User login
Hospitalists innovate in ICU management
With intensive care units stretched to their limits – and beyond – during the COVID-19 pandemic, hospitalists became more central than ever in orchestrating the response.
At SHM Converge, the annual conference of the Society of Hospital Medicine, two hospitalists shared how their teams helped to develop new critical care units and strategies for best managing and allocating care to COVID patients in the ICU.
“The pandemic has been a selective pressure on us as a specialty,” said Jason Stein, MD, SFHM, a full-time clinical hospitalist at Roper Hospital, a 332-bed facility in Charleston, S.C.
Dr. Stein explained how hospitalists at Roper helped create the Progressive Care Unit – a negative-pressure unit with 12 high-flow oxygen beds overseen by a hospital medicine team, with the help of a respiratory therapist, pharmacist, and nurses. Patients in this unit had escalating acuity – quickly increasing oxygen needs – or deescalating acuity, such as ICU transfers, Dr. Stein said. Cardiac catheterization space was converted for the unit, which was intended to preserve beds in the hospital ICU for patients needing mechanical ventilation or vasoactive medication.
Interdisciplinary rounds – to assess oxygen and inflammatory marker trends, and run through a COVID care checklist – took place every day at 10 a.m.
“Consistency was the key,” Dr. Stein said.
At Weill Cornell Medical Center in New York, hospitalists helped build the COVID Recovery Unit, which was dedicated to the care of patients coming out of the ICU, said Vishwas Anand Singh, MD, MS, FHM, cochief of hospital medicine at New York Presbyterian–Lower Manhattan Hospital.
“The pandemic created an unprecedented need for critical care, and post-ICU care,” Dr. Singh said. “After extubation, patients remain very complicated and they have unique needs.”
The 30-bed COVID Recovery Unit – converted from a behavioral health unit – was designed to meet those needs. It was staffed by one lead hospitalist, 3 hospitalist physicians, 3 advanced practitioners, about 12 nurses and a neurologist, psychiatrist, and neuropsychologist.
The idea was to integrate medical care with careful attention to rehab and neuropsychological needs, Dr. Singh said. To be in the unit, patients had to be medically stable but with ongoing medical and rehabilitation needs and able to tolerate about half an hour of physical or occupational therapy each day.
The space was set up so that patients could interact with each other as well as staff, and this ability to share their experiences of trauma and recovery “led to an improved sense of psychological well-being and to healing,” according to Dr. Singh. Group therapy and meditation were also held several times a week.
“All this together, we thought we were really meeting the need for a lot of these patients from medical to psychosocial,” he said.
New York Presbyterian––Lower Manhattan Hospital also established a program called ICU Outreach to give hospitalists a “bird’s eye view” of the ICU in order to help move patients from unit to unit for optimized care. One hospitalist acted as a bridge between the ICU, the floors, and the emergency room.
The hospitalist on duty touched based with the ICU each day at 10 a.m., assessed the available beds, compiled a list of patients being discharged, met with all of the hospitalists and individual teams in inpatient and emergency services, and compiled a list of “watchers” – the sickest patients who needed help being managed.
The broad perspective was important, Dr. Singh said.
“We quickly found that each individual team or provider only knew the patients they were caring for, and the ICU Outreach person knew the whole big picture and could put the pieces together,” he said. “They could answer who was next in line for a bed, who benefited from a goals of care discussion, who could be managed on the floor with assistance. And this bridge, having this person fill this role, allowed the intensivists to focus on the patients they had in the unit.”
Palliative care and patient flow
Dr. Singh also described how hospitalists played an important role in palliative care for COVID patients. The hospital medicine team offered hospitalist palliative care services, which included COVIDtalk, a course on communicating about end of life, which helped to expand the pool of palliative care providers. Those trained were taught that these difficult conversations had to be honest and clear, with the goals of care addressed very early in the admission, should a patient decompensate soon after arrival.
A palliative “rapid response team” included a virtual hospitalist, a palliative care nurse practitioner, and a virtual psychiatrist – a team available 24 hours a day to have longer conversations so that clinicians could better tend to their patients when the in-person palliative care service was stretched thin, or at off hours like the middle of the night.
These innovations not only helped serve patients and families better, but also gave hospitalists training and experience in palliative care.
At Roper Hospital, Dr. Stein explained how hospitalists helped improve management of COVID patient flow. Depending on the time of day and the staffing on duty, there could be considerable confusion about where patients should go after the ED, or the COVID progressive unit, or the floor.
Hospitalists helped develop hospitalwide algorithms for escalating and deescalating acuity, Dr. Stein said, providing a “shared mental model for where a patient should go.”
“There are many ways hospitalists can and did rise to meet the unique demands of COVID,” Dr. Singh said, “whether it was innovating a new unit or service or work flow or leading a multidisciplinary team to extend or support other services that may have been strained.”
With intensive care units stretched to their limits – and beyond – during the COVID-19 pandemic, hospitalists became more central than ever in orchestrating the response.
At SHM Converge, the annual conference of the Society of Hospital Medicine, two hospitalists shared how their teams helped to develop new critical care units and strategies for best managing and allocating care to COVID patients in the ICU.
“The pandemic has been a selective pressure on us as a specialty,” said Jason Stein, MD, SFHM, a full-time clinical hospitalist at Roper Hospital, a 332-bed facility in Charleston, S.C.
Dr. Stein explained how hospitalists at Roper helped create the Progressive Care Unit – a negative-pressure unit with 12 high-flow oxygen beds overseen by a hospital medicine team, with the help of a respiratory therapist, pharmacist, and nurses. Patients in this unit had escalating acuity – quickly increasing oxygen needs – or deescalating acuity, such as ICU transfers, Dr. Stein said. Cardiac catheterization space was converted for the unit, which was intended to preserve beds in the hospital ICU for patients needing mechanical ventilation or vasoactive medication.
Interdisciplinary rounds – to assess oxygen and inflammatory marker trends, and run through a COVID care checklist – took place every day at 10 a.m.
“Consistency was the key,” Dr. Stein said.
At Weill Cornell Medical Center in New York, hospitalists helped build the COVID Recovery Unit, which was dedicated to the care of patients coming out of the ICU, said Vishwas Anand Singh, MD, MS, FHM, cochief of hospital medicine at New York Presbyterian–Lower Manhattan Hospital.
“The pandemic created an unprecedented need for critical care, and post-ICU care,” Dr. Singh said. “After extubation, patients remain very complicated and they have unique needs.”
The 30-bed COVID Recovery Unit – converted from a behavioral health unit – was designed to meet those needs. It was staffed by one lead hospitalist, 3 hospitalist physicians, 3 advanced practitioners, about 12 nurses and a neurologist, psychiatrist, and neuropsychologist.
The idea was to integrate medical care with careful attention to rehab and neuropsychological needs, Dr. Singh said. To be in the unit, patients had to be medically stable but with ongoing medical and rehabilitation needs and able to tolerate about half an hour of physical or occupational therapy each day.
The space was set up so that patients could interact with each other as well as staff, and this ability to share their experiences of trauma and recovery “led to an improved sense of psychological well-being and to healing,” according to Dr. Singh. Group therapy and meditation were also held several times a week.
“All this together, we thought we were really meeting the need for a lot of these patients from medical to psychosocial,” he said.
New York Presbyterian––Lower Manhattan Hospital also established a program called ICU Outreach to give hospitalists a “bird’s eye view” of the ICU in order to help move patients from unit to unit for optimized care. One hospitalist acted as a bridge between the ICU, the floors, and the emergency room.
The hospitalist on duty touched based with the ICU each day at 10 a.m., assessed the available beds, compiled a list of patients being discharged, met with all of the hospitalists and individual teams in inpatient and emergency services, and compiled a list of “watchers” – the sickest patients who needed help being managed.
The broad perspective was important, Dr. Singh said.
“We quickly found that each individual team or provider only knew the patients they were caring for, and the ICU Outreach person knew the whole big picture and could put the pieces together,” he said. “They could answer who was next in line for a bed, who benefited from a goals of care discussion, who could be managed on the floor with assistance. And this bridge, having this person fill this role, allowed the intensivists to focus on the patients they had in the unit.”
Palliative care and patient flow
Dr. Singh also described how hospitalists played an important role in palliative care for COVID patients. The hospital medicine team offered hospitalist palliative care services, which included COVIDtalk, a course on communicating about end of life, which helped to expand the pool of palliative care providers. Those trained were taught that these difficult conversations had to be honest and clear, with the goals of care addressed very early in the admission, should a patient decompensate soon after arrival.
A palliative “rapid response team” included a virtual hospitalist, a palliative care nurse practitioner, and a virtual psychiatrist – a team available 24 hours a day to have longer conversations so that clinicians could better tend to their patients when the in-person palliative care service was stretched thin, or at off hours like the middle of the night.
These innovations not only helped serve patients and families better, but also gave hospitalists training and experience in palliative care.
At Roper Hospital, Dr. Stein explained how hospitalists helped improve management of COVID patient flow. Depending on the time of day and the staffing on duty, there could be considerable confusion about where patients should go after the ED, or the COVID progressive unit, or the floor.
Hospitalists helped develop hospitalwide algorithms for escalating and deescalating acuity, Dr. Stein said, providing a “shared mental model for where a patient should go.”
“There are many ways hospitalists can and did rise to meet the unique demands of COVID,” Dr. Singh said, “whether it was innovating a new unit or service or work flow or leading a multidisciplinary team to extend or support other services that may have been strained.”
With intensive care units stretched to their limits – and beyond – during the COVID-19 pandemic, hospitalists became more central than ever in orchestrating the response.
At SHM Converge, the annual conference of the Society of Hospital Medicine, two hospitalists shared how their teams helped to develop new critical care units and strategies for best managing and allocating care to COVID patients in the ICU.
“The pandemic has been a selective pressure on us as a specialty,” said Jason Stein, MD, SFHM, a full-time clinical hospitalist at Roper Hospital, a 332-bed facility in Charleston, S.C.
Dr. Stein explained how hospitalists at Roper helped create the Progressive Care Unit – a negative-pressure unit with 12 high-flow oxygen beds overseen by a hospital medicine team, with the help of a respiratory therapist, pharmacist, and nurses. Patients in this unit had escalating acuity – quickly increasing oxygen needs – or deescalating acuity, such as ICU transfers, Dr. Stein said. Cardiac catheterization space was converted for the unit, which was intended to preserve beds in the hospital ICU for patients needing mechanical ventilation or vasoactive medication.
Interdisciplinary rounds – to assess oxygen and inflammatory marker trends, and run through a COVID care checklist – took place every day at 10 a.m.
“Consistency was the key,” Dr. Stein said.
At Weill Cornell Medical Center in New York, hospitalists helped build the COVID Recovery Unit, which was dedicated to the care of patients coming out of the ICU, said Vishwas Anand Singh, MD, MS, FHM, cochief of hospital medicine at New York Presbyterian–Lower Manhattan Hospital.
“The pandemic created an unprecedented need for critical care, and post-ICU care,” Dr. Singh said. “After extubation, patients remain very complicated and they have unique needs.”
