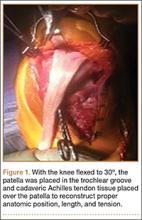
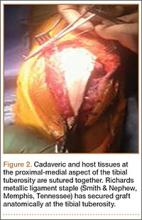
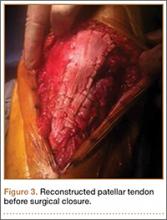
User login
Intra-Articular Dislocation of the Patella With Associated Hoffa Fracture in a Skeletally Immature Patient
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
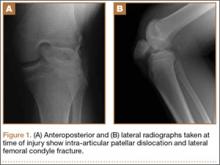
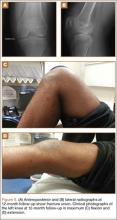
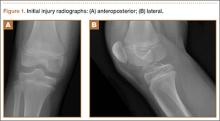
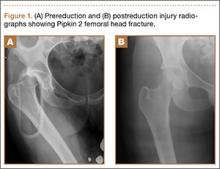
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
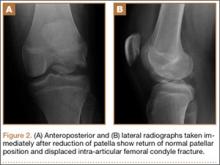
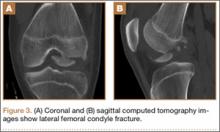
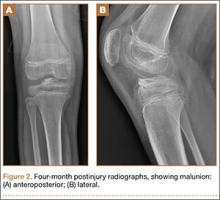
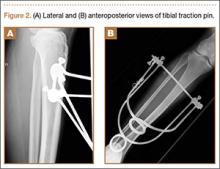
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
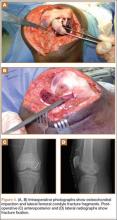
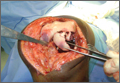
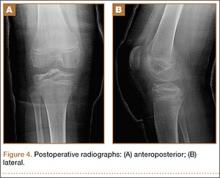
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
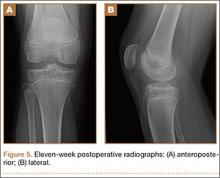
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
Primary Apocrine Adenocarcinoma of the Axilla
Primary apocrine adenocarcinoma (AA) is a rare cutaneous malignancy, with most of the available information about this disease consolidated from anecdotal evidence of single case reports and small case series with fewer than 30 patients.1-11 Although certain histologic and immunohistochemical features have been suggested to be useful in the diagnosis of AA, there is no clear consensus on the required pathologic criteria.1,5,6,9,10,12,13 Additionally, the clinical presentation of AA is highly variable, which further adds to the challenge of making an accurate diagnosis.1-3,5,9,10,13
Apocrine adenocarcinoma usually arises in areas of high apocrine gland density such as the axillae or anogenital region.2,4,6 It also has been reported in areas such as the scalp, ear canal, eyelids, chest, nipples, arms, wrists, and fingers.4,8,10,14-16 Apocrine adenocarcinoma in unusual locations such as the eyelid and ear canal is thought to arise from modified apocrine glands such as the Moll glands of the eyelid and the ceruminous glands of the ear canal.9,10 The presence of ectopic apocrine glands may lead to AA in atypical sites such as the wrists and fingers.5,16 The areola is an apocrine-dense area; therefore, AA may present on the nipples or within supernumerary nipples anywhere along the milk lines.4
Apocrine adenocarcinoma clinically presents as an asymptomatic to slightly painful, slowly growing, and erythematous to violaceous nodule or tumor.4,6,9 However, in a minority of cases the initial presentation consists of a cystic or ulcerated mass with overlying granulation tissue and purulent discharge.6,9,11 A wide time frame from the onset of symptoms to diagnosis has been reported, ranging from weeks to decades.4,6-8 The conventional treatment of AA is wide local excision.2,4,6,9 Although AA often presents with local lymph node metastasis at the time of diagnosis, there is no consensus on the use of sentinel lymph node biopsy (SLNB), nodal dissection, or adjuvant chemoradiation therapy.1,3,8,9
We report the case of a 49-year-old man with primary AA of the left axilla; the clinical and histologic features of AA as well as the appropriate diagnostic and treatment modalities also are provided.
Case Report
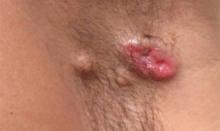
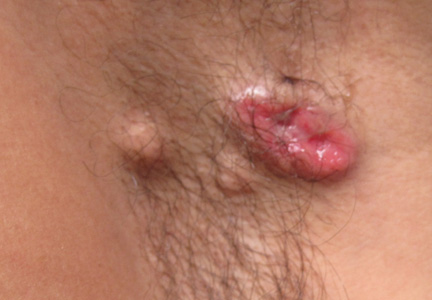
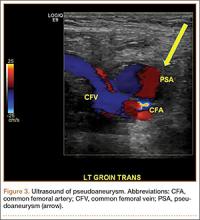
A 49-year-old man with a slowly growing tender mass of the left axilla of 1 year’s duration was referred to our dermatology clinic for evaluation. A review of systems revealed loss of appetite, fatigue, and a 4-month history of unintentional weight loss (15–20 lb). The patient had a history of hepatitis C virus, intravenous drug use, alcohol abuse, and cigarette smoking (1 pack daily) for many years. Additionally, the patient reported a paternal family history of numerous visceral malignancies. Examination of the left axilla revealed a 1.5×5-cm ulcerated tumor that produced serosanguineous discharge and was tender to palpation (Figure 1). Two 1-cm, firm, freely mobile subcutaneous nodules with no overlying skin changes were palpable at the medial border of the ulcerated nodule. There was no additional cervical or axillary lymphadenopathy, and a breast examination was normal.
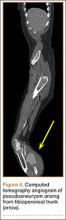
The differential diagnosis included primary squamous cell carcinoma or adnexal neoplasm, primary breast carcinoma, lymphoma, scrofuloderma, atypical mycobacterial infection, and cutaneous metastasis from an internal malignancy. Two 4-mm punch biopsies were performed and sent for routine histopathology and bacterial, fungal, and mycobacterial tissue cultures. To exclude a primary visceral malignancy or metastasis, computed tomography of the chest, abdomen, and pelvis; positron emission tomography (PET) from the base of the skull to the thighs; colonoscopy; magnetic resonance imaging of the brain; esophagogastroduodenoscopy; and mammography were conducted. Prominent left axillary lymphadenopathy was noted on computed tomography. Additionally, PET identified extranodal spread in the left axilla, left lateral chest wall, and the left sternocleidomastoid region. Furthermore, a 1-cm hypermetabolic nodule involving the right rectus abdominus muscle was noted on the PET scan. Based on their appearance, the nodules most likely represented metastasis from a primary skin malignancy. The rest of the studies were unremarkable. Serum tumor markers including prostate-specific antigen, cancer antigen 19-9, and carcinoembryonic antigen were within reference range. Immunostaining for estrogen receptor, progesterone receptor, and ERBB2 (formerly HER2/neu) was negative. The only abnormalities noted on serum chemistries were slight elevations in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and the a-fetoprotein tumor marker, which was attributed to chronic hepatitis C infection. Bacterial, fungal, and mycobacterial tissue cultures also were negative. These results ruled out infection and suggested against a primary visceral malignancy with cutaneous metastasis.
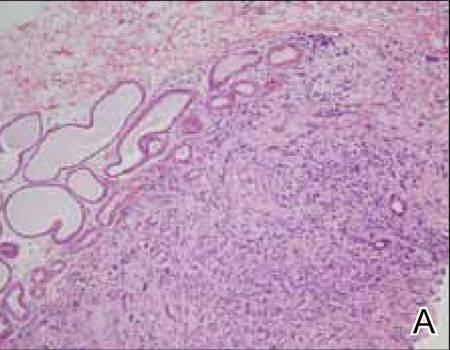
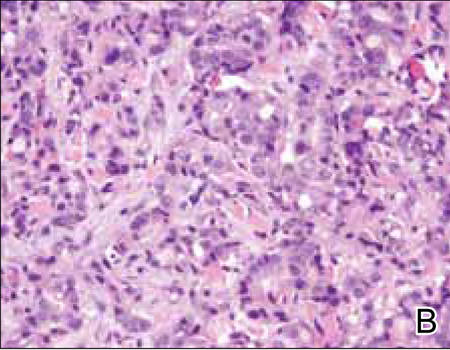
Histopathology revealed a moderately differentiated adenocarcinoma adjacent to healthy-appearing apocrine glands (Figure 2A). The normal glands were composed of cuboidal cells with abundant eosinophilic cytoplasm and prominent nuclei. The cells were arranged in a single layer in a glandular formation with prominent decapitation secretion. Adjacent to the normal apocrine glandular tissue was a focus of malignant epithelioid cells that extended to the lateral and inferior margins. The neoplastic cells were cuboidal to angulated in appearance with prominent nuclei and seemed to form ill-defined tubular or glandular structures that partially resembled apocrine glands (Figure 2B). Decapitation secretion is a feature of apocrine differentiation. Examination of additional tissue sections of the tumor did not reveal remarkable decapitation secretion in contrast to the adjacent healthy apocrine glands. Rather, a solid sheet arrangement was primarily noted in several sections (Figure 2B). Neither frequent mitoses nor prominent cellular atypia were seen, and there was no evidence of lymphatic, perineural, or vascular invasion.
  |
Immunohistochemically, tumor cells reacted strongly to cytokeratin AE1/AE3 and CAM5.2, stains used to identify various cytokeratins present in epithelial tissue. Staining for epithelial membrane antigen and carcinoembryonic antigen revealed focal glandular differentiation, which further supported the epithelial origin of the neoplastic cells. Gross cystic disease fluid protein 15 (GCDFP-15) is a marker of apocrine differentiation and may indicate a carcinoma of apocrine or eccrine origin. In our case, staining for GCDFP-15 was negative in the cutaneous sections but highlighted tumor cells in 6 of 13 ipsilateral lymph nodes from locoregional metastasis. The cellular and structural morphology, immunohistochemistry, and absence of an alternative primary visceral malignancy supported the diagnosis of primary AA.
Initially the patient was not considered to be a candidate for surgery due to the rapid growth of the tumor with metastases, fatigue, weight loss, and pain. Therefore, radiation therapy was started. The patient responded well to treatment with controlled pain and resolution of the palpable mass of the left axilla. Moreover, a follow-up PET scan revealed no residual tumor and persistent, albeit decreased, axillary lymphadenopathy. As the patient’s clinical status had improved, excision of the left axillary tumor with lymph node dissection was performed 10 months after initial presentation.
In this case, the differential diagnosis consisted of various cutaneous neoplasms, primary mammary carcinoma, cutaneous metastasis, and infection. Diagnostic imaging and laboratory testing failed to identify any primary internal malignancies. Similarly, the negative cultures ruled out an infectious process. Furthermore, the axillary mass was noted to be separate from the breast tissue on physical examination and mammography. Histologically, the tumor showed features that were suggestive of an anaplastic process as well as decapitation secretion and glandular formation that clearly resembled apocrine differentiation.
Comment
Apocrine adenocarcinoma arises from apocrine sweat glands and therefore is mostly reported in areas of high apocrine gland density such as the axillae and the anogenital region.2,4,6 However, AA also has been reported in unusual locations,1,5,10,14-16 and they may arise from a pre-existing nevus sebaceous or from supernumerary nipples, which can occur anywhere along the milk lines.4,15 Apocrine adenocarcinoma most commonly arises in individuals aged 40 to 50 years.3,17 A slight male predominance has been reported but no racial predilection.1,4-6 Although a few reports have described the development of AAs within pre-existing benign tumors such as apocrine adenomas, apocrine hyperplasias, cylindromas, and nevi sebaceous, they usually are thought to arise de novo.4-6
Clinical Presentation
Apocrine adenocarcinoma is highly variable in its clinical manifestation.1,6 Most cases arise as erythematous to violaceous, firm, solitary nodules. Nonetheless, AA also can present as erythematous patches of skin resembling erysipelas and ulcerated nodules with overlying granulation tissue and purulent exudate.4,6,9,11 Although AA typically is slow growing and indolent, the time frame reported from onset to diagnosis ranges from weeks to decades.1,6,7 Most cases present asymptomatically; when symptoms do occur, the most common ones are tenderness, purulent discharge, and restricted range of motion from extremely large tumors.3,9 Incidence of lymph node metastasis is reported at 40% to 50% at the time of presentation.4,6 Additionally, AA has a high rate of local recurrence, but extranodal metastasis rarely is seen.2,6 When metastasis does occur, it is via lymphatic and hematogenous spread.6,9 Metastatic dissemination of AA may occur in the liver, lungs, bone, brain, and parotid glands, as well as the skin via intraepidermal pagetoid spread.4,6,9,13
Histopathology
The histologic characteristics essential to the diagnosis of primary AA are anaplastic differentiation and apocrine origin.1,2,9,10,17 Apocrine units include coiled secretory glands that reside in the deep dermis connecting to a straight duct that empties into the isthmus of the hair follicle.9,13 These secretory glands have a single row of cuboidal secretory cells lining the tubular component and stratified squamous epithelium lining the straight intradermal component that opens onto the hair follicle.9 Contractile myoepithelial cells surround the secretory cell layer of the gland.9,13
The cuboidal secretory cells of the apocrine gland have abundant eosinophilic cytoplasm1,4,9 and are further characterized by glandular arrangement and decapitation secretion, 2 features that are strongly suggestive of apocrine differentiation.4-6 In contrast, the tumor cells of AA can be characterized by hyperchromatic nuclei, nuclear pleomorphism, mitotic figures, and a lack of decapitation secretion.1,2,6 In malignancy, erratic or poorly differentiated ductal structures may be seen,1,3-6 including papillary, cordlike, solid, or complex glandular patterns that can potentially invade the adjacent tissue without a clearly recognizable myoepithelial layer that contains them.1,3,4,6 Moreover, AA may progress with lymphatic, vascular, or neural invasion.1,13
Various stains may be used in immunohistochemical analysis to aid in the diagnosis of AA.1,5 Cytokeratin AE1/AE3, CAM5.2, epithelial membrane antigen, smooth muscle antigen, periodic acid–Schiff positivity with diastase resistance, and GCDFP-15 are useful in supporting the diagnosis of AA.2,6,10,17 Cytokeratin AE1/AE3 and CAM5.2 stain various cytokeratins to confirm the epithelial origin of the tissue.2 Epithelial membrane antigen is an antigen present on the apical surface of glandular epithelial cells that also has been used to identify epithelial cells in AA.2 Additionally, smooth muscle actin may be used to detect the myoepithelial layer of cells surrounding the apocrine glands.17 The lack of a continuous layer surrounding the secretory cells suggests invasion into the adjacent tissue.1,9,17 Periodic acid–Schiff staining with diastase resistance can be used to identify the mucin stored in the intracytoplasmic granules of apocrine cells and the lumen.3 Some stains such as GCDFP-15 may highlight cells of multiple origins (eg, apocrine and eccrine).10 However, there is the possibility that poorly differentiated AAs would fail to be identified as such even with well-established apocrine markers, which may explain the differential GCDFP-15 staining patterns in our patient’s skin and lymph node sections.1,5 Therefore, there is not a single perfect set of immunohistological criteria to aid in the diagnosis of AA.6,10,12 Fundamentally, diagnosis requires detection of primary apocrine differentiation with features such as invasion or spread to adjacent tissue to suggest malignancy and rule out an alternate primary malignant process.1,2,9,10,17
Treatment and Prognosis
Primary treatment of AA consists of wide local excision with adjuvant options that include chemotherapy and radiation.2,6 Due to the high rate of lymph node metastases at presentation (40%–50%), SLNB is recommended. A positive SLNB should be followed with complete axillary lymphadenectomy4,6; however, there is a lack of consensus regarding the role of SLNB and lymph node dissection in detecting subclinical lymph node disease, which might improve local recurrence rate and prognosis.6 Similarly, research shows variable results with adjunctive treatment such as chemotherapy or radiation therapy.6,9,13 Adjuvant treatment with chemotherapy or radiation therapy should be considered in cases with large tumor size; perineural, lymphatic, or vascular invasion; or when complete removal of the tumor is not possible due to location or size.2,6 However, neither the role nor the efficacy of such treatments in AA is well established.6,9,13
There is little information in the literature regarding the prognosis of AA. Although no specific or well-documented prognostic criteria exist, it is generally believed that patients with well-differentiated AA will have higher cure rates or lower rates of local recurrence and lymph node metastasis than patients with poorly differentiated neoplasms.3,6,10 A few small case series with long-term follow-up of patients ranging from 2 to 10 years have shown that prognosis may be favorable for AA patients despite local recurrence and regional lymph node metastasis.1,5
Conclusion
Primary AA is a rare cutaneous neoplasm that most commonly occurs in the axillae and the anogenital region. Apocrine adenocarcinoma presents with highly variable clinical and histopathological findings that make diagnosis a challenge. Clinicians should keep this entity in their differential diagnosis for patients who present with nodules arising in apocrine gland–bearing skin. Ultimately, histopathology is critical to diagnosis, and special stains are often required. To make the diagnosis, a tissue biopsy demonstrating apocrine differentiation and anaplastic features to suggest a malignant process are required. Additionally, a careful workup to rule out other diagnoses should be performed. Testing modalities that detect the presence of useful markers such as apocrine or epithelial origin should be used, and the presence of positive findings should support the diagnosis of AA. However, immunohistochemical findings should be used in the context of the patient’s clinical presentation and other available data. Treatment includes wide local excision, and lymphadenectomy is recommended in the setting of nodal spread. For aggressive tumors or metastases, excision may be followed by radiation therapy and chemotherapy.
1. Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
2. Cham PM, Niehans GA, Foman N, et al. Primary cutaneous apocrine carcinoma presenting as carcinoma erysipeloides [published online ahead of print November 6, 2007]. Br J Dermatol. 2008;158:194-196.
3. Chamberlain RS, Huber K, White JC, et al. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22:131-135.
4. Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
5. Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
6. Katagiri Y, Ansai S. Two cases of cutaneous apocrine ductal carcinoma of the axilla. case report and review of the literature. Dermatology. 1999;199:332-337.
7. Maury G, Guillot B, Bessis D, et al. Unusual axillary apocrine carcinoma of the skin: histological diagnostic difficulties [article in French] [published online ahead of print July 7, 2010]. Ann Dermatol Venereol. 2010;137:555-559.
8. Alex G. Apocrine adenocarcinoma of the nipple: a case report. Cases J. 2008;1:88.
9. MacNeill KN, Riddell RH, Ghazarian D. Perianal apocrine adenocarcinoma arising in a benign apocrine adenoma; first case report and review of the literature. J Clin Pathol. 2005;58:217-219.
10. Shintaku M, Tsuta K, Yoshida H, et al. Apocrine adenocarcinoma of the eyelid with aggressive biological behavior: report of a case. Pathol Int. 2002;52:169-173.
11. Zehr KJ, Rubin M, Ratner L. Apocrine adenocarcinoma presenting as a large ulcerated axillary mass. Dermatol Surg. 1997;23:585-587.
12. Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:141-142.
13. Hernandez JM, Copeland EM 3rd. Infiltrating apocrine adenocarcinoma with extramammary pagetoid spread. Am Surg. 2007;73:307-309.
14. Dhawan SS, Nanda VS, Grekin S, et al. Apocrine adenocarcinoma: case report and review of the literature. J Dermatol Surg Oncol. 1990;16:468-470.
15. Hügel H, Requena L. Ductal carcinoma arising from a syringocystadenoma papilliferum in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 2003;25:490-493.
16. Stout AP, Cooley SG. Carcinoma of sweat glands. Cancer. 1951;4:521-536.
17. Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms—part 2: an approach to tumours of cutaneous sweat glands [published online ahead of print August 1, 2006]. J Clin Pathol. 2007;60:145-159.
