User login
Ceramides
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
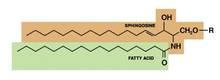
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at sknews@elsevier.com.
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at sknews@elsevier.com.
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at sknews@elsevier.com.
Knee Pain After Falling Off Ladder
ANSWER
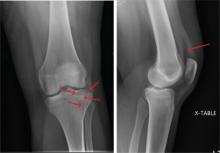
The radiograph shows a lucency within the lateral tibial plateau and tibial metaphysis, consistent with a fracture. It is mildly depressed and slightly comminuted.
Fluid collection is also evident on the lateral view, likely reflecting a lipohemarthrosis. The patient was placed in a knee immobilizer and made non–weight-bearing. She was instructed to follow up with an orthopedist when she returned home (as she was visiting from out of town).
ANSWER
The radiograph shows a lucency within the lateral tibial plateau and tibial metaphysis, consistent with a fracture. It is mildly depressed and slightly comminuted.
Fluid collection is also evident on the lateral view, likely reflecting a lipohemarthrosis. The patient was placed in a knee immobilizer and made non–weight-bearing. She was instructed to follow up with an orthopedist when she returned home (as she was visiting from out of town).
ANSWER
The radiograph shows a lucency within the lateral tibial plateau and tibial metaphysis, consistent with a fracture. It is mildly depressed and slightly comminuted.
Fluid collection is also evident on the lateral view, likely reflecting a lipohemarthrosis. The patient was placed in a knee immobilizer and made non–weight-bearing. She was instructed to follow up with an orthopedist when she returned home (as she was visiting from out of town).
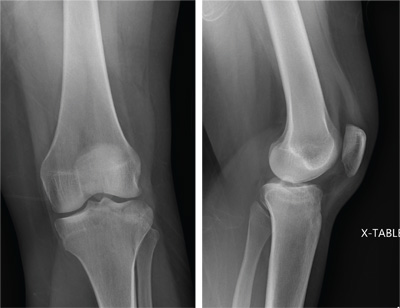
A 25-year-old woman presents for evaluation of left knee pain secondary to a fall. She states she was descending a ladder when she missed a step while still several feet above the ground. She landed on her left foot, awkwardly twisting her leg. She now has swelling and pain in her knee and difficulty bearing weight on that leg. Her medical history is unremarkable. Examination reveals a moderate amount of swelling that limits her ability to flex her left knee. She has diffuse tenderness throughout the knee. Because of the swelling and the patient’s severe discomfort, instability tests are not performed. She has good distal pulses and sensation. Radiographs of the knee are obtained. What is your impression?
The Flu, or a Problem with His Pacemaker?
ANSWER
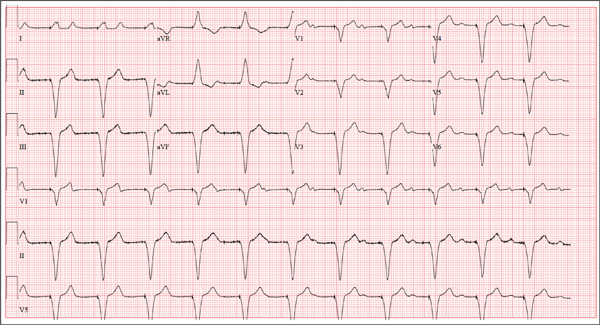
This ECG is remarkable for ventricular pacing at a rate of 70 beats/min, with an underlying sinus rhythm at the same rate as the pacemaker but dissociated from ventricular pacing. Ventricular pacing is evidenced by the presence of a pacing spike before each QRS complex, and the fact that each QRS complex in all leads is wide (200 ms) and does not demonstrate variability within an ECG lead. The T waves are similar in each lead as well. A left-axis deviation of –83° is attributable to pacing from the right ventricle.
What is interesting to note is that P waves are visible and are at a rate very close to that of the ventricular paced beats; however, they show no association with the pacing spike or the QRS complexes. This is most evident in lead V1 and the rhythm strip of lead I, which shows the P waves marching through the QRS and T-wave complexes without being associated with any ventricular conduction. This is an unusual situation in which the sinus rate and the paced ventricular rate are very similar.
Interrogation of the pacemaker generator revealed that the programming had been inadvertently changed from DDDR at a rate of 60 beats/min to VVI at a rate of 70 beats/min. After the device was reprogrammed to its original settings, the patient’s symptoms resolved.
ANSWER
This ECG is remarkable for ventricular pacing at a rate of 70 beats/min, with an underlying sinus rhythm at the same rate as the pacemaker but dissociated from ventricular pacing. Ventricular pacing is evidenced by the presence of a pacing spike before each QRS complex, and the fact that each QRS complex in all leads is wide (200 ms) and does not demonstrate variability within an ECG lead. The T waves are similar in each lead as well. A left-axis deviation of –83° is attributable to pacing from the right ventricle.
What is interesting to note is that P waves are visible and are at a rate very close to that of the ventricular paced beats; however, they show no association with the pacing spike or the QRS complexes. This is most evident in lead V1 and the rhythm strip of lead I, which shows the P waves marching through the QRS and T-wave complexes without being associated with any ventricular conduction. This is an unusual situation in which the sinus rate and the paced ventricular rate are very similar.
Interrogation of the pacemaker generator revealed that the programming had been inadvertently changed from DDDR at a rate of 60 beats/min to VVI at a rate of 70 beats/min. After the device was reprogrammed to its original settings, the patient’s symptoms resolved.
ANSWER
This ECG is remarkable for ventricular pacing at a rate of 70 beats/min, with an underlying sinus rhythm at the same rate as the pacemaker but dissociated from ventricular pacing. Ventricular pacing is evidenced by the presence of a pacing spike before each QRS complex, and the fact that each QRS complex in all leads is wide (200 ms) and does not demonstrate variability within an ECG lead. The T waves are similar in each lead as well. A left-axis deviation of –83° is attributable to pacing from the right ventricle.
What is interesting to note is that P waves are visible and are at a rate very close to that of the ventricular paced beats; however, they show no association with the pacing spike or the QRS complexes. This is most evident in lead V1 and the rhythm strip of lead I, which shows the P waves marching through the QRS and T-wave complexes without being associated with any ventricular conduction. This is an unusual situation in which the sinus rate and the paced ventricular rate are very similar.
Interrogation of the pacemaker generator revealed that the programming had been inadvertently changed from DDDR at a rate of 60 beats/min to VVI at a rate of 70 beats/min. After the device was reprogrammed to its original settings, the patient’s symptoms resolved.
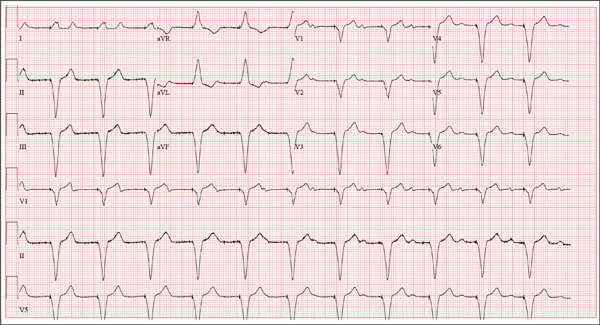
A 75-year-old man presents to your office with complaints of shortness of breath. He states he has had “the flu” for the past week, but it doesn’t seem to be getting any better. His shortness of breath has persisted without change, and he is concerned he may be developing pneumonia. He denies having a productive cough, fevers, chills, or night sweats. Medical history is remarkable for GERD, hyperlipidemia, hypertension, and complete heart block with implantation of a dual-chamber permanent pacemaker in 2010. He has had several surgeries, including a right inguinal hernia repair and an appendectomy. Family history is positive for breast cancer, colon cancer, and stroke. There is no family history of cardiac or pulmonary disease. Social history reveals a retired accountant who lives at home with his wife. He has an occasional brandy in the evening and has never smoked. His current medications include metoprolol, rosu¬\vastatin, and omeprazole. He has no known drug allergies. The review of systems is unremarkable, with the exception of the shortness of breath. The patient is concerned, however, that since his pacemaker was interrogated one week ago, he hasn’t “felt the same.” Physical examination reveals a blood pressure of 130/70 mm Hg; pulse, 70 beats/min; respiratory rate, 16 breaths/min-1; temperature, 36.6°C; and O2 saturation, 97% on room air. The patient’s weight is 105 kg. The cardiovascular exam reveals a regular rate of 70 beats/min, and a grade II/VI early systolic murmur best heard at the left upper sternal border and without radiation. There are no rubs, gallops, or bruits. The pulmonary exam reveals scattered crackles in the right lower chest, which clear with coughing. There are no rhonchi or bronchial breath sounds. All other exams yield normal results. The patient provides a copy of an interrogation report from one year ago, which states his pacemaker is programmed DDDR at a rate of 60 beats/min, with an upper tracking and sensing rate of 130 beats/min, a paced AV delay of 150 ms, and a sensed AV delay of 120 ms. Given the patient’s concern about his most recent interrogation, you call an experienced practitioner to determine whether the patient’s device is functioning appropriately. While waiting, you obtain an ECG, which reveals the following: a ventricular rate of 70 beats/min; PR interval, not measurable; QRS duration, 200 ms; QT/QTc interval, 500/540 ms; no P axis; R axis, –83°; and T axis, 71°. What is your interpretation, and is there any concern regarding his pacemaker function?
All of Her Friends Say She Has Ringworm
ANSWER
The correct answer is pityriasis rosea (PR; choice “d”), which is commonly seen in patients ages 10 to 35 and is about twice as likely to occur in women as in men.
Lichen planus (LP; choice “a”) can mimic PR but lacks the peculiar centripetal scale and oval shape. Furthermore, it does not present with a herald patch.
Guttate psoriasis (choice “b”) could easily be confused for PR. However, it displays heavier white uniform scales with a salmon-pink base, tends to have a distinctly round configuration, and does not involve the appearance of a herald patch.
Secondary syphilis (choice “c”) can usually be ruled out by the sexual history, but also by the lack of a herald patch and the absence of centripetal scaling. Highly variable in appearance, the lesions of secondary syphilis are often seen on the palms.
DISCUSSION
PR was first described in 1860 by Camille Gibert, who used the term pityriasis to describe the fine scale seen with this condition, and chose the term rosea to denote the rosy or pink color.
For a variety of reasons, PR is assumed to be a viral exanthema since, as with many such eruptions, its incidence clusters in the fall and spring, it occurs in close contacts and families, and it is commonly seen in immunocompromised patients. In addition, acquiring the condition appears to confer lifelong immunity.
However, the jury is still out with regard to the exact virus responsible for the disease. Human herpesviruses 6 and 7 are the strongest candidates in terms of antibody production, but no herpesviral particles have been detected in tissue samples.
The so-called herald patch appears initially, in a majority of cases, as a salmon-pink patch that can become as large as 5 to 10 cm, on the trunk or arms. The smaller oval lesions begin to appear within a week or two, averaging 1 to 2 cm in diameter; most display the characteristic “centripetal” scaling, clearly sparing the lesions’ periphery and serving as an essentially pathognomic finding.
On darker-skinned patients, the lesions (including the herald patch) will tend to be brown to black. The examiner must then look for the other characteristic aspects of PR, including the oval (as opposed to round) shape, the long axes of which will often parallel the skin tension lines on the back to produce what is termed the “Christmas tree pattern.” In the author’s experience, the most consistent diagnostic finding is the centripetal scaling seen in at least a few lesions.
Since secondary syphilis is a major item in the differential, obtaining a careful sexual history is essential. If this is uncertain, or if the lesions are not a good fit for PR, obtaining a punch biopsy and serum rapid plasma reagin is necessary. The biopsy in secondary syphilis will show an infiltrate largely composed of plasma cells.
TREATMENT
Once the diagnosis of PR is made, patient education is essential. Affected patients should be reassured about the benign and self-limiting nature of the problem, but also about the likelihood that the condition will persist for up to nine weeks. Darker-skinned patients need to understand that the hyperpigmentation will last for months after the condition has resolved.
Relief of the itching experienced by 75% of PR patients can be achieved with topical steroids (eg, triamcinolone 0.1% cream) and oral antihistamines at bedtime (eg, hydroxyzine 25 to 50 mg) and/or during the daytime (cetirizine 10 mg/d), plus the liberal use of soothing OTC lotions (eg, those containing camphor and menthol). Systemic steroids appear to prolong the condition and are not terribly helpful in controlling the symptoms. In severe cases, phototherapy (narrow-band UVB) can be useful in controlling the itching.
ANSWER
The correct answer is pityriasis rosea (PR; choice “d”), which is commonly seen in patients ages 10 to 35 and is about twice as likely to occur in women as in men.
Lichen planus (LP; choice “a”) can mimic PR but lacks the peculiar centripetal scale and oval shape. Furthermore, it does not present with a herald patch.
Guttate psoriasis (choice “b”) could easily be confused for PR. However, it displays heavier white uniform scales with a salmon-pink base, tends to have a distinctly round configuration, and does not involve the appearance of a herald patch.
Secondary syphilis (choice “c”) can usually be ruled out by the sexual history, but also by the lack of a herald patch and the absence of centripetal scaling. Highly variable in appearance, the lesions of secondary syphilis are often seen on the palms.
DISCUSSION
PR was first described in 1860 by Camille Gibert, who used the term pityriasis to describe the fine scale seen with this condition, and chose the term rosea to denote the rosy or pink color.
For a variety of reasons, PR is assumed to be a viral exanthema since, as with many such eruptions, its incidence clusters in the fall and spring, it occurs in close contacts and families, and it is commonly seen in immunocompromised patients. In addition, acquiring the condition appears to confer lifelong immunity.
However, the jury is still out with regard to the exact virus responsible for the disease. Human herpesviruses 6 and 7 are the strongest candidates in terms of antibody production, but no herpesviral particles have been detected in tissue samples.
The so-called herald patch appears initially, in a majority of cases, as a salmon-pink patch that can become as large as 5 to 10 cm, on the trunk or arms. The smaller oval lesions begin to appear within a week or two, averaging 1 to 2 cm in diameter; most display the characteristic “centripetal” scaling, clearly sparing the lesions’ periphery and serving as an essentially pathognomic finding.
On darker-skinned patients, the lesions (including the herald patch) will tend to be brown to black. The examiner must then look for the other characteristic aspects of PR, including the oval (as opposed to round) shape, the long axes of which will often parallel the skin tension lines on the back to produce what is termed the “Christmas tree pattern.” In the author’s experience, the most consistent diagnostic finding is the centripetal scaling seen in at least a few lesions.
Since secondary syphilis is a major item in the differential, obtaining a careful sexual history is essential. If this is uncertain, or if the lesions are not a good fit for PR, obtaining a punch biopsy and serum rapid plasma reagin is necessary. The biopsy in secondary syphilis will show an infiltrate largely composed of plasma cells.
TREATMENT
Once the diagnosis of PR is made, patient education is essential. Affected patients should be reassured about the benign and self-limiting nature of the problem, but also about the likelihood that the condition will persist for up to nine weeks. Darker-skinned patients need to understand that the hyperpigmentation will last for months after the condition has resolved.
Relief of the itching experienced by 75% of PR patients can be achieved with topical steroids (eg, triamcinolone 0.1% cream) and oral antihistamines at bedtime (eg, hydroxyzine 25 to 50 mg) and/or during the daytime (cetirizine 10 mg/d), plus the liberal use of soothing OTC lotions (eg, those containing camphor and menthol). Systemic steroids appear to prolong the condition and are not terribly helpful in controlling the symptoms. In severe cases, phototherapy (narrow-band UVB) can be useful in controlling the itching.
ANSWER
The correct answer is pityriasis rosea (PR; choice “d”), which is commonly seen in patients ages 10 to 35 and is about twice as likely to occur in women as in men.
Lichen planus (LP; choice “a”) can mimic PR but lacks the peculiar centripetal scale and oval shape. Furthermore, it does not present with a herald patch.
Guttate psoriasis (choice “b”) could easily be confused for PR. However, it displays heavier white uniform scales with a salmon-pink base, tends to have a distinctly round configuration, and does not involve the appearance of a herald patch.
Secondary syphilis (choice “c”) can usually be ruled out by the sexual history, but also by the lack of a herald patch and the absence of centripetal scaling. Highly variable in appearance, the lesions of secondary syphilis are often seen on the palms.
DISCUSSION
PR was first described in 1860 by Camille Gibert, who used the term pityriasis to describe the fine scale seen with this condition, and chose the term rosea to denote the rosy or pink color.
For a variety of reasons, PR is assumed to be a viral exanthema since, as with many such eruptions, its incidence clusters in the fall and spring, it occurs in close contacts and families, and it is commonly seen in immunocompromised patients. In addition, acquiring the condition appears to confer lifelong immunity.
However, the jury is still out with regard to the exact virus responsible for the disease. Human herpesviruses 6 and 7 are the strongest candidates in terms of antibody production, but no herpesviral particles have been detected in tissue samples.
The so-called herald patch appears initially, in a majority of cases, as a salmon-pink patch that can become as large as 5 to 10 cm, on the trunk or arms. The smaller oval lesions begin to appear within a week or two, averaging 1 to 2 cm in diameter; most display the characteristic “centripetal” scaling, clearly sparing the lesions’ periphery and serving as an essentially pathognomic finding.
On darker-skinned patients, the lesions (including the herald patch) will tend to be brown to black. The examiner must then look for the other characteristic aspects of PR, including the oval (as opposed to round) shape, the long axes of which will often parallel the skin tension lines on the back to produce what is termed the “Christmas tree pattern.” In the author’s experience, the most consistent diagnostic finding is the centripetal scaling seen in at least a few lesions.
Since secondary syphilis is a major item in the differential, obtaining a careful sexual history is essential. If this is uncertain, or if the lesions are not a good fit for PR, obtaining a punch biopsy and serum rapid plasma reagin is necessary. The biopsy in secondary syphilis will show an infiltrate largely composed of plasma cells.
TREATMENT
Once the diagnosis of PR is made, patient education is essential. Affected patients should be reassured about the benign and self-limiting nature of the problem, but also about the likelihood that the condition will persist for up to nine weeks. Darker-skinned patients need to understand that the hyperpigmentation will last for months after the condition has resolved.
Relief of the itching experienced by 75% of PR patients can be achieved with topical steroids (eg, triamcinolone 0.1% cream) and oral antihistamines at bedtime (eg, hydroxyzine 25 to 50 mg) and/or during the daytime (cetirizine 10 mg/d), plus the liberal use of soothing OTC lotions (eg, those containing camphor and menthol). Systemic steroids appear to prolong the condition and are not terribly helpful in controlling the symptoms. In severe cases, phototherapy (narrow-band UVB) can be useful in controlling the itching.
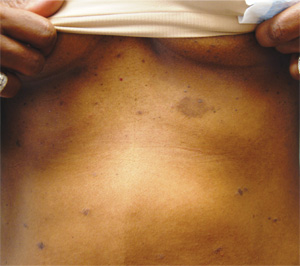
Three weeks ago, a 25-year-old woman noticed an asymp¬tomatic lesion of unknown origin on her chest. Since then, smaller versions have appeared in “crops” on her trunk, arms, and lower neck. Friends were unanimous in their opinion that she had “ringworm,” so she consulted her pharmacist, who recommended clotrimazole cream. Despite her use of it, however, the lesions continue to increase in number. Her original lesion has become less red and scaly, though. The patient has felt fine from the outset and maintains that she is “quite healthy” in other respects. Employed as an IT technician, she denies any exposure to children, pets, or sexually transmitted diseases. The patient, who is African-American, has type V skin. Her original lesion—located on her left inframammary chest—is dark brown, macular, oval to round, and measures about 3.8 cm. On her trunk, arms, and lower neck, 15 to 20 oval, papulosquamous lesions are seen; these are widely scattered, all hyperpigmented (brown), and average 1.5 cm in diameter. Several of these smaller lesions have scaly centers that spare the peripheral margins. The long axes of her oval back lesions are parallel with natural lines of cleavage in the skin.
