User login
DAPA-HF: Dapagliflozin slows T2D onset in heart failure patients
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
FROM ADA 2020
Smart phones boosted compliance for cardiac device data transmission
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
FROM HEART RHYTHM 2020
CV outcomes of SGLT2 inhibitors and GLP-1 agonists compared in real-world study
Drug adherence, healthcare use, medical costs, and heart failure rates were better among patients with type 2 diabetes who were newly prescribed a sodium-glucose cotransporter 2 (SGLT2) inhibitor than a glucagon-like peptide 1 (GLP-1) receptor agonist in a real-world, observational study.
Composite cardiovascular (CV) outcomes were similar between the two drug classes.
Insiya Poonawalla, PhD, a researcher at Humana Healthcare Research, Flower Mound, Texas, reported the study results in an oral presentation on June 12 at the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
The investigators matched more than 10,000 patients with type 2 diabetes — half initiated on an SGLT2 inhibitor and half initiated on a GLP-1 agonist — from the Humana database of insurance claims data.
“These findings suggest potential benefits” of SGLT2 inhibitors, “particularly where risk related to heart failure is an important consideration,” Poonawalla said, but as always, any benefits need to be weighed against any risks.
And “while this study provides a pretty complete and current picture of claims until 2018,” it has limitations inherent to observational data (such as possible errors or omissions in the claims data), she conceded.
Mikhail Kosiborod, MD, invited to comment on the research, said this preliminary study was likely too short and small to definitively demonstrate differences in composite CV outcomes between the two drug classes, but he noted that the overall findings are not unexpected.
And often, the particular CV risk profile of an individual patient will point to one or the other of these drug classes as a best fit, he noted.
Too soon to alter clinical practice
Kosiborod, from Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, said he nevertheless feels “it would be a bit premature to use these findings as a guide to change clinical practice.”
“The study is relatively small in scope and likely underpowered to examine CV outcomes,” he said in an email interview.
Larger population-based studies and ideally head-to-head randomized controlled trials of various type 2 diabetes agents could compare these two drug classes more definitively, he asserted.
In the meantime, safety profiles of both medication classes “have been well established — in tens of thousands of patients in clinical trials and millions of patients prescribed these therapies in clinical practice,” he noted.
In general, the drugs in both classes are well-tolerated and safe for most patients with type 2 diabetes when used appropriately.
“Certainly, patients with type 2 diabetes and established CV disease (or at high risk for CV complications) are ideal candidates for either an SGLT2 inhibitor or a GLP-1 receptor agonist,” Kosiborod said.
“Given the data we have from outcome trials, an SGLT2 inhibitor would be a better initial strategy in a patient with type 2 diabetes and heart failure (especially heart failure with reduced ejection fraction) and/or diabetic kidney disease,” he continued.
On the other hand, “a GLP-1 receptor agonist may be a better initial strategy in a type 2 diabetes patient with (or at very high risk for) atherosclerotic cardiovascular disease (ASCVD), especially if there is concomitant obesity contributing to the disease process.”
Limited comparisons of these two newer drug classes
“Real-world evidence comparing these two therapeutic classes based on CV outcomes is limited,” Poonawalla said at the start of her presentation, and relative treatment persistence, utilization, and cost data are even less well studied.
To investigate this, the researchers identified patients aged 19 to 89 years who were newly prescribed one of these two types of antidiabetic agents during January 1, 2015 through June 30, 2017.
Poonawalla and senior study author Phil Schwab, PhD, research lead, Humana Healthcare Research, Louisville, Kentucky, clarified the study design and findings in an email to this new organization.
The team matched 5507 patients initiated on a GLP-1 agonist with 5507 patients newly prescribed an SGLT2 inhibitor.
Patients were a mean age of 65 years and 53% were women.
More than a third (37%) had established ASCVD, including myocardial infarction (MI) (7.9%) and stroke (9.8%), and 11.5% had heart failure.
About two thirds were receiving metformin and about a third were receiving insulin.
In the GLP-1 agonist group, more than half of patients were prescribed liraglutide (57%), followed by dulaglutide (33%), exenatide, and lixisenatide (two patients).
In the SGLT2 inhibitor group, close to 70% received canagliflozin, about a quarter received empagliflozin, and the rest received dapagliflozin.
During up to 3.5 years of follow-up, a similar percentage of patients in each group had either an MI, stroke, or died (the primary composite CV outcome) (hazard ratio [HR], 0.98; 95% CI, 0.89 - 1.07).
However, more patients in the GLP-1 agonist group had heart failure or died (the secondary composite CV outcome), driven by a higher rate of heart failure in this group.
But after adjusting for time to events there was no significant between-group difference in the secondary composite CV outcome (HR, 1.09; 95% CI, 0.99 - 1.21).
During the 12-months after the initial prescription, patients who were started on a GLP-1 agonist versus an SGLT2 inhibitor had higher mean monthly medical costs, which included hospitalizations, emergency department (ED) visits, and outpatient visits ($904 vs $834; P < .001).
They also had higher pharmacy costs, which covered all drugs ($891 vs $783; P < .001).
And they were more likely to discontinue treatment (HR, 1.15; 95% CI, 1.10 - 1.21), be hospitalized (14.4% vs 11.9%; P < .001), or visit the ED (27.4% vs 23.5%; P < .001).
“Not too surprising” and “somewhat reassuring”
Overall, Kosiborod did not find the results surprising.
Given the sample size and follow-up time, event rates were probably quite low and insufficient to draw firm conclusions about the composite CV outcomes, he reiterated.
However, given the comparable effects of these two drug types on major adverse cardiac events (MACE) in similar patient populations with type 2 diabetes, it is not too surprising that there were no significant differences in these outcomes.
It was also “somewhat reassuring” to see that heart failure rates were lower with SGLT2 inhibitors, “as one would expect,” he said, because these agents “have been shown to significantly reduce the risk of hospitalization for heart failure in multiple outcome trials, whereas GLP-1 receptor agonists’ beneficial CV effects appear to be more limited to MACE reduction.”
The higher rates of discontinuation with GLP-1 receptor agonists “is also not a surprise, since patients experience more gastrointestinal tolerability issues with these agents (mainly nausea),” which can be mitigated in the majority of patients with appropriate education and close follow up — but is not done consistently.
Similarly, “the cost differences are also expected, since GLP-1 receptor agonists tend to be more expensive.”
On the other hand, the higher rates of hospitalizations with GLP-1 agonists compared to SGLT2 inhibitors “requires further exploration and confirmation,” Kosiborod said.
But he suspects this may be due to residual confounding, “since GLP-1 agonists are typically initiated later in the type 2 diabetes treatment algorithm,” so these patients could have lengthier, more difficult-to-manage type 2 diabetes with more comorbidities despite the propensity matching.
Poonawalla and Schwab are employed by Humana. Kosiborod has disclosed research support from AstraZeneca and Boehringer Ingelheim; honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and consulting fees from Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, GlaxoSmithKline, Glytec, Intarcia, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi Aventis .
This article first appeared on Medscape.com
Drug adherence, healthcare use, medical costs, and heart failure rates were better among patients with type 2 diabetes who were newly prescribed a sodium-glucose cotransporter 2 (SGLT2) inhibitor than a glucagon-like peptide 1 (GLP-1) receptor agonist in a real-world, observational study.
Composite cardiovascular (CV) outcomes were similar between the two drug classes.
Insiya Poonawalla, PhD, a researcher at Humana Healthcare Research, Flower Mound, Texas, reported the study results in an oral presentation on June 12 at the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
The investigators matched more than 10,000 patients with type 2 diabetes — half initiated on an SGLT2 inhibitor and half initiated on a GLP-1 agonist — from the Humana database of insurance claims data.
“These findings suggest potential benefits” of SGLT2 inhibitors, “particularly where risk related to heart failure is an important consideration,” Poonawalla said, but as always, any benefits need to be weighed against any risks.
And “while this study provides a pretty complete and current picture of claims until 2018,” it has limitations inherent to observational data (such as possible errors or omissions in the claims data), she conceded.
Mikhail Kosiborod, MD, invited to comment on the research, said this preliminary study was likely too short and small to definitively demonstrate differences in composite CV outcomes between the two drug classes, but he noted that the overall findings are not unexpected.
And often, the particular CV risk profile of an individual patient will point to one or the other of these drug classes as a best fit, he noted.
Too soon to alter clinical practice
Kosiborod, from Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, said he nevertheless feels “it would be a bit premature to use these findings as a guide to change clinical practice.”
“The study is relatively small in scope and likely underpowered to examine CV outcomes,” he said in an email interview.
Larger population-based studies and ideally head-to-head randomized controlled trials of various type 2 diabetes agents could compare these two drug classes more definitively, he asserted.
In the meantime, safety profiles of both medication classes “have been well established — in tens of thousands of patients in clinical trials and millions of patients prescribed these therapies in clinical practice,” he noted.
In general, the drugs in both classes are well-tolerated and safe for most patients with type 2 diabetes when used appropriately.
“Certainly, patients with type 2 diabetes and established CV disease (or at high risk for CV complications) are ideal candidates for either an SGLT2 inhibitor or a GLP-1 receptor agonist,” Kosiborod said.
“Given the data we have from outcome trials, an SGLT2 inhibitor would be a better initial strategy in a patient with type 2 diabetes and heart failure (especially heart failure with reduced ejection fraction) and/or diabetic kidney disease,” he continued.
On the other hand, “a GLP-1 receptor agonist may be a better initial strategy in a type 2 diabetes patient with (or at very high risk for) atherosclerotic cardiovascular disease (ASCVD), especially if there is concomitant obesity contributing to the disease process.”
Limited comparisons of these two newer drug classes
“Real-world evidence comparing these two therapeutic classes based on CV outcomes is limited,” Poonawalla said at the start of her presentation, and relative treatment persistence, utilization, and cost data are even less well studied.
To investigate this, the researchers identified patients aged 19 to 89 years who were newly prescribed one of these two types of antidiabetic agents during January 1, 2015 through June 30, 2017.
Poonawalla and senior study author Phil Schwab, PhD, research lead, Humana Healthcare Research, Louisville, Kentucky, clarified the study design and findings in an email to this new organization.
The team matched 5507 patients initiated on a GLP-1 agonist with 5507 patients newly prescribed an SGLT2 inhibitor.
Patients were a mean age of 65 years and 53% were women.
More than a third (37%) had established ASCVD, including myocardial infarction (MI) (7.9%) and stroke (9.8%), and 11.5% had heart failure.
About two thirds were receiving metformin and about a third were receiving insulin.
In the GLP-1 agonist group, more than half of patients were prescribed liraglutide (57%), followed by dulaglutide (33%), exenatide, and lixisenatide (two patients).
In the SGLT2 inhibitor group, close to 70% received canagliflozin, about a quarter received empagliflozin, and the rest received dapagliflozin.
During up to 3.5 years of follow-up, a similar percentage of patients in each group had either an MI, stroke, or died (the primary composite CV outcome) (hazard ratio [HR], 0.98; 95% CI, 0.89 - 1.07).
However, more patients in the GLP-1 agonist group had heart failure or died (the secondary composite CV outcome), driven by a higher rate of heart failure in this group.
But after adjusting for time to events there was no significant between-group difference in the secondary composite CV outcome (HR, 1.09; 95% CI, 0.99 - 1.21).
During the 12-months after the initial prescription, patients who were started on a GLP-1 agonist versus an SGLT2 inhibitor had higher mean monthly medical costs, which included hospitalizations, emergency department (ED) visits, and outpatient visits ($904 vs $834; P < .001).
They also had higher pharmacy costs, which covered all drugs ($891 vs $783; P < .001).
And they were more likely to discontinue treatment (HR, 1.15; 95% CI, 1.10 - 1.21), be hospitalized (14.4% vs 11.9%; P < .001), or visit the ED (27.4% vs 23.5%; P < .001).
“Not too surprising” and “somewhat reassuring”
Overall, Kosiborod did not find the results surprising.
Given the sample size and follow-up time, event rates were probably quite low and insufficient to draw firm conclusions about the composite CV outcomes, he reiterated.
However, given the comparable effects of these two drug types on major adverse cardiac events (MACE) in similar patient populations with type 2 diabetes, it is not too surprising that there were no significant differences in these outcomes.
It was also “somewhat reassuring” to see that heart failure rates were lower with SGLT2 inhibitors, “as one would expect,” he said, because these agents “have been shown to significantly reduce the risk of hospitalization for heart failure in multiple outcome trials, whereas GLP-1 receptor agonists’ beneficial CV effects appear to be more limited to MACE reduction.”
The higher rates of discontinuation with GLP-1 receptor agonists “is also not a surprise, since patients experience more gastrointestinal tolerability issues with these agents (mainly nausea),” which can be mitigated in the majority of patients with appropriate education and close follow up — but is not done consistently.
Similarly, “the cost differences are also expected, since GLP-1 receptor agonists tend to be more expensive.”
On the other hand, the higher rates of hospitalizations with GLP-1 agonists compared to SGLT2 inhibitors “requires further exploration and confirmation,” Kosiborod said.
But he suspects this may be due to residual confounding, “since GLP-1 agonists are typically initiated later in the type 2 diabetes treatment algorithm,” so these patients could have lengthier, more difficult-to-manage type 2 diabetes with more comorbidities despite the propensity matching.
Poonawalla and Schwab are employed by Humana. Kosiborod has disclosed research support from AstraZeneca and Boehringer Ingelheim; honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and consulting fees from Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, GlaxoSmithKline, Glytec, Intarcia, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi Aventis .
This article first appeared on Medscape.com
Drug adherence, healthcare use, medical costs, and heart failure rates were better among patients with type 2 diabetes who were newly prescribed a sodium-glucose cotransporter 2 (SGLT2) inhibitor than a glucagon-like peptide 1 (GLP-1) receptor agonist in a real-world, observational study.
Composite cardiovascular (CV) outcomes were similar between the two drug classes.
Insiya Poonawalla, PhD, a researcher at Humana Healthcare Research, Flower Mound, Texas, reported the study results in an oral presentation on June 12 at the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
The investigators matched more than 10,000 patients with type 2 diabetes — half initiated on an SGLT2 inhibitor and half initiated on a GLP-1 agonist — from the Humana database of insurance claims data.
“These findings suggest potential benefits” of SGLT2 inhibitors, “particularly where risk related to heart failure is an important consideration,” Poonawalla said, but as always, any benefits need to be weighed against any risks.
And “while this study provides a pretty complete and current picture of claims until 2018,” it has limitations inherent to observational data (such as possible errors or omissions in the claims data), she conceded.
Mikhail Kosiborod, MD, invited to comment on the research, said this preliminary study was likely too short and small to definitively demonstrate differences in composite CV outcomes between the two drug classes, but he noted that the overall findings are not unexpected.
And often, the particular CV risk profile of an individual patient will point to one or the other of these drug classes as a best fit, he noted.
Too soon to alter clinical practice
Kosiborod, from Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, said he nevertheless feels “it would be a bit premature to use these findings as a guide to change clinical practice.”
“The study is relatively small in scope and likely underpowered to examine CV outcomes,” he said in an email interview.
Larger population-based studies and ideally head-to-head randomized controlled trials of various type 2 diabetes agents could compare these two drug classes more definitively, he asserted.
In the meantime, safety profiles of both medication classes “have been well established — in tens of thousands of patients in clinical trials and millions of patients prescribed these therapies in clinical practice,” he noted.
In general, the drugs in both classes are well-tolerated and safe for most patients with type 2 diabetes when used appropriately.
“Certainly, patients with type 2 diabetes and established CV disease (or at high risk for CV complications) are ideal candidates for either an SGLT2 inhibitor or a GLP-1 receptor agonist,” Kosiborod said.
“Given the data we have from outcome trials, an SGLT2 inhibitor would be a better initial strategy in a patient with type 2 diabetes and heart failure (especially heart failure with reduced ejection fraction) and/or diabetic kidney disease,” he continued.
On the other hand, “a GLP-1 receptor agonist may be a better initial strategy in a type 2 diabetes patient with (or at very high risk for) atherosclerotic cardiovascular disease (ASCVD), especially if there is concomitant obesity contributing to the disease process.”
Limited comparisons of these two newer drug classes
“Real-world evidence comparing these two therapeutic classes based on CV outcomes is limited,” Poonawalla said at the start of her presentation, and relative treatment persistence, utilization, and cost data are even less well studied.
To investigate this, the researchers identified patients aged 19 to 89 years who were newly prescribed one of these two types of antidiabetic agents during January 1, 2015 through June 30, 2017.
Poonawalla and senior study author Phil Schwab, PhD, research lead, Humana Healthcare Research, Louisville, Kentucky, clarified the study design and findings in an email to this new organization.
The team matched 5507 patients initiated on a GLP-1 agonist with 5507 patients newly prescribed an SGLT2 inhibitor.
Patients were a mean age of 65 years and 53% were women.
More than a third (37%) had established ASCVD, including myocardial infarction (MI) (7.9%) and stroke (9.8%), and 11.5% had heart failure.
About two thirds were receiving metformin and about a third were receiving insulin.
In the GLP-1 agonist group, more than half of patients were prescribed liraglutide (57%), followed by dulaglutide (33%), exenatide, and lixisenatide (two patients).
In the SGLT2 inhibitor group, close to 70% received canagliflozin, about a quarter received empagliflozin, and the rest received dapagliflozin.
During up to 3.5 years of follow-up, a similar percentage of patients in each group had either an MI, stroke, or died (the primary composite CV outcome) (hazard ratio [HR], 0.98; 95% CI, 0.89 - 1.07).
However, more patients in the GLP-1 agonist group had heart failure or died (the secondary composite CV outcome), driven by a higher rate of heart failure in this group.
But after adjusting for time to events there was no significant between-group difference in the secondary composite CV outcome (HR, 1.09; 95% CI, 0.99 - 1.21).
During the 12-months after the initial prescription, patients who were started on a GLP-1 agonist versus an SGLT2 inhibitor had higher mean monthly medical costs, which included hospitalizations, emergency department (ED) visits, and outpatient visits ($904 vs $834; P < .001).
They also had higher pharmacy costs, which covered all drugs ($891 vs $783; P < .001).
And they were more likely to discontinue treatment (HR, 1.15; 95% CI, 1.10 - 1.21), be hospitalized (14.4% vs 11.9%; P < .001), or visit the ED (27.4% vs 23.5%; P < .001).
“Not too surprising” and “somewhat reassuring”
Overall, Kosiborod did not find the results surprising.
Given the sample size and follow-up time, event rates were probably quite low and insufficient to draw firm conclusions about the composite CV outcomes, he reiterated.
However, given the comparable effects of these two drug types on major adverse cardiac events (MACE) in similar patient populations with type 2 diabetes, it is not too surprising that there were no significant differences in these outcomes.
It was also “somewhat reassuring” to see that heart failure rates were lower with SGLT2 inhibitors, “as one would expect,” he said, because these agents “have been shown to significantly reduce the risk of hospitalization for heart failure in multiple outcome trials, whereas GLP-1 receptor agonists’ beneficial CV effects appear to be more limited to MACE reduction.”
The higher rates of discontinuation with GLP-1 receptor agonists “is also not a surprise, since patients experience more gastrointestinal tolerability issues with these agents (mainly nausea),” which can be mitigated in the majority of patients with appropriate education and close follow up — but is not done consistently.
Similarly, “the cost differences are also expected, since GLP-1 receptor agonists tend to be more expensive.”
On the other hand, the higher rates of hospitalizations with GLP-1 agonists compared to SGLT2 inhibitors “requires further exploration and confirmation,” Kosiborod said.
But he suspects this may be due to residual confounding, “since GLP-1 agonists are typically initiated later in the type 2 diabetes treatment algorithm,” so these patients could have lengthier, more difficult-to-manage type 2 diabetes with more comorbidities despite the propensity matching.
Poonawalla and Schwab are employed by Humana. Kosiborod has disclosed research support from AstraZeneca and Boehringer Ingelheim; honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and consulting fees from Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, GlaxoSmithKline, Glytec, Intarcia, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi Aventis .
This article first appeared on Medscape.com
FROM ADA 2020
Half of young adults with diabetes have diastolic dysfunction
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
FROM ADA 2020
Key clinical point: .
Major finding: Tissue Doppler echocardiography detected diastolic dysfunction in 58% of patients with type 2 diabetes and 47% of type 1 patients.
Study details: SEARCH for Diabetes in Youth study, with 479 American adolescents and young adults with diabetes.
Disclosures: SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
Source: Shah AS et al. ADA 2020, Abstract 58-OR.
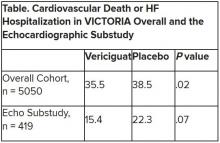
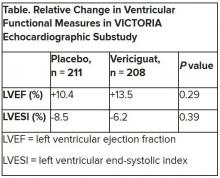
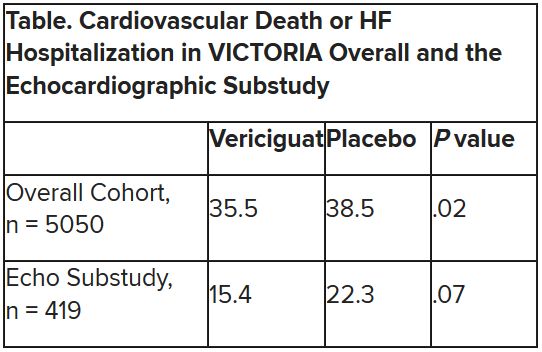
Mortality differs by LVEF between women and men
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
FROM ESC HEART FAILURE 2020
VICTORIA results deepen mystery of vericiguat in low-EF heart failure
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2020
Aspirin and warfarin together leads to increased bleeding without reducing thrombotic events
Background: Current guidelines recommend against using aspirin in combination with warfarin for patients with AFib, unless the patient has another indication for aspirin such as recent percutaneous coronary intervention (PCI) or a mechanical heart valve. These recommendations are based on limited clinical trial data that showed an increased risk of adverse events with combination therapy without clinical benefit. Despite these recommendations, recent studies have shown that aspirin use without a clinical indication remains common in patients taking warfarin for AFib. The prevalence of aspirin use without a clinical indication in patients taking warfarin for VTE is less well studied.
Study design: Registry-based cohort study.
Setting: Six anticoagulation clinics in Michigan.
Synopsis: Of the 6,539 patients included in the study, 2,453 patients (37.5%) were taking both warfarin and aspirin without an indication for aspirin therapy; 3,688 propensity score–matched patients (1,844 in each group) were compared to assess rates of bleeding and rates of observed thrombosis at 1 year in patients taking warfarin alone versus warfarin plus aspirin. Patients treated with warfarin plus aspirin experienced more bleeding events than did patients on warfarin monotherapy (95% confidence interval, 23.8%-28.3% vs. 95% CI, 18.3%-22.3%; P less than .001). Rates of observed thrombosis were similar between the two groups (95% CI, 1.6%-3.1% vs. 95% CI, 2.0%-3.6%; P = .40). This study demonstrates that aspirin use without a clinical indication remains common in patients taking warfarin for AFib or VTE, and that reducing inappropriate aspirin use in this patient population may help prevent adverse outcomes.
Bottom line: Use of aspirin without a clinical indication in patients taking warfarin is common and is associated with an increased risk of bleeding without significant clinical benefit.
Citation: Schaefer JK et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019 Mar 4;179(4):533-41.
Dr. Wachter is an associate medical director at Duke Regional Hospital and an assistant professor of medicine at Duke University.
Background: Current guidelines recommend against using aspirin in combination with warfarin for patients with AFib, unless the patient has another indication for aspirin such as recent percutaneous coronary intervention (PCI) or a mechanical heart valve. These recommendations are based on limited clinical trial data that showed an increased risk of adverse events with combination therapy without clinical benefit. Despite these recommendations, recent studies have shown that aspirin use without a clinical indication remains common in patients taking warfarin for AFib. The prevalence of aspirin use without a clinical indication in patients taking warfarin for VTE is less well studied.
Study design: Registry-based cohort study.
Setting: Six anticoagulation clinics in Michigan.
Synopsis: Of the 6,539 patients included in the study, 2,453 patients (37.5%) were taking both warfarin and aspirin without an indication for aspirin therapy; 3,688 propensity score–matched patients (1,844 in each group) were compared to assess rates of bleeding and rates of observed thrombosis at 1 year in patients taking warfarin alone versus warfarin plus aspirin. Patients treated with warfarin plus aspirin experienced more bleeding events than did patients on warfarin monotherapy (95% confidence interval, 23.8%-28.3% vs. 95% CI, 18.3%-22.3%; P less than .001). Rates of observed thrombosis were similar between the two groups (95% CI, 1.6%-3.1% vs. 95% CI, 2.0%-3.6%; P = .40). This study demonstrates that aspirin use without a clinical indication remains common in patients taking warfarin for AFib or VTE, and that reducing inappropriate aspirin use in this patient population may help prevent adverse outcomes.
Bottom line: Use of aspirin without a clinical indication in patients taking warfarin is common and is associated with an increased risk of bleeding without significant clinical benefit.
Citation: Schaefer JK et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019 Mar 4;179(4):533-41.
Dr. Wachter is an associate medical director at Duke Regional Hospital and an assistant professor of medicine at Duke University.
Background: Current guidelines recommend against using aspirin in combination with warfarin for patients with AFib, unless the patient has another indication for aspirin such as recent percutaneous coronary intervention (PCI) or a mechanical heart valve. These recommendations are based on limited clinical trial data that showed an increased risk of adverse events with combination therapy without clinical benefit. Despite these recommendations, recent studies have shown that aspirin use without a clinical indication remains common in patients taking warfarin for AFib. The prevalence of aspirin use without a clinical indication in patients taking warfarin for VTE is less well studied.
Study design: Registry-based cohort study.
Setting: Six anticoagulation clinics in Michigan.
Synopsis: Of the 6,539 patients included in the study, 2,453 patients (37.5%) were taking both warfarin and aspirin without an indication for aspirin therapy; 3,688 propensity score–matched patients (1,844 in each group) were compared to assess rates of bleeding and rates of observed thrombosis at 1 year in patients taking warfarin alone versus warfarin plus aspirin. Patients treated with warfarin plus aspirin experienced more bleeding events than did patients on warfarin monotherapy (95% confidence interval, 23.8%-28.3% vs. 95% CI, 18.3%-22.3%; P less than .001). Rates of observed thrombosis were similar between the two groups (95% CI, 1.6%-3.1% vs. 95% CI, 2.0%-3.6%; P = .40). This study demonstrates that aspirin use without a clinical indication remains common in patients taking warfarin for AFib or VTE, and that reducing inappropriate aspirin use in this patient population may help prevent adverse outcomes.
Bottom line: Use of aspirin without a clinical indication in patients taking warfarin is common and is associated with an increased risk of bleeding without significant clinical benefit.
Citation: Schaefer JK et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019 Mar 4;179(4):533-41.
Dr. Wachter is an associate medical director at Duke Regional Hospital and an assistant professor of medicine at Duke University.
I’m getting old (and it’s costing me)
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
How old is too old for statins?
ILLUSTRATIVE CASE
Ms. M is a 76-year-old woman with well-controlled type 2 diabetes mellitus for 10 years and well-controlled mild hypertension. She is otherwise healthy, and her mother lived to age 95. Ms. M has never smoked, has no previous history of vascular/cardiovascular disease, and drinks 1 glass of wine 2 to 3 times per week. Based on the American College of Cardiology (ACC) calculator, she was started on atorvastatin years ago. Is continued use of the medication of any benefit at her current age?
The 2018 American Heart Association (AHA)/ACC/Multi-Society cholesterol guidelines do not provide primary prevention recommendations for those older than age 75 years.3 Up to age 75, the guidelines recommend that patients with type 2 diabetes and a low-density lipoprotein cholesterol (LDL-C) level ≥ 70 mg/dL, as well as those without diabetes but with an LDL-C ≥ 70 mg/dL and a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥ 10%, be started on medium-intensity statin therapy.
A 2018 consensus panel review of the current literature, sponsored by the National Institute on Aging and the National Heart, Lung, and Blood Institute, concluded that there was insufficient evidence regarding the benefits and harms of statins in older adults, especially those with comorbidities, and that there was a paucity of evidence about statin therapy outcomes (both adverse and beneficial) relevant to older adults.4
A review of all guidelines published since 2013 revealed that only the United Kingdom’s 2014 National Institute for Health and Care Excellence (NICE) guideline provides a strong, risk-based recommendation for initiating primary prevention with statins in patients > 75 years old.5 These recommendations are based on the QRISK2 calculator (which has since been updated to the QRISK3), which assigns everyone ages > 75 years a > 10% 10-year risk score. This provides a universal statin indication for anyone in the 76-to-84 age range.6
Both the ACC/AHA and US Preventive Services Task Force guidelines clearly state that there are too few data and inadequate evidence in people older than 75 for a strong, risk-based statin recommendation.5 The Canadian Cardiovascular Society guideline takes a similar stance, emphasizing that the recommended Framingham risk model is not well validated in people > 75 years.5
STUDY SUMMARIES
Two different looks at statin use in the elderly
A retrospective cohort study (N = 46,864; median follow-up, 5.6 years) examined whether statin treatment is associated with a reduction in atherosclerotic disease and mortality in old and very old adults with and without type 2 diabetes.1 Patients were enrolled from a large, anonymized national database in Spain. The researchers looked only at first-time users of statins and those without a statin prescription within the past 18 months.
Patients with previous ASCVD, type 1 diabetes, previous lipid-lowering treatment, dementia, cancer, or paralysis were excluded, as were those who were in residential care, were on dialysis, or had received an organ transplant. Patients were stratified by age (75-84 years and ≥ 85 years), diabetes status (with or without type 2 diabetes), and statin use (nonuser or new user).
Continue to: Results
Results. For patients with type 2 diabetes, the risk of ASCVD (a composite of coronary heart disease and stroke) was lower among those who took statins than among those who did not in the 75-to-84 group (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.65-0.89; 1-year number needed to treat [NNT] = 164). Among those who took statins, there was also lower all-cause mortality (HR = 0.84; 95% CI, 0.75-0.94; 1-year NNT = 306). In those ages ≥ 85 years with diabetes, the statin group did not have a lower risk of ASCVD (HR = 0.82; 95% CI, 0.53-1.26) or all-cause mortality (HR = 1.05; 95% CI, 0.86-1.28).
For patients ages 75 to 84 years without diabetes, there was no difference in risk between groups for ASCVD (HR = 0.94; 95% CI, 0.86–1.04) or all-cause mortality (HR = 0.98; 95% CI, 0.91-1.05). In those ages ≥ 85 years without diabetes, there was also no difference between groups for ASCVD (HR = 1; 95% CI, 0.80-1.24) or for all-cause mortality (HR = 1; 95% CI, 0.90-1.11).
A 2019 meta-analysis of randomized controlled trials (RCTs) (n = 134,537) and RCT summary data (n = 12,705) evaluated the safety and efficacy of statin therapy in patients ages ≥ 55 years.2 In the group of patients ages > 75 years (n = 14,483; median follow-up, 4.9 years), each 1 mmol/L reduction in LDL-C was associated with significant decreased risk for major vascular events (risk ratio [RR] = 0.82; 95% CI, 0.70-0.95) and for major coronary events (RR = 0.82; 95% CI, 0.70-0.96).
In subgroup analysis by the presence or absence of previous vascular disease, there was a decreased risk per 1 mmol/L LDL-C reduction of major vascular events in patients with previous vascular disease (RR = 0.85; 95% CI, 0.73-0.98); however, there was not a significant effect in patients without previous vascular disease (RR = 0.92; 95% CI, 0.73-1.16).
WHAT’S NEW
Statins may be unnecessary in older adults without ASCVD or T2DM
Statin therapy reduces the risk of ASCVD and mortality in patients ages 75 to 84 with type 2 diabetes and in patients > 75 years with known vascular disease. However, statin therapy seems to provide no benefit in patients ages > 75 years without ASCVD or in patients ages ≥ 85 years without ASCVD, regardless of type 2 diabetes status.
Continue to: CAVEATS
CAVEATS
Retrospective cohort design leaves cause and effect equivocal
Even though the first study was large (with more than 46,000 patients) and the median follow-up was 5.6 years, it was a retrospective cohort study. While there is clearly an association between statin therapy and reduced ASCVD and all-cause mortality in patients with diabetes ages 75 to 84 years, cause and effect cannot be unequivocally stated. However, the meta-analysis, which included RCTs, confirms the benefit of statins in secondary prevention for older patients.
The cohort study did not look at adverse effects from statin therapy in this age group, but the data from the 2019 meta-analysis did not reveal any significant risk of myopathy.
CHALLENGES TO IMPLEMENTATION
Guidelines are lacking and discontinuing meds requires discussion
The lack of supporting guidelines to treat this age group with statins remains the largest barrier to implementation. Many patients may already be taking a statin, so a discussion about discontinuing medication will need to be initiated.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
3. Stone NJ, Grundy SM. The 2018 AHA/ACC/Multi-Society cholesterol guidelines: looking at past, present and future. Prog Cardiovasc Dis. 2019;62:375-383.
4. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults—moving towards evidence-based decision-making. J Am Geriatr Soc. 2018;66:2188-2196.
5. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85-94.
6. ClinRisk. Welcome to the QRISK®3-2018 risk calculator. www.qrisk.org/three/. Accessed May 27, 2020.
ILLUSTRATIVE CASE
Ms. M is a 76-year-old woman with well-controlled type 2 diabetes mellitus for 10 years and well-controlled mild hypertension. She is otherwise healthy, and her mother lived to age 95. Ms. M has never smoked, has no previous history of vascular/cardiovascular disease, and drinks 1 glass of wine 2 to 3 times per week. Based on the American College of Cardiology (ACC) calculator, she was started on atorvastatin years ago. Is continued use of the medication of any benefit at her current age?
The 2018 American Heart Association (AHA)/ACC/Multi-Society cholesterol guidelines do not provide primary prevention recommendations for those older than age 75 years.3 Up to age 75, the guidelines recommend that patients with type 2 diabetes and a low-density lipoprotein cholesterol (LDL-C) level ≥ 70 mg/dL, as well as those without diabetes but with an LDL-C ≥ 70 mg/dL and a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥ 10%, be started on medium-intensity statin therapy.
A 2018 consensus panel review of the current literature, sponsored by the National Institute on Aging and the National Heart, Lung, and Blood Institute, concluded that there was insufficient evidence regarding the benefits and harms of statins in older adults, especially those with comorbidities, and that there was a paucity of evidence about statin therapy outcomes (both adverse and beneficial) relevant to older adults.4
A review of all guidelines published since 2013 revealed that only the United Kingdom’s 2014 National Institute for Health and Care Excellence (NICE) guideline provides a strong, risk-based recommendation for initiating primary prevention with statins in patients > 75 years old.5 These recommendations are based on the QRISK2 calculator (which has since been updated to the QRISK3), which assigns everyone ages > 75 years a > 10% 10-year risk score. This provides a universal statin indication for anyone in the 76-to-84 age range.6
Both the ACC/AHA and US Preventive Services Task Force guidelines clearly state that there are too few data and inadequate evidence in people older than 75 for a strong, risk-based statin recommendation.5 The Canadian Cardiovascular Society guideline takes a similar stance, emphasizing that the recommended Framingham risk model is not well validated in people > 75 years.5
STUDY SUMMARIES
Two different looks at statin use in the elderly
A retrospective cohort study (N = 46,864; median follow-up, 5.6 years) examined whether statin treatment is associated with a reduction in atherosclerotic disease and mortality in old and very old adults with and without type 2 diabetes.1 Patients were enrolled from a large, anonymized national database in Spain. The researchers looked only at first-time users of statins and those without a statin prescription within the past 18 months.
Patients with previous ASCVD, type 1 diabetes, previous lipid-lowering treatment, dementia, cancer, or paralysis were excluded, as were those who were in residential care, were on dialysis, or had received an organ transplant. Patients were stratified by age (75-84 years and ≥ 85 years), diabetes status (with or without type 2 diabetes), and statin use (nonuser or new user).
Continue to: Results
Results. For patients with type 2 diabetes, the risk of ASCVD (a composite of coronary heart disease and stroke) was lower among those who took statins than among those who did not in the 75-to-84 group (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.65-0.89; 1-year number needed to treat [NNT] = 164). Among those who took statins, there was also lower all-cause mortality (HR = 0.84; 95% CI, 0.75-0.94; 1-year NNT = 306). In those ages ≥ 85 years with diabetes, the statin group did not have a lower risk of ASCVD (HR = 0.82; 95% CI, 0.53-1.26) or all-cause mortality (HR = 1.05; 95% CI, 0.86-1.28).
For patients ages 75 to 84 years without diabetes, there was no difference in risk between groups for ASCVD (HR = 0.94; 95% CI, 0.86–1.04) or all-cause mortality (HR = 0.98; 95% CI, 0.91-1.05). In those ages ≥ 85 years without diabetes, there was also no difference between groups for ASCVD (HR = 1; 95% CI, 0.80-1.24) or for all-cause mortality (HR = 1; 95% CI, 0.90-1.11).
A 2019 meta-analysis of randomized controlled trials (RCTs) (n = 134,537) and RCT summary data (n = 12,705) evaluated the safety and efficacy of statin therapy in patients ages ≥ 55 years.2 In the group of patients ages > 75 years (n = 14,483; median follow-up, 4.9 years), each 1 mmol/L reduction in LDL-C was associated with significant decreased risk for major vascular events (risk ratio [RR] = 0.82; 95% CI, 0.70-0.95) and for major coronary events (RR = 0.82; 95% CI, 0.70-0.96).
In subgroup analysis by the presence or absence of previous vascular disease, there was a decreased risk per 1 mmol/L LDL-C reduction of major vascular events in patients with previous vascular disease (RR = 0.85; 95% CI, 0.73-0.98); however, there was not a significant effect in patients without previous vascular disease (RR = 0.92; 95% CI, 0.73-1.16).
WHAT’S NEW
Statins may be unnecessary in older adults without ASCVD or T2DM
Statin therapy reduces the risk of ASCVD and mortality in patients ages 75 to 84 with type 2 diabetes and in patients > 75 years with known vascular disease. However, statin therapy seems to provide no benefit in patients ages > 75 years without ASCVD or in patients ages ≥ 85 years without ASCVD, regardless of type 2 diabetes status.
Continue to: CAVEATS
CAVEATS
Retrospective cohort design leaves cause and effect equivocal
Even though the first study was large (with more than 46,000 patients) and the median follow-up was 5.6 years, it was a retrospective cohort study. While there is clearly an association between statin therapy and reduced ASCVD and all-cause mortality in patients with diabetes ages 75 to 84 years, cause and effect cannot be unequivocally stated. However, the meta-analysis, which included RCTs, confirms the benefit of statins in secondary prevention for older patients.
The cohort study did not look at adverse effects from statin therapy in this age group, but the data from the 2019 meta-analysis did not reveal any significant risk of myopathy.
CHALLENGES TO IMPLEMENTATION
Guidelines are lacking and discontinuing meds requires discussion
The lack of supporting guidelines to treat this age group with statins remains the largest barrier to implementation. Many patients may already be taking a statin, so a discussion about discontinuing medication will need to be initiated.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Ms. M is a 76-year-old woman with well-controlled type 2 diabetes mellitus for 10 years and well-controlled mild hypertension. She is otherwise healthy, and her mother lived to age 95. Ms. M has never smoked, has no previous history of vascular/cardiovascular disease, and drinks 1 glass of wine 2 to 3 times per week. Based on the American College of Cardiology (ACC) calculator, she was started on atorvastatin years ago. Is continued use of the medication of any benefit at her current age?
The 2018 American Heart Association (AHA)/ACC/Multi-Society cholesterol guidelines do not provide primary prevention recommendations for those older than age 75 years.3 Up to age 75, the guidelines recommend that patients with type 2 diabetes and a low-density lipoprotein cholesterol (LDL-C) level ≥ 70 mg/dL, as well as those without diabetes but with an LDL-C ≥ 70 mg/dL and a 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥ 10%, be started on medium-intensity statin therapy.
A 2018 consensus panel review of the current literature, sponsored by the National Institute on Aging and the National Heart, Lung, and Blood Institute, concluded that there was insufficient evidence regarding the benefits and harms of statins in older adults, especially those with comorbidities, and that there was a paucity of evidence about statin therapy outcomes (both adverse and beneficial) relevant to older adults.4
A review of all guidelines published since 2013 revealed that only the United Kingdom’s 2014 National Institute for Health and Care Excellence (NICE) guideline provides a strong, risk-based recommendation for initiating primary prevention with statins in patients > 75 years old.5 These recommendations are based on the QRISK2 calculator (which has since been updated to the QRISK3), which assigns everyone ages > 75 years a > 10% 10-year risk score. This provides a universal statin indication for anyone in the 76-to-84 age range.6
Both the ACC/AHA and US Preventive Services Task Force guidelines clearly state that there are too few data and inadequate evidence in people older than 75 for a strong, risk-based statin recommendation.5 The Canadian Cardiovascular Society guideline takes a similar stance, emphasizing that the recommended Framingham risk model is not well validated in people > 75 years.5
STUDY SUMMARIES
Two different looks at statin use in the elderly
A retrospective cohort study (N = 46,864; median follow-up, 5.6 years) examined whether statin treatment is associated with a reduction in atherosclerotic disease and mortality in old and very old adults with and without type 2 diabetes.1 Patients were enrolled from a large, anonymized national database in Spain. The researchers looked only at first-time users of statins and those without a statin prescription within the past 18 months.
Patients with previous ASCVD, type 1 diabetes, previous lipid-lowering treatment, dementia, cancer, or paralysis were excluded, as were those who were in residential care, were on dialysis, or had received an organ transplant. Patients were stratified by age (75-84 years and ≥ 85 years), diabetes status (with or without type 2 diabetes), and statin use (nonuser or new user).
Continue to: Results
Results. For patients with type 2 diabetes, the risk of ASCVD (a composite of coronary heart disease and stroke) was lower among those who took statins than among those who did not in the 75-to-84 group (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.65-0.89; 1-year number needed to treat [NNT] = 164). Among those who took statins, there was also lower all-cause mortality (HR = 0.84; 95% CI, 0.75-0.94; 1-year NNT = 306). In those ages ≥ 85 years with diabetes, the statin group did not have a lower risk of ASCVD (HR = 0.82; 95% CI, 0.53-1.26) or all-cause mortality (HR = 1.05; 95% CI, 0.86-1.28).
For patients ages 75 to 84 years without diabetes, there was no difference in risk between groups for ASCVD (HR = 0.94; 95% CI, 0.86–1.04) or all-cause mortality (HR = 0.98; 95% CI, 0.91-1.05). In those ages ≥ 85 years without diabetes, there was also no difference between groups for ASCVD (HR = 1; 95% CI, 0.80-1.24) or for all-cause mortality (HR = 1; 95% CI, 0.90-1.11).
A 2019 meta-analysis of randomized controlled trials (RCTs) (n = 134,537) and RCT summary data (n = 12,705) evaluated the safety and efficacy of statin therapy in patients ages ≥ 55 years.2 In the group of patients ages > 75 years (n = 14,483; median follow-up, 4.9 years), each 1 mmol/L reduction in LDL-C was associated with significant decreased risk for major vascular events (risk ratio [RR] = 0.82; 95% CI, 0.70-0.95) and for major coronary events (RR = 0.82; 95% CI, 0.70-0.96).
In subgroup analysis by the presence or absence of previous vascular disease, there was a decreased risk per 1 mmol/L LDL-C reduction of major vascular events in patients with previous vascular disease (RR = 0.85; 95% CI, 0.73-0.98); however, there was not a significant effect in patients without previous vascular disease (RR = 0.92; 95% CI, 0.73-1.16).
WHAT’S NEW
Statins may be unnecessary in older adults without ASCVD or T2DM
Statin therapy reduces the risk of ASCVD and mortality in patients ages 75 to 84 with type 2 diabetes and in patients > 75 years with known vascular disease. However, statin therapy seems to provide no benefit in patients ages > 75 years without ASCVD or in patients ages ≥ 85 years without ASCVD, regardless of type 2 diabetes status.
Continue to: CAVEATS
CAVEATS
Retrospective cohort design leaves cause and effect equivocal
Even though the first study was large (with more than 46,000 patients) and the median follow-up was 5.6 years, it was a retrospective cohort study. While there is clearly an association between statin therapy and reduced ASCVD and all-cause mortality in patients with diabetes ages 75 to 84 years, cause and effect cannot be unequivocally stated. However, the meta-analysis, which included RCTs, confirms the benefit of statins in secondary prevention for older patients.
The cohort study did not look at adverse effects from statin therapy in this age group, but the data from the 2019 meta-analysis did not reveal any significant risk of myopathy.
CHALLENGES TO IMPLEMENTATION
Guidelines are lacking and discontinuing meds requires discussion
The lack of supporting guidelines to treat this age group with statins remains the largest barrier to implementation. Many patients may already be taking a statin, so a discussion about discontinuing medication will need to be initiated.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
3. Stone NJ, Grundy SM. The 2018 AHA/ACC/Multi-Society cholesterol guidelines: looking at past, present and future. Prog Cardiovasc Dis. 2019;62:375-383.
4. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults—moving towards evidence-based decision-making. J Am Geriatr Soc. 2018;66:2188-2196.
5. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85-94.
6. ClinRisk. Welcome to the QRISK®3-2018 risk calculator. www.qrisk.org/three/. Accessed May 27, 2020.
1. Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
3. Stone NJ, Grundy SM. The 2018 AHA/ACC/Multi-Society cholesterol guidelines: looking at past, present and future. Prog Cardiovasc Dis. 2019;62:375-383.
4. Singh S, Zieman S, Go AS, et al. Statins for primary prevention in older adults—moving towards evidence-based decision-making. J Am Geriatr Soc. 2018;66:2188-2196.
5. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85-94.
6. ClinRisk. Welcome to the QRISK®3-2018 risk calculator. www.qrisk.org/three/. Accessed May 27, 2020.
PRACTICE CHANGER
Do not start a statin in patients ages ≥ 75 years who do not have known vascular disease or type 2 diabetes; start or continue a statin in all patients ages 75 to 84 with type 2 diabetes to prevent cardiovascular events and mortality; and start or continue a statin in patients ages > 75 years who have known vascular occlusive disease.
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis of randomized controlled trials and a retrospective cohort study.
Ramos R, Comas-Cufi M, Marti-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359.1
Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.2
Cardiology societies unite to denounce racist violence
The death of George Floyd and other African Americans spurred the Association of Black Cardiologists, the American Heart Association, and the American College of Cardiology to join forces and issue an urgent letter denouncing recent and ongoing events.
Starting off by acknowledging that these are “difficult and disturbing times,” the presidents of the three societies tied the violence into the bigger public health picture. “Like cardiovascular disease, acts of violence and racism are core causes of psychosocial stress that promote poor well-being and cardiovascular health, especially for communities of color.”
“It’s not just one quick solution, one quick letter. It’s more of an ongoing project to raise awareness and have really defined projects. We want to have goals, tactics, and measurable outcomes. We want to make sure it’s not just a banner on the wall,” Athena Poppas, MD, president of the American College of Cardiology and one of three physicians signing the letter, said in an interview.
The Association of Black Cardiologists drafted the statement and asked the AHA and ACC if they wanted to sign on. “It felt important to join them and follow their lead,” she said. “There is a clear link between psychosocial stress and discrimination and health equity in the communities.”
Interestingly, the ABC and ACC have an existing partnership, one that included creating a “Campaign for the Future” a little more than a year ago. One of the focuses is on reducing health disparities and starting a diversity and inclusion task force that later became a committee. The groups held a joint board of trustees meeting at Morehouse University, Atlanta, in January 2020. Thinking about that time, Dr. Poppas added, “who knew what was about to transpire over the next few months?”
The letter is only one component of an ongoing effort to “find concrete ways to make change, both within the college and within our profession,” added Dr. Poppas, chief of cardiology and professor of medicine at Brown University, Providence, R.I., and director of the Lifespan Cardiovascular Institute of Rhode Island, Miriam Hospitals, and Newport Hospitals. “Thereby, there is good data that you affect health equity in the population as well.”
“We DENOUNCE incidents of racism and violence that continue to ravage our communities,” the society leaders wrote in the letter. “Given that heart disease and stroke are the leading causes of death for communities of color, particularly African Americans who have the lowest life expectancy of all racial/ethnic groups living in the United States, we are extremely disturbed by violent acts that cut to the core of the lives of our community.”
Other societies released similar statements. For example, the American College of Physicians expressed “grave concern” about recent events and the American Medical Association released a statement entitled “Police brutality must stop.”
A cardiologist speaks out
“Thank you to my organizations, the Association of Black Cardiologists and the American College of Cardiology, for taking a stand,” Travis C. Batts, MD, said in a video statement posted to YouTube on June 2, 2020.
“As an African American male who has sons, brothers, and friends who are also African American, I oftentimes have angst, particularly with my sons. Despite what I do to create an environment that cultivates education and puts them in the right position, there are some people who would stop just at how they look when they approach them,” Dr. Batts said.
“I always have that fear as a father that at some point they may engage with law enforcement – and it may not turn out the way we want it to,” said Dr. Batts, chairman of medical sub-specialties and medical director of the cardiology clinic at Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base, Tex. He also is an associate professor of cardiovascular medicine for the Uniformed Services University of the Health Sciences, Bethesda, Md., and is an adjunct assistant professor at Texas A&M University. He went on in the video to describe how a personal encounter with police years ago changed his life.
The urgent letter from the cardiology societies speaks to health care disparities, Dr. Batts said, “but it doesn’t stop there. It talks about their goals to balance these issues that we see as a pervasive problem in our community.”
The societies point out that George Floyd’s death is not an isolated incident. “Mr. Floyd’s death comes on the heels of other recent incidents caught on camera. In another 2020 incident, Ahmaud Arbery was shot and killed while jogging in his hometown of Brunswick, Ga. Christian Cooper is fortunately alive and well to speak to the Memorial Day incident in New York’s Central Park where he was accused of threatening the life of a woman while bird watching.” They added that “another senseless death involves officers entering the Louisville, Kent., home of emergency medical technician Breonna Taylor.”
Dr. Batts said this portion of the statement was particularly poignant: “We stand and link arms in solidarity with efforts to dismantle systems that maintain excess morbidity and mortality, especially among vulnerable populations and those historically oppressed. Indeed, our collective vast membership, many of whom are at the front lines of clinical health care, has taken an oath to decisively and with kindness, compassion and grace act to relieve suffering related to ‘I can’t breathe’ in order to preserve life.”
A Positive Response
The response to the urgent letter has been “overwhelmingly positive,” Dr. Poppas said. “This isn’t political, per se. This is really about justice, about health equity, and about being moral and conscious human beings. People I hadn’t heard from in years said, ‘thank you for doing this.’ ” The comments on social media were “almost uniformly positive,” she added. “There is always one or two people who feel this isn’t what cardiology is about.”
“Although making a statement is important, so is doing the hard work to make change,” Dr. Poppas said. The goal involves “rolling up our sleeves and spending the time, the money and the energy to make changes – so 5-10 years from now, it looks different.”
In addition to Dr. Poppas, Michelle A. Albert, MD, MPH, president of the Association of Black Cardiologists and Robert A. Harrington, MD, president of the American Heart Association, signed the letter. Dr. Pappas and Dr. Batts had no relevant disclosures.
The death of George Floyd and other African Americans spurred the Association of Black Cardiologists, the American Heart Association, and the American College of Cardiology to join forces and issue an urgent letter denouncing recent and ongoing events.
Starting off by acknowledging that these are “difficult and disturbing times,” the presidents of the three societies tied the violence into the bigger public health picture. “Like cardiovascular disease, acts of violence and racism are core causes of psychosocial stress that promote poor well-being and cardiovascular health, especially for communities of color.”
“It’s not just one quick solution, one quick letter. It’s more of an ongoing project to raise awareness and have really defined projects. We want to have goals, tactics, and measurable outcomes. We want to make sure it’s not just a banner on the wall,” Athena Poppas, MD, president of the American College of Cardiology and one of three physicians signing the letter, said in an interview.
The Association of Black Cardiologists drafted the statement and asked the AHA and ACC if they wanted to sign on. “It felt important to join them and follow their lead,” she said. “There is a clear link between psychosocial stress and discrimination and health equity in the communities.”
Interestingly, the ABC and ACC have an existing partnership, one that included creating a “Campaign for the Future” a little more than a year ago. One of the focuses is on reducing health disparities and starting a diversity and inclusion task force that later became a committee. The groups held a joint board of trustees meeting at Morehouse University, Atlanta, in January 2020. Thinking about that time, Dr. Poppas added, “who knew what was about to transpire over the next few months?”
The letter is only one component of an ongoing effort to “find concrete ways to make change, both within the college and within our profession,” added Dr. Poppas, chief of cardiology and professor of medicine at Brown University, Providence, R.I., and director of the Lifespan Cardiovascular Institute of Rhode Island, Miriam Hospitals, and Newport Hospitals. “Thereby, there is good data that you affect health equity in the population as well.”
“We DENOUNCE incidents of racism and violence that continue to ravage our communities,” the society leaders wrote in the letter. “Given that heart disease and stroke are the leading causes of death for communities of color, particularly African Americans who have the lowest life expectancy of all racial/ethnic groups living in the United States, we are extremely disturbed by violent acts that cut to the core of the lives of our community.”
Other societies released similar statements. For example, the American College of Physicians expressed “grave concern” about recent events and the American Medical Association released a statement entitled “Police brutality must stop.”
A cardiologist speaks out
“Thank you to my organizations, the Association of Black Cardiologists and the American College of Cardiology, for taking a stand,” Travis C. Batts, MD, said in a video statement posted to YouTube on June 2, 2020.
“As an African American male who has sons, brothers, and friends who are also African American, I oftentimes have angst, particularly with my sons. Despite what I do to create an environment that cultivates education and puts them in the right position, there are some people who would stop just at how they look when they approach them,” Dr. Batts said.
“I always have that fear as a father that at some point they may engage with law enforcement – and it may not turn out the way we want it to,” said Dr. Batts, chairman of medical sub-specialties and medical director of the cardiology clinic at Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base, Tex. He also is an associate professor of cardiovascular medicine for the Uniformed Services University of the Health Sciences, Bethesda, Md., and is an adjunct assistant professor at Texas A&M University. He went on in the video to describe how a personal encounter with police years ago changed his life.
The urgent letter from the cardiology societies speaks to health care disparities, Dr. Batts said, “but it doesn’t stop there. It talks about their goals to balance these issues that we see as a pervasive problem in our community.”
The societies point out that George Floyd’s death is not an isolated incident. “Mr. Floyd’s death comes on the heels of other recent incidents caught on camera. In another 2020 incident, Ahmaud Arbery was shot and killed while jogging in his hometown of Brunswick, Ga. Christian Cooper is fortunately alive and well to speak to the Memorial Day incident in New York’s Central Park where he was accused of threatening the life of a woman while bird watching.” They added that “another senseless death involves officers entering the Louisville, Kent., home of emergency medical technician Breonna Taylor.”
Dr. Batts said this portion of the statement was particularly poignant: “We stand and link arms in solidarity with efforts to dismantle systems that maintain excess morbidity and mortality, especially among vulnerable populations and those historically oppressed. Indeed, our collective vast membership, many of whom are at the front lines of clinical health care, has taken an oath to decisively and with kindness, compassion and grace act to relieve suffering related to ‘I can’t breathe’ in order to preserve life.”
A Positive Response
The response to the urgent letter has been “overwhelmingly positive,” Dr. Poppas said. “This isn’t political, per se. This is really about justice, about health equity, and about being moral and conscious human beings. People I hadn’t heard from in years said, ‘thank you for doing this.’ ” The comments on social media were “almost uniformly positive,” she added. “There is always one or two people who feel this isn’t what cardiology is about.”
“Although making a statement is important, so is doing the hard work to make change,” Dr. Poppas said. The goal involves “rolling up our sleeves and spending the time, the money and the energy to make changes – so 5-10 years from now, it looks different.”
In addition to Dr. Poppas, Michelle A. Albert, MD, MPH, president of the Association of Black Cardiologists and Robert A. Harrington, MD, president of the American Heart Association, signed the letter. Dr. Pappas and Dr. Batts had no relevant disclosures.
The death of George Floyd and other African Americans spurred the Association of Black Cardiologists, the American Heart Association, and the American College of Cardiology to join forces and issue an urgent letter denouncing recent and ongoing events.
Starting off by acknowledging that these are “difficult and disturbing times,” the presidents of the three societies tied the violence into the bigger public health picture. “Like cardiovascular disease, acts of violence and racism are core causes of psychosocial stress that promote poor well-being and cardiovascular health, especially for communities of color.”
“It’s not just one quick solution, one quick letter. It’s more of an ongoing project to raise awareness and have really defined projects. We want to have goals, tactics, and measurable outcomes. We want to make sure it’s not just a banner on the wall,” Athena Poppas, MD, president of the American College of Cardiology and one of three physicians signing the letter, said in an interview.
The Association of Black Cardiologists drafted the statement and asked the AHA and ACC if they wanted to sign on. “It felt important to join them and follow their lead,” she said. “There is a clear link between psychosocial stress and discrimination and health equity in the communities.”
Interestingly, the ABC and ACC have an existing partnership, one that included creating a “Campaign for the Future” a little more than a year ago. One of the focuses is on reducing health disparities and starting a diversity and inclusion task force that later became a committee. The groups held a joint board of trustees meeting at Morehouse University, Atlanta, in January 2020. Thinking about that time, Dr. Poppas added, “who knew what was about to transpire over the next few months?”
The letter is only one component of an ongoing effort to “find concrete ways to make change, both within the college and within our profession,” added Dr. Poppas, chief of cardiology and professor of medicine at Brown University, Providence, R.I., and director of the Lifespan Cardiovascular Institute of Rhode Island, Miriam Hospitals, and Newport Hospitals. “Thereby, there is good data that you affect health equity in the population as well.”
“We DENOUNCE incidents of racism and violence that continue to ravage our communities,” the society leaders wrote in the letter. “Given that heart disease and stroke are the leading causes of death for communities of color, particularly African Americans who have the lowest life expectancy of all racial/ethnic groups living in the United States, we are extremely disturbed by violent acts that cut to the core of the lives of our community.”
Other societies released similar statements. For example, the American College of Physicians expressed “grave concern” about recent events and the American Medical Association released a statement entitled “Police brutality must stop.”
A cardiologist speaks out
“Thank you to my organizations, the Association of Black Cardiologists and the American College of Cardiology, for taking a stand,” Travis C. Batts, MD, said in a video statement posted to YouTube on June 2, 2020.
“As an African American male who has sons, brothers, and friends who are also African American, I oftentimes have angst, particularly with my sons. Despite what I do to create an environment that cultivates education and puts them in the right position, there are some people who would stop just at how they look when they approach them,” Dr. Batts said.
“I always have that fear as a father that at some point they may engage with law enforcement – and it may not turn out the way we want it to,” said Dr. Batts, chairman of medical sub-specialties and medical director of the cardiology clinic at Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base, Tex. He also is an associate professor of cardiovascular medicine for the Uniformed Services University of the Health Sciences, Bethesda, Md., and is an adjunct assistant professor at Texas A&M University. He went on in the video to describe how a personal encounter with police years ago changed his life.
The urgent letter from the cardiology societies speaks to health care disparities, Dr. Batts said, “but it doesn’t stop there. It talks about their goals to balance these issues that we see as a pervasive problem in our community.”
The societies point out that George Floyd’s death is not an isolated incident. “Mr. Floyd’s death comes on the heels of other recent incidents caught on camera. In another 2020 incident, Ahmaud Arbery was shot and killed while jogging in his hometown of Brunswick, Ga. Christian Cooper is fortunately alive and well to speak to the Memorial Day incident in New York’s Central Park where he was accused of threatening the life of a woman while bird watching.” They added that “another senseless death involves officers entering the Louisville, Kent., home of emergency medical technician Breonna Taylor.”
Dr. Batts said this portion of the statement was particularly poignant: “We stand and link arms in solidarity with efforts to dismantle systems that maintain excess morbidity and mortality, especially among vulnerable populations and those historically oppressed. Indeed, our collective vast membership, many of whom are at the front lines of clinical health care, has taken an oath to decisively and with kindness, compassion and grace act to relieve suffering related to ‘I can’t breathe’ in order to preserve life.”
A Positive Response
The response to the urgent letter has been “overwhelmingly positive,” Dr. Poppas said. “This isn’t political, per se. This is really about justice, about health equity, and about being moral and conscious human beings. People I hadn’t heard from in years said, ‘thank you for doing this.’ ” The comments on social media were “almost uniformly positive,” she added. “There is always one or two people who feel this isn’t what cardiology is about.”
“Although making a statement is important, so is doing the hard work to make change,” Dr. Poppas said. The goal involves “rolling up our sleeves and spending the time, the money and the energy to make changes – so 5-10 years from now, it looks different.”
In addition to Dr. Poppas, Michelle A. Albert, MD, MPH, president of the Association of Black Cardiologists and Robert A. Harrington, MD, president of the American Heart Association, signed the letter. Dr. Pappas and Dr. Batts had no relevant disclosures.