User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
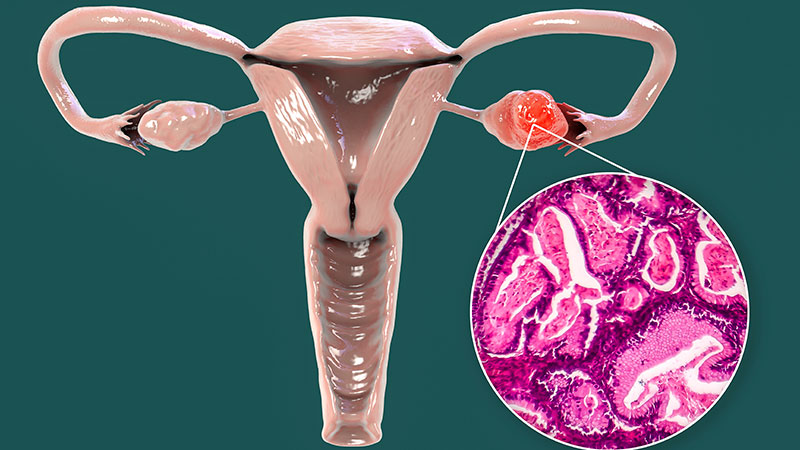
PATINA Trial Shifts Paradigm in HER2+/ER+ Breast Cancer Treatment, Prolonging Survival With Targeted Combination Therapy
This is a transcript of a video essay, which can be found on Medscape.
I’m here with you today to talk about what I think was one of the most important trials reported at the December San Antonio Breast Cancer Symposium meeting, the PATINA trial.
This is a trial that was not on our radar as we were looking forward to the meeting. In fact, it wasn’t on the agenda because the results didn’t become available until about a week and a half before the meeting kicked off. Kudos to the authors for getting these data out there, and to the organizers for recognizing the importance and finding a way to add this to the program.
The PATINA trial enrolled patients whose tumors were both HER2 positive and ER positive. That is about half of our patients with HER2-positive disease.
Almost all of our trials looking at HER2-targeted therapies did not allow patients to continue antiestrogen therapy. Patients could have had antiestrogen therapy before they came to those HER2-focused trials. Some did, some may not have. It was not a requirement, but they could not continue it.
The same is true for patients with ER-positive disease. If your disease was ER positive and HER2 positive, you were excluded from all of our recent trials focusing on ER-positive disease. That includes those looking at the benefit of cyclin-dependent kinase inhibitors.
It also includes those looking at PI3 kinase inhibitors, AKT inhibitors, and selective estrogen receptor downregulators in their oral formulations. We›ve had to pick: Do we want to focus on HER2 or do we want to focus on ER? The PATINA trial results are not only important for practice, but they also show us the problem in that dichotomy.
PATINA enrolled patients who were receiving their first chemotherapy and HER2-targeted therapy for metastatic disease. Once they had received at least four cycles of combined therapy, they could receive additional chemotherapy, but they could also move into a maintenance phase if their disease was responding or stable, continuing HER2-targeted therapy alone without chemotherapy.
At that point, hormone therapy was reintroduced. This is a common practice for many of us. Those patients were then randomized to either palbociclib or not. This was a large effort, with 518 patients in this randomized trial. The expectations of progression-free survival were based on the results of the CLEOPATRA trial.
The trial assumed about a 15-month progression-free survival in those randomized to the control arm. What was actually observed was a 29-month progression-free survival. Two things might have contributed to this difference.
First, the CLEOPATRA trial did not allow patients to receive concurrent hormone therapy, and that may have had a major impact on its own. Also, CLEOPATRA reported the PFS for all of the patients enrolled. To get into PATINA, you had to be responding or stable to your initial combined modality therapy. Those patients with really resistant disease who progressed early were excluded, and that may have had an impact as well.
With the addition of palbociclib, that 29-month progression-free survival became 44 months. Stop and think about this. There was almost a 4-year period of time where patients were on trastuzumab and pertuzumab, an aromatase inhibitor, and a cyclin-dependent kinase inhibitor. No chemotherapy, much less day-to-day toxicities — not no toxicity, but less of the day-to-day toxicities that patients are really troubled by.
We don’t yet have mature overall survival data. Those will be coming. You can imagine with progression-free survival nearing 4 years, overall survival data will be some months or years hence until there are enough events for us to look at that evaluation.
Realizing that there are going to be issues with insurance approval and regulatory approvals, I would like to take these results into account for my patients in that situation.
It also challenges those of us who are developing clinical trials and drugs to realize that studying targets in isolation is needed early in the development of new agents. To get the maximum benefit for our patients, you need to put those building blocks back together and stop this forced dichotomy.
That doesn’t serve our patients well and it’s not where we will need to be in the future.
Kathy D. Miller, Professor of Medicine, Indiana University School of Medicine; Co-Director, Breast Cancer Program, Indiana University Simon Cancer Center, Indianapolis, Indiana, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This is a transcript of a video essay, which can be found on Medscape.
I’m here with you today to talk about what I think was one of the most important trials reported at the December San Antonio Breast Cancer Symposium meeting, the PATINA trial.
This is a trial that was not on our radar as we were looking forward to the meeting. In fact, it wasn’t on the agenda because the results didn’t become available until about a week and a half before the meeting kicked off. Kudos to the authors for getting these data out there, and to the organizers for recognizing the importance and finding a way to add this to the program.
The PATINA trial enrolled patients whose tumors were both HER2 positive and ER positive. That is about half of our patients with HER2-positive disease.
Almost all of our trials looking at HER2-targeted therapies did not allow patients to continue antiestrogen therapy. Patients could have had antiestrogen therapy before they came to those HER2-focused trials. Some did, some may not have. It was not a requirement, but they could not continue it.
The same is true for patients with ER-positive disease. If your disease was ER positive and HER2 positive, you were excluded from all of our recent trials focusing on ER-positive disease. That includes those looking at the benefit of cyclin-dependent kinase inhibitors.
It also includes those looking at PI3 kinase inhibitors, AKT inhibitors, and selective estrogen receptor downregulators in their oral formulations. We›ve had to pick: Do we want to focus on HER2 or do we want to focus on ER? The PATINA trial results are not only important for practice, but they also show us the problem in that dichotomy.
PATINA enrolled patients who were receiving their first chemotherapy and HER2-targeted therapy for metastatic disease. Once they had received at least four cycles of combined therapy, they could receive additional chemotherapy, but they could also move into a maintenance phase if their disease was responding or stable, continuing HER2-targeted therapy alone without chemotherapy.
At that point, hormone therapy was reintroduced. This is a common practice for many of us. Those patients were then randomized to either palbociclib or not. This was a large effort, with 518 patients in this randomized trial. The expectations of progression-free survival were based on the results of the CLEOPATRA trial.
The trial assumed about a 15-month progression-free survival in those randomized to the control arm. What was actually observed was a 29-month progression-free survival. Two things might have contributed to this difference.
First, the CLEOPATRA trial did not allow patients to receive concurrent hormone therapy, and that may have had a major impact on its own. Also, CLEOPATRA reported the PFS for all of the patients enrolled. To get into PATINA, you had to be responding or stable to your initial combined modality therapy. Those patients with really resistant disease who progressed early were excluded, and that may have had an impact as well.
With the addition of palbociclib, that 29-month progression-free survival became 44 months. Stop and think about this. There was almost a 4-year period of time where patients were on trastuzumab and pertuzumab, an aromatase inhibitor, and a cyclin-dependent kinase inhibitor. No chemotherapy, much less day-to-day toxicities — not no toxicity, but less of the day-to-day toxicities that patients are really troubled by.
We don’t yet have mature overall survival data. Those will be coming. You can imagine with progression-free survival nearing 4 years, overall survival data will be some months or years hence until there are enough events for us to look at that evaluation.
Realizing that there are going to be issues with insurance approval and regulatory approvals, I would like to take these results into account for my patients in that situation.
It also challenges those of us who are developing clinical trials and drugs to realize that studying targets in isolation is needed early in the development of new agents. To get the maximum benefit for our patients, you need to put those building blocks back together and stop this forced dichotomy.
That doesn’t serve our patients well and it’s not where we will need to be in the future.
Kathy D. Miller, Professor of Medicine, Indiana University School of Medicine; Co-Director, Breast Cancer Program, Indiana University Simon Cancer Center, Indianapolis, Indiana, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This is a transcript of a video essay, which can be found on Medscape.
I’m here with you today to talk about what I think was one of the most important trials reported at the December San Antonio Breast Cancer Symposium meeting, the PATINA trial.
This is a trial that was not on our radar as we were looking forward to the meeting. In fact, it wasn’t on the agenda because the results didn’t become available until about a week and a half before the meeting kicked off. Kudos to the authors for getting these data out there, and to the organizers for recognizing the importance and finding a way to add this to the program.
The PATINA trial enrolled patients whose tumors were both HER2 positive and ER positive. That is about half of our patients with HER2-positive disease.
Almost all of our trials looking at HER2-targeted therapies did not allow patients to continue antiestrogen therapy. Patients could have had antiestrogen therapy before they came to those HER2-focused trials. Some did, some may not have. It was not a requirement, but they could not continue it.
The same is true for patients with ER-positive disease. If your disease was ER positive and HER2 positive, you were excluded from all of our recent trials focusing on ER-positive disease. That includes those looking at the benefit of cyclin-dependent kinase inhibitors.
It also includes those looking at PI3 kinase inhibitors, AKT inhibitors, and selective estrogen receptor downregulators in their oral formulations. We›ve had to pick: Do we want to focus on HER2 or do we want to focus on ER? The PATINA trial results are not only important for practice, but they also show us the problem in that dichotomy.
PATINA enrolled patients who were receiving their first chemotherapy and HER2-targeted therapy for metastatic disease. Once they had received at least four cycles of combined therapy, they could receive additional chemotherapy, but they could also move into a maintenance phase if their disease was responding or stable, continuing HER2-targeted therapy alone without chemotherapy.
At that point, hormone therapy was reintroduced. This is a common practice for many of us. Those patients were then randomized to either palbociclib or not. This was a large effort, with 518 patients in this randomized trial. The expectations of progression-free survival were based on the results of the CLEOPATRA trial.
The trial assumed about a 15-month progression-free survival in those randomized to the control arm. What was actually observed was a 29-month progression-free survival. Two things might have contributed to this difference.
First, the CLEOPATRA trial did not allow patients to receive concurrent hormone therapy, and that may have had a major impact on its own. Also, CLEOPATRA reported the PFS for all of the patients enrolled. To get into PATINA, you had to be responding or stable to your initial combined modality therapy. Those patients with really resistant disease who progressed early were excluded, and that may have had an impact as well.
With the addition of palbociclib, that 29-month progression-free survival became 44 months. Stop and think about this. There was almost a 4-year period of time where patients were on trastuzumab and pertuzumab, an aromatase inhibitor, and a cyclin-dependent kinase inhibitor. No chemotherapy, much less day-to-day toxicities — not no toxicity, but less of the day-to-day toxicities that patients are really troubled by.
We don’t yet have mature overall survival data. Those will be coming. You can imagine with progression-free survival nearing 4 years, overall survival data will be some months or years hence until there are enough events for us to look at that evaluation.
Realizing that there are going to be issues with insurance approval and regulatory approvals, I would like to take these results into account for my patients in that situation.
It also challenges those of us who are developing clinical trials and drugs to realize that studying targets in isolation is needed early in the development of new agents. To get the maximum benefit for our patients, you need to put those building blocks back together and stop this forced dichotomy.
That doesn’t serve our patients well and it’s not where we will need to be in the future.
Kathy D. Miller, Professor of Medicine, Indiana University School of Medicine; Co-Director, Breast Cancer Program, Indiana University Simon Cancer Center, Indianapolis, Indiana, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Last Month in Oncology: FDA Cancer News Roundup
Last month, the United States Food and Drug Administration (FDA) approved two new drugs and two biosimilars as well as halted commercialization for a hemophilia treatment.
Here’s a deeper look of what happened last month.
New Drugs
1. The FDA has approved mirdametinib (Gomekli, SpringWorks Therapeutics, Inc.) for adult and pediatric patients 2 years or older with neurofibromatosis type 1 and symptomatic plexiform neurofibromas that are not amenable to complete resection.
Approval for this agent was based on overall response rate findings from a multicenter, single-arm, phase 2b trial. The trial, which enrolled 58 adults and 56 pediatric patients with this rare disease, reported confirmed overall response rates of 41% among adults and 52% among children.
Adverse reactions occurring in at least 25% of adults included rash, diarrhea, nausea, musculoskeletal pain, vomiting, and fatigue. Mirdametinib can also cause ocular toxicity. Treatment should be withheld, discontinued, or the dosage reduced based on the severity of these adverse reactions, according to the FDA notice.
2. The FDA has approved vimseltinib (Romvimza, Deciphera Pharmaceuticals, LLC) to treat adult patients with symptomatic tenosynovial giant cell tumors who will not benefit from surgical resection.
Vimseltinib was approved based on findings from the MOTION trial, which included 123 patients randomly assigned 2:1 to vimseltinib 30 mg twice weekly or to placebo for 24 weeks. At 25 weeks, the objective response rate was 40% in the vimseltinib arm and 0% in the placebo arm. The median duration of response was not reached in the vimseltinib arm. Patients receiving vimseltinib also demonstrated significant improvements in active range of motion, physical functioning, and pain at this time. After another 6 months of follow-up, 58% of responders had a duration of response of 9 months or longer.
Treatment-emergent adverse events in MOTION were largely of grade 1 or 2. The most common adverse reactions, occurring in at least 20% of patients, included increased aspartate aminotransferase, periorbital edema, fatigue, rash, and cholesterol.
New or Expanded Indications
1. The FDA has approved a supplemental Biologics License Application for brentuximab vedotin (Adcetris, Seagen Inc.), in combination with lenalidomide and rituximab, for adults with relapsed or refractory large B-cell lymphoma, after at least two prior lines of therapy, who are ineligible for stem cell transplant or chimeric antigen receptor T-cell therapy. This includes patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, DLBCL arising from indolent lymphoma, or high-grade B-cell lymphoma.
Approval was based on the randomized, double-blind, placebo-controlled ECHELON-3 trial, which randomly assigned patients 1:1 to receive lenalidomide and rituximab plus either brentuximab vedotin or placebo until disease progression or unacceptable toxicity. Researchers reported a median overall survival of 13.8 months in the treatment group vs 8.5 months in the placebo group (hazard ratio, 0.63).
2. The FDA has approved the Biologics License Application for Ospomyv and Xbryk (Samsung Bioepis Co.) — biosimilars referencing denosumab (Prolia and Xgeva, respectively) — to treat osteoporosis and cancer-related bone loss.
Ospomyv and Xbryk have been approved for use in all indications of the approved reference drugs. Specifically, Xbryk is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors or multiple myeloma, and Ospomyv is indicated in several populations of patients with osteoporosis at high risk for fracture.
“The FDA approval of Ospomyv and Xbryk marks a key step in improving patient access and alleviating treatment cost for patients with osteoporosis and cancer-related bone loss in the United States,” Byoungin Jung, vice president at Samsung Bioepis, said in the news release.
Drug Commercialization Halt
Pfizer announced last month that it will halt the global development and commercialization of its hemophilia gene therapy fidanacogene elaparvovec (Beqvez). The company cited several reasons for the discontinuation, including low demand from patients and doctors.
Beqvez is a one-time therapy approved in the United States last April to treat adults with moderate to severe hemophilia B, a rare bleeding disorder that affects almost 4 in 100,000 men in the United States.
The significant price tag is one reason hematologists have cited for the low uptake. Another barrier is that “we don’t know the long-term outcomes” associated with the drug, pediatric hematologist Ben Samelson-Jones, MD, PhD, of the Perelman School of Medicine at the University of Pennsylvania and Children’s Hospital of Philadelphia, Philadelphia, told this news organization earlier this year.
Other issues include the prospect of newer treatment advances in the hemophilia space and logistical challenges. “There’s just a lot of logistics to getting an institution ready to provide this type of therapy,” Samelson-Jones added.
A version of this article first appeared on Medscape.com.
Last month, the United States Food and Drug Administration (FDA) approved two new drugs and two biosimilars as well as halted commercialization for a hemophilia treatment.
Here’s a deeper look of what happened last month.
New Drugs
1. The FDA has approved mirdametinib (Gomekli, SpringWorks Therapeutics, Inc.) for adult and pediatric patients 2 years or older with neurofibromatosis type 1 and symptomatic plexiform neurofibromas that are not amenable to complete resection.
Approval for this agent was based on overall response rate findings from a multicenter, single-arm, phase 2b trial. The trial, which enrolled 58 adults and 56 pediatric patients with this rare disease, reported confirmed overall response rates of 41% among adults and 52% among children.
Adverse reactions occurring in at least 25% of adults included rash, diarrhea, nausea, musculoskeletal pain, vomiting, and fatigue. Mirdametinib can also cause ocular toxicity. Treatment should be withheld, discontinued, or the dosage reduced based on the severity of these adverse reactions, according to the FDA notice.
2. The FDA has approved vimseltinib (Romvimza, Deciphera Pharmaceuticals, LLC) to treat adult patients with symptomatic tenosynovial giant cell tumors who will not benefit from surgical resection.
Vimseltinib was approved based on findings from the MOTION trial, which included 123 patients randomly assigned 2:1 to vimseltinib 30 mg twice weekly or to placebo for 24 weeks. At 25 weeks, the objective response rate was 40% in the vimseltinib arm and 0% in the placebo arm. The median duration of response was not reached in the vimseltinib arm. Patients receiving vimseltinib also demonstrated significant improvements in active range of motion, physical functioning, and pain at this time. After another 6 months of follow-up, 58% of responders had a duration of response of 9 months or longer.
Treatment-emergent adverse events in MOTION were largely of grade 1 or 2. The most common adverse reactions, occurring in at least 20% of patients, included increased aspartate aminotransferase, periorbital edema, fatigue, rash, and cholesterol.
New or Expanded Indications
1. The FDA has approved a supplemental Biologics License Application for brentuximab vedotin (Adcetris, Seagen Inc.), in combination with lenalidomide and rituximab, for adults with relapsed or refractory large B-cell lymphoma, after at least two prior lines of therapy, who are ineligible for stem cell transplant or chimeric antigen receptor T-cell therapy. This includes patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, DLBCL arising from indolent lymphoma, or high-grade B-cell lymphoma.
Approval was based on the randomized, double-blind, placebo-controlled ECHELON-3 trial, which randomly assigned patients 1:1 to receive lenalidomide and rituximab plus either brentuximab vedotin or placebo until disease progression or unacceptable toxicity. Researchers reported a median overall survival of 13.8 months in the treatment group vs 8.5 months in the placebo group (hazard ratio, 0.63).
2. The FDA has approved the Biologics License Application for Ospomyv and Xbryk (Samsung Bioepis Co.) — biosimilars referencing denosumab (Prolia and Xgeva, respectively) — to treat osteoporosis and cancer-related bone loss.
Ospomyv and Xbryk have been approved for use in all indications of the approved reference drugs. Specifically, Xbryk is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors or multiple myeloma, and Ospomyv is indicated in several populations of patients with osteoporosis at high risk for fracture.
“The FDA approval of Ospomyv and Xbryk marks a key step in improving patient access and alleviating treatment cost for patients with osteoporosis and cancer-related bone loss in the United States,” Byoungin Jung, vice president at Samsung Bioepis, said in the news release.
Drug Commercialization Halt
Pfizer announced last month that it will halt the global development and commercialization of its hemophilia gene therapy fidanacogene elaparvovec (Beqvez). The company cited several reasons for the discontinuation, including low demand from patients and doctors.
Beqvez is a one-time therapy approved in the United States last April to treat adults with moderate to severe hemophilia B, a rare bleeding disorder that affects almost 4 in 100,000 men in the United States.
The significant price tag is one reason hematologists have cited for the low uptake. Another barrier is that “we don’t know the long-term outcomes” associated with the drug, pediatric hematologist Ben Samelson-Jones, MD, PhD, of the Perelman School of Medicine at the University of Pennsylvania and Children’s Hospital of Philadelphia, Philadelphia, told this news organization earlier this year.
Other issues include the prospect of newer treatment advances in the hemophilia space and logistical challenges. “There’s just a lot of logistics to getting an institution ready to provide this type of therapy,” Samelson-Jones added.
A version of this article first appeared on Medscape.com.
Last month, the United States Food and Drug Administration (FDA) approved two new drugs and two biosimilars as well as halted commercialization for a hemophilia treatment.
Here’s a deeper look of what happened last month.
New Drugs
1. The FDA has approved mirdametinib (Gomekli, SpringWorks Therapeutics, Inc.) for adult and pediatric patients 2 years or older with neurofibromatosis type 1 and symptomatic plexiform neurofibromas that are not amenable to complete resection.
Approval for this agent was based on overall response rate findings from a multicenter, single-arm, phase 2b trial. The trial, which enrolled 58 adults and 56 pediatric patients with this rare disease, reported confirmed overall response rates of 41% among adults and 52% among children.
Adverse reactions occurring in at least 25% of adults included rash, diarrhea, nausea, musculoskeletal pain, vomiting, and fatigue. Mirdametinib can also cause ocular toxicity. Treatment should be withheld, discontinued, or the dosage reduced based on the severity of these adverse reactions, according to the FDA notice.
2. The FDA has approved vimseltinib (Romvimza, Deciphera Pharmaceuticals, LLC) to treat adult patients with symptomatic tenosynovial giant cell tumors who will not benefit from surgical resection.
Vimseltinib was approved based on findings from the MOTION trial, which included 123 patients randomly assigned 2:1 to vimseltinib 30 mg twice weekly or to placebo for 24 weeks. At 25 weeks, the objective response rate was 40% in the vimseltinib arm and 0% in the placebo arm. The median duration of response was not reached in the vimseltinib arm. Patients receiving vimseltinib also demonstrated significant improvements in active range of motion, physical functioning, and pain at this time. After another 6 months of follow-up, 58% of responders had a duration of response of 9 months or longer.
Treatment-emergent adverse events in MOTION were largely of grade 1 or 2. The most common adverse reactions, occurring in at least 20% of patients, included increased aspartate aminotransferase, periorbital edema, fatigue, rash, and cholesterol.
New or Expanded Indications
1. The FDA has approved a supplemental Biologics License Application for brentuximab vedotin (Adcetris, Seagen Inc.), in combination with lenalidomide and rituximab, for adults with relapsed or refractory large B-cell lymphoma, after at least two prior lines of therapy, who are ineligible for stem cell transplant or chimeric antigen receptor T-cell therapy. This includes patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, DLBCL arising from indolent lymphoma, or high-grade B-cell lymphoma.
Approval was based on the randomized, double-blind, placebo-controlled ECHELON-3 trial, which randomly assigned patients 1:1 to receive lenalidomide and rituximab plus either brentuximab vedotin or placebo until disease progression or unacceptable toxicity. Researchers reported a median overall survival of 13.8 months in the treatment group vs 8.5 months in the placebo group (hazard ratio, 0.63).
2. The FDA has approved the Biologics License Application for Ospomyv and Xbryk (Samsung Bioepis Co.) — biosimilars referencing denosumab (Prolia and Xgeva, respectively) — to treat osteoporosis and cancer-related bone loss.
Ospomyv and Xbryk have been approved for use in all indications of the approved reference drugs. Specifically, Xbryk is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumors or multiple myeloma, and Ospomyv is indicated in several populations of patients with osteoporosis at high risk for fracture.
“The FDA approval of Ospomyv and Xbryk marks a key step in improving patient access and alleviating treatment cost for patients with osteoporosis and cancer-related bone loss in the United States,” Byoungin Jung, vice president at Samsung Bioepis, said in the news release.
Drug Commercialization Halt
Pfizer announced last month that it will halt the global development and commercialization of its hemophilia gene therapy fidanacogene elaparvovec (Beqvez). The company cited several reasons for the discontinuation, including low demand from patients and doctors.
Beqvez is a one-time therapy approved in the United States last April to treat adults with moderate to severe hemophilia B, a rare bleeding disorder that affects almost 4 in 100,000 men in the United States.
The significant price tag is one reason hematologists have cited for the low uptake. Another barrier is that “we don’t know the long-term outcomes” associated with the drug, pediatric hematologist Ben Samelson-Jones, MD, PhD, of the Perelman School of Medicine at the University of Pennsylvania and Children’s Hospital of Philadelphia, Philadelphia, told this news organization earlier this year.
Other issues include the prospect of newer treatment advances in the hemophilia space and logistical challenges. “There’s just a lot of logistics to getting an institution ready to provide this type of therapy,” Samelson-Jones added.
A version of this article first appeared on Medscape.com.
New Biomarkers Identified for Treatment Response in IBD
BERLIN — presented at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Differences uncovered in multiple messenger RNAs, proteins, metabolites, and gut microbiota were associated with responders and nonresponders to biologics and Janus kinase inhibitors, suggesting the potential for predictive biomarkers in IBD, including Crohn’s disease (CD) and ulcerative colitis (UC).
“With further work we hope to confirm these findings and evaluate their clinical relevance in identifying patients most likely to respond to tailored therapeutic interventions,” said Montserrat Baldan-Martin, PhD, a researcher at the University Hospital of the Princess, Madrid, Spain.
The treatment of IBD is challenging because of the heterogeneity across clinical, immunological, molecular, genetic, and microbiologic features, with one third of patients failing to respond to any one treatment.
Baldan-Martin and colleagues wanted to find predictive biomarkers of response by examining the differences across multi-omics profiles relative to different therapies.
The study analyzed 127 patients with IBD (57 with CD and 70 with UC) before and after 14 weeks of treatment with one of the following: anti–tumor necrosis factors (TNFs), ustekinumab, vedolizumab, or tofacitinib. Patient response to treatment was evaluated using endoscopic criteria that categorized them as responders or nonresponders to the different therapies.
In addition, molecular data from various biologic samples — serum, urine, extracellular vesicles, intestinal biopsies, and stool — were tested using transcriptomics, proteomics, metabolomics, and metagenomics.
Clear Differences
“The most significant differences were seen in gene expression within intestinal tissue of responder and nonresponder patients with ulcerative colitis taking vedolizumab,” Baldan-Martin reported.
Proteomic analysis revealed that a total of 1377 proteins were identified across all groups (CD, UC, and the four drug classes/therapies). Responders and nonresponders for each therapy expressed different proteins in serum extracellular vesicles and intestinal tissues.
For example, patients with CD who responded to anti-TNF therapies had 138 different proteins from those of anti-TNF nonresponders, while patients with UC who responded to anti-TNF therapies had 218 different proteins from those of anti-TNF nonresponders, reported Baldan-Martin.
Also, we observed almost no proteins “in common between ulcerative colitis responders versus nonresponders for all treatments,” she noted. And we “saw only three proteins in common with Crohn’s disease patients [on different drugs].”
Metabolomic analysis identified deregulation of 24 serum lipoproteins in CD responders to ustekinumab, compared with nonresponders.
“We observed greater differences in the lipoproteins in serum than metabolites in serum and urine,” Baldan-Martin added.
Analysis of biologic pathways also highlighted enrichment in ketone and butyrate metabolism, mitochondrial electron transport chain activity, carnitine synthesis, and fatty acid oxidation pathways, while metagenomic analysis revealed the greatest microbial differences in UC responders and nonresponders to anti-TNF therapies.
Baldan-Martin said research was ongoing with a new cohort of patients that aims to validate some of the biomarkers and help identify the patients most likely to respond to tailored therapeutic interventions.
“One of the challenges is integrating results from different omics approaches to create a more holistic understanding of the disease,” she said, adding that she hopes the research “will potentially open doors for early detection through multi-panel biomarkers.”
Session moderator Mark Samaan, MD, consultant gastroenterologist at Guy’s and St Thomas’ NHS Foundation Trust, London, England, said that “the findings related to nonresponse to specific drugs in UC and CD were interesting. With longitudinal follow-up, we’d hope this might help us pick out patients less likely to respond and who show early nonresponse to specific drugs based on serum, urine, and fecal sampling.”
“It’s very helpful to know if someone is a nonresponder within 14 weeks because we can then move the patient on to something else relatively quickly,” he added.
Baldan-Martin and Samaan declared no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — presented at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Differences uncovered in multiple messenger RNAs, proteins, metabolites, and gut microbiota were associated with responders and nonresponders to biologics and Janus kinase inhibitors, suggesting the potential for predictive biomarkers in IBD, including Crohn’s disease (CD) and ulcerative colitis (UC).
“With further work we hope to confirm these findings and evaluate their clinical relevance in identifying patients most likely to respond to tailored therapeutic interventions,” said Montserrat Baldan-Martin, PhD, a researcher at the University Hospital of the Princess, Madrid, Spain.
The treatment of IBD is challenging because of the heterogeneity across clinical, immunological, molecular, genetic, and microbiologic features, with one third of patients failing to respond to any one treatment.
Baldan-Martin and colleagues wanted to find predictive biomarkers of response by examining the differences across multi-omics profiles relative to different therapies.
The study analyzed 127 patients with IBD (57 with CD and 70 with UC) before and after 14 weeks of treatment with one of the following: anti–tumor necrosis factors (TNFs), ustekinumab, vedolizumab, or tofacitinib. Patient response to treatment was evaluated using endoscopic criteria that categorized them as responders or nonresponders to the different therapies.
In addition, molecular data from various biologic samples — serum, urine, extracellular vesicles, intestinal biopsies, and stool — were tested using transcriptomics, proteomics, metabolomics, and metagenomics.
Clear Differences
“The most significant differences were seen in gene expression within intestinal tissue of responder and nonresponder patients with ulcerative colitis taking vedolizumab,” Baldan-Martin reported.
Proteomic analysis revealed that a total of 1377 proteins were identified across all groups (CD, UC, and the four drug classes/therapies). Responders and nonresponders for each therapy expressed different proteins in serum extracellular vesicles and intestinal tissues.
For example, patients with CD who responded to anti-TNF therapies had 138 different proteins from those of anti-TNF nonresponders, while patients with UC who responded to anti-TNF therapies had 218 different proteins from those of anti-TNF nonresponders, reported Baldan-Martin.
Also, we observed almost no proteins “in common between ulcerative colitis responders versus nonresponders for all treatments,” she noted. And we “saw only three proteins in common with Crohn’s disease patients [on different drugs].”
Metabolomic analysis identified deregulation of 24 serum lipoproteins in CD responders to ustekinumab, compared with nonresponders.
“We observed greater differences in the lipoproteins in serum than metabolites in serum and urine,” Baldan-Martin added.
Analysis of biologic pathways also highlighted enrichment in ketone and butyrate metabolism, mitochondrial electron transport chain activity, carnitine synthesis, and fatty acid oxidation pathways, while metagenomic analysis revealed the greatest microbial differences in UC responders and nonresponders to anti-TNF therapies.
Baldan-Martin said research was ongoing with a new cohort of patients that aims to validate some of the biomarkers and help identify the patients most likely to respond to tailored therapeutic interventions.
“One of the challenges is integrating results from different omics approaches to create a more holistic understanding of the disease,” she said, adding that she hopes the research “will potentially open doors for early detection through multi-panel biomarkers.”
Session moderator Mark Samaan, MD, consultant gastroenterologist at Guy’s and St Thomas’ NHS Foundation Trust, London, England, said that “the findings related to nonresponse to specific drugs in UC and CD were interesting. With longitudinal follow-up, we’d hope this might help us pick out patients less likely to respond and who show early nonresponse to specific drugs based on serum, urine, and fecal sampling.”
“It’s very helpful to know if someone is a nonresponder within 14 weeks because we can then move the patient on to something else relatively quickly,” he added.
Baldan-Martin and Samaan declared no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — presented at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Differences uncovered in multiple messenger RNAs, proteins, metabolites, and gut microbiota were associated with responders and nonresponders to biologics and Janus kinase inhibitors, suggesting the potential for predictive biomarkers in IBD, including Crohn’s disease (CD) and ulcerative colitis (UC).
“With further work we hope to confirm these findings and evaluate their clinical relevance in identifying patients most likely to respond to tailored therapeutic interventions,” said Montserrat Baldan-Martin, PhD, a researcher at the University Hospital of the Princess, Madrid, Spain.
The treatment of IBD is challenging because of the heterogeneity across clinical, immunological, molecular, genetic, and microbiologic features, with one third of patients failing to respond to any one treatment.
Baldan-Martin and colleagues wanted to find predictive biomarkers of response by examining the differences across multi-omics profiles relative to different therapies.
The study analyzed 127 patients with IBD (57 with CD and 70 with UC) before and after 14 weeks of treatment with one of the following: anti–tumor necrosis factors (TNFs), ustekinumab, vedolizumab, or tofacitinib. Patient response to treatment was evaluated using endoscopic criteria that categorized them as responders or nonresponders to the different therapies.
In addition, molecular data from various biologic samples — serum, urine, extracellular vesicles, intestinal biopsies, and stool — were tested using transcriptomics, proteomics, metabolomics, and metagenomics.
Clear Differences
“The most significant differences were seen in gene expression within intestinal tissue of responder and nonresponder patients with ulcerative colitis taking vedolizumab,” Baldan-Martin reported.
Proteomic analysis revealed that a total of 1377 proteins were identified across all groups (CD, UC, and the four drug classes/therapies). Responders and nonresponders for each therapy expressed different proteins in serum extracellular vesicles and intestinal tissues.
For example, patients with CD who responded to anti-TNF therapies had 138 different proteins from those of anti-TNF nonresponders, while patients with UC who responded to anti-TNF therapies had 218 different proteins from those of anti-TNF nonresponders, reported Baldan-Martin.
Also, we observed almost no proteins “in common between ulcerative colitis responders versus nonresponders for all treatments,” she noted. And we “saw only three proteins in common with Crohn’s disease patients [on different drugs].”
Metabolomic analysis identified deregulation of 24 serum lipoproteins in CD responders to ustekinumab, compared with nonresponders.
“We observed greater differences in the lipoproteins in serum than metabolites in serum and urine,” Baldan-Martin added.
Analysis of biologic pathways also highlighted enrichment in ketone and butyrate metabolism, mitochondrial electron transport chain activity, carnitine synthesis, and fatty acid oxidation pathways, while metagenomic analysis revealed the greatest microbial differences in UC responders and nonresponders to anti-TNF therapies.
Baldan-Martin said research was ongoing with a new cohort of patients that aims to validate some of the biomarkers and help identify the patients most likely to respond to tailored therapeutic interventions.
“One of the challenges is integrating results from different omics approaches to create a more holistic understanding of the disease,” she said, adding that she hopes the research “will potentially open doors for early detection through multi-panel biomarkers.”
Session moderator Mark Samaan, MD, consultant gastroenterologist at Guy’s and St Thomas’ NHS Foundation Trust, London, England, said that “the findings related to nonresponse to specific drugs in UC and CD were interesting. With longitudinal follow-up, we’d hope this might help us pick out patients less likely to respond and who show early nonresponse to specific drugs based on serum, urine, and fecal sampling.”
“It’s very helpful to know if someone is a nonresponder within 14 weeks because we can then move the patient on to something else relatively quickly,” he added.
Baldan-Martin and Samaan declared no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM ECCO 2025
Not All Plant-based Diets Are Equal in IBD Risk Mitigation
BERLIN — according to the results of a large cohort study.
The study, which included both Crohn’s disease (CD) and ulcerative colitis (UC), also showed that diet quality may affect disease progression and surgery risk for individuals already diagnosed with IBD.
“Not all plant-based foods are equal — they don’t all have the same effect on health outcomes,” said study researcher, Judith Wellens, MD, PhD, gastroenterology resident at Leuven University Hospital in Belgium.
“We need to look at what people are eating more carefully because it isn’t black and white, with all plant-based food being good and animal-based food being bad,” said Wellens, who presented the data at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Although she advocates for plant-based diets, Wellens stressed that “they need to be individualized to ensure the overall dietary quality is good. Just cutting out meat products is not very helpful. We think it is the unhealthy additions to some plant-based diets that drive the IBD risk.”
Is It the Plants or the Processed Ingredients?
“Preclinical studies have already taught us that plant-based diets alter the gut microbiota in a beneficial way. However, many diets promoted for IBD — for example the Crohn’s disease exclusion diet — contain ingredients that are animal based. This is confusing for patients and for clinicians,” said Wellens.
To look more closely at the question, she and her colleagues analyzed data for 187,888 participants from the UK Biobank and 341,539 participants from across eight European countries from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. None of the participants had IBD at baseline.
Based on participant 24-hour dietary recalls, the researchers constructed plant-based diet indices (PDIs) with diets categorized as healthy (eg, whole grains, fruits, vegetables, legumes, and vegetarian protein alternatives) or unhealthy (eg, emulsifiers, refined grains, fries, fruit juices, sweets, desserts, sugar-sweetened beverages, and processed foods).
The primary outcome was the incidence of IBD (either CD or UC), whereas the secondary outcome was IBD-related surgery, thereby marking disease progression. Cox regression analysis estimated IBD risk and progression. Incidences of IBD were similar between the two cohorts.
In the UK Biobank cohort, 925 participants developed IBD over a median follow-up of 11.6 years. Participants who followed a healthy PDI had a 25% reduced IBD risk, whereas those who followed an unhealthy PDI had a 48% increased risk for disease development. Both CD and UC showed similar outcomes.
The EPIC cohort had a longer median follow-up time of 14.5 years, during which 548 people developed IBD. Healthy PDIs were linked to a 29% reduced risk for IBD, whereas unhealthy PDIs were associated with a 54% increased risk.
A healthy PDI halved the risk for surgery in participants from the UK Biobank, whereas an unhealthy PDI was associated with a twofold higher risk for surgery.
There were no significant associations between PDIs and other outcomes, such as cardiovascular disease, diabetes, or all-cause mortality.
The researchers also looked at the interactions between genetics and plant-based diets, but those results were not presented at the meeting.
However, Wellens said in an interview that people with a moderate to high risk for IBD based on their polygenetic risk score showed increased odds for IBD risk.
“We don’t test people for their genetic risk of IBD, but if people have close relatives with IBD, then there is probably an increased genetic risk of its development,” she added.
Commenting on the findings, James Lindsay, PhD, professor of inflammatory bowel disease, Queen Mary University of London in England, said that several recent epidemiological studies have highlighted “the negative impact of ultra-processed foods on increasing the risk of developing Crohn’s disease.”
Based on these studies, “one might assume that plant-based diets would be protective,” he said, however, the current study shows us “that plant-based diets are not all equal and there are unhealthy aspects to some.”
“Of course, showing that a diet is associated with an outcome is not the same as knowing that changing a diet will reduce the risk,” Lindsay added. “That requires a well-designed, carefully controlled trial.”
Wellens and Lindsay reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — according to the results of a large cohort study.
The study, which included both Crohn’s disease (CD) and ulcerative colitis (UC), also showed that diet quality may affect disease progression and surgery risk for individuals already diagnosed with IBD.
“Not all plant-based foods are equal — they don’t all have the same effect on health outcomes,” said study researcher, Judith Wellens, MD, PhD, gastroenterology resident at Leuven University Hospital in Belgium.
“We need to look at what people are eating more carefully because it isn’t black and white, with all plant-based food being good and animal-based food being bad,” said Wellens, who presented the data at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Although she advocates for plant-based diets, Wellens stressed that “they need to be individualized to ensure the overall dietary quality is good. Just cutting out meat products is not very helpful. We think it is the unhealthy additions to some plant-based diets that drive the IBD risk.”
Is It the Plants or the Processed Ingredients?
“Preclinical studies have already taught us that plant-based diets alter the gut microbiota in a beneficial way. However, many diets promoted for IBD — for example the Crohn’s disease exclusion diet — contain ingredients that are animal based. This is confusing for patients and for clinicians,” said Wellens.
To look more closely at the question, she and her colleagues analyzed data for 187,888 participants from the UK Biobank and 341,539 participants from across eight European countries from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. None of the participants had IBD at baseline.
Based on participant 24-hour dietary recalls, the researchers constructed plant-based diet indices (PDIs) with diets categorized as healthy (eg, whole grains, fruits, vegetables, legumes, and vegetarian protein alternatives) or unhealthy (eg, emulsifiers, refined grains, fries, fruit juices, sweets, desserts, sugar-sweetened beverages, and processed foods).
The primary outcome was the incidence of IBD (either CD or UC), whereas the secondary outcome was IBD-related surgery, thereby marking disease progression. Cox regression analysis estimated IBD risk and progression. Incidences of IBD were similar between the two cohorts.
In the UK Biobank cohort, 925 participants developed IBD over a median follow-up of 11.6 years. Participants who followed a healthy PDI had a 25% reduced IBD risk, whereas those who followed an unhealthy PDI had a 48% increased risk for disease development. Both CD and UC showed similar outcomes.
The EPIC cohort had a longer median follow-up time of 14.5 years, during which 548 people developed IBD. Healthy PDIs were linked to a 29% reduced risk for IBD, whereas unhealthy PDIs were associated with a 54% increased risk.
A healthy PDI halved the risk for surgery in participants from the UK Biobank, whereas an unhealthy PDI was associated with a twofold higher risk for surgery.
There were no significant associations between PDIs and other outcomes, such as cardiovascular disease, diabetes, or all-cause mortality.
The researchers also looked at the interactions between genetics and plant-based diets, but those results were not presented at the meeting.
However, Wellens said in an interview that people with a moderate to high risk for IBD based on their polygenetic risk score showed increased odds for IBD risk.
“We don’t test people for their genetic risk of IBD, but if people have close relatives with IBD, then there is probably an increased genetic risk of its development,” she added.
Commenting on the findings, James Lindsay, PhD, professor of inflammatory bowel disease, Queen Mary University of London in England, said that several recent epidemiological studies have highlighted “the negative impact of ultra-processed foods on increasing the risk of developing Crohn’s disease.”
Based on these studies, “one might assume that plant-based diets would be protective,” he said, however, the current study shows us “that plant-based diets are not all equal and there are unhealthy aspects to some.”
“Of course, showing that a diet is associated with an outcome is not the same as knowing that changing a diet will reduce the risk,” Lindsay added. “That requires a well-designed, carefully controlled trial.”
Wellens and Lindsay reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — according to the results of a large cohort study.
The study, which included both Crohn’s disease (CD) and ulcerative colitis (UC), also showed that diet quality may affect disease progression and surgery risk for individuals already diagnosed with IBD.
“Not all plant-based foods are equal — they don’t all have the same effect on health outcomes,” said study researcher, Judith Wellens, MD, PhD, gastroenterology resident at Leuven University Hospital in Belgium.
“We need to look at what people are eating more carefully because it isn’t black and white, with all plant-based food being good and animal-based food being bad,” said Wellens, who presented the data at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Although she advocates for plant-based diets, Wellens stressed that “they need to be individualized to ensure the overall dietary quality is good. Just cutting out meat products is not very helpful. We think it is the unhealthy additions to some plant-based diets that drive the IBD risk.”
Is It the Plants or the Processed Ingredients?
“Preclinical studies have already taught us that plant-based diets alter the gut microbiota in a beneficial way. However, many diets promoted for IBD — for example the Crohn’s disease exclusion diet — contain ingredients that are animal based. This is confusing for patients and for clinicians,” said Wellens.
To look more closely at the question, she and her colleagues analyzed data for 187,888 participants from the UK Biobank and 341,539 participants from across eight European countries from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. None of the participants had IBD at baseline.
Based on participant 24-hour dietary recalls, the researchers constructed plant-based diet indices (PDIs) with diets categorized as healthy (eg, whole grains, fruits, vegetables, legumes, and vegetarian protein alternatives) or unhealthy (eg, emulsifiers, refined grains, fries, fruit juices, sweets, desserts, sugar-sweetened beverages, and processed foods).
The primary outcome was the incidence of IBD (either CD or UC), whereas the secondary outcome was IBD-related surgery, thereby marking disease progression. Cox regression analysis estimated IBD risk and progression. Incidences of IBD were similar between the two cohorts.
In the UK Biobank cohort, 925 participants developed IBD over a median follow-up of 11.6 years. Participants who followed a healthy PDI had a 25% reduced IBD risk, whereas those who followed an unhealthy PDI had a 48% increased risk for disease development. Both CD and UC showed similar outcomes.
The EPIC cohort had a longer median follow-up time of 14.5 years, during which 548 people developed IBD. Healthy PDIs were linked to a 29% reduced risk for IBD, whereas unhealthy PDIs were associated with a 54% increased risk.
A healthy PDI halved the risk for surgery in participants from the UK Biobank, whereas an unhealthy PDI was associated with a twofold higher risk for surgery.
There were no significant associations between PDIs and other outcomes, such as cardiovascular disease, diabetes, or all-cause mortality.
The researchers also looked at the interactions between genetics and plant-based diets, but those results were not presented at the meeting.
However, Wellens said in an interview that people with a moderate to high risk for IBD based on their polygenetic risk score showed increased odds for IBD risk.
“We don’t test people for their genetic risk of IBD, but if people have close relatives with IBD, then there is probably an increased genetic risk of its development,” she added.
Commenting on the findings, James Lindsay, PhD, professor of inflammatory bowel disease, Queen Mary University of London in England, said that several recent epidemiological studies have highlighted “the negative impact of ultra-processed foods on increasing the risk of developing Crohn’s disease.”
Based on these studies, “one might assume that plant-based diets would be protective,” he said, however, the current study shows us “that plant-based diets are not all equal and there are unhealthy aspects to some.”
“Of course, showing that a diet is associated with an outcome is not the same as knowing that changing a diet will reduce the risk,” Lindsay added. “That requires a well-designed, carefully controlled trial.”
Wellens and Lindsay reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM ECCO 2025
Model May Predict Which UC Patients Risk Rehospitalization
a preliminary modeling study suggests.
“Absence of a gastroenterologist consultation within the year prior to admission, male sex, shorter length of hospital stay, and narcotic prescription at the time of discharge were independently associated with the risk for 90-day rehospitalization for a UC-related indication,” study author Sanjay Murthy, MD, associate professor of gastroenterology at the University of Ottawa, Ontario, Canada, and staff gastroenterologist at the Inflammatory Bowel Disease Centre at The Ottawa Hospital, said in an interview.
“While some hospital readmissions are likely unavoidable, a subset of them, particularly readmissions that occur soon after discharge, may be preventable with early and intensive postdischarge outpatient management,” he said. “Identifying those who are at high risk for early readmission is a rational first step toward applying targeted outpatient interventions that reduce this risk.”
The study was published in The Journal of the Canadian Association of Gastroenterology.
Major Predictor Variables
The researchers conducted a retrospective study in adults with UC who were admitted to The Ottawa Hospital between 2009 and 2016 for a UC flare or UC-related complication, excluding bowel cancer. Using medical records and administrative health databases, they derived and validated a multivariable logistic regression model of 90-day UC-related rehospitalization risk.
Participants’ mean age at UC diagnosis was 35.3 years and 50.4% were men. In the year before the index hospitalization, 138 (55.6%) participants had a gastroenterologist visit, whereas 41 (16.5%) were hospitalized.
During the index hospitalization, 42 (16.9%) patients were newly diagnosed with UC, and 25 (10.1%) underwent intra-abdominal surgery. At discharge, 34 (13.7%) patients were prescribed an outpatient narcotic. The mean length of hospital stay was 9.97 days. Twenty-seven individuals (10.9%) were rehospitalized within 90 days of discharge.
Out of 35 variables, the model identified the following four as significant predictors of 90-day rehospitalization: gastroenterologist consultation within the prior year (adjusted odds ratio [aOR], 0.09), male sex (aOR, 3.77), length of hospital stay (aOR, 0.93), and discharge with narcotics prescription (aOR, 5.94).
The model had 77.8% sensitivity, 80.9% specificity, 33% positive predictive value, and 96.7% negative predictive value for predicting high vs low risk for 90-day hospital readmission.
The researchers noted several study limitations. The cohort was relatively small, which limited the statistical power for model building and identifying variable associations with the outcome. In addition, the study was conducted in a single tertiary care center, which limits its generalizability. Retrospective data may have affected the accuracy of the measurements, and information on some relevant variables was not available.
Nevertheless, Murthy said, “optimally applying our prediction model at the point of hospital discharge would have classified only about a quarter of individuals in our cohort as being at high-risk for 90-day readmission and potentially needing targeted early outpatient intervention, and this would have captured close to 80% of individuals who were destined for early readmission.”
“However, our research is still preliminary and requires considerably more work to ensure that the findings are suitable for application to clinical practice,” he added. “In the meantime, practitioners may reflect on the potential importance of the major predictor variables identified in our study within their practices.”
Careful Follow-Up Key
Rajiv Bhuta, MD, assistant professor of clinical gastroenterology and hepatology at Temple University and a gastroenterologist at Temple University Hospital, both in Philadelphia, Pennsylvania, commented on the study but was not involved in it.
“The model performed fairly well (c-statistic of 0.78) using four variables: Gastroenterologist consultation within the prior year (protective), male sex (higher risk), length of stay (marginally protective), and narcotic prescription at discharge (higher risk). These are intuitive predictors that align with prior literature on UC hospitalizations,” said Bhuta.
“From a clinical perspective, this type of tool could be useful for targeting high-risk patients for early outpatient interventions (eg, close gastroenterology follow-up and pain management strategies). The negative predictive value (96.7%) suggests that it is particularly good at identifying patients at low risk for rehospitalization, which may help prioritize resource allocation more efficiently. However, practical implementation will require external validation and integration into electronic medical records to automatically flag high-risk patients at discharge.”
In addition, Bhuta noted, “the study only examines patient data through 2016. Why have the last 8 years been excluded? Given the small sample size and the sea change in available inflammatory bowel disease therapies since 2016, there could be significantly different findings with more current data.”
Furthermore, there is a lack of specific data supporting the protective effect of a gastroenterology visit in the previous year, and the readmission rate was lower than that reported by others (10% vs 20%), which, he said “may skew their findings.”
“The strong protective effect of prior gastroenterologist visits underscores the importance of specialty proactive disease management in these complex patients,” Bhuta continued. “Narcotic prescriptions at discharge may indicate inadequate disease activity control, thus making these patients important targets for close follow-up. Narcotics are generally not required once successful disease control has been achieved with steroids or biologics.
“While promising, this tool should not yet replace clinical judgment until it undergoes external validation,” he concluded. “In the meantime, clinicians should focus on structured outpatient follow-up and careful discharge planning to minimize UC-related rehospitalizations.”
This study was funded by a grant provided to Murthy by the department of medicine at the University of Ottawa. Murthy and Bhuta declared having no relevant financial relationships.
A version of this article appeared on Medscape.com .
a preliminary modeling study suggests.
“Absence of a gastroenterologist consultation within the year prior to admission, male sex, shorter length of hospital stay, and narcotic prescription at the time of discharge were independently associated with the risk for 90-day rehospitalization for a UC-related indication,” study author Sanjay Murthy, MD, associate professor of gastroenterology at the University of Ottawa, Ontario, Canada, and staff gastroenterologist at the Inflammatory Bowel Disease Centre at The Ottawa Hospital, said in an interview.
“While some hospital readmissions are likely unavoidable, a subset of them, particularly readmissions that occur soon after discharge, may be preventable with early and intensive postdischarge outpatient management,” he said. “Identifying those who are at high risk for early readmission is a rational first step toward applying targeted outpatient interventions that reduce this risk.”
The study was published in The Journal of the Canadian Association of Gastroenterology.
Major Predictor Variables
The researchers conducted a retrospective study in adults with UC who were admitted to The Ottawa Hospital between 2009 and 2016 for a UC flare or UC-related complication, excluding bowel cancer. Using medical records and administrative health databases, they derived and validated a multivariable logistic regression model of 90-day UC-related rehospitalization risk.
Participants’ mean age at UC diagnosis was 35.3 years and 50.4% were men. In the year before the index hospitalization, 138 (55.6%) participants had a gastroenterologist visit, whereas 41 (16.5%) were hospitalized.
During the index hospitalization, 42 (16.9%) patients were newly diagnosed with UC, and 25 (10.1%) underwent intra-abdominal surgery. At discharge, 34 (13.7%) patients were prescribed an outpatient narcotic. The mean length of hospital stay was 9.97 days. Twenty-seven individuals (10.9%) were rehospitalized within 90 days of discharge.
Out of 35 variables, the model identified the following four as significant predictors of 90-day rehospitalization: gastroenterologist consultation within the prior year (adjusted odds ratio [aOR], 0.09), male sex (aOR, 3.77), length of hospital stay (aOR, 0.93), and discharge with narcotics prescription (aOR, 5.94).
The model had 77.8% sensitivity, 80.9% specificity, 33% positive predictive value, and 96.7% negative predictive value for predicting high vs low risk for 90-day hospital readmission.
The researchers noted several study limitations. The cohort was relatively small, which limited the statistical power for model building and identifying variable associations with the outcome. In addition, the study was conducted in a single tertiary care center, which limits its generalizability. Retrospective data may have affected the accuracy of the measurements, and information on some relevant variables was not available.
Nevertheless, Murthy said, “optimally applying our prediction model at the point of hospital discharge would have classified only about a quarter of individuals in our cohort as being at high-risk for 90-day readmission and potentially needing targeted early outpatient intervention, and this would have captured close to 80% of individuals who were destined for early readmission.”
“However, our research is still preliminary and requires considerably more work to ensure that the findings are suitable for application to clinical practice,” he added. “In the meantime, practitioners may reflect on the potential importance of the major predictor variables identified in our study within their practices.”
Careful Follow-Up Key
Rajiv Bhuta, MD, assistant professor of clinical gastroenterology and hepatology at Temple University and a gastroenterologist at Temple University Hospital, both in Philadelphia, Pennsylvania, commented on the study but was not involved in it.
“The model performed fairly well (c-statistic of 0.78) using four variables: Gastroenterologist consultation within the prior year (protective), male sex (higher risk), length of stay (marginally protective), and narcotic prescription at discharge (higher risk). These are intuitive predictors that align with prior literature on UC hospitalizations,” said Bhuta.
“From a clinical perspective, this type of tool could be useful for targeting high-risk patients for early outpatient interventions (eg, close gastroenterology follow-up and pain management strategies). The negative predictive value (96.7%) suggests that it is particularly good at identifying patients at low risk for rehospitalization, which may help prioritize resource allocation more efficiently. However, practical implementation will require external validation and integration into electronic medical records to automatically flag high-risk patients at discharge.”
In addition, Bhuta noted, “the study only examines patient data through 2016. Why have the last 8 years been excluded? Given the small sample size and the sea change in available inflammatory bowel disease therapies since 2016, there could be significantly different findings with more current data.”
Furthermore, there is a lack of specific data supporting the protective effect of a gastroenterology visit in the previous year, and the readmission rate was lower than that reported by others (10% vs 20%), which, he said “may skew their findings.”
“The strong protective effect of prior gastroenterologist visits underscores the importance of specialty proactive disease management in these complex patients,” Bhuta continued. “Narcotic prescriptions at discharge may indicate inadequate disease activity control, thus making these patients important targets for close follow-up. Narcotics are generally not required once successful disease control has been achieved with steroids or biologics.
“While promising, this tool should not yet replace clinical judgment until it undergoes external validation,” he concluded. “In the meantime, clinicians should focus on structured outpatient follow-up and careful discharge planning to minimize UC-related rehospitalizations.”
This study was funded by a grant provided to Murthy by the department of medicine at the University of Ottawa. Murthy and Bhuta declared having no relevant financial relationships.
A version of this article appeared on Medscape.com .
a preliminary modeling study suggests.
“Absence of a gastroenterologist consultation within the year prior to admission, male sex, shorter length of hospital stay, and narcotic prescription at the time of discharge were independently associated with the risk for 90-day rehospitalization for a UC-related indication,” study author Sanjay Murthy, MD, associate professor of gastroenterology at the University of Ottawa, Ontario, Canada, and staff gastroenterologist at the Inflammatory Bowel Disease Centre at The Ottawa Hospital, said in an interview.
“While some hospital readmissions are likely unavoidable, a subset of them, particularly readmissions that occur soon after discharge, may be preventable with early and intensive postdischarge outpatient management,” he said. “Identifying those who are at high risk for early readmission is a rational first step toward applying targeted outpatient interventions that reduce this risk.”
The study was published in The Journal of the Canadian Association of Gastroenterology.
Major Predictor Variables
The researchers conducted a retrospective study in adults with UC who were admitted to The Ottawa Hospital between 2009 and 2016 for a UC flare or UC-related complication, excluding bowel cancer. Using medical records and administrative health databases, they derived and validated a multivariable logistic regression model of 90-day UC-related rehospitalization risk.
Participants’ mean age at UC diagnosis was 35.3 years and 50.4% were men. In the year before the index hospitalization, 138 (55.6%) participants had a gastroenterologist visit, whereas 41 (16.5%) were hospitalized.
During the index hospitalization, 42 (16.9%) patients were newly diagnosed with UC, and 25 (10.1%) underwent intra-abdominal surgery. At discharge, 34 (13.7%) patients were prescribed an outpatient narcotic. The mean length of hospital stay was 9.97 days. Twenty-seven individuals (10.9%) were rehospitalized within 90 days of discharge.
Out of 35 variables, the model identified the following four as significant predictors of 90-day rehospitalization: gastroenterologist consultation within the prior year (adjusted odds ratio [aOR], 0.09), male sex (aOR, 3.77), length of hospital stay (aOR, 0.93), and discharge with narcotics prescription (aOR, 5.94).
The model had 77.8% sensitivity, 80.9% specificity, 33% positive predictive value, and 96.7% negative predictive value for predicting high vs low risk for 90-day hospital readmission.
The researchers noted several study limitations. The cohort was relatively small, which limited the statistical power for model building and identifying variable associations with the outcome. In addition, the study was conducted in a single tertiary care center, which limits its generalizability. Retrospective data may have affected the accuracy of the measurements, and information on some relevant variables was not available.
Nevertheless, Murthy said, “optimally applying our prediction model at the point of hospital discharge would have classified only about a quarter of individuals in our cohort as being at high-risk for 90-day readmission and potentially needing targeted early outpatient intervention, and this would have captured close to 80% of individuals who were destined for early readmission.”
“However, our research is still preliminary and requires considerably more work to ensure that the findings are suitable for application to clinical practice,” he added. “In the meantime, practitioners may reflect on the potential importance of the major predictor variables identified in our study within their practices.”
Careful Follow-Up Key
Rajiv Bhuta, MD, assistant professor of clinical gastroenterology and hepatology at Temple University and a gastroenterologist at Temple University Hospital, both in Philadelphia, Pennsylvania, commented on the study but was not involved in it.
“The model performed fairly well (c-statistic of 0.78) using four variables: Gastroenterologist consultation within the prior year (protective), male sex (higher risk), length of stay (marginally protective), and narcotic prescription at discharge (higher risk). These are intuitive predictors that align with prior literature on UC hospitalizations,” said Bhuta.
“From a clinical perspective, this type of tool could be useful for targeting high-risk patients for early outpatient interventions (eg, close gastroenterology follow-up and pain management strategies). The negative predictive value (96.7%) suggests that it is particularly good at identifying patients at low risk for rehospitalization, which may help prioritize resource allocation more efficiently. However, practical implementation will require external validation and integration into electronic medical records to automatically flag high-risk patients at discharge.”
In addition, Bhuta noted, “the study only examines patient data through 2016. Why have the last 8 years been excluded? Given the small sample size and the sea change in available inflammatory bowel disease therapies since 2016, there could be significantly different findings with more current data.”
Furthermore, there is a lack of specific data supporting the protective effect of a gastroenterology visit in the previous year, and the readmission rate was lower than that reported by others (10% vs 20%), which, he said “may skew their findings.”
“The strong protective effect of prior gastroenterologist visits underscores the importance of specialty proactive disease management in these complex patients,” Bhuta continued. “Narcotic prescriptions at discharge may indicate inadequate disease activity control, thus making these patients important targets for close follow-up. Narcotics are generally not required once successful disease control has been achieved with steroids or biologics.
“While promising, this tool should not yet replace clinical judgment until it undergoes external validation,” he concluded. “In the meantime, clinicians should focus on structured outpatient follow-up and careful discharge planning to minimize UC-related rehospitalizations.”
This study was funded by a grant provided to Murthy by the department of medicine at the University of Ottawa. Murthy and Bhuta declared having no relevant financial relationships.
A version of this article appeared on Medscape.com .
FROM THE JOURNAL OF THE CANADIAN ASSOCIATION OF GASTROENTEROLOGY
More Layoffs at VA and Other Health Agencies
The large-scale layoffs in the federal government that began in January continue, as the US Department of Veterans Affairs (VA) announced the dismissal of > 1400 employees in “non-mission critical roles,” including those “related to DEI” (diversity, equity, inclusion) on Feb. 24. According to VA, those fired are bargaining-unit probationary employees who have served > 1 year in a competitive service appointment or who have served > 2 years in an excepted service appointment.
The agency says the “personnel moves” will save > $83 million annually, which will be redirected back toward health care, benefits and services for VA beneficiaries.
Of the nearly 40,000 probationary employees in the department, the majority were exempt, the VA says, because they serve in mission-critical positions—primarily those supporting benefits and services for VA beneficiaries, such as Veterans Crisis Line responders. VA employees who elected to participate in the Office of Personnel Management’s (OPM) deferred resignation program are also exempt. As an “additional safeguard,” the VA says the first Senior Executive Service (SES) or SES-equivalent leader in a dismissed employee’s chain of command can request the employee be exempted from removal.
The latest cuts follow the dismissal of > 1000 employees announced Feb. 13. In that case, the VA expected to save > $98 million annually, also to be “redirected back” toward health care, benefits, and services. VA insists it continues to hire for mission-critical positions that are exempt from the federal hiring freeze.
Layoffs are also impacting other federal public health agencies. Although the White House has not released figures, a ProPublica investigation details the impact of the layoffs on organ transplant and maternal mortality programs. Other layoffs that have been reported include :
- About 750 workers at the Centers for Disease Control and Prevention
- More than 1000 staffers at the National Institutes of Health
- “Dozens” at the Centers for Medicare and Medicaid Services
- “Scores” at the US Food and Drug Administration
- Downsizing at the VA EHR Modernization Integration Office
“By gutting essential health staff, hiding vital public health data, and silencing health experts, these actions have left every American family more vulnerable to deadly disease outbreaks, unsafe food and water, and preventable deaths,” the American Public Health Association said in a press release. “This is also not just an attack on federal institutions – it's a direct attack on every parent trying to protect their child from disease, every worker relying on public health safeguards and every family depending on rapid responses to outbreaks and emergencies.” American Public Health Association also announced that is suing the Department of Government Efficiency for violating federal transparency laws. “It is unfathomable that anybody thinks these cuts have value and are doing anything other than being performative.”
In 2024, the VA had planned to trim its 458,000-member workforce by about 2%, or 10,000 employees, through attrition (with most of the reduction coming from VHA). VHA Chief Financial Officer Laura Duke told reporters in March 2024 that the reduction was needed because the agency had far exceeded its hiring goals last year, and was also seeing higher-than-expected retention rates.
“These and other recent personnel decisions are extraordinarily difficult, but VA is focused on allocating its resources to help as many veterans, families, caregivers, and survivors as possible,” VA Secretary Doug Collins said. “These moves will not hurt VA health care, benefits or beneficiaries. In fact, veterans are going to notice a change for the better. In the coming weeks and months, VA will be announcing plans to put these resources to work helping the department fulfill its core mission: providing the best possible care and benefits to veterans, their families, caregivers and survivors.”
Senate Veterans’ Affairs Committee Ranking Member Richard Blumenthal (D-CT) and a group of 35 Democratic senators signed a letter earlier in February calling for Sec. Collins to immediately reinstate the terminated VA employees. “[W]e were outraged,” the letter said, “by the Administration’s abrupt and indiscriminate termination of tens of thousands of workers across almost every government agency, including more than 1000 Department of Veterans Affairs (VA) employees. We were further disturbed by the manner in which you publicly celebrated this reprehensible announcement—a clear departure from the assurances provided throughout your confirmation process to never ‘balance budgets on the back of veterans’ benefits’ and to always ‘put the veteran first.’”
Blumenthal also notes that the “continued mass terminations” come at a time when the VA faces critical staffing shortages and increased demand for its services. The senators detailed the effects the cuts were having, including how openings for new clinics were delayed because the VA cannot hire the necessary staff to open their doors; service lines at VA hospitals and clinics halted; beds and operating rooms at VA facilities suspended; support lines for caregivers reduced; Veterans Crisis Line employees fired; and suicide prevention training sessions postponed or canceled.
The large-scale layoffs in the federal government that began in January continue, as the US Department of Veterans Affairs (VA) announced the dismissal of > 1400 employees in “non-mission critical roles,” including those “related to DEI” (diversity, equity, inclusion) on Feb. 24. According to VA, those fired are bargaining-unit probationary employees who have served > 1 year in a competitive service appointment or who have served > 2 years in an excepted service appointment.
The agency says the “personnel moves” will save > $83 million annually, which will be redirected back toward health care, benefits and services for VA beneficiaries.
Of the nearly 40,000 probationary employees in the department, the majority were exempt, the VA says, because they serve in mission-critical positions—primarily those supporting benefits and services for VA beneficiaries, such as Veterans Crisis Line responders. VA employees who elected to participate in the Office of Personnel Management’s (OPM) deferred resignation program are also exempt. As an “additional safeguard,” the VA says the first Senior Executive Service (SES) or SES-equivalent leader in a dismissed employee’s chain of command can request the employee be exempted from removal.
The latest cuts follow the dismissal of > 1000 employees announced Feb. 13. In that case, the VA expected to save > $98 million annually, also to be “redirected back” toward health care, benefits, and services. VA insists it continues to hire for mission-critical positions that are exempt from the federal hiring freeze.
Layoffs are also impacting other federal public health agencies. Although the White House has not released figures, a ProPublica investigation details the impact of the layoffs on organ transplant and maternal mortality programs. Other layoffs that have been reported include :
- About 750 workers at the Centers for Disease Control and Prevention
- More than 1000 staffers at the National Institutes of Health
- “Dozens” at the Centers for Medicare and Medicaid Services
- “Scores” at the US Food and Drug Administration
- Downsizing at the VA EHR Modernization Integration Office
“By gutting essential health staff, hiding vital public health data, and silencing health experts, these actions have left every American family more vulnerable to deadly disease outbreaks, unsafe food and water, and preventable deaths,” the American Public Health Association said in a press release. “This is also not just an attack on federal institutions – it's a direct attack on every parent trying to protect their child from disease, every worker relying on public health safeguards and every family depending on rapid responses to outbreaks and emergencies.” American Public Health Association also announced that is suing the Department of Government Efficiency for violating federal transparency laws. “It is unfathomable that anybody thinks these cuts have value and are doing anything other than being performative.”
In 2024, the VA had planned to trim its 458,000-member workforce by about 2%, or 10,000 employees, through attrition (with most of the reduction coming from VHA). VHA Chief Financial Officer Laura Duke told reporters in March 2024 that the reduction was needed because the agency had far exceeded its hiring goals last year, and was also seeing higher-than-expected retention rates.
“These and other recent personnel decisions are extraordinarily difficult, but VA is focused on allocating its resources to help as many veterans, families, caregivers, and survivors as possible,” VA Secretary Doug Collins said. “These moves will not hurt VA health care, benefits or beneficiaries. In fact, veterans are going to notice a change for the better. In the coming weeks and months, VA will be announcing plans to put these resources to work helping the department fulfill its core mission: providing the best possible care and benefits to veterans, their families, caregivers and survivors.”
Senate Veterans’ Affairs Committee Ranking Member Richard Blumenthal (D-CT) and a group of 35 Democratic senators signed a letter earlier in February calling for Sec. Collins to immediately reinstate the terminated VA employees. “[W]e were outraged,” the letter said, “by the Administration’s abrupt and indiscriminate termination of tens of thousands of workers across almost every government agency, including more than 1000 Department of Veterans Affairs (VA) employees. We were further disturbed by the manner in which you publicly celebrated this reprehensible announcement—a clear departure from the assurances provided throughout your confirmation process to never ‘balance budgets on the back of veterans’ benefits’ and to always ‘put the veteran first.’”
Blumenthal also notes that the “continued mass terminations” come at a time when the VA faces critical staffing shortages and increased demand for its services. The senators detailed the effects the cuts were having, including how openings for new clinics were delayed because the VA cannot hire the necessary staff to open their doors; service lines at VA hospitals and clinics halted; beds and operating rooms at VA facilities suspended; support lines for caregivers reduced; Veterans Crisis Line employees fired; and suicide prevention training sessions postponed or canceled.
The large-scale layoffs in the federal government that began in January continue, as the US Department of Veterans Affairs (VA) announced the dismissal of > 1400 employees in “non-mission critical roles,” including those “related to DEI” (diversity, equity, inclusion) on Feb. 24. According to VA, those fired are bargaining-unit probationary employees who have served > 1 year in a competitive service appointment or who have served > 2 years in an excepted service appointment.
The agency says the “personnel moves” will save > $83 million annually, which will be redirected back toward health care, benefits and services for VA beneficiaries.
Of the nearly 40,000 probationary employees in the department, the majority were exempt, the VA says, because they serve in mission-critical positions—primarily those supporting benefits and services for VA beneficiaries, such as Veterans Crisis Line responders. VA employees who elected to participate in the Office of Personnel Management’s (OPM) deferred resignation program are also exempt. As an “additional safeguard,” the VA says the first Senior Executive Service (SES) or SES-equivalent leader in a dismissed employee’s chain of command can request the employee be exempted from removal.
The latest cuts follow the dismissal of > 1000 employees announced Feb. 13. In that case, the VA expected to save > $98 million annually, also to be “redirected back” toward health care, benefits, and services. VA insists it continues to hire for mission-critical positions that are exempt from the federal hiring freeze.
Layoffs are also impacting other federal public health agencies. Although the White House has not released figures, a ProPublica investigation details the impact of the layoffs on organ transplant and maternal mortality programs. Other layoffs that have been reported include :
- About 750 workers at the Centers for Disease Control and Prevention
- More than 1000 staffers at the National Institutes of Health
- “Dozens” at the Centers for Medicare and Medicaid Services
- “Scores” at the US Food and Drug Administration
- Downsizing at the VA EHR Modernization Integration Office
“By gutting essential health staff, hiding vital public health data, and silencing health experts, these actions have left every American family more vulnerable to deadly disease outbreaks, unsafe food and water, and preventable deaths,” the American Public Health Association said in a press release. “This is also not just an attack on federal institutions – it's a direct attack on every parent trying to protect their child from disease, every worker relying on public health safeguards and every family depending on rapid responses to outbreaks and emergencies.” American Public Health Association also announced that is suing the Department of Government Efficiency for violating federal transparency laws. “It is unfathomable that anybody thinks these cuts have value and are doing anything other than being performative.”
In 2024, the VA had planned to trim its 458,000-member workforce by about 2%, or 10,000 employees, through attrition (with most of the reduction coming from VHA). VHA Chief Financial Officer Laura Duke told reporters in March 2024 that the reduction was needed because the agency had far exceeded its hiring goals last year, and was also seeing higher-than-expected retention rates.
“These and other recent personnel decisions are extraordinarily difficult, but VA is focused on allocating its resources to help as many veterans, families, caregivers, and survivors as possible,” VA Secretary Doug Collins said. “These moves will not hurt VA health care, benefits or beneficiaries. In fact, veterans are going to notice a change for the better. In the coming weeks and months, VA will be announcing plans to put these resources to work helping the department fulfill its core mission: providing the best possible care and benefits to veterans, their families, caregivers and survivors.”
Senate Veterans’ Affairs Committee Ranking Member Richard Blumenthal (D-CT) and a group of 35 Democratic senators signed a letter earlier in February calling for Sec. Collins to immediately reinstate the terminated VA employees. “[W]e were outraged,” the letter said, “by the Administration’s abrupt and indiscriminate termination of tens of thousands of workers across almost every government agency, including more than 1000 Department of Veterans Affairs (VA) employees. We were further disturbed by the manner in which you publicly celebrated this reprehensible announcement—a clear departure from the assurances provided throughout your confirmation process to never ‘balance budgets on the back of veterans’ benefits’ and to always ‘put the veteran first.’”
Blumenthal also notes that the “continued mass terminations” come at a time when the VA faces critical staffing shortages and increased demand for its services. The senators detailed the effects the cuts were having, including how openings for new clinics were delayed because the VA cannot hire the necessary staff to open their doors; service lines at VA hospitals and clinics halted; beds and operating rooms at VA facilities suspended; support lines for caregivers reduced; Veterans Crisis Line employees fired; and suicide prevention training sessions postponed or canceled.
ASCO Updates Treatment Guidance for Newly Diagnosed, Advanced Ovarian Cancer
The American Society of Clinical Oncology (ASCO) has released updated guidelines for neoadjuvant chemotherapy in newly diagnosed advanced ovarian cancer, introducing changes in patient selection and treatment strategies. The changes reflect emerging evidence on racial disparities, treatment outcomes, and quality of life considerations.
The publication of the new guidance follows dramatic shifts in treatment patterns over the past decade.
“There had been a big shift in how we were treating patients in the United States,” explained Stephanie Gaillard, MD, PhD, one of the authors of the updated guidelines. “We saw a substantial drop in the number of patients undergoing primary cytoreductive surgery for ovarian cancer from about 70% of patients in 2010 to only about 37% in 2021.”
The new guidelines maintain the recommendation for platinum/taxane-based neoadjuvant chemotherapy but introduce modifications regarding timing and duration.
“It’s still a recommendation that gynecologic oncologists are involved in determining whether someone is eligible for primary cytoreductive surgery or should undergo neoadjuvant chemotherapy first,” Gaillard noted. “We emphasize that patients who are eligible for primary cytoreductive surgery should undergo surgery as opposed to receiving neoadjuvant chemotherapy.”
Alexander Melamed, MD, MPH, a gynecologic oncologist at Massachusetts General Hospital, Boston, who was not involved in authoring the updated guidelines, noted that additional evidence-based guidance is needed to individualize treatment plans. He pointed to four completed trials comparing neoadjuvant chemotherapy with cytoreductive surgery, noting: “When these trials have been pooled together in meta-analyses, there was a higher risk of mortality associated with primary cytoreductive surgery and a higher risk of severe complications.”
The updated guidelines take this higher risk for mortality with primary cytoreductive surgery into consideration, and patients who are not eligible for primary surgery would receive neoadjuvant chemotherapy, Gaillard noted.
Changes in Patient Selection
The 2025 guidelines describe a more nuanced approach for selecting patients for neoadjuvant chemotherapy vs primary cytoreductive surgery. While the 2016 ASCO guidelines primarily focused on disease burden and surgical resectability when selecting patients for neoadjuvant chemotherapy, the new recommendations incorporate additional factors.
The guidelines discuss recent findings showing that Black patients experience a 38% lower likelihood of undergoing cytoreductive surgery than non-Black patients. In addition, compared with non-Hispanic White women, Asian and Black women more frequently receive neoadjuvant chemotherapy with interval debulking surgery rather than primary cytoreductive surgery. According to the authors, these differences persist even after accounting for clinical factors, suggesting that structural barriers to healthcare access may play a role.
The guidelines discuss how affordability, availability, and accessibility mediate racial disparities in ovarian cancer care. According to the authors, structural inequities in healthcare access influence treatment quality for minority patients. Non-White patients face greater challenges in accessing gynecologic oncology consultations and standard-of-care combination therapy, leading to poorer survival outcomes, the guidelines say.
According to Melamed, the guidelines serve as an important tool for promoting healthcare equity. “Having recommendations and standards is incredibly important for achieving equity because once there is consensus on a best practice, it doesn’t matter if you’re rich, poor, or a patient of a particular racial or ethnic group — if you have the disease, you ought to have access to that standard,” he said.
The 2016 ASCO guidelines focused primarily on disease burden and surgical resectability, whereas the 2024 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for ovarian cancer focus more on oncologic outcomes and surgical considerations. Based on the NCCN guidelines, treatment selection for ovarian cancer is primarily determined by the histologic subtype, stage of disease, and whether the patient is a candidate for primary surgery. The 2025 ASCO guidelines, on the other hand, emphasize the importance of quality-of-life outcomes during treatment selection. The authors of the updated ASCO guidelines acknowledged that treatment decisions should consider both the duration and quality of life, particularly for elderly patients or those with multiple comorbidities.
Treatment Timing and Duration
The guidelines maintain the recommendations for platinum/taxane-based neoadjuvant chemotherapy described in the previous ASCO guidelines but introduce modifications regarding treatment timing and duration. The optimal window for interval cytoreductive surgery now falls after three to four chemotherapy cycles, allowing more individualized approaches based on patient response and tolerance.
In addition, postsurgical chemotherapy protocols have become more flexible. Rather than mandating a fixed number of cycles, the guidelines encourage tailoring treatment duration to individual patient factors including response assessment, performance status, and quality-of-life considerations.
The updated guidelines also emphasize the importance of genetic and molecular testing at diagnosis, which Melamed identifies as “absolutely central to treatment and deciding who receives maintenance therapy.” This is also recommended by the NCCN guidelines.
However, he highlighted the following practical challenge in molecular testing after neoadjuvant chemotherapy. “Probably 20% of patients have an exceptional response to neoadjuvant therapy, such that there is insufficient tissue at the time of their cytoreduction to do somatic testing,” he said.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
A notable difference between the 2016 and 2025 guidelines is the inclusion of HIPEC in the updated guidelines.
Commenting to this news organization, Gaillard explained the nuanced approach to HIPEC: “The committee discussed HIPEC extensively. We recognize that it may not be available at many centers and requires specially trained staff and dedicated resources. The reason for including HIPEC in the guidelines is to highlight that there have been studies that show a potential overall survival benefit.”
Melamed considers the recommendation of HIPEC to be one of the strongest aspects of the updated guidelines. “There have been two large trials and one smaller one that have shown that for patients treated with neoadjuvant chemotherapy, the addition of HIPEC appears to improve overall survival,” he explained.
Implementation Strategies
The authors acknowledged that barriers to healthcare delivery present significant challenges to the implementation of the guidelines. Limited access to gynecologic oncologists in rural areas, insurance coverage gaps, and varying surgical expertise across institutions complicate the delivery of optimal care. The guidelines also emphasize the need for solutions to ensure equitable access to recommended treatments.
Melamed noted that the decentralized structure of the healthcare system in the United States complicates the uniform adoption of guidelines, particularly in resource-limited settings, adding that “geographic region and local resources and expertise influence both access to treatment and outcomes.”
Although both the updated ASCO guidelines and NCCN guidelines emphasize the importance of evaluation by a gynecologic oncologist for determining the most appropriate treatment strategy, the scarcity of gynecologic oncologists is one of the most significant barriers to accessing optimal care, according to Gaillard. She proposes telemedicine consultations and enhanced communication between medical oncologists and gynecologic oncologists to ensure equitable access.
Gaillard also commented on the challenges in implementing a multidisciplinary treatment approach, the importance of which is emphasized in the updated guidelines.
“There can be a limited availability of the multidisciplinary team to be involved in this decision-making,” she said. “Ideally, patient assessment by a gynecologic oncologist would happen in person, but recognizing that availability is limited, it doesn’t necessarily have to. Sometimes, it can just be a conversation between a medical oncologist and a gynecologic oncologist detailing a treatment plan together.”
Looking Ahead
Gaillard noted that ovarian cancer is a very active field of research and that the guidelines may need to be updated again in the near future to incorporate novel treatment approaches.
“Newer and more effective targeted therapies based on tumor profiling are being developed,” she said. “These will hopefully move earlier in the treatment course for patients. Maybe we will not use chemotherapy in the future because we will have more directed and targeted therapies.”
She also emphasized the importance of early diagnosis in shaping future treatment guidelines for ovarian cancer.
“Neoadjuvant chemotherapy is predominantly used in situations where patients have very advanced disease and may not benefit from primary cytoreductive surgery,” she noted. “If we develop better diagnostic tools that will allow us to diagnose patients earlier, then we may not need to use neoadjuvant chemotherapy.”
All funding for the administration of the guideline development project was provided by ASCO. Gaillard reported receiving consulting or advisory fees from Verastem, Merck, AstraZeneca, and Compugen; research funding from AstraZeneca, Tesaro, Compugen, Genentech/Roche, Clovis Oncology, Tempest Therapeutics, Blueprint Pharmaceutic, Immunogen, Volastra Therapeutics, and Beigene; and patents, royalties, or other intellectual property from US Patent Nos 10,258,604 and 10,905,659, licensed by Duke University to Sermonix. Melamed reported receiving research funding from the National Cancer Institute and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has released updated guidelines for neoadjuvant chemotherapy in newly diagnosed advanced ovarian cancer, introducing changes in patient selection and treatment strategies. The changes reflect emerging evidence on racial disparities, treatment outcomes, and quality of life considerations.
The publication of the new guidance follows dramatic shifts in treatment patterns over the past decade.
“There had been a big shift in how we were treating patients in the United States,” explained Stephanie Gaillard, MD, PhD, one of the authors of the updated guidelines. “We saw a substantial drop in the number of patients undergoing primary cytoreductive surgery for ovarian cancer from about 70% of patients in 2010 to only about 37% in 2021.”
The new guidelines maintain the recommendation for platinum/taxane-based neoadjuvant chemotherapy but introduce modifications regarding timing and duration.
“It’s still a recommendation that gynecologic oncologists are involved in determining whether someone is eligible for primary cytoreductive surgery or should undergo neoadjuvant chemotherapy first,” Gaillard noted. “We emphasize that patients who are eligible for primary cytoreductive surgery should undergo surgery as opposed to receiving neoadjuvant chemotherapy.”
Alexander Melamed, MD, MPH, a gynecologic oncologist at Massachusetts General Hospital, Boston, who was not involved in authoring the updated guidelines, noted that additional evidence-based guidance is needed to individualize treatment plans. He pointed to four completed trials comparing neoadjuvant chemotherapy with cytoreductive surgery, noting: “When these trials have been pooled together in meta-analyses, there was a higher risk of mortality associated with primary cytoreductive surgery and a higher risk of severe complications.”
The updated guidelines take this higher risk for mortality with primary cytoreductive surgery into consideration, and patients who are not eligible for primary surgery would receive neoadjuvant chemotherapy, Gaillard noted.
Changes in Patient Selection
The 2025 guidelines describe a more nuanced approach for selecting patients for neoadjuvant chemotherapy vs primary cytoreductive surgery. While the 2016 ASCO guidelines primarily focused on disease burden and surgical resectability when selecting patients for neoadjuvant chemotherapy, the new recommendations incorporate additional factors.
The guidelines discuss recent findings showing that Black patients experience a 38% lower likelihood of undergoing cytoreductive surgery than non-Black patients. In addition, compared with non-Hispanic White women, Asian and Black women more frequently receive neoadjuvant chemotherapy with interval debulking surgery rather than primary cytoreductive surgery. According to the authors, these differences persist even after accounting for clinical factors, suggesting that structural barriers to healthcare access may play a role.
The guidelines discuss how affordability, availability, and accessibility mediate racial disparities in ovarian cancer care. According to the authors, structural inequities in healthcare access influence treatment quality for minority patients. Non-White patients face greater challenges in accessing gynecologic oncology consultations and standard-of-care combination therapy, leading to poorer survival outcomes, the guidelines say.
According to Melamed, the guidelines serve as an important tool for promoting healthcare equity. “Having recommendations and standards is incredibly important for achieving equity because once there is consensus on a best practice, it doesn’t matter if you’re rich, poor, or a patient of a particular racial or ethnic group — if you have the disease, you ought to have access to that standard,” he said.
The 2016 ASCO guidelines focused primarily on disease burden and surgical resectability, whereas the 2024 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for ovarian cancer focus more on oncologic outcomes and surgical considerations. Based on the NCCN guidelines, treatment selection for ovarian cancer is primarily determined by the histologic subtype, stage of disease, and whether the patient is a candidate for primary surgery. The 2025 ASCO guidelines, on the other hand, emphasize the importance of quality-of-life outcomes during treatment selection. The authors of the updated ASCO guidelines acknowledged that treatment decisions should consider both the duration and quality of life, particularly for elderly patients or those with multiple comorbidities.
Treatment Timing and Duration
The guidelines maintain the recommendations for platinum/taxane-based neoadjuvant chemotherapy described in the previous ASCO guidelines but introduce modifications regarding treatment timing and duration. The optimal window for interval cytoreductive surgery now falls after three to four chemotherapy cycles, allowing more individualized approaches based on patient response and tolerance.
In addition, postsurgical chemotherapy protocols have become more flexible. Rather than mandating a fixed number of cycles, the guidelines encourage tailoring treatment duration to individual patient factors including response assessment, performance status, and quality-of-life considerations.
The updated guidelines also emphasize the importance of genetic and molecular testing at diagnosis, which Melamed identifies as “absolutely central to treatment and deciding who receives maintenance therapy.” This is also recommended by the NCCN guidelines.
However, he highlighted the following practical challenge in molecular testing after neoadjuvant chemotherapy. “Probably 20% of patients have an exceptional response to neoadjuvant therapy, such that there is insufficient tissue at the time of their cytoreduction to do somatic testing,” he said.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
A notable difference between the 2016 and 2025 guidelines is the inclusion of HIPEC in the updated guidelines.
Commenting to this news organization, Gaillard explained the nuanced approach to HIPEC: “The committee discussed HIPEC extensively. We recognize that it may not be available at many centers and requires specially trained staff and dedicated resources. The reason for including HIPEC in the guidelines is to highlight that there have been studies that show a potential overall survival benefit.”
Melamed considers the recommendation of HIPEC to be one of the strongest aspects of the updated guidelines. “There have been two large trials and one smaller one that have shown that for patients treated with neoadjuvant chemotherapy, the addition of HIPEC appears to improve overall survival,” he explained.
Implementation Strategies
The authors acknowledged that barriers to healthcare delivery present significant challenges to the implementation of the guidelines. Limited access to gynecologic oncologists in rural areas, insurance coverage gaps, and varying surgical expertise across institutions complicate the delivery of optimal care. The guidelines also emphasize the need for solutions to ensure equitable access to recommended treatments.
Melamed noted that the decentralized structure of the healthcare system in the United States complicates the uniform adoption of guidelines, particularly in resource-limited settings, adding that “geographic region and local resources and expertise influence both access to treatment and outcomes.”
Although both the updated ASCO guidelines and NCCN guidelines emphasize the importance of evaluation by a gynecologic oncologist for determining the most appropriate treatment strategy, the scarcity of gynecologic oncologists is one of the most significant barriers to accessing optimal care, according to Gaillard. She proposes telemedicine consultations and enhanced communication between medical oncologists and gynecologic oncologists to ensure equitable access.
Gaillard also commented on the challenges in implementing a multidisciplinary treatment approach, the importance of which is emphasized in the updated guidelines.
“There can be a limited availability of the multidisciplinary team to be involved in this decision-making,” she said. “Ideally, patient assessment by a gynecologic oncologist would happen in person, but recognizing that availability is limited, it doesn’t necessarily have to. Sometimes, it can just be a conversation between a medical oncologist and a gynecologic oncologist detailing a treatment plan together.”
Looking Ahead
Gaillard noted that ovarian cancer is a very active field of research and that the guidelines may need to be updated again in the near future to incorporate novel treatment approaches.
“Newer and more effective targeted therapies based on tumor profiling are being developed,” she said. “These will hopefully move earlier in the treatment course for patients. Maybe we will not use chemotherapy in the future because we will have more directed and targeted therapies.”
She also emphasized the importance of early diagnosis in shaping future treatment guidelines for ovarian cancer.
“Neoadjuvant chemotherapy is predominantly used in situations where patients have very advanced disease and may not benefit from primary cytoreductive surgery,” she noted. “If we develop better diagnostic tools that will allow us to diagnose patients earlier, then we may not need to use neoadjuvant chemotherapy.”
All funding for the administration of the guideline development project was provided by ASCO. Gaillard reported receiving consulting or advisory fees from Verastem, Merck, AstraZeneca, and Compugen; research funding from AstraZeneca, Tesaro, Compugen, Genentech/Roche, Clovis Oncology, Tempest Therapeutics, Blueprint Pharmaceutic, Immunogen, Volastra Therapeutics, and Beigene; and patents, royalties, or other intellectual property from US Patent Nos 10,258,604 and 10,905,659, licensed by Duke University to Sermonix. Melamed reported receiving research funding from the National Cancer Institute and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has released updated guidelines for neoadjuvant chemotherapy in newly diagnosed advanced ovarian cancer, introducing changes in patient selection and treatment strategies. The changes reflect emerging evidence on racial disparities, treatment outcomes, and quality of life considerations.
The publication of the new guidance follows dramatic shifts in treatment patterns over the past decade.
“There had been a big shift in how we were treating patients in the United States,” explained Stephanie Gaillard, MD, PhD, one of the authors of the updated guidelines. “We saw a substantial drop in the number of patients undergoing primary cytoreductive surgery for ovarian cancer from about 70% of patients in 2010 to only about 37% in 2021.”
The new guidelines maintain the recommendation for platinum/taxane-based neoadjuvant chemotherapy but introduce modifications regarding timing and duration.
“It’s still a recommendation that gynecologic oncologists are involved in determining whether someone is eligible for primary cytoreductive surgery or should undergo neoadjuvant chemotherapy first,” Gaillard noted. “We emphasize that patients who are eligible for primary cytoreductive surgery should undergo surgery as opposed to receiving neoadjuvant chemotherapy.”
Alexander Melamed, MD, MPH, a gynecologic oncologist at Massachusetts General Hospital, Boston, who was not involved in authoring the updated guidelines, noted that additional evidence-based guidance is needed to individualize treatment plans. He pointed to four completed trials comparing neoadjuvant chemotherapy with cytoreductive surgery, noting: “When these trials have been pooled together in meta-analyses, there was a higher risk of mortality associated with primary cytoreductive surgery and a higher risk of severe complications.”
The updated guidelines take this higher risk for mortality with primary cytoreductive surgery into consideration, and patients who are not eligible for primary surgery would receive neoadjuvant chemotherapy, Gaillard noted.
Changes in Patient Selection
The 2025 guidelines describe a more nuanced approach for selecting patients for neoadjuvant chemotherapy vs primary cytoreductive surgery. While the 2016 ASCO guidelines primarily focused on disease burden and surgical resectability when selecting patients for neoadjuvant chemotherapy, the new recommendations incorporate additional factors.
The guidelines discuss recent findings showing that Black patients experience a 38% lower likelihood of undergoing cytoreductive surgery than non-Black patients. In addition, compared with non-Hispanic White women, Asian and Black women more frequently receive neoadjuvant chemotherapy with interval debulking surgery rather than primary cytoreductive surgery. According to the authors, these differences persist even after accounting for clinical factors, suggesting that structural barriers to healthcare access may play a role.
The guidelines discuss how affordability, availability, and accessibility mediate racial disparities in ovarian cancer care. According to the authors, structural inequities in healthcare access influence treatment quality for minority patients. Non-White patients face greater challenges in accessing gynecologic oncology consultations and standard-of-care combination therapy, leading to poorer survival outcomes, the guidelines say.
According to Melamed, the guidelines serve as an important tool for promoting healthcare equity. “Having recommendations and standards is incredibly important for achieving equity because once there is consensus on a best practice, it doesn’t matter if you’re rich, poor, or a patient of a particular racial or ethnic group — if you have the disease, you ought to have access to that standard,” he said.
The 2016 ASCO guidelines focused primarily on disease burden and surgical resectability, whereas the 2024 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for ovarian cancer focus more on oncologic outcomes and surgical considerations. Based on the NCCN guidelines, treatment selection for ovarian cancer is primarily determined by the histologic subtype, stage of disease, and whether the patient is a candidate for primary surgery. The 2025 ASCO guidelines, on the other hand, emphasize the importance of quality-of-life outcomes during treatment selection. The authors of the updated ASCO guidelines acknowledged that treatment decisions should consider both the duration and quality of life, particularly for elderly patients or those with multiple comorbidities.
Treatment Timing and Duration
The guidelines maintain the recommendations for platinum/taxane-based neoadjuvant chemotherapy described in the previous ASCO guidelines but introduce modifications regarding treatment timing and duration. The optimal window for interval cytoreductive surgery now falls after three to four chemotherapy cycles, allowing more individualized approaches based on patient response and tolerance.
In addition, postsurgical chemotherapy protocols have become more flexible. Rather than mandating a fixed number of cycles, the guidelines encourage tailoring treatment duration to individual patient factors including response assessment, performance status, and quality-of-life considerations.
The updated guidelines also emphasize the importance of genetic and molecular testing at diagnosis, which Melamed identifies as “absolutely central to treatment and deciding who receives maintenance therapy.” This is also recommended by the NCCN guidelines.
However, he highlighted the following practical challenge in molecular testing after neoadjuvant chemotherapy. “Probably 20% of patients have an exceptional response to neoadjuvant therapy, such that there is insufficient tissue at the time of their cytoreduction to do somatic testing,” he said.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
A notable difference between the 2016 and 2025 guidelines is the inclusion of HIPEC in the updated guidelines.
Commenting to this news organization, Gaillard explained the nuanced approach to HIPEC: “The committee discussed HIPEC extensively. We recognize that it may not be available at many centers and requires specially trained staff and dedicated resources. The reason for including HIPEC in the guidelines is to highlight that there have been studies that show a potential overall survival benefit.”
Melamed considers the recommendation of HIPEC to be one of the strongest aspects of the updated guidelines. “There have been two large trials and one smaller one that have shown that for patients treated with neoadjuvant chemotherapy, the addition of HIPEC appears to improve overall survival,” he explained.
Implementation Strategies
The authors acknowledged that barriers to healthcare delivery present significant challenges to the implementation of the guidelines. Limited access to gynecologic oncologists in rural areas, insurance coverage gaps, and varying surgical expertise across institutions complicate the delivery of optimal care. The guidelines also emphasize the need for solutions to ensure equitable access to recommended treatments.
Melamed noted that the decentralized structure of the healthcare system in the United States complicates the uniform adoption of guidelines, particularly in resource-limited settings, adding that “geographic region and local resources and expertise influence both access to treatment and outcomes.”
Although both the updated ASCO guidelines and NCCN guidelines emphasize the importance of evaluation by a gynecologic oncologist for determining the most appropriate treatment strategy, the scarcity of gynecologic oncologists is one of the most significant barriers to accessing optimal care, according to Gaillard. She proposes telemedicine consultations and enhanced communication between medical oncologists and gynecologic oncologists to ensure equitable access.
Gaillard also commented on the challenges in implementing a multidisciplinary treatment approach, the importance of which is emphasized in the updated guidelines.
“There can be a limited availability of the multidisciplinary team to be involved in this decision-making,” she said. “Ideally, patient assessment by a gynecologic oncologist would happen in person, but recognizing that availability is limited, it doesn’t necessarily have to. Sometimes, it can just be a conversation between a medical oncologist and a gynecologic oncologist detailing a treatment plan together.”
Looking Ahead
Gaillard noted that ovarian cancer is a very active field of research and that the guidelines may need to be updated again in the near future to incorporate novel treatment approaches.
“Newer and more effective targeted therapies based on tumor profiling are being developed,” she said. “These will hopefully move earlier in the treatment course for patients. Maybe we will not use chemotherapy in the future because we will have more directed and targeted therapies.”
She also emphasized the importance of early diagnosis in shaping future treatment guidelines for ovarian cancer.
“Neoadjuvant chemotherapy is predominantly used in situations where patients have very advanced disease and may not benefit from primary cytoreductive surgery,” she noted. “If we develop better diagnostic tools that will allow us to diagnose patients earlier, then we may not need to use neoadjuvant chemotherapy.”
All funding for the administration of the guideline development project was provided by ASCO. Gaillard reported receiving consulting or advisory fees from Verastem, Merck, AstraZeneca, and Compugen; research funding from AstraZeneca, Tesaro, Compugen, Genentech/Roche, Clovis Oncology, Tempest Therapeutics, Blueprint Pharmaceutic, Immunogen, Volastra Therapeutics, and Beigene; and patents, royalties, or other intellectual property from US Patent Nos 10,258,604 and 10,905,659, licensed by Duke University to Sermonix. Melamed reported receiving research funding from the National Cancer Institute and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Choosing the Ideal Endoscopic Enteral Access Method: AGA Practice Update
At least 250,000 US hospitalized patients a year require enteral support using an artificial pathway into the gastrointestinal (GI) tract to deliver nutrition or medication. In light of this,
Covering indications, placement techniques, and management, the comprehensive document is a response to the increasing use of enteral access devices in chronic GI conditions. The update, published in Gastroenterology, addresses patient factors complicating placement decision-making such as thrombocytopenia, use of dual antiplatelet therapy, or performance of percutaneous access in the setting of cirrhosis.
“We provide clinical recommendations in these various scenarios understanding that the final decision-making is in the hands of the provider and care team,” said first author Dejan Micic, MD, a gastroenterologist and associate professor at University of Chicago Medical Center in Illinois at the time of the update (since relocated to Loyola University Medical Center in Chicago). “We hope this can serve a day-to-day purpose for clinical gastroenterologists and can be referenced as they encounter individuals with or needing an enteral access device.”
Traditionally, enteral access was reserved for patients with severe malnutrition or those unable to maintain oral intake. Recent recommendations emphasize early nutritional intervention including prehabilitation before major surgery, adjunctive therapy for oncology patients, and in specific inflammatory conditions such as Crohn’s disease. “These shifts recognize the role of enteral nutrition not only in preventing malnutrition but also as a therapeutic strategy,” Micic said in an interview.
There is, however, variability in the use of devices including the selection of appropriate units, technical aspects of placement, and subsequent management. “Such variability can lead to complications, suboptimal patient outcomes, and inefficiencies in care delivery,” Micic said.
He added that enteral access has been historically underemphasized in GI endoscopic training. “While procedural skill in placing devices such as percutaneous endoscopic gastrostomy, or PEG, tubes is often taught, a comprehensive understanding of the broader clinical context — such as proper patient selection, prevention of complications, and postplacement care — is not always thoroughly covered.”
The current update aims to bridge knowledge gaps with evidence-based-guidance. “It also underscores the importance of interdisciplinary collaboration with dietitians, nurses, and care givers to achieve the best outcomes for patients,” Micic said.
Commenting on the update but not involved with creating it, Shirley C. Paski, MD, MS, a gastroenterologist at the Cleveland Clinic, Ohio, called it timely, adding: “As GI training is becoming more subspecialized and interventional radiology has been able to provide enteral access, gastroenterology training in enteral access has declined to where some fellows are graduating with limited enteral access experience.”
Yet malnutrition remains a common consequence when GI disease is severe, chronic, or refractory to treatment, or in the setting of postsurgical anatomy, she added. “Enteral nutrition is increasingly being considered a therapeutic or adjunct treatment in some cases of Crohn’s disease or small intestinal bacterial overgrowth. Gastroenterologists need the endoscopic skill to secure enteral access tubes, particularly in more challenging anatomy.”
Also commenting on the document but not involved in it, Steven Shamah, MD, director of Endoscopy at Northwell Lenox Hill Hospital in New York City, said: “This should serve as a concise review for any general hospitalist or gastroenterologist to understand what we have and when we should offer the proper feeding tube options.” He stressed, however, that all gastroenterologists should be trained in the placing of all of tube options.
“The axiom ‘If the gut works, we should use it’ is something that I was taught when I was a medical student and it still holds true,” Shamah continued. “There’s been a jump in interventional procedures to assure continuity of the GI tract even in progressive malignancy. So there’s a rise in moving away from intravenous nutrition and a rise in tube-delivered enteral nutrition.” Options for reducing reflux and aspiration will likely take on more importance, he said.
Tubing Options
According to Micic and colleagues, recent data suggest a favorable safety profile of enteral feeding tubes placed endoscopically compared with surgical or radiologic placement. The illustrated AGA document outlines such approaches as synthetic flexible tubes placed into the stomach or small bowel via the oral (orogastric and oroenteral) or nasal routes (nasogastric [NG] and nasojejunal [NJ]) and percutaneous tubes accessing the stomach. The choice of tube, access point, delivery site, and feeding method varies with indication, expected duration of use, and patient anatomy, the authors stressed.
The update notes that NG and NJ tubes can be used immediately after confirmation of placement, most often with abdominal radiography. PEG tubes can be used immediately for medications and after 4 hours for tube feedings. A multidisciplinary team approach after placement provides improved patient care. “Dietitians assist with formula choice, volume, free water needs, and delivery method, and nurses and advanced practice clinicians assist with tube site assessment and troubleshooting,” the authors wrote.
Complications can occur but should be infrequent, Micic said. “Frankly, most complications can be predicted based on the duration of use and prevented with appropriate monitoring.” Common complications include tube dislodgement, clogging, site infections, buried bumper syndrome, and aspiration. “Minimizing these risks requires a thorough understanding of patient-specific factors, careful technique during placement, and ongoing monitoring after the device is in use,” he added.
Paski said the update aligns with established guidelines for enteral access but also offers suggestions to mitigate the risk of tube placement in patients in whom placement has traditionally been more challenging. “This is a helpful addition to the literature because if enteral access cannot be obtained in a patient unable to meet their needs orally, total paternal nutrition is the next and much more invasive step for nutrition support.”
She called the practice update a concise, comprehensive reference for trainees and experienced gastroenterologists to optimize placement conditions and reduce complication risk, noting that training in nutrition is suboptimal in many GI fellowships.
Becoming familiar with common and advanced enteral access techniques is within the armamentarium of all practicing gastroenterologists, the authors stated. Because malnutrition affects nearly all GI disorders, “understanding common routes of enteral access and the basic principles of nutrition support promotes the initiation of optimal enteral nutrition, mitigating the impact of malnutrition, and improving prognosis for patients at nutritional risk,” they wrote.
Micic served on the advisory board for Ironwood Pharmaceuticals and is on the speaker’s bureau for Takeda Pharmaceuticals. One coauthor served as a consultant for Merit Medical, Circa Scientific, and Aspero Medical. Paski and Shamah had disclosed no competing interests relevant to their comments.
A version of this article appeared on Medscape.com.
At least 250,000 US hospitalized patients a year require enteral support using an artificial pathway into the gastrointestinal (GI) tract to deliver nutrition or medication. In light of this,
Covering indications, placement techniques, and management, the comprehensive document is a response to the increasing use of enteral access devices in chronic GI conditions. The update, published in Gastroenterology, addresses patient factors complicating placement decision-making such as thrombocytopenia, use of dual antiplatelet therapy, or performance of percutaneous access in the setting of cirrhosis.
“We provide clinical recommendations in these various scenarios understanding that the final decision-making is in the hands of the provider and care team,” said first author Dejan Micic, MD, a gastroenterologist and associate professor at University of Chicago Medical Center in Illinois at the time of the update (since relocated to Loyola University Medical Center in Chicago). “We hope this can serve a day-to-day purpose for clinical gastroenterologists and can be referenced as they encounter individuals with or needing an enteral access device.”
Traditionally, enteral access was reserved for patients with severe malnutrition or those unable to maintain oral intake. Recent recommendations emphasize early nutritional intervention including prehabilitation before major surgery, adjunctive therapy for oncology patients, and in specific inflammatory conditions such as Crohn’s disease. “These shifts recognize the role of enteral nutrition not only in preventing malnutrition but also as a therapeutic strategy,” Micic said in an interview.
There is, however, variability in the use of devices including the selection of appropriate units, technical aspects of placement, and subsequent management. “Such variability can lead to complications, suboptimal patient outcomes, and inefficiencies in care delivery,” Micic said.
He added that enteral access has been historically underemphasized in GI endoscopic training. “While procedural skill in placing devices such as percutaneous endoscopic gastrostomy, or PEG, tubes is often taught, a comprehensive understanding of the broader clinical context — such as proper patient selection, prevention of complications, and postplacement care — is not always thoroughly covered.”
The current update aims to bridge knowledge gaps with evidence-based-guidance. “It also underscores the importance of interdisciplinary collaboration with dietitians, nurses, and care givers to achieve the best outcomes for patients,” Micic said.
Commenting on the update but not involved with creating it, Shirley C. Paski, MD, MS, a gastroenterologist at the Cleveland Clinic, Ohio, called it timely, adding: “As GI training is becoming more subspecialized and interventional radiology has been able to provide enteral access, gastroenterology training in enteral access has declined to where some fellows are graduating with limited enteral access experience.”
Yet malnutrition remains a common consequence when GI disease is severe, chronic, or refractory to treatment, or in the setting of postsurgical anatomy, she added. “Enteral nutrition is increasingly being considered a therapeutic or adjunct treatment in some cases of Crohn’s disease or small intestinal bacterial overgrowth. Gastroenterologists need the endoscopic skill to secure enteral access tubes, particularly in more challenging anatomy.”
Also commenting on the document but not involved in it, Steven Shamah, MD, director of Endoscopy at Northwell Lenox Hill Hospital in New York City, said: “This should serve as a concise review for any general hospitalist or gastroenterologist to understand what we have and when we should offer the proper feeding tube options.” He stressed, however, that all gastroenterologists should be trained in the placing of all of tube options.
“The axiom ‘If the gut works, we should use it’ is something that I was taught when I was a medical student and it still holds true,” Shamah continued. “There’s been a jump in interventional procedures to assure continuity of the GI tract even in progressive malignancy. So there’s a rise in moving away from intravenous nutrition and a rise in tube-delivered enteral nutrition.” Options for reducing reflux and aspiration will likely take on more importance, he said.
Tubing Options
According to Micic and colleagues, recent data suggest a favorable safety profile of enteral feeding tubes placed endoscopically compared with surgical or radiologic placement. The illustrated AGA document outlines such approaches as synthetic flexible tubes placed into the stomach or small bowel via the oral (orogastric and oroenteral) or nasal routes (nasogastric [NG] and nasojejunal [NJ]) and percutaneous tubes accessing the stomach. The choice of tube, access point, delivery site, and feeding method varies with indication, expected duration of use, and patient anatomy, the authors stressed.
The update notes that NG and NJ tubes can be used immediately after confirmation of placement, most often with abdominal radiography. PEG tubes can be used immediately for medications and after 4 hours for tube feedings. A multidisciplinary team approach after placement provides improved patient care. “Dietitians assist with formula choice, volume, free water needs, and delivery method, and nurses and advanced practice clinicians assist with tube site assessment and troubleshooting,” the authors wrote.
Complications can occur but should be infrequent, Micic said. “Frankly, most complications can be predicted based on the duration of use and prevented with appropriate monitoring.” Common complications include tube dislodgement, clogging, site infections, buried bumper syndrome, and aspiration. “Minimizing these risks requires a thorough understanding of patient-specific factors, careful technique during placement, and ongoing monitoring after the device is in use,” he added.
Paski said the update aligns with established guidelines for enteral access but also offers suggestions to mitigate the risk of tube placement in patients in whom placement has traditionally been more challenging. “This is a helpful addition to the literature because if enteral access cannot be obtained in a patient unable to meet their needs orally, total paternal nutrition is the next and much more invasive step for nutrition support.”
She called the practice update a concise, comprehensive reference for trainees and experienced gastroenterologists to optimize placement conditions and reduce complication risk, noting that training in nutrition is suboptimal in many GI fellowships.
Becoming familiar with common and advanced enteral access techniques is within the armamentarium of all practicing gastroenterologists, the authors stated. Because malnutrition affects nearly all GI disorders, “understanding common routes of enteral access and the basic principles of nutrition support promotes the initiation of optimal enteral nutrition, mitigating the impact of malnutrition, and improving prognosis for patients at nutritional risk,” they wrote.
Micic served on the advisory board for Ironwood Pharmaceuticals and is on the speaker’s bureau for Takeda Pharmaceuticals. One coauthor served as a consultant for Merit Medical, Circa Scientific, and Aspero Medical. Paski and Shamah had disclosed no competing interests relevant to their comments.
A version of this article appeared on Medscape.com.
At least 250,000 US hospitalized patients a year require enteral support using an artificial pathway into the gastrointestinal (GI) tract to deliver nutrition or medication. In light of this,
Covering indications, placement techniques, and management, the comprehensive document is a response to the increasing use of enteral access devices in chronic GI conditions. The update, published in Gastroenterology, addresses patient factors complicating placement decision-making such as thrombocytopenia, use of dual antiplatelet therapy, or performance of percutaneous access in the setting of cirrhosis.
“We provide clinical recommendations in these various scenarios understanding that the final decision-making is in the hands of the provider and care team,” said first author Dejan Micic, MD, a gastroenterologist and associate professor at University of Chicago Medical Center in Illinois at the time of the update (since relocated to Loyola University Medical Center in Chicago). “We hope this can serve a day-to-day purpose for clinical gastroenterologists and can be referenced as they encounter individuals with or needing an enteral access device.”
Traditionally, enteral access was reserved for patients with severe malnutrition or those unable to maintain oral intake. Recent recommendations emphasize early nutritional intervention including prehabilitation before major surgery, adjunctive therapy for oncology patients, and in specific inflammatory conditions such as Crohn’s disease. “These shifts recognize the role of enteral nutrition not only in preventing malnutrition but also as a therapeutic strategy,” Micic said in an interview.
There is, however, variability in the use of devices including the selection of appropriate units, technical aspects of placement, and subsequent management. “Such variability can lead to complications, suboptimal patient outcomes, and inefficiencies in care delivery,” Micic said.
He added that enteral access has been historically underemphasized in GI endoscopic training. “While procedural skill in placing devices such as percutaneous endoscopic gastrostomy, or PEG, tubes is often taught, a comprehensive understanding of the broader clinical context — such as proper patient selection, prevention of complications, and postplacement care — is not always thoroughly covered.”
The current update aims to bridge knowledge gaps with evidence-based-guidance. “It also underscores the importance of interdisciplinary collaboration with dietitians, nurses, and care givers to achieve the best outcomes for patients,” Micic said.
Commenting on the update but not involved with creating it, Shirley C. Paski, MD, MS, a gastroenterologist at the Cleveland Clinic, Ohio, called it timely, adding: “As GI training is becoming more subspecialized and interventional radiology has been able to provide enteral access, gastroenterology training in enteral access has declined to where some fellows are graduating with limited enteral access experience.”
Yet malnutrition remains a common consequence when GI disease is severe, chronic, or refractory to treatment, or in the setting of postsurgical anatomy, she added. “Enteral nutrition is increasingly being considered a therapeutic or adjunct treatment in some cases of Crohn’s disease or small intestinal bacterial overgrowth. Gastroenterologists need the endoscopic skill to secure enteral access tubes, particularly in more challenging anatomy.”
Also commenting on the document but not involved in it, Steven Shamah, MD, director of Endoscopy at Northwell Lenox Hill Hospital in New York City, said: “This should serve as a concise review for any general hospitalist or gastroenterologist to understand what we have and when we should offer the proper feeding tube options.” He stressed, however, that all gastroenterologists should be trained in the placing of all of tube options.
“The axiom ‘If the gut works, we should use it’ is something that I was taught when I was a medical student and it still holds true,” Shamah continued. “There’s been a jump in interventional procedures to assure continuity of the GI tract even in progressive malignancy. So there’s a rise in moving away from intravenous nutrition and a rise in tube-delivered enteral nutrition.” Options for reducing reflux and aspiration will likely take on more importance, he said.
Tubing Options
According to Micic and colleagues, recent data suggest a favorable safety profile of enteral feeding tubes placed endoscopically compared with surgical or radiologic placement. The illustrated AGA document outlines such approaches as synthetic flexible tubes placed into the stomach or small bowel via the oral (orogastric and oroenteral) or nasal routes (nasogastric [NG] and nasojejunal [NJ]) and percutaneous tubes accessing the stomach. The choice of tube, access point, delivery site, and feeding method varies with indication, expected duration of use, and patient anatomy, the authors stressed.
The update notes that NG and NJ tubes can be used immediately after confirmation of placement, most often with abdominal radiography. PEG tubes can be used immediately for medications and after 4 hours for tube feedings. A multidisciplinary team approach after placement provides improved patient care. “Dietitians assist with formula choice, volume, free water needs, and delivery method, and nurses and advanced practice clinicians assist with tube site assessment and troubleshooting,” the authors wrote.
Complications can occur but should be infrequent, Micic said. “Frankly, most complications can be predicted based on the duration of use and prevented with appropriate monitoring.” Common complications include tube dislodgement, clogging, site infections, buried bumper syndrome, and aspiration. “Minimizing these risks requires a thorough understanding of patient-specific factors, careful technique during placement, and ongoing monitoring after the device is in use,” he added.
Paski said the update aligns with established guidelines for enteral access but also offers suggestions to mitigate the risk of tube placement in patients in whom placement has traditionally been more challenging. “This is a helpful addition to the literature because if enteral access cannot be obtained in a patient unable to meet their needs orally, total paternal nutrition is the next and much more invasive step for nutrition support.”
She called the practice update a concise, comprehensive reference for trainees and experienced gastroenterologists to optimize placement conditions and reduce complication risk, noting that training in nutrition is suboptimal in many GI fellowships.
Becoming familiar with common and advanced enteral access techniques is within the armamentarium of all practicing gastroenterologists, the authors stated. Because malnutrition affects nearly all GI disorders, “understanding common routes of enteral access and the basic principles of nutrition support promotes the initiation of optimal enteral nutrition, mitigating the impact of malnutrition, and improving prognosis for patients at nutritional risk,” they wrote.
Micic served on the advisory board for Ironwood Pharmaceuticals and is on the speaker’s bureau for Takeda Pharmaceuticals. One coauthor served as a consultant for Merit Medical, Circa Scientific, and Aspero Medical. Paski and Shamah had disclosed no competing interests relevant to their comments.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Open Clinical Trials for Patients With Chronic Obstructive Pulmonary Disease
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
Patients With Asthma and COPD At Increased Cancer Risk From Microplastics
Individuals with asthma and chronic obstructive pulmonary disease (COPD) were more vulnerable than healthy controls to epithelial cell changes caused by microplastics exposure, based on data from a new simulation study.
Microplastic fibers present in the ambient air can be inhaled into the lungs and promote a range of complications including oxidative stress, local injury, and cytotoxicity, but data on the effects of microplastic fibers on individuals with obstructive lung diseases are limited, wrote Magdalena Poplinska-Goryca, MD, of the Medical University of Warsaw, Warsaw, Poland, and colleagues.
In a study published in Scientific Reports, the researchers identified 10 adults aged ≥ 18 years with asthma, eight adults aged ≥ 40 years with COPD, and 11 healthy adult controls. Individuals with more serious conditions such as severe asthma or COPD, unstable or uncontrolled disease, concomitant malignancies, or chronic or acute lung disease were excluded.
The researchers obtained nasal epithelial cells from all participants, and exposed these cells to microplastic fibers created by the researchers in a laboratory setting. Overall, asthmatic and COPD airway epithelial cells showed a different reaction to microplastic fibers stimulation compared to healthy epithelial cells. The most significant response was associated with Th2 inflammation, modulation of stress response, and carcinogenesis. No differences in cytotoxic or minor inflammatory effects on epithelial cells of patients with asthma or COPD were noted compared with healthy controls.
In addition, flow cytometric analysis showed increased CD24+ epithelial cells in asthma patients compared to controls after microplastics exposure.
“Many of the gene candidates selected from RNA-Seq analysis are related to cancer (upregulated in many cancer types according to the literature), and the activation of CD24 on primarily ciliated asthmatic epithelial cells after microplastic stimulation further supports this theory,” the researchers wrote.
The findings were limited by several factors including the use of nasal rather than bronchial epithelial cells, which would have yielded more information, the researchers noted. Also, patients with severe asthma and COPD were excluded, they said, because of the impact of oral steroid and antibiotic use by this patient group on epithelial cell immunology that could bias the results of epithelial response to microplastic fiber exposure.
However, the results suggest that “the structural impairment of the airway epithelium in obstructive diseases enhances the impact of microplastic particles compared to healthy epithelium,” the researchers concluded.
Current and Future Implications
The current study is important in addressing the increasing environmental presence of microplastics and their potential impact on respiratory health, said Seyedmohammad Pourshahid, MD, assistant professor of thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia, in an interview.
“By examining how microplastics interact with airway epithelial cells, particularly in individuals with asthma and COPD, the research aims to elucidate mechanisms that could contribute to disease progression or exacerbation,” he said.
“The study’s findings that microplastics did not induce a strong inflammatory response, unlike other pollutants such as PM2.5, were unexpected; instead, microplastics appeared to influence pathways related to airway remodeling and oxidative stress,” Pourshahid noted. “This suggests that microplastics may affect respiratory health through mechanisms distinct from traditional pollutants,” he said.
“While preliminary, this research highlights the potential role of environmental microplastic exposure in respiratory diseases,” Pourshahid told this news organization. “Clinicians should be aware of emerging environmental factors that could impact patient health, especially in individuals with asthma and COPD. This awareness may inform patient education and advocacy for reducing exposure to airborne microplastics,” he said.
More studies are needed to explore the long-term effects of microplastic exposure on respiratory health, particularly in vulnerable populations, said Pourshahid. Research with in vivo models is necessary to confirm the findings and assess potential clinical implications to confirm these findings and assess potential clinical implications, he said. “Understanding the prevalence and sources of daily microplastic exposure can inform public health strategies to mitigate risks,” he added.
The study was supported by the Jakub Potocki Foundation. Paplińska-Goryca and Pourshahid had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Individuals with asthma and chronic obstructive pulmonary disease (COPD) were more vulnerable than healthy controls to epithelial cell changes caused by microplastics exposure, based on data from a new simulation study.
Microplastic fibers present in the ambient air can be inhaled into the lungs and promote a range of complications including oxidative stress, local injury, and cytotoxicity, but data on the effects of microplastic fibers on individuals with obstructive lung diseases are limited, wrote Magdalena Poplinska-Goryca, MD, of the Medical University of Warsaw, Warsaw, Poland, and colleagues.
In a study published in Scientific Reports, the researchers identified 10 adults aged ≥ 18 years with asthma, eight adults aged ≥ 40 years with COPD, and 11 healthy adult controls. Individuals with more serious conditions such as severe asthma or COPD, unstable or uncontrolled disease, concomitant malignancies, or chronic or acute lung disease were excluded.
The researchers obtained nasal epithelial cells from all participants, and exposed these cells to microplastic fibers created by the researchers in a laboratory setting. Overall, asthmatic and COPD airway epithelial cells showed a different reaction to microplastic fibers stimulation compared to healthy epithelial cells. The most significant response was associated with Th2 inflammation, modulation of stress response, and carcinogenesis. No differences in cytotoxic or minor inflammatory effects on epithelial cells of patients with asthma or COPD were noted compared with healthy controls.
In addition, flow cytometric analysis showed increased CD24+ epithelial cells in asthma patients compared to controls after microplastics exposure.
“Many of the gene candidates selected from RNA-Seq analysis are related to cancer (upregulated in many cancer types according to the literature), and the activation of CD24 on primarily ciliated asthmatic epithelial cells after microplastic stimulation further supports this theory,” the researchers wrote.
The findings were limited by several factors including the use of nasal rather than bronchial epithelial cells, which would have yielded more information, the researchers noted. Also, patients with severe asthma and COPD were excluded, they said, because of the impact of oral steroid and antibiotic use by this patient group on epithelial cell immunology that could bias the results of epithelial response to microplastic fiber exposure.
However, the results suggest that “the structural impairment of the airway epithelium in obstructive diseases enhances the impact of microplastic particles compared to healthy epithelium,” the researchers concluded.
Current and Future Implications
The current study is important in addressing the increasing environmental presence of microplastics and their potential impact on respiratory health, said Seyedmohammad Pourshahid, MD, assistant professor of thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia, in an interview.
“By examining how microplastics interact with airway epithelial cells, particularly in individuals with asthma and COPD, the research aims to elucidate mechanisms that could contribute to disease progression or exacerbation,” he said.
“The study’s findings that microplastics did not induce a strong inflammatory response, unlike other pollutants such as PM2.5, were unexpected; instead, microplastics appeared to influence pathways related to airway remodeling and oxidative stress,” Pourshahid noted. “This suggests that microplastics may affect respiratory health through mechanisms distinct from traditional pollutants,” he said.
“While preliminary, this research highlights the potential role of environmental microplastic exposure in respiratory diseases,” Pourshahid told this news organization. “Clinicians should be aware of emerging environmental factors that could impact patient health, especially in individuals with asthma and COPD. This awareness may inform patient education and advocacy for reducing exposure to airborne microplastics,” he said.
More studies are needed to explore the long-term effects of microplastic exposure on respiratory health, particularly in vulnerable populations, said Pourshahid. Research with in vivo models is necessary to confirm the findings and assess potential clinical implications to confirm these findings and assess potential clinical implications, he said. “Understanding the prevalence and sources of daily microplastic exposure can inform public health strategies to mitigate risks,” he added.
The study was supported by the Jakub Potocki Foundation. Paplińska-Goryca and Pourshahid had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Individuals with asthma and chronic obstructive pulmonary disease (COPD) were more vulnerable than healthy controls to epithelial cell changes caused by microplastics exposure, based on data from a new simulation study.
Microplastic fibers present in the ambient air can be inhaled into the lungs and promote a range of complications including oxidative stress, local injury, and cytotoxicity, but data on the effects of microplastic fibers on individuals with obstructive lung diseases are limited, wrote Magdalena Poplinska-Goryca, MD, of the Medical University of Warsaw, Warsaw, Poland, and colleagues.
In a study published in Scientific Reports, the researchers identified 10 adults aged ≥ 18 years with asthma, eight adults aged ≥ 40 years with COPD, and 11 healthy adult controls. Individuals with more serious conditions such as severe asthma or COPD, unstable or uncontrolled disease, concomitant malignancies, or chronic or acute lung disease were excluded.
The researchers obtained nasal epithelial cells from all participants, and exposed these cells to microplastic fibers created by the researchers in a laboratory setting. Overall, asthmatic and COPD airway epithelial cells showed a different reaction to microplastic fibers stimulation compared to healthy epithelial cells. The most significant response was associated with Th2 inflammation, modulation of stress response, and carcinogenesis. No differences in cytotoxic or minor inflammatory effects on epithelial cells of patients with asthma or COPD were noted compared with healthy controls.
In addition, flow cytometric analysis showed increased CD24+ epithelial cells in asthma patients compared to controls after microplastics exposure.
“Many of the gene candidates selected from RNA-Seq analysis are related to cancer (upregulated in many cancer types according to the literature), and the activation of CD24 on primarily ciliated asthmatic epithelial cells after microplastic stimulation further supports this theory,” the researchers wrote.
The findings were limited by several factors including the use of nasal rather than bronchial epithelial cells, which would have yielded more information, the researchers noted. Also, patients with severe asthma and COPD were excluded, they said, because of the impact of oral steroid and antibiotic use by this patient group on epithelial cell immunology that could bias the results of epithelial response to microplastic fiber exposure.
However, the results suggest that “the structural impairment of the airway epithelium in obstructive diseases enhances the impact of microplastic particles compared to healthy epithelium,” the researchers concluded.
Current and Future Implications
The current study is important in addressing the increasing environmental presence of microplastics and their potential impact on respiratory health, said Seyedmohammad Pourshahid, MD, assistant professor of thoracic medicine and surgery at the Lewis Katz School of Medicine at Temple University, Philadelphia, in an interview.
“By examining how microplastics interact with airway epithelial cells, particularly in individuals with asthma and COPD, the research aims to elucidate mechanisms that could contribute to disease progression or exacerbation,” he said.
“The study’s findings that microplastics did not induce a strong inflammatory response, unlike other pollutants such as PM2.5, were unexpected; instead, microplastics appeared to influence pathways related to airway remodeling and oxidative stress,” Pourshahid noted. “This suggests that microplastics may affect respiratory health through mechanisms distinct from traditional pollutants,” he said.
“While preliminary, this research highlights the potential role of environmental microplastic exposure in respiratory diseases,” Pourshahid told this news organization. “Clinicians should be aware of emerging environmental factors that could impact patient health, especially in individuals with asthma and COPD. This awareness may inform patient education and advocacy for reducing exposure to airborne microplastics,” he said.
More studies are needed to explore the long-term effects of microplastic exposure on respiratory health, particularly in vulnerable populations, said Pourshahid. Research with in vivo models is necessary to confirm the findings and assess potential clinical implications to confirm these findings and assess potential clinical implications, he said. “Understanding the prevalence and sources of daily microplastic exposure can inform public health strategies to mitigate risks,” he added.
The study was supported by the Jakub Potocki Foundation. Paplińska-Goryca and Pourshahid had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.