User login
Legislators disagree on new drug-pricing proposal
Legislators sparred about the best way to lower drug prices during a Sept. 25 hearing, bickering along partisan lines over new legislation by U.S. House Speaker Nancy Pelosi, (D-Calif.) that would require Medicare to negotiate drug prices with manufacturers.
The more than 4-hour hearing by the House Committee on Energy & Commerce Subcommittee on Health centered on HR 3, the “Lower Drug Costs Now Act of 2019,” introduced by Speaker Pelosi on Sept. 24, which would compel the Centers for Medicare & Medicaid Services to make deals with manufacturers on the maximum, reasonable price for the top 250 highest-cost drugs. If a drug maker refused to participate in the negotiation, the company would face a steep noncompliance fee, according to the bill, while manufacturers that overcharged Medicare or failed to offer the negotiated price would be subject to a civil penalty equal to 10 times the cost difference. The legislation includes a $2,000 out-of-pocket limit for Medicare patients.
If enacted, the legislation would transform the pricing landscape of medications and allow more families to access needed treatments, Speaker Pelosi said during the hearing.
“What it does, as you know; it ends the ban – imagine, there’s a ban on negotiating for lower drug prices,” she said. “So, it ends the ban for the [U.S. Department of Health and Human Services] Secretary now to have the opportunity to negotiate for lower prices. But, the even better news is that these drug prices will be lower not just for Medicare recipients, which was one of the original proposals, but for everyone. It will stop companies from ripping us off by charging five times, four times, three times what is charged in other countries.”
However, Republican legislators spent much of the hearing criticizing the bill, claiming the legislation was crafted behind closed doors by Democrats who made no efforts to gain Republican feedback.
“There is no debate that Republicans and Democrats want to work together to lower drug cost for consumers,” Ranking Member Greg Walden (R-Ore.) said during the hearing. “Madam chair, I have to strongly express my great frustration about the decision to sabotage both the tradition of this committee and the bipartisan work that you know was well underway to tackle high-cost drugs. ... We’ve worked in a bipartisan way up until now. I thought we were headed in a good faith down that same path until the speaker’s office dropped this partisan plan on our [progress]. This is partisan politics at its worst, and it’s an avoidable failure.”
Amid the partisan squabbling, legislators heard differing views from economists about the logistics of the legislation and whether the pricing negotiation model makes sense for the United States.
Gerard F. Anderson, PhD, a professor at Johns Hopkins University and director of the Johns Hopkins Center for Hospital Finance and Management in Baltimore told congressional leaders that negotiation between the government and drug makers is both possible and results in lower prices, even for drugs without therapeutic competition. He noted that the Medicaid program currently negotiates prices for supplemental rebates and that the Veterans Administration and the Department of Defense routinely negotiate discounts greater than the federal supply schedule. Dr. Anderson estimated that the Veterans Administration and the Department of Defense pay an average of 30%-40% less than Medicare prescription drug plans for the same medications.
“Allowing the federal government to negotiate prices for expensive drugs without competition in both the public and private sectors will be more effective in lowering drug prices for everyone,” Dr. Anderson said during testimony. “ Imposing financial penalties for drug companies will bring them to the table. International prices can be used to determine if the drug company is negotiating in good faith.”
But Benedic Ippolito, PhD, a research fellow for the American Enterprise Institute, Washington, raised concerns the legislation may cause reduced innovation. He said the United States accounts for about 60% of drug spending in the developed world, and that, because the market is so large, changes in spending will have first-order implications for the types of future drugs available, Dr. Ippolito said during testimony.
“Consider the incentives associated with the [bill’s] negotiation process,” he said. “Drugs that have no competitors would be subject to aggressive rate regulation by the [HHS] Secretary. The same is not true of drugs with at least one such competitor. It is entirely possible that being a second market entrant could prove substantially more profitable than bringing a novel therapeutic to market. Thus, the proposal could substantially depress incentives to pursue path-breaking drugs.”
Outside the hearing, Speaker Pelosi’s proposed bill is being praised by some physician groups, including the American College of Physicians. In a statement, ACP President Robert McLean, MD, said the college was pleased that the bill focuses on keeping drugs affordable for patients and includes provisions that would support research into new therapies and treatment options.
“ACP specifically supports the provisions that would allow Medicare to negotiate prices with manufacturers,” Dr. McLean said in the statement. “The College has longstanding policy supporting the ability of Medicare to leverage its purchasing power and directly negotiate with manufacturers for drug prices. Although ACP does not have policy on several of the specific provisions in the bill, we are supportive of its overall goals and direction, particularly the emphasis on negotiation and transparency in drug pricing.
Meanwhile, Senate Finance Chairman Charles E. Grassley, (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) released the statutory text of their own drug-pricing legislation on Sept. 25, titled “the Prescription Drug Pricing Reduction Act of 2019 (S-2543).” The bill calls for a number of changes to the Medicare Part D program, such as reduced beneficiary cost sharing and linking drug price increases to the rate of inflation. The legislation would also make more information available regarding pharmacy benefit manager practices and change how Medicare calculates Part B prescription drug payment amounts to lower spending and out-of-pocket costs for patients.
“President Trump has called on Congress to work together on a bipartisan bill to lower prescription drug prices, [and] there’s only one bipartisan bill in Congress to lower prescription drug prices that’s passed the committee process,” Chairman Grassley said in a statement. “This is it. Making prescription drugs more affordable consistently ranks as a top issue for Americans from every corner of the country. I encourage my colleagues on both sides of the aisle to come to the table and work with us to get a bill passed and signed into law.”
Legislators sparred about the best way to lower drug prices during a Sept. 25 hearing, bickering along partisan lines over new legislation by U.S. House Speaker Nancy Pelosi, (D-Calif.) that would require Medicare to negotiate drug prices with manufacturers.
The more than 4-hour hearing by the House Committee on Energy & Commerce Subcommittee on Health centered on HR 3, the “Lower Drug Costs Now Act of 2019,” introduced by Speaker Pelosi on Sept. 24, which would compel the Centers for Medicare & Medicaid Services to make deals with manufacturers on the maximum, reasonable price for the top 250 highest-cost drugs. If a drug maker refused to participate in the negotiation, the company would face a steep noncompliance fee, according to the bill, while manufacturers that overcharged Medicare or failed to offer the negotiated price would be subject to a civil penalty equal to 10 times the cost difference. The legislation includes a $2,000 out-of-pocket limit for Medicare patients.
If enacted, the legislation would transform the pricing landscape of medications and allow more families to access needed treatments, Speaker Pelosi said during the hearing.
“What it does, as you know; it ends the ban – imagine, there’s a ban on negotiating for lower drug prices,” she said. “So, it ends the ban for the [U.S. Department of Health and Human Services] Secretary now to have the opportunity to negotiate for lower prices. But, the even better news is that these drug prices will be lower not just for Medicare recipients, which was one of the original proposals, but for everyone. It will stop companies from ripping us off by charging five times, four times, three times what is charged in other countries.”
However, Republican legislators spent much of the hearing criticizing the bill, claiming the legislation was crafted behind closed doors by Democrats who made no efforts to gain Republican feedback.
“There is no debate that Republicans and Democrats want to work together to lower drug cost for consumers,” Ranking Member Greg Walden (R-Ore.) said during the hearing. “Madam chair, I have to strongly express my great frustration about the decision to sabotage both the tradition of this committee and the bipartisan work that you know was well underway to tackle high-cost drugs. ... We’ve worked in a bipartisan way up until now. I thought we were headed in a good faith down that same path until the speaker’s office dropped this partisan plan on our [progress]. This is partisan politics at its worst, and it’s an avoidable failure.”
Amid the partisan squabbling, legislators heard differing views from economists about the logistics of the legislation and whether the pricing negotiation model makes sense for the United States.
Gerard F. Anderson, PhD, a professor at Johns Hopkins University and director of the Johns Hopkins Center for Hospital Finance and Management in Baltimore told congressional leaders that negotiation between the government and drug makers is both possible and results in lower prices, even for drugs without therapeutic competition. He noted that the Medicaid program currently negotiates prices for supplemental rebates and that the Veterans Administration and the Department of Defense routinely negotiate discounts greater than the federal supply schedule. Dr. Anderson estimated that the Veterans Administration and the Department of Defense pay an average of 30%-40% less than Medicare prescription drug plans for the same medications.
“Allowing the federal government to negotiate prices for expensive drugs without competition in both the public and private sectors will be more effective in lowering drug prices for everyone,” Dr. Anderson said during testimony. “ Imposing financial penalties for drug companies will bring them to the table. International prices can be used to determine if the drug company is negotiating in good faith.”
But Benedic Ippolito, PhD, a research fellow for the American Enterprise Institute, Washington, raised concerns the legislation may cause reduced innovation. He said the United States accounts for about 60% of drug spending in the developed world, and that, because the market is so large, changes in spending will have first-order implications for the types of future drugs available, Dr. Ippolito said during testimony.
“Consider the incentives associated with the [bill’s] negotiation process,” he said. “Drugs that have no competitors would be subject to aggressive rate regulation by the [HHS] Secretary. The same is not true of drugs with at least one such competitor. It is entirely possible that being a second market entrant could prove substantially more profitable than bringing a novel therapeutic to market. Thus, the proposal could substantially depress incentives to pursue path-breaking drugs.”
Outside the hearing, Speaker Pelosi’s proposed bill is being praised by some physician groups, including the American College of Physicians. In a statement, ACP President Robert McLean, MD, said the college was pleased that the bill focuses on keeping drugs affordable for patients and includes provisions that would support research into new therapies and treatment options.
“ACP specifically supports the provisions that would allow Medicare to negotiate prices with manufacturers,” Dr. McLean said in the statement. “The College has longstanding policy supporting the ability of Medicare to leverage its purchasing power and directly negotiate with manufacturers for drug prices. Although ACP does not have policy on several of the specific provisions in the bill, we are supportive of its overall goals and direction, particularly the emphasis on negotiation and transparency in drug pricing.
