User login
Is neuroimaging necessary to evaluate syncope?
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
Click for Credit: Suicide in Medicaid youth; persistent back pain; more
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
More chest compression–only CPR leads to increased survival rates
according to a Swedish study of out-of-hospital cardiac arrests and subsequent CPR.
“These findings support continuous endorsement of chest compression–only CPR as an option in future CPR guidelines because it is associated with higher CPR rates and survival in out-of-hospital cardiac arrests,” wrote Gabriel Riva, MD, of the Karolinska Institutet, Stockholm, and his coauthors. The study was published in Circulation.
To determine changes in the rate and type of CPR performed before emergency medical services (EMS) arrival, the researchers compared all bystander-witnessed out-of-hospital cardiac arrests (OHCAs) reported in Sweden between 2000 and 2017. In all, 30,445 patients were included; the time periods compared were 2000-2005, 2006-2010, and 2011-2017. Patients were categorized as receiving either no CPR (NO-CPR), standard CPR (S-CPR), or chest compression–only CPR (CO-CPR). In 2005, CO-CPR was introduced in national CPR guidelines as an option for bystanders; in 2010, it was recommended for anyone untrained in CPR.
The proportion of patients who received CPR in general increased from 41% in 2000-2005 to 59% in 2006-2010 to 68% in 2011-2017. S-CPR changed from 35% to 45% to 38% over the three periods, while CO-CPR increased from 5% to 14% to 30%. In regard to 30-day survival rates, the S-CPR group saw an increase from 9% to 13% to 16% and the CO-CPR group increased from 8% to 12% to 14%, compared with 4% to 6% to 7% for the NO-CPR group.
The authors noted the limitations of their study, including the results being based on register data and therefore subject to misclassification and missing data. In addition, missing data negated any reporting on the neurological function of survivors; analyzing witnessed OHCAs only also meant the findings could not be validated for nonwitnessed OHCA.
The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
SOURCE: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
according to a Swedish study of out-of-hospital cardiac arrests and subsequent CPR.
“These findings support continuous endorsement of chest compression–only CPR as an option in future CPR guidelines because it is associated with higher CPR rates and survival in out-of-hospital cardiac arrests,” wrote Gabriel Riva, MD, of the Karolinska Institutet, Stockholm, and his coauthors. The study was published in Circulation.
To determine changes in the rate and type of CPR performed before emergency medical services (EMS) arrival, the researchers compared all bystander-witnessed out-of-hospital cardiac arrests (OHCAs) reported in Sweden between 2000 and 2017. In all, 30,445 patients were included; the time periods compared were 2000-2005, 2006-2010, and 2011-2017. Patients were categorized as receiving either no CPR (NO-CPR), standard CPR (S-CPR), or chest compression–only CPR (CO-CPR). In 2005, CO-CPR was introduced in national CPR guidelines as an option for bystanders; in 2010, it was recommended for anyone untrained in CPR.
The proportion of patients who received CPR in general increased from 41% in 2000-2005 to 59% in 2006-2010 to 68% in 2011-2017. S-CPR changed from 35% to 45% to 38% over the three periods, while CO-CPR increased from 5% to 14% to 30%. In regard to 30-day survival rates, the S-CPR group saw an increase from 9% to 13% to 16% and the CO-CPR group increased from 8% to 12% to 14%, compared with 4% to 6% to 7% for the NO-CPR group.
The authors noted the limitations of their study, including the results being based on register data and therefore subject to misclassification and missing data. In addition, missing data negated any reporting on the neurological function of survivors; analyzing witnessed OHCAs only also meant the findings could not be validated for nonwitnessed OHCA.
The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
SOURCE: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
according to a Swedish study of out-of-hospital cardiac arrests and subsequent CPR.
“These findings support continuous endorsement of chest compression–only CPR as an option in future CPR guidelines because it is associated with higher CPR rates and survival in out-of-hospital cardiac arrests,” wrote Gabriel Riva, MD, of the Karolinska Institutet, Stockholm, and his coauthors. The study was published in Circulation.
To determine changes in the rate and type of CPR performed before emergency medical services (EMS) arrival, the researchers compared all bystander-witnessed out-of-hospital cardiac arrests (OHCAs) reported in Sweden between 2000 and 2017. In all, 30,445 patients were included; the time periods compared were 2000-2005, 2006-2010, and 2011-2017. Patients were categorized as receiving either no CPR (NO-CPR), standard CPR (S-CPR), or chest compression–only CPR (CO-CPR). In 2005, CO-CPR was introduced in national CPR guidelines as an option for bystanders; in 2010, it was recommended for anyone untrained in CPR.
The proportion of patients who received CPR in general increased from 41% in 2000-2005 to 59% in 2006-2010 to 68% in 2011-2017. S-CPR changed from 35% to 45% to 38% over the three periods, while CO-CPR increased from 5% to 14% to 30%. In regard to 30-day survival rates, the S-CPR group saw an increase from 9% to 13% to 16% and the CO-CPR group increased from 8% to 12% to 14%, compared with 4% to 6% to 7% for the NO-CPR group.
The authors noted the limitations of their study, including the results being based on register data and therefore subject to misclassification and missing data. In addition, missing data negated any reporting on the neurological function of survivors; analyzing witnessed OHCAs only also meant the findings could not be validated for nonwitnessed OHCA.
The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
SOURCE: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
FROM CIRCULATION
Key clinical point: Since chest compression-only CPR was introduced and recommended as an alternative for bystanders witnessing a cardiac arrest, CPR rates and survival rates have increased.
Major finding: From 2001-2005 to 2011-2017, 30-day survival rates increased from 9% to 16% for the standard CPR group and from 8% to 14% for the chest compression–only group, compared with 4%-7% for the no CPR group.
Study details: An observational nationwide cohort study of 30,445 Swedish patients who suffered out-of-hospital cardiac arrest.
Disclosures: The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
Source: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
Management of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
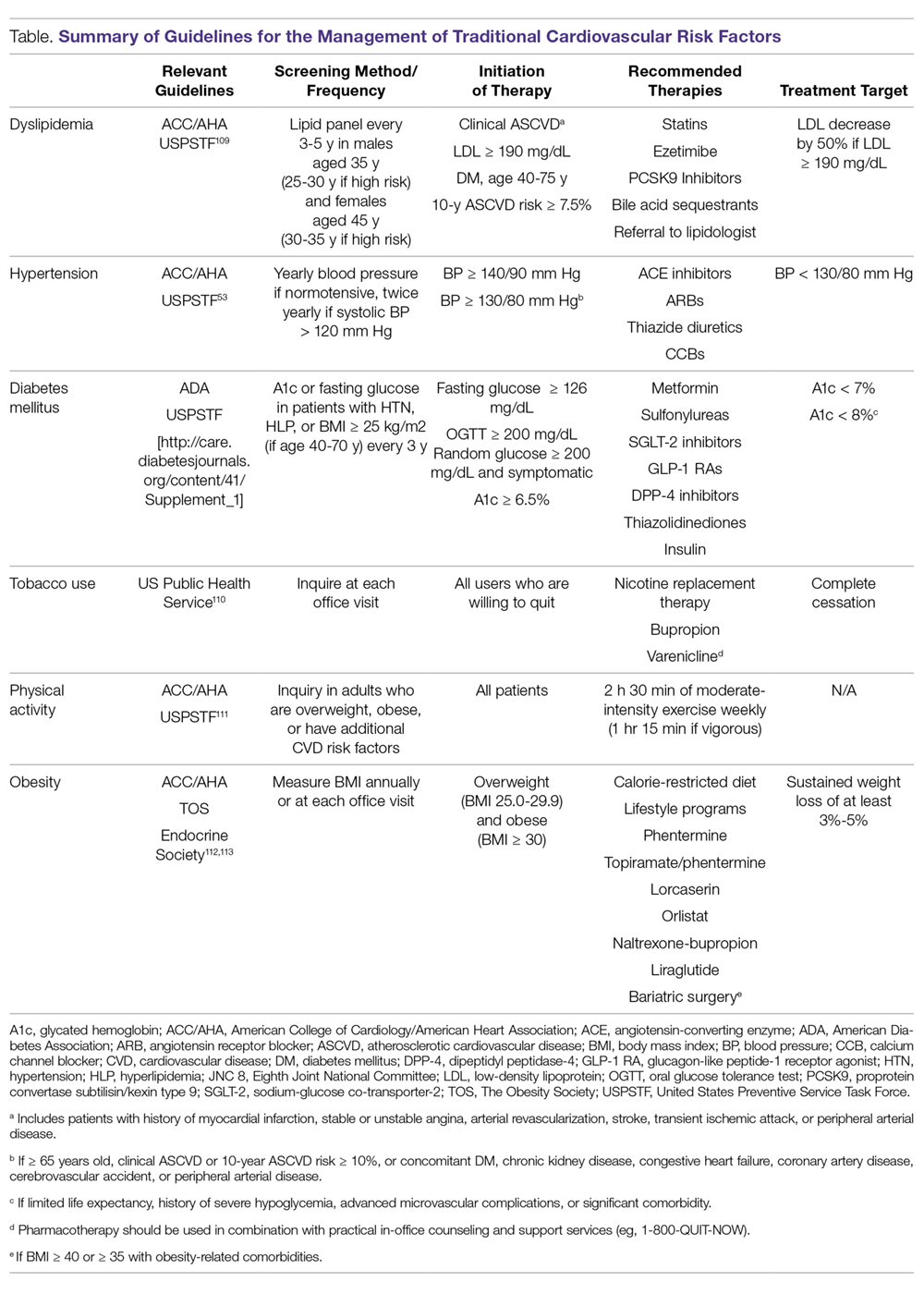
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
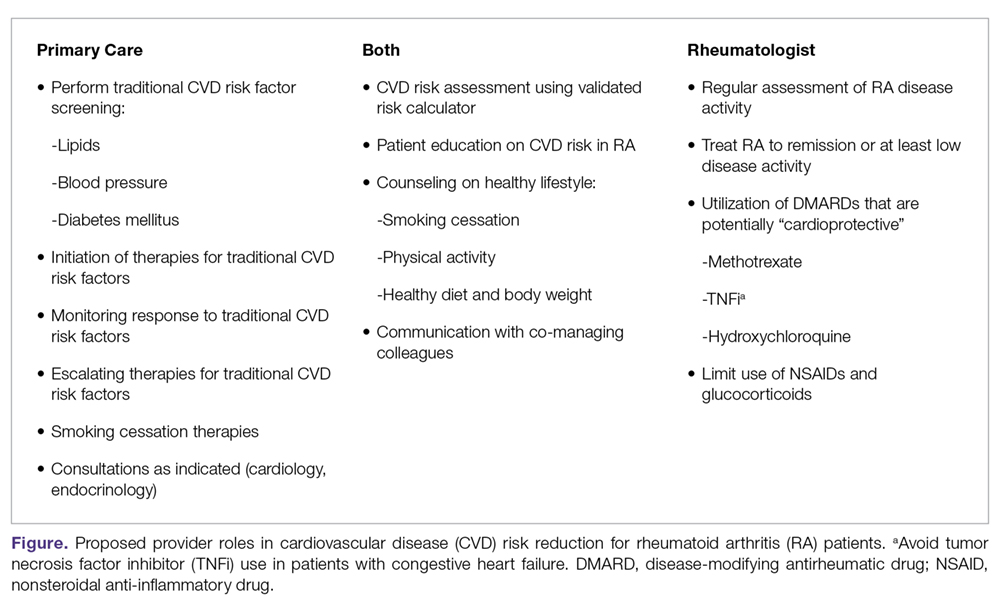
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rh
Abstract
- Objective: To review the management of traditional and nontraditional CVD cardiovascular disease risk factors in rheumatoid arthritis (RA).
- Methods: Literature review of the management of CVD risk in RA.
- Results: Because of the increased risk of CVD events and CVD mortality among RA patients, aggressive management of CVD risk is essential. Providers should follow national guidelines for the management of traditional CVD risk factors, including dyslipidemia, hypertension, and diabetes mellitus. Similar efforts are needed in counseling on lifestyle modifications, including smoking cessation, regular exercise, and maintaining a healthy body weight. Because higher RA disease activity is also linked with CVD risk, aggressive treatment of RA to a target of low disease activity or remission is critical. Furthermore, the selection of potentially “cardioprotective” agents such as methotrexate and tumor necrosis factor inhibitors, while limiting use of nonsteroidal anti-inflammatory drugs and glucocorticoids, are strategies that could be employed by rheumatologists to help mitigate CVD risk in their patients with RA.
- Conclusion: Routine assessment of CVD risk, management of traditional CVD risk factors, counseling on healthy lifestyle habits, and aggressive treatment of RA are essential to minimize CVD risk in this population.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment; cardiovascular risk management.
Editor’s note: This article is part 2 of a 2-part article. “Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the January/February 2019 issue.
Rheumatoid arthritis (RA) is a systemic autoimmune condition that contributes to an increased risk for cardiovascular disease (CVD) among affected patients. In persons with RA, the risk of incident CVD and CVD mortality are increased by approximately 50% compared with the general population.1,2 To minimize CVD risk in this population, providers must routinely assess for CVD risk factors3 and aggressively manage both traditional and nontraditional CVD risk factors.