The 30-bed COVID Recovery Unit – converted from a behavioral health unit – was designed to meet those needs. It was staffed by one lead hospitalist, 3 hospitalist physicians, 3 advanced practitioners, about 12 nurses and a neurologist, psychiatrist, and neuropsychologist.
The idea was to integrate medical care with careful attention to rehab and neuropsychological needs, Dr. Singh said. To be in the unit, patients had to be medically stable but with ongoing medical and rehabilitation needs and able to tolerate about half an hour of physical or occupational therapy each day.
The space was set up so that patients could interact with each other as well as staff, and this ability to share their experiences of trauma and recovery “led to an improved sense of psychological well-being and to healing,” according to Dr. Singh. Group therapy and meditation were also held several times a week.
“All this together, we thought we were really meeting the need for a lot of these patients from medical to psychosocial,” he said.
New York Presbyterian––Lower Manhattan Hospital also established a program called ICU Outreach to give hospitalists a “bird’s eye view” of the ICU in order to help move patients from unit to unit for optimized care. One hospitalist acted as a bridge between the ICU, the floors, and the emergency room.
The hospitalist on duty touched based with the ICU each day at 10 a.m., assessed the available beds, compiled a list of patients being discharged, met with all of the hospitalists and individual teams in inpatient and emergency services, and compiled a list of “watchers” – the sickest patients who needed help being managed.
The broad perspective was important, Dr. Singh said.
“We quickly found that each individual team or provider only knew the patients they were caring for, and the ICU Outreach person knew the whole big picture and could put the pieces together,” he said. “They could answer who was next in line for a bed, who benefited from a goals of care discussion, who could be managed on the floor with assistance. And this bridge, having this person fill this role, allowed the intensivists to focus on the patients they had in the unit.”
Palliative care and patient flow
Dr. Singh also described how hospitalists played an important role in palliative care for COVID patients. The hospital medicine team offered hospitalist palliative care services, which included COVIDtalk, a course on communicating about end of life, which helped to expand the pool of palliative care providers. Those trained were taught that these difficult conversations had to be honest and clear, with the goals of care addressed very early in the admission, should a patient decompensate soon after arrival.
A palliative “rapid response team” included a virtual hospitalist, a palliative care nurse practitioner, and a virtual psychiatrist – a team available 24 hours a day to have longer conversations so that clinicians could better tend to their patients when the in-person palliative care service was stretched thin, or at off hours like the middle of the night.
These innovations not only helped serve patients and families better, but also gave hospitalists training and experience in palliative care.
At Roper Hospital, Dr. Stein explained how hospitalists helped improve management of COVID patient flow. Depending on the time of day and the staffing on duty, there could be considerable confusion about where patients should go after the ED, or the COVID progressive unit, or the floor.
Hospitalists helped develop hospitalwide algorithms for escalating and deescalating acuity, Dr. Stein said, providing a “shared mental model for where a patient should go.”
“There are many ways hospitalists can and did rise to meet the unique demands of COVID,” Dr. Singh said, “whether it was innovating a new unit or service or work flow or leading a multidisciplinary team to extend or support other services that may have been strained.”
FROM SHM CONVERGE 2021
COVID-19: One Patient at a Time
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; james.colbert@bcbsma.com.
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; james.colbert@bcbsma.com.
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; james.colbert@bcbsma.com.
Financial disclosures: None.
Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 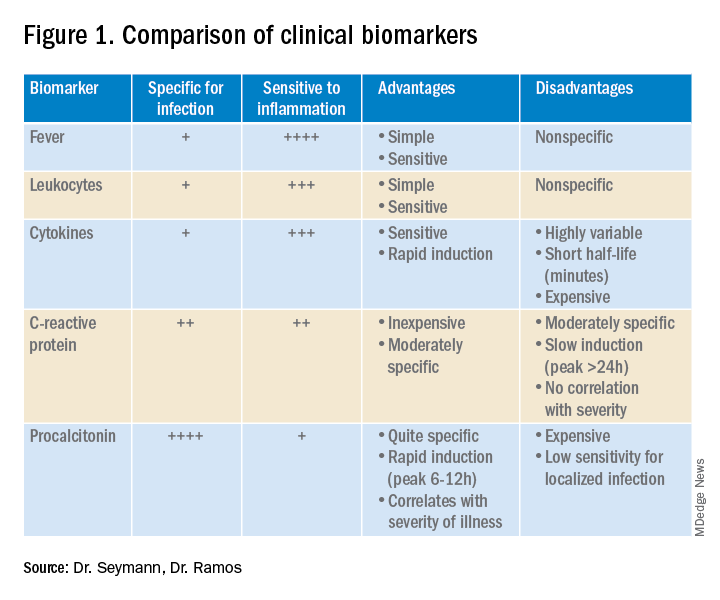
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
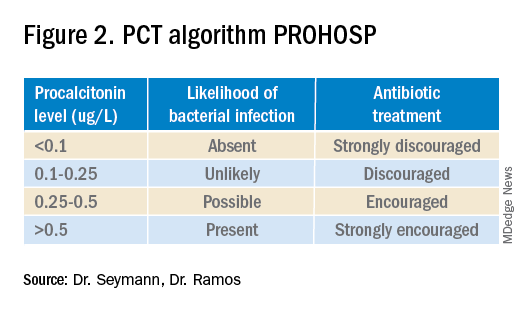
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.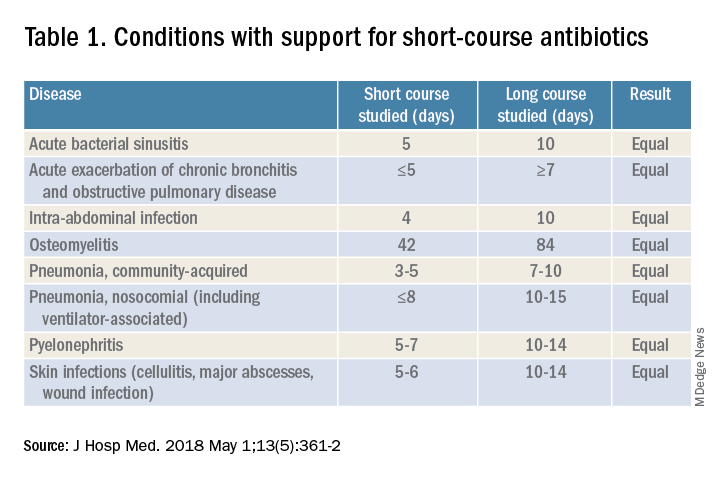
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.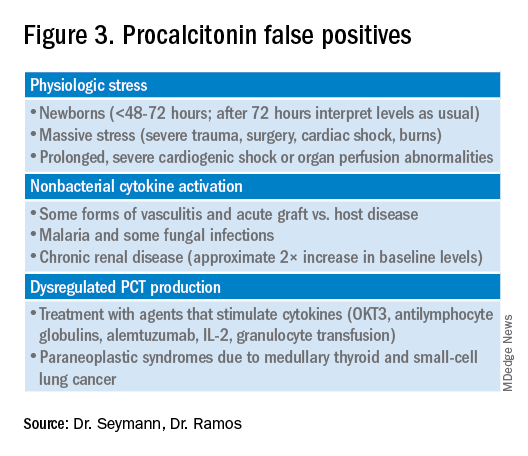
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Evidence or anecdote: Clinical judgment in COVID care
As the COVID-19 pandemic continues and evidence evolves, clinical judgment is the bottom line for clinical care, according to Adarsh Bhimraj, MD, of the Cleveland Clinic, and James Walter, MD, of Northwestern Medicine, Chicago.
In a debate/discussion presented at SHM Converge, the annual conference of the Society of Hospital Medicine, Dr. Bhimraj and Dr. Walter took sides in a friendly debate on the value of remdesivir and tocilizumab for hospitalized COVID-19 patients.
Dr. Bhimraj argued for the use of remdesivir or tocilizumab in patients hospitalized with COVID-19 pneumonia, and Dr. Walter presented the case against their use.
Referendum on remdesivir
The main sources referenced by the presenters regarding remdesivir were the WHO Solidarity Trial (N Engl J Med. 2021 Feb 11. doi: 10.1056/NEJMoa2023184) and the Adaptive Covid-19 Treatment Trial (ACCT) final report (N Engl J Med. 2020 Nov 5. doi: 10.1056/NEJMoa2007764).
“The ‘debate’ is partly artificial,” and meant to illustrate how clinicians can use their own clinical faculties and reasoning to make an informed decision when treating COVID-19 patients, Dr. Bhimraj said.
The ACCT trial compared remdesivir with placebo in patients with severe enough COVID-19 to require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation. The primary outcome in the study was time to recovery, and “the devil is in the details,” Dr. Bhimraj said. The outcomes clinicians should look for in studies are those that matter to patients, such as death, disability, and discomfort, he noted. Disease-oriented endpoints are easier to measure, but not always meaningful for patients, he said. The study showed an average 5-day decrease in illness, “but the fact is that it did not show a mortality benefit,” he noted.
Another large, open-label study of remdesivir across 30 countries showed no survival benefit associated with the drug, compared with standard of care, said Dr. Bhimraj. Patients treated with remdesivir remained in the hospital longer, but Dr. Bhimraj said he believed that was a bias. “I think the physicians kept the patients in the hospital longer to give the treatment rather than the treatments themselves prolonging the treatment duration,” he said.
In conclusion for remdesivir, “the solid data show that there is an early recovery,” he said. “At least for severe disease, even if there is no mortality benefit, there is a role. I argue that, if someone asks if you want to use remdesivir in severe COVID-19 patients, say yes, especially if you value people getting out of the hospital sooner. In a crisis situation, there is a role for remdesivir.”
Dr. Walter discussed the “con” side of using remdesivir. “We can start with a predata hypothesis, but integrate new data about the efficacy into a postdata hypothesis,” he said.
Dr. Walter made several points against the use of remdesivir in hospitalized COVID-19 patients. First, it has not shown any improvement in mortality and may increase the length of hospital stay, he noted.
Data from the ACCT-1 trial and the WHO solidarity trial, showed “no signal of mortality benefit at all,” he said. In addition, the World Health Organization, American College of Physicians, and National Institutes of Health all recommend against remdesivir for patients who require mechanical ventilation or extracorporeal membrane oxygenation, he said. The efficacy when used with steroids remains unclear, and long-term safety data are lacking, he added.
Taking on tocilizumab
Tocilizumab, an anti-inflammatory agent, has demonstrated an impact on several surrogate markers, notably C-reactive protein, temperature, and oxygenation. Dr. Bhimraj said. He reviewed data from eight published studies on the use of tocilizumab in COVID-19 patients.
Arguably, some trials may not have been powered adequately, and in combination, some trials show an effect on clinical deterioration, if not a mortality benefit, he said.
Consequently, in the context of COVID-19, tocilizumab “should be used early in the disease process, especially if steroids are not working,” said Dr. Bhimraj. Despite the limited evidence, “there is a niche population where this might be beneficial,” he said.
By contrast, Dr. Walter took the position of skepticism about the value of tocilizumab for COVID-19 patients.
