User login
Human papillomavirus vaccine: Safe, effective, underused
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
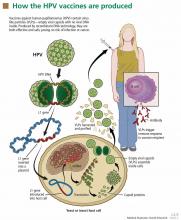
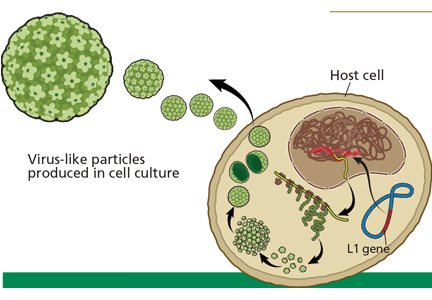
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
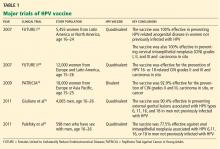
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
KEY POINTS
- Two HPV vaccines are available: a quadrivalent vaccine against HVP types 6, 11, 16, and 18, and a bivalent vaccine against types 16 and 18.
- HPV causes cervical cancer, genital warts, oropharyngeal cancer, anal cancer, and recurrent respiratory papillomatosis, creating a considerable economic and health burden.
- The host immune response to natural HPV infection is slow and weak. In contrast, HPV vaccine induces a strong and long-lasting immune response.
- The HPV vaccines have greater than 90% efficacy in preventing cervical dysplasia and genital warts that are caused by the HPV types the vaccine contains. They are as safe as other common prophylactic vaccines.
- HPV vaccination has been challenged by public controversy over the vaccine’s safety, teenage sexuality, mandatory legislation, and the cost of the vaccine.
A short story of the short QT syndrome
Sudden cardiac death in a young person is a devastating event that has puzzled physicians for decades. In recent years, many of the underlying cardiac pathologies have been identified. These include structural abnormalities such as hypertrophic cardiomyopathy and nonstructural disorders associated with unstable rhythms that lead to sudden cardiac death.
The best known of these “channelopathies” are the long QT syndromes, which result from abnormal potassium and sodium channels in myocytes. Recently, interest has been growing in a disorder that may carry a similarly grim prognosis but that has an opposite finding on electrocardiography (ECG).
Short QT syndrome is a recently described heterogeneous genetic channelopathy that causes both atrial and ventricular arrhythmias and that has been documented to cause sudden cardiac death.
In 1996, a 37-year-old woman from Spain died suddenly; ECG several days earlier had shown a short QT interval of 266 ms.1 Two years later, an unrelated 17-year-old American woman undergoing laparoscopic cholecystectomy suddenly developed atrial fibrillation with a rapid ventricular response.1 Her QT interval was 225 ms. Her brother had a QT interval of 240 ms, and her mother’s was 230 ms. The patient’s maternal grandfather had a history of atrial fibrillation, and his QT interval was 245 ms. These cases led to the description of this new clinical syndrome (see below).2
CLINICAL FEATURES
Short QT syndrome has been associated with both atrial and ventricular arrhythmias. Atrial fibrillation, polymorphic ventricular tachycardia, and ventricular fibrillation have all been well described. Patients who have symptoms usually present with palpitations, presyncope, syncope, or sudden or aborted cardiac death.3,4
ELECTROCARDIOGRAPHIC FEATURES
The primary finding on ECG is a short QT interval. However, others have been noted (Figure 1):
Short or absent ST segment
This finding is not merely a consequence of the short QT interval. In 10 patients with short QT syndrome, the distance from the J point to the peak T wave ranged from 80 to 120 ms. In 12 healthy people whose QT interval was less than 320 ms, this distance ranged from 150 ms to 240 ms.5
Tall and peaked T wave
A tall and peaked T wave is a common feature in short QT syndrome. However, it was also evident in people with short QT intervals who had no other features of the syndrome.5
QT response to heart rate
Normally, the QT interval is inversely related to the heart rate, but this is not true in short QT syndrome: the QT interval remains relatively fixed with changes in heart rate.6,7 This feature is less helpful in the office setting but may be found with Holter monitoring by measuring the QT interval at different heart rates.
BUT WHAT IS CONSIDERED A SHORT QT INTERVAL?
In clinical practice, the QT interval is corrected for the heart rate by the Bazett formula:
Corrected QT (QTc) = [QT interval/square root of the RR interval]
Review of ECGs from large populations in Finland (n = 10,822), Japan (n = 12,149), the United States (n = 79,743), and Switzerland (n = 41,676) revealed that a QTc value of 350 ms in males and 365 ms in females was 2.0 standard deviations (SD) below the mean.8–11 However, a QTc less than the 2.0 SD cutoff did not necessarily equal arrhythmogenic potential. This was illustrated in a 29-year follow-up study of Finnish patients with QTc values as short as 320 ms, in whom no arrhythmias were documented.8 Conversely, some patients with purported short QT syndrome had QTc intervals as long as 381 ms.12
Similar problems with uncertainty of values have plagued the diagnosis of long QT syndrome.13 The lack of reference ranges and the overlap between healthy and affected people called for the development of a scoring system that involves criteria based on ECG and on the clinical evaluation.14,15
ESTABLISHING THE DIAGNOSIS OF SHORT QT SYNDROME
Clearly, the diagnosis of short QT syndrome can be challenging to establish. The first step is to rule out other causes of a short QT interval.
Differential diagnosis of short QT interval
In addition to genetic channelopathies, other causes of short QT interval must be ruled out before entertaining the diagnosis of short QT syndrome.
- Hypercalcemia is the most important of these: there is usually an accompanying prolonged PR interval and a wide QRS complex16
- Hyperkalemia17
- Acidosis17
- Increased vagal tone17
- After ventricular fibrillation (thought to be related to increased intracellular calcium)18
- Digitalis use19
- Androgen use.20
Interestingly, a shorter-than-expected QT interval was noted in patients with chronic fatigue syndrome.21
Which interval to use: QT or QTc?
Unfortunately, most population-based studies that searched for a short QT interval on ECG have used QTc as the main search parameter.8–11 As already mentioned, in patients with short QT syndrome, the QT interval is, uniquely, not shortened if the heart beats faster. In contrast, the QTc often overestimates the QT interval in patients with short QT syndrome, especially when the heart rate is in the 80s to 90s.16
In a review of cases of short QT syndrome worldwide, Bjerregaard et al22 found that the QT interval ranged from 210 ms to 340 ms with a mean ± 2 SD of 282 ± 62 ms. On the other hand, the QTc ranged from 248 ms to 345 ms with a mean ± 2 SD of 305 ± 42 ms.
Therefore, correction formulas (such as the Bazett formula) do not perform well in ruling in the diagnosis of short QT syndrome—and they do even worse in ruling it out.16,22
To establish a diagnosis of short QT syndrome in someone with prior evidence of atrial or ventricular fibrillation, a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient.22 In borderline cases in which the QT interval is slightly longer, some experts recommend other tests, although strong evidence validating their predictive value does not exist. These tests include genotyping, analysis of T wave morphology, and electrophysiologic studies.16
Recently, Gollob et al23 proposed a scoring system for short QT syndrome (Table 1). After reviewing the literature and comparing the diagnostic markers, the investigators determined diagnostic criteria that, when applied to the previously reported cases, were able to identify 58 (95.08%) of 61 patients with short QT syndrome (ie, a sensitivity of 95%).
For patients with intermediate probability, the authors recommended continued medical and ECG surveillance as well as ECGs for first-degree relatives, to further clarify the diagnosis.
Again, a principal caveat about this system is that it relies on the QTc interval rather than the QT interval to diagnose short QT syndrome.
THE SCOPE OF THE DISEASE
In a recent review of the literature, Gollob et al23 found a total of 61 cases of short QT syndrome reported in English. The cohort was predominantly male (75.4%), and most of the symptomatic patients presented during late adolescence and early adulthood. However, there have been reports of infants (4 and 8 months old), and of a man who presented for the first time at the age of 70. Of note, the authors only considered short QT syndrome types 1, 2, and 3 (see below) in their search for cases.
Whether the syndrome is truly this rare or, rather, whether many physicians are not aware of it is still to be determined. In addition, it is possible that incorrectly measuring the QT interval contributes to the lack of identification of this entity. Both of these factors were implicated in the rarity of reported long QT syndrome early after its discovery.14,15
MUTATIONS IN CARDIAC ION CHANNELS
Five distinct genetic defects have been associated with short QT syndrome. As in long QT syndrome, these give rise to subtypes of short QT syndrome, which are numbered 1 to 5 (see below).
The cardiac action potential
To understand how the mutations shorten the QT interval, we will briefly review of the cardiac myocyte action potential.24 In nonpacemaker cells of the heart, the activation of the cell membrane initiates a series of changes in ion channels that allow the movement of ions along an electrical gradient. This movement occurs in five phases and is repeated with every cardiac cycle (Figure 2).
In phase 0, the cardiac cell rapidly depolarizes.
Repolarization occurs in phases 1, 2, and 3 and is largely a function of potassium ions leaving the cell. During phase 2, calcium and sodium ions enter the cell and balance the outward potassium flow, creating the “flat” portion of the repolarization curve. Phase 3 is the main phase of repolarization in which the membrane potential rapidly falls back to its resting state (–90 mV). During phases 1 and 2, the cell membrane is completely refractory to stimulation, whereas phase 3 is divided into three parts:
- The effective refractory period: the cell is able to generate a potential that is too weak to be propagated
- The relative refractory period: the cell can respond to a stimulus that is stronger than normal
- The supernormal phase: the last small portion of phase 3, in which a less-than-normal stimulus can yield a response in the cell.
In phase 4, the cell is completely repolarized, and the cycle can start again.
Five types of short QT syndrome
Short QT syndrome 1. In 2004, Brugada et al25 identified the first mutation that causes abnormal shortening of the action potential duration. In contrast to the mutations that underlie long QT syndrome, this mutation actually causes a gain of function in the gene coding the rapidly acting delayed potassium current (IKr) channel proteins KCNH2 or HERG. Potassium leaving at a more rapid rate causes the cell to repolarize more quickly and shortens the QT interval. The clinical syndrome associated with KCNH2 gene gain-of-function mutation is called short QT syndrome 1.
Short QT syndromes 2 and 3. Other IK (potassium channel) proteins have been implicated as well. Gain-of-function mutations in the KCNQ1 and KCNJ2 genes are believed to account for short QT syndromes 2 and 3, respectively. KCNQ1 codes for the IKs protein, and KCNJ2 codes for the IK1 protein.26,27
Short QT syndromes 4 and 5 were identified by Antzelevitch et al,28 who described several patients who had a combination of channel abnormalities and ECG findings. Their ECGs showed “Brugada-syndrome-like” ST elevation in the right precordial leads, but with a short QT interval. These new syndromes were found to be associated with genetic abnormalities distinct from those of Brugada syndrome and other short QT syndromes. These abnormalities involved loss-of-function mutations in the CACNA1C gene (which codes for the alpha-1 subunit of the L-type cardiac calcium channel) and in the CACNB2 gene (which codes for the beta-2b subunit of the same channel). The two defects correspond to the clinical syndromes short QT syndrome 4 and short QT syndrome 5, respectively.28
MECHANISM OF ARRHYTHMOGENESIS IN SHORT QT SYNDROME
The myocardium is made of different layers: the epicardium, the endocardium, and the middle layer of myocytes composed mainly of M cells. Cells in the different layers differ in the concentration of their channels and can be affected differently in various syndromes. When cells in one or two of the layers repolarize at a rate different from cells in another layer, they create different degrees of refractoriness, which establishes the potential for reentry circuits to form.
It is believed that in short QT syndrome the endocardial cells and M cells repolarize faster than the epicardial cells, predisposing to reentry and arrhythmias. This accentuation of “transmural dispersion of repolarization” accounts for arrhythmogenesis in short QT syndrome as well as in long QT syndrome and the Brugada syndromes. The difference between these syndromes appears to be the layer or area of the myocardium that is affected more by the channelopathy (the M cells in long QT syndrome and the epicardium of the right ventricle in the Brugada syndrome).29
WHEN TO THINK OF SHORT QT SYNDROME
In any survivor of sudden cardiac death, the QT interval should be thoroughly scrutinized, and family members should undergo ECG. Patients in whom a short QT interval is incidentally discovered and for which other reasons are ruled out (see differential diagnosis) should be encouraged to have family members undergo ECG. Other potential patients are young people who develop atrial fibrillation and patients who have idiopathic ventricular fibrillation.4
TREATMENT AND PROGNOSIS
Evidence-based recommendations for the management of short QT syndrome do not yet exist, mainly because the number of patients identified to date is small.
Implantable cardioverter-defibrillators
Although placing an implantable cardioverter-defibrillator (ICD) seems to be warranted in patients who experience ventricular fibrillation, ventricular tachycardia, or aborted cardiac death, or in patients who have a family history of the same symptoms, the best management option is less clear for patients who have no symptoms and no family history.30 In addition, some patients may not want an ICD or may even not qualify for this therapy.
A unique problem with ICDs in short QT syndrome stems from one of the syndrome’s main features on ECG: the tall and peaked T wave that closely follows the R wave can sometimes be interpreted as a short R-R interval, provoking an inappropriate shock from the ICD.31
For the above reasons, we strongly encourage consulting a center with expertise in QT-interval-related disorders before placing an ICD in a patient suspected of having short QT syndrome.
Antiarrhythmic drugs
Prolongation of the QT interval (and the effective refractory period) with drugs has been an interesting area of research. Gaita et al32 studied the effect of four antiarrhythmics—flecainide (Tambocor), sotalol (Betapace), ibutilide (Corvert), and quinidine—in six patients with short QT syndrome. Only quinidine was associated with significant QT prolongation, from 263 ± 12 ms to 362 ms ± 25 ms. This resulted in a longer ventricular effective refractory period (> 200 ms), and ventricular fibrillation was no longer inducible during provocative testing.
In a recent study of long-term outcomes of 53 patients with short QT syndrome, Giustetto et al33 noticed that none of the patients taking quinidine, including those with a history of cardiac arrest, had any further arrhythmsic events. On the other hand, the incidence of arrhythmic events during the follow-up was 4.9% per year in patients not taking this drug. Quinidine had a stronger effect on the QT interval in patients with the HERG mutation than in those without.
RESEARCH MAY LEAD TO A BETTER UNDERSTANDING OF OTHER DISEASES
The short QT syndrome is one of the most recently recognized cardiac channelopathies associated with malignant arrhythmias. As with long QT syndrome, research in short QT syndrome may lead to a better understanding of the pathogenesis of more common but still poorly understood arrhythmias such as lone atrial fibrillation and idiopathic ventricular fibrillation.
- The Short QT Syndrome http://www.shortqtsyndrome.org/short_qt_history.htm. Accessed October 30, 2012.
- Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000; 94:99–102.
- Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006; 27:2440–2447.
- Viskin S, Zeltser D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004; 1:587–591.
- Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009; 6:267–271.
- Redpath CJ, Green MS, Birnie DH, Gollob MH. Rapid genetic testing facilitating the diagnosis of short QT syndrome. Can J Cardiol 2009; 25:e133–e135.
- Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005; 16:54–58.
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007; 116:714–720.
- Funada A, Hayashi K, Ino H, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol 2008; 31:270–274.
- Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007; 40:228–234.
- Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009; 6:652–657.
- Itoh H, Sakaguchi T, Ashihara T, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol 2009; 137:83–85.
- Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992; 327:846–852.
- Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399–411.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993; 88:782–784.
- Bjerregaard P, Nallapaneni H, Gussak I. Short QT interval in clinical practice. J Electrocardiol 2010; 43:390–395.
- Maury P, Extramiana F, Sbragia P, et al. Short QT syndrome. Update on a recent entity. Arch Cardiovasc Dis 2008; 101:779–786.
- Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med 1990; 227:211–213.
- Cheng TO. Digitalis administration: an underappreciated but common cause of short QT interval. Circulation 2004; 109:e152.
- Hancox JC, Choisy SC, James AF. Short QT interval linked to androgen misuse: wider significance and possible basis. Ann Noninvasive Electrocardiol 2009; 14:311–312.
- Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I. Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 2006; 39:389–394.
- Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm 2008; 5:AB43.
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol 2011; 57:802–812.
- Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J 1994; 21:30–41.
- Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004; 109:30–35.
- Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004; 109:2394–2397.
- Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005; 96:800–807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007; 115:442–449.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 2007; 4:964–972.
- Lunati M, Bongiorni MG, Boriani G, et al. Linee guida AIAC 2006 all’impianto di pacemaker, dispositivi per la resincronizzazione cardiaca (CRT) e defibrillatori automatici impiantabili (ICD). GIAC 2005; 8:1–58.
- Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003; 14:1273–1277.
- Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004; 43:1494–1499.
- Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol 2011; 58:587–595.
Sudden cardiac death in a young person is a devastating event that has puzzled physicians for decades. In recent years, many of the underlying cardiac pathologies have been identified. These include structural abnormalities such as hypertrophic cardiomyopathy and nonstructural disorders associated with unstable rhythms that lead to sudden cardiac death.
The best known of these “channelopathies” are the long QT syndromes, which result from abnormal potassium and sodium channels in myocytes. Recently, interest has been growing in a disorder that may carry a similarly grim prognosis but that has an opposite finding on electrocardiography (ECG).
Short QT syndrome is a recently described heterogeneous genetic channelopathy that causes both atrial and ventricular arrhythmias and that has been documented to cause sudden cardiac death.
In 1996, a 37-year-old woman from Spain died suddenly; ECG several days earlier had shown a short QT interval of 266 ms.1 Two years later, an unrelated 17-year-old American woman undergoing laparoscopic cholecystectomy suddenly developed atrial fibrillation with a rapid ventricular response.1 Her QT interval was 225 ms. Her brother had a QT interval of 240 ms, and her mother’s was 230 ms. The patient’s maternal grandfather had a history of atrial fibrillation, and his QT interval was 245 ms. These cases led to the description of this new clinical syndrome (see below).2
CLINICAL FEATURES
Short QT syndrome has been associated with both atrial and ventricular arrhythmias. Atrial fibrillation, polymorphic ventricular tachycardia, and ventricular fibrillation have all been well described. Patients who have symptoms usually present with palpitations, presyncope, syncope, or sudden or aborted cardiac death.3,4
ELECTROCARDIOGRAPHIC FEATURES
The primary finding on ECG is a short QT interval. However, others have been noted (Figure 1):
Short or absent ST segment
This finding is not merely a consequence of the short QT interval. In 10 patients with short QT syndrome, the distance from the J point to the peak T wave ranged from 80 to 120 ms. In 12 healthy people whose QT interval was less than 320 ms, this distance ranged from 150 ms to 240 ms.5
Tall and peaked T wave
A tall and peaked T wave is a common feature in short QT syndrome. However, it was also evident in people with short QT intervals who had no other features of the syndrome.5
QT response to heart rate
Normally, the QT interval is inversely related to the heart rate, but this is not true in short QT syndrome: the QT interval remains relatively fixed with changes in heart rate.6,7 This feature is less helpful in the office setting but may be found with Holter monitoring by measuring the QT interval at different heart rates.
BUT WHAT IS CONSIDERED A SHORT QT INTERVAL?
In clinical practice, the QT interval is corrected for the heart rate by the Bazett formula:
Corrected QT (QTc) = [QT interval/square root of the RR interval]
Review of ECGs from large populations in Finland (n = 10,822), Japan (n = 12,149), the United States (n = 79,743), and Switzerland (n = 41,676) revealed that a QTc value of 350 ms in males and 365 ms in females was 2.0 standard deviations (SD) below the mean.8–11 However, a QTc less than the 2.0 SD cutoff did not necessarily equal arrhythmogenic potential. This was illustrated in a 29-year follow-up study of Finnish patients with QTc values as short as 320 ms, in whom no arrhythmias were documented.8 Conversely, some patients with purported short QT syndrome had QTc intervals as long as 381 ms.12
Similar problems with uncertainty of values have plagued the diagnosis of long QT syndrome.13 The lack of reference ranges and the overlap between healthy and affected people called for the development of a scoring system that involves criteria based on ECG and on the clinical evaluation.14,15
ESTABLISHING THE DIAGNOSIS OF SHORT QT SYNDROME
Clearly, the diagnosis of short QT syndrome can be challenging to establish. The first step is to rule out other causes of a short QT interval.
Differential diagnosis of short QT interval
In addition to genetic channelopathies, other causes of short QT interval must be ruled out before entertaining the diagnosis of short QT syndrome.
- Hypercalcemia is the most important of these: there is usually an accompanying prolonged PR interval and a wide QRS complex16
- Hyperkalemia17
- Acidosis17
- Increased vagal tone17
- After ventricular fibrillation (thought to be related to increased intracellular calcium)18
- Digitalis use19
- Androgen use.20
Interestingly, a shorter-than-expected QT interval was noted in patients with chronic fatigue syndrome.21
Which interval to use: QT or QTc?
Unfortunately, most population-based studies that searched for a short QT interval on ECG have used QTc as the main search parameter.8–11 As already mentioned, in patients with short QT syndrome, the QT interval is, uniquely, not shortened if the heart beats faster. In contrast, the QTc often overestimates the QT interval in patients with short QT syndrome, especially when the heart rate is in the 80s to 90s.16
In a review of cases of short QT syndrome worldwide, Bjerregaard et al22 found that the QT interval ranged from 210 ms to 340 ms with a mean ± 2 SD of 282 ± 62 ms. On the other hand, the QTc ranged from 248 ms to 345 ms with a mean ± 2 SD of 305 ± 42 ms.
Therefore, correction formulas (such as the Bazett formula) do not perform well in ruling in the diagnosis of short QT syndrome—and they do even worse in ruling it out.16,22
To establish a diagnosis of short QT syndrome in someone with prior evidence of atrial or ventricular fibrillation, a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient.22 In borderline cases in which the QT interval is slightly longer, some experts recommend other tests, although strong evidence validating their predictive value does not exist. These tests include genotyping, analysis of T wave morphology, and electrophysiologic studies.16
Recently, Gollob et al23 proposed a scoring system for short QT syndrome (Table 1). After reviewing the literature and comparing the diagnostic markers, the investigators determined diagnostic criteria that, when applied to the previously reported cases, were able to identify 58 (95.08%) of 61 patients with short QT syndrome (ie, a sensitivity of 95%).
For patients with intermediate probability, the authors recommended continued medical and ECG surveillance as well as ECGs for first-degree relatives, to further clarify the diagnosis.
Again, a principal caveat about this system is that it relies on the QTc interval rather than the QT interval to diagnose short QT syndrome.
THE SCOPE OF THE DISEASE
In a recent review of the literature, Gollob et al23 found a total of 61 cases of short QT syndrome reported in English. The cohort was predominantly male (75.4%), and most of the symptomatic patients presented during late adolescence and early adulthood. However, there have been reports of infants (4 and 8 months old), and of a man who presented for the first time at the age of 70. Of note, the authors only considered short QT syndrome types 1, 2, and 3 (see below) in their search for cases.
Whether the syndrome is truly this rare or, rather, whether many physicians are not aware of it is still to be determined. In addition, it is possible that incorrectly measuring the QT interval contributes to the lack of identification of this entity. Both of these factors were implicated in the rarity of reported long QT syndrome early after its discovery.14,15
MUTATIONS IN CARDIAC ION CHANNELS
Five distinct genetic defects have been associated with short QT syndrome. As in long QT syndrome, these give rise to subtypes of short QT syndrome, which are numbered 1 to 5 (see below).
The cardiac action potential
To understand how the mutations shorten the QT interval, we will briefly review of the cardiac myocyte action potential.24 In nonpacemaker cells of the heart, the activation of the cell membrane initiates a series of changes in ion channels that allow the movement of ions along an electrical gradient. This movement occurs in five phases and is repeated with every cardiac cycle (Figure 2).
In phase 0, the cardiac cell rapidly depolarizes.
Repolarization occurs in phases 1, 2, and 3 and is largely a function of potassium ions leaving the cell. During phase 2, calcium and sodium ions enter the cell and balance the outward potassium flow, creating the “flat” portion of the repolarization curve. Phase 3 is the main phase of repolarization in which the membrane potential rapidly falls back to its resting state (–90 mV). During phases 1 and 2, the cell membrane is completely refractory to stimulation, whereas phase 3 is divided into three parts:
- The effective refractory period: the cell is able to generate a potential that is too weak to be propagated
- The relative refractory period: the cell can respond to a stimulus that is stronger than normal
- The supernormal phase: the last small portion of phase 3, in which a less-than-normal stimulus can yield a response in the cell.
In phase 4, the cell is completely repolarized, and the cycle can start again.