Managing Traditional Risk Factors
As in the general population, identification and management of traditional CVD risk factors are crucial to minimize CVD risk in the RA population. A prospective study of 201 RA patients demonstrated that traditional CVD risk factors were in fact more predictive of endothelial dysfunction and carotid atherosclerosis than were disease-related inflammatory markers in RA.4 Management of traditional risk factors is detailed in the following sections, and recommendations for managing all traditional CVD risk factors are summarized in the Table.
Dyslipidemia
The role of dyslipidemia in atherogenesis is well established, and as a result, lipid levels are nearly universally included in CVD risk stratification tools. However, the interpretation of lipid levels in the context of RA is challenging because of the effects of systemic inflammation on their absolute values. Compared to the general population, patients with RA have lower total cholesterol (TC) and low-density lipoprotein (LDL) levels independent of lipid-lowering therapy.5,6 Despite this, RA patients are at increased risk for CVD. There is even some evidence to suggest a “lipid paradox” in RA, whereby lower TC (< 4 mmol/L) and LDL levels suggest an increased risk of CVD.7,8 In contrast to LDL, higher levels of high-density lipoprotein (HDL) are typically associated with reduced CVD risk, as in the general population.8,9 Interestingly, in a cohort of 16,085 RA patients and 48,499 age- and sex-matched controls, there was no significant difference in the relationship between LDL and CVD risk, suggesting that quantitative lipid levels alone may not entirely explain the CVD mortality gap in RA.9 As such, there is substantial interest in lipoprotein function within the context of CVD risk in RA. Recent investigations have identified impaired HDL function, with reduced cholesterol efflux capacity and antioxidant properties, as well as increased scavenger receptor expression and foam cell formation, in patients with RA.10,11 More research is needed to elucidate how these alterations affect CVD morbidity and mortality and how their measurement could be integrated into improved CVD risk assessment.
Meta-analyses of randomized controlled trials have estimated that lipid-lowering therapy with HMG-CoA reductase inhibitors (statins) reduces the risk of CVD by 25% to 30%; as such, statin therapy has become the standard of care for reduction of CVD risk in the general population.12 Benefits for primary prevention of CVD in RA have also been observed; statin therapy was associated with a reduced risk of CVD events (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.20-0.98) and all-cause mortality (HR, 0.43; 95% CI, 0.20-0.92) in a population-based cohort study.13 Statins appear to have similar lipid-lowering effects and result in similar CVD risk reduction when used for primary or secondary prevention in RA patients compared to non-RA controls.14-16 Additionally, anti-inflammatory properties of statins may act in synergy with disease-modifying antirheumatic drugs (DMARDs) to improve RA disease activity. In a small study of RA patients, statin therapy improved subjective and objective markers of RA disease activity in conjunction with methotrexate.17
While statins provide robust reduction in CVD risk, some individuals cannot tolerate statin therapy or do not achieve goal LDL levels with statin therapy. Select non-statin LDL-cholesterol-lowering agents have shown promise for reducing CVD events in the general population.18 Ezetimibe, which inhibits cholesterol absorption in the small intestine, very modestly reduced CVD events when added to atorvastatin (relative risk [RR], 0.94; 95% CI, 0.89-0.99) in a double-blind randomized controlled trial.19 Novel monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibit the internalization of surface LDL receptors, promoting LDL clearance. Two PCSK-9 inhibitors, alirocumab and evolocumab, were approved by the US Food and Drug Administration (FDA) after randomized controlled trials demonstrated their efficacy in lowering LDL by approximately 60% and reducing CVD events by approximately 15% in patients on maximum-tolerated statin therapy.20-22 To date, non-statin LDL-cholesterol-lowering agents have been subject to limited study in RA.23
Identification and management of dyslipidemia offers an opportunity for substantial CVD risk reduction at the RA population level. Unfortunately, current rates of lipid screening are inadequate in this high-risk group. In a study of 3298 Medicare patients with RA, less than half of RA patients with an indication underwent appropriate lipid screening.24 Additionally, statins are often underutilized for both primary and secondary prevention in RA patients. Only 27% of RA patients meeting National Cholesterol Education Program Adult Treatment Panel III criteria were initiated on statin therapy in a population-based cohort study.25 Among patients discharged after a first myocardial infarction (MI), the odds of receiving lipid-lowering therapy were 31% lower for RA patients (odds ratio [OR], 0.69; 95% CI, 0.58-0.82).26 Similar to the general population, adherence to statins in RA patients appears to be poor.27-30 This raises particular concern considering that a population-based cohort study of RA patients demonstrated a 67% increased risk of MI associated with statin discontinuation, regardless of prior MI status.27 Providers—rheumatologists, primary care providers, and cardiologists alike—need to remain vigilant in efforts to assess CVD risk to identify patients who will benefit from lipid-lowering therapy and to emphasize the importance to patients of statin adherence. Novel models of health-care delivery, health technologies, and patient engagement in care may prove useful for improving lipid screening and management in RA.
Tobacco Use
Cigarette smoking is a shared risk factor for both CVD and RA. Large cohort studies have identified a dose-dependent increased risk of incident RA, particularly seropositive RA, among smokers.31-34 Tobacco smoking has also been associated with increased levels of inflammation and RA disease activity.35 The consequences of tobacco use in the general population are staggering. Among individuals over the age of 30 years, tobacco use is responsible for 12% of all deaths and 10% of all CVD deaths.36 Similar findings are observed in RA; a recent meta-analysis estimated there is a 50% increased risk of CVD events in RA related to smoking tobacco.37 In the general population, smoking cessation markedly lowers CVD risk, and over time CVD risk may approach that of nonsmokers.38,39 Thus, regular counseling and interventions to facilitate smoking cessation are critical to reducing CVD risk in RA patients. RA-specific smoking cessation programs have been proposed, but have yet to outperform standard smoking cessation programs.40
Diabetes Mellitus
It is estimated that almost 10% of the US population has diabetes mellitus (DM), which in isolation portends substantial CVD risk.41 There is an increased prevalence of DM in RA, perhaps owing to factors such as physical inactivity and chronic glucocorticoid use, though a higher level of RA disease activity itself has been associated with increased insulin resistance.42-45 In a cohort of 100 RA patients who were neither obese nor diabetic, RA patients had significantly higher fasting blood glucose and insulin levels than age- and sex-matched controls. These findings were even more pronounced in RA patients with higher levels of disease activity.44 Similar to the general population, DM is associated with poor CVD outcomes in RA.37 Therefore, both appropriate management of diabetes and control of RA disease activity are vitally important to minimize CVD risk related to DM.
Hypertension
Though not a universal finding, there may be an increased prevalence of hypertension in RA patients.31,46 Nonsteroidal anti-inflammatory drug (NSAID) and glucocorticoid use may play a role in the development of hypertension, while DMARDs appear to exert a less substantial effect on blood pressure.47,48 At least one study found that DMARD initiation (particularly for methotrexate and hydroxychloroquine) was associated with significant, albeit small, declines in both systolic and diastolic blood pressure over the first 6 months of treatment.49
Despite its potentially higher prevalence in this population, hypertension is both underdiagnosed and undertreated in RA patients.24,50-52 This is an important deficiency to target because, as in the general population, hypertension is associated with an increased risk of MI (RR, 1.84; 95% CI, 1.38-2.46) and composite CVD outcomes (RR, 2.24; 95% CI, 1.42-3.06) in RA.37 Thresholds for initiation and escalation of antihypertensive therapy are not specific to the RA population; thus, diagnosis and management of hypertension should be informed by the American College of Cardiology/American Heart Association guidelines, treating those with in-office blood pressures exceeding 140/90 mm Hg (> 130/80 mm Hg if aged > 65 years or with concomitant CVD, DM, chronic kidney disease, or 10-year atherosclerotic cardiovascular disease risk > 10%), typically with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, or thiazide diuretics as comorbidities may dictate or allow.53 Also, the use of NSAIDs and glucocorticoids should be minimized, particularly in those with concomitant hypertension.
Physical Activity
Likely due to factors such as articular pain and stiffness, as well as physical limitations, RA patients are more sedentary than the general population.54,55 In a study of objectively assessed sedentary behavior in RA patients, greater average sedentary time per day and greater number of sedentary bouts (> 20 min) were associated with increased 10-year risk of CVD as assessed by the QRISK2.56 Conversely, the beneficial effects of exercise are well documented. Light to moderate physical activity has been associated with improved cardiovascular outcomes, greater physical function, higher levels of HDL, as well as reduced systemic inflammation and disease activity, and improved endothelial function in RA patients.57-61 While there has been concern that physical activity may result in accelerated joint damage, even high-intensity exercise was shown to be safe without causing significant progression of joint damage.58
Obesity, Weight Loss, and Diet
While obesity is clearly associated with CVD risk in the general population, this relationship is much more complex in RA, as underweight RA patients are also at higher risk for CVD and CVD-related mortality.62-64 One potential explanation for this finding is that pathological weight loss resulting in an underweight body mass index (BMI) is an independent predictor of CVD. In a study of US Veterans with RA, higher rates of weight loss (> 3 kg/m2/year) were associated with increased CVD mortality (HR, 2.27; 95% CI, 1.61-3.19) independent of BMI.65 Systemic inflammation in RA can lead to “rheumatoid cachexia,” characterized by decreased muscle mass, increased adiposity, and increased CVD risk despite a normal or potentially decreased BMI.66 Practitioners should be mindful of not only current body weight, but also patients’ weight trajectories when counseling on lifestyle practices such as healthy diet and regular exercise in RA patients. For obese individuals with RA, healthy weight loss should be encouraged. Interestingly, bariatric surgery in RA patients may improve RA disease activity in addition to its known effects on body weight and DM.67
Counseling on healthy diet with a focus on limiting foods high in saturated- and trans-fatty acids and high glycemic index foods, and increasing consumption of fruits, vegetables, and mono-unsaturated fatty acids is a well-accepted and common practice to help minimize CVD risk in the general population.68 No studies to date have investigated the effect of specific diets on CVD risk in RA patients, and thus we recommend adherence to general population recommendations.
Managing RA-related CVD Risk Factors
Disease Activity
In addition to traditional risk factors, several studies have identified associations between the level of RA disease activity and risk of CVD. In a cohort of US Veterans with RA, CVD-related mortality increased in a dose-dependent manner with higher disease activity categories. In stark contrast, the CVD mortality rates of those in remission paralleled the rates from the general population (standardized mortality ratio [SMR], 0.68; 95% CI, 0.37-1.27).69 In a separate cohort of 1157 RA patients without prior CVD, achieving low disease activity was associated with a lower risk of incident CVD events (HR, 0.65; 95% CI, 0.43-0.99).70 Additionally, high disease activity has been associated with surrogate markers of CVD and other CVD risk factors including NT-proBNP and systolic blood pressure.71,72 While no randomized controlled trial data is available to inform this recommendation, observational data suggest RA should be aggressively treated (ideally to achieve and maintain remission or low disease activity) to minimize CVD risk. While keeping this treatment goal in mind, the differential effects of specific RA therapies on CVD must also be considered.
Glucocorticoids and NSAIDs
With the expanding repertoire of DMARDs available and more aggressive treatment approaches, the role of glucocorticoids and NSAIDs in RA treatment is decreasing over time. While their efficacy for improving pain and stiffness is well established, concern regarding their contribution to CVD risk in RA patients is warranted.
Glucocorticoids are known to have detrimental effects on traditional CVD risk factors such as hypertension, insulin resistance, and dyslipidemia in the general population, as well as in RA patients.73,74 In a meta-analysis of predominantly observational studies of RA patients, glucocorticoid use was associated with an increased risk of CVD events (RR, 1.47; 95% CI, 1.34-1.60), including MI, congestive heart failure (CHF), and cerebrovascular accident (CVA).75 Evidence is conflicting in regards to a clear dose threshold that leads to increased CVD risk with glucocorticoids, though higher doses are associated with greater risk.76-81 As RA patients requiring glucocorticoids typically have higher disease activity, confounding by indication remains a complicating factor in assessing the relative contributions of glucocorticoid use and RA disease activity to elevated CVD risk in many analyses.
The increased CVD risk with NSAID use is not specific to RA and has been well established in the general population.82-84 In the previously mentioned meta-analysis, an increased overall risk of CVD events was observed with NSAID use in RA (RR, 1.18; 95% CI, 1.01-1.38). It should be noted that cyclo-oxygenase 2 (COX-2) inhibitors, in particular rofecoxib (now removed from the market), appeared to drive the majority of this risk (RR, 1.36; 95% CI, 1.10-1.67 in COX-2 inhibitors and RR 1.08, 95% CI, 0.94-1.24 in nonselective NSAIDs), suggesting a potential differential risk among NSAIDs.75 While naproxen has been thought to carry the lowest risk of CVD based on initial studies, this has not been universally observed, including in a recent randomized controlled trial of more than 24,000 RA and osteoarthritis patients.82,85,86
Providers should use the lowest possible dose and duration of glucocorticoids and NSAIDs to achieve symptom relief, with continual efforts to taper or discontinue. Candidates for glucocorticoid and NSAID therapy should be selected carefully, and use of these therapies should be avoided in those with prior CVD or at high risk for CVD based on traditional CVD risk factors. Most importantly, providers should focus on utilizing DMARDs for the management of RA, which more effectively treat RA as well as reduce CVD risk.
