User login
Contralateral Constrictor Dose Predicts Swallowing Function After Radiation for Head and Neck Cancer
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
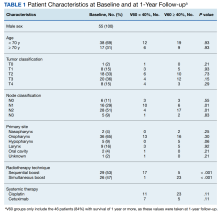
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
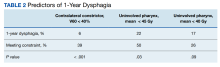
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
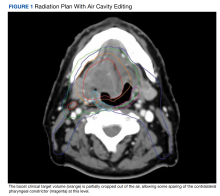
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
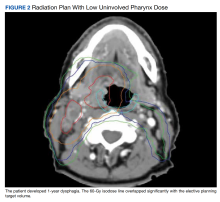
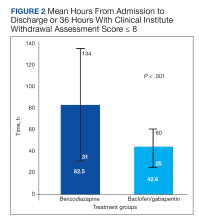
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Longitudinal Dynamic in Weight Loss Impacts Clinical Outcomes for Veterans Undergoing Curative Surgery for Colorectal Cancer
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
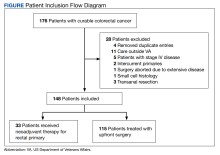
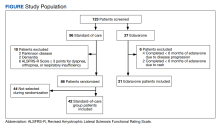
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
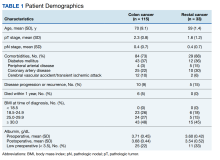
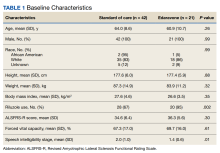
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
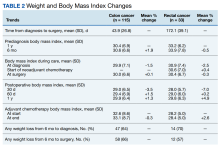
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
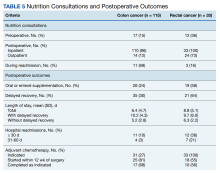
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
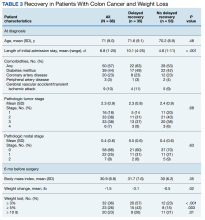
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs= -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
Disparities in Melanoma Demographics, Tumor Stage, and Metastases in Hispanic and Latino Patients: A Retrospective Study
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.
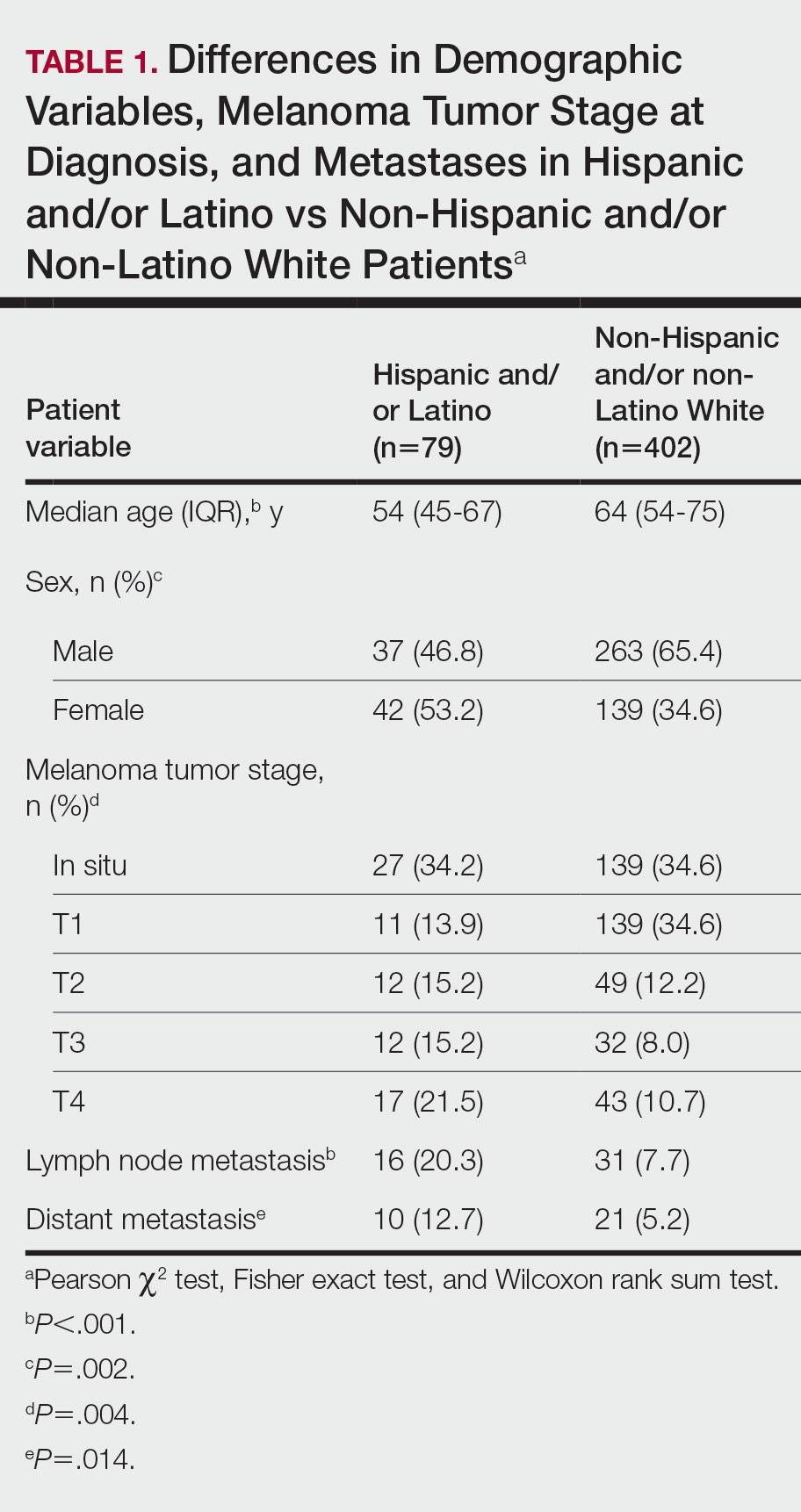
The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).
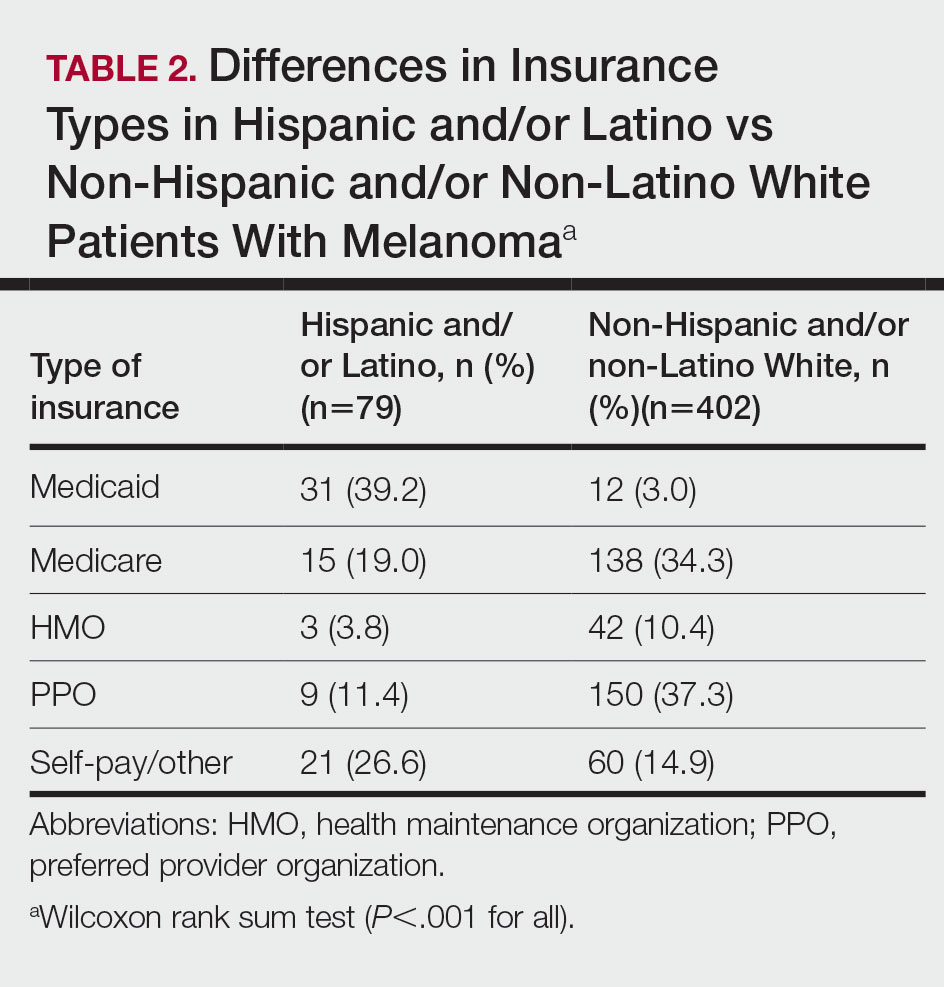
This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
Practice Points
- Hispanic and/or Latino patients often present with more advanced-stage melanomas and have decreased survival rates compared with non-Hispanic and/or non-Latino White patients.
- More education and awareness on the risk for melanoma as well as sun-protective behaviors in the Hispanic and/or Latino population is needed among both health care providers and patients to prevent diagnosis of melanoma in later stages and improve outcomes.
Artificial Intelligence vs Medical Providers in the Dermoscopic Diagnosis of Melanoma
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.
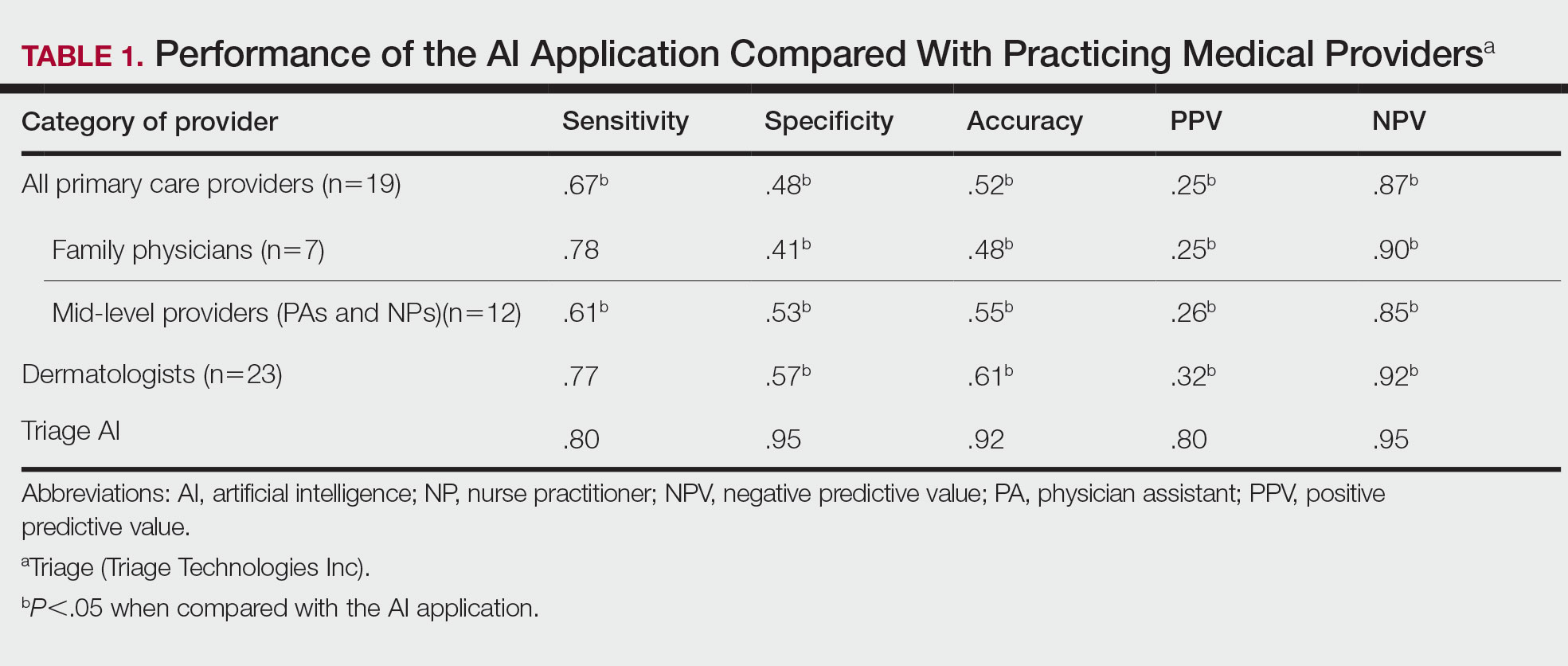
Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.
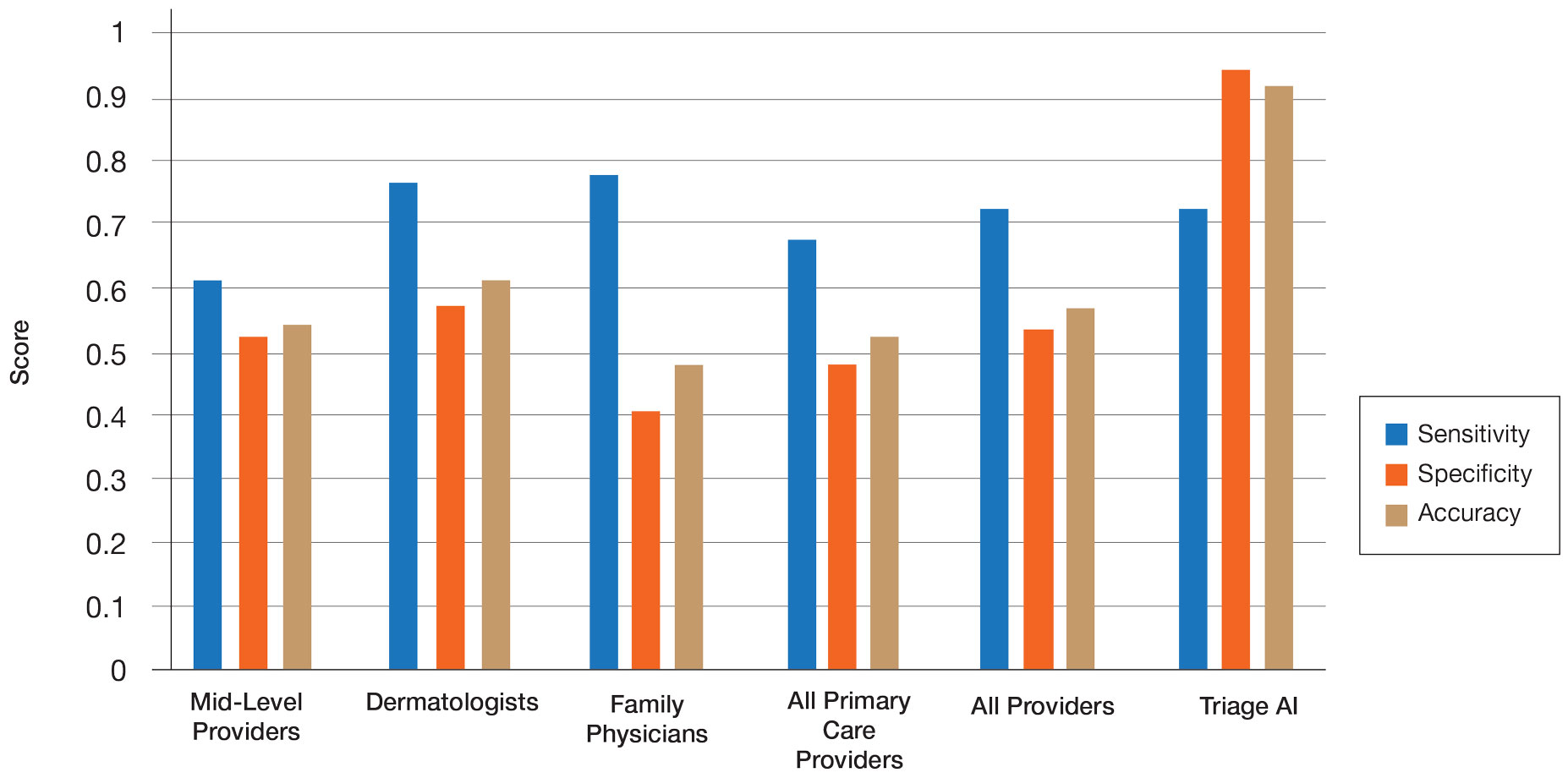
Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.
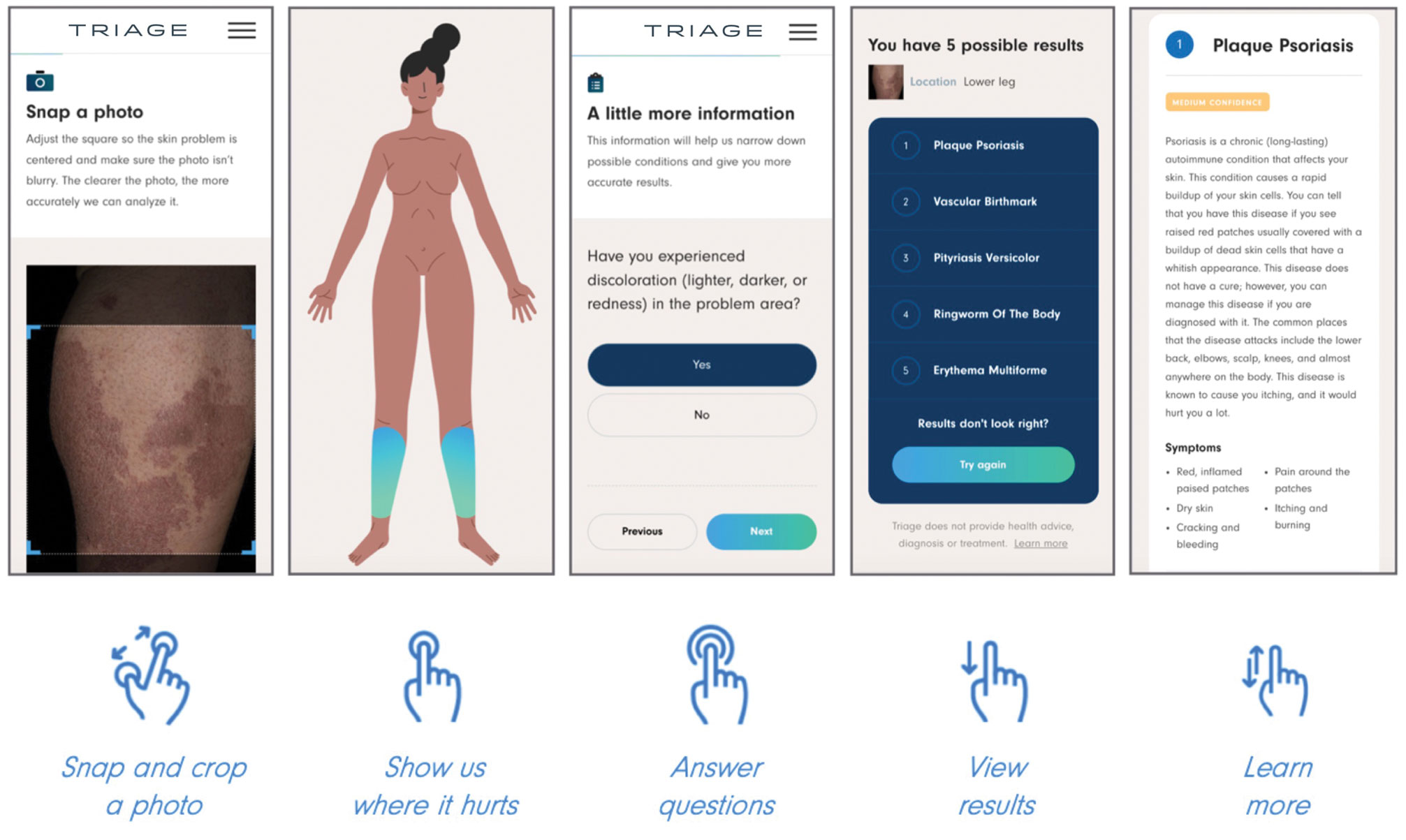
The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.
In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.

Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.

Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.

The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.

In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.

Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.

Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.

The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.

In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
Practice Points
- Artificial intelligence (AI) has the potential to facilitate the diagnosis of pigmented lesions and expedite the management of malignant melanoma.
- Further studies should be done to see if the high diagnostic accuracy of the AI application we studied translates to a decrease in unnecessary biopsies or expedited referral for pigmented lesions.
- The large variability of formal dermoscopy training among board-certified dermatologists may contribute to the decreased ability to identify pigmented lesions with dermoscopic imaging compared to AI.
Assessment of IV Edaravone Use in the Management of Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
Evaluation of Gabapentin and Baclofen Combination for Inpatient Management of Alcohol Withdrawal Syndrome
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7-9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
Methods
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
Results
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28).
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome.
Additionally, this study examined multiple secondary outcomes (Table 2).
Discussion
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate recordkeeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
Conclusions
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
1. National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. Updated April 2021. Accessed February 2, 2023. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder
2. Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3-4):175-177. doi:10.1080/19371918.2013.758987
3. Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. Accessed January 26, 2023. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis
4. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07. doi:10.7860/JCDR/2015/13407.6538
5. Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144-151. doi:10.1001/jama.278.2.144
6. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649-655.
7. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. doi:10.1111/j.1530-0277.2009.00986.x
8. Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics. 2018;59(5):496-505. doi:10.1016/j.psym.2018.03.002
9. Bates RE, Leung JG, Morgan RJ 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542-549. Published 2020 Aug 19. doi:10.1016/j.mayocpiqo.2020.06.002
10. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
11. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. doi:10.1177/1060028015585849
12. Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi:10.3389/fpsyt.2018.00773
13. Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. Published 2019 Nov 6. doi:10.1002/14651858.CD008502.pub6
Battlefield Acupuncture vs Ketorolac for Treating Pain in the Emergency Department
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
Acute pain is a primary symptom for many patients who present to the emergency department (ED). The ED team is challenged with relieving pain while limiting harm from medications.1 A 2017 National Health Interview Survey showed that compared with nonveterans, more veterans reported pain in the previous 3 months, and the rate of severe pain was 40% higher in the veteran group especially among those who served during the era of wars in Afghanistan and Iraq.2
The American College of Emergency Physicians guidelines pain management guidelines recommend patient-centered shared decision making that includes patient education about treatment goals and expectations, and short- and long-term risks, as well as a preference toward pharmacologic treatment with nonopioid analgesics except for patients with severe pain or pain refractory to other drug and treatment modalities.3 There is a lack of evidence regarding superior efficacy of either opioid or nonopioid analgesics; therefore, the use of nonopioid analgesics, such as oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) or central analgesics, such as acetaminophen, is preferred for treating acute pain to mitigate adverse effects (AEs) and risks associated with opioid use.1,3,4 The US Department of Veterans Affairs (VA) and Department of Defense (DoD) guideline on managing opioid therapy for chronic pain, updated in 2017 and 2022, similarly recommends alternatives to opioids for mild-to-moderate acute pain and encourages multimodal pain care.5 However, use of other pharmacologic treatments, such as NSAIDs, is limited by AE profiles, patient contraindications, and severity of acute pain etiologies. There is a need for the expanded use of nonpharmacologic treatments for addressing pain in the veteran population.
The American College of Emergency Physicians guidelines recommend nonpharmacologic modalities, such as applying heat or cold, physical therapy, cognitive behavioral therapy, and acupuncture.3 A 2014 study reported that 37% to 46% of active duty and reserve military personnel use complementary and alternative medicine (CAM) for a variety of ailments, and there is increasing interest in the use of CAM as adjuncts to traditional therapies.6 According to one study, some CAM therapies are used significantly more by military personnel than used by civilians.7 However, the percentage of the veteran population using acupuncture in this study was small, and more information is needed to assess its use.
Auricular acupuncture originated in traditional Chinese medicine.8 Contemporary auricular acupuncture experts view this modality as a self-contained microsystem mapping portions of the ear to specific parts of the body and internal organs. The analgesic effects may be mediated through the central nervous system by local release of endorphins through nerve fiber activation and neurotransmitters—including serotonin, dopamine, and norepinephrine—leading to pre- and postsynaptic suppression of pain transmission.
Battlefield acupuncture (BFA) uses 5 set points anatomically located on each ear.9 Practitioners use small semipermanent, dartlike acupuncture needles. Patients could experience pain relief in a few minutes, which can last minutes, hours, days, weeks, or months depending on the pathology of the pain. This procedure developed in 2001 has been studied for different pain types and has shown benefit when used for postsurgical pain, chronic spinal cord injury−related neuropathic pain, and general chronic pain, as well as for other indications, such as insomnia, depression, and weight loss.8,10-13 In 2018, a randomized controlled trial compared postintervention numeric rating scale (NRS) pain scores in patients presenting to the ED with acute or acute-on-chronic lower back pain who received BFA as an adjunct to standard care vs standard care alone.14 Patients receiving BFA as an adjunct to standard care were found to have mean postintervention pain scores 1.7 points lower than those receiving standard care alone. This study demonstrated that BFA was feasible and well tolerated for lower back pain in the ED as an adjunct to standard care. The study was limited by the adjunct use of BFA rather than as monotherapy and by the practitioners’ discretion regarding standard care, which was not defined by the study’s authors.
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, offers several CAM modalities, such as exercise/movement therapy, chiropractic, art/music therapy, and relaxation workshops, which are widely used by veterans. Recent evidence suggests BFA could reduce pain scores as an adjunct or an alternative to pharmacologic therapy. We are interested in how CAM therapies, such as BFA, can help avoid AEs associated with opioid or NSAID therapy.
At the JBVAMC ED, ketorolac 15 mg is the preferred first-line treatment of acute, noncancer pain, based on the results of previous studies. In 2018 BFA was offered first to veterans presenting with acute or acute-on-chronic pain to the ED; however, its effectiveness for pain reduction vs ketorolac has not been evaluated in this patient population. Limited literature is available on BFA and its use in the ED. To our knowledge, this was the first observational study assessing the difference between a single session of BFA vs a single dose of ketorolac in treating noncancer acute or acute-on-chronic pain in the ED.
Methods
This study was a retrospective chart review of patients who presented to the JBVAMC ED with acute pain or acute-on-chronic pain, who received ketorolac or BFA. The study population was generated from a list of all IV and intramuscular (IM) ketorolac unit dose orders verified from June 1, 2018, through August 30, 2019, and a list of all BFA procedure notes signed from June 1, 2018, through August 30, 2019. Patients were included in the study if they had documented administration of IV or IM ketorolac or BFA between June 1, 2018, and August 30, 2019. Patients who received ketorolac doses other than 15 mg, the intervention was administered outside of the ED, received adjunct treatment in addition to the treatment intervention in the ED, had no baseline NRS pain score documented before the intervention, had an NRS pain score of < 4, had no postintervention NRS pain score documented within 6 hours, had a treatment indication other than pain, or had active cancer were excluded. As in previous JBVAMC studies, we used NRS pain score cutoffs (mild, moderate, severe, and very severe) based on Woo and colleagues’ meta-analysis and excluded scores < 4.15
Endpoints
The primary endpoint was the mean difference in NRS pain score before and after the intervention, determined by comparing the NRS pain score documented at triage to the ED with the first documented NRS pain score at least 30 minutes to 6 hours after treatment administration. The secondary endpoints included the number of patients prescribed pain medication at discharge, the number of patients who were discharged with no medications, and the number of patients admitted to the hospital. The safety endpoint included any AEs of the intervention. Subgroup analyses were performed comparing the mean difference in NRS pain score among subgroups classified by severity of baseline NRS pain score and pain location.
Statistical Analysis
Baseline characteristics and endpoints were analyzed using descriptive statistics. Categorical data were analyzed using Fisher exact test and z test for proportions, and continuous data were compared using t test and paired t test. An 80% power calculation determined that 84 patients per group were needed to detect a statistically significant difference in pain score reduction of 1.3 at a type-1 error rate of 0.05. The sample size was based on a calculation performed in a previously published study that compared IV ketorolac at 3 single-dose regimens for treating acute pain in the ED.16 The 1.3 pain score reduction is considered the minimum clinically significant difference in pain that could be detected with the NRS.17
Results
Sixty-one patients received BFA during the study period: 31 were excluded (26 received adjunct treatment in the ED, 2 had active cancer documented, 2 had an indication other than pain, and 1 received BFA outside of the ED), leaving 30 patients in the BFA cohort. During the study period, 1299 patients received ketorolac.
Baseline characteristics were similar between the 2 groups except for the average baseline NRS pain score, which was statistically significantly higher in the BFA vs ketorolac group (8.7 vs 7.7, respectively; P = .02). The mean age was 51 years in the BFA group and 48 years in the ketorolac group. Most patients in each cohort were male: 80% in the BFA group and 71% in the ketorolac group. The most common types of pain documented as the chief ED presentation included back, lower extremity, and head.
Endpoints
The mean difference in NRS pain score was 3.9 for the BFA group and 5.1 for the ketorolac group. Both were clinically and statistically significant reductions (P = .03 and P < .01), but the difference between the intervention groups in NRS score reduction was not statistically significant (P = .07).
For the secondary endpoint of outpatient prescriptions written at discharge, there was no significant difference between the groups except for oral NSAIDs, which were more likely to be prescribed to patients who received ketorolac (P = .01).
Subgroup Analysis
An analysis was performed for subgroups classified by baseline NRS pain score (mild: 4; moderate, 5 - 6; severe, 7 - 9; and very severe, 10). Data for mild pain was limited because a small number of patients received interventions. For moderate pain, the mean difference in NRS pain score for BFA and ketorolac was 3.5 and 3.8, respectively; for severe pain, 3.4 and 5.3; and for very severe pain, 4.6 and 6.4. There was a larger difference in the preintervention and postintervention NRS pain scores within severe pain and very severe pain groups.
Discussion
Both interventions resulted in a significant reduction in the mean NRS pain score of about 4 to 5 points within their group, and BFA resulted in a similar NRS pain score reduction compared with ketorolac 15 mg. Because the baseline NRS pain scores were significantly different between the BFA and ketorolac groups,
In this study, more patients in the BFA group presented to the ED with lower extremity pain, such as gout or neuropathy, compared with the ketorolac group; however, BFA did not result in a significantly different pain score reduction in this subgroup compared with ketorolac. Patients receiving BFA were more likely to receive topical analgesics or muscle relaxants at discharge; whereas those receiving ketorolac were significantly more likely to receive oral NSAIDs. Patients in this study also were more likely to be admitted to the hospital if they received ketorolac; however, for these patients, pain was secondary to their chief presentation, and the admitting physician’s familiarity with ketorolac might have been the reason for choosing this intervention. Reasons for the admissions were surgical observation, psychiatric stabilization, kidney/gallstones, rule out of acute coronary syndrome, pneumonia, and proctitis in the ketorolac group, and suicidal ideations in the BFA group.
Limitations
As a limited number of patients received BFA at JBVAMC, the study was not sufficiently powered to detect a difference in the primary outcome. Because BFA required a consultation to be entered in the electronic health record, in addition to time needed to perform the procedure, practitioners might have preferred IV/IM ketorolac during busy times in the ED, potentially leading to underrepresentation in the BFA group. Prescribing preferences might have differed among the rotating physicians, timing of the documentation of the NRS pain score could have differed based on the treatment intervention, and the investigators were unable to control or accurately assess whether patients had taken an analgesic medication before presenting to the ED.
Conclusions
NRS pain score reduction with BFA did not differ compared with ketorolac 15 mg for treating acute and acute-on-chronic pain in the ED. Although this study was underpowered, these results add to the limited existing literature, suggesting that both interventions could result in clinically significant pain score reductions for patients presenting to the ED with severe and very severe pain, making BFA a viable nonpharmacologic option. Future studies could include investigating the benefit of BFA in the veteran population by studying larger samples in the ED, surveying patients after their interventions to identify rates AEs, and exploring the use of BFA for chronic pain in the outpatient setting.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
1. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. doi:10.1016/j.annemergmed.2012.06.013
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Motov S, Strayer R, Hayes BD, et al. The treatment of acute pain in the emergency department: a white paper position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2018;54(5):731-736. doi:10.1016/j.jemermed.2018.01.020
4. Samcam I, Papa L. Acute pain management in the emergency department. In: Prostran M, ed. Pain Management. IntechOpen; 2016. doi:10.5772/62861
5. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the use of opioids in the management of chronic pain. Accessed February 15, 2023. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
6. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population surveys. Med Care. 2014;52(12 suppl 5):S83-590. doi:10.1097/MLR.0000000000000227
7. Goertz C, Marriott BP, Finch FD, et al. Military report more complementary and alternative medicine use than civilians. J Altern Complement Med. 2013;19(6):509-517. doi:10.1089/acm.2012.0108
8. King HC, Hickey AH, Connelly C. Auricular acupuncture: a brief introduction for military providers. Mil Med. 2013;178(8):867-874. doi:10.7205/MILMED-D-13-00075
9. Niemtzow RC. Battlefield acupuncture. Medical Acupunct. 2007;19(4):225-228. doi:10.1089/acu.2007.0603
10. Collinsworth KM, Goss DL. Battlefield acupuncture and physical therapy versus physical therapy alone after shoulder surgery. Med Acupunct. 2019;31(4):228-238. doi:10.1089/acu.2019.1372
11. Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med. 2017;40(4):432-438. doi:10.1080/10790268.2016.1141489
12. Federman DG, Radhakrishnan K, Gabriel L, Poulin LM, Kravetz JD. Group battlefield acupuncture in primary care for veterans with pain. South Med J. 2018;111(10):619-624. doi:10.14423/SMJ.0000000000000877
13. Garner BK, Hopkinson SG, Ketz AK, Landis CA, Trego LL. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Med Acupunct. 2018;30(5):262-272. doi:10.1089/acu.2018.1294
14. Fox LM, Murakami M, Danesh H, Manini AF. Battlefield acupuncture to treat low back pain in the emergency department. Am J Emerg Med. 2018; 36:1045-1048. doi:10.1016/j.ajem.2018.02.038
15. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
16. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177-184. doi:10.1016/j.annemergmed.2016.10.014
17. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390-392. doi:10.1111/j.1553-2712.2003.tb01355.
When a patient with chronic alcohol use abruptly stops drinking
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3
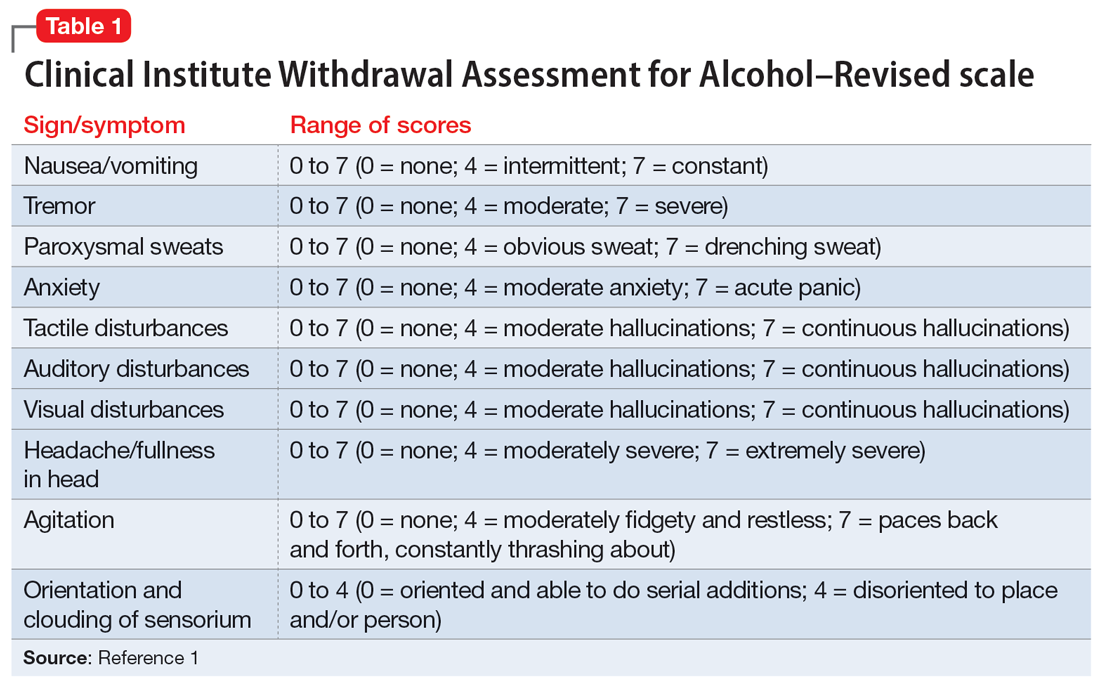
Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-
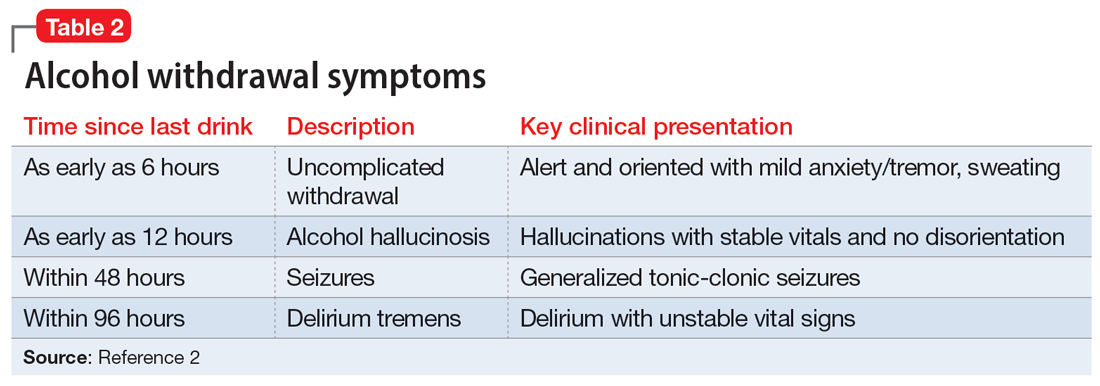
If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.
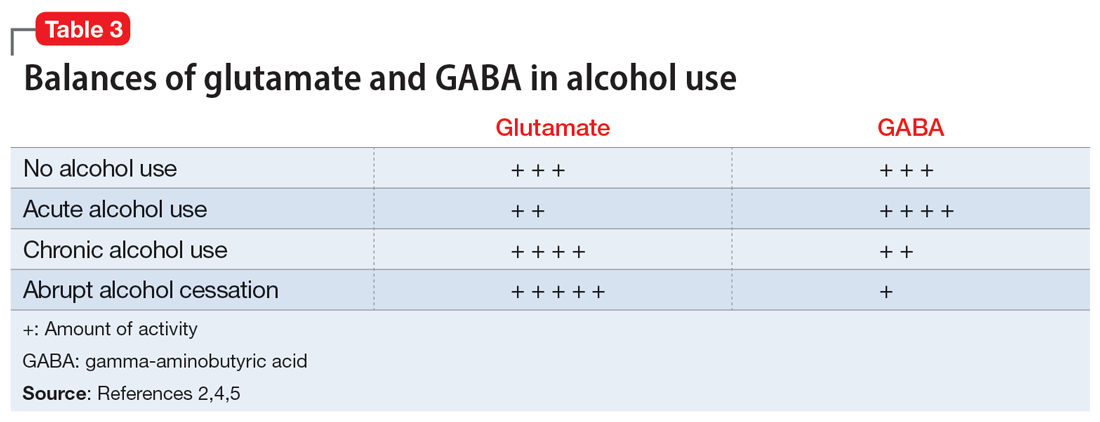
EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation.Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence.Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation.Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence.Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation.Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence.Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
Premedical Student Interest in and Exposure to Dermatology at Howard University
Diversity of health care professionals improves medical outcomes and quality of life in patients. 1 There is a lack of diversity in dermatology, with only 4.2% of dermatologists identifying as Hispanic and 3% identifying as African American, 2 possibly due to a lack of early exposure to dermatology among high school and undergraduate students, a low number of underrepresented students in medical school, a lack of formal mentorship programs geared to underrepresented students, and implicit biases. 1-4 Furthermore, the field is competitive, with many more applicants than available positions. In 2022, there were 851 applicants competing for 492 residency positions in dermatology. 5 Thus, it is important to educate young students about dermatology and understand root causes as to why the number of u nderrepresented in medicine (UiM) dermatologists remains stagnant.
According to Pritchett et al,4 it is crucial for dermatologists to interact with high school and college students to foster an early interest in dermatology. Many racial minority students do not progress from high school to college and then from college to medical school, which leaves a substantially reduced number of eligible UiM applicants who can progress into dermatology.6 Increasing the amount of UiM students going to medical school requires early mediation. Collaborating with pre-existing premedical school organizations through presentations and workshops is another way to promote an early interest in dermatology.4 Special consideration should be given to students who are UiM.
Among the general medical school curriculum, requirements for exposure to dermatology are not high. In one study, the median number of clinical and preclinical hours required was 10. Furthermore, 20% of 33 medical schools did not require preclinical dermatology hours (hours done before medical school rotations begin and in an academic setting), 36% required no clinical hours (rotational hours), 8% required no dermatology hours whatsoever, and only 10% required clinical dermatology rotation.3 Based on these findings, it is clear that dermatology is not well incorporated into medical school curricula. Furthermore, curricula have historically neglected to display adequate representation of skin of color.7 As a result, medical students generally have limited exposure to dermatology3 and are exposed even less to presentations of dermatologic issues in historically marginalized populations.7
Given the paucity of research on UiM students’ perceptions of dermatology prior to medical school, our cross-sectional survey study sought to evaluate the level of interest in dermatology of UiM premedical undergraduates. This survey specifically evaluated exposure to dermatology, preconceived notions about the field, and mentorship opportunities. By understanding these factors, dermatologists and dermatology residency programs can use this information to create mentorship opportunities and better adjust existing programs to meet students’ needs.
Methods
A 19-question multiple-choice survey was administered electronically (SurveyMonkey) in May 2020 to premedical students at Howard University (Washington, DC). One screening question was used: “What is your major?” Those who considered themselves a science major and/or with premedical interest were allowed to complete the survey. All students surveyed were members of the Health Professions Society at Howard University. Students who were interested in pursuing medical school were invited to respond. Approval for this study was obtained from the Howard University institutional review board (FWA00000891).
The survey was divided into 3 sections: Demographics, Exposure to Medicine and Dermatology, and Perceptions of Dermatology. The Demographics section addressed gender, age, and race/ethnicity. The Exposure to Medicine and Dermatology section addressed interest in attending medical school, shadowing experience, exposure to dermatology, and mentoring. The Perceptions of Dermatology section addressed preconceived notions about the field (eg, “dermatology is interesting and exciting”).
Statistical Analysis—The data represented are percentages based on the number of respondents who answered each question. Answers in response to “Please enter any comments” were organized into themes, and the number of respondents who discussed each theme was quantified into a table.
Results
A total of 271 survey invitations were sent to premedical students at Howard University. Students were informed of the study protocol and asked to consent before proceeding to have their responses anonymously collected. Based on the screening question, 152 participants qualified for the survey, and 152 participants completed it (response rate, 56%; completion rate, 100%). Participants were asked to complete the survey only once.