Notably, decades of research show that tocilizumab has shown no benefit in patients with sepsis or septic shock, or those with acute respiratory distress syndrome, which have similarities to COVID-19 (JAMA. 2020 Sep 3. doi: 10.1001/jama.2020.17052).
He cited a research letter published in JAMA in September 2020, which showed that cytokine levels were in fact lower in critically ill patients with COVID-19, compared with those who had conditions including sepsis with and without ARDS.
Dr. Walter also cited data on the questionable benefit of tocilizumab when used with steroids and the negligible impact on mortality in hospitalized COVID-19 patients seen in the RECOVERY trial.
Limited data mean that therapeutic decisions related to COVID-19 are more nuanced, but they can be made, the presenters agreed.
Ultimately, when trying to decide whether a drug is efficacious, futile, or harmful, “What we have to do is consider the grand totality of the evidence,” Dr. Bhimraj emphasized.
Dr. Bhimraj and Dr. Walter had no relevant financial conflicts to disclose.
As the COVID-19 pandemic continues and evidence evolves, clinical judgment is the bottom line for clinical care, according to Adarsh Bhimraj, MD, of the Cleveland Clinic, and James Walter, MD, of Northwestern Medicine, Chicago.
In a debate/discussion presented at SHM Converge, the annual conference of the Society of Hospital Medicine, Dr. Bhimraj and Dr. Walter took sides in a friendly debate on the value of remdesivir and tocilizumab for hospitalized COVID-19 patients.
Dr. Bhimraj argued for the use of remdesivir or tocilizumab in patients hospitalized with COVID-19 pneumonia, and Dr. Walter presented the case against their use.
Referendum on remdesivir
The main sources referenced by the presenters regarding remdesivir were the WHO Solidarity Trial (N Engl J Med. 2021 Feb 11. doi: 10.1056/NEJMoa2023184) and the Adaptive Covid-19 Treatment Trial (ACCT) final report (N Engl J Med. 2020 Nov 5. doi: 10.1056/NEJMoa2007764).
“The ‘debate’ is partly artificial,” and meant to illustrate how clinicians can use their own clinical faculties and reasoning to make an informed decision when treating COVID-19 patients, Dr. Bhimraj said.
The ACCT trial compared remdesivir with placebo in patients with severe enough COVID-19 to require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation. The primary outcome in the study was time to recovery, and “the devil is in the details,” Dr. Bhimraj said. The outcomes clinicians should look for in studies are those that matter to patients, such as death, disability, and discomfort, he noted. Disease-oriented endpoints are easier to measure, but not always meaningful for patients, he said. The study showed an average 5-day decrease in illness, “but the fact is that it did not show a mortality benefit,” he noted.
Another large, open-label study of remdesivir across 30 countries showed no survival benefit associated with the drug, compared with standard of care, said Dr. Bhimraj. Patients treated with remdesivir remained in the hospital longer, but Dr. Bhimraj said he believed that was a bias. “I think the physicians kept the patients in the hospital longer to give the treatment rather than the treatments themselves prolonging the treatment duration,” he said.
In conclusion for remdesivir, “the solid data show that there is an early recovery,” he said. “At least for severe disease, even if there is no mortality benefit, there is a role. I argue that, if someone asks if you want to use remdesivir in severe COVID-19 patients, say yes, especially if you value people getting out of the hospital sooner. In a crisis situation, there is a role for remdesivir.”
Dr. Walter discussed the “con” side of using remdesivir. “We can start with a predata hypothesis, but integrate new data about the efficacy into a postdata hypothesis,” he said.
Dr. Walter made several points against the use of remdesivir in hospitalized COVID-19 patients. First, it has not shown any improvement in mortality and may increase the length of hospital stay, he noted.
Data from the ACCT-1 trial and the WHO solidarity trial, showed “no signal of mortality benefit at all,” he said. In addition, the World Health Organization, American College of Physicians, and National Institutes of Health all recommend against remdesivir for patients who require mechanical ventilation or extracorporeal membrane oxygenation, he said. The efficacy when used with steroids remains unclear, and long-term safety data are lacking, he added.
Taking on tocilizumab
Tocilizumab, an anti-inflammatory agent, has demonstrated an impact on several surrogate markers, notably C-reactive protein, temperature, and oxygenation. Dr. Bhimraj said. He reviewed data from eight published studies on the use of tocilizumab in COVID-19 patients.
Arguably, some trials may not have been powered adequately, and in combination, some trials show an effect on clinical deterioration, if not a mortality benefit, he said.
Consequently, in the context of COVID-19, tocilizumab “should be used early in the disease process, especially if steroids are not working,” said Dr. Bhimraj. Despite the limited evidence, “there is a niche population where this might be beneficial,” he said.
By contrast, Dr. Walter took the position of skepticism about the value of tocilizumab for COVID-19 patients.
Notably, decades of research show that tocilizumab has shown no benefit in patients with sepsis or septic shock, or those with acute respiratory distress syndrome, which have similarities to COVID-19 (JAMA. 2020 Sep 3. doi: 10.1001/jama.2020.17052).
He cited a research letter published in JAMA in September 2020, which showed that cytokine levels were in fact lower in critically ill patients with COVID-19, compared with those who had conditions including sepsis with and without ARDS.
Dr. Walter also cited data on the questionable benefit of tocilizumab when used with steroids and the negligible impact on mortality in hospitalized COVID-19 patients seen in the RECOVERY trial.
Limited data mean that therapeutic decisions related to COVID-19 are more nuanced, but they can be made, the presenters agreed.
Ultimately, when trying to decide whether a drug is efficacious, futile, or harmful, “What we have to do is consider the grand totality of the evidence,” Dr. Bhimraj emphasized.
Dr. Bhimraj and Dr. Walter had no relevant financial conflicts to disclose.
As the COVID-19 pandemic continues and evidence evolves, clinical judgment is the bottom line for clinical care, according to Adarsh Bhimraj, MD, of the Cleveland Clinic, and James Walter, MD, of Northwestern Medicine, Chicago.
In a debate/discussion presented at SHM Converge, the annual conference of the Society of Hospital Medicine, Dr. Bhimraj and Dr. Walter took sides in a friendly debate on the value of remdesivir and tocilizumab for hospitalized COVID-19 patients.
Dr. Bhimraj argued for the use of remdesivir or tocilizumab in patients hospitalized with COVID-19 pneumonia, and Dr. Walter presented the case against their use.
Referendum on remdesivir
The main sources referenced by the presenters regarding remdesivir were the WHO Solidarity Trial (N Engl J Med. 2021 Feb 11. doi: 10.1056/NEJMoa2023184) and the Adaptive Covid-19 Treatment Trial (ACCT) final report (N Engl J Med. 2020 Nov 5. doi: 10.1056/NEJMoa2007764).
“The ‘debate’ is partly artificial,” and meant to illustrate how clinicians can use their own clinical faculties and reasoning to make an informed decision when treating COVID-19 patients, Dr. Bhimraj said.
The ACCT trial compared remdesivir with placebo in patients with severe enough COVID-19 to require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation. The primary outcome in the study was time to recovery, and “the devil is in the details,” Dr. Bhimraj said. The outcomes clinicians should look for in studies are those that matter to patients, such as death, disability, and discomfort, he noted. Disease-oriented endpoints are easier to measure, but not always meaningful for patients, he said. The study showed an average 5-day decrease in illness, “but the fact is that it did not show a mortality benefit,” he noted.
Another large, open-label study of remdesivir across 30 countries showed no survival benefit associated with the drug, compared with standard of care, said Dr. Bhimraj. Patients treated with remdesivir remained in the hospital longer, but Dr. Bhimraj said he believed that was a bias. “I think the physicians kept the patients in the hospital longer to give the treatment rather than the treatments themselves prolonging the treatment duration,” he said.
In conclusion for remdesivir, “the solid data show that there is an early recovery,” he said. “At least for severe disease, even if there is no mortality benefit, there is a role. I argue that, if someone asks if you want to use remdesivir in severe COVID-19 patients, say yes, especially if you value people getting out of the hospital sooner. In a crisis situation, there is a role for remdesivir.”
Dr. Walter discussed the “con” side of using remdesivir. “We can start with a predata hypothesis, but integrate new data about the efficacy into a postdata hypothesis,” he said.
Dr. Walter made several points against the use of remdesivir in hospitalized COVID-19 patients. First, it has not shown any improvement in mortality and may increase the length of hospital stay, he noted.
Data from the ACCT-1 trial and the WHO solidarity trial, showed “no signal of mortality benefit at all,” he said. In addition, the World Health Organization, American College of Physicians, and National Institutes of Health all recommend against remdesivir for patients who require mechanical ventilation or extracorporeal membrane oxygenation, he said. The efficacy when used with steroids remains unclear, and long-term safety data are lacking, he added.
Taking on tocilizumab
Tocilizumab, an anti-inflammatory agent, has demonstrated an impact on several surrogate markers, notably C-reactive protein, temperature, and oxygenation. Dr. Bhimraj said. He reviewed data from eight published studies on the use of tocilizumab in COVID-19 patients.
Arguably, some trials may not have been powered adequately, and in combination, some trials show an effect on clinical deterioration, if not a mortality benefit, he said.
Consequently, in the context of COVID-19, tocilizumab “should be used early in the disease process, especially if steroids are not working,” said Dr. Bhimraj. Despite the limited evidence, “there is a niche population where this might be beneficial,” he said.
By contrast, Dr. Walter took the position of skepticism about the value of tocilizumab for COVID-19 patients.
Notably, decades of research show that tocilizumab has shown no benefit in patients with sepsis or septic shock, or those with acute respiratory distress syndrome, which have similarities to COVID-19 (JAMA. 2020 Sep 3. doi: 10.1001/jama.2020.17052).
He cited a research letter published in JAMA in September 2020, which showed that cytokine levels were in fact lower in critically ill patients with COVID-19, compared with those who had conditions including sepsis with and without ARDS.
Dr. Walter also cited data on the questionable benefit of tocilizumab when used with steroids and the negligible impact on mortality in hospitalized COVID-19 patients seen in the RECOVERY trial.
Limited data mean that therapeutic decisions related to COVID-19 are more nuanced, but they can be made, the presenters agreed.
Ultimately, when trying to decide whether a drug is efficacious, futile, or harmful, “What we have to do is consider the grand totality of the evidence,” Dr. Bhimraj emphasized.
Dr. Bhimraj and Dr. Walter had no relevant financial conflicts to disclose.
FROM SHM CONVERGE 2021
Short-term oxygen prescriptions lead to inappropriate long-term use
In past posts for this news organization, I’ve railed against the cost of inappropriate prescriptions for oxygen. A recent review recommended against prescribing oxygen for patients with isolated exertional or nocturnal desaturations, and recently published randomized trials found no demonstrable benefit to oxygen use in the absence of resting hypoxemia. a common practice in clinics where I’ve worked. However, oxygen prescriptions at hospital discharge are a far more pernicious cause of wasted resources.
Prescriptions at hospital discharge, sometimes referred to as short-term oxygen therapy (STOT), account for a large proportion of total oxygen use. Past data have shown that the term “STOT” is a misnomer, as most patients provided with oxygen at discharge are never reevaluated and become long-term oxygen users. The high cost of durable medical equipment related to oxygen delivery prompted the American Thoracic Society and American College of Chest Physicians to recommend postdischarge reassessment of oxygen needs in their Choosing Wisely campaign for adult pulmonary medicine.
A recent study published in the Annals of the American Thoracic Society (Ann ATS) highlights the benefits available if we decide to “choose wisely.” The authors studied patients covered by Veterans Affairs and discharged on STOT between 2006 and 2011. Only 43.6% (287/659) had complete reassessment (oxygen testing at rest and with ambulation) within 90 days. Of those, 124 (43.2%) were eligible for discontinuation via Centers for Medicare & Medicaid Services guidelines. A total of 70.7% (466/659) were tested at rest, and only 15.7% (73/466) had resting hypoxemia. If one accepts the results of the recently published Long-Term Oxygen Treatment Trial, this means that 84.3% (393/466) would be eligible for oxygen discontinuation.
The Ann ATS study provides a blueprint for how we might improve these dismal numbers. There were five separate sites reviewed in their paper. At one site, reassessment occurred in 78.5% of STOT patients and 100% had oxygen discontinued when appropriate. What was their secret? An automatic alert system and a dedicated clinic, coordinator, and respiratory therapist. Also, among the 124 patients who had a full reassessment and no longer qualified for oxygen, 86.3% had it discontinued.
There are countless reasons why STOT is common, but discontinuation is not. Most COPD exacerbations are managed by nonpulmonologists on general medicine wards prior to discharge. In my experience, these physicians are reluctant to release a patient with exertional hypoxia without STOT. They also assume that the pulmonary clinic will do its job during the obligatory outpatient follow-up appointment they schedule with us. At the follow-up, the patient and physician are reluctant to stop therapy because of psychological dependence and therapeutic overconfidence, respectively.
In summary, STOT following hospitalization comprises the majority of all oxygen prescriptions. Historically, the United States provides far more oxygen than other developed countries, and only CMS reimbursement changes have bent the “overprescription” curve. The Ann ATS study shows that a well-designed program at the hospital level can put oxygen decisions back in the hands of providers.
Let’s “choose wisely” and follow what works, or we’ll have only ourselves to blame when reimbursement decisions are taken out of our hands.
A version of this article first appeared on Medscape.com.
In past posts for this news organization, I’ve railed against the cost of inappropriate prescriptions for oxygen. A recent review recommended against prescribing oxygen for patients with isolated exertional or nocturnal desaturations, and recently published randomized trials found no demonstrable benefit to oxygen use in the absence of resting hypoxemia. a common practice in clinics where I’ve worked. However, oxygen prescriptions at hospital discharge are a far more pernicious cause of wasted resources.
Prescriptions at hospital discharge, sometimes referred to as short-term oxygen therapy (STOT), account for a large proportion of total oxygen use. Past data have shown that the term “STOT” is a misnomer, as most patients provided with oxygen at discharge are never reevaluated and become long-term oxygen users. The high cost of durable medical equipment related to oxygen delivery prompted the American Thoracic Society and American College of Chest Physicians to recommend postdischarge reassessment of oxygen needs in their Choosing Wisely campaign for adult pulmonary medicine.
A recent study published in the Annals of the American Thoracic Society (Ann ATS) highlights the benefits available if we decide to “choose wisely.” The authors studied patients covered by Veterans Affairs and discharged on STOT between 2006 and 2011. Only 43.6% (287/659) had complete reassessment (oxygen testing at rest and with ambulation) within 90 days. Of those, 124 (43.2%) were eligible for discontinuation via Centers for Medicare & Medicaid Services guidelines. A total of 70.7% (466/659) were tested at rest, and only 15.7% (73/466) had resting hypoxemia. If one accepts the results of the recently published Long-Term Oxygen Treatment Trial, this means that 84.3% (393/466) would be eligible for oxygen discontinuation.
The Ann ATS study provides a blueprint for how we might improve these dismal numbers. There were five separate sites reviewed in their paper. At one site, reassessment occurred in 78.5% of STOT patients and 100% had oxygen discontinued when appropriate. What was their secret? An automatic alert system and a dedicated clinic, coordinator, and respiratory therapist. Also, among the 124 patients who had a full reassessment and no longer qualified for oxygen, 86.3% had it discontinued.
There are countless reasons why STOT is common, but discontinuation is not. Most COPD exacerbations are managed by nonpulmonologists on general medicine wards prior to discharge. In my experience, these physicians are reluctant to release a patient with exertional hypoxia without STOT. They also assume that the pulmonary clinic will do its job during the obligatory outpatient follow-up appointment they schedule with us. At the follow-up, the patient and physician are reluctant to stop therapy because of psychological dependence and therapeutic overconfidence, respectively.
In summary, STOT following hospitalization comprises the majority of all oxygen prescriptions. Historically, the United States provides far more oxygen than other developed countries, and only CMS reimbursement changes have bent the “overprescription” curve. The Ann ATS study shows that a well-designed program at the hospital level can put oxygen decisions back in the hands of providers.
Let’s “choose wisely” and follow what works, or we’ll have only ourselves to blame when reimbursement decisions are taken out of our hands.
A version of this article first appeared on Medscape.com.
In past posts for this news organization, I’ve railed against the cost of inappropriate prescriptions for oxygen. A recent review recommended against prescribing oxygen for patients with isolated exertional or nocturnal desaturations, and recently published randomized trials found no demonstrable benefit to oxygen use in the absence of resting hypoxemia. a common practice in clinics where I’ve worked. However, oxygen prescriptions at hospital discharge are a far more pernicious cause of wasted resources.
Prescriptions at hospital discharge, sometimes referred to as short-term oxygen therapy (STOT), account for a large proportion of total oxygen use. Past data have shown that the term “STOT” is a misnomer, as most patients provided with oxygen at discharge are never reevaluated and become long-term oxygen users. The high cost of durable medical equipment related to oxygen delivery prompted the American Thoracic Society and American College of Chest Physicians to recommend postdischarge reassessment of oxygen needs in their Choosing Wisely campaign for adult pulmonary medicine.
A recent study published in the Annals of the American Thoracic Society (Ann ATS) highlights the benefits available if we decide to “choose wisely.” The authors studied patients covered by Veterans Affairs and discharged on STOT between 2006 and 2011. Only 43.6% (287/659) had complete reassessment (oxygen testing at rest and with ambulation) within 90 days. Of those, 124 (43.2%) were eligible for discontinuation via Centers for Medicare & Medicaid Services guidelines. A total of 70.7% (466/659) were tested at rest, and only 15.7% (73/466) had resting hypoxemia. If one accepts the results of the recently published Long-Term Oxygen Treatment Trial, this means that 84.3% (393/466) would be eligible for oxygen discontinuation.
The Ann ATS study provides a blueprint for how we might improve these dismal numbers. There were five separate sites reviewed in their paper. At one site, reassessment occurred in 78.5% of STOT patients and 100% had oxygen discontinued when appropriate. What was their secret? An automatic alert system and a dedicated clinic, coordinator, and respiratory therapist. Also, among the 124 patients who had a full reassessment and no longer qualified for oxygen, 86.3% had it discontinued.
There are countless reasons why STOT is common, but discontinuation is not. Most COPD exacerbations are managed by nonpulmonologists on general medicine wards prior to discharge. In my experience, these physicians are reluctant to release a patient with exertional hypoxia without STOT. They also assume that the pulmonary clinic will do its job during the obligatory outpatient follow-up appointment they schedule with us. At the follow-up, the patient and physician are reluctant to stop therapy because of psychological dependence and therapeutic overconfidence, respectively.
In summary, STOT following hospitalization comprises the majority of all oxygen prescriptions. Historically, the United States provides far more oxygen than other developed countries, and only CMS reimbursement changes have bent the “overprescription” curve. The Ann ATS study shows that a well-designed program at the hospital level can put oxygen decisions back in the hands of providers.
Let’s “choose wisely” and follow what works, or we’ll have only ourselves to blame when reimbursement decisions are taken out of our hands.
A version of this article first appeared on Medscape.com.
New guidelines advise expanded use of high-flow nasal oxygen for patients with ARDS
Hospitalized patients with acute respiratory failure can benefit from high-flow nasal oxygen in certain settings, according to a new clinical guideline from the American College of Physicians.
High-flow nasal oxygen (HFNO) has demonstrated advantages including improved oxygenation and ventilation, wrote Arianne K. Baldomero, MD, of Minneapolis Veterans Affairs Health Care System and the University of Minnesota, Minneapolis, and colleagues. “However, the comparative benefits and harms of HFNO in clinical outcomes, including mortality, intubation, hospital length of stay, patient comfort, clearance of airway secretions, and reduced work of breathing are not well known.”
In the guideline, published in Annals of Internal Medicine, the authors recommend the use of high-flow nasal oxygen in hospitalized patients for initial or postextubation management of acute respiratory failure. The target population includes those patients treated in hospital wards, EDs, intermediate/step-down units, and ICUs.
Use of HFNO therapy as a form of noninvasive respiratory support for hospitalized patients has increased in recent years. The treatment involves delivering warm, humidified oxygen via nasal cannula at a flow level higher than the patient’s inspiratory flow.
Potential benefits of HFNO include greater patient comfort, improved compliance, and psychological benefits, according to the authors. HFNO also can be used as respiratory support in critically ill patients for a number of indications including respiratory failure or support post extubation; however, treatment of patients with COVID-19 and related conditions were not considered in the guideline.
The guideline was based on evidence comparing HFNO with conventional oxygen therapy (COT) and noninvasive ventilation (NIV). The authors reviewed 29 randomized, controlled trials that showed clinically meaningful outcomes in HFNO patients, as well as similar rates of, or reductions in, mortality, intubations, and hospital-acquired pneumonia, and increased reports of patient comfort. Data also supported the safety of HFNO with few, if any, contraindications other than problems with fitting the nasal cannula.
Across several trials comparing HFNO and NIV for initial management of acute respiratory failure, HFNO reduced all-cause mortality, intubation, and hospital-acquired pneumonia, although the authors categorized the results as “low-certainty evidence.” HFNO was not more effective than NIV for postextubation management. Based trials comparing HFNO and COT for postextubation management, the authors concluded that HFNO may reduce rates of reintubation and improve patient comfort, also with low-certainty evidence.
The research was limited by a lack of studies comparing HFNO with NIV or COT for acute respiratory failure in patients who were post lung transplantation, or for those with pulmonary embolism, pulmonary arterial hypertension, or asthma, the authors said. Other limitations included the variation in study design, study populations, and treatment protocols across the included studies. Additional research is needed to better identify the patients most likely to benefit from HFNO, according to type of acute respiratory failure.
Despite these limitations, the results support the guideline recommendation for HFNO in cases of acute respiratory failure and postextubation management. However, “broad applicability, including required clinician and health system experience and resource use, remains unknown,” the authors concluded.
