User login
At 5 years, iFR found as effective and safe as FFR for guiding PCI intervention
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
The rate of major adverse cardiac events (MACE) over 5 years is similar whether revascularization is guided by instantaneous wave-free ratio (iFR) or fractional flow reserve (FFR), according to long-term results of the iFR-SWEDEHEART study.
“The results are about the same as reported at 12 months. There were no significant differences in any outcome we evaluated,” according to Matthias Götberg, MD, PhD.
When the initial results of the noninferiority iFR-SWEDEHEART trial were published after 1 year of follow-up, the primary MACE endpoint of death from any-cause nonfatal myocardial infarction, or unplanned revascularization, was met by 6.7% and 6.1% of those randomized to iFR or FFR, respectively.
These outcomes were not significantly different and placed iFR well within the predefined boundaries of noninferiority (P = .007).
In this new and final follow-up of iFR-SWEDEHEART, which evaluated the same 2,019 patients who were alive at 1 year (none were lost to follow-up), the MACE endpoint was met by 21.5% and 19.9% of those managed with iFR and FFR, respectively. The hazard ratio (1.09) had a wide 95% confidence interval (0.90-1.31) that did not approach statistical significance.
No differences seen across outcomes
When broken down into the MACE components, there were no differences between iFR and FFR, respectively, for all-cause death (9.4% vs. 7.9%), MI (5.8% vs. 5.7%) or unplanned revascularization (11.6% vs. 11.3%).
Across predefined subgroups, such as those defined by age, gender, stable versus unstable angina, and presence of risk factors such as diabetes, hypertension, hyperlipidemia, and smoking, there were also no significant differences in outcome.
At the time iFR-SWEDEHART was initiated, FFR had already been accepted as more effective than angiographic assessment to identify lesion ischemia and the need for percutaneous intervention (PCI). The iFR-SWEDEHEART trial tested iFR, a relatively new technology at the time, as a noninferior alternative. Unlike FFR, which requires adenosine to dilate the vessel, adding cost and patient discomfort, iFR measures the resting pressure gradient across the coronary lesion, and it is generally easier to perform.
“The advantage of iFR is that it provides an instantaneous lesion assessment without the need for adenosine,” Dr. Götberg explained in presenting the results at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
When the procedural results were compared in the published study at 1 year, it was noted that the mean number of lesions evaluated per patient was higher (1.55 vs. 1.43; P = .002), but the proportion of lesions found functionally significant was lower (29.2% vs. 36.8%; P < .0001) among those randomized to iFR than in the FFR group.
While most other procedural characteristics, such as PCI access route, fluoroscopy time, and contrast use did not differ significantly, fewer stents were placed in patients managed with iFR (1.58 vs. 1.73; P = .048), and a reduction in the average procedural time of a few minutes approached significance (P = .09).
Patient discomfort is greater with FFR
Patient discomfort measured during the procedure did differ, according to Dr. Götberg, an interventional cardiologist at Skåne University Hospital, Lund, Sweden.
Only about 30% in the FFR group reported no discomfort. Most of the others reported mild or moderate discomfort, but nearly 10% characterized the discomfort as severe. In the iFR group, more than 95% reported no discomfort. All of the remaining patients reported discomfort level as mild.
Because differences in MACE would be most likely to occur in the first year after revascularization, the similarity of the 1- and 5-year results were expected, according to Dr. Götberg. However, a 5-year follow-up was considered prudent given the relatively limited experience with iFR when the study was designed. This technique is now well established and widely used.
The study supports the premise that quicker and easier-to-obtain results with iFR are obtained without sacrificing greater relative risk of failing to identify a vulnerable lesion, according to Dr. Götberg.
Nevertheless, iFR and FFR “are not an exact match,” according to Jennifer A. Rymer, MD, an interventional cardiologist and assistant professor of medicine at Duke University, Durham, N.C. Although she called this trial an “excellent” demonstration of comparable utility in distinguishing lesions that do not require intervention from those that do, she implied that some clinicians might still prefer FFR for other reasons.
For example, FFR provides information about coronary flow reserve and microvascular resistance that are relevant to the underlying pathophysiology in a diseased vessel, according to Shmuel Banai, MD, head of interventional cardiology, Tel Aviv Medical Center. Recognizing that this information is not as readily generated by iFR, he is among those who plan to continue to use FFR despite these results.
However, for those who are now routinely performing iFR for the purposes of guiding revascularization, “these data are reassuring,” said David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta. The 5-year data essentially eliminate the likelihood that iFR relative to FFR increases the risk of missing functionally significant lesions for revascularization procedures.
Dr. Götberg reports financial relationships with Abbott, Boston Scientific, Medtronic, and Phillips Healthcare. Dr. Rymer reports no potential financial conflicts of interest. Dr. Banai has a financial relationship with Neovasc. Dr. Kandzari reports financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
Statins’ effects on CVD outweigh risk for diabetes in RA
The use of statins by patients with rheumatoid arthritis appears to provide an overall net benefit on cardiovascular disease outcomes that outweighs the risk of type 2 diabetes mellitus (T2DM) seen with the drugs in the general population, according to evidence from a cohort study of more than 16,000 people in the United Kingdom that was presented at the virtual annual meeting of the American College of Rheumatology.
“Our study emphasizes that RA patients should be assessed for statin initiation to improve CVD risk,” lead study author Gulsen Ozen, MD, a third-year resident at the University of Nebraska, Omaha, said in an interview. Because the risk of T2DM with statin use is no worse in patients with RA than in the general population, statin initiation “is actually a great opportunity to address the risk factors for T2DM such as activity and exercise, obesity and weight loss, and [use of glucocorticoids], which have other important health effects,” she said.
“Also, importantly, even if [patients] develop T2DM, statins still work on CVD and mortality outcomes as in patients without diabetes,” Dr. Ozen added. “Given all, the benefits of statins way outweigh the hazards.”
Dr. Ozen said this was the first large cohort study to evaluate CVD mortality and T2DM risks with statins in patients with RA, a claim with which rheumatologist Elena Myasoedova, MD, PhD, of the Mayo Clinic in Rochester, Minn., concurred.
Dr. Myasoedova, professor of rheumatology and epidemiology at Mayo, said in an interview that the study was “methodologically rigorous” using time-conditional propensity score (TCPS) matching and a prevalent new-user design, “thus addressing the immortal time bias” found in the design of studies in which patients enter a cohort but do not start a treatment before developing the outcome of interest and are assigned to the untreated group or when the period of delay from when patients enter the cohort to when they are treated is excluded from the analysis. An earlier study from the same authors did not use TCPS matching, she said.
“The study findings suggest that patients with RA can benefit from statin use in terms of CVD outcomes and mortality but physicians should use vigilance regarding increased T2DM risk and discuss this possibility with patients,” Dr. Myasoedova said. “Identifying patients who are at higher risk of developing T2DM after statin initiation would be important to personalize the approach to statin therapy.”
Study details
The study accessed records from the U.K. Clinical Practice Research Datalink and linked Hospital Episode Statistics and Office of National Statistics databases. It analyzed adult patients with RA who were diagnosed during 1989-2018 in two cohorts: One for CVD and all-cause mortality, consisting of 1,768 statin initiators and 3,528 TCPS-matched nonusers; and a T2DM cohort with 3,608 statin initiators and 7,208 TCPS-matched nonusers.
In the entire cohort, statin use was associated with a 32% reduction in CV events (composite endpoint of the nonfatal or fatal MI, stroke, hospitalized heart failure, or CVD mortality), a 54% reduction in all-cause mortality, and a 33% increase in risk for T2DM, Dr. Ozen said. Results were similar in both sexes, although CV event reduction with statins in men did not reach statistical significance, likely because of a smaller sample size, she said.
Patients with and without a history of CVD had a similar reduction in CV events and all-cause mortality, and risk for T2DM increased with statins, but the latter reached statistical significance only in patients without a history of CVD, Dr. Ozen said.
Patients with RA who are at risk for T2DM and who are taking statins require blood glucose monitoring, which is typically done in patients with RA on disease-modifying antirheumatic drugs, and hemoglobin A1c testing when glucose levels are impaired, she said. “Any concerns for T2DM would be also communicated by the primary care providers of the patients to initiate further assessment and management,” she said.
But Dr. Ozen noted that confusion exists among primary care physicians and rheumatologists about who’s responsible for prescribing statins in these patients. “I would like to remind you that instead of assigning this role to a certain specialty, just good communication could improve this care gap of statin underutilization in RA,” she said. “Also, for rheumatologists, given that all-cause mortality reduction with statins was as high as CV event reduction, statins may be reducing other causes of mortality through improving disease activity.”
Bristol-Myers Squibb provided funding for the study. Dr. Ozen and Dr. Myasoedova have no relevant disclosures.
The use of statins by patients with rheumatoid arthritis appears to provide an overall net benefit on cardiovascular disease outcomes that outweighs the risk of type 2 diabetes mellitus (T2DM) seen with the drugs in the general population, according to evidence from a cohort study of more than 16,000 people in the United Kingdom that was presented at the virtual annual meeting of the American College of Rheumatology.
“Our study emphasizes that RA patients should be assessed for statin initiation to improve CVD risk,” lead study author Gulsen Ozen, MD, a third-year resident at the University of Nebraska, Omaha, said in an interview. Because the risk of T2DM with statin use is no worse in patients with RA than in the general population, statin initiation “is actually a great opportunity to address the risk factors for T2DM such as activity and exercise, obesity and weight loss, and [use of glucocorticoids], which have other important health effects,” she said.
“Also, importantly, even if [patients] develop T2DM, statins still work on CVD and mortality outcomes as in patients without diabetes,” Dr. Ozen added. “Given all, the benefits of statins way outweigh the hazards.”
Dr. Ozen said this was the first large cohort study to evaluate CVD mortality and T2DM risks with statins in patients with RA, a claim with which rheumatologist Elena Myasoedova, MD, PhD, of the Mayo Clinic in Rochester, Minn., concurred.
Dr. Myasoedova, professor of rheumatology and epidemiology at Mayo, said in an interview that the study was “methodologically rigorous” using time-conditional propensity score (TCPS) matching and a prevalent new-user design, “thus addressing the immortal time bias” found in the design of studies in which patients enter a cohort but do not start a treatment before developing the outcome of interest and are assigned to the untreated group or when the period of delay from when patients enter the cohort to when they are treated is excluded from the analysis. An earlier study from the same authors did not use TCPS matching, she said.
“The study findings suggest that patients with RA can benefit from statin use in terms of CVD outcomes and mortality but physicians should use vigilance regarding increased T2DM risk and discuss this possibility with patients,” Dr. Myasoedova said. “Identifying patients who are at higher risk of developing T2DM after statin initiation would be important to personalize the approach to statin therapy.”
Study details
The study accessed records from the U.K. Clinical Practice Research Datalink and linked Hospital Episode Statistics and Office of National Statistics databases. It analyzed adult patients with RA who were diagnosed during 1989-2018 in two cohorts: One for CVD and all-cause mortality, consisting of 1,768 statin initiators and 3,528 TCPS-matched nonusers; and a T2DM cohort with 3,608 statin initiators and 7,208 TCPS-matched nonusers.
In the entire cohort, statin use was associated with a 32% reduction in CV events (composite endpoint of the nonfatal or fatal MI, stroke, hospitalized heart failure, or CVD mortality), a 54% reduction in all-cause mortality, and a 33% increase in risk for T2DM, Dr. Ozen said. Results were similar in both sexes, although CV event reduction with statins in men did not reach statistical significance, likely because of a smaller sample size, she said.
Patients with and without a history of CVD had a similar reduction in CV events and all-cause mortality, and risk for T2DM increased with statins, but the latter reached statistical significance only in patients without a history of CVD, Dr. Ozen said.
Patients with RA who are at risk for T2DM and who are taking statins require blood glucose monitoring, which is typically done in patients with RA on disease-modifying antirheumatic drugs, and hemoglobin A1c testing when glucose levels are impaired, she said. “Any concerns for T2DM would be also communicated by the primary care providers of the patients to initiate further assessment and management,” she said.
But Dr. Ozen noted that confusion exists among primary care physicians and rheumatologists about who’s responsible for prescribing statins in these patients. “I would like to remind you that instead of assigning this role to a certain specialty, just good communication could improve this care gap of statin underutilization in RA,” she said. “Also, for rheumatologists, given that all-cause mortality reduction with statins was as high as CV event reduction, statins may be reducing other causes of mortality through improving disease activity.”
Bristol-Myers Squibb provided funding for the study. Dr. Ozen and Dr. Myasoedova have no relevant disclosures.
The use of statins by patients with rheumatoid arthritis appears to provide an overall net benefit on cardiovascular disease outcomes that outweighs the risk of type 2 diabetes mellitus (T2DM) seen with the drugs in the general population, according to evidence from a cohort study of more than 16,000 people in the United Kingdom that was presented at the virtual annual meeting of the American College of Rheumatology.
“Our study emphasizes that RA patients should be assessed for statin initiation to improve CVD risk,” lead study author Gulsen Ozen, MD, a third-year resident at the University of Nebraska, Omaha, said in an interview. Because the risk of T2DM with statin use is no worse in patients with RA than in the general population, statin initiation “is actually a great opportunity to address the risk factors for T2DM such as activity and exercise, obesity and weight loss, and [use of glucocorticoids], which have other important health effects,” she said.
“Also, importantly, even if [patients] develop T2DM, statins still work on CVD and mortality outcomes as in patients without diabetes,” Dr. Ozen added. “Given all, the benefits of statins way outweigh the hazards.”
Dr. Ozen said this was the first large cohort study to evaluate CVD mortality and T2DM risks with statins in patients with RA, a claim with which rheumatologist Elena Myasoedova, MD, PhD, of the Mayo Clinic in Rochester, Minn., concurred.
Dr. Myasoedova, professor of rheumatology and epidemiology at Mayo, said in an interview that the study was “methodologically rigorous” using time-conditional propensity score (TCPS) matching and a prevalent new-user design, “thus addressing the immortal time bias” found in the design of studies in which patients enter a cohort but do not start a treatment before developing the outcome of interest and are assigned to the untreated group or when the period of delay from when patients enter the cohort to when they are treated is excluded from the analysis. An earlier study from the same authors did not use TCPS matching, she said.
“The study findings suggest that patients with RA can benefit from statin use in terms of CVD outcomes and mortality but physicians should use vigilance regarding increased T2DM risk and discuss this possibility with patients,” Dr. Myasoedova said. “Identifying patients who are at higher risk of developing T2DM after statin initiation would be important to personalize the approach to statin therapy.”
Study details
The study accessed records from the U.K. Clinical Practice Research Datalink and linked Hospital Episode Statistics and Office of National Statistics databases. It analyzed adult patients with RA who were diagnosed during 1989-2018 in two cohorts: One for CVD and all-cause mortality, consisting of 1,768 statin initiators and 3,528 TCPS-matched nonusers; and a T2DM cohort with 3,608 statin initiators and 7,208 TCPS-matched nonusers.
In the entire cohort, statin use was associated with a 32% reduction in CV events (composite endpoint of the nonfatal or fatal MI, stroke, hospitalized heart failure, or CVD mortality), a 54% reduction in all-cause mortality, and a 33% increase in risk for T2DM, Dr. Ozen said. Results were similar in both sexes, although CV event reduction with statins in men did not reach statistical significance, likely because of a smaller sample size, she said.
Patients with and without a history of CVD had a similar reduction in CV events and all-cause mortality, and risk for T2DM increased with statins, but the latter reached statistical significance only in patients without a history of CVD, Dr. Ozen said.
Patients with RA who are at risk for T2DM and who are taking statins require blood glucose monitoring, which is typically done in patients with RA on disease-modifying antirheumatic drugs, and hemoglobin A1c testing when glucose levels are impaired, she said. “Any concerns for T2DM would be also communicated by the primary care providers of the patients to initiate further assessment and management,” she said.
But Dr. Ozen noted that confusion exists among primary care physicians and rheumatologists about who’s responsible for prescribing statins in these patients. “I would like to remind you that instead of assigning this role to a certain specialty, just good communication could improve this care gap of statin underutilization in RA,” she said. “Also, for rheumatologists, given that all-cause mortality reduction with statins was as high as CV event reduction, statins may be reducing other causes of mortality through improving disease activity.”
Bristol-Myers Squibb provided funding for the study. Dr. Ozen and Dr. Myasoedova have no relevant disclosures.
FROM ACR 2021
Validated scoring system identifies low-risk syncope patients
ILLUSTRATIVE CASE
A 30-year-old woman presented to the ED after she “passed out” while standing at a concert. She lost consciousness for 10 seconds. After she revived, her friends drove her to the ED. She is healthy, with no chronic medical conditions, no medication use, and no drug or alcohol use. Should she be admitted to the hospital for observation?
Syncope, a transient loss of consciousness followed by spontaneous complete recovery, accounts for 1% of ED visits.2 Approximately 10% of patients presenting to the ED will have a serious underlying condition identified and among 3% to 5% of these patients with syncope, the serious condition will be identified only after they leave the ED.1 Most patients have a benign course, but more than half of all patients presenting to the ED with syncope will be hospitalized, costing $2.4 billion annually.2
Because of the high hospitalization rate of patients with syncope, a practical and accurate tool to risk-stratify patients is vital. Other tools, such as the San Francisco Syncope Rule, Short-Term Prognosis of Syncope, and Risk Stratification of Syncope in the Emergency Department, lack validation or are excessively complex, with extensive lab work or testing.3
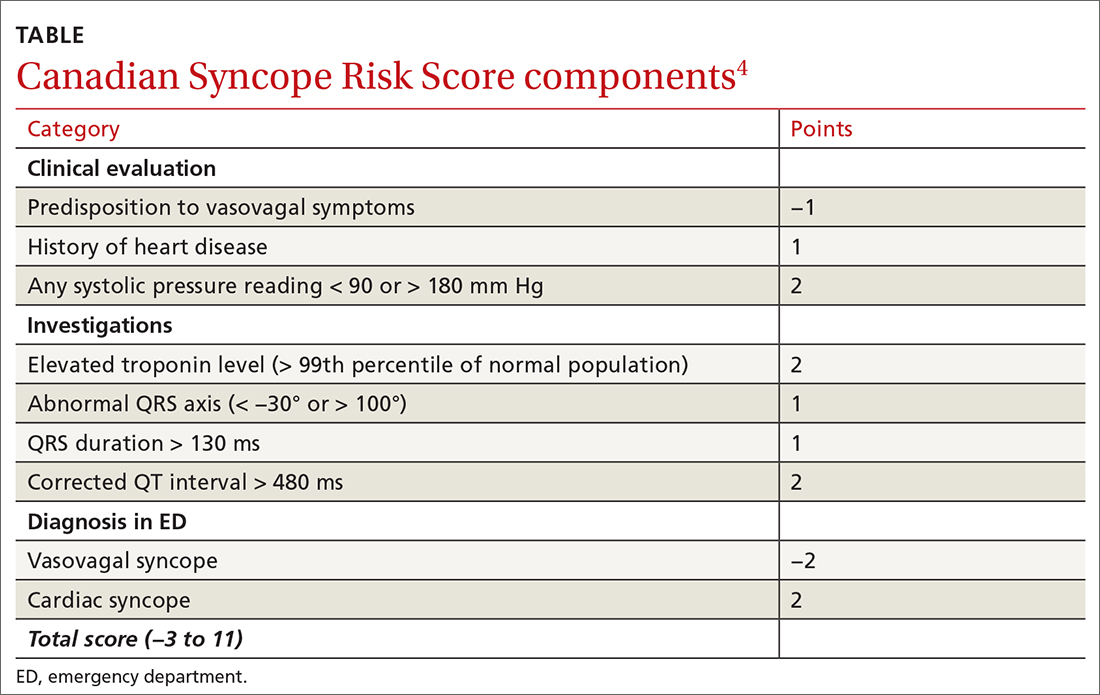
The CSRS was previously derived from a large, multisite consecutive cohort, and was internally validated and reported according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guideline statement.4 Patients are assigned points based on clinical findings, test results, and the diagnosis given in the ED (TABLE4). The scoring system is used to stratify patients as very low (−3, −2), low (−1, 0), medium (1, 2, 3), high (4, 5), or very high (≥6) risk.4
STUDY SUMMARY
Less than 1% of very low– and low-risk patients had serious 30-day outcomes
This multisite Canadian prospective validation cohort study enrolled patients age ≥ 16 years who presented to the ED within 24 hours of syncope. Both discharged and hospitalized patients were included.1
Patients were excluded if they had loss of consciousness for > 5 minutes, mental status changes at presentation, history of current or previous seizure, or head trauma
ED physicians confirmed patient eligibility, obtained verbal consent, and completed the data collection form. In addition, research assistants sought to identify eligible patients who were not previously enrolled by reviewing all ED visits during the study period.
Continue to: To examine 30-day outcomes...
To examine 30-day outcomes, researchers reviewed all available patient medical record
A total of 4131 patients made up the validation cohort. A serious condition was identified during the initial ED visit in 160 patients (3.9%), who were excluded from the study, and 152 patients (3.7%) were lost to follow-up. Of the 3819 patients included in the final analysis, troponin was not measured in 1566 patients (41%), and an electrocardiogram was not obtained in 114 patients (3%). A serious outcome within 30 days was experienced by 139 patients (3.6%; 95% CI, 3.1%-4.3%). There was good correlation to the model-predicted serious outcome probability of 3.2% (95% CI, 2.7%-3.8%).1
Three of 1631 (0.2%) patients classified as very low risk and 9 of 1254 (0.7%) low-risk patients experienced a serious outcome, and no patients died. In the group classified as medium risk, 55 of 687 (8%) patients experienced a serious outcome, and there was 1 death. In the high-risk group, 32 of 167 (19.2%) patients experienced a serious outcome, and there were 5 deaths. In the group classified as very high risk, 40 of 78 (51.3%) patients experienced a serious outcome, and there were 7 deaths. The CSRS was able to identify very low– or low-risk patients (score of −1 or better) with a sensitivity of 97.8% (95% CI, 93.8%-99.6%) and a specificity of 44.3% (95% CI, 42.7%-45.9%).1
WHAT’S NEW
This scoring system offers a validated method to risk-stratify ED patients
Previous recommendations from the American College of Cardiology/American Heart Associationsuggested determining disposition of ED patients by using clinical judgment based on a list of risk factors such as age, chronic conditions, and medications. However, there was no scoring system.3 This new scoring system allows physicians to send home very low– and low-risk patients with reassurance that the likelihood of a serious outcome is less than 1%. High-risk and very high–risk patients should be admitted to the hospital for further evaluation. Most moderate-risk patients (8% risk of serious outcome but 0.1% risk of death) can also be discharged after providers have a risk/benefit discussion, including precautions for signs of arrhythmia or need for urgent return to the hospital.
CAVEATS
The study does not translate to all clinical settings
Because this study was done in EDs, the scoring system cannot necessarily be applied to urgent care or outpatient settings. However, 41% of the patients in the study did not have troponin testing performed. Therefore, physicians could consider using the scoring system in settings where this lab test is not immediately available.
Continue to: This scoring system was also only...
This scoring system was also only validated with adult patients presenting within 24 hours of their syncopal episode. It is unknown how it may predict the outcomes of patients who present > 24 hours after syncope.
CHALLENGES TO IMPLEMENTATION
Clinicians may not be awareof the CSRS scoring system
The main challenge to implementation is practitioner awareness of the CSRS scoring system and how to use it appropriately, as there are several different syncopal scoring systems that may already be in use. Additionally, depending on the electronic health record used, the CSRS scoring system may not be embedded. Using and documenting scores may also be a challenge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Thiruganasambandamoorthy V, Sivilotti MLA, Le Sage N, et al. Multicenter emergency department validation of the Canadian Syncope Risk Score. JAMA Intern Med. 2020;180:737-744. doi:10.1001/jamainternmed.2020.0288
2. Probst MA, Kanzaria HK, Gbedemah M, et al. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33:998-1001. doi:10.1016/j.ajem.2015.04.030
3. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi:10.1016/j.jacc.2017.03.002
4. Thiruganasambandamoorthy V, Kwong K, Wells GA, et al. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ. 2016;188:E289-E298. doi:10.1503/cmaj.151469
ILLUSTRATIVE CASE
A 30-year-old woman presented to the ED after she “passed out” while standing at a concert. She lost consciousness for 10 seconds. After she revived, her friends drove her to the ED. She is healthy, with no chronic medical conditions, no medication use, and no drug or alcohol use. Should she be admitted to the hospital for observation?
Syncope, a transient loss of consciousness followed by spontaneous complete recovery, accounts for 1% of ED visits.2 Approximately 10% of patients presenting to the ED will have a serious underlying condition identified and among 3% to 5% of these patients with syncope, the serious condition will be identified only after they leave the ED.1 Most patients have a benign course, but more than half of all patients presenting to the ED with syncope will be hospitalized, costing $2.4 billion annually.2
Because of the high hospitalization rate of patients with syncope, a practical and accurate tool to risk-stratify patients is vital. Other tools, such as the San Francisco Syncope Rule, Short-Term Prognosis of Syncope, and Risk Stratification of Syncope in the Emergency Department, lack validation or are excessively complex, with extensive lab work or testing.3
The CSRS was previously derived from a large, multisite consecutive cohort, and was internally validated and reported according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guideline statement.4 Patients are assigned points based on clinical findings, test results, and the diagnosis given in the ED (TABLE4). The scoring system is used to stratify patients as very low (−3, −2), low (−1, 0), medium (1, 2, 3), high (4, 5), or very high (≥6) risk.4
STUDY SUMMARY
Less than 1% of very low– and low-risk patients had serious 30-day outcomes
This multisite Canadian prospective validation cohort study enrolled patients age ≥ 16 years who presented to the ED within 24 hours of syncope. Both discharged and hospitalized patients were included.1
Patients were excluded if they had loss of consciousness for > 5 minutes, mental status changes at presentation, history of current or previous seizure, or head trauma
ED physicians confirmed patient eligibility, obtained verbal consent, and completed the data collection form. In addition, research assistants sought to identify eligible patients who were not previously enrolled by reviewing all ED visits during the study period.
Continue to: To examine 30-day outcomes...
To examine 30-day outcomes, researchers reviewed all available patient medical record
A total of 4131 patients made up the validation cohort. A serious condition was identified during the initial ED visit in 160 patients (3.9%), who were excluded from the study, and 152 patients (3.7%) were lost to follow-up. Of the 3819 patients included in the final analysis, troponin was not measured in 1566 patients (41%), and an electrocardiogram was not obtained in 114 patients (3%). A serious outcome within 30 days was experienced by 139 patients (3.6%; 95% CI, 3.1%-4.3%). There was good correlation to the model-predicted serious outcome probability of 3.2% (95% CI, 2.7%-3.8%).1
Three of 1631 (0.2%) patients classified as very low risk and 9 of 1254 (0.7%) low-risk patients experienced a serious outcome, and no patients died. In the group classified as medium risk, 55 of 687 (8%) patients experienced a serious outcome, and there was 1 death. In the high-risk group, 32 of 167 (19.2%) patients experienced a serious outcome, and there were 5 deaths. In the group classified as very high risk, 40 of 78 (51.3%) patients experienced a serious outcome, and there were 7 deaths. The CSRS was able to identify very low– or low-risk patients (score of −1 or better) with a sensitivity of 97.8% (95% CI, 93.8%-99.6%) and a specificity of 44.3% (95% CI, 42.7%-45.9%).1
WHAT’S NEW
This scoring system offers a validated method to risk-stratify ED patients
Previous recommendations from the American College of Cardiology/American Heart Associationsuggested determining disposition of ED patients by using clinical judgment based on a list of risk factors such as age, chronic conditions, and medications. However, there was no scoring system.3 This new scoring system allows physicians to send home very low– and low-risk patients with reassurance that the likelihood of a serious outcome is less than 1%. High-risk and very high–risk patients should be admitted to the hospital for further evaluation. Most moderate-risk patients (8% risk of serious outcome but 0.1% risk of death) can also be discharged after providers have a risk/benefit discussion, including precautions for signs of arrhythmia or need for urgent return to the hospital.
CAVEATS
The study does not translate to all clinical settings
Because this study was done in EDs, the scoring system cannot necessarily be applied to urgent care or outpatient settings. However, 41% of the patients in the study did not have troponin testing performed. Therefore, physicians could consider using the scoring system in settings where this lab test is not immediately available.
Continue to: This scoring system was also only...
This scoring system was also only validated with adult patients presenting within 24 hours of their syncopal episode. It is unknown how it may predict the outcomes of patients who present > 24 hours after syncope.
CHALLENGES TO IMPLEMENTATION
Clinicians may not be awareof the CSRS scoring system
The main challenge to implementation is practitioner awareness of the CSRS scoring system and how to use it appropriately, as there are several different syncopal scoring systems that may already be in use. Additionally, depending on the electronic health record used, the CSRS scoring system may not be embedded. Using and documenting scores may also be a challenge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 30-year-old woman presented to the ED after she “passed out” while standing at a concert. She lost consciousness for 10 seconds. After she revived, her friends drove her to the ED. She is healthy, with no chronic medical conditions, no medication use, and no drug or alcohol use. Should she be admitted to the hospital for observation?
Syncope, a transient loss of consciousness followed by spontaneous complete recovery, accounts for 1% of ED visits.2 Approximately 10% of patients presenting to the ED will have a serious underlying condition identified and among 3% to 5% of these patients with syncope, the serious condition will be identified only after they leave the ED.1 Most patients have a benign course, but more than half of all patients presenting to the ED with syncope will be hospitalized, costing $2.4 billion annually.2
Because of the high hospitalization rate of patients with syncope, a practical and accurate tool to risk-stratify patients is vital. Other tools, such as the San Francisco Syncope Rule, Short-Term Prognosis of Syncope, and Risk Stratification of Syncope in the Emergency Department, lack validation or are excessively complex, with extensive lab work or testing.3
The CSRS was previously derived from a large, multisite consecutive cohort, and was internally validated and reported according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guideline statement.4 Patients are assigned points based on clinical findings, test results, and the diagnosis given in the ED (TABLE4). The scoring system is used to stratify patients as very low (−3, −2), low (−1, 0), medium (1, 2, 3), high (4, 5), or very high (≥6) risk.4
STUDY SUMMARY
Less than 1% of very low– and low-risk patients had serious 30-day outcomes
This multisite Canadian prospective validation cohort study enrolled patients age ≥ 16 years who presented to the ED within 24 hours of syncope. Both discharged and hospitalized patients were included.1
Patients were excluded if they had loss of consciousness for > 5 minutes, mental status changes at presentation, history of current or previous seizure, or head trauma
ED physicians confirmed patient eligibility, obtained verbal consent, and completed the data collection form. In addition, research assistants sought to identify eligible patients who were not previously enrolled by reviewing all ED visits during the study period.
Continue to: To examine 30-day outcomes...