Five types of short QT syndrome
Short QT syndrome 1. In 2004, Brugada et al25 identified the first mutation that causes abnormal shortening of the action potential duration. In contrast to the mutations that underlie long QT syndrome, this mutation actually causes a gain of function in the gene coding the rapidly acting delayed potassium current (IKr) channel proteins KCNH2 or HERG. Potassium leaving at a more rapid rate causes the cell to repolarize more quickly and shortens the QT interval. The clinical syndrome associated with KCNH2 gene gain-of-function mutation is called short QT syndrome 1.
Short QT syndromes 2 and 3. Other IK (potassium channel) proteins have been implicated as well. Gain-of-function mutations in the KCNQ1 and KCNJ2 genes are believed to account for short QT syndromes 2 and 3, respectively. KCNQ1 codes for the IKs protein, and KCNJ2 codes for the IK1 protein.26,27
Short QT syndromes 4 and 5 were identified by Antzelevitch et al,28 who described several patients who had a combination of channel abnormalities and ECG findings. Their ECGs showed “Brugada-syndrome-like” ST elevation in the right precordial leads, but with a short QT interval. These new syndromes were found to be associated with genetic abnormalities distinct from those of Brugada syndrome and other short QT syndromes. These abnormalities involved loss-of-function mutations in the CACNA1C gene (which codes for the alpha-1 subunit of the L-type cardiac calcium channel) and in the CACNB2 gene (which codes for the beta-2b subunit of the same channel). The two defects correspond to the clinical syndromes short QT syndrome 4 and short QT syndrome 5, respectively.28
MECHANISM OF ARRHYTHMOGENESIS IN SHORT QT SYNDROME
The myocardium is made of different layers: the epicardium, the endocardium, and the middle layer of myocytes composed mainly of M cells. Cells in the different layers differ in the concentration of their channels and can be affected differently in various syndromes. When cells in one or two of the layers repolarize at a rate different from cells in another layer, they create different degrees of refractoriness, which establishes the potential for reentry circuits to form.
It is believed that in short QT syndrome the endocardial cells and M cells repolarize faster than the epicardial cells, predisposing to reentry and arrhythmias. This accentuation of “transmural dispersion of repolarization” accounts for arrhythmogenesis in short QT syndrome as well as in long QT syndrome and the Brugada syndromes. The difference between these syndromes appears to be the layer or area of the myocardium that is affected more by the channelopathy (the M cells in long QT syndrome and the epicardium of the right ventricle in the Brugada syndrome).29
WHEN TO THINK OF SHORT QT SYNDROME
In any survivor of sudden cardiac death, the QT interval should be thoroughly scrutinized, and family members should undergo ECG. Patients in whom a short QT interval is incidentally discovered and for which other reasons are ruled out (see differential diagnosis) should be encouraged to have family members undergo ECG. Other potential patients are young people who develop atrial fibrillation and patients who have idiopathic ventricular fibrillation.4
TREATMENT AND PROGNOSIS
Evidence-based recommendations for the management of short QT syndrome do not yet exist, mainly because the number of patients identified to date is small.
Implantable cardioverter-defibrillators
Although placing an implantable cardioverter-defibrillator (ICD) seems to be warranted in patients who experience ventricular fibrillation, ventricular tachycardia, or aborted cardiac death, or in patients who have a family history of the same symptoms, the best management option is less clear for patients who have no symptoms and no family history.30 In addition, some patients may not want an ICD or may even not qualify for this therapy.
A unique problem with ICDs in short QT syndrome stems from one of the syndrome’s main features on ECG: the tall and peaked T wave that closely follows the R wave can sometimes be interpreted as a short R-R interval, provoking an inappropriate shock from the ICD.31
For the above reasons, we strongly encourage consulting a center with expertise in QT-interval-related disorders before placing an ICD in a patient suspected of having short QT syndrome.
Antiarrhythmic drugs
Prolongation of the QT interval (and the effective refractory period) with drugs has been an interesting area of research. Gaita et al32 studied the effect of four antiarrhythmics—flecainide (Tambocor), sotalol (Betapace), ibutilide (Corvert), and quinidine—in six patients with short QT syndrome. Only quinidine was associated with significant QT prolongation, from 263 ± 12 ms to 362 ms ± 25 ms. This resulted in a longer ventricular effective refractory period (> 200 ms), and ventricular fibrillation was no longer inducible during provocative testing.
In a recent study of long-term outcomes of 53 patients with short QT syndrome, Giustetto et al33 noticed that none of the patients taking quinidine, including those with a history of cardiac arrest, had any further arrhythmsic events. On the other hand, the incidence of arrhythmic events during the follow-up was 4.9% per year in patients not taking this drug. Quinidine had a stronger effect on the QT interval in patients with the HERG mutation than in those without.
RESEARCH MAY LEAD TO A BETTER UNDERSTANDING OF OTHER DISEASES
The short QT syndrome is one of the most recently recognized cardiac channelopathies associated with malignant arrhythmias. As with long QT syndrome, research in short QT syndrome may lead to a better understanding of the pathogenesis of more common but still poorly understood arrhythmias such as lone atrial fibrillation and idiopathic ventricular fibrillation.
Sudden cardiac death in a young person is a devastating event that has puzzled physicians for decades. In recent years, many of the underlying cardiac pathologies have been identified. These include structural abnormalities such as hypertrophic cardiomyopathy and nonstructural disorders associated with unstable rhythms that lead to sudden cardiac death.
The best known of these “channelopathies” are the long QT syndromes, which result from abnormal potassium and sodium channels in myocytes. Recently, interest has been growing in a disorder that may carry a similarly grim prognosis but that has an opposite finding on electrocardiography (ECG).
Short QT syndrome is a recently described heterogeneous genetic channelopathy that causes both atrial and ventricular arrhythmias and that has been documented to cause sudden cardiac death.
In 1996, a 37-year-old woman from Spain died suddenly; ECG several days earlier had shown a short QT interval of 266 ms.1 Two years later, an unrelated 17-year-old American woman undergoing laparoscopic cholecystectomy suddenly developed atrial fibrillation with a rapid ventricular response.1 Her QT interval was 225 ms. Her brother had a QT interval of 240 ms, and her mother’s was 230 ms. The patient’s maternal grandfather had a history of atrial fibrillation, and his QT interval was 245 ms. These cases led to the description of this new clinical syndrome (see below).2
CLINICAL FEATURES
Short QT syndrome has been associated with both atrial and ventricular arrhythmias. Atrial fibrillation, polymorphic ventricular tachycardia, and ventricular fibrillation have all been well described. Patients who have symptoms usually present with palpitations, presyncope, syncope, or sudden or aborted cardiac death.3,4
ELECTROCARDIOGRAPHIC FEATURES
The primary finding on ECG is a short QT interval. However, others have been noted (Figure 1):
Short or absent ST segment
This finding is not merely a consequence of the short QT interval. In 10 patients with short QT syndrome, the distance from the J point to the peak T wave ranged from 80 to 120 ms. In 12 healthy people whose QT interval was less than 320 ms, this distance ranged from 150 ms to 240 ms.5
Tall and peaked T wave
A tall and peaked T wave is a common feature in short QT syndrome. However, it was also evident in people with short QT intervals who had no other features of the syndrome.5
QT response to heart rate
Normally, the QT interval is inversely related to the heart rate, but this is not true in short QT syndrome: the QT interval remains relatively fixed with changes in heart rate.6,7 This feature is less helpful in the office setting but may be found with Holter monitoring by measuring the QT interval at different heart rates.
BUT WHAT IS CONSIDERED A SHORT QT INTERVAL?
In clinical practice, the QT interval is corrected for the heart rate by the Bazett formula:
Corrected QT (QTc) = [QT interval/square root of the RR interval]
Review of ECGs from large populations in Finland (n = 10,822), Japan (n = 12,149), the United States (n = 79,743), and Switzerland (n = 41,676) revealed that a QTc value of 350 ms in males and 365 ms in females was 2.0 standard deviations (SD) below the mean.8–11 However, a QTc less than the 2.0 SD cutoff did not necessarily equal arrhythmogenic potential. This was illustrated in a 29-year follow-up study of Finnish patients with QTc values as short as 320 ms, in whom no arrhythmias were documented.8 Conversely, some patients with purported short QT syndrome had QTc intervals as long as 381 ms.12
Similar problems with uncertainty of values have plagued the diagnosis of long QT syndrome.13 The lack of reference ranges and the overlap between healthy and affected people called for the development of a scoring system that involves criteria based on ECG and on the clinical evaluation.14,15
ESTABLISHING THE DIAGNOSIS OF SHORT QT SYNDROME
Clearly, the diagnosis of short QT syndrome can be challenging to establish. The first step is to rule out other causes of a short QT interval.
Differential diagnosis of short QT interval
In addition to genetic channelopathies, other causes of short QT interval must be ruled out before entertaining the diagnosis of short QT syndrome.
- Hypercalcemia is the most important of these: there is usually an accompanying prolonged PR interval and a wide QRS complex16
- Hyperkalemia17
- Acidosis17
- Increased vagal tone17
- After ventricular fibrillation (thought to be related to increased intracellular calcium)18
- Digitalis use19
- Androgen use.20
Interestingly, a shorter-than-expected QT interval was noted in patients with chronic fatigue syndrome.21
Which interval to use: QT or QTc?
Unfortunately, most population-based studies that searched for a short QT interval on ECG have used QTc as the main search parameter.8–11 As already mentioned, in patients with short QT syndrome, the QT interval is, uniquely, not shortened if the heart beats faster. In contrast, the QTc often overestimates the QT interval in patients with short QT syndrome, especially when the heart rate is in the 80s to 90s.16
In a review of cases of short QT syndrome worldwide, Bjerregaard et al22 found that the QT interval ranged from 210 ms to 340 ms with a mean ± 2 SD of 282 ± 62 ms. On the other hand, the QTc ranged from 248 ms to 345 ms with a mean ± 2 SD of 305 ± 42 ms.
Therefore, correction formulas (such as the Bazett formula) do not perform well in ruling in the diagnosis of short QT syndrome—and they do even worse in ruling it out.16,22
To establish a diagnosis of short QT syndrome in someone with prior evidence of atrial or ventricular fibrillation, a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient.22 In borderline cases in which the QT interval is slightly longer, some experts recommend other tests, although strong evidence validating their predictive value does not exist. These tests include genotyping, analysis of T wave morphology, and electrophysiologic studies.16
Recently, Gollob et al23 proposed a scoring system for short QT syndrome (Table 1). After reviewing the literature and comparing the diagnostic markers, the investigators determined diagnostic criteria that, when applied to the previously reported cases, were able to identify 58 (95.08%) of 61 patients with short QT syndrome (ie, a sensitivity of 95%).
For patients with intermediate probability, the authors recommended continued medical and ECG surveillance as well as ECGs for first-degree relatives, to further clarify the diagnosis.
Again, a principal caveat about this system is that it relies on the QTc interval rather than the QT interval to diagnose short QT syndrome.
THE SCOPE OF THE DISEASE
In a recent review of the literature, Gollob et al23 found a total of 61 cases of short QT syndrome reported in English. The cohort was predominantly male (75.4%), and most of the symptomatic patients presented during late adolescence and early adulthood. However, there have been reports of infants (4 and 8 months old), and of a man who presented for the first time at the age of 70. Of note, the authors only considered short QT syndrome types 1, 2, and 3 (see below) in their search for cases.
Whether the syndrome is truly this rare or, rather, whether many physicians are not aware of it is still to be determined. In addition, it is possible that incorrectly measuring the QT interval contributes to the lack of identification of this entity. Both of these factors were implicated in the rarity of reported long QT syndrome early after its discovery.14,15
MUTATIONS IN CARDIAC ION CHANNELS
Five distinct genetic defects have been associated with short QT syndrome. As in long QT syndrome, these give rise to subtypes of short QT syndrome, which are numbered 1 to 5 (see below).
The cardiac action potential
To understand how the mutations shorten the QT interval, we will briefly review of the cardiac myocyte action potential.24 In nonpacemaker cells of the heart, the activation of the cell membrane initiates a series of changes in ion channels that allow the movement of ions along an electrical gradient. This movement occurs in five phases and is repeated with every cardiac cycle (Figure 2).
In phase 0, the cardiac cell rapidly depolarizes.
Repolarization occurs in phases 1, 2, and 3 and is largely a function of potassium ions leaving the cell. During phase 2, calcium and sodium ions enter the cell and balance the outward potassium flow, creating the “flat” portion of the repolarization curve. Phase 3 is the main phase of repolarization in which the membrane potential rapidly falls back to its resting state (–90 mV). During phases 1 and 2, the cell membrane is completely refractory to stimulation, whereas phase 3 is divided into three parts:
- The effective refractory period: the cell is able to generate a potential that is too weak to be propagated
- The relative refractory period: the cell can respond to a stimulus that is stronger than normal
- The supernormal phase: the last small portion of phase 3, in which a less-than-normal stimulus can yield a response in the cell.
In phase 4, the cell is completely repolarized, and the cycle can start again.
Five types of short QT syndrome
Short QT syndrome 1. In 2004, Brugada et al25 identified the first mutation that causes abnormal shortening of the action potential duration. In contrast to the mutations that underlie long QT syndrome, this mutation actually causes a gain of function in the gene coding the rapidly acting delayed potassium current (IKr) channel proteins KCNH2 or HERG. Potassium leaving at a more rapid rate causes the cell to repolarize more quickly and shortens the QT interval. The clinical syndrome associated with KCNH2 gene gain-of-function mutation is called short QT syndrome 1.
Short QT syndromes 2 and 3. Other IK (potassium channel) proteins have been implicated as well. Gain-of-function mutations in the KCNQ1 and KCNJ2 genes are believed to account for short QT syndromes 2 and 3, respectively. KCNQ1 codes for the IKs protein, and KCNJ2 codes for the IK1 protein.26,27
Short QT syndromes 4 and 5 were identified by Antzelevitch et al,28 who described several patients who had a combination of channel abnormalities and ECG findings. Their ECGs showed “Brugada-syndrome-like” ST elevation in the right precordial leads, but with a short QT interval. These new syndromes were found to be associated with genetic abnormalities distinct from those of Brugada syndrome and other short QT syndromes. These abnormalities involved loss-of-function mutations in the CACNA1C gene (which codes for the alpha-1 subunit of the L-type cardiac calcium channel) and in the CACNB2 gene (which codes for the beta-2b subunit of the same channel). The two defects correspond to the clinical syndromes short QT syndrome 4 and short QT syndrome 5, respectively.28
MECHANISM OF ARRHYTHMOGENESIS IN SHORT QT SYNDROME
The myocardium is made of different layers: the epicardium, the endocardium, and the middle layer of myocytes composed mainly of M cells. Cells in the different layers differ in the concentration of their channels and can be affected differently in various syndromes. When cells in one or two of the layers repolarize at a rate different from cells in another layer, they create different degrees of refractoriness, which establishes the potential for reentry circuits to form.
It is believed that in short QT syndrome the endocardial cells and M cells repolarize faster than the epicardial cells, predisposing to reentry and arrhythmias. This accentuation of “transmural dispersion of repolarization” accounts for arrhythmogenesis in short QT syndrome as well as in long QT syndrome and the Brugada syndromes. The difference between these syndromes appears to be the layer or area of the myocardium that is affected more by the channelopathy (the M cells in long QT syndrome and the epicardium of the right ventricle in the Brugada syndrome).29
WHEN TO THINK OF SHORT QT SYNDROME
In any survivor of sudden cardiac death, the QT interval should be thoroughly scrutinized, and family members should undergo ECG. Patients in whom a short QT interval is incidentally discovered and for which other reasons are ruled out (see differential diagnosis) should be encouraged to have family members undergo ECG. Other potential patients are young people who develop atrial fibrillation and patients who have idiopathic ventricular fibrillation.4
TREATMENT AND PROGNOSIS
Evidence-based recommendations for the management of short QT syndrome do not yet exist, mainly because the number of patients identified to date is small.
Implantable cardioverter-defibrillators
Although placing an implantable cardioverter-defibrillator (ICD) seems to be warranted in patients who experience ventricular fibrillation, ventricular tachycardia, or aborted cardiac death, or in patients who have a family history of the same symptoms, the best management option is less clear for patients who have no symptoms and no family history.30 In addition, some patients may not want an ICD or may even not qualify for this therapy.
A unique problem with ICDs in short QT syndrome stems from one of the syndrome’s main features on ECG: the tall and peaked T wave that closely follows the R wave can sometimes be interpreted as a short R-R interval, provoking an inappropriate shock from the ICD.31
For the above reasons, we strongly encourage consulting a center with expertise in QT-interval-related disorders before placing an ICD in a patient suspected of having short QT syndrome.
Antiarrhythmic drugs
Prolongation of the QT interval (and the effective refractory period) with drugs has been an interesting area of research. Gaita et al32 studied the effect of four antiarrhythmics—flecainide (Tambocor), sotalol (Betapace), ibutilide (Corvert), and quinidine—in six patients with short QT syndrome. Only quinidine was associated with significant QT prolongation, from 263 ± 12 ms to 362 ms ± 25 ms. This resulted in a longer ventricular effective refractory period (> 200 ms), and ventricular fibrillation was no longer inducible during provocative testing.
In a recent study of long-term outcomes of 53 patients with short QT syndrome, Giustetto et al33 noticed that none of the patients taking quinidine, including those with a history of cardiac arrest, had any further arrhythmsic events. On the other hand, the incidence of arrhythmic events during the follow-up was 4.9% per year in patients not taking this drug. Quinidine had a stronger effect on the QT interval in patients with the HERG mutation than in those without.
RESEARCH MAY LEAD TO A BETTER UNDERSTANDING OF OTHER DISEASES
The short QT syndrome is one of the most recently recognized cardiac channelopathies associated with malignant arrhythmias. As with long QT syndrome, research in short QT syndrome may lead to a better understanding of the pathogenesis of more common but still poorly understood arrhythmias such as lone atrial fibrillation and idiopathic ventricular fibrillation.
- The Short QT Syndrome http://www.shortqtsyndrome.org/short_qt_history.htm. Accessed October 30, 2012.
- Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000; 94:99–102.
- Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006; 27:2440–2447.
- Viskin S, Zeltser D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004; 1:587–591.
- Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009; 6:267–271.
- Redpath CJ, Green MS, Birnie DH, Gollob MH. Rapid genetic testing facilitating the diagnosis of short QT syndrome. Can J Cardiol 2009; 25:e133–e135.
- Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005; 16:54–58.
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007; 116:714–720.
- Funada A, Hayashi K, Ino H, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol 2008; 31:270–274.
- Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007; 40:228–234.
- Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009; 6:652–657.
- Itoh H, Sakaguchi T, Ashihara T, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol 2009; 137:83–85.
- Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992; 327:846–852.
- Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399–411.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993; 88:782–784.
- Bjerregaard P, Nallapaneni H, Gussak I. Short QT interval in clinical practice. J Electrocardiol 2010; 43:390–395.
- Maury P, Extramiana F, Sbragia P, et al. Short QT syndrome. Update on a recent entity. Arch Cardiovasc Dis 2008; 101:779–786.
- Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med 1990; 227:211–213.
- Cheng TO. Digitalis administration: an underappreciated but common cause of short QT interval. Circulation 2004; 109:e152.
- Hancox JC, Choisy SC, James AF. Short QT interval linked to androgen misuse: wider significance and possible basis. Ann Noninvasive Electrocardiol 2009; 14:311–312.
- Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I. Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 2006; 39:389–394.
- Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm 2008; 5:AB43.
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol 2011; 57:802–812.
- Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J 1994; 21:30–41.
- Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004; 109:30–35.
- Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004; 109:2394–2397.
- Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005; 96:800–807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007; 115:442–449.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 2007; 4:964–972.
- Lunati M, Bongiorni MG, Boriani G, et al. Linee guida AIAC 2006 all’impianto di pacemaker, dispositivi per la resincronizzazione cardiaca (CRT) e defibrillatori automatici impiantabili (ICD). GIAC 2005; 8:1–58.
- Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003; 14:1273–1277.
- Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004; 43:1494–1499.
- Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol 2011; 58:587–595.
- The Short QT Syndrome http://www.shortqtsyndrome.org/short_qt_history.htm. Accessed October 30, 2012.
- Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology 2000; 94:99–102.
- Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006; 27:2440–2447.
- Viskin S, Zeltser D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm 2004; 1:587–591.
- Anttonen O, Junttila MJ, Maury P, et al. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm 2009; 6:267–271.
- Redpath CJ, Green MS, Birnie DH, Gollob MH. Rapid genetic testing facilitating the diagnosis of short QT syndrome. Can J Cardiol 2009; 25:e133–e135.
- Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol 2005; 16:54–58.
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 2007; 116:714–720.
- Funada A, Hayashi K, Ino H, et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol 2008; 31:270–274.
- Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007; 40:228–234.
- Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm 2009; 6:652–657.
- Itoh H, Sakaguchi T, Ashihara T, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol 2009; 137:83–85.
- Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992; 327:846–852.
- Schwartz PJ. Idiopathic long QT syndrome: progress and questions. Am Heart J 1985; 109:399–411.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993; 88:782–784.
- Bjerregaard P, Nallapaneni H, Gussak I. Short QT interval in clinical practice. J Electrocardiol 2010; 43:390–395.
- Maury P, Extramiana F, Sbragia P, et al. Short QT syndrome. Update on a recent entity. Arch Cardiovasc Dis 2008; 101:779–786.
- Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med 1990; 227:211–213.
- Cheng TO. Digitalis administration: an underappreciated but common cause of short QT interval. Circulation 2004; 109:e152.
- Hancox JC, Choisy SC, James AF. Short QT interval linked to androgen misuse: wider significance and possible basis. Ann Noninvasive Electrocardiol 2009; 14:311–312.
- Naschitz J, Fields M, Isseroff H, Sharif D, Sabo E, Rosner I. Shortened QT interval: a distinctive feature of the dysautonomia of chronic fatigue syndrome. J Electrocardiol 2006; 39:389–394.
- Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm 2008; 5:AB43.
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol 2011; 57:802–812.
- Shih HT. Anatomy of the action potential in the heart. Tex Heart Inst J 1994; 21:30–41.
- Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 2004; 109:30–35.
- Bellocq C, van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation 2004; 109:2394–2397.
- Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res 2005; 96:800–807.
- Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007; 115:442–449.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 2007; 4:964–972.
- Lunati M, Bongiorni MG, Boriani G, et al. Linee guida AIAC 2006 all’impianto di pacemaker, dispositivi per la resincronizzazione cardiaca (CRT) e defibrillatori automatici impiantabili (ICD). GIAC 2005; 8:1–58.
- Schimpf R, Wolpert C, Bianchi F, et al. Congenital short QT syndrome and implantable cardioverter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol 2003; 14:1273–1277.
- Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol 2004; 43:1494–1499.
- Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol 2011; 58:587–595.
KEY POINTS
- Short QT syndrome is a genetic disease described initially in young patients who had atrial fibrillation or who died suddenly with no apparent structural heart disease.
- The diagnosis is established by the finding of a short QT interval. However, other factors including personal and family history are also important in establishing the diagnosis.
- The current recommendations for managing patients with short QT syndrome are not evidence-based. We encourage consultation with centers that have special interest in QT-interval-related disorders.
- Placement of an implantable cardioverter-defibrillator is considered the standard of care, especially in survivors of sudden cardiac death, ventricular fibrillation, or ventricular tachycardia. Unfortunately, a higher incidence of inappropriate shocks adds to the challenges of managing this potentially deadly disease.
Advanced heart failure: Transplantation, LVADs, and beyond
Patients with advanced heart failure far outnumber the hearts available for transplantation. Partly as a consequence of this shortage, left-ventricular assist devices (LVADs) are being used more widely.
This article is an update on options for managing severe, advanced heart failure, with special attention to new developments and continuing challenges in heart transplantation and LVADs.
THE PREVALENCE OF HEART FAILURE
About 2.6% of the US population age 20 and older have heart failure—some 5.8 million people. Of these, about half have systolic heart failure.1 Patients with systolic heart failure can be classified by degree of severity under two systems:
The New York Heart Association (NYHA) classifies patients by their functional status, from I (no limitation in activities) to IV (symptoms at rest). NYHA class III (symptoms with minimal exertion) is sometimes further broken down into IIIa and IIIb, with the latter defined as having a recent history of dyspnea at rest.
The joint American College of Cardiology and American Heart Association (ACC/AHA) classification uses four stages, from A (high risk of developing heart failure, ie, having risk factors such as family history of heart disease, hypertension, or diabetes) to D (advanced heart disease despite treatment). Patients in stage D tend to be recurrently hospitalized despite cardiac resynchronization therapy and drug therapy, and they cannot be safely discharged without specialized interventions. The options for these patients are limited: either end-of-life care or extraordinary measures such as heart transplantation, long-term treatment with inotropic drugs, permanent mechanical circulatory support, or experimental therapies.2
The estimated number of people in ACC/AHA stage D or NYHA class IV is 15,600 to 156,000. The approximate number of heart transplants performed in the United States each year is 2,100.3
WHICH AMBULATORY PATIENTS ARE MOST AT RISK?
The range for the estimated number of patients with advanced heart failure (NYHA class IIIb or IV) is wide (see above) because these patients may be hard to recognize. The most debilitated patients are obvious: they tend to be in the intensive care unit with end-organ failure. However, it is a challenge to recognize patients at extremely high risk who are still ambulatory.
The European Society of Cardiology4 developed a definition of advanced chronic heart failure that can help identify patients who are candidates for the transplant list and for an LVAD. All the following features must be present despite optimal therapy that includes diuretics, inhibitors of the renin-angiotensin-aldosterone system, and beta-blockers, unless these are poorly tolerated or contraindicated, and cardiac resynchronization therapy if indicated:
- Severe symptoms, with dyspnea or fatigue at rest or with minimal exertion (NYHA class III or IV)
- Episodes of fluid retention (pulmonary or systemic congestion, peripheral edema) or of reduced cardiac output at rest (peripheral hypoperfusion)
- Objective evidence of severe cardiac dysfunction (at least one of the following): left ventricular ejection fraction less than 30%, pseudonormal or restrictive mitral inflow pattern on Doppler echocardiography, high left or right ventricular filling pressure (or both left and right filling pressures), and elevated B-type natriuretic peptides
- Severely impaired functional capacity demonstrated by one of the following: inability to exercise, 6-minute walk test distance less than 300 m (or less in women or patients who are age 75 and older), or peak oxygen intake less than 12 to 14 mL/kg/min
- One or more hospitalizations for heart failure in the past 6 months.
Treadmill exercise time is an easily performed test. Hsich et al5 found that the longer patients can walk, the lower their risk of death, and that this variable is about as predictive of survival in patients with systolic left ventricular dysfunction as peak oxygen consumption, which is much more cumbersome to measure.
The Seattle Heart Failure Model gives an estimate of prognosis for ambulatory patients with advanced heart failure. Available at http://depts.washington.edu/shfm/, it is based on age, sex, NYHA class, weight, ejection fraction, blood pressure, medications, a few laboratory values, and other clinical information. The model has been validated in numerous cohorts,6 but it may underestimate risk and is currently being tested in clinical trials (REVIVE-IT and ROADMAP; see at www.clinicaltrials.gov).
Recurrent hospitalization is a simple predictor of risk. A study of about 7,000 patients worldwide found that after hospitalization with acute decompensated heart failure, the strongest predictor of death within 6 months was readmission for any reason within 30 days of the index hospitalization (Starling RC, unpublished observation, 2011). Any patient with heart failure who is repeatedly hospitalized should have a consultation with a heart failure specialist.
INOTROPIC THERAPY FOR BRIDGING
Inotropic drugs, which include intravenous dobutamine (Dobutrex) and milrinone (Primacor), are used to help maintain end-organ function until a patient can obtain a heart transplant or LVAD.
Inotropic therapy should not be viewed as an alternative to heart transplantation or device implantation. We inform patients that inotropic therapy is purely palliative and may actually increase the risk of death, which is about 50% at 6 months and nearly 100% at 1 year. A patient on inotropic therapy who is not a candidate for a transplant or for an assist device should be referred to a hospice program.7
CARDIAC TRANSPLANTATION: SUCCESSES, CHALLENGES
Survival rates after heart transplantation are now excellent. The 1-year survival rate is about 90%, the 5-year rate is about 70%, but only about 20% survive 20 years or longer.8,9 The prognosis is not as good as for combined heart-lung transplantation patients.
Age is an important factor and is a contentious issue: some medical centers will not offer transplantation to patients over age 65. Others regard age as just another risk factor, like renal dysfunction or diabetes.
Quality of life after heart transplantation is excellent: patients are usually able to return to work, regardless of their profession.
The leading cause of death after heart transplantation is malignancy, followed by coronary artery vasculopathy, then by graft failure. Some patients develop left ventricular dysfunction and heart failure of unknown cause. Others develop antibody-mediated rejection; in recent years this has been more promptly recognized, but treatment remains a challenge.
Acute rejection, which used to be one of the main causes of death, now has an extremely low incidence because of modern drug therapies. In a US observational study currently being conducted in about 200 patients receiving a heart transplant (details on CTOT-05 at www.clinicaltrials.gov), the incidence of moderate rejection during the first year is less than 10% (Starling RC, unpublished observation). But several concerns remain.
Adverse effects of immunosuppressive drugs continue to be problematic. These include infection, malignancy, osteoporosis, chronic kidney toxicity, hypertension, and neuropathy.
Renal dysfunction is one of the largest issues. About 10% of heart transplant recipients develop stage 4 kidney disease (with a glomerular filtration rate < 30 mL/min) and need kidney transplantation or renal replacement therapy because of the use of calcineurin inhibitors for immunosuppression.10
Coronary artery vasculopathy was the largest problem when heart transplantation began and continues to be a major concern and focus of research.11,12 Case 1 (below) is an example of the problem.
Case 1: Poor outcome despite an ideal scenario
A 57-year-old businessman had dilated cardiomyopathy and progressive heart failure for 10 years. He was listed for transplantation in 2008 and was given an LVAD (HeartMate II, Thoratec Corp, Pleasanton, CA) as a bridge until a donor heart became available.
In 2009, he received a heart transplant under ideal conditions: the donor was a large 30-year-old man who died of a gunshot wound to the head in the same city in which the patient and transplant hospital were located. Cardiopulmonary resuscitation was not performed, and the cold ischemic time was just a little more than 3 hours. Immune indicators were ideal with a negative prospective cross-match.
Laboratory results after transplantation included creatinine 1.7 mg/dL (normal 0.6–1.2 mg/dL), low-density lipoprotein cholesterol 75 mg/dL, high-density lipoprotein cholesterol 64 mg/dL, and triglycerides 90 mg/dL.
The patient was given immunosuppressive therapy with cyclosporine (Neoral), mycophenolate (CellCept), and prednisone. Because his creatinine level was high, he was also perioperatively given basiliximab (Simulect), a monoclonal antibody to the alpha chain (CD25) of the interleukin-2 receptor. (In a patient who has poor renal function, basilixumab may help by enabling us to delay the use of calcineurin inhibitors.) He also received simvastatin (Zocor) 10 mg.
Per Cleveland Clinic protocol, he underwent 13 biopsy procedures during his first year, and each was normal (grade 0 or 1R). Evaluation by cardiac catheterization at 1 year showed some irregularities in the left anterior descending artery, but a stent was not deemed necessary. Also, per protocol, he underwent intravascular ultrasonography, which revealed abnormal thickness in the intima and media, indicating that coronary artery disease was developing, although it was nonobstructive.
Two months after this checkup, the patient collapsed and suddenly died while shopping. At autopsy, his left anterior descending artery was found to be severely obstructed.
Coronary artery vasculopathy is still a major problem
This case shows that coronary artery vasculopathy may develop despite an ideal transplantation scenario. It remains a large concern and a focus of research.
Coronary vasculopathy develops in 30% to 40% of heart transplant recipients within 5 years, and the incidence has not been reduced by much over the years. However, probably fewer than 5% of these patients die or even need bypass surgery or stenting, and the problem is managed the same as native atherosclerosis. We perform routine annual cardiac catheterizations or stress tests, or both, and place stents in severely blocked arteries.
THE DONOR SHORTAGE: CHANGING HOW HEARTS ARE ALLOCATED
The number of patients receiving a heart transplant in the United States—about 2,000 per year—has not increased in the past decade. The European Union also has great difficulty obtaining hearts for people in need, and almost every transplant candidate there gets mechanical support for some time. The gap between those listed for transplant and the number transplanted each year continues to widen in both the United States and Europe.
All transplant candidates are assigned a status by the United Network of Organ Sharing (UNOS) based on their medical condition. The highest status, 1A, goes to patients who are seriously ill, in the hospital, on high doses of inotropic drugs (specific dosages are defined) and mechanical circulatory support such as an LVAD, and expected to live less than 1 month without a transplant. Status 1B patients are stable on lower-dose inotropic therapy or on mechanical support, and can be in the hospital or at home. Status 2 patients are stable and ambulatory and are not on inotropic drugs.
In July 2006, UNOS changed the rules on how patients are prioritized for obtaining an organ. The new rules are based both on severity of illness (see above) and geographic proximity to the donor heart—local, within 500 miles (“zone A”) or within 500 to 1,000 miles (“zone B”). The order of priority for donor hearts is:
- Local, status 1A
- Local, status 1B
- Zone A, status 1A
- Zone A, status 1B
- Local, status 2
- Zone B, status 1A
- Zone B, status 1B
- Zone A, status 2.
As a result of the change, donor hearts that become available in a particular hospital do not necessarily go to a patient in that state. Another result is that status 2 patients, who were previously the most common transplant recipients, now have much less access because all status 1 patients within 500 miles are given higher priority. Since the change, only 8% of hearts nationwide go to status 2 patients, which is 67% fewer than before. At the same time, organs allocated to status 1A patients have increased by 26%, and their death rates have fallen.3
The new allocation system is a positive change for the sickest patients, providing quicker access and a reduction in waiting-list mortality.13 The drawback is that status 2 patients who are less ill are less likely to ever receive an organ until their condition worsens.
Heart transplant outcomes are publicly reported
The Scientific Registry of Transplant Recipients publicly reports heart transplant outcomes (www.srtr.org). For any transplant center, the public can learn the number of patients waiting for a transplant, the death rate on the waiting list, the number of transplants performed in the previous 12 months, the waiting time in months, and observed and risk-adjusted expected survival rates. A center that deviates from the expected survival rates by 10% or more may be audited and could lose its certification.
Also listed on the Web site is the percentage of patients who receive a support device before receiving a transplant. This can vary widely between institutions and may reflect the organ availability at the transplant center (waiting times) or the preferences and expertise of the transplantation team. We believe that the mortality rate on the waiting list will be reduced with appropriate use of LVADs as a bridge to transplantation when indicated. We have now transitioned to the use of the improved continuous-flow LVADs and rarely maintain patients on continuous inotropic therapy at home to await a donor organ.
MECHANICAL CIRCULATORY SUPPORT: BRIDGE OR DESTINATION?
Mechanical circulatory support devices are increasingly being used to sustain patients with advanced heart failure. Currently at Cleveland Clinic, more LVADs are implanted than hearts are transplanted.
Mechanical circulatory support is indicated for patients who are listed for transplant to keep them functioning as well as possible while they are waiting (bridge to transplant). For others it is “destination therapy”: they are not candidates for a transplant, but a device may improve and prolong the rest of their life.
Case 2: A good outcome despite a poor prognosis
A 71-year-old man was rejected for transplantation by his local hospital because of his age and also because he had pulmonary artery hypertension (78/42 mm Hg; reference range 15–30/5–15 mm Hg) and creatinine elevation (3.0 mg/dL; reference range 0.6–1.5 mg/dL). Nevertheless, he did well on a mechanical device and was accepted for transplantation by Cleveland Clinic. He received a transplant and is still alive and active 14 years later.
Comment. Determining that a patient is not a good transplantation candidate is often impossible. Putting the patient on mechanical support for a period of time can often help clarify whether transplantation is advisable. Probably most patients who receive mechanical support do so as a bridge to decision: most are acutely ill and many have organ dysfunction, pulmonary hypertension, and renal insufficiency. After a period of support, they can be assessed for suitability for transplantation.
LVADs continue to improve
Many devices are available for mechanical circulatory support.14 In addition to LVADs, there are right-ventricular assist devices (RVADs), and devices that simultaneously support both ventricles (BiVADs). Total artificial hearts are also available, as are acute temporary percutaneous devices. These temporary devices—TandemHeart (CardiacAssist, Pittsburgh, PA) and Impella (Abiomed, Danvers, MD)—can be used before a long-term mechanical device can be surgically implanted.
LVADs are of three types:
- Pulsatile volume-displacement pumps, which mimic the pumping action of the natural heart. These early devices were large and placed in the abdomen.
- Continuous axial-flow pumps, which do not have a “heartbeat.” These are quieter and lighter than the early pumps, and use a turbine that spins at 8,000 to 10,000 rpm.
- Continuous centrifugal-flow pumps. These have a rotor spinning at 2,000 to 3,000 rpm, and most of them are magnetically powered and suspended.
The superiority of LVADs over medical therapy was clearly shown even in early studies that used pulsatile LVADs.15 The results of such studies and the increased durability of the devices have led to their rapidly expanded use.
The newer continuous-flow pumps offer significant improvements over the old pulsatile-flow pumps, being smaller, lighter, quieter, and more durable (Table 1). A 2007 study of 133 patients on a continuous axial-flow LVAD (HeartMate II) found that 76% were still alive after 6 months, and patients had significant improvement in functional status and quality of life.16 In a postapproval study based on registry data, HeartMate II was found superior to pulsatile pumps in terms of survival up to 12 months, percentage of patients reaching transplant, and cardiac recovery. Adverse event rates were similar or lower for HeartMate II.17
Another study compared a continuousflow with a pulsatile-flow LVAD for patients who were ineligible for transplantation. Survival at 2 years was 58% with the continuousflow device vs 24% with the pulsatile-flow device (P = .008).18 Since then, postmarket data of patients who received an LVAD showed that 85% are still alive at 1 year.19 This study can be viewed as supporting the use of LVADs as destination therapy.
Quality of life for patients receiving an LVAD has been excellent. When biventricular pacemakers for resynchronization therapy first became available, distances on the 6-minute walk test improved by 39 m, which was deemed a big improvement. LVAD devices have increased the 6-minute walk distance by 156 m.20
Adverse events with LVADs have improved, but continue to be of concern
Infections can arise in the blood stream, in the device pocket, or especially where the driveline exits the skin. As devices have become smaller, driveline diameters have become smaller as well, allowing for a better seal at the skin and making this less of a problem. Some centers report the incidence of driveline infections as less than 20%, but they continue to be a focus of concern.18
Stroke rates continue to improve, although patients still require intensive lifelong anticoagulation. The target international normalized ratio varies by device manufacturer, ranging from 1.7 to 2.5.
Bleeding. Acquired von Willebrand syndrome can develop in patients who have an LVAD, with the gastrointestinal system being the most frequent site of bleeding.21
Device thrombosis occurs very rarely (2%–3%) but is very serious and may require pump exchange.
Mechanical malfunction. As duration of therapy lengthens, problems are arising with aging devices, such as broken wires or short circuits. New-generation pumps have markedly improved durability and reliability.
Good data are kept on device outcomes
The Interagency for Mechanically Assisted Circulatory Support (INTERMACS) maintains a national registry of patients with a mechanical circulatory support device to treat advanced heart failure. It was jointly established in 2006 by the National Heart, Lung, and Blood Institute, Centers for Medicare and Medicaid Services (CMS), the US Food and Drug Administration, and others. Reporting to INTERMACS is required for CMS reimbursement.
The INTERMACS database now has about 4,500 patients at 126 medical centers and is yielding useful information that is published in annual reports.22 The 2011 report focused on the experience with mechanical circulatory support as destination therapy and showed that patients who receive continuousflow pumps have significantly better survival rates than those with pulsatile-flow pumps.23 An earlier report showed that the level of illness at the time of implantation predicts survival24; this information now drives cardiologists to try to improve patient status with a temporary support device or intra-aortic balloon pump before implanting a durable device. The sickest patients (INTERMACS level 1) have the poorest outcomes, and centers now do fewer implantations in patients in this category. We have learned this important lesson from the INTERMACS registry.
The new devices have received a lot of media attention, and patient accrual has increased steadily as the devices have been approved.
On November 20, 2012, the US Food and Drug Administration approved the HeartWare Ventricular Assist System (HeartWare, Framingham, MA) for heart failure patients awaiting a transplant.
FUTURE DIRECTIONS
PROCEED II is an ongoing global clinical trial comparing the outcomes with donor hearts transported in standard cold storage to those transported in an experimental transport device that pumps the heart under physiologic conditions. If proven effective, this device could allow long-distance transport of donor hearts and expand the donor population.
A prospective, randomized study is now enrolling patients to evaluate induction therapy with rituximab (Rituxan) plus conventional immunosuppression (tacrolimus [Prograf], mycophenolate, steroid taper) vs placebo induction plus conventional immunosuppression. The study will enroll 400 patients (200 to each treatment arm) at 25 sites and will have a 36-month accrual period with 12-month follow-up (see http://clinicaltrials.gov/show/NCT01278745). The study is based on data in primates that found that eliminating B cells with an anti-CD20 drug before transplantation markedly reduced the incidence of coronary artery vasculopathy.
- Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 1221:e46–e215.
- Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:1977–2016.
- 2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD.
- Metra M, Ponikowski P, Dickstein K, et al; Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9:684–694.
- Hsich E, Gorodeski EZ, Starling RC, Blackstone EH, Ishwaran H, Lauer MS. Importance of treadmill exercise time as an initial prognostic screening tool in patients with systolic left ventricular dysfunction. Circulation 2009; 119:3189–3197.
- Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail 2010; 3:706–714.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324.
- Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth official adult heart transplant report—2009. J Heart Lung Transplant 2009; 28:1007–1022.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report—2010. J Heart Lung Transplant 2010; 29:1089–1103.
- Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349:931–940.
- Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1999; 340:272–277. Erratum in: N Engl J Med 1999; 340:976.
- Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiactransplant recipients. N Engl J Med 2003; 349:847–858.
- Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail 2012; 5:249–258.
- Baughman KL, Jarcho JA. Bridge to life—cardiac mechanical support. N Engl J Med 2007; 357:846–849.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Miller LW, Pagani FD, Russell SD, et al; HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357:885–896.
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011; 57:1890–1898.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011; 92:1406–1413.
- Starling RC. Improved quantity and quality of life: a winning combination to treat advanced heart failure. J Am Coll Cardiol 2010; 55:1835–1836.
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010; 56:1207–1213.
- Kirklin JK, Naftel DC, Kormos RL, et al. The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant 2012; 31:117–126.
- Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 2011; 30:115–123.
- Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant 2010; 29:1–10.
SUGGESTED READING
Costanzo MR, Dipchand A, Starling R, et al; International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29:914–956.
Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant 2006; 25:1024–1042.
Slaughter MS, Pagani FD, Rogers JG, et al; HeartMate II Clinical Investigators. Clinical management of continuous flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010; 29(4 suppl):S1–S39.
Patients with advanced heart failure far outnumber the hearts available for transplantation. Partly as a consequence of this shortage, left-ventricular assist devices (LVADs) are being used more widely.
This article is an update on options for managing severe, advanced heart failure, with special attention to new developments and continuing challenges in heart transplantation and LVADs.
THE PREVALENCE OF HEART FAILURE
About 2.6% of the US population age 20 and older have heart failure—some 5.8 million people. Of these, about half have systolic heart failure.1 Patients with systolic heart failure can be classified by degree of severity under two systems:
The New York Heart Association (NYHA) classifies patients by their functional status, from I (no limitation in activities) to IV (symptoms at rest). NYHA class III (symptoms with minimal exertion) is sometimes further broken down into IIIa and IIIb, with the latter defined as having a recent history of dyspnea at rest.
The joint American College of Cardiology and American Heart Association (ACC/AHA) classification uses four stages, from A (high risk of developing heart failure, ie, having risk factors such as family history of heart disease, hypertension, or diabetes) to D (advanced heart disease despite treatment). Patients in stage D tend to be recurrently hospitalized despite cardiac resynchronization therapy and drug therapy, and they cannot be safely discharged without specialized interventions. The options for these patients are limited: either end-of-life care or extraordinary measures such as heart transplantation, long-term treatment with inotropic drugs, permanent mechanical circulatory support, or experimental therapies.2
The estimated number of people in ACC/AHA stage D or NYHA class IV is 15,600 to 156,000. The approximate number of heart transplants performed in the United States each year is 2,100.3
WHICH AMBULATORY PATIENTS ARE MOST AT RISK?
The range for the estimated number of patients with advanced heart failure (NYHA class IIIb or IV) is wide (see above) because these patients may be hard to recognize. The most debilitated patients are obvious: they tend to be in the intensive care unit with end-organ failure. However, it is a challenge to recognize patients at extremely high risk who are still ambulatory.
The European Society of Cardiology4 developed a definition of advanced chronic heart failure that can help identify patients who are candidates for the transplant list and for an LVAD. All the following features must be present despite optimal therapy that includes diuretics, inhibitors of the renin-angiotensin-aldosterone system, and beta-blockers, unless these are poorly tolerated or contraindicated, and cardiac resynchronization therapy if indicated:
- Severe symptoms, with dyspnea or fatigue at rest or with minimal exertion (NYHA class III or IV)
- Episodes of fluid retention (pulmonary or systemic congestion, peripheral edema) or of reduced cardiac output at rest (peripheral hypoperfusion)
- Objective evidence of severe cardiac dysfunction (at least one of the following): left ventricular ejection fraction less than 30%, pseudonormal or restrictive mitral inflow pattern on Doppler echocardiography, high left or right ventricular filling pressure (or both left and right filling pressures), and elevated B-type natriuretic peptides
- Severely impaired functional capacity demonstrated by one of the following: inability to exercise, 6-minute walk test distance less than 300 m (or less in women or patients who are age 75 and older), or peak oxygen intake less than 12 to 14 mL/kg/min
- One or more hospitalizations for heart failure in the past 6 months.
Treadmill exercise time is an easily performed test. Hsich et al5 found that the longer patients can walk, the lower their risk of death, and that this variable is about as predictive of survival in patients with systolic left ventricular dysfunction as peak oxygen consumption, which is much more cumbersome to measure.
The Seattle Heart Failure Model gives an estimate of prognosis for ambulatory patients with advanced heart failure. Available at http://depts.washington.edu/shfm/, it is based on age, sex, NYHA class, weight, ejection fraction, blood pressure, medications, a few laboratory values, and other clinical information. The model has been validated in numerous cohorts,6 but it may underestimate risk and is currently being tested in clinical trials (REVIVE-IT and ROADMAP; see at www.clinicaltrials.gov).
Recurrent hospitalization is a simple predictor of risk. A study of about 7,000 patients worldwide found that after hospitalization with acute decompensated heart failure, the strongest predictor of death within 6 months was readmission for any reason within 30 days of the index hospitalization (Starling RC, unpublished observation, 2011). Any patient with heart failure who is repeatedly hospitalized should have a consultation with a heart failure specialist.
INOTROPIC THERAPY FOR BRIDGING
Inotropic drugs, which include intravenous dobutamine (Dobutrex) and milrinone (Primacor), are used to help maintain end-organ function until a patient can obtain a heart transplant or LVAD.
Inotropic therapy should not be viewed as an alternative to heart transplantation or device implantation. We inform patients that inotropic therapy is purely palliative and may actually increase the risk of death, which is about 50% at 6 months and nearly 100% at 1 year. A patient on inotropic therapy who is not a candidate for a transplant or for an assist device should be referred to a hospice program.7
CARDIAC TRANSPLANTATION: SUCCESSES, CHALLENGES
Survival rates after heart transplantation are now excellent. The 1-year survival rate is about 90%, the 5-year rate is about 70%, but only about 20% survive 20 years or longer.8,9 The prognosis is not as good as for combined heart-lung transplantation patients.
Age is an important factor and is a contentious issue: some medical centers will not offer transplantation to patients over age 65. Others regard age as just another risk factor, like renal dysfunction or diabetes.
Quality of life after heart transplantation is excellent: patients are usually able to return to work, regardless of their profession.
The leading cause of death after heart transplantation is malignancy, followed by coronary artery vasculopathy, then by graft failure. Some patients develop left ventricular dysfunction and heart failure of unknown cause. Others develop antibody-mediated rejection; in recent years this has been more promptly recognized, but treatment remains a challenge.
Acute rejection, which used to be one of the main causes of death, now has an extremely low incidence because of modern drug therapies. In a US observational study currently being conducted in about 200 patients receiving a heart transplant (details on CTOT-05 at www.clinicaltrials.gov), the incidence of moderate rejection during the first year is less than 10% (Starling RC, unpublished observation). But several concerns remain.
Adverse effects of immunosuppressive drugs continue to be problematic. These include infection, malignancy, osteoporosis, chronic kidney toxicity, hypertension, and neuropathy.
Renal dysfunction is one of the largest issues. About 10% of heart transplant recipients develop stage 4 kidney disease (with a glomerular filtration rate < 30 mL/min) and need kidney transplantation or renal replacement therapy because of the use of calcineurin inhibitors for immunosuppression.10
Coronary artery vasculopathy was the largest problem when heart transplantation began and continues to be a major concern and focus of research.11,12 Case 1 (below) is an example of the problem.
Case 1: Poor outcome despite an ideal scenario
A 57-year-old businessman had dilated cardiomyopathy and progressive heart failure for 10 years. He was listed for transplantation in 2008 and was given an LVAD (HeartMate II, Thoratec Corp, Pleasanton, CA) as a bridge until a donor heart became available.