Primary apocrine adenocarcinoma (AA) is a rare cutaneous malignancy, with most of the available information about this disease consolidated from anecdotal evidence of single case reports and small case series with fewer than 30 patients.1-11 Although certain histologic and immunohistochemical features have been suggested to be useful in the diagnosis of AA, there is no clear consensus on the required pathologic criteria.1,5,6,9,10,12,13 Additionally, the clinical presentation of AA is highly variable, which further adds to the challenge of making an accurate diagnosis.1-3,5,9,10,13
Apocrine adenocarcinoma usually arises in areas of high apocrine gland density such as the axillae or anogenital region.2,4,6 It also has been reported in areas such as the scalp, ear canal, eyelids, chest, nipples, arms, wrists, and fingers.4,8,10,14-16 Apocrine adenocarcinoma in unusual locations such as the eyelid and ear canal is thought to arise from modified apocrine glands such as the Moll glands of the eyelid and the ceruminous glands of the ear canal.9,10 The presence of ectopic apocrine glands may lead to AA in atypical sites such as the wrists and fingers.5,16 The areola is an apocrine-dense area; therefore, AA may present on the nipples or within supernumerary nipples anywhere along the milk lines.4
Apocrine adenocarcinoma clinically presents as an asymptomatic to slightly painful, slowly growing, and erythematous to violaceous nodule or tumor.4,6,9 However, in a minority of cases the initial presentation consists of a cystic or ulcerated mass with overlying granulation tissue and purulent discharge.6,9,11 A wide time frame from the onset of symptoms to diagnosis has been reported, ranging from weeks to decades.4,6-8 The conventional treatment of AA is wide local excision.2,4,6,9 Although AA often presents with local lymph node metastasis at the time of diagnosis, there is no consensus on the use of sentinel lymph node biopsy (SLNB), nodal dissection, or adjuvant chemoradiation therapy.1,3,8,9
We report the case of a 49-year-old man with primary AA of the left axilla; the clinical and histologic features of AA as well as the appropriate diagnostic and treatment modalities also are provided.
Case Report
A 49-year-old man with a slowly growing tender mass of the left axilla of 1 year’s duration was referred to our dermatology clinic for evaluation. A review of systems revealed loss of appetite, fatigue, and a 4-month history of unintentional weight loss (15–20 lb). The patient had a history of hepatitis C virus, intravenous drug use, alcohol abuse, and cigarette smoking (1 pack daily) for many years. Additionally, the patient reported a paternal family history of numerous visceral malignancies. Examination of the left axilla revealed a 1.5×5-cm ulcerated tumor that produced serosanguineous discharge and was tender to palpation (Figure 1). Two 1-cm, firm, freely mobile subcutaneous nodules with no overlying skin changes were palpable at the medial border of the ulcerated nodule. There was no additional cervical or axillary lymphadenopathy, and a breast examination was normal.
The differential diagnosis included primary squamous cell carcinoma or adnexal neoplasm, primary breast carcinoma, lymphoma, scrofuloderma, atypical mycobacterial infection, and cutaneous metastasis from an internal malignancy. Two 4-mm punch biopsies were performed and sent for routine histopathology and bacterial, fungal, and mycobacterial tissue cultures. To exclude a primary visceral malignancy or metastasis, computed tomography of the chest, abdomen, and pelvis; positron emission tomography (PET) from the base of the skull to the thighs; colonoscopy; magnetic resonance imaging of the brain; esophagogastroduodenoscopy; and mammography were conducted. Prominent left axillary lymphadenopathy was noted on computed tomography. Additionally, PET identified extranodal spread in the left axilla, left lateral chest wall, and the left sternocleidomastoid region. Furthermore, a 1-cm hypermetabolic nodule involving the right rectus abdominus muscle was noted on the PET scan. Based on their appearance, the nodules most likely represented metastasis from a primary skin malignancy. The rest of the studies were unremarkable. Serum tumor markers including prostate-specific antigen, cancer antigen 19-9, and carcinoembryonic antigen were within reference range. Immunostaining for estrogen receptor, progesterone receptor, and ERBB2 (formerly HER2/neu) was negative. The only abnormalities noted on serum chemistries were slight elevations in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and the a-fetoprotein tumor marker, which was attributed to chronic hepatitis C infection. Bacterial, fungal, and mycobacterial tissue cultures also were negative. These results ruled out infection and suggested against a primary visceral malignancy with cutaneous metastasis.
Histopathology revealed a moderately differentiated adenocarcinoma adjacent to healthy-appearing apocrine glands (Figure 2A). The normal glands were composed of cuboidal cells with abundant eosinophilic cytoplasm and prominent nuclei. The cells were arranged in a single layer in a glandular formation with prominent decapitation secretion. Adjacent to the normal apocrine glandular tissue was a focus of malignant epithelioid cells that extended to the lateral and inferior margins. The neoplastic cells were cuboidal to angulated in appearance with prominent nuclei and seemed to form ill-defined tubular or glandular structures that partially resembled apocrine glands (Figure 2B). Decapitation secretion is a feature of apocrine differentiation. Examination of additional tissue sections of the tumor did not reveal remarkable decapitation secretion in contrast to the adjacent healthy apocrine glands. Rather, a solid sheet arrangement was primarily noted in several sections (Figure 2B). Neither frequent mitoses nor prominent cellular atypia were seen, and there was no evidence of lymphatic, perineural, or vascular invasion.
  |
Immunohistochemically, tumor cells reacted strongly to cytokeratin AE1/AE3 and CAM5.2, stains used to identify various cytokeratins present in epithelial tissue. Staining for epithelial membrane antigen and carcinoembryonic antigen revealed focal glandular differentiation, which further supported the epithelial origin of the neoplastic cells. Gross cystic disease fluid protein 15 (GCDFP-15) is a marker of apocrine differentiation and may indicate a carcinoma of apocrine or eccrine origin. In our case, staining for GCDFP-15 was negative in the cutaneous sections but highlighted tumor cells in 6 of 13 ipsilateral lymph nodes from locoregional metastasis. The cellular and structural morphology, immunohistochemistry, and absence of an alternative primary visceral malignancy supported the diagnosis of primary AA.
Initially the patient was not considered to be a candidate for surgery due to the rapid growth of the tumor with metastases, fatigue, weight loss, and pain. Therefore, radiation therapy was started. The patient responded well to treatment with controlled pain and resolution of the palpable mass of the left axilla. Moreover, a follow-up PET scan revealed no residual tumor and persistent, albeit decreased, axillary lymphadenopathy. As the patient’s clinical status had improved, excision of the left axillary tumor with lymph node dissection was performed 10 months after initial presentation.
In this case, the differential diagnosis consisted of various cutaneous neoplasms, primary mammary carcinoma, cutaneous metastasis, and infection. Diagnostic imaging and laboratory testing failed to identify any primary internal malignancies. Similarly, the negative cultures ruled out an infectious process. Furthermore, the axillary mass was noted to be separate from the breast tissue on physical examination and mammography. Histologically, the tumor showed features that were suggestive of an anaplastic process as well as decapitation secretion and glandular formation that clearly resembled apocrine differentiation.
Comment
Apocrine adenocarcinoma arises from apocrine sweat glands and therefore is mostly reported in areas of high apocrine gland density such as the axillae and the anogenital region.2,4,6 However, AA also has been reported in unusual locations,1,5,10,14-16 and they may arise from a pre-existing nevus sebaceous or from supernumerary nipples, which can occur anywhere along the milk lines.4,15 Apocrine adenocarcinoma most commonly arises in individuals aged 40 to 50 years.3,17 A slight male predominance has been reported but no racial predilection.1,4-6 Although a few reports have described the development of AAs within pre-existing benign tumors such as apocrine adenomas, apocrine hyperplasias, cylindromas, and nevi sebaceous, they usually are thought to arise de novo.4-6
Clinical Presentation
Apocrine adenocarcinoma is highly variable in its clinical manifestation.1,6 Most cases arise as erythematous to violaceous, firm, solitary nodules. Nonetheless, AA also can present as erythematous patches of skin resembling erysipelas and ulcerated nodules with overlying granulation tissue and purulent exudate.4,6,9,11 Although AA typically is slow growing and indolent, the time frame reported from onset to diagnosis ranges from weeks to decades.1,6,7 Most cases present asymptomatically; when symptoms do occur, the most common ones are tenderness, purulent discharge, and restricted range of motion from extremely large tumors.3,9 Incidence of lymph node metastasis is reported at 40% to 50% at the time of presentation.4,6 Additionally, AA has a high rate of local recurrence, but extranodal metastasis rarely is seen.2,6 When metastasis does occur, it is via lymphatic and hematogenous spread.6,9 Metastatic dissemination of AA may occur in the liver, lungs, bone, brain, and parotid glands, as well as the skin via intraepidermal pagetoid spread.4,6,9,13
Histopathology
The histologic characteristics essential to the diagnosis of primary AA are anaplastic differentiation and apocrine origin.1,2,9,10,17 Apocrine units include coiled secretory glands that reside in the deep dermis connecting to a straight duct that empties into the isthmus of the hair follicle.9,13 These secretory glands have a single row of cuboidal secretory cells lining the tubular component and stratified squamous epithelium lining the straight intradermal component that opens onto the hair follicle.9 Contractile myoepithelial cells surround the secretory cell layer of the gland.9,13
The cuboidal secretory cells of the apocrine gland have abundant eosinophilic cytoplasm1,4,9 and are further characterized by glandular arrangement and decapitation secretion, 2 features that are strongly suggestive of apocrine differentiation.4-6 In contrast, the tumor cells of AA can be characterized by hyperchromatic nuclei, nuclear pleomorphism, mitotic figures, and a lack of decapitation secretion.1,2,6 In malignancy, erratic or poorly differentiated ductal structures may be seen,1,3-6 including papillary, cordlike, solid, or complex glandular patterns that can potentially invade the adjacent tissue without a clearly recognizable myoepithelial layer that contains them.1,3,4,6 Moreover, AA may progress with lymphatic, vascular, or neural invasion.1,13
Various stains may be used in immunohistochemical analysis to aid in the diagnosis of AA.1,5 Cytokeratin AE1/AE3, CAM5.2, epithelial membrane antigen, smooth muscle antigen, periodic acid–Schiff positivity with diastase resistance, and GCDFP-15 are useful in supporting the diagnosis of AA.2,6,10,17 Cytokeratin AE1/AE3 and CAM5.2 stain various cytokeratins to confirm the epithelial origin of the tissue.2 Epithelial membrane antigen is an antigen present on the apical surface of glandular epithelial cells that also has been used to identify epithelial cells in AA.2 Additionally, smooth muscle actin may be used to detect the myoepithelial layer of cells surrounding the apocrine glands.17 The lack of a continuous layer surrounding the secretory cells suggests invasion into the adjacent tissue.1,9,17 Periodic acid–Schiff staining with diastase resistance can be used to identify the mucin stored in the intracytoplasmic granules of apocrine cells and the lumen.3 Some stains such as GCDFP-15 may highlight cells of multiple origins (eg, apocrine and eccrine).10 However, there is the possibility that poorly differentiated AAs would fail to be identified as such even with well-established apocrine markers, which may explain the differential GCDFP-15 staining patterns in our patient’s skin and lymph node sections.1,5 Therefore, there is not a single perfect set of immunohistological criteria to aid in the diagnosis of AA.6,10,12 Fundamentally, diagnosis requires detection of primary apocrine differentiation with features such as invasion or spread to adjacent tissue to suggest malignancy and rule out an alternate primary malignant process.1,2,9,10,17
Treatment and Prognosis
Primary treatment of AA consists of wide local excision with adjuvant options that include chemotherapy and radiation.2,6 Due to the high rate of lymph node metastases at presentation (40%–50%), SLNB is recommended. A positive SLNB should be followed with complete axillary lymphadenectomy4,6; however, there is a lack of consensus regarding the role of SLNB and lymph node dissection in detecting subclinical lymph node disease, which might improve local recurrence rate and prognosis.6 Similarly, research shows variable results with adjunctive treatment such as chemotherapy or radiation therapy.6,9,13 Adjuvant treatment with chemotherapy or radiation therapy should be considered in cases with large tumor size; perineural, lymphatic, or vascular invasion; or when complete removal of the tumor is not possible due to location or size.2,6 However, neither the role nor the efficacy of such treatments in AA is well established.6,9,13
There is little information in the literature regarding the prognosis of AA. Although no specific or well-documented prognostic criteria exist, it is generally believed that patients with well-differentiated AA will have higher cure rates or lower rates of local recurrence and lymph node metastasis than patients with poorly differentiated neoplasms.3,6,10 A few small case series with long-term follow-up of patients ranging from 2 to 10 years have shown that prognosis may be favorable for AA patients despite local recurrence and regional lymph node metastasis.1,5
Conclusion
Primary AA is a rare cutaneous neoplasm that most commonly occurs in the axillae and the anogenital region. Apocrine adenocarcinoma presents with highly variable clinical and histopathological findings that make diagnosis a challenge. Clinicians should keep this entity in their differential diagnosis for patients who present with nodules arising in apocrine gland–bearing skin. Ultimately, histopathology is critical to diagnosis, and special stains are often required. To make the diagnosis, a tissue biopsy demonstrating apocrine differentiation and anaplastic features to suggest a malignant process are required. Additionally, a careful workup to rule out other diagnoses should be performed. Testing modalities that detect the presence of useful markers such as apocrine or epithelial origin should be used, and the presence of positive findings should support the diagnosis of AA. However, immunohistochemical findings should be used in the context of the patient’s clinical presentation and other available data. Treatment includes wide local excision, and lymphadenectomy is recommended in the setting of nodal spread. For aggressive tumors or metastases, excision may be followed by radiation therapy and chemotherapy.
Primary apocrine adenocarcinoma (AA) is a rare cutaneous malignancy, with most of the available information about this disease consolidated from anecdotal evidence of single case reports and small case series with fewer than 30 patients.1-11 Although certain histologic and immunohistochemical features have been suggested to be useful in the diagnosis of AA, there is no clear consensus on the required pathologic criteria.1,5,6,9,10,12,13 Additionally, the clinical presentation of AA is highly variable, which further adds to the challenge of making an accurate diagnosis.1-3,5,9,10,13
Apocrine adenocarcinoma usually arises in areas of high apocrine gland density such as the axillae or anogenital region.2,4,6 It also has been reported in areas such as the scalp, ear canal, eyelids, chest, nipples, arms, wrists, and fingers.4,8,10,14-16 Apocrine adenocarcinoma in unusual locations such as the eyelid and ear canal is thought to arise from modified apocrine glands such as the Moll glands of the eyelid and the ceruminous glands of the ear canal.9,10 The presence of ectopic apocrine glands may lead to AA in atypical sites such as the wrists and fingers.5,16 The areola is an apocrine-dense area; therefore, AA may present on the nipples or within supernumerary nipples anywhere along the milk lines.4
Apocrine adenocarcinoma clinically presents as an asymptomatic to slightly painful, slowly growing, and erythematous to violaceous nodule or tumor.4,6,9 However, in a minority of cases the initial presentation consists of a cystic or ulcerated mass with overlying granulation tissue and purulent discharge.6,9,11 A wide time frame from the onset of symptoms to diagnosis has been reported, ranging from weeks to decades.4,6-8 The conventional treatment of AA is wide local excision.2,4,6,9 Although AA often presents with local lymph node metastasis at the time of diagnosis, there is no consensus on the use of sentinel lymph node biopsy (SLNB), nodal dissection, or adjuvant chemoradiation therapy.1,3,8,9
We report the case of a 49-year-old man with primary AA of the left axilla; the clinical and histologic features of AA as well as the appropriate diagnostic and treatment modalities also are provided.
Case Report
A 49-year-old man with a slowly growing tender mass of the left axilla of 1 year’s duration was referred to our dermatology clinic for evaluation. A review of systems revealed loss of appetite, fatigue, and a 4-month history of unintentional weight loss (15–20 lb). The patient had a history of hepatitis C virus, intravenous drug use, alcohol abuse, and cigarette smoking (1 pack daily) for many years. Additionally, the patient reported a paternal family history of numerous visceral malignancies. Examination of the left axilla revealed a 1.5×5-cm ulcerated tumor that produced serosanguineous discharge and was tender to palpation (Figure 1). Two 1-cm, firm, freely mobile subcutaneous nodules with no overlying skin changes were palpable at the medial border of the ulcerated nodule. There was no additional cervical or axillary lymphadenopathy, and a breast examination was normal.
The differential diagnosis included primary squamous cell carcinoma or adnexal neoplasm, primary breast carcinoma, lymphoma, scrofuloderma, atypical mycobacterial infection, and cutaneous metastasis from an internal malignancy. Two 4-mm punch biopsies were performed and sent for routine histopathology and bacterial, fungal, and mycobacterial tissue cultures. To exclude a primary visceral malignancy or metastasis, computed tomography of the chest, abdomen, and pelvis; positron emission tomography (PET) from the base of the skull to the thighs; colonoscopy; magnetic resonance imaging of the brain; esophagogastroduodenoscopy; and mammography were conducted. Prominent left axillary lymphadenopathy was noted on computed tomography. Additionally, PET identified extranodal spread in the left axilla, left lateral chest wall, and the left sternocleidomastoid region. Furthermore, a 1-cm hypermetabolic nodule involving the right rectus abdominus muscle was noted on the PET scan. Based on their appearance, the nodules most likely represented metastasis from a primary skin malignancy. The rest of the studies were unremarkable. Serum tumor markers including prostate-specific antigen, cancer antigen 19-9, and carcinoembryonic antigen were within reference range. Immunostaining for estrogen receptor, progesterone receptor, and ERBB2 (formerly HER2/neu) was negative. The only abnormalities noted on serum chemistries were slight elevations in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and the a-fetoprotein tumor marker, which was attributed to chronic hepatitis C infection. Bacterial, fungal, and mycobacterial tissue cultures also were negative. These results ruled out infection and suggested against a primary visceral malignancy with cutaneous metastasis.
Histopathology revealed a moderately differentiated adenocarcinoma adjacent to healthy-appearing apocrine glands (Figure 2A). The normal glands were composed of cuboidal cells with abundant eosinophilic cytoplasm and prominent nuclei. The cells were arranged in a single layer in a glandular formation with prominent decapitation secretion. Adjacent to the normal apocrine glandular tissue was a focus of malignant epithelioid cells that extended to the lateral and inferior margins. The neoplastic cells were cuboidal to angulated in appearance with prominent nuclei and seemed to form ill-defined tubular or glandular structures that partially resembled apocrine glands (Figure 2B). Decapitation secretion is a feature of apocrine differentiation. Examination of additional tissue sections of the tumor did not reveal remarkable decapitation secretion in contrast to the adjacent healthy apocrine glands. Rather, a solid sheet arrangement was primarily noted in several sections (Figure 2B). Neither frequent mitoses nor prominent cellular atypia were seen, and there was no evidence of lymphatic, perineural, or vascular invasion.
  |
Immunohistochemically, tumor cells reacted strongly to cytokeratin AE1/AE3 and CAM5.2, stains used to identify various cytokeratins present in epithelial tissue. Staining for epithelial membrane antigen and carcinoembryonic antigen revealed focal glandular differentiation, which further supported the epithelial origin of the neoplastic cells. Gross cystic disease fluid protein 15 (GCDFP-15) is a marker of apocrine differentiation and may indicate a carcinoma of apocrine or eccrine origin. In our case, staining for GCDFP-15 was negative in the cutaneous sections but highlighted tumor cells in 6 of 13 ipsilateral lymph nodes from locoregional metastasis. The cellular and structural morphology, immunohistochemistry, and absence of an alternative primary visceral malignancy supported the diagnosis of primary AA.