ACO Insider: An Rx for rising health spending
With the looming federal "sequestration" threatening drastic spending cuts, our nation’s leaders are finally confronting the main drivers of our deficit dilemma: government "entitlement" programs such as Social Security, Medicare, and Medicaid.
Meanwhile, there is broad consensus that many of our runaway health care costs are avoidable. Our current fee-for-service health care payment system rewards higher-intensity care in greater volume, with no consequence for lack of coordination. It is a significant reason that our health care system is fragmented, inefficient, and too costly.
Federal government receipts total approximately 19% of our nation’s gross domestic product. Yet if our health care spending trends remain unchecked, by 2035 Medicare and Medicaid alone are predicted to consume 13% of GDP. By 2080, Medicare and Medicaid will consume all federal taxes, while total public and private health spending will claim almost 50% of GDP. We will have to borrow to pay for the rest of the federal government’s obligations: defense, education, transportation, etc.
As of 2012, our nation is already $16 trillion in the hole and counting. Sticking with the status quo would be a disastrous choice.
However, if medical providers work together and accept new payment incentives that reward value instead of volume, we can help fix America’s broken health care system.
That cannot be done remotely in Washington. It requires health care providers in each community cooperating to increase health care quality and cut cumulative costs.
Quality, savings, and patient satisfaction all must be achieved for providers to receive incentive payments under the new health care payment model, called "value-based reimbursement."
There is plenty of waste to be found and eliminated. Last summer, the Institute of Medicine concluded that America wastes about 30% of its health care spending – some $750 billion a year – on unneeded care, excessive paperwork, fraud, and other inefficiencies.
With basic health care becoming unaffordable for many ordinary working families and individuals, that amount of waste is unacceptable.
Although no one can hope to eradicate it overnight, it’s time somebody did something about it. America is asking physicians to step up and form teams, teams such as accountable care organizations.
By doing so, you can help ensure access, improve patient care, promote efficiency, stretch health care dollars, and make patients more of a partner in their treatment. ACOs typically receive 50% of the savings they create, which should be considered compensation to you for professional services.
As healers with a calling to serve, you have an opportunity to do your part to enhance patient care while helping to improve our nation’s fiscal health. Besides empowering, and paying, physicians to regain control of the physician/patient relationship, your patients, your profession, and your nation need you.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at bbobbitt@smithlaw.com, or at 919-821-6612.
With the looming federal "sequestration" threatening drastic spending cuts, our nation’s leaders are finally confronting the main drivers of our deficit dilemma: government "entitlement" programs such as Social Security, Medicare, and Medicaid.
Meanwhile, there is broad consensus that many of our runaway health care costs are avoidable. Our current fee-for-service health care payment system rewards higher-intensity care in greater volume, with no consequence for lack of coordination. It is a significant reason that our health care system is fragmented, inefficient, and too costly.
Federal government receipts total approximately 19% of our nation’s gross domestic product. Yet if our health care spending trends remain unchecked, by 2035 Medicare and Medicaid alone are predicted to consume 13% of GDP. By 2080, Medicare and Medicaid will consume all federal taxes, while total public and private health spending will claim almost 50% of GDP. We will have to borrow to pay for the rest of the federal government’s obligations: defense, education, transportation, etc.
As of 2012, our nation is already $16 trillion in the hole and counting. Sticking with the status quo would be a disastrous choice.
However, if medical providers work together and accept new payment incentives that reward value instead of volume, we can help fix America’s broken health care system.
That cannot be done remotely in Washington. It requires health care providers in each community cooperating to increase health care quality and cut cumulative costs.
Quality, savings, and patient satisfaction all must be achieved for providers to receive incentive payments under the new health care payment model, called "value-based reimbursement."
There is plenty of waste to be found and eliminated. Last summer, the Institute of Medicine concluded that America wastes about 30% of its health care spending – some $750 billion a year – on unneeded care, excessive paperwork, fraud, and other inefficiencies.
With basic health care becoming unaffordable for many ordinary working families and individuals, that amount of waste is unacceptable.
Although no one can hope to eradicate it overnight, it’s time somebody did something about it. America is asking physicians to step up and form teams, teams such as accountable care organizations.
By doing so, you can help ensure access, improve patient care, promote efficiency, stretch health care dollars, and make patients more of a partner in their treatment. ACOs typically receive 50% of the savings they create, which should be considered compensation to you for professional services.
As healers with a calling to serve, you have an opportunity to do your part to enhance patient care while helping to improve our nation’s fiscal health. Besides empowering, and paying, physicians to regain control of the physician/patient relationship, your patients, your profession, and your nation need you.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at bbobbitt@smithlaw.com, or at 919-821-6612.
With the looming federal "sequestration" threatening drastic spending cuts, our nation’s leaders are finally confronting the main drivers of our deficit dilemma: government "entitlement" programs such as Social Security, Medicare, and Medicaid.
Meanwhile, there is broad consensus that many of our runaway health care costs are avoidable. Our current fee-for-service health care payment system rewards higher-intensity care in greater volume, with no consequence for lack of coordination. It is a significant reason that our health care system is fragmented, inefficient, and too costly.
Federal government receipts total approximately 19% of our nation’s gross domestic product. Yet if our health care spending trends remain unchecked, by 2035 Medicare and Medicaid alone are predicted to consume 13% of GDP. By 2080, Medicare and Medicaid will consume all federal taxes, while total public and private health spending will claim almost 50% of GDP. We will have to borrow to pay for the rest of the federal government’s obligations: defense, education, transportation, etc.
As of 2012, our nation is already $16 trillion in the hole and counting. Sticking with the status quo would be a disastrous choice.
However, if medical providers work together and accept new payment incentives that reward value instead of volume, we can help fix America’s broken health care system.
That cannot be done remotely in Washington. It requires health care providers in each community cooperating to increase health care quality and cut cumulative costs.
Quality, savings, and patient satisfaction all must be achieved for providers to receive incentive payments under the new health care payment model, called "value-based reimbursement."
There is plenty of waste to be found and eliminated. Last summer, the Institute of Medicine concluded that America wastes about 30% of its health care spending – some $750 billion a year – on unneeded care, excessive paperwork, fraud, and other inefficiencies.
With basic health care becoming unaffordable for many ordinary working families and individuals, that amount of waste is unacceptable.
Although no one can hope to eradicate it overnight, it’s time somebody did something about it. America is asking physicians to step up and form teams, teams such as accountable care organizations.
By doing so, you can help ensure access, improve patient care, promote efficiency, stretch health care dollars, and make patients more of a partner in their treatment. ACOs typically receive 50% of the savings they create, which should be considered compensation to you for professional services.
As healers with a calling to serve, you have an opportunity to do your part to enhance patient care while helping to improve our nation’s fiscal health. Besides empowering, and paying, physicians to regain control of the physician/patient relationship, your patients, your profession, and your nation need you.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at bbobbitt@smithlaw.com, or at 919-821-6612.
Third drug approved for metastatic, treatment-resistant GIST
Regorafenib, a multikinase inhibitor, has been approved as a treatment for locally advanced, unresectable, or metastatic gastrointestinal stromal tumor in people who have been treated with imatinib and sunitinib, the other two treatments approved for GIST, the Food and Drug Administration announced on Feb. 26.
Regorafenib was first approved in September as a treatment for metastatic colorectal cancer, and "provides an important new treatment option for patients with GIST in which other approved drugs are no longer effective," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. The recommended dose is 160 mg orally once a day for the first 21 days of each 28-day cycle, according to the prescribing information for regorafenib, which is marketed as Stivarga by Bayer HealthCare Pharmaceuticals.
Approval was based on the interim results of the phase III GRID (GIST – Regorafenib In Progressive Disease) study, comparing placebo plus best supportive care (BSC) to regorafenib plus BSC in 199 patients with locally advanced, unresectable, or metastatic GIST, previously treated with imatinib and sunitinib, according to the FDA statement, as well as the statement issued by the manufacturer. The median progression-free survival (the primary endpoint) was 4.8 months among those on regorafenib, compared with 0.8 months among those on placebo, a statistically significant difference (Lancet 381;9863:295-302). At the time of the planned interim analysis, there was no statistically significant difference in overall survival.
The most common adverse events associated with treatment, reported by at least 30% of those treated, included hand-foot syndrome, diarrhea, mucositis, dysphonia, asthenia/fatigue, hypertension, reduced appetite and food intake, and rash. Serious adverse events, affecting less than 1% of patients, included hepatotoxicity, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks, and intestinal perforations. The regorafenib label includes a boxed warning about the risk of hepatotoxicity associated with treatment, noting that severe and sometimes fatal hepatotoxicity has been reported in clinical trials, and that hepatic function should be monitored before and during treatment.
Regorafenib inhibits multiple kinases that are involved in normal cellular functions, as well as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment, according to the manufacturer.
Regorafenib was the focus of the FDA’s priority review program, which evaluates the drug in 6 months instead of the usual 12 months, and is designated for products "that may provide safe and effective therapy when no satisfactory alternative therapy exists, or offer significant improvement compared to marketed products," according to the FDA statement.
The FDA cites a National Cancer Institute estimate that 3,300-6,000 new cases of GIST are diagnosed every year in the United States, affecting mostly older adults. The previously approved colorectal cancer indication is for people who have metastatic colorectal cancer, who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.
Imatinib (Gleevec) and sunitinib (Sutent) are both orally administered kinase inhibitors.
Regorafenib, a multikinase inhibitor, has been approved as a treatment for locally advanced, unresectable, or metastatic gastrointestinal stromal tumor in people who have been treated with imatinib and sunitinib, the other two treatments approved for GIST, the Food and Drug Administration announced on Feb. 26.
Regorafenib was first approved in September as a treatment for metastatic colorectal cancer, and "provides an important new treatment option for patients with GIST in which other approved drugs are no longer effective," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. The recommended dose is 160 mg orally once a day for the first 21 days of each 28-day cycle, according to the prescribing information for regorafenib, which is marketed as Stivarga by Bayer HealthCare Pharmaceuticals.
Approval was based on the interim results of the phase III GRID (GIST – Regorafenib In Progressive Disease) study, comparing placebo plus best supportive care (BSC) to regorafenib plus BSC in 199 patients with locally advanced, unresectable, or metastatic GIST, previously treated with imatinib and sunitinib, according to the FDA statement, as well as the statement issued by the manufacturer. The median progression-free survival (the primary endpoint) was 4.8 months among those on regorafenib, compared with 0.8 months among those on placebo, a statistically significant difference (Lancet 381;9863:295-302). At the time of the planned interim analysis, there was no statistically significant difference in overall survival.
The most common adverse events associated with treatment, reported by at least 30% of those treated, included hand-foot syndrome, diarrhea, mucositis, dysphonia, asthenia/fatigue, hypertension, reduced appetite and food intake, and rash. Serious adverse events, affecting less than 1% of patients, included hepatotoxicity, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks, and intestinal perforations. The regorafenib label includes a boxed warning about the risk of hepatotoxicity associated with treatment, noting that severe and sometimes fatal hepatotoxicity has been reported in clinical trials, and that hepatic function should be monitored before and during treatment.
Regorafenib inhibits multiple kinases that are involved in normal cellular functions, as well as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment, according to the manufacturer.
Regorafenib was the focus of the FDA’s priority review program, which evaluates the drug in 6 months instead of the usual 12 months, and is designated for products "that may provide safe and effective therapy when no satisfactory alternative therapy exists, or offer significant improvement compared to marketed products," according to the FDA statement.
The FDA cites a National Cancer Institute estimate that 3,300-6,000 new cases of GIST are diagnosed every year in the United States, affecting mostly older adults. The previously approved colorectal cancer indication is for people who have metastatic colorectal cancer, who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.
Imatinib (Gleevec) and sunitinib (Sutent) are both orally administered kinase inhibitors.
Regorafenib, a multikinase inhibitor, has been approved as a treatment for locally advanced, unresectable, or metastatic gastrointestinal stromal tumor in people who have been treated with imatinib and sunitinib, the other two treatments approved for GIST, the Food and Drug Administration announced on Feb. 26.
Regorafenib was first approved in September as a treatment for metastatic colorectal cancer, and "provides an important new treatment option for patients with GIST in which other approved drugs are no longer effective," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. The recommended dose is 160 mg orally once a day for the first 21 days of each 28-day cycle, according to the prescribing information for regorafenib, which is marketed as Stivarga by Bayer HealthCare Pharmaceuticals.
Approval was based on the interim results of the phase III GRID (GIST – Regorafenib In Progressive Disease) study, comparing placebo plus best supportive care (BSC) to regorafenib plus BSC in 199 patients with locally advanced, unresectable, or metastatic GIST, previously treated with imatinib and sunitinib, according to the FDA statement, as well as the statement issued by the manufacturer. The median progression-free survival (the primary endpoint) was 4.8 months among those on regorafenib, compared with 0.8 months among those on placebo, a statistically significant difference (Lancet 381;9863:295-302). At the time of the planned interim analysis, there was no statistically significant difference in overall survival.
The most common adverse events associated with treatment, reported by at least 30% of those treated, included hand-foot syndrome, diarrhea, mucositis, dysphonia, asthenia/fatigue, hypertension, reduced appetite and food intake, and rash. Serious adverse events, affecting less than 1% of patients, included hepatotoxicity, severe bleeding, blistering and peeling of skin, very high blood pressures requiring emergency treatment, heart attacks, and intestinal perforations. The regorafenib label includes a boxed warning about the risk of hepatotoxicity associated with treatment, noting that severe and sometimes fatal hepatotoxicity has been reported in clinical trials, and that hepatic function should be monitored before and during treatment.
Regorafenib inhibits multiple kinases that are involved in normal cellular functions, as well as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment, according to the manufacturer.
Regorafenib was the focus of the FDA’s priority review program, which evaluates the drug in 6 months instead of the usual 12 months, and is designated for products "that may provide safe and effective therapy when no satisfactory alternative therapy exists, or offer significant improvement compared to marketed products," according to the FDA statement.
The FDA cites a National Cancer Institute estimate that 3,300-6,000 new cases of GIST are diagnosed every year in the United States, affecting mostly older adults. The previously approved colorectal cancer indication is for people who have metastatic colorectal cancer, who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.
Imatinib (Gleevec) and sunitinib (Sutent) are both orally administered kinase inhibitors.
The EHR Report Podcast: Optimal Use
Despite all the discussion of meaningful use of EHRs to earn federal incentives, what physicians seem most frustrated with is the lack of optimal use of their EHRs. In this podcast, Dr. Skolnik and Dr. Notte talk about what you can do to optimize your EHR and make your interactions with it easier and more effective throughout the clinical day.
To download the podcast, right-click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Despite all the discussion of meaningful use of EHRs to earn federal incentives, what physicians seem most frustrated with is the lack of optimal use of their EHRs. In this podcast, Dr. Skolnik and Dr. Notte talk about what you can do to optimize your EHR and make your interactions with it easier and more effective throughout the clinical day.
To download the podcast, right-click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Despite all the discussion of meaningful use of EHRs to earn federal incentives, what physicians seem most frustrated with is the lack of optimal use of their EHRs. In this podcast, Dr. Skolnik and Dr. Notte talk about what you can do to optimize your EHR and make your interactions with it easier and more effective throughout the clinical day.
To download the podcast, right-click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Survey of Academic PHM Programs in the US
Pediatric hospital medicine (PHM) is a relatively new field that has been growing rapidly over the past 20 years.[1] The field has been increasingly recognized for its contributions to high‐quality patient care, patient safety, systems improvement, medical education, and research.[2, 3, 4, 5, 6, 7, 8, 9] However, there appears to be significant variation among programs, even in basic factors such as how clinical effort is defined, the extent of in‐house coverage provided, and the scope of clinical services provided, and there exists a paucity of data describing these variations.[8]
Most previously published work did not specifically focus on academic programs,[2, 3, 8, 9] and specifically targeted hospital leadership,[2] practicing hospitalists,[3] residents,[7] and pediatric residency or clerkship directors,[4, 7] rather than hospitalist directors.[9] Furthermore, previous work focused on specific aspects of PHM programs such as education,[4, 7] value,[2] work environment,[9] and clinical practice,[3] rather than a more comprehensive approach.
We conducted a survey of academic PHM programs to learn about the current state and variation among programs across multiple domains (organizational, administrative, and financial). We speculated that:
- Many institutions currently lacking an academic PHM program were planning on starting a program in the next 3 years.
- Variability exists in hospitalist workload among programs.
- In programs providing clinical coverage at more than 1 site, variability exists in the relationship between the main site and satellite site(s) in terms of decision making, scheduling, and reporting of performance.
METHODS
Sample
We used the online American Medical Association Fellowship and Residency Electronic Interactive Database (FREIDA) to identify all 198 accredited pediatric residency training programs in the United States. A total of 246 hospitals were affiliated with these programs, and all of these were targeted for the survey. In addition, academic PHM program leaders were targeted directly with email invitations through the American Academy of Pediatrics (AAP) Section on Hospital Medicine LISTSERV.
Survey Instrument
A 49‐question online survey on the administrative, organizational, and financial aspects of academic PHM programs was developed with the input of academic PHM hospital leaders from Cincinnati Children's Hospital Medical Center and St. Louis Children's Hospital. First, the survey questions were developed de novo by the researchers. Then, multiple hospitalist leaders from each institution took the survey and gave feedback on content and structure. Using this feedback, changes were made and then tested by the leaders taking the new version of the survey. This process was repeated for 3 cycles until consensus was reached by the researchers on the final version of the survey. The survey contained questions that asked if the program provided coverage at a single site or at multiple sites and utilized a combination of open‐ended and fixed‐choice questions. For some questions, more than 1 answer was permitted. For the purposes of this survey, we utilized the following definitions adapted from the Society of Hospital Medicine. A hospitalist was defined as a physician who specializes in the practice of hospital medicine.[10] An academic PHM program was defined as any hospitalist practice associated with a pediatric residency program.[11] A nocturnist was defined as a hospitalist who predominantly works a schedule providing night coverage.[12]
Survey Administration
SurveyMonkey, an online survey software, was used to administer the survey. In June 2011, letters were mailed to all 246 hospitals affiliated with an accredited pediatric residency program as described above. These were addressed to either the hospital medicine director (if identified using the institutions Web site) or pediatric residency director. The letter asked the recipient to either participate in the survey or forward the survey to the physician best able to answer the survey. The letters included a description of the study and a link to the online survey. Of note, there was no follow‐up on this process. We also distributed the direct link to the survey and a copy of the letter utilizing the AAP Section on Hospital Medicine LISTSERV. Two reminders were sent through the LISTSERV in the month after the initial request. All respondents were informed that they would receive the deidentified raw data as an incentive to participate in the survey. Respondents were defined as those answering the first question, Does your program have an academic hospitalist program?
Statistical Analysis
Completed survey responses were extracted to Microsoft Excel (Microsoft Corp., Redmond, WA) for data analysis. Basic statistics were utilized to determine response rates for each question. Data were stratified for program type (single site or at multiple sites). For some questions, data were further stratified for the main site of multiple‐site programs for comparison to single‐site programs. In a few instances, more than 1 physician from a particular program responded to the survey. For these, the most appropriate respondent (PHM director, residency director, senior hospitalist) was identified utilizing the programs' publicly available Web site; only that physician's answers were used in the analysis.