Methotrexate
Methotrexate (MTX), a mainstay in the treatment of RA, is a conventional DMARD observed to improve overall survival and mitigate CVD risk in multiple RA cohorts.75,87,88 In a recent meta-analysis comprised of 236,525 RA patients and 5410 CVD events, MTX use was associated with a 28% reduction in overall CVD events across 8 studies (RR, 0.72; 95% CI, 0.57-0.91), substantiating similar findings in a prior meta-analysis.75,88 MTX use was specifically associated with a decreased risk of MI (RR, 0.81; 95% CI, 0.68-0.96). Case-control and cohort studies have cited a 20% to 50% reduced risk of CHF with MTX use.89,90 The potential cardioprotective effect of MTX appears to be both multifactorial and complex, likely mediated through both direct and indirect mechanisms. MTX directly promotes anti-atherogenic lipoprotein function, improves endothelial function, and scavenges free radicals.91,92 Indirectly, MTX likely reduces CVD risk by effectively reducing RA disease activity. Based on these and other data, MTX remains the cornerstone of DMARD therapy in RA patients when targeting CVD risk reduction.
Hydroxychloroquine
Emerging evidence suggests that hydroxychloroquine (HCQ), an antimalarial most often utilized in combination with alternative DMARDs in RA, prevents DM and has beneficial effects on lipid profiles. A recent meta-analysis compiled 3 homogenous observational studies that investigated the effect of HCQ on incident DM. RA patients ever exposed to HCQ had a 40% lower incidence of DM (HR, 0.59; 95% CI, 0.49-0.70).93 Increased duration of HCQ use was shown to further reduce risk of incident DM.94 The aforementioned meta-analysis also pooled 5 studies investigating the effect of HCQ on lipid profiles, with favorable mean differences in TC (–9.82 mg/dL), LDL (–10.61 mg/dL), HDL (4.13 mg/dL), and triglycerides (–19.15 mg/dL) in HCQ users compared to non-users.93 Given these favorable changes to traditional CVD risk factors, it is not surprising that in a retrospective study of 1266 RA patients without prior CVD, HCQ was associated with significantly lower risk of incident CVD. While external validation of these findings is needed, HCQ is an attractive conventional DMARD to be used in RA for CVD risk reduction. Moreover, its combination with MTX and sulfasalazine also shows promise for CVD risk reduction.95,96
TNF Inhibitors
Tumor necrosis factor (TNF) inhibitors are often the initial biologic DMARD therapy used in RA patients not responding to conventional DMARDs. In the previously described meta-analysis, TNF inhibitors were associated with similar reductions in CVD events as MTX (RR, 0.70; 95% CI, 0.54-0.90).75 Of note, there was a trend toward reduced risk of CHF (RR, 0.75; 95% CI, 0.49-1.15) in this same meta-analysis, an area of concern with TNF inhibitor use due to a prior randomized controlled trial demonstrating worsening clinical status in patients with existing moderate-to-severe CHF treated with high-dose infliximab.97 Current RA treatment guidelines recommend avoiding TNF inhibitor use in individuals with CHF.98
Aside from the risk of CHF exacerbation, TNF inhibitors appear to be cardioprotective. Similar to MTX, the mechanism by which TNF inhibition reduces cardiovascular risk is complex and likely due to both direct and indirect mechanisms. Substantial research has been conducted on the effect of TNF inhibition on lipids, with a recent meta-analysis demonstrating increases in HDL and TC, with stable LDL and atherogenic index over treatment follow-up.99 A subsequent meta-analysis not limited to RA patients yielded similar results.100 In addition to quantitative lipid changes, alteration of lipoprotein function, improvement in myocardial function, reduced aortic stiffness, improved blood pressure, and reduced RA disease activity may also be responsible for cardioprotective benefits of these agents.101,102
Non-TNF Biologic and Traditional Synthetic DMARDs
Tocilizumab, an IL-6 inhibitor, can potently increase LDL levels, but it does not appear to increase the risk of CVD events and may actually promote more favorable anti-atherogenic lipoprotein function.103-106 Although these quantitative lipid changes received significant attention in the wake of early reports detailing this effect, similar lipid changes appear to accompany other DMARDs including TNF inhibitors and tofacitinib.107 There have been few studies evaluating the risk of CVD with other non-TNF inhibitor biologic DMARDs and traditional synthetic DMARDs, warranting future study.
Conclusion
To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessments of CVD risk,3 and aggressively manage both traditional and nontraditional CVD risk factors. The differential roles in this effort may not be clear; thus, we have proposed a co-management strategy detailed in the Figure. Clear communication between providers is of the utmost importance to ensure effective management of CVD risk.
Given limited evidence for RA-specific CVD risk assessments and traditional risk factor treatment targets, management should follow pertinent national guidelines. The importance of lifestyle counseling should not be overlooked, with a focus on smoking cessation, healthy diet and body weight, and regular aerobic exercise. Finally, rheumatologists should aggressively manage RA using a treat-to-target approach, minimize the use of glucocorticoids and NSAIDs, and preferentially select DMARDs that have been associated with lower CVD risk. Through this comprehensive approach, recent trends of improved CVD outcomes in RA will hopefully become more widespread.108
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
1. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
3. Johnson TM, Mikuls TR, England BR. Assessment of cardiovascular risk in rheumatoid arthritis. J Clin Outcomes Manage. 2019;26:41-47.
4. Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: A six-year prospective study. Arthritis Res Ther. 2013;15:R203.
5. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310-1314.
6. Liao KP, Cai T, Gainer VS, et al. Lipid and lipoprotein levels and trend in rheumatoid arthritis compared to the general population. Arthritis Care Res (Hoboken). 2013;65:2046-2050.
7. Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482-487.
8. Zhang J, Chen L, Delzell E, et al. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad Med J. 2014;90:722-729.
9. Liao KP, Liu J, Lu B, et al. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67:2004-2010.
10. Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157-1162.
11. Voloshyna I, Modayil S, Littlefield MJ, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). 2013 238:1192-1197.
12. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816.
13. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. 2012;39:32-40.
14. An J, Alemao E, Reynolds K, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol. 2016;43:1989-1996.
15. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: An explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford). 2011;50:324-329.
16. Semb AG, Kvien TK, DeMicco DA, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836-2846.
17. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38:229-235.
18. Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92-125.
19. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
21. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
22. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
23. Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852-858.
24. Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221-1230.
25. Akkara Veetil BM, Myasoedova E, Matteson EL, et al. Use of lipid-lowering agents in rheumatoid arthritis: A population-based cohort study. J Rheumatol. 2013;40:1082-1088.
26. Lindhardsen J, Ahlehoff O, Gislason GH, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2012;71:1496-1501.
27. De Vera MA, Choi H, Abrahamowicz M, et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: A population-based cohort study. Ann Rheum Dis. 2011;70:1020-1024.
28. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 2013;158:526-534.
29. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: A cohort study. Ann Intern Med. 2017;167:221-227.
30. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574-580.
31. Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Joint Bone Spine. 2011;78:179-183.
32. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50:2005-2013.
33. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1,503.e9.
34. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
35. Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: A cross-sectional analysis of US veterans. Rheumatology (Oxford). 2016;55:1969-1977.
36. World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, World Health Organization, 2012.
37. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10:e0117952.
38. Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2010. 6, Cardiovascular Diseases. Available from: https://ncbi.nlm.nih.gov/books/NBK53012/
39. Mons U, Muezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551.
40. Aimer P, Treharne GJ, Stebbings S, Frampton C, Cameron V, Kirby S, et al. Efficacy of a rheumatoid arthritis-specific smoking cessation program: A randomized controlled pilot trial. Arthritis Care Res (Hoboken). 2017;69:28-37.
41. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
42. Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: A systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33:115-121.
43. Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem. 2010;43:661-335.
44. Arias de la Rosa I, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med. 2018;84(1):61-77.
45. Wilson JC, Sarsour K, Gale S, et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018 Jun 1. doi: 10.1002/acr.23611.
46. Chung CP, Giles JT, Petri M, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544.
47. Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059-1066.
48. Snowden S, Nelson R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol Rev. 2011;19:184-191.
49. Baker JF, Sauer B, Teng CC, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol. 2018;24:203-209.
50. Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482.
51. Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed “out-of-office” in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated “white coat” phenomenon. Arthritis Res Ther. 2013;15:R142.
52. van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:1210-1216.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127-248.
54. Lee J, Dunlop D, Ehrlich-Jones L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488-493.
55. Sokka T, Hakkinen A, Kautiainen H, et al. Physical inactivity in patients with rheumatoid arthritis: Data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59:42-50.
56. Fenton SAM, Veldhuijzen van Zanten JJCS, Kitas GD, et al. Sedentary behaviour is associated with increased long-term cardiovascular risk in patients with rheumatoid arthritis independently of moderate-to-vigorous physical activity. BMC Musculoskelet Disord. 2017;18:131,017-1473-9.
57. Byram KW, Oeser AM, Linton MF, et al. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol. 2018 May 25. 9.
58. de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424.
59. Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, et al. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825.
60. Khoja SS, Almeida GJ, Chester Wasko M, et al. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:424-431.
61. Metsios GS, Koutedakis Y, Veldhuijzen van Zanten JJ, et al. Cardiorespiratory fitness levels and their association with cardiovascular profile in patients with rheumatoid arthritis: A cross-sectional study. Rheumatology (Oxford). 2015;54:2215-2220.
62. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-1629.
63. Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457.
64. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:1471-1479.
65. England BR, Baker JF, Sayles H, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:11-18.
66. Dessein PH, Solomon A, Hollan I. Metabolic abnormalities in patients with inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:901-915.
67. Sparks JA, Halperin F, Karlson JC, et al. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67:1619-1626.
68. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659-669.
69. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
70. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
71. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
72. Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: A post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342-1345.
73. Hafstrom I, Rohani M, Deneberg S, et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34:1810-1816.
74. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894.
75. Roubille C. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2003;74:480-489.
76. Ajeganova S, Svensson B, Hafstrom I, BARFOT Study Group. Low-dose prednisolone treatment of early rheumatoid arthritis and late cardiovascular outcome and survival: 10-year follow-up of a 2-year randomised trial. BMJ Open. 2014;4:e004259,2013-004259.
77. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
78. del Rincon I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:264-272.
79. Davis JM,3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;56:820-830.
80. Zhang J, Xie F, Yun H, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813-1818.
81. Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576-582.
82. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: A nationwide cohort study. Ann Rheum Dis. 2014;73:1515-1521.
83. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: A nationwide cohort study. Circulation. 2011;123:2226-2235.
84. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141-149.
85. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ. 2011;342:c7086.
86. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519-2529.
87. Wasko MC, Dasgupta A, Hubert Het al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342.
88. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370.
89. Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford). 2005;44:677-680.
90. Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601-1606.
91. Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015;67:1155-1164.
92. Zimmerman MC, Clemens DL, Duryee MJ, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588-593.
93. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98-103.
94. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187-193.
95. Charles-Schoeman C, Wang X, Lee YY, et al. Association of triple therapy with improvement in cholesterol profiles over two-year followup in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheumatol. 2016;68:577-586.
96. Charles-Schoeman C, Yin Lee Y, Shahbazian A, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46-57.
97. Chung ES, Packer M, Lo KH, , Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133-3140.
98. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1-26.
99. Daien CI, Duny Y, Barnetche Tet al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862-868.
100. Di Minno MN, Ambrosino P, Peluso R, et al. Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-alpha blockers: A meta-analysis of prospective studies. Ann Med. 2014;46:73-83.
101. Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868-872.
102. O’Neill F, Charakida M, Topham E, et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart. 2017;103:766-773.
103. Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222-232.
104. Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 2015;67:372-380.
105. Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812.
106. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702.
107. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117-127.
108. Myasoedova E, Gabriel SE, Matteson EL, et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732-739.
109. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
110. Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med. 2008;35:158-176.
111. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
112. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
113. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985-3023.
Point-of-Care versus Central Laboratory Glucose Testing in Postoperative Cardiac Surgery Patients
From the Maine Medical Center, Portland, ME (Dr. Kramer, Ms. Palmeri, Dr. Robich, Mr. Groom, Dr. Hayes, Ms. Janoushek, Dr. Rappold, Dr. Swarz, and Dr. Quinn), and the Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, Portland, ME (Dr. Lucas).
Abstract
- Objective. To determine the accuracy of the glucometer currently used for point-of-care testing (POCT) of blood glucose in our cardiothoracic surgery intensive care unit (CTICU).
- Design. Prospective cohort study.
- Setting. Tertiary care community hospital affiliated with a school of medicine.
- Participants. Coronary artery bypass graft (CABG) surgery patients.
