User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Sport climbing tied to improved posture in Parkinson’s disease
In a randomized controlled study, those who participated in scaling a wall using ropes and fixed anchors were less stooped at 12 weeks than was a control group that participated in some form of unsupervised physical activity.
The results underscore that it is never too late to learn a new sport or type of movement – and that this type of intervention may have big health payoffs, said study investigator Heidemarie Zach, MD, associate professor of neurology, Medical University of Vienna, Austria.
“There’s no hurdle too high over which you can’t climb, or burden you can’t conquer,” said Dr. Zach. “As long as you can walk independently and walk up a stair, you can go climbing.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Common feature of Parkinson’s disease
The analysis is part of a larger project that included a 2021 study showing a reduced Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) score by almost 13 points in patients who participated in sport climbing. The activity was also significantly associated with improved bradykinesia, rigidity, and tremor.
The current analysis focused on stooped posture, which in addition to motor symptoms is a common feature of Parkinson’s disease. This postural deformity can result in significant discomfort, pain, and decreased quality of life.
Pharmaceutical treatments are mostly ineffective for postural deformities, the researchers noted. Physical therapy may help improve symptoms, but only a few randomized studies have examined improved posture in patients with Parkinson’s disease using physiotherapy in general and alternative sports in particular.
Sport climbing is “really unique” in Parkinson’s disease, said Dr. Zach, who has yet to come across other research on this intervention. A climber herself, she recommended it to one of her patients: A 79-year old man with Parkinson’s disease who was a walker and hiker, and who ended up loving the sport. She called him her “pilot patient.”
The single-center study included 48 adult participants up to age 78 years (mean age, 65) with mild to moderate Parkinson’s disease. Most were at Hoehn & Yahr stage 2, with some at stage 3. All had no previous climbing experience. Exclusion criteria included having a condition other than Parkinson’s disease.
The researchers randomly assigned participants to a sport climbing course or to a control group.
The sport climbing group had a 90-minute climbing session each week for 12 weeks in an indoor gym. Under the supervision of an instructor, they were harnessed and connected to ropes with mats placed on the ground for safety.
The climbing wall was about 15 meters (50 feet) high. Participants typically started at 2 or 3 meters (6.5 to 9.5 feet) and worked their way up, Dr. Zach noted.
Those in the control group were asked to participate for 12 weeks in unsupervised physical activity, as recommended by the World Health Organization and the European Physiotherapy Guidelines for Parkinson’s Disease. This included at least 2.5 hours of moderate-intensity activity or 75 minutes of vigorous activity each week.
Whole-body workout
The primary outcome was improvement in posture, measured using a “simple” but highly reliable tool, said Dr. Zach. While the patients stood with their backs straight against a wall, researchers measured the distance in centimeters between the C7 sagittal vertical axis (C7SVA) and the wall.
The mean C7SVA at baseline did not significantly differ between the two groups, at 8.2 cm for the climbing group versus 7.7 cm for the control group. However, results showed only sport climbing was associated with significantly lessened forward flexion of the cervical spine.
The climbing group showed a decrease of the C7SVA by 1.7 cm (95% confidence interval [CI], 0.8-2.6 cm). “So climbers were more erect and less stooped after 12 weeks,” Dr. Zach said.
She noted that the mean difference in the control group was 0.5 cm (95% confidence interval [CI], –0.2 to 1.3 cm), which “is almost nothing.”
There did not seem to be any predictor, such as age, sex, or body mass index, for what patient subgroups benefit the most from the intervention, Dr. Zach noted.
In explaining why climbing helps posture, she said it is akin to “a whole-body workout.” The activity increases upper-body strength by using back and shoulder girdle muscles, as well as joint flexibility, Dr. Zach noted. Movements involved in climbing, such as repeated reaching for a distant hold, stretch the muscles of the hip flexors and hip.
As these movements reduce rigidity, the climbing action may also promote an upright posture. And as wall climbing involves planning and executing movements, it trains spatial body awareness, an important component of maintaining and correcting posture, she said.
Dr. Zach noted a motivational group dynamic likely also contributed to the success of the intervention. “They were cheering each other at the bottom” of the climbing wall, she said.
The results show that posture can be added to the improvements in Parkinson’s disease already documented from climbing, including improved motor symptoms, rigidity, and tremor, she said. The next step on the research agenda is to show whether the intervention has a positive impact on gait, Dr. Zach added.
‘Quite adventurous’
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer at the American Parkinson Disease Association, said she welcomes “any new idea” to help patients with Parkinson’s disease – and that sport climbing sounds “quite adventurous.”
“The general concept that you’re asking the body to move in a novel way is a good thing for everyone and especially for people with Parkinson’s disease,” said Dr. Gilbert, who was not involved with the research.
She noted that in Parkinson’s disease, an ideal exercise intervention includes a combination of four modalities: stretching, balance, aerobics, and strengthening. Rope climbing involves many of these, in addition to a cognitive element, Dr. Gilbert said. It’s also important that patients with Parkinson’s disease participate in an activity they enjoy, she added.
However, she stressed that safety has to be “weighed,” especially for patients with stage 3 Parkinson’s disease, who often have balance problems. “It may be difficult to climb a rope if you have balance problems,” Dr. Gilbert said. “The intervention needs to be tailored to the existing disability, and perhaps this activity is more a reasonable thing for patients at milder stages.”
Dr. Zach and Dr. Gilbert have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, those who participated in scaling a wall using ropes and fixed anchors were less stooped at 12 weeks than was a control group that participated in some form of unsupervised physical activity.
The results underscore that it is never too late to learn a new sport or type of movement – and that this type of intervention may have big health payoffs, said study investigator Heidemarie Zach, MD, associate professor of neurology, Medical University of Vienna, Austria.
“There’s no hurdle too high over which you can’t climb, or burden you can’t conquer,” said Dr. Zach. “As long as you can walk independently and walk up a stair, you can go climbing.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Common feature of Parkinson’s disease
The analysis is part of a larger project that included a 2021 study showing a reduced Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) score by almost 13 points in patients who participated in sport climbing. The activity was also significantly associated with improved bradykinesia, rigidity, and tremor.
The current analysis focused on stooped posture, which in addition to motor symptoms is a common feature of Parkinson’s disease. This postural deformity can result in significant discomfort, pain, and decreased quality of life.
Pharmaceutical treatments are mostly ineffective for postural deformities, the researchers noted. Physical therapy may help improve symptoms, but only a few randomized studies have examined improved posture in patients with Parkinson’s disease using physiotherapy in general and alternative sports in particular.
Sport climbing is “really unique” in Parkinson’s disease, said Dr. Zach, who has yet to come across other research on this intervention. A climber herself, she recommended it to one of her patients: A 79-year old man with Parkinson’s disease who was a walker and hiker, and who ended up loving the sport. She called him her “pilot patient.”
The single-center study included 48 adult participants up to age 78 years (mean age, 65) with mild to moderate Parkinson’s disease. Most were at Hoehn & Yahr stage 2, with some at stage 3. All had no previous climbing experience. Exclusion criteria included having a condition other than Parkinson’s disease.
The researchers randomly assigned participants to a sport climbing course or to a control group.
The sport climbing group had a 90-minute climbing session each week for 12 weeks in an indoor gym. Under the supervision of an instructor, they were harnessed and connected to ropes with mats placed on the ground for safety.
The climbing wall was about 15 meters (50 feet) high. Participants typically started at 2 or 3 meters (6.5 to 9.5 feet) and worked their way up, Dr. Zach noted.