Initially the patient was not considered to be a candidate for surgery due to the rapid growth of the tumor with metastases, fatigue, weight loss, and pain. Therefore, radiation therapy was started. The patient responded well to treatment with controlled pain and resolution of the palpable mass of the left axilla. Moreover, a follow-up PET scan revealed no residual tumor and persistent, albeit decreased, axillary lymphadenopathy. As the patient’s clinical status had improved, excision of the left axillary tumor with lymph node dissection was performed 10 months after initial presentation.
In this case, the differential diagnosis consisted of various cutaneous neoplasms, primary mammary carcinoma, cutaneous metastasis, and infection. Diagnostic imaging and laboratory testing failed to identify any primary internal malignancies. Similarly, the negative cultures ruled out an infectious process. Furthermore, the axillary mass was noted to be separate from the breast tissue on physical examination and mammography. Histologically, the tumor showed features that were suggestive of an anaplastic process as well as decapitation secretion and glandular formation that clearly resembled apocrine differentiation.
Comment
Apocrine adenocarcinoma arises from apocrine sweat glands and therefore is mostly reported in areas of high apocrine gland density such as the axillae and the anogenital region.2,4,6 However, AA also has been reported in unusual locations,1,5,10,14-16 and they may arise from a pre-existing nevus sebaceous or from supernumerary nipples, which can occur anywhere along the milk lines.4,15 Apocrine adenocarcinoma most commonly arises in individuals aged 40 to 50 years.3,17 A slight male predominance has been reported but no racial predilection.1,4-6 Although a few reports have described the development of AAs within pre-existing benign tumors such as apocrine adenomas, apocrine hyperplasias, cylindromas, and nevi sebaceous, they usually are thought to arise de novo.4-6
Clinical Presentation
Apocrine adenocarcinoma is highly variable in its clinical manifestation.1,6 Most cases arise as erythematous to violaceous, firm, solitary nodules. Nonetheless, AA also can present as erythematous patches of skin resembling erysipelas and ulcerated nodules with overlying granulation tissue and purulent exudate.4,6,9,11 Although AA typically is slow growing and indolent, the time frame reported from onset to diagnosis ranges from weeks to decades.1,6,7 Most cases present asymptomatically; when symptoms do occur, the most common ones are tenderness, purulent discharge, and restricted range of motion from extremely large tumors.3,9 Incidence of lymph node metastasis is reported at 40% to 50% at the time of presentation.4,6 Additionally, AA has a high rate of local recurrence, but extranodal metastasis rarely is seen.2,6 When metastasis does occur, it is via lymphatic and hematogenous spread.6,9 Metastatic dissemination of AA may occur in the liver, lungs, bone, brain, and parotid glands, as well as the skin via intraepidermal pagetoid spread.4,6,9,13
Histopathology
The histologic characteristics essential to the diagnosis of primary AA are anaplastic differentiation and apocrine origin.1,2,9,10,17 Apocrine units include coiled secretory glands that reside in the deep dermis connecting to a straight duct that empties into the isthmus of the hair follicle.9,13 These secretory glands have a single row of cuboidal secretory cells lining the tubular component and stratified squamous epithelium lining the straight intradermal component that opens onto the hair follicle.9 Contractile myoepithelial cells surround the secretory cell layer of the gland.9,13
The cuboidal secretory cells of the apocrine gland have abundant eosinophilic cytoplasm1,4,9 and are further characterized by glandular arrangement and decapitation secretion, 2 features that are strongly suggestive of apocrine differentiation.4-6 In contrast, the tumor cells of AA can be characterized by hyperchromatic nuclei, nuclear pleomorphism, mitotic figures, and a lack of decapitation secretion.1,2,6 In malignancy, erratic or poorly differentiated ductal structures may be seen,1,3-6 including papillary, cordlike, solid, or complex glandular patterns that can potentially invade the adjacent tissue without a clearly recognizable myoepithelial layer that contains them.1,3,4,6 Moreover, AA may progress with lymphatic, vascular, or neural invasion.1,13
Various stains may be used in immunohistochemical analysis to aid in the diagnosis of AA.1,5 Cytokeratin AE1/AE3, CAM5.2, epithelial membrane antigen, smooth muscle antigen, periodic acid–Schiff positivity with diastase resistance, and GCDFP-15 are useful in supporting the diagnosis of AA.2,6,10,17 Cytokeratin AE1/AE3 and CAM5.2 stain various cytokeratins to confirm the epithelial origin of the tissue.2 Epithelial membrane antigen is an antigen present on the apical surface of glandular epithelial cells that also has been used to identify epithelial cells in AA.2 Additionally, smooth muscle actin may be used to detect the myoepithelial layer of cells surrounding the apocrine glands.17 The lack of a continuous layer surrounding the secretory cells suggests invasion into the adjacent tissue.1,9,17 Periodic acid–Schiff staining with diastase resistance can be used to identify the mucin stored in the intracytoplasmic granules of apocrine cells and the lumen.3 Some stains such as GCDFP-15 may highlight cells of multiple origins (eg, apocrine and eccrine).10 However, there is the possibility that poorly differentiated AAs would fail to be identified as such even with well-established apocrine markers, which may explain the differential GCDFP-15 staining patterns in our patient’s skin and lymph node sections.1,5 Therefore, there is not a single perfect set of immunohistological criteria to aid in the diagnosis of AA.6,10,12 Fundamentally, diagnosis requires detection of primary apocrine differentiation with features such as invasion or spread to adjacent tissue to suggest malignancy and rule out an alternate primary malignant process.1,2,9,10,17
Treatment and Prognosis
Primary treatment of AA consists of wide local excision with adjuvant options that include chemotherapy and radiation.2,6 Due to the high rate of lymph node metastases at presentation (40%–50%), SLNB is recommended. A positive SLNB should be followed with complete axillary lymphadenectomy4,6; however, there is a lack of consensus regarding the role of SLNB and lymph node dissection in detecting subclinical lymph node disease, which might improve local recurrence rate and prognosis.6 Similarly, research shows variable results with adjunctive treatment such as chemotherapy or radiation therapy.6,9,13 Adjuvant treatment with chemotherapy or radiation therapy should be considered in cases with large tumor size; perineural, lymphatic, or vascular invasion; or when complete removal of the tumor is not possible due to location or size.2,6 However, neither the role nor the efficacy of such treatments in AA is well established.6,9,13
There is little information in the literature regarding the prognosis of AA. Although no specific or well-documented prognostic criteria exist, it is generally believed that patients with well-differentiated AA will have higher cure rates or lower rates of local recurrence and lymph node metastasis than patients with poorly differentiated neoplasms.3,6,10 A few small case series with long-term follow-up of patients ranging from 2 to 10 years have shown that prognosis may be favorable for AA patients despite local recurrence and regional lymph node metastasis.1,5
Conclusion
Primary AA is a rare cutaneous neoplasm that most commonly occurs in the axillae and the anogenital region. Apocrine adenocarcinoma presents with highly variable clinical and histopathological findings that make diagnosis a challenge. Clinicians should keep this entity in their differential diagnosis for patients who present with nodules arising in apocrine gland–bearing skin. Ultimately, histopathology is critical to diagnosis, and special stains are often required. To make the diagnosis, a tissue biopsy demonstrating apocrine differentiation and anaplastic features to suggest a malignant process are required. Additionally, a careful workup to rule out other diagnoses should be performed. Testing modalities that detect the presence of useful markers such as apocrine or epithelial origin should be used, and the presence of positive findings should support the diagnosis of AA. However, immunohistochemical findings should be used in the context of the patient’s clinical presentation and other available data. Treatment includes wide local excision, and lymphadenectomy is recommended in the setting of nodal spread. For aggressive tumors or metastases, excision may be followed by radiation therapy and chemotherapy.
1. Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
2. Cham PM, Niehans GA, Foman N, et al. Primary cutaneous apocrine carcinoma presenting as carcinoma erysipeloides [published online ahead of print November 6, 2007]. Br J Dermatol. 2008;158:194-196.
3. Chamberlain RS, Huber K, White JC, et al. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22:131-135.
4. Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
5. Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
6. Katagiri Y, Ansai S. Two cases of cutaneous apocrine ductal carcinoma of the axilla. case report and review of the literature. Dermatology. 1999;199:332-337.
7. Maury G, Guillot B, Bessis D, et al. Unusual axillary apocrine carcinoma of the skin: histological diagnostic difficulties [article in French] [published online ahead of print July 7, 2010]. Ann Dermatol Venereol. 2010;137:555-559.
8. Alex G. Apocrine adenocarcinoma of the nipple: a case report. Cases J. 2008;1:88.
9. MacNeill KN, Riddell RH, Ghazarian D. Perianal apocrine adenocarcinoma arising in a benign apocrine adenoma; first case report and review of the literature. J Clin Pathol. 2005;58:217-219.
10. Shintaku M, Tsuta K, Yoshida H, et al. Apocrine adenocarcinoma of the eyelid with aggressive biological behavior: report of a case. Pathol Int. 2002;52:169-173.
11. Zehr KJ, Rubin M, Ratner L. Apocrine adenocarcinoma presenting as a large ulcerated axillary mass. Dermatol Surg. 1997;23:585-587.
12. Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:141-142.
13. Hernandez JM, Copeland EM 3rd. Infiltrating apocrine adenocarcinoma with extramammary pagetoid spread. Am Surg. 2007;73:307-309.
14. Dhawan SS, Nanda VS, Grekin S, et al. Apocrine adenocarcinoma: case report and review of the literature. J Dermatol Surg Oncol. 1990;16:468-470.
15. Hügel H, Requena L. Ductal carcinoma arising from a syringocystadenoma papilliferum in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 2003;25:490-493.
16. Stout AP, Cooley SG. Carcinoma of sweat glands. Cancer. 1951;4:521-536.
17. Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms—part 2: an approach to tumours of cutaneous sweat glands [published online ahead of print August 1, 2006]. J Clin Pathol. 2007;60:145-159.
1. Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
2. Cham PM, Niehans GA, Foman N, et al. Primary cutaneous apocrine carcinoma presenting as carcinoma erysipeloides [published online ahead of print November 6, 2007]. Br J Dermatol. 2008;158:194-196.
3. Chamberlain RS, Huber K, White JC, et al. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22:131-135.
4. Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
5. Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
6. Katagiri Y, Ansai S. Two cases of cutaneous apocrine ductal carcinoma of the axilla. case report and review of the literature. Dermatology. 1999;199:332-337.
7. Maury G, Guillot B, Bessis D, et al. Unusual axillary apocrine carcinoma of the skin: histological diagnostic difficulties [article in French] [published online ahead of print July 7, 2010]. Ann Dermatol Venereol. 2010;137:555-559.
8. Alex G. Apocrine adenocarcinoma of the nipple: a case report. Cases J. 2008;1:88.
9. MacNeill KN, Riddell RH, Ghazarian D. Perianal apocrine adenocarcinoma arising in a benign apocrine adenoma; first case report and review of the literature. J Clin Pathol. 2005;58:217-219.
10. Shintaku M, Tsuta K, Yoshida H, et al. Apocrine adenocarcinoma of the eyelid with aggressive biological behavior: report of a case. Pathol Int. 2002;52:169-173.
11. Zehr KJ, Rubin M, Ratner L. Apocrine adenocarcinoma presenting as a large ulcerated axillary mass. Dermatol Surg. 1997;23:585-587.
12. Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:141-142.
13. Hernandez JM, Copeland EM 3rd. Infiltrating apocrine adenocarcinoma with extramammary pagetoid spread. Am Surg. 2007;73:307-309.
14. Dhawan SS, Nanda VS, Grekin S, et al. Apocrine adenocarcinoma: case report and review of the literature. J Dermatol Surg Oncol. 1990;16:468-470.
15. Hügel H, Requena L. Ductal carcinoma arising from a syringocystadenoma papilliferum in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 2003;25:490-493.
16. Stout AP, Cooley SG. Carcinoma of sweat glands. Cancer. 1951;4:521-536.
17. Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms—part 2: an approach to tumours of cutaneous sweat glands [published online ahead of print August 1, 2006]. J Clin Pathol. 2007;60:145-159.
Practice Points
- Primary apocrine adenocarcinoma (AA) is a rare cutaneous malignancy with metastatic potential.
It arises in areas of high apocrine gland density including the axillae and anogenital region. - Apocrine adenocarcinoma must be differentiated from various infections and cutaneous metastases from internal malignancies.
- Primary apocrine differentiation with invasion to adjacent tissue is a key histopathologic feature of AA.
Significant response to lacosamide in a patient with severe chemotherapy-induced peripheral neuropathy
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Young Man With Headache, Confusion, and Hearing Loss
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 25-year-old male was found wandering naked in his room with the shower running after failing to come to work on a Monday morning. When found, he was able to talk and follow some commands but was confused about what was happening. He had experienced a right periorbital headache with nausea and vomiting for several days before admission. A computed tomography (CT) scan of the head at an outside hospital was negative.
Related: Pain, Anxiety, and Dementia: A Catastrophic Outcome
The patient had no history of tick or insect bites, skin rash, chest pain, shortness of breath, trauma, or illicit drug or alcohol use. He smoked a half pack of cigarettes per day. The patient had spent time in the military in the Middle East and North Africa 3 years earlier and had 3 tattoos. Over the past few months, he had been noted to be more aggressive, including having gotten into a bar fight. His past medical history was significant only for documented hearing loss in the right ear per reports from the air base.
On examination, the patient’s temperature was 97.3°F, heart rate 47 bpm, respirations 20 breathes/min, and blood pressure 97/60 mm Hg. His neck was supple, and the remainder of the general examination was normal. The neurologic examination revealed the patient to be awake and alert but apathetic, irritable, and refusing to talk after a few minutes. He was slow to respond, spoke loudly, and had a poor attention span. The patient was disoriented to time and place and remembered 0/5 objects at 5 minutes.
Blood tests revealed 12,500/μL white blood cell (WBC) count (increased mononuclear cells, 13.7%); hemoglobin, 14.6 g/dL; platelet count 194,000/μL; and hematocrit, 43.6%. Electrolytes, general chemistries, vitamin B12, thyroid-stimulating hormone, copper, erythrocyte sedimentation rate, and urinalysis were all normal. The CT head scan was normal, and the urine drug screen and alcohol levels were negative.
The initial audiologic evaluation revealed absent acoustic reflexes bilaterally at 500 Hz, 1 KHz, 2 KHz, and 4 KHz. The brain stem auditory evoked potentials showed no replicable waveforms from the right ear and a wave I present in the left ear with no other replicable waveforms.
A very broad differential diagnosis was considered at this point (Table 1). Lumbar puncture was performed with an opening pressure of 17.5 cm H2O. There were 10 WBC/μL with 12% segmented polymorphonuclear cells and 83% lymphocytes, 30 red blood cells/μL, glucose of 71 mg/dL, and protein of 221 mg/dL. The Venereal Disease Research Laboratory Test was nonreactive; cryptococcal antigen, acid-fast stain, and bacterial and fungal cultures were negative. The electroencephalogram (EEG) showed mild diffuse slowing with frontal intermittent rhythmic delta activity. The magnetic resonance imaging (MRI) was significant for leptomeningeal and pachymeningeal enhancement with a small area of restricted diffusion in the splenium of the corpus callosum (Figure 1). Other cerebral spinal fluid and serum studies were negative or nonreactive (Table 2).
The patient completed a 2-week course of ceftriaxone 1 gram q 12 hours, vancomycin 1,000 mg q 12 hours, acyclovir 700 mg q 8 hours, and doxycycline 100 mg bid without any notable clinical change. A repeat lumbar puncture was acellular and had a protein of 254 mg/dL.
Two days later he worsened, becoming more withdrawn, unable to speak, irritable, and unwilling to be examined. He refused to get out of bed even with his family members present. A repeat MRI at this time showed continued meningeal enhancement, enlargement of the previously seen corpus callosal lesion, a new white matter lesion in the right parietal region, and a dark hole in the corpus callosum on the sagittal T1 image (Figures 2A and 2B). Audiometric testing showed profound hearing loss at low and high frequencies, with severe loss at middle frequencies in both ears.
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Diagnosis
At this point, a diagnosis must explain encephalitis/encephalopathy, hearing loss, and MRI findings of meningeal enhancement and lesions in the corpus callosum and right parietal white matter. The main differential at this point included acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), infection, and vasculitis/vasculopathy (especially primary central nervous system vasculitis). Acute disseminated encephalomyelitis usually has large, asymmetric lesions in the subcortical white matter and gray white junction, with corpus callosal lesions being unusual. Meningeal enhancement is very rare, and hearing loss would be unusual as well.1
Encephalopathy would be unusual in MS and if seen is usually associated with large confluent lesions (the Marburg variant). Meningeal enhancement would be rare on MRI, and the location of the corpus callosal lesion as shown on the T1- sagittal MRI would be atypical for both MS and ADEM (Figure 2B). Hearing loss has been described in MS, with a 4% to 5% incidence, often as the first manifestation and usually with full recovery.2 With the extensive evaluation and treatment in this case, infection was unlikely at this point. Primary central nervous system vasculitis remained a definite possibility and could explain most of the findings. However, there have been no reports of hearing loss or corpus callosal lesions in the literature with this latter condition.
The presence of encephalopathy and hearing loss, in addition to the location of the corpus callosal lesion as demonstrated on the sagittal T1-weighted MRI (Figure 2B) suggested the need for an ophthalmologic consult with dilated retinoscopy and fluorescein angiography (Figure 3). A retinal examination showed branch retinal artery occlusions with cotton wool spots (infarctions). Fluorescein angiography showed branch retinal artery occlusions and arteriolar wall hyperfluoresence in one area. This demonstrated the final feature of the triad of encephalopathy, hearing loss, and branch retinal artery occlusions, confirming the diagnosis of Susac syndrome (SS).
Discussion and Literature Review
The patient was treated with a 3-day course of IV methylprednisolone 1 g daily for 3 days, followed by oral prednisone 60 mg daily for 1 week, followed by a slow taper thereafter. Both his cognition and behavior improved by the second day of treatment, and this continued during his hospital stay. After a short stay in the rehabilitation unit, he was transferred to a facility closer to his home. Mental status improved almost to baseline, but he got minimal if any improvement in his hearing function. Despite the branch retinal occlusions, he had no noticeable deficit in his visual function.
John O. Susac, MD, first described 2 women with a triad of encephalopathy, hearing loss, and branch retinal artery occlusions as a syndrome that subsequently was named after him.3 The syndrome most frequently affects women aged 20 to 40 years. Headaches consistent with migraines occur at onset in a majority of patients.4 Encephalopathy may be acute or subacute and mild to severe. Symptoms can include mood changes, personality change, bizarre behavior, hallucinations, memory and cognitive difficulties, ataxia, seizures, corticospinal tract signs, and myoclonus.5,6 The retinopathy may cause scintillating scotomata or segmental loss of vision but may also be asymptomatic due to occlusions in very distal, branch arteries. Hearing loss may be acute and severe or insidious and mild. Audiometry shows low-to-mid frequency hearing loss.
Related: Infliximab-Induced Complications
Hearing loss is usually permanent and, if severe, may require a cochlear implant.7 The disease course is variable and unpredictable. It may be monophasic, lasting under 2 years. This is often the case if encephalopathy occurs in the first 2 years. Susac syndrome can also have a polycyclic course, with remissions lasting up to 18 years. A chronic continuous course has also been described.8 All 3 components of the triad are not always present, and those without encephalopathy are more likely to have a polycyclic or chronic continuous course. The differential diagnosis is broad, as in the present case.