Research catches up with practice
The guidelines are important at this time because “the medical literature over the past 3-4 years is catching up to what hospitalists, pulmonologists, and critical care specialists have been doing clinically over the past 6-8 years with perceived better results, Jacqueline W. Fincher, MD, MACP, President of the American College of Physicians, said in an interview.
“HFNO has been used to a varying degree over the last 6-8 years by physicians with much-perceived improved benefit in patients who are hypoxemic on usual noninvasive therapy or conventional oxygen therapy with the impending need for intubation or post extubation,” Dr. Fincher said. “During the COVID pandemic particularly with the attack on the respiratory system with COVID pneumonia and frequently associated ARDS [acute respiratory distress syndrome], the use of HFNO has been enormously helpful in trying to keep patients well oxygenated without having to intubate or reintubate them.
“We now have the medical literature that supports what has been seen clinically to make the recommendations and guidelines based on the scientific evidence,” Dr. Fincher added. “If we can avoid intubation associated with the patient being sedated, unable to eat, talk, or meaningfully participate in their care or get the patient off the ventilator sooner for the same reasons, then we have significantly improved the quality of their care, decreased their risk of infection, decreased their days in the ICU and the hospital, we will have succeeded in providing the best care possible. The availability of HFNO, with much greater comfort to the patient than being intubated, is a great tool in the toolbox of respiratory care.”
Dr. Fincher said she was not surprised by any of the recommendations. “We knew the use of HFNO helped but we were surprised by the evidence of the degree to which it is enormously helpful to patients.
“The good news is that HFNO is readily available at most hospitals, but it really requires an intensive care unit and a team of physicians, nurses, and respiratory therapists to be familiar with its use and work closely together to monitor the patient for significant changes in their respiratory status to titrate therapy,” she noted.
Looking ahead, some areas in need of more research that might impact updates to the guidelines include “What are some areas in need of more research that might impact future updates to these guidelines? Specifics on whether initiating HFNO earlier in the course of the patient’s hypoxemic illness is better or worse, as well as the use of HFNO outside of the ICU setting,” Dr. Fincher said. “The needed monitoring of the patient to know whether their respiratory status was deteriorating and how fast would be critical along with the specific indications for titration of the HFNO.”
The evidence review was commissioned and funded by the ACP. The data come from work supported by and conducted at the Minneapolis VA Health Care System. Lead author Dr. Baldomero was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences.
Hospitalized patients with acute respiratory failure can benefit from high-flow nasal oxygen in certain settings, according to a new clinical guideline from the American College of Physicians.
High-flow nasal oxygen (HFNO) has demonstrated advantages including improved oxygenation and ventilation, wrote Arianne K. Baldomero, MD, of Minneapolis Veterans Affairs Health Care System and the University of Minnesota, Minneapolis, and colleagues. “However, the comparative benefits and harms of HFNO in clinical outcomes, including mortality, intubation, hospital length of stay, patient comfort, clearance of airway secretions, and reduced work of breathing are not well known.”
In the guideline, published in Annals of Internal Medicine, the authors recommend the use of high-flow nasal oxygen in hospitalized patients for initial or postextubation management of acute respiratory failure. The target population includes those patients treated in hospital wards, EDs, intermediate/step-down units, and ICUs.
Use of HFNO therapy as a form of noninvasive respiratory support for hospitalized patients has increased in recent years. The treatment involves delivering warm, humidified oxygen via nasal cannula at a flow level higher than the patient’s inspiratory flow.
Potential benefits of HFNO include greater patient comfort, improved compliance, and psychological benefits, according to the authors. HFNO also can be used as respiratory support in critically ill patients for a number of indications including respiratory failure or support post extubation; however, treatment of patients with COVID-19 and related conditions were not considered in the guideline.
The guideline was based on evidence comparing HFNO with conventional oxygen therapy (COT) and noninvasive ventilation (NIV). The authors reviewed 29 randomized, controlled trials that showed clinically meaningful outcomes in HFNO patients, as well as similar rates of, or reductions in, mortality, intubations, and hospital-acquired pneumonia, and increased reports of patient comfort. Data also supported the safety of HFNO with few, if any, contraindications other than problems with fitting the nasal cannula.
Across several trials comparing HFNO and NIV for initial management of acute respiratory failure, HFNO reduced all-cause mortality, intubation, and hospital-acquired pneumonia, although the authors categorized the results as “low-certainty evidence.” HFNO was not more effective than NIV for postextubation management. Based trials comparing HFNO and COT for postextubation management, the authors concluded that HFNO may reduce rates of reintubation and improve patient comfort, also with low-certainty evidence.
The research was limited by a lack of studies comparing HFNO with NIV or COT for acute respiratory failure in patients who were post lung transplantation, or for those with pulmonary embolism, pulmonary arterial hypertension, or asthma, the authors said. Other limitations included the variation in study design, study populations, and treatment protocols across the included studies. Additional research is needed to better identify the patients most likely to benefit from HFNO, according to type of acute respiratory failure.
Despite these limitations, the results support the guideline recommendation for HFNO in cases of acute respiratory failure and postextubation management. However, “broad applicability, including required clinician and health system experience and resource use, remains unknown,” the authors concluded.
Research catches up with practice
The guidelines are important at this time because “the medical literature over the past 3-4 years is catching up to what hospitalists, pulmonologists, and critical care specialists have been doing clinically over the past 6-8 years with perceived better results, Jacqueline W. Fincher, MD, MACP, President of the American College of Physicians, said in an interview.
“HFNO has been used to a varying degree over the last 6-8 years by physicians with much-perceived improved benefit in patients who are hypoxemic on usual noninvasive therapy or conventional oxygen therapy with the impending need for intubation or post extubation,” Dr. Fincher said. “During the COVID pandemic particularly with the attack on the respiratory system with COVID pneumonia and frequently associated ARDS [acute respiratory distress syndrome], the use of HFNO has been enormously helpful in trying to keep patients well oxygenated without having to intubate or reintubate them.
“We now have the medical literature that supports what has been seen clinically to make the recommendations and guidelines based on the scientific evidence,” Dr. Fincher added. “If we can avoid intubation associated with the patient being sedated, unable to eat, talk, or meaningfully participate in their care or get the patient off the ventilator sooner for the same reasons, then we have significantly improved the quality of their care, decreased their risk of infection, decreased their days in the ICU and the hospital, we will have succeeded in providing the best care possible. The availability of HFNO, with much greater comfort to the patient than being intubated, is a great tool in the toolbox of respiratory care.”
Dr. Fincher said she was not surprised by any of the recommendations. “We knew the use of HFNO helped but we were surprised by the evidence of the degree to which it is enormously helpful to patients.
“The good news is that HFNO is readily available at most hospitals, but it really requires an intensive care unit and a team of physicians, nurses, and respiratory therapists to be familiar with its use and work closely together to monitor the patient for significant changes in their respiratory status to titrate therapy,” she noted.
Looking ahead, some areas in need of more research that might impact updates to the guidelines include “What are some areas in need of more research that might impact future updates to these guidelines? Specifics on whether initiating HFNO earlier in the course of the patient’s hypoxemic illness is better or worse, as well as the use of HFNO outside of the ICU setting,” Dr. Fincher said. “The needed monitoring of the patient to know whether their respiratory status was deteriorating and how fast would be critical along with the specific indications for titration of the HFNO.”
The evidence review was commissioned and funded by the ACP. The data come from work supported by and conducted at the Minneapolis VA Health Care System. Lead author Dr. Baldomero was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences.
Hospitalized patients with acute respiratory failure can benefit from high-flow nasal oxygen in certain settings, according to a new clinical guideline from the American College of Physicians.
High-flow nasal oxygen (HFNO) has demonstrated advantages including improved oxygenation and ventilation, wrote Arianne K. Baldomero, MD, of Minneapolis Veterans Affairs Health Care System and the University of Minnesota, Minneapolis, and colleagues. “However, the comparative benefits and harms of HFNO in clinical outcomes, including mortality, intubation, hospital length of stay, patient comfort, clearance of airway secretions, and reduced work of breathing are not well known.”
In the guideline, published in Annals of Internal Medicine, the authors recommend the use of high-flow nasal oxygen in hospitalized patients for initial or postextubation management of acute respiratory failure. The target population includes those patients treated in hospital wards, EDs, intermediate/step-down units, and ICUs.
Use of HFNO therapy as a form of noninvasive respiratory support for hospitalized patients has increased in recent years. The treatment involves delivering warm, humidified oxygen via nasal cannula at a flow level higher than the patient’s inspiratory flow.
Potential benefits of HFNO include greater patient comfort, improved compliance, and psychological benefits, according to the authors. HFNO also can be used as respiratory support in critically ill patients for a number of indications including respiratory failure or support post extubation; however, treatment of patients with COVID-19 and related conditions were not considered in the guideline.
The guideline was based on evidence comparing HFNO with conventional oxygen therapy (COT) and noninvasive ventilation (NIV). The authors reviewed 29 randomized, controlled trials that showed clinically meaningful outcomes in HFNO patients, as well as similar rates of, or reductions in, mortality, intubations, and hospital-acquired pneumonia, and increased reports of patient comfort. Data also supported the safety of HFNO with few, if any, contraindications other than problems with fitting the nasal cannula.
Across several trials comparing HFNO and NIV for initial management of acute respiratory failure, HFNO reduced all-cause mortality, intubation, and hospital-acquired pneumonia, although the authors categorized the results as “low-certainty evidence.” HFNO was not more effective than NIV for postextubation management. Based trials comparing HFNO and COT for postextubation management, the authors concluded that HFNO may reduce rates of reintubation and improve patient comfort, also with low-certainty evidence.
The research was limited by a lack of studies comparing HFNO with NIV or COT for acute respiratory failure in patients who were post lung transplantation, or for those with pulmonary embolism, pulmonary arterial hypertension, or asthma, the authors said. Other limitations included the variation in study design, study populations, and treatment protocols across the included studies. Additional research is needed to better identify the patients most likely to benefit from HFNO, according to type of acute respiratory failure.
Despite these limitations, the results support the guideline recommendation for HFNO in cases of acute respiratory failure and postextubation management. However, “broad applicability, including required clinician and health system experience and resource use, remains unknown,” the authors concluded.
Research catches up with practice
The guidelines are important at this time because “the medical literature over the past 3-4 years is catching up to what hospitalists, pulmonologists, and critical care specialists have been doing clinically over the past 6-8 years with perceived better results, Jacqueline W. Fincher, MD, MACP, President of the American College of Physicians, said in an interview.
“HFNO has been used to a varying degree over the last 6-8 years by physicians with much-perceived improved benefit in patients who are hypoxemic on usual noninvasive therapy or conventional oxygen therapy with the impending need for intubation or post extubation,” Dr. Fincher said. “During the COVID pandemic particularly with the attack on the respiratory system with COVID pneumonia and frequently associated ARDS [acute respiratory distress syndrome], the use of HFNO has been enormously helpful in trying to keep patients well oxygenated without having to intubate or reintubate them.