To examine 30-day outcomes, researchers reviewed all available patient medical record
A total of 4131 patients made up the validation cohort. A serious condition was identified during the initial ED visit in 160 patients (3.9%), who were excluded from the study, and 152 patients (3.7%) were lost to follow-up. Of the 3819 patients included in the final analysis, troponin was not measured in 1566 patients (41%), and an electrocardiogram was not obtained in 114 patients (3%). A serious outcome within 30 days was experienced by 139 patients (3.6%; 95% CI, 3.1%-4.3%). There was good correlation to the model-predicted serious outcome probability of 3.2% (95% CI, 2.7%-3.8%).1
Three of 1631 (0.2%) patients classified as very low risk and 9 of 1254 (0.7%) low-risk patients experienced a serious outcome, and no patients died. In the group classified as medium risk, 55 of 687 (8%) patients experienced a serious outcome, and there was 1 death. In the high-risk group, 32 of 167 (19.2%) patients experienced a serious outcome, and there were 5 deaths. In the group classified as very high risk, 40 of 78 (51.3%) patients experienced a serious outcome, and there were 7 deaths. The CSRS was able to identify very low– or low-risk patients (score of −1 or better) with a sensitivity of 97.8% (95% CI, 93.8%-99.6%) and a specificity of 44.3% (95% CI, 42.7%-45.9%).1
WHAT’S NEW
This scoring system offers a validated method to risk-stratify ED patients
Previous recommendations from the American College of Cardiology/American Heart Associationsuggested determining disposition of ED patients by using clinical judgment based on a list of risk factors such as age, chronic conditions, and medications. However, there was no scoring system.3 This new scoring system allows physicians to send home very low– and low-risk patients with reassurance that the likelihood of a serious outcome is less than 1%. High-risk and very high–risk patients should be admitted to the hospital for further evaluation. Most moderate-risk patients (8% risk of serious outcome but 0.1% risk of death) can also be discharged after providers have a risk/benefit discussion, including precautions for signs of arrhythmia or need for urgent return to the hospital.
CAVEATS
The study does not translate to all clinical settings
Because this study was done in EDs, the scoring system cannot necessarily be applied to urgent care or outpatient settings. However, 41% of the patients in the study did not have troponin testing performed. Therefore, physicians could consider using the scoring system in settings where this lab test is not immediately available.
Continue to: This scoring system was also only...
This scoring system was also only validated with adult patients presenting within 24 hours of their syncopal episode. It is unknown how it may predict the outcomes of patients who present > 24 hours after syncope.
CHALLENGES TO IMPLEMENTATION
Clinicians may not be awareof the CSRS scoring system
The main challenge to implementation is practitioner awareness of the CSRS scoring system and how to use it appropriately, as there are several different syncopal scoring systems that may already be in use. Additionally, depending on the electronic health record used, the CSRS scoring system may not be embedded. Using and documenting scores may also be a challenge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Thiruganasambandamoorthy V, Sivilotti MLA, Le Sage N, et al. Multicenter emergency department validation of the Canadian Syncope Risk Score. JAMA Intern Med. 2020;180:737-744. doi:10.1001/jamainternmed.2020.0288
2. Probst MA, Kanzaria HK, Gbedemah M, et al. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33:998-1001. doi:10.1016/j.ajem.2015.04.030
3. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi:10.1016/j.jacc.2017.03.002
4. Thiruganasambandamoorthy V, Kwong K, Wells GA, et al. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ. 2016;188:E289-E298. doi:10.1503/cmaj.151469
1. Thiruganasambandamoorthy V, Sivilotti MLA, Le Sage N, et al. Multicenter emergency department validation of the Canadian Syncope Risk Score. JAMA Intern Med. 2020;180:737-744. doi:10.1001/jamainternmed.2020.0288
2. Probst MA, Kanzaria HK, Gbedemah M, et al. National trends in resource utilization associated with ED visits for syncope. Am J Emerg Med. 2015;33:998-1001. doi:10.1016/j.ajem.2015.04.030
3. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi:10.1016/j.jacc.2017.03.002
4. Thiruganasambandamoorthy V, Kwong K, Wells GA, et al. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ. 2016;188:E289-E298. doi:10.1503/cmaj.151469
PRACTICE CHANGER
Physicians should use the Canadian Syncope Risk Score (CSRS) to identify and send home very low– and low-risk patients from the emergency department (ED) after a syncopal episode.
STRENGTH OF RECOMMENDATION
A: Validated clinical decision rule based on a prospective cohort study1
Thiruganasambandamoorthy V, Sivilotti MLA, Le Sage N, et al. Multicenter emergency department validation of the Canadian Syncope Risk Score. JAMA Intern Med. 2020;180:737-744. doi:10.1001/jamainternmed.2020.0288
Tips and tools to help refine your approach to chest pain
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3
First challenge: The scale of the differential Dx
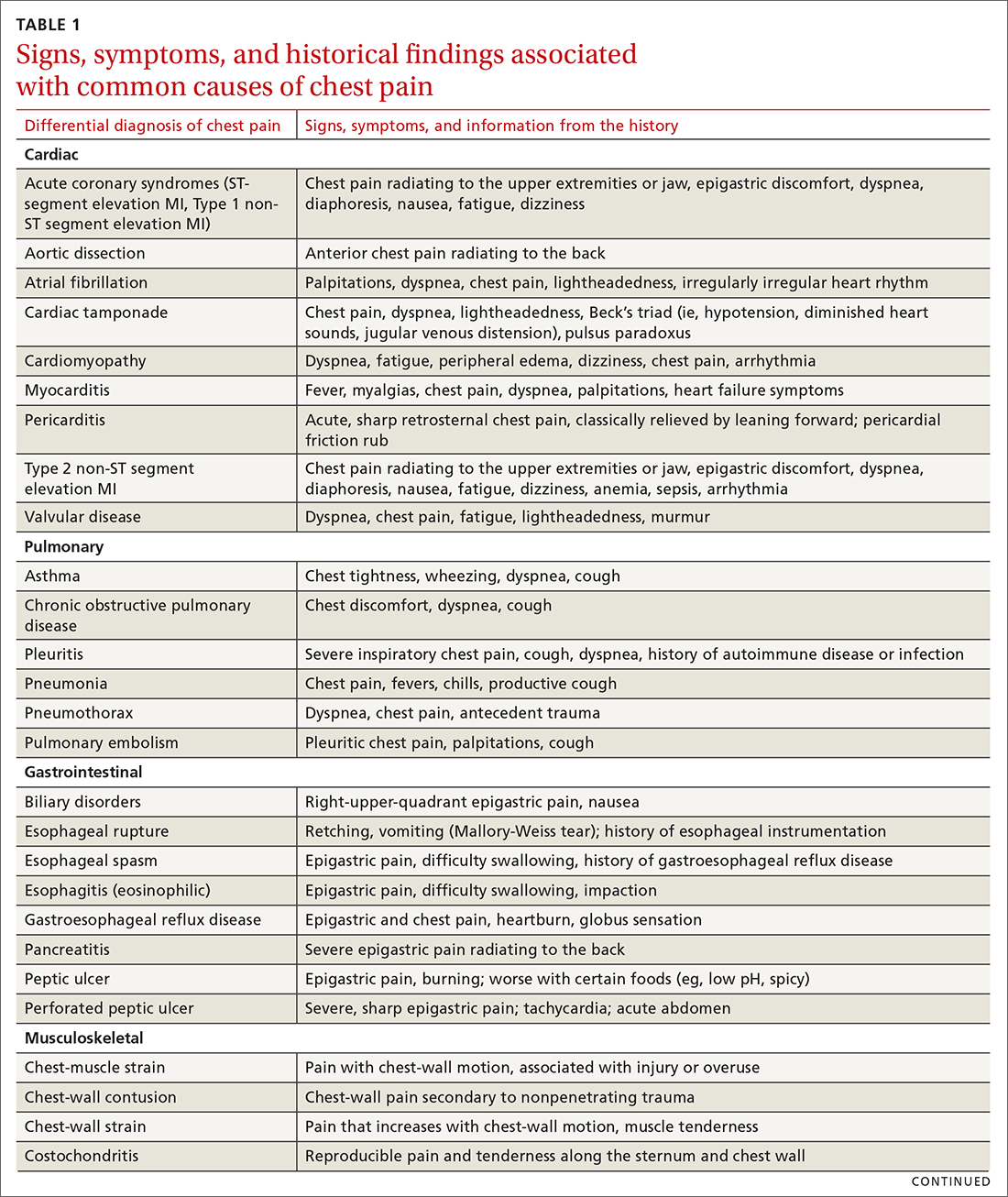
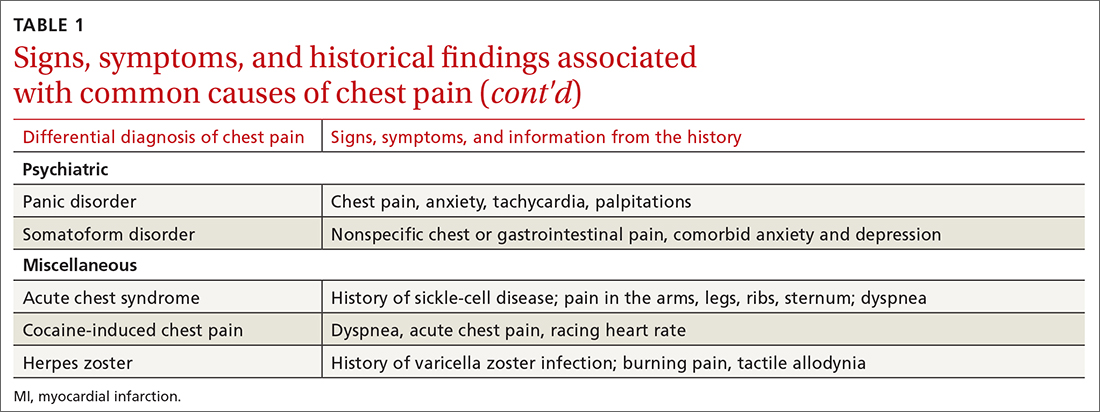
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
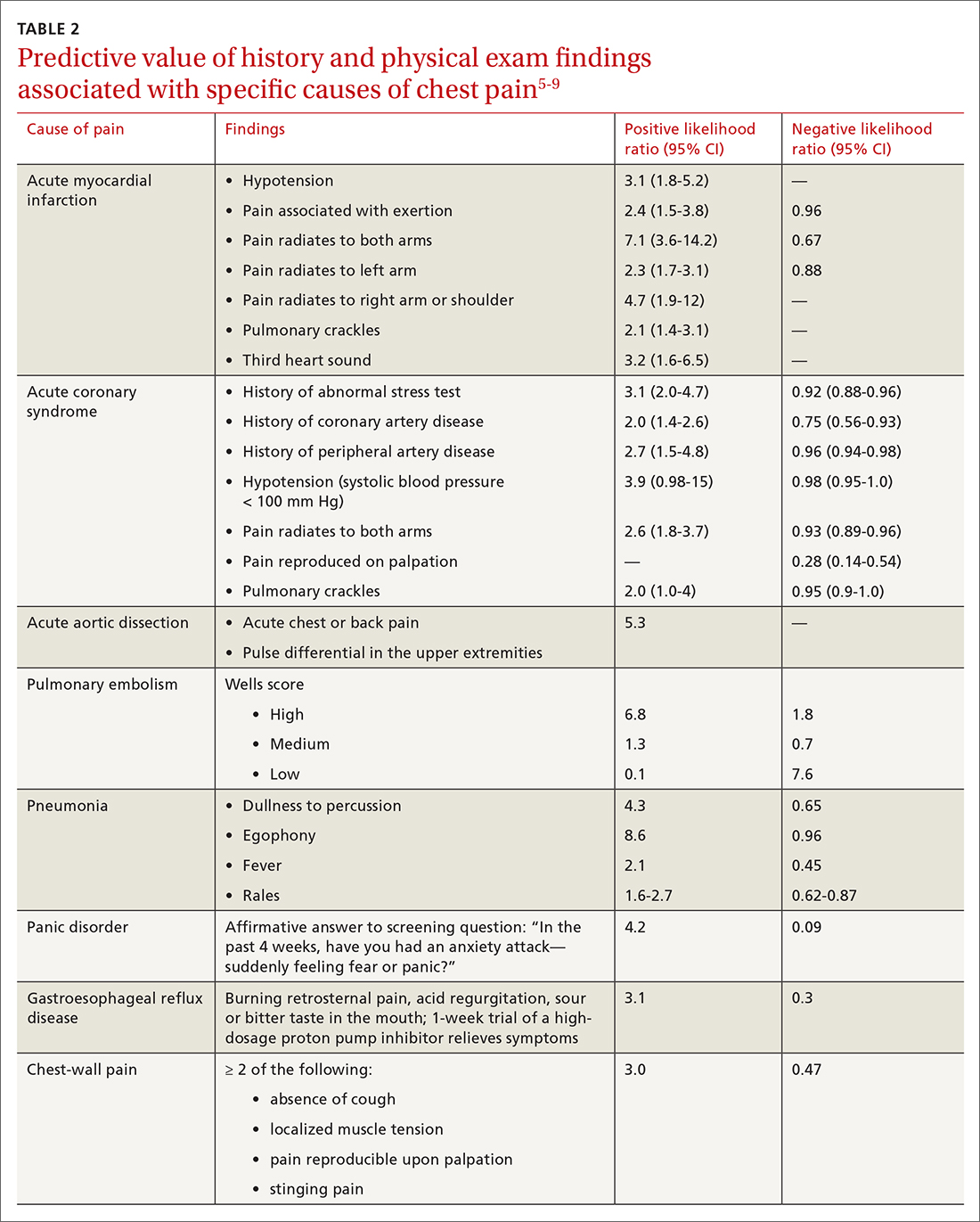
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
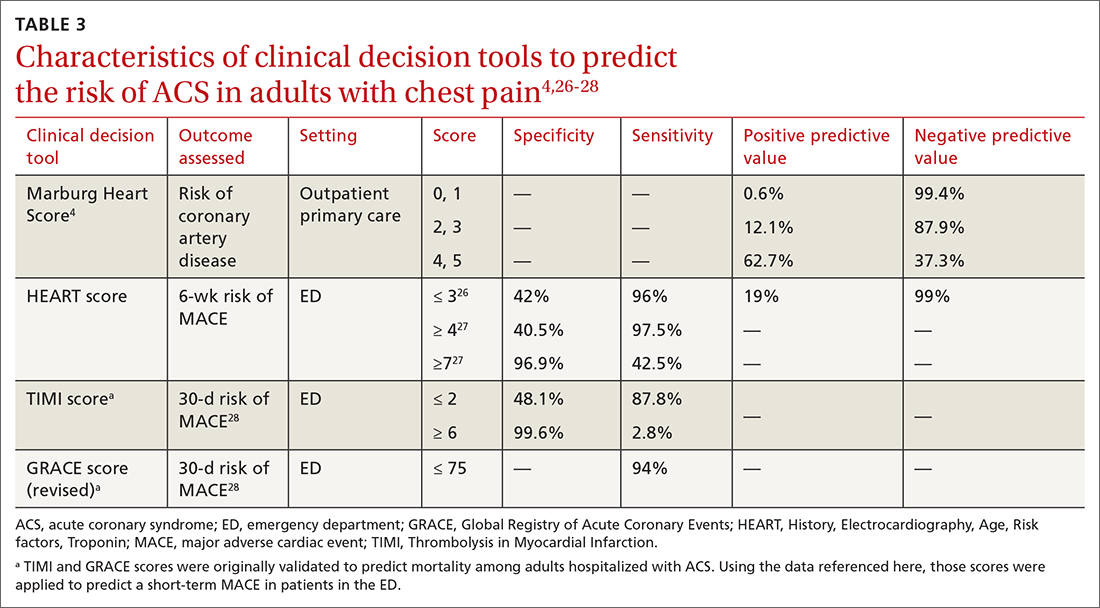
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
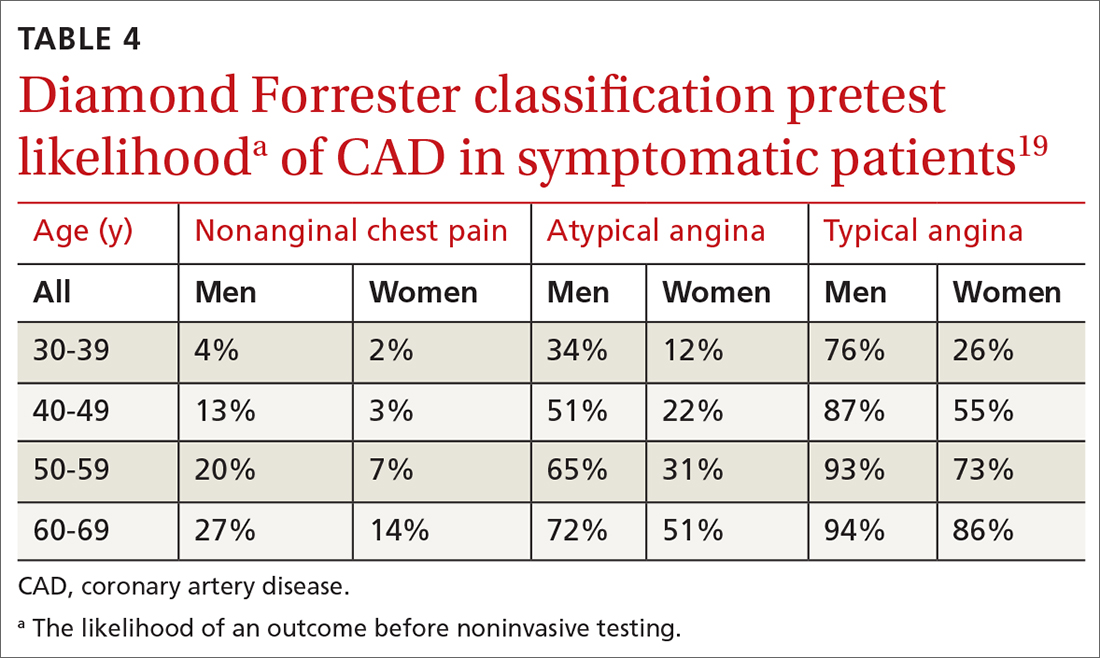
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
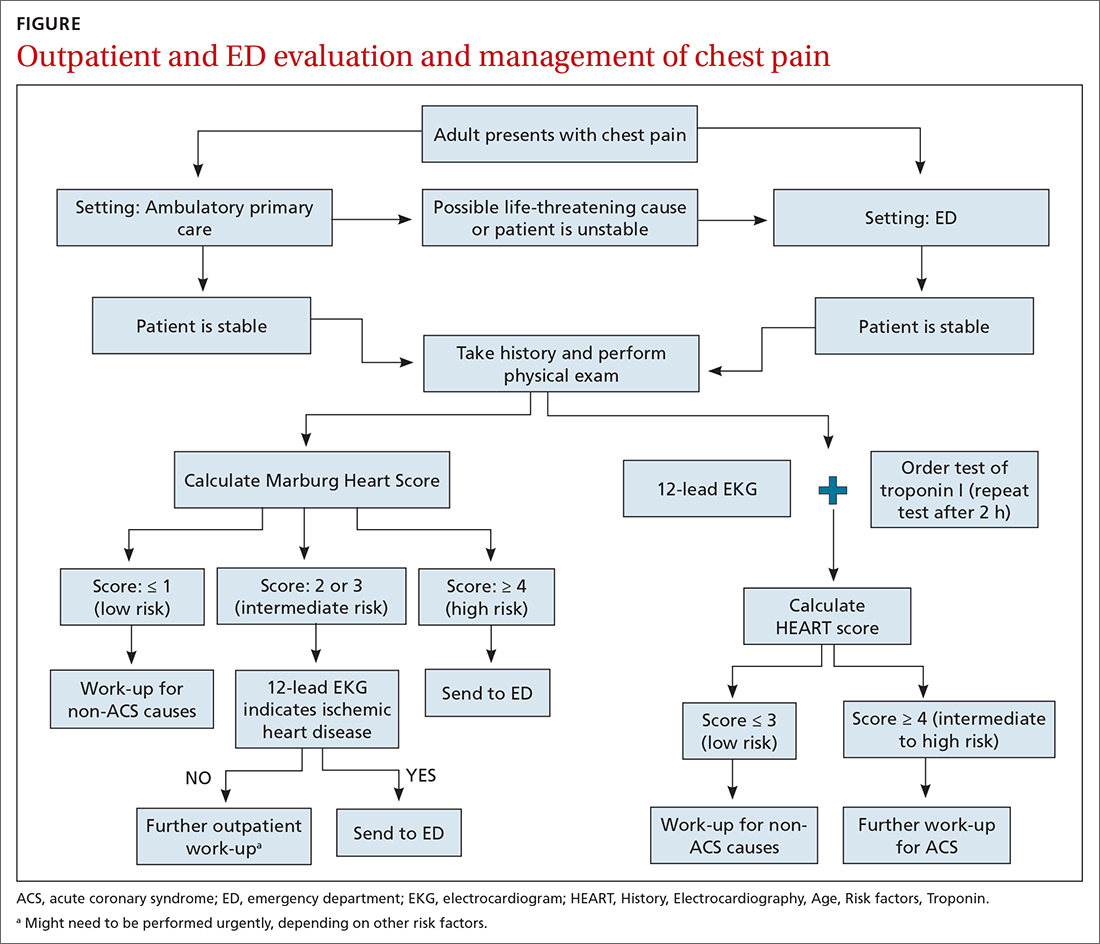
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; Anne_Mounsey@med.unc.edu
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3
First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; Anne_Mounsey@med.unc.edu
One of the most concerning and challenging patient complaints presented to physicians is chest pain. Chest pain is a ubiquitous complaint in primary care settings and in the emergency department (ED), accounting for 8 million ED visits and 0.4% of all primary care visits in North America annually.1,2
Despite the great number of chest-pain encounters, early identification of life-threatening causes and prompt treatment remain a challenge. In this article, we examine how the approach to a complaint of chest pain in a primary care practice (and, likewise, in the ED) must first, rest on the clinical evaluation and second, employ risk-stratification tools to aid in evaluation, appropriate diagnosis, triage, and treatment.
Chest pain by the numbers
Acute coronary syndrome (ACS) is the cause of chest pain in 5.1% of patients with chest pain who present to the ED, compared with 1.5% to 3.1% of chest-pain patients seen in ambulatory care.1,3 “Nonspecific chest pain” is the most frequent diagnosis of chest pain in the ED for all age groups (47.5% to 55.8%).3 In contrast, the most common cause of chest pain in primary care is musculoskeletal (36%), followed by gastrointestinal disease (18% to 19%); serious cardiac causes (15%), including ACS (1.5%); nonspecific causes (16%); psychiatric causes (8%); and pulmonary causes (5% to 10%).4 Among patients seen in the ED because of chest pain, 57.4% are discharged, 30.6% are admitted for further evaluation, and 0.4% die in the ED or after admission.3
First challenge: The scale of the differential Dx
The differential diagnosis of chest pain is broad. It includes life-threatening causes, such as ACS (from ST-segment elevation myocardial infarction [STEMI], Type 1 non-STEMI, and unstable angina), acute aortic dissection, pulmonary embolism (PE), esophageal rupture, and tension pneumothorax, as well as non-life-threatening causes (TABLE 1).
History and physical exam guide early decisions
Triage assessment of the patient with chest pain, including vital signs, general appearance, and basic symptom questions, can guide you as to whether they require transfer to a higher level of care. Although an individual’s findings cannot, alone, accurately exclude or diagnose ACS, the findings can be used in combination in clinical decision tools to distinguish noncardiac chest pain from ACS.
History. Features in the history (TABLE 25-9) that are most helpful at increasing the probability (ie, a positive likelihood ratio [LR] ≥ 2) of chest pain being caused by ACS are:
- pain radiating to both arms or the right arm
- pain that is worse upon exertion
- a history of peripheral artery disease or coronary artery disease (CAD)
- a previously abnormal stress test.
The presence of any prior normal stress test is unhelpful: Such patients have a similar risk of a 30-day adverse cardiac event as a patient who has never had a stress test.5
Continue to: A history of tobacco use...
A history of tobacco use, hyperlipidemia, hypertension, obesity, acute myocardial infarction (AMI), coronary artery bypass grafting, or a family history of CAD does not significantly increase the risk of ACS.6 However, exploring each of these risk factors further is important, because genetic links between these risk factors can lead to an increased risk of CAD (eg, familial hypercholesterolemia).7
A history of normal or near-normal coronary angiography (< 25% stenosis) is associated with a lower likelihood of ACS, because 98% of such patients are free of AMI and 90% are without single-vessel coronary disease nearly 10 years out.6 A history of coronary artery bypass grafting is not necessarily predictive of ACS (LR = 1-3).5,6
Historical features classically associated with ACS, but that have an LR < 2, are pain radiating to the neck or jaw, nausea or vomiting, dyspnea, and pain that is relieved with nitroglycerin.5,6 Pain described as pleuritic, sharp, positional, or reproduced with palpation is less likely due to AMI.5
Physical exam findings are not independently diagnostic when evaluating chest pain. However, a third heart sound is the most likely finding associated with AMI and hypotension is the clinical sign most likely associated with ACS.5
Consider the diagnosis of PE in all patients with chest pain. In PE, chest pain might be associated with dyspnea, presyncope, syncope, or hemoptysis.8 On examination, 40% of patients have tachycardia.8 If PE is suspected; the patient should be risk-stratified using a validated prediction rule (see the discussion of PE that follows).
Continue to: Other historical features...
Other historical features or physical exam findings correlate with aortic dissection, pneumonia, and psychiatric causes of chest pain (TABLE 25-9).
Useful EKG findings
Among patients in whom ACS or PE is suspected, 12-lead electrocardiography (EKG) should be performed.
AMI. EKG findings most predictive of AMI are new ST-segment elevation or depression > 1 mm (LR = 6-54), new left bundle branch block (LR = 6.3), Q wave (positive LR = 3.9), and prominent, wide-based (hyperacute) T wave (LR = 3.1).10
ACS. Useful EKG findings to predict ACS are ST-segment depression (LR = 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia, defined as ST-segment depression, T-wave inversion, or Q wave (LR = 3.6 [95% CI, 1.6-5.7]).10
PE. The most common abnormal finding on EKG in the setting of PE is sinus tachycardia.
Continue to: Right ventricular strain
Right ventricular strain. Other findings that reflect right ventricular strain, but are much less common, are complete or incomplete right bundle branch block, prominent S wave in lead I, Q wave in lead III, and T-wave inversion in lead III (S1Q3T3; the McGinn-White sign) and in leads V1-V4.8
The utility of troponin and high-sensitivity troponin testing
Clinical evaluation and EKG findings are unable to diagnose or exclude ACS without the use of the cardiac biomarker troponin. In the past decade, high-sensitivity troponin assays have been used to stratify patients at risk of ACS.11,12 Many protocols now exist using short interval (2-3 hours), high-sensitivity troponin testing to identify patients at low risk of myocardial infarction who can be safely discharged from the ED after 2 normal tests of the troponin level.13-16
An elevated troponin value alone, however, is not a specific indicator of ACS; troponin can be elevated in the settings of myocardial ischemia related to increased oxygen demand (Type 2 non-STEMI) and decreased renal clearance. Consideration of the rate of rising and falling levels of troponin, its absolute value > 99th percentile, and other findings is critical to interpreting an elevated troponin level.17 Studies in which the HEART score (History, Electrocardiography, Age, Risk factors, Troponin) was combined with high-sensitivity troponin measurement show that this pairing is promising in reducing unnecessary admissions for chest pain.18 (For a description of this tool, see the discussion of the HEART score that follows.) Carlton and colleagues18 showed that a HEART score ≤ 3 and a negative high-sensitivity troponin I level had a negative predictive value of ≥ 99.5% for AMI.
Clinical decision tools: Who needs care? Who can go home?
Given the varied presentations of patients with life-threatening causes of chest pain, it is challenging to confidently determine who is safe to send home after initial assessment. Guidance in 2014 from the American Heart Association and American College of Cardiology recommends risk-stratifying patients for ACS using clinical decision tools to help guide management.19,20 The American College of Physicians, in its 2015 guidelines, also recommends using a clinical decision tool to assess patients when there is suspicion of PE.21 Clinical application of these tools identifies patients at low risk of life-threatening conditions and can help avoid unnecessary intervention and a higher level of care.
Tools for investigating ACS
The Marburg Heart Score22 assesses the likelihood of CAD in ambulatory settings while the HEART score assesses the risk of major adverse cardiac events in ED patients.23 The Diamond Forrester criteria can be used to assess the pretest probability of CAD in both settings.24
Continue to: Marburg Heart Score
Marburg Heart Score. Validated in patients older than 35 years of age in 2 different outpatient populations in 201022 and 2012,25 the Marburg score is determined by answering 5 questions:
- Female ≥ 65 years? Or male ≥ 55 years of age? (No, 0; Yes, +1)
- Known CAD, cerebrovascular disease, or peripheral vascular disease? (No, 0; Yes, +1)
- Is pain worse with exercise? (No, 0; Yes, +1)
- Is pain reproducible with palpation? (No, +1, Yes, 0)
- Does the patient assume that the pain is cardiac in nature? (No, 0; Yes, +1)
A Marburg Heart Score of 0 or 1 means CAD is highly unlikely in a patient with chest pain (negative predictive value = 99%-100%; positive predictive value = 0.6%)4 (TABLE 34,26-28). A score of ≤ 2 has a negative predictive value of 98%. A Marburg Heart Score of 4 or 5 has a relatively low positive predictive value (63%).4
This tool does not accurately diagnose acute MI, but it does help identify patients at low risk of ACS, thus reducing unnecessary subsequent testing. Although no clinical decision tool can rule out AMI with absolute certainty, the Marburg Heart Score is considered one of the most extensively tested and sensitive tools to predict low risk of CAD in outpatient primary care.29
INTERCHEST rule (in outpatient primary care) is a newer prediction rule using data from 5 primary care–based studies of chest pain.30 For a score ≤ 2, the negative predictive value for CAD causing chest pain is 97% to 98% and the positive predictive value is 43%. INTERCHEST incorporates studies used to validate the Marburg Heart Score, but has not been validated beyond initial pooled studies. Concerns have been raised about the quality of these pooled studies, however, and this rule has not been widely accepted for clinical use at this time.29
The HEART score has been validated in patients older than 12 years in multiple institutions and across multiple ED populations.23,31,32 It is widely used in the ED to assess a patient’s risk of major adverse cardiac events (MACE) over the next 6 weeks. MACE is defined as AMI, percutaneous coronary intervention, coronary artery bypass grafting, or death.
Continue to: The HEART score...
The HEART score is calculated based on 5 components:
- History of chest pain (slightly [0], moderately [+1], or highly [+2]) suspicious for ACS)
- EKG (normal [0], nonspecific ST changes [+1], significant ST deviations [+2])
- Age (< 45 y [0], 45-64 y [+1], ≥ 65 y [+2])
- Risk factors (none [0], 1 or 2 [+1], ≥ 3 or a history of atherosclerotic disease [+2]) a
- Initial troponin assay, standard sensitivity (≤ normal [0], 1-3× normal [+1], > 3× normal [+2]).
For patients with a HEART score of 0-3 (ie, at low risk), the pooled positive predictive value of a MACE was determined to be 0.19 (95% CI, 0.14-0.24), and the negative predictive value was 0.99 (95% CI, 0.98-0.99)—making it an effective tool to rule out a MACE over the short term26 (TABLE 34,26-28).
Because the HEART Score was published in 2008, multiple systematic reviews and meta-analyses have compared it to the TIMI (Thrombolysis in Myocardial Infarction) and GRACE (Global Registry of Acute Coronary Events) scores for predicting short-term (30-day to 6-week) MACE in ED patients.27,28,33,34 These studies have all shown that the HEART score is relatively superior to the TIMI and GRACE tools.
Characteristics of these tools are summarized in TABLE 3.4,26-28
Diamond Forrester classification (in ED and outpatient settings). This tool uses 3 criteria—substernal chest pain, pain that increases upon exertion or with stress, and pain relieved by nitroglycerin or rest—to classify chest pain as typical angina (all 3 criteria), atypical angina (2 criteria), or noncardiac chest pain (0 criteria or 1 criterion).24 Pretest probability (ie, the likelihood of an outcome before noninvasive testing) of the pain being due to CAD can then be determined from the type of chest pain and the patient’s gender and age19 (TABLE 419). Recent studies have found that the Diamond Forrester criteria might overestimate the probability of CAD.35
Continue to: Noninvasive imaging-based diagnostic methods
Noninvasive imaging-based diagnostic methods
Positron-emission tomography stress testing, stress echocardiography, myocardial perfusion scanning, exercise treadmill testing. The first 3 of these imaging tests have a sensitivity and specificity ranging from 74% to 87%36; exercise treadmill testing is less sensitive (68%) and specific (77%).37
In a patient with a very low (< 5%) probability of CAD, a positive stress test (of any modality) is likely to be a false-positive; conversely, in a patient with a very high (> 90%) probability of CAD, a negative stress test is likely to be a false-negative.19 The American Heart Association, therefore, does not recommend any of these modalities for patients who have a < 5% or > 90% probability of CAD.19
Noninvasive testing to rule out ACS in low- and intermediate-risk patients who present to the ED with chest pain provides no clinical benefit over clinical evaluation alone.38 Therefore, these tests are rarely used in the initial evaluation of chest pain in an acute setting.
Coronary artery calcium score (CACS), coronary computed tomography angiography (CCTA). These tests have demonstrated promise in the risk stratification of chest pain, given their high sensitivity and negative predictive value in low- and intermediate-risk patients.39,40 However, their application remains unclear in the evaluation of acute chest pain: Appropriate-use criteria do not favor CACS or CCTA alone to evaluate acute chest pain when there is suspicion of ACS.41 The Choosing Wisely initiative (for “avoiding unnecessary medical tests, treatments, and procedures”; www.choosingwisely.org) recommends against CCTA for high-risk patients presenting to the ED with acute chest pain.42
Cardiac magnetic resonance imaging does not have an established role in the evaluation of patients with suspected ACS.43
Continue to: Tools for investigating PE
Tools for investigating PE
Three clinical decision tools have been validated to predict the risk of PE: the Wells score, the Geneva score, and Pulmonary Embolism Rule Out Criteria (PERC).44,45
Wells score is more sensitive than the Geneva score and has been validated in ambulatory1 and ED46-48 settings. Based on Wells criteria, high-risk patients need further evaluation with imaging. In low-risk patients, a normal D-dimer level effectively excludes PE, with a < 1% risk of subsequent thromboembolism in the following 3 months. Positive predictive value of the Wells decision tool is low because it is intended to rule out, not confirm, PE.
PERC can be used in a low-probability setting (defined as the treating physician arriving at the conclusion that PE is not the most likely diagnosis and can be excluded with a negative D-dimer test). In that setting, if the patient meets the 8 clinical variables in PERC, the diagnosis of PE is, effectively, ruled out.48
Summing up: Evaluation of chest pain guided by risk of CAD
Patients who present in an outpatient setting with a potentially life-threatening cause of chest pain (TABLE 1) and patients with unstable vital signs should be sent to the ED for urgent evaluation. In the remaining outpatients, use the Marburg Heart Score or Diamond Forrester classification to assess the likelihood that pain is due to CAD (in the ED, the HEART score can be used for this purpose) (FIGURE).
When the risk is low. No further cardiac testing is indicated in patients with a risk of CAD < 5%, based on a Marburg score of 0 or 1, or on Diamond Forrester criteria; an abnormal stress test is likely to be a false-positive.19
Continue to: Moderate risk
Moderate risk. However, further testing is indicated, with a stress test (with or without myocardial imaging), in patients whose risk of CAD is 5% to 70%, based on the Diamond Forrester classification or an intermediate Marburg Heart Score (ie, a score of 2 or 3 but a normal EKG). This further testing can be performed urgently in patients who have multiple other risk factors that are not assessed by the Marburg Heart Score.
High risk. In patients whose risk is > 70%, invasive testing with angiography should be considered.35,49
EKG abnormalities. Patients with a Marburg Score of 2 or 3 and an abnormal EKG should be sent to the ED (FIGURE). There, patients with a HEART score < 4 and a negative 2-3–hour troponin test have a < 1% chance of ACS and can be safely discharged.31
CORRESPONDENCE
Anne Mounsey, MD, UNC Family Medicine, 590 Manning Drive, Chapel Hill, NC 27599; Anne_Mounsey@med.unc.edu
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
1. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018;36:1-12. doi: 10.1016/j.ccl.2017.08.001
2. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. Accessed February 16, 2021. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
3. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029-1032. doi: 10.1001/jamainternmed.2016.2498
4. Ebell MH. Evaluation of chest pain in primary care patients. Am Fam Physician. 2011;83:603-605.
5. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547-564. doi: 10.1161/CIRCULATIONAHA.116.021886
6. Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome? The rational clinical examination systematic review. JAMA. 2015;314:1955-1965. doi: 10.1001/jama.2015.12735
7. Kolminsky J, Choxi R, Mahmoud AR, et al. Familial hypercholesterolemia: cardiovascular risk stratification and clinical management. American College of Cardiology. June 1, 2020. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2020/06/01/13/54/familial-hypercholesterolemia
8. Konstantinides SV, Meyer G, Becattini C, et al; . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
9. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-182.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868-877. doi: 10.1056/NEJMoa0903515
12. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858-867. doi: 10.1056/NEJMoa0900428
13. Tada M, Azuma H, Yamada N, et al. A comprehensive validation of very early rule-out strategies for non-ST-segment elevation myocardial infarction in emergency departments: protocol for a multicentre prospective cohort study. BMJ Open. 2019;9:e026985. doi: 10.1136/bmjopen-2018-026985
14. Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211-1218. doi: 10.1001/archinternmed.2012.3698
15. Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481-2488. doi: 10.1016/S0140-6736(15)00391-8
16. Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913-1924. doi: 10.1001/jama.2017.17488
17. Vasile VC, Jaffe AS. High-sensitivity cardiac troponin in the evaluation of possible AMI. American College of Cardiology. July 16, 2018. Accessed September 28, 2021. www.acc.org/latest-in-cardiology/articles/2018/07/16/09/17/high-sensitivity-cardiac-troponin-in-the-evaluation-of-possible-am
18. Carlton EW, Khattab A, Greaves K. Identifying patients suitable for discharge after a single-presentation high-sensitivity troponin result: a comparison of five established risk scores and two high-sensitivity assays. Ann Emerg Med. 2015;66:635-645.e1. doi: 10.1016/j.annemergmed.2015.07.006
19. Qaseem A, Fihn SD, Williams S, et al; . Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/Preventative Cardiovascular nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157:729-734. doi: 10.7326/0003-4819-157-10-201211200-00010
20. Amsterdam EA, Wenger NK, Brindis RG, et al; . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-2394. doi: 10.1161/CIR.0000000000000133
21. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163:701-711. doi: 10.7326/M14-1772
22. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300. doi: 10.1503/cmaj.100212
23. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196. doi: 10.1007/BF03086144
24. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350-1358. doi: 10.1056/NEJM197906143002402
25. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e21. doi: 10.3399/bjgp12X649106
26. Laureano-Phillips J, Robinson RD, Aryal S, et al. HEART score risk stratification of low-risk chest pain patients in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2019;74:187-203. doi: 10.1016/j.annemergmed.2018.12.010
27. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26:140-151. doi: 10.1111/acem.13649
28. Sakamoto JT, Liu N, Koh ZX, et al. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016;221:759-764. doi: 10.1016/j.ijcard.2016.07.147
29. Harskamp RE, Laeven SC, Himmelreich JCL, et al. Chest pain in general practice: a systematic review of prediction rules. BMJ Open. 2019;9:e027081. doi: 10.1136/bmjopen-2018-027081
30. Aerts M, Minalu G, Bösner S, et al. Internal Working Group on Chest Pain in Primary Care (INTERCHEST). Pooled individual patient data from five countries were used to derive a clinical prediction rule for coronary artery disease in primary care. J. Clin Epidemiol. 2017;81:120-128. doi: 10.1016/j.jclinepi.2016.09.011
31. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients in the emergency department. Int J Cardiol. 2013;168:2153-2158. doi: 10.1016/j.ijcard.2013.01.255
32. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164-169. doi: 10.1097/HPC.0b013e3181ec36d8
33. Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656-661. doi: 10.1016/j.ijcard.2016.10.080
34. Reaney PDW, Elliott HI, Noman A, et al. Risk stratifying chest pain patients in the emergency department using HEART, GRACE and TIMI scores, with a single contemporary troponin result, to predict major adverse cardiac events. Emerg Med J. 2018;35:420-427. doi: 10.1136/emermed-2017-207172
35. Bittencourt MS, Hulten E, Polonsky TS, et al. European Society of Cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the Diamond Forrester score: The Partners Registry. Circulation. 2016;134:201-211. doi: 10.1161/CIRCULATIONAHA.116.023396
36. Mordi IR, Badar AA, Irving RJ, et al. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427-437. doi: 10.2147/VHRM.S106838
37. Borque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. doi: 10.1016/j.jcmg.2015.09.006
38. Reinhardt SW, Lin C-J, Novak E, et al. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178:212-219. doi: 10.1001/jamainternmed.2017.7360
39. Fernandez-Friera L, Garcia-Alvarez A, Bagheriannejad-Esfahani F, et al. Diagnostic value of coronary artery calcium scoring in low-intermediate risk patients evaluated in the emergency department for acute coronary syndrome. Am J Cardiol. 2011;107:17-23. doi: 10.1016/j.amjcard.2010.08.037
40. Linde JJ, H, Hansen TF, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J AM Coll Cardiol 2020;75:453-463. doi: 10.1016/j.jacc.2019.12.012
41. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525-e555. doi: 10.1161/CIR.0b013e3181fcae66
42. Society of Cardiovascular Computed Tomography. Five things physicians and patients should question. Choosing Wisely Campaign. February 21, 2013. Accessed September 28, 2021. www.choosingwisely.org/wp-content/uploads/2015/02/SCCT-Choosing-Wisely-List.pdf
43. Hamm CW, Bassand J-P, Agewall S, et al; . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999-3054. doi: 10.1093/eurheartj/ehr236
44. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
45. Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:957-970. doi: 10.1111/j.1538-7836.2010.03801.x
46. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in the emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247-1255. doi: 10.1111/j.1538-7836.2004.00790.x
47. Hendriksen JMT, Geersing G-J, Lucassen WAM, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015;351:h4438. doi: 10.1136/bmj.h4438
48. Shen J-H, Chen H-L, Chen J-R, et al. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482-492. doi: 10.1007/s11239-015-1250-2
49. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventative Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. doi: 10.1016/j.jacc.2012.07.013
PRACTICE RECOMMENDATIONS
› Use the highly sensitive Marburg Heart Score to rule out coronary artery disease as a cause of chest pain in the ambulatory care setting. B
› Consider a prior normal stress test result nonpredictive of outcome in a patient presenting with chest pain. Patients with such a history of testing have a risk of a 30-day adverse cardiac event that is similar to the risk seen in patients who have never had a stress test. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
a Risk factors include hypertension, hypercholesterolemia, diabetes, obesity (body mass index > 30), smoking (current, or smoking cessation for ≤ 3 mo), and family history of CAD (ie, parent or sibling affected before 65 years of age). Atherosclerotic disease includes history of AMI, percutaneous coronary intervention or coronary artery bypass grafting, stroke, or peripheral artery disease.
FAVOR III China: QFR-guided PCI shows advantage over angiography
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV have coronary plaque
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Antihypertensives tied to lower Alzheimer’s disease pathology
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANA 2021
How applicable is ISCHEMIA trial to U.S. clinical practice?
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
The applicability of the results of the ISCHEMIA trial to real-world clinical practice in the United States has been called into question by a new study showing that less than a third of U.S. patients with stable ischemic heart disease (IHD) who currently undergo intervention would meet the trial’s inclusion criteria.
The ISCHEMIA trial, first reported in 2019, showed that, for stable patients with moderate to severe ischemia, an invasive approach using percutaneous coronary intervention (PCI) did not significantly reduce major cardiovascular events after a median 3.2 years of follow-up, compared with a conservative medical strategy.
For the current study, a group of interventionalists analyzed contemporary U.S. data on patients undergoing PCI and found that a large proportion of patients receiving PCI for stable ischemic heart disease in the United States would not have met criteria of the ISCHEMIA trial population.
The study was published online Nov. 1 in JACC: Cardiovascular Interventions).
“While ISCHEMIA was a very well-conducted trial, our results show that it only applies to about one-third of stable IHD patients undergoing intervention in U.S. clinical practice in the real world. In this group, while ISCHEMIA did not show a reduction in event rate in the intervention group, there was a reduction in symptoms,” lead author Saurav Chatterjee, MD, Long Island Jewish Medical Center, New York, told this news organization.
“But ISCHEMIA did not really answer the question for 67% of stable IHD in current U.S. practice. We may be abIe to defer PCI in these patients, but we don’t know that from the ISCHEMIA trial, as these patients were not included in the trial,” Chatterjee said.
“There is some concern that people will accept the ISCHEMIA results as being universal, but we cannot apply these results to all stable IHD patients who currently undergo intervention,” he added. “We believe that patients who do not fall into the ISCHEMIA population need a nuanced individual approach, taking into account symptom burden and patient preferences.”
In the new report, Dr. Chatterjee and associates note that the applicability of the ISCHEMIA findings to contemporary practice has been questioned by some, because of the exclusion of a significant proportion of patients that are routinely considered for revascularization, both within and outside of the United States.
They point out that the ISCHEMIA trial recruited 16.5% of its participants from the United States, and the proportion of patients in contemporary U.S. practice that would have qualified for the trial is not clear.
They therefore examined the proportion of stable IHD patients meeting inclusion criteria for the ISCHEMIA trial in a U.S. nationwide PCI registry.
The researchers used data from the National Cardiovascular Data Registry (NCDR) CathPCI Registry, which includes patients undergoing PCI at 1,662 institutions and accounts for more than 90% of PCI-capable hospitals in the United States.
All PCI procedures performed at institutions participating in the NCDR CathPCI Registry from October 2017 to June 2019 were identified. Patients presenting with acute coronary syndrome (ACS), cardiogenic shock, or cardiac arrest were excluded, as there is significant evidence in favor of revascularization in these groups, and they were not included in the ISCHEMIA trial.
Subsequently, all remaining stable IHD patients were classified into one of four groups.
- ISCHEMIA-like: These patients had intermediate- or high-risk findings on a stress test but no high-risk features that would have excluded enrollment into the ISCHEMIA trial
- High risk: This group comprised patients with stable IHD and left ventricular ejection fraction less than 35%, significant unprotected left main stenosis (>50%), preexisting dialysis, recent heart-failure exacerbation, or heart transplant. These patients would have met exclusion criteria for the ISCHEMIA trial
- Low risk: This group included patients with stable and negative or low-risk findings on stress test and would have met exclusion criteria for the ISCHEMIA trial
- Not classifiable: This group comprised patients with stable IHD not fitting any of the other cohorts, including no stress test or extent of ischemia not reported on stress testing. These patients would have not had enough information to clearly meet inclusion or exclusion criteria for the ISCHEMIA trial
Results showed that during the study period 927,011 patients underwent PCI as recorded in the NCDR CathPCI Registry. Of these, 58% had ACS, cardiogenic shock, or cardiac arrest and were excluded; the remaining 388,212 patients who underwent PCI for stable IHD comprised the study population.
Of these, 125,302 (32.28%) had a moderate- or high-risk stress test without high-risk anatomic or clinical features and met ISCHEMIA trial inclusion criteria.
Among stable IHD patients not meeting ISCHEMIA trial inclusion criteria, 71,852 (18.51%) had high-risk criteria that would have excluded them from the ISCHEMIA trial, a total of 67,159 (17.29%) patients had low-risk criteria that would have excluded them from the ISCHEMIA trial, and 123,899 (31.92%) were unclassifiable, either owing to lack of stress testing or the extent of ischemia not being reported on stress testing.
The authors suggest that the unclassifiable patients appear to represent a “higher-risk” population than those closely resembling the ISCHEMIA trial population, with more prior myocardial infarction and heart failure.
ISCHEMIA investigators respond
In an accompanying editorial, ISCHEMIA investigators David J. Maron, MD, Stanford (Calif.) University, and Sripal Bangalore, MD, and Judith S. Hochman, MD, New York University, argue that many of the patients highlighted by Dr. Chatterjee and associates were excluded from the ISCHEMIA trial for good reason.
They explain that ISCHEMIA was designed under the premise that prior stable IHD strategy trials such as COURAGE and BARI 2D included lower-risk patients, and the remaining gap was to evaluate the utility of invasive management in those at higher risk with moderate or severe stress-induced ischemia.
They point out that, among the NCDR patients with stable IHD in the current study by Dr. Chatterjee and associates who did not meet ISCHEMIA entry criteria, 18.5% had high-risk features, including 35.2% with left main coronary artery disease, 43.7% with left ventricular systolic dysfunction, and 16.8% with end-stage renal disease.
Although ISCHEMIA results do not apply to patients who were excluded from the trial, there is little controversy regarding the benefit of revascularization in patients with stable IHD with left main coronary artery disease or left ventricular ejection fraction <35%, which is why they were excluded from ISCHEMIA, the editorialists note.
They also report that patients with end-stage renal disease, who were also designated as not meeting ISCHEMIA inclusion criteria, were included in the companion ISCHEMIA CKD trial.
They further point out that, at the other end of the risk spectrum, 17.3% of stable IHD patients in the current analysis had negative or low-risk functional testing, and these patients were excluded from ISCHEMIA because they were shown in COURAGE and BARI 2D to not benefit from revascularization, and they do not meet guideline recommendations for elective PCI in the absence of symptoms.
Of the 31.9% of stable IHD patients who had missing data on ischemic burden, the ISCHEMIA investigators say that some of these would have qualified for the trial, although it is not possible to say how many. They suggest a conservative estimate of 50%.
Taking these arguments into account, the editorialists recalculated the proportion of NCDR PCI patients with stable IHD who would have been included in ISCHEMIA as between 62.1% and 68.6% of patients.
They say the current NCDR analysis by Dr. Chatterjee and associates should be interpreted as indicating, at worst, that the ISCHEMIA trial results apply to only 32% of patients undergoing elective PCI in the United States, and at best “that the results apply to a far higher proportion, excluding only those at high risk (18.5%) or with unacceptable symptoms despite maximal medical therapy (percentage unknown), for whom PCI is clearly indicated.”
The editorialists conclude: “The purpose of the analysis by Chatterjee et al. is to inform the cardiovascular community of the proportion of patients with stable IHD in clinical practice who would have been excluded from ISCHEMIA without regard for the logic of each exclusion criterion. The purpose of this editorial is to provide context for the analysis, admittedly from the perspective of ISCHEMIA investigators, with the hope that this helps readers clearly see the relevance of the trial to patients under their care.”
They add: “For practical and ethical reasons, ISCHEMIA excluded stable patients with high-risk features, angina inadequately controlled by medication, and low-risk features who do not meet evidence-based guidelines for revascularization. That leaves a large percentage of patients for whom the ISCHEMIA trial is highly relevant; exactly what percentage on the basis of NCDR data is hard to say.”
The ISCHEMIA trial was supported by the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
FFR-guided PCI falls short vs. surgery in multivessel disease: FAME 3
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
SUGAR trial finds superior stent for those with diabetes and CAD
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Superiority shown on TLF endpoint
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021