User login
Five reasons sacubitril/valsartan should not be approved for HFpEF
In an ideal world, people could afford sacubitril/valsartan (Entresto), and clinicians would be allowed to prescribe it using clinical judgment as their guide. The imprimatur of an “[Food and Drug Administration]–labeled indication” would be unnecessary.
This is not our world. Guideline writers, third-party payers, and FDA regulators now play major roles in clinical decisions.
The angiotensin receptor neprilysin inhibitor is approved for use in patients with heart failure with reduced ejection fraction (HFrEF). In December 2020, an FDA advisory committee voted 12-1 in support of a vaguely worded question: Does PARAGON-HF provide sufficient evidence to support any indication for the drug in patients with heart failure with preserved ejection fraction (HFpEF)? The committee did not reach a consensus on what that indication should be.
Before I list five reasons why I hope the FDA does not approve the drug for any indication in patients with HFpEF, let’s review the seminal trial.
PARAGON-HF
PARAGON-HF randomly assigned slightly more than 4,800 patients with symptomatic HFpEF (left ventricular ejection fraction [LVEF] ≥45%) to sacubitril/valsartan or valsartan alone. The primary endpoint was total hospitalizations for heart failure (HHF) and death because of cardiovascular (CV) events.
Sacubitril/valsartan reduced the rate of the primary endpoint by 13% (rate ratio, 0.87; 95% confidence interval, 0.75-1.01; P = .06). There were 894 primary endpoint events in the sacubitril/valsartan arm, compared with 1,009 events in the valsartan arm.
The lower rate of events in the sacubitril/valsartan arm was driven by fewer hospitalizations for heart failure. CV death was essentially the same in both arms (204 deaths in the sacubitril/valsartan group versus 212 deaths in the valsartan group).
A note on the patients: the investigators screened more than 10,000 patients and enrolled less than half of them. The mean age was 73 years; 52% of patients were women, but only 2% were Black. The mean LVEF was 57%; 95% of patients had hypertension and were receiving diuretics at baseline.
Now to the five reasons not to approve the drug for this indication.
1. Uncertainty of benefit in HFpEF
A P value for the primary endpoint greater than the threshold of .05 suggests some degree of uncertainty. A nice way of describing this uncertainty is with a Bayesian analysis. Whereas a P value tells you the chance of seeing these results if the drug has no benefit, the Bayesian approach tells you the chance of drug benefit given the trial results.
By email, James Brophy, MD, a senior scientist in the Centre for Outcomes Research and Evaluation at McGill University, Montreal, showed me a Bayesian calculation of PARAGON-HF. He estimated a 38% chance that sacubitril/valsartan had a clinically meaningful 15% reduction in the primary endpoint, a 3% chance that it worsens outcomes, and a 58% chance that it is essentially no better than valsartan.
The take-home is that, in PARAGON-HF, a best-case scenario involving select high-risk patients with run-in periods and trial-level follow-up, there is substantial uncertainty as to whether the drug is any better than a generic standard.
2. Modest effect size in PARAGON-HF
Let’s assume the benefit seen in PARAGON-HF is not caused by chance. Was the effect clinically significant?
For context, consider the large effect size that sacubitril/valsartan had versus enalapril for patients with HFrEF.
In PARADIGM-HF, sacubitril/valsartan led to a 20% reduction in the composite primary endpoint. Importantly, this included equal reductions in both HHF and CV death. All-cause death was also significantly reduced in the active arm.
Because patients with HFpEF have a similarly poor prognosis as those with HFrEF, a truly beneficial drug should reduce not only HHF but also CV death and overall death. The lack of effect on these “harder” endpoints in PARAGON-HF points to a far more modest effect size for sacubitril/valsartan in HFpEF.
What’s more, even the signal of reduced HHF in PARAGON-HF is tenuous. The PARAGON-HF authors chose total HHF, whereas previous trials in patients with HFpEF used first HHF as their primary endpoint. Had PARAGON-HF followed the methods of prior trials, first HHF would not have made statistical significance (hazard ratio, 0.90; 95% CI, 0.79-1.04)
3. Subgroups not compelling
Proponents highlight the possibility that sacubitril/valsartan exerted a heterogenous effect in two subgroups.
In women, sacubitril/valsartan resulted in a 27% reduction in the primary endpoint (HR, 0.73; 95% CI, 0.59-0.90), whereas men showed no significant difference (HR, 1.03; 95% CI, 0.85-1.25). And the drug seemed to have little benefit over valsartan in patients with a median LVEF greater than 57%.
The problem with subgroups is that, if you look at enough of them, some can be positive on the basis of chance alone. For instance, patients enrolled in western Europe had an outsized benefit from sacubitril/valsartan, compared with patients from other areas.
FDA reviewers noted: “It is possible that the heterogeneity of treatment effect observed in the subgroups by gender and LVEF in PARAGON-HF is a chance finding.”
By email, clinical trial expert Sanjay Kaul, MD, from Cedars-Sinai Medical Center in Los Angeles, expressed serious concern with the subgroup analyses in PARAGON-HF because the sex interaction was confined to HHF alone. There was no interaction for other outcomes, such as CV death, all-cause mortality, renal endpoints, blood pressure, or lowering of N-terminal of the prohormone brain natriuretic peptide.
Similarly, the interaction with ejection fraction was confined to total HHF; it was not seen with New York Heart Association class improvement, all-cause mortality, quality of life, renal endpoints, or time to first event.
Dr. Kaul also emphasized something cardiologists know well, “that ejection fraction is not a static variable and is expected to change during the course of the trial.” This point makes it hard to believe that a partially subjective measurement, such as LVEF, could be a precise modifier of benefit.
4. Approval would stop research
If the FDA approves sacubitril/valsartan for patients with HFpEF, there is a near-zero chance we will learn whether there are subsets of patients who benefit more or less from the drug.
It will be the defibrillator problem all over again. Namely, while the average effect of a defibrillator is to reduce mortality in patients with HFrEF, in approximately 9 of 10 patients the implanted device is never used. Efforts to find subgroups that are most likely to need (or not need) an implantable defibrillator have been impossible because industry has no incentive to fund trials that may narrow the number of patients who qualify for their product.
It will be the same with sacubitril/valsartan. This is not nefarious; it is merely a limitation of industry funding of trials.
5. Opportunity costs
The category of HFpEF is vast.
FDA approval – even for a subset of these patients – would have huge cost implications. I understand cost issues are considered outside the purview of the FDA, but health care spending isn’t infinite. Money spent covering this costly drug is money not available for other things.
Despite this nation’s wealth, we struggle to provide even basic care to large numbers of people. Approval of an expensive drug with no or modest benefit will only exacerbate these stark disparities.
Conclusion
Given our current system of health care delivery, my pragmatic answer is for the FDA to say no to sacubitril/valsartan for HFpEF.
If you believe the drug has outsized benefits in women or those with mild impairment of systolic function, the way to answer these questions is not with subgroup analyses from a trial that did not reach statistical significance in its primary endpoint, but with more randomized trials. Isn’t that what “exploratory” subgroups are for?
Holding off on an indication for HFpEF will force proponents to define a subset of patients who garner a clear and substantial benefit from sacubitril/valsartan.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. MDedge is part of the Medscape Professional Network.
A version of this article first appeared on Medscape.com.
In an ideal world, people could afford sacubitril/valsartan (Entresto), and clinicians would be allowed to prescribe it using clinical judgment as their guide. The imprimatur of an “[Food and Drug Administration]–labeled indication” would be unnecessary.
This is not our world. Guideline writers, third-party payers, and FDA regulators now play major roles in clinical decisions.
The angiotensin receptor neprilysin inhibitor is approved for use in patients with heart failure with reduced ejection fraction (HFrEF). In December 2020, an FDA advisory committee voted 12-1 in support of a vaguely worded question: Does PARAGON-HF provide sufficient evidence to support any indication for the drug in patients with heart failure with preserved ejection fraction (HFpEF)? The committee did not reach a consensus on what that indication should be.
Before I list five reasons why I hope the FDA does not approve the drug for any indication in patients with HFpEF, let’s review the seminal trial.
PARAGON-HF
PARAGON-HF randomly assigned slightly more than 4,800 patients with symptomatic HFpEF (left ventricular ejection fraction [LVEF] ≥45%) to sacubitril/valsartan or valsartan alone. The primary endpoint was total hospitalizations for heart failure (HHF) and death because of cardiovascular (CV) events.
Sacubitril/valsartan reduced the rate of the primary endpoint by 13% (rate ratio, 0.87; 95% confidence interval, 0.75-1.01; P = .06). There were 894 primary endpoint events in the sacubitril/valsartan arm, compared with 1,009 events in the valsartan arm.
The lower rate of events in the sacubitril/valsartan arm was driven by fewer hospitalizations for heart failure. CV death was essentially the same in both arms (204 deaths in the sacubitril/valsartan group versus 212 deaths in the valsartan group).
A note on the patients: the investigators screened more than 10,000 patients and enrolled less than half of them. The mean age was 73 years; 52% of patients were women, but only 2% were Black. The mean LVEF was 57%; 95% of patients had hypertension and were receiving diuretics at baseline.
Now to the five reasons not to approve the drug for this indication.
1. Uncertainty of benefit in HFpEF
A P value for the primary endpoint greater than the threshold of .05 suggests some degree of uncertainty. A nice way of describing this uncertainty is with a Bayesian analysis. Whereas a P value tells you the chance of seeing these results if the drug has no benefit, the Bayesian approach tells you the chance of drug benefit given the trial results.
By email, James Brophy, MD, a senior scientist in the Centre for Outcomes Research and Evaluation at McGill University, Montreal, showed me a Bayesian calculation of PARAGON-HF. He estimated a 38% chance that sacubitril/valsartan had a clinically meaningful 15% reduction in the primary endpoint, a 3% chance that it worsens outcomes, and a 58% chance that it is essentially no better than valsartan.
The take-home is that, in PARAGON-HF, a best-case scenario involving select high-risk patients with run-in periods and trial-level follow-up, there is substantial uncertainty as to whether the drug is any better than a generic standard.
2. Modest effect size in PARAGON-HF
Let’s assume the benefit seen in PARAGON-HF is not caused by chance. Was the effect clinically significant?
For context, consider the large effect size that sacubitril/valsartan had versus enalapril for patients with HFrEF.
In PARADIGM-HF, sacubitril/valsartan led to a 20% reduction in the composite primary endpoint. Importantly, this included equal reductions in both HHF and CV death. All-cause death was also significantly reduced in the active arm.
Because patients with HFpEF have a similarly poor prognosis as those with HFrEF, a truly beneficial drug should reduce not only HHF but also CV death and overall death. The lack of effect on these “harder” endpoints in PARAGON-HF points to a far more modest effect size for sacubitril/valsartan in HFpEF.
What’s more, even the signal of reduced HHF in PARAGON-HF is tenuous. The PARAGON-HF authors chose total HHF, whereas previous trials in patients with HFpEF used first HHF as their primary endpoint. Had PARAGON-HF followed the methods of prior trials, first HHF would not have made statistical significance (hazard ratio, 0.90; 95% CI, 0.79-1.04)
3. Subgroups not compelling
Proponents highlight the possibility that sacubitril/valsartan exerted a heterogenous effect in two subgroups.
In women, sacubitril/valsartan resulted in a 27% reduction in the primary endpoint (HR, 0.73; 95% CI, 0.59-0.90), whereas men showed no significant difference (HR, 1.03; 95% CI, 0.85-1.25). And the drug seemed to have little benefit over valsartan in patients with a median LVEF greater than 57%.
The problem with subgroups is that, if you look at enough of them, some can be positive on the basis of chance alone. For instance, patients enrolled in western Europe had an outsized benefit from sacubitril/valsartan, compared with patients from other areas.
FDA reviewers noted: “It is possible that the heterogeneity of treatment effect observed in the subgroups by gender and LVEF in PARAGON-HF is a chance finding.”
By email, clinical trial expert Sanjay Kaul, MD, from Cedars-Sinai Medical Center in Los Angeles, expressed serious concern with the subgroup analyses in PARAGON-HF because the sex interaction was confined to HHF alone. There was no interaction for other outcomes, such as CV death, all-cause mortality, renal endpoints, blood pressure, or lowering of N-terminal of the prohormone brain natriuretic peptide.
Similarly, the interaction with ejection fraction was confined to total HHF; it was not seen with New York Heart Association class improvement, all-cause mortality, quality of life, renal endpoints, or time to first event.
Dr. Kaul also emphasized something cardiologists know well, “that ejection fraction is not a static variable and is expected to change during the course of the trial.” This point makes it hard to believe that a partially subjective measurement, such as LVEF, could be a precise modifier of benefit.
4. Approval would stop research
If the FDA approves sacubitril/valsartan for patients with HFpEF, there is a near-zero chance we will learn whether there are subsets of patients who benefit more or less from the drug.
It will be the defibrillator problem all over again. Namely, while the average effect of a defibrillator is to reduce mortality in patients with HFrEF, in approximately 9 of 10 patients the implanted device is never used. Efforts to find subgroups that are most likely to need (or not need) an implantable defibrillator have been impossible because industry has no incentive to fund trials that may narrow the number of patients who qualify for their product.
It will be the same with sacubitril/valsartan. This is not nefarious; it is merely a limitation of industry funding of trials.
5. Opportunity costs
The category of HFpEF is vast.
FDA approval – even for a subset of these patients – would have huge cost implications. I understand cost issues are considered outside the purview of the FDA, but health care spending isn’t infinite. Money spent covering this costly drug is money not available for other things.
Despite this nation’s wealth, we struggle to provide even basic care to large numbers of people. Approval of an expensive drug with no or modest benefit will only exacerbate these stark disparities.
Conclusion
Given our current system of health care delivery, my pragmatic answer is for the FDA to say no to sacubitril/valsartan for HFpEF.
If you believe the drug has outsized benefits in women or those with mild impairment of systolic function, the way to answer these questions is not with subgroup analyses from a trial that did not reach statistical significance in its primary endpoint, but with more randomized trials. Isn’t that what “exploratory” subgroups are for?
Holding off on an indication for HFpEF will force proponents to define a subset of patients who garner a clear and substantial benefit from sacubitril/valsartan.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. MDedge is part of the Medscape Professional Network.
A version of this article first appeared on Medscape.com.
In an ideal world, people could afford sacubitril/valsartan (Entresto), and clinicians would be allowed to prescribe it using clinical judgment as their guide. The imprimatur of an “[Food and Drug Administration]–labeled indication” would be unnecessary.
This is not our world. Guideline writers, third-party payers, and FDA regulators now play major roles in clinical decisions.
The angiotensin receptor neprilysin inhibitor is approved for use in patients with heart failure with reduced ejection fraction (HFrEF). In December 2020, an FDA advisory committee voted 12-1 in support of a vaguely worded question: Does PARAGON-HF provide sufficient evidence to support any indication for the drug in patients with heart failure with preserved ejection fraction (HFpEF)? The committee did not reach a consensus on what that indication should be.
Before I list five reasons why I hope the FDA does not approve the drug for any indication in patients with HFpEF, let’s review the seminal trial.
PARAGON-HF
PARAGON-HF randomly assigned slightly more than 4,800 patients with symptomatic HFpEF (left ventricular ejection fraction [LVEF] ≥45%) to sacubitril/valsartan or valsartan alone. The primary endpoint was total hospitalizations for heart failure (HHF) and death because of cardiovascular (CV) events.
Sacubitril/valsartan reduced the rate of the primary endpoint by 13% (rate ratio, 0.87; 95% confidence interval, 0.75-1.01; P = .06). There were 894 primary endpoint events in the sacubitril/valsartan arm, compared with 1,009 events in the valsartan arm.
The lower rate of events in the sacubitril/valsartan arm was driven by fewer hospitalizations for heart failure. CV death was essentially the same in both arms (204 deaths in the sacubitril/valsartan group versus 212 deaths in the valsartan group).
