User login
MDedge latest news is breaking news from medical conferences, journals, guidelines, the FDA and CDC.
Vasculitis Patients Need Multiple COVID Vaccine Boosters
People with vasculitis may need at least three or four vaccinations for COVID-19 before they start to show an immune response against SARS-CoV-2 infection, new research has suggested.
In a longitudinal retrospective study, serum antibody neutralization against the Omicron variant of the virus and its descendants was found to be “largely absent” after the first two doses of COVID-19 vaccine had been given to patients. But increasing neutralizing antibody titers were seen after both the third and fourth vaccine boosters had been administered.
Results also showed that the more recently people had been treated with the B cell–depleting therapy rituximab, the lower the levels of immunogenicity that were achieved, and thus protection against SARS-CoV-2.
“Our results have significant implications for individuals treated with rituximab in the post-Omicron era, highlighting the value of additive boosters in affirming increasing protection in clinically vulnerable populations,” the team behind the work at the University of Cambridge in England, has reported in Science Advances.
Moreover, because the use of rituximab reduced the neutralization of not just wild-type (WT) Omicron but also the Omicron-descendant variants BA.1, BA.2, BA.4, and XBB, this highlights “the urgent need for additional adjunctive strategies to enhance vaccine-induced immunity as well as preferential access for such patients to updated vaccines using spike from now circulating Omicron lineages,” the team added.
Studying Humoral Responses to SARS-CoV-2 Vaccines
Corresponding author Ravindra K. Gupta, BMBCh, MA, MPH, PhD, told this news organization that studying humoral responses to SARS-CoV-2 vaccines in immunocompromised individuals such as those with vasculitis was important for two main reasons.
“It is really important at individual level for their own health, of course, but also because we know that variants of concern have often evolved and developed within patients and can then spread in wider populations,” he said.
Gupta, who is professor of clinical microbiology at the Cambridge Institute for Therapeutic Immunology & Infectious Disease added: “We believe that the variants of concern that we’re having to deal with right now, including Omicron, have come from such [immunocompromised] individuals.”
Omicron “was a big shift,” Gupta noted. “It had a lot of new mutations on it, so it was almost like a new strain of the virus.” Few studies have looked at the longitudinal immunogenicity proffered by COVID vaccines in the post-Omicron era, particularly in those with vasculitis who are often treated with immunosuppressive drugs, including rituximab.
Two-Pronged Study Approach
For the study, a population of immunocompromised individuals diagnosed with vasculitis who had been treated with rituximab in the past 5 years was identified. Just over half (58%) had received adenovirus-based AZD1222/ChAdOx1 nCoV-19 (AstraZeneca-Oxford; AZN) and 37% BNT162b2 (Pfizer-BioNTech; mRNA) as their primary vaccines. Patients with antineutrophil cytoplasmic antibody–associated vasculitis comprised the majority of those who received rituximab (83%), compared with less than half of those who did not take rituximab (48%).
A two-pronged approach was taken with the researchers first measuring neutralizing antibody titers before and 30 days after four successive COVID vaccinations in a group of 32 individuals with available samples. They then performed a cross-sectional, case-control study in 95 individuals to look at neutralizing antibody titers and antibody-dependent cell-mediated cytotoxicity (ADCC) in individuals who had (n = 64) and had not (n = 31) been treated with rituximab in the past 5 years and had samples available after their third and fourth COVID vaccinations.
The first analysis was done to see how people were responding to vaccination over time. “That told us that there was a problem with the first two doses and that we got some response after doses three and four, but the response was uniformly quite poor against the new variants of concern,” Gupta said.
A human embryonic kidney cell model had been used to determine individuals’ neutralizing antibody titers in response to WT, BA.1, BA.2, BA.4, and XBB pseudotyped viruses. After the first and second COVID vaccinations, the geometric mean titer (GMT) against each variant barely increased from a baseline of 40.0. The greatest increases in GMT was seen with the WT virus, at 43.7, 90.7, 256.3, and 394.2, after the first, second, third and fourth doses, respectively. The lowest increases in GMT were seen with the XBB variant, with respective values of 40.0, 40.8, 45.7, and 53.9.
Incremental Benefit Offers Some ‘Reassurance’
Vasculitis specialist Rona Smith, MA, MB BChir, MD, who was one of the authors of the paper, told this news organization separately that the results showed there was “an incremental benefit of having COVID vaccinations,” which “offers a little bit of reassurance” that there can be an immune response in people with vasculitis.
Although results of the cross-sectional study showed that there was a significant dampening effect of rituximab treatment on the immune response, “I don’t think it’s an isolated effect in our [vasculitis] patients,” Smith suggested, adding the results were “probably still relevant to patients who receive routine dosing of rituximab for other conditions.”
Neutralizing antibody titers were consistently lower among individuals who had been treated with rituximab vs those who had not, with treatment in the past 18 months found to significantly impair immunogenicity.
The ADCC response was better preserved than the neutralizing antibody response, Gupta said, although it was still significantly lower in the rituximab-treated than in the non–rituximab-treated patients.
When to Vaccinate in Vasculitis?
Regarding when to give vaccines to people with vasculitis, Smith said: “Current recommendations are that patients should receive any vaccines that they’re offered routinely, whether that be COVID vaccines, flu vaccines, pneumococcal vaccines.”
As for the timing of those vaccinations, she observed that the current thinking was that vaccinations should “ideally be at least 1 month before a rituximab treatment, and ideally 3-4 [months] after their last dose. However, as many patients are on a 6-month dosing cycle, it can be difficult for some of them to find a suitable time window to have the COVID vaccine when it is offered.”
Additional precautions, such as wearing masks in crowded places and avoiding visits to acutely unwell friends or relatives, may still be prudent, Smith acknowledged, but he was clear that people should not be locking themselves away as they did during the COVID-19 pandemic.
When advising patients, “our general recommendation is that it is better to have a vaccine than not, but we can’t guarantee how well you will respond to it, but some response is better than none,” Smith said.
The study was independently supported. Gupta had no relevant financial relationships to disclose. Smith was a coauthor of the paper and has received research grant funding from Union Therapeutics, GlaxoSmithKline/Vir Biotechnology, Addenbrooke’s Charitable Trust, and Vasculitis UK. Another coauthor reported receiving research grants from CSL Vifor, Roche, and GlaxoSmithKline and advisory board, consultancy, and lecture fees from Roche and CSL Vifor.
A version of this article first appeared on Medscape.com.
People with vasculitis may need at least three or four vaccinations for COVID-19 before they start to show an immune response against SARS-CoV-2 infection, new research has suggested.
In a longitudinal retrospective study, serum antibody neutralization against the Omicron variant of the virus and its descendants was found to be “largely absent” after the first two doses of COVID-19 vaccine had been given to patients. But increasing neutralizing antibody titers were seen after both the third and fourth vaccine boosters had been administered.
Results also showed that the more recently people had been treated with the B cell–depleting therapy rituximab, the lower the levels of immunogenicity that were achieved, and thus protection against SARS-CoV-2.
“Our results have significant implications for individuals treated with rituximab in the post-Omicron era, highlighting the value of additive boosters in affirming increasing protection in clinically vulnerable populations,” the team behind the work at the University of Cambridge in England, has reported in Science Advances.
Moreover, because the use of rituximab reduced the neutralization of not just wild-type (WT) Omicron but also the Omicron-descendant variants BA.1, BA.2, BA.4, and XBB, this highlights “the urgent need for additional adjunctive strategies to enhance vaccine-induced immunity as well as preferential access for such patients to updated vaccines using spike from now circulating Omicron lineages,” the team added.
Studying Humoral Responses to SARS-CoV-2 Vaccines
Corresponding author Ravindra K. Gupta, BMBCh, MA, MPH, PhD, told this news organization that studying humoral responses to SARS-CoV-2 vaccines in immunocompromised individuals such as those with vasculitis was important for two main reasons.
“It is really important at individual level for their own health, of course, but also because we know that variants of concern have often evolved and developed within patients and can then spread in wider populations,” he said.
Gupta, who is professor of clinical microbiology at the Cambridge Institute for Therapeutic Immunology & Infectious Disease added: “We believe that the variants of concern that we’re having to deal with right now, including Omicron, have come from such [immunocompromised] individuals.”
Omicron “was a big shift,” Gupta noted. “It had a lot of new mutations on it, so it was almost like a new strain of the virus.” Few studies have looked at the longitudinal immunogenicity proffered by COVID vaccines in the post-Omicron era, particularly in those with vasculitis who are often treated with immunosuppressive drugs, including rituximab.
Two-Pronged Study Approach
For the study, a population of immunocompromised individuals diagnosed with vasculitis who had been treated with rituximab in the past 5 years was identified. Just over half (58%) had received adenovirus-based AZD1222/ChAdOx1 nCoV-19 (AstraZeneca-Oxford; AZN) and 37% BNT162b2 (Pfizer-BioNTech; mRNA) as their primary vaccines. Patients with antineutrophil cytoplasmic antibody–associated vasculitis comprised the majority of those who received rituximab (83%), compared with less than half of those who did not take rituximab (48%).
A two-pronged approach was taken with the researchers first measuring neutralizing antibody titers before and 30 days after four successive COVID vaccinations in a group of 32 individuals with available samples. They then performed a cross-sectional, case-control study in 95 individuals to look at neutralizing antibody titers and antibody-dependent cell-mediated cytotoxicity (ADCC) in individuals who had (n = 64) and had not (n = 31) been treated with rituximab in the past 5 years and had samples available after their third and fourth COVID vaccinations.
The first analysis was done to see how people were responding to vaccination over time. “That told us that there was a problem with the first two doses and that we got some response after doses three and four, but the response was uniformly quite poor against the new variants of concern,” Gupta said.
A human embryonic kidney cell model had been used to determine individuals’ neutralizing antibody titers in response to WT, BA.1, BA.2, BA.4, and XBB pseudotyped viruses. After the first and second COVID vaccinations, the geometric mean titer (GMT) against each variant barely increased from a baseline of 40.0. The greatest increases in GMT was seen with the WT virus, at 43.7, 90.7, 256.3, and 394.2, after the first, second, third and fourth doses, respectively. The lowest increases in GMT were seen with the XBB variant, with respective values of 40.0, 40.8, 45.7, and 53.9.
Incremental Benefit Offers Some ‘Reassurance’
Vasculitis specialist Rona Smith, MA, MB BChir, MD, who was one of the authors of the paper, told this news organization separately that the results showed there was “an incremental benefit of having COVID vaccinations,” which “offers a little bit of reassurance” that there can be an immune response in people with vasculitis.
Although results of the cross-sectional study showed that there was a significant dampening effect of rituximab treatment on the immune response, “I don’t think it’s an isolated effect in our [vasculitis] patients,” Smith suggested, adding the results were “probably still relevant to patients who receive routine dosing of rituximab for other conditions.”
Neutralizing antibody titers were consistently lower among individuals who had been treated with rituximab vs those who had not, with treatment in the past 18 months found to significantly impair immunogenicity.
The ADCC response was better preserved than the neutralizing antibody response, Gupta said, although it was still significantly lower in the rituximab-treated than in the non–rituximab-treated patients.
When to Vaccinate in Vasculitis?
Regarding when to give vaccines to people with vasculitis, Smith said: “Current recommendations are that patients should receive any vaccines that they’re offered routinely, whether that be COVID vaccines, flu vaccines, pneumococcal vaccines.”
As for the timing of those vaccinations, she observed that the current thinking was that vaccinations should “ideally be at least 1 month before a rituximab treatment, and ideally 3-4 [months] after their last dose. However, as many patients are on a 6-month dosing cycle, it can be difficult for some of them to find a suitable time window to have the COVID vaccine when it is offered.”
Additional precautions, such as wearing masks in crowded places and avoiding visits to acutely unwell friends or relatives, may still be prudent, Smith acknowledged, but he was clear that people should not be locking themselves away as they did during the COVID-19 pandemic.
When advising patients, “our general recommendation is that it is better to have a vaccine than not, but we can’t guarantee how well you will respond to it, but some response is better than none,” Smith said.
The study was independently supported. Gupta had no relevant financial relationships to disclose. Smith was a coauthor of the paper and has received research grant funding from Union Therapeutics, GlaxoSmithKline/Vir Biotechnology, Addenbrooke’s Charitable Trust, and Vasculitis UK. Another coauthor reported receiving research grants from CSL Vifor, Roche, and GlaxoSmithKline and advisory board, consultancy, and lecture fees from Roche and CSL Vifor.
A version of this article first appeared on Medscape.com.
People with vasculitis may need at least three or four vaccinations for COVID-19 before they start to show an immune response against SARS-CoV-2 infection, new research has suggested.
In a longitudinal retrospective study, serum antibody neutralization against the Omicron variant of the virus and its descendants was found to be “largely absent” after the first two doses of COVID-19 vaccine had been given to patients. But increasing neutralizing antibody titers were seen after both the third and fourth vaccine boosters had been administered.
Results also showed that the more recently people had been treated with the B cell–depleting therapy rituximab, the lower the levels of immunogenicity that were achieved, and thus protection against SARS-CoV-2.
“Our results have significant implications for individuals treated with rituximab in the post-Omicron era, highlighting the value of additive boosters in affirming increasing protection in clinically vulnerable populations,” the team behind the work at the University of Cambridge in England, has reported in Science Advances.
Moreover, because the use of rituximab reduced the neutralization of not just wild-type (WT) Omicron but also the Omicron-descendant variants BA.1, BA.2, BA.4, and XBB, this highlights “the urgent need for additional adjunctive strategies to enhance vaccine-induced immunity as well as preferential access for such patients to updated vaccines using spike from now circulating Omicron lineages,” the team added.
Studying Humoral Responses to SARS-CoV-2 Vaccines
Corresponding author Ravindra K. Gupta, BMBCh, MA, MPH, PhD, told this news organization that studying humoral responses to SARS-CoV-2 vaccines in immunocompromised individuals such as those with vasculitis was important for two main reasons.
“It is really important at individual level for their own health, of course, but also because we know that variants of concern have often evolved and developed within patients and can then spread in wider populations,” he said.
Gupta, who is professor of clinical microbiology at the Cambridge Institute for Therapeutic Immunology & Infectious Disease added: “We believe that the variants of concern that we’re having to deal with right now, including Omicron, have come from such [immunocompromised] individuals.”
Omicron “was a big shift,” Gupta noted. “It had a lot of new mutations on it, so it was almost like a new strain of the virus.” Few studies have looked at the longitudinal immunogenicity proffered by COVID vaccines in the post-Omicron era, particularly in those with vasculitis who are often treated with immunosuppressive drugs, including rituximab.
Two-Pronged Study Approach
For the study, a population of immunocompromised individuals diagnosed with vasculitis who had been treated with rituximab in the past 5 years was identified. Just over half (58%) had received adenovirus-based AZD1222/ChAdOx1 nCoV-19 (AstraZeneca-Oxford; AZN) and 37% BNT162b2 (Pfizer-BioNTech; mRNA) as their primary vaccines. Patients with antineutrophil cytoplasmic antibody–associated vasculitis comprised the majority of those who received rituximab (83%), compared with less than half of those who did not take rituximab (48%).
A two-pronged approach was taken with the researchers first measuring neutralizing antibody titers before and 30 days after four successive COVID vaccinations in a group of 32 individuals with available samples. They then performed a cross-sectional, case-control study in 95 individuals to look at neutralizing antibody titers and antibody-dependent cell-mediated cytotoxicity (ADCC) in individuals who had (n = 64) and had not (n = 31) been treated with rituximab in the past 5 years and had samples available after their third and fourth COVID vaccinations.
The first analysis was done to see how people were responding to vaccination over time. “That told us that there was a problem with the first two doses and that we got some response after doses three and four, but the response was uniformly quite poor against the new variants of concern,” Gupta said.
A human embryonic kidney cell model had been used to determine individuals’ neutralizing antibody titers in response to WT, BA.1, BA.2, BA.4, and XBB pseudotyped viruses. After the first and second COVID vaccinations, the geometric mean titer (GMT) against each variant barely increased from a baseline of 40.0. The greatest increases in GMT was seen with the WT virus, at 43.7, 90.7, 256.3, and 394.2, after the first, second, third and fourth doses, respectively. The lowest increases in GMT were seen with the XBB variant, with respective values of 40.0, 40.8, 45.7, and 53.9.
Incremental Benefit Offers Some ‘Reassurance’
Vasculitis specialist Rona Smith, MA, MB BChir, MD, who was one of the authors of the paper, told this news organization separately that the results showed there was “an incremental benefit of having COVID vaccinations,” which “offers a little bit of reassurance” that there can be an immune response in people with vasculitis.
Although results of the cross-sectional study showed that there was a significant dampening effect of rituximab treatment on the immune response, “I don’t think it’s an isolated effect in our [vasculitis] patients,” Smith suggested, adding the results were “probably still relevant to patients who receive routine dosing of rituximab for other conditions.”
Neutralizing antibody titers were consistently lower among individuals who had been treated with rituximab vs those who had not, with treatment in the past 18 months found to significantly impair immunogenicity.
The ADCC response was better preserved than the neutralizing antibody response, Gupta said, although it was still significantly lower in the rituximab-treated than in the non–rituximab-treated patients.
When to Vaccinate in Vasculitis?
Regarding when to give vaccines to people with vasculitis, Smith said: “Current recommendations are that patients should receive any vaccines that they’re offered routinely, whether that be COVID vaccines, flu vaccines, pneumococcal vaccines.”
As for the timing of those vaccinations, she observed that the current thinking was that vaccinations should “ideally be at least 1 month before a rituximab treatment, and ideally 3-4 [months] after their last dose. However, as many patients are on a 6-month dosing cycle, it can be difficult for some of them to find a suitable time window to have the COVID vaccine when it is offered.”
Additional precautions, such as wearing masks in crowded places and avoiding visits to acutely unwell friends or relatives, may still be prudent, Smith acknowledged, but he was clear that people should not be locking themselves away as they did during the COVID-19 pandemic.
When advising patients, “our general recommendation is that it is better to have a vaccine than not, but we can’t guarantee how well you will respond to it, but some response is better than none,” Smith said.
The study was independently supported. Gupta had no relevant financial relationships to disclose. Smith was a coauthor of the paper and has received research grant funding from Union Therapeutics, GlaxoSmithKline/Vir Biotechnology, Addenbrooke’s Charitable Trust, and Vasculitis UK. Another coauthor reported receiving research grants from CSL Vifor, Roche, and GlaxoSmithKline and advisory board, consultancy, and lecture fees from Roche and CSL Vifor.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
New Five-Type Index Provides Doctors Guide for Long COVID
A new analysis of long-COVID patients has identified five distinct subtypes that researchers say will help doctors diagnose the condition.
The new five-type index, developed by federal researchers with the National Institutes of Health’s RECOVER COVID Initiative, identified the most common symptoms in 14,000 people with long COVID, with data from an additional 4000 people added to the updated 2024 index.
By using the index, physicians and researchers can better understand the condition, which is difficult to treat and diagnose because no standard definitions or therapies have been developed. Doctors can use the index to offer more targeted care and help patients manage their symptoms more effectively.
The index may also help researchers find more treatments for long COVID. Because long COVID can affect so many different parts of the body, it will take time to fully understand how to treat it, but studies like this are making progress in the right direction, experts said.
This new index uses an updated point system, where points are allotted to each symptom in a list of the 44 most reported symptoms in people with likely long COVID based on how often they occur. Among people in the study with prior COVID infection, 2213 (18%) met the threshold for long COVID.
The 44 most common symptoms were then distributed among 5 subtypes, with each representing a difference in impact on quality of life and overall health. The most common symptoms were fatigue (85.8%), postexertional malaise (87.4%), and postexertional soreness (75.0%) — where persistent fatigue and discomfort occur after physical or mental exertion — dizziness (65.8%), brain fog (63.8%), gastrointestinal symptoms (59.3%), and palpitations (58%).
For those with prior COVID infection, symptoms were more prevalent in all cases.
Subtype 1
Those grouped into subtype 1 did not report a high incidence of impact on quality of life, physical health, or daily function. Only 21% of people in subtype 1 reported a “poor or fair quality of life.”
A change in smell or taste — usually a symptom that’s bothersome but doesn’t seriously impact overall health — was most present in subtype 1, with 100% of people in subtype 1 reporting it.
The only other symptoms in over 50% of people with subtype 1— which were 490 of the 2213 with prior COVID infection — were fatigue (66%), postexertional malaise (53%), and postexertional soreness (55%).
Though these two symptoms can certainly impact quality of life, they became much more prevalent in other subtypes.
Subtype 2
The prevalence of possibly debilitating symptoms like postexertional malaise (94%), fatigue (81%), and chronic cough (100%) rose dramatically in people grouped into subtype 2.
Plus, 25% of people in subtype 2 reported a “poor or fair quality of life. Postexertional malaise, I think, is probably one of the most debilitating of the symptoms. When somebody comes in and tells me that they’re tired and I think they might have long COVID, the first thing I try to do is see if it is postexertional malaise vs just postinfectious fatigue,” said Lisa Sanders, MD, medical director of Yale’s Long Covid Multidisciplinary Care Center in New Haven, Connecticut.
Postinfectious fatigue usually resolves much more quickly than postexertional malaise. The latter accounts for several symptoms as also associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). ME/CFS is a chronic illness that causes severe fatigue and makes it difficult for sufferers to perform routine, daily activities.
“Postexertional malaise is an additive symptom of ME/CFS, and that can take a long time to resolve,” Sanders added.
The similarity between these two symptoms highlights the importance that physicians must place in scrutinizing symptoms to a high degree when they suspect a patient of having long COVID, experts said. By doing so, clinicians can unveil the mask of overlapping symptoms between long COVID symptoms and symptoms of other illnesses.
Subtype 3
About 37% of people grouped in subtype 3 reported a poor or fair quality of life, a significant rise from subtypes 1 and 2.
Fatigue symptoms were reported by 92%, whereas 82% reported postexertional soreness, and 70% reported dizziness. Additionally, 100% of people in subtype 3 reported brain fog as a symptom.
Sanders said these symptoms are also common in people with postural orthostatic tachycardia syndrome. This condition results from a reduced volume of blood returning to the heart after standing up, which leads to an abnormally fast heart rate. Palpitations and fainting can then occur.
Brain fog can be especially debilitating in people who are used to multitasking. With brain fog, people accustomed to easily alternating between tasks or doing multiple tasks at once can only do one thing at a time. This can cause stress and an overload of thoughts, even precipitating a change in careers if severe enough.
Though brain fog tends to resolve within 6-9 months after infection, it can last up to 18 months or more. Experts say doctors should always be on the lookout if a patient complains they have trouble concentrating or multitasking in the months after a COVID infection. A neurological exam and cognitive testing can identify abnormalities in brain function.
Subtype 4
About 40% of people in the study grouped into subtype 4 reported a poor or fair quality of life, a modest increase from those with subtype 3. About 65% reported symptoms of brain fog and 92% reported palpitations.
Dizziness was also prevalent at 71%, whereas 60% reported gastrointestinal issues, and 36% said they experienced fever, sweats, and chills.
Nearly 700 of the 2213 people fell into this subtype group, by far the highest number.
Subtype 5
A whopping 66% of people in subtype 5 reported a poor to fair quality of life. These people usually reported multisystem symptoms.
In terms of prevalence rises across the spectrum of 44 common long-COVID symptoms, 99% reported shortness of breath; 98%, postexertional soreness; 94%, dizziness; 92%, postexertional malaise; 80%, GI problems; 78%, weakness; and 69%, chest pain.
A higher proportion of Hispanic and multiracial participants were classified as having subtype 5. Also, according to the study, “higher proportions of unvaccinated participants and those with SARS-CoV-2 infection before circulation of the Omicron variant were in subtype 5.”
This suggests the severity of the Delta variant of COVID-19 be linked to some of the worst long COVID symptoms, but further study would have to be done to conclusively determine may be just a correlation.
When Do Symptoms Resolve?
According to Sanders, around 17 million Americans are thought to have long COVID. Although 90%-100% of people typically recover within 3 years, that still leaves possibly around 5% of those who don’t recover.
“What people usually say is, ‘I got COVID, and I never quite recovered,” Sanders said.
“Five percent of 17 million turns out to be a lot. It’s a lot of suffering,” she added. “I would say that the most common symptoms are fatigue, brain fog, anosmia or dysgeusia, and sleep disorders,” as evidenced by the high percentage of people in certain subtypes of the study reporting a poor quality of life.
A version of this article first appeared on Medscape.com.
A new analysis of long-COVID patients has identified five distinct subtypes that researchers say will help doctors diagnose the condition.
The new five-type index, developed by federal researchers with the National Institutes of Health’s RECOVER COVID Initiative, identified the most common symptoms in 14,000 people with long COVID, with data from an additional 4000 people added to the updated 2024 index.
By using the index, physicians and researchers can better understand the condition, which is difficult to treat and diagnose because no standard definitions or therapies have been developed. Doctors can use the index to offer more targeted care and help patients manage their symptoms more effectively.
The index may also help researchers find more treatments for long COVID. Because long COVID can affect so many different parts of the body, it will take time to fully understand how to treat it, but studies like this are making progress in the right direction, experts said.
This new index uses an updated point system, where points are allotted to each symptom in a list of the 44 most reported symptoms in people with likely long COVID based on how often they occur. Among people in the study with prior COVID infection, 2213 (18%) met the threshold for long COVID.
The 44 most common symptoms were then distributed among 5 subtypes, with each representing a difference in impact on quality of life and overall health. The most common symptoms were fatigue (85.8%), postexertional malaise (87.4%), and postexertional soreness (75.0%) — where persistent fatigue and discomfort occur after physical or mental exertion — dizziness (65.8%), brain fog (63.8%), gastrointestinal symptoms (59.3%), and palpitations (58%).
For those with prior COVID infection, symptoms were more prevalent in all cases.
Subtype 1
Those grouped into subtype 1 did not report a high incidence of impact on quality of life, physical health, or daily function. Only 21% of people in subtype 1 reported a “poor or fair quality of life.”
A change in smell or taste — usually a symptom that’s bothersome but doesn’t seriously impact overall health — was most present in subtype 1, with 100% of people in subtype 1 reporting it.
The only other symptoms in over 50% of people with subtype 1— which were 490 of the 2213 with prior COVID infection — were fatigue (66%), postexertional malaise (53%), and postexertional soreness (55%).
Though these two symptoms can certainly impact quality of life, they became much more prevalent in other subtypes.
Subtype 2
The prevalence of possibly debilitating symptoms like postexertional malaise (94%), fatigue (81%), and chronic cough (100%) rose dramatically in people grouped into subtype 2.
Plus, 25% of people in subtype 2 reported a “poor or fair quality of life. Postexertional malaise, I think, is probably one of the most debilitating of the symptoms. When somebody comes in and tells me that they’re tired and I think they might have long COVID, the first thing I try to do is see if it is postexertional malaise vs just postinfectious fatigue,” said Lisa Sanders, MD, medical director of Yale’s Long Covid Multidisciplinary Care Center in New Haven, Connecticut.
Postinfectious fatigue usually resolves much more quickly than postexertional malaise. The latter accounts for several symptoms as also associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). ME/CFS is a chronic illness that causes severe fatigue and makes it difficult for sufferers to perform routine, daily activities.
“Postexertional malaise is an additive symptom of ME/CFS, and that can take a long time to resolve,” Sanders added.
The similarity between these two symptoms highlights the importance that physicians must place in scrutinizing symptoms to a high degree when they suspect a patient of having long COVID, experts said. By doing so, clinicians can unveil the mask of overlapping symptoms between long COVID symptoms and symptoms of other illnesses.
Subtype 3
About 37% of people grouped in subtype 3 reported a poor or fair quality of life, a significant rise from subtypes 1 and 2.
Fatigue symptoms were reported by 92%, whereas 82% reported postexertional soreness, and 70% reported dizziness. Additionally, 100% of people in subtype 3 reported brain fog as a symptom.
Sanders said these symptoms are also common in people with postural orthostatic tachycardia syndrome. This condition results from a reduced volume of blood returning to the heart after standing up, which leads to an abnormally fast heart rate. Palpitations and fainting can then occur.
Brain fog can be especially debilitating in people who are used to multitasking. With brain fog, people accustomed to easily alternating between tasks or doing multiple tasks at once can only do one thing at a time. This can cause stress and an overload of thoughts, even precipitating a change in careers if severe enough.
Though brain fog tends to resolve within 6-9 months after infection, it can last up to 18 months or more. Experts say doctors should always be on the lookout if a patient complains they have trouble concentrating or multitasking in the months after a COVID infection. A neurological exam and cognitive testing can identify abnormalities in brain function.
Subtype 4
About 40% of people in the study grouped into subtype 4 reported a poor or fair quality of life, a modest increase from those with subtype 3. About 65% reported symptoms of brain fog and 92% reported palpitations.
Dizziness was also prevalent at 71%, whereas 60% reported gastrointestinal issues, and 36% said they experienced fever, sweats, and chills.
Nearly 700 of the 2213 people fell into this subtype group, by far the highest number.
Subtype 5
A whopping 66% of people in subtype 5 reported a poor to fair quality of life. These people usually reported multisystem symptoms.
In terms of prevalence rises across the spectrum of 44 common long-COVID symptoms, 99% reported shortness of breath; 98%, postexertional soreness; 94%, dizziness; 92%, postexertional malaise; 80%, GI problems; 78%, weakness; and 69%, chest pain.
A higher proportion of Hispanic and multiracial participants were classified as having subtype 5. Also, according to the study, “higher proportions of unvaccinated participants and those with SARS-CoV-2 infection before circulation of the Omicron variant were in subtype 5.”
This suggests the severity of the Delta variant of COVID-19 be linked to some of the worst long COVID symptoms, but further study would have to be done to conclusively determine may be just a correlation.
When Do Symptoms Resolve?
According to Sanders, around 17 million Americans are thought to have long COVID. Although 90%-100% of people typically recover within 3 years, that still leaves possibly around 5% of those who don’t recover.
“What people usually say is, ‘I got COVID, and I never quite recovered,” Sanders said.
“Five percent of 17 million turns out to be a lot. It’s a lot of suffering,” she added. “I would say that the most common symptoms are fatigue, brain fog, anosmia or dysgeusia, and sleep disorders,” as evidenced by the high percentage of people in certain subtypes of the study reporting a poor quality of life.
A version of this article first appeared on Medscape.com.
A new analysis of long-COVID patients has identified five distinct subtypes that researchers say will help doctors diagnose the condition.
The new five-type index, developed by federal researchers with the National Institutes of Health’s RECOVER COVID Initiative, identified the most common symptoms in 14,000 people with long COVID, with data from an additional 4000 people added to the updated 2024 index.
By using the index, physicians and researchers can better understand the condition, which is difficult to treat and diagnose because no standard definitions or therapies have been developed. Doctors can use the index to offer more targeted care and help patients manage their symptoms more effectively.
The index may also help researchers find more treatments for long COVID. Because long COVID can affect so many different parts of the body, it will take time to fully understand how to treat it, but studies like this are making progress in the right direction, experts said.
This new index uses an updated point system, where points are allotted to each symptom in a list of the 44 most reported symptoms in people with likely long COVID based on how often they occur. Among people in the study with prior COVID infection, 2213 (18%) met the threshold for long COVID.
The 44 most common symptoms were then distributed among 5 subtypes, with each representing a difference in impact on quality of life and overall health. The most common symptoms were fatigue (85.8%), postexertional malaise (87.4%), and postexertional soreness (75.0%) — where persistent fatigue and discomfort occur after physical or mental exertion — dizziness (65.8%), brain fog (63.8%), gastrointestinal symptoms (59.3%), and palpitations (58%).
For those with prior COVID infection, symptoms were more prevalent in all cases.
Subtype 1
Those grouped into subtype 1 did not report a high incidence of impact on quality of life, physical health, or daily function. Only 21% of people in subtype 1 reported a “poor or fair quality of life.”
A change in smell or taste — usually a symptom that’s bothersome but doesn’t seriously impact overall health — was most present in subtype 1, with 100% of people in subtype 1 reporting it.
The only other symptoms in over 50% of people with subtype 1— which were 490 of the 2213 with prior COVID infection — were fatigue (66%), postexertional malaise (53%), and postexertional soreness (55%).
Though these two symptoms can certainly impact quality of life, they became much more prevalent in other subtypes.
Subtype 2
The prevalence of possibly debilitating symptoms like postexertional malaise (94%), fatigue (81%), and chronic cough (100%) rose dramatically in people grouped into subtype 2.
Plus, 25% of people in subtype 2 reported a “poor or fair quality of life. Postexertional malaise, I think, is probably one of the most debilitating of the symptoms. When somebody comes in and tells me that they’re tired and I think they might have long COVID, the first thing I try to do is see if it is postexertional malaise vs just postinfectious fatigue,” said Lisa Sanders, MD, medical director of Yale’s Long Covid Multidisciplinary Care Center in New Haven, Connecticut.
Postinfectious fatigue usually resolves much more quickly than postexertional malaise. The latter accounts for several symptoms as also associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). ME/CFS is a chronic illness that causes severe fatigue and makes it difficult for sufferers to perform routine, daily activities.
“Postexertional malaise is an additive symptom of ME/CFS, and that can take a long time to resolve,” Sanders added.
The similarity between these two symptoms highlights the importance that physicians must place in scrutinizing symptoms to a high degree when they suspect a patient of having long COVID, experts said. By doing so, clinicians can unveil the mask of overlapping symptoms between long COVID symptoms and symptoms of other illnesses.
Subtype 3
About 37% of people grouped in subtype 3 reported a poor or fair quality of life, a significant rise from subtypes 1 and 2.
Fatigue symptoms were reported by 92%, whereas 82% reported postexertional soreness, and 70% reported dizziness. Additionally, 100% of people in subtype 3 reported brain fog as a symptom.
Sanders said these symptoms are also common in people with postural orthostatic tachycardia syndrome. This condition results from a reduced volume of blood returning to the heart after standing up, which leads to an abnormally fast heart rate. Palpitations and fainting can then occur.
Brain fog can be especially debilitating in people who are used to multitasking. With brain fog, people accustomed to easily alternating between tasks or doing multiple tasks at once can only do one thing at a time. This can cause stress and an overload of thoughts, even precipitating a change in careers if severe enough.
Though brain fog tends to resolve within 6-9 months after infection, it can last up to 18 months or more. Experts say doctors should always be on the lookout if a patient complains they have trouble concentrating or multitasking in the months after a COVID infection. A neurological exam and cognitive testing can identify abnormalities in brain function.
Subtype 4
About 40% of people in the study grouped into subtype 4 reported a poor or fair quality of life, a modest increase from those with subtype 3. About 65% reported symptoms of brain fog and 92% reported palpitations.
Dizziness was also prevalent at 71%, whereas 60% reported gastrointestinal issues, and 36% said they experienced fever, sweats, and chills.
Nearly 700 of the 2213 people fell into this subtype group, by far the highest number.
Subtype 5
A whopping 66% of people in subtype 5 reported a poor to fair quality of life. These people usually reported multisystem symptoms.
In terms of prevalence rises across the spectrum of 44 common long-COVID symptoms, 99% reported shortness of breath; 98%, postexertional soreness; 94%, dizziness; 92%, postexertional malaise; 80%, GI problems; 78%, weakness; and 69%, chest pain.
A higher proportion of Hispanic and multiracial participants were classified as having subtype 5. Also, according to the study, “higher proportions of unvaccinated participants and those with SARS-CoV-2 infection before circulation of the Omicron variant were in subtype 5.”
This suggests the severity of the Delta variant of COVID-19 be linked to some of the worst long COVID symptoms, but further study would have to be done to conclusively determine may be just a correlation.
When Do Symptoms Resolve?
According to Sanders, around 17 million Americans are thought to have long COVID. Although 90%-100% of people typically recover within 3 years, that still leaves possibly around 5% of those who don’t recover.
“What people usually say is, ‘I got COVID, and I never quite recovered,” Sanders said.
“Five percent of 17 million turns out to be a lot. It’s a lot of suffering,” she added. “I would say that the most common symptoms are fatigue, brain fog, anosmia or dysgeusia, and sleep disorders,” as evidenced by the high percentage of people in certain subtypes of the study reporting a poor quality of life.
A version of this article first appeared on Medscape.com.
FROM JAMA
Next-Gen Sequencing Tumor Testing Remains Low in Prostate and Urothelial Cancer Cases
This article is a based on a video essay. The transcript has been edited for clarity.
I’d like to discuss what I think is a very interesting analysis that we need to see much more of. It’s perhaps not surprising, but the data, I think, are sobering. The paper was published in JAMA Network Open, entitled, “Trends and Disparities in Next-Generation Sequencing in Metastatic Prostate and Urothelial Cancers.”
As I think most of the listening audience is aware, we are in the midst of an ongoing — I would argue, accelerating — revolution in our understanding of cancer, its development and treatments, based upon our characterization at the molecular level of individual cancers.
This, of course, is changing the treatment paradigms and the drugs that we might have available in the first-, second-, and third-line settings. The question to be asked is, how are we, at a clinical level, keeping up with all of these changes, like those approved by the US Food and Drug Administration, and new diagnostic testing with a variety of molecular platforms?
This particular analysis looked at that specific question in metastatic prostate cancer and urothelial malignancies, obviously including bladder cancer. With the new approvals — including tumor agnostic testing, very specific testing, and very molecularly based drugs that are approved for particular abnormalities — they looked at the percentages of patients and the potential disparities in terms of the testing that has been performed.
There were 11,927 patients with prostate cancer. There were 6490 patients with advanced urothelial malignancies; the majority of these were male, but there were females included in this group.
The researchers looked at 2015 vs 2022 data. It’s not 2024 data, but it goes all the way to the end of 2022, so, not that long ago. In the metastatic prostate cancer group, 19% of patients had undergone molecular testing or next-generation sequencing in 2015.
By 2022, that number had increased, but only to 27%. Three out of four patients with metastatic prostate cancer had not undergone testing to know whether they were potential candidates for specific therapies. I won’t even get into the question of potential germline abnormalities that might be observed that are relevant for other discussions.
Among patients with urothelial cancer, in 2015, 14% had undergone such testing. By 2022, this number was substantially increased to 46.6%, but still, that’s less than 1 out of 2 patients. More than 50% of patients had not undergone the testing, and yet we have therapy that might be available for these populations based on such testing.
I should add that the population of Black, African American, and Hispanic patients was actually considerably lower, percentage-wise, than the numbers that I’ve quoted.
Clearly, there are explanations. There are socioeconomic explanations and insurance coverage explanations. However, the bottom line is that we have therapies available today, and we’ll have more in the future, that are based on knowledge of this testing.
Based on these data, which most recently included 2022 — we’ll see where we are in 2024 and 2025, and with other types — more than half of patients are not getting the testing to know if this is relevant for them and their care.
These are major questions that need to be addressed. Hopefully, answers will be forthcoming and we will see in the future that these percentages will be much higher for the benefit of our patients.
Dr Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, has disclosed the following relevant financial relationships with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This article is a based on a video essay. The transcript has been edited for clarity.
I’d like to discuss what I think is a very interesting analysis that we need to see much more of. It’s perhaps not surprising, but the data, I think, are sobering. The paper was published in JAMA Network Open, entitled, “Trends and Disparities in Next-Generation Sequencing in Metastatic Prostate and Urothelial Cancers.”
As I think most of the listening audience is aware, we are in the midst of an ongoing — I would argue, accelerating — revolution in our understanding of cancer, its development and treatments, based upon our characterization at the molecular level of individual cancers.
This, of course, is changing the treatment paradigms and the drugs that we might have available in the first-, second-, and third-line settings. The question to be asked is, how are we, at a clinical level, keeping up with all of these changes, like those approved by the US Food and Drug Administration, and new diagnostic testing with a variety of molecular platforms?
This particular analysis looked at that specific question in metastatic prostate cancer and urothelial malignancies, obviously including bladder cancer. With the new approvals — including tumor agnostic testing, very specific testing, and very molecularly based drugs that are approved for particular abnormalities — they looked at the percentages of patients and the potential disparities in terms of the testing that has been performed.
There were 11,927 patients with prostate cancer. There were 6490 patients with advanced urothelial malignancies; the majority of these were male, but there were females included in this group.
The researchers looked at 2015 vs 2022 data. It’s not 2024 data, but it goes all the way to the end of 2022, so, not that long ago. In the metastatic prostate cancer group, 19% of patients had undergone molecular testing or next-generation sequencing in 2015.
By 2022, that number had increased, but only to 27%. Three out of four patients with metastatic prostate cancer had not undergone testing to know whether they were potential candidates for specific therapies. I won’t even get into the question of potential germline abnormalities that might be observed that are relevant for other discussions.
Among patients with urothelial cancer, in 2015, 14% had undergone such testing. By 2022, this number was substantially increased to 46.6%, but still, that’s less than 1 out of 2 patients. More than 50% of patients had not undergone the testing, and yet we have therapy that might be available for these populations based on such testing.
I should add that the population of Black, African American, and Hispanic patients was actually considerably lower, percentage-wise, than the numbers that I’ve quoted.
Clearly, there are explanations. There are socioeconomic explanations and insurance coverage explanations. However, the bottom line is that we have therapies available today, and we’ll have more in the future, that are based on knowledge of this testing.
Based on these data, which most recently included 2022 — we’ll see where we are in 2024 and 2025, and with other types — more than half of patients are not getting the testing to know if this is relevant for them and their care.
These are major questions that need to be addressed. Hopefully, answers will be forthcoming and we will see in the future that these percentages will be much higher for the benefit of our patients.
Dr Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, has disclosed the following relevant financial relationships with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This article is a based on a video essay. The transcript has been edited for clarity.
I’d like to discuss what I think is a very interesting analysis that we need to see much more of. It’s perhaps not surprising, but the data, I think, are sobering. The paper was published in JAMA Network Open, entitled, “Trends and Disparities in Next-Generation Sequencing in Metastatic Prostate and Urothelial Cancers.”
As I think most of the listening audience is aware, we are in the midst of an ongoing — I would argue, accelerating — revolution in our understanding of cancer, its development and treatments, based upon our characterization at the molecular level of individual cancers.
This, of course, is changing the treatment paradigms and the drugs that we might have available in the first-, second-, and third-line settings. The question to be asked is, how are we, at a clinical level, keeping up with all of these changes, like those approved by the US Food and Drug Administration, and new diagnostic testing with a variety of molecular platforms?
This particular analysis looked at that specific question in metastatic prostate cancer and urothelial malignancies, obviously including bladder cancer. With the new approvals — including tumor agnostic testing, very specific testing, and very molecularly based drugs that are approved for particular abnormalities — they looked at the percentages of patients and the potential disparities in terms of the testing that has been performed.
There were 11,927 patients with prostate cancer. There were 6490 patients with advanced urothelial malignancies; the majority of these were male, but there were females included in this group.
The researchers looked at 2015 vs 2022 data. It’s not 2024 data, but it goes all the way to the end of 2022, so, not that long ago. In the metastatic prostate cancer group, 19% of patients had undergone molecular testing or next-generation sequencing in 2015.
By 2022, that number had increased, but only to 27%. Three out of four patients with metastatic prostate cancer had not undergone testing to know whether they were potential candidates for specific therapies. I won’t even get into the question of potential germline abnormalities that might be observed that are relevant for other discussions.
Among patients with urothelial cancer, in 2015, 14% had undergone such testing. By 2022, this number was substantially increased to 46.6%, but still, that’s less than 1 out of 2 patients. More than 50% of patients had not undergone the testing, and yet we have therapy that might be available for these populations based on such testing.
I should add that the population of Black, African American, and Hispanic patients was actually considerably lower, percentage-wise, than the numbers that I’ve quoted.
Clearly, there are explanations. There are socioeconomic explanations and insurance coverage explanations. However, the bottom line is that we have therapies available today, and we’ll have more in the future, that are based on knowledge of this testing.
Based on these data, which most recently included 2022 — we’ll see where we are in 2024 and 2025, and with other types — more than half of patients are not getting the testing to know if this is relevant for them and their care.
These are major questions that need to be addressed. Hopefully, answers will be forthcoming and we will see in the future that these percentages will be much higher for the benefit of our patients.
Dr Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, has disclosed the following relevant financial relationships with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
Navigating Esophageal Dysfunction in Immune and Infectious Disorders: AGA Clinical Practice Update
“Many different disorders can lead to esophageal dysfunction, which is characterized by symptoms including dysphagia, odynophagia, chest pain and heartburn. These symptoms can be caused either by immune or infectious conditions and can either be localized to the esophagus or part of a larger systemic process,” co–first author Emily McGowan, MD, PhD, with the division of allergy and immunology, University of Virginia School of Medicine, Charlottesville, said in an AGA podcast.
However, without a “high index of suspicion,” these conditions can be overlooked, leading to delays in diagnosis and unnecessary procedures. “With this clinical practice update, we wanted to help providers more readily recognize these conditions so that patients can be diagnosed and treated earlier in the course of their disease,” McGowan explained.
“This is a fantastic review that highlights how many different systemic disorders can affect the esophagus,” Scott Gabbard, MD, gastroenterologist and section head at the Center for Neurogastroenterology and Motility, Cleveland Clinic, Ohio, who wasn’t involved in the review, said in an interview.
“Honestly, for the practicing gastroenterologist, this is one of those reviews that I could envision someone either saving to his or her desktop for reference or printing it and pinning it next to his or her desk,” Gabbard said.
Best Practice Advice
The clinical practice update is published in Clinical Gastroenterology and Hepatology. It includes 10 “best practice advice” statements and a table highlighting “important” considerations when evaluating patients with esophageal dysfunction.
The review authors note that esophageal dysfunction may result from localized infections — most commonly Candida, herpes simplex virus, and cytomegalovirus — or systemic immune-mediated diseases, such as systemic sclerosis (SSc), mixed connective tissue disease (MCTD), and eosinophilic esophagitis (EoE).
They advise clinicians to identify if there are risks for inflammatory or infectious possibilities for a patient’s esophageal symptoms and investigate for these disorders as a potential cause of esophageal dysfunction.
Once esophageal infection is identified, it’s important to identify whether accompanying signs and symptoms point to immunocompromise leading to a more systemic infection. Consultation with an infectious disease expert is recommended to guide appropriate treatment, the authors said.
If symptoms fail to improve after therapy for infectious esophagitis, the patient should be evaluated for refractory infection or additional underlying sources of esophageal and immunologic dysfunction is advised.
It’s also important to recognize that patients with EoE who continue to have symptoms of esophageal dysfunction despite histologic and endoscopic disease remission, may develop a motility disorder and evaluation of esophageal motility may be warranted, the authors said.
In patients with histologic and endoscopic features of lymphocytic esophagitis, treatment of lymphocytic-related inflammation with proton-pump inhibitor (PPI) therapy or swallowed topical corticosteroids and esophageal dilation as needed should be considered.
In patients who present with esophageal symptoms in the setting of hypereosinophilia (absolute eosinophil count > 1500 cells/uL), the authors advise further workup of non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis should be considered, with consultation with an allergy/immunology specialist if helpful.
In patients with rheumatologic diseases, especially SSc and MCTD, it’s important to be aware that esophageal symptoms can occur because of involvement of the esophageal muscle layer, resulting in dysmotility and/or incompetence of the lower esophageal sphincter, they said.
In the setting of Crohn’s disease, some patients can develop esophageal involvement from inflammation, stricturing, or fistulizing changes with granulomas seen histologically. Esophageal manifestations of Crohn’s disease tend to occur in patients with active intestinal disease.
In patients with dermatologic diseases of lichen planus or bullous disorders, dysphagia can occur because of endoscopically visible esophageal mucosal involvement. Esophageal lichen planus, in particular, can occur without skin involvement and can be difficult to define on esophageal histopathology.
The authors also advise clinicians to consider infectious and inflammatory causes of secondary achalasia during initial evaluation.
“Achalasia and EoE might coexist more commonly than what gastroenterologists think, especially in younger patients,” co–first author Chanakyaram Reddy, MD, a gastroenterologist with Baylor University Medical Center, Dallas, Texas, said in the AGA podcast.
He noted that in a recent population-based study, the estimated relative risk of EoE was over 30-fold higher in patients with achalasia aged ≤ 40 years.
“In any suspected achalasia case, it would be wise to obtain biopsies throughout the entire esophagus when the patient is off confounding medications such as PPI therapy to establish if significant esophageal eosinophilia is coexistent,” Reddy said.
“If EoE-level eosinophilia is found, it would be reasonable to consider treating medically for EoE prior to committing to achalasia-specific interventions, which often involve permanent disruption of the esophageal muscle layer,” he added.
Gabbard said this review helps the clinician think beyond gastroesophageal reflux disease (GERD) — the most common cause of esophageal dysfunction — and consider other causes for esophageal dysfunction.
“We are seeing more complex disorders affect the esophagus. It’s not just GERD and you absolutely need a high index of suspicion because you can find varying disorders to blame for many esophageal symptoms that could otherwise be thought to be just reflux,” he said.
This research had no commercial funding. Disclosures for the authors are listed with the original article. Gabbard had no relevant disclosures.
A version of this article appeared on Medscape.com.
“Many different disorders can lead to esophageal dysfunction, which is characterized by symptoms including dysphagia, odynophagia, chest pain and heartburn. These symptoms can be caused either by immune or infectious conditions and can either be localized to the esophagus or part of a larger systemic process,” co–first author Emily McGowan, MD, PhD, with the division of allergy and immunology, University of Virginia School of Medicine, Charlottesville, said in an AGA podcast.
However, without a “high index of suspicion,” these conditions can be overlooked, leading to delays in diagnosis and unnecessary procedures. “With this clinical practice update, we wanted to help providers more readily recognize these conditions so that patients can be diagnosed and treated earlier in the course of their disease,” McGowan explained.
“This is a fantastic review that highlights how many different systemic disorders can affect the esophagus,” Scott Gabbard, MD, gastroenterologist and section head at the Center for Neurogastroenterology and Motility, Cleveland Clinic, Ohio, who wasn’t involved in the review, said in an interview.
“Honestly, for the practicing gastroenterologist, this is one of those reviews that I could envision someone either saving to his or her desktop for reference or printing it and pinning it next to his or her desk,” Gabbard said.
Best Practice Advice
The clinical practice update is published in Clinical Gastroenterology and Hepatology. It includes 10 “best practice advice” statements and a table highlighting “important” considerations when evaluating patients with esophageal dysfunction.
The review authors note that esophageal dysfunction may result from localized infections — most commonly Candida, herpes simplex virus, and cytomegalovirus — or systemic immune-mediated diseases, such as systemic sclerosis (SSc), mixed connective tissue disease (MCTD), and eosinophilic esophagitis (EoE).
They advise clinicians to identify if there are risks for inflammatory or infectious possibilities for a patient’s esophageal symptoms and investigate for these disorders as a potential cause of esophageal dysfunction.
Once esophageal infection is identified, it’s important to identify whether accompanying signs and symptoms point to immunocompromise leading to a more systemic infection. Consultation with an infectious disease expert is recommended to guide appropriate treatment, the authors said.
If symptoms fail to improve after therapy for infectious esophagitis, the patient should be evaluated for refractory infection or additional underlying sources of esophageal and immunologic dysfunction is advised.
It’s also important to recognize that patients with EoE who continue to have symptoms of esophageal dysfunction despite histologic and endoscopic disease remission, may develop a motility disorder and evaluation of esophageal motility may be warranted, the authors said.
In patients with histologic and endoscopic features of lymphocytic esophagitis, treatment of lymphocytic-related inflammation with proton-pump inhibitor (PPI) therapy or swallowed topical corticosteroids and esophageal dilation as needed should be considered.
In patients who present with esophageal symptoms in the setting of hypereosinophilia (absolute eosinophil count > 1500 cells/uL), the authors advise further workup of non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis should be considered, with consultation with an allergy/immunology specialist if helpful.
In patients with rheumatologic diseases, especially SSc and MCTD, it’s important to be aware that esophageal symptoms can occur because of involvement of the esophageal muscle layer, resulting in dysmotility and/or incompetence of the lower esophageal sphincter, they said.
In the setting of Crohn’s disease, some patients can develop esophageal involvement from inflammation, stricturing, or fistulizing changes with granulomas seen histologically. Esophageal manifestations of Crohn’s disease tend to occur in patients with active intestinal disease.
In patients with dermatologic diseases of lichen planus or bullous disorders, dysphagia can occur because of endoscopically visible esophageal mucosal involvement. Esophageal lichen planus, in particular, can occur without skin involvement and can be difficult to define on esophageal histopathology.
The authors also advise clinicians to consider infectious and inflammatory causes of secondary achalasia during initial evaluation.
“Achalasia and EoE might coexist more commonly than what gastroenterologists think, especially in younger patients,” co–first author Chanakyaram Reddy, MD, a gastroenterologist with Baylor University Medical Center, Dallas, Texas, said in the AGA podcast.
He noted that in a recent population-based study, the estimated relative risk of EoE was over 30-fold higher in patients with achalasia aged ≤ 40 years.
“In any suspected achalasia case, it would be wise to obtain biopsies throughout the entire esophagus when the patient is off confounding medications such as PPI therapy to establish if significant esophageal eosinophilia is coexistent,” Reddy said.
“If EoE-level eosinophilia is found, it would be reasonable to consider treating medically for EoE prior to committing to achalasia-specific interventions, which often involve permanent disruption of the esophageal muscle layer,” he added.
Gabbard said this review helps the clinician think beyond gastroesophageal reflux disease (GERD) — the most common cause of esophageal dysfunction — and consider other causes for esophageal dysfunction.
“We are seeing more complex disorders affect the esophagus. It’s not just GERD and you absolutely need a high index of suspicion because you can find varying disorders to blame for many esophageal symptoms that could otherwise be thought to be just reflux,” he said.
This research had no commercial funding. Disclosures for the authors are listed with the original article. Gabbard had no relevant disclosures.
A version of this article appeared on Medscape.com.
“Many different disorders can lead to esophageal dysfunction, which is characterized by symptoms including dysphagia, odynophagia, chest pain and heartburn. These symptoms can be caused either by immune or infectious conditions and can either be localized to the esophagus or part of a larger systemic process,” co–first author Emily McGowan, MD, PhD, with the division of allergy and immunology, University of Virginia School of Medicine, Charlottesville, said in an AGA podcast.
However, without a “high index of suspicion,” these conditions can be overlooked, leading to delays in diagnosis and unnecessary procedures. “With this clinical practice update, we wanted to help providers more readily recognize these conditions so that patients can be diagnosed and treated earlier in the course of their disease,” McGowan explained.
“This is a fantastic review that highlights how many different systemic disorders can affect the esophagus,” Scott Gabbard, MD, gastroenterologist and section head at the Center for Neurogastroenterology and Motility, Cleveland Clinic, Ohio, who wasn’t involved in the review, said in an interview.
“Honestly, for the practicing gastroenterologist, this is one of those reviews that I could envision someone either saving to his or her desktop for reference or printing it and pinning it next to his or her desk,” Gabbard said.
Best Practice Advice
The clinical practice update is published in Clinical Gastroenterology and Hepatology. It includes 10 “best practice advice” statements and a table highlighting “important” considerations when evaluating patients with esophageal dysfunction.
The review authors note that esophageal dysfunction may result from localized infections — most commonly Candida, herpes simplex virus, and cytomegalovirus — or systemic immune-mediated diseases, such as systemic sclerosis (SSc), mixed connective tissue disease (MCTD), and eosinophilic esophagitis (EoE).
They advise clinicians to identify if there are risks for inflammatory or infectious possibilities for a patient’s esophageal symptoms and investigate for these disorders as a potential cause of esophageal dysfunction.
Once esophageal infection is identified, it’s important to identify whether accompanying signs and symptoms point to immunocompromise leading to a more systemic infection. Consultation with an infectious disease expert is recommended to guide appropriate treatment, the authors said.
If symptoms fail to improve after therapy for infectious esophagitis, the patient should be evaluated for refractory infection or additional underlying sources of esophageal and immunologic dysfunction is advised.
It’s also important to recognize that patients with EoE who continue to have symptoms of esophageal dysfunction despite histologic and endoscopic disease remission, may develop a motility disorder and evaluation of esophageal motility may be warranted, the authors said.
In patients with histologic and endoscopic features of lymphocytic esophagitis, treatment of lymphocytic-related inflammation with proton-pump inhibitor (PPI) therapy or swallowed topical corticosteroids and esophageal dilation as needed should be considered.
In patients who present with esophageal symptoms in the setting of hypereosinophilia (absolute eosinophil count > 1500 cells/uL), the authors advise further workup of non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis should be considered, with consultation with an allergy/immunology specialist if helpful.
In patients with rheumatologic diseases, especially SSc and MCTD, it’s important to be aware that esophageal symptoms can occur because of involvement of the esophageal muscle layer, resulting in dysmotility and/or incompetence of the lower esophageal sphincter, they said.
In the setting of Crohn’s disease, some patients can develop esophageal involvement from inflammation, stricturing, or fistulizing changes with granulomas seen histologically. Esophageal manifestations of Crohn’s disease tend to occur in patients with active intestinal disease.
In patients with dermatologic diseases of lichen planus or bullous disorders, dysphagia can occur because of endoscopically visible esophageal mucosal involvement. Esophageal lichen planus, in particular, can occur without skin involvement and can be difficult to define on esophageal histopathology.
The authors also advise clinicians to consider infectious and inflammatory causes of secondary achalasia during initial evaluation.
“Achalasia and EoE might coexist more commonly than what gastroenterologists think, especially in younger patients,” co–first author Chanakyaram Reddy, MD, a gastroenterologist with Baylor University Medical Center, Dallas, Texas, said in the AGA podcast.
He noted that in a recent population-based study, the estimated relative risk of EoE was over 30-fold higher in patients with achalasia aged ≤ 40 years.
“In any suspected achalasia case, it would be wise to obtain biopsies throughout the entire esophagus when the patient is off confounding medications such as PPI therapy to establish if significant esophageal eosinophilia is coexistent,” Reddy said.
“If EoE-level eosinophilia is found, it would be reasonable to consider treating medically for EoE prior to committing to achalasia-specific interventions, which often involve permanent disruption of the esophageal muscle layer,” he added.
Gabbard said this review helps the clinician think beyond gastroesophageal reflux disease (GERD) — the most common cause of esophageal dysfunction — and consider other causes for esophageal dysfunction.
“We are seeing more complex disorders affect the esophagus. It’s not just GERD and you absolutely need a high index of suspicion because you can find varying disorders to blame for many esophageal symptoms that could otherwise be thought to be just reflux,” he said.
This research had no commercial funding. Disclosures for the authors are listed with the original article. Gabbard had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Finding and Following Your Passion
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
Over the last year, I have been reading more about professional identity and professional branding, all of which have evolved in the setting of social media. However, the root of it remains constant — finding the intersection(s) of what you love. A common problem, especially as a trainee and early-career gastroenterologist, is that you may have many interests: various disease processes, innovation, medical education, leadership development, and much more. Since becoming faculty, I continue to define and refine my professional niche, trying to distinguish my “interests” from “passions.” It is a journey that my mentors advise me not to rush through and I am enjoying every moment of it!
In this issue’s “In Focus,” Dr. Hamza Salim, Dr. Anni Chowdhury, and Dr. Lavanya Viswanathan provide a practical guide for the clinical evaluation of chronic constipation and a systematic approach to treatment.
In the first of a two-part series in the “Short Clinical Review” section, Dr. Christopher Velez and Dr. Kara J. Jencks discuss the health inequities among sexual and gender minority (SGM) patients, particularly with disorders of brain-gut interaction (DBGI). They review common SGM terminology, sample verbiage for trauma-informed care, and case presentations to help guide our approach to providing care for SGM patients with DGBI.
The transition from trainee to early faculty may be difficult for those who are interested in research but struggle with the change from being a part of a research team to running one. In the “Early Career” section, Dr. Lauren Feld and colleagues describes her experience establishing a research lab as an early-career academic, from creating a niche to time management and mentorship.
The Federal Trade Commission’s noncompete ban made big news in April 2024 but there is still a lot of gray area for physicians. Dr. Timothy Craig Allen explains the ruling, what it means to physicians, the status of it today, and what the future may hold. Lastly, for “Private Practice Perspectives” in collaboration with Digestive Health Physicians Alliance, I interview Dr. Vasu Appalaneni on her use of artificial intelligence in private practice.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Polyethylene glycol was first used in the 1940s and 1950s to understand the physiology of the intestines, and first published as a compound for colonoscopy bowel preparation in 1981.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Best Practices When Using POEM to Treat Achalasia: AGA Clinical Update
The American Gastroenterological Association (AGA) has released
“Any patient suspected to have achalasia, or difficulty swallowing for that matter, should undergo a comprehensive diagnostic workup, and that should include clinical history, review of medication, as well as tests. The diagnosis should not be based on isolated tests but on the clinical picture as a whole,” first author Dennis Yang, MD, AGAF, with the Center for Interventional Endoscopy, AdventHealth, Orlando, Florida, noted in an AGA podcast about the update.
The clinical practice update, published in Gastroenterology, includes 12 “best practice advice” statements.
Since its introduction to clinical practice more than a decade ago, POEM has matured and gained widespread acceptance because of its efficacy and safety profile.
POEM has at least similar outcomes to laparoscopic Heller myotomy and pneumatic dilation for type I and type II achalasia with better results for those with type III achalasia, Yang noted.
“However, besides disease phenotype, we need to remember that choosing the right treatment for the patient is going to be based on multiple factors including patient characteristics as well as local expertise,” Yang added.
In terms of technical considerations, the update states that both anterior and posterior tunnel approaches demonstrate comparable success and postprocedure reflux rates. Tunnel orientation should be tailored to the patient’s surgical history and endoscopist’s preference.
It further states that optimal length of the myotomy in the esophagus and cardia, as it pertains to treatment efficacy and risk for postprocedure reflux, remains to be determined.
Adjunct techniques, including real-time intraprocedure functional luminal impedance planimetry, may be considered to tailor or confirm the adequacy of the myotomy.
Same-day discharge after POEM can be considered in select patients who meet discharge criteria. Patients with advanced age, significant comorbidities, poor social support, and/or access to specialized care should be considered for hospital admission, irrespective of symptoms.
The update notes that specific guidelines on the role and extent of antibiotic prophylaxis before and after POEM are lacking. A single dose of antibiotics at the time of POEM “may be sufficient” for antibiotic prophylaxis.
In terms of immediate post-POEM care, the update notes that the clinical impact of routine esophagram or endoscopy immediately post-POEM remains unclear. Testing can be considered based on local practice preferences and in cases in which intraprocedural events or postprocedural findings warrant further evaluation.
Proton pump inhibitors are recommended immediately following POEM, as gastroesophageal reflux disease (GERD) is common following POEM, occurring in up to 65% of cases.
Routine endoscopic surveillance is advised to monitor GERD, disease progression, and esophageal cancer risk, which is significantly higher in achalasia patients.
“Just like diabetes and hypertension, we need to remember that achalasia is a chronic disease and long-term postprocedural surveillance is strongly encouraged to monitor disease progression as well as potential complications of reflux,” Yang said.
He noted that surveillance should be considered irrespective of patient symptoms because many of these patients may remain asymptomatic.
“Primary gastroenterologists should have a very low threshold in referring the patient back to the POEM endoscopist or any specialized esophageal center because the ideology of symptoms in these patients can be quite difficult to tease out and often require comprehensive diagnostic workup,” Yang said.
Evidence for POEM in esophagogastric outflow obstruction and other nonachalasia spastic motility disorders is limited and should only be considered on a case-by-case basis after other less invasive approaches have been exhausted, the update states.
For perspective on the POEM clinical practice update, this news organization spoke with Mouen Khashab, MD, director of therapeutic endoscopy, Johns Hopkins University, Baltimore, Maryland.
“The document is very well written and comprehensive,” Khashab said.
However, Khashab said he would have liked to see greater emphasis on the value or role of a short myotomy in the esophagus and cardia.
“There is level I evidence that the short esophageal myotomy is equivalent to a long esophageal myotomy for type I and II achalasia. When you do a short myotomy, you save procedure time and there is potentially a lower incidence of blown-out myotomy or BOM,” Khashab said.
Khashab also noted that a long myotomy on the gastric side “likely increases the risk of reflux disease, and therefore a limited myotomy on the gastric side likely also is advantageous.”
This research had no commercial funding. Yang serves as a consultant for Boston Scientific, Olympus, FujiFilm, Microtech, Medtronic, 3D-Matrix, and Neptune Medical, and has received research support from Microtech and 3D-Matrix. Khashab had no relevant disclosures.
A version of this article appeared on Medscape.com .
The American Gastroenterological Association (AGA) has released
“Any patient suspected to have achalasia, or difficulty swallowing for that matter, should undergo a comprehensive diagnostic workup, and that should include clinical history, review of medication, as well as tests. The diagnosis should not be based on isolated tests but on the clinical picture as a whole,” first author Dennis Yang, MD, AGAF, with the Center for Interventional Endoscopy, AdventHealth, Orlando, Florida, noted in an AGA podcast about the update.
The clinical practice update, published in Gastroenterology, includes 12 “best practice advice” statements.
Since its introduction to clinical practice more than a decade ago, POEM has matured and gained widespread acceptance because of its efficacy and safety profile.
POEM has at least similar outcomes to laparoscopic Heller myotomy and pneumatic dilation for type I and type II achalasia with better results for those with type III achalasia, Yang noted.
“However, besides disease phenotype, we need to remember that choosing the right treatment for the patient is going to be based on multiple factors including patient characteristics as well as local expertise,” Yang added.
In terms of technical considerations, the update states that both anterior and posterior tunnel approaches demonstrate comparable success and postprocedure reflux rates. Tunnel orientation should be tailored to the patient’s surgical history and endoscopist’s preference.
It further states that optimal length of the myotomy in the esophagus and cardia, as it pertains to treatment efficacy and risk for postprocedure reflux, remains to be determined.
Adjunct techniques, including real-time intraprocedure functional luminal impedance planimetry, may be considered to tailor or confirm the adequacy of the myotomy.
Same-day discharge after POEM can be considered in select patients who meet discharge criteria. Patients with advanced age, significant comorbidities, poor social support, and/or access to specialized care should be considered for hospital admission, irrespective of symptoms.
The update notes that specific guidelines on the role and extent of antibiotic prophylaxis before and after POEM are lacking. A single dose of antibiotics at the time of POEM “may be sufficient” for antibiotic prophylaxis.
In terms of immediate post-POEM care, the update notes that the clinical impact of routine esophagram or endoscopy immediately post-POEM remains unclear. Testing can be considered based on local practice preferences and in cases in which intraprocedural events or postprocedural findings warrant further evaluation.
Proton pump inhibitors are recommended immediately following POEM, as gastroesophageal reflux disease (GERD) is common following POEM, occurring in up to 65% of cases.
Routine endoscopic surveillance is advised to monitor GERD, disease progression, and esophageal cancer risk, which is significantly higher in achalasia patients.
“Just like diabetes and hypertension, we need to remember that achalasia is a chronic disease and long-term postprocedural surveillance is strongly encouraged to monitor disease progression as well as potential complications of reflux,” Yang said.
He noted that surveillance should be considered irrespective of patient symptoms because many of these patients may remain asymptomatic.
“Primary gastroenterologists should have a very low threshold in referring the patient back to the POEM endoscopist or any specialized esophageal center because the ideology of symptoms in these patients can be quite difficult to tease out and often require comprehensive diagnostic workup,” Yang said.
Evidence for POEM in esophagogastric outflow obstruction and other nonachalasia spastic motility disorders is limited and should only be considered on a case-by-case basis after other less invasive approaches have been exhausted, the update states.
For perspective on the POEM clinical practice update, this news organization spoke with Mouen Khashab, MD, director of therapeutic endoscopy, Johns Hopkins University, Baltimore, Maryland.
“The document is very well written and comprehensive,” Khashab said.
However, Khashab said he would have liked to see greater emphasis on the value or role of a short myotomy in the esophagus and cardia.
“There is level I evidence that the short esophageal myotomy is equivalent to a long esophageal myotomy for type I and II achalasia. When you do a short myotomy, you save procedure time and there is potentially a lower incidence of blown-out myotomy or BOM,” Khashab said.
Khashab also noted that a long myotomy on the gastric side “likely increases the risk of reflux disease, and therefore a limited myotomy on the gastric side likely also is advantageous.”
This research had no commercial funding. Yang serves as a consultant for Boston Scientific, Olympus, FujiFilm, Microtech, Medtronic, 3D-Matrix, and Neptune Medical, and has received research support from Microtech and 3D-Matrix. Khashab had no relevant disclosures.
A version of this article appeared on Medscape.com .
The American Gastroenterological Association (AGA) has released
“Any patient suspected to have achalasia, or difficulty swallowing for that matter, should undergo a comprehensive diagnostic workup, and that should include clinical history, review of medication, as well as tests. The diagnosis should not be based on isolated tests but on the clinical picture as a whole,” first author Dennis Yang, MD, AGAF, with the Center for Interventional Endoscopy, AdventHealth, Orlando, Florida, noted in an AGA podcast about the update.
The clinical practice update, published in Gastroenterology, includes 12 “best practice advice” statements.
Since its introduction to clinical practice more than a decade ago, POEM has matured and gained widespread acceptance because of its efficacy and safety profile.
POEM has at least similar outcomes to laparoscopic Heller myotomy and pneumatic dilation for type I and type II achalasia with better results for those with type III achalasia, Yang noted.
“However, besides disease phenotype, we need to remember that choosing the right treatment for the patient is going to be based on multiple factors including patient characteristics as well as local expertise,” Yang added.
In terms of technical considerations, the update states that both anterior and posterior tunnel approaches demonstrate comparable success and postprocedure reflux rates. Tunnel orientation should be tailored to the patient’s surgical history and endoscopist’s preference.
It further states that optimal length of the myotomy in the esophagus and cardia, as it pertains to treatment efficacy and risk for postprocedure reflux, remains to be determined.
Adjunct techniques, including real-time intraprocedure functional luminal impedance planimetry, may be considered to tailor or confirm the adequacy of the myotomy.
Same-day discharge after POEM can be considered in select patients who meet discharge criteria. Patients with advanced age, significant comorbidities, poor social support, and/or access to specialized care should be considered for hospital admission, irrespective of symptoms.
The update notes that specific guidelines on the role and extent of antibiotic prophylaxis before and after POEM are lacking. A single dose of antibiotics at the time of POEM “may be sufficient” for antibiotic prophylaxis.
In terms of immediate post-POEM care, the update notes that the clinical impact of routine esophagram or endoscopy immediately post-POEM remains unclear. Testing can be considered based on local practice preferences and in cases in which intraprocedural events or postprocedural findings warrant further evaluation.
Proton pump inhibitors are recommended immediately following POEM, as gastroesophageal reflux disease (GERD) is common following POEM, occurring in up to 65% of cases.
Routine endoscopic surveillance is advised to monitor GERD, disease progression, and esophageal cancer risk, which is significantly higher in achalasia patients.
“Just like diabetes and hypertension, we need to remember that achalasia is a chronic disease and long-term postprocedural surveillance is strongly encouraged to monitor disease progression as well as potential complications of reflux,” Yang said.
He noted that surveillance should be considered irrespective of patient symptoms because many of these patients may remain asymptomatic.
“Primary gastroenterologists should have a very low threshold in referring the patient back to the POEM endoscopist or any specialized esophageal center because the ideology of symptoms in these patients can be quite difficult to tease out and often require comprehensive diagnostic workup,” Yang said.
Evidence for POEM in esophagogastric outflow obstruction and other nonachalasia spastic motility disorders is limited and should only be considered on a case-by-case basis after other less invasive approaches have been exhausted, the update states.
For perspective on the POEM clinical practice update, this news organization spoke with Mouen Khashab, MD, director of therapeutic endoscopy, Johns Hopkins University, Baltimore, Maryland.
“The document is very well written and comprehensive,” Khashab said.
However, Khashab said he would have liked to see greater emphasis on the value or role of a short myotomy in the esophagus and cardia.
“There is level I evidence that the short esophageal myotomy is equivalent to a long esophageal myotomy for type I and II achalasia. When you do a short myotomy, you save procedure time and there is potentially a lower incidence of blown-out myotomy or BOM,” Khashab said.
Khashab also noted that a long myotomy on the gastric side “likely increases the risk of reflux disease, and therefore a limited myotomy on the gastric side likely also is advantageous.”
This research had no commercial funding. Yang serves as a consultant for Boston Scientific, Olympus, FujiFilm, Microtech, Medtronic, 3D-Matrix, and Neptune Medical, and has received research support from Microtech and 3D-Matrix. Khashab had no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM GASTROENTEROLOGY
Journal Highlights: October-December 2024
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus
Reed CC et al. Daily or Twice Daily Treatment with Topical Steroids Results in Similar Responses in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Nov. doi: 10.1016/j.cgh.2024.10.016.
Patel RV et al. Functional Lumen Imaging Probe Provides an Accurate Assessment of Esophageal Diameter in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.032.
Stomach
Shah SC et al. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.001.
IBD
Griffiths BJ et al. Hypercoagulation after Hospital Discharge in Acute Severe Ulcerative Colitis: A Prospective Study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.031.
Liver
Lassailly G et al. Resolution of MASH with no worsening of fibrosis after bariatric surgery improves 15-year survival: a prospective cohort study. Clin Gastroenterol Hepatol. 2024 Dec. doi: 10.1016/j.cgh.2024.10.025.
Norman JS et al. Model for Urgency for Liver Transplantation in Hepatocellular Carcinoma: A Practical Model to Prioritize Patients With Hepatocellular Carcinoma on the Liver Transplant Waiting List. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.11.015.
Davis JPE et al. AGA Clinical Practice Update on Management of Portal Vein Thrombosis in Patients With Cirrhosis: Expert Review. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.10.038.
Pancreas
Drewes AM et al. Pain in Chronic Pancreatitis: Navigating the Maze of Blocked Tubes and Tangled Wires. Gastroenterology. 2024 Dec. doi: 10.1053/j.gastro.2024.11.026.
Endoscopy
Kindel TL et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity; Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.10.003.
Schmidt KA et al. Understanding Patients’ Current Acceptability of Artificial Intelligence During Colonoscopy for Polyp Detection: A Single-Center Study. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250905.
Chandramouli S et al. Endoscopic Surveillance Patterns and Management of Helicobacter pylori in Newly Diagnosed Gastric Intestinal Metaplasia. Techniques and Innovations in Gastrointestinal Endoscopy. 2024 Dec. doi: 10.1016/j.tige.2024.250904.
Practice Management
Tsai C et al. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct. doi: 10.1016/j.cgh.2024.09.015.
Mintz KM et al. Incorporating a GI Dietitian into Your GI Practice. Gastroenterology. 2024 Nov. doi: 10.1053/j.gastro.2024.10.022.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
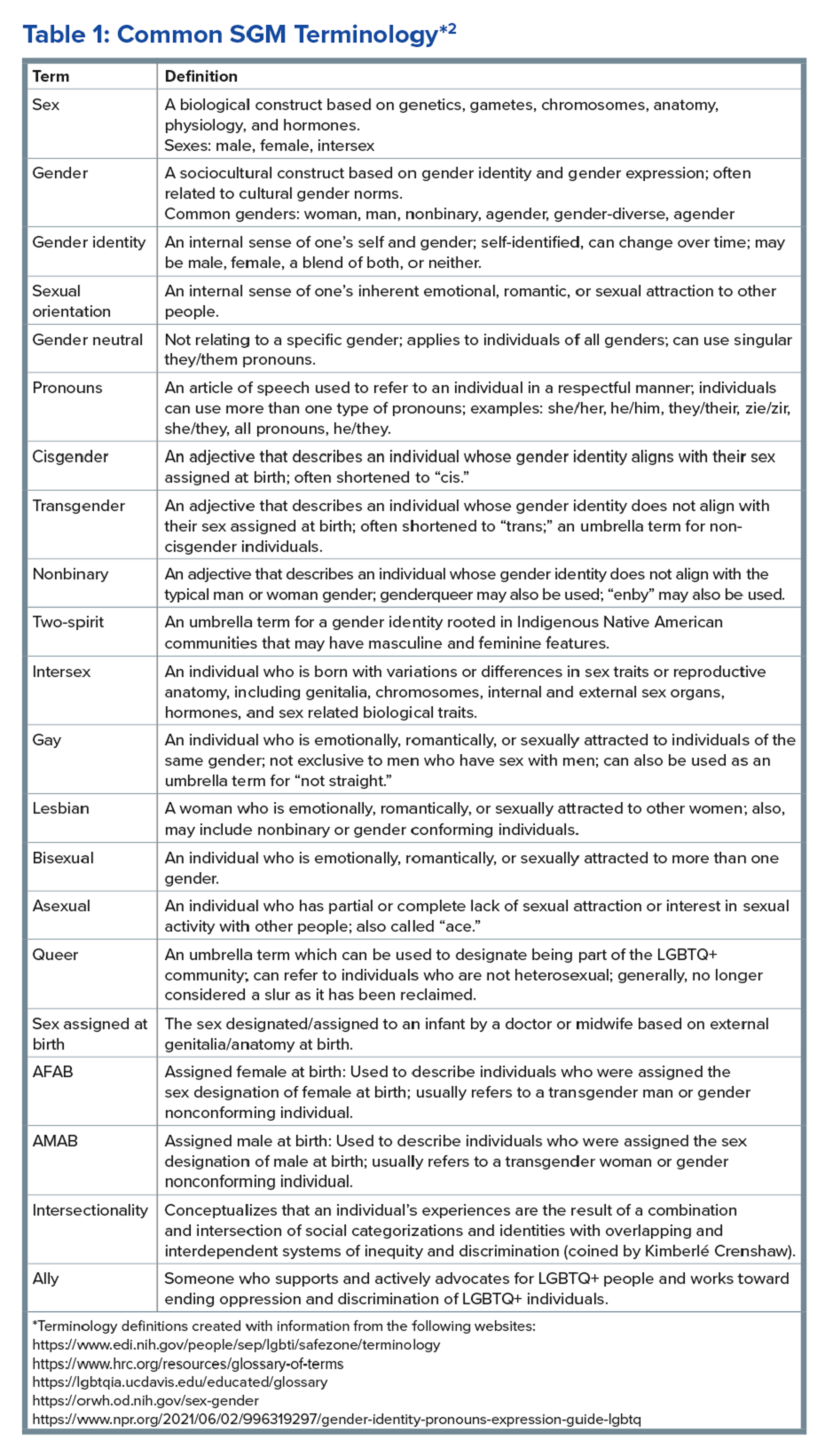
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
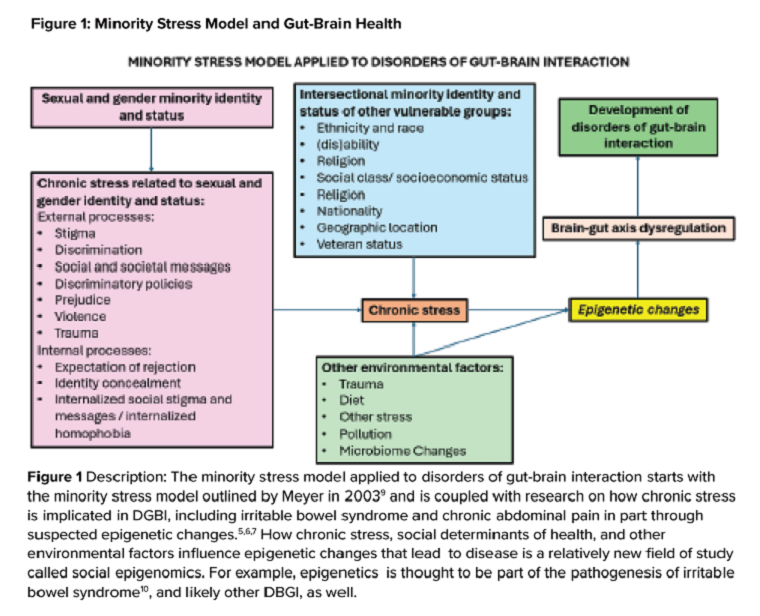
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Immunotherapy Reduces Skin Cancer Precursors
TOPLINE:
Immune checkpoint inhibitors (ICIs) show promise for field cancerization, based on their ability to reduce actinic keratoses (AKs) in a new study.
METHODOLOGY:
- This prospective cohort study included 23 immunocompetent participants (26.1% women; mean age, 69.7 years) from Australia who received ICIs for any cancer between April 2022 and November 2023.
- The most frequently prescribed ICI regimen was a combination of nivolumab and ipilimumab (34.8%), followed by nivolumab monotherapy (26.1%) and cemiplimab (21.7%) or pembrolizumab (17.4%) monotherapy.
- More than half of the patients received ICI therapy for skin cancer (melanoma, 30.4%; cutaneous squamous cell carcinoma, 26.1%); 34.8% had lung cancer; two had other carcinomas.
- The primary outcome was the number of AKs at 12 months after starting ICI therapy; the secondary outcome was the number of keratinocyte carcinomas (KCs) excised 12 months before and after ICI therapy.
TAKEAWAY:
- At 12 months, one patient had complete resolution from AK, and the mean number of AKs significantly decreased from 47.2 at baseline to 14.3 (P < .001).
- Younger patients (66.7% vs 33.3%; P = .007) and those with a history of blistering sunburn (100% vs 0; P = .005) were more likely to experience ≥ 65% reduction in AK count.
- KC incidence in the year before ICI therapy vs the year after initiation dropped from 42 to 17 cases, respectively, and the number of cutaneous squamous cell carcinomas decreased from 16 to 5.
- Adverse events occurred in 11 participants (47.8%), with maculopapular rash or pruritus the most common.
IN PRACTICE:
“This pilot cohort study highlights the potential association of ICI therapy, originally used in cancer treatment, with significant reduction of clinical AKs,” the authors wrote. These findings, they said, “underscore ICIs’ potential as a novel approach to mitigating field cancerization in high-risk populations.”
SOURCE:
Charlotte Cox, MD, MPhil, MPHTM, BMSt, University of Queensland, Brisbane, Australia, led the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations included interrater reliability issues in AK counting. Not all patients completed the follow-up period, and observations about changes after stopping ICI therapy were limited. Surveillance bias could be present in KC reporting.
DISCLOSURES:
This work was supported by grants from the Metro South Health SERTA project and by the French Society of Dermatology, La Ligue Contre le Cancer, the Collège des Enseignants en Dermatologie de France, and the European Association of Dermatology and Venereology. Cox received personal fees from the University of Queensland scholarship funds during this work. Some authors reported receiving personal fees and support from pharmaceutical and cosmetic companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Immune checkpoint inhibitors (ICIs) show promise for field cancerization, based on their ability to reduce actinic keratoses (AKs) in a new study.
METHODOLOGY:
- This prospective cohort study included 23 immunocompetent participants (26.1% women; mean age, 69.7 years) from Australia who received ICIs for any cancer between April 2022 and November 2023.
- The most frequently prescribed ICI regimen was a combination of nivolumab and ipilimumab (34.8%), followed by nivolumab monotherapy (26.1%) and cemiplimab (21.7%) or pembrolizumab (17.4%) monotherapy.
- More than half of the patients received ICI therapy for skin cancer (melanoma, 30.4%; cutaneous squamous cell carcinoma, 26.1%); 34.8% had lung cancer; two had other carcinomas.
- The primary outcome was the number of AKs at 12 months after starting ICI therapy; the secondary outcome was the number of keratinocyte carcinomas (KCs) excised 12 months before and after ICI therapy.
TAKEAWAY:
- At 12 months, one patient had complete resolution from AK, and the mean number of AKs significantly decreased from 47.2 at baseline to 14.3 (P < .001).
- Younger patients (66.7% vs 33.3%; P = .007) and those with a history of blistering sunburn (100% vs 0; P = .005) were more likely to experience ≥ 65% reduction in AK count.
- KC incidence in the year before ICI therapy vs the year after initiation dropped from 42 to 17 cases, respectively, and the number of cutaneous squamous cell carcinomas decreased from 16 to 5.
- Adverse events occurred in 11 participants (47.8%), with maculopapular rash or pruritus the most common.
IN PRACTICE:
“This pilot cohort study highlights the potential association of ICI therapy, originally used in cancer treatment, with significant reduction of clinical AKs,” the authors wrote. These findings, they said, “underscore ICIs’ potential as a novel approach to mitigating field cancerization in high-risk populations.”
SOURCE:
Charlotte Cox, MD, MPhil, MPHTM, BMSt, University of Queensland, Brisbane, Australia, led the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations included interrater reliability issues in AK counting. Not all patients completed the follow-up period, and observations about changes after stopping ICI therapy were limited. Surveillance bias could be present in KC reporting.
DISCLOSURES:
This work was supported by grants from the Metro South Health SERTA project and by the French Society of Dermatology, La Ligue Contre le Cancer, the Collège des Enseignants en Dermatologie de France, and the European Association of Dermatology and Venereology. Cox received personal fees from the University of Queensland scholarship funds during this work. Some authors reported receiving personal fees and support from pharmaceutical and cosmetic companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Immune checkpoint inhibitors (ICIs) show promise for field cancerization, based on their ability to reduce actinic keratoses (AKs) in a new study.
METHODOLOGY:
- This prospective cohort study included 23 immunocompetent participants (26.1% women; mean age, 69.7 years) from Australia who received ICIs for any cancer between April 2022 and November 2023.
- The most frequently prescribed ICI regimen was a combination of nivolumab and ipilimumab (34.8%), followed by nivolumab monotherapy (26.1%) and cemiplimab (21.7%) or pembrolizumab (17.4%) monotherapy.
- More than half of the patients received ICI therapy for skin cancer (melanoma, 30.4%; cutaneous squamous cell carcinoma, 26.1%); 34.8% had lung cancer; two had other carcinomas.
- The primary outcome was the number of AKs at 12 months after starting ICI therapy; the secondary outcome was the number of keratinocyte carcinomas (KCs) excised 12 months before and after ICI therapy.
TAKEAWAY:
- At 12 months, one patient had complete resolution from AK, and the mean number of AKs significantly decreased from 47.2 at baseline to 14.3 (P < .001).
- Younger patients (66.7% vs 33.3%; P = .007) and those with a history of blistering sunburn (100% vs 0; P = .005) were more likely to experience ≥ 65% reduction in AK count.
- KC incidence in the year before ICI therapy vs the year after initiation dropped from 42 to 17 cases, respectively, and the number of cutaneous squamous cell carcinomas decreased from 16 to 5.
- Adverse events occurred in 11 participants (47.8%), with maculopapular rash or pruritus the most common.
IN PRACTICE:
“This pilot cohort study highlights the potential association of ICI therapy, originally used in cancer treatment, with significant reduction of clinical AKs,” the authors wrote. These findings, they said, “underscore ICIs’ potential as a novel approach to mitigating field cancerization in high-risk populations.”
SOURCE:
Charlotte Cox, MD, MPhil, MPHTM, BMSt, University of Queensland, Brisbane, Australia, led the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations included interrater reliability issues in AK counting. Not all patients completed the follow-up period, and observations about changes after stopping ICI therapy were limited. Surveillance bias could be present in KC reporting.
DISCLOSURES:
This work was supported by grants from the Metro South Health SERTA project and by the French Society of Dermatology, La Ligue Contre le Cancer, the Collège des Enseignants en Dermatologie de France, and the European Association of Dermatology and Venereology. Cox received personal fees from the University of Queensland scholarship funds during this work. Some authors reported receiving personal fees and support from pharmaceutical and cosmetic companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Promise for CAR T-Cell Therapies in Solid Tumors?
Chimeric antigen receptor (CAR) T-cell therapy has shown efficacy in blood cancers — with six CAR T-cell products now approved by the Food and Drug Administration (FDA) to treat six hematologic malignancies.
For solid tumors, however, the efficacy of CAR T-cell treatments has been limited and progress “more incremental,” Christian Hinrichs, MD, with Rutgers Cancer Institute in New Brunswick, New Jersey, told this news organization. Currently, there are no CAR T-cell therapies approved in the United States to treat solid tumors.
Why have CAR T-cell therapies been less effective against solid tumors?
Perhaps the biggest hurdle is the ability to identify and selectively target specific molecular structures in cancer cells without causing severe toxicity by injuring healthy cells, Hinrichs and coauthors wrote in a recent JAMA review.
CAR T-cells are made up of “T cells genetically engineered to express a synthetic receptor that recognizes a tumor cell-surface protein,” Hinrichs and colleagues explained. But identifying cell surface antigens that are exclusive to solid tumor cells has been a challenge, which means CAR T-cell therapies end up affecting both tumor and healthy tissues.
“This makes it difficult to target and kill all the tumor cells without causing severe toxicity from injury to healthy cells,” Hinrichs explained.
Other common obstacles include challenges penetrating the dense extracellular matrix of solid tumors and the need to overcome inhibitory cells and molecules in the tumor microenvironment.
Despite the challenges and slow progress, some “promising results” have begun to emerge in the solid tumor, CAR T-cell space, Hinrichs said.
A recent phase 1-2 study, for instance, found that 63% (17 of 27) of pediatric patients with heavily pretreated neuroblastoma achieved an overall response with an investigational CAR T-cell therapy, GD2-CART01.
In a recent phase 1 trial, 38 of 98 patients with gastrointestinal cancers (39%) achieved partial or complete responses after receiving an investigational CAR T-cell treatment directed at Claudin18.2. However, the responses were short overall and could have been related to the chemotherapy given before the CAR T-cell infusion.
Another phase 1 trial found that a GPC3-targeted CAR T-cell therapy led to an objective response rate in half (12 of 24) of heavily treated patients with advanced hepatocellular carcinoma, with a disease control rate of almost 91%.
Outside of CAR-T cell therapies, other cell-based treatments have shown promise against solid tumors, including two T-cell therapies recently approved by the FDA.
Last February, the FDA approved the tumor-infiltrating lymphocyte (TIL) therapy lifileucel (Amtagvi) for advanced melanoma. In August, the agency approved the T-cell receptor (TCR) therapy afamitresgene autoleucel for advanced synovial sarcoma.
“Response rates for these cellular therapies are in the 30% range, but already there is clear data that there’s durability for some patients, which is very exciting because previously treated patients really have very few treatment options,” Jennifer Brudno, MD, with the National Cancer Institute and coauthor of the JAMA review, said in a journal podcast.
Several cell-based agents are in early trials to treat a range of solid tumors.
Hinrichs and colleagues previously reported findings from a phase 2 clinical trial of TIL therapy for human papillomavirus (HPV) — associated cancers including cervical, oropharyngeal, and anal cancers. Responses occurred in 5 of 18 patients with cervical cancer and 2 of 11 patients with noncervical cancers. “Two of the patients with cervical cancer had complete responses that are ongoing years after a single infusion of cells,” Hinrichs told this news organization.
Hinrichs was also involved in a phase 1 trial of gene-engineered TCR T-cells targeting HPV E7 for HPV-associated cancers reported tumor responses in 6 of 12 patients, including 4 of 8 with tumors refractory to checkpoint blockade immunotherapy. A phase 2 trial is now open at Rutgers Cancer Institute, as is an early trial testing a new TCR T-cell therapy targeting Kita-Kyushu Lung Cancer Antigen-1 to treat metastatic gastric, lung, breast, and cervical cancers.
Despite the encouraging findings, for CAR T-cell and other cell-based therapies to be successful against solid tumors, “we need to develop more treatments directed against antigens that are expressed by most or all the cells in a tumor but not by critical healthy tissues,” Hinrichs said.
“It may also be important to increase the potency of therapeutic cells and develop more sophisticated methods of antigen targeting that can better distinguish between tumors and healthy tissues,” he noted.
Brudno reported being an unpaid scientific advisory board member for and receiving travel expenses from Kyverna Therapeutics. Hinrichs reported receiving personal fees from Neogene Therapeutics, Capstan Therapeutics, GlaxoSmithKline, Vir Biotechnology, and PACT Pharma; equity from Scarlet TCR (company officer); and sponsored research agreements from T-Cure Biosciences and Neogene Therapeutics outside the submitted work. He also holds several patents related to cellular therapies.
A version of this article first appeared on Medscape.com.
Chimeric antigen receptor (CAR) T-cell therapy has shown efficacy in blood cancers — with six CAR T-cell products now approved by the Food and Drug Administration (FDA) to treat six hematologic malignancies.
For solid tumors, however, the efficacy of CAR T-cell treatments has been limited and progress “more incremental,” Christian Hinrichs, MD, with Rutgers Cancer Institute in New Brunswick, New Jersey, told this news organization. Currently, there are no CAR T-cell therapies approved in the United States to treat solid tumors.
Why have CAR T-cell therapies been less effective against solid tumors?
Perhaps the biggest hurdle is the ability to identify and selectively target specific molecular structures in cancer cells without causing severe toxicity by injuring healthy cells, Hinrichs and coauthors wrote in a recent JAMA review.
CAR T-cells are made up of “T cells genetically engineered to express a synthetic receptor that recognizes a tumor cell-surface protein,” Hinrichs and colleagues explained. But identifying cell surface antigens that are exclusive to solid tumor cells has been a challenge, which means CAR T-cell therapies end up affecting both tumor and healthy tissues.
“This makes it difficult to target and kill all the tumor cells without causing severe toxicity from injury to healthy cells,” Hinrichs explained.
Other common obstacles include challenges penetrating the dense extracellular matrix of solid tumors and the need to overcome inhibitory cells and molecules in the tumor microenvironment.
Despite the challenges and slow progress, some “promising results” have begun to emerge in the solid tumor, CAR T-cell space, Hinrichs said.
A recent phase 1-2 study, for instance, found that 63% (17 of 27) of pediatric patients with heavily pretreated neuroblastoma achieved an overall response with an investigational CAR T-cell therapy, GD2-CART01.
In a recent phase 1 trial, 38 of 98 patients with gastrointestinal cancers (39%) achieved partial or complete responses after receiving an investigational CAR T-cell treatment directed at Claudin18.2. However, the responses were short overall and could have been related to the chemotherapy given before the CAR T-cell infusion.
Another phase 1 trial found that a GPC3-targeted CAR T-cell therapy led to an objective response rate in half (12 of 24) of heavily treated patients with advanced hepatocellular carcinoma, with a disease control rate of almost 91%.
Outside of CAR-T cell therapies, other cell-based treatments have shown promise against solid tumors, including two T-cell therapies recently approved by the FDA.
Last February, the FDA approved the tumor-infiltrating lymphocyte (TIL) therapy lifileucel (Amtagvi) for advanced melanoma. In August, the agency approved the T-cell receptor (TCR) therapy afamitresgene autoleucel for advanced synovial sarcoma.
“Response rates for these cellular therapies are in the 30% range, but already there is clear data that there’s durability for some patients, which is very exciting because previously treated patients really have very few treatment options,” Jennifer Brudno, MD, with the National Cancer Institute and coauthor of the JAMA review, said in a journal podcast.
Several cell-based agents are in early trials to treat a range of solid tumors.
Hinrichs and colleagues previously reported findings from a phase 2 clinical trial of TIL therapy for human papillomavirus (HPV) — associated cancers including cervical, oropharyngeal, and anal cancers. Responses occurred in 5 of 18 patients with cervical cancer and 2 of 11 patients with noncervical cancers. “Two of the patients with cervical cancer had complete responses that are ongoing years after a single infusion of cells,” Hinrichs told this news organization.
Hinrichs was also involved in a phase 1 trial of gene-engineered TCR T-cells targeting HPV E7 for HPV-associated cancers reported tumor responses in 6 of 12 patients, including 4 of 8 with tumors refractory to checkpoint blockade immunotherapy. A phase 2 trial is now open at Rutgers Cancer Institute, as is an early trial testing a new TCR T-cell therapy targeting Kita-Kyushu Lung Cancer Antigen-1 to treat metastatic gastric, lung, breast, and cervical cancers.
Despite the encouraging findings, for CAR T-cell and other cell-based therapies to be successful against solid tumors, “we need to develop more treatments directed against antigens that are expressed by most or all the cells in a tumor but not by critical healthy tissues,” Hinrichs said.
“It may also be important to increase the potency of therapeutic cells and develop more sophisticated methods of antigen targeting that can better distinguish between tumors and healthy tissues,” he noted.
Brudno reported being an unpaid scientific advisory board member for and receiving travel expenses from Kyverna Therapeutics. Hinrichs reported receiving personal fees from Neogene Therapeutics, Capstan Therapeutics, GlaxoSmithKline, Vir Biotechnology, and PACT Pharma; equity from Scarlet TCR (company officer); and sponsored research agreements from T-Cure Biosciences and Neogene Therapeutics outside the submitted work. He also holds several patents related to cellular therapies.
A version of this article first appeared on Medscape.com.
Chimeric antigen receptor (CAR) T-cell therapy has shown efficacy in blood cancers — with six CAR T-cell products now approved by the Food and Drug Administration (FDA) to treat six hematologic malignancies.
For solid tumors, however, the efficacy of CAR T-cell treatments has been limited and progress “more incremental,” Christian Hinrichs, MD, with Rutgers Cancer Institute in New Brunswick, New Jersey, told this news organization. Currently, there are no CAR T-cell therapies approved in the United States to treat solid tumors.
Why have CAR T-cell therapies been less effective against solid tumors?
Perhaps the biggest hurdle is the ability to identify and selectively target specific molecular structures in cancer cells without causing severe toxicity by injuring healthy cells, Hinrichs and coauthors wrote in a recent JAMA review.
CAR T-cells are made up of “T cells genetically engineered to express a synthetic receptor that recognizes a tumor cell-surface protein,” Hinrichs and colleagues explained. But identifying cell surface antigens that are exclusive to solid tumor cells has been a challenge, which means CAR T-cell therapies end up affecting both tumor and healthy tissues.
“This makes it difficult to target and kill all the tumor cells without causing severe toxicity from injury to healthy cells,” Hinrichs explained.
Other common obstacles include challenges penetrating the dense extracellular matrix of solid tumors and the need to overcome inhibitory cells and molecules in the tumor microenvironment.
Despite the challenges and slow progress, some “promising results” have begun to emerge in the solid tumor, CAR T-cell space, Hinrichs said.
A recent phase 1-2 study, for instance, found that 63% (17 of 27) of pediatric patients with heavily pretreated neuroblastoma achieved an overall response with an investigational CAR T-cell therapy, GD2-CART01.
In a recent phase 1 trial, 38 of 98 patients with gastrointestinal cancers (39%) achieved partial or complete responses after receiving an investigational CAR T-cell treatment directed at Claudin18.2. However, the responses were short overall and could have been related to the chemotherapy given before the CAR T-cell infusion.
Another phase 1 trial found that a GPC3-targeted CAR T-cell therapy led to an objective response rate in half (12 of 24) of heavily treated patients with advanced hepatocellular carcinoma, with a disease control rate of almost 91%.
Outside of CAR-T cell therapies, other cell-based treatments have shown promise against solid tumors, including two T-cell therapies recently approved by the FDA.
Last February, the FDA approved the tumor-infiltrating lymphocyte (TIL) therapy lifileucel (Amtagvi) for advanced melanoma. In August, the agency approved the T-cell receptor (TCR) therapy afamitresgene autoleucel for advanced synovial sarcoma.
“Response rates for these cellular therapies are in the 30% range, but already there is clear data that there’s durability for some patients, which is very exciting because previously treated patients really have very few treatment options,” Jennifer Brudno, MD, with the National Cancer Institute and coauthor of the JAMA review, said in a journal podcast.
Several cell-based agents are in early trials to treat a range of solid tumors.
Hinrichs and colleagues previously reported findings from a phase 2 clinical trial of TIL therapy for human papillomavirus (HPV) — associated cancers including cervical, oropharyngeal, and anal cancers. Responses occurred in 5 of 18 patients with cervical cancer and 2 of 11 patients with noncervical cancers. “Two of the patients with cervical cancer had complete responses that are ongoing years after a single infusion of cells,” Hinrichs told this news organization.
Hinrichs was also involved in a phase 1 trial of gene-engineered TCR T-cells targeting HPV E7 for HPV-associated cancers reported tumor responses in 6 of 12 patients, including 4 of 8 with tumors refractory to checkpoint blockade immunotherapy. A phase 2 trial is now open at Rutgers Cancer Institute, as is an early trial testing a new TCR T-cell therapy targeting Kita-Kyushu Lung Cancer Antigen-1 to treat metastatic gastric, lung, breast, and cervical cancers.
Despite the encouraging findings, for CAR T-cell and other cell-based therapies to be successful against solid tumors, “we need to develop more treatments directed against antigens that are expressed by most or all the cells in a tumor but not by critical healthy tissues,” Hinrichs said.
“It may also be important to increase the potency of therapeutic cells and develop more sophisticated methods of antigen targeting that can better distinguish between tumors and healthy tissues,” he noted.
Brudno reported being an unpaid scientific advisory board member for and receiving travel expenses from Kyverna Therapeutics. Hinrichs reported receiving personal fees from Neogene Therapeutics, Capstan Therapeutics, GlaxoSmithKline, Vir Biotechnology, and PACT Pharma; equity from Scarlet TCR (company officer); and sponsored research agreements from T-Cure Biosciences and Neogene Therapeutics outside the submitted work. He also holds several patents related to cellular therapies.
A version of this article first appeared on Medscape.com.





