User login
Psychoactive supplements: What to tell patients
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
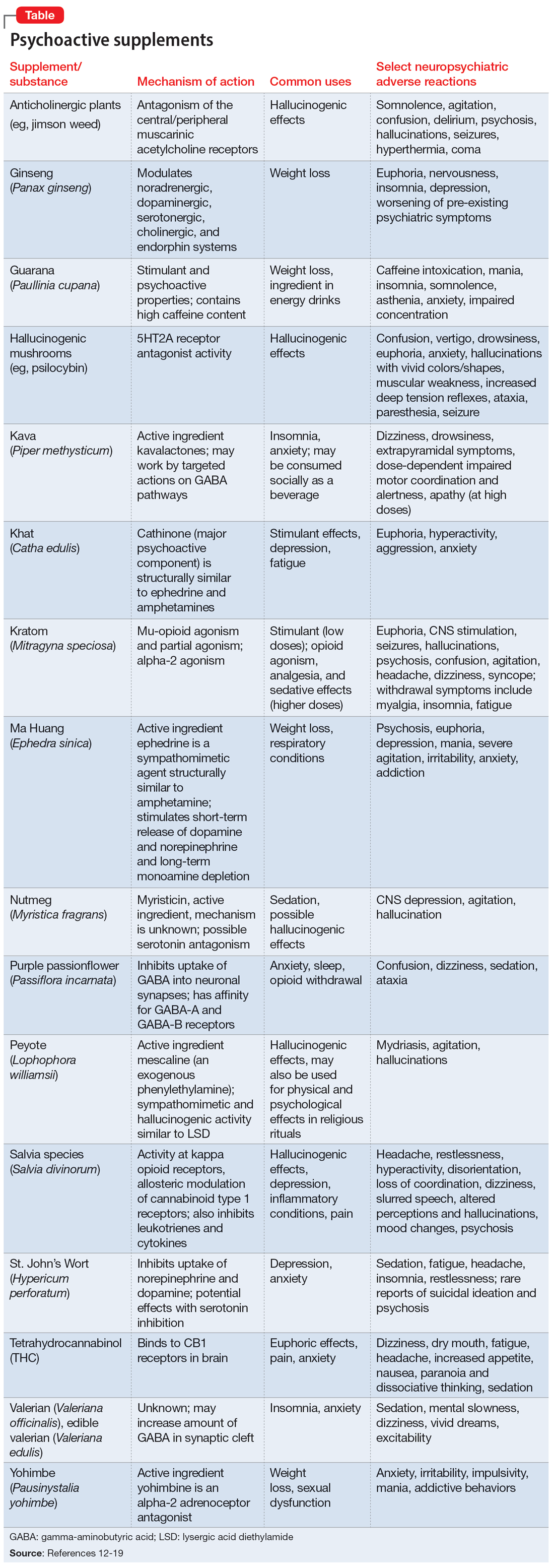
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20
The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20

The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20

The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
Increased anxiety and depression after menstruation
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
Prodromal symptoms of schizophrenia: What to look for
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
Generic stimulant shortage update: From bad to worse
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) just completed my first semester of medical school. An important lesson imparted in my coursework so far has been to remain a staunch advocate for patients. Yet compared to the rigors of medical school, over the past year it has been far more difficult to help patients locate generic Adderall. Physicians were already overburdened with administrative responsibilities stretching into burnout territory well before the shortage, and now this! Unlike paper prescriptions of old, which patients could take to any pharmacy, e-prescribing apps require selection of a specific pharmacy, and controlled substances such as stimulants require 2-factor authentication. But if the designated pharmacy does not have the medication in stock, the entire process must be repeated with an alternative pharmacy, long after the visit has concluded.
To add insult to injury, the generic stimulant shortage has grown even worse. As of February 2023, generic Adderall remained hard to find and generic Concerta was also in short supply. How did this happen? In 1985, Bulow et al¹ coined the game theory concept of “strategic substitutes,” where (for example) as beef becomes less readily accessible, consumers may switch to eating chicken as their protein. Unable to locate generic Adderall, many patients have turned to generic Concerta as a substitute psychostimulant to continue management of their attention-deficit/hyperactivity disorder.
In addition to the increase in demand, compounding the shortage is that one of the manufacturers of generic Concerta has discontinued production.² Branded methylphenidates and amphetamines, which are much more expensive than their generic counterparts, have remained in ample supply, but many insurers require trials of generics before considering coverage for more expensive brands.
Our approach to this situation
Each morning we call our local and chain pharmacies to take a census of their supply of generic stimulants. Some pharmacies refuse to release this information. Despite these census reports, we have found cases where patients have been turned away from pharmacies when they are not “regular customers,” while patients whom the pharmacies know retain access as “members.” Hence, a patient is unlikely to obtain these medications if their regular pharmacy is out of stock.
We want to share a workaround that has been effective. After unsuccessfully searching for generic stimulants at the patient’s regular pharmacy, I (RLP) write “dispense as written” for the closest branded version and file a prior authorization with the patient’s insurance company, noting “patient unable to trial any generic amphetamines or methylphenidates due to current nationwide shortage.” Even with the most difficult insurers, the response has been “a temporary 3-month authorization has been granted,” which is at least a small victory for our desperate patients and busy prescribers who are both struggling to negotiate a fragmented health care system.
1. Bulow JI, Geanakoplos JD, Klemperer PD. Multimarket oligopoly: strategic substitutes and complements. Journal of Political Economy. 1985;93(3):488-511. https://doi.org/10.1086/261312
2. US Food & Drug Administration. FDA Drug Shortages. Accessed January 7, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Methylphenidate+Hydrochloride+Extended+Release+Tablets&st=d
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) just completed my first semester of medical school. An important lesson imparted in my coursework so far has been to remain a staunch advocate for patients. Yet compared to the rigors of medical school, over the past year it has been far more difficult to help patients locate generic Adderall. Physicians were already overburdened with administrative responsibilities stretching into burnout territory well before the shortage, and now this! Unlike paper prescriptions of old, which patients could take to any pharmacy, e-prescribing apps require selection of a specific pharmacy, and controlled substances such as stimulants require 2-factor authentication. But if the designated pharmacy does not have the medication in stock, the entire process must be repeated with an alternative pharmacy, long after the visit has concluded.
To add insult to injury, the generic stimulant shortage has grown even worse. As of February 2023, generic Adderall remained hard to find and generic Concerta was also in short supply. How did this happen? In 1985, Bulow et al¹ coined the game theory concept of “strategic substitutes,” where (for example) as beef becomes less readily accessible, consumers may switch to eating chicken as their protein. Unable to locate generic Adderall, many patients have turned to generic Concerta as a substitute psychostimulant to continue management of their attention-deficit/hyperactivity disorder.
In addition to the increase in demand, compounding the shortage is that one of the manufacturers of generic Concerta has discontinued production.² Branded methylphenidates and amphetamines, which are much more expensive than their generic counterparts, have remained in ample supply, but many insurers require trials of generics before considering coverage for more expensive brands.
Our approach to this situation
Each morning we call our local and chain pharmacies to take a census of their supply of generic stimulants. Some pharmacies refuse to release this information. Despite these census reports, we have found cases where patients have been turned away from pharmacies when they are not “regular customers,” while patients whom the pharmacies know retain access as “members.” Hence, a patient is unlikely to obtain these medications if their regular pharmacy is out of stock.
We want to share a workaround that has been effective. After unsuccessfully searching for generic stimulants at the patient’s regular pharmacy, I (RLP) write “dispense as written” for the closest branded version and file a prior authorization with the patient’s insurance company, noting “patient unable to trial any generic amphetamines or methylphenidates due to current nationwide shortage.” Even with the most difficult insurers, the response has been “a temporary 3-month authorization has been granted,” which is at least a small victory for our desperate patients and busy prescribers who are both struggling to negotiate a fragmented health care system.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) just completed my first semester of medical school. An important lesson imparted in my coursework so far has been to remain a staunch advocate for patients. Yet compared to the rigors of medical school, over the past year it has been far more difficult to help patients locate generic Adderall. Physicians were already overburdened with administrative responsibilities stretching into burnout territory well before the shortage, and now this! Unlike paper prescriptions of old, which patients could take to any pharmacy, e-prescribing apps require selection of a specific pharmacy, and controlled substances such as stimulants require 2-factor authentication. But if the designated pharmacy does not have the medication in stock, the entire process must be repeated with an alternative pharmacy, long after the visit has concluded.
To add insult to injury, the generic stimulant shortage has grown even worse. As of February 2023, generic Adderall remained hard to find and generic Concerta was also in short supply. How did this happen? In 1985, Bulow et al¹ coined the game theory concept of “strategic substitutes,” where (for example) as beef becomes less readily accessible, consumers may switch to eating chicken as their protein. Unable to locate generic Adderall, many patients have turned to generic Concerta as a substitute psychostimulant to continue management of their attention-deficit/hyperactivity disorder.
In addition to the increase in demand, compounding the shortage is that one of the manufacturers of generic Concerta has discontinued production.² Branded methylphenidates and amphetamines, which are much more expensive than their generic counterparts, have remained in ample supply, but many insurers require trials of generics before considering coverage for more expensive brands.
Our approach to this situation
Each morning we call our local and chain pharmacies to take a census of their supply of generic stimulants. Some pharmacies refuse to release this information. Despite these census reports, we have found cases where patients have been turned away from pharmacies when they are not “regular customers,” while patients whom the pharmacies know retain access as “members.” Hence, a patient is unlikely to obtain these medications if their regular pharmacy is out of stock.
We want to share a workaround that has been effective. After unsuccessfully searching for generic stimulants at the patient’s regular pharmacy, I (RLP) write “dispense as written” for the closest branded version and file a prior authorization with the patient’s insurance company, noting “patient unable to trial any generic amphetamines or methylphenidates due to current nationwide shortage.” Even with the most difficult insurers, the response has been “a temporary 3-month authorization has been granted,” which is at least a small victory for our desperate patients and busy prescribers who are both struggling to negotiate a fragmented health care system.
1. Bulow JI, Geanakoplos JD, Klemperer PD. Multimarket oligopoly: strategic substitutes and complements. Journal of Political Economy. 1985;93(3):488-511. https://doi.org/10.1086/261312
2. US Food & Drug Administration. FDA Drug Shortages. Accessed January 7, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Methylphenidate+Hydrochloride+Extended+Release+Tablets&st=d
1. Bulow JI, Geanakoplos JD, Klemperer PD. Multimarket oligopoly: strategic substitutes and complements. Journal of Political Economy. 1985;93(3):488-511. https://doi.org/10.1086/261312
2. US Food & Drug Administration. FDA Drug Shortages. Accessed January 7, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Methylphenidate+Hydrochloride+Extended+Release+Tablets&st=d
Evolutions in endoscopy
Dear colleagues,
We continue our theme of highlighting innovations in gastroenterology by exploring how endoscopy continues to blur the lines with surgery. In this issue of Perspectives, Dr. RJ Sealock, assistant professor of medicine at the Baylor College of Medicine, and Dr. Thiru Muniraj, associate professor of medicine at the Yale School of Medicine share their experiences performing minimally invasive alternatives to surgery, discussing both sides of gastrointestinal perforations – treating and creating. Dr. Sealock describes how we can “MacGyver” traditional surgical wound vacs to treat Boerhaave's, while Dr. Muniraj shows how lumen-apposing metal stents allow us to treat acute cholecystitis in poor surgical candidates.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Endoscopic vacuum therapy for GI perforation
BY ROBERT JAY SEALOCK, MD
Gastrointestinal endoscopy has evolved from a diagnostic modality into a therapeutic tool used to treat a wide variety of luminal pathology. Endoscopic closure of full thickness injuries is a field that has rapidly expanded because of advanced endoscopic tissue resection and the need for subsequent defect closure as well as technological advances in closure devices such an endoscopic suturing platforms and large over-the-scope clips.
Prior to the advent of closure devices, endoscopic means of treating full thickness defects included through-the-scope (TTS) clips and fully covered metal stents. Given the small size, TTS clips are useful for mucosal closure but are limited in their ability to achieve full thickness closure. Fully covered metal stents utilized particularly for upper GI tract perforations and leaks are intended to divert gastrointestinal content away from the site of injury, thereby allowing secondary intention healing. Stents have several limitations, including frequent downstream migration and an inability to create a “watertight” seal in minimizing wound contamination. For decades, our surgical colleagues have utilized negative pressure wound therapy or vacuum therapy to expedite large wound closure. Given their familiarity with the technique, surgeons began adapting vacuum therapy for the treatment of postsurgical anastomotic leaks and fistulas particularly within the rectum.1 Eventually, the same technique was applied to the treatment of upper GI tract anastomotic leaks.2 Endoscopic vacuum therapy (EVT) overcomes many of the limitations of traditional endoscopic closure or diversion using covered stents through the use of suction to promote granulation tissue and aspirate infected wound contents.3
The approach to full thickness luminal injury must be individualized, but for a majority of indications EVT can be considered as a first-line approach. In our own experience, EVT closure can be achieved in more than 80% of patients with a variety of injuries such as iatrogenic endoscopic perforations (e.g., esophageal perforation during Savary dilation), surgical defects (sleeve gastrectomy leaks), and spontaneous perforations (e.g., Boerhaave syndrome). The initial step is endoscopic assessment of the luminal injury as well as the extraluminal cavity. In some situations, it is necessary to manually clean the defect cavity of necrotic material and food.