In 2009, he received a heart transplant under ideal conditions: the donor was a large 30-year-old man who died of a gunshot wound to the head in the same city in which the patient and transplant hospital were located. Cardiopulmonary resuscitation was not performed, and the cold ischemic time was just a little more than 3 hours. Immune indicators were ideal with a negative prospective cross-match.
Laboratory results after transplantation included creatinine 1.7 mg/dL (normal 0.6–1.2 mg/dL), low-density lipoprotein cholesterol 75 mg/dL, high-density lipoprotein cholesterol 64 mg/dL, and triglycerides 90 mg/dL.
The patient was given immunosuppressive therapy with cyclosporine (Neoral), mycophenolate (CellCept), and prednisone. Because his creatinine level was high, he was also perioperatively given basiliximab (Simulect), a monoclonal antibody to the alpha chain (CD25) of the interleukin-2 receptor. (In a patient who has poor renal function, basilixumab may help by enabling us to delay the use of calcineurin inhibitors.) He also received simvastatin (Zocor) 10 mg.
Per Cleveland Clinic protocol, he underwent 13 biopsy procedures during his first year, and each was normal (grade 0 or 1R). Evaluation by cardiac catheterization at 1 year showed some irregularities in the left anterior descending artery, but a stent was not deemed necessary. Also, per protocol, he underwent intravascular ultrasonography, which revealed abnormal thickness in the intima and media, indicating that coronary artery disease was developing, although it was nonobstructive.
Two months after this checkup, the patient collapsed and suddenly died while shopping. At autopsy, his left anterior descending artery was found to be severely obstructed.
Coronary artery vasculopathy is still a major problem
This case shows that coronary artery vasculopathy may develop despite an ideal transplantation scenario. It remains a large concern and a focus of research.
Coronary vasculopathy develops in 30% to 40% of heart transplant recipients within 5 years, and the incidence has not been reduced by much over the years. However, probably fewer than 5% of these patients die or even need bypass surgery or stenting, and the problem is managed the same as native atherosclerosis. We perform routine annual cardiac catheterizations or stress tests, or both, and place stents in severely blocked arteries.
THE DONOR SHORTAGE: CHANGING HOW HEARTS ARE ALLOCATED
The number of patients receiving a heart transplant in the United States—about 2,000 per year—has not increased in the past decade. The European Union also has great difficulty obtaining hearts for people in need, and almost every transplant candidate there gets mechanical support for some time. The gap between those listed for transplant and the number transplanted each year continues to widen in both the United States and Europe.
All transplant candidates are assigned a status by the United Network of Organ Sharing (UNOS) based on their medical condition. The highest status, 1A, goes to patients who are seriously ill, in the hospital, on high doses of inotropic drugs (specific dosages are defined) and mechanical circulatory support such as an LVAD, and expected to live less than 1 month without a transplant. Status 1B patients are stable on lower-dose inotropic therapy or on mechanical support, and can be in the hospital or at home. Status 2 patients are stable and ambulatory and are not on inotropic drugs.
In July 2006, UNOS changed the rules on how patients are prioritized for obtaining an organ. The new rules are based both on severity of illness (see above) and geographic proximity to the donor heart—local, within 500 miles (“zone A”) or within 500 to 1,000 miles (“zone B”). The order of priority for donor hearts is:
- Local, status 1A
- Local, status 1B
- Zone A, status 1A
- Zone A, status 1B
- Local, status 2
- Zone B, status 1A
- Zone B, status 1B
- Zone A, status 2.
As a result of the change, donor hearts that become available in a particular hospital do not necessarily go to a patient in that state. Another result is that status 2 patients, who were previously the most common transplant recipients, now have much less access because all status 1 patients within 500 miles are given higher priority. Since the change, only 8% of hearts nationwide go to status 2 patients, which is 67% fewer than before. At the same time, organs allocated to status 1A patients have increased by 26%, and their death rates have fallen.3
The new allocation system is a positive change for the sickest patients, providing quicker access and a reduction in waiting-list mortality.13 The drawback is that status 2 patients who are less ill are less likely to ever receive an organ until their condition worsens.
Heart transplant outcomes are publicly reported
The Scientific Registry of Transplant Recipients publicly reports heart transplant outcomes (www.srtr.org). For any transplant center, the public can learn the number of patients waiting for a transplant, the death rate on the waiting list, the number of transplants performed in the previous 12 months, the waiting time in months, and observed and risk-adjusted expected survival rates. A center that deviates from the expected survival rates by 10% or more may be audited and could lose its certification.
Also listed on the Web site is the percentage of patients who receive a support device before receiving a transplant. This can vary widely between institutions and may reflect the organ availability at the transplant center (waiting times) or the preferences and expertise of the transplantation team. We believe that the mortality rate on the waiting list will be reduced with appropriate use of LVADs as a bridge to transplantation when indicated. We have now transitioned to the use of the improved continuous-flow LVADs and rarely maintain patients on continuous inotropic therapy at home to await a donor organ.
MECHANICAL CIRCULATORY SUPPORT: BRIDGE OR DESTINATION?
Mechanical circulatory support devices are increasingly being used to sustain patients with advanced heart failure. Currently at Cleveland Clinic, more LVADs are implanted than hearts are transplanted.
Mechanical circulatory support is indicated for patients who are listed for transplant to keep them functioning as well as possible while they are waiting (bridge to transplant). For others it is “destination therapy”: they are not candidates for a transplant, but a device may improve and prolong the rest of their life.
Case 2: A good outcome despite a poor prognosis
A 71-year-old man was rejected for transplantation by his local hospital because of his age and also because he had pulmonary artery hypertension (78/42 mm Hg; reference range 15–30/5–15 mm Hg) and creatinine elevation (3.0 mg/dL; reference range 0.6–1.5 mg/dL). Nevertheless, he did well on a mechanical device and was accepted for transplantation by Cleveland Clinic. He received a transplant and is still alive and active 14 years later.
Comment. Determining that a patient is not a good transplantation candidate is often impossible. Putting the patient on mechanical support for a period of time can often help clarify whether transplantation is advisable. Probably most patients who receive mechanical support do so as a bridge to decision: most are acutely ill and many have organ dysfunction, pulmonary hypertension, and renal insufficiency. After a period of support, they can be assessed for suitability for transplantation.
LVADs continue to improve
Many devices are available for mechanical circulatory support.14 In addition to LVADs, there are right-ventricular assist devices (RVADs), and devices that simultaneously support both ventricles (BiVADs). Total artificial hearts are also available, as are acute temporary percutaneous devices. These temporary devices—TandemHeart (CardiacAssist, Pittsburgh, PA) and Impella (Abiomed, Danvers, MD)—can be used before a long-term mechanical device can be surgically implanted.
LVADs are of three types:
- Pulsatile volume-displacement pumps, which mimic the pumping action of the natural heart. These early devices were large and placed in the abdomen.
- Continuous axial-flow pumps, which do not have a “heartbeat.” These are quieter and lighter than the early pumps, and use a turbine that spins at 8,000 to 10,000 rpm.
- Continuous centrifugal-flow pumps. These have a rotor spinning at 2,000 to 3,000 rpm, and most of them are magnetically powered and suspended.
The superiority of LVADs over medical therapy was clearly shown even in early studies that used pulsatile LVADs.15 The results of such studies and the increased durability of the devices have led to their rapidly expanded use.
The newer continuous-flow pumps offer significant improvements over the old pulsatile-flow pumps, being smaller, lighter, quieter, and more durable (Table 1). A 2007 study of 133 patients on a continuous axial-flow LVAD (HeartMate II) found that 76% were still alive after 6 months, and patients had significant improvement in functional status and quality of life.16 In a postapproval study based on registry data, HeartMate II was found superior to pulsatile pumps in terms of survival up to 12 months, percentage of patients reaching transplant, and cardiac recovery. Adverse event rates were similar or lower for HeartMate II.17
Another study compared a continuousflow with a pulsatile-flow LVAD for patients who were ineligible for transplantation. Survival at 2 years was 58% with the continuousflow device vs 24% with the pulsatile-flow device (P = .008).18 Since then, postmarket data of patients who received an LVAD showed that 85% are still alive at 1 year.19 This study can be viewed as supporting the use of LVADs as destination therapy.
Quality of life for patients receiving an LVAD has been excellent. When biventricular pacemakers for resynchronization therapy first became available, distances on the 6-minute walk test improved by 39 m, which was deemed a big improvement. LVAD devices have increased the 6-minute walk distance by 156 m.20
Adverse events with LVADs have improved, but continue to be of concern
Infections can arise in the blood stream, in the device pocket, or especially where the driveline exits the skin. As devices have become smaller, driveline diameters have become smaller as well, allowing for a better seal at the skin and making this less of a problem. Some centers report the incidence of driveline infections as less than 20%, but they continue to be a focus of concern.18
Stroke rates continue to improve, although patients still require intensive lifelong anticoagulation. The target international normalized ratio varies by device manufacturer, ranging from 1.7 to 2.5.
Bleeding. Acquired von Willebrand syndrome can develop in patients who have an LVAD, with the gastrointestinal system being the most frequent site of bleeding.21
Device thrombosis occurs very rarely (2%–3%) but is very serious and may require pump exchange.
Mechanical malfunction. As duration of therapy lengthens, problems are arising with aging devices, such as broken wires or short circuits. New-generation pumps have markedly improved durability and reliability.
Good data are kept on device outcomes
The Interagency for Mechanically Assisted Circulatory Support (INTERMACS) maintains a national registry of patients with a mechanical circulatory support device to treat advanced heart failure. It was jointly established in 2006 by the National Heart, Lung, and Blood Institute, Centers for Medicare and Medicaid Services (CMS), the US Food and Drug Administration, and others. Reporting to INTERMACS is required for CMS reimbursement.
The INTERMACS database now has about 4,500 patients at 126 medical centers and is yielding useful information that is published in annual reports.22 The 2011 report focused on the experience with mechanical circulatory support as destination therapy and showed that patients who receive continuousflow pumps have significantly better survival rates than those with pulsatile-flow pumps.23 An earlier report showed that the level of illness at the time of implantation predicts survival24; this information now drives cardiologists to try to improve patient status with a temporary support device or intra-aortic balloon pump before implanting a durable device. The sickest patients (INTERMACS level 1) have the poorest outcomes, and centers now do fewer implantations in patients in this category. We have learned this important lesson from the INTERMACS registry.
The new devices have received a lot of media attention, and patient accrual has increased steadily as the devices have been approved.
On November 20, 2012, the US Food and Drug Administration approved the HeartWare Ventricular Assist System (HeartWare, Framingham, MA) for heart failure patients awaiting a transplant.
FUTURE DIRECTIONS
PROCEED II is an ongoing global clinical trial comparing the outcomes with donor hearts transported in standard cold storage to those transported in an experimental transport device that pumps the heart under physiologic conditions. If proven effective, this device could allow long-distance transport of donor hearts and expand the donor population.
A prospective, randomized study is now enrolling patients to evaluate induction therapy with rituximab (Rituxan) plus conventional immunosuppression (tacrolimus [Prograf], mycophenolate, steroid taper) vs placebo induction plus conventional immunosuppression. The study will enroll 400 patients (200 to each treatment arm) at 25 sites and will have a 36-month accrual period with 12-month follow-up (see http://clinicaltrials.gov/show/NCT01278745). The study is based on data in primates that found that eliminating B cells with an anti-CD20 drug before transplantation markedly reduced the incidence of coronary artery vasculopathy.
Patients with advanced heart failure far outnumber the hearts available for transplantation. Partly as a consequence of this shortage, left-ventricular assist devices (LVADs) are being used more widely.
This article is an update on options for managing severe, advanced heart failure, with special attention to new developments and continuing challenges in heart transplantation and LVADs.
THE PREVALENCE OF HEART FAILURE
About 2.6% of the US population age 20 and older have heart failure—some 5.8 million people. Of these, about half have systolic heart failure.1 Patients with systolic heart failure can be classified by degree of severity under two systems:
The New York Heart Association (NYHA) classifies patients by their functional status, from I (no limitation in activities) to IV (symptoms at rest). NYHA class III (symptoms with minimal exertion) is sometimes further broken down into IIIa and IIIb, with the latter defined as having a recent history of dyspnea at rest.
The joint American College of Cardiology and American Heart Association (ACC/AHA) classification uses four stages, from A (high risk of developing heart failure, ie, having risk factors such as family history of heart disease, hypertension, or diabetes) to D (advanced heart disease despite treatment). Patients in stage D tend to be recurrently hospitalized despite cardiac resynchronization therapy and drug therapy, and they cannot be safely discharged without specialized interventions. The options for these patients are limited: either end-of-life care or extraordinary measures such as heart transplantation, long-term treatment with inotropic drugs, permanent mechanical circulatory support, or experimental therapies.2
The estimated number of people in ACC/AHA stage D or NYHA class IV is 15,600 to 156,000. The approximate number of heart transplants performed in the United States each year is 2,100.3
WHICH AMBULATORY PATIENTS ARE MOST AT RISK?
The range for the estimated number of patients with advanced heart failure (NYHA class IIIb or IV) is wide (see above) because these patients may be hard to recognize. The most debilitated patients are obvious: they tend to be in the intensive care unit with end-organ failure. However, it is a challenge to recognize patients at extremely high risk who are still ambulatory.
The European Society of Cardiology4 developed a definition of advanced chronic heart failure that can help identify patients who are candidates for the transplant list and for an LVAD. All the following features must be present despite optimal therapy that includes diuretics, inhibitors of the renin-angiotensin-aldosterone system, and beta-blockers, unless these are poorly tolerated or contraindicated, and cardiac resynchronization therapy if indicated:
- Severe symptoms, with dyspnea or fatigue at rest or with minimal exertion (NYHA class III or IV)
- Episodes of fluid retention (pulmonary or systemic congestion, peripheral edema) or of reduced cardiac output at rest (peripheral hypoperfusion)
- Objective evidence of severe cardiac dysfunction (at least one of the following): left ventricular ejection fraction less than 30%, pseudonormal or restrictive mitral inflow pattern on Doppler echocardiography, high left or right ventricular filling pressure (or both left and right filling pressures), and elevated B-type natriuretic peptides
- Severely impaired functional capacity demonstrated by one of the following: inability to exercise, 6-minute walk test distance less than 300 m (or less in women or patients who are age 75 and older), or peak oxygen intake less than 12 to 14 mL/kg/min
- One or more hospitalizations for heart failure in the past 6 months.
Treadmill exercise time is an easily performed test. Hsich et al5 found that the longer patients can walk, the lower their risk of death, and that this variable is about as predictive of survival in patients with systolic left ventricular dysfunction as peak oxygen consumption, which is much more cumbersome to measure.
The Seattle Heart Failure Model gives an estimate of prognosis for ambulatory patients with advanced heart failure. Available at http://depts.washington.edu/shfm/, it is based on age, sex, NYHA class, weight, ejection fraction, blood pressure, medications, a few laboratory values, and other clinical information. The model has been validated in numerous cohorts,6 but it may underestimate risk and is currently being tested in clinical trials (REVIVE-IT and ROADMAP; see at www.clinicaltrials.gov).
Recurrent hospitalization is a simple predictor of risk. A study of about 7,000 patients worldwide found that after hospitalization with acute decompensated heart failure, the strongest predictor of death within 6 months was readmission for any reason within 30 days of the index hospitalization (Starling RC, unpublished observation, 2011). Any patient with heart failure who is repeatedly hospitalized should have a consultation with a heart failure specialist.
INOTROPIC THERAPY FOR BRIDGING
Inotropic drugs, which include intravenous dobutamine (Dobutrex) and milrinone (Primacor), are used to help maintain end-organ function until a patient can obtain a heart transplant or LVAD.
Inotropic therapy should not be viewed as an alternative to heart transplantation or device implantation. We inform patients that inotropic therapy is purely palliative and may actually increase the risk of death, which is about 50% at 6 months and nearly 100% at 1 year. A patient on inotropic therapy who is not a candidate for a transplant or for an assist device should be referred to a hospice program.7
CARDIAC TRANSPLANTATION: SUCCESSES, CHALLENGES
Survival rates after heart transplantation are now excellent. The 1-year survival rate is about 90%, the 5-year rate is about 70%, but only about 20% survive 20 years or longer.8,9 The prognosis is not as good as for combined heart-lung transplantation patients.
Age is an important factor and is a contentious issue: some medical centers will not offer transplantation to patients over age 65. Others regard age as just another risk factor, like renal dysfunction or diabetes.
Quality of life after heart transplantation is excellent: patients are usually able to return to work, regardless of their profession.
The leading cause of death after heart transplantation is malignancy, followed by coronary artery vasculopathy, then by graft failure. Some patients develop left ventricular dysfunction and heart failure of unknown cause. Others develop antibody-mediated rejection; in recent years this has been more promptly recognized, but treatment remains a challenge.
Acute rejection, which used to be one of the main causes of death, now has an extremely low incidence because of modern drug therapies. In a US observational study currently being conducted in about 200 patients receiving a heart transplant (details on CTOT-05 at www.clinicaltrials.gov), the incidence of moderate rejection during the first year is less than 10% (Starling RC, unpublished observation). But several concerns remain.
Adverse effects of immunosuppressive drugs continue to be problematic. These include infection, malignancy, osteoporosis, chronic kidney toxicity, hypertension, and neuropathy.
Renal dysfunction is one of the largest issues. About 10% of heart transplant recipients develop stage 4 kidney disease (with a glomerular filtration rate < 30 mL/min) and need kidney transplantation or renal replacement therapy because of the use of calcineurin inhibitors for immunosuppression.10
Coronary artery vasculopathy was the largest problem when heart transplantation began and continues to be a major concern and focus of research.11,12 Case 1 (below) is an example of the problem.
Case 1: Poor outcome despite an ideal scenario
A 57-year-old businessman had dilated cardiomyopathy and progressive heart failure for 10 years. He was listed for transplantation in 2008 and was given an LVAD (HeartMate II, Thoratec Corp, Pleasanton, CA) as a bridge until a donor heart became available.
In 2009, he received a heart transplant under ideal conditions: the donor was a large 30-year-old man who died of a gunshot wound to the head in the same city in which the patient and transplant hospital were located. Cardiopulmonary resuscitation was not performed, and the cold ischemic time was just a little more than 3 hours. Immune indicators were ideal with a negative prospective cross-match.
Laboratory results after transplantation included creatinine 1.7 mg/dL (normal 0.6–1.2 mg/dL), low-density lipoprotein cholesterol 75 mg/dL, high-density lipoprotein cholesterol 64 mg/dL, and triglycerides 90 mg/dL.
The patient was given immunosuppressive therapy with cyclosporine (Neoral), mycophenolate (CellCept), and prednisone. Because his creatinine level was high, he was also perioperatively given basiliximab (Simulect), a monoclonal antibody to the alpha chain (CD25) of the interleukin-2 receptor. (In a patient who has poor renal function, basilixumab may help by enabling us to delay the use of calcineurin inhibitors.) He also received simvastatin (Zocor) 10 mg.
Per Cleveland Clinic protocol, he underwent 13 biopsy procedures during his first year, and each was normal (grade 0 or 1R). Evaluation by cardiac catheterization at 1 year showed some irregularities in the left anterior descending artery, but a stent was not deemed necessary. Also, per protocol, he underwent intravascular ultrasonography, which revealed abnormal thickness in the intima and media, indicating that coronary artery disease was developing, although it was nonobstructive.
Two months after this checkup, the patient collapsed and suddenly died while shopping. At autopsy, his left anterior descending artery was found to be severely obstructed.
Coronary artery vasculopathy is still a major problem
This case shows that coronary artery vasculopathy may develop despite an ideal transplantation scenario. It remains a large concern and a focus of research.
Coronary vasculopathy develops in 30% to 40% of heart transplant recipients within 5 years, and the incidence has not been reduced by much over the years. However, probably fewer than 5% of these patients die or even need bypass surgery or stenting, and the problem is managed the same as native atherosclerosis. We perform routine annual cardiac catheterizations or stress tests, or both, and place stents in severely blocked arteries.
THE DONOR SHORTAGE: CHANGING HOW HEARTS ARE ALLOCATED
The number of patients receiving a heart transplant in the United States—about 2,000 per year—has not increased in the past decade. The European Union also has great difficulty obtaining hearts for people in need, and almost every transplant candidate there gets mechanical support for some time. The gap between those listed for transplant and the number transplanted each year continues to widen in both the United States and Europe.
All transplant candidates are assigned a status by the United Network of Organ Sharing (UNOS) based on their medical condition. The highest status, 1A, goes to patients who are seriously ill, in the hospital, on high doses of inotropic drugs (specific dosages are defined) and mechanical circulatory support such as an LVAD, and expected to live less than 1 month without a transplant. Status 1B patients are stable on lower-dose inotropic therapy or on mechanical support, and can be in the hospital or at home. Status 2 patients are stable and ambulatory and are not on inotropic drugs.
In July 2006, UNOS changed the rules on how patients are prioritized for obtaining an organ. The new rules are based both on severity of illness (see above) and geographic proximity to the donor heart—local, within 500 miles (“zone A”) or within 500 to 1,000 miles (“zone B”). The order of priority for donor hearts is:
- Local, status 1A
- Local, status 1B
- Zone A, status 1A
- Zone A, status 1B
- Local, status 2
- Zone B, status 1A
- Zone B, status 1B
- Zone A, status 2.
As a result of the change, donor hearts that become available in a particular hospital do not necessarily go to a patient in that state. Another result is that status 2 patients, who were previously the most common transplant recipients, now have much less access because all status 1 patients within 500 miles are given higher priority. Since the change, only 8% of hearts nationwide go to status 2 patients, which is 67% fewer than before. At the same time, organs allocated to status 1A patients have increased by 26%, and their death rates have fallen.3
The new allocation system is a positive change for the sickest patients, providing quicker access and a reduction in waiting-list mortality.13 The drawback is that status 2 patients who are less ill are less likely to ever receive an organ until their condition worsens.
Heart transplant outcomes are publicly reported
The Scientific Registry of Transplant Recipients publicly reports heart transplant outcomes (www.srtr.org). For any transplant center, the public can learn the number of patients waiting for a transplant, the death rate on the waiting list, the number of transplants performed in the previous 12 months, the waiting time in months, and observed and risk-adjusted expected survival rates. A center that deviates from the expected survival rates by 10% or more may be audited and could lose its certification.
Also listed on the Web site is the percentage of patients who receive a support device before receiving a transplant. This can vary widely between institutions and may reflect the organ availability at the transplant center (waiting times) or the preferences and expertise of the transplantation team. We believe that the mortality rate on the waiting list will be reduced with appropriate use of LVADs as a bridge to transplantation when indicated. We have now transitioned to the use of the improved continuous-flow LVADs and rarely maintain patients on continuous inotropic therapy at home to await a donor organ.
MECHANICAL CIRCULATORY SUPPORT: BRIDGE OR DESTINATION?
Mechanical circulatory support devices are increasingly being used to sustain patients with advanced heart failure. Currently at Cleveland Clinic, more LVADs are implanted than hearts are transplanted.
Mechanical circulatory support is indicated for patients who are listed for transplant to keep them functioning as well as possible while they are waiting (bridge to transplant). For others it is “destination therapy”: they are not candidates for a transplant, but a device may improve and prolong the rest of their life.
Case 2: A good outcome despite a poor prognosis
A 71-year-old man was rejected for transplantation by his local hospital because of his age and also because he had pulmonary artery hypertension (78/42 mm Hg; reference range 15–30/5–15 mm Hg) and creatinine elevation (3.0 mg/dL; reference range 0.6–1.5 mg/dL). Nevertheless, he did well on a mechanical device and was accepted for transplantation by Cleveland Clinic. He received a transplant and is still alive and active 14 years later.
Comment. Determining that a patient is not a good transplantation candidate is often impossible. Putting the patient on mechanical support for a period of time can often help clarify whether transplantation is advisable. Probably most patients who receive mechanical support do so as a bridge to decision: most are acutely ill and many have organ dysfunction, pulmonary hypertension, and renal insufficiency. After a period of support, they can be assessed for suitability for transplantation.
LVADs continue to improve
Many devices are available for mechanical circulatory support.14 In addition to LVADs, there are right-ventricular assist devices (RVADs), and devices that simultaneously support both ventricles (BiVADs). Total artificial hearts are also available, as are acute temporary percutaneous devices. These temporary devices—TandemHeart (CardiacAssist, Pittsburgh, PA) and Impella (Abiomed, Danvers, MD)—can be used before a long-term mechanical device can be surgically implanted.
LVADs are of three types:
- Pulsatile volume-displacement pumps, which mimic the pumping action of the natural heart. These early devices were large and placed in the abdomen.