Those in the control group were asked to participate for 12 weeks in unsupervised physical activity, as recommended by the World Health Organization and the European Physiotherapy Guidelines for Parkinson’s Disease. This included at least 2.5 hours of moderate-intensity activity or 75 minutes of vigorous activity each week.
Whole-body workout
The primary outcome was improvement in posture, measured using a “simple” but highly reliable tool, said Dr. Zach. While the patients stood with their backs straight against a wall, researchers measured the distance in centimeters between the C7 sagittal vertical axis (C7SVA) and the wall.
The mean C7SVA at baseline did not significantly differ between the two groups, at 8.2 cm for the climbing group versus 7.7 cm for the control group. However, results showed only sport climbing was associated with significantly lessened forward flexion of the cervical spine.
The climbing group showed a decrease of the C7SVA by 1.7 cm (95% confidence interval [CI], 0.8-2.6 cm). “So climbers were more erect and less stooped after 12 weeks,” Dr. Zach said.
She noted that the mean difference in the control group was 0.5 cm (95% confidence interval [CI], –0.2 to 1.3 cm), which “is almost nothing.”
There did not seem to be any predictor, such as age, sex, or body mass index, for what patient subgroups benefit the most from the intervention, Dr. Zach noted.
In explaining why climbing helps posture, she said it is akin to “a whole-body workout.” The activity increases upper-body strength by using back and shoulder girdle muscles, as well as joint flexibility, Dr. Zach noted. Movements involved in climbing, such as repeated reaching for a distant hold, stretch the muscles of the hip flexors and hip.
As these movements reduce rigidity, the climbing action may also promote an upright posture. And as wall climbing involves planning and executing movements, it trains spatial body awareness, an important component of maintaining and correcting posture, she said.
Dr. Zach noted a motivational group dynamic likely also contributed to the success of the intervention. “They were cheering each other at the bottom” of the climbing wall, she said.
The results show that posture can be added to the improvements in Parkinson’s disease already documented from climbing, including improved motor symptoms, rigidity, and tremor, she said. The next step on the research agenda is to show whether the intervention has a positive impact on gait, Dr. Zach added.
‘Quite adventurous’
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer at the American Parkinson Disease Association, said she welcomes “any new idea” to help patients with Parkinson’s disease – and that sport climbing sounds “quite adventurous.”
“The general concept that you’re asking the body to move in a novel way is a good thing for everyone and especially for people with Parkinson’s disease,” said Dr. Gilbert, who was not involved with the research.
She noted that in Parkinson’s disease, an ideal exercise intervention includes a combination of four modalities: stretching, balance, aerobics, and strengthening. Rope climbing involves many of these, in addition to a cognitive element, Dr. Gilbert said. It’s also important that patients with Parkinson’s disease participate in an activity they enjoy, she added.
However, she stressed that safety has to be “weighed,” especially for patients with stage 3 Parkinson’s disease, who often have balance problems. “It may be difficult to climb a rope if you have balance problems,” Dr. Gilbert said. “The intervention needs to be tailored to the existing disability, and perhaps this activity is more a reasonable thing for patients at milder stages.”
Dr. Zach and Dr. Gilbert have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, those who participated in scaling a wall using ropes and fixed anchors were less stooped at 12 weeks than was a control group that participated in some form of unsupervised physical activity.
The results underscore that it is never too late to learn a new sport or type of movement – and that this type of intervention may have big health payoffs, said study investigator Heidemarie Zach, MD, associate professor of neurology, Medical University of Vienna, Austria.
“There’s no hurdle too high over which you can’t climb, or burden you can’t conquer,” said Dr. Zach. “As long as you can walk independently and walk up a stair, you can go climbing.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Common feature of Parkinson’s disease
The analysis is part of a larger project that included a 2021 study showing a reduced Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) score by almost 13 points in patients who participated in sport climbing. The activity was also significantly associated with improved bradykinesia, rigidity, and tremor.
The current analysis focused on stooped posture, which in addition to motor symptoms is a common feature of Parkinson’s disease. This postural deformity can result in significant discomfort, pain, and decreased quality of life.
Pharmaceutical treatments are mostly ineffective for postural deformities, the researchers noted. Physical therapy may help improve symptoms, but only a few randomized studies have examined improved posture in patients with Parkinson’s disease using physiotherapy in general and alternative sports in particular.
Sport climbing is “really unique” in Parkinson’s disease, said Dr. Zach, who has yet to come across other research on this intervention. A climber herself, she recommended it to one of her patients: A 79-year old man with Parkinson’s disease who was a walker and hiker, and who ended up loving the sport. She called him her “pilot patient.”
The single-center study included 48 adult participants up to age 78 years (mean age, 65) with mild to moderate Parkinson’s disease. Most were at Hoehn & Yahr stage 2, with some at stage 3. All had no previous climbing experience. Exclusion criteria included having a condition other than Parkinson’s disease.
The researchers randomly assigned participants to a sport climbing course or to a control group.
The sport climbing group had a 90-minute climbing session each week for 12 weeks in an indoor gym. Under the supervision of an instructor, they were harnessed and connected to ropes with mats placed on the ground for safety.
The climbing wall was about 15 meters (50 feet) high. Participants typically started at 2 or 3 meters (6.5 to 9.5 feet) and worked their way up, Dr. Zach noted.
Those in the control group were asked to participate for 12 weeks in unsupervised physical activity, as recommended by the World Health Organization and the European Physiotherapy Guidelines for Parkinson’s Disease. This included at least 2.5 hours of moderate-intensity activity or 75 minutes of vigorous activity each week.
Whole-body workout
The primary outcome was improvement in posture, measured using a “simple” but highly reliable tool, said Dr. Zach. While the patients stood with their backs straight against a wall, researchers measured the distance in centimeters between the C7 sagittal vertical axis (C7SVA) and the wall.
The mean C7SVA at baseline did not significantly differ between the two groups, at 8.2 cm for the climbing group versus 7.7 cm for the control group. However, results showed only sport climbing was associated with significantly lessened forward flexion of the cervical spine.
The climbing group showed a decrease of the C7SVA by 1.7 cm (95% confidence interval [CI], 0.8-2.6 cm). “So climbers were more erect and less stooped after 12 weeks,” Dr. Zach said.
She noted that the mean difference in the control group was 0.5 cm (95% confidence interval [CI], –0.2 to 1.3 cm), which “is almost nothing.”
There did not seem to be any predictor, such as age, sex, or body mass index, for what patient subgroups benefit the most from the intervention, Dr. Zach noted.
In explaining why climbing helps posture, she said it is akin to “a whole-body workout.” The activity increases upper-body strength by using back and shoulder girdle muscles, as well as joint flexibility, Dr. Zach noted. Movements involved in climbing, such as repeated reaching for a distant hold, stretch the muscles of the hip flexors and hip.
As these movements reduce rigidity, the climbing action may also promote an upright posture. And as wall climbing involves planning and executing movements, it trains spatial body awareness, an important component of maintaining and correcting posture, she said.
Dr. Zach noted a motivational group dynamic likely also contributed to the success of the intervention. “They were cheering each other at the bottom” of the climbing wall, she said.
The results show that posture can be added to the improvements in Parkinson’s disease already documented from climbing, including improved motor symptoms, rigidity, and tremor, she said. The next step on the research agenda is to show whether the intervention has a positive impact on gait, Dr. Zach added.
‘Quite adventurous’
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer at the American Parkinson Disease Association, said she welcomes “any new idea” to help patients with Parkinson’s disease – and that sport climbing sounds “quite adventurous.”
