User login
Perianal North American Blastomycosis
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
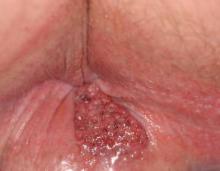
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
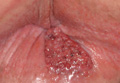
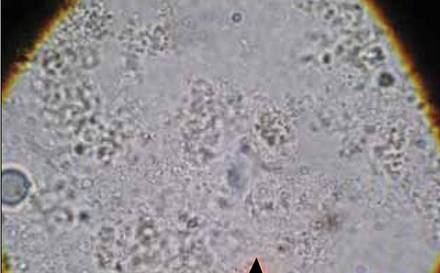
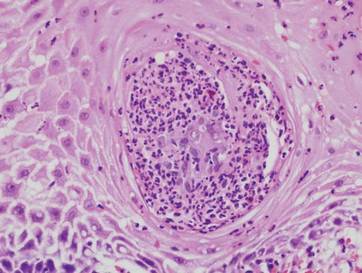
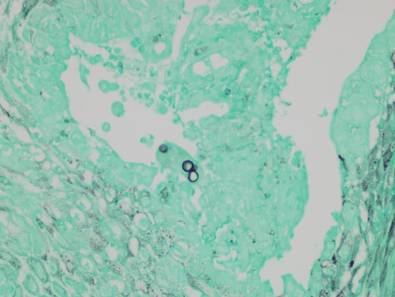
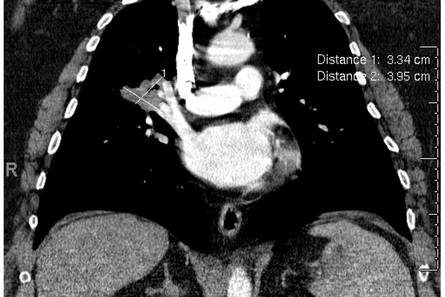
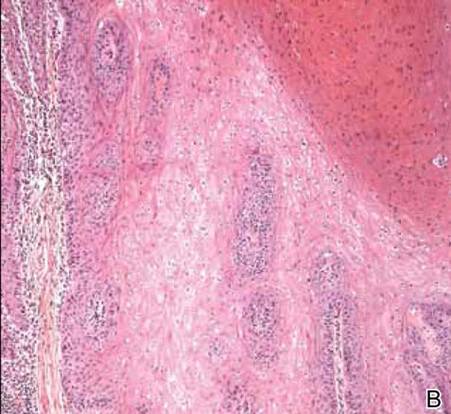
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
 |  |
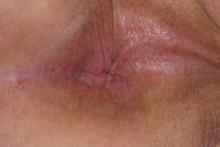
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
 | 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
 |  |
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
 | 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
 |  |
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
 | 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
Practice Points
- Cutaneous North American blastomycosis usually occurs in a periorificial distribution.
- The perianal region should be included in the periorificial regions considered in North American blastomycosis infections.
From the Washington Office
Last month’s edition of this column ended by calling Fellows’ attention to a new Web-based tool developed by the ACS Division of Advocacy and Health Policy to assist them in avoiding significant penalties in Medicare physician payment. This new online tool was highlighted in an e-mail sent to Fellows on June 24, 2015.
Based on follow-up inquiries since received, I would like to delve deeper into the specifics of how to successfully participate in the Physician Quality Reporting System (PQRS) and hopefully assist Fellows in avoiding penalties of up to 9% in their Medicare physician payment in the year 2017 secondary to failure to successfully participate in the current law Medicare quality programs in the current calendar year of 2015.
Despite the much publicized, and laudable, permanent repeal of the Sustainable Growth Rate (SGR), current law quality programs are still in effect. Medicare oversees several programs that offer physicians incentives for successful participation and/or penalties for failure to nonparticipation. These programs include the PQRS, the Value-Based Payment Modifier (VM) and the Electronic Health Record (EHR) Incentive Program, also known as the “EHR Meaningful Use program.”
Calendar year 2014 was the last year that physicians could earn incentives for some of these programs. Failure to participate in the Medicare quality programs leads to the potential for penalties that are applied 2 years after the performance period. Penalties in 2015 already are being assessed based on how successfully physicians participated in 2013. Thus, performance in 2015 will impact payment in 2017. Specifically, failure to participate in the programs in 2015 could result in a total penalty of 9% applied in 2017.
The College has developed resources to assist Fellows in being successful reporters. For most Fellows, the options found in the Surgeon Specific Registry (SSR) will be applicable. The SSR, formerly known as the ACS Case Log system, allows surgeons to track their cases and outcomes in a convenient and confidential manner. The SSR can also be utilized to comply with the regulatory requirements of submitting PQRS data as they have been approved to provide PQRS registry-based reporting for 2015. Use of the SSR is offered free of charge to ACS surgeon members and is available to nonmember surgeons for an annual fee.
The SSR offers a total of three options for surgeons to utilize to participate in PQRS reporting. Those options are: 1) General Surgery Measures Group; 2) Individual Measure reporting, which includes options for surgical specialties; and 3) Trauma Measures Option through the SSR’s Qualified Clinical Data Registry (QCDR). The deadline for submitting calendar year 2015 patient information in the SSR is January 31, 2016. The SSR will submit the PQRS data to Centers for Medicare & Medicaid Services (CMS).
For those surgeons for whom it could be applicable, the General Surgery Measures Group option is perhaps the least onerous in its requirements. Surgeons need report on a minimum of 20 patients, at least 11 of whom must be Medicare Part B patients. Should this option be selected, ALL seven of the included measures along with all nine risk factor variables must be reported for each of the 20 patients.
Surgeons may also choose to report individual measures data through the SSR. Those choosing this option are required to report on nine measures in three National Quality Strategy (NQS) categories, called “Domains.” One of the measures selected must further be designated as a “cross-cutting measure,” for example the documentation of current medications in the medical record, medication reconciliation, advanced care plan, or tobacco-use screening and cessation, as mentioned above. However, individual measures data must be entered for at least 50% of the provider’s Medicare Part B patients in order to be successful using this option. In order to assist one in determining whether this option is suitable for reporting, I would refer Fellows to the ACS website, www.facs.org/quality-programs/ssr/pqrs/options for a more expansive list of the individual measures, their “domains,” and whether or not they are designated as “cross-cutting.”
The SSR also provides the opportunity to leverage measures applicable to trauma surgery for successful PQRS reporting via the 2015 PQRS Trauma QCDR., which allow providers to submit non-PQRS measures, for example, measures not contained in the approved measure set or a measure that may be in the set but has substantive differences in the manner in which it is reported by the QCDR. The SSR Trauma QCDR includes 10 non-PQRS measures and one PQRS measure in this reporting option. Those choosing this option must report on 9 of the 11 designated measures, including 2 outcomes measures across three of the NQS domains. Reports must be completed on 50% of the surgeon’s Medicare Part B patients that meet the measurement requirements. One can also view the complete list of measures included in the Trauma Measures Option at the ACS website referenced above.
Lastly, for bariatric surgeons, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) has also been approved as a QCDR for PQRS for 2015 reporting. MBSAQIP participants have the opportunity to voluntarily elect that their QCDR quality measures be submitted for PQRS participation. Metabolic and bariatric surgeons will receive reports of their QCDR measure results such that they can track their results. MBSAQIP will submit approved QCDR measures on behalf of participants who elect to have such done on their behalf. Specifics on the approved MBSAQIP QCDR quality measures are available at www.facs.org/quality-programs/mbsaqip/resources/data-registry.
As always, ACS staff in both Washington and Chicago are available to answer questions and assist members in participating in the 2015 PQRS program:
• General PQRS questions: ACS Division of Advocacy and Health Policy, 202-337-6701 or QualityDC@facs.org.
• Specific SSR questions: ACS Division of Research and Optimal Patient Care, 312-202-5000 or ssr@facs.org.
• Information on MBSAQIP: ACS Division of Research and Optimal Patient Care, 312-202-5000 or rkrapikas@facs.org.
I highly encourage all Fellows to invest the time necessary to successfully participate in PQRS and thereby avoid penalties in their 2017 Medicare payment.
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington.
Last month’s edition of this column ended by calling Fellows’ attention to a new Web-based tool developed by the ACS Division of Advocacy and Health Policy to assist them in avoiding significant penalties in Medicare physician payment. This new online tool was highlighted in an e-mail sent to Fellows on June 24, 2015.
Based on follow-up inquiries since received, I would like to delve deeper into the specifics of how to successfully participate in the Physician Quality Reporting System (PQRS) and hopefully assist Fellows in avoiding penalties of up to 9% in their Medicare physician payment in the year 2017 secondary to failure to successfully participate in the current law Medicare quality programs in the current calendar year of 2015.
Despite the much publicized, and laudable, permanent repeal of the Sustainable Growth Rate (SGR), current law quality programs are still in effect. Medicare oversees several programs that offer physicians incentives for successful participation and/or penalties for failure to nonparticipation. These programs include the PQRS, the Value-Based Payment Modifier (VM) and the Electronic Health Record (EHR) Incentive Program, also known as the “EHR Meaningful Use program.”
Calendar year 2014 was the last year that physicians could earn incentives for some of these programs. Failure to participate in the Medicare quality programs leads to the potential for penalties that are applied 2 years after the performance period. Penalties in 2015 already are being assessed based on how successfully physicians participated in 2013. Thus, performance in 2015 will impact payment in 2017. Specifically, failure to participate in the programs in 2015 could result in a total penalty of 9% applied in 2017.
The College has developed resources to assist Fellows in being successful reporters. For most Fellows, the options found in the Surgeon Specific Registry (SSR) will be applicable. The SSR, formerly known as the ACS Case Log system, allows surgeons to track their cases and outcomes in a convenient and confidential manner. The SSR can also be utilized to comply with the regulatory requirements of submitting PQRS data as they have been approved to provide PQRS registry-based reporting for 2015. Use of the SSR is offered free of charge to ACS surgeon members and is available to nonmember surgeons for an annual fee.
The SSR offers a total of three options for surgeons to utilize to participate in PQRS reporting. Those options are: 1) General Surgery Measures Group; 2) Individual Measure reporting, which includes options for surgical specialties; and 3) Trauma Measures Option through the SSR’s Qualified Clinical Data Registry (QCDR). The deadline for submitting calendar year 2015 patient information in the SSR is January 31, 2016. The SSR will submit the PQRS data to Centers for Medicare & Medicaid Services (CMS).
For those surgeons for whom it could be applicable, the General Surgery Measures Group option is perhaps the least onerous in its requirements. Surgeons need report on a minimum of 20 patients, at least 11 of whom must be Medicare Part B patients. Should this option be selected, ALL seven of the included measures along with all nine risk factor variables must be reported for each of the 20 patients.
Surgeons may also choose to report individual measures data through the SSR. Those choosing this option are required to report on nine measures in three National Quality Strategy (NQS) categories, called “Domains.” One of the measures selected must further be designated as a “cross-cutting measure,” for example the documentation of current medications in the medical record, medication reconciliation, advanced care plan, or tobacco-use screening and cessation, as mentioned above. However, individual measures data must be entered for at least 50% of the provider’s Medicare Part B patients in order to be successful using this option. In order to assist one in determining whether this option is suitable for reporting, I would refer Fellows to the ACS website, www.facs.org/quality-programs/ssr/pqrs/options for a more expansive list of the individual measures, their “domains,” and whether or not they are designated as “cross-cutting.”
The SSR also provides the opportunity to leverage measures applicable to trauma surgery for successful PQRS reporting via the 2015 PQRS Trauma QCDR., which allow providers to submit non-PQRS measures, for example, measures not contained in the approved measure set or a measure that may be in the set but has substantive differences in the manner in which it is reported by the QCDR. The SSR Trauma QCDR includes 10 non-PQRS measures and one PQRS measure in this reporting option. Those choosing this option must report on 9 of the 11 designated measures, including 2 outcomes measures across three of the NQS domains. Reports must be completed on 50% of the surgeon’s Medicare Part B patients that meet the measurement requirements. One can also view the complete list of measures included in the Trauma Measures Option at the ACS website referenced above.
Lastly, for bariatric surgeons, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) has also been approved as a QCDR for PQRS for 2015 reporting. MBSAQIP participants have the opportunity to voluntarily elect that their QCDR quality measures be submitted for PQRS participation. Metabolic and bariatric surgeons will receive reports of their QCDR measure results such that they can track their results. MBSAQIP will submit approved QCDR measures on behalf of participants who elect to have such done on their behalf. Specifics on the approved MBSAQIP QCDR quality measures are available at www.facs.org/quality-programs/mbsaqip/resources/data-registry.
As always, ACS staff in both Washington and Chicago are available to answer questions and assist members in participating in the 2015 PQRS program:
• General PQRS questions: ACS Division of Advocacy and Health Policy, 202-337-6701 or QualityDC@facs.org.
• Specific SSR questions: ACS Division of Research and Optimal Patient Care, 312-202-5000 or ssr@facs.org.
• Information on MBSAQIP: ACS Division of Research and Optimal Patient Care, 312-202-5000 or rkrapikas@facs.org.
I highly encourage all Fellows to invest the time necessary to successfully participate in PQRS and thereby avoid penalties in their 2017 Medicare payment.
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington.
Last month’s edition of this column ended by calling Fellows’ attention to a new Web-based tool developed by the ACS Division of Advocacy and Health Policy to assist them in avoiding significant penalties in Medicare physician payment. This new online tool was highlighted in an e-mail sent to Fellows on June 24, 2015.
Based on follow-up inquiries since received, I would like to delve deeper into the specifics of how to successfully participate in the Physician Quality Reporting System (PQRS) and hopefully assist Fellows in avoiding penalties of up to 9% in their Medicare physician payment in the year 2017 secondary to failure to successfully participate in the current law Medicare quality programs in the current calendar year of 2015.
Despite the much publicized, and laudable, permanent repeal of the Sustainable Growth Rate (SGR), current law quality programs are still in effect. Medicare oversees several programs that offer physicians incentives for successful participation and/or penalties for failure to nonparticipation. These programs include the PQRS, the Value-Based Payment Modifier (VM) and the Electronic Health Record (EHR) Incentive Program, also known as the “EHR Meaningful Use program.”
Calendar year 2014 was the last year that physicians could earn incentives for some of these programs. Failure to participate in the Medicare quality programs leads to the potential for penalties that are applied 2 years after the performance period. Penalties in 2015 already are being assessed based on how successfully physicians participated in 2013. Thus, performance in 2015 will impact payment in 2017. Specifically, failure to participate in the programs in 2015 could result in a total penalty of 9% applied in 2017.
The College has developed resources to assist Fellows in being successful reporters. For most Fellows, the options found in the Surgeon Specific Registry (SSR) will be applicable. The SSR, formerly known as the ACS Case Log system, allows surgeons to track their cases and outcomes in a convenient and confidential manner. The SSR can also be utilized to comply with the regulatory requirements of submitting PQRS data as they have been approved to provide PQRS registry-based reporting for 2015. Use of the SSR is offered free of charge to ACS surgeon members and is available to nonmember surgeons for an annual fee.
The SSR offers a total of three options for surgeons to utilize to participate in PQRS reporting. Those options are: 1) General Surgery Measures Group; 2) Individual Measure reporting, which includes options for surgical specialties; and 3) Trauma Measures Option through the SSR’s Qualified Clinical Data Registry (QCDR). The deadline for submitting calendar year 2015 patient information in the SSR is January 31, 2016. The SSR will submit the PQRS data to Centers for Medicare & Medicaid Services (CMS).
For those surgeons for whom it could be applicable, the General Surgery Measures Group option is perhaps the least onerous in its requirements. Surgeons need report on a minimum of 20 patients, at least 11 of whom must be Medicare Part B patients. Should this option be selected, ALL seven of the included measures along with all nine risk factor variables must be reported for each of the 20 patients.
Surgeons may also choose to report individual measures data through the SSR. Those choosing this option are required to report on nine measures in three National Quality Strategy (NQS) categories, called “Domains.” One of the measures selected must further be designated as a “cross-cutting measure,” for example the documentation of current medications in the medical record, medication reconciliation, advanced care plan, or tobacco-use screening and cessation, as mentioned above. However, individual measures data must be entered for at least 50% of the provider’s Medicare Part B patients in order to be successful using this option. In order to assist one in determining whether this option is suitable for reporting, I would refer Fellows to the ACS website, www.facs.org/quality-programs/ssr/pqrs/options for a more expansive list of the individual measures, their “domains,” and whether or not they are designated as “cross-cutting.”
The SSR also provides the opportunity to leverage measures applicable to trauma surgery for successful PQRS reporting via the 2015 PQRS Trauma QCDR., which allow providers to submit non-PQRS measures, for example, measures not contained in the approved measure set or a measure that may be in the set but has substantive differences in the manner in which it is reported by the QCDR. The SSR Trauma QCDR includes 10 non-PQRS measures and one PQRS measure in this reporting option. Those choosing this option must report on 9 of the 11 designated measures, including 2 outcomes measures across three of the NQS domains. Reports must be completed on 50% of the surgeon’s Medicare Part B patients that meet the measurement requirements. One can also view the complete list of measures included in the Trauma Measures Option at the ACS website referenced above.
Lastly, for bariatric surgeons, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) has also been approved as a QCDR for PQRS for 2015 reporting. MBSAQIP participants have the opportunity to voluntarily elect that their QCDR quality measures be submitted for PQRS participation. Metabolic and bariatric surgeons will receive reports of their QCDR measure results such that they can track their results. MBSAQIP will submit approved QCDR measures on behalf of participants who elect to have such done on their behalf. Specifics on the approved MBSAQIP QCDR quality measures are available at www.facs.org/quality-programs/mbsaqip/resources/data-registry.
As always, ACS staff in both Washington and Chicago are available to answer questions and assist members in participating in the 2015 PQRS program:
• General PQRS questions: ACS Division of Advocacy and Health Policy, 202-337-6701 or QualityDC@facs.org.
• Specific SSR questions: ACS Division of Research and Optimal Patient Care, 312-202-5000 or ssr@facs.org.
• Information on MBSAQIP: ACS Division of Research and Optimal Patient Care, 312-202-5000 or rkrapikas@facs.org.
I highly encourage all Fellows to invest the time necessary to successfully participate in PQRS and thereby avoid penalties in their 2017 Medicare payment.
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy for the Division of Advocacy and Health Policy in the ACS offices in Washington.
The Rural Surgeon: The burden of transfer
I have been on both ends of the phone call. I began my career at a several-hundred bed community hospital in a town without a university medical center. We took all the local knife-and-gun club incidents, and whatever other surgical emergencies might arise, while receiving phone calls from nearly every point of the magnetic compass. It’s easy to recall the sagging feeling when you realize more serious work is coming in on the helicopter from Podunk, USA, and you’re not off call for another few hours. These memories linger as now I’m the one making the phone calls. When I state that I am the one, I mean the one and only; this is solo general surgery practice and I’m the only general surgeon in my county.
When I do speak with colleagues kind enough to accept our patients, I feel relieved. But the burden of transfer doesn’t travel away with the patient in the ambulance or on the airplane. There always seem to be questions or looks of concern lately, and I don’t just mean from other medical professionals. Maybe it is an Internet thing, but everyone is a critic these days. We are being watched by more than partners and employers, more than payers and agencies, more than our government bean counters. Families, allied health professionals, and even nonclinical staff all have opinions about which patients stay and which leaves our 14-bed, critical access hospital. Gods may have once walked these halls, but nowadays it’s just me!
Of course, any interested party can also criticize my decisions to keep any particular patient; why would anybody restrict their furrowed glare only to transfers? When we keep patients at the edge of our practice, or perform a procedure that is only done rarely locally, we incite more than just the volume debate on the ACS Communities. Goodness – my wife has heard about cases I have done via town chitter-chatter before I even get home!
How does one deal with being whipsawed? This phenomenon is defined in the business world as being subjected to two difficult situations or opposing pressures at the same time. If you transfer, you are criticized. The only thing that changes are the critics if you keep and care for that very same patient! For many rural colleagues, being whipsawed is on the short list of job dissatisfaction drivers; somewhere behind the heavyweight champ of being asked to be in two different places at the same time.
Transferring a patient rarely leads to the lasting criticism that keeping an ill patient locally can. Obviously keeping a patient extends the time period where others can knowingly shake their heads in disbelief. That extra time allows us to educate staff and others as to why a patient with more than simple hernia or appendicitis is being admitted to our little hospital. We can detail why this is a really good thing for everyone – including the patient!
So many of our locals are elderly, and when we keep one for serious surgical illness, so much goes into that decision besides just the patient’s age and comorbid conditions. Immediate family, friends, or existing social support all must be examined and understood. A significant number of geriatric couples are only “independent” together; send one off for surgery a hundred or more miles away and the remaining spouse suffers measurably. Sometimes there is no local family, as nuclear members live in neighboring states or even overseas. I’m always surprised when my patients have trouble even arranging rides to and from our facilities for the routine procedures we do regularly. I think to myself, what will they do when the inevitable happens?
Our geography plays a serious role for those patients who don’t drive any appreciable distances. The mountains to our east are difficult to negotiate and west, well, you’ll get wet rather quickly. Going north and south on Highway 101 can be tricky during summer and dangerous any time in bad weather. I talk to some patients about sending them to Portland and I get looks in response like I’m proposing surgical care in some exotic foreign capital. Urban anxiety, traffic, and unfamiliarity with our largest metropolis make the 300-mile journey untenable for many of our patients; and the TV show isn’t helping our cause! Even cases that define themselves from the get-go as major university referrals return afterward and ask us to assume their postoperative care. Our patients often can’t make the trip to follow-up with the experts who provided their life-saving care.
Stretching our surgical muscles is obviously important for all ACS members. In bigger facilities you can see and sometimes scrub into fascinating cases in other subspecialties, or at least participate in discussions about such in the surgery lounge. I won’t attach a photo of the desk space that serves as my lounge, dictation station, bathroom, and locker. Let’s just say it’s probably not quite the same as many Fellows are used to.
For the rural solo practitioner, a bigger case, done perhaps with a medical student or just scrub technicians, may not be done as slickly as it would be by a surgical team approaching the same at the university. If the case can be done safely though, it pays dividends. After all, it could be tonight when a major car wreck happens, or a hemodynamically unstable abdominal sepsis case presents, and we are forced to do a serious case – perhaps surgery at the edge of my comfort zone or something we don’t do with frequency. Keeping some bigger cases makes those scenarios just a bit less scary.
I have been recruited as an advocate for our College, trying to influence those in our nation’s capital to reexamine the 96-hour rule as it applies to critical access hospitals. A phone call to my senior senator’s staff leads to a conference call and follow-up I remain involved with – a first in my professional career. One issue that resonated with D.C. staffers was recruiting my successor. How do we entice the young surgeon to a rural practice if all we do are lumps and bumps, appendectomies, and inguinal hernias? Regionalization of surgical care may be coming but that can’t excite our younger and future colleagues. In each of the last 2 years my third-year medical students parked here for their first rotation and got hustled into the OR to assist with emergency surgery. The enthusiasm was palpable and energizing, but one was a case that raised some eyebrows: pneumatosis intestinalis requiring two small bowel resections with anastomoses and an open abdomen in an elderly male. This fellow did great; I see him doing his grocery shopping these days. My perspective is that case enabled this year’s day 1 emergency, making the surgery safer here in rural America.
When we call to transfer a patient, please understand real thought and a piece of who we are as surgeons accompanies that patient. Transfer is very rarely a reflex action. Also, realize that not every case we keep is a weak fastball over the middle of the plate; sometimes we do real work here at the limit of our comfort zone, but we do so for myriad good reasons.
Dr. Levine is a general surgeon practicing in coastal southwestern Oregon. Despite growing up in Brooklyn and on Long Island in New York, he has been a practicing rural surgeon since 1999. Folks barely even notice the accent anymore!
I have been on both ends of the phone call. I began my career at a several-hundred bed community hospital in a town without a university medical center. We took all the local knife-and-gun club incidents, and whatever other surgical emergencies might arise, while receiving phone calls from nearly every point of the magnetic compass. It’s easy to recall the sagging feeling when you realize more serious work is coming in on the helicopter from Podunk, USA, and you’re not off call for another few hours. These memories linger as now I’m the one making the phone calls. When I state that I am the one, I mean the one and only; this is solo general surgery practice and I’m the only general surgeon in my county.
When I do speak with colleagues kind enough to accept our patients, I feel relieved. But the burden of transfer doesn’t travel away with the patient in the ambulance or on the airplane. There always seem to be questions or looks of concern lately, and I don’t just mean from other medical professionals. Maybe it is an Internet thing, but everyone is a critic these days. We are being watched by more than partners and employers, more than payers and agencies, more than our government bean counters. Families, allied health professionals, and even nonclinical staff all have opinions about which patients stay and which leaves our 14-bed, critical access hospital. Gods may have once walked these halls, but nowadays it’s just me!
Of course, any interested party can also criticize my decisions to keep any particular patient; why would anybody restrict their furrowed glare only to transfers? When we keep patients at the edge of our practice, or perform a procedure that is only done rarely locally, we incite more than just the volume debate on the ACS Communities. Goodness – my wife has heard about cases I have done via town chitter-chatter before I even get home!
How does one deal with being whipsawed? This phenomenon is defined in the business world as being subjected to two difficult situations or opposing pressures at the same time. If you transfer, you are criticized. The only thing that changes are the critics if you keep and care for that very same patient! For many rural colleagues, being whipsawed is on the short list of job dissatisfaction drivers; somewhere behind the heavyweight champ of being asked to be in two different places at the same time.
Transferring a patient rarely leads to the lasting criticism that keeping an ill patient locally can. Obviously keeping a patient extends the time period where others can knowingly shake their heads in disbelief. That extra time allows us to educate staff and others as to why a patient with more than simple hernia or appendicitis is being admitted to our little hospital. We can detail why this is a really good thing for everyone – including the patient!
So many of our locals are elderly, and when we keep one for serious surgical illness, so much goes into that decision besides just the patient’s age and comorbid conditions. Immediate family, friends, or existing social support all must be examined and understood. A significant number of geriatric couples are only “independent” together; send one off for surgery a hundred or more miles away and the remaining spouse suffers measurably. Sometimes there is no local family, as nuclear members live in neighboring states or even overseas. I’m always surprised when my patients have trouble even arranging rides to and from our facilities for the routine procedures we do regularly. I think to myself, what will they do when the inevitable happens?
Our geography plays a serious role for those patients who don’t drive any appreciable distances. The mountains to our east are difficult to negotiate and west, well, you’ll get wet rather quickly. Going north and south on Highway 101 can be tricky during summer and dangerous any time in bad weather. I talk to some patients about sending them to Portland and I get looks in response like I’m proposing surgical care in some exotic foreign capital. Urban anxiety, traffic, and unfamiliarity with our largest metropolis make the 300-mile journey untenable for many of our patients; and the TV show isn’t helping our cause! Even cases that define themselves from the get-go as major university referrals return afterward and ask us to assume their postoperative care. Our patients often can’t make the trip to follow-up with the experts who provided their life-saving care.
Stretching our surgical muscles is obviously important for all ACS members. In bigger facilities you can see and sometimes scrub into fascinating cases in other subspecialties, or at least participate in discussions about such in the surgery lounge. I won’t attach a photo of the desk space that serves as my lounge, dictation station, bathroom, and locker. Let’s just say it’s probably not quite the same as many Fellows are used to.
For the rural solo practitioner, a bigger case, done perhaps with a medical student or just scrub technicians, may not be done as slickly as it would be by a surgical team approaching the same at the university. If the case can be done safely though, it pays dividends. After all, it could be tonight when a major car wreck happens, or a hemodynamically unstable abdominal sepsis case presents, and we are forced to do a serious case – perhaps surgery at the edge of my comfort zone or something we don’t do with frequency. Keeping some bigger cases makes those scenarios just a bit less scary.
I have been recruited as an advocate for our College, trying to influence those in our nation’s capital to reexamine the 96-hour rule as it applies to critical access hospitals. A phone call to my senior senator’s staff leads to a conference call and follow-up I remain involved with – a first in my professional career. One issue that resonated with D.C. staffers was recruiting my successor. How do we entice the young surgeon to a rural practice if all we do are lumps and bumps, appendectomies, and inguinal hernias? Regionalization of surgical care may be coming but that can’t excite our younger and future colleagues. In each of the last 2 years my third-year medical students parked here for their first rotation and got hustled into the OR to assist with emergency surgery. The enthusiasm was palpable and energizing, but one was a case that raised some eyebrows: pneumatosis intestinalis requiring two small bowel resections with anastomoses and an open abdomen in an elderly male. This fellow did great; I see him doing his grocery shopping these days. My perspective is that case enabled this year’s day 1 emergency, making the surgery safer here in rural America.
When we call to transfer a patient, please understand real thought and a piece of who we are as surgeons accompanies that patient. Transfer is very rarely a reflex action. Also, realize that not every case we keep is a weak fastball over the middle of the plate; sometimes we do real work here at the limit of our comfort zone, but we do so for myriad good reasons.
Dr. Levine is a general surgeon practicing in coastal southwestern Oregon. Despite growing up in Brooklyn and on Long Island in New York, he has been a practicing rural surgeon since 1999. Folks barely even notice the accent anymore!
I have been on both ends of the phone call. I began my career at a several-hundred bed community hospital in a town without a university medical center. We took all the local knife-and-gun club incidents, and whatever other surgical emergencies might arise, while receiving phone calls from nearly every point of the magnetic compass. It’s easy to recall the sagging feeling when you realize more serious work is coming in on the helicopter from Podunk, USA, and you’re not off call for another few hours. These memories linger as now I’m the one making the phone calls. When I state that I am the one, I mean the one and only; this is solo general surgery practice and I’m the only general surgeon in my county.
When I do speak with colleagues kind enough to accept our patients, I feel relieved. But the burden of transfer doesn’t travel away with the patient in the ambulance or on the airplane. There always seem to be questions or looks of concern lately, and I don’t just mean from other medical professionals. Maybe it is an Internet thing, but everyone is a critic these days. We are being watched by more than partners and employers, more than payers and agencies, more than our government bean counters. Families, allied health professionals, and even nonclinical staff all have opinions about which patients stay and which leaves our 14-bed, critical access hospital. Gods may have once walked these halls, but nowadays it’s just me!
Of course, any interested party can also criticize my decisions to keep any particular patient; why would anybody restrict their furrowed glare only to transfers? When we keep patients at the edge of our practice, or perform a procedure that is only done rarely locally, we incite more than just the volume debate on the ACS Communities. Goodness – my wife has heard about cases I have done via town chitter-chatter before I even get home!
How does one deal with being whipsawed? This phenomenon is defined in the business world as being subjected to two difficult situations or opposing pressures at the same time. If you transfer, you are criticized. The only thing that changes are the critics if you keep and care for that very same patient! For many rural colleagues, being whipsawed is on the short list of job dissatisfaction drivers; somewhere behind the heavyweight champ of being asked to be in two different places at the same time.
Transferring a patient rarely leads to the lasting criticism that keeping an ill patient locally can. Obviously keeping a patient extends the time period where others can knowingly shake their heads in disbelief. That extra time allows us to educate staff and others as to why a patient with more than simple hernia or appendicitis is being admitted to our little hospital. We can detail why this is a really good thing for everyone – including the patient!
So many of our locals are elderly, and when we keep one for serious surgical illness, so much goes into that decision besides just the patient’s age and comorbid conditions. Immediate family, friends, or existing social support all must be examined and understood. A significant number of geriatric couples are only “independent” together; send one off for surgery a hundred or more miles away and the remaining spouse suffers measurably. Sometimes there is no local family, as nuclear members live in neighboring states or even overseas. I’m always surprised when my patients have trouble even arranging rides to and from our facilities for the routine procedures we do regularly. I think to myself, what will they do when the inevitable happens?
Our geography plays a serious role for those patients who don’t drive any appreciable distances. The mountains to our east are difficult to negotiate and west, well, you’ll get wet rather quickly. Going north and south on Highway 101 can be tricky during summer and dangerous any time in bad weather. I talk to some patients about sending them to Portland and I get looks in response like I’m proposing surgical care in some exotic foreign capital. Urban anxiety, traffic, and unfamiliarity with our largest metropolis make the 300-mile journey untenable for many of our patients; and the TV show isn’t helping our cause! Even cases that define themselves from the get-go as major university referrals return afterward and ask us to assume their postoperative care. Our patients often can’t make the trip to follow-up with the experts who provided their life-saving care.
Stretching our surgical muscles is obviously important for all ACS members. In bigger facilities you can see and sometimes scrub into fascinating cases in other subspecialties, or at least participate in discussions about such in the surgery lounge. I won’t attach a photo of the desk space that serves as my lounge, dictation station, bathroom, and locker. Let’s just say it’s probably not quite the same as many Fellows are used to.
For the rural solo practitioner, a bigger case, done perhaps with a medical student or just scrub technicians, may not be done as slickly as it would be by a surgical team approaching the same at the university. If the case can be done safely though, it pays dividends. After all, it could be tonight when a major car wreck happens, or a hemodynamically unstable abdominal sepsis case presents, and we are forced to do a serious case – perhaps surgery at the edge of my comfort zone or something we don’t do with frequency. Keeping some bigger cases makes those scenarios just a bit less scary.
I have been recruited as an advocate for our College, trying to influence those in our nation’s capital to reexamine the 96-hour rule as it applies to critical access hospitals. A phone call to my senior senator’s staff leads to a conference call and follow-up I remain involved with – a first in my professional career. One issue that resonated with D.C. staffers was recruiting my successor. How do we entice the young surgeon to a rural practice if all we do are lumps and bumps, appendectomies, and inguinal hernias? Regionalization of surgical care may be coming but that can’t excite our younger and future colleagues. In each of the last 2 years my third-year medical students parked here for their first rotation and got hustled into the OR to assist with emergency surgery. The enthusiasm was palpable and energizing, but one was a case that raised some eyebrows: pneumatosis intestinalis requiring two small bowel resections with anastomoses and an open abdomen in an elderly male. This fellow did great; I see him doing his grocery shopping these days. My perspective is that case enabled this year’s day 1 emergency, making the surgery safer here in rural America.
When we call to transfer a patient, please understand real thought and a piece of who we are as surgeons accompanies that patient. Transfer is very rarely a reflex action. Also, realize that not every case we keep is a weak fastball over the middle of the plate; sometimes we do real work here at the limit of our comfort zone, but we do so for myriad good reasons.
Dr. Levine is a general surgeon practicing in coastal southwestern Oregon. Despite growing up in Brooklyn and on Long Island in New York, he has been a practicing rural surgeon since 1999. Folks barely even notice the accent anymore!
Verrucous Carcinoma on the Lower Extremities
To the Editor:
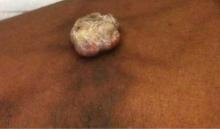
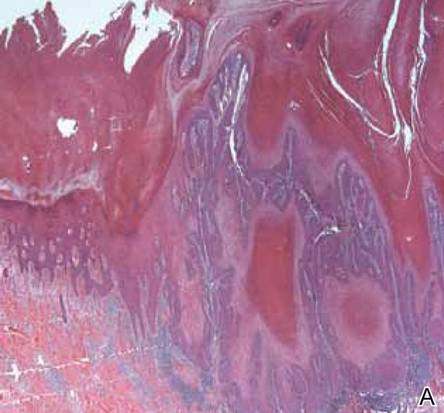
A 38-year-old black man presented with a slowly enlarging growth on the left thigh of 7 years’ duration. The lesion would occasionally scrape off but always recurred. He reported that the tumor developed in the area of a prior nevus. He reported no direct trauma to the area, chronic inflammation, or similar lesions elsewhere. His medical history included gastroesophageal reflux disease and inactive sarcoidosis. Physical examination revealed a 3×3×1-cm exophytic, hyperkeratotic, erythematous nodule with surrounding stellate and branching hyperpigmentation on the anterior aspect of the thigh (Figure 1). Pathologic examination demonstrated hyperkeratosis with an endophytic proliferation of mildly atypical keratinocytes with broad blunted rete ridges (Figure 2). Complete excision of the lesion was performed.
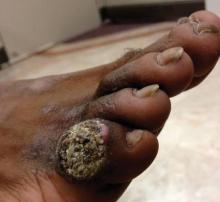
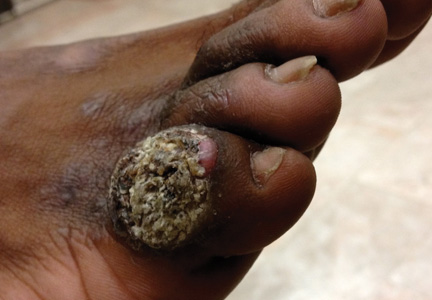
A 33-year-old black man presented with a rapidly growing lesion on the right fifth toe of 3 months’ duration. The patient originally believed the initial small papule was a corn, and after attempts to shave it down with a razor blade, the lesion grew rapidly into a large painful tumor. He reported no prior trauma to the area or history of a similar lesion. Physical examination revealed a 2×2×0.5-cm hyperkeratotic, papillated, hard nodule with a heaped-up border and no ulceration or drainage (Figure 3). A shave biopsy of the lesion was obtained. Microscopic examination revealed hyperkeratosis, parakeratosis, and papillomatosis with deep extension of mildly atypical keratinocytes into the dermis. Small toe amputation was performed by an orthopedic surgeon.
  |
Verrucous carcinoma, first described by Ackerman1 in 1948, is an uncommon, low-grade, well-differentiated variant of squamous cell carcinoma. It presents as a slow-growing, bulky, exophytic tumor with a broad base. The tumor can ulcerate or present with surface sinus tracts that drain foul-smelling material. Typically, the tumor occurs in the fifth to sixth decades of life, with men outnumbering women by a ratio of 5.3 to 1.2 The prevalence of verrucous carcinoma in black individuals is unknown. A review of nonmelanoma skin cancers in skin of color identifies squamous cell carcinoma as the most common cutaneous carcinoma but does not report on the rare verrucous variant.3
Verrucous carcinoma is found in a variety of mucosal and skin surfaces. Verrucous carcinoma of the oral cavity, found most commonly on the buccal mucosa, is known as florid oral papillomatosis or Ackerman carcinoma. Cutaneous verrucous carcinoma is referred to as carcinoma cuniculatum or epithelioma cuniculatum and is predominantly located on the plantar surface of the foot. It is less commonly reported on the palm, scalp, face, extremities, and back. Verrucous carcinoma found in the anogenital area is referred to as the Buschke-Löwenstein tumor.4
Histologically, the lesion shows minimal cytologic atypia. Topped by an undulating keratinized mass, the deep margin of the tumor advances as a broad bulbous projection, compressing the underlying connective tissue in a bulldozing manner. Typically there also are keratin-filled sinuses and intraepidermal microabscesses.1
Human papillomavirus types 6, 11, 16, and 18 may be involved in the induction of the tumor. Human papillomavirus types 6 and 11 are frequently associated with the Buschke-Löwenstein tumor,2,4 while carcinoma cuniculatum is most commonly associated with human papillomavirus 16.5-7 In several cases of verrucous carcinoma, the tumor was reported to arise from preexisting lesions with chronic inflammation, such as a chronic ulcer, inflamed cyst, or burn scar.2 Ackerman carcinoma has been associated with the use of snuff, chewing tobacco, and betel nuts.
Morbidity and mortality from verrucous carcinoma arises from local invasion and infiltration into adjacent bone. The tumor rarely metastasizes, with regional lymph nodes being the only reported site of metastasis.8 The treatment of cutaneous verrucous carcinoma is complete surgical excision. Mohs micrographic surgery is preferred because it minimizes recurrence risk.4 Radiation therapy is contraindicated because it has been reported to cause the tumor to become more aggressive.9,10 Although local recurrence may occur, the prognosis is usually favorable.
1. Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
2. Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin). a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
3. Jackson BA. Nonmelanoma skin cancer in persons of color. Semin Cutan Med Surg. 2009;28:93-95.
4. Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21; quiz 22-24.
5. Assaf C, Steinhoff M, Petrov I, et al. Verrucous carcinoma of the axilla: case report and review. J Cutan Pathol. 2004;31:199-204.
6. Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
7. Miyamoto T, Sasaoka R, Hagari Y, et al. Association of cutaneous verrucous carcinoma with human papillomavirus type 16. Br J Dermatol. 1999;140:168-169.
8. Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases [published online ahead of print July 11, 2008]. Oral Oncol. 2009;45:47-51.
9. Perez CA, Krans FT, Evans JC, et al. Anaplastic transformation in verrucous carcinoma of the oral cavity after radiation therapy. Radiology. 1966;86:108-115.
10. Proffett SD, Spooner TR, Kosek JC. Origin of undifferentiated neoplasm from verrucous epidermal carcinoma of oral cavity following irradiation. Cancer. 1970;26:389-393.
To the Editor:
A 38-year-old black man presented with a slowly enlarging growth on the left thigh of 7 years’ duration. The lesion would occasionally scrape off but always recurred. He reported that the tumor developed in the area of a prior nevus. He reported no direct trauma to the area, chronic inflammation, or similar lesions elsewhere. His medical history included gastroesophageal reflux disease and inactive sarcoidosis. Physical examination revealed a 3×3×1-cm exophytic, hyperkeratotic, erythematous nodule with surrounding stellate and branching hyperpigmentation on the anterior aspect of the thigh (Figure 1). Pathologic examination demonstrated hyperkeratosis with an endophytic proliferation of mildly atypical keratinocytes with broad blunted rete ridges (Figure 2). Complete excision of the lesion was performed.
A 33-year-old black man presented with a rapidly growing lesion on the right fifth toe of 3 months’ duration. The patient originally believed the initial small papule was a corn, and after attempts to shave it down with a razor blade, the lesion grew rapidly into a large painful tumor. He reported no prior trauma to the area or history of a similar lesion. Physical examination revealed a 2×2×0.5-cm hyperkeratotic, papillated, hard nodule with a heaped-up border and no ulceration or drainage (Figure 3). A shave biopsy of the lesion was obtained. Microscopic examination revealed hyperkeratosis, parakeratosis, and papillomatosis with deep extension of mildly atypical keratinocytes into the dermis. Small toe amputation was performed by an orthopedic surgeon.
  |
Verrucous carcinoma, first described by Ackerman1 in 1948, is an uncommon, low-grade, well-differentiated variant of squamous cell carcinoma. It presents as a slow-growing, bulky, exophytic tumor with a broad base. The tumor can ulcerate or present with surface sinus tracts that drain foul-smelling material. Typically, the tumor occurs in the fifth to sixth decades of life, with men outnumbering women by a ratio of 5.3 to 1.2 The prevalence of verrucous carcinoma in black individuals is unknown. A review of nonmelanoma skin cancers in skin of color identifies squamous cell carcinoma as the most common cutaneous carcinoma but does not report on the rare verrucous variant.3
Verrucous carcinoma is found in a variety of mucosal and skin surfaces. Verrucous carcinoma of the oral cavity, found most commonly on the buccal mucosa, is known as florid oral papillomatosis or Ackerman carcinoma. Cutaneous verrucous carcinoma is referred to as carcinoma cuniculatum or epithelioma cuniculatum and is predominantly located on the plantar surface of the foot. It is less commonly reported on the palm, scalp, face, extremities, and back. Verrucous carcinoma found in the anogenital area is referred to as the Buschke-Löwenstein tumor.4
Histologically, the lesion shows minimal cytologic atypia. Topped by an undulating keratinized mass, the deep margin of the tumor advances as a broad bulbous projection, compressing the underlying connective tissue in a bulldozing manner. Typically there also are keratin-filled sinuses and intraepidermal microabscesses.1
Human papillomavirus types 6, 11, 16, and 18 may be involved in the induction of the tumor. Human papillomavirus types 6 and 11 are frequently associated with the Buschke-Löwenstein tumor,2,4 while carcinoma cuniculatum is most commonly associated with human papillomavirus 16.5-7 In several cases of verrucous carcinoma, the tumor was reported to arise from preexisting lesions with chronic inflammation, such as a chronic ulcer, inflamed cyst, or burn scar.2 Ackerman carcinoma has been associated with the use of snuff, chewing tobacco, and betel nuts.
Morbidity and mortality from verrucous carcinoma arises from local invasion and infiltration into adjacent bone. The tumor rarely metastasizes, with regional lymph nodes being the only reported site of metastasis.8 The treatment of cutaneous verrucous carcinoma is complete surgical excision. Mohs micrographic surgery is preferred because it minimizes recurrence risk.4 Radiation therapy is contraindicated because it has been reported to cause the tumor to become more aggressive.9,10 Although local recurrence may occur, the prognosis is usually favorable.
To the Editor:
A 38-year-old black man presented with a slowly enlarging growth on the left thigh of 7 years’ duration. The lesion would occasionally scrape off but always recurred. He reported that the tumor developed in the area of a prior nevus. He reported no direct trauma to the area, chronic inflammation, or similar lesions elsewhere. His medical history included gastroesophageal reflux disease and inactive sarcoidosis. Physical examination revealed a 3×3×1-cm exophytic, hyperkeratotic, erythematous nodule with surrounding stellate and branching hyperpigmentation on the anterior aspect of the thigh (Figure 1). Pathologic examination demonstrated hyperkeratosis with an endophytic proliferation of mildly atypical keratinocytes with broad blunted rete ridges (Figure 2). Complete excision of the lesion was performed.
A 33-year-old black man presented with a rapidly growing lesion on the right fifth toe of 3 months’ duration. The patient originally believed the initial small papule was a corn, and after attempts to shave it down with a razor blade, the lesion grew rapidly into a large painful tumor. He reported no prior trauma to the area or history of a similar lesion. Physical examination revealed a 2×2×0.5-cm hyperkeratotic, papillated, hard nodule with a heaped-up border and no ulceration or drainage (Figure 3). A shave biopsy of the lesion was obtained. Microscopic examination revealed hyperkeratosis, parakeratosis, and papillomatosis with deep extension of mildly atypical keratinocytes into the dermis. Small toe amputation was performed by an orthopedic surgeon.
  |
Verrucous carcinoma, first described by Ackerman1 in 1948, is an uncommon, low-grade, well-differentiated variant of squamous cell carcinoma. It presents as a slow-growing, bulky, exophytic tumor with a broad base. The tumor can ulcerate or present with surface sinus tracts that drain foul-smelling material. Typically, the tumor occurs in the fifth to sixth decades of life, with men outnumbering women by a ratio of 5.3 to 1.2 The prevalence of verrucous carcinoma in black individuals is unknown. A review of nonmelanoma skin cancers in skin of color identifies squamous cell carcinoma as the most common cutaneous carcinoma but does not report on the rare verrucous variant.3
Verrucous carcinoma is found in a variety of mucosal and skin surfaces. Verrucous carcinoma of the oral cavity, found most commonly on the buccal mucosa, is known as florid oral papillomatosis or Ackerman carcinoma. Cutaneous verrucous carcinoma is referred to as carcinoma cuniculatum or epithelioma cuniculatum and is predominantly located on the plantar surface of the foot. It is less commonly reported on the palm, scalp, face, extremities, and back. Verrucous carcinoma found in the anogenital area is referred to as the Buschke-Löwenstein tumor.4
Histologically, the lesion shows minimal cytologic atypia. Topped by an undulating keratinized mass, the deep margin of the tumor advances as a broad bulbous projection, compressing the underlying connective tissue in a bulldozing manner. Typically there also are keratin-filled sinuses and intraepidermal microabscesses.1
Human papillomavirus types 6, 11, 16, and 18 may be involved in the induction of the tumor. Human papillomavirus types 6 and 11 are frequently associated with the Buschke-Löwenstein tumor,2,4 while carcinoma cuniculatum is most commonly associated with human papillomavirus 16.5-7 In several cases of verrucous carcinoma, the tumor was reported to arise from preexisting lesions with chronic inflammation, such as a chronic ulcer, inflamed cyst, or burn scar.2 Ackerman carcinoma has been associated with the use of snuff, chewing tobacco, and betel nuts.
Morbidity and mortality from verrucous carcinoma arises from local invasion and infiltration into adjacent bone. The tumor rarely metastasizes, with regional lymph nodes being the only reported site of metastasis.8 The treatment of cutaneous verrucous carcinoma is complete surgical excision. Mohs micrographic surgery is preferred because it minimizes recurrence risk.4 Radiation therapy is contraindicated because it has been reported to cause the tumor to become more aggressive.9,10 Although local recurrence may occur, the prognosis is usually favorable.
1. Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
2. Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin). a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
3. Jackson BA. Nonmelanoma skin cancer in persons of color. Semin Cutan Med Surg. 2009;28:93-95.
4. Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21; quiz 22-24.
5. Assaf C, Steinhoff M, Petrov I, et al. Verrucous carcinoma of the axilla: case report and review. J Cutan Pathol. 2004;31:199-204.
6. Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
7. Miyamoto T, Sasaoka R, Hagari Y, et al. Association of cutaneous verrucous carcinoma with human papillomavirus type 16. Br J Dermatol. 1999;140:168-169.
8. Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases [published online ahead of print July 11, 2008]. Oral Oncol. 2009;45:47-51.
9. Perez CA, Krans FT, Evans JC, et al. Anaplastic transformation in verrucous carcinoma of the oral cavity after radiation therapy. Radiology. 1966;86:108-115.
10. Proffett SD, Spooner TR, Kosek JC. Origin of undifferentiated neoplasm from verrucous epidermal carcinoma of oral cavity following irradiation. Cancer. 1970;26:389-393.
1. Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
2. Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin). a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
3. Jackson BA. Nonmelanoma skin cancer in persons of color. Semin Cutan Med Surg. 2009;28:93-95.
4. Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21; quiz 22-24.
5. Assaf C, Steinhoff M, Petrov I, et al. Verrucous carcinoma of the axilla: case report and review. J Cutan Pathol. 2004;31:199-204.
6. Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
7. Miyamoto T, Sasaoka R, Hagari Y, et al. Association of cutaneous verrucous carcinoma with human papillomavirus type 16. Br J Dermatol. 1999;140:168-169.
8. Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases [published online ahead of print July 11, 2008]. Oral Oncol. 2009;45:47-51.
9. Perez CA, Krans FT, Evans JC, et al. Anaplastic transformation in verrucous carcinoma of the oral cavity after radiation therapy. Radiology. 1966;86:108-115.
10. Proffett SD, Spooner TR, Kosek JC. Origin of undifferentiated neoplasm from verrucous epidermal carcinoma of oral cavity following irradiation. Cancer. 1970;26:389-393.
Sweet Syndrome Presenting With an Unusual Morphology
To the Editor:
Sweet syndrome is a neutrophilic dermatosis that typically presents as an acute onset of multiple, painful, sharply demarcated, small (measuring a few centimeters), raised, red plaques that occasionally present with superimposed pustules, vesicles, or bullae on the face, neck, upper chest, back, and extremities. Patients are often febrile and may have mucosal and systemic involvement.1 Although 71% of cases are idiopathic, others are associated with malignancy; autoimmune disorders; infections; pregnancy; and rarely medications, especially all-trans-retinoic acid, granulocyte colony-stimulating factor, vaccines, and antibiotics.1,2 We present a case of Sweet syndrome induced by trimethoprim-sulfamethoxazole (TMP-SMX) with an unusual clinical presentation.
A 71-year-old man with a medical history of nonmelanoma skin cancer initiated a course of TMP-SMX for a wound infection of the lower leg following Mohs micrographic surgery. Eight days later, he developed a painful eruption preceded by 1 day of fever, malaise, blurry vision, and myalgia. Trimethoprim-sulfamethoxazole was discontinued. Physical examination revealed ill-defined, discrete and coalescing, 1- to 6-mm edematous erythematous papules studded with pustules involving the scalp, face, neck, back (Figure 1), and extremities. The patient also had conjunctival erythema and an elevated temperature (38.3°C). Laboratory workup revealed an elevated white blood cell count (11,300/mL [reference range, 4500–11,000/µL]), blood urea nitrogen level (33 mg/µL [reference range, 7–20 mg/dL]), and creatinine level (2.00 mg/dL [reference range, 0.6–1.2 mg/dL]). Liver function tests were normal. A biopsy demonstrated marked papillary dermal edema with a dense, bandlike, superficial dermal neutrophilic infiltrate (Figure 2). A few neutrophils were present in the epidermis with formation of minute intraepidermal pustules. The patient was diagnosed with Sweet syndrome and treated with intravenous methylprednisolone 60 mg 3 times daily (1.5 mg/kg body weight) tapered over 17 days and triamcinolone acetonide ointment 0.1% twice daily. His fever and leukocytosis resolved within 1 day and the eruption improved within 2 days with residual desquamation that cleared by 3 weeks.
 |  |
Morphologically, our case resembled acute generalized exanthematous pustulosis (AGEP), which presents with edematous erythema studded with pustules.3 Although fever and leukocytosis are often present in both AGEP and Sweet syndrome, our patient’s pain, malaise, and myalgia favored Sweet syndrome, as did his conjunctivitis, which is unusual in AGEP.1,3 Histologically, our case was characteristic for Sweet syndrome, which presents with marked papillary dermal edema and a dense neutrophilic dermal infiltrate with neutrophil exocytosis and spongiform pustules in 21% of cases.1 Acute generalized exanthematous pustulosis, characterized by spongiform pustules and a perivascular neutrophilic infiltrate, does not exhibit the dense dermal neutrophilic infiltrate of Sweet syndrome.3 Mecca et al4 also reported a case displaying overlapping features of Sweet syndrome and AGEP. The patient presented with photodistributed papules and pinpoint pustules on an erythematous base favoring a diagnosis of AGEP with histologic findings compatible with Sweet syndrome. The authors suggested a clinicopathologic continuum may exist among drug-related neutrophilic dermatoses.4
In conclusion, we present a case of TMP-SMX–induced Sweet syndrome that morphologically resembled AGEP. It is important to recognize that Sweet syndrome may present in this unusual manner, as it may have notable internal involvement, and responds rapidly to systemic steroids, whereas AGEP has minimal systemic involvement and clears spontaneously.
1. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Kluger N, Marque M, Stoebner PE, et al. Possible drug-induced Sweet’s syndrome due to trimethoprim-sulfamethoxazole. Acta Derm Venereol. 2008;88:637-638.
3. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
4. Mecca P, Tobin E, Andrew Carlson J. Photo-distributed neutrophilic drug eruption and adult respiratory distress syndrome associated with antidepressant therapy. J Cutan Pathol. 2004;31:189-194.
To the Editor:
Sweet syndrome is a neutrophilic dermatosis that typically presents as an acute onset of multiple, painful, sharply demarcated, small (measuring a few centimeters), raised, red plaques that occasionally present with superimposed pustules, vesicles, or bullae on the face, neck, upper chest, back, and extremities. Patients are often febrile and may have mucosal and systemic involvement.1 Although 71% of cases are idiopathic, others are associated with malignancy; autoimmune disorders; infections; pregnancy; and rarely medications, especially all-trans-retinoic acid, granulocyte colony-stimulating factor, vaccines, and antibiotics.1,2 We present a case of Sweet syndrome induced by trimethoprim-sulfamethoxazole (TMP-SMX) with an unusual clinical presentation.
A 71-year-old man with a medical history of nonmelanoma skin cancer initiated a course of TMP-SMX for a wound infection of the lower leg following Mohs micrographic surgery. Eight days later, he developed a painful eruption preceded by 1 day of fever, malaise, blurry vision, and myalgia. Trimethoprim-sulfamethoxazole was discontinued. Physical examination revealed ill-defined, discrete and coalescing, 1- to 6-mm edematous erythematous papules studded with pustules involving the scalp, face, neck, back (Figure 1), and extremities. The patient also had conjunctival erythema and an elevated temperature (38.3°C). Laboratory workup revealed an elevated white blood cell count (11,300/mL [reference range, 4500–11,000/µL]), blood urea nitrogen level (33 mg/µL [reference range, 7–20 mg/dL]), and creatinine level (2.00 mg/dL [reference range, 0.6–1.2 mg/dL]). Liver function tests were normal. A biopsy demonstrated marked papillary dermal edema with a dense, bandlike, superficial dermal neutrophilic infiltrate (Figure 2). A few neutrophils were present in the epidermis with formation of minute intraepidermal pustules. The patient was diagnosed with Sweet syndrome and treated with intravenous methylprednisolone 60 mg 3 times daily (1.5 mg/kg body weight) tapered over 17 days and triamcinolone acetonide ointment 0.1% twice daily. His fever and leukocytosis resolved within 1 day and the eruption improved within 2 days with residual desquamation that cleared by 3 weeks.
 |  |
Morphologically, our case resembled acute generalized exanthematous pustulosis (AGEP), which presents with edematous erythema studded with pustules.3 Although fever and leukocytosis are often present in both AGEP and Sweet syndrome, our patient’s pain, malaise, and myalgia favored Sweet syndrome, as did his conjunctivitis, which is unusual in AGEP.1,3 Histologically, our case was characteristic for Sweet syndrome, which presents with marked papillary dermal edema and a dense neutrophilic dermal infiltrate with neutrophil exocytosis and spongiform pustules in 21% of cases.1 Acute generalized exanthematous pustulosis, characterized by spongiform pustules and a perivascular neutrophilic infiltrate, does not exhibit the dense dermal neutrophilic infiltrate of Sweet syndrome.3 Mecca et al4 also reported a case displaying overlapping features of Sweet syndrome and AGEP. The patient presented with photodistributed papules and pinpoint pustules on an erythematous base favoring a diagnosis of AGEP with histologic findings compatible with Sweet syndrome. The authors suggested a clinicopathologic continuum may exist among drug-related neutrophilic dermatoses.4
In conclusion, we present a case of TMP-SMX–induced Sweet syndrome that morphologically resembled AGEP. It is important to recognize that Sweet syndrome may present in this unusual manner, as it may have notable internal involvement, and responds rapidly to systemic steroids, whereas AGEP has minimal systemic involvement and clears spontaneously.
To the Editor:
Sweet syndrome is a neutrophilic dermatosis that typically presents as an acute onset of multiple, painful, sharply demarcated, small (measuring a few centimeters), raised, red plaques that occasionally present with superimposed pustules, vesicles, or bullae on the face, neck, upper chest, back, and extremities. Patients are often febrile and may have mucosal and systemic involvement.1 Although 71% of cases are idiopathic, others are associated with malignancy; autoimmune disorders; infections; pregnancy; and rarely medications, especially all-trans-retinoic acid, granulocyte colony-stimulating factor, vaccines, and antibiotics.1,2 We present a case of Sweet syndrome induced by trimethoprim-sulfamethoxazole (TMP-SMX) with an unusual clinical presentation.
A 71-year-old man with a medical history of nonmelanoma skin cancer initiated a course of TMP-SMX for a wound infection of the lower leg following Mohs micrographic surgery. Eight days later, he developed a painful eruption preceded by 1 day of fever, malaise, blurry vision, and myalgia. Trimethoprim-sulfamethoxazole was discontinued. Physical examination revealed ill-defined, discrete and coalescing, 1- to 6-mm edematous erythematous papules studded with pustules involving the scalp, face, neck, back (Figure 1), and extremities. The patient also had conjunctival erythema and an elevated temperature (38.3°C). Laboratory workup revealed an elevated white blood cell count (11,300/mL [reference range, 4500–11,000/µL]), blood urea nitrogen level (33 mg/µL [reference range, 7–20 mg/dL]), and creatinine level (2.00 mg/dL [reference range, 0.6–1.2 mg/dL]). Liver function tests were normal. A biopsy demonstrated marked papillary dermal edema with a dense, bandlike, superficial dermal neutrophilic infiltrate (Figure 2). A few neutrophils were present in the epidermis with formation of minute intraepidermal pustules. The patient was diagnosed with Sweet syndrome and treated with intravenous methylprednisolone 60 mg 3 times daily (1.5 mg/kg body weight) tapered over 17 days and triamcinolone acetonide ointment 0.1% twice daily. His fever and leukocytosis resolved within 1 day and the eruption improved within 2 days with residual desquamation that cleared by 3 weeks.
 |  |
Morphologically, our case resembled acute generalized exanthematous pustulosis (AGEP), which presents with edematous erythema studded with pustules.3 Although fever and leukocytosis are often present in both AGEP and Sweet syndrome, our patient’s pain, malaise, and myalgia favored Sweet syndrome, as did his conjunctivitis, which is unusual in AGEP.1,3 Histologically, our case was characteristic for Sweet syndrome, which presents with marked papillary dermal edema and a dense neutrophilic dermal infiltrate with neutrophil exocytosis and spongiform pustules in 21% of cases.1 Acute generalized exanthematous pustulosis, characterized by spongiform pustules and a perivascular neutrophilic infiltrate, does not exhibit the dense dermal neutrophilic infiltrate of Sweet syndrome.3 Mecca et al4 also reported a case displaying overlapping features of Sweet syndrome and AGEP. The patient presented with photodistributed papules and pinpoint pustules on an erythematous base favoring a diagnosis of AGEP with histologic findings compatible with Sweet syndrome. The authors suggested a clinicopathologic continuum may exist among drug-related neutrophilic dermatoses.4
In conclusion, we present a case of TMP-SMX–induced Sweet syndrome that morphologically resembled AGEP. It is important to recognize that Sweet syndrome may present in this unusual manner, as it may have notable internal involvement, and responds rapidly to systemic steroids, whereas AGEP has minimal systemic involvement and clears spontaneously.
1. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Kluger N, Marque M, Stoebner PE, et al. Possible drug-induced Sweet’s syndrome due to trimethoprim-sulfamethoxazole. Acta Derm Venereol. 2008;88:637-638.
3. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
4. Mecca P, Tobin E, Andrew Carlson J. Photo-distributed neutrophilic drug eruption and adult respiratory distress syndrome associated with antidepressant therapy. J Cutan Pathol. 2004;31:189-194.
1. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Kluger N, Marque M, Stoebner PE, et al. Possible drug-induced Sweet’s syndrome due to trimethoprim-sulfamethoxazole. Acta Derm Venereol. 2008;88:637-638.
3. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
4. Mecca P, Tobin E, Andrew Carlson J. Photo-distributed neutrophilic drug eruption and adult respiratory distress syndrome associated with antidepressant therapy. J Cutan Pathol. 2004;31:189-194.
LISTEN NOW: Scott Sears, MD, MBA, Explains How GME Programs Could Be Better Aligned
SCOTT SEARS, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians, discusses how
GME programs could be better aligned with the shifting reality in medicine.
SCOTT SEARS, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians, discusses how
GME programs could be better aligned with the shifting reality in medicine.
SCOTT SEARS, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians, discusses how
GME programs could be better aligned with the shifting reality in medicine.
LISTEN NOW: Ruth Ann Crystal, MD, Pursues Documentary Film "Kitchen Table Deliveries"
Excerpts of The Hospitalist's interview with Dr. Ruth Ann Crystal, who is attempting to create a website with videos of historical medical practice.
Excerpts of The Hospitalist's interview with Dr. Ruth Ann Crystal, who is attempting to create a website with videos of historical medical practice.
Excerpts of The Hospitalist's interview with Dr. Ruth Ann Crystal, who is attempting to create a website with videos of historical medical practice.
Education doesn’t ensure appropriate use of VTE prophylaxis

Photo courtesy of the CDC
In a single-center study, researchers found that educating healthcare providers about the need for venous thromboembolism (VTE) prophylaxis did not ensure that patients received appropriate treatment.
Before the educational program was introduced, 36% of patients who were at risk of VTE were not receiving VTE prophylaxis. After the program, 26% of at-risk patients were not receiving prophylaxis.
The researchers reported these findings in the Canadian Journal of Cardiology.
The team carried out chart reviews of patients in a university-affiliated, tertiary care cardiology center, which included a clinical teaching unit and a coronary care unit.
Audits were conducted 3 and 5 months before the introduction of an educational program on VTE prophylaxis protocol, followed by a second series of audits 3 and 5 months after protocol initiation.
In each set of audits, conducted over 2 months, 3 independent groups consisting of a physician and a nonphysician healthcare provider (nursing, pharmacy) each reviewed the data. Discrepancies were settled by the senior investigators.
In the first set of audits, 173 charts for patients considered at high risk for VTE were evaluated. The second set of audits included 247 patients.
Prior to the educational program, including a guideline-based protocol, 36% of all patients who were considered at risk for VTE did not receive prophylaxis.
Three months after the program was initiated, 21% of patients were still not being treated according to the recommended guidelines, and that percentage rose to 28% at 5 months post-protocol.
“Awareness and education surrounding VTE prophylaxis is challenging in the inpatient teaching unit model due to a number of factors, including the high turnover of senior and junior physicians as well as nursing staff,” said study author Colette Seifer, of the University of Manitoba in Winnipeg, Manitoba, Canada.
“A single time point intervention is unlikely to result in a sustained improvement in VTE prophylaxis rates.”
However, Seifer and her colleagues believe that automated alerts and checklists incorporated into electronic patient records or used via innovative software programs have the potential to improve compliance rates. ![]()

Photo courtesy of the CDC
In a single-center study, researchers found that educating healthcare providers about the need for venous thromboembolism (VTE) prophylaxis did not ensure that patients received appropriate treatment.
Before the educational program was introduced, 36% of patients who were at risk of VTE were not receiving VTE prophylaxis. After the program, 26% of at-risk patients were not receiving prophylaxis.
The researchers reported these findings in the Canadian Journal of Cardiology.
The team carried out chart reviews of patients in a university-affiliated, tertiary care cardiology center, which included a clinical teaching unit and a coronary care unit.
Audits were conducted 3 and 5 months before the introduction of an educational program on VTE prophylaxis protocol, followed by a second series of audits 3 and 5 months after protocol initiation.
In each set of audits, conducted over 2 months, 3 independent groups consisting of a physician and a nonphysician healthcare provider (nursing, pharmacy) each reviewed the data. Discrepancies were settled by the senior investigators.
In the first set of audits, 173 charts for patients considered at high risk for VTE were evaluated. The second set of audits included 247 patients.
Prior to the educational program, including a guideline-based protocol, 36% of all patients who were considered at risk for VTE did not receive prophylaxis.
Three months after the program was initiated, 21% of patients were still not being treated according to the recommended guidelines, and that percentage rose to 28% at 5 months post-protocol.
“Awareness and education surrounding VTE prophylaxis is challenging in the inpatient teaching unit model due to a number of factors, including the high turnover of senior and junior physicians as well as nursing staff,” said study author Colette Seifer, of the University of Manitoba in Winnipeg, Manitoba, Canada.
“A single time point intervention is unlikely to result in a sustained improvement in VTE prophylaxis rates.”
However, Seifer and her colleagues believe that automated alerts and checklists incorporated into electronic patient records or used via innovative software programs have the potential to improve compliance rates. ![]()

Photo courtesy of the CDC
In a single-center study, researchers found that educating healthcare providers about the need for venous thromboembolism (VTE) prophylaxis did not ensure that patients received appropriate treatment.
Before the educational program was introduced, 36% of patients who were at risk of VTE were not receiving VTE prophylaxis. After the program, 26% of at-risk patients were not receiving prophylaxis.
The researchers reported these findings in the Canadian Journal of Cardiology.
The team carried out chart reviews of patients in a university-affiliated, tertiary care cardiology center, which included a clinical teaching unit and a coronary care unit.
Audits were conducted 3 and 5 months before the introduction of an educational program on VTE prophylaxis protocol, followed by a second series of audits 3 and 5 months after protocol initiation.
In each set of audits, conducted over 2 months, 3 independent groups consisting of a physician and a nonphysician healthcare provider (nursing, pharmacy) each reviewed the data. Discrepancies were settled by the senior investigators.
In the first set of audits, 173 charts for patients considered at high risk for VTE were evaluated. The second set of audits included 247 patients.
Prior to the educational program, including a guideline-based protocol, 36% of all patients who were considered at risk for VTE did not receive prophylaxis.
Three months after the program was initiated, 21% of patients were still not being treated according to the recommended guidelines, and that percentage rose to 28% at 5 months post-protocol.
“Awareness and education surrounding VTE prophylaxis is challenging in the inpatient teaching unit model due to a number of factors, including the high turnover of senior and junior physicians as well as nursing staff,” said study author Colette Seifer, of the University of Manitoba in Winnipeg, Manitoba, Canada.
“A single time point intervention is unlikely to result in a sustained improvement in VTE prophylaxis rates.”
However, Seifer and her colleagues believe that automated alerts and checklists incorporated into electronic patient records or used via innovative software programs have the potential to improve compliance rates. ![]()
How religion affects well-being in cancer patients

Photo by Petr Kratochvil
Three meta-analyses shed new light on the role religion and spirituality play in cancer patients’ mental, social, and physical well-being.
The analyses, published in Cancer, indicate that religion and spirituality have significant associations with patients’ health.
But investigators observed wide variability among studies with regard to how different dimensions of religion and spirituality relate to different aspects of health.
In the first analysis, the investigators focused on physical health. Patients reporting greater overall religiousness and spirituality reported better physical health, greater ability to perform their usual daily tasks, and fewer physical symptoms of cancer and treatment.
“These relationships were particularly strong in patients who experienced greater emotional aspects of religion and spirituality, including a sense of meaning and purpose in life as well as a connection to a source larger than oneself,” said study author Heather Jim, PhD, of the Moffitt Cancer Center in Tampa, Florida.
Dr Jim noted that patients who reported greater cognitive aspects of religion and spirituality, such as the ability to integrate the cancer into their religious or spiritual beliefs, also reported better physical health. However, physical health was not related to behavioral aspects of religion and spiritualty, such as church attendance, prayer, or meditation.
In the second analysis, the investigators examined patients’ mental health. The team discovered that emotional aspects of religion and spirituality were more strongly associated with positive mental health than behavioral or cognitive aspects of religion and spirituality.
“Spiritual well-being was, unsurprisingly, associated with less anxiety, depression, or distress,” said study author John Salsman, PhD, of Wake Forest School of Medicine in Winston-Salem, North Carolina.
“Also, greater levels of spiritual distress and a sense of disconnectedness with God or a religious community was associated with greater psychological distress or poorer emotional well-being.”
The third analysis pertained to social health, or patients’ capacity to retain social roles and relationships in the face of illness. Religion and spirituality, as well as each of its dimensions, had modest but reliable links with social health.
“When we took a closer look, we found that patients with stronger spiritual well-being, more benign images of God (such as perceptions of a benevolent God rather than an angry or distant God), or stronger beliefs (such as convictions that a personal God can be called upon for assistance) reported better social health,” said study author Allen Sherman, PhD, of the University of Arkansas for Medical Sciences in Little Rock. “In contrast, those who struggled with their faith fared more poorly.”
The investigators believe future research should focus on how relationships between religious or spiritual involvement and health change over time and whether support services designed to enhance particular aspects of religion and spirituality in interested patients might help improve their well-being.
“In addition, some patients struggle with the religious or spiritual significance of their cancer, which is normal,” Dr Jim said. “How they resolve their struggle may impact their health, but more research is needed to better understand and support these patients.” ![]()

Photo by Petr Kratochvil
Three meta-analyses shed new light on the role religion and spirituality play in cancer patients’ mental, social, and physical well-being.
The analyses, published in Cancer, indicate that religion and spirituality have significant associations with patients’ health.
But investigators observed wide variability among studies with regard to how different dimensions of religion and spirituality relate to different aspects of health.
In the first analysis, the investigators focused on physical health. Patients reporting greater overall religiousness and spirituality reported better physical health, greater ability to perform their usual daily tasks, and fewer physical symptoms of cancer and treatment.
“These relationships were particularly strong in patients who experienced greater emotional aspects of religion and spirituality, including a sense of meaning and purpose in life as well as a connection to a source larger than oneself,” said study author Heather Jim, PhD, of the Moffitt Cancer Center in Tampa, Florida.
Dr Jim noted that patients who reported greater cognitive aspects of religion and spirituality, such as the ability to integrate the cancer into their religious or spiritual beliefs, also reported better physical health. However, physical health was not related to behavioral aspects of religion and spiritualty, such as church attendance, prayer, or meditation.
In the second analysis, the investigators examined patients’ mental health. The team discovered that emotional aspects of religion and spirituality were more strongly associated with positive mental health than behavioral or cognitive aspects of religion and spirituality.
“Spiritual well-being was, unsurprisingly, associated with less anxiety, depression, or distress,” said study author John Salsman, PhD, of Wake Forest School of Medicine in Winston-Salem, North Carolina.
“Also, greater levels of spiritual distress and a sense of disconnectedness with God or a religious community was associated with greater psychological distress or poorer emotional well-being.”
The third analysis pertained to social health, or patients’ capacity to retain social roles and relationships in the face of illness. Religion and spirituality, as well as each of its dimensions, had modest but reliable links with social health.
“When we took a closer look, we found that patients with stronger spiritual well-being, more benign images of God (such as perceptions of a benevolent God rather than an angry or distant God), or stronger beliefs (such as convictions that a personal God can be called upon for assistance) reported better social health,” said study author Allen Sherman, PhD, of the University of Arkansas for Medical Sciences in Little Rock. “In contrast, those who struggled with their faith fared more poorly.”
The investigators believe future research should focus on how relationships between religious or spiritual involvement and health change over time and whether support services designed to enhance particular aspects of religion and spirituality in interested patients might help improve their well-being.
“In addition, some patients struggle with the religious or spiritual significance of their cancer, which is normal,” Dr Jim said. “How they resolve their struggle may impact their health, but more research is needed to better understand and support these patients.” ![]()

Photo by Petr Kratochvil
Three meta-analyses shed new light on the role religion and spirituality play in cancer patients’ mental, social, and physical well-being.
The analyses, published in Cancer, indicate that religion and spirituality have significant associations with patients’ health.
But investigators observed wide variability among studies with regard to how different dimensions of religion and spirituality relate to different aspects of health.
In the first analysis, the investigators focused on physical health. Patients reporting greater overall religiousness and spirituality reported better physical health, greater ability to perform their usual daily tasks, and fewer physical symptoms of cancer and treatment.
“These relationships were particularly strong in patients who experienced greater emotional aspects of religion and spirituality, including a sense of meaning and purpose in life as well as a connection to a source larger than oneself,” said study author Heather Jim, PhD, of the Moffitt Cancer Center in Tampa, Florida.
Dr Jim noted that patients who reported greater cognitive aspects of religion and spirituality, such as the ability to integrate the cancer into their religious or spiritual beliefs, also reported better physical health. However, physical health was not related to behavioral aspects of religion and spiritualty, such as church attendance, prayer, or meditation.
In the second analysis, the investigators examined patients’ mental health. The team discovered that emotional aspects of religion and spirituality were more strongly associated with positive mental health than behavioral or cognitive aspects of religion and spirituality.
“Spiritual well-being was, unsurprisingly, associated with less anxiety, depression, or distress,” said study author John Salsman, PhD, of Wake Forest School of Medicine in Winston-Salem, North Carolina.
“Also, greater levels of spiritual distress and a sense of disconnectedness with God or a religious community was associated with greater psychological distress or poorer emotional well-being.”
The third analysis pertained to social health, or patients’ capacity to retain social roles and relationships in the face of illness. Religion and spirituality, as well as each of its dimensions, had modest but reliable links with social health.
“When we took a closer look, we found that patients with stronger spiritual well-being, more benign images of God (such as perceptions of a benevolent God rather than an angry or distant God), or stronger beliefs (such as convictions that a personal God can be called upon for assistance) reported better social health,” said study author Allen Sherman, PhD, of the University of Arkansas for Medical Sciences in Little Rock. “In contrast, those who struggled with their faith fared more poorly.”
The investigators believe future research should focus on how relationships between religious or spiritual involvement and health change over time and whether support services designed to enhance particular aspects of religion and spirituality in interested patients might help improve their well-being.
“In addition, some patients struggle with the religious or spiritual significance of their cancer, which is normal,” Dr Jim said. “How they resolve their struggle may impact their health, but more research is needed to better understand and support these patients.” ![]()
Impact of an Inpatient PN Program
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
© 2015 Society of Hospital Medicine