Meanwhile, Senate Finance Chairman Charles E. Grassley, (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) released the statutory text of their own drug-pricing legislation on Sept. 25, titled “the Prescription Drug Pricing Reduction Act of 2019 (S-2543).” The bill calls for a number of changes to the Medicare Part D program, such as reduced beneficiary cost sharing and linking drug price increases to the rate of inflation. The legislation would also make more information available regarding pharmacy benefit manager practices and change how Medicare calculates Part B prescription drug payment amounts to lower spending and out-of-pocket costs for patients.
“President Trump has called on Congress to work together on a bipartisan bill to lower prescription drug prices, [and] there’s only one bipartisan bill in Congress to lower prescription drug prices that’s passed the committee process,” Chairman Grassley said in a statement. “This is it. Making prescription drugs more affordable consistently ranks as a top issue for Americans from every corner of the country. I encourage my colleagues on both sides of the aisle to come to the table and work with us to get a bill passed and signed into law.”
Legislators sparred about the best way to lower drug prices during a Sept. 25 hearing, bickering along partisan lines over new legislation by U.S. House Speaker Nancy Pelosi, (D-Calif.) that would require Medicare to negotiate drug prices with manufacturers.
The more than 4-hour hearing by the House Committee on Energy & Commerce Subcommittee on Health centered on HR 3, the “Lower Drug Costs Now Act of 2019,” introduced by Speaker Pelosi on Sept. 24, which would compel the Centers for Medicare & Medicaid Services to make deals with manufacturers on the maximum, reasonable price for the top 250 highest-cost drugs. If a drug maker refused to participate in the negotiation, the company would face a steep noncompliance fee, according to the bill, while manufacturers that overcharged Medicare or failed to offer the negotiated price would be subject to a civil penalty equal to 10 times the cost difference. The legislation includes a $2,000 out-of-pocket limit for Medicare patients.
If enacted, the legislation would transform the pricing landscape of medications and allow more families to access needed treatments, Speaker Pelosi said during the hearing.
“What it does, as you know; it ends the ban – imagine, there’s a ban on negotiating for lower drug prices,” she said. “So, it ends the ban for the [U.S. Department of Health and Human Services] Secretary now to have the opportunity to negotiate for lower prices. But, the even better news is that these drug prices will be lower not just for Medicare recipients, which was one of the original proposals, but for everyone. It will stop companies from ripping us off by charging five times, four times, three times what is charged in other countries.”
However, Republican legislators spent much of the hearing criticizing the bill, claiming the legislation was crafted behind closed doors by Democrats who made no efforts to gain Republican feedback.
“There is no debate that Republicans and Democrats want to work together to lower drug cost for consumers,” Ranking Member Greg Walden (R-Ore.) said during the hearing. “Madam chair, I have to strongly express my great frustration about the decision to sabotage both the tradition of this committee and the bipartisan work that you know was well underway to tackle high-cost drugs. ... We’ve worked in a bipartisan way up until now. I thought we were headed in a good faith down that same path until the speaker’s office dropped this partisan plan on our [progress]. This is partisan politics at its worst, and it’s an avoidable failure.”
Amid the partisan squabbling, legislators heard differing views from economists about the logistics of the legislation and whether the pricing negotiation model makes sense for the United States.
Gerard F. Anderson, PhD, a professor at Johns Hopkins University and director of the Johns Hopkins Center for Hospital Finance and Management in Baltimore told congressional leaders that negotiation between the government and drug makers is both possible and results in lower prices, even for drugs without therapeutic competition. He noted that the Medicaid program currently negotiates prices for supplemental rebates and that the Veterans Administration and the Department of Defense routinely negotiate discounts greater than the federal supply schedule. Dr. Anderson estimated that the Veterans Administration and the Department of Defense pay an average of 30%-40% less than Medicare prescription drug plans for the same medications.
“Allowing the federal government to negotiate prices for expensive drugs without competition in both the public and private sectors will be more effective in lowering drug prices for everyone,” Dr. Anderson said during testimony. “ Imposing financial penalties for drug companies will bring them to the table. International prices can be used to determine if the drug company is negotiating in good faith.”
But Benedic Ippolito, PhD, a research fellow for the American Enterprise Institute, Washington, raised concerns the legislation may cause reduced innovation. He said the United States accounts for about 60% of drug spending in the developed world, and that, because the market is so large, changes in spending will have first-order implications for the types of future drugs available, Dr. Ippolito said during testimony.
“Consider the incentives associated with the [bill’s] negotiation process,” he said. “Drugs that have no competitors would be subject to aggressive rate regulation by the [HHS] Secretary. The same is not true of drugs with at least one such competitor. It is entirely possible that being a second market entrant could prove substantially more profitable than bringing a novel therapeutic to market. Thus, the proposal could substantially depress incentives to pursue path-breaking drugs.”
Outside the hearing, Speaker Pelosi’s proposed bill is being praised by some physician groups, including the American College of Physicians. In a statement, ACP President Robert McLean, MD, said the college was pleased that the bill focuses on keeping drugs affordable for patients and includes provisions that would support research into new therapies and treatment options.
“ACP specifically supports the provisions that would allow Medicare to negotiate prices with manufacturers,” Dr. McLean said in the statement. “The College has longstanding policy supporting the ability of Medicare to leverage its purchasing power and directly negotiate with manufacturers for drug prices. Although ACP does not have policy on several of the specific provisions in the bill, we are supportive of its overall goals and direction, particularly the emphasis on negotiation and transparency in drug pricing.
Meanwhile, Senate Finance Chairman Charles E. Grassley, (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) released the statutory text of their own drug-pricing legislation on Sept. 25, titled “the Prescription Drug Pricing Reduction Act of 2019 (S-2543).” The bill calls for a number of changes to the Medicare Part D program, such as reduced beneficiary cost sharing and linking drug price increases to the rate of inflation. The legislation would also make more information available regarding pharmacy benefit manager practices and change how Medicare calculates Part B prescription drug payment amounts to lower spending and out-of-pocket costs for patients.
“President Trump has called on Congress to work together on a bipartisan bill to lower prescription drug prices, [and] there’s only one bipartisan bill in Congress to lower prescription drug prices that’s passed the committee process,” Chairman Grassley said in a statement. “This is it. Making prescription drugs more affordable consistently ranks as a top issue for Americans from every corner of the country. I encourage my colleagues on both sides of the aisle to come to the table and work with us to get a bill passed and signed into law.”
CMS weighs extension of Oncology Care Model
WASHINGTON – Federal officials are considering how they might extend a test of payment approaches meant to spur more coordinated cancer care beyond the program’s current 2021 end date.
Alexandra Chong, PhD, the team lead for the Oncology Care Model at the Center for Medicare and Medicaid Innovation (CMMI), said her agency found strong support for sustaining this kind of effort at “practice transformation.” The Oncology Care Model, which kicked off in 2016, involves about 175 practices and 10 insurers.
“As we look forward, we certainly recognize and appreciate the importance of the impact that [the Oncology Care Model] made and the desire for a continuation, either in an iteration of the current model or a different model,” Dr. Chong said during a panel discussion at a policy summit sponsored by the National Comprehensive Cancer Network (NCCN).
Dr. Chong said the Centers for Medicare & Medicaid Services is aware that there is “a lot of interest” in the future of the program. “We have heard from our practices that this model has been an opportunity for them to provide the care that they really want to provide,” Dr. Chong said.
A fellow panelist at the NCCN event, Kerin Adelson, MD, chief quality officer of Yale’s Smilow Cancer Hospital, New Haven, Conn., pressed for continuation of this kind of a payment test. Smilow has used revenue from the Oncology Care Model program to develop dashboards to measure individual clinicians’ patterns of care and share data with them, Dr. Adelson said.
The changes have been “transformative,” including allowing for hiring of additional support staff, Dr. Adelson said.
“I am terrified about what’s going to happen at the end of the program if there is not another model to transition to,” Dr. Adelson said. “We will potentially be looking at layoffs of these people who are so incredible and have done so much for the care we are giving.”
In May 2019, some of Dr. Chong’s CMMI colleagues published a report in the Journal of the National Cancer Institute about the experiences of clinics participating in the Oncology Care Model. For instance, the U.S. Oncology Network used Monthly Enhanced Oncology Services [MEOS] payments through the program for new hires such as navigators, social workers, additional advanced practice providers, “and even data analysts,” according to the report.
Still, there have been “growing pains” with the start-up of the model, Dr. Chong said. Fellow participants on the NCCN panel cited the introduction of costly new drugs as one wrinkle in the early days of the Oncology Care Model. Another is that physicians in practices signed up for the program sometimes are unaware of it.
The American Society of Clinical Oncology also is advocating for a continuation of a model along the lines of the Oncology Care Model.
“It would be good to see it continue. We’re certainly supportive of continuing to work on this model and refine it and see where it goes,” Stephen S. Grubbs, MD, ASCO’s vice president for clinical affairs, said in an interview.
Dr. Grubbs said that CMS’s Oncology Care Model could not be greatly expanded in its current form, as it imposes a steep administrative burden for both practices who participate, as well as for CMMI. The current model also does not serve smaller and rural practices well, he said.
ASCO is refining its own proposal for an alternative payment approach. The group plans to present its Patient-Centered Oncology Payment (PCOP) model, first published in 2015, before an influential federal advisory group, the Physician-Focused Payment Model Technical Advisory Committee (PTAC), at a meeting in 2020.
WASHINGTON – Federal officials are considering how they might extend a test of payment approaches meant to spur more coordinated cancer care beyond the program’s current 2021 end date.
Alexandra Chong, PhD, the team lead for the Oncology Care Model at the Center for Medicare and Medicaid Innovation (CMMI), said her agency found strong support for sustaining this kind of effort at “practice transformation.” The Oncology Care Model, which kicked off in 2016, involves about 175 practices and 10 insurers.