Once the cavity is cleaned and the size of the defect is assessed, the EVT device is manufactured at the bedside using commonly available materials and tools. A wound vacuum polyurethane sponge is affixed to a nasogastric tube, trimmed to the desired shape and size, and placed either within the defect cavity or within the GI lumen next to the defect opening.4 The EVT device is exchanged at an interval of 3-5 days, which allows the promotion of granulation tissue and subsequent downsizing as the cavity shrinks. In our series, an average number of five exchanges was necessary to achieve closure, with an average time to closure of 25 days.
Most experts would recommend initially placing the EVT device within the defect cavity. Once the cavity size can no longer accommodate the device, complete closure is achieved via intraluminal placement. The use of constant negative pressure (typically 150 mm to 175 mm Hg) prevents migration or dislodgement of the device.
For those who use EVT, there is some satisfaction from assembling and tailoring your own device, much like the protagonist in the 1980s television series “MacGyver,” who would manufacture devices out of readily available materials to address difficult and life-threatening situations. This need for self-assembly also has fostered ingenuity and creativity in the field, which can be found in social media and peer-reviewed sources.5 For some, however, the need to assemble your own device may be a deterrent. There is certainly an opportunity for commercialization and innovation, thereby putting Food and Drug Administration–approved devices into the hands of endoscopists. EVT is also a time- and labor-intensive therapy without specific reimbursement codes. Despite these limitations we continue to use and advocate for EVT given its clinical success in a population of patients with complex luminal injuries.
Dr. Sealock is assistant professor of medicine, department of gastroenterology and hepatology, Baylor College of Medicine, Houston. He receives research funding from AbbVie and is a consultant to ConMed and Ambu.
References
1. Weidenhagen R et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: A new method. Surg Endosc Other Interv Tech. 2008;22(8):1818-25. doi: 10.1007/s00464-007-9706-x.
2. Wedemeyer J et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2008;67(4):708-11. doi: 10.1016/j.gie.2007.10.064.
3. Mennigen R et al. Comparison of endoscopic vacuum therapy versus stent for anastomotic leak after esophagectomy. J Gastrointest Surg. 2015;19(7):1229-35.
4. Abdulsada M et al. Endoluminal vacuum therapy of esophageal perforations. VideoGIE. 2020;5(1):8-10. doi: 10.1016/j.vgie.2019.10.004
5. de Moura DTH et al. Cost-effective modified endoscopic vacuum therapy for the treatment of gastrointestinal transmural defects: Step-by-step process of manufacturing and its advantages. VideoGIE. 2021 Sep 4;6(12):523-8. doi: 10.1016/j.vgie.2021.08.002.
LAMS for gallbladder drainage
BY THIRU MUNIRAJ, MD, PHD, FACG, FRCP
Surgical cholecystectomy is the gold standard of treatment for acute cholecystitis (AC).1 The morbidity and mortality rates remain high in high-risk surgical patients, such as those with cirrhosis, coagulopathy, advanced malignancy, severe cardiopulmonary conditions, or poor performance status. Percutaneous gallbladder drainage (PT-GBD) typically has been performed as an alternative in these cases. Endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) is rapidly becoming a preferred alternative treatment to surgery in the case of AC at expert centers.
Since Baron and Topazian introduced EUS-GBD using a double pigtail stent in 2007, the procedure has evolved with the introduction of dedicated newly developed short, bi-flanged, covered lumen-apposing metal stents (LAMS) that have revolutionized this procedure as a single-step technique with excellent efficacy and safety outcomes. Although EUS-GBD is widely adopted among endosonographers, several skilled ERCP [endoscopic retrograde cholangiopancreatography] endoscopists still perform endoscopic transpapillary gallbladder drainage (ET-GBD) with ERCP as an alternative for high-risk surgical patients with AC. However, three-way comparative studies and randomized trials between PT-GBD, ETGBD, and EUS-GBD have clearly shown that EUS-GBD with LAMS is the most effective and safer alternative with the lowest rate of recurrent cholecystitis.2,3 The recent Tokyo Guidelines 2018 now suggest EUS-GBD as one of the viable options for AC treatment.4
In my institution, we offer EUS-GBD for nonsurgical candidates with AC with and without gallstones. In addition to its excellent benefits on quality of life through avoidance of an external percutaneous drain, EUS-GBD offers the ability to remove gallstones endoscopically using irrigation, suction, basket, and direct electrohydraulic lithotripsy. Moreover, EUS-GBD allows direct visualization and mucosal evaluation of the gallbladder when dysplasia or malignancy is suspected. The other indications where I perform EUS-GBD drainage are conversion of PT-GBD to EUS-GBD and as a backdoor alternate to failed ERCP where the cystic duct is patent and EUS-bile duct drainage is not amenable. In nonoperative malignant biliary stricture patients with indwelling metal biliary stents covering the cystic duct, I have a low threshold to perform a prophylactic EUS-GBD if the gallbladder is distended.
I perform EUS-GBD procedures under propofol intravenous anesthesia with the patient in the left lateral position on the fluoroscopy table. I choose the site to create the fistula for EUS-GBD either in the duodenal bulb or gastric antrum, whichever seems safer and easier to deploy the LAMS stent without torquing the endoscope much. In case of inadvertent complications such as stent maldeployment, the gastric site is often very forgiving. My preferred stent for EUS-GBD is 10 mm x 10 mm LAMS with hot cautery, as this seems to be the ideal size. We can choose a 10 mm x 15 mm stent if a larger stone removal is expected. I never choose smaller LAMS stents (6 mm and 8 mm), as the saddle length is not enough to bridge the thickened gallbladder wall and the thick gastric antral wall. In patients with calculous cholecystitis, I prefer to place a 7Fr 4cm pigtail plastic stent within the lumen of LAMS to ensure patency, especially if it is a gastric site, as food occlusion is more common. Unlike with pseudocyst drainage, these LAMS for EUS-GBD can be left indefinitely without removal. I avoid EUS-GBD in patients who have large-volume ascites or are too sick to tolerate anesthesia. Although a subsequent cholecystectomy post EUS-GBD is doable, I have a clear discussion with the surgeon before choosing this approach over ERCP ET-GBD in case future surgery is still an option. This is more important in patients who are awaiting liver transplantation.
The first step in establishing a program for EUS-GBD is to establish strong collaboration with your surgeons. In our institution, once our surgeons determine that patients with AC are high risk for surgery, they initiate a multidisciplinary discussion and reach out to advanced endoscopists at the same time or before consulting interventional radiology. The key to establishing a successful EUS-GBD program is to get “buy-in” from the surgeons and create a “signature” pathway for AC in your own institution.
EUS-GBD to drain the gallbladder in nonsurgical patients is one of my favorite procedures. Until the currently available LAMS secures an on-label indication for AC, we must wait and watch to see if there are enough advanced endoscopists ready to take over the challenge of all nonsurgical cholecystitis gallbladders – especially during late-night calls – rather than requesting PT-GBD. Soon, EUS-GBD will consign PT-GBD to centers without access to advanced endoscopists who perform EUS-guided interventions and limit ERCP transpapillary ET-GBD to patients with coagulopathy or large ascites.
Dr. Muniraj is associate professor of medicine, Yale School of Medicine, New Haven, Conn., and a consultant to Boston Scientific.
References
1. Endo I et al. Optimal treatment strategy for acute cholecystitis based on predictive factors: Japan-Taiwan multicenter cohort study. J Hepatobiliary Pancreat Sci. 2017. 24(6):346-61.
2. Siddiqui A et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an international, multicenter study. Surg Endosc. 2019;33(4):1260-70.
3. Teoh AYB et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut. 2020;69(6):1085-91.
4. Mori Y et al. Tokyo Guidelines 2018: Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):87-95.
Dear colleagues,
We continue our theme of highlighting innovations in gastroenterology by exploring how endoscopy continues to blur the lines with surgery. In this issue of Perspectives, Dr. RJ Sealock, assistant professor of medicine at the Baylor College of Medicine, and Dr. Thiru Muniraj, associate professor of medicine at the Yale School of Medicine share their experiences performing minimally invasive alternatives to surgery, discussing both sides of gastrointestinal perforations – treating and creating. Dr. Sealock describes how we can “MacGyver” traditional surgical wound vacs to treat Boerhaave's, while Dr. Muniraj shows how lumen-apposing metal stents allow us to treat acute cholecystitis in poor surgical candidates.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Endoscopic vacuum therapy for GI perforation
BY ROBERT JAY SEALOCK, MD
Gastrointestinal endoscopy has evolved from a diagnostic modality into a therapeutic tool used to treat a wide variety of luminal pathology. Endoscopic closure of full thickness injuries is a field that has rapidly expanded because of advanced endoscopic tissue resection and the need for subsequent defect closure as well as technological advances in closure devices such an endoscopic suturing platforms and large over-the-scope clips.
Prior to the advent of closure devices, endoscopic means of treating full thickness defects included through-the-scope (TTS) clips and fully covered metal stents. Given the small size, TTS clips are useful for mucosal closure but are limited in their ability to achieve full thickness closure. Fully covered metal stents utilized particularly for upper GI tract perforations and leaks are intended to divert gastrointestinal content away from the site of injury, thereby allowing secondary intention healing. Stents have several limitations, including frequent downstream migration and an inability to create a “watertight” seal in minimizing wound contamination. For decades, our surgical colleagues have utilized negative pressure wound therapy or vacuum therapy to expedite large wound closure. Given their familiarity with the technique, surgeons began adapting vacuum therapy for the treatment of postsurgical anastomotic leaks and fistulas particularly within the rectum.1 Eventually, the same technique was applied to the treatment of upper GI tract anastomotic leaks.2 Endoscopic vacuum therapy (EVT) overcomes many of the limitations of traditional endoscopic closure or diversion using covered stents through the use of suction to promote granulation tissue and aspirate infected wound contents.3
The approach to full thickness luminal injury must be individualized, but for a majority of indications EVT can be considered as a first-line approach. In our own experience, EVT closure can be achieved in more than 80% of patients with a variety of injuries such as iatrogenic endoscopic perforations (e.g., esophageal perforation during Savary dilation), surgical defects (sleeve gastrectomy leaks), and spontaneous perforations (e.g., Boerhaave syndrome). The initial step is endoscopic assessment of the luminal injury as well as the extraluminal cavity. In some situations, it is necessary to manually clean the defect cavity of necrotic material and food.
Once the cavity is cleaned and the size of the defect is assessed, the EVT device is manufactured at the bedside using commonly available materials and tools. A wound vacuum polyurethane sponge is affixed to a nasogastric tube, trimmed to the desired shape and size, and placed either within the defect cavity or within the GI lumen next to the defect opening.4 The EVT device is exchanged at an interval of 3-5 days, which allows the promotion of granulation tissue and subsequent downsizing as the cavity shrinks. In our series, an average number of five exchanges was necessary to achieve closure, with an average time to closure of 25 days.
Most experts would recommend initially placing the EVT device within the defect cavity. Once the cavity size can no longer accommodate the device, complete closure is achieved via intraluminal placement. The use of constant negative pressure (typically 150 mm to 175 mm Hg) prevents migration or dislodgement of the device.
For those who use EVT, there is some satisfaction from assembling and tailoring your own device, much like the protagonist in the 1980s television series “MacGyver,” who would manufacture devices out of readily available materials to address difficult and life-threatening situations. This need for self-assembly also has fostered ingenuity and creativity in the field, which can be found in social media and peer-reviewed sources.5 For some, however, the need to assemble your own device may be a deterrent. There is certainly an opportunity for commercialization and innovation, thereby putting Food and Drug Administration–approved devices into the hands of endoscopists. EVT is also a time- and labor-intensive therapy without specific reimbursement codes. Despite these limitations we continue to use and advocate for EVT given its clinical success in a population of patients with complex luminal injuries.
Dr. Sealock is assistant professor of medicine, department of gastroenterology and hepatology, Baylor College of Medicine, Houston. He receives research funding from AbbVie and is a consultant to ConMed and Ambu.
References
1. Weidenhagen R et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: A new method. Surg Endosc Other Interv Tech. 2008;22(8):1818-25. doi: 10.1007/s00464-007-9706-x.
2. Wedemeyer J et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2008;67(4):708-11. doi: 10.1016/j.gie.2007.10.064.
3. Mennigen R et al. Comparison of endoscopic vacuum therapy versus stent for anastomotic leak after esophagectomy. J Gastrointest Surg. 2015;19(7):1229-35.
4. Abdulsada M et al. Endoluminal vacuum therapy of esophageal perforations. VideoGIE. 2020;5(1):8-10. doi: 10.1016/j.vgie.2019.10.004
5. de Moura DTH et al. Cost-effective modified endoscopic vacuum therapy for the treatment of gastrointestinal transmural defects: Step-by-step process of manufacturing and its advantages. VideoGIE. 2021 Sep 4;6(12):523-8. doi: 10.1016/j.vgie.2021.08.002.
LAMS for gallbladder drainage
BY THIRU MUNIRAJ, MD, PHD, FACG, FRCP
Surgical cholecystectomy is the gold standard of treatment for acute cholecystitis (AC).1 The morbidity and mortality rates remain high in high-risk surgical patients, such as those with cirrhosis, coagulopathy, advanced malignancy, severe cardiopulmonary conditions, or poor performance status. Percutaneous gallbladder drainage (PT-GBD) typically has been performed as an alternative in these cases. Endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) is rapidly becoming a preferred alternative treatment to surgery in the case of AC at expert centers.
Since Baron and Topazian introduced EUS-GBD using a double pigtail stent in 2007, the procedure has evolved with the introduction of dedicated newly developed short, bi-flanged, covered lumen-apposing metal stents (LAMS) that have revolutionized this procedure as a single-step technique with excellent efficacy and safety outcomes. Although EUS-GBD is widely adopted among endosonographers, several skilled ERCP [endoscopic retrograde cholangiopancreatography] endoscopists still perform endoscopic transpapillary gallbladder drainage (ET-GBD) with ERCP as an alternative for high-risk surgical patients with AC. However, three-way comparative studies and randomized trials between PT-GBD, ETGBD, and EUS-GBD have clearly shown that EUS-GBD with LAMS is the most effective and safer alternative with the lowest rate of recurrent cholecystitis.2,3 The recent Tokyo Guidelines 2018 now suggest EUS-GBD as one of the viable options for AC treatment.4
In my institution, we offer EUS-GBD for nonsurgical candidates with AC with and without gallstones. In addition to its excellent benefits on quality of life through avoidance of an external percutaneous drain, EUS-GBD offers the ability to remove gallstones endoscopically using irrigation, suction, basket, and direct electrohydraulic lithotripsy. Moreover, EUS-GBD allows direct visualization and mucosal evaluation of the gallbladder when dysplasia or malignancy is suspected. The other indications where I perform EUS-GBD drainage are conversion of PT-GBD to EUS-GBD and as a backdoor alternate to failed ERCP where the cystic duct is patent and EUS-bile duct drainage is not amenable. In nonoperative malignant biliary stricture patients with indwelling metal biliary stents covering the cystic duct, I have a low threshold to perform a prophylactic EUS-GBD if the gallbladder is distended.
I perform EUS-GBD procedures under propofol intravenous anesthesia with the patient in the left lateral position on the fluoroscopy table. I choose the site to create the fistula for EUS-GBD either in the duodenal bulb or gastric antrum, whichever seems safer and easier to deploy the LAMS stent without torquing the endoscope much. In case of inadvertent complications such as stent maldeployment, the gastric site is often very forgiving. My preferred stent for EUS-GBD is 10 mm x 10 mm LAMS with hot cautery, as this seems to be the ideal size. We can choose a 10 mm x 15 mm stent if a larger stone removal is expected. I never choose smaller LAMS stents (6 mm and 8 mm), as the saddle length is not enough to bridge the thickened gallbladder wall and the thick gastric antral wall. In patients with calculous cholecystitis, I prefer to place a 7Fr 4cm pigtail plastic stent within the lumen of LAMS to ensure patency, especially if it is a gastric site, as food occlusion is more common. Unlike with pseudocyst drainage, these LAMS for EUS-GBD can be left indefinitely without removal. I avoid EUS-GBD in patients who have large-volume ascites or are too sick to tolerate anesthesia. Although a subsequent cholecystectomy post EUS-GBD is doable, I have a clear discussion with the surgeon before choosing this approach over ERCP ET-GBD in case future surgery is still an option. This is more important in patients who are awaiting liver transplantation.
The first step in establishing a program for EUS-GBD is to establish strong collaboration with your surgeons. In our institution, once our surgeons determine that patients with AC are high risk for surgery, they initiate a multidisciplinary discussion and reach out to advanced endoscopists at the same time or before consulting interventional radiology. The key to establishing a successful EUS-GBD program is to get “buy-in” from the surgeons and create a “signature” pathway for AC in your own institution.
EUS-GBD to drain the gallbladder in nonsurgical patients is one of my favorite procedures. Until the currently available LAMS secures an on-label indication for AC, we must wait and watch to see if there are enough advanced endoscopists ready to take over the challenge of all nonsurgical cholecystitis gallbladders – especially during late-night calls – rather than requesting PT-GBD. Soon, EUS-GBD will consign PT-GBD to centers without access to advanced endoscopists who perform EUS-guided interventions and limit ERCP transpapillary ET-GBD to patients with coagulopathy or large ascites.
Dr. Muniraj is associate professor of medicine, Yale School of Medicine, New Haven, Conn., and a consultant to Boston Scientific.
References
1. Endo I et al. Optimal treatment strategy for acute cholecystitis based on predictive factors: Japan-Taiwan multicenter cohort study. J Hepatobiliary Pancreat Sci. 2017. 24(6):346-61.
2. Siddiqui A et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an international, multicenter study. Surg Endosc. 2019;33(4):1260-70.
3. Teoh AYB et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut. 2020;69(6):1085-91.
4. Mori Y et al. Tokyo Guidelines 2018: Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):87-95.
Dear colleagues,
We continue our theme of highlighting innovations in gastroenterology by exploring how endoscopy continues to blur the lines with surgery. In this issue of Perspectives, Dr. RJ Sealock, assistant professor of medicine at the Baylor College of Medicine, and Dr. Thiru Muniraj, associate professor of medicine at the Yale School of Medicine share their experiences performing minimally invasive alternatives to surgery, discussing both sides of gastrointestinal perforations – treating and creating. Dr. Sealock describes how we can “MacGyver” traditional surgical wound vacs to treat Boerhaave's, while Dr. Muniraj shows how lumen-apposing metal stents allow us to treat acute cholecystitis in poor surgical candidates.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Endoscopic vacuum therapy for GI perforation
BY ROBERT JAY SEALOCK, MD
Gastrointestinal endoscopy has evolved from a diagnostic modality into a therapeutic tool used to treat a wide variety of luminal pathology. Endoscopic closure of full thickness injuries is a field that has rapidly expanded because of advanced endoscopic tissue resection and the need for subsequent defect closure as well as technological advances in closure devices such an endoscopic suturing platforms and large over-the-scope clips.
Prior to the advent of closure devices, endoscopic means of treating full thickness defects included through-the-scope (TTS) clips and fully covered metal stents. Given the small size, TTS clips are useful for mucosal closure but are limited in their ability to achieve full thickness closure. Fully covered metal stents utilized particularly for upper GI tract perforations and leaks are intended to divert gastrointestinal content away from the site of injury, thereby allowing secondary intention healing. Stents have several limitations, including frequent downstream migration and an inability to create a “watertight” seal in minimizing wound contamination. For decades, our surgical colleagues have utilized negative pressure wound therapy or vacuum therapy to expedite large wound closure. Given their familiarity with the technique, surgeons began adapting vacuum therapy for the treatment of postsurgical anastomotic leaks and fistulas particularly within the rectum.1 Eventually, the same technique was applied to the treatment of upper GI tract anastomotic leaks.2 Endoscopic vacuum therapy (EVT) overcomes many of the limitations of traditional endoscopic closure or diversion using covered stents through the use of suction to promote granulation tissue and aspirate infected wound contents.3
The approach to full thickness luminal injury must be individualized, but for a majority of indications EVT can be considered as a first-line approach. In our own experience, EVT closure can be achieved in more than 80% of patients with a variety of injuries such as iatrogenic endoscopic perforations (e.g., esophageal perforation during Savary dilation), surgical defects (sleeve gastrectomy leaks), and spontaneous perforations (e.g., Boerhaave syndrome). The initial step is endoscopic assessment of the luminal injury as well as the extraluminal cavity. In some situations, it is necessary to manually clean the defect cavity of necrotic material and food.
Once the cavity is cleaned and the size of the defect is assessed, the EVT device is manufactured at the bedside using commonly available materials and tools. A wound vacuum polyurethane sponge is affixed to a nasogastric tube, trimmed to the desired shape and size, and placed either within the defect cavity or within the GI lumen next to the defect opening.4 The EVT device is exchanged at an interval of 3-5 days, which allows the promotion of granulation tissue and subsequent downsizing as the cavity shrinks. In our series, an average number of five exchanges was necessary to achieve closure, with an average time to closure of 25 days.
Most experts would recommend initially placing the EVT device within the defect cavity. Once the cavity size can no longer accommodate the device, complete closure is achieved via intraluminal placement. The use of constant negative pressure (typically 150 mm to 175 mm Hg) prevents migration or dislodgement of the device.
For those who use EVT, there is some satisfaction from assembling and tailoring your own device, much like the protagonist in the 1980s television series “MacGyver,” who would manufacture devices out of readily available materials to address difficult and life-threatening situations. This need for self-assembly also has fostered ingenuity and creativity in the field, which can be found in social media and peer-reviewed sources.5 For some, however, the need to assemble your own device may be a deterrent. There is certainly an opportunity for commercialization and innovation, thereby putting Food and Drug Administration–approved devices into the hands of endoscopists. EVT is also a time- and labor-intensive therapy without specific reimbursement codes. Despite these limitations we continue to use and advocate for EVT given its clinical success in a population of patients with complex luminal injuries.
Dr. Sealock is assistant professor of medicine, department of gastroenterology and hepatology, Baylor College of Medicine, Houston. He receives research funding from AbbVie and is a consultant to ConMed and Ambu.
References
1. Weidenhagen R et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: A new method. Surg Endosc Other Interv Tech. 2008;22(8):1818-25. doi: 10.1007/s00464-007-9706-x.
2. Wedemeyer J et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc. 2008;67(4):708-11. doi: 10.1016/j.gie.2007.10.064.
3. Mennigen R et al. Comparison of endoscopic vacuum therapy versus stent for anastomotic leak after esophagectomy. J Gastrointest Surg. 2015;19(7):1229-35.
4. Abdulsada M et al. Endoluminal vacuum therapy of esophageal perforations. VideoGIE. 2020;5(1):8-10. doi: 10.1016/j.vgie.2019.10.004
5. de Moura DTH et al. Cost-effective modified endoscopic vacuum therapy for the treatment of gastrointestinal transmural defects: Step-by-step process of manufacturing and its advantages. VideoGIE. 2021 Sep 4;6(12):523-8. doi: 10.1016/j.vgie.2021.08.002.
LAMS for gallbladder drainage
BY THIRU MUNIRAJ, MD, PHD, FACG, FRCP
Surgical cholecystectomy is the gold standard of treatment for acute cholecystitis (AC).1 The morbidity and mortality rates remain high in high-risk surgical patients, such as those with cirrhosis, coagulopathy, advanced malignancy, severe cardiopulmonary conditions, or poor performance status. Percutaneous gallbladder drainage (PT-GBD) typically has been performed as an alternative in these cases. Endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) is rapidly becoming a preferred alternative treatment to surgery in the case of AC at expert centers.
Since Baron and Topazian introduced EUS-GBD using a double pigtail stent in 2007, the procedure has evolved with the introduction of dedicated newly developed short, bi-flanged, covered lumen-apposing metal stents (LAMS) that have revolutionized this procedure as a single-step technique with excellent efficacy and safety outcomes. Although EUS-GBD is widely adopted among endosonographers, several skilled ERCP [endoscopic retrograde cholangiopancreatography] endoscopists still perform endoscopic transpapillary gallbladder drainage (ET-GBD) with ERCP as an alternative for high-risk surgical patients with AC. However, three-way comparative studies and randomized trials between PT-GBD, ETGBD, and EUS-GBD have clearly shown that EUS-GBD with LAMS is the most effective and safer alternative with the lowest rate of recurrent cholecystitis.2,3 The recent Tokyo Guidelines 2018 now suggest EUS-GBD as one of the viable options for AC treatment.4
In my institution, we offer EUS-GBD for nonsurgical candidates with AC with and without gallstones. In addition to its excellent benefits on quality of life through avoidance of an external percutaneous drain, EUS-GBD offers the ability to remove gallstones endoscopically using irrigation, suction, basket, and direct electrohydraulic lithotripsy. Moreover, EUS-GBD allows direct visualization and mucosal evaluation of the gallbladder when dysplasia or malignancy is suspected. The other indications where I perform EUS-GBD drainage are conversion of PT-GBD to EUS-GBD and as a backdoor alternate to failed ERCP where the cystic duct is patent and EUS-bile duct drainage is not amenable. In nonoperative malignant biliary stricture patients with indwelling metal biliary stents covering the cystic duct, I have a low threshold to perform a prophylactic EUS-GBD if the gallbladder is distended.
I perform EUS-GBD procedures under propofol intravenous anesthesia with the patient in the left lateral position on the fluoroscopy table. I choose the site to create the fistula for EUS-GBD either in the duodenal bulb or gastric antrum, whichever seems safer and easier to deploy the LAMS stent without torquing the endoscope much. In case of inadvertent complications such as stent maldeployment, the gastric site is often very forgiving. My preferred stent for EUS-GBD is 10 mm x 10 mm LAMS with hot cautery, as this seems to be the ideal size. We can choose a 10 mm x 15 mm stent if a larger stone removal is expected. I never choose smaller LAMS stents (6 mm and 8 mm), as the saddle length is not enough to bridge the thickened gallbladder wall and the thick gastric antral wall. In patients with calculous cholecystitis, I prefer to place a 7Fr 4cm pigtail plastic stent within the lumen of LAMS to ensure patency, especially if it is a gastric site, as food occlusion is more common. Unlike with pseudocyst drainage, these LAMS for EUS-GBD can be left indefinitely without removal. I avoid EUS-GBD in patients who have large-volume ascites or are too sick to tolerate anesthesia. Although a subsequent cholecystectomy post EUS-GBD is doable, I have a clear discussion with the surgeon before choosing this approach over ERCP ET-GBD in case future surgery is still an option. This is more important in patients who are awaiting liver transplantation.
The first step in establishing a program for EUS-GBD is to establish strong collaboration with your surgeons. In our institution, once our surgeons determine that patients with AC are high risk for surgery, they initiate a multidisciplinary discussion and reach out to advanced endoscopists at the same time or before consulting interventional radiology. The key to establishing a successful EUS-GBD program is to get “buy-in” from the surgeons and create a “signature” pathway for AC in your own institution.
EUS-GBD to drain the gallbladder in nonsurgical patients is one of my favorite procedures. Until the currently available LAMS secures an on-label indication for AC, we must wait and watch to see if there are enough advanced endoscopists ready to take over the challenge of all nonsurgical cholecystitis gallbladders – especially during late-night calls – rather than requesting PT-GBD. Soon, EUS-GBD will consign PT-GBD to centers without access to advanced endoscopists who perform EUS-guided interventions and limit ERCP transpapillary ET-GBD to patients with coagulopathy or large ascites.
Dr. Muniraj is associate professor of medicine, Yale School of Medicine, New Haven, Conn., and a consultant to Boston Scientific.
References
1. Endo I et al. Optimal treatment strategy for acute cholecystitis based on predictive factors: Japan-Taiwan multicenter cohort study. J Hepatobiliary Pancreat Sci. 2017. 24(6):346-61.
2. Siddiqui A et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an international, multicenter study. Surg Endosc. 2019;33(4):1260-70.
3. Teoh AYB et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut. 2020;69(6):1085-91.
4. Mori Y et al. Tokyo Guidelines 2018: Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):87-95.
Enhancing CRC awareness and screening uptake
Each March, we celebrate National Colorectal Cancer Awareness Month to raise awareness of this common, deadly, and preventable form of cancer and advocate for increased screening uptake and investment in related research. Enhancing awareness is particularly important for those estimated 20 million average-risk individuals between the ages of 45 and 49 who became newly eligible for screening under the revised 2021 U.S. Preventive Services Task Force CRC screening guidelines, given alarming increases in early-onset CRC incidence. But as we know, awareness of CRC and screening eligibility alone is not enough to improve outcomes without addressing the many other patient, provider, and system-level barriers to screening uptake. Indeed, even before health care delivery disruptions related to the COVID-19 pandemic, CRC screening was underutilized, and inequities in screening uptake and downstream outcomes existed.
While there is not space here for a full discussion of these important topics, I refer you to our Gastroenterology Data Trends 2022 supplement (https://cdn.mdedge.com/files/s3fs-public/aga_data_trends_2022_web.pdf), which includes two excellent articles by Dr. Rachel Issaka of the University of Washington (“The Impact of COVID-19 on Colorectal Cancer Screening Programs”) and Dr. Aasma Shaukat of NYU (“Early Onset Colorectal Cancer: Trends in Incidence and Screening”).
In our March issue, we highlight the AGA’s decade-long advocacy efforts to close the “colonoscopy loophole” and reduce financial barriers to colorectal cancer screening. From AGA’s flagship journals, we report on the first Delphi-based consensus recommendations on early-onset colorectal cancer and highlight a study out of Italy comparing two computer-aided optical diagnosis systems for detection of small, leave-in-situ colon polyps. In our March Member Spotlight, we introduce you to gastroenterologist Christina Tennyson, MD, who shares the rewards and challenges of practicing gastroenterology in a rural area and explains how she incorporates “lifestyle medicine” into her clinical practice. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column on endoscopic innovation in management of GI perforation and acute cholecystitis.
We hope you enjoy these stories and all the exciting content featured in our March issue!
Megan A. Adams, MD, JD, MSc
Each March, we celebrate National Colorectal Cancer Awareness Month to raise awareness of this common, deadly, and preventable form of cancer and advocate for increased screening uptake and investment in related research. Enhancing awareness is particularly important for those estimated 20 million average-risk individuals between the ages of 45 and 49 who became newly eligible for screening under the revised 2021 U.S. Preventive Services Task Force CRC screening guidelines, given alarming increases in early-onset CRC incidence. But as we know, awareness of CRC and screening eligibility alone is not enough to improve outcomes without addressing the many other patient, provider, and system-level barriers to screening uptake. Indeed, even before health care delivery disruptions related to the COVID-19 pandemic, CRC screening was underutilized, and inequities in screening uptake and downstream outcomes existed.
While there is not space here for a full discussion of these important topics, I refer you to our Gastroenterology Data Trends 2022 supplement (https://cdn.mdedge.com/files/s3fs-public/aga_data_trends_2022_web.pdf), which includes two excellent articles by Dr. Rachel Issaka of the University of Washington (“The Impact of COVID-19 on Colorectal Cancer Screening Programs”) and Dr. Aasma Shaukat of NYU (“Early Onset Colorectal Cancer: Trends in Incidence and Screening”).
In our March issue, we highlight the AGA’s decade-long advocacy efforts to close the “colonoscopy loophole” and reduce financial barriers to colorectal cancer screening. From AGA’s flagship journals, we report on the first Delphi-based consensus recommendations on early-onset colorectal cancer and highlight a study out of Italy comparing two computer-aided optical diagnosis systems for detection of small, leave-in-situ colon polyps. In our March Member Spotlight, we introduce you to gastroenterologist Christina Tennyson, MD, who shares the rewards and challenges of practicing gastroenterology in a rural area and explains how she incorporates “lifestyle medicine” into her clinical practice. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column on endoscopic innovation in management of GI perforation and acute cholecystitis.
We hope you enjoy these stories and all the exciting content featured in our March issue!
Megan A. Adams, MD, JD, MSc
Each March, we celebrate National Colorectal Cancer Awareness Month to raise awareness of this common, deadly, and preventable form of cancer and advocate for increased screening uptake and investment in related research. Enhancing awareness is particularly important for those estimated 20 million average-risk individuals between the ages of 45 and 49 who became newly eligible for screening under the revised 2021 U.S. Preventive Services Task Force CRC screening guidelines, given alarming increases in early-onset CRC incidence. But as we know, awareness of CRC and screening eligibility alone is not enough to improve outcomes without addressing the many other patient, provider, and system-level barriers to screening uptake. Indeed, even before health care delivery disruptions related to the COVID-19 pandemic, CRC screening was underutilized, and inequities in screening uptake and downstream outcomes existed.
While there is not space here for a full discussion of these important topics, I refer you to our Gastroenterology Data Trends 2022 supplement (https://cdn.mdedge.com/files/s3fs-public/aga_data_trends_2022_web.pdf), which includes two excellent articles by Dr. Rachel Issaka of the University of Washington (“The Impact of COVID-19 on Colorectal Cancer Screening Programs”) and Dr. Aasma Shaukat of NYU (“Early Onset Colorectal Cancer: Trends in Incidence and Screening”).
In our March issue, we highlight the AGA’s decade-long advocacy efforts to close the “colonoscopy loophole” and reduce financial barriers to colorectal cancer screening. From AGA’s flagship journals, we report on the first Delphi-based consensus recommendations on early-onset colorectal cancer and highlight a study out of Italy comparing two computer-aided optical diagnosis systems for detection of small, leave-in-situ colon polyps. In our March Member Spotlight, we introduce you to gastroenterologist Christina Tennyson, MD, who shares the rewards and challenges of practicing gastroenterology in a rural area and explains how she incorporates “lifestyle medicine” into her clinical practice. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column on endoscopic innovation in management of GI perforation and acute cholecystitis.
We hope you enjoy these stories and all the exciting content featured in our March issue!
Megan A. Adams, MD, JD, MSc
The Evolving Role for Transplantation in Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) has served as a paradigm of progress among the non-Hodgkin lymphomas over the past 30 years. It was originally defined within the Kiel classification as centrocytic lymphoma, then renamed MCL once the characteristic translocation and resulting cyclin D1 overexpression were identified. These diagnostic markers allowed for the characterization of MCL subtypes as well as the initiation of MCL-focused clinical trials which, in turn, led to regulatory approval of more effective regimens, new therapeutic agents, and an improvement in overall survival (OS) from around 3 years to more than 10 years for many patients.
Despite this progress, virtually all patients relapse, and a cure remains elusive for most. In younger (< 65 to 70 years), medically-fit patients who are transplant-eligible and have symptomatic MCL, a standard of care has been induction chemoimmunotherapy containing high-dose cytarabine followed by ASCT consolidation. For example, a clinical trial of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) alternating with R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin; 3 cycles each) showed a significant benefit over R-CHOP x 6 cycles; at a median follow-up of 10.6 years, the time-to-treatment failure was 8.4 v 3.9 years. In another trial, all patients received induction R-DHAP (with cisplatin or an alternative platinum agent) x 4 cycles followed by ASCT. Those patients randomized to post-ASCT maintenance rituximab for 3 years had significantly improved, 4-year progression-free survival (PFS) as compared with observation only (83% vs 64%, p < 0.001); maintenance also significantly improved OS.
Although ASCT consolidation followed by maintenance became widely adopted on the basis of these and other clinical trials, important questions remain:
First, MCL is biologically and clinically quite heterogeneous. Several prognostic tools such as the MCL International Prognostic Index (MIPI) scoring system and biomarkers are available to define lower- versus higher-risk subtypes, but none is routinely used for treatment planning. About 15% of MCL patients present with a highly-aggressive blastoid or pleomorphic variant that usually carries a TP53 mutation or deletion. Given the short survival and limited benefit from dose-intensive chemotherapy and ASCT in TP53-mutated MCL, should transplant be avoided in these patients?
Second, if deep remission is achieved following front-line therapy, defined as positron emission tomography (PET) negative and measurable residual disease (MRD) negative, will high-dose chemotherapy and ASCT provide additional benefits or only toxicity? This question is being addressed by the ongoing ECOG 4151 study, a risk-adapted trial in which post-induction MRD-negative patients are randomized to standard ASCT consolidation plus maintenance rituximab vs maintenance only.
Bruton tyrosine kinase inhibitors (BTKi) are now among the most used agents for relapsed MCL. Recent clinical trials testing the integration of a BTKi into first- or second-line therapy have shown increased response rates and variable clinical outcomes and toxicities for the combinations, depending upon the chemotherapy- and non-chemotherapy backbones utilized, as well as the BTKi. Combinations with the BCL2 inhibitor venetoclax plus chemotherapy or BTKi are also showing promise.
The activity of BTKi in MCL led the European MCL Network (EMCL) to design the 3-arm TRIANGLE study to analyze the potential of ibrutinib to improve outcomes when given in conjunction with standard ASCT consolidation, and the ability to replace the need for ASCT. The TRIANGLE results were presented by Dr. Martin Dreyling in the Plenary Session at the December 2022 American Society of Hematology (ASH) Annual Meeting. Transplant-eligible MCL patients < 65 years of age were randomized to the EMCL’s established front-line therapy of alternating R-CHOP/R-DHAP plus ASCT; the same regimen plus oral ibrutinib given with the R-CHOP induction cycles and then post-ASCT ibrutinib maintenance therapy for 2 years (Arm A+I); or the A+I regimen minus ASCT (Arm I). Maintenance rituximab was allowed in each arm, on the basis of the treating centers’ institutional guidelines. Overall, 54%-58% of patients in each study arm received rituximab maintenance, with no differential benefit in efficacy noted for those so treated.
The results showed that 94%-98% of patients responded by the end of induction (defined as R-chemo and ASCT), with complete remissions in 36%-45% (from computerized tomography imaging, not PET scan). With a median follow-up of 31 months, failure-free survival (FFS; the primary study endpoint) was significantly improved for A+I vs A (3 year FFS of 88% vs 72%, respectively; p = 0.0008). In a subgroup analysis, FFS was notably improved for A+I in patients with high-level TP53 overexpression by immunohistochemistry. Toxicity did not differ during the induction and ASCT periods among the 3 arms regarding cytopenia, gastrointestinal disorders, and infections. However, neutropenia and infections were increased in the ibrutinib-containing arms during maintenance therapy—especially for Arm A+I.
The authors concluded that ASCT plus ibrutinib (Arm A+I) is superior to ASCT only (Arm A), and that Arm A is not superior to ibrutinib without ASCT (Arm I). No decision can yet be made regarding A+I versus I for which FFS to date remains very similar; however, the authors favor ibrutinib without ASCT due to lower toxicity. OS is trending to favor the ibrutinib arms, but longer follow-up will be needed to fully assess.
Should ASCT consolidation now be replaced by ibrutinib-containing induction R-CHOP/R-DHAP and maintenance ibrutinib, with or without maintenance rituximab? A definitive answer will require the fully-published TRIANGLE results, as well as ongoing analysis with longer follow-up. However, it seems very likely that ASCT indeed will be replaced by the new approach. TP53-mutated MCL should be treated with ibrutinib plus R-CHOP/R-DHAP and ibrutinib maintenance as validated in this trial.
Many centers have begun using a second-generation BTKi, acalabrutinib or zanubrutinib, rather than ibrutinib due to equivalent response rates with more favorable side effect profiles and fewer treatment discontinuations. Caution is warranted regarding simply adding a BTKi to one’s favored MCL induction regimen and foregoing ASCT—pending additional studies and the safety of such alternative approaches.
These are indeed exciting times of therapeutic progress, as they have been improving outcomes and providing longer survival outcomes for MCL patients. Targeted agents facilitate this shift to less intensive and chemotherapy-free regimens that provide enhanced response and mitigate short- and longer-term toxicities. More results will be forthcoming for MRD as a treatment endpoint, guiding maintenance therapy, and for risk-adapted treatment of newly-diagnosed and relapsing patients (based upon MCL subtype and biomarker profiles). Enrolling patients into clinical trials is strongly encouraged as the best mechanism to help answer emerging questions in the field and open the pathway to continued progress.
Mantle cell lymphoma (MCL) has served as a paradigm of progress among the non-Hodgkin lymphomas over the past 30 years. It was originally defined within the Kiel classification as centrocytic lymphoma, then renamed MCL once the characteristic translocation and resulting cyclin D1 overexpression were identified. These diagnostic markers allowed for the characterization of MCL subtypes as well as the initiation of MCL-focused clinical trials which, in turn, led to regulatory approval of more effective regimens, new therapeutic agents, and an improvement in overall survival (OS) from around 3 years to more than 10 years for many patients.
Despite this progress, virtually all patients relapse, and a cure remains elusive for most. In younger (< 65 to 70 years), medically-fit patients who are transplant-eligible and have symptomatic MCL, a standard of care has been induction chemoimmunotherapy containing high-dose cytarabine followed by ASCT consolidation. For example, a clinical trial of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) alternating with R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin; 3 cycles each) showed a significant benefit over R-CHOP x 6 cycles; at a median follow-up of 10.6 years, the time-to-treatment failure was 8.4 v 3.9 years. In another trial, all patients received induction R-DHAP (with cisplatin or an alternative platinum agent) x 4 cycles followed by ASCT. Those patients randomized to post-ASCT maintenance rituximab for 3 years had significantly improved, 4-year progression-free survival (PFS) as compared with observation only (83% vs 64%, p < 0.001); maintenance also significantly improved OS.
Although ASCT consolidation followed by maintenance became widely adopted on the basis of these and other clinical trials, important questions remain:
First, MCL is biologically and clinically quite heterogeneous. Several prognostic tools such as the MCL International Prognostic Index (MIPI) scoring system and biomarkers are available to define lower- versus higher-risk subtypes, but none is routinely used for treatment planning. About 15% of MCL patients present with a highly-aggressive blastoid or pleomorphic variant that usually carries a TP53 mutation or deletion. Given the short survival and limited benefit from dose-intensive chemotherapy and ASCT in TP53-mutated MCL, should transplant be avoided in these patients?
Second, if deep remission is achieved following front-line therapy, defined as positron emission tomography (PET) negative and measurable residual disease (MRD) negative, will high-dose chemotherapy and ASCT provide additional benefits or only toxicity? This question is being addressed by the ongoing ECOG 4151 study, a risk-adapted trial in which post-induction MRD-negative patients are randomized to standard ASCT consolidation plus maintenance rituximab vs maintenance only.
Bruton tyrosine kinase inhibitors (BTKi) are now among the most used agents for relapsed MCL. Recent clinical trials testing the integration of a BTKi into first- or second-line therapy have shown increased response rates and variable clinical outcomes and toxicities for the combinations, depending upon the chemotherapy- and non-chemotherapy backbones utilized, as well as the BTKi. Combinations with the BCL2 inhibitor venetoclax plus chemotherapy or BTKi are also showing promise.
The activity of BTKi in MCL led the European MCL Network (EMCL) to design the 3-arm TRIANGLE study to analyze the potential of ibrutinib to improve outcomes when given in conjunction with standard ASCT consolidation, and the ability to replace the need for ASCT. The TRIANGLE results were presented by Dr. Martin Dreyling in the Plenary Session at the December 2022 American Society of Hematology (ASH) Annual Meeting. Transplant-eligible MCL patients < 65 years of age were randomized to the EMCL’s established front-line therapy of alternating R-CHOP/R-DHAP plus ASCT; the same regimen plus oral ibrutinib given with the R-CHOP induction cycles and then post-ASCT ibrutinib maintenance therapy for 2 years (Arm A+I); or the A+I regimen minus ASCT (Arm I). Maintenance rituximab was allowed in each arm, on the basis of the treating centers’ institutional guidelines. Overall, 54%-58% of patients in each study arm received rituximab maintenance, with no differential benefit in efficacy noted for those so treated.
The results showed that 94%-98% of patients responded by the end of induction (defined as R-chemo and ASCT), with complete remissions in 36%-45% (from computerized tomography imaging, not PET scan). With a median follow-up of 31 months, failure-free survival (FFS; the primary study endpoint) was significantly improved for A+I vs A (3 year FFS of 88% vs 72%, respectively; p = 0.0008). In a subgroup analysis, FFS was notably improved for A+I in patients with high-level TP53 overexpression by immunohistochemistry. Toxicity did not differ during the induction and ASCT periods among the 3 arms regarding cytopenia, gastrointestinal disorders, and infections. However, neutropenia and infections were increased in the ibrutinib-containing arms during maintenance therapy—especially for Arm A+I.
The authors concluded that ASCT plus ibrutinib (Arm A+I) is superior to ASCT only (Arm A), and that Arm A is not superior to ibrutinib without ASCT (Arm I). No decision can yet be made regarding A+I versus I for which FFS to date remains very similar; however, the authors favor ibrutinib without ASCT due to lower toxicity. OS is trending to favor the ibrutinib arms, but longer follow-up will be needed to fully assess.
Should ASCT consolidation now be replaced by ibrutinib-containing induction R-CHOP/R-DHAP and maintenance ibrutinib, with or without maintenance rituximab? A definitive answer will require the fully-published TRIANGLE results, as well as ongoing analysis with longer follow-up. However, it seems very likely that ASCT indeed will be replaced by the new approach. TP53-mutated MCL should be treated with ibrutinib plus R-CHOP/R-DHAP and ibrutinib maintenance as validated in this trial.
Many centers have begun using a second-generation BTKi, acalabrutinib or zanubrutinib, rather than ibrutinib due to equivalent response rates with more favorable side effect profiles and fewer treatment discontinuations. Caution is warranted regarding simply adding a BTKi to one’s favored MCL induction regimen and foregoing ASCT—pending additional studies and the safety of such alternative approaches.
These are indeed exciting times of therapeutic progress, as they have been improving outcomes and providing longer survival outcomes for MCL patients. Targeted agents facilitate this shift to less intensive and chemotherapy-free regimens that provide enhanced response and mitigate short- and longer-term toxicities. More results will be forthcoming for MRD as a treatment endpoint, guiding maintenance therapy, and for risk-adapted treatment of newly-diagnosed and relapsing patients (based upon MCL subtype and biomarker profiles). Enrolling patients into clinical trials is strongly encouraged as the best mechanism to help answer emerging questions in the field and open the pathway to continued progress.
Mantle cell lymphoma (MCL) has served as a paradigm of progress among the non-Hodgkin lymphomas over the past 30 years. It was originally defined within the Kiel classification as centrocytic lymphoma, then renamed MCL once the characteristic translocation and resulting cyclin D1 overexpression were identified. These diagnostic markers allowed for the characterization of MCL subtypes as well as the initiation of MCL-focused clinical trials which, in turn, led to regulatory approval of more effective regimens, new therapeutic agents, and an improvement in overall survival (OS) from around 3 years to more than 10 years for many patients.
Despite this progress, virtually all patients relapse, and a cure remains elusive for most. In younger (< 65 to 70 years), medically-fit patients who are transplant-eligible and have symptomatic MCL, a standard of care has been induction chemoimmunotherapy containing high-dose cytarabine followed by ASCT consolidation. For example, a clinical trial of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) alternating with R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin; 3 cycles each) showed a significant benefit over R-CHOP x 6 cycles; at a median follow-up of 10.6 years, the time-to-treatment failure was 8.4 v 3.9 years. In another trial, all patients received induction R-DHAP (with cisplatin or an alternative platinum agent) x 4 cycles followed by ASCT. Those patients randomized to post-ASCT maintenance rituximab for 3 years had significantly improved, 4-year progression-free survival (PFS) as compared with observation only (83% vs 64%, p < 0.001); maintenance also significantly improved OS.
Although ASCT consolidation followed by maintenance became widely adopted on the basis of these and other clinical trials, important questions remain:
First, MCL is biologically and clinically quite heterogeneous. Several prognostic tools such as the MCL International Prognostic Index (MIPI) scoring system and biomarkers are available to define lower- versus higher-risk subtypes, but none is routinely used for treatment planning. About 15% of MCL patients present with a highly-aggressive blastoid or pleomorphic variant that usually carries a TP53 mutation or deletion. Given the short survival and limited benefit from dose-intensive chemotherapy and ASCT in TP53-mutated MCL, should transplant be avoided in these patients?
Second, if deep remission is achieved following front-line therapy, defined as positron emission tomography (PET) negative and measurable residual disease (MRD) negative, will high-dose chemotherapy and ASCT provide additional benefits or only toxicity? This question is being addressed by the ongoing ECOG 4151 study, a risk-adapted trial in which post-induction MRD-negative patients are randomized to standard ASCT consolidation plus maintenance rituximab vs maintenance only.
Bruton tyrosine kinase inhibitors (BTKi) are now among the most used agents for relapsed MCL. Recent clinical trials testing the integration of a BTKi into first- or second-line therapy have shown increased response rates and variable clinical outcomes and toxicities for the combinations, depending upon the chemotherapy- and non-chemotherapy backbones utilized, as well as the BTKi. Combinations with the BCL2 inhibitor venetoclax plus chemotherapy or BTKi are also showing promise.
The activity of BTKi in MCL led the European MCL Network (EMCL) to design the 3-arm TRIANGLE study to analyze the potential of ibrutinib to improve outcomes when given in conjunction with standard ASCT consolidation, and the ability to replace the need for ASCT. The TRIANGLE results were presented by Dr. Martin Dreyling in the Plenary Session at the December 2022 American Society of Hematology (ASH) Annual Meeting. Transplant-eligible MCL patients < 65 years of age were randomized to the EMCL’s established front-line therapy of alternating R-CHOP/R-DHAP plus ASCT; the same regimen plus oral ibrutinib given with the R-CHOP induction cycles and then post-ASCT ibrutinib maintenance therapy for 2 years (Arm A+I); or the A+I regimen minus ASCT (Arm I). Maintenance rituximab was allowed in each arm, on the basis of the treating centers’ institutional guidelines. Overall, 54%-58% of patients in each study arm received rituximab maintenance, with no differential benefit in efficacy noted for those so treated.
The results showed that 94%-98% of patients responded by the end of induction (defined as R-chemo and ASCT), with complete remissions in 36%-45% (from computerized tomography imaging, not PET scan). With a median follow-up of 31 months, failure-free survival (FFS; the primary study endpoint) was significantly improved for A+I vs A (3 year FFS of 88% vs 72%, respectively; p = 0.0008). In a subgroup analysis, FFS was notably improved for A+I in patients with high-level TP53 overexpression by immunohistochemistry. Toxicity did not differ during the induction and ASCT periods among the 3 arms regarding cytopenia, gastrointestinal disorders, and infections. However, neutropenia and infections were increased in the ibrutinib-containing arms during maintenance therapy—especially for Arm A+I.
The authors concluded that ASCT plus ibrutinib (Arm A+I) is superior to ASCT only (Arm A), and that Arm A is not superior to ibrutinib without ASCT (Arm I). No decision can yet be made regarding A+I versus I for which FFS to date remains very similar; however, the authors favor ibrutinib without ASCT due to lower toxicity. OS is trending to favor the ibrutinib arms, but longer follow-up will be needed to fully assess.
Should ASCT consolidation now be replaced by ibrutinib-containing induction R-CHOP/R-DHAP and maintenance ibrutinib, with or without maintenance rituximab? A definitive answer will require the fully-published TRIANGLE results, as well as ongoing analysis with longer follow-up. However, it seems very likely that ASCT indeed will be replaced by the new approach. TP53-mutated MCL should be treated with ibrutinib plus R-CHOP/R-DHAP and ibrutinib maintenance as validated in this trial.
Many centers have begun using a second-generation BTKi, acalabrutinib or zanubrutinib, rather than ibrutinib due to equivalent response rates with more favorable side effect profiles and fewer treatment discontinuations. Caution is warranted regarding simply adding a BTKi to one’s favored MCL induction regimen and foregoing ASCT—pending additional studies and the safety of such alternative approaches.
These are indeed exciting times of therapeutic progress, as they have been improving outcomes and providing longer survival outcomes for MCL patients. Targeted agents facilitate this shift to less intensive and chemotherapy-free regimens that provide enhanced response and mitigate short- and longer-term toxicities. More results will be forthcoming for MRD as a treatment endpoint, guiding maintenance therapy, and for risk-adapted treatment of newly-diagnosed and relapsing patients (based upon MCL subtype and biomarker profiles). Enrolling patients into clinical trials is strongly encouraged as the best mechanism to help answer emerging questions in the field and open the pathway to continued progress.
Treatment of Axial Psoriatic Arthritis
Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that may involve several different domains, including peripheral joints, entheses, nails, axial skeleton, and skin. A recent increased awareness of PsA has accompanied a large increase in available therapeutic options. In addition to traditional disease-modifying antirheumatic drugs (DMARDs), new biologics and targeted small molecules have now been shown to be effective in PsA. These agents include those targeting pathways involving tumor necrosis factor (TNF), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), interleukins (IL) 12, 17, 23, janus kinase (JAK), and phosphodiesterase 4 (PDE4). These agents have demonstrated efficacy in outcome measures developed for peripheral arthritis, such as the American College of Rheumatology 20 (ACR20) response. However, an ongoing question is whether these agents are equally effective in axial disease. Based on our experience and the existing literature, we believe that some of these agents, including PDE4 and IL-23 inhibitors, are not effective for axial disease.
Moll and Wright’s original description of PsA estimated that 5% of patients with PsA had axial disease1; however, they were describing patients in whom axial arthritis was the predominant, or the only, manifestation. There are many patients for whom axial symptoms are just one of several domains of disease activity. With this in mind, and depending on the cohort studied, the estimated overall prevalence of axial disease ranges from 7% to 32% in patients with PsA.2 This is in contrast to peripheral arthritis, a domain that occurs in most patients with PsA and is the most common manifestation of PsA.2 We believe there are differences in axial and peripheral response among some of the drugs used to treat PsA; therefore it is critical to consider both the presence and magnitude of axial involvement.
An absence of axial PsA–specific clinical trials complicates navigating this treatment domain. Most considerations regarding treatment options for axial disease in PsA are extrapolated from ankylosing spondylitis (AS) trials and experience, as is the case for the TNF and JAK inhibitors. To our knowledge, only one high-quality randomized trial, MAXIMISE, looked specifically at the treatment of axial PsA, in this case with the IL-17 inhibitor secukinumab.3 This trial demonstrated efficacy of secukinumab in reducing symptoms and acute phase reactants in patients with PsA who were categorized as having active axial disease using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Other than conclusions drawn from AS trials and from this single axial PsA randomized controlled trial, data on the treatment of axial PsA are drawn entirely from observational and post-hoc analyses. As there are no consensus criteria for axial PsA, the cohorts included in these data may vary. This heterogeneity showcases the diversity in patients with PsA with axial disease but complicates the generalizability of the findings to individual patients.
Another challenge in understanding axial response to medication is the lack of specific, validated outcome measures for axial PsA. The BASDAI and, more recently, the Assessment in Ankylosing Spondylitis (ASAS) and the Ankylosing Spondylitis Disease Activity Score (ASDAS), all developed specifically for AS, are often used to measure treatment response. The BASDAI incorporates patient-reported symptoms which include fatigue, peripheral joint pain/swelling, tenderness, and morning stiffness not specifically localized to the back. The ASDAS also includes a C-reactive protein measurement.
When used to assess response in PsA, however, these patient-reported outcomes may not be precise enough to separate the impact of axial disease or symptoms from that of peripheral disease. Only question 2 on the BASDAI specifically addresses axial complaints: “How would you describe the overall level of AS-related pain you have had in your neck, back, or hips?” Even this question is vulnerable to confounding from noninflammatory causes of back pain. Although these issues exist with patient-reported outcomes, objective spinal mobility measures used in evaluation of AS, including the modified Schober test, lumbar side flexion, and cervical rotation, have been demonstrated also to perform well in axial PsA.4
This was corroborated in the INSPIRE study, which showed adequate interobserver reliability in primary AS that was equally reproducible in axial PsA, with most measures, including occiput to wall, modified Schober test, cervical rotation, lateral bending, and hip mobility, performing in a “good to excellent” manner.5 Therefore, the inclusion of these objective measures in future therapeutic studies may enhance the external validation of available data.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has established therapeutic guidelines for psoriatic disease based on currently available literature and data. Similar to previous iterations of guidelines, GRAPPA continues to recommend agents with TNF inhibition or IL-17 inhibition for patients with PsA with axial disease who have failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and/or glucocorticoid injections. Newly recommended in the latest iteration of the GRAPPA guidelines, based on the efficacy of these agents in AS, is the use of JAK inhibitors for axial PsA.6
Although TNF, IL-17, and JAK targeted therapies have demonstrated more likely benefit, albeit subject to the trial limitations previously discussed, the question remains whether agents targeting PDE4 and IL-23 are an effective option for axial PsA. Studies of both PDE4 and IL-23 inhibitors in AS have not demonstrated adequate benefit, which, importantly, contrasts with the previously mentioned and recommended therapies. Additionally, there are no primary randomized control trials that have directly evaluated the efficacy of IL-23 therapy in axial PsA.
Existing data about potential benefit come from post-hoc analyses of the PSUMMIT 1 and 2 trials7-10 with ustekinumab (which inhibits IL-12 and IL-23) and the DISCOVER trials11-13 with guselkumab (a pure IL-23 inhibitor). However, these analyses relied on a physician-reported diagnosis of axial disease and not on prespecified entry criteria. This lack of uniform diagnostic criteria may introduce bias into the interpretation of the results and limit external validation. All patients in these trials had a significant burden of peripheral arthritis; therefore it is hard to know whether, even in patients with physician-reported axial disease, improvement in general outcome measures were due to true amelioration of axial disease or were confounded by improvement in peripheral and skin domains. The analysis of these trials did look specifically at patient answers to BASDAI question 2 regarding level of neck, back, or hip pain. However, it remains difficult to be certain that the results are truly a reflection of axial symptoms and are not driven by patient-perceived improvement in other disease domains and an overall positive trajectory in well-being.
In our years of practice, when we turned to biologic agents, the IL-23 inhibitors and the IL-12/23 inhibitor have not been as effective in patients with PsA who have axial-predominant symptoms. The lack of efficacy of these agents in AS, in contrast to their benefit in psoriatic skin and peripheral joint disease, raises questions about the pathophysiologic role of IL-23 in axial disease, which is yet to be fully understood. For patients with a significant burden of axial pain, in concordance with the consensus from GRAPPA,6 our strategy is to start with TNF, IL-17, or JAK targeted therapies, with the choice based on patient-specific factors, including patient comorbidities, patient administration preference, and insurance coverage. We do believe it is reasonable to try IL-23–targeted therapies in patients who have mild axial symptoms when their predominant symptoms are in other domains, such as the peripheral joints or skin. In our opinion, more convincing data supporting IL-23 inhibition are required to move this into the forefront of axial-predominant PsA therapy. Clearly the investigation of axial disease in PsA lags behind that of peripheral and skin domains. Specific classification criteria for axial PsA, as are being currently developed by GRAPPA, should facilitate more focused therapeutic trials that can better inform optimal treatment of patients with this subset of disease.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55-78. doi:10.1016/0049-0172(73)90035-8
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi:10.1016/j.rdc.2015.07.001
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590. doi:10.1136/annrheumdis-2020-218808
- Fernández-Sueiro JL, Willisch A, Pértega-Díaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum. 2009;61(3):386-392. doi:10.1002/art.24280
- Gladman DD, Inman RD, Cook RJ, et al. International spondyloarthritis interobserver reliability exercise—the INSPIRE study: I. Assessment of spinal measures. J Rheumatol. 2007;34(8):1733-1739.
- Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022;49(6 suppl 1):52-54. doi:10.3899/jrheum.211331
- McInnes IB, Kavanaugh A, Gottlieb AB, et al; PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. doi:10.1016/S0140-6736(13)60594-2
- Ritchlin C, Rahman P, Kavanaugh A, et al; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990-999. doi:10.1136/annrheumdis-2013-204655
- Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984-1988. doi:10.1136/annrheumdis-2015-209068
- McInnes IB, Chakravarty SD, Apaolaza I, et al. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: data from PSUMMIT 1 and PSUMMIT 2. RMD Open. 2019;5(2):e000990. doi:10.1136/rmdopen-2019-000990
- Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER-1 Study Group. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. doi:10.1016/S0140-6736(20)30265-8
- Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. doi:10.1016/S0140-6736(20)30263-4
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 discover-1 and discover-2 studies. Lancet Rheumatol. 2021;3(10). doi:https://doi.org/10.1016/S2665-9913(21)00105-3
Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that may involve several different domains, including peripheral joints, entheses, nails, axial skeleton, and skin. A recent increased awareness of PsA has accompanied a large increase in available therapeutic options. In addition to traditional disease-modifying antirheumatic drugs (DMARDs), new biologics and targeted small molecules have now been shown to be effective in PsA. These agents include those targeting pathways involving tumor necrosis factor (TNF), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), interleukins (IL) 12, 17, 23, janus kinase (JAK), and phosphodiesterase 4 (PDE4). These agents have demonstrated efficacy in outcome measures developed for peripheral arthritis, such as the American College of Rheumatology 20 (ACR20) response. However, an ongoing question is whether these agents are equally effective in axial disease. Based on our experience and the existing literature, we believe that some of these agents, including PDE4 and IL-23 inhibitors, are not effective for axial disease.
Moll and Wright’s original description of PsA estimated that 5% of patients with PsA had axial disease1; however, they were describing patients in whom axial arthritis was the predominant, or the only, manifestation. There are many patients for whom axial symptoms are just one of several domains of disease activity. With this in mind, and depending on the cohort studied, the estimated overall prevalence of axial disease ranges from 7% to 32% in patients with PsA.2 This is in contrast to peripheral arthritis, a domain that occurs in most patients with PsA and is the most common manifestation of PsA.2 We believe there are differences in axial and peripheral response among some of the drugs used to treat PsA; therefore it is critical to consider both the presence and magnitude of axial involvement.
An absence of axial PsA–specific clinical trials complicates navigating this treatment domain. Most considerations regarding treatment options for axial disease in PsA are extrapolated from ankylosing spondylitis (AS) trials and experience, as is the case for the TNF and JAK inhibitors. To our knowledge, only one high-quality randomized trial, MAXIMISE, looked specifically at the treatment of axial PsA, in this case with the IL-17 inhibitor secukinumab.3 This trial demonstrated efficacy of secukinumab in reducing symptoms and acute phase reactants in patients with PsA who were categorized as having active axial disease using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Other than conclusions drawn from AS trials and from this single axial PsA randomized controlled trial, data on the treatment of axial PsA are drawn entirely from observational and post-hoc analyses. As there are no consensus criteria for axial PsA, the cohorts included in these data may vary. This heterogeneity showcases the diversity in patients with PsA with axial disease but complicates the generalizability of the findings to individual patients.
Another challenge in understanding axial response to medication is the lack of specific, validated outcome measures for axial PsA. The BASDAI and, more recently, the Assessment in Ankylosing Spondylitis (ASAS) and the Ankylosing Spondylitis Disease Activity Score (ASDAS), all developed specifically for AS, are often used to measure treatment response. The BASDAI incorporates patient-reported symptoms which include fatigue, peripheral joint pain/swelling, tenderness, and morning stiffness not specifically localized to the back. The ASDAS also includes a C-reactive protein measurement.
When used to assess response in PsA, however, these patient-reported outcomes may not be precise enough to separate the impact of axial disease or symptoms from that of peripheral disease. Only question 2 on the BASDAI specifically addresses axial complaints: “How would you describe the overall level of AS-related pain you have had in your neck, back, or hips?” Even this question is vulnerable to confounding from noninflammatory causes of back pain. Although these issues exist with patient-reported outcomes, objective spinal mobility measures used in evaluation of AS, including the modified Schober test, lumbar side flexion, and cervical rotation, have been demonstrated also to perform well in axial PsA.4
This was corroborated in the INSPIRE study, which showed adequate interobserver reliability in primary AS that was equally reproducible in axial PsA, with most measures, including occiput to wall, modified Schober test, cervical rotation, lateral bending, and hip mobility, performing in a “good to excellent” manner.5 Therefore, the inclusion of these objective measures in future therapeutic studies may enhance the external validation of available data.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has established therapeutic guidelines for psoriatic disease based on currently available literature and data. Similar to previous iterations of guidelines, GRAPPA continues to recommend agents with TNF inhibition or IL-17 inhibition for patients with PsA with axial disease who have failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and/or glucocorticoid injections. Newly recommended in the latest iteration of the GRAPPA guidelines, based on the efficacy of these agents in AS, is the use of JAK inhibitors for axial PsA.6
Although TNF, IL-17, and JAK targeted therapies have demonstrated more likely benefit, albeit subject to the trial limitations previously discussed, the question remains whether agents targeting PDE4 and IL-23 are an effective option for axial PsA. Studies of both PDE4 and IL-23 inhibitors in AS have not demonstrated adequate benefit, which, importantly, contrasts with the previously mentioned and recommended therapies. Additionally, there are no primary randomized control trials that have directly evaluated the efficacy of IL-23 therapy in axial PsA.
Existing data about potential benefit come from post-hoc analyses of the PSUMMIT 1 and 2 trials7-10 with ustekinumab (which inhibits IL-12 and IL-23) and the DISCOVER trials11-13 with guselkumab (a pure IL-23 inhibitor). However, these analyses relied on a physician-reported diagnosis of axial disease and not on prespecified entry criteria. This lack of uniform diagnostic criteria may introduce bias into the interpretation of the results and limit external validation. All patients in these trials had a significant burden of peripheral arthritis; therefore it is hard to know whether, even in patients with physician-reported axial disease, improvement in general outcome measures were due to true amelioration of axial disease or were confounded by improvement in peripheral and skin domains. The analysis of these trials did look specifically at patient answers to BASDAI question 2 regarding level of neck, back, or hip pain. However, it remains difficult to be certain that the results are truly a reflection of axial symptoms and are not driven by patient-perceived improvement in other disease domains and an overall positive trajectory in well-being.
In our years of practice, when we turned to biologic agents, the IL-23 inhibitors and the IL-12/23 inhibitor have not been as effective in patients with PsA who have axial-predominant symptoms. The lack of efficacy of these agents in AS, in contrast to their benefit in psoriatic skin and peripheral joint disease, raises questions about the pathophysiologic role of IL-23 in axial disease, which is yet to be fully understood. For patients with a significant burden of axial pain, in concordance with the consensus from GRAPPA,6 our strategy is to start with TNF, IL-17, or JAK targeted therapies, with the choice based on patient-specific factors, including patient comorbidities, patient administration preference, and insurance coverage. We do believe it is reasonable to try IL-23–targeted therapies in patients who have mild axial symptoms when their predominant symptoms are in other domains, such as the peripheral joints or skin. In our opinion, more convincing data supporting IL-23 inhibition are required to move this into the forefront of axial-predominant PsA therapy. Clearly the investigation of axial disease in PsA lags behind that of peripheral and skin domains. Specific classification criteria for axial PsA, as are being currently developed by GRAPPA, should facilitate more focused therapeutic trials that can better inform optimal treatment of patients with this subset of disease.
Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that may involve several different domains, including peripheral joints, entheses, nails, axial skeleton, and skin. A recent increased awareness of PsA has accompanied a large increase in available therapeutic options. In addition to traditional disease-modifying antirheumatic drugs (DMARDs), new biologics and targeted small molecules have now been shown to be effective in PsA. These agents include those targeting pathways involving tumor necrosis factor (TNF), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), interleukins (IL) 12, 17, 23, janus kinase (JAK), and phosphodiesterase 4 (PDE4). These agents have demonstrated efficacy in outcome measures developed for peripheral arthritis, such as the American College of Rheumatology 20 (ACR20) response. However, an ongoing question is whether these agents are equally effective in axial disease. Based on our experience and the existing literature, we believe that some of these agents, including PDE4 and IL-23 inhibitors, are not effective for axial disease.
Moll and Wright’s original description of PsA estimated that 5% of patients with PsA had axial disease1; however, they were describing patients in whom axial arthritis was the predominant, or the only, manifestation. There are many patients for whom axial symptoms are just one of several domains of disease activity. With this in mind, and depending on the cohort studied, the estimated overall prevalence of axial disease ranges from 7% to 32% in patients with PsA.2 This is in contrast to peripheral arthritis, a domain that occurs in most patients with PsA and is the most common manifestation of PsA.2 We believe there are differences in axial and peripheral response among some of the drugs used to treat PsA; therefore it is critical to consider both the presence and magnitude of axial involvement.
An absence of axial PsA–specific clinical trials complicates navigating this treatment domain. Most considerations regarding treatment options for axial disease in PsA are extrapolated from ankylosing spondylitis (AS) trials and experience, as is the case for the TNF and JAK inhibitors. To our knowledge, only one high-quality randomized trial, MAXIMISE, looked specifically at the treatment of axial PsA, in this case with the IL-17 inhibitor secukinumab.3 This trial demonstrated efficacy of secukinumab in reducing symptoms and acute phase reactants in patients with PsA who were categorized as having active axial disease using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). Other than conclusions drawn from AS trials and from this single axial PsA randomized controlled trial, data on the treatment of axial PsA are drawn entirely from observational and post-hoc analyses. As there are no consensus criteria for axial PsA, the cohorts included in these data may vary. This heterogeneity showcases the diversity in patients with PsA with axial disease but complicates the generalizability of the findings to individual patients.
Another challenge in understanding axial response to medication is the lack of specific, validated outcome measures for axial PsA. The BASDAI and, more recently, the Assessment in Ankylosing Spondylitis (ASAS) and the Ankylosing Spondylitis Disease Activity Score (ASDAS), all developed specifically for AS, are often used to measure treatment response. The BASDAI incorporates patient-reported symptoms which include fatigue, peripheral joint pain/swelling, tenderness, and morning stiffness not specifically localized to the back. The ASDAS also includes a C-reactive protein measurement.
When used to assess response in PsA, however, these patient-reported outcomes may not be precise enough to separate the impact of axial disease or symptoms from that of peripheral disease. Only question 2 on the BASDAI specifically addresses axial complaints: “How would you describe the overall level of AS-related pain you have had in your neck, back, or hips?” Even this question is vulnerable to confounding from noninflammatory causes of back pain. Although these issues exist with patient-reported outcomes, objective spinal mobility measures used in evaluation of AS, including the modified Schober test, lumbar side flexion, and cervical rotation, have been demonstrated also to perform well in axial PsA.4
This was corroborated in the INSPIRE study, which showed adequate interobserver reliability in primary AS that was equally reproducible in axial PsA, with most measures, including occiput to wall, modified Schober test, cervical rotation, lateral bending, and hip mobility, performing in a “good to excellent” manner.5 Therefore, the inclusion of these objective measures in future therapeutic studies may enhance the external validation of available data.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has established therapeutic guidelines for psoriatic disease based on currently available literature and data. Similar to previous iterations of guidelines, GRAPPA continues to recommend agents with TNF inhibition or IL-17 inhibition for patients with PsA with axial disease who have failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and/or glucocorticoid injections. Newly recommended in the latest iteration of the GRAPPA guidelines, based on the efficacy of these agents in AS, is the use of JAK inhibitors for axial PsA.6
Although TNF, IL-17, and JAK targeted therapies have demonstrated more likely benefit, albeit subject to the trial limitations previously discussed, the question remains whether agents targeting PDE4 and IL-23 are an effective option for axial PsA. Studies of both PDE4 and IL-23 inhibitors in AS have not demonstrated adequate benefit, which, importantly, contrasts with the previously mentioned and recommended therapies. Additionally, there are no primary randomized control trials that have directly evaluated the efficacy of IL-23 therapy in axial PsA.
Existing data about potential benefit come from post-hoc analyses of the PSUMMIT 1 and 2 trials7-10 with ustekinumab (which inhibits IL-12 and IL-23) and the DISCOVER trials11-13 with guselkumab (a pure IL-23 inhibitor). However, these analyses relied on a physician-reported diagnosis of axial disease and not on prespecified entry criteria. This lack of uniform diagnostic criteria may introduce bias into the interpretation of the results and limit external validation. All patients in these trials had a significant burden of peripheral arthritis; therefore it is hard to know whether, even in patients with physician-reported axial disease, improvement in general outcome measures were due to true amelioration of axial disease or were confounded by improvement in peripheral and skin domains. The analysis of these trials did look specifically at patient answers to BASDAI question 2 regarding level of neck, back, or hip pain. However, it remains difficult to be certain that the results are truly a reflection of axial symptoms and are not driven by patient-perceived improvement in other disease domains and an overall positive trajectory in well-being.
In our years of practice, when we turned to biologic agents, the IL-23 inhibitors and the IL-12/23 inhibitor have not been as effective in patients with PsA who have axial-predominant symptoms. The lack of efficacy of these agents in AS, in contrast to their benefit in psoriatic skin and peripheral joint disease, raises questions about the pathophysiologic role of IL-23 in axial disease, which is yet to be fully understood. For patients with a significant burden of axial pain, in concordance with the consensus from GRAPPA,6 our strategy is to start with TNF, IL-17, or JAK targeted therapies, with the choice based on patient-specific factors, including patient comorbidities, patient administration preference, and insurance coverage. We do believe it is reasonable to try IL-23–targeted therapies in patients who have mild axial symptoms when their predominant symptoms are in other domains, such as the peripheral joints or skin. In our opinion, more convincing data supporting IL-23 inhibition are required to move this into the forefront of axial-predominant PsA therapy. Clearly the investigation of axial disease in PsA lags behind that of peripheral and skin domains. Specific classification criteria for axial PsA, as are being currently developed by GRAPPA, should facilitate more focused therapeutic trials that can better inform optimal treatment of patients with this subset of disease.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55-78. doi:10.1016/0049-0172(73)90035-8
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi:10.1016/j.rdc.2015.07.001
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590. doi:10.1136/annrheumdis-2020-218808
- Fernández-Sueiro JL, Willisch A, Pértega-Díaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum. 2009;61(3):386-392. doi:10.1002/art.24280
- Gladman DD, Inman RD, Cook RJ, et al. International spondyloarthritis interobserver reliability exercise—the INSPIRE study: I. Assessment of spinal measures. J Rheumatol. 2007;34(8):1733-1739.
- Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022;49(6 suppl 1):52-54. doi:10.3899/jrheum.211331
- McInnes IB, Kavanaugh A, Gottlieb AB, et al; PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. doi:10.1016/S0140-6736(13)60594-2
- Ritchlin C, Rahman P, Kavanaugh A, et al; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990-999. doi:10.1136/annrheumdis-2013-204655
- Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984-1988. doi:10.1136/annrheumdis-2015-209068
- McInnes IB, Chakravarty SD, Apaolaza I, et al. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: data from PSUMMIT 1 and PSUMMIT 2. RMD Open. 2019;5(2):e000990. doi:10.1136/rmdopen-2019-000990
- Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER-1 Study Group. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. doi:10.1016/S0140-6736(20)30265-8
- Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. doi:10.1016/S0140-6736(20)30263-4
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 discover-1 and discover-2 studies. Lancet Rheumatol. 2021;3(10). doi:https://doi.org/10.1016/S2665-9913(21)00105-3
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55-78. doi:10.1016/0049-0172(73)90035-8
- Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545-568. doi:10.1016/j.rdc.2015.07.001
- Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590. doi:10.1136/annrheumdis-2020-218808
- Fernández-Sueiro JL, Willisch A, Pértega-Díaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum. 2009;61(3):386-392. doi:10.1002/art.24280
- Gladman DD, Inman RD, Cook RJ, et al. International spondyloarthritis interobserver reliability exercise—the INSPIRE study: I. Assessment of spinal measures. J Rheumatol. 2007;34(8):1733-1739.
- Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022;49(6 suppl 1):52-54. doi:10.3899/jrheum.211331
- McInnes IB, Kavanaugh A, Gottlieb AB, et al; PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. doi:10.1016/S0140-6736(13)60594-2
- Ritchlin C, Rahman P, Kavanaugh A, et al; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990-999. doi:10.1136/annrheumdis-2013-204655
- Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016;75(11):1984-1988. doi:10.1136/annrheumdis-2015-209068
- McInnes IB, Chakravarty SD, Apaolaza I, et al. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: data from PSUMMIT 1 and PSUMMIT 2. RMD Open. 2019;5(2):e000990. doi:10.1136/rmdopen-2019-000990
- Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER-1 Study Group. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115-1125. doi:10.1016/S0140-6736(20)30265-8
- Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER-2 Study Group. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126-1136. doi:10.1016/S0140-6736(20)30263-4
- Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 discover-1 and discover-2 studies. Lancet Rheumatol. 2021;3(10). doi:https://doi.org/10.1016/S2665-9913(21)00105-3
Culinary medicine guides rural GI doctor’s career
Someone once told Christina Tennyson, MD, that clinical medicine was a grind. Instead of veering away from the profession, she dove in. Medicine will always have its frustrations, she acknowledged.
However, “finding areas that interest me and incorporating those into clinical practice has really helped me enjoy the practice of medicine,” said Dr. Tennyson, who works at Augusta Health in Fishersville, Va.
It has also inspired her to think outside the box in her gastroenterology practice. What her patients eat and the lifestyle choices they make is a central focus of her work.
Q: Why did you choose GI?
Dr. Tennyson: I always had an interest in nutrition. During training at medical school at NYU [New York University], I also really loved learning all I could about internal medicine. I worked with a great surgical team as a student and enjoyed being in the operating room. Although I knew I didn’t want to enter surgery, the experience encouraged me to pursue gastroenterology as it involved nutrition, internal medicine, and procedures as well as my favorite organ, the small bowel. I worked with some great mentors in gastroenterology, such as Dr. David Metz and Dr. Dave Katzka, at the University of Pennsylvania as a resident. I enjoyed taking care of patients with both acute and chronic conditions as well as the mix of doing procedures and seeing patients in the office. It also provided me the opportunity to incorporate nutrition into my clinical practice.
Q: What gives you the most joy in your day-to-day practice?
Dr. Tennyson: I enjoy helping my patients make meaningful lifestyle changes that can positively impact digestive health and well-being. I try to address topics related to lifestyle medicine in most of my clinical visits including eating more fiber/plants, exercise, positive relationships, and stress management. Many of the conditions we treat as gastroenterologists can benefit from addressing aspects of lifestyle along with our conventional medical therapies. I reinforce that attention to these areas can make a difference. I enjoy sharing recipes, books, and websites that I have found helpful.
Q: How has your job changed since you first began your career?
Dr. Tennyson: After fellowship, I joined the faculty at Columbia University and worked at the Celiac Disease Center seeing patients, teaching, and performing clinical research under the mentorship of Peter Green, MD, and Suzanne Lewis, MD. It was a great opportunity to learn and practice in a tertiary center. I later switched roles and joined a general multispecialty community practice in Brooklyn [N.Y.] affiliated with an academic medical center. After practicing in New York for 10 years, I left my clinical practice and performed locums work for several years in underserved rural areas. I enjoyed working in rural areas and took a permanent position at a community hospital in Virginia’s Shenandoah Valley.
Q: Describe your biggest practice-related challenge and what you are doing to address it.
Dr. Tennyson: The small, rural community hospital where I currently work does not have the same resources and staffing as urban tertiary centers. While we are taking care of the community in our general gastroenterology practice, we’ve also launched an integrated care model in our hospital. We have collaborated with behavioral health, dietitians, nurses, health coaches, exercise physiologists as well as other members of the community, including farmers and a chef. We have performed some innovative, engaging programs, including fermentation workshops, cooking classes, farm walks, and mindfulness programs.
Q: What are you most proud of accomplishing?
Dr. Tennyson: I am proud that I took a nontraditional path after training to do what I enjoy and find rewarding. I received certification during GI fellowship at Mount Sinai [N.Y.] as a physician nutrition specialist. I later completed a fellowship in integrative medicine at the University of Arizona, received certification in lifestyle medicine, and completed coursework in culinary medicine. I really enjoyed doing locums work taking care of patients in other parts of the country, like Mississippi or Maine. I’ve enjoyed working in more rural areas and bringing some innovative programs to the community.
Q: What teacher or mentor had the greatest impact on you?
Dr. Tennyson: Dr. Anthony Grieco while I was a student at NYU. He is an astute clinician, always listened to his patients, loved clinical medicine, and had an endless fund of knowledge. I wanted to be a doctor like him. During my fellowship at Mount Sinai, I was also exposed to many great mentors including Dr. Lloyd Mayer, Dr. Jerome Waye, Dr. Steve Itzkowitz, and Dr. Blair Lewis who encouraged my interest in nutrition and small bowel diseases.
Lightning round
What's your superpower?
Finding fun in mundane things
Favorite movie to quote?
The Princess Bride
What is your favorite form of exercise?
A hike in the woods
Name one thing on your bucket list.
Galapagos Islands trip before my kids grow up
Cats or dogs?
Dogs
Summer or winter?
Summer
Someone once told Christina Tennyson, MD, that clinical medicine was a grind. Instead of veering away from the profession, she dove in. Medicine will always have its frustrations, she acknowledged.
However, “finding areas that interest me and incorporating those into clinical practice has really helped me enjoy the practice of medicine,” said Dr. Tennyson, who works at Augusta Health in Fishersville, Va.
It has also inspired her to think outside the box in her gastroenterology practice. What her patients eat and the lifestyle choices they make is a central focus of her work.
Q: Why did you choose GI?
Dr. Tennyson: I always had an interest in nutrition. During training at medical school at NYU [New York University], I also really loved learning all I could about internal medicine. I worked with a great surgical team as a student and enjoyed being in the operating room. Although I knew I didn’t want to enter surgery, the experience encouraged me to pursue gastroenterology as it involved nutrition, internal medicine, and procedures as well as my favorite organ, the small bowel. I worked with some great mentors in gastroenterology, such as Dr. David Metz and Dr. Dave Katzka, at the University of Pennsylvania as a resident. I enjoyed taking care of patients with both acute and chronic conditions as well as the mix of doing procedures and seeing patients in the office. It also provided me the opportunity to incorporate nutrition into my clinical practice.
Q: What gives you the most joy in your day-to-day practice?
Dr. Tennyson: I enjoy helping my patients make meaningful lifestyle changes that can positively impact digestive health and well-being. I try to address topics related to lifestyle medicine in most of my clinical visits including eating more fiber/plants, exercise, positive relationships, and stress management. Many of the conditions we treat as gastroenterologists can benefit from addressing aspects of lifestyle along with our conventional medical therapies. I reinforce that attention to these areas can make a difference. I enjoy sharing recipes, books, and websites that I have found helpful.
Q: How has your job changed since you first began your career?
Dr. Tennyson: After fellowship, I joined the faculty at Columbia University and worked at the Celiac Disease Center seeing patients, teaching, and performing clinical research under the mentorship of Peter Green, MD, and Suzanne Lewis, MD. It was a great opportunity to learn and practice in a tertiary center. I later switched roles and joined a general multispecialty community practice in Brooklyn [N.Y.] affiliated with an academic medical center. After practicing in New York for 10 years, I left my clinical practice and performed locums work for several years in underserved rural areas. I enjoyed working in rural areas and took a permanent position at a community hospital in Virginia’s Shenandoah Valley.
Q: Describe your biggest practice-related challenge and what you are doing to address it.
Dr. Tennyson: The small, rural community hospital where I currently work does not have the same resources and staffing as urban tertiary centers. While we are taking care of the community in our general gastroenterology practice, we’ve also launched an integrated care model in our hospital. We have collaborated with behavioral health, dietitians, nurses, health coaches, exercise physiologists as well as other members of the community, including farmers and a chef. We have performed some innovative, engaging programs, including fermentation workshops, cooking classes, farm walks, and mindfulness programs.
Q: What are you most proud of accomplishing?
Dr. Tennyson: I am proud that I took a nontraditional path after training to do what I enjoy and find rewarding. I received certification during GI fellowship at Mount Sinai [N.Y.] as a physician nutrition specialist. I later completed a fellowship in integrative medicine at the University of Arizona, received certification in lifestyle medicine, and completed coursework in culinary medicine. I really enjoyed doing locums work taking care of patients in other parts of the country, like Mississippi or Maine. I’ve enjoyed working in more rural areas and bringing some innovative programs to the community.
Q: What teacher or mentor had the greatest impact on you?
Dr. Tennyson: Dr. Anthony Grieco while I was a student at NYU. He is an astute clinician, always listened to his patients, loved clinical medicine, and had an endless fund of knowledge. I wanted to be a doctor like him. During my fellowship at Mount Sinai, I was also exposed to many great mentors including Dr. Lloyd Mayer, Dr. Jerome Waye, Dr. Steve Itzkowitz, and Dr. Blair Lewis who encouraged my interest in nutrition and small bowel diseases.
Lightning round
What's your superpower?
Finding fun in mundane things
Favorite movie to quote?
The Princess Bride
What is your favorite form of exercise?
A hike in the woods
Name one thing on your bucket list.
Galapagos Islands trip before my kids grow up
Cats or dogs?
Dogs
Summer or winter?
Summer
Someone once told Christina Tennyson, MD, that clinical medicine was a grind. Instead of veering away from the profession, she dove in. Medicine will always have its frustrations, she acknowledged.
However, “finding areas that interest me and incorporating those into clinical practice has really helped me enjoy the practice of medicine,” said Dr. Tennyson, who works at Augusta Health in Fishersville, Va.
It has also inspired her to think outside the box in her gastroenterology practice. What her patients eat and the lifestyle choices they make is a central focus of her work.
Q: Why did you choose GI?
Dr. Tennyson: I always had an interest in nutrition. During training at medical school at NYU [New York University], I also really loved learning all I could about internal medicine. I worked with a great surgical team as a student and enjoyed being in the operating room. Although I knew I didn’t want to enter surgery, the experience encouraged me to pursue gastroenterology as it involved nutrition, internal medicine, and procedures as well as my favorite organ, the small bowel. I worked with some great mentors in gastroenterology, such as Dr. David Metz and Dr. Dave Katzka, at the University of Pennsylvania as a resident. I enjoyed taking care of patients with both acute and chronic conditions as well as the mix of doing procedures and seeing patients in the office. It also provided me the opportunity to incorporate nutrition into my clinical practice.
Q: What gives you the most joy in your day-to-day practice?
Dr. Tennyson: I enjoy helping my patients make meaningful lifestyle changes that can positively impact digestive health and well-being. I try to address topics related to lifestyle medicine in most of my clinical visits including eating more fiber/plants, exercise, positive relationships, and stress management. Many of the conditions we treat as gastroenterologists can benefit from addressing aspects of lifestyle along with our conventional medical therapies. I reinforce that attention to these areas can make a difference. I enjoy sharing recipes, books, and websites that I have found helpful.
Q: How has your job changed since you first began your career?
Dr. Tennyson: After fellowship, I joined the faculty at Columbia University and worked at the Celiac Disease Center seeing patients, teaching, and performing clinical research under the mentorship of Peter Green, MD, and Suzanne Lewis, MD. It was a great opportunity to learn and practice in a tertiary center. I later switched roles and joined a general multispecialty community practice in Brooklyn [N.Y.] affiliated with an academic medical center. After practicing in New York for 10 years, I left my clinical practice and performed locums work for several years in underserved rural areas. I enjoyed working in rural areas and took a permanent position at a community hospital in Virginia’s Shenandoah Valley.
Q: Describe your biggest practice-related challenge and what you are doing to address it.
Dr. Tennyson: The small, rural community hospital where I currently work does not have the same resources and staffing as urban tertiary centers. While we are taking care of the community in our general gastroenterology practice, we’ve also launched an integrated care model in our hospital. We have collaborated with behavioral health, dietitians, nurses, health coaches, exercise physiologists as well as other members of the community, including farmers and a chef. We have performed some innovative, engaging programs, including fermentation workshops, cooking classes, farm walks, and mindfulness programs.
Q: What are you most proud of accomplishing?
Dr. Tennyson: I am proud that I took a nontraditional path after training to do what I enjoy and find rewarding. I received certification during GI fellowship at Mount Sinai [N.Y.] as a physician nutrition specialist. I later completed a fellowship in integrative medicine at the University of Arizona, received certification in lifestyle medicine, and completed coursework in culinary medicine. I really enjoyed doing locums work taking care of patients in other parts of the country, like Mississippi or Maine. I’ve enjoyed working in more rural areas and bringing some innovative programs to the community.
Q: What teacher or mentor had the greatest impact on you?
Dr. Tennyson: Dr. Anthony Grieco while I was a student at NYU. He is an astute clinician, always listened to his patients, loved clinical medicine, and had an endless fund of knowledge. I wanted to be a doctor like him. During my fellowship at Mount Sinai, I was also exposed to many great mentors including Dr. Lloyd Mayer, Dr. Jerome Waye, Dr. Steve Itzkowitz, and Dr. Blair Lewis who encouraged my interest in nutrition and small bowel diseases.
Lightning round
What's your superpower?
Finding fun in mundane things
Favorite movie to quote?
The Princess Bride
What is your favorite form of exercise?
A hike in the woods
Name one thing on your bucket list.
Galapagos Islands trip before my kids grow up
Cats or dogs?
Dogs
Summer or winter?
Summer
Old drug verapamil may have new use in type 1 diabetes
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.