“The general concept that you’re asking the body to move in a novel way is a good thing for everyone and especially for people with Parkinson’s disease,” said Dr. Gilbert, who was not involved with the research.
She noted that in Parkinson’s disease, an ideal exercise intervention includes a combination of four modalities: stretching, balance, aerobics, and strengthening. Rope climbing involves many of these, in addition to a cognitive element, Dr. Gilbert said. It’s also important that patients with Parkinson’s disease participate in an activity they enjoy, she added.
However, she stressed that safety has to be “weighed,” especially for patients with stage 3 Parkinson’s disease, who often have balance problems. “It may be difficult to climb a rope if you have balance problems,” Dr. Gilbert said. “The intervention needs to be tailored to the existing disability, and perhaps this activity is more a reasonable thing for patients at milder stages.”
Dr. Zach and Dr. Gilbert have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS 2022
Weight gain linked to cancer survival in men and women
Cancer cachexia is a syndrome of weight loss that frequently occurs during cancer treatment. Consequences can include skeletal muscle loss, fatigue, functional impairment, worse quality of life, and worse survival. On the other hand, weight gain during cancer treatment has been tied to better survival.
Few studies have examined the relationship between weight gain and outcomes by sex.
“The finding that weight gain occurred in subsets of males and females is a new observation. The fact that weight gain occurs in cancer patients during anticancer treatment could confound results of clinical [trials] evaluating novel anticachexia treatments. Simultaneously studying longitudinal body weights and serum and cellular biomarkers in cancer patients might provide insights into mechanisms involved in cachexia. Increased understanding of mechanisms driving cachexia could lead to new therapeutic strategies,” said study coauthor Philip Bonomi, MD, who is an oncologist at Rush Medical College, Chicago.
“This data, although it appears to be very basic, is critically important, especially as we consider our novel interventions in the treatment of cancer cachexia,” said Eric Roeland, MD, during his presentation of the study at the annual meeting of European Society for Medical Oncology. Dr. Roeland is a medical oncologist at Oregon Health & Science University, Portland.
Dr. Roeland is also the lead author of cancer cachexia guidelines published by the American Society of Clinical Oncology in 2020. The guidelines suggest that dietary counseling can be offered to patients, but warns against routine use of enteral feeding tubes and parenteral nutrition. Although no specific drug can be recommended for cancer cachexia, progesterone analogs and corticosteroids used over the short term (weeks) can be used on a trial base to improve appetite and weight gain. While not approved in the United States, anamorelin was approved in 2020 in Japan for cancer cachexia in NSCLC, gastric cancer, pancreatic cancer, and colorectal cancer.
The new study should raise awareness of the importance of adverse effects of cancer treatments, said Karin Jordan, MD, University Hospital Heidelberg (Germany). She served as a discussant following the presentation. “As a medical oncologist, we focus a bit too much on the benefits of antineoplastic therapy, both on cure and on the survival benefit. But what is also very, very important to do is a balanced oncology treatment to focus on the risks of oncology therapies,” she said.
The study is limited by its retrospective nature and potential for bias. “The hypothesis that weight gain leads to improved survival is not really proven as it likewise may be the other way around,” Dr. Jordan said.
However, in oncology research, a phenomenon called the “obesity paradox” is increasingly catching the interest of investigators. Observational studies have shown that overweight patients with certain cancers (specifically, colorectal, endometrial and lung cancer). actually have improved overall survival as compared with normal-weight patients.
Details from the new study
The researchers pooled data 1,030 patients who participated in three phase 3 clinical trials conducted between 2005 and 2011. The patients all received platinum-based chemotherapy as part of control arms. 304 were female and 726 were male. The median age was 62. 16.7% were Asian, the mean body mass index was 24.6 kg/m2, 88.5% had stage 4 disease, 36.9% had adenocarcinoma, and 86.3% were current or former smokers.
Males and females had similar magnitudes and rate of weight gain over the course of treatment. Any weight gain was associated with improved overall survival in both males (12.7 vs. 8.0 months; hazard ratio, 0.60; P < .001) and females (16.2 vs. 10.1 months; HR, 0.65; P = .0028). Patients who had a weight gain of 2.5% of body weight or more saw an improvement in overall survival in both males (14.0 vs. 8.2 months; HR, 0.57; P < .001) and females (16.7 vs. 11.3 months; HR, 0.61; P = .0041).
Patients with a weight gain of 5% or more was associated with improved survival in males (13.6 vs. 8.9 months; HR, 0.62; P = .0001), but there was no statistically significant association in females (16.7 vs. 12.6 months; HR, 0.69; P = .1107).
Regardless of weight-gain status, males had lower survival rates than females. All of the associations were independent of smoking status.
The study was funded by Pfizer. Dr. Bonomi has received honoraria from Pfizer and Helsinn for participation in scientific advisory boards. Dr. Jordan has consulted for Amgen, Hexal, Riemser, Helsinn, Voluntis, Pfizer, and BD Solution. She has received research funding from Deutsche Krebshilfe. She has received honoraria from MSD, Merck, Amgen, Hexal, Riemser, Helsinn, Voluntis, Pfizer, Pomme-med, PharmaMar, arttemoi, OnkoUpdate, Stemline, and Roche.
Cancer cachexia is a syndrome of weight loss that frequently occurs during cancer treatment. Consequences can include skeletal muscle loss, fatigue, functional impairment, worse quality of life, and worse survival. On the other hand, weight gain during cancer treatment has been tied to better survival.
Few studies have examined the relationship between weight gain and outcomes by sex.
“The finding that weight gain occurred in subsets of males and females is a new observation. The fact that weight gain occurs in cancer patients during anticancer treatment could confound results of clinical [trials] evaluating novel anticachexia treatments. Simultaneously studying longitudinal body weights and serum and cellular biomarkers in cancer patients might provide insights into mechanisms involved in cachexia. Increased understanding of mechanisms driving cachexia could lead to new therapeutic strategies,” said study coauthor Philip Bonomi, MD, who is an oncologist at Rush Medical College, Chicago.
“This data, although it appears to be very basic, is critically important, especially as we consider our novel interventions in the treatment of cancer cachexia,” said Eric Roeland, MD, during his presentation of the study at the annual meeting of European Society for Medical Oncology. Dr. Roeland is a medical oncologist at Oregon Health & Science University, Portland.
Dr. Roeland is also the lead author of cancer cachexia guidelines published by the American Society of Clinical Oncology in 2020. The guidelines suggest that dietary counseling can be offered to patients, but warns against routine use of enteral feeding tubes and parenteral nutrition. Although no specific drug can be recommended for cancer cachexia, progesterone analogs and corticosteroids used over the short term (weeks) can be used on a trial base to improve appetite and weight gain. While not approved in the United States, anamorelin was approved in 2020 in Japan for cancer cachexia in NSCLC, gastric cancer, pancreatic cancer, and colorectal cancer.
The new study should raise awareness of the importance of adverse effects of cancer treatments, said Karin Jordan, MD, University Hospital Heidelberg (Germany). She served as a discussant following the presentation. “As a medical oncologist, we focus a bit too much on the benefits of antineoplastic therapy, both on cure and on the survival benefit. But what is also very, very important to do is a balanced oncology treatment to focus on the risks of oncology therapies,” she said.
The study is limited by its retrospective nature and potential for bias. “The hypothesis that weight gain leads to improved survival is not really proven as it likewise may be the other way around,” Dr. Jordan said.
