User login
Sarcoidosis, complete heart block, and warm autoimmune hemolytic anemia in a young woman
Sarcoidosis is a multisystem granulomatous disease that affects 10-40 people per 100,000 in the United States and Europe, with an increased prevalence among blacks compared with whites.1 The clinical presentation of sarcoidosis is variable. Sarcoidosis frequently involves the lungs and can have numerous extrapulmonary manifestations including skin, joint, cardiac, and eye lesions. We present a rare case of sarcoidosis with concurrent third-degree heart block and warm autoimmune hemolytic anemia and discuss possible mechanisms behind this presentation.
Click on the PDF icon at the top of this introduction to read the full article.
Sarcoidosis is a multisystem granulomatous disease that affects 10-40 people per 100,000 in the United States and Europe, with an increased prevalence among blacks compared with whites.1 The clinical presentation of sarcoidosis is variable. Sarcoidosis frequently involves the lungs and can have numerous extrapulmonary manifestations including skin, joint, cardiac, and eye lesions. We present a rare case of sarcoidosis with concurrent third-degree heart block and warm autoimmune hemolytic anemia and discuss possible mechanisms behind this presentation.
Click on the PDF icon at the top of this introduction to read the full article.
Sarcoidosis is a multisystem granulomatous disease that affects 10-40 people per 100,000 in the United States and Europe, with an increased prevalence among blacks compared with whites.1 The clinical presentation of sarcoidosis is variable. Sarcoidosis frequently involves the lungs and can have numerous extrapulmonary manifestations including skin, joint, cardiac, and eye lesions. We present a rare case of sarcoidosis with concurrent third-degree heart block and warm autoimmune hemolytic anemia and discuss possible mechanisms behind this presentation.
Click on the PDF icon at the top of this introduction to read the full article.
Distant skin metastases as primary presentation of gastric cancer
Distant gastric metastasis to the skin is uncommonly a presenting symptom, although nonspecific paraneoplastic syndromes with dermatologic manifestation including diffuse seborrheic keratoses (Leser-Trelat sign), tripe palms, and acanthosis nigricans have been described in the literature. We report here the case of a 49-year-old woman with gastric adenocarcinoma who presented with cutaneous metastasis as an initial symptom. In our case, metastatic skin lesions responded significantly to EOX chemotherapy (epirubicin+oxaliplatin+capecitabine) despite progression of systemic disease. In similar presentations, a high index of clinical suspicion and skin biopsy are important.
Click on the PDF icon at the top of this introduction to read the full article.
Distant gastric metastasis to the skin is uncommonly a presenting symptom, although nonspecific paraneoplastic syndromes with dermatologic manifestation including diffuse seborrheic keratoses (Leser-Trelat sign), tripe palms, and acanthosis nigricans have been described in the literature. We report here the case of a 49-year-old woman with gastric adenocarcinoma who presented with cutaneous metastasis as an initial symptom. In our case, metastatic skin lesions responded significantly to EOX chemotherapy (epirubicin+oxaliplatin+capecitabine) despite progression of systemic disease. In similar presentations, a high index of clinical suspicion and skin biopsy are important.
Click on the PDF icon at the top of this introduction to read the full article.
Distant gastric metastasis to the skin is uncommonly a presenting symptom, although nonspecific paraneoplastic syndromes with dermatologic manifestation including diffuse seborrheic keratoses (Leser-Trelat sign), tripe palms, and acanthosis nigricans have been described in the literature. We report here the case of a 49-year-old woman with gastric adenocarcinoma who presented with cutaneous metastasis as an initial symptom. In our case, metastatic skin lesions responded significantly to EOX chemotherapy (epirubicin+oxaliplatin+capecitabine) despite progression of systemic disease. In similar presentations, a high index of clinical suspicion and skin biopsy are important.
Click on the PDF icon at the top of this introduction to read the full article.
Total Hip Arthroplasty After Contralateral Hip Disarticulation: A Challenging “Simple Primary”
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
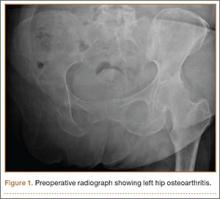
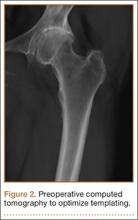
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
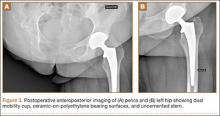
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Cholinergic Urticaria With Anaphylaxis: Hazardous Duty of a Deployed US Marine
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.
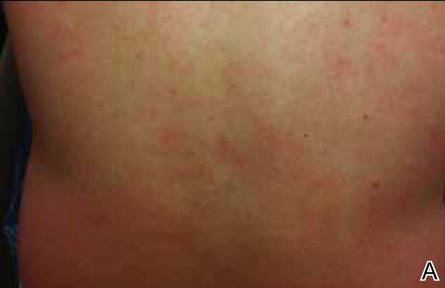 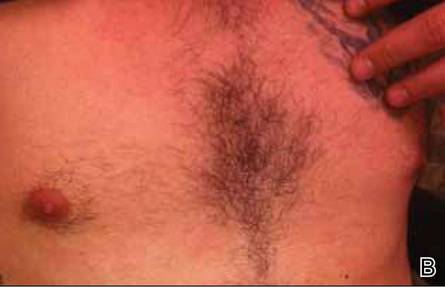 Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.
  Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
Cholinergic urticaria (CU) is a condition that primarily affects young adults. It can severely limit their activity levels and therefore job performance. Rarely, this condition can be associated with anaphylaxis, requiring a high index of suspicion by the clinician to ensure proper evaluation and treatment to prevent future respiratory compromise. We present the case of a 27-year-old US Marine with CU and anaphylaxis confirmed by a water challenge test in a warm bath.
Case Report
An otherwise healthy 27-year-old white man who was a US Marine presented with a concern of hives that appeared during strenuous exercise when he was deployed in Afghanistan approximately 1 year earlier. He initially began to experience urticarial lesions when taking warm showers with concomitant shortness of breath and wheezing. He reported no history of hives or asthma. Despite using diphenhydramine as needed to control symptoms for several months, he noted that the episodes of urticaria occurred with light-headedness, dizziness, or vomiting even with mild physical activity or common daily activities. Symptoms typically would resolve 30 to 90 minutes after he stopped exercising or cooled off. Over the course of approximately 1 year, the patient was prescribed a variety of sedating and nonsedating antihistamines (eg, diphenhydramine, hydroxyzine, doxepin, cetirizine, loratadine, fexofenadine, montelukast) by primary care while deployed, some of which mitigated his symptoms during warm showers and outdoor activities but not during exercise.
After returning from his deployment, the patient was initially referred to the dermatology department. No lesions were noted on physical examination. Based on his history, he was advised to avoid strenuous exercise and activity. During subsequent visits to dermatology an exercise challenge test was considered but not initiated due to lack of facilities to provide appropriate airway monitoring. The allergy department was consulted and the patient also was prescribed leukotriene receptor antagonists in addition to the antihistamines he was already taking. It was decided that a water challenge test in a warm bath would be performed in lieu of an exercise challenge to confirm a diagnosis of CU versus CU with anaphylaxis. If the patient did not have a reaction to the water challenge test, an exercise challenge would be offered.
After stopping treatment with antihistamines and leukotriene receptor antagonists for 1 week, a water challenge test was performed. A heparin lock was placed in the untested left arm for intravenous access. The right arm was immersed in a warm bath for 5 minutes without incident. After confirming no reaction, the arm was immersed for another 5 minutes, after which the patient reported flushing, warmth, and itching with visible 2- to 3-mm urticarial lesions on the back (Figure, A) and chest (Figure, B). The arm was subsequently removed from the water. No lesions were noted on either of the arms. The patient developed a cough after removing the arm from the water and his peak expiratory flow rate dropped from 520 to 440 L/min. After 5 minutes his peak expiratory flow rate recovered to 500 L/min and the coughing subsided. He also reported mild nausea and a headache. He was rapidly cooled with ice to abort any further reaction. An epinephrine autoinjector was on hand but was not used due to rapidly resolving symptoms. The diagnosis of CU with anaphylaxis was confirmed.
  Erythematous eruption with 2- to 3-mm urticarial lesions on the back (A) and chest (B). |
Comment
Urticaria is a heterogeneous group of disorders that includes both cholinergic and exercise-induced variants. Cholinergic urticaria affects as many as 11.2% of young adults aged 15 to 35 years, with a peak incidence of 20% between 26 and 28 years of age.1 Clinical presentation consists of wheals (central swelling with peripheral erythema) that are 1 to 5 mm in diameter2 with associated itching/burning that typically resolves within 1 to 24 hours. Cholinergic urticaria is the result of a rise in core body temperature independent of exercise status; it is distinguished from exercise-induced urticaria, which occurs in response to vigorous exercise and is not related to a rise in body temperature.3 In particular, these forms of urticaria can severely impact the lives and careers of young servicemen and servicewomen who are routinely deployed to warm environments.
A provocation test is recommended by Magerl et al4 in patients with a suspected diagnosis of CU. First, an exercise challenge test using a bicycle, treadmill, or similar equipment is recommended, with the patient exercising for 15 minutes beyond the start of sweating. Readings for urticaria are made immediately following the test and 10 minutes later. If the test is positive, a water challenge test in a hot bath (42°C) is then recommended for 15 minutes beyond an increase of 1°C in baseline core body temperature.4 One study demonstrated that 43% (13/30) of patients with CU experienced bronchial hyperresponsiveness on methacholine challenge testing.5 These findings suggest a possible utility in testing CU patients for potential disease-related respiratory compromise. A practical limitation of this study was that it did not examine a link between bronchial hyperresponsiveness and anaphylaxis during cholinergic urticarial flares. An exercise challenge test was not performed in our patient due to a history of wheezing and shortness of breath with exercise; instead we went directly to the water challenge test. We felt that limited immersion in the water (ie, only 1 arm) further minimized the risk for anaphylaxis compared with full-body immersion.
Any activity that raises core body temperature in a patient with CU can induce onset of lesions. One case report described a patient who experienced symptoms while undergoing hemodialysis, which resolved when the dialysate temperature was decreased from the normal 36.5°C to 35°C.6 However, most cases are triggered by daily activities or work. The mainstay of treatment of CU is nonsedating antihistamines. Cetirizine has demonstrated particular efficacy.7 For unresponsive cases, treatments include scopolamine butylbromide8,9; ketotifen10; combinations of cetirizine, montelukast, and propanolol11; and danazol.12
Conclusion
Cholinergic urticaria is mostly prevalent among young adults, with highest incidence in the late 20s. Active duty servicemen and servicewomen are among those who are at the greatest risk for developing CU, especially those deployed to tropical environments. Frequently, CU is associated with bronchial hyperresponsiveness and also can be associated with anaphylaxis, as was seen in our patient. Care must be taken before provocative tests are conducted in these patients and should be done in a controlled environment in which airway compromise can be properly assessed and treated if anaphylaxis were to occur.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
1. Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
2. Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines. an EAACI position paper. Allergy. 1997;52:504-513.
3. Zuberbier T, Asero R, Bindslev-Jensen C, et al; Dermatology Section of the European Academy of Allergology and Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-1426.
4. Magerl M, Borzova E, Giménez-Arnau A, et al; EAACI/GA2LEN/EDF/UNEV. The definition and diagnostic testing of physical and cholinergic urticarias—EAACI/GA2LEN/EDF/UNEV consensus panel recommendations [published online ahead of print September 30, 2009]. Allergy. 2009;64:1715-1721.
5. Petalas K, Kontou-Fili K, Gratziou C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann Allergy Asthma Immunol. 2009;102:416-421.
6. Morel V, Hauser C. Generalized cholinergic heat urticaria induced by hemodialysis. Kidney Int. 2006;70:230.
7. Zuberbier T, Aberer W, Burtin B, et al. Efficacy of cetirizine in cholinergic urticaria. Acta Derm Venereol. 1995;75:147-149.
8. Tsunemi Y, Ihn H, Saeki H, et al. Cholinergic urticaria successfully treated with scopolamine butylbromide. Int J Dermatol. 2003;42:850.
9. Ujiie H, Shimizu T, Natsuga K, et al. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin Exp Dermatol. 2006;31:588-589.
10. McClean SP, Arreaza EE, Lett-Brown MA, et al. Refractory cholinergic urticaria successfully treated with ketotifen. J Allergy Clin Immunol. 1989;83:738-741.
11. Feinberg JH, Toner CB. Successful treatment of disabling cholinergic urticaria. Mil Med. 2008;173:217-220.
12. La Shell MS, England RW. Severe refractory cholinergic urticaria treated with danazol. J Drugs Dermatol. 2006;5:664-667.
Practice Points
- Cholinergic urticaria can be a life-threatening condition and should be diagnosed in a controlled clinical setting where airway can be maintained.
- Cholinergic urticaria can be a profession-limiting condition, affecting people whose work involves exposure to heat or physical activity such as members of the military.
Diagnosis and Treatment of Leprosy Type 1 (Reversal) Reaction
Leprosy is a chronic granulomatous infection caused by the organism Mycobacterium leprae that primarily affects the skin and peripheral nerves.1 The organism is thought to be transmitted from person to person via the nasal secretions of infected individuals and is known to have a long incubation period, lasting 2 to 6 years.2 Leprosy has several distinct clinical presentations depending on the host immune response to the infection.3 Treatment typically involves antimicrobials (eg, clofazimine, dapsone, rifampin). Once treatment has begun, an important aspect of patient care is the recognition and treatment of leprosy reactions. Leprosy reactions are acute inflammatory complications that typically occur during the treatment course but also may occur in untreated disease. Type 1 (reversal) and type 2 (erythema nodosum leprosum) reactions are the 2 main types of leprosy reactions, which may affect 30% to 50% of all leprosy patients combined.4 Vasculonecrotic reactions (Lucio leprosy phenomenon) in leprosy are much less common.
We report a case of a 44-year-old man who repeatedly developed physical findings consistent with type 1 reactions after undergoing multiple treatments for leprosy. A discussion of leprosy, as well as its clinical manifestations, treatment options, and management of reversal reactions, also will be provided.