“We now have the medical literature that supports what has been seen clinically to make the recommendations and guidelines based on the scientific evidence,” Dr. Fincher added. “If we can avoid intubation associated with the patient being sedated, unable to eat, talk, or meaningfully participate in their care or get the patient off the ventilator sooner for the same reasons, then we have significantly improved the quality of their care, decreased their risk of infection, decreased their days in the ICU and the hospital, we will have succeeded in providing the best care possible. The availability of HFNO, with much greater comfort to the patient than being intubated, is a great tool in the toolbox of respiratory care.”
Dr. Fincher said she was not surprised by any of the recommendations. “We knew the use of HFNO helped but we were surprised by the evidence of the degree to which it is enormously helpful to patients.
“The good news is that HFNO is readily available at most hospitals, but it really requires an intensive care unit and a team of physicians, nurses, and respiratory therapists to be familiar with its use and work closely together to monitor the patient for significant changes in their respiratory status to titrate therapy,” she noted.
Looking ahead, some areas in need of more research that might impact updates to the guidelines include “What are some areas in need of more research that might impact future updates to these guidelines? Specifics on whether initiating HFNO earlier in the course of the patient’s hypoxemic illness is better or worse, as well as the use of HFNO outside of the ICU setting,” Dr. Fincher said. “The needed monitoring of the patient to know whether their respiratory status was deteriorating and how fast would be critical along with the specific indications for titration of the HFNO.”
The evidence review was commissioned and funded by the ACP. The data come from work supported by and conducted at the Minneapolis VA Health Care System. Lead author Dr. Baldomero was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences.
FROM THE ANNALS OF INTERNAL MEDICINE
Early palliative care consultation in the medical ICU
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Reclaiming patient-centered care from the grip of COVID-19
Over a year has passed since the first case of COVID-19 was reported in the United States, with over 114 million cases now reported worldwide, and over 2.5 million deaths at the time of this writing (Dong E, et al. Lancet Infect Dis. doi: 10.1016/S1473-3099[20]30120-1). While our vaccination efforts here in the United States have provided a much-needed glimmer of hope, it has been bittersweet, as we recently surpassed the grim milestone of 500,000 COVID-19-related deaths.
The infectious nature of SARS-CoV-2, coupled with the lack of adequate PPE early in the pandemic, led to radical changes in most hospital visitor policies. Rather than welcoming families into the care setting as we have been accustomed, we were forced to restrict access. While well-intentioned, the impact of this on patients, their families – and as we later learned, ourselves – has been devastating. Patients found themselves alone in an unfamiliar environment, infected with a disease there was no effective treatment for, hearing dismal news regarding inpatient and ICU mortality rates on news networks, and families could not see for themselves how their loved ones were progressing in their hospital course.
The impact on patient-centered care
The impact of this pandemic on patients and health care providers alike cannot be overstated. Arguably, one of the greatest challenges created by COVID-19 has been its direct assault on the core values of patient-centered care that we have spent decades striving to promote and embody.
Since its identification as a quality gap by the Institute of Medicine in 2001, the definition of patient-centered care has been tweaked over the past 20 years (Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001). Most frameworks include the active participation of patients and their families as part of the health care team, encouraging and facilitating the presence of family members in the care setting, and focusing on patients’ physical comfort and emotional well-being as fundamental tenets of patient centeredness (NEJM Catalyst: What is Patient-Centered Care? Explore the definition, benefits, and examples of patient-centered care. How does patient-centered care translate to new delivery models? January 1,2017).
Families, the “F” in the ABCDEF Bundle, have been recognized as an integral part of care in the ICU setting (Ely EW. Crit Care Med. 2017;45[2]:321). While engagement of family members began with our recognition of their role in emotionally supporting patients and efforts to improve communication, we have also seen the impact of family participation on reducing ICU delirium through frequent re-orientation and encouragement of early mobility (McKenzie J, et al. Australas J Ageing. 2020;39:21). In fact, a recent study has suggested that family members could play an even more active role in detecting and assessing ICU delirium using objective assessment tools (Fiest K, et al. Crit Care Med. 2020;48[7]:954). Post-ICU PTSD has been well described in both ICU survivors as well as in their family members, with evidence that family participation in care of patients during their ICU stay leads to its reduction (Amass TH, et al. Crit Care Med. 2020[Feb];48[2]:176).
The emotional toll
Comforting patients and families in times of distress and suffering is something that comes naturally to many in critical care, and our training further improves our ability to do this effectively. No amount of training, however, could have prepared us for the degree and volume of suffering we bore witness to this past year and the resulting moral injury many are still dealing with. We were present for families’ most intimate moments, holding phones and tablets up to patients so their families could say their goodbyes, listening to the “I love yous,” “I’ll miss yous,” “I’m sorrys,” and “Please don’t gos.” Nurses held patients’ hands as they took their last breaths so they wouldn’t die alone and worked to move husbands and wives into the same room so they could be together in their final moments. Entrenched in each of our identities is the role of healer, and we found ourselves questioning our effectiveness in rising to meet suffering on a scale we had never seen before. Little did we understand that while our paradigms were reinforcing the benefits of patient-centered care for patients and their families, that framework was also serving to facilitate our role as healers – that without it, we all suffer.
Rising to the challenge
These unprecedented circumstances led to creative efforts to bridge some of these barriers. Health systems created photo lanyards that providers wore over their PPE so patients could identify their health care team and connect with them on a more human level. Video conferencing technology was brought to the patient bedside using smartphones and tablets to assist them in communicating with their families. Doctors and nurses coordinated multiple calls throughout the day to ensure families felt included in the care plans and were always abreast of any new developments.
All these initiatives were our way of attempting to alleviate some of the suffering we were witnessing, and in some ways felt complicit in. It is in hindsight that we can look back and question if we could have done things differently. We treated family as visitors, when in fact, they are fundamental members of the care team who play an active and critical role in patient care. This was, in part, driven by national unpreparedness when it came to PPE supplies, in addition to misinformation and inconsistent messaging early in the pandemic with regards to the mechanism of transmission of disease from various health organizations. While we did our best given the circumstances, we must not allow this experience to lead us away from the tenets we know to be essential to patient, family, and health care provider well-being.
All in health care met the call to action – nurses, physicians, advanced practice providers, respiratory therapists, nutritionists, pharmacists, physical therapists, patient transporters, environmental service workers, and all others who kept our hospitals and patient care facilities open through this pandemic and embarked on what amounted to a collective, global, ongoing “code-blue alert,” resuscitating patient after patient, hotspot after hotspot, region after region, and country after country. We expanded hospital bed capacities, created ICU beds where there were none, developed novel process protocols, and learned in real time what seemed to help (or not) in treating this novel disease, all while participating in incredible international scientific collaboration and information sharing that has contributed in getting the collective “us” through this first year of the pandemic. We did what we were trained and called to do.
Preparing for the future
There will inevitably be another public health crisis, and we must advocate for better preparedness next time, insisting on overall stronger public health systems and pandemic preparedness. We must address our PPE stores and supply chains. We must have disaster preparedness plans that go beyond the scope of mass casualty events and bioterrorism. Beyond physical recovery, we must tend to the factors that impact patients’ long-term recovery, with attention to emotional and psychological well-being. We must advocate for all of this now, while the memories are fresh and before the impact of this collective suffering begins to fade. It can never again be acceptable to exclude families from the health care setting. We must advocate for our patients and for the resources, systems, processes, and support that will allow us to do better.
Dr. Hegab is Associate Director, Pulmonary Hypertension Program, Medical Director, Pulmonary Embolism Response Team, Division of Pulmonary and Critical Care Medicine, Henry Ford Hospital; and Assistant Professor, Wayne State University School of Medicine, Detroit.
Over a year has passed since the first case of COVID-19 was reported in the United States, with over 114 million cases now reported worldwide, and over 2.5 million deaths at the time of this writing (Dong E, et al. Lancet Infect Dis. doi: 10.1016/S1473-3099[20]30120-1). While our vaccination efforts here in the United States have provided a much-needed glimmer of hope, it has been bittersweet, as we recently surpassed the grim milestone of 500,000 COVID-19-related deaths.
The infectious nature of SARS-CoV-2, coupled with the lack of adequate PPE early in the pandemic, led to radical changes in most hospital visitor policies. Rather than welcoming families into the care setting as we have been accustomed, we were forced to restrict access. While well-intentioned, the impact of this on patients, their families – and as we later learned, ourselves – has been devastating. Patients found themselves alone in an unfamiliar environment, infected with a disease there was no effective treatment for, hearing dismal news regarding inpatient and ICU mortality rates on news networks, and families could not see for themselves how their loved ones were progressing in their hospital course.
The impact on patient-centered care
The impact of this pandemic on patients and health care providers alike cannot be overstated. Arguably, one of the greatest challenges created by COVID-19 has been its direct assault on the core values of patient-centered care that we have spent decades striving to promote and embody.
Since its identification as a quality gap by the Institute of Medicine in 2001, the definition of patient-centered care has been tweaked over the past 20 years (Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001). Most frameworks include the active participation of patients and their families as part of the health care team, encouraging and facilitating the presence of family members in the care setting, and focusing on patients’ physical comfort and emotional well-being as fundamental tenets of patient centeredness (NEJM Catalyst: What is Patient-Centered Care? Explore the definition, benefits, and examples of patient-centered care. How does patient-centered care translate to new delivery models? January 1,2017).
Families, the “F” in the ABCDEF Bundle, have been recognized as an integral part of care in the ICU setting (Ely EW. Crit Care Med. 2017;45[2]:321). While engagement of family members began with our recognition of their role in emotionally supporting patients and efforts to improve communication, we have also seen the impact of family participation on reducing ICU delirium through frequent re-orientation and encouragement of early mobility (McKenzie J, et al. Australas J Ageing. 2020;39:21). In fact, a recent study has suggested that family members could play an even more active role in detecting and assessing ICU delirium using objective assessment tools (Fiest K, et al. Crit Care Med. 2020;48[7]:954). Post-ICU PTSD has been well described in both ICU survivors as well as in their family members, with evidence that family participation in care of patients during their ICU stay leads to its reduction (Amass TH, et al. Crit Care Med. 2020[Feb];48[2]:176).
The emotional toll
Comforting patients and families in times of distress and suffering is something that comes naturally to many in critical care, and our training further improves our ability to do this effectively. No amount of training, however, could have prepared us for the degree and volume of suffering we bore witness to this past year and the resulting moral injury many are still dealing with. We were present for families’ most intimate moments, holding phones and tablets up to patients so their families could say their goodbyes, listening to the “I love yous,” “I’ll miss yous,” “I’m sorrys,” and “Please don’t gos.” Nurses held patients’ hands as they took their last breaths so they wouldn’t die alone and worked to move husbands and wives into the same room so they could be together in their final moments. Entrenched in each of our identities is the role of healer, and we found ourselves questioning our effectiveness in rising to meet suffering on a scale we had never seen before. Little did we understand that while our paradigms were reinforcing the benefits of patient-centered care for patients and their families, that framework was also serving to facilitate our role as healers – that without it, we all suffer.
