User login
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
20-year-old woman • 2 syncopal episodes • nausea • dizziness • Dx?
THE CASE
A 20-year-old woman presented to clinic with a chief complaint of 2 syncopal episodes within 10 minutes of each other. She reported that in both cases, she felt nauseated and dizzy before losing consciousness. She lost consciousness for a few seconds during the first episode and a few minutes during the second episode. Both episodes were unwitnessed.
The patient denied any fasting, vomiting, diarrhea, palpitations, chest pain, incontinence, oral trauma, headaches, fevers, chills, or tremors. Her last menstrual period started 3 days prior to presentation. The patient was taking sertraline 25 mg once daily for anxiety and depression and norethindrone acetate–ethinyl estradiol tablets 20 µg daily for birth control. She also was finishing a 7-day course of metronidazole for bacterial vaginosis. She reported having started the sertraline about 10 days prior to the syncopal episodes. She denied any personal history of drug or alcohol use, syncope, seizures, or any other medical conditions. Family history was negative for any cardiac or neurologic conditions.
The patient appeared euvolemic on exam. Overall, the review of the respiratory, cardiac, and neurologic systems was unremarkable. An electrocardiogram, obtained in clinic, showed a normal sinus rhythm and QT interval. Orthostatic blood pressure and heart rate measurements were as follows: supine, 122/83 mm Hg and 67 beats/min; seated, 118/87 mm Hg and 60 beats/min; and standing, 123/83 mm Hg and 95 beats/min. In addition to the increase in pulse between sitting and standing, the patient reported feeling nauseated when transitioning to a standing position.
Laboratory work-up included a comprehensive metabolic panel, complete blood count, and thyroid-stimulating hormone test. The results showed mild erythrocytosis with a hematocrit and hemoglobin of 46.1% and 15.6 g/dL respectively, as well as mild hypercalcemia (10.4 mg/dL).
THE DIAGNOSIS
An increase in heart rate of more than 30 beats/min when the patient went from a sitting to a standing position pointed to a diagnosis of postural orthostatic tachycardia syndrome (POTS). This prompted us to stop the sertraline.
DISCUSSION
POTS is a type of intolerance to orthostasis related to a significant increase in pulse without resulting hypotension upon standing. Other symptoms that accompany this change in position include dizziness, lightheadedness, blurry vision, and fatigue. Syncope occurs in about 40% of patients with POTS, which may be more frequent than for patients with orthostatic hypotension.1
The overall prevalence of POTS is 0.2% to 1%; however, it is generally seen in a 5:1 female-to-male ratio.2,3 POTS is often idiopathic. That said, it can also be caused by medication adverse effects, hypovolemia, and stressors, including vaccinations, viral infections, trauma, and emotional triggers. On physical exam, this patient did not appear to be hypovolemic, and she reported normal oral intake prior to this visit. Since the patient had started taking sertraline about 10 days prior to her syncopal episodes, we suspected POTS secondary to sertraline use was the likely etiology in this otherwise healthy young woman.
Continue to: Syncope could indicate a larger cardiovascular problem
Syncope could indicate a larger cardiovascular problem
The differential diagnosis of dizziness with loss of consciousness includes anemia, vasovagal syncope, orthostatic hypotension, dehydration, electrolyte imbalance, arrhythmia, prolonged QT syndrome, cardiac valve or structure abnormality, and seizure. Most of these differentials can be ruled out from basic laboratory tests or cardiac imaging. In POTS, the diagnostic work-up is essentially normal compared to other causes of syncope. Orthostatic hypotension, for example, is similar; however, there is an additional change in the arterial blood pressure.
Unintended adverse effects
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, are known to have fewer cardiovascular adverse effects compared to older antidepressants such as tricyclic antidepressants and monoamine oxidase inhibitors.4 However, case reports have shown an association between SSRIs and syncope.4-6 SSRIs have also been tied to increased heart rate variability.7
Nearly 2 weeks after stopping sertraline, our patient presented to clinic and was given a diagnosis of streptococcal pharyngitis. She said she’d had no additional syncopal episodes. Twenty days after sertraline cessation, the patient returned for follow-up. Her blood pressure and heart rate were as follows: supine, 112/68 mm Hg and 61 beats/min; seated, 113/74 mm Hg and 87 beats/min; and standing, 108/74 mm Hg and 78 beats/min.
Thus, after cessation of sertraline, her orthostatic heart rate changes were smaller than when she was first examined. Her vital signs showed an increase in pulse of 26 beats/min between lying and sitting, without any reports of nausea. She had no further complaints of dizziness or syncopal episodes.
THE TAKEAWAY
We don’t always know how a patient will respond to a newly prescribed medication or lifestyle change. A proper review of a patient’s history and medication use is a pivotal first step in making any diagnosis.
CORRESPONDENCE
Courtney Lynn Dominguez, MD, 4220 North Roxboro Street, Durham, NC 27704; courtney.dominguez@duke.edu
1. Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245-249. doi: 10.1016/j.amjmed.2009.09.018
2. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3-11. doi: 10.1016/j.autneu.2018.02.005
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2018;285:352-366. doi:10.1111/joim.12852
4. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-390.
5. Feder R. Bradycardia and syncope induced by fluoxetine. J Clin Psychiatry. 1991;52:139.
6. Ellison JM, Milofsky JE, Ely E. Fluoxetine-induced bradycardia and syncope in two patients. J Clin Psychiatry. 1990;51:385-386.
7. Tucker P, Adamson P, Miranda R Jr, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370-376. doi: 10.1097/00004714-199710000-00006
THE CASE
A 20-year-old woman presented to clinic with a chief complaint of 2 syncopal episodes within 10 minutes of each other. She reported that in both cases, she felt nauseated and dizzy before losing consciousness. She lost consciousness for a few seconds during the first episode and a few minutes during the second episode. Both episodes were unwitnessed.
The patient denied any fasting, vomiting, diarrhea, palpitations, chest pain, incontinence, oral trauma, headaches, fevers, chills, or tremors. Her last menstrual period started 3 days prior to presentation. The patient was taking sertraline 25 mg once daily for anxiety and depression and norethindrone acetate–ethinyl estradiol tablets 20 µg daily for birth control. She also was finishing a 7-day course of metronidazole for bacterial vaginosis. She reported having started the sertraline about 10 days prior to the syncopal episodes. She denied any personal history of drug or alcohol use, syncope, seizures, or any other medical conditions. Family history was negative for any cardiac or neurologic conditions.
The patient appeared euvolemic on exam. Overall, the review of the respiratory, cardiac, and neurologic systems was unremarkable. An electrocardiogram, obtained in clinic, showed a normal sinus rhythm and QT interval. Orthostatic blood pressure and heart rate measurements were as follows: supine, 122/83 mm Hg and 67 beats/min; seated, 118/87 mm Hg and 60 beats/min; and standing, 123/83 mm Hg and 95 beats/min. In addition to the increase in pulse between sitting and standing, the patient reported feeling nauseated when transitioning to a standing position.
Laboratory work-up included a comprehensive metabolic panel, complete blood count, and thyroid-stimulating hormone test. The results showed mild erythrocytosis with a hematocrit and hemoglobin of 46.1% and 15.6 g/dL respectively, as well as mild hypercalcemia (10.4 mg/dL).
THE DIAGNOSIS
An increase in heart rate of more than 30 beats/min when the patient went from a sitting to a standing position pointed to a diagnosis of postural orthostatic tachycardia syndrome (POTS). This prompted us to stop the sertraline.
DISCUSSION
POTS is a type of intolerance to orthostasis related to a significant increase in pulse without resulting hypotension upon standing. Other symptoms that accompany this change in position include dizziness, lightheadedness, blurry vision, and fatigue. Syncope occurs in about 40% of patients with POTS, which may be more frequent than for patients with orthostatic hypotension.1
The overall prevalence of POTS is 0.2% to 1%; however, it is generally seen in a 5:1 female-to-male ratio.2,3 POTS is often idiopathic. That said, it can also be caused by medication adverse effects, hypovolemia, and stressors, including vaccinations, viral infections, trauma, and emotional triggers. On physical exam, this patient did not appear to be hypovolemic, and she reported normal oral intake prior to this visit. Since the patient had started taking sertraline about 10 days prior to her syncopal episodes, we suspected POTS secondary to sertraline use was the likely etiology in this otherwise healthy young woman.
Continue to: Syncope could indicate a larger cardiovascular problem
Syncope could indicate a larger cardiovascular problem
The differential diagnosis of dizziness with loss of consciousness includes anemia, vasovagal syncope, orthostatic hypotension, dehydration, electrolyte imbalance, arrhythmia, prolonged QT syndrome, cardiac valve or structure abnormality, and seizure. Most of these differentials can be ruled out from basic laboratory tests or cardiac imaging. In POTS, the diagnostic work-up is essentially normal compared to other causes of syncope. Orthostatic hypotension, for example, is similar; however, there is an additional change in the arterial blood pressure.
Unintended adverse effects
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, are known to have fewer cardiovascular adverse effects compared to older antidepressants such as tricyclic antidepressants and monoamine oxidase inhibitors.4 However, case reports have shown an association between SSRIs and syncope.4-6 SSRIs have also been tied to increased heart rate variability.7
Nearly 2 weeks after stopping sertraline, our patient presented to clinic and was given a diagnosis of streptococcal pharyngitis. She said she’d had no additional syncopal episodes. Twenty days after sertraline cessation, the patient returned for follow-up. Her blood pressure and heart rate were as follows: supine, 112/68 mm Hg and 61 beats/min; seated, 113/74 mm Hg and 87 beats/min; and standing, 108/74 mm Hg and 78 beats/min.
Thus, after cessation of sertraline, her orthostatic heart rate changes were smaller than when she was first examined. Her vital signs showed an increase in pulse of 26 beats/min between lying and sitting, without any reports of nausea. She had no further complaints of dizziness or syncopal episodes.
THE TAKEAWAY
We don’t always know how a patient will respond to a newly prescribed medication or lifestyle change. A proper review of a patient’s history and medication use is a pivotal first step in making any diagnosis.
CORRESPONDENCE
Courtney Lynn Dominguez, MD, 4220 North Roxboro Street, Durham, NC 27704; courtney.dominguez@duke.edu
THE CASE
A 20-year-old woman presented to clinic with a chief complaint of 2 syncopal episodes within 10 minutes of each other. She reported that in both cases, she felt nauseated and dizzy before losing consciousness. She lost consciousness for a few seconds during the first episode and a few minutes during the second episode. Both episodes were unwitnessed.
The patient denied any fasting, vomiting, diarrhea, palpitations, chest pain, incontinence, oral trauma, headaches, fevers, chills, or tremors. Her last menstrual period started 3 days prior to presentation. The patient was taking sertraline 25 mg once daily for anxiety and depression and norethindrone acetate–ethinyl estradiol tablets 20 µg daily for birth control. She also was finishing a 7-day course of metronidazole for bacterial vaginosis. She reported having started the sertraline about 10 days prior to the syncopal episodes. She denied any personal history of drug or alcohol use, syncope, seizures, or any other medical conditions. Family history was negative for any cardiac or neurologic conditions.
The patient appeared euvolemic on exam. Overall, the review of the respiratory, cardiac, and neurologic systems was unremarkable. An electrocardiogram, obtained in clinic, showed a normal sinus rhythm and QT interval. Orthostatic blood pressure and heart rate measurements were as follows: supine, 122/83 mm Hg and 67 beats/min; seated, 118/87 mm Hg and 60 beats/min; and standing, 123/83 mm Hg and 95 beats/min. In addition to the increase in pulse between sitting and standing, the patient reported feeling nauseated when transitioning to a standing position.
Laboratory work-up included a comprehensive metabolic panel, complete blood count, and thyroid-stimulating hormone test. The results showed mild erythrocytosis with a hematocrit and hemoglobin of 46.1% and 15.6 g/dL respectively, as well as mild hypercalcemia (10.4 mg/dL).
THE DIAGNOSIS
An increase in heart rate of more than 30 beats/min when the patient went from a sitting to a standing position pointed to a diagnosis of postural orthostatic tachycardia syndrome (POTS). This prompted us to stop the sertraline.
DISCUSSION
POTS is a type of intolerance to orthostasis related to a significant increase in pulse without resulting hypotension upon standing. Other symptoms that accompany this change in position include dizziness, lightheadedness, blurry vision, and fatigue. Syncope occurs in about 40% of patients with POTS, which may be more frequent than for patients with orthostatic hypotension.1
The overall prevalence of POTS is 0.2% to 1%; however, it is generally seen in a 5:1 female-to-male ratio.2,3 POTS is often idiopathic. That said, it can also be caused by medication adverse effects, hypovolemia, and stressors, including vaccinations, viral infections, trauma, and emotional triggers. On physical exam, this patient did not appear to be hypovolemic, and she reported normal oral intake prior to this visit. Since the patient had started taking sertraline about 10 days prior to her syncopal episodes, we suspected POTS secondary to sertraline use was the likely etiology in this otherwise healthy young woman.
Continue to: Syncope could indicate a larger cardiovascular problem
Syncope could indicate a larger cardiovascular problem
The differential diagnosis of dizziness with loss of consciousness includes anemia, vasovagal syncope, orthostatic hypotension, dehydration, electrolyte imbalance, arrhythmia, prolonged QT syndrome, cardiac valve or structure abnormality, and seizure. Most of these differentials can be ruled out from basic laboratory tests or cardiac imaging. In POTS, the diagnostic work-up is essentially normal compared to other causes of syncope. Orthostatic hypotension, for example, is similar; however, there is an additional change in the arterial blood pressure.
Unintended adverse effects
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, are known to have fewer cardiovascular adverse effects compared to older antidepressants such as tricyclic antidepressants and monoamine oxidase inhibitors.4 However, case reports have shown an association between SSRIs and syncope.4-6 SSRIs have also been tied to increased heart rate variability.7
Nearly 2 weeks after stopping sertraline, our patient presented to clinic and was given a diagnosis of streptococcal pharyngitis. She said she’d had no additional syncopal episodes. Twenty days after sertraline cessation, the patient returned for follow-up. Her blood pressure and heart rate were as follows: supine, 112/68 mm Hg and 61 beats/min; seated, 113/74 mm Hg and 87 beats/min; and standing, 108/74 mm Hg and 78 beats/min.
Thus, after cessation of sertraline, her orthostatic heart rate changes were smaller than when she was first examined. Her vital signs showed an increase in pulse of 26 beats/min between lying and sitting, without any reports of nausea. She had no further complaints of dizziness or syncopal episodes.
THE TAKEAWAY
We don’t always know how a patient will respond to a newly prescribed medication or lifestyle change. A proper review of a patient’s history and medication use is a pivotal first step in making any diagnosis.
CORRESPONDENCE
Courtney Lynn Dominguez, MD, 4220 North Roxboro Street, Durham, NC 27704; courtney.dominguez@duke.edu
1. Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245-249. doi: 10.1016/j.amjmed.2009.09.018
2. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3-11. doi: 10.1016/j.autneu.2018.02.005
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2018;285:352-366. doi:10.1111/joim.12852
4. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-390.
5. Feder R. Bradycardia and syncope induced by fluoxetine. J Clin Psychiatry. 1991;52:139.
6. Ellison JM, Milofsky JE, Ely E. Fluoxetine-induced bradycardia and syncope in two patients. J Clin Psychiatry. 1990;51:385-386.
7. Tucker P, Adamson P, Miranda R Jr, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370-376. doi: 10.1097/00004714-199710000-00006
1. Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245-249. doi: 10.1016/j.amjmed.2009.09.018
2. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3-11. doi: 10.1016/j.autneu.2018.02.005
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2018;285:352-366. doi:10.1111/joim.12852
4. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-390.
5. Feder R. Bradycardia and syncope induced by fluoxetine. J Clin Psychiatry. 1991;52:139.
6. Ellison JM, Milofsky JE, Ely E. Fluoxetine-induced bradycardia and syncope in two patients. J Clin Psychiatry. 1990;51:385-386.
7. Tucker P, Adamson P, Miranda R Jr, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370-376. doi: 10.1097/00004714-199710000-00006
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
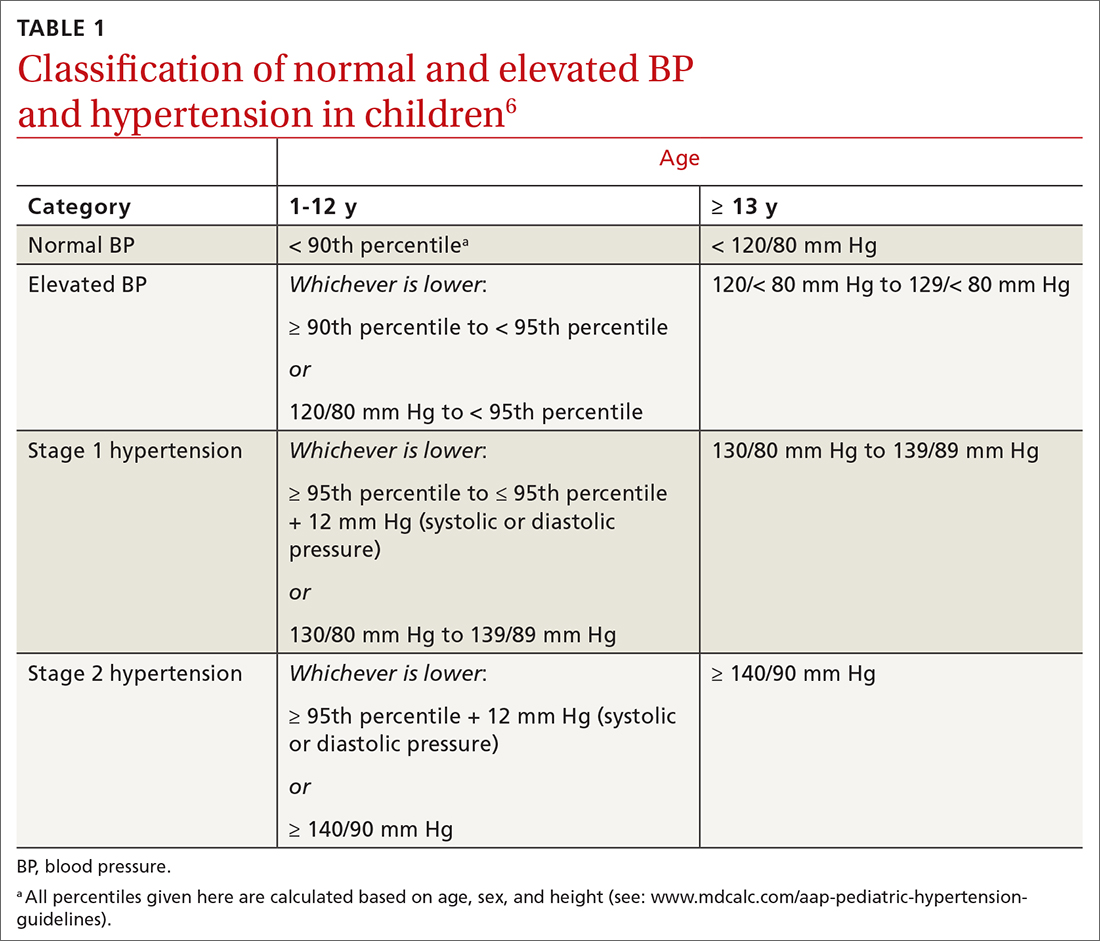
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16
Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
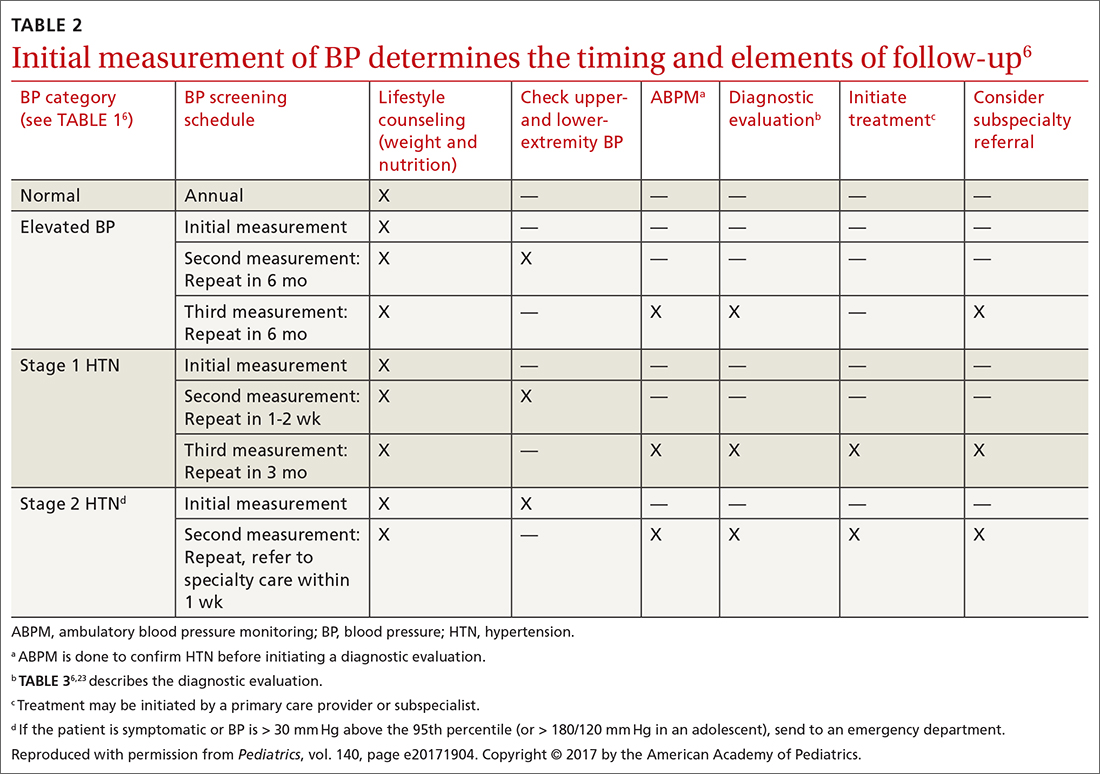
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
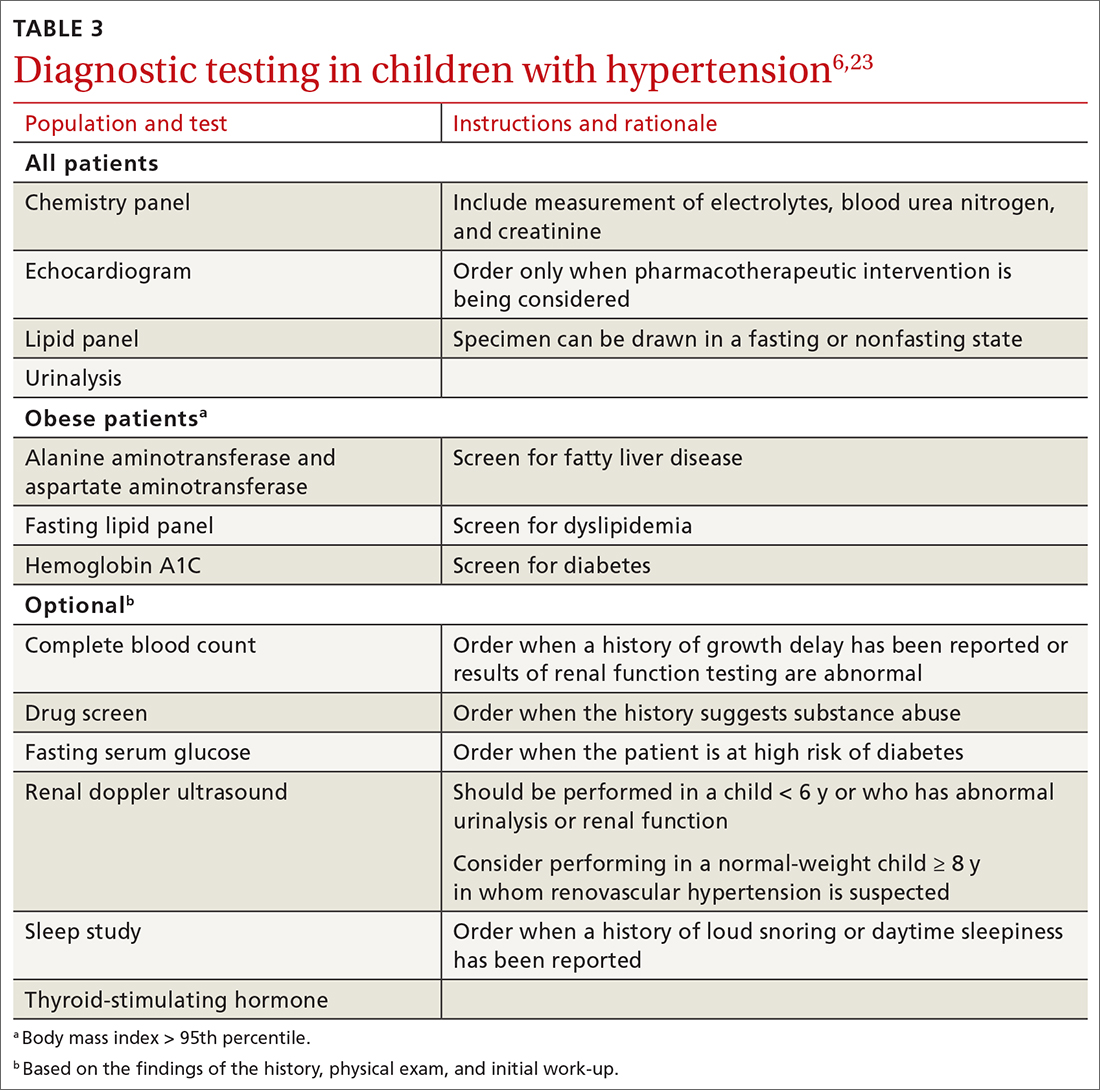
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
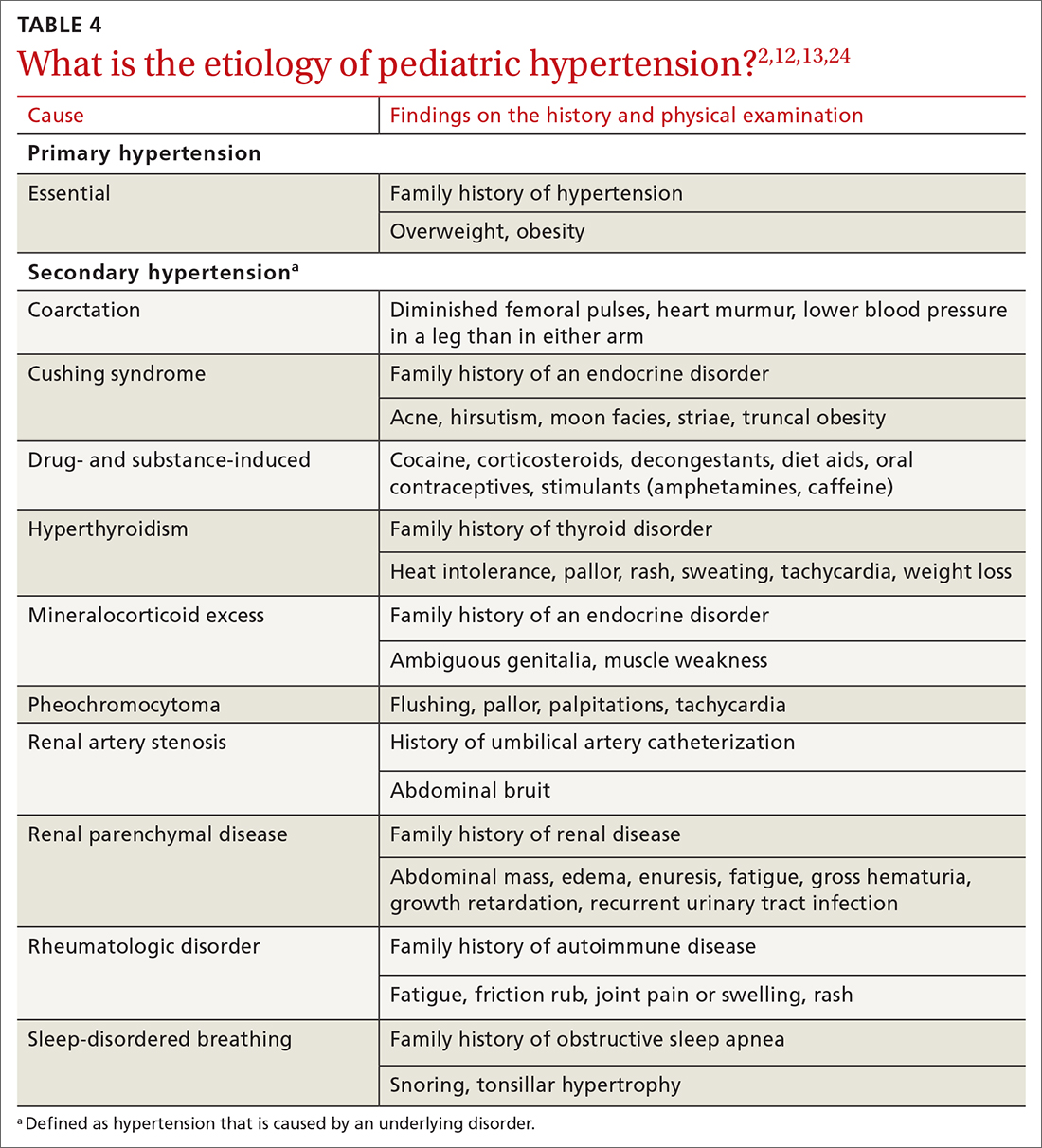
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.
Mapping out the treatment plan
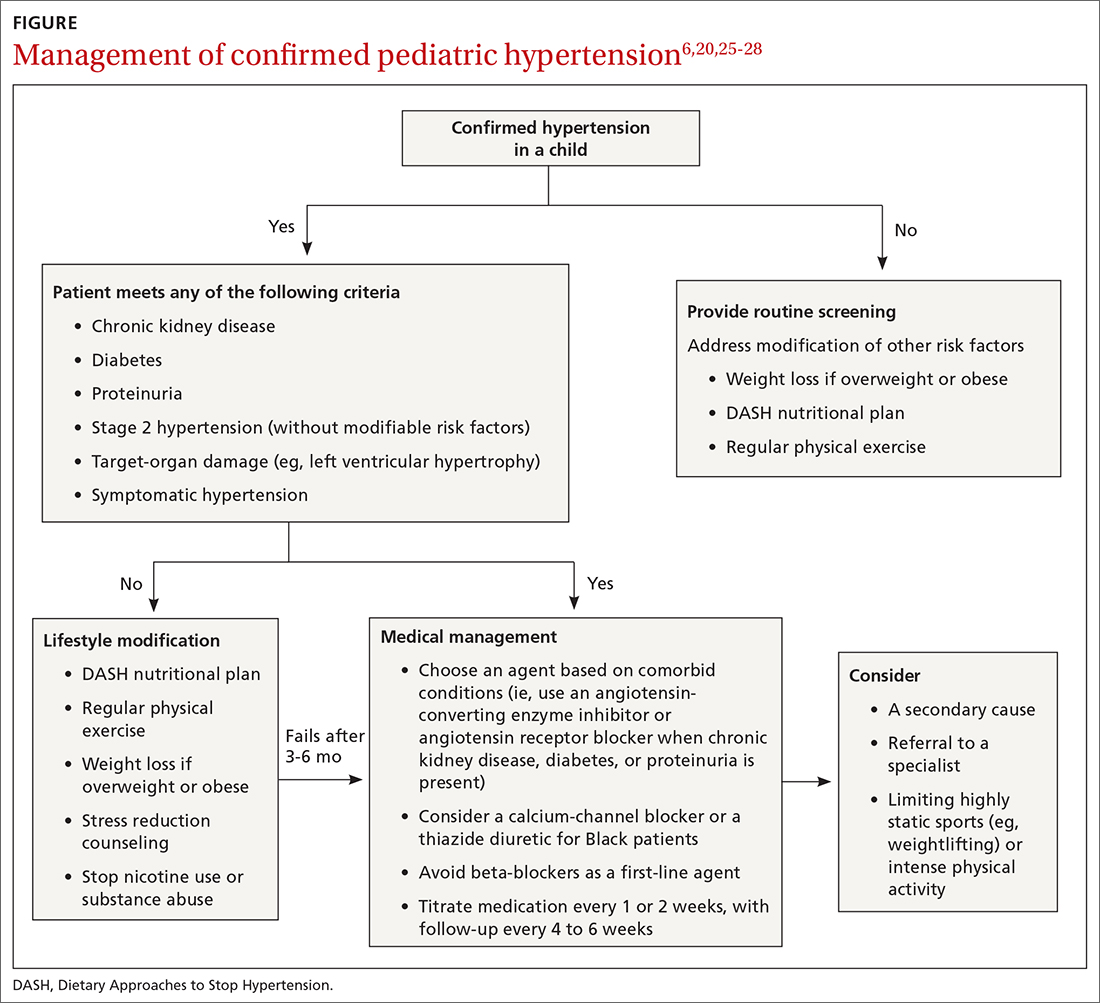
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; dustinksmith@yahoo.com
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16
Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.
Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; dustinksmith@yahoo.com
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16
Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.
Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; dustinksmith@yahoo.com
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Healthy with obesity? The latest study casts doubt
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
compared with people without obesity and or adverse metabolic profiles, new research suggests.
The latest data on this controversial subject come from an analysis of nearly 400,000 people in the U.K. Biobank. Although the data also showed that metabolically healthy obesity poses less risk than “metabolically unhealthy” obesity, the risk of progression from healthy to unhealthy within 3-5 years was high.
“People with metabolically healthy obesity are not ‘healthy’ as they are at higher risk of atherosclerotic cardiovascular disease [ASCVD], heart failure, and respiratory diseases, compared with nonobese people with a normal metabolic profile. As such, weight management could be beneficial to all people with obesity irrespective of metabolic profile,” Ziyi Zhou and colleagues wrote in their report, published June 10, 2021, in Diabetologia.
Moreover, they advised avoiding the term metabolically healthy obesity entirely in clinical medicine “as it is misleading, and different strategies for risk stratification should be explored.”
In interviews, two experts provided somewhat different takes on the study and the overall subject.
‘Lifestyle should be explored with every single patient regardless of their weight’
Yoni Freedhoff, MD, medical director of the Bariatric Medical Institute, Ottawa, said “clinicians and patients need to be aware that obesity increases a person’s risk of various medical problems, and in turn this might lead to more frequent screening. This increased screening might be analogous to that of a person with a strong familial history of cancer who of course we would never describe as being ‘unhealthy’ as a consequence of their increased risk.”
In addition to screening, “lifestyle should be explored with every single patient regardless of their weight, and if a person’s weight is not affecting their health or their quality of life, a clinician need only let the patient know that, were they to want to discuss weight management options in the future, that they’d be there for them,” said Dr. Freedhoff.
‘Metabolically healthy obesity’ has had many definitions
Matthias Schulze, DrPH, head of the molecular epidemiology at the German Institute of Human Nutrition, Potsdam, and professor at the University of Potsdam, pointed out that the way metabolically healthy obesity is defined and the outcomes assessed make a difference.
In the current study, the term is defined as having a body mass index of at least 30 kg/m2 and at least four of six metabolically healthy criteria: blood pressure, C-reactive protein, triacylglycerols, LDL cholesterol, HDL cholesterol, and hemoglobin A1c.
In May 2021, Dr. Schulze and associates reported in JAMA Network Open on a different definition that they found to identify individuals who do not have an increased risk of cardiovascular disease death and total mortality. Interestingly, they also used the U.K. Biobank as their validation cohort.
“We derived a new definition of metabolic health ... that is different from those used in [the current] article. Importantly, we included a measure of body fat distribution, waist-to-hip ratio. On the other side, we investigated only mortality outcomes and we can therefore not exclude the possibility that other outcomes may still be related. [For example], a higher diabetes risk may still be present among those we have defined as having metabolically healthy obesity.”
Dr. Schulze also said that several previous studies and meta-analyses have suggested that “previous common definitions of metabolically healthy obesity do not identify a subgroup without risk, or being at risk comparable to normal-weight metabolically healthy. Thus, this study confirms this conclusion. [But] this doesn’t rule out that there are better ways of defining subgroups.”
Clinically, he said “given that we investigated only mortality, we cannot conclude that our ‘metabolically healthy obesity’ group doesn’t require intervention.”
Higher rates of diabetes, ASCVD, heart failure, death
The current population-based study included 381,363 U.K. Biobank participants who were followed up for a median 11.2 years. Overall, about 55% did not have obesity or metabolic abnormalities, 9% had metabolically healthy obesity, 20% were metabolically unhealthy but did not have obesity, and 16% had metabolically unhealthy obesity as defined by the investigators.
The investigators adjusted the data for several potential confounders, including age, sex, ethnicity, education, socioeconomic status, smoking status, physical activity, and dietary factors.
Compared with individuals without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher rates of incident diabetes (hazard ratio, 4.32), ASCVD (HR, 1.18), myocardial infarction (HR, 1.23), stroke (HR, 1.10), heart failure (HR, 1.76), respiratory diseases (HR, 1.28), and chronic obstructive pulmonary disease (HR, 1.19).
In general, rates of cardiovascular and respiratory outcomes were highest in metabolically unhealthy obesity, followed by those without obesity but with metabolic abnormalities and those with metabolically healthy obesity. However, for incident and fatal heart failure and incident respiratory diseases, those with metabolically healthy obesity had higher rates than did those without obesity but with metabolic abnormalities.
Compared with those without obesity or metabolic abnormalities, those with metabolically healthy obesity had significantly higher all-cause mortality rates (HR, 1.22). And, compared with those without obesity (regardless of metabolic status) at baseline, those with metabolically healthy obesity were significantly more likely to have diabetes (HR, 2.06), heart failure (HR, 1.6), and respiratory diseases (HR, 1.2), but not ASCVD. The association was also significant for all-cause and heart failure mortality (HR, 1.12 and 1.44, respectively), but not for other causes of death.
Progression from metabolically healthy to unhealthy is common
Among 8,512 participants for whom longitudinal data were available for a median of 4.4 years, half of those with metabolically healthy obesity remained in that category, 20% no longer had obesity, and more than a quarter transitioned to metabolically unhealthy obesity. Compared with those without obesity or metabolic abnormalities throughout, those who transitioned from metabolically healthy to metabolically unhealthy had significantly higher rates of incident ASCVD (HR, 2.46) and all-cause mortality (HR, 3.07).
But those who remained in the metabolically healthy obesity category throughout did not have significantly increased risks for the adverse outcomes measured.
Ms. Zhou and colleagues noted that the data demonstrate heterogeneity among people with obesity, which offers the potential to stratify risk based on prognosis. For example, “people with [metabolically unhealthy obesity] were at a higher risk of mortality and morbidity than everyone else, and thus they should be prioritized for intervention.”
However, they add, “Obesity is associated with a wide range of diseases, and using a single label or categorical risk algorithm is unlikely to be effective compared with prediction algorithms based on disease-specific and continuous risk markers.”
Ms. Zhou has no disclosures. One coauthor has relationships with numerous pharmaceutical companies; the rest have none. Dr. Freedhoff has served as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health. He is a speaker or a member of a speakers bureau for Obesity Canada and Novo Nordisk, received research grant from Novo Nordisk, and received income of at least $250 from WebMD, CTV, and Random House. Dr/ Schulze has received grants from German Federal Ministry of Education and Research.
FROM DIABETOLOGIA
Third COVID-19 vaccine dose helped some transplant recipients
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
Simple risk assessment predicts post-PCI ischemic events
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
A patient’s risk for ischemic events, but not bleeding, after percutaneous coronary intervention (PCI) can be predicted simply based on whether they have one or more guideline-based standardized risk criteria, a large-scale real-world analysis suggests.
Haoyu Wang, MD, and colleagues showed that having at least one high-risk feature, as outlined in the 2018 European Society of Cardiology and European Association for Cardiothoracic Surgery (ESC/EACTS) Guidelines on Myocardial Revascularization, was associated with an increased risk for target vessel failure by 48% and for a patient-oriented composite outcome by 44%.
Moreover, they showed that implantation of at least three stents and the presence of diabetes and diffuse multivessel disease were the only high-risk features from the guidelines that were independent predictors of the two outcomes.
The study of more than 10,000 PCI patients also showed that determining whether patients were at high bleeding risk (HBR) did not modify their ischemic risk.
This, said Dr. Wang, from the National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, underscores the importance of applying the high ischemic risk (HIR) criteria from the ESC/EACTS guidelines when tailoring dual antiplatelet therapy (DAPT).
The research was presented at the European Atherosclerosis Society 2021 Virtual Congress on June 2, and published online in the Journal of Atherosclerosis and Thrombosis.
Dr. Wang told theheart.org | Medscape Cardiology that they conducted the study to determine which – HIR or HBR – is “most important to balance when treating patients undergoing PCI and then having dual antiplatelet therapy.”
The results showed that when patients have both a HIR and HBR, it is the ESC/EACTS guideline HIR criteria that have “a higher impact” than the bleeding risk, and that this can be “used to guide our choice of the duration of dual anti-platelet therapy.”
“Maybe we can extend, or use more potent, P2Y12 inhibitors” in those situations, he said.
S. Lale Tokgözoglu, MD, PhD, professor of cardiology, Hacettepe University, Ankara, Turkey, who was not involved in the study, said the HIR assessment “performed well,” adding that the HBR score might have been expected to attenuate its “prognostic advantage.”
She told this news organization that the results “are interesting since previous observations have suggested that Asian patients may be more prone to medication side effects and bleeding.”
These findings emphasize the importance of assessing HIR in daily PCI practice and confirm that it “performs well in different populations in real life,” added Dr. Tokgözoglu, a former president of the EAS.
The ESC/EACTS guidelines aimed to standardize the definition of HIR, Dr. Wang said during the presentation.
They set out 10 high-risk features for ischemic events for patients undergoing revascularization, which included patient medical history, comorbid conditions, and the characteristics of the PCI procedure.
Although the goals of the criteria are to inform decision-making and stimulate research, Dr. Wang said that their “prevalence and prognostic association with clinical outcomes are yet to be established in real-world PCI practice.”
Alongside, the Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score was developed to predict out-of-hospital bleeding in patients receiving DAPT after stent implantation.
Although a PRECISE-DAPT score of at least 25 constitutes a patient at high bleeding risk, Dr. Wang pointed out that such patients are typically also at risk for ischemic events after PCI, and it is “unclear” whether being at HBR modifies this risk.
To investigate further, they used the prospective, real-world Fuwai PCI registry to collate an all-comer patient population with unselected use of drug-eluting stents at the National Center for Cardiovascular Diseases at Fuwai Hospital.
They excluded individuals who were treated with balloon angioplasty alone, bioresorbable scaffolds, or bare metal stents, leaving a total population of 10,167 patients who were treated in 2013.
In that cohort, 5,149 patients (50.6%) met at least one risk criterion from the ESC/EACTS guidelines (HIR patients) and 5,018 (49.4%) met none of the risk criteria (non-HIR patients).
The most common criteria were implantation of at least three stents (23.5%); total stent length greater than 60 mm (20.2%); diffuse multivessel disease, especially in diabetic patients (18.5%); and a history of ST-segment elevation myocardial infarction (13.9%).
HIR patients were significantly older than non-HIR patients (average age, 58.86 vs. 57.77 years; P < .001), were more likely to have diabetes mellitus (42.6% vs. 16.9%; P < .001); and were more likely to have already had a myocardial infarction (32.2% vs. 5.2%; P < .001).
HIR patients also had higher average PRECISE-ADAPT scores than those without HIR (11.22 vs. 9.94; P < .001), and were conversely less likely to have the left anterior descending artery as the target vessel than non-HIR patients (86.0% vs. 94.6%; P < .001).
Cox regression analysis taking into account a range of patient and clinical factors revealed that HIR patients were significantly more likely than their non-HIR counterparts to experience target vessel failure (hazard ratio, 1.48; 95% confidence interval, 1.25-1.74; P < .001).
They were also significantly more likely to have a patient-oriented composite outcome, defined as all-cause death, any myocardial infarction, or any revascularization (HR, 1.44; 95% CI, 1.28-1.63; P < .001).
There was also a significantly higher risk for cardiac death in HIR than in non-HIR patients (HR, 1.95; 95% CI, 1.16-3.29; P = .012).
However, there was no significant association between HIR status and clinically relevant bleeding (HR, 0.84; 95% CI, 0.66-1.06; P = .143).
When the researchers looked at individual ischemic risk features, they found that, on fully adjusted analyses, only two were independent predictors of target vessel failure and the patient-oriented composite outcome.
Having at least three stents implanted was significantly associated with target vessel failure (HR, 1.36; 95% CI, 1.02-1.80; P = .038), and borderline significantly associated with the patient oriented composite outcome (HR, 1.23; 95% CI, 1.00-1.53; P = .056).
Diffuse multivessel disease, especially in diabetic patients, was significantly associated with both target vessel failure (HR, 1.24; 95% CI, 1.02-1.51; P = .035) and with the patient-oriented composite outcome (HR, 1.20; 95% CI, 1.04-1.39; P = .012).
Neither risk feature was significantly associated with clinically relevant bleeding, Dr. Wang noted.
Stratifying the patients by HBR status, the team found that rates of target vessel failure, the patient-oriented composite outcome, cardiac death, myocardial infarction, and definite/probable stent thrombosis were higher in patients with both HIR and HBR than those with neither HIR nor HBR (P < .001).
Further stratifying patients by PRECISE-ADAPT scores – 10 or less indicating very low risk, 11-17 indicating low risk, 18-24 indicating moderate risk, and at least 25 indicating high risk – showed that HIR features had a consistent effect on ischemic and bleeding outcomes, regardless of bleeding risk.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
FDA: More metformin extended-release tablets recalled
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
FROM THE FOOD AND DRUG ADMINISTRATION
Eat two fruits a day, ward off diabetes?
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
A new study supports the recommendation of eating two servings of fruit a day for health benefits – in this case a lower risk of diabetes.
Adults who ate two servings of fruit a day had 36% lower odds of developing diabetes within 5 years compared to those who ate less than a half serving of fruit a day, after adjusting for confounders, in a population-based Australian study.
The findings by Nicola P. Bondonno, PhD, and colleagues, based on data from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab), were published online June 2 in the Journal of Clinical Endocrinology & Metabolism.
The study also showed that a higher fruit intake was associated with higher insulin sensitivity and lower pancreatic beta-cell function in a dose-response manner.
And a higher intake of apples – but not citrus fruit or bananas, the two other fruits studied – was associated with lower post-load serum insulin levels.
“This indicates that people who consumed more fruit [especially apples] had to produce less insulin to lower their blood glucose levels,” Dr. Bondonno, from the Institute for Nutrition Research, Edith Cowan University, Perth, Australia, explained in a statement from the Endocrine Society.
“This is important since high levels of circulating insulin (hyperinsulinemia) can damage blood vessels” and this is “related not only to diabetes, but also to high blood pressure, obesity, and heart disease,” she observed.
Fruit juice doesn’t have same effect
The study supports the recommendation of the Australian Dietary Guidelines – 2 servings of fruit a day, where one serving is 150 grams, which corresponds to a medium-sized apple, orange, or banana – Dr. Bondonno clarified in an email.
However, fruit juice was not associated with better glucose or insulin levels, or lower risk of diabetes, possibly because of its relatively high glycemic load and fewer beneficial fibers, the researchers speculate; added data suggest that even juice with added fiber does not trigger satiety.
The study findings “support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate [type 2 diabetes] risk,” Dr. Bondonno and colleagues summarize.
“Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower [type 2 diabetes] incidence,” they conclude.
Lower 5-year odds of diabetes
It is not clear how eating fruit may confer protection against developing diabetes, the researchers write.
They aimed to examine how consumption of total fruit, individual fruit, and fruit juice is related to glucose tolerance, insulin sensitivity, and incident diabetes at 5 years and 12 years in participants in the nationally representative AusDiab study.
They identified 7,675 adults aged 25 and older without diabetes who had undergone blood tests and completed a food frequency questionnaire in 1999-2000.
Participants had indicated how often they ate 10 different types of fruit, any type of fruit juice, and other foods on a scale of 0 (never) to 10 (three or more times/day).
Researchers divided participants into quartiles based on their median fruit consumption: 62 (range 0-95) g/day, 122 (95-162) g/day, 230 (162-283) g/day, and 372 (283-961) g/day.
The most commonly consumed fruit was apples (23% of total fruit intake), followed by bananas (20%) and citrus fruit (18%). Other fruits each accounted for less than 8% of total fruit intake, so they were not studied separately.
Participants in each quartile had a similar mean age (54 years) and body mass index (27 kg/m2).
However, compared with participants in quartile 1 (low fruit intake), those in quartiles 3 and 4 (moderate and high fruit intakes, respectively) were more likely to be female, do at least 150 minutes of physical activity a week, and less likely to smoke. They also ate more vegetables and less red meat and processed meat, but they consumed more sugar.
Of 4,674 participants who had 5-year follow-up, 179 participants developed diabetes.
Compared to participants with a low fruit intake (quartile 1), those with a moderate fruit intake (quartile 3) had a 36% lower odds of developing diabetes within 5 years (odds ratio, 0.64; 95% confidence interval, 0.44-0.92) after adjusting for age, sex, physical activity, education, socioeconomic status, income, body mass index, smoking, cardiovascular disease, parental history of diabetes, and consumption of alcohol, vegetables, red meat, processed meat, and calories.
Of the 3,518 participants with 12-year follow-up, 247 participants had diabetes, but there were no significant associations between fruit consumption and this longer-term risk of diabetes, possibly due to the small number of participants and events.
The study was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Dr. Bondonno has reported no relevant financial disclosures. Disclosures of the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
Are left atrial thrombi that defy preprocedure anticoagulation predictable?
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
Three or more weeks of oral anticoagulation (OAC) sometimes isn’t up to the job of clearing any potentially embolic left atrial (LA) thrombi before procedures like cardioversion or catheter ablation in patients with atrial fibrillation (AF). Such OAC-defiant LA thrombi aren’t common, nor are they rare enough to ignore, suggests a new meta-analysis that might also have identified features that predispose to them.
Such predictors of LA clots that persist despite OAC could potentially guide selective use of transesophageal echocardiography (TEE) instead of more routine policies to either use or not use TEE for thrombus rule-out before rhythm-control procedures, researchers propose.
Their prevalence was about 2.7% among the study’s more than 14,000 patients who received at least 3 weeks of OAC with either vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) before undergoing TEE.
But OAC-resistant LA thrombi were two- to four-times as common in patients with than without certain features, including AF other than paroxysmal and higher CHADS2 and CHA2DS2-VASc stroke risk-stratification scores.
“TEE imaging in select patients at an elevated risk of LA thrombus, despite anticoagulation status, may be a reasonable approach to minimize the risk of thromboembolic complications following cardioversion or catheter ablation,” propose the study’s authors, led by Antony Lurie, BMSC, Population Health Research Institute, Hamilton, Ont. Their report was published in the June 15 issue of the Journal of the American College of Cardiology.
Guidelines don’t encourage TEE before cardioversion in patients who have been on OAC for at least 3 weeks, the group notes, and policies on TEE use before AF ablation vary widely regardless of anticoagulation status.
The current study suggests that 3 weeks of OAC isn’t enough for a substantial number of patients, who might be put at thromboembolic risk if TEE were to be skipped before rhythm-control procedures.
Conversely, many patients unlikely to have LA thrombi get preprocedure TEE anyway. That can happen “irrespective of how long they’ve been anticoagulated, their pattern of atrial fibrillation, or their stroke risk,” senior author Jorge A. Wong, MD, MPH, Population Health Research Institute and McMaster University, Hamilton, Ont., told this news organization.
But “TEE is an invasive imaging modality, so it is associated with small element of risk.” The current study, Dr. Wong said, points to potential risk-stratification tools clinicians might use to guide more selective TEE screening.
“At sites where TEEs are done all the time for patients undergoing ablation, one could use several of these risk markers to perhaps tailor use of TEE in individuals,” Dr. Wong said. “For example, in people with paroxysmal atrial fibrillation, we found that the risk of left atrial appendage clot was approximately 1% or less.” Screening by TEE might reasonably be avoided in such patients.
“Fortunately, continued oral anticoagulation already yields low peri-procedural stroke rates,” observes an accompanying editorial from Paulus Kirchhof, MD, and Christoph Sinning, MD, from the University Heart & Vascular Center and German Centre of Cardiovascular Research, Hamburg.
“Based on this new analysis of existing data, a risk-based use of TEE imaging in anticoagulated patients could enable further improvement in the safe delivery of rhythm control interventions in patients with AF,” the editorialists agree.
The meta-analysis covered 10 prospective and 25 retrospective studies with a total of 14,653 patients that reported whether LA thrombus was present in patients with AF or atrial flutter (AFL) who underwent TEE after at least 3 weeks of VKA or DOAC therapy. Reports for 30 of the studies identified patients by rhythm-control procedure, and the remaining five didn’t specify TEE indications.
The weighted mean prevalence of LA thrombus at TEE was 2.73% (95% confidence interval, 1.95%-3.80%). The finding was not significantly changed in separate sensitivity analyses, the report says, including one limited to studies with low risk of bias and others excluding patients with valvular AF, interrupted OAC, heparin bridging, or subtherapeutic anticoagulation, respectively.
Patients treated with VKA and DOACs showed similar prevalences of LA thrombi, with means of 2.80% and 3.12%, respectively (P = .674). The prevalence was significantly higher in patients:
- with nonparoxysmal than with paroxysmal AF/AFL (4.81% vs. 1.03%; P < .001)
- undergoing cardioversion than ablation (5.55% vs. 1.65; P < .001)
- with CHA2DS2-VASc scores of at least 3 than with scores of 2 or less (6.31% vs. 1.06%; P < .001).
A limitation of the study, observe Dr. Kirchhof and Dr. Sinning, “is that all patients had a clinical indication for a TEE, which might be a selection bias. When a thrombus was found on TEE, clinical judgment led to postponing of the procedure,” thereby avoiding potential thromboembolism.
“Thus, the paper cannot demonstrate that presence of a thrombus on TEE is related to peri-procedural ischemic stroke,” they write.
The literature puts the risk for stroke or systemic embolism at well under 1% for patients anticoagulated with either VKA or DOACs for at least 3 weeks prior to cardioversion, in contrast to the nearly 3% prevalence of LA appendage thrombus by TEE in the current analysis, Dr. Wong observed.
“So we’re seeing a lot more left atrial appendage thrombus than we would see stroke,” but there wasn’t a way to determine whether that increases the stroke risk, he agreed.Dr. Wong, Dr. Lurie, and the other authors report no relevant conflicts. Dr. Kirchhof discloses receiving partial support “from several drug and device companies active in atrial fibrillation” and to being listed as inventor on two AF-related patents held by the University of Birmingham. Dr. Sinning reports no relevant relationships.
A version of this article first appeared on Medscape.com.
More evidence links COVID vaccines to rare cases of myocarditis in youth
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.