“As we look forward, we certainly recognize and appreciate the importance of the impact that [the Oncology Care Model] made and the desire for a continuation, either in an iteration of the current model or a different model,” Dr. Chong said during a panel discussion at a policy summit sponsored by the National Comprehensive Cancer Network (NCCN).
Dr. Chong said the Centers for Medicare & Medicaid Services is aware that there is “a lot of interest” in the future of the program. “We have heard from our practices that this model has been an opportunity for them to provide the care that they really want to provide,” Dr. Chong said.
A fellow panelist at the NCCN event, Kerin Adelson, MD, chief quality officer of Yale’s Smilow Cancer Hospital, New Haven, Conn., pressed for continuation of this kind of a payment test. Smilow has used revenue from the Oncology Care Model program to develop dashboards to measure individual clinicians’ patterns of care and share data with them, Dr. Adelson said.
The changes have been “transformative,” including allowing for hiring of additional support staff, Dr. Adelson said.
“I am terrified about what’s going to happen at the end of the program if there is not another model to transition to,” Dr. Adelson said. “We will potentially be looking at layoffs of these people who are so incredible and have done so much for the care we are giving.”
In May 2019, some of Dr. Chong’s CMMI colleagues published a report in the Journal of the National Cancer Institute about the experiences of clinics participating in the Oncology Care Model. For instance, the U.S. Oncology Network used Monthly Enhanced Oncology Services [MEOS] payments through the program for new hires such as navigators, social workers, additional advanced practice providers, “and even data analysts,” according to the report.
Still, there have been “growing pains” with the start-up of the model, Dr. Chong said. Fellow participants on the NCCN panel cited the introduction of costly new drugs as one wrinkle in the early days of the Oncology Care Model. Another is that physicians in practices signed up for the program sometimes are unaware of it.
The American Society of Clinical Oncology also is advocating for a continuation of a model along the lines of the Oncology Care Model.
“It would be good to see it continue. We’re certainly supportive of continuing to work on this model and refine it and see where it goes,” Stephen S. Grubbs, MD, ASCO’s vice president for clinical affairs, said in an interview.
Dr. Grubbs said that CMS’s Oncology Care Model could not be greatly expanded in its current form, as it imposes a steep administrative burden for both practices who participate, as well as for CMMI. The current model also does not serve smaller and rural practices well, he said.
ASCO is refining its own proposal for an alternative payment approach. The group plans to present its Patient-Centered Oncology Payment (PCOP) model, first published in 2015, before an influential federal advisory group, the Physician-Focused Payment Model Technical Advisory Committee (PTAC), at a meeting in 2020.
WASHINGTON – Federal officials are considering how they might extend a test of payment approaches meant to spur more coordinated cancer care beyond the program’s current 2021 end date.
Alexandra Chong, PhD, the team lead for the Oncology Care Model at the Center for Medicare and Medicaid Innovation (CMMI), said her agency found strong support for sustaining this kind of effort at “practice transformation.” The Oncology Care Model, which kicked off in 2016, involves about 175 practices and 10 insurers.
“As we look forward, we certainly recognize and appreciate the importance of the impact that [the Oncology Care Model] made and the desire for a continuation, either in an iteration of the current model or a different model,” Dr. Chong said during a panel discussion at a policy summit sponsored by the National Comprehensive Cancer Network (NCCN).
Dr. Chong said the Centers for Medicare & Medicaid Services is aware that there is “a lot of interest” in the future of the program. “We have heard from our practices that this model has been an opportunity for them to provide the care that they really want to provide,” Dr. Chong said.
A fellow panelist at the NCCN event, Kerin Adelson, MD, chief quality officer of Yale’s Smilow Cancer Hospital, New Haven, Conn., pressed for continuation of this kind of a payment test. Smilow has used revenue from the Oncology Care Model program to develop dashboards to measure individual clinicians’ patterns of care and share data with them, Dr. Adelson said.
The changes have been “transformative,” including allowing for hiring of additional support staff, Dr. Adelson said.
“I am terrified about what’s going to happen at the end of the program if there is not another model to transition to,” Dr. Adelson said. “We will potentially be looking at layoffs of these people who are so incredible and have done so much for the care we are giving.”
In May 2019, some of Dr. Chong’s CMMI colleagues published a report in the Journal of the National Cancer Institute about the experiences of clinics participating in the Oncology Care Model. For instance, the U.S. Oncology Network used Monthly Enhanced Oncology Services [MEOS] payments through the program for new hires such as navigators, social workers, additional advanced practice providers, “and even data analysts,” according to the report.
Still, there have been “growing pains” with the start-up of the model, Dr. Chong said. Fellow participants on the NCCN panel cited the introduction of costly new drugs as one wrinkle in the early days of the Oncology Care Model. Another is that physicians in practices signed up for the program sometimes are unaware of it.
The American Society of Clinical Oncology also is advocating for a continuation of a model along the lines of the Oncology Care Model.
“It would be good to see it continue. We’re certainly supportive of continuing to work on this model and refine it and see where it goes,” Stephen S. Grubbs, MD, ASCO’s vice president for clinical affairs, said in an interview.
Dr. Grubbs said that CMS’s Oncology Care Model could not be greatly expanded in its current form, as it imposes a steep administrative burden for both practices who participate, as well as for CMMI. The current model also does not serve smaller and rural practices well, he said.
ASCO is refining its own proposal for an alternative payment approach. The group plans to present its Patient-Centered Oncology Payment (PCOP) model, first published in 2015, before an influential federal advisory group, the Physician-Focused Payment Model Technical Advisory Committee (PTAC), at a meeting in 2020.
REPORTING FROM NCCN POLICY SUMMIT 2019
Cancer drug prices higher in U.S. than Europe; don’t correlate with clinical benefit
BARCELONA – Cancer drug prices are significantly higher in the United States than in Europe, and they don’t correlate with clinical benefit in either region, according to an analysis of drug costs and value-related scores between 2009 and 2018.
Of 63 cancer drugs approved by the Food and Drug Administration between 2009 and 2017 and by the European Medicines Agency as of December 31, 2018, 46 (73%) were approved for solid tumors, and 17 (27%) for hematologic malignancies. Median cancer drug prices in Europe were 52% lower than U.S. prices, Kerstin N. Vokinger, MD, Academic Chair For Health Policy at the Institute For Primary Care, University Hospital Zürich, and colleagues found.
The findings were released via press release at the European Society for Medical Oncology (ESMO) Congress and will be part of a poster discussion session there.
The investigators found no association between monthly treatment cost and the American Society of Clinical Oncology Value Framework (ASCO-VF) or the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) scores in any country. They also found no association between the price differential in the United States and Europe and either ASCO-VF (P = .599) or ESMO-MBCS (P = .321) scores.
For the analysis, they assessed U.S. average sales prices or, when those weren’t available, wholesale acquisition costs as of Feb. 1, 2019, and compared them with comparable currency-adjusted ex-factory drug costs in England, France, Germany, and Switzerland.
ASCO-VF and ESMO-MCBS scores were then assessed based on the highest score from pivotal trials supporting each solid tumor drug.
The median monthly cost for drugs with low-benefit scores on ESMO-MCBS ranged from $4,361-$5,273 in the European countries, compared with $12,436 in the United States, according to the release.
“Drug costs were not associated with clinical benefit score in any of the countries we looked at. For example, some of the more expensive drugs for prostate and lung cancer in Switzerland had lower ESMO-MCBS scores, while cheaper drugs had higher scores,” said Dr. Vokinger, who also is affiliated with the Program on Regulation, Therapeutics, and Law at Harvard Medical School, Boston. “It is important that drug pricing is aligned with clinical value and that our limited resources are spent on innovative medicines that offer improved outcomes.”
Barbara Kiesewetter, MD, of the Medical University of Vienna and a member of the ESMO-MCBS Working Group, said the findings underscore the importance of using the scoring systems in clinical practice to assist in treatment-related decision making.
The ESMO-MCBS, for example, is available online, easy to use, and helps explain “the factors that are used to grade the clinical benefit of medicines.
“It’s very important to have this validated score not only for daily decision making, but to influence reimbursement decisions and reduce treatment disparities,” she said, also noting in the release that “[c]ost is one of the main reasons why patients are denied access to the newer anticancer drugs.”
“By showing which drugs are most likely to be worth the higher cost, we can hopefully improve access to the drugs with greatest value so that patients receive standardized, optimal therapy wherever they live,” she said.
The study was funded by the Swiss Cancer League. The authors reported having no conflicts of interest.
SOURCE: Vokinger KN et al. ESMO 2019, Abstract 1631PD-PR.
BARCELONA – Cancer drug prices are significantly higher in the United States than in Europe, and they don’t correlate with clinical benefit in either region, according to an analysis of drug costs and value-related scores between 2009 and 2018.
Of 63 cancer drugs approved by the Food and Drug Administration between 2009 and 2017 and by the European Medicines Agency as of December 31, 2018, 46 (73%) were approved for solid tumors, and 17 (27%) for hematologic malignancies. Median cancer drug prices in Europe were 52% lower than U.S. prices, Kerstin N. Vokinger, MD, Academic Chair For Health Policy at the Institute For Primary Care, University Hospital Zürich, and colleagues found.
The findings were released via press release at the European Society for Medical Oncology (ESMO) Congress and will be part of a poster discussion session there.
The investigators found no association between monthly treatment cost and the American Society of Clinical Oncology Value Framework (ASCO-VF) or the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) scores in any country. They also found no association between the price differential in the United States and Europe and either ASCO-VF (P = .599) or ESMO-MBCS (P = .321) scores.
For the analysis, they assessed U.S. average sales prices or, when those weren’t available, wholesale acquisition costs as of Feb. 1, 2019, and compared them with comparable currency-adjusted ex-factory drug costs in England, France, Germany, and Switzerland.