However, in oncology research, a phenomenon called the “obesity paradox” is increasingly catching the interest of investigators. Observational studies have shown that overweight patients with certain cancers (specifically, colorectal, endometrial and lung cancer). actually have improved overall survival as compared with normal-weight patients.
Details from the new study
The researchers pooled data 1,030 patients who participated in three phase 3 clinical trials conducted between 2005 and 2011. The patients all received platinum-based chemotherapy as part of control arms. 304 were female and 726 were male. The median age was 62. 16.7% were Asian, the mean body mass index was 24.6 kg/m2, 88.5% had stage 4 disease, 36.9% had adenocarcinoma, and 86.3% were current or former smokers.
Males and females had similar magnitudes and rate of weight gain over the course of treatment. Any weight gain was associated with improved overall survival in both males (12.7 vs. 8.0 months; hazard ratio, 0.60; P < .001) and females (16.2 vs. 10.1 months; HR, 0.65; P = .0028). Patients who had a weight gain of 2.5% of body weight or more saw an improvement in overall survival in both males (14.0 vs. 8.2 months; HR, 0.57; P < .001) and females (16.7 vs. 11.3 months; HR, 0.61; P = .0041).
Patients with a weight gain of 5% or more was associated with improved survival in males (13.6 vs. 8.9 months; HR, 0.62; P = .0001), but there was no statistically significant association in females (16.7 vs. 12.6 months; HR, 0.69; P = .1107).
Regardless of weight-gain status, males had lower survival rates than females. All of the associations were independent of smoking status.
The study was funded by Pfizer. Dr. Bonomi has received honoraria from Pfizer and Helsinn for participation in scientific advisory boards. Dr. Jordan has consulted for Amgen, Hexal, Riemser, Helsinn, Voluntis, Pfizer, and BD Solution. She has received research funding from Deutsche Krebshilfe. She has received honoraria from MSD, Merck, Amgen, Hexal, Riemser, Helsinn, Voluntis, Pfizer, Pomme-med, PharmaMar, arttemoi, OnkoUpdate, Stemline, and Roche.
Cancer cachexia is a syndrome of weight loss that frequently occurs during cancer treatment. Consequences can include skeletal muscle loss, fatigue, functional impairment, worse quality of life, and worse survival. On the other hand, weight gain during cancer treatment has been tied to better survival.
Few studies have examined the relationship between weight gain and outcomes by sex.
“The finding that weight gain occurred in subsets of males and females is a new observation. The fact that weight gain occurs in cancer patients during anticancer treatment could confound results of clinical [trials] evaluating novel anticachexia treatments. Simultaneously studying longitudinal body weights and serum and cellular biomarkers in cancer patients might provide insights into mechanisms involved in cachexia. Increased understanding of mechanisms driving cachexia could lead to new therapeutic strategies,” said study coauthor Philip Bonomi, MD, who is an oncologist at Rush Medical College, Chicago.
“This data, although it appears to be very basic, is critically important, especially as we consider our novel interventions in the treatment of cancer cachexia,” said Eric Roeland, MD, during his presentation of the study at the annual meeting of European Society for Medical Oncology. Dr. Roeland is a medical oncologist at Oregon Health & Science University, Portland.
Dr. Roeland is also the lead author of cancer cachexia guidelines published by the American Society of Clinical Oncology in 2020. The guidelines suggest that dietary counseling can be offered to patients, but warns against routine use of enteral feeding tubes and parenteral nutrition. Although no specific drug can be recommended for cancer cachexia, progesterone analogs and corticosteroids used over the short term (weeks) can be used on a trial base to improve appetite and weight gain. While not approved in the United States, anamorelin was approved in 2020 in Japan for cancer cachexia in NSCLC, gastric cancer, pancreatic cancer, and colorectal cancer.
The new study should raise awareness of the importance of adverse effects of cancer treatments, said Karin Jordan, MD, University Hospital Heidelberg (Germany). She served as a discussant following the presentation. “As a medical oncologist, we focus a bit too much on the benefits of antineoplastic therapy, both on cure and on the survival benefit. But what is also very, very important to do is a balanced oncology treatment to focus on the risks of oncology therapies,” she said.
The study is limited by its retrospective nature and potential for bias. “The hypothesis that weight gain leads to improved survival is not really proven as it likewise may be the other way around,” Dr. Jordan said.
However, in oncology research, a phenomenon called the “obesity paradox” is increasingly catching the interest of investigators. Observational studies have shown that overweight patients with certain cancers (specifically, colorectal, endometrial and lung cancer). actually have improved overall survival as compared with normal-weight patients.
Details from the new study
The researchers pooled data 1,030 patients who participated in three phase 3 clinical trials conducted between 2005 and 2011. The patients all received platinum-based chemotherapy as part of control arms. 304 were female and 726 were male. The median age was 62. 16.7% were Asian, the mean body mass index was 24.6 kg/m2, 88.5% had stage 4 disease, 36.9% had adenocarcinoma, and 86.3% were current or former smokers.
Males and females had similar magnitudes and rate of weight gain over the course of treatment. Any weight gain was associated with improved overall survival in both males (12.7 vs. 8.0 months; hazard ratio, 0.60; P < .001) and females (16.2 vs. 10.1 months; HR, 0.65; P = .0028). Patients who had a weight gain of 2.5% of body weight or more saw an improvement in overall survival in both males (14.0 vs. 8.2 months; HR, 0.57; P < .001) and females (16.7 vs. 11.3 months; HR, 0.61; P = .0041).
Patients with a weight gain of 5% or more was associated with improved survival in males (13.6 vs. 8.9 months; HR, 0.62; P = .0001), but there was no statistically significant association in females (16.7 vs. 12.6 months; HR, 0.69; P = .1107).
Regardless of weight-gain status, males had lower survival rates than females. All of the associations were independent of smoking status.
The study was funded by Pfizer. Dr. Bonomi has received honoraria from Pfizer and Helsinn for participation in scientific advisory boards. Dr. Jordan has consulted for Amgen, Hexal, Riemser, Helsinn, Voluntis, Pfizer, and BD Solution. She has received research funding from Deutsche Krebshilfe. She has received honoraria from MSD, Merck, Amgen, Hexal, Riemser, Helsinn, Voluntis, Pfizer, Pomme-med, PharmaMar, arttemoi, OnkoUpdate, Stemline, and Roche.
FROM ESMO CONGRESS 2022
Is vitamin B12 protective against Parkinson’s disease?
A high baseline intake of vitamin B12 is linked to lower risk of developing Parkinson’s disease, new research suggests. “The results leave the door open for the possibility that vitamin B12 may have a beneficial effect in protecting against Parkinson’s disease,” said lead author Mario H. Flores, PhD, a research fellow at Harvard T.H. Chan School of Public Health, Boston.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
B vitamins and Parkinson’s disease
Previous preclinical studies have suggested that B vitamins protect against Parkinson’s disease by decreasing plasma homocysteine levels and through other neuroprotective effects. However, there have been only two epidemiologic studies of B vitamins in Parkinson’s disease – and their results were inconsistent, Dr. Flores noted.
The new study included 80,965 women from the Nurses’ Health Study and 48,837 men from the Health Professionals Follow-up Study. All completed a food frequency questionnaire at baseline and every 4 years.
Researchers collected information on dietary, supplemental, and total intake of folate, vitamin B6, and vitamin B12 over the course of about 30 years up to 2012. They estimated hazard ratios and 95% confidence intervals for Parkinson’s disease according to quintiles of cumulative average intake.
During follow-up, 495 women and 621 men were diagnosed with Parkinson’s disease.
