User login
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
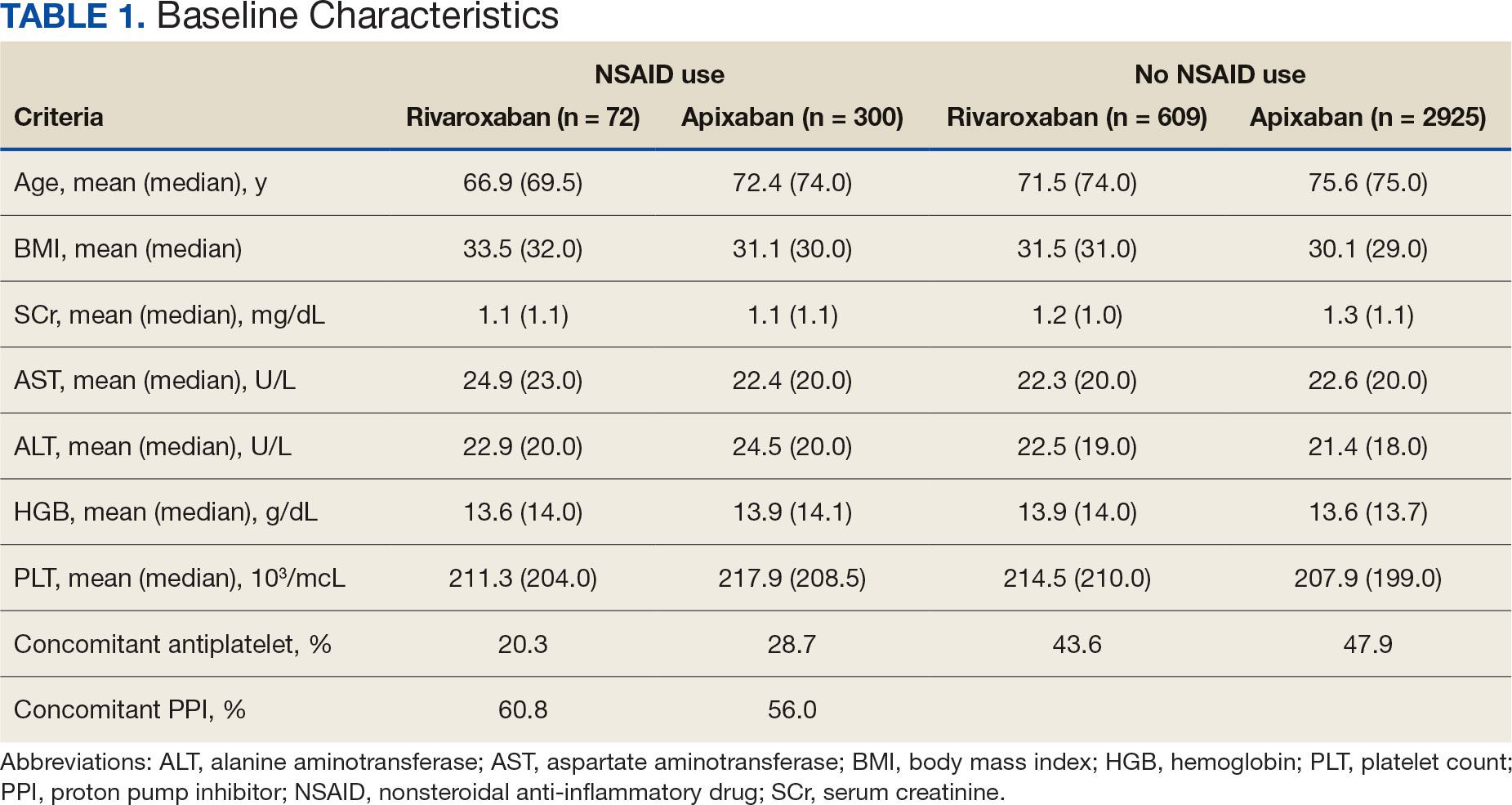
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.
A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
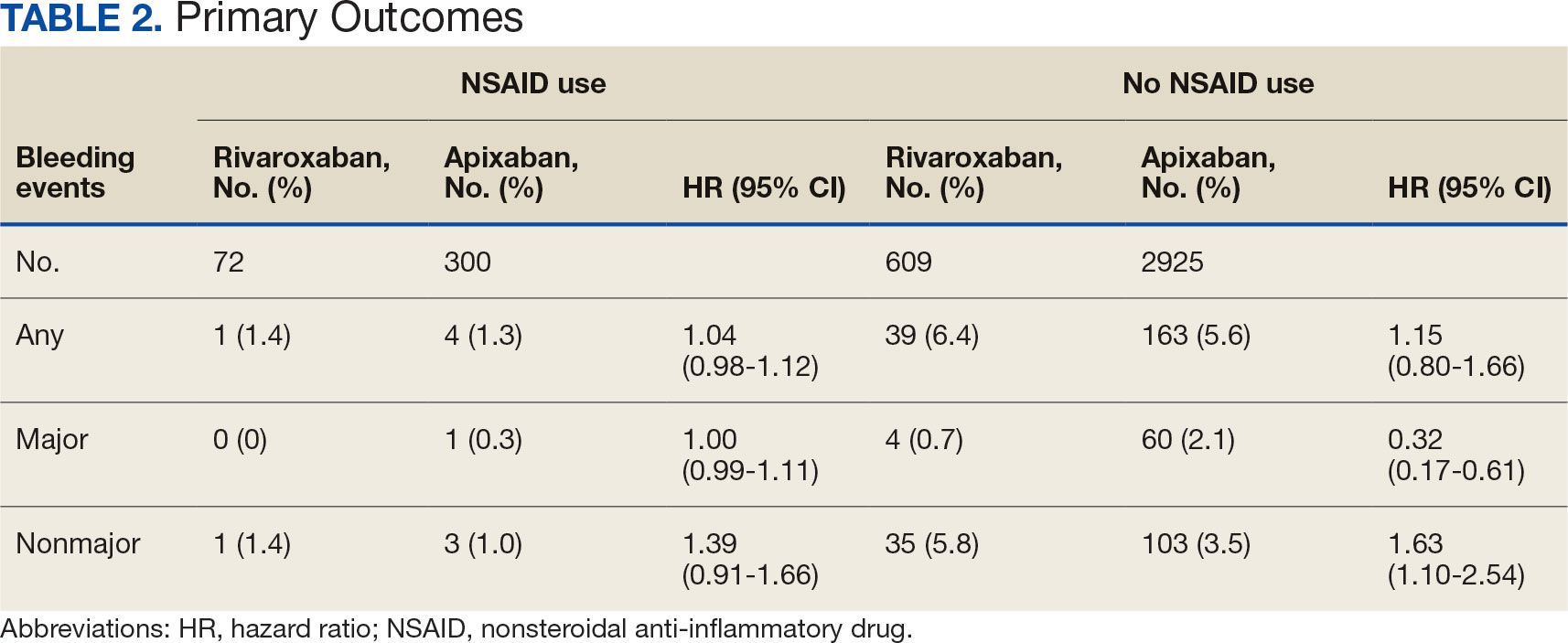
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).
The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Study Finds Association Between Statins and Glaucoma
Adults with high cholesterol taking statins may have a significantly higher risk of developing glaucoma than those not taking the cholesterol-lowering drugs, an observational study of a large research database found.
The study, published in Ophthalmology Glaucoma, analyzed electronic health records of 79,742 adults with hyperlipidemia in the All of Us Research Program database from 2017 to 2022. The repository is maintained by the National Institutes of Health and provides data for research into precision medicine.
The 6365 statin users in the study population had a 47% greater unadjusted prevalence of glaucoma than nonusers of the drugs (P < .001) and a 13% greater prevalence in models that adjusted for potential confounding variables (P = .02). The researchers also found statin users had significantly higher levels of low-density lipoprotein cholesterol (LDL-C), but even patients with optimal levels of LDL-C had higher rates of glaucoma.
‘A Little Unusual’
Drawing any clinically relevant conclusions from this latest study would be premature, said Victoria Tseng, MD, PhD, an assistant professor at UCLA Stein Eye Institute and Doheny Eye Centers UCLA, and the senior author of the study. “I certainly would not be telling my patients on statins to stop their statins.”
Tseng acknowledged her group’s finding runs counter to previous studies that found statins may help prevent glaucoma or at least have no effect on the eye disease, although the association between cholesterol and glaucoma has been well established.
A 2019 analysis of nearly 137,000 participants in three population studies found no connection between statin use and the risk for primary open-angle glaucoma. A 2012 study of more than 500,000 people with high cholesterol found statin use was associated with a significant reduction in the risk for open-angle glaucoma.
“It’s a little unusual that we found the opposite,” Tseng said in an interview.
One explanation is the observational nature of the AoU analysis Tseng’s group conducted. “We don’t know what these people look like or how well the data were collected, so we’re going off of what’s there in the database,” she said.
Another explanation could be the nature of hyperlipidemia itself, she said. “There have definitely been studies that suggest increased cholesterol levels are associated with an increased risk of glaucoma. Presumably, you’re not going to be taking a statin unless your cholesterol is a little worse.”
While the study analysis attempted to control for cholesterol levels, Tseng noted, “there could be some residual confounding from that.”
Statin users in the study had an average LDL-C level of 144.9 mg/dL vs 136.3 mg/dL in the population not taking any cholesterol medication (P < .001). Statin users with optimal LDL-C, defined as less than 100 mg/dL, had a 39% greater adjusted prevalence of glaucoma (P = .02), while those with high LDL-C (160-189 mg/dL) had a 37% greater adjusted prevalence (P = .005).
Age was another factor in the risk for glaucoma, the study found. Statin users aged 60-69 years had an adjusted rate of glaucoma 28% greater than that for nonusers (P = .05).
Laboratory studies may help clarify the relationships between statins and glaucoma, Tseng said. That could include putting statins directly on the optic nerve of laboratory mice and further investigating how statins affect the mechanisms that influence eye pressure, a key driver of glaucoma. From a population study perspective, a randomized trial of glaucoma patients comparing the effect of statins and other cholesterol-lowering medications with nonuse may provide answers.
Database Strengths and Limitations
The study “adds to the somewhat mixed literature on the potential association between statins and glaucoma,” Sophia Wang, MD, MS, a glaucoma specialist at Stanford Byers Eye Institute in Palo Alto, California, said in an interview.
The AoU research cohort is a “notable strength” of the new paper, added Wang, who has used the AoU database to study the relationship between blood pressure, blood pressure medications, and glaucoma.
“The population is especially large and diverse, with a large proportion of participants from backgrounds that are traditionally underrepresented in research,” she said. And The inclusion of both medical records and survey data means the health information on the cohort is detailed and longitudinal.
“The authors make excellent use here of the data by including in their analyses results of laboratory investigations — LDL-C, notably — which wouldn’t be readily available in other types of datasets such as claims datasets,” she said.
However, the database has limitations as well, including its reliance on coding, which is prone to errors, to determine glaucoma diagnosis and missing information on eye examinations. In addition, the study used one LDL-C measurement rather than multiple measurements, Wang pointed out, “and we know that LDL-C can vary over time.”
The study was funded by Research to Prevent Blindness. Tseng and Wang reported no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Adults with high cholesterol taking statins may have a significantly higher risk of developing glaucoma than those not taking the cholesterol-lowering drugs, an observational study of a large research database found.
The study, published in Ophthalmology Glaucoma, analyzed electronic health records of 79,742 adults with hyperlipidemia in the All of Us Research Program database from 2017 to 2022. The repository is maintained by the National Institutes of Health and provides data for research into precision medicine.
The 6365 statin users in the study population had a 47% greater unadjusted prevalence of glaucoma than nonusers of the drugs (P < .001) and a 13% greater prevalence in models that adjusted for potential confounding variables (P = .02). The researchers also found statin users had significantly higher levels of low-density lipoprotein cholesterol (LDL-C), but even patients with optimal levels of LDL-C had higher rates of glaucoma.
‘A Little Unusual’
Drawing any clinically relevant conclusions from this latest study would be premature, said Victoria Tseng, MD, PhD, an assistant professor at UCLA Stein Eye Institute and Doheny Eye Centers UCLA, and the senior author of the study. “I certainly would not be telling my patients on statins to stop their statins.”
Tseng acknowledged her group’s finding runs counter to previous studies that found statins may help prevent glaucoma or at least have no effect on the eye disease, although the association between cholesterol and glaucoma has been well established.
A 2019 analysis of nearly 137,000 participants in three population studies found no connection between statin use and the risk for primary open-angle glaucoma. A 2012 study of more than 500,000 people with high cholesterol found statin use was associated with a significant reduction in the risk for open-angle glaucoma.
“It’s a little unusual that we found the opposite,” Tseng said in an interview.
One explanation is the observational nature of the AoU analysis Tseng’s group conducted. “We don’t know what these people look like or how well the data were collected, so we’re going off of what’s there in the database,” she said.
Another explanation could be the nature of hyperlipidemia itself, she said. “There have definitely been studies that suggest increased cholesterol levels are associated with an increased risk of glaucoma. Presumably, you’re not going to be taking a statin unless your cholesterol is a little worse.”
While the study analysis attempted to control for cholesterol levels, Tseng noted, “there could be some residual confounding from that.”
Statin users in the study had an average LDL-C level of 144.9 mg/dL vs 136.3 mg/dL in the population not taking any cholesterol medication (P < .001). Statin users with optimal LDL-C, defined as less than 100 mg/dL, had a 39% greater adjusted prevalence of glaucoma (P = .02), while those with high LDL-C (160-189 mg/dL) had a 37% greater adjusted prevalence (P = .005).
Age was another factor in the risk for glaucoma, the study found. Statin users aged 60-69 years had an adjusted rate of glaucoma 28% greater than that for nonusers (P = .05).
Laboratory studies may help clarify the relationships between statins and glaucoma, Tseng said. That could include putting statins directly on the optic nerve of laboratory mice and further investigating how statins affect the mechanisms that influence eye pressure, a key driver of glaucoma. From a population study perspective, a randomized trial of glaucoma patients comparing the effect of statins and other cholesterol-lowering medications with nonuse may provide answers.
Database Strengths and Limitations
The study “adds to the somewhat mixed literature on the potential association between statins and glaucoma,” Sophia Wang, MD, MS, a glaucoma specialist at Stanford Byers Eye Institute in Palo Alto, California, said in an interview.
The AoU research cohort is a “notable strength” of the new paper, added Wang, who has used the AoU database to study the relationship between blood pressure, blood pressure medications, and glaucoma.
“The population is especially large and diverse, with a large proportion of participants from backgrounds that are traditionally underrepresented in research,” she said. And The inclusion of both medical records and survey data means the health information on the cohort is detailed and longitudinal.
“The authors make excellent use here of the data by including in their analyses results of laboratory investigations — LDL-C, notably — which wouldn’t be readily available in other types of datasets such as claims datasets,” she said.
However, the database has limitations as well, including its reliance on coding, which is prone to errors, to determine glaucoma diagnosis and missing information on eye examinations. In addition, the study used one LDL-C measurement rather than multiple measurements, Wang pointed out, “and we know that LDL-C can vary over time.”
The study was funded by Research to Prevent Blindness. Tseng and Wang reported no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Adults with high cholesterol taking statins may have a significantly higher risk of developing glaucoma than those not taking the cholesterol-lowering drugs, an observational study of a large research database found.
The study, published in Ophthalmology Glaucoma, analyzed electronic health records of 79,742 adults with hyperlipidemia in the All of Us Research Program database from 2017 to 2022. The repository is maintained by the National Institutes of Health and provides data for research into precision medicine.
The 6365 statin users in the study population had a 47% greater unadjusted prevalence of glaucoma than nonusers of the drugs (P < .001) and a 13% greater prevalence in models that adjusted for potential confounding variables (P = .02). The researchers also found statin users had significantly higher levels of low-density lipoprotein cholesterol (LDL-C), but even patients with optimal levels of LDL-C had higher rates of glaucoma.
‘A Little Unusual’
Drawing any clinically relevant conclusions from this latest study would be premature, said Victoria Tseng, MD, PhD, an assistant professor at UCLA Stein Eye Institute and Doheny Eye Centers UCLA, and the senior author of the study. “I certainly would not be telling my patients on statins to stop their statins.”
Tseng acknowledged her group’s finding runs counter to previous studies that found statins may help prevent glaucoma or at least have no effect on the eye disease, although the association between cholesterol and glaucoma has been well established.
A 2019 analysis of nearly 137,000 participants in three population studies found no connection between statin use and the risk for primary open-angle glaucoma. A 2012 study of more than 500,000 people with high cholesterol found statin use was associated with a significant reduction in the risk for open-angle glaucoma.
“It’s a little unusual that we found the opposite,” Tseng said in an interview.
One explanation is the observational nature of the AoU analysis Tseng’s group conducted. “We don’t know what these people look like or how well the data were collected, so we’re going off of what’s there in the database,” she said.
Another explanation could be the nature of hyperlipidemia itself, she said. “There have definitely been studies that suggest increased cholesterol levels are associated with an increased risk of glaucoma. Presumably, you’re not going to be taking a statin unless your cholesterol is a little worse.”
While the study analysis attempted to control for cholesterol levels, Tseng noted, “there could be some residual confounding from that.”
Statin users in the study had an average LDL-C level of 144.9 mg/dL vs 136.3 mg/dL in the population not taking any cholesterol medication (P < .001). Statin users with optimal LDL-C, defined as less than 100 mg/dL, had a 39% greater adjusted prevalence of glaucoma (P = .02), while those with high LDL-C (160-189 mg/dL) had a 37% greater adjusted prevalence (P = .005).
Age was another factor in the risk for glaucoma, the study found. Statin users aged 60-69 years had an adjusted rate of glaucoma 28% greater than that for nonusers (P = .05).
Laboratory studies may help clarify the relationships between statins and glaucoma, Tseng said. That could include putting statins directly on the optic nerve of laboratory mice and further investigating how statins affect the mechanisms that influence eye pressure, a key driver of glaucoma. From a population study perspective, a randomized trial of glaucoma patients comparing the effect of statins and other cholesterol-lowering medications with nonuse may provide answers.
Database Strengths and Limitations
The study “adds to the somewhat mixed literature on the potential association between statins and glaucoma,” Sophia Wang, MD, MS, a glaucoma specialist at Stanford Byers Eye Institute in Palo Alto, California, said in an interview.
The AoU research cohort is a “notable strength” of the new paper, added Wang, who has used the AoU database to study the relationship between blood pressure, blood pressure medications, and glaucoma.
“The population is especially large and diverse, with a large proportion of participants from backgrounds that are traditionally underrepresented in research,” she said. And The inclusion of both medical records and survey data means the health information on the cohort is detailed and longitudinal.
“The authors make excellent use here of the data by including in their analyses results of laboratory investigations — LDL-C, notably — which wouldn’t be readily available in other types of datasets such as claims datasets,” she said.
However, the database has limitations as well, including its reliance on coding, which is prone to errors, to determine glaucoma diagnosis and missing information on eye examinations. In addition, the study used one LDL-C measurement rather than multiple measurements, Wang pointed out, “and we know that LDL-C can vary over time.”
The study was funded by Research to Prevent Blindness. Tseng and Wang reported no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
FROM OPHTHALMOLOGY GLAUCOMA
Drugs to Target Lp(a): What’s Coming
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here at the American Heart Association Scientific Sessions. It’s a very exciting meeting, but one of the interesting topics that we’re going to be talking about is lipoprotein(a) [Lp(a)] . It’s definitely one of the hottest sessions of the meeting.
Joining me to discuss this topic is Dr Steve Nicholls, who is arguably one of the leading experts in the world on lipids. He’s a professor of medicine at Monash University in Australia. Welcome. Thanks, Steve.
Stephen J. Nicholls, MBBS, PhD: Thanks for having me.
O’Donoghue: There are two phase 2 studies that we’ll circle back to that are being presented here at the American Heart Association meeting. These are for novel therapeutics that lower Lp(a). Perhaps taking a step back, we know that there’s a large body of evidence to support the concept that Lp(a) plays a causal role in heart disease and atherogenesis, but to date we haven’t had any effective therapies to really lower it.
Thinking about the therapeutics specifically that are on the horizon, perhaps we could start there. Which one is furthest along in development, and how does that look in terms of its ability to lower Lp(a)?
Pelacarsen, an ASO
Nicholls: Most of the therapies are injectable. Most of them are nucleic acid–based therapies, and the one that’s most advanced is an agent called pelacarsen. Pelacarsen is an antisense oligonucleotide (ASO), and it has gone all the way through its early phase 2 studies. It has a fully enrolled cardiovascular outcome trial.
We’re all eagerly awaiting the results of that study sometime in the next year or so. That will be the first large-scale clinical trial that will give us some clinical validation to ask the question of whether substantive lowering of Lp(a) will lower cardiovascular risk, with an agent that in early studies looks like it lowers Lp(a) about 80%.
O’Donoghue: Which is tremendous, because again, we really don’t have any effective therapies right now. I guess one of the big questions is, how much do we need to lower Lp(a) for that to translate into meaningful clinical benefit? What’s your sense there?
Nicholls: Well, we simply don’t know. We’ve tried to look to genetics to try and give us some sort of sense in terms of what that looks like. Lp(a) is a little tricky because the assays and the numbers that get spit out can be tricky in terms of trying to compare apples and apples in different studies.
We think that it’s probably at least a 50- to 75-mg/dL lowering of Lp(a) using the old units. We think that pelacarsen would hit that, and so our hope is that that would translate to a 15%-20% reduction in major cardiovascular events, but again, we’ve never asked this question before.
We have data from PCSK9 inhibitor trials showing that lesser reductions in Lp(a) of 25%-30% with both evolocumab and alirocumab contributed to the clinical benefit that we saw in those studies. Those agents were really good at lowering low-density lipoprotein (LDL) cholesterol, but Lp(a) lowering seemed to matter. One would be very hopeful that if a 25%-30% lowering of Lp(a) is useful, then an 80% or greater lowering of Lp(a) should be really useful.
The siRNAs
O’Donoghue: In addition to the ASO pelacarsen that you mentioned, there are several therapeutics in the pipeline, including three small interfering (si) RNAs that are at least in phase 2 and phase 3 testing at this point in time. There’s olpasiran, which in phase 2 testing led to more than a 95% reduction in Lp(a), and then lepodisiran , which has now moved into phase 3 testing, albeit we haven’t seen yet the phase 2 results.
What is your sense of lepodisiran and its efficacy?
Nicholls: What’s been really quite striking about the siRNAs is the even more profound degree of lowering of Lp(a) that we’re seeing. We’re seeing 90% and greater lowering of Lp(a) in all of those programs. We’re seeing some differences between the programs in terms of the durability of that effect.
I think it would be fair to say that with zerlasiran we’re starting to see perhaps that lowering effect starts to taper off a little bit more quickly than the other two. I think that may have some implications in terms of what dosing regimens may look like in the future.
Even so, we’re talking about therapies that may be dosed 3- to 6-monthly, or even with the potential for being less frequent than that with lepodisiran. Again, I think the phase 2 data will be really important in terms of giving us more information.
O’Donoghue: For the lepodisiran results, I was really quite struck that even though it was small numbers, single dose administered, it really looked like the duration of effect persisted at the higher doses up to about a year.
Nicholls: It looks pretty promising. We’ve launched the ACCLAIM study, the large cardiovascular outcome trial of lepodisiran, with a 6-monthly regimen. We are hopeful that more information may be able to give us the opportunity for even less frequent administration.
That has really important implications for patients where adherence is a particular issue. They may just simply want to come into the clinic. You know, once or twice a year, very much like we’re seeing with inclisiran, and that may be a really effective approach for many patients.
O’Donoghue: You alluded to the zerlasiran results, which were presented here at the American Heart Association meeting, and that even though it led to a robust reduction in Lp(a), it looked like the durability component was maybe a little bit shorter than for some of the other siRNAs that are currently being evaluated.
What’s your sense of that?
Nicholls: It probably is. The implications clinically, at least in an outcome trial when they ultimately get to that point, probably aren’t that important. They’ll probably just have slightly more frequent administration. That may become a bigger issue when it gets out into the clinic.
The nice thing is that if all of these agents appear to be effective, are well tolerated, and get out to the clinic, then clinicians and patients are going to have a lot of choice.
O’Donoghue: I think more competition is always good news for the field, ultimately. I think to your point, especially for a drug that might be self-administered, ultimately, whether it’s once a month or once every 3 months, it doesn’t probably make much difference. I think different choices are needed for different patients.
Perhaps that’s a perfect segue to talk about the oral Lp(a) inhibitor that is also being developed. You presented these results for muvalaplin.
Muvalaplin, an Oral Small Molecule
Nicholls: In terms of frequency of administration, we’re talking about a daily oral therapeutic. For patients who don’t want an injectable and are happy to take a tablet every day, muvalaplin has the potential to be a really good option for them.
Muvalaplin is an oral small-molecule inhibitor. It essentially prevents apolipoprotein(a) [apo(a)] from binding to apolipoprotein B (apo B). We presented phase 1 data at the European Society of Cardiology meeting last year, showing probably Lp(a) lowering on the order of about 65%. Here, we’re going to show that that’s a little bit more. It looks like it’s probably at least 70% lowering using a standard Lp(a) assay. Using an assay that looks specifically at intact Lp(a) particles, it’s probably well in excess of 80%.
Those are really good results. The safety and tolerability with muvalaplin look really good. Again, we’ll need to see that agent move forward into a large outcome trial and we’ve yet to hear about that, at least for now.
O’Donoghue: It’s an interesting challenge that you faced in terms of the assay because, as you say, it really disrupts the apo(a) from binding to the apo B particle, and hence, a traditional assay that just measures apo(a), regardless of whether or not it’s bound to an apo B particle, may be a conservative estimate.
Nicholls: It may, in particular, because we know that apo(a) ultimately then binds to the drug. That assay is measuring what we think is nonfunctional apo(a) in addition to functional apo(a). It’s measuring functional apo(a) that’s still on an actual Lp(a) particle, but if it’s bound to muvalaplin, we think to some degree that’s probably unfair to count that. That’s why trying to develop other assays to try and understand the full effect of the drug is really important in terms of trying to understand how we develop that and move that forward.
O’Donoghue: Is there any evidence yet that the apo(a) particle that is not bound to apo B is in fact nonfunctional as you described it?
Nicholls: We think that’s likely to be the case, but I think there continues to be research in that space to try and settle that question once and for all.
O’Donoghue: Again, I think it’s a really exciting time in this field. Right now, we have three ongoing phase 3 trials. We have the pelacarsen trial that is still in follow-up, and fingers crossed, maybe will report out next year. Olpasiran is also in phase 3 testing, completed enrollment, and also is in the follow-up period. We also have lepodisiran, the ACCLAIM trial, as you mentioned. For people who are perhaps watching and looking to enroll their patients, this trial is still ongoing right now in terms of enrollment.
Nicholls: It is, and what’s nice about the ACCLAIM study is that it includes both primary and secondary prevention patients. For the first time in a big outcome trial, patients with high Lp(a) levels but who have yet to have a clinical event can actually get into a clinical trial.
I’m sure, like you, my clinic is full of patients with high Lp(a) who are really desperate to get into these trials. Many of those primary prevention patients just simply haven’t qualified, so that’s really good news.
The step beyond that, if we’re talking about even less frequent administration, is gene editing. We’re seeing those studies with CRISPR move forward to try to evaluate whether a single gene-editing approach at Lp(a) will be all that you need, which is even a more amazing concept, but that’s a study that needs more work.
O’Donoghue: An exciting space though, for sure. As a final thought, you mentioned the patients in your clinic who you have identified as having high Lp(a). What are you doing right now in your practice for managing those patients? I think there are many practitioners out there who struggle with whether they should really measure their patients’ Lp(a), and whether they want to know that information.
Nicholls: Yeah, it’s really hard. The answer is yes, we do want to know it. We know it’s a great risk enhancer. We know that a patient with a high Lp(a) is somebody whom I want to more intensively treat their other risk factors. I’m aiming for a lower LDL. I’m being much tighter with blood pressure control.
I think there’s some argument from observational data at least that aspirin remains a consideration, particularly in patients where you think there’s a particularly high risk associated with that high Lp(a). I think there are things we absolutely can do today, but we can’t do anything if you don’t know the numbers.
It starts with testing, and then we can move on to what we can do today, and then hopefully in the not-too-distant future, we’ll have specific therapies that really enable for us to address Lp(a) quite definitively.
O’Donoghue: Thanks again for taking the time. This was a very helpful discussion.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Michelle loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. Dr O’Donoghue, Senior Investigator, TIMI Study Group; Associate Professor of Medicine, Harvard Medical School; Associate Physician, Brigham and Women’s Hospital, Boston, Massachusetts, disclosed ties to Janssen; Novartis; CVS Minute Clinic; Merck & Co.; GlaxoSmithKline; Eisai Inc.; AstraZeneca Pharmaceuticals LP; Janssen Pharmaceuticals; Medicines Company; and Amgen. The opinions expressed in this article do not necessarily reflect the views and opinions of Brigham and Women’s Hospital. Stephen J. Nicholls, MBBS, PhD, Director, Victorian Heart Institute, Monash University; Director, Victorian Heart Hospital, Monash Health, Melbourne, Australia, has disclosed ties with Akcea Therapeutics; Amgen; AstraZeneca; Boehringer Ingelheim; CSL Behring; Eli Lilly and Company; Esperion Therapeutics; Kowa Pharmaceuticals; Merck; Novo Nordisk; Pfizer; Sanofi Regeneron; Daichii Sankyo; Vaxxinity; Cyclarity; CSL Sequirus; Takeda; Anthera Pharmaceuticals; Cerenis Therapeutics; Infraredx; New Amsterdam Pharma; Novartis; and Resverlogix.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here at the American Heart Association Scientific Sessions. It’s a very exciting meeting, but one of the interesting topics that we’re going to be talking about is lipoprotein(a) [Lp(a)] . It’s definitely one of the hottest sessions of the meeting.
Joining me to discuss this topic is Dr Steve Nicholls, who is arguably one of the leading experts in the world on lipids. He’s a professor of medicine at Monash University in Australia. Welcome. Thanks, Steve.
Stephen J. Nicholls, MBBS, PhD: Thanks for having me.
O’Donoghue: There are two phase 2 studies that we’ll circle back to that are being presented here at the American Heart Association meeting. These are for novel therapeutics that lower Lp(a). Perhaps taking a step back, we know that there’s a large body of evidence to support the concept that Lp(a) plays a causal role in heart disease and atherogenesis, but to date we haven’t had any effective therapies to really lower it.
Thinking about the therapeutics specifically that are on the horizon, perhaps we could start there. Which one is furthest along in development, and how does that look in terms of its ability to lower Lp(a)?
Pelacarsen, an ASO
Nicholls: Most of the therapies are injectable. Most of them are nucleic acid–based therapies, and the one that’s most advanced is an agent called pelacarsen. Pelacarsen is an antisense oligonucleotide (ASO), and it has gone all the way through its early phase 2 studies. It has a fully enrolled cardiovascular outcome trial.
We’re all eagerly awaiting the results of that study sometime in the next year or so. That will be the first large-scale clinical trial that will give us some clinical validation to ask the question of whether substantive lowering of Lp(a) will lower cardiovascular risk, with an agent that in early studies looks like it lowers Lp(a) about 80%.
O’Donoghue: Which is tremendous, because again, we really don’t have any effective therapies right now. I guess one of the big questions is, how much do we need to lower Lp(a) for that to translate into meaningful clinical benefit? What’s your sense there?
Nicholls: Well, we simply don’t know. We’ve tried to look to genetics to try and give us some sort of sense in terms of what that looks like. Lp(a) is a little tricky because the assays and the numbers that get spit out can be tricky in terms of trying to compare apples and apples in different studies.
We think that it’s probably at least a 50- to 75-mg/dL lowering of Lp(a) using the old units. We think that pelacarsen would hit that, and so our hope is that that would translate to a 15%-20% reduction in major cardiovascular events, but again, we’ve never asked this question before.
We have data from PCSK9 inhibitor trials showing that lesser reductions in Lp(a) of 25%-30% with both evolocumab and alirocumab contributed to the clinical benefit that we saw in those studies. Those agents were really good at lowering low-density lipoprotein (LDL) cholesterol, but Lp(a) lowering seemed to matter. One would be very hopeful that if a 25%-30% lowering of Lp(a) is useful, then an 80% or greater lowering of Lp(a) should be really useful.
The siRNAs
O’Donoghue: In addition to the ASO pelacarsen that you mentioned, there are several therapeutics in the pipeline, including three small interfering (si) RNAs that are at least in phase 2 and phase 3 testing at this point in time. There’s olpasiran, which in phase 2 testing led to more than a 95% reduction in Lp(a), and then lepodisiran , which has now moved into phase 3 testing, albeit we haven’t seen yet the phase 2 results.
What is your sense of lepodisiran and its efficacy?
Nicholls: What’s been really quite striking about the siRNAs is the even more profound degree of lowering of Lp(a) that we’re seeing. We’re seeing 90% and greater lowering of Lp(a) in all of those programs. We’re seeing some differences between the programs in terms of the durability of that effect.
I think it would be fair to say that with zerlasiran we’re starting to see perhaps that lowering effect starts to taper off a little bit more quickly than the other two. I think that may have some implications in terms of what dosing regimens may look like in the future.
Even so, we’re talking about therapies that may be dosed 3- to 6-monthly, or even with the potential for being less frequent than that with lepodisiran. Again, I think the phase 2 data will be really important in terms of giving us more information.
O’Donoghue: For the lepodisiran results, I was really quite struck that even though it was small numbers, single dose administered, it really looked like the duration of effect persisted at the higher doses up to about a year.
Nicholls: It looks pretty promising. We’ve launched the ACCLAIM study, the large cardiovascular outcome trial of lepodisiran, with a 6-monthly regimen. We are hopeful that more information may be able to give us the opportunity for even less frequent administration.
That has really important implications for patients where adherence is a particular issue. They may just simply want to come into the clinic. You know, once or twice a year, very much like we’re seeing with inclisiran, and that may be a really effective approach for many patients.
O’Donoghue: You alluded to the zerlasiran results, which were presented here at the American Heart Association meeting, and that even though it led to a robust reduction in Lp(a), it looked like the durability component was maybe a little bit shorter than for some of the other siRNAs that are currently being evaluated.
What’s your sense of that?
Nicholls: It probably is. The implications clinically, at least in an outcome trial when they ultimately get to that point, probably aren’t that important. They’ll probably just have slightly more frequent administration. That may become a bigger issue when it gets out into the clinic.
The nice thing is that if all of these agents appear to be effective, are well tolerated, and get out to the clinic, then clinicians and patients are going to have a lot of choice.
O’Donoghue: I think more competition is always good news for the field, ultimately. I think to your point, especially for a drug that might be self-administered, ultimately, whether it’s once a month or once every 3 months, it doesn’t probably make much difference. I think different choices are needed for different patients.
Perhaps that’s a perfect segue to talk about the oral Lp(a) inhibitor that is also being developed. You presented these results for muvalaplin.
Muvalaplin, an Oral Small Molecule
Nicholls: In terms of frequency of administration, we’re talking about a daily oral therapeutic. For patients who don’t want an injectable and are happy to take a tablet every day, muvalaplin has the potential to be a really good option for them.
Muvalaplin is an oral small-molecule inhibitor. It essentially prevents apolipoprotein(a) [apo(a)] from binding to apolipoprotein B (apo B). We presented phase 1 data at the European Society of Cardiology meeting last year, showing probably Lp(a) lowering on the order of about 65%. Here, we’re going to show that that’s a little bit more. It looks like it’s probably at least 70% lowering using a standard Lp(a) assay. Using an assay that looks specifically at intact Lp(a) particles, it’s probably well in excess of 80%.
Those are really good results. The safety and tolerability with muvalaplin look really good. Again, we’ll need to see that agent move forward into a large outcome trial and we’ve yet to hear about that, at least for now.
O’Donoghue: It’s an interesting challenge that you faced in terms of the assay because, as you say, it really disrupts the apo(a) from binding to the apo B particle, and hence, a traditional assay that just measures apo(a), regardless of whether or not it’s bound to an apo B particle, may be a conservative estimate.
Nicholls: It may, in particular, because we know that apo(a) ultimately then binds to the drug. That assay is measuring what we think is nonfunctional apo(a) in addition to functional apo(a). It’s measuring functional apo(a) that’s still on an actual Lp(a) particle, but if it’s bound to muvalaplin, we think to some degree that’s probably unfair to count that. That’s why trying to develop other assays to try and understand the full effect of the drug is really important in terms of trying to understand how we develop that and move that forward.
O’Donoghue: Is there any evidence yet that the apo(a) particle that is not bound to apo B is in fact nonfunctional as you described it?
Nicholls: We think that’s likely to be the case, but I think there continues to be research in that space to try and settle that question once and for all.
O’Donoghue: Again, I think it’s a really exciting time in this field. Right now, we have three ongoing phase 3 trials. We have the pelacarsen trial that is still in follow-up, and fingers crossed, maybe will report out next year. Olpasiran is also in phase 3 testing, completed enrollment, and also is in the follow-up period. We also have lepodisiran, the ACCLAIM trial, as you mentioned. For people who are perhaps watching and looking to enroll their patients, this trial is still ongoing right now in terms of enrollment.
Nicholls: It is, and what’s nice about the ACCLAIM study is that it includes both primary and secondary prevention patients. For the first time in a big outcome trial, patients with high Lp(a) levels but who have yet to have a clinical event can actually get into a clinical trial.
I’m sure, like you, my clinic is full of patients with high Lp(a) who are really desperate to get into these trials. Many of those primary prevention patients just simply haven’t qualified, so that’s really good news.
The step beyond that, if we’re talking about even less frequent administration, is gene editing. We’re seeing those studies with CRISPR move forward to try to evaluate whether a single gene-editing approach at Lp(a) will be all that you need, which is even a more amazing concept, but that’s a study that needs more work.
O’Donoghue: An exciting space though, for sure. As a final thought, you mentioned the patients in your clinic who you have identified as having high Lp(a). What are you doing right now in your practice for managing those patients? I think there are many practitioners out there who struggle with whether they should really measure their patients’ Lp(a), and whether they want to know that information.
Nicholls: Yeah, it’s really hard. The answer is yes, we do want to know it. We know it’s a great risk enhancer. We know that a patient with a high Lp(a) is somebody whom I want to more intensively treat their other risk factors. I’m aiming for a lower LDL. I’m being much tighter with blood pressure control.
I think there’s some argument from observational data at least that aspirin remains a consideration, particularly in patients where you think there’s a particularly high risk associated with that high Lp(a). I think there are things we absolutely can do today, but we can’t do anything if you don’t know the numbers.
It starts with testing, and then we can move on to what we can do today, and then hopefully in the not-too-distant future, we’ll have specific therapies that really enable for us to address Lp(a) quite definitively.
O’Donoghue: Thanks again for taking the time. This was a very helpful discussion.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Michelle loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. Dr O’Donoghue, Senior Investigator, TIMI Study Group; Associate Professor of Medicine, Harvard Medical School; Associate Physician, Brigham and Women’s Hospital, Boston, Massachusetts, disclosed ties to Janssen; Novartis; CVS Minute Clinic; Merck & Co.; GlaxoSmithKline; Eisai Inc.; AstraZeneca Pharmaceuticals LP; Janssen Pharmaceuticals; Medicines Company; and Amgen. The opinions expressed in this article do not necessarily reflect the views and opinions of Brigham and Women’s Hospital. Stephen J. Nicholls, MBBS, PhD, Director, Victorian Heart Institute, Monash University; Director, Victorian Heart Hospital, Monash Health, Melbourne, Australia, has disclosed ties with Akcea Therapeutics; Amgen; AstraZeneca; Boehringer Ingelheim; CSL Behring; Eli Lilly and Company; Esperion Therapeutics; Kowa Pharmaceuticals; Merck; Novo Nordisk; Pfizer; Sanofi Regeneron; Daichii Sankyo; Vaxxinity; Cyclarity; CSL Sequirus; Takeda; Anthera Pharmaceuticals; Cerenis Therapeutics; Infraredx; New Amsterdam Pharma; Novartis; and Resverlogix.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here at the American Heart Association Scientific Sessions. It’s a very exciting meeting, but one of the interesting topics that we’re going to be talking about is lipoprotein(a) [Lp(a)] . It’s definitely one of the hottest sessions of the meeting.
Joining me to discuss this topic is Dr Steve Nicholls, who is arguably one of the leading experts in the world on lipids. He’s a professor of medicine at Monash University in Australia. Welcome. Thanks, Steve.
Stephen J. Nicholls, MBBS, PhD: Thanks for having me.
O’Donoghue: There are two phase 2 studies that we’ll circle back to that are being presented here at the American Heart Association meeting. These are for novel therapeutics that lower Lp(a). Perhaps taking a step back, we know that there’s a large body of evidence to support the concept that Lp(a) plays a causal role in heart disease and atherogenesis, but to date we haven’t had any effective therapies to really lower it.
Thinking about the therapeutics specifically that are on the horizon, perhaps we could start there. Which one is furthest along in development, and how does that look in terms of its ability to lower Lp(a)?
Pelacarsen, an ASO
Nicholls: Most of the therapies are injectable. Most of them are nucleic acid–based therapies, and the one that’s most advanced is an agent called pelacarsen. Pelacarsen is an antisense oligonucleotide (ASO), and it has gone all the way through its early phase 2 studies. It has a fully enrolled cardiovascular outcome trial.
We’re all eagerly awaiting the results of that study sometime in the next year or so. That will be the first large-scale clinical trial that will give us some clinical validation to ask the question of whether substantive lowering of Lp(a) will lower cardiovascular risk, with an agent that in early studies looks like it lowers Lp(a) about 80%.
O’Donoghue: Which is tremendous, because again, we really don’t have any effective therapies right now. I guess one of the big questions is, how much do we need to lower Lp(a) for that to translate into meaningful clinical benefit? What’s your sense there?
Nicholls: Well, we simply don’t know. We’ve tried to look to genetics to try and give us some sort of sense in terms of what that looks like. Lp(a) is a little tricky because the assays and the numbers that get spit out can be tricky in terms of trying to compare apples and apples in different studies.
We think that it’s probably at least a 50- to 75-mg/dL lowering of Lp(a) using the old units. We think that pelacarsen would hit that, and so our hope is that that would translate to a 15%-20% reduction in major cardiovascular events, but again, we’ve never asked this question before.
We have data from PCSK9 inhibitor trials showing that lesser reductions in Lp(a) of 25%-30% with both evolocumab and alirocumab contributed to the clinical benefit that we saw in those studies. Those agents were really good at lowering low-density lipoprotein (LDL) cholesterol, but Lp(a) lowering seemed to matter. One would be very hopeful that if a 25%-30% lowering of Lp(a) is useful, then an 80% or greater lowering of Lp(a) should be really useful.
The siRNAs
O’Donoghue: In addition to the ASO pelacarsen that you mentioned, there are several therapeutics in the pipeline, including three small interfering (si) RNAs that are at least in phase 2 and phase 3 testing at this point in time. There’s olpasiran, which in phase 2 testing led to more than a 95% reduction in Lp(a), and then lepodisiran , which has now moved into phase 3 testing, albeit we haven’t seen yet the phase 2 results.
What is your sense of lepodisiran and its efficacy?
Nicholls: What’s been really quite striking about the siRNAs is the even more profound degree of lowering of Lp(a) that we’re seeing. We’re seeing 90% and greater lowering of Lp(a) in all of those programs. We’re seeing some differences between the programs in terms of the durability of that effect.
I think it would be fair to say that with zerlasiran we’re starting to see perhaps that lowering effect starts to taper off a little bit more quickly than the other two. I think that may have some implications in terms of what dosing regimens may look like in the future.
Even so, we’re talking about therapies that may be dosed 3- to 6-monthly, or even with the potential for being less frequent than that with lepodisiran. Again, I think the phase 2 data will be really important in terms of giving us more information.
O’Donoghue: For the lepodisiran results, I was really quite struck that even though it was small numbers, single dose administered, it really looked like the duration of effect persisted at the higher doses up to about a year.
Nicholls: It looks pretty promising. We’ve launched the ACCLAIM study, the large cardiovascular outcome trial of lepodisiran, with a 6-monthly regimen. We are hopeful that more information may be able to give us the opportunity for even less frequent administration.
That has really important implications for patients where adherence is a particular issue. They may just simply want to come into the clinic. You know, once or twice a year, very much like we’re seeing with inclisiran, and that may be a really effective approach for many patients.
O’Donoghue: You alluded to the zerlasiran results, which were presented here at the American Heart Association meeting, and that even though it led to a robust reduction in Lp(a), it looked like the durability component was maybe a little bit shorter than for some of the other siRNAs that are currently being evaluated.
What’s your sense of that?
Nicholls: It probably is. The implications clinically, at least in an outcome trial when they ultimately get to that point, probably aren’t that important. They’ll probably just have slightly more frequent administration. That may become a bigger issue when it gets out into the clinic.
The nice thing is that if all of these agents appear to be effective, are well tolerated, and get out to the clinic, then clinicians and patients are going to have a lot of choice.
O’Donoghue: I think more competition is always good news for the field, ultimately. I think to your point, especially for a drug that might be self-administered, ultimately, whether it’s once a month or once every 3 months, it doesn’t probably make much difference. I think different choices are needed for different patients.
Perhaps that’s a perfect segue to talk about the oral Lp(a) inhibitor that is also being developed. You presented these results for muvalaplin.
Muvalaplin, an Oral Small Molecule
Nicholls: In terms of frequency of administration, we’re talking about a daily oral therapeutic. For patients who don’t want an injectable and are happy to take a tablet every day, muvalaplin has the potential to be a really good option for them.
Muvalaplin is an oral small-molecule inhibitor. It essentially prevents apolipoprotein(a) [apo(a)] from binding to apolipoprotein B (apo B). We presented phase 1 data at the European Society of Cardiology meeting last year, showing probably Lp(a) lowering on the order of about 65%. Here, we’re going to show that that’s a little bit more. It looks like it’s probably at least 70% lowering using a standard Lp(a) assay. Using an assay that looks specifically at intact Lp(a) particles, it’s probably well in excess of 80%.
Those are really good results. The safety and tolerability with muvalaplin look really good. Again, we’ll need to see that agent move forward into a large outcome trial and we’ve yet to hear about that, at least for now.
O’Donoghue: It’s an interesting challenge that you faced in terms of the assay because, as you say, it really disrupts the apo(a) from binding to the apo B particle, and hence, a traditional assay that just measures apo(a), regardless of whether or not it’s bound to an apo B particle, may be a conservative estimate.
Nicholls: It may, in particular, because we know that apo(a) ultimately then binds to the drug. That assay is measuring what we think is nonfunctional apo(a) in addition to functional apo(a). It’s measuring functional apo(a) that’s still on an actual Lp(a) particle, but if it’s bound to muvalaplin, we think to some degree that’s probably unfair to count that. That’s why trying to develop other assays to try and understand the full effect of the drug is really important in terms of trying to understand how we develop that and move that forward.
O’Donoghue: Is there any evidence yet that the apo(a) particle that is not bound to apo B is in fact nonfunctional as you described it?
Nicholls: We think that’s likely to be the case, but I think there continues to be research in that space to try and settle that question once and for all.
O’Donoghue: Again, I think it’s a really exciting time in this field. Right now, we have three ongoing phase 3 trials. We have the pelacarsen trial that is still in follow-up, and fingers crossed, maybe will report out next year. Olpasiran is also in phase 3 testing, completed enrollment, and also is in the follow-up period. We also have lepodisiran, the ACCLAIM trial, as you mentioned. For people who are perhaps watching and looking to enroll their patients, this trial is still ongoing right now in terms of enrollment.
Nicholls: It is, and what’s nice about the ACCLAIM study is that it includes both primary and secondary prevention patients. For the first time in a big outcome trial, patients with high Lp(a) levels but who have yet to have a clinical event can actually get into a clinical trial.
I’m sure, like you, my clinic is full of patients with high Lp(a) who are really desperate to get into these trials. Many of those primary prevention patients just simply haven’t qualified, so that’s really good news.
The step beyond that, if we’re talking about even less frequent administration, is gene editing. We’re seeing those studies with CRISPR move forward to try to evaluate whether a single gene-editing approach at Lp(a) will be all that you need, which is even a more amazing concept, but that’s a study that needs more work.
O’Donoghue: An exciting space though, for sure. As a final thought, you mentioned the patients in your clinic who you have identified as having high Lp(a). What are you doing right now in your practice for managing those patients? I think there are many practitioners out there who struggle with whether they should really measure their patients’ Lp(a), and whether they want to know that information.
Nicholls: Yeah, it’s really hard. The answer is yes, we do want to know it. We know it’s a great risk enhancer. We know that a patient with a high Lp(a) is somebody whom I want to more intensively treat their other risk factors. I’m aiming for a lower LDL. I’m being much tighter with blood pressure control.
I think there’s some argument from observational data at least that aspirin remains a consideration, particularly in patients where you think there’s a particularly high risk associated with that high Lp(a). I think there are things we absolutely can do today, but we can’t do anything if you don’t know the numbers.
It starts with testing, and then we can move on to what we can do today, and then hopefully in the not-too-distant future, we’ll have specific therapies that really enable for us to address Lp(a) quite definitively.
O’Donoghue: Thanks again for taking the time. This was a very helpful discussion.
Michelle O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Michelle loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. Dr O’Donoghue, Senior Investigator, TIMI Study Group; Associate Professor of Medicine, Harvard Medical School; Associate Physician, Brigham and Women’s Hospital, Boston, Massachusetts, disclosed ties to Janssen; Novartis; CVS Minute Clinic; Merck & Co.; GlaxoSmithKline; Eisai Inc.; AstraZeneca Pharmaceuticals LP; Janssen Pharmaceuticals; Medicines Company; and Amgen. The opinions expressed in this article do not necessarily reflect the views and opinions of Brigham and Women’s Hospital. Stephen J. Nicholls, MBBS, PhD, Director, Victorian Heart Institute, Monash University; Director, Victorian Heart Hospital, Monash Health, Melbourne, Australia, has disclosed ties with Akcea Therapeutics; Amgen; AstraZeneca; Boehringer Ingelheim; CSL Behring; Eli Lilly and Company; Esperion Therapeutics; Kowa Pharmaceuticals; Merck; Novo Nordisk; Pfizer; Sanofi Regeneron; Daichii Sankyo; Vaxxinity; Cyclarity; CSL Sequirus; Takeda; Anthera Pharmaceuticals; Cerenis Therapeutics; Infraredx; New Amsterdam Pharma; Novartis; and Resverlogix.
A version of this article appeared on Medscape.com.
Flu Shot Reminders Improve Use in Heart Attack Survivors
, showed the NUDGE FLU series of clinical trials.
Influenza has the potential to be a dangerous infection on its own, but it increases the risk for cardiovascular events among people with a history of heart attack, said the study’s lead author, Ankeet Bhatt, MD, a cardiologist at Kaiser Permanente San Francisco Medical Center, San Francisco.
“Yearly influenza vaccines help prevent influenza infection and, in patients with a heart attack, are potentially cardioprotective,” he said during his presentation at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago. The NUDGE FLU results were simultaneously published online in JAMA Cardiology.
In Denmark, where the trials were conducted, about 80% of older adults get flu shots, but only about 40% of younger adults with chronic diseases do, Bhatt reported. In the United States, about 45% of adults and 55% of children received at least one dose of the flu vaccine in the 2023/24 flu season, according to the US Centers for Disease Control and Prevention (CDC).
The NUDGE FLU Trials
Bhatt and his colleagues conducted three related clinical trials during the 2022/23 and 2023/24 flu seasons: NUDGE-FLU and NUDGE-FLU-2 targeted older adults, whereas NUDGE-FLU-CHRONIC targeted younger adults with chronic diseases. Nearly 2 million people were involved in the three trials.
Participants were randomized to receive one of a series of different behavioral-science-informed letters, delivered through a government-run electronic communication system, or no reminder.
People who received any of the nudges had higher rates of vaccination; among heart attack survivors, there was a 1.8% improvement and among adults without a history of heart attack, there was a 1.3% improvement. But a nudge that explained the potential cardiovascular benefits of flu shots was even more effective, leading to a 3.9% increase among people with a history of heart attack and a 2% increase among those with no heart attack history.
“A simple sentence resulted in a durable improvement in the vaccination rate,” said Bhatt.
The effect was even greater among those who had not been vaccinated in the previous flu season. Among heart attack survivors, nearly 14% more people got the vaccine compared with just 1.5% more survivors who were previously vaccinated. And it was most effective among younger adults who had experienced a recent heart attack, resulting in a 26% increase.
“The impact was larger in patients with a history of acute myocardial infarction, in those who were vaccine-hesitant, and in younger people” — all groups with the most to gain from vaccination in terms of cardiovascular protection — Bhatt reported.
About 25% of people in the United States are unsure about whether to get a flu shot, said Orly Vardeny, PharmD, professor of medicine at the University of Minnesota Medical School in Minneapolis, who was not involved in the study. The fact that previously unvaccinated people were convinced by the nudges is reassuring. “That’s the group where this intervention is most likely to move the needle,” she said.
Around half of all people hospitalized for flu in the United States have cardiovascular disease, CDC data showed, so “even a small increase in the number of patients who get vaccinated has substantial public health benefits,” Vardeny said.
The NUDGE FLU series showed that nudges like this should be employed as a simple tool to improve vaccination rates, but the system would be much more difficult to implement in the United States, Bhatt said.
Denmark has a national health service and a preexisting government electronic communication system, whereas the US system is privately run and more fractured. It would be possible to make it work, he pointed out, but would take some effort.
A version of this article first appeared on Medscape.com.
, showed the NUDGE FLU series of clinical trials.
Influenza has the potential to be a dangerous infection on its own, but it increases the risk for cardiovascular events among people with a history of heart attack, said the study’s lead author, Ankeet Bhatt, MD, a cardiologist at Kaiser Permanente San Francisco Medical Center, San Francisco.
“Yearly influenza vaccines help prevent influenza infection and, in patients with a heart attack, are potentially cardioprotective,” he said during his presentation at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago. The NUDGE FLU results were simultaneously published online in JAMA Cardiology.
In Denmark, where the trials were conducted, about 80% of older adults get flu shots, but only about 40% of younger adults with chronic diseases do, Bhatt reported. In the United States, about 45% of adults and 55% of children received at least one dose of the flu vaccine in the 2023/24 flu season, according to the US Centers for Disease Control and Prevention (CDC).
The NUDGE FLU Trials
Bhatt and his colleagues conducted three related clinical trials during the 2022/23 and 2023/24 flu seasons: NUDGE-FLU and NUDGE-FLU-2 targeted older adults, whereas NUDGE-FLU-CHRONIC targeted younger adults with chronic diseases. Nearly 2 million people were involved in the three trials.
Participants were randomized to receive one of a series of different behavioral-science-informed letters, delivered through a government-run electronic communication system, or no reminder.
People who received any of the nudges had higher rates of vaccination; among heart attack survivors, there was a 1.8% improvement and among adults without a history of heart attack, there was a 1.3% improvement. But a nudge that explained the potential cardiovascular benefits of flu shots was even more effective, leading to a 3.9% increase among people with a history of heart attack and a 2% increase among those with no heart attack history.
“A simple sentence resulted in a durable improvement in the vaccination rate,” said Bhatt.
The effect was even greater among those who had not been vaccinated in the previous flu season. Among heart attack survivors, nearly 14% more people got the vaccine compared with just 1.5% more survivors who were previously vaccinated. And it was most effective among younger adults who had experienced a recent heart attack, resulting in a 26% increase.
“The impact was larger in patients with a history of acute myocardial infarction, in those who were vaccine-hesitant, and in younger people” — all groups with the most to gain from vaccination in terms of cardiovascular protection — Bhatt reported.
About 25% of people in the United States are unsure about whether to get a flu shot, said Orly Vardeny, PharmD, professor of medicine at the University of Minnesota Medical School in Minneapolis, who was not involved in the study. The fact that previously unvaccinated people were convinced by the nudges is reassuring. “That’s the group where this intervention is most likely to move the needle,” she said.
Around half of all people hospitalized for flu in the United States have cardiovascular disease, CDC data showed, so “even a small increase in the number of patients who get vaccinated has substantial public health benefits,” Vardeny said.
The NUDGE FLU series showed that nudges like this should be employed as a simple tool to improve vaccination rates, but the system would be much more difficult to implement in the United States, Bhatt said.
Denmark has a national health service and a preexisting government electronic communication system, whereas the US system is privately run and more fractured. It would be possible to make it work, he pointed out, but would take some effort.
A version of this article first appeared on Medscape.com.
, showed the NUDGE FLU series of clinical trials.
Influenza has the potential to be a dangerous infection on its own, but it increases the risk for cardiovascular events among people with a history of heart attack, said the study’s lead author, Ankeet Bhatt, MD, a cardiologist at Kaiser Permanente San Francisco Medical Center, San Francisco.
“Yearly influenza vaccines help prevent influenza infection and, in patients with a heart attack, are potentially cardioprotective,” he said during his presentation at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago. The NUDGE FLU results were simultaneously published online in JAMA Cardiology.
In Denmark, where the trials were conducted, about 80% of older adults get flu shots, but only about 40% of younger adults with chronic diseases do, Bhatt reported. In the United States, about 45% of adults and 55% of children received at least one dose of the flu vaccine in the 2023/24 flu season, according to the US Centers for Disease Control and Prevention (CDC).
The NUDGE FLU Trials
Bhatt and his colleagues conducted three related clinical trials during the 2022/23 and 2023/24 flu seasons: NUDGE-FLU and NUDGE-FLU-2 targeted older adults, whereas NUDGE-FLU-CHRONIC targeted younger adults with chronic diseases. Nearly 2 million people were involved in the three trials.
Participants were randomized to receive one of a series of different behavioral-science-informed letters, delivered through a government-run electronic communication system, or no reminder.
People who received any of the nudges had higher rates of vaccination; among heart attack survivors, there was a 1.8% improvement and among adults without a history of heart attack, there was a 1.3% improvement. But a nudge that explained the potential cardiovascular benefits of flu shots was even more effective, leading to a 3.9% increase among people with a history of heart attack and a 2% increase among those with no heart attack history.
“A simple sentence resulted in a durable improvement in the vaccination rate,” said Bhatt.
The effect was even greater among those who had not been vaccinated in the previous flu season. Among heart attack survivors, nearly 14% more people got the vaccine compared with just 1.5% more survivors who were previously vaccinated. And it was most effective among younger adults who had experienced a recent heart attack, resulting in a 26% increase.
“The impact was larger in patients with a history of acute myocardial infarction, in those who were vaccine-hesitant, and in younger people” — all groups with the most to gain from vaccination in terms of cardiovascular protection — Bhatt reported.
About 25% of people in the United States are unsure about whether to get a flu shot, said Orly Vardeny, PharmD, professor of medicine at the University of Minnesota Medical School in Minneapolis, who was not involved in the study. The fact that previously unvaccinated people were convinced by the nudges is reassuring. “That’s the group where this intervention is most likely to move the needle,” she said.
Around half of all people hospitalized for flu in the United States have cardiovascular disease, CDC data showed, so “even a small increase in the number of patients who get vaccinated has substantial public health benefits,” Vardeny said.
The NUDGE FLU series showed that nudges like this should be employed as a simple tool to improve vaccination rates, but the system would be much more difficult to implement in the United States, Bhatt said.
Denmark has a national health service and a preexisting government electronic communication system, whereas the US system is privately run and more fractured. It would be possible to make it work, he pointed out, but would take some effort.
A version of this article first appeared on Medscape.com.
FROM AHA 2024
New Investigation Casts Doubt on Landmark Ticagrelor Trial
New questions about the landmark trial that launched the antiplatelet drug ticagrelor worldwide are being raised after an investigation uncovered more information about how the PLATO study was conducted.
Peter Doshi, PhD, senior editor at The BMJ, obtained primary records for the trial and unpublished data through a Freedom of Information Act request, and has detailed inconsistencies and omissions in data reporting from the 2009 trial originally published in The New England Journal of Medicine (NEJM). The new investigation into the Platelet Inhibition and Patient Outcomes (PLATO) trial is published in The BMJ.
The findings come as generic versions of ticagrelor (Brilinta) are expected to become available soon in the United States. Ticagrelor is the only P2Y12 inhibitor still under patent, and in 2022, the United States spent more than $750 million on it, according to the report.
PLATO, sponsored by ticagrelor manufacturer AstraZeneca, included more than 18,000 patients in 43 countries. Investigators reported that ticagrelor reduced deaths from vascular causes, heart attack, or stroke compared with clopidogrel (Plavix). However, in a subgroup analysis, among US patients, there were more deaths in the ticagrelor group, and AstraZeneca failed its first bid for approval from the US Food and Drug Administration (FDA).
Failed First Bid for FDA Approval
AstraZeneca resubmitted its application, which was met with objections by some FDA staff members, including medical officer Thomas Marciniak, who called the resubmission “the worst in my experience regarding completeness of the submissions and the sponsor responding completely and accurately to requests,” Doshi reports.
Despite the objections, the FDA in 2011 approved ticagrelor for acute coronary syndrome, kicking off intense controversy over the trial, as several other studies have failed to replicate PLATO’s positive results.
Doubts have grown about its apparent advantage over cheaper, off-patent P2Y12 inhibitors such as clopidogrel and prasugrel.
“Critics said it was noteworthy that ticagrelor failed in the US,” Doshi writes, “the only high enrolling country where sites were not monitored by the sponsor itself.” Doshi’s report points out that critics of the trial “highlight that AstraZeneca itself carried out the data monitoring for PLATO except for sites that were monitored by third party contract research organizations. In the four countries exclusively monitored by non-sponsor personnel—Georgia, Israel, Russia, and the US—ticagrelor fared worse.”
Victor Serebruany, MD, from Johns Hopkins University, said he was initially impressed by the trial results but became skeptical after noticing inconsistencies and anomalies in the data. He filed a complaint with the US District Court in the District of Columbia, suggesting that the cardiovascular events in the study “may have been manipulated.”
US Department of Justice Investigation
The US Department of Justice (DOJ) opened an investigation in 2013 and closed it in 2014 with no further action. Serebruany continues to publish critiques of the trial 15 years later but told The BMJ he has little hope that the questions will be resolved unless the DOJ re-engages with an investigation.
Doshi also points out discrepancies in the data reported. In the 2009 paper, published as an intent-to-treat analysis, investigators said there were 905 total deaths from any cause among all randomized patients. “An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths ‘discovered after withdrawal of consent,’” he reports.
The NEJM responded to Doshi that while it didn’t dispute the error in the number of deaths, it was uncertain about publishing a correction, citing new — not yet published — guidelines from the International Committee of Medical Journal Editors. NEJM Editor-in-Chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice.”
Doshi’s investigation includes an interview with Eric Bates, MD, professor of internal medicine at the University of Michigan in Ann Arbor, and a co-author of the US guidelines that recommend ticagrelor, who said he was “increasingly disturbed by how trial after trial came out as being not dramatically positive in any way.” Bates is now calling for a review of ticagrelor’s recommendation in guidelines, according to the report.
AstraZeneca declined to be interviewed for the BMJ investigation, according to Doshi, and a spokesperson from the company told the journal by email that they have “nothing to add,” directing editors to its 2014 public statement after the DOJ’s investigation into PLATO. The BMJ said PLATO trial co-chairs Robert A. Harrington, MD, and Lars Wallentin, MD, did not respond to The BMJ’s requests for comment.
Will the Guidelines Be Changed Now?
“I know and have worked with Drs Wallentin and Harrington,” Bates told Medscape Medical News, “and find them to be honest, intelligent clinical scientists with the highest ethical standards who manage conflicts of interest as well as can be done in the clinical research arena, where industry support is required to develop new knowledge,” he said.
“If there is a concern that AstraZeneca was manipulating the dataset and FDA submission, that is an important issue,” Bates said. “The US paradox and the failure of any other antiplatelet trial to find a comparative mortality advantage are two unexplained issues with PLATO that provide good fodder for conspiracy theories. I agree with the NEJM that this trial is 15 years old and may not be worth readjudicating in the current treatment era.”
Other calls for revisiting guidelines have come after disappointing postlicensure studies have repeatedly demonstrated that ticagrelor has “similar efficacy to clopidogrel but with increased bleeding and [dyspnea],” Doshi reports.
“My concern is the marketing spin by AstraZeneca and the promotion of ticagrelor by six to eight ‘thought leaders’ consistently funded by AstraZeneca over the past 10 years,” said Bates. “They have flooded the literature with supportive subset and post hoc analyses, review articles, and ‘meta-analyses’ flawed by selection and intellectual bias, and public interviews that consistently discount the findings of the many subsequent randomized controlled trials that have not supported the superiority of ticagrelor over clopidogrel or prasugrel.”
A version of this article first appeared on Medscape.com.
New questions about the landmark trial that launched the antiplatelet drug ticagrelor worldwide are being raised after an investigation uncovered more information about how the PLATO study was conducted.
Peter Doshi, PhD, senior editor at The BMJ, obtained primary records for the trial and unpublished data through a Freedom of Information Act request, and has detailed inconsistencies and omissions in data reporting from the 2009 trial originally published in The New England Journal of Medicine (NEJM). The new investigation into the Platelet Inhibition and Patient Outcomes (PLATO) trial is published in The BMJ.
The findings come as generic versions of ticagrelor (Brilinta) are expected to become available soon in the United States. Ticagrelor is the only P2Y12 inhibitor still under patent, and in 2022, the United States spent more than $750 million on it, according to the report.
PLATO, sponsored by ticagrelor manufacturer AstraZeneca, included more than 18,000 patients in 43 countries. Investigators reported that ticagrelor reduced deaths from vascular causes, heart attack, or stroke compared with clopidogrel (Plavix). However, in a subgroup analysis, among US patients, there were more deaths in the ticagrelor group, and AstraZeneca failed its first bid for approval from the US Food and Drug Administration (FDA).
Failed First Bid for FDA Approval
AstraZeneca resubmitted its application, which was met with objections by some FDA staff members, including medical officer Thomas Marciniak, who called the resubmission “the worst in my experience regarding completeness of the submissions and the sponsor responding completely and accurately to requests,” Doshi reports.
Despite the objections, the FDA in 2011 approved ticagrelor for acute coronary syndrome, kicking off intense controversy over the trial, as several other studies have failed to replicate PLATO’s positive results.
Doubts have grown about its apparent advantage over cheaper, off-patent P2Y12 inhibitors such as clopidogrel and prasugrel.
“Critics said it was noteworthy that ticagrelor failed in the US,” Doshi writes, “the only high enrolling country where sites were not monitored by the sponsor itself.” Doshi’s report points out that critics of the trial “highlight that AstraZeneca itself carried out the data monitoring for PLATO except for sites that were monitored by third party contract research organizations. In the four countries exclusively monitored by non-sponsor personnel—Georgia, Israel, Russia, and the US—ticagrelor fared worse.”
Victor Serebruany, MD, from Johns Hopkins University, said he was initially impressed by the trial results but became skeptical after noticing inconsistencies and anomalies in the data. He filed a complaint with the US District Court in the District of Columbia, suggesting that the cardiovascular events in the study “may have been manipulated.”
US Department of Justice Investigation
The US Department of Justice (DOJ) opened an investigation in 2013 and closed it in 2014 with no further action. Serebruany continues to publish critiques of the trial 15 years later but told The BMJ he has little hope that the questions will be resolved unless the DOJ re-engages with an investigation.
Doshi also points out discrepancies in the data reported. In the 2009 paper, published as an intent-to-treat analysis, investigators said there were 905 total deaths from any cause among all randomized patients. “An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths ‘discovered after withdrawal of consent,’” he reports.
The NEJM responded to Doshi that while it didn’t dispute the error in the number of deaths, it was uncertain about publishing a correction, citing new — not yet published — guidelines from the International Committee of Medical Journal Editors. NEJM Editor-in-Chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice.”
Doshi’s investigation includes an interview with Eric Bates, MD, professor of internal medicine at the University of Michigan in Ann Arbor, and a co-author of the US guidelines that recommend ticagrelor, who said he was “increasingly disturbed by how trial after trial came out as being not dramatically positive in any way.” Bates is now calling for a review of ticagrelor’s recommendation in guidelines, according to the report.
AstraZeneca declined to be interviewed for the BMJ investigation, according to Doshi, and a spokesperson from the company told the journal by email that they have “nothing to add,” directing editors to its 2014 public statement after the DOJ’s investigation into PLATO. The BMJ said PLATO trial co-chairs Robert A. Harrington, MD, and Lars Wallentin, MD, did not respond to The BMJ’s requests for comment.
Will the Guidelines Be Changed Now?
“I know and have worked with Drs Wallentin and Harrington,” Bates told Medscape Medical News, “and find them to be honest, intelligent clinical scientists with the highest ethical standards who manage conflicts of interest as well as can be done in the clinical research arena, where industry support is required to develop new knowledge,” he said.
“If there is a concern that AstraZeneca was manipulating the dataset and FDA submission, that is an important issue,” Bates said. “The US paradox and the failure of any other antiplatelet trial to find a comparative mortality advantage are two unexplained issues with PLATO that provide good fodder for conspiracy theories. I agree with the NEJM that this trial is 15 years old and may not be worth readjudicating in the current treatment era.”
Other calls for revisiting guidelines have come after disappointing postlicensure studies have repeatedly demonstrated that ticagrelor has “similar efficacy to clopidogrel but with increased bleeding and [dyspnea],” Doshi reports.
“My concern is the marketing spin by AstraZeneca and the promotion of ticagrelor by six to eight ‘thought leaders’ consistently funded by AstraZeneca over the past 10 years,” said Bates. “They have flooded the literature with supportive subset and post hoc analyses, review articles, and ‘meta-analyses’ flawed by selection and intellectual bias, and public interviews that consistently discount the findings of the many subsequent randomized controlled trials that have not supported the superiority of ticagrelor over clopidogrel or prasugrel.”
A version of this article first appeared on Medscape.com.
New questions about the landmark trial that launched the antiplatelet drug ticagrelor worldwide are being raised after an investigation uncovered more information about how the PLATO study was conducted.
Peter Doshi, PhD, senior editor at The BMJ, obtained primary records for the trial and unpublished data through a Freedom of Information Act request, and has detailed inconsistencies and omissions in data reporting from the 2009 trial originally published in The New England Journal of Medicine (NEJM). The new investigation into the Platelet Inhibition and Patient Outcomes (PLATO) trial is published in The BMJ.
The findings come as generic versions of ticagrelor (Brilinta) are expected to become available soon in the United States. Ticagrelor is the only P2Y12 inhibitor still under patent, and in 2022, the United States spent more than $750 million on it, according to the report.
PLATO, sponsored by ticagrelor manufacturer AstraZeneca, included more than 18,000 patients in 43 countries. Investigators reported that ticagrelor reduced deaths from vascular causes, heart attack, or stroke compared with clopidogrel (Plavix). However, in a subgroup analysis, among US patients, there were more deaths in the ticagrelor group, and AstraZeneca failed its first bid for approval from the US Food and Drug Administration (FDA).
Failed First Bid for FDA Approval
AstraZeneca resubmitted its application, which was met with objections by some FDA staff members, including medical officer Thomas Marciniak, who called the resubmission “the worst in my experience regarding completeness of the submissions and the sponsor responding completely and accurately to requests,” Doshi reports.
Despite the objections, the FDA in 2011 approved ticagrelor for acute coronary syndrome, kicking off intense controversy over the trial, as several other studies have failed to replicate PLATO’s positive results.
Doubts have grown about its apparent advantage over cheaper, off-patent P2Y12 inhibitors such as clopidogrel and prasugrel.
“Critics said it was noteworthy that ticagrelor failed in the US,” Doshi writes, “the only high enrolling country where sites were not monitored by the sponsor itself.” Doshi’s report points out that critics of the trial “highlight that AstraZeneca itself carried out the data monitoring for PLATO except for sites that were monitored by third party contract research organizations. In the four countries exclusively monitored by non-sponsor personnel—Georgia, Israel, Russia, and the US—ticagrelor fared worse.”
Victor Serebruany, MD, from Johns Hopkins University, said he was initially impressed by the trial results but became skeptical after noticing inconsistencies and anomalies in the data. He filed a complaint with the US District Court in the District of Columbia, suggesting that the cardiovascular events in the study “may have been manipulated.”
US Department of Justice Investigation
The US Department of Justice (DOJ) opened an investigation in 2013 and closed it in 2014 with no further action. Serebruany continues to publish critiques of the trial 15 years later but told The BMJ he has little hope that the questions will be resolved unless the DOJ re-engages with an investigation.
Doshi also points out discrepancies in the data reported. In the 2009 paper, published as an intent-to-treat analysis, investigators said there were 905 total deaths from any cause among all randomized patients. “An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths ‘discovered after withdrawal of consent,’” he reports.
The NEJM responded to Doshi that while it didn’t dispute the error in the number of deaths, it was uncertain about publishing a correction, citing new — not yet published — guidelines from the International Committee of Medical Journal Editors. NEJM Editor-in-Chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice.”
Doshi’s investigation includes an interview with Eric Bates, MD, professor of internal medicine at the University of Michigan in Ann Arbor, and a co-author of the US guidelines that recommend ticagrelor, who said he was “increasingly disturbed by how trial after trial came out as being not dramatically positive in any way.” Bates is now calling for a review of ticagrelor’s recommendation in guidelines, according to the report.
AstraZeneca declined to be interviewed for the BMJ investigation, according to Doshi, and a spokesperson from the company told the journal by email that they have “nothing to add,” directing editors to its 2014 public statement after the DOJ’s investigation into PLATO. The BMJ said PLATO trial co-chairs Robert A. Harrington, MD, and Lars Wallentin, MD, did not respond to The BMJ’s requests for comment.
Will the Guidelines Be Changed Now?
“I know and have worked with Drs Wallentin and Harrington,” Bates told Medscape Medical News, “and find them to be honest, intelligent clinical scientists with the highest ethical standards who manage conflicts of interest as well as can be done in the clinical research arena, where industry support is required to develop new knowledge,” he said.
“If there is a concern that AstraZeneca was manipulating the dataset and FDA submission, that is an important issue,” Bates said. “The US paradox and the failure of any other antiplatelet trial to find a comparative mortality advantage are two unexplained issues with PLATO that provide good fodder for conspiracy theories. I agree with the NEJM that this trial is 15 years old and may not be worth readjudicating in the current treatment era.”
Other calls for revisiting guidelines have come after disappointing postlicensure studies have repeatedly demonstrated that ticagrelor has “similar efficacy to clopidogrel but with increased bleeding and [dyspnea],” Doshi reports.
“My concern is the marketing spin by AstraZeneca and the promotion of ticagrelor by six to eight ‘thought leaders’ consistently funded by AstraZeneca over the past 10 years,” said Bates. “They have flooded the literature with supportive subset and post hoc analyses, review articles, and ‘meta-analyses’ flawed by selection and intellectual bias, and public interviews that consistently discount the findings of the many subsequent randomized controlled trials that have not supported the superiority of ticagrelor over clopidogrel or prasugrel.”
A version of this article first appeared on Medscape.com.
FROM THE BMJ
NT-proBNP May Predict Atrial Fibrillation Risk Early
TOPLINE:
Elevated levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a key biomarker for diagnosing heart failure, show a nearly fourfold increased risk for atrial fibrillation (AF) in at-risk individuals. The utility of this biomarker was particularly evident in older adults and when serum-based measurements were used.
METHODOLOGY:
- Researchers conducted a meta-analysis of prospective cohort, case-cohort, or nested case-control studies to examine the association between NT-proBNP and the incidence of AF.
- They also explored the potential of NT-proBNP in improving risk prediction models for AF.
- Overall, 136,089 adults were included from 16 cohorts, and 8017 cases of incident AF were reported over a median follow-up of 4-20 years.
- Most of the included cohorts were from Europe (n = 12), followed by America (n = 3) and Asia (n = 1).
- The accuracy of the risk prediction models was evaluated using C-indexes, with values in the range of 0.50-0.70, low accuracy; 0.70-0.90, moderate accuracy; and > 0.90, high accuracy.
TAKEAWAY:
- Elevated NT-proBNP levels showed a strong association with the risk for AF, with individuals in the highest quintile of NT-proBNP facing a 3.84-fold higher risk for incident AF (pooled relative risk [RR], 3.84; 95% CI, 3.03-4.87) than those in the lowest quintile.
- The risk increased by 9% for each 10 pg/mL increase in NT-proBNP (RR, 1.09; 95% CI, 1.04-1.14), with a significant nonlinear dose-response association found between NT-proBNP and the risk for AF (P for nonlinearity < .001).
- The association was stronger in the subgroups of older adults and when the biomarker was measured in serum samples.
- The addition of NT-proBNP to traditional risk prediction models for AF may improve predictive accuracy, with the ΔC-indexes ranging from 0.010 to 0.060.
IN PRACTICE:
“The significance of NT-proBNP in enhancing AF risk stratification deserves greater attention, with potential expansion to routine health screening,” the authors wrote.
SOURCE:
The study was led by Wanyue Wang, Department of Epidemiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, and was published online on December 06, 2024, in Heart.
LIMITATIONS:
Significant heterogeneity was observed in this meta-analysis, with the subgroup articles only providing exploratory and indicative findings. Due to the observational nature of this study, residual confounding could not be excluded. None of the prospective studies included differentiated subtypes of AF, such as paroxysmal and asymptomatic forms, which might have influenced the observed outcomes.
DISCLOSURES:
This study was supported by grants from the National Key Research and Development Program of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and National High Level Hospital Clinical Research Funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a key biomarker for diagnosing heart failure, show a nearly fourfold increased risk for atrial fibrillation (AF) in at-risk individuals. The utility of this biomarker was particularly evident in older adults and when serum-based measurements were used.
METHODOLOGY:
- Researchers conducted a meta-analysis of prospective cohort, case-cohort, or nested case-control studies to examine the association between NT-proBNP and the incidence of AF.
- They also explored the potential of NT-proBNP in improving risk prediction models for AF.
- Overall, 136,089 adults were included from 16 cohorts, and 8017 cases of incident AF were reported over a median follow-up of 4-20 years.
- Most of the included cohorts were from Europe (n = 12), followed by America (n = 3) and Asia (n = 1).
- The accuracy of the risk prediction models was evaluated using C-indexes, with values in the range of 0.50-0.70, low accuracy; 0.70-0.90, moderate accuracy; and > 0.90, high accuracy.
TAKEAWAY:
- Elevated NT-proBNP levels showed a strong association with the risk for AF, with individuals in the highest quintile of NT-proBNP facing a 3.84-fold higher risk for incident AF (pooled relative risk [RR], 3.84; 95% CI, 3.03-4.87) than those in the lowest quintile.
- The risk increased by 9% for each 10 pg/mL increase in NT-proBNP (RR, 1.09; 95% CI, 1.04-1.14), with a significant nonlinear dose-response association found between NT-proBNP and the risk for AF (P for nonlinearity < .001).
- The association was stronger in the subgroups of older adults and when the biomarker was measured in serum samples.
- The addition of NT-proBNP to traditional risk prediction models for AF may improve predictive accuracy, with the ΔC-indexes ranging from 0.010 to 0.060.
IN PRACTICE:
“The significance of NT-proBNP in enhancing AF risk stratification deserves greater attention, with potential expansion to routine health screening,” the authors wrote.
SOURCE:
The study was led by Wanyue Wang, Department of Epidemiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, and was published online on December 06, 2024, in Heart.
LIMITATIONS:
Significant heterogeneity was observed in this meta-analysis, with the subgroup articles only providing exploratory and indicative findings. Due to the observational nature of this study, residual confounding could not be excluded. None of the prospective studies included differentiated subtypes of AF, such as paroxysmal and asymptomatic forms, which might have influenced the observed outcomes.
DISCLOSURES:
This study was supported by grants from the National Key Research and Development Program of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and National High Level Hospital Clinical Research Funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a key biomarker for diagnosing heart failure, show a nearly fourfold increased risk for atrial fibrillation (AF) in at-risk individuals. The utility of this biomarker was particularly evident in older adults and when serum-based measurements were used.
METHODOLOGY:
- Researchers conducted a meta-analysis of prospective cohort, case-cohort, or nested case-control studies to examine the association between NT-proBNP and the incidence of AF.
- They also explored the potential of NT-proBNP in improving risk prediction models for AF.
- Overall, 136,089 adults were included from 16 cohorts, and 8017 cases of incident AF were reported over a median follow-up of 4-20 years.
- Most of the included cohorts were from Europe (n = 12), followed by America (n = 3) and Asia (n = 1).
- The accuracy of the risk prediction models was evaluated using C-indexes, with values in the range of 0.50-0.70, low accuracy; 0.70-0.90, moderate accuracy; and > 0.90, high accuracy.
TAKEAWAY:
- Elevated NT-proBNP levels showed a strong association with the risk for AF, with individuals in the highest quintile of NT-proBNP facing a 3.84-fold higher risk for incident AF (pooled relative risk [RR], 3.84; 95% CI, 3.03-4.87) than those in the lowest quintile.
- The risk increased by 9% for each 10 pg/mL increase in NT-proBNP (RR, 1.09; 95% CI, 1.04-1.14), with a significant nonlinear dose-response association found between NT-proBNP and the risk for AF (P for nonlinearity < .001).
- The association was stronger in the subgroups of older adults and when the biomarker was measured in serum samples.
- The addition of NT-proBNP to traditional risk prediction models for AF may improve predictive accuracy, with the ΔC-indexes ranging from 0.010 to 0.060.
IN PRACTICE:
“The significance of NT-proBNP in enhancing AF risk stratification deserves greater attention, with potential expansion to routine health screening,” the authors wrote.
SOURCE:
The study was led by Wanyue Wang, Department of Epidemiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, and was published online on December 06, 2024, in Heart.
LIMITATIONS:
Significant heterogeneity was observed in this meta-analysis, with the subgroup articles only providing exploratory and indicative findings. Due to the observational nature of this study, residual confounding could not be excluded. None of the prospective studies included differentiated subtypes of AF, such as paroxysmal and asymptomatic forms, which might have influenced the observed outcomes.
DISCLOSURES:
This study was supported by grants from the National Key Research and Development Program of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Natural Science Foundation of China, and National High Level Hospital Clinical Research Funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Obesity Medications: Could Coverage Offset Obesity Care Costs?
The question may seem simple: , such as cardiovascular disease and diabetes?
It’s a question that’s getting an increased amount of attention.
And for good reason — more than two in five US adults have obesity, according to the Centers for Disease Control and Prevention, and costs to treat obesity, in 2019 dollars, approached $173 billion, including productivity losses. Adults with obesity have annual healthcare costs of $1861 more than those at healthier weights.
Among recent developments:
- A proposed new rule, announced on November 26 by the Biden administration, expands coverage of anti-obesity medication for Americans who have Medicare and Medicaid. If it takes effect, an estimated 3.4 million Medicare recipients and about 4 million adult Medicaid enrollees could get access to the medications.
- As Medicare coverage goes, private insurers often follow. Observers predict that if the Centers for Medicare & Medicaid Services (CMS) covers anti-obesity drugs, more private employers may soon do the same. Recently, however, some private plans have done the opposite and dropped coverage of the pricey GLP-1s, which can cost $1000 a month or more out-of-pocket, citing excess costs for their company.
- Among the analyses about the value of weight loss on healthcare cost savings is a report published on December 5 in JAMA Network Open. Emory University experts looked at privately insured adults and adult Medicare beneficiaries with a body mass index (BMI) of ≥ 25 (classified as overweight). The conclusion: Projected annual savings from weight loss among US adults with obesity were substantial for both employee-based insurance and Medicare recipients.
- Besides helping obesity and obesity-related conditions, access to GLP-1s could have a favorable effect on productivity, others claim. That’s one focus of a 5-year partnership between the University of Manchester in England, and Eli Lilly and Company. Called SURMOUNT-REAL UK, the study will evaluate the effectiveness of tirzepatide in weight loss, diabetes prevention, and prevention of obesity-related complications in adults with obesity. It also aims to look at changes in health-related quality of life with weight loss and with changes in employment status and sick days.
CMS Proposal
In a statement announcing the proposal for Medicare and Medicaid to offer weight loss drugs, the White House noted that “tens of millions of Americans struggle with obesity” but that currently Medicare only covers the anti-obesity medications for certain conditions such as diabetes. The new proposal would expand that access to those with obesity. As of August, just 13 states cover GLP-1s in Medicaid programs, and North Carolina was the latest to do so.
Organizations advocating for health equity and recognition that obesity is a chronic disease came out in strong support of the proposal.
Kenneth E. Thorpe, PhD, a health policy expert at Emory University in Atlanta, who coauthored the recent analysis finding that weight loss offsets healthcare costs on an individual basis, told this news organization: “If finalized, this broad new coverage [by Medicare and Medicaid] would have a profound impact on the ability of Americans to access these novel medications that could significantly reduce obesity-related healthcare spending and improve overall health.”
The proposal “is modernizing the coverage of Medicare and Medicaid for obesity treatment,” agreed John Cawley, PhD, professor of economics and public policy at Cornell University in Ithaca, New York, who has researched the direct medical costs of obesity in the United States. “In this HHS rule, they talk about the scientific and medical consensus that having obesity is a chronic condition.”
The proposal requires a 60-day comment period that ends January 27, 2025, taking the timeline into the beginning of the Trump administration. Cawley and others pointed out that Trump’s pick for Health and Human Services Secretary, Robert F. Kennedy Jr, has been an outspoken opponent of the anti-obesity medicines, suggesting instead that Americans simply eat better.
Expert Analyses: Emory, Cornell, Southern California
So would paying for the pricey GLP-1s be smart in the long term? Analyses don’t agree.
Weight loss among those with obesity produces healthcare cost savings, said Thorpe and Peter Joski, MSPH, an associate research professor at Emory University. The two compared annual healthcare spending among privately insured adults and adult Medicare beneficiaries with a BMI of ≥ 25, using data from the Medical Expenditure Panel Survey — Household Component from April 1 to June 20, 2024.
The researchers looked at 3774 adults insured with Medicare and 13,435 with employer-sponsored insurance. Overall, those with private insurance with a weight loss of 5% spent an estimated average of $670 less on healthcare. Those with a weight loss of 25% spent an estimated $2849 less on healthcare. Among those with Medicare who had one or more comorbidities, a 5% weight loss reduced spending by $1262 on average; a 25% loss reduced it by an estimated $5442, or 31%.
Thorpe called the savings substantial. In an email interview, Thorpe said, “So yes, weight loss for people living with obesity does lower healthcare costs, as my research shows, but it also lowers other costs as well.”
These include costs associated with disability, workers’ compensation, presenteeism/absenteeism, and everyday costs, he said. He contends that “those other costs should factor into decisions about preventing and treating obesity of payors and policymakers and enhance the case for cost-effectiveness of treating obesity.”
Other research suggests it’s important to target the use of the anti-obesity medications to the BMI range that would get the most benefit. For people just barely above the BMI threshold of 30, no cost savings are expected, Cawley found in his research. But he has found substantial cost reduction if the BMI was 35-40.
However, as Cawley pointed out, as the drugs get cheaper and more options become available, the entire scenario is expected to shift.
The Congressional Budget Office View
In October, the nonpartisan Congressional Budget Office issued a report, “How Would Authorizing Medicare to Cover Anti-Obesity Medications Affect the Federal Budget?” Among the conclusions: Covering the anti-obesity medications would increase federal spending, on net, by about $35 billion from 2026 to 2034. Total direct federal costs of covering the medication would increase from $1.6 billion in 2026 to $7.1 billion in 2034. And it said total savings from improved health of the beneficiaries would be small, less than $50 million in 2026 and rising to $1 billion in 2034.
Covering the medications would cost $5600 per user in 2026, then down to $4300 in 2034. The offset of savings per user would be about $50 in 2026, then $650 in 2034.
Expert Analysis: USC Schaeffer Center
“The costs offsets come over time,” said Alison Sexton Ward, PhD, an economist at the University of Southern California’s Leonard D. Schaeffer Center, Los Angeles, and an expert on the topic. “If we look at the average annual medical cost over a lifetime, we do see cost offsets there.”
However, treating obesity means people will live longer, “and living longer costs more,” she said.
She took issue with some of the calculations in the CBO report, such as not considering the effect of semaglutide’s patent expiring in 2033.
In a white paper published in April 2023, Sexton Ward and her coauthors modeled potential social benefits and medical cost offsets from granting access to the newer weight loss drugs. The cumulative social benefits of providing coverage over the next decade would reach nearly $1 trillion, they said. Benefits would increase if private insurance expanded coverage. “In the first 10 years alone, covering weight loss therapies would save Medicare $175 billion-$245 billion, depending on whether private insurance joins Medicare in providing coverage for younger populations.”
While much focus is on Medicare coverage, Sexton Ward and others pointed out the need to expand coverage to younger ages, with the aim of preventing or delaying obesity-related complications.
Lilly UK Trial
A spokesperson for Lilly declined to comment further on the UK study, explaining that the study was just launching.
Besides tracking weight loss, researchers will evaluate the effect of the weight loss on sick days from work and employment. Obesity is shown to affect a person’s ability to work, leading to more absenteeism, so treating the obesity may improve productivity.
Beyond Health: The Value of Weight Loss
“I love the idea of studying whether access to obesity medications helps people stay employed and do their job,” said Cristy Gallagher, associate director of Research and Policy at STOP Obesity Alliance at the Milken Institute School of Public Health, George Washington University, Washington, DC. The alliance includes more than 50 organizations advocating for adult obesity treatment.
“One of our big arguments is [that] access to care, and to obesity care, will also help other conditions — comorbidities like heart disease and diabetes.”
However, access to the anti-obesity medications, by itself, is not enough, Gallagher said. Other components, such as intensive behavioral therapy and guidance about diet and exercise, are needed, she said. So, too, for those who need it, is access to bariatric surgery, she said. And medication access should include other options besides the GLP-1s, she said. “Not every medication is right for everybody.”
Cawley, Gallagher, Thorpe, and Sexton Ward had no disclosures.
A version of this article appeared on Medscape.com.
The question may seem simple: , such as cardiovascular disease and diabetes?
It’s a question that’s getting an increased amount of attention.
And for good reason — more than two in five US adults have obesity, according to the Centers for Disease Control and Prevention, and costs to treat obesity, in 2019 dollars, approached $173 billion, including productivity losses. Adults with obesity have annual healthcare costs of $1861 more than those at healthier weights.
Among recent developments:
- A proposed new rule, announced on November 26 by the Biden administration, expands coverage of anti-obesity medication for Americans who have Medicare and Medicaid. If it takes effect, an estimated 3.4 million Medicare recipients and about 4 million adult Medicaid enrollees could get access to the medications.
- As Medicare coverage goes, private insurers often follow. Observers predict that if the Centers for Medicare & Medicaid Services (CMS) covers anti-obesity drugs, more private employers may soon do the same. Recently, however, some private plans have done the opposite and dropped coverage of the pricey GLP-1s, which can cost $1000 a month or more out-of-pocket, citing excess costs for their company.
- Among the analyses about the value of weight loss on healthcare cost savings is a report published on December 5 in JAMA Network Open. Emory University experts looked at privately insured adults and adult Medicare beneficiaries with a body mass index (BMI) of ≥ 25 (classified as overweight). The conclusion: Projected annual savings from weight loss among US adults with obesity were substantial for both employee-based insurance and Medicare recipients.
- Besides helping obesity and obesity-related conditions, access to GLP-1s could have a favorable effect on productivity, others claim. That’s one focus of a 5-year partnership between the University of Manchester in England, and Eli Lilly and Company. Called SURMOUNT-REAL UK, the study will evaluate the effectiveness of tirzepatide in weight loss, diabetes prevention, and prevention of obesity-related complications in adults with obesity. It also aims to look at changes in health-related quality of life with weight loss and with changes in employment status and sick days.
CMS Proposal
In a statement announcing the proposal for Medicare and Medicaid to offer weight loss drugs, the White House noted that “tens of millions of Americans struggle with obesity” but that currently Medicare only covers the anti-obesity medications for certain conditions such as diabetes. The new proposal would expand that access to those with obesity. As of August, just 13 states cover GLP-1s in Medicaid programs, and North Carolina was the latest to do so.
Organizations advocating for health equity and recognition that obesity is a chronic disease came out in strong support of the proposal.
Kenneth E. Thorpe, PhD, a health policy expert at Emory University in Atlanta, who coauthored the recent analysis finding that weight loss offsets healthcare costs on an individual basis, told this news organization: “If finalized, this broad new coverage [by Medicare and Medicaid] would have a profound impact on the ability of Americans to access these novel medications that could significantly reduce obesity-related healthcare spending and improve overall health.”
The proposal “is modernizing the coverage of Medicare and Medicaid for obesity treatment,” agreed John Cawley, PhD, professor of economics and public policy at Cornell University in Ithaca, New York, who has researched the direct medical costs of obesity in the United States. “In this HHS rule, they talk about the scientific and medical consensus that having obesity is a chronic condition.”
The proposal requires a 60-day comment period that ends January 27, 2025, taking the timeline into the beginning of the Trump administration. Cawley and others pointed out that Trump’s pick for Health and Human Services Secretary, Robert F. Kennedy Jr, has been an outspoken opponent of the anti-obesity medicines, suggesting instead that Americans simply eat better.
Expert Analyses: Emory, Cornell, Southern California
So would paying for the pricey GLP-1s be smart in the long term? Analyses don’t agree.
Weight loss among those with obesity produces healthcare cost savings, said Thorpe and Peter Joski, MSPH, an associate research professor at Emory University. The two compared annual healthcare spending among privately insured adults and adult Medicare beneficiaries with a BMI of ≥ 25, using data from the Medical Expenditure Panel Survey — Household Component from April 1 to June 20, 2024.
The researchers looked at 3774 adults insured with Medicare and 13,435 with employer-sponsored insurance. Overall, those with private insurance with a weight loss of 5% spent an estimated average of $670 less on healthcare. Those with a weight loss of 25% spent an estimated $2849 less on healthcare. Among those with Medicare who had one or more comorbidities, a 5% weight loss reduced spending by $1262 on average; a 25% loss reduced it by an estimated $5442, or 31%.
Thorpe called the savings substantial. In an email interview, Thorpe said, “So yes, weight loss for people living with obesity does lower healthcare costs, as my research shows, but it also lowers other costs as well.”
These include costs associated with disability, workers’ compensation, presenteeism/absenteeism, and everyday costs, he said. He contends that “those other costs should factor into decisions about preventing and treating obesity of payors and policymakers and enhance the case for cost-effectiveness of treating obesity.”
Other research suggests it’s important to target the use of the anti-obesity medications to the BMI range that would get the most benefit. For people just barely above the BMI threshold of 30, no cost savings are expected, Cawley found in his research. But he has found substantial cost reduction if the BMI was 35-40.
However, as Cawley pointed out, as the drugs get cheaper and more options become available, the entire scenario is expected to shift.
The Congressional Budget Office View
In October, the nonpartisan Congressional Budget Office issued a report, “How Would Authorizing Medicare to Cover Anti-Obesity Medications Affect the Federal Budget?” Among the conclusions: Covering the anti-obesity medications would increase federal spending, on net, by about $35 billion from 2026 to 2034. Total direct federal costs of covering the medication would increase from $1.6 billion in 2026 to $7.1 billion in 2034. And it said total savings from improved health of the beneficiaries would be small, less than $50 million in 2026 and rising to $1 billion in 2034.
Covering the medications would cost $5600 per user in 2026, then down to $4300 in 2034. The offset of savings per user would be about $50 in 2026, then $650 in 2034.
Expert Analysis: USC Schaeffer Center
“The costs offsets come over time,” said Alison Sexton Ward, PhD, an economist at the University of Southern California’s Leonard D. Schaeffer Center, Los Angeles, and an expert on the topic. “If we look at the average annual medical cost over a lifetime, we do see cost offsets there.”
However, treating obesity means people will live longer, “and living longer costs more,” she said.
She took issue with some of the calculations in the CBO report, such as not considering the effect of semaglutide’s patent expiring in 2033.
In a white paper published in April 2023, Sexton Ward and her coauthors modeled potential social benefits and medical cost offsets from granting access to the newer weight loss drugs. The cumulative social benefits of providing coverage over the next decade would reach nearly $1 trillion, they said. Benefits would increase if private insurance expanded coverage. “In the first 10 years alone, covering weight loss therapies would save Medicare $175 billion-$245 billion, depending on whether private insurance joins Medicare in providing coverage for younger populations.”
While much focus is on Medicare coverage, Sexton Ward and others pointed out the need to expand coverage to younger ages, with the aim of preventing or delaying obesity-related complications.
Lilly UK Trial
A spokesperson for Lilly declined to comment further on the UK study, explaining that the study was just launching.
Besides tracking weight loss, researchers will evaluate the effect of the weight loss on sick days from work and employment. Obesity is shown to affect a person’s ability to work, leading to more absenteeism, so treating the obesity may improve productivity.
Beyond Health: The Value of Weight Loss
“I love the idea of studying whether access to obesity medications helps people stay employed and do their job,” said Cristy Gallagher, associate director of Research and Policy at STOP Obesity Alliance at the Milken Institute School of Public Health, George Washington University, Washington, DC. The alliance includes more than 50 organizations advocating for adult obesity treatment.
“One of our big arguments is [that] access to care, and to obesity care, will also help other conditions — comorbidities like heart disease and diabetes.”
However, access to the anti-obesity medications, by itself, is not enough, Gallagher said. Other components, such as intensive behavioral therapy and guidance about diet and exercise, are needed, she said. So, too, for those who need it, is access to bariatric surgery, she said. And medication access should include other options besides the GLP-1s, she said. “Not every medication is right for everybody.”
Cawley, Gallagher, Thorpe, and Sexton Ward had no disclosures.
A version of this article appeared on Medscape.com.
The question may seem simple: , such as cardiovascular disease and diabetes?
It’s a question that’s getting an increased amount of attention.
And for good reason — more than two in five US adults have obesity, according to the Centers for Disease Control and Prevention, and costs to treat obesity, in 2019 dollars, approached $173 billion, including productivity losses. Adults with obesity have annual healthcare costs of $1861 more than those at healthier weights.
Among recent developments:
- A proposed new rule, announced on November 26 by the Biden administration, expands coverage of anti-obesity medication for Americans who have Medicare and Medicaid. If it takes effect, an estimated 3.4 million Medicare recipients and about 4 million adult Medicaid enrollees could get access to the medications.
- As Medicare coverage goes, private insurers often follow. Observers predict that if the Centers for Medicare & Medicaid Services (CMS) covers anti-obesity drugs, more private employers may soon do the same. Recently, however, some private plans have done the opposite and dropped coverage of the pricey GLP-1s, which can cost $1000 a month or more out-of-pocket, citing excess costs for their company.
- Among the analyses about the value of weight loss on healthcare cost savings is a report published on December 5 in JAMA Network Open. Emory University experts looked at privately insured adults and adult Medicare beneficiaries with a body mass index (BMI) of ≥ 25 (classified as overweight). The conclusion: Projected annual savings from weight loss among US adults with obesity were substantial for both employee-based insurance and Medicare recipients.
- Besides helping obesity and obesity-related conditions, access to GLP-1s could have a favorable effect on productivity, others claim. That’s one focus of a 5-year partnership between the University of Manchester in England, and Eli Lilly and Company. Called SURMOUNT-REAL UK, the study will evaluate the effectiveness of tirzepatide in weight loss, diabetes prevention, and prevention of obesity-related complications in adults with obesity. It also aims to look at changes in health-related quality of life with weight loss and with changes in employment status and sick days.
CMS Proposal
In a statement announcing the proposal for Medicare and Medicaid to offer weight loss drugs, the White House noted that “tens of millions of Americans struggle with obesity” but that currently Medicare only covers the anti-obesity medications for certain conditions such as diabetes. The new proposal would expand that access to those with obesity. As of August, just 13 states cover GLP-1s in Medicaid programs, and North Carolina was the latest to do so.
Organizations advocating for health equity and recognition that obesity is a chronic disease came out in strong support of the proposal.
Kenneth E. Thorpe, PhD, a health policy expert at Emory University in Atlanta, who coauthored the recent analysis finding that weight loss offsets healthcare costs on an individual basis, told this news organization: “If finalized, this broad new coverage [by Medicare and Medicaid] would have a profound impact on the ability of Americans to access these novel medications that could significantly reduce obesity-related healthcare spending and improve overall health.”
The proposal “is modernizing the coverage of Medicare and Medicaid for obesity treatment,” agreed John Cawley, PhD, professor of economics and public policy at Cornell University in Ithaca, New York, who has researched the direct medical costs of obesity in the United States. “In this HHS rule, they talk about the scientific and medical consensus that having obesity is a chronic condition.”
The proposal requires a 60-day comment period that ends January 27, 2025, taking the timeline into the beginning of the Trump administration. Cawley and others pointed out that Trump’s pick for Health and Human Services Secretary, Robert F. Kennedy Jr, has been an outspoken opponent of the anti-obesity medicines, suggesting instead that Americans simply eat better.
Expert Analyses: Emory, Cornell, Southern California
So would paying for the pricey GLP-1s be smart in the long term? Analyses don’t agree.
Weight loss among those with obesity produces healthcare cost savings, said Thorpe and Peter Joski, MSPH, an associate research professor at Emory University. The two compared annual healthcare spending among privately insured adults and adult Medicare beneficiaries with a BMI of ≥ 25, using data from the Medical Expenditure Panel Survey — Household Component from April 1 to June 20, 2024.
The researchers looked at 3774 adults insured with Medicare and 13,435 with employer-sponsored insurance. Overall, those with private insurance with a weight loss of 5% spent an estimated average of $670 less on healthcare. Those with a weight loss of 25% spent an estimated $2849 less on healthcare. Among those with Medicare who had one or more comorbidities, a 5% weight loss reduced spending by $1262 on average; a 25% loss reduced it by an estimated $5442, or 31%.
Thorpe called the savings substantial. In an email interview, Thorpe said, “So yes, weight loss for people living with obesity does lower healthcare costs, as my research shows, but it also lowers other costs as well.”
These include costs associated with disability, workers’ compensation, presenteeism/absenteeism, and everyday costs, he said. He contends that “those other costs should factor into decisions about preventing and treating obesity of payors and policymakers and enhance the case for cost-effectiveness of treating obesity.”
Other research suggests it’s important to target the use of the anti-obesity medications to the BMI range that would get the most benefit. For people just barely above the BMI threshold of 30, no cost savings are expected, Cawley found in his research. But he has found substantial cost reduction if the BMI was 35-40.
However, as Cawley pointed out, as the drugs get cheaper and more options become available, the entire scenario is expected to shift.
The Congressional Budget Office View
In October, the nonpartisan Congressional Budget Office issued a report, “How Would Authorizing Medicare to Cover Anti-Obesity Medications Affect the Federal Budget?” Among the conclusions: Covering the anti-obesity medications would increase federal spending, on net, by about $35 billion from 2026 to 2034. Total direct federal costs of covering the medication would increase from $1.6 billion in 2026 to $7.1 billion in 2034. And it said total savings from improved health of the beneficiaries would be small, less than $50 million in 2026 and rising to $1 billion in 2034.
Covering the medications would cost $5600 per user in 2026, then down to $4300 in 2034. The offset of savings per user would be about $50 in 2026, then $650 in 2034.
Expert Analysis: USC Schaeffer Center
“The costs offsets come over time,” said Alison Sexton Ward, PhD, an economist at the University of Southern California’s Leonard D. Schaeffer Center, Los Angeles, and an expert on the topic. “If we look at the average annual medical cost over a lifetime, we do see cost offsets there.”
However, treating obesity means people will live longer, “and living longer costs more,” she said.
She took issue with some of the calculations in the CBO report, such as not considering the effect of semaglutide’s patent expiring in 2033.
In a white paper published in April 2023, Sexton Ward and her coauthors modeled potential social benefits and medical cost offsets from granting access to the newer weight loss drugs. The cumulative social benefits of providing coverage over the next decade would reach nearly $1 trillion, they said. Benefits would increase if private insurance expanded coverage. “In the first 10 years alone, covering weight loss therapies would save Medicare $175 billion-$245 billion, depending on whether private insurance joins Medicare in providing coverage for younger populations.”
While much focus is on Medicare coverage, Sexton Ward and others pointed out the need to expand coverage to younger ages, with the aim of preventing or delaying obesity-related complications.
Lilly UK Trial
A spokesperson for Lilly declined to comment further on the UK study, explaining that the study was just launching.
Besides tracking weight loss, researchers will evaluate the effect of the weight loss on sick days from work and employment. Obesity is shown to affect a person’s ability to work, leading to more absenteeism, so treating the obesity may improve productivity.
Beyond Health: The Value of Weight Loss
“I love the idea of studying whether access to obesity medications helps people stay employed and do their job,” said Cristy Gallagher, associate director of Research and Policy at STOP Obesity Alliance at the Milken Institute School of Public Health, George Washington University, Washington, DC. The alliance includes more than 50 organizations advocating for adult obesity treatment.
“One of our big arguments is [that] access to care, and to obesity care, will also help other conditions — comorbidities like heart disease and diabetes.”
However, access to the anti-obesity medications, by itself, is not enough, Gallagher said. Other components, such as intensive behavioral therapy and guidance about diet and exercise, are needed, she said. So, too, for those who need it, is access to bariatric surgery, she said. And medication access should include other options besides the GLP-1s, she said. “Not every medication is right for everybody.”
Cawley, Gallagher, Thorpe, and Sexton Ward had no disclosures.
A version of this article appeared on Medscape.com.
Clopidogrel Tops Aspirin Post-PCI, Even in High-Risk Cases
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The beneficial effect of clopidogrel monotherapy over aspirin monotherapy in patients who underwent percutaneous coronary intervention (PCI) and remained event free for 6-18 months on dual antiplatelet therapy (DAPT) is consistent, regardless of bleeding risk or PCI complexity, according to a post hoc analysis of the HOST-EXAM trial.
METHODOLOGY:
- The HOST-EXAM Extended study conducted across 37 sites in South Korea included patients who underwent PCI with drug-eluting stents and remained free of clinical events for 6-18 months post-PCI, while receiving DAPT.
- This post hoc analysis of the HOST-EXAM Extended study compared the effectiveness of long-term daily clopidogrel (75 mg) with that of aspirin monotherapy (100 mg) after PCI, according to bleeding risk and procedural complexity in 3974 patients (mean age, 63 years; 75% men) who were followed for up to 5.9 years.
- High bleeding risk was reported in 866 patients, and 849 patients underwent complex PCI.
- Patients were classified into four distinct risk groups: No bleeding risk and noncomplex PCI, no bleeding risk and complex PCI, high bleeding risk and noncomplex PCI, and high bleeding risk and complex PCI.
- The co-primary endpoints were thrombotic composite events (cardiovascular death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and definite/probable stent thrombosis) and any bleeding event.
TAKEAWAY:
- Thrombotic composite events (hazard ratio [HR], 2.15; P < .001) and any bleeding event (HR, 3.64; P < .001) were more frequent in patients with a high bleeding risk than in those without.
- However, there was no difference in the risk for thrombotic composite events or any bleeding event by PCI complexity.
- The long-term benefits of clopidogrel monotherapy over aspirin monotherapy were seen in all patients, regardless of bleeding risks (P for interaction = .38 for thrombotic composite events and P for interaction = .20 for any bleeding event) or PCI complexity (P for interaction = .12 for thrombotic composite events and P for interaction = .62 for any bleeding event).
- The greatest risk reduction in thrombotic composite events with clopidogrel monotherapy occurred in patients with a high bleeding risk who underwent complex PCI (HR, 0.46; P = .03).
IN PRACTICE:
“[In this study], no significant interaction was found between treatment arms and risk groups, denoting that the beneficial impact of clopidogrel monotherapy was consistent regardless of HBR [high bleeding risk] or PCI complexity,” the authors wrote.
SOURCE:
This study was led by Jeehoon Kang, MD, Seoul National University College of Medicine and Seoul National University Hospital, Seoul, Republic of Korea. It was published online on November 27, 2024, in JAMA Cardiology.
LIMITATIONS:
As this study is a post hoc analysis, the findings should be considered primarily hypothesis generating. This study was conducted exclusively in an East Asian population and may not be generalizable to other ethnic groups. The definitions of high bleeding risk and complex PCI used in this analysis were not prespecified in the study protocol of the HOST-EXAM trial. Certain criteria defining high bleeding risk were not analyzed as they fell under the exclusion criteria of the HOST-EXAM trial or were not recorded in the study case report form.
DISCLOSURES:
This study was supported by grants from the Patient-Centered Clinical Research Coordinating Center and Seoul National University Hospital. One author reported receiving grants and personal fees from various pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
When Is the Best Time to Deliver for Pregnant Patients With Chronic Hypertension?
TOPLINE:
Among pregnant patients with chronic hypertension, delivery at 39 weeks of gestation provides an optimal balance between stillbirth risk and neonatal outcomes. Analysis of 227,977 term singleton deliveries shows consistent findings across different patient subgroups.
METHODOLOGY:
- A population-based retrospective cohort study analyzed 227,977 nonanomalous singleton term births in the United States from 2014 to 2018 among patients with chronic hypertension.
- Researchers excluded pregnancies with superimposed preeclampsia, eclampsia, pregestational diabetes, and deliveries occurring before 37 weeks or at 43 or more weeks of gestation.
- Analysis compared rates of stillbirth, infant death within 1 year of life, and neonatal morbidity at each week of term pregnancy.
- Neonatal morbidity was defined as a composite of neonatal intensive care unit admission, ventilation for 6 hours or longer, a low 5-minute Apgar score (≤ 3), and seizures.
TAKEAWAY:
- The rate of stillbirth per 10,000 ongoing pregnancies increased with gestational age and was lowest at 38 weeks (6.5; 95% CI, 5.4-7.7).
- Rates of infant death and neonatal morbidity were lowest at 40 weeks (18.0/10,000 live births; 95% CI, 13.7-23.6) and 39 weeks (637/10,000 live births; 95% CI, 619-654), respectively.
- At 39 weeks of gestation, the risk for delivery was lower (651/10,000; 95% CI, 633-670) than the composite risk for expectant management (750/10,000; 95% CI, 720-781).
- According to the authors, findings were consistent for non-Hispanic Black patients and pregnancies complicated by fetal growth restriction.
IN PRACTICE:
“To prevent one case of stillbirth, infant death, or neonatal morbidity, an estimated 101 patients with chronic hypertension would need to deliver at 39 weeks of gestation as opposed to 40 weeks. Given the approximately 45,000 patients with chronic hypertension who deliver at term each year in the United States, a policy of delivery at 39 weeks of gestation theoretically would prevent 450 adverse perinatal events per year,” wrote the authors of the study.
SOURCE:
The study was led by Ira Hamilton, James Liu, Labeena Wajahat, and Robert Rossi, University of Cincinnati College of Medicine in Cincinnati. It was published online in O&G Open.
LIMITATIONS:
According to the authors, the study could not stratify chronic hypertension based on medication use, number of medications, or degree of control. The researchers note that exact timing of delivery in weeks and days was not reported, limiting precise understanding of optimal delivery timing. Additionally, the study could not examine rates of neonatal morbidity and mortality in patients who developed superimposed preeclampsia during expectant management.
DISCLOSURES:
The authors did not report any potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among pregnant patients with chronic hypertension, delivery at 39 weeks of gestation provides an optimal balance between stillbirth risk and neonatal outcomes. Analysis of 227,977 term singleton deliveries shows consistent findings across different patient subgroups.
METHODOLOGY:
- A population-based retrospective cohort study analyzed 227,977 nonanomalous singleton term births in the United States from 2014 to 2018 among patients with chronic hypertension.
- Researchers excluded pregnancies with superimposed preeclampsia, eclampsia, pregestational diabetes, and deliveries occurring before 37 weeks or at 43 or more weeks of gestation.
- Analysis compared rates of stillbirth, infant death within 1 year of life, and neonatal morbidity at each week of term pregnancy.
- Neonatal morbidity was defined as a composite of neonatal intensive care unit admission, ventilation for 6 hours or longer, a low 5-minute Apgar score (≤ 3), and seizures.
TAKEAWAY:
- The rate of stillbirth per 10,000 ongoing pregnancies increased with gestational age and was lowest at 38 weeks (6.5; 95% CI, 5.4-7.7).
- Rates of infant death and neonatal morbidity were lowest at 40 weeks (18.0/10,000 live births; 95% CI, 13.7-23.6) and 39 weeks (637/10,000 live births; 95% CI, 619-654), respectively.
- At 39 weeks of gestation, the risk for delivery was lower (651/10,000; 95% CI, 633-670) than the composite risk for expectant management (750/10,000; 95% CI, 720-781).
- According to the authors, findings were consistent for non-Hispanic Black patients and pregnancies complicated by fetal growth restriction.
IN PRACTICE:
“To prevent one case of stillbirth, infant death, or neonatal morbidity, an estimated 101 patients with chronic hypertension would need to deliver at 39 weeks of gestation as opposed to 40 weeks. Given the approximately 45,000 patients with chronic hypertension who deliver at term each year in the United States, a policy of delivery at 39 weeks of gestation theoretically would prevent 450 adverse perinatal events per year,” wrote the authors of the study.
SOURCE:
The study was led by Ira Hamilton, James Liu, Labeena Wajahat, and Robert Rossi, University of Cincinnati College of Medicine in Cincinnati. It was published online in O&G Open.
LIMITATIONS:
According to the authors, the study could not stratify chronic hypertension based on medication use, number of medications, or degree of control. The researchers note that exact timing of delivery in weeks and days was not reported, limiting precise understanding of optimal delivery timing. Additionally, the study could not examine rates of neonatal morbidity and mortality in patients who developed superimposed preeclampsia during expectant management.
DISCLOSURES:
The authors did not report any potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Among pregnant patients with chronic hypertension, delivery at 39 weeks of gestation provides an optimal balance between stillbirth risk and neonatal outcomes. Analysis of 227,977 term singleton deliveries shows consistent findings across different patient subgroups.
METHODOLOGY:
- A population-based retrospective cohort study analyzed 227,977 nonanomalous singleton term births in the United States from 2014 to 2018 among patients with chronic hypertension.
- Researchers excluded pregnancies with superimposed preeclampsia, eclampsia, pregestational diabetes, and deliveries occurring before 37 weeks or at 43 or more weeks of gestation.
- Analysis compared rates of stillbirth, infant death within 1 year of life, and neonatal morbidity at each week of term pregnancy.
- Neonatal morbidity was defined as a composite of neonatal intensive care unit admission, ventilation for 6 hours or longer, a low 5-minute Apgar score (≤ 3), and seizures.
TAKEAWAY:
- The rate of stillbirth per 10,000 ongoing pregnancies increased with gestational age and was lowest at 38 weeks (6.5; 95% CI, 5.4-7.7).
- Rates of infant death and neonatal morbidity were lowest at 40 weeks (18.0/10,000 live births; 95% CI, 13.7-23.6) and 39 weeks (637/10,000 live births; 95% CI, 619-654), respectively.
- At 39 weeks of gestation, the risk for delivery was lower (651/10,000; 95% CI, 633-670) than the composite risk for expectant management (750/10,000; 95% CI, 720-781).
- According to the authors, findings were consistent for non-Hispanic Black patients and pregnancies complicated by fetal growth restriction.
IN PRACTICE:
“To prevent one case of stillbirth, infant death, or neonatal morbidity, an estimated 101 patients with chronic hypertension would need to deliver at 39 weeks of gestation as opposed to 40 weeks. Given the approximately 45,000 patients with chronic hypertension who deliver at term each year in the United States, a policy of delivery at 39 weeks of gestation theoretically would prevent 450 adverse perinatal events per year,” wrote the authors of the study.
SOURCE:
The study was led by Ira Hamilton, James Liu, Labeena Wajahat, and Robert Rossi, University of Cincinnati College of Medicine in Cincinnati. It was published online in O&G Open.
LIMITATIONS:
According to the authors, the study could not stratify chronic hypertension based on medication use, number of medications, or degree of control. The researchers note that exact timing of delivery in weeks and days was not reported, limiting precise understanding of optimal delivery timing. Additionally, the study could not examine rates of neonatal morbidity and mortality in patients who developed superimposed preeclampsia during expectant management.
DISCLOSURES:
The authors did not report any potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cutaneous Lupus Associated with Greater Risk for Atherosclerotic Cardiovascular Disease
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.