- Measurements. Blood glucose levels obtained via POCT with a glucometer using fingerstick and radial artery blood samples were compared with values obtained via central laboratory testing of radial artery blood samples (gold standard) in 106 CABG patients on continuous insulin infusions (CII) upon arrival to the CTICU from the operating room and 102 CABG patients on CII in the CTICU 6 hours later.
- Results. Fingerstick POCT and central lab blood glucose values correlated well (r = 0.83 for admission and 0.86 for 6-hour values), but the mean values were significantly different as determined by paired t-tests. Upon arrival, the fingerstick POCT mean value was 120.9 mg/dL, while the central laboratory value was 127.9 mg/dL (P value = 0.03). At the 6-hour time point, the mean value for fingerstick POCT was 129.7 mg/dL compared to a central laboratory value of 137.3 (P value = 0.02).
- Conclusion. The blood glucose POCT values correlated well with central laboratory values, but the values were statistically significantly different. Nevertheless, accurate clinical decisions were made despite the inaccuracies of POCT glucose testing, as experienced bedside nurses were able to use the glucometer successfully and safely. The device’s results informed them when the blood glucose was out of a prescibed range and the direction of the change, and they were able to adjust the CII accordingly.
Keywords: quality improvement; glucose management; point-of-care testing; critical care.
Achieving glycemic control in patients with and without diabetes during coronary artery bypass graft (CABG) surgery is associated with reduced perioperative morbidity and mortality and improved long-term survival.1 Hyperglycemia has detrimental effects on the cardiovascular system and insulin has beneficial effects on the ischemic myocardium.2 The current recommendations of the Society of Thoracic Surgery regarding blood glucose management include the use of continuous insulin infusions (CII) during and after surgery in the critical care unit,3 keeping blood glucose in a moderate range. Glucometers are commonly used in the critical care perioperative setting for point-of-care testing (POCT) for timely determinations of blood glucose levels for patients on CII.
POCT for glucose monitoring is a valuable tool for managing patients with diabetes in the outpatient setting. Evolving from urinary test strips that depended on a colorimetric model, glucometers now incoroporate digital technology that allows patients to determine their blood glucose using a drop of blood from a fingerstick. The US Food and Drug Administration’s approval for most glucose POCT technology includes home use by diabetic patients and use in the hospital setting, with the exception of critically ill patients, who may be affected by hypoxemia, poor capillary perfusion, tissue edema, severe anemia4 or other pathophysiologic states that could impact the accuracy of the devices. For example, poor peripheral perfusion related to shock or vasoconstrictors and interstitial edema are variables that could contribute to an erroneous reading. Therefore, many glucometers used in the critical care setting are being used off-label. Because much of the current POCT technology for glucose monitoring may provide erroneous results in certain ranges and in some clinical settings, the safety of most glucometers has been called into question.5,6
Given the concern regarding the potential inaccuracies of commonly used glucometers in the critical care setting, we undertook a quality improvement project to analyze the clinical performance of the glucometer currently used in our critically ill postoperative cardiac surgery population. The cardiac surgery division policy at our institution is to place all patients, both diabetic and nondiabetic, on a CII intraoperatively and to continue the infusion for at least 24 to 48 hours postoperatively. The CII start rate is determined utilizing the division’s Insulin Start Chart, and then the CII is adjusted according to the nomogram through the postoperative course. Both the Insulin Start Chart and nomogram have been previously described by Kramer et al.7
Currently, POCT of glucose in all post cardiac surgery patients is done hourly or more frequently in the first 24 to 48 hours after surgery in order to adjust the CII. In patients undergoing the stress of cardiac surgery, the action of insulin is counter-regulated by glucagon, epinephrine, norepinephrine, cortisol, and growth hormone. The resulting varying degrees of insulin resistance in this population of patients requires close monitoring of blood glucose, keeping it in a prescribed range, which in our center is 110 to 150 mg/dL, both in diabetic and nondiabetic patients. Frequent laboratory and POCT determinations of glucose are made. Providers and bedside nurses adjust the CII according to central laboratory values, POCT values, and trends, as previously described.7
Methods
Setting
Maine Medical Center is a 600-bed tertiary care teaching hospital. It is a level 1 trauma center where 1000 cardiac surgical operations are performed annually. POCT glucose monitoring is relied upon to monitor blood glucose and adjust the CII accordingly. This project, which did not require any additional procedures outside of the standard of care for this population of patients, was reviewed by the Institutional Review Board, who determined that this activity does not meet either the definition of research as specified under 45 CFR 46.102 (d) or the definition of clinical investigation as specified in 21 CFR 56.102 (c).
Patients
Using central laboratory glucose values drawn from the radial artery as the gold standard, we created a registry of consecutive postoperative cardiac surgery patients who had undergone CABG surgery and had blood glucose determinations from both POCT (fingerstick and radial artery samples) and central laboratory testing (radial artery sample) during a 7-month period (May 2016 through February 2017). To be included in the registry, patients had to (1) be postoperative following isolated CABG or CABG plus Maze procedure; (2) have been on cardiopulmonary bypass (CPB); (3) have radial arterial lines; and (4) be on a CII. A total of 116 patients qualified according to the inclusion criteria. Patients missing glucose results in 1 or more of the variables were excluded from data analysis.
Measurements and Variables
Using a POCT glucometer (FreeStyle Precision Pro, Abbott Laboratories, Abbott Park, IL), blood glucose conentrations were measured on samples obtained from both fingerstick and radial artery. Concurrently, radial arterial blood was sent to the central laboratory for glucose measurement. Blood glucose values were compared in CABG patients on CII upon arrival to the cardiothoracic surgery intensive care unit (CTICU) from the operating room and CABG patients on CII 6 hours after arrival in the CTICU. During the 6-hour interval, blood glucose levels were tested hourly or more frequently, allowing nurses to identify trends in blood glucose changes in order to keep blood glucose in the prescribed goal range of 110 to 150 mg/dL. At each of these 2 time points, on arrival to CTICU and 6 hours later, blood glucose values obtained with radial artery POCT and fingerstick POCT were compared with values obtained with central laboratory testing of radial artery samples. The amount of blood required was 1 drop each for POCT fingerstick and POCT radial artery and 2 mL for central lab testing.
Patient characteristics were identified from the electronic medical record. The variables recorded were type of operation, time on CPB, time of CTICU arrival, temperature, vasoconstrictor infusions (norepinephrine, vasopressin, phenylephrine), preoperative diagnosis of diabetes mellitus, preoperative HbA1c, and hemoglobin/hematocrit. Hemoglobin/hematocrit was only available at the time of the patient’s arrival to CTICU. The study was completed within the confines of our center’s standard of care protocol for postoperative cardiac surgical patients.
Analysis
We used standard statistical techniques to describe the study population, including proportions for categorical variables and means (standard deviations) for continuous variables. Correlation and regression techniques were used to describe the relationship between POCT and laboratory (gold standard) tests, both measured as continuous variables, and paired t-tests with Bonferroni correction were used to compare the central tendency and range of these comparisons. We calculated the differences between the gold standard measure and the POCT measure as an indication of outliers (ie, cases in which the 2 tests gave markedly different results). We examined plots to ascertain at which levels of the gold standard test these outliers occurred. An interim analysis was done at the halfway point and submitted to the Institutional Review Board, but no correction to the P value was done based on this analysis, which was largely qualitative. We used Bonferroni correction to declare a P value of 0.025 statistically significant with the 2-way comparisons of both fingerstick and radial artery values to central laboratory values. When the data was stratified by a clinical characteristic creating a 4-way comparison, we used Bonferroni correction to declare a P value of 0.0125 to be statistically significant when comparing both fingerstick and radial artery values to central laboratory values.
Results
Glucose POCT evaluations were carried out on 116 consecutive patients who underwent CABG surgery with or without a Maze procedure on CPB with a CII and an arterial line. Due to missing glucose results in 1 or more of the variables, 10 patients were excluded from data analysis for the time point of arrival in the CTICU and 14 patients were excluded from data analysis for the time point of 6 hours post CTICU arrival. This gave a final count of 106 CABG patients for CTICU arrival data analysis and 102 CABG patients for the 6 hours after CTICU arrival data analysis.
Patients ranged in age from 43 to 85 years, with a mean of age of 66 years, 22% were were women, 41% were diabetic, and 18% had peripheral vascular disease (Table 1). The average preoperative HbA1c was 6.4% ± 1.3% (range, 4.6% to 11.1%). Mean time on CBP for the group was 101 ± 31 minutes (range, 43 to 233 minutes). Postoperative mean hematocrit and hemoglobin were 32.5% and 11.4 g/dL, respectively. The average core temperature of patients on arrival was 36.0°C, which rose to an average of 36.6°C 6 hours later. A vasoconstrictor drip was infusing on 52% of patients upon CTICU arrival; 65% had a vasoconstrictor drip infusing 6 hours after arrival to the CTICU. Hemoglobin results were available only upon CTICU arrival as they are not routinely checked at 6 hours; 74 (64%) patients had a hemoglobin < 12 g/dL.
Compared to central laboratory testing, which we are defining as the gold standard, fingerstick POCT performed better on arrival, while radial artery POCT performed better at 6 hours (Table 2). At CTICU arrival, the mean blood glucose value for fingerstick POCT was 121 ± 24.1 mg/dL, 116 ± 27.2 mg/dL for radial artery POCT, and 128 ± 23.5 mg/dL for central lab testing. The difference in mean blood glucose between the fingerstick POCT and central lab testing was not statistically significant (P = 0.032), while the difference in mean blood glucose between radial artery POCT and central lab testing was statistically significant (P = 0.001). At 6 hours post arrival to the CTICU, the mean fingerstick POCT blood glucose value was 130 ± 23.9 mg/dL, compared to the mean central lab testing value of 137 ± 22.4 mg/dL; this difference was statistically significant (P = 0.019), while the radial artery POCT blood glucose value (133 ± 24.6 mg/dL) was not significantly different from the central lab testing value.
Blood glucose values from fingerstick POCT and central laboratory testing correlated well (r = 0.83 for admission and 0.86 for 6-hour values), as did radial artery POCT and central lab values (r = 0.87 for admission and 0.90 for 6-hour values) (Figures 1, 2, 3, and 4). Comparing individual values for fingerstick POCT and central lab testing, within-person differences between the 2 values ranged from –45 to 25 mg/dL, with 21% of pairs discrepant by 20 mg/dL or more (Figure 1); results were similar at 6 hours (Figure 2), with slightly less discrepancy.
The differences between radial artery POCT and central lab testing values at CTICU arrival ranged from –43 to 80 mg/dL, with 24% of pairs discrepant by 20 mg/dL or more (Figure 3). At 6 hours post CTICU arrival, the difference between radial artery POCT and central lab testing values ranged from –130 to 27 mg/dL, with 11% of pairs discrepant by 20 mg/dL or more (Figure 4). Ninety-two percent of central laboratory values were either close to (± 20) or within the moderate glycemic control target range (110–150 mg/dL).
When the patient cohort was stratified by anemia, diabetes, body temperature, and receipt of vasoconstrictor, there were no significant differences between mean fingerstick POCT and central lab testing values for any strata on CTICU arrival, while there were significant differences between radial artery POCT and central lab testing means for both vasoconstrictor strata as well as for patients with core temperature > 36.1°C (Table 2). At 6 hours, there were no statistically significant differences when stratified for receipt of vasoconstrictor or presence of diabetes. Stratification for anemia or core body temperature was not done for patients at the 6-hour post CTICU arrival time because no hemoglobin value was available and all patients except 1 reached a core temperature of 36.1°C.
Although we measured POCT values obtained using 2 different blood sample sources, fingerstick POCT performed better than radial artery POCT testing with regard to the mean values when compared with the central lab. However, radial artery POCT performed better with regard to correlation with the central lab value. In other words, fingerstick POCT values were less significantly different than radial artery POCT values when compared with the central lab, while radial artery POCT values correlated better with values from the central lab. In spite of this unexplained variability in differences and correlation, the blood glucose values stayed in the target goal range (Figures 1-4).
Discussion
The accuracy of glucose POCT in the critical care setting has been called into question.4,5 The clinical demands of glucose management using CII include timely and accurate guidance in postoperaptive cardiac surgery, in this case, CABG. A previous study compared POCT and central laboratory blood glucose values in medical intensive care unit patients,8 but not in patients who have had CABG surgery. Another study has reviewed the difference in glucose values from POCT and central lab analysis in the critically ill population, but not in the post cardiac surgical population.9 We have shown that the POCT blood glucose values correlate well with the clinical lab values, but the values are statistically different. Our study adds an additional observation in that, although the POCT inconsistencies were statistically significant, they were not clinically significant. That is, POCT of blood glucose was inaccurate, but it still helped guide care by providing enough information to keep the blood glucose in range (most of the time) and allowing the bedside nurse to detect trends and make appropriate adjustments to the infusion. However, given these inconsistencies, we recommend a low threshold for sending additional samples to the central lab to double-check the glucose values, especially when they are outside the prescribed range. Our analysis provides some measure of reassurance with regard to current postoperative CABG glucose management by showing that the limitations of the blood glucose meter do not jeopardize the safety of patients. Nonetheless, we look forward to advances in the accuracy of POCT blood glucose technology so that critical care patients can be better managed when blood glucose is outside the prescribed range.
This analysis of 116 CABG patients points out both the inaccuracy and the utility of a representative POCT glucometer (in this case, the FreeStyle Precision Pro) used at the bedside to manage CIIs in postoperative CABG patients, keeping the blood glucose level in the moderate control range (110-150 mg/dL). The correlation plot shows that in this population the bedside nurses were able to keep blood glucose in range most of the time, in spite of the inaccuracy of POCT of blood glucose, given that the error of the test fits in the wide margin of 40 mg/dL. The fact that the 6-hour values were slightly less variable than the admission values indicates that sequential determinations of blood glucose over the 6-hour period to detect trends allowed good clinical management even in the face of such inaccuracy. The correlation allows the inaccurate number (blood glucose value) to indicate direction, and frequent determinations allow the bedside nurse to keep that number in the prescribed range most of the time in this population of patients.
Conclusion
We have found that glucometer blood glucose determinations in our center used on a homogenous population (CABG surgery) utilizing a single type of glucometer correlated well with those of the central lab, but were not always accurate. In spite of the inaccuracies, experienced bedside nurses were able to use the instrument successfully and safely, as it informed them if the blood glucose was in or out of a predetermined range and in which direction it was going.
Acknowledgment: The authors are indebted to the nurses of the Cardiothoracic Surgery Intensive Care Unit at Maine Medical Center for their support and assistance, without which this analysis would not have been possible.
Corresponding author: Robert S. Kramer, MD, Division of Cardiothoracic Surgery, Maine Medical Center Cardiovascular Institute, 22 Bramhall St., Portland ME 04102; kramer@mmc.org.
Financial disclosures: None.
1. Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021.
2. Lazar H. Glycemic control during coronary artery bypass graft surgery. ISRN Cardiol. 2012;2012:292490.
3. Lazar HL, McDonnell M, Chipkin SR, et al; Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons Practice Guideline Series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663-669.
4. US Food and Drug Administration. Blood Glucose Monitoring Test Systems for Prescription Point of Care Use. Guidance for Industry and Food and Drug Administration Staff,.www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM380325.pdf. Accessed March 8, 2019.
5. Finkielman JD, Oyen LJ, Afess B. Agreement between bedside blood and plasma glucose measurement in the ICU Setting. Chest. 2005;127:1749-1511.
6. Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38:471-476.
7. Kramer R, Groom R, Weldner D, et al. Glycemic control reduces deep sternal wound infection: a multidisciplinary approach. Arch Surg. 2008;143:451-456.
8. Peterson JR, Graves DF, Tacker DH, et al. Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clinica Chimica Acta. 2008;396:10-13.
9. Cook A, Laughlin D, Moore M, et al. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. Am J Crit Care. 2009;18:65-72.
From the Maine Medical Center, Portland, ME (Dr. Kramer, Ms. Palmeri, Dr. Robich, Mr. Groom, Dr. Hayes, Ms. Janoushek, Dr. Rappold, Dr. Swarz, and Dr. Quinn), and the Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, Portland, ME (Dr. Lucas).
Abstract
- Objective. To determine the accuracy of the glucometer currently used for point-of-care testing (POCT) of blood glucose in our cardiothoracic surgery intensive care unit (CTICU).
- Design. Prospective cohort study.
- Setting. Tertiary care community hospital affiliated with a school of medicine.
- Participants. Coronary artery bypass graft (CABG) surgery patients.
- Measurements. Blood glucose levels obtained via POCT with a glucometer using fingerstick and radial artery blood samples were compared with values obtained via central laboratory testing of radial artery blood samples (gold standard) in 106 CABG patients on continuous insulin infusions (CII) upon arrival to the CTICU from the operating room and 102 CABG patients on CII in the CTICU 6 hours later.
- Results. Fingerstick POCT and central lab blood glucose values correlated well (r = 0.83 for admission and 0.86 for 6-hour values), but the mean values were significantly different as determined by paired t-tests. Upon arrival, the fingerstick POCT mean value was 120.9 mg/dL, while the central laboratory value was 127.9 mg/dL (P value = 0.03). At the 6-hour time point, the mean value for fingerstick POCT was 129.7 mg/dL compared to a central laboratory value of 137.3 (P value = 0.02).
- Conclusion. The blood glucose POCT values correlated well with central laboratory values, but the values were statistically significantly different. Nevertheless, accurate clinical decisions were made despite the inaccuracies of POCT glucose testing, as experienced bedside nurses were able to use the glucometer successfully and safely. The device’s results informed them when the blood glucose was out of a prescibed range and the direction of the change, and they were able to adjust the CII accordingly.
Keywords: quality improvement; glucose management; point-of-care testing; critical care.
Achieving glycemic control in patients with and without diabetes during coronary artery bypass graft (CABG) surgery is associated with reduced perioperative morbidity and mortality and improved long-term survival.1 Hyperglycemia has detrimental effects on the cardiovascular system and insulin has beneficial effects on the ischemic myocardium.2 The current recommendations of the Society of Thoracic Surgery regarding blood glucose management include the use of continuous insulin infusions (CII) during and after surgery in the critical care unit,3 keeping blood glucose in a moderate range. Glucometers are commonly used in the critical care perioperative setting for point-of-care testing (POCT) for timely determinations of blood glucose levels for patients on CII.
POCT for glucose monitoring is a valuable tool for managing patients with diabetes in the outpatient setting. Evolving from urinary test strips that depended on a colorimetric model, glucometers now incoroporate digital technology that allows patients to determine their blood glucose using a drop of blood from a fingerstick. The US Food and Drug Administration’s approval for most glucose POCT technology includes home use by diabetic patients and use in the hospital setting, with the exception of critically ill patients, who may be affected by hypoxemia, poor capillary perfusion, tissue edema, severe anemia4 or other pathophysiologic states that could impact the accuracy of the devices. For example, poor peripheral perfusion related to shock or vasoconstrictors and interstitial edema are variables that could contribute to an erroneous reading. Therefore, many glucometers used in the critical care setting are being used off-label. Because much of the current POCT technology for glucose monitoring may provide erroneous results in certain ranges and in some clinical settings, the safety of most glucometers has been called into question.5,6
Given the concern regarding the potential inaccuracies of commonly used glucometers in the critical care setting, we undertook a quality improvement project to analyze the clinical performance of the glucometer currently used in our critically ill postoperative cardiac surgery population. The cardiac surgery division policy at our institution is to place all patients, both diabetic and nondiabetic, on a CII intraoperatively and to continue the infusion for at least 24 to 48 hours postoperatively. The CII start rate is determined utilizing the division’s Insulin Start Chart, and then the CII is adjusted according to the nomogram through the postoperative course. Both the Insulin Start Chart and nomogram have been previously described by Kramer et al.7
Currently, POCT of glucose in all post cardiac surgery patients is done hourly or more frequently in the first 24 to 48 hours after surgery in order to adjust the CII. In patients undergoing the stress of cardiac surgery, the action of insulin is counter-regulated by glucagon, epinephrine, norepinephrine, cortisol, and growth hormone. The resulting varying degrees of insulin resistance in this population of patients requires close monitoring of blood glucose, keeping it in a prescribed range, which in our center is 110 to 150 mg/dL, both in diabetic and nondiabetic patients. Frequent laboratory and POCT determinations of glucose are made. Providers and bedside nurses adjust the CII according to central laboratory values, POCT values, and trends, as previously described.7
Methods
Setting
Maine Medical Center is a 600-bed tertiary care teaching hospital. It is a level 1 trauma center where 1000 cardiac surgical operations are performed annually. POCT glucose monitoring is relied upon to monitor blood glucose and adjust the CII accordingly. This project, which did not require any additional procedures outside of the standard of care for this population of patients, was reviewed by the Institutional Review Board, who determined that this activity does not meet either the definition of research as specified under 45 CFR 46.102 (d) or the definition of clinical investigation as specified in 21 CFR 56.102 (c).
Patients
Using central laboratory glucose values drawn from the radial artery as the gold standard, we created a registry of consecutive postoperative cardiac surgery patients who had undergone CABG surgery and had blood glucose determinations from both POCT (fingerstick and radial artery samples) and central laboratory testing (radial artery sample) during a 7-month period (May 2016 through February 2017). To be included in the registry, patients had to (1) be postoperative following isolated CABG or CABG plus Maze procedure; (2) have been on cardiopulmonary bypass (CPB); (3) have radial arterial lines; and (4) be on a CII. A total of 116 patients qualified according to the inclusion criteria. Patients missing glucose results in 1 or more of the variables were excluded from data analysis.
Measurements and Variables
Using a POCT glucometer (FreeStyle Precision Pro, Abbott Laboratories, Abbott Park, IL), blood glucose conentrations were measured on samples obtained from both fingerstick and radial artery. Concurrently, radial arterial blood was sent to the central laboratory for glucose measurement. Blood glucose values were compared in CABG patients on CII upon arrival to the cardiothoracic surgery intensive care unit (CTICU) from the operating room and CABG patients on CII 6 hours after arrival in the CTICU. During the 6-hour interval, blood glucose levels were tested hourly or more frequently, allowing nurses to identify trends in blood glucose changes in order to keep blood glucose in the prescribed goal range of 110 to 150 mg/dL. At each of these 2 time points, on arrival to CTICU and 6 hours later, blood glucose values obtained with radial artery POCT and fingerstick POCT were compared with values obtained with central laboratory testing of radial artery samples. The amount of blood required was 1 drop each for POCT fingerstick and POCT radial artery and 2 mL for central lab testing.
Patient characteristics were identified from the electronic medical record. The variables recorded were type of operation, time on CPB, time of CTICU arrival, temperature, vasoconstrictor infusions (norepinephrine, vasopressin, phenylephrine), preoperative diagnosis of diabetes mellitus, preoperative HbA1c, and hemoglobin/hematocrit. Hemoglobin/hematocrit was only available at the time of the patient’s arrival to CTICU. The study was completed within the confines of our center’s standard of care protocol for postoperative cardiac surgical patients.
Analysis
We used standard statistical techniques to describe the study population, including proportions for categorical variables and means (standard deviations) for continuous variables. Correlation and regression techniques were used to describe the relationship between POCT and laboratory (gold standard) tests, both measured as continuous variables, and paired t-tests with Bonferroni correction were used to compare the central tendency and range of these comparisons. We calculated the differences between the gold standard measure and the POCT measure as an indication of outliers (ie, cases in which the 2 tests gave markedly different results). We examined plots to ascertain at which levels of the gold standard test these outliers occurred. An interim analysis was done at the halfway point and submitted to the Institutional Review Board, but no correction to the P value was done based on this analysis, which was largely qualitative. We used Bonferroni correction to declare a P value of 0.025 statistically significant with the 2-way comparisons of both fingerstick and radial artery values to central laboratory values. When the data was stratified by a clinical characteristic creating a 4-way comparison, we used Bonferroni correction to declare a P value of 0.0125 to be statistically significant when comparing both fingerstick and radial artery values to central laboratory values.
Results
Glucose POCT evaluations were carried out on 116 consecutive patients who underwent CABG surgery with or without a Maze procedure on CPB with a CII and an arterial line. Due to missing glucose results in 1 or more of the variables, 10 patients were excluded from data analysis for the time point of arrival in the CTICU and 14 patients were excluded from data analysis for the time point of 6 hours post CTICU arrival. This gave a final count of 106 CABG patients for CTICU arrival data analysis and 102 CABG patients for the 6 hours after CTICU arrival data analysis.
Patients ranged in age from 43 to 85 years, with a mean of age of 66 years, 22% were were women, 41% were diabetic, and 18% had peripheral vascular disease (Table 1). The average preoperative HbA1c was 6.4% ± 1.3% (range, 4.6% to 11.1%). Mean time on CBP for the group was 101 ± 31 minutes (range, 43 to 233 minutes). Postoperative mean hematocrit and hemoglobin were 32.5% and 11.4 g/dL, respectively. The average core temperature of patients on arrival was 36.0°C, which rose to an average of 36.6°C 6 hours later. A vasoconstrictor drip was infusing on 52% of patients upon CTICU arrival; 65% had a vasoconstrictor drip infusing 6 hours after arrival to the CTICU. Hemoglobin results were available only upon CTICU arrival as they are not routinely checked at 6 hours; 74 (64%) patients had a hemoglobin < 12 g/dL.
Compared to central laboratory testing, which we are defining as the gold standard, fingerstick POCT performed better on arrival, while radial artery POCT performed better at 6 hours (Table 2). At CTICU arrival, the mean blood glucose value for fingerstick POCT was 121 ± 24.1 mg/dL, 116 ± 27.2 mg/dL for radial artery POCT, and 128 ± 23.5 mg/dL for central lab testing. The difference in mean blood glucose between the fingerstick POCT and central lab testing was not statistically significant (P = 0.032), while the difference in mean blood glucose between radial artery POCT and central lab testing was statistically significant (P = 0.001). At 6 hours post arrival to the CTICU, the mean fingerstick POCT blood glucose value was 130 ± 23.9 mg/dL, compared to the mean central lab testing value of 137 ± 22.4 mg/dL; this difference was statistically significant (P = 0.019), while the radial artery POCT blood glucose value (133 ± 24.6 mg/dL) was not significantly different from the central lab testing value.