The investigators adjusted for potential confounders, including age, year, smoking status, physical activity, intake of alcohol or caffeine, hormone use (in women), intake of dairy and flavonoids, and Mediterranean diet score.
Analyses of cumulative average total folate, B6, and B12 intake were not associated with Parkinson’s disease risk. “The results of the primary analysis of cumulative intake were not significant for any of the vitamins we looked at,” said Dr. Flores.
Researchers also conducted secondary analyses, including assessment of how the most recent intake of B vitamins related to Parkinson’s disease risk. This analysis also did not find a significant association.
However, when examining baseline intake of vitamin B12, “we saw some suggestion for a potential inverse association with Parkinson’s disease,” Dr. Flores said.
Among individuals with higher total intake of vitamin B12, there was a lower risk for Parkinson’s disease (pooled hazard ratio for top vs. bottom quintile, 0.74; 95% confidence interval [CI], 0.60-0.89; P for trend, .001). Intake from both diet and supplements contributed to this inverse association, the investigators noted.
Dietary sources of vitamin B12 include poultry, meat, fish, and dairy products; however, the main sources in this study were multivitamins/supplements and enriched foods such as cereals, said Dr. Flores.
Several limitations
In an attempt to overcome risk for reverse causality, the researchers examined B12 intake during four lagged exposure periods: 8-, 12-, 16- and 20-year lags. They found a significant relationship between intake for the 20-year lag time and development of Parkinson’s disease.
Overall, the study results provide support for a possible protective effect of early intake of vitamin B12 on the development of Parkinson’s disease, Dr. Flores noted.
In addition to being involved in the regulation of homocysteine levels, vitamin B12 may help regulate leucine-rich repeat kinase 2 (LRRK2), an enzyme implicated in the pathogenesis of Parkinson’s disease, he said.
However, the study did not examine how B12 deficiency might relate to risk for Parkinson’s disease, which “is something worth looking at in future studies,” said Dr. Flores.
He added that although findings from a single study cannot translate into recommendations on ideal vitamin B12 intake to prevent or delay Parkinson’s disease onset, the median intake in the highest quintile that the study linked to less Parkinson’s disease risk was 18 mcg/d at baseline. The amount in multivitamins can vary from 5 to 25 mcg.
Dr. Flores said a limitation of the study was that it included U.S. health care professionals, “most of whom arguably have very good nutritional status.”
As well, assessment of vitamin B intake was self-reported, so there might have been measurement error – and there may have been an unmeasured confounding factor that could explain the associations.
Dr. Flores also stressed that the effect of B12 on Parkinson’s disease risk “was very modest,” and the results need to be confirmed in other studies “ideally looking at circulating levels of vitamin B12.”
Not ready to recommend
Commenting on the research, Michael S. Okun, MD, medical adviser at the Parkinson’s Foundation and professor and director of the Norman Fixel Institute for Neurological Diseases at the University of Florida, Gainesville, noted that other recent studies have suggested high-dose B12 may be preventive and a possible treatment in Parkinson’s disease.
“Although only a secondary aim of the current study, there was a reported potential benefit” in this new study, too, said Dr. Okun, who was not involved with the research.
However, the evidence is still not strong enough to change prescribing habits, he noted. “We do not recommend high-dose B12 either for those at genetic risk of Parkinson’s or those already with the disease,” Dr. Okun said.
He added that because multiple recent studies have questioned the beneficial effects for multivitamin combinations used to prevent neurologic diseases, “it wasn’t surprising to see results showing a lack of protection against later-onset Parkinson’s disease with [cumulative] folate, B6, and B12 intake” in the current study.
The study was supported by the NIH. Dr. Flores and Dr. Okun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A high baseline intake of vitamin B12 is linked to lower risk of developing Parkinson’s disease, new research suggests. “The results leave the door open for the possibility that vitamin B12 may have a beneficial effect in protecting against Parkinson’s disease,” said lead author Mario H. Flores, PhD, a research fellow at Harvard T.H. Chan School of Public Health, Boston.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
B vitamins and Parkinson’s disease
Previous preclinical studies have suggested that B vitamins protect against Parkinson’s disease by decreasing plasma homocysteine levels and through other neuroprotective effects. However, there have been only two epidemiologic studies of B vitamins in Parkinson’s disease – and their results were inconsistent, Dr. Flores noted.
The new study included 80,965 women from the Nurses’ Health Study and 48,837 men from the Health Professionals Follow-up Study. All completed a food frequency questionnaire at baseline and every 4 years.
Researchers collected information on dietary, supplemental, and total intake of folate, vitamin B6, and vitamin B12 over the course of about 30 years up to 2012. They estimated hazard ratios and 95% confidence intervals for Parkinson’s disease according to quintiles of cumulative average intake.
During follow-up, 495 women and 621 men were diagnosed with Parkinson’s disease.
The investigators adjusted for potential confounders, including age, year, smoking status, physical activity, intake of alcohol or caffeine, hormone use (in women), intake of dairy and flavonoids, and Mediterranean diet score.
Analyses of cumulative average total folate, B6, and B12 intake were not associated with Parkinson’s disease risk. “The results of the primary analysis of cumulative intake were not significant for any of the vitamins we looked at,” said Dr. Flores.
Researchers also conducted secondary analyses, including assessment of how the most recent intake of B vitamins related to Parkinson’s disease risk. This analysis also did not find a significant association.
However, when examining baseline intake of vitamin B12, “we saw some suggestion for a potential inverse association with Parkinson’s disease,” Dr. Flores said.
Among individuals with higher total intake of vitamin B12, there was a lower risk for Parkinson’s disease (pooled hazard ratio for top vs. bottom quintile, 0.74; 95% confidence interval [CI], 0.60-0.89; P for trend, .001). Intake from both diet and supplements contributed to this inverse association, the investigators noted.
Dietary sources of vitamin B12 include poultry, meat, fish, and dairy products; however, the main sources in this study were multivitamins/supplements and enriched foods such as cereals, said Dr. Flores.
Several limitations
In an attempt to overcome risk for reverse causality, the researchers examined B12 intake during four lagged exposure periods: 8-, 12-, 16- and 20-year lags. They found a significant relationship between intake for the 20-year lag time and development of Parkinson’s disease.
Overall, the study results provide support for a possible protective effect of early intake of vitamin B12 on the development of Parkinson’s disease, Dr. Flores noted.
In addition to being involved in the regulation of homocysteine levels, vitamin B12 may help regulate leucine-rich repeat kinase 2 (LRRK2), an enzyme implicated in the pathogenesis of Parkinson’s disease, he said.
However, the study did not examine how B12 deficiency might relate to risk for Parkinson’s disease, which “is something worth looking at in future studies,” said Dr. Flores.
He added that although findings from a single study cannot translate into recommendations on ideal vitamin B12 intake to prevent or delay Parkinson’s disease onset, the median intake in the highest quintile that the study linked to less Parkinson’s disease risk was 18 mcg/d at baseline. The amount in multivitamins can vary from 5 to 25 mcg.
Dr. Flores said a limitation of the study was that it included U.S. health care professionals, “most of whom arguably have very good nutritional status.”
As well, assessment of vitamin B intake was self-reported, so there might have been measurement error – and there may have been an unmeasured confounding factor that could explain the associations.
Dr. Flores also stressed that the effect of B12 on Parkinson’s disease risk “was very modest,” and the results need to be confirmed in other studies “ideally looking at circulating levels of vitamin B12.”
Not ready to recommend
Commenting on the research, Michael S. Okun, MD, medical adviser at the Parkinson’s Foundation and professor and director of the Norman Fixel Institute for Neurological Diseases at the University of Florida, Gainesville, noted that other recent studies have suggested high-dose B12 may be preventive and a possible treatment in Parkinson’s disease.
“Although only a secondary aim of the current study, there was a reported potential benefit” in this new study, too, said Dr. Okun, who was not involved with the research.
However, the evidence is still not strong enough to change prescribing habits, he noted. “We do not recommend high-dose B12 either for those at genetic risk of Parkinson’s or those already with the disease,” Dr. Okun said.
He added that because multiple recent studies have questioned the beneficial effects for multivitamin combinations used to prevent neurologic diseases, “it wasn’t surprising to see results showing a lack of protection against later-onset Parkinson’s disease with [cumulative] folate, B6, and B12 intake” in the current study.
The study was supported by the NIH. Dr. Flores and Dr. Okun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A high baseline intake of vitamin B12 is linked to lower risk of developing Parkinson’s disease, new research suggests. “The results leave the door open for the possibility that vitamin B12 may have a beneficial effect in protecting against Parkinson’s disease,” said lead author Mario H. Flores, PhD, a research fellow at Harvard T.H. Chan School of Public Health, Boston.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
B vitamins and Parkinson’s disease
Previous preclinical studies have suggested that B vitamins protect against Parkinson’s disease by decreasing plasma homocysteine levels and through other neuroprotective effects. However, there have been only two epidemiologic studies of B vitamins in Parkinson’s disease – and their results were inconsistent, Dr. Flores noted.
The new study included 80,965 women from the Nurses’ Health Study and 48,837 men from the Health Professionals Follow-up Study. All completed a food frequency questionnaire at baseline and every 4 years.
Researchers collected information on dietary, supplemental, and total intake of folate, vitamin B6, and vitamin B12 over the course of about 30 years up to 2012. They estimated hazard ratios and 95% confidence intervals for Parkinson’s disease according to quintiles of cumulative average intake.
During follow-up, 495 women and 621 men were diagnosed with Parkinson’s disease.
The investigators adjusted for potential confounders, including age, year, smoking status, physical activity, intake of alcohol or caffeine, hormone use (in women), intake of dairy and flavonoids, and Mediterranean diet score.
Analyses of cumulative average total folate, B6, and B12 intake were not associated with Parkinson’s disease risk. “The results of the primary analysis of cumulative intake were not significant for any of the vitamins we looked at,” said Dr. Flores.
Researchers also conducted secondary analyses, including assessment of how the most recent intake of B vitamins related to Parkinson’s disease risk. This analysis also did not find a significant association.
However, when examining baseline intake of vitamin B12, “we saw some suggestion for a potential inverse association with Parkinson’s disease,” Dr. Flores said.
Among individuals with higher total intake of vitamin B12, there was a lower risk for Parkinson’s disease (pooled hazard ratio for top vs. bottom quintile, 0.74; 95% confidence interval [CI], 0.60-0.89; P for trend, .001). Intake from both diet and supplements contributed to this inverse association, the investigators noted.
Dietary sources of vitamin B12 include poultry, meat, fish, and dairy products; however, the main sources in this study were multivitamins/supplements and enriched foods such as cereals, said Dr. Flores.
Several limitations
In an attempt to overcome risk for reverse causality, the researchers examined B12 intake during four lagged exposure periods: 8-, 12-, 16- and 20-year lags. They found a significant relationship between intake for the 20-year lag time and development of Parkinson’s disease.
Overall, the study results provide support for a possible protective effect of early intake of vitamin B12 on the development of Parkinson’s disease, Dr. Flores noted.
In addition to being involved in the regulation of homocysteine levels, vitamin B12 may help regulate leucine-rich repeat kinase 2 (LRRK2), an enzyme implicated in the pathogenesis of Parkinson’s disease, he said.
However, the study did not examine how B12 deficiency might relate to risk for Parkinson’s disease, which “is something worth looking at in future studies,” said Dr. Flores.
He added that although findings from a single study cannot translate into recommendations on ideal vitamin B12 intake to prevent or delay Parkinson’s disease onset, the median intake in the highest quintile that the study linked to less Parkinson’s disease risk was 18 mcg/d at baseline. The amount in multivitamins can vary from 5 to 25 mcg.
Dr. Flores said a limitation of the study was that it included U.S. health care professionals, “most of whom arguably have very good nutritional status.”
As well, assessment of vitamin B intake was self-reported, so there might have been measurement error – and there may have been an unmeasured confounding factor that could explain the associations.
Dr. Flores also stressed that the effect of B12 on Parkinson’s disease risk “was very modest,” and the results need to be confirmed in other studies “ideally looking at circulating levels of vitamin B12.”
Not ready to recommend
Commenting on the research, Michael S. Okun, MD, medical adviser at the Parkinson’s Foundation and professor and director of the Norman Fixel Institute for Neurological Diseases at the University of Florida, Gainesville, noted that other recent studies have suggested high-dose B12 may be preventive and a possible treatment in Parkinson’s disease.
“Although only a secondary aim of the current study, there was a reported potential benefit” in this new study, too, said Dr. Okun, who was not involved with the research.
However, the evidence is still not strong enough to change prescribing habits, he noted. “We do not recommend high-dose B12 either for those at genetic risk of Parkinson’s or those already with the disease,” Dr. Okun said.
He added that because multiple recent studies have questioned the beneficial effects for multivitamin combinations used to prevent neurologic diseases, “it wasn’t surprising to see results showing a lack of protection against later-onset Parkinson’s disease with [cumulative] folate, B6, and B12 intake” in the current study.
The study was supported by the NIH. Dr. Flores and Dr. Okun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS 2022
Legacy of neutral renal denervation trial recast by long-term outcomes: SYMPLICITY HTN-3
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.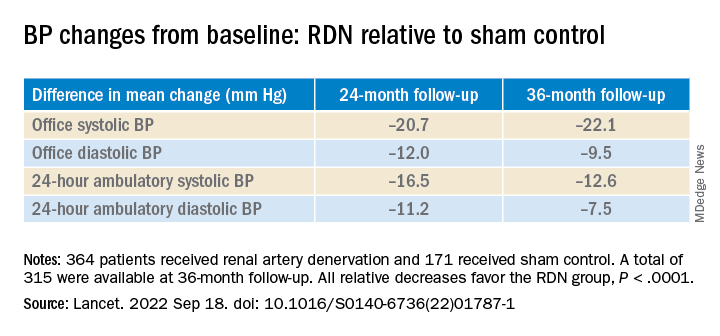
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Progress of the AGA Equity Project
In May 2022, the Digestive Disease Week (DDW) conference was held in person again for the first time in 3 years. Two years prior in July 2020 AGA launched the Equity Project, a six-point strategic plan to achieve equity and eradicate health disparities in digestive diseases.
President John Inadomi elected to focus his AGA Presidential Plenary session on updates in gastrointestinal and hepatic health disparities, and opened with a powerful testimony on his personal experiences encountering racism, and recognizing the need to translate spoken intentions into action.
This served as the perfect segue to the second plenary presentation in which an update was given on the progress of the Equity Project by co-chairs Byron Cryer, MD, and Sandra Quezada, MD, MS. Dr. Cryer described the vision of the Equity Project, including: a just world, free of inequities in access and health care delivery; state-of-the-art and well-funded research of multicultural populations; a diverse physician and scientist workforce and leadership; recognition of achievements of people of color; membership and staff committed to self-awareness and eliminating unconscious bias; and an engaged, large, diverse, vocal, and culturally- and socially aware early career membership.
Concrete action items were identified by a coalition of AGA members with diverse representation across specialties, practice settings, and identities. AGA staff and constituency programs have been critical in the execution of each action item. Key performance indicators were selected to gauge progress and hold the organization accountable in implementation of project tactics. These metrics demonstrate that the first 2 years of the Equity Project have been very productive. Salient accomplishments include three congressional briefings on health disparities topics, increased education and dialogue on diversity, equity, and inclusion (DEI) through podcasts, career development workshops and DDW sessions, fundraising of over $300,000 to support health disparities research, dedicated DEI sections and section editors for Gastroenterology and Clinical Gastroenterology and Hepatology, and the creation of a guide for GI fellowship program directors to promote equity and mitigate bias in the fellowship selection process.
Although the Equity Project is entering its third and final implementation year, the spirit and values of the Equity Project will live on. Excellence in equity requires ongoing, focused dedication – AGA is committed to ensuring that equity, diversity, and inclusion are inherently embedded through the fabric of the organization, and continuously integrated and assessed in all of the organization’s future strategic initiatives.
Dr. Quezada is an associate professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore. She reports being on the People of Color Advisory Board for Janssen. Dr. Cryer is chief of internal medicine and the Ralph Tompsett Endowed Chair in Medicine at Baylor University Medical Center, Dallas, and a professor of internal medicine at Texas A&M School of Medicine. He has no relevant conflicts of interest. These remarks were made during the AGA Presidential Plenary at DDW 2022.
In May 2022, the Digestive Disease Week (DDW) conference was held in person again for the first time in 3 years. Two years prior in July 2020 AGA launched the Equity Project, a six-point strategic plan to achieve equity and eradicate health disparities in digestive diseases.
President John Inadomi elected to focus his AGA Presidential Plenary session on updates in gastrointestinal and hepatic health disparities, and opened with a powerful testimony on his personal experiences encountering racism, and recognizing the need to translate spoken intentions into action.
This served as the perfect segue to the second plenary presentation in which an update was given on the progress of the Equity Project by co-chairs Byron Cryer, MD, and Sandra Quezada, MD, MS. Dr. Cryer described the vision of the Equity Project, including: a just world, free of inequities in access and health care delivery; state-of-the-art and well-funded research of multicultural populations; a diverse physician and scientist workforce and leadership; recognition of achievements of people of color; membership and staff committed to self-awareness and eliminating unconscious bias; and an engaged, large, diverse, vocal, and culturally- and socially aware early career membership.
Concrete action items were identified by a coalition of AGA members with diverse representation across specialties, practice settings, and identities. AGA staff and constituency programs have been critical in the execution of each action item. Key performance indicators were selected to gauge progress and hold the organization accountable in implementation of project tactics. These metrics demonstrate that the first 2 years of the Equity Project have been very productive. Salient accomplishments include three congressional briefings on health disparities topics, increased education and dialogue on diversity, equity, and inclusion (DEI) through podcasts, career development workshops and DDW sessions, fundraising of over $300,000 to support health disparities research, dedicated DEI sections and section editors for Gastroenterology and Clinical Gastroenterology and Hepatology, and the creation of a guide for GI fellowship program directors to promote equity and mitigate bias in the fellowship selection process.
Although the Equity Project is entering its third and final implementation year, the spirit and values of the Equity Project will live on. Excellence in equity requires ongoing, focused dedication – AGA is committed to ensuring that equity, diversity, and inclusion are inherently embedded through the fabric of the organization, and continuously integrated and assessed in all of the organization’s future strategic initiatives.
Dr. Quezada is an associate professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore. She reports being on the People of Color Advisory Board for Janssen. Dr. Cryer is chief of internal medicine and the Ralph Tompsett Endowed Chair in Medicine at Baylor University Medical Center, Dallas, and a professor of internal medicine at Texas A&M School of Medicine. He has no relevant conflicts of interest. These remarks were made during the AGA Presidential Plenary at DDW 2022.
In May 2022, the Digestive Disease Week (DDW) conference was held in person again for the first time in 3 years. Two years prior in July 2020 AGA launched the Equity Project, a six-point strategic plan to achieve equity and eradicate health disparities in digestive diseases.
President John Inadomi elected to focus his AGA Presidential Plenary session on updates in gastrointestinal and hepatic health disparities, and opened with a powerful testimony on his personal experiences encountering racism, and recognizing the need to translate spoken intentions into action.
This served as the perfect segue to the second plenary presentation in which an update was given on the progress of the Equity Project by co-chairs Byron Cryer, MD, and Sandra Quezada, MD, MS. Dr. Cryer described the vision of the Equity Project, including: a just world, free of inequities in access and health care delivery; state-of-the-art and well-funded research of multicultural populations; a diverse physician and scientist workforce and leadership; recognition of achievements of people of color; membership and staff committed to self-awareness and eliminating unconscious bias; and an engaged, large, diverse, vocal, and culturally- and socially aware early career membership.
Concrete action items were identified by a coalition of AGA members with diverse representation across specialties, practice settings, and identities. AGA staff and constituency programs have been critical in the execution of each action item. Key performance indicators were selected to gauge progress and hold the organization accountable in implementation of project tactics. These metrics demonstrate that the first 2 years of the Equity Project have been very productive. Salient accomplishments include three congressional briefings on health disparities topics, increased education and dialogue on diversity, equity, and inclusion (DEI) through podcasts, career development workshops and DDW sessions, fundraising of over $300,000 to support health disparities research, dedicated DEI sections and section editors for Gastroenterology and Clinical Gastroenterology and Hepatology, and the creation of a guide for GI fellowship program directors to promote equity and mitigate bias in the fellowship selection process.
Although the Equity Project is entering its third and final implementation year, the spirit and values of the Equity Project will live on. Excellence in equity requires ongoing, focused dedication – AGA is committed to ensuring that equity, diversity, and inclusion are inherently embedded through the fabric of the organization, and continuously integrated and assessed in all of the organization’s future strategic initiatives.
Dr. Quezada is an associate professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore. She reports being on the People of Color Advisory Board for Janssen. Dr. Cryer is chief of internal medicine and the Ralph Tompsett Endowed Chair in Medicine at Baylor University Medical Center, Dallas, and a professor of internal medicine at Texas A&M School of Medicine. He has no relevant conflicts of interest. These remarks were made during the AGA Presidential Plenary at DDW 2022.
Updates in eosinophilic gastrointestinal diseases
Eosinophilic gastrointestinal diseases (EGIDs) are characterized by GI signs or symptoms occurring along with tissue eosinophilia. Eosinophilic esophagitis (EoE) is the more commonly recognized EGID as endoscopic and histopathologic diagnostic criteria have long been established. Because of a lack of consensus on biopsy protocols, poorly understood histopathologic diagnostic criteria, and vague, nonspecific gastrointestinal complaints, patients with non-EoE EGIDs go unrecognized for years. Because of this, there is increasing emphasis on better defining rare, distal eosinophilic gastrointestinal diseases (i.e., eosinophilic gastritis, enteritis, and colitis).
EGID nomenclature was standardized in 2022 in part to minimize vague terminology (i.e., eosinophilic gastroenteritis) and to provide more specific information about the location of eosinophilic disease. The 2022 nomenclature suggest that EGID be used as the umbrella term for all GI luminal eosinophilia (without a known cause) but with emphasis on the site of specific eosinophilic involvement (i.e., eosinophilic gastritis or eosinophilic gastritis and colitis). Importantly, there is much work to be done to adequately identify patients suffering from EGIDs. Symptoms are variable, ranging from abdominal pain, bloating, and nausea seen in proximal disease to loose stools and hematochezia in more distal involvement. Signs of disease, such as iron or other nutrient deficiencies and protein loss, may also occur. Endoscopic findings can vary from erythema, granularity, erosions, ulcerations, and blunting to even normal-appearing tissue. In eosinophilic gastritis, Ikuo Hirano, MD, and colleagues demonstrated that increasing endoscopic inflammatory findings in the stomach correlate with assessment of disease severity. Regardless of endoscopic findings, numerous biopsies are needed for the diagnosis of EGIDs because, as already established in EoE, eosinophil involvement is patchy. Nirmala Gonsalves, MD, and Evan Dellon, MD, found that a minimum of four biopsies each in the gastric antrum, gastric body, and small bowel are needed to detect disease. Optimal biopsy patterns have not yet been determined for eosinophilic ileitis or colitis.
Despite these advances, there is more work to be performed. Although these disease states are termed “eosinophilic,” the immunopathology driving these diseases is multifactorial, involving lymphocytes and mast cells and creating different phenotypes of disease in a similar fashion to inflammatory bowel disease. Current therapies being studied include eosinophil-depleting medications along with others targeting T2 immune pathways. Patients may need multiple therapeutic options, and personalized medicine will soon play a larger role in defining treatments. For now, researchers are fervently working on improved methods to identify, phenotype, and treat these morbid disorders.
Dr. Peterson is associate professor of gastroenterology at University of Utah Health, Salt Lake City. She has no relevant conflicts of interest. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Eosinophilic gastrointestinal diseases (EGIDs) are characterized by GI signs or symptoms occurring along with tissue eosinophilia. Eosinophilic esophagitis (EoE) is the more commonly recognized EGID as endoscopic and histopathologic diagnostic criteria have long been established. Because of a lack of consensus on biopsy protocols, poorly understood histopathologic diagnostic criteria, and vague, nonspecific gastrointestinal complaints, patients with non-EoE EGIDs go unrecognized for years. Because of this, there is increasing emphasis on better defining rare, distal eosinophilic gastrointestinal diseases (i.e., eosinophilic gastritis, enteritis, and colitis).
EGID nomenclature was standardized in 2022 in part to minimize vague terminology (i.e., eosinophilic gastroenteritis) and to provide more specific information about the location of eosinophilic disease. The 2022 nomenclature suggest that EGID be used as the umbrella term for all GI luminal eosinophilia (without a known cause) but with emphasis on the site of specific eosinophilic involvement (i.e., eosinophilic gastritis or eosinophilic gastritis and colitis). Importantly, there is much work to be done to adequately identify patients suffering from EGIDs. Symptoms are variable, ranging from abdominal pain, bloating, and nausea seen in proximal disease to loose stools and hematochezia in more distal involvement. Signs of disease, such as iron or other nutrient deficiencies and protein loss, may also occur. Endoscopic findings can vary from erythema, granularity, erosions, ulcerations, and blunting to even normal-appearing tissue. In eosinophilic gastritis, Ikuo Hirano, MD, and colleagues demonstrated that increasing endoscopic inflammatory findings in the stomach correlate with assessment of disease severity. Regardless of endoscopic findings, numerous biopsies are needed for the diagnosis of EGIDs because, as already established in EoE, eosinophil involvement is patchy. Nirmala Gonsalves, MD, and Evan Dellon, MD, found that a minimum of four biopsies each in the gastric antrum, gastric body, and small bowel are needed to detect disease. Optimal biopsy patterns have not yet been determined for eosinophilic ileitis or colitis.
Despite these advances, there is more work to be performed. Although these disease states are termed “eosinophilic,” the immunopathology driving these diseases is multifactorial, involving lymphocytes and mast cells and creating different phenotypes of disease in a similar fashion to inflammatory bowel disease. Current therapies being studied include eosinophil-depleting medications along with others targeting T2 immune pathways. Patients may need multiple therapeutic options, and personalized medicine will soon play a larger role in defining treatments. For now, researchers are fervently working on improved methods to identify, phenotype, and treat these morbid disorders.
Dr. Peterson is associate professor of gastroenterology at University of Utah Health, Salt Lake City. She has no relevant conflicts of interest. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Eosinophilic gastrointestinal diseases (EGIDs) are characterized by GI signs or symptoms occurring along with tissue eosinophilia. Eosinophilic esophagitis (EoE) is the more commonly recognized EGID as endoscopic and histopathologic diagnostic criteria have long been established. Because of a lack of consensus on biopsy protocols, poorly understood histopathologic diagnostic criteria, and vague, nonspecific gastrointestinal complaints, patients with non-EoE EGIDs go unrecognized for years. Because of this, there is increasing emphasis on better defining rare, distal eosinophilic gastrointestinal diseases (i.e., eosinophilic gastritis, enteritis, and colitis).
EGID nomenclature was standardized in 2022 in part to minimize vague terminology (i.e., eosinophilic gastroenteritis) and to provide more specific information about the location of eosinophilic disease. The 2022 nomenclature suggest that EGID be used as the umbrella term for all GI luminal eosinophilia (without a known cause) but with emphasis on the site of specific eosinophilic involvement (i.e., eosinophilic gastritis or eosinophilic gastritis and colitis). Importantly, there is much work to be done to adequately identify patients suffering from EGIDs. Symptoms are variable, ranging from abdominal pain, bloating, and nausea seen in proximal disease to loose stools and hematochezia in more distal involvement. Signs of disease, such as iron or other nutrient deficiencies and protein loss, may also occur. Endoscopic findings can vary from erythema, granularity, erosions, ulcerations, and blunting to even normal-appearing tissue. In eosinophilic gastritis, Ikuo Hirano, MD, and colleagues demonstrated that increasing endoscopic inflammatory findings in the stomach correlate with assessment of disease severity. Regardless of endoscopic findings, numerous biopsies are needed for the diagnosis of EGIDs because, as already established in EoE, eosinophil involvement is patchy. Nirmala Gonsalves, MD, and Evan Dellon, MD, found that a minimum of four biopsies each in the gastric antrum, gastric body, and small bowel are needed to detect disease. Optimal biopsy patterns have not yet been determined for eosinophilic ileitis or colitis.
Despite these advances, there is more work to be performed. Although these disease states are termed “eosinophilic,” the immunopathology driving these diseases is multifactorial, involving lymphocytes and mast cells and creating different phenotypes of disease in a similar fashion to inflammatory bowel disease. Current therapies being studied include eosinophil-depleting medications along with others targeting T2 immune pathways. Patients may need multiple therapeutic options, and personalized medicine will soon play a larger role in defining treatments. For now, researchers are fervently working on improved methods to identify, phenotype, and treat these morbid disorders.
Dr. Peterson is associate professor of gastroenterology at University of Utah Health, Salt Lake City. She has no relevant conflicts of interest. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Post-PCI FFR in multivessel disease predicts target vessel failure: FAME 3 analysis
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
Risk by FFR is continuous variable
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
FROM TCT 2022
Unsure on the best T2D drug choice? Let patients decide
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
AT EASD 2022
Early age at hysterectomy ups type 2 diabetes risk
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
FROM EASD 2022
Amulet, Watchman 2.5 LAAO outcomes neck and neck at 3 years
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM TCT 2022















