User login
Dementia Deemed Highly Preventable: Here’s How
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
One in Ten Chronic Pain Patients May Develop Opioid Use Disorder
TOPLINE:
Nearly 10% of patients with chronic pain treated with opioids develop opioid use disorder, whereas 30% show signs and symptoms of dependence, highlighting the need for monitoring and alternative pain management strategies.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis using MEDLINE, Embase, and PsycINFO databases from inception to January 27, 2021.
- The studies analyzed were predominantly from the United States (n = 115) as well as high-income countries such as the United Kingdom (n = 5), France (n = 3), Spain (n = 4), Germany (n = 4), and Australia (n = 2).
- A total of 148 studies from various settings with over 4.3 million participants were included, focusing on patients aged ≥ 12 years with chronic non-cancer pain of ≥ 3 months duration, treated with opioid analgesics.
- Problematic opioid use was categorized into four categories: dependence and opioid use disorder, signs and symptoms of dependence and opioid use disorder, aberrant behavior, and at risk for dependence and opioid use disorder.
TAKEAWAY:
- The pooled prevalence of dependence and opioid use disorder was 9.3% (95% CI, 5.7%-14.8%), with significant heterogeneity across studies.
- Signs and symptoms of dependence were observed in 29.6% (95% CI, 22.1%-38.3%) of patients, indicating a high prevalence of problematic opioid use.
- Aberrant behavior was reported in 22% (95% CI, 17.4%-27.3%) of patients, highlighting the need for careful monitoring and intervention.
- The prevalence of patients at risk of developing dependence was 12.4% (95% CI, 4.3%-30.7%), suggesting the importance of early identification and prevention strategies.
IN PRACTICE:
“Clinicians and policymakers need a more accurate estimate of the prevalence of problematic opioid use in pain patients so that they can gauge the true extent of the problem, change prescribing guidance if necessary, and develop and implement effective interventions to manage the problem,” Kyla H. Thomas, PhD, the lead author, noted in a press release. Knowing the size of the problem is a necessary step to managing it, she added.
SOURCE:
The study was led by Dr. Thomas, Population Health Sciences, Bristol Medical School, University of Bristol in England. It was published online, in Addiction.
LIMITATIONS:
The study’s high heterogeneity across included studies suggests caution in interpreting the findings. The reliance on self-reported data and varying definitions of problematic opioid use may affect the accuracy of prevalence estimates. Most studies were conducted in high-income countries, limiting the generalizability to other settings.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR). Dr. Thomas reported receiving financial support from the NIHR for this study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly 10% of patients with chronic pain treated with opioids develop opioid use disorder, whereas 30% show signs and symptoms of dependence, highlighting the need for monitoring and alternative pain management strategies.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis using MEDLINE, Embase, and PsycINFO databases from inception to January 27, 2021.
- The studies analyzed were predominantly from the United States (n = 115) as well as high-income countries such as the United Kingdom (n = 5), France (n = 3), Spain (n = 4), Germany (n = 4), and Australia (n = 2).
- A total of 148 studies from various settings with over 4.3 million participants were included, focusing on patients aged ≥ 12 years with chronic non-cancer pain of ≥ 3 months duration, treated with opioid analgesics.
- Problematic opioid use was categorized into four categories: dependence and opioid use disorder, signs and symptoms of dependence and opioid use disorder, aberrant behavior, and at risk for dependence and opioid use disorder.
TAKEAWAY:
- The pooled prevalence of dependence and opioid use disorder was 9.3% (95% CI, 5.7%-14.8%), with significant heterogeneity across studies.
- Signs and symptoms of dependence were observed in 29.6% (95% CI, 22.1%-38.3%) of patients, indicating a high prevalence of problematic opioid use.
- Aberrant behavior was reported in 22% (95% CI, 17.4%-27.3%) of patients, highlighting the need for careful monitoring and intervention.
- The prevalence of patients at risk of developing dependence was 12.4% (95% CI, 4.3%-30.7%), suggesting the importance of early identification and prevention strategies.
IN PRACTICE:
“Clinicians and policymakers need a more accurate estimate of the prevalence of problematic opioid use in pain patients so that they can gauge the true extent of the problem, change prescribing guidance if necessary, and develop and implement effective interventions to manage the problem,” Kyla H. Thomas, PhD, the lead author, noted in a press release. Knowing the size of the problem is a necessary step to managing it, she added.
SOURCE:
The study was led by Dr. Thomas, Population Health Sciences, Bristol Medical School, University of Bristol in England. It was published online, in Addiction.
LIMITATIONS:
The study’s high heterogeneity across included studies suggests caution in interpreting the findings. The reliance on self-reported data and varying definitions of problematic opioid use may affect the accuracy of prevalence estimates. Most studies were conducted in high-income countries, limiting the generalizability to other settings.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR). Dr. Thomas reported receiving financial support from the NIHR for this study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly 10% of patients with chronic pain treated with opioids develop opioid use disorder, whereas 30% show signs and symptoms of dependence, highlighting the need for monitoring and alternative pain management strategies.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis using MEDLINE, Embase, and PsycINFO databases from inception to January 27, 2021.
- The studies analyzed were predominantly from the United States (n = 115) as well as high-income countries such as the United Kingdom (n = 5), France (n = 3), Spain (n = 4), Germany (n = 4), and Australia (n = 2).
- A total of 148 studies from various settings with over 4.3 million participants were included, focusing on patients aged ≥ 12 years with chronic non-cancer pain of ≥ 3 months duration, treated with opioid analgesics.
- Problematic opioid use was categorized into four categories: dependence and opioid use disorder, signs and symptoms of dependence and opioid use disorder, aberrant behavior, and at risk for dependence and opioid use disorder.
TAKEAWAY:
- The pooled prevalence of dependence and opioid use disorder was 9.3% (95% CI, 5.7%-14.8%), with significant heterogeneity across studies.
- Signs and symptoms of dependence were observed in 29.6% (95% CI, 22.1%-38.3%) of patients, indicating a high prevalence of problematic opioid use.
- Aberrant behavior was reported in 22% (95% CI, 17.4%-27.3%) of patients, highlighting the need for careful monitoring and intervention.
- The prevalence of patients at risk of developing dependence was 12.4% (95% CI, 4.3%-30.7%), suggesting the importance of early identification and prevention strategies.
IN PRACTICE:
“Clinicians and policymakers need a more accurate estimate of the prevalence of problematic opioid use in pain patients so that they can gauge the true extent of the problem, change prescribing guidance if necessary, and develop and implement effective interventions to manage the problem,” Kyla H. Thomas, PhD, the lead author, noted in a press release. Knowing the size of the problem is a necessary step to managing it, she added.
SOURCE:
The study was led by Dr. Thomas, Population Health Sciences, Bristol Medical School, University of Bristol in England. It was published online, in Addiction.
LIMITATIONS:
The study’s high heterogeneity across included studies suggests caution in interpreting the findings. The reliance on self-reported data and varying definitions of problematic opioid use may affect the accuracy of prevalence estimates. Most studies were conducted in high-income countries, limiting the generalizability to other settings.
DISCLOSURES:
The study was funded by the National Institute for Health and Care Research (NIHR). Dr. Thomas reported receiving financial support from the NIHR for this study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FIT Screening Cuts Colorectal Cancer Mortality by One Third
TOPLINE:
METHODOLOGY:
- In the United States, annual FIT screening is recommended among average-risk adults to reduce the risk for death from CRC, but evidence on its effectiveness is limited.
- Researchers performed a nested case-control study within two large, demographically diverse health systems with long-standing programs of mailing FITs to promote CRC screening efforts.
- They compared 1103 adults who had died of CRC between 2011 and 2017 (cases) with 9608 matched, randomly selected people who were alive and free of CRC (controls).
- Analyses focused on FIT screening completed within 5 years before CRC diagnosis for cases or the corresponding date for controls.
- The primary outcome measured was CRC death overall and by tumor location; secondary analyses assessed CRC death by race and ethnicity.
TAKEAWAY:
- In regression analysis, completing one or more FIT screenings was associated with a 33% lower risk for CRC death overall.
- There was a 42% lower risk for death from left colon and rectum cancers but no significant reduction in mortality from right colon cancers.
- The benefits of FIT screening were observed across racial and ethnic groups, with significant mortality reductions of 63% in non-Hispanic Asian, 42% in non-Hispanic Black, and 29% in non-Hispanic White individuals.
IN PRACTICE:
“The findings support the use of strategies for coordinated and equitable large-scale population-based delivery of FIT screening with follow-up of abnormal screening results to help avert preventable premature CRC deaths,” the authors wrote.
SOURCE:
The study, with first author Chyke A. Doubeni, MD, MPH, Center for Health Equity, The Ohio State University Wexner Medical Center, Columbus, Ohio, was published online in JAMA Network Open.
LIMITATIONS:
Almost one half of study subjects had completed two or more FITs, but the case-control design was not suitable for assessing the impact of repeated screening. The study was conducted prior to the US Preventive Services Task Force recommendation to start screening at age 45 years, so the findings may not directly apply to adults aged 45-49 years.
DISCLOSURES:
The study was funded by the National Cancer Institute. Dr. Doubeni reported receiving royalties from UpToDate, and additional authors reported receiving grants outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the United States, annual FIT screening is recommended among average-risk adults to reduce the risk for death from CRC, but evidence on its effectiveness is limited.
- Researchers performed a nested case-control study within two large, demographically diverse health systems with long-standing programs of mailing FITs to promote CRC screening efforts.
- They compared 1103 adults who had died of CRC between 2011 and 2017 (cases) with 9608 matched, randomly selected people who were alive and free of CRC (controls).
- Analyses focused on FIT screening completed within 5 years before CRC diagnosis for cases or the corresponding date for controls.
- The primary outcome measured was CRC death overall and by tumor location; secondary analyses assessed CRC death by race and ethnicity.
TAKEAWAY:
- In regression analysis, completing one or more FIT screenings was associated with a 33% lower risk for CRC death overall.
- There was a 42% lower risk for death from left colon and rectum cancers but no significant reduction in mortality from right colon cancers.
- The benefits of FIT screening were observed across racial and ethnic groups, with significant mortality reductions of 63% in non-Hispanic Asian, 42% in non-Hispanic Black, and 29% in non-Hispanic White individuals.
IN PRACTICE:
“The findings support the use of strategies for coordinated and equitable large-scale population-based delivery of FIT screening with follow-up of abnormal screening results to help avert preventable premature CRC deaths,” the authors wrote.
SOURCE:
The study, with first author Chyke A. Doubeni, MD, MPH, Center for Health Equity, The Ohio State University Wexner Medical Center, Columbus, Ohio, was published online in JAMA Network Open.
LIMITATIONS:
Almost one half of study subjects had completed two or more FITs, but the case-control design was not suitable for assessing the impact of repeated screening. The study was conducted prior to the US Preventive Services Task Force recommendation to start screening at age 45 years, so the findings may not directly apply to adults aged 45-49 years.