- Continuous axial-flow pumps, which do not have a “heartbeat.” These are quieter and lighter than the early pumps, and use a turbine that spins at 8,000 to 10,000 rpm.
- Continuous centrifugal-flow pumps. These have a rotor spinning at 2,000 to 3,000 rpm, and most of them are magnetically powered and suspended.
The superiority of LVADs over medical therapy was clearly shown even in early studies that used pulsatile LVADs.15 The results of such studies and the increased durability of the devices have led to their rapidly expanded use.
The newer continuous-flow pumps offer significant improvements over the old pulsatile-flow pumps, being smaller, lighter, quieter, and more durable (Table 1). A 2007 study of 133 patients on a continuous axial-flow LVAD (HeartMate II) found that 76% were still alive after 6 months, and patients had significant improvement in functional status and quality of life.16 In a postapproval study based on registry data, HeartMate II was found superior to pulsatile pumps in terms of survival up to 12 months, percentage of patients reaching transplant, and cardiac recovery. Adverse event rates were similar or lower for HeartMate II.17
Another study compared a continuousflow with a pulsatile-flow LVAD for patients who were ineligible for transplantation. Survival at 2 years was 58% with the continuousflow device vs 24% with the pulsatile-flow device (P = .008).18 Since then, postmarket data of patients who received an LVAD showed that 85% are still alive at 1 year.19 This study can be viewed as supporting the use of LVADs as destination therapy.
Quality of life for patients receiving an LVAD has been excellent. When biventricular pacemakers for resynchronization therapy first became available, distances on the 6-minute walk test improved by 39 m, which was deemed a big improvement. LVAD devices have increased the 6-minute walk distance by 156 m.20
Adverse events with LVADs have improved, but continue to be of concern
Infections can arise in the blood stream, in the device pocket, or especially where the driveline exits the skin. As devices have become smaller, driveline diameters have become smaller as well, allowing for a better seal at the skin and making this less of a problem. Some centers report the incidence of driveline infections as less than 20%, but they continue to be a focus of concern.18
Stroke rates continue to improve, although patients still require intensive lifelong anticoagulation. The target international normalized ratio varies by device manufacturer, ranging from 1.7 to 2.5.
Bleeding. Acquired von Willebrand syndrome can develop in patients who have an LVAD, with the gastrointestinal system being the most frequent site of bleeding.21
Device thrombosis occurs very rarely (2%–3%) but is very serious and may require pump exchange.
Mechanical malfunction. As duration of therapy lengthens, problems are arising with aging devices, such as broken wires or short circuits. New-generation pumps have markedly improved durability and reliability.
Good data are kept on device outcomes
The Interagency for Mechanically Assisted Circulatory Support (INTERMACS) maintains a national registry of patients with a mechanical circulatory support device to treat advanced heart failure. It was jointly established in 2006 by the National Heart, Lung, and Blood Institute, Centers for Medicare and Medicaid Services (CMS), the US Food and Drug Administration, and others. Reporting to INTERMACS is required for CMS reimbursement.
The INTERMACS database now has about 4,500 patients at 126 medical centers and is yielding useful information that is published in annual reports.22 The 2011 report focused on the experience with mechanical circulatory support as destination therapy and showed that patients who receive continuousflow pumps have significantly better survival rates than those with pulsatile-flow pumps.23 An earlier report showed that the level of illness at the time of implantation predicts survival24; this information now drives cardiologists to try to improve patient status with a temporary support device or intra-aortic balloon pump before implanting a durable device. The sickest patients (INTERMACS level 1) have the poorest outcomes, and centers now do fewer implantations in patients in this category. We have learned this important lesson from the INTERMACS registry.
The new devices have received a lot of media attention, and patient accrual has increased steadily as the devices have been approved.
On November 20, 2012, the US Food and Drug Administration approved the HeartWare Ventricular Assist System (HeartWare, Framingham, MA) for heart failure patients awaiting a transplant.
FUTURE DIRECTIONS
PROCEED II is an ongoing global clinical trial comparing the outcomes with donor hearts transported in standard cold storage to those transported in an experimental transport device that pumps the heart under physiologic conditions. If proven effective, this device could allow long-distance transport of donor hearts and expand the donor population.
A prospective, randomized study is now enrolling patients to evaluate induction therapy with rituximab (Rituxan) plus conventional immunosuppression (tacrolimus [Prograf], mycophenolate, steroid taper) vs placebo induction plus conventional immunosuppression. The study will enroll 400 patients (200 to each treatment arm) at 25 sites and will have a 36-month accrual period with 12-month follow-up (see http://clinicaltrials.gov/show/NCT01278745). The study is based on data in primates that found that eliminating B cells with an anti-CD20 drug before transplantation markedly reduced the incidence of coronary artery vasculopathy.
- Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 1221:e46–e215.
- Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:1977–2016.
- 2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD.
- Metra M, Ponikowski P, Dickstein K, et al; Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9:684–694.
- Hsich E, Gorodeski EZ, Starling RC, Blackstone EH, Ishwaran H, Lauer MS. Importance of treadmill exercise time as an initial prognostic screening tool in patients with systolic left ventricular dysfunction. Circulation 2009; 119:3189–3197.
- Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail 2010; 3:706–714.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324.
- Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth official adult heart transplant report—2009. J Heart Lung Transplant 2009; 28:1007–1022.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report—2010. J Heart Lung Transplant 2010; 29:1089–1103.
- Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349:931–940.
- Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1999; 340:272–277. Erratum in: N Engl J Med 1999; 340:976.
- Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiactransplant recipients. N Engl J Med 2003; 349:847–858.
- Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail 2012; 5:249–258.
- Baughman KL, Jarcho JA. Bridge to life—cardiac mechanical support. N Engl J Med 2007; 357:846–849.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Miller LW, Pagani FD, Russell SD, et al; HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357:885–896.
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011; 57:1890–1898.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011; 92:1406–1413.
- Starling RC. Improved quantity and quality of life: a winning combination to treat advanced heart failure. J Am Coll Cardiol 2010; 55:1835–1836.
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010; 56:1207–1213.
- Kirklin JK, Naftel DC, Kormos RL, et al. The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant 2012; 31:117–126.
- Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 2011; 30:115–123.
- Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant 2010; 29:1–10.
SUGGESTED READING
Costanzo MR, Dipchand A, Starling R, et al; International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29:914–956.
Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant 2006; 25:1024–1042.
Slaughter MS, Pagani FD, Rogers JG, et al; HeartMate II Clinical Investigators. Clinical management of continuous flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010; 29(4 suppl):S1–S39.
- Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 1221:e46–e215.
- Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:1977–2016.
- 2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD.
- Metra M, Ponikowski P, Dickstein K, et al; Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9:684–694.
- Hsich E, Gorodeski EZ, Starling RC, Blackstone EH, Ishwaran H, Lauer MS. Importance of treadmill exercise time as an initial prognostic screening tool in patients with systolic left ventricular dysfunction. Circulation 2009; 119:3189–3197.
- Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail 2010; 3:706–714.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324.
- Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth official adult heart transplant report—2009. J Heart Lung Transplant 2009; 28:1007–1022.
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report—2010. J Heart Lung Transplant 2010; 29:1089–1103.
- Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349:931–940.
- Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1999; 340:272–277. Erratum in: N Engl J Med 1999; 340:976.
- Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiactransplant recipients. N Engl J Med 2003; 349:847–858.
- Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail 2012; 5:249–258.
- Baughman KL, Jarcho JA. Bridge to life—cardiac mechanical support. N Engl J Med 2007; 357:846–849.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Miller LW, Pagani FD, Russell SD, et al; HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357:885–896.
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011; 57:1890–1898.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011; 92:1406–1413.
- Starling RC. Improved quantity and quality of life: a winning combination to treat advanced heart failure. J Am Coll Cardiol 2010; 55:1835–1836.
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010; 56:1207–1213.
- Kirklin JK, Naftel DC, Kormos RL, et al. The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant 2012; 31:117–126.
- Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 2011; 30:115–123.
- Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant 2010; 29:1–10.
SUGGESTED READING
Costanzo MR, Dipchand A, Starling R, et al; International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29:914–956.
Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant 2006; 25:1024–1042.
Slaughter MS, Pagani FD, Rogers JG, et al; HeartMate II Clinical Investigators. Clinical management of continuous flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010; 29(4 suppl):S1–S39.
KEY POINTS
- After heart transplantation, survival rates are high and quality of life is excellent, although coronary artery disease, renal dysfunction, and the need for immunosuppressive drugs are ongoing challenges.
- Changes in donor heart allocation made in 2006 more strongly favor the sickest patients and have reduced the rate of mortality on the waiting list.
- Continuous-flow left-ventricular assist devices offer many advantages over the older pulsatile-flow devices, including improved outcomes, smaller size, less noise, and greater durability.
- Inotropic therapy is purely palliative and should not be viewed as an alternative to heart transplantation or device implantation.
When do Raynaud symptoms merit a workup for autoimmune rheumatic disease?
Indications that Raynaud phenomenon may be the presenting manifestation of a systemic autoimmune rheumatic disease are older age at onset (ie, over age 30), male sex, asymmetric involvement, and prolonged and painful attacks that can be severe enough to cause ischemic digital ulceration or gangrene (Figure 1).
Hence, chronic and severe digital ischemia causing ulceration or infarction differentiates secondary from primary Raynaud phenomenon and should prompt an investigation for an autoimmune rheumatic process. When taking the history, the clinician should seek clues to an underlying autoimmune condition, such as arthralgia, heartburn, dysphagia, shortness of breath, cough, and should examine the patient for telltale signs such as puffy hands and fingers, sclerodactyly, digital pitting scars, loss of fingertip pulp tissue, telangiectasias, and calcinosis.
CLUES TO PRIMARY VS SECONDARY RAYNAUD PHENOMENON
A diagnostic algorithm of digital ischemia (Figure 2) illustrates the range of presentations and possible causes. In Raynaud phenomenon, cold temperature and emotional stress provoke reversible color changes of the fingers and toes. Intense vasospasm of the digital arteries produces three well-defined phases1: white (pallor resulting from vasospasm), blue (dusky cyanosis due to deoxygenation of static venous blood) (Figure 1), and red (reactive hyperemia after the restoration of blood flow). However, only about 60% of patients have all three color changes. The attacks are associated with paresthesias, an uncomfortable feeling of coldness in the fingers, and ischemic pain.
Primary Raynaud phenomenon
Primary or idiopathic Raynaud phenomenon is seen in 5% to 10% of the general population. It more commonly affects women ages 15 to 30, is generally mild, involves the digits symmetrically, and is sometimes familial. An increase in alpha-2 adrenergic responses in the digital vessels leads to arterial vasospasm, an exaggerated physiologic response to cold temperatures.2 Geographic variability in prevalence likely represents differences in mean outdoor temperatures,3 which is in part why attacks of primary Raynaud phenomenon tend to be worse in the winter months.4
Secondary Raynaud phenomenon
Raynaud phenomenon also often occurs in certain autoimmune rheumatic diseases (secondary Raynaud phenomenon): for example, it is seen in scleroderma (90% to 95% of patients), mixed connective tissue disease (85%), systemic lupus erythematosus (40%), antisynthetase syndrome (40%), and sometimes in patients with other autoimmune rheumatic diseases. It may also be seen in hematologic disorders (cryoglobulinemia, cryofibrinogenemia, paraproteinemias, cold agglutinin disease, and polycythemia rubra vera), and it can also result from environmental and occupational exposures (frostbite, use of vibrating tools) and from exposure to certain drugs and toxins, such as polyvinyl chloride (Figure 2).
Acrocyanosis, a benign neurohormonal condition, should be included in the differential diagnosis for Raynaud phenomenon. Raynaud phenomenon is episodic, whereas acrocyanosis leads to persistent cyanosis of the acral body parts (fingers, toes) that is exacerbated by cold temperatures. However, the trophic skin changes, localized pain, and ulceration are not seen in acrocyanosis.
NAILFOLD CAPILLAROSCOPY: A KEY PART OF THE WORKUP
Nailfold capillaroscopy should be part of the evaluation of patients with Raynaud phenomenon (Figure 3), as it is one of the most reliable tests for distinguishing between primary and secondary Raynaud phenomenon.5 The sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis increases significantly with the addition of nailfold capillary abnormalities.6,7
A stereomicroscope or videocapillaroscope is usually recommended to evaluate nailfold capillary morphology,5 but if such equipment is not available, a regular ophthalmoscope (with the lens set at 20 diopters or higher for better resolution) can serve the purpose at the bedside.8 A drop of mineral oil is placed on the nailfold to improve the image resolution, as it makes the horny layer of the cuticle transparent.
Abnormal patterns include dilated and enlarged capillary loops, disorganized capillaries, “dropouts” (avascular areas), microhemorrhages, and arborized capillaries (Figure 3).5 At no additional cost, the presence of these microvascular changes would add to the suspicion of secondary Raynaud phenomenon (negative predictive value of 93%).9 In addition, evolving capillaroscopic changes can be seen during follow-up visits, indicating the progressive nature of the microvasculopathy seen in these autoimmune rheumatic diseases.10
ADDITIONAL TESTING
If an underlying autoimmune rheumatic disease is suspected, laboratory testing should include a complete blood cell count, an erythrocyte sedimentation rate, and an antinuclear antibody (ANA) assay. If the ANA assay is negative, no further testing is usually necessary; however, a positive test should alert the clinician to consider an underlying autoimmune rheumatic process (negative predictive value of 93%).9 In a patient presenting with Raynaud phenomenon, a positive ANA test (even in the absence of other symptoms) warrants more frequent follow-up, urinalysis, and perhaps referral to a rheumatologist.
In the case of a positive ANA test, before ordering additional autoantibody tests, it is useful to consider the relevant non-Raynaud clinical manifestations. Indiscriminate ordering of a battery of autoantibodies should be avoided because of significant added cost and because it is not likely to provide additional information to guide management.
On the other hand, these more specific antibody tests may be of value in confirming the diagnosis suggested by the clinical profile of specific autoimmune rheumatic diseases, eg, anti-double-stranded DNA11 and anti-Smith12 antibodies for lupus, anti-topoisomerase I (Scl-70) and anti-centromere antibodies for scleroderma, 13 and anti-synthetase (eg, anti-Jo-1) antibodies for autoimmune myositis.14,15
- Raynaud M. On local asphyxia and symmetrical gangrene of the extremities (1862), and new research on the nature and treatment of local asphyxia of the extremities (1872).Barlow T, trans. Selected monographs (121). London: New Sydenham Society, 1988.
- Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol 2005; 17:752–760.
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5-region comparison. J Rheumatol 1997; 24:879–889.
- Wigley FM. Clinical practice. Raynaud’s phenomenon. N Engl J Med 2002; 347:1001–1018.
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19:437–452.
- Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44:735–736.
- Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25:754–757.
- Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis 2001; 60:407–409.
- Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158:595–600.
- Wong ML, Highton J, Palmer DG. Sequential nailfold capillary microscopy in scleroderma and related disorders. Ann Rheum Dis 1988; 47:53–61.
- Weinstein A, Bordwell B, Stone B, Tibbetts C, Rothfield NF. Antibodies to native DNA and serum complement (C3) levels. Application to diagnosis and classification of systemic lupus erythematosus. Am J Med 1983; 74:206–216.
- Craft J. Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992; 18:311–335.
- Weiner ES, Hildebrandt S, Senécal JL, et al. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud’s disease. A prospective study. Arthritis Rheum 1991; 34:68–77.
- Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest 1990; 85:468–475.
- Ghirardello A, Zampieri S, Tarricone E, et al. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 2006; 39:217–221.
Indications that Raynaud phenomenon may be the presenting manifestation of a systemic autoimmune rheumatic disease are older age at onset (ie, over age 30), male sex, asymmetric involvement, and prolonged and painful attacks that can be severe enough to cause ischemic digital ulceration or gangrene (Figure 1).
Hence, chronic and severe digital ischemia causing ulceration or infarction differentiates secondary from primary Raynaud phenomenon and should prompt an investigation for an autoimmune rheumatic process. When taking the history, the clinician should seek clues to an underlying autoimmune condition, such as arthralgia, heartburn, dysphagia, shortness of breath, cough, and should examine the patient for telltale signs such as puffy hands and fingers, sclerodactyly, digital pitting scars, loss of fingertip pulp tissue, telangiectasias, and calcinosis.
CLUES TO PRIMARY VS SECONDARY RAYNAUD PHENOMENON
A diagnostic algorithm of digital ischemia (Figure 2) illustrates the range of presentations and possible causes. In Raynaud phenomenon, cold temperature and emotional stress provoke reversible color changes of the fingers and toes. Intense vasospasm of the digital arteries produces three well-defined phases1: white (pallor resulting from vasospasm), blue (dusky cyanosis due to deoxygenation of static venous blood) (Figure 1), and red (reactive hyperemia after the restoration of blood flow). However, only about 60% of patients have all three color changes. The attacks are associated with paresthesias, an uncomfortable feeling of coldness in the fingers, and ischemic pain.
Primary Raynaud phenomenon
Primary or idiopathic Raynaud phenomenon is seen in 5% to 10% of the general population. It more commonly affects women ages 15 to 30, is generally mild, involves the digits symmetrically, and is sometimes familial. An increase in alpha-2 adrenergic responses in the digital vessels leads to arterial vasospasm, an exaggerated physiologic response to cold temperatures.2 Geographic variability in prevalence likely represents differences in mean outdoor temperatures,3 which is in part why attacks of primary Raynaud phenomenon tend to be worse in the winter months.4
Secondary Raynaud phenomenon
Raynaud phenomenon also often occurs in certain autoimmune rheumatic diseases (secondary Raynaud phenomenon): for example, it is seen in scleroderma (90% to 95% of patients), mixed connective tissue disease (85%), systemic lupus erythematosus (40%), antisynthetase syndrome (40%), and sometimes in patients with other autoimmune rheumatic diseases. It may also be seen in hematologic disorders (cryoglobulinemia, cryofibrinogenemia, paraproteinemias, cold agglutinin disease, and polycythemia rubra vera), and it can also result from environmental and occupational exposures (frostbite, use of vibrating tools) and from exposure to certain drugs and toxins, such as polyvinyl chloride (Figure 2).
Acrocyanosis, a benign neurohormonal condition, should be included in the differential diagnosis for Raynaud phenomenon. Raynaud phenomenon is episodic, whereas acrocyanosis leads to persistent cyanosis of the acral body parts (fingers, toes) that is exacerbated by cold temperatures. However, the trophic skin changes, localized pain, and ulceration are not seen in acrocyanosis.
NAILFOLD CAPILLAROSCOPY: A KEY PART OF THE WORKUP
Nailfold capillaroscopy should be part of the evaluation of patients with Raynaud phenomenon (Figure 3), as it is one of the most reliable tests for distinguishing between primary and secondary Raynaud phenomenon.5 The sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis increases significantly with the addition of nailfold capillary abnormalities.6,7
A stereomicroscope or videocapillaroscope is usually recommended to evaluate nailfold capillary morphology,5 but if such equipment is not available, a regular ophthalmoscope (with the lens set at 20 diopters or higher for better resolution) can serve the purpose at the bedside.8 A drop of mineral oil is placed on the nailfold to improve the image resolution, as it makes the horny layer of the cuticle transparent.
Abnormal patterns include dilated and enlarged capillary loops, disorganized capillaries, “dropouts” (avascular areas), microhemorrhages, and arborized capillaries (Figure 3).5 At no additional cost, the presence of these microvascular changes would add to the suspicion of secondary Raynaud phenomenon (negative predictive value of 93%).9 In addition, evolving capillaroscopic changes can be seen during follow-up visits, indicating the progressive nature of the microvasculopathy seen in these autoimmune rheumatic diseases.10
ADDITIONAL TESTING
If an underlying autoimmune rheumatic disease is suspected, laboratory testing should include a complete blood cell count, an erythrocyte sedimentation rate, and an antinuclear antibody (ANA) assay. If the ANA assay is negative, no further testing is usually necessary; however, a positive test should alert the clinician to consider an underlying autoimmune rheumatic process (negative predictive value of 93%).9 In a patient presenting with Raynaud phenomenon, a positive ANA test (even in the absence of other symptoms) warrants more frequent follow-up, urinalysis, and perhaps referral to a rheumatologist.
In the case of a positive ANA test, before ordering additional autoantibody tests, it is useful to consider the relevant non-Raynaud clinical manifestations. Indiscriminate ordering of a battery of autoantibodies should be avoided because of significant added cost and because it is not likely to provide additional information to guide management.
On the other hand, these more specific antibody tests may be of value in confirming the diagnosis suggested by the clinical profile of specific autoimmune rheumatic diseases, eg, anti-double-stranded DNA11 and anti-Smith12 antibodies for lupus, anti-topoisomerase I (Scl-70) and anti-centromere antibodies for scleroderma, 13 and anti-synthetase (eg, anti-Jo-1) antibodies for autoimmune myositis.14,15
Indications that Raynaud phenomenon may be the presenting manifestation of a systemic autoimmune rheumatic disease are older age at onset (ie, over age 30), male sex, asymmetric involvement, and prolonged and painful attacks that can be severe enough to cause ischemic digital ulceration or gangrene (Figure 1).
Hence, chronic and severe digital ischemia causing ulceration or infarction differentiates secondary from primary Raynaud phenomenon and should prompt an investigation for an autoimmune rheumatic process. When taking the history, the clinician should seek clues to an underlying autoimmune condition, such as arthralgia, heartburn, dysphagia, shortness of breath, cough, and should examine the patient for telltale signs such as puffy hands and fingers, sclerodactyly, digital pitting scars, loss of fingertip pulp tissue, telangiectasias, and calcinosis.
CLUES TO PRIMARY VS SECONDARY RAYNAUD PHENOMENON
A diagnostic algorithm of digital ischemia (Figure 2) illustrates the range of presentations and possible causes. In Raynaud phenomenon, cold temperature and emotional stress provoke reversible color changes of the fingers and toes. Intense vasospasm of the digital arteries produces three well-defined phases1: white (pallor resulting from vasospasm), blue (dusky cyanosis due to deoxygenation of static venous blood) (Figure 1), and red (reactive hyperemia after the restoration of blood flow). However, only about 60% of patients have all three color changes. The attacks are associated with paresthesias, an uncomfortable feeling of coldness in the fingers, and ischemic pain.
Primary Raynaud phenomenon
Primary or idiopathic Raynaud phenomenon is seen in 5% to 10% of the general population. It more commonly affects women ages 15 to 30, is generally mild, involves the digits symmetrically, and is sometimes familial. An increase in alpha-2 adrenergic responses in the digital vessels leads to arterial vasospasm, an exaggerated physiologic response to cold temperatures.2 Geographic variability in prevalence likely represents differences in mean outdoor temperatures,3 which is in part why attacks of primary Raynaud phenomenon tend to be worse in the winter months.4
Secondary Raynaud phenomenon
Raynaud phenomenon also often occurs in certain autoimmune rheumatic diseases (secondary Raynaud phenomenon): for example, it is seen in scleroderma (90% to 95% of patients), mixed connective tissue disease (85%), systemic lupus erythematosus (40%), antisynthetase syndrome (40%), and sometimes in patients with other autoimmune rheumatic diseases. It may also be seen in hematologic disorders (cryoglobulinemia, cryofibrinogenemia, paraproteinemias, cold agglutinin disease, and polycythemia rubra vera), and it can also result from environmental and occupational exposures (frostbite, use of vibrating tools) and from exposure to certain drugs and toxins, such as polyvinyl chloride (Figure 2).
Acrocyanosis, a benign neurohormonal condition, should be included in the differential diagnosis for Raynaud phenomenon. Raynaud phenomenon is episodic, whereas acrocyanosis leads to persistent cyanosis of the acral body parts (fingers, toes) that is exacerbated by cold temperatures. However, the trophic skin changes, localized pain, and ulceration are not seen in acrocyanosis.
NAILFOLD CAPILLAROSCOPY: A KEY PART OF THE WORKUP
Nailfold capillaroscopy should be part of the evaluation of patients with Raynaud phenomenon (Figure 3), as it is one of the most reliable tests for distinguishing between primary and secondary Raynaud phenomenon.5 The sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis increases significantly with the addition of nailfold capillary abnormalities.6,7
A stereomicroscope or videocapillaroscope is usually recommended to evaluate nailfold capillary morphology,5 but if such equipment is not available, a regular ophthalmoscope (with the lens set at 20 diopters or higher for better resolution) can serve the purpose at the bedside.8 A drop of mineral oil is placed on the nailfold to improve the image resolution, as it makes the horny layer of the cuticle transparent.