Demographics—Eighty-four percent of respondents identified as science majors, and the remaining 16% identified as nonscience premedical. Ninety-four percent of participants identified as Black or African American; 3% as Asian or Asian American; and the remaining 3% as Other. Most respondents were female (82%), 16% were male, and 2% were either nonbinary or preferred not to answer. Ninety-nine percent were aged 18 to 24 years, and 1% were aged 25 to 34 years (Table 1).
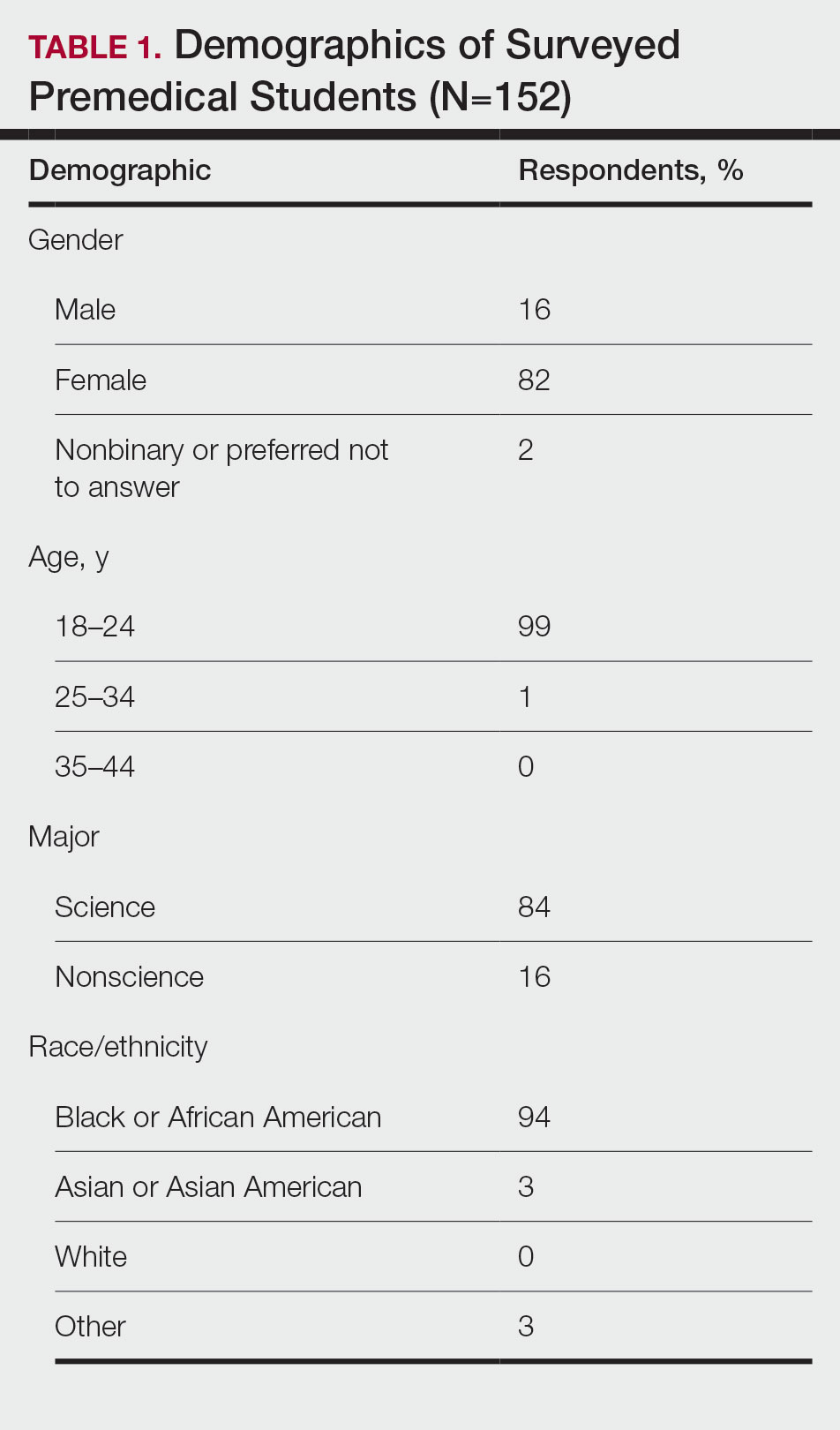
Exposure to Medicine and Dermatology—Ninety-three percent of participants planned on attending medical school, and most students developed an interest in medicine from an early age. Ninety-six percent cited that they became interested in medicine prior to beginning their undergraduate education, and 4% developed an interest as freshmen or sophomores. When asked what led to their interest in medicine, family influence had the single greatest impact on students’ decision to pursue medicine (33%). Classes/school were the second most influential factor (24%), followed by volunteering (15%), shadowing (13%), other (7%), and peer influence (3%)(Figure 1).
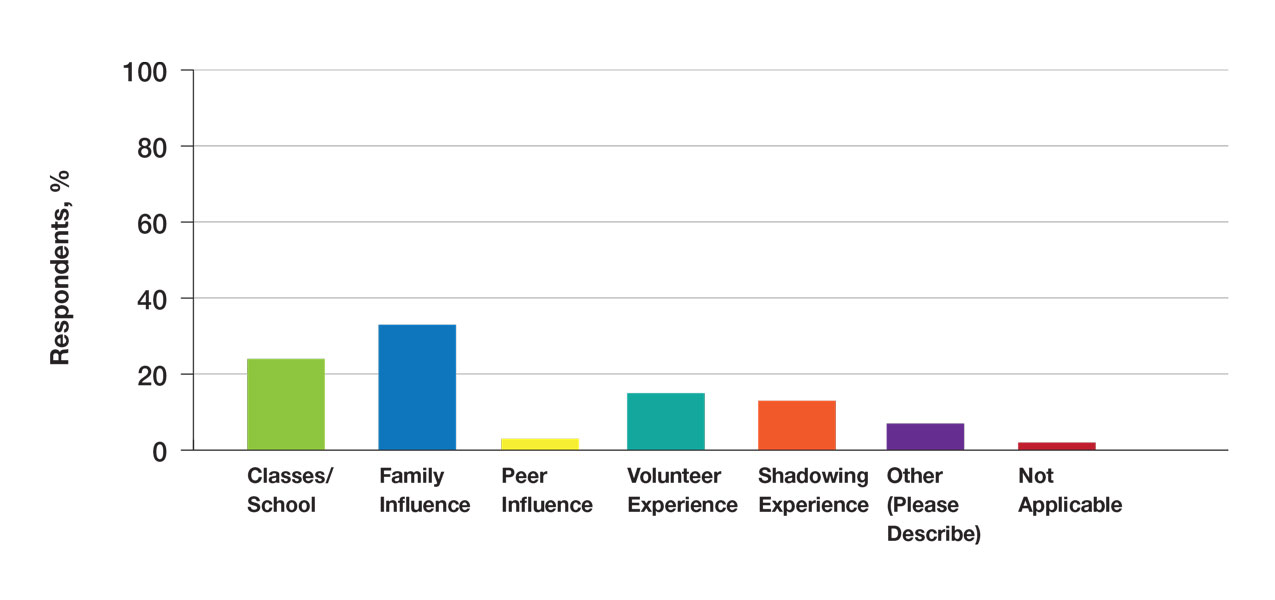
Many (56%) premedical students surveyed had shadowing experience to varying degrees. Approximately 18% had fewer than 8 hours of shadowing experience, 24% had 8 to 40 hours, and 14% had more than 40 hours. However, many (43%) premedical students had no shadowing experience (Figure 2). Similarly, 30% of premedical students responded to having a physician as a mentor.
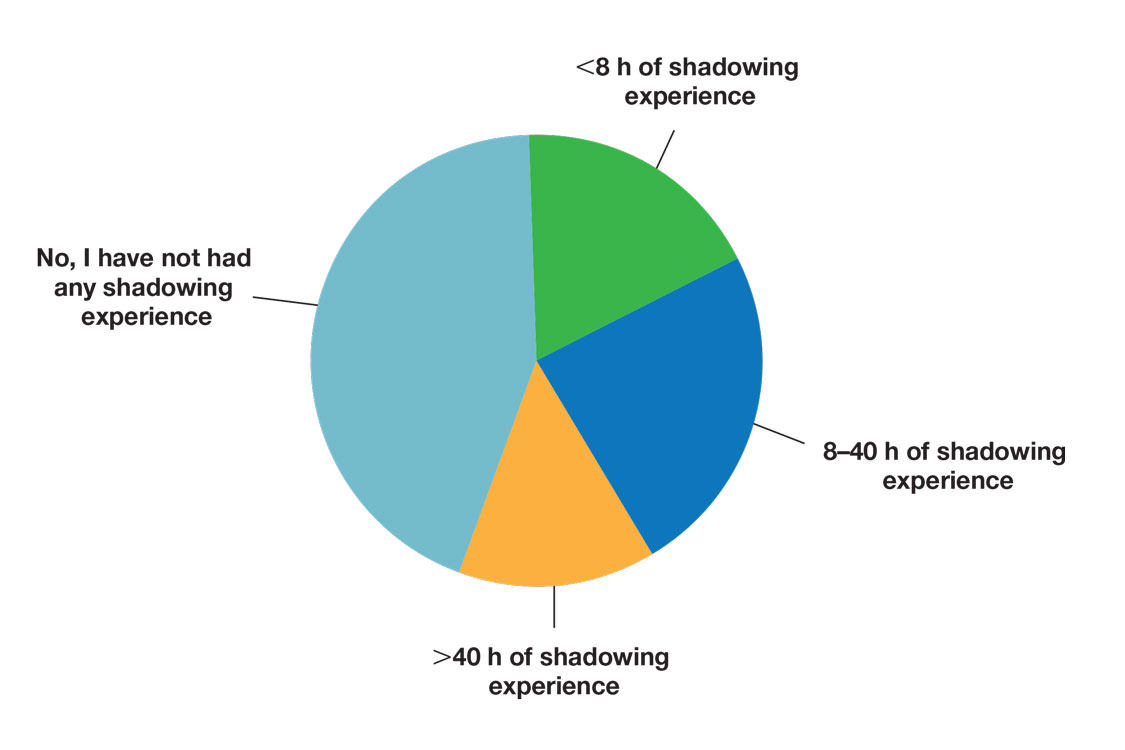
Regarding exposure to dermatology, 42% of premedical students had none. However, 58% of students had exposure to dermatology by being a patient themselves, 40% through seeing a dermatologist with a family member, 21% through seeing a dermatologist on television or social media, 5% through shadowing or volunteering, 3% through mentorship, and 1% through dermatology research (Figure 3).
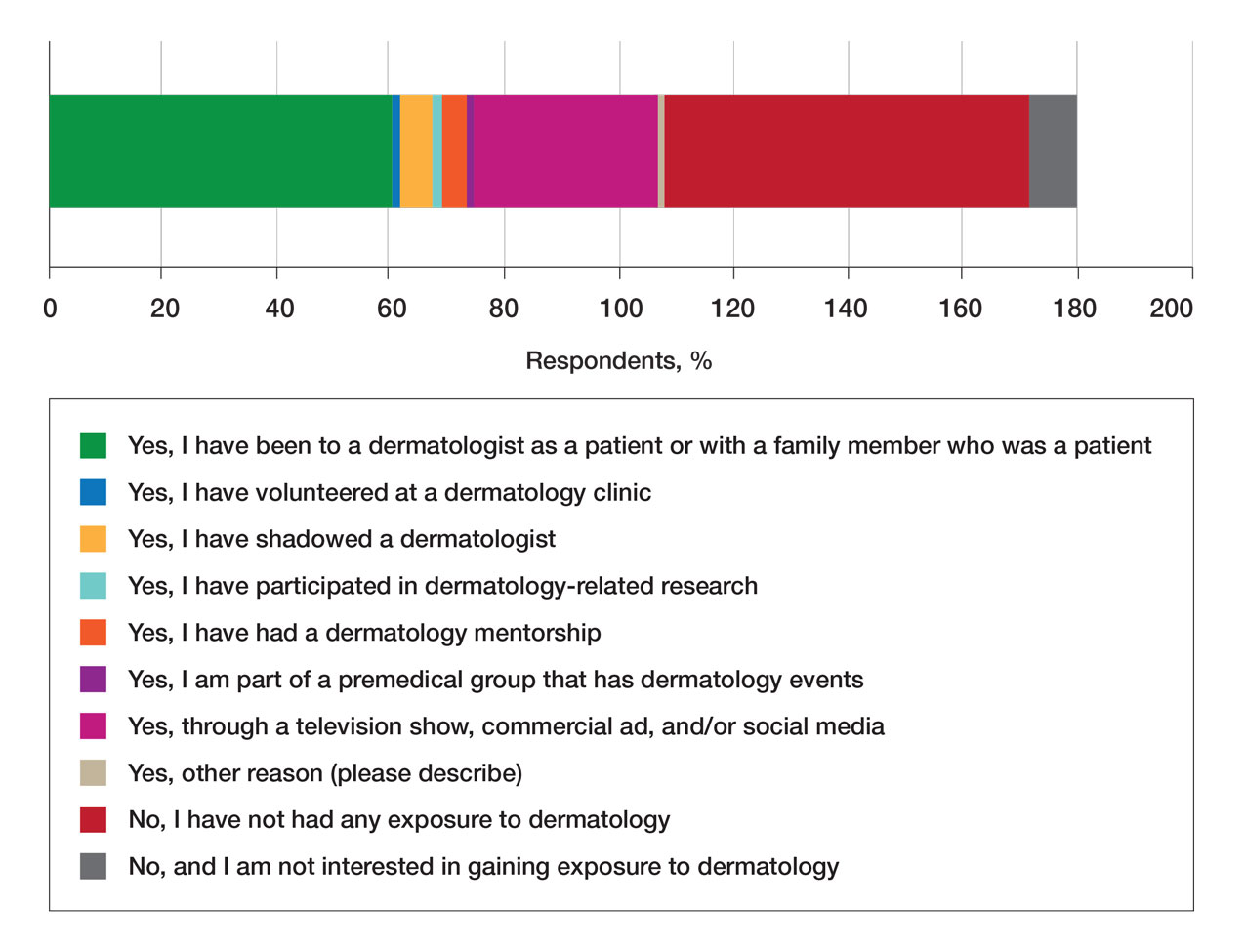
Of students who said they were interested in dermatology (32%), 16% developed their interest before undergraduate education, while 9% developed interest in their freshman or sophomore year and 7% in their junior or senior year of undergraduate education. Three percent of respondents indicated that they had a dermatology mentorship.
Perceptions of Dermatology—To further evaluate the level of interest that UiM premedical students have in the field of dermatology, students were asked how much they agree or disagree on whether the field of dermatology is interesting. Sixty-three percent of the students agreed that the field of dermatology is interesting, 34% remained uncertain, and 3% disagreed. Additionally, students were asked whether they would consider dermatology as a career; 54% of respondents would consider dermatology as a career, 30% remained uncertain, and 16% would not consider dermatology as a career choice.
Nearly all (95%) students agreed that dermatologists do valuable work that goes beyond the scope of cosmetic procedures such as neuromodulators, fillers, chemical peels, and lasers. Some students also noted they had personal experiences interacting with a dermatologist. For example, one student described visiting the dermatologist many times to get a treatment regimen for their eczema.
Overall themes from the survey are depicted in Table 2. Major themes found in the comments included the desire for more dermatology-related opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients and students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty.
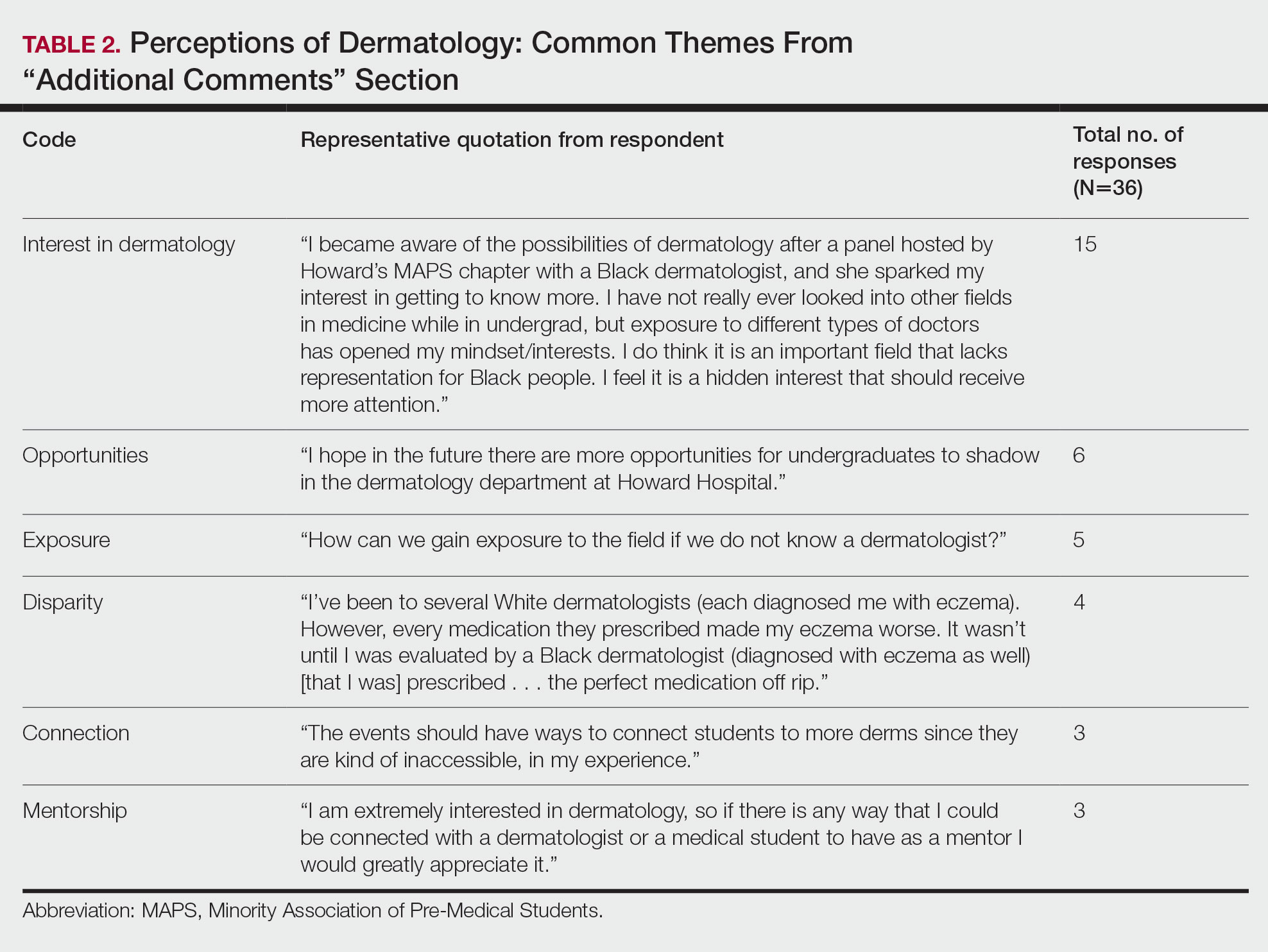
Comment
Interest in Dermatology—In this cross-sectional survey study of 152 UiM undergraduate students, it was found that many students were interested in dermatology as a career, and more than 70% would be interested in attending events that increased exposure to the field of dermatology. Of the students who had any exposure to dermatology, less than 5% had shadowed an actual dermatologist. The survey showed that there is great potential interest in exposing UiM undergraduate students to the field of dermatology. We found that UiM students are interested in learning more about dermatology, with 80% indicating that they would be willing to participate in dermatology-focused events if they were available. Overall, students mentioned a lack of opportunities, mentorship, exposure, and connections in dermatology despite their interest in the field.
Racial Disparities in Dermatology—Additionally, students discussed disparities they encountered with dermatology due to a lack of patient-provider race concordance and the perceived difference in care when encountering a race-concordant dermatologist. One student noted that they went to multiple White dermatologists for their eczema, and “it wasn’t until I was evaluated by a Black dermatologist (diagnosed with eczema as well) [that I was] prescribed . . . the perfect medication.” Another student noted how a Black dermatologist sparked their interest in getting to know more about the field and remarked that they “think it is an important field that lacks representation for Black people.” This research stresses the need for more dermatology mentorship among UiM undergraduates.
Family Influence on Career Selection—The majority of UiM students in our study became interested in medicine because of family, which is consistent with other studies. In a cross-sectional survey of 300 Pakistani students (150 medical and 150 nonmedical), 87% of students stated that their family had an influence on their career selection.8 In another study of 15 junior doctors in Sierra Leone, the most common reasons for pursuing medicine were the desire to help and familial and peer influence.9 This again showcases how family can have a positive impact on career selection for medical professionals and highlights the need for early intervention.
Shadowing—One way in which student exposure to dermatology can be effectively increased is by shadowing. In a study evaluating a 30-week shadowing program at the Pediatric Continuity Clinic in Los Angeles, California, a greater proportion of premedical students believed they had a good understanding of the job of a resident physician after the program’s completion compared to before starting the program (an increase from 78% to 100%).10 The proportion of students reporting a good understanding of the patient-physician relationship after completing the program also increased from 33% to 78%. Furthermore, 72% of the residents stated that having the undergraduates in the clinic was a positive experience.10 Thus, increasing shadowing opportunities is one extremely effective way to increase student knowledge and awareness of and exposure to dermatology.
Dermatology Mentors—Although 32% of students were interested in dermatology, 3% of students had mentorship in dermatology. In prior studies, it has been shown that mentorship is of great importance in student success and interest in pursuing a specialty. A report from the Association of American Medical Colleges 2019 Medical School Graduation Questionnaire found that the third most influential factor (52.1%) in specialty selection was role model influence.11 In fact, having a role model is consistently one of the top 3 influences on student specialty choice and interest in the last 5 years of survey research. Some studies also have shown mentorship as a positive influence in specialty interest at the undergraduate and graduate levels. A study on an undergraduate student interest group noted that surgeon mentorship and exposure were positive factors to students’ interests in surgery.12 In fact, the Association of American Medical Colleges noted that some surgical specialties, such as orthopedic surgery, had 45% of respondents who were interested in the specialty before medical school pursue their initial preference in medical school.13 Another survey corroborated these findings; more orthopedic-bound students compared with other specialties indicated they were more likely to pursue their field because of experiences prior to medical school.14
One of the reasons students might not have been exposed to as many opportunities for mentorship in dermatology is because the specialty is one of the smaller fields in medicine and tends to be concentrated in more well-resourced metropolitan areas.15 Dermatologists make up only 1.3% of the physician workforce.16 Because there might not be as much exposure to the field, students might also explore their interests in dermatology through other fields, such as through shadowing and observing primary care physicians who often treat patients with dermatologic issues. Skin diseases are a common reason for primary care visits, and one study suggested dermatologic diseases can make up approximately 8.4% of visits in primary care.17
Moreover, only 1% of medical schools require an elective in dermatology.18 With exposure being a crucial component to pursuing the specialty, it also is important to pursue formal mentorship within the specialty itself. One study noted that formal mentorship in dermatology was important for most (67%) respondents when considering the specialty; however, 39% of respondents mentioned receiving mentorship in the past. In fact, dermatology was one of the top 3 specialties for which respondents agreed that formal mentorship was important.19
Mentorship also has been shown to provide students with a variety of opportunities to develop personally and professionally. Some of these opportunities include increased confidence in their personal and professional success, increased desire to pursue a career in a field of interest, networking opportunities, career coaching, and support and research guidance.20 A research study among medical students at Albert Einstein College of Medicine in New York, New York, found that US Medical Licensing Examination Step 1 scores, clinical grades, and the chance of not matching were important factors preventing them from applying to dermatology.21
Factors in Dermatology Residency Selection—A survey was conducted wherein 95 of 114 dermatology program directors expressed that among the top 5 criteria for dermatology resident selection were Step 1 scores and clinical grades, supporting the notion that academic factors were given a great emphasis during residency selection.22 Furthermore, among underrepresented minority medical students, a lack of diversity, the belief that minority students are seen negatively by residencies, socioeconomic factors, and not having mentors were major reasons for being dissuaded from applying to dermatology.21 These results showcase the heightened importance of mentors for underrepresented minority medical students in particular.
In graduate medical education, resources such as wikis, social networking sites, and blogs provide media through which trainees can communicate, exchange ideas, and enhance their medical knowledge.23,24 A survey of 9606 osteopathic medical students showed that 35% of 992 respondents had used social media to learn more about residencies, and 10% believed that social media had influenced their choice of residency.25 Given the impact social media has on recruitment, it also can be employed in a similar manner by dermatologists and dermatology residency programs to attract younger students to the field.
Access to More Opportunities to Learn About Dermatology—Besides shadowing and mentorship, other avenues of exposure to dermatology are possible and should be considered. In our study, 80% of students agreed that they would attend an event that increases exposure to dermatology if held by the premedical group, which suggests that students are eager to learn more about the field and want access to more opportunities, which could include learning procedures such as suturing or how to use a dermatoscope, attending guest speaker events, or participating in Learn2Derm volunteer events.
Learn2Derm was a skin cancer prevention fair first organized by medical students at George Washington University in Washington, DC. Students and residents sought to deliver sunscreens to underserved areas in Washington, DC, as well as teach residents about the importance of skin health. Participating in such events could be an excellent opportunity for all students to gain exposure to important topics in dermatology.26
General Opinions of Dermatology—General opinions about dermatology and medicine were collected from the students through the optional “Additional Comments” section. Major themes found in the comments included the desire for more opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients/students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty. From these themes, it can be gleaned that students are open to and eager for more opportunities to gain exposure and connections, and increasing the number of minority dermatologists is of importance.
Limitations—An important limitation of this study was the potential for selection bias, as the sample was chosen from a population at one university, which is not representative of the general population. Further, we only sampled students who were premedical and likely from a UiM racial group due to the demographics of the student population at the university, but given that the goal of the survey was to understand exposure to dermatology in underrepresented groups, we believe it was the appropriate population to target. Additionally, results were not compared with other more represented racial groups to see if these findings were unique to UiM undergraduate students.
Conclusion
Among premedical students, dermatology is an area of great interest with minimal opportunities available for exposure and learning because it is a smaller specialty with fewer experiences available for shadowing and mentorship. Although most UiM premedical students who were surveyed were exposed to the field through either the media or being a dermatology patient, fewer were exposed to the field through clinical experiences (such as shadowing) or mentorship. Most respondents found dermatology to be interesting and have considered pursuing it as a career. In particular, race-concordant mentoring in dermatologic care was valued by many students in garnering their interest in the field.
Most UiM students wanted more exposure to dermatology-related opportunities as well as mentorship and connections. Increasing shadowing, research, pipeline programs, and general events geared to dermatology are some modalities that could help improve exposure to dermatology for UiM students, especially for those interested in pursuing the field. This increased exposure can help positively influence more UiM students to pursue dermatology and help close the diversity gap in the field. Additionally, many were interested in attending potential dermatology informational events.
Given the fact that dermatology is a small field and mentorship may be hard to access, increasing informational events may be a more reasonable approach to inspiring and supporting interest. These events could include learning how to use certain tools and techniques, guest speaker events, or participating in educational volunteer efforts such as Learn2Derm.26
Future research should focus on identifying beneficial factors of UiM premedical students who retain an interest in dermatology throughout their careers and actually apply to dermatology programs and become dermatologists. Those who do not apply to the specialty can be identified to understand potential dissuading factors and obstacles. Ultimately, more research and development of exposure opportunities, including mentorship programs and informational events, can be used to close the gap and improve diversity and health outcomes in dermatology.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Bae G, Qiu M, Reese E, et al. Changes in sex and ethnic diversity in dermatology residents over multiple decades. JAMA Dermatol. 2016;152:92-94.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; 2022. Accessed March 19, 2023. https://www.nrmp.org/wp-content/uploads/2022/11/2022-Main-Match-Results-and-Data-Final-Revised.pdf
- 6. Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315.
- Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196.
- Saad SM, Fatima SS, Faruqi AA. Students’ views regarding selecting medicine as a profession. J Pak Med Assoc. 2011;61:832-836.
- Woodward A, Thomas S, Jalloh M, et al. Reasons to pursue a career in medicine: a qualitative study in Sierra Leone. Global Health Res Policy. 2017;2:34.
- Thang C, Barnette NM, Patel KS, et al. Association of shadowing program for undergraduate premedical students with improvements in understanding medical education and training. Cureus. 2019;11:E6396.
- Murphy B. The 11 factors that influence med student specialty choice. American Medical Association. December 1, 2020. Accessed March 14, 2023. https://www.ama-assn.org/residents-students/specialty-profiles/11-factors-influence-med-student-specialty-choice
- Vakayil V, Chandrashekar M, Hedberg J, et al. An undergraduate surgery interest group: introducing premedical students to the practice of surgery. Adv Med Educ Pract. 2020;13:339-349.
- 2021 Report on Residents Executive Summary. Association of American Medical Colleges; 2021. Accessed March 14, 2023. https://www.aamc.org/data-reports/students-residents/data/report-residents/2021/executive-summary
- Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94:e78.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Active Physicians With a U.S. Doctor of Medicine (U.S. MD) Degree by Specialty, 2019. Association of American Medical Colleges; 2019. Accessed March 14, 2023. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
- Rübsam ML, Esch M, Baum E, et al. Diagnosing skin disease in primary care: a qualitative study of GPs’ approaches. Fam Pract. 2015;32:591-595.
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470.
- Mylona E, Brubaker L, Williams VN, et al. Does formal mentoring for faculty members matter? a survey of clinical faculty members. Med Educ. 2016;50:670-681.
- Ratnapalan S. Mentoring in medicine. Can Fam Physician. 2010;56:198.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760.
- Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37:411-416.
- McGowan BS, Wasko M, Vartabedian BS, et al. Understanding the factors that influence the adoption and meaningful use of social media by physicians to share medical information. J Med Internet Res. 2012;14:e117.
- Schweitzer J, Hannan A, Coren J. The role of social networking web sites in influencing residency decisions. J Am Osteopath Assoc. 2012;112:673-679.
- Medical students lead event addressing disparity in skin cancer morbidity and mortality. Dermatology News. August 19, 2021. Accessed March 14, 2023. https://www.mdedge.com/dermatology/article/244488/diversity-medicine/medical-students-lead-event-addressing-disparity-skin
Diversity of health care professionals improves medical outcomes and quality of life in patients. 1 There is a lack of diversity in dermatology, with only 4.2% of dermatologists identifying as Hispanic and 3% identifying as African American, 2 possibly due to a lack of early exposure to dermatology among high school and undergraduate students, a low number of underrepresented students in medical school, a lack of formal mentorship programs geared to underrepresented students, and implicit biases. 1-4 Furthermore, the field is competitive, with many more applicants than available positions. In 2022, there were 851 applicants competing for 492 residency positions in dermatology. 5 Thus, it is important to educate young students about dermatology and understand root causes as to why the number of u nderrepresented in medicine (UiM) dermatologists remains stagnant.
According to Pritchett et al,4 it is crucial for dermatologists to interact with high school and college students to foster an early interest in dermatology. Many racial minority students do not progress from high school to college and then from college to medical school, which leaves a substantially reduced number of eligible UiM applicants who can progress into dermatology.6 Increasing the amount of UiM students going to medical school requires early mediation. Collaborating with pre-existing premedical school organizations through presentations and workshops is another way to promote an early interest in dermatology.4 Special consideration should be given to students who are UiM.
Among the general medical school curriculum, requirements for exposure to dermatology are not high. In one study, the median number of clinical and preclinical hours required was 10. Furthermore, 20% of 33 medical schools did not require preclinical dermatology hours (hours done before medical school rotations begin and in an academic setting), 36% required no clinical hours (rotational hours), 8% required no dermatology hours whatsoever, and only 10% required clinical dermatology rotation.3 Based on these findings, it is clear that dermatology is not well incorporated into medical school curricula. Furthermore, curricula have historically neglected to display adequate representation of skin of color.7 As a result, medical students generally have limited exposure to dermatology3 and are exposed even less to presentations of dermatologic issues in historically marginalized populations.7
Given the paucity of research on UiM students’ perceptions of dermatology prior to medical school, our cross-sectional survey study sought to evaluate the level of interest in dermatology of UiM premedical undergraduates. This survey specifically evaluated exposure to dermatology, preconceived notions about the field, and mentorship opportunities. By understanding these factors, dermatologists and dermatology residency programs can use this information to create mentorship opportunities and better adjust existing programs to meet students’ needs.
Methods
A 19-question multiple-choice survey was administered electronically (SurveyMonkey) in May 2020 to premedical students at Howard University (Washington, DC). One screening question was used: “What is your major?” Those who considered themselves a science major and/or with premedical interest were allowed to complete the survey. All students surveyed were members of the Health Professions Society at Howard University. Students who were interested in pursuing medical school were invited to respond. Approval for this study was obtained from the Howard University institutional review board (FWA00000891).
The survey was divided into 3 sections: Demographics, Exposure to Medicine and Dermatology, and Perceptions of Dermatology. The Demographics section addressed gender, age, and race/ethnicity. The Exposure to Medicine and Dermatology section addressed interest in attending medical school, shadowing experience, exposure to dermatology, and mentoring. The Perceptions of Dermatology section addressed preconceived notions about the field (eg, “dermatology is interesting and exciting”).
Statistical Analysis—The data represented are percentages based on the number of respondents who answered each question. Answers in response to “Please enter any comments” were organized into themes, and the number of respondents who discussed each theme was quantified into a table.
Results
A total of 271 survey invitations were sent to premedical students at Howard University. Students were informed of the study protocol and asked to consent before proceeding to have their responses anonymously collected. Based on the screening question, 152 participants qualified for the survey, and 152 participants completed it (response rate, 56%; completion rate, 100%). Participants were asked to complete the survey only once.
Demographics—Eighty-four percent of respondents identified as science majors, and the remaining 16% identified as nonscience premedical. Ninety-four percent of participants identified as Black or African American; 3% as Asian or Asian American; and the remaining 3% as Other. Most respondents were female (82%), 16% were male, and 2% were either nonbinary or preferred not to answer. Ninety-nine percent were aged 18 to 24 years, and 1% were aged 25 to 34 years (Table 1).

Exposure to Medicine and Dermatology—Ninety-three percent of participants planned on attending medical school, and most students developed an interest in medicine from an early age. Ninety-six percent cited that they became interested in medicine prior to beginning their undergraduate education, and 4% developed an interest as freshmen or sophomores. When asked what led to their interest in medicine, family influence had the single greatest impact on students’ decision to pursue medicine (33%). Classes/school were the second most influential factor (24%), followed by volunteering (15%), shadowing (13%), other (7%), and peer influence (3%)(Figure 1).

Many (56%) premedical students surveyed had shadowing experience to varying degrees. Approximately 18% had fewer than 8 hours of shadowing experience, 24% had 8 to 40 hours, and 14% had more than 40 hours. However, many (43%) premedical students had no shadowing experience (Figure 2). Similarly, 30% of premedical students responded to having a physician as a mentor.

Regarding exposure to dermatology, 42% of premedical students had none. However, 58% of students had exposure to dermatology by being a patient themselves, 40% through seeing a dermatologist with a family member, 21% through seeing a dermatologist on television or social media, 5% through shadowing or volunteering, 3% through mentorship, and 1% through dermatology research (Figure 3).

Of students who said they were interested in dermatology (32%), 16% developed their interest before undergraduate education, while 9% developed interest in their freshman or sophomore year and 7% in their junior or senior year of undergraduate education. Three percent of respondents indicated that they had a dermatology mentorship.
Perceptions of Dermatology—To further evaluate the level of interest that UiM premedical students have in the field of dermatology, students were asked how much they agree or disagree on whether the field of dermatology is interesting. Sixty-three percent of the students agreed that the field of dermatology is interesting, 34% remained uncertain, and 3% disagreed. Additionally, students were asked whether they would consider dermatology as a career; 54% of respondents would consider dermatology as a career, 30% remained uncertain, and 16% would not consider dermatology as a career choice.
Nearly all (95%) students agreed that dermatologists do valuable work that goes beyond the scope of cosmetic procedures such as neuromodulators, fillers, chemical peels, and lasers. Some students also noted they had personal experiences interacting with a dermatologist. For example, one student described visiting the dermatologist many times to get a treatment regimen for their eczema.
Overall themes from the survey are depicted in Table 2. Major themes found in the comments included the desire for more dermatology-related opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients and students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty.

Comment
Interest in Dermatology—In this cross-sectional survey study of 152 UiM undergraduate students, it was found that many students were interested in dermatology as a career, and more than 70% would be interested in attending events that increased exposure to the field of dermatology. Of the students who had any exposure to dermatology, less than 5% had shadowed an actual dermatologist. The survey showed that there is great potential interest in exposing UiM undergraduate students to the field of dermatology. We found that UiM students are interested in learning more about dermatology, with 80% indicating that they would be willing to participate in dermatology-focused events if they were available. Overall, students mentioned a lack of opportunities, mentorship, exposure, and connections in dermatology despite their interest in the field.
Racial Disparities in Dermatology—Additionally, students discussed disparities they encountered with dermatology due to a lack of patient-provider race concordance and the perceived difference in care when encountering a race-concordant dermatologist. One student noted that they went to multiple White dermatologists for their eczema, and “it wasn’t until I was evaluated by a Black dermatologist (diagnosed with eczema as well) [that I was] prescribed . . . the perfect medication.” Another student noted how a Black dermatologist sparked their interest in getting to know more about the field and remarked that they “think it is an important field that lacks representation for Black people.” This research stresses the need for more dermatology mentorship among UiM undergraduates.
Family Influence on Career Selection—The majority of UiM students in our study became interested in medicine because of family, which is consistent with other studies. In a cross-sectional survey of 300 Pakistani students (150 medical and 150 nonmedical), 87% of students stated that their family had an influence on their career selection.8 In another study of 15 junior doctors in Sierra Leone, the most common reasons for pursuing medicine were the desire to help and familial and peer influence.9 This again showcases how family can have a positive impact on career selection for medical professionals and highlights the need for early intervention.
Shadowing—One way in which student exposure to dermatology can be effectively increased is by shadowing. In a study evaluating a 30-week shadowing program at the Pediatric Continuity Clinic in Los Angeles, California, a greater proportion of premedical students believed they had a good understanding of the job of a resident physician after the program’s completion compared to before starting the program (an increase from 78% to 100%).10 The proportion of students reporting a good understanding of the patient-physician relationship after completing the program also increased from 33% to 78%. Furthermore, 72% of the residents stated that having the undergraduates in the clinic was a positive experience.10 Thus, increasing shadowing opportunities is one extremely effective way to increase student knowledge and awareness of and exposure to dermatology.
Dermatology Mentors—Although 32% of students were interested in dermatology, 3% of students had mentorship in dermatology. In prior studies, it has been shown that mentorship is of great importance in student success and interest in pursuing a specialty. A report from the Association of American Medical Colleges 2019 Medical School Graduation Questionnaire found that the third most influential factor (52.1%) in specialty selection was role model influence.11 In fact, having a role model is consistently one of the top 3 influences on student specialty choice and interest in the last 5 years of survey research. Some studies also have shown mentorship as a positive influence in specialty interest at the undergraduate and graduate levels. A study on an undergraduate student interest group noted that surgeon mentorship and exposure were positive factors to students’ interests in surgery.12 In fact, the Association of American Medical Colleges noted that some surgical specialties, such as orthopedic surgery, had 45% of respondents who were interested in the specialty before medical school pursue their initial preference in medical school.13 Another survey corroborated these findings; more orthopedic-bound students compared with other specialties indicated they were more likely to pursue their field because of experiences prior to medical school.14
One of the reasons students might not have been exposed to as many opportunities for mentorship in dermatology is because the specialty is one of the smaller fields in medicine and tends to be concentrated in more well-resourced metropolitan areas.15 Dermatologists make up only 1.3% of the physician workforce.16 Because there might not be as much exposure to the field, students might also explore their interests in dermatology through other fields, such as through shadowing and observing primary care physicians who often treat patients with dermatologic issues. Skin diseases are a common reason for primary care visits, and one study suggested dermatologic diseases can make up approximately 8.4% of visits in primary care.17
Moreover, only 1% of medical schools require an elective in dermatology.18 With exposure being a crucial component to pursuing the specialty, it also is important to pursue formal mentorship within the specialty itself. One study noted that formal mentorship in dermatology was important for most (67%) respondents when considering the specialty; however, 39% of respondents mentioned receiving mentorship in the past. In fact, dermatology was one of the top 3 specialties for which respondents agreed that formal mentorship was important.19
Mentorship also has been shown to provide students with a variety of opportunities to develop personally and professionally. Some of these opportunities include increased confidence in their personal and professional success, increased desire to pursue a career in a field of interest, networking opportunities, career coaching, and support and research guidance.20 A research study among medical students at Albert Einstein College of Medicine in New York, New York, found that US Medical Licensing Examination Step 1 scores, clinical grades, and the chance of not matching were important factors preventing them from applying to dermatology.21
Factors in Dermatology Residency Selection—A survey was conducted wherein 95 of 114 dermatology program directors expressed that among the top 5 criteria for dermatology resident selection were Step 1 scores and clinical grades, supporting the notion that academic factors were given a great emphasis during residency selection.22 Furthermore, among underrepresented minority medical students, a lack of diversity, the belief that minority students are seen negatively by residencies, socioeconomic factors, and not having mentors were major reasons for being dissuaded from applying to dermatology.21 These results showcase the heightened importance of mentors for underrepresented minority medical students in particular.
In graduate medical education, resources such as wikis, social networking sites, and blogs provide media through which trainees can communicate, exchange ideas, and enhance their medical knowledge.23,24 A survey of 9606 osteopathic medical students showed that 35% of 992 respondents had used social media to learn more about residencies, and 10% believed that social media had influenced their choice of residency.25 Given the impact social media has on recruitment, it also can be employed in a similar manner by dermatologists and dermatology residency programs to attract younger students to the field.
Access to More Opportunities to Learn About Dermatology—Besides shadowing and mentorship, other avenues of exposure to dermatology are possible and should be considered. In our study, 80% of students agreed that they would attend an event that increases exposure to dermatology if held by the premedical group, which suggests that students are eager to learn more about the field and want access to more opportunities, which could include learning procedures such as suturing or how to use a dermatoscope, attending guest speaker events, or participating in Learn2Derm volunteer events.
Learn2Derm was a skin cancer prevention fair first organized by medical students at George Washington University in Washington, DC. Students and residents sought to deliver sunscreens to underserved areas in Washington, DC, as well as teach residents about the importance of skin health. Participating in such events could be an excellent opportunity for all students to gain exposure to important topics in dermatology.26
General Opinions of Dermatology—General opinions about dermatology and medicine were collected from the students through the optional “Additional Comments” section. Major themes found in the comments included the desire for more opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients/students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty. From these themes, it can be gleaned that students are open to and eager for more opportunities to gain exposure and connections, and increasing the number of minority dermatologists is of importance.
Limitations—An important limitation of this study was the potential for selection bias, as the sample was chosen from a population at one university, which is not representative of the general population. Further, we only sampled students who were premedical and likely from a UiM racial group due to the demographics of the student population at the university, but given that the goal of the survey was to understand exposure to dermatology in underrepresented groups, we believe it was the appropriate population to target. Additionally, results were not compared with other more represented racial groups to see if these findings were unique to UiM undergraduate students.
Conclusion
Among premedical students, dermatology is an area of great interest with minimal opportunities available for exposure and learning because it is a smaller specialty with fewer experiences available for shadowing and mentorship. Although most UiM premedical students who were surveyed were exposed to the field through either the media or being a dermatology patient, fewer were exposed to the field through clinical experiences (such as shadowing) or mentorship. Most respondents found dermatology to be interesting and have considered pursuing it as a career. In particular, race-concordant mentoring in dermatologic care was valued by many students in garnering their interest in the field.
Most UiM students wanted more exposure to dermatology-related opportunities as well as mentorship and connections. Increasing shadowing, research, pipeline programs, and general events geared to dermatology are some modalities that could help improve exposure to dermatology for UiM students, especially for those interested in pursuing the field. This increased exposure can help positively influence more UiM students to pursue dermatology and help close the diversity gap in the field. Additionally, many were interested in attending potential dermatology informational events.
Given the fact that dermatology is a small field and mentorship may be hard to access, increasing informational events may be a more reasonable approach to inspiring and supporting interest. These events could include learning how to use certain tools and techniques, guest speaker events, or participating in educational volunteer efforts such as Learn2Derm.26
Future research should focus on identifying beneficial factors of UiM premedical students who retain an interest in dermatology throughout their careers and actually apply to dermatology programs and become dermatologists. Those who do not apply to the specialty can be identified to understand potential dissuading factors and obstacles. Ultimately, more research and development of exposure opportunities, including mentorship programs and informational events, can be used to close the gap and improve diversity and health outcomes in dermatology.
Diversity of health care professionals improves medical outcomes and quality of life in patients. 1 There is a lack of diversity in dermatology, with only 4.2% of dermatologists identifying as Hispanic and 3% identifying as African American, 2 possibly due to a lack of early exposure to dermatology among high school and undergraduate students, a low number of underrepresented students in medical school, a lack of formal mentorship programs geared to underrepresented students, and implicit biases. 1-4 Furthermore, the field is competitive, with many more applicants than available positions. In 2022, there were 851 applicants competing for 492 residency positions in dermatology. 5 Thus, it is important to educate young students about dermatology and understand root causes as to why the number of u nderrepresented in medicine (UiM) dermatologists remains stagnant.
According to Pritchett et al,4 it is crucial for dermatologists to interact with high school and college students to foster an early interest in dermatology. Many racial minority students do not progress from high school to college and then from college to medical school, which leaves a substantially reduced number of eligible UiM applicants who can progress into dermatology.6 Increasing the amount of UiM students going to medical school requires early mediation. Collaborating with pre-existing premedical school organizations through presentations and workshops is another way to promote an early interest in dermatology.4 Special consideration should be given to students who are UiM.
Among the general medical school curriculum, requirements for exposure to dermatology are not high. In one study, the median number of clinical and preclinical hours required was 10. Furthermore, 20% of 33 medical schools did not require preclinical dermatology hours (hours done before medical school rotations begin and in an academic setting), 36% required no clinical hours (rotational hours), 8% required no dermatology hours whatsoever, and only 10% required clinical dermatology rotation.3 Based on these findings, it is clear that dermatology is not well incorporated into medical school curricula. Furthermore, curricula have historically neglected to display adequate representation of skin of color.7 As a result, medical students generally have limited exposure to dermatology3 and are exposed even less to presentations of dermatologic issues in historically marginalized populations.7
Given the paucity of research on UiM students’ perceptions of dermatology prior to medical school, our cross-sectional survey study sought to evaluate the level of interest in dermatology of UiM premedical undergraduates. This survey specifically evaluated exposure to dermatology, preconceived notions about the field, and mentorship opportunities. By understanding these factors, dermatologists and dermatology residency programs can use this information to create mentorship opportunities and better adjust existing programs to meet students’ needs.
Methods
A 19-question multiple-choice survey was administered electronically (SurveyMonkey) in May 2020 to premedical students at Howard University (Washington, DC). One screening question was used: “What is your major?” Those who considered themselves a science major and/or with premedical interest were allowed to complete the survey. All students surveyed were members of the Health Professions Society at Howard University. Students who were interested in pursuing medical school were invited to respond. Approval for this study was obtained from the Howard University institutional review board (FWA00000891).
The survey was divided into 3 sections: Demographics, Exposure to Medicine and Dermatology, and Perceptions of Dermatology. The Demographics section addressed gender, age, and race/ethnicity. The Exposure to Medicine and Dermatology section addressed interest in attending medical school, shadowing experience, exposure to dermatology, and mentoring. The Perceptions of Dermatology section addressed preconceived notions about the field (eg, “dermatology is interesting and exciting”).
Statistical Analysis—The data represented are percentages based on the number of respondents who answered each question. Answers in response to “Please enter any comments” were organized into themes, and the number of respondents who discussed each theme was quantified into a table.
Results
A total of 271 survey invitations were sent to premedical students at Howard University. Students were informed of the study protocol and asked to consent before proceeding to have their responses anonymously collected. Based on the screening question, 152 participants qualified for the survey, and 152 participants completed it (response rate, 56%; completion rate, 100%). Participants were asked to complete the survey only once.
Demographics—Eighty-four percent of respondents identified as science majors, and the remaining 16% identified as nonscience premedical. Ninety-four percent of participants identified as Black or African American; 3% as Asian or Asian American; and the remaining 3% as Other. Most respondents were female (82%), 16% were male, and 2% were either nonbinary or preferred not to answer. Ninety-nine percent were aged 18 to 24 years, and 1% were aged 25 to 34 years (Table 1).

Exposure to Medicine and Dermatology—Ninety-three percent of participants planned on attending medical school, and most students developed an interest in medicine from an early age. Ninety-six percent cited that they became interested in medicine prior to beginning their undergraduate education, and 4% developed an interest as freshmen or sophomores. When asked what led to their interest in medicine, family influence had the single greatest impact on students’ decision to pursue medicine (33%). Classes/school were the second most influential factor (24%), followed by volunteering (15%), shadowing (13%), other (7%), and peer influence (3%)(Figure 1).

Many (56%) premedical students surveyed had shadowing experience to varying degrees. Approximately 18% had fewer than 8 hours of shadowing experience, 24% had 8 to 40 hours, and 14% had more than 40 hours. However, many (43%) premedical students had no shadowing experience (Figure 2). Similarly, 30% of premedical students responded to having a physician as a mentor.

Regarding exposure to dermatology, 42% of premedical students had none. However, 58% of students had exposure to dermatology by being a patient themselves, 40% through seeing a dermatologist with a family member, 21% through seeing a dermatologist on television or social media, 5% through shadowing or volunteering, 3% through mentorship, and 1% through dermatology research (Figure 3).

Of students who said they were interested in dermatology (32%), 16% developed their interest before undergraduate education, while 9% developed interest in their freshman or sophomore year and 7% in their junior or senior year of undergraduate education. Three percent of respondents indicated that they had a dermatology mentorship.
Perceptions of Dermatology—To further evaluate the level of interest that UiM premedical students have in the field of dermatology, students were asked how much they agree or disagree on whether the field of dermatology is interesting. Sixty-three percent of the students agreed that the field of dermatology is interesting, 34% remained uncertain, and 3% disagreed. Additionally, students were asked whether they would consider dermatology as a career; 54% of respondents would consider dermatology as a career, 30% remained uncertain, and 16% would not consider dermatology as a career choice.
Nearly all (95%) students agreed that dermatologists do valuable work that goes beyond the scope of cosmetic procedures such as neuromodulators, fillers, chemical peels, and lasers. Some students also noted they had personal experiences interacting with a dermatologist. For example, one student described visiting the dermatologist many times to get a treatment regimen for their eczema.
Overall themes from the survey are depicted in Table 2. Major themes found in the comments included the desire for more dermatology-related opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients and students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty.

Comment
Interest in Dermatology—In this cross-sectional survey study of 152 UiM undergraduate students, it was found that many students were interested in dermatology as a career, and more than 70% would be interested in attending events that increased exposure to the field of dermatology. Of the students who had any exposure to dermatology, less than 5% had shadowed an actual dermatologist. The survey showed that there is great potential interest in exposing UiM undergraduate students to the field of dermatology. We found that UiM students are interested in learning more about dermatology, with 80% indicating that they would be willing to participate in dermatology-focused events if they were available. Overall, students mentioned a lack of opportunities, mentorship, exposure, and connections in dermatology despite their interest in the field.
Racial Disparities in Dermatology—Additionally, students discussed disparities they encountered with dermatology due to a lack of patient-provider race concordance and the perceived difference in care when encountering a race-concordant dermatologist. One student noted that they went to multiple White dermatologists for their eczema, and “it wasn’t until I was evaluated by a Black dermatologist (diagnosed with eczema as well) [that I was] prescribed . . . the perfect medication.” Another student noted how a Black dermatologist sparked their interest in getting to know more about the field and remarked that they “think it is an important field that lacks representation for Black people.” This research stresses the need for more dermatology mentorship among UiM undergraduates.
Family Influence on Career Selection—The majority of UiM students in our study became interested in medicine because of family, which is consistent with other studies. In a cross-sectional survey of 300 Pakistani students (150 medical and 150 nonmedical), 87% of students stated that their family had an influence on their career selection.8 In another study of 15 junior doctors in Sierra Leone, the most common reasons for pursuing medicine were the desire to help and familial and peer influence.9 This again showcases how family can have a positive impact on career selection for medical professionals and highlights the need for early intervention.
Shadowing—One way in which student exposure to dermatology can be effectively increased is by shadowing. In a study evaluating a 30-week shadowing program at the Pediatric Continuity Clinic in Los Angeles, California, a greater proportion of premedical students believed they had a good understanding of the job of a resident physician after the program’s completion compared to before starting the program (an increase from 78% to 100%).10 The proportion of students reporting a good understanding of the patient-physician relationship after completing the program also increased from 33% to 78%. Furthermore, 72% of the residents stated that having the undergraduates in the clinic was a positive experience.10 Thus, increasing shadowing opportunities is one extremely effective way to increase student knowledge and awareness of and exposure to dermatology.
Dermatology Mentors—Although 32% of students were interested in dermatology, 3% of students had mentorship in dermatology. In prior studies, it has been shown that mentorship is of great importance in student success and interest in pursuing a specialty. A report from the Association of American Medical Colleges 2019 Medical School Graduation Questionnaire found that the third most influential factor (52.1%) in specialty selection was role model influence.11 In fact, having a role model is consistently one of the top 3 influences on student specialty choice and interest in the last 5 years of survey research. Some studies also have shown mentorship as a positive influence in specialty interest at the undergraduate and graduate levels. A study on an undergraduate student interest group noted that surgeon mentorship and exposure were positive factors to students’ interests in surgery.12 In fact, the Association of American Medical Colleges noted that some surgical specialties, such as orthopedic surgery, had 45% of respondents who were interested in the specialty before medical school pursue their initial preference in medical school.13 Another survey corroborated these findings; more orthopedic-bound students compared with other specialties indicated they were more likely to pursue their field because of experiences prior to medical school.14
One of the reasons students might not have been exposed to as many opportunities for mentorship in dermatology is because the specialty is one of the smaller fields in medicine and tends to be concentrated in more well-resourced metropolitan areas.15 Dermatologists make up only 1.3% of the physician workforce.16 Because there might not be as much exposure to the field, students might also explore their interests in dermatology through other fields, such as through shadowing and observing primary care physicians who often treat patients with dermatologic issues. Skin diseases are a common reason for primary care visits, and one study suggested dermatologic diseases can make up approximately 8.4% of visits in primary care.17
Moreover, only 1% of medical schools require an elective in dermatology.18 With exposure being a crucial component to pursuing the specialty, it also is important to pursue formal mentorship within the specialty itself. One study noted that formal mentorship in dermatology was important for most (67%) respondents when considering the specialty; however, 39% of respondents mentioned receiving mentorship in the past. In fact, dermatology was one of the top 3 specialties for which respondents agreed that formal mentorship was important.19
Mentorship also has been shown to provide students with a variety of opportunities to develop personally and professionally. Some of these opportunities include increased confidence in their personal and professional success, increased desire to pursue a career in a field of interest, networking opportunities, career coaching, and support and research guidance.20 A research study among medical students at Albert Einstein College of Medicine in New York, New York, found that US Medical Licensing Examination Step 1 scores, clinical grades, and the chance of not matching were important factors preventing them from applying to dermatology.21
Factors in Dermatology Residency Selection—A survey was conducted wherein 95 of 114 dermatology program directors expressed that among the top 5 criteria for dermatology resident selection were Step 1 scores and clinical grades, supporting the notion that academic factors were given a great emphasis during residency selection.22 Furthermore, among underrepresented minority medical students, a lack of diversity, the belief that minority students are seen negatively by residencies, socioeconomic factors, and not having mentors were major reasons for being dissuaded from applying to dermatology.21 These results showcase the heightened importance of mentors for underrepresented minority medical students in particular.
In graduate medical education, resources such as wikis, social networking sites, and blogs provide media through which trainees can communicate, exchange ideas, and enhance their medical knowledge.23,24 A survey of 9606 osteopathic medical students showed that 35% of 992 respondents had used social media to learn more about residencies, and 10% believed that social media had influenced their choice of residency.25 Given the impact social media has on recruitment, it also can be employed in a similar manner by dermatologists and dermatology residency programs to attract younger students to the field.
Access to More Opportunities to Learn About Dermatology—Besides shadowing and mentorship, other avenues of exposure to dermatology are possible and should be considered. In our study, 80% of students agreed that they would attend an event that increases exposure to dermatology if held by the premedical group, which suggests that students are eager to learn more about the field and want access to more opportunities, which could include learning procedures such as suturing or how to use a dermatoscope, attending guest speaker events, or participating in Learn2Derm volunteer events.
Learn2Derm was a skin cancer prevention fair first organized by medical students at George Washington University in Washington, DC. Students and residents sought to deliver sunscreens to underserved areas in Washington, DC, as well as teach residents about the importance of skin health. Participating in such events could be an excellent opportunity for all students to gain exposure to important topics in dermatology.26
General Opinions of Dermatology—General opinions about dermatology and medicine were collected from the students through the optional “Additional Comments” section. Major themes found in the comments included the desire for more opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients/students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty. From these themes, it can be gleaned that students are open to and eager for more opportunities to gain exposure and connections, and increasing the number of minority dermatologists is of importance.
Limitations—An important limitation of this study was the potential for selection bias, as the sample was chosen from a population at one university, which is not representative of the general population. Further, we only sampled students who were premedical and likely from a UiM racial group due to the demographics of the student population at the university, but given that the goal of the survey was to understand exposure to dermatology in underrepresented groups, we believe it was the appropriate population to target. Additionally, results were not compared with other more represented racial groups to see if these findings were unique to UiM undergraduate students.
Conclusion
Among premedical students, dermatology is an area of great interest with minimal opportunities available for exposure and learning because it is a smaller specialty with fewer experiences available for shadowing and mentorship. Although most UiM premedical students who were surveyed were exposed to the field through either the media or being a dermatology patient, fewer were exposed to the field through clinical experiences (such as shadowing) or mentorship. Most respondents found dermatology to be interesting and have considered pursuing it as a career. In particular, race-concordant mentoring in dermatologic care was valued by many students in garnering their interest in the field.
Most UiM students wanted more exposure to dermatology-related opportunities as well as mentorship and connections. Increasing shadowing, research, pipeline programs, and general events geared to dermatology are some modalities that could help improve exposure to dermatology for UiM students, especially for those interested in pursuing the field. This increased exposure can help positively influence more UiM students to pursue dermatology and help close the diversity gap in the field. Additionally, many were interested in attending potential dermatology informational events.
Given the fact that dermatology is a small field and mentorship may be hard to access, increasing informational events may be a more reasonable approach to inspiring and supporting interest. These events could include learning how to use certain tools and techniques, guest speaker events, or participating in educational volunteer efforts such as Learn2Derm.26
Future research should focus on identifying beneficial factors of UiM premedical students who retain an interest in dermatology throughout their careers and actually apply to dermatology programs and become dermatologists. Those who do not apply to the specialty can be identified to understand potential dissuading factors and obstacles. Ultimately, more research and development of exposure opportunities, including mentorship programs and informational events, can be used to close the gap and improve diversity and health outcomes in dermatology.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Bae G, Qiu M, Reese E, et al. Changes in sex and ethnic diversity in dermatology residents over multiple decades. JAMA Dermatol. 2016;152:92-94.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; 2022. Accessed March 19, 2023. https://www.nrmp.org/wp-content/uploads/2022/11/2022-Main-Match-Results-and-Data-Final-Revised.pdf
- 6. Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315.
- Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196.
- Saad SM, Fatima SS, Faruqi AA. Students’ views regarding selecting medicine as a profession. J Pak Med Assoc. 2011;61:832-836.
- Woodward A, Thomas S, Jalloh M, et al. Reasons to pursue a career in medicine: a qualitative study in Sierra Leone. Global Health Res Policy. 2017;2:34.
- Thang C, Barnette NM, Patel KS, et al. Association of shadowing program for undergraduate premedical students with improvements in understanding medical education and training. Cureus. 2019;11:E6396.
- Murphy B. The 11 factors that influence med student specialty choice. American Medical Association. December 1, 2020. Accessed March 14, 2023. https://www.ama-assn.org/residents-students/specialty-profiles/11-factors-influence-med-student-specialty-choice
- Vakayil V, Chandrashekar M, Hedberg J, et al. An undergraduate surgery interest group: introducing premedical students to the practice of surgery. Adv Med Educ Pract. 2020;13:339-349.
- 2021 Report on Residents Executive Summary. Association of American Medical Colleges; 2021. Accessed March 14, 2023. https://www.aamc.org/data-reports/students-residents/data/report-residents/2021/executive-summary
- Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94:e78.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Active Physicians With a U.S. Doctor of Medicine (U.S. MD) Degree by Specialty, 2019. Association of American Medical Colleges; 2019. Accessed March 14, 2023. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
- Rübsam ML, Esch M, Baum E, et al. Diagnosing skin disease in primary care: a qualitative study of GPs’ approaches. Fam Pract. 2015;32:591-595.
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470.
- Mylona E, Brubaker L, Williams VN, et al. Does formal mentoring for faculty members matter? a survey of clinical faculty members. Med Educ. 2016;50:670-681.
- Ratnapalan S. Mentoring in medicine. Can Fam Physician. 2010;56:198.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760.
- Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37:411-416.
- McGowan BS, Wasko M, Vartabedian BS, et al. Understanding the factors that influence the adoption and meaningful use of social media by physicians to share medical information. J Med Internet Res. 2012;14:e117.
- Schweitzer J, Hannan A, Coren J. The role of social networking web sites in influencing residency decisions. J Am Osteopath Assoc. 2012;112:673-679.
- Medical students lead event addressing disparity in skin cancer morbidity and mortality. Dermatology News. August 19, 2021. Accessed March 14, 2023. https://www.mdedge.com/dermatology/article/244488/diversity-medicine/medical-students-lead-event-addressing-disparity-skin
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Bae G, Qiu M, Reese E, et al. Changes in sex and ethnic diversity in dermatology residents over multiple decades. JAMA Dermatol. 2016;152:92-94.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; 2022. Accessed March 19, 2023. https://www.nrmp.org/wp-content/uploads/2022/11/2022-Main-Match-Results-and-Data-Final-Revised.pdf
- 6. Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315.
- Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196.
- Saad SM, Fatima SS, Faruqi AA. Students’ views regarding selecting medicine as a profession. J Pak Med Assoc. 2011;61:832-836.
- Woodward A, Thomas S, Jalloh M, et al. Reasons to pursue a career in medicine: a qualitative study in Sierra Leone. Global Health Res Policy. 2017;2:34.
- Thang C, Barnette NM, Patel KS, et al. Association of shadowing program for undergraduate premedical students with improvements in understanding medical education and training. Cureus. 2019;11:E6396.
- Murphy B. The 11 factors that influence med student specialty choice. American Medical Association. December 1, 2020. Accessed March 14, 2023. https://www.ama-assn.org/residents-students/specialty-profiles/11-factors-influence-med-student-specialty-choice
- Vakayil V, Chandrashekar M, Hedberg J, et al. An undergraduate surgery interest group: introducing premedical students to the practice of surgery. Adv Med Educ Pract. 2020;13:339-349.
- 2021 Report on Residents Executive Summary. Association of American Medical Colleges; 2021. Accessed March 14, 2023. https://www.aamc.org/data-reports/students-residents/data/report-residents/2021/executive-summary
- Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94:e78.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Active Physicians With a U.S. Doctor of Medicine (U.S. MD) Degree by Specialty, 2019. Association of American Medical Colleges; 2019. Accessed March 14, 2023. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
- Rübsam ML, Esch M, Baum E, et al. Diagnosing skin disease in primary care: a qualitative study of GPs’ approaches. Fam Pract. 2015;32:591-595.
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470.
- Mylona E, Brubaker L, Williams VN, et al. Does formal mentoring for faculty members matter? a survey of clinical faculty members. Med Educ. 2016;50:670-681.
- Ratnapalan S. Mentoring in medicine. Can Fam Physician. 2010;56:198.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760.
- Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37:411-416.
- McGowan BS, Wasko M, Vartabedian BS, et al. Understanding the factors that influence the adoption and meaningful use of social media by physicians to share medical information. J Med Internet Res. 2012;14:e117.
- Schweitzer J, Hannan A, Coren J. The role of social networking web sites in influencing residency decisions. J Am Osteopath Assoc. 2012;112:673-679.
- Medical students lead event addressing disparity in skin cancer morbidity and mortality. Dermatology News. August 19, 2021. Accessed March 14, 2023. https://www.mdedge.com/dermatology/article/244488/diversity-medicine/medical-students-lead-event-addressing-disparity-skin
Practice Points
- Many premedical students desire more exposure to dermatology than they have been receiving, particularly in mentorship and shadowing. Most exposure has been through social media or as patients in a dermatology clinic.
- Diverse mentorship and diversity of dermatology care are important to underrepresented in medicine premedical students and needs to be further incorporated.
Relationships Between Residence Characteristics and Nursing Home Compare Database Quality Measures
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18
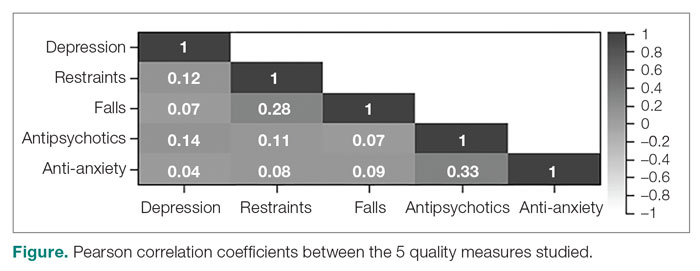
Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).
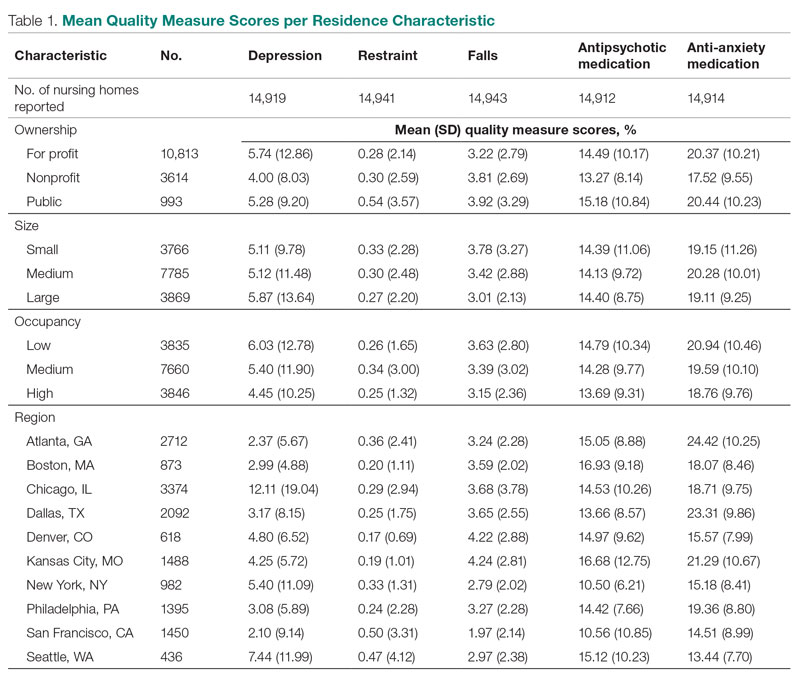
Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.
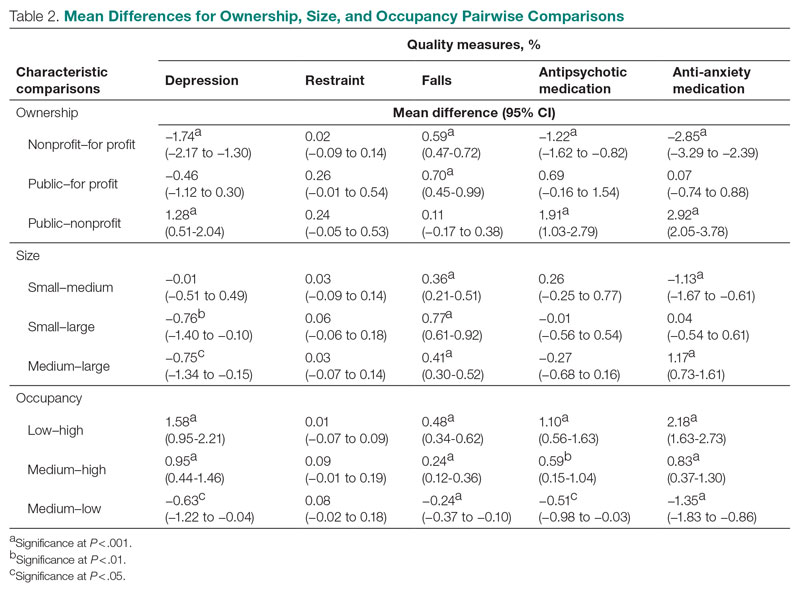
Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.
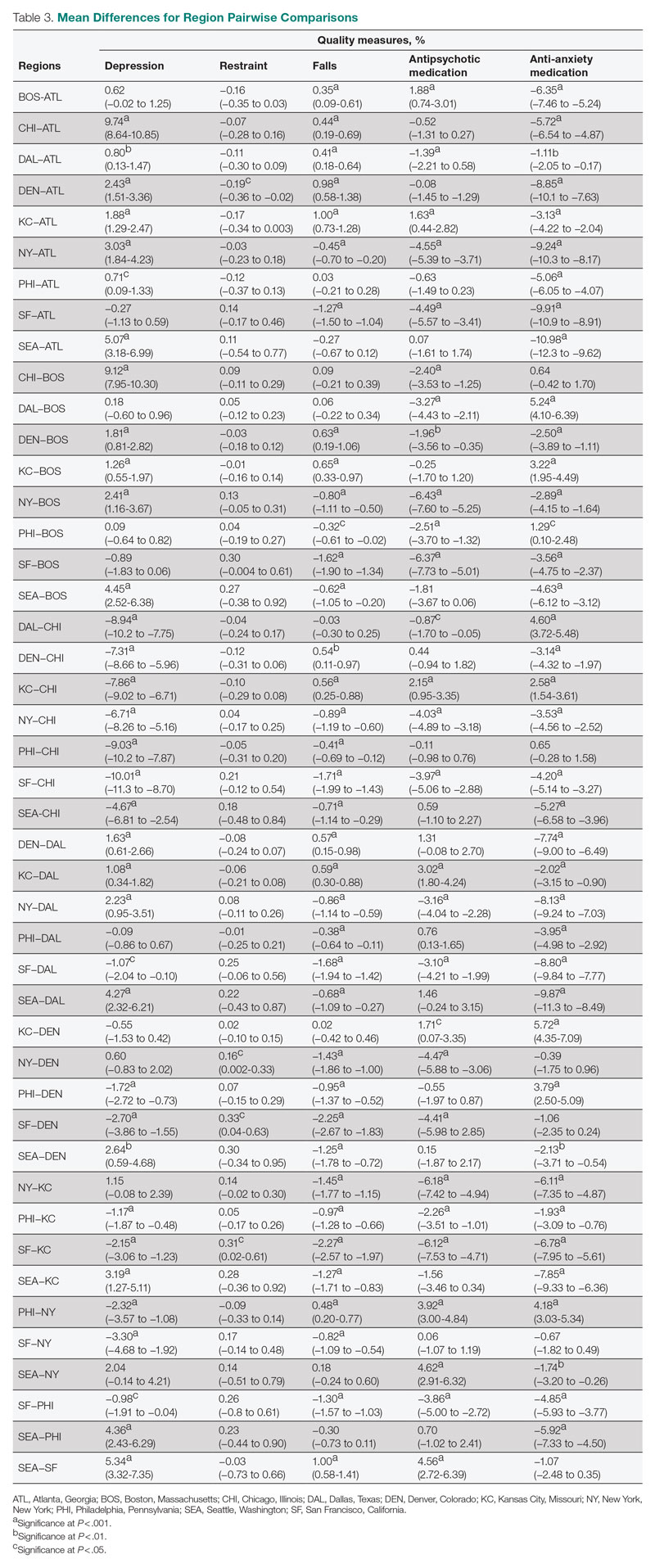
Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, puckett.brian@huskers.unl.edu.
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, puckett.brian@huskers.unl.edu.
Disclosures: None reported.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, puckett.brian@huskers.unl.edu.
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.