DISCLOSURES:
The study was funded by the National Cancer Institute. Dr. Doubeni reported receiving royalties from UpToDate, and additional authors reported receiving grants outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the United States, annual FIT screening is recommended among average-risk adults to reduce the risk for death from CRC, but evidence on its effectiveness is limited.
- Researchers performed a nested case-control study within two large, demographically diverse health systems with long-standing programs of mailing FITs to promote CRC screening efforts.
- They compared 1103 adults who had died of CRC between 2011 and 2017 (cases) with 9608 matched, randomly selected people who were alive and free of CRC (controls).
- Analyses focused on FIT screening completed within 5 years before CRC diagnosis for cases or the corresponding date for controls.
- The primary outcome measured was CRC death overall and by tumor location; secondary analyses assessed CRC death by race and ethnicity.
TAKEAWAY:
- In regression analysis, completing one or more FIT screenings was associated with a 33% lower risk for CRC death overall.
- There was a 42% lower risk for death from left colon and rectum cancers but no significant reduction in mortality from right colon cancers.
- The benefits of FIT screening were observed across racial and ethnic groups, with significant mortality reductions of 63% in non-Hispanic Asian, 42% in non-Hispanic Black, and 29% in non-Hispanic White individuals.
IN PRACTICE:
“The findings support the use of strategies for coordinated and equitable large-scale population-based delivery of FIT screening with follow-up of abnormal screening results to help avert preventable premature CRC deaths,” the authors wrote.
SOURCE:
The study, with first author Chyke A. Doubeni, MD, MPH, Center for Health Equity, The Ohio State University Wexner Medical Center, Columbus, Ohio, was published online in JAMA Network Open.
LIMITATIONS:
Almost one half of study subjects had completed two or more FITs, but the case-control design was not suitable for assessing the impact of repeated screening. The study was conducted prior to the US Preventive Services Task Force recommendation to start screening at age 45 years, so the findings may not directly apply to adults aged 45-49 years.
DISCLOSURES:
The study was funded by the National Cancer Institute. Dr. Doubeni reported receiving royalties from UpToDate, and additional authors reported receiving grants outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Colorectal Cancer: New Primary Care Method Predicts Onset Within Next 2 Years
TOPLINE:
Up to 16% of primary care patients are non-compliant with FIT, which is the gold standard for predicting CRC.
METHODOLOGY:
- This study was retrospective cohort of 50,387 UK Biobank participants reporting a CRC symptom in primary care at age ≥ 40 years.
- The novel method, called an integrated risk model, used a combination of a polygenic risk score from genetic testing, symptoms, and patient characteristics to identify patients likely to develop CRC in the next 2 years.
- The primary outcome was the risk model’s performance in classifying a CRC case according to a statistical metric, the receiver operating characteristic area under the curve. Values range from 0 to 1, where 1 indicates perfect discriminative power and 0.5 indicates no discriminative power.
TAKEAWAY:
- The cohort of 50,387 participants was found to have 438 cases of CRC and 49,949 controls without CRC within 2 years of symptom reporting. CRC cases were diagnosed by hospital records, cancer registries, or death records.
- Increased risk of a CRC diagnosis was identified by a combination of six variables: older age at index date of symptom, higher polygenic risk score, which included 201 variants, male sex, previous smoking, rectal bleeding, and change in bowel habit.
- The polygenic risk score alone had good ability to distinguish cases from controls because 1.45% of participants in the highest quintile and 0.42% in the lowest quintile were later diagnosed with CRC.
- The variables were used to calculate an integrated risk model, which estimated the cross-sectional risk (in 80% of the final cohort) of a subsequent CRC diagnosis within 2 years. The highest scoring integrated risk model in this study was found to have a receiver operating characteristic area under the curve value of 0.76 with a 95% CI of 0.71-0.81. (A value of this magnitude indicates moderate discriminative ability to distinguish cases from controls because it falls between 0.7 and 0.8. A higher value [above 0.8] is considered strong and a lower value [< 0.7] is considered weak.)
IN PRACTICE:
The authors concluded, “The [integrated risk model] developed in this study predicts, with good accuracy, which patients presenting with CRC symptoms in a primary care setting are likely to be diagnosed with CRC within the next 2 years.”
The integrated risk model has “potential to be immediately actionable in the clinical setting … [by] inform[ing] patient triage, improving early diagnostic rates and health outcomes and reducing pressure on diagnostic secondary care services.”
SOURCE:
The corresponding author is Harry D. Green of the University of Exeter, England. The study (2024 Aug 1. doi: 10.1038/s41431-024-01654-3) appeared in the European Journal of Human Genetics.
LIMITATIONS:
Limitations included an observational design and the inability of the integrated risk model to outperform FIT, which has a receiver operating characteristic area under the curve of 0.95.
DISCLOSURES:
None of the authors reported competing interests. The funding sources included the National Institute for Health and Care Research and others.
A version of this article first appeared on Medscape.com.
TOPLINE:
Up to 16% of primary care patients are non-compliant with FIT, which is the gold standard for predicting CRC.
METHODOLOGY:
- This study was retrospective cohort of 50,387 UK Biobank participants reporting a CRC symptom in primary care at age ≥ 40 years.
- The novel method, called an integrated risk model, used a combination of a polygenic risk score from genetic testing, symptoms, and patient characteristics to identify patients likely to develop CRC in the next 2 years.
- The primary outcome was the risk model’s performance in classifying a CRC case according to a statistical metric, the receiver operating characteristic area under the curve. Values range from 0 to 1, where 1 indicates perfect discriminative power and 0.5 indicates no discriminative power.
TAKEAWAY:
- The cohort of 50,387 participants was found to have 438 cases of CRC and 49,949 controls without CRC within 2 years of symptom reporting. CRC cases were diagnosed by hospital records, cancer registries, or death records.
- Increased risk of a CRC diagnosis was identified by a combination of six variables: older age at index date of symptom, higher polygenic risk score, which included 201 variants, male sex, previous smoking, rectal bleeding, and change in bowel habit.
- The polygenic risk score alone had good ability to distinguish cases from controls because 1.45% of participants in the highest quintile and 0.42% in the lowest quintile were later diagnosed with CRC.
- The variables were used to calculate an integrated risk model, which estimated the cross-sectional risk (in 80% of the final cohort) of a subsequent CRC diagnosis within 2 years. The highest scoring integrated risk model in this study was found to have a receiver operating characteristic area under the curve value of 0.76 with a 95% CI of 0.71-0.81. (A value of this magnitude indicates moderate discriminative ability to distinguish cases from controls because it falls between 0.7 and 0.8. A higher value [above 0.8] is considered strong and a lower value [< 0.7] is considered weak.)
IN PRACTICE:
The authors concluded, “The [integrated risk model] developed in this study predicts, with good accuracy, which patients presenting with CRC symptoms in a primary care setting are likely to be diagnosed with CRC within the next 2 years.”
The integrated risk model has “potential to be immediately actionable in the clinical setting … [by] inform[ing] patient triage, improving early diagnostic rates and health outcomes and reducing pressure on diagnostic secondary care services.”
SOURCE:
The corresponding author is Harry D. Green of the University of Exeter, England. The study (2024 Aug 1. doi: 10.1038/s41431-024-01654-3) appeared in the European Journal of Human Genetics.
LIMITATIONS:
Limitations included an observational design and the inability of the integrated risk model to outperform FIT, which has a receiver operating characteristic area under the curve of 0.95.
DISCLOSURES:
None of the authors reported competing interests. The funding sources included the National Institute for Health and Care Research and others.
A version of this article first appeared on Medscape.com.
TOPLINE:
Up to 16% of primary care patients are non-compliant with FIT, which is the gold standard for predicting CRC.
METHODOLOGY:
- This study was retrospective cohort of 50,387 UK Biobank participants reporting a CRC symptom in primary care at age ≥ 40 years.
- The novel method, called an integrated risk model, used a combination of a polygenic risk score from genetic testing, symptoms, and patient characteristics to identify patients likely to develop CRC in the next 2 years.
- The primary outcome was the risk model’s performance in classifying a CRC case according to a statistical metric, the receiver operating characteristic area under the curve. Values range from 0 to 1, where 1 indicates perfect discriminative power and 0.5 indicates no discriminative power.
TAKEAWAY:
- The cohort of 50,387 participants was found to have 438 cases of CRC and 49,949 controls without CRC within 2 years of symptom reporting. CRC cases were diagnosed by hospital records, cancer registries, or death records.
- Increased risk of a CRC diagnosis was identified by a combination of six variables: older age at index date of symptom, higher polygenic risk score, which included 201 variants, male sex, previous smoking, rectal bleeding, and change in bowel habit.
- The polygenic risk score alone had good ability to distinguish cases from controls because 1.45% of participants in the highest quintile and 0.42% in the lowest quintile were later diagnosed with CRC.
- The variables were used to calculate an integrated risk model, which estimated the cross-sectional risk (in 80% of the final cohort) of a subsequent CRC diagnosis within 2 years. The highest scoring integrated risk model in this study was found to have a receiver operating characteristic area under the curve value of 0.76 with a 95% CI of 0.71-0.81. (A value of this magnitude indicates moderate discriminative ability to distinguish cases from controls because it falls between 0.7 and 0.8. A higher value [above 0.8] is considered strong and a lower value [< 0.7] is considered weak.)
IN PRACTICE:
The authors concluded, “The [integrated risk model] developed in this study predicts, with good accuracy, which patients presenting with CRC symptoms in a primary care setting are likely to be diagnosed with CRC within the next 2 years.”
The integrated risk model has “potential to be immediately actionable in the clinical setting … [by] inform[ing] patient triage, improving early diagnostic rates and health outcomes and reducing pressure on diagnostic secondary care services.”
SOURCE:
The corresponding author is Harry D. Green of the University of Exeter, England. The study (2024 Aug 1. doi: 10.1038/s41431-024-01654-3) appeared in the European Journal of Human Genetics.
LIMITATIONS:
Limitations included an observational design and the inability of the integrated risk model to outperform FIT, which has a receiver operating characteristic area under the curve of 0.95.
DISCLOSURES:
None of the authors reported competing interests. The funding sources included the National Institute for Health and Care Research and others.
A version of this article first appeared on Medscape.com.
Stool-Based Methylation Test May Improve CRC Screening
based on a prospective, real-world study.
These findings suggest that the mSDC2 assay could improve the efficacy and resource utilization of existing screening programs, reported co–lead authors Shengbing Zhao, MD and Zixuan He, MD, of Naval Medical University, Shanghai, China, and colleagues.
“Conventional risk-stratification strategies, such as fecal immunochemical test (FIT) and life risk factors, are still criticized for being inferior at identifying early-stage CRC and ACN, and their real-world performance is probably further weakened by the low annual participation rate and compliance of subsequent colonoscopy,” the investigators wrote in Gastroenterology. Recent case studies have reported “high diagnostic performance” using stool-based testing for mSDC2, which is “the most accurate single-targeted gene” for colorectal neoplasia, according to the investigators; however, real-world outcomes have yet to be demonstrated, prompting the present study. The prospective, multicenter, community-based trial compared the diagnostic performance of the mSDC2 test against FIT and Asia-Pacific Colorectal Screening (APCS) scores.
The primary outcome was detection of ACN. Secondary outcomes included detection of CRC, early-stage CRC, ACN, colorectal neoplasia (CN), and clinically relevant serrated polyp (CRSP). Screening strategies were also compared in terms of cost-effectiveness and impact on colonoscopy workload.The final dataset included 10,360 participants aged 45-75 years who underwent screening between 2020 and 2022.
After determining APCS scores, stool samples were analyzed for mSDC2 and FIT markers. Based on risk stratification results, participants were invited to undergo colonoscopy. A total of 3,381 participants completed colonoscopy, with 1914 from the increased-risk population and 1467 from the average-risk population. Participants who tested positive for mSDC2 had significantly higher detection rates for all measured outcomes than those who tested negative (all, P < .05). For example, the detection rate for ACN was 26.6% in mSDC2-positive participants, compared with 9.3% in mSDC2-negative participants, with a relative risk of 2.87 (95% CI, 2.39-3.44). For CRC, the detection rate was 4.2% in mSDC2-positive participants vs 0.1% in mSDC2-negative participants, yielding a relative risk of 29.73 (95% CI, 10.29-85.91). Performance held steady across subgroups.The mSDC2 test demonstrated cost-effectiveness by significantly reducing the number of colonoscopies needed to detect one case of ACN or CRC. Specifically, the number of colonoscopies needed to screen for ACN and CRC was reduced by 56.2% and 81.5%, respectively. Parallel combinations of mSDC2 with APCS or FIT enhanced both diagnostic performance and cost-effectiveness.
“This study further illustrates that the mSDC2 test consistently improves predictive abilities for CN, CRSP, ACN, and CRC, which is not influenced by subgroups of lesion location or risk factors, even under the risk stratification by FIT or APCS,” the investigators wrote. “The excellent diagnostic ability of mSDC2 in premalignant lesions, early-stage CRC, and early-onset CRC indicates a promising value in early detection and prevention of CRC ... the mSDC2 test or a parallel combination of multiple screening methods might be promising to improve real-world CRC screening performance and reduce colonoscopy workload in community practice.”The study was supported by the National Key Research and Development Program of China, Deep Blue Project of Naval Medical University, the Creative Biosciences, and others. The investigators reported no conflicts of interest.
based on a prospective, real-world study.
These findings suggest that the mSDC2 assay could improve the efficacy and resource utilization of existing screening programs, reported co–lead authors Shengbing Zhao, MD and Zixuan He, MD, of Naval Medical University, Shanghai, China, and colleagues.
“Conventional risk-stratification strategies, such as fecal immunochemical test (FIT) and life risk factors, are still criticized for being inferior at identifying early-stage CRC and ACN, and their real-world performance is probably further weakened by the low annual participation rate and compliance of subsequent colonoscopy,” the investigators wrote in Gastroenterology. Recent case studies have reported “high diagnostic performance” using stool-based testing for mSDC2, which is “the most accurate single-targeted gene” for colorectal neoplasia, according to the investigators; however, real-world outcomes have yet to be demonstrated, prompting the present study. The prospective, multicenter, community-based trial compared the diagnostic performance of the mSDC2 test against FIT and Asia-Pacific Colorectal Screening (APCS) scores.
The primary outcome was detection of ACN. Secondary outcomes included detection of CRC, early-stage CRC, ACN, colorectal neoplasia (CN), and clinically relevant serrated polyp (CRSP). Screening strategies were also compared in terms of cost-effectiveness and impact on colonoscopy workload.The final dataset included 10,360 participants aged 45-75 years who underwent screening between 2020 and 2022.
After determining APCS scores, stool samples were analyzed for mSDC2 and FIT markers. Based on risk stratification results, participants were invited to undergo colonoscopy. A total of 3,381 participants completed colonoscopy, with 1914 from the increased-risk population and 1467 from the average-risk population. Participants who tested positive for mSDC2 had significantly higher detection rates for all measured outcomes than those who tested negative (all, P < .05). For example, the detection rate for ACN was 26.6% in mSDC2-positive participants, compared with 9.3% in mSDC2-negative participants, with a relative risk of 2.87 (95% CI, 2.39-3.44). For CRC, the detection rate was 4.2% in mSDC2-positive participants vs 0.1% in mSDC2-negative participants, yielding a relative risk of 29.73 (95% CI, 10.29-85.91). Performance held steady across subgroups.The mSDC2 test demonstrated cost-effectiveness by significantly reducing the number of colonoscopies needed to detect one case of ACN or CRC. Specifically, the number of colonoscopies needed to screen for ACN and CRC was reduced by 56.2% and 81.5%, respectively. Parallel combinations of mSDC2 with APCS or FIT enhanced both diagnostic performance and cost-effectiveness.
“This study further illustrates that the mSDC2 test consistently improves predictive abilities for CN, CRSP, ACN, and CRC, which is not influenced by subgroups of lesion location or risk factors, even under the risk stratification by FIT or APCS,” the investigators wrote. “The excellent diagnostic ability of mSDC2 in premalignant lesions, early-stage CRC, and early-onset CRC indicates a promising value in early detection and prevention of CRC ... the mSDC2 test or a parallel combination of multiple screening methods might be promising to improve real-world CRC screening performance and reduce colonoscopy workload in community practice.”The study was supported by the National Key Research and Development Program of China, Deep Blue Project of Naval Medical University, the Creative Biosciences, and others. The investigators reported no conflicts of interest.
based on a prospective, real-world study.
These findings suggest that the mSDC2 assay could improve the efficacy and resource utilization of existing screening programs, reported co–lead authors Shengbing Zhao, MD and Zixuan He, MD, of Naval Medical University, Shanghai, China, and colleagues.
“Conventional risk-stratification strategies, such as fecal immunochemical test (FIT) and life risk factors, are still criticized for being inferior at identifying early-stage CRC and ACN, and their real-world performance is probably further weakened by the low annual participation rate and compliance of subsequent colonoscopy,” the investigators wrote in Gastroenterology. Recent case studies have reported “high diagnostic performance” using stool-based testing for mSDC2, which is “the most accurate single-targeted gene” for colorectal neoplasia, according to the investigators; however, real-world outcomes have yet to be demonstrated, prompting the present study. The prospective, multicenter, community-based trial compared the diagnostic performance of the mSDC2 test against FIT and Asia-Pacific Colorectal Screening (APCS) scores.
The primary outcome was detection of ACN. Secondary outcomes included detection of CRC, early-stage CRC, ACN, colorectal neoplasia (CN), and clinically relevant serrated polyp (CRSP). Screening strategies were also compared in terms of cost-effectiveness and impact on colonoscopy workload.The final dataset included 10,360 participants aged 45-75 years who underwent screening between 2020 and 2022.
After determining APCS scores, stool samples were analyzed for mSDC2 and FIT markers. Based on risk stratification results, participants were invited to undergo colonoscopy. A total of 3,381 participants completed colonoscopy, with 1914 from the increased-risk population and 1467 from the average-risk population. Participants who tested positive for mSDC2 had significantly higher detection rates for all measured outcomes than those who tested negative (all, P < .05). For example, the detection rate for ACN was 26.6% in mSDC2-positive participants, compared with 9.3% in mSDC2-negative participants, with a relative risk of 2.87 (95% CI, 2.39-3.44). For CRC, the detection rate was 4.2% in mSDC2-positive participants vs 0.1% in mSDC2-negative participants, yielding a relative risk of 29.73 (95% CI, 10.29-85.91). Performance held steady across subgroups.The mSDC2 test demonstrated cost-effectiveness by significantly reducing the number of colonoscopies needed to detect one case of ACN or CRC. Specifically, the number of colonoscopies needed to screen for ACN and CRC was reduced by 56.2% and 81.5%, respectively. Parallel combinations of mSDC2 with APCS or FIT enhanced both diagnostic performance and cost-effectiveness.
“This study further illustrates that the mSDC2 test consistently improves predictive abilities for CN, CRSP, ACN, and CRC, which is not influenced by subgroups of lesion location or risk factors, even under the risk stratification by FIT or APCS,” the investigators wrote. “The excellent diagnostic ability of mSDC2 in premalignant lesions, early-stage CRC, and early-onset CRC indicates a promising value in early detection and prevention of CRC ... the mSDC2 test or a parallel combination of multiple screening methods might be promising to improve real-world CRC screening performance and reduce colonoscopy workload in community practice.”The study was supported by the National Key Research and Development Program of China, Deep Blue Project of Naval Medical University, the Creative Biosciences, and others. The investigators reported no conflicts of interest.
FROM GASTROENTEROLOGY
These Four Factors Account for 18 Years of Life Expectancy
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
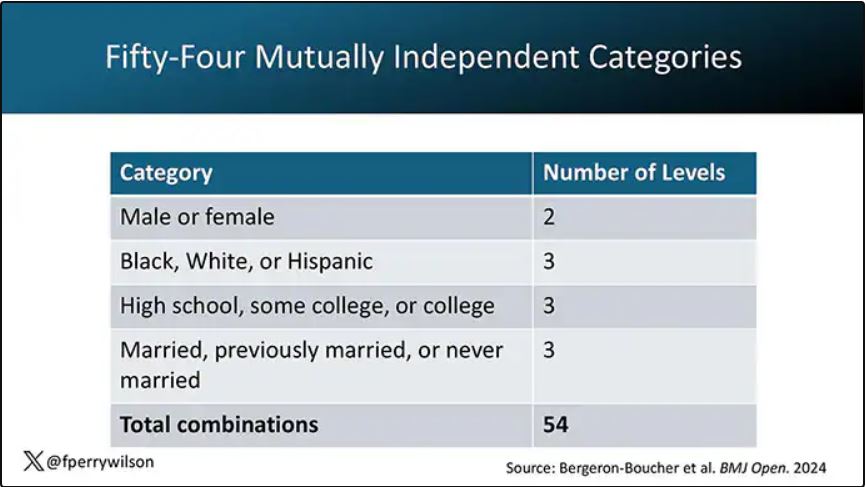
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
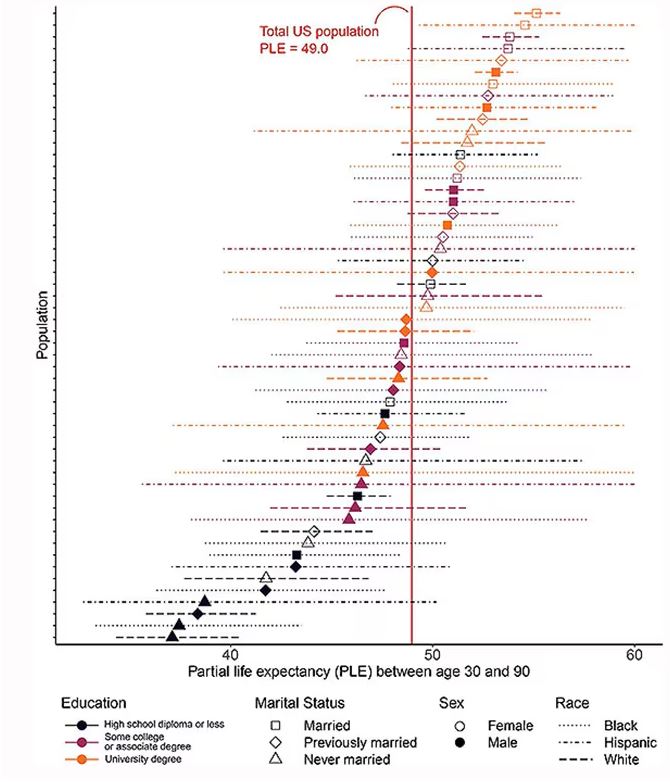
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
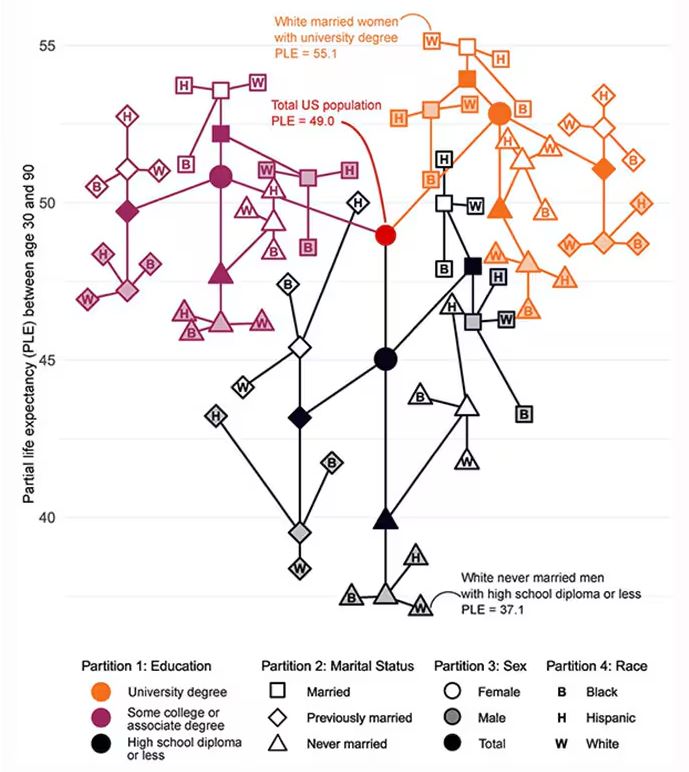
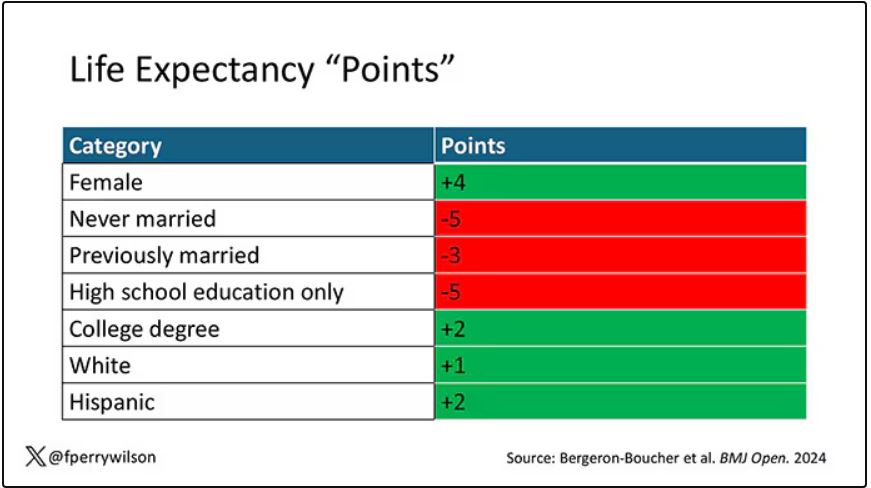
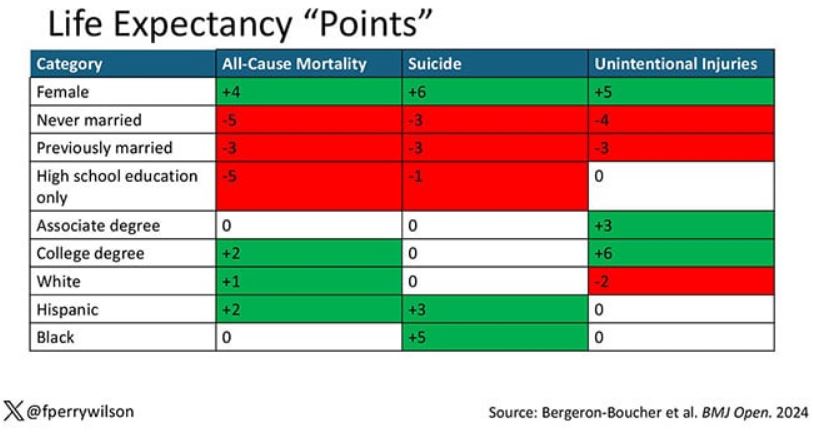
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
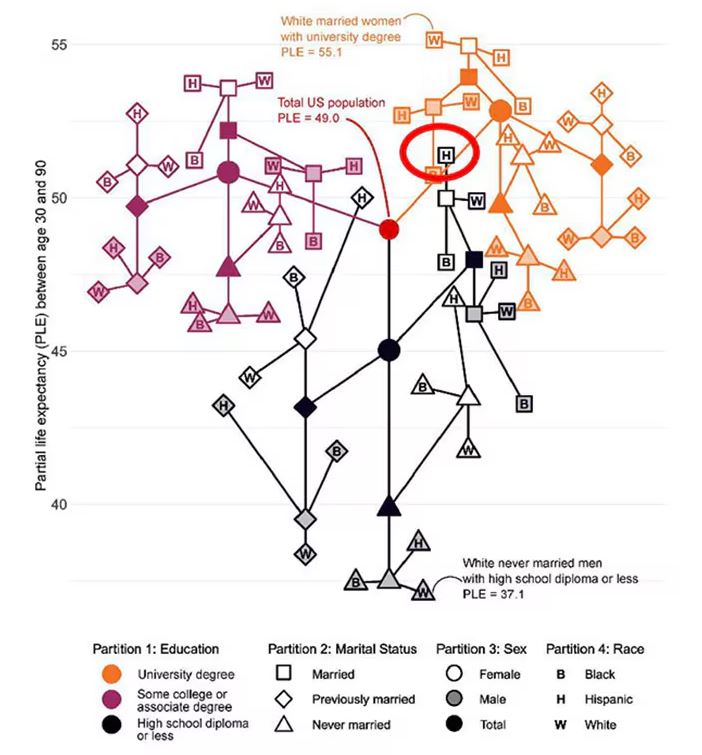
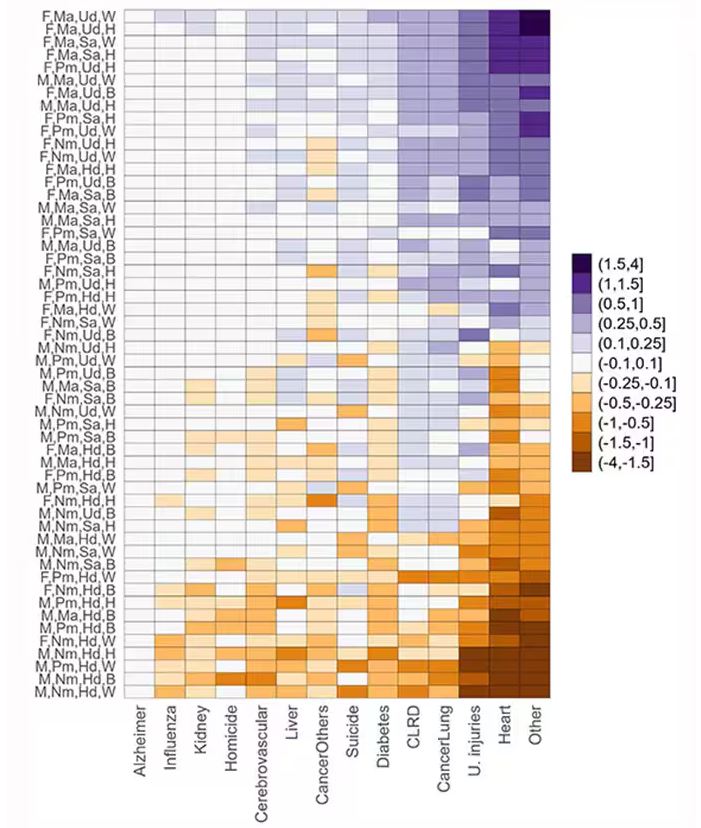
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis Overuse Linked to Increased Risk for Head and Neck Cancer
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Light Therapy, Phototherapy, Photobiomodulation: New Ways to Heal With Light
A surprising therapy is showing promise for chronic pain, vision loss, and muscle recovery, among other conditions.
It’s not a pill, an injection, or surgery.
It’s light.
Yes, light. The thing that appears when you open the curtains, flip a switch, or strike a match.
Light illuminates our world and helps us see. Early human trials suggest it may help us heal in new ways as well.
“Phototherapy is still in its infancy,” said Mohab Ibrahim, MD, PhD, a professor of anesthesiology at the University of Arizona, Tucson, who studies the effects of light on chronic pain. “There are so many questions, a lot of things we do not understand yet. But that’s where it gets interesting. What we can conclude is that different colors of light can influence different biological functions.”
This growing field goes by several names. Light therapy. Phototherapy. Photobiomodulation.
It leverages known effects of light on human health — such as skin exposure to ultraviolet light producing vitamin D or blue light’s power to regulate human body clocks — to take light as medicine in surprising new directions.
New Science, Old Idea
The science is young, but the concept of using light to restore health is thousands of years old.
Hippocrates prescribed sunbathing to patients at his medical center on the Greek island of Kos in 400 BC. Florence Nightingale promoted sunshine, along with fresh air, as prerequisites for recovery in hospitals during the Civil War. A Danish doctor, Niels Finsen, won the Nobel Prize in 1903 for developing ultraviolet lamps to treat a tuberculosis-related skin condition. And worried parents of the 1930s sat their babies in front of mercury arc lamps, bought at the drugstore, to discourage rickets.
Today, light therapy is widely used in medicine for newborn jaundice, psoriasis, and seasonal affective disorder and in light-activated treatments for cancers of the esophagus and lungs, as well as for actinic keratosis, a skin condition that can lead to cancer.
But researchers are finding that light may be capable of far more, particularly in conditions with few treatment options or where available drugs have unwanted side effects.
How Red Light Could Restore Vision
When 100 midlife and older adults, aged 53-91, with the dry form of age-related macular degeneration (AMD) were treated with an experimental red-light therapy or a sham therapy, the light treatment group showed signs of improved vision, as measured on a standard eye chart.
Volunteers received the therapy three times a week for 3-5 weeks, every 4 months for 2 years. By the study’s end, 67% of those treated with light could read an additional five letters on the chart, and 20% could read 10 or more. About 7% developed geographic atrophy — the most advanced, vision-threatening stage of dry AMD — compared with 24% in the sham group.
The study, called LIGHTSITE III, was conducted at 10 ophthalmology centers across the United States. The device they used — the Valeda Light Delivery System from medical device company LumiThera — is available in Europe and now being reviewed by the Food and Drug Administration (FDA).
Exposure to red light at the wavelengths used in the study likely revitalizes failing mitochondria — the power plants inside cells — so they produce more energy, the researchers say.
“This is the first therapy for dry AMD that’s actually shown a benefit in improving vision,” said study coauthor Richard Rosen, MD, chair of ophthalmology at the Icahn School of Medicine at Mount Sinai and chief of Retinal Services at the New York Eye and Ear Infirmary in New York City. “Supplements called AREDS can reduce progression, and in wet AMD we can improve vision loss with injections. But in dry AMD, none of the treatments studied in the past have improved it.”
AMD develops when the eyes can’t break down natural by-products, which glom together as clumps of protein called drusen. Drusen can lodge under the retina, eventually damaging tissue.
“Retinal epithelial cells, a single layer of cells that cares for the photoreceptors in the eyes, are there for life,” Dr. Rosen said. “They have a tremendous capacity to repair themselves, but things [such as aging and smoking] get in the way.”
“I’m proposing,” Dr. Rosen said, “that by boosting energy levels in cells [with red light], we’re improving normal repair mechanisms.”
Lab studies support this idea.
In a 2017 mouse study from the University College London Institute of Ophthalmology in England, retinal function improved by 25% in old mice exposed to red light. And a 2019 study from the Ophthalmological Research Foundation, Oviedo, Spain, found that exposure to blue light harmed the mitochondria in retina cells, while red light somewhat counteracted the losses.
If cleared by the FDA — which the company anticipated could happen in 2024 — LumiThera’s light delivery device will likely be most useful in the beginning stages of dry AMD, Dr. Rosen said. “I think treatment of early dry AMD will be huge.”
Eventually, light therapy may also be valuable in treating or managing glaucoma and diabetic retinopathy.
For now, Dr. Rosen recommended that clinicians and consumers with AMD skip over-the-counter (OTC) red-light therapy devices currently on the market.
“We don’t know what kind of light the devices produce,” he said. “The wavelengths can vary. The eyes are delicate. Experimenting on your own may be hazardous to your vision.”
Green Light for Pain Relief
On his way to the pharmacy to pick up pain relievers for a headache, Dr. Ibrahim passed Gene C. Reid Park in Tucson. Recalling how his brother eased headaches by sitting in his backyard, Dr. Ibrahim pulled over.
“Reid Park is probably one of the greenest areas of Tucson,” said Dr. Ibrahim, who also serves as medical director of the Comprehensive Center for Pain & Addiction at Banner-University Medical Center Phoenix in Arizona. “I spent a half hour or 40 minutes there, and my headache felt better.”
Being outdoors in a green space may be soothing for lots of reasons, like the quiet or the fresh air. But there’s also sunlight reflected off and shining through greenery. The experience inspired Dr. Ibrahim to take a closer look at the effects of green light on chronic pain.
In his 2021 study of 29 people with migraines, participants reported that, after daily exposure to green light for 10 weeks, the number of days per month when they had headaches fell from 7.9 to 2.4 for those who had episodic migraines and from 22.3 to 9.4 for those with chronic migraines. In another 2021 study, 21 people with fibromyalgia who had green light therapy for 10 weeks said their average, self-reported pain intensity fell from 8.4 to 4.9 on a 10-point scale used at the University of Arizona’s pain clinic.
Volunteers in both studies got their light therapy at home, switching on green LED lights while they listened to music, read a book, relaxed, or exercised for 1 or 2 hours daily. The lights were within their field of vision, but they did not look directly at them.
Dr. Ibrahim now has funding from the Department of Defense and Department of Veterans Affairs to find out why green light alters pain perception.
“What we know is that the visual system is connected to certain areas of the brain that also modulate pain,” he said. “We are trying to understand the connection.”
Padma Gulur, MD, a professor of anesthesiology and population health and director of Pain Management Strategy and Opioid Surveillance at Duke University, Durham, North Carolina, saw similar results in a 2023 study of 45 people with fibromyalgia. But instead of using a light source, volunteers wore glasses with clear, green, or blue lenses for 4 hours a day.
After 2 weeks, 33% in the green lens group reduced their use of opioids by 10% or more, compared with 11% in the blue lens group and 8% who wore clear lenses. Previous studies have found green light affects levels of the feel-good brain chemical serotonin and stimulates the body’s own opioid system, the authors noted.
“Green light helps your body control and reduce pain,” Dr. Gulur said. It “seems to help with pain relief by affecting the body’s natural pain management system. This effect appears to play a crucial role in antinociception — reducing the sensation of pain; antiallodynia — preventing normal, nonpainful stimuli from causing pain; and antihyperalgesia — reducing heightened sensitivity to pain.”
Light therapy could help pain patients reduce their dose of opioids or even forgo the drugs altogether, Dr. Gulur said. “It is our hope this will become a useful adjuvant therapy to manage pain.”
In the University of Arizona studies, some patients on green-light therapy stopped their medications completely. Even if they didn’t, other benefits appeared. “They had improved quality of life, decreased depression and anxiety, and improved sleep,” Dr. Ibrahim said.
But not just any green light or green-tinted glasses will work, both researchers said. “We have found there are specific frequencies of green light that give this benefit,” Dr. Gulur said. “OTC products may not be helpful for that reason.”
While Dr. Ibrahim said it could be possible for healthcare practitioners and consumers to consult his studies and put together an inexpensive green-light device at home while carefully following the protocol participants used in the studies , it would first be a good idea for patients to talk with their family doctor or a pain specialist.
“A headache is not always just a headache,” Dr. Ibrahim said. “It could be some other abnormality that needs diagnosis and treatment. If you have long-lasting pain or pain that’s getting worse, it’s always better to discuss it with your physician.”
Helping Muscles Recover With Red Light
Intense exercise — whether it’s a sprint at the end of a morning run, an extra set of biceps curls, or a weekend of all-day DIY home improvement projects — can temporarily damage muscle, causing soreness, inflammation, and even swelling. Phototherapy with red and near-infrared light is widely used by sports trainers, physical therapists, and athletes to aid in recovery. It may even work better than a trendy plunge in an ice bath, according to a 2019 Texas State University review.
But how does it work? Jamie Ghigiarelli, PhD, professor of Allied Health & Kinesiology at Hofstra University in Hempstead, New York, looked closely at signs of inflammation and muscle damage in 12 athletes to find out.
Study participants overtaxed their muscles with rounds of chin-ups, high-speed sprints, and repeated bench presses. Afterward, they relaxed in a full-body red-light therapy bed or in a similar bed without lights.
The results, published in 2020, showed that blood levels of creatine kinase — an enzyme that’s elevated by muscle damage — were 18% lower 1-3 days after exercising for the light-bed group than for the control group.
“Photobiomodulation seems to help with muscle recovery,” Dr. Ghigiarelli said.
Red light at wavelengths from 650 to 820 nm can enter muscle cells, where it is absorbed by mitochondria and boosts their energy production, he said. At the time of his research, some exercise science researchers and athletes thought using light therapy before an event might also increase athletic performance, but according to Dr. Ghigiarelli, that use has not panned out.
Handheld red light and near-infrared light devices for muscle recovery are widely available, but it’s important to do your homework before buying one.
“You want to choose a device with the right energy production — the right wavelength of light, the right power — to be safe and effective,” he said.
For details, he recommends consulting a 2019 paper in The Brazilian Journal of Physical Therapy called “Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions.”
The paper, from the Laboratory of Phototherapy and Innovative Technologies in Health at the Universidade Nove de Julho in Sao Paulo, Brazil, recommends that for small muscle groups like the biceps or triceps, use red-light lasers or LED devices with a wavelength of 640 nm for red light or 950 nm for infrared light, at a power of 50-200 mW per diode for single-probe device types, at a dose of 20-60 J, given 5-10 minutes after exercise.
A version of this article appeared on Medscape.com.
A surprising therapy is showing promise for chronic pain, vision loss, and muscle recovery, among other conditions.
It’s not a pill, an injection, or surgery.
It’s light.
Yes, light. The thing that appears when you open the curtains, flip a switch, or strike a match.
Light illuminates our world and helps us see. Early human trials suggest it may help us heal in new ways as well.
“Phototherapy is still in its infancy,” said Mohab Ibrahim, MD, PhD, a professor of anesthesiology at the University of Arizona, Tucson, who studies the effects of light on chronic pain. “There are so many questions, a lot of things we do not understand yet. But that’s where it gets interesting. What we can conclude is that different colors of light can influence different biological functions.”
This growing field goes by several names. Light therapy. Phototherapy. Photobiomodulation.
It leverages known effects of light on human health — such as skin exposure to ultraviolet light producing vitamin D or blue light’s power to regulate human body clocks — to take light as medicine in surprising new directions.
New Science, Old Idea
The science is young, but the concept of using light to restore health is thousands of years old.
Hippocrates prescribed sunbathing to patients at his medical center on the Greek island of Kos in 400 BC. Florence Nightingale promoted sunshine, along with fresh air, as prerequisites for recovery in hospitals during the Civil War. A Danish doctor, Niels Finsen, won the Nobel Prize in 1903 for developing ultraviolet lamps to treat a tuberculosis-related skin condition. And worried parents of the 1930s sat their babies in front of mercury arc lamps, bought at the drugstore, to discourage rickets.
Today, light therapy is widely used in medicine for newborn jaundice, psoriasis, and seasonal affective disorder and in light-activated treatments for cancers of the esophagus and lungs, as well as for actinic keratosis, a skin condition that can lead to cancer.
But researchers are finding that light may be capable of far more, particularly in conditions with few treatment options or where available drugs have unwanted side effects.
How Red Light Could Restore Vision
When 100 midlife and older adults, aged 53-91, with the dry form of age-related macular degeneration (AMD) were treated with an experimental red-light therapy or a sham therapy, the light treatment group showed signs of improved vision, as measured on a standard eye chart.
Volunteers received the therapy three times a week for 3-5 weeks, every 4 months for 2 years. By the study’s end, 67% of those treated with light could read an additional five letters on the chart, and 20% could read 10 or more. About 7% developed geographic atrophy — the most advanced, vision-threatening stage of dry AMD — compared with 24% in the sham group.
The study, called LIGHTSITE III, was conducted at 10 ophthalmology centers across the United States. The device they used — the Valeda Light Delivery System from medical device company LumiThera — is available in Europe and now being reviewed by the Food and Drug Administration (FDA).
Exposure to red light at the wavelengths used in the study likely revitalizes failing mitochondria — the power plants inside cells — so they produce more energy, the researchers say.
“This is the first therapy for dry AMD that’s actually shown a benefit in improving vision,” said study coauthor Richard Rosen, MD, chair of ophthalmology at the Icahn School of Medicine at Mount Sinai and chief of Retinal Services at the New York Eye and Ear Infirmary in New York City. “Supplements called AREDS can reduce progression, and in wet AMD we can improve vision loss with injections. But in dry AMD, none of the treatments studied in the past have improved it.”
AMD develops when the eyes can’t break down natural by-products, which glom together as clumps of protein called drusen. Drusen can lodge under the retina, eventually damaging tissue.
“Retinal epithelial cells, a single layer of cells that cares for the photoreceptors in the eyes, are there for life,” Dr. Rosen said. “They have a tremendous capacity to repair themselves, but things [such as aging and smoking] get in the way.”
“I’m proposing,” Dr. Rosen said, “that by boosting energy levels in cells [with red light], we’re improving normal repair mechanisms.”
Lab studies support this idea.
In a 2017 mouse study from the University College London Institute of Ophthalmology in England, retinal function improved by 25% in old mice exposed to red light. And a 2019 study from the Ophthalmological Research Foundation, Oviedo, Spain, found that exposure to blue light harmed the mitochondria in retina cells, while red light somewhat counteracted the losses.
If cleared by the FDA — which the company anticipated could happen in 2024 — LumiThera’s light delivery device will likely be most useful in the beginning stages of dry AMD, Dr. Rosen said. “I think treatment of early dry AMD will be huge.”
Eventually, light therapy may also be valuable in treating or managing glaucoma and diabetic retinopathy.
For now, Dr. Rosen recommended that clinicians and consumers with AMD skip over-the-counter (OTC) red-light therapy devices currently on the market.
“We don’t know what kind of light the devices produce,” he said. “The wavelengths can vary. The eyes are delicate. Experimenting on your own may be hazardous to your vision.”
Green Light for Pain Relief
On his way to the pharmacy to pick up pain relievers for a headache, Dr. Ibrahim passed Gene C. Reid Park in Tucson. Recalling how his brother eased headaches by sitting in his backyard, Dr. Ibrahim pulled over.
“Reid Park is probably one of the greenest areas of Tucson,” said Dr. Ibrahim, who also serves as medical director of the Comprehensive Center for Pain & Addiction at Banner-University Medical Center Phoenix in Arizona. “I spent a half hour or 40 minutes there, and my headache felt better.”
Being outdoors in a green space may be soothing for lots of reasons, like the quiet or the fresh air. But there’s also sunlight reflected off and shining through greenery. The experience inspired Dr. Ibrahim to take a closer look at the effects of green light on chronic pain.
In his 2021 study of 29 people with migraines, participants reported that, after daily exposure to green light for 10 weeks, the number of days per month when they had headaches fell from 7.9 to 2.4 for those who had episodic migraines and from 22.3 to 9.4 for those with chronic migraines. In another 2021 study, 21 people with fibromyalgia who had green light therapy for 10 weeks said their average, self-reported pain intensity fell from 8.4 to 4.9 on a 10-point scale used at the University of Arizona’s pain clinic.
Volunteers in both studies got their light therapy at home, switching on green LED lights while they listened to music, read a book, relaxed, or exercised for 1 or 2 hours daily. The lights were within their field of vision, but they did not look directly at them.
Dr. Ibrahim now has funding from the Department of Defense and Department of Veterans Affairs to find out why green light alters pain perception.
“What we know is that the visual system is connected to certain areas of the brain that also modulate pain,” he said. “We are trying to understand the connection.”
Padma Gulur, MD, a professor of anesthesiology and population health and director of Pain Management Strategy and Opioid Surveillance at Duke University, Durham, North Carolina, saw similar results in a 2023 study of 45 people with fibromyalgia. But instead of using a light source, volunteers wore glasses with clear, green, or blue lenses for 4 hours a day.
After 2 weeks, 33% in the green lens group reduced their use of opioids by 10% or more, compared with 11% in the blue lens group and 8% who wore clear lenses. Previous studies have found green light affects levels of the feel-good brain chemical serotonin and stimulates the body’s own opioid system, the authors noted.
“Green light helps your body control and reduce pain,” Dr. Gulur said. It “seems to help with pain relief by affecting the body’s natural pain management system. This effect appears to play a crucial role in antinociception — reducing the sensation of pain; antiallodynia — preventing normal, nonpainful stimuli from causing pain; and antihyperalgesia — reducing heightened sensitivity to pain.”
Light therapy could help pain patients reduce their dose of opioids or even forgo the drugs altogether, Dr. Gulur said. “It is our hope this will become a useful adjuvant therapy to manage pain.”
In the University of Arizona studies, some patients on green-light therapy stopped their medications completely. Even if they didn’t, other benefits appeared. “They had improved quality of life, decreased depression and anxiety, and improved sleep,” Dr. Ibrahim said.
But not just any green light or green-tinted glasses will work, both researchers said. “We have found there are specific frequencies of green light that give this benefit,” Dr. Gulur said. “OTC products may not be helpful for that reason.”
While Dr. Ibrahim said it could be possible for healthcare practitioners and consumers to consult his studies and put together an inexpensive green-light device at home while carefully following the protocol participants used in the studies , it would first be a good idea for patients to talk with their family doctor or a pain specialist.
“A headache is not always just a headache,” Dr. Ibrahim said. “It could be some other abnormality that needs diagnosis and treatment. If you have long-lasting pain or pain that’s getting worse, it’s always better to discuss it with your physician.”
Helping Muscles Recover With Red Light
Intense exercise — whether it’s a sprint at the end of a morning run, an extra set of biceps curls, or a weekend of all-day DIY home improvement projects — can temporarily damage muscle, causing soreness, inflammation, and even swelling. Phototherapy with red and near-infrared light is widely used by sports trainers, physical therapists, and athletes to aid in recovery. It may even work better than a trendy plunge in an ice bath, according to a 2019 Texas State University review.
But how does it work? Jamie Ghigiarelli, PhD, professor of Allied Health & Kinesiology at Hofstra University in Hempstead, New York, looked closely at signs of inflammation and muscle damage in 12 athletes to find out.
Study participants overtaxed their muscles with rounds of chin-ups, high-speed sprints, and repeated bench presses. Afterward, they relaxed in a full-body red-light therapy bed or in a similar bed without lights.
The results, published in 2020, showed that blood levels of creatine kinase — an enzyme that’s elevated by muscle damage — were 18% lower 1-3 days after exercising for the light-bed group than for the control group.
“Photobiomodulation seems to help with muscle recovery,” Dr. Ghigiarelli said.
Red light at wavelengths from 650 to 820 nm can enter muscle cells, where it is absorbed by mitochondria and boosts their energy production, he said. At the time of his research, some exercise science researchers and athletes thought using light therapy before an event might also increase athletic performance, but according to Dr. Ghigiarelli, that use has not panned out.
Handheld red light and near-infrared light devices for muscle recovery are widely available, but it’s important to do your homework before buying one.
“You want to choose a device with the right energy production — the right wavelength of light, the right power — to be safe and effective,” he said.
For details, he recommends consulting a 2019 paper in The Brazilian Journal of Physical Therapy called “Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions.”
The paper, from the Laboratory of Phototherapy and Innovative Technologies in Health at the Universidade Nove de Julho in Sao Paulo, Brazil, recommends that for small muscle groups like the biceps or triceps, use red-light lasers or LED devices with a wavelength of 640 nm for red light or 950 nm for infrared light, at a power of 50-200 mW per diode for single-probe device types, at a dose of 20-60 J, given 5-10 minutes after exercise.
A version of this article appeared on Medscape.com.
A surprising therapy is showing promise for chronic pain, vision loss, and muscle recovery, among other conditions.
It’s not a pill, an injection, or surgery.
It’s light.
Yes, light. The thing that appears when you open the curtains, flip a switch, or strike a match.
Light illuminates our world and helps us see. Early human trials suggest it may help us heal in new ways as well.
“Phototherapy is still in its infancy,” said Mohab Ibrahim, MD, PhD, a professor of anesthesiology at the University of Arizona, Tucson, who studies the effects of light on chronic pain. “There are so many questions, a lot of things we do not understand yet. But that’s where it gets interesting. What we can conclude is that different colors of light can influence different biological functions.”
This growing field goes by several names. Light therapy. Phototherapy. Photobiomodulation.
It leverages known effects of light on human health — such as skin exposure to ultraviolet light producing vitamin D or blue light’s power to regulate human body clocks — to take light as medicine in surprising new directions.
New Science, Old Idea
The science is young, but the concept of using light to restore health is thousands of years old.
Hippocrates prescribed sunbathing to patients at his medical center on the Greek island of Kos in 400 BC. Florence Nightingale promoted sunshine, along with fresh air, as prerequisites for recovery in hospitals during the Civil War. A Danish doctor, Niels Finsen, won the Nobel Prize in 1903 for developing ultraviolet lamps to treat a tuberculosis-related skin condition. And worried parents of the 1930s sat their babies in front of mercury arc lamps, bought at the drugstore, to discourage rickets.
Today, light therapy is widely used in medicine for newborn jaundice, psoriasis, and seasonal affective disorder and in light-activated treatments for cancers of the esophagus and lungs, as well as for actinic keratosis, a skin condition that can lead to cancer.
But researchers are finding that light may be capable of far more, particularly in conditions with few treatment options or where available drugs have unwanted side effects.
How Red Light Could Restore Vision
When 100 midlife and older adults, aged 53-91, with the dry form of age-related macular degeneration (AMD) were treated with an experimental red-light therapy or a sham therapy, the light treatment group showed signs of improved vision, as measured on a standard eye chart.
Volunteers received the therapy three times a week for 3-5 weeks, every 4 months for 2 years. By the study’s end, 67% of those treated with light could read an additional five letters on the chart, and 20% could read 10 or more. About 7% developed geographic atrophy — the most advanced, vision-threatening stage of dry AMD — compared with 24% in the sham group.
The study, called LIGHTSITE III, was conducted at 10 ophthalmology centers across the United States. The device they used — the Valeda Light Delivery System from medical device company LumiThera — is available in Europe and now being reviewed by the Food and Drug Administration (FDA).
Exposure to red light at the wavelengths used in the study likely revitalizes failing mitochondria — the power plants inside cells — so they produce more energy, the researchers say.
“This is the first therapy for dry AMD that’s actually shown a benefit in improving vision,” said study coauthor Richard Rosen, MD, chair of ophthalmology at the Icahn School of Medicine at Mount Sinai and chief of Retinal Services at the New York Eye and Ear Infirmary in New York City. “Supplements called AREDS can reduce progression, and in wet AMD we can improve vision loss with injections. But in dry AMD, none of the treatments studied in the past have improved it.”
AMD develops when the eyes can’t break down natural by-products, which glom together as clumps of protein called drusen. Drusen can lodge under the retina, eventually damaging tissue.
“Retinal epithelial cells, a single layer of cells that cares for the photoreceptors in the eyes, are there for life,” Dr. Rosen said. “They have a tremendous capacity to repair themselves, but things [such as aging and smoking] get in the way.”
“I’m proposing,” Dr. Rosen said, “that by boosting energy levels in cells [with red light], we’re improving normal repair mechanisms.”
Lab studies support this idea.
In a 2017 mouse study from the University College London Institute of Ophthalmology in England, retinal function improved by 25% in old mice exposed to red light. And a 2019 study from the Ophthalmological Research Foundation, Oviedo, Spain, found that exposure to blue light harmed the mitochondria in retina cells, while red light somewhat counteracted the losses.
If cleared by the FDA — which the company anticipated could happen in 2024 — LumiThera’s light delivery device will likely be most useful in the beginning stages of dry AMD, Dr. Rosen said. “I think treatment of early dry AMD will be huge.”
Eventually, light therapy may also be valuable in treating or managing glaucoma and diabetic retinopathy.
For now, Dr. Rosen recommended that clinicians and consumers with AMD skip over-the-counter (OTC) red-light therapy devices currently on the market.
“We don’t know what kind of light the devices produce,” he said. “The wavelengths can vary. The eyes are delicate. Experimenting on your own may be hazardous to your vision.”
Green Light for Pain Relief
On his way to the pharmacy to pick up pain relievers for a headache, Dr. Ibrahim passed Gene C. Reid Park in Tucson. Recalling how his brother eased headaches by sitting in his backyard, Dr. Ibrahim pulled over.
“Reid Park is probably one of the greenest areas of Tucson,” said Dr. Ibrahim, who also serves as medical director of the Comprehensive Center for Pain & Addiction at Banner-University Medical Center Phoenix in Arizona. “I spent a half hour or 40 minutes there, and my headache felt better.”
Being outdoors in a green space may be soothing for lots of reasons, like the quiet or the fresh air. But there’s also sunlight reflected off and shining through greenery. The experience inspired Dr. Ibrahim to take a closer look at the effects of green light on chronic pain.
In his 2021 study of 29 people with migraines, participants reported that, after daily exposure to green light for 10 weeks, the number of days per month when they had headaches fell from 7.9 to 2.4 for those who had episodic migraines and from 22.3 to 9.4 for those with chronic migraines. In another 2021 study, 21 people with fibromyalgia who had green light therapy for 10 weeks said their average, self-reported pain intensity fell from 8.4 to 4.9 on a 10-point scale used at the University of Arizona’s pain clinic.
Volunteers in both studies got their light therapy at home, switching on green LED lights while they listened to music, read a book, relaxed, or exercised for 1 or 2 hours daily. The lights were within their field of vision, but they did not look directly at them.
Dr. Ibrahim now has funding from the Department of Defense and Department of Veterans Affairs to find out why green light alters pain perception.
“What we know is that the visual system is connected to certain areas of the brain that also modulate pain,” he said. “We are trying to understand the connection.”
Padma Gulur, MD, a professor of anesthesiology and population health and director of Pain Management Strategy and Opioid Surveillance at Duke University, Durham, North Carolina, saw similar results in a 2023 study of 45 people with fibromyalgia. But instead of using a light source, volunteers wore glasses with clear, green, or blue lenses for 4 hours a day.
After 2 weeks, 33% in the green lens group reduced their use of opioids by 10% or more, compared with 11% in the blue lens group and 8% who wore clear lenses. Previous studies have found green light affects levels of the feel-good brain chemical serotonin and stimulates the body’s own opioid system, the authors noted.
“Green light helps your body control and reduce pain,” Dr. Gulur said. It “seems to help with pain relief by affecting the body’s natural pain management system. This effect appears to play a crucial role in antinociception — reducing the sensation of pain; antiallodynia — preventing normal, nonpainful stimuli from causing pain; and antihyperalgesia — reducing heightened sensitivity to pain.”
Light therapy could help pain patients reduce their dose of opioids or even forgo the drugs altogether, Dr. Gulur said. “It is our hope this will become a useful adjuvant therapy to manage pain.”
In the University of Arizona studies, some patients on green-light therapy stopped their medications completely. Even if they didn’t, other benefits appeared. “They had improved quality of life, decreased depression and anxiety, and improved sleep,” Dr. Ibrahim said.
But not just any green light or green-tinted glasses will work, both researchers said. “We have found there are specific frequencies of green light that give this benefit,” Dr. Gulur said. “OTC products may not be helpful for that reason.”
While Dr. Ibrahim said it could be possible for healthcare practitioners and consumers to consult his studies and put together an inexpensive green-light device at home while carefully following the protocol participants used in the studies , it would first be a good idea for patients to talk with their family doctor or a pain specialist.
“A headache is not always just a headache,” Dr. Ibrahim said. “It could be some other abnormality that needs diagnosis and treatment. If you have long-lasting pain or pain that’s getting worse, it’s always better to discuss it with your physician.”
Helping Muscles Recover With Red Light
Intense exercise — whether it’s a sprint at the end of a morning run, an extra set of biceps curls, or a weekend of all-day DIY home improvement projects — can temporarily damage muscle, causing soreness, inflammation, and even swelling. Phototherapy with red and near-infrared light is widely used by sports trainers, physical therapists, and athletes to aid in recovery. It may even work better than a trendy plunge in an ice bath, according to a 2019 Texas State University review.
But how does it work? Jamie Ghigiarelli, PhD, professor of Allied Health & Kinesiology at Hofstra University in Hempstead, New York, looked closely at signs of inflammation and muscle damage in 12 athletes to find out.
Study participants overtaxed their muscles with rounds of chin-ups, high-speed sprints, and repeated bench presses. Afterward, they relaxed in a full-body red-light therapy bed or in a similar bed without lights.
The results, published in 2020, showed that blood levels of creatine kinase — an enzyme that’s elevated by muscle damage — were 18% lower 1-3 days after exercising for the light-bed group than for the control group.
“Photobiomodulation seems to help with muscle recovery,” Dr. Ghigiarelli said.
Red light at wavelengths from 650 to 820 nm can enter muscle cells, where it is absorbed by mitochondria and boosts their energy production, he said. At the time of his research, some exercise science researchers and athletes thought using light therapy before an event might also increase athletic performance, but according to Dr. Ghigiarelli, that use has not panned out.
Handheld red light and near-infrared light devices for muscle recovery are widely available, but it’s important to do your homework before buying one.
“You want to choose a device with the right energy production — the right wavelength of light, the right power — to be safe and effective,” he said.
For details, he recommends consulting a 2019 paper in The Brazilian Journal of Physical Therapy called “Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions.”
The paper, from the Laboratory of Phototherapy and Innovative Technologies in Health at the Universidade Nove de Julho in Sao Paulo, Brazil, recommends that for small muscle groups like the biceps or triceps, use red-light lasers or LED devices with a wavelength of 640 nm for red light or 950 nm for infrared light, at a power of 50-200 mW per diode for single-probe device types, at a dose of 20-60 J, given 5-10 minutes after exercise.
A version of this article appeared on Medscape.com.
Navigating Election Anxiety: How Worry Affects the Brain
Once again, America is deeply divided before a national election, with people on each side convinced of the horrors that will be visited upon us if the other side wins.
’Tis the season — and regrettably, not to be jolly but to be worried.
As a neuroscientist, I am especially aware of the deleterious mental and physical impact of chronic worry on our citizenry. That’s because worry is not “all in your head.” We modern humans live in a world of worry which appears to be progressively growing.
Flight or Fight
Worry stems from the brain’s rather remarkable ability to foresee and reflexively respond to threat. Our “fight or flight” brain machinery probably arose in our vertebrate ancestors more than 300 million years ago. The fact that we have machinery akin to that possessed by lizards or tigers or shrews is testimony to its crucial contribution to our species’ survival.
As the phrase “fight or flight” suggests, a brain that senses trouble immediately biases certain body and brain functions. As it shifts into a higher-alert mode, it increases the energy supplies in our blood and supports other changes that facilitate faster and stronger reactions, while it shuts down less essential processes which do not contribute to hiding, fighting, or running like hell.
This hyperreactive response is initiated in the amygdala in the anterior brain, which identifies “what’s happening” as immediately or potentially threatening. The now-activated amygdala generates a response in the hypothalamus that provokes an immediate increase of adrenaline and cortisol in the body, and cortisol and noradrenaline in the brain. Both sharply speed up our physical and neurologic reactivity. In the brain, that is achieved by increasing the level of excitability of neurons across the forebrain. Depending on the perceived level of threat, an excitable brain will be just a little or a lot more “on alert,” just a little or a lot faster to respond, and just a little or a lot better at remembering the specific “warning” events that trigger this lizard-brain response.
Alas, this machinery was designed to be engaged every so often when a potentially dangerous surprise arises in life. When the worry and stress are persistent, the brain experiences a kind of neurologic “burn-out” of its fight versus flight machinery.
Dangers of Nonstop Anxiety and Stress
A consistently stressed-out brain turns down its production and release of noradrenaline, and the brain becomes less attentive, less engaged. This sets the brain on the path to an anxiety (and then a depressive) disorder, and, in the longer term, to cognitive losses in memory and executive control systems, and to emotional distortions that can lead to substance abuse or other addictions.
Our political distress is but one source of persistent worry and stress. Worry is a modern plague. The head counts of individuals seeking psychiatric or psychological health are at an all-time high in the United States. Near-universal low-level stressors, such as 2 years of COVID, insecurities about the changing demands of our professional and private lives, and a deeply divided body politic are unequivocally affecting American brain health.
The brain also collaborates in our body’s response to stress. Its regulation of hormonal responses and its autonomic nervous system’s mediated responses contribute to elevated blood sugar levels, to craving high-sugar foods, to elevated blood pressure, and to weaker immune responses. This all contributes to higher risks for cardiovascular and other dietary- and immune system–related disease. And ultimately, to shorter lifespans.
Strategies to Address Neurologic Changes Arising From Chronic Stress
There are many things you can try to bring your worry back to a manageable (and even productive) level.
- Engage in a “reset” strategy several times a day to bring your amygdala and locus coeruleus back under control. It takes a minute (or five) of calm, positive meditation to take your brain to a happy, optimistic place. Or use a mindfulness exercise to quiet down that overactive amygdala.
- Talk to people. Keeping your worries to yourself can compound them. Hashing through your concerns with a family member, friend, professional coach, or therapist can help put them in perspective and may allow you to come up with strategies to identify and neurologically respond to your sources of stress.
- Exercise, both physically and mentally. Do what works for you, whether it’s a run, a long walk, pumping iron, playing racquetball — anything that promotes physical release. Exercise your brain too. Engage in a project or activity that is mentally demanding. Personally, I like to garden and do online brain exercises. There’s nothing quite like yanking out weeds or hitting a new personal best at a cognitive exercise for me to notch a sense of accomplishment to counterbalance the unresolved issues driving my worry.
- Accept the uncertainty. Life is full of uncertainty. To paraphrase from Yale theologian Reinhold Niebuhr’s “Serenity Prayer”: Have the serenity to accept what you cannot help, the courage to change what you can, and the wisdom to recognize one from the other.
And, please, be assured that you’ll make it through this election season.
Dr. Merzenich, professor emeritus, Department of Neuroscience, University of California San Francisco, disclosed ties with Posit Science. He is often credited with discovering lifelong plasticity, with being the first to harness plasticity for human benefit (in his co-invention of the cochlear implant), and for pioneering the field of plasticity-based computerized brain exercise. He is a Kavli Laureate in Neuroscience, and he has been honored by each of the US National Academies of Sciences, Engineering, and Medicine. He may be most widely known for a series of specials on the brain on public television. His current focus is BrainHQ, a brain exercise app.
A version of this article appeared on Medscape.com.
Once again, America is deeply divided before a national election, with people on each side convinced of the horrors that will be visited upon us if the other side wins.
’Tis the season — and regrettably, not to be jolly but to be worried.
As a neuroscientist, I am especially aware of the deleterious mental and physical impact of chronic worry on our citizenry. That’s because worry is not “all in your head.” We modern humans live in a world of worry which appears to be progressively growing.
Flight or Fight
Worry stems from the brain’s rather remarkable ability to foresee and reflexively respond to threat. Our “fight or flight” brain machinery probably arose in our vertebrate ancestors more than 300 million years ago. The fact that we have machinery akin to that possessed by lizards or tigers or shrews is testimony to its crucial contribution to our species’ survival.
As the phrase “fight or flight” suggests, a brain that senses trouble immediately biases certain body and brain functions. As it shifts into a higher-alert mode, it increases the energy supplies in our blood and supports other changes that facilitate faster and stronger reactions, while it shuts down less essential processes which do not contribute to hiding, fighting, or running like hell.
This hyperreactive response is initiated in the amygdala in the anterior brain, which identifies “what’s happening” as immediately or potentially threatening. The now-activated amygdala generates a response in the hypothalamus that provokes an immediate increase of adrenaline and cortisol in the body, and cortisol and noradrenaline in the brain. Both sharply speed up our physical and neurologic reactivity. In the brain, that is achieved by increasing the level of excitability of neurons across the forebrain. Depending on the perceived level of threat, an excitable brain will be just a little or a lot more “on alert,” just a little or a lot faster to respond, and just a little or a lot better at remembering the specific “warning” events that trigger this lizard-brain response.
Alas, this machinery was designed to be engaged every so often when a potentially dangerous surprise arises in life. When the worry and stress are persistent, the brain experiences a kind of neurologic “burn-out” of its fight versus flight machinery.
Dangers of Nonstop Anxiety and Stress
A consistently stressed-out brain turns down its production and release of noradrenaline, and the brain becomes less attentive, less engaged. This sets the brain on the path to an anxiety (and then a depressive) disorder, and, in the longer term, to cognitive losses in memory and executive control systems, and to emotional distortions that can lead to substance abuse or other addictions.
Our political distress is but one source of persistent worry and stress. Worry is a modern plague. The head counts of individuals seeking psychiatric or psychological health are at an all-time high in the United States. Near-universal low-level stressors, such as 2 years of COVID, insecurities about the changing demands of our professional and private lives, and a deeply divided body politic are unequivocally affecting American brain health.
The brain also collaborates in our body’s response to stress. Its regulation of hormonal responses and its autonomic nervous system’s mediated responses contribute to elevated blood sugar levels, to craving high-sugar foods, to elevated blood pressure, and to weaker immune responses. This all contributes to higher risks for cardiovascular and other dietary- and immune system–related disease. And ultimately, to shorter lifespans.
Strategies to Address Neurologic Changes Arising From Chronic Stress
There are many things you can try to bring your worry back to a manageable (and even productive) level.
- Engage in a “reset” strategy several times a day to bring your amygdala and locus coeruleus back under control. It takes a minute (or five) of calm, positive meditation to take your brain to a happy, optimistic place. Or use a mindfulness exercise to quiet down that overactive amygdala.
- Talk to people. Keeping your worries to yourself can compound them. Hashing through your concerns with a family member, friend, professional coach, or therapist can help put them in perspective and may allow you to come up with strategies to identify and neurologically respond to your sources of stress.
- Exercise, both physically and mentally. Do what works for you, whether it’s a run, a long walk, pumping iron, playing racquetball — anything that promotes physical release. Exercise your brain too. Engage in a project or activity that is mentally demanding. Personally, I like to garden and do online brain exercises. There’s nothing quite like yanking out weeds or hitting a new personal best at a cognitive exercise for me to notch a sense of accomplishment to counterbalance the unresolved issues driving my worry.
- Accept the uncertainty. Life is full of uncertainty. To paraphrase from Yale theologian Reinhold Niebuhr’s “Serenity Prayer”: Have the serenity to accept what you cannot help, the courage to change what you can, and the wisdom to recognize one from the other.
And, please, be assured that you’ll make it through this election season.
Dr. Merzenich, professor emeritus, Department of Neuroscience, University of California San Francisco, disclosed ties with Posit Science. He is often credited with discovering lifelong plasticity, with being the first to harness plasticity for human benefit (in his co-invention of the cochlear implant), and for pioneering the field of plasticity-based computerized brain exercise. He is a Kavli Laureate in Neuroscience, and he has been honored by each of the US National Academies of Sciences, Engineering, and Medicine. He may be most widely known for a series of specials on the brain on public television. His current focus is BrainHQ, a brain exercise app.
A version of this article appeared on Medscape.com.
Once again, America is deeply divided before a national election, with people on each side convinced of the horrors that will be visited upon us if the other side wins.
’Tis the season — and regrettably, not to be jolly but to be worried.
As a neuroscientist, I am especially aware of the deleterious mental and physical impact of chronic worry on our citizenry. That’s because worry is not “all in your head.” We modern humans live in a world of worry which appears to be progressively growing.
Flight or Fight
Worry stems from the brain’s rather remarkable ability to foresee and reflexively respond to threat. Our “fight or flight” brain machinery probably arose in our vertebrate ancestors more than 300 million years ago. The fact that we have machinery akin to that possessed by lizards or tigers or shrews is testimony to its crucial contribution to our species’ survival.
As the phrase “fight or flight” suggests, a brain that senses trouble immediately biases certain body and brain functions. As it shifts into a higher-alert mode, it increases the energy supplies in our blood and supports other changes that facilitate faster and stronger reactions, while it shuts down less essential processes which do not contribute to hiding, fighting, or running like hell.
This hyperreactive response is initiated in the amygdala in the anterior brain, which identifies “what’s happening” as immediately or potentially threatening. The now-activated amygdala generates a response in the hypothalamus that provokes an immediate increase of adrenaline and cortisol in the body, and cortisol and noradrenaline in the brain. Both sharply speed up our physical and neurologic reactivity. In the brain, that is achieved by increasing the level of excitability of neurons across the forebrain. Depending on the perceived level of threat, an excitable brain will be just a little or a lot more “on alert,” just a little or a lot faster to respond, and just a little or a lot better at remembering the specific “warning” events that trigger this lizard-brain response.
Alas, this machinery was designed to be engaged every so often when a potentially dangerous surprise arises in life. When the worry and stress are persistent, the brain experiences a kind of neurologic “burn-out” of its fight versus flight machinery.
Dangers of Nonstop Anxiety and Stress
A consistently stressed-out brain turns down its production and release of noradrenaline, and the brain becomes less attentive, less engaged. This sets the brain on the path to an anxiety (and then a depressive) disorder, and, in the longer term, to cognitive losses in memory and executive control systems, and to emotional distortions that can lead to substance abuse or other addictions.
Our political distress is but one source of persistent worry and stress. Worry is a modern plague. The head counts of individuals seeking psychiatric or psychological health are at an all-time high in the United States. Near-universal low-level stressors, such as 2 years of COVID, insecurities about the changing demands of our professional and private lives, and a deeply divided body politic are unequivocally affecting American brain health.
The brain also collaborates in our body’s response to stress. Its regulation of hormonal responses and its autonomic nervous system’s mediated responses contribute to elevated blood sugar levels, to craving high-sugar foods, to elevated blood pressure, and to weaker immune responses. This all contributes to higher risks for cardiovascular and other dietary- and immune system–related disease. And ultimately, to shorter lifespans.
Strategies to Address Neurologic Changes Arising From Chronic Stress
There are many things you can try to bring your worry back to a manageable (and even productive) level.
- Engage in a “reset” strategy several times a day to bring your amygdala and locus coeruleus back under control. It takes a minute (or five) of calm, positive meditation to take your brain to a happy, optimistic place. Or use a mindfulness exercise to quiet down that overactive amygdala.
- Talk to people. Keeping your worries to yourself can compound them. Hashing through your concerns with a family member, friend, professional coach, or therapist can help put them in perspective and may allow you to come up with strategies to identify and neurologically respond to your sources of stress.
- Exercise, both physically and mentally. Do what works for you, whether it’s a run, a long walk, pumping iron, playing racquetball — anything that promotes physical release. Exercise your brain too. Engage in a project or activity that is mentally demanding. Personally, I like to garden and do online brain exercises. There’s nothing quite like yanking out weeds or hitting a new personal best at a cognitive exercise for me to notch a sense of accomplishment to counterbalance the unresolved issues driving my worry.
- Accept the uncertainty. Life is full of uncertainty. To paraphrase from Yale theologian Reinhold Niebuhr’s “Serenity Prayer”: Have the serenity to accept what you cannot help, the courage to change what you can, and the wisdom to recognize one from the other.
And, please, be assured that you’ll make it through this election season.
Dr. Merzenich, professor emeritus, Department of Neuroscience, University of California San Francisco, disclosed ties with Posit Science. He is often credited with discovering lifelong plasticity, with being the first to harness plasticity for human benefit (in his co-invention of the cochlear implant), and for pioneering the field of plasticity-based computerized brain exercise. He is a Kavli Laureate in Neuroscience, and he has been honored by each of the US National Academies of Sciences, Engineering, and Medicine. He may be most widely known for a series of specials on the brain on public television. His current focus is BrainHQ, a brain exercise app.
A version of this article appeared on Medscape.com.
Immunotherapy May Be Overused in Dying Patients With Cancer
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.