Blood glucose values from fingerstick POCT and central laboratory testing correlated well (r = 0.83 for admission and 0.86 for 6-hour values), as did radial artery POCT and central lab values (r = 0.87 for admission and 0.90 for 6-hour values) (Figures 1, 2, 3, and 4). Comparing individual values for fingerstick POCT and central lab testing, within-person differences between the 2 values ranged from –45 to 25 mg/dL, with 21% of pairs discrepant by 20 mg/dL or more (Figure 1); results were similar at 6 hours (Figure 2), with slightly less discrepancy.
The differences between radial artery POCT and central lab testing values at CTICU arrival ranged from –43 to 80 mg/dL, with 24% of pairs discrepant by 20 mg/dL or more (Figure 3). At 6 hours post CTICU arrival, the difference between radial artery POCT and central lab testing values ranged from –130 to 27 mg/dL, with 11% of pairs discrepant by 20 mg/dL or more (Figure 4). Ninety-two percent of central laboratory values were either close to (± 20) or within the moderate glycemic control target range (110–150 mg/dL).
When the patient cohort was stratified by anemia, diabetes, body temperature, and receipt of vasoconstrictor, there were no significant differences between mean fingerstick POCT and central lab testing values for any strata on CTICU arrival, while there were significant differences between radial artery POCT and central lab testing means for both vasoconstrictor strata as well as for patients with core temperature > 36.1°C (Table 2). At 6 hours, there were no statistically significant differences when stratified for receipt of vasoconstrictor or presence of diabetes. Stratification for anemia or core body temperature was not done for patients at the 6-hour post CTICU arrival time because no hemoglobin value was available and all patients except 1 reached a core temperature of 36.1°C.
Although we measured POCT values obtained using 2 different blood sample sources, fingerstick POCT performed better than radial artery POCT testing with regard to the mean values when compared with the central lab. However, radial artery POCT performed better with regard to correlation with the central lab value. In other words, fingerstick POCT values were less significantly different than radial artery POCT values when compared with the central lab, while radial artery POCT values correlated better with values from the central lab. In spite of this unexplained variability in differences and correlation, the blood glucose values stayed in the target goal range (Figures 1-4).
Discussion
The accuracy of glucose POCT in the critical care setting has been called into question.4,5 The clinical demands of glucose management using CII include timely and accurate guidance in postoperaptive cardiac surgery, in this case, CABG. A previous study compared POCT and central laboratory blood glucose values in medical intensive care unit patients,8 but not in patients who have had CABG surgery. Another study has reviewed the difference in glucose values from POCT and central lab analysis in the critically ill population, but not in the post cardiac surgical population.9 We have shown that the POCT blood glucose values correlate well with the clinical lab values, but the values are statistically different. Our study adds an additional observation in that, although the POCT inconsistencies were statistically significant, they were not clinically significant. That is, POCT of blood glucose was inaccurate, but it still helped guide care by providing enough information to keep the blood glucose in range (most of the time) and allowing the bedside nurse to detect trends and make appropriate adjustments to the infusion. However, given these inconsistencies, we recommend a low threshold for sending additional samples to the central lab to double-check the glucose values, especially when they are outside the prescribed range. Our analysis provides some measure of reassurance with regard to current postoperative CABG glucose management by showing that the limitations of the blood glucose meter do not jeopardize the safety of patients. Nonetheless, we look forward to advances in the accuracy of POCT blood glucose technology so that critical care patients can be better managed when blood glucose is outside the prescribed range.
This analysis of 116 CABG patients points out both the inaccuracy and the utility of a representative POCT glucometer (in this case, the FreeStyle Precision Pro) used at the bedside to manage CIIs in postoperative CABG patients, keeping the blood glucose level in the moderate control range (110-150 mg/dL). The correlation plot shows that in this population the bedside nurses were able to keep blood glucose in range most of the time, in spite of the inaccuracy of POCT of blood glucose, given that the error of the test fits in the wide margin of 40 mg/dL. The fact that the 6-hour values were slightly less variable than the admission values indicates that sequential determinations of blood glucose over the 6-hour period to detect trends allowed good clinical management even in the face of such inaccuracy. The correlation allows the inaccurate number (blood glucose value) to indicate direction, and frequent determinations allow the bedside nurse to keep that number in the prescribed range most of the time in this population of patients.
Conclusion
We have found that glucometer blood glucose determinations in our center used on a homogenous population (CABG surgery) utilizing a single type of glucometer correlated well with those of the central lab, but were not always accurate. In spite of the inaccuracies, experienced bedside nurses were able to use the instrument successfully and safely, as it informed them if the blood glucose was in or out of a predetermined range and in which direction it was going.
Acknowledgment: The authors are indebted to the nurses of the Cardiothoracic Surgery Intensive Care Unit at Maine Medical Center for their support and assistance, without which this analysis would not have been possible.
Corresponding author: Robert S. Kramer, MD, Division of Cardiothoracic Surgery, Maine Medical Center Cardiovascular Institute, 22 Bramhall St., Portland ME 04102; kramer@mmc.org.
Financial disclosures: None.
From the Maine Medical Center, Portland, ME (Dr. Kramer, Ms. Palmeri, Dr. Robich, Mr. Groom, Dr. Hayes, Ms. Janoushek, Dr. Rappold, Dr. Swarz, and Dr. Quinn), and the Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, Portland, ME (Dr. Lucas).
Abstract
- Objective. To determine the accuracy of the glucometer currently used for point-of-care testing (POCT) of blood glucose in our cardiothoracic surgery intensive care unit (CTICU).
- Design. Prospective cohort study.
- Setting. Tertiary care community hospital affiliated with a school of medicine.
- Participants. Coronary artery bypass graft (CABG) surgery patients.
- Measurements. Blood glucose levels obtained via POCT with a glucometer using fingerstick and radial artery blood samples were compared with values obtained via central laboratory testing of radial artery blood samples (gold standard) in 106 CABG patients on continuous insulin infusions (CII) upon arrival to the CTICU from the operating room and 102 CABG patients on CII in the CTICU 6 hours later.
- Results. Fingerstick POCT and central lab blood glucose values correlated well (r = 0.83 for admission and 0.86 for 6-hour values), but the mean values were significantly different as determined by paired t-tests. Upon arrival, the fingerstick POCT mean value was 120.9 mg/dL, while the central laboratory value was 127.9 mg/dL (P value = 0.03). At the 6-hour time point, the mean value for fingerstick POCT was 129.7 mg/dL compared to a central laboratory value of 137.3 (P value = 0.02).
- Conclusion. The blood glucose POCT values correlated well with central laboratory values, but the values were statistically significantly different. Nevertheless, accurate clinical decisions were made despite the inaccuracies of POCT glucose testing, as experienced bedside nurses were able to use the glucometer successfully and safely. The device’s results informed them when the blood glucose was out of a prescibed range and the direction of the change, and they were able to adjust the CII accordingly.
Keywords: quality improvement; glucose management; point-of-care testing; critical care.
Achieving glycemic control in patients with and without diabetes during coronary artery bypass graft (CABG) surgery is associated with reduced perioperative morbidity and mortality and improved long-term survival.1 Hyperglycemia has detrimental effects on the cardiovascular system and insulin has beneficial effects on the ischemic myocardium.2 The current recommendations of the Society of Thoracic Surgery regarding blood glucose management include the use of continuous insulin infusions (CII) during and after surgery in the critical care unit,3 keeping blood glucose in a moderate range. Glucometers are commonly used in the critical care perioperative setting for point-of-care testing (POCT) for timely determinations of blood glucose levels for patients on CII.
POCT for glucose monitoring is a valuable tool for managing patients with diabetes in the outpatient setting. Evolving from urinary test strips that depended on a colorimetric model, glucometers now incoroporate digital technology that allows patients to determine their blood glucose using a drop of blood from a fingerstick. The US Food and Drug Administration’s approval for most glucose POCT technology includes home use by diabetic patients and use in the hospital setting, with the exception of critically ill patients, who may be affected by hypoxemia, poor capillary perfusion, tissue edema, severe anemia4 or other pathophysiologic states that could impact the accuracy of the devices. For example, poor peripheral perfusion related to shock or vasoconstrictors and interstitial edema are variables that could contribute to an erroneous reading. Therefore, many glucometers used in the critical care setting are being used off-label. Because much of the current POCT technology for glucose monitoring may provide erroneous results in certain ranges and in some clinical settings, the safety of most glucometers has been called into question.5,6
Given the concern regarding the potential inaccuracies of commonly used glucometers in the critical care setting, we undertook a quality improvement project to analyze the clinical performance of the glucometer currently used in our critically ill postoperative cardiac surgery population. The cardiac surgery division policy at our institution is to place all patients, both diabetic and nondiabetic, on a CII intraoperatively and to continue the infusion for at least 24 to 48 hours postoperatively. The CII start rate is determined utilizing the division’s Insulin Start Chart, and then the CII is adjusted according to the nomogram through the postoperative course. Both the Insulin Start Chart and nomogram have been previously described by Kramer et al.7
Currently, POCT of glucose in all post cardiac surgery patients is done hourly or more frequently in the first 24 to 48 hours after surgery in order to adjust the CII. In patients undergoing the stress of cardiac surgery, the action of insulin is counter-regulated by glucagon, epinephrine, norepinephrine, cortisol, and growth hormone. The resulting varying degrees of insulin resistance in this population of patients requires close monitoring of blood glucose, keeping it in a prescribed range, which in our center is 110 to 150 mg/dL, both in diabetic and nondiabetic patients. Frequent laboratory and POCT determinations of glucose are made. Providers and bedside nurses adjust the CII according to central laboratory values, POCT values, and trends, as previously described.7
Methods
Setting
Maine Medical Center is a 600-bed tertiary care teaching hospital. It is a level 1 trauma center where 1000 cardiac surgical operations are performed annually. POCT glucose monitoring is relied upon to monitor blood glucose and adjust the CII accordingly. This project, which did not require any additional procedures outside of the standard of care for this population of patients, was reviewed by the Institutional Review Board, who determined that this activity does not meet either the definition of research as specified under 45 CFR 46.102 (d) or the definition of clinical investigation as specified in 21 CFR 56.102 (c).
Patients
Using central laboratory glucose values drawn from the radial artery as the gold standard, we created a registry of consecutive postoperative cardiac surgery patients who had undergone CABG surgery and had blood glucose determinations from both POCT (fingerstick and radial artery samples) and central laboratory testing (radial artery sample) during a 7-month period (May 2016 through February 2017). To be included in the registry, patients had to (1) be postoperative following isolated CABG or CABG plus Maze procedure; (2) have been on cardiopulmonary bypass (CPB); (3) have radial arterial lines; and (4) be on a CII. A total of 116 patients qualified according to the inclusion criteria. Patients missing glucose results in 1 or more of the variables were excluded from data analysis.
Measurements and Variables
Using a POCT glucometer (FreeStyle Precision Pro, Abbott Laboratories, Abbott Park, IL), blood glucose conentrations were measured on samples obtained from both fingerstick and radial artery. Concurrently, radial arterial blood was sent to the central laboratory for glucose measurement. Blood glucose values were compared in CABG patients on CII upon arrival to the cardiothoracic surgery intensive care unit (CTICU) from the operating room and CABG patients on CII 6 hours after arrival in the CTICU. During the 6-hour interval, blood glucose levels were tested hourly or more frequently, allowing nurses to identify trends in blood glucose changes in order to keep blood glucose in the prescribed goal range of 110 to 150 mg/dL. At each of these 2 time points, on arrival to CTICU and 6 hours later, blood glucose values obtained with radial artery POCT and fingerstick POCT were compared with values obtained with central laboratory testing of radial artery samples. The amount of blood required was 1 drop each for POCT fingerstick and POCT radial artery and 2 mL for central lab testing.
Patient characteristics were identified from the electronic medical record. The variables recorded were type of operation, time on CPB, time of CTICU arrival, temperature, vasoconstrictor infusions (norepinephrine, vasopressin, phenylephrine), preoperative diagnosis of diabetes mellitus, preoperative HbA1c, and hemoglobin/hematocrit. Hemoglobin/hematocrit was only available at the time of the patient’s arrival to CTICU. The study was completed within the confines of our center’s standard of care protocol for postoperative cardiac surgical patients.
Analysis
We used standard statistical techniques to describe the study population, including proportions for categorical variables and means (standard deviations) for continuous variables. Correlation and regression techniques were used to describe the relationship between POCT and laboratory (gold standard) tests, both measured as continuous variables, and paired t-tests with Bonferroni correction were used to compare the central tendency and range of these comparisons. We calculated the differences between the gold standard measure and the POCT measure as an indication of outliers (ie, cases in which the 2 tests gave markedly different results). We examined plots to ascertain at which levels of the gold standard test these outliers occurred. An interim analysis was done at the halfway point and submitted to the Institutional Review Board, but no correction to the P value was done based on this analysis, which was largely qualitative. We used Bonferroni correction to declare a P value of 0.025 statistically significant with the 2-way comparisons of both fingerstick and radial artery values to central laboratory values. When the data was stratified by a clinical characteristic creating a 4-way comparison, we used Bonferroni correction to declare a P value of 0.0125 to be statistically significant when comparing both fingerstick and radial artery values to central laboratory values.
