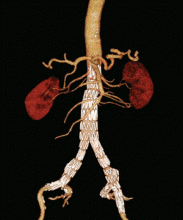
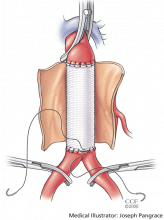
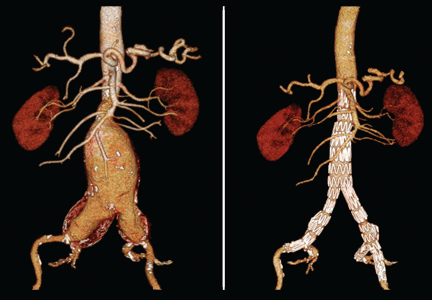
User login
Nausea and vomiting in advanced cancer: the Cleveland Clinic protocol
Nausea and vomiting are common and distressing symptoms in advanced cancer. Both are multifactorial and cause significant morbidity, nutritional failure, and reduced quality of life. Assessment includes a detailed history, physical examination and investigations for reversible causes. Assessment and management will be influenced by performance status, prognosis, and goals of care. Several drug classes are effective with some having the added benefit of multiple routes of administration. It is our institution’s practice to recommend metoclopramide as the first drug with haloperidol as an alternative antiemetic.
Click on the PDF icon at the top of this introduction to read the full article.
Nausea and vomiting are common and distressing symptoms in advanced cancer. Both are multifactorial and cause significant morbidity, nutritional failure, and reduced quality of life. Assessment includes a detailed history, physical examination and investigations for reversible causes. Assessment and management will be influenced by performance status, prognosis, and goals of care. Several drug classes are effective with some having the added benefit of multiple routes of administration. It is our institution’s practice to recommend metoclopramide as the first drug with haloperidol as an alternative antiemetic.
Click on the PDF icon at the top of this introduction to read the full article.
Nausea and vomiting are common and distressing symptoms in advanced cancer. Both are multifactorial and cause significant morbidity, nutritional failure, and reduced quality of life. Assessment includes a detailed history, physical examination and investigations for reversible causes. Assessment and management will be influenced by performance status, prognosis, and goals of care. Several drug classes are effective with some having the added benefit of multiple routes of administration. It is our institution’s practice to recommend metoclopramide as the first drug with haloperidol as an alternative antiemetic.
Click on the PDF icon at the top of this introduction to read the full article.
Initiating palliative care conversations: lessons from Jewish bioethics
What are the ethical responsibilities of the medical staff (doctors, nurses, social workers, and chaplains) regarding thepreservation of meaningful life for their patients who are approaching the end of life (EOL)? In particular, what is the staff’sethical responsibility to initiate a conversation with their patient regarding palliative care? By subjecting traditional Jewish teachings to an ethical analysis and then exploring the underlying universal principles, we will suggest a general ethical duty toinform patients of the different care options, especially in a manner that preserves hope. The principle that we can derive from Jewish bioethics teaches that the medical staff has a responsibility to help our patients live in a way that is consistent with how they understand their task or responsibility in life. For some patients, the best way to preserve a meaningful life in which they can fulfill their sense of purpose in the time that remains is to focus on palliation. For this reason, although palliative and supportive care are provided from the time of diagnosis, it is critical we make sure our patients realize that they have the opportunity to make a decision between either pursuing additional active treatments or choosing to focus primarily on palliative therapies to maximize quality of life. The Jewish tradition and our experience in spiritual care suggest the importance of helping patients preserve hope while, simultaneously, honestly acknowledging their situation. Staff members can play a vital role in helping patients make the most of this new period of their lives.
Click on the PDF icon at the top of this introduction to read the full article.
What are the ethical responsibilities of the medical staff (doctors, nurses, social workers, and chaplains) regarding thepreservation of meaningful life for their patients who are approaching the end of life (EOL)? In particular, what is the staff’sethical responsibility to initiate a conversation with their patient regarding palliative care? By subjecting traditional Jewish teachings to an ethical analysis and then exploring the underlying universal principles, we will suggest a general ethical duty toinform patients of the different care options, especially in a manner that preserves hope. The principle that we can derive from Jewish bioethics teaches that the medical staff has a responsibility to help our patients live in a way that is consistent with how they understand their task or responsibility in life. For some patients, the best way to preserve a meaningful life in which they can fulfill their sense of purpose in the time that remains is to focus on palliation. For this reason, although palliative and supportive care are provided from the time of diagnosis, it is critical we make sure our patients realize that they have the opportunity to make a decision between either pursuing additional active treatments or choosing to focus primarily on palliative therapies to maximize quality of life. The Jewish tradition and our experience in spiritual care suggest the importance of helping patients preserve hope while, simultaneously, honestly acknowledging their situation. Staff members can play a vital role in helping patients make the most of this new period of their lives.
Click on the PDF icon at the top of this introduction to read the full article.
What are the ethical responsibilities of the medical staff (doctors, nurses, social workers, and chaplains) regarding thepreservation of meaningful life for their patients who are approaching the end of life (EOL)? In particular, what is the staff’sethical responsibility to initiate a conversation with their patient regarding palliative care? By subjecting traditional Jewish teachings to an ethical analysis and then exploring the underlying universal principles, we will suggest a general ethical duty toinform patients of the different care options, especially in a manner that preserves hope. The principle that we can derive from Jewish bioethics teaches that the medical staff has a responsibility to help our patients live in a way that is consistent with how they understand their task or responsibility in life. For some patients, the best way to preserve a meaningful life in which they can fulfill their sense of purpose in the time that remains is to focus on palliation. For this reason, although palliative and supportive care are provided from the time of diagnosis, it is critical we make sure our patients realize that they have the opportunity to make a decision between either pursuing additional active treatments or choosing to focus primarily on palliative therapies to maximize quality of life. The Jewish tradition and our experience in spiritual care suggest the importance of helping patients preserve hope while, simultaneously, honestly acknowledging their situation. Staff members can play a vital role in helping patients make the most of this new period of their lives.
Click on the PDF icon at the top of this introduction to read the full article.
A Randomized, Controlled Trial of Panax quinquefolius Extract (CVT-E002) to Reduce Respiratory Infection in Patients With Chronic Lymphocytic Leukemia
Kevin P. High, MD, MS; Doug Case, PhD; MD, David Hurd, MD; Bayard Powell, MD; Glenn Lesser, MD; Ann R. Falsey, MD; Robert Siegel, MD; Joanna Metzner-Sadurski, MD; John C. Krauss, MD; Bernard Chinnasami, MD, George Sanders, MD, Steven Rousey, MD, Edward G. Shaw, MD
Abstract
Background
Chronic lymphocytic leukemia (CLL) patients are at high risk for acute respiratory illness (ARI).
Objective
We evaluated the safety and efficacy of a proprietary extract of Panax quinquefolius, CVT-E002, in reducing ARI.
Methods
This was a double-blind, placebo-controlled, randomized trial of 293 subjects with early-stage, untreated CLL conducted January–March 2009.
Results
ARI was common, occurring on about 10% of days during the study period. There were no significant differences of the 2 a priori primary end points: ARI days (8.5 ± 17.2 for CVT-E002 vs 6.8 ± 13.3 for placebo) and severe ARI days (2.9 ± 9.5 for CVT-E002 vs 2.6 ± 9.8 for placebo). However, 51% of CVT-E002 vs 56% of placebo recipients experienced at least 1 ARI (difference, −5%; 95% confidence interval [CI], −16% to 7%); more intense ARI occurred in 32% of CVT-E002 vs 39% of placebo recipients (difference, −7%; 95% CI, −18% to 4%), and symptom-specific evaluation showed reduced moderate to severe sore throat (P = .004) and a lower rate of grade ≥3 toxicities (P = .02) in CVT-E002 recipients. Greater seroconversion (4-fold increases in antibody titer) vs 9 common viral pathogens was documented in CVT-E002 recipients (16% vs 7%, P = .04).
Limitations
Serologic evaluation of antibody titers was not tied to a specific illness, but covered the entire study period.
Conclusion
CVT-E002 was well tolerated. It did not reduce the number of ARI days or antibiotic use; however, there was a trend toward reduced rates of moderate to severe ARI and significantly less sore throat, suggesting that the increased rate of seroconversion most likely reflects CVT-E002-enhanced antibody responses.
*For a PDF of the full article and accompanying commentary by Paul Sloan, click on the links to the left of this introduction.
Kevin P. High, MD, MS; Doug Case, PhD; MD, David Hurd, MD; Bayard Powell, MD; Glenn Lesser, MD; Ann R. Falsey, MD; Robert Siegel, MD; Joanna Metzner-Sadurski, MD; John C. Krauss, MD; Bernard Chinnasami, MD, George Sanders, MD, Steven Rousey, MD, Edward G. Shaw, MD
Abstract
Background
Chronic lymphocytic leukemia (CLL) patients are at high risk for acute respiratory illness (ARI).
Objective
We evaluated the safety and efficacy of a proprietary extract of Panax quinquefolius, CVT-E002, in reducing ARI.
Methods
This was a double-blind, placebo-controlled, randomized trial of 293 subjects with early-stage, untreated CLL conducted January–March 2009.
Results
ARI was common, occurring on about 10% of days during the study period. There were no significant differences of the 2 a priori primary end points: ARI days (8.5 ± 17.2 for CVT-E002 vs 6.8 ± 13.3 for placebo) and severe ARI days (2.9 ± 9.5 for CVT-E002 vs 2.6 ± 9.8 for placebo). However, 51% of CVT-E002 vs 56% of placebo recipients experienced at least 1 ARI (difference, −5%; 95% confidence interval [CI], −16% to 7%); more intense ARI occurred in 32% of CVT-E002 vs 39% of placebo recipients (difference, −7%; 95% CI, −18% to 4%), and symptom-specific evaluation showed reduced moderate to severe sore throat (P = .004) and a lower rate of grade ≥3 toxicities (P = .02) in CVT-E002 recipients. Greater seroconversion (4-fold increases in antibody titer) vs 9 common viral pathogens was documented in CVT-E002 recipients (16% vs 7%, P = .04).
Limitations
Serologic evaluation of antibody titers was not tied to a specific illness, but covered the entire study period.
Conclusion
CVT-E002 was well tolerated. It did not reduce the number of ARI days or antibiotic use; however, there was a trend toward reduced rates of moderate to severe ARI and significantly less sore throat, suggesting that the increased rate of seroconversion most likely reflects CVT-E002-enhanced antibody responses.
*For a PDF of the full article and accompanying commentary by Paul Sloan, click on the links to the left of this introduction.
Kevin P. High, MD, MS; Doug Case, PhD; MD, David Hurd, MD; Bayard Powell, MD; Glenn Lesser, MD; Ann R. Falsey, MD; Robert Siegel, MD; Joanna Metzner-Sadurski, MD; John C. Krauss, MD; Bernard Chinnasami, MD, George Sanders, MD, Steven Rousey, MD, Edward G. Shaw, MD
Abstract
Background
Chronic lymphocytic leukemia (CLL) patients are at high risk for acute respiratory illness (ARI).
Objective
We evaluated the safety and efficacy of a proprietary extract of Panax quinquefolius, CVT-E002, in reducing ARI.
Methods
This was a double-blind, placebo-controlled, randomized trial of 293 subjects with early-stage, untreated CLL conducted January–March 2009.
Results
ARI was common, occurring on about 10% of days during the study period. There were no significant differences of the 2 a priori primary end points: ARI days (8.5 ± 17.2 for CVT-E002 vs 6.8 ± 13.3 for placebo) and severe ARI days (2.9 ± 9.5 for CVT-E002 vs 2.6 ± 9.8 for placebo). However, 51% of CVT-E002 vs 56% of placebo recipients experienced at least 1 ARI (difference, −5%; 95% confidence interval [CI], −16% to 7%); more intense ARI occurred in 32% of CVT-E002 vs 39% of placebo recipients (difference, −7%; 95% CI, −18% to 4%), and symptom-specific evaluation showed reduced moderate to severe sore throat (P = .004) and a lower rate of grade ≥3 toxicities (P = .02) in CVT-E002 recipients. Greater seroconversion (4-fold increases in antibody titer) vs 9 common viral pathogens was documented in CVT-E002 recipients (16% vs 7%, P = .04).
Limitations
Serologic evaluation of antibody titers was not tied to a specific illness, but covered the entire study period.
Conclusion
CVT-E002 was well tolerated. It did not reduce the number of ARI days or antibiotic use; however, there was a trend toward reduced rates of moderate to severe ARI and significantly less sore throat, suggesting that the increased rate of seroconversion most likely reflects CVT-E002-enhanced antibody responses.
*For a PDF of the full article and accompanying commentary by Paul Sloan, click on the links to the left of this introduction.
Septic shock: The initial moments and beyond
Considerably fewer patients who develop sepsis are dying of it now, thanks to a number of studies of how to reverse sepsis-induced tissue hypoxia.1 The greatest strides in improving outcomes have been attributed to better early management, which includes prompt recognition of sepsis, rapid initiation of antimicrobial therapy, elimination of the source of infection, and early goal-directed therapy. Thus, even though the incidence of severe sepsis and septic shock is increasing,2,3 the Surviving Sepsis Campaign has documented a significant decrease in unadjusted mortality rates (37% to 30.8%) associated with the bundled approach in the management of sepsis.4 (We will talk about this later in the article.)
This review will summarize the evidence for the early management of septic shock and will evaluate the various treatment decisions beyond the initial phases of resuscitation.
INFLAMMATION AND VASODILATION
Sepsis syndrome starts with an infection that leads to a proinflammatory state with a complex interaction between anti-inflammatory and proinflammatory mediators, enhanced coagulation, and impaired fibrinolysis.5,6
Sepsis induces vasodilation by way of inappropriate activation of vasodilatory mechanisms (increased synthesis of nitric oxide and vasopressin deficiency) and failure of vasoconstrictor mechanisms (activation of ATP-sensitive potassium channels in vascular smooth muscle).7 Thus, the hemodynamic abnormalities are multifactorial, and the resultant tissue hypoperfusion further contributes to the proinflammatory and procoagulant state, precipitating multiorgan dysfunction and, often, death.
DEFINITIONS
- Sepsis—infection together with systemic manifestation of inflammatory response
- Severe sepsis—sepsis plus induced organ dysfunction or evidence of tissue hypoperfusion
- Septic shock—sepsis-induced hypotension persisting despite adequate fluid resuscitation.
EARLY MANAGEMENT OF SEPTIC SHOCK
Early in the course of septic shock, the physician’s job is to:
- Recognize it promptly
- Begin empiric antibiotic therapy quickly
- Eliminate the source of infection, if applicable, eg, by removing an infected central venous catheter
- Give fluid resuscitation, titrated to specific goals
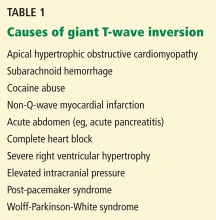
- Give vasopressor therapy to maintain blood pressure, organ perfusion, and oxygen delivery (Table 1).
The line between “early” and “late” is not clear. Traditionally, it has been drawn at 6 hours from presentation, and this cutoff was used in some of the studies we will discuss here.
Recognizing severe sepsis early in its course
The diagnosis of severe sepsis may be challenging, since up to 40% of patients may present with cryptic shock. These patients may not be hemodynamically compromised but may show evidence of tissue hypoxia, eg, an elevated serum lactate concentration or a low central venous oxygen saturation (Scvo2), or both.8 In view of this, much effort has gone into finding a biomarker that, in addition to clinical features, can help identify patients in an early stage of sepsis.
Procalcitonin levels rise in response to severe bacterial infection,9 and they correlate with sepsis-related organ failure scores and outcomes.10,11 Thus, the serum procalcitonin level may help in assessing the severity of sepsis, especially when combined with standard clinical and laboratory variables. However, controversy exists about the threshold to use in making decisions about antibiotic therapy and the value of this test in differentiating severe noninfectious inflammatory reactions from infectious causes of shock.12 Therefore, it is not widely used in clinical practice.
Serum lactate has been used for decades as a marker of tissue hypoperfusion. It is typically elevated in patients with severe sepsis and septic shock, and although the hyperlactatemia could be a result of global hypoperfusion, it can also be secondary to sepsis-induced mitochondrial dysfunction,13 impaired pyruvate dehydrogenase activity,14 increased aerobic glycolysis by catecholamine-stimulated sodium-potassium pump hyperactivity,15 and even impaired clearance.16
But whatever the mechanism, elevated lactate in severe sepsis and septic shock predicts a poor outcome and may help guide aggressive resuscitation. In fact, early lactate clearance (ie, normalization of an elevated value on repeat testing within the first 6 hours) is associated with better outcomes in patients with severe sepsis and septic shock.17,18
Panels of biomarkers. A literature search revealed over 3,000 papers on 178 different biomarkers in sepsis.19 Many of these biomarkers lack sufficient specificity and sensitivity for clinical use, and thus some investigators have suggested using a panel of them to enhance their predictive ability. Shapiro et al20 evaluated 971 patients admitted to the emergency department with suspected infection and discovered that a panel of three biomarkers (neutrophil gelatinase-associated lipocalin, protein C, and interleukin-1 receptor antagonist) was highly predictive of severe sepsis, septic shock, and death.
Starting empiric antibiotic therapy early
As soon as severe sepsis and septic shock are recognized, it is imperative that adequate empiric antibiotic treatment be started, along with infectious source control if applicable.21 The Surviving Sepsis Campaign guidelines recommend starting intravenous antibiotics as early as possible—within the first hour of recognition of severe sepsis with or without septic shock.22
Kumar et al,23 in a multicenter retrospective study of patients with septic shock, found that each hour of delay in giving appropriate antimicrobial agents in the first 6 hours from the onset of hypotension was associated with a 7.6% decrease in the in-hospital survival rate.
In a similar study,24 the same investigators analyzed data from 5,715 septic shock patients regarding the impact of starting the right antimicrobial therapy. Appropriate antimicrobial agents (ie, those having in vitro activity against the isolated pathogens) were given in 80.1% of cases, and the survival rate in those who received appropriate antibiotics was drastically higher than in those who received inappropriate ones (52.0% vs 10.3%, P < .0001).
In addition, two recent studies evaluated the importance of early empiric antibiotic therapy in conjunction with resuscitative protocols.25,26 In a preplanned analysis of early antimicrobial use in a study comparing lactate clearance and Scvo2 as goals of therapy, Puskarich et al26 found that fewer patients who received antibiotics before shock was recognized (according to formal criteria) died. Similarly, in a retrospective study in patients presenting to the emergency department and treated with early goal-directed therapy (defined below), Gaieski et al25 found that the mortality rate was drastically lower when antibiotics were started within 1 hour of either triage or initiation of early goal-directed therapy.
In short, it is imperative to promptly start the most appropriate broad-spectrum antibiotics to target the most likely pathogens based on site of infection, patient risk of multidrug-resistant pathogens, and local susceptibility patterns.
Goal-directed resuscitative therapy
As with antimicrobial therapy, resuscitative therapy should be started early and directed at defined goals.
Rivers et al27 conducted a randomized, controlled study in patients with severe sepsis or septic shock presenting to an emergency department of an urban teaching hospital. The patients were at high risk and had either persistent hypotension after a fluid challenge or serum lactate levels of 4 mmol/L or higher.
Two hundred sixty patients were randomized to receive either early goal-directed therapy in a protocol aimed at maximizing the intravascular volume and correcting global tissue hypoxia or standard therapy in the first 6 hours after presentation. The goals in the goal-directed therapy group were:
- Central venous pressure 8 to 12 mm Hg (achieved with aggressive fluid resuscitation with crystalloids)
- Mean arterial blood pressure greater than 65 mm Hg (maintained with vasoactive drugs, if necessary)
- Scvo2 above 70%. To achieve this third goal, packed red blood cells were infused to reach a target hematocrit of greater than 30%. For patients with a hematocrit higher than 30% but still with an Scvo2 less than 70%, inotropic agents were added and titrated to the Scvo2 goal of 70%.
Goal-directed therapy reduced the in-hospital mortality rate by 16% (the mortality rates were 30.5% in the goal-directed group and 46.5% in the standard therapy group, P = .009) and also reduced the 28- and 60-day mortality rates by similar proportions.27
Subsequent studies of a protocol for early recognition and treatment of sepsis have concluded that early aggressive fluid resuscitation decreases the ensuing need for vasopressor support.28 A resuscitation strategy based on early goal-directed therapy is a major component of the initial resuscitation bundle recommended by the Surviving Sepsis Campaign.22 (A “bundle” refers to the implementation of a core set of recommendations involving the simultaneous adaptation of a number of interventions.)
Areas of debate. However, concerns have been raised about the design of the study by Rivers et al and the mortality rate in the control group, which was higher than one would expect from the patients’ Acute Physiology and Chronic Health Evaluation II (APACHE II) scores.29 In particular, the bundled approach they used precludes the ability to differentiate which interventions were responsible for the outcome benefits. Indeed, there were two major interventions in the early goal-directed therapy group: a protocol for achieving the goals described and the use of Scvo2 as a goal.
Aggressive fluid resuscitation is considered the most critical aspect of all the major interventions, and there is little argument on its value. The debate centers on central venous pressure as a preload marker, since after the publication of the early goal-directed therapy trial,27 several studies showed that central venous pressure may not be a valid measure to predict fluid responsiveness (discussed later in this paper).30,31
The choice of colloids or crystalloids for fluid resuscitation is another area of debate. Clinical evidence suggests that albumin is equivalent to normal saline in a heterogeneous intensive care unit population,32 but subgroup analyses suggest albumin may be superior in patients with septic shock.33 Studies are ongoing (NCT00707122, NCT01337934, and NCT00318942). The use of hydroxyethyl starch in severe sepsis is associated with higher rates of acute renal failure and need for renal replacement therapy than Ringer’s lactate,34 and is generally not recommended. This is further substantiated by two recent randomized controlled studies, which found that the use of hydroxyethyl starch for fluid resuscitation in severe sepsis, compared with crystalloids, did not reduce the mortality rate (and even increased it in one study), and was associated with more need for renal replacement therapy.35,36
The use of Scvo2 is yet another topic of debate, and other monitoring variables have been evaluated. A recent study assessed the noninferiority of incorporating venous lactate clearance into the early goal-directed therapy protocol vs Scvo2.37 Both groups had identical goals for central venous pressure and mean arterial pressure but differed in the use of lactate clearance (defined as at least a 10% decline) or Scvo2 (> 70%) as the goal for improving tissue hypoxia. There were no significant differences between groups in their in-hospital mortality rates (17% in the lactate clearance group vs 23% in the Scvo2 group; criteria for noninferiority met). This suggests that lactate may be an alternative to Scvo2 as a goal in early goal-directed therapy. However, a secondary analysis of the data revealed a lack of concordance in achieving lactate clearance and Scvo2 goals, which suggests that these parameters may be measuring distinct physiologic processes.38 Since the hemodynamic profiles of septic shock patients are complex, it may be prudent to use both of these markers of resuscitation until further studies are completed.
Given the debate, a number of prospective randomized trials are under way to evaluate resuscitative interventions. These include the Protocolized Care for Early Septic Shock trial (NCT00510835), the Australasian Resuscitation in Sepsis Evaluation trial (NCT00975793), and the Protocolised Management of Sepsis (ProMISe) trial in the United Kingdom (ISRCTN 36307479). These three trials will evaluate, collectively, close to 4,000 patients and will provide considerable insights into resuscitative interventions in septic shock.
Vasopressors: Which one to use?
If fluid therapy does not restore perfusion, vasopressors should be promptly initiated, as the longer that hypotension goes on, the lower the survival rate.39
But which vasopressor should be used? The early goal-directed therapy protocol used in the study by Rivers et al27 did not specify which vasopressor should be used to keep the mean arterial pressure above 65 mm Hg.
The Surviving Sepsis Campaign22 recommends norepinephrine as the first-choice vasopressor, with dopamine as an alternative only in selected patients, such as those with absolute or relative bradycardia.
The guidelines also recommend epinephrine to be added to or substituted for norepinephrine when an additional catecholamine is needed to maintain adequate blood pressure.22 Furthermore, vasopressin at a dose of 0.03 units/min can be added to norepinephrine with the intent of raising the blood pressure or decreasing the norepinephrine requirement. Higher doses of vasopressin should be reserved for salvage therapy.
Regarding phenylephrine, the guidelines recommend against its use except when norepinephrine use is associated with significant tachyarrhythmias, cardiac output is known to be higher, or as a salvage therapy.22
This is a topic of debate, with recent clinical studies offering further insight.
De Backer et al40 compared the effects of dopamine vs norepinephrine for the treatment of shock in 1,679 patients, 62% of whom had septic shock. Overall, there was a trend towards better outcomes with norepinephrine, but no significant difference in mortality rates at 28 days (52.5% with dopamine vs 48.5% with norepinephrine, P = .10). Importantly, fewer patients who were randomized to norepinephrine developed arrhythmias (12.4% vs 24.1%, P < .001), and the norepinephrine group required fewer days of study drug (11.0 vs 12.5, P = .01) and open-label vasopressors (12.6 vs 14.2, P = .007). Of note, patients with cardiogenic shock randomized to norepinephrine had a significantly lower mortality rate than those randomized to dopamine. Although no significant difference in outcome was found between the two vasopressors in the subgroup of patients with septic shock, the overall improvements in secondary surrogate markers suggest that norepinephrine should be the first-line agent.
Norepinephrine has also been compared with “secondary” vasopressors. Annane et al,41 in a prospective multicenter randomized controlled study, evaluated the effect of norepinephrine plus dobutamine vs epinephrine alone in managing septic shock. There was no significant difference in the primary outcome measure of 28-day mortality (34% with norepinephrine plus dobutamine vs 40% with epinephrine alone, P = .31). However, the study was powered to evaluate for an absolute risk reduction of 20% in the mortality rate, which would be a big reduction. A smaller reduction in the mortality rate, which would not have been statistically significant in this study, might still be considered clinically significant. Furthermore, the group randomized to norepinephrine plus dobutamine had more vasopressor-free days (20 days vs 22 days, P = .05) and less acidosis on days 1 to 4 than the group randomized to epinephrine.
Norepinephrine was also compared with phenylephrine as a first-line vasopressor in a randomized controlled trial in 32 patients with septic shock. No difference was found in cardiopulmonary performance, global oxygen transport, or regional hemodynamics between phenylephrine and norepinephrine.42
While encouraging, these preliminary data need to be verified in a larger randomized controlled trial with concrete outcome measures before being clinically adapted. Taken together, the above studies suggest that norepinephrine should be the initial vasopressor of choice for patients with septic shock.
CONTINUED MANAGEMENT OF SEPTIC SHOCK
How to manage septic shock after the initial stages is much less defined.
Uncertainty persists about the importance of achieving the early goals of resuscitation in patients who did not reach them in the initial 6 hours of treatment. Although there are data suggesting that extending the goals beyond the initial 6 hours may be beneficial, clinicians should use caution when interpreting these results in light of the observational design of the studies.43,44 For the purpose of this discussion, “continued management” of septic shock will mean after the first 6 hours and after all the early goals are met.
The clinical decisions necessary after the initial stages of resuscitation include:
- Whether further fluid resuscitation is needed
- Assessment for further and additional hemodynamic therapies
- Consideration of adjunctive therapies
- Reevaluation of antibiotic choices (Table 2).
Is more fluid needed? How can we tell?
There is considerable debate about the ideal method for assessing fluid responsiveness. In fact, one of the criticisms of the early goal-directed therapy study27 was that it used central venous pressure as a marker of fluid responsiveness.
Several studies have shown that central venous pressure or pulmonary artery occlusion pressure may not be valid measures of fluid responsiveness.45 In fact, in a retrospective study of 150 volume challenges, the area under the receiver-operating-characteristics curve of central venous pressure as a marker of fluid responsiveness was only 0.58. (Recall that the closer the area under the curve is to 1.0, the better the test; a value of 0.50 is the same as chance.) The area under the curve for pulmonary artery occlusion pressure was 0.63.46
In contrast, several dynamic indices have been proposed to better guide fluid resuscitation in mechanically ventilated patients.31 These are based on changes in stroke volume, aortic blood flow, or arterial pulse pressure in response to the ventilator cycle or passive leg-raising. A detailed review of these markers can be found elsewhere,31 but taken together, they have a sensitivity and specificity of over 90% for predicting fluid responsiveness. Clinicians may consider using dynamic markers of fluid responsiveness to determine when to give additional fluids, particularly after the first 6 hours of shock, in which data supporting the use of central venous pressure are lacking.
Optimal use of fluids is particularly important, since some studies suggest that “overresuscitation” has negative consequences. In a multicenter observational study of 1,177 patients with sepsis, after adjusting for a number of comorbidities and baseline severity of illness, the cumulative fluid balance in the first 72 hours after the onset of sepsis was independently associated with a worse mortality rate.47
Furthermore, in a retrospective analysis of a randomized controlled trial of vasopressin in conjunction with norepinephrine for septic shock, patients in the highest quartile of fluid balance (more fluid in than out) at 12 hours and 4 days after presentation had significantly higher mortality rates than those in the lowest two quartiles.48 The worse outcome with a positive fluid balance might be explained by worsening oxygenation and prolonged mechanical ventilation, as demonstrated by the Fluid and Catheter Treatment Trial in patients with acute lung injury or acute respiratory distress syndrome (ALI/ARDS).49 Indeed, when fluid balance in patients with septic shockinduced ALI/ARDS was evaluated, patients with both adequate initial fluid resuscitation and conservative late fluid management had a lower mortality rate than those with either one alone.50
In view of these findings, especially beyond the initial hours of resuscitation, clinicians should remember that further unnecessary fluid administration may have detrimental effects. Therefore, given the superior predictive abilities of dynamic markers of fluid responsiveness, these should be used to determine the need for further fluid boluses.
In cases in which patients are no longer fluid-responsive and need increasing levels of hemodynamic support, clinicians still have a number of options. These include increasing the current vasopressor dose or starting an additional therapy such as an alternative catecholamine vasopressor, vasopressin, inotropic therapy, or an adjunctive therapy such as a corticosteroid. The intervention could also be a combination of the above choices.
Adding catecholamines
The optimal time point or vasopressor dose at which to consider initiating additional therapies is unknown. However, the Vasopressin and Septic Shock Trial (VASST) provides some insight.51
This study compared two strategies: escalating doses of norepinephrine vs adding vasopressin to norepinephrine. Overall, adding vasopressin showed no benefit in terms of a lower mortality rate. However, in the subgroup of patients with norepinephrine requirements of 5 to 14 μg/min at study enrollment (ie, a low dose, reflecting less-severe sepsis) vasopressin was associated with a lower 28-day mortality rate (26.5% vs 35.7%, P = .05) and 90-day mortality rate (35.8% vs 46.1%, P = .04). Benefit was also noted in patients with other markers of lower disease severity such as low lactate levels or having received a single vasopressor at baseline.51
Although subgroup analyses should not generally be used to guide treatment decisions, a prospective trial may never be done to evaluate adding vasopressin to catecholamines earlier vs later. Thus, clinicians who choose to use vasopressin may consider starting this therapy when catecholamine doses are relatively low or before profound hyperlactatemia from prolonged tissue hypoxia has developed.
There is less evidence to guide clinicians who are considering adding a different catecholamine. The theoretical concerns of splanchnic ischemia and cardiac arrhythmia associated with higher doses of catecholamines are usually the impetus to limit a single catecholamine to a “maximum” dose. However, studies that have evaluated combination catecholamine therapies have generally studied combinations of vasopressors with inotropes and lacked standardization in their protocols, thus making them difficult to interpret.52–54 One could also argue that additional catecholamine therapies, which all function similarly, may have additive effects and cause even more adverse effects. As such, adding another vasopressor should be reserved for patients experiencing noticeable adverse effects (such as tachycardia) on first-line therapy.
Inotropic support
Left ventricular function should be assessed in all patients who continue to be hypotensive despite adequate fluid resuscitation and vasopressor therapy. In a study of patients with septic shock in whom echocardiography was performed daily for the first 3 days of hemodynamic support, new-onset left ventricular hypokinesia was found in 26 (39%) of 67 patients on presentation and in an additional 14 patients (21%) after at least 24 hours of norepinephrine.55 Adding inotropic support with dobutamine or epinephrine led to decreases in vasopressor dose and enhanced left ventricular ejection fraction.
In short, left ventricular hypokinesia is common in septic shock, may occur at presentation or after a period of vasopressor support, and is usually correctable with the addition of inotropic support.
Corticosteroids
Beyond hemodynamic support with fluids and catecholamines or vasopressin (or both), clinicians should also consider adjunctive corticosteroid therapy. However, for many years the issue has been controversial for patients with severe sepsis and septic shock.
Annane et al56 conducted a large, multicenter, randomized, double-blind, placebocontrolled trial to assess the effect of low doses of corticosteroids in patients with refractory septic shock. Overall, the 28-day mortality rate was 61% in the treatment group and 55% in the placebo group, which was not statistically significant (adjusted odds ratio 0.65, 95% confidence interval 0.39–1.07, P value .09). However, when separated by response to cosyntropin stimulation, those with a change in cortisol of 9 ug/dL or less (nonresponders) randomized to receive corticosteroids had significantly higher survival rates in the short term (28 days) and the long term (1 year). The positive results of this study led to the adoption of low-dose hydrocortisone as standard practice in most patients with septic shock.57
But then, to evaluate the effects of corticosteroids in a broader intensive-care population with septic shock, another trial was designed: the Corticosteroid Therapy of Septic Shock (CORTICUS) trial.58 Surprisingly, this multicenter, randomized, double-blind, placebo-controlled trial found no significant difference in survival between the group that received hydrocortisone and the placebo group, regardless of response to a cosyntropin stimulation test.
Taking into account the above studies and other randomized controlled trials, the 2012 Surviving Sepsis Campaign guidelines and the International Task Force for the Diagnosis and Management of Corticosteroid Insufficiency in Critically Ill Adult Patients recommend intravenous hydrocortisone therapy in adults with septic shock whose blood pressure responds poorly to fluid resuscitation and vasopressor therapy. These consensus statements do not recommend the cosyntropin stimulation test to identify patients with septic shock who should receive corticosteroids.22,59 The guidelines, however, do not explicitly define poor response to initial therapy.
Of note, in the Annane study, which found a lower mortality rate with corticosteroids, the patients were severely ill, with a mean baseline norepinephrine dose of 1.1 μg/kg/min. In contrast, in the CORTICUS study (which found no benefit of hydrocortisone), patients had lower baseline vasopressor doses, with a mean norepinephrine dose of 0.5 μg/kg/min.
While corticosteroids are associated with a higher rate of shock reversal 7 days after initiation, 59 this has not translated into a consistent reduction in the death rate. If a clinician is considering adding corticosteroids to decrease the risk of death, it would seem prudent to add this therapy in patients receiving norepinephrine in doses above 0.5 μg/kg/min.
The ideal sequence and combination of the above therapies including fluids, catecholamine vasopressors, vasopressin, inotropes, and vasopressors have not been elucidated. However, some preliminary evidence suggests an advantage with the combination of vasopressin and corticosteroids. In a subgroup analysis of the VASST study, in patients who received corticosteroids, the combination of vasopressin plus norepinephrine was associated with a lower 28-day mortality rate than with norepinephrine alone (35.9% vs 44.7%, P = .03).60 These findings have been replicated in other studies,61,62 prompting suggestions for a study of vasopressin with and without corticosteroids in patients on norepinephrine to elucidate the role of each therapy individually and in combination.
Tight glycemic control
As with corticosteroids, the pendulum for tight glycemic control in critically ill patients has swung widely in recent years. Enthusiasm was high at first after the publication of a study by van den Berghe et al, which described a 3.4% absolute reduction in mortality with intensive insulin therapy to maintain blood glucose at or below 110 mg/dL.63 However, the significant benefits found in this study were never replicated.
In fact, recent evidence suggests that tight glycemic control is associated with no benefit and a higher risk of hypoglycemia.34,64 In the largest randomized controlled trial of this topic, with more than 6,000 patients, intensive insulin therapy with a target blood glucose level of 81 to 108 mg/dL was associated with a significantly higher mortality rate (odds ratio 1.14, 95% confidence interval 1.02–1.28, P = .02) than with a target glucose level of less than 180 mg/dL.65 Furthermore, in a recent follow-up analysis,66 moderate hypoglycemia (serum glucose 41–70 mg/dL) and severe hypoglycemia (serum glucose < 41 mg/dL) were associated with a higher rate of death in a dose-response relationship.66
Taking this information together, clinicians should be aware that there is no additional benefit in lowering blood glucose below the range of 140 to 180 mg/dL, and that doing so may be harmful.
Drotecogin alfa
Drotecogin alfa (Xigris) was another adjunctive therapy that has fallen from favor. It was approved for the treatment of severe sepsis in light of promising findings in initial studies.67
However, on October 25, 2011, drotecogin alfa was voluntarily withdrawn from the market by the manufacturer after another study found no beneficial effect on the mortality rates at 28 days or at 90 days.68 Furthermore, no difference could be found regarding any predetermined primary or secondary outcome measures.
Continued antibiotic therapy
The decision whether to continue initial empiric antimicrobial coverage, broaden it, or de-escalate must be faced for all patients with septic shock, and is ultimately clinical.
The serum procalcitonin level has been proposed to guide antibiotic discontinuation in several clinical settings, although there are still questions about the safety of such an approach. The largest randomized trial published to date reported that a procalcitoninguided strategy to treat suspected bacterial infections in nonsurgical patients could reduce antibiotic exposure with no apparent adverse outcomes.69 On the other hand, other data discourage the use of procalcitonin-guided antimicrobial escalation, as this approach did not improve survival and worsened organ function and length of stay in the intensive care unit.70
The Surviving Sepsis Campaign guidelines recommend combination antibiotic therapy for no longer than 3 to 5 days and limiting the duration of antibiotics in most cases to 7 to 10 days.22
TRIALS ARE ONGOING
The understanding of the pathophysiology and treatment of sepsis has greatly advanced over the last decade. Adoption of evidence-based protocols for managing patients with septic shock has improved outcomes. Nevertheless, many multicenter trials are being conducted worldwide to look into some of the most controversial therapies, and their results will guide therapy in the future.
- Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011; 140:1223–1231.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310.
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med 2003; 168:165–172.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222–231.
- Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Med 2004; 30:1032–1040.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348:138–150.
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001; 345:588–595.
- Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med 1996; 14:218–225.
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 34:515–518.
- Muller B, Becker KL, Schachinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000; 28:977–983.
- Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care (London, England) 1999; 3:45–50.
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007; 7:210–217.
- Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002; 360:219–223.
- Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock 1996; 6:89–94.
- Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 2005; 365:871–875.
- Levraut J, Ciebiera JP, Chave S, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than over-production. Am J Respir Crit Care Med 1998; 157:1021–1026.
- Arnold RC, Shapiro NI, Jones AE, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009; 32:35–39.
- Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004; 32:1637–1642.
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010; 14:R15.
- Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 2009; 37:96–104.
- Marshall JC, al Naqbi A. Principles of source control in the management of sepsis. Crit Care Clin 2009; 25:753–768,viii–ix.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136:1237–1248.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–1053.
- Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39:2066–2071.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006; 34:2707–2713.
- Schmidt GA. Counterpoint: adherence to early goal-directed therapy: does it really matter? No. Both risks and benefits require further study. Chest 2010; 138:480–483; discussion 483–484.
- Jain RK, Antonio BL, Bowton DL, Houle TT, MacGregor DA. Variability in central venous pressure measurements and the potential impact on fluid management. Shock 2009; 33:253–257.
- Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest 2008; 133:252–263.
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011; 37:86–96.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010; 303:739–746.
- Puskarich MA, Trzciak S, Shapiro NI, Kline JA, Jones AE. Concordance and prognostic value of central venous oxygen saturation and lactate clearance in emergency department patients with septic shock. Acad Emerg Med 2011; 19:S159–S160.
- Dunser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med 2009; 35:1225–1233.
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007; 370:676–684.
- Morelli A, Ertmer C, Rehberg S, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care (London, England) 2008; 12:R143.
- Coba V, Whitmill M, Mooney R, et al. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med 2011 Jan 10[Epub ahead of print].
- Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodriguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011; 36:542–547.
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134:172–178.
- Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007; 35:64–68.
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34:344–353.
- Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–265.
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009; 136:102–109.
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887.
- Vincent JL, Roman A, Kahn RJ. Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med 1990; 18:689–693.
- Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med 1997; 23:282–287.
- Redl-Wenzl EM, Armbruster C, Edelmann G, et al. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med 1993; 19:151–154.
- Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008; 36:1701–1706.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871.
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004; 32:858–873.
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124.
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36:1937–1949.
- Russell JA, Walley KR, Gordon AC, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med 2009; 37:811–818.
- Bauer SR, Lam SW, Cha SS, Oyen LJ. Effect of corticosteroids on arginine vasopressin-containing vasopressor therapy for septic shock: a case control study. J Crit Care 2008; 23:500–506.
- Torgersen C, Luckner G, Schroder DC, et al. Concomitant arginine-vasopressin and hydrocortisone therapy in severe septic shock: association with mortality. Intensive Care Med 2011; 37:1432–1437.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009; 35:1738–1748.
- Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367:1108–1118.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344:699–709.
- Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012; 366:2055–2064.
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2009; 375:463–474.
- Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011; 39:2048–2058.
Considerably fewer patients who develop sepsis are dying of it now, thanks to a number of studies of how to reverse sepsis-induced tissue hypoxia.1 The greatest strides in improving outcomes have been attributed to better early management, which includes prompt recognition of sepsis, rapid initiation of antimicrobial therapy, elimination of the source of infection, and early goal-directed therapy. Thus, even though the incidence of severe sepsis and septic shock is increasing,2,3 the Surviving Sepsis Campaign has documented a significant decrease in unadjusted mortality rates (37% to 30.8%) associated with the bundled approach in the management of sepsis.4 (We will talk about this later in the article.)
This review will summarize the evidence for the early management of septic shock and will evaluate the various treatment decisions beyond the initial phases of resuscitation.
INFLAMMATION AND VASODILATION
Sepsis syndrome starts with an infection that leads to a proinflammatory state with a complex interaction between anti-inflammatory and proinflammatory mediators, enhanced coagulation, and impaired fibrinolysis.5,6
Sepsis induces vasodilation by way of inappropriate activation of vasodilatory mechanisms (increased synthesis of nitric oxide and vasopressin deficiency) and failure of vasoconstrictor mechanisms (activation of ATP-sensitive potassium channels in vascular smooth muscle).7 Thus, the hemodynamic abnormalities are multifactorial, and the resultant tissue hypoperfusion further contributes to the proinflammatory and procoagulant state, precipitating multiorgan dysfunction and, often, death.
DEFINITIONS
- Sepsis—infection together with systemic manifestation of inflammatory response
- Severe sepsis—sepsis plus induced organ dysfunction or evidence of tissue hypoperfusion
- Septic shock—sepsis-induced hypotension persisting despite adequate fluid resuscitation.
EARLY MANAGEMENT OF SEPTIC SHOCK
Early in the course of septic shock, the physician’s job is to:
- Recognize it promptly
- Begin empiric antibiotic therapy quickly
- Eliminate the source of infection, if applicable, eg, by removing an infected central venous catheter
- Give fluid resuscitation, titrated to specific goals
- Give vasopressor therapy to maintain blood pressure, organ perfusion, and oxygen delivery (Table 1).
The line between “early” and “late” is not clear. Traditionally, it has been drawn at 6 hours from presentation, and this cutoff was used in some of the studies we will discuss here.
Recognizing severe sepsis early in its course
The diagnosis of severe sepsis may be challenging, since up to 40% of patients may present with cryptic shock. These patients may not be hemodynamically compromised but may show evidence of tissue hypoxia, eg, an elevated serum lactate concentration or a low central venous oxygen saturation (Scvo2), or both.8 In view of this, much effort has gone into finding a biomarker that, in addition to clinical features, can help identify patients in an early stage of sepsis.
Procalcitonin levels rise in response to severe bacterial infection,9 and they correlate with sepsis-related organ failure scores and outcomes.10,11 Thus, the serum procalcitonin level may help in assessing the severity of sepsis, especially when combined with standard clinical and laboratory variables. However, controversy exists about the threshold to use in making decisions about antibiotic therapy and the value of this test in differentiating severe noninfectious inflammatory reactions from infectious causes of shock.12 Therefore, it is not widely used in clinical practice.
Serum lactate has been used for decades as a marker of tissue hypoperfusion. It is typically elevated in patients with severe sepsis and septic shock, and although the hyperlactatemia could be a result of global hypoperfusion, it can also be secondary to sepsis-induced mitochondrial dysfunction,13 impaired pyruvate dehydrogenase activity,14 increased aerobic glycolysis by catecholamine-stimulated sodium-potassium pump hyperactivity,15 and even impaired clearance.16
But whatever the mechanism, elevated lactate in severe sepsis and septic shock predicts a poor outcome and may help guide aggressive resuscitation. In fact, early lactate clearance (ie, normalization of an elevated value on repeat testing within the first 6 hours) is associated with better outcomes in patients with severe sepsis and septic shock.17,18
Panels of biomarkers. A literature search revealed over 3,000 papers on 178 different biomarkers in sepsis.19 Many of these biomarkers lack sufficient specificity and sensitivity for clinical use, and thus some investigators have suggested using a panel of them to enhance their predictive ability. Shapiro et al20 evaluated 971 patients admitted to the emergency department with suspected infection and discovered that a panel of three biomarkers (neutrophil gelatinase-associated lipocalin, protein C, and interleukin-1 receptor antagonist) was highly predictive of severe sepsis, septic shock, and death.
Starting empiric antibiotic therapy early
As soon as severe sepsis and septic shock are recognized, it is imperative that adequate empiric antibiotic treatment be started, along with infectious source control if applicable.21 The Surviving Sepsis Campaign guidelines recommend starting intravenous antibiotics as early as possible—within the first hour of recognition of severe sepsis with or without septic shock.22
Kumar et al,23 in a multicenter retrospective study of patients with septic shock, found that each hour of delay in giving appropriate antimicrobial agents in the first 6 hours from the onset of hypotension was associated with a 7.6% decrease in the in-hospital survival rate.
In a similar study,24 the same investigators analyzed data from 5,715 septic shock patients regarding the impact of starting the right antimicrobial therapy. Appropriate antimicrobial agents (ie, those having in vitro activity against the isolated pathogens) were given in 80.1% of cases, and the survival rate in those who received appropriate antibiotics was drastically higher than in those who received inappropriate ones (52.0% vs 10.3%, P < .0001).
In addition, two recent studies evaluated the importance of early empiric antibiotic therapy in conjunction with resuscitative protocols.25,26 In a preplanned analysis of early antimicrobial use in a study comparing lactate clearance and Scvo2 as goals of therapy, Puskarich et al26 found that fewer patients who received antibiotics before shock was recognized (according to formal criteria) died. Similarly, in a retrospective study in patients presenting to the emergency department and treated with early goal-directed therapy (defined below), Gaieski et al25 found that the mortality rate was drastically lower when antibiotics were started within 1 hour of either triage or initiation of early goal-directed therapy.
In short, it is imperative to promptly start the most appropriate broad-spectrum antibiotics to target the most likely pathogens based on site of infection, patient risk of multidrug-resistant pathogens, and local susceptibility patterns.
Goal-directed resuscitative therapy
As with antimicrobial therapy, resuscitative therapy should be started early and directed at defined goals.
Rivers et al27 conducted a randomized, controlled study in patients with severe sepsis or septic shock presenting to an emergency department of an urban teaching hospital. The patients were at high risk and had either persistent hypotension after a fluid challenge or serum lactate levels of 4 mmol/L or higher.
Two hundred sixty patients were randomized to receive either early goal-directed therapy in a protocol aimed at maximizing the intravascular volume and correcting global tissue hypoxia or standard therapy in the first 6 hours after presentation. The goals in the goal-directed therapy group were:
- Central venous pressure 8 to 12 mm Hg (achieved with aggressive fluid resuscitation with crystalloids)
- Mean arterial blood pressure greater than 65 mm Hg (maintained with vasoactive drugs, if necessary)
- Scvo2 above 70%. To achieve this third goal, packed red blood cells were infused to reach a target hematocrit of greater than 30%. For patients with a hematocrit higher than 30% but still with an Scvo2 less than 70%, inotropic agents were added and titrated to the Scvo2 goal of 70%.
Goal-directed therapy reduced the in-hospital mortality rate by 16% (the mortality rates were 30.5% in the goal-directed group and 46.5% in the standard therapy group, P = .009) and also reduced the 28- and 60-day mortality rates by similar proportions.27
Subsequent studies of a protocol for early recognition and treatment of sepsis have concluded that early aggressive fluid resuscitation decreases the ensuing need for vasopressor support.28 A resuscitation strategy based on early goal-directed therapy is a major component of the initial resuscitation bundle recommended by the Surviving Sepsis Campaign.22 (A “bundle” refers to the implementation of a core set of recommendations involving the simultaneous adaptation of a number of interventions.)
Areas of debate. However, concerns have been raised about the design of the study by Rivers et al and the mortality rate in the control group, which was higher than one would expect from the patients’ Acute Physiology and Chronic Health Evaluation II (APACHE II) scores.29 In particular, the bundled approach they used precludes the ability to differentiate which interventions were responsible for the outcome benefits. Indeed, there were two major interventions in the early goal-directed therapy group: a protocol for achieving the goals described and the use of Scvo2 as a goal.
Aggressive fluid resuscitation is considered the most critical aspect of all the major interventions, and there is little argument on its value. The debate centers on central venous pressure as a preload marker, since after the publication of the early goal-directed therapy trial,27 several studies showed that central venous pressure may not be a valid measure to predict fluid responsiveness (discussed later in this paper).30,31
The choice of colloids or crystalloids for fluid resuscitation is another area of debate. Clinical evidence suggests that albumin is equivalent to normal saline in a heterogeneous intensive care unit population,32 but subgroup analyses suggest albumin may be superior in patients with septic shock.33 Studies are ongoing (NCT00707122, NCT01337934, and NCT00318942). The use of hydroxyethyl starch in severe sepsis is associated with higher rates of acute renal failure and need for renal replacement therapy than Ringer’s lactate,34 and is generally not recommended. This is further substantiated by two recent randomized controlled studies, which found that the use of hydroxyethyl starch for fluid resuscitation in severe sepsis, compared with crystalloids, did not reduce the mortality rate (and even increased it in one study), and was associated with more need for renal replacement therapy.35,36
The use of Scvo2 is yet another topic of debate, and other monitoring variables have been evaluated. A recent study assessed the noninferiority of incorporating venous lactate clearance into the early goal-directed therapy protocol vs Scvo2.37 Both groups had identical goals for central venous pressure and mean arterial pressure but differed in the use of lactate clearance (defined as at least a 10% decline) or Scvo2 (> 70%) as the goal for improving tissue hypoxia. There were no significant differences between groups in their in-hospital mortality rates (17% in the lactate clearance group vs 23% in the Scvo2 group; criteria for noninferiority met). This suggests that lactate may be an alternative to Scvo2 as a goal in early goal-directed therapy. However, a secondary analysis of the data revealed a lack of concordance in achieving lactate clearance and Scvo2 goals, which suggests that these parameters may be measuring distinct physiologic processes.38 Since the hemodynamic profiles of septic shock patients are complex, it may be prudent to use both of these markers of resuscitation until further studies are completed.
Given the debate, a number of prospective randomized trials are under way to evaluate resuscitative interventions. These include the Protocolized Care for Early Septic Shock trial (NCT00510835), the Australasian Resuscitation in Sepsis Evaluation trial (NCT00975793), and the Protocolised Management of Sepsis (ProMISe) trial in the United Kingdom (ISRCTN 36307479). These three trials will evaluate, collectively, close to 4,000 patients and will provide considerable insights into resuscitative interventions in septic shock.
Vasopressors: Which one to use?
If fluid therapy does not restore perfusion, vasopressors should be promptly initiated, as the longer that hypotension goes on, the lower the survival rate.39
But which vasopressor should be used? The early goal-directed therapy protocol used in the study by Rivers et al27 did not specify which vasopressor should be used to keep the mean arterial pressure above 65 mm Hg.
The Surviving Sepsis Campaign22 recommends norepinephrine as the first-choice vasopressor, with dopamine as an alternative only in selected patients, such as those with absolute or relative bradycardia.
The guidelines also recommend epinephrine to be added to or substituted for norepinephrine when an additional catecholamine is needed to maintain adequate blood pressure.22 Furthermore, vasopressin at a dose of 0.03 units/min can be added to norepinephrine with the intent of raising the blood pressure or decreasing the norepinephrine requirement. Higher doses of vasopressin should be reserved for salvage therapy.
Regarding phenylephrine, the guidelines recommend against its use except when norepinephrine use is associated with significant tachyarrhythmias, cardiac output is known to be higher, or as a salvage therapy.22
This is a topic of debate, with recent clinical studies offering further insight.
De Backer et al40 compared the effects of dopamine vs norepinephrine for the treatment of shock in 1,679 patients, 62% of whom had septic shock. Overall, there was a trend towards better outcomes with norepinephrine, but no significant difference in mortality rates at 28 days (52.5% with dopamine vs 48.5% with norepinephrine, P = .10). Importantly, fewer patients who were randomized to norepinephrine developed arrhythmias (12.4% vs 24.1%, P < .001), and the norepinephrine group required fewer days of study drug (11.0 vs 12.5, P = .01) and open-label vasopressors (12.6 vs 14.2, P = .007). Of note, patients with cardiogenic shock randomized to norepinephrine had a significantly lower mortality rate than those randomized to dopamine. Although no significant difference in outcome was found between the two vasopressors in the subgroup of patients with septic shock, the overall improvements in secondary surrogate markers suggest that norepinephrine should be the first-line agent.
Norepinephrine has also been compared with “secondary” vasopressors. Annane et al,41 in a prospective multicenter randomized controlled study, evaluated the effect of norepinephrine plus dobutamine vs epinephrine alone in managing septic shock. There was no significant difference in the primary outcome measure of 28-day mortality (34% with norepinephrine plus dobutamine vs 40% with epinephrine alone, P = .31). However, the study was powered to evaluate for an absolute risk reduction of 20% in the mortality rate, which would be a big reduction. A smaller reduction in the mortality rate, which would not have been statistically significant in this study, might still be considered clinically significant. Furthermore, the group randomized to norepinephrine plus dobutamine had more vasopressor-free days (20 days vs 22 days, P = .05) and less acidosis on days 1 to 4 than the group randomized to epinephrine.
Norepinephrine was also compared with phenylephrine as a first-line vasopressor in a randomized controlled trial in 32 patients with septic shock. No difference was found in cardiopulmonary performance, global oxygen transport, or regional hemodynamics between phenylephrine and norepinephrine.42
While encouraging, these preliminary data need to be verified in a larger randomized controlled trial with concrete outcome measures before being clinically adapted. Taken together, the above studies suggest that norepinephrine should be the initial vasopressor of choice for patients with septic shock.
CONTINUED MANAGEMENT OF SEPTIC SHOCK
How to manage septic shock after the initial stages is much less defined.
Uncertainty persists about the importance of achieving the early goals of resuscitation in patients who did not reach them in the initial 6 hours of treatment. Although there are data suggesting that extending the goals beyond the initial 6 hours may be beneficial, clinicians should use caution when interpreting these results in light of the observational design of the studies.43,44 For the purpose of this discussion, “continued management” of septic shock will mean after the first 6 hours and after all the early goals are met.
The clinical decisions necessary after the initial stages of resuscitation include:
- Whether further fluid resuscitation is needed
- Assessment for further and additional hemodynamic therapies
- Consideration of adjunctive therapies
- Reevaluation of antibiotic choices (Table 2).
Is more fluid needed? How can we tell?
There is considerable debate about the ideal method for assessing fluid responsiveness. In fact, one of the criticisms of the early goal-directed therapy study27 was that it used central venous pressure as a marker of fluid responsiveness.
Several studies have shown that central venous pressure or pulmonary artery occlusion pressure may not be valid measures of fluid responsiveness.45 In fact, in a retrospective study of 150 volume challenges, the area under the receiver-operating-characteristics curve of central venous pressure as a marker of fluid responsiveness was only 0.58. (Recall that the closer the area under the curve is to 1.0, the better the test; a value of 0.50 is the same as chance.) The area under the curve for pulmonary artery occlusion pressure was 0.63.46
In contrast, several dynamic indices have been proposed to better guide fluid resuscitation in mechanically ventilated patients.31 These are based on changes in stroke volume, aortic blood flow, or arterial pulse pressure in response to the ventilator cycle or passive leg-raising. A detailed review of these markers can be found elsewhere,31 but taken together, they have a sensitivity and specificity of over 90% for predicting fluid responsiveness. Clinicians may consider using dynamic markers of fluid responsiveness to determine when to give additional fluids, particularly after the first 6 hours of shock, in which data supporting the use of central venous pressure are lacking.
Optimal use of fluids is particularly important, since some studies suggest that “overresuscitation” has negative consequences. In a multicenter observational study of 1,177 patients with sepsis, after adjusting for a number of comorbidities and baseline severity of illness, the cumulative fluid balance in the first 72 hours after the onset of sepsis was independently associated with a worse mortality rate.47
Furthermore, in a retrospective analysis of a randomized controlled trial of vasopressin in conjunction with norepinephrine for septic shock, patients in the highest quartile of fluid balance (more fluid in than out) at 12 hours and 4 days after presentation had significantly higher mortality rates than those in the lowest two quartiles.48 The worse outcome with a positive fluid balance might be explained by worsening oxygenation and prolonged mechanical ventilation, as demonstrated by the Fluid and Catheter Treatment Trial in patients with acute lung injury or acute respiratory distress syndrome (ALI/ARDS).49 Indeed, when fluid balance in patients with septic shockinduced ALI/ARDS was evaluated, patients with both adequate initial fluid resuscitation and conservative late fluid management had a lower mortality rate than those with either one alone.50
In view of these findings, especially beyond the initial hours of resuscitation, clinicians should remember that further unnecessary fluid administration may have detrimental effects. Therefore, given the superior predictive abilities of dynamic markers of fluid responsiveness, these should be used to determine the need for further fluid boluses.
In cases in which patients are no longer fluid-responsive and need increasing levels of hemodynamic support, clinicians still have a number of options. These include increasing the current vasopressor dose or starting an additional therapy such as an alternative catecholamine vasopressor, vasopressin, inotropic therapy, or an adjunctive therapy such as a corticosteroid. The intervention could also be a combination of the above choices.
Adding catecholamines
The optimal time point or vasopressor dose at which to consider initiating additional therapies is unknown. However, the Vasopressin and Septic Shock Trial (VASST) provides some insight.51
This study compared two strategies: escalating doses of norepinephrine vs adding vasopressin to norepinephrine. Overall, adding vasopressin showed no benefit in terms of a lower mortality rate. However, in the subgroup of patients with norepinephrine requirements of 5 to 14 μg/min at study enrollment (ie, a low dose, reflecting less-severe sepsis) vasopressin was associated with a lower 28-day mortality rate (26.5% vs 35.7%, P = .05) and 90-day mortality rate (35.8% vs 46.1%, P = .04). Benefit was also noted in patients with other markers of lower disease severity such as low lactate levels or having received a single vasopressor at baseline.51
Although subgroup analyses should not generally be used to guide treatment decisions, a prospective trial may never be done to evaluate adding vasopressin to catecholamines earlier vs later. Thus, clinicians who choose to use vasopressin may consider starting this therapy when catecholamine doses are relatively low or before profound hyperlactatemia from prolonged tissue hypoxia has developed.
There is less evidence to guide clinicians who are considering adding a different catecholamine. The theoretical concerns of splanchnic ischemia and cardiac arrhythmia associated with higher doses of catecholamines are usually the impetus to limit a single catecholamine to a “maximum” dose. However, studies that have evaluated combination catecholamine therapies have generally studied combinations of vasopressors with inotropes and lacked standardization in their protocols, thus making them difficult to interpret.52–54 One could also argue that additional catecholamine therapies, which all function similarly, may have additive effects and cause even more adverse effects. As such, adding another vasopressor should be reserved for patients experiencing noticeable adverse effects (such as tachycardia) on first-line therapy.
Inotropic support
Left ventricular function should be assessed in all patients who continue to be hypotensive despite adequate fluid resuscitation and vasopressor therapy. In a study of patients with septic shock in whom echocardiography was performed daily for the first 3 days of hemodynamic support, new-onset left ventricular hypokinesia was found in 26 (39%) of 67 patients on presentation and in an additional 14 patients (21%) after at least 24 hours of norepinephrine.55 Adding inotropic support with dobutamine or epinephrine led to decreases in vasopressor dose and enhanced left ventricular ejection fraction.
In short, left ventricular hypokinesia is common in septic shock, may occur at presentation or after a period of vasopressor support, and is usually correctable with the addition of inotropic support.
Corticosteroids
Beyond hemodynamic support with fluids and catecholamines or vasopressin (or both), clinicians should also consider adjunctive corticosteroid therapy. However, for many years the issue has been controversial for patients with severe sepsis and septic shock.
Annane et al56 conducted a large, multicenter, randomized, double-blind, placebocontrolled trial to assess the effect of low doses of corticosteroids in patients with refractory septic shock. Overall, the 28-day mortality rate was 61% in the treatment group and 55% in the placebo group, which was not statistically significant (adjusted odds ratio 0.65, 95% confidence interval 0.39–1.07, P value .09). However, when separated by response to cosyntropin stimulation, those with a change in cortisol of 9 ug/dL or less (nonresponders) randomized to receive corticosteroids had significantly higher survival rates in the short term (28 days) and the long term (1 year). The positive results of this study led to the adoption of low-dose hydrocortisone as standard practice in most patients with septic shock.57
But then, to evaluate the effects of corticosteroids in a broader intensive-care population with septic shock, another trial was designed: the Corticosteroid Therapy of Septic Shock (CORTICUS) trial.58 Surprisingly, this multicenter, randomized, double-blind, placebo-controlled trial found no significant difference in survival between the group that received hydrocortisone and the placebo group, regardless of response to a cosyntropin stimulation test.
Taking into account the above studies and other randomized controlled trials, the 2012 Surviving Sepsis Campaign guidelines and the International Task Force for the Diagnosis and Management of Corticosteroid Insufficiency in Critically Ill Adult Patients recommend intravenous hydrocortisone therapy in adults with septic shock whose blood pressure responds poorly to fluid resuscitation and vasopressor therapy. These consensus statements do not recommend the cosyntropin stimulation test to identify patients with septic shock who should receive corticosteroids.22,59 The guidelines, however, do not explicitly define poor response to initial therapy.
Of note, in the Annane study, which found a lower mortality rate with corticosteroids, the patients were severely ill, with a mean baseline norepinephrine dose of 1.1 μg/kg/min. In contrast, in the CORTICUS study (which found no benefit of hydrocortisone), patients had lower baseline vasopressor doses, with a mean norepinephrine dose of 0.5 μg/kg/min.
While corticosteroids are associated with a higher rate of shock reversal 7 days after initiation, 59 this has not translated into a consistent reduction in the death rate. If a clinician is considering adding corticosteroids to decrease the risk of death, it would seem prudent to add this therapy in patients receiving norepinephrine in doses above 0.5 μg/kg/min.
The ideal sequence and combination of the above therapies including fluids, catecholamine vasopressors, vasopressin, inotropes, and vasopressors have not been elucidated. However, some preliminary evidence suggests an advantage with the combination of vasopressin and corticosteroids. In a subgroup analysis of the VASST study, in patients who received corticosteroids, the combination of vasopressin plus norepinephrine was associated with a lower 28-day mortality rate than with norepinephrine alone (35.9% vs 44.7%, P = .03).60 These findings have been replicated in other studies,61,62 prompting suggestions for a study of vasopressin with and without corticosteroids in patients on norepinephrine to elucidate the role of each therapy individually and in combination.
Tight glycemic control
As with corticosteroids, the pendulum for tight glycemic control in critically ill patients has swung widely in recent years. Enthusiasm was high at first after the publication of a study by van den Berghe et al, which described a 3.4% absolute reduction in mortality with intensive insulin therapy to maintain blood glucose at or below 110 mg/dL.63 However, the significant benefits found in this study were never replicated.
In fact, recent evidence suggests that tight glycemic control is associated with no benefit and a higher risk of hypoglycemia.34,64 In the largest randomized controlled trial of this topic, with more than 6,000 patients, intensive insulin therapy with a target blood glucose level of 81 to 108 mg/dL was associated with a significantly higher mortality rate (odds ratio 1.14, 95% confidence interval 1.02–1.28, P = .02) than with a target glucose level of less than 180 mg/dL.65 Furthermore, in a recent follow-up analysis,66 moderate hypoglycemia (serum glucose 41–70 mg/dL) and severe hypoglycemia (serum glucose < 41 mg/dL) were associated with a higher rate of death in a dose-response relationship.66
Taking this information together, clinicians should be aware that there is no additional benefit in lowering blood glucose below the range of 140 to 180 mg/dL, and that doing so may be harmful.
Drotecogin alfa
Drotecogin alfa (Xigris) was another adjunctive therapy that has fallen from favor. It was approved for the treatment of severe sepsis in light of promising findings in initial studies.67
However, on October 25, 2011, drotecogin alfa was voluntarily withdrawn from the market by the manufacturer after another study found no beneficial effect on the mortality rates at 28 days or at 90 days.68 Furthermore, no difference could be found regarding any predetermined primary or secondary outcome measures.
Continued antibiotic therapy
The decision whether to continue initial empiric antimicrobial coverage, broaden it, or de-escalate must be faced for all patients with septic shock, and is ultimately clinical.
The serum procalcitonin level has been proposed to guide antibiotic discontinuation in several clinical settings, although there are still questions about the safety of such an approach. The largest randomized trial published to date reported that a procalcitoninguided strategy to treat suspected bacterial infections in nonsurgical patients could reduce antibiotic exposure with no apparent adverse outcomes.69 On the other hand, other data discourage the use of procalcitonin-guided antimicrobial escalation, as this approach did not improve survival and worsened organ function and length of stay in the intensive care unit.70
The Surviving Sepsis Campaign guidelines recommend combination antibiotic therapy for no longer than 3 to 5 days and limiting the duration of antibiotics in most cases to 7 to 10 days.22
TRIALS ARE ONGOING
The understanding of the pathophysiology and treatment of sepsis has greatly advanced over the last decade. Adoption of evidence-based protocols for managing patients with septic shock has improved outcomes. Nevertheless, many multicenter trials are being conducted worldwide to look into some of the most controversial therapies, and their results will guide therapy in the future.
Considerably fewer patients who develop sepsis are dying of it now, thanks to a number of studies of how to reverse sepsis-induced tissue hypoxia.1 The greatest strides in improving outcomes have been attributed to better early management, which includes prompt recognition of sepsis, rapid initiation of antimicrobial therapy, elimination of the source of infection, and early goal-directed therapy. Thus, even though the incidence of severe sepsis and septic shock is increasing,2,3 the Surviving Sepsis Campaign has documented a significant decrease in unadjusted mortality rates (37% to 30.8%) associated with the bundled approach in the management of sepsis.4 (We will talk about this later in the article.)
This review will summarize the evidence for the early management of septic shock and will evaluate the various treatment decisions beyond the initial phases of resuscitation.
INFLAMMATION AND VASODILATION
Sepsis syndrome starts with an infection that leads to a proinflammatory state with a complex interaction between anti-inflammatory and proinflammatory mediators, enhanced coagulation, and impaired fibrinolysis.5,6
Sepsis induces vasodilation by way of inappropriate activation of vasodilatory mechanisms (increased synthesis of nitric oxide and vasopressin deficiency) and failure of vasoconstrictor mechanisms (activation of ATP-sensitive potassium channels in vascular smooth muscle).7 Thus, the hemodynamic abnormalities are multifactorial, and the resultant tissue hypoperfusion further contributes to the proinflammatory and procoagulant state, precipitating multiorgan dysfunction and, often, death.
DEFINITIONS
- Sepsis—infection together with systemic manifestation of inflammatory response
- Severe sepsis—sepsis plus induced organ dysfunction or evidence of tissue hypoperfusion
- Septic shock—sepsis-induced hypotension persisting despite adequate fluid resuscitation.
EARLY MANAGEMENT OF SEPTIC SHOCK
Early in the course of septic shock, the physician’s job is to:
- Recognize it promptly
- Begin empiric antibiotic therapy quickly
- Eliminate the source of infection, if applicable, eg, by removing an infected central venous catheter
- Give fluid resuscitation, titrated to specific goals
- Give vasopressor therapy to maintain blood pressure, organ perfusion, and oxygen delivery (Table 1).
The line between “early” and “late” is not clear. Traditionally, it has been drawn at 6 hours from presentation, and this cutoff was used in some of the studies we will discuss here.
Recognizing severe sepsis early in its course
The diagnosis of severe sepsis may be challenging, since up to 40% of patients may present with cryptic shock. These patients may not be hemodynamically compromised but may show evidence of tissue hypoxia, eg, an elevated serum lactate concentration or a low central venous oxygen saturation (Scvo2), or both.8 In view of this, much effort has gone into finding a biomarker that, in addition to clinical features, can help identify patients in an early stage of sepsis.
Procalcitonin levels rise in response to severe bacterial infection,9 and they correlate with sepsis-related organ failure scores and outcomes.10,11 Thus, the serum procalcitonin level may help in assessing the severity of sepsis, especially when combined with standard clinical and laboratory variables. However, controversy exists about the threshold to use in making decisions about antibiotic therapy and the value of this test in differentiating severe noninfectious inflammatory reactions from infectious causes of shock.12 Therefore, it is not widely used in clinical practice.
Serum lactate has been used for decades as a marker of tissue hypoperfusion. It is typically elevated in patients with severe sepsis and septic shock, and although the hyperlactatemia could be a result of global hypoperfusion, it can also be secondary to sepsis-induced mitochondrial dysfunction,13 impaired pyruvate dehydrogenase activity,14 increased aerobic glycolysis by catecholamine-stimulated sodium-potassium pump hyperactivity,15 and even impaired clearance.16
But whatever the mechanism, elevated lactate in severe sepsis and septic shock predicts a poor outcome and may help guide aggressive resuscitation. In fact, early lactate clearance (ie, normalization of an elevated value on repeat testing within the first 6 hours) is associated with better outcomes in patients with severe sepsis and septic shock.17,18
Panels of biomarkers. A literature search revealed over 3,000 papers on 178 different biomarkers in sepsis.19 Many of these biomarkers lack sufficient specificity and sensitivity for clinical use, and thus some investigators have suggested using a panel of them to enhance their predictive ability. Shapiro et al20 evaluated 971 patients admitted to the emergency department with suspected infection and discovered that a panel of three biomarkers (neutrophil gelatinase-associated lipocalin, protein C, and interleukin-1 receptor antagonist) was highly predictive of severe sepsis, septic shock, and death.
Starting empiric antibiotic therapy early
As soon as severe sepsis and septic shock are recognized, it is imperative that adequate empiric antibiotic treatment be started, along with infectious source control if applicable.21 The Surviving Sepsis Campaign guidelines recommend starting intravenous antibiotics as early as possible—within the first hour of recognition of severe sepsis with or without septic shock.22
Kumar et al,23 in a multicenter retrospective study of patients with septic shock, found that each hour of delay in giving appropriate antimicrobial agents in the first 6 hours from the onset of hypotension was associated with a 7.6% decrease in the in-hospital survival rate.
In a similar study,24 the same investigators analyzed data from 5,715 septic shock patients regarding the impact of starting the right antimicrobial therapy. Appropriate antimicrobial agents (ie, those having in vitro activity against the isolated pathogens) were given in 80.1% of cases, and the survival rate in those who received appropriate antibiotics was drastically higher than in those who received inappropriate ones (52.0% vs 10.3%, P < .0001).
In addition, two recent studies evaluated the importance of early empiric antibiotic therapy in conjunction with resuscitative protocols.25,26 In a preplanned analysis of early antimicrobial use in a study comparing lactate clearance and Scvo2 as goals of therapy, Puskarich et al26 found that fewer patients who received antibiotics before shock was recognized (according to formal criteria) died. Similarly, in a retrospective study in patients presenting to the emergency department and treated with early goal-directed therapy (defined below), Gaieski et al25 found that the mortality rate was drastically lower when antibiotics were started within 1 hour of either triage or initiation of early goal-directed therapy.
In short, it is imperative to promptly start the most appropriate broad-spectrum antibiotics to target the most likely pathogens based on site of infection, patient risk of multidrug-resistant pathogens, and local susceptibility patterns.
Goal-directed resuscitative therapy
As with antimicrobial therapy, resuscitative therapy should be started early and directed at defined goals.
Rivers et al27 conducted a randomized, controlled study in patients with severe sepsis or septic shock presenting to an emergency department of an urban teaching hospital. The patients were at high risk and had either persistent hypotension after a fluid challenge or serum lactate levels of 4 mmol/L or higher.
Two hundred sixty patients were randomized to receive either early goal-directed therapy in a protocol aimed at maximizing the intravascular volume and correcting global tissue hypoxia or standard therapy in the first 6 hours after presentation. The goals in the goal-directed therapy group were:
- Central venous pressure 8 to 12 mm Hg (achieved with aggressive fluid resuscitation with crystalloids)
- Mean arterial blood pressure greater than 65 mm Hg (maintained with vasoactive drugs, if necessary)
- Scvo2 above 70%. To achieve this third goal, packed red blood cells were infused to reach a target hematocrit of greater than 30%. For patients with a hematocrit higher than 30% but still with an Scvo2 less than 70%, inotropic agents were added and titrated to the Scvo2 goal of 70%.
Goal-directed therapy reduced the in-hospital mortality rate by 16% (the mortality rates were 30.5% in the goal-directed group and 46.5% in the standard therapy group, P = .009) and also reduced the 28- and 60-day mortality rates by similar proportions.27
Subsequent studies of a protocol for early recognition and treatment of sepsis have concluded that early aggressive fluid resuscitation decreases the ensuing need for vasopressor support.28 A resuscitation strategy based on early goal-directed therapy is a major component of the initial resuscitation bundle recommended by the Surviving Sepsis Campaign.22 (A “bundle” refers to the implementation of a core set of recommendations involving the simultaneous adaptation of a number of interventions.)
Areas of debate. However, concerns have been raised about the design of the study by Rivers et al and the mortality rate in the control group, which was higher than one would expect from the patients’ Acute Physiology and Chronic Health Evaluation II (APACHE II) scores.29 In particular, the bundled approach they used precludes the ability to differentiate which interventions were responsible for the outcome benefits. Indeed, there were two major interventions in the early goal-directed therapy group: a protocol for achieving the goals described and the use of Scvo2 as a goal.
Aggressive fluid resuscitation is considered the most critical aspect of all the major interventions, and there is little argument on its value. The debate centers on central venous pressure as a preload marker, since after the publication of the early goal-directed therapy trial,27 several studies showed that central venous pressure may not be a valid measure to predict fluid responsiveness (discussed later in this paper).30,31
The choice of colloids or crystalloids for fluid resuscitation is another area of debate. Clinical evidence suggests that albumin is equivalent to normal saline in a heterogeneous intensive care unit population,32 but subgroup analyses suggest albumin may be superior in patients with septic shock.33 Studies are ongoing (NCT00707122, NCT01337934, and NCT00318942). The use of hydroxyethyl starch in severe sepsis is associated with higher rates of acute renal failure and need for renal replacement therapy than Ringer’s lactate,34 and is generally not recommended. This is further substantiated by two recent randomized controlled studies, which found that the use of hydroxyethyl starch for fluid resuscitation in severe sepsis, compared with crystalloids, did not reduce the mortality rate (and even increased it in one study), and was associated with more need for renal replacement therapy.35,36
The use of Scvo2 is yet another topic of debate, and other monitoring variables have been evaluated. A recent study assessed the noninferiority of incorporating venous lactate clearance into the early goal-directed therapy protocol vs Scvo2.37 Both groups had identical goals for central venous pressure and mean arterial pressure but differed in the use of lactate clearance (defined as at least a 10% decline) or Scvo2 (> 70%) as the goal for improving tissue hypoxia. There were no significant differences between groups in their in-hospital mortality rates (17% in the lactate clearance group vs 23% in the Scvo2 group; criteria for noninferiority met). This suggests that lactate may be an alternative to Scvo2 as a goal in early goal-directed therapy. However, a secondary analysis of the data revealed a lack of concordance in achieving lactate clearance and Scvo2 goals, which suggests that these parameters may be measuring distinct physiologic processes.38 Since the hemodynamic profiles of septic shock patients are complex, it may be prudent to use both of these markers of resuscitation until further studies are completed.
Given the debate, a number of prospective randomized trials are under way to evaluate resuscitative interventions. These include the Protocolized Care for Early Septic Shock trial (NCT00510835), the Australasian Resuscitation in Sepsis Evaluation trial (NCT00975793), and the Protocolised Management of Sepsis (ProMISe) trial in the United Kingdom (ISRCTN 36307479). These three trials will evaluate, collectively, close to 4,000 patients and will provide considerable insights into resuscitative interventions in septic shock.
Vasopressors: Which one to use?
If fluid therapy does not restore perfusion, vasopressors should be promptly initiated, as the longer that hypotension goes on, the lower the survival rate.39
But which vasopressor should be used? The early goal-directed therapy protocol used in the study by Rivers et al27 did not specify which vasopressor should be used to keep the mean arterial pressure above 65 mm Hg.
The Surviving Sepsis Campaign22 recommends norepinephrine as the first-choice vasopressor, with dopamine as an alternative only in selected patients, such as those with absolute or relative bradycardia.
The guidelines also recommend epinephrine to be added to or substituted for norepinephrine when an additional catecholamine is needed to maintain adequate blood pressure.22 Furthermore, vasopressin at a dose of 0.03 units/min can be added to norepinephrine with the intent of raising the blood pressure or decreasing the norepinephrine requirement. Higher doses of vasopressin should be reserved for salvage therapy.
Regarding phenylephrine, the guidelines recommend against its use except when norepinephrine use is associated with significant tachyarrhythmias, cardiac output is known to be higher, or as a salvage therapy.22
This is a topic of debate, with recent clinical studies offering further insight.
De Backer et al40 compared the effects of dopamine vs norepinephrine for the treatment of shock in 1,679 patients, 62% of whom had septic shock. Overall, there was a trend towards better outcomes with norepinephrine, but no significant difference in mortality rates at 28 days (52.5% with dopamine vs 48.5% with norepinephrine, P = .10). Importantly, fewer patients who were randomized to norepinephrine developed arrhythmias (12.4% vs 24.1%, P < .001), and the norepinephrine group required fewer days of study drug (11.0 vs 12.5, P = .01) and open-label vasopressors (12.6 vs 14.2, P = .007). Of note, patients with cardiogenic shock randomized to norepinephrine had a significantly lower mortality rate than those randomized to dopamine. Although no significant difference in outcome was found between the two vasopressors in the subgroup of patients with septic shock, the overall improvements in secondary surrogate markers suggest that norepinephrine should be the first-line agent.
Norepinephrine has also been compared with “secondary” vasopressors. Annane et al,41 in a prospective multicenter randomized controlled study, evaluated the effect of norepinephrine plus dobutamine vs epinephrine alone in managing septic shock. There was no significant difference in the primary outcome measure of 28-day mortality (34% with norepinephrine plus dobutamine vs 40% with epinephrine alone, P = .31). However, the study was powered to evaluate for an absolute risk reduction of 20% in the mortality rate, which would be a big reduction. A smaller reduction in the mortality rate, which would not have been statistically significant in this study, might still be considered clinically significant. Furthermore, the group randomized to norepinephrine plus dobutamine had more vasopressor-free days (20 days vs 22 days, P = .05) and less acidosis on days 1 to 4 than the group randomized to epinephrine.
Norepinephrine was also compared with phenylephrine as a first-line vasopressor in a randomized controlled trial in 32 patients with septic shock. No difference was found in cardiopulmonary performance, global oxygen transport, or regional hemodynamics between phenylephrine and norepinephrine.42
While encouraging, these preliminary data need to be verified in a larger randomized controlled trial with concrete outcome measures before being clinically adapted. Taken together, the above studies suggest that norepinephrine should be the initial vasopressor of choice for patients with septic shock.
CONTINUED MANAGEMENT OF SEPTIC SHOCK
How to manage septic shock after the initial stages is much less defined.
Uncertainty persists about the importance of achieving the early goals of resuscitation in patients who did not reach them in the initial 6 hours of treatment. Although there are data suggesting that extending the goals beyond the initial 6 hours may be beneficial, clinicians should use caution when interpreting these results in light of the observational design of the studies.43,44 For the purpose of this discussion, “continued management” of septic shock will mean after the first 6 hours and after all the early goals are met.
The clinical decisions necessary after the initial stages of resuscitation include:
- Whether further fluid resuscitation is needed
- Assessment for further and additional hemodynamic therapies
- Consideration of adjunctive therapies
- Reevaluation of antibiotic choices (Table 2).
Is more fluid needed? How can we tell?
There is considerable debate about the ideal method for assessing fluid responsiveness. In fact, one of the criticisms of the early goal-directed therapy study27 was that it used central venous pressure as a marker of fluid responsiveness.
Several studies have shown that central venous pressure or pulmonary artery occlusion pressure may not be valid measures of fluid responsiveness.45 In fact, in a retrospective study of 150 volume challenges, the area under the receiver-operating-characteristics curve of central venous pressure as a marker of fluid responsiveness was only 0.58. (Recall that the closer the area under the curve is to 1.0, the better the test; a value of 0.50 is the same as chance.) The area under the curve for pulmonary artery occlusion pressure was 0.63.46
In contrast, several dynamic indices have been proposed to better guide fluid resuscitation in mechanically ventilated patients.31 These are based on changes in stroke volume, aortic blood flow, or arterial pulse pressure in response to the ventilator cycle or passive leg-raising. A detailed review of these markers can be found elsewhere,31 but taken together, they have a sensitivity and specificity of over 90% for predicting fluid responsiveness. Clinicians may consider using dynamic markers of fluid responsiveness to determine when to give additional fluids, particularly after the first 6 hours of shock, in which data supporting the use of central venous pressure are lacking.
Optimal use of fluids is particularly important, since some studies suggest that “overresuscitation” has negative consequences. In a multicenter observational study of 1,177 patients with sepsis, after adjusting for a number of comorbidities and baseline severity of illness, the cumulative fluid balance in the first 72 hours after the onset of sepsis was independently associated with a worse mortality rate.47
Furthermore, in a retrospective analysis of a randomized controlled trial of vasopressin in conjunction with norepinephrine for septic shock, patients in the highest quartile of fluid balance (more fluid in than out) at 12 hours and 4 days after presentation had significantly higher mortality rates than those in the lowest two quartiles.48 The worse outcome with a positive fluid balance might be explained by worsening oxygenation and prolonged mechanical ventilation, as demonstrated by the Fluid and Catheter Treatment Trial in patients with acute lung injury or acute respiratory distress syndrome (ALI/ARDS).49 Indeed, when fluid balance in patients with septic shockinduced ALI/ARDS was evaluated, patients with both adequate initial fluid resuscitation and conservative late fluid management had a lower mortality rate than those with either one alone.50
In view of these findings, especially beyond the initial hours of resuscitation, clinicians should remember that further unnecessary fluid administration may have detrimental effects. Therefore, given the superior predictive abilities of dynamic markers of fluid responsiveness, these should be used to determine the need for further fluid boluses.
In cases in which patients are no longer fluid-responsive and need increasing levels of hemodynamic support, clinicians still have a number of options. These include increasing the current vasopressor dose or starting an additional therapy such as an alternative catecholamine vasopressor, vasopressin, inotropic therapy, or an adjunctive therapy such as a corticosteroid. The intervention could also be a combination of the above choices.
Adding catecholamines
The optimal time point or vasopressor dose at which to consider initiating additional therapies is unknown. However, the Vasopressin and Septic Shock Trial (VASST) provides some insight.51
This study compared two strategies: escalating doses of norepinephrine vs adding vasopressin to norepinephrine. Overall, adding vasopressin showed no benefit in terms of a lower mortality rate. However, in the subgroup of patients with norepinephrine requirements of 5 to 14 μg/min at study enrollment (ie, a low dose, reflecting less-severe sepsis) vasopressin was associated with a lower 28-day mortality rate (26.5% vs 35.7%, P = .05) and 90-day mortality rate (35.8% vs 46.1%, P = .04). Benefit was also noted in patients with other markers of lower disease severity such as low lactate levels or having received a single vasopressor at baseline.51
Although subgroup analyses should not generally be used to guide treatment decisions, a prospective trial may never be done to evaluate adding vasopressin to catecholamines earlier vs later. Thus, clinicians who choose to use vasopressin may consider starting this therapy when catecholamine doses are relatively low or before profound hyperlactatemia from prolonged tissue hypoxia has developed.
There is less evidence to guide clinicians who are considering adding a different catecholamine. The theoretical concerns of splanchnic ischemia and cardiac arrhythmia associated with higher doses of catecholamines are usually the impetus to limit a single catecholamine to a “maximum” dose. However, studies that have evaluated combination catecholamine therapies have generally studied combinations of vasopressors with inotropes and lacked standardization in their protocols, thus making them difficult to interpret.52–54 One could also argue that additional catecholamine therapies, which all function similarly, may have additive effects and cause even more adverse effects. As such, adding another vasopressor should be reserved for patients experiencing noticeable adverse effects (such as tachycardia) on first-line therapy.
Inotropic support
Left ventricular function should be assessed in all patients who continue to be hypotensive despite adequate fluid resuscitation and vasopressor therapy. In a study of patients with septic shock in whom echocardiography was performed daily for the first 3 days of hemodynamic support, new-onset left ventricular hypokinesia was found in 26 (39%) of 67 patients on presentation and in an additional 14 patients (21%) after at least 24 hours of norepinephrine.55 Adding inotropic support with dobutamine or epinephrine led to decreases in vasopressor dose and enhanced left ventricular ejection fraction.
In short, left ventricular hypokinesia is common in septic shock, may occur at presentation or after a period of vasopressor support, and is usually correctable with the addition of inotropic support.
Corticosteroids
Beyond hemodynamic support with fluids and catecholamines or vasopressin (or both), clinicians should also consider adjunctive corticosteroid therapy. However, for many years the issue has been controversial for patients with severe sepsis and septic shock.
Annane et al56 conducted a large, multicenter, randomized, double-blind, placebocontrolled trial to assess the effect of low doses of corticosteroids in patients with refractory septic shock. Overall, the 28-day mortality rate was 61% in the treatment group and 55% in the placebo group, which was not statistically significant (adjusted odds ratio 0.65, 95% confidence interval 0.39–1.07, P value .09). However, when separated by response to cosyntropin stimulation, those with a change in cortisol of 9 ug/dL or less (nonresponders) randomized to receive corticosteroids had significantly higher survival rates in the short term (28 days) and the long term (1 year). The positive results of this study led to the adoption of low-dose hydrocortisone as standard practice in most patients with septic shock.57
But then, to evaluate the effects of corticosteroids in a broader intensive-care population with septic shock, another trial was designed: the Corticosteroid Therapy of Septic Shock (CORTICUS) trial.58 Surprisingly, this multicenter, randomized, double-blind, placebo-controlled trial found no significant difference in survival between the group that received hydrocortisone and the placebo group, regardless of response to a cosyntropin stimulation test.
Taking into account the above studies and other randomized controlled trials, the 2012 Surviving Sepsis Campaign guidelines and the International Task Force for the Diagnosis and Management of Corticosteroid Insufficiency in Critically Ill Adult Patients recommend intravenous hydrocortisone therapy in adults with septic shock whose blood pressure responds poorly to fluid resuscitation and vasopressor therapy. These consensus statements do not recommend the cosyntropin stimulation test to identify patients with septic shock who should receive corticosteroids.22,59 The guidelines, however, do not explicitly define poor response to initial therapy.
Of note, in the Annane study, which found a lower mortality rate with corticosteroids, the patients were severely ill, with a mean baseline norepinephrine dose of 1.1 μg/kg/min. In contrast, in the CORTICUS study (which found no benefit of hydrocortisone), patients had lower baseline vasopressor doses, with a mean norepinephrine dose of 0.5 μg/kg/min.
While corticosteroids are associated with a higher rate of shock reversal 7 days after initiation, 59 this has not translated into a consistent reduction in the death rate. If a clinician is considering adding corticosteroids to decrease the risk of death, it would seem prudent to add this therapy in patients receiving norepinephrine in doses above 0.5 μg/kg/min.
The ideal sequence and combination of the above therapies including fluids, catecholamine vasopressors, vasopressin, inotropes, and vasopressors have not been elucidated. However, some preliminary evidence suggests an advantage with the combination of vasopressin and corticosteroids. In a subgroup analysis of the VASST study, in patients who received corticosteroids, the combination of vasopressin plus norepinephrine was associated with a lower 28-day mortality rate than with norepinephrine alone (35.9% vs 44.7%, P = .03).60 These findings have been replicated in other studies,61,62 prompting suggestions for a study of vasopressin with and without corticosteroids in patients on norepinephrine to elucidate the role of each therapy individually and in combination.
Tight glycemic control
As with corticosteroids, the pendulum for tight glycemic control in critically ill patients has swung widely in recent years. Enthusiasm was high at first after the publication of a study by van den Berghe et al, which described a 3.4% absolute reduction in mortality with intensive insulin therapy to maintain blood glucose at or below 110 mg/dL.63 However, the significant benefits found in this study were never replicated.
In fact, recent evidence suggests that tight glycemic control is associated with no benefit and a higher risk of hypoglycemia.34,64 In the largest randomized controlled trial of this topic, with more than 6,000 patients, intensive insulin therapy with a target blood glucose level of 81 to 108 mg/dL was associated with a significantly higher mortality rate (odds ratio 1.14, 95% confidence interval 1.02–1.28, P = .02) than with a target glucose level of less than 180 mg/dL.65 Furthermore, in a recent follow-up analysis,66 moderate hypoglycemia (serum glucose 41–70 mg/dL) and severe hypoglycemia (serum glucose < 41 mg/dL) were associated with a higher rate of death in a dose-response relationship.66
Taking this information together, clinicians should be aware that there is no additional benefit in lowering blood glucose below the range of 140 to 180 mg/dL, and that doing so may be harmful.
Drotecogin alfa
Drotecogin alfa (Xigris) was another adjunctive therapy that has fallen from favor. It was approved for the treatment of severe sepsis in light of promising findings in initial studies.67
However, on October 25, 2011, drotecogin alfa was voluntarily withdrawn from the market by the manufacturer after another study found no beneficial effect on the mortality rates at 28 days or at 90 days.68 Furthermore, no difference could be found regarding any predetermined primary or secondary outcome measures.
Continued antibiotic therapy
The decision whether to continue initial empiric antimicrobial coverage, broaden it, or de-escalate must be faced for all patients with septic shock, and is ultimately clinical.
The serum procalcitonin level has been proposed to guide antibiotic discontinuation in several clinical settings, although there are still questions about the safety of such an approach. The largest randomized trial published to date reported that a procalcitoninguided strategy to treat suspected bacterial infections in nonsurgical patients could reduce antibiotic exposure with no apparent adverse outcomes.69 On the other hand, other data discourage the use of procalcitonin-guided antimicrobial escalation, as this approach did not improve survival and worsened organ function and length of stay in the intensive care unit.70
The Surviving Sepsis Campaign guidelines recommend combination antibiotic therapy for no longer than 3 to 5 days and limiting the duration of antibiotics in most cases to 7 to 10 days.22
TRIALS ARE ONGOING
The understanding of the pathophysiology and treatment of sepsis has greatly advanced over the last decade. Adoption of evidence-based protocols for managing patients with septic shock has improved outcomes. Nevertheless, many multicenter trials are being conducted worldwide to look into some of the most controversial therapies, and their results will guide therapy in the future.
- Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011; 140:1223–1231.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310.
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med 2003; 168:165–172.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222–231.
- Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Med 2004; 30:1032–1040.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348:138–150.
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001; 345:588–595.
- Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med 1996; 14:218–225.
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 34:515–518.
- Muller B, Becker KL, Schachinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000; 28:977–983.
- Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care (London, England) 1999; 3:45–50.
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007; 7:210–217.
- Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002; 360:219–223.
- Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock 1996; 6:89–94.
- Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 2005; 365:871–875.
- Levraut J, Ciebiera JP, Chave S, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than over-production. Am J Respir Crit Care Med 1998; 157:1021–1026.
- Arnold RC, Shapiro NI, Jones AE, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009; 32:35–39.
- Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004; 32:1637–1642.
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010; 14:R15.
- Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 2009; 37:96–104.
- Marshall JC, al Naqbi A. Principles of source control in the management of sepsis. Crit Care Clin 2009; 25:753–768,viii–ix.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136:1237–1248.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–1053.
- Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39:2066–2071.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006; 34:2707–2713.
- Schmidt GA. Counterpoint: adherence to early goal-directed therapy: does it really matter? No. Both risks and benefits require further study. Chest 2010; 138:480–483; discussion 483–484.
- Jain RK, Antonio BL, Bowton DL, Houle TT, MacGregor DA. Variability in central venous pressure measurements and the potential impact on fluid management. Shock 2009; 33:253–257.
- Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest 2008; 133:252–263.
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011; 37:86–96.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010; 303:739–746.
- Puskarich MA, Trzciak S, Shapiro NI, Kline JA, Jones AE. Concordance and prognostic value of central venous oxygen saturation and lactate clearance in emergency department patients with septic shock. Acad Emerg Med 2011; 19:S159–S160.
- Dunser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med 2009; 35:1225–1233.
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007; 370:676–684.
- Morelli A, Ertmer C, Rehberg S, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care (London, England) 2008; 12:R143.
- Coba V, Whitmill M, Mooney R, et al. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med 2011 Jan 10[Epub ahead of print].
- Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodriguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011; 36:542–547.
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134:172–178.
- Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007; 35:64–68.
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34:344–353.
- Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–265.
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009; 136:102–109.
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887.
- Vincent JL, Roman A, Kahn RJ. Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med 1990; 18:689–693.
- Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med 1997; 23:282–287.
- Redl-Wenzl EM, Armbruster C, Edelmann G, et al. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med 1993; 19:151–154.
- Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008; 36:1701–1706.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871.
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004; 32:858–873.
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124.
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36:1937–1949.
- Russell JA, Walley KR, Gordon AC, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med 2009; 37:811–818.
- Bauer SR, Lam SW, Cha SS, Oyen LJ. Effect of corticosteroids on arginine vasopressin-containing vasopressor therapy for septic shock: a case control study. J Crit Care 2008; 23:500–506.
- Torgersen C, Luckner G, Schroder DC, et al. Concomitant arginine-vasopressin and hydrocortisone therapy in severe septic shock: association with mortality. Intensive Care Med 2011; 37:1432–1437.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009; 35:1738–1748.
- Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367:1108–1118.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344:699–709.
- Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012; 366:2055–2064.
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2009; 375:463–474.
- Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011; 39:2048–2058.
- Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011; 140:1223–1231.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310.
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med 2003; 168:165–172.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222–231.
- Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Med 2004; 30:1032–1040.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348:138–150.
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001; 345:588–595.
- Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med 1996; 14:218–225.
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 34:515–518.
- Muller B, Becker KL, Schachinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000; 28:977–983.
- Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care (London, England) 1999; 3:45–50.
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007; 7:210–217.
- Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002; 360:219–223.
- Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock 1996; 6:89–94.
- Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 2005; 365:871–875.
- Levraut J, Ciebiera JP, Chave S, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than over-production. Am J Respir Crit Care Med 1998; 157:1021–1026.
- Arnold RC, Shapiro NI, Jones AE, et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock 2009; 32:35–39.
- Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004; 32:1637–1642.
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010; 14:R15.
- Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 2009; 37:96–104.
- Marshall JC, al Naqbi A. Principles of source control in the management of sepsis. Crit Care Clin 2009; 25:753–768,viii–ix.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136:1237–1248.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–1053.
- Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39:2066–2071.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006; 34:2707–2713.
- Schmidt GA. Counterpoint: adherence to early goal-directed therapy: does it really matter? No. Both risks and benefits require further study. Chest 2010; 138:480–483; discussion 483–484.
- Jain RK, Antonio BL, Bowton DL, Houle TT, MacGregor DA. Variability in central venous pressure measurements and the potential impact on fluid management. Shock 2009; 33:253–257.
- Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest 2008; 133:252–263.
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011; 37:86–96.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010; 303:739–746.
- Puskarich MA, Trzciak S, Shapiro NI, Kline JA, Jones AE. Concordance and prognostic value of central venous oxygen saturation and lactate clearance in emergency department patients with septic shock. Acad Emerg Med 2011; 19:S159–S160.
- Dunser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med 2009; 35:1225–1233.
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007; 370:676–684.
- Morelli A, Ertmer C, Rehberg S, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care (London, England) 2008; 12:R143.
- Coba V, Whitmill M, Mooney R, et al. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med 2011 Jan 10[Epub ahead of print].
- Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodriguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011; 36:542–547.
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134:172–178.
- Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007; 35:64–68.
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34:344–353.
- Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–265.
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
- Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009; 136:102–109.
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887.
- Vincent JL, Roman A, Kahn RJ. Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med 1990; 18:689–693.
- Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med 1997; 23:282–287.
- Redl-Wenzl EM, Armbruster C, Edelmann G, et al. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med 1993; 19:151–154.
- Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008; 36:1701–1706.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871.
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004; 32:858–873.
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124.
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36:1937–1949.
- Russell JA, Walley KR, Gordon AC, et al. Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med 2009; 37:811–818.
- Bauer SR, Lam SW, Cha SS, Oyen LJ. Effect of corticosteroids on arginine vasopressin-containing vasopressor therapy for septic shock: a case control study. J Crit Care 2008; 23:500–506.
- Torgersen C, Luckner G, Schroder DC, et al. Concomitant arginine-vasopressin and hydrocortisone therapy in severe septic shock: association with mortality. Intensive Care Med 2011; 37:1432–1437.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009; 35:1738–1748.
- Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367:1108–1118.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344:699–709.
- Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012; 366:2055–2064.
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2009; 375:463–474.
- Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011; 39:2048–2058.
KEY POINTS
- Managing septic shock in the first 6 hours involves prompt recognition, empiric antibiotic therapy, elimination of the source of infection (if applicable), fluid resuscitation titrated to specific goals, and vasopressor therapy.
- A number of biomarkers have been proposed to help recognize septic shock early in its course.
- A delay in starting appropriate antibiotic treatment is associated with higher risk of death.
- The ideal measure of the adequacy of fluid resuscitation remains a topic of study and debate.
- Preliminary studies suggest that norepinephrine should be the initial vasopressor.
- Management after the first 6 hours is less well defined. Decisions in this period include whether to give further fluid resuscitation, further and additional hemodynamic therapies, adjunctive therapies, and antibiotics.
Frailty in older adults: Implications for end-of-life care
As people get older, they have more things wrong with them. And the more things they have wrong with them, the more likely they are to die. But everyone accumulates deficits at a different rate, and not all people of the same age have the same short-term risk of dying. This variable susceptibility to death and other adverse outcomes in older people of the same age is called frailty.1
Frailty poses special challenges to how we organize and deliver health care. These challenges are sometimes seen most starkly when people are most frail, especially as they approach the end of life.
In this paper, we will review how frailty is conceptualized and defined, consider how frailty affects the care of people at the end of their lives, and suggest practices that can make end-of-life care better for frail older adults.
DEFINING FRAILTY
As with all complex systems, when frail people become acutely unwell their highest-order functions fail first. Thus, cognitive impairment, functional decline, impaired mobility, and social withdrawal are hallmark presentations of the further accumulation of deficits in vulnerable seniors.
Delirium and falls are important clues that a person’s resilience is becoming compromised and that the person is at risk of further insults in a downward spiral or acceleration of things going wrong.1,2 Frailty is associated with poor health outcomes, from disability to institutionalization and death.3
This idea of frailty as vulnerability arising from dysregulation of multiple physiologic systems is reasonably non-controversial. Even so, there are competing views on how to systematically quantify those who are at an increased risk of adverse sequelae.
Quantifying frailty is particularly important if it can tell us if a patient is at high risk of further decline and death. As frailty advances, it is appropriate to shift the focus of care to palliation, with the goal of optimizing quality of life and easing symptoms.4 Identifying someone as frail can aid decision-making in the setting of critical illness, where the system commonly defaults to an “always do everything” mode without considering the ramifications of such an approach. Furthermore, without a routine means of measuring frailty, it is often left to critical care units or rapid-response medical teams to initiate a discussion about whether an aggressive course of care is appropriate or desired.5,6
Frailty as a syndrome
Fried et al7 defined frailty as a syndrome arising from the “physiologic triad” of sarcopenia and immune and neuroendocrine dysregulation. Patients are considered frail if they have three or more of the following five criteria:
- Reduced activity
- Slowing of mobility
- Weight loss
- Diminished handgrip strength
- Exhaustion.
Someone who has only one or two of these items is said to be “pre-frail”; someone with none is said to be “robust.”
The frailty index
An alternative viewpoint is that frailty is a state arising from the accumulation of deficits, which can be counted in a frailty index.
The frailty index is based on the concept that frailty is a consequence of interacting physical, psychological, and social factors. As deficits accumulate, people become increasingly vulnerable to adverse outcomes.
The frailty index is calculated as the number of deficits the patient has, divided by the number of deficits considered. For example, in a frailty index based on a comprehensive geriatric assessment, an individual with impairments in 4 of 10 domains and with 10 of 24 possible comorbidities would have 14 of 34 possible deficits, for a frailty index of 0.41.8
A criticism of the frailty index is that it includes functional dependence as a deficit. The criticism stems from the view that frailty should be seen as occurring prior to disability. According to this view, including dependence in instrumental and basic activities of daily living as a deficit confuses disability with frailty.
Proponents of the frailty index counter that frailty is not “all or none” and needs to be graded. The frailty index can distinguish between people with and without disability by means of the number of deficits that they have, which is most important. For example, a person disabled by a paraplegic injury would have a lower frailty index score and therefore would be considered less frail than a person with advanced cancer affecting multiple body systems. (This is assuming the person who has suffered the injury resulting in paraplegia doesn’t have a concomitant condition such as renal failure or heart disease. In the absence of other health insults, such patients are less at risk of further morbidity or death than the patient with advanced cancer until they get another health insult or insults added to their frailty.)
In any case, functional capacity is fundamental in medical decision-making and when estimating prognoses. An example is the use of the Eastern Cooperative Oncology Group’s functional status measure.9,10
Sum of physical and psychological stressors
Consensus is growing for the concept that frail people are made more vulnerable by the combination of both physical and psychological stressors. This is particularly important to bear in mind for patients who may appear physically robust but whose total health burden makes them vulnerable to further insults.
For example, think of a relatively young overweight patient with hypertension, diabetes, dyslipidemia, and ischemic white matter changes (which can manifest as low mood and even mild vascular cognitive impairment). In such a patient, an acute illness could result in cognitive and functional decline that can be permanent.
Balance of assets and deficits
About 20 years ago, we used the metaphor of a balance beam to describe how frailty comes about in older adults. In this view, there is an interplay of physiological and functional health determinants. Assets such as health, resources, and caregivers are balanced against deficits such as illnesses, dependency on others, and support burden.8
For the most part, later concepts of frailty have focused on the individual, with social factors construed separately as social vulnerability.11
Tools for assessing frailty in people who are not yet disabled
Several tools exist to clinically assess frailty in people who are not yet disabled.
The FRAIL scale.12 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging formulated a scale for measuring frailty as a “pre-disability state.” The FRAIL scale consists of five easily remembered items:
- Fatigue
- Resistance (inability to climb one flight of stairs)
- Ambulation (inability to walk one block)
- Illnesses (more than five)
- Loss of weight (> 5%).
Like the “reduced activity” criterion of the frailty syndrome mentioned above (in practical terms, described as the inability to do heavy household chores),13 the FRAIL scale seems to blur the distinction between disability (here, the inability to climb stairs or to walk a block) and “pre-disability,” to an uncertain end. It also seems to blend the notion of a state and a syndrome; these points will need to be clarified in due course.
The Tilburg Frailty Indicator14 was constructed around the multidimensional viewpoint of frailty, beyond disease or disability state, to identify frail community-dwelling older individuals. The first part of this two-part questionnaire consists of 10 questions on frailty determinants and medical comorbidities, while the second part contains physical, psychological, and social variables strongly associated with frailty, as well as information about disability in walking and balance. Interestingly, although it includes both social and physical factors, it does not include cognition.
The Clinical Frailty Scale was developed as a practical approach to assess frailty using physical and functional indicators of health and illness burden. The descriptors for this 7-point scale guide clinicians in quantifying the degree of frailty present. It ranges from 1 (very fit) to 7 (severely frail).7 The higher the score, the higher the risks of death or institutionalization. Even mild frailty is associated with a 50% 5-year mortality rate in community-dwelling older adults (Figure 1).8
The Edmonton Frail Scale,15 like the Clinical Frailty Scale, was developed to be practical and usable at the bedside. It is based on the following domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance.
In a community-based sample, the Edmonton Frail Scale compared favorably with the clinical assessment of geriatric specialists who completed a comprehensive evaluation (Pearson’s correlation coefficient 0.64, P < .001).15
FRAILTY AS A PROGNOSTIC INDICATOR
Using frailty scales to aid in prognostication can be useful to clinicians. Survival prognostication is inherently challenging in individuals with multiple comorbidities and variable trajectories of decline, but it remains a vital clinical skill for all clinicians. Framing these difficult discussions in the context of degree of frailty provides a unifying concept, beyond a single-system construct, for care providers, patients, and their loved ones.
Patients nearing the end of their lives need this kind of clarity and support. Regardless of their diagnoses, patients typically want to know when they are at high risk of dying, as do their families and caregivers. People in general look for such information so that they can align medical decision-making congruently with predicted prognosis.16,17 They also use it to plan for the final chapter of their life and their death.
The frailty index is strongly correlated with risk of death
The frailty index is strongly correlated with the risk of death, with a correlation coefficient greater than 0.95. As such, an individual’s frailty index score is considered an estimate of biologic age, which has greater correlation with associated morbidity and death than does chronological age.18,19 In the general population, more than 99% of people have a frailty index value of less than 0.7. As people approach this value, the chance of survival is greatly diminished; indeed, one report suggested that of those who have a frailty index value of more than 0.5 (based on a comprehensive geriatric assessment), 100% are dead by about 20 months later.20,21
In short, there is a limit to which deficits can be added before the system fails. In this sense, the frailty index is akin to the concept of physiologic reserve. Reserve is finite, and as a system loses redundancy it can no longer survive new stresses.
What does this information mean for individual patients?
Even so, prognostication for individual patients remains probabilistic. Any patient has a chance to improve, stabilize, worsen, or die. However, a patient can reach an upper limit of frailty. At that point, instead of accumulating another deficit, death is much more likely. Similarly, although improvement can happen, the chance of improvement is low, and the improvement is typically modest.
Framing survival possibilities in terms of the number of things that people have wrong with them and the chance of death or of change (and the extent of change) makes sense to physicians, patients, and families. Being able to do so offers a much greater opportunity for realistic discussions of the likely outcomes of medical care than the foreseeable scenario of a junior doctor asking a senior citizen, “If your heart stops, do you want us to save your life?”
Understanding prognosis in the face of not just disease but also frailty can also help us focus not on disease but on health consequences of illness. Can the person think? Walk? Care for herself or himself? Interact with others? These questions need to be considered when end-of-life decisions are being discussed.22
Since making predictions about survival is most challenging when multiple comorbidities are present, using the concept of accumulating deficits to better define the slope of decline can be very helpful when discussing “the road ahead” with patients and their families. Visually mapping out the slope of decline and how it is accelerating as conditions progress and deficits accumulate can aid in medical decision-making. Looking individually at the deficits themselves and associated markers of progression can also help with prognostic discussions.
For example, a patient with chronic obstructive pulmonary disease could very well be unaware of the progression and ultimately terminal prognosis of this disease. The slope of clinical decline can be initially shallow, with saw-tooth fluctuations from acute exacerbations that seemingly “resolve to baseline” when antibiotic and steroid courses are completed. Talking with these patients and their families about heralding markers, such as more hospitalizations and cognitive decline with acute exacerbations, can clarify the steepening slope of decline and the way comorbidities interact.
FRAILTY AND END-OF-LIFE CARE
Frailty is progressive, and as it worsens, integrating a palliative approach is appropriate, with a focus on optimizing quality of life and relieving symptoms.4 This principle holds true regardless of the care setting, from acute care hospitals to hospice facilities and long-term care residences.
The principles of end-of-life care are applicable to frail individuals with progressive conditions from the time of diagnosis throughout the course of decline. As the population ages, more people suffer and die from progressive chronic conditions such as cerebrovascular disease, respiratory disease, and dementia.23 An interdisciplinary team approach can ensure all components of palliation are effectively delivered, such as easing symptoms, providing psychosocial and spiritual support, and improving quality of life.24
Pain management
Pain is widely underassessed and undertreated in older patients. Its management at the end of life is particularly challenging if the patient’s language is compromised, as in dementia.23,25,26
A recent cross-sectional analysis of self-reported pain in a longitudinal study of community-dwelling older adults showed an independent association between moderate or higher pain and frailty. The authors propose that persistent pain goes beyond physical discomfort in that it may contribute to homeostenosis (progressive diminishment of homeostatic reserve) and directly worsen frailty.27
Examine the medication list
In palliative care, medical interventions focus on optimizing quality of life.
This especially includes reexamining long medication lists that increase the chance of adverse drug effects.28 Many patients are on disease-modifying medications that may or may not help control symptoms—and might well exacerbate them. For example, betablockers for ischemic heart disease and angiotensin-converting enzyme inhibitors for diabetic nephropathy both can cause hypotension-induced lassitude or even falls due to orthostasis.
A sensible approach is to keep the drugs that may still contribute to quality of life, while discontinuing other drugs that may be causing side effects or that are unlikely to provide meaningful benefit in terms of prevention in patients who have limited life expectancy.29 Discontinuing ineffective, poorly tolerated, and duplicated medications also makes it easier to introduce new medications to manage symptoms—there will be fewer drug interactions, and fewer pills to take, an increasingly important issue in the setting of gastrointestinal symptoms such as dysphagia and gastroparesis or compliance issues as frequently encountered when cognitive impairment is present.29,30
In managing symptoms, start low and go slow—but get there
In managing symptoms in frail elderly patients we use the same classes of medications as in younger patients. The trick is to use appropriate doses.
The concept of “start low and go slow” is key, but so is “get there”—ie, reach the therapeutic goal. The principal drugs for symptom control, such as opioids for pain and dyspnea and anxiolytics for anxiety and restlessness, are associated with a higher rate of and more severe adverse effects in frail older adults.
Even so, most frail older adults appear to be undertreated in this regard, particularly if they are cognitively impaired.23 This fact, coupled with the reality that behavioral symptoms associated with advanced dementia can represent unmet care needs including undertreated pain, highlights the critical need to control symptoms optimally in frail seniors.
This is particularly relevant for those who can no longer verbally articulate their symptoms. Nonverbal pain scales and vigilant assessment of behavioral signs of pain are paramount skills for clinicians providing palliative care to patients with cognitive impairment. Caregivers and loved ones should be included in the assessment process.26,31
Adjunctive therapies for pain control
Maximizing adjunctive therapies can optimize pain control in this drug-sensitive population. Heat and cold packs to affected areas, acupuncture, massage therapy, and structured exercise regimens are some options that can improve quality of life. Cognitive behavioral therapy may offer coping strategies, provided the patient can participate in this process from a cognitive perspective.26,27 Topical preparations are often well tolerated and may include medicinal ingredients that are helpful without systemic effects, such as anti-inflammatory drugs or analgesics.
Optimal use of nonopioid drugs may help reduce the need for narcotics, particularly in the presence of musculoskeletal pain. An example is acetaminophen in regular doses—we would recommend no more than 3 g per day. Acetaminophen is preferred for older adults rather than nonsteroidal anti-inflammatory drugs, given the potential gastric and cardiovascular side effects of the latter medications.
Antidepressants and anticonvulsants such as gabapentin can also be considered as adjuncts for pain control, particularly in the setting of neuropathic pain, with careful monitoring for tolerance.25
When opioids are used, vigilance for constipation is essential.
Establishing goals of care
Goals of care need to be established incrementally along the course of clinical decline and as early as possible so that palliative support can be promptly implemented as symptoms worsen.32
End-of-life care can still include treatments with curative intent, depending on the overall prognosis and the state of the underlying terminal illness. On the other hand, frail older adults who are subjected to invasive treatments that are unlikely to provide cure, such as Whipple surgery, need special intervention postoperatively if they are not to suffer complications such as persisting delirium and functional decline.33
In this regard, geriatric palliative care is frequently about not “crossing a threshold.” Patients may still be receiving active management and be hospitalized for acute exacerbations of progressing chronic conditions, such as chronic obstructive pulmonary disease and heart failure, while palliative principles are introduced and increasingly become the focus of care.
To align goals of care with frailty burden, it is crucial to quantify frailty and to review the patient’s comorbidities. Particularly when dementia is present, lack of communication between the patient’s doctors or between the doctor and the family about disease burden can lead to inappropriately aggressive care.
Many family members and even clinicians do not recognize that advanced dementia is terminal.34,35 In light of this, a palliative approach to care may not even be considered as an appropriate plan when hallmark complications associated with progressing cognitive decline occur, such as aspiration pneumonia or dehydration. Education about dementia and other conditions with progressive organ failure should be done as soon as possible after diagnosis and readdressed at intervals throughout the patient’s clinical decline.
Earlier discussions also ensure that patients themselves can be involved in decision-making more often before cognitive impairment advances to the point where proxy discussions take over.16
BETTER PALLIATIVE CARE FOR ALL
Palliative care, developed initially to provide holistic and timely symptom-based care for patients with noncurable cancer, should also be available and offered to patients with nonmalignant, life-limiting diseases.23,36,37 Meeting this standard of geriatric care is not easy, given the burden of frailty in this population. Needed are multimodal palliative efforts across the spectrum of settings, from the home to the hospital and nursing home.23
To do this, we need to embrace the complexity posed by each person’s presentation and view care through the frailty lens. This will give us a common language in which to engage in a conversation with the same goal in mind: optimizing quality of life.
Furthermore, quantifying frailty can help minimize interventions that are futile or burdensome, that are not expected to ease symptoms, and that can worsen cognition and function. At the end of a patient’s life, we do not want to add to his or her frailty burden but rather minimize the morbidity associated with it.
The concept of frailty assessment is therefore essential for the timely delivery of holistic palliative care in geriatric patients who have progressive and ultimately terminal conditions.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Michel JP, Bonin-Guillame S, Gold G, Herrmann F. Cognition and frailty: possible interrelations. In:Carey JR, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Berlin Heidelberg: Springer; 2005:119–124. Research and Perspectives in Longevity.
- Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med 2005; 72:1105–1112.
- Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can’t do anymore that I wish I could . . . “. JAMA 2006; 296:2245–2253.
- Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–286.
- McDermid RC, Bagshaw SM. ICU and critical care outreach for the elderly. Best Pract Res Clin Anaesthesiol 2011; 25:439–449.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ 1994; 150:489–495.
- Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011; 78:138–149.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 2008; 3:e2232.
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9:71–72.
- Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res 2011; 23:209–216.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11:344–355.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011; 15:301.
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56:898–903.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127:494–496.
- Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010; 58:318–323.
- Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127:840–848.
- Davies E, Higginson IJ; WHO Europe. Better Palliative Care for Older People. Milan, Italy: Tipolitografia Trabella Sr; 2004.
- Raudonis BM, Daniel K. Frailty: an indication for palliative care. Geriatr Nurs 2010; 31:379–384.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1436.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6):S205–S224.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012; 60:113–117.
- Hanlon JT, Perera S, Sevick MA, Rodriguez KL, Jaffe EJ. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc 2010; 11:579–583.
- Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc 2007; 55:590–595.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med 2007; 10:185–209.
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer 2012; 20:507–513.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213:245–252.
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538.
- Arcand M, Monette J, Monette M, et al. Educating nursing home staff about the progression of dementia and the comfort care option: impact on family satisfaction with end-of-life care. J Am Med Dir Assoc 2009; 10:50–55.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164:321–326.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17:1144–1163.
As people get older, they have more things wrong with them. And the more things they have wrong with them, the more likely they are to die. But everyone accumulates deficits at a different rate, and not all people of the same age have the same short-term risk of dying. This variable susceptibility to death and other adverse outcomes in older people of the same age is called frailty.1
Frailty poses special challenges to how we organize and deliver health care. These challenges are sometimes seen most starkly when people are most frail, especially as they approach the end of life.
In this paper, we will review how frailty is conceptualized and defined, consider how frailty affects the care of people at the end of their lives, and suggest practices that can make end-of-life care better for frail older adults.
DEFINING FRAILTY
As with all complex systems, when frail people become acutely unwell their highest-order functions fail first. Thus, cognitive impairment, functional decline, impaired mobility, and social withdrawal are hallmark presentations of the further accumulation of deficits in vulnerable seniors.
Delirium and falls are important clues that a person’s resilience is becoming compromised and that the person is at risk of further insults in a downward spiral or acceleration of things going wrong.1,2 Frailty is associated with poor health outcomes, from disability to institutionalization and death.3
This idea of frailty as vulnerability arising from dysregulation of multiple physiologic systems is reasonably non-controversial. Even so, there are competing views on how to systematically quantify those who are at an increased risk of adverse sequelae.
Quantifying frailty is particularly important if it can tell us if a patient is at high risk of further decline and death. As frailty advances, it is appropriate to shift the focus of care to palliation, with the goal of optimizing quality of life and easing symptoms.4 Identifying someone as frail can aid decision-making in the setting of critical illness, where the system commonly defaults to an “always do everything” mode without considering the ramifications of such an approach. Furthermore, without a routine means of measuring frailty, it is often left to critical care units or rapid-response medical teams to initiate a discussion about whether an aggressive course of care is appropriate or desired.5,6
Frailty as a syndrome
Fried et al7 defined frailty as a syndrome arising from the “physiologic triad” of sarcopenia and immune and neuroendocrine dysregulation. Patients are considered frail if they have three or more of the following five criteria:
- Reduced activity
- Slowing of mobility
- Weight loss
- Diminished handgrip strength
- Exhaustion.
Someone who has only one or two of these items is said to be “pre-frail”; someone with none is said to be “robust.”
The frailty index
An alternative viewpoint is that frailty is a state arising from the accumulation of deficits, which can be counted in a frailty index.
The frailty index is based on the concept that frailty is a consequence of interacting physical, psychological, and social factors. As deficits accumulate, people become increasingly vulnerable to adverse outcomes.
The frailty index is calculated as the number of deficits the patient has, divided by the number of deficits considered. For example, in a frailty index based on a comprehensive geriatric assessment, an individual with impairments in 4 of 10 domains and with 10 of 24 possible comorbidities would have 14 of 34 possible deficits, for a frailty index of 0.41.8
A criticism of the frailty index is that it includes functional dependence as a deficit. The criticism stems from the view that frailty should be seen as occurring prior to disability. According to this view, including dependence in instrumental and basic activities of daily living as a deficit confuses disability with frailty.
Proponents of the frailty index counter that frailty is not “all or none” and needs to be graded. The frailty index can distinguish between people with and without disability by means of the number of deficits that they have, which is most important. For example, a person disabled by a paraplegic injury would have a lower frailty index score and therefore would be considered less frail than a person with advanced cancer affecting multiple body systems. (This is assuming the person who has suffered the injury resulting in paraplegia doesn’t have a concomitant condition such as renal failure or heart disease. In the absence of other health insults, such patients are less at risk of further morbidity or death than the patient with advanced cancer until they get another health insult or insults added to their frailty.)
In any case, functional capacity is fundamental in medical decision-making and when estimating prognoses. An example is the use of the Eastern Cooperative Oncology Group’s functional status measure.9,10
Sum of physical and psychological stressors
Consensus is growing for the concept that frail people are made more vulnerable by the combination of both physical and psychological stressors. This is particularly important to bear in mind for patients who may appear physically robust but whose total health burden makes them vulnerable to further insults.
For example, think of a relatively young overweight patient with hypertension, diabetes, dyslipidemia, and ischemic white matter changes (which can manifest as low mood and even mild vascular cognitive impairment). In such a patient, an acute illness could result in cognitive and functional decline that can be permanent.
Balance of assets and deficits
About 20 years ago, we used the metaphor of a balance beam to describe how frailty comes about in older adults. In this view, there is an interplay of physiological and functional health determinants. Assets such as health, resources, and caregivers are balanced against deficits such as illnesses, dependency on others, and support burden.8
For the most part, later concepts of frailty have focused on the individual, with social factors construed separately as social vulnerability.11
Tools for assessing frailty in people who are not yet disabled
Several tools exist to clinically assess frailty in people who are not yet disabled.
The FRAIL scale.12 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging formulated a scale for measuring frailty as a “pre-disability state.” The FRAIL scale consists of five easily remembered items:
- Fatigue
- Resistance (inability to climb one flight of stairs)
- Ambulation (inability to walk one block)
- Illnesses (more than five)
- Loss of weight (> 5%).
Like the “reduced activity” criterion of the frailty syndrome mentioned above (in practical terms, described as the inability to do heavy household chores),13 the FRAIL scale seems to blur the distinction between disability (here, the inability to climb stairs or to walk a block) and “pre-disability,” to an uncertain end. It also seems to blend the notion of a state and a syndrome; these points will need to be clarified in due course.
The Tilburg Frailty Indicator14 was constructed around the multidimensional viewpoint of frailty, beyond disease or disability state, to identify frail community-dwelling older individuals. The first part of this two-part questionnaire consists of 10 questions on frailty determinants and medical comorbidities, while the second part contains physical, psychological, and social variables strongly associated with frailty, as well as information about disability in walking and balance. Interestingly, although it includes both social and physical factors, it does not include cognition.
The Clinical Frailty Scale was developed as a practical approach to assess frailty using physical and functional indicators of health and illness burden. The descriptors for this 7-point scale guide clinicians in quantifying the degree of frailty present. It ranges from 1 (very fit) to 7 (severely frail).7 The higher the score, the higher the risks of death or institutionalization. Even mild frailty is associated with a 50% 5-year mortality rate in community-dwelling older adults (Figure 1).8
The Edmonton Frail Scale,15 like the Clinical Frailty Scale, was developed to be practical and usable at the bedside. It is based on the following domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance.
In a community-based sample, the Edmonton Frail Scale compared favorably with the clinical assessment of geriatric specialists who completed a comprehensive evaluation (Pearson’s correlation coefficient 0.64, P < .001).15
FRAILTY AS A PROGNOSTIC INDICATOR
Using frailty scales to aid in prognostication can be useful to clinicians. Survival prognostication is inherently challenging in individuals with multiple comorbidities and variable trajectories of decline, but it remains a vital clinical skill for all clinicians. Framing these difficult discussions in the context of degree of frailty provides a unifying concept, beyond a single-system construct, for care providers, patients, and their loved ones.
Patients nearing the end of their lives need this kind of clarity and support. Regardless of their diagnoses, patients typically want to know when they are at high risk of dying, as do their families and caregivers. People in general look for such information so that they can align medical decision-making congruently with predicted prognosis.16,17 They also use it to plan for the final chapter of their life and their death.
The frailty index is strongly correlated with risk of death
The frailty index is strongly correlated with the risk of death, with a correlation coefficient greater than 0.95. As such, an individual’s frailty index score is considered an estimate of biologic age, which has greater correlation with associated morbidity and death than does chronological age.18,19 In the general population, more than 99% of people have a frailty index value of less than 0.7. As people approach this value, the chance of survival is greatly diminished; indeed, one report suggested that of those who have a frailty index value of more than 0.5 (based on a comprehensive geriatric assessment), 100% are dead by about 20 months later.20,21
In short, there is a limit to which deficits can be added before the system fails. In this sense, the frailty index is akin to the concept of physiologic reserve. Reserve is finite, and as a system loses redundancy it can no longer survive new stresses.
What does this information mean for individual patients?
Even so, prognostication for individual patients remains probabilistic. Any patient has a chance to improve, stabilize, worsen, or die. However, a patient can reach an upper limit of frailty. At that point, instead of accumulating another deficit, death is much more likely. Similarly, although improvement can happen, the chance of improvement is low, and the improvement is typically modest.
Framing survival possibilities in terms of the number of things that people have wrong with them and the chance of death or of change (and the extent of change) makes sense to physicians, patients, and families. Being able to do so offers a much greater opportunity for realistic discussions of the likely outcomes of medical care than the foreseeable scenario of a junior doctor asking a senior citizen, “If your heart stops, do you want us to save your life?”
Understanding prognosis in the face of not just disease but also frailty can also help us focus not on disease but on health consequences of illness. Can the person think? Walk? Care for herself or himself? Interact with others? These questions need to be considered when end-of-life decisions are being discussed.22
Since making predictions about survival is most challenging when multiple comorbidities are present, using the concept of accumulating deficits to better define the slope of decline can be very helpful when discussing “the road ahead” with patients and their families. Visually mapping out the slope of decline and how it is accelerating as conditions progress and deficits accumulate can aid in medical decision-making. Looking individually at the deficits themselves and associated markers of progression can also help with prognostic discussions.
For example, a patient with chronic obstructive pulmonary disease could very well be unaware of the progression and ultimately terminal prognosis of this disease. The slope of clinical decline can be initially shallow, with saw-tooth fluctuations from acute exacerbations that seemingly “resolve to baseline” when antibiotic and steroid courses are completed. Talking with these patients and their families about heralding markers, such as more hospitalizations and cognitive decline with acute exacerbations, can clarify the steepening slope of decline and the way comorbidities interact.
FRAILTY AND END-OF-LIFE CARE
Frailty is progressive, and as it worsens, integrating a palliative approach is appropriate, with a focus on optimizing quality of life and relieving symptoms.4 This principle holds true regardless of the care setting, from acute care hospitals to hospice facilities and long-term care residences.
The principles of end-of-life care are applicable to frail individuals with progressive conditions from the time of diagnosis throughout the course of decline. As the population ages, more people suffer and die from progressive chronic conditions such as cerebrovascular disease, respiratory disease, and dementia.23 An interdisciplinary team approach can ensure all components of palliation are effectively delivered, such as easing symptoms, providing psychosocial and spiritual support, and improving quality of life.24
Pain management
Pain is widely underassessed and undertreated in older patients. Its management at the end of life is particularly challenging if the patient’s language is compromised, as in dementia.23,25,26
A recent cross-sectional analysis of self-reported pain in a longitudinal study of community-dwelling older adults showed an independent association between moderate or higher pain and frailty. The authors propose that persistent pain goes beyond physical discomfort in that it may contribute to homeostenosis (progressive diminishment of homeostatic reserve) and directly worsen frailty.27
Examine the medication list
In palliative care, medical interventions focus on optimizing quality of life.
This especially includes reexamining long medication lists that increase the chance of adverse drug effects.28 Many patients are on disease-modifying medications that may or may not help control symptoms—and might well exacerbate them. For example, betablockers for ischemic heart disease and angiotensin-converting enzyme inhibitors for diabetic nephropathy both can cause hypotension-induced lassitude or even falls due to orthostasis.
A sensible approach is to keep the drugs that may still contribute to quality of life, while discontinuing other drugs that may be causing side effects or that are unlikely to provide meaningful benefit in terms of prevention in patients who have limited life expectancy.29 Discontinuing ineffective, poorly tolerated, and duplicated medications also makes it easier to introduce new medications to manage symptoms—there will be fewer drug interactions, and fewer pills to take, an increasingly important issue in the setting of gastrointestinal symptoms such as dysphagia and gastroparesis or compliance issues as frequently encountered when cognitive impairment is present.29,30
In managing symptoms, start low and go slow—but get there
In managing symptoms in frail elderly patients we use the same classes of medications as in younger patients. The trick is to use appropriate doses.
The concept of “start low and go slow” is key, but so is “get there”—ie, reach the therapeutic goal. The principal drugs for symptom control, such as opioids for pain and dyspnea and anxiolytics for anxiety and restlessness, are associated with a higher rate of and more severe adverse effects in frail older adults.
Even so, most frail older adults appear to be undertreated in this regard, particularly if they are cognitively impaired.23 This fact, coupled with the reality that behavioral symptoms associated with advanced dementia can represent unmet care needs including undertreated pain, highlights the critical need to control symptoms optimally in frail seniors.
This is particularly relevant for those who can no longer verbally articulate their symptoms. Nonverbal pain scales and vigilant assessment of behavioral signs of pain are paramount skills for clinicians providing palliative care to patients with cognitive impairment. Caregivers and loved ones should be included in the assessment process.26,31
Adjunctive therapies for pain control
Maximizing adjunctive therapies can optimize pain control in this drug-sensitive population. Heat and cold packs to affected areas, acupuncture, massage therapy, and structured exercise regimens are some options that can improve quality of life. Cognitive behavioral therapy may offer coping strategies, provided the patient can participate in this process from a cognitive perspective.26,27 Topical preparations are often well tolerated and may include medicinal ingredients that are helpful without systemic effects, such as anti-inflammatory drugs or analgesics.
Optimal use of nonopioid drugs may help reduce the need for narcotics, particularly in the presence of musculoskeletal pain. An example is acetaminophen in regular doses—we would recommend no more than 3 g per day. Acetaminophen is preferred for older adults rather than nonsteroidal anti-inflammatory drugs, given the potential gastric and cardiovascular side effects of the latter medications.
Antidepressants and anticonvulsants such as gabapentin can also be considered as adjuncts for pain control, particularly in the setting of neuropathic pain, with careful monitoring for tolerance.25
When opioids are used, vigilance for constipation is essential.
Establishing goals of care
Goals of care need to be established incrementally along the course of clinical decline and as early as possible so that palliative support can be promptly implemented as symptoms worsen.32
End-of-life care can still include treatments with curative intent, depending on the overall prognosis and the state of the underlying terminal illness. On the other hand, frail older adults who are subjected to invasive treatments that are unlikely to provide cure, such as Whipple surgery, need special intervention postoperatively if they are not to suffer complications such as persisting delirium and functional decline.33
In this regard, geriatric palliative care is frequently about not “crossing a threshold.” Patients may still be receiving active management and be hospitalized for acute exacerbations of progressing chronic conditions, such as chronic obstructive pulmonary disease and heart failure, while palliative principles are introduced and increasingly become the focus of care.
To align goals of care with frailty burden, it is crucial to quantify frailty and to review the patient’s comorbidities. Particularly when dementia is present, lack of communication between the patient’s doctors or between the doctor and the family about disease burden can lead to inappropriately aggressive care.
Many family members and even clinicians do not recognize that advanced dementia is terminal.34,35 In light of this, a palliative approach to care may not even be considered as an appropriate plan when hallmark complications associated with progressing cognitive decline occur, such as aspiration pneumonia or dehydration. Education about dementia and other conditions with progressive organ failure should be done as soon as possible after diagnosis and readdressed at intervals throughout the patient’s clinical decline.
Earlier discussions also ensure that patients themselves can be involved in decision-making more often before cognitive impairment advances to the point where proxy discussions take over.16
BETTER PALLIATIVE CARE FOR ALL
Palliative care, developed initially to provide holistic and timely symptom-based care for patients with noncurable cancer, should also be available and offered to patients with nonmalignant, life-limiting diseases.23,36,37 Meeting this standard of geriatric care is not easy, given the burden of frailty in this population. Needed are multimodal palliative efforts across the spectrum of settings, from the home to the hospital and nursing home.23
To do this, we need to embrace the complexity posed by each person’s presentation and view care through the frailty lens. This will give us a common language in which to engage in a conversation with the same goal in mind: optimizing quality of life.
Furthermore, quantifying frailty can help minimize interventions that are futile or burdensome, that are not expected to ease symptoms, and that can worsen cognition and function. At the end of a patient’s life, we do not want to add to his or her frailty burden but rather minimize the morbidity associated with it.
The concept of frailty assessment is therefore essential for the timely delivery of holistic palliative care in geriatric patients who have progressive and ultimately terminal conditions.
As people get older, they have more things wrong with them. And the more things they have wrong with them, the more likely they are to die. But everyone accumulates deficits at a different rate, and not all people of the same age have the same short-term risk of dying. This variable susceptibility to death and other adverse outcomes in older people of the same age is called frailty.1
Frailty poses special challenges to how we organize and deliver health care. These challenges are sometimes seen most starkly when people are most frail, especially as they approach the end of life.
In this paper, we will review how frailty is conceptualized and defined, consider how frailty affects the care of people at the end of their lives, and suggest practices that can make end-of-life care better for frail older adults.
DEFINING FRAILTY
As with all complex systems, when frail people become acutely unwell their highest-order functions fail first. Thus, cognitive impairment, functional decline, impaired mobility, and social withdrawal are hallmark presentations of the further accumulation of deficits in vulnerable seniors.
Delirium and falls are important clues that a person’s resilience is becoming compromised and that the person is at risk of further insults in a downward spiral or acceleration of things going wrong.1,2 Frailty is associated with poor health outcomes, from disability to institutionalization and death.3
This idea of frailty as vulnerability arising from dysregulation of multiple physiologic systems is reasonably non-controversial. Even so, there are competing views on how to systematically quantify those who are at an increased risk of adverse sequelae.
Quantifying frailty is particularly important if it can tell us if a patient is at high risk of further decline and death. As frailty advances, it is appropriate to shift the focus of care to palliation, with the goal of optimizing quality of life and easing symptoms.4 Identifying someone as frail can aid decision-making in the setting of critical illness, where the system commonly defaults to an “always do everything” mode without considering the ramifications of such an approach. Furthermore, without a routine means of measuring frailty, it is often left to critical care units or rapid-response medical teams to initiate a discussion about whether an aggressive course of care is appropriate or desired.5,6
Frailty as a syndrome
Fried et al7 defined frailty as a syndrome arising from the “physiologic triad” of sarcopenia and immune and neuroendocrine dysregulation. Patients are considered frail if they have three or more of the following five criteria:
- Reduced activity
- Slowing of mobility
- Weight loss
- Diminished handgrip strength
- Exhaustion.
Someone who has only one or two of these items is said to be “pre-frail”; someone with none is said to be “robust.”
The frailty index
An alternative viewpoint is that frailty is a state arising from the accumulation of deficits, which can be counted in a frailty index.
The frailty index is based on the concept that frailty is a consequence of interacting physical, psychological, and social factors. As deficits accumulate, people become increasingly vulnerable to adverse outcomes.
The frailty index is calculated as the number of deficits the patient has, divided by the number of deficits considered. For example, in a frailty index based on a comprehensive geriatric assessment, an individual with impairments in 4 of 10 domains and with 10 of 24 possible comorbidities would have 14 of 34 possible deficits, for a frailty index of 0.41.8
A criticism of the frailty index is that it includes functional dependence as a deficit. The criticism stems from the view that frailty should be seen as occurring prior to disability. According to this view, including dependence in instrumental and basic activities of daily living as a deficit confuses disability with frailty.
Proponents of the frailty index counter that frailty is not “all or none” and needs to be graded. The frailty index can distinguish between people with and without disability by means of the number of deficits that they have, which is most important. For example, a person disabled by a paraplegic injury would have a lower frailty index score and therefore would be considered less frail than a person with advanced cancer affecting multiple body systems. (This is assuming the person who has suffered the injury resulting in paraplegia doesn’t have a concomitant condition such as renal failure or heart disease. In the absence of other health insults, such patients are less at risk of further morbidity or death than the patient with advanced cancer until they get another health insult or insults added to their frailty.)
In any case, functional capacity is fundamental in medical decision-making and when estimating prognoses. An example is the use of the Eastern Cooperative Oncology Group’s functional status measure.9,10
Sum of physical and psychological stressors
Consensus is growing for the concept that frail people are made more vulnerable by the combination of both physical and psychological stressors. This is particularly important to bear in mind for patients who may appear physically robust but whose total health burden makes them vulnerable to further insults.
For example, think of a relatively young overweight patient with hypertension, diabetes, dyslipidemia, and ischemic white matter changes (which can manifest as low mood and even mild vascular cognitive impairment). In such a patient, an acute illness could result in cognitive and functional decline that can be permanent.
Balance of assets and deficits
About 20 years ago, we used the metaphor of a balance beam to describe how frailty comes about in older adults. In this view, there is an interplay of physiological and functional health determinants. Assets such as health, resources, and caregivers are balanced against deficits such as illnesses, dependency on others, and support burden.8
For the most part, later concepts of frailty have focused on the individual, with social factors construed separately as social vulnerability.11
Tools for assessing frailty in people who are not yet disabled
Several tools exist to clinically assess frailty in people who are not yet disabled.
The FRAIL scale.12 The Geriatric Advisory Panel of the International Academy of Nutrition and Aging formulated a scale for measuring frailty as a “pre-disability state.” The FRAIL scale consists of five easily remembered items:
- Fatigue
- Resistance (inability to climb one flight of stairs)
- Ambulation (inability to walk one block)
- Illnesses (more than five)
- Loss of weight (> 5%).
Like the “reduced activity” criterion of the frailty syndrome mentioned above (in practical terms, described as the inability to do heavy household chores),13 the FRAIL scale seems to blur the distinction between disability (here, the inability to climb stairs or to walk a block) and “pre-disability,” to an uncertain end. It also seems to blend the notion of a state and a syndrome; these points will need to be clarified in due course.
The Tilburg Frailty Indicator14 was constructed around the multidimensional viewpoint of frailty, beyond disease or disability state, to identify frail community-dwelling older individuals. The first part of this two-part questionnaire consists of 10 questions on frailty determinants and medical comorbidities, while the second part contains physical, psychological, and social variables strongly associated with frailty, as well as information about disability in walking and balance. Interestingly, although it includes both social and physical factors, it does not include cognition.
The Clinical Frailty Scale was developed as a practical approach to assess frailty using physical and functional indicators of health and illness burden. The descriptors for this 7-point scale guide clinicians in quantifying the degree of frailty present. It ranges from 1 (very fit) to 7 (severely frail).7 The higher the score, the higher the risks of death or institutionalization. Even mild frailty is associated with a 50% 5-year mortality rate in community-dwelling older adults (Figure 1).8
The Edmonton Frail Scale,15 like the Clinical Frailty Scale, was developed to be practical and usable at the bedside. It is based on the following domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and functional performance.
In a community-based sample, the Edmonton Frail Scale compared favorably with the clinical assessment of geriatric specialists who completed a comprehensive evaluation (Pearson’s correlation coefficient 0.64, P < .001).15
FRAILTY AS A PROGNOSTIC INDICATOR
Using frailty scales to aid in prognostication can be useful to clinicians. Survival prognostication is inherently challenging in individuals with multiple comorbidities and variable trajectories of decline, but it remains a vital clinical skill for all clinicians. Framing these difficult discussions in the context of degree of frailty provides a unifying concept, beyond a single-system construct, for care providers, patients, and their loved ones.
Patients nearing the end of their lives need this kind of clarity and support. Regardless of their diagnoses, patients typically want to know when they are at high risk of dying, as do their families and caregivers. People in general look for such information so that they can align medical decision-making congruently with predicted prognosis.16,17 They also use it to plan for the final chapter of their life and their death.
The frailty index is strongly correlated with risk of death
The frailty index is strongly correlated with the risk of death, with a correlation coefficient greater than 0.95. As such, an individual’s frailty index score is considered an estimate of biologic age, which has greater correlation with associated morbidity and death than does chronological age.18,19 In the general population, more than 99% of people have a frailty index value of less than 0.7. As people approach this value, the chance of survival is greatly diminished; indeed, one report suggested that of those who have a frailty index value of more than 0.5 (based on a comprehensive geriatric assessment), 100% are dead by about 20 months later.20,21
In short, there is a limit to which deficits can be added before the system fails. In this sense, the frailty index is akin to the concept of physiologic reserve. Reserve is finite, and as a system loses redundancy it can no longer survive new stresses.
What does this information mean for individual patients?
Even so, prognostication for individual patients remains probabilistic. Any patient has a chance to improve, stabilize, worsen, or die. However, a patient can reach an upper limit of frailty. At that point, instead of accumulating another deficit, death is much more likely. Similarly, although improvement can happen, the chance of improvement is low, and the improvement is typically modest.
Framing survival possibilities in terms of the number of things that people have wrong with them and the chance of death or of change (and the extent of change) makes sense to physicians, patients, and families. Being able to do so offers a much greater opportunity for realistic discussions of the likely outcomes of medical care than the foreseeable scenario of a junior doctor asking a senior citizen, “If your heart stops, do you want us to save your life?”
Understanding prognosis in the face of not just disease but also frailty can also help us focus not on disease but on health consequences of illness. Can the person think? Walk? Care for herself or himself? Interact with others? These questions need to be considered when end-of-life decisions are being discussed.22
Since making predictions about survival is most challenging when multiple comorbidities are present, using the concept of accumulating deficits to better define the slope of decline can be very helpful when discussing “the road ahead” with patients and their families. Visually mapping out the slope of decline and how it is accelerating as conditions progress and deficits accumulate can aid in medical decision-making. Looking individually at the deficits themselves and associated markers of progression can also help with prognostic discussions.
For example, a patient with chronic obstructive pulmonary disease could very well be unaware of the progression and ultimately terminal prognosis of this disease. The slope of clinical decline can be initially shallow, with saw-tooth fluctuations from acute exacerbations that seemingly “resolve to baseline” when antibiotic and steroid courses are completed. Talking with these patients and their families about heralding markers, such as more hospitalizations and cognitive decline with acute exacerbations, can clarify the steepening slope of decline and the way comorbidities interact.
FRAILTY AND END-OF-LIFE CARE
Frailty is progressive, and as it worsens, integrating a palliative approach is appropriate, with a focus on optimizing quality of life and relieving symptoms.4 This principle holds true regardless of the care setting, from acute care hospitals to hospice facilities and long-term care residences.
The principles of end-of-life care are applicable to frail individuals with progressive conditions from the time of diagnosis throughout the course of decline. As the population ages, more people suffer and die from progressive chronic conditions such as cerebrovascular disease, respiratory disease, and dementia.23 An interdisciplinary team approach can ensure all components of palliation are effectively delivered, such as easing symptoms, providing psychosocial and spiritual support, and improving quality of life.24
Pain management
Pain is widely underassessed and undertreated in older patients. Its management at the end of life is particularly challenging if the patient’s language is compromised, as in dementia.23,25,26
A recent cross-sectional analysis of self-reported pain in a longitudinal study of community-dwelling older adults showed an independent association between moderate or higher pain and frailty. The authors propose that persistent pain goes beyond physical discomfort in that it may contribute to homeostenosis (progressive diminishment of homeostatic reserve) and directly worsen frailty.27
Examine the medication list
In palliative care, medical interventions focus on optimizing quality of life.
This especially includes reexamining long medication lists that increase the chance of adverse drug effects.28 Many patients are on disease-modifying medications that may or may not help control symptoms—and might well exacerbate them. For example, betablockers for ischemic heart disease and angiotensin-converting enzyme inhibitors for diabetic nephropathy both can cause hypotension-induced lassitude or even falls due to orthostasis.
A sensible approach is to keep the drugs that may still contribute to quality of life, while discontinuing other drugs that may be causing side effects or that are unlikely to provide meaningful benefit in terms of prevention in patients who have limited life expectancy.29 Discontinuing ineffective, poorly tolerated, and duplicated medications also makes it easier to introduce new medications to manage symptoms—there will be fewer drug interactions, and fewer pills to take, an increasingly important issue in the setting of gastrointestinal symptoms such as dysphagia and gastroparesis or compliance issues as frequently encountered when cognitive impairment is present.29,30
In managing symptoms, start low and go slow—but get there
In managing symptoms in frail elderly patients we use the same classes of medications as in younger patients. The trick is to use appropriate doses.
The concept of “start low and go slow” is key, but so is “get there”—ie, reach the therapeutic goal. The principal drugs for symptom control, such as opioids for pain and dyspnea and anxiolytics for anxiety and restlessness, are associated with a higher rate of and more severe adverse effects in frail older adults.
Even so, most frail older adults appear to be undertreated in this regard, particularly if they are cognitively impaired.23 This fact, coupled with the reality that behavioral symptoms associated with advanced dementia can represent unmet care needs including undertreated pain, highlights the critical need to control symptoms optimally in frail seniors.
This is particularly relevant for those who can no longer verbally articulate their symptoms. Nonverbal pain scales and vigilant assessment of behavioral signs of pain are paramount skills for clinicians providing palliative care to patients with cognitive impairment. Caregivers and loved ones should be included in the assessment process.26,31
Adjunctive therapies for pain control
Maximizing adjunctive therapies can optimize pain control in this drug-sensitive population. Heat and cold packs to affected areas, acupuncture, massage therapy, and structured exercise regimens are some options that can improve quality of life. Cognitive behavioral therapy may offer coping strategies, provided the patient can participate in this process from a cognitive perspective.26,27 Topical preparations are often well tolerated and may include medicinal ingredients that are helpful without systemic effects, such as anti-inflammatory drugs or analgesics.
Optimal use of nonopioid drugs may help reduce the need for narcotics, particularly in the presence of musculoskeletal pain. An example is acetaminophen in regular doses—we would recommend no more than 3 g per day. Acetaminophen is preferred for older adults rather than nonsteroidal anti-inflammatory drugs, given the potential gastric and cardiovascular side effects of the latter medications.
Antidepressants and anticonvulsants such as gabapentin can also be considered as adjuncts for pain control, particularly in the setting of neuropathic pain, with careful monitoring for tolerance.25
When opioids are used, vigilance for constipation is essential.
Establishing goals of care
Goals of care need to be established incrementally along the course of clinical decline and as early as possible so that palliative support can be promptly implemented as symptoms worsen.32
End-of-life care can still include treatments with curative intent, depending on the overall prognosis and the state of the underlying terminal illness. On the other hand, frail older adults who are subjected to invasive treatments that are unlikely to provide cure, such as Whipple surgery, need special intervention postoperatively if they are not to suffer complications such as persisting delirium and functional decline.33
In this regard, geriatric palliative care is frequently about not “crossing a threshold.” Patients may still be receiving active management and be hospitalized for acute exacerbations of progressing chronic conditions, such as chronic obstructive pulmonary disease and heart failure, while palliative principles are introduced and increasingly become the focus of care.
To align goals of care with frailty burden, it is crucial to quantify frailty and to review the patient’s comorbidities. Particularly when dementia is present, lack of communication between the patient’s doctors or between the doctor and the family about disease burden can lead to inappropriately aggressive care.
Many family members and even clinicians do not recognize that advanced dementia is terminal.34,35 In light of this, a palliative approach to care may not even be considered as an appropriate plan when hallmark complications associated with progressing cognitive decline occur, such as aspiration pneumonia or dehydration. Education about dementia and other conditions with progressive organ failure should be done as soon as possible after diagnosis and readdressed at intervals throughout the patient’s clinical decline.
Earlier discussions also ensure that patients themselves can be involved in decision-making more often before cognitive impairment advances to the point where proxy discussions take over.16
BETTER PALLIATIVE CARE FOR ALL
Palliative care, developed initially to provide holistic and timely symptom-based care for patients with noncurable cancer, should also be available and offered to patients with nonmalignant, life-limiting diseases.23,36,37 Meeting this standard of geriatric care is not easy, given the burden of frailty in this population. Needed are multimodal palliative efforts across the spectrum of settings, from the home to the hospital and nursing home.23
To do this, we need to embrace the complexity posed by each person’s presentation and view care through the frailty lens. This will give us a common language in which to engage in a conversation with the same goal in mind: optimizing quality of life.
Furthermore, quantifying frailty can help minimize interventions that are futile or burdensome, that are not expected to ease symptoms, and that can worsen cognition and function. At the end of a patient’s life, we do not want to add to his or her frailty burden but rather minimize the morbidity associated with it.
The concept of frailty assessment is therefore essential for the timely delivery of holistic palliative care in geriatric patients who have progressive and ultimately terminal conditions.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Michel JP, Bonin-Guillame S, Gold G, Herrmann F. Cognition and frailty: possible interrelations. In:Carey JR, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Berlin Heidelberg: Springer; 2005:119–124. Research and Perspectives in Longevity.
- Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med 2005; 72:1105–1112.
- Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can’t do anymore that I wish I could . . . “. JAMA 2006; 296:2245–2253.
- Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–286.
- McDermid RC, Bagshaw SM. ICU and critical care outreach for the elderly. Best Pract Res Clin Anaesthesiol 2011; 25:439–449.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ 1994; 150:489–495.
- Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011; 78:138–149.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 2008; 3:e2232.
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9:71–72.
- Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res 2011; 23:209–216.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11:344–355.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011; 15:301.
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56:898–903.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127:494–496.
- Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010; 58:318–323.
- Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127:840–848.
- Davies E, Higginson IJ; WHO Europe. Better Palliative Care for Older People. Milan, Italy: Tipolitografia Trabella Sr; 2004.
- Raudonis BM, Daniel K. Frailty: an indication for palliative care. Geriatr Nurs 2010; 31:379–384.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1436.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6):S205–S224.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012; 60:113–117.
- Hanlon JT, Perera S, Sevick MA, Rodriguez KL, Jaffe EJ. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc 2010; 11:579–583.
- Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc 2007; 55:590–595.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med 2007; 10:185–209.
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer 2012; 20:507–513.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213:245–252.
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538.
- Arcand M, Monette J, Monette M, et al. Educating nursing home staff about the progression of dementia and the comfort care option: impact on family satisfaction with end-of-life care. J Am Med Dir Assoc 2009; 10:50–55.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164:321–326.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17:1144–1163.
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27:17–26.
- Michel JP, Bonin-Guillame S, Gold G, Herrmann F. Cognition and frailty: possible interrelations. In:Carey JR, Robine JM, Michel JP, Christen Y, editors. Longevity and Frailty. Berlin Heidelberg: Springer; 2005:119–124. Research and Perspectives in Longevity.
- Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med 2005; 72:1105–1112.
- Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can’t do anymore that I wish I could . . . “. JAMA 2006; 296:2245–2253.
- Abellan van Kan G, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med 2010; 26:275–286.
- McDermid RC, Bagshaw SM. ICU and critical care outreach for the elderly. Best Pract Res Clin Anaesthesiol 2011; 25:439–449.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ 1994; 150:489–495.
- Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 2011; 78:138–149.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655.
- Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS One 2008; 3:e2232.
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9:71–72.
- Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res 2011; 23:209–216.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11:344–355.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011; 15:301.
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56:898–903.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–727.
- Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev 2006; 127:494–496.
- Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010; 58:318–323.
- Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127:840–848.
- Davies E, Higginson IJ; WHO Europe. Better Palliative Care for Older People. Milan, Italy: Tipolitografia Trabella Sr; 2004.
- Raudonis BM, Daniel K. Frailty: an indication for palliative care. Geriatr Nurs 2010; 31:379–384.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1436.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6):S205–S224.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012; 60:113–117.
- Hanlon JT, Perera S, Sevick MA, Rodriguez KL, Jaffe EJ. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc 2010; 11:579–583.
- Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc 2007; 55:590–595.
- Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med 2006; 166:605–609.
- Kapo J, Morrison LJ, Liao S. Palliative care for the older adult. J Palliat Med 2007; 10:185–209.
- Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer 2012; 20:507–513.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213:245–252.
- Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361:1529–1538.
- Arcand M, Monette J, Monette M, et al. Educating nursing home staff about the progression of dementia and the comfort care option: impact on family satisfaction with end-of-life care. J Am Med Dir Assoc 2009; 10:50–55.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004; 164:321–326.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17:1144–1163.
KEY POINTS
- Frail older adults are more susceptible to delirium, functional decline, impaired mobility, falls, social withdrawal, and death.
- Evaluating the health care needs of people who are frail requires assessment of their cognition, function, mobility, balance, and social circumstances, in addition to understanding their medical problems.
- When people are so frail that they cannot withstand interventions that can cause significant injury, such as surgery or chemotherapy, then appropriate end-of-life care should focus on maintaining their highest-order functions.
- End-of-life care can include curative treatments of some episodes if they threaten cognition, mobility, or function or cause pain and suffering, even in the context of an overall palliative care plan.
A 67-year old man with an abdominal aortic aneurysm
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
Cervical cancer screening: What’s new and what’s coming?
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
KEY POINTS
- The new guidelines still recommend starting screening with cytologic (Papanicolaou) testing at age 21, but now recommend repeating the test less often, ie, every 3 years rather than every 2 years for women age 21 to 29.
- Women age 30 and older who are screened by combined cytologic and HPV testing should be rescreened every 5 years if both tests are negative (instead of every 3 years, as previously recommended). Alternatively, they can be screened by cytology alone every 3 years.
- We can stop screening women at age 65 if they have had adequate screening until then and no history of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in the past 20 years. Once screening is discontinued, it should not resume, even if the patient has a new sexual partner.
- Screening should not change after HPV vaccination.
- When women have negative cytology but positive HPV results, tests for the HPV 16 and 18 genotypes can help to identify those at higher risk of developing CIN2+.
Peer-reviewers for 2012
We thank those who reviewed manuscripts submitted to the Cleveland Clinic Journal of Medicine in the year ending December 31, 2012. Reviewing papers for scientific journals is an arduous task and involves considerable time and effort. We are grateful to these reviewers for contributing their expertise this past year.
—Brian F. Mandell, MD, PhD, Editor in Chief
We thank those who reviewed manuscripts submitted to the Cleveland Clinic Journal of Medicine in the year ending December 31, 2012. Reviewing papers for scientific journals is an arduous task and involves considerable time and effort. We are grateful to these reviewers for contributing their expertise this past year.
—Brian F. Mandell, MD, PhD, Editor in Chief
We thank those who reviewed manuscripts submitted to the Cleveland Clinic Journal of Medicine in the year ending December 31, 2012. Reviewing papers for scientific journals is an arduous task and involves considerable time and effort. We are grateful to these reviewers for contributing their expertise this past year.
—Brian F. Mandell, MD, PhD, Editor in Chief
Sore throat, odynophagia, hoarseness, and a muffled, high-pitched voice
A 55-year-old man presents to the emergency department with a sore throat, odynophagia, hoarseness, and a high-pitched muffled voice for 1 day. He is otherwise healthy, with no fever, chills, or exposure to sick contacts.
Q: Which is the most likely diagnosis?
- Retropharyngeal abscess
- Croup
- Streptococcal pharyngitis (“strep throat”)
- Acute epiglottitis
- Viral upper-respiratory infection
A: The correct answer is acute epiglottitis.
Acute epiglottitis is a cellulitis of the epiglottis and adjacent tissues that, without treatment, can progress to life-threatening airway obstruction.
In children, this entity most often occurs between ages 2 and 4. Previously, it was mainly associated with Haemophilus influenzae type B infection, but since the start of vaccination for this organism, epiglottitis has become less common.
In recent years, Streptococcus pneumoniae and beta-hemolytic streptococci have become the main culprits in epiglottitis in children. In adults, common organisms include H influenzae, S pneumoniae, Staphylococcus aureus, and betahemolytic streptococci. Epiglottitis may also be caused by herpes simplex, varicella-zoster, and influenza viruses. Noninfectious causes include ingestion of a foreign body and thermal injury.
Common signs and symptoms of epiglottitis include sore throat, a high-pitched and muffled voice, labored breathing, and respiratory distress.1 Since airway obstruction can be remarkably precipitous, it is important to obtain early consultation with an otolaryngologist for laryngoscopy and visualization of the erythematous, swollen epiglottis. Signs of severe upper-airway obstruction may be absent until late in the disease process, making early diagnosis crucial.
On physical examination, the oropharynx will often appear benign. Currently, the preferred diagnostic test for epiglottitis is visualization by laryngoscopy, which has close to 100% sensitivity and specificity.2 By comparison, lateral neck radiography has poor sensitivity (38% to 88%) and specificity (78%).3,4 Bedside ultrasonography has been reported as capable of revealing the “alphabet P sign” in the longitudinal view through the thyrohyoid membrane.5
At triage, if clinical suspicion of total airway obstruction is high, the patient may be taken directly to the operating room for visualization of the epiglottis and, if necessary, for endotracheal intubation. When the risk is considered low or moderate, lateral neck radiography may be helpful,6 as it will sometimes reveal a swollen epiglottis (ie, “thumb sign”), thickened arytenoepiglottic folds, and obliteration of the epiglottic vallecula. If the radiograph is normal and clinical suspicion is still present, laryngoscopy can be done.
For low-risk, acute epiglottitis, closely monitoring the patient in an intensive care unit is recommended. For severe, high-risk cases, securing the airway is of the utmost importance. In addition to securing the airway, empiric antimicrobial therapy—such as with cefotaxime or ceftriaxone plus clindamycin or vancomycin—is often warranted in both low-risk and high-risk cases. The use of corticosteroids in epiglottitis has not been studied in a randomized, controlled trial, and, though common, it remains controversial.7 While undergoing treatment, patients should be closely monitored for respiratory compromise.
HOW OUR PATIENT WAS MANAGED
Our patient had a high-pitched, muffled voice and prominent, tender anterior cervical lymph nodes. No drooling or stridor was noted. The oropharynx appeared normal. Lateral soft-tissue radiography of the neck (Figure 1) revealed several key findings:
- A swollen epiglottis (ie, the “thumb sign”) consistent with acute epiglottitis and a narrowed airway
- Swollen arytenoids
- A shallow V-shaped epiglottic vallecula.
For comparison, Figure 2 shows a normal lateral soft-tissue radiograph of the neck from a different patient.
Our patient was referred to an otolaryngologist, who noted a severely inflamed epiglottis on laryngoscopy.
The patient was given intravenous vancomycin, ceftriaxone, and high-dose corticosteroids and was admitted to the intensive care unit. After his symptoms improved, repeat laryngoscopy revealed markedly diminished inflammation. He was discharged home with an additional course of oral antibiotics.
- Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am 2007; 21:449–469.
- Cheung CS, Man SY, Graham CA, et al. Adult epiglottitis: 6 years experience in a university teaching hospital in Hong Kong. Eur J Emerg Med 2009; 16:221–226.
- Stankiewicz JA, Bowes AK. Croup and epiglottitis: a radiologic study. Laryngoscope 1985; 95:1159–1160.
- Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol 1998; 27:332–336.
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011; 29:359.e1–359.e3.
- Ragosta KG, Orr R, Detweiler MJ. Revisiting epiglottitis: a protocol—the value of lateral neck radiographs. J Am Osteopath Assoc 1997; 97:227–229.
- Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep 2008; 10:200–204.
A 55-year-old man presents to the emergency department with a sore throat, odynophagia, hoarseness, and a high-pitched muffled voice for 1 day. He is otherwise healthy, with no fever, chills, or exposure to sick contacts.
Q: Which is the most likely diagnosis?
- Retropharyngeal abscess
- Croup
- Streptococcal pharyngitis (“strep throat”)
- Acute epiglottitis
- Viral upper-respiratory infection
A: The correct answer is acute epiglottitis.
Acute epiglottitis is a cellulitis of the epiglottis and adjacent tissues that, without treatment, can progress to life-threatening airway obstruction.
In children, this entity most often occurs between ages 2 and 4. Previously, it was mainly associated with Haemophilus influenzae type B infection, but since the start of vaccination for this organism, epiglottitis has become less common.
In recent years, Streptococcus pneumoniae and beta-hemolytic streptococci have become the main culprits in epiglottitis in children. In adults, common organisms include H influenzae, S pneumoniae, Staphylococcus aureus, and betahemolytic streptococci. Epiglottitis may also be caused by herpes simplex, varicella-zoster, and influenza viruses. Noninfectious causes include ingestion of a foreign body and thermal injury.
Common signs and symptoms of epiglottitis include sore throat, a high-pitched and muffled voice, labored breathing, and respiratory distress.1 Since airway obstruction can be remarkably precipitous, it is important to obtain early consultation with an otolaryngologist for laryngoscopy and visualization of the erythematous, swollen epiglottis. Signs of severe upper-airway obstruction may be absent until late in the disease process, making early diagnosis crucial.
On physical examination, the oropharynx will often appear benign. Currently, the preferred diagnostic test for epiglottitis is visualization by laryngoscopy, which has close to 100% sensitivity and specificity.2 By comparison, lateral neck radiography has poor sensitivity (38% to 88%) and specificity (78%).3,4 Bedside ultrasonography has been reported as capable of revealing the “alphabet P sign” in the longitudinal view through the thyrohyoid membrane.5
At triage, if clinical suspicion of total airway obstruction is high, the patient may be taken directly to the operating room for visualization of the epiglottis and, if necessary, for endotracheal intubation. When the risk is considered low or moderate, lateral neck radiography may be helpful,6 as it will sometimes reveal a swollen epiglottis (ie, “thumb sign”), thickened arytenoepiglottic folds, and obliteration of the epiglottic vallecula. If the radiograph is normal and clinical suspicion is still present, laryngoscopy can be done.
For low-risk, acute epiglottitis, closely monitoring the patient in an intensive care unit is recommended. For severe, high-risk cases, securing the airway is of the utmost importance. In addition to securing the airway, empiric antimicrobial therapy—such as with cefotaxime or ceftriaxone plus clindamycin or vancomycin—is often warranted in both low-risk and high-risk cases. The use of corticosteroids in epiglottitis has not been studied in a randomized, controlled trial, and, though common, it remains controversial.7 While undergoing treatment, patients should be closely monitored for respiratory compromise.
HOW OUR PATIENT WAS MANAGED
Our patient had a high-pitched, muffled voice and prominent, tender anterior cervical lymph nodes. No drooling or stridor was noted. The oropharynx appeared normal. Lateral soft-tissue radiography of the neck (Figure 1) revealed several key findings:
- A swollen epiglottis (ie, the “thumb sign”) consistent with acute epiglottitis and a narrowed airway
- Swollen arytenoids
- A shallow V-shaped epiglottic vallecula.
For comparison, Figure 2 shows a normal lateral soft-tissue radiograph of the neck from a different patient.
Our patient was referred to an otolaryngologist, who noted a severely inflamed epiglottis on laryngoscopy.
The patient was given intravenous vancomycin, ceftriaxone, and high-dose corticosteroids and was admitted to the intensive care unit. After his symptoms improved, repeat laryngoscopy revealed markedly diminished inflammation. He was discharged home with an additional course of oral antibiotics.
A 55-year-old man presents to the emergency department with a sore throat, odynophagia, hoarseness, and a high-pitched muffled voice for 1 day. He is otherwise healthy, with no fever, chills, or exposure to sick contacts.
Q: Which is the most likely diagnosis?
- Retropharyngeal abscess
- Croup
- Streptococcal pharyngitis (“strep throat”)
- Acute epiglottitis
- Viral upper-respiratory infection
A: The correct answer is acute epiglottitis.
Acute epiglottitis is a cellulitis of the epiglottis and adjacent tissues that, without treatment, can progress to life-threatening airway obstruction.
In children, this entity most often occurs between ages 2 and 4. Previously, it was mainly associated with Haemophilus influenzae type B infection, but since the start of vaccination for this organism, epiglottitis has become less common.
In recent years, Streptococcus pneumoniae and beta-hemolytic streptococci have become the main culprits in epiglottitis in children. In adults, common organisms include H influenzae, S pneumoniae, Staphylococcus aureus, and betahemolytic streptococci. Epiglottitis may also be caused by herpes simplex, varicella-zoster, and influenza viruses. Noninfectious causes include ingestion of a foreign body and thermal injury.
Common signs and symptoms of epiglottitis include sore throat, a high-pitched and muffled voice, labored breathing, and respiratory distress.1 Since airway obstruction can be remarkably precipitous, it is important to obtain early consultation with an otolaryngologist for laryngoscopy and visualization of the erythematous, swollen epiglottis. Signs of severe upper-airway obstruction may be absent until late in the disease process, making early diagnosis crucial.
On physical examination, the oropharynx will often appear benign. Currently, the preferred diagnostic test for epiglottitis is visualization by laryngoscopy, which has close to 100% sensitivity and specificity.2 By comparison, lateral neck radiography has poor sensitivity (38% to 88%) and specificity (78%).3,4 Bedside ultrasonography has been reported as capable of revealing the “alphabet P sign” in the longitudinal view through the thyrohyoid membrane.5
At triage, if clinical suspicion of total airway obstruction is high, the patient may be taken directly to the operating room for visualization of the epiglottis and, if necessary, for endotracheal intubation. When the risk is considered low or moderate, lateral neck radiography may be helpful,6 as it will sometimes reveal a swollen epiglottis (ie, “thumb sign”), thickened arytenoepiglottic folds, and obliteration of the epiglottic vallecula. If the radiograph is normal and clinical suspicion is still present, laryngoscopy can be done.
For low-risk, acute epiglottitis, closely monitoring the patient in an intensive care unit is recommended. For severe, high-risk cases, securing the airway is of the utmost importance. In addition to securing the airway, empiric antimicrobial therapy—such as with cefotaxime or ceftriaxone plus clindamycin or vancomycin—is often warranted in both low-risk and high-risk cases. The use of corticosteroids in epiglottitis has not been studied in a randomized, controlled trial, and, though common, it remains controversial.7 While undergoing treatment, patients should be closely monitored for respiratory compromise.
HOW OUR PATIENT WAS MANAGED
Our patient had a high-pitched, muffled voice and prominent, tender anterior cervical lymph nodes. No drooling or stridor was noted. The oropharynx appeared normal. Lateral soft-tissue radiography of the neck (Figure 1) revealed several key findings:
- A swollen epiglottis (ie, the “thumb sign”) consistent with acute epiglottitis and a narrowed airway
- Swollen arytenoids
- A shallow V-shaped epiglottic vallecula.
For comparison, Figure 2 shows a normal lateral soft-tissue radiograph of the neck from a different patient.
Our patient was referred to an otolaryngologist, who noted a severely inflamed epiglottis on laryngoscopy.
The patient was given intravenous vancomycin, ceftriaxone, and high-dose corticosteroids and was admitted to the intensive care unit. After his symptoms improved, repeat laryngoscopy revealed markedly diminished inflammation. He was discharged home with an additional course of oral antibiotics.
- Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am 2007; 21:449–469.
- Cheung CS, Man SY, Graham CA, et al. Adult epiglottitis: 6 years experience in a university teaching hospital in Hong Kong. Eur J Emerg Med 2009; 16:221–226.
- Stankiewicz JA, Bowes AK. Croup and epiglottitis: a radiologic study. Laryngoscope 1985; 95:1159–1160.
- Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol 1998; 27:332–336.
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011; 29:359.e1–359.e3.
- Ragosta KG, Orr R, Detweiler MJ. Revisiting epiglottitis: a protocol—the value of lateral neck radiographs. J Am Osteopath Assoc 1997; 97:227–229.
- Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep 2008; 10:200–204.
- Alcaide ML, Bisno AL. Pharyngitis and epiglottitis. Infect Dis Clin North Am 2007; 21:449–469.
- Cheung CS, Man SY, Graham CA, et al. Adult epiglottitis: 6 years experience in a university teaching hospital in Hong Kong. Eur J Emerg Med 2009; 16:221–226.
- Stankiewicz JA, Bowes AK. Croup and epiglottitis: a radiologic study. Laryngoscope 1985; 95:1159–1160.
- Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol 1998; 27:332–336.
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011; 29:359.e1–359.e3.
- Ragosta KG, Orr R, Detweiler MJ. Revisiting epiglottitis: a protocol—the value of lateral neck radiographs. J Am Osteopath Assoc 1997; 97:227–229.
- Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep 2008; 10:200–204.
Giant inverted T waves
A 48-year-old man with hypertension was being evaluated for a noncardiac issue (progressive multifocal leukoencephalopathy). He had been an active runner and did not have any cardiovascular symptoms at the time. The electrocardiogram (ECG) shown in Figure 1 was a routine study done as a part of that evaluation. His cardiovascular examination was unremarkable, without murmur, S3, or S4. His pulse was regular at 72 beats per minute, and his blood pressure was 112/76 mm Hg.
Q: Which of the following electrocardiographic findings suggest left ventricular hypertrophy?
- Sum of the S wave in V1 and the R wave in V6 ≥ 35 mm
- Sum of the S wave in V3 and the R wave in aVL > 28 mm (men)
- Sum of the S wave in V3 and the R wave in aVL > 20 mm (women)
- All of the above
A: The correct answer is all of the above.1,2
Our patient’s ECG shows sinus bradycardia and left ventricular hypertrophy, suggested by prominent voltage (sum of S in V1 and R in V6 ≥ 35 mm) and supported by ST-segment and T-wave changes in the lateral and midprecordial leads. Classic changes of left ventricular hypertrophy often include increased voltage and downsloping ST-segment depression with negative T waves in V5 and V6 (secondary repolarization changes or “strain” pattern).
Notable on this tracing are the large, asymmetric negative T waves in leads V3 through V6. Giant T waves are defined as negative T waves with voltage greater than 10 mm.3 Although there is no specific pattern of ventricular hypertrophy on an ECG that establishes the diagnosis of hypertrophic cardiomyopathy, left ventricular hypertrophy with T waves of this quality suggest the possibility of hypertrophic cardiomyopathy with apical hypertrophy.
Q: What are the other causes of giant negative T waves?
- Subarachnoid hemorrhage
- Complete heart block
- Non-Q-wave myocardial infarction
- All of the above
A: The correct answer is all of the above. Additional causes of dramatic T-wave inversion are listed in Table 1. Clinically, non-Q-wave myocardial infarction with T-wave changes and acute central nervous system injury are probably the most commonly seen.4
Echocardiography in this patient revealed severe apical hypertrophy of the ventricle with distal cavity obliteration. The left ventricular outflow-tract gradient was normal. The mitral valve appeared normal, and there was no resting systolic anterior motion.
Cardiac magnetic resonance imaging showed the apical variant of hypertrophic cardiomyopathy but no evidence of left ventricular noncompaction, which is a differential diagnosis of apical hypertrophic obstructive cardiomyopathy. This disease was first described in Japan by Yamaguchi et al5 and Sakamoto et al6 and is regarded as a subgroup of nonobstructive hypertrophic cardiomyopathy. The prognosis of apical hypertrophic cardiomyopathy with regard to sudden cardiac death is believed to be better than that of other forms of hypertrophic cardiomyopathy.3
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. 1949. Ann Noninvasive Electrocardiol 2001; 6:343–368.
- Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987; 75:565–572.
- Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:638–645.
- Jacobson D, Schrire V. Giant T wave inversion. Br Heart J 1966; 28:768–775.
- Yamaguchi H, Ishimura T, Nishiyama S, et al. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol 1979; 44:401–412.
- Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasonocardiotomographic study. Jpn Heart J 1976; 17:611–629.
A 48-year-old man with hypertension was being evaluated for a noncardiac issue (progressive multifocal leukoencephalopathy). He had been an active runner and did not have any cardiovascular symptoms at the time. The electrocardiogram (ECG) shown in Figure 1 was a routine study done as a part of that evaluation. His cardiovascular examination was unremarkable, without murmur, S3, or S4. His pulse was regular at 72 beats per minute, and his blood pressure was 112/76 mm Hg.
Q: Which of the following electrocardiographic findings suggest left ventricular hypertrophy?
- Sum of the S wave in V1 and the R wave in V6 ≥ 35 mm
- Sum of the S wave in V3 and the R wave in aVL > 28 mm (men)
- Sum of the S wave in V3 and the R wave in aVL > 20 mm (women)
- All of the above
A: The correct answer is all of the above.1,2
Our patient’s ECG shows sinus bradycardia and left ventricular hypertrophy, suggested by prominent voltage (sum of S in V1 and R in V6 ≥ 35 mm) and supported by ST-segment and T-wave changes in the lateral and midprecordial leads. Classic changes of left ventricular hypertrophy often include increased voltage and downsloping ST-segment depression with negative T waves in V5 and V6 (secondary repolarization changes or “strain” pattern).
Notable on this tracing are the large, asymmetric negative T waves in leads V3 through V6. Giant T waves are defined as negative T waves with voltage greater than 10 mm.3 Although there is no specific pattern of ventricular hypertrophy on an ECG that establishes the diagnosis of hypertrophic cardiomyopathy, left ventricular hypertrophy with T waves of this quality suggest the possibility of hypertrophic cardiomyopathy with apical hypertrophy.
Q: What are the other causes of giant negative T waves?
- Subarachnoid hemorrhage
- Complete heart block
- Non-Q-wave myocardial infarction
- All of the above
A: The correct answer is all of the above. Additional causes of dramatic T-wave inversion are listed in Table 1. Clinically, non-Q-wave myocardial infarction with T-wave changes and acute central nervous system injury are probably the most commonly seen.4
Echocardiography in this patient revealed severe apical hypertrophy of the ventricle with distal cavity obliteration. The left ventricular outflow-tract gradient was normal. The mitral valve appeared normal, and there was no resting systolic anterior motion.
Cardiac magnetic resonance imaging showed the apical variant of hypertrophic cardiomyopathy but no evidence of left ventricular noncompaction, which is a differential diagnosis of apical hypertrophic obstructive cardiomyopathy. This disease was first described in Japan by Yamaguchi et al5 and Sakamoto et al6 and is regarded as a subgroup of nonobstructive hypertrophic cardiomyopathy. The prognosis of apical hypertrophic cardiomyopathy with regard to sudden cardiac death is believed to be better than that of other forms of hypertrophic cardiomyopathy.3
A 48-year-old man with hypertension was being evaluated for a noncardiac issue (progressive multifocal leukoencephalopathy). He had been an active runner and did not have any cardiovascular symptoms at the time. The electrocardiogram (ECG) shown in Figure 1 was a routine study done as a part of that evaluation. His cardiovascular examination was unremarkable, without murmur, S3, or S4. His pulse was regular at 72 beats per minute, and his blood pressure was 112/76 mm Hg.
Q: Which of the following electrocardiographic findings suggest left ventricular hypertrophy?
- Sum of the S wave in V1 and the R wave in V6 ≥ 35 mm
- Sum of the S wave in V3 and the R wave in aVL > 28 mm (men)
- Sum of the S wave in V3 and the R wave in aVL > 20 mm (women)
- All of the above
A: The correct answer is all of the above.1,2
Our patient’s ECG shows sinus bradycardia and left ventricular hypertrophy, suggested by prominent voltage (sum of S in V1 and R in V6 ≥ 35 mm) and supported by ST-segment and T-wave changes in the lateral and midprecordial leads. Classic changes of left ventricular hypertrophy often include increased voltage and downsloping ST-segment depression with negative T waves in V5 and V6 (secondary repolarization changes or “strain” pattern).
Notable on this tracing are the large, asymmetric negative T waves in leads V3 through V6. Giant T waves are defined as negative T waves with voltage greater than 10 mm.3 Although there is no specific pattern of ventricular hypertrophy on an ECG that establishes the diagnosis of hypertrophic cardiomyopathy, left ventricular hypertrophy with T waves of this quality suggest the possibility of hypertrophic cardiomyopathy with apical hypertrophy.
Q: What are the other causes of giant negative T waves?
- Subarachnoid hemorrhage
- Complete heart block
- Non-Q-wave myocardial infarction
- All of the above
A: The correct answer is all of the above. Additional causes of dramatic T-wave inversion are listed in Table 1. Clinically, non-Q-wave myocardial infarction with T-wave changes and acute central nervous system injury are probably the most commonly seen.4
Echocardiography in this patient revealed severe apical hypertrophy of the ventricle with distal cavity obliteration. The left ventricular outflow-tract gradient was normal. The mitral valve appeared normal, and there was no resting systolic anterior motion.
Cardiac magnetic resonance imaging showed the apical variant of hypertrophic cardiomyopathy but no evidence of left ventricular noncompaction, which is a differential diagnosis of apical hypertrophic obstructive cardiomyopathy. This disease was first described in Japan by Yamaguchi et al5 and Sakamoto et al6 and is regarded as a subgroup of nonobstructive hypertrophic cardiomyopathy. The prognosis of apical hypertrophic cardiomyopathy with regard to sudden cardiac death is believed to be better than that of other forms of hypertrophic cardiomyopathy.3
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. 1949. Ann Noninvasive Electrocardiol 2001; 6:343–368.
- Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987; 75:565–572.
- Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:638–645.
- Jacobson D, Schrire V. Giant T wave inversion. Br Heart J 1966; 28:768–775.
- Yamaguchi H, Ishimura T, Nishiyama S, et al. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol 1979; 44:401–412.
- Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasonocardiotomographic study. Jpn Heart J 1976; 17:611–629.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. 1949. Ann Noninvasive Electrocardiol 2001; 6:343–368.
- Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation 1987; 75:565–572.
- Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:638–645.
- Jacobson D, Schrire V. Giant T wave inversion. Br Heart J 1966; 28:768–775.
- Yamaguchi H, Ishimura T, Nishiyama S, et al. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol 1979; 44:401–412.
- Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasonocardiotomographic study. Jpn Heart J 1976; 17:611–629.