A note on the patients: the investigators screened more than 10,000 patients and enrolled less than half of them. The mean age was 73 years; 52% of patients were women, but only 2% were Black. The mean LVEF was 57%; 95% of patients had hypertension and were receiving diuretics at baseline.
Now to the five reasons not to approve the drug for this indication.
1. Uncertainty of benefit in HFpEF
A P value for the primary endpoint greater than the threshold of .05 suggests some degree of uncertainty. A nice way of describing this uncertainty is with a Bayesian analysis. Whereas a P value tells you the chance of seeing these results if the drug has no benefit, the Bayesian approach tells you the chance of drug benefit given the trial results.
By email, James Brophy, MD, a senior scientist in the Centre for Outcomes Research and Evaluation at McGill University, Montreal, showed me a Bayesian calculation of PARAGON-HF. He estimated a 38% chance that sacubitril/valsartan had a clinically meaningful 15% reduction in the primary endpoint, a 3% chance that it worsens outcomes, and a 58% chance that it is essentially no better than valsartan.
The take-home is that, in PARAGON-HF, a best-case scenario involving select high-risk patients with run-in periods and trial-level follow-up, there is substantial uncertainty as to whether the drug is any better than a generic standard.
2. Modest effect size in PARAGON-HF
Let’s assume the benefit seen in PARAGON-HF is not caused by chance. Was the effect clinically significant?
For context, consider the large effect size that sacubitril/valsartan had versus enalapril for patients with HFrEF.
In PARADIGM-HF, sacubitril/valsartan led to a 20% reduction in the composite primary endpoint. Importantly, this included equal reductions in both HHF and CV death. All-cause death was also significantly reduced in the active arm.
Because patients with HFpEF have a similarly poor prognosis as those with HFrEF, a truly beneficial drug should reduce not only HHF but also CV death and overall death. The lack of effect on these “harder” endpoints in PARAGON-HF points to a far more modest effect size for sacubitril/valsartan in HFpEF.
What’s more, even the signal of reduced HHF in PARAGON-HF is tenuous. The PARAGON-HF authors chose total HHF, whereas previous trials in patients with HFpEF used first HHF as their primary endpoint. Had PARAGON-HF followed the methods of prior trials, first HHF would not have made statistical significance (hazard ratio, 0.90; 95% CI, 0.79-1.04)
3. Subgroups not compelling
Proponents highlight the possibility that sacubitril/valsartan exerted a heterogenous effect in two subgroups.
In women, sacubitril/valsartan resulted in a 27% reduction in the primary endpoint (HR, 0.73; 95% CI, 0.59-0.90), whereas men showed no significant difference (HR, 1.03; 95% CI, 0.85-1.25). And the drug seemed to have little benefit over valsartan in patients with a median LVEF greater than 57%.
The problem with subgroups is that, if you look at enough of them, some can be positive on the basis of chance alone. For instance, patients enrolled in western Europe had an outsized benefit from sacubitril/valsartan, compared with patients from other areas.
FDA reviewers noted: “It is possible that the heterogeneity of treatment effect observed in the subgroups by gender and LVEF in PARAGON-HF is a chance finding.”
By email, clinical trial expert Sanjay Kaul, MD, from Cedars-Sinai Medical Center in Los Angeles, expressed serious concern with the subgroup analyses in PARAGON-HF because the sex interaction was confined to HHF alone. There was no interaction for other outcomes, such as CV death, all-cause mortality, renal endpoints, blood pressure, or lowering of N-terminal of the prohormone brain natriuretic peptide.
Similarly, the interaction with ejection fraction was confined to total HHF; it was not seen with New York Heart Association class improvement, all-cause mortality, quality of life, renal endpoints, or time to first event.
Dr. Kaul also emphasized something cardiologists know well, “that ejection fraction is not a static variable and is expected to change during the course of the trial.” This point makes it hard to believe that a partially subjective measurement, such as LVEF, could be a precise modifier of benefit.
4. Approval would stop research
If the FDA approves sacubitril/valsartan for patients with HFpEF, there is a near-zero chance we will learn whether there are subsets of patients who benefit more or less from the drug.
It will be the defibrillator problem all over again. Namely, while the average effect of a defibrillator is to reduce mortality in patients with HFrEF, in approximately 9 of 10 patients the implanted device is never used. Efforts to find subgroups that are most likely to need (or not need) an implantable defibrillator have been impossible because industry has no incentive to fund trials that may narrow the number of patients who qualify for their product.
It will be the same with sacubitril/valsartan. This is not nefarious; it is merely a limitation of industry funding of trials.
5. Opportunity costs
The category of HFpEF is vast.
FDA approval – even for a subset of these patients – would have huge cost implications. I understand cost issues are considered outside the purview of the FDA, but health care spending isn’t infinite. Money spent covering this costly drug is money not available for other things.
Despite this nation’s wealth, we struggle to provide even basic care to large numbers of people. Approval of an expensive drug with no or modest benefit will only exacerbate these stark disparities.
Conclusion
Given our current system of health care delivery, my pragmatic answer is for the FDA to say no to sacubitril/valsartan for HFpEF.
If you believe the drug has outsized benefits in women or those with mild impairment of systolic function, the way to answer these questions is not with subgroup analyses from a trial that did not reach statistical significance in its primary endpoint, but with more randomized trials. Isn’t that what “exploratory” subgroups are for?
Holding off on an indication for HFpEF will force proponents to define a subset of patients who garner a clear and substantial benefit from sacubitril/valsartan.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He espouses a conservative approach to medical practice. He participates in clinical research and writes often about the state of medical evidence. MDedge is part of the Medscape Professional Network.
A version of this article first appeared on Medscape.com.
Can ‘big’ be healthy? Yes – and no
While many people were committing to their New Year’s resolutions to lose weight, in January 2020 Cosmopolitan UK magazine released covers portraying 11 women of different shapes and sizes, with the headline, “This is healthy!” Each version of the cover features one or more of the 11 women wearing athletic gear and makeup, some of whom are caught mid-action – boxing, doing yoga, or simply rejoicing in being who they are. Seeing these, I was reminded of a patient I cared for as an intern.
Janet Spears (not her real name) was thin. Standing barely 5 feet 3 inches, she weighed 110 pounds. For those out there who think of size in terms of body mass index (BMI), it was about 20 kg/m2, solidly in the “normal” category. At the age of 62, despite this healthy BMI, she had so much plaque in her arteries that she needed surgery to improve blood flow to her foot.
Admittedly, whenever I had read about people with high cholesterol, type 2 diabetes, or atherosclerosis, I pictured bigger people. But when I met Ms. Spears, I realized that one’s health cannot necessarily be inferred from physical appearance.
As a bariatric surgeon board certified in obesity medicine, I’ve probably spent more time thinking and learning about obesity than most people – and yet I still didn’t know what to make of the Cosmopolitan covers.
I saw the reaction on Twitter before I saw the magazines themselves, and I quickly observed a number of people decrying the covers, suggesting that they promote obesity:
Multiple people suggested that this was inappropriate, especially in the context of the COVID-19 pandemic and the fact that people with obesity are at risk for worse outcomes, compared with those without obesity. (As an aside, these comments suggest that people did not read the associated article, which is about fitness and body image more than it is about obesity.)
Does size reflect health?
Putting the pandemic aside for a moment, the question the magazine covers raise is whether physical appearance reflects health. That’s what got me thinking about Ms. Spears, who, though appearing healthy, was sick enough that she needed to have major surgery. This whole conversation hinges, of course, on one’s definition of health.
A common knee-jerk response, especially from physicians, would be to say that obesity is by definition unhealthy. Some researchers have suggested though that a segment of people with obesity fall into a category called metabolically healthy obesity, which is typically characterized by a limited set of data such as cholesterol, blood sugar, and blood pressure. Indeed, some people with obesity have normal values in those categories.
Being metabolically healthy, however, does not preclude other medical problems associated with obesity, including joint pain, cancer, and mood disorders, among other issues. So even those who have metabolically healthy obesity are not necessarily immune to the many other obesity-related conditions.
What about body positivity?
As I delved further into the conversation about these covers, I saw people embracing the idea of promoting different-sized bodies. With almost two thirds of the U.S. population having overweight or obesity, one might argue that it’s high time magazine covers and the media reflect the reality in our hometowns. Unrealistic images in the media are associated with negative self-image and disordered eating, so perhaps embracing the shapes of real people may help us all have healthier attitudes toward our bodies.
That said, this idea can be taken too far. The Health at Every Size movement, which some might consider to be the ultimate body-positivity movement, espouses the idea that size and health are completely unrelated. That crosses a line between what we know to be true – that, at a population level, higher weight is associated with more medical problems – and fake news.
Another idea to consider is fitness, as opposed to health. Fitness can be defined multiple ways, but if we consider it to be measured exercise capacity, those who are more fit have a longer life expectancy than those with lower fitness levels at a given BMI. While some feel that the Cosmopolitan covers promote obesity and are therefore irresponsible, it’s at least as likely that highlighting people with obesity being active may inspire others with obesity to do the same.
Now let’s bring the pandemic back into the picture. As much as we all wish that it was over, with uncontrolled spread in every state and record numbers of people dying, COVID-19 is still very much a part of our reality. Having obesity increases the risk of having a severe case of COVID-19 if infected. Patients with obesity are also more likely than those without obesity to be hospitalized, require intensive care, and die with COVID-19.
Guiding the conversation
Pandemic or not, the truth is that obesity is related to multiple medical problems. That does not mean that every person with obesity has medical problems. The musician Lizzo, for example, is someone with obesity who considers herself to be healthy. She posts images and videos of working out and shares her personal fitness routine with her millions of fans. As a physician, I worry about the medical conditions – metabolic or otherwise – that someone like her may develop. But I love how she embraces who she is while striving to be healthier.
Most of the critical comments I have seen about the Cosmopolitan covers have, at best, bordered on fat shaming; others are solidly in that category. And the vitriol aimed at the larger models is despicable. It seems that conversations about obesity often vacillate from one extreme (fat shaming) to the other (extreme body positivity).
Although it may not sell magazines, I would love to see more nuanced, fact-based discussions, both in the media and in our clinics. We can start by acknowledging the fact that people of different sizes can be healthy. The truth is that we can’t tell very much about a person’s health from their outward appearance, and we should probably stop trying to make such inferences.
Assessment of health is most accurately judged by each person with their medical team, not by observers who use media images as part of their own propaganda machine, pushing one extreme view or another. As physicians, we have the opportunity and the responsibility to support our patients in the pursuit of health, without shame or judgment. Maybe that’s a New Year’s resolution worth committing to.
Arghavan Salles, MD, PhD, is a bariatric surgeon.
A version of this article first appeared on Medscape.com.
While many people were committing to their New Year’s resolutions to lose weight, in January 2020 Cosmopolitan UK magazine released covers portraying 11 women of different shapes and sizes, with the headline, “This is healthy!” Each version of the cover features one or more of the 11 women wearing athletic gear and makeup, some of whom are caught mid-action – boxing, doing yoga, or simply rejoicing in being who they are. Seeing these, I was reminded of a patient I cared for as an intern.
Janet Spears (not her real name) was thin. Standing barely 5 feet 3 inches, she weighed 110 pounds. For those out there who think of size in terms of body mass index (BMI), it was about 20 kg/m2, solidly in the “normal” category. At the age of 62, despite this healthy BMI, she had so much plaque in her arteries that she needed surgery to improve blood flow to her foot.
Admittedly, whenever I had read about people with high cholesterol, type 2 diabetes, or atherosclerosis, I pictured bigger people. But when I met Ms. Spears, I realized that one’s health cannot necessarily be inferred from physical appearance.
As a bariatric surgeon board certified in obesity medicine, I’ve probably spent more time thinking and learning about obesity than most people – and yet I still didn’t know what to make of the Cosmopolitan covers.
I saw the reaction on Twitter before I saw the magazines themselves, and I quickly observed a number of people decrying the covers, suggesting that they promote obesity:
Multiple people suggested that this was inappropriate, especially in the context of the COVID-19 pandemic and the fact that people with obesity are at risk for worse outcomes, compared with those without obesity. (As an aside, these comments suggest that people did not read the associated article, which is about fitness and body image more than it is about obesity.)
Does size reflect health?
Putting the pandemic aside for a moment, the question the magazine covers raise is whether physical appearance reflects health. That’s what got me thinking about Ms. Spears, who, though appearing healthy, was sick enough that she needed to have major surgery. This whole conversation hinges, of course, on one’s definition of health.
A common knee-jerk response, especially from physicians, would be to say that obesity is by definition unhealthy. Some researchers have suggested though that a segment of people with obesity fall into a category called metabolically healthy obesity, which is typically characterized by a limited set of data such as cholesterol, blood sugar, and blood pressure. Indeed, some people with obesity have normal values in those categories.
Being metabolically healthy, however, does not preclude other medical problems associated with obesity, including joint pain, cancer, and mood disorders, among other issues. So even those who have metabolically healthy obesity are not necessarily immune to the many other obesity-related conditions.
What about body positivity?
As I delved further into the conversation about these covers, I saw people embracing the idea of promoting different-sized bodies. With almost two thirds of the U.S. population having overweight or obesity, one might argue that it’s high time magazine covers and the media reflect the reality in our hometowns. Unrealistic images in the media are associated with negative self-image and disordered eating, so perhaps embracing the shapes of real people may help us all have healthier attitudes toward our bodies.
That said, this idea can be taken too far. The Health at Every Size movement, which some might consider to be the ultimate body-positivity movement, espouses the idea that size and health are completely unrelated. That crosses a line between what we know to be true – that, at a population level, higher weight is associated with more medical problems – and fake news.
Another idea to consider is fitness, as opposed to health. Fitness can be defined multiple ways, but if we consider it to be measured exercise capacity, those who are more fit have a longer life expectancy than those with lower fitness levels at a given BMI. While some feel that the Cosmopolitan covers promote obesity and are therefore irresponsible, it’s at least as likely that highlighting people with obesity being active may inspire others with obesity to do the same.
Now let’s bring the pandemic back into the picture. As much as we all wish that it was over, with uncontrolled spread in every state and record numbers of people dying, COVID-19 is still very much a part of our reality. Having obesity increases the risk of having a severe case of COVID-19 if infected. Patients with obesity are also more likely than those without obesity to be hospitalized, require intensive care, and die with COVID-19.
Guiding the conversation
Pandemic or not, the truth is that obesity is related to multiple medical problems. That does not mean that every person with obesity has medical problems. The musician Lizzo, for example, is someone with obesity who considers herself to be healthy. She posts images and videos of working out and shares her personal fitness routine with her millions of fans. As a physician, I worry about the medical conditions – metabolic or otherwise – that someone like her may develop. But I love how she embraces who she is while striving to be healthier.
Most of the critical comments I have seen about the Cosmopolitan covers have, at best, bordered on fat shaming; others are solidly in that category. And the vitriol aimed at the larger models is despicable. It seems that conversations about obesity often vacillate from one extreme (fat shaming) to the other (extreme body positivity).
Although it may not sell magazines, I would love to see more nuanced, fact-based discussions, both in the media and in our clinics. We can start by acknowledging the fact that people of different sizes can be healthy. The truth is that we can’t tell very much about a person’s health from their outward appearance, and we should probably stop trying to make such inferences.
Assessment of health is most accurately judged by each person with their medical team, not by observers who use media images as part of their own propaganda machine, pushing one extreme view or another. As physicians, we have the opportunity and the responsibility to support our patients in the pursuit of health, without shame or judgment. Maybe that’s a New Year’s resolution worth committing to.
Arghavan Salles, MD, PhD, is a bariatric surgeon.
A version of this article first appeared on Medscape.com.
While many people were committing to their New Year’s resolutions to lose weight, in January 2020 Cosmopolitan UK magazine released covers portraying 11 women of different shapes and sizes, with the headline, “This is healthy!” Each version of the cover features one or more of the 11 women wearing athletic gear and makeup, some of whom are caught mid-action – boxing, doing yoga, or simply rejoicing in being who they are. Seeing these, I was reminded of a patient I cared for as an intern.
Janet Spears (not her real name) was thin. Standing barely 5 feet 3 inches, she weighed 110 pounds. For those out there who think of size in terms of body mass index (BMI), it was about 20 kg/m2, solidly in the “normal” category. At the age of 62, despite this healthy BMI, she had so much plaque in her arteries that she needed surgery to improve blood flow to her foot.
Admittedly, whenever I had read about people with high cholesterol, type 2 diabetes, or atherosclerosis, I pictured bigger people. But when I met Ms. Spears, I realized that one’s health cannot necessarily be inferred from physical appearance.
As a bariatric surgeon board certified in obesity medicine, I’ve probably spent more time thinking and learning about obesity than most people – and yet I still didn’t know what to make of the Cosmopolitan covers.
I saw the reaction on Twitter before I saw the magazines themselves, and I quickly observed a number of people decrying the covers, suggesting that they promote obesity:
Multiple people suggested that this was inappropriate, especially in the context of the COVID-19 pandemic and the fact that people with obesity are at risk for worse outcomes, compared with those without obesity. (As an aside, these comments suggest that people did not read the associated article, which is about fitness and body image more than it is about obesity.)
Does size reflect health?
Putting the pandemic aside for a moment, the question the magazine covers raise is whether physical appearance reflects health. That’s what got me thinking about Ms. Spears, who, though appearing healthy, was sick enough that she needed to have major surgery. This whole conversation hinges, of course, on one’s definition of health.
A common knee-jerk response, especially from physicians, would be to say that obesity is by definition unhealthy. Some researchers have suggested though that a segment of people with obesity fall into a category called metabolically healthy obesity, which is typically characterized by a limited set of data such as cholesterol, blood sugar, and blood pressure. Indeed, some people with obesity have normal values in those categories.
Being metabolically healthy, however, does not preclude other medical problems associated with obesity, including joint pain, cancer, and mood disorders, among other issues. So even those who have metabolically healthy obesity are not necessarily immune to the many other obesity-related conditions.
What about body positivity?
As I delved further into the conversation about these covers, I saw people embracing the idea of promoting different-sized bodies. With almost two thirds of the U.S. population having overweight or obesity, one might argue that it’s high time magazine covers and the media reflect the reality in our hometowns. Unrealistic images in the media are associated with negative self-image and disordered eating, so perhaps embracing the shapes of real people may help us all have healthier attitudes toward our bodies.
That said, this idea can be taken too far. The Health at Every Size movement, which some might consider to be the ultimate body-positivity movement, espouses the idea that size and health are completely unrelated. That crosses a line between what we know to be true – that, at a population level, higher weight is associated with more medical problems – and fake news.
Another idea to consider is fitness, as opposed to health. Fitness can be defined multiple ways, but if we consider it to be measured exercise capacity, those who are more fit have a longer life expectancy than those with lower fitness levels at a given BMI. While some feel that the Cosmopolitan covers promote obesity and are therefore irresponsible, it’s at least as likely that highlighting people with obesity being active may inspire others with obesity to do the same.
Now let’s bring the pandemic back into the picture. As much as we all wish that it was over, with uncontrolled spread in every state and record numbers of people dying, COVID-19 is still very much a part of our reality. Having obesity increases the risk of having a severe case of COVID-19 if infected. Patients with obesity are also more likely than those without obesity to be hospitalized, require intensive care, and die with COVID-19.
Guiding the conversation
Pandemic or not, the truth is that obesity is related to multiple medical problems. That does not mean that every person with obesity has medical problems. The musician Lizzo, for example, is someone with obesity who considers herself to be healthy. She posts images and videos of working out and shares her personal fitness routine with her millions of fans. As a physician, I worry about the medical conditions – metabolic or otherwise – that someone like her may develop. But I love how she embraces who she is while striving to be healthier.
Most of the critical comments I have seen about the Cosmopolitan covers have, at best, bordered on fat shaming; others are solidly in that category. And the vitriol aimed at the larger models is despicable. It seems that conversations about obesity often vacillate from one extreme (fat shaming) to the other (extreme body positivity).
Although it may not sell magazines, I would love to see more nuanced, fact-based discussions, both in the media and in our clinics. We can start by acknowledging the fact that people of different sizes can be healthy. The truth is that we can’t tell very much about a person’s health from their outward appearance, and we should probably stop trying to make such inferences.
Assessment of health is most accurately judged by each person with their medical team, not by observers who use media images as part of their own propaganda machine, pushing one extreme view or another. As physicians, we have the opportunity and the responsibility to support our patients in the pursuit of health, without shame or judgment. Maybe that’s a New Year’s resolution worth committing to.
Arghavan Salles, MD, PhD, is a bariatric surgeon.
A version of this article first appeared on Medscape.com.
Intraoperative rupture of ovarian cancer: Does it worsen outcomes?
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.
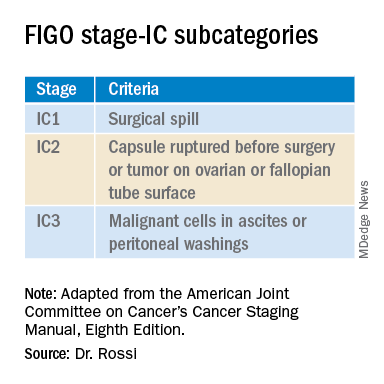
In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.

In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
Intact removal of an ovarian cyst is a well-established gynecologic surgical principle because ovarian cancer is definitively diagnosed only in retrospect (after ovarian extraction) and intraoperative cyst rupture upstages an otherwise nonmetastatic cancer to stage IC. This lumps cancers that are ruptured during surgical extraction together with those that have spontaneously ruptured or have surface excrescences. The theoretical rationale for this “lumping” is that contact between malignant cells from the ruptured cyst may take hold on peritoneal surfaces resulting in development of metastases. To offset this theoretical risk, it has been recommended that all stage IC ovarian cancer is treated with chemotherapy, whereas low-grade stage IA and IB cancers generally are not. No conscientious surgeon wants their surgical intervention to be the cause of a patient needing toxic chemotherapy. But is the contact between malignant cyst fluid and the peritoneum truly as bad as a spontaneous breach of the surface of the tumor? Or is cyst rupture a confounder for other adverse prognostic features, such as histologic cell type and dense pelvic attachments? If ovarian cyst rupture is an independent risk factor for patients with stage I ovarian cancer, strategies should be employed to avoid this occurrence, and we should understand how to counsel and treat patients in whom this has occurred.

In 2017 the International Federation of Gynecology and Obstetrics (FIGO) staging of epithelial ovarian cancer subcategorized stage IC. This group encompasses women with contact between malignant cells and the peritoneum in the absence of other extraovarian disease. The table includes these distinct groupings. Stage IC1 includes patients in whom intraoperative spill occurred. Stage IC2 includes women with preoperative cyst rupture, and or microscopic or macroscopic surface involvement because the data support that these cases carry a poorer prognosis, compared with those with intraoperative rupture (IC1).1 The final subcategory, IC3, includes women who have washings (obtained at the onset of surgery, prior to manipulation of the tumor) that were positive for malignant cells, denoting preexisting contact between the tumor and peritoneum and a phenotypically more aggressive tumor.
The clinical significance of ovarian cancer capsule rupture has been evaluated in multiple studies with some mixed results.1 Consistently, it is reported that preoperative rupture, surface or capsular involvement, and preexisting peritoneal circulation of metastatic cells all portend a poorer prognosis; however, it is less clear that iatrogenic surgical rupture has the same deleterious association. In a large retrospective series from Japan, the authors evaluated 15,163 cases of stage I ovarian cancer and identified 7,227 cases of iatrogenic (intraoperative) cyst rupture.2 These cases were significantly more likely to occur among clear cell cancers, and were more likely to occur in younger patients. Worse prognosis was associated with cell type (clear cell cancers), but non–clear cell cancers (such as serous, mucinous, and endometrioid) did not have a higher hazard ratio for death when intraoperative rupture occurred. But why would intraoperative cyst rupture result in worse prognosis for only one histologic cell type? The authors hypothesized that perhaps rupture was more likely to occur during extraction of these clear cell tumors because they were associated with dense adhesions from associated endometriosis, and perhaps an adverse biologic phenomenon associated with infiltrative endometriosis is driving the behavior of this cancer.
The Japanese study also looked at the effect of chemotherapy on these same patients’ outcomes. Interestingly, the addition of chemotherapy did not improve survival for the patients with stage IC1 cancers, which was in contrast to the improved survival seen when chemotherapy was given to those with spontaneous rupture or ovarian surface involvement (IC2, IC3). These data support differentiating the subgroups of stage IC cancer in treatment decision-making, and suggest that adjuvant chemotherapy might be avoided for patients with nonclear cell stage IC1 ovarian cancer. While the outcomes are worse for patients with ruptured clear cell cancers, current therapeutic options for clear cell cancers are limited because of their known resistance to traditional agents, and outcomes for women with clear cell cancer can be worse across all stages.
While cyst rupture may not always negatively affect prognosis, the goal of surgery remains an intact removal, which influences decisions regarding surgical approach. Most adnexal masses are removed via minimally invasive surgery (MIS). MIS is associated with benefits of morbidity and cost, and therefore should be considered wherever feasible. However, MIS is associated with an increased risk of ovarian cyst rupture, likely because of the rigid instrumentation used when approaching a curved structure, in addition to the disparity in size of the pathology, compared with the extraction site incision.3 When weighing the benefits and risks of different surgical approaches, it is important to gauge the probability of malignancy. Not all complex ovarian masses associated with elevations in tumor markers are malignant, and certainly most that are associated with normal tumor markers are not. If the preoperative clinical data suggest that the mass is more likely to be malignant (e.g., mostly solid, vascular tumors with very elevated tumor markers), consideration might be made to abandoning a purely minimally invasive approach to a hand-assisted MIS or laparotomy approach. However, it would seem that abandoning an MIS approach to remove every ovarian cyst is unwise given that there is clear patient benefit with MIS and, as discussed above, most cases of iatrogenic malignant cyst rupture are unavoidable even with laparotomy, and do not necessarily independently portend poorer survival or mandate chemotherapy.
Surgeons should be both nuanced and flexible and apply some basic rules of thumb when approaching the diagnostically uncertain adnexal mass. Peritoneal washings should be obtained at the commencement of the case to discriminate those cases of true stage IC3. The peritoneum parallel to the ovarian vessel should be extensively opened to a level above the pelvic brim. In order to do this, the physiological attachments between the sigmoid colon or cecum and the suspensory ligament of the ovary may need to be carefully mobilized. This allows for retroperitoneal identification of the ureter and skeletonization of the ovarian vessels at least 2 cm proximal to their insertion into the ovary and avoidance of contact with the ovary itself (which may have a fragile capsule) or incomplete ovarian resection. If the ovary remains invested close to the sidewall or colonic structures and the appropriate peritoneal and retroperitoneal mobilization has not occurred, the surgeon may unavoidably rupture the ovarian cyst as they try to “hug” the ovary with their bites of tissue in an attempt to avoid visceral injury. There is little role for an ovarian cystectomy in a postmenopausal woman undergoing surgery for a complex adnexal mass, particularly if she has elevated tumor markers, because the process of performing ovarian cystectomy commonly invokes cyst rupture or fragmentation. Ovarian cystectomy should be reserved for premenopausal women with adnexal masses at low suspicion for malignancy. If the adnexa appears densely adherent to adjacent structures – for example, associated with infiltrative endometriosis – consideration for laparotomy or a hand-assisted approach may be necessary; in such cases, even open surgery can result in cyst rupture, and the morbidity of conversion to laparotomy should be weighed for individual cases.
Finally, retrieval of the ovarian specimen should occur intact without morcellation. There should be no uncontained morcellation of adnexal structures during retrieval of even normal-appearing ovaries. The preferred retrieval method is to place the adnexa in an appropriately sized retrieval bag, after which contained morcellation or drainage can occur to facilitate removal through a laparoscopic incision. Contained morcellation is very difficult for large solid masses through a laparoscopic port site; in these cases, extension of the incision may be necessary.
While operative spill of an ovarian cancer does upstage nonmetastatic ovarian cancer, it is unclear that, in most cases, this is independently associated with worse prognosis, and chemotherapy may not always be of added value. However, best surgical practice should always include strategies to minimize the chance of rupture when approaching adnexal masses, particularly those at highest likelihood of malignancy.
References
1. Kim HS et al. Eur J Surg Oncol. 2013 Mar 39(3):279-89.
2. Matsuo K et al. Obstet Gynecol. 2019 Nov;134(5):1017-26.
3. Matsuo K et al. JAMA Oncol. 2020 Jul 1;6(7):1110-3.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
ctDNA outperforms CEA in colorectal cancer
Despite standard blood monitoring and routine imaging, patients with colorectal cancer (CRC) tend to have multiple, incurable metastases when relapse occurs.
A new study indicates that postsurgical circulating tumor DNA (ctDNA) testing may improve our ability to predict relapse in patients with stage I-III CRC.
ctDNA testing outperformed carcinoembryonic antigen (CEA) testing in predicting relapse-free survival, and ctDNA was detected about 8 months prior to relapse detection via CT.
Tenna V. Henriksen, of Aarhus University in Denmark, presented these results at the 2021 Gastrointestinal Cancers Symposium (abstract 11).
The multi-institutional study included 260 patients with CRC – 4 with stage I disease, 90 with stage II, and 166 with stage III disease.
Patients were monitored with plasma ctDNA testing within 2 months of primary surgery. Some patients were monitored with follow-up ctDNA sampling every 3 months for an additional 3 years.
Individual tumors and matched germline DNA were interrogated with whole-exome sequencing, and somatic single nucleotide variants were identified. Personalized multiplex PCR assays were developed to track tumor-specific single nucleotide variants via the Signatera® ctDNA assay.
The researchers retrospectively assessed ctDNA’s performance in:
- Stratifying the postoperative risk of relapse.
- Quantifying the benefit of adjuvant chemotherapy in patients who had or did not have ctDNA in plasma.
- Efficiently detecting relapse, in comparison with standard surveillance tests.
Surveillance CT scans were performed at 12 and 36 months but not at the frequency recommended in NCCN guidelines.
In all, 165 patients received adjuvant chemotherapy. The decision to give chemotherapy was made on a clinical basis by treating physicians who were blinded to the results of ctDNA testing.
Results
Of the 260 patients analyzed, 48 relapsed. The median follow-up was 28.4 months overall and 29.9 months in the nonrelapse cases.
The researchers assessed postoperative ctDNA status prior to adjuvant chemotherapy in 218 patients and after adjuvant chemotherapy in 108 patients.
In the prechemotherapy group, 20 patients were ctDNA positive, and 80% of them relapsed. In contrast, 13% of the 198 ctDNA-negative patients relapsed. The hazard ratio (HR) for relapse-free survival was 11 (95% confidence interval, 5.9-21; P < .0001).
After adjuvant chemotherapy, 12.5% of the ctDNA-negative patients relapsed, compared with 83.3% of the ctDNA-positive patients. The HR for relapse-free survival was 12 (95% CI, 4.9-27; P < .0001).
Results from longitudinal ctDNA testing in 202 patients suggested that serial sampling is more useful than sampling at a single point in time. The recurrence rate was 3.4% among patients who remained persistently ctDNA negative, compared with 89.3% in patients who were ctDNA positive. The HR for relapse-free survival was 51 (95% CI, 20-125; P < .0001).
In a subgroup of 29 patients with clinical recurrence detected by CT imaging, ctDNA detection occurred a median of 8.1 months earlier than radiologic relapse.
Among the 197 patients who had serial CEA and ctDNA measurements, longitudinal CEA testing correlated with relapse-free survival (HR, 4.9; 95% CI, 3.2-15, P < .0001) but not nearly as well as ctDNA testing (HR, 95.7; 95% CI, 28-322, P < .0001) in a univariable analysis.
In a multivariable analysis, the HR for relapse-free survival was 1.8 (95% CI, 0.77-4.0; P = .184) for longitudinal CEA and 80.55 (95% CI, 23.1-281; P < .0001) for longitudinal ctDNA.
“[W]hen we pit them against each other in a multivariable analysis, we can see that all the predictive power is in the ctDNA samples,” Ms. Henriksen said. “This indicates that ctDNA is a stronger biomarker compared to CEA, at least with relapse-free survival.”
Availability is not actionability
Study discussant Michael J. Overman, MD, of MD Anderson Cancer Center in Houston, acknowledged that this research substantiates the ability of postoperative ctDNA detection to risk stratify patients with stage III CRC, the predominant stage of participants in the study.
The current results reinforce the researchers’ previously published work (JAMA Oncol. 2019;5[8]:1124-31) and affirm similar findings by other groups (JAMA Oncol. 2019;5[12]:1710-17 and JAMA Oncol. 2019;5[8]:1118-23).
However, Dr. Overman cautioned that “availability is not the same as actionability.”
He also said the “tumor-informed mutation” approach utilized in the Signatera assay differs from the simpler “panel-based” approach, which is also undergoing clinical testing and offers the additional opportunity to test potentially actionable epigenetic targets such as DNA methylation.
Furthermore, the practicality of integrating the tumor-informed mutation approach into the time constraints required in clinical practice was not evaluated in the current analysis.
Dr. Overman pointed out that 16 of the 20 ctDNA-positive patients who received adjuvant chemotherapy sustained a recurrence, so the chemotherapy benefit was lower than expected.
Finally, although ctDNA outperformed CEA in detecting relapse, the greatest impact of ctDNA is its potential to inform the clinician’s decision to escalate and deescalate treatment with impact on survival – a potential that remains unfulfilled.
Next steps
Ms. Henriksen closed her presentation with the perspective that, for serial ctDNA monitoring to be implemented in clinical settings, testing in randomized clinical trials will be needed.
In Denmark, the IMPROVE-IT study is enrolling patients with stage I or low-risk stage II CRC. In this trial, ctDNA-positive patients will receive adjuvant chemotherapy, and ctDNA-negative patients will have longitudinal ctDNA testing but no adjuvant chemotherapy.
A second study, IMPROVE-IT2, will assess the value of ctDNA to direct intensified radiologic surveillance to improve the application of potentially curative treatment for patients with stage II (high-risk) or stage III CRC. Patients with negative ctDNA tests will be followed with longitudinal ctDNA testing only.
In his talk, Dr. Overman highlighted several prospective studies assessing the value of ctDNA testing.
One of these is the ongoing COBRA study (NRG-GI-005), which uses a ctDNA assay (Guardant Lunar-1) for patients with stage IIA CRC for whom standard adjuvant chemotherapy is not indicated.
The patients in COBRA are randomized to active surveillance or assay-directed therapy. Patients assigned to assay-directed therapy have samples analyzed for ctDNA, which would guide the decision about adjuvant chemotherapy. If the postoperative sample is ctDNA positive and the patient accepts adjuvant chemotherapy, the patient could receive one of two standard adjuvant chemotherapy regimens. If ctDNA negative, the patient would be followed with active surveillance alone.
Dr. Overman also highlighted the planned CIRCULATE US trial (NRG-GI008). The aim of this trial is to test intensified adjuvant treatment for stage III CRC patients with positive ctDNA tests. It will employ the Signatera assay.
Ideally, these ongoing trials will provide the evidence base needed for clinicians to optimize adjuvant therapy and surveillance using ctDNA technology.
The current study was sponsored by the Danish Council for Independent Research, The Novo Nordisk Foundation, The Danish Cancer Society, and Natera Inc. Ms. Henriksen disclosed no conflicts of interest. Dr. Overman disclosed relationships with Array BioPharma, Bristol-Myers Squibb, Gritstone Oncology, Janssen, MedImmune, Merck, Novartis, Pfizer, Promega, Roche/Genentech, and Spectrum Pharmaceuticals.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Despite standard blood monitoring and routine imaging, patients with colorectal cancer (CRC) tend to have multiple, incurable metastases when relapse occurs.
A new study indicates that postsurgical circulating tumor DNA (ctDNA) testing may improve our ability to predict relapse in patients with stage I-III CRC.
ctDNA testing outperformed carcinoembryonic antigen (CEA) testing in predicting relapse-free survival, and ctDNA was detected about 8 months prior to relapse detection via CT.
Tenna V. Henriksen, of Aarhus University in Denmark, presented these results at the 2021 Gastrointestinal Cancers Symposium (abstract 11).
The multi-institutional study included 260 patients with CRC – 4 with stage I disease, 90 with stage II, and 166 with stage III disease.
Patients were monitored with plasma ctDNA testing within 2 months of primary surgery. Some patients were monitored with follow-up ctDNA sampling every 3 months for an additional 3 years.
Individual tumors and matched germline DNA were interrogated with whole-exome sequencing, and somatic single nucleotide variants were identified. Personalized multiplex PCR assays were developed to track tumor-specific single nucleotide variants via the Signatera® ctDNA assay.
The researchers retrospectively assessed ctDNA’s performance in:
- Stratifying the postoperative risk of relapse.
- Quantifying the benefit of adjuvant chemotherapy in patients who had or did not have ctDNA in plasma.
- Efficiently detecting relapse, in comparison with standard surveillance tests.
Surveillance CT scans were performed at 12 and 36 months but not at the frequency recommended in NCCN guidelines.
In all, 165 patients received adjuvant chemotherapy. The decision to give chemotherapy was made on a clinical basis by treating physicians who were blinded to the results of ctDNA testing.
Results
Of the 260 patients analyzed, 48 relapsed. The median follow-up was 28.4 months overall and 29.9 months in the nonrelapse cases.
The researchers assessed postoperative ctDNA status prior to adjuvant chemotherapy in 218 patients and after adjuvant chemotherapy in 108 patients.
In the prechemotherapy group, 20 patients were ctDNA positive, and 80% of them relapsed. In contrast, 13% of the 198 ctDNA-negative patients relapsed. The hazard ratio (HR) for relapse-free survival was 11 (95% confidence interval, 5.9-21; P < .0001).
After adjuvant chemotherapy, 12.5% of the ctDNA-negative patients relapsed, compared with 83.3% of the ctDNA-positive patients. The HR for relapse-free survival was 12 (95% CI, 4.9-27; P < .0001).
Results from longitudinal ctDNA testing in 202 patients suggested that serial sampling is more useful than sampling at a single point in time. The recurrence rate was 3.4% among patients who remained persistently ctDNA negative, compared with 89.3% in patients who were ctDNA positive. The HR for relapse-free survival was 51 (95% CI, 20-125; P < .0001).
In a subgroup of 29 patients with clinical recurrence detected by CT imaging, ctDNA detection occurred a median of 8.1 months earlier than radiologic relapse.
Among the 197 patients who had serial CEA and ctDNA measurements, longitudinal CEA testing correlated with relapse-free survival (HR, 4.9; 95% CI, 3.2-15, P < .0001) but not nearly as well as ctDNA testing (HR, 95.7; 95% CI, 28-322, P < .0001) in a univariable analysis.
In a multivariable analysis, the HR for relapse-free survival was 1.8 (95% CI, 0.77-4.0; P = .184) for longitudinal CEA and 80.55 (95% CI, 23.1-281; P < .0001) for longitudinal ctDNA.
“[W]hen we pit them against each other in a multivariable analysis, we can see that all the predictive power is in the ctDNA samples,” Ms. Henriksen said. “This indicates that ctDNA is a stronger biomarker compared to CEA, at least with relapse-free survival.”
Availability is not actionability
Study discussant Michael J. Overman, MD, of MD Anderson Cancer Center in Houston, acknowledged that this research substantiates the ability of postoperative ctDNA detection to risk stratify patients with stage III CRC, the predominant stage of participants in the study.
The current results reinforce the researchers’ previously published work (JAMA Oncol. 2019;5[8]:1124-31) and affirm similar findings by other groups (JAMA Oncol. 2019;5[12]:1710-17 and JAMA Oncol. 2019;5[8]:1118-23).
However, Dr. Overman cautioned that “availability is not the same as actionability.”
He also said the “tumor-informed mutation” approach utilized in the Signatera assay differs from the simpler “panel-based” approach, which is also undergoing clinical testing and offers the additional opportunity to test potentially actionable epigenetic targets such as DNA methylation.
Furthermore, the practicality of integrating the tumor-informed mutation approach into the time constraints required in clinical practice was not evaluated in the current analysis.
Dr. Overman pointed out that 16 of the 20 ctDNA-positive patients who received adjuvant chemotherapy sustained a recurrence, so the chemotherapy benefit was lower than expected.
Finally, although ctDNA outperformed CEA in detecting relapse, the greatest impact of ctDNA is its potential to inform the clinician’s decision to escalate and deescalate treatment with impact on survival – a potential that remains unfulfilled.
Next steps
Ms. Henriksen closed her presentation with the perspective that, for serial ctDNA monitoring to be implemented in clinical settings, testing in randomized clinical trials will be needed.
In Denmark, the IMPROVE-IT study is enrolling patients with stage I or low-risk stage II CRC. In this trial, ctDNA-positive patients will receive adjuvant chemotherapy, and ctDNA-negative patients will have longitudinal ctDNA testing but no adjuvant chemotherapy.
A second study, IMPROVE-IT2, will assess the value of ctDNA to direct intensified radiologic surveillance to improve the application of potentially curative treatment for patients with stage II (high-risk) or stage III CRC. Patients with negative ctDNA tests will be followed with longitudinal ctDNA testing only.
In his talk, Dr. Overman highlighted several prospective studies assessing the value of ctDNA testing.
One of these is the ongoing COBRA study (NRG-GI-005), which uses a ctDNA assay (Guardant Lunar-1) for patients with stage IIA CRC for whom standard adjuvant chemotherapy is not indicated.
The patients in COBRA are randomized to active surveillance or assay-directed therapy. Patients assigned to assay-directed therapy have samples analyzed for ctDNA, which would guide the decision about adjuvant chemotherapy. If the postoperative sample is ctDNA positive and the patient accepts adjuvant chemotherapy, the patient could receive one of two standard adjuvant chemotherapy regimens. If ctDNA negative, the patient would be followed with active surveillance alone.
Dr. Overman also highlighted the planned CIRCULATE US trial (NRG-GI008). The aim of this trial is to test intensified adjuvant treatment for stage III CRC patients with positive ctDNA tests. It will employ the Signatera assay.
Ideally, these ongoing trials will provide the evidence base needed for clinicians to optimize adjuvant therapy and surveillance using ctDNA technology.
The current study was sponsored by the Danish Council for Independent Research, The Novo Nordisk Foundation, The Danish Cancer Society, and Natera Inc. Ms. Henriksen disclosed no conflicts of interest. Dr. Overman disclosed relationships with Array BioPharma, Bristol-Myers Squibb, Gritstone Oncology, Janssen, MedImmune, Merck, Novartis, Pfizer, Promega, Roche/Genentech, and Spectrum Pharmaceuticals.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Despite standard blood monitoring and routine imaging, patients with colorectal cancer (CRC) tend to have multiple, incurable metastases when relapse occurs.
A new study indicates that postsurgical circulating tumor DNA (ctDNA) testing may improve our ability to predict relapse in patients with stage I-III CRC.
ctDNA testing outperformed carcinoembryonic antigen (CEA) testing in predicting relapse-free survival, and ctDNA was detected about 8 months prior to relapse detection via CT.
Tenna V. Henriksen, of Aarhus University in Denmark, presented these results at the 2021 Gastrointestinal Cancers Symposium (abstract 11).
The multi-institutional study included 260 patients with CRC – 4 with stage I disease, 90 with stage II, and 166 with stage III disease.
Patients were monitored with plasma ctDNA testing within 2 months of primary surgery. Some patients were monitored with follow-up ctDNA sampling every 3 months for an additional 3 years.
Individual tumors and matched germline DNA were interrogated with whole-exome sequencing, and somatic single nucleotide variants were identified. Personalized multiplex PCR assays were developed to track tumor-specific single nucleotide variants via the Signatera® ctDNA assay.
The researchers retrospectively assessed ctDNA’s performance in:
- Stratifying the postoperative risk of relapse.
- Quantifying the benefit of adjuvant chemotherapy in patients who had or did not have ctDNA in plasma.
- Efficiently detecting relapse, in comparison with standard surveillance tests.
Surveillance CT scans were performed at 12 and 36 months but not at the frequency recommended in NCCN guidelines.
In all, 165 patients received adjuvant chemotherapy. The decision to give chemotherapy was made on a clinical basis by treating physicians who were blinded to the results of ctDNA testing.
Results
Of the 260 patients analyzed, 48 relapsed. The median follow-up was 28.4 months overall and 29.9 months in the nonrelapse cases.
The researchers assessed postoperative ctDNA status prior to adjuvant chemotherapy in 218 patients and after adjuvant chemotherapy in 108 patients.
In the prechemotherapy group, 20 patients were ctDNA positive, and 80% of them relapsed. In contrast, 13% of the 198 ctDNA-negative patients relapsed. The hazard ratio (HR) for relapse-free survival was 11 (95% confidence interval, 5.9-21; P < .0001).
After adjuvant chemotherapy, 12.5% of the ctDNA-negative patients relapsed, compared with 83.3% of the ctDNA-positive patients. The HR for relapse-free survival was 12 (95% CI, 4.9-27; P < .0001).
Results from longitudinal ctDNA testing in 202 patients suggested that serial sampling is more useful than sampling at a single point in time. The recurrence rate was 3.4% among patients who remained persistently ctDNA negative, compared with 89.3% in patients who were ctDNA positive. The HR for relapse-free survival was 51 (95% CI, 20-125; P < .0001).
In a subgroup of 29 patients with clinical recurrence detected by CT imaging, ctDNA detection occurred a median of 8.1 months earlier than radiologic relapse.
Among the 197 patients who had serial CEA and ctDNA measurements, longitudinal CEA testing correlated with relapse-free survival (HR, 4.9; 95% CI, 3.2-15, P < .0001) but not nearly as well as ctDNA testing (HR, 95.7; 95% CI, 28-322, P < .0001) in a univariable analysis.
In a multivariable analysis, the HR for relapse-free survival was 1.8 (95% CI, 0.77-4.0; P = .184) for longitudinal CEA and 80.55 (95% CI, 23.1-281; P < .0001) for longitudinal ctDNA.
“[W]hen we pit them against each other in a multivariable analysis, we can see that all the predictive power is in the ctDNA samples,” Ms. Henriksen said. “This indicates that ctDNA is a stronger biomarker compared to CEA, at least with relapse-free survival.”
Availability is not actionability
Study discussant Michael J. Overman, MD, of MD Anderson Cancer Center in Houston, acknowledged that this research substantiates the ability of postoperative ctDNA detection to risk stratify patients with stage III CRC, the predominant stage of participants in the study.
The current results reinforce the researchers’ previously published work (JAMA Oncol. 2019;5[8]:1124-31) and affirm similar findings by other groups (JAMA Oncol. 2019;5[12]:1710-17 and JAMA Oncol. 2019;5[8]:1118-23).
However, Dr. Overman cautioned that “availability is not the same as actionability.”
He also said the “tumor-informed mutation” approach utilized in the Signatera assay differs from the simpler “panel-based” approach, which is also undergoing clinical testing and offers the additional opportunity to test potentially actionable epigenetic targets such as DNA methylation.
Furthermore, the practicality of integrating the tumor-informed mutation approach into the time constraints required in clinical practice was not evaluated in the current analysis.
Dr. Overman pointed out that 16 of the 20 ctDNA-positive patients who received adjuvant chemotherapy sustained a recurrence, so the chemotherapy benefit was lower than expected.
Finally, although ctDNA outperformed CEA in detecting relapse, the greatest impact of ctDNA is its potential to inform the clinician’s decision to escalate and deescalate treatment with impact on survival – a potential that remains unfulfilled.
Next steps
Ms. Henriksen closed her presentation with the perspective that, for serial ctDNA monitoring to be implemented in clinical settings, testing in randomized clinical trials will be needed.
In Denmark, the IMPROVE-IT study is enrolling patients with stage I or low-risk stage II CRC. In this trial, ctDNA-positive patients will receive adjuvant chemotherapy, and ctDNA-negative patients will have longitudinal ctDNA testing but no adjuvant chemotherapy.
A second study, IMPROVE-IT2, will assess the value of ctDNA to direct intensified radiologic surveillance to improve the application of potentially curative treatment for patients with stage II (high-risk) or stage III CRC. Patients with negative ctDNA tests will be followed with longitudinal ctDNA testing only.
In his talk, Dr. Overman highlighted several prospective studies assessing the value of ctDNA testing.
One of these is the ongoing COBRA study (NRG-GI-005), which uses a ctDNA assay (Guardant Lunar-1) for patients with stage IIA CRC for whom standard adjuvant chemotherapy is not indicated.
The patients in COBRA are randomized to active surveillance or assay-directed therapy. Patients assigned to assay-directed therapy have samples analyzed for ctDNA, which would guide the decision about adjuvant chemotherapy. If the postoperative sample is ctDNA positive and the patient accepts adjuvant chemotherapy, the patient could receive one of two standard adjuvant chemotherapy regimens. If ctDNA negative, the patient would be followed with active surveillance alone.
Dr. Overman also highlighted the planned CIRCULATE US trial (NRG-GI008). The aim of this trial is to test intensified adjuvant treatment for stage III CRC patients with positive ctDNA tests. It will employ the Signatera assay.
Ideally, these ongoing trials will provide the evidence base needed for clinicians to optimize adjuvant therapy and surveillance using ctDNA technology.
The current study was sponsored by the Danish Council for Independent Research, The Novo Nordisk Foundation, The Danish Cancer Society, and Natera Inc. Ms. Henriksen disclosed no conflicts of interest. Dr. Overman disclosed relationships with Array BioPharma, Bristol-Myers Squibb, Gritstone Oncology, Janssen, MedImmune, Merck, Novartis, Pfizer, Promega, Roche/Genentech, and Spectrum Pharmaceuticals.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM GI Cancers Symposium 2021
Colonoscopy prep suggestions for those who hate it
A 61-year-old man is seen for a primary care visit. He has a history of colonic polyps (tubular adenoma) on two previous colonoscopies (at age 50 and 55). He has been on an appropriate 5-year schedule, but is overdue for his colonoscopy. He did not follow up with messages from his gastroenterologist for scheduling his colonoscopy last year. He explains he really hates the whole preparation for colonoscopy, but does realize he needs to follow up, and is willing to do so now. What do you recommend for colonoscopy prep?
A) Diet as usual until 5 p.m. day before, then clear liquid diet. Start GoLYTELY (1 gallon) night before procedure.
B) Low-fiber diet X2 days, clear liquid diet day before procedure, GoLYTELY (1 gallon) night before procedure.
C) Low residue diet X3 days, SUPREP the night before the procedure.
D) Low residue diet X2 days, followed by clear liquid diet the day before the procedure, SUPREP the night before the procedure.
It is common for patients to be reluctant to follow recommendations for colonoscopy due to dreading the prep. I would recommend choice C here, as the least difficult bowel preparation for colonoscopy.
Gastroenterologists are usually the ones to recommend the bowel prep that they want their patients to follow.
Major diet change for several days before colonoscopy is difficult for many patients. Standard advice is that patients eat only low-fiber foods starting 3 days before the procedure. Patients are advised to switch to a completely clear liquid diet 1-2 days before the colonoscopy.
Are there more tolerable diets to offer patients?
Soweid and colleagues randomized 200 patients to a low residue diet for the three meals the day before colonoscopy vs. clear liquid diet.1 The low residue diet allowed patients to eat meat, eggs, cheese, bread, rice, and ice cream. Not surprisingly, patients tolerated the low residue diet better with statistically significantly less nausea, vomiting, weakness, headache, sleep difficulties, and hunger. The patients in the low residue diet group also had better bowel prep than did those in the clear liquid diet group (81% vs. 52%, P less than 0.001).1
In a recent meta- analysis, low residue diets were comparable to clear liquid diets in regard to adequacy of bowel prep and for detection of polyps.2 Patients who followed low residue diets had statistically significantly less headaches, nausea, vomiting, and hunger. Very importantly, patients who followed low residue diets showed an increased willingness to repeat it, compared with those who followed a clear liquid diet (P less than .005; odds ratio, 2.23; 95% confidence interval, 1.28-3.89).2
What alternatives to GoLYTELY exist?
Another part of the bowel prep that patients struggle with is drinking a gallon of GoLYTELY (polyethylene glycol/electrolytes). Drinking that amount of this nasty stuff is never welcome.
There are a number of lower-volume alternatives that are as effective as GoLYTELY. Sarvepalli and colleagues did a retrospective study of 75,874 patients who had a colonoscopy in the Cleveland Clinic health system.3 The choice of bowel prep was not associated with adenoma detection.
Patients who lower volume preparations (2 quarts) SUPREP, MoviPrep, Osmoprep and HalfLytely had varying results of rates of inadequate bowel prep compared with patients who took GoLYTELY. Results for patients taking SUPREP and MoviPrep were statistically significantly better than for patients taking GoLYTELY. Results for patients taking OsmoPrep were not statistically different from those for patients taking GoLYTELY. Rates of inadequate bowel prep were statistically higher, meaning worse, for patients taking HalfLytely vs. patients taking GoLYTELY.3
Gu and colleagues did a prospective study of bowel prep outcomes from 4,339 colonoscopies, involving 75 different endoscopists.4 There was a wide range of bowel preps used, including low- and high-volume bowel preps. The low-volume preparations, SUPREP (P less than .001), MoviPrep (P less than .004) and MiraLAX with Gatorade (P less than .001), were superior to GoLYTELY for bowel cleansing. This was based on scoring via the Boston Bowel Preparation Scale. All were better tolerated than GoLYTELY.
Myth: All patients need a clear liquid diet and GoLYTELY for their bowel prep.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Soweid AM et al. A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy 2010;42:633-8.
2. Zhang X et al. Low-[residue] diet versus clear-liquid diet for bowel preparation before colonoscopy: meta-analysis and trial sequential analysis of randomized controlled trials. Gastrointest Endosc. 2020 Sep;92(3):508-18.
3. Sarvepalli S et al. Comparative effectiveness of commercial bowel preparations in ambulatory patients presenting for screening or surveillance colonoscopy. Dig Dis Sci. 2020 Jul 20. doi: 10.1007/s10620-020-06492-z.
4. Gu P et al. Comparing the real-world effectiveness of competing colonoscopy preparations: results of a prospective trial. Am J Gastroenterol. 2019;114(2):305-14.
A 61-year-old man is seen for a primary care visit. He has a history of colonic polyps (tubular adenoma) on two previous colonoscopies (at age 50 and 55). He has been on an appropriate 5-year schedule, but is overdue for his colonoscopy. He did not follow up with messages from his gastroenterologist for scheduling his colonoscopy last year. He explains he really hates the whole preparation for colonoscopy, but does realize he needs to follow up, and is willing to do so now. What do you recommend for colonoscopy prep?
A) Diet as usual until 5 p.m. day before, then clear liquid diet. Start GoLYTELY (1 gallon) night before procedure.
B) Low-fiber diet X2 days, clear liquid diet day before procedure, GoLYTELY (1 gallon) night before procedure.
C) Low residue diet X3 days, SUPREP the night before the procedure.
D) Low residue diet X2 days, followed by clear liquid diet the day before the procedure, SUPREP the night before the procedure.
It is common for patients to be reluctant to follow recommendations for colonoscopy due to dreading the prep. I would recommend choice C here, as the least difficult bowel preparation for colonoscopy.
Gastroenterologists are usually the ones to recommend the bowel prep that they want their patients to follow.
Major diet change for several days before colonoscopy is difficult for many patients. Standard advice is that patients eat only low-fiber foods starting 3 days before the procedure. Patients are advised to switch to a completely clear liquid diet 1-2 days before the colonoscopy.
Are there more tolerable diets to offer patients?
Soweid and colleagues randomized 200 patients to a low residue diet for the three meals the day before colonoscopy vs. clear liquid diet.1 The low residue diet allowed patients to eat meat, eggs, cheese, bread, rice, and ice cream. Not surprisingly, patients tolerated the low residue diet better with statistically significantly less nausea, vomiting, weakness, headache, sleep difficulties, and hunger. The patients in the low residue diet group also had better bowel prep than did those in the clear liquid diet group (81% vs. 52%, P less than 0.001).1
In a recent meta- analysis, low residue diets were comparable to clear liquid diets in regard to adequacy of bowel prep and for detection of polyps.2 Patients who followed low residue diets had statistically significantly less headaches, nausea, vomiting, and hunger. Very importantly, patients who followed low residue diets showed an increased willingness to repeat it, compared with those who followed a clear liquid diet (P less than .005; odds ratio, 2.23; 95% confidence interval, 1.28-3.89).2
What alternatives to GoLYTELY exist?
Another part of the bowel prep that patients struggle with is drinking a gallon of GoLYTELY (polyethylene glycol/electrolytes). Drinking that amount of this nasty stuff is never welcome.
There are a number of lower-volume alternatives that are as effective as GoLYTELY. Sarvepalli and colleagues did a retrospective study of 75,874 patients who had a colonoscopy in the Cleveland Clinic health system.3 The choice of bowel prep was not associated with adenoma detection.
Patients who lower volume preparations (2 quarts) SUPREP, MoviPrep, Osmoprep and HalfLytely had varying results of rates of inadequate bowel prep compared with patients who took GoLYTELY. Results for patients taking SUPREP and MoviPrep were statistically significantly better than for patients taking GoLYTELY. Results for patients taking OsmoPrep were not statistically different from those for patients taking GoLYTELY. Rates of inadequate bowel prep were statistically higher, meaning worse, for patients taking HalfLytely vs. patients taking GoLYTELY.3
Gu and colleagues did a prospective study of bowel prep outcomes from 4,339 colonoscopies, involving 75 different endoscopists.4 There was a wide range of bowel preps used, including low- and high-volume bowel preps. The low-volume preparations, SUPREP (P less than .001), MoviPrep (P less than .004) and MiraLAX with Gatorade (P less than .001), were superior to GoLYTELY for bowel cleansing. This was based on scoring via the Boston Bowel Preparation Scale. All were better tolerated than GoLYTELY.
Myth: All patients need a clear liquid diet and GoLYTELY for their bowel prep.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Soweid AM et al. A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy 2010;42:633-8.
2. Zhang X et al. Low-[residue] diet versus clear-liquid diet for bowel preparation before colonoscopy: meta-analysis and trial sequential analysis of randomized controlled trials. Gastrointest Endosc. 2020 Sep;92(3):508-18.
3. Sarvepalli S et al. Comparative effectiveness of commercial bowel preparations in ambulatory patients presenting for screening or surveillance colonoscopy. Dig Dis Sci. 2020 Jul 20. doi: 10.1007/s10620-020-06492-z.
4. Gu P et al. Comparing the real-world effectiveness of competing colonoscopy preparations: results of a prospective trial. Am J Gastroenterol. 2019;114(2):305-14.
A 61-year-old man is seen for a primary care visit. He has a history of colonic polyps (tubular adenoma) on two previous colonoscopies (at age 50 and 55). He has been on an appropriate 5-year schedule, but is overdue for his colonoscopy. He did not follow up with messages from his gastroenterologist for scheduling his colonoscopy last year. He explains he really hates the whole preparation for colonoscopy, but does realize he needs to follow up, and is willing to do so now. What do you recommend for colonoscopy prep?
A) Diet as usual until 5 p.m. day before, then clear liquid diet. Start GoLYTELY (1 gallon) night before procedure.
B) Low-fiber diet X2 days, clear liquid diet day before procedure, GoLYTELY (1 gallon) night before procedure.
C) Low residue diet X3 days, SUPREP the night before the procedure.
D) Low residue diet X2 days, followed by clear liquid diet the day before the procedure, SUPREP the night before the procedure.
It is common for patients to be reluctant to follow recommendations for colonoscopy due to dreading the prep. I would recommend choice C here, as the least difficult bowel preparation for colonoscopy.
Gastroenterologists are usually the ones to recommend the bowel prep that they want their patients to follow.
Major diet change for several days before colonoscopy is difficult for many patients. Standard advice is that patients eat only low-fiber foods starting 3 days before the procedure. Patients are advised to switch to a completely clear liquid diet 1-2 days before the colonoscopy.
Are there more tolerable diets to offer patients?
Soweid and colleagues randomized 200 patients to a low residue diet for the three meals the day before colonoscopy vs. clear liquid diet.1 The low residue diet allowed patients to eat meat, eggs, cheese, bread, rice, and ice cream. Not surprisingly, patients tolerated the low residue diet better with statistically significantly less nausea, vomiting, weakness, headache, sleep difficulties, and hunger. The patients in the low residue diet group also had better bowel prep than did those in the clear liquid diet group (81% vs. 52%, P less than 0.001).1
In a recent meta- analysis, low residue diets were comparable to clear liquid diets in regard to adequacy of bowel prep and for detection of polyps.2 Patients who followed low residue diets had statistically significantly less headaches, nausea, vomiting, and hunger. Very importantly, patients who followed low residue diets showed an increased willingness to repeat it, compared with those who followed a clear liquid diet (P less than .005; odds ratio, 2.23; 95% confidence interval, 1.28-3.89).2
What alternatives to GoLYTELY exist?
Another part of the bowel prep that patients struggle with is drinking a gallon of GoLYTELY (polyethylene glycol/electrolytes). Drinking that amount of this nasty stuff is never welcome.
There are a number of lower-volume alternatives that are as effective as GoLYTELY. Sarvepalli and colleagues did a retrospective study of 75,874 patients who had a colonoscopy in the Cleveland Clinic health system.3 The choice of bowel prep was not associated with adenoma detection.
Patients who lower volume preparations (2 quarts) SUPREP, MoviPrep, Osmoprep and HalfLytely had varying results of rates of inadequate bowel prep compared with patients who took GoLYTELY. Results for patients taking SUPREP and MoviPrep were statistically significantly better than for patients taking GoLYTELY. Results for patients taking OsmoPrep were not statistically different from those for patients taking GoLYTELY. Rates of inadequate bowel prep were statistically higher, meaning worse, for patients taking HalfLytely vs. patients taking GoLYTELY.3
Gu and colleagues did a prospective study of bowel prep outcomes from 4,339 colonoscopies, involving 75 different endoscopists.4 There was a wide range of bowel preps used, including low- and high-volume bowel preps. The low-volume preparations, SUPREP (P less than .001), MoviPrep (P less than .004) and MiraLAX with Gatorade (P less than .001), were superior to GoLYTELY for bowel cleansing. This was based on scoring via the Boston Bowel Preparation Scale. All were better tolerated than GoLYTELY.
Myth: All patients need a clear liquid diet and GoLYTELY for their bowel prep.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Soweid AM et al. A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy 2010;42:633-8.
2. Zhang X et al. Low-[residue] diet versus clear-liquid diet for bowel preparation before colonoscopy: meta-analysis and trial sequential analysis of randomized controlled trials. Gastrointest Endosc. 2020 Sep;92(3):508-18.
3. Sarvepalli S et al. Comparative effectiveness of commercial bowel preparations in ambulatory patients presenting for screening or surveillance colonoscopy. Dig Dis Sci. 2020 Jul 20. doi: 10.1007/s10620-020-06492-z.
4. Gu P et al. Comparing the real-world effectiveness of competing colonoscopy preparations: results of a prospective trial. Am J Gastroenterol. 2019;114(2):305-14.
Reproductive psychiatry in 2021: Old questions and new challenges
Across this period of the pandemic, we’ve spent considerable attention focusing on adaptations to clinical care for pregnant and postpartum women across the board. From the start, this has included a shift to telemedicine for the majority of our patients who come to see us with psychiatric disorders either before, during, or after pregnancy.
Specific issues for perinatal patients since the early days of COVID-19 have included the shifts in women’s plans with respect to delivery as well as the limitation on women’s ability to configure the types of support that they had originally planned on with family, friends, and others during delivery. Telemedicine again helped, at least in part, to fill that void by having online digital support by individuals or groups for both pregnant and postpartum women. These supports were always available, but quickly scaled up during the first 6-9 months of the pandemic and have likely seen their greatest increase in participation in the history of support groups for pregnant and postpartum women.
Similarly, at our own center, we have seen a dramatic increase across the last 10 months in requests for consultation by women with psychiatric disorders who have hopes and plans to conceive, to those who are pregnant or post partum and who are trying to sustain emotional well-being despite the added burden of the pandemic. As we heard similar stories regarding interactions with perinatal patients from reproductive psychiatrists across the country, my colleagues and I had to set up an additional resource, Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital, Boston, which has been mentioned in previous columns, which has only grown during the last 6 months of the pandemic. We have colleagues across the country joining us from 2 p.m. to 3 p.m. on Wednesdays after our own faculty rounds, where we perform case reviews of our own patients, and invite our colleagues to share cases that are then reviewed with expert panelists together with our own faculty in a collaborative environment. Feedback from the community of clinicians has indicated that these virtual rounds have been invaluable to their efforts in taking care of women with perinatal psychiatric issues, particularly during the pandemic.
Of particular note during consultations on our service is the number of women coming to see us for questions about the reproductive safety of the medications on which they are maintained. Hundreds of women present to the center each year for the most up-to-date information regarding the reproductive safety of the most commonly used psychiatric medications in reproductive-age women, including antidepressants (SSRIs, serotonin norepinephrine reuptake inhibitor), mood stabilizers, lithium, lamotrigine, and atypical antipsychotics, as well as other medicines used to treat symptoms that have been a particular issue during the pandemic, such as insomnia and anxiety (benzodiazepines, nonbenzodiazepines, sedative hypnotics, and medicines such as gabapentin).
While consultation regarding risk of fetal exposure to psychotropics has been the cornerstone of our clinical work for 25 years, it has taken on a particularly critical dimension during the pandemic given the wish that women stay euthymic during the pandemic to limit the possibility of patients needing to present in a clinical space that would increase their risk for COVID-19, and to also minimize their risk for postpartum depression. (Psychiatric disorder during pregnancy remains the strongest predictor of emergence or worsening of underlying illness during the postpartum period.)
It is also noteworthy that, during a pandemic year, publications in reproductive psychiatry have been numerous, and we continue to make an effort at our own center to keep up with this and to share with our colleagues our impression of that literature using the weekly blog at womensmentalhealth.org. Last year brought the largest audience to the blog and visits to womensmentalhealth.org in the history of our center.
A case recently at our center presents a unique opportunity to review a confusing question in reproductive psychiatry over the last 15 years. A woman with a longstanding history of mixed anxiety and depression recently came to see us on a regimen of escitalopram and low-dose benzodiazepine. She was doing well, and she and her husband of 4 years were hopeful about starting to try to conceive despite the pandemic. We reviewed the reproductive safety data of the medicines on which she was maintained and made plans to follow-up as her plans galvanized. She notified me several months later that she had become pregnant but had experienced an early miscarriage. The patient was obviously upset and, as she reflected on her decision to maintain treatment with SSRI during her attempts to conceive and across a very early pregnancy, she queried about the extent to which her SSRI use might have contributed to her miscarriage.
The question about the possible association of antidepressant use during pregnancy and increased risk for miscarriage goes back at least 15 years when there were reports of an increased risk of miscarriage in women taking SSRIs during pregnancy. In that early work, there was an apparent increase in miscarriage in women taking SSRIs relative to a control group, but the rate did not exceed the prevalence of miscarriage in the general population. Since those early reports, we are lucky to have had multiple investigators look very closely at this issue, including one meta-analysis of 11 studies done approximately 8 years ago that failed to show an increased risk of miscarriage in the setting of first trimester exposure to SSRIs.
What has been most problematic methodologically, however, has been the failure to account for the potential role of depression in models that predict risk. A subsequent large epidemiologic study from Denmark evaluating over a million women has looked at this question further. The authors found a slightly increased risk of spontaneous abortion associated with the use of antidepressants (12.0% in women with antidepressant exposure vs. 11.1% in women with no exposure). However, looking only at women with a diagnosis of depression, the adjusted risk ratio for spontaneous abortion after any antidepressant exposure was 1.00 (95% confidence interval, 0.80-1.24). Thus, the researchers concluded that exposure to depression – but not exposure to antidepressant – is associated with a slightly higher risk of miscarriage.
Even more recently, a follow-up study examining this question supports the large epidemiologic study by Kjaersgaard and colleagues. For most readers, this effectively answers this very important question for women about rates of miscarriage associated with fetal exposure to SSRIs.
For the patient who presented at the center, reassuring her with this information felt particularly good, especially within the context of the pandemic. After several months of trying to conceive, she again became pregnant and delivered without difficulty. What was palpable in that clinical scenario, as it relates to the practice of reproductive psychiatry during the pandemic, is the even-greater emotional valence that questions about using psychiatric medications during pregnancy has taken on across these past months. While attention and thoughtful consideration about the relative risks of using psychiatric medications during pregnancy should be standard clinical practice, the level of anxiety associated with decisions to sustain or to discontinue treatment during pregnancy seems to have increased for some patients during the pandemic.
Even as the COVID-19 vaccine initiative across the United States is rolled out, 2021 will continue to be a complicated time for women and families. We still need to be vigilant. In addition to screening for perinatal depression during pregnancy and the postpartum period, we should be equally mindful of screening and treating perinatal anxiety, particularly during this challenging time. The challenge to keep pregnant and postpartum women well is perhaps even greater now, 10 months into the pandemic, than it was when we were in crisis mode in March 2020. As clinicians, we need to mobilize the spectrum of both pharmacologic and nonpharmacologic treatment options to sustain emotional well-being among women planning to conceive as well as those who are pregnant or postpartum as we navigate our way to safer times.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Across this period of the pandemic, we’ve spent considerable attention focusing on adaptations to clinical care for pregnant and postpartum women across the board. From the start, this has included a shift to telemedicine for the majority of our patients who come to see us with psychiatric disorders either before, during, or after pregnancy.
Specific issues for perinatal patients since the early days of COVID-19 have included the shifts in women’s plans with respect to delivery as well as the limitation on women’s ability to configure the types of support that they had originally planned on with family, friends, and others during delivery. Telemedicine again helped, at least in part, to fill that void by having online digital support by individuals or groups for both pregnant and postpartum women. These supports were always available, but quickly scaled up during the first 6-9 months of the pandemic and have likely seen their greatest increase in participation in the history of support groups for pregnant and postpartum women.
Similarly, at our own center, we have seen a dramatic increase across the last 10 months in requests for consultation by women with psychiatric disorders who have hopes and plans to conceive, to those who are pregnant or post partum and who are trying to sustain emotional well-being despite the added burden of the pandemic. As we heard similar stories regarding interactions with perinatal patients from reproductive psychiatrists across the country, my colleagues and I had to set up an additional resource, Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital, Boston, which has been mentioned in previous columns, which has only grown during the last 6 months of the pandemic. We have colleagues across the country joining us from 2 p.m. to 3 p.m. on Wednesdays after our own faculty rounds, where we perform case reviews of our own patients, and invite our colleagues to share cases that are then reviewed with expert panelists together with our own faculty in a collaborative environment. Feedback from the community of clinicians has indicated that these virtual rounds have been invaluable to their efforts in taking care of women with perinatal psychiatric issues, particularly during the pandemic.
Of particular note during consultations on our service is the number of women coming to see us for questions about the reproductive safety of the medications on which they are maintained. Hundreds of women present to the center each year for the most up-to-date information regarding the reproductive safety of the most commonly used psychiatric medications in reproductive-age women, including antidepressants (SSRIs, serotonin norepinephrine reuptake inhibitor), mood stabilizers, lithium, lamotrigine, and atypical antipsychotics, as well as other medicines used to treat symptoms that have been a particular issue during the pandemic, such as insomnia and anxiety (benzodiazepines, nonbenzodiazepines, sedative hypnotics, and medicines such as gabapentin).
While consultation regarding risk of fetal exposure to psychotropics has been the cornerstone of our clinical work for 25 years, it has taken on a particularly critical dimension during the pandemic given the wish that women stay euthymic during the pandemic to limit the possibility of patients needing to present in a clinical space that would increase their risk for COVID-19, and to also minimize their risk for postpartum depression. (Psychiatric disorder during pregnancy remains the strongest predictor of emergence or worsening of underlying illness during the postpartum period.)
It is also noteworthy that, during a pandemic year, publications in reproductive psychiatry have been numerous, and we continue to make an effort at our own center to keep up with this and to share with our colleagues our impression of that literature using the weekly blog at womensmentalhealth.org. Last year brought the largest audience to the blog and visits to womensmentalhealth.org in the history of our center.
A case recently at our center presents a unique opportunity to review a confusing question in reproductive psychiatry over the last 15 years. A woman with a longstanding history of mixed anxiety and depression recently came to see us on a regimen of escitalopram and low-dose benzodiazepine. She was doing well, and she and her husband of 4 years were hopeful about starting to try to conceive despite the pandemic. We reviewed the reproductive safety data of the medicines on which she was maintained and made plans to follow-up as her plans galvanized. She notified me several months later that she had become pregnant but had experienced an early miscarriage. The patient was obviously upset and, as she reflected on her decision to maintain treatment with SSRI during her attempts to conceive and across a very early pregnancy, she queried about the extent to which her SSRI use might have contributed to her miscarriage.
The question about the possible association of antidepressant use during pregnancy and increased risk for miscarriage goes back at least 15 years when there were reports of an increased risk of miscarriage in women taking SSRIs during pregnancy. In that early work, there was an apparent increase in miscarriage in women taking SSRIs relative to a control group, but the rate did not exceed the prevalence of miscarriage in the general population. Since those early reports, we are lucky to have had multiple investigators look very closely at this issue, including one meta-analysis of 11 studies done approximately 8 years ago that failed to show an increased risk of miscarriage in the setting of first trimester exposure to SSRIs.
What has been most problematic methodologically, however, has been the failure to account for the potential role of depression in models that predict risk. A subsequent large epidemiologic study from Denmark evaluating over a million women has looked at this question further. The authors found a slightly increased risk of spontaneous abortion associated with the use of antidepressants (12.0% in women with antidepressant exposure vs. 11.1% in women with no exposure). However, looking only at women with a diagnosis of depression, the adjusted risk ratio for spontaneous abortion after any antidepressant exposure was 1.00 (95% confidence interval, 0.80-1.24). Thus, the researchers concluded that exposure to depression – but not exposure to antidepressant – is associated with a slightly higher risk of miscarriage.
Even more recently, a follow-up study examining this question supports the large epidemiologic study by Kjaersgaard and colleagues. For most readers, this effectively answers this very important question for women about rates of miscarriage associated with fetal exposure to SSRIs.
For the patient who presented at the center, reassuring her with this information felt particularly good, especially within the context of the pandemic. After several months of trying to conceive, she again became pregnant and delivered without difficulty. What was palpable in that clinical scenario, as it relates to the practice of reproductive psychiatry during the pandemic, is the even-greater emotional valence that questions about using psychiatric medications during pregnancy has taken on across these past months. While attention and thoughtful consideration about the relative risks of using psychiatric medications during pregnancy should be standard clinical practice, the level of anxiety associated with decisions to sustain or to discontinue treatment during pregnancy seems to have increased for some patients during the pandemic.
Even as the COVID-19 vaccine initiative across the United States is rolled out, 2021 will continue to be a complicated time for women and families. We still need to be vigilant. In addition to screening for perinatal depression during pregnancy and the postpartum period, we should be equally mindful of screening and treating perinatal anxiety, particularly during this challenging time. The challenge to keep pregnant and postpartum women well is perhaps even greater now, 10 months into the pandemic, than it was when we were in crisis mode in March 2020. As clinicians, we need to mobilize the spectrum of both pharmacologic and nonpharmacologic treatment options to sustain emotional well-being among women planning to conceive as well as those who are pregnant or postpartum as we navigate our way to safer times.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Across this period of the pandemic, we’ve spent considerable attention focusing on adaptations to clinical care for pregnant and postpartum women across the board. From the start, this has included a shift to telemedicine for the majority of our patients who come to see us with psychiatric disorders either before, during, or after pregnancy.
Specific issues for perinatal patients since the early days of COVID-19 have included the shifts in women’s plans with respect to delivery as well as the limitation on women’s ability to configure the types of support that they had originally planned on with family, friends, and others during delivery. Telemedicine again helped, at least in part, to fill that void by having online digital support by individuals or groups for both pregnant and postpartum women. These supports were always available, but quickly scaled up during the first 6-9 months of the pandemic and have likely seen their greatest increase in participation in the history of support groups for pregnant and postpartum women.
Similarly, at our own center, we have seen a dramatic increase across the last 10 months in requests for consultation by women with psychiatric disorders who have hopes and plans to conceive, to those who are pregnant or post partum and who are trying to sustain emotional well-being despite the added burden of the pandemic. As we heard similar stories regarding interactions with perinatal patients from reproductive psychiatrists across the country, my colleagues and I had to set up an additional resource, Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital, Boston, which has been mentioned in previous columns, which has only grown during the last 6 months of the pandemic. We have colleagues across the country joining us from 2 p.m. to 3 p.m. on Wednesdays after our own faculty rounds, where we perform case reviews of our own patients, and invite our colleagues to share cases that are then reviewed with expert panelists together with our own faculty in a collaborative environment. Feedback from the community of clinicians has indicated that these virtual rounds have been invaluable to their efforts in taking care of women with perinatal psychiatric issues, particularly during the pandemic.
Of particular note during consultations on our service is the number of women coming to see us for questions about the reproductive safety of the medications on which they are maintained. Hundreds of women present to the center each year for the most up-to-date information regarding the reproductive safety of the most commonly used psychiatric medications in reproductive-age women, including antidepressants (SSRIs, serotonin norepinephrine reuptake inhibitor), mood stabilizers, lithium, lamotrigine, and atypical antipsychotics, as well as other medicines used to treat symptoms that have been a particular issue during the pandemic, such as insomnia and anxiety (benzodiazepines, nonbenzodiazepines, sedative hypnotics, and medicines such as gabapentin).
While consultation regarding risk of fetal exposure to psychotropics has been the cornerstone of our clinical work for 25 years, it has taken on a particularly critical dimension during the pandemic given the wish that women stay euthymic during the pandemic to limit the possibility of patients needing to present in a clinical space that would increase their risk for COVID-19, and to also minimize their risk for postpartum depression. (Psychiatric disorder during pregnancy remains the strongest predictor of emergence or worsening of underlying illness during the postpartum period.)
It is also noteworthy that, during a pandemic year, publications in reproductive psychiatry have been numerous, and we continue to make an effort at our own center to keep up with this and to share with our colleagues our impression of that literature using the weekly blog at womensmentalhealth.org. Last year brought the largest audience to the blog and visits to womensmentalhealth.org in the history of our center.
A case recently at our center presents a unique opportunity to review a confusing question in reproductive psychiatry over the last 15 years. A woman with a longstanding history of mixed anxiety and depression recently came to see us on a regimen of escitalopram and low-dose benzodiazepine. She was doing well, and she and her husband of 4 years were hopeful about starting to try to conceive despite the pandemic. We reviewed the reproductive safety data of the medicines on which she was maintained and made plans to follow-up as her plans galvanized. She notified me several months later that she had become pregnant but had experienced an early miscarriage. The patient was obviously upset and, as she reflected on her decision to maintain treatment with SSRI during her attempts to conceive and across a very early pregnancy, she queried about the extent to which her SSRI use might have contributed to her miscarriage.
The question about the possible association of antidepressant use during pregnancy and increased risk for miscarriage goes back at least 15 years when there were reports of an increased risk of miscarriage in women taking SSRIs during pregnancy. In that early work, there was an apparent increase in miscarriage in women taking SSRIs relative to a control group, but the rate did not exceed the prevalence of miscarriage in the general population. Since those early reports, we are lucky to have had multiple investigators look very closely at this issue, including one meta-analysis of 11 studies done approximately 8 years ago that failed to show an increased risk of miscarriage in the setting of first trimester exposure to SSRIs.
What has been most problematic methodologically, however, has been the failure to account for the potential role of depression in models that predict risk. A subsequent large epidemiologic study from Denmark evaluating over a million women has looked at this question further. The authors found a slightly increased risk of spontaneous abortion associated with the use of antidepressants (12.0% in women with antidepressant exposure vs. 11.1% in women with no exposure). However, looking only at women with a diagnosis of depression, the adjusted risk ratio for spontaneous abortion after any antidepressant exposure was 1.00 (95% confidence interval, 0.80-1.24). Thus, the researchers concluded that exposure to depression – but not exposure to antidepressant – is associated with a slightly higher risk of miscarriage.
Even more recently, a follow-up study examining this question supports the large epidemiologic study by Kjaersgaard and colleagues. For most readers, this effectively answers this very important question for women about rates of miscarriage associated with fetal exposure to SSRIs.
For the patient who presented at the center, reassuring her with this information felt particularly good, especially within the context of the pandemic. After several months of trying to conceive, she again became pregnant and delivered without difficulty. What was palpable in that clinical scenario, as it relates to the practice of reproductive psychiatry during the pandemic, is the even-greater emotional valence that questions about using psychiatric medications during pregnancy has taken on across these past months. While attention and thoughtful consideration about the relative risks of using psychiatric medications during pregnancy should be standard clinical practice, the level of anxiety associated with decisions to sustain or to discontinue treatment during pregnancy seems to have increased for some patients during the pandemic.
Even as the COVID-19 vaccine initiative across the United States is rolled out, 2021 will continue to be a complicated time for women and families. We still need to be vigilant. In addition to screening for perinatal depression during pregnancy and the postpartum period, we should be equally mindful of screening and treating perinatal anxiety, particularly during this challenging time. The challenge to keep pregnant and postpartum women well is perhaps even greater now, 10 months into the pandemic, than it was when we were in crisis mode in March 2020. As clinicians, we need to mobilize the spectrum of both pharmacologic and nonpharmacologic treatment options to sustain emotional well-being among women planning to conceive as well as those who are pregnant or postpartum as we navigate our way to safer times.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
On receiving the COVID-19 vaccine
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
The state of inpatient COVID-19 care
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.
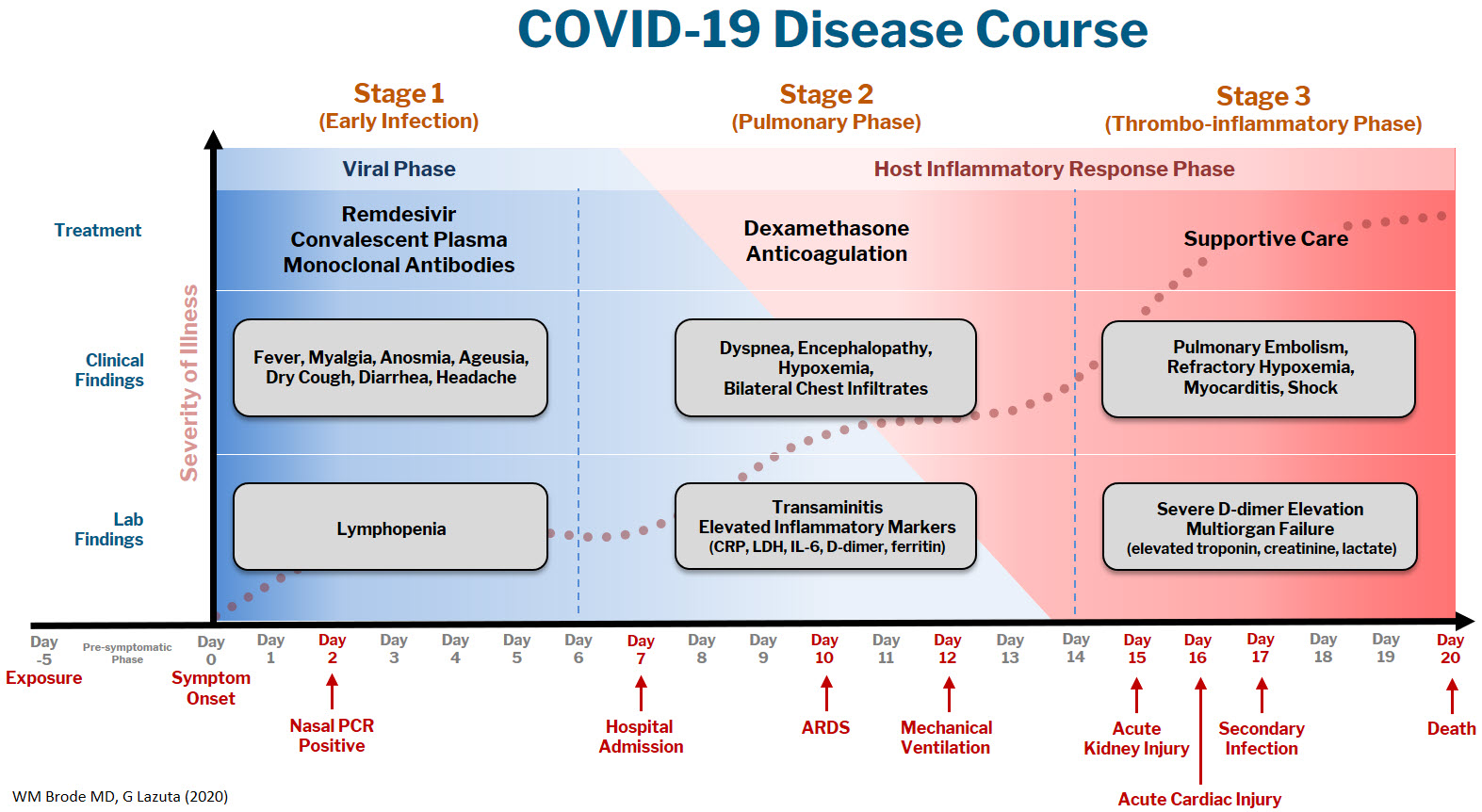
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
A brief evidence-based review of everything we have learned
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
How COVID-19 will continue to alter patient visits
Finding the current domestic and global situations too disheartening to write about, I have decided for the moment to take the long view in hopes of finding something to stimulate your imaginations. It appears that we have several vaccines effective against SARS-CoV-2 if not in your hands at the moment at least in someone’s freezer or at the very least somewhere near beginning of their journey in the production pipeline. It may be a year of more but thanks to the vaccines and herd immunity there will be a time when parents may feel more comfortable about bringing their children into your office. How are you going to dial back your office routine to something even vaguely familiar?
To keep your office afloat financially you have probably been forced to adopt and adapt telemedicine strategies to your practice style. Prior to the pandemic you may have been among the few who were actively experimenting with practicing remotely. But, it is more likely that you had given little serious thought to how you would manage your patients without them being physically present.
You probably carried in your mind a list of symptoms and complaints which you had promised yourself that you would never treat without first laying eyes and hands on the patient. You may have even codified this list into a set of guidelines that you included in the office manual for your nurses, assistants, and receptionists. You may have looked askance at some of your colleagues whom you felt too often treated their patients (and yours when they were covering) based on what seemed to be scanty information gleaned from a phone call. The impropriety of this kind of clinical behavior may have even come up at staff meetings or at least been the topic of hallway discussions.
How did your list of complaints that demanded an in-person visit evolve? I suspect that in large part it was formed as you modeled the behavior of your mentors and teachers. In some cases you may have heard of tragic cases in which a child had died or suffered serious consequences of being treated without an in-person evaluation. In many cases you were following a tradition or ethic that said treating in certain circumstances without an exam just wasn’t done.
Have the realities of the pandemic forced you to alter your list of must-see-before-I’ll-treat complaints? Have you found yourself calling in antibiotic prescriptions for children with ear pain who 1 year ago you would have told to come in for an office visit? Are you treating “strep throats” without a rapid strep test or culture? How many stimulant prescriptions have you refilled for children who haven’t been reevaluated in the office in over a year? How are you going to manage the tsunami of requests for sports physicals once the junior high and high school teams are allowed to return to action? You probably won’t have the time to examine all of the sports candidates who show up in your office with crumpled forms recently retrieved from crumb-filled backpacks.
Where are you going to reset the bar as the pandemic lifts and the barriers that have prevented patients from coming to your office over the last year or year and a half recede? Have you realized that many of your office visits in prepandemic times were unnecessary? How many children with otitis really needed to be followed up with an ear recheck visit? Which children with sore throats and a fever needed to be examined? Was a yearly exam really necessary for a high school sophomore who wanted to play basketball? Has your comfort zone widened to include more patient complaints that can be managed without a face to face encounter? Where will telemedicine fit into the mix?
At some time in the next 12 months you will have to recalibrate and reset the bar. It will probably be a gradual process that in large part can be molded by the responses of the families who may have also come to realize that seeing you in the office isn’t quite as necessary as you both may have thought it was.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com
Finding the current domestic and global situations too disheartening to write about, I have decided for the moment to take the long view in hopes of finding something to stimulate your imaginations. It appears that we have several vaccines effective against SARS-CoV-2 if not in your hands at the moment at least in someone’s freezer or at the very least somewhere near beginning of their journey in the production pipeline. It may be a year of more but thanks to the vaccines and herd immunity there will be a time when parents may feel more comfortable about bringing their children into your office. How are you going to dial back your office routine to something even vaguely familiar?
To keep your office afloat financially you have probably been forced to adopt and adapt telemedicine strategies to your practice style. Prior to the pandemic you may have been among the few who were actively experimenting with practicing remotely. But, it is more likely that you had given little serious thought to how you would manage your patients without them being physically present.
You probably carried in your mind a list of symptoms and complaints which you had promised yourself that you would never treat without first laying eyes and hands on the patient. You may have even codified this list into a set of guidelines that you included in the office manual for your nurses, assistants, and receptionists. You may have looked askance at some of your colleagues whom you felt too often treated their patients (and yours when they were covering) based on what seemed to be scanty information gleaned from a phone call. The impropriety of this kind of clinical behavior may have even come up at staff meetings or at least been the topic of hallway discussions.
How did your list of complaints that demanded an in-person visit evolve? I suspect that in large part it was formed as you modeled the behavior of your mentors and teachers. In some cases you may have heard of tragic cases in which a child had died or suffered serious consequences of being treated without an in-person evaluation. In many cases you were following a tradition or ethic that said treating in certain circumstances without an exam just wasn’t done.
Have the realities of the pandemic forced you to alter your list of must-see-before-I’ll-treat complaints? Have you found yourself calling in antibiotic prescriptions for children with ear pain who 1 year ago you would have told to come in for an office visit? Are you treating “strep throats” without a rapid strep test or culture? How many stimulant prescriptions have you refilled for children who haven’t been reevaluated in the office in over a year? How are you going to manage the tsunami of requests for sports physicals once the junior high and high school teams are allowed to return to action? You probably won’t have the time to examine all of the sports candidates who show up in your office with crumpled forms recently retrieved from crumb-filled backpacks.
Where are you going to reset the bar as the pandemic lifts and the barriers that have prevented patients from coming to your office over the last year or year and a half recede? Have you realized that many of your office visits in prepandemic times were unnecessary? How many children with otitis really needed to be followed up with an ear recheck visit? Which children with sore throats and a fever needed to be examined? Was a yearly exam really necessary for a high school sophomore who wanted to play basketball? Has your comfort zone widened to include more patient complaints that can be managed without a face to face encounter? Where will telemedicine fit into the mix?
At some time in the next 12 months you will have to recalibrate and reset the bar. It will probably be a gradual process that in large part can be molded by the responses of the families who may have also come to realize that seeing you in the office isn’t quite as necessary as you both may have thought it was.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com
Finding the current domestic and global situations too disheartening to write about, I have decided for the moment to take the long view in hopes of finding something to stimulate your imaginations. It appears that we have several vaccines effective against SARS-CoV-2 if not in your hands at the moment at least in someone’s freezer or at the very least somewhere near beginning of their journey in the production pipeline. It may be a year of more but thanks to the vaccines and herd immunity there will be a time when parents may feel more comfortable about bringing their children into your office. How are you going to dial back your office routine to something even vaguely familiar?
To keep your office afloat financially you have probably been forced to adopt and adapt telemedicine strategies to your practice style. Prior to the pandemic you may have been among the few who were actively experimenting with practicing remotely. But, it is more likely that you had given little serious thought to how you would manage your patients without them being physically present.
You probably carried in your mind a list of symptoms and complaints which you had promised yourself that you would never treat without first laying eyes and hands on the patient. You may have even codified this list into a set of guidelines that you included in the office manual for your nurses, assistants, and receptionists. You may have looked askance at some of your colleagues whom you felt too often treated their patients (and yours when they were covering) based on what seemed to be scanty information gleaned from a phone call. The impropriety of this kind of clinical behavior may have even come up at staff meetings or at least been the topic of hallway discussions.
How did your list of complaints that demanded an in-person visit evolve? I suspect that in large part it was formed as you modeled the behavior of your mentors and teachers. In some cases you may have heard of tragic cases in which a child had died or suffered serious consequences of being treated without an in-person evaluation. In many cases you were following a tradition or ethic that said treating in certain circumstances without an exam just wasn’t done.
Have the realities of the pandemic forced you to alter your list of must-see-before-I’ll-treat complaints? Have you found yourself calling in antibiotic prescriptions for children with ear pain who 1 year ago you would have told to come in for an office visit? Are you treating “strep throats” without a rapid strep test or culture? How many stimulant prescriptions have you refilled for children who haven’t been reevaluated in the office in over a year? How are you going to manage the tsunami of requests for sports physicals once the junior high and high school teams are allowed to return to action? You probably won’t have the time to examine all of the sports candidates who show up in your office with crumpled forms recently retrieved from crumb-filled backpacks.
Where are you going to reset the bar as the pandemic lifts and the barriers that have prevented patients from coming to your office over the last year or year and a half recede? Have you realized that many of your office visits in prepandemic times were unnecessary? How many children with otitis really needed to be followed up with an ear recheck visit? Which children with sore throats and a fever needed to be examined? Was a yearly exam really necessary for a high school sophomore who wanted to play basketball? Has your comfort zone widened to include more patient complaints that can be managed without a face to face encounter? Where will telemedicine fit into the mix?
At some time in the next 12 months you will have to recalibrate and reset the bar. It will probably be a gradual process that in large part can be molded by the responses of the families who may have also come to realize that seeing you in the office isn’t quite as necessary as you both may have thought it was.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com
Schools, COVID-19, and Jan. 6, 2021
The first weeks of 2021 have us considering how best to face compound challenges and we expect parents will be looking to their pediatricians for guidance. There are daily stories of the COVID-19 death toll, an abstraction made real by tragic stories of shattered families. Most families are approaching the first anniversary of their children being in virtual school, with growing concerns about the quality of virtual education, loss of socialization and group activities, and additional risks facing poor and vulnerable children. There are real concerns about the future impact of children spending so much time every day on their screens for school, extracurricular activities, social time, and relaxation. While the COVID-19 vaccines promise a return to “normal” sometime in 2021, in-person school may not return until late in the spring or next fall.
After the events of Jan. 6, families face an additional challenge: Discussing the violent invasion of the U.S. Capitol by the president’s supporters. This event was shocking, frightening, and confusing for most, and continues to be heavily covered in the news and online. There is a light in all this darkness. We have the opportunity to talk with our children – and to share explanations, perspectives, values, and even the discomfort of the unknowns – about COVID-19, use of the Internet, and the violence of Jan 6. We will consider how parents can approach this challenge for three age groups. With each group, parents will need to be calm and curious and will need time to give their children their full attention. We are all living through history. When parents can be fully present with their children, even for short periods at meals or at bedtime, it will help all to get their balance back and start to make sense of the extraordinary events we have been facing.
The youngest children (aged 3-6 years), those who were in preschool or kindergarten before the pandemic, need the most from their parents during this time. If they are attending school virtually, their online school days are likely short and challenging. Children at this age are mastering behavior rather than cognitive tasks. They are learning how to manage their bodies in space (stay in their seats!), how to be patient and kind (take turns!), and how to manage frustration (math is hard, try again!). Without the physical presence of their teacher and classmates, these lessons are tougher to internalize. Given their age-appropriate short attention spans, they often walk away from a screen, even if it’s class time. They are more likely to be paying attention to their parents, responding to the emotional climate at home. Even if they are not watching news websites themselves, they are likely to have overheard or noticed the news about recent events. Parents of young children should take care to turn off the television or their own computer, as repeated frightening videos of the insurrection can cause their children to worry that these events continue to unfold. These children need their parents’ undivided attention, even just for a little while. Play a board game with them (good chance to stay in their seats, take turns, and manage losing). Or get them outside for some physical play. While playing, parents can ask what they have seen, heard, or understand about what happened in the Capitol. Then they can correct misperceptions that might be frightening and offer reasonable reassurances in language these young children can understand. They might tell their children that sometimes people get angry when they have lost, and even adults can behave badly and make mistakes. They can focus on who the helpers are, and what they could do to help also. They could write letters of appreciation to their elected officials or to the Capitol police who were so brave in protecting others. If their children are curious, parents can find books or videos that are age appropriate about the Constitution and how elections work in a democracy. Parents don’t need to be able to answer every question, watching “Schoolhouse Rock” videos on YouTube together is a wonderful way to complement their online school and support their healthy development.
School-aged children (7-12 years) are developmentally focused on mastery experiences, whether they are social, academic, or athletic. They may be better equipped to pay attention and do homework than their younger siblings, but they will miss building friendships and having a real audience for their efforts as they build emotional maturity. They are prone to worry and distress about the big events that they can understand, at least in concrete terms, but have never faced before. These children usually have been able to use social media and online games to stay connected to friends, but they are less likely than their older siblings to independently exercise or explore new interests without a parent or teacher to guide and support them. These children are likely to be spending a lot of their time online on websites their parents don’t know about, and most likely to be curious about the events of Jan. 6. Parents should close their own device and invite their school-age children to show them what they are working on in school. Be curious about all of it, even how they are doing gym or music class. Then ask about what they have seen or heard about the election and its aftermath at school, from friends, or on their own. Let them be the teachers about what happened and how they learned about it. Parents can correct misinformation or offer reliable sources of information they can investigate together. What they will need is validation of the difficult feelings that events like these can cause; that is, acknowledgment, acceptance, and understanding of big feelings, without trying to just make those feelings go away. Parents might help them to be curious about what can make people get angry, break laws, and even hurt others, and how we protest injustices in a democracy. These children may be ready to take a deeper dive into history, via a good film or documentary, with their parents’ company for discussion afterward. Be their audience and model curiosity and patience, all the while validating the feelings that might arise.
Teenagers are developmentally focused on building their own identities, cultivating independence, and deeper relationships beyond their family. While they may be well equipped to manage online learning and to stay connected to their friends and teachers through electronic means, they are also facing considerable challenge, as their ability to explore new interests, build new relationships, and be meaningfully independent has been profoundly restrained over the past year. And they are facing other losses, as milestones like proms, performances, and competitions have been altered or missed. Parents still know when their teenager is most likely to talk, and they should check in with them during those times. They can ask them about what classes are working online and which ones aren’t, and what extracurriculars are still possible. They should not be discouraged if their teenager only offers cursory responses, it matters that they are showing up and showing interest. The election and its aftermath provide a meaningful matter to discuss; parents can find out if it is being discussed by any teachers or friends. What do they think triggered the events of Jan. 6? Who should be held responsible? How to reasonably protest injustice? What does a society do when citizens can’t agree on facts? More than offering reassurance, parents should be curious about their adolescent’s developing identity and their values, how they are thinking about complex issues around free speech and justice. It is a wonderful opportunity for parents to learn about their adolescent’s emerging identity, to be tolerant of their autonomy, and an opportunity to offer their perspective and values.
At every age, parents need to be present by listening and drawing their children out without distraction. Now is a time to build relationships and to use the difficult events of the day to shed light on deeper issues and values. This is hard, but far better than having children deal with these issues in darkness or alone.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
The first weeks of 2021 have us considering how best to face compound challenges and we expect parents will be looking to their pediatricians for guidance. There are daily stories of the COVID-19 death toll, an abstraction made real by tragic stories of shattered families. Most families are approaching the first anniversary of their children being in virtual school, with growing concerns about the quality of virtual education, loss of socialization and group activities, and additional risks facing poor and vulnerable children. There are real concerns about the future impact of children spending so much time every day on their screens for school, extracurricular activities, social time, and relaxation. While the COVID-19 vaccines promise a return to “normal” sometime in 2021, in-person school may not return until late in the spring or next fall.
After the events of Jan. 6, families face an additional challenge: Discussing the violent invasion of the U.S. Capitol by the president’s supporters. This event was shocking, frightening, and confusing for most, and continues to be heavily covered in the news and online. There is a light in all this darkness. We have the opportunity to talk with our children – and to share explanations, perspectives, values, and even the discomfort of the unknowns – about COVID-19, use of the Internet, and the violence of Jan 6. We will consider how parents can approach this challenge for three age groups. With each group, parents will need to be calm and curious and will need time to give their children their full attention. We are all living through history. When parents can be fully present with their children, even for short periods at meals or at bedtime, it will help all to get their balance back and start to make sense of the extraordinary events we have been facing.
The youngest children (aged 3-6 years), those who were in preschool or kindergarten before the pandemic, need the most from their parents during this time. If they are attending school virtually, their online school days are likely short and challenging. Children at this age are mastering behavior rather than cognitive tasks. They are learning how to manage their bodies in space (stay in their seats!), how to be patient and kind (take turns!), and how to manage frustration (math is hard, try again!). Without the physical presence of their teacher and classmates, these lessons are tougher to internalize. Given their age-appropriate short attention spans, they often walk away from a screen, even if it’s class time. They are more likely to be paying attention to their parents, responding to the emotional climate at home. Even if they are not watching news websites themselves, they are likely to have overheard or noticed the news about recent events. Parents of young children should take care to turn off the television or their own computer, as repeated frightening videos of the insurrection can cause their children to worry that these events continue to unfold. These children need their parents’ undivided attention, even just for a little while. Play a board game with them (good chance to stay in their seats, take turns, and manage losing). Or get them outside for some physical play. While playing, parents can ask what they have seen, heard, or understand about what happened in the Capitol. Then they can correct misperceptions that might be frightening and offer reasonable reassurances in language these young children can understand. They might tell their children that sometimes people get angry when they have lost, and even adults can behave badly and make mistakes. They can focus on who the helpers are, and what they could do to help also. They could write letters of appreciation to their elected officials or to the Capitol police who were so brave in protecting others. If their children are curious, parents can find books or videos that are age appropriate about the Constitution and how elections work in a democracy. Parents don’t need to be able to answer every question, watching “Schoolhouse Rock” videos on YouTube together is a wonderful way to complement their online school and support their healthy development.
School-aged children (7-12 years) are developmentally focused on mastery experiences, whether they are social, academic, or athletic. They may be better equipped to pay attention and do homework than their younger siblings, but they will miss building friendships and having a real audience for their efforts as they build emotional maturity. They are prone to worry and distress about the big events that they can understand, at least in concrete terms, but have never faced before. These children usually have been able to use social media and online games to stay connected to friends, but they are less likely than their older siblings to independently exercise or explore new interests without a parent or teacher to guide and support them. These children are likely to be spending a lot of their time online on websites their parents don’t know about, and most likely to be curious about the events of Jan. 6. Parents should close their own device and invite their school-age children to show them what they are working on in school. Be curious about all of it, even how they are doing gym or music class. Then ask about what they have seen or heard about the election and its aftermath at school, from friends, or on their own. Let them be the teachers about what happened and how they learned about it. Parents can correct misinformation or offer reliable sources of information they can investigate together. What they will need is validation of the difficult feelings that events like these can cause; that is, acknowledgment, acceptance, and understanding of big feelings, without trying to just make those feelings go away. Parents might help them to be curious about what can make people get angry, break laws, and even hurt others, and how we protest injustices in a democracy. These children may be ready to take a deeper dive into history, via a good film or documentary, with their parents’ company for discussion afterward. Be their audience and model curiosity and patience, all the while validating the feelings that might arise.
Teenagers are developmentally focused on building their own identities, cultivating independence, and deeper relationships beyond their family. While they may be well equipped to manage online learning and to stay connected to their friends and teachers through electronic means, they are also facing considerable challenge, as their ability to explore new interests, build new relationships, and be meaningfully independent has been profoundly restrained over the past year. And they are facing other losses, as milestones like proms, performances, and competitions have been altered or missed. Parents still know when their teenager is most likely to talk, and they should check in with them during those times. They can ask them about what classes are working online and which ones aren’t, and what extracurriculars are still possible. They should not be discouraged if their teenager only offers cursory responses, it matters that they are showing up and showing interest. The election and its aftermath provide a meaningful matter to discuss; parents can find out if it is being discussed by any teachers or friends. What do they think triggered the events of Jan. 6? Who should be held responsible? How to reasonably protest injustice? What does a society do when citizens can’t agree on facts? More than offering reassurance, parents should be curious about their adolescent’s developing identity and their values, how they are thinking about complex issues around free speech and justice. It is a wonderful opportunity for parents to learn about their adolescent’s emerging identity, to be tolerant of their autonomy, and an opportunity to offer their perspective and values.
At every age, parents need to be present by listening and drawing their children out without distraction. Now is a time to build relationships and to use the difficult events of the day to shed light on deeper issues and values. This is hard, but far better than having children deal with these issues in darkness or alone.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
The first weeks of 2021 have us considering how best to face compound challenges and we expect parents will be looking to their pediatricians for guidance. There are daily stories of the COVID-19 death toll, an abstraction made real by tragic stories of shattered families. Most families are approaching the first anniversary of their children being in virtual school, with growing concerns about the quality of virtual education, loss of socialization and group activities, and additional risks facing poor and vulnerable children. There are real concerns about the future impact of children spending so much time every day on their screens for school, extracurricular activities, social time, and relaxation. While the COVID-19 vaccines promise a return to “normal” sometime in 2021, in-person school may not return until late in the spring or next fall.
After the events of Jan. 6, families face an additional challenge: Discussing the violent invasion of the U.S. Capitol by the president’s supporters. This event was shocking, frightening, and confusing for most, and continues to be heavily covered in the news and online. There is a light in all this darkness. We have the opportunity to talk with our children – and to share explanations, perspectives, values, and even the discomfort of the unknowns – about COVID-19, use of the Internet, and the violence of Jan 6. We will consider how parents can approach this challenge for three age groups. With each group, parents will need to be calm and curious and will need time to give their children their full attention. We are all living through history. When parents can be fully present with their children, even for short periods at meals or at bedtime, it will help all to get their balance back and start to make sense of the extraordinary events we have been facing.
The youngest children (aged 3-6 years), those who were in preschool or kindergarten before the pandemic, need the most from their parents during this time. If they are attending school virtually, their online school days are likely short and challenging. Children at this age are mastering behavior rather than cognitive tasks. They are learning how to manage their bodies in space (stay in their seats!), how to be patient and kind (take turns!), and how to manage frustration (math is hard, try again!). Without the physical presence of their teacher and classmates, these lessons are tougher to internalize. Given their age-appropriate short attention spans, they often walk away from a screen, even if it’s class time. They are more likely to be paying attention to their parents, responding to the emotional climate at home. Even if they are not watching news websites themselves, they are likely to have overheard or noticed the news about recent events. Parents of young children should take care to turn off the television or their own computer, as repeated frightening videos of the insurrection can cause their children to worry that these events continue to unfold. These children need their parents’ undivided attention, even just for a little while. Play a board game with them (good chance to stay in their seats, take turns, and manage losing). Or get them outside for some physical play. While playing, parents can ask what they have seen, heard, or understand about what happened in the Capitol. Then they can correct misperceptions that might be frightening and offer reasonable reassurances in language these young children can understand. They might tell their children that sometimes people get angry when they have lost, and even adults can behave badly and make mistakes. They can focus on who the helpers are, and what they could do to help also. They could write letters of appreciation to their elected officials or to the Capitol police who were so brave in protecting others. If their children are curious, parents can find books or videos that are age appropriate about the Constitution and how elections work in a democracy. Parents don’t need to be able to answer every question, watching “Schoolhouse Rock” videos on YouTube together is a wonderful way to complement their online school and support their healthy development.
School-aged children (7-12 years) are developmentally focused on mastery experiences, whether they are social, academic, or athletic. They may be better equipped to pay attention and do homework than their younger siblings, but they will miss building friendships and having a real audience for their efforts as they build emotional maturity. They are prone to worry and distress about the big events that they can understand, at least in concrete terms, but have never faced before. These children usually have been able to use social media and online games to stay connected to friends, but they are less likely than their older siblings to independently exercise or explore new interests without a parent or teacher to guide and support them. These children are likely to be spending a lot of their time online on websites their parents don’t know about, and most likely to be curious about the events of Jan. 6. Parents should close their own device and invite their school-age children to show them what they are working on in school. Be curious about all of it, even how they are doing gym or music class. Then ask about what they have seen or heard about the election and its aftermath at school, from friends, or on their own. Let them be the teachers about what happened and how they learned about it. Parents can correct misinformation or offer reliable sources of information they can investigate together. What they will need is validation of the difficult feelings that events like these can cause; that is, acknowledgment, acceptance, and understanding of big feelings, without trying to just make those feelings go away. Parents might help them to be curious about what can make people get angry, break laws, and even hurt others, and how we protest injustices in a democracy. These children may be ready to take a deeper dive into history, via a good film or documentary, with their parents’ company for discussion afterward. Be their audience and model curiosity and patience, all the while validating the feelings that might arise.
Teenagers are developmentally focused on building their own identities, cultivating independence, and deeper relationships beyond their family. While they may be well equipped to manage online learning and to stay connected to their friends and teachers through electronic means, they are also facing considerable challenge, as their ability to explore new interests, build new relationships, and be meaningfully independent has been profoundly restrained over the past year. And they are facing other losses, as milestones like proms, performances, and competitions have been altered or missed. Parents still know when their teenager is most likely to talk, and they should check in with them during those times. They can ask them about what classes are working online and which ones aren’t, and what extracurriculars are still possible. They should not be discouraged if their teenager only offers cursory responses, it matters that they are showing up and showing interest. The election and its aftermath provide a meaningful matter to discuss; parents can find out if it is being discussed by any teachers or friends. What do they think triggered the events of Jan. 6? Who should be held responsible? How to reasonably protest injustice? What does a society do when citizens can’t agree on facts? More than offering reassurance, parents should be curious about their adolescent’s developing identity and their values, how they are thinking about complex issues around free speech and justice. It is a wonderful opportunity for parents to learn about their adolescent’s emerging identity, to be tolerant of their autonomy, and an opportunity to offer their perspective and values.
At every age, parents need to be present by listening and drawing their children out without distraction. Now is a time to build relationships and to use the difficult events of the day to shed light on deeper issues and values. This is hard, but far better than having children deal with these issues in darkness or alone.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at pdnews@mdedge.com.