Abnormal patterns include dilated and enlarged capillary loops, disorganized capillaries, “dropouts” (avascular areas), microhemorrhages, and arborized capillaries (Figure 3).5 At no additional cost, the presence of these microvascular changes would add to the suspicion of secondary Raynaud phenomenon (negative predictive value of 93%).9 In addition, evolving capillaroscopic changes can be seen during follow-up visits, indicating the progressive nature of the microvasculopathy seen in these autoimmune rheumatic diseases.10
ADDITIONAL TESTING
If an underlying autoimmune rheumatic disease is suspected, laboratory testing should include a complete blood cell count, an erythrocyte sedimentation rate, and an antinuclear antibody (ANA) assay. If the ANA assay is negative, no further testing is usually necessary; however, a positive test should alert the clinician to consider an underlying autoimmune rheumatic process (negative predictive value of 93%).9 In a patient presenting with Raynaud phenomenon, a positive ANA test (even in the absence of other symptoms) warrants more frequent follow-up, urinalysis, and perhaps referral to a rheumatologist.
In the case of a positive ANA test, before ordering additional autoantibody tests, it is useful to consider the relevant non-Raynaud clinical manifestations. Indiscriminate ordering of a battery of autoantibodies should be avoided because of significant added cost and because it is not likely to provide additional information to guide management.
On the other hand, these more specific antibody tests may be of value in confirming the diagnosis suggested by the clinical profile of specific autoimmune rheumatic diseases, eg, anti-double-stranded DNA11 and anti-Smith12 antibodies for lupus, anti-topoisomerase I (Scl-70) and anti-centromere antibodies for scleroderma, 13 and anti-synthetase (eg, anti-Jo-1) antibodies for autoimmune myositis.14,15
- Raynaud M. On local asphyxia and symmetrical gangrene of the extremities (1862), and new research on the nature and treatment of local asphyxia of the extremities (1872).Barlow T, trans. Selected monographs (121). London: New Sydenham Society, 1988.
- Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol 2005; 17:752–760.
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5-region comparison. J Rheumatol 1997; 24:879–889.
- Wigley FM. Clinical practice. Raynaud’s phenomenon. N Engl J Med 2002; 347:1001–1018.
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19:437–452.
- Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44:735–736.
- Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25:754–757.
- Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis 2001; 60:407–409.
- Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158:595–600.
- Wong ML, Highton J, Palmer DG. Sequential nailfold capillary microscopy in scleroderma and related disorders. Ann Rheum Dis 1988; 47:53–61.
- Weinstein A, Bordwell B, Stone B, Tibbetts C, Rothfield NF. Antibodies to native DNA and serum complement (C3) levels. Application to diagnosis and classification of systemic lupus erythematosus. Am J Med 1983; 74:206–216.
- Craft J. Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992; 18:311–335.
- Weiner ES, Hildebrandt S, Senécal JL, et al. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud’s disease. A prospective study. Arthritis Rheum 1991; 34:68–77.
- Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest 1990; 85:468–475.
- Ghirardello A, Zampieri S, Tarricone E, et al. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 2006; 39:217–221.
- Raynaud M. On local asphyxia and symmetrical gangrene of the extremities (1862), and new research on the nature and treatment of local asphyxia of the extremities (1872).Barlow T, trans. Selected monographs (121). London: New Sydenham Society, 1988.
- Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol 2005; 17:752–760.
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5-region comparison. J Rheumatol 1997; 24:879–889.
- Wigley FM. Clinical practice. Raynaud’s phenomenon. N Engl J Med 2002; 347:1001–1018.
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19:437–452.
- Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44:735–736.
- Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25:754–757.
- Anders HJ, Sigl T, Schattenkirchner M. Differentiation between primary and secondary Raynaud’s phenomenon: a prospective study comparing nailfold capillaroscopy using an ophthalmoscope or stereomicroscope. Ann Rheum Dis 2001; 60:407–409.
- Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158:595–600.
- Wong ML, Highton J, Palmer DG. Sequential nailfold capillary microscopy in scleroderma and related disorders. Ann Rheum Dis 1988; 47:53–61.
- Weinstein A, Bordwell B, Stone B, Tibbetts C, Rothfield NF. Antibodies to native DNA and serum complement (C3) levels. Application to diagnosis and classification of systemic lupus erythematosus. Am J Med 1983; 74:206–216.
- Craft J. Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992; 18:311–335.
- Weiner ES, Hildebrandt S, Senécal JL, et al. Prognostic significance of anticentromere antibodies and anti-topoisomerase I antibodies in Raynaud’s disease. A prospective study. Arthritis Rheum 1991; 34:68–77.
- Miller FW, Twitty SA, Biswas T, Plotz PH. Origin and regulation of a disease-specific autoantibody response. Antigenic epitopes, spectrotype stability, and isotype restriction of anti-Jo-1 autoantibodies. J Clin Invest 1990; 85:468–475.
- Ghirardello A, Zampieri S, Tarricone E, et al. Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 2006; 39:217–221.
Right upper-abdominal pain in a 97-year-old
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
A 47-year-old man with chest and neck pain
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
A guideline is like a prescription
Before we write a prescription, we review the patient’s diagnosis, the known evidence, and our own experience. Then we discuss the risks and benefits with the patient. If he or she chooses to fill the prescription, we are responsible for the outcome. But patients may choose to not take the medication, feeling that the accumulated evidence does not apply to them or not fully understanding the balance of potential benefit and harm.
When a group of expert physicians writes practice guidelines, they review the literature and their own experience and then summarize key practices that they believe should be followed or avoided. These guidelines are offered to practicing physicians to accept or reject. Unlike the physician writing an individual prescription, the authors of the guidelines are not held directly responsible for the outcome in a specific patient.
Guidelines seem to be accepted on an academic level, not as a prescriptive approach to care but as a way of evaluating the relevant evidence and its practical application. But many practicing clinicians fear that guidelines are leading to the algorithmic practice of medicine and to reimbursement according to adherence to the guidelines, regardless of patient outcomes.
In this issue, Roland Moskowitz, an expert in osteoarthritis, comments on the 2012 American College of Rheumatology “recommendations” for the treatment of this disease.1 He notes that these recommendations are not a cookbook, points out areas in which his own practices differ from them, and emphasizes the need to individualize our recommendations. For instance, he notes that some of his patients have relief of pain after hyaluronan injections into their osteoarthritic knees, even though the guidelines do not recommend this therapy1 and structured reviews suggest it has little benefit (in groups of patients) beyond that of placebo injections. This apparent paradox suggests that this therapy is not appropriate for everyone, but also that it should not be removed from our toolkit. Certainly, the patient’s response to an injection (outcome) should be evaluated before repeating this therapy.
We should not worship the idol of guidelines alone, but neither should we ignore them and make decisions only on the basis of anecdote and experience. When making individual treatment decisions, we must assess external validity before applying pooled trial data. And administrators need to understand that clinicians may choose to not follow practice guidelines in individual patients for very valid reasons.
Most studies of the impact of guidelines have focused on how well physicians comply with them, not on patient outcomes. Compliance—of patients with physicians’ advice and of physicians with guidelines—is a complicated process. Compliance should not be considered a cookbook expectation—for patients or for doctors.
- Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64:465–474.
Before we write a prescription, we review the patient’s diagnosis, the known evidence, and our own experience. Then we discuss the risks and benefits with the patient. If he or she chooses to fill the prescription, we are responsible for the outcome. But patients may choose to not take the medication, feeling that the accumulated evidence does not apply to them or not fully understanding the balance of potential benefit and harm.
When a group of expert physicians writes practice guidelines, they review the literature and their own experience and then summarize key practices that they believe should be followed or avoided. These guidelines are offered to practicing physicians to accept or reject. Unlike the physician writing an individual prescription, the authors of the guidelines are not held directly responsible for the outcome in a specific patient.
Guidelines seem to be accepted on an academic level, not as a prescriptive approach to care but as a way of evaluating the relevant evidence and its practical application. But many practicing clinicians fear that guidelines are leading to the algorithmic practice of medicine and to reimbursement according to adherence to the guidelines, regardless of patient outcomes.
In this issue, Roland Moskowitz, an expert in osteoarthritis, comments on the 2012 American College of Rheumatology “recommendations” for the treatment of this disease.1 He notes that these recommendations are not a cookbook, points out areas in which his own practices differ from them, and emphasizes the need to individualize our recommendations. For instance, he notes that some of his patients have relief of pain after hyaluronan injections into their osteoarthritic knees, even though the guidelines do not recommend this therapy1 and structured reviews suggest it has little benefit (in groups of patients) beyond that of placebo injections. This apparent paradox suggests that this therapy is not appropriate for everyone, but also that it should not be removed from our toolkit. Certainly, the patient’s response to an injection (outcome) should be evaluated before repeating this therapy.
We should not worship the idol of guidelines alone, but neither should we ignore them and make decisions only on the basis of anecdote and experience. When making individual treatment decisions, we must assess external validity before applying pooled trial data. And administrators need to understand that clinicians may choose to not follow practice guidelines in individual patients for very valid reasons.
Most studies of the impact of guidelines have focused on how well physicians comply with them, not on patient outcomes. Compliance—of patients with physicians’ advice and of physicians with guidelines—is a complicated process. Compliance should not be considered a cookbook expectation—for patients or for doctors.
Before we write a prescription, we review the patient’s diagnosis, the known evidence, and our own experience. Then we discuss the risks and benefits with the patient. If he or she chooses to fill the prescription, we are responsible for the outcome. But patients may choose to not take the medication, feeling that the accumulated evidence does not apply to them or not fully understanding the balance of potential benefit and harm.
When a group of expert physicians writes practice guidelines, they review the literature and their own experience and then summarize key practices that they believe should be followed or avoided. These guidelines are offered to practicing physicians to accept or reject. Unlike the physician writing an individual prescription, the authors of the guidelines are not held directly responsible for the outcome in a specific patient.
Guidelines seem to be accepted on an academic level, not as a prescriptive approach to care but as a way of evaluating the relevant evidence and its practical application. But many practicing clinicians fear that guidelines are leading to the algorithmic practice of medicine and to reimbursement according to adherence to the guidelines, regardless of patient outcomes.
In this issue, Roland Moskowitz, an expert in osteoarthritis, comments on the 2012 American College of Rheumatology “recommendations” for the treatment of this disease.1 He notes that these recommendations are not a cookbook, points out areas in which his own practices differ from them, and emphasizes the need to individualize our recommendations. For instance, he notes that some of his patients have relief of pain after hyaluronan injections into their osteoarthritic knees, even though the guidelines do not recommend this therapy1 and structured reviews suggest it has little benefit (in groups of patients) beyond that of placebo injections. This apparent paradox suggests that this therapy is not appropriate for everyone, but also that it should not be removed from our toolkit. Certainly, the patient’s response to an injection (outcome) should be evaluated before repeating this therapy.
We should not worship the idol of guidelines alone, but neither should we ignore them and make decisions only on the basis of anecdote and experience. When making individual treatment decisions, we must assess external validity before applying pooled trial data. And administrators need to understand that clinicians may choose to not follow practice guidelines in individual patients for very valid reasons.
Most studies of the impact of guidelines have focused on how well physicians comply with them, not on patient outcomes. Compliance—of patients with physicians’ advice and of physicians with guidelines—is a complicated process. Compliance should not be considered a cookbook expectation—for patients or for doctors.
- Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64:465–474.
- Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64:465–474.
The 2012 ACR guidelines for osteoarthritis: Not a cookbook
“When I see a patient with arthritis coming in the front door, I leave by the back door.”
—Sir William Ostler
Fortunately for today’s physicians treating patients with osteoarthritis, we need not be as pessimistic as Osler was more than a century ago when he uttered his now-famous words. Still, there is no magic bullet for the contemporary clinician treating an elderly patient with osteoarthritis. Instead, there are many imperfect bullets, and choosing between them is always a balancing act between benefit and risk from various agents: nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics such as acetaminophen and tramadol, opioids, and supplements such as glucosamine and chondroitin sulfate.
So there was great interest when, in 2012,1 the American College of Rheumatology (ACR) updated its previous guidelines (from 2000) on drug and nondrug therapies for osteoarthritis of the hip and the knee2 and added new recommendations on osteoarthritis of the hand.
Revising the guidelines was appropriate, since new therapies have become available. But, as the guideline authors state, with osteoarthritis, as with other diseases, guidelines cannot be a “cookbook.”
The treatment approach differs depending on the patient’s clinical presentation and on the preferences of the patient and the physician. Often, more than one approach is possible, and more than one approach may be appropriate in a given patient at a given time. The guideline authors also point out that some physicians may disagree with some of the recommendations.
I wish to review here several of the key recommendations. But I also provide some of my personal perspective and experience after 4 decades of treating patients with osteoarthritis.
HOW THE GUIDELINES WERE MADE
The new ACR guidelines were developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, a formal process to develop recommendations that are as evidence-based as possible.3
The authors are outstanding experts in the field of osteoarthritis from throughout the United States and Canada. Further, the recommendations were voted on by a “technical expert panel” representing the fields of rheumatology, orthopedics, physical medicine, and rehabilitation, from both academic medicine and private practice. This representation provides a balance of input from the types of clinicians frequently involved in managing osteoarthritis.
The initial literature searches for drug therapies were conducted during late 2008, and those for nonpharmacologic treatments were conducted during the second and third quarters of 2009. The goal of the literature searches was to identify the most current systematic reviews and meta-analyses that would provide reliable estimates of benefits of intervention for the prespecified clinically relevant outcomes of pain and function, as well as data on safety.
Recommendations: For or against, strong or weak—and the informed patient’s perspective
Therapies received the following possible recommendations:
- Strong recommendation to use
- Weak (or conditional) recommendation to use
- No recommendation
- Weak (or conditional) recommendation not to use
- Strong recommendation not to use.
A strong recommendation required high-quality evidence and evidence of a large difference between desirable and undesirable effects of the treatment. A conditional recommendation was based on the absence of high-quality evidence, evidence of only a small difference between desirable and undesirable effects of the treatment, or both.
One interesting feature of these recommendations is that they took into account how informed patients might themselves evaluate the data with their medical condition.
For instance, if a therapy received a strong favorable recommendation, we can assume that most informed patients would choose to receive it, and we can shape our interaction with the patient accordingly. A conditional recommendation means that most informed patients would choose the treatment—but many would not, and physicians should keep the patient’s values and preferences in mind.
I admit I had a problem with the meaning of the word “conditional” in the context of these guidelines. When evaluating a treatment, the term “weak” is readily understood and clearer. By using the word “weak,” one is making a positive statement in support of use but letting you know that the data and recommendation are weak. The word “conditional” is less readily defined and does not necessarily imply support for use.
Recommendations were drafted after discussion of the evidence at each meeting of the technical expert panel. Consensus was defined as 75% or more of the members of the panel voting to either strongly or conditionally recommend using a therapy, to either strongly or conditionally recommend not using it, or to choose not to make a recommendation on its use.
OSTEOARTHRITIS OF THE HAND: NO STRONG RECOMMENDATIONS
The technical expert panel gave no strong recommendations for any nondrug or drug treatment for osteoarthritis of the hand.
Conditional recommendations for nondrug treatments
The panel conditionally recommended the following:
- All patients with osteoarthritis of the hand should be evaluated either by their primary physician or by an occupational or physical therapist, particularly with respect to ability to perform activities of daily living.
- Assistive devices such as jar openers, key turners, and pull tabs for zippers should be recommended, as needed.
- Patients should be instructed in joint protection and in the use of thermal treatments (eg, heating pads, ultrasound devices, hot packs, and ice packs).
Comments. Appliances are often beneficial in patients who have involvement of the first carpometacarpal (trapeziometacarpal) joint. Although over-the-counter thumb splints are an option, referral to an occupational therapist for splint prescription is advantageous to achieve a comfortable fit and, importantly, for instructions to the patient on how to avoid joint trauma.
It would be unrealistic to expect primary care physicians and internists to have the expertise to make detailed recommendations about orthopedic appliances. Accordingly, referral to an occupational therapist or an orthopedist is advisable for these situations. It is important, however, that the physician be aware of what treatments are available and most effective, and of the indications for referral.
As for heat treatment, elastic stretch gloves may relieve symptoms through their warming and massaging effects.4
Some drugs for hand osteoarthritis got conditional recommendations in favor
The expert panel gave conditional recommendations in favor of:
- Topical capsaicin
- Topical NSAIDs
- Oral NSAIDs (including both nonselective and selective agents)
- Tramadol (Ultram)
- Topical rather than oral NSAIDs for patients age 75 and older.
Other drug treatments got conditional recommendations against their use
The expert panel gave conditional recommendations against using:
- Intra-articular injections, and in particular, corticosteroid injections in the trapeziometacarpal (first carpometacarpal) joint
- Opioid analgesics
- Oral methotrexate or sulfasalazine in patients with erosive inflammatory interphalangeal osteoarthritis.
No recommendation for or against
- Hydroxychloroquine.
Comments—Intra-articular injections, opioids, and oral NSAIDs
I differ with these recommendations on several points.
Although the guidelines committee conditionally recommended against using intra-articular therapies for hand osteoarthritis, I find that intra-articular corticosteroid injections are often effective, particularly in patients who have inflammatory forms of the disease, ie, “erosive inflammatory osteoarthritis.” Most nonspecialist physicians probably have limited experience in giving injections into small joints, and referral to a rheumatologist or orthopedist would be appropriate.
I disagree as well with the conditional recommendation that intra-articular corticosteroid injections not be used for involvement of the trapeziometacarpal (first carpometacarpal) joint. I find that many patients with osteoarthritis of this joint experience improvement with intra-articular corticosteroid injections.
I agree that there are limited data on the use of intra-articular hyaluronan injections in this situation and do not routinely use them in this joint.
Opioid analgesics also received a conditional recommendation against their use. The same caveats apply here as for these drugs elsewhere.5 If used, opioids should be used at the lowest dose possible and for as short a time as possible. If the physician is uncomfortable prescribing opioids for patients with osteoarthritis, referral to a pain specialist is recommended.
I disagree to some extent with the conditional recommendation that people age 75 and older should use topical rather than oral NSAIDs. I understand the recommendation, given that older people have a higher frequency of gastrointestinal, renal, and cardiac disease and are best served by avoiding NSAIDs. However, we all see patients over age 75 who are physiologically younger than their numerical age. Accordingly, I feel that the judgment of the physician plays a role in whether NSAIDs are reasonable for some older patients.
The committee recommended not using oral methotrexate or sulfasalazine in patients with erosive inflammatory interphalangeal osteoarthritis. I have used oral hydroxychloroquine off-label in such patients and find that they respond in a very rewarding fashion.
Given that this is an off-label use of hydroxychloroquine, the drug should be used only with appropriate consideration and after discussion with the patient about toxicity, especially about the risk of ocular manifestations.
OSTEOARTHRITIS OF THE KNEE
Some nondrug therapies got strong recommendations
The expert panel strongly recommended:
- Exercise (aerobic, resistance, land-based, and aquatic)
- Weight loss (for patients who are overweight).
Other nondrug therapies got conditional recommendations
The panel conditionally recommended:
- Self-management programs
- Manual therapy in combination with supervised exercise
- Psychosocial interventions
- Medially directed patellar taping
- Medially wedged insoles (if the patient has lateral compartment osteoarthritis)
- Laterally wedged subtalar strapped insoles (if the patient has medial compartment osteoarthritis)
- Heat therapy
- Walking aids, as needed
- Tai chi
- Chinese acupuncture
- Transcutaneous electrical nerve stimulation.
Comments. The ACR panel appropriately noted that Chinese acupuncture or transcutaneous electrical stimulation should be recommended only if the patient has chronic moderate to severe pain and is a candidate for total knee arthroplasty but is unwilling to undergo the procedure or has comorbid medical conditions that rule out surgery.
Nondrug therapies for knee osteoarthritis that got no recommendation for or against
- Balance exercise
- Laterally wedged insoles
- Manual therapy alone
- Knee braces
- Laterally directed patellar taping.
Comments. It was somewhat surprising that there were no recommendations about laterally wedged insoles or knee braces. Laterally wedged insoles have been recommended for patients who have medial compartment knee osteoarthritis6; being thinner at the instep and thicker at the outer edge of the foot, they reduce load on the medial aspect of the knee. One has to be cautious in using knee wedging in patients who have concomitant ankle or hip angle deformities, lest these joints be compromised.
Some of these treatments would be out of the realm of the nonspecialist physician.
Conditional recommendations for initial drug therapy for knee osteoarthritis
The panel conditionally recommended that patients who have osteoarthritis of the knee use one of the following:
- Acetaminophen (contained in Tylenol and a host of other products)
- Oral NSAIDs
- Topical NSAIDs (with a strong recommendation for topical NSAIDs rather than oral NSAIDs in patients age 75 and older)
- Tramadol
- Intra-articular corticosteroid injections.
Comments. In the past, it was recommended that acetaminophen in full doses of up to 4,000 mg per day be considered.7 Current dogma, however, is that doses of acetaminophen should not exceed 3,000 mg per day to avoid damaging the liver. This concern led the US Food and Drug Administration (FDA) in 2011 to advise that the maximum daily dose be limited.8 The ACR panel recommended that patients be counseled to avoid all other products that contain acetaminophen, which is especially cogent, given the presence of this agent in many over-the-counter medications.9
The panel conditionally recommended that people age 75 and older use topical rather than oral NSAIDs. As mentioned earlier, a specific age limit does not take into account that many people age 75 and older may actually be physiologically younger than some in their 50s or 60s. Accordingly, it is recommended that the physician use judgment in this regard so that NSAIDs will not be denied to patients for whom they might be of significant value.
Strong recommendation for gastric protection in patients at risk on NSAIDs
If a patient with knee osteoarthritis has a history of a symptomatic or complicated upper gastrointestinal ulcer but has not had an upper gastrointestinal bleed in the past year and the physician chooses to prescribe an oral NSAID, the expert panel strongly recommended using either a cyclooxygenase (COX)-2-selective inhibitor or a nonselective NSAID in combination with a proton pump inhibitor.
Comment. The suggestion that patients who have had a complicated upper gastrointestinal ulcer in the past year could be considered for treatment with a COX-2-selective inhibitor or nonselective NSAID in combination with a proton pump inhibitor seemed a bit aggressive. My own inclination would be to avoid both nonselective and selective inhibitors in this situation. Alternative agents such as acetaminophen in full doses, tramadol, intra-articular hyaluronan injections, and intra-articular corticosteroid injections seem preferable with respect to safety in such patients.
The suggestion that a proton pump inhibitor be used whenever an NSAID is given for chronic management of knee or hip osteoarthritis is reasonable.10,11 Although some studies have suggested that chronic use of proton pump inhibitors may predispose to osteopenia or osteoporosis, others have not, and gastric protection should be considered in patients at gastrointestinal risk.
Strong recommendation against ibuprofen in patients taking aspirin
The ACR panel strongly recommended that ibuprofen (Advil) not be prescribed to patients with knee osteoarthritis who are using aspirin in low doses for cardioprotection, and strongly recommended using another nonselective NSAID plus a proton pump inhibitor instead. The panel also strongly recommended against using a COX-2-selective inhibitor in this situation.12,13
Comment. The rationale for these recommendations is that ibuprofen may render aspirin ineffective as a cardioprotective agent. Ibuprofen interferes with the aspirin-binding site on platelets, so that the protective effect of aspirin is lost.14,15 Celecoxib (Celebrex)16 and diclofenac (Voltaren) have binding sites different from that of aspirin, although the ACR recommends against using COX-2-selective inhibitors such as celecoxib in the situation and gives no recommendation about other NSAIDs.
No recommendations for or against
The panel issued no recommendations for or against the following treatments for patients with knee osteoarthritis:
- Intra-articular hyaluronan injections
- Duloxetine (Cymbalta)
- Opioid analgesics.
Comments on knee injections
Intra-articular injections of corticosteroids or hyaluronan are commonly used for knee osteoarthritis. As noted, corticosteroid injections received a conditional recommendation, while hyaluronan injections received no recommendation for or against.
How often to inject corticosteroids? In general, too-frequent injection of corticosteroids is to be avoided, in view of the risk of promoting joint breakdown. There is no “magic” number of injections that is safe, although more than 4 per year in the same joint should generally be avoided. In some situations, however, repeat injections may be reasonable if alternative therapies are associated with higher risk.
Raynauld et al,17 in a randomized, double-blind, placebo-controlled trial, demonstrated that intra-articular corticosteroid injections at 3-month intervals for 2 years were not deleterious to knees.
My philosophy is generally not to inject on a regular basis, but to be selective and be guided by the patient’s clinical condition and response to prior injections.
Are hyaluronan injections effective? Although experts differ in their enthusiasm for intra-articular hyaluronan injections in the knee, I have found that many patients benefit from this treatment. Multiple studies have found it efficacious and safe overall.18–21 However, some systematic reviews have called its efficacy into question.7
Although differences in efficacy have been noted, this therapy was approved as being useful in patients with knee osteoarthritis in the Osteoarthritis Research Society International (OARSI) recommendations.7 The effect sizes were smaller in later assessments.22
Hyaluronan injections do not pose the risk of joint breakdown that corticosteroid injections do, but their clinical efficacy is not as dramatic. Adverse reactions to most intraarticular hyaluronans are limited, with slight increases in pain and stiffness after injection. Significant inflammatory reactions characterized as “postinjection flares” are more commonly seen with high-molecular-weight crosslinked preparations. These reactions can be severe and can mimic joint infection clinically. Joint aspiration with synovial fluid analysis and culture may be necessary to exclude infection. Response to aspiration and nonsteroidal inflammatory agents or intra-articular corticosteroids is usually excellent.
Ultrasonographic guidance. As with intraarticular injections in other areas, ultrasonographic guidance is becoming more common, as it allows for more accurate drug administration.
Pes anserine bursitis must be ruled out as a cause of the patient’s knee symptoms—misdiagnosis is not uncommon. The bursa is located on the medial aspect of the tibia, and inflammation of the bursa is a common cause of pain in this area. Local steroid injection is extremely effective in symptomatic therapy. Physical therapy and NSAIDs may be adequate to treat milder cases.
Conditional recommendation against glucosamine, chondroitin, capsaicin
The ACR panel conditionally recommended that patients with knee osteoarthritis not use:
- Chondroitin sulfate
- Glucosamine
- Topical capsaicin.
Comment. Evidence is mixed about the efficacy of glucosamine and chondroitin sulfate, which are so-called nutraceuticals. Some studies found them useful23–25 but some did not,26 and a meta-analysis concluded that they do not help.27 The OARSI guidelines published in 2008 stated that these agents may relieve symptoms of osteoarthritis of the knee.7 The OARSI update published in 2010 found that glucosamine was effective, but less so than in previous studies.22 If glucosamine is effective, some studies suggest that glucosamine sulfate is more effective than glucosamine hydrochloride.22
The same OARSI review revealed that chondroitin sulfate relieved pain but with heterogeneous, dissimilar effect sizes. Of interest was the finding that the 5-year incidence of total knee replacement was lower in patients treated with glucosamine sulfate 1,500 mg/day than with placebo. Also, the rate of decline of joint space narrowing was reported to be reduced in chondroitin sulfate-treated patients.22
In practice, a conditional recommendation against a treatment means that most informed patients would not want the treatment, but some would. Accordingly, if patients still want to take chondroitin or glucosamine after being informed of the limited evidence of benefit, I feel a trial of their use is reasonable.
OSTEOARTHRITIS OF THE HIP
Indications for therapy of osteoarthritis of the hip are similar to those for osteoarthritis of the knee.
As in the knee, nonpharmacologic therapies are important. Loss of weight for overweight patients is extremely important; supervised exercise is especially valuable. Use of canes or crutches as needed is conditionally recommended.
Pharmacologic management is similar to that of osteoarthritis of the knee, with particular use of acetaminophen, NSAIDs, tramadol, and intra-articular corticosteroid injections.
Comment. Intra-articular injection of corticosteroids into the hip would be out of the realm of most nonspecialist practices. Although some rheumatologists are expert in such injections, this treatment is generally best left to an orthopedist or invasive radiologist. The use of ultrasonographic guidance is becoming more frequent, with many rheumatologists having developed expertise in this approach to the knee and the hip. Since most studies were in patients with osteoarthritis of the knee, fewer data are available as to the efficacy of these agents in patients with hip osteoarthritis.
Fewer data are available also with respect to the benefit of chondroitin sulfate and glucosamine in patients with osteoarthritis of the hip. Total joint replacement is extremely effective if conservative therapy does not help.
FIRST, DO NO HARM
Guidelines from the ACR,1,2 the European League Against Rheumatism (EULAR),28,29 the American Academy of Orthopedic Surgeons (AAOS),30 and the OARSI7,22 all differ somewhat, owing to the different evidence available at the time each guideline was developed and to different geographic and cultural backgrounds.
The compositions of these various panels also differ sufficiently to affect their overall recommendations. For example, the EULAR panel consisted of only rheumatologists and an orthopedic surgeon; for the hand osteoarthritis recommendations they added a physiatrist and two allied health professionals.28,29 The OARSI panel included two primary care physicians in addition to rheumatologists and an orthopedic surgeon.7 The ACR was the only professional society to include primary care physicians, physiatrists, and geriatricians along with rheumatologists, an orthopedic surgeon, and physical and occupational therapists.
Although it is to be expected that there will not be universal agreement on all points of management of osteoarthritis by diverse groups, it is essential that input from all these experts representing various subspecialties be recognized. Therapeutic approaches will vary depending on patient characteristics and the experience of the treating physician. As long as therapy is based on reasonable supportive data, beneficial effects can be anticipated. Therapies that received conditional recommendations are not to be discounted if a reasonable percent of patients respond in positive fashion. Obviously, strong recommendations are more likely to be universally accepted since the likelihood that they will be beneficial is stronger.
In any approach to therapy, the caveat primum non nocere—first, do no harm—must always be kept in mind.
- Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64:465–474.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000; 43:1905–1915.
- Atkins D, Best D, Briss PA, et al; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; 328:1490.
- Askari A, Moskowitz RW, Ryan C. Stretch gloves. A study of objective and subjective effectiveness in arthritis of the hands. Arthritis Rheum 1974; 17:263–265.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Fang MA, Taylor CE, Nouvong A, Masih S, Kao KC, Perell KL. Effects of footwear on medial compartment knee osteoarthritis. J Rehabil Res Dev 2006; 43:427–434.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008; 16:137–162.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure. January 13, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm. Accessed November 28, 2012.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med 2010; 77:19–27.
- Bolten WW. Rational use of nonsteroidal anti-inflammatory drugs and proton pump inhibitors in combination for rheumatic diseases. Orthopedic Research and Reviews 2010; 2:75–84.
- Graham DY, Agrawal NM, Campbell DR, et al; NSAID-Associated Gastric Ulcer Prevention Study Group. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med 2002; 162:169–175.
- American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Antiinflammatory Drugs. Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum 2008; 59:1058–1073.
- Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation 2007; 115:1634–1642.
- Ellison J, Dager W. Recent FDA warning of the concomitant use of aspirin and ibuprofen and the effects on platelet aggregation. Prev Cardiol 2007; 10:61–63.
- Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ. The interaction of ibuprofen and diclofenac with aspirin in healthy volunteers. Br J Pharmacol 2009; 157:931–934.
- Wilner KD, Rushing M, Walden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002; 42:1027–1030.
- Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003; 48:370–377.
- Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis 2012; 71:1454–1460.
- Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012; 26:257–268.
- Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2004; 86-A:538–545.
- Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Visco-supplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012; 157:180–191.
- Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010; 18:476–499.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treat-ment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007; 15:981–1000.
- Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005; 2:CD002946.
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum 2007; 56:2267–2277.
- Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006; 354:795–808.
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675.
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2005; 64:669–681.
- Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007; 66:377–388.
- American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (non-arthroplasty). Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
“When I see a patient with arthritis coming in the front door, I leave by the back door.”
—Sir William Ostler
Fortunately for today’s physicians treating patients with osteoarthritis, we need not be as pessimistic as Osler was more than a century ago when he uttered his now-famous words. Still, there is no magic bullet for the contemporary clinician treating an elderly patient with osteoarthritis. Instead, there are many imperfect bullets, and choosing between them is always a balancing act between benefit and risk from various agents: nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics such as acetaminophen and tramadol, opioids, and supplements such as glucosamine and chondroitin sulfate.
So there was great interest when, in 2012,1 the American College of Rheumatology (ACR) updated its previous guidelines (from 2000) on drug and nondrug therapies for osteoarthritis of the hip and the knee2 and added new recommendations on osteoarthritis of the hand.
Revising the guidelines was appropriate, since new therapies have become available. But, as the guideline authors state, with osteoarthritis, as with other diseases, guidelines cannot be a “cookbook.”
The treatment approach differs depending on the patient’s clinical presentation and on the preferences of the patient and the physician. Often, more than one approach is possible, and more than one approach may be appropriate in a given patient at a given time. The guideline authors also point out that some physicians may disagree with some of the recommendations.
I wish to review here several of the key recommendations. But I also provide some of my personal perspective and experience after 4 decades of treating patients with osteoarthritis.
HOW THE GUIDELINES WERE MADE
The new ACR guidelines were developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, a formal process to develop recommendations that are as evidence-based as possible.3
The authors are outstanding experts in the field of osteoarthritis from throughout the United States and Canada. Further, the recommendations were voted on by a “technical expert panel” representing the fields of rheumatology, orthopedics, physical medicine, and rehabilitation, from both academic medicine and private practice. This representation provides a balance of input from the types of clinicians frequently involved in managing osteoarthritis.
The initial literature searches for drug therapies were conducted during late 2008, and those for nonpharmacologic treatments were conducted during the second and third quarters of 2009. The goal of the literature searches was to identify the most current systematic reviews and meta-analyses that would provide reliable estimates of benefits of intervention for the prespecified clinically relevant outcomes of pain and function, as well as data on safety.
Recommendations: For or against, strong or weak—and the informed patient’s perspective
Therapies received the following possible recommendations:
- Strong recommendation to use
- Weak (or conditional) recommendation to use
- No recommendation
- Weak (or conditional) recommendation not to use
- Strong recommendation not to use.
A strong recommendation required high-quality evidence and evidence of a large difference between desirable and undesirable effects of the treatment. A conditional recommendation was based on the absence of high-quality evidence, evidence of only a small difference between desirable and undesirable effects of the treatment, or both.
One interesting feature of these recommendations is that they took into account how informed patients might themselves evaluate the data with their medical condition.
For instance, if a therapy received a strong favorable recommendation, we can assume that most informed patients would choose to receive it, and we can shape our interaction with the patient accordingly. A conditional recommendation means that most informed patients would choose the treatment—but many would not, and physicians should keep the patient’s values and preferences in mind.
I admit I had a problem with the meaning of the word “conditional” in the context of these guidelines. When evaluating a treatment, the term “weak” is readily understood and clearer. By using the word “weak,” one is making a positive statement in support of use but letting you know that the data and recommendation are weak. The word “conditional” is less readily defined and does not necessarily imply support for use.
Recommendations were drafted after discussion of the evidence at each meeting of the technical expert panel. Consensus was defined as 75% or more of the members of the panel voting to either strongly or conditionally recommend using a therapy, to either strongly or conditionally recommend not using it, or to choose not to make a recommendation on its use.
OSTEOARTHRITIS OF THE HAND: NO STRONG RECOMMENDATIONS
The technical expert panel gave no strong recommendations for any nondrug or drug treatment for osteoarthritis of the hand.
Conditional recommendations for nondrug treatments
The panel conditionally recommended the following:
- All patients with osteoarthritis of the hand should be evaluated either by their primary physician or by an occupational or physical therapist, particularly with respect to ability to perform activities of daily living.
- Assistive devices such as jar openers, key turners, and pull tabs for zippers should be recommended, as needed.
- Patients should be instructed in joint protection and in the use of thermal treatments (eg, heating pads, ultrasound devices, hot packs, and ice packs).
Comments. Appliances are often beneficial in patients who have involvement of the first carpometacarpal (trapeziometacarpal) joint. Although over-the-counter thumb splints are an option, referral to an occupational therapist for splint prescription is advantageous to achieve a comfortable fit and, importantly, for instructions to the patient on how to avoid joint trauma.
It would be unrealistic to expect primary care physicians and internists to have the expertise to make detailed recommendations about orthopedic appliances. Accordingly, referral to an occupational therapist or an orthopedist is advisable for these situations. It is important, however, that the physician be aware of what treatments are available and most effective, and of the indications for referral.
As for heat treatment, elastic stretch gloves may relieve symptoms through their warming and massaging effects.4
Some drugs for hand osteoarthritis got conditional recommendations in favor
The expert panel gave conditional recommendations in favor of:
- Topical capsaicin
- Topical NSAIDs
- Oral NSAIDs (including both nonselective and selective agents)
- Tramadol (Ultram)
- Topical rather than oral NSAIDs for patients age 75 and older.
Other drug treatments got conditional recommendations against their use
The expert panel gave conditional recommendations against using:
- Intra-articular injections, and in particular, corticosteroid injections in the trapeziometacarpal (first carpometacarpal) joint
- Opioid analgesics
- Oral methotrexate or sulfasalazine in patients with erosive inflammatory interphalangeal osteoarthritis.
No recommendation for or against
- Hydroxychloroquine.
Comments—Intra-articular injections, opioids, and oral NSAIDs
I differ with these recommendations on several points.
Although the guidelines committee conditionally recommended against using intra-articular therapies for hand osteoarthritis, I find that intra-articular corticosteroid injections are often effective, particularly in patients who have inflammatory forms of the disease, ie, “erosive inflammatory osteoarthritis.” Most nonspecialist physicians probably have limited experience in giving injections into small joints, and referral to a rheumatologist or orthopedist would be appropriate.
I disagree as well with the conditional recommendation that intra-articular corticosteroid injections not be used for involvement of the trapeziometacarpal (first carpometacarpal) joint. I find that many patients with osteoarthritis of this joint experience improvement with intra-articular corticosteroid injections.
I agree that there are limited data on the use of intra-articular hyaluronan injections in this situation and do not routinely use them in this joint.
Opioid analgesics also received a conditional recommendation against their use. The same caveats apply here as for these drugs elsewhere.5 If used, opioids should be used at the lowest dose possible and for as short a time as possible. If the physician is uncomfortable prescribing opioids for patients with osteoarthritis, referral to a pain specialist is recommended.
I disagree to some extent with the conditional recommendation that people age 75 and older should use topical rather than oral NSAIDs. I understand the recommendation, given that older people have a higher frequency of gastrointestinal, renal, and cardiac disease and are best served by avoiding NSAIDs. However, we all see patients over age 75 who are physiologically younger than their numerical age. Accordingly, I feel that the judgment of the physician plays a role in whether NSAIDs are reasonable for some older patients.
The committee recommended not using oral methotrexate or sulfasalazine in patients with erosive inflammatory interphalangeal osteoarthritis. I have used oral hydroxychloroquine off-label in such patients and find that they respond in a very rewarding fashion.
Given that this is an off-label use of hydroxychloroquine, the drug should be used only with appropriate consideration and after discussion with the patient about toxicity, especially about the risk of ocular manifestations.
OSTEOARTHRITIS OF THE KNEE
Some nondrug therapies got strong recommendations
The expert panel strongly recommended:
- Exercise (aerobic, resistance, land-based, and aquatic)
- Weight loss (for patients who are overweight).
Other nondrug therapies got conditional recommendations
The panel conditionally recommended:
- Self-management programs
- Manual therapy in combination with supervised exercise
- Psychosocial interventions
- Medially directed patellar taping
- Medially wedged insoles (if the patient has lateral compartment osteoarthritis)
- Laterally wedged subtalar strapped insoles (if the patient has medial compartment osteoarthritis)
- Heat therapy
- Walking aids, as needed
- Tai chi
- Chinese acupuncture
- Transcutaneous electrical nerve stimulation.
Comments. The ACR panel appropriately noted that Chinese acupuncture or transcutaneous electrical stimulation should be recommended only if the patient has chronic moderate to severe pain and is a candidate for total knee arthroplasty but is unwilling to undergo the procedure or has comorbid medical conditions that rule out surgery.
Nondrug therapies for knee osteoarthritis that got no recommendation for or against
- Balance exercise
- Laterally wedged insoles
- Manual therapy alone
- Knee braces
- Laterally directed patellar taping.
Comments. It was somewhat surprising that there were no recommendations about laterally wedged insoles or knee braces. Laterally wedged insoles have been recommended for patients who have medial compartment knee osteoarthritis6; being thinner at the instep and thicker at the outer edge of the foot, they reduce load on the medial aspect of the knee. One has to be cautious in using knee wedging in patients who have concomitant ankle or hip angle deformities, lest these joints be compromised.
Some of these treatments would be out of the realm of the nonspecialist physician.
Conditional recommendations for initial drug therapy for knee osteoarthritis
The panel conditionally recommended that patients who have osteoarthritis of the knee use one of the following:
- Acetaminophen (contained in Tylenol and a host of other products)
- Oral NSAIDs
- Topical NSAIDs (with a strong recommendation for topical NSAIDs rather than oral NSAIDs in patients age 75 and older)
- Tramadol
- Intra-articular corticosteroid injections.
Comments. In the past, it was recommended that acetaminophen in full doses of up to 4,000 mg per day be considered.7 Current dogma, however, is that doses of acetaminophen should not exceed 3,000 mg per day to avoid damaging the liver. This concern led the US Food and Drug Administration (FDA) in 2011 to advise that the maximum daily dose be limited.8 The ACR panel recommended that patients be counseled to avoid all other products that contain acetaminophen, which is especially cogent, given the presence of this agent in many over-the-counter medications.9
The panel conditionally recommended that people age 75 and older use topical rather than oral NSAIDs. As mentioned earlier, a specific age limit does not take into account that many people age 75 and older may actually be physiologically younger than some in their 50s or 60s. Accordingly, it is recommended that the physician use judgment in this regard so that NSAIDs will not be denied to patients for whom they might be of significant value.
Strong recommendation for gastric protection in patients at risk on NSAIDs
If a patient with knee osteoarthritis has a history of a symptomatic or complicated upper gastrointestinal ulcer but has not had an upper gastrointestinal bleed in the past year and the physician chooses to prescribe an oral NSAID, the expert panel strongly recommended using either a cyclooxygenase (COX)-2-selective inhibitor or a nonselective NSAID in combination with a proton pump inhibitor.
Comment. The suggestion that patients who have had a complicated upper gastrointestinal ulcer in the past year could be considered for treatment with a COX-2-selective inhibitor or nonselective NSAID in combination with a proton pump inhibitor seemed a bit aggressive. My own inclination would be to avoid both nonselective and selective inhibitors in this situation. Alternative agents such as acetaminophen in full doses, tramadol, intra-articular hyaluronan injections, and intra-articular corticosteroid injections seem preferable with respect to safety in such patients.
The suggestion that a proton pump inhibitor be used whenever an NSAID is given for chronic management of knee or hip osteoarthritis is reasonable.10,11 Although some studies have suggested that chronic use of proton pump inhibitors may predispose to osteopenia or osteoporosis, others have not, and gastric protection should be considered in patients at gastrointestinal risk.
Strong recommendation against ibuprofen in patients taking aspirin
The ACR panel strongly recommended that ibuprofen (Advil) not be prescribed to patients with knee osteoarthritis who are using aspirin in low doses for cardioprotection, and strongly recommended using another nonselective NSAID plus a proton pump inhibitor instead. The panel also strongly recommended against using a COX-2-selective inhibitor in this situation.12,13
Comment. The rationale for these recommendations is that ibuprofen may render aspirin ineffective as a cardioprotective agent. Ibuprofen interferes with the aspirin-binding site on platelets, so that the protective effect of aspirin is lost.14,15 Celecoxib (Celebrex)16 and diclofenac (Voltaren) have binding sites different from that of aspirin, although the ACR recommends against using COX-2-selective inhibitors such as celecoxib in the situation and gives no recommendation about other NSAIDs.
No recommendations for or against
The panel issued no recommendations for or against the following treatments for patients with knee osteoarthritis:
- Intra-articular hyaluronan injections
- Duloxetine (Cymbalta)
- Opioid analgesics.
Comments on knee injections
Intra-articular injections of corticosteroids or hyaluronan are commonly used for knee osteoarthritis. As noted, corticosteroid injections received a conditional recommendation, while hyaluronan injections received no recommendation for or against.
How often to inject corticosteroids? In general, too-frequent injection of corticosteroids is to be avoided, in view of the risk of promoting joint breakdown. There is no “magic” number of injections that is safe, although more than 4 per year in the same joint should generally be avoided. In some situations, however, repeat injections may be reasonable if alternative therapies are associated with higher risk.
Raynauld et al,17 in a randomized, double-blind, placebo-controlled trial, demonstrated that intra-articular corticosteroid injections at 3-month intervals for 2 years were not deleterious to knees.
My philosophy is generally not to inject on a regular basis, but to be selective and be guided by the patient’s clinical condition and response to prior injections.
Are hyaluronan injections effective? Although experts differ in their enthusiasm for intra-articular hyaluronan injections in the knee, I have found that many patients benefit from this treatment. Multiple studies have found it efficacious and safe overall.18–21 However, some systematic reviews have called its efficacy into question.7
Although differences in efficacy have been noted, this therapy was approved as being useful in patients with knee osteoarthritis in the Osteoarthritis Research Society International (OARSI) recommendations.7 The effect sizes were smaller in later assessments.22
Hyaluronan injections do not pose the risk of joint breakdown that corticosteroid injections do, but their clinical efficacy is not as dramatic. Adverse reactions to most intraarticular hyaluronans are limited, with slight increases in pain and stiffness after injection. Significant inflammatory reactions characterized as “postinjection flares” are more commonly seen with high-molecular-weight crosslinked preparations. These reactions can be severe and can mimic joint infection clinically. Joint aspiration with synovial fluid analysis and culture may be necessary to exclude infection. Response to aspiration and nonsteroidal inflammatory agents or intra-articular corticosteroids is usually excellent.
Ultrasonographic guidance. As with intraarticular injections in other areas, ultrasonographic guidance is becoming more common, as it allows for more accurate drug administration.
Pes anserine bursitis must be ruled out as a cause of the patient’s knee symptoms—misdiagnosis is not uncommon. The bursa is located on the medial aspect of the tibia, and inflammation of the bursa is a common cause of pain in this area. Local steroid injection is extremely effective in symptomatic therapy. Physical therapy and NSAIDs may be adequate to treat milder cases.
Conditional recommendation against glucosamine, chondroitin, capsaicin
The ACR panel conditionally recommended that patients with knee osteoarthritis not use:
- Chondroitin sulfate
- Glucosamine
- Topical capsaicin.
Comment. Evidence is mixed about the efficacy of glucosamine and chondroitin sulfate, which are so-called nutraceuticals. Some studies found them useful23–25 but some did not,26 and a meta-analysis concluded that they do not help.27 The OARSI guidelines published in 2008 stated that these agents may relieve symptoms of osteoarthritis of the knee.7 The OARSI update published in 2010 found that glucosamine was effective, but less so than in previous studies.22 If glucosamine is effective, some studies suggest that glucosamine sulfate is more effective than glucosamine hydrochloride.22
The same OARSI review revealed that chondroitin sulfate relieved pain but with heterogeneous, dissimilar effect sizes. Of interest was the finding that the 5-year incidence of total knee replacement was lower in patients treated with glucosamine sulfate 1,500 mg/day than with placebo. Also, the rate of decline of joint space narrowing was reported to be reduced in chondroitin sulfate-treated patients.22
In practice, a conditional recommendation against a treatment means that most informed patients would not want the treatment, but some would. Accordingly, if patients still want to take chondroitin or glucosamine after being informed of the limited evidence of benefit, I feel a trial of their use is reasonable.
OSTEOARTHRITIS OF THE HIP
Indications for therapy of osteoarthritis of the hip are similar to those for osteoarthritis of the knee.
As in the knee, nonpharmacologic therapies are important. Loss of weight for overweight patients is extremely important; supervised exercise is especially valuable. Use of canes or crutches as needed is conditionally recommended.
Pharmacologic management is similar to that of osteoarthritis of the knee, with particular use of acetaminophen, NSAIDs, tramadol, and intra-articular corticosteroid injections.
Comment. Intra-articular injection of corticosteroids into the hip would be out of the realm of most nonspecialist practices. Although some rheumatologists are expert in such injections, this treatment is generally best left to an orthopedist or invasive radiologist. The use of ultrasonographic guidance is becoming more frequent, with many rheumatologists having developed expertise in this approach to the knee and the hip. Since most studies were in patients with osteoarthritis of the knee, fewer data are available as to the efficacy of these agents in patients with hip osteoarthritis.
Fewer data are available also with respect to the benefit of chondroitin sulfate and glucosamine in patients with osteoarthritis of the hip. Total joint replacement is extremely effective if conservative therapy does not help.
FIRST, DO NO HARM
Guidelines from the ACR,1,2 the European League Against Rheumatism (EULAR),28,29 the American Academy of Orthopedic Surgeons (AAOS),30 and the OARSI7,22 all differ somewhat, owing to the different evidence available at the time each guideline was developed and to different geographic and cultural backgrounds.
The compositions of these various panels also differ sufficiently to affect their overall recommendations. For example, the EULAR panel consisted of only rheumatologists and an orthopedic surgeon; for the hand osteoarthritis recommendations they added a physiatrist and two allied health professionals.28,29 The OARSI panel included two primary care physicians in addition to rheumatologists and an orthopedic surgeon.7 The ACR was the only professional society to include primary care physicians, physiatrists, and geriatricians along with rheumatologists, an orthopedic surgeon, and physical and occupational therapists.
Although it is to be expected that there will not be universal agreement on all points of management of osteoarthritis by diverse groups, it is essential that input from all these experts representing various subspecialties be recognized. Therapeutic approaches will vary depending on patient characteristics and the experience of the treating physician. As long as therapy is based on reasonable supportive data, beneficial effects can be anticipated. Therapies that received conditional recommendations are not to be discounted if a reasonable percent of patients respond in positive fashion. Obviously, strong recommendations are more likely to be universally accepted since the likelihood that they will be beneficial is stronger.
In any approach to therapy, the caveat primum non nocere—first, do no harm—must always be kept in mind.
“When I see a patient with arthritis coming in the front door, I leave by the back door.”
—Sir William Ostler
Fortunately for today’s physicians treating patients with osteoarthritis, we need not be as pessimistic as Osler was more than a century ago when he uttered his now-famous words. Still, there is no magic bullet for the contemporary clinician treating an elderly patient with osteoarthritis. Instead, there are many imperfect bullets, and choosing between them is always a balancing act between benefit and risk from various agents: nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics such as acetaminophen and tramadol, opioids, and supplements such as glucosamine and chondroitin sulfate.
So there was great interest when, in 2012,1 the American College of Rheumatology (ACR) updated its previous guidelines (from 2000) on drug and nondrug therapies for osteoarthritis of the hip and the knee2 and added new recommendations on osteoarthritis of the hand.
Revising the guidelines was appropriate, since new therapies have become available. But, as the guideline authors state, with osteoarthritis, as with other diseases, guidelines cannot be a “cookbook.”
The treatment approach differs depending on the patient’s clinical presentation and on the preferences of the patient and the physician. Often, more than one approach is possible, and more than one approach may be appropriate in a given patient at a given time. The guideline authors also point out that some physicians may disagree with some of the recommendations.
I wish to review here several of the key recommendations. But I also provide some of my personal perspective and experience after 4 decades of treating patients with osteoarthritis.
HOW THE GUIDELINES WERE MADE
The new ACR guidelines were developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, a formal process to develop recommendations that are as evidence-based as possible.3
The authors are outstanding experts in the field of osteoarthritis from throughout the United States and Canada. Further, the recommendations were voted on by a “technical expert panel” representing the fields of rheumatology, orthopedics, physical medicine, and rehabilitation, from both academic medicine and private practice. This representation provides a balance of input from the types of clinicians frequently involved in managing osteoarthritis.
The initial literature searches for drug therapies were conducted during late 2008, and those for nonpharmacologic treatments were conducted during the second and third quarters of 2009. The goal of the literature searches was to identify the most current systematic reviews and meta-analyses that would provide reliable estimates of benefits of intervention for the prespecified clinically relevant outcomes of pain and function, as well as data on safety.
Recommendations: For or against, strong or weak—and the informed patient’s perspective
Therapies received the following possible recommendations:
- Strong recommendation to use
- Weak (or conditional) recommendation to use
- No recommendation
- Weak (or conditional) recommendation not to use
- Strong recommendation not to use.
A strong recommendation required high-quality evidence and evidence of a large difference between desirable and undesirable effects of the treatment. A conditional recommendation was based on the absence of high-quality evidence, evidence of only a small difference between desirable and undesirable effects of the treatment, or both.
One interesting feature of these recommendations is that they took into account how informed patients might themselves evaluate the data with their medical condition.
For instance, if a therapy received a strong favorable recommendation, we can assume that most informed patients would choose to receive it, and we can shape our interaction with the patient accordingly. A conditional recommendation means that most informed patients would choose the treatment—but many would not, and physicians should keep the patient’s values and preferences in mind.
I admit I had a problem with the meaning of the word “conditional” in the context of these guidelines. When evaluating a treatment, the term “weak” is readily understood and clearer. By using the word “weak,” one is making a positive statement in support of use but letting you know that the data and recommendation are weak. The word “conditional” is less readily defined and does not necessarily imply support for use.
Recommendations were drafted after discussion of the evidence at each meeting of the technical expert panel. Consensus was defined as 75% or more of the members of the panel voting to either strongly or conditionally recommend using a therapy, to either strongly or conditionally recommend not using it, or to choose not to make a recommendation on its use.
OSTEOARTHRITIS OF THE HAND: NO STRONG RECOMMENDATIONS
The technical expert panel gave no strong recommendations for any nondrug or drug treatment for osteoarthritis of the hand.
Conditional recommendations for nondrug treatments
The panel conditionally recommended the following:
- All patients with osteoarthritis of the hand should be evaluated either by their primary physician or by an occupational or physical therapist, particularly with respect to ability to perform activities of daily living.
- Assistive devices such as jar openers, key turners, and pull tabs for zippers should be recommended, as needed.
- Patients should be instructed in joint protection and in the use of thermal treatments (eg, heating pads, ultrasound devices, hot packs, and ice packs).
Comments. Appliances are often beneficial in patients who have involvement of the first carpometacarpal (trapeziometacarpal) joint. Although over-the-counter thumb splints are an option, referral to an occupational therapist for splint prescription is advantageous to achieve a comfortable fit and, importantly, for instructions to the patient on how to avoid joint trauma.
It would be unrealistic to expect primary care physicians and internists to have the expertise to make detailed recommendations about orthopedic appliances. Accordingly, referral to an occupational therapist or an orthopedist is advisable for these situations. It is important, however, that the physician be aware of what treatments are available and most effective, and of the indications for referral.
As for heat treatment, elastic stretch gloves may relieve symptoms through their warming and massaging effects.4
Some drugs for hand osteoarthritis got conditional recommendations in favor
The expert panel gave conditional recommendations in favor of:
- Topical capsaicin
- Topical NSAIDs
- Oral NSAIDs (including both nonselective and selective agents)
- Tramadol (Ultram)
- Topical rather than oral NSAIDs for patients age 75 and older.
Other drug treatments got conditional recommendations against their use
The expert panel gave conditional recommendations against using:
- Intra-articular injections, and in particular, corticosteroid injections in the trapeziometacarpal (first carpometacarpal) joint
- Opioid analgesics
- Oral methotrexate or sulfasalazine in patients with erosive inflammatory interphalangeal osteoarthritis.
No recommendation for or against
- Hydroxychloroquine.
Comments—Intra-articular injections, opioids, and oral NSAIDs
I differ with these recommendations on several points.
Although the guidelines committee conditionally recommended against using intra-articular therapies for hand osteoarthritis, I find that intra-articular corticosteroid injections are often effective, particularly in patients who have inflammatory forms of the disease, ie, “erosive inflammatory osteoarthritis.” Most nonspecialist physicians probably have limited experience in giving injections into small joints, and referral to a rheumatologist or orthopedist would be appropriate.
I disagree as well with the conditional recommendation that intra-articular corticosteroid injections not be used for involvement of the trapeziometacarpal (first carpometacarpal) joint. I find that many patients with osteoarthritis of this joint experience improvement with intra-articular corticosteroid injections.
I agree that there are limited data on the use of intra-articular hyaluronan injections in this situation and do not routinely use them in this joint.
Opioid analgesics also received a conditional recommendation against their use. The same caveats apply here as for these drugs elsewhere.5 If used, opioids should be used at the lowest dose possible and for as short a time as possible. If the physician is uncomfortable prescribing opioids for patients with osteoarthritis, referral to a pain specialist is recommended.
I disagree to some extent with the conditional recommendation that people age 75 and older should use topical rather than oral NSAIDs. I understand the recommendation, given that older people have a higher frequency of gastrointestinal, renal, and cardiac disease and are best served by avoiding NSAIDs. However, we all see patients over age 75 who are physiologically younger than their numerical age. Accordingly, I feel that the judgment of the physician plays a role in whether NSAIDs are reasonable for some older patients.
The committee recommended not using oral methotrexate or sulfasalazine in patients with erosive inflammatory interphalangeal osteoarthritis. I have used oral hydroxychloroquine off-label in such patients and find that they respond in a very rewarding fashion.
Given that this is an off-label use of hydroxychloroquine, the drug should be used only with appropriate consideration and after discussion with the patient about toxicity, especially about the risk of ocular manifestations.
OSTEOARTHRITIS OF THE KNEE
Some nondrug therapies got strong recommendations
The expert panel strongly recommended:
- Exercise (aerobic, resistance, land-based, and aquatic)
- Weight loss (for patients who are overweight).
Other nondrug therapies got conditional recommendations
The panel conditionally recommended:
- Self-management programs
- Manual therapy in combination with supervised exercise
- Psychosocial interventions
- Medially directed patellar taping
- Medially wedged insoles (if the patient has lateral compartment osteoarthritis)
- Laterally wedged subtalar strapped insoles (if the patient has medial compartment osteoarthritis)
- Heat therapy
- Walking aids, as needed
- Tai chi
- Chinese acupuncture
- Transcutaneous electrical nerve stimulation.
Comments. The ACR panel appropriately noted that Chinese acupuncture or transcutaneous electrical stimulation should be recommended only if the patient has chronic moderate to severe pain and is a candidate for total knee arthroplasty but is unwilling to undergo the procedure or has comorbid medical conditions that rule out surgery.
Nondrug therapies for knee osteoarthritis that got no recommendation for or against
- Balance exercise
- Laterally wedged insoles
- Manual therapy alone
- Knee braces
- Laterally directed patellar taping.
Comments. It was somewhat surprising that there were no recommendations about laterally wedged insoles or knee braces. Laterally wedged insoles have been recommended for patients who have medial compartment knee osteoarthritis6; being thinner at the instep and thicker at the outer edge of the foot, they reduce load on the medial aspect of the knee. One has to be cautious in using knee wedging in patients who have concomitant ankle or hip angle deformities, lest these joints be compromised.
Some of these treatments would be out of the realm of the nonspecialist physician.
Conditional recommendations for initial drug therapy for knee osteoarthritis
The panel conditionally recommended that patients who have osteoarthritis of the knee use one of the following:
- Acetaminophen (contained in Tylenol and a host of other products)
- Oral NSAIDs
- Topical NSAIDs (with a strong recommendation for topical NSAIDs rather than oral NSAIDs in patients age 75 and older)
- Tramadol
- Intra-articular corticosteroid injections.
Comments. In the past, it was recommended that acetaminophen in full doses of up to 4,000 mg per day be considered.7 Current dogma, however, is that doses of acetaminophen should not exceed 3,000 mg per day to avoid damaging the liver. This concern led the US Food and Drug Administration (FDA) in 2011 to advise that the maximum daily dose be limited.8 The ACR panel recommended that patients be counseled to avoid all other products that contain acetaminophen, which is especially cogent, given the presence of this agent in many over-the-counter medications.9
The panel conditionally recommended that people age 75 and older use topical rather than oral NSAIDs. As mentioned earlier, a specific age limit does not take into account that many people age 75 and older may actually be physiologically younger than some in their 50s or 60s. Accordingly, it is recommended that the physician use judgment in this regard so that NSAIDs will not be denied to patients for whom they might be of significant value.
Strong recommendation for gastric protection in patients at risk on NSAIDs
If a patient with knee osteoarthritis has a history of a symptomatic or complicated upper gastrointestinal ulcer but has not had an upper gastrointestinal bleed in the past year and the physician chooses to prescribe an oral NSAID, the expert panel strongly recommended using either a cyclooxygenase (COX)-2-selective inhibitor or a nonselective NSAID in combination with a proton pump inhibitor.
Comment. The suggestion that patients who have had a complicated upper gastrointestinal ulcer in the past year could be considered for treatment with a COX-2-selective inhibitor or nonselective NSAID in combination with a proton pump inhibitor seemed a bit aggressive. My own inclination would be to avoid both nonselective and selective inhibitors in this situation. Alternative agents such as acetaminophen in full doses, tramadol, intra-articular hyaluronan injections, and intra-articular corticosteroid injections seem preferable with respect to safety in such patients.
The suggestion that a proton pump inhibitor be used whenever an NSAID is given for chronic management of knee or hip osteoarthritis is reasonable.10,11 Although some studies have suggested that chronic use of proton pump inhibitors may predispose to osteopenia or osteoporosis, others have not, and gastric protection should be considered in patients at gastrointestinal risk.
Strong recommendation against ibuprofen in patients taking aspirin
The ACR panel strongly recommended that ibuprofen (Advil) not be prescribed to patients with knee osteoarthritis who are using aspirin in low doses for cardioprotection, and strongly recommended using another nonselective NSAID plus a proton pump inhibitor instead. The panel also strongly recommended against using a COX-2-selective inhibitor in this situation.12,13
Comment. The rationale for these recommendations is that ibuprofen may render aspirin ineffective as a cardioprotective agent. Ibuprofen interferes with the aspirin-binding site on platelets, so that the protective effect of aspirin is lost.14,15 Celecoxib (Celebrex)16 and diclofenac (Voltaren) have binding sites different from that of aspirin, although the ACR recommends against using COX-2-selective inhibitors such as celecoxib in the situation and gives no recommendation about other NSAIDs.
No recommendations for or against
The panel issued no recommendations for or against the following treatments for patients with knee osteoarthritis:
- Intra-articular hyaluronan injections
- Duloxetine (Cymbalta)
- Opioid analgesics.
Comments on knee injections
Intra-articular injections of corticosteroids or hyaluronan are commonly used for knee osteoarthritis. As noted, corticosteroid injections received a conditional recommendation, while hyaluronan injections received no recommendation for or against.
How often to inject corticosteroids? In general, too-frequent injection of corticosteroids is to be avoided, in view of the risk of promoting joint breakdown. There is no “magic” number of injections that is safe, although more than 4 per year in the same joint should generally be avoided. In some situations, however, repeat injections may be reasonable if alternative therapies are associated with higher risk.
Raynauld et al,17 in a randomized, double-blind, placebo-controlled trial, demonstrated that intra-articular corticosteroid injections at 3-month intervals for 2 years were not deleterious to knees.
My philosophy is generally not to inject on a regular basis, but to be selective and be guided by the patient’s clinical condition and response to prior injections.
Are hyaluronan injections effective? Although experts differ in their enthusiasm for intra-articular hyaluronan injections in the knee, I have found that many patients benefit from this treatment. Multiple studies have found it efficacious and safe overall.18–21 However, some systematic reviews have called its efficacy into question.7
Although differences in efficacy have been noted, this therapy was approved as being useful in patients with knee osteoarthritis in the Osteoarthritis Research Society International (OARSI) recommendations.7 The effect sizes were smaller in later assessments.22
Hyaluronan injections do not pose the risk of joint breakdown that corticosteroid injections do, but their clinical efficacy is not as dramatic. Adverse reactions to most intraarticular hyaluronans are limited, with slight increases in pain and stiffness after injection. Significant inflammatory reactions characterized as “postinjection flares” are more commonly seen with high-molecular-weight crosslinked preparations. These reactions can be severe and can mimic joint infection clinically. Joint aspiration with synovial fluid analysis and culture may be necessary to exclude infection. Response to aspiration and nonsteroidal inflammatory agents or intra-articular corticosteroids is usually excellent.
Ultrasonographic guidance. As with intraarticular injections in other areas, ultrasonographic guidance is becoming more common, as it allows for more accurate drug administration.
Pes anserine bursitis must be ruled out as a cause of the patient’s knee symptoms—misdiagnosis is not uncommon. The bursa is located on the medial aspect of the tibia, and inflammation of the bursa is a common cause of pain in this area. Local steroid injection is extremely effective in symptomatic therapy. Physical therapy and NSAIDs may be adequate to treat milder cases.
Conditional recommendation against glucosamine, chondroitin, capsaicin
The ACR panel conditionally recommended that patients with knee osteoarthritis not use:
- Chondroitin sulfate
- Glucosamine
- Topical capsaicin.
Comment. Evidence is mixed about the efficacy of glucosamine and chondroitin sulfate, which are so-called nutraceuticals. Some studies found them useful23–25 but some did not,26 and a meta-analysis concluded that they do not help.27 The OARSI guidelines published in 2008 stated that these agents may relieve symptoms of osteoarthritis of the knee.7 The OARSI update published in 2010 found that glucosamine was effective, but less so than in previous studies.22 If glucosamine is effective, some studies suggest that glucosamine sulfate is more effective than glucosamine hydrochloride.22
The same OARSI review revealed that chondroitin sulfate relieved pain but with heterogeneous, dissimilar effect sizes. Of interest was the finding that the 5-year incidence of total knee replacement was lower in patients treated with glucosamine sulfate 1,500 mg/day than with placebo. Also, the rate of decline of joint space narrowing was reported to be reduced in chondroitin sulfate-treated patients.22
In practice, a conditional recommendation against a treatment means that most informed patients would not want the treatment, but some would. Accordingly, if patients still want to take chondroitin or glucosamine after being informed of the limited evidence of benefit, I feel a trial of their use is reasonable.
OSTEOARTHRITIS OF THE HIP
Indications for therapy of osteoarthritis of the hip are similar to those for osteoarthritis of the knee.
As in the knee, nonpharmacologic therapies are important. Loss of weight for overweight patients is extremely important; supervised exercise is especially valuable. Use of canes or crutches as needed is conditionally recommended.
Pharmacologic management is similar to that of osteoarthritis of the knee, with particular use of acetaminophen, NSAIDs, tramadol, and intra-articular corticosteroid injections.
Comment. Intra-articular injection of corticosteroids into the hip would be out of the realm of most nonspecialist practices. Although some rheumatologists are expert in such injections, this treatment is generally best left to an orthopedist or invasive radiologist. The use of ultrasonographic guidance is becoming more frequent, with many rheumatologists having developed expertise in this approach to the knee and the hip. Since most studies were in patients with osteoarthritis of the knee, fewer data are available as to the efficacy of these agents in patients with hip osteoarthritis.
Fewer data are available also with respect to the benefit of chondroitin sulfate and glucosamine in patients with osteoarthritis of the hip. Total joint replacement is extremely effective if conservative therapy does not help.
FIRST, DO NO HARM
Guidelines from the ACR,1,2 the European League Against Rheumatism (EULAR),28,29 the American Academy of Orthopedic Surgeons (AAOS),30 and the OARSI7,22 all differ somewhat, owing to the different evidence available at the time each guideline was developed and to different geographic and cultural backgrounds.
The compositions of these various panels also differ sufficiently to affect their overall recommendations. For example, the EULAR panel consisted of only rheumatologists and an orthopedic surgeon; for the hand osteoarthritis recommendations they added a physiatrist and two allied health professionals.28,29 The OARSI panel included two primary care physicians in addition to rheumatologists and an orthopedic surgeon.7 The ACR was the only professional society to include primary care physicians, physiatrists, and geriatricians along with rheumatologists, an orthopedic surgeon, and physical and occupational therapists.
Although it is to be expected that there will not be universal agreement on all points of management of osteoarthritis by diverse groups, it is essential that input from all these experts representing various subspecialties be recognized. Therapeutic approaches will vary depending on patient characteristics and the experience of the treating physician. As long as therapy is based on reasonable supportive data, beneficial effects can be anticipated. Therapies that received conditional recommendations are not to be discounted if a reasonable percent of patients respond in positive fashion. Obviously, strong recommendations are more likely to be universally accepted since the likelihood that they will be beneficial is stronger.
In any approach to therapy, the caveat primum non nocere—first, do no harm—must always be kept in mind.
- Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64:465–474.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000; 43:1905–1915.
- Atkins D, Best D, Briss PA, et al; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; 328:1490.
- Askari A, Moskowitz RW, Ryan C. Stretch gloves. A study of objective and subjective effectiveness in arthritis of the hands. Arthritis Rheum 1974; 17:263–265.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Fang MA, Taylor CE, Nouvong A, Masih S, Kao KC, Perell KL. Effects of footwear on medial compartment knee osteoarthritis. J Rehabil Res Dev 2006; 43:427–434.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008; 16:137–162.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure. January 13, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm. Accessed November 28, 2012.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med 2010; 77:19–27.
- Bolten WW. Rational use of nonsteroidal anti-inflammatory drugs and proton pump inhibitors in combination for rheumatic diseases. Orthopedic Research and Reviews 2010; 2:75–84.
- Graham DY, Agrawal NM, Campbell DR, et al; NSAID-Associated Gastric Ulcer Prevention Study Group. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med 2002; 162:169–175.
- American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Antiinflammatory Drugs. Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum 2008; 59:1058–1073.
- Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation 2007; 115:1634–1642.
- Ellison J, Dager W. Recent FDA warning of the concomitant use of aspirin and ibuprofen and the effects on platelet aggregation. Prev Cardiol 2007; 10:61–63.
- Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ. The interaction of ibuprofen and diclofenac with aspirin in healthy volunteers. Br J Pharmacol 2009; 157:931–934.
- Wilner KD, Rushing M, Walden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002; 42:1027–1030.
- Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003; 48:370–377.
- Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis 2012; 71:1454–1460.
- Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012; 26:257–268.
- Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2004; 86-A:538–545.
- Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Visco-supplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012; 157:180–191.
- Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010; 18:476–499.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treat-ment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007; 15:981–1000.
- Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005; 2:CD002946.
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum 2007; 56:2267–2277.
- Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006; 354:795–808.
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675.
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2005; 64:669–681.
- Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007; 66:377–388.
- American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (non-arthroplasty). Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
- Hochberg MC, Altman RD, April KT, et al; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64:465–474.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000; 43:1905–1915.
- Atkins D, Best D, Briss PA, et al; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; 328:1490.
- Askari A, Moskowitz RW, Ryan C. Stretch gloves. A study of objective and subjective effectiveness in arthritis of the hands. Arthritis Rheum 1974; 17:263–265.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- Fang MA, Taylor CE, Nouvong A, Masih S, Kao KC, Perell KL. Effects of footwear on medial compartment knee osteoarthritis. J Rehabil Res Dev 2006; 43:427–434.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008; 16:137–162.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure. January 13, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm. Accessed November 28, 2012.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med 2010; 77:19–27.
- Bolten WW. Rational use of nonsteroidal anti-inflammatory drugs and proton pump inhibitors in combination for rheumatic diseases. Orthopedic Research and Reviews 2010; 2:75–84.
- Graham DY, Agrawal NM, Campbell DR, et al; NSAID-Associated Gastric Ulcer Prevention Study Group. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med 2002; 162:169–175.
- American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Antiinflammatory Drugs. Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum 2008; 59:1058–1073.
- Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation 2007; 115:1634–1642.
- Ellison J, Dager W. Recent FDA warning of the concomitant use of aspirin and ibuprofen and the effects on platelet aggregation. Prev Cardiol 2007; 10:61–63.
- Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ. The interaction of ibuprofen and diclofenac with aspirin in healthy volunteers. Br J Pharmacol 2009; 157:931–934.
- Wilner KD, Rushing M, Walden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002; 42:1027–1030.
- Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003; 48:370–377.
- Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis 2012; 71:1454–1460.
- Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012; 26:257–268.
- Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2004; 86-A:538–545.
- Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Visco-supplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012; 157:180–191.
- Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010; 18:476–499.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treat-ment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007; 15:981–1000.
- Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005; 2:CD002946.
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum 2007; 56:2267–2277.
- Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006; 354:795–808.
- Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675.
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2005; 64:669–681.
- Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007; 66:377–388.
- American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (non-arthroplasty). Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
Correction: Androgen deficiency in older men
In an article in the November 2012 issue (McGill JJ, Shoskes DA, Sabanegh ES Jr. Androgen deficiency in older men: Indications, advantages, and pitfalls of testosterone replacement therapy. Cleve Clin J Med 2012; 79:797–806), the final sentence of the abstract was omitted. The missing sentence should read as follows: “This article reviews androgen decline in men, focusing on those over age 40, and covers symptoms, indications, contraindications, diagnosis, treatments, and the risks and benefits of treatment.” The online version of the article has been corrected.
In an article in the November 2012 issue (McGill JJ, Shoskes DA, Sabanegh ES Jr. Androgen deficiency in older men: Indications, advantages, and pitfalls of testosterone replacement therapy. Cleve Clin J Med 2012; 79:797–806), the final sentence of the abstract was omitted. The missing sentence should read as follows: “This article reviews androgen decline in men, focusing on those over age 40, and covers symptoms, indications, contraindications, diagnosis, treatments, and the risks and benefits of treatment.” The online version of the article has been corrected.
In an article in the November 2012 issue (McGill JJ, Shoskes DA, Sabanegh ES Jr. Androgen deficiency in older men: Indications, advantages, and pitfalls of testosterone replacement therapy. Cleve Clin J Med 2012; 79:797–806), the final sentence of the abstract was omitted. The missing sentence should read as follows: “This article reviews androgen decline in men, focusing on those over age 40, and covers symptoms, indications, contraindications, diagnosis, treatments, and the risks and benefits of treatment.” The online version of the article has been corrected.