User login
Potential dapagliflozin benefit post MI is not a ‘mandate’
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
AT AHA 2023
In MI with anemia, results may favor liberal transfusion: MINT
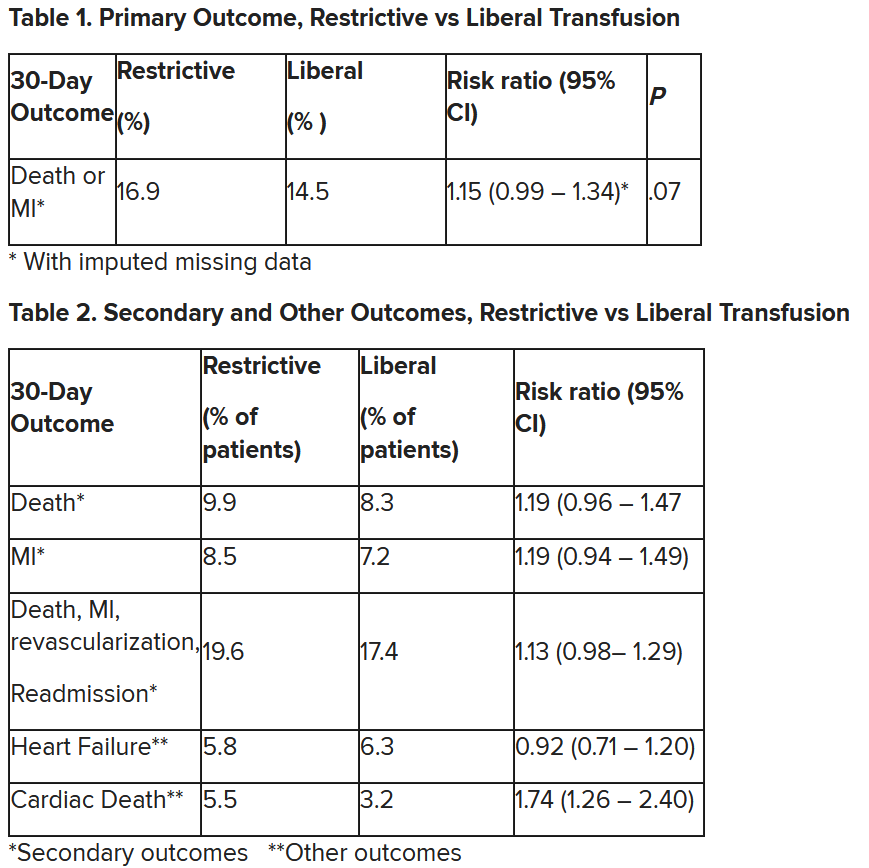
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Short aspirin therapy noninferior to DAPT for 1 year after PCI for ACS
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
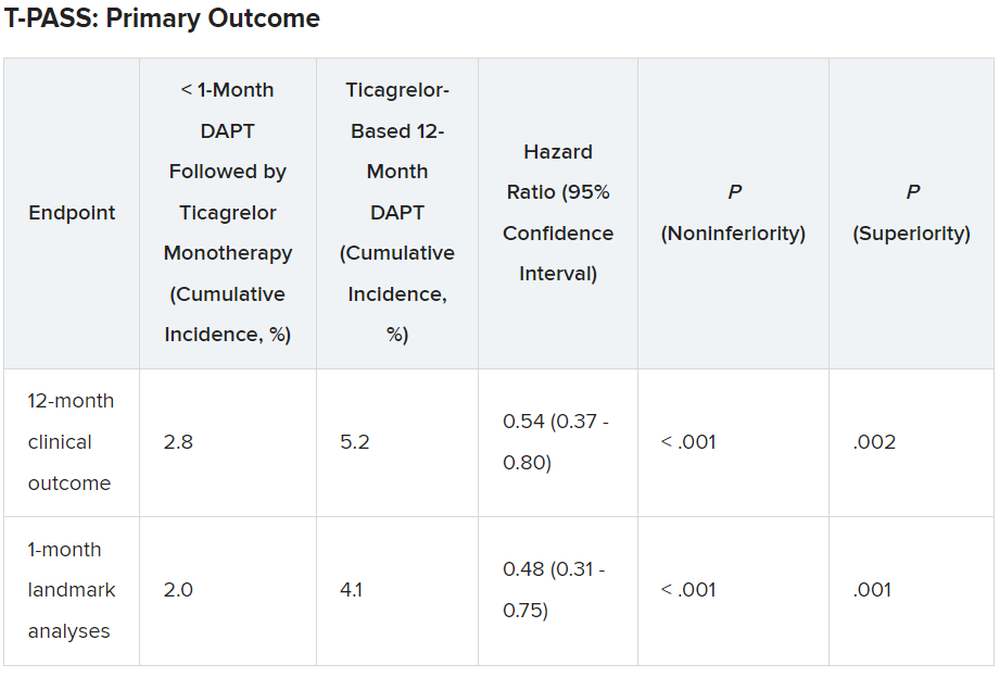
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Marijuana use dramatically increases risk of heart problems, stroke
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
FROM AHA 2023
Chinese medicine improves outcomes in STEMI patients
TOPLINE:
Tongxinluo, a traditional Chinese medicine made from extracts of multiple plants and insects, significantly reduced myocardial infarction (MI), stroke, and related events when used alongside guideline-directed treatments in patients with ST-segment elevation myocardial infarction (STEMI), results of a large trial suggest.
METHODOLOGY:
- The double-blind China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction (CTS-AMI) included 3,777 adult patients, mean age 61 years, 76.9% men, with STEMI at 124 centers in China.
- Researchers randomly assigned patients to receive oral Tongxinluo using a loading dose of eight capsules (2.08 g) followed by a maintenance dose of four capsules (1.04 g) or oral placebo three times a day for 12 months.
- Doctors were instructed to also provide STEMI guideline-directed treatments, which include dual antiplatelet therapy and coronary reperfusion (percutaneous coronary intervention or thrombolysis).
- The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) at 30 days, a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke.
TAKEAWAY:
- (relative risk, 0.64; 95% confidence interval, 0.47-0.88; risk difference, –1.8%; 95% CI, –3.2% to –0.6%; P = .006).
- Individual components of MACCEs were also significantly lower in the Tongxinluo group, including 30-day cardiac death (3.0% vs. 4.2%; RR, 0.70; 95% CI, 0.50-0.99; P = .04) and myocardial reinfarction (0% vs. 0.5%; RR, 0.35; 95% CI, 0.13-0.99; P = .003), but there was no significant difference in 30-day stroke rate.
- At 1 year, the Tongxinluo group had a lower MACCE rate than did the placebo group (5.3% vs. 8.3%; hazard ratio, 0.64; 95% CI, 0.49-0.82; P = .001), an almost significant lower rate of all-cause death (5.1% vs. 6.6%; HR, 0.77; 95% CI, 0.59-1.01; P = .06), and lower rates of other outcomes.
- Rates of nonfatal serious adverse events were similar (2.2% in the Tongxinluo and 2.8% in the placebo groups; P = .25), but the Tongxinluo group had more adverse drug reactions (2.1% vs 1.1%; P = .02), which were mainly driven by symptoms in the digestive system such as stomach discomfort and nausea.
IN PRACTICE:
Unlike most traditional Chinese medicine research, the design of this study incorporated all key elements of randomized clinical trials, including placebo control and blinding in addition to randomization, so it “may serve as a model for future clinical trials to evaluate the safety and efficacy of traditional Chinese medicine,” the authors conclude.
In an accompanying editorial, Richard G. Bach, MD, cardiovascular division, Washington University School of Medicine, St. Louis, expressed skepticism about whether the benefits of Tongxinluo can be extrapolated to populations outside China that have distinct genetic backgrounds, lipid profiles, and diets. He also stressed that the active ingredients and mechanisms of action of Tongxinluo are unknown and noted reports suggesting that traditional Chinese medicines can contain undeclared material, heavy metals, and other adulterants associated with potentially toxic effects.
In an Editor’s Note, Gregory Curfman, MD, said in making a judgment about the validity of this research, “the editors were faced with the task of walking a fine line between skepticism and plausibility.” Though all patients should receive beta-blockers and an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker after STEMI, use of these drugs was suboptimal in these patients. Still, the benefits of Chinese medicine are plausible, said Dr. Curfman, noting research on the malaria drug artemisinin, which was isolated from a traditional Chinese medicine, was awarded the Nobel Prize.
SOURCE:
The research was led by Yuejin Yang, MD, PhD, department of cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, and colleagues. It was published online on Oct. 24 in JAMA. The results were previously reported at the American Heart Association Scientific Sessions 2022.
LIMITATIONS:
Despite the demonstrated clinical benefit of Tongxinluo, its active ingredients and the exact mechanisms of action have not been established. Use of guideline-directed medical therapy was suboptimal, with only 64% of patients prescribed beta-blockers and 51%-52% prescribed an ACE inhibitor or ARB during hospitalization, which may have affected the magnitude of the benefit of Tongxinluo. As study patients were all Chinese, the generalizability to other populations, especially in countries with higher adherence to guideline-directed medical therapy, is unknown.
DISCLOSURES:
The study received funding from the National Key Research and Development Program of China and a research grant from Shijiazhuang Yiling Pharmaceutical. Yuejin Yang reported receiving grants from Shijiazhuang Yiling Pharmaceutical and the National Key Research and Development Program of China; in addition, he has a patent related to the mechanisms of Tongxinluo in alleviating rat myocardial reperfusion injury and a patent related to the mechanisms of Tongxinluo on enhancing the protective effects of exosomes derived from mesenchymal stem cells in rat acute myocardial infarction. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Tongxinluo, a traditional Chinese medicine made from extracts of multiple plants and insects, significantly reduced myocardial infarction (MI), stroke, and related events when used alongside guideline-directed treatments in patients with ST-segment elevation myocardial infarction (STEMI), results of a large trial suggest.
METHODOLOGY:
- The double-blind China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction (CTS-AMI) included 3,777 adult patients, mean age 61 years, 76.9% men, with STEMI at 124 centers in China.
- Researchers randomly assigned patients to receive oral Tongxinluo using a loading dose of eight capsules (2.08 g) followed by a maintenance dose of four capsules (1.04 g) or oral placebo three times a day for 12 months.
- Doctors were instructed to also provide STEMI guideline-directed treatments, which include dual antiplatelet therapy and coronary reperfusion (percutaneous coronary intervention or thrombolysis).
- The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) at 30 days, a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke.
TAKEAWAY:
- (relative risk, 0.64; 95% confidence interval, 0.47-0.88; risk difference, –1.8%; 95% CI, –3.2% to –0.6%; P = .006).
- Individual components of MACCEs were also significantly lower in the Tongxinluo group, including 30-day cardiac death (3.0% vs. 4.2%; RR, 0.70; 95% CI, 0.50-0.99; P = .04) and myocardial reinfarction (0% vs. 0.5%; RR, 0.35; 95% CI, 0.13-0.99; P = .003), but there was no significant difference in 30-day stroke rate.
- At 1 year, the Tongxinluo group had a lower MACCE rate than did the placebo group (5.3% vs. 8.3%; hazard ratio, 0.64; 95% CI, 0.49-0.82; P = .001), an almost significant lower rate of all-cause death (5.1% vs. 6.6%; HR, 0.77; 95% CI, 0.59-1.01; P = .06), and lower rates of other outcomes.
- Rates of nonfatal serious adverse events were similar (2.2% in the Tongxinluo and 2.8% in the placebo groups; P = .25), but the Tongxinluo group had more adverse drug reactions (2.1% vs 1.1%; P = .02), which were mainly driven by symptoms in the digestive system such as stomach discomfort and nausea.
IN PRACTICE:
Unlike most traditional Chinese medicine research, the design of this study incorporated all key elements of randomized clinical trials, including placebo control and blinding in addition to randomization, so it “may serve as a model for future clinical trials to evaluate the safety and efficacy of traditional Chinese medicine,” the authors conclude.
In an accompanying editorial, Richard G. Bach, MD, cardiovascular division, Washington University School of Medicine, St. Louis, expressed skepticism about whether the benefits of Tongxinluo can be extrapolated to populations outside China that have distinct genetic backgrounds, lipid profiles, and diets. He also stressed that the active ingredients and mechanisms of action of Tongxinluo are unknown and noted reports suggesting that traditional Chinese medicines can contain undeclared material, heavy metals, and other adulterants associated with potentially toxic effects.
In an Editor’s Note, Gregory Curfman, MD, said in making a judgment about the validity of this research, “the editors were faced with the task of walking a fine line between skepticism and plausibility.” Though all patients should receive beta-blockers and an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker after STEMI, use of these drugs was suboptimal in these patients. Still, the benefits of Chinese medicine are plausible, said Dr. Curfman, noting research on the malaria drug artemisinin, which was isolated from a traditional Chinese medicine, was awarded the Nobel Prize.
SOURCE:
The research was led by Yuejin Yang, MD, PhD, department of cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, and colleagues. It was published online on Oct. 24 in JAMA. The results were previously reported at the American Heart Association Scientific Sessions 2022.
LIMITATIONS:
Despite the demonstrated clinical benefit of Tongxinluo, its active ingredients and the exact mechanisms of action have not been established. Use of guideline-directed medical therapy was suboptimal, with only 64% of patients prescribed beta-blockers and 51%-52% prescribed an ACE inhibitor or ARB during hospitalization, which may have affected the magnitude of the benefit of Tongxinluo. As study patients were all Chinese, the generalizability to other populations, especially in countries with higher adherence to guideline-directed medical therapy, is unknown.
DISCLOSURES:
The study received funding from the National Key Research and Development Program of China and a research grant from Shijiazhuang Yiling Pharmaceutical. Yuejin Yang reported receiving grants from Shijiazhuang Yiling Pharmaceutical and the National Key Research and Development Program of China; in addition, he has a patent related to the mechanisms of Tongxinluo in alleviating rat myocardial reperfusion injury and a patent related to the mechanisms of Tongxinluo on enhancing the protective effects of exosomes derived from mesenchymal stem cells in rat acute myocardial infarction. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Tongxinluo, a traditional Chinese medicine made from extracts of multiple plants and insects, significantly reduced myocardial infarction (MI), stroke, and related events when used alongside guideline-directed treatments in patients with ST-segment elevation myocardial infarction (STEMI), results of a large trial suggest.
METHODOLOGY:
- The double-blind China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction (CTS-AMI) included 3,777 adult patients, mean age 61 years, 76.9% men, with STEMI at 124 centers in China.
- Researchers randomly assigned patients to receive oral Tongxinluo using a loading dose of eight capsules (2.08 g) followed by a maintenance dose of four capsules (1.04 g) or oral placebo three times a day for 12 months.
- Doctors were instructed to also provide STEMI guideline-directed treatments, which include dual antiplatelet therapy and coronary reperfusion (percutaneous coronary intervention or thrombolysis).
- The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) at 30 days, a composite of cardiac death, myocardial reinfarction, emergent coronary revascularization, and stroke.
TAKEAWAY:
- (relative risk, 0.64; 95% confidence interval, 0.47-0.88; risk difference, –1.8%; 95% CI, –3.2% to –0.6%; P = .006).
- Individual components of MACCEs were also significantly lower in the Tongxinluo group, including 30-day cardiac death (3.0% vs. 4.2%; RR, 0.70; 95% CI, 0.50-0.99; P = .04) and myocardial reinfarction (0% vs. 0.5%; RR, 0.35; 95% CI, 0.13-0.99; P = .003), but there was no significant difference in 30-day stroke rate.
- At 1 year, the Tongxinluo group had a lower MACCE rate than did the placebo group (5.3% vs. 8.3%; hazard ratio, 0.64; 95% CI, 0.49-0.82; P = .001), an almost significant lower rate of all-cause death (5.1% vs. 6.6%; HR, 0.77; 95% CI, 0.59-1.01; P = .06), and lower rates of other outcomes.
- Rates of nonfatal serious adverse events were similar (2.2% in the Tongxinluo and 2.8% in the placebo groups; P = .25), but the Tongxinluo group had more adverse drug reactions (2.1% vs 1.1%; P = .02), which were mainly driven by symptoms in the digestive system such as stomach discomfort and nausea.
IN PRACTICE:
Unlike most traditional Chinese medicine research, the design of this study incorporated all key elements of randomized clinical trials, including placebo control and blinding in addition to randomization, so it “may serve as a model for future clinical trials to evaluate the safety and efficacy of traditional Chinese medicine,” the authors conclude.
In an accompanying editorial, Richard G. Bach, MD, cardiovascular division, Washington University School of Medicine, St. Louis, expressed skepticism about whether the benefits of Tongxinluo can be extrapolated to populations outside China that have distinct genetic backgrounds, lipid profiles, and diets. He also stressed that the active ingredients and mechanisms of action of Tongxinluo are unknown and noted reports suggesting that traditional Chinese medicines can contain undeclared material, heavy metals, and other adulterants associated with potentially toxic effects.
