User login
AHA: Physical activity best first-line for high BP, cholesterol
The optimal first step to address mild to moderately elevated blood pressure and cholesterol in otherwise healthy adults is a “prescription” to sit less and move more, the American Heart Association says in a new scientific statement.
“The current American Heart Association guidelines for diagnosing high blood pressure and cholesterol recognize that otherwise healthy individuals with mildly or moderately elevated levels of these cardiovascular risk factors should actively attempt to reduce these risks,” Bethany Barone Gibbs, PhD, chair of the statement writing group, said in an AHA news release.
“The first treatment strategy for many of these patients should be healthy lifestyle changes beginning with increasing physical activity,” said Dr. Gibbs, from the University of Pittsburgh.
The 12-page AHA scientific statement – Physical Activity as a Critical Component of First-Line Treatment for Elevated Blood Pressure or Cholesterol: Who, What, and How? – was published online June 2 in Hypertension.
Every little bit helps
According to the AHA, about 21% of American adults have systolic blood pressure between 120 and 139 mm Hg, or diastolic blood pressure between 80 and 89 mm Hg, which meets the criteria for lifestyle-only treatment for elevated BP outlined in the American College of Cardiology (ACC)/AHA high blood pressure guideline.
In addition, about 28% of American adults have LDL cholesterol above 70 mg/dL and otherwise meet the low-risk criteria for heart disease or stroke. These individuals would meet the criteria for lifestyle-only treatment outlined in the 2018 ACC/AHA cholesterol treatment guidelines, which include increased physical activity, weight loss, better diet, smoking cessation, and moderating alcohol intake.
“Of the recommended lifestyle changes, increasing physical activity has extensive benefits, including improving both blood pressure and blood cholesterol, that are comparable, superior, or complementary to other healthy lifestyle changes,” the writing group says.
“Physical activity assessment and prescription are an excellent lifestyle behavior treatment option for all patients, including for the large population of mild-moderate-risk patients with elevated blood pressure and blood cholesterol,” they note.
Research has shown that increasing physical activity can lead to clinically meaningful 3 or 4 mm Hg reductions in systolic and diastolic blood pressure, and 3 to 6 mg/dL decreases in LDL cholesterol, the authors point out.
Previous evidence also shows that physically active people have a 21% lower risk of developing cardiovascular disease and a 36% lower risk for death from cardiovascular diseases than those who are not physically active.
Physical activity also has benefits beyond heart health, including a lower risk for some cancers; improved bone, brain, and mental health; and better sleep, they note.
The U.S. Department of Health and Human Services 2018 physical activity guidelines advise Americans to log 150 minutes of moderate-intensity aerobic exercise or 75 minutes of vigorous aerobic activity each week and to participate in two or more weekly strength training sessions.
However, there is no minimum amount of time to receive benefits from physical activity.
“Every little bit of activity is better than none. Even small initial increases of 5 to 10 minutes a day can yield health benefits,” Dr. Gibbs said.
Translational advice for clinicians
The AHA statement encourages clinicians to ask patients about their physical activity at every interaction; provide ideas and resources to help patients improve and sustain regular life-long physical activity; and encourage and celebrate small increases in activity, such as walking more or taking the stairs, to help with motivation.
“In our world where physical activity is increasingly engineered out of our lives and the overwhelming default is to sit – and even more so now as the nation and the world is practicing quarantine and isolation to reduce the spread of coronavirus – the message that we must be relentless in our pursuit to ‘sit less and move more’ throughout the day is more important than ever,” said Dr. Gibbs.
The statement was prepared by a volunteer writing group on behalf of the AHA Council on Lifestyle and Cardiometabolic Health; the Council on Cardiovascular and Stroke Nursing; and the Council on Clinical Cardiology.
This research had no commercial funding. A list of disclosures for the writing group is available with the original article.
A version of this article first appeared on Medscape.com.
The optimal first step to address mild to moderately elevated blood pressure and cholesterol in otherwise healthy adults is a “prescription” to sit less and move more, the American Heart Association says in a new scientific statement.
“The current American Heart Association guidelines for diagnosing high blood pressure and cholesterol recognize that otherwise healthy individuals with mildly or moderately elevated levels of these cardiovascular risk factors should actively attempt to reduce these risks,” Bethany Barone Gibbs, PhD, chair of the statement writing group, said in an AHA news release.
“The first treatment strategy for many of these patients should be healthy lifestyle changes beginning with increasing physical activity,” said Dr. Gibbs, from the University of Pittsburgh.
The 12-page AHA scientific statement – Physical Activity as a Critical Component of First-Line Treatment for Elevated Blood Pressure or Cholesterol: Who, What, and How? – was published online June 2 in Hypertension.
Every little bit helps
According to the AHA, about 21% of American adults have systolic blood pressure between 120 and 139 mm Hg, or diastolic blood pressure between 80 and 89 mm Hg, which meets the criteria for lifestyle-only treatment for elevated BP outlined in the American College of Cardiology (ACC)/AHA high blood pressure guideline.
In addition, about 28% of American adults have LDL cholesterol above 70 mg/dL and otherwise meet the low-risk criteria for heart disease or stroke. These individuals would meet the criteria for lifestyle-only treatment outlined in the 2018 ACC/AHA cholesterol treatment guidelines, which include increased physical activity, weight loss, better diet, smoking cessation, and moderating alcohol intake.
“Of the recommended lifestyle changes, increasing physical activity has extensive benefits, including improving both blood pressure and blood cholesterol, that are comparable, superior, or complementary to other healthy lifestyle changes,” the writing group says.
“Physical activity assessment and prescription are an excellent lifestyle behavior treatment option for all patients, including for the large population of mild-moderate-risk patients with elevated blood pressure and blood cholesterol,” they note.
Research has shown that increasing physical activity can lead to clinically meaningful 3 or 4 mm Hg reductions in systolic and diastolic blood pressure, and 3 to 6 mg/dL decreases in LDL cholesterol, the authors point out.
Previous evidence also shows that physically active people have a 21% lower risk of developing cardiovascular disease and a 36% lower risk for death from cardiovascular diseases than those who are not physically active.
Physical activity also has benefits beyond heart health, including a lower risk for some cancers; improved bone, brain, and mental health; and better sleep, they note.
The U.S. Department of Health and Human Services 2018 physical activity guidelines advise Americans to log 150 minutes of moderate-intensity aerobic exercise or 75 minutes of vigorous aerobic activity each week and to participate in two or more weekly strength training sessions.
However, there is no minimum amount of time to receive benefits from physical activity.
“Every little bit of activity is better than none. Even small initial increases of 5 to 10 minutes a day can yield health benefits,” Dr. Gibbs said.
Translational advice for clinicians
The AHA statement encourages clinicians to ask patients about their physical activity at every interaction; provide ideas and resources to help patients improve and sustain regular life-long physical activity; and encourage and celebrate small increases in activity, such as walking more or taking the stairs, to help with motivation.
“In our world where physical activity is increasingly engineered out of our lives and the overwhelming default is to sit – and even more so now as the nation and the world is practicing quarantine and isolation to reduce the spread of coronavirus – the message that we must be relentless in our pursuit to ‘sit less and move more’ throughout the day is more important than ever,” said Dr. Gibbs.
The statement was prepared by a volunteer writing group on behalf of the AHA Council on Lifestyle and Cardiometabolic Health; the Council on Cardiovascular and Stroke Nursing; and the Council on Clinical Cardiology.
This research had no commercial funding. A list of disclosures for the writing group is available with the original article.
A version of this article first appeared on Medscape.com.
The optimal first step to address mild to moderately elevated blood pressure and cholesterol in otherwise healthy adults is a “prescription” to sit less and move more, the American Heart Association says in a new scientific statement.
“The current American Heart Association guidelines for diagnosing high blood pressure and cholesterol recognize that otherwise healthy individuals with mildly or moderately elevated levels of these cardiovascular risk factors should actively attempt to reduce these risks,” Bethany Barone Gibbs, PhD, chair of the statement writing group, said in an AHA news release.
“The first treatment strategy for many of these patients should be healthy lifestyle changes beginning with increasing physical activity,” said Dr. Gibbs, from the University of Pittsburgh.
The 12-page AHA scientific statement – Physical Activity as a Critical Component of First-Line Treatment for Elevated Blood Pressure or Cholesterol: Who, What, and How? – was published online June 2 in Hypertension.
Every little bit helps
According to the AHA, about 21% of American adults have systolic blood pressure between 120 and 139 mm Hg, or diastolic blood pressure between 80 and 89 mm Hg, which meets the criteria for lifestyle-only treatment for elevated BP outlined in the American College of Cardiology (ACC)/AHA high blood pressure guideline.
In addition, about 28% of American adults have LDL cholesterol above 70 mg/dL and otherwise meet the low-risk criteria for heart disease or stroke. These individuals would meet the criteria for lifestyle-only treatment outlined in the 2018 ACC/AHA cholesterol treatment guidelines, which include increased physical activity, weight loss, better diet, smoking cessation, and moderating alcohol intake.
“Of the recommended lifestyle changes, increasing physical activity has extensive benefits, including improving both blood pressure and blood cholesterol, that are comparable, superior, or complementary to other healthy lifestyle changes,” the writing group says.
“Physical activity assessment and prescription are an excellent lifestyle behavior treatment option for all patients, including for the large population of mild-moderate-risk patients with elevated blood pressure and blood cholesterol,” they note.
Research has shown that increasing physical activity can lead to clinically meaningful 3 or 4 mm Hg reductions in systolic and diastolic blood pressure, and 3 to 6 mg/dL decreases in LDL cholesterol, the authors point out.
Previous evidence also shows that physically active people have a 21% lower risk of developing cardiovascular disease and a 36% lower risk for death from cardiovascular diseases than those who are not physically active.
Physical activity also has benefits beyond heart health, including a lower risk for some cancers; improved bone, brain, and mental health; and better sleep, they note.
The U.S. Department of Health and Human Services 2018 physical activity guidelines advise Americans to log 150 minutes of moderate-intensity aerobic exercise or 75 minutes of vigorous aerobic activity each week and to participate in two or more weekly strength training sessions.
However, there is no minimum amount of time to receive benefits from physical activity.
“Every little bit of activity is better than none. Even small initial increases of 5 to 10 minutes a day can yield health benefits,” Dr. Gibbs said.
Translational advice for clinicians
The AHA statement encourages clinicians to ask patients about their physical activity at every interaction; provide ideas and resources to help patients improve and sustain regular life-long physical activity; and encourage and celebrate small increases in activity, such as walking more or taking the stairs, to help with motivation.
“In our world where physical activity is increasingly engineered out of our lives and the overwhelming default is to sit – and even more so now as the nation and the world is practicing quarantine and isolation to reduce the spread of coronavirus – the message that we must be relentless in our pursuit to ‘sit less and move more’ throughout the day is more important than ever,” said Dr. Gibbs.
The statement was prepared by a volunteer writing group on behalf of the AHA Council on Lifestyle and Cardiometabolic Health; the Council on Cardiovascular and Stroke Nursing; and the Council on Clinical Cardiology.
This research had no commercial funding. A list of disclosures for the writing group is available with the original article.
A version of this article first appeared on Medscape.com.
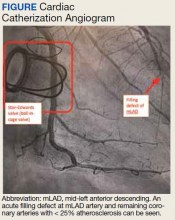
First risk score to predict bleeding risk after TAVR
(TAVR).
“Despite the TAVR iterations, we recognize that bleeding remains a very important and perhaps also neglected issue. Indeed, no specifically developed standard algorithm existed before this to assess bleeding risk post-TAVR,” lead author Eliano Pio Navarese, MD, PhD, said in an interview.
Although bleeding rates can be as high as 9% at 30 days and between 3% and 11% in the first year, only a few studies have applied existing scores to TAVR patients, he noted.
The PREDICT-TAVR score includes six common variables and can be calculated by hand using a simple nomogram or a web-based calculator, with a dedicated website in the works, said Dr. Navarese, Nicolaus Copernicus University and SIRO MEDICINE Network, Bydgoszcz, Poland, and the University of Alberta, Edmonton.
A strength of the score is that machine-learning methods were used and the choice of variables optimized through recursive feature elimination and cross validation to remove the weakest variables, he said. Artificial intelligence, including use of random forest, naïve Bayes, and logistic regression classifiers, was also applied to the algorithms and the results cross-checked with standard multivariate analysis.
“It was a tremendous effort in terms of the analytics conducted,” Dr. Navarese said. “This is not a simple score but the integration of the most sophisticated machine learning methods and algorithms.”
Details are published in the June 14 issue of JACC: Cardiovascular Interventions.
The six variables used to calculate 30-day bleeding risk after TAVR and the points assigned to each are:
- blood hemoglobin (0-10 points)
- serum iron concentration (0-5 points)
- common femoral artery diameter (0-3 points)
- (0-3 points)
- dual antiplatelet therapy (DAPT; 0-2 points)
- oral anticoagulation therapy (0-2 points)
The six items were selected among 104 baseline variables from 5,185 consecutive patients undergoing transfemoral TAVR in the prospective RISPEVA (Registro Italiano GISE sull’Impianto di Valvola Aortica Percutanea) registry between March 2012 and December 2019, then validated in 5,043 patients in the prospective POL-TAVI (Polish Registry of Transcatheter Aortic Valve Implantation) between January 2013 and December 2019.
In the derivation cohort, 216 patients (4.2%) experienced bleeding events at 1 year, with 169 events (78%) occurring during the first 30 days.
PREDICT-TAVR exhibited high discriminatory power for bleeding events at 30 days, as reflected by an area under the curve (AUC) of 0.80 (95% confidence interval, 0.75-0.83). Internal validation by optimism bootstrap-corrected AUC was consistent at 0.79 (95% CI, 0.75-0.83).
PREDICT-TAVR also outperformed scores not developed for TAVR, such as the PARIS score for patients undergoing percutaneous coronary intervention (AUC, 0.69) and the well-validated HAS-BLED for patients receiving anticoagulation (AUC, 0.58; P < .001 for both).
In the validation cohort, the AUC for bleeding complications at 30 days was 0.78 (95% CI, 0.72-0.82) versus an AUC of 0.68 for PARIS and 0.66 for HAS-BLED.
A HAS-BLED score of 4 predicted a higher rate of severe bleeding and mortality in the year after transfemoral TAVR in the 2018 Japanese OCEAN-TAVI study.
Bleeding events by risk categories
Risk score quartiles identified as low risk were 8 points or less, as moderate risk were 8 to less than 10 points, as high risk were 10 to less than 12 points, and as very-high-risk score were above 12 points.
In the derivation cohort, 30-day bleeding events across quartiles were 0.8%, 1.1%, 2.5%, and 8.5%, respectively (overall P < .001).
Compared with the lowest quartile, bleeding risk was numerically higher for the second quartile (odds ratio, 1.75) and significantly higher in the third (OR, 2.0) and fourth (OR, 2.49) quartiles (P < .001 for both).
A landmark cumulative-event analysis showed a significantly greater risk of bleeding for the two highest quartiles up to 30 days; however, these differences were no longer significant from 30 days to 1 year, likely because of a limited number of events, the authors suggest. Similar results were seen in the validation cohort.
The number of patients in the high- and very-high-risk groups isn’t trivial, and bleeding rates reached as high as 12.6% in the highest quartile, Dr. Navarese observed. Guidelines recommend DAPT for 3 to 6 months after TAVR; however, emerging data, including a recent meta-analysis, suggest monotherapy may be a very good option.
“So, if you had a high bleeding risk and are considering postprocedural DAPT or anticoagulation, I would think twice rather than administering dual antiplatelet therapy or anticoagulation for a long time, or at least, I would consider the impact of this score on this choice,” he said.
Subgroup analyses showed AUCs ranging from 0.77 to 0.81 for subgroups such as age older than 80 years, diabetes, obesity, female sex, previous PCI, and New York Heart Association class III or IV.
Serum iron showed the highest AUC in the primary PREDICT-TAVR model; however, should iron levels be unavailable, a simplified score modeled without iron levels retained predictive power, yielding AUCs for 30-day bleeding of 0.78 in the derivation cohort and 0.75 in the validation cohort.
“PREDICT-TAVR score can impact clinical practice, not only selecting the optimal thrombotic regimen in certain high bleeding-risk populations but also to treat pre-TAVR anemia and iron deficiencies, which may affect outcomes,” Dr. Navarese said. “Of course, future prospective biological and clinical investigations are needed to elucidate the score and the role of the score’s treatable risk traits in reducing post-TAVR bleeding complications.”
Commenting for this news organization, Sunil Rao, MD, Duke University, Durham, N.C., said anemia is a covariant in many risk models for bleeding and vascular complications in PCI and acute coronary syndrome, but hemoglobin and iron levels are collinear.
“The problem I think is when you throw hemoglobin and iron in the same model, just by play of chance, one variable can knock out the other one,” he said. “So I don’t know necessarily if we need to start measuring iron on everyone. We certainly should be measuring hemoglobin, which I think most people will have, and if a patient has pre-existing anemia, that should be a red flag for us.”
Age and Society of Thoracic Surgeons (STS) risk score did not reach statistical significance in the model – likely reflecting the high-/extremely-high-risk patient population with an average STS score of 7.7 and average age of 82 years – but may become more important as TAVR is applied more widely, Dr. Rao and Zachary Wegermann, MD, Duke Clinical Research Institute, write in an accompanying editorial.
They also point out that the study was limited by a low rate of bleeding events, and, importantly, the score can’t distinguish between minor or major bleeding.
“It’s worth trying to repeat the analyses in lower-risk patients because we may find other covariates that are important,” Dr. Rao said in an interview. “The other thing we need to get to is probably being a little bit more sophisticated. The variables included in these models are the ones that are measured; they’re also the ones that are clinically apparent.”
“But there’s a whole area of genomic medicine, proteomic medicine, metabolomic medicine that, as it starts developing and becomes more and more sophisticated, my suspicion is that we’re going to get even more precise and accurate about patients’ risk, and it’s going to become more individualized, rather than just measuring variables like age and lab values,” he said.
In the meantime, having variables documented in the electronic health record, with hard stops deployed if variables aren’t measured, is “a step in the right direction,” he added.
Dr. Navarese has received research grants from Abbott, Amgen, and Medtronic and received lecture fees and honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron, outside the submitted work. Dr. Rao and Dr. Wegermann report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
(TAVR).
“Despite the TAVR iterations, we recognize that bleeding remains a very important and perhaps also neglected issue. Indeed, no specifically developed standard algorithm existed before this to assess bleeding risk post-TAVR,” lead author Eliano Pio Navarese, MD, PhD, said in an interview.
Although bleeding rates can be as high as 9% at 30 days and between 3% and 11% in the first year, only a few studies have applied existing scores to TAVR patients, he noted.
The PREDICT-TAVR score includes six common variables and can be calculated by hand using a simple nomogram or a web-based calculator, with a dedicated website in the works, said Dr. Navarese, Nicolaus Copernicus University and SIRO MEDICINE Network, Bydgoszcz, Poland, and the University of Alberta, Edmonton.
A strength of the score is that machine-learning methods were used and the choice of variables optimized through recursive feature elimination and cross validation to remove the weakest variables, he said. Artificial intelligence, including use of random forest, naïve Bayes, and logistic regression classifiers, was also applied to the algorithms and the results cross-checked with standard multivariate analysis.
“It was a tremendous effort in terms of the analytics conducted,” Dr. Navarese said. “This is not a simple score but the integration of the most sophisticated machine learning methods and algorithms.”
Details are published in the June 14 issue of JACC: Cardiovascular Interventions.
The six variables used to calculate 30-day bleeding risk after TAVR and the points assigned to each are:
- blood hemoglobin (0-10 points)
- serum iron concentration (0-5 points)
- common femoral artery diameter (0-3 points)
- (0-3 points)
- dual antiplatelet therapy (DAPT; 0-2 points)
- oral anticoagulation therapy (0-2 points)
The six items were selected among 104 baseline variables from 5,185 consecutive patients undergoing transfemoral TAVR in the prospective RISPEVA (Registro Italiano GISE sull’Impianto di Valvola Aortica Percutanea) registry between March 2012 and December 2019, then validated in 5,043 patients in the prospective POL-TAVI (Polish Registry of Transcatheter Aortic Valve Implantation) between January 2013 and December 2019.
In the derivation cohort, 216 patients (4.2%) experienced bleeding events at 1 year, with 169 events (78%) occurring during the first 30 days.
PREDICT-TAVR exhibited high discriminatory power for bleeding events at 30 days, as reflected by an area under the curve (AUC) of 0.80 (95% confidence interval, 0.75-0.83). Internal validation by optimism bootstrap-corrected AUC was consistent at 0.79 (95% CI, 0.75-0.83).
PREDICT-TAVR also outperformed scores not developed for TAVR, such as the PARIS score for patients undergoing percutaneous coronary intervention (AUC, 0.69) and the well-validated HAS-BLED for patients receiving anticoagulation (AUC, 0.58; P < .001 for both).
In the validation cohort, the AUC for bleeding complications at 30 days was 0.78 (95% CI, 0.72-0.82) versus an AUC of 0.68 for PARIS and 0.66 for HAS-BLED.
A HAS-BLED score of 4 predicted a higher rate of severe bleeding and mortality in the year after transfemoral TAVR in the 2018 Japanese OCEAN-TAVI study.
Bleeding events by risk categories
Risk score quartiles identified as low risk were 8 points or less, as moderate risk were 8 to less than 10 points, as high risk were 10 to less than 12 points, and as very-high-risk score were above 12 points.
In the derivation cohort, 30-day bleeding events across quartiles were 0.8%, 1.1%, 2.5%, and 8.5%, respectively (overall P < .001).
Compared with the lowest quartile, bleeding risk was numerically higher for the second quartile (odds ratio, 1.75) and significantly higher in the third (OR, 2.0) and fourth (OR, 2.49) quartiles (P < .001 for both).
A landmark cumulative-event analysis showed a significantly greater risk of bleeding for the two highest quartiles up to 30 days; however, these differences were no longer significant from 30 days to 1 year, likely because of a limited number of events, the authors suggest. Similar results were seen in the validation cohort.
The number of patients in the high- and very-high-risk groups isn’t trivial, and bleeding rates reached as high as 12.6% in the highest quartile, Dr. Navarese observed. Guidelines recommend DAPT for 3 to 6 months after TAVR; however, emerging data, including a recent meta-analysis, suggest monotherapy may be a very good option.
“So, if you had a high bleeding risk and are considering postprocedural DAPT or anticoagulation, I would think twice rather than administering dual antiplatelet therapy or anticoagulation for a long time, or at least, I would consider the impact of this score on this choice,” he said.
Subgroup analyses showed AUCs ranging from 0.77 to 0.81 for subgroups such as age older than 80 years, diabetes, obesity, female sex, previous PCI, and New York Heart Association class III or IV.
Serum iron showed the highest AUC in the primary PREDICT-TAVR model; however, should iron levels be unavailable, a simplified score modeled without iron levels retained predictive power, yielding AUCs for 30-day bleeding of 0.78 in the derivation cohort and 0.75 in the validation cohort.
“PREDICT-TAVR score can impact clinical practice, not only selecting the optimal thrombotic regimen in certain high bleeding-risk populations but also to treat pre-TAVR anemia and iron deficiencies, which may affect outcomes,” Dr. Navarese said. “Of course, future prospective biological and clinical investigations are needed to elucidate the score and the role of the score’s treatable risk traits in reducing post-TAVR bleeding complications.”
Commenting for this news organization, Sunil Rao, MD, Duke University, Durham, N.C., said anemia is a covariant in many risk models for bleeding and vascular complications in PCI and acute coronary syndrome, but hemoglobin and iron levels are collinear.
“The problem I think is when you throw hemoglobin and iron in the same model, just by play of chance, one variable can knock out the other one,” he said. “So I don’t know necessarily if we need to start measuring iron on everyone. We certainly should be measuring hemoglobin, which I think most people will have, and if a patient has pre-existing anemia, that should be a red flag for us.”
Age and Society of Thoracic Surgeons (STS) risk score did not reach statistical significance in the model – likely reflecting the high-/extremely-high-risk patient population with an average STS score of 7.7 and average age of 82 years – but may become more important as TAVR is applied more widely, Dr. Rao and Zachary Wegermann, MD, Duke Clinical Research Institute, write in an accompanying editorial.
They also point out that the study was limited by a low rate of bleeding events, and, importantly, the score can’t distinguish between minor or major bleeding.
“It’s worth trying to repeat the analyses in lower-risk patients because we may find other covariates that are important,” Dr. Rao said in an interview. “The other thing we need to get to is probably being a little bit more sophisticated. The variables included in these models are the ones that are measured; they’re also the ones that are clinically apparent.”
“But there’s a whole area of genomic medicine, proteomic medicine, metabolomic medicine that, as it starts developing and becomes more and more sophisticated, my suspicion is that we’re going to get even more precise and accurate about patients’ risk, and it’s going to become more individualized, rather than just measuring variables like age and lab values,” he said.
In the meantime, having variables documented in the electronic health record, with hard stops deployed if variables aren’t measured, is “a step in the right direction,” he added.
Dr. Navarese has received research grants from Abbott, Amgen, and Medtronic and received lecture fees and honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron, outside the submitted work. Dr. Rao and Dr. Wegermann report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
(TAVR).
“Despite the TAVR iterations, we recognize that bleeding remains a very important and perhaps also neglected issue. Indeed, no specifically developed standard algorithm existed before this to assess bleeding risk post-TAVR,” lead author Eliano Pio Navarese, MD, PhD, said in an interview.
Although bleeding rates can be as high as 9% at 30 days and between 3% and 11% in the first year, only a few studies have applied existing scores to TAVR patients, he noted.
The PREDICT-TAVR score includes six common variables and can be calculated by hand using a simple nomogram or a web-based calculator, with a dedicated website in the works, said Dr. Navarese, Nicolaus Copernicus University and SIRO MEDICINE Network, Bydgoszcz, Poland, and the University of Alberta, Edmonton.
A strength of the score is that machine-learning methods were used and the choice of variables optimized through recursive feature elimination and cross validation to remove the weakest variables, he said. Artificial intelligence, including use of random forest, naïve Bayes, and logistic regression classifiers, was also applied to the algorithms and the results cross-checked with standard multivariate analysis.
“It was a tremendous effort in terms of the analytics conducted,” Dr. Navarese said. “This is not a simple score but the integration of the most sophisticated machine learning methods and algorithms.”
Details are published in the June 14 issue of JACC: Cardiovascular Interventions.
The six variables used to calculate 30-day bleeding risk after TAVR and the points assigned to each are:
- blood hemoglobin (0-10 points)
- serum iron concentration (0-5 points)
- common femoral artery diameter (0-3 points)
- (0-3 points)
- dual antiplatelet therapy (DAPT; 0-2 points)
- oral anticoagulation therapy (0-2 points)
The six items were selected among 104 baseline variables from 5,185 consecutive patients undergoing transfemoral TAVR in the prospective RISPEVA (Registro Italiano GISE sull’Impianto di Valvola Aortica Percutanea) registry between March 2012 and December 2019, then validated in 5,043 patients in the prospective POL-TAVI (Polish Registry of Transcatheter Aortic Valve Implantation) between January 2013 and December 2019.
In the derivation cohort, 216 patients (4.2%) experienced bleeding events at 1 year, with 169 events (78%) occurring during the first 30 days.
PREDICT-TAVR exhibited high discriminatory power for bleeding events at 30 days, as reflected by an area under the curve (AUC) of 0.80 (95% confidence interval, 0.75-0.83). Internal validation by optimism bootstrap-corrected AUC was consistent at 0.79 (95% CI, 0.75-0.83).
PREDICT-TAVR also outperformed scores not developed for TAVR, such as the PARIS score for patients undergoing percutaneous coronary intervention (AUC, 0.69) and the well-validated HAS-BLED for patients receiving anticoagulation (AUC, 0.58; P < .001 for both).
In the validation cohort, the AUC for bleeding complications at 30 days was 0.78 (95% CI, 0.72-0.82) versus an AUC of 0.68 for PARIS and 0.66 for HAS-BLED.
A HAS-BLED score of 4 predicted a higher rate of severe bleeding and mortality in the year after transfemoral TAVR in the 2018 Japanese OCEAN-TAVI study.
Bleeding events by risk categories
Risk score quartiles identified as low risk were 8 points or less, as moderate risk were 8 to less than 10 points, as high risk were 10 to less than 12 points, and as very-high-risk score were above 12 points.
In the derivation cohort, 30-day bleeding events across quartiles were 0.8%, 1.1%, 2.5%, and 8.5%, respectively (overall P < .001).
Compared with the lowest quartile, bleeding risk was numerically higher for the second quartile (odds ratio, 1.75) and significantly higher in the third (OR, 2.0) and fourth (OR, 2.49) quartiles (P < .001 for both).
A landmark cumulative-event analysis showed a significantly greater risk of bleeding for the two highest quartiles up to 30 days; however, these differences were no longer significant from 30 days to 1 year, likely because of a limited number of events, the authors suggest. Similar results were seen in the validation cohort.
The number of patients in the high- and very-high-risk groups isn’t trivial, and bleeding rates reached as high as 12.6% in the highest quartile, Dr. Navarese observed. Guidelines recommend DAPT for 3 to 6 months after TAVR; however, emerging data, including a recent meta-analysis, suggest monotherapy may be a very good option.
“So, if you had a high bleeding risk and are considering postprocedural DAPT or anticoagulation, I would think twice rather than administering dual antiplatelet therapy or anticoagulation for a long time, or at least, I would consider the impact of this score on this choice,” he said.
Subgroup analyses showed AUCs ranging from 0.77 to 0.81 for subgroups such as age older than 80 years, diabetes, obesity, female sex, previous PCI, and New York Heart Association class III or IV.
Serum iron showed the highest AUC in the primary PREDICT-TAVR model; however, should iron levels be unavailable, a simplified score modeled without iron levels retained predictive power, yielding AUCs for 30-day bleeding of 0.78 in the derivation cohort and 0.75 in the validation cohort.
“PREDICT-TAVR score can impact clinical practice, not only selecting the optimal thrombotic regimen in certain high bleeding-risk populations but also to treat pre-TAVR anemia and iron deficiencies, which may affect outcomes,” Dr. Navarese said. “Of course, future prospective biological and clinical investigations are needed to elucidate the score and the role of the score’s treatable risk traits in reducing post-TAVR bleeding complications.”
Commenting for this news organization, Sunil Rao, MD, Duke University, Durham, N.C., said anemia is a covariant in many risk models for bleeding and vascular complications in PCI and acute coronary syndrome, but hemoglobin and iron levels are collinear.
“The problem I think is when you throw hemoglobin and iron in the same model, just by play of chance, one variable can knock out the other one,” he said. “So I don’t know necessarily if we need to start measuring iron on everyone. We certainly should be measuring hemoglobin, which I think most people will have, and if a patient has pre-existing anemia, that should be a red flag for us.”
Age and Society of Thoracic Surgeons (STS) risk score did not reach statistical significance in the model – likely reflecting the high-/extremely-high-risk patient population with an average STS score of 7.7 and average age of 82 years – but may become more important as TAVR is applied more widely, Dr. Rao and Zachary Wegermann, MD, Duke Clinical Research Institute, write in an accompanying editorial.
They also point out that the study was limited by a low rate of bleeding events, and, importantly, the score can’t distinguish between minor or major bleeding.
“It’s worth trying to repeat the analyses in lower-risk patients because we may find other covariates that are important,” Dr. Rao said in an interview. “The other thing we need to get to is probably being a little bit more sophisticated. The variables included in these models are the ones that are measured; they’re also the ones that are clinically apparent.”
“But there’s a whole area of genomic medicine, proteomic medicine, metabolomic medicine that, as it starts developing and becomes more and more sophisticated, my suspicion is that we’re going to get even more precise and accurate about patients’ risk, and it’s going to become more individualized, rather than just measuring variables like age and lab values,” he said.
In the meantime, having variables documented in the electronic health record, with hard stops deployed if variables aren’t measured, is “a step in the right direction,” he added.
Dr. Navarese has received research grants from Abbott, Amgen, and Medtronic and received lecture fees and honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron, outside the submitted work. Dr. Rao and Dr. Wegermann report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Evidence builds for iPhone 12 interference with cardiac devices
Further evidence that powerful magnets in some Apple iPhones can interfere with cardiac implantable electronic devices (CIEDs) comes from a small study that also suggests some devices are more susceptible than others.
The iPhone 12 Pro Max with MagSafe technology interfered with CIEDs implanted in three consecutive patients presenting to an electrophysiology lab and in 8 of 11 implantable cardioverter defibrillators (ICDs) and pacemakers (72.7%) still in their original packaging.
The results, published in the Journal of the American Heart Association, are consistent with a widely publicized single-patient report this February and evidence of electromagnetic interference with fitness wristbands and e-cigarettes.
The MagSafe technology supports wireless charging and is optimized by a ring-shaped array of magnets. Although magnet mode activation has been shown to occur in CIEDs with exposure to a magnetic field as low as 10 gauss, the field strength of the iPhone 12 Pro Max can be greater than 50 G when in direct contact, the researchers determined.
“If this becomes a standard in a lot of the new smartphones or companies start to use stronger magnets ... then we will see more and more of these consumer electronic and device interactions,” senior author Michael Wu, MD, Brown University, Providence, R.I., told this news organization.
In a May advisory on these device interactions, the U.S. Food and Drug Administration also cautioned that the number of consumer electronics with strong magnets is expected to increase over time.
That trend appears to be already underway, with Forbes reporting in February that the MagSafe batteries will be “getting stronger” as part of upgrades to the iPhone 13 and Bloomberg reporting in advance of Apple’s annual developers conference this week that an upgraded version of MagSafe is in the works to support wireless charging for its iPad. MagSafe has not been used previously in iPads.
Although Apple has acknowledged that the iPhone 12 contains more magnets than previous iPhone models, it says “they’re not expected to pose a greater risk of magnetic interference to medical devices than prior iPhone models.” The company maintains a page that specifically warns about the potential for interactions and advises that consumers keep the iPhone and MagSafe accessories more than 15 cm (6 inches) away from medical devices.
Older-generation iPhones have not shown this risk, with only one case of interference reported with the iPhone 6 and an Apple Watch in 1,352 tests among 148 patients with CIEDs and leads from four different manufacturers.
In the present study, magnet reversion mode was triggered in all three patients when the iPhone 12 Pro Max was placed on the skin over the device.
The phone inhibited tachycardia therapies in Medtronic’s Amplia MRI Quad CRT-D and Abbott’s 1231-40 Fortify VR device.
The Boston Scientific V273 Intua CRT-P device, however, “appeared to be less susceptible, as we were only able to elicit transient temporary asynchronous pacing but no sustained response by the iPhone 12 Pro Max magnet,” Dr. Wu and colleagues note.
Among the 11 ex vivo CIEDs tested, placing the iPhone 12 Pro Max directly over the packaged device inhibited tachytherapies in Medtronic’s Visia AF MRI ICD and Abbott’s Fortify Assura DR ICD and Ellipse DR ICD.
The phone also led to asynchronous pacing in Medtronic’s Azure, Advisa MRI, and Adapta pacemakers and in Abbott’s Assurity MRI pacemaker.
Boston Scientific devices again “appeared to be less susceptible, as no clear magnet interference” was noted in the Dynagen ICD, Emblem MRI S-ICD, or Accolade MRI pacemaker, Dr. Wu reported. There was temporary asynchronous pacing but no sustained response in the company’s U125 Valitude pacemaker.
Using the Medtronic Visia AF MRI ICD, the researchers found that the iPhone 12 Pro Max was able to trigger magnet reversion mode at a distance up to 1.5 cm (0.6 inch) from the anterior aspect of the device ex vivo.
The difference in magnet response to the iPhone 12 Pro Max among the different devices is likely due to different hall-sensor magnet sensitivity, as all of the devices were susceptible to a standard donut magnet, Dr. Wu noted. Boston Scientific’s Accolade MRI pacemaker, for example, requires a magnet stronger than 70 G to activate magnet mode, according to the product manual.
“Even so, sometimes with our test, we were able to trigger a brief response,” he said. “The response isn’t as lasting as some of the other companies, but with the small sample size, I can only speculate and suggest that maybe it’s possible. But we always want a formal study through the company or other agencies to really pinpoint which company has more susceptible devices.”
As to whether manufacturers should build CIEDs less susceptible to today’s stronger magnets, Dr. Wu said it’s worth exploring, but there are pros and cons.
Although magnets in consumer devices have the potential to inhibit lifesaving therapies, a magnet is also very useful in certain medical settings, such as a quick way to ensure pacing without worrying about electrocautery noise during surgery or to deactivate a defibrillator if there’s noise resulting in inappropriate shocks.
“It would require an overhaul of a lot of the devices going forward, and I think that’s something that’s worth exploring, especially now that a lot of devices are using wireless communication, Bluetooth, and other communication technology,” he said.
Even though the study is small, Dr. Wu said, it does represent many of the available devices and has clinical implications, given that people often put their smartphones in a breast pocket.
“This report highlights the importance of public awareness regarding an interaction between CIEDs and a recently released smartphone model with magnetic charging capability,” Dr. Wu and colleagues conclude.
Apple was contacted for comment but had not responded at press time.
The authors reported no study funding or relevant conflicts of interests.
A version of this article first appeared on Medscape.com.
Further evidence that powerful magnets in some Apple iPhones can interfere with cardiac implantable electronic devices (CIEDs) comes from a small study that also suggests some devices are more susceptible than others.
The iPhone 12 Pro Max with MagSafe technology interfered with CIEDs implanted in three consecutive patients presenting to an electrophysiology lab and in 8 of 11 implantable cardioverter defibrillators (ICDs) and pacemakers (72.7%) still in their original packaging.
The results, published in the Journal of the American Heart Association, are consistent with a widely publicized single-patient report this February and evidence of electromagnetic interference with fitness wristbands and e-cigarettes.
The MagSafe technology supports wireless charging and is optimized by a ring-shaped array of magnets. Although magnet mode activation has been shown to occur in CIEDs with exposure to a magnetic field as low as 10 gauss, the field strength of the iPhone 12 Pro Max can be greater than 50 G when in direct contact, the researchers determined.
“If this becomes a standard in a lot of the new smartphones or companies start to use stronger magnets ... then we will see more and more of these consumer electronic and device interactions,” senior author Michael Wu, MD, Brown University, Providence, R.I., told this news organization.
In a May advisory on these device interactions, the U.S. Food and Drug Administration also cautioned that the number of consumer electronics with strong magnets is expected to increase over time.
That trend appears to be already underway, with Forbes reporting in February that the MagSafe batteries will be “getting stronger” as part of upgrades to the iPhone 13 and Bloomberg reporting in advance of Apple’s annual developers conference this week that an upgraded version of MagSafe is in the works to support wireless charging for its iPad. MagSafe has not been used previously in iPads.
Although Apple has acknowledged that the iPhone 12 contains more magnets than previous iPhone models, it says “they’re not expected to pose a greater risk of magnetic interference to medical devices than prior iPhone models.” The company maintains a page that specifically warns about the potential for interactions and advises that consumers keep the iPhone and MagSafe accessories more than 15 cm (6 inches) away from medical devices.
Older-generation iPhones have not shown this risk, with only one case of interference reported with the iPhone 6 and an Apple Watch in 1,352 tests among 148 patients with CIEDs and leads from four different manufacturers.
In the present study, magnet reversion mode was triggered in all three patients when the iPhone 12 Pro Max was placed on the skin over the device.
The phone inhibited tachycardia therapies in Medtronic’s Amplia MRI Quad CRT-D and Abbott’s 1231-40 Fortify VR device.
The Boston Scientific V273 Intua CRT-P device, however, “appeared to be less susceptible, as we were only able to elicit transient temporary asynchronous pacing but no sustained response by the iPhone 12 Pro Max magnet,” Dr. Wu and colleagues note.
Among the 11 ex vivo CIEDs tested, placing the iPhone 12 Pro Max directly over the packaged device inhibited tachytherapies in Medtronic’s Visia AF MRI ICD and Abbott’s Fortify Assura DR ICD and Ellipse DR ICD.
The phone also led to asynchronous pacing in Medtronic’s Azure, Advisa MRI, and Adapta pacemakers and in Abbott’s Assurity MRI pacemaker.
Boston Scientific devices again “appeared to be less susceptible, as no clear magnet interference” was noted in the Dynagen ICD, Emblem MRI S-ICD, or Accolade MRI pacemaker, Dr. Wu reported. There was temporary asynchronous pacing but no sustained response in the company’s U125 Valitude pacemaker.
Using the Medtronic Visia AF MRI ICD, the researchers found that the iPhone 12 Pro Max was able to trigger magnet reversion mode at a distance up to 1.5 cm (0.6 inch) from the anterior aspect of the device ex vivo.
The difference in magnet response to the iPhone 12 Pro Max among the different devices is likely due to different hall-sensor magnet sensitivity, as all of the devices were susceptible to a standard donut magnet, Dr. Wu noted. Boston Scientific’s Accolade MRI pacemaker, for example, requires a magnet stronger than 70 G to activate magnet mode, according to the product manual.
“Even so, sometimes with our test, we were able to trigger a brief response,” he said. “The response isn’t as lasting as some of the other companies, but with the small sample size, I can only speculate and suggest that maybe it’s possible. But we always want a formal study through the company or other agencies to really pinpoint which company has more susceptible devices.”
As to whether manufacturers should build CIEDs less susceptible to today’s stronger magnets, Dr. Wu said it’s worth exploring, but there are pros and cons.
Although magnets in consumer devices have the potential to inhibit lifesaving therapies, a magnet is also very useful in certain medical settings, such as a quick way to ensure pacing without worrying about electrocautery noise during surgery or to deactivate a defibrillator if there’s noise resulting in inappropriate shocks.
“It would require an overhaul of a lot of the devices going forward, and I think that’s something that’s worth exploring, especially now that a lot of devices are using wireless communication, Bluetooth, and other communication technology,” he said.
Even though the study is small, Dr. Wu said, it does represent many of the available devices and has clinical implications, given that people often put their smartphones in a breast pocket.
“This report highlights the importance of public awareness regarding an interaction between CIEDs and a recently released smartphone model with magnetic charging capability,” Dr. Wu and colleagues conclude.
Apple was contacted for comment but had not responded at press time.
The authors reported no study funding or relevant conflicts of interests.
A version of this article first appeared on Medscape.com.
Further evidence that powerful magnets in some Apple iPhones can interfere with cardiac implantable electronic devices (CIEDs) comes from a small study that also suggests some devices are more susceptible than others.
The iPhone 12 Pro Max with MagSafe technology interfered with CIEDs implanted in three consecutive patients presenting to an electrophysiology lab and in 8 of 11 implantable cardioverter defibrillators (ICDs) and pacemakers (72.7%) still in their original packaging.
The results, published in the Journal of the American Heart Association, are consistent with a widely publicized single-patient report this February and evidence of electromagnetic interference with fitness wristbands and e-cigarettes.
The MagSafe technology supports wireless charging and is optimized by a ring-shaped array of magnets. Although magnet mode activation has been shown to occur in CIEDs with exposure to a magnetic field as low as 10 gauss, the field strength of the iPhone 12 Pro Max can be greater than 50 G when in direct contact, the researchers determined.
“If this becomes a standard in a lot of the new smartphones or companies start to use stronger magnets ... then we will see more and more of these consumer electronic and device interactions,” senior author Michael Wu, MD, Brown University, Providence, R.I., told this news organization.
In a May advisory on these device interactions, the U.S. Food and Drug Administration also cautioned that the number of consumer electronics with strong magnets is expected to increase over time.
That trend appears to be already underway, with Forbes reporting in February that the MagSafe batteries will be “getting stronger” as part of upgrades to the iPhone 13 and Bloomberg reporting in advance of Apple’s annual developers conference this week that an upgraded version of MagSafe is in the works to support wireless charging for its iPad. MagSafe has not been used previously in iPads.
Although Apple has acknowledged that the iPhone 12 contains more magnets than previous iPhone models, it says “they’re not expected to pose a greater risk of magnetic interference to medical devices than prior iPhone models.” The company maintains a page that specifically warns about the potential for interactions and advises that consumers keep the iPhone and MagSafe accessories more than 15 cm (6 inches) away from medical devices.
Older-generation iPhones have not shown this risk, with only one case of interference reported with the iPhone 6 and an Apple Watch in 1,352 tests among 148 patients with CIEDs and leads from four different manufacturers.
In the present study, magnet reversion mode was triggered in all three patients when the iPhone 12 Pro Max was placed on the skin over the device.
The phone inhibited tachycardia therapies in Medtronic’s Amplia MRI Quad CRT-D and Abbott’s 1231-40 Fortify VR device.
The Boston Scientific V273 Intua CRT-P device, however, “appeared to be less susceptible, as we were only able to elicit transient temporary asynchronous pacing but no sustained response by the iPhone 12 Pro Max magnet,” Dr. Wu and colleagues note.
Among the 11 ex vivo CIEDs tested, placing the iPhone 12 Pro Max directly over the packaged device inhibited tachytherapies in Medtronic’s Visia AF MRI ICD and Abbott’s Fortify Assura DR ICD and Ellipse DR ICD.
The phone also led to asynchronous pacing in Medtronic’s Azure, Advisa MRI, and Adapta pacemakers and in Abbott’s Assurity MRI pacemaker.
Boston Scientific devices again “appeared to be less susceptible, as no clear magnet interference” was noted in the Dynagen ICD, Emblem MRI S-ICD, or Accolade MRI pacemaker, Dr. Wu reported. There was temporary asynchronous pacing but no sustained response in the company’s U125 Valitude pacemaker.
Using the Medtronic Visia AF MRI ICD, the researchers found that the iPhone 12 Pro Max was able to trigger magnet reversion mode at a distance up to 1.5 cm (0.6 inch) from the anterior aspect of the device ex vivo.
The difference in magnet response to the iPhone 12 Pro Max among the different devices is likely due to different hall-sensor magnet sensitivity, as all of the devices were susceptible to a standard donut magnet, Dr. Wu noted. Boston Scientific’s Accolade MRI pacemaker, for example, requires a magnet stronger than 70 G to activate magnet mode, according to the product manual.
“Even so, sometimes with our test, we were able to trigger a brief response,” he said. “The response isn’t as lasting as some of the other companies, but with the small sample size, I can only speculate and suggest that maybe it’s possible. But we always want a formal study through the company or other agencies to really pinpoint which company has more susceptible devices.”
As to whether manufacturers should build CIEDs less susceptible to today’s stronger magnets, Dr. Wu said it’s worth exploring, but there are pros and cons.
Although magnets in consumer devices have the potential to inhibit lifesaving therapies, a magnet is also very useful in certain medical settings, such as a quick way to ensure pacing without worrying about electrocautery noise during surgery or to deactivate a defibrillator if there’s noise resulting in inappropriate shocks.
“It would require an overhaul of a lot of the devices going forward, and I think that’s something that’s worth exploring, especially now that a lot of devices are using wireless communication, Bluetooth, and other communication technology,” he said.
Even though the study is small, Dr. Wu said, it does represent many of the available devices and has clinical implications, given that people often put their smartphones in a breast pocket.
“This report highlights the importance of public awareness regarding an interaction between CIEDs and a recently released smartphone model with magnetic charging capability,” Dr. Wu and colleagues conclude.
Apple was contacted for comment but had not responded at press time.
The authors reported no study funding or relevant conflicts of interests.
A version of this article first appeared on Medscape.com.
LDCT lung cancer screening may ID aortic stenosis risk
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Red meat intake tied to higher coronary heart disease risk
Increased intake of meat was linked to the risk of coronary heart disease, and substituting plant protein for red or processed meat appeared to reduce that risk, in a study from pooled cohorts totaling more than a million persons.
“We know that red and processed meat intake has been associated with higher risks of fatal coronary heart disease,” said Laila Al-Shaar, PhD, of Penn State University, Hershey. However, very few studies have evaluated substitution of alternative protein sources for red and processed meat in relation to fatal CHD risk, she said.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, Dr. Al-Shaar and colleagues reviewed individual-level data from the Pooling Project of Prospective Studies of Diet and Cancer, which included 16 prospective cohorts totaling 1,364,211 participants. The average age of the participants was 57 years, and 40% were men. Individuals with a history of cancer or cardiovascular disease were excluded. The participants were followed for 7-32 years. Diet was assessed in each cohort using baselines questionnaires, and cases were identified through medical records.
Total red meat included processed meat and unprocessed red meat; animal protein sources included seafood, poultry, eggs, and low- and high-fat dairy products; and plant protein sources included nuts and beans.
The researchers identified 51,176 fatal CHD cases during the study period. After controlling for dietary and nondietary factors, they found that an increase of 100 g per day of total red meat intake was associated with a 7% increased risk of fatal coronary heart disease (relative risk, 1.07).
However, substituting 200 calories (kcal) per day from nuts, low- and high-fat dairy products, and poultry for 200 calories per day from total red meat was associated with a 6%-14% lower risk of fatal CHD, Dr. Al-Shaar added at the meeting sponsored by the American Heart Association.
These associations were stronger when substituting the alternative protein sources for processed meat, especially among women; risk was reduced by 17%-24%, on the basis of 14,888 cases.
The researchers also found that substituting 200 calories per day from eggs for 200 calories per day for total red meat and unprocessed red meat was associated with 8% and 14% higher risk of fatal CHD, respectively; but this substitution of eggs for processed meat was not significant (4%).
“When we did the association by gender, the results were even stronger in women,” said Dr. Al-Shaar. However, “these are very preliminary results” that should be interpreted with caution, and more analysis is needed, she said. “We are planning to include other cohorts with other protein sources such as soy protein,” she noted. However, the results provide additional evidence that consumption of red and processed meat contributes to an increased risk of coronary heart disease, and that substituting some red and processed meat with nuts, dairy products, or poultry may reduce this risk, she concluded.
Women especially benefit from red meat reduction
The study is important because of the continuing interest in various sources of dietary protein intake, Linda Van Horn, PhD, RD, of Northwestern University, Chicago, said in an interview.
“The investigators studied associations of substituting other animal and plant protein sources for total red meat, unprocessed red meat, and processed meat in relation to risk of fatal CHD,” she said.
The researchers found that swapping as little as 200 calories per day of total red meat for nuts, low- or high-fat dairy products, or poultry were associated with a 6%-14% reduced risk of fatal CHD, said Dr. Van Horn. “Alternatively, if those 200 calories per day for red meat were substituted with eggs, they saw as much as 14% higher risk of fatal CHD,” she noted.
The message for both consumers and clinicians is that the findings from this large study support recommendations for plant-based and lean animal sources of protein instead of red and processed meat or eggs, as these sources “offer significantly lower risk for CHD mortality,” Dr. Van Horn said. “This may be especially true for women, but the total population is likely to benefit from this approach,” she said.
Additional research is needed, Dr. Van Horn emphasized. “Prospective lifetime data, starting in utero and over the life course, are needed to better establish recommended dietary patterns at every age and among all ethnicities and diverse socioeconomic groups,” she said.
Dr. Al-Shaar had no financial conflicts to disclose. Dr. Van Horn had no financial conflicts to disclose.
Increased intake of meat was linked to the risk of coronary heart disease, and substituting plant protein for red or processed meat appeared to reduce that risk, in a study from pooled cohorts totaling more than a million persons.
“We know that red and processed meat intake has been associated with higher risks of fatal coronary heart disease,” said Laila Al-Shaar, PhD, of Penn State University, Hershey. However, very few studies have evaluated substitution of alternative protein sources for red and processed meat in relation to fatal CHD risk, she said.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, Dr. Al-Shaar and colleagues reviewed individual-level data from the Pooling Project of Prospective Studies of Diet and Cancer, which included 16 prospective cohorts totaling 1,364,211 participants. The average age of the participants was 57 years, and 40% were men. Individuals with a history of cancer or cardiovascular disease were excluded. The participants were followed for 7-32 years. Diet was assessed in each cohort using baselines questionnaires, and cases were identified through medical records.
Total red meat included processed meat and unprocessed red meat; animal protein sources included seafood, poultry, eggs, and low- and high-fat dairy products; and plant protein sources included nuts and beans.
The researchers identified 51,176 fatal CHD cases during the study period. After controlling for dietary and nondietary factors, they found that an increase of 100 g per day of total red meat intake was associated with a 7% increased risk of fatal coronary heart disease (relative risk, 1.07).
However, substituting 200 calories (kcal) per day from nuts, low- and high-fat dairy products, and poultry for 200 calories per day from total red meat was associated with a 6%-14% lower risk of fatal CHD, Dr. Al-Shaar added at the meeting sponsored by the American Heart Association.
These associations were stronger when substituting the alternative protein sources for processed meat, especially among women; risk was reduced by 17%-24%, on the basis of 14,888 cases.
The researchers also found that substituting 200 calories per day from eggs for 200 calories per day for total red meat and unprocessed red meat was associated with 8% and 14% higher risk of fatal CHD, respectively; but this substitution of eggs for processed meat was not significant (4%).
“When we did the association by gender, the results were even stronger in women,” said Dr. Al-Shaar. However, “these are very preliminary results” that should be interpreted with caution, and more analysis is needed, she said. “We are planning to include other cohorts with other protein sources such as soy protein,” she noted. However, the results provide additional evidence that consumption of red and processed meat contributes to an increased risk of coronary heart disease, and that substituting some red and processed meat with nuts, dairy products, or poultry may reduce this risk, she concluded.
Women especially benefit from red meat reduction
The study is important because of the continuing interest in various sources of dietary protein intake, Linda Van Horn, PhD, RD, of Northwestern University, Chicago, said in an interview.
“The investigators studied associations of substituting other animal and plant protein sources for total red meat, unprocessed red meat, and processed meat in relation to risk of fatal CHD,” she said.
The researchers found that swapping as little as 200 calories per day of total red meat for nuts, low- or high-fat dairy products, or poultry were associated with a 6%-14% reduced risk of fatal CHD, said Dr. Van Horn. “Alternatively, if those 200 calories per day for red meat were substituted with eggs, they saw as much as 14% higher risk of fatal CHD,” she noted.
The message for both consumers and clinicians is that the findings from this large study support recommendations for plant-based and lean animal sources of protein instead of red and processed meat or eggs, as these sources “offer significantly lower risk for CHD mortality,” Dr. Van Horn said. “This may be especially true for women, but the total population is likely to benefit from this approach,” she said.
Additional research is needed, Dr. Van Horn emphasized. “Prospective lifetime data, starting in utero and over the life course, are needed to better establish recommended dietary patterns at every age and among all ethnicities and diverse socioeconomic groups,” she said.
Dr. Al-Shaar had no financial conflicts to disclose. Dr. Van Horn had no financial conflicts to disclose.
Increased intake of meat was linked to the risk of coronary heart disease, and substituting plant protein for red or processed meat appeared to reduce that risk, in a study from pooled cohorts totaling more than a million persons.
“We know that red and processed meat intake has been associated with higher risks of fatal coronary heart disease,” said Laila Al-Shaar, PhD, of Penn State University, Hershey. However, very few studies have evaluated substitution of alternative protein sources for red and processed meat in relation to fatal CHD risk, she said.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, Dr. Al-Shaar and colleagues reviewed individual-level data from the Pooling Project of Prospective Studies of Diet and Cancer, which included 16 prospective cohorts totaling 1,364,211 participants. The average age of the participants was 57 years, and 40% were men. Individuals with a history of cancer or cardiovascular disease were excluded. The participants were followed for 7-32 years. Diet was assessed in each cohort using baselines questionnaires, and cases were identified through medical records.
Total red meat included processed meat and unprocessed red meat; animal protein sources included seafood, poultry, eggs, and low- and high-fat dairy products; and plant protein sources included nuts and beans.
The researchers identified 51,176 fatal CHD cases during the study period. After controlling for dietary and nondietary factors, they found that an increase of 100 g per day of total red meat intake was associated with a 7% increased risk of fatal coronary heart disease (relative risk, 1.07).
However, substituting 200 calories (kcal) per day from nuts, low- and high-fat dairy products, and poultry for 200 calories per day from total red meat was associated with a 6%-14% lower risk of fatal CHD, Dr. Al-Shaar added at the meeting sponsored by the American Heart Association.
These associations were stronger when substituting the alternative protein sources for processed meat, especially among women; risk was reduced by 17%-24%, on the basis of 14,888 cases.
The researchers also found that substituting 200 calories per day from eggs for 200 calories per day for total red meat and unprocessed red meat was associated with 8% and 14% higher risk of fatal CHD, respectively; but this substitution of eggs for processed meat was not significant (4%).
“When we did the association by gender, the results were even stronger in women,” said Dr. Al-Shaar. However, “these are very preliminary results” that should be interpreted with caution, and more analysis is needed, she said. “We are planning to include other cohorts with other protein sources such as soy protein,” she noted. However, the results provide additional evidence that consumption of red and processed meat contributes to an increased risk of coronary heart disease, and that substituting some red and processed meat with nuts, dairy products, or poultry may reduce this risk, she concluded.
Women especially benefit from red meat reduction
The study is important because of the continuing interest in various sources of dietary protein intake, Linda Van Horn, PhD, RD, of Northwestern University, Chicago, said in an interview.
“The investigators studied associations of substituting other animal and plant protein sources for total red meat, unprocessed red meat, and processed meat in relation to risk of fatal CHD,” she said.
The researchers found that swapping as little as 200 calories per day of total red meat for nuts, low- or high-fat dairy products, or poultry were associated with a 6%-14% reduced risk of fatal CHD, said Dr. Van Horn. “Alternatively, if those 200 calories per day for red meat were substituted with eggs, they saw as much as 14% higher risk of fatal CHD,” she noted.
The message for both consumers and clinicians is that the findings from this large study support recommendations for plant-based and lean animal sources of protein instead of red and processed meat or eggs, as these sources “offer significantly lower risk for CHD mortality,” Dr. Van Horn said. “This may be especially true for women, but the total population is likely to benefit from this approach,” she said.
Additional research is needed, Dr. Van Horn emphasized. “Prospective lifetime data, starting in utero and over the life course, are needed to better establish recommended dietary patterns at every age and among all ethnicities and diverse socioeconomic groups,” she said.
Dr. Al-Shaar had no financial conflicts to disclose. Dr. Van Horn had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2021
Thinking Outside the ‘Cage’
A 74-year-old male veteran presented at an urgent care clinic in Aguadilla, Puerto Rico, with a sharp, nonradiating, left-sided precordial chest pain that started while cleaning his house and gardening. The patient described the pain as 9 on the 10-point Wong-Baker FACES Pain Rating Scale, lasting about 5 to 10 minutes and was alleviated with rest. The patient’s medical history consisted of multiple comorbidities, including a mitral valve replacement with a Star-Edwards valve (ball in cage) in 1987. The electrocardiogram performed at the clinic showed no acute ischemic changes. Due to the persistent pain, the patient was transferred to Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, for further evaluation and management. On arrival, the patient had an international normalized ratio (INR) of 2.22; elevated high-sensitive troponin enzyme readings of 56 ng/L at 6:38 PM (0h); 61 ng/L at 7:38 PM (1h); and 83 ng/L at 9:47 PM (3h), reference range, 0-22 ng/L, and changes that prompted admission to the cardiac critical care unit. Two days later, a follow-up enzyme level was 52 ng/L. Cardiac catheterization revealed an acute filling defect at mid-left anterior descending artery and remaining coronary arteries with < 25% atherosclerosis (Figure). A myocardial perfusion study was performed for myocardial viability. The results showed a small, reversible perfusion defect involving the apical-septal wall with the remaining left ventricular myocardium appearing viable. Aspirin was added to the patient’s anticoagulation regimen of warfarin. Once target INR was reached, the patient was discharged home without recurrence of angina.
- What is your diagnosis?
- How would you treat this patient?
Acute coronary syndrome (ACS) consists of clinical suspicion of myocardial ischemia or laboratory confirmation of myocardial infarction (MI). ACS includes 3 major entities: non-ST elevation MI (NSTEMI), unstable angina, and ST-elevation MI (STEMI). ACS usually occurs as a result of a reduced supply of oxygenated blood to the myocardium, which is caused by restriction or occlusion of at least 1 of the coronary arteries. This alteration in blood flow is commonly secondary to a rupture of an atherosclerotic plaque or spontaneous dissection of a coronary artery. In rare cases, this reduction in blood flow is caused by a coronary embolism (CE) arising from a prosthetic heart valve.1,2
One of the first descriptions of CE was provided by Rudolf Virchow in the 1850s from postmortem autopsy findings.3 At that time, these coronary findings were associated with intracardiac mural thrombus or infective endocarditis. During the 1940s, CE was described in living patients who had survived a MI, and outcomes were not as catastrophic as originally believed. In the 1960s, a higher than usual association between prosthetic valves and CE was suspected and later confirmed by the invention and implementation of coronary angiography. Multiple studies have been published that confirm the association between prosthetic valves (especially in the mitral position), atrial fibrillation (AF), and a higher than usual rate of CEs.4,5
Discussion
The prevalence of this disease has varied during the years. Data from autopsies of patients with ACS and evidence of thromboembolic material in coronary arteries originally estimated a prevalence as high as 13%.6,7 After the invention of diagnostic angiography, consensus studies have established the prevalence to be approximately 3% in patient with ACS.1 The prevalence may be higher in patient with significant risk factors that may increase the probability of CEs, like prosthetic heart valves and AF.2
In 2015 Shibata and colleagues proposed a scoring system for the diagnosis of CE. The scoring system consisted of major and minor criteria.6 Diagnosis of CE is established by ≥ 2 major criteria; 1 major and 2 minor; or ≥ 3 minor criteria. This scoring system increases the diagnostic probability of the disease.1,6
The major criteria are angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components (met by this patient); concomitant coronary emboli in multiple coronary vascular territories; concomitant systemic embolization without left ventricular thrombus attributable to acute MI; histological evidence of venous origin of coronary embolic material; and evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography, computed tomography, or magnetic resonance imaging.1,6 The minor criteria are 25% stenosis on coronary angiography except for the culprit lesion (met by this patient); presence of emboli risk factors, such as prosthetic heart valve (met by this patient); and AF.1,6
Management of CE remains controversial; aspiration of thrombus may be considered in the acute setting and with evidence of a heavy thrombus formation. This may allow for restoration of flow and retrieval of thrombus formation for histopathologic evaluation. However, it is important to mention that in the setting of STEMI, aspiration has been shown to increase risk of stroke and lead to increased morbidity. If aspiration of thrombus provides good restoration of flow, there is no need for further percutaneous intervention. Benefits of aspiration in low thrombus burden are not well established and do not provide any additional benefit compared with those of anticoagulation.6-11
Anticoagulation should be initiated in patients with AF and low bleeding risk, even when CHA2DS2-VASc (congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, aged 65 to 74 years, sex category) score is low. In patients with prolonged immobilization, recent surgery, pregnancy, use of oral contraceptives/tamoxifen, or other reversible risks, 3 months of anticoagulation has been shown to be sufficient. In the setting of active cancer or known thrombophilia, prolonged anticoagulation is recommended. Thrombophilia testing is not recommended in the setting of CE.1
The America College of Cardiology/American Heart Association guidelines for valvular heart disease recommend that patients with mechanical prosthetic aortic valves should be started on a vitamin K antagonist with a target INR of 2 to 3. (Class 1A). Prosthetic mitral and high thromboembolic valves require a higher INR target above 3.0. The addition of antiplatelet agents, such as aspirin in doses of 75 to 100 mg, should be started to decrease risk of thromboembolic disease in all patients with prosthetic heart valves.12
CE is not a common cause of ACS. Nevertheless, it was considered in the differential diagnosis of this patient, and diagnostic criteria were reviewed. This patient met the diagnostic criteria for a definitive diagnosis of CE. These included 1 major and 2 minor criteria: angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components; < 25% stenosis on coronary angiography except for the culprit lesion; and presence of emboli risk factors (prosthetic heart valve).
CE is rare, and review of the literature reveals that it accounts for < 3% of all ACS cases. Despite its rarity, it is important to recognize its risk factors, which include prosthetic heart valves, valvuloplasty, vasculitis, AF, left ventricular aneurysm, and endocarditis. The difference in treatment between CE and the most frequently encountered etiologies of ACS reveals the importance in recognizing this syndrome. Management of CE remains controversial. Nevertheless, when the culprit lesion is located in a distal portion of the vessel involved, as was seen in our patient, and in cases where there is a low thrombi burden, anticoagulation instead of thrombectomy is usually preferred. Patients with prosthetic mechanical valves have a high incidence of thromboembolism. This sometimes leads to thrombi formation in uncommon locations. Guidelines of therapy in these patients recommend that all prosthetic mechanical valves should be treated with both antiplatelet and anticoagulation therapies to reduce the risk of thrombi formation.
Conclusion
Physicians involved in diagnosing ACS should be aware of the risk factors for CE and always consider it while evaluating patients and developing the differential diagnosis.
1. Raphael CE, Heit JA, Reeder GS, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(2):172-180. doi:10.1016/j.jcin.2017.08.057
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST-segment-elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. doi:10.1161/CIRCINTERVENTIONS.117.005587
3. Oakley C, Yusuf R, Hollman A. Coronary embolism and angina in mitral stenosis. Br Heart J. 1961;23(4):357-369. doi:10.1136/hrt.23.4.357
4. Charles RG, Epstein EJ. Diagnosis of coronary embolism: a review. J R Soc Med. 1983;76(10):863-869.
5. Bawell MB, Moragues V, Shrader EL. Coronary embolism. Circulation. 1956;14(6):1159-1163. doi:10.1161/01.cir.14.6.1159
6. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241-250. doi:10.1161/CIRCULATIONAHA.114.015134
7. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88(2):155-161. doi:10.7326/0003-4819-88-2-155
8. Lacunza-Ruiz FJ, Muñoz-Esparza C, García-de-Lara J. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc Interv. 2014;7(10):e127-e128. doi:10.1016/j.jcin.2014.02.025
9. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387(10014):127-135. doi:10.1016/S0140-6736(15)00448-1
10. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction [published correction appears in N Engl J Med. 2014 Aug 21;371(8):786]. N Engl J Med. 2013;369(17):1587-1597. doi:10.1056/NEJMoa1308789
11. Kalçık M, Yesin M, Gürsoy MO, Karakoyun S, Özkan M. Treatment strategies for prosthetic valve thrombosis-derived coronary embolism. JACC Cardiovasc Interv. 2015;8(5):756-757. doi:10.1016/j.jcin.2014.11.019
12. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi:10.1161/CIR.0000000000000503
A 74-year-old male veteran presented at an urgent care clinic in Aguadilla, Puerto Rico, with a sharp, nonradiating, left-sided precordial chest pain that started while cleaning his house and gardening. The patient described the pain as 9 on the 10-point Wong-Baker FACES Pain Rating Scale, lasting about 5 to 10 minutes and was alleviated with rest. The patient’s medical history consisted of multiple comorbidities, including a mitral valve replacement with a Star-Edwards valve (ball in cage) in 1987. The electrocardiogram performed at the clinic showed no acute ischemic changes. Due to the persistent pain, the patient was transferred to Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, for further evaluation and management. On arrival, the patient had an international normalized ratio (INR) of 2.22; elevated high-sensitive troponin enzyme readings of 56 ng/L at 6:38 PM (0h); 61 ng/L at 7:38 PM (1h); and 83 ng/L at 9:47 PM (3h), reference range, 0-22 ng/L, and changes that prompted admission to the cardiac critical care unit. Two days later, a follow-up enzyme level was 52 ng/L. Cardiac catheterization revealed an acute filling defect at mid-left anterior descending artery and remaining coronary arteries with < 25% atherosclerosis (Figure). A myocardial perfusion study was performed for myocardial viability. The results showed a small, reversible perfusion defect involving the apical-septal wall with the remaining left ventricular myocardium appearing viable. Aspirin was added to the patient’s anticoagulation regimen of warfarin. Once target INR was reached, the patient was discharged home without recurrence of angina.
- What is your diagnosis?
- How would you treat this patient?
Acute coronary syndrome (ACS) consists of clinical suspicion of myocardial ischemia or laboratory confirmation of myocardial infarction (MI). ACS includes 3 major entities: non-ST elevation MI (NSTEMI), unstable angina, and ST-elevation MI (STEMI). ACS usually occurs as a result of a reduced supply of oxygenated blood to the myocardium, which is caused by restriction or occlusion of at least 1 of the coronary arteries. This alteration in blood flow is commonly secondary to a rupture of an atherosclerotic plaque or spontaneous dissection of a coronary artery. In rare cases, this reduction in blood flow is caused by a coronary embolism (CE) arising from a prosthetic heart valve.1,2
One of the first descriptions of CE was provided by Rudolf Virchow in the 1850s from postmortem autopsy findings.3 At that time, these coronary findings were associated with intracardiac mural thrombus or infective endocarditis. During the 1940s, CE was described in living patients who had survived a MI, and outcomes were not as catastrophic as originally believed. In the 1960s, a higher than usual association between prosthetic valves and CE was suspected and later confirmed by the invention and implementation of coronary angiography. Multiple studies have been published that confirm the association between prosthetic valves (especially in the mitral position), atrial fibrillation (AF), and a higher than usual rate of CEs.4,5
Discussion
The prevalence of this disease has varied during the years. Data from autopsies of patients with ACS and evidence of thromboembolic material in coronary arteries originally estimated a prevalence as high as 13%.6,7 After the invention of diagnostic angiography, consensus studies have established the prevalence to be approximately 3% in patient with ACS.1 The prevalence may be higher in patient with significant risk factors that may increase the probability of CEs, like prosthetic heart valves and AF.2
In 2015 Shibata and colleagues proposed a scoring system for the diagnosis of CE. The scoring system consisted of major and minor criteria.6 Diagnosis of CE is established by ≥ 2 major criteria; 1 major and 2 minor; or ≥ 3 minor criteria. This scoring system increases the diagnostic probability of the disease.1,6
The major criteria are angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components (met by this patient); concomitant coronary emboli in multiple coronary vascular territories; concomitant systemic embolization without left ventricular thrombus attributable to acute MI; histological evidence of venous origin of coronary embolic material; and evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography, computed tomography, or magnetic resonance imaging.1,6 The minor criteria are 25% stenosis on coronary angiography except for the culprit lesion (met by this patient); presence of emboli risk factors, such as prosthetic heart valve (met by this patient); and AF.1,6
Management of CE remains controversial; aspiration of thrombus may be considered in the acute setting and with evidence of a heavy thrombus formation. This may allow for restoration of flow and retrieval of thrombus formation for histopathologic evaluation. However, it is important to mention that in the setting of STEMI, aspiration has been shown to increase risk of stroke and lead to increased morbidity. If aspiration of thrombus provides good restoration of flow, there is no need for further percutaneous intervention. Benefits of aspiration in low thrombus burden are not well established and do not provide any additional benefit compared with those of anticoagulation.6-11
Anticoagulation should be initiated in patients with AF and low bleeding risk, even when CHA2DS2-VASc (congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, aged 65 to 74 years, sex category) score is low. In patients with prolonged immobilization, recent surgery, pregnancy, use of oral contraceptives/tamoxifen, or other reversible risks, 3 months of anticoagulation has been shown to be sufficient. In the setting of active cancer or known thrombophilia, prolonged anticoagulation is recommended. Thrombophilia testing is not recommended in the setting of CE.1
The America College of Cardiology/American Heart Association guidelines for valvular heart disease recommend that patients with mechanical prosthetic aortic valves should be started on a vitamin K antagonist with a target INR of 2 to 3. (Class 1A). Prosthetic mitral and high thromboembolic valves require a higher INR target above 3.0. The addition of antiplatelet agents, such as aspirin in doses of 75 to 100 mg, should be started to decrease risk of thromboembolic disease in all patients with prosthetic heart valves.12
CE is not a common cause of ACS. Nevertheless, it was considered in the differential diagnosis of this patient, and diagnostic criteria were reviewed. This patient met the diagnostic criteria for a definitive diagnosis of CE. These included 1 major and 2 minor criteria: angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components; < 25% stenosis on coronary angiography except for the culprit lesion; and presence of emboli risk factors (prosthetic heart valve).
CE is rare, and review of the literature reveals that it accounts for < 3% of all ACS cases. Despite its rarity, it is important to recognize its risk factors, which include prosthetic heart valves, valvuloplasty, vasculitis, AF, left ventricular aneurysm, and endocarditis. The difference in treatment between CE and the most frequently encountered etiologies of ACS reveals the importance in recognizing this syndrome. Management of CE remains controversial. Nevertheless, when the culprit lesion is located in a distal portion of the vessel involved, as was seen in our patient, and in cases where there is a low thrombi burden, anticoagulation instead of thrombectomy is usually preferred. Patients with prosthetic mechanical valves have a high incidence of thromboembolism. This sometimes leads to thrombi formation in uncommon locations. Guidelines of therapy in these patients recommend that all prosthetic mechanical valves should be treated with both antiplatelet and anticoagulation therapies to reduce the risk of thrombi formation.
Conclusion
Physicians involved in diagnosing ACS should be aware of the risk factors for CE and always consider it while evaluating patients and developing the differential diagnosis.
A 74-year-old male veteran presented at an urgent care clinic in Aguadilla, Puerto Rico, with a sharp, nonradiating, left-sided precordial chest pain that started while cleaning his house and gardening. The patient described the pain as 9 on the 10-point Wong-Baker FACES Pain Rating Scale, lasting about 5 to 10 minutes and was alleviated with rest. The patient’s medical history consisted of multiple comorbidities, including a mitral valve replacement with a Star-Edwards valve (ball in cage) in 1987. The electrocardiogram performed at the clinic showed no acute ischemic changes. Due to the persistent pain, the patient was transferred to Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, for further evaluation and management. On arrival, the patient had an international normalized ratio (INR) of 2.22; elevated high-sensitive troponin enzyme readings of 56 ng/L at 6:38 PM (0h); 61 ng/L at 7:38 PM (1h); and 83 ng/L at 9:47 PM (3h), reference range, 0-22 ng/L, and changes that prompted admission to the cardiac critical care unit. Two days later, a follow-up enzyme level was 52 ng/L. Cardiac catheterization revealed an acute filling defect at mid-left anterior descending artery and remaining coronary arteries with < 25% atherosclerosis (Figure). A myocardial perfusion study was performed for myocardial viability. The results showed a small, reversible perfusion defect involving the apical-septal wall with the remaining left ventricular myocardium appearing viable. Aspirin was added to the patient’s anticoagulation regimen of warfarin. Once target INR was reached, the patient was discharged home without recurrence of angina.
- What is your diagnosis?
- How would you treat this patient?
Acute coronary syndrome (ACS) consists of clinical suspicion of myocardial ischemia or laboratory confirmation of myocardial infarction (MI). ACS includes 3 major entities: non-ST elevation MI (NSTEMI), unstable angina, and ST-elevation MI (STEMI). ACS usually occurs as a result of a reduced supply of oxygenated blood to the myocardium, which is caused by restriction or occlusion of at least 1 of the coronary arteries. This alteration in blood flow is commonly secondary to a rupture of an atherosclerotic plaque or spontaneous dissection of a coronary artery. In rare cases, this reduction in blood flow is caused by a coronary embolism (CE) arising from a prosthetic heart valve.1,2
One of the first descriptions of CE was provided by Rudolf Virchow in the 1850s from postmortem autopsy findings.3 At that time, these coronary findings were associated with intracardiac mural thrombus or infective endocarditis. During the 1940s, CE was described in living patients who had survived a MI, and outcomes were not as catastrophic as originally believed. In the 1960s, a higher than usual association between prosthetic valves and CE was suspected and later confirmed by the invention and implementation of coronary angiography. Multiple studies have been published that confirm the association between prosthetic valves (especially in the mitral position), atrial fibrillation (AF), and a higher than usual rate of CEs.4,5
Discussion
The prevalence of this disease has varied during the years. Data from autopsies of patients with ACS and evidence of thromboembolic material in coronary arteries originally estimated a prevalence as high as 13%.6,7 After the invention of diagnostic angiography, consensus studies have established the prevalence to be approximately 3% in patient with ACS.1 The prevalence may be higher in patient with significant risk factors that may increase the probability of CEs, like prosthetic heart valves and AF.2
In 2015 Shibata and colleagues proposed a scoring system for the diagnosis of CE. The scoring system consisted of major and minor criteria.6 Diagnosis of CE is established by ≥ 2 major criteria; 1 major and 2 minor; or ≥ 3 minor criteria. This scoring system increases the diagnostic probability of the disease.1,6
The major criteria are angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components (met by this patient); concomitant coronary emboli in multiple coronary vascular territories; concomitant systemic embolization without left ventricular thrombus attributable to acute MI; histological evidence of venous origin of coronary embolic material; and evidence of an embolic source based on transthoracic echocardiography, transesophageal echocardiography, computed tomography, or magnetic resonance imaging.1,6 The minor criteria are 25% stenosis on coronary angiography except for the culprit lesion (met by this patient); presence of emboli risk factors, such as prosthetic heart valve (met by this patient); and AF.1,6
Management of CE remains controversial; aspiration of thrombus may be considered in the acute setting and with evidence of a heavy thrombus formation. This may allow for restoration of flow and retrieval of thrombus formation for histopathologic evaluation. However, it is important to mention that in the setting of STEMI, aspiration has been shown to increase risk of stroke and lead to increased morbidity. If aspiration of thrombus provides good restoration of flow, there is no need for further percutaneous intervention. Benefits of aspiration in low thrombus burden are not well established and do not provide any additional benefit compared with those of anticoagulation.6-11
Anticoagulation should be initiated in patients with AF and low bleeding risk, even when CHA2DS2-VASc (congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, aged 65 to 74 years, sex category) score is low. In patients with prolonged immobilization, recent surgery, pregnancy, use of oral contraceptives/tamoxifen, or other reversible risks, 3 months of anticoagulation has been shown to be sufficient. In the setting of active cancer or known thrombophilia, prolonged anticoagulation is recommended. Thrombophilia testing is not recommended in the setting of CE.1
The America College of Cardiology/American Heart Association guidelines for valvular heart disease recommend that patients with mechanical prosthetic aortic valves should be started on a vitamin K antagonist with a target INR of 2 to 3. (Class 1A). Prosthetic mitral and high thromboembolic valves require a higher INR target above 3.0. The addition of antiplatelet agents, such as aspirin in doses of 75 to 100 mg, should be started to decrease risk of thromboembolic disease in all patients with prosthetic heart valves.12
CE is not a common cause of ACS. Nevertheless, it was considered in the differential diagnosis of this patient, and diagnostic criteria were reviewed. This patient met the diagnostic criteria for a definitive diagnosis of CE. These included 1 major and 2 minor criteria: angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components; < 25% stenosis on coronary angiography except for the culprit lesion; and presence of emboli risk factors (prosthetic heart valve).
CE is rare, and review of the literature reveals that it accounts for < 3% of all ACS cases. Despite its rarity, it is important to recognize its risk factors, which include prosthetic heart valves, valvuloplasty, vasculitis, AF, left ventricular aneurysm, and endocarditis. The difference in treatment between CE and the most frequently encountered etiologies of ACS reveals the importance in recognizing this syndrome. Management of CE remains controversial. Nevertheless, when the culprit lesion is located in a distal portion of the vessel involved, as was seen in our patient, and in cases where there is a low thrombi burden, anticoagulation instead of thrombectomy is usually preferred. Patients with prosthetic mechanical valves have a high incidence of thromboembolism. This sometimes leads to thrombi formation in uncommon locations. Guidelines of therapy in these patients recommend that all prosthetic mechanical valves should be treated with both antiplatelet and anticoagulation therapies to reduce the risk of thrombi formation.
Conclusion
Physicians involved in diagnosing ACS should be aware of the risk factors for CE and always consider it while evaluating patients and developing the differential diagnosis.
1. Raphael CE, Heit JA, Reeder GS, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(2):172-180. doi:10.1016/j.jcin.2017.08.057
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST-segment-elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. doi:10.1161/CIRCINTERVENTIONS.117.005587
3. Oakley C, Yusuf R, Hollman A. Coronary embolism and angina in mitral stenosis. Br Heart J. 1961;23(4):357-369. doi:10.1136/hrt.23.4.357
4. Charles RG, Epstein EJ. Diagnosis of coronary embolism: a review. J R Soc Med. 1983;76(10):863-869.
5. Bawell MB, Moragues V, Shrader EL. Coronary embolism. Circulation. 1956;14(6):1159-1163. doi:10.1161/01.cir.14.6.1159
6. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241-250. doi:10.1161/CIRCULATIONAHA.114.015134
7. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88(2):155-161. doi:10.7326/0003-4819-88-2-155
8. Lacunza-Ruiz FJ, Muñoz-Esparza C, García-de-Lara J. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc Interv. 2014;7(10):e127-e128. doi:10.1016/j.jcin.2014.02.025
9. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387(10014):127-135. doi:10.1016/S0140-6736(15)00448-1
10. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction [published correction appears in N Engl J Med. 2014 Aug 21;371(8):786]. N Engl J Med. 2013;369(17):1587-1597. doi:10.1056/NEJMoa1308789
11. Kalçık M, Yesin M, Gürsoy MO, Karakoyun S, Özkan M. Treatment strategies for prosthetic valve thrombosis-derived coronary embolism. JACC Cardiovasc Interv. 2015;8(5):756-757. doi:10.1016/j.jcin.2014.11.019
12. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi:10.1161/CIR.0000000000000503
1. Raphael CE, Heit JA, Reeder GS, et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(2):172-180. doi:10.1016/j.jcin.2017.08.057
2. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST-segment-elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. doi:10.1161/CIRCINTERVENTIONS.117.005587
3. Oakley C, Yusuf R, Hollman A. Coronary embolism and angina in mitral stenosis. Br Heart J. 1961;23(4):357-369. doi:10.1136/hrt.23.4.357
4. Charles RG, Epstein EJ. Diagnosis of coronary embolism: a review. J R Soc Med. 1983;76(10):863-869.
5. Bawell MB, Moragues V, Shrader EL. Coronary embolism. Circulation. 1956;14(6):1159-1163. doi:10.1161/01.cir.14.6.1159
6. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241-250. doi:10.1161/CIRCULATIONAHA.114.015134
7. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88(2):155-161. doi:10.7326/0003-4819-88-2-155
8. Lacunza-Ruiz FJ, Muñoz-Esparza C, García-de-Lara J. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc Interv. 2014;7(10):e127-e128. doi:10.1016/j.jcin.2014.02.025
9. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387(10014):127-135. doi:10.1016/S0140-6736(15)00448-1
10. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction [published correction appears in N Engl J Med. 2014 Aug 21;371(8):786]. N Engl J Med. 2013;369(17):1587-1597. doi:10.1056/NEJMoa1308789
11. Kalçık M, Yesin M, Gürsoy MO, Karakoyun S, Özkan M. Treatment strategies for prosthetic valve thrombosis-derived coronary embolism. JACC Cardiovasc Interv. 2015;8(5):756-757. doi:10.1016/j.jcin.2014.11.019
12. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi:10.1161/CIR.0000000000000503
Revised dispatch system boosts bystander CPR in those with limited English
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
FROM JAMA NETWORK OPEN
FDA approves ‘game changer’ semaglutide for weight loss
The U.S. Food and Drug Administration has approved a 2.4 mg/week subcutaneous dose of the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy, Novo Nordisk) for weight loss.
Specifically, this drug format and dosage are approved as an adjunct to a reduced-calorie diet and increased physical activity to treat adults who have obesity (body mass index [BMI] ≥ 30 kg/m2) or are overweight (BMI ≥ 27 kg/m2) with at least one weight-related comorbidity.
Semaglutide “induces weight loss by reducing hunger, increasing feelings of fullness, and thereby helping people eat less and reduce their calorie intake,” according to a company statement.
Novo Nordisk plans to launch Wegovy later this month in the United States. The prescribing information can be found here.
This weight-loss drug is currently under review by the European Medicines Agency.
Several experts told Medscape that they believe the approval of this drug – as long as it is reimbursed – has the potential to change the paradigm of care when it comes to weight loss.
‘Game changer’ drug tested in STEP clinical trial program
The favorable FDA ruling is based on results from the Semaglutide Treatment Effect in People With Obesity (STEP) program of four phase 3 clinical trials that tested the drug’s safety and efficacy in more than 4,500 adults with overweight or obesity obesity who were randomized to receive a reduced a calorie meal plan and increased physical activity (placebo) or this lifestyle intervention plus semaglutide.
The four 68-week trials of subcutaneous semaglutide 2.4 mg/week versus placebo were published in February and March 2021.
As previously reported by this news organization, all trials were in adults with overweight or obesity:
- was in 1,961 adults (N Engl J Med. 2021 March 18;384:989-1002).
- was in 1,210 adults who also had diabetes (Lancet. 2021 Mar 13;397;971-84).
- was in 611 adults, where those in the treatment group also underwent an intensive lifestyle intervention (JAMA. 2021 Feb 24;325:1403-13.
- was in 803 adults who had reached a target dose of 2.4 mg semaglutide after a 20-week run-in (and the trial examined further weight loss in the subsequent 48 weeks) (JAMA 2021 Mar 23;325:1414-25).
In the STEP 1, 2, and 4 trials of individuals with overweight and obesity, those in the semaglutide groups attained a 15%-18% weight loss over 68 weeks.
The dosage was well-tolerated. The most common side effects were gastrointestinal, and they were transient and mild or moderate in severity.
The side effects, contraindications, and a black box warning about thyroid C-cell tumors are spelled out in the prescribing information.
A coauthor of the STEP 1 trial, Rachel Batterham, MBBS, PhD, of the Centre for Obesity Research at University College London, said at the time of publication: “The findings of this study represent a major breakthrough for improving the health of people with obesity.”
“No other drug has come close to producing this level of weight loss – this really is a gamechanger. For the first time, people can achieve through drugs what was only possible through weight-loss surgery,” she added.
Welcome Addition, But Will Insurance Coverage, Price Thwart Access?
Thomas A. Wadden, PhD, from the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, and lead author of STEP 3, commented in an email to this news organization that “semaglutide 2.4 mg appears to be the breakthrough in weight management that healthcare providers and their patients with obesity have been waiting for.”
The mean 15% weight loss at 68 weeks is nearly twice what is seen with other FDA-approved anti-obesity medications, he noted, and moreover, 70% of patients taking semaglutide lost at least 10% of their initial weight, which is associated with clinically meaningful improvements in obesity-related type 2 diabetes, hypertension, obstructive sleep apnea, and impaired quality of life.
And “nearly one-third of users are likely to lose 20% or more of their starting weight, an outcome which eludes traditional diet and exercise interventions and which approaches weight losses produced by the most widely performed bariatric surgery, sleeve gastrectomy (with mean losses of 25% of initial weight at 1 year).” Dr. Wadden stressed.
Thus “the efficacy of semaglutide 2.4 mg, combined with its favorable safety profile, makes this medication a potential game changer,” he summarized, echoing Dr. Batterham.
However, insurance coverage and price could block uptake.
“I hope that the millions of people – in the U.S. and worldwide – who could benefit from this medication eventually will have access to it,” said Dr. Wadden. “In the U.S., the coverage of anti-obesity medications by insurers and employers will need to improve to ensure this happens, and the medication must be reasonably priced. These changes are critical to making this medication the game changer it could be.”
“This approval is an important development,” Scott Kahan, MD, director of the National Center for Weight and Wellness, Washington, who was not involved in the clinical trials of this drug, similarly wrote in an email.
“In a field with relatively few medication options, the availability of additional obesity pharmacotherapy agents is welcome,” he said. “In particular, semaglutide has shown impressive efficacy and safety data; as such it should be a valuable clinical option for many patients.”
However, it is concerning that “access to obesity treatments has traditionally been a challenge,” Dr. Kahan warned. “Novo Nordisk’s other obesity medication, Saxenda, has been a valuable tool, but one that exceedingly few patients are able to utilize due to minimal insurance reimbursement and very high cost.”
“It remains to be seen how accessible semaglutide will be for patients,” according to Dr. Kahan, “Still, if the challenge of limited coverage and high cost can be mitigated, this medication has a chance to significantly change the current paradigm of care, which until till now has included minimal use of pharmacotherapy outside specialty clinics,” he maintains.
Lower-dose injectable and pill already approved for diabetes
Subcutaneous semaglutide at doses up to 1 mg/week (Ozempic, Novo Nordisk), which comes as prefilled pens at doses of 0.5 mg or 1.0 mg, is already approved for the treatment of type 2 diabetes.
The company is also applying for approval for a higher dose of semaglutide, 2 mg/week, for use in type 2 diabetes, and has just resubmitted its label expansion application to the FDA, after the agency issued a refusal to file letter in March.
And in September 2019, the FDA approved oral semaglutide (Rybelsus, Novo Nordisk), in doses of 7 and 14 mg/day, to improve glycemic control in type 2 diabetes, making it the first GLP-1 receptor agonist available in tablet form.
CVOT and oral format trials for obesity on the horizon
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial will shed light on cardiovascular outcomes after 2.5-5 years in patients with cardiovascular disease and overweight or obesity but without type 2 diabetes. Participants will receive semaglutide in doses up to a maximum of 2.4 mg/week, or placebo, as an adjunct to lifestyle recommendations focused on cardiovascular risk reduction. The study is expected to complete in 2023.
And Novo Nordisk plans to initiate a global 68-week phase 3 trial in the second half of 2021 on the efficacy and safety of oral semaglutide 50 mg compared with placebo in 1000 people with obesity or overweight and comorbidities.
A version of this article first appeared on Medscape.com.
This article was updated 6/7/21.
The U.S. Food and Drug Administration has approved a 2.4 mg/week subcutaneous dose of the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy, Novo Nordisk) for weight loss.
Specifically, this drug format and dosage are approved as an adjunct to a reduced-calorie diet and increased physical activity to treat adults who have obesity (body mass index [BMI] ≥ 30 kg/m2) or are overweight (BMI ≥ 27 kg/m2) with at least one weight-related comorbidity.
Semaglutide “induces weight loss by reducing hunger, increasing feelings of fullness, and thereby helping people eat less and reduce their calorie intake,” according to a company statement.
Novo Nordisk plans to launch Wegovy later this month in the United States. The prescribing information can be found here.
This weight-loss drug is currently under review by the European Medicines Agency.
Several experts told Medscape that they believe the approval of this drug – as long as it is reimbursed – has the potential to change the paradigm of care when it comes to weight loss.
‘Game changer’ drug tested in STEP clinical trial program
The favorable FDA ruling is based on results from the Semaglutide Treatment Effect in People With Obesity (STEP) program of four phase 3 clinical trials that tested the drug’s safety and efficacy in more than 4,500 adults with overweight or obesity obesity who were randomized to receive a reduced a calorie meal plan and increased physical activity (placebo) or this lifestyle intervention plus semaglutide.
The four 68-week trials of subcutaneous semaglutide 2.4 mg/week versus placebo were published in February and March 2021.
As previously reported by this news organization, all trials were in adults with overweight or obesity:
- was in 1,961 adults (N Engl J Med. 2021 March 18;384:989-1002).
- was in 1,210 adults who also had diabetes (Lancet. 2021 Mar 13;397;971-84).
- was in 611 adults, where those in the treatment group also underwent an intensive lifestyle intervention (JAMA. 2021 Feb 24;325:1403-13.
- was in 803 adults who had reached a target dose of 2.4 mg semaglutide after a 20-week run-in (and the trial examined further weight loss in the subsequent 48 weeks) (JAMA 2021 Mar 23;325:1414-25).
In the STEP 1, 2, and 4 trials of individuals with overweight and obesity, those in the semaglutide groups attained a 15%-18% weight loss over 68 weeks.
The dosage was well-tolerated. The most common side effects were gastrointestinal, and they were transient and mild or moderate in severity.
The side effects, contraindications, and a black box warning about thyroid C-cell tumors are spelled out in the prescribing information.
A coauthor of the STEP 1 trial, Rachel Batterham, MBBS, PhD, of the Centre for Obesity Research at University College London, said at the time of publication: “The findings of this study represent a major breakthrough for improving the health of people with obesity.”
“No other drug has come close to producing this level of weight loss – this really is a gamechanger. For the first time, people can achieve through drugs what was only possible through weight-loss surgery,” she added.
Welcome Addition, But Will Insurance Coverage, Price Thwart Access?
Thomas A. Wadden, PhD, from the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, and lead author of STEP 3, commented in an email to this news organization that “semaglutide 2.4 mg appears to be the breakthrough in weight management that healthcare providers and their patients with obesity have been waiting for.”
The mean 15% weight loss at 68 weeks is nearly twice what is seen with other FDA-approved anti-obesity medications, he noted, and moreover, 70% of patients taking semaglutide lost at least 10% of their initial weight, which is associated with clinically meaningful improvements in obesity-related type 2 diabetes, hypertension, obstructive sleep apnea, and impaired quality of life.
And “nearly one-third of users are likely to lose 20% or more of their starting weight, an outcome which eludes traditional diet and exercise interventions and which approaches weight losses produced by the most widely performed bariatric surgery, sleeve gastrectomy (with mean losses of 25% of initial weight at 1 year).” Dr. Wadden stressed.
Thus “the efficacy of semaglutide 2.4 mg, combined with its favorable safety profile, makes this medication a potential game changer,” he summarized, echoing Dr. Batterham.
However, insurance coverage and price could block uptake.
“I hope that the millions of people – in the U.S. and worldwide – who could benefit from this medication eventually will have access to it,” said Dr. Wadden. “In the U.S., the coverage of anti-obesity medications by insurers and employers will need to improve to ensure this happens, and the medication must be reasonably priced. These changes are critical to making this medication the game changer it could be.”
“This approval is an important development,” Scott Kahan, MD, director of the National Center for Weight and Wellness, Washington, who was not involved in the clinical trials of this drug, similarly wrote in an email.
“In a field with relatively few medication options, the availability of additional obesity pharmacotherapy agents is welcome,” he said. “In particular, semaglutide has shown impressive efficacy and safety data; as such it should be a valuable clinical option for many patients.”
However, it is concerning that “access to obesity treatments has traditionally been a challenge,” Dr. Kahan warned. “Novo Nordisk’s other obesity medication, Saxenda, has been a valuable tool, but one that exceedingly few patients are able to utilize due to minimal insurance reimbursement and very high cost.”
“It remains to be seen how accessible semaglutide will be for patients,” according to Dr. Kahan, “Still, if the challenge of limited coverage and high cost can be mitigated, this medication has a chance to significantly change the current paradigm of care, which until till now has included minimal use of pharmacotherapy outside specialty clinics,” he maintains.
Lower-dose injectable and pill already approved for diabetes
Subcutaneous semaglutide at doses up to 1 mg/week (Ozempic, Novo Nordisk), which comes as prefilled pens at doses of 0.5 mg or 1.0 mg, is already approved for the treatment of type 2 diabetes.
The company is also applying for approval for a higher dose of semaglutide, 2 mg/week, for use in type 2 diabetes, and has just resubmitted its label expansion application to the FDA, after the agency issued a refusal to file letter in March.
And in September 2019, the FDA approved oral semaglutide (Rybelsus, Novo Nordisk), in doses of 7 and 14 mg/day, to improve glycemic control in type 2 diabetes, making it the first GLP-1 receptor agonist available in tablet form.
CVOT and oral format trials for obesity on the horizon
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial will shed light on cardiovascular outcomes after 2.5-5 years in patients with cardiovascular disease and overweight or obesity but without type 2 diabetes. Participants will receive semaglutide in doses up to a maximum of 2.4 mg/week, or placebo, as an adjunct to lifestyle recommendations focused on cardiovascular risk reduction. The study is expected to complete in 2023.
And Novo Nordisk plans to initiate a global 68-week phase 3 trial in the second half of 2021 on the efficacy and safety of oral semaglutide 50 mg compared with placebo in 1000 people with obesity or overweight and comorbidities.
A version of this article first appeared on Medscape.com.
This article was updated 6/7/21.
The U.S. Food and Drug Administration has approved a 2.4 mg/week subcutaneous dose of the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy, Novo Nordisk) for weight loss.
Specifically, this drug format and dosage are approved as an adjunct to a reduced-calorie diet and increased physical activity to treat adults who have obesity (body mass index [BMI] ≥ 30 kg/m2) or are overweight (BMI ≥ 27 kg/m2) with at least one weight-related comorbidity.
Semaglutide “induces weight loss by reducing hunger, increasing feelings of fullness, and thereby helping people eat less and reduce their calorie intake,” according to a company statement.
Novo Nordisk plans to launch Wegovy later this month in the United States. The prescribing information can be found here.
This weight-loss drug is currently under review by the European Medicines Agency.
Several experts told Medscape that they believe the approval of this drug – as long as it is reimbursed – has the potential to change the paradigm of care when it comes to weight loss.
‘Game changer’ drug tested in STEP clinical trial program
The favorable FDA ruling is based on results from the Semaglutide Treatment Effect in People With Obesity (STEP) program of four phase 3 clinical trials that tested the drug’s safety and efficacy in more than 4,500 adults with overweight or obesity obesity who were randomized to receive a reduced a calorie meal plan and increased physical activity (placebo) or this lifestyle intervention plus semaglutide.
The four 68-week trials of subcutaneous semaglutide 2.4 mg/week versus placebo were published in February and March 2021.
As previously reported by this news organization, all trials were in adults with overweight or obesity:
- was in 1,961 adults (N Engl J Med. 2021 March 18;384:989-1002).
- was in 1,210 adults who also had diabetes (Lancet. 2021 Mar 13;397;971-84).
- was in 611 adults, where those in the treatment group also underwent an intensive lifestyle intervention (JAMA. 2021 Feb 24;325:1403-13.
- was in 803 adults who had reached a target dose of 2.4 mg semaglutide after a 20-week run-in (and the trial examined further weight loss in the subsequent 48 weeks) (JAMA 2021 Mar 23;325:1414-25).
In the STEP 1, 2, and 4 trials of individuals with overweight and obesity, those in the semaglutide groups attained a 15%-18% weight loss over 68 weeks.
The dosage was well-tolerated. The most common side effects were gastrointestinal, and they were transient and mild or moderate in severity.
The side effects, contraindications, and a black box warning about thyroid C-cell tumors are spelled out in the prescribing information.
A coauthor of the STEP 1 trial, Rachel Batterham, MBBS, PhD, of the Centre for Obesity Research at University College London, said at the time of publication: “The findings of this study represent a major breakthrough for improving the health of people with obesity.”
“No other drug has come close to producing this level of weight loss – this really is a gamechanger. For the first time, people can achieve through drugs what was only possible through weight-loss surgery,” she added.
Welcome Addition, But Will Insurance Coverage, Price Thwart Access?
Thomas A. Wadden, PhD, from the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, and lead author of STEP 3, commented in an email to this news organization that “semaglutide 2.4 mg appears to be the breakthrough in weight management that healthcare providers and their patients with obesity have been waiting for.”
The mean 15% weight loss at 68 weeks is nearly twice what is seen with other FDA-approved anti-obesity medications, he noted, and moreover, 70% of patients taking semaglutide lost at least 10% of their initial weight, which is associated with clinically meaningful improvements in obesity-related type 2 diabetes, hypertension, obstructive sleep apnea, and impaired quality of life.
And “nearly one-third of users are likely to lose 20% or more of their starting weight, an outcome which eludes traditional diet and exercise interventions and which approaches weight losses produced by the most widely performed bariatric surgery, sleeve gastrectomy (with mean losses of 25% of initial weight at 1 year).” Dr. Wadden stressed.
Thus “the efficacy of semaglutide 2.4 mg, combined with its favorable safety profile, makes this medication a potential game changer,” he summarized, echoing Dr. Batterham.
However, insurance coverage and price could block uptake.
“I hope that the millions of people – in the U.S. and worldwide – who could benefit from this medication eventually will have access to it,” said Dr. Wadden. “In the U.S., the coverage of anti-obesity medications by insurers and employers will need to improve to ensure this happens, and the medication must be reasonably priced. These changes are critical to making this medication the game changer it could be.”
“This approval is an important development,” Scott Kahan, MD, director of the National Center for Weight and Wellness, Washington, who was not involved in the clinical trials of this drug, similarly wrote in an email.
“In a field with relatively few medication options, the availability of additional obesity pharmacotherapy agents is welcome,” he said. “In particular, semaglutide has shown impressive efficacy and safety data; as such it should be a valuable clinical option for many patients.”
However, it is concerning that “access to obesity treatments has traditionally been a challenge,” Dr. Kahan warned. “Novo Nordisk’s other obesity medication, Saxenda, has been a valuable tool, but one that exceedingly few patients are able to utilize due to minimal insurance reimbursement and very high cost.”
“It remains to be seen how accessible semaglutide will be for patients,” according to Dr. Kahan, “Still, if the challenge of limited coverage and high cost can be mitigated, this medication has a chance to significantly change the current paradigm of care, which until till now has included minimal use of pharmacotherapy outside specialty clinics,” he maintains.
Lower-dose injectable and pill already approved for diabetes
Subcutaneous semaglutide at doses up to 1 mg/week (Ozempic, Novo Nordisk), which comes as prefilled pens at doses of 0.5 mg or 1.0 mg, is already approved for the treatment of type 2 diabetes.
The company is also applying for approval for a higher dose of semaglutide, 2 mg/week, for use in type 2 diabetes, and has just resubmitted its label expansion application to the FDA, after the agency issued a refusal to file letter in March.
And in September 2019, the FDA approved oral semaglutide (Rybelsus, Novo Nordisk), in doses of 7 and 14 mg/day, to improve glycemic control in type 2 diabetes, making it the first GLP-1 receptor agonist available in tablet form.
CVOT and oral format trials for obesity on the horizon
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial will shed light on cardiovascular outcomes after 2.5-5 years in patients with cardiovascular disease and overweight or obesity but without type 2 diabetes. Participants will receive semaglutide in doses up to a maximum of 2.4 mg/week, or placebo, as an adjunct to lifestyle recommendations focused on cardiovascular risk reduction. The study is expected to complete in 2023.
And Novo Nordisk plans to initiate a global 68-week phase 3 trial in the second half of 2021 on the efficacy and safety of oral semaglutide 50 mg compared with placebo in 1000 people with obesity or overweight and comorbidities.
A version of this article first appeared on Medscape.com.
This article was updated 6/7/21.
Medication in heart failure: Pro tips on therapy with the ‘four pillars of survival’
On the medication front, there are now “four pillars of survival” in the setting of heart failure with reduced ejection fraction (EF), a cardiologist told hospitalists recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
The quartet of drugs are beta blockers, angiotensin receptor–neprilysin inhibitors, mineralocorticoid receptor antagonists, and the newest addition – sodium-glucose cotransporter 2 inhibitors.
“If we use all four of these medications, the absolute risk reduction [in mortality] is 25% over a 2-year period,” said cardiologist Celeste T. Williams, MD, of Henry Ford Hospital, Detroit. “So it is very important that we use these medications,” she said.
But managing the medications, she said, can be challenging. Dr. Williams offered these tips about the use of medication in heart failure.
Beta blockers are crucial players
“Beta blockers save lives,” Dr. Williams said, “but there’s always a debate about how much we should titrate beta blockers.”
How can you determine the proper titration? Focus on heart rates, she recommended. “We know that higher heart rates in heart failure patients are associated with worse outcomes. There was subgroup analysis in the BEAUTIFUL study that looked at 5,300 patients with EF less than 40% who had CAD [coronary artery disease]. They found that patients with heart rates greater than 70 had a 34% increased risk of cardiovascular death and a 53% increased risk of heart failure hospitalization compared to heart rates less than 70.”
Focus on getting your patient’s heart rate lower than 70 while maintaining their blood pressure, she said.
“Another question we have is, ‘When these patients come into hospitals, what should we do with the beta blocker? Should we continue it? Should we stop it?’ If you can, you always want to continue the beta blocker or the ACE [angiotensin-converting enzyme] inhibitor, because studies have shown us that the likelihood for patients to be on these medications 90 days later is dismal,” she said. “But you also need to look at the patient. If the patient is in cardiogenic shock, their beta blocker should be stopped.”
Consider multiple factors when titrating various medications
“In the hospital, we always will look at hemodynamic compromise in the patient. Is the patient in cardiogenic shock?” Dr. Williams said. “We also must think about compliance concerns. Are the patients even taking their medication? And if they are taking their medications, are they tolerating standard medical therapy? Are they hypotensive? Are they only able to tolerate minimal meds? Have you seen that their creatine continues to rise? Or are they having poor diuresis with the rise in diuretics?”
All these questions are useful, she said, as you determine whether you should titrate medication yourself or refer the patient to an advanced heart failure specialist.
Understand when to stick with guideline-directed medical therapy
Dr. Williams said another question often arises: “If your patient’s EF recovers, should you stop guideline-directed medical therapy [GDMT]?” She highlighted a TRED-HF study that evaluated patients who had recovered from dilated, nonischemic cardiomyopathy and were receiving GDMT. “They withdrew GDMT for half of the patients and looked at their echoes 6 months later. They found that 40% of the patients relapsed. Their EFs went below 40% again. Stopping medications is not the best idea for most of these patients.”
However, she said, there are scenarios in which GDMT may be withdrawn, such as for patients with tachycardia-induced cardiomyopathies whose EF recovers after ablation, those whose EF recovers after alcoholic cardiomyopathy, and those who receive valve replacements. “We need to remember that a lot of the patients who develop stage C heart failure have risk factors. Even though their heart failure has recovered, they have risks that need to be treated, and you can use the same medications that you use for heart failure to control their risk. Therefore, you would not get into trouble by withdrawing their medications.”
She added: “If you’re unable to titrate GDMT because the blood pressure is too soft, the creatine continues to rise, or the patient just has a lot of heart failure symptoms, this is indicative that the patient is sicker than they may appear.” At this point, defer to a heart failure specialist, she said.
Consider ivabradine as an add-on when appropriate
In some cases, a heart rate of less than 70 bpm will not be achieved even with GDMT and maximum tolerated doses, Dr. Williams said. “If they’re in sinus, you can add on a medication called ivabradine, which was studied in the SHIFT study. This looked at patients with EF of less than 35% who had class 2-3 heart failure in sinus rhythm. They had to have a hospitalization within the last 12 months. The patients were randomized to either ivabradine or placebo. The primary outcome was [cardiovascular] death or heart failure hospitalization. They found that patients who had ivabradine had a decrease in heart failure hospitalization.”
The lesson, she said, is that “ivabradine is a great medication to add on to patients who are still tachycardic in sinus when you cannot titrate up the beta blocker.”
Dr. Williams reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On the medication front, there are now “four pillars of survival” in the setting of heart failure with reduced ejection fraction (EF), a cardiologist told hospitalists recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
The quartet of drugs are beta blockers, angiotensin receptor–neprilysin inhibitors, mineralocorticoid receptor antagonists, and the newest addition – sodium-glucose cotransporter 2 inhibitors.
“If we use all four of these medications, the absolute risk reduction [in mortality] is 25% over a 2-year period,” said cardiologist Celeste T. Williams, MD, of Henry Ford Hospital, Detroit. “So it is very important that we use these medications,” she said.
But managing the medications, she said, can be challenging. Dr. Williams offered these tips about the use of medication in heart failure.
Beta blockers are crucial players
“Beta blockers save lives,” Dr. Williams said, “but there’s always a debate about how much we should titrate beta blockers.”
How can you determine the proper titration? Focus on heart rates, she recommended. “We know that higher heart rates in heart failure patients are associated with worse outcomes. There was subgroup analysis in the BEAUTIFUL study that looked at 5,300 patients with EF less than 40% who had CAD [coronary artery disease]. They found that patients with heart rates greater than 70 had a 34% increased risk of cardiovascular death and a 53% increased risk of heart failure hospitalization compared to heart rates less than 70.”
Focus on getting your patient’s heart rate lower than 70 while maintaining their blood pressure, she said.
“Another question we have is, ‘When these patients come into hospitals, what should we do with the beta blocker? Should we continue it? Should we stop it?’ If you can, you always want to continue the beta blocker or the ACE [angiotensin-converting enzyme] inhibitor, because studies have shown us that the likelihood for patients to be on these medications 90 days later is dismal,” she said. “But you also need to look at the patient. If the patient is in cardiogenic shock, their beta blocker should be stopped.”
Consider multiple factors when titrating various medications
“In the hospital, we always will look at hemodynamic compromise in the patient. Is the patient in cardiogenic shock?” Dr. Williams said. “We also must think about compliance concerns. Are the patients even taking their medication? And if they are taking their medications, are they tolerating standard medical therapy? Are they hypotensive? Are they only able to tolerate minimal meds? Have you seen that their creatine continues to rise? Or are they having poor diuresis with the rise in diuretics?”
All these questions are useful, she said, as you determine whether you should titrate medication yourself or refer the patient to an advanced heart failure specialist.
Understand when to stick with guideline-directed medical therapy
Dr. Williams said another question often arises: “If your patient’s EF recovers, should you stop guideline-directed medical therapy [GDMT]?” She highlighted a TRED-HF study that evaluated patients who had recovered from dilated, nonischemic cardiomyopathy and were receiving GDMT. “They withdrew GDMT for half of the patients and looked at their echoes 6 months later. They found that 40% of the patients relapsed. Their EFs went below 40% again. Stopping medications is not the best idea for most of these patients.”
However, she said, there are scenarios in which GDMT may be withdrawn, such as for patients with tachycardia-induced cardiomyopathies whose EF recovers after ablation, those whose EF recovers after alcoholic cardiomyopathy, and those who receive valve replacements. “We need to remember that a lot of the patients who develop stage C heart failure have risk factors. Even though their heart failure has recovered, they have risks that need to be treated, and you can use the same medications that you use for heart failure to control their risk. Therefore, you would not get into trouble by withdrawing their medications.”
She added: “If you’re unable to titrate GDMT because the blood pressure is too soft, the creatine continues to rise, or the patient just has a lot of heart failure symptoms, this is indicative that the patient is sicker than they may appear.” At this point, defer to a heart failure specialist, she said.
Consider ivabradine as an add-on when appropriate
In some cases, a heart rate of less than 70 bpm will not be achieved even with GDMT and maximum tolerated doses, Dr. Williams said. “If they’re in sinus, you can add on a medication called ivabradine, which was studied in the SHIFT study. This looked at patients with EF of less than 35% who had class 2-3 heart failure in sinus rhythm. They had to have a hospitalization within the last 12 months. The patients were randomized to either ivabradine or placebo. The primary outcome was [cardiovascular] death or heart failure hospitalization. They found that patients who had ivabradine had a decrease in heart failure hospitalization.”
The lesson, she said, is that “ivabradine is a great medication to add on to patients who are still tachycardic in sinus when you cannot titrate up the beta blocker.”
Dr. Williams reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On the medication front, there are now “four pillars of survival” in the setting of heart failure with reduced ejection fraction (EF), a cardiologist told hospitalists recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
The quartet of drugs are beta blockers, angiotensin receptor–neprilysin inhibitors, mineralocorticoid receptor antagonists, and the newest addition – sodium-glucose cotransporter 2 inhibitors.
“If we use all four of these medications, the absolute risk reduction [in mortality] is 25% over a 2-year period,” said cardiologist Celeste T. Williams, MD, of Henry Ford Hospital, Detroit. “So it is very important that we use these medications,” she said.
But managing the medications, she said, can be challenging. Dr. Williams offered these tips about the use of medication in heart failure.
Beta blockers are crucial players
“Beta blockers save lives,” Dr. Williams said, “but there’s always a debate about how much we should titrate beta blockers.”
How can you determine the proper titration? Focus on heart rates, she recommended. “We know that higher heart rates in heart failure patients are associated with worse outcomes. There was subgroup analysis in the BEAUTIFUL study that looked at 5,300 patients with EF less than 40% who had CAD [coronary artery disease]. They found that patients with heart rates greater than 70 had a 34% increased risk of cardiovascular death and a 53% increased risk of heart failure hospitalization compared to heart rates less than 70.”
Focus on getting your patient’s heart rate lower than 70 while maintaining their blood pressure, she said.
“Another question we have is, ‘When these patients come into hospitals, what should we do with the beta blocker? Should we continue it? Should we stop it?’ If you can, you always want to continue the beta blocker or the ACE [angiotensin-converting enzyme] inhibitor, because studies have shown us that the likelihood for patients to be on these medications 90 days later is dismal,” she said. “But you also need to look at the patient. If the patient is in cardiogenic shock, their beta blocker should be stopped.”
Consider multiple factors when titrating various medications
“In the hospital, we always will look at hemodynamic compromise in the patient. Is the patient in cardiogenic shock?” Dr. Williams said. “We also must think about compliance concerns. Are the patients even taking their medication? And if they are taking their medications, are they tolerating standard medical therapy? Are they hypotensive? Are they only able to tolerate minimal meds? Have you seen that their creatine continues to rise? Or are they having poor diuresis with the rise in diuretics?”
All these questions are useful, she said, as you determine whether you should titrate medication yourself or refer the patient to an advanced heart failure specialist.
Understand when to stick with guideline-directed medical therapy
Dr. Williams said another question often arises: “If your patient’s EF recovers, should you stop guideline-directed medical therapy [GDMT]?” She highlighted a TRED-HF study that evaluated patients who had recovered from dilated, nonischemic cardiomyopathy and were receiving GDMT. “They withdrew GDMT for half of the patients and looked at their echoes 6 months later. They found that 40% of the patients relapsed. Their EFs went below 40% again. Stopping medications is not the best idea for most of these patients.”
However, she said, there are scenarios in which GDMT may be withdrawn, such as for patients with tachycardia-induced cardiomyopathies whose EF recovers after ablation, those whose EF recovers after alcoholic cardiomyopathy, and those who receive valve replacements. “We need to remember that a lot of the patients who develop stage C heart failure have risk factors. Even though their heart failure has recovered, they have risks that need to be treated, and you can use the same medications that you use for heart failure to control their risk. Therefore, you would not get into trouble by withdrawing their medications.”
She added: “If you’re unable to titrate GDMT because the blood pressure is too soft, the creatine continues to rise, or the patient just has a lot of heart failure symptoms, this is indicative that the patient is sicker than they may appear.” At this point, defer to a heart failure specialist, she said.
Consider ivabradine as an add-on when appropriate
In some cases, a heart rate of less than 70 bpm will not be achieved even with GDMT and maximum tolerated doses, Dr. Williams said. “If they’re in sinus, you can add on a medication called ivabradine, which was studied in the SHIFT study. This looked at patients with EF of less than 35% who had class 2-3 heart failure in sinus rhythm. They had to have a hospitalization within the last 12 months. The patients were randomized to either ivabradine or placebo. The primary outcome was [cardiovascular] death or heart failure hospitalization. They found that patients who had ivabradine had a decrease in heart failure hospitalization.”
The lesson, she said, is that “ivabradine is a great medication to add on to patients who are still tachycardic in sinus when you cannot titrate up the beta blocker.”
Dr. Williams reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A1c below prediabetes cutoff linked to subclinical atherosclerosis
, according to an analysis of data on almost 4,000 middle-aged individuals.
“If one looks at the incidence of generalized subclinical atherosclerosis, we are not talking small numbers,” senior study author Valentin Fuster, MD, PhD, said in an interview. “We are talking about between 45% and 82% of this middle-age population that already has atherosclerotic disease subclinically.
“Actually,” he added, “the disease was extensive in 5%-30% of these individuals of middle age.”
The study included 3,973 participants from the Progression of Early Subclinical Atherosclerosis study who did not have diabetes. A1c showed an association with the prevalence and multiterritorial extent of subclinical atherosclerosis as measured by two-dimensional ultrasound and coronary artery calcium score (CACS; P < .001). For example, those with A1c above 6.1% (133 participants) had a 33.1% rate of generalized subclinical atherosclerosis, compared with 4.9% for those with A1c below 4.8% (243), the lowest-score group in the study.
Patients in the subprediabetes band, between 5.0% and 5.5%, had significantly higher rates of generalized subclinical atherosclerosis than did the lowest-score group: 8% in the 4.9%-5.0% group (375 participants); 9.9% in the 5.1%-5.2% range (687); 10.3% in the 5.3%-5.4% group (928); and 11.5% in the 5.5%-5.6% group (842).
Those in the 5.1%-5.2% and 5/3%-5.4% A1c groups had a 27% greater chance of having subclinical atherosclerosis, while those in the 5.5%-5.6% group had a 36% greater risk, according to an odds ratio analysis adjusted for established cardiovascular risk factors. The risks were even higher for patients with prediabetes, the researchers reported in the Journal of the American College of Cardiology.
A call for earlier intervention
Notably, the study found that fasting plasma glucose testing did not yield a similar association between A1c and atherosclerosis.
“The message is that we all talk about people when they are close to the development of cardiovascular events, and here we are talking about people who we should pay attention to much earlier,” said Dr. Fuster, physician-in-chief at Icahn School of Medicine at Mount Sinai in New York and director of the National Center for Cardiovascular Investigation in Madrid, where the observational study originated said. “People should be sensitized to HbA1c much more than they would’ve been in the past, and I think this study actually validates that.”
Christie Ballantyne, MD, noted in an interview that these findings support the utility of A1c for predicting CVD risk.
“I think more and more we should be ordering a HbA1c” during routine physical exams, Dr. Ballantyne said. “You don’t have to be obese to get it; there are lots of people, maybe they’re slightly overweight. It’s a reasonable test to be getting when you get to middle age and older to get an idea for assessing for both developing diabetes and also the presence of atherosclerosis and the risk for having cardiovascular events.”
Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center in Houston, coauthored an editorial comment on the study.
Clinicians typically start to manage CVD and diabetes risk “late in the process,” Dr. Ballantyne said. This study suggested that earlier use of antidiabetes therapies, namely peptide-1 agonists and semisynthetic glucagon-like peptide-2 inhibitors, may be warranted in patients with intermediate risk of CVD.
“It’s just more data for the rationale that, perhaps we could end up doing trials to show we can take high-risk people and prevent them from getting both heart disease and diabetes,” Dr. Ballantyne added. “Could we start a little earlier with better precision?”
These finding don’t yet call for a change in how cardiologists and endocrinologists manage patients on the cusp of prediabetes, said Paul S. Jellinger, MD, of Hollywood, Fla., and a professor at the University of Miami. “The endpoint of subclinical atherosclerosis does not necessarily translate into the harder endpoint of CVD events, although there is certainly reason to believe it does,” he said in an interview, noting that he’s often used CACS to stratify atherosclerotic CVD risk in patients.
“I will now consider extending that assessment to patients with lower A1c levels,” he said.
If future studies validate this finding, he said, “serious consideration will have to be made for treating the very large numbers of patients with A1c levels in the prediabetic range and below with antidiabetic agents that have ASCVD prevention properties while lowering A1c. We have those agents today.”
The Progression of Early Subclinical Atherosclerosis study received funding from the National Center for Cardiovascular Investigation in Madrid, Santander Bank, and the Carlos III Health Institute in Madrid. Dr. Fuster had no disclosures. Dr. Ballantyne disclosed receiving research funding through his institution from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostic; and has served as a consultant for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic and Sanofi-Synthélabo.
Dr. Jellinger had no disclosures.
, according to an analysis of data on almost 4,000 middle-aged individuals.
“If one looks at the incidence of generalized subclinical atherosclerosis, we are not talking small numbers,” senior study author Valentin Fuster, MD, PhD, said in an interview. “We are talking about between 45% and 82% of this middle-age population that already has atherosclerotic disease subclinically.
“Actually,” he added, “the disease was extensive in 5%-30% of these individuals of middle age.”
The study included 3,973 participants from the Progression of Early Subclinical Atherosclerosis study who did not have diabetes. A1c showed an association with the prevalence and multiterritorial extent of subclinical atherosclerosis as measured by two-dimensional ultrasound and coronary artery calcium score (CACS; P < .001). For example, those with A1c above 6.1% (133 participants) had a 33.1% rate of generalized subclinical atherosclerosis, compared with 4.9% for those with A1c below 4.8% (243), the lowest-score group in the study.
Patients in the subprediabetes band, between 5.0% and 5.5%, had significantly higher rates of generalized subclinical atherosclerosis than did the lowest-score group: 8% in the 4.9%-5.0% group (375 participants); 9.9% in the 5.1%-5.2% range (687); 10.3% in the 5.3%-5.4% group (928); and 11.5% in the 5.5%-5.6% group (842).
Those in the 5.1%-5.2% and 5/3%-5.4% A1c groups had a 27% greater chance of having subclinical atherosclerosis, while those in the 5.5%-5.6% group had a 36% greater risk, according to an odds ratio analysis adjusted for established cardiovascular risk factors. The risks were even higher for patients with prediabetes, the researchers reported in the Journal of the American College of Cardiology.
A call for earlier intervention
Notably, the study found that fasting plasma glucose testing did not yield a similar association between A1c and atherosclerosis.
“The message is that we all talk about people when they are close to the development of cardiovascular events, and here we are talking about people who we should pay attention to much earlier,” said Dr. Fuster, physician-in-chief at Icahn School of Medicine at Mount Sinai in New York and director of the National Center for Cardiovascular Investigation in Madrid, where the observational study originated said. “People should be sensitized to HbA1c much more than they would’ve been in the past, and I think this study actually validates that.”
Christie Ballantyne, MD, noted in an interview that these findings support the utility of A1c for predicting CVD risk.
“I think more and more we should be ordering a HbA1c” during routine physical exams, Dr. Ballantyne said. “You don’t have to be obese to get it; there are lots of people, maybe they’re slightly overweight. It’s a reasonable test to be getting when you get to middle age and older to get an idea for assessing for both developing diabetes and also the presence of atherosclerosis and the risk for having cardiovascular events.”
Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center in Houston, coauthored an editorial comment on the study.
Clinicians typically start to manage CVD and diabetes risk “late in the process,” Dr. Ballantyne said. This study suggested that earlier use of antidiabetes therapies, namely peptide-1 agonists and semisynthetic glucagon-like peptide-2 inhibitors, may be warranted in patients with intermediate risk of CVD.
“It’s just more data for the rationale that, perhaps we could end up doing trials to show we can take high-risk people and prevent them from getting both heart disease and diabetes,” Dr. Ballantyne added. “Could we start a little earlier with better precision?”
These finding don’t yet call for a change in how cardiologists and endocrinologists manage patients on the cusp of prediabetes, said Paul S. Jellinger, MD, of Hollywood, Fla., and a professor at the University of Miami. “The endpoint of subclinical atherosclerosis does not necessarily translate into the harder endpoint of CVD events, although there is certainly reason to believe it does,” he said in an interview, noting that he’s often used CACS to stratify atherosclerotic CVD risk in patients.
“I will now consider extending that assessment to patients with lower A1c levels,” he said.
If future studies validate this finding, he said, “serious consideration will have to be made for treating the very large numbers of patients with A1c levels in the prediabetic range and below with antidiabetic agents that have ASCVD prevention properties while lowering A1c. We have those agents today.”
The Progression of Early Subclinical Atherosclerosis study received funding from the National Center for Cardiovascular Investigation in Madrid, Santander Bank, and the Carlos III Health Institute in Madrid. Dr. Fuster had no disclosures. Dr. Ballantyne disclosed receiving research funding through his institution from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostic; and has served as a consultant for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic and Sanofi-Synthélabo.
Dr. Jellinger had no disclosures.
, according to an analysis of data on almost 4,000 middle-aged individuals.
“If one looks at the incidence of generalized subclinical atherosclerosis, we are not talking small numbers,” senior study author Valentin Fuster, MD, PhD, said in an interview. “We are talking about between 45% and 82% of this middle-age population that already has atherosclerotic disease subclinically.
“Actually,” he added, “the disease was extensive in 5%-30% of these individuals of middle age.”
The study included 3,973 participants from the Progression of Early Subclinical Atherosclerosis study who did not have diabetes. A1c showed an association with the prevalence and multiterritorial extent of subclinical atherosclerosis as measured by two-dimensional ultrasound and coronary artery calcium score (CACS; P < .001). For example, those with A1c above 6.1% (133 participants) had a 33.1% rate of generalized subclinical atherosclerosis, compared with 4.9% for those with A1c below 4.8% (243), the lowest-score group in the study.
Patients in the subprediabetes band, between 5.0% and 5.5%, had significantly higher rates of generalized subclinical atherosclerosis than did the lowest-score group: 8% in the 4.9%-5.0% group (375 participants); 9.9% in the 5.1%-5.2% range (687); 10.3% in the 5.3%-5.4% group (928); and 11.5% in the 5.5%-5.6% group (842).
Those in the 5.1%-5.2% and 5/3%-5.4% A1c groups had a 27% greater chance of having subclinical atherosclerosis, while those in the 5.5%-5.6% group had a 36% greater risk, according to an odds ratio analysis adjusted for established cardiovascular risk factors. The risks were even higher for patients with prediabetes, the researchers reported in the Journal of the American College of Cardiology.
A call for earlier intervention
Notably, the study found that fasting plasma glucose testing did not yield a similar association between A1c and atherosclerosis.
“The message is that we all talk about people when they are close to the development of cardiovascular events, and here we are talking about people who we should pay attention to much earlier,” said Dr. Fuster, physician-in-chief at Icahn School of Medicine at Mount Sinai in New York and director of the National Center for Cardiovascular Investigation in Madrid, where the observational study originated said. “People should be sensitized to HbA1c much more than they would’ve been in the past, and I think this study actually validates that.”
Christie Ballantyne, MD, noted in an interview that these findings support the utility of A1c for predicting CVD risk.
“I think more and more we should be ordering a HbA1c” during routine physical exams, Dr. Ballantyne said. “You don’t have to be obese to get it; there are lots of people, maybe they’re slightly overweight. It’s a reasonable test to be getting when you get to middle age and older to get an idea for assessing for both developing diabetes and also the presence of atherosclerosis and the risk for having cardiovascular events.”
Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center in Houston, coauthored an editorial comment on the study.
Clinicians typically start to manage CVD and diabetes risk “late in the process,” Dr. Ballantyne said. This study suggested that earlier use of antidiabetes therapies, namely peptide-1 agonists and semisynthetic glucagon-like peptide-2 inhibitors, may be warranted in patients with intermediate risk of CVD.
“It’s just more data for the rationale that, perhaps we could end up doing trials to show we can take high-risk people and prevent them from getting both heart disease and diabetes,” Dr. Ballantyne added. “Could we start a little earlier with better precision?”
These finding don’t yet call for a change in how cardiologists and endocrinologists manage patients on the cusp of prediabetes, said Paul S. Jellinger, MD, of Hollywood, Fla., and a professor at the University of Miami. “The endpoint of subclinical atherosclerosis does not necessarily translate into the harder endpoint of CVD events, although there is certainly reason to believe it does,” he said in an interview, noting that he’s often used CACS to stratify atherosclerotic CVD risk in patients.
“I will now consider extending that assessment to patients with lower A1c levels,” he said.
If future studies validate this finding, he said, “serious consideration will have to be made for treating the very large numbers of patients with A1c levels in the prediabetic range and below with antidiabetic agents that have ASCVD prevention properties while lowering A1c. We have those agents today.”
The Progression of Early Subclinical Atherosclerosis study received funding from the National Center for Cardiovascular Investigation in Madrid, Santander Bank, and the Carlos III Health Institute in Madrid. Dr. Fuster had no disclosures. Dr. Ballantyne disclosed receiving research funding through his institution from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostic; and has served as a consultant for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic and Sanofi-Synthélabo.
Dr. Jellinger had no disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY