or less could predict the need for transfusion. For all patients, a cutoff of 35% or less had a sensitivity of 94%, specificity of 77%, positive-predictive value of 5%, and negative predictive value of 99.9%.
In patients who had an abdominal CT, a cutoff hematocrit of 35% or less had a sensitivity of 90%, specificity of 76%, positive predictive value of 21%, and negative predictive value of 99%.
These results led the researchers to conclude that this hematocrit cutoff may be a reliable screening tool.
“Admission hematocrit can be done rapidly in the trauma bay, is relatively inexpensive, causes minimal harm, and can aid in critical decision-making and rapid identification of occult bleeding,” said study author Jamie Golden, MD, of CHLA.
“Our results show that a hematocrit level of less than 35% on admission predicts a greater likelihood for the need of transfusion in pediatric blunt trauma patients.”
The researchers stressed that a doctor's concern in the face of clinical signs of hemorrhagic shock should always take priority over lab data. However, a repeat hematocrit can be quickly and easily performed if clinically indicated.
They added that the results of their study require validation in a prospective, multicenter study. ![]()
Study reveals tumor suppressor in AML
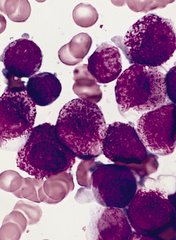
The protein-coding gene hnRNP K acts as a tumor suppressor in acute myeloid leukemia (AML), according to research published in Cancer Cell.
Investigators found that AML patients who carried a partial deletion of chromosome 9 also experienced a significant decrease in hnRNP K expression.
This deletion, 9q21.32, along with the decreased levels of hnRNP K, led to reduced survival and increased tumor formation.
“Our data implicates hnRNP K in the development of blood disorders and suggests it acts as a tumor suppressor,” said Sean Post, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Both in vivo and in vitro results indicate that hnRNP K achieves this through regulation of key genetic pathways. Our study found that hnRNP K expression must be maintained for proper cellular regulation and to prevent tumor formation.”
Dr Post and his colleagues examined hnRNP K’s role in tumorigenesis by generating a mouse model harboring an Hnrnpk knockout allele.
They found that Hnrnpk haploinsufficiency resulted in reduced survival, increased tumor formation, genomic instability, and the development of transplantable hematopoietic neoplasms with myeloproliferation.
“Our findings showed that Hnrnpk haploinsufficiency led to tumor development by deregulating cell proliferation and differentiation programs through control of key cellular pathways, which suggests these pathways may be exploited by targeted therapies,” Dr Post said.
Specifically, he and his colleagues found that reduced hnRNP K expression attenuated p21 activation, downregulated C/EBP levels, and activated STAT3 signaling.
The investigators also analyzed samples from AML patients who harbored 9q21.32 and found a significant decrease in hnRNP K expression.
“It was clear that these changes in AML patients with the 9q21.32 deletion resulted in a tumor suppressor gene involved in blood cancer development,” Dr Post said. ![]()

The protein-coding gene hnRNP K acts as a tumor suppressor in acute myeloid leukemia (AML), according to research published in Cancer Cell.
Investigators found that AML patients who carried a partial deletion of chromosome 9 also experienced a significant decrease in hnRNP K expression.
This deletion, 9q21.32, along with the decreased levels of hnRNP K, led to reduced survival and increased tumor formation.
“Our data implicates hnRNP K in the development of blood disorders and suggests it acts as a tumor suppressor,” said Sean Post, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Both in vivo and in vitro results indicate that hnRNP K achieves this through regulation of key genetic pathways. Our study found that hnRNP K expression must be maintained for proper cellular regulation and to prevent tumor formation.”
Dr Post and his colleagues examined hnRNP K’s role in tumorigenesis by generating a mouse model harboring an Hnrnpk knockout allele.
They found that Hnrnpk haploinsufficiency resulted in reduced survival, increased tumor formation, genomic instability, and the development of transplantable hematopoietic neoplasms with myeloproliferation.
“Our findings showed that Hnrnpk haploinsufficiency led to tumor development by deregulating cell proliferation and differentiation programs through control of key cellular pathways, which suggests these pathways may be exploited by targeted therapies,” Dr Post said.
Specifically, he and his colleagues found that reduced hnRNP K expression attenuated p21 activation, downregulated C/EBP levels, and activated STAT3 signaling.
The investigators also analyzed samples from AML patients who harbored 9q21.32 and found a significant decrease in hnRNP K expression.
“It was clear that these changes in AML patients with the 9q21.32 deletion resulted in a tumor suppressor gene involved in blood cancer development,” Dr Post said. ![]()

The protein-coding gene hnRNP K acts as a tumor suppressor in acute myeloid leukemia (AML), according to research published in Cancer Cell.
Investigators found that AML patients who carried a partial deletion of chromosome 9 also experienced a significant decrease in hnRNP K expression.
This deletion, 9q21.32, along with the decreased levels of hnRNP K, led to reduced survival and increased tumor formation.
“Our data implicates hnRNP K in the development of blood disorders and suggests it acts as a tumor suppressor,” said Sean Post, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Both in vivo and in vitro results indicate that hnRNP K achieves this through regulation of key genetic pathways. Our study found that hnRNP K expression must be maintained for proper cellular regulation and to prevent tumor formation.”
Dr Post and his colleagues examined hnRNP K’s role in tumorigenesis by generating a mouse model harboring an Hnrnpk knockout allele.
They found that Hnrnpk haploinsufficiency resulted in reduced survival, increased tumor formation, genomic instability, and the development of transplantable hematopoietic neoplasms with myeloproliferation.
“Our findings showed that Hnrnpk haploinsufficiency led to tumor development by deregulating cell proliferation and differentiation programs through control of key cellular pathways, which suggests these pathways may be exploited by targeted therapies,” Dr Post said.
Specifically, he and his colleagues found that reduced hnRNP K expression attenuated p21 activation, downregulated C/EBP levels, and activated STAT3 signaling.
The investigators also analyzed samples from AML patients who harbored 9q21.32 and found a significant decrease in hnRNP K expression.
“It was clear that these changes in AML patients with the 9q21.32 deletion resulted in a tumor suppressor gene involved in blood cancer development,” Dr Post said. ![]()
Occupational Contact Dermatitis From Carbapenems
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.
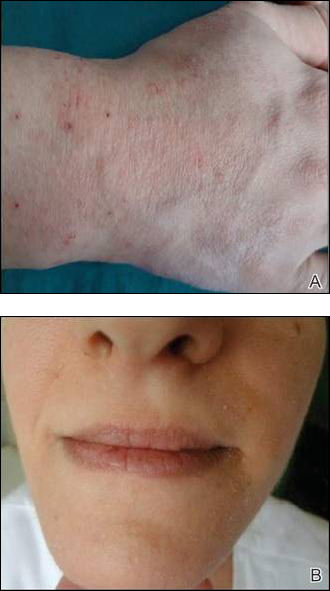
One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.
Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.

One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.
Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.

One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.
Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.