In an Editor’s Note, Gregory Curfman, MD, said in making a judgment about the validity of this research, “the editors were faced with the task of walking a fine line between skepticism and plausibility.” Though all patients should receive beta-blockers and an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker after STEMI, use of these drugs was suboptimal in these patients. Still, the benefits of Chinese medicine are plausible, said Dr. Curfman, noting research on the malaria drug artemisinin, which was isolated from a traditional Chinese medicine, was awarded the Nobel Prize.
SOURCE:
The research was led by Yuejin Yang, MD, PhD, department of cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, and colleagues. It was published online on Oct. 24 in JAMA. The results were previously reported at the American Heart Association Scientific Sessions 2022.
LIMITATIONS:
Despite the demonstrated clinical benefit of Tongxinluo, its active ingredients and the exact mechanisms of action have not been established. Use of guideline-directed medical therapy was suboptimal, with only 64% of patients prescribed beta-blockers and 51%-52% prescribed an ACE inhibitor or ARB during hospitalization, which may have affected the magnitude of the benefit of Tongxinluo. As study patients were all Chinese, the generalizability to other populations, especially in countries with higher adherence to guideline-directed medical therapy, is unknown.
DISCLOSURES:
The study received funding from the National Key Research and Development Program of China and a research grant from Shijiazhuang Yiling Pharmaceutical. Yuejin Yang reported receiving grants from Shijiazhuang Yiling Pharmaceutical and the National Key Research and Development Program of China; in addition, he has a patent related to the mechanisms of Tongxinluo in alleviating rat myocardial reperfusion injury and a patent related to the mechanisms of Tongxinluo on enhancing the protective effects of exosomes derived from mesenchymal stem cells in rat acute myocardial infarction. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
This drug works, but wait till you hear what’s in it
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
Common meds link to sudden cardiac arrest in type 2 diabetes
HAMBURG, Germany – , shows the first such analysis of real-world, primary care data.
People with type 2 diabetes who do not have a history of CVD have almost three times the risk of SCA if they take antipsychotic medications and nearly double the risk if they take certain antibiotics that prolong the QT interval, notably, macrolides and fluoroquinolones.
“These data show that commonly prescribed drugs - antipsychotic medications, used by about 3% of people with type 2 diabetes, and antibiotics, taken by 5% to 10%, convey an increased risk of sudden cardiac arrest in those without a history of cardiovascular disease,” said Peter Harms, MSc, who presented the study at the annual meeting of the European Association for the Study of Diabetes. Another drug associated with an increase in SCA among patients with diabetes was domperidone, an antinausea medication.
“Perhaps these drugs could be avoided in some cases, and GPs should be more aware of the possible consequences of their use,” he added. “If the patient has type 2 diabetes, then maybe it’s better to avoid some of these medications and try and cope without them, or at least find an alternative antibiotic.”
Mr. Harms, an epidemiologist from Amsterdam University Medical Centers, highlighted that their study was unique because the investigators drew upon primary care data. “These data are extensive, and we find a lot of associations which are very real.”
SCA is associated with 50% of all cardiac deaths and accounts for 20% of all mortality in high-income countries. Of those people who experience SCA, 80% of cases prove fatal.
“As the name suggests, it is difficult to predict because it is sudden, especially in people without a cardiovascular disease history,” Mr. Harms pointed out in an interview with this news organization. He highlighted that “around half of those who experience SCA, often between the ages of 40 and 60 years, have never seen a cardiologist, but many do have type 2 diabetes.
“We need to better understand how to recognize people at risk of SCA, know who to watch and how to prevent these events,” he emphasized.
Vladimira Fejfarova, MD, comoderated the session and commented on the study. “From the clinical point of view, it’s necessary to evaluate risk factors that can contribute to sudden cardiac arrest.”
Overall, the researchers found that, among people with type 2 diabetes who do not have a history of CVD, hypoglycemia, severe hypertension, dyslipidemia, and use of QTc-prolonging medications are associated with SCA risk. Among people with type 2 diabetes and CVD, albuminuria and heart failure are associated with SCA risk.
Dr. Fejfarova added: “With type 2 diabetes and also type 1, we need to look more at adverse events, especially when treating infections with macrolides, but also mycotic infections, because antimycotic drugs are known to influence QT intervals that could contribute to sudden cardiac arrest.
“We need to be more cautious with prescribing certain antibiotics that have these side effects in our patients with diabetes,” asserted Dr. Fejfarova, from the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Type 2 diabetes doubles the risk of SCA
The researcher decided to investigate the population of people with type 2 diabetes because their risk of SCD is around twice that of those without type 2 diabetes. Because these patients have relatively frequent checkups with general practitioners, Mr. Harms turned to primary care databases that contained comprehensive and relatively routine information on risk indicators.
Longitudinal associations between clinical characteristics of 3,919 patients with type 2 diabetes – both those with and those without a history of CVD – and SCA (a total of 689 patients) were determined.
Cases were found in the AmsteRdam REsuscitation STtudies (ARREST) registry of out-of-hospital resuscitation attempts by emergency medical services in the Dutch region of Noord-Holland from 2010 to 2019. Case patients were matched with up to five control patients. The control group comprised people with type 2 diabetes who had not experienced an SCA. Control patients were sourced from the same primary care practices who were of similar age and sex. Clinical measurements, including blood pressure and blood glucose readings, medication use, and medical history for the 5 years leading up to an SCA, were obtained from general practice records. A multivariable analysis was performed, and results were stratified for people with and for those without a history of CVD.
Of particular interest were drugs that interfere with cardiac function, including some prokinetic, antibiotic, and antipsychotic medications. All of the drugs are known to be associated with a change in QTc prolongation. Examples include domperidone (QTc-prolonging prokinetic), macrolides and fluoroquinolones (QTc-prolonging antibiotics), and haloperidol (a QTc-prolonging antipsychotic).
Antibiotic and antipsychotic use might contribute to SCA in T2D
Case patients and control patients were similar in age, hemoglobin A1c level, and other characteristics with the exception that more patients with SCA had a history of CVD (40.0% vs. 29.4%).
“Looking at the associations in the overall population, insulin use was strongly associated with SCA risk [hazard ratio, 2.38] and perhaps this was an indicator of severity of type 2 diabetes,” remarked Mr. Harms. “Also, unsurprisingly, a history of arrhythmia [HR, 1.68] and, more surprisingly, prokinetic drug use [HR, 1.66; 95% confidence interval, 1.20-2.31], specifically those known for QTc-prolongation, were associated with SCA.”
Among people who had experienced an SCA and who did not have a history of CVD (337 case patients/2,023 control patients), QTc-prolonging antipsychotic medication use was associated with SCA at an HR of 2.87, and antibiotic medication use was associated with SCA at an HR of 1.66. A low fasting glucose level (< 4.5 mmol/mol) was associated with SCA at an HR of 2.5; severely high systolic blood pressure (> 180 mm Hg) was associated with SCA at an HR of 2.21; low HDL cholesterol level, with an HR of 1.35; and high LDL cholesterol level (> 2.6 mmol/L), with an HR of 1.64.
Among people with a history of CVD (352 case patients/1,207 control patients), associations between albuminuria and SCA were moderate (HR, 1.54) and severe (HR, 1.55); heart failure was associated with SCA at an HR of 1.85 (95% CI, 1.50-2.29).
Comoderator Dr. Fejfarova added that, in addition to the findings from Dr. Harms’ study, other research presented in the same session highlighted the importance of checking patients for the presence of arrhythmias that could lead to the development of atrioventricular blocks, sinus node diseases, and SCA.
Mr. Harms and Dr. Fejfarova have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HAMBURG, Germany – , shows the first such analysis of real-world, primary care data.
People with type 2 diabetes who do not have a history of CVD have almost three times the risk of SCA if they take antipsychotic medications and nearly double the risk if they take certain antibiotics that prolong the QT interval, notably, macrolides and fluoroquinolones.
“These data show that commonly prescribed drugs - antipsychotic medications, used by about 3% of people with type 2 diabetes, and antibiotics, taken by 5% to 10%, convey an increased risk of sudden cardiac arrest in those without a history of cardiovascular disease,” said Peter Harms, MSc, who presented the study at the annual meeting of the European Association for the Study of Diabetes. Another drug associated with an increase in SCA among patients with diabetes was domperidone, an antinausea medication.
“Perhaps these drugs could be avoided in some cases, and GPs should be more aware of the possible consequences of their use,” he added. “If the patient has type 2 diabetes, then maybe it’s better to avoid some of these medications and try and cope without them, or at least find an alternative antibiotic.”
Mr. Harms, an epidemiologist from Amsterdam University Medical Centers, highlighted that their study was unique because the investigators drew upon primary care data. “These data are extensive, and we find a lot of associations which are very real.”
SCA is associated with 50% of all cardiac deaths and accounts for 20% of all mortality in high-income countries. Of those people who experience SCA, 80% of cases prove fatal.
“As the name suggests, it is difficult to predict because it is sudden, especially in people without a cardiovascular disease history,” Mr. Harms pointed out in an interview with this news organization. He highlighted that “around half of those who experience SCA, often between the ages of 40 and 60 years, have never seen a cardiologist, but many do have type 2 diabetes.
“We need to better understand how to recognize people at risk of SCA, know who to watch and how to prevent these events,” he emphasized.
Vladimira Fejfarova, MD, comoderated the session and commented on the study. “From the clinical point of view, it’s necessary to evaluate risk factors that can contribute to sudden cardiac arrest.”
Overall, the researchers found that, among people with type 2 diabetes who do not have a history of CVD, hypoglycemia, severe hypertension, dyslipidemia, and use of QTc-prolonging medications are associated with SCA risk. Among people with type 2 diabetes and CVD, albuminuria and heart failure are associated with SCA risk.
Dr. Fejfarova added: “With type 2 diabetes and also type 1, we need to look more at adverse events, especially when treating infections with macrolides, but also mycotic infections, because antimycotic drugs are known to influence QT intervals that could contribute to sudden cardiac arrest.
“We need to be more cautious with prescribing certain antibiotics that have these side effects in our patients with diabetes,” asserted Dr. Fejfarova, from the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Type 2 diabetes doubles the risk of SCA
The researcher decided to investigate the population of people with type 2 diabetes because their risk of SCD is around twice that of those without type 2 diabetes. Because these patients have relatively frequent checkups with general practitioners, Mr. Harms turned to primary care databases that contained comprehensive and relatively routine information on risk indicators.
Longitudinal associations between clinical characteristics of 3,919 patients with type 2 diabetes – both those with and those without a history of CVD – and SCA (a total of 689 patients) were determined.
Cases were found in the AmsteRdam REsuscitation STtudies (ARREST) registry of out-of-hospital resuscitation attempts by emergency medical services in the Dutch region of Noord-Holland from 2010 to 2019. Case patients were matched with up to five control patients. The control group comprised people with type 2 diabetes who had not experienced an SCA. Control patients were sourced from the same primary care practices who were of similar age and sex. Clinical measurements, including blood pressure and blood glucose readings, medication use, and medical history for the 5 years leading up to an SCA, were obtained from general practice records. A multivariable analysis was performed, and results were stratified for people with and for those without a history of CVD.
Of particular interest were drugs that interfere with cardiac function, including some prokinetic, antibiotic, and antipsychotic medications. All of the drugs are known to be associated with a change in QTc prolongation. Examples include domperidone (QTc-prolonging prokinetic), macrolides and fluoroquinolones (QTc-prolonging antibiotics), and haloperidol (a QTc-prolonging antipsychotic).
Antibiotic and antipsychotic use might contribute to SCA in T2D
Case patients and control patients were similar in age, hemoglobin A1c level, and other characteristics with the exception that more patients with SCA had a history of CVD (40.0% vs. 29.4%).
“Looking at the associations in the overall population, insulin use was strongly associated with SCA risk [hazard ratio, 2.38] and perhaps this was an indicator of severity of type 2 diabetes,” remarked Mr. Harms. “Also, unsurprisingly, a history of arrhythmia [HR, 1.68] and, more surprisingly, prokinetic drug use [HR, 1.66; 95% confidence interval, 1.20-2.31], specifically those known for QTc-prolongation, were associated with SCA.”
Among people who had experienced an SCA and who did not have a history of CVD (337 case patients/2,023 control patients), QTc-prolonging antipsychotic medication use was associated with SCA at an HR of 2.87, and antibiotic medication use was associated with SCA at an HR of 1.66. A low fasting glucose level (< 4.5 mmol/mol) was associated with SCA at an HR of 2.5; severely high systolic blood pressure (> 180 mm Hg) was associated with SCA at an HR of 2.21; low HDL cholesterol level, with an HR of 1.35; and high LDL cholesterol level (> 2.6 mmol/L), with an HR of 1.64.
Among people with a history of CVD (352 case patients/1,207 control patients), associations between albuminuria and SCA were moderate (HR, 1.54) and severe (HR, 1.55); heart failure was associated with SCA at an HR of 1.85 (95% CI, 1.50-2.29).
Comoderator Dr. Fejfarova added that, in addition to the findings from Dr. Harms’ study, other research presented in the same session highlighted the importance of checking patients for the presence of arrhythmias that could lead to the development of atrioventricular blocks, sinus node diseases, and SCA.
Mr. Harms and Dr. Fejfarova have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HAMBURG, Germany – , shows the first such analysis of real-world, primary care data.
People with type 2 diabetes who do not have a history of CVD have almost three times the risk of SCA if they take antipsychotic medications and nearly double the risk if they take certain antibiotics that prolong the QT interval, notably, macrolides and fluoroquinolones.
“These data show that commonly prescribed drugs - antipsychotic medications, used by about 3% of people with type 2 diabetes, and antibiotics, taken by 5% to 10%, convey an increased risk of sudden cardiac arrest in those without a history of cardiovascular disease,” said Peter Harms, MSc, who presented the study at the annual meeting of the European Association for the Study of Diabetes. Another drug associated with an increase in SCA among patients with diabetes was domperidone, an antinausea medication.
“Perhaps these drugs could be avoided in some cases, and GPs should be more aware of the possible consequences of their use,” he added. “If the patient has type 2 diabetes, then maybe it’s better to avoid some of these medications and try and cope without them, or at least find an alternative antibiotic.”
Mr. Harms, an epidemiologist from Amsterdam University Medical Centers, highlighted that their study was unique because the investigators drew upon primary care data. “These data are extensive, and we find a lot of associations which are very real.”
SCA is associated with 50% of all cardiac deaths and accounts for 20% of all mortality in high-income countries. Of those people who experience SCA, 80% of cases prove fatal.
“As the name suggests, it is difficult to predict because it is sudden, especially in people without a cardiovascular disease history,” Mr. Harms pointed out in an interview with this news organization. He highlighted that “around half of those who experience SCA, often between the ages of 40 and 60 years, have never seen a cardiologist, but many do have type 2 diabetes.
“We need to better understand how to recognize people at risk of SCA, know who to watch and how to prevent these events,” he emphasized.
Vladimira Fejfarova, MD, comoderated the session and commented on the study. “From the clinical point of view, it’s necessary to evaluate risk factors that can contribute to sudden cardiac arrest.”
Overall, the researchers found that, among people with type 2 diabetes who do not have a history of CVD, hypoglycemia, severe hypertension, dyslipidemia, and use of QTc-prolonging medications are associated with SCA risk. Among people with type 2 diabetes and CVD, albuminuria and heart failure are associated with SCA risk.
Dr. Fejfarova added: “With type 2 diabetes and also type 1, we need to look more at adverse events, especially when treating infections with macrolides, but also mycotic infections, because antimycotic drugs are known to influence QT intervals that could contribute to sudden cardiac arrest.
“We need to be more cautious with prescribing certain antibiotics that have these side effects in our patients with diabetes,” asserted Dr. Fejfarova, from the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague.
Type 2 diabetes doubles the risk of SCA
The researcher decided to investigate the population of people with type 2 diabetes because their risk of SCD is around twice that of those without type 2 diabetes. Because these patients have relatively frequent checkups with general practitioners, Mr. Harms turned to primary care databases that contained comprehensive and relatively routine information on risk indicators.
Longitudinal associations between clinical characteristics of 3,919 patients with type 2 diabetes – both those with and those without a history of CVD – and SCA (a total of 689 patients) were determined.
Cases were found in the AmsteRdam REsuscitation STtudies (ARREST) registry of out-of-hospital resuscitation attempts by emergency medical services in the Dutch region of Noord-Holland from 2010 to 2019. Case patients were matched with up to five control patients. The control group comprised people with type 2 diabetes who had not experienced an SCA. Control patients were sourced from the same primary care practices who were of similar age and sex. Clinical measurements, including blood pressure and blood glucose readings, medication use, and medical history for the 5 years leading up to an SCA, were obtained from general practice records. A multivariable analysis was performed, and results were stratified for people with and for those without a history of CVD.
Of particular interest were drugs that interfere with cardiac function, including some prokinetic, antibiotic, and antipsychotic medications. All of the drugs are known to be associated with a change in QTc prolongation. Examples include domperidone (QTc-prolonging prokinetic), macrolides and fluoroquinolones (QTc-prolonging antibiotics), and haloperidol (a QTc-prolonging antipsychotic).
Antibiotic and antipsychotic use might contribute to SCA in T2D
Case patients and control patients were similar in age, hemoglobin A1c level, and other characteristics with the exception that more patients with SCA had a history of CVD (40.0% vs. 29.4%).
“Looking at the associations in the overall population, insulin use was strongly associated with SCA risk [hazard ratio, 2.38] and perhaps this was an indicator of severity of type 2 diabetes,” remarked Mr. Harms. “Also, unsurprisingly, a history of arrhythmia [HR, 1.68] and, more surprisingly, prokinetic drug use [HR, 1.66; 95% confidence interval, 1.20-2.31], specifically those known for QTc-prolongation, were associated with SCA.”
Among people who had experienced an SCA and who did not have a history of CVD (337 case patients/2,023 control patients), QTc-prolonging antipsychotic medication use was associated with SCA at an HR of 2.87, and antibiotic medication use was associated with SCA at an HR of 1.66. A low fasting glucose level (< 4.5 mmol/mol) was associated with SCA at an HR of 2.5; severely high systolic blood pressure (> 180 mm Hg) was associated with SCA at an HR of 2.21; low HDL cholesterol level, with an HR of 1.35; and high LDL cholesterol level (> 2.6 mmol/L), with an HR of 1.64.
Among people with a history of CVD (352 case patients/1,207 control patients), associations between albuminuria and SCA were moderate (HR, 1.54) and severe (HR, 1.55); heart failure was associated with SCA at an HR of 1.85 (95% CI, 1.50-2.29).
Comoderator Dr. Fejfarova added that, in addition to the findings from Dr. Harms’ study, other research presented in the same session highlighted the importance of checking patients for the presence of arrhythmias that could lead to the development of atrioventricular blocks, sinus node diseases, and SCA.
Mr. Harms and Dr. Fejfarova have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2023
CMS ‘million hearts’ CVD risk reduction model works
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CKD linked to cardiac arrest in Hispanic, Latinx patients
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
Depression tied to higher all-cause and cardiovascular mortality
In a large prospective study, a graded higher risk of all-cause mortality and mortality from cardiovascular disease (CVD) and ischemic heart disease (IHD) emerged in adults with moderate to severe depressive symptoms, compared with those with no such symptoms.
Participants with mild depressive symptoms had a 35%-49% higher risk of all-cause and CVD mortality, respectively, while for those with moderate to severe depressive symptoms, the risk of all-cause, CVD, and IHD mortality was 62%, 79%, and 121% higher, respectively.
“This information highlights the importance for clinicians to identify patients with depressive symptoms and help them engage in treatment,” lead author Zefeng Zhang, MD, PhD, of the division for heart disease and stroke prevention at the U.S. Centers for Disease Control and Prevention, Atlanta, said in an interview.
The study appears in JAMA Network Open.
A nonclassic risk factor for CVD death
This graded positive association between depressive symptoms and CVD death was observed in data from the National Health and Nutrition Examination Survey 2005-2018, which were linked with the National Death Index through 2019 for adults aged 20 and older. Data analysis occurred from March 1 to May 26, 2023. According to the authors, their analyses extend findings from previous research by assessing these associations in a large, diverse, and nationally representative sample. Using more nuanced CVD-related causes of death, depressive symptoms emerged as a nontraditional risk factor for CVD mortality.
The study
In a total cohort of 23,694, about half male, mean overall age 44.7 years, prevalences of mild and moderate to severe depression were 14.9% and 7.2%, respectively, with depressive symptoms assessed by the nine-item Patient Health Questionnaire asking about symptoms over the past 2 weeks.
Adults with depression had significantly lower CV health scores in six of the American Heart Association Life’s Essential 8 metrics for heart health. For all-cause mortality, hazard ratios were 1.35 (95% confidence interval, 1.07-1.72) for mild depressive symptoms vs. none and 1.62 (95% CI, 1.24-2.12) for moderate to severe depressive symptoms vs. none.
The corresponding hazard ratios were 1.49 (95% CI, 1.11-2.0) and 1.79 (95% CI,1.22-2.62) for CVD mortality and 0.96 (95% CI, 0.58-1.60) and 2.21 (95% CI, 1.24-3.91) for IHD death, with associations largely consistent across subgroups.
At the highest severity of depressive symptoms (almost daily for past 2 weeks), feeling tired or having little energy, poor appetite or overeating, and having little interest in doing things were significantly associated with all-cause and CVD mortality after adjusting for potential confounders.
Approximately 11%-16% of the positive associations could be explained by lifestyle factors such as excess alcohol consumption, overeating, and inactivity as per the AHA’s Life’s Essential 8 metrics.
“Taken together with the body of literature on associations between depression and CVD mortality, these findings can support public health efforts to develop a comprehensive, nationwide strategy to improve well-being, including both mental and cardiovascular health,” Dr. Zhang and associates wrote.
This research was funded by the U.S. Centers for Disease Control and Prevention. The authors had no conflicts of interest to disclose.
In a large prospective study, a graded higher risk of all-cause mortality and mortality from cardiovascular disease (CVD) and ischemic heart disease (IHD) emerged in adults with moderate to severe depressive symptoms, compared with those with no such symptoms.
Participants with mild depressive symptoms had a 35%-49% higher risk of all-cause and CVD mortality, respectively, while for those with moderate to severe depressive symptoms, the risk of all-cause, CVD, and IHD mortality was 62%, 79%, and 121% higher, respectively.
“This information highlights the importance for clinicians to identify patients with depressive symptoms and help them engage in treatment,” lead author Zefeng Zhang, MD, PhD, of the division for heart disease and stroke prevention at the U.S. Centers for Disease Control and Prevention, Atlanta, said in an interview.
The study appears in JAMA Network Open.
A nonclassic risk factor for CVD death
This graded positive association between depressive symptoms and CVD death was observed in data from the National Health and Nutrition Examination Survey 2005-2018, which were linked with the National Death Index through 2019 for adults aged 20 and older. Data analysis occurred from March 1 to May 26, 2023. According to the authors, their analyses extend findings from previous research by assessing these associations in a large, diverse, and nationally representative sample. Using more nuanced CVD-related causes of death, depressive symptoms emerged as a nontraditional risk factor for CVD mortality.
The study
In a total cohort of 23,694, about half male, mean overall age 44.7 years, prevalences of mild and moderate to severe depression were 14.9% and 7.2%, respectively, with depressive symptoms assessed by the nine-item Patient Health Questionnaire asking about symptoms over the past 2 weeks.
Adults with depression had significantly lower CV health scores in six of the American Heart Association Life’s Essential 8 metrics for heart health. For all-cause mortality, hazard ratios were 1.35 (95% confidence interval, 1.07-1.72) for mild depressive symptoms vs. none and 1.62 (95% CI, 1.24-2.12) for moderate to severe depressive symptoms vs. none.
The corresponding hazard ratios were 1.49 (95% CI, 1.11-2.0) and 1.79 (95% CI,1.22-2.62) for CVD mortality and 0.96 (95% CI, 0.58-1.60) and 2.21 (95% CI, 1.24-3.91) for IHD death, with associations largely consistent across subgroups.
At the highest severity of depressive symptoms (almost daily for past 2 weeks), feeling tired or having little energy, poor appetite or overeating, and having little interest in doing things were significantly associated with all-cause and CVD mortality after adjusting for potential confounders.
Approximately 11%-16% of the positive associations could be explained by lifestyle factors such as excess alcohol consumption, overeating, and inactivity as per the AHA’s Life’s Essential 8 metrics.
“Taken together with the body of literature on associations between depression and CVD mortality, these findings can support public health efforts to develop a comprehensive, nationwide strategy to improve well-being, including both mental and cardiovascular health,” Dr. Zhang and associates wrote.
This research was funded by the U.S. Centers for Disease Control and Prevention. The authors had no conflicts of interest to disclose.
In a large prospective study, a graded higher risk of all-cause mortality and mortality from cardiovascular disease (CVD) and ischemic heart disease (IHD) emerged in adults with moderate to severe depressive symptoms, compared with those with no such symptoms.
Participants with mild depressive symptoms had a 35%-49% higher risk of all-cause and CVD mortality, respectively, while for those with moderate to severe depressive symptoms, the risk of all-cause, CVD, and IHD mortality was 62%, 79%, and 121% higher, respectively.
“This information highlights the importance for clinicians to identify patients with depressive symptoms and help them engage in treatment,” lead author Zefeng Zhang, MD, PhD, of the division for heart disease and stroke prevention at the U.S. Centers for Disease Control and Prevention, Atlanta, said in an interview.
The study appears in JAMA Network Open.
A nonclassic risk factor for CVD death
This graded positive association between depressive symptoms and CVD death was observed in data from the National Health and Nutrition Examination Survey 2005-2018, which were linked with the National Death Index through 2019 for adults aged 20 and older. Data analysis occurred from March 1 to May 26, 2023. According to the authors, their analyses extend findings from previous research by assessing these associations in a large, diverse, and nationally representative sample. Using more nuanced CVD-related causes of death, depressive symptoms emerged as a nontraditional risk factor for CVD mortality.
The study
In a total cohort of 23,694, about half male, mean overall age 44.7 years, prevalences of mild and moderate to severe depression were 14.9% and 7.2%, respectively, with depressive symptoms assessed by the nine-item Patient Health Questionnaire asking about symptoms over the past 2 weeks.
Adults with depression had significantly lower CV health scores in six of the American Heart Association Life’s Essential 8 metrics for heart health. For all-cause mortality, hazard ratios were 1.35 (95% confidence interval, 1.07-1.72) for mild depressive symptoms vs. none and 1.62 (95% CI, 1.24-2.12) for moderate to severe depressive symptoms vs. none.
The corresponding hazard ratios were 1.49 (95% CI, 1.11-2.0) and 1.79 (95% CI,1.22-2.62) for CVD mortality and 0.96 (95% CI, 0.58-1.60) and 2.21 (95% CI, 1.24-3.91) for IHD death, with associations largely consistent across subgroups.
At the highest severity of depressive symptoms (almost daily for past 2 weeks), feeling tired or having little energy, poor appetite or overeating, and having little interest in doing things were significantly associated with all-cause and CVD mortality after adjusting for potential confounders.
Approximately 11%-16% of the positive associations could be explained by lifestyle factors such as excess alcohol consumption, overeating, and inactivity as per the AHA’s Life’s Essential 8 metrics.
“Taken together with the body of literature on associations between depression and CVD mortality, these findings can support public health efforts to develop a comprehensive, nationwide strategy to improve well-being, including both mental and cardiovascular health,” Dr. Zhang and associates wrote.
This research was funded by the U.S. Centers for Disease Control and Prevention. The authors had no conflicts of interest to disclose.
FROM JAMA NETWORK OPEN