Case Report
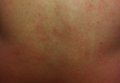
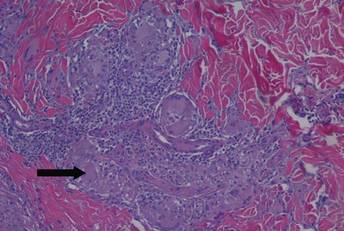
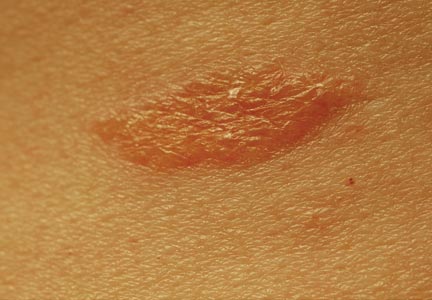
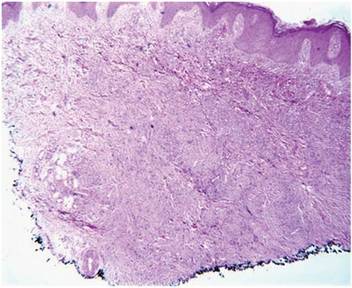
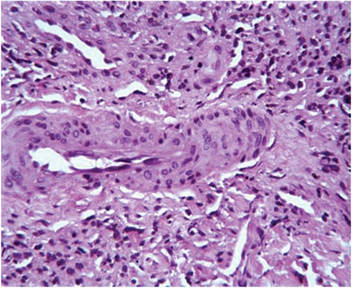
A healthy 44-year-old man presented with a several month history of elevated, erythematous to yellow, anesthetic papules and plaques on the trunk (Figure 1). No other systemic symptoms were noted. Biopsies of multiple skin lesions showed noncaseating granulomas with preferential extension in a perineural pattern and tracking along the arrector pili muscle (Figure 2). The cutaneous nerves appeared to be slightly enlarged. The patient reported a history of living in Louisiana and growing up with armadillos in the backyard, often filling the holes that they dug, but he denied having direct contact with or eating armadillos. In childhood, the patient traveled across the border to Mexico a few times but only for the day. He spent several months in the Middle East (ie, Diego Garcia, Saudi Arabia) more than 10 years prior to presentation, and he spent 2 weeks in Korea approximately 2 years prior to presentation but did not travel off the US air base. He had never traveled to South America or Africa. The clinical and histopathologic findings were consistent with Hansen disease (leprosy) and were determined to be tuberculoid in type given the limited clinical presentation, tuberculoid granulomas on biopsy, and no visible organisms on histopathologic analysis.
|
The patient initially was started on rifampin but was unable to tolerate treatment due to subsequent hepatotoxicity. He then was transitioned to a dual regimen of clofazimine and dapsone, which he tolerated well for the full 12-month treatment course. The cutaneous lesions quickly resolved after starting treatment, leaving a fine “cigarette paper–like” atrophy of the skin. After 12 months, it was subsequently presumed that the patient’s disease had been cured and treatment was stopped.
Nine months later, the patient noted new papules and plaques beginning to reappear in the truncal region. He was seen in clinic and a repeat biopsy was conducted, revealing perineural inflammation and noncaseating granulomas that were similar to the initial biopsies. Fite staining showed no acid-fast bacilli. Polymerase chain reaction was negative for M leprae. Nevertheless, a diagnosis of recurrent leprosy was made based on the patient’s clinical manifestations. He initially was started on dapsone, minocycline, and levofloxacin but was unable to tolerate the minocycline due to subsequent vertigo. After 1 month of treatment with dapsone and levofloxacin, the patient was clinically clear of all skin lesions and a repeat 12-month course of treatment was completed.
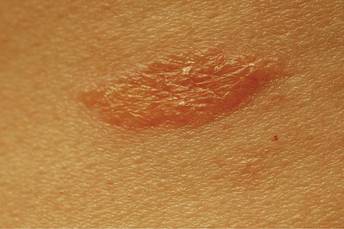
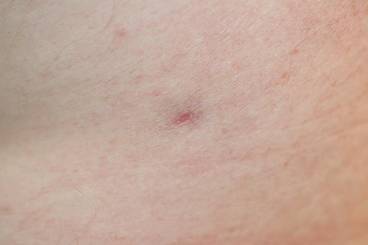
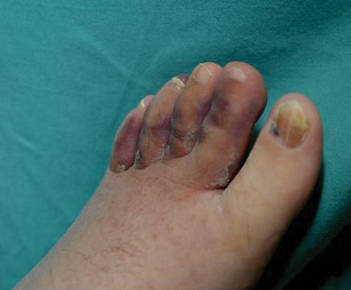
One year after completing the second 12-month treatment course, the patient again developed recurrent, indurated, erythematous papules and plaques on the trunk. Expert consultation from the National Hansen’s Disease (Leprosy) Program determined that the patient was experiencing a type 1 (reversal) reaction, not recurrent disease. Intralesional triamcinolone acetonide (10 mg/cc) was subsequently administered within the individual lesions. After a few treatments, the patient experienced notable regression of the lesions and has since been free of recurrent reactions (Figures 3 and 4).
|
Comment
Mycobacterium leprae
Mycobacterium leprae is an obligate intracellular bacillus that is confined to humans, armadillos of specific locales, and sphagnum moss. It is an acid-fast bacillus that is microscopically indistinguishable from other mycobacteria and is best detected on Fite staining of tissue sections.5
Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14
Treatment Options
Treatment of leprosy typically involves multidrug therapy. There are several effective chemotherapeutic agents against M leprae, including dapsone, clofazimine, ofloxacin, and minocycline.15 The World Health Organization recommendations for treatment are based on the classification of patient disease as either multibacillary or paucibacillary.16 Currently, patients are classified as multibacillary if they have 6 or more skin lesions and paucibacillary if they have fewer than 6 lesions.5 World Health Organization recommendations for paucibacillary leprosy include monthly doses of rifampin along with daily doses of dapsone for 6 months. Multibacillary patients usually are treated with a combination of rifampin, dapsone, and clofazimine for 12 months.1
Management of Reversal Reactions
Leprosy reactions can occur in all leprosy patients most commonly during multidrug therapy and represent a delayed hypersensitivity response to M leprae antigens.17 Type 1 and 2 reactions together affect 40% to 50% of all patients at least once during their disease course. Type 1 reactions occur in patients in the tuberculoid and borderline portion of the spectrum. These reactions manifest as erythema and induration of existing plaques. Frequently, progressive neuritis leads to sensory and motor neuropathy. These type 1 reactions typically develop gradually and may last for several weeks.4 Type 2 reactions occur in patients with borderline lepromatous leprosy and lepromatous leprosy and are characterized by the appearance of tender, erythematous, subcutaneous nodules. They are often accompanied by systemic symptoms such as malaise, fever, edema, arthralgia, and weight loss. Organ systems including the joints, eyes, testes, and nervous system also may be affected.18 The natural course of a type 2 reaction is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
All leprosy reactions are believed to be immunologically mediated; however, the mechanism responsible for each reaction type remains poorly defined. The histology of type 1 reactions is that of a delayed-type hypersensitivity response with CD4+ T cells, macrophages, and expression of IL-2 in lesions. In type 1 reactions, increases in cytokines including IL-1, IL-2, IL-12, IFN-γ, and tumor necrosis factor a have been documented both locally within the skin and systemically in the serum. However, studies have not been able to differentiate if this enhanced TH1 response is related to an immunological versus an inflammatory process.19
Type 2 leprosy reactions occur in patients with poor cellular immunity to M leprae. The acute lesions typically are characterized by a neutrophilic infiltrate superimposed on a chronic lepromatous pattern, and there is a systemic inflammatory response to immune complex deposition. It has been proposed that type 2 leprosy reactions are a type of Arthus reaction characterized by deposition of an immunoglobulin-antigen complex in vascular endothelium with subsequent complement activation. Both immunoglobulin and complement have been demonstrated in the reactive nodules of type 2 reactions, and serum complement is decreased in these patients, supporting this pathogenic process.4 Other studies have identified possible immune cell activation in type 2 reactions, including increases in TH2-related cytokines.19
These immunologic reactions can ultimately lead to impaired motor, sensory, and autonomic nerve function if allowed to progress.20 As a result, anesthetic limbs are subjected to repeated trauma, infection, and pressure necrosis that may lead to limb deformity. Autonomic nerve dysfunction may lead to loss of the corneal reflex, which can result in blindness. Common motor findings include wrist and foot drop as well as clawing of the hand from damage to the nerves of the upper extremity.20
Treatment of both type 1 and 2 leprosy reactions is imperative, as these inflammatory reactions are responsible for a great deal of the permanent nerve damage, deformity, and disability that is associated with leprosy.21 Oral and intralesional corticosteroids typically are highly effective for the clinical treatment of type 1 and 2 leprosy reactions given their anti-inflammatory properties. Our patient’s type 1 leprosy reaction responded well to intralesional corticosteroid injections. Thalidomide also has proven to be highly effective in treating type 2 reactions and was used frequently prior to realization of its teratogenic effects. It is now prohibited for use in women of childbearing age but is still routinely used in many countries for the treatment of type 2 reactions in men and postmenopausal women. Other therapies for type 2 reactions that have been used with some success include cyclosporine, azathioprine, and pentoxifylline.4
Conclusion
In summary, we present a unique case of multiple cutaneous reversal reactions in a patient with leprosy years after successful antimicrobial therapy. Proper recognition of this phenomenon is important to avoid overtreatment for mistaken recurrent disease. Although rare in the United States, leprosy should be considered in the differential diagnosis of patients presenting with hypoesthetic or anesthetic skin lesions, chronic annular dermatitis, papular or nodular granulomatous skin lesions, diffuse cutaneous infiltrative disease, peripheral neuropathy, and a history of travel to regions where the disease is known to be endemic. Additionally, if left untreated, M leprae infection and subsequent type 1 or type 2 reactions can lead to devastating neurologic and cutaneous sequelae. Prompt recognition and treatment of these reactions is imperative to prevent these long-term complications.
1. Kalisiak M, Yeung R, Dytoc M. Dermacase: leprosy. Can Fam Physician. 2009;55:55-56.
2. Kustner EC, Cruz MP, Dansis CP, et al. Lepromatous leprosy: a review and case report. Med Oral Patol Cir Bucal. 2006;11:474-479.
3. Nunez-Gussman J, Hwang L, Hsu S. Targetoid erythematous plaques: an unusual morphological presentation of multibacillary Hansen’s disease. Eur J Dermatol. 2001;11:65-67.
4. Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
5. Gelber RH. Leprosy (Hansen’s disease). In: Fauci AS, Kasper DL, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:1021-1027.
6. Simon HB. Infections due to mycobacterium. In: Dale DC, Federman DD, Antman K, et al, eds. ACP Medicine. New York, NY: WebMD Professional Publishing; 2004:1703-1720.
7. Baker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748-759.
8. Ishii N. Recent advances in the treatment of leprosy. Dermatol Online J. 2003;9:5. http://dermatology.cdlib.org/92/reviews/leprosy/ishii.html. Accessed March 16, 2015.
9. World Health Organization. Global leprosy situation, 2012. Weekly Epidemiological Rec. 2013;88:365-380. http://www.who.int/wer/2007/wer8835.pdf. Accessed March 16, 2015.
10. Gelber RH. Hansen disease. West J Med. 1993;158:583-590.
11. Deps PD, Alves L, Gripp CG, et al. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol. 2008;74:338-342.
12. Booth AV, Kovich OI. Lepromatous leprosy. Dermatol Online J. 2007;13:9.
13. Yens DA, Asters DJ, Teitel A. Subcutaneous nodules and joint deformity in leprosy. Clin Rheumatol. 2003;9:181-186.
14. Panezai S, Saleh FG. Leprosy and peripheral neuropathy. J Clin Neuromuscul Dis. 2004;5:138-145.
15. Sasaki S, Takeshita F, Okuda K, et al. Mycobacterium leprae and leprosy. Microbiol Immunol. 2001;45:729-736.
16. Kumar A, Girdhar A, Chakma J, et al. WHO Multidrug Therapy for Leprosy: Epidemiology of default in treatment in Agra District, Uttar Pradesh, India. BioMed Research International. doi:10.1155/2015/705804.
17. Fiallo P, Clapasson A, Favre A, et al. Overexpression of vascular endothelial growth factor and its endothelial cell receptor KDR in type I leprosy reaction. Am J Med Hyg. 2002;66:180-185.
18. Sales AM, de Matos HJ, Nery JAC, et al. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243-248.
19. Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type I (reversal) reactions. Am J Trop Med Hyg. 2002;66:409-415.
20. Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ. 2004;170:71-78.
21. Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health. 1999;4:493-498.
Leprosy is a chronic granulomatous infection caused by the organism Mycobacterium leprae that primarily affects the skin and peripheral nerves.1 The organism is thought to be transmitted from person to person via the nasal secretions of infected individuals and is known to have a long incubation period, lasting 2 to 6 years.2 Leprosy has several distinct clinical presentations depending on the host immune response to the infection.3 Treatment typically involves antimicrobials (eg, clofazimine, dapsone, rifampin). Once treatment has begun, an important aspect of patient care is the recognition and treatment of leprosy reactions. Leprosy reactions are acute inflammatory complications that typically occur during the treatment course but also may occur in untreated disease. Type 1 (reversal) and type 2 (erythema nodosum leprosum) reactions are the 2 main types of leprosy reactions, which may affect 30% to 50% of all leprosy patients combined.4 Vasculonecrotic reactions (Lucio leprosy phenomenon) in leprosy are much less common.
We report a case of a 44-year-old man who repeatedly developed physical findings consistent with type 1 reactions after undergoing multiple treatments for leprosy. A discussion of leprosy, as well as its clinical manifestations, treatment options, and management of reversal reactions, also will be provided.
Case Report
A healthy 44-year-old man presented with a several month history of elevated, erythematous to yellow, anesthetic papules and plaques on the trunk (Figure 1). No other systemic symptoms were noted. Biopsies of multiple skin lesions showed noncaseating granulomas with preferential extension in a perineural pattern and tracking along the arrector pili muscle (Figure 2). The cutaneous nerves appeared to be slightly enlarged. The patient reported a history of living in Louisiana and growing up with armadillos in the backyard, often filling the holes that they dug, but he denied having direct contact with or eating armadillos. In childhood, the patient traveled across the border to Mexico a few times but only for the day. He spent several months in the Middle East (ie, Diego Garcia, Saudi Arabia) more than 10 years prior to presentation, and he spent 2 weeks in Korea approximately 2 years prior to presentation but did not travel off the US air base. He had never traveled to South America or Africa. The clinical and histopathologic findings were consistent with Hansen disease (leprosy) and were determined to be tuberculoid in type given the limited clinical presentation, tuberculoid granulomas on biopsy, and no visible organisms on histopathologic analysis.
|
The patient initially was started on rifampin but was unable to tolerate treatment due to subsequent hepatotoxicity. He then was transitioned to a dual regimen of clofazimine and dapsone, which he tolerated well for the full 12-month treatment course. The cutaneous lesions quickly resolved after starting treatment, leaving a fine “cigarette paper–like” atrophy of the skin. After 12 months, it was subsequently presumed that the patient’s disease had been cured and treatment was stopped.
Nine months later, the patient noted new papules and plaques beginning to reappear in the truncal region. He was seen in clinic and a repeat biopsy was conducted, revealing perineural inflammation and noncaseating granulomas that were similar to the initial biopsies. Fite staining showed no acid-fast bacilli. Polymerase chain reaction was negative for M leprae. Nevertheless, a diagnosis of recurrent leprosy was made based on the patient’s clinical manifestations. He initially was started on dapsone, minocycline, and levofloxacin but was unable to tolerate the minocycline due to subsequent vertigo. After 1 month of treatment with dapsone and levofloxacin, the patient was clinically clear of all skin lesions and a repeat 12-month course of treatment was completed.
One year after completing the second 12-month treatment course, the patient again developed recurrent, indurated, erythematous papules and plaques on the trunk. Expert consultation from the National Hansen’s Disease (Leprosy) Program determined that the patient was experiencing a type 1 (reversal) reaction, not recurrent disease. Intralesional triamcinolone acetonide (10 mg/cc) was subsequently administered within the individual lesions. After a few treatments, the patient experienced notable regression of the lesions and has since been free of recurrent reactions (Figures 3 and 4).

|
Comment
Mycobacterium leprae
Mycobacterium leprae is an obligate intracellular bacillus that is confined to humans, armadillos of specific locales, and sphagnum moss. It is an acid-fast bacillus that is microscopically indistinguishable from other mycobacteria and is best detected on Fite staining of tissue sections.5
Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14
Treatment Options
Treatment of leprosy typically involves multidrug therapy. There are several effective chemotherapeutic agents against M leprae, including dapsone, clofazimine, ofloxacin, and minocycline.15 The World Health Organization recommendations for treatment are based on the classification of patient disease as either multibacillary or paucibacillary.16 Currently, patients are classified as multibacillary if they have 6 or more skin lesions and paucibacillary if they have fewer than 6 lesions.5 World Health Organization recommendations for paucibacillary leprosy include monthly doses of rifampin along with daily doses of dapsone for 6 months. Multibacillary patients usually are treated with a combination of rifampin, dapsone, and clofazimine for 12 months.1
Management of Reversal Reactions
Leprosy reactions can occur in all leprosy patients most commonly during multidrug therapy and represent a delayed hypersensitivity response to M leprae antigens.17 Type 1 and 2 reactions together affect 40% to 50% of all patients at least once during their disease course. Type 1 reactions occur in patients in the tuberculoid and borderline portion of the spectrum. These reactions manifest as erythema and induration of existing plaques. Frequently, progressive neuritis leads to sensory and motor neuropathy. These type 1 reactions typically develop gradually and may last for several weeks.4 Type 2 reactions occur in patients with borderline lepromatous leprosy and lepromatous leprosy and are characterized by the appearance of tender, erythematous, subcutaneous nodules. They are often accompanied by systemic symptoms such as malaise, fever, edema, arthralgia, and weight loss. Organ systems including the joints, eyes, testes, and nervous system also may be affected.18 The natural course of a type 2 reaction is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
All leprosy reactions are believed to be immunologically mediated; however, the mechanism responsible for each reaction type remains poorly defined. The histology of type 1 reactions is that of a delayed-type hypersensitivity response with CD4+ T cells, macrophages, and expression of IL-2 in lesions. In type 1 reactions, increases in cytokines including IL-1, IL-2, IL-12, IFN-γ, and tumor necrosis factor a have been documented both locally within the skin and systemically in the serum. However, studies have not been able to differentiate if this enhanced TH1 response is related to an immunological versus an inflammatory process.19
Type 2 leprosy reactions occur in patients with poor cellular immunity to M leprae. The acute lesions typically are characterized by a neutrophilic infiltrate superimposed on a chronic lepromatous pattern, and there is a systemic inflammatory response to immune complex deposition. It has been proposed that type 2 leprosy reactions are a type of Arthus reaction characterized by deposition of an immunoglobulin-antigen complex in vascular endothelium with subsequent complement activation. Both immunoglobulin and complement have been demonstrated in the reactive nodules of type 2 reactions, and serum complement is decreased in these patients, supporting this pathogenic process.4 Other studies have identified possible immune cell activation in type 2 reactions, including increases in TH2-related cytokines.19
These immunologic reactions can ultimately lead to impaired motor, sensory, and autonomic nerve function if allowed to progress.20 As a result, anesthetic limbs are subjected to repeated trauma, infection, and pressure necrosis that may lead to limb deformity. Autonomic nerve dysfunction may lead to loss of the corneal reflex, which can result in blindness. Common motor findings include wrist and foot drop as well as clawing of the hand from damage to the nerves of the upper extremity.20
Treatment of both type 1 and 2 leprosy reactions is imperative, as these inflammatory reactions are responsible for a great deal of the permanent nerve damage, deformity, and disability that is associated with leprosy.21 Oral and intralesional corticosteroids typically are highly effective for the clinical treatment of type 1 and 2 leprosy reactions given their anti-inflammatory properties. Our patient’s type 1 leprosy reaction responded well to intralesional corticosteroid injections. Thalidomide also has proven to be highly effective in treating type 2 reactions and was used frequently prior to realization of its teratogenic effects. It is now prohibited for use in women of childbearing age but is still routinely used in many countries for the treatment of type 2 reactions in men and postmenopausal women. Other therapies for type 2 reactions that have been used with some success include cyclosporine, azathioprine, and pentoxifylline.4
Conclusion
In summary, we present a unique case of multiple cutaneous reversal reactions in a patient with leprosy years after successful antimicrobial therapy. Proper recognition of this phenomenon is important to avoid overtreatment for mistaken recurrent disease. Although rare in the United States, leprosy should be considered in the differential diagnosis of patients presenting with hypoesthetic or anesthetic skin lesions, chronic annular dermatitis, papular or nodular granulomatous skin lesions, diffuse cutaneous infiltrative disease, peripheral neuropathy, and a history of travel to regions where the disease is known to be endemic. Additionally, if left untreated, M leprae infection and subsequent type 1 or type 2 reactions can lead to devastating neurologic and cutaneous sequelae. Prompt recognition and treatment of these reactions is imperative to prevent these long-term complications.
Leprosy is a chronic granulomatous infection caused by the organism Mycobacterium leprae that primarily affects the skin and peripheral nerves.1 The organism is thought to be transmitted from person to person via the nasal secretions of infected individuals and is known to have a long incubation period, lasting 2 to 6 years.2 Leprosy has several distinct clinical presentations depending on the host immune response to the infection.3 Treatment typically involves antimicrobials (eg, clofazimine, dapsone, rifampin). Once treatment has begun, an important aspect of patient care is the recognition and treatment of leprosy reactions. Leprosy reactions are acute inflammatory complications that typically occur during the treatment course but also may occur in untreated disease. Type 1 (reversal) and type 2 (erythema nodosum leprosum) reactions are the 2 main types of leprosy reactions, which may affect 30% to 50% of all leprosy patients combined.4 Vasculonecrotic reactions (Lucio leprosy phenomenon) in leprosy are much less common.
We report a case of a 44-year-old man who repeatedly developed physical findings consistent with type 1 reactions after undergoing multiple treatments for leprosy. A discussion of leprosy, as well as its clinical manifestations, treatment options, and management of reversal reactions, also will be provided.
Case Report
A healthy 44-year-old man presented with a several month history of elevated, erythematous to yellow, anesthetic papules and plaques on the trunk (Figure 1). No other systemic symptoms were noted. Biopsies of multiple skin lesions showed noncaseating granulomas with preferential extension in a perineural pattern and tracking along the arrector pili muscle (Figure 2). The cutaneous nerves appeared to be slightly enlarged. The patient reported a history of living in Louisiana and growing up with armadillos in the backyard, often filling the holes that they dug, but he denied having direct contact with or eating armadillos. In childhood, the patient traveled across the border to Mexico a few times but only for the day. He spent several months in the Middle East (ie, Diego Garcia, Saudi Arabia) more than 10 years prior to presentation, and he spent 2 weeks in Korea approximately 2 years prior to presentation but did not travel off the US air base. He had never traveled to South America or Africa. The clinical and histopathologic findings were consistent with Hansen disease (leprosy) and were determined to be tuberculoid in type given the limited clinical presentation, tuberculoid granulomas on biopsy, and no visible organisms on histopathologic analysis.
|
The patient initially was started on rifampin but was unable to tolerate treatment due to subsequent hepatotoxicity. He then was transitioned to a dual regimen of clofazimine and dapsone, which he tolerated well for the full 12-month treatment course. The cutaneous lesions quickly resolved after starting treatment, leaving a fine “cigarette paper–like” atrophy of the skin. After 12 months, it was subsequently presumed that the patient’s disease had been cured and treatment was stopped.
Nine months later, the patient noted new papules and plaques beginning to reappear in the truncal region. He was seen in clinic and a repeat biopsy was conducted, revealing perineural inflammation and noncaseating granulomas that were similar to the initial biopsies. Fite staining showed no acid-fast bacilli. Polymerase chain reaction was negative for M leprae. Nevertheless, a diagnosis of recurrent leprosy was made based on the patient’s clinical manifestations. He initially was started on dapsone, minocycline, and levofloxacin but was unable to tolerate the minocycline due to subsequent vertigo. After 1 month of treatment with dapsone and levofloxacin, the patient was clinically clear of all skin lesions and a repeat 12-month course of treatment was completed.
One year after completing the second 12-month treatment course, the patient again developed recurrent, indurated, erythematous papules and plaques on the trunk. Expert consultation from the National Hansen’s Disease (Leprosy) Program determined that the patient was experiencing a type 1 (reversal) reaction, not recurrent disease. Intralesional triamcinolone acetonide (10 mg/cc) was subsequently administered within the individual lesions. After a few treatments, the patient experienced notable regression of the lesions and has since been free of recurrent reactions (Figures 3 and 4).

|
Comment
Mycobacterium leprae
Mycobacterium leprae is an obligate intracellular bacillus that is confined to humans, armadillos of specific locales, and sphagnum moss. It is an acid-fast bacillus that is microscopically indistinguishable from other mycobacteria and is best detected on Fite staining of tissue sections.5
Mycobacterium leprae has 2 unique properties. It is thermolabile, growing best at 27°C to 30°C. Given its thermal sensitivity, M leprae has a preference for peripheral tissues including the skin, peripheral nerves, and the mucosa of the upper airways. It also may affect other tissues such as the bones and some viscera.2 The other unique quality of M leprae is its slow replication, with a generation time of 12 to 14 days. Because of the slow growth of M leprae, the incubation period in humans typically ranges from 2 to 6 years, with the minimal incubation period being 2 to 3 years and the maximum incubation period being as long as 40 years.6
Perhaps the greatest challenge to investigators is the fact that M leprae cannot be grown via normal laboratory culture methods. A possible explanation is reductive evolution, which may have led to a number of inactivated (pseudogenes) in the genome of this organism. In fact, close genetic examination of this organism has led to the conclusion that only half of the genome of M leprae is actually functional. This gene decay may explain the specific host tropism of M leprae as well as the inability to culture this organism in a laboratory setting.5,7
Incidence
Leprosy is primarily a disease of developing countries. More than 80% of the world’s cases of leprosy occur in India, China, Myanmar, Indonesia, Brazil, Nigeria, Madagascar, and Nepal. Although Africa has the highest prevalence, Asia is known to have had the most cases.5 In contrast, leprosy is largely absent from Europe, Canada, and the United States, except as imported cases or scattered cases along the southern border of the United States. In the United States, for example, fewer than 100 cases of leprosy are diagnosed each year, with almost all cases identified in immigrants from endemic areas.6
The global burden of leprosy, defined as the number of new cases detected annually, is stabilizing, which can be attributed in large part to the World Health Organization’s commitment in 1991 to eliminate leprosy as a public health concern by the year 2000 by implementing worldwide treatment regimes. Elimination was defined as a prevalence of less than 1 case per 10,000 persons.8 By 2012, only 3 of 122 countries had not achieved this standard, which is evidence of the program’s success.9
Disease Transmission
There is still some uncertainty involving the mode by which leprosy is transmitted. The most widely held view is that M leprae infection occurs primarily via nasal secretions.10 Transmission is thought to be respiratory, as large numbers of bacilli typically are found in the nasal secretions of untreated patients with multibacillary disease.6 Although nasal secretions often are regarded as the most common mode of M leprae transmission, other possible modes of transmission also may be important, including direct dermal inoculation and vector transmission, though neither has been proven.10 Finally, studies involving patients with confirmed exposure to armadillos have demonstrated a 2-fold increase in the incidence of leprosy versus the general population.11 Because this topic remains controversial, additional studies are needed to ascertain the mechanism of transmission of leprosy between humans and armadillos to confirm the evidence of this study.
Classification
Clinical manifestations of leprosy vary in accordance with the immune response of the host, with the more severe forms of the disease presenting in patients with the least immunity to M leprae.12 Traditionally, patient disease is classified using the Ridley-Jopling scale, which includes tuberculoid, borderline tuberculoid, borderline, borderline lepromatous, and lepromatous types of leprosy.
Tuberculoid leprosy, as noted in our patient, is characterized by a high degree of cellular immunity, a low antigen load, a small number or absence of acid-fast bacilli in skin lesions, and a predominance of helper T cells. Skin lesions in tuberculoid leprosy usually consist of 1 to 2 large hypopigmented or erythematous anesthetic lesions with raised margins and possible overlying scale.13 In tuberculoid leprosy, neural involvement often is asymmetrical and localized and may be the sole clinical finding.10
In stark contrast, lepromatous leprosy is characterized by low cellular immunity, a large antigen load, numerous acid-fast bacilli in tissues, and a predominance of suppressor T cells. Patients with lepromatous leprosy develop widespread disease that includes cutaneous findings of diffuse erythematous macules, nodules, and papules. Disease also can be demonstrated in the upper respiratory tract, anterior chambers of the eyes, testes, lymph nodes, periosteum, and superficial sensory and motor nerves of patients with lepromatous leprosy.12 Neural involvement typically is more symmetrical and diffuse than in patients with tuberculoid leprosy.10
The spectrum of disease between tuberculoid leprosy and lepromatous leprosy includes borderline tuberculoid leprosy, borderline leprosy, and borderline lepromatous leprosy.14 The clinical presentation of borderline leprosy also varies according to the patient’s immune response. Skin lesions vary in number and usually are associated with loss of sensation. Bacilli spreading throughout the bloodstream can lead to more diffuse systemic involvement. Clinical improvement of borderline leprosy to the tuberculoid type often is seen with treatment. Disease progression or deterioration to the lepromatous type can occur with immune system compromise.14
Treatment Options
Treatment of leprosy typically involves multidrug therapy. There are several effective chemotherapeutic agents against M leprae, including dapsone, clofazimine, ofloxacin, and minocycline.15 The World Health Organization recommendations for treatment are based on the classification of patient disease as either multibacillary or paucibacillary.16 Currently, patients are classified as multibacillary if they have 6 or more skin lesions and paucibacillary if they have fewer than 6 lesions.5 World Health Organization recommendations for paucibacillary leprosy include monthly doses of rifampin along with daily doses of dapsone for 6 months. Multibacillary patients usually are treated with a combination of rifampin, dapsone, and clofazimine for 12 months.1
Management of Reversal Reactions
Leprosy reactions can occur in all leprosy patients most commonly during multidrug therapy and represent a delayed hypersensitivity response to M leprae antigens.17 Type 1 and 2 reactions together affect 40% to 50% of all patients at least once during their disease course. Type 1 reactions occur in patients in the tuberculoid and borderline portion of the spectrum. These reactions manifest as erythema and induration of existing plaques. Frequently, progressive neuritis leads to sensory and motor neuropathy. These type 1 reactions typically develop gradually and may last for several weeks.4 Type 2 reactions occur in patients with borderline lepromatous leprosy and lepromatous leprosy and are characterized by the appearance of tender, erythematous, subcutaneous nodules. They are often accompanied by systemic symptoms such as malaise, fever, edema, arthralgia, and weight loss. Organ systems including the joints, eyes, testes, and nervous system also may be affected.18 The natural course of a type 2 reaction is 1 to 2 weeks, but many patients experience multiple recurrences over several months.
All leprosy reactions are believed to be immunologically mediated; however, the mechanism responsible for each reaction type remains poorly defined. The histology of type 1 reactions is that of a delayed-type hypersensitivity response with CD4+ T cells, macrophages, and expression of IL-2 in lesions. In type 1 reactions, increases in cytokines including IL-1, IL-2, IL-12, IFN-γ, and tumor necrosis factor a have been documented both locally within the skin and systemically in the serum. However, studies have not been able to differentiate if this enhanced TH1 response is related to an immunological versus an inflammatory process.19
Type 2 leprosy reactions occur in patients with poor cellular immunity to M leprae. The acute lesions typically are characterized by a neutrophilic infiltrate superimposed on a chronic lepromatous pattern, and there is a systemic inflammatory response to immune complex deposition. It has been proposed that type 2 leprosy reactions are a type of Arthus reaction characterized by deposition of an immunoglobulin-antigen complex in vascular endothelium with subsequent complement activation. Both immunoglobulin and complement have been demonstrated in the reactive nodules of type 2 reactions, and serum complement is decreased in these patients, supporting this pathogenic process.4 Other studies have identified possible immune cell activation in type 2 reactions, including increases in TH2-related cytokines.19
These immunologic reactions can ultimately lead to impaired motor, sensory, and autonomic nerve function if allowed to progress.20 As a result, anesthetic limbs are subjected to repeated trauma, infection, and pressure necrosis that may lead to limb deformity. Autonomic nerve dysfunction may lead to loss of the corneal reflex, which can result in blindness. Common motor findings include wrist and foot drop as well as clawing of the hand from damage to the nerves of the upper extremity.20
Treatment of both type 1 and 2 leprosy reactions is imperative, as these inflammatory reactions are responsible for a great deal of the permanent nerve damage, deformity, and disability that is associated with leprosy.21 Oral and intralesional corticosteroids typically are highly effective for the clinical treatment of type 1 and 2 leprosy reactions given their anti-inflammatory properties. Our patient’s type 1 leprosy reaction responded well to intralesional corticosteroid injections. Thalidomide also has proven to be highly effective in treating type 2 reactions and was used frequently prior to realization of its teratogenic effects. It is now prohibited for use in women of childbearing age but is still routinely used in many countries for the treatment of type 2 reactions in men and postmenopausal women. Other therapies for type 2 reactions that have been used with some success include cyclosporine, azathioprine, and pentoxifylline.4
Conclusion
In summary, we present a unique case of multiple cutaneous reversal reactions in a patient with leprosy years after successful antimicrobial therapy. Proper recognition of this phenomenon is important to avoid overtreatment for mistaken recurrent disease. Although rare in the United States, leprosy should be considered in the differential diagnosis of patients presenting with hypoesthetic or anesthetic skin lesions, chronic annular dermatitis, papular or nodular granulomatous skin lesions, diffuse cutaneous infiltrative disease, peripheral neuropathy, and a history of travel to regions where the disease is known to be endemic. Additionally, if left untreated, M leprae infection and subsequent type 1 or type 2 reactions can lead to devastating neurologic and cutaneous sequelae. Prompt recognition and treatment of these reactions is imperative to prevent these long-term complications.
1. Kalisiak M, Yeung R, Dytoc M. Dermacase: leprosy. Can Fam Physician. 2009;55:55-56.
2. Kustner EC, Cruz MP, Dansis CP, et al. Lepromatous leprosy: a review and case report. Med Oral Patol Cir Bucal. 2006;11:474-479.
3. Nunez-Gussman J, Hwang L, Hsu S. Targetoid erythematous plaques: an unusual morphological presentation of multibacillary Hansen’s disease. Eur J Dermatol. 2001;11:65-67.
4. Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
5. Gelber RH. Leprosy (Hansen’s disease). In: Fauci AS, Kasper DL, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:1021-1027.
6. Simon HB. Infections due to mycobacterium. In: Dale DC, Federman DD, Antman K, et al, eds. ACP Medicine. New York, NY: WebMD Professional Publishing; 2004:1703-1720.
7. Baker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748-759.
8. Ishii N. Recent advances in the treatment of leprosy. Dermatol Online J. 2003;9:5. http://dermatology.cdlib.org/92/reviews/leprosy/ishii.html. Accessed March 16, 2015.
9. World Health Organization. Global leprosy situation, 2012. Weekly Epidemiological Rec. 2013;88:365-380. http://www.who.int/wer/2007/wer8835.pdf. Accessed March 16, 2015.
10. Gelber RH. Hansen disease. West J Med. 1993;158:583-590.
11. Deps PD, Alves L, Gripp CG, et al. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol. 2008;74:338-342.
12. Booth AV, Kovich OI. Lepromatous leprosy. Dermatol Online J. 2007;13:9.
13. Yens DA, Asters DJ, Teitel A. Subcutaneous nodules and joint deformity in leprosy. Clin Rheumatol. 2003;9:181-186.
14. Panezai S, Saleh FG. Leprosy and peripheral neuropathy. J Clin Neuromuscul Dis. 2004;5:138-145.
15. Sasaki S, Takeshita F, Okuda K, et al. Mycobacterium leprae and leprosy. Microbiol Immunol. 2001;45:729-736.
16. Kumar A, Girdhar A, Chakma J, et al. WHO Multidrug Therapy for Leprosy: Epidemiology of default in treatment in Agra District, Uttar Pradesh, India. BioMed Research International. doi:10.1155/2015/705804.
17. Fiallo P, Clapasson A, Favre A, et al. Overexpression of vascular endothelial growth factor and its endothelial cell receptor KDR in type I leprosy reaction. Am J Med Hyg. 2002;66:180-185.
18. Sales AM, de Matos HJ, Nery JAC, et al. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243-248.
19. Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type I (reversal) reactions. Am J Trop Med Hyg. 2002;66:409-415.
20. Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ. 2004;170:71-78.
21. Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health. 1999;4:493-498.
1. Kalisiak M, Yeung R, Dytoc M. Dermacase: leprosy. Can Fam Physician. 2009;55:55-56.
2. Kustner EC, Cruz MP, Dansis CP, et al. Lepromatous leprosy: a review and case report. Med Oral Patol Cir Bucal. 2006;11:474-479.
3. Nunez-Gussman J, Hwang L, Hsu S. Targetoid erythematous plaques: an unusual morphological presentation of multibacillary Hansen’s disease. Eur J Dermatol. 2001;11:65-67.
4. Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
5. Gelber RH. Leprosy (Hansen’s disease). In: Fauci AS, Kasper DL, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill; 2008:1021-1027.
6. Simon HB. Infections due to mycobacterium. In: Dale DC, Federman DD, Antman K, et al, eds. ACP Medicine. New York, NY: WebMD Professional Publishing; 2004:1703-1720.
7. Baker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748-759.
8. Ishii N. Recent advances in the treatment of leprosy. Dermatol Online J. 2003;9:5. http://dermatology.cdlib.org/92/reviews/leprosy/ishii.html. Accessed March 16, 2015.
9. World Health Organization. Global leprosy situation, 2012. Weekly Epidemiological Rec. 2013;88:365-380. http://www.who.int/wer/2007/wer8835.pdf. Accessed March 16, 2015.
10. Gelber RH. Hansen disease. West J Med. 1993;158:583-590.
11. Deps PD, Alves L, Gripp CG, et al. Contact with armadillos increases the risk of leprosy in Brazil: a case control study. Indian J Dermatol Venereol Leprol. 2008;74:338-342.
12. Booth AV, Kovich OI. Lepromatous leprosy. Dermatol Online J. 2007;13:9.
13. Yens DA, Asters DJ, Teitel A. Subcutaneous nodules and joint deformity in leprosy. Clin Rheumatol. 2003;9:181-186.
14. Panezai S, Saleh FG. Leprosy and peripheral neuropathy. J Clin Neuromuscul Dis. 2004;5:138-145.
15. Sasaki S, Takeshita F, Okuda K, et al. Mycobacterium leprae and leprosy. Microbiol Immunol. 2001;45:729-736.
16. Kumar A, Girdhar A, Chakma J, et al. WHO Multidrug Therapy for Leprosy: Epidemiology of default in treatment in Agra District, Uttar Pradesh, India. BioMed Research International. doi:10.1155/2015/705804.
17. Fiallo P, Clapasson A, Favre A, et al. Overexpression of vascular endothelial growth factor and its endothelial cell receptor KDR in type I leprosy reaction. Am J Med Hyg. 2002;66:180-185.
18. Sales AM, de Matos HJ, Nery JAC, et al. Double-blind trial of the efficacy of pentoxifylline vs thalidomide for the treatment of type II reaction in leprosy. Braz J Med Biol Res. 2007;40:243-248.
19. Lockwood DN, Colston MJ, Khanolkar-Young SR. The detection of Mycobacterium leprae protein and carbohydrate antigens in skin and nerve from leprosy patients with type I (reversal) reactions. Am J Trop Med Hyg. 2002;66:409-415.
20. Boggild AK, Keystone JS, Kain KC. Leprosy: a primer for Canadian physicians. CMAJ. 2004;170:71-78.
21. Rook GA, Baker R. Cortisol metabolism, cortisol sensitivity and the pathogenesis of leprosy reactions. Trop Med Int Health. 1999;4:493-498.
Practice Points
- Reversal reactions are not uncommonly witnessed in patients undergoing treatment of leprosy.
- Leprosy has several distinct clinical presentations ranging from tuberculoid leprosy to lepromatous leprosy with the extent of disease generally depending on the host’s immune response to the infection.
Mucocutaneous Presentation of Kaposi Sarcoma in an Asymptomatic Human Immunodeficiency Virus–Positive Man
Case Report
A 45-year-old man presented with persistent swelling and “black-and-blue” lesions on the legs, feet, and toes of 6 months’ duration. The painless lesions first appeared on the left lower leg and then began to appear on the right leg in recent months. Three weeks prior to presentation, he developed swelling of the left lower leg during hospitalization for a lumbar laminectomy. A venous ultrasound was negative for a deep vein thrombosis. He denied trauma or history of bleeding diathesis. He did not report symptoms of dyspnea, angina, or claudication, and a review of systems was unremarkable.
The patient’s medical history included spinal stenosis, chronic back pain, osteoarthritis, and anxiety. His medications included oxycodone, zolpidem, and alprazolam. In addition to a recent lumbar laminectomy, he had undergone extensive dental work in the last 6 months. The patient denied the use of cigarettes, alcohol, or intravenous drugs.
Physical examination revealed scattered, purple, segmented patches on the dorsal and plantar aspects of the feet, both calves, both heels, and toes (Figure 1). Mild nonpitting edema was present below the left knee along with edema on the dorsum of the left foot. The distribution of the lesions was initially suggestive of cholesterol embolization syndrome; however, both the femoral and posterior tibial pulses were symmetric and palpable (+2). Well-demarcated violaceous plaques with central clearing and a rustlike discoloration were noted on the hard and soft palates. Cervical lymphadenopathy was not present.
|
Laboratory tests including a repeat venous ultrasound of the left lower leg revealed no evidence of deep vein thrombosis. Ankle brachial index revealed no abnormalities and blood flow to the lower legs was adequate. Computed tomography scans of the chest, abdomen, and pelvis were unremarkable except for mild splenomegaly and moderate cardiomegaly. Lastly, human immunodeficiency virus (HIV) 1 and HIV-2 enzyme immunoassay was reactive.
Histopathologic examination of a punch biopsy from the right fourth toe was representative of the plaque stage of Kaposi sarcoma (KS) with a diffuse collection of extravasated erythrocytes and neoplastic vascular proliferation among a background of numerous plasma cells and hemosiderophages (Figure 2). Higher magnification illustrated the promontory sign, whereby native vessels encroach on neoplastic slitlike vascular spaces (Figure 3). A final diagnosis of AIDS-related KS was made. The patient was referred to an infectious disease specialist for evaluation of his CD4 levels and HIV management.
Comment
Kaposi sarcoma is a neoplastic proliferation of the blood vessels in the skin characterized by the formation of violaceous macules and papules that often appear on a single distal extremity, such as the foot. Over time the lesions can develop on the opposite extremity and coalesce into poorly demarcated plaques and nodules with accompanying stasis and lymphedema of the involved extremities.1 Evolution of the lesions depends on the KS subtype. The most common clinical variant of KS is the classic form, which primarily is seen in those of Mediterranean, Eastern European, or Ashkenazi Jewish descent, with a predilection for men and older adults.1,2 The endemic form of KS, or African KS, is more aggressive with rapid visceral involvement and rare skin lesions; it is common among prepubertal children in sub-Saharan Africa with no predilection for either sex.2 In the setting of severe immune suppression, organ transplantation, or chemotherapy, a third KS subtype known as iatrogenic KS can occur. The clinical course of iatrogenic KS may range from scattered cutaneous lesions to diffuse involvement secondary to increased dosages and long-term use of immunosuppressive agents.2
Our patient had AIDS-related or epidemic KS. AIDS-related KS is largely predominant among homosexual men, but due to the awareness of safe sexual practices and the introduction of highly active antiretroviral therapy (HAART), KS incidence in the United States has declined.1,2 However, despite recent advances in HIV therapy, AIDS-related KS is still the most common neoplasm seen in AIDS patients and is the presenting manifestation of AIDS in up to 30% of cases.3 Up to 22% of cases first appear on the gingiva, hard palate, and tongue, with concomitant dysphagia and airway obstruction in severe cases.4,5 More advanced cases of AIDS-related KS can present with initial symptoms such as abdominal pain, melena, dyspnea, lymphadenopathy, and weight loss, which suggests involvement of the gastrointestinal tract, lungs, and other organ systems.
Regardless of the subtype, the etiology of KS currently is thought to be secondary to infection with human herpesvirus 8 (HHV-8), also known as Kaposi sarcoma–associated herpesvirus (KSHV).1 Human immunodeficiency virus infection can enhance KSHV expression through the HIV transactivator protein, which activates KSHV oncogenes and angiogenic growth factors to promote the development of KS lesions.2,6Likewise, KSHV enhances HIV upregulation through latency-associated nuclear antigen, a protein that interacts with HIV Tat protein to further activate long terminal repeats of HIV-1.2
The differential diagnosis of KS is broad. The slightly elevated, pinkish reddish discolorations of KS may resemble verruca plana and/or squamous cell carcinoma on visual observation, whereas nodular KS may resemble giant cell granuloma, pyogenic granuloma, or hemangiopericytoma.4,7 Cases of KS with lymph node involvement may include a differential diagnosis of lymphoma, angiosarcoma, and bacillary angiomatosis.7 Other vascular pathologies that may be considered in the differential diagnosis include vascular tumors (eg, spindle cell hemangioma), fibrohistiocytic tumors (eg, dermatofibrosarcoma protuberans), and a collection of spindle cell mesenchymal tumors.8
Kaposi sarcoma progresses through several histologic stages beginning with the patch stage, then progressing to the plaque stage, and finally culminating in the nodular stage. The patch stage is the first stage in KS progression and a crowded dermis can be seen with the formation of slitlike vascular spaces lined by endothelial cells with red blood cell extravasation into the lumens of newly formed vascular channels, hence demonstrating the promontory sign.8 In the plaque stage, the promontory sign still is present and there is a greater presence of slitlike spaces, giving the micrograph an overall sievelike appearance. Erythrocytes can be found residing within the clear cytoplasm of spindled endothelial cells, leading to the development of autolumination. Finally, the nodular stage is characterized by a neoplastic proliferation of monomorphic spindle cells that form fasciclelike nests in the dermis.8 To distinguish KS from other angioproliferative tumors, one can stain for HHV-8 latent nuclear antigen-1, which is found in the nuclei of infected endothelial cells.1,8,9
Kaposi sarcoma is treated through a variety of mechanisms depending on the subtype. Classic KS lesions often can be observed as they a follow a benign and nonaggressive course.1 Highly active antiretroviral therapy is the mainstay of care in AIDS-related KS and has led to regression of lesions and a remarkable decline in the incidence of KS.3 The HAART regimen consists of a protease inhibitor or nonnucleoside reverse-transcriptase inhibitor with the addition of 2 nucleoside reverse-transcriptase inhibitors. More advanced or refractory cases of KS often require dual treatment with HAART and a chemotherapy agent such as pegylated liposomal doxorubicin. Combination therapy has been shown to result in stronger therapeutic responses and lower relapse rates in contrast to HAART alone.7 Patients also may consider other treatment modalities to manage KS lesions such as surgical removal of lesions, laser therapy, paclitaxel, interferon alfa, oral etoposide, thalidomide, and topical therapies such as imiquimod cream 5% and alitretinoin.1,7
Conclusion
Kaposi sarcoma is a rare but concerning dermatologic condition that signals the need for further diagnostic evaluation. Coexpression of viruses such as HIV and HHV-8 can result in a more virulent and rapid progression of KS to encompass both mucous membrane and systemic involvement. Our patient’s lesions were the first presenting sign of HIV infection despite being asymptomatic at the time of diagnosis, which is alarming in the sense that more than 21% of HIV-infected individuals in the United States have not been clinically diagnosed.10 Inquiry of HIV risk factors and routine screening for HIV should be performed in refractory cases of skin disease as an underlying clue to further investigate the immune system. We present our unique case of mucocutaneous development of KS in an asymptomatic HIV-positive man to stress the importance of KS recognition and management.
1. Jan MM, Laskas JW, Griffin TD. Eruptive Kaposi sarcoma: an unusual presentation in an HIV-negative patient. Cutis. 2011;87:34-38.
2. Geraminejad P, Memar O, Aronson I, et al. Kaposi’s sarcoma and other manifestations of human herpesvirus 8. J Am Acad Dermatol. 2002;47:641-655.
3. Kharkar V, Gutte RM, Khopkar U, et al. Kaposi’s sarcoma: a presenting manifestation of HIV infection in an Indian. Indian J Dermatol Venereol Leprol. 2009;75:391-393.
4. Naidu A, Havard DB, Ray JM, et al. Oral and maxillofacial pathology case of the month. Kaposi’s sarcoma. Tex Dent J. 2011;128:376-377, 382-383.
5. Lawson G, Matar N, Kesch S, et al. Laryngeal Kaposi sarcoma: case report and literature review. B-ENT. 2010;6:285-288.
6. Sullivan RJ, Pantanowitz L, Casper C, et al. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma–associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis. 2008;47:1209-1215.
7. Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma [published online ahead of print March 4, 2011]. Cancer Lett. 2011;305:150-162.
8. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
9. Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342.
10. Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007 [published correction appears in JAMA. 2011;306:1548]. JAMA. 2011;305:1450-1459.
Case Report
A 45-year-old man presented with persistent swelling and “black-and-blue” lesions on the legs, feet, and toes of 6 months’ duration. The painless lesions first appeared on the left lower leg and then began to appear on the right leg in recent months. Three weeks prior to presentation, he developed swelling of the left lower leg during hospitalization for a lumbar laminectomy. A venous ultrasound was negative for a deep vein thrombosis. He denied trauma or history of bleeding diathesis. He did not report symptoms of dyspnea, angina, or claudication, and a review of systems was unremarkable.
The patient’s medical history included spinal stenosis, chronic back pain, osteoarthritis, and anxiety. His medications included oxycodone, zolpidem, and alprazolam. In addition to a recent lumbar laminectomy, he had undergone extensive dental work in the last 6 months. The patient denied the use of cigarettes, alcohol, or intravenous drugs.
Physical examination revealed scattered, purple, segmented patches on the dorsal and plantar aspects of the feet, both calves, both heels, and toes (Figure 1). Mild nonpitting edema was present below the left knee along with edema on the dorsum of the left foot. The distribution of the lesions was initially suggestive of cholesterol embolization syndrome; however, both the femoral and posterior tibial pulses were symmetric and palpable (+2). Well-demarcated violaceous plaques with central clearing and a rustlike discoloration were noted on the hard and soft palates. Cervical lymphadenopathy was not present.
|
Laboratory tests including a repeat venous ultrasound of the left lower leg revealed no evidence of deep vein thrombosis. Ankle brachial index revealed no abnormalities and blood flow to the lower legs was adequate. Computed tomography scans of the chest, abdomen, and pelvis were unremarkable except for mild splenomegaly and moderate cardiomegaly. Lastly, human immunodeficiency virus (HIV) 1 and HIV-2 enzyme immunoassay was reactive.
Histopathologic examination of a punch biopsy from the right fourth toe was representative of the plaque stage of Kaposi sarcoma (KS) with a diffuse collection of extravasated erythrocytes and neoplastic vascular proliferation among a background of numerous plasma cells and hemosiderophages (Figure 2). Higher magnification illustrated the promontory sign, whereby native vessels encroach on neoplastic slitlike vascular spaces (Figure 3). A final diagnosis of AIDS-related KS was made. The patient was referred to an infectious disease specialist for evaluation of his CD4 levels and HIV management.
Comment
Kaposi sarcoma is a neoplastic proliferation of the blood vessels in the skin characterized by the formation of violaceous macules and papules that often appear on a single distal extremity, such as the foot. Over time the lesions can develop on the opposite extremity and coalesce into poorly demarcated plaques and nodules with accompanying stasis and lymphedema of the involved extremities.1 Evolution of the lesions depends on the KS subtype. The most common clinical variant of KS is the classic form, which primarily is seen in those of Mediterranean, Eastern European, or Ashkenazi Jewish descent, with a predilection for men and older adults.1,2 The endemic form of KS, or African KS, is more aggressive with rapid visceral involvement and rare skin lesions; it is common among prepubertal children in sub-Saharan Africa with no predilection for either sex.2 In the setting of severe immune suppression, organ transplantation, or chemotherapy, a third KS subtype known as iatrogenic KS can occur. The clinical course of iatrogenic KS may range from scattered cutaneous lesions to diffuse involvement secondary to increased dosages and long-term use of immunosuppressive agents.2
Our patient had AIDS-related or epidemic KS. AIDS-related KS is largely predominant among homosexual men, but due to the awareness of safe sexual practices and the introduction of highly active antiretroviral therapy (HAART), KS incidence in the United States has declined.1,2 However, despite recent advances in HIV therapy, AIDS-related KS is still the most common neoplasm seen in AIDS patients and is the presenting manifestation of AIDS in up to 30% of cases.3 Up to 22% of cases first appear on the gingiva, hard palate, and tongue, with concomitant dysphagia and airway obstruction in severe cases.4,5 More advanced cases of AIDS-related KS can present with initial symptoms such as abdominal pain, melena, dyspnea, lymphadenopathy, and weight loss, which suggests involvement of the gastrointestinal tract, lungs, and other organ systems.
Regardless of the subtype, the etiology of KS currently is thought to be secondary to infection with human herpesvirus 8 (HHV-8), also known as Kaposi sarcoma–associated herpesvirus (KSHV).1 Human immunodeficiency virus infection can enhance KSHV expression through the HIV transactivator protein, which activates KSHV oncogenes and angiogenic growth factors to promote the development of KS lesions.2,6Likewise, KSHV enhances HIV upregulation through latency-associated nuclear antigen, a protein that interacts with HIV Tat protein to further activate long terminal repeats of HIV-1.2
The differential diagnosis of KS is broad. The slightly elevated, pinkish reddish discolorations of KS may resemble verruca plana and/or squamous cell carcinoma on visual observation, whereas nodular KS may resemble giant cell granuloma, pyogenic granuloma, or hemangiopericytoma.4,7 Cases of KS with lymph node involvement may include a differential diagnosis of lymphoma, angiosarcoma, and bacillary angiomatosis.7 Other vascular pathologies that may be considered in the differential diagnosis include vascular tumors (eg, spindle cell hemangioma), fibrohistiocytic tumors (eg, dermatofibrosarcoma protuberans), and a collection of spindle cell mesenchymal tumors.8
Kaposi sarcoma progresses through several histologic stages beginning with the patch stage, then progressing to the plaque stage, and finally culminating in the nodular stage. The patch stage is the first stage in KS progression and a crowded dermis can be seen with the formation of slitlike vascular spaces lined by endothelial cells with red blood cell extravasation into the lumens of newly formed vascular channels, hence demonstrating the promontory sign.8 In the plaque stage, the promontory sign still is present and there is a greater presence of slitlike spaces, giving the micrograph an overall sievelike appearance. Erythrocytes can be found residing within the clear cytoplasm of spindled endothelial cells, leading to the development of autolumination. Finally, the nodular stage is characterized by a neoplastic proliferation of monomorphic spindle cells that form fasciclelike nests in the dermis.8 To distinguish KS from other angioproliferative tumors, one can stain for HHV-8 latent nuclear antigen-1, which is found in the nuclei of infected endothelial cells.1,8,9
Kaposi sarcoma is treated through a variety of mechanisms depending on the subtype. Classic KS lesions often can be observed as they a follow a benign and nonaggressive course.1 Highly active antiretroviral therapy is the mainstay of care in AIDS-related KS and has led to regression of lesions and a remarkable decline in the incidence of KS.3 The HAART regimen consists of a protease inhibitor or nonnucleoside reverse-transcriptase inhibitor with the addition of 2 nucleoside reverse-transcriptase inhibitors. More advanced or refractory cases of KS often require dual treatment with HAART and a chemotherapy agent such as pegylated liposomal doxorubicin. Combination therapy has been shown to result in stronger therapeutic responses and lower relapse rates in contrast to HAART alone.7 Patients also may consider other treatment modalities to manage KS lesions such as surgical removal of lesions, laser therapy, paclitaxel, interferon alfa, oral etoposide, thalidomide, and topical therapies such as imiquimod cream 5% and alitretinoin.1,7
Conclusion
Kaposi sarcoma is a rare but concerning dermatologic condition that signals the need for further diagnostic evaluation. Coexpression of viruses such as HIV and HHV-8 can result in a more virulent and rapid progression of KS to encompass both mucous membrane and systemic involvement. Our patient’s lesions were the first presenting sign of HIV infection despite being asymptomatic at the time of diagnosis, which is alarming in the sense that more than 21% of HIV-infected individuals in the United States have not been clinically diagnosed.10 Inquiry of HIV risk factors and routine screening for HIV should be performed in refractory cases of skin disease as an underlying clue to further investigate the immune system. We present our unique case of mucocutaneous development of KS in an asymptomatic HIV-positive man to stress the importance of KS recognition and management.
Case Report
A 45-year-old man presented with persistent swelling and “black-and-blue” lesions on the legs, feet, and toes of 6 months’ duration. The painless lesions first appeared on the left lower leg and then began to appear on the right leg in recent months. Three weeks prior to presentation, he developed swelling of the left lower leg during hospitalization for a lumbar laminectomy. A venous ultrasound was negative for a deep vein thrombosis. He denied trauma or history of bleeding diathesis. He did not report symptoms of dyspnea, angina, or claudication, and a review of systems was unremarkable.
The patient’s medical history included spinal stenosis, chronic back pain, osteoarthritis, and anxiety. His medications included oxycodone, zolpidem, and alprazolam. In addition to a recent lumbar laminectomy, he had undergone extensive dental work in the last 6 months. The patient denied the use of cigarettes, alcohol, or intravenous drugs.
Physical examination revealed scattered, purple, segmented patches on the dorsal and plantar aspects of the feet, both calves, both heels, and toes (Figure 1). Mild nonpitting edema was present below the left knee along with edema on the dorsum of the left foot. The distribution of the lesions was initially suggestive of cholesterol embolization syndrome; however, both the femoral and posterior tibial pulses were symmetric and palpable (+2). Well-demarcated violaceous plaques with central clearing and a rustlike discoloration were noted on the hard and soft palates. Cervical lymphadenopathy was not present.
|
Laboratory tests including a repeat venous ultrasound of the left lower leg revealed no evidence of deep vein thrombosis. Ankle brachial index revealed no abnormalities and blood flow to the lower legs was adequate. Computed tomography scans of the chest, abdomen, and pelvis were unremarkable except for mild splenomegaly and moderate cardiomegaly. Lastly, human immunodeficiency virus (HIV) 1 and HIV-2 enzyme immunoassay was reactive.
Histopathologic examination of a punch biopsy from the right fourth toe was representative of the plaque stage of Kaposi sarcoma (KS) with a diffuse collection of extravasated erythrocytes and neoplastic vascular proliferation among a background of numerous plasma cells and hemosiderophages (Figure 2). Higher magnification illustrated the promontory sign, whereby native vessels encroach on neoplastic slitlike vascular spaces (Figure 3). A final diagnosis of AIDS-related KS was made. The patient was referred to an infectious disease specialist for evaluation of his CD4 levels and HIV management.
Comment
Kaposi sarcoma is a neoplastic proliferation of the blood vessels in the skin characterized by the formation of violaceous macules and papules that often appear on a single distal extremity, such as the foot. Over time the lesions can develop on the opposite extremity and coalesce into poorly demarcated plaques and nodules with accompanying stasis and lymphedema of the involved extremities.1 Evolution of the lesions depends on the KS subtype. The most common clinical variant of KS is the classic form, which primarily is seen in those of Mediterranean, Eastern European, or Ashkenazi Jewish descent, with a predilection for men and older adults.1,2 The endemic form of KS, or African KS, is more aggressive with rapid visceral involvement and rare skin lesions; it is common among prepubertal children in sub-Saharan Africa with no predilection for either sex.2 In the setting of severe immune suppression, organ transplantation, or chemotherapy, a third KS subtype known as iatrogenic KS can occur. The clinical course of iatrogenic KS may range from scattered cutaneous lesions to diffuse involvement secondary to increased dosages and long-term use of immunosuppressive agents.2
Our patient had AIDS-related or epidemic KS. AIDS-related KS is largely predominant among homosexual men, but due to the awareness of safe sexual practices and the introduction of highly active antiretroviral therapy (HAART), KS incidence in the United States has declined.1,2 However, despite recent advances in HIV therapy, AIDS-related KS is still the most common neoplasm seen in AIDS patients and is the presenting manifestation of AIDS in up to 30% of cases.3 Up to 22% of cases first appear on the gingiva, hard palate, and tongue, with concomitant dysphagia and airway obstruction in severe cases.4,5 More advanced cases of AIDS-related KS can present with initial symptoms such as abdominal pain, melena, dyspnea, lymphadenopathy, and weight loss, which suggests involvement of the gastrointestinal tract, lungs, and other organ systems.
Regardless of the subtype, the etiology of KS currently is thought to be secondary to infection with human herpesvirus 8 (HHV-8), also known as Kaposi sarcoma–associated herpesvirus (KSHV).1 Human immunodeficiency virus infection can enhance KSHV expression through the HIV transactivator protein, which activates KSHV oncogenes and angiogenic growth factors to promote the development of KS lesions.2,6Likewise, KSHV enhances HIV upregulation through latency-associated nuclear antigen, a protein that interacts with HIV Tat protein to further activate long terminal repeats of HIV-1.2
The differential diagnosis of KS is broad. The slightly elevated, pinkish reddish discolorations of KS may resemble verruca plana and/or squamous cell carcinoma on visual observation, whereas nodular KS may resemble giant cell granuloma, pyogenic granuloma, or hemangiopericytoma.4,7 Cases of KS with lymph node involvement may include a differential diagnosis of lymphoma, angiosarcoma, and bacillary angiomatosis.7 Other vascular pathologies that may be considered in the differential diagnosis include vascular tumors (eg, spindle cell hemangioma), fibrohistiocytic tumors (eg, dermatofibrosarcoma protuberans), and a collection of spindle cell mesenchymal tumors.8
Kaposi sarcoma progresses through several histologic stages beginning with the patch stage, then progressing to the plaque stage, and finally culminating in the nodular stage. The patch stage is the first stage in KS progression and a crowded dermis can be seen with the formation of slitlike vascular spaces lined by endothelial cells with red blood cell extravasation into the lumens of newly formed vascular channels, hence demonstrating the promontory sign.8 In the plaque stage, the promontory sign still is present and there is a greater presence of slitlike spaces, giving the micrograph an overall sievelike appearance. Erythrocytes can be found residing within the clear cytoplasm of spindled endothelial cells, leading to the development of autolumination. Finally, the nodular stage is characterized by a neoplastic proliferation of monomorphic spindle cells that form fasciclelike nests in the dermis.8 To distinguish KS from other angioproliferative tumors, one can stain for HHV-8 latent nuclear antigen-1, which is found in the nuclei of infected endothelial cells.1,8,9
Kaposi sarcoma is treated through a variety of mechanisms depending on the subtype. Classic KS lesions often can be observed as they a follow a benign and nonaggressive course.1 Highly active antiretroviral therapy is the mainstay of care in AIDS-related KS and has led to regression of lesions and a remarkable decline in the incidence of KS.3 The HAART regimen consists of a protease inhibitor or nonnucleoside reverse-transcriptase inhibitor with the addition of 2 nucleoside reverse-transcriptase inhibitors. More advanced or refractory cases of KS often require dual treatment with HAART and a chemotherapy agent such as pegylated liposomal doxorubicin. Combination therapy has been shown to result in stronger therapeutic responses and lower relapse rates in contrast to HAART alone.7 Patients also may consider other treatment modalities to manage KS lesions such as surgical removal of lesions, laser therapy, paclitaxel, interferon alfa, oral etoposide, thalidomide, and topical therapies such as imiquimod cream 5% and alitretinoin.1,7
Conclusion
Kaposi sarcoma is a rare but concerning dermatologic condition that signals the need for further diagnostic evaluation. Coexpression of viruses such as HIV and HHV-8 can result in a more virulent and rapid progression of KS to encompass both mucous membrane and systemic involvement. Our patient’s lesions were the first presenting sign of HIV infection despite being asymptomatic at the time of diagnosis, which is alarming in the sense that more than 21% of HIV-infected individuals in the United States have not been clinically diagnosed.10 Inquiry of HIV risk factors and routine screening for HIV should be performed in refractory cases of skin disease as an underlying clue to further investigate the immune system. We present our unique case of mucocutaneous development of KS in an asymptomatic HIV-positive man to stress the importance of KS recognition and management.
1. Jan MM, Laskas JW, Griffin TD. Eruptive Kaposi sarcoma: an unusual presentation in an HIV-negative patient. Cutis. 2011;87:34-38.
2. Geraminejad P, Memar O, Aronson I, et al. Kaposi’s sarcoma and other manifestations of human herpesvirus 8. J Am Acad Dermatol. 2002;47:641-655.
3. Kharkar V, Gutte RM, Khopkar U, et al. Kaposi’s sarcoma: a presenting manifestation of HIV infection in an Indian. Indian J Dermatol Venereol Leprol. 2009;75:391-393.
4. Naidu A, Havard DB, Ray JM, et al. Oral and maxillofacial pathology case of the month. Kaposi’s sarcoma. Tex Dent J. 2011;128:376-377, 382-383.
5. Lawson G, Matar N, Kesch S, et al. Laryngeal Kaposi sarcoma: case report and literature review. B-ENT. 2010;6:285-288.
6. Sullivan RJ, Pantanowitz L, Casper C, et al. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma–associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis. 2008;47:1209-1215.
7. Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma [published online ahead of print March 4, 2011]. Cancer Lett. 2011;305:150-162.
8. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
9. Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342.
10. Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007 [published correction appears in JAMA. 2011;306:1548]. JAMA. 2011;305:1450-1459.
1. Jan MM, Laskas JW, Griffin TD. Eruptive Kaposi sarcoma: an unusual presentation in an HIV-negative patient. Cutis. 2011;87:34-38.
2. Geraminejad P, Memar O, Aronson I, et al. Kaposi’s sarcoma and other manifestations of human herpesvirus 8. J Am Acad Dermatol. 2002;47:641-655.
3. Kharkar V, Gutte RM, Khopkar U, et al. Kaposi’s sarcoma: a presenting manifestation of HIV infection in an Indian. Indian J Dermatol Venereol Leprol. 2009;75:391-393.
4. Naidu A, Havard DB, Ray JM, et al. Oral and maxillofacial pathology case of the month. Kaposi’s sarcoma. Tex Dent J. 2011;128:376-377, 382-383.
5. Lawson G, Matar N, Kesch S, et al. Laryngeal Kaposi sarcoma: case report and literature review. B-ENT. 2010;6:285-288.
6. Sullivan RJ, Pantanowitz L, Casper C, et al. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma–associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis. 2008;47:1209-1215.
7. Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma [published online ahead of print March 4, 2011]. Cancer Lett. 2011;305:150-162.
8. Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
9. Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335-342.
10. Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007 [published correction appears in JAMA. 2011;306:1548]. JAMA. 2011;305:1450-1459.
Practice Points
- Kaposi sarcoma is a rare malignant proliferation of endothelial cells with many subtypes.
- Kaposi sarcoma in patients with coexpression of human immunodeficiency virus and human herpesvirus 8 often have a more virulent and rapid progression of disease.
Metastatic melanoma masquerading as disseminated sporotrichosis
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Anxiety tied to fear of falling • fatigue • difficulty concentrating • Dx?
THE CASE
A 21-year-old college student was referred to us by the counseling center at our university for a psychiatric evaluation after 11 psychotherapy sessions over 3 months had failed to reduce her feelings of anxiety and panic.
During our evaluation, the patient described feeling “not quite right” for many months. She had been experiencing mental fogginess, fatigue, and worsening concentration/ memory. Her anxiety, which had been gradually increasing, was the result of being unsure about her gait. She first noticed this while walking down some bleachers; she felt dizzy, was afraid of falling, and couldn’t walk down without assistance. All episodes of “panic” occurred in situations where she experienced disequilibrium, unsteady gait, and fear of falling. She grew fearful of driving or going anywhere without assistance.
The patient had celiac disease that was well controlled with a gluten-free diet. She had no personal or family psychiatric history and no history of substance abuse.
THE DIAGNOSIS
Physical exam and lab studies, including a complete blood count, comprehensive metabolic panel, and thyrotropin and folate levels, were normal. Her homocysteine level was 11.8 μmol/L (reference range, 5.4-11.9 μmol/L) and vitamin B12 level was 292 pg/mL (reference range, 200-1100 pg/mL). Her lab report included a note that read, “Although the reference range for vitamin B12 is 200 to 1100 pg/mL, it has been reported that between 5% and 10% of patients with values between 200 and 400 pg/mL may experience neuropsychiatric and hematologic abnormalities due to occult B12 deficiency; <1% of patients with values >400 pg/mL will have symptoms.”
Based on this vitamin B12 level, the patient’s symptoms, and her borderline high homocysteine level, we diagnosed vitamin B12 deficiency.
DISCUSSION
There are no recommendations by the US Preventive Services Task Force or any other major US medical society for routine vitamin B12 screening.1 In Canada, the Medical Services Commission of the British Columbia Ministry of Health recommends B12 screening for patients who present with macrocytic anemia or unexplained neurologic symptoms (eg, paresthesia, numbness, poor motor coordination, memory lapses, or cognitive or personality changes).2
Vitamin B12 deficiency can be caused by numerous conditions, including those that cause malabsorption (such as gastric bypass). It can also be caused by diseases such as human immunodeficiency virus infection or Crohn’s disease, long-term adherence to a vegetarian or vegan diet, or by any other lack of dietary intake.1 The condition can cause hematologic-related signs and symptoms such as megaloblastic anemia, fatigue, and syncope. It also can have neurologic manifestations, including paresthesia, weakness, motor disturbances (including gait abnormalities), vision loss, and a wide range of cognitive and behavioral changes.1 Anemia is uncommon because since 1998, the US Food and Drug Administration has required fortification of all enriched grain and cereal products with folic acid; thus, vitamin B12 deficiency may proceed without anemia revealing its presence.1
A controversial topic. Vitamin B12 deficiency is a complicated and controversial subject. Specifically, there is uncertainty about the clinical importance of lower serum levels of vitamin B12 (200-400 pg/mL), their impact on well-being, and the need for treatment. In addition to measuring a patient’s serum B12 level, testing a second biomarker (such as homocysteine or methylmalonic acid) can be helpful in establishing a diagnosis of B12 deficiency.1 Levels of each of these are elevated in patients with B12 deficiency.1
Although vitamin B12 deficiency has been well studied in older patients,3 little has been published about the condition in young adults. National Health and Nutrition Examination Survey (NHANES) data from 2000 to 2004 shows that almost 40% of people ages 19 to 30 years have a B12 level <400 pg/mL.1 How many of these individuals are at risk of complications of B12 deficiency is unknown.
B12 supplementation might improve depression, anxiety
B12 supplementation is inexpensive and has no significant adverse effects.1 It can be administered orally, parenterally (intramuscularly or subcutaneously), or intranasally.1 A common oral regimen is 1 mg/d; parental regimens vary widely, but might include a 1-mg injection once a week for 8 weeks, then once a month for life.1
Some evidence suggests B12 supplementation may improve symptoms of depression and anxiety. A Pakistani study randomized 73 patients with depression and “low normal” B12 levels (190-300 pg/mL) to an antidepressant only (equivalent to imipramine 100-250 mg/d or fluoxetine 20-40 mg/d) or an antidepressant plus parenteral B12 (1000 mcg once a week).4 At 3 months follow-up, 100% of the treatment group showed at least a 20% reduction in their Hamilton Depression Rating Scale (HAM-D) score, compared to 69% in the control arm (P<.001).4
A Swedish study analyzed the effects of several B vitamins, including 0.5 mg/d of B12 vs placebo on mood in 65 celiac patients on a gluten-free diet who had borderline/low normal B12 levels (>191 pg/mL).5 Patients who scored low on a measure of psychological well-being at the beginning of the study and who received B12 experienced significant improvements in anxiety and depressed mood compared to those who received placebo.
Our patient
Because neurologic and psychiatric symptoms require assured compliance and urgent treatment, our patient received vitamin B12 parenterally as cyanocobalamin 1 mg/mL. She was given this dosage intramuscularly once a day for 5 days, then once a week for 4 weeks. She will continue to receive it once a month indefinitely.
The patient was advised that if she wished to switch to oral therapy, she could do so after several months of parenteral treatment, as long as she had close follow-up with frequent B12 measurements to assure that she was absorbing oral therapy. Her anxiety and mood symptoms resolved within one month, and her disequilibrium was almost entirely resolved within 3 months of treatment.
THE TAKEAWAY
Although more common in older patients, vitamin B12 deficiency can also affect younger patients. “Low normal” B12 levels (200-400 pg/mL) may affect psychological well-being.
Consider testing serum B12 and a second biomarker—such as homocysteine or methylmalonic acid, if indicated—in patients who present with depressed mood, anxiety, cognitive symptoms, and/or fatigue. Vitamin B12 supplementation can be administered orally and has no major adverse effects.
1. Centers for Disease Control and Prevention. Why vitamin B12 deficiency should be on your radar screen. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/b12/. Accessed February 26, 2015.
2. Guidelines and Protocols Advisory Committee, Medical Services Commission, British Columbia Ministry of Health. Cobalamin (vitamin B12) Deficiency - Investigation & Management. British Columbia Ministry of Health Web site. Available at: http://www.bcguidelines.ca/guideline_cobalamin.html. Accessed March 13, 2015.
3. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197-1204.
4. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44-48
5. Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Ailment Pharmacol Ther. 2009;29:811-816.
THE CASE
A 21-year-old college student was referred to us by the counseling center at our university for a psychiatric evaluation after 11 psychotherapy sessions over 3 months had failed to reduce her feelings of anxiety and panic.
During our evaluation, the patient described feeling “not quite right” for many months. She had been experiencing mental fogginess, fatigue, and worsening concentration/ memory. Her anxiety, which had been gradually increasing, was the result of being unsure about her gait. She first noticed this while walking down some bleachers; she felt dizzy, was afraid of falling, and couldn’t walk down without assistance. All episodes of “panic” occurred in situations where she experienced disequilibrium, unsteady gait, and fear of falling. She grew fearful of driving or going anywhere without assistance.
The patient had celiac disease that was well controlled with a gluten-free diet. She had no personal or family psychiatric history and no history of substance abuse.
THE DIAGNOSIS
Physical exam and lab studies, including a complete blood count, comprehensive metabolic panel, and thyrotropin and folate levels, were normal. Her homocysteine level was 11.8 μmol/L (reference range, 5.4-11.9 μmol/L) and vitamin B12 level was 292 pg/mL (reference range, 200-1100 pg/mL). Her lab report included a note that read, “Although the reference range for vitamin B12 is 200 to 1100 pg/mL, it has been reported that between 5% and 10% of patients with values between 200 and 400 pg/mL may experience neuropsychiatric and hematologic abnormalities due to occult B12 deficiency; <1% of patients with values >400 pg/mL will have symptoms.”
Based on this vitamin B12 level, the patient’s symptoms, and her borderline high homocysteine level, we diagnosed vitamin B12 deficiency.
DISCUSSION
There are no recommendations by the US Preventive Services Task Force or any other major US medical society for routine vitamin B12 screening.1 In Canada, the Medical Services Commission of the British Columbia Ministry of Health recommends B12 screening for patients who present with macrocytic anemia or unexplained neurologic symptoms (eg, paresthesia, numbness, poor motor coordination, memory lapses, or cognitive or personality changes).2
Vitamin B12 deficiency can be caused by numerous conditions, including those that cause malabsorption (such as gastric bypass). It can also be caused by diseases such as human immunodeficiency virus infection or Crohn’s disease, long-term adherence to a vegetarian or vegan diet, or by any other lack of dietary intake.1 The condition can cause hematologic-related signs and symptoms such as megaloblastic anemia, fatigue, and syncope. It also can have neurologic manifestations, including paresthesia, weakness, motor disturbances (including gait abnormalities), vision loss, and a wide range of cognitive and behavioral changes.1 Anemia is uncommon because since 1998, the US Food and Drug Administration has required fortification of all enriched grain and cereal products with folic acid; thus, vitamin B12 deficiency may proceed without anemia revealing its presence.1
A controversial topic. Vitamin B12 deficiency is a complicated and controversial subject. Specifically, there is uncertainty about the clinical importance of lower serum levels of vitamin B12 (200-400 pg/mL), their impact on well-being, and the need for treatment. In addition to measuring a patient’s serum B12 level, testing a second biomarker (such as homocysteine or methylmalonic acid) can be helpful in establishing a diagnosis of B12 deficiency.1 Levels of each of these are elevated in patients with B12 deficiency.1
Although vitamin B12 deficiency has been well studied in older patients,3 little has been published about the condition in young adults. National Health and Nutrition Examination Survey (NHANES) data from 2000 to 2004 shows that almost 40% of people ages 19 to 30 years have a B12 level <400 pg/mL.1 How many of these individuals are at risk of complications of B12 deficiency is unknown.
B12 supplementation might improve depression, anxiety
B12 supplementation is inexpensive and has no significant adverse effects.1 It can be administered orally, parenterally (intramuscularly or subcutaneously), or intranasally.1 A common oral regimen is 1 mg/d; parental regimens vary widely, but might include a 1-mg injection once a week for 8 weeks, then once a month for life.1
Some evidence suggests B12 supplementation may improve symptoms of depression and anxiety. A Pakistani study randomized 73 patients with depression and “low normal” B12 levels (190-300 pg/mL) to an antidepressant only (equivalent to imipramine 100-250 mg/d or fluoxetine 20-40 mg/d) or an antidepressant plus parenteral B12 (1000 mcg once a week).4 At 3 months follow-up, 100% of the treatment group showed at least a 20% reduction in their Hamilton Depression Rating Scale (HAM-D) score, compared to 69% in the control arm (P<.001).4
A Swedish study analyzed the effects of several B vitamins, including 0.5 mg/d of B12 vs placebo on mood in 65 celiac patients on a gluten-free diet who had borderline/low normal B12 levels (>191 pg/mL).5 Patients who scored low on a measure of psychological well-being at the beginning of the study and who received B12 experienced significant improvements in anxiety and depressed mood compared to those who received placebo.
Our patient
Because neurologic and psychiatric symptoms require assured compliance and urgent treatment, our patient received vitamin B12 parenterally as cyanocobalamin 1 mg/mL. She was given this dosage intramuscularly once a day for 5 days, then once a week for 4 weeks. She will continue to receive it once a month indefinitely.
The patient was advised that if she wished to switch to oral therapy, she could do so after several months of parenteral treatment, as long as she had close follow-up with frequent B12 measurements to assure that she was absorbing oral therapy. Her anxiety and mood symptoms resolved within one month, and her disequilibrium was almost entirely resolved within 3 months of treatment.
THE TAKEAWAY
Although more common in older patients, vitamin B12 deficiency can also affect younger patients. “Low normal” B12 levels (200-400 pg/mL) may affect psychological well-being.
Consider testing serum B12 and a second biomarker—such as homocysteine or methylmalonic acid, if indicated—in patients who present with depressed mood, anxiety, cognitive symptoms, and/or fatigue. Vitamin B12 supplementation can be administered orally and has no major adverse effects.
THE CASE
A 21-year-old college student was referred to us by the counseling center at our university for a psychiatric evaluation after 11 psychotherapy sessions over 3 months had failed to reduce her feelings of anxiety and panic.
During our evaluation, the patient described feeling “not quite right” for many months. She had been experiencing mental fogginess, fatigue, and worsening concentration/ memory. Her anxiety, which had been gradually increasing, was the result of being unsure about her gait. She first noticed this while walking down some bleachers; she felt dizzy, was afraid of falling, and couldn’t walk down without assistance. All episodes of “panic” occurred in situations where she experienced disequilibrium, unsteady gait, and fear of falling. She grew fearful of driving or going anywhere without assistance.
The patient had celiac disease that was well controlled with a gluten-free diet. She had no personal or family psychiatric history and no history of substance abuse.
THE DIAGNOSIS
Physical exam and lab studies, including a complete blood count, comprehensive metabolic panel, and thyrotropin and folate levels, were normal. Her homocysteine level was 11.8 μmol/L (reference range, 5.4-11.9 μmol/L) and vitamin B12 level was 292 pg/mL (reference range, 200-1100 pg/mL). Her lab report included a note that read, “Although the reference range for vitamin B12 is 200 to 1100 pg/mL, it has been reported that between 5% and 10% of patients with values between 200 and 400 pg/mL may experience neuropsychiatric and hematologic abnormalities due to occult B12 deficiency; <1% of patients with values >400 pg/mL will have symptoms.”
Based on this vitamin B12 level, the patient’s symptoms, and her borderline high homocysteine level, we diagnosed vitamin B12 deficiency.
DISCUSSION
There are no recommendations by the US Preventive Services Task Force or any other major US medical society for routine vitamin B12 screening.1 In Canada, the Medical Services Commission of the British Columbia Ministry of Health recommends B12 screening for patients who present with macrocytic anemia or unexplained neurologic symptoms (eg, paresthesia, numbness, poor motor coordination, memory lapses, or cognitive or personality changes).2
Vitamin B12 deficiency can be caused by numerous conditions, including those that cause malabsorption (such as gastric bypass). It can also be caused by diseases such as human immunodeficiency virus infection or Crohn’s disease, long-term adherence to a vegetarian or vegan diet, or by any other lack of dietary intake.1 The condition can cause hematologic-related signs and symptoms such as megaloblastic anemia, fatigue, and syncope. It also can have neurologic manifestations, including paresthesia, weakness, motor disturbances (including gait abnormalities), vision loss, and a wide range of cognitive and behavioral changes.1 Anemia is uncommon because since 1998, the US Food and Drug Administration has required fortification of all enriched grain and cereal products with folic acid; thus, vitamin B12 deficiency may proceed without anemia revealing its presence.1
A controversial topic. Vitamin B12 deficiency is a complicated and controversial subject. Specifically, there is uncertainty about the clinical importance of lower serum levels of vitamin B12 (200-400 pg/mL), their impact on well-being, and the need for treatment. In addition to measuring a patient’s serum B12 level, testing a second biomarker (such as homocysteine or methylmalonic acid) can be helpful in establishing a diagnosis of B12 deficiency.1 Levels of each of these are elevated in patients with B12 deficiency.1
Although vitamin B12 deficiency has been well studied in older patients,3 little has been published about the condition in young adults. National Health and Nutrition Examination Survey (NHANES) data from 2000 to 2004 shows that almost 40% of people ages 19 to 30 years have a B12 level <400 pg/mL.1 How many of these individuals are at risk of complications of B12 deficiency is unknown.
B12 supplementation might improve depression, anxiety
B12 supplementation is inexpensive and has no significant adverse effects.1 It can be administered orally, parenterally (intramuscularly or subcutaneously), or intranasally.1 A common oral regimen is 1 mg/d; parental regimens vary widely, but might include a 1-mg injection once a week for 8 weeks, then once a month for life.1
Some evidence suggests B12 supplementation may improve symptoms of depression and anxiety. A Pakistani study randomized 73 patients with depression and “low normal” B12 levels (190-300 pg/mL) to an antidepressant only (equivalent to imipramine 100-250 mg/d or fluoxetine 20-40 mg/d) or an antidepressant plus parenteral B12 (1000 mcg once a week).4 At 3 months follow-up, 100% of the treatment group showed at least a 20% reduction in their Hamilton Depression Rating Scale (HAM-D) score, compared to 69% in the control arm (P<.001).4
A Swedish study analyzed the effects of several B vitamins, including 0.5 mg/d of B12 vs placebo on mood in 65 celiac patients on a gluten-free diet who had borderline/low normal B12 levels (>191 pg/mL).5 Patients who scored low on a measure of psychological well-being at the beginning of the study and who received B12 experienced significant improvements in anxiety and depressed mood compared to those who received placebo.
Our patient
Because neurologic and psychiatric symptoms require assured compliance and urgent treatment, our patient received vitamin B12 parenterally as cyanocobalamin 1 mg/mL. She was given this dosage intramuscularly once a day for 5 days, then once a week for 4 weeks. She will continue to receive it once a month indefinitely.
The patient was advised that if she wished to switch to oral therapy, she could do so after several months of parenteral treatment, as long as she had close follow-up with frequent B12 measurements to assure that she was absorbing oral therapy. Her anxiety and mood symptoms resolved within one month, and her disequilibrium was almost entirely resolved within 3 months of treatment.
THE TAKEAWAY
Although more common in older patients, vitamin B12 deficiency can also affect younger patients. “Low normal” B12 levels (200-400 pg/mL) may affect psychological well-being.
Consider testing serum B12 and a second biomarker—such as homocysteine or methylmalonic acid, if indicated—in patients who present with depressed mood, anxiety, cognitive symptoms, and/or fatigue. Vitamin B12 supplementation can be administered orally and has no major adverse effects.
1. Centers for Disease Control and Prevention. Why vitamin B12 deficiency should be on your radar screen. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/b12/. Accessed February 26, 2015.
2. Guidelines and Protocols Advisory Committee, Medical Services Commission, British Columbia Ministry of Health. Cobalamin (vitamin B12) Deficiency - Investigation & Management. British Columbia Ministry of Health Web site. Available at: http://www.bcguidelines.ca/guideline_cobalamin.html. Accessed March 13, 2015.
3. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197-1204.
4. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44-48
5. Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Ailment Pharmacol Ther. 2009;29:811-816.
1. Centers for Disease Control and Prevention. Why vitamin B12 deficiency should be on your radar screen. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/b12/. Accessed February 26, 2015.
2. Guidelines and Protocols Advisory Committee, Medical Services Commission, British Columbia Ministry of Health. Cobalamin (vitamin B12) Deficiency - Investigation & Management. British Columbia Ministry of Health Web site. Available at: http://www.bcguidelines.ca/guideline_cobalamin.html. Accessed March 13, 2015.
3. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197-1204.
4. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44-48
5. Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Ailment Pharmacol Ther. 2009;29:811-816.
Headache • fatigue • blurred vision • Dx?
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
Case Report: Spontaneous Retroperitoneal Bleeding Masking as Left Lower Quadrant Abdominal Pain
Case
A 76-year-old woman with worsening intermittent lower left quadrant abdominal pain was brought to the ED by her daughter, who stated that her mother’s pain had started 2 days prior to presentation. The patient described the pain, which was present with movement and at rest, as sharp and radiating to the left hip, left lower back, and left inner thigh. She denied shortness of breath, chest pain, fever, chills, nausea, and vomiting. The patient initially reported having diarrhea, but further inquiry revealed that her stools had been softer than usual but not watery or more frequent.
One week before presentation, the patient had been treated at another hospital for myocardial infarction (MI) and new-onset atrial fibrillation. During this hospital stay, she had declined diagnostic cardiac catheterization and was started on warfarin and enoxaparin at discharge. Other than a history of hypertension, the patient had been in good health prior to the MI and atrial fibrillation. Her regular medications included metoprolol, lisinopril, and aspirin.
On physical examination, she was alert and oriented, but appeared to be in mild distress. The patient was an obese, elderly female with a body mass index of 35.2 kg/m2. Her vital signs were: temperature, 98.4˚F; blood pressure, 180/74 mm Hg; heart rate, 69 beats/minute; and respiratory rate, 18 breaths/minute. No respiratory distress was noted, and her oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat examination was normal, with a normocephalic and atraumatic head. The patient’s neck was supple and without jugular vein distension or tracheal deviation. A cardiopulmonary examination revealed breath sounds with normal effort and no rales, wheezes, or rhonchi; no murmurs, rubs, or gallops were heard on auscultation. The patient’s abdomen was soft, without rebound tenderness, distention, or guarding, and bowel sounds were normal and appreciated on auscultation. She had tenderness to palpation in the left lower quadrant.
On musculoskeletal examination, the patient’s hips had normal range of motion and there was equal +5/5 strength bilaterally in the hips and lower extremities. The remainder of the examination of her lower extremities was without rash, erythema, or edema, and there were no lesions or rashes elsewhere on the skin. No focal deficits were noted on neurological examination.
The patient was admitted to the hospital with a request for cardiology and surgical consultations. Upon inpatient admission and evaluation, the warfarin and enoxaparin were stopped, and the surgical team elected for conservative management of the retroperitoneal bleed. The patient had stable Hgb levels on serial complete blood-cell counts.
A renal ultrasound was ordered to investigate the low-density lesion in the left kidney revealed a left renal cyst. It is, however, unlikely the renal cyst was contributory to the retroperitoneal bleed. As the patient remained hemodynamically stable and her pain abated, she was discharged on hospital day 4.
Discussion
Overview
Spontaneous retroperitoneal bleeding is a rare but potentially life-threatening occurrence that is most commonly seen in patients on anticoagulation therapy, those with bleeding disorders, and those on hemodialysis.1 Patients with spontaneous retroperitoneal bleeding present with a variety of symptoms including abdominal pain, hip and upper thigh pain, back pain, and hypotension.2,3 In one observational cohort study, the nonspecific nature of symptoms led to misdiagnosis in 10.1% of the cases.3 The patient in this case exhibited several of the abovementioned symptoms and complaints.
The literature concerning spontaneous retroperitoneal bleeding is limited and consists of mainly case reports and case series. Warfarin, clopidogrel, unfractionated and low-molecular weight heparin use have all been reported in patients with retroperitoneal bleeding,4 and various studies have placed the incidence of this distinct entity to be between 0.6% to 6.6%.5,6
Spontaneous Versus Iatrogenic Retroperitoneal Bleeding
By definition, spontaneous retroperitoneal bleeding occurs without trauma, surgery, invasive procedures, and abdominal aortic aneurysm.3 Iatrogenic retroperitoneal bleeding, however, is a rare but known complication of catheterization procedures involving the femoral region, with an increased risk in female patients.7 Based on this increased incidence, the patient in this case was specifically asked whether she had undergone any procedures during her latest admission, including cardiac catheterization, which she denied.
In contrast, spontaneous retroperitoneal hemorrhage has an unclear pathogenesis. Some authors have suggested unrecognized minor trauma such as coughing as a possible cause.8 The patient in this case was asked on hospital day 2 if she had experienced a history of cough the week prior to presentation to the ED, to which she stated she had not.
Risk Factors
More recent case reports focus on renal or adrenal tumors as a potential source of spontaneous retroperitoneal bleeding. Although this patient did have a renal cyst in left kidney, its contribution to the retroperitoneal bleed is unknown and thought to be only incidental.
A retrospective chart review of 119 identified patients with spontaneous retroperitoneal bleeds sought to identify reliable predictors of early diagnosis. Ivascu et al2 found that elderly patients on both anticoagulation and antiplatelet therapy were at the highest risk. Shah et al9 echoed the sentiment of severity in their retrospective review which showed that those on combined anticoagulant and antiplatelet therapy were more likely to require intensive care unit (ICU) admission and had longer ICU stays.
Conclusion
Unlike hemorrhages in other locations in the body, retroperitoneal bleeding can be difficult to diagnose since patients often present with nonspecific symptoms such as lower abdominal pain. Clinicians should therefore maintain a high index of suspicion, especially in patients who are on anticoagulation therapy, who have a coagulopathy, or who are on hemodialysis. Delay or failure to diagnose this condition may lead to significant morbidity and mortality.
Dr Lui is a resident, department of emergency medicine, Henry Ford Wyandotte Hospital, Wyandotte, Michigan. Dr Boehm is the emergency medicine residency program director in the graduate medical education department, Saint Mary Mercy Hospital, livonia, Michigan; and is an emergency physician at the Emergency physicians Medical Group, ann arbor, Michigan.
- Bhasin HK, Dana CL. Spontaneous retroperitoneal hemorrhage in chronically hemodialyzed patients. Nephron. 1978; 22(4-6):322-327.
- Ivascu FA, Janczyk RJ, Bair HA, Bendick PJ, Howells GA. Spontaneous retroperitoneal hemorrhage. Am J Surg. 2005;189(3):345-347.
- Sunga, KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emer Med. 2012;43(2):e157-e161.