Rising to the challenge
These unprecedented circumstances led to creative efforts to bridge some of these barriers. Health systems created photo lanyards that providers wore over their PPE so patients could identify their health care team and connect with them on a more human level. Video conferencing technology was brought to the patient bedside using smartphones and tablets to assist them in communicating with their families. Doctors and nurses coordinated multiple calls throughout the day to ensure families felt included in the care plans and were always abreast of any new developments.
All these initiatives were our way of attempting to alleviate some of the suffering we were witnessing, and in some ways felt complicit in. It is in hindsight that we can look back and question if we could have done things differently. We treated family as visitors, when in fact, they are fundamental members of the care team who play an active and critical role in patient care. This was, in part, driven by national unpreparedness when it came to PPE supplies, in addition to misinformation and inconsistent messaging early in the pandemic with regards to the mechanism of transmission of disease from various health organizations. While we did our best given the circumstances, we must not allow this experience to lead us away from the tenets we know to be essential to patient, family, and health care provider well-being.
All in health care met the call to action – nurses, physicians, advanced practice providers, respiratory therapists, nutritionists, pharmacists, physical therapists, patient transporters, environmental service workers, and all others who kept our hospitals and patient care facilities open through this pandemic and embarked on what amounted to a collective, global, ongoing “code-blue alert,” resuscitating patient after patient, hotspot after hotspot, region after region, and country after country. We expanded hospital bed capacities, created ICU beds where there were none, developed novel process protocols, and learned in real time what seemed to help (or not) in treating this novel disease, all while participating in incredible international scientific collaboration and information sharing that has contributed in getting the collective “us” through this first year of the pandemic. We did what we were trained and called to do.
Preparing for the future
There will inevitably be another public health crisis, and we must advocate for better preparedness next time, insisting on overall stronger public health systems and pandemic preparedness. We must address our PPE stores and supply chains. We must have disaster preparedness plans that go beyond the scope of mass casualty events and bioterrorism. Beyond physical recovery, we must tend to the factors that impact patients’ long-term recovery, with attention to emotional and psychological well-being. We must advocate for all of this now, while the memories are fresh and before the impact of this collective suffering begins to fade. It can never again be acceptable to exclude families from the health care setting. We must advocate for our patients and for the resources, systems, processes, and support that will allow us to do better.
Dr. Hegab is Associate Director, Pulmonary Hypertension Program, Medical Director, Pulmonary Embolism Response Team, Division of Pulmonary and Critical Care Medicine, Henry Ford Hospital; and Assistant Professor, Wayne State University School of Medicine, Detroit.
Over a year has passed since the first case of COVID-19 was reported in the United States, with over 114 million cases now reported worldwide, and over 2.5 million deaths at the time of this writing (Dong E, et al. Lancet Infect Dis. doi: 10.1016/S1473-3099[20]30120-1). While our vaccination efforts here in the United States have provided a much-needed glimmer of hope, it has been bittersweet, as we recently surpassed the grim milestone of 500,000 COVID-19-related deaths.
The infectious nature of SARS-CoV-2, coupled with the lack of adequate PPE early in the pandemic, led to radical changes in most hospital visitor policies. Rather than welcoming families into the care setting as we have been accustomed, we were forced to restrict access. While well-intentioned, the impact of this on patients, their families – and as we later learned, ourselves – has been devastating. Patients found themselves alone in an unfamiliar environment, infected with a disease there was no effective treatment for, hearing dismal news regarding inpatient and ICU mortality rates on news networks, and families could not see for themselves how their loved ones were progressing in their hospital course.
The impact on patient-centered care
The impact of this pandemic on patients and health care providers alike cannot be overstated. Arguably, one of the greatest challenges created by COVID-19 has been its direct assault on the core values of patient-centered care that we have spent decades striving to promote and embody.
Since its identification as a quality gap by the Institute of Medicine in 2001, the definition of patient-centered care has been tweaked over the past 20 years (Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001). Most frameworks include the active participation of patients and their families as part of the health care team, encouraging and facilitating the presence of family members in the care setting, and focusing on patients’ physical comfort and emotional well-being as fundamental tenets of patient centeredness (NEJM Catalyst: What is Patient-Centered Care? Explore the definition, benefits, and examples of patient-centered care. How does patient-centered care translate to new delivery models? January 1,2017).
Families, the “F” in the ABCDEF Bundle, have been recognized as an integral part of care in the ICU setting (Ely EW. Crit Care Med. 2017;45[2]:321). While engagement of family members began with our recognition of their role in emotionally supporting patients and efforts to improve communication, we have also seen the impact of family participation on reducing ICU delirium through frequent re-orientation and encouragement of early mobility (McKenzie J, et al. Australas J Ageing. 2020;39:21). In fact, a recent study has suggested that family members could play an even more active role in detecting and assessing ICU delirium using objective assessment tools (Fiest K, et al. Crit Care Med. 2020;48[7]:954). Post-ICU PTSD has been well described in both ICU survivors as well as in their family members, with evidence that family participation in care of patients during their ICU stay leads to its reduction (Amass TH, et al. Crit Care Med. 2020[Feb];48[2]:176).
The emotional toll
Comforting patients and families in times of distress and suffering is something that comes naturally to many in critical care, and our training further improves our ability to do this effectively. No amount of training, however, could have prepared us for the degree and volume of suffering we bore witness to this past year and the resulting moral injury many are still dealing with. We were present for families’ most intimate moments, holding phones and tablets up to patients so their families could say their goodbyes, listening to the “I love yous,” “I’ll miss yous,” “I’m sorrys,” and “Please don’t gos.” Nurses held patients’ hands as they took their last breaths so they wouldn’t die alone and worked to move husbands and wives into the same room so they could be together in their final moments. Entrenched in each of our identities is the role of healer, and we found ourselves questioning our effectiveness in rising to meet suffering on a scale we had never seen before. Little did we understand that while our paradigms were reinforcing the benefits of patient-centered care for patients and their families, that framework was also serving to facilitate our role as healers – that without it, we all suffer.
Rising to the challenge
These unprecedented circumstances led to creative efforts to bridge some of these barriers. Health systems created photo lanyards that providers wore over their PPE so patients could identify their health care team and connect with them on a more human level. Video conferencing technology was brought to the patient bedside using smartphones and tablets to assist them in communicating with their families. Doctors and nurses coordinated multiple calls throughout the day to ensure families felt included in the care plans and were always abreast of any new developments.
All these initiatives were our way of attempting to alleviate some of the suffering we were witnessing, and in some ways felt complicit in. It is in hindsight that we can look back and question if we could have done things differently. We treated family as visitors, when in fact, they are fundamental members of the care team who play an active and critical role in patient care. This was, in part, driven by national unpreparedness when it came to PPE supplies, in addition to misinformation and inconsistent messaging early in the pandemic with regards to the mechanism of transmission of disease from various health organizations. While we did our best given the circumstances, we must not allow this experience to lead us away from the tenets we know to be essential to patient, family, and health care provider well-being.
All in health care met the call to action – nurses, physicians, advanced practice providers, respiratory therapists, nutritionists, pharmacists, physical therapists, patient transporters, environmental service workers, and all others who kept our hospitals and patient care facilities open through this pandemic and embarked on what amounted to a collective, global, ongoing “code-blue alert,” resuscitating patient after patient, hotspot after hotspot, region after region, and country after country. We expanded hospital bed capacities, created ICU beds where there were none, developed novel process protocols, and learned in real time what seemed to help (or not) in treating this novel disease, all while participating in incredible international scientific collaboration and information sharing that has contributed in getting the collective “us” through this first year of the pandemic. We did what we were trained and called to do.
Preparing for the future
There will inevitably be another public health crisis, and we must advocate for better preparedness next time, insisting on overall stronger public health systems and pandemic preparedness. We must address our PPE stores and supply chains. We must have disaster preparedness plans that go beyond the scope of mass casualty events and bioterrorism. Beyond physical recovery, we must tend to the factors that impact patients’ long-term recovery, with attention to emotional and psychological well-being. We must advocate for all of this now, while the memories are fresh and before the impact of this collective suffering begins to fade. It can never again be acceptable to exclude families from the health care setting. We must advocate for our patients and for the resources, systems, processes, and support that will allow us to do better.
Dr. Hegab is Associate Director, Pulmonary Hypertension Program, Medical Director, Pulmonary Embolism Response Team, Division of Pulmonary and Critical Care Medicine, Henry Ford Hospital; and Assistant Professor, Wayne State University School of Medicine, Detroit.
Delirium risk factors identified in ICU cancer patients
Hematology-oncology patients who receive treatment in the intensive care unit often develop delirium, and according to new findings, mechanical ventilation, high-dose corticosteroid use, and brain metastases were identified as independent risk factors.
Roughly half of all hematology-oncology patients who were admitted to the ICU experienced delirium, explained lead author Rachel Klosko, PharmD, PGY-2 cardiology pharmacy resident at the Ohio State University, Columbus.
“Delirium was associated with increased mortality, an increase in hospital length of stay, and increased length of stay in the ICU,” she said.
Dr. Klosko presented the study results at the at the Critical Care Congress sponsored by the Society of Critical Care Medicine (SCCM), which was held virtually this year.
Delirium is an acute and fluctuating disturbance of consciousness and cognition and fluctuates in severity. Critically ill patients are subject to numerous risk factors for delirium. “It can occur in independently of any known neurological disorder,” said Dr. Klosko, adding that its occurrence has been associated with poorer outcomes in ICU patients.
In this study, Dr. Klosko and colleagues sought to determine the incidence of delirium in cancer patients who were admitted to the ICU, as well as identify the associated risk factors and recognize potential consequences of the development of delirium in this patient population.
They conducted a single center, retrospective, cohort study that evaluated patients between the ages of 18 and 89 years who were admitted to the hematology-oncology medical or surgical ICU between July 1, 2018, and June 30, 2019.
The study’s primary endpoint was the incidence of delirium within 7 days of ICU admission, defined as two positive Confusion Assessment Method for the ICU (CAM-ICU) assessments within 24 hours. Patients identified with delirium were compared to those without it, for the evaluation of secondary endpoints that included hospital mortality and ICU and hospital length of stay. The researchers also sought to identify independent risk factors for delirium in this population.
A total of 244 patients were included in the final analysis. Of this group, 125 (51.2%) experienced delirium during their stay in the ICU, and 119 (48.8%) did not.
Mortality in the delirium group was significantly higher at 32.8% vs. 15.1% (P = .001). In addition, the delirium group was associated with significantly higher ICU length of stay (6 days vs. 3 days, P < .001) and hospital length of stay (21 days vs. 12 days, P < .001).
“When comparing the baseline characteristics between the two groups, the delirium group had a longer hospital length prior to ICU admission, a higher SOFA score, a higher rate of brain metastases, a higher rate of shock, and higher receipt of high-dose steroids, benzodiazepines, and immunotherapy,” said Dr. Klosko.
After multivariable regression, four variables were included in the final model. Among patients with delirium, the SOFA score increased by 25% (odds ratio[OR] 1.25, P < .001), while the odds of delirium were almost four times higher among those treated with high-dose corticosteroids (OR 3.79, P = .004). Delirium was also eight times higher (OR 8.48, P < .001) among those who received mechanical ventilation and five times higher in (OR 5.38, P = .015) in patients with brain metastases.