Human Subjects Protection
This study was determined to be exempt from review by the Cincinnati Children's Hospital Medical Center and Washington University in St. Louis institutional review boards. All potential responders received written information about the survey. Survey design allowed for anonymous responses with voluntary documentation of program name and responders' contact information. The willingness to respond was qualified as implied consent. Data were deidentified prior to analysis and prior to sharing with the survey participants.
RESULTS
Response Rates
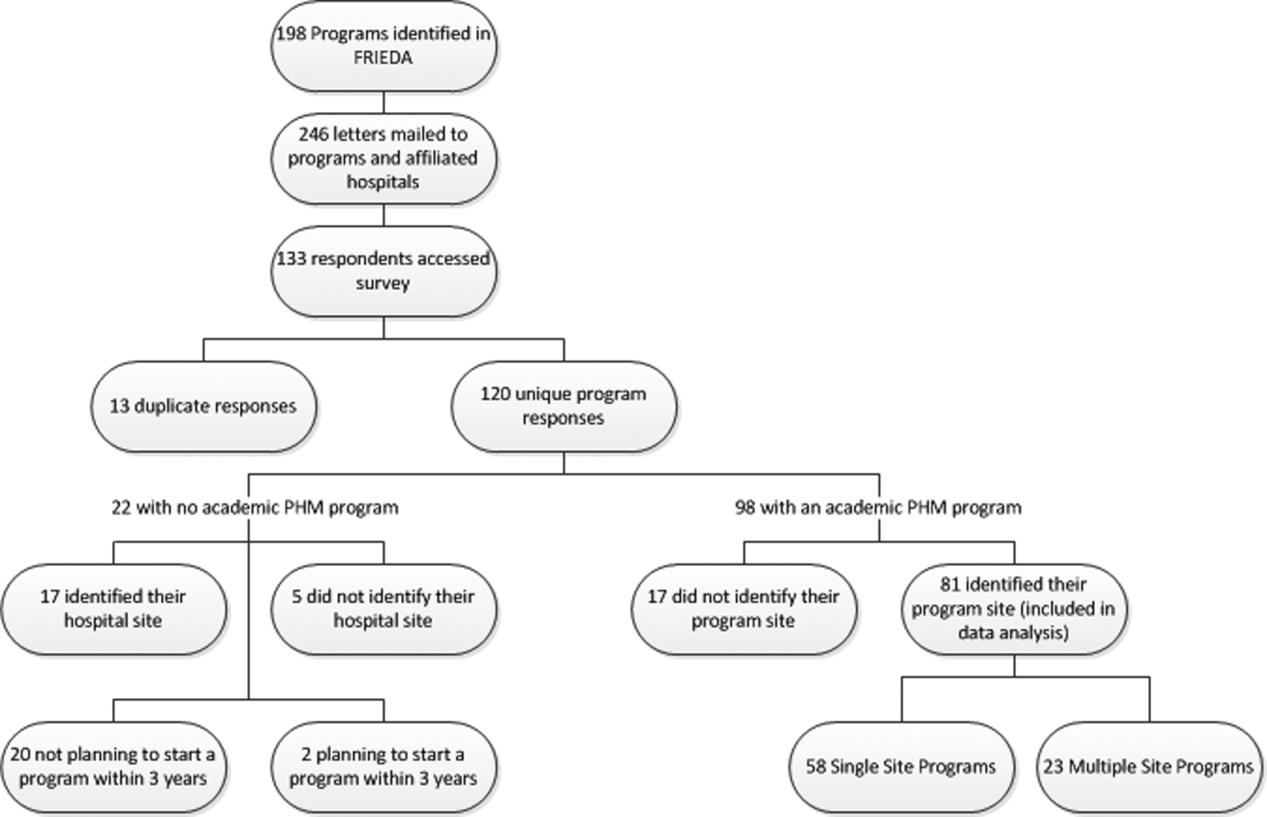
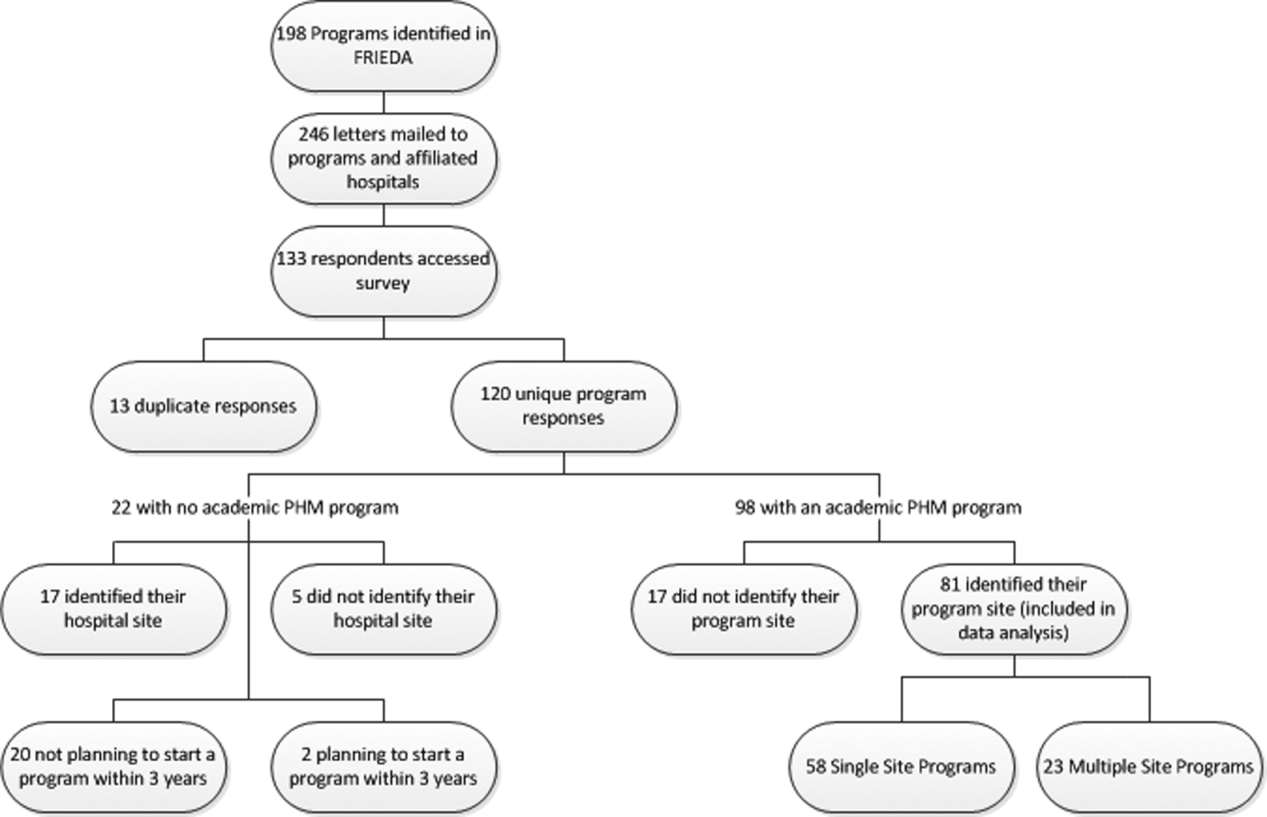
A total of 133 responses were received. Duplicate responses from the same program (13/133) were eliminated from the analysis. This yielded an overall response rate of 48.8% (120/246). A total of 81.7% (98/120) of institutions reported having an academic PHM program. Of the 18.3% (22/120) of institutions reporting not having a program, 9.1% (2/22) reported planning on starting a program in the next 3 years. Of the 98 respondents with an academic PHM program, 17 answered only the first survey question, Does your program have an academic hospitalist program? The remaining 81 completed surveys were left for further analysis. All of these respondents identified their program, and therefore we are certain that there were no duplicate responses in the analytic dataset. Of these, 23 (28%) indicated that their programs provided clinical care at multiple sites, and 58 (72%) indicated that their program provided care at a single site (Figure 1).
Administrative
Respondents reported wide variation for the definition of a 1.0 full‐time employee (FTE) hospitalist in their group. This included the units used (hours/year, weeks/year, shifts/year) as well as actual physician workload (Table 1). Weeks/year was the most common unit utilized by programs to define workload (66% of single‐site programs, 48% of multiple‐site programs), followed by hours/year (19%, 22%) and shifts/year (14%, 22%). The mean and median workload per FTE is represented (Table 1). The large ranges and the standard deviations from the mean indicate variability in workload per FTE (Table 1).
| Single‐Site Program | Multiple‐Site Programs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Programs | Mean | Median | SD | Range | % Programs | Mean | Median | SD | Range | |
| ||||||||||
| Weeks on service | 66 | 27.14 | 26 | 8.1 | 1246 | 48 | 27.2 | 24 | 9.6 | 1736 |
| Hours/year | 19 | 1886.25 | 1880 | 231.2 | 16002300 | 22 | 1767.33 | 1738 | 109.0 | 16641944 |
| Shifts/year* | 14 | 183 | 191 | 52.2 | 182240 | 22 | 191 | 184 | 38.3 | 155214 |
Scheduled in‐house hospitalist coverage also varied. Daytime coverage was defined as until 3 to 5 pm, evening coverage was defined a until 10 pm to midnight, and 24‐hour coverage was defined a 24/7. Programs reported plans to increase in‐house coverage with the implementation of the 2011 Accreditation Council for Graduate Medical Education (ACGME) resident work hours restrictions.[13] Among single‐site programs, there was a planned 50% increase in day/evening coverage (14% to 21%), with a planned decrease in day‐only coverage, and no change in 24/7 coverage (Table 2). Among the main sites of multiple‐site programs, there was a planned 50% increase in 24/7 in‐house coverage (35% to 52%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 3). Among the satellite sites of multiple‐site programs, there was a planned 9% increase in 24/7 coverage (41% to 50%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 2). Most programs reported that all hospitalists share night coverage (87% single site, 89% multiple sites) (Table 2). Multiple‐site programs were more likely than single‐site programs to use nocturnists, moonlighters, and incentives for those providing evening or night coverage (Table 2).
| Single Site (n=58) | Main Site of Multiple‐Site Programs (n=23) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| ||||
| Organizational | ||||
| Night shifts | .79 (46/58) | .83 (19/23) | ||
| All share nights | .87 (40/46) | .89 (17/19) | ||
| Nocturnists | .09 (4/46) | .26 (5/19) | ||
| Moonlighters | .04 (2/46) | .12 (2/19) | ||
| Night shift incentives | .74 (43/58) | .78 (18/23) | ||
| Financial | .12 (5/43) | .28 (5/18) | ||
| Time | .12 (5/43) | .22 (4/18) | ||
| No incentives | .79 (34/43) | .61 (11/18) | ||
| In‐house hospitalist coverage pre July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .35 (8/23) | ||
| Day and evening | .14 (8/58) | .17 (4/23) | ||
| Day only | .57 (33/58) | .48 (11/23) | ||
| In‐house hospitalist coverage post July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .52 (12/23) | ||
| Day and evening | .21 (12/58) | .17 (4/23) | ||
| Day only | .50 (29/58) | .30 (7/23) | ||
| Administrative | ||||
| Own division | .32 (18/57) | .98 (57/58) | .74 (17/23) | 1.0 (23/23) |
| Part of another division | .68 (39/57) | .26 (6/23) | ||
| Financial | ||||
| Revenues>expenses | .26 (14/53) | .91 (53/58) | .04 (1/23) | .04 (19/23) |
| Incentives supplement base salary | .45 (25/55) | .95 (55/58) | .48 (10/21) | .91 (21/23) |
| Metrics used to determine incentivesb | .47 (27/58) | .52 (12/23) | ||
| RVUs/MD | .85 (23/27) | .83 (10/12) | ||
| Costs/discharge | .19 (5/27) | .08 (1/12) | ||
| Financial reportingb | .81 (47/58) | .04 (19/23) | ||
| Charges | .64 (30/47) | .68 (13/19) | ||
| Collections | .66 (31/47) | .68 (13/19) | ||
| RVUs | .77 (36/47) | .47 (9/19) | ||
| Main Site (n=23) | Satellite Sites (n=51) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| In‐house hospitalist coverage pre July 2011 | 1.0 (23/23) | .80 (41/51) | ||
| 24/7 | .35 (8/23) | .41 (17/41) | ||
| Day and evening | .17 (4/23) | .10 (4/41) | ||
| Day only | .48 (11/23) | .49 (20/41) | ||
| In‐house hospitalist coverage post July 2011 | 1.0 (23/23) | |||
| 24/7 | .52 (12/23) | .50 (19/38) | .75 (38/51) | |
| Day and evening | .17 (4/23) | .11 (4/38) | ||
| Day only | .30 (7/23) | .39 (15/38) | ||
| Night shift coverage | .83 (19/23) | .78 (18/23) | ||
| All share nights | .89 (17/19) | .94 (17/18) | ||
| Nocturnists | .26 (5/19) | .22 (4/18) | ||
| Moonlighters | .12 (2/19) | .17 (3/18) | ||
The vast majority of multiple‐site programs reported that their different clinical sites are considered parts of a single hospitalist program (96%), and that there is a designated medical director for each site (83%). However, only 70% of multiple‐site programs report that decisions concerning physician coverage are made as a group, and only 65% report that scheduling is done centrally. In addition, there is variability in how quality, safety, and patient satisfaction is reported (group vs site). The majority of programs report sharing revenues and expenses among the sites (Table 4).
| Proportion | Response Rate | |
|---|---|---|
| Sites regularly collaborate on: | 1.0 (23/23) | |
| Quality improvement projects | .74 (17/23) | |
| Safety initiatives | .74 (17/23) | |
| Research | .48 (11/23) | |
| Have a designated hospitalist medical director for each site | .83 (19/23) | 1.0 (23/23) |
| Different sites considered parts of a single hospitalist program | .96 (22/23) | 1.0 (23/23) |
| Make decisions on program/coverage/hour changes as a group | .70 (16/23) | 1.0 (23/23) |
| Scheduling done centrally | .65 (15/23) | 1.0 (23/23) |
| Report or track the following as individual sites: | ||
| Quality measures | .43 (9/21) | .91 (21/23) |
| Safety measures | .48 (10/21) | .91 (21/23) |
| Patient satisfaction | .50 (10/20) | .87 (20/23) |
| Report or track the following as a group: | ||
| Quality measures | .33 (7/21) | .91 (21/23) |
| Safety measures | .33 (7/21) | .91 (21/23) |
| Patient satisfaction | .30 (6/20) | .87 (20/23) |
| Report or track the following as both individual sites and as a group: | ||
| Quality measures | .24 (5/21) | .91 (21/23) |
| Safety measures | .19 (4/21) | .91 (21/23) |
| Patient satisfaction | .25 (4/20) | .87 (20/23) |
| Sites share revenues and expenses | .67 (14/21) | .91 (21/23) |
Organizational
Of the single‐site programs that answered the question Is your hospital medicine program considered its own division or a section within another division? 32% reported that their programs were considered its own division, and 68% reported that they were a part of another division, predominately (62%) general pediatrics, but also a few (6% combined) within emergency medicine, critical care, physical medicine and rehabilitation, and infectious diseases. Of the multiple‐site programs, a majority of 74% programs were their own division, and 26% were part of another division (Table 2). Respondents reported that their satellite sites included pediatric units in small community hospitals, small pediatric hospitals, large nonpediatric hospitals with pediatric units, rehabilitation facilities, and Shriner orthopedic hospitals.
Financial
Of the single‐site programs that answered the question Do patient revenues produced by your hospitalist group cover all expenses? only 26% reported that revenues exceeded expenses. Of the multiple‐site programs responding to this question, only 4% reported that the main site of their programs had revenues greater than expenses (Table 2). Programs used a combination of metrics to report revenue, and relative value unit (RVU)/medical doctor (MD) is the most commonly used metric to determine incentive pay (Table 2).
DISCUSSION
Our study demonstrates that academic PHM programs are common, which is consistent with previous data.[4, 7, 9, 14] The data support our belief that more institutions are planning on starting PHM programs. However, there exist much variability in a variety of program factors.[2, 3, 8, 9, 14] The fact that up to 35% of categorical pediatric residents are considering a career as a hospitalist further highlights the need for better data on PHM programs.[7]
We demonstrated that variability existed in hospitalist workload at academic PHM programs. We found considerable variation in the workload per hospitalist (large ranges and standard deviations), as well as variability in how an FTE is defined (hours/year, weeks/year, shifts/year) (Table 1). In addition, survey respondents might have interpreted certain questions differently, and this might have led to increased variability in the data. For example, the question concerning the definition of an FTE was worded as A clinical FTE is defined as. Some of the reported variation in workload might be partially explained by hospitalists having additional nonclinical responsibilities within hospital medicine or another field, including protected time for quality improvement, medical education, research, or administrative activities. Furthermore, some hospitalists might have clinical responsibilities outside of hospital medicine. Given that most PHM programs lack a formal internal definition of what it means to be a hospitalist,[7] it is not surprising to find such variation between programs. The variability in the extent of in‐house coverage provided by academic PHM programs, as well as institutional plans for increased coverage with the 2011 residency work‐hours restrictions is also described, and is consistent with other recently published data.[14] This is likely to continue, as 70% of academic PHM programs reported an anticipated increase in coverage in the near future,[14] suggesting that academic hospitalists are being used to help fill gaps in coverage left by changes in resident staffing.
Our data describe the percentage of academic programs that have a distinct division of hospital medicine. The fact that multisite programs were more likely to report being a distinct division might reflect the increased complexities of providing care at more than 1 site, requiring a greater infrastructure. This might be important in institutional planning as well as academic and financial expectations of academic pediatric hospitalists.
We also demonstrated that programs with multiple sites differ as far as the degree of integration of the various sites, with variation reported in decision making, scheduling, and how quality, safety, and patient satisfaction are reported (Table 4). Whether or not increased integration between the various clinical sites of a multiple‐site program is associated with better performance and/or physician satisfaction are questions that need to be answered. However, academic PHM directors would likely agree that there are great challenges inherent in managing these programs. These challenges include professional integration (do hospitalists based at satellite sites feel that they are academically supported?), clinical work/expectations (fewer resources and fewer learners at satellite sites likely affects workload), and administrative issues (physician scheduling likely becomes more complex as the number of sites increases). As programs continue to grow and provide clinical services in multiple geographic sites, it will become more important to understand how the different sites are coordinated to identify and develop best practices.
Older studies have described that the majority of PHM programs (70%78%) reported that professional revenues do not cover expenses, unfortunately these results were not stratified for program type (academic vs community).[2, 9]
Our study describes that few academic PHM programs (26% of single site, 4% of multiple‐site programs) report revenues (defined in our survey as only the collections from professional billing) in excess of expenses. This is consistent with prior studies that have included both academic and community PHM programs.[2] Therefore, it appears to be common for PHM programs to require institutional funding to cover all program expenses, as collections from professional billing are not generally adequate for this purpose. We believe that this is a critical point for both hospitalists and administrators to understand. However, it is equally important that they be transparent about the importance and value of the nonrevenue‐generating work performed by PHM programs. It has been reported that the vast majority of pediatric hospitalists are highly involved in education, quality improvement work, practice guideline development, and other work that is vitally important to institutions.[3] Furthermore, although one might expect PHM leaders to believe that their programs add value beyond the professional revenue collected,[9] even hospital leadership has been reported to perceive that PHM programs add value in several ways, including increased patient satisfaction (94%), increased referring MD satisfaction (90%), decreased length of stay (81%), and decreased costs (62%).[2] Pediatric residency and clerkship directors report that pediatric hospitalists are more accessible than other faculty (84% vs 64%) and are associated with an increase in the practice of evidence‐based medicine (76% vs 61%).[4] Therefore, there is strong evidence supporting that pediatric hospitalist programs provide important value that is not evident on a balance sheet.
In addition, our data also indicate that programs currently use a variety of metrics in combination to report productivity, and there is no accepted gold standard for measuring the performance of a hospitalist or hospitalist program (Table 2). Given that hospitalists generally cannot control how many patients they see, and given the fact that hospitalists are strongly perceived to provide value to their institutions beyond generating clinical revenue, metrics such as RVUs and charges likely do not accurately represent actual productivity.[2] Furthermore, it is likely that the metrics currently used underestimate actual productivity as they are not designed to take into account confounding factors that might affect hospitalist productivity. For example, consider an academic hospitalist who has clinical responsibilities divided between direct patient care and supervisory patient care (such as a team with some combination of residents, medical students, and physician extenders). When providing direct patient care, the hospitalist is likely responsible or all of the tasks usually performed by residents, including writing all patient notes and prescriptions, all communication with families, nurses, specialists, and primary care providers; and discharge planning. Conversely, when providing supervisory care, it is likely that the tasks are divided among the team members, and the hospitalist has the additional responsibility for providing teaching. However, the hospitalist might be responsible for more complex and acute patients. These factors are not adequately measured by RVUs or professional billing. Furthermore, these metrics do not capture the differences in providing in‐house daytime versus evening/night coverage, and do not measure the work performed while being on call when outside of the hospital. It is important for PHM programs and leaders to develop a better representation of the value provided by hospitalists, and for institutional leaders to understand this value, because previous work has suggested that the majority of hospital leaders do not plan to phase out the subsidy of hospitalists over time, as they do not anticipate the program(s) will be able to covercosts.[2] Given the realities of decreasing reimbursement and healthcare reform, it is unlikely to become more common for PHM programs to generate enough professional revenue to cover expenses.
The main strength of this descriptive study is the comprehensive nature of the survey, including many previously unreported data. In addition, the data are consistent with previously published work, which validates the quality of the data.
This study has several limitations including a low response rate and the exclusion of some hospitals or programs because they provided insufficient data for analysis. However, a post hoc analysis demonstrated that the majority of the institutions reporting that they did not have an academic PHM program (18/22), and those that were excluded due to insufficient data (12/17) were either smaller residency programs (<60 residents) or hospitals that were not the main site of a residency program. Therefore, our data likely are a good representation of academic PHM programs at larger academic institutions. Another potential weakness is that, although PHM program directors and pediatric residency directors were targeted, the respondent might not have been the person with the best knowledge of the program, which could have produced inaccurate data, particularly in terms of finances. However, the general consistency of our findings with previous work, particularly the high percentage of institutions with academic PHM programs,[4, 7, 9, 14] the low percentage of programs with revenues greater than expenses,[2, 9] and the trend toward increased in‐house coverage associated with the 2011 ACGME work‐hour restrictions,[14] supports the validity of our other results. In addition, survey respondents might have interpreted certain questions differently, specifically the questions concerning the definition of an FTE, and this might have led to increased variability in the data.
CONCLUSIONS
Academic PHM programs exist in the vast majority of academic centers, and more institutions are planning on starting programs in the next few years. There appears to be variability in a number of program factors, including hospitalist workload, in‐house coverage, and whether the program is a separate division or a section within another academic division. Many programs are currently providing care at more than 1 site. Programs uncommonly reported that their revenues exceeded their expenses. These data are the most comprehensive data existing for academic PHM programs.
Acknowledgment
Disclosure: Nothing to report.
- . Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107–112.
- , , . Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- , . Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179–186.
- , , . Hospitalists' involvement in pediatrics training: perspectives from pediatric residency program and clerkship directors. Acad Med. 2009;84(11):1617–1621.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , , , . Pediatric hospitalists' influences on education and career plans. J Hosp Med. 2012;7(4):282–286.
- , . Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7(4):350–357.
- , , , . Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(33):33–39.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition7(4):299–303.
Pediatric hospital medicine (PHM) is a relatively new field that has been growing rapidly over the past 20 years.[1] The field has been increasingly recognized for its contributions to high‐quality patient care, patient safety, systems improvement, medical education, and research.[2, 3, 4, 5, 6, 7, 8, 9] However, there appears to be significant variation among programs, even in basic factors such as how clinical effort is defined, the extent of in‐house coverage provided, and the scope of clinical services provided, and there exists a paucity of data describing these variations.[8]
Most previously published work did not specifically focus on academic programs,[2, 3, 8, 9] and specifically targeted hospital leadership,[2] practicing hospitalists,[3] residents,[7] and pediatric residency or clerkship directors,[4, 7] rather than hospitalist directors.[9] Furthermore, previous work focused on specific aspects of PHM programs such as education,[4, 7] value,[2] work environment,[9] and clinical practice,[3] rather than a more comprehensive approach.
We conducted a survey of academic PHM programs to learn about the current state and variation among programs across multiple domains (organizational, administrative, and financial). We speculated that:
- Many institutions currently lacking an academic PHM program were planning on starting a program in the next 3 years.
- Variability exists in hospitalist workload among programs.
- In programs providing clinical coverage at more than 1 site, variability exists in the relationship between the main site and satellite site(s) in terms of decision making, scheduling, and reporting of performance.
METHODS
Sample
We used the online American Medical Association Fellowship and Residency Electronic Interactive Database (FREIDA) to identify all 198 accredited pediatric residency training programs in the United States. A total of 246 hospitals were affiliated with these programs, and all of these were targeted for the survey. In addition, academic PHM program leaders were targeted directly with email invitations through the American Academy of Pediatrics (AAP) Section on Hospital Medicine LISTSERV.
Survey Instrument
A 49‐question online survey on the administrative, organizational, and financial aspects of academic PHM programs was developed with the input of academic PHM hospital leaders from Cincinnati Children's Hospital Medical Center and St. Louis Children's Hospital. First, the survey questions were developed de novo by the researchers. Then, multiple hospitalist leaders from each institution took the survey and gave feedback on content and structure. Using this feedback, changes were made and then tested by the leaders taking the new version of the survey. This process was repeated for 3 cycles until consensus was reached by the researchers on the final version of the survey. The survey contained questions that asked if the program provided coverage at a single site or at multiple sites and utilized a combination of open‐ended and fixed‐choice questions. For some questions, more than 1 answer was permitted. For the purposes of this survey, we utilized the following definitions adapted from the Society of Hospital Medicine. A hospitalist was defined as a physician who specializes in the practice of hospital medicine.[10] An academic PHM program was defined as any hospitalist practice associated with a pediatric residency program.[11] A nocturnist was defined as a hospitalist who predominantly works a schedule providing night coverage.[12]
Survey Administration
SurveyMonkey, an online survey software, was used to administer the survey. In June 2011, letters were mailed to all 246 hospitals affiliated with an accredited pediatric residency program as described above. These were addressed to either the hospital medicine director (if identified using the institutions Web site) or pediatric residency director. The letter asked the recipient to either participate in the survey or forward the survey to the physician best able to answer the survey. The letters included a description of the study and a link to the online survey. Of note, there was no follow‐up on this process. We also distributed the direct link to the survey and a copy of the letter utilizing the AAP Section on Hospital Medicine LISTSERV. Two reminders were sent through the LISTSERV in the month after the initial request. All respondents were informed that they would receive the deidentified raw data as an incentive to participate in the survey. Respondents were defined as those answering the first question, Does your program have an academic hospitalist program?
Statistical Analysis
Completed survey responses were extracted to Microsoft Excel (Microsoft Corp., Redmond, WA) for data analysis. Basic statistics were utilized to determine response rates for each question. Data were stratified for program type (single site or at multiple sites). For some questions, data were further stratified for the main site of multiple‐site programs for comparison to single‐site programs. In a few instances, more than 1 physician from a particular program responded to the survey. For these, the most appropriate respondent (PHM director, residency director, senior hospitalist) was identified utilizing the programs' publicly available Web site; only that physician's answers were used in the analysis.
Human Subjects Protection
This study was determined to be exempt from review by the Cincinnati Children's Hospital Medical Center and Washington University in St. Louis institutional review boards. All potential responders received written information about the survey. Survey design allowed for anonymous responses with voluntary documentation of program name and responders' contact information. The willingness to respond was qualified as implied consent. Data were deidentified prior to analysis and prior to sharing with the survey participants.
RESULTS
Response Rates
A total of 133 responses were received. Duplicate responses from the same program (13/133) were eliminated from the analysis. This yielded an overall response rate of 48.8% (120/246). A total of 81.7% (98/120) of institutions reported having an academic PHM program. Of the 18.3% (22/120) of institutions reporting not having a program, 9.1% (2/22) reported planning on starting a program in the next 3 years. Of the 98 respondents with an academic PHM program, 17 answered only the first survey question, Does your program have an academic hospitalist program? The remaining 81 completed surveys were left for further analysis. All of these respondents identified their program, and therefore we are certain that there were no duplicate responses in the analytic dataset. Of these, 23 (28%) indicated that their programs provided clinical care at multiple sites, and 58 (72%) indicated that their program provided care at a single site (Figure 1).

Administrative
Respondents reported wide variation for the definition of a 1.0 full‐time employee (FTE) hospitalist in their group. This included the units used (hours/year, weeks/year, shifts/year) as well as actual physician workload (Table 1). Weeks/year was the most common unit utilized by programs to define workload (66% of single‐site programs, 48% of multiple‐site programs), followed by hours/year (19%, 22%) and shifts/year (14%, 22%). The mean and median workload per FTE is represented (Table 1). The large ranges and the standard deviations from the mean indicate variability in workload per FTE (Table 1).
| Single‐Site Program | Multiple‐Site Programs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Programs | Mean | Median | SD | Range | % Programs | Mean | Median | SD | Range | |
| ||||||||||
| Weeks on service | 66 | 27.14 | 26 | 8.1 | 1246 | 48 | 27.2 | 24 | 9.6 | 1736 |
| Hours/year | 19 | 1886.25 | 1880 | 231.2 | 16002300 | 22 | 1767.33 | 1738 | 109.0 | 16641944 |
| Shifts/year* | 14 | 183 | 191 | 52.2 | 182240 | 22 | 191 | 184 | 38.3 | 155214 |
Scheduled in‐house hospitalist coverage also varied. Daytime coverage was defined as until 3 to 5 pm, evening coverage was defined a until 10 pm to midnight, and 24‐hour coverage was defined a 24/7. Programs reported plans to increase in‐house coverage with the implementation of the 2011 Accreditation Council for Graduate Medical Education (ACGME) resident work hours restrictions.[13] Among single‐site programs, there was a planned 50% increase in day/evening coverage (14% to 21%), with a planned decrease in day‐only coverage, and no change in 24/7 coverage (Table 2). Among the main sites of multiple‐site programs, there was a planned 50% increase in 24/7 in‐house coverage (35% to 52%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 3). Among the satellite sites of multiple‐site programs, there was a planned 9% increase in 24/7 coverage (41% to 50%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 2). Most programs reported that all hospitalists share night coverage (87% single site, 89% multiple sites) (Table 2). Multiple‐site programs were more likely than single‐site programs to use nocturnists, moonlighters, and incentives for those providing evening or night coverage (Table 2).
| Single Site (n=58) | Main Site of Multiple‐Site Programs (n=23) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| ||||
| Organizational | ||||
| Night shifts | .79 (46/58) | .83 (19/23) | ||
| All share nights | .87 (40/46) | .89 (17/19) | ||
| Nocturnists | .09 (4/46) | .26 (5/19) | ||
| Moonlighters | .04 (2/46) | .12 (2/19) | ||
| Night shift incentives | .74 (43/58) | .78 (18/23) | ||
| Financial | .12 (5/43) | .28 (5/18) | ||
| Time | .12 (5/43) | .22 (4/18) | ||
| No incentives | .79 (34/43) | .61 (11/18) | ||
| In‐house hospitalist coverage pre July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .35 (8/23) | ||
| Day and evening | .14 (8/58) | .17 (4/23) | ||
| Day only | .57 (33/58) | .48 (11/23) | ||
| In‐house hospitalist coverage post July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .52 (12/23) | ||
| Day and evening | .21 (12/58) | .17 (4/23) | ||
| Day only | .50 (29/58) | .30 (7/23) | ||
| Administrative | ||||
| Own division | .32 (18/57) | .98 (57/58) | .74 (17/23) | 1.0 (23/23) |
| Part of another division | .68 (39/57) | .26 (6/23) | ||
| Financial | ||||
| Revenues>expenses | .26 (14/53) | .91 (53/58) | .04 (1/23) | .04 (19/23) |
| Incentives supplement base salary | .45 (25/55) | .95 (55/58) | .48 (10/21) | .91 (21/23) |
| Metrics used to determine incentivesb | .47 (27/58) | .52 (12/23) | ||
| RVUs/MD | .85 (23/27) | .83 (10/12) | ||
| Costs/discharge | .19 (5/27) | .08 (1/12) | ||
| Financial reportingb | .81 (47/58) | .04 (19/23) | ||
| Charges | .64 (30/47) | .68 (13/19) | ||
| Collections | .66 (31/47) | .68 (13/19) | ||
| RVUs | .77 (36/47) | .47 (9/19) | ||
| Main Site (n=23) | Satellite Sites (n=51) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| In‐house hospitalist coverage pre July 2011 | 1.0 (23/23) | .80 (41/51) | ||
| 24/7 | .35 (8/23) | .41 (17/41) | ||
| Day and evening | .17 (4/23) | .10 (4/41) | ||
| Day only | .48 (11/23) | .49 (20/41) | ||
| In‐house hospitalist coverage post July 2011 | 1.0 (23/23) | |||
| 24/7 | .52 (12/23) | .50 (19/38) | .75 (38/51) | |
| Day and evening | .17 (4/23) | .11 (4/38) | ||
| Day only | .30 (7/23) | .39 (15/38) | ||
| Night shift coverage | .83 (19/23) | .78 (18/23) | ||
| All share nights | .89 (17/19) | .94 (17/18) | ||
| Nocturnists | .26 (5/19) | .22 (4/18) | ||
| Moonlighters | .12 (2/19) | .17 (3/18) | ||
The vast majority of multiple‐site programs reported that their different clinical sites are considered parts of a single hospitalist program (96%), and that there is a designated medical director for each site (83%). However, only 70% of multiple‐site programs report that decisions concerning physician coverage are made as a group, and only 65% report that scheduling is done centrally. In addition, there is variability in how quality, safety, and patient satisfaction is reported (group vs site). The majority of programs report sharing revenues and expenses among the sites (Table 4).
| Proportion | Response Rate | |
|---|---|---|
| Sites regularly collaborate on: | 1.0 (23/23) | |
| Quality improvement projects | .74 (17/23) | |
| Safety initiatives | .74 (17/23) | |
| Research | .48 (11/23) | |
| Have a designated hospitalist medical director for each site | .83 (19/23) | 1.0 (23/23) |
| Different sites considered parts of a single hospitalist program | .96 (22/23) | 1.0 (23/23) |
| Make decisions on program/coverage/hour changes as a group | .70 (16/23) | 1.0 (23/23) |
| Scheduling done centrally | .65 (15/23) | 1.0 (23/23) |
| Report or track the following as individual sites: | ||
| Quality measures | .43 (9/21) | .91 (21/23) |
| Safety measures | .48 (10/21) | .91 (21/23) |
| Patient satisfaction | .50 (10/20) | .87 (20/23) |
| Report or track the following as a group: | ||
| Quality measures | .33 (7/21) | .91 (21/23) |
| Safety measures | .33 (7/21) | .91 (21/23) |
| Patient satisfaction | .30 (6/20) | .87 (20/23) |
| Report or track the following as both individual sites and as a group: | ||
| Quality measures | .24 (5/21) | .91 (21/23) |
| Safety measures | .19 (4/21) | .91 (21/23) |
| Patient satisfaction | .25 (4/20) | .87 (20/23) |
| Sites share revenues and expenses | .67 (14/21) | .91 (21/23) |
Organizational
Of the single‐site programs that answered the question Is your hospital medicine program considered its own division or a section within another division? 32% reported that their programs were considered its own division, and 68% reported that they were a part of another division, predominately (62%) general pediatrics, but also a few (6% combined) within emergency medicine, critical care, physical medicine and rehabilitation, and infectious diseases. Of the multiple‐site programs, a majority of 74% programs were their own division, and 26% were part of another division (Table 2). Respondents reported that their satellite sites included pediatric units in small community hospitals, small pediatric hospitals, large nonpediatric hospitals with pediatric units, rehabilitation facilities, and Shriner orthopedic hospitals.
Financial
Of the single‐site programs that answered the question Do patient revenues produced by your hospitalist group cover all expenses? only 26% reported that revenues exceeded expenses. Of the multiple‐site programs responding to this question, only 4% reported that the main site of their programs had revenues greater than expenses (Table 2). Programs used a combination of metrics to report revenue, and relative value unit (RVU)/medical doctor (MD) is the most commonly used metric to determine incentive pay (Table 2).
DISCUSSION
Our study demonstrates that academic PHM programs are common, which is consistent with previous data.[4, 7, 9, 14] The data support our belief that more institutions are planning on starting PHM programs. However, there exist much variability in a variety of program factors.[2, 3, 8, 9, 14] The fact that up to 35% of categorical pediatric residents are considering a career as a hospitalist further highlights the need for better data on PHM programs.[7]
We demonstrated that variability existed in hospitalist workload at academic PHM programs. We found considerable variation in the workload per hospitalist (large ranges and standard deviations), as well as variability in how an FTE is defined (hours/year, weeks/year, shifts/year) (Table 1). In addition, survey respondents might have interpreted certain questions differently, and this might have led to increased variability in the data. For example, the question concerning the definition of an FTE was worded as A clinical FTE is defined as. Some of the reported variation in workload might be partially explained by hospitalists having additional nonclinical responsibilities within hospital medicine or another field, including protected time for quality improvement, medical education, research, or administrative activities. Furthermore, some hospitalists might have clinical responsibilities outside of hospital medicine. Given that most PHM programs lack a formal internal definition of what it means to be a hospitalist,[7] it is not surprising to find such variation between programs. The variability in the extent of in‐house coverage provided by academic PHM programs, as well as institutional plans for increased coverage with the 2011 residency work‐hours restrictions is also described, and is consistent with other recently published data.[14] This is likely to continue, as 70% of academic PHM programs reported an anticipated increase in coverage in the near future,[14] suggesting that academic hospitalists are being used to help fill gaps in coverage left by changes in resident staffing.
Our data describe the percentage of academic programs that have a distinct division of hospital medicine. The fact that multisite programs were more likely to report being a distinct division might reflect the increased complexities of providing care at more than 1 site, requiring a greater infrastructure. This might be important in institutional planning as well as academic and financial expectations of academic pediatric hospitalists.
We also demonstrated that programs with multiple sites differ as far as the degree of integration of the various sites, with variation reported in decision making, scheduling, and how quality, safety, and patient satisfaction are reported (Table 4). Whether or not increased integration between the various clinical sites of a multiple‐site program is associated with better performance and/or physician satisfaction are questions that need to be answered. However, academic PHM directors would likely agree that there are great challenges inherent in managing these programs. These challenges include professional integration (do hospitalists based at satellite sites feel that they are academically supported?), clinical work/expectations (fewer resources and fewer learners at satellite sites likely affects workload), and administrative issues (physician scheduling likely becomes more complex as the number of sites increases). As programs continue to grow and provide clinical services in multiple geographic sites, it will become more important to understand how the different sites are coordinated to identify and develop best practices.
Older studies have described that the majority of PHM programs (70%78%) reported that professional revenues do not cover expenses, unfortunately these results were not stratified for program type (academic vs community).[2, 9]
Our study describes that few academic PHM programs (26% of single site, 4% of multiple‐site programs) report revenues (defined in our survey as only the collections from professional billing) in excess of expenses. This is consistent with prior studies that have included both academic and community PHM programs.[2] Therefore, it appears to be common for PHM programs to require institutional funding to cover all program expenses, as collections from professional billing are not generally adequate for this purpose. We believe that this is a critical point for both hospitalists and administrators to understand. However, it is equally important that they be transparent about the importance and value of the nonrevenue‐generating work performed by PHM programs. It has been reported that the vast majority of pediatric hospitalists are highly involved in education, quality improvement work, practice guideline development, and other work that is vitally important to institutions.[3] Furthermore, although one might expect PHM leaders to believe that their programs add value beyond the professional revenue collected,[9] even hospital leadership has been reported to perceive that PHM programs add value in several ways, including increased patient satisfaction (94%), increased referring MD satisfaction (90%), decreased length of stay (81%), and decreased costs (62%).[2] Pediatric residency and clerkship directors report that pediatric hospitalists are more accessible than other faculty (84% vs 64%) and are associated with an increase in the practice of evidence‐based medicine (76% vs 61%).[4] Therefore, there is strong evidence supporting that pediatric hospitalist programs provide important value that is not evident on a balance sheet.
In addition, our data also indicate that programs currently use a variety of metrics in combination to report productivity, and there is no accepted gold standard for measuring the performance of a hospitalist or hospitalist program (Table 2). Given that hospitalists generally cannot control how many patients they see, and given the fact that hospitalists are strongly perceived to provide value to their institutions beyond generating clinical revenue, metrics such as RVUs and charges likely do not accurately represent actual productivity.[2] Furthermore, it is likely that the metrics currently used underestimate actual productivity as they are not designed to take into account confounding factors that might affect hospitalist productivity. For example, consider an academic hospitalist who has clinical responsibilities divided between direct patient care and supervisory patient care (such as a team with some combination of residents, medical students, and physician extenders). When providing direct patient care, the hospitalist is likely responsible or all of the tasks usually performed by residents, including writing all patient notes and prescriptions, all communication with families, nurses, specialists, and primary care providers; and discharge planning. Conversely, when providing supervisory care, it is likely that the tasks are divided among the team members, and the hospitalist has the additional responsibility for providing teaching. However, the hospitalist might be responsible for more complex and acute patients. These factors are not adequately measured by RVUs or professional billing. Furthermore, these metrics do not capture the differences in providing in‐house daytime versus evening/night coverage, and do not measure the work performed while being on call when outside of the hospital. It is important for PHM programs and leaders to develop a better representation of the value provided by hospitalists, and for institutional leaders to understand this value, because previous work has suggested that the majority of hospital leaders do not plan to phase out the subsidy of hospitalists over time, as they do not anticipate the program(s) will be able to covercosts.[2] Given the realities of decreasing reimbursement and healthcare reform, it is unlikely to become more common for PHM programs to generate enough professional revenue to cover expenses.
The main strength of this descriptive study is the comprehensive nature of the survey, including many previously unreported data. In addition, the data are consistent with previously published work, which validates the quality of the data.
This study has several limitations including a low response rate and the exclusion of some hospitals or programs because they provided insufficient data for analysis. However, a post hoc analysis demonstrated that the majority of the institutions reporting that they did not have an academic PHM program (18/22), and those that were excluded due to insufficient data (12/17) were either smaller residency programs (<60 residents) or hospitals that were not the main site of a residency program. Therefore, our data likely are a good representation of academic PHM programs at larger academic institutions. Another potential weakness is that, although PHM program directors and pediatric residency directors were targeted, the respondent might not have been the person with the best knowledge of the program, which could have produced inaccurate data, particularly in terms of finances. However, the general consistency of our findings with previous work, particularly the high percentage of institutions with academic PHM programs,[4, 7, 9, 14] the low percentage of programs with revenues greater than expenses,[2, 9] and the trend toward increased in‐house coverage associated with the 2011 ACGME work‐hour restrictions,[14] supports the validity of our other results. In addition, survey respondents might have interpreted certain questions differently, specifically the questions concerning the definition of an FTE, and this might have led to increased variability in the data.
CONCLUSIONS
Academic PHM programs exist in the vast majority of academic centers, and more institutions are planning on starting programs in the next few years. There appears to be variability in a number of program factors, including hospitalist workload, in‐house coverage, and whether the program is a separate division or a section within another academic division. Many programs are currently providing care at more than 1 site. Programs uncommonly reported that their revenues exceeded their expenses. These data are the most comprehensive data existing for academic PHM programs.
Acknowledgment
Disclosure: Nothing to report.
Pediatric hospital medicine (PHM) is a relatively new field that has been growing rapidly over the past 20 years.[1] The field has been increasingly recognized for its contributions to high‐quality patient care, patient safety, systems improvement, medical education, and research.[2, 3, 4, 5, 6, 7, 8, 9] However, there appears to be significant variation among programs, even in basic factors such as how clinical effort is defined, the extent of in‐house coverage provided, and the scope of clinical services provided, and there exists a paucity of data describing these variations.[8]
Most previously published work did not specifically focus on academic programs,[2, 3, 8, 9] and specifically targeted hospital leadership,[2] practicing hospitalists,[3] residents,[7] and pediatric residency or clerkship directors,[4, 7] rather than hospitalist directors.[9] Furthermore, previous work focused on specific aspects of PHM programs such as education,[4, 7] value,[2] work environment,[9] and clinical practice,[3] rather than a more comprehensive approach.
We conducted a survey of academic PHM programs to learn about the current state and variation among programs across multiple domains (organizational, administrative, and financial). We speculated that:
- Many institutions currently lacking an academic PHM program were planning on starting a program in the next 3 years.
- Variability exists in hospitalist workload among programs.
- In programs providing clinical coverage at more than 1 site, variability exists in the relationship between the main site and satellite site(s) in terms of decision making, scheduling, and reporting of performance.
METHODS
Sample
We used the online American Medical Association Fellowship and Residency Electronic Interactive Database (FREIDA) to identify all 198 accredited pediatric residency training programs in the United States. A total of 246 hospitals were affiliated with these programs, and all of these were targeted for the survey. In addition, academic PHM program leaders were targeted directly with email invitations through the American Academy of Pediatrics (AAP) Section on Hospital Medicine LISTSERV.
Survey Instrument
A 49‐question online survey on the administrative, organizational, and financial aspects of academic PHM programs was developed with the input of academic PHM hospital leaders from Cincinnati Children's Hospital Medical Center and St. Louis Children's Hospital. First, the survey questions were developed de novo by the researchers. Then, multiple hospitalist leaders from each institution took the survey and gave feedback on content and structure. Using this feedback, changes were made and then tested by the leaders taking the new version of the survey. This process was repeated for 3 cycles until consensus was reached by the researchers on the final version of the survey. The survey contained questions that asked if the program provided coverage at a single site or at multiple sites and utilized a combination of open‐ended and fixed‐choice questions. For some questions, more than 1 answer was permitted. For the purposes of this survey, we utilized the following definitions adapted from the Society of Hospital Medicine. A hospitalist was defined as a physician who specializes in the practice of hospital medicine.[10] An academic PHM program was defined as any hospitalist practice associated with a pediatric residency program.[11] A nocturnist was defined as a hospitalist who predominantly works a schedule providing night coverage.[12]
Survey Administration
SurveyMonkey, an online survey software, was used to administer the survey. In June 2011, letters were mailed to all 246 hospitals affiliated with an accredited pediatric residency program as described above. These were addressed to either the hospital medicine director (if identified using the institutions Web site) or pediatric residency director. The letter asked the recipient to either participate in the survey or forward the survey to the physician best able to answer the survey. The letters included a description of the study and a link to the online survey. Of note, there was no follow‐up on this process. We also distributed the direct link to the survey and a copy of the letter utilizing the AAP Section on Hospital Medicine LISTSERV. Two reminders were sent through the LISTSERV in the month after the initial request. All respondents were informed that they would receive the deidentified raw data as an incentive to participate in the survey. Respondents were defined as those answering the first question, Does your program have an academic hospitalist program?
Statistical Analysis
Completed survey responses were extracted to Microsoft Excel (Microsoft Corp., Redmond, WA) for data analysis. Basic statistics were utilized to determine response rates for each question. Data were stratified for program type (single site or at multiple sites). For some questions, data were further stratified for the main site of multiple‐site programs for comparison to single‐site programs. In a few instances, more than 1 physician from a particular program responded to the survey. For these, the most appropriate respondent (PHM director, residency director, senior hospitalist) was identified utilizing the programs' publicly available Web site; only that physician's answers were used in the analysis.
Human Subjects Protection
This study was determined to be exempt from review by the Cincinnati Children's Hospital Medical Center and Washington University in St. Louis institutional review boards. All potential responders received written information about the survey. Survey design allowed for anonymous responses with voluntary documentation of program name and responders' contact information. The willingness to respond was qualified as implied consent. Data were deidentified prior to analysis and prior to sharing with the survey participants.
RESULTS
Response Rates
A total of 133 responses were received. Duplicate responses from the same program (13/133) were eliminated from the analysis. This yielded an overall response rate of 48.8% (120/246). A total of 81.7% (98/120) of institutions reported having an academic PHM program. Of the 18.3% (22/120) of institutions reporting not having a program, 9.1% (2/22) reported planning on starting a program in the next 3 years. Of the 98 respondents with an academic PHM program, 17 answered only the first survey question, Does your program have an academic hospitalist program? The remaining 81 completed surveys were left for further analysis. All of these respondents identified their program, and therefore we are certain that there were no duplicate responses in the analytic dataset. Of these, 23 (28%) indicated that their programs provided clinical care at multiple sites, and 58 (72%) indicated that their program provided care at a single site (Figure 1).

Administrative
Respondents reported wide variation for the definition of a 1.0 full‐time employee (FTE) hospitalist in their group. This included the units used (hours/year, weeks/year, shifts/year) as well as actual physician workload (Table 1). Weeks/year was the most common unit utilized by programs to define workload (66% of single‐site programs, 48% of multiple‐site programs), followed by hours/year (19%, 22%) and shifts/year (14%, 22%). The mean and median workload per FTE is represented (Table 1). The large ranges and the standard deviations from the mean indicate variability in workload per FTE (Table 1).
| Single‐Site Program | Multiple‐Site Programs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Programs | Mean | Median | SD | Range | % Programs | Mean | Median | SD | Range | |
| ||||||||||
| Weeks on service | 66 | 27.14 | 26 | 8.1 | 1246 | 48 | 27.2 | 24 | 9.6 | 1736 |
| Hours/year | 19 | 1886.25 | 1880 | 231.2 | 16002300 | 22 | 1767.33 | 1738 | 109.0 | 16641944 |
| Shifts/year* | 14 | 183 | 191 | 52.2 | 182240 | 22 | 191 | 184 | 38.3 | 155214 |
Scheduled in‐house hospitalist coverage also varied. Daytime coverage was defined as until 3 to 5 pm, evening coverage was defined a until 10 pm to midnight, and 24‐hour coverage was defined a 24/7. Programs reported plans to increase in‐house coverage with the implementation of the 2011 Accreditation Council for Graduate Medical Education (ACGME) resident work hours restrictions.[13] Among single‐site programs, there was a planned 50% increase in day/evening coverage (14% to 21%), with a planned decrease in day‐only coverage, and no change in 24/7 coverage (Table 2). Among the main sites of multiple‐site programs, there was a planned 50% increase in 24/7 in‐house coverage (35% to 52%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 3). Among the satellite sites of multiple‐site programs, there was a planned 9% increase in 24/7 coverage (41% to 50%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 2). Most programs reported that all hospitalists share night coverage (87% single site, 89% multiple sites) (Table 2). Multiple‐site programs were more likely than single‐site programs to use nocturnists, moonlighters, and incentives for those providing evening or night coverage (Table 2).
| Single Site (n=58) | Main Site of Multiple‐Site Programs (n=23) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| ||||
| Organizational | ||||
| Night shifts | .79 (46/58) | .83 (19/23) | ||
| All share nights | .87 (40/46) | .89 (17/19) | ||
| Nocturnists | .09 (4/46) | .26 (5/19) | ||
| Moonlighters | .04 (2/46) | .12 (2/19) | ||
| Night shift incentives | .74 (43/58) | .78 (18/23) | ||
| Financial | .12 (5/43) | .28 (5/18) | ||
| Time | .12 (5/43) | .22 (4/18) | ||
| No incentives | .79 (34/43) | .61 (11/18) | ||
| In‐house hospitalist coverage pre July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .35 (8/23) | ||
| Day and evening | .14 (8/58) | .17 (4/23) | ||
| Day only | .57 (33/58) | .48 (11/23) | ||
| In‐house hospitalist coverage post July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .52 (12/23) | ||
| Day and evening | .21 (12/58) | .17 (4/23) | ||
| Day only | .50 (29/58) | .30 (7/23) | ||
| Administrative | ||||
| Own division | .32 (18/57) | .98 (57/58) | .74 (17/23) | 1.0 (23/23) |
| Part of another division | .68 (39/57) | .26 (6/23) | ||
| Financial | ||||
| Revenues>expenses | .26 (14/53) | .91 (53/58) | .04 (1/23) | .04 (19/23) |
| Incentives supplement base salary | .45 (25/55) | .95 (55/58) | .48 (10/21) | .91 (21/23) |
| Metrics used to determine incentivesb | .47 (27/58) | .52 (12/23) | ||
| RVUs/MD | .85 (23/27) | .83 (10/12) | ||
| Costs/discharge | .19 (5/27) | .08 (1/12) | ||
| Financial reportingb | .81 (47/58) | .04 (19/23) | ||
| Charges | .64 (30/47) | .68 (13/19) | ||
| Collections | .66 (31/47) | .68 (13/19) | ||
| RVUs | .77 (36/47) | .47 (9/19) | ||
| Main Site (n=23) | Satellite Sites (n=51) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| In‐house hospitalist coverage pre July 2011 | 1.0 (23/23) | .80 (41/51) | ||
| 24/7 | .35 (8/23) | .41 (17/41) | ||
| Day and evening | .17 (4/23) | .10 (4/41) | ||
| Day only | .48 (11/23) | .49 (20/41) | ||
| In‐house hospitalist coverage post July 2011 | 1.0 (23/23) | |||
| 24/7 | .52 (12/23) | .50 (19/38) | .75 (38/51) | |
| Day and evening | .17 (4/23) | .11 (4/38) | ||
| Day only | .30 (7/23) | .39 (15/38) | ||
| Night shift coverage | .83 (19/23) | .78 (18/23) | ||
| All share nights | .89 (17/19) | .94 (17/18) | ||
| Nocturnists | .26 (5/19) | .22 (4/18) | ||
| Moonlighters | .12 (2/19) | .17 (3/18) | ||
The vast majority of multiple‐site programs reported that their different clinical sites are considered parts of a single hospitalist program (96%), and that there is a designated medical director for each site (83%). However, only 70% of multiple‐site programs report that decisions concerning physician coverage are made as a group, and only 65% report that scheduling is done centrally. In addition, there is variability in how quality, safety, and patient satisfaction is reported (group vs site). The majority of programs report sharing revenues and expenses among the sites (Table 4).
| Proportion | Response Rate | |
|---|---|---|
| Sites regularly collaborate on: | 1.0 (23/23) | |
| Quality improvement projects | .74 (17/23) | |
| Safety initiatives | .74 (17/23) | |
| Research | .48 (11/23) | |
| Have a designated hospitalist medical director for each site | .83 (19/23) | 1.0 (23/23) |
| Different sites considered parts of a single hospitalist program | .96 (22/23) | 1.0 (23/23) |
| Make decisions on program/coverage/hour changes as a group | .70 (16/23) | 1.0 (23/23) |
| Scheduling done centrally | .65 (15/23) | 1.0 (23/23) |
| Report or track the following as individual sites: | ||
| Quality measures | .43 (9/21) | .91 (21/23) |
| Safety measures | .48 (10/21) | .91 (21/23) |
| Patient satisfaction | .50 (10/20) | .87 (20/23) |
| Report or track the following as a group: | ||
| Quality measures | .33 (7/21) | .91 (21/23) |
| Safety measures | .33 (7/21) | .91 (21/23) |
| Patient satisfaction | .30 (6/20) | .87 (20/23) |
| Report or track the following as both individual sites and as a group: | ||
| Quality measures | .24 (5/21) | .91 (21/23) |
| Safety measures | .19 (4/21) | .91 (21/23) |
| Patient satisfaction | .25 (4/20) | .87 (20/23) |
| Sites share revenues and expenses | .67 (14/21) | .91 (21/23) |
Organizational
Of the single‐site programs that answered the question Is your hospital medicine program considered its own division or a section within another division? 32% reported that their programs were considered its own division, and 68% reported that they were a part of another division, predominately (62%) general pediatrics, but also a few (6% combined) within emergency medicine, critical care, physical medicine and rehabilitation, and infectious diseases. Of the multiple‐site programs, a majority of 74% programs were their own division, and 26% were part of another division (Table 2). Respondents reported that their satellite sites included pediatric units in small community hospitals, small pediatric hospitals, large nonpediatric hospitals with pediatric units, rehabilitation facilities, and Shriner orthopedic hospitals.
Financial
Of the single‐site programs that answered the question Do patient revenues produced by your hospitalist group cover all expenses? only 26% reported that revenues exceeded expenses. Of the multiple‐site programs responding to this question, only 4% reported that the main site of their programs had revenues greater than expenses (Table 2). Programs used a combination of metrics to report revenue, and relative value unit (RVU)/medical doctor (MD) is the most commonly used metric to determine incentive pay (Table 2).
DISCUSSION
Our study demonstrates that academic PHM programs are common, which is consistent with previous data.[4, 7, 9, 14] The data support our belief that more institutions are planning on starting PHM programs. However, there exist much variability in a variety of program factors.[2, 3, 8, 9, 14] The fact that up to 35% of categorical pediatric residents are considering a career as a hospitalist further highlights the need for better data on PHM programs.[7]
We demonstrated that variability existed in hospitalist workload at academic PHM programs. We found considerable variation in the workload per hospitalist (large ranges and standard deviations), as well as variability in how an FTE is defined (hours/year, weeks/year, shifts/year) (Table 1). In addition, survey respondents might have interpreted certain questions differently, and this might have led to increased variability in the data. For example, the question concerning the definition of an FTE was worded as A clinical FTE is defined as. Some of the reported variation in workload might be partially explained by hospitalists having additional nonclinical responsibilities within hospital medicine or another field, including protected time for quality improvement, medical education, research, or administrative activities. Furthermore, some hospitalists might have clinical responsibilities outside of hospital medicine. Given that most PHM programs lack a formal internal definition of what it means to be a hospitalist,[7] it is not surprising to find such variation between programs. The variability in the extent of in‐house coverage provided by academic PHM programs, as well as institutional plans for increased coverage with the 2011 residency work‐hours restrictions is also described, and is consistent with other recently published data.[14] This is likely to continue, as 70% of academic PHM programs reported an anticipated increase in coverage in the near future,[14] suggesting that academic hospitalists are being used to help fill gaps in coverage left by changes in resident staffing.
Our data describe the percentage of academic programs that have a distinct division of hospital medicine. The fact that multisite programs were more likely to report being a distinct division might reflect the increased complexities of providing care at more than 1 site, requiring a greater infrastructure. This might be important in institutional planning as well as academic and financial expectations of academic pediatric hospitalists.
We also demonstrated that programs with multiple sites differ as far as the degree of integration of the various sites, with variation reported in decision making, scheduling, and how quality, safety, and patient satisfaction are reported (Table 4). Whether or not increased integration between the various clinical sites of a multiple‐site program is associated with better performance and/or physician satisfaction are questions that need to be answered. However, academic PHM directors would likely agree that there are great challenges inherent in managing these programs. These challenges include professional integration (do hospitalists based at satellite sites feel that they are academically supported?), clinical work/expectations (fewer resources and fewer learners at satellite sites likely affects workload), and administrative issues (physician scheduling likely becomes more complex as the number of sites increases). As programs continue to grow and provide clinical services in multiple geographic sites, it will become more important to understand how the different sites are coordinated to identify and develop best practices.
Older studies have described that the majority of PHM programs (70%78%) reported that professional revenues do not cover expenses, unfortunately these results were not stratified for program type (academic vs community).[2, 9]
Our study describes that few academic PHM programs (26% of single site, 4% of multiple‐site programs) report revenues (defined in our survey as only the collections from professional billing) in excess of expenses. This is consistent with prior studies that have included both academic and community PHM programs.[2] Therefore, it appears to be common for PHM programs to require institutional funding to cover all program expenses, as collections from professional billing are not generally adequate for this purpose. We believe that this is a critical point for both hospitalists and administrators to understand. However, it is equally important that they be transparent about the importance and value of the nonrevenue‐generating work performed by PHM programs. It has been reported that the vast majority of pediatric hospitalists are highly involved in education, quality improvement work, practice guideline development, and other work that is vitally important to institutions.[3] Furthermore, although one might expect PHM leaders to believe that their programs add value beyond the professional revenue collected,[9] even hospital leadership has been reported to perceive that PHM programs add value in several ways, including increased patient satisfaction (94%), increased referring MD satisfaction (90%), decreased length of stay (81%), and decreased costs (62%).[2] Pediatric residency and clerkship directors report that pediatric hospitalists are more accessible than other faculty (84% vs 64%) and are associated with an increase in the practice of evidence‐based medicine (76% vs 61%).[4] Therefore, there is strong evidence supporting that pediatric hospitalist programs provide important value that is not evident on a balance sheet.
In addition, our data also indicate that programs currently use a variety of metrics in combination to report productivity, and there is no accepted gold standard for measuring the performance of a hospitalist or hospitalist program (Table 2). Given that hospitalists generally cannot control how many patients they see, and given the fact that hospitalists are strongly perceived to provide value to their institutions beyond generating clinical revenue, metrics such as RVUs and charges likely do not accurately represent actual productivity.[2] Furthermore, it is likely that the metrics currently used underestimate actual productivity as they are not designed to take into account confounding factors that might affect hospitalist productivity. For example, consider an academic hospitalist who has clinical responsibilities divided between direct patient care and supervisory patient care (such as a team with some combination of residents, medical students, and physician extenders). When providing direct patient care, the hospitalist is likely responsible or all of the tasks usually performed by residents, including writing all patient notes and prescriptions, all communication with families, nurses, specialists, and primary care providers; and discharge planning. Conversely, when providing supervisory care, it is likely that the tasks are divided among the team members, and the hospitalist has the additional responsibility for providing teaching. However, the hospitalist might be responsible for more complex and acute patients. These factors are not adequately measured by RVUs or professional billing. Furthermore, these metrics do not capture the differences in providing in‐house daytime versus evening/night coverage, and do not measure the work performed while being on call when outside of the hospital. It is important for PHM programs and leaders to develop a better representation of the value provided by hospitalists, and for institutional leaders to understand this value, because previous work has suggested that the majority of hospital leaders do not plan to phase out the subsidy of hospitalists over time, as they do not anticipate the program(s) will be able to covercosts.[2] Given the realities of decreasing reimbursement and healthcare reform, it is unlikely to become more common for PHM programs to generate enough professional revenue to cover expenses.
The main strength of this descriptive study is the comprehensive nature of the survey, including many previously unreported data. In addition, the data are consistent with previously published work, which validates the quality of the data.
This study has several limitations including a low response rate and the exclusion of some hospitals or programs because they provided insufficient data for analysis. However, a post hoc analysis demonstrated that the majority of the institutions reporting that they did not have an academic PHM program (18/22), and those that were excluded due to insufficient data (12/17) were either smaller residency programs (<60 residents) or hospitals that were not the main site of a residency program. Therefore, our data likely are a good representation of academic PHM programs at larger academic institutions. Another potential weakness is that, although PHM program directors and pediatric residency directors were targeted, the respondent might not have been the person with the best knowledge of the program, which could have produced inaccurate data, particularly in terms of finances. However, the general consistency of our findings with previous work, particularly the high percentage of institutions with academic PHM programs,[4, 7, 9, 14] the low percentage of programs with revenues greater than expenses,[2, 9] and the trend toward increased in‐house coverage associated with the 2011 ACGME work‐hour restrictions,[14] supports the validity of our other results. In addition, survey respondents might have interpreted certain questions differently, specifically the questions concerning the definition of an FTE, and this might have led to increased variability in the data.
CONCLUSIONS
Academic PHM programs exist in the vast majority of academic centers, and more institutions are planning on starting programs in the next few years. There appears to be variability in a number of program factors, including hospitalist workload, in‐house coverage, and whether the program is a separate division or a section within another academic division. Many programs are currently providing care at more than 1 site. Programs uncommonly reported that their revenues exceeded their expenses. These data are the most comprehensive data existing for academic PHM programs.
Acknowledgment
Disclosure: Nothing to report.
- . Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107–112.
- , , . Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- , . Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179–186.
- , , . Hospitalists' involvement in pediatrics training: perspectives from pediatric residency program and clerkship directors. Acad Med. 2009;84(11):1617–1621.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , , , . Pediatric hospitalists' influences on education and career plans. J Hosp Med. 2012;7(4):282–286.
- , . Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7(4):350–357.
- , , , . Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(33):33–39.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition7(4):299–303.
- . Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107–112.
- , , . Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- , . Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179–186.
- , , . Hospitalists' involvement in pediatrics training: perspectives from pediatric residency program and clerkship directors. Acad Med. 2009;84(11):1617–1621.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , , , . Pediatric hospitalists' influences on education and career plans. J Hosp Med. 2012;7(4):282–286.
- , . Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7(4):350–357.
- , , , . Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(33):33–39.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition7(4):299–303.
Copyright © 2013 Society of Hospital Medicine
Preventing the intergenerational transmission of trauma
Intergenerational trauma often proves to be a prevailing feature of family systems.
The trauma of the Nazi concentration camps, for example, can be re-experienced in the lives of the children of camp survivors. Even the grandchildren of Holocaust survivors have been found to suffer from the effects of trauma. These effects manifest through characteristics such as increased suspiciousness of others, anger, and irritability in these individuals compared with controls (J. Relig. Health 2011;50:321-9).
Such intergenerational trauma has been found among urban American Indian and Alaska Native populations who have been involved in culturally specific sobriety maintenance programs (Am. Indian Alsk. Native Ment. Health Res. 2011;18:17-40). Likewise, a body of research supports the notion that untreated intergenerational trauma tied to generations of slavery in the United States continues to negatively affect many in the black community.
Other kinds of trauma can be passed down through the generations, as well. Take the trauma of a combat soldier; victim of or prisoner of war; survivor of a mass shooting or of child abuse; witness of genocide; or survivor of colonial suppression, slavery, or political totalitarianism. People who have experienced these traumas can pass down the consequences to subsequent generations.
We know that people who suffer trauma firsthand often develop posttraumatic stress disorder symptoms (PTSD) symptoms such as fearfulness, nightmares, flashbacks, sorrow, and difficulty with emotional closeness. However, it also is clear that compared with controls, the children of veterans with PTSD have shown an inability to experience appropriate emotional responses to situations and difficulty in solving problems effectively both within and outside the family unit (Aust. N.Z. J. Psychiatry 2001;35:345-51).
The trauma of childhood abuse also is transmitted down through the influences of the other members of the family, especially their children.
Another group known to suffer from the effects of intergenerational trauma is the children of alcoholics. This is a group that has demonstrated an increased need to care for others and keep secrets. They might use lying as a normal coping style and sometimes experience difficulty being children. Such behaviors are understood as a direct consequence of the experience of the family dysfunction. The question about trauma is: How do the symptoms of PTSD get "passed down" through the next generations, when the younger family members were not exposed to any trauma?
Various mechanisms have been considered, with individual psychological mechanisms and family dynamics being the most commonly cited mechanisms. Other factors have been suggested, such as the role of cultural and societal factors in the perpetuation of symptoms. Children and young adults might develop retaliatory fantasies "to right the wrongs done to their families." These types of beliefs and fantasies fuel many sectarian struggles around the world.
Individual psychological mechanisms commonly considered to be important are projection and identification. The parent with PTSD projects unwanted aspects of himself onto the child, who takes up the projection and identifies with it; this is called projective identification. Fear of the cold or the dark in the father then becomes the child’s fear instead. Children who are closest to the traumatized parent will be most affected.
Other postulated mechanisms focus on affect regulation. Parents who have difficulty with emotional regulation will have difficulty bonding appropriately with their child. On the other hand, emotional numbing might be present, which interferes with the development of a strong bond between parent and child.
One study of male Vietnam veterans found that "emotional numbing" and the quality of their relationship with their children remained significant even after investigators controlled for numerous factors, including the fathers’ family-of-origin stressors, combat exposure, depression, and substance abuse (J. Trauma Stress 2002;15:351-7). In other words, the children then suffer from secondary trauma.
Trauma-affected families also might have difficulty setting appropriate boundaries between parent and child so that the child becomes the caregiver of sorts and protector of the parent. The fears of the parent can become the fears of the child. It might be confusing for the child when a parent says: "Shh! Did you hear that noise," implying that "they" will get us, without really specifying the who and why, thus depriving the child of a rational explanation of his or her own experiences.
However, sometimes, trauma is not transmitted intergenerationally, a series of meta-analyses shows (Attach. Hum. Dev. 2008;10:105-21). Instead, these families are able to develop resilience and adapt well in the face of adversity – and achieve posttraumatic growth. How do we help the families with trauma become these resilient families?
Here is a list of nine points that can help guide the family psychiatrist:
1. The ability to regulate emotions, especially negative affect, is key to maintaining an understandable emotional climate for others in the family. Frequent unexplained emotional outbursts are difficult for other family members to understand. For children, it is especially important for them to understand that any emotional dysregulation is not caused by their behavior but by the parent’s experience of prior trauma.
2. The family should have an understanding of the meaning and cause of the traumatic events.
The traumatic events must be symbolized in a way that allows conversation and discussion about the past. The mention of wartime trauma can be phrased in a way that allows for the experience of pain, and then recovery, with hope and resilience as the message. A narrative story is important, with a good ending that the parent has survived, has overcome difficulties, and is here in the present with the child.
3. The parent must have "worked through" the trauma to the extent that he can internally symbolize his experiences enough to be able to talk about them and relay them to his
offspring in a coherent narrative with a positive message.
4. Open communication about the trauma prevents any unsymbolized, unspoken aspects of the trauma from being driven into unconsciousness, where they become dark fearful secrets that haunt the imagination and awaken the children, even as adults, at night.
5. Being able to access public accounts of the traumatic events is helpful to widen the family’s understanding of how others are affected, thus reducing the fearfulness of being alone with the trauma. Families should be encouraged to access these sources in order to understand the global aspects of trauma and the associated suffering and recovery.
6. For many families, having suffered trauma means that they must always be prepared for disaster. This, too, can be framed in a positive way, more like the scout motto of "be prepared," rather than the fearful posture of the survivalist.
7. A family fleeing from trauma might experience displacement through immigration and have no sense of home. This can be modulated by reestablishing and developing a new sense of community, and developing strong social and family rootedness. Sometimes, this involves a religious or spiritual group affiliation.
8. Family members who have suffered trauma often can identify skills that helped them survive. Hope, education, community, art – these values can be transmitted as the positive legacy of trauma. Helping families identify with positive resilient features of surviving trauma does not mean forgetting about the trauma but identifying the aspects that help the family go forward, enabling them to develop a narrative that allows recovery and growth.
9. If the child or other family members develop ongoing secondary PTSD or have enduring feelings of survivor guilt, persecution, and so on that are not resolved by family intervention, individual assessment might be needed.
In conclusion, despite the many illuminating case reports and anecdotes about the intergenerational transmission of trauma (for example, see J. Marital Fam. Ther. 2004;30:45-59), the message to families must be resilience focused. The question for these families becomes: "What did you do to manage the trauma and survive?"
Using a narrative framework, we can help these families identify the factors that can contribute to resilience, and build a future for the family that does not transmit traumatic symptoms but rather transmits the ability to move forward, despite traumatic symptoms.
E-mail Dr. Heru at cpnews@elsevier.com.
Intergenerational trauma often proves to be a prevailing feature of family systems.
The trauma of the Nazi concentration camps, for example, can be re-experienced in the lives of the children of camp survivors. Even the grandchildren of Holocaust survivors have been found to suffer from the effects of trauma. These effects manifest through characteristics such as increased suspiciousness of others, anger, and irritability in these individuals compared with controls (J. Relig. Health 2011;50:321-9).
Such intergenerational trauma has been found among urban American Indian and Alaska Native populations who have been involved in culturally specific sobriety maintenance programs (Am. Indian Alsk. Native Ment. Health Res. 2011;18:17-40). Likewise, a body of research supports the notion that untreated intergenerational trauma tied to generations of slavery in the United States continues to negatively affect many in the black community.
Other kinds of trauma can be passed down through the generations, as well. Take the trauma of a combat soldier; victim of or prisoner of war; survivor of a mass shooting or of child abuse; witness of genocide; or survivor of colonial suppression, slavery, or political totalitarianism. People who have experienced these traumas can pass down the consequences to subsequent generations.
We know that people who suffer trauma firsthand often develop posttraumatic stress disorder symptoms (PTSD) symptoms such as fearfulness, nightmares, flashbacks, sorrow, and difficulty with emotional closeness. However, it also is clear that compared with controls, the children of veterans with PTSD have shown an inability to experience appropriate emotional responses to situations and difficulty in solving problems effectively both within and outside the family unit (Aust. N.Z. J. Psychiatry 2001;35:345-51).
The trauma of childhood abuse also is transmitted down through the influences of the other members of the family, especially their children.
Another group known to suffer from the effects of intergenerational trauma is the children of alcoholics. This is a group that has demonstrated an increased need to care for others and keep secrets. They might use lying as a normal coping style and sometimes experience difficulty being children. Such behaviors are understood as a direct consequence of the experience of the family dysfunction. The question about trauma is: How do the symptoms of PTSD get "passed down" through the next generations, when the younger family members were not exposed to any trauma?
Various mechanisms have been considered, with individual psychological mechanisms and family dynamics being the most commonly cited mechanisms. Other factors have been suggested, such as the role of cultural and societal factors in the perpetuation of symptoms. Children and young adults might develop retaliatory fantasies "to right the wrongs done to their families." These types of beliefs and fantasies fuel many sectarian struggles around the world.
Individual psychological mechanisms commonly considered to be important are projection and identification. The parent with PTSD projects unwanted aspects of himself onto the child, who takes up the projection and identifies with it; this is called projective identification. Fear of the cold or the dark in the father then becomes the child’s fear instead. Children who are closest to the traumatized parent will be most affected.
Other postulated mechanisms focus on affect regulation. Parents who have difficulty with emotional regulation will have difficulty bonding appropriately with their child. On the other hand, emotional numbing might be present, which interferes with the development of a strong bond between parent and child.
One study of male Vietnam veterans found that "emotional numbing" and the quality of their relationship with their children remained significant even after investigators controlled for numerous factors, including the fathers’ family-of-origin stressors, combat exposure, depression, and substance abuse (J. Trauma Stress 2002;15:351-7). In other words, the children then suffer from secondary trauma.
Trauma-affected families also might have difficulty setting appropriate boundaries between parent and child so that the child becomes the caregiver of sorts and protector of the parent. The fears of the parent can become the fears of the child. It might be confusing for the child when a parent says: "Shh! Did you hear that noise," implying that "they" will get us, without really specifying the who and why, thus depriving the child of a rational explanation of his or her own experiences.
However, sometimes, trauma is not transmitted intergenerationally, a series of meta-analyses shows (Attach. Hum. Dev. 2008;10:105-21). Instead, these families are able to develop resilience and adapt well in the face of adversity – and achieve posttraumatic growth. How do we help the families with trauma become these resilient families?
Here is a list of nine points that can help guide the family psychiatrist:
1. The ability to regulate emotions, especially negative affect, is key to maintaining an understandable emotional climate for others in the family. Frequent unexplained emotional outbursts are difficult for other family members to understand. For children, it is especially important for them to understand that any emotional dysregulation is not caused by their behavior but by the parent’s experience of prior trauma.
2. The family should have an understanding of the meaning and cause of the traumatic events.
The traumatic events must be symbolized in a way that allows conversation and discussion about the past. The mention of wartime trauma can be phrased in a way that allows for the experience of pain, and then recovery, with hope and resilience as the message. A narrative story is important, with a good ending that the parent has survived, has overcome difficulties, and is here in the present with the child.
3. The parent must have "worked through" the trauma to the extent that he can internally symbolize his experiences enough to be able to talk about them and relay them to his
offspring in a coherent narrative with a positive message.
4. Open communication about the trauma prevents any unsymbolized, unspoken aspects of the trauma from being driven into unconsciousness, where they become dark fearful secrets that haunt the imagination and awaken the children, even as adults, at night.
5. Being able to access public accounts of the traumatic events is helpful to widen the family’s understanding of how others are affected, thus reducing the fearfulness of being alone with the trauma. Families should be encouraged to access these sources in order to understand the global aspects of trauma and the associated suffering and recovery.
6. For many families, having suffered trauma means that they must always be prepared for disaster. This, too, can be framed in a positive way, more like the scout motto of "be prepared," rather than the fearful posture of the survivalist.
7. A family fleeing from trauma might experience displacement through immigration and have no sense of home. This can be modulated by reestablishing and developing a new sense of community, and developing strong social and family rootedness. Sometimes, this involves a religious or spiritual group affiliation.
8. Family members who have suffered trauma often can identify skills that helped them survive. Hope, education, community, art – these values can be transmitted as the positive legacy of trauma. Helping families identify with positive resilient features of surviving trauma does not mean forgetting about the trauma but identifying the aspects that help the family go forward, enabling them to develop a narrative that allows recovery and growth.
9. If the child or other family members develop ongoing secondary PTSD or have enduring feelings of survivor guilt, persecution, and so on that are not resolved by family intervention, individual assessment might be needed.
In conclusion, despite the many illuminating case reports and anecdotes about the intergenerational transmission of trauma (for example, see J. Marital Fam. Ther. 2004;30:45-59), the message to families must be resilience focused. The question for these families becomes: "What did you do to manage the trauma and survive?"
Using a narrative framework, we can help these families identify the factors that can contribute to resilience, and build a future for the family that does not transmit traumatic symptoms but rather transmits the ability to move forward, despite traumatic symptoms.
E-mail Dr. Heru at cpnews@elsevier.com.
Intergenerational trauma often proves to be a prevailing feature of family systems.
The trauma of the Nazi concentration camps, for example, can be re-experienced in the lives of the children of camp survivors. Even the grandchildren of Holocaust survivors have been found to suffer from the effects of trauma. These effects manifest through characteristics such as increased suspiciousness of others, anger, and irritability in these individuals compared with controls (J. Relig. Health 2011;50:321-9).
Such intergenerational trauma has been found among urban American Indian and Alaska Native populations who have been involved in culturally specific sobriety maintenance programs (Am. Indian Alsk. Native Ment. Health Res. 2011;18:17-40). Likewise, a body of research supports the notion that untreated intergenerational trauma tied to generations of slavery in the United States continues to negatively affect many in the black community.
Other kinds of trauma can be passed down through the generations, as well. Take the trauma of a combat soldier; victim of or prisoner of war; survivor of a mass shooting or of child abuse; witness of genocide; or survivor of colonial suppression, slavery, or political totalitarianism. People who have experienced these traumas can pass down the consequences to subsequent generations.
We know that people who suffer trauma firsthand often develop posttraumatic stress disorder symptoms (PTSD) symptoms such as fearfulness, nightmares, flashbacks, sorrow, and difficulty with emotional closeness. However, it also is clear that compared with controls, the children of veterans with PTSD have shown an inability to experience appropriate emotional responses to situations and difficulty in solving problems effectively both within and outside the family unit (Aust. N.Z. J. Psychiatry 2001;35:345-51).
The trauma of childhood abuse also is transmitted down through the influences of the other members of the family, especially their children.
Another group known to suffer from the effects of intergenerational trauma is the children of alcoholics. This is a group that has demonstrated an increased need to care for others and keep secrets. They might use lying as a normal coping style and sometimes experience difficulty being children. Such behaviors are understood as a direct consequence of the experience of the family dysfunction. The question about trauma is: How do the symptoms of PTSD get "passed down" through the next generations, when the younger family members were not exposed to any trauma?
Various mechanisms have been considered, with individual psychological mechanisms and family dynamics being the most commonly cited mechanisms. Other factors have been suggested, such as the role of cultural and societal factors in the perpetuation of symptoms. Children and young adults might develop retaliatory fantasies "to right the wrongs done to their families." These types of beliefs and fantasies fuel many sectarian struggles around the world.
Individual psychological mechanisms commonly considered to be important are projection and identification. The parent with PTSD projects unwanted aspects of himself onto the child, who takes up the projection and identifies with it; this is called projective identification. Fear of the cold or the dark in the father then becomes the child’s fear instead. Children who are closest to the traumatized parent will be most affected.
Other postulated mechanisms focus on affect regulation. Parents who have difficulty with emotional regulation will have difficulty bonding appropriately with their child. On the other hand, emotional numbing might be present, which interferes with the development of a strong bond between parent and child.
One study of male Vietnam veterans found that "emotional numbing" and the quality of their relationship with their children remained significant even after investigators controlled for numerous factors, including the fathers’ family-of-origin stressors, combat exposure, depression, and substance abuse (J. Trauma Stress 2002;15:351-7). In other words, the children then suffer from secondary trauma.
Trauma-affected families also might have difficulty setting appropriate boundaries between parent and child so that the child becomes the caregiver of sorts and protector of the parent. The fears of the parent can become the fears of the child. It might be confusing for the child when a parent says: "Shh! Did you hear that noise," implying that "they" will get us, without really specifying the who and why, thus depriving the child of a rational explanation of his or her own experiences.
However, sometimes, trauma is not transmitted intergenerationally, a series of meta-analyses shows (Attach. Hum. Dev. 2008;10:105-21). Instead, these families are able to develop resilience and adapt well in the face of adversity – and achieve posttraumatic growth. How do we help the families with trauma become these resilient families?
Here is a list of nine points that can help guide the family psychiatrist:
1. The ability to regulate emotions, especially negative affect, is key to maintaining an understandable emotional climate for others in the family. Frequent unexplained emotional outbursts are difficult for other family members to understand. For children, it is especially important for them to understand that any emotional dysregulation is not caused by their behavior but by the parent’s experience of prior trauma.
2. The family should have an understanding of the meaning and cause of the traumatic events.
The traumatic events must be symbolized in a way that allows conversation and discussion about the past. The mention of wartime trauma can be phrased in a way that allows for the experience of pain, and then recovery, with hope and resilience as the message. A narrative story is important, with a good ending that the parent has survived, has overcome difficulties, and is here in the present with the child.
3. The parent must have "worked through" the trauma to the extent that he can internally symbolize his experiences enough to be able to talk about them and relay them to his
offspring in a coherent narrative with a positive message.
4. Open communication about the trauma prevents any unsymbolized, unspoken aspects of the trauma from being driven into unconsciousness, where they become dark fearful secrets that haunt the imagination and awaken the children, even as adults, at night.
5. Being able to access public accounts of the traumatic events is helpful to widen the family’s understanding of how others are affected, thus reducing the fearfulness of being alone with the trauma. Families should be encouraged to access these sources in order to understand the global aspects of trauma and the associated suffering and recovery.
6. For many families, having suffered trauma means that they must always be prepared for disaster. This, too, can be framed in a positive way, more like the scout motto of "be prepared," rather than the fearful posture of the survivalist.
7. A family fleeing from trauma might experience displacement through immigration and have no sense of home. This can be modulated by reestablishing and developing a new sense of community, and developing strong social and family rootedness. Sometimes, this involves a religious or spiritual group affiliation.
8. Family members who have suffered trauma often can identify skills that helped them survive. Hope, education, community, art – these values can be transmitted as the positive legacy of trauma. Helping families identify with positive resilient features of surviving trauma does not mean forgetting about the trauma but identifying the aspects that help the family go forward, enabling them to develop a narrative that allows recovery and growth.
9. If the child or other family members develop ongoing secondary PTSD or have enduring feelings of survivor guilt, persecution, and so on that are not resolved by family intervention, individual assessment might be needed.
In conclusion, despite the many illuminating case reports and anecdotes about the intergenerational transmission of trauma (for example, see J. Marital Fam. Ther. 2004;30:45-59), the message to families must be resilience focused. The question for these families becomes: "What did you do to manage the trauma and survive?"
Using a narrative framework, we can help these families identify the factors that can contribute to resilience, and build a future for the family that does not transmit traumatic symptoms but rather transmits the ability to move forward, despite traumatic symptoms.
E-mail Dr. Heru at cpnews@elsevier.com.
How Should Physicians Assess and Manage Pressure Ulcers in the Hospitalized Patient?
The Case
An 85-year-old woman with stroke, functional quadriplegia, and diabetes mellitus presents with altered mental status. She is febrile (38.5°C) with leukocytosis (14,400 cells/mm3) and has a 5 cm x 4 cm x 2 cm Stage III malodorous sacral ulcer without surrounding erythema, tunneling, or pain. The ulcer base is partially covered by green slough. How should this pressure ulcer be evaluated and treated?
Overview
Pressure ulcers in vulnerable populations, such as the elderly and those with limited mobility, are exceedingly common. In the acute-care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with 2.5 million cases treated annually at an estimated cost of $11 billion per year.1,2 Moreover, as of Oct. 1, 2008, the Centers for Medicare & Medicaid Services (CMS) guideline states that hospitals will no longer receive additional payment when a hospitalized patient develops Stage III or IV pressure ulcers that are not present on admission.
A pressure ulcer is a localized injury to skin and underlying soft tissue over a bony prominence due to sustained external pressure.3 Prolonged pressure on these weight-bearing areas leads to reduced blood flow, ischemia, cell death, and necrosis of local tissues.4 Risk factors for developing pressure ulcers include increased external pressure, shear, friction, moisture, poor perfusion, immobility, incontinence, malnutrition, and impaired mental status.4 Inadequately treated pressure ulcers can lead to pain, tunneling, fistula formation, disfigurement, infection, prolonged hospitalization, lower quality of life, and increased mortality.4
Because of the significant morbidities and high costs associated with the care of pressure ulcers in acute care, hospitalists must be familiar with the assessment and treatment of pressure ulcers in vulnerable patients.
Review of the Data
The management of pressure ulcers in the hospitalized patient starts with a comprehensive assessment of the patient’s medical comorbidities, risk factors, and wound-staging. Considerations must be given to differentiate an infected pressure ulcer from a noninfected ulcer. These evaluations then guide the appropriate treatments of pressure ulcers, including the prevention of progression or formation of new ulcers, debridement, application of wound dressing, and antibiotic use.
Assessing pressure ulcer stage. The National Pressure Ulcer Advisory Panel (NPUAP) Classification System is the most commonly used staging tool. It describes four stages of pressure ulcers (see Table 1).3 A Stage 1 pressure ulcer is characterized by intact skin with nonblanchable erythema and may be discolored, painful, soft, firm, and warmer or cooler compared to adjacent area. A Stage II pressure ulcer presents with partial thickness skin loss with a shallow red-pink wound bed without slough, or as an intact or ruptured serum-filled blister. Stage II pressure ulcers do not include skin tears, tape burns, macerations, or excoriations. A Stage III pressure ulcer has full thickness skin loss with or without visible subcutaneous fat. Bone, tendon, or muscle are not exposed or directly palpable. Slough may be present but it does not obscure the depth of ulcer. Deep ulcers can develop in anatomical regions with high adiposity, such as the pelvic girdle. A Stage IV pressure ulcer has full thickness tissue loss with exposed and palpable bone, tendon, or muscle. Slough, eschar, undermining, and tunneling may be present. The depth of a Stage IV ulcer varies depending on anatomical location and adiposity. Stage IV ulcers also create a nidus for osteomyelitis.
NPUAP describes two additional categories of pressure ulcers: unstageable and deep tissue injury.3 An unstageable ulcer has full thickness skin or tissue loss of unknown depth because the wound base is completely obscured by slough or eschar. The ulcer can only be accurately categorized as Stage III or IV after sufficient slough or eschar is removed to identify wound depth. Lastly, suspected deep tissue injury describes a localized area of discolored intact skin (purple or maroon) or blood-filled blister due to damage of underlying tissue from pressure or shear.
Diagnosing infected pressure ulcers. Pressure ulcer infection delays wound healing and increases risks for sepsis, cellulitis, osteomyelitis, and death.5,6 Clinical evidence of soft tissue involvement, such as erythema, warmth, tenderness, foul odor, or purulent discharge, and systemic inflammatory response (fever, tachycardia, or leukocytosis) are suggestive of a wound infection.3,5 However, these clinical signs may be absent and thus make the distinction between chronic wound and infected pressure ulcer difficult.7 Delayed healing with friable granulation tissue and increased pain in a treated wound may be the only signs of a pressure ulcer infection.3,5,7
Routine laboratory tests (i.e. white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) are neither sensitive nor specific in diagnosing wound infection. Moreover, because pressure ulcers are typically colonized with ≥105 organisms/mL of normal skin flora and bacteria from adjacent gastrointestinal or urogenital environments, swab cultures identify colonizing organisms and are not recommended as a diagnostic test for pressure ulcer microbiologic evaluation.5,6 If microbiological data are needed to guide antibiotic use, cultures of blood, bone, or deep tissue biopsied from a surgically debrided wound should be used.5 Importantly, a higher index of suspicion should be maintained for infection of Stage III or IV pressure ulcers because they are more commonly infected than Stage I or II ulcers.3
Prevention. The prevention of wound progression is essential in treating acute, chronic, or infected pressure ulcers. Although management guidelines are limited by few high-quality, randomized controlled trials, NPUAP recommends a number of prevention strategies targeting risk factors that contribute to pressure ulcer development.2,3,8
For all bed-bound and chair-bound persons with impaired ability to self-reposition, risk assessment for pressure ulcer should be done on admission and repeated every 24 hours. The presence of such risk factors as immobility, shear, friction, moisture, incontinence, and malnutrition should be used to guide preventive treatments. Pressure relief on an ulcer can be achieved by repositioning the immobile patient at one- to two-hour intervals. Pressure-redistributing support surfaces (static, overlays, or dynamic) reduce tissue pressure and decrease overall incidence of pressure ulcers. Due to a lack of relative efficacy data, the selection of a support surface should be determined by the patient’s individual needs in order to reduce pressure and shear.3 For instance, dynamic support is an appropriate surface for an immobile patient with multiple or nonhealing ulcers. Shearing force and friction can be reduced by limiting head-of-bed elevation to <30° and using such transfer aids as bed linens while repositioning patients. The use of pillows, foam wedges, or other devices should be used to eliminate direct contact of bony prominences or reduce pressure on heels.8
Skin care should be optimized to limit excessive dryness or moisture. This includes using moisturizers for dry skin, particularly for the sacrum, and implementing bowel and bladder programs and absorbent underpads in patients with bowel or bladder incontinence.2 Given that patients with pressure ulcers are in a catabolic state, those who are nutritionally compromised may benefit from nutritional supplementation.3 Lastly, appropriate use of local and systemic pain regimen for painful pressure ulcers can improve patient cooperation in repositioning, dressing change, and quality of life.
Debridement. Wound debridement removes necrotic tissue often present in infected or chronic pressure ulcers, reduces risk for further infection, and promotes granulation tissue formation and wound healing. Debridement, however, is not indicated for ulcers of an ischemic limb or dry eschar of the heel, due to propensity for complications.3,4 The five common debridement methods are sharp, mechanical, autolytic, enzymatic, and biosurgical. The debridement method of choice is determined by clinician preference and availability.4
Sharp debridement results in rapid removal of large amounts of nonviable necrotic tissues and eschar using sharp instruments and, therefore, is indicated if wound infection or sepsis is present. Mechanical debridement by wet-to-dry dressing or whirlpool nonselectively removes granulation tissue and, thus, should be used cautiously. Autolytic debridement uses occlusive dressings (i.e. hydrocolloid or hydrogel) to maintain a moist wound environment in order to optimize the body’s inherent ability to selectively self-digest necrotic tissues. Enzymatic debridement with concentrated topical proteolytic enzymes (i.e. collagenase) digests necrotic tissues and achieves faster debridement than autolysis while being less invasive than surgical intervention. Biosurgery utilizes maggots (i.e. Lucilia sericata) that produce enzymes to effectively debride necrotic tissues.
Wound care and dressing. Pressure ulcers should be cleansed with each dressing change using such physiologic solutions as normal saline. Cleansing with antimicrobial solutions for ulcers with large necrotic debris or infection needs to be thoughtfully administered due to the potential impairment on wound healing.4 Wound dressing should maintain a moist wound environment to allow epithelialization and limit excessive exudates in order to prevent maceration. Although there are many categories of moisture retentive dressings, their comparative effectiveness remain unclear.4 Table 2 summarizes characteristics of common wound dressings and their applications.
Antibiotic use. Topical antibiotics are appropriate for Stage III or IV ulcers with signs of local infection, including periwound erythema and friable granulation tissue.4 The Agency for Health Care Policy and Research recommends a two-week trial of a topical antibiotic, such as silver sulfadiazine, for pressure ulcers that fail to heal after two to four weeks of optimal care.6 Systemic antibiotics should be used for patients who demonstrate evidence of systemic infection including sepsis, osteomyelitis, or cellulitis with associated fever and leukocytosis. The choice of systemic antibiotics should be based on cultures from blood, bone, or deep tissue biopsied from a surgically debrided wound.4,6
Back to the Case
The patient was hospitalized for altered mental status. She was at high risk for the progression of her sacral ulcer and the development of new pressure ulcers due to immobility, incontinence, malnutrition, and impaired mental status. The sacral wound was a chronic, Stage III pressure ulcer without evidence of local tissue infection. However, the presence of leukocytosis and fever were suggestive of an underlying infection. Her urine analysis was consistent with a urinary tract infection.
Trimethoprim/sulfamethoxazole was administered with subsequent resolution of leukocytosis, fever, and delirium. The sacral ulcer was cleansed with normal saline and covered with hydrocolloid dressing every 72 hours in order to maintain a moist wound environment and facilitate autolysis. Preventive interventions guided by her risk factors for pressure ulcer were implemented. Interventions included:
- Daily skin and wound assessment;
- Pressure relief with repositioning every two hours;
- Use of a dynamic support surface;
- Head-of-bed elevation of no more than <30° to reduce shear and friction;
- Use of transfer aids;
- Use of devices to eliminate direct contact of bony prominences;
- Optimizing skin care with moisturizers for dry skin and frequent changing of absorbent under pads for incontinence; and
- Consulting nutrition service to optimize nutritional intake.
Bottom Line
Assessments of pressure ulcer stage, wound infection, and risk factors guide targeted therapeutic interventions that facilitate wound healing and prevent new pressure ulcer formation.
Dr. Prager is a fellow in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai School of Medicine in New York City. Dr. Ko is a hospitalist and an assistant professor in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai.
References
- Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Washington, D.C.: National Pressure Ulcer Advisory Panel; 2009.
- Bates-Jensen BM. Chapter 58. Pressure Ulcers. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill; 2009.
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35(11):1390-1396.
- Agency for Health Care Policy and Research (AHCPR). Treatment of Pressure Ulcers. Clinical Practice Guideline Number 15. U.S. Department of Health and Human Services. 1994.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605-611.
- National Pressure Ulcer Advisory Panel. Pressure Ulcer Prevention Points. National Pressure Ulcer Advisory Panel website. Available at: http://www.npuap.org/resources/educational-and-clinical-resources/pressure-ulcer-prevention-points/. Accessed Aug. 1, 2012.
- Reuben DB, Herr KA, Pacala JT, et al. Skin Ulcers. In: Geriatrics At Your Fingertips. 12th ed. New York: The American Geriatrics Society; 2010.
The Case
An 85-year-old woman with stroke, functional quadriplegia, and diabetes mellitus presents with altered mental status. She is febrile (38.5°C) with leukocytosis (14,400 cells/mm3) and has a 5 cm x 4 cm x 2 cm Stage III malodorous sacral ulcer without surrounding erythema, tunneling, or pain. The ulcer base is partially covered by green slough. How should this pressure ulcer be evaluated and treated?
Overview
Pressure ulcers in vulnerable populations, such as the elderly and those with limited mobility, are exceedingly common. In the acute-care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with 2.5 million cases treated annually at an estimated cost of $11 billion per year.1,2 Moreover, as of Oct. 1, 2008, the Centers for Medicare & Medicaid Services (CMS) guideline states that hospitals will no longer receive additional payment when a hospitalized patient develops Stage III or IV pressure ulcers that are not present on admission.
A pressure ulcer is a localized injury to skin and underlying soft tissue over a bony prominence due to sustained external pressure.3 Prolonged pressure on these weight-bearing areas leads to reduced blood flow, ischemia, cell death, and necrosis of local tissues.4 Risk factors for developing pressure ulcers include increased external pressure, shear, friction, moisture, poor perfusion, immobility, incontinence, malnutrition, and impaired mental status.4 Inadequately treated pressure ulcers can lead to pain, tunneling, fistula formation, disfigurement, infection, prolonged hospitalization, lower quality of life, and increased mortality.4
Because of the significant morbidities and high costs associated with the care of pressure ulcers in acute care, hospitalists must be familiar with the assessment and treatment of pressure ulcers in vulnerable patients.
Review of the Data
The management of pressure ulcers in the hospitalized patient starts with a comprehensive assessment of the patient’s medical comorbidities, risk factors, and wound-staging. Considerations must be given to differentiate an infected pressure ulcer from a noninfected ulcer. These evaluations then guide the appropriate treatments of pressure ulcers, including the prevention of progression or formation of new ulcers, debridement, application of wound dressing, and antibiotic use.
Assessing pressure ulcer stage. The National Pressure Ulcer Advisory Panel (NPUAP) Classification System is the most commonly used staging tool. It describes four stages of pressure ulcers (see Table 1).3 A Stage 1 pressure ulcer is characterized by intact skin with nonblanchable erythema and may be discolored, painful, soft, firm, and warmer or cooler compared to adjacent area. A Stage II pressure ulcer presents with partial thickness skin loss with a shallow red-pink wound bed without slough, or as an intact or ruptured serum-filled blister. Stage II pressure ulcers do not include skin tears, tape burns, macerations, or excoriations. A Stage III pressure ulcer has full thickness skin loss with or without visible subcutaneous fat. Bone, tendon, or muscle are not exposed or directly palpable. Slough may be present but it does not obscure the depth of ulcer. Deep ulcers can develop in anatomical regions with high adiposity, such as the pelvic girdle. A Stage IV pressure ulcer has full thickness tissue loss with exposed and palpable bone, tendon, or muscle. Slough, eschar, undermining, and tunneling may be present. The depth of a Stage IV ulcer varies depending on anatomical location and adiposity. Stage IV ulcers also create a nidus for osteomyelitis.
NPUAP describes two additional categories of pressure ulcers: unstageable and deep tissue injury.3 An unstageable ulcer has full thickness skin or tissue loss of unknown depth because the wound base is completely obscured by slough or eschar. The ulcer can only be accurately categorized as Stage III or IV after sufficient slough or eschar is removed to identify wound depth. Lastly, suspected deep tissue injury describes a localized area of discolored intact skin (purple or maroon) or blood-filled blister due to damage of underlying tissue from pressure or shear.
Diagnosing infected pressure ulcers. Pressure ulcer infection delays wound healing and increases risks for sepsis, cellulitis, osteomyelitis, and death.5,6 Clinical evidence of soft tissue involvement, such as erythema, warmth, tenderness, foul odor, or purulent discharge, and systemic inflammatory response (fever, tachycardia, or leukocytosis) are suggestive of a wound infection.3,5 However, these clinical signs may be absent and thus make the distinction between chronic wound and infected pressure ulcer difficult.7 Delayed healing with friable granulation tissue and increased pain in a treated wound may be the only signs of a pressure ulcer infection.3,5,7
Routine laboratory tests (i.e. white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) are neither sensitive nor specific in diagnosing wound infection. Moreover, because pressure ulcers are typically colonized with ≥105 organisms/mL of normal skin flora and bacteria from adjacent gastrointestinal or urogenital environments, swab cultures identify colonizing organisms and are not recommended as a diagnostic test for pressure ulcer microbiologic evaluation.5,6 If microbiological data are needed to guide antibiotic use, cultures of blood, bone, or deep tissue biopsied from a surgically debrided wound should be used.5 Importantly, a higher index of suspicion should be maintained for infection of Stage III or IV pressure ulcers because they are more commonly infected than Stage I or II ulcers.3
Prevention. The prevention of wound progression is essential in treating acute, chronic, or infected pressure ulcers. Although management guidelines are limited by few high-quality, randomized controlled trials, NPUAP recommends a number of prevention strategies targeting risk factors that contribute to pressure ulcer development.2,3,8
For all bed-bound and chair-bound persons with impaired ability to self-reposition, risk assessment for pressure ulcer should be done on admission and repeated every 24 hours. The presence of such risk factors as immobility, shear, friction, moisture, incontinence, and malnutrition should be used to guide preventive treatments. Pressure relief on an ulcer can be achieved by repositioning the immobile patient at one- to two-hour intervals. Pressure-redistributing support surfaces (static, overlays, or dynamic) reduce tissue pressure and decrease overall incidence of pressure ulcers. Due to a lack of relative efficacy data, the selection of a support surface should be determined by the patient’s individual needs in order to reduce pressure and shear.3 For instance, dynamic support is an appropriate surface for an immobile patient with multiple or nonhealing ulcers. Shearing force and friction can be reduced by limiting head-of-bed elevation to <30° and using such transfer aids as bed linens while repositioning patients. The use of pillows, foam wedges, or other devices should be used to eliminate direct contact of bony prominences or reduce pressure on heels.8
Skin care should be optimized to limit excessive dryness or moisture. This includes using moisturizers for dry skin, particularly for the sacrum, and implementing bowel and bladder programs and absorbent underpads in patients with bowel or bladder incontinence.2 Given that patients with pressure ulcers are in a catabolic state, those who are nutritionally compromised may benefit from nutritional supplementation.3 Lastly, appropriate use of local and systemic pain regimen for painful pressure ulcers can improve patient cooperation in repositioning, dressing change, and quality of life.
Debridement. Wound debridement removes necrotic tissue often present in infected or chronic pressure ulcers, reduces risk for further infection, and promotes granulation tissue formation and wound healing. Debridement, however, is not indicated for ulcers of an ischemic limb or dry eschar of the heel, due to propensity for complications.3,4 The five common debridement methods are sharp, mechanical, autolytic, enzymatic, and biosurgical. The debridement method of choice is determined by clinician preference and availability.4
Sharp debridement results in rapid removal of large amounts of nonviable necrotic tissues and eschar using sharp instruments and, therefore, is indicated if wound infection or sepsis is present. Mechanical debridement by wet-to-dry dressing or whirlpool nonselectively removes granulation tissue and, thus, should be used cautiously. Autolytic debridement uses occlusive dressings (i.e. hydrocolloid or hydrogel) to maintain a moist wound environment in order to optimize the body’s inherent ability to selectively self-digest necrotic tissues. Enzymatic debridement with concentrated topical proteolytic enzymes (i.e. collagenase) digests necrotic tissues and achieves faster debridement than autolysis while being less invasive than surgical intervention. Biosurgery utilizes maggots (i.e. Lucilia sericata) that produce enzymes to effectively debride necrotic tissues.
Wound care and dressing. Pressure ulcers should be cleansed with each dressing change using such physiologic solutions as normal saline. Cleansing with antimicrobial solutions for ulcers with large necrotic debris or infection needs to be thoughtfully administered due to the potential impairment on wound healing.4 Wound dressing should maintain a moist wound environment to allow epithelialization and limit excessive exudates in order to prevent maceration. Although there are many categories of moisture retentive dressings, their comparative effectiveness remain unclear.4 Table 2 summarizes characteristics of common wound dressings and their applications.
Antibiotic use. Topical antibiotics are appropriate for Stage III or IV ulcers with signs of local infection, including periwound erythema and friable granulation tissue.4 The Agency for Health Care Policy and Research recommends a two-week trial of a topical antibiotic, such as silver sulfadiazine, for pressure ulcers that fail to heal after two to four weeks of optimal care.6 Systemic antibiotics should be used for patients who demonstrate evidence of systemic infection including sepsis, osteomyelitis, or cellulitis with associated fever and leukocytosis. The choice of systemic antibiotics should be based on cultures from blood, bone, or deep tissue biopsied from a surgically debrided wound.4,6
Back to the Case
The patient was hospitalized for altered mental status. She was at high risk for the progression of her sacral ulcer and the development of new pressure ulcers due to immobility, incontinence, malnutrition, and impaired mental status. The sacral wound was a chronic, Stage III pressure ulcer without evidence of local tissue infection. However, the presence of leukocytosis and fever were suggestive of an underlying infection. Her urine analysis was consistent with a urinary tract infection.
Trimethoprim/sulfamethoxazole was administered with subsequent resolution of leukocytosis, fever, and delirium. The sacral ulcer was cleansed with normal saline and covered with hydrocolloid dressing every 72 hours in order to maintain a moist wound environment and facilitate autolysis. Preventive interventions guided by her risk factors for pressure ulcer were implemented. Interventions included:
- Daily skin and wound assessment;
- Pressure relief with repositioning every two hours;
- Use of a dynamic support surface;
- Head-of-bed elevation of no more than <30° to reduce shear and friction;
- Use of transfer aids;
- Use of devices to eliminate direct contact of bony prominences;
- Optimizing skin care with moisturizers for dry skin and frequent changing of absorbent under pads for incontinence; and
- Consulting nutrition service to optimize nutritional intake.
Bottom Line
Assessments of pressure ulcer stage, wound infection, and risk factors guide targeted therapeutic interventions that facilitate wound healing and prevent new pressure ulcer formation.
Dr. Prager is a fellow in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai School of Medicine in New York City. Dr. Ko is a hospitalist and an assistant professor in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai.
References
- Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Washington, D.C.: National Pressure Ulcer Advisory Panel; 2009.
- Bates-Jensen BM. Chapter 58. Pressure Ulcers. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill; 2009.
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35(11):1390-1396.
- Agency for Health Care Policy and Research (AHCPR). Treatment of Pressure Ulcers. Clinical Practice Guideline Number 15. U.S. Department of Health and Human Services. 1994.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605-611.
- National Pressure Ulcer Advisory Panel. Pressure Ulcer Prevention Points. National Pressure Ulcer Advisory Panel website. Available at: http://www.npuap.org/resources/educational-and-clinical-resources/pressure-ulcer-prevention-points/. Accessed Aug. 1, 2012.
- Reuben DB, Herr KA, Pacala JT, et al. Skin Ulcers. In: Geriatrics At Your Fingertips. 12th ed. New York: The American Geriatrics Society; 2010.
The Case
An 85-year-old woman with stroke, functional quadriplegia, and diabetes mellitus presents with altered mental status. She is febrile (38.5°C) with leukocytosis (14,400 cells/mm3) and has a 5 cm x 4 cm x 2 cm Stage III malodorous sacral ulcer without surrounding erythema, tunneling, or pain. The ulcer base is partially covered by green slough. How should this pressure ulcer be evaluated and treated?
Overview
Pressure ulcers in vulnerable populations, such as the elderly and those with limited mobility, are exceedingly common. In the acute-care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with 2.5 million cases treated annually at an estimated cost of $11 billion per year.1,2 Moreover, as of Oct. 1, 2008, the Centers for Medicare & Medicaid Services (CMS) guideline states that hospitals will no longer receive additional payment when a hospitalized patient develops Stage III or IV pressure ulcers that are not present on admission.
A pressure ulcer is a localized injury to skin and underlying soft tissue over a bony prominence due to sustained external pressure.3 Prolonged pressure on these weight-bearing areas leads to reduced blood flow, ischemia, cell death, and necrosis of local tissues.4 Risk factors for developing pressure ulcers include increased external pressure, shear, friction, moisture, poor perfusion, immobility, incontinence, malnutrition, and impaired mental status.4 Inadequately treated pressure ulcers can lead to pain, tunneling, fistula formation, disfigurement, infection, prolonged hospitalization, lower quality of life, and increased mortality.4
Because of the significant morbidities and high costs associated with the care of pressure ulcers in acute care, hospitalists must be familiar with the assessment and treatment of pressure ulcers in vulnerable patients.
Review of the Data
The management of pressure ulcers in the hospitalized patient starts with a comprehensive assessment of the patient’s medical comorbidities, risk factors, and wound-staging. Considerations must be given to differentiate an infected pressure ulcer from a noninfected ulcer. These evaluations then guide the appropriate treatments of pressure ulcers, including the prevention of progression or formation of new ulcers, debridement, application of wound dressing, and antibiotic use.
Assessing pressure ulcer stage. The National Pressure Ulcer Advisory Panel (NPUAP) Classification System is the most commonly used staging tool. It describes four stages of pressure ulcers (see Table 1).3 A Stage 1 pressure ulcer is characterized by intact skin with nonblanchable erythema and may be discolored, painful, soft, firm, and warmer or cooler compared to adjacent area. A Stage II pressure ulcer presents with partial thickness skin loss with a shallow red-pink wound bed without slough, or as an intact or ruptured serum-filled blister. Stage II pressure ulcers do not include skin tears, tape burns, macerations, or excoriations. A Stage III pressure ulcer has full thickness skin loss with or without visible subcutaneous fat. Bone, tendon, or muscle are not exposed or directly palpable. Slough may be present but it does not obscure the depth of ulcer. Deep ulcers can develop in anatomical regions with high adiposity, such as the pelvic girdle. A Stage IV pressure ulcer has full thickness tissue loss with exposed and palpable bone, tendon, or muscle. Slough, eschar, undermining, and tunneling may be present. The depth of a Stage IV ulcer varies depending on anatomical location and adiposity. Stage IV ulcers also create a nidus for osteomyelitis.
NPUAP describes two additional categories of pressure ulcers: unstageable and deep tissue injury.3 An unstageable ulcer has full thickness skin or tissue loss of unknown depth because the wound base is completely obscured by slough or eschar. The ulcer can only be accurately categorized as Stage III or IV after sufficient slough or eschar is removed to identify wound depth. Lastly, suspected deep tissue injury describes a localized area of discolored intact skin (purple or maroon) or blood-filled blister due to damage of underlying tissue from pressure or shear.
Diagnosing infected pressure ulcers. Pressure ulcer infection delays wound healing and increases risks for sepsis, cellulitis, osteomyelitis, and death.5,6 Clinical evidence of soft tissue involvement, such as erythema, warmth, tenderness, foul odor, or purulent discharge, and systemic inflammatory response (fever, tachycardia, or leukocytosis) are suggestive of a wound infection.3,5 However, these clinical signs may be absent and thus make the distinction between chronic wound and infected pressure ulcer difficult.7 Delayed healing with friable granulation tissue and increased pain in a treated wound may be the only signs of a pressure ulcer infection.3,5,7
Routine laboratory tests (i.e. white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) are neither sensitive nor specific in diagnosing wound infection. Moreover, because pressure ulcers are typically colonized with ≥105 organisms/mL of normal skin flora and bacteria from adjacent gastrointestinal or urogenital environments, swab cultures identify colonizing organisms and are not recommended as a diagnostic test for pressure ulcer microbiologic evaluation.5,6 If microbiological data are needed to guide antibiotic use, cultures of blood, bone, or deep tissue biopsied from a surgically debrided wound should be used.5 Importantly, a higher index of suspicion should be maintained for infection of Stage III or IV pressure ulcers because they are more commonly infected than Stage I or II ulcers.3
Prevention. The prevention of wound progression is essential in treating acute, chronic, or infected pressure ulcers. Although management guidelines are limited by few high-quality, randomized controlled trials, NPUAP recommends a number of prevention strategies targeting risk factors that contribute to pressure ulcer development.2,3,8
For all bed-bound and chair-bound persons with impaired ability to self-reposition, risk assessment for pressure ulcer should be done on admission and repeated every 24 hours. The presence of such risk factors as immobility, shear, friction, moisture, incontinence, and malnutrition should be used to guide preventive treatments. Pressure relief on an ulcer can be achieved by repositioning the immobile patient at one- to two-hour intervals. Pressure-redistributing support surfaces (static, overlays, or dynamic) reduce tissue pressure and decrease overall incidence of pressure ulcers. Due to a lack of relative efficacy data, the selection of a support surface should be determined by the patient’s individual needs in order to reduce pressure and shear.3 For instance, dynamic support is an appropriate surface for an immobile patient with multiple or nonhealing ulcers. Shearing force and friction can be reduced by limiting head-of-bed elevation to <30° and using such transfer aids as bed linens while repositioning patients. The use of pillows, foam wedges, or other devices should be used to eliminate direct contact of bony prominences or reduce pressure on heels.8
Skin care should be optimized to limit excessive dryness or moisture. This includes using moisturizers for dry skin, particularly for the sacrum, and implementing bowel and bladder programs and absorbent underpads in patients with bowel or bladder incontinence.2 Given that patients with pressure ulcers are in a catabolic state, those who are nutritionally compromised may benefit from nutritional supplementation.3 Lastly, appropriate use of local and systemic pain regimen for painful pressure ulcers can improve patient cooperation in repositioning, dressing change, and quality of life.
Debridement. Wound debridement removes necrotic tissue often present in infected or chronic pressure ulcers, reduces risk for further infection, and promotes granulation tissue formation and wound healing. Debridement, however, is not indicated for ulcers of an ischemic limb or dry eschar of the heel, due to propensity for complications.3,4 The five common debridement methods are sharp, mechanical, autolytic, enzymatic, and biosurgical. The debridement method of choice is determined by clinician preference and availability.4
Sharp debridement results in rapid removal of large amounts of nonviable necrotic tissues and eschar using sharp instruments and, therefore, is indicated if wound infection or sepsis is present. Mechanical debridement by wet-to-dry dressing or whirlpool nonselectively removes granulation tissue and, thus, should be used cautiously. Autolytic debridement uses occlusive dressings (i.e. hydrocolloid or hydrogel) to maintain a moist wound environment in order to optimize the body’s inherent ability to selectively self-digest necrotic tissues. Enzymatic debridement with concentrated topical proteolytic enzymes (i.e. collagenase) digests necrotic tissues and achieves faster debridement than autolysis while being less invasive than surgical intervention. Biosurgery utilizes maggots (i.e. Lucilia sericata) that produce enzymes to effectively debride necrotic tissues.
Wound care and dressing. Pressure ulcers should be cleansed with each dressing change using such physiologic solutions as normal saline. Cleansing with antimicrobial solutions for ulcers with large necrotic debris or infection needs to be thoughtfully administered due to the potential impairment on wound healing.4 Wound dressing should maintain a moist wound environment to allow epithelialization and limit excessive exudates in order to prevent maceration. Although there are many categories of moisture retentive dressings, their comparative effectiveness remain unclear.4 Table 2 summarizes characteristics of common wound dressings and their applications.
Antibiotic use. Topical antibiotics are appropriate for Stage III or IV ulcers with signs of local infection, including periwound erythema and friable granulation tissue.4 The Agency for Health Care Policy and Research recommends a two-week trial of a topical antibiotic, such as silver sulfadiazine, for pressure ulcers that fail to heal after two to four weeks of optimal care.6 Systemic antibiotics should be used for patients who demonstrate evidence of systemic infection including sepsis, osteomyelitis, or cellulitis with associated fever and leukocytosis. The choice of systemic antibiotics should be based on cultures from blood, bone, or deep tissue biopsied from a surgically debrided wound.4,6
Back to the Case
The patient was hospitalized for altered mental status. She was at high risk for the progression of her sacral ulcer and the development of new pressure ulcers due to immobility, incontinence, malnutrition, and impaired mental status. The sacral wound was a chronic, Stage III pressure ulcer without evidence of local tissue infection. However, the presence of leukocytosis and fever were suggestive of an underlying infection. Her urine analysis was consistent with a urinary tract infection.
Trimethoprim/sulfamethoxazole was administered with subsequent resolution of leukocytosis, fever, and delirium. The sacral ulcer was cleansed with normal saline and covered with hydrocolloid dressing every 72 hours in order to maintain a moist wound environment and facilitate autolysis. Preventive interventions guided by her risk factors for pressure ulcer were implemented. Interventions included:
- Daily skin and wound assessment;
- Pressure relief with repositioning every two hours;
- Use of a dynamic support surface;
- Head-of-bed elevation of no more than <30° to reduce shear and friction;
- Use of transfer aids;
- Use of devices to eliminate direct contact of bony prominences;
- Optimizing skin care with moisturizers for dry skin and frequent changing of absorbent under pads for incontinence; and
- Consulting nutrition service to optimize nutritional intake.
Bottom Line
Assessments of pressure ulcer stage, wound infection, and risk factors guide targeted therapeutic interventions that facilitate wound healing and prevent new pressure ulcer formation.
Dr. Prager is a fellow in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai School of Medicine in New York City. Dr. Ko is a hospitalist and an assistant professor in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai.
References
- Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Washington, D.C.: National Pressure Ulcer Advisory Panel; 2009.
- Bates-Jensen BM. Chapter 58. Pressure Ulcers. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill; 2009.
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35(11):1390-1396.
- Agency for Health Care Policy and Research (AHCPR). Treatment of Pressure Ulcers. Clinical Practice Guideline Number 15. U.S. Department of Health and Human Services. 1994.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605-611.
- National Pressure Ulcer Advisory Panel. Pressure Ulcer Prevention Points. National Pressure Ulcer Advisory Panel website. Available at: http://www.npuap.org/resources/educational-and-clinical-resources/pressure-ulcer-prevention-points/. Accessed Aug. 1, 2012.
- Reuben DB, Herr KA, Pacala JT, et al. Skin Ulcers. In: Geriatrics At Your Fingertips. 12th ed. New York: The American Geriatrics Society; 2010.