- Ernits M, Mohan PS, Fares LG 2nd, Hardy H 3rd. A retroperitoneal bleed induced by enoxaparin therapy. Am Surg. 2005;71(5):430-433.
- Mant MJ, O’Brien BD, Thong KL, Hammond GW, Birtwhistle RV, Grace MG. Haemorrhagic complications of heparin therapy. Lancet. 1977;1(8022):1133-1135.
- Forfar JC. A 7-year analysis of haemorrhage in patients on long-term anticoagulant treatment. Br Heart J. 1979;42(2):128-132.
- Sajnani N, Bogart DB. Retroperitoneal hemorrhage as a complication of percutaneous intervention: report of 2 cases and review of the literature. Open Cardiovasc Med J. 2013;7:16-22.
- Berná JD, Zuazu I, Madrigal M, García-Medina V, Fernández C, Guirado F. Conservative treatment of large rectus sheath hematoma in patients undergoing anticoagulant therapy. Abdom Imaging. 2000;25(3):230-234.
- Shah, RD, Nagar S, Shanley CJ, Janczyk RJ. Factors affecting the severity of spontaneous retroperitoneal hemorrhage in anticoagulated patients. Am J Surg.
Case
A 76-year-old woman with worsening intermittent lower left quadrant abdominal pain was brought to the ED by her daughter, who stated that her mother’s pain had started 2 days prior to presentation. The patient described the pain, which was present with movement and at rest, as sharp and radiating to the left hip, left lower back, and left inner thigh. She denied shortness of breath, chest pain, fever, chills, nausea, and vomiting. The patient initially reported having diarrhea, but further inquiry revealed that her stools had been softer than usual but not watery or more frequent.
One week before presentation, the patient had been treated at another hospital for myocardial infarction (MI) and new-onset atrial fibrillation. During this hospital stay, she had declined diagnostic cardiac catheterization and was started on warfarin and enoxaparin at discharge. Other than a history of hypertension, the patient had been in good health prior to the MI and atrial fibrillation. Her regular medications included metoprolol, lisinopril, and aspirin.
On physical examination, she was alert and oriented, but appeared to be in mild distress. The patient was an obese, elderly female with a body mass index of 35.2 kg/m2. Her vital signs were: temperature, 98.4˚F; blood pressure, 180/74 mm Hg; heart rate, 69 beats/minute; and respiratory rate, 18 breaths/minute. No respiratory distress was noted, and her oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat examination was normal, with a normocephalic and atraumatic head. The patient’s neck was supple and without jugular vein distension or tracheal deviation. A cardiopulmonary examination revealed breath sounds with normal effort and no rales, wheezes, or rhonchi; no murmurs, rubs, or gallops were heard on auscultation. The patient’s abdomen was soft, without rebound tenderness, distention, or guarding, and bowel sounds were normal and appreciated on auscultation. She had tenderness to palpation in the left lower quadrant.
On musculoskeletal examination, the patient’s hips had normal range of motion and there was equal +5/5 strength bilaterally in the hips and lower extremities. The remainder of the examination of her lower extremities was without rash, erythema, or edema, and there were no lesions or rashes elsewhere on the skin. No focal deficits were noted on neurological examination.
The patient was admitted to the hospital with a request for cardiology and surgical consultations. Upon inpatient admission and evaluation, the warfarin and enoxaparin were stopped, and the surgical team elected for conservative management of the retroperitoneal bleed. The patient had stable Hgb levels on serial complete blood-cell counts.
A renal ultrasound was ordered to investigate the low-density lesion in the left kidney revealed a left renal cyst. It is, however, unlikely the renal cyst was contributory to the retroperitoneal bleed. As the patient remained hemodynamically stable and her pain abated, she was discharged on hospital day 4.
Discussion
Overview
Spontaneous retroperitoneal bleeding is a rare but potentially life-threatening occurrence that is most commonly seen in patients on anticoagulation therapy, those with bleeding disorders, and those on hemodialysis.1 Patients with spontaneous retroperitoneal bleeding present with a variety of symptoms including abdominal pain, hip and upper thigh pain, back pain, and hypotension.2,3 In one observational cohort study, the nonspecific nature of symptoms led to misdiagnosis in 10.1% of the cases.3 The patient in this case exhibited several of the abovementioned symptoms and complaints.
The literature concerning spontaneous retroperitoneal bleeding is limited and consists of mainly case reports and case series. Warfarin, clopidogrel, unfractionated and low-molecular weight heparin use have all been reported in patients with retroperitoneal bleeding,4 and various studies have placed the incidence of this distinct entity to be between 0.6% to 6.6%.5,6
Spontaneous Versus Iatrogenic Retroperitoneal Bleeding
By definition, spontaneous retroperitoneal bleeding occurs without trauma, surgery, invasive procedures, and abdominal aortic aneurysm.3 Iatrogenic retroperitoneal bleeding, however, is a rare but known complication of catheterization procedures involving the femoral region, with an increased risk in female patients.7 Based on this increased incidence, the patient in this case was specifically asked whether she had undergone any procedures during her latest admission, including cardiac catheterization, which she denied.
In contrast, spontaneous retroperitoneal hemorrhage has an unclear pathogenesis. Some authors have suggested unrecognized minor trauma such as coughing as a possible cause.8 The patient in this case was asked on hospital day 2 if she had experienced a history of cough the week prior to presentation to the ED, to which she stated she had not.
Risk Factors
More recent case reports focus on renal or adrenal tumors as a potential source of spontaneous retroperitoneal bleeding. Although this patient did have a renal cyst in left kidney, its contribution to the retroperitoneal bleed is unknown and thought to be only incidental.
A retrospective chart review of 119 identified patients with spontaneous retroperitoneal bleeds sought to identify reliable predictors of early diagnosis. Ivascu et al2 found that elderly patients on both anticoagulation and antiplatelet therapy were at the highest risk. Shah et al9 echoed the sentiment of severity in their retrospective review which showed that those on combined anticoagulant and antiplatelet therapy were more likely to require intensive care unit (ICU) admission and had longer ICU stays.
Conclusion
Unlike hemorrhages in other locations in the body, retroperitoneal bleeding can be difficult to diagnose since patients often present with nonspecific symptoms such as lower abdominal pain. Clinicians should therefore maintain a high index of suspicion, especially in patients who are on anticoagulation therapy, who have a coagulopathy, or who are on hemodialysis. Delay or failure to diagnose this condition may lead to significant morbidity and mortality.
Dr Lui is a resident, department of emergency medicine, Henry Ford Wyandotte Hospital, Wyandotte, Michigan. Dr Boehm is the emergency medicine residency program director in the graduate medical education department, Saint Mary Mercy Hospital, livonia, Michigan; and is an emergency physician at the Emergency physicians Medical Group, ann arbor, Michigan.
Case
A 76-year-old woman with worsening intermittent lower left quadrant abdominal pain was brought to the ED by her daughter, who stated that her mother’s pain had started 2 days prior to presentation. The patient described the pain, which was present with movement and at rest, as sharp and radiating to the left hip, left lower back, and left inner thigh. She denied shortness of breath, chest pain, fever, chills, nausea, and vomiting. The patient initially reported having diarrhea, but further inquiry revealed that her stools had been softer than usual but not watery or more frequent.
One week before presentation, the patient had been treated at another hospital for myocardial infarction (MI) and new-onset atrial fibrillation. During this hospital stay, she had declined diagnostic cardiac catheterization and was started on warfarin and enoxaparin at discharge. Other than a history of hypertension, the patient had been in good health prior to the MI and atrial fibrillation. Her regular medications included metoprolol, lisinopril, and aspirin.
On physical examination, she was alert and oriented, but appeared to be in mild distress. The patient was an obese, elderly female with a body mass index of 35.2 kg/m2. Her vital signs were: temperature, 98.4˚F; blood pressure, 180/74 mm Hg; heart rate, 69 beats/minute; and respiratory rate, 18 breaths/minute. No respiratory distress was noted, and her oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat examination was normal, with a normocephalic and atraumatic head. The patient’s neck was supple and without jugular vein distension or tracheal deviation. A cardiopulmonary examination revealed breath sounds with normal effort and no rales, wheezes, or rhonchi; no murmurs, rubs, or gallops were heard on auscultation. The patient’s abdomen was soft, without rebound tenderness, distention, or guarding, and bowel sounds were normal and appreciated on auscultation. She had tenderness to palpation in the left lower quadrant.
On musculoskeletal examination, the patient’s hips had normal range of motion and there was equal +5/5 strength bilaterally in the hips and lower extremities. The remainder of the examination of her lower extremities was without rash, erythema, or edema, and there were no lesions or rashes elsewhere on the skin. No focal deficits were noted on neurological examination.
The patient was admitted to the hospital with a request for cardiology and surgical consultations. Upon inpatient admission and evaluation, the warfarin and enoxaparin were stopped, and the surgical team elected for conservative management of the retroperitoneal bleed. The patient had stable Hgb levels on serial complete blood-cell counts.
A renal ultrasound was ordered to investigate the low-density lesion in the left kidney revealed a left renal cyst. It is, however, unlikely the renal cyst was contributory to the retroperitoneal bleed. As the patient remained hemodynamically stable and her pain abated, she was discharged on hospital day 4.
Discussion
Overview
Spontaneous retroperitoneal bleeding is a rare but potentially life-threatening occurrence that is most commonly seen in patients on anticoagulation therapy, those with bleeding disorders, and those on hemodialysis.1 Patients with spontaneous retroperitoneal bleeding present with a variety of symptoms including abdominal pain, hip and upper thigh pain, back pain, and hypotension.2,3 In one observational cohort study, the nonspecific nature of symptoms led to misdiagnosis in 10.1% of the cases.3 The patient in this case exhibited several of the abovementioned symptoms and complaints.
The literature concerning spontaneous retroperitoneal bleeding is limited and consists of mainly case reports and case series. Warfarin, clopidogrel, unfractionated and low-molecular weight heparin use have all been reported in patients with retroperitoneal bleeding,4 and various studies have placed the incidence of this distinct entity to be between 0.6% to 6.6%.5,6
Spontaneous Versus Iatrogenic Retroperitoneal Bleeding
By definition, spontaneous retroperitoneal bleeding occurs without trauma, surgery, invasive procedures, and abdominal aortic aneurysm.3 Iatrogenic retroperitoneal bleeding, however, is a rare but known complication of catheterization procedures involving the femoral region, with an increased risk in female patients.7 Based on this increased incidence, the patient in this case was specifically asked whether she had undergone any procedures during her latest admission, including cardiac catheterization, which she denied.
In contrast, spontaneous retroperitoneal hemorrhage has an unclear pathogenesis. Some authors have suggested unrecognized minor trauma such as coughing as a possible cause.8 The patient in this case was asked on hospital day 2 if she had experienced a history of cough the week prior to presentation to the ED, to which she stated she had not.
Risk Factors
More recent case reports focus on renal or adrenal tumors as a potential source of spontaneous retroperitoneal bleeding. Although this patient did have a renal cyst in left kidney, its contribution to the retroperitoneal bleed is unknown and thought to be only incidental.
A retrospective chart review of 119 identified patients with spontaneous retroperitoneal bleeds sought to identify reliable predictors of early diagnosis. Ivascu et al2 found that elderly patients on both anticoagulation and antiplatelet therapy were at the highest risk. Shah et al9 echoed the sentiment of severity in their retrospective review which showed that those on combined anticoagulant and antiplatelet therapy were more likely to require intensive care unit (ICU) admission and had longer ICU stays.
Conclusion
Unlike hemorrhages in other locations in the body, retroperitoneal bleeding can be difficult to diagnose since patients often present with nonspecific symptoms such as lower abdominal pain. Clinicians should therefore maintain a high index of suspicion, especially in patients who are on anticoagulation therapy, who have a coagulopathy, or who are on hemodialysis. Delay or failure to diagnose this condition may lead to significant morbidity and mortality.
Dr Lui is a resident, department of emergency medicine, Henry Ford Wyandotte Hospital, Wyandotte, Michigan. Dr Boehm is the emergency medicine residency program director in the graduate medical education department, Saint Mary Mercy Hospital, livonia, Michigan; and is an emergency physician at the Emergency physicians Medical Group, ann arbor, Michigan.
- Bhasin HK, Dana CL. Spontaneous retroperitoneal hemorrhage in chronically hemodialyzed patients. Nephron. 1978; 22(4-6):322-327.
- Ivascu FA, Janczyk RJ, Bair HA, Bendick PJ, Howells GA. Spontaneous retroperitoneal hemorrhage. Am J Surg. 2005;189(3):345-347.
- Sunga, KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emer Med. 2012;43(2):e157-e161.
- Ernits M, Mohan PS, Fares LG 2nd, Hardy H 3rd. A retroperitoneal bleed induced by enoxaparin therapy. Am Surg. 2005;71(5):430-433.
- Mant MJ, O’Brien BD, Thong KL, Hammond GW, Birtwhistle RV, Grace MG. Haemorrhagic complications of heparin therapy. Lancet. 1977;1(8022):1133-1135.
- Forfar JC. A 7-year analysis of haemorrhage in patients on long-term anticoagulant treatment. Br Heart J. 1979;42(2):128-132.
- Sajnani N, Bogart DB. Retroperitoneal hemorrhage as a complication of percutaneous intervention: report of 2 cases and review of the literature. Open Cardiovasc Med J. 2013;7:16-22.
- Berná JD, Zuazu I, Madrigal M, García-Medina V, Fernández C, Guirado F. Conservative treatment of large rectus sheath hematoma in patients undergoing anticoagulant therapy. Abdom Imaging. 2000;25(3):230-234.
- Shah, RD, Nagar S, Shanley CJ, Janczyk RJ. Factors affecting the severity of spontaneous retroperitoneal hemorrhage in anticoagulated patients. Am J Surg.
- Bhasin HK, Dana CL. Spontaneous retroperitoneal hemorrhage in chronically hemodialyzed patients. Nephron. 1978; 22(4-6):322-327.
- Ivascu FA, Janczyk RJ, Bair HA, Bendick PJ, Howells GA. Spontaneous retroperitoneal hemorrhage. Am J Surg. 2005;189(3):345-347.
- Sunga, KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emer Med. 2012;43(2):e157-e161.
- Ernits M, Mohan PS, Fares LG 2nd, Hardy H 3rd. A retroperitoneal bleed induced by enoxaparin therapy. Am Surg. 2005;71(5):430-433.
- Mant MJ, O’Brien BD, Thong KL, Hammond GW, Birtwhistle RV, Grace MG. Haemorrhagic complications of heparin therapy. Lancet. 1977;1(8022):1133-1135.
- Forfar JC. A 7-year analysis of haemorrhage in patients on long-term anticoagulant treatment. Br Heart J. 1979;42(2):128-132.
- Sajnani N, Bogart DB. Retroperitoneal hemorrhage as a complication of percutaneous intervention: report of 2 cases and review of the literature. Open Cardiovasc Med J. 2013;7:16-22.
- Berná JD, Zuazu I, Madrigal M, García-Medina V, Fernández C, Guirado F. Conservative treatment of large rectus sheath hematoma in patients undergoing anticoagulant therapy. Abdom Imaging. 2000;25(3):230-234.
- Shah, RD, Nagar S, Shanley CJ, Janczyk RJ. Factors affecting the severity of spontaneous retroperitoneal hemorrhage in anticoagulated patients. Am J Surg.