Results
Glucose POCT evaluations were carried out on 116 consecutive patients who underwent CABG surgery with or without a Maze procedure on CPB with a CII and an arterial line. Due to missing glucose results in 1 or more of the variables, 10 patients were excluded from data analysis for the time point of arrival in the CTICU and 14 patients were excluded from data analysis for the time point of 6 hours post CTICU arrival. This gave a final count of 106 CABG patients for CTICU arrival data analysis and 102 CABG patients for the 6 hours after CTICU arrival data analysis.
Patients ranged in age from 43 to 85 years, with a mean of age of 66 years, 22% were were women, 41% were diabetic, and 18% had peripheral vascular disease (Table 1). The average preoperative HbA1c was 6.4% ± 1.3% (range, 4.6% to 11.1%). Mean time on CBP for the group was 101 ± 31 minutes (range, 43 to 233 minutes). Postoperative mean hematocrit and hemoglobin were 32.5% and 11.4 g/dL, respectively. The average core temperature of patients on arrival was 36.0°C, which rose to an average of 36.6°C 6 hours later. A vasoconstrictor drip was infusing on 52% of patients upon CTICU arrival; 65% had a vasoconstrictor drip infusing 6 hours after arrival to the CTICU. Hemoglobin results were available only upon CTICU arrival as they are not routinely checked at 6 hours; 74 (64%) patients had a hemoglobin < 12 g/dL.
Compared to central laboratory testing, which we are defining as the gold standard, fingerstick POCT performed better on arrival, while radial artery POCT performed better at 6 hours (Table 2). At CTICU arrival, the mean blood glucose value for fingerstick POCT was 121 ± 24.1 mg/dL, 116 ± 27.2 mg/dL for radial artery POCT, and 128 ± 23.5 mg/dL for central lab testing. The difference in mean blood glucose between the fingerstick POCT and central lab testing was not statistically significant (P = 0.032), while the difference in mean blood glucose between radial artery POCT and central lab testing was statistically significant (P = 0.001). At 6 hours post arrival to the CTICU, the mean fingerstick POCT blood glucose value was 130 ± 23.9 mg/dL, compared to the mean central lab testing value of 137 ± 22.4 mg/dL; this difference was statistically significant (P = 0.019), while the radial artery POCT blood glucose value (133 ± 24.6 mg/dL) was not significantly different from the central lab testing value.
Blood glucose values from fingerstick POCT and central laboratory testing correlated well (r = 0.83 for admission and 0.86 for 6-hour values), as did radial artery POCT and central lab values (r = 0.87 for admission and 0.90 for 6-hour values) (Figures 1, 2, 3, and 4). Comparing individual values for fingerstick POCT and central lab testing, within-person differences between the 2 values ranged from –45 to 25 mg/dL, with 21% of pairs discrepant by 20 mg/dL or more (Figure 1); results were similar at 6 hours (Figure 2), with slightly less discrepancy.
The differences between radial artery POCT and central lab testing values at CTICU arrival ranged from –43 to 80 mg/dL, with 24% of pairs discrepant by 20 mg/dL or more (Figure 3). At 6 hours post CTICU arrival, the difference between radial artery POCT and central lab testing values ranged from –130 to 27 mg/dL, with 11% of pairs discrepant by 20 mg/dL or more (Figure 4). Ninety-two percent of central laboratory values were either close to (± 20) or within the moderate glycemic control target range (110–150 mg/dL).
When the patient cohort was stratified by anemia, diabetes, body temperature, and receipt of vasoconstrictor, there were no significant differences between mean fingerstick POCT and central lab testing values for any strata on CTICU arrival, while there were significant differences between radial artery POCT and central lab testing means for both vasoconstrictor strata as well as for patients with core temperature > 36.1°C (Table 2). At 6 hours, there were no statistically significant differences when stratified for receipt of vasoconstrictor or presence of diabetes. Stratification for anemia or core body temperature was not done for patients at the 6-hour post CTICU arrival time because no hemoglobin value was available and all patients except 1 reached a core temperature of 36.1°C.
Although we measured POCT values obtained using 2 different blood sample sources, fingerstick POCT performed better than radial artery POCT testing with regard to the mean values when compared with the central lab. However, radial artery POCT performed better with regard to correlation with the central lab value. In other words, fingerstick POCT values were less significantly different than radial artery POCT values when compared with the central lab, while radial artery POCT values correlated better with values from the central lab. In spite of this unexplained variability in differences and correlation, the blood glucose values stayed in the target goal range (Figures 1-4).
Discussion
The accuracy of glucose POCT in the critical care setting has been called into question.4,5 The clinical demands of glucose management using CII include timely and accurate guidance in postoperaptive cardiac surgery, in this case, CABG. A previous study compared POCT and central laboratory blood glucose values in medical intensive care unit patients,8 but not in patients who have had CABG surgery. Another study has reviewed the difference in glucose values from POCT and central lab analysis in the critically ill population, but not in the post cardiac surgical population.9 We have shown that the POCT blood glucose values correlate well with the clinical lab values, but the values are statistically different. Our study adds an additional observation in that, although the POCT inconsistencies were statistically significant, they were not clinically significant. That is, POCT of blood glucose was inaccurate, but it still helped guide care by providing enough information to keep the blood glucose in range (most of the time) and allowing the bedside nurse to detect trends and make appropriate adjustments to the infusion. However, given these inconsistencies, we recommend a low threshold for sending additional samples to the central lab to double-check the glucose values, especially when they are outside the prescribed range. Our analysis provides some measure of reassurance with regard to current postoperative CABG glucose management by showing that the limitations of the blood glucose meter do not jeopardize the safety of patients. Nonetheless, we look forward to advances in the accuracy of POCT blood glucose technology so that critical care patients can be better managed when blood glucose is outside the prescribed range.
This analysis of 116 CABG patients points out both the inaccuracy and the utility of a representative POCT glucometer (in this case, the FreeStyle Precision Pro) used at the bedside to manage CIIs in postoperative CABG patients, keeping the blood glucose level in the moderate control range (110-150 mg/dL). The correlation plot shows that in this population the bedside nurses were able to keep blood glucose in range most of the time, in spite of the inaccuracy of POCT of blood glucose, given that the error of the test fits in the wide margin of 40 mg/dL. The fact that the 6-hour values were slightly less variable than the admission values indicates that sequential determinations of blood glucose over the 6-hour period to detect trends allowed good clinical management even in the face of such inaccuracy. The correlation allows the inaccurate number (blood glucose value) to indicate direction, and frequent determinations allow the bedside nurse to keep that number in the prescribed range most of the time in this population of patients.
Conclusion
We have found that glucometer blood glucose determinations in our center used on a homogenous population (CABG surgery) utilizing a single type of glucometer correlated well with those of the central lab, but were not always accurate. In spite of the inaccuracies, experienced bedside nurses were able to use the instrument successfully and safely, as it informed them if the blood glucose was in or out of a predetermined range and in which direction it was going.
Acknowledgment: The authors are indebted to the nurses of the Cardiothoracic Surgery Intensive Care Unit at Maine Medical Center for their support and assistance, without which this analysis would not have been possible.
Corresponding author: Robert S. Kramer, MD, Division of Cardiothoracic Surgery, Maine Medical Center Cardiovascular Institute, 22 Bramhall St., Portland ME 04102; kramer@mmc.org.
Financial disclosures: None.
1. Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021.
2. Lazar H. Glycemic control during coronary artery bypass graft surgery. ISRN Cardiol. 2012;2012:292490.
3. Lazar HL, McDonnell M, Chipkin SR, et al; Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons Practice Guideline Series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663-669.
4. US Food and Drug Administration. Blood Glucose Monitoring Test Systems for Prescription Point of Care Use. Guidance for Industry and Food and Drug Administration Staff,.www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM380325.pdf. Accessed March 8, 2019.
5. Finkielman JD, Oyen LJ, Afess B. Agreement between bedside blood and plasma glucose measurement in the ICU Setting. Chest. 2005;127:1749-1511.
6. Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38:471-476.
7. Kramer R, Groom R, Weldner D, et al. Glycemic control reduces deep sternal wound infection: a multidisciplinary approach. Arch Surg. 2008;143:451-456.
8. Peterson JR, Graves DF, Tacker DH, et al. Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clinica Chimica Acta. 2008;396:10-13.
9. Cook A, Laughlin D, Moore M, et al. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. Am J Crit Care. 2009;18:65-72.
1. Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021.
2. Lazar H. Glycemic control during coronary artery bypass graft surgery. ISRN Cardiol. 2012;2012:292490.
3. Lazar HL, McDonnell M, Chipkin SR, et al; Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons Practice Guideline Series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663-669.
4. US Food and Drug Administration. Blood Glucose Monitoring Test Systems for Prescription Point of Care Use. Guidance for Industry and Food and Drug Administration Staff,.www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM380325.pdf. Accessed March 8, 2019.
5. Finkielman JD, Oyen LJ, Afess B. Agreement between bedside blood and plasma glucose measurement in the ICU Setting. Chest. 2005;127:1749-1511.
6. Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38:471-476.
7. Kramer R, Groom R, Weldner D, et al. Glycemic control reduces deep sternal wound infection: a multidisciplinary approach. Arch Surg. 2008;143:451-456.
8. Peterson JR, Graves DF, Tacker DH, et al. Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clinica Chimica Acta. 2008;396:10-13.
9. Cook A, Laughlin D, Moore M, et al. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. Am J Crit Care. 2009;18:65-72.
Ticagrelor reversal agent looks promising
NEW ORLEANS – A novel targeted ticagrelor reversal agent demonstrated rapid and sustained reversal of the potent antiplatelet agent in a phase 1 proof-of-concept study, Deepak L. Bhatt, MD, reported at the annual meeting of the American College of Cardiology.
“Hopefully the FDA will view this as something that really is a breakthrough,” commented Dr. Bhatt, executive director of interventional cardiology programs at Brigham and Women’s Hospital and professor of medicine at Harvard University, both in Boston.
Why a breakthrough? Because despite recent major advances in the ability to reverse the action of the direct-acting oral anticoagulants and thereby greatly improve their safety margin, there have been no parallel developments with regard to the potent antiplatelet agents ticagrelor (Brilinta), prasugrel (Effient), and clopidogrel. The effects of these antiplatelet drugs take 3-5 days to dissipate after they’ve been stopped, which is highly problematic when they’ve induced catastrophic bleeding or a patient requires emergent or urgent surgery, the cardiologist explained.
“The ability to reverse tigracelor’s antiplatelet effects rapidly could distinguish it from other antiplatelet agents such as prasugrel or even generic clopidogrel and, for that matter, even aspirin,” Dr. Bhatt said.
The ticagrelor reversal agent, known for now as PB2452, is an intravenously administered recombinant human immunoglobulin G1 monoclonal antibody antigen-binding fragment. It binds specifically and with high affinity to ticagrelor and its active metabolite. In the phase 1, placebo-controlled, double-blind study conducted in 64 healthy volunteers pretreated with ticagrelor for 48 hours, it reversed oral ticagrelor’s antiplatelet effects within 5 minutes and, with prolonged infusion, showed sustained effect for at least 20 hours.
The only adverse events observed in blinded assessment were minor injection site issues.
PB2452 is specific to ticagrelor and will not reverse the activity of other potent antiplatelet agents. Indeed, because of their chemical structure, neither prasugrel nor clopidogrel is reversible, according to Dr. Bhatt.
He said the developmental game plan for the ticagrelor reversal agent is initially to get it approved by the Food and Drug Administration for ticagrelor-related catastrophic bleeding, such as intracranial hemorrhage, since there is a recognized major unmet need in such situations. But as shown in the phase 1 study, BP2452 is potentially titratable by varying the size of the initial bolus dose and the dosing and duration of the subsequent infusion. So after initial approval for catastrophic bleeding, it makes sense to branch out and conduct further studies establishing the reversal agent’s value for prevention of bleeding complications caused by ticagrelor. An example might be a patient on ticagrelor because she recently received a stent in her left main coronary artery who falls and breaks her hip, and her surgeon says she needs surgery right away.
“If someone on ticagrelor came in with an intracranial hemorrhage, you’d want rapid reversal and have it sustained for as many days as the neurologist advises, whereas maybe if someone came in on ticagrelor after placement of a left main stent and you needed to do a lumbar puncture, you’d want to reverse the antiplatelet effect for the LP, and then if things go smoothly you’d want to get the ticagrelor back on board so the stent doesn’t thrombose. But that type of more precise dosing will require further work,” according to the cardiologist.
Discussant Barbara S. Wiggins, PharmD, commented, “We’ve been fortunate to have reversal agents come out for oral anticoagulants, but in terms of antiplatelet activity we’ve not been able to be successful with platelet transfusions. So having a reversal agent added to our armamentarium certainly is something that’s desirable.”
The phase 1 study of PB2452 indicates the monoclonal antibody checks the key boxes one looks for in a reversal agent: quick onset, long duration of effect, lack of a rebound in platelet activity after drug cessation, and potential for tailored titration. Of course, data on efficacy outcomes will also be necessary, noted Dr. Wiggins, a clinical pharmacologist at the Medical University of South Carolina, Charleston.
She added that she was favorably impressed that Dr. Bhatt and his coinvestigators went to the trouble of convincingly demonstrating reversal of ticagrelor’s antiplatelet effects using three different assays: light transmission aggregometry, which is considered the standard, as well as the point-of-care VerifyNow P2Y12 assay and the modified CY-QUANT assay.
The phase 1 study was funded by PhaseBio Pharmaceuticals. Dr. Bhatt reported the company provided a research grant directly to Brigham and Women’s Hospital.
Simultaneous with Dr. Bhatt’s presentation, the study results were published online (N Engl J Med. 2019 Mar 17. doi: 10.1056/NEJMoa1901778).
NEW ORLEANS – A novel targeted ticagrelor reversal agent demonstrated rapid and sustained reversal of the potent antiplatelet agent in a phase 1 proof-of-concept study, Deepak L. Bhatt, MD, reported at the annual meeting of the American College of Cardiology.
“Hopefully the FDA will view this as something that really is a breakthrough,” commented Dr. Bhatt, executive director of interventional cardiology programs at Brigham and Women’s Hospital and professor of medicine at Harvard University, both in Boston.
Why a breakthrough? Because despite recent major advances in the ability to reverse the action of the direct-acting oral anticoagulants and thereby greatly improve their safety margin, there have been no parallel developments with regard to the potent antiplatelet agents ticagrelor (Brilinta), prasugrel (Effient), and clopidogrel. The effects of these antiplatelet drugs take 3-5 days to dissipate after they’ve been stopped, which is highly problematic when they’ve induced catastrophic bleeding or a patient requires emergent or urgent surgery, the cardiologist explained.
“The ability to reverse tigracelor’s antiplatelet effects rapidly could distinguish it from other antiplatelet agents such as prasugrel or even generic clopidogrel and, for that matter, even aspirin,” Dr. Bhatt said.
The ticagrelor reversal agent, known for now as PB2452, is an intravenously administered recombinant human immunoglobulin G1 monoclonal antibody antigen-binding fragment. It binds specifically and with high affinity to ticagrelor and its active metabolite. In the phase 1, placebo-controlled, double-blind study conducted in 64 healthy volunteers pretreated with ticagrelor for 48 hours, it reversed oral ticagrelor’s antiplatelet effects within 5 minutes and, with prolonged infusion, showed sustained effect for at least 20 hours.
The only adverse events observed in blinded assessment were minor injection site issues.
PB2452 is specific to ticagrelor and will not reverse the activity of other potent antiplatelet agents. Indeed, because of their chemical structure, neither prasugrel nor clopidogrel is reversible, according to Dr. Bhatt.
He said the developmental game plan for the ticagrelor reversal agent is initially to get it approved by the Food and Drug Administration for ticagrelor-related catastrophic bleeding, such as intracranial hemorrhage, since there is a recognized major unmet need in such situations. But as shown in the phase 1 study, BP2452 is potentially titratable by varying the size of the initial bolus dose and the dosing and duration of the subsequent infusion. So after initial approval for catastrophic bleeding, it makes sense to branch out and conduct further studies establishing the reversal agent’s value for prevention of bleeding complications caused by ticagrelor. An example might be a patient on ticagrelor because she recently received a stent in her left main coronary artery who falls and breaks her hip, and her surgeon says she needs surgery right away.
“If someone on ticagrelor came in with an intracranial hemorrhage, you’d want rapid reversal and have it sustained for as many days as the neurologist advises, whereas maybe if someone came in on ticagrelor after placement of a left main stent and you needed to do a lumbar puncture, you’d want to reverse the antiplatelet effect for the LP, and then if things go smoothly you’d want to get the ticagrelor back on board so the stent doesn’t thrombose. But that type of more precise dosing will require further work,” according to the cardiologist.
Discussant Barbara S. Wiggins, PharmD, commented, “We’ve been fortunate to have reversal agents come out for oral anticoagulants, but in terms of antiplatelet activity we’ve not been able to be successful with platelet transfusions. So having a reversal agent added to our armamentarium certainly is something that’s desirable.”
The phase 1 study of PB2452 indicates the monoclonal antibody checks the key boxes one looks for in a reversal agent: quick onset, long duration of effect, lack of a rebound in platelet activity after drug cessation, and potential for tailored titration. Of course, data on efficacy outcomes will also be necessary, noted Dr. Wiggins, a clinical pharmacologist at the Medical University of South Carolina, Charleston.
She added that she was favorably impressed that Dr. Bhatt and his coinvestigators went to the trouble of convincingly demonstrating reversal of ticagrelor’s antiplatelet effects using three different assays: light transmission aggregometry, which is considered the standard, as well as the point-of-care VerifyNow P2Y12 assay and the modified CY-QUANT assay.
The phase 1 study was funded by PhaseBio Pharmaceuticals. Dr. Bhatt reported the company provided a research grant directly to Brigham and Women’s Hospital.
Simultaneous with Dr. Bhatt’s presentation, the study results were published online (N Engl J Med. 2019 Mar 17. doi: 10.1056/NEJMoa1901778).
NEW ORLEANS – A novel targeted ticagrelor reversal agent demonstrated rapid and sustained reversal of the potent antiplatelet agent in a phase 1 proof-of-concept study, Deepak L. Bhatt, MD, reported at the annual meeting of the American College of Cardiology.
“Hopefully the FDA will view this as something that really is a breakthrough,” commented Dr. Bhatt, executive director of interventional cardiology programs at Brigham and Women’s Hospital and professor of medicine at Harvard University, both in Boston.
Why a breakthrough? Because despite recent major advances in the ability to reverse the action of the direct-acting oral anticoagulants and thereby greatly improve their safety margin, there have been no parallel developments with regard to the potent antiplatelet agents ticagrelor (Brilinta), prasugrel (Effient), and clopidogrel. The effects of these antiplatelet drugs take 3-5 days to dissipate after they’ve been stopped, which is highly problematic when they’ve induced catastrophic bleeding or a patient requires emergent or urgent surgery, the cardiologist explained.
“The ability to reverse tigracelor’s antiplatelet effects rapidly could distinguish it from other antiplatelet agents such as prasugrel or even generic clopidogrel and, for that matter, even aspirin,” Dr. Bhatt said.
The ticagrelor reversal agent, known for now as PB2452, is an intravenously administered recombinant human immunoglobulin G1 monoclonal antibody antigen-binding fragment. It binds specifically and with high affinity to ticagrelor and its active metabolite. In the phase 1, placebo-controlled, double-blind study conducted in 64 healthy volunteers pretreated with ticagrelor for 48 hours, it reversed oral ticagrelor’s antiplatelet effects within 5 minutes and, with prolonged infusion, showed sustained effect for at least 20 hours.
The only adverse events observed in blinded assessment were minor injection site issues.
PB2452 is specific to ticagrelor and will not reverse the activity of other potent antiplatelet agents. Indeed, because of their chemical structure, neither prasugrel nor clopidogrel is reversible, according to Dr. Bhatt.
He said the developmental game plan for the ticagrelor reversal agent is initially to get it approved by the Food and Drug Administration for ticagrelor-related catastrophic bleeding, such as intracranial hemorrhage, since there is a recognized major unmet need in such situations. But as shown in the phase 1 study, BP2452 is potentially titratable by varying the size of the initial bolus dose and the dosing and duration of the subsequent infusion. So after initial approval for catastrophic bleeding, it makes sense to branch out and conduct further studies establishing the reversal agent’s value for prevention of bleeding complications caused by ticagrelor. An example might be a patient on ticagrelor because she recently received a stent in her left main coronary artery who falls and breaks her hip, and her surgeon says she needs surgery right away.
“If someone on ticagrelor came in with an intracranial hemorrhage, you’d want rapid reversal and have it sustained for as many days as the neurologist advises, whereas maybe if someone came in on ticagrelor after placement of a left main stent and you needed to do a lumbar puncture, you’d want to reverse the antiplatelet effect for the LP, and then if things go smoothly you’d want to get the ticagrelor back on board so the stent doesn’t thrombose. But that type of more precise dosing will require further work,” according to the cardiologist.
Discussant Barbara S. Wiggins, PharmD, commented, “We’ve been fortunate to have reversal agents come out for oral anticoagulants, but in terms of antiplatelet activity we’ve not been able to be successful with platelet transfusions. So having a reversal agent added to our armamentarium certainly is something that’s desirable.”
The phase 1 study of PB2452 indicates the monoclonal antibody checks the key boxes one looks for in a reversal agent: quick onset, long duration of effect, lack of a rebound in platelet activity after drug cessation, and potential for tailored titration. Of course, data on efficacy outcomes will also be necessary, noted Dr. Wiggins, a clinical pharmacologist at the Medical University of South Carolina, Charleston.
She added that she was favorably impressed that Dr. Bhatt and his coinvestigators went to the trouble of convincingly demonstrating reversal of ticagrelor’s antiplatelet effects using three different assays: light transmission aggregometry, which is considered the standard, as well as the point-of-care VerifyNow P2Y12 assay and the modified CY-QUANT assay.
The phase 1 study was funded by PhaseBio Pharmaceuticals. Dr. Bhatt reported the company provided a research grant directly to Brigham and Women’s Hospital.
Simultaneous with Dr. Bhatt’s presentation, the study results were published online (N Engl J Med. 2019 Mar 17. doi: 10.1056/NEJMoa1901778).
REPORTING FROM ACC 19
Key clinical point: Oral ticagrelor’s antiplatelet effect was reversed within 5 minutes by a novel targeted monoclonal antibody.
Major finding: A novel targeted monoclonal antibody reversed oral ticagrelor’s antiplatelet effects within 5 minutes and, with prolonged infusion, showed sustained effect for at least 20 hours.
Study details: This phase 1 study included 64 healthy subjects pretreated with 48 hours of ticagrelor before receiving various doses of the reversal agent or placebo.
Disclosures: The study was funded by PhaseBio Pharmaceuticals, which provided a research grant directly to Brigham and Women’s Hospital.
The 2018 AHA/ACC cholesterol guidelines: What’s changed?
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
Some cardiac devices vulnerable to cybersecurity threats
Implantable cardiac devices made by Medtronic have cybersecurity vulnerabilities, according to a safety communication from the Food and Drug Administration. That said, so far the FDA is unaware of any reports of harm related to these vulnerabilities, and the agency still advises doctors and patients to continue using the devices as intended and in accordance with device labeling.
The Conexus wireless telemetry protocol used with Medtronic’s implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators, as well as with certain models of Medtronic’s CareLink Programmer and the MyCareLink Monitor, lacks encryption, authentication, or authorization, which leaves the devices open to exploitation. Such exploitation “could allow unauthorized individuals ... to access and potentially manipulate an implantable device, home monitor, or clinic programmer,” the agency said in its safety communication.
The FDA provides several recommendations in the safety communication, including obtaining these devices “directly from the manufacturer to ensure integrity of the system” and operating “the programmers within well-managed networks.”
Implantable cardiac devices made by Medtronic have cybersecurity vulnerabilities, according to a safety communication from the Food and Drug Administration. That said, so far the FDA is unaware of any reports of harm related to these vulnerabilities, and the agency still advises doctors and patients to continue using the devices as intended and in accordance with device labeling.
The Conexus wireless telemetry protocol used with Medtronic’s implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators, as well as with certain models of Medtronic’s CareLink Programmer and the MyCareLink Monitor, lacks encryption, authentication, or authorization, which leaves the devices open to exploitation. Such exploitation “could allow unauthorized individuals ... to access and potentially manipulate an implantable device, home monitor, or clinic programmer,” the agency said in its safety communication.
The FDA provides several recommendations in the safety communication, including obtaining these devices “directly from the manufacturer to ensure integrity of the system” and operating “the programmers within well-managed networks.”
Implantable cardiac devices made by Medtronic have cybersecurity vulnerabilities, according to a safety communication from the Food and Drug Administration. That said, so far the FDA is unaware of any reports of harm related to these vulnerabilities, and the agency still advises doctors and patients to continue using the devices as intended and in accordance with device labeling.
The Conexus wireless telemetry protocol used with Medtronic’s implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators, as well as with certain models of Medtronic’s CareLink Programmer and the MyCareLink Monitor, lacks encryption, authentication, or authorization, which leaves the devices open to exploitation. Such exploitation “could allow unauthorized individuals ... to access and potentially manipulate an implantable device, home monitor, or clinic programmer,” the agency said in its safety communication.
The FDA provides several recommendations in the safety communication, including obtaining these devices “directly from the manufacturer to ensure integrity of the system” and operating “the programmers within well-managed networks.”
Occurrence of pulmonary embolisms in hospitalized patients nearly doubled during 2004-2015
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
REPORTING FROM ACC 2019
Increased sudden death risk in HIV linked to cardiac fibrosis
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
REPORTING FROM CROI 2019