Dr. Klosko noted that the main limitations for this study were that it was a single center retrospective analysis, and that patients were reviewed within the first 7 days of ICU admission. “This potentially missed patients who developed delirium outside of this time frame,” she said. In addition, “too few patients received high-dose benzodiazepines,” and “none of the patients received continuous neuromuscular blockade.”
However, in “contrast to these limitations, this is the largest study to date that has analyzed delirium in this population,” Dr. Klosko said.
Commenting on the study, Brenda Pun, DNP, RN, director of data quality at the Vanderbilt Critical Illness, Brain Dysfunction, and Survivorship Center, Nashville, Tenn., pointed out that the goal of this study was to describe delirium in this specific population. “But I will take a step backward and say that they are just confirming that these patients look like other ICU patients in many regards,” she said.
She explained that the sicker patients are, the higher the rates of delirium. “We have implemented strategies to lower these rates, and they have improved,” Dr. Pun said. “Ten years ago, I would say that 80% of patients who were on a ventilator would have delirium but now the rates are around 50% and that’s what we are typically seeing now.”
Dr. Pun emphasized that this study shows that delirium is like the “canary in the coal mine” or a red flag. “It’s a sign that something is wrong and that we need to pay attention, because the patient’s outcome may be worse,” she said. “So this is saying that we need to see if there is something that can be changed or modified to decrease the incidence of delirium—these are important questions.”
There was no outside sponsor. The authors had no disclosures. Dr. Pun has no disclosures.
Hematology-oncology patients who receive treatment in the intensive care unit often develop delirium, and according to new findings, mechanical ventilation, high-dose corticosteroid use, and brain metastases were identified as independent risk factors.
Roughly half of all hematology-oncology patients who were admitted to the ICU experienced delirium, explained lead author Rachel Klosko, PharmD, PGY-2 cardiology pharmacy resident at the Ohio State University, Columbus.
“Delirium was associated with increased mortality, an increase in hospital length of stay, and increased length of stay in the ICU,” she said.
Dr. Klosko presented the study results at the at the Critical Care Congress sponsored by the Society of Critical Care Medicine (SCCM), which was held virtually this year.
Delirium is an acute and fluctuating disturbance of consciousness and cognition and fluctuates in severity. Critically ill patients are subject to numerous risk factors for delirium. “It can occur in independently of any known neurological disorder,” said Dr. Klosko, adding that its occurrence has been associated with poorer outcomes in ICU patients.
In this study, Dr. Klosko and colleagues sought to determine the incidence of delirium in cancer patients who were admitted to the ICU, as well as identify the associated risk factors and recognize potential consequences of the development of delirium in this patient population.
They conducted a single center, retrospective, cohort study that evaluated patients between the ages of 18 and 89 years who were admitted to the hematology-oncology medical or surgical ICU between July 1, 2018, and June 30, 2019.
The study’s primary endpoint was the incidence of delirium within 7 days of ICU admission, defined as two positive Confusion Assessment Method for the ICU (CAM-ICU) assessments within 24 hours. Patients identified with delirium were compared to those without it, for the evaluation of secondary endpoints that included hospital mortality and ICU and hospital length of stay. The researchers also sought to identify independent risk factors for delirium in this population.
A total of 244 patients were included in the final analysis. Of this group, 125 (51.2%) experienced delirium during their stay in the ICU, and 119 (48.8%) did not.
Mortality in the delirium group was significantly higher at 32.8% vs. 15.1% (P = .001). In addition, the delirium group was associated with significantly higher ICU length of stay (6 days vs. 3 days, P < .001) and hospital length of stay (21 days vs. 12 days, P < .001).
“When comparing the baseline characteristics between the two groups, the delirium group had a longer hospital length prior to ICU admission, a higher SOFA score, a higher rate of brain metastases, a higher rate of shock, and higher receipt of high-dose steroids, benzodiazepines, and immunotherapy,” said Dr. Klosko.
After multivariable regression, four variables were included in the final model. Among patients with delirium, the SOFA score increased by 25% (odds ratio[OR] 1.25, P < .001), while the odds of delirium were almost four times higher among those treated with high-dose corticosteroids (OR 3.79, P = .004). Delirium was also eight times higher (OR 8.48, P < .001) among those who received mechanical ventilation and five times higher in (OR 5.38, P = .015) in patients with brain metastases.
Dr. Klosko noted that the main limitations for this study were that it was a single center retrospective analysis, and that patients were reviewed within the first 7 days of ICU admission. “This potentially missed patients who developed delirium outside of this time frame,” she said. In addition, “too few patients received high-dose benzodiazepines,” and “none of the patients received continuous neuromuscular blockade.”
However, in “contrast to these limitations, this is the largest study to date that has analyzed delirium in this population,” Dr. Klosko said.
Commenting on the study, Brenda Pun, DNP, RN, director of data quality at the Vanderbilt Critical Illness, Brain Dysfunction, and Survivorship Center, Nashville, Tenn., pointed out that the goal of this study was to describe delirium in this specific population. “But I will take a step backward and say that they are just confirming that these patients look like other ICU patients in many regards,” she said.
She explained that the sicker patients are, the higher the rates of delirium. “We have implemented strategies to lower these rates, and they have improved,” Dr. Pun said. “Ten years ago, I would say that 80% of patients who were on a ventilator would have delirium but now the rates are around 50% and that’s what we are typically seeing now.”
Dr. Pun emphasized that this study shows that delirium is like the “canary in the coal mine” or a red flag. “It’s a sign that something is wrong and that we need to pay attention, because the patient’s outcome may be worse,” she said. “So this is saying that we need to see if there is something that can be changed or modified to decrease the incidence of delirium—these are important questions.”
There was no outside sponsor. The authors had no disclosures. Dr. Pun has no disclosures.
Hematology-oncology patients who receive treatment in the intensive care unit often develop delirium, and according to new findings, mechanical ventilation, high-dose corticosteroid use, and brain metastases were identified as independent risk factors.
Roughly half of all hematology-oncology patients who were admitted to the ICU experienced delirium, explained lead author Rachel Klosko, PharmD, PGY-2 cardiology pharmacy resident at the Ohio State University, Columbus.
“Delirium was associated with increased mortality, an increase in hospital length of stay, and increased length of stay in the ICU,” she said.
Dr. Klosko presented the study results at the at the Critical Care Congress sponsored by the Society of Critical Care Medicine (SCCM), which was held virtually this year.
Delirium is an acute and fluctuating disturbance of consciousness and cognition and fluctuates in severity. Critically ill patients are subject to numerous risk factors for delirium. “It can occur in independently of any known neurological disorder,” said Dr. Klosko, adding that its occurrence has been associated with poorer outcomes in ICU patients.
In this study, Dr. Klosko and colleagues sought to determine the incidence of delirium in cancer patients who were admitted to the ICU, as well as identify the associated risk factors and recognize potential consequences of the development of delirium in this patient population.
They conducted a single center, retrospective, cohort study that evaluated patients between the ages of 18 and 89 years who were admitted to the hematology-oncology medical or surgical ICU between July 1, 2018, and June 30, 2019.
The study’s primary endpoint was the incidence of delirium within 7 days of ICU admission, defined as two positive Confusion Assessment Method for the ICU (CAM-ICU) assessments within 24 hours. Patients identified with delirium were compared to those without it, for the evaluation of secondary endpoints that included hospital mortality and ICU and hospital length of stay. The researchers also sought to identify independent risk factors for delirium in this population.
A total of 244 patients were included in the final analysis. Of this group, 125 (51.2%) experienced delirium during their stay in the ICU, and 119 (48.8%) did not.
Mortality in the delirium group was significantly higher at 32.8% vs. 15.1% (P = .001). In addition, the delirium group was associated with significantly higher ICU length of stay (6 days vs. 3 days, P < .001) and hospital length of stay (21 days vs. 12 days, P < .001).
“When comparing the baseline characteristics between the two groups, the delirium group had a longer hospital length prior to ICU admission, a higher SOFA score, a higher rate of brain metastases, a higher rate of shock, and higher receipt of high-dose steroids, benzodiazepines, and immunotherapy,” said Dr. Klosko.
After multivariable regression, four variables were included in the final model. Among patients with delirium, the SOFA score increased by 25% (odds ratio[OR] 1.25, P < .001), while the odds of delirium were almost four times higher among those treated with high-dose corticosteroids (OR 3.79, P = .004). Delirium was also eight times higher (OR 8.48, P < .001) among those who received mechanical ventilation and five times higher in (OR 5.38, P = .015) in patients with brain metastases.
Dr. Klosko noted that the main limitations for this study were that it was a single center retrospective analysis, and that patients were reviewed within the first 7 days of ICU admission. “This potentially missed patients who developed delirium outside of this time frame,” she said. In addition, “too few patients received high-dose benzodiazepines,” and “none of the patients received continuous neuromuscular blockade.”
However, in “contrast to these limitations, this is the largest study to date that has analyzed delirium in this population,” Dr. Klosko said.
Commenting on the study, Brenda Pun, DNP, RN, director of data quality at the Vanderbilt Critical Illness, Brain Dysfunction, and Survivorship Center, Nashville, Tenn., pointed out that the goal of this study was to describe delirium in this specific population. “But I will take a step backward and say that they are just confirming that these patients look like other ICU patients in many regards,” she said.
She explained that the sicker patients are, the higher the rates of delirium. “We have implemented strategies to lower these rates, and they have improved,” Dr. Pun said. “Ten years ago, I would say that 80% of patients who were on a ventilator would have delirium but now the rates are around 50% and that’s what we are typically seeing now.”
Dr. Pun emphasized that this study shows that delirium is like the “canary in the coal mine” or a red flag. “It’s a sign that something is wrong and that we need to pay attention, because the patient’s outcome may be worse,” she said. “So this is saying that we need to see if there is something that can be changed or modified to decrease the incidence of delirium—these are important questions.”
There was no outside sponsor. The authors had no disclosures. Dr. Pun has no disclosures.
FROM CCC50
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
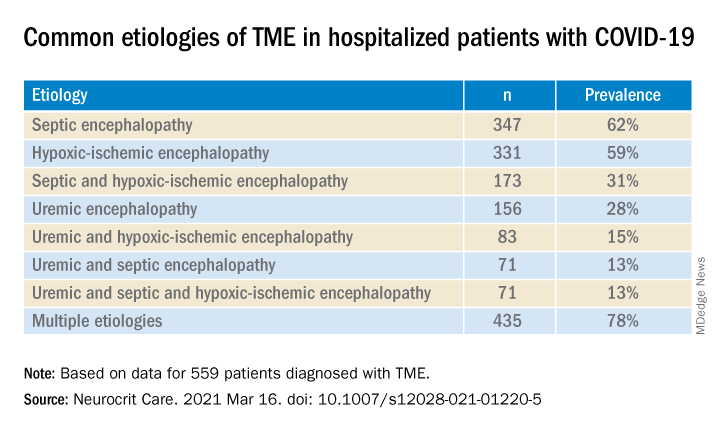
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE









