User login
Coffee lowers heart failure risk in unique study
Higher coffee consumption is associated with a lower risk of heart failure, according to a machine learning–based algorithm that analyzed data from three large observational trials.
“Coffee consumption actually was predictive on top of known risk factors originally identified from those three trials.” The study is significant because it underscores the potential of big data for individualizing patient management, lead investigator David Kao, MD, said in an interview. “We in fact adjusted for the scores that are commonly used to predict heart disease, and coffee consumption remained a predictor even on top of that.”
The study used supervised machine learning to analyze data on diet and other variables from three well-known observational studies: Framingham Heart Study (FHS), Cardiovascular Heart Study (CHS), and ARIC (Atherosclerosis Risk in Communities). The goal of the study, published online on Feb. 9, 2021*, was to identify potential novel risk factors for incident coronary heart disease, stroke, and heart failure.
“The main difference of the relationship between coffee and heart disease, compared with prior analyses, is that we’re able to find it in these well-known and well-accepted studies that have helped us find risk factors before,” Dr. Kao said
The study included 2,732 FHS participants aged 30-62 years, 3,704 CHS patients aged 65 and older, and 14,925 ARIC subjects aged 45-64, all of whom had no history of cardiovascular disease events when they enrolled. Primary outcomes for the machine-learning study were times to incident coronary heart disease, heart failure, and stroke.
Mathematics, not hypotheses
To compensate for variations in methodologies between the three observational trials, the study used 204 data measurements collected at the first FHS exam, including 16 dietary variables and for which similar data were collected for the other two studies.
The machine-learning model used what’s known as a random forest analysis to identify the leading potential risk factors from among the 204 variables. To confirm findings between studies, the authors used a technique called “data harmonization” to smooth variations in the methodologies of the trials, not only with participant age and duration and date of the trials, but also in how data on coffee consumption were gathered. For example, FHS collected that data as cups per day, whereas CHS and ARIC collected that as monthly, weekly, and daily consumption. The study converted the coffee consumption data from CHS and ARIC to cups per day to conform to FHS data.
Random forest analysis is a type of machine learning that randomly creates a cluster of decision trees – the “forest” – to determine which variables, such as dietary factors, are important in predicting a result. The analysis uses mathematics, not hypotheses, to identify important variables.
Heart failure and risk reduced
In this study, the analysis determined that each cup of caffeinated coffee daily was linked with a 5% reduction in the risk of heart failure (hazard ratio, 0.95; P = .02) and 6% reduction in stroke risk (HR, 0.94; P = .02), but had no significant impact on risk for coronary heart disease or cardiovascular disease.
When the data were adjusted for the FHS CVD risk score, increasing coffee consumption remained significantly associated with an identical lower risk of heart failure (P = .03) but not stroke (P = .33).
While the study supports an association between coffee consumption and heart failure risk, it doesn’t establish causation, noted Alice H. Lichtenstein, DSc, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston. “The authors could not rule out the possibility that caffeinated coffee intake was a proxy for other heart-healthy lifestyle behaviors,” Dr. Lichtenstein said. “Perhaps the best message from the study is that there appears to be no adverse effects of drinking moderate amounts of caffeinated coffee, and there may be benefits.”
She added a note of caution. “This result does not suggest coffee intake should be increased, nor does it give license to increasing coffee drinks with a lot of added cream and sugar.”
Machine learning mines observational trials
Dr. Kao explained the rationale for applying a machine-learning algorithm to the three observational trials. “When these trials were designed in general, they had an idea of what they were looking for in terms of what might be a risk factor,” said Dr. Kao, of the University of Colorado at Denver, Aurora. “What we were interested in doing was to look for risk factors that nobody really thought about ahead of time and let the data show us what might be a predictor without any bias of what we imagined to be true.”
He described the role of machine learning in extracting and “filtering” data from the trials. “Machine learning allows us to look at a very large number of factors or variables and identify the most important ones in predicting a specific outcome,” he said. This study evaluated the 204 variables and focused on dietary factors because they’re modifiable.
“We looked at them in these different studies where we could, and coffee was the one that was reproducible in all of them,” he said. “Machine learning helped filter down these very large numbers of variables in ways you can’t do with traditional statistics. It’s useful in studies like this because they gather thousands and thousands of variables that generally nobody uses, but these methods allow you to actually do something with them – to determine which ones are most important.”
He added: “These methods I think will take us toward personalized medicine where you’re really individualizing a plan for keeping a patient healthy. We still have a lot of work to do, but there’s a lot of promise for really helping each of us to figure out the ways we can become the healthiest that we can be.”
The study was supported with funding from the National Heart, Lung, and Blood Institute and the American Heart Association. Dr. Kao and coauthors, as well as Dr. Lichtenstein, had no relevant financial relationships to disclose.
*Correction, 2/10/21: An earlier version of this article misstated the study's publication date.
Higher coffee consumption is associated with a lower risk of heart failure, according to a machine learning–based algorithm that analyzed data from three large observational trials.
“Coffee consumption actually was predictive on top of known risk factors originally identified from those three trials.” The study is significant because it underscores the potential of big data for individualizing patient management, lead investigator David Kao, MD, said in an interview. “We in fact adjusted for the scores that are commonly used to predict heart disease, and coffee consumption remained a predictor even on top of that.”
The study used supervised machine learning to analyze data on diet and other variables from three well-known observational studies: Framingham Heart Study (FHS), Cardiovascular Heart Study (CHS), and ARIC (Atherosclerosis Risk in Communities). The goal of the study, published online on Feb. 9, 2021*, was to identify potential novel risk factors for incident coronary heart disease, stroke, and heart failure.
“The main difference of the relationship between coffee and heart disease, compared with prior analyses, is that we’re able to find it in these well-known and well-accepted studies that have helped us find risk factors before,” Dr. Kao said
The study included 2,732 FHS participants aged 30-62 years, 3,704 CHS patients aged 65 and older, and 14,925 ARIC subjects aged 45-64, all of whom had no history of cardiovascular disease events when they enrolled. Primary outcomes for the machine-learning study were times to incident coronary heart disease, heart failure, and stroke.
Mathematics, not hypotheses
To compensate for variations in methodologies between the three observational trials, the study used 204 data measurements collected at the first FHS exam, including 16 dietary variables and for which similar data were collected for the other two studies.
The machine-learning model used what’s known as a random forest analysis to identify the leading potential risk factors from among the 204 variables. To confirm findings between studies, the authors used a technique called “data harmonization” to smooth variations in the methodologies of the trials, not only with participant age and duration and date of the trials, but also in how data on coffee consumption were gathered. For example, FHS collected that data as cups per day, whereas CHS and ARIC collected that as monthly, weekly, and daily consumption. The study converted the coffee consumption data from CHS and ARIC to cups per day to conform to FHS data.
Random forest analysis is a type of machine learning that randomly creates a cluster of decision trees – the “forest” – to determine which variables, such as dietary factors, are important in predicting a result. The analysis uses mathematics, not hypotheses, to identify important variables.
Heart failure and risk reduced
In this study, the analysis determined that each cup of caffeinated coffee daily was linked with a 5% reduction in the risk of heart failure (hazard ratio, 0.95; P = .02) and 6% reduction in stroke risk (HR, 0.94; P = .02), but had no significant impact on risk for coronary heart disease or cardiovascular disease.
When the data were adjusted for the FHS CVD risk score, increasing coffee consumption remained significantly associated with an identical lower risk of heart failure (P = .03) but not stroke (P = .33).
While the study supports an association between coffee consumption and heart failure risk, it doesn’t establish causation, noted Alice H. Lichtenstein, DSc, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston. “The authors could not rule out the possibility that caffeinated coffee intake was a proxy for other heart-healthy lifestyle behaviors,” Dr. Lichtenstein said. “Perhaps the best message from the study is that there appears to be no adverse effects of drinking moderate amounts of caffeinated coffee, and there may be benefits.”
She added a note of caution. “This result does not suggest coffee intake should be increased, nor does it give license to increasing coffee drinks with a lot of added cream and sugar.”
Machine learning mines observational trials
Dr. Kao explained the rationale for applying a machine-learning algorithm to the three observational trials. “When these trials were designed in general, they had an idea of what they were looking for in terms of what might be a risk factor,” said Dr. Kao, of the University of Colorado at Denver, Aurora. “What we were interested in doing was to look for risk factors that nobody really thought about ahead of time and let the data show us what might be a predictor without any bias of what we imagined to be true.”
He described the role of machine learning in extracting and “filtering” data from the trials. “Machine learning allows us to look at a very large number of factors or variables and identify the most important ones in predicting a specific outcome,” he said. This study evaluated the 204 variables and focused on dietary factors because they’re modifiable.
“We looked at them in these different studies where we could, and coffee was the one that was reproducible in all of them,” he said. “Machine learning helped filter down these very large numbers of variables in ways you can’t do with traditional statistics. It’s useful in studies like this because they gather thousands and thousands of variables that generally nobody uses, but these methods allow you to actually do something with them – to determine which ones are most important.”
He added: “These methods I think will take us toward personalized medicine where you’re really individualizing a plan for keeping a patient healthy. We still have a lot of work to do, but there’s a lot of promise for really helping each of us to figure out the ways we can become the healthiest that we can be.”
The study was supported with funding from the National Heart, Lung, and Blood Institute and the American Heart Association. Dr. Kao and coauthors, as well as Dr. Lichtenstein, had no relevant financial relationships to disclose.
*Correction, 2/10/21: An earlier version of this article misstated the study's publication date.
Higher coffee consumption is associated with a lower risk of heart failure, according to a machine learning–based algorithm that analyzed data from three large observational trials.
“Coffee consumption actually was predictive on top of known risk factors originally identified from those three trials.” The study is significant because it underscores the potential of big data for individualizing patient management, lead investigator David Kao, MD, said in an interview. “We in fact adjusted for the scores that are commonly used to predict heart disease, and coffee consumption remained a predictor even on top of that.”
The study used supervised machine learning to analyze data on diet and other variables from three well-known observational studies: Framingham Heart Study (FHS), Cardiovascular Heart Study (CHS), and ARIC (Atherosclerosis Risk in Communities). The goal of the study, published online on Feb. 9, 2021*, was to identify potential novel risk factors for incident coronary heart disease, stroke, and heart failure.
“The main difference of the relationship between coffee and heart disease, compared with prior analyses, is that we’re able to find it in these well-known and well-accepted studies that have helped us find risk factors before,” Dr. Kao said
The study included 2,732 FHS participants aged 30-62 years, 3,704 CHS patients aged 65 and older, and 14,925 ARIC subjects aged 45-64, all of whom had no history of cardiovascular disease events when they enrolled. Primary outcomes for the machine-learning study were times to incident coronary heart disease, heart failure, and stroke.
Mathematics, not hypotheses
To compensate for variations in methodologies between the three observational trials, the study used 204 data measurements collected at the first FHS exam, including 16 dietary variables and for which similar data were collected for the other two studies.
The machine-learning model used what’s known as a random forest analysis to identify the leading potential risk factors from among the 204 variables. To confirm findings between studies, the authors used a technique called “data harmonization” to smooth variations in the methodologies of the trials, not only with participant age and duration and date of the trials, but also in how data on coffee consumption were gathered. For example, FHS collected that data as cups per day, whereas CHS and ARIC collected that as monthly, weekly, and daily consumption. The study converted the coffee consumption data from CHS and ARIC to cups per day to conform to FHS data.
Random forest analysis is a type of machine learning that randomly creates a cluster of decision trees – the “forest” – to determine which variables, such as dietary factors, are important in predicting a result. The analysis uses mathematics, not hypotheses, to identify important variables.
Heart failure and risk reduced
In this study, the analysis determined that each cup of caffeinated coffee daily was linked with a 5% reduction in the risk of heart failure (hazard ratio, 0.95; P = .02) and 6% reduction in stroke risk (HR, 0.94; P = .02), but had no significant impact on risk for coronary heart disease or cardiovascular disease.
When the data were adjusted for the FHS CVD risk score, increasing coffee consumption remained significantly associated with an identical lower risk of heart failure (P = .03) but not stroke (P = .33).
While the study supports an association between coffee consumption and heart failure risk, it doesn’t establish causation, noted Alice H. Lichtenstein, DSc, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston. “The authors could not rule out the possibility that caffeinated coffee intake was a proxy for other heart-healthy lifestyle behaviors,” Dr. Lichtenstein said. “Perhaps the best message from the study is that there appears to be no adverse effects of drinking moderate amounts of caffeinated coffee, and there may be benefits.”
She added a note of caution. “This result does not suggest coffee intake should be increased, nor does it give license to increasing coffee drinks with a lot of added cream and sugar.”
Machine learning mines observational trials
Dr. Kao explained the rationale for applying a machine-learning algorithm to the three observational trials. “When these trials were designed in general, they had an idea of what they were looking for in terms of what might be a risk factor,” said Dr. Kao, of the University of Colorado at Denver, Aurora. “What we were interested in doing was to look for risk factors that nobody really thought about ahead of time and let the data show us what might be a predictor without any bias of what we imagined to be true.”
He described the role of machine learning in extracting and “filtering” data from the trials. “Machine learning allows us to look at a very large number of factors or variables and identify the most important ones in predicting a specific outcome,” he said. This study evaluated the 204 variables and focused on dietary factors because they’re modifiable.
“We looked at them in these different studies where we could, and coffee was the one that was reproducible in all of them,” he said. “Machine learning helped filter down these very large numbers of variables in ways you can’t do with traditional statistics. It’s useful in studies like this because they gather thousands and thousands of variables that generally nobody uses, but these methods allow you to actually do something with them – to determine which ones are most important.”
He added: “These methods I think will take us toward personalized medicine where you’re really individualizing a plan for keeping a patient healthy. We still have a lot of work to do, but there’s a lot of promise for really helping each of us to figure out the ways we can become the healthiest that we can be.”
The study was supported with funding from the National Heart, Lung, and Blood Institute and the American Heart Association. Dr. Kao and coauthors, as well as Dr. Lichtenstein, had no relevant financial relationships to disclose.
*Correction, 2/10/21: An earlier version of this article misstated the study's publication date.
FROM CIRCULATION: HEART FAILURE
Truncus Bicaroticus With Arteria Lusoria: A Rare Combination of Aortic Root Anatomy Complicating Cardiac Catheterization
While most patients with arteria lusoria and common carotid trunk conditions are asymptomatic, discovery of such anomalies periprocedurally may affect the cardiac catheterization access site, catheter selection, and additional imaging.
Branching of the great vessels from the aorta normally progresses with the brachiocephalic trunk as the first takeoff followed by the left common carotid and left subclavian artery in approximately 85% of cases.1 Variants of great vessel branching patterns include the so-called bovine arch, arteria lusoria or aberrant right subclavian artery (ARSA), aberrant origin of the vertebral arteries, and truncus bicaroticus, or common origin of the carotid arteries (COCA). These aberrancies are quite rare, some with an incidence of < 1%.1,2
These vascular anomalies become clinically relevant when they pose difficulty for operators in surgical and interventional specialties, necessitating unique approaches, catheters, and techniques to overcome. We present a case of concomitant aortic arch abnormalities during a diagnostic workup for transcatheter aortic valve replacement (TAVR) in a patient with previous coronary artery bypass grafting (CABG).
Case Presentation
A 66-year-old woman with coronary artery disease (CAD) status post-CABG and stage D1 aortic stenosis (AS) presented with exertional dyspnea. She was referred for coronary angiography as part of a workup for TAVR. Echocardiography confirmed severe AS with a peak velocity of 4.1 m/s, mean pressure gradient of 50 mm Hg, and an aortic valve area of 0.7 cm2. The patient was scheduled for cardiac catheterization with anticipated left radial artery approach for intubation and opacification of the left internal mammary artery (LIMA). However, this approach was abandoned during the procedure due to discovery of aberrant left radial artery anatomy, and the procedure was completed via femoral access.
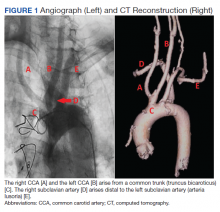
Subsequent coronary angiography revealed 3-vessel CAD, patent saphenous vein grafts (SVG) to the right coronary artery (RCA) and a diagonal branch vessel with an occluded SVG to the left circumflex. Difficulty was encountered when engaging the left subclavian artery using a JR 4.0 diagnostic catheter for LIMA angiography. Nonselective angiography of the aortic arch was performed and demonstrated an uncommon anatomical variant (Figure 1, left). The right common carotid artery (CCA) [A] and the left CCA [B] arose from a single trunk, consistent with truncus bicaroticus or COCA [C]. The right subclavian artery [D] originated distal to the left subclavian artery otherwise known as arteria lusoria or ARSA forming an incomplete vascular ring [E]. Selective engagement of the left subclavian artery remained problematic even with the use of specialty arch catheters (Headhunter and LIMA catheters). The procedure concluded without confirming patency of the LIMA graft. A total of 145 mL of Omnipaque (iohexol injection) contrast was used for the procedure, and no adverse events occurred.
Same-day access of the ipsilateral ulnar artery was not pursued because of the risk of hand ischemia. The patient underwent repeat catheterization utilizing left ulnar artery access after adequate recovery time from the initial left radial approach. Selective LIMA angiography was achieved and demonstrated a patent LIMA to LAD graft. A computed tomography (CT) aorta for purposes of TAVR planning was able to reconstruct the aortic arch vasculature (Figure 1, right) confirming the presence of both ARSA and COCA. The patient went on to undergo successful TAVR with subsequent improvement of clinical symptoms.
Discussion
Arteria lusoria is defined as an anomalous right subclavian artery arising distal to the origin of the left subclavian artery on the aortic arch. It has an estimated incidence of 0.5 to 2% and occurs as a consequence of abnormal embryologic involution of the right fourth aortic arch and right proximal dorsal aorta. This causes the origin of the right subclavian artery to shift onto the descending aorta and cross the mediastinum from left to right, passing behind the esophagus and the trachea.1,3-5
ARSA is often associated with other anatomic abnormalities, including COCA, right-sided aortic arch, interrupted aortic arch, aortic coarctation, tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, atrial septal defects, and ventricular septal defects.Underlying genetic disorders, such as Edwards, Down, DiGeorge syndromes, aneurysms, and arterioesophageal fistulae can accompany these vascular malformations.6
COCA, such as we encountered, is the presence of a single branch from the aorta giving off both right and left common carotid arteries. It has an incidence of < 0.1% in isolation and is discovered most often in cadaveric dissections or incidentally on imaging.1 Its embryologic origin results from the third pair of cervical aortic arches persisting as a common bicarotid trunk.1,4,5 The combination of ARSA and COCA is rare. Of the 0.5 to 2% of ARSA cases discovered, only 20% of those cases present with associated COCA for a combined prevalence estimated at < 0.05%.7
The majority of patients with either anatomic abnormality are asymptomatic. However, a few classic clinical manifestations have been described. ARSA can rarely present with dysphagia lusoria, a condition resulting from an incomplete vascular ring formed by the abnormal course of the right subclavian compressing the esophagus. Although not seen in our patient, it should be considered in the differential diagnosis for dysphagia.1,2,7 Ortner syndrome can result from right laryngeal nerve compression and palsy resultant from the aberrant course of the right subclavian artery.8 Another clinically relevant feature of ARSA is the presence of a diverticulum of Kommerell or dilatation at the origin of the right subclavian artery. It is a type of retroesophageal diverticulum resulting from persistence of a segment of the right sixth aortic arch.9 Finally, the spatial arrangement of ARSA increases risk for injury during head and neck surgical procedures, such as thyroidectomy, tracheotomy, and lymph node dissection of the right paratracheal fossa.6 Although the incidence is not well described, COCA has been described in several case reports as causing tracheal compression with dyspnea and in some cases, ischemic stroke.4,5,10
Diagnosis
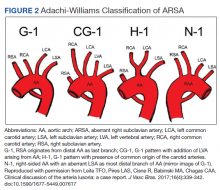
The diagnosis of ARSA and COCA is often made incidentally on diagnostic imaging studies such as endovascular imaging, CT angiography, magnetic resonance (MR) angiography, postmortem cadaveric dissections, or, as in our case, during cardiac catheterization.11,12 A classification system for aortic arch branching patterns exists published by Adachi and Williams.6 The classification includes ARSA and differentiates it into 4 subtypes (Figure 2). Our patient exhibited type H-1, indicating ARSA as the distal most branch of the aortic arch with coexistence of COCA.6 The primary clinical implication of ARSA and COCA in our case was increased difficulty and complexity when performing coronary angiography. Available literature has well characterized the challenges operators encounter when cannulating aberrant great vessel anatomy, often electing to perform nonselective aortography to define a patient’s anatomy.7,9,13 A comparison of diagnostic imaging techniques for vascular rings such as ARSA have shown MR, CT, and endovascular angiography to be the most reliable modalities to delineate vascular anatomy.14
Methods
Due to the presence of CABG in our patient, left radial and ulnar artery approaches were used rather than a right radial artery approach. Engagement of the LIMA is performed most commonly with left radial or femoral artery access using an internal mammary catheter that has a more steeply angled tip (80º-85º) compared with the standard JR catheter. An accessory left radial artery anatomic variant was encountered in our case precluding left radial approach. In addition, abnormal takeoffs of the great vessels thwarted multiple attempts at intubation of the LSA (Figure 1, right). Some data suggest CT imaging can be of assistance in establishing patency of bypass grafts in CABG patients.15 This can be considered an option if branch-vessel anatomy remains unclear. Our patient exhibited several risk factors for stroke, including female gender, hypertension, and prior CABG. These and other risk factors may influence clinical decisions such as continued catheter manipulation, choice of catheter type, and further contrast studies.16
Nonselective angiography in these cases often can require excessive iodinated contrast, exposing the patient to increased risk of contrast-induced nephropathy (CIN).7,17 Although the amount of contrast used in our case was average for diagnostic catheterization,the patient went on to undergo a second catheterization and CT angiography to establish LIMA graft patency.17 CT imaging reconstruction elucidated her aberrant branch-vessel anatomy. Patients are at increased risk of CIN with contrast loads < 200 mL per study, and this effect is compounded when the patient is elderly, has diabetes mellitus, and/or antecedent renal disease.18 Attention to the patient’s preoperative glomerular filtration rate, avoidance of nephrotoxic agents, and intraoperative left ventricular end-diastolic pressure during cardiac catheterization with postcontrast administration of IV isotonic fluids have been shown to prevent CIN.19,20 In the POSEIDON trial, fluid administration on a sliding scale based on the left ventricular end-diastolic pressure resulted in lower absolute risk of CIN postcatheterization vs standard postprocedure hydration in cardiac catheterization.21 Further, the now widespread use of low and iso-osmolar contrast agents further reduces the risk of CIN.22
For cardiac catheter laboratory operators, it is important to note that ARSA is more frequently encountered due to increased use of the transradial approach to coronary angiography.11 It should be suspected when accessing the ascending aorta proves exceptionally challenging and the catheter has a predilection for entering the descending aorta.11 While more technically demanding, 2 cases described by Allen and colleagues exhibited safe and successful entry into the ascending aorta with catheter rotation and hydrophilic support wires indicating the right radial approach is feasible despite presence of ARSA.12 Several patient-initiated maneuvers can be utilized to aid in accessing the ascending aorta. For example, deep inspiration to reduce the angulation between the aortic arch and ARSA. The use of curved catheters, such as Amplatz left, internal mammary catheter, or Simmons catheter may be considered to cannulate the ascending aorta if ARSA is encountered. Complications associated with a transradial approach include dissection and intramural hematoma. Minor bleeds and vasospasm also can occur secondary to increased procedural duration.6,8
Treatment
ARSA and COCA are considered normal anatomic variants and no treatment is indicated if the patient is asymptomatic. If symptoms are present, they often arise from aneurysmal or occlusive complications of the vascular anatomy. In patients with isolated ARSA and mild dysphasia or reflux symptoms, the use of prokinetics and antireflux medications may provide relief. It is important to note the coexistence of ARSA and COCA is more likely to produce esophageal compression compared to ARSA alone due to formation of a more complete vascular ring. Surgical management has been described in severe cases of ARSA involving risk of aneurysm rupture, right upper limb ischemia, or compression of the esophagus or trachea.
Several surgical approaches have been described, including simple ligation and division of ARSA and reimplantation of the RSA into the right CCA or ascending aorta.5 A recent review of 180 cases of ARSA diagnosed on CT angiography with concomitant common carotid trunk in half of studied individuals focused on a hybrid open and intravascular procedure. This procedure involved a double transposition or bypass (LSA to left common carotid artery and ARSA to the right CCA) followed by implantation of a thoracic stent graft. Few cases are eligible for these procedures or require them for definitive treatment.23
Conclusions
Recognition of aortic arch anatomical variants such as our case of ARSA with concomitant COCA may influence clinician decisions in various specialties, such as interventional cardiology, interventional neurology, cardiothoracic surgery, and gastroenterology. While most patients with these conditions are asymptomatic, some may present with dysphagia, dyspnea, and/or stroke symptoms. In our practice, discovery of such anomalies periprocedurally may affect cardiac catheterization access site, catheter selection, and additional imaging. The presence of arteria lusoria can be of critical importance when encountering a patient with myocardial infarction as switching from transradial to transfemoral approach may be required to gain access to the ascending aorta. Overall, transradial coronary angiography and percutaneous coronary intervention is not contraindicated in the setting of ARSA/COCA and can be safely performed by an experienced operator.
It is important for surgical specialists to be aware of the coexistence of anomalies where the discovery of one aberrancy can signal coexistent variant anatomy. If aortic arch anatomy is unclear, it is useful to perform nonselective angiography and/or further imaging with CT angiography. Knowledge of abnormal aortic arch anatomy can decrease fluoroscopy time and contrast load administered, thereby reducing potential periprocedural adverse events.
1. Kurt MA, An I, Ikiz I. A case with coincidence of aberrant right subclavian artery and common origin of the carotid arteries. Ann Anat. 1997;179(2):175-176. doi:10.1016/s0940-9602(97)80100-8
2. Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med. 1966;97(2):438-446. doi:10.2214/ajr.97.2.438
3. Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10(2):176-184. doi:10.3348/kjr.2009.10.2.176
4. Ozateş M, Nazaroglu H, Uyar A. MR angiography in diagnosis of aberrant right subclavian artery associated with common carotid trunk. Eur Radiol. 2000;10(9):1503. doi:10.1007/s003300000335
5. Poultsides GA, Lolis ED, Vasquez J, Drezner AD, Venieratos D. Common origins of carotid and subclavian arterial systems: report of a rare aortic arch variant. Ann Vasc Surg. 2004;18(5):597-600. doi:10.1007/s10016-004-0060-3
6. Leite TFO, Pires LAS, Cisne R, Babinski MA, Chagas CAA. Clinical discussion of the arteria lusoria: a case report. J Vasc Bras. 2017;16(4):339-342. doi:10.1590/1677-5449.007617
7. Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-1012. doi:10.1007/s00247-007-0574-2
8. Rafiq A, Chutani S, Krim NR. Incidental finding of arteria lusoria during transradial coronary catheterization: significance in interventional cardiology. Catheter Cardiovasc Interv. 2018;91(7):1283-1286. doi:10.1002/ccd.27439
9. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26-S44. doi:10.21037/cdt.2017.10.15
10. Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Neurol. 2010;3(1):33-34.
11. Valsecchi O, Vassileva A, Musumeci G, et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc Interv. 2006;67(6):870-878. doi:10.1002/ccd.20732
12. Allen D, Bews H, Vo M, Kass M, Jassal DS, Ravandi A. Arteria lusoria: an anomalous finding during right transradial coronary intervention. Case Rep Cardiol. 2016;2016:8079856. doi:10.1155/2016/8079856
13. Fineschi M, Iadanza A, Sinicropi G, Pierli C. Images in cardiology: angiographic evidence of aberrant right subclavian artery associated with common carotid trunk. Heart. 2002;88(2):158. doi:10.1136/heart.88.2.158
14. van Son JA, Julsrud PR, Hagler DJ, et al. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57(3):604-610. doi:10.1016/0003-4975(94)90552-5
15. Lee R, Lim J, Kaw G, Wan G, Ng K, Ho KT. Comprehensive noninvasive evaluation of bypass grafts and native coronary arteries in patients after coronary bypass surgery: accuracy of 64-slice multidetector computed tomography compared to invasive coronary angiography. J Cardiovasc Med (Hagerstown). 2010;11(2):81-90. doi:10.2459/JCM.0b013e32832f3e2e
16. Hamon M, Baron JC, Viader F, Hamon M. Periprocedural stroke and cardiac catheterization. Circulation. 2008;118(6): 678-683. doi:10.1161/CIRCULATIONAHA.108.784504
17. Hwang JR, D’Alfonso S, Kostuk WJ, et al. Contrast volume use in manual vs automated contrast injection systems for diagnostic coronary angiography and percutaneous coronary interventions. Can J Cardiol. 2013;29(3):372-376. doi:10.1016/j.cjca.2012.11.023
18. Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150(6):1237-1242.
19. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-728. doi:10.1148/radiol.13122276
20. American College of Radiology. ACR Manual on Contrast Media 2020. American College of Radiology; 2020:33-34. Accessed January 15, 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
21. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823. doi:10.1016/S0140-6736(14)60689-9
22. Aoun J, Nicolas D, Brown JR, Jaber BL. Maximum allowable contrast dose and prevention of acute kidney injury following cardiovascular procedures. Curr Opin Nephrol Hypertens. 2018;27(2):121-129. doi:10.1097/MNH.0000000000000389
23. Settembre N, Saba C, Bouziane Z, Jeannon F, Mandry D, Malikov S. Hybrid treatment of the aberrant right subclavian artery (arteria lusoria): feasibility study on 180 angio-CTs. Ann Vasc Surg. 2017;44:229-233. doi:10.1016/j.avsg.2017.03.172
While most patients with arteria lusoria and common carotid trunk conditions are asymptomatic, discovery of such anomalies periprocedurally may affect the cardiac catheterization access site, catheter selection, and additional imaging.
While most patients with arteria lusoria and common carotid trunk conditions are asymptomatic, discovery of such anomalies periprocedurally may affect the cardiac catheterization access site, catheter selection, and additional imaging.
Branching of the great vessels from the aorta normally progresses with the brachiocephalic trunk as the first takeoff followed by the left common carotid and left subclavian artery in approximately 85% of cases.1 Variants of great vessel branching patterns include the so-called bovine arch, arteria lusoria or aberrant right subclavian artery (ARSA), aberrant origin of the vertebral arteries, and truncus bicaroticus, or common origin of the carotid arteries (COCA). These aberrancies are quite rare, some with an incidence of < 1%.1,2
These vascular anomalies become clinically relevant when they pose difficulty for operators in surgical and interventional specialties, necessitating unique approaches, catheters, and techniques to overcome. We present a case of concomitant aortic arch abnormalities during a diagnostic workup for transcatheter aortic valve replacement (TAVR) in a patient with previous coronary artery bypass grafting (CABG).
Case Presentation
A 66-year-old woman with coronary artery disease (CAD) status post-CABG and stage D1 aortic stenosis (AS) presented with exertional dyspnea. She was referred for coronary angiography as part of a workup for TAVR. Echocardiography confirmed severe AS with a peak velocity of 4.1 m/s, mean pressure gradient of 50 mm Hg, and an aortic valve area of 0.7 cm2. The patient was scheduled for cardiac catheterization with anticipated left radial artery approach for intubation and opacification of the left internal mammary artery (LIMA). However, this approach was abandoned during the procedure due to discovery of aberrant left radial artery anatomy, and the procedure was completed via femoral access.
Subsequent coronary angiography revealed 3-vessel CAD, patent saphenous vein grafts (SVG) to the right coronary artery (RCA) and a diagonal branch vessel with an occluded SVG to the left circumflex. Difficulty was encountered when engaging the left subclavian artery using a JR 4.0 diagnostic catheter for LIMA angiography. Nonselective angiography of the aortic arch was performed and demonstrated an uncommon anatomical variant (Figure 1, left). The right common carotid artery (CCA) [A] and the left CCA [B] arose from a single trunk, consistent with truncus bicaroticus or COCA [C]. The right subclavian artery [D] originated distal to the left subclavian artery otherwise known as arteria lusoria or ARSA forming an incomplete vascular ring [E]. Selective engagement of the left subclavian artery remained problematic even with the use of specialty arch catheters (Headhunter and LIMA catheters). The procedure concluded without confirming patency of the LIMA graft. A total of 145 mL of Omnipaque (iohexol injection) contrast was used for the procedure, and no adverse events occurred.
Same-day access of the ipsilateral ulnar artery was not pursued because of the risk of hand ischemia. The patient underwent repeat catheterization utilizing left ulnar artery access after adequate recovery time from the initial left radial approach. Selective LIMA angiography was achieved and demonstrated a patent LIMA to LAD graft. A computed tomography (CT) aorta for purposes of TAVR planning was able to reconstruct the aortic arch vasculature (Figure 1, right) confirming the presence of both ARSA and COCA. The patient went on to undergo successful TAVR with subsequent improvement of clinical symptoms.
Discussion
Arteria lusoria is defined as an anomalous right subclavian artery arising distal to the origin of the left subclavian artery on the aortic arch. It has an estimated incidence of 0.5 to 2% and occurs as a consequence of abnormal embryologic involution of the right fourth aortic arch and right proximal dorsal aorta. This causes the origin of the right subclavian artery to shift onto the descending aorta and cross the mediastinum from left to right, passing behind the esophagus and the trachea.1,3-5
ARSA is often associated with other anatomic abnormalities, including COCA, right-sided aortic arch, interrupted aortic arch, aortic coarctation, tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, atrial septal defects, and ventricular septal defects.Underlying genetic disorders, such as Edwards, Down, DiGeorge syndromes, aneurysms, and arterioesophageal fistulae can accompany these vascular malformations.6
COCA, such as we encountered, is the presence of a single branch from the aorta giving off both right and left common carotid arteries. It has an incidence of < 0.1% in isolation and is discovered most often in cadaveric dissections or incidentally on imaging.1 Its embryologic origin results from the third pair of cervical aortic arches persisting as a common bicarotid trunk.1,4,5 The combination of ARSA and COCA is rare. Of the 0.5 to 2% of ARSA cases discovered, only 20% of those cases present with associated COCA for a combined prevalence estimated at < 0.05%.7
The majority of patients with either anatomic abnormality are asymptomatic. However, a few classic clinical manifestations have been described. ARSA can rarely present with dysphagia lusoria, a condition resulting from an incomplete vascular ring formed by the abnormal course of the right subclavian compressing the esophagus. Although not seen in our patient, it should be considered in the differential diagnosis for dysphagia.1,2,7 Ortner syndrome can result from right laryngeal nerve compression and palsy resultant from the aberrant course of the right subclavian artery.8 Another clinically relevant feature of ARSA is the presence of a diverticulum of Kommerell or dilatation at the origin of the right subclavian artery. It is a type of retroesophageal diverticulum resulting from persistence of a segment of the right sixth aortic arch.9 Finally, the spatial arrangement of ARSA increases risk for injury during head and neck surgical procedures, such as thyroidectomy, tracheotomy, and lymph node dissection of the right paratracheal fossa.6 Although the incidence is not well described, COCA has been described in several case reports as causing tracheal compression with dyspnea and in some cases, ischemic stroke.4,5,10
Diagnosis
The diagnosis of ARSA and COCA is often made incidentally on diagnostic imaging studies such as endovascular imaging, CT angiography, magnetic resonance (MR) angiography, postmortem cadaveric dissections, or, as in our case, during cardiac catheterization.11,12 A classification system for aortic arch branching patterns exists published by Adachi and Williams.6 The classification includes ARSA and differentiates it into 4 subtypes (Figure 2). Our patient exhibited type H-1, indicating ARSA as the distal most branch of the aortic arch with coexistence of COCA.6 The primary clinical implication of ARSA and COCA in our case was increased difficulty and complexity when performing coronary angiography. Available literature has well characterized the challenges operators encounter when cannulating aberrant great vessel anatomy, often electing to perform nonselective aortography to define a patient’s anatomy.7,9,13 A comparison of diagnostic imaging techniques for vascular rings such as ARSA have shown MR, CT, and endovascular angiography to be the most reliable modalities to delineate vascular anatomy.14
Methods
Due to the presence of CABG in our patient, left radial and ulnar artery approaches were used rather than a right radial artery approach. Engagement of the LIMA is performed most commonly with left radial or femoral artery access using an internal mammary catheter that has a more steeply angled tip (80º-85º) compared with the standard JR catheter. An accessory left radial artery anatomic variant was encountered in our case precluding left radial approach. In addition, abnormal takeoffs of the great vessels thwarted multiple attempts at intubation of the LSA (Figure 1, right). Some data suggest CT imaging can be of assistance in establishing patency of bypass grafts in CABG patients.15 This can be considered an option if branch-vessel anatomy remains unclear. Our patient exhibited several risk factors for stroke, including female gender, hypertension, and prior CABG. These and other risk factors may influence clinical decisions such as continued catheter manipulation, choice of catheter type, and further contrast studies.16
Nonselective angiography in these cases often can require excessive iodinated contrast, exposing the patient to increased risk of contrast-induced nephropathy (CIN).7,17 Although the amount of contrast used in our case was average for diagnostic catheterization,the patient went on to undergo a second catheterization and CT angiography to establish LIMA graft patency.17 CT imaging reconstruction elucidated her aberrant branch-vessel anatomy. Patients are at increased risk of CIN with contrast loads < 200 mL per study, and this effect is compounded when the patient is elderly, has diabetes mellitus, and/or antecedent renal disease.18 Attention to the patient’s preoperative glomerular filtration rate, avoidance of nephrotoxic agents, and intraoperative left ventricular end-diastolic pressure during cardiac catheterization with postcontrast administration of IV isotonic fluids have been shown to prevent CIN.19,20 In the POSEIDON trial, fluid administration on a sliding scale based on the left ventricular end-diastolic pressure resulted in lower absolute risk of CIN postcatheterization vs standard postprocedure hydration in cardiac catheterization.21 Further, the now widespread use of low and iso-osmolar contrast agents further reduces the risk of CIN.22
For cardiac catheter laboratory operators, it is important to note that ARSA is more frequently encountered due to increased use of the transradial approach to coronary angiography.11 It should be suspected when accessing the ascending aorta proves exceptionally challenging and the catheter has a predilection for entering the descending aorta.11 While more technically demanding, 2 cases described by Allen and colleagues exhibited safe and successful entry into the ascending aorta with catheter rotation and hydrophilic support wires indicating the right radial approach is feasible despite presence of ARSA.12 Several patient-initiated maneuvers can be utilized to aid in accessing the ascending aorta. For example, deep inspiration to reduce the angulation between the aortic arch and ARSA. The use of curved catheters, such as Amplatz left, internal mammary catheter, or Simmons catheter may be considered to cannulate the ascending aorta if ARSA is encountered. Complications associated with a transradial approach include dissection and intramural hematoma. Minor bleeds and vasospasm also can occur secondary to increased procedural duration.6,8
Treatment
ARSA and COCA are considered normal anatomic variants and no treatment is indicated if the patient is asymptomatic. If symptoms are present, they often arise from aneurysmal or occlusive complications of the vascular anatomy. In patients with isolated ARSA and mild dysphasia or reflux symptoms, the use of prokinetics and antireflux medications may provide relief. It is important to note the coexistence of ARSA and COCA is more likely to produce esophageal compression compared to ARSA alone due to formation of a more complete vascular ring. Surgical management has been described in severe cases of ARSA involving risk of aneurysm rupture, right upper limb ischemia, or compression of the esophagus or trachea.
Several surgical approaches have been described, including simple ligation and division of ARSA and reimplantation of the RSA into the right CCA or ascending aorta.5 A recent review of 180 cases of ARSA diagnosed on CT angiography with concomitant common carotid trunk in half of studied individuals focused on a hybrid open and intravascular procedure. This procedure involved a double transposition or bypass (LSA to left common carotid artery and ARSA to the right CCA) followed by implantation of a thoracic stent graft. Few cases are eligible for these procedures or require them for definitive treatment.23
Conclusions
Recognition of aortic arch anatomical variants such as our case of ARSA with concomitant COCA may influence clinician decisions in various specialties, such as interventional cardiology, interventional neurology, cardiothoracic surgery, and gastroenterology. While most patients with these conditions are asymptomatic, some may present with dysphagia, dyspnea, and/or stroke symptoms. In our practice, discovery of such anomalies periprocedurally may affect cardiac catheterization access site, catheter selection, and additional imaging. The presence of arteria lusoria can be of critical importance when encountering a patient with myocardial infarction as switching from transradial to transfemoral approach may be required to gain access to the ascending aorta. Overall, transradial coronary angiography and percutaneous coronary intervention is not contraindicated in the setting of ARSA/COCA and can be safely performed by an experienced operator.
It is important for surgical specialists to be aware of the coexistence of anomalies where the discovery of one aberrancy can signal coexistent variant anatomy. If aortic arch anatomy is unclear, it is useful to perform nonselective angiography and/or further imaging with CT angiography. Knowledge of abnormal aortic arch anatomy can decrease fluoroscopy time and contrast load administered, thereby reducing potential periprocedural adverse events.
Branching of the great vessels from the aorta normally progresses with the brachiocephalic trunk as the first takeoff followed by the left common carotid and left subclavian artery in approximately 85% of cases.1 Variants of great vessel branching patterns include the so-called bovine arch, arteria lusoria or aberrant right subclavian artery (ARSA), aberrant origin of the vertebral arteries, and truncus bicaroticus, or common origin of the carotid arteries (COCA). These aberrancies are quite rare, some with an incidence of < 1%.1,2
These vascular anomalies become clinically relevant when they pose difficulty for operators in surgical and interventional specialties, necessitating unique approaches, catheters, and techniques to overcome. We present a case of concomitant aortic arch abnormalities during a diagnostic workup for transcatheter aortic valve replacement (TAVR) in a patient with previous coronary artery bypass grafting (CABG).
Case Presentation
A 66-year-old woman with coronary artery disease (CAD) status post-CABG and stage D1 aortic stenosis (AS) presented with exertional dyspnea. She was referred for coronary angiography as part of a workup for TAVR. Echocardiography confirmed severe AS with a peak velocity of 4.1 m/s, mean pressure gradient of 50 mm Hg, and an aortic valve area of 0.7 cm2. The patient was scheduled for cardiac catheterization with anticipated left radial artery approach for intubation and opacification of the left internal mammary artery (LIMA). However, this approach was abandoned during the procedure due to discovery of aberrant left radial artery anatomy, and the procedure was completed via femoral access.
Subsequent coronary angiography revealed 3-vessel CAD, patent saphenous vein grafts (SVG) to the right coronary artery (RCA) and a diagonal branch vessel with an occluded SVG to the left circumflex. Difficulty was encountered when engaging the left subclavian artery using a JR 4.0 diagnostic catheter for LIMA angiography. Nonselective angiography of the aortic arch was performed and demonstrated an uncommon anatomical variant (Figure 1, left). The right common carotid artery (CCA) [A] and the left CCA [B] arose from a single trunk, consistent with truncus bicaroticus or COCA [C]. The right subclavian artery [D] originated distal to the left subclavian artery otherwise known as arteria lusoria or ARSA forming an incomplete vascular ring [E]. Selective engagement of the left subclavian artery remained problematic even with the use of specialty arch catheters (Headhunter and LIMA catheters). The procedure concluded without confirming patency of the LIMA graft. A total of 145 mL of Omnipaque (iohexol injection) contrast was used for the procedure, and no adverse events occurred.
Same-day access of the ipsilateral ulnar artery was not pursued because of the risk of hand ischemia. The patient underwent repeat catheterization utilizing left ulnar artery access after adequate recovery time from the initial left radial approach. Selective LIMA angiography was achieved and demonstrated a patent LIMA to LAD graft. A computed tomography (CT) aorta for purposes of TAVR planning was able to reconstruct the aortic arch vasculature (Figure 1, right) confirming the presence of both ARSA and COCA. The patient went on to undergo successful TAVR with subsequent improvement of clinical symptoms.
Discussion
Arteria lusoria is defined as an anomalous right subclavian artery arising distal to the origin of the left subclavian artery on the aortic arch. It has an estimated incidence of 0.5 to 2% and occurs as a consequence of abnormal embryologic involution of the right fourth aortic arch and right proximal dorsal aorta. This causes the origin of the right subclavian artery to shift onto the descending aorta and cross the mediastinum from left to right, passing behind the esophagus and the trachea.1,3-5
ARSA is often associated with other anatomic abnormalities, including COCA, right-sided aortic arch, interrupted aortic arch, aortic coarctation, tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, atrial septal defects, and ventricular septal defects.Underlying genetic disorders, such as Edwards, Down, DiGeorge syndromes, aneurysms, and arterioesophageal fistulae can accompany these vascular malformations.6
COCA, such as we encountered, is the presence of a single branch from the aorta giving off both right and left common carotid arteries. It has an incidence of < 0.1% in isolation and is discovered most often in cadaveric dissections or incidentally on imaging.1 Its embryologic origin results from the third pair of cervical aortic arches persisting as a common bicarotid trunk.1,4,5 The combination of ARSA and COCA is rare. Of the 0.5 to 2% of ARSA cases discovered, only 20% of those cases present with associated COCA for a combined prevalence estimated at < 0.05%.7
The majority of patients with either anatomic abnormality are asymptomatic. However, a few classic clinical manifestations have been described. ARSA can rarely present with dysphagia lusoria, a condition resulting from an incomplete vascular ring formed by the abnormal course of the right subclavian compressing the esophagus. Although not seen in our patient, it should be considered in the differential diagnosis for dysphagia.1,2,7 Ortner syndrome can result from right laryngeal nerve compression and palsy resultant from the aberrant course of the right subclavian artery.8 Another clinically relevant feature of ARSA is the presence of a diverticulum of Kommerell or dilatation at the origin of the right subclavian artery. It is a type of retroesophageal diverticulum resulting from persistence of a segment of the right sixth aortic arch.9 Finally, the spatial arrangement of ARSA increases risk for injury during head and neck surgical procedures, such as thyroidectomy, tracheotomy, and lymph node dissection of the right paratracheal fossa.6 Although the incidence is not well described, COCA has been described in several case reports as causing tracheal compression with dyspnea and in some cases, ischemic stroke.4,5,10
Diagnosis
The diagnosis of ARSA and COCA is often made incidentally on diagnostic imaging studies such as endovascular imaging, CT angiography, magnetic resonance (MR) angiography, postmortem cadaveric dissections, or, as in our case, during cardiac catheterization.11,12 A classification system for aortic arch branching patterns exists published by Adachi and Williams.6 The classification includes ARSA and differentiates it into 4 subtypes (Figure 2). Our patient exhibited type H-1, indicating ARSA as the distal most branch of the aortic arch with coexistence of COCA.6 The primary clinical implication of ARSA and COCA in our case was increased difficulty and complexity when performing coronary angiography. Available literature has well characterized the challenges operators encounter when cannulating aberrant great vessel anatomy, often electing to perform nonselective aortography to define a patient’s anatomy.7,9,13 A comparison of diagnostic imaging techniques for vascular rings such as ARSA have shown MR, CT, and endovascular angiography to be the most reliable modalities to delineate vascular anatomy.14
Methods
Due to the presence of CABG in our patient, left radial and ulnar artery approaches were used rather than a right radial artery approach. Engagement of the LIMA is performed most commonly with left radial or femoral artery access using an internal mammary catheter that has a more steeply angled tip (80º-85º) compared with the standard JR catheter. An accessory left radial artery anatomic variant was encountered in our case precluding left radial approach. In addition, abnormal takeoffs of the great vessels thwarted multiple attempts at intubation of the LSA (Figure 1, right). Some data suggest CT imaging can be of assistance in establishing patency of bypass grafts in CABG patients.15 This can be considered an option if branch-vessel anatomy remains unclear. Our patient exhibited several risk factors for stroke, including female gender, hypertension, and prior CABG. These and other risk factors may influence clinical decisions such as continued catheter manipulation, choice of catheter type, and further contrast studies.16
Nonselective angiography in these cases often can require excessive iodinated contrast, exposing the patient to increased risk of contrast-induced nephropathy (CIN).7,17 Although the amount of contrast used in our case was average for diagnostic catheterization,the patient went on to undergo a second catheterization and CT angiography to establish LIMA graft patency.17 CT imaging reconstruction elucidated her aberrant branch-vessel anatomy. Patients are at increased risk of CIN with contrast loads < 200 mL per study, and this effect is compounded when the patient is elderly, has diabetes mellitus, and/or antecedent renal disease.18 Attention to the patient’s preoperative glomerular filtration rate, avoidance of nephrotoxic agents, and intraoperative left ventricular end-diastolic pressure during cardiac catheterization with postcontrast administration of IV isotonic fluids have been shown to prevent CIN.19,20 In the POSEIDON trial, fluid administration on a sliding scale based on the left ventricular end-diastolic pressure resulted in lower absolute risk of CIN postcatheterization vs standard postprocedure hydration in cardiac catheterization.21 Further, the now widespread use of low and iso-osmolar contrast agents further reduces the risk of CIN.22
For cardiac catheter laboratory operators, it is important to note that ARSA is more frequently encountered due to increased use of the transradial approach to coronary angiography.11 It should be suspected when accessing the ascending aorta proves exceptionally challenging and the catheter has a predilection for entering the descending aorta.11 While more technically demanding, 2 cases described by Allen and colleagues exhibited safe and successful entry into the ascending aorta with catheter rotation and hydrophilic support wires indicating the right radial approach is feasible despite presence of ARSA.12 Several patient-initiated maneuvers can be utilized to aid in accessing the ascending aorta. For example, deep inspiration to reduce the angulation between the aortic arch and ARSA. The use of curved catheters, such as Amplatz left, internal mammary catheter, or Simmons catheter may be considered to cannulate the ascending aorta if ARSA is encountered. Complications associated with a transradial approach include dissection and intramural hematoma. Minor bleeds and vasospasm also can occur secondary to increased procedural duration.6,8
Treatment
ARSA and COCA are considered normal anatomic variants and no treatment is indicated if the patient is asymptomatic. If symptoms are present, they often arise from aneurysmal or occlusive complications of the vascular anatomy. In patients with isolated ARSA and mild dysphasia or reflux symptoms, the use of prokinetics and antireflux medications may provide relief. It is important to note the coexistence of ARSA and COCA is more likely to produce esophageal compression compared to ARSA alone due to formation of a more complete vascular ring. Surgical management has been described in severe cases of ARSA involving risk of aneurysm rupture, right upper limb ischemia, or compression of the esophagus or trachea.
Several surgical approaches have been described, including simple ligation and division of ARSA and reimplantation of the RSA into the right CCA or ascending aorta.5 A recent review of 180 cases of ARSA diagnosed on CT angiography with concomitant common carotid trunk in half of studied individuals focused on a hybrid open and intravascular procedure. This procedure involved a double transposition or bypass (LSA to left common carotid artery and ARSA to the right CCA) followed by implantation of a thoracic stent graft. Few cases are eligible for these procedures or require them for definitive treatment.23
Conclusions
Recognition of aortic arch anatomical variants such as our case of ARSA with concomitant COCA may influence clinician decisions in various specialties, such as interventional cardiology, interventional neurology, cardiothoracic surgery, and gastroenterology. While most patients with these conditions are asymptomatic, some may present with dysphagia, dyspnea, and/or stroke symptoms. In our practice, discovery of such anomalies periprocedurally may affect cardiac catheterization access site, catheter selection, and additional imaging. The presence of arteria lusoria can be of critical importance when encountering a patient with myocardial infarction as switching from transradial to transfemoral approach may be required to gain access to the ascending aorta. Overall, transradial coronary angiography and percutaneous coronary intervention is not contraindicated in the setting of ARSA/COCA and can be safely performed by an experienced operator.
It is important for surgical specialists to be aware of the coexistence of anomalies where the discovery of one aberrancy can signal coexistent variant anatomy. If aortic arch anatomy is unclear, it is useful to perform nonselective angiography and/or further imaging with CT angiography. Knowledge of abnormal aortic arch anatomy can decrease fluoroscopy time and contrast load administered, thereby reducing potential periprocedural adverse events.
1. Kurt MA, An I, Ikiz I. A case with coincidence of aberrant right subclavian artery and common origin of the carotid arteries. Ann Anat. 1997;179(2):175-176. doi:10.1016/s0940-9602(97)80100-8
2. Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med. 1966;97(2):438-446. doi:10.2214/ajr.97.2.438
3. Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10(2):176-184. doi:10.3348/kjr.2009.10.2.176
4. Ozateş M, Nazaroglu H, Uyar A. MR angiography in diagnosis of aberrant right subclavian artery associated with common carotid trunk. Eur Radiol. 2000;10(9):1503. doi:10.1007/s003300000335
5. Poultsides GA, Lolis ED, Vasquez J, Drezner AD, Venieratos D. Common origins of carotid and subclavian arterial systems: report of a rare aortic arch variant. Ann Vasc Surg. 2004;18(5):597-600. doi:10.1007/s10016-004-0060-3
6. Leite TFO, Pires LAS, Cisne R, Babinski MA, Chagas CAA. Clinical discussion of the arteria lusoria: a case report. J Vasc Bras. 2017;16(4):339-342. doi:10.1590/1677-5449.007617
7. Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-1012. doi:10.1007/s00247-007-0574-2
8. Rafiq A, Chutani S, Krim NR. Incidental finding of arteria lusoria during transradial coronary catheterization: significance in interventional cardiology. Catheter Cardiovasc Interv. 2018;91(7):1283-1286. doi:10.1002/ccd.27439
9. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26-S44. doi:10.21037/cdt.2017.10.15
10. Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Neurol. 2010;3(1):33-34.
11. Valsecchi O, Vassileva A, Musumeci G, et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc Interv. 2006;67(6):870-878. doi:10.1002/ccd.20732
12. Allen D, Bews H, Vo M, Kass M, Jassal DS, Ravandi A. Arteria lusoria: an anomalous finding during right transradial coronary intervention. Case Rep Cardiol. 2016;2016:8079856. doi:10.1155/2016/8079856
13. Fineschi M, Iadanza A, Sinicropi G, Pierli C. Images in cardiology: angiographic evidence of aberrant right subclavian artery associated with common carotid trunk. Heart. 2002;88(2):158. doi:10.1136/heart.88.2.158
14. van Son JA, Julsrud PR, Hagler DJ, et al. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57(3):604-610. doi:10.1016/0003-4975(94)90552-5
15. Lee R, Lim J, Kaw G, Wan G, Ng K, Ho KT. Comprehensive noninvasive evaluation of bypass grafts and native coronary arteries in patients after coronary bypass surgery: accuracy of 64-slice multidetector computed tomography compared to invasive coronary angiography. J Cardiovasc Med (Hagerstown). 2010;11(2):81-90. doi:10.2459/JCM.0b013e32832f3e2e
16. Hamon M, Baron JC, Viader F, Hamon M. Periprocedural stroke and cardiac catheterization. Circulation. 2008;118(6): 678-683. doi:10.1161/CIRCULATIONAHA.108.784504
17. Hwang JR, D’Alfonso S, Kostuk WJ, et al. Contrast volume use in manual vs automated contrast injection systems for diagnostic coronary angiography and percutaneous coronary interventions. Can J Cardiol. 2013;29(3):372-376. doi:10.1016/j.cjca.2012.11.023
18. Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150(6):1237-1242.
19. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-728. doi:10.1148/radiol.13122276
20. American College of Radiology. ACR Manual on Contrast Media 2020. American College of Radiology; 2020:33-34. Accessed January 15, 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
21. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823. doi:10.1016/S0140-6736(14)60689-9
22. Aoun J, Nicolas D, Brown JR, Jaber BL. Maximum allowable contrast dose and prevention of acute kidney injury following cardiovascular procedures. Curr Opin Nephrol Hypertens. 2018;27(2):121-129. doi:10.1097/MNH.0000000000000389
23. Settembre N, Saba C, Bouziane Z, Jeannon F, Mandry D, Malikov S. Hybrid treatment of the aberrant right subclavian artery (arteria lusoria): feasibility study on 180 angio-CTs. Ann Vasc Surg. 2017;44:229-233. doi:10.1016/j.avsg.2017.03.172
1. Kurt MA, An I, Ikiz I. A case with coincidence of aberrant right subclavian artery and common origin of the carotid arteries. Ann Anat. 1997;179(2):175-176. doi:10.1016/s0940-9602(97)80100-8
2. Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med. 1966;97(2):438-446. doi:10.2214/ajr.97.2.438
3. Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10(2):176-184. doi:10.3348/kjr.2009.10.2.176
4. Ozateş M, Nazaroglu H, Uyar A. MR angiography in diagnosis of aberrant right subclavian artery associated with common carotid trunk. Eur Radiol. 2000;10(9):1503. doi:10.1007/s003300000335
5. Poultsides GA, Lolis ED, Vasquez J, Drezner AD, Venieratos D. Common origins of carotid and subclavian arterial systems: report of a rare aortic arch variant. Ann Vasc Surg. 2004;18(5):597-600. doi:10.1007/s10016-004-0060-3
6. Leite TFO, Pires LAS, Cisne R, Babinski MA, Chagas CAA. Clinical discussion of the arteria lusoria: a case report. J Vasc Bras. 2017;16(4):339-342. doi:10.1590/1677-5449.007617
7. Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-1012. doi:10.1007/s00247-007-0574-2
8. Rafiq A, Chutani S, Krim NR. Incidental finding of arteria lusoria during transradial coronary catheterization: significance in interventional cardiology. Catheter Cardiovasc Interv. 2018;91(7):1283-1286. doi:10.1002/ccd.27439
9. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26-S44. doi:10.21037/cdt.2017.10.15
10. Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Neurol. 2010;3(1):33-34.
11. Valsecchi O, Vassileva A, Musumeci G, et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc Interv. 2006;67(6):870-878. doi:10.1002/ccd.20732
12. Allen D, Bews H, Vo M, Kass M, Jassal DS, Ravandi A. Arteria lusoria: an anomalous finding during right transradial coronary intervention. Case Rep Cardiol. 2016;2016:8079856. doi:10.1155/2016/8079856
13. Fineschi M, Iadanza A, Sinicropi G, Pierli C. Images in cardiology: angiographic evidence of aberrant right subclavian artery associated with common carotid trunk. Heart. 2002;88(2):158. doi:10.1136/heart.88.2.158
14. van Son JA, Julsrud PR, Hagler DJ, et al. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57(3):604-610. doi:10.1016/0003-4975(94)90552-5
15. Lee R, Lim J, Kaw G, Wan G, Ng K, Ho KT. Comprehensive noninvasive evaluation of bypass grafts and native coronary arteries in patients after coronary bypass surgery: accuracy of 64-slice multidetector computed tomography compared to invasive coronary angiography. J Cardiovasc Med (Hagerstown). 2010;11(2):81-90. doi:10.2459/JCM.0b013e32832f3e2e
16. Hamon M, Baron JC, Viader F, Hamon M. Periprocedural stroke and cardiac catheterization. Circulation. 2008;118(6): 678-683. doi:10.1161/CIRCULATIONAHA.108.784504
17. Hwang JR, D’Alfonso S, Kostuk WJ, et al. Contrast volume use in manual vs automated contrast injection systems for diagnostic coronary angiography and percutaneous coronary interventions. Can J Cardiol. 2013;29(3):372-376. doi:10.1016/j.cjca.2012.11.023
18. Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150(6):1237-1242.
19. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-728. doi:10.1148/radiol.13122276
20. American College of Radiology. ACR Manual on Contrast Media 2020. American College of Radiology; 2020:33-34. Accessed January 15, 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
21. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823. doi:10.1016/S0140-6736(14)60689-9
22. Aoun J, Nicolas D, Brown JR, Jaber BL. Maximum allowable contrast dose and prevention of acute kidney injury following cardiovascular procedures. Curr Opin Nephrol Hypertens. 2018;27(2):121-129. doi:10.1097/MNH.0000000000000389
23. Settembre N, Saba C, Bouziane Z, Jeannon F, Mandry D, Malikov S. Hybrid treatment of the aberrant right subclavian artery (arteria lusoria): feasibility study on 180 angio-CTs. Ann Vasc Surg. 2017;44:229-233. doi:10.1016/j.avsg.2017.03.172
A Case Series of Catheter-Directed Thrombolysis With Mechanical Thrombectomy for Treating Severe Deep Vein Thrombosis
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
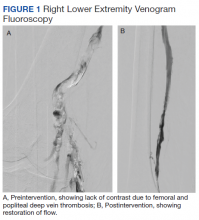
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Cardiac activity not uncommon after lifesaving measures stop
Among critically ill patients pulseless after planned withdrawal of life-sustaining therapies, cardiac activity restarted in 14% of cases, research shows.
Reassuringly, most resumption of heart activity happened in the first 1-2 minutes and most lasted 1 or 2 seconds.
“The reason we wanted to look at death determination specifically is we know that the stories persist about people coming back to life following death, and that’s not just in the public, it’s in the medical community as well,” lead author Sonny Dhanani, MD, of Children’s Hospital of Eastern Ontario, Ottawa, said in an interview.
“We thought that if we provided scientific evidence of whether this happened or not, we might dispel some myths and misunderstanding, which would hopefully promote organ donation.”
About 70% of organ donations occur after brain death, but an increasing number follow circulatory determination of death, he noted. Most protocols recommend 5 minutes of apnea and pulselessness by arterial catheter monitor before declaring death. But practices vary from 10 minutes in some European countries to 75 seconds in infant heart donors at one Colorado hospital.
Reports of patients recovering 10 minutes after pulselessness have raised concerns about the Lazarus phenomenon, or autoresuscitation, but are based in patients after cardiopulmonary resuscitation was terminated.
The present study, known as Death Prediction and Physiology after Removal of Therapy (DePParRT), enrolled patients at 20 intensive care sites in Canada, the Czech Republic, and the Netherlands, only if surrogate decision-makers agreed on withdrawal of life-sustaining measures without CPR and imminent death was anticipated.
As reported Jan. 28 in the New England Journal of Medicine, physicians observed resumption of circulation or cardiac activity prospectively in 1% of 631 patients based on bedside ECG, arterial pressure catheter monitors, palpated arterial pulse, breaths, or physical movements.
A retrospective review of data from 480 patients with complete ECG and arterial waveforms and at least 5 minutes of continuous waveform monitoring after pulselessness showed resumption of cardiac activity in 14% of patients.
The longest period of pulselessness before the heart showed signs of activity again was 4 minutes and 20 seconds. “So that was a reassuring number, because that’s within our 5-minute window that we currently use,” Dr. Dhanani said.
Importantly, “nobody woke up, nobody ended up being resuscitated, and all of these individuals died. And I think that’s going to be very helpful in this context,” he added.
In all, there were 77 cessations and resumptions in 67 of the 480 patients. The median duration of resumed cardiac activity was 3.9 seconds but, notably, ranged from 1 second to 13 minutes and 14 seconds.
“Though surprising, I think maybe not unreasonable,” observed Dr. Dhanani. “The heart is a very robust organ, and we maybe should anticipate these things happening, where at the end of life the heart may restart for minutes.”
In this situation, it’s important to wait the 13 minutes for the heart to stop again and then “wait another 5 minutes to make sure it doesn’t restart before determining death,” he said. “I think that’s where this study is going to now inform policy makers and guidelines, especially in the context of donations.”
The findings will be taken as strong support for the 5-minute window, said Robert Truog, MD, director of the Harvard Medical School Center for Bioethics and the Frances Glessner Lee Professor of Medical Ethics, Anaesthesia, and Pediatrics, Boston.
“I think it’s a safe point, I think people will refer to it, and it will be used to support the 5-minute window, and that’s probably reasonable,” he told this news organization. “Certainly, if it’s read in Europe it will cut the time from 10 minutes to 5 minutes, and that’s a good thing because 10 minutes is a very long time to wait.”
He noted that the 5-minute window provides reasonable assurance to the public and, with new technologies, permits most organs to be usable for donation after cardiac death. That said, there’s nothing magical about the number.
“In some ways I see this paper as providing interesting data but not actually providing an answer, because from the patient’s perspective and from the recipient’s perspective, waiting until the heart has made its last squeeze may not be the most relevant ethical question,” Dr. Truog said. “It may be, once we know this patient is not going to have return of cardiorespiratory function, is not going to wake up, that’s the point at which we ought to focus on organ preservation and organ retrieval, and that can be much sooner than 5 minutes.”
Dr. Dhanani and colleagues note that the generalizability of the results might be limited because patients without arterial pressure catheters were excluded, and 24% of enrolled patients could not be included in the retrospective waveform analysis owing to incomplete data.
“Our study definition of cardiac activity used an arbitrary threshold of pulse pressure (less than 5 mm Hg) that does not imply meaningful circulation,” they add. “This conservative consensus definition may have been partially responsible for the ostensibly high incidence (14%) of transient resumptions of cardiac activity identified through waveform adjudication.”
The study was supported by the Canadian Institutes for Health Research as part of the Canadian Donation and Transplantation Research Program, CHEO Research Institute, and Karel Pavlík Foundation. Dr. Dhanani has consulted for Canadian Blood Services. Dr. Truog reports no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Among critically ill patients pulseless after planned withdrawal of life-sustaining therapies, cardiac activity restarted in 14% of cases, research shows.
Reassuringly, most resumption of heart activity happened in the first 1-2 minutes and most lasted 1 or 2 seconds.
“The reason we wanted to look at death determination specifically is we know that the stories persist about people coming back to life following death, and that’s not just in the public, it’s in the medical community as well,” lead author Sonny Dhanani, MD, of Children’s Hospital of Eastern Ontario, Ottawa, said in an interview.
“We thought that if we provided scientific evidence of whether this happened or not, we might dispel some myths and misunderstanding, which would hopefully promote organ donation.”
About 70% of organ donations occur after brain death, but an increasing number follow circulatory determination of death, he noted. Most protocols recommend 5 minutes of apnea and pulselessness by arterial catheter monitor before declaring death. But practices vary from 10 minutes in some European countries to 75 seconds in infant heart donors at one Colorado hospital.
Reports of patients recovering 10 minutes after pulselessness have raised concerns about the Lazarus phenomenon, or autoresuscitation, but are based in patients after cardiopulmonary resuscitation was terminated.
The present study, known as Death Prediction and Physiology after Removal of Therapy (DePParRT), enrolled patients at 20 intensive care sites in Canada, the Czech Republic, and the Netherlands, only if surrogate decision-makers agreed on withdrawal of life-sustaining measures without CPR and imminent death was anticipated.
As reported Jan. 28 in the New England Journal of Medicine, physicians observed resumption of circulation or cardiac activity prospectively in 1% of 631 patients based on bedside ECG, arterial pressure catheter monitors, palpated arterial pulse, breaths, or physical movements.
A retrospective review of data from 480 patients with complete ECG and arterial waveforms and at least 5 minutes of continuous waveform monitoring after pulselessness showed resumption of cardiac activity in 14% of patients.
The longest period of pulselessness before the heart showed signs of activity again was 4 minutes and 20 seconds. “So that was a reassuring number, because that’s within our 5-minute window that we currently use,” Dr. Dhanani said.
Importantly, “nobody woke up, nobody ended up being resuscitated, and all of these individuals died. And I think that’s going to be very helpful in this context,” he added.
In all, there were 77 cessations and resumptions in 67 of the 480 patients. The median duration of resumed cardiac activity was 3.9 seconds but, notably, ranged from 1 second to 13 minutes and 14 seconds.
“Though surprising, I think maybe not unreasonable,” observed Dr. Dhanani. “The heart is a very robust organ, and we maybe should anticipate these things happening, where at the end of life the heart may restart for minutes.”
In this situation, it’s important to wait the 13 minutes for the heart to stop again and then “wait another 5 minutes to make sure it doesn’t restart before determining death,” he said. “I think that’s where this study is going to now inform policy makers and guidelines, especially in the context of donations.”
The findings will be taken as strong support for the 5-minute window, said Robert Truog, MD, director of the Harvard Medical School Center for Bioethics and the Frances Glessner Lee Professor of Medical Ethics, Anaesthesia, and Pediatrics, Boston.
“I think it’s a safe point, I think people will refer to it, and it will be used to support the 5-minute window, and that’s probably reasonable,” he told this news organization. “Certainly, if it’s read in Europe it will cut the time from 10 minutes to 5 minutes, and that’s a good thing because 10 minutes is a very long time to wait.”
He noted that the 5-minute window provides reasonable assurance to the public and, with new technologies, permits most organs to be usable for donation after cardiac death. That said, there’s nothing magical about the number.
“In some ways I see this paper as providing interesting data but not actually providing an answer, because from the patient’s perspective and from the recipient’s perspective, waiting until the heart has made its last squeeze may not be the most relevant ethical question,” Dr. Truog said. “It may be, once we know this patient is not going to have return of cardiorespiratory function, is not going to wake up, that’s the point at which we ought to focus on organ preservation and organ retrieval, and that can be much sooner than 5 minutes.”
Dr. Dhanani and colleagues note that the generalizability of the results might be limited because patients without arterial pressure catheters were excluded, and 24% of enrolled patients could not be included in the retrospective waveform analysis owing to incomplete data.
“Our study definition of cardiac activity used an arbitrary threshold of pulse pressure (less than 5 mm Hg) that does not imply meaningful circulation,” they add. “This conservative consensus definition may have been partially responsible for the ostensibly high incidence (14%) of transient resumptions of cardiac activity identified through waveform adjudication.”
The study was supported by the Canadian Institutes for Health Research as part of the Canadian Donation and Transplantation Research Program, CHEO Research Institute, and Karel Pavlík Foundation. Dr. Dhanani has consulted for Canadian Blood Services. Dr. Truog reports no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Among critically ill patients pulseless after planned withdrawal of life-sustaining therapies, cardiac activity restarted in 14% of cases, research shows.
Reassuringly, most resumption of heart activity happened in the first 1-2 minutes and most lasted 1 or 2 seconds.
“The reason we wanted to look at death determination specifically is we know that the stories persist about people coming back to life following death, and that’s not just in the public, it’s in the medical community as well,” lead author Sonny Dhanani, MD, of Children’s Hospital of Eastern Ontario, Ottawa, said in an interview.
“We thought that if we provided scientific evidence of whether this happened or not, we might dispel some myths and misunderstanding, which would hopefully promote organ donation.”
About 70% of organ donations occur after brain death, but an increasing number follow circulatory determination of death, he noted. Most protocols recommend 5 minutes of apnea and pulselessness by arterial catheter monitor before declaring death. But practices vary from 10 minutes in some European countries to 75 seconds in infant heart donors at one Colorado hospital.
Reports of patients recovering 10 minutes after pulselessness have raised concerns about the Lazarus phenomenon, or autoresuscitation, but are based in patients after cardiopulmonary resuscitation was terminated.
The present study, known as Death Prediction and Physiology after Removal of Therapy (DePParRT), enrolled patients at 20 intensive care sites in Canada, the Czech Republic, and the Netherlands, only if surrogate decision-makers agreed on withdrawal of life-sustaining measures without CPR and imminent death was anticipated.
As reported Jan. 28 in the New England Journal of Medicine, physicians observed resumption of circulation or cardiac activity prospectively in 1% of 631 patients based on bedside ECG, arterial pressure catheter monitors, palpated arterial pulse, breaths, or physical movements.
A retrospective review of data from 480 patients with complete ECG and arterial waveforms and at least 5 minutes of continuous waveform monitoring after pulselessness showed resumption of cardiac activity in 14% of patients.
The longest period of pulselessness before the heart showed signs of activity again was 4 minutes and 20 seconds. “So that was a reassuring number, because that’s within our 5-minute window that we currently use,” Dr. Dhanani said.
Importantly, “nobody woke up, nobody ended up being resuscitated, and all of these individuals died. And I think that’s going to be very helpful in this context,” he added.
In all, there were 77 cessations and resumptions in 67 of the 480 patients. The median duration of resumed cardiac activity was 3.9 seconds but, notably, ranged from 1 second to 13 minutes and 14 seconds.
“Though surprising, I think maybe not unreasonable,” observed Dr. Dhanani. “The heart is a very robust organ, and we maybe should anticipate these things happening, where at the end of life the heart may restart for minutes.”
In this situation, it’s important to wait the 13 minutes for the heart to stop again and then “wait another 5 minutes to make sure it doesn’t restart before determining death,” he said. “I think that’s where this study is going to now inform policy makers and guidelines, especially in the context of donations.”
The findings will be taken as strong support for the 5-minute window, said Robert Truog, MD, director of the Harvard Medical School Center for Bioethics and the Frances Glessner Lee Professor of Medical Ethics, Anaesthesia, and Pediatrics, Boston.
“I think it’s a safe point, I think people will refer to it, and it will be used to support the 5-minute window, and that’s probably reasonable,” he told this news organization. “Certainly, if it’s read in Europe it will cut the time from 10 minutes to 5 minutes, and that’s a good thing because 10 minutes is a very long time to wait.”
He noted that the 5-minute window provides reasonable assurance to the public and, with new technologies, permits most organs to be usable for donation after cardiac death. That said, there’s nothing magical about the number.
“In some ways I see this paper as providing interesting data but not actually providing an answer, because from the patient’s perspective and from the recipient’s perspective, waiting until the heart has made its last squeeze may not be the most relevant ethical question,” Dr. Truog said. “It may be, once we know this patient is not going to have return of cardiorespiratory function, is not going to wake up, that’s the point at which we ought to focus on organ preservation and organ retrieval, and that can be much sooner than 5 minutes.”
Dr. Dhanani and colleagues note that the generalizability of the results might be limited because patients without arterial pressure catheters were excluded, and 24% of enrolled patients could not be included in the retrospective waveform analysis owing to incomplete data.
“Our study definition of cardiac activity used an arbitrary threshold of pulse pressure (less than 5 mm Hg) that does not imply meaningful circulation,” they add. “This conservative consensus definition may have been partially responsible for the ostensibly high incidence (14%) of transient resumptions of cardiac activity identified through waveform adjudication.”
The study was supported by the Canadian Institutes for Health Research as part of the Canadian Donation and Transplantation Research Program, CHEO Research Institute, and Karel Pavlík Foundation. Dr. Dhanani has consulted for Canadian Blood Services. Dr. Truog reports no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Oily fish linked to lower risk of diabetes in largest study to date
People who report regularly eating oily fish had a significantly reduced risk for developing type 2 diabetes in a prospective, observational study of nearly 400,000 UK residents.
The results also show a significant, but weaker, positive link between regular use of fish oil supplements and a drop in the incidence of type 2 diabetes, Qibin Qi, PhD, and colleagues wrote in a report published in Diabetes Care. Their analysis failed to show a significant link between consumption of non-oily fish and type 2 diabetes onset.
The study is notable for being “the largest so far” to examine the link between fish consumption and type 2 diabetes incidence, and the first to establish a clear, significant association between regularly eating oily fish and a drop in the incidence of diabetes, said Dr. Qi, an epidemiologist at Albert Einstein College of Medicine in New York.
“At present, it is prudent to recommend fresh oily fish as a part of a healthy dietary pattern instead of fish oil supplements for diabetes prevention,” said Dr. Qi and coauthors.
The study included just over 392,000 adults without type 2 diabetes or cardiovascular disease at baseline enrolled in the UK Biobank. Median follow-up was just over 10 years, during which 7,262 participants developed diabetes.
Participants who ate either one, or two or more, servings of oily fish weekly each had a significant 22% lower rate of incident type 2 diabetes than that of those who ate no oily fish, after adjustment for multiple confounders. Those who reported regularly taking a fish oil supplement had a significant 9% lower incidence of type 2 diabetes than that of those who didn’t.
Evidence growing to add oily fish to diet to prevent type 2 diabetes
“Many current dietary guidelines recommend consumption of two servings of fish, preferably oily, per week, primarily based on cardiovascular benefits,” Dr. Qi said in an interview.
“No prior statements recommended oily fish for prevention of type 2 diabetes,” he explained, adding: “Our findings support future recommendations, but the evidence is not strong enough to make a [formal] recommendation now. We need evidence from clinical trials.”
Jason Wu, PhD, an epidemiologist at the University of New South Wales in Sydney, Australia, who specializes in this field but was not involved with the current study, said it “is a very well-conducted study, and certainly generates important new evidence supporting the potential benefits of regular consumption of oily fish.”
But he agrees that the evidence remains too preliminary for any official recommendations on eating oily fish for preventing the development of type 2 diabetes, including targeting advice to high-risk subgroups such as those with prediabetes or people who are obese.
Before any groups make recommendations, “we need to thoroughly review all the literature in this space to appraise the overall body of evidence,” Dr. Wu noted in an interview.
Oily fish: Solid evidence for prevention of CVD events
In contrast, the case for including oily fish in the diet to prevent CVD events seems settled. In 2018, a panel assembled by the American Heart Association to address the issue released a statement that concluded: “Current scientific evidence strongly supports the recommendation that seafood be an integral component of a heart-healthy dietary pattern.” It added that “a large body of evidence supports the recommendation to consume nonfried seafood, especially species higher in long-chain n-3 fatty acids, one to two times per week for cardiovascular benefits, including reduced risk of cardiac death, coronary heart disease, and ischemic stroke.”
The statement highlighted that “cold-water oily fish such as salmon, anchovies, herring, mackerel (Atlantic and Pacific), tuna (bluefin and albacore), and sardines have the highest levels” of long-chain n-3 fatty acids, notably eicosapentaenoic acid and docosahexaenoic acid, also collectively known as omega-3 fatty acids.
These fish types were among the oily fishes tallied in the UK Biobank data used by Dr. Qi and colleagues.
The case for fish oil supplements for preventing CVD events is much rockier, as summarized in a 2019 editorial, with some studies reporting no discernible effect while others indicate efficacy.
A second commentary from December 2020 highlighted how results from the REDUCE-IT trial showed clear benefit for preventing CVD using a highly purified form of fish oil, icosapent ethyl (Vascepa, Amarin). However, findings from two other recent reports, the STRENGTH and OMENI studies, failed to show CVD benefits from more conventional fish oil formulations.
Composite CVD and diabetes prevention effects?
The new findings by Dr. Qi and colleagues “highlight the need to specifically test the effect of fish oil supplements on glucose metabolism in people who cannot or choose not to regularly eat oily fish,” said Dr. Wu, a researcher at the George Institute for Global Health in Newtown, Australia.
“If eventually there is really strong evidence that fish, fish oil, or both have independent effects on both CVD and type 2 diabetes” it would be reasonable to integrate both outcomes into a single, composite, efficacy endpoint for the purpose of future studies, he added.
Dr. Qi agreed on both points. “A randomized, controlled trial of fish oil on type 2 diabetes as a primary outcome is needed. Most existing data are based on secondary analyses in the randomized trials for CVD,” he explained.
But, he added, “our results suggest a potential beneficial effect from fish oil supplements,” which implies that these may be “better than nothing” for people who can’t add oily fish to their regular diet.
The means by which fish and fish oil might slow or stop progression to type 2 diabetes remains uncertain.
The mechanisms for preventing both diabetes and CVD events may overlap, Dr. Qi noted, such as anti-inflammatory effects and improved insulin sensitivity, both of which have been observed in animal studies.
Evidence is “still lacking from human studies,” he explained, but if such mechanisms were at play, Dr. Wu said that would “add biologic plausibility” to a possible causal link between oily fish consumption and diabetes prevention.
“But we can’t assume that omega-3 fatty acids alone will have the same effect as oily fish, which obviously contains many other components.”
The study received no commercial funding. Dr. Qi and Dr. Wu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who report regularly eating oily fish had a significantly reduced risk for developing type 2 diabetes in a prospective, observational study of nearly 400,000 UK residents.
The results also show a significant, but weaker, positive link between regular use of fish oil supplements and a drop in the incidence of type 2 diabetes, Qibin Qi, PhD, and colleagues wrote in a report published in Diabetes Care. Their analysis failed to show a significant link between consumption of non-oily fish and type 2 diabetes onset.
The study is notable for being “the largest so far” to examine the link between fish consumption and type 2 diabetes incidence, and the first to establish a clear, significant association between regularly eating oily fish and a drop in the incidence of diabetes, said Dr. Qi, an epidemiologist at Albert Einstein College of Medicine in New York.
“At present, it is prudent to recommend fresh oily fish as a part of a healthy dietary pattern instead of fish oil supplements for diabetes prevention,” said Dr. Qi and coauthors.
The study included just over 392,000 adults without type 2 diabetes or cardiovascular disease at baseline enrolled in the UK Biobank. Median follow-up was just over 10 years, during which 7,262 participants developed diabetes.
Participants who ate either one, or two or more, servings of oily fish weekly each had a significant 22% lower rate of incident type 2 diabetes than that of those who ate no oily fish, after adjustment for multiple confounders. Those who reported regularly taking a fish oil supplement had a significant 9% lower incidence of type 2 diabetes than that of those who didn’t.
Evidence growing to add oily fish to diet to prevent type 2 diabetes
“Many current dietary guidelines recommend consumption of two servings of fish, preferably oily, per week, primarily based on cardiovascular benefits,” Dr. Qi said in an interview.
“No prior statements recommended oily fish for prevention of type 2 diabetes,” he explained, adding: “Our findings support future recommendations, but the evidence is not strong enough to make a [formal] recommendation now. We need evidence from clinical trials.”
Jason Wu, PhD, an epidemiologist at the University of New South Wales in Sydney, Australia, who specializes in this field but was not involved with the current study, said it “is a very well-conducted study, and certainly generates important new evidence supporting the potential benefits of regular consumption of oily fish.”
But he agrees that the evidence remains too preliminary for any official recommendations on eating oily fish for preventing the development of type 2 diabetes, including targeting advice to high-risk subgroups such as those with prediabetes or people who are obese.
Before any groups make recommendations, “we need to thoroughly review all the literature in this space to appraise the overall body of evidence,” Dr. Wu noted in an interview.
Oily fish: Solid evidence for prevention of CVD events
In contrast, the case for including oily fish in the diet to prevent CVD events seems settled. In 2018, a panel assembled by the American Heart Association to address the issue released a statement that concluded: “Current scientific evidence strongly supports the recommendation that seafood be an integral component of a heart-healthy dietary pattern.” It added that “a large body of evidence supports the recommendation to consume nonfried seafood, especially species higher in long-chain n-3 fatty acids, one to two times per week for cardiovascular benefits, including reduced risk of cardiac death, coronary heart disease, and ischemic stroke.”
The statement highlighted that “cold-water oily fish such as salmon, anchovies, herring, mackerel (Atlantic and Pacific), tuna (bluefin and albacore), and sardines have the highest levels” of long-chain n-3 fatty acids, notably eicosapentaenoic acid and docosahexaenoic acid, also collectively known as omega-3 fatty acids.
These fish types were among the oily fishes tallied in the UK Biobank data used by Dr. Qi and colleagues.
The case for fish oil supplements for preventing CVD events is much rockier, as summarized in a 2019 editorial, with some studies reporting no discernible effect while others indicate efficacy.
A second commentary from December 2020 highlighted how results from the REDUCE-IT trial showed clear benefit for preventing CVD using a highly purified form of fish oil, icosapent ethyl (Vascepa, Amarin). However, findings from two other recent reports, the STRENGTH and OMENI studies, failed to show CVD benefits from more conventional fish oil formulations.
Composite CVD and diabetes prevention effects?
The new findings by Dr. Qi and colleagues “highlight the need to specifically test the effect of fish oil supplements on glucose metabolism in people who cannot or choose not to regularly eat oily fish,” said Dr. Wu, a researcher at the George Institute for Global Health in Newtown, Australia.
“If eventually there is really strong evidence that fish, fish oil, or both have independent effects on both CVD and type 2 diabetes” it would be reasonable to integrate both outcomes into a single, composite, efficacy endpoint for the purpose of future studies, he added.
Dr. Qi agreed on both points. “A randomized, controlled trial of fish oil on type 2 diabetes as a primary outcome is needed. Most existing data are based on secondary analyses in the randomized trials for CVD,” he explained.
But, he added, “our results suggest a potential beneficial effect from fish oil supplements,” which implies that these may be “better than nothing” for people who can’t add oily fish to their regular diet.
The means by which fish and fish oil might slow or stop progression to type 2 diabetes remains uncertain.
The mechanisms for preventing both diabetes and CVD events may overlap, Dr. Qi noted, such as anti-inflammatory effects and improved insulin sensitivity, both of which have been observed in animal studies.
Evidence is “still lacking from human studies,” he explained, but if such mechanisms were at play, Dr. Wu said that would “add biologic plausibility” to a possible causal link between oily fish consumption and diabetes prevention.
“But we can’t assume that omega-3 fatty acids alone will have the same effect as oily fish, which obviously contains many other components.”
The study received no commercial funding. Dr. Qi and Dr. Wu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who report regularly eating oily fish had a significantly reduced risk for developing type 2 diabetes in a prospective, observational study of nearly 400,000 UK residents.
The results also show a significant, but weaker, positive link between regular use of fish oil supplements and a drop in the incidence of type 2 diabetes, Qibin Qi, PhD, and colleagues wrote in a report published in Diabetes Care. Their analysis failed to show a significant link between consumption of non-oily fish and type 2 diabetes onset.
The study is notable for being “the largest so far” to examine the link between fish consumption and type 2 diabetes incidence, and the first to establish a clear, significant association between regularly eating oily fish and a drop in the incidence of diabetes, said Dr. Qi, an epidemiologist at Albert Einstein College of Medicine in New York.
“At present, it is prudent to recommend fresh oily fish as a part of a healthy dietary pattern instead of fish oil supplements for diabetes prevention,” said Dr. Qi and coauthors.
The study included just over 392,000 adults without type 2 diabetes or cardiovascular disease at baseline enrolled in the UK Biobank. Median follow-up was just over 10 years, during which 7,262 participants developed diabetes.
Participants who ate either one, or two or more, servings of oily fish weekly each had a significant 22% lower rate of incident type 2 diabetes than that of those who ate no oily fish, after adjustment for multiple confounders. Those who reported regularly taking a fish oil supplement had a significant 9% lower incidence of type 2 diabetes than that of those who didn’t.
Evidence growing to add oily fish to diet to prevent type 2 diabetes
“Many current dietary guidelines recommend consumption of two servings of fish, preferably oily, per week, primarily based on cardiovascular benefits,” Dr. Qi said in an interview.
“No prior statements recommended oily fish for prevention of type 2 diabetes,” he explained, adding: “Our findings support future recommendations, but the evidence is not strong enough to make a [formal] recommendation now. We need evidence from clinical trials.”
Jason Wu, PhD, an epidemiologist at the University of New South Wales in Sydney, Australia, who specializes in this field but was not involved with the current study, said it “is a very well-conducted study, and certainly generates important new evidence supporting the potential benefits of regular consumption of oily fish.”
But he agrees that the evidence remains too preliminary for any official recommendations on eating oily fish for preventing the development of type 2 diabetes, including targeting advice to high-risk subgroups such as those with prediabetes or people who are obese.
Before any groups make recommendations, “we need to thoroughly review all the literature in this space to appraise the overall body of evidence,” Dr. Wu noted in an interview.
Oily fish: Solid evidence for prevention of CVD events
In contrast, the case for including oily fish in the diet to prevent CVD events seems settled. In 2018, a panel assembled by the American Heart Association to address the issue released a statement that concluded: “Current scientific evidence strongly supports the recommendation that seafood be an integral component of a heart-healthy dietary pattern.” It added that “a large body of evidence supports the recommendation to consume nonfried seafood, especially species higher in long-chain n-3 fatty acids, one to two times per week for cardiovascular benefits, including reduced risk of cardiac death, coronary heart disease, and ischemic stroke.”
The statement highlighted that “cold-water oily fish such as salmon, anchovies, herring, mackerel (Atlantic and Pacific), tuna (bluefin and albacore), and sardines have the highest levels” of long-chain n-3 fatty acids, notably eicosapentaenoic acid and docosahexaenoic acid, also collectively known as omega-3 fatty acids.
These fish types were among the oily fishes tallied in the UK Biobank data used by Dr. Qi and colleagues.
The case for fish oil supplements for preventing CVD events is much rockier, as summarized in a 2019 editorial, with some studies reporting no discernible effect while others indicate efficacy.
A second commentary from December 2020 highlighted how results from the REDUCE-IT trial showed clear benefit for preventing CVD using a highly purified form of fish oil, icosapent ethyl (Vascepa, Amarin). However, findings from two other recent reports, the STRENGTH and OMENI studies, failed to show CVD benefits from more conventional fish oil formulations.
Composite CVD and diabetes prevention effects?
The new findings by Dr. Qi and colleagues “highlight the need to specifically test the effect of fish oil supplements on glucose metabolism in people who cannot or choose not to regularly eat oily fish,” said Dr. Wu, a researcher at the George Institute for Global Health in Newtown, Australia.
“If eventually there is really strong evidence that fish, fish oil, or both have independent effects on both CVD and type 2 diabetes” it would be reasonable to integrate both outcomes into a single, composite, efficacy endpoint for the purpose of future studies, he added.
Dr. Qi agreed on both points. “A randomized, controlled trial of fish oil on type 2 diabetes as a primary outcome is needed. Most existing data are based on secondary analyses in the randomized trials for CVD,” he explained.
But, he added, “our results suggest a potential beneficial effect from fish oil supplements,” which implies that these may be “better than nothing” for people who can’t add oily fish to their regular diet.
The means by which fish and fish oil might slow or stop progression to type 2 diabetes remains uncertain.
The mechanisms for preventing both diabetes and CVD events may overlap, Dr. Qi noted, such as anti-inflammatory effects and improved insulin sensitivity, both of which have been observed in animal studies.
Evidence is “still lacking from human studies,” he explained, but if such mechanisms were at play, Dr. Wu said that would “add biologic plausibility” to a possible causal link between oily fish consumption and diabetes prevention.
“But we can’t assume that omega-3 fatty acids alone will have the same effect as oily fish, which obviously contains many other components.”
The study received no commercial funding. Dr. Qi and Dr. Wu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PURE: High refined-grain intake boosts death, CVD events
That’s one finding from an assessment of a more than 137,000 people in 21 countries that documented a clear link between a high level of consumption of refined grains and a significantly increased risk for death from any cause or major cardiovascular disease (CVD) event during a median follow-up of 9.5 years.
The results showed that people who reported eating at least 350 g (seven servings) of refined grain daily had a significant 29% increased risk of either death or a major CVD event (MI, stroke, or heart failure), compared with those who consumed less than one serving per day (fewer than 50 g) of refined grain after adjustment for multiple potential confounders, according to a report from the Prospective Urban Rural Epidemiology (PURE) study published in the BMJ on Feb. 3, 2021.
The analysis also showed no significant association between levels of whole grains or white rice in the diet and CVD events. Rice was considered a separate grain in the analysis because nearly two-thirds of the PURE study population reside in Asia, where rice is a staple food.
The findings show that “reduction in the quantity of refined grains and sugar, and improvement in the quality of carbohydrates is essential for better health outcomes, although we do not suggest complete elimination of refined grains,” said Mahshid Dehghan, PhD, lead investigator for this report and a researcher in nutrition epidemiology at the Population Health Research Institute of McMaster University, Hamilton, Ont.
‘Widely applicable’ results from large, diverse study
Although prior evidence had already shown the CVD risk from eating larger amounts of refined grains, “our findings are robust and more widely applicable because our large study recorded over 9,000 deaths and 3,500 major CVD events across a broad range of refined grain intake, and in a variety of different settings and cultures with varying dietary patterns,” Dr. Dehghan said in an interview.
“This is an important paper, with the strength of data from diverse countries. The associations are robust,” commented Dariush Mozaffarian, MD, DrPH, professor and dean of the Friedman School of Nutrition Science and Policy at Tufts University, Boston, who was not involved in the new report.
“The public and the public health community think about added sugar in food as harmful, but starch has gotten a free pass,” he said in an interview. Recently revised U.S. dietary guidelines recommend that refined grains constitute less than half of a person’s carbohydrate consumption, but that limitation remains set too high, Dr. Mozaffarian cautioned. A much safer daily consumption limit would cap refined grains to no more than one serving a day.
The data for the current PURE analysis came from more than 148,000 people aged 35-70 years at entry in 21 geographically and economically diverse countries. Excluding patients with known CVD at baseline left a cohort of 137,130 people.
The results showed no significant association between the quantity of whole grains consumed and the main outcome, nor a link between higher amounts of white rice consumption and the main outcome.
“Our findings suggest that intake of up to 350 g of cooked rice daily may not pose a significant health risk,” said Dr. Dehghan.
Refined grains produce a glucose surge
Dr. Dehghan and associates speculated that possible explanations for their findings are that “varieties of rice such as long-grain rice and especially parboiled white rice may have both a definite glycemic advantage and an overall nutritional advantage over refined wheat products. Also, depending on the culture and the nature of the rice eaten, rice may be displacing less desirable foods.”
In contrast, refined grains undergo “rapid action by digestive enzymes and quick absorption from the small intestines [that] could lead to an increase in postprandial blood glucose concentrations. The rise in glucose concentrations increases the insulin concentrations, which leads to hypoglycemia, lipolysis, and the stimulation of hunger and food intake,” the authors wrote.
“It’s similar to eating sugar, or candy,” noted Dr. Mozaffarian, as refined grain “is 100% glucose.” Whole grains differ by entering the gut packaged in cell structures that slow digestion and avoid delivering sugar in an unnaturally rapid way.
“We are providing new evidence, and we hope that dietary guidelines in North America encourage individuals to lower their refined grain and sugar intake,” Dr. Dehghan said.
PURE has received partial funding with unrestricted grants from several drug companies. Dr. Dehghan had no disclosures. Dr. Mozaffarian has been an adviser to or has received personal fees from several food companies, but had no relevant disclosures.
That’s one finding from an assessment of a more than 137,000 people in 21 countries that documented a clear link between a high level of consumption of refined grains and a significantly increased risk for death from any cause or major cardiovascular disease (CVD) event during a median follow-up of 9.5 years.
The results showed that people who reported eating at least 350 g (seven servings) of refined grain daily had a significant 29% increased risk of either death or a major CVD event (MI, stroke, or heart failure), compared with those who consumed less than one serving per day (fewer than 50 g) of refined grain after adjustment for multiple potential confounders, according to a report from the Prospective Urban Rural Epidemiology (PURE) study published in the BMJ on Feb. 3, 2021.
The analysis also showed no significant association between levels of whole grains or white rice in the diet and CVD events. Rice was considered a separate grain in the analysis because nearly two-thirds of the PURE study population reside in Asia, where rice is a staple food.
The findings show that “reduction in the quantity of refined grains and sugar, and improvement in the quality of carbohydrates is essential for better health outcomes, although we do not suggest complete elimination of refined grains,” said Mahshid Dehghan, PhD, lead investigator for this report and a researcher in nutrition epidemiology at the Population Health Research Institute of McMaster University, Hamilton, Ont.
‘Widely applicable’ results from large, diverse study
Although prior evidence had already shown the CVD risk from eating larger amounts of refined grains, “our findings are robust and more widely applicable because our large study recorded over 9,000 deaths and 3,500 major CVD events across a broad range of refined grain intake, and in a variety of different settings and cultures with varying dietary patterns,” Dr. Dehghan said in an interview.
“This is an important paper, with the strength of data from diverse countries. The associations are robust,” commented Dariush Mozaffarian, MD, DrPH, professor and dean of the Friedman School of Nutrition Science and Policy at Tufts University, Boston, who was not involved in the new report.
“The public and the public health community think about added sugar in food as harmful, but starch has gotten a free pass,” he said in an interview. Recently revised U.S. dietary guidelines recommend that refined grains constitute less than half of a person’s carbohydrate consumption, but that limitation remains set too high, Dr. Mozaffarian cautioned. A much safer daily consumption limit would cap refined grains to no more than one serving a day.
The data for the current PURE analysis came from more than 148,000 people aged 35-70 years at entry in 21 geographically and economically diverse countries. Excluding patients with known CVD at baseline left a cohort of 137,130 people.
The results showed no significant association between the quantity of whole grains consumed and the main outcome, nor a link between higher amounts of white rice consumption and the main outcome.
“Our findings suggest that intake of up to 350 g of cooked rice daily may not pose a significant health risk,” said Dr. Dehghan.
Refined grains produce a glucose surge
Dr. Dehghan and associates speculated that possible explanations for their findings are that “varieties of rice such as long-grain rice and especially parboiled white rice may have both a definite glycemic advantage and an overall nutritional advantage over refined wheat products. Also, depending on the culture and the nature of the rice eaten, rice may be displacing less desirable foods.”
In contrast, refined grains undergo “rapid action by digestive enzymes and quick absorption from the small intestines [that] could lead to an increase in postprandial blood glucose concentrations. The rise in glucose concentrations increases the insulin concentrations, which leads to hypoglycemia, lipolysis, and the stimulation of hunger and food intake,” the authors wrote.
“It’s similar to eating sugar, or candy,” noted Dr. Mozaffarian, as refined grain “is 100% glucose.” Whole grains differ by entering the gut packaged in cell structures that slow digestion and avoid delivering sugar in an unnaturally rapid way.
“We are providing new evidence, and we hope that dietary guidelines in North America encourage individuals to lower their refined grain and sugar intake,” Dr. Dehghan said.
PURE has received partial funding with unrestricted grants from several drug companies. Dr. Dehghan had no disclosures. Dr. Mozaffarian has been an adviser to or has received personal fees from several food companies, but had no relevant disclosures.
That’s one finding from an assessment of a more than 137,000 people in 21 countries that documented a clear link between a high level of consumption of refined grains and a significantly increased risk for death from any cause or major cardiovascular disease (CVD) event during a median follow-up of 9.5 years.
The results showed that people who reported eating at least 350 g (seven servings) of refined grain daily had a significant 29% increased risk of either death or a major CVD event (MI, stroke, or heart failure), compared with those who consumed less than one serving per day (fewer than 50 g) of refined grain after adjustment for multiple potential confounders, according to a report from the Prospective Urban Rural Epidemiology (PURE) study published in the BMJ on Feb. 3, 2021.
The analysis also showed no significant association between levels of whole grains or white rice in the diet and CVD events. Rice was considered a separate grain in the analysis because nearly two-thirds of the PURE study population reside in Asia, where rice is a staple food.
The findings show that “reduction in the quantity of refined grains and sugar, and improvement in the quality of carbohydrates is essential for better health outcomes, although we do not suggest complete elimination of refined grains,” said Mahshid Dehghan, PhD, lead investigator for this report and a researcher in nutrition epidemiology at the Population Health Research Institute of McMaster University, Hamilton, Ont.
‘Widely applicable’ results from large, diverse study
Although prior evidence had already shown the CVD risk from eating larger amounts of refined grains, “our findings are robust and more widely applicable because our large study recorded over 9,000 deaths and 3,500 major CVD events across a broad range of refined grain intake, and in a variety of different settings and cultures with varying dietary patterns,” Dr. Dehghan said in an interview.
“This is an important paper, with the strength of data from diverse countries. The associations are robust,” commented Dariush Mozaffarian, MD, DrPH, professor and dean of the Friedman School of Nutrition Science and Policy at Tufts University, Boston, who was not involved in the new report.
“The public and the public health community think about added sugar in food as harmful, but starch has gotten a free pass,” he said in an interview. Recently revised U.S. dietary guidelines recommend that refined grains constitute less than half of a person’s carbohydrate consumption, but that limitation remains set too high, Dr. Mozaffarian cautioned. A much safer daily consumption limit would cap refined grains to no more than one serving a day.
The data for the current PURE analysis came from more than 148,000 people aged 35-70 years at entry in 21 geographically and economically diverse countries. Excluding patients with known CVD at baseline left a cohort of 137,130 people.
The results showed no significant association between the quantity of whole grains consumed and the main outcome, nor a link between higher amounts of white rice consumption and the main outcome.
“Our findings suggest that intake of up to 350 g of cooked rice daily may not pose a significant health risk,” said Dr. Dehghan.
Refined grains produce a glucose surge
Dr. Dehghan and associates speculated that possible explanations for their findings are that “varieties of rice such as long-grain rice and especially parboiled white rice may have both a definite glycemic advantage and an overall nutritional advantage over refined wheat products. Also, depending on the culture and the nature of the rice eaten, rice may be displacing less desirable foods.”
In contrast, refined grains undergo “rapid action by digestive enzymes and quick absorption from the small intestines [that] could lead to an increase in postprandial blood glucose concentrations. The rise in glucose concentrations increases the insulin concentrations, which leads to hypoglycemia, lipolysis, and the stimulation of hunger and food intake,” the authors wrote.
“It’s similar to eating sugar, or candy,” noted Dr. Mozaffarian, as refined grain “is 100% glucose.” Whole grains differ by entering the gut packaged in cell structures that slow digestion and avoid delivering sugar in an unnaturally rapid way.
“We are providing new evidence, and we hope that dietary guidelines in North America encourage individuals to lower their refined grain and sugar intake,” Dr. Dehghan said.
PURE has received partial funding with unrestricted grants from several drug companies. Dr. Dehghan had no disclosures. Dr. Mozaffarian has been an adviser to or has received personal fees from several food companies, but had no relevant disclosures.
Complete PCI beats culprit-lesion-only PCI in STEMI patients with multivessel CAD
Background: Previous trials have shown a reduction in composite outcomes if STEMI patients undergo staged PCI of nonculprit lesions discovered incidentally at the time of primary PCI for STEMI. However, no randomized trial has had the power to assess if staged PCI of nonculprit lesions reduces cardiovascular death or MI.
Study design: Prospective randomized clinical trial.
Setting: PCI-capable centers in 31 countries.
Synopsis: In this study, if multivessel disease was identified during primary PCI for STEMI, patients were randomized to either culprit-lesion-only PCI or complete revascularization with staged PCI of all suitable nonculprit lesions (either during the index hospitalization or up to 45 days after randomization).
Overall, 4,041 patients from 140 centers were randomized with median 3-year follow-up. The complete revascularization group had lower rates of the primary composite outcome of death from cardiovascular disease or new MI (absolute reduction, 2.7%; 7.8% vs. 10.5%; number needed to treat, 37; hazard ratio, 0.74; 95% confidence interval, 0.60-0.91; P = .004). This finding was driven by lower incidence of new MI in the complete revascularization group – the incidence of death was similar between the groups. A coprimary composite outcome of death from cardiovascular causes, new MI, or ischemia-driven revascularization also favored complete revascularization, with an absolute risk reduction of 7.8% (8.9% vs. 16.7%; NNT, 13; HR, 0.51; 95% CI, 0.43-0.61; P less than .001). No statistically significant differences between groups were noted for the safety outcomes of major bleeding, stroke, stent thrombosis, or contrast-induced kidney injury.
Bottom line: Patients with STEMI who have multivessel disease incidentally discovered during primary PCI have a lower incidence of new MI and ischemia-driven revascularization when they undergo complete revascularization of all suitable lesions, as opposed to PCI of only their culprit lesion.
Citation: Mehta SR et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019 Oct 10;381:1411-21.
Dr. Porter is chief quality and safety resident at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Previous trials have shown a reduction in composite outcomes if STEMI patients undergo staged PCI of nonculprit lesions discovered incidentally at the time of primary PCI for STEMI. However, no randomized trial has had the power to assess if staged PCI of nonculprit lesions reduces cardiovascular death or MI.
Study design: Prospective randomized clinical trial.
Setting: PCI-capable centers in 31 countries.
Synopsis: In this study, if multivessel disease was identified during primary PCI for STEMI, patients were randomized to either culprit-lesion-only PCI or complete revascularization with staged PCI of all suitable nonculprit lesions (either during the index hospitalization or up to 45 days after randomization).
Overall, 4,041 patients from 140 centers were randomized with median 3-year follow-up. The complete revascularization group had lower rates of the primary composite outcome of death from cardiovascular disease or new MI (absolute reduction, 2.7%; 7.8% vs. 10.5%; number needed to treat, 37; hazard ratio, 0.74; 95% confidence interval, 0.60-0.91; P = .004). This finding was driven by lower incidence of new MI in the complete revascularization group – the incidence of death was similar between the groups. A coprimary composite outcome of death from cardiovascular causes, new MI, or ischemia-driven revascularization also favored complete revascularization, with an absolute risk reduction of 7.8% (8.9% vs. 16.7%; NNT, 13; HR, 0.51; 95% CI, 0.43-0.61; P less than .001). No statistically significant differences between groups were noted for the safety outcomes of major bleeding, stroke, stent thrombosis, or contrast-induced kidney injury.
Bottom line: Patients with STEMI who have multivessel disease incidentally discovered during primary PCI have a lower incidence of new MI and ischemia-driven revascularization when they undergo complete revascularization of all suitable lesions, as opposed to PCI of only their culprit lesion.
Citation: Mehta SR et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019 Oct 10;381:1411-21.
Dr. Porter is chief quality and safety resident at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Previous trials have shown a reduction in composite outcomes if STEMI patients undergo staged PCI of nonculprit lesions discovered incidentally at the time of primary PCI for STEMI. However, no randomized trial has had the power to assess if staged PCI of nonculprit lesions reduces cardiovascular death or MI.
Study design: Prospective randomized clinical trial.
Setting: PCI-capable centers in 31 countries.
Synopsis: In this study, if multivessel disease was identified during primary PCI for STEMI, patients were randomized to either culprit-lesion-only PCI or complete revascularization with staged PCI of all suitable nonculprit lesions (either during the index hospitalization or up to 45 days after randomization).
Overall, 4,041 patients from 140 centers were randomized with median 3-year follow-up. The complete revascularization group had lower rates of the primary composite outcome of death from cardiovascular disease or new MI (absolute reduction, 2.7%; 7.8% vs. 10.5%; number needed to treat, 37; hazard ratio, 0.74; 95% confidence interval, 0.60-0.91; P = .004). This finding was driven by lower incidence of new MI in the complete revascularization group – the incidence of death was similar between the groups. A coprimary composite outcome of death from cardiovascular causes, new MI, or ischemia-driven revascularization also favored complete revascularization, with an absolute risk reduction of 7.8% (8.9% vs. 16.7%; NNT, 13; HR, 0.51; 95% CI, 0.43-0.61; P less than .001). No statistically significant differences between groups were noted for the safety outcomes of major bleeding, stroke, stent thrombosis, or contrast-induced kidney injury.
Bottom line: Patients with STEMI who have multivessel disease incidentally discovered during primary PCI have a lower incidence of new MI and ischemia-driven revascularization when they undergo complete revascularization of all suitable lesions, as opposed to PCI of only their culprit lesion.
Citation: Mehta SR et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019 Oct 10;381:1411-21.
Dr. Porter is chief quality and safety resident at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Microthrombi, necrosis seen in COVID-19 hearts on autopsy
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
An update on Aspirin for Cardioprevention
Read More
Read More
Read More
Study flags cardiovascular disease in men with breast cancer
.
Among 24 male breast cancer patients evaluated over a decade in the Washington area, 88% were obese or overweight, 58% had hypertension, and 54% had hyperlipidemia.
Tachyarrhythmia existed in 8% of the men before cancer treatment and developed in 13% during treatment.
Two patients had preexisting heart failure, two patients developed the disease after treatment, and another two patients experienced a decline in left ventricular ejection fraction during the course of their cancer treatment.
“Our hope is that treating male breast cancer patients becomes a multidisciplinary approach where oncologists recruit their cardio-oncologist counterparts to mitigate cardiovascular risk factors, so patients live a long and healthy life after cancer treatment,” said Michael Ibrahim, one of the study authors and a 4th-year medical student at Georgetown University in Washington.
The data were presented Jan. 25 as part of the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient virtual course, which is hosting live sessions Feb. 5-6.
Although the association between cardiovascular disease and breast cancer is well documented in female breast cancer patients, there is little evidence in their male counterparts, especially African Americans, Mr. Ibrahim noted.
To provide some context, Mr. Ibrahim highlighted a 2018 report in nearly 3,500 female breast cancer patients, ages 40-79, in whom 52% were obese/overweight, 35% had hypertension, and 28% had hyperlipidemia.
Diabetes was present in 7.5% of the women, which was roughly equivalent to the 8% found among the men, Mr. Ibrahim said. The men were of similar age (38-79 years), with 42% being African American, 29% White, 4% Hispanic, and 25% another ethnicity.
Importantly, half of the men had a family history of breast cancer, and two were positive for a mutation in the BRCA gene.
A 2017 in-depth review of male breast cancer cites advancing age, hormonal imbalance, radiation exposure, and family history of breast cancer as key risk factors for the development of the disease, but the “most relevant risk factor” is a mutation in the BRCA2 gene.
Male breast cancer accounts for less than 1% of all breast cancers, but the incidence is rising and, in some patient groups, reaching 15% over their lifetimes, the paper notes. Additionally, these patients are at special risk for developing a second cancer.
Remarkably, 25% of men in the D.C. cohort were diagnosed with a second primary malignancy, 13% a third primary cancer, and 4% a fourth primary cancer, Mr. Ibrahim reported. “This goes to show that male breast cancer patients should routinely undergo cancer screening,” he said.
The initial diagnosis was invasive ductal carcinoma in 79% of the men, with the remaining ductal carcinoma in situ. All patients underwent mastectomy, 17% had anthracycline chemotherapy, 8% received HER2-targeted therapy, 16% had radiation, and 71% received hormone therapy.
In terms of cardiovascular management, statins were the most prescribed medication (46%), followed by antiplatelet therapy (42%) and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (38%).
An implantable cardioverter defibrillator/pacemaker was the most common intervention (16%), followed by bypass surgery in 8% and coronary angioplasty in 4%.
Mr. Ibrahim noted that the study was limited by the small sample size and that further research is needed to understand the risk of preexisting cardiovascular disease on long-term outcomes as well as the cardiotoxic effects of chemoradiation in male breast cancer patients.
In a statement, Mr. Ibrahim reiterated the need for a multidisciplinary cancer care team to evaluate patients’ cardiovascular risk prior to and through cancer treatment.
“On a more personal level, cancer patients are already surprised by their cancer diagnosis,” he added. “Similar to the pretreatment consultation with radiation oncology, breast surgery, and medical oncology, an upfront cardiovascular risk assessment provides greater comfort and further minimizes psychological surprise with cardiovascular complications going into cancer treatment.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Among 24 male breast cancer patients evaluated over a decade in the Washington area, 88% were obese or overweight, 58% had hypertension, and 54% had hyperlipidemia.
Tachyarrhythmia existed in 8% of the men before cancer treatment and developed in 13% during treatment.
Two patients had preexisting heart failure, two patients developed the disease after treatment, and another two patients experienced a decline in left ventricular ejection fraction during the course of their cancer treatment.
“Our hope is that treating male breast cancer patients becomes a multidisciplinary approach where oncologists recruit their cardio-oncologist counterparts to mitigate cardiovascular risk factors, so patients live a long and healthy life after cancer treatment,” said Michael Ibrahim, one of the study authors and a 4th-year medical student at Georgetown University in Washington.
The data were presented Jan. 25 as part of the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient virtual course, which is hosting live sessions Feb. 5-6.
Although the association between cardiovascular disease and breast cancer is well documented in female breast cancer patients, there is little evidence in their male counterparts, especially African Americans, Mr. Ibrahim noted.
To provide some context, Mr. Ibrahim highlighted a 2018 report in nearly 3,500 female breast cancer patients, ages 40-79, in whom 52% were obese/overweight, 35% had hypertension, and 28% had hyperlipidemia.
Diabetes was present in 7.5% of the women, which was roughly equivalent to the 8% found among the men, Mr. Ibrahim said. The men were of similar age (38-79 years), with 42% being African American, 29% White, 4% Hispanic, and 25% another ethnicity.
Importantly, half of the men had a family history of breast cancer, and two were positive for a mutation in the BRCA gene.
A 2017 in-depth review of male breast cancer cites advancing age, hormonal imbalance, radiation exposure, and family history of breast cancer as key risk factors for the development of the disease, but the “most relevant risk factor” is a mutation in the BRCA2 gene.
Male breast cancer accounts for less than 1% of all breast cancers, but the incidence is rising and, in some patient groups, reaching 15% over their lifetimes, the paper notes. Additionally, these patients are at special risk for developing a second cancer.
Remarkably, 25% of men in the D.C. cohort were diagnosed with a second primary malignancy, 13% a third primary cancer, and 4% a fourth primary cancer, Mr. Ibrahim reported. “This goes to show that male breast cancer patients should routinely undergo cancer screening,” he said.
The initial diagnosis was invasive ductal carcinoma in 79% of the men, with the remaining ductal carcinoma in situ. All patients underwent mastectomy, 17% had anthracycline chemotherapy, 8% received HER2-targeted therapy, 16% had radiation, and 71% received hormone therapy.
In terms of cardiovascular management, statins were the most prescribed medication (46%), followed by antiplatelet therapy (42%) and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (38%).
An implantable cardioverter defibrillator/pacemaker was the most common intervention (16%), followed by bypass surgery in 8% and coronary angioplasty in 4%.
Mr. Ibrahim noted that the study was limited by the small sample size and that further research is needed to understand the risk of preexisting cardiovascular disease on long-term outcomes as well as the cardiotoxic effects of chemoradiation in male breast cancer patients.
In a statement, Mr. Ibrahim reiterated the need for a multidisciplinary cancer care team to evaluate patients’ cardiovascular risk prior to and through cancer treatment.
“On a more personal level, cancer patients are already surprised by their cancer diagnosis,” he added. “Similar to the pretreatment consultation with radiation oncology, breast surgery, and medical oncology, an upfront cardiovascular risk assessment provides greater comfort and further minimizes psychological surprise with cardiovascular complications going into cancer treatment.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Among 24 male breast cancer patients evaluated over a decade in the Washington area, 88% were obese or overweight, 58% had hypertension, and 54% had hyperlipidemia.
Tachyarrhythmia existed in 8% of the men before cancer treatment and developed in 13% during treatment.
Two patients had preexisting heart failure, two patients developed the disease after treatment, and another two patients experienced a decline in left ventricular ejection fraction during the course of their cancer treatment.
“Our hope is that treating male breast cancer patients becomes a multidisciplinary approach where oncologists recruit their cardio-oncologist counterparts to mitigate cardiovascular risk factors, so patients live a long and healthy life after cancer treatment,” said Michael Ibrahim, one of the study authors and a 4th-year medical student at Georgetown University in Washington.
The data were presented Jan. 25 as part of the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient virtual course, which is hosting live sessions Feb. 5-6.
Although the association between cardiovascular disease and breast cancer is well documented in female breast cancer patients, there is little evidence in their male counterparts, especially African Americans, Mr. Ibrahim noted.
To provide some context, Mr. Ibrahim highlighted a 2018 report in nearly 3,500 female breast cancer patients, ages 40-79, in whom 52% were obese/overweight, 35% had hypertension, and 28% had hyperlipidemia.
Diabetes was present in 7.5% of the women, which was roughly equivalent to the 8% found among the men, Mr. Ibrahim said. The men were of similar age (38-79 years), with 42% being African American, 29% White, 4% Hispanic, and 25% another ethnicity.
Importantly, half of the men had a family history of breast cancer, and two were positive for a mutation in the BRCA gene.
A 2017 in-depth review of male breast cancer cites advancing age, hormonal imbalance, radiation exposure, and family history of breast cancer as key risk factors for the development of the disease, but the “most relevant risk factor” is a mutation in the BRCA2 gene.
Male breast cancer accounts for less than 1% of all breast cancers, but the incidence is rising and, in some patient groups, reaching 15% over their lifetimes, the paper notes. Additionally, these patients are at special risk for developing a second cancer.
Remarkably, 25% of men in the D.C. cohort were diagnosed with a second primary malignancy, 13% a third primary cancer, and 4% a fourth primary cancer, Mr. Ibrahim reported. “This goes to show that male breast cancer patients should routinely undergo cancer screening,” he said.
The initial diagnosis was invasive ductal carcinoma in 79% of the men, with the remaining ductal carcinoma in situ. All patients underwent mastectomy, 17% had anthracycline chemotherapy, 8% received HER2-targeted therapy, 16% had radiation, and 71% received hormone therapy.
In terms of cardiovascular management, statins were the most prescribed medication (46%), followed by antiplatelet therapy (42%) and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (38%).
An implantable cardioverter defibrillator/pacemaker was the most common intervention (16%), followed by bypass surgery in 8% and coronary angioplasty in 4%.
Mr. Ibrahim noted that the study was limited by the small sample size and that further research is needed to understand the risk of preexisting cardiovascular disease on long-term outcomes as well as the cardiotoxic effects of chemoradiation in male breast cancer patients.
In a statement, Mr. Ibrahim reiterated the need for a multidisciplinary cancer care team to evaluate patients’ cardiovascular risk prior to and through cancer treatment.
“On a more personal level, cancer patients are already surprised by their cancer diagnosis,” he added. “Similar to the pretreatment consultation with radiation oncology, breast surgery, and medical oncology, an upfront cardiovascular risk assessment provides greater comfort and further minimizes psychological surprise with cardiovascular complications going into cancer treatment.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.