A cerebral spinal fluid evaluation often shows an elevated protein of 100 to 3,000 mg/dL, a mild lymphocytic pleocytosis (5-30 cells/mm), and oligoclonal bands may be present. Antiendothelial antibodies are present in the serum but not specific (also seen in systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and juvenile dermatomyositis).8 The EEG usually shows diffuse slowing. The MRI is almost always abnormal, and studies have shown virtually 100% have corpus callosal lesions. These occur in the central region of the corpus callosum, consistent with infarction. Demyelinating lesions, with MS or ADEM, on the other hand, tend to occur on the inferior surface of the corpus callosum. If SS is suspected, a sagittal fluid- attenuated inversion recovery (FLAIR) MRI should be obtained to look for these changes. About one-third or more of MRIs show leptomeningeal enhancement, and other lesions can be found scattered throughout the white matter, cerebellum, brain stem, and gray matter.9
Because relatively few cases have been described, SS etiology remains obscure at this time. The disease has an affinity for small precapillary arterioles, of > 100 μm in diameter. The pathology shows necrosis and inflammatory changes of the endothelial cells, making them the primary site of the immune attack. This immune-mediated injury leads to narrowing and occlusion of the microvasculature, with resulting ischemia of the brain, retina, and cochlea. This pathology is very similar to that of juvenile dermatomyositis, which involves muscle, skin, and the gastrointestinal tract.10
Treatment approaches are based on treatments for juvenile dermatomyositis. It is suggested that the patient be given pulse methylprednisolone therapy of 1 g per day for 3 days followed by prednisone 60 mg to 80 mg per day for 4 weeks. Newer recommendations suggest giving IV immunoglobulin in the first week as well, followed by additional courses every month for 6 months. Cyclophosphamide or mycophenolate mofetil should be considered for long-term treatment with consideration of etanercept, cyclosporine, or rituximab in those who fail to respond.10 Aggressive treatment is suggested, because this is a self-limiting disorder, but the deficits tend to be permanent.
Related: Rituximab and Primary Sjögren Syndrome
This patient was atypical, because SS primarily affects young females. Review of the literature indicates that men account for about 25% of patients.8 The presentation, however, was not unusual and demonstrated the difficulty in making this diagnosis. In this patient with encephalopathy, the unusual feature was hearing loss, but it must be kept in mind that both hearing loss and visual changes can be difficult to identify in a confused patient. Brain stem auditory evoked potentials may be helpful in investigating hearing loss in noncooperative patients. An MRI may show centrally located corpus callosal lesions. If SS is suspected, sagittal FLAIR images, which often are not routinely done, should be obtained.
The most helpful evaluation is a dilated direct retinoscopy, which will usually show the branch retinal artery occlusions, and if not, fluorescein angiography will usually show a change. The presence of Gass plaques, yellow-white retinal arterial wall plaques from lipid deposition into the damaged arterial wall, with hyperfluoresence on fluorescein angiography is considered pathognomonic of SS.8 Establishing the diagnosis of SS as soon as possible is critical, because early treatment may lessen the degree of permanent disability.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
1. Eckstein C, Saidha S, Levy M. A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol. 2012;259(5):801-816.
2. Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124(4):245-249.
3. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-316.
4. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine (Baltimore). 1998;77(1):3-11.
5. Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591-593.
6. Hahn JS, Lannin WC, Sarwal MM. Microangiopathy of brain, retina, and inner ear (Susac’s syndrome) in an adolescent female presenting as acute disseminated encephalomyelitis. Pediatrics. 2004;114(1):276-281.
7. Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome—a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30(1):34-40.
8. Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol. 2011;22(6):472-476.
9. Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology. 2003;61(12): 1783-1787.
10. Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci. 2007;257(1-2):215-220.
Arthroscopic Treatment of Tibial Spine Malunion With Resorbable Screws
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
Anterior tibial spine fractures are rare, occurring with an incidence of 3 per 100,000 per year.1,2 Historically, this fracture has occurred more frequently in children,3-5 and was considered a condition of skeletal immaturity and the pediatric equivalent of an anterior cruciate ligament (ACL) rupture.6 However, recent literature indicates that this fracture is more common in the adult population than previously thought.7 The tibial spine is an attachment point for the ACL and an avulsion may produce ACL laxity,8 predisposing to further symptomatic laxity and premature osteoarthritis. Nearly 40% of these fractures are associated with concomitant injuries to surrounding structures.9
Meyers and McKeever10,11 originally classified these fractures into 3 groups on the basis of displacement. Type I fractures present with no significant displacement of the anterior margin, type II involve displacement and are hinged, while type III have complete displacement.10,11 More recently, a type IV fracture has been added, involving comminution of the displaced fragment. Nondisplaced fractures are commonly treated with immobilization in varying degrees of extension; this allows the femoral condyles to compress and to reduce the fracture while arthroscopic or open reduction is the preferred method for displaced fractures of the tibial spine.2,4,8,10
We report the case of an 11-year-old boy with a tibial spine fracture that failed conservative management. He developed a subsequent malunion with impingement anteriorly of the tibial spine on the notch, and residual instability of the ACL. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old Caucasian boy was referred to our office for evaluation of right knee injury. He sustained the injury approximately 3 months earlier, and it was determined that he had a tibial spine fracture. Conservative management with immobilization in extension and activity modification was undertaken; however, he was referred for further evaluation because of healing in a malreduced position and residual ACL laxity. Physical examination showed a grade 2A Lachman test (contralateral limb with negative Lachman examination), negative McMurray test, and pain with forced hyperextension; range-of-motion examination showed lack of the terminal 5º of extension. Magnetic resonance and computed tomography imaging from an outside facility showed a skeletally immature individual with a large tibial spine fracture that had healed in a malunited position with the fragment extended on a posterior hinge, creating a large prominence anteriorly (Figures 1A, 1B). Magnetic resonance imaging showed that the ACL fibers were likely to remain intact but would lack appropriate tension secondary to the displacement of the tibial insertion.
Because of healing in a displaced position, lack of terminal extension, ACL laxity, and subjective complaints of pain, we discussed surgery with the patient and his parents (Figures 2A, 2B). Four months after the initial injury, the patient underwent surgery for a right tibial spine malunion arthroscopic takedown and repair, as well as an intraoperative evaluation of the ACL. Standard arthroscopy was performed, using anterolateral and anteromedial arthroscopic portals, and an accessory medial peripatellar portal. During surgery, a large prominence was noted in the region of the anterior tibial spine (Figure 3A). The ACL fibers maintained a slack position secondary to the elevation of the tibial insertion point, and intraoperative Lachman examination showed anterior translation of the tibia on the femur as the slack was removed from the ACL. During surgery, impingement of the anterior tibial spine along the femoral notch was shown to be significant by taking the knee into near-full extension (Figure 3B). A cam-like effect was noted at the time of impingement with the posterior soft tissues relaxing to accommodate slight further extension.
Based on these findings, we chose to take down the malunited fracture and repair it (Figure 3C). PDS suture (Ethicon, Somerville, New Jersey) was temporarily placed along the intermeniscal ligament and anterior horns of the medial and lateral menisci, using a system of spinal needles to facilitate suture passage. Surgical clamps were hung from the suture to provide traction on the sutures throughout the case, allowing the intermeniscal ligament and menisci to recede anteriorly to improve working space and aid in preventing iatrogenic injury. These sutures were removed at the conclusion of the case. Using a combination of curettes, elevator, and small shaver, we were able to meticulously remove interposed malunited callus to allow for mobilization of the displaced fragment. After removal of the excess bone formation, a typical donor site was created, allowing the displaced spine fragment to be hinged into appropriate alignment (Figure 3D). We were able to maintain a posterior cortical hinge to facilitate this process.
Then, we placed Kirschner wires (K-wires) across the fracture in an antegrade fashion, anterior to the trochlea and notch, using an accessory medial peripatellar starting point percutaneously, under direct visualization to avoid iatrogenic chondral injury. The tibial spine fragment was temporarily maintained in a reduced position with an arthroscopic probe and pinned in place with two 0.062-in K-wires. The fracture was stabilized with 8 resorbable 1.6-mm poly-L-lactic/polyglycolic acid (PLLA/PGA) nails, in varying lengths from 18 mm to 22 mm. Excellent fixation was obtained, and range of motion was tested from 0º to 80º, without movement of the fracture site (Figure 3E). Fluoroscopy with multi-axial views verified adequate fixation and reduction. Further, we examined and noted a taut ACL after fixation. The patient was placed in a long leg cast for 3 weeks at 30º, based upon intraoperative determination of the position of least tension on the fracture fragment.
At 3-week follow-up, the patient was progressing well and transitioned from a long leg cast to a hinged knee brace, to allow for early range of motion. Radiographs showed appropriate alignment of the tibial spine fracture with no significant loss of fixation (Figures 4A, 4B). Physical therapy was initiated between 0º and 30º, and flexion was progressively increased over the course of the first 3 weeks. Active and active-assist, closed-chain activities were maintained. Seven weeks postoperatively, the patient displayed continued clinical progression. Radiographs showed interval healing with slight lucency over the anterolateral aspect of the fracture fragment, likely related to the early resorptive process of healing. Physical examination showed movement between 0º and 120º, stable Lachman test, and stable anterior drawer. Crutches were discontinued and hinged knee brace was converted to an ACL brace. By the 11th week, motion had increased to 140º, and radiographs continued to show acceptable alignment and healing (Figures 5A, 5B). The patient was released to return to play as tolerated; however, an ACL brace was recommended during his initial return to provide additional support.
Discussion
In this report, we present an approach for arthroscopic reduction of a malunited tibial spine fracture using resorbable PLLA/PGA nails. The number of polyglycolic nails employed is individualized per case, dependent on the surface area and the quality of the bone within the fractured fragment. Preoperative templating allows for measurements from the fractured fragment to the level of the proximal tibial physis. Based on these measurements, nails are chosen to maximize fixation length and avoid the physis. Despite studies that have examined the effect of transphyseal K-wire pinning or drilling on subsequent growth, there is no consensus about optimal technique. Experiments in animal models indicate that drill injuries destroying less than 8% to 9% of the physis do not impact total bone growth.12,13 Further, temporary crossing of the physeal plate for internal fixation of dislocated joint injuries has not been shown to result in bone bridging or growth disturbance.14,15
Each nail is 1.6 mm in diameter, leaving a small footprint. The nails are used judiciously to provide effective stabilization of the fragment and to maintain a cost-conscious approach. An accessory superomedial peripatellar portal allows an appropriate angle for nail placement. This portal allows access to all regions of the fractured fragment, while an anteromedial and anterolateral portal are used as working and camera portals, respectively. Nails are placed to provide an axis perpendicular to the fracture line to allow appropriate compression. By virtue of the shape of the typical fragment in a tibial spine fracture, the nails vary in insertion angle.
The occurrence of anterior tibial spine fractures is rare, and while several techniques have been described to repair this fracture, there remains a great deal of uncertainty regarding the best course of treatment. A review of the literature finds arthroscopic and open approaches, as well as techniques employing K-wire fixation, metal screw fixation, staple fixation, absorbable fixation, and fixation with sutures passed through the tibial tunnel.16-18
Avulsion fractures of the tibial eminence were treated with open fixation until McLennan8 first reported the benefits of reduction with an arthroscope. Open reduction and internal fixation provide the benefit of direct visualization,9 while arthroscopic reduction offers decreased morbidity and an accelerated recovery of knee functions,8 despite the fact that a higher rate of range-of-motion deficits were seen in patients treated arthroscopically.19 We feel that with proper early rehabilitation to achieve range of motion, the risk of this can be minimal.
Various arthroscopic approaches that improve the accuracy of the reduction and decrease surgical invasiveness have been described. Suture and screw fixation are among the most common methods, and both have resulted in positive outcomes.20-24 Suture fixation of the tibial eminence is technically demanding but offers secure fixation without the need for follow-up hardware removal. Screw fixation results in secure fixation; however, numerous hardware-related issues may necessitate removal. Furthermore, in skeletally immature patients, screw fixation may disturb the growth plate if it crosses an open physis.9
Hunter and Willis25 retrospectively reviewed patients with tibial eminence fractures treated with either screw or suture fixation and found a 44% reoperation rate in the screw-fixation group. Removal was often recommended as a result of hardware-related issues. There was a 13% reoperation rate in the suture-fixation group, which resulted largely from stiffness.25 In a recent review, Gans and colleagues19 reviewed 6 publications comparing screw and suture fixation of tibial eminence fractures and found 82.4% of screw patients had laxity on both the anterior drawer and Lachman tests, compared with 18.8% in the suture-fixation group. This study also noted a slightly higher rate of arthrofibrosis in patients treated with suture fixation.19 Biomechanical studies indicate that suture fixation imparts greater strength under cyclic-loading conditions;26 however, there does not appear to be a difference in ultimate force required for fixation failure.27
Ultimately, both suture and screw fixation result in secure methods of fixation; however, there are often greater issues with screw fixation because of the persistent hardware. Metal has been the most popular method for fracture fixation, and while biodegradable materials have been alluring, adverse tissue reactions have slowed implementation. However, these implants have become increasingly sophisticated, thereby reducing disadvantages.28 Previous biodegradable devices were often composed of a single polymer, and many caused adverse reactions by degrading too quickly or provided no real advantages because they degraded too slowly.29 As the number of polymers approved for internal use and surgical applications continues to rise, so too will the benefits of employing this technology. Furthermore, by including multiple polymers in these implants, one is better able to control the degradation rate, limiting the tissue response.
In this study, we employed PLLA/PGA nails. Studies of PGA implants indicate this molecule degrades at a fast rate resulting in adverse tissue reactions. Adverse reactions in studies of PLLA implants are less frequent because of their slower rate of degradation.29,30 Combining these monomers results in appropriate strength and a controlled degradation rate, reducing the likelihood of adverse reactions. Furthermore, numerous studies have reported that inflammatory responses in children are rare and mild in nature.31,32 Absorbable implants have displayed efficacy in numerous orthopedic settings33-36 and are beneficial in procedures that are not suitable for repeated surgeries, such as reconstruction of the ACL.37 There is some concern about the use of absorbable implants in synovial joints. Polyglycolic acid use in synovial joints may cause foreign-body reactions and may increase the risk of intra-articular dissemination of polymeric debris;38 however, use of a multipolymer construct decreases the likelihood of this occurrence.
Polyglycolic nails confer the advantage over nonresorbable screw fixation because further procedure for hardware removal is not required. Although suture fixation has proved to be beneficial over nonresorbable screw fixation, implantation of resorbable nails appears to have several advantages. In Dr. Estes’ experience, placement of resorbable screws through an accessory superomedial portal is far less technically demanding than placement of suture through the fracture fragment. Further, as sutures are passed from the extra-articular to the intra-articular region of the joint, capsular layers of the knee may inadvertently be bound up in the fixation, predisposing to arthrofibrosis.
At the same time, biodegradable devices are often more costly than alternative forms of treatment; however, a true cost-to-benefit analysis requires consideration of other factors. One of the benefits of biodegradable hardware is that there is no need for follow-up hardware removal. Reports have indicated that up to 91% of patients thought that hardware removal was the most negative aspect of metal implants.39 It is estimated that if the removal rate for metallic implants is higher than 19% to 54%, resorbable implants would be more cost-effective.40 The cost of sutures and screws is variable, however; they are invariably less expensive than biodegradable nails. A study of fracture patients determined that biodegradable implants were cheaper on average after considering the cost of implant removal.40 Ultimately, the hardware choice depends on numerous factors, including surgeon’s discretion; however, biodegradable hardware should not be discounted for financial reasons because the difference in cost is likely negligible.
Conclusion
The approach described in this report offers efficient and secure fixation with resorbable hardware without a reduction in range of motion. Resorbable implants may prove beneficial in the treatment of tibial eminence fractures by offering robust fixation without the concerns associated with permanent hardware.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
1. Hargrove R, Parsons S, Payne R. Anterior tibial spine fracture – an easy fracture to miss. Accid Emerg Nurs. 2004;12(3):173-175.
2. Aderinto J, Walmsley P, Keating JF. Fractures of the tibial spine: epidemiology and outcome. Knee. 2008;15(3):164-167.
3. Driessen MJ, Winkelman PA. Fractures of the intercondylar eminence of the tibia in childhood. Neth J Surg. 1984;36(3):69-72.
4. Zaricznyj B. Avulsion fracture of the tibial eminence: treatment by open reduction and pinning. J Bone Joint Surg Am. 1977;59(8):1111-1114.
5. Molander ML, Wallin G, Wikstad I. Fracture of the intercondylar eminence of the tibia: a review of 35 patients. J Bone Joint Surg Br. 1981;63(1):89-91.
6. Kieser DC, Gwynne-Jones D, Dreyer S. Displaced tibial intercondylar eminence fractures. J Orthop Surg. 2011;19(3):292-296.
7. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop. 2005;(434):207-212.
8. McLennan JG. The role of arthroscopic surgery in the treatment of fractures of the intercondylar eminence of the tibia. J Bone Joint Surg Br. 1982;64(4):477-480.
9. Lafrance RM, Giordano B, Goldblatt J, Voloshin I, Maloney M. Pediatric tibial eminence fractures: evaluation and management. J Am Acad Orthop Surg. 2010;18(7):395-405.
10. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41(2):209-220.
11. Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52(8):1677-1684.
12. Garcés GL, Mugica-Garay I, López-González Coviella N, Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop. 1994;14(2):225-228.
13. Janarv PM, Wikström B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18(2):149-154.
14. Boelitz R, Dallek M, Meenen NM, Jungbluth KH. Reaction of the epiphyseal groove to groove-crossing bore-wire osteosynthesis. Results of a histomorphologic small animal study. Unfallchirurgie. 1994;20(3):131-137.
15. Yung PS, Lam CY, Ng BK, Lam TP, Cheng JC. Percutaneous transphyseal intramedullary Kirschner wire pinning: a safe and effective procedure for treatment of displaced diaphyseal forearm fracture in children. J Pediatr Orthop. 2004;24(1):7-12.
16. Bong MR, Romero A, Kubiak E, et al. Suture versus screw fixation of displaced tibial eminence fractures: a biomechanical comparison. Arthroscopy. 2005;21(10):1172-1176.
17. Vega JR, Irribarra LA, Baar AK, Iñiguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
18. Shepley RW. Arthroscopic treatment of type III tibial spine fractures using absorbable fixation. Orthopedics. 2004;27(7):767-769.
19. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2013;42(7):1743-1750.
20. Delcogliano A, Chiossi S, Caporaso A, Menghi A, Rinonapoli G. Tibial intercondylar eminence fractures in adults: arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):255-259.
21. Mulhall KJ, Dowdall J, Grannell M, McCabe JP. Tibial spine fractures: an analysis of outcome in surgically treated type III injuries. Injury. 1999;30(4):289-292.
22. Geissler WB, Matthews DE. Arthroscopic suture fixation of displaced tibial eminence fractures. Orthopedics. 1993;16(3):331-333.
23. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
24. Reynders P, Reynders K, Broos P. Pediatric and adolescent tibial eminence fractures: arthroscopic cannulated screw fixation. J Trauma. 2002;53(1):49-54.
25. Hunter RE, Willis JA. Arthroscopic fixation of avulsion fractures of the tibial eminence: technique and outcome. Arthroscopy. 2004;20(2):113-121.
26. Eggers AK, Becker C, Weimann A, et al. Biomechanical evaluation of different fixation methods for tibial eminence fractures. Am J Sports Med. 2007;35(3):404-410.
27. Mahar AT, Duncan D, Oka R, Lowry A, Gillingham B, Chambers H. Biomechanical comparison of four different fixation techniques for pediatric tibial eminence avulsion fractures. J Pediatr Orthop. 2008;28(2):159-162.
28. Toro C, Robiony M, Zerman N, Politi M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005;54(4):199-206.
29. Andriano KP, Pohjonen T, Törmälä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater.1994;5(2):133-140.
30. Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
31. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
32. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93-102.
33. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med. 2013;5(5):1531-1537.
34. Singh G, Mohammad S, Chak RK, Lepcha N, Singh N, Malkunje LR. Bio-resorbable plates as effective implant in paediatric mandibular fracture. J Maxillofac Oral Surg. 2012;11(4):400-406.
35. Sakamoto Y, Shimizu Y, Nagasao T, Kishi K. Combined use of resorbable poly-L-lactic acid-polyglycolic acid implant and bone cement for treating large orbital floor fractures. J Plast Reconstr Aesthet Surg. 2014;67(3):e88-e90.
36. Benz G, Kallieris D, Seeböck T, McIntosh A, Daum R. Bioresorbable pins and screws in paediatric traumatology. Eur J Pediatr Surg. 1994;4(2):103-107.
37. Gaweda K, Walawski J, Weglowski R, Krzyzanowski W. Comparison of bioabsorbable interference screws and posts for distal fixation in anterior cruciate ligament reconstruction. Int Orthop. 2009;33(1):123-127.
38. Böstman OM. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg Br. 1998;80(2):333-338.
39. Mittal R, Morley J, Dinopoulos H, Drakoulakis EG, Vermani E, Giannoudis PV. Use of bio-resorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005;36(2):333-338.
40. Böstman OM. Metallic or absorbable fracture fixation devices. A cost minimization analysis. Clin Orthop. 1996;(329):233-239.
Popliteal Artery Pseudoaneurysm: An Unusual Complication of Tibial Traction
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
Recurrent Patellar Tendon Rupture in a Patient After Intramedullary Nailing of the Tibia: Reconstruction Using an Achilles Tendon Allograft
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
Ruptures of the patellar tendon usually occur in patients under age 40 years, with men having a higher incidence than women.1 History of local steroid injection,2,3 total knee arthroplasty,4-8 anterior cruciate ligament reconstruction with central third patellar tendon autograft,9-11 and a variety of systemic diseases are associated with an increased tendency to rupture.12-15 Primary acute ruptures of the patellar tendon can be difficult to repair because of the quality of remaining tissues. In cases of chronic tendon ruptures subject to delayed treatment, additional complications such as tissue contracture and scar-tissue formation are likely to exist.15-17
Complications after intramedullary (IM) nailing of the tibia include infection, compartment syndrome, deep vein thrombosis, thermal necrosis of the bone with alteration of its endosteal architecture, failure of the hardware, malunion, and nonunion.18 The most common complaint after IM nailing of the tibia is chronic anterior knee pain and symptoms similar to tendonitis; incidences as high as 86% have been reported.18-20 Extensive review of the literature found only 2 reports of patellar tendon rupture after IM nailing of the tibia; both cases used a patellar tendon–splitting approach. The first report described patellar tendon rupture 8 years after IM nailing of the tibia during a forced deep-flexion movement.21 Radiographic examination showed the IM nail positioned proud relative to the tibial plateau, impinging upon the patellar tendon. An intraoperative examination confirmed the radiographic findings and found rupture of the patellar tendon to be consistent with the exposed tip of the IM nail. The second report described patellar tendon rupture 2 months postoperatively in a patient with Ehlers-Danlos syndrome, a hereditary disorder characterized by alterations to muscle/tendon tissue and hyperextensible skin.22
Patellar tendon rupture after IM nailing of the tibia is a rare complication. Patellar tendon re-rupture after primary repair in a patient with history of IM tibial nailing has not been reported. This case outlines the progression of such a patient with a recurrent patellar tendon rupture that was successfully reconstructed using an Achilles tendon allograft. The patient’s surgical history of IM tibial nailing through a mid-patellar tendon–splitting approach 4 years prior to initial tendon rupture is noteworthy and potentially predisposed the patient to injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 44-year-old woman, 5 ft, 3 in tall, and weighing 129 lb (body mass index, 22.8), with a history of osteoporosis and transverse myelitis, presented with pain and persistent swelling about the left knee. Her baseline ambulatory status required crutches because of decreased sensation and strength in her lower extremity in conjunction with a foot drop; she had mild quadriceps and hamstring muscle weakness but otherwise normal knee function. The patient had been seen 4 years earlier at our facility for IM fixation of a distal tibia fracture through a patellar tendon–splitting approach. The fracture was well healed and showed no signs of complication or nail migration; the nail was not proud.
Initially, the patient was admitted to another hospital through the emergency department for swelling and pain about the left knee. She was believed to have an infection and was placed on antibiotics by the primary care team. An orthopedic evaluation showed induration, edema, and warmth in the patellar tendon region of the left knee. Magnetic resonance imaging (MRI) showed a full-thickness patellar tendon rupture. Aspiration of the knee was performed and cultures were negative; white blood cell, erythrocyte sedimentation rate, and C-reactive protein values were normal. The risks and benefits of various treatments were discussed, and surgical intervention was elected to repair the patellar tendon.
Intraoperative findings showed a massive midsubstance rupture of the patellar tendon, accompanied by medial and lateral retinacular tears and a quadriceps tendon partial rupture; the central aspect of the quadriceps tendon attaching to the patella remained intact. The patella was retracted proximally; no evidence of active infection was present. Good-quality tissue remained attached to both the tibial tuberosity and the inferior pole of the patella. A No. 2 FiberWire suture (Arthrex, Inc, Naples, Florida) was used to run whip stitches in the distal end of the patellar tendon and a second No. 2 FiberWire suture was used to run whip stitches in the proximal aspect of the patellar tendon rupture. The 4 ends of the sutures were tied together, thus re-approximating the distal and proximal ends of the ruptured patellar tendon. No bone drilling was used because the midsubstance tear was amenable to good repair with reasonable expectation of healing based on tissue quality. The quadriceps tendon, which was partially torn, was repaired with a No. 1 Vicryl suture (Ethicon, Somerville, New Jersey). The medial and lateral retinacula were also repaired with a No. 1 Vicryl suture. The suturing scheme effectively re-approximated the knee extensor mechanism, and the patient was placed in a knee immobilizer that permitted no flexion for 6 weeks postoperatively.
After 3 months of gradual improvement with physical therapy, the patient returned for a follow-up visit, concerned that her knee function was beginning to decline. Physical examination showed patella alta with a thinned and diminutive palpable tendon in the patellar tendon region. She was capable of active flexion to 90º and extension to 50º, but beyond 50º, she was unable to actively extend; she was capable of full passive extension. MRI showed a repeat full-thickness patellar tendon tear with retraction from the inferior pole of the patella; previous tears to the quadriceps tendon were healed. Because of the recurrent nature of the injury, the patient’s physical examination, MRI findings, and anticipated poor quality of remaining tendon tissue, patellar tendon reconstruction using a cadaveric Achilles tendon allograft was recommended. The patient chose surgery for potential improvement in knee range of motion, active extension, and ambulation.
The previous anterior midline incision was used and carried down through the subcutaneous tissues where a complete rupture of the patellar tendon was identified. A limited amount of good-quality tendon tissue remained at the medial aspect of the tibial tuberosity. The remaining tissue located at the patella’s inferior pole was nonviable for use in surgical repair. Retinacular contractures were released to bring the patella distally; the trochlear groove was used as the anatomic landmark for the patella resting position. During reconstruction, the knee was placed into 30° of flexion, with the patella located in the trochlear groove, and the cadaveric Achilles tendon was placed on the midline of the patella, where measurements were done to assess proper length and tension (Figure 1).
The patient’s remaining native tissue on the medial aspect of the tibial tuberosity was used to augment the Achilles tendon graft medially. The cadaveric Achilles tendon graft was primarily used to replace the central and lateral aspects of the patellar tendon. Additionally, the calcaneal bone segment at the end of the Achilles tendon graft was removed prior to use. Cadaveric and host tissues at the medial aspect of the tibial tuberosity were sutured together with a No. 1 Vicryl suture (Figure 2). The distal aspect of the cadaveric Achilles tendon was used to re-approximate the patient’s native patellar tendon insertion at the tibial tuberosity. To supplement the graft anchor, a Richards metallic ligament staple (Smith & Nephew, Memphis, Tennessee) was used to fix the distal aspect of the Achilles tendon graft into the tibial tuberosity.
Proper tensioning of the graft was performed by visualizing patella tracking during the arc-of-knee motion and properly suturing the graft to allow for functional range. The proximal aspect of the cadaveric Achilles tendon was sutured into host tissues surrounding the superior pole of the patellar and quadriceps tendon. The edges of the graft were sutured with supplemental No. 1 Vicryl sutures (Figure 3).
Before surgical closure, knee range of motion was checked and noted to be 0º to 100º. The repaired construct was stable and uncompromised throughout the entire range of motion. Patella tracking was central and significantly improved; knee stability was normal to varus and valgus stress.
The patient was placed in a knee immobilizer for 6 weeks before range of motion was allowed. Seven months postoperatively, the patient returned for a follow-up visit, ambulating with 2 forearm crutches, which was her baseline ambulatory status. Physical examination revealed passive range of motion from 0º to 130º, an extension lag of 10º, and 4/5 quadriceps strength. It was recommended the patient continue physical therapy to improve strength and range of motion.
Conclusion
This is the first report in the literature documenting a recurrent patellar tendon rupture after primary repair in a patient with a history of IM tibial nailing. It is also the first report of a cadaveric Achilles tendon allograft used as a solution to this problem. Complete reconstruction of the patellar tendon using an Achilles tendon allograft is a method commonly used for ruptures after total knee arthroplasty.4-7,23,24 This case report highlights the utility of a cadaveric Achilles tendon in the setting of a recurrent patellar tendon rupture with poor remaining tissue quality.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
1. Scott WN, Insall JN. Injuries of the knee. In: Rockwood CA Jr, Green DP, Bucholz RW, eds. Fractures in Adults. 3rd ed. Philadelphia, PA: JB Lippincott; 1991: 1799-1914.
2. Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12(4):300-301.
3. Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1(4):31-37.
4. Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty. A report of seven cases. J Bone Joint Surg Am. 1992;74(7):974-979.
5. Emerson RH Jr, Head WC, Malinin TI. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop.1990;(260):154-161.
6. Gustillo RB, Thompson R. Quadriceps and patellar tendon ruptures following total knee arthroplasty. In: Rand JA, Dorr LD, eds. Total Arthroplasty of the Knee: Proceedings of the Knee Society, 1985-1986. Rockville, MD: Aspen; 1987: 41-70.
7. Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop. 1989;(244):233-238.
8. Schoderbek RJ, Brown TE, Mulhall KJ, et al. Extensor mechanism disruption after total knee arthroplasty. Clin Orthop. 2006;446:176-185.
9. Bonamo JJ, Krinik RM, Sporn AA. Rupture of the patellar ligament after use of the central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am. 1984;66(8):1294-1297.
10. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
11. Mickelsen PL, Morgan SJ, Johnson WA, Ferrari JD. Patellar tendon rupture 3 years after anterior cruciate ligament reconstruction with a central one third bone-patellar tendon-bone graft. Arthroscopy. 2001;17(6):648-652.
12. Morgan J, McCarty DJ. Tendon ruptures in patients with systemic lupus erythematosus treated with corticosteroids. Arthritis Rheum. 1974;17(6):1033-1036.
13. Webb LX, Toby EB. Bilateral rupture of the patellar tendon in an otherwise healthy male patient following minor trauma. J Trauma. 1986;26(11):1045-1048.
14. Greis PE, Holmstrom MC, Lahav A. Surgical treatment options for patella tendon rupture, Part I: Acute. Orthopedics. 2005;28(7):672-679.
15. Greis PE, Lahav A, Holstrom MC. Surgical treatment options for patella tendon rupture, part II: chronic. Orthopedics. 2005;28(8):765-769.
16. Lewis PB, Rue JP, Bach BR Jr. Chronic patellar tendon rupture: surgical reconstruction technique using 2 Achilles tendon allografts. J Knee Surg. 2008;21(12):130-135.
17. McNally PD, Marcelli EA. Achilles tendon allograft of a chronic patellar tendon rupture. Arthroscopy. 1998;14(3):340-344.
18. Katsoulis E, Court-Brown C, Giannoudis PV. Incidence and atieology of anterior knee pain after intramedullary nailing of the femur and tibia. J Bone Joint Surg Br. 2006;88(5):576-580.
19. Brumback RJ, Uwagie-Ero S, Lakatos RP, et al. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70(1):1453-1462.
20. Koval KJ, Clapper MF, Brumback RJ, et al. Complications of reamed intramedullary nailing of the tibia. J Orthop Trauma. 1991;5(2):184-189.
21. Kretzler JE, Curtin SL, Wegner DA, Baumgaertner MR, Galloway MT. Patella tendon rupture: a late complication of a tibial nail. Orthopedics. 1995;18(11):1109-1111.
22. Moroney P, McCarthy T, Borton D. Patellar tendon rupture post reamed intra-medullary tibial nail in a patient with Ehlers-Danlos syndrome. A case report. Eur J Orthop Surg Traumatol. 2004;14(1):50-51.
23. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
24. Falconiero RP, Pallis MP. Chronic rupture of a patellar tendon: a technique for reconstruction with Achilles allograft. Arthroscopy. 1996;12(5):623-626.
Chest pain • shortness of breath • fever and nausea • Dx?
THE CASE
A 38-year-old Hispanic man was brought to the emergency department (ED) after losing consciousness and falling at home, striking his elbow, head, and neck. For the past week, he’d had palpitations, shortness of breath, mild swelling of the lower extremities, fever, nausea, and fatigue. He had also been experiencing squeezing chest pain that worsened with exertion and was only partially relieved by nitroglycerin.
The patient did not have any rashes and denied having contact with anyone who was sick. He said that he’d been bitten by mosquitos during recent outdoor activities. His medical history included hypertension, hemorrhagic basal ganglia stroke, hyperlipidemia, sleep apnea, metabolic syndrome, and gout. The patient denied smoking or using illicit drugs.
In the ED, his temperature was 101°F, heart rate was 112 beats/min, blood pressure was 175/100 mm Hg, and respiratory rate was 18 breaths/min. His head and neck exam was normal, with no neck stiffness. A lung exam revealed bilateral basal crackles, and a neurologic exam showed residual right-sided weakness due to the hemorrhagic stroke one year ago.
Lab test results revealed the following: white blood cell (WBC) count, 13,000/mm3 with relative monocytosis (14%); lymphocytosis (44%) with normal neutrophils and no bands; hemoglobin, 12 g/dL; hematocrit, 36/mm3; and platelets, 300,000/mm3. Liver function tests were within normal limits. Urinalysis was unremarkable. His troponin I level was elevated at 1.385 ng/dL. In addition to the tachycardia, his electrocardiogram (EKG) showed left axis deviation, left atrial enlargement, left anterior fascicular block, and diffuse nonspecific ST and T wave abnormalities. Chest x-ray was unremarkable except for cardiomegaly. A computed tomography (CT) scan of his head showed residual changes from the previous stroke.
The patient was admitted with a provisional diagnosis of systemic inflammatory response syndrome (SIRS), syncope, non–ST elevation myocardial infarction (NSTEMI), and acute heart failure. The patient had continuous EKG monitoring and serial assessments of his troponin levels. He was also given aspirin, metoprolol 25 mg BID, lisinopril 10 mg/d, furosemide 40 mg IV, isosorbide mononitrate 60 mg/d, and atorvastatin 40 mg/d.
The patient’s cardiac enzymes subsequently decreased. A left heart catheterization was performed, which showed minimum irregularities of the left anterior descending artery (< 20% narrowing) and an ejection fraction (EF) of 35%, without any evidence of obstructive coronary artery disease (CAD). An echocardiogram revealed systolic dysfunction, with an EF of 35% to 40% and global hypokinesis without any apical ballooning or pericardial effusion. (An echocardiogram performed 6 months earlier had shown normal systolic function, an EF of 60% to 65%, and no wall motion abnormalities.) Blood, urine, and fungal cultures were negative; stool studies for ova and parasites were also negative. A lower extremity venous Doppler was negative for deep vein thrombosis.
THE DIAGNOSIS
Because our patient had SIRS, troponinemia, acute systolic dysfunction, and global hypokinesis without any evidence of obstructive CAD, we considered a diagnosis of viral myocarditis. Serologic studies for echovirus, coxsackievirus B, parvovirus B19, adenovirus, and human herpesvirus 6 (HHV-6) all came back negative. However, an enzyme-linked immunosorbent assay (ELISA) for West Nile virus (WNV) was positive. WNV infection was confirmed with a positive plaque reduction neutralization test and a positive qualitative polymerase chain reaction (PCR) assay, which established a diagnosis of WNV myocarditis.
DISCUSSION
While most individuals infected with WNV are asymptomatic, 20% to 40% of patients will exhibit symptoms.1-4 Typical presentations of WNV infection include West Nile fever and neuroinvasive disease. West Nile fever is a self-limited illness characterized by a low-grade fever, headache, malaise, back pain, myalgia, and anorexia for 3 to 6 days.2 Neuroinvasive disease caused by WNV may present as encephalitis, meningitis, or flaccid paralysis.5 Atypical presentations of the virus include rhabdomyolysis,6 fatal hemorrhagic fever with multi-organ failure and palpable purpura,7 hepatitis,8 pancreatitis,9 central diabetes insipidus,10 and myocarditis.11
Although WNV has been linked to myocarditismin animals,12 few human cases of WNV myocarditis11,13 or cardiomyopathy14 have been reported. Viral myocarditis often leads to the development of dilated cardiomyopathy, and myocardial damage may result from direct virus-induced cytotoxicity, T cell-mediated immune response to the virus, or apoptosis.15 Some research suggests that immune-mediated mechanisms play a primary role in myocardial damage. Caforio et al16 found that anti-alpha myosin antibodies were present in 34% of myocarditis patients. In a follow-up study, these antibodies were shown to persist for up to 6 months, which far surpasses the viral cardiac replication timeline of 2 to 3 weeks,17 suggesting that damage occurring after that time is primarily an autoimmune process.
The differential diagnosis for WNV myocarditis includes myocardial stunning from demand ischemia related to SIRS, Takotsubo cardiomyopathy (stress cardiomyopathy), and Dressler’s syndrome. For our patient, myocardial stunning from demand ischemia was less likely because he had no obstructive coronary disease or focal hypokinesis. In addition, the persistence of left ventricular systolic dysfunction and global hypokinesis demonstrated in a repeat echocardiogram during follow-up 6 months later reinforced the likelihood of myocarditis.
The patient’s chest pain with syncope, elevated troponin level, and nonspecific EKG changes in the absence of obstructive CAD raised the possibility of Takotsubo cardiomyopathy. The characteristic echocardiogram finding in Takotsubo cardiomyopathy is transient apical ballooning with akinesis or hypokinesis in the apical and/or mid ventricular regions (typical variant) or isolated midventricular hypokinesis (apical sparing variant). Our patient’s echocardiogram did not show any of these focal wall motion abnormalities, but instead showed global hypokinesis. In addition, the persistence of systolic dysfunction during the repeat echocardiogram and the patient’s lack of psychological distress made the diagnosis of Takotsubo cardiomyopathy unlikely.
Dressler’s syndrome, which is also known as post-myocardial infarction (MI) syndrome, typically presents weeks to months after MI as pleuritic chest pain with a pericardial rub, elevated inflammatory markers, typical EKG changes (diffuse ST-segment elevation and PR-segment depression), and pericardial effusion. This did not fit our patient’s presentation.
Supportive care for heart failure is the mainstay of treatment
The standard treatment for WNV myocarditis is supportive care. Diuretics are used as needed for fluid overload, along with angiotensin-converting enzyme inhibitors and beta-blockers for cardiomyopathy with decreased EF.
Our patient’s dyspnea improved with treatment of furosemide 40 mg IV BID, and his blood pressure was controlled with metoprolol 25 mg BID and lisinopril 10 mg BID. His chest pain and fever resolved when his blood pressure improved. He was discharged home after 7 days on the furosemide, metoprolol, and lisinopril, in addition to isosorbide mononitrate 30 mg/d, atorvastatin 40 mg/d, and aspirin 325 mg/d. An echocardiogram performed 6 months later showed persistent systolic dysfunction, with an EF of 35% and global wall motion abnormalities.
THE TAKEAWAY
In addition to acute coronary syndrome, consider alternate etiologies in patients who present with chest pain and elevated cardiac biomarkers, particularly if diagnostic work-up is negative for obstructive coronary artery disease. WNV myocarditis should be considered as a diagnosis when a patient’s symptoms suggest acute coronary syndrome but are accompanied by fever, headache, and other constitutional symptoms, especially during mosquito season or a WNV outbreak.
1. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814.
2. Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272-277.
3. Brown JA, Factor DL, Tkachenko N, et al. West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis. 2007;7:479-488.
4. Zou S, Foster GA, Dodd RY, et al. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354-1361.
5. Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300.
6. Montgomery SP, Chow CC, Smith SW, et al. Rhabdomyolysis in patients with west nile encephalitis and meningitis. Vector Borne Zoonotic Dis. 2005;5:252-257.
7. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42:1527-1535.
8. Georges AJ, Lesbordes JL, Georges-Courbot MC, et al. Fatal hepatitis from West Nile virus. Ann Inst Pasteur Virol. 1988;138:237.
9. Perelman A, Stern J. Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg. 1974;23:1150-1152.
10. Sherman-Weber S, Axelrod P. Central diabetes insipidus complicating West Nile encephalitis. Clin Infect Dis. 2004;38:1042-1043.
11. Pergam SA, DeLong CE, Echevarria L, et al. Myocarditis in West Nile virus infection. Am J Trop Med Hyg. 2006;75:1232-1233.
12. van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637-657.
13. Kushawaha A, Jadonath S, Mobarakai N. West nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147.
14. Khouzam RN. Significant cardiomyopathy secondary to West Nile virus infection. South Med J. 2009;102:527-528.
15. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100.
16. Caforio AL, Goldman JH, Haven AJ, et al. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270-275.
17. Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11-18.
THE CASE
A 38-year-old Hispanic man was brought to the emergency department (ED) after losing consciousness and falling at home, striking his elbow, head, and neck. For the past week, he’d had palpitations, shortness of breath, mild swelling of the lower extremities, fever, nausea, and fatigue. He had also been experiencing squeezing chest pain that worsened with exertion and was only partially relieved by nitroglycerin.
The patient did not have any rashes and denied having contact with anyone who was sick. He said that he’d been bitten by mosquitos during recent outdoor activities. His medical history included hypertension, hemorrhagic basal ganglia stroke, hyperlipidemia, sleep apnea, metabolic syndrome, and gout. The patient denied smoking or using illicit drugs.
In the ED, his temperature was 101°F, heart rate was 112 beats/min, blood pressure was 175/100 mm Hg, and respiratory rate was 18 breaths/min. His head and neck exam was normal, with no neck stiffness. A lung exam revealed bilateral basal crackles, and a neurologic exam showed residual right-sided weakness due to the hemorrhagic stroke one year ago.
Lab test results revealed the following: white blood cell (WBC) count, 13,000/mm3 with relative monocytosis (14%); lymphocytosis (44%) with normal neutrophils and no bands; hemoglobin, 12 g/dL; hematocrit, 36/mm3; and platelets, 300,000/mm3. Liver function tests were within normal limits. Urinalysis was unremarkable. His troponin I level was elevated at 1.385 ng/dL. In addition to the tachycardia, his electrocardiogram (EKG) showed left axis deviation, left atrial enlargement, left anterior fascicular block, and diffuse nonspecific ST and T wave abnormalities. Chest x-ray was unremarkable except for cardiomegaly. A computed tomography (CT) scan of his head showed residual changes from the previous stroke.
The patient was admitted with a provisional diagnosis of systemic inflammatory response syndrome (SIRS), syncope, non–ST elevation myocardial infarction (NSTEMI), and acute heart failure. The patient had continuous EKG monitoring and serial assessments of his troponin levels. He was also given aspirin, metoprolol 25 mg BID, lisinopril 10 mg/d, furosemide 40 mg IV, isosorbide mononitrate 60 mg/d, and atorvastatin 40 mg/d.
The patient’s cardiac enzymes subsequently decreased. A left heart catheterization was performed, which showed minimum irregularities of the left anterior descending artery (< 20% narrowing) and an ejection fraction (EF) of 35%, without any evidence of obstructive coronary artery disease (CAD). An echocardiogram revealed systolic dysfunction, with an EF of 35% to 40% and global hypokinesis without any apical ballooning or pericardial effusion. (An echocardiogram performed 6 months earlier had shown normal systolic function, an EF of 60% to 65%, and no wall motion abnormalities.) Blood, urine, and fungal cultures were negative; stool studies for ova and parasites were also negative. A lower extremity venous Doppler was negative for deep vein thrombosis.
THE DIAGNOSIS
Because our patient had SIRS, troponinemia, acute systolic dysfunction, and global hypokinesis without any evidence of obstructive CAD, we considered a diagnosis of viral myocarditis. Serologic studies for echovirus, coxsackievirus B, parvovirus B19, adenovirus, and human herpesvirus 6 (HHV-6) all came back negative. However, an enzyme-linked immunosorbent assay (ELISA) for West Nile virus (WNV) was positive. WNV infection was confirmed with a positive plaque reduction neutralization test and a positive qualitative polymerase chain reaction (PCR) assay, which established a diagnosis of WNV myocarditis.
DISCUSSION
While most individuals infected with WNV are asymptomatic, 20% to 40% of patients will exhibit symptoms.1-4 Typical presentations of WNV infection include West Nile fever and neuroinvasive disease. West Nile fever is a self-limited illness characterized by a low-grade fever, headache, malaise, back pain, myalgia, and anorexia for 3 to 6 days.2 Neuroinvasive disease caused by WNV may present as encephalitis, meningitis, or flaccid paralysis.5 Atypical presentations of the virus include rhabdomyolysis,6 fatal hemorrhagic fever with multi-organ failure and palpable purpura,7 hepatitis,8 pancreatitis,9 central diabetes insipidus,10 and myocarditis.11
Although WNV has been linked to myocarditismin animals,12 few human cases of WNV myocarditis11,13 or cardiomyopathy14 have been reported. Viral myocarditis often leads to the development of dilated cardiomyopathy, and myocardial damage may result from direct virus-induced cytotoxicity, T cell-mediated immune response to the virus, or apoptosis.15 Some research suggests that immune-mediated mechanisms play a primary role in myocardial damage. Caforio et al16 found that anti-alpha myosin antibodies were present in 34% of myocarditis patients. In a follow-up study, these antibodies were shown to persist for up to 6 months, which far surpasses the viral cardiac replication timeline of 2 to 3 weeks,17 suggesting that damage occurring after that time is primarily an autoimmune process.
The differential diagnosis for WNV myocarditis includes myocardial stunning from demand ischemia related to SIRS, Takotsubo cardiomyopathy (stress cardiomyopathy), and Dressler’s syndrome. For our patient, myocardial stunning from demand ischemia was less likely because he had no obstructive coronary disease or focal hypokinesis. In addition, the persistence of left ventricular systolic dysfunction and global hypokinesis demonstrated in a repeat echocardiogram during follow-up 6 months later reinforced the likelihood of myocarditis.
The patient’s chest pain with syncope, elevated troponin level, and nonspecific EKG changes in the absence of obstructive CAD raised the possibility of Takotsubo cardiomyopathy. The characteristic echocardiogram finding in Takotsubo cardiomyopathy is transient apical ballooning with akinesis or hypokinesis in the apical and/or mid ventricular regions (typical variant) or isolated midventricular hypokinesis (apical sparing variant). Our patient’s echocardiogram did not show any of these focal wall motion abnormalities, but instead showed global hypokinesis. In addition, the persistence of systolic dysfunction during the repeat echocardiogram and the patient’s lack of psychological distress made the diagnosis of Takotsubo cardiomyopathy unlikely.
Dressler’s syndrome, which is also known as post-myocardial infarction (MI) syndrome, typically presents weeks to months after MI as pleuritic chest pain with a pericardial rub, elevated inflammatory markers, typical EKG changes (diffuse ST-segment elevation and PR-segment depression), and pericardial effusion. This did not fit our patient’s presentation.
Supportive care for heart failure is the mainstay of treatment
The standard treatment for WNV myocarditis is supportive care. Diuretics are used as needed for fluid overload, along with angiotensin-converting enzyme inhibitors and beta-blockers for cardiomyopathy with decreased EF.
Our patient’s dyspnea improved with treatment of furosemide 40 mg IV BID, and his blood pressure was controlled with metoprolol 25 mg BID and lisinopril 10 mg BID. His chest pain and fever resolved when his blood pressure improved. He was discharged home after 7 days on the furosemide, metoprolol, and lisinopril, in addition to isosorbide mononitrate 30 mg/d, atorvastatin 40 mg/d, and aspirin 325 mg/d. An echocardiogram performed 6 months later showed persistent systolic dysfunction, with an EF of 35% and global wall motion abnormalities.
THE TAKEAWAY
In addition to acute coronary syndrome, consider alternate etiologies in patients who present with chest pain and elevated cardiac biomarkers, particularly if diagnostic work-up is negative for obstructive coronary artery disease. WNV myocarditis should be considered as a diagnosis when a patient’s symptoms suggest acute coronary syndrome but are accompanied by fever, headache, and other constitutional symptoms, especially during mosquito season or a WNV outbreak.
THE CASE
A 38-year-old Hispanic man was brought to the emergency department (ED) after losing consciousness and falling at home, striking his elbow, head, and neck. For the past week, he’d had palpitations, shortness of breath, mild swelling of the lower extremities, fever, nausea, and fatigue. He had also been experiencing squeezing chest pain that worsened with exertion and was only partially relieved by nitroglycerin.
The patient did not have any rashes and denied having contact with anyone who was sick. He said that he’d been bitten by mosquitos during recent outdoor activities. His medical history included hypertension, hemorrhagic basal ganglia stroke, hyperlipidemia, sleep apnea, metabolic syndrome, and gout. The patient denied smoking or using illicit drugs.
In the ED, his temperature was 101°F, heart rate was 112 beats/min, blood pressure was 175/100 mm Hg, and respiratory rate was 18 breaths/min. His head and neck exam was normal, with no neck stiffness. A lung exam revealed bilateral basal crackles, and a neurologic exam showed residual right-sided weakness due to the hemorrhagic stroke one year ago.
Lab test results revealed the following: white blood cell (WBC) count, 13,000/mm3 with relative monocytosis (14%); lymphocytosis (44%) with normal neutrophils and no bands; hemoglobin, 12 g/dL; hematocrit, 36/mm3; and platelets, 300,000/mm3. Liver function tests were within normal limits. Urinalysis was unremarkable. His troponin I level was elevated at 1.385 ng/dL. In addition to the tachycardia, his electrocardiogram (EKG) showed left axis deviation, left atrial enlargement, left anterior fascicular block, and diffuse nonspecific ST and T wave abnormalities. Chest x-ray was unremarkable except for cardiomegaly. A computed tomography (CT) scan of his head showed residual changes from the previous stroke.
The patient was admitted with a provisional diagnosis of systemic inflammatory response syndrome (SIRS), syncope, non–ST elevation myocardial infarction (NSTEMI), and acute heart failure. The patient had continuous EKG monitoring and serial assessments of his troponin levels. He was also given aspirin, metoprolol 25 mg BID, lisinopril 10 mg/d, furosemide 40 mg IV, isosorbide mononitrate 60 mg/d, and atorvastatin 40 mg/d.
The patient’s cardiac enzymes subsequently decreased. A left heart catheterization was performed, which showed minimum irregularities of the left anterior descending artery (< 20% narrowing) and an ejection fraction (EF) of 35%, without any evidence of obstructive coronary artery disease (CAD). An echocardiogram revealed systolic dysfunction, with an EF of 35% to 40% and global hypokinesis without any apical ballooning or pericardial effusion. (An echocardiogram performed 6 months earlier had shown normal systolic function, an EF of 60% to 65%, and no wall motion abnormalities.) Blood, urine, and fungal cultures were negative; stool studies for ova and parasites were also negative. A lower extremity venous Doppler was negative for deep vein thrombosis.
THE DIAGNOSIS
Because our patient had SIRS, troponinemia, acute systolic dysfunction, and global hypokinesis without any evidence of obstructive CAD, we considered a diagnosis of viral myocarditis. Serologic studies for echovirus, coxsackievirus B, parvovirus B19, adenovirus, and human herpesvirus 6 (HHV-6) all came back negative. However, an enzyme-linked immunosorbent assay (ELISA) for West Nile virus (WNV) was positive. WNV infection was confirmed with a positive plaque reduction neutralization test and a positive qualitative polymerase chain reaction (PCR) assay, which established a diagnosis of WNV myocarditis.
DISCUSSION
While most individuals infected with WNV are asymptomatic, 20% to 40% of patients will exhibit symptoms.1-4 Typical presentations of WNV infection include West Nile fever and neuroinvasive disease. West Nile fever is a self-limited illness characterized by a low-grade fever, headache, malaise, back pain, myalgia, and anorexia for 3 to 6 days.2 Neuroinvasive disease caused by WNV may present as encephalitis, meningitis, or flaccid paralysis.5 Atypical presentations of the virus include rhabdomyolysis,6 fatal hemorrhagic fever with multi-organ failure and palpable purpura,7 hepatitis,8 pancreatitis,9 central diabetes insipidus,10 and myocarditis.11
Although WNV has been linked to myocarditismin animals,12 few human cases of WNV myocarditis11,13 or cardiomyopathy14 have been reported. Viral myocarditis often leads to the development of dilated cardiomyopathy, and myocardial damage may result from direct virus-induced cytotoxicity, T cell-mediated immune response to the virus, or apoptosis.15 Some research suggests that immune-mediated mechanisms play a primary role in myocardial damage. Caforio et al16 found that anti-alpha myosin antibodies were present in 34% of myocarditis patients. In a follow-up study, these antibodies were shown to persist for up to 6 months, which far surpasses the viral cardiac replication timeline of 2 to 3 weeks,17 suggesting that damage occurring after that time is primarily an autoimmune process.
The differential diagnosis for WNV myocarditis includes myocardial stunning from demand ischemia related to SIRS, Takotsubo cardiomyopathy (stress cardiomyopathy), and Dressler’s syndrome. For our patient, myocardial stunning from demand ischemia was less likely because he had no obstructive coronary disease or focal hypokinesis. In addition, the persistence of left ventricular systolic dysfunction and global hypokinesis demonstrated in a repeat echocardiogram during follow-up 6 months later reinforced the likelihood of myocarditis.
The patient’s chest pain with syncope, elevated troponin level, and nonspecific EKG changes in the absence of obstructive CAD raised the possibility of Takotsubo cardiomyopathy. The characteristic echocardiogram finding in Takotsubo cardiomyopathy is transient apical ballooning with akinesis or hypokinesis in the apical and/or mid ventricular regions (typical variant) or isolated midventricular hypokinesis (apical sparing variant). Our patient’s echocardiogram did not show any of these focal wall motion abnormalities, but instead showed global hypokinesis. In addition, the persistence of systolic dysfunction during the repeat echocardiogram and the patient’s lack of psychological distress made the diagnosis of Takotsubo cardiomyopathy unlikely.
Dressler’s syndrome, which is also known as post-myocardial infarction (MI) syndrome, typically presents weeks to months after MI as pleuritic chest pain with a pericardial rub, elevated inflammatory markers, typical EKG changes (diffuse ST-segment elevation and PR-segment depression), and pericardial effusion. This did not fit our patient’s presentation.
Supportive care for heart failure is the mainstay of treatment
The standard treatment for WNV myocarditis is supportive care. Diuretics are used as needed for fluid overload, along with angiotensin-converting enzyme inhibitors and beta-blockers for cardiomyopathy with decreased EF.
Our patient’s dyspnea improved with treatment of furosemide 40 mg IV BID, and his blood pressure was controlled with metoprolol 25 mg BID and lisinopril 10 mg BID. His chest pain and fever resolved when his blood pressure improved. He was discharged home after 7 days on the furosemide, metoprolol, and lisinopril, in addition to isosorbide mononitrate 30 mg/d, atorvastatin 40 mg/d, and aspirin 325 mg/d. An echocardiogram performed 6 months later showed persistent systolic dysfunction, with an EF of 35% and global wall motion abnormalities.
THE TAKEAWAY
In addition to acute coronary syndrome, consider alternate etiologies in patients who present with chest pain and elevated cardiac biomarkers, particularly if diagnostic work-up is negative for obstructive coronary artery disease. WNV myocarditis should be considered as a diagnosis when a patient’s symptoms suggest acute coronary syndrome but are accompanied by fever, headache, and other constitutional symptoms, especially during mosquito season or a WNV outbreak.
1. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814.
2. Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272-277.
3. Brown JA, Factor DL, Tkachenko N, et al. West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis. 2007;7:479-488.
4. Zou S, Foster GA, Dodd RY, et al. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354-1361.
5. Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300.
6. Montgomery SP, Chow CC, Smith SW, et al. Rhabdomyolysis in patients with west nile encephalitis and meningitis. Vector Borne Zoonotic Dis. 2005;5:252-257.
7. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42:1527-1535.
8. Georges AJ, Lesbordes JL, Georges-Courbot MC, et al. Fatal hepatitis from West Nile virus. Ann Inst Pasteur Virol. 1988;138:237.
9. Perelman A, Stern J. Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg. 1974;23:1150-1152.
10. Sherman-Weber S, Axelrod P. Central diabetes insipidus complicating West Nile encephalitis. Clin Infect Dis. 2004;38:1042-1043.
11. Pergam SA, DeLong CE, Echevarria L, et al. Myocarditis in West Nile virus infection. Am J Trop Med Hyg. 2006;75:1232-1233.
12. van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637-657.
13. Kushawaha A, Jadonath S, Mobarakai N. West nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147.
14. Khouzam RN. Significant cardiomyopathy secondary to West Nile virus infection. South Med J. 2009;102:527-528.
15. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100.
16. Caforio AL, Goldman JH, Haven AJ, et al. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270-275.
17. Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11-18.
1. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807-1814.
2. Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272-277.
3. Brown JA, Factor DL, Tkachenko N, et al. West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis. 2007;7:479-488.
4. Zou S, Foster GA, Dodd RY, et al. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354-1361.
5. Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300.
6. Montgomery SP, Chow CC, Smith SW, et al. Rhabdomyolysis in patients with west nile encephalitis and meningitis. Vector Borne Zoonotic Dis. 2005;5:252-257.
7. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42:1527-1535.
8. Georges AJ, Lesbordes JL, Georges-Courbot MC, et al. Fatal hepatitis from West Nile virus. Ann Inst Pasteur Virol. 1988;138:237.
9. Perelman A, Stern J. Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg. 1974;23:1150-1152.
10. Sherman-Weber S, Axelrod P. Central diabetes insipidus complicating West Nile encephalitis. Clin Infect Dis. 2004;38:1042-1043.
11. Pergam SA, DeLong CE, Echevarria L, et al. Myocarditis in West Nile virus infection. Am J Trop Med Hyg. 2006;75:1232-1233.
12. van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637-657.
13. Kushawaha A, Jadonath S, Mobarakai N. West nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147.
14. Khouzam RN. Significant cardiomyopathy secondary to West Nile virus infection. South Med J. 2009;102:527-528.
15. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091-1100.
16. Caforio AL, Goldman JH, Haven AJ, et al. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270-275.
17. Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11-18.
Mind the Gap: Case Study in Toxicology
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
An 8-month-old boy with a history of hypotonia, developmental delay, and seizure disorder refractory to multiple anticonvulsant medications, was presented to the ED with a 2-week history of intermittent fever and poor oral intake. His current medications included sodium bromide 185 mg orally twice daily for his seizure disorder.
On physical examination, the boy appeared small for his age, with diffuse hypotonia and diminished reflexes. He was able to track with his eyes but was otherwise unresponsive. No rash was present. Results of initial laboratory studies were: sodium 144 mEq/L; potassium, 4.8 mEq/L; chloride, 179 mEq/L; bicarbonate, 21 mEq/L; blood urea nitrogen, 6 mg/dL; creatinine, 0.1 mg/dL; and glucose, 63 mg/dL. His anion gap (AG) was −56.
What does the anion gap represent?
The AG is a valuable clinical calculation derived from the measured extracellular electrolytes and provides an index of acid-base status.1 Due to the necessity of electroneutrality, the sum of positive charges (cations) in the extracellular fluid must be balanced exactly with the sum of negative charges (anions). However, to routinely measure all of the cations and anions in the serum would be time-consuming and is also unnecessary. Because most clinical laboratories commonly only measure one relevant cation (sodium) and two anions (chloride and bicarbonate), the positive and negative sums are not completely balanced. The AG therefore refers to this difference (ie, AG = Na – [Cl + HCO3]).
Of course, electroneutrality exists in vivo, and is accomplished by the presence of unmeasured anions (UA) (eg, lactate and phosphate) and unmeasured cations (UC) (eg, potassium and calcium) not accounted for in the AG (ie, AG = UA – UC). In other words, the sum of measured plus the unmeasured anions must equal the sum of the measured plus unmeasured cations.
What causes a low or negative anion gap?
While most healthcare providers are well versed in the clinical significance of an elevated AG (eg, MUDPILES [methanol, uremia, diabetic ketoacidosis, propylene glycol or phenformin, iron or isoniazid, lactate, ethylene glycol, salicylates]), the meaning of a low or negative AG is underappreciated. There are several scenarios that could potentially yield a low or negative AG, including decreased concentration of UA, increased concentrations of nonsodium cations (UC), and overestimation of serum chloride.
Decreased Concentration of Unmeasured Anions. This most commonly occurs by two mechanisms: dilution of the extracellular fluid or hypoalbuminemia. The addition of water to the extracellular fluid will cause a proportionate dilution of all the measured electrolytes. Since the concentration of measured cations is higher than the measured anions, there is a small and relatively insignificant decrease in the AG.
Alternatively, hypoalbuminemia results in a low AG due to the change in UA; albumin is negatively charged. At physiologic pH, the overwhelming majority of serum proteins are anionic and counter-balanced by the positive charge of sodium. Albumin, the most abundant serum protein, accounts for approximately 75% of the normal AG. Hypoalbuminemic states, such as cirrhosis or nephrotic syndrome, can therefore cause low AG due to the retention of chloride to replace the lost negative charge. The albumin concentration can be corrected to calculate the AG.2
Nonsodium Cations. There are a number of clinical conditions that result in the retention of nonsodium cations. For example, the excess positively charged paraproteins associated with IgG myeloma raise the UC concentration, resulting in a low AG. Similarly, elevations of unmeasured cationic electrolytes, such as calcium and magnesium, may also result in a lower AG. Significant changes in AG, though, are caused only by profound (and often life-threatening) hypercalcemia or hypermagnesemia.
Overestimation of Serum Chloride. Overestimation of serum chloride most commonly occurs in the clinical scenario of bromide exposure. In normal physiologic conditions, chloride is the only halide present in the extracellular fluid. With intake of brominated products, chloride may be partially replaced by bromide. As there is greater renal tubular avidity for bromide, chronic ingestion of bromide results in a gradual rise in serum bromide concentrations with a proportional fall in chloride. However, and more importantly, bromide interferes with a number of laboratory techniques measuring chloride concentrations, resulting in a spuriously elevated chloride, or pseudohyperchloremia. Because the measured sodium and bicarbonate concentrations will remain unchanged, this falsely elevated chloride measurement will result in a negative AG.
What causes the falsely elevated chloride?
All of the current laboratory techniques for measurement of serum chloride concentration can potentially result in a falsely elevated value. However, the degree of pseudohyperchloremia will depend on the specific assay used for measurement. The ion-selective electrode method used by many common laboratory analyzers appears to have the greatest interference on chloride measurement in the presence of bromide. This is simply due to the molecular similarity of bromide and chloride. Conversely, the coulometry method, often used as a reference standard, has the least interference of current laboratory methods.3 This is because coulometry does not completely rely on molecular structure to measure concentration, but rather it measures the amount of energy produced or consumed in an electrolysis reaction. Iodide, another halide compound, has also been described as a cause of pseudohyperchloremia, whereas fluoride does not seem to have significant interference.4
How are patients exposed to bromide salts?
Bromide salts, specifically sodium bromide, are infrequently used to treat seizure disorders, but are generally reserved for patients with epilepsy refractory to other, less toxic anticonvulsant medications. During the era when bromide salts were more commonly used to treat epilepsy, bromide intoxication, or bromism, was frequently observed.
Bromism may manifest as a constellation of nonspecific neurological and psychiatric symptoms. These most commonly include headache, weakness, agitation, confusion, and hallucinations. In more severe cases of bromism, stupor and coma may occur.3,5
Although bromide salts are no longer commonly prescribed, a number of products still contain brominated ingredients. Symptoms of bromide intoxication can occur with chronic use of a cough syrup containing dextromethorphan hydrobromide as well as the brominated vegetable oils found in some soft drinks.5
How is bromism treated?
The treatment of bromism involves preventing further exposure to bromide and promoting bromide excretion. Bromide has a long half-life (10-12 days), and in patients with normal renal function, it is possible to reduce this half-life to approximately 3 days with hydration and diuresis with sodium chloride.3 Alternatively, in patients with impaired renal function or severe intoxication, hemodialysis has been used effectively.5
Case Conclusion
The patient was admitted for observation and treated with intravenous sodium chloride. After consultation with his neurologist, he was discharged home in the care of his parents, who were advised to continue him on sodium bromide 185 mg orally twice daily since his seizures were refractory to other anticonvulsant medications.
Dr Repplinger is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
- Emmett M, Narins RG. Clinical use of the anion gap. Medicine (Baltimore). 1977;56(1):38-54.
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807-1810.
- Vasuyattakul S, Lertpattanasuwan N, Vareesangthip K, Nimmannit S, Nilwarangkur S. A negative aniongap as a clue to diagnose bromide intoxication.Nephron. 1995;69(3):311-313.
- Yamamoto K, Kobayashi H, Kobayashi T, MurakamiS. False hyperchloremia in bromism. J Anesth.1991;5(1):88-91.
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremiaand decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol. 1992;12(4):268-270.
Case Report: Sudden Chest Pain Following an Asthma Attack
Case
A 20-year-old woman with a history of asthma and cigarette smoking presented to the ED twice within the same week. At the initial presentation, the patient stated that she had been experiencing symptoms of an upper respiratory infection over the past 4 days which had progressed to difficulty breathing. She further noted that these symptoms were also consistent with childhood asthma exacerbations. In addition to childhood asthma, the patient’s medical history included chronic dermatitis, for which she had been taking oral doxycycline daily.
The patient was treated with intravenous (IV) corticosteroids and an albuterol/atrovent nebulizer. Based on significant response to treatment and improvement, she was discharged on a 5-day steroid burst and albuterol meter-dose inhaler. She was also counseled to continue taking doxycycline daily for the chronic dermatitis.
Two days after discharge, the patient returned to the ED with episodes of coughing followed by worsening shortness of breath and sharp chest pain that radiated into her neck and back, which she described as unbearable. Neither the pain nor shortness of breath responded to albuterol.
The patient’s vital signs at this second presentation were: blood pressure, 144/88 mm Hg; heart rate, 98 beats/minute; respiratory rate, 28 breaths/minute; and temperature, 97.8˚ F. Oxygen (O2) saturation was 94% on room air. On physical examination, the patient was uncomfortable and in mild distress. She was visibly tachypneic with diminished breath sounds, but no wheezing, rales, or rhonchi were appreciated on auscultation. Cardiac auscultation was likewise normal. The patient had a visceral sense of pain on palpation of her anterior chest wall, but no crepitus was appreciated. Both an electrocardiogram and chest X-ray (CXR) were normal, but laboratory evaluation revealed a white blood cell count of 18.4 u/L (4.0-11.0).
An assessment based on Well’s criteria showed the patient to be at a moderate risk for pulmonary embolism (PE), and a computed tomography angiography (CTA) of the chest was obtained. The CTA was negative for PE but showed significant pneumomediastinum (Figures 1 and 2). Upon repeat examination, crepitus was palpated on the anterior right neck, correlating with the CTA findings.
The patient was admitted to the inpatient ward for observation and continued support, and she was given IV analgesics and supplemental O2 via nasal cannula. Due to the considerable amount of air surrounding her heart, she was considered at high-risk for disease progression. Since she had been on continuous doxycycline therapy, the inpatient team performed a gastrografin swallow study to rule out esophageal perforation. The results of this test were negative, further supporting asthma exacerbation as the etiology for the pneumomediastinum. As cigarette smoking was a contributing risk factor, she was also counseled on tobacco cessation.
During her inpatient stay, the patient completed the 5-day course of oral corticosteroids that had been prescribed at her first ED visit, and was weaned-off supplemental O2. After an uncomplicated clinical course and full resolution of symptoms, she was discharged on hospital day 3 with an albuterol hydrofluoroalkane inhaler to be used as needed; she was also advised to continue taking doxycycline as prescribed for her dermatologic condition. The patient was followed in the outpatient setting on a routine basis; to date, she has remained asymptomatic with no sequelae.
Pneumomediastinum
Also referred to as mediastinal emphysema, pneumomediastinum manifests as free air surrounding the mediastinal structures.1-7 This condition most commonly occurs in middle-aged men; though rare, 25% of all cases have been linked to existing pulmonary disease.1,6,8,9 Patients with pneumomediastinum typically present with pleuritic chest pain (often radiating to the neck and back), dyspnea, dysphonia, and dysphagia.1,2,5,7-9
Risk Factors
Pneumomediastinum is usually associated with asthma exacerbations; however, it has also been linked to increases in intrathoracic pressure, extension of subcutaneous emphysema from superior structures (eg, cervical region), esophageal lesions, as well as use and/or withdrawal symptoms of certain drugs (eg, cocaine).1,2,4,6,8
When considering primary pulmonary disease, the pathophysiological process is driven by a pressure gradient between the pulmonary interstitium and the alveoli. The force transference causes alveolar rupture, allowing air to escape into the interstitium.9 In asthma, obstruction in the minor airways leads to air-trapping and barotrauma of distal airways, resulting in the aforementioned alveolar rupture.2 Once in the lung interstitium, the free air flows along the pressure gradient through the hilum from the pulmonary parenchyma in a centripetal direction before entering the mediastinum (Macklin effect).2,9
Pneumomediastinum is not exclusive to asthma and subsequent alveolar barotrauma. There are reported instances of gas-producing microorganisms within the mediastinum as well as perforated mucosal structures—specifically the esophagus (of which doxycycline is a risk factor) and tracheobronchial tree, which have also manifested as mediastinal emphysema.6,9
One of the most life-threatening causes of pneumomediastinum is Boerhaave syndrome in which there is a spontaneous rupture of the esophagus.3 In addition, as previously mentioned, numerous illicit drugs (eg, cocaine, marijuana, ecstasy) have been implicated in pneumomediastinum—with cocaine posing the greatest risk.8,9 Perna et al8 relate cocaine-related pneumomediastinum to the direct toxic effects of the drug on the lung parenchyma in the acute phases of use and not due to pressure variations resulting from the Valsalva maneuver necessary to inhale the drug.
Predisposing risk factors for mediastinal emphysema include preexisting pulmonary disease (eg, chronic obstructive pulmonary disease, asthma) and a history of smoking.5,6,8,9 Precipitating risks include recent exercise, significant upper respiratory infection, forceful straining (eg, cough, emesis, childbirth), and diabetic ketoacidosis.6,8,9 Unlikely sources that have also been implicated are those related to surgical procedures, such as arthroscopy, adenotonsillectomy, instrumentation, and recreational activities (eg, scuba diving, playing wind instruments).3,6
Diagnosis
The diagnosis is often made clinically and confirmed radiographically.2,7 Physical examination findings vary by the extent of the mediastinal emphysema. Common findings include the Hamman sign, which has been described as classic “crunching, crackling, bubbling, and rasping sounds synchronous with peak heart beat” on auscultation.2,5,8 Air from the mediastinum may dissect into the skin, particularly in the superior structures of the neck and face, resulting in a subcutaneous emphysema described as a palpable crunching sound.2
Imaging
Obtaining a plain CXR is an acceptable standard of practice. Pneumomediastinum has been described as either a “classic hyperlucency” or, as Chiu et al5 have described, as “lucent streaks or bubbles of gas that outline mediastinal structures” on a posteroanterior and lateral film, implying damage to luminal structures of the intrathoracic cavity.2,4,5,7,9 This lucency has been further described as an outline of the inner pleura of mediastinal structures, which is a defined crease lateral to the pulmonary artery and arch of the aorta along the cardiac border (best visualized on a lateral view image).5,7 A scant amount of air, however, may not be visualized on a plain film.2,4,5,9 Therefore, the literature supports a thoracic CT as the gold standard for its greater sensitivity in visualizing a small pneumomediastinum.2,9
Treatment
Since pneumomediastinum is typically a self-limited disease, treatment is often conservative management with specific consideration to the patient’s age, severity of symptoms, underlying cause, and comorbidities.2,10 Treatment should include rest, supplemental O2, and analgesia.5,7,9 Hospitalization for an observation period of 2 to 5 days is recommended, with one study reporting an average length of stay as 3 days or until resolution of symptoms.8,9
Disposition is best determined by the patient’s clinical course; however, the risk of progression warrants observation for serial follow-up examinations and possibly repeat imaging as well as access to acute intervention, if required. Though rare, several serious comorbid conditions have been reported, including hypertensive episodes, bilateral pneumothoraxes, and even cardiac compression (or tension pneumomediastinum) limiting cardiac output and leading to cardiac arrest.2,4 Following discharge, there has been no clinical study reporting recurrence of pneumomediastinum; therefore, close clinical follow-up is not necessarily indicated.4,9
Conclusion
Pneumomediastinum is a rare complication of acute and chronic pulmonary and extrapulmonary diseases. As seen in this case presentation, the condition is typically associated with asthma exacerbations. Patients often present with chest pain, dyspnea, dysphonia, and dysphagia. Although diagnosis may be confirmed using plain CRX, CTA is the preferred modality due to its higher sensitivity in visualizing mild pneumomediastinum. While the risk of mortality is present, the condition generally follows a stable clinical course with minimal risk of long-term morbidity.
Major Howell is a physician assistant, department of emergency medicine, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio. Captain Pennington is an emergency medicine physician, department of emergency medicine, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio.
Acknowledgement:The authors wish to extend special thanks to Dr Nurani Kester, department of emergency medicine, San Antonio Military Medical Center, Fort Sam Houston, Texas; and to Dr Luke Simonet, department of radiology, David Grant United States Air Force Medical Center, Travis Air Force Base, California.
Disclosure: The authors report no conflicts of interest.
- Dajer-Fadel WL, Argüero-Sanchez R, Ibarra-Pérez C, Navarro-Reynoso F. Systemic review of spontaneous pneumomediastinium: a survey of 22 years’ data. Asian Cardiovasc Thorac Ann. 2014;22(8):997-1002.
- Karakaya Z, Demir S, Sagay SS, Karakaya O, Ozdinç S. Bilateral spontaneous pneumothorax, pneumomediastinum, and subcutaenous emphyesema: rare and fatal complications of asthma. Case Rep Emerg Med. 2012;2012:242579.
- Kogan I, Celli B. Pneumomediastinum in a 63-year-old woman with asthma exacerbation. Chest. 2000;117(6):1778-1781.
- Momin AU, Chung DA, John LC. Childhood asthma predisposes to spontaneous pneumomediastinum. Emerg Med J. 2004;21(5):630-631.
- Chiu CY, Wong KS, Yao TC, Huang JL. Asthmatic versus non-asthmatic spontaneous pneumomediastinum in children. Asian Pac J Allergy Immunol. 2005;23(1):19-22.
- Akinyemi R, Ogah O, Akisanya C, et al. Pneumomediastinum and subcutaneous emphysema complicating acute exacerbation of bronchial asthma. Ann Ib Postgrad Med. 2007:5(2):78-79.
- Firinci F, Ozgürler F, Doğan M, Koçyigit A, Mete E. Spontaneous pneumomediastinum in childhood: report of an adolescent case diagnose with asthma. Tuberk Toraks. 2014;62(3):253-254.
- Perna V, Vilá E, Guelbenzu J, Amat, I. Penumomediastinum: is this really a benign entity? When it can be considered as spontaenous? Our experience in 47 adult patients? Eur J Cardiothorac Surg. 2010;37(3):573-575.
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31(6):1110-1114.
- Mahajan P, AI Maslamani NJ, Purayil, NK. Rare case of pneumorrhachis, pneumomediastinum, pneumothorax, and surgical emphysema secondary to bronchial asthma. Int Med Case Rep J. 2014;7:35-39.
Case
A 20-year-old woman with a history of asthma and cigarette smoking presented to the ED twice within the same week. At the initial presentation, the patient stated that she had been experiencing symptoms of an upper respiratory infection over the past 4 days which had progressed to difficulty breathing. She further noted that these symptoms were also consistent with childhood asthma exacerbations. In addition to childhood asthma, the patient’s medical history included chronic dermatitis, for which she had been taking oral doxycycline daily.
The patient was treated with intravenous (IV) corticosteroids and an albuterol/atrovent nebulizer. Based on significant response to treatment and improvement, she was discharged on a 5-day steroid burst and albuterol meter-dose inhaler. She was also counseled to continue taking doxycycline daily for the chronic dermatitis.
Two days after discharge, the patient returned to the ED with episodes of coughing followed by worsening shortness of breath and sharp chest pain that radiated into her neck and back, which she described as unbearable. Neither the pain nor shortness of breath responded to albuterol.
The patient’s vital signs at this second presentation were: blood pressure, 144/88 mm Hg; heart rate, 98 beats/minute; respiratory rate, 28 breaths/minute; and temperature, 97.8˚ F. Oxygen (O2) saturation was 94% on room air. On physical examination, the patient was uncomfortable and in mild distress. She was visibly tachypneic with diminished breath sounds, but no wheezing, rales, or rhonchi were appreciated on auscultation. Cardiac auscultation was likewise normal. The patient had a visceral sense of pain on palpation of her anterior chest wall, but no crepitus was appreciated. Both an electrocardiogram and chest X-ray (CXR) were normal, but laboratory evaluation revealed a white blood cell count of 18.4 u/L (4.0-11.0).
An assessment based on Well’s criteria showed the patient to be at a moderate risk for pulmonary embolism (PE), and a computed tomography angiography (CTA) of the chest was obtained. The CTA was negative for PE but showed significant pneumomediastinum (Figures 1 and 2). Upon repeat examination, crepitus was palpated on the anterior right neck, correlating with the CTA findings.
The patient was admitted to the inpatient ward for observation and continued support, and she was given IV analgesics and supplemental O2 via nasal cannula. Due to the considerable amount of air surrounding her heart, she was considered at high-risk for disease progression. Since she had been on continuous doxycycline therapy, the inpatient team performed a gastrografin swallow study to rule out esophageal perforation. The results of this test were negative, further supporting asthma exacerbation as the etiology for the pneumomediastinum. As cigarette smoking was a contributing risk factor, she was also counseled on tobacco cessation.
During her inpatient stay, the patient completed the 5-day course of oral corticosteroids that had been prescribed at her first ED visit, and was weaned-off supplemental O2. After an uncomplicated clinical course and full resolution of symptoms, she was discharged on hospital day 3 with an albuterol hydrofluoroalkane inhaler to be used as needed; she was also advised to continue taking doxycycline as prescribed for her dermatologic condition. The patient was followed in the outpatient setting on a routine basis; to date, she has remained asymptomatic with no sequelae.
Pneumomediastinum
Also referred to as mediastinal emphysema, pneumomediastinum manifests as free air surrounding the mediastinal structures.1-7 This condition most commonly occurs in middle-aged men; though rare, 25% of all cases have been linked to existing pulmonary disease.1,6,8,9 Patients with pneumomediastinum typically present with pleuritic chest pain (often radiating to the neck and back), dyspnea, dysphonia, and dysphagia.1,2,5,7-9
Risk Factors
Pneumomediastinum is usually associated with asthma exacerbations; however, it has also been linked to increases in intrathoracic pressure, extension of subcutaneous emphysema from superior structures (eg, cervical region), esophageal lesions, as well as use and/or withdrawal symptoms of certain drugs (eg, cocaine).1,2,4,6,8
When considering primary pulmonary disease, the pathophysiological process is driven by a pressure gradient between the pulmonary interstitium and the alveoli. The force transference causes alveolar rupture, allowing air to escape into the interstitium.9 In asthma, obstruction in the minor airways leads to air-trapping and barotrauma of distal airways, resulting in the aforementioned alveolar rupture.2 Once in the lung interstitium, the free air flows along the pressure gradient through the hilum from the pulmonary parenchyma in a centripetal direction before entering the mediastinum (Macklin effect).2,9
Pneumomediastinum is not exclusive to asthma and subsequent alveolar barotrauma. There are reported instances of gas-producing microorganisms within the mediastinum as well as perforated mucosal structures—specifically the esophagus (of which doxycycline is a risk factor) and tracheobronchial tree, which have also manifested as mediastinal emphysema.6,9
One of the most life-threatening causes of pneumomediastinum is Boerhaave syndrome in which there is a spontaneous rupture of the esophagus.3 In addition, as previously mentioned, numerous illicit drugs (eg, cocaine, marijuana, ecstasy) have been implicated in pneumomediastinum—with cocaine posing the greatest risk.8,9 Perna et al8 relate cocaine-related pneumomediastinum to the direct toxic effects of the drug on the lung parenchyma in the acute phases of use and not due to pressure variations resulting from the Valsalva maneuver necessary to inhale the drug.
Predisposing risk factors for mediastinal emphysema include preexisting pulmonary disease (eg, chronic obstructive pulmonary disease, asthma) and a history of smoking.5,6,8,9 Precipitating risks include recent exercise, significant upper respiratory infection, forceful straining (eg, cough, emesis, childbirth), and diabetic ketoacidosis.6,8,9 Unlikely sources that have also been implicated are those related to surgical procedures, such as arthroscopy, adenotonsillectomy, instrumentation, and recreational activities (eg, scuba diving, playing wind instruments).3,6
Diagnosis
The diagnosis is often made clinically and confirmed radiographically.2,7 Physical examination findings vary by the extent of the mediastinal emphysema. Common findings include the Hamman sign, which has been described as classic “crunching, crackling, bubbling, and rasping sounds synchronous with peak heart beat” on auscultation.2,5,8 Air from the mediastinum may dissect into the skin, particularly in the superior structures of the neck and face, resulting in a subcutaneous emphysema described as a palpable crunching sound.2
Imaging
Obtaining a plain CXR is an acceptable standard of practice. Pneumomediastinum has been described as either a “classic hyperlucency” or, as Chiu et al5 have described, as “lucent streaks or bubbles of gas that outline mediastinal structures” on a posteroanterior and lateral film, implying damage to luminal structures of the intrathoracic cavity.2,4,5,7,9 This lucency has been further described as an outline of the inner pleura of mediastinal structures, which is a defined crease lateral to the pulmonary artery and arch of the aorta along the cardiac border (best visualized on a lateral view image).5,7 A scant amount of air, however, may not be visualized on a plain film.2,4,5,9 Therefore, the literature supports a thoracic CT as the gold standard for its greater sensitivity in visualizing a small pneumomediastinum.2,9
Treatment
Since pneumomediastinum is typically a self-limited disease, treatment is often conservative management with specific consideration to the patient’s age, severity of symptoms, underlying cause, and comorbidities.2,10 Treatment should include rest, supplemental O2, and analgesia.5,7,9 Hospitalization for an observation period of 2 to 5 days is recommended, with one study reporting an average length of stay as 3 days or until resolution of symptoms.8,9
Disposition is best determined by the patient’s clinical course; however, the risk of progression warrants observation for serial follow-up examinations and possibly repeat imaging as well as access to acute intervention, if required. Though rare, several serious comorbid conditions have been reported, including hypertensive episodes, bilateral pneumothoraxes, and even cardiac compression (or tension pneumomediastinum) limiting cardiac output and leading to cardiac arrest.2,4 Following discharge, there has been no clinical study reporting recurrence of pneumomediastinum; therefore, close clinical follow-up is not necessarily indicated.4,9
Conclusion
Pneumomediastinum is a rare complication of acute and chronic pulmonary and extrapulmonary diseases. As seen in this case presentation, the condition is typically associated with asthma exacerbations. Patients often present with chest pain, dyspnea, dysphonia, and dysphagia. Although diagnosis may be confirmed using plain CRX, CTA is the preferred modality due to its higher sensitivity in visualizing mild pneumomediastinum. While the risk of mortality is present, the condition generally follows a stable clinical course with minimal risk of long-term morbidity.
Major Howell is a physician assistant, department of emergency medicine, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio. Captain Pennington is an emergency medicine physician, department of emergency medicine, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio.
Acknowledgement:The authors wish to extend special thanks to Dr Nurani Kester, department of emergency medicine, San Antonio Military Medical Center, Fort Sam Houston, Texas; and to Dr Luke Simonet, department of radiology, David Grant United States Air Force Medical Center, Travis Air Force Base, California.
Disclosure: The authors report no conflicts of interest.
Case
A 20-year-old woman with a history of asthma and cigarette smoking presented to the ED twice within the same week. At the initial presentation, the patient stated that she had been experiencing symptoms of an upper respiratory infection over the past 4 days which had progressed to difficulty breathing. She further noted that these symptoms were also consistent with childhood asthma exacerbations. In addition to childhood asthma, the patient’s medical history included chronic dermatitis, for which she had been taking oral doxycycline daily.
The patient was treated with intravenous (IV) corticosteroids and an albuterol/atrovent nebulizer. Based on significant response to treatment and improvement, she was discharged on a 5-day steroid burst and albuterol meter-dose inhaler. She was also counseled to continue taking doxycycline daily for the chronic dermatitis.
Two days after discharge, the patient returned to the ED with episodes of coughing followed by worsening shortness of breath and sharp chest pain that radiated into her neck and back, which she described as unbearable. Neither the pain nor shortness of breath responded to albuterol.
The patient’s vital signs at this second presentation were: blood pressure, 144/88 mm Hg; heart rate, 98 beats/minute; respiratory rate, 28 breaths/minute; and temperature, 97.8˚ F. Oxygen (O2) saturation was 94% on room air. On physical examination, the patient was uncomfortable and in mild distress. She was visibly tachypneic with diminished breath sounds, but no wheezing, rales, or rhonchi were appreciated on auscultation. Cardiac auscultation was likewise normal. The patient had a visceral sense of pain on palpation of her anterior chest wall, but no crepitus was appreciated. Both an electrocardiogram and chest X-ray (CXR) were normal, but laboratory evaluation revealed a white blood cell count of 18.4 u/L (4.0-11.0).
An assessment based on Well’s criteria showed the patient to be at a moderate risk for pulmonary embolism (PE), and a computed tomography angiography (CTA) of the chest was obtained. The CTA was negative for PE but showed significant pneumomediastinum (Figures 1 and 2). Upon repeat examination, crepitus was palpated on the anterior right neck, correlating with the CTA findings.
The patient was admitted to the inpatient ward for observation and continued support, and she was given IV analgesics and supplemental O2 via nasal cannula. Due to the considerable amount of air surrounding her heart, she was considered at high-risk for disease progression. Since she had been on continuous doxycycline therapy, the inpatient team performed a gastrografin swallow study to rule out esophageal perforation. The results of this test were negative, further supporting asthma exacerbation as the etiology for the pneumomediastinum. As cigarette smoking was a contributing risk factor, she was also counseled on tobacco cessation.
During her inpatient stay, the patient completed the 5-day course of oral corticosteroids that had been prescribed at her first ED visit, and was weaned-off supplemental O2. After an uncomplicated clinical course and full resolution of symptoms, she was discharged on hospital day 3 with an albuterol hydrofluoroalkane inhaler to be used as needed; she was also advised to continue taking doxycycline as prescribed for her dermatologic condition. The patient was followed in the outpatient setting on a routine basis; to date, she has remained asymptomatic with no sequelae.
Pneumomediastinum
Also referred to as mediastinal emphysema, pneumomediastinum manifests as free air surrounding the mediastinal structures.1-7 This condition most commonly occurs in middle-aged men; though rare, 25% of all cases have been linked to existing pulmonary disease.1,6,8,9 Patients with pneumomediastinum typically present with pleuritic chest pain (often radiating to the neck and back), dyspnea, dysphonia, and dysphagia.1,2,5,7-9
Risk Factors
Pneumomediastinum is usually associated with asthma exacerbations; however, it has also been linked to increases in intrathoracic pressure, extension of subcutaneous emphysema from superior structures (eg, cervical region), esophageal lesions, as well as use and/or withdrawal symptoms of certain drugs (eg, cocaine).1,2,4,6,8
When considering primary pulmonary disease, the pathophysiological process is driven by a pressure gradient between the pulmonary interstitium and the alveoli. The force transference causes alveolar rupture, allowing air to escape into the interstitium.9 In asthma, obstruction in the minor airways leads to air-trapping and barotrauma of distal airways, resulting in the aforementioned alveolar rupture.2 Once in the lung interstitium, the free air flows along the pressure gradient through the hilum from the pulmonary parenchyma in a centripetal direction before entering the mediastinum (Macklin effect).2,9
Pneumomediastinum is not exclusive to asthma and subsequent alveolar barotrauma. There are reported instances of gas-producing microorganisms within the mediastinum as well as perforated mucosal structures—specifically the esophagus (of which doxycycline is a risk factor) and tracheobronchial tree, which have also manifested as mediastinal emphysema.6,9
One of the most life-threatening causes of pneumomediastinum is Boerhaave syndrome in which there is a spontaneous rupture of the esophagus.3 In addition, as previously mentioned, numerous illicit drugs (eg, cocaine, marijuana, ecstasy) have been implicated in pneumomediastinum—with cocaine posing the greatest risk.8,9 Perna et al8 relate cocaine-related pneumomediastinum to the direct toxic effects of the drug on the lung parenchyma in the acute phases of use and not due to pressure variations resulting from the Valsalva maneuver necessary to inhale the drug.
Predisposing risk factors for mediastinal emphysema include preexisting pulmonary disease (eg, chronic obstructive pulmonary disease, asthma) and a history of smoking.5,6,8,9 Precipitating risks include recent exercise, significant upper respiratory infection, forceful straining (eg, cough, emesis, childbirth), and diabetic ketoacidosis.6,8,9 Unlikely sources that have also been implicated are those related to surgical procedures, such as arthroscopy, adenotonsillectomy, instrumentation, and recreational activities (eg, scuba diving, playing wind instruments).3,6
Diagnosis
The diagnosis is often made clinically and confirmed radiographically.2,7 Physical examination findings vary by the extent of the mediastinal emphysema. Common findings include the Hamman sign, which has been described as classic “crunching, crackling, bubbling, and rasping sounds synchronous with peak heart beat” on auscultation.2,5,8 Air from the mediastinum may dissect into the skin, particularly in the superior structures of the neck and face, resulting in a subcutaneous emphysema described as a palpable crunching sound.2
Imaging
Obtaining a plain CXR is an acceptable standard of practice. Pneumomediastinum has been described as either a “classic hyperlucency” or, as Chiu et al5 have described, as “lucent streaks or bubbles of gas that outline mediastinal structures” on a posteroanterior and lateral film, implying damage to luminal structures of the intrathoracic cavity.2,4,5,7,9 This lucency has been further described as an outline of the inner pleura of mediastinal structures, which is a defined crease lateral to the pulmonary artery and arch of the aorta along the cardiac border (best visualized on a lateral view image).5,7 A scant amount of air, however, may not be visualized on a plain film.2,4,5,9 Therefore, the literature supports a thoracic CT as the gold standard for its greater sensitivity in visualizing a small pneumomediastinum.2,9
Treatment
Since pneumomediastinum is typically a self-limited disease, treatment is often conservative management with specific consideration to the patient’s age, severity of symptoms, underlying cause, and comorbidities.2,10 Treatment should include rest, supplemental O2, and analgesia.5,7,9 Hospitalization for an observation period of 2 to 5 days is recommended, with one study reporting an average length of stay as 3 days or until resolution of symptoms.8,9
Disposition is best determined by the patient’s clinical course; however, the risk of progression warrants observation for serial follow-up examinations and possibly repeat imaging as well as access to acute intervention, if required. Though rare, several serious comorbid conditions have been reported, including hypertensive episodes, bilateral pneumothoraxes, and even cardiac compression (or tension pneumomediastinum) limiting cardiac output and leading to cardiac arrest.2,4 Following discharge, there has been no clinical study reporting recurrence of pneumomediastinum; therefore, close clinical follow-up is not necessarily indicated.4,9
Conclusion
Pneumomediastinum is a rare complication of acute and chronic pulmonary and extrapulmonary diseases. As seen in this case presentation, the condition is typically associated with asthma exacerbations. Patients often present with chest pain, dyspnea, dysphonia, and dysphagia. Although diagnosis may be confirmed using plain CRX, CTA is the preferred modality due to its higher sensitivity in visualizing mild pneumomediastinum. While the risk of mortality is present, the condition generally follows a stable clinical course with minimal risk of long-term morbidity.
Major Howell is a physician assistant, department of emergency medicine, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio. Captain Pennington is an emergency medicine physician, department of emergency medicine, Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio.
Acknowledgement:The authors wish to extend special thanks to Dr Nurani Kester, department of emergency medicine, San Antonio Military Medical Center, Fort Sam Houston, Texas; and to Dr Luke Simonet, department of radiology, David Grant United States Air Force Medical Center, Travis Air Force Base, California.
Disclosure: The authors report no conflicts of interest.
- Dajer-Fadel WL, Argüero-Sanchez R, Ibarra-Pérez C, Navarro-Reynoso F. Systemic review of spontaneous pneumomediastinium: a survey of 22 years’ data. Asian Cardiovasc Thorac Ann. 2014;22(8):997-1002.
- Karakaya Z, Demir S, Sagay SS, Karakaya O, Ozdinç S. Bilateral spontaneous pneumothorax, pneumomediastinum, and subcutaenous emphyesema: rare and fatal complications of asthma. Case Rep Emerg Med. 2012;2012:242579.
- Kogan I, Celli B. Pneumomediastinum in a 63-year-old woman with asthma exacerbation. Chest. 2000;117(6):1778-1781.
- Momin AU, Chung DA, John LC. Childhood asthma predisposes to spontaneous pneumomediastinum. Emerg Med J. 2004;21(5):630-631.
- Chiu CY, Wong KS, Yao TC, Huang JL. Asthmatic versus non-asthmatic spontaneous pneumomediastinum in children. Asian Pac J Allergy Immunol. 2005;23(1):19-22.
- Akinyemi R, Ogah O, Akisanya C, et al. Pneumomediastinum and subcutaneous emphysema complicating acute exacerbation of bronchial asthma. Ann Ib Postgrad Med. 2007:5(2):78-79.
- Firinci F, Ozgürler F, Doğan M, Koçyigit A, Mete E. Spontaneous pneumomediastinum in childhood: report of an adolescent case diagnose with asthma. Tuberk Toraks. 2014;62(3):253-254.
- Perna V, Vilá E, Guelbenzu J, Amat, I. Penumomediastinum: is this really a benign entity? When it can be considered as spontaenous? Our experience in 47 adult patients? Eur J Cardiothorac Surg. 2010;37(3):573-575.
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31(6):1110-1114.
- Mahajan P, AI Maslamani NJ, Purayil, NK. Rare case of pneumorrhachis, pneumomediastinum, pneumothorax, and surgical emphysema secondary to bronchial asthma. Int Med Case Rep J. 2014;7:35-39.
- Dajer-Fadel WL, Argüero-Sanchez R, Ibarra-Pérez C, Navarro-Reynoso F. Systemic review of spontaneous pneumomediastinium: a survey of 22 years’ data. Asian Cardiovasc Thorac Ann. 2014;22(8):997-1002.
- Karakaya Z, Demir S, Sagay SS, Karakaya O, Ozdinç S. Bilateral spontaneous pneumothorax, pneumomediastinum, and subcutaenous emphyesema: rare and fatal complications of asthma. Case Rep Emerg Med. 2012;2012:242579.
- Kogan I, Celli B. Pneumomediastinum in a 63-year-old woman with asthma exacerbation. Chest. 2000;117(6):1778-1781.
- Momin AU, Chung DA, John LC. Childhood asthma predisposes to spontaneous pneumomediastinum. Emerg Med J. 2004;21(5):630-631.
- Chiu CY, Wong KS, Yao TC, Huang JL. Asthmatic versus non-asthmatic spontaneous pneumomediastinum in children. Asian Pac J Allergy Immunol. 2005;23(1):19-22.
- Akinyemi R, Ogah O, Akisanya C, et al. Pneumomediastinum and subcutaneous emphysema complicating acute exacerbation of bronchial asthma. Ann Ib Postgrad Med. 2007:5(2):78-79.
- Firinci F, Ozgürler F, Doğan M, Koçyigit A, Mete E. Spontaneous pneumomediastinum in childhood: report of an adolescent case diagnose with asthma. Tuberk Toraks. 2014;62(3):253-254.
- Perna V, Vilá E, Guelbenzu J, Amat, I. Penumomediastinum: is this really a benign entity? When it can be considered as spontaenous? Our experience in 47 adult patients? Eur J Cardiothorac Surg. 2010;37(3):573-575.
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31(6):1110-1114.
- Mahajan P, AI Maslamani NJ, Purayil, NK. Rare case of pneumorrhachis, pneumomediastinum, pneumothorax, and surgical emphysema secondary to bronchial asthma. Int Med Case Rep J. 2014;7:35-39.