User login
Chromoblastomycosis
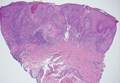
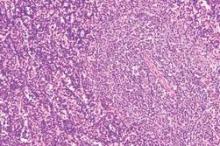
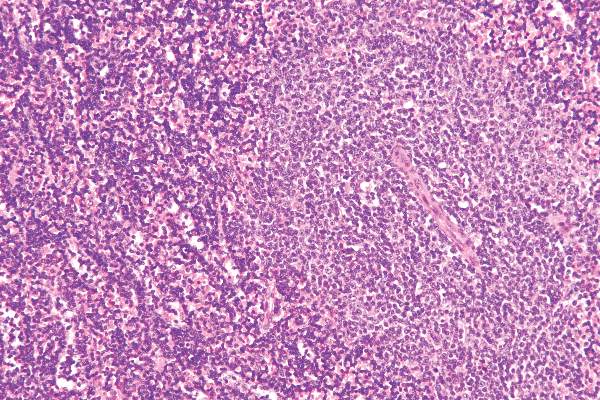
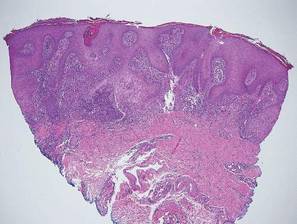
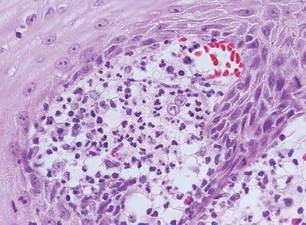
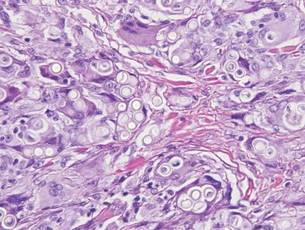
Chromoblastomycosis is a chronic fungal infection of the skin and subcutaneous tissues that demonstrates characteristic Medlar or sclerotic bodies that resemble copper pennies on histopathology.1 Cutaneous infection often results from direct inoculation, such as from a wood splinter. Clinically, the lesion typically is a pink papule that progresses to a verrucous plaque on the legs of farmers or rural workers in the tropics or subtropics. There usually are no associated constitutional symptoms. Several dematiaceous (darkly pigmented) fungi cause chromoblastomycosis, including Fonsecaea compacta, Cladophialophora carrionii, Rhinocladiella aquaspersa, Phialophora verrucosa, and Fonsecaea pedrosoi. Cellular division occurs by internal septation rather than budding. Skin biopsy can confirm the diagnosis.1 Chromoblastomycosis is histopathologically characterized by pseudoepitheli- omatous hyperplasia (Figure 1) with histiocytes and neutrophils surrounding distinct copper-colored Medlar bodies (6–12 μm)(Figure 2), which are fungal spores.1-3 Several conditions demonstrate pseudoepitheliomatous hyperplasia with intraepidermal pustules and can be remembered by the mnemonic “here come big green leafy vegetables”: halogenoderma, chromoblastomycosis, blastomycosis, granuloma inguinale, leishmaniasis, and pemphigus vegetans.2 Treatment of chromoblastomycosis can be challenging, as no standard treatment has been established and therapy can be complicated by low cure rates and high relapse rates, especially in chronic and extensive disease. Treatment can include cryotherapy or surgical excision for small lesions in combination with systemic antifungals.4 Itraconazole (200–400 mg daily) for at least 6 months has been reported to have up to a 90% cure rate with mild to moderate disease and 44% with severe disease.5 Combination oral antifungal treatment with itraconazole and terbinafine has been recommended.6 There are reports of progression of chromoblastomycosis to squamous cell carcinoma, which is rare and occurred after long-standing, inadequately treated lesions.7

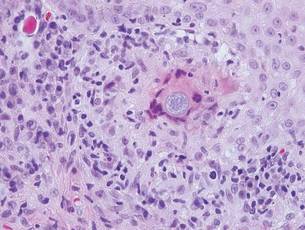
Blastomycosis also presents with pseudoepitheliomatous hyperplasia, as seen in chromoblastomycosis, but organisms typically are few in number and demonstrate a thick, asymmetrical, refractile wall and a dark nucleus. Although chromoblastomycosis and blastomycosis are similar in size (8–15 μm), the broad-based budding of blastomycosis (Figure 3) is a key feature and the yeast are not pigmented.1-3 Blastomycosis is caused by Blastomyces dermatitidis and is endemic to the Mississippi and Ohio River valleys, Great Lakes region, and Southeastern United States. Cutaneous infection typically occurs from inhalation of the dimorphic fungi into the lungs and occasional dissemination involving the skin, causing papulopustules and thick, crusted, warty plaques with central ulceration. Rarely, primary cutaneous blastomycosis can occur from direct inoculation, typically in a laboratory. Treatment of disseminated blastomycosis includes systemic antifungals.1
Coccidioidomycosis is characterized by large spherules (10–80 μm) with refractile walls and granular gray cytoplasm.2,3 Coccidioidomycosis spherules occasionally contain endospores2 and often are noticeably larger than surrounding histiocyte nuclei (Figure 4), whereas chromoblastomycosis, blastomycosis, cryptococcosis, and lobomycosis are more similar in size to histiocyte nuclei. Coccidioidomycosis is caused by Coccidioides immitis, a highly virulent dimorphic fungus found in the Southwestern United States, northern Mexico, and Central and South America. Pulmonary infection occurs by inhalation of arthroconidia, often from soil, and is asymptomatic in most patients; however, immunocompromised patients are predisposed to disseminated cutaneous infection. Facial lesions are most common and can present as papules, pustules, plaques, abscesses, sinus tracts, and/or ulcerations. Treatment of disseminated infection requires systemic antifungals; amphotericin B has proven most effective.1
Cryptococcosis is characterized by vacuoles with small (2–20 μm), central, pleomorphic yeast (Figure 5). The vacuole is due to a gelati- nous capsule that stains red with mucicarmine and blue with Alcian blue.2,3 Cryptococcosis is caused by Cryptococcus neoformans and is associated with pigeon droppings. Disseminated infection in patients with human immunodefi- ciency virus often presents as umbilicated molluscumlike lesions and portends a poor prognosis with a mortality rate of up to 80%.8 Disseminated infection necessitates aggressive treatment with systemic antifungals.1
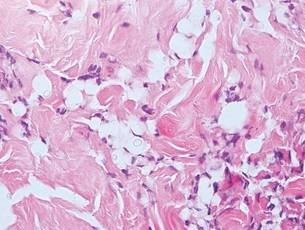
Lobomycosis demonstrates thick-walled, refractile spherules with surrounding histiocytes and multinucleated giant cells. The yeast of lobomycosis (6–12 μm) is of similar size to chromoblastomycosis and blastomycosis, but linear chains resembling a child’s pop beads are characteristic of this condition (Figure 6).2,3 Lobomycosis is caused by Lacazia loboi and is acquired most frequently through contact with dolphins in Central and South America. Clinically, lesions present as slow-growing, keloidlike nodules, often on the face, ears, and distal extremities. Surgical treatment may be required given that oral antifungals typically are ineffective.1
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Fernandez-Flores A, Saeb-Lima M, Arenas-Guzman R. Morphological findings of deep cutaneous fungal infections. Am J Dermatopathol. 2014;36:531-556.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Queiroz-Telles F, McGinnis MR, Salkin I, et al. Subcutaneous mycoses. Infect Dis Clin North Am. 2003;17:59-85.
- Bonifaz A, Paredes-Solís, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Rojas OC, González GM, Moreno-Treviño M, et al. Chromoblastomycosis by Cladophialophora carrionii associated with squamous cell carcinoma and review of published reports. Mycopathologia. 2015;179:153-157.
- Durden FM, Elewski B. Cutaneous involvement with Cryptococcus neoformans in AIDS. J Am Acad Dermatol. 1994;30:844-848.
Chromoblastomycosis is a chronic fungal infection of the skin and subcutaneous tissues that demonstrates characteristic Medlar or sclerotic bodies that resemble copper pennies on histopathology.1 Cutaneous infection often results from direct inoculation, such as from a wood splinter. Clinically, the lesion typically is a pink papule that progresses to a verrucous plaque on the legs of farmers or rural workers in the tropics or subtropics. There usually are no associated constitutional symptoms. Several dematiaceous (darkly pigmented) fungi cause chromoblastomycosis, including Fonsecaea compacta, Cladophialophora carrionii, Rhinocladiella aquaspersa, Phialophora verrucosa, and Fonsecaea pedrosoi. Cellular division occurs by internal septation rather than budding. Skin biopsy can confirm the diagnosis.1 Chromoblastomycosis is histopathologically characterized by pseudoepitheli- omatous hyperplasia (Figure 1) with histiocytes and neutrophils surrounding distinct copper-colored Medlar bodies (6–12 μm)(Figure 2), which are fungal spores.1-3 Several conditions demonstrate pseudoepitheliomatous hyperplasia with intraepidermal pustules and can be remembered by the mnemonic “here come big green leafy vegetables”: halogenoderma, chromoblastomycosis, blastomycosis, granuloma inguinale, leishmaniasis, and pemphigus vegetans.2 Treatment of chromoblastomycosis can be challenging, as no standard treatment has been established and therapy can be complicated by low cure rates and high relapse rates, especially in chronic and extensive disease. Treatment can include cryotherapy or surgical excision for small lesions in combination with systemic antifungals.4 Itraconazole (200–400 mg daily) for at least 6 months has been reported to have up to a 90% cure rate with mild to moderate disease and 44% with severe disease.5 Combination oral antifungal treatment with itraconazole and terbinafine has been recommended.6 There are reports of progression of chromoblastomycosis to squamous cell carcinoma, which is rare and occurred after long-standing, inadequately treated lesions.7


Blastomycosis also presents with pseudoepitheliomatous hyperplasia, as seen in chromoblastomycosis, but organisms typically are few in number and demonstrate a thick, asymmetrical, refractile wall and a dark nucleus. Although chromoblastomycosis and blastomycosis are similar in size (8–15 μm), the broad-based budding of blastomycosis (Figure 3) is a key feature and the yeast are not pigmented.1-3 Blastomycosis is caused by Blastomyces dermatitidis and is endemic to the Mississippi and Ohio River valleys, Great Lakes region, and Southeastern United States. Cutaneous infection typically occurs from inhalation of the dimorphic fungi into the lungs and occasional dissemination involving the skin, causing papulopustules and thick, crusted, warty plaques with central ulceration. Rarely, primary cutaneous blastomycosis can occur from direct inoculation, typically in a laboratory. Treatment of disseminated blastomycosis includes systemic antifungals.1

Coccidioidomycosis is characterized by large spherules (10–80 μm) with refractile walls and granular gray cytoplasm.2,3 Coccidioidomycosis spherules occasionally contain endospores2 and often are noticeably larger than surrounding histiocyte nuclei (Figure 4), whereas chromoblastomycosis, blastomycosis, cryptococcosis, and lobomycosis are more similar in size to histiocyte nuclei. Coccidioidomycosis is caused by Coccidioides immitis, a highly virulent dimorphic fungus found in the Southwestern United States, northern Mexico, and Central and South America. Pulmonary infection occurs by inhalation of arthroconidia, often from soil, and is asymptomatic in most patients; however, immunocompromised patients are predisposed to disseminated cutaneous infection. Facial lesions are most common and can present as papules, pustules, plaques, abscesses, sinus tracts, and/or ulcerations. Treatment of disseminated infection requires systemic antifungals; amphotericin B has proven most effective.1

Cryptococcosis is characterized by vacuoles with small (2–20 μm), central, pleomorphic yeast (Figure 5). The vacuole is due to a gelati- nous capsule that stains red with mucicarmine and blue with Alcian blue.2,3 Cryptococcosis is caused by Cryptococcus neoformans and is associated with pigeon droppings. Disseminated infection in patients with human immunodefi- ciency virus often presents as umbilicated molluscumlike lesions and portends a poor prognosis with a mortality rate of up to 80%.8 Disseminated infection necessitates aggressive treatment with systemic antifungals.1

Lobomycosis demonstrates thick-walled, refractile spherules with surrounding histiocytes and multinucleated giant cells. The yeast of lobomycosis (6–12 μm) is of similar size to chromoblastomycosis and blastomycosis, but linear chains resembling a child’s pop beads are characteristic of this condition (Figure 6).2,3 Lobomycosis is caused by Lacazia loboi and is acquired most frequently through contact with dolphins in Central and South America. Clinically, lesions present as slow-growing, keloidlike nodules, often on the face, ears, and distal extremities. Surgical treatment may be required given that oral antifungals typically are ineffective.1

Chromoblastomycosis is a chronic fungal infection of the skin and subcutaneous tissues that demonstrates characteristic Medlar or sclerotic bodies that resemble copper pennies on histopathology.1 Cutaneous infection often results from direct inoculation, such as from a wood splinter. Clinically, the lesion typically is a pink papule that progresses to a verrucous plaque on the legs of farmers or rural workers in the tropics or subtropics. There usually are no associated constitutional symptoms. Several dematiaceous (darkly pigmented) fungi cause chromoblastomycosis, including Fonsecaea compacta, Cladophialophora carrionii, Rhinocladiella aquaspersa, Phialophora verrucosa, and Fonsecaea pedrosoi. Cellular division occurs by internal septation rather than budding. Skin biopsy can confirm the diagnosis.1 Chromoblastomycosis is histopathologically characterized by pseudoepitheli- omatous hyperplasia (Figure 1) with histiocytes and neutrophils surrounding distinct copper-colored Medlar bodies (6–12 μm)(Figure 2), which are fungal spores.1-3 Several conditions demonstrate pseudoepitheliomatous hyperplasia with intraepidermal pustules and can be remembered by the mnemonic “here come big green leafy vegetables”: halogenoderma, chromoblastomycosis, blastomycosis, granuloma inguinale, leishmaniasis, and pemphigus vegetans.2 Treatment of chromoblastomycosis can be challenging, as no standard treatment has been established and therapy can be complicated by low cure rates and high relapse rates, especially in chronic and extensive disease. Treatment can include cryotherapy or surgical excision for small lesions in combination with systemic antifungals.4 Itraconazole (200–400 mg daily) for at least 6 months has been reported to have up to a 90% cure rate with mild to moderate disease and 44% with severe disease.5 Combination oral antifungal treatment with itraconazole and terbinafine has been recommended.6 There are reports of progression of chromoblastomycosis to squamous cell carcinoma, which is rare and occurred after long-standing, inadequately treated lesions.7


Blastomycosis also presents with pseudoepitheliomatous hyperplasia, as seen in chromoblastomycosis, but organisms typically are few in number and demonstrate a thick, asymmetrical, refractile wall and a dark nucleus. Although chromoblastomycosis and blastomycosis are similar in size (8–15 μm), the broad-based budding of blastomycosis (Figure 3) is a key feature and the yeast are not pigmented.1-3 Blastomycosis is caused by Blastomyces dermatitidis and is endemic to the Mississippi and Ohio River valleys, Great Lakes region, and Southeastern United States. Cutaneous infection typically occurs from inhalation of the dimorphic fungi into the lungs and occasional dissemination involving the skin, causing papulopustules and thick, crusted, warty plaques with central ulceration. Rarely, primary cutaneous blastomycosis can occur from direct inoculation, typically in a laboratory. Treatment of disseminated blastomycosis includes systemic antifungals.1

Coccidioidomycosis is characterized by large spherules (10–80 μm) with refractile walls and granular gray cytoplasm.2,3 Coccidioidomycosis spherules occasionally contain endospores2 and often are noticeably larger than surrounding histiocyte nuclei (Figure 4), whereas chromoblastomycosis, blastomycosis, cryptococcosis, and lobomycosis are more similar in size to histiocyte nuclei. Coccidioidomycosis is caused by Coccidioides immitis, a highly virulent dimorphic fungus found in the Southwestern United States, northern Mexico, and Central and South America. Pulmonary infection occurs by inhalation of arthroconidia, often from soil, and is asymptomatic in most patients; however, immunocompromised patients are predisposed to disseminated cutaneous infection. Facial lesions are most common and can present as papules, pustules, plaques, abscesses, sinus tracts, and/or ulcerations. Treatment of disseminated infection requires systemic antifungals; amphotericin B has proven most effective.1

Cryptococcosis is characterized by vacuoles with small (2–20 μm), central, pleomorphic yeast (Figure 5). The vacuole is due to a gelati- nous capsule that stains red with mucicarmine and blue with Alcian blue.2,3 Cryptococcosis is caused by Cryptococcus neoformans and is associated with pigeon droppings. Disseminated infection in patients with human immunodefi- ciency virus often presents as umbilicated molluscumlike lesions and portends a poor prognosis with a mortality rate of up to 80%.8 Disseminated infection necessitates aggressive treatment with systemic antifungals.1

Lobomycosis demonstrates thick-walled, refractile spherules with surrounding histiocytes and multinucleated giant cells. The yeast of lobomycosis (6–12 μm) is of similar size to chromoblastomycosis and blastomycosis, but linear chains resembling a child’s pop beads are characteristic of this condition (Figure 6).2,3 Lobomycosis is caused by Lacazia loboi and is acquired most frequently through contact with dolphins in Central and South America. Clinically, lesions present as slow-growing, keloidlike nodules, often on the face, ears, and distal extremities. Surgical treatment may be required given that oral antifungals typically are ineffective.1

- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Fernandez-Flores A, Saeb-Lima M, Arenas-Guzman R. Morphological findings of deep cutaneous fungal infections. Am J Dermatopathol. 2014;36:531-556.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Queiroz-Telles F, McGinnis MR, Salkin I, et al. Subcutaneous mycoses. Infect Dis Clin North Am. 2003;17:59-85.
- Bonifaz A, Paredes-Solís, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Rojas OC, González GM, Moreno-Treviño M, et al. Chromoblastomycosis by Cladophialophora carrionii associated with squamous cell carcinoma and review of published reports. Mycopathologia. 2015;179:153-157.
- Durden FM, Elewski B. Cutaneous involvement with Cryptococcus neoformans in AIDS. J Am Acad Dermatol. 1994;30:844-848.
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Fernandez-Flores A, Saeb-Lima M, Arenas-Guzman R. Morphological findings of deep cutaneous fungal infections. Am J Dermatopathol. 2014;36:531-556.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Queiroz-Telles F, McGinnis MR, Salkin I, et al. Subcutaneous mycoses. Infect Dis Clin North Am. 2003;17:59-85.
- Bonifaz A, Paredes-Solís, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Rojas OC, González GM, Moreno-Treviño M, et al. Chromoblastomycosis by Cladophialophora carrionii associated with squamous cell carcinoma and review of published reports. Mycopathologia. 2015;179:153-157.
- Durden FM, Elewski B. Cutaneous involvement with Cryptococcus neoformans in AIDS. J Am Acad Dermatol. 1994;30:844-848.
The Surgical M&M Conference: Balancing a Blame-Free Environment with Individual Responsibility
The traditional Surgical Morbidity and Mortality Conference that I remember so well from my residency days has changed. Not everything has changed, however. Usually the most senior resident involved still presents the case along with a discussion of the operation performed and the complication. There is invariably a discussion of the central question, “What should have been done differently?” Residents still occasionally get nervous before presenting a case as I did years ago, and there is still the occasional disagreement over how a surgical issue was handled. But there are subtle differences notable in the M&M discussions of today.
Rather than focusing on who did something wrong, there is today more often a focus on the “systems issues” in the case. In other words, if a pneumothorax was missed after a central line placement, the discussion today is much more commonly focused on the systems that should have been in place to ensure that such an abnormality was noted and acted upon. In years past, the focus was squarely on identifying which resident shirked his or her responsibility to review the film.
This current “blame-free” environment is the hallmark of a “learning organization” that aims to use the review process to improve performance. Mistakes are viewed as opportunities for learning and improving the system. And the nonpunitive analysis goes a long way toward improving morale among the residents and certainly encourages teamwork and identification of mechanisms to avoid errors within a hospital or service. These are all good things. But I worry that perhaps there is a tendency to go too far with avoiding individual responsibility.
Sometimes it is easy to talk about things “just happening” in large medical systems of today. Many surgeons are accustomed to dictating operative reports in the passive voice. For example, I find myself routinely stating, “the patient was prepped and draped,” “an incision was made,” and “exposure was obtained.” All these statements suggest that things happened and, perhaps “mistakes were made,” but there is little attribution to a specific actor. Unfortunately, it can be easy to also talk about patient care in a similarly abstract manner in which it is hard to identify who did what to whom.
The central question, I believe, is whether this new focus on the system and the team is ultimately better for patient care. We do want all members of the operating room team, for example, to feel responsible for speaking up when something does not seem right. We want every person involved in a patient’s care to feel comfortable with stopping an incorrect intervention. Surgeons, in particular, should not be upset by having the medical student question which side of the patient is being operated upon. Hierarchy should never stand in the way of speaking up to avoid an error being made. Nevertheless, we must not completely eliminate the sense of personal responsibility that each individual caregiver should feel toward ensuring the well-being of the patient.
In 1937, Chicago surgeon Max Thorek, M.D., wrote a pioneering book entitled, Surgical Errors and Safeguards. Dr. Thorek wrote, “While it is human to err, it is inhuman not to try, if possible, to protect those who entrust their lives into our hands from avoidable failures and danger.” I believe that this philosophy continues to be embodied in the Surgical M&M conference.
One of the central components of the M&M discussion has not changed. After all of the discussion about systems and corporate responsibility, I believe that the most common statement that I have heard from the treating surgeon is, “My error was that I should have done ... ” Although some observers might see this ascription of the individual role of the surgeon to be anachronistic, I believe that it captures the reality of the situation that even though patients are operated upon by teams, it is most commonly an individual relationship with a specific surgeon that has prompted the patient to go ahead with the surgery. We must not lose sight of the importance of that individual relationship and the responsibility that the individual surgeon has in influencing patient choice. In many ways, although the tenor of the Surgical M&M conference has changed the old question of “What could I have done differently?” remains of central importance to ensuring that surgeons take responsibility for their patients’ well-being.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
The traditional Surgical Morbidity and Mortality Conference that I remember so well from my residency days has changed. Not everything has changed, however. Usually the most senior resident involved still presents the case along with a discussion of the operation performed and the complication. There is invariably a discussion of the central question, “What should have been done differently?” Residents still occasionally get nervous before presenting a case as I did years ago, and there is still the occasional disagreement over how a surgical issue was handled. But there are subtle differences notable in the M&M discussions of today.
Rather than focusing on who did something wrong, there is today more often a focus on the “systems issues” in the case. In other words, if a pneumothorax was missed after a central line placement, the discussion today is much more commonly focused on the systems that should have been in place to ensure that such an abnormality was noted and acted upon. In years past, the focus was squarely on identifying which resident shirked his or her responsibility to review the film.
This current “blame-free” environment is the hallmark of a “learning organization” that aims to use the review process to improve performance. Mistakes are viewed as opportunities for learning and improving the system. And the nonpunitive analysis goes a long way toward improving morale among the residents and certainly encourages teamwork and identification of mechanisms to avoid errors within a hospital or service. These are all good things. But I worry that perhaps there is a tendency to go too far with avoiding individual responsibility.
Sometimes it is easy to talk about things “just happening” in large medical systems of today. Many surgeons are accustomed to dictating operative reports in the passive voice. For example, I find myself routinely stating, “the patient was prepped and draped,” “an incision was made,” and “exposure was obtained.” All these statements suggest that things happened and, perhaps “mistakes were made,” but there is little attribution to a specific actor. Unfortunately, it can be easy to also talk about patient care in a similarly abstract manner in which it is hard to identify who did what to whom.
The central question, I believe, is whether this new focus on the system and the team is ultimately better for patient care. We do want all members of the operating room team, for example, to feel responsible for speaking up when something does not seem right. We want every person involved in a patient’s care to feel comfortable with stopping an incorrect intervention. Surgeons, in particular, should not be upset by having the medical student question which side of the patient is being operated upon. Hierarchy should never stand in the way of speaking up to avoid an error being made. Nevertheless, we must not completely eliminate the sense of personal responsibility that each individual caregiver should feel toward ensuring the well-being of the patient.
In 1937, Chicago surgeon Max Thorek, M.D., wrote a pioneering book entitled, Surgical Errors and Safeguards. Dr. Thorek wrote, “While it is human to err, it is inhuman not to try, if possible, to protect those who entrust their lives into our hands from avoidable failures and danger.” I believe that this philosophy continues to be embodied in the Surgical M&M conference.
One of the central components of the M&M discussion has not changed. After all of the discussion about systems and corporate responsibility, I believe that the most common statement that I have heard from the treating surgeon is, “My error was that I should have done ... ” Although some observers might see this ascription of the individual role of the surgeon to be anachronistic, I believe that it captures the reality of the situation that even though patients are operated upon by teams, it is most commonly an individual relationship with a specific surgeon that has prompted the patient to go ahead with the surgery. We must not lose sight of the importance of that individual relationship and the responsibility that the individual surgeon has in influencing patient choice. In many ways, although the tenor of the Surgical M&M conference has changed the old question of “What could I have done differently?” remains of central importance to ensuring that surgeons take responsibility for their patients’ well-being.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
The traditional Surgical Morbidity and Mortality Conference that I remember so well from my residency days has changed. Not everything has changed, however. Usually the most senior resident involved still presents the case along with a discussion of the operation performed and the complication. There is invariably a discussion of the central question, “What should have been done differently?” Residents still occasionally get nervous before presenting a case as I did years ago, and there is still the occasional disagreement over how a surgical issue was handled. But there are subtle differences notable in the M&M discussions of today.
Rather than focusing on who did something wrong, there is today more often a focus on the “systems issues” in the case. In other words, if a pneumothorax was missed after a central line placement, the discussion today is much more commonly focused on the systems that should have been in place to ensure that such an abnormality was noted and acted upon. In years past, the focus was squarely on identifying which resident shirked his or her responsibility to review the film.
This current “blame-free” environment is the hallmark of a “learning organization” that aims to use the review process to improve performance. Mistakes are viewed as opportunities for learning and improving the system. And the nonpunitive analysis goes a long way toward improving morale among the residents and certainly encourages teamwork and identification of mechanisms to avoid errors within a hospital or service. These are all good things. But I worry that perhaps there is a tendency to go too far with avoiding individual responsibility.
Sometimes it is easy to talk about things “just happening” in large medical systems of today. Many surgeons are accustomed to dictating operative reports in the passive voice. For example, I find myself routinely stating, “the patient was prepped and draped,” “an incision was made,” and “exposure was obtained.” All these statements suggest that things happened and, perhaps “mistakes were made,” but there is little attribution to a specific actor. Unfortunately, it can be easy to also talk about patient care in a similarly abstract manner in which it is hard to identify who did what to whom.
The central question, I believe, is whether this new focus on the system and the team is ultimately better for patient care. We do want all members of the operating room team, for example, to feel responsible for speaking up when something does not seem right. We want every person involved in a patient’s care to feel comfortable with stopping an incorrect intervention. Surgeons, in particular, should not be upset by having the medical student question which side of the patient is being operated upon. Hierarchy should never stand in the way of speaking up to avoid an error being made. Nevertheless, we must not completely eliminate the sense of personal responsibility that each individual caregiver should feel toward ensuring the well-being of the patient.
In 1937, Chicago surgeon Max Thorek, M.D., wrote a pioneering book entitled, Surgical Errors and Safeguards. Dr. Thorek wrote, “While it is human to err, it is inhuman not to try, if possible, to protect those who entrust their lives into our hands from avoidable failures and danger.” I believe that this philosophy continues to be embodied in the Surgical M&M conference.
One of the central components of the M&M discussion has not changed. After all of the discussion about systems and corporate responsibility, I believe that the most common statement that I have heard from the treating surgeon is, “My error was that I should have done ... ” Although some observers might see this ascription of the individual role of the surgeon to be anachronistic, I believe that it captures the reality of the situation that even though patients are operated upon by teams, it is most commonly an individual relationship with a specific surgeon that has prompted the patient to go ahead with the surgery. We must not lose sight of the importance of that individual relationship and the responsibility that the individual surgeon has in influencing patient choice. In many ways, although the tenor of the Surgical M&M conference has changed the old question of “What could I have done differently?” remains of central importance to ensuring that surgeons take responsibility for their patients’ well-being.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Your College: A Remarkable Organization
Peering out from 28 floors above the busy, early morning streets of downtown Chicago, I was entranced by the view. The rising sun in the east created a shimmering, iridescent play of light on the waters of Lake Michigan that extended as far as the eye could see. The room I was in also commanded my attention. Several rows of desks, each with a computer screen, faced a single elevated line of chairs for the leaders of the deliberations that were about to take place. Above this row on the front wall of this imposing room is emblazoned the seal of our College with its mission statement, “The ACS is dedicated to improving the care of the surgical patient and to safeguarding standards of care in an optimal and ethical practice environment,” to remind those in the room of the ultimate purpose in serving this professional organization. So the Regents room and view appeared to me, a newcomer to these meetings of the leadership of the American College of Surgeons.
I hope I can convince you in the paragraphs that follow that this mission and its execution by Regents, Governors, and Fellows of the ACS, are every bit as noble as the architecture of the room and the view it affords.
I have been a Fellow of the American College of Surgeons since 1980. My membership in this extraordinary association has provided me many benefits and numerous opportunities. In the early years, I significantly expanded the knowledge base I had gained in residency by attending every fall Clinical Congress and consuming as many educational offerings as time would permit during this nearly week-long learning marathon. After a few years, I was granted the privilege of being on the instructor end of several of these educational exchanges. At that time in my career, it appeared to me that the ACS’s main and almost sole purpose was to provide continuing education for surgeons who attended the annual Clinical Congress. I have subsequently found that it is so much more.
In 2005, I was invited to represent one of my specialist surgical societies as a Governor. During my 6-year term, I came to realize that the key purpose of the Board of Governors (BOG) is to provide an effective communication conduit between the Fellows and the sole policy-making body of the ACS, the Board of Regents (BOR). In recent years, most of the Regents have attended the annual BOG meeting in order to facilitate this interaction.
In 2012, I was elected First Vice-President of the ACS. Now as an officer of the College, I was invited for 2 years to attend all BOR meetings and to be in the mainstream of all communications relating to strategy and policy. These opportunities provided me with an intimate, inside look at how this large organization of nearly 80,000 members functions to serve the entire surgical profession including each of its many specialties. What I learned about the internal workings of the leadership and those who dedicate their time to this work has reinforced my own commitment to the ACS. It is a remarkable organization.
This brings us back to the well-designed and impressive Regents’ room high above Chicago. The hum of numerous disjointed conversations ceased as the Chair of the ACS Board of Regents called the June 2012 meeting to order. My attention is now focused on the proceedings rather than on the beauty of Lake Michigan below. Although every surgical specialty is represented among the 22 Regents, all discussion was invariably directed toward the betterment of the surgical profession as a whole rather than about any specific specialty’s interests.
The Regents are dedicated servants of the ACS. In addition to three one-and-a-half day meetings annually, each of which requires hours of reading in preparation, most of the Regents serve on at least two committees of the Board. Regents are nominated by Fellows, advisory councils, and committees, and are elected by the much larger BOG which represents every state and Canadian province, several countries, and many surgery specialist societies. In addition to assuring that all surgical specialties are represented, bylaws of the ACS state that the President of the ACS and two Canadian Fellows must be among the BOR membership. Based on my 2-year experience, the BOG has exhibited considerable wisdom in their choice of Regents.
The officers of the ACS (President-Elect, First and Second Vice-Presidents and Vice-Presidents-Elect, Secretary, Treasurer), and the officers of the BOG (Chair, Vice-Chair, and Secretary/Treasurer) attend all BOR meetings and serve in an advisory capacity. Also in attendance and providing essential input are executive members of the ACS staff and, representing the younger ACS membership, the chairs of the Resident Associates Society (RAS) and the Young Fellows Association (YAF). Although only Regents can vote and are therefore responsible for establishing ACS policy, I discovered they welcome participation from all in attendance. I always felt that my contributions and those of other non-voting attendees were thoughtfully and carefully considered.
Much of the preparatory work for BOR meetings is done in the committees that meet just prior to the full Board meeting. I had the pleasure of being on the Honors Committee that selects Honorary Fellows of the ACS from regions throughout the world and selects Fellows for special awards such as the Distinguished Service Award, and on the Members Services Liaison Committee that concentrates on expanding ACS membership and on more fully informing the Fellows of BOR activities. Among several other important committees are the Central Judiciary Committee that is responsible for disciplining Fellows who breach the ethical standards of our College and the Finance Committee that assures responsible fiscal stewardship of the ACS. Deliberations of all of the committees are brought before the full Board for final approval
Although the BOR has been the policy-making body since the founding of the ACS 102 years ago, the structure of our society has evolved considerably, especially during the past 2 decades. The ACS is organized around five major Divisions: Advocacy and Health Policy, Education, Integrated Communications, Member Services, and Research and Optimal Patient Care. The Directors of these Divisions report on a regular basis to the BOR to keep the Regents’ knowledge up-to-date and to assist them in determining the strategic direction of the ACS. Much of the discussion, modifications, and innovations center around these Divisions, also represented as pillars in the recent BOG re-organization. I trust you are aware of the many achievements that have resulted: NSQIP, legislative elimination of the flawed Sustainable Growth Rate (SGR) formula, reorganization of the Clinical Congress, and a re-emphasis on global surgery and the Operation Giving Back Program to name but a few.
Finally, a key role of the BOR is to select the Executive Director of the ACS who manages the day-to-day operations of the College with the Board’s strategic guidance. The ACS has been blessed with a number of excellent Directors, none more visionary and competent than the present Director, David Hoyt, MD, FACS, who is 1 year into his second 5-year term.
I hope that this discussion provides you with a better understanding of the role and functioning of the BOR and the College of which you are a member. The grandeur of the BOR room appropriately parallels the excellence of what takes place within it.
Take time to visit the next time you are in Chicago. I am certain the ACS staff would be pleased and proud to meet you, show you around, and have you experience what I have tried to describe in this brief discourse.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
Peering out from 28 floors above the busy, early morning streets of downtown Chicago, I was entranced by the view. The rising sun in the east created a shimmering, iridescent play of light on the waters of Lake Michigan that extended as far as the eye could see. The room I was in also commanded my attention. Several rows of desks, each with a computer screen, faced a single elevated line of chairs for the leaders of the deliberations that were about to take place. Above this row on the front wall of this imposing room is emblazoned the seal of our College with its mission statement, “The ACS is dedicated to improving the care of the surgical patient and to safeguarding standards of care in an optimal and ethical practice environment,” to remind those in the room of the ultimate purpose in serving this professional organization. So the Regents room and view appeared to me, a newcomer to these meetings of the leadership of the American College of Surgeons.
I hope I can convince you in the paragraphs that follow that this mission and its execution by Regents, Governors, and Fellows of the ACS, are every bit as noble as the architecture of the room and the view it affords.
I have been a Fellow of the American College of Surgeons since 1980. My membership in this extraordinary association has provided me many benefits and numerous opportunities. In the early years, I significantly expanded the knowledge base I had gained in residency by attending every fall Clinical Congress and consuming as many educational offerings as time would permit during this nearly week-long learning marathon. After a few years, I was granted the privilege of being on the instructor end of several of these educational exchanges. At that time in my career, it appeared to me that the ACS’s main and almost sole purpose was to provide continuing education for surgeons who attended the annual Clinical Congress. I have subsequently found that it is so much more.
In 2005, I was invited to represent one of my specialist surgical societies as a Governor. During my 6-year term, I came to realize that the key purpose of the Board of Governors (BOG) is to provide an effective communication conduit between the Fellows and the sole policy-making body of the ACS, the Board of Regents (BOR). In recent years, most of the Regents have attended the annual BOG meeting in order to facilitate this interaction.
In 2012, I was elected First Vice-President of the ACS. Now as an officer of the College, I was invited for 2 years to attend all BOR meetings and to be in the mainstream of all communications relating to strategy and policy. These opportunities provided me with an intimate, inside look at how this large organization of nearly 80,000 members functions to serve the entire surgical profession including each of its many specialties. What I learned about the internal workings of the leadership and those who dedicate their time to this work has reinforced my own commitment to the ACS. It is a remarkable organization.
This brings us back to the well-designed and impressive Regents’ room high above Chicago. The hum of numerous disjointed conversations ceased as the Chair of the ACS Board of Regents called the June 2012 meeting to order. My attention is now focused on the proceedings rather than on the beauty of Lake Michigan below. Although every surgical specialty is represented among the 22 Regents, all discussion was invariably directed toward the betterment of the surgical profession as a whole rather than about any specific specialty’s interests.
The Regents are dedicated servants of the ACS. In addition to three one-and-a-half day meetings annually, each of which requires hours of reading in preparation, most of the Regents serve on at least two committees of the Board. Regents are nominated by Fellows, advisory councils, and committees, and are elected by the much larger BOG which represents every state and Canadian province, several countries, and many surgery specialist societies. In addition to assuring that all surgical specialties are represented, bylaws of the ACS state that the President of the ACS and two Canadian Fellows must be among the BOR membership. Based on my 2-year experience, the BOG has exhibited considerable wisdom in their choice of Regents.
The officers of the ACS (President-Elect, First and Second Vice-Presidents and Vice-Presidents-Elect, Secretary, Treasurer), and the officers of the BOG (Chair, Vice-Chair, and Secretary/Treasurer) attend all BOR meetings and serve in an advisory capacity. Also in attendance and providing essential input are executive members of the ACS staff and, representing the younger ACS membership, the chairs of the Resident Associates Society (RAS) and the Young Fellows Association (YAF). Although only Regents can vote and are therefore responsible for establishing ACS policy, I discovered they welcome participation from all in attendance. I always felt that my contributions and those of other non-voting attendees were thoughtfully and carefully considered.
Much of the preparatory work for BOR meetings is done in the committees that meet just prior to the full Board meeting. I had the pleasure of being on the Honors Committee that selects Honorary Fellows of the ACS from regions throughout the world and selects Fellows for special awards such as the Distinguished Service Award, and on the Members Services Liaison Committee that concentrates on expanding ACS membership and on more fully informing the Fellows of BOR activities. Among several other important committees are the Central Judiciary Committee that is responsible for disciplining Fellows who breach the ethical standards of our College and the Finance Committee that assures responsible fiscal stewardship of the ACS. Deliberations of all of the committees are brought before the full Board for final approval
Although the BOR has been the policy-making body since the founding of the ACS 102 years ago, the structure of our society has evolved considerably, especially during the past 2 decades. The ACS is organized around five major Divisions: Advocacy and Health Policy, Education, Integrated Communications, Member Services, and Research and Optimal Patient Care. The Directors of these Divisions report on a regular basis to the BOR to keep the Regents’ knowledge up-to-date and to assist them in determining the strategic direction of the ACS. Much of the discussion, modifications, and innovations center around these Divisions, also represented as pillars in the recent BOG re-organization. I trust you are aware of the many achievements that have resulted: NSQIP, legislative elimination of the flawed Sustainable Growth Rate (SGR) formula, reorganization of the Clinical Congress, and a re-emphasis on global surgery and the Operation Giving Back Program to name but a few.
Finally, a key role of the BOR is to select the Executive Director of the ACS who manages the day-to-day operations of the College with the Board’s strategic guidance. The ACS has been blessed with a number of excellent Directors, none more visionary and competent than the present Director, David Hoyt, MD, FACS, who is 1 year into his second 5-year term.
I hope that this discussion provides you with a better understanding of the role and functioning of the BOR and the College of which you are a member. The grandeur of the BOR room appropriately parallels the excellence of what takes place within it.
Take time to visit the next time you are in Chicago. I am certain the ACS staff would be pleased and proud to meet you, show you around, and have you experience what I have tried to describe in this brief discourse.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
Peering out from 28 floors above the busy, early morning streets of downtown Chicago, I was entranced by the view. The rising sun in the east created a shimmering, iridescent play of light on the waters of Lake Michigan that extended as far as the eye could see. The room I was in also commanded my attention. Several rows of desks, each with a computer screen, faced a single elevated line of chairs for the leaders of the deliberations that were about to take place. Above this row on the front wall of this imposing room is emblazoned the seal of our College with its mission statement, “The ACS is dedicated to improving the care of the surgical patient and to safeguarding standards of care in an optimal and ethical practice environment,” to remind those in the room of the ultimate purpose in serving this professional organization. So the Regents room and view appeared to me, a newcomer to these meetings of the leadership of the American College of Surgeons.
I hope I can convince you in the paragraphs that follow that this mission and its execution by Regents, Governors, and Fellows of the ACS, are every bit as noble as the architecture of the room and the view it affords.
I have been a Fellow of the American College of Surgeons since 1980. My membership in this extraordinary association has provided me many benefits and numerous opportunities. In the early years, I significantly expanded the knowledge base I had gained in residency by attending every fall Clinical Congress and consuming as many educational offerings as time would permit during this nearly week-long learning marathon. After a few years, I was granted the privilege of being on the instructor end of several of these educational exchanges. At that time in my career, it appeared to me that the ACS’s main and almost sole purpose was to provide continuing education for surgeons who attended the annual Clinical Congress. I have subsequently found that it is so much more.
In 2005, I was invited to represent one of my specialist surgical societies as a Governor. During my 6-year term, I came to realize that the key purpose of the Board of Governors (BOG) is to provide an effective communication conduit between the Fellows and the sole policy-making body of the ACS, the Board of Regents (BOR). In recent years, most of the Regents have attended the annual BOG meeting in order to facilitate this interaction.
In 2012, I was elected First Vice-President of the ACS. Now as an officer of the College, I was invited for 2 years to attend all BOR meetings and to be in the mainstream of all communications relating to strategy and policy. These opportunities provided me with an intimate, inside look at how this large organization of nearly 80,000 members functions to serve the entire surgical profession including each of its many specialties. What I learned about the internal workings of the leadership and those who dedicate their time to this work has reinforced my own commitment to the ACS. It is a remarkable organization.
This brings us back to the well-designed and impressive Regents’ room high above Chicago. The hum of numerous disjointed conversations ceased as the Chair of the ACS Board of Regents called the June 2012 meeting to order. My attention is now focused on the proceedings rather than on the beauty of Lake Michigan below. Although every surgical specialty is represented among the 22 Regents, all discussion was invariably directed toward the betterment of the surgical profession as a whole rather than about any specific specialty’s interests.
The Regents are dedicated servants of the ACS. In addition to three one-and-a-half day meetings annually, each of which requires hours of reading in preparation, most of the Regents serve on at least two committees of the Board. Regents are nominated by Fellows, advisory councils, and committees, and are elected by the much larger BOG which represents every state and Canadian province, several countries, and many surgery specialist societies. In addition to assuring that all surgical specialties are represented, bylaws of the ACS state that the President of the ACS and two Canadian Fellows must be among the BOR membership. Based on my 2-year experience, the BOG has exhibited considerable wisdom in their choice of Regents.
The officers of the ACS (President-Elect, First and Second Vice-Presidents and Vice-Presidents-Elect, Secretary, Treasurer), and the officers of the BOG (Chair, Vice-Chair, and Secretary/Treasurer) attend all BOR meetings and serve in an advisory capacity. Also in attendance and providing essential input are executive members of the ACS staff and, representing the younger ACS membership, the chairs of the Resident Associates Society (RAS) and the Young Fellows Association (YAF). Although only Regents can vote and are therefore responsible for establishing ACS policy, I discovered they welcome participation from all in attendance. I always felt that my contributions and those of other non-voting attendees were thoughtfully and carefully considered.
Much of the preparatory work for BOR meetings is done in the committees that meet just prior to the full Board meeting. I had the pleasure of being on the Honors Committee that selects Honorary Fellows of the ACS from regions throughout the world and selects Fellows for special awards such as the Distinguished Service Award, and on the Members Services Liaison Committee that concentrates on expanding ACS membership and on more fully informing the Fellows of BOR activities. Among several other important committees are the Central Judiciary Committee that is responsible for disciplining Fellows who breach the ethical standards of our College and the Finance Committee that assures responsible fiscal stewardship of the ACS. Deliberations of all of the committees are brought before the full Board for final approval
Although the BOR has been the policy-making body since the founding of the ACS 102 years ago, the structure of our society has evolved considerably, especially during the past 2 decades. The ACS is organized around five major Divisions: Advocacy and Health Policy, Education, Integrated Communications, Member Services, and Research and Optimal Patient Care. The Directors of these Divisions report on a regular basis to the BOR to keep the Regents’ knowledge up-to-date and to assist them in determining the strategic direction of the ACS. Much of the discussion, modifications, and innovations center around these Divisions, also represented as pillars in the recent BOG re-organization. I trust you are aware of the many achievements that have resulted: NSQIP, legislative elimination of the flawed Sustainable Growth Rate (SGR) formula, reorganization of the Clinical Congress, and a re-emphasis on global surgery and the Operation Giving Back Program to name but a few.
Finally, a key role of the BOR is to select the Executive Director of the ACS who manages the day-to-day operations of the College with the Board’s strategic guidance. The ACS has been blessed with a number of excellent Directors, none more visionary and competent than the present Director, David Hoyt, MD, FACS, who is 1 year into his second 5-year term.
I hope that this discussion provides you with a better understanding of the role and functioning of the BOR and the College of which you are a member. The grandeur of the BOR room appropriately parallels the excellence of what takes place within it.
Take time to visit the next time you are in Chicago. I am certain the ACS staff would be pleased and proud to meet you, show you around, and have you experience what I have tried to describe in this brief discourse.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
From the Washington Office: Avoid Medicare Penalties
In the August edition of this column, I wrote at length about the requirement for surgeons to successfully report Medicare quality data in the current calendar year of 2015 in order to avoid Medicare payment penalties of up to 9 percent in 2017. It is absolutely imperative that surgeons take the time necessary to comply with the requirements of Medicare’s three current law quality programs in order to avoid the penalties associated with such.
Even though the MACRA legislation passed earlier this year mandates significant changes in the way Medicare payment updates to physicians are calculated, those changes will not go into effect until 2019. In the meantime, penalties remain in effect for Medicare’s three current law quality programs: PQRS (Physician Quality Reporting System), VBM (Value-Based Modifier) and EHR-MU (Electronic Health Record-Meaningful Use).
While it is certainly understandable that one could deem this requirement to be an unnecessary administrative burden taking time away from otherwise already busy and complex lives, successful compliance is not as daunting as one might imagine. Specifically, only one key action is necessary to avoid the Medicare penalties otherwise imposed by both PQRS and the VBM. That key action is compliance with the requirements of PQRS. Additionally, there are several resources available to you through the College’s website specifically designed to facilitate successful reporting in the most efficient way possible and minimize the time on task necessary to comply.
As was recently communicated to all Fellows in an e-mail communication from Dr. Hoyt, the ACS Surgeon Specific Registry (SSR) allows surgeons to track their cases and also facilitates compliance with the regulatory requirements of PQRS. Registration for the SSR can be found at: https://www.facs.org/quality-programs/ssr
The SSR allows surgeons to report on:
1) PQRS General Surgery Measures Group
2) PQRS Individual Measures
3) ACS SSR QCDR – Trauma Measures Option
Surgeons can utilize any of the three options to meet the requirements for PQRS compliance. A list of all the reportable measures available for each of the above can be found at: https://www.facs.org/quality-programs/ssr/pqrs/options.
For those surgeons for whom it could be applicable, the PQRS General Surgery Measure Group option is perhaps the least onerous. With this option, surgeons need to report on only twenty patients, eleven of whom must be Medicare Part B patients. Should this option be selected, Fellows need to be certain to complete the information by reporting on ALL seven of the included measures along with all nine risk factor variables for each of the twenty patients.
The deadline for submission of calendar year 2015 data into the SSR is January 31, 2016. The SSR will submit PQRS data on behalf of surgeons to Centers for Medicare and Medicaid Services (CMS).
The SSR is free of charge to ACS members.
Links to additional resources which provide further information include:
1) Glossary of Terms: https://www.facs.org/advocacy/regulatory/medicare-penalties/glossary
2) “How to Avoid Medicare Penalties” – summary document: https://www.facs.org/advocacy/regulatory/medicare-penalties
3) Step by Step Flowchart of Participation in Medicare Quality Programs: https://www.facs.org/advocacy/quality/medicare-programs
As always, ACS staff in both the Washington and Chicago offices are available to answer questions and assist members in participating in the 2015 PQRS program:
General PQRS questions: ACS Division of Advocacy and Health Policy, 202/337-6701 or QualityDC@facs.org.
Specific SSR questions: ACS Division of Research and Optimal Patient Care, 312/202-5000 or ssr@facs.org.
In closing, I will again highly encourage all Fellows to invest the time necessary to successfully comply with the PQRS requirement through the SSR and thereby avoid penalties of up to 9 percent in their 2017 Medicare payment.
Until next month...
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington.
In the August edition of this column, I wrote at length about the requirement for surgeons to successfully report Medicare quality data in the current calendar year of 2015 in order to avoid Medicare payment penalties of up to 9 percent in 2017. It is absolutely imperative that surgeons take the time necessary to comply with the requirements of Medicare’s three current law quality programs in order to avoid the penalties associated with such.
Even though the MACRA legislation passed earlier this year mandates significant changes in the way Medicare payment updates to physicians are calculated, those changes will not go into effect until 2019. In the meantime, penalties remain in effect for Medicare’s three current law quality programs: PQRS (Physician Quality Reporting System), VBM (Value-Based Modifier) and EHR-MU (Electronic Health Record-Meaningful Use).
While it is certainly understandable that one could deem this requirement to be an unnecessary administrative burden taking time away from otherwise already busy and complex lives, successful compliance is not as daunting as one might imagine. Specifically, only one key action is necessary to avoid the Medicare penalties otherwise imposed by both PQRS and the VBM. That key action is compliance with the requirements of PQRS. Additionally, there are several resources available to you through the College’s website specifically designed to facilitate successful reporting in the most efficient way possible and minimize the time on task necessary to comply.
As was recently communicated to all Fellows in an e-mail communication from Dr. Hoyt, the ACS Surgeon Specific Registry (SSR) allows surgeons to track their cases and also facilitates compliance with the regulatory requirements of PQRS. Registration for the SSR can be found at: https://www.facs.org/quality-programs/ssr
The SSR allows surgeons to report on:
1) PQRS General Surgery Measures Group
2) PQRS Individual Measures
3) ACS SSR QCDR – Trauma Measures Option
Surgeons can utilize any of the three options to meet the requirements for PQRS compliance. A list of all the reportable measures available for each of the above can be found at: https://www.facs.org/quality-programs/ssr/pqrs/options.
For those surgeons for whom it could be applicable, the PQRS General Surgery Measure Group option is perhaps the least onerous. With this option, surgeons need to report on only twenty patients, eleven of whom must be Medicare Part B patients. Should this option be selected, Fellows need to be certain to complete the information by reporting on ALL seven of the included measures along with all nine risk factor variables for each of the twenty patients.
The deadline for submission of calendar year 2015 data into the SSR is January 31, 2016. The SSR will submit PQRS data on behalf of surgeons to Centers for Medicare and Medicaid Services (CMS).
The SSR is free of charge to ACS members.
Links to additional resources which provide further information include:
1) Glossary of Terms: https://www.facs.org/advocacy/regulatory/medicare-penalties/glossary
2) “How to Avoid Medicare Penalties” – summary document: https://www.facs.org/advocacy/regulatory/medicare-penalties
3) Step by Step Flowchart of Participation in Medicare Quality Programs: https://www.facs.org/advocacy/quality/medicare-programs
As always, ACS staff in both the Washington and Chicago offices are available to answer questions and assist members in participating in the 2015 PQRS program:
General PQRS questions: ACS Division of Advocacy and Health Policy, 202/337-6701 or QualityDC@facs.org.
Specific SSR questions: ACS Division of Research and Optimal Patient Care, 312/202-5000 or ssr@facs.org.
In closing, I will again highly encourage all Fellows to invest the time necessary to successfully comply with the PQRS requirement through the SSR and thereby avoid penalties of up to 9 percent in their 2017 Medicare payment.
Until next month...
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington.
In the August edition of this column, I wrote at length about the requirement for surgeons to successfully report Medicare quality data in the current calendar year of 2015 in order to avoid Medicare payment penalties of up to 9 percent in 2017. It is absolutely imperative that surgeons take the time necessary to comply with the requirements of Medicare’s three current law quality programs in order to avoid the penalties associated with such.
Even though the MACRA legislation passed earlier this year mandates significant changes in the way Medicare payment updates to physicians are calculated, those changes will not go into effect until 2019. In the meantime, penalties remain in effect for Medicare’s three current law quality programs: PQRS (Physician Quality Reporting System), VBM (Value-Based Modifier) and EHR-MU (Electronic Health Record-Meaningful Use).
While it is certainly understandable that one could deem this requirement to be an unnecessary administrative burden taking time away from otherwise already busy and complex lives, successful compliance is not as daunting as one might imagine. Specifically, only one key action is necessary to avoid the Medicare penalties otherwise imposed by both PQRS and the VBM. That key action is compliance with the requirements of PQRS. Additionally, there are several resources available to you through the College’s website specifically designed to facilitate successful reporting in the most efficient way possible and minimize the time on task necessary to comply.
As was recently communicated to all Fellows in an e-mail communication from Dr. Hoyt, the ACS Surgeon Specific Registry (SSR) allows surgeons to track their cases and also facilitates compliance with the regulatory requirements of PQRS. Registration for the SSR can be found at: https://www.facs.org/quality-programs/ssr
The SSR allows surgeons to report on:
1) PQRS General Surgery Measures Group
2) PQRS Individual Measures
3) ACS SSR QCDR – Trauma Measures Option
Surgeons can utilize any of the three options to meet the requirements for PQRS compliance. A list of all the reportable measures available for each of the above can be found at: https://www.facs.org/quality-programs/ssr/pqrs/options.
For those surgeons for whom it could be applicable, the PQRS General Surgery Measure Group option is perhaps the least onerous. With this option, surgeons need to report on only twenty patients, eleven of whom must be Medicare Part B patients. Should this option be selected, Fellows need to be certain to complete the information by reporting on ALL seven of the included measures along with all nine risk factor variables for each of the twenty patients.
The deadline for submission of calendar year 2015 data into the SSR is January 31, 2016. The SSR will submit PQRS data on behalf of surgeons to Centers for Medicare and Medicaid Services (CMS).
The SSR is free of charge to ACS members.
Links to additional resources which provide further information include:
1) Glossary of Terms: https://www.facs.org/advocacy/regulatory/medicare-penalties/glossary
2) “How to Avoid Medicare Penalties” – summary document: https://www.facs.org/advocacy/regulatory/medicare-penalties
3) Step by Step Flowchart of Participation in Medicare Quality Programs: https://www.facs.org/advocacy/quality/medicare-programs
As always, ACS staff in both the Washington and Chicago offices are available to answer questions and assist members in participating in the 2015 PQRS program:
General PQRS questions: ACS Division of Advocacy and Health Policy, 202/337-6701 or QualityDC@facs.org.
Specific SSR questions: ACS Division of Research and Optimal Patient Care, 312/202-5000 or ssr@facs.org.
In closing, I will again highly encourage all Fellows to invest the time necessary to successfully comply with the PQRS requirement through the SSR and thereby avoid penalties of up to 9 percent in their 2017 Medicare payment.
Until next month...
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington.
We hold the pen, but who writes the story?
Mrs. J, a physically frail but mentally sharp 75-year-old with known metastatic gastric cancer was admitted to the hospital 2 days ago with a small bowel obstruction. Despite appropriate conservative management, her symptoms are worsening. Her prior cancer treatment consisted of gastric resection with reconstruction and chemo and radiation therapy. The probability of identifying a treatable cause for her bowel obstruction during exploratory laparotomy is believed to be small.
Mr. S, a debilitated 58-year-old previously treated with primary chemotherapy and radiation for cancer at the base of his tongue, presents to your office with severe pain due to recurrent disease. The cancer is potentially resectable, but it will require an extensive resection necessitating complex free flap reconstruction in this previously irradiated field.
Is an operation indicated in either/both of these patients? The risk of causing harm with these operations may outweigh the potential benefits, so how do you decide?
Surgery residents have a lot to learn during their residency training. Not only must they gain a mastery of the pathophysiology of surgical disease, they must learn a multitude of operations while they hone their manual dexterity skills. And they must learn how to take care of a multitude of patients.
Less understood and explicitly taught is how to determine whether an operation is appropriate for this specific patient. Understanding the pathophysiology of the patient’s illness is not enough; it requires an ability to effectively communicate with the patient, to understand that person’s hopes and goals, and then honestly determine whether an operation is in fact indicated. It may sound like the antithesis of surgical training, but learning when not to operate is as important as learning when to do so.
Sometimes it’s easy. When the underlying condition is easily treatable by an operation and without it the previously healthy patient will likely die, operation is usually warranted and accepted. For the critically ill patient who will not survive transfer to the operating room and induction of anesthesia, an operation would be impossible.
As illustrated by the patients described at the beginning of this piece, the decision making can be a bit more complicated.
These are the type of patients the surgeon intuitively believes will not do well, but they are referred for an operation and what surgeons do, is ... operate. “To cut is to cure,” is the old adage, not “To cut is to care.”
These are some of the toughest decisions a surgeon can make and are the ones surgeons seem to remember. The enormous responsibility that accompanies the decision to take someone to the operating room and through a potentially difficult postoperative period can be burdensome for the surgeon and potentially fraught with suffering for all.
Understanding how to address goals of care with patients and families can make these decisions easier. Yet these communication skills are not necessarily emphasized during surgical training, and in fact, they are not the forte of many physicians in general, which has led to the growth of the specialty of palliative medicine. Palliative medicine specialists are trained experts in these communication techniques.
One of the cardinal goals of palliative medicine is to help patients and families think about and clarify their treatment goals. Asking questions about “code status” is not the same as exploring someone’s overall treatment goals. Goals can range from wanting to stay alive no matter in what condition to wanting to be kept comfortable at home surrounded by loved ones even if it means a potentially shorter lifespan. By having patients clarify their ultimate goals it may become apparent that a high-risk operation is not the best way to proceed. Perhaps aggressive pain management and arranging effective home support better meets the patient’s overall goals.
You don’t have to be a palliative medicine specialist to have these conversations with patients, but it does require specific communication skills, which can be taught.
For example, many clinicians start their patient encounters by giving a brief overview of the current situation or skip straight to discussions concerning the various treatment options. But are you sure you and your patient are really starting from the same place? You can’t assume that the patient/family truly understands the medical condition, no matter what may be implied in the medical record or the referring physician’s notes. And you can’t assume a patient wants an operation just because he or she shows up in your office.
A more effective way to start the conversation is to begin by asking patients what they understand about their conditions. This will ensure your subsequent discussion corrects any misinformation and better clarifies their understanding of the situation. Starting your encounter in this fashion is critical and can avoid misunderstandings that can lead to treatments the patients do not actually want, and mistrust should complications arise.
An elective rotation with palliative medicine providers to learn these skills can be a great addition to surgical residency training. These conversations can be some of the most meaningful patient interactions a physician can experience. Incorporating an elective rotation with a palliative medicine team into surgical residency training can add value to residency training and have long-lasting benefit for future surgeons, and ultimately, for their patients, as they venture on in their surgical careers.
Nadine B. Semer, M.D., MPH, FACS, is board certified in general surgery, plastic surgery, and palliative medicine. As a reconstructive plastic surgeon, she has worked not only in the United States, but has had the privilege of taking her skills to underserved and resource-poor areas throughout the world. She currently is practicing palliative medicine full time, and is an assistant professor at UT Southwestern Medical School, in Dallas, based at Parkland Hospital.
Mrs. J, a physically frail but mentally sharp 75-year-old with known metastatic gastric cancer was admitted to the hospital 2 days ago with a small bowel obstruction. Despite appropriate conservative management, her symptoms are worsening. Her prior cancer treatment consisted of gastric resection with reconstruction and chemo and radiation therapy. The probability of identifying a treatable cause for her bowel obstruction during exploratory laparotomy is believed to be small.
Mr. S, a debilitated 58-year-old previously treated with primary chemotherapy and radiation for cancer at the base of his tongue, presents to your office with severe pain due to recurrent disease. The cancer is potentially resectable, but it will require an extensive resection necessitating complex free flap reconstruction in this previously irradiated field.
Is an operation indicated in either/both of these patients? The risk of causing harm with these operations may outweigh the potential benefits, so how do you decide?
Surgery residents have a lot to learn during their residency training. Not only must they gain a mastery of the pathophysiology of surgical disease, they must learn a multitude of operations while they hone their manual dexterity skills. And they must learn how to take care of a multitude of patients.
Less understood and explicitly taught is how to determine whether an operation is appropriate for this specific patient. Understanding the pathophysiology of the patient’s illness is not enough; it requires an ability to effectively communicate with the patient, to understand that person’s hopes and goals, and then honestly determine whether an operation is in fact indicated. It may sound like the antithesis of surgical training, but learning when not to operate is as important as learning when to do so.
Sometimes it’s easy. When the underlying condition is easily treatable by an operation and without it the previously healthy patient will likely die, operation is usually warranted and accepted. For the critically ill patient who will not survive transfer to the operating room and induction of anesthesia, an operation would be impossible.
As illustrated by the patients described at the beginning of this piece, the decision making can be a bit more complicated.
These are the type of patients the surgeon intuitively believes will not do well, but they are referred for an operation and what surgeons do, is ... operate. “To cut is to cure,” is the old adage, not “To cut is to care.”
These are some of the toughest decisions a surgeon can make and are the ones surgeons seem to remember. The enormous responsibility that accompanies the decision to take someone to the operating room and through a potentially difficult postoperative period can be burdensome for the surgeon and potentially fraught with suffering for all.
Understanding how to address goals of care with patients and families can make these decisions easier. Yet these communication skills are not necessarily emphasized during surgical training, and in fact, they are not the forte of many physicians in general, which has led to the growth of the specialty of palliative medicine. Palliative medicine specialists are trained experts in these communication techniques.
One of the cardinal goals of palliative medicine is to help patients and families think about and clarify their treatment goals. Asking questions about “code status” is not the same as exploring someone’s overall treatment goals. Goals can range from wanting to stay alive no matter in what condition to wanting to be kept comfortable at home surrounded by loved ones even if it means a potentially shorter lifespan. By having patients clarify their ultimate goals it may become apparent that a high-risk operation is not the best way to proceed. Perhaps aggressive pain management and arranging effective home support better meets the patient’s overall goals.
You don’t have to be a palliative medicine specialist to have these conversations with patients, but it does require specific communication skills, which can be taught.
For example, many clinicians start their patient encounters by giving a brief overview of the current situation or skip straight to discussions concerning the various treatment options. But are you sure you and your patient are really starting from the same place? You can’t assume that the patient/family truly understands the medical condition, no matter what may be implied in the medical record or the referring physician’s notes. And you can’t assume a patient wants an operation just because he or she shows up in your office.
A more effective way to start the conversation is to begin by asking patients what they understand about their conditions. This will ensure your subsequent discussion corrects any misinformation and better clarifies their understanding of the situation. Starting your encounter in this fashion is critical and can avoid misunderstandings that can lead to treatments the patients do not actually want, and mistrust should complications arise.
An elective rotation with palliative medicine providers to learn these skills can be a great addition to surgical residency training. These conversations can be some of the most meaningful patient interactions a physician can experience. Incorporating an elective rotation with a palliative medicine team into surgical residency training can add value to residency training and have long-lasting benefit for future surgeons, and ultimately, for their patients, as they venture on in their surgical careers.
Nadine B. Semer, M.D., MPH, FACS, is board certified in general surgery, plastic surgery, and palliative medicine. As a reconstructive plastic surgeon, she has worked not only in the United States, but has had the privilege of taking her skills to underserved and resource-poor areas throughout the world. She currently is practicing palliative medicine full time, and is an assistant professor at UT Southwestern Medical School, in Dallas, based at Parkland Hospital.
Mrs. J, a physically frail but mentally sharp 75-year-old with known metastatic gastric cancer was admitted to the hospital 2 days ago with a small bowel obstruction. Despite appropriate conservative management, her symptoms are worsening. Her prior cancer treatment consisted of gastric resection with reconstruction and chemo and radiation therapy. The probability of identifying a treatable cause for her bowel obstruction during exploratory laparotomy is believed to be small.
Mr. S, a debilitated 58-year-old previously treated with primary chemotherapy and radiation for cancer at the base of his tongue, presents to your office with severe pain due to recurrent disease. The cancer is potentially resectable, but it will require an extensive resection necessitating complex free flap reconstruction in this previously irradiated field.
Is an operation indicated in either/both of these patients? The risk of causing harm with these operations may outweigh the potential benefits, so how do you decide?
Surgery residents have a lot to learn during their residency training. Not only must they gain a mastery of the pathophysiology of surgical disease, they must learn a multitude of operations while they hone their manual dexterity skills. And they must learn how to take care of a multitude of patients.
Less understood and explicitly taught is how to determine whether an operation is appropriate for this specific patient. Understanding the pathophysiology of the patient’s illness is not enough; it requires an ability to effectively communicate with the patient, to understand that person’s hopes and goals, and then honestly determine whether an operation is in fact indicated. It may sound like the antithesis of surgical training, but learning when not to operate is as important as learning when to do so.
Sometimes it’s easy. When the underlying condition is easily treatable by an operation and without it the previously healthy patient will likely die, operation is usually warranted and accepted. For the critically ill patient who will not survive transfer to the operating room and induction of anesthesia, an operation would be impossible.
As illustrated by the patients described at the beginning of this piece, the decision making can be a bit more complicated.
These are the type of patients the surgeon intuitively believes will not do well, but they are referred for an operation and what surgeons do, is ... operate. “To cut is to cure,” is the old adage, not “To cut is to care.”
These are some of the toughest decisions a surgeon can make and are the ones surgeons seem to remember. The enormous responsibility that accompanies the decision to take someone to the operating room and through a potentially difficult postoperative period can be burdensome for the surgeon and potentially fraught with suffering for all.
Understanding how to address goals of care with patients and families can make these decisions easier. Yet these communication skills are not necessarily emphasized during surgical training, and in fact, they are not the forte of many physicians in general, which has led to the growth of the specialty of palliative medicine. Palliative medicine specialists are trained experts in these communication techniques.
One of the cardinal goals of palliative medicine is to help patients and families think about and clarify their treatment goals. Asking questions about “code status” is not the same as exploring someone’s overall treatment goals. Goals can range from wanting to stay alive no matter in what condition to wanting to be kept comfortable at home surrounded by loved ones even if it means a potentially shorter lifespan. By having patients clarify their ultimate goals it may become apparent that a high-risk operation is not the best way to proceed. Perhaps aggressive pain management and arranging effective home support better meets the patient’s overall goals.
You don’t have to be a palliative medicine specialist to have these conversations with patients, but it does require specific communication skills, which can be taught.
For example, many clinicians start their patient encounters by giving a brief overview of the current situation or skip straight to discussions concerning the various treatment options. But are you sure you and your patient are really starting from the same place? You can’t assume that the patient/family truly understands the medical condition, no matter what may be implied in the medical record or the referring physician’s notes. And you can’t assume a patient wants an operation just because he or she shows up in your office.
A more effective way to start the conversation is to begin by asking patients what they understand about their conditions. This will ensure your subsequent discussion corrects any misinformation and better clarifies their understanding of the situation. Starting your encounter in this fashion is critical and can avoid misunderstandings that can lead to treatments the patients do not actually want, and mistrust should complications arise.
An elective rotation with palliative medicine providers to learn these skills can be a great addition to surgical residency training. These conversations can be some of the most meaningful patient interactions a physician can experience. Incorporating an elective rotation with a palliative medicine team into surgical residency training can add value to residency training and have long-lasting benefit for future surgeons, and ultimately, for their patients, as they venture on in their surgical careers.
Nadine B. Semer, M.D., MPH, FACS, is board certified in general surgery, plastic surgery, and palliative medicine. As a reconstructive plastic surgeon, she has worked not only in the United States, but has had the privilege of taking her skills to underserved and resource-poor areas throughout the world. She currently is practicing palliative medicine full time, and is an assistant professor at UT Southwestern Medical School, in Dallas, based at Parkland Hospital.
Five epigenetic biomarkers define three CLL subgroups
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
FROM LEUKEMIA
Key clinical point: A new strategy allows CLL patients to be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes.
Major finding: Epigenetic classification was the strongest predictor of time to treatment (P <.001), along with Binet stage (P <.001); these findings were corroborated in a validation series (n = 97).
Data source: A prediction model formulated using five epigenetic biomarkers that was able to classify CLL patients accurately into the three subgroups.
Disclosures: The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
What Is Your Diagnosis? Fixed Cutaneous Sporotrichosis
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
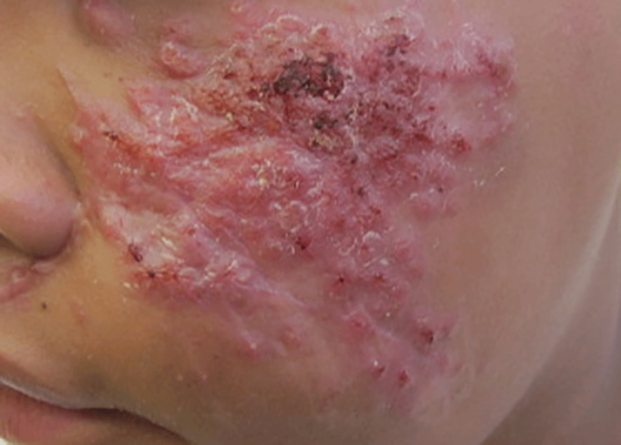
A 13-year-old adolescent boy presented with erythematous, tender, scaly, indurated nodules coalescing into plaques on the left cheek and periocular region. He denied any vision changes, the extraocular muscles were intact, and he was afebrile. Two weeks prior to presentation, the patient was hospitalized after an all-terrain vehicle accident that resulted in an extensive midfacial avulsion of the left cheek. The wound was cleaned and repaired by an otorhinolaryngologist. Three days later, he developed swelling and erythema of the left cheek, which was treated by his primary care provider with oral cephalexin, then trimethoprim-sulfamethoxazole for postsurgical wound infection. After completing his antibiotic course, he noticed continued worsening of the wound with increased edema, erythema, and tenderness. He was then referred to our clinic for further evaluation.
What’s Eating You? Ant-Induced Alopecia (Pheidole)
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Practice Points
- Ant-induced alopecia should be considered in the differential diagnosis for patients from endemic regions (eg, Iran, Turkey) with new-onset localized hair loss or in patients recently visiting those areas with a concordant history.
- Ant-induced alopecia is thought to result from mechanical and/or chemical breakage, most commonly caused by Pheidole ants, leaving follicles intact and allowing for hair regrowth without treatment through the normal hair cycle.
Complex picture emerges of prescription opioid abuse
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
FROM JAMA
Key clinical point: The percentage of nonmedical use of prescription opioids declined during the last decade, but the prevalence of use disorders, the frequency of abuse, and related mortality all increased.
Major finding: The 1-year prevalence of opioid use disorders rose from 0.6% to 0.9%, that of high-frequency use increased from 0.3% to 0.4%, and that of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000.
Data source: An analysis of time trends in prescription opioid use, based on two nationally representative data sets involving 472,200 adults.
Disclosures: The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
Spironolactone for Adult Female Acne
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.