ASCO-VF and ESMO-MCBS scores were then assessed based on the highest score from pivotal trials supporting each solid tumor drug.
The median monthly cost for drugs with low-benefit scores on ESMO-MCBS ranged from $4,361-$5,273 in the European countries, compared with $12,436 in the United States, according to the release.
“Drug costs were not associated with clinical benefit score in any of the countries we looked at. For example, some of the more expensive drugs for prostate and lung cancer in Switzerland had lower ESMO-MCBS scores, while cheaper drugs had higher scores,” said Dr. Vokinger, who also is affiliated with the Program on Regulation, Therapeutics, and Law at Harvard Medical School, Boston. “It is important that drug pricing is aligned with clinical value and that our limited resources are spent on innovative medicines that offer improved outcomes.”
Barbara Kiesewetter, MD, of the Medical University of Vienna and a member of the ESMO-MCBS Working Group, said the findings underscore the importance of using the scoring systems in clinical practice to assist in treatment-related decision making.
The ESMO-MCBS, for example, is available online, easy to use, and helps explain “the factors that are used to grade the clinical benefit of medicines.
“It’s very important to have this validated score not only for daily decision making, but to influence reimbursement decisions and reduce treatment disparities,” she said, also noting in the release that “[c]ost is one of the main reasons why patients are denied access to the newer anticancer drugs.”
“By showing which drugs are most likely to be worth the higher cost, we can hopefully improve access to the drugs with greatest value so that patients receive standardized, optimal therapy wherever they live,” she said.
The study was funded by the Swiss Cancer League. The authors reported having no conflicts of interest.
SOURCE: Vokinger KN et al. ESMO 2019, Abstract 1631PD-PR.
BARCELONA – Cancer drug prices are significantly higher in the United States than in Europe, and they don’t correlate with clinical benefit in either region, according to an analysis of drug costs and value-related scores between 2009 and 2018.
Of 63 cancer drugs approved by the Food and Drug Administration between 2009 and 2017 and by the European Medicines Agency as of December 31, 2018, 46 (73%) were approved for solid tumors, and 17 (27%) for hematologic malignancies. Median cancer drug prices in Europe were 52% lower than U.S. prices, Kerstin N. Vokinger, MD, Academic Chair For Health Policy at the Institute For Primary Care, University Hospital Zürich, and colleagues found.
The findings were released via press release at the European Society for Medical Oncology (ESMO) Congress and will be part of a poster discussion session there.
The investigators found no association between monthly treatment cost and the American Society of Clinical Oncology Value Framework (ASCO-VF) or the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) scores in any country. They also found no association between the price differential in the United States and Europe and either ASCO-VF (P = .599) or ESMO-MBCS (P = .321) scores.
For the analysis, they assessed U.S. average sales prices or, when those weren’t available, wholesale acquisition costs as of Feb. 1, 2019, and compared them with comparable currency-adjusted ex-factory drug costs in England, France, Germany, and Switzerland.
ASCO-VF and ESMO-MCBS scores were then assessed based on the highest score from pivotal trials supporting each solid tumor drug.
The median monthly cost for drugs with low-benefit scores on ESMO-MCBS ranged from $4,361-$5,273 in the European countries, compared with $12,436 in the United States, according to the release.
“Drug costs were not associated with clinical benefit score in any of the countries we looked at. For example, some of the more expensive drugs for prostate and lung cancer in Switzerland had lower ESMO-MCBS scores, while cheaper drugs had higher scores,” said Dr. Vokinger, who also is affiliated with the Program on Regulation, Therapeutics, and Law at Harvard Medical School, Boston. “It is important that drug pricing is aligned with clinical value and that our limited resources are spent on innovative medicines that offer improved outcomes.”
Barbara Kiesewetter, MD, of the Medical University of Vienna and a member of the ESMO-MCBS Working Group, said the findings underscore the importance of using the scoring systems in clinical practice to assist in treatment-related decision making.
The ESMO-MCBS, for example, is available online, easy to use, and helps explain “the factors that are used to grade the clinical benefit of medicines.
“It’s very important to have this validated score not only for daily decision making, but to influence reimbursement decisions and reduce treatment disparities,” she said, also noting in the release that “[c]ost is one of the main reasons why patients are denied access to the newer anticancer drugs.”
“By showing which drugs are most likely to be worth the higher cost, we can hopefully improve access to the drugs with greatest value so that patients receive standardized, optimal therapy wherever they live,” she said.
The study was funded by the Swiss Cancer League. The authors reported having no conflicts of interest.
SOURCE: Vokinger KN et al. ESMO 2019, Abstract 1631PD-PR.
REPORTING FROM ESMO 2019
Happiness in my solo practice
I don’t want to rule the world.
Some doctors do, albeit in a non–Attila-the-Hun sort of way. They want to have offices on every street corner, in every suburb of a given city, sometimes more than one city. Like the Starbucks of medicine.
That’s not me. I’m happy in my little one-office world.
Maybe I just don’t have the ambition, or the business mindset, or whatever it takes to want to do that. I understand it’s all part of wanting to be successful, and obviously those doctors are more driven in that direction than I am. The more offices, the more patients can be seen, and the more money you make.
It’s not quite that simple, though. No one can be in more than one place at the same time, so to see more patients at more places you need more doctors. To pay more doctors requires more money, which in turn requires more patients.
There’s nothing wrong with taking over the world (or at least a suburb) if you like that sort of thing. But to me, more money brings more headaches. More offices to rent, more staff to hire, more people to handle billing, IT, HR, payroll, accounting, contracts, and so on.
You can have it. I’ve taken over all the world I want, in my case a 1,200-square-foot suite on the second floor of a small-to-medium-size medical building. To some that may sound unambitious, but to me, it’s perfect.
I know where my Keurig, Sodastream, and office supplies are. Except for my secretary and her cheerfully rambunctious young daughter, I don’t have to worry about sharing stuff here, or if anyone wants a different carpet color, or what’s going on at a satellite office halfway across town.
If other doctors want to try and take over the world, more power to them, but I’m happy with this. Enough is as good as a feast.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t want to rule the world.
Some doctors do, albeit in a non–Attila-the-Hun sort of way. They want to have offices on every street corner, in every suburb of a given city, sometimes more than one city. Like the Starbucks of medicine.
That’s not me. I’m happy in my little one-office world.
Maybe I just don’t have the ambition, or the business mindset, or whatever it takes to want to do that. I understand it’s all part of wanting to be successful, and obviously those doctors are more driven in that direction than I am. The more offices, the more patients can be seen, and the more money you make.
It’s not quite that simple, though. No one can be in more than one place at the same time, so to see more patients at more places you need more doctors. To pay more doctors requires more money, which in turn requires more patients.
There’s nothing wrong with taking over the world (or at least a suburb) if you like that sort of thing. But to me, more money brings more headaches. More offices to rent, more staff to hire, more people to handle billing, IT, HR, payroll, accounting, contracts, and so on.
You can have it. I’ve taken over all the world I want, in my case a 1,200-square-foot suite on the second floor of a small-to-medium-size medical building. To some that may sound unambitious, but to me, it’s perfect.
I know where my Keurig, Sodastream, and office supplies are. Except for my secretary and her cheerfully rambunctious young daughter, I don’t have to worry about sharing stuff here, or if anyone wants a different carpet color, or what’s going on at a satellite office halfway across town.
If other doctors want to try and take over the world, more power to them, but I’m happy with this. Enough is as good as a feast.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t want to rule the world.
Some doctors do, albeit in a non–Attila-the-Hun sort of way. They want to have offices on every street corner, in every suburb of a given city, sometimes more than one city. Like the Starbucks of medicine.
That’s not me. I’m happy in my little one-office world.
Maybe I just don’t have the ambition, or the business mindset, or whatever it takes to want to do that. I understand it’s all part of wanting to be successful, and obviously those doctors are more driven in that direction than I am. The more offices, the more patients can be seen, and the more money you make.
It’s not quite that simple, though. No one can be in more than one place at the same time, so to see more patients at more places you need more doctors. To pay more doctors requires more money, which in turn requires more patients.
There’s nothing wrong with taking over the world (or at least a suburb) if you like that sort of thing. But to me, more money brings more headaches. More offices to rent, more staff to hire, more people to handle billing, IT, HR, payroll, accounting, contracts, and so on.
You can have it. I’ve taken over all the world I want, in my case a 1,200-square-foot suite on the second floor of a small-to-medium-size medical building. To some that may sound unambitious, but to me, it’s perfect.
I know where my Keurig, Sodastream, and office supplies are. Except for my secretary and her cheerfully rambunctious young daughter, I don’t have to worry about sharing stuff here, or if anyone wants a different carpet color, or what’s going on at a satellite office halfway across town.
If other doctors want to try and take over the world, more power to them, but I’m happy with this. Enough is as good as a feast.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Multiple Myeloma Research Consortium, cancer centers add new leaders
Hearn Jay Cho, MD, PhD, has been appointed chief medical officer of the Multiple Myeloma Research Foundation (MMRF). In this role, Dr. Cho will develop a clinical research strategy and accelerate drug development programs for the MMRF. He will also lead the Multiple Myeloma Research Consortium, a group of 25 research centers focused on multiple myeloma.
Dr. Cho will continue on as an associate professor of medicine at the Icahn School of Medicine at Mt. Sinai in New York, and as an attending physician with the multiple myeloma service at the Mt. Sinai Tisch Cancer Institute. He will also continue to manage his lab at Mt. Sinai.
Michael Jay Styler, MD, has joined the department of hematology/oncology as an associate professor in the academic clinician track as part of the bone marrow transplant program at Fox Chase Cancer Center in Philadelphia.
Dr. Styler was previously medical director of the transplant center, apheresis center, and collection center at Hahnemann University Hospital in Philadelphia, as well as medical director of the stem cell transplant program and the clinical service chief of hematology/oncology. Dr. Styler was also an associate professor at Drexel University, Philadelphia, where he served as division director of hematology/oncology and associate fellowship director of the hematology/oncology program.
Jaspreet Chahal, MD, has joined the Oncology Institute of Hope and Innovation, which has locations in Arizona, California, and Nevada. Dr. Chahal will serve patients in Tucson and Green Valley, Ariz.
Dr. Chahal has a doctor of medicine degree from St. George’s University in West Indies, Grenada. She completed her internship in hematology and oncology at Hofstra University, Hempstead, N.Y., and her residency in internal medicine at the University of Arizona in Tucson.
Marcin Chwistek, MD, has been appointed editor in chief of AAHPM Quarterly, which is published by the American Academy of Hospice and Palliative Medicine. Dr. Chwistek is an associate professor in the department of hematology/oncology at Fox Chase Cancer Center in Philadelphia, where he is also director of the pain and palliative care program.
As editor in chief of AAHPM Quarterly, Dr. Chwistek will work with other members of the editorial board to develop content related to hospice care and palliative medicine. Dr. Chwistek previously served as the associate editor in chief of AAHPM Quarterly and writes a column that appears in every issue.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com , and you could be featured in Movers in Medicine.
Hearn Jay Cho, MD, PhD, has been appointed chief medical officer of the Multiple Myeloma Research Foundation (MMRF). In this role, Dr. Cho will develop a clinical research strategy and accelerate drug development programs for the MMRF. He will also lead the Multiple Myeloma Research Consortium, a group of 25 research centers focused on multiple myeloma.
Dr. Cho will continue on as an associate professor of medicine at the Icahn School of Medicine at Mt. Sinai in New York, and as an attending physician with the multiple myeloma service at the Mt. Sinai Tisch Cancer Institute. He will also continue to manage his lab at Mt. Sinai.
Michael Jay Styler, MD, has joined the department of hematology/oncology as an associate professor in the academic clinician track as part of the bone marrow transplant program at Fox Chase Cancer Center in Philadelphia.
Dr. Styler was previously medical director of the transplant center, apheresis center, and collection center at Hahnemann University Hospital in Philadelphia, as well as medical director of the stem cell transplant program and the clinical service chief of hematology/oncology. Dr. Styler was also an associate professor at Drexel University, Philadelphia, where he served as division director of hematology/oncology and associate fellowship director of the hematology/oncology program.
Jaspreet Chahal, MD, has joined the Oncology Institute of Hope and Innovation, which has locations in Arizona, California, and Nevada. Dr. Chahal will serve patients in Tucson and Green Valley, Ariz.
Dr. Chahal has a doctor of medicine degree from St. George’s University in West Indies, Grenada. She completed her internship in hematology and oncology at Hofstra University, Hempstead, N.Y., and her residency in internal medicine at the University of Arizona in Tucson.
Marcin Chwistek, MD, has been appointed editor in chief of AAHPM Quarterly, which is published by the American Academy of Hospice and Palliative Medicine. Dr. Chwistek is an associate professor in the department of hematology/oncology at Fox Chase Cancer Center in Philadelphia, where he is also director of the pain and palliative care program.
As editor in chief of AAHPM Quarterly, Dr. Chwistek will work with other members of the editorial board to develop content related to hospice care and palliative medicine. Dr. Chwistek previously served as the associate editor in chief of AAHPM Quarterly and writes a column that appears in every issue.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com , and you could be featured in Movers in Medicine.
Hearn Jay Cho, MD, PhD, has been appointed chief medical officer of the Multiple Myeloma Research Foundation (MMRF). In this role, Dr. Cho will develop a clinical research strategy and accelerate drug development programs for the MMRF. He will also lead the Multiple Myeloma Research Consortium, a group of 25 research centers focused on multiple myeloma.
Dr. Cho will continue on as an associate professor of medicine at the Icahn School of Medicine at Mt. Sinai in New York, and as an attending physician with the multiple myeloma service at the Mt. Sinai Tisch Cancer Institute. He will also continue to manage his lab at Mt. Sinai.
Michael Jay Styler, MD, has joined the department of hematology/oncology as an associate professor in the academic clinician track as part of the bone marrow transplant program at Fox Chase Cancer Center in Philadelphia.
Dr. Styler was previously medical director of the transplant center, apheresis center, and collection center at Hahnemann University Hospital in Philadelphia, as well as medical director of the stem cell transplant program and the clinical service chief of hematology/oncology. Dr. Styler was also an associate professor at Drexel University, Philadelphia, where he served as division director of hematology/oncology and associate fellowship director of the hematology/oncology program.
Jaspreet Chahal, MD, has joined the Oncology Institute of Hope and Innovation, which has locations in Arizona, California, and Nevada. Dr. Chahal will serve patients in Tucson and Green Valley, Ariz.
Dr. Chahal has a doctor of medicine degree from St. George’s University in West Indies, Grenada. She completed her internship in hematology and oncology at Hofstra University, Hempstead, N.Y., and her residency in internal medicine at the University of Arizona in Tucson.
Marcin Chwistek, MD, has been appointed editor in chief of AAHPM Quarterly, which is published by the American Academy of Hospice and Palliative Medicine. Dr. Chwistek is an associate professor in the department of hematology/oncology at Fox Chase Cancer Center in Philadelphia, where he is also director of the pain and palliative care program.
As editor in chief of AAHPM Quarterly, Dr. Chwistek will work with other members of the editorial board to develop content related to hospice care and palliative medicine. Dr. Chwistek previously served as the associate editor in chief of AAHPM Quarterly and writes a column that appears in every issue.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com , and you could be featured in Movers in Medicine.
Don’t let legal considerations drive your decision making about a new job offer
SAN DIEGO – When weighing an offer to join a dermatology practice, don’t allow legal considerations to drive your decision making, Mathew M. Avram, MD, JD, advised at the annual Masters of Aesthetics Symposium.
“Determine your professional interests and follow them accordingly,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “Your decision as to whether to join a particular practice should not be based upon a contract. Make certain to determine the business end of your deal with any new employer first: the compensation, the work hours, etc. Decide that upfront before you get into the legal details.”
The way he sees it, your relationship with your employer will always be paramount. “This is very important,” said Dr. Avram, who practiced law before he became a dermatologist. “A good contract with a bad employer will not save you from an unhappy employment experience. Few contracts ever end up in litigation, and you cannot sue your way to a happier work experience. Trust your intuition about people; trust your underlying interest in potentially working with this person, and work from there.”
Whether you choose to work in an academic practice, a private practice, or a hybrid, the key issues to address include compensation, benefits, work hours, job responsibilities, and future partnership possibilities. “As an employee, you have the greatest leverage prior to signing an agreement,” he said. “This is the time to ask for what you want.” This may include special requests such as asking the employer to purchase lasers or other special equipment for your office, or to set aside dedicated clinical time for cosmetic procedures, so that you can build a cosmetic practice.
If you’re mulling over a job offer in academics, consider asking for an academic title, what the scope of your authority is, and about your ability to hire or prevent the hiring of others. “Let’s say you’re starting a laser center in your academic center,” said Dr. Avram, who is a past president of the American Society for Laser Medicine and Surgery. “You may not want to find out after you’ve signed your contract that they’ve hired five other people to do the same thing.”
If you’re considering a job offer from a private practice, ask for specifics about bonuses, partnership track, scope of practice, and device purchases. “You want to have that decided before you sign,” he said. “But these are business issues, not legal issues. An agreement needs to be made between you and your employer as to these issues. Once they’ve been agreed upon, it’s time to proceed with a contract. This is where legal advice becomes helpful.” The trick is to negotiate in good faith while maintaining your relationship with your new employer. “Do not destroy your relationship over legal points, but do not cave on crucial issues for fear of upsetting your new employer,” he said.
In law school, Dr. Avram learned that there is no such thing as a standard employment contract. Any contract can be amended, no matter how large or small the institution. “You can amend a mortgage agreement, so you can certainly amend a physician employment contract,” he said. “If the employer is not going to put their commitments in writing, they are probably not going to honor that commitment to you. Written agreements supersede all preceding oral agreements. Each key term needs to be stated explicitly. If not, you have lost all leverage to enforce your initial agreement.”
Key provisions are restrictive covenants and at-will employment. Dr. Avram defined restrictive covenants as contractual agreements that attempt to restrict an employee so as to limit that person’s ability to compete. “This can include noncompete clauses, nonsolicitation agreements, and confidentiality agreements,” he said. “A noncompete agreement prohibits a doctor from competing against their former practice within a specific geographic area for a period of time after the employment has ended. The nonsolicitation agreement restricts the manner and time during which a physician can solicit patients or employees from a practice after termination of employment. A confidentiality agreement is usually indefinite as to time and it restricts the employee from disclosing confidential practice information.”
State laws, he continued, govern restrictive covenants. Some states prohibit them. States that allow them limit their scope to prevent undue burden on the employee’s ability to make a living after termination. “Restrictive covenants in cities need to be narrower than those in rural areas,” he said. “Overly broad restrictive covenants may be unenforceable. Courts can ‘blue line’ covenants to make them more reasonable in scope.”
Next, Dr. Avram discussed involuntary termination in the workplace. Termination with cause means that termination can only result from violation of policy or ethics code violation or significantly poor performance. “In the absence of these circumstances, termination cannot legally proceed,” he said. “This protects the employee from an arbitrary termination.”
On the other hand, at-will employees can be terminated without cause. “It’s a much easier standard to terminate an at-will employee, as long as the reason is not illegal,” he said. “In academic centers, your employment as a physician may require a for-cause termination, whereas your administrative title may be without cause.”
One way to avoid potential legal trouble is to abide by your employer’s rules governing a physician’s interactions with industry. “It is foolish to break those rules,” Dr. Avram said. “Transparency ahead of time is always your best policy. Remember: Your day job is far more valuable to you than part-time work with industry. Also, know that pharma payments are publicly reported by the Centers for Medicare & Medicaid Services.” When it comes to establishing contracts with industry, “read them carefully and have your academic center approve them, if applicable,” he said. “Watch out for noncompete clauses, and some contracts require that you do not speak negatively about your findings. That’s something you should try to avoid.”
Dr. Avram also advised clinicians not to discuss intellectual property with industry representatives. “Do not give away your intellectual property to industry when you’re consulting with them,” he said. “It is easier to do than you might think. If you have a great idea, keep it to yourself or get it protected. This is much easier for an institution where they want you to do these things. They take ownership of a lot of it, but they make it easier to do. Otherwise, you have to go through the expensive process of getting a patent.”
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, InMode, and Zalea, and intellectual property rights with Cytrellis.
SAN DIEGO – When weighing an offer to join a dermatology practice, don’t allow legal considerations to drive your decision making, Mathew M. Avram, MD, JD, advised at the annual Masters of Aesthetics Symposium.
“Determine your professional interests and follow them accordingly,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “Your decision as to whether to join a particular practice should not be based upon a contract. Make certain to determine the business end of your deal with any new employer first: the compensation, the work hours, etc. Decide that upfront before you get into the legal details.”
The way he sees it, your relationship with your employer will always be paramount. “This is very important,” said Dr. Avram, who practiced law before he became a dermatologist. “A good contract with a bad employer will not save you from an unhappy employment experience. Few contracts ever end up in litigation, and you cannot sue your way to a happier work experience. Trust your intuition about people; trust your underlying interest in potentially working with this person, and work from there.”
Whether you choose to work in an academic practice, a private practice, or a hybrid, the key issues to address include compensation, benefits, work hours, job responsibilities, and future partnership possibilities. “As an employee, you have the greatest leverage prior to signing an agreement,” he said. “This is the time to ask for what you want.” This may include special requests such as asking the employer to purchase lasers or other special equipment for your office, or to set aside dedicated clinical time for cosmetic procedures, so that you can build a cosmetic practice.
If you’re mulling over a job offer in academics, consider asking for an academic title, what the scope of your authority is, and about your ability to hire or prevent the hiring of others. “Let’s say you’re starting a laser center in your academic center,” said Dr. Avram, who is a past president of the American Society for Laser Medicine and Surgery. “You may not want to find out after you’ve signed your contract that they’ve hired five other people to do the same thing.”
If you’re considering a job offer from a private practice, ask for specifics about bonuses, partnership track, scope of practice, and device purchases. “You want to have that decided before you sign,” he said. “But these are business issues, not legal issues. An agreement needs to be made between you and your employer as to these issues. Once they’ve been agreed upon, it’s time to proceed with a contract. This is where legal advice becomes helpful.” The trick is to negotiate in good faith while maintaining your relationship with your new employer. “Do not destroy your relationship over legal points, but do not cave on crucial issues for fear of upsetting your new employer,” he said.
In law school, Dr. Avram learned that there is no such thing as a standard employment contract. Any contract can be amended, no matter how large or small the institution. “You can amend a mortgage agreement, so you can certainly amend a physician employment contract,” he said. “If the employer is not going to put their commitments in writing, they are probably not going to honor that commitment to you. Written agreements supersede all preceding oral agreements. Each key term needs to be stated explicitly. If not, you have lost all leverage to enforce your initial agreement.”
Key provisions are restrictive covenants and at-will employment. Dr. Avram defined restrictive covenants as contractual agreements that attempt to restrict an employee so as to limit that person’s ability to compete. “This can include noncompete clauses, nonsolicitation agreements, and confidentiality agreements,” he said. “A noncompete agreement prohibits a doctor from competing against their former practice within a specific geographic area for a period of time after the employment has ended. The nonsolicitation agreement restricts the manner and time during which a physician can solicit patients or employees from a practice after termination of employment. A confidentiality agreement is usually indefinite as to time and it restricts the employee from disclosing confidential practice information.”
State laws, he continued, govern restrictive covenants. Some states prohibit them. States that allow them limit their scope to prevent undue burden on the employee’s ability to make a living after termination. “Restrictive covenants in cities need to be narrower than those in rural areas,” he said. “Overly broad restrictive covenants may be unenforceable. Courts can ‘blue line’ covenants to make them more reasonable in scope.”
Next, Dr. Avram discussed involuntary termination in the workplace. Termination with cause means that termination can only result from violation of policy or ethics code violation or significantly poor performance. “In the absence of these circumstances, termination cannot legally proceed,” he said. “This protects the employee from an arbitrary termination.”
On the other hand, at-will employees can be terminated without cause. “It’s a much easier standard to terminate an at-will employee, as long as the reason is not illegal,” he said. “In academic centers, your employment as a physician may require a for-cause termination, whereas your administrative title may be without cause.”
One way to avoid potential legal trouble is to abide by your employer’s rules governing a physician’s interactions with industry. “It is foolish to break those rules,” Dr. Avram said. “Transparency ahead of time is always your best policy. Remember: Your day job is far more valuable to you than part-time work with industry. Also, know that pharma payments are publicly reported by the Centers for Medicare & Medicaid Services.” When it comes to establishing contracts with industry, “read them carefully and have your academic center approve them, if applicable,” he said. “Watch out for noncompete clauses, and some contracts require that you do not speak negatively about your findings. That’s something you should try to avoid.”
Dr. Avram also advised clinicians not to discuss intellectual property with industry representatives. “Do not give away your intellectual property to industry when you’re consulting with them,” he said. “It is easier to do than you might think. If you have a great idea, keep it to yourself or get it protected. This is much easier for an institution where they want you to do these things. They take ownership of a lot of it, but they make it easier to do. Otherwise, you have to go through the expensive process of getting a patent.”
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, InMode, and Zalea, and intellectual property rights with Cytrellis.
SAN DIEGO – When weighing an offer to join a dermatology practice, don’t allow legal considerations to drive your decision making, Mathew M. Avram, MD, JD, advised at the annual Masters of Aesthetics Symposium.
“Determine your professional interests and follow them accordingly,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “Your decision as to whether to join a particular practice should not be based upon a contract. Make certain to determine the business end of your deal with any new employer first: the compensation, the work hours, etc. Decide that upfront before you get into the legal details.”
The way he sees it, your relationship with your employer will always be paramount. “This is very important,” said Dr. Avram, who practiced law before he became a dermatologist. “A good contract with a bad employer will not save you from an unhappy employment experience. Few contracts ever end up in litigation, and you cannot sue your way to a happier work experience. Trust your intuition about people; trust your underlying interest in potentially working with this person, and work from there.”
Whether you choose to work in an academic practice, a private practice, or a hybrid, the key issues to address include compensation, benefits, work hours, job responsibilities, and future partnership possibilities. “As an employee, you have the greatest leverage prior to signing an agreement,” he said. “This is the time to ask for what you want.” This may include special requests such as asking the employer to purchase lasers or other special equipment for your office, or to set aside dedicated clinical time for cosmetic procedures, so that you can build a cosmetic practice.
If you’re mulling over a job offer in academics, consider asking for an academic title, what the scope of your authority is, and about your ability to hire or prevent the hiring of others. “Let’s say you’re starting a laser center in your academic center,” said Dr. Avram, who is a past president of the American Society for Laser Medicine and Surgery. “You may not want to find out after you’ve signed your contract that they’ve hired five other people to do the same thing.”
If you’re considering a job offer from a private practice, ask for specifics about bonuses, partnership track, scope of practice, and device purchases. “You want to have that decided before you sign,” he said. “But these are business issues, not legal issues. An agreement needs to be made between you and your employer as to these issues. Once they’ve been agreed upon, it’s time to proceed with a contract. This is where legal advice becomes helpful.” The trick is to negotiate in good faith while maintaining your relationship with your new employer. “Do not destroy your relationship over legal points, but do not cave on crucial issues for fear of upsetting your new employer,” he said.
In law school, Dr. Avram learned that there is no such thing as a standard employment contract. Any contract can be amended, no matter how large or small the institution. “You can amend a mortgage agreement, so you can certainly amend a physician employment contract,” he said. “If the employer is not going to put their commitments in writing, they are probably not going to honor that commitment to you. Written agreements supersede all preceding oral agreements. Each key term needs to be stated explicitly. If not, you have lost all leverage to enforce your initial agreement.”
Key provisions are restrictive covenants and at-will employment. Dr. Avram defined restrictive covenants as contractual agreements that attempt to restrict an employee so as to limit that person’s ability to compete. “This can include noncompete clauses, nonsolicitation agreements, and confidentiality agreements,” he said. “A noncompete agreement prohibits a doctor from competing against their former practice within a specific geographic area for a period of time after the employment has ended. The nonsolicitation agreement restricts the manner and time during which a physician can solicit patients or employees from a practice after termination of employment. A confidentiality agreement is usually indefinite as to time and it restricts the employee from disclosing confidential practice information.”
State laws, he continued, govern restrictive covenants. Some states prohibit them. States that allow them limit their scope to prevent undue burden on the employee’s ability to make a living after termination. “Restrictive covenants in cities need to be narrower than those in rural areas,” he said. “Overly broad restrictive covenants may be unenforceable. Courts can ‘blue line’ covenants to make them more reasonable in scope.”
Next, Dr. Avram discussed involuntary termination in the workplace. Termination with cause means that termination can only result from violation of policy or ethics code violation or significantly poor performance. “In the absence of these circumstances, termination cannot legally proceed,” he said. “This protects the employee from an arbitrary termination.”
On the other hand, at-will employees can be terminated without cause. “It’s a much easier standard to terminate an at-will employee, as long as the reason is not illegal,” he said. “In academic centers, your employment as a physician may require a for-cause termination, whereas your administrative title may be without cause.”
One way to avoid potential legal trouble is to abide by your employer’s rules governing a physician’s interactions with industry. “It is foolish to break those rules,” Dr. Avram said. “Transparency ahead of time is always your best policy. Remember: Your day job is far more valuable to you than part-time work with industry. Also, know that pharma payments are publicly reported by the Centers for Medicare & Medicaid Services.” When it comes to establishing contracts with industry, “read them carefully and have your academic center approve them, if applicable,” he said. “Watch out for noncompete clauses, and some contracts require that you do not speak negatively about your findings. That’s something you should try to avoid.”
Dr. Avram also advised clinicians not to discuss intellectual property with industry representatives. “Do not give away your intellectual property to industry when you’re consulting with them,” he said. “It is easier to do than you might think. If you have a great idea, keep it to yourself or get it protected. This is much easier for an institution where they want you to do these things. They take ownership of a lot of it, but they make it easier to do. Otherwise, you have to go through the expensive process of getting a patent.”
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, InMode, and Zalea, and intellectual property rights with Cytrellis.
EXPERT ANALYSIS FROM MOAS 2019
Women’s residency and subspecialty choices diverging
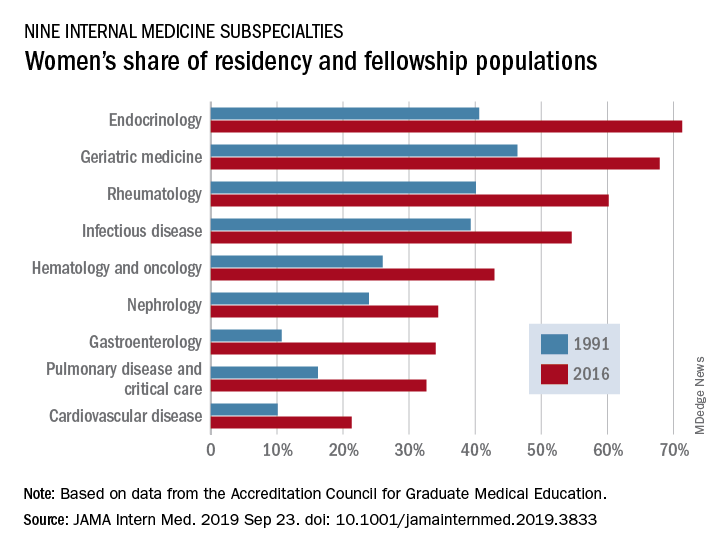
Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.
FROM JAMA INTERNAL MEDICINE
Automatic reenrollment helps keep people insured
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
FROM JAMA INTERNAL MEDICINE
Business case for interoperability remains elusive
In a recent survey issued by the Center for Connected Medicine that asked, “What is needed most to push interoperability forward in health care?” 53% of the 100 IT and business leader participants at hospitals and health systems answered “senior leadership commitment to interoperability as a top strategic priority.”
That interoperability continues to be an elusive target despite being a regulatory emphasis for more than a decade comes as no surprise.
“You have to put interoperability into the context of the challenges of being a leader in health care delivery organization or a hospital,” Robert Bart, MD, chief medical information officer of the Health Services Division at the University of Pittsburgh Medical Center, said in an interview. UPMC operates the Center for Connected Medicine in partnership with GE Healthcare and Nokia.
“For many health care delivery systems, the operational margin is extremely small,” he continued. “From a strategic perspective, they may be prioritizing things or opportunities that directly contribute to the bottom line and the financial success or even financial viability of the organization. “Interoperability certainly helps health care overall in the U.S., but whether it contributes directly to the financial bottom line of any given specific organization might vary significantly.”
But Dr. Bart said it really is not the financial incentives, a staple in the early days of the meaningful use program to spur adoption, that will get electronic health records and other health IT into a more interoperable space.
“I am not sure that financial incentives – unless they are significantly different in amounts than they currently are – are going to be the sole single reason why organizations prioritize interoperability higher,” he said.
Rather, he sees two key components that will drive interoperability.
First, he cited efforts by the Centers for Medicare & Medicaid Services to bring ownership of health data to the individual as an important driver.
“I think that is a better direction to push as opposed to financial incentives because I think that is really where we philosophically and then therefore operationally need to get to,” Dr. Bart said.
The second would take a lot more involvement from government that likely is more difficult to achieve: getting a more clear definition of health IT standards.
“We have accomplished some forward movement in interoperability, but we probably have far more road in front of us than we have left behind us as it relates to interoperability,” he said. “A fair amount of that is related to standards which, unfortunately, oftentimes are left up to interpretation. So when it comes to operationalizing these standards between different vendors or different health care systems, they sometimes don’t match up and add even more challenge to the exchange of information.”
And with industry not taking an active lead in solving some of the problems related to the standardization of data needed to drive interoperability, he suggested government should play a bigger role.
“I would like the government to take a stronger hand in helping health care define the standards so the standards are actually much more executable and there is a lot less interpretation, which I think would ease the ability for interoperability to flourish a lot more,” he said.
The move to value-based care could also provide added incentive to drive interoperability, Dr. Bart said.
Value-based care is “certainly not going to raise more barriers toward interoperability and potentially could lower some as organizations recognize that it may be a path to decreasing the costs of repeating tests that will not improve the quality of care delivered,” he said.
But overall, it would appear that getting to an interoperable point is going to be a challenge.
The report notes that “beyond tasks required by regulation or related to basic functioning (such as sharing data within their own system), interoperability still presents a challenge to organizations. Fewer than 4 in 10 report success with sharing data with other health systems, effectiveness in tapping into unstructured data, and effectiveness in reducing the cost of care.”
SOURCE: A report issued by the Center for Connected Medicine called “Improving Health Care Interoperability: Are We Making Progress?”
In a recent survey issued by the Center for Connected Medicine that asked, “What is needed most to push interoperability forward in health care?” 53% of the 100 IT and business leader participants at hospitals and health systems answered “senior leadership commitment to interoperability as a top strategic priority.”
That interoperability continues to be an elusive target despite being a regulatory emphasis for more than a decade comes as no surprise.
“You have to put interoperability into the context of the challenges of being a leader in health care delivery organization or a hospital,” Robert Bart, MD, chief medical information officer of the Health Services Division at the University of Pittsburgh Medical Center, said in an interview. UPMC operates the Center for Connected Medicine in partnership with GE Healthcare and Nokia.
“For many health care delivery systems, the operational margin is extremely small,” he continued. “From a strategic perspective, they may be prioritizing things or opportunities that directly contribute to the bottom line and the financial success or even financial viability of the organization. “Interoperability certainly helps health care overall in the U.S., but whether it contributes directly to the financial bottom line of any given specific organization might vary significantly.”
But Dr. Bart said it really is not the financial incentives, a staple in the early days of the meaningful use program to spur adoption, that will get electronic health records and other health IT into a more interoperable space.
“I am not sure that financial incentives – unless they are significantly different in amounts than they currently are – are going to be the sole single reason why organizations prioritize interoperability higher,” he said.
Rather, he sees two key components that will drive interoperability.
First, he cited efforts by the Centers for Medicare & Medicaid Services to bring ownership of health data to the individual as an important driver.
“I think that is a better direction to push as opposed to financial incentives because I think that is really where we philosophically and then therefore operationally need to get to,” Dr. Bart said.
The second would take a lot more involvement from government that likely is more difficult to achieve: getting a more clear definition of health IT standards.
“We have accomplished some forward movement in interoperability, but we probably have far more road in front of us than we have left behind us as it relates to interoperability,” he said. “A fair amount of that is related to standards which, unfortunately, oftentimes are left up to interpretation. So when it comes to operationalizing these standards between different vendors or different health care systems, they sometimes don’t match up and add even more challenge to the exchange of information.”
And with industry not taking an active lead in solving some of the problems related to the standardization of data needed to drive interoperability, he suggested government should play a bigger role.
“I would like the government to take a stronger hand in helping health care define the standards so the standards are actually much more executable and there is a lot less interpretation, which I think would ease the ability for interoperability to flourish a lot more,” he said.
The move to value-based care could also provide added incentive to drive interoperability, Dr. Bart said.
Value-based care is “certainly not going to raise more barriers toward interoperability and potentially could lower some as organizations recognize that it may be a path to decreasing the costs of repeating tests that will not improve the quality of care delivered,” he said.
But overall, it would appear that getting to an interoperable point is going to be a challenge.
The report notes that “beyond tasks required by regulation or related to basic functioning (such as sharing data within their own system), interoperability still presents a challenge to organizations. Fewer than 4 in 10 report success with sharing data with other health systems, effectiveness in tapping into unstructured data, and effectiveness in reducing the cost of care.”
SOURCE: A report issued by the Center for Connected Medicine called “Improving Health Care Interoperability: Are We Making Progress?”
In a recent survey issued by the Center for Connected Medicine that asked, “What is needed most to push interoperability forward in health care?” 53% of the 100 IT and business leader participants at hospitals and health systems answered “senior leadership commitment to interoperability as a top strategic priority.”
That interoperability continues to be an elusive target despite being a regulatory emphasis for more than a decade comes as no surprise.
“You have to put interoperability into the context of the challenges of being a leader in health care delivery organization or a hospital,” Robert Bart, MD, chief medical information officer of the Health Services Division at the University of Pittsburgh Medical Center, said in an interview. UPMC operates the Center for Connected Medicine in partnership with GE Healthcare and Nokia.
“For many health care delivery systems, the operational margin is extremely small,” he continued. “From a strategic perspective, they may be prioritizing things or opportunities that directly contribute to the bottom line and the financial success or even financial viability of the organization. “Interoperability certainly helps health care overall in the U.S., but whether it contributes directly to the financial bottom line of any given specific organization might vary significantly.”
But Dr. Bart said it really is not the financial incentives, a staple in the early days of the meaningful use program to spur adoption, that will get electronic health records and other health IT into a more interoperable space.
“I am not sure that financial incentives – unless they are significantly different in amounts than they currently are – are going to be the sole single reason why organizations prioritize interoperability higher,” he said.
Rather, he sees two key components that will drive interoperability.
First, he cited efforts by the Centers for Medicare & Medicaid Services to bring ownership of health data to the individual as an important driver.
“I think that is a better direction to push as opposed to financial incentives because I think that is really where we philosophically and then therefore operationally need to get to,” Dr. Bart said.
The second would take a lot more involvement from government that likely is more difficult to achieve: getting a more clear definition of health IT standards.
“We have accomplished some forward movement in interoperability, but we probably have far more road in front of us than we have left behind us as it relates to interoperability,” he said. “A fair amount of that is related to standards which, unfortunately, oftentimes are left up to interpretation. So when it comes to operationalizing these standards between different vendors or different health care systems, they sometimes don’t match up and add even more challenge to the exchange of information.”
And with industry not taking an active lead in solving some of the problems related to the standardization of data needed to drive interoperability, he suggested government should play a bigger role.
“I would like the government to take a stronger hand in helping health care define the standards so the standards are actually much more executable and there is a lot less interpretation, which I think would ease the ability for interoperability to flourish a lot more,” he said.
The move to value-based care could also provide added incentive to drive interoperability, Dr. Bart said.
Value-based care is “certainly not going to raise more barriers toward interoperability and potentially could lower some as organizations recognize that it may be a path to decreasing the costs of repeating tests that will not improve the quality of care delivered,” he said.
But overall, it would appear that getting to an interoperable point is going to be a challenge.
The report notes that “beyond tasks required by regulation or related to basic functioning (such as sharing data within their own system), interoperability still presents a challenge to organizations. Fewer than 4 in 10 report success with sharing data with other health systems, effectiveness in tapping into unstructured data, and effectiveness in reducing the cost of care.”
SOURCE: A report issued by the Center for Connected Medicine called “Improving Health Care Interoperability: Are We Making Progress?”
Legislative Conference 2019
Unreasonable step therapy and prior authorizations, overly expensive generic drugs, opposition to a 5-year Medicare pay freeze, surprise medical billing, and the risks and benefits of sunscreen use for the prevention of skin cancer.
These were just some of the hot button issues that 156 of my fellow We also were joined by 49 patients and practice administrators for this terrific meeting filled with classes and speakers. Members of Congress joined us at various sessions and social occasions with good food, fine wine, and great conversations. Radio personality and CNN host Michael Smerconish gave a very funny speech at dinner and had comments about civility in politics. The comradery was excellent and the intellectual food for thought was extraordinary – right up the alley of dermatologists who are also political junkies.
Most congressional representatives are not well versed in health care topics. As a result, we spent a good deal of time on education – speaking to junior staffers who are expected to keep the members of Congress updated on what they need to know about medical and, specifically, dermatologic issues. As many refer to Washington as “the swamp,” you can consider the House members to be the “big gators,” and the staffers the ”little gators.” Taking that analogy further, lobbying (or educating) then logically becomes “gator wrestling.”
Most of the time we met with little gators, although this year we also met with 84 big gators. We took turns telling them true stories based on our patients’ problems with abuses within our health care system. I think these stories are effective. This is your representative government in action!
On the last day of the conference, we made personal calls by state on the hill offices. Some groups, like Ohio, were nine strong! Everyone gets to speak. This year’s meeting was attended by a total of 31 dermatology residents, and the residents from Ohio State and Cleveland Clinic who participated in our state meetings were terrific. In all, we covered 228 offices – 157 Congressional and 71 Senate.
Rep. John Joyce, MD, FAAD (R-PA-13) was on hand and is the first dermatologist elected for a full term to Congress. Anyone at the meeting could have spent all the time they wanted with him discussing our issues. He is most knowledgeable and a great asset for our specialty. Dr. Joyce is recognized as a dermatologist by his fellow representatives, and even by Speaker Nancy Pelosi and President Trump. For 30 years, Dr. Joyce has been a proud member of the American Academy of Dermatology, as is his wife Dr. Alice Joyce, who also is a dermatologist and continues to run their practice, Altoona Dermatology Associates.
Dr. Joyce is a true asset to dermatology. As an individual, I advocate supporting his campaign financially if you get the chance, beyond just what SkinPAC can give him.
In sum, the AADA Legislative Conference is a lot of productive fun. You get to network with all the leaders of the AAD, as well as many of the leaders of the United States. If you donate $5,000 to SkinPAC, they will pay your way to the conference. If you contribute $1,000, you get to go to the high-donor dinner (The same goes for a $50 donation from residents, no typo!). What’s not to like about that? Most dermatologists like a good party. Next year’s meeting is Sept. 13-15, 2020. See you there!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Unreasonable step therapy and prior authorizations, overly expensive generic drugs, opposition to a 5-year Medicare pay freeze, surprise medical billing, and the risks and benefits of sunscreen use for the prevention of skin cancer.
These were just some of the hot button issues that 156 of my fellow We also were joined by 49 patients and practice administrators for this terrific meeting filled with classes and speakers. Members of Congress joined us at various sessions and social occasions with good food, fine wine, and great conversations. Radio personality and CNN host Michael Smerconish gave a very funny speech at dinner and had comments about civility in politics. The comradery was excellent and the intellectual food for thought was extraordinary – right up the alley of dermatologists who are also political junkies.
Most congressional representatives are not well versed in health care topics. As a result, we spent a good deal of time on education – speaking to junior staffers who are expected to keep the members of Congress updated on what they need to know about medical and, specifically, dermatologic issues. As many refer to Washington as “the swamp,” you can consider the House members to be the “big gators,” and the staffers the ”little gators.” Taking that analogy further, lobbying (or educating) then logically becomes “gator wrestling.”
Most of the time we met with little gators, although this year we also met with 84 big gators. We took turns telling them true stories based on our patients’ problems with abuses within our health care system. I think these stories are effective. This is your representative government in action!
On the last day of the conference, we made personal calls by state on the hill offices. Some groups, like Ohio, were nine strong! Everyone gets to speak. This year’s meeting was attended by a total of 31 dermatology residents, and the residents from Ohio State and Cleveland Clinic who participated in our state meetings were terrific. In all, we covered 228 offices – 157 Congressional and 71 Senate.
Rep. John Joyce, MD, FAAD (R-PA-13) was on hand and is the first dermatologist elected for a full term to Congress. Anyone at the meeting could have spent all the time they wanted with him discussing our issues. He is most knowledgeable and a great asset for our specialty. Dr. Joyce is recognized as a dermatologist by his fellow representatives, and even by Speaker Nancy Pelosi and President Trump. For 30 years, Dr. Joyce has been a proud member of the American Academy of Dermatology, as is his wife Dr. Alice Joyce, who also is a dermatologist and continues to run their practice, Altoona Dermatology Associates.
Dr. Joyce is a true asset to dermatology. As an individual, I advocate supporting his campaign financially if you get the chance, beyond just what SkinPAC can give him.
In sum, the AADA Legislative Conference is a lot of productive fun. You get to network with all the leaders of the AAD, as well as many of the leaders of the United States. If you donate $5,000 to SkinPAC, they will pay your way to the conference. If you contribute $1,000, you get to go to the high-donor dinner (The same goes for a $50 donation from residents, no typo!). What’s not to like about that? Most dermatologists like a good party. Next year’s meeting is Sept. 13-15, 2020. See you there!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Unreasonable step therapy and prior authorizations, overly expensive generic drugs, opposition to a 5-year Medicare pay freeze, surprise medical billing, and the risks and benefits of sunscreen use for the prevention of skin cancer.
These were just some of the hot button issues that 156 of my fellow We also were joined by 49 patients and practice administrators for this terrific meeting filled with classes and speakers. Members of Congress joined us at various sessions and social occasions with good food, fine wine, and great conversations. Radio personality and CNN host Michael Smerconish gave a very funny speech at dinner and had comments about civility in politics. The comradery was excellent and the intellectual food for thought was extraordinary – right up the alley of dermatologists who are also political junkies.
Most congressional representatives are not well versed in health care topics. As a result, we spent a good deal of time on education – speaking to junior staffers who are expected to keep the members of Congress updated on what they need to know about medical and, specifically, dermatologic issues. As many refer to Washington as “the swamp,” you can consider the House members to be the “big gators,” and the staffers the ”little gators.” Taking that analogy further, lobbying (or educating) then logically becomes “gator wrestling.”
Most of the time we met with little gators, although this year we also met with 84 big gators. We took turns telling them true stories based on our patients’ problems with abuses within our health care system. I think these stories are effective. This is your representative government in action!
On the last day of the conference, we made personal calls by state on the hill offices. Some groups, like Ohio, were nine strong! Everyone gets to speak. This year’s meeting was attended by a total of 31 dermatology residents, and the residents from Ohio State and Cleveland Clinic who participated in our state meetings were terrific. In all, we covered 228 offices – 157 Congressional and 71 Senate.
Rep. John Joyce, MD, FAAD (R-PA-13) was on hand and is the first dermatologist elected for a full term to Congress. Anyone at the meeting could have spent all the time they wanted with him discussing our issues. He is most knowledgeable and a great asset for our specialty. Dr. Joyce is recognized as a dermatologist by his fellow representatives, and even by Speaker Nancy Pelosi and President Trump. For 30 years, Dr. Joyce has been a proud member of the American Academy of Dermatology, as is his wife Dr. Alice Joyce, who also is a dermatologist and continues to run their practice, Altoona Dermatology Associates.
Dr. Joyce is a true asset to dermatology. As an individual, I advocate supporting his campaign financially if you get the chance, beyond just what SkinPAC can give him.
In sum, the AADA Legislative Conference is a lot of productive fun. You get to network with all the leaders of the AAD, as well as many of the leaders of the United States. If you donate $5,000 to SkinPAC, they will pay your way to the conference. If you contribute $1,000, you get to go to the high-donor dinner (The same goes for a $50 donation from residents, no typo!). What’s not to like about that? Most dermatologists like a good party. Next year’s meeting is Sept. 13-15, 2020. See you there!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.




















