User login
How do you manage common inpatient oncologic emergencies?
Three routinely encountered emergencies in the inpatient setting
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
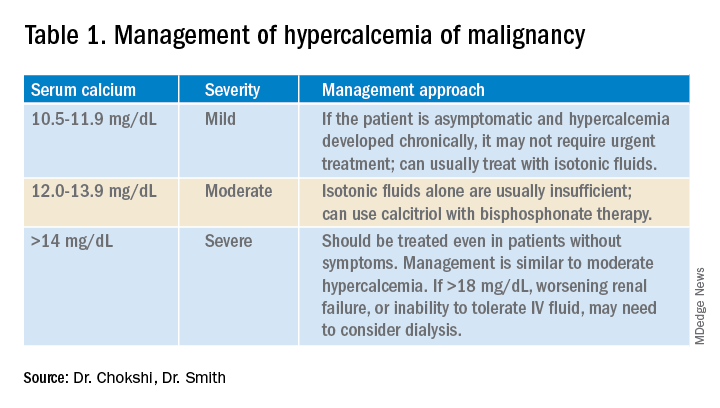
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).
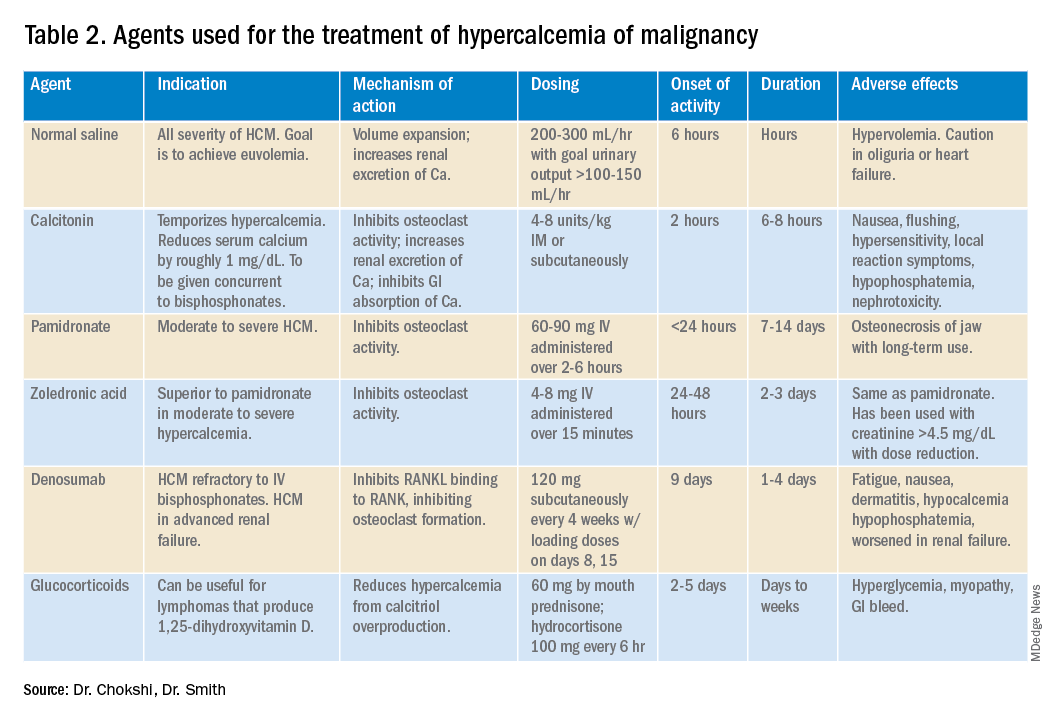
For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12
Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge
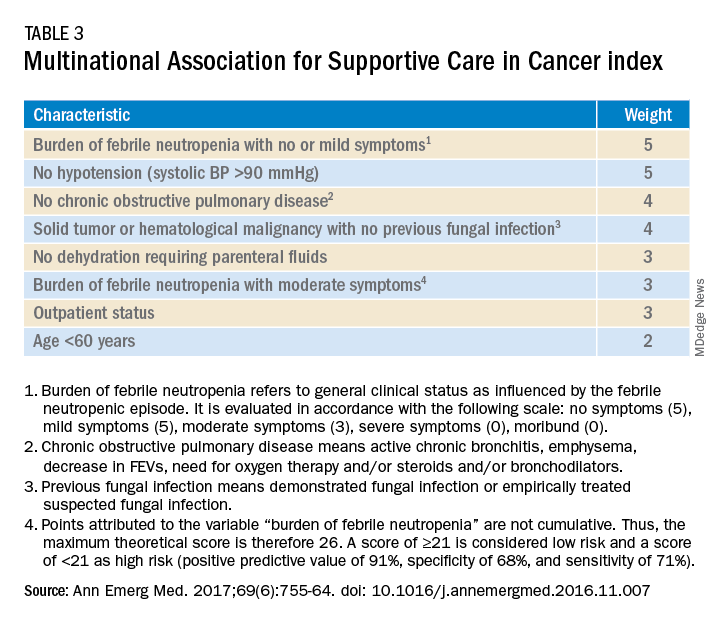
Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways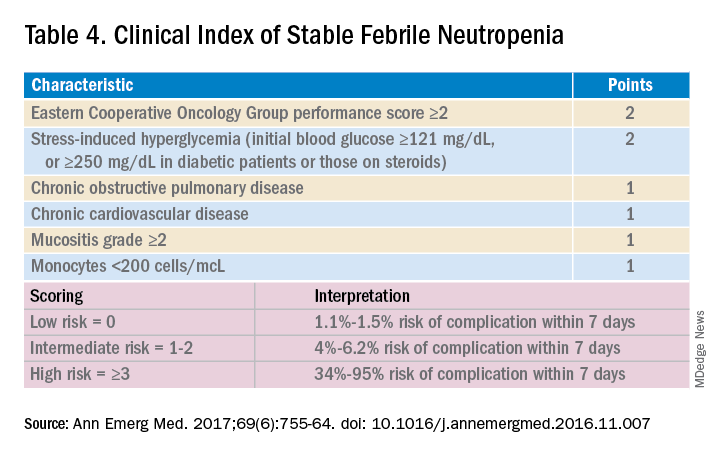
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
Three routinely encountered emergencies in the inpatient setting
Three routinely encountered emergencies in the inpatient setting
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).

For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12

Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge

Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
In 2016, there were an estimated 15,338,988 people living with cancer in the United States.1 As such, it is important that hospitalists be proficient in managing oncologic emergencies that can arise during the natural history of cancer or from its treatment. This article will review three emergencies that are routinely encountered in the inpatient setting: malignant spinal cord compression (MSCC), hypercalcemia of malignancy (HCM), and febrile neutropenia (FN).
Case
Mr. Williams is a 56-year-old man with newly diagnosed metastatic prostate cancer, diabetes mellitus, peptic ulcer disease, and hypertension. He is admitted with back pain and lower extremity weakness worsening over 2 weeks. He denies loss of sensation or bowel and bladder incontinence and can walk. MRI confirms cord compression at T10. What initial and subsequent steroid doses would be of most benefit to administer?
Malignant spinal cord compression
Treatment of MSCC usually aims to preserve function rather than reverse established deficits. MSCC from epidural tumor metastasis develops in 5%-14% of all cancer cases,2 with back pain as the most common symptom. Nearly 60%-85% of patients have weakness at the time of diagnosis,3 and unfortunately, nearly two-thirds of patients will be nonambulatory at presentation.
While timely steroid administration in addition to definitive treatment may maintain ambulatory capacity at 1 year after therapy,4 there is no consensus on the optimal loading and maintenance dose and duration of steroids.
Overview of the data
Although there are no formal guidelines on optimal steroid dosing for MSCC, it is common practice for dexamethasone to be initially dosed at 10 mg followed by 4 mg every 4-6 hours.5 The use of higher doses of dexamethasone may result in improvement in neurologic deficits, but has higher risks for toxicity and is not universally supported in the literature.
A study conducted by Vecht and colleagues demonstrated few differences between initial high-dose and low-dose dexamethasone.6 Intravenous administration of either 10 mg or 100 mg dexamethasone, both followed by total 16 mg of dexamethasone orally per day, showed no significant difference in mobility or survival between the groups.
In a prospective study by Heimdal and colleagues that evaluated the relationship between dexamethasone dose and toxicity, higher doses of steroids had no meaningful impact on neurological symptoms and resulted in more severe side effects.7 Patients were either given 96-mg IV loading dose, gradually tapered over 2 weeks, or enrolled in the low-dose group in which they received 4-mg IV dexamethasone four times per day with a taper over 2 weeks. The high-dose group experienced side effects in 28.6% of patients, with 14.3% experiencing serious side effects. Meanwhile, 7.9% of the low-dose group exhibited some side effects, with none experiencing serious adverse effects.The high-dose group did not experience a significant increase in mobility (57.1 vs. 57.9%).
Key takeaways
Dexamthasone 10-mg oral or IV followed by 4 mg every 4-6 hours until definitive treatment is started is associated with improved neurologic outcomes and minimal adverse side effects. Higher doses of steroids are unlikely to offer more benefit. In patients with paraplegia or autonomic dysfunction, the ability to restore neurologic function is reduced and the burdens of steroid treatment may outweigh its benefits.5
Case continued
Mr. Williams completed treatment for MSCC but was still complaining of extreme lethargy and noticed an increase in thirst and no bowel movement in 5 days. His serum calcium was 14 mg/dL.
Hypercalcemia of malignancy
HCM is the most common paraneoplastic syndrome, observed in nearly 30% of patients with advanced cancer. It is a poor prognostic indicator, and approximately half of all patients with HCM will die within 30 days.8 Cancer is the most common reason for hypercalcemia in the inpatient setting9 and is most often associated with multiple myeloma, non–small cell lung cancer, breast cancer, renal cell carcinoma, non-Hodgkins lymphoma, and leukemia.
Hypercalcemia most often presents with cognitive changes and lethargy, anorexia, nausea, constipation, polyuria and polydipsia, and renal failure. Bradycardia and shortened QT interval are seen more with severe hypercalcemia.
Management of hypercalcemia of malignancy
Management of HCM depends on corrected calcium or ionized calcium levels, chronicity, degree of symptoms, and presence of renal failure. In general, mild asymptomatic hypercalcemia can be managed with outpatient care. Serum calcium greater than 14 mg/dL should be treated regardless of symptoms (Table 1).

For mild to moderate HCM, management involves saline administration to achieve euvolemia and calcitonin, which has temporizing effects. Early administration of IV bisphosphonates for moderate to severe HCM is beneficial because onset of action is 24-48 hours. Furosemide for management of HCM has fallen out of favor unless the patient develops hypervolemia. Denosumab has been Food and Drug Administration–approved for HCM refractory to bisphosphonate therapy and can manage HCM in 64% of patients who did not respond adequately to bisphosphonate therapy.10 Because it can be used in advanced renal failure without dose adjustment, it is first-line therapy in this population, although the risk for hypocalcemia is increased in renal failure. For patients with serum calcium greater than 18 mg/dL, worsening renal failure, or inability to tolerate IV fluids, dialysis with a low-calcium bath should be considered (Table 2).
Zoledronic acid versus pamidronate
A single dose of zoledronic acid normalizes the serum calcium concentration in 88% of patients, compared with 70% of those who received pamidronate, in a pooled analysis of two phase 3 trials.11 The median duration of normocalcemia was longer for those receiving zoledronic acid (32-43 days vs. 18 days). The efficacy of the 4-mg and 8-mg zoledronic acid doses were similar, but the 4-mg dose was recommended because of renal toxicity and increased mortality associated with the higher dose.Despite this data, many specialists maintain that pamidronate, which is less expensive, is of similar clinical efficacy to ZA.12

Key takeaways
Management of HCM should be determined by the severity of the calcium level. The mainstay of treatment includes hydration with normal saline, calcitonin ,and bisphosphonate therapy; zoledronic acid is preferred over pamidronate. For patients refractory to bisphosphonates or patients with renal insufficiency, denosumab should be used.
Case continued: Febrile neutropenia
Febrile neutropenia is defined as a single oral temperature of 100.9° F or a temperature of 100.4° F sustained over a 1-hour period in a patient with absolute neutrophil count (ANC) less than 1,000 cells/mL or ANC expected to decrease to less than 500 cells/mL within a 48-hour period.13 Up to 30% of patients with solid tumors develop febrile neutropenia after chemotherapy, and nearly 80% of patients with hematologic malignancy or after hematopoietic stem cell therapy (HSCT) experience it.
Even though an infectious etiology is identified in only 30%-40% of cases, all patients with febrile neutropenia should initially receive at least empiric gram-negative coverage. The mortality rate is nearly 70% in neutropenic patients who do not receive empiric antibiotics and is reduced to 4%-20% with antibiotics.14
Risk stratification for febrile neutropenia and early discharge

Talcott’s Rules, the Multinational Association for Supportive Care in Cancer (MASCC) score, and the Clinical Index of Stable Febrile Neutropenia (CISNE) are validated tools to determine low-risk febrile neutropenia patients (Tables 3 and 4). The Infectious Diseases Society of America guidelines validated the use of MASCC in 2002 but found that CISNE had better performance than other tools. Coyne and colleagues conducted a retrospective cohort study to assess these two risk stratification tools in the ED and found that the CISNE was 98.3% specific for identifying adverse outcomes, whereas the MASCC was 54.2% specific.15
A study by Talcott and colleagues used Talcott’s Rules to identify low-risk febrile neutropenia patients, who were randomized to early discharge with home intravenous antibiotics versus continued inpatient management. There were no significant differences in the primary outcomes, defined as any change in clinical status requiring medical evaluation.16 Another study suggested that discharge after 24 hours based on clinical stability with outpatient oral antibiotics were noninferior to standard inpatient and intravenous antibiotic therapy.17 A Cochrane review in 2013 of 22 randomized controlled trials determined that oral antibiotics were an acceptable treatment for low-risk patients.18
Key takeaways
Though the MASCC is highly sensitive in identifying low-risk febrile neutropenia patients, it should be used with clinical caution because up to 11% of patients characterized as low risk developed severe complications.19 If a low-risk patient with solid tumor malignancy has adequate home support, lives within an hour of the hospital, and has access to follow-up within 72 hours, oral antibiotics and early discharge can be considered.
Dr. Chokshi is assistant professor in the division of hospital medicine at Mount Sinai Hospital, New York. Dr. Smith is associate professor in the division of hematology/oncology at Mount Sinai Hospital.
QUIZ
Mrs. Smith is a 64-year-old woman with endometrial cancer with temperature of 100.4° F at home. She takes no antibiotics, has no other medical history, and was sent in from clinic and admitted for further management. She feels well, and preliminary infectious workup is negative. She has been afebrile for more than 24 hours, and her ANC is 600 cells/mL.
Her son’s soccer game is tomorrow, and she would like to be present. Her family is involved in her care. Under what conditions can she be discharged?
A. She should not be discharged until full course of empiric intravenous antibiotics is completed.
B. Consider discharge in another 24 hours if she remains afebrile.
C. Discharge if low risk by MASCC or CISNE, with oral doses of levofloxacin or moxifloxacin or oral ciprofloxacin and amoxicillin/clavulanic acid.
Answer: C. The patient has a solid tumor malignancy, is low risk by both MASCC and CISNE, and can most likely be discharged if she is clinically stable or improved. A 7-day course of antibiotics is recommended with close follow-up.
References
1. SEER. Cancer of Any Site - Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/all.html. Accessed 2019 Jul 17.
2. Kwok Y et al. Clinical Approach to Metastatic Epidural Spinal Cord Compression. Hematol Oncol Clin North Am. 2006;20(6):1297-305.
3. Helweg-Larsen S et al. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46(5):1163-9.
4. Sørensen P et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30(1):22-7.
5. Skeoch G et al. Corticosteroid treatment for metastatic spinal cord compression: A review. Global Spine J. 2017;7(3):272-9.
6. Vecht C et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39(9):1255-7.
7. Heimdal K et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-4.
8. Ralston S et al. Cancer-associated hypercalcemia: Morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499-504.
9. Lindner G et al. Hypercalcemia in the ED: Prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657-60.
10. Hu M et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst. 2013;105(18):1417-20.
11. Major P et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558-67.
12. Stewart A. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-9.
13. Freifeld A et al. Executive summary: Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427-31.
14. Baden L et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882-913.
15. Coyne C et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med. 2017;69(6):755-64.
16. Talcott J et al. Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol. 2011;29(30):3977-83.
17. Innes H et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89(1):43-9.
18. Vidal L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst. Rev. 2013.
19. Taplitz RA et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-53.
Unneeded meds at discharge could cause harm
A significant number of patients leave the hospital with inappropriate drugs because of a lack of medication reconciliation at discharge, new research shows.
Proton pump inhibitors – known to have adverse effects, such as fractures, osteoporosis, and progressive kidney disease – make up 30% of inappropriate prescriptions at discharge.
“These medications can have a significant toxic effect, especially in the long term,” said Harsh Patel, MD, from Medical City Healthcare in Fort Worth, Tex.
And “when we interviewed patients, they were unable to recall ever partaking in a pulmonary function test or endoscopy to warrant the medications,” he said in an interview.
For their retrospective chart review, Dr. Patel and colleagues assessed patients admitted to the ICU in 13 hospitals over a 6-month period in northern Texas. Of the 12,930 patients, 2,557 had not previously received but were prescribed during their hospital stay a bronchodilator, a proton pump inhibitor, or an H2 receptor agonist.
Of those 2,557 patients, 26.8% were discharged on a proton pump inhibitor, 8.4% on an H2 receptor agonist, and 5.49% on a bronchodilator.
There were no corresponding diseases or diagnoses to justify continued use, Dr. Patel said during his presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
Button fatigue
The problem stems from a technology disconnect when patients are transferred from the ICU to the general population.
Doctors expect that the medications will be reconciled at discharge, said one of the study investigators, Prashanth Reddy, MD, from Medical City Las Colinas (Tex.).
But in some instances, clinicians unfamiliar with the case click through the electronic health record to get the patient “out of the ICU to the floor,” he explained. “They don’t always know what medications to keep.”
“They may have button fatigue, so they just accept and continue,” Dr. Reddy said in an interviews.
In light of these findings, the team has kick-started a project to improve transition out of the ICU and minimize overprescription at discharge.
“This is the kind of a problem where we thought we could have some influence,” said Dr. Reddy.
One solution would be to put “stop orders” on potentially harmful medications. “But we don’t want to increase button fatigue even more, so we have to find a happy medium,” he said. “It’s going to take a while to formulate the best path on this.”
The inclusion of pharmacy residents in rounds could make a difference. “When we rounded with pharmacy residents, these issues got addressed,” Dr. Patel said. The pharmacy residents often asked: “Can we go over the meds? Does this person really need all this?”
Medication reconciliations not only have a positive effect on a patient’s health, they can also cut costs by eliminating unneeded drugs. And “patients are always happy to hear we’re taking them off a drug,” Dr. Patel added.
He said he remembers one of his mentors telling him that, if he could get his patients down to five medications, “then you’ve achieved success as a physician.”
“I’m still working toward that,” he said. “The end goal should sometimes be, less is more.”
COPD patients overprescribed home oxygen
In addition to medications, home oxygen therapy is often prescribed when patients are discharged from the hospital.
A study of 69 patients who were continued on home oxygen therapy after hospitalization for an exacerbation of chronic obstructive pulmonary disease was presented by Analisa Taylor, MD, from the University of Illinois at Chicago.
Despite guideline recommendations that patients be reassessed within 90 days of discharge, only 38 patients in the cohort were reassessed, and “28 were considered eligible for discontinuation,” she said during her presentation.
However, “of those, only four were ultimately discontinued,” she reported.
The reason for this gap needs to be examined, noted Dr. Taylor, suggesting that “perhaps clinical inertia plays a role in the continuation of previously prescribed therapy despite a lack of ongoing clinical benefit.”
A version of this article originally appeared on Medscape.com.
A significant number of patients leave the hospital with inappropriate drugs because of a lack of medication reconciliation at discharge, new research shows.
Proton pump inhibitors – known to have adverse effects, such as fractures, osteoporosis, and progressive kidney disease – make up 30% of inappropriate prescriptions at discharge.
“These medications can have a significant toxic effect, especially in the long term,” said Harsh Patel, MD, from Medical City Healthcare in Fort Worth, Tex.
And “when we interviewed patients, they were unable to recall ever partaking in a pulmonary function test or endoscopy to warrant the medications,” he said in an interview.
For their retrospective chart review, Dr. Patel and colleagues assessed patients admitted to the ICU in 13 hospitals over a 6-month period in northern Texas. Of the 12,930 patients, 2,557 had not previously received but were prescribed during their hospital stay a bronchodilator, a proton pump inhibitor, or an H2 receptor agonist.
Of those 2,557 patients, 26.8% were discharged on a proton pump inhibitor, 8.4% on an H2 receptor agonist, and 5.49% on a bronchodilator.
There were no corresponding diseases or diagnoses to justify continued use, Dr. Patel said during his presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
Button fatigue
The problem stems from a technology disconnect when patients are transferred from the ICU to the general population.
Doctors expect that the medications will be reconciled at discharge, said one of the study investigators, Prashanth Reddy, MD, from Medical City Las Colinas (Tex.).
But in some instances, clinicians unfamiliar with the case click through the electronic health record to get the patient “out of the ICU to the floor,” he explained. “They don’t always know what medications to keep.”
“They may have button fatigue, so they just accept and continue,” Dr. Reddy said in an interviews.
In light of these findings, the team has kick-started a project to improve transition out of the ICU and minimize overprescription at discharge.
“This is the kind of a problem where we thought we could have some influence,” said Dr. Reddy.
One solution would be to put “stop orders” on potentially harmful medications. “But we don’t want to increase button fatigue even more, so we have to find a happy medium,” he said. “It’s going to take a while to formulate the best path on this.”
The inclusion of pharmacy residents in rounds could make a difference. “When we rounded with pharmacy residents, these issues got addressed,” Dr. Patel said. The pharmacy residents often asked: “Can we go over the meds? Does this person really need all this?”
Medication reconciliations not only have a positive effect on a patient’s health, they can also cut costs by eliminating unneeded drugs. And “patients are always happy to hear we’re taking them off a drug,” Dr. Patel added.
He said he remembers one of his mentors telling him that, if he could get his patients down to five medications, “then you’ve achieved success as a physician.”
“I’m still working toward that,” he said. “The end goal should sometimes be, less is more.”
COPD patients overprescribed home oxygen
In addition to medications, home oxygen therapy is often prescribed when patients are discharged from the hospital.
A study of 69 patients who were continued on home oxygen therapy after hospitalization for an exacerbation of chronic obstructive pulmonary disease was presented by Analisa Taylor, MD, from the University of Illinois at Chicago.
Despite guideline recommendations that patients be reassessed within 90 days of discharge, only 38 patients in the cohort were reassessed, and “28 were considered eligible for discontinuation,” she said during her presentation.
However, “of those, only four were ultimately discontinued,” she reported.
The reason for this gap needs to be examined, noted Dr. Taylor, suggesting that “perhaps clinical inertia plays a role in the continuation of previously prescribed therapy despite a lack of ongoing clinical benefit.”
A version of this article originally appeared on Medscape.com.
A significant number of patients leave the hospital with inappropriate drugs because of a lack of medication reconciliation at discharge, new research shows.
Proton pump inhibitors – known to have adverse effects, such as fractures, osteoporosis, and progressive kidney disease – make up 30% of inappropriate prescriptions at discharge.
“These medications can have a significant toxic effect, especially in the long term,” said Harsh Patel, MD, from Medical City Healthcare in Fort Worth, Tex.
And “when we interviewed patients, they were unable to recall ever partaking in a pulmonary function test or endoscopy to warrant the medications,” he said in an interview.
For their retrospective chart review, Dr. Patel and colleagues assessed patients admitted to the ICU in 13 hospitals over a 6-month period in northern Texas. Of the 12,930 patients, 2,557 had not previously received but were prescribed during their hospital stay a bronchodilator, a proton pump inhibitor, or an H2 receptor agonist.
Of those 2,557 patients, 26.8% were discharged on a proton pump inhibitor, 8.4% on an H2 receptor agonist, and 5.49% on a bronchodilator.
There were no corresponding diseases or diagnoses to justify continued use, Dr. Patel said during his presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
Button fatigue
The problem stems from a technology disconnect when patients are transferred from the ICU to the general population.
Doctors expect that the medications will be reconciled at discharge, said one of the study investigators, Prashanth Reddy, MD, from Medical City Las Colinas (Tex.).
But in some instances, clinicians unfamiliar with the case click through the electronic health record to get the patient “out of the ICU to the floor,” he explained. “They don’t always know what medications to keep.”
“They may have button fatigue, so they just accept and continue,” Dr. Reddy said in an interviews.
In light of these findings, the team has kick-started a project to improve transition out of the ICU and minimize overprescription at discharge.
“This is the kind of a problem where we thought we could have some influence,” said Dr. Reddy.
One solution would be to put “stop orders” on potentially harmful medications. “But we don’t want to increase button fatigue even more, so we have to find a happy medium,” he said. “It’s going to take a while to formulate the best path on this.”
The inclusion of pharmacy residents in rounds could make a difference. “When we rounded with pharmacy residents, these issues got addressed,” Dr. Patel said. The pharmacy residents often asked: “Can we go over the meds? Does this person really need all this?”
Medication reconciliations not only have a positive effect on a patient’s health, they can also cut costs by eliminating unneeded drugs. And “patients are always happy to hear we’re taking them off a drug,” Dr. Patel added.
He said he remembers one of his mentors telling him that, if he could get his patients down to five medications, “then you’ve achieved success as a physician.”
“I’m still working toward that,” he said. “The end goal should sometimes be, less is more.”
COPD patients overprescribed home oxygen
In addition to medications, home oxygen therapy is often prescribed when patients are discharged from the hospital.
A study of 69 patients who were continued on home oxygen therapy after hospitalization for an exacerbation of chronic obstructive pulmonary disease was presented by Analisa Taylor, MD, from the University of Illinois at Chicago.
Despite guideline recommendations that patients be reassessed within 90 days of discharge, only 38 patients in the cohort were reassessed, and “28 were considered eligible for discontinuation,” she said during her presentation.
However, “of those, only four were ultimately discontinued,” she reported.
The reason for this gap needs to be examined, noted Dr. Taylor, suggesting that “perhaps clinical inertia plays a role in the continuation of previously prescribed therapy despite a lack of ongoing clinical benefit.”
A version of this article originally appeared on Medscape.com.
FROM CHEST 2020
Score predicts risk for ventilation in COVID-19 patients
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
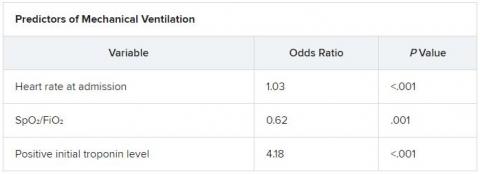
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
A new scoring system can predict whether COVID-19 patients will require invasive mechanical ventilation, researchers report.
The score uses three variables to predict future risk: heart rate; the ratio of oxygen saturation (SpO2) to fraction of inspired oxygen (FiO2); and a positive troponin I level.
“What excites us is it’s a really benign tool,” said Muhtadi Alnababteh, MD, from the Medstar Washington (D.C.) Hospital Center. “For the first two variables you only need to look at vital signs, no labs or invasive diagnostics.”
“The third part is a simple lab, which is performed universally and can be done in any hospital,” he told this news organization. “We know that even rural hospitals can do this.”
For their retrospective analysis, Dr. Alnababteh and his colleagues assessed 265 adults with confirmed COVID-19 infection who were admitted to a single tertiary care center in March and April. They looked at demographic characteristics, lab results, and clinical and outcome information.
Ultimately, 54 of these patients required invasive mechanical ventilation.
On multiple-regression analysis, the researchers determined that three variables independently predicted the need for invasive mechanical ventilation.
Calibration of the model was good (Hosmer–Lemeshow score, 6.3; P = .39), as was predictive ability (area under the curve, 0.80).
The risk for invasive mechanical ventilation increased as the number of positive variables increased (P < .001), from 15.4% for those with one positive variable, to 29.0% for those with two, to 60.5% for those with three positive variables.
The team established cutoff points for each variable and developed a points-based scoring system to predict risk.
It was an initial surprise that troponin – a cardiac marker – would be a risk factor. “Originally, we thought COVID-19 only affects the lung,” Dr. Alnababteh explained during his presentation at CHEST 2020. Later studies, however, showed it can cause myocarditis symptoms.
The case for looking at cardiac markers was made when a study of young athletes who recovered from COVID-19 after experiencing mild or no symptoms showed that 15% had signs of myocarditis on cardiac MRI.
“If mild COVID disease in young patients caused cardiac injury, you can imagine what it can do to older patients with severe disease,” Alnababteh said.
This tool will help triage patients who are not sick enough for the ICU but are known to be at high risk for ventilation. “It’s one of the biggest decisions you have to make: Where do you send your patient? This score helps determine that,” he said.
The researchers are now working to validate the score and evaluate how it performs, he reported.
Existing scores evaluated for COVID-19 outcome prediction
The MuLBSTA score can also be used to predict outcomes in patients with COVID-19.
A retrospective evaluation of 163 patients was presented at CHEST 2020 by Jurgena Tusha, MD, from Wayne State University in Detroit.
Patients who survived their illness had a mean MuLBSTA score of 8.67, whereas patients who died had a mean score of 13.60.
The score “correlated significantly with mortality, ventilator support, and length of stay, which may be used to provide guidance to screen patients and make further clinical decisions,” Dr. Tusha said in a press release.
“Further studies are required to validate this study in larger patient cohorts,” she added.
The three-variable scoring system is easier to use than the MuLBSTA, and more specific, said Dr. Alnababteh.
“The main difference between our study and the MuLBSTA study is that we came up with a novel score for COVID-19 patients,” he said. “Our study score doesn’t require chest x-rays or blood cultures, and the outcome is need for invasive mechanical ventilation, not mortality.”
A version of this article originally appeared on Medscape.com.
Certain statins linked to lower mortality risk in patients admitted for sepsis
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
FROM CHEST 2020
Bronchoscopy can be conducted safely patients with severe COVID-19
Bronchoscopy with intermittent apnea can be conducted safely for both patients with severe COVID-19 and health care workers, a recent study has found. In addition, the high rate of superinfection in these patients indicates that bronchoalveolar lavage (BAL) should sent to the lab if there is any suspicion for secondary pneumonia.
Those are two key findings from a single-center retrospective study led by Stephanie H. Chang, MD, that was published in CHEST.
“While there is a risk of aerosolization and transmission of COVID-19 with bronchoscopy, this can be mitigated with bronchoscopy under intermittent apnea and appropriate PPE [personal protective equipment] in a negative-pressure room, with no significant adverse patient outcomes and a 0% rate of transmission to health care workers,” Dr. Chang, a thoracic surgeon in the department of cardiothoracic surgery at New York University Langone Health, said in an interview. “In appropriate clinical scenarios that will significantly impact patient care, bronchoscopy can be and should be safely performed in patients with COVID-19.”
Although a recent statement from the American Association for Bronchoscopy & Interventional Pulmonology indicates that bronchoscopy is relatively contraindicated in patients with suspected and confirmed COVID-19 infections, it does support use of the procedure in a subset of such patients. It reads: “The only role for bronchoscopy would be when less invasive testing to confirm COVID-19 are inconclusive, suspicion for an alternative diagnosis that would impact clinical management is suspected, or an urgent lifesaving intervention.”
For the current study, Dr. Chang and colleagues retrospectively studied the records of 412 patients with confirmed COVID-19 who were admitted to NYU Langone Health’s Manhattan campus between March 13 and April 24, 2020. If these, 321 required intubation and 107 (33%) underwent bronchoscopy, with a total of 241 bronchoscopies being performed.
Primary outcomes of interest were patient and health care provider safety, defined as freedom from periprocedural complications and COVID-19 transmission, respectively. Secondary outcomes included secondary infection with bacterial or fungal pneumonia.
The bronchoscopy team included six cardiothoracic surgeons and four cardiothoracic surgery residents. Each procedure was performed by a sole bronchoscopist in a negative-pressure room, with a bedside nurse immediately available outside of the room. The bronchoscopist wore full PPE, which consisted of hair cover, a fitted N95 mask, a face shield, gown, and gloves. Each patient was preoxygenated for 2 minutes with a fraction of inspired oxygen at 1.0 in order to maximize apneic time. For patients who were not on sedation and/or neuromuscular blockade, periprocedural anesthesia with propofol and rocuronium was employed to decrease the risk of spontaneous breathing leading to aerosolization.
The bronchoscope used in all cases was the disposable Ambu aScope and a corresponding monitor. The device was used to clear all secretions, clot, or mucus plugs, and to collect bronchoalveolar lavage (BAL) samples. If oxygen saturation decreased below 90%, the bronchoscopist interrupted the procedure and reconnected the patient to the ventilator. After an additional period of preoxygenation, bronchoscopy was then completed.
The mean age of the 107 patients was 62 years, and 81% were male. Dr. Chang and colleagues reported that, of the 241 bronchoscopies performed, no periprocedural complication of severe hypoxia requiring bag-valve ventilation, pneumothorax, or intraprocedural arrhythmias occurred, and that three patients required endotracheal tube advancement or replacement for dislodgement during the procedure.
About half of patients (51%) received a BAL, and 35 (65%) had a positive culture. Among 23 patients who had a negative tracheal culture, 8 patients had a positive BAL, which indicated a 35% diagnostic yield for patients with negative tracheal aspirates. In addition, three patients had differing cultures between the BAL and tracheal aspirate. One was growing Pseudomonas and Klebsiella in the tracheal aspirate with Enterococcus in the BAL, while the other two patients were growing an extra pathogen (Escherichia coli or Serratia) in the BAL.
“The most surprising data was the 65% rate of secondary infection with BAL, which is significantly higher than the rate in standard patients with acute respiratory distress syndrome,” Dr. Chang said. “Additionally, the high rate of bronchoscopy (33% in intubated patients) is also significantly higher than that of standard viral ARDS patients. This increased rate of superimposed infection and need for bronchoscopy may be due to the abnormally thick secretions seen in patients with COVID-19.”
Of the 10 cardiothoracic surgery team members, 1 resident was COVID-19 positive by reverse transcriptase polymerase chain reaction (rtPCR) prior to performing any bronchoscopies. The remaining nine team members tested negative for COVID-19 via nasal pharyngeal swab for rtPCR assay, with at least one negative test performed 2 weeks after the last bronchoscopy performed during the study period.
“The use of apnea was well tolerated by the patients and likely contributed to the lack of transmission of COVID-19 to the health care providers,” Dr. Chang said. “Additionally, this work demonstrates a higher rate of superinfection with bacterial or fungal pneumonia, compared to other reports. It is also the only one that describes the false negative rate for negative tracheal aspirates, which is the current recommended diagnostic test for secondary pneumonia in patients with COVID-19.” She acknowledged certain limitation of the study, including its retrospective design. “Thus, the clinical impact of bronchoscopy on patient outcomes cannot be accurately assessed.”
The authors reported having no financial disclosures.
SOURCE: Chang S et al. CHEST. 2020 Oct 8. doi: 10.1016/j.chest.2020.09.263.
Bronchoscopy with intermittent apnea can be conducted safely for both patients with severe COVID-19 and health care workers, a recent study has found. In addition, the high rate of superinfection in these patients indicates that bronchoalveolar lavage (BAL) should sent to the lab if there is any suspicion for secondary pneumonia.
Those are two key findings from a single-center retrospective study led by Stephanie H. Chang, MD, that was published in CHEST.
“While there is a risk of aerosolization and transmission of COVID-19 with bronchoscopy, this can be mitigated with bronchoscopy under intermittent apnea and appropriate PPE [personal protective equipment] in a negative-pressure room, with no significant adverse patient outcomes and a 0% rate of transmission to health care workers,” Dr. Chang, a thoracic surgeon in the department of cardiothoracic surgery at New York University Langone Health, said in an interview. “In appropriate clinical scenarios that will significantly impact patient care, bronchoscopy can be and should be safely performed in patients with COVID-19.”
Although a recent statement from the American Association for Bronchoscopy & Interventional Pulmonology indicates that bronchoscopy is relatively contraindicated in patients with suspected and confirmed COVID-19 infections, it does support use of the procedure in a subset of such patients. It reads: “The only role for bronchoscopy would be when less invasive testing to confirm COVID-19 are inconclusive, suspicion for an alternative diagnosis that would impact clinical management is suspected, or an urgent lifesaving intervention.”
For the current study, Dr. Chang and colleagues retrospectively studied the records of 412 patients with confirmed COVID-19 who were admitted to NYU Langone Health’s Manhattan campus between March 13 and April 24, 2020. If these, 321 required intubation and 107 (33%) underwent bronchoscopy, with a total of 241 bronchoscopies being performed.
Primary outcomes of interest were patient and health care provider safety, defined as freedom from periprocedural complications and COVID-19 transmission, respectively. Secondary outcomes included secondary infection with bacterial or fungal pneumonia.
The bronchoscopy team included six cardiothoracic surgeons and four cardiothoracic surgery residents. Each procedure was performed by a sole bronchoscopist in a negative-pressure room, with a bedside nurse immediately available outside of the room. The bronchoscopist wore full PPE, which consisted of hair cover, a fitted N95 mask, a face shield, gown, and gloves. Each patient was preoxygenated for 2 minutes with a fraction of inspired oxygen at 1.0 in order to maximize apneic time. For patients who were not on sedation and/or neuromuscular blockade, periprocedural anesthesia with propofol and rocuronium was employed to decrease the risk of spontaneous breathing leading to aerosolization.
The bronchoscope used in all cases was the disposable Ambu aScope and a corresponding monitor. The device was used to clear all secretions, clot, or mucus plugs, and to collect bronchoalveolar lavage (BAL) samples. If oxygen saturation decreased below 90%, the bronchoscopist interrupted the procedure and reconnected the patient to the ventilator. After an additional period of preoxygenation, bronchoscopy was then completed.
The mean age of the 107 patients was 62 years, and 81% were male. Dr. Chang and colleagues reported that, of the 241 bronchoscopies performed, no periprocedural complication of severe hypoxia requiring bag-valve ventilation, pneumothorax, or intraprocedural arrhythmias occurred, and that three patients required endotracheal tube advancement or replacement for dislodgement during the procedure.
About half of patients (51%) received a BAL, and 35 (65%) had a positive culture. Among 23 patients who had a negative tracheal culture, 8 patients had a positive BAL, which indicated a 35% diagnostic yield for patients with negative tracheal aspirates. In addition, three patients had differing cultures between the BAL and tracheal aspirate. One was growing Pseudomonas and Klebsiella in the tracheal aspirate with Enterococcus in the BAL, while the other two patients were growing an extra pathogen (Escherichia coli or Serratia) in the BAL.
“The most surprising data was the 65% rate of secondary infection with BAL, which is significantly higher than the rate in standard patients with acute respiratory distress syndrome,” Dr. Chang said. “Additionally, the high rate of bronchoscopy (33% in intubated patients) is also significantly higher than that of standard viral ARDS patients. This increased rate of superimposed infection and need for bronchoscopy may be due to the abnormally thick secretions seen in patients with COVID-19.”
Of the 10 cardiothoracic surgery team members, 1 resident was COVID-19 positive by reverse transcriptase polymerase chain reaction (rtPCR) prior to performing any bronchoscopies. The remaining nine team members tested negative for COVID-19 via nasal pharyngeal swab for rtPCR assay, with at least one negative test performed 2 weeks after the last bronchoscopy performed during the study period.
“The use of apnea was well tolerated by the patients and likely contributed to the lack of transmission of COVID-19 to the health care providers,” Dr. Chang said. “Additionally, this work demonstrates a higher rate of superinfection with bacterial or fungal pneumonia, compared to other reports. It is also the only one that describes the false negative rate for negative tracheal aspirates, which is the current recommended diagnostic test for secondary pneumonia in patients with COVID-19.” She acknowledged certain limitation of the study, including its retrospective design. “Thus, the clinical impact of bronchoscopy on patient outcomes cannot be accurately assessed.”
The authors reported having no financial disclosures.
SOURCE: Chang S et al. CHEST. 2020 Oct 8. doi: 10.1016/j.chest.2020.09.263.
Bronchoscopy with intermittent apnea can be conducted safely for both patients with severe COVID-19 and health care workers, a recent study has found. In addition, the high rate of superinfection in these patients indicates that bronchoalveolar lavage (BAL) should sent to the lab if there is any suspicion for secondary pneumonia.
Those are two key findings from a single-center retrospective study led by Stephanie H. Chang, MD, that was published in CHEST.
“While there is a risk of aerosolization and transmission of COVID-19 with bronchoscopy, this can be mitigated with bronchoscopy under intermittent apnea and appropriate PPE [personal protective equipment] in a negative-pressure room, with no significant adverse patient outcomes and a 0% rate of transmission to health care workers,” Dr. Chang, a thoracic surgeon in the department of cardiothoracic surgery at New York University Langone Health, said in an interview. “In appropriate clinical scenarios that will significantly impact patient care, bronchoscopy can be and should be safely performed in patients with COVID-19.”
Although a recent statement from the American Association for Bronchoscopy & Interventional Pulmonology indicates that bronchoscopy is relatively contraindicated in patients with suspected and confirmed COVID-19 infections, it does support use of the procedure in a subset of such patients. It reads: “The only role for bronchoscopy would be when less invasive testing to confirm COVID-19 are inconclusive, suspicion for an alternative diagnosis that would impact clinical management is suspected, or an urgent lifesaving intervention.”
For the current study, Dr. Chang and colleagues retrospectively studied the records of 412 patients with confirmed COVID-19 who were admitted to NYU Langone Health’s Manhattan campus between March 13 and April 24, 2020. If these, 321 required intubation and 107 (33%) underwent bronchoscopy, with a total of 241 bronchoscopies being performed.
Primary outcomes of interest were patient and health care provider safety, defined as freedom from periprocedural complications and COVID-19 transmission, respectively. Secondary outcomes included secondary infection with bacterial or fungal pneumonia.
The bronchoscopy team included six cardiothoracic surgeons and four cardiothoracic surgery residents. Each procedure was performed by a sole bronchoscopist in a negative-pressure room, with a bedside nurse immediately available outside of the room. The bronchoscopist wore full PPE, which consisted of hair cover, a fitted N95 mask, a face shield, gown, and gloves. Each patient was preoxygenated for 2 minutes with a fraction of inspired oxygen at 1.0 in order to maximize apneic time. For patients who were not on sedation and/or neuromuscular blockade, periprocedural anesthesia with propofol and rocuronium was employed to decrease the risk of spontaneous breathing leading to aerosolization.
The bronchoscope used in all cases was the disposable Ambu aScope and a corresponding monitor. The device was used to clear all secretions, clot, or mucus plugs, and to collect bronchoalveolar lavage (BAL) samples. If oxygen saturation decreased below 90%, the bronchoscopist interrupted the procedure and reconnected the patient to the ventilator. After an additional period of preoxygenation, bronchoscopy was then completed.
The mean age of the 107 patients was 62 years, and 81% were male. Dr. Chang and colleagues reported that, of the 241 bronchoscopies performed, no periprocedural complication of severe hypoxia requiring bag-valve ventilation, pneumothorax, or intraprocedural arrhythmias occurred, and that three patients required endotracheal tube advancement or replacement for dislodgement during the procedure.
About half of patients (51%) received a BAL, and 35 (65%) had a positive culture. Among 23 patients who had a negative tracheal culture, 8 patients had a positive BAL, which indicated a 35% diagnostic yield for patients with negative tracheal aspirates. In addition, three patients had differing cultures between the BAL and tracheal aspirate. One was growing Pseudomonas and Klebsiella in the tracheal aspirate with Enterococcus in the BAL, while the other two patients were growing an extra pathogen (Escherichia coli or Serratia) in the BAL.
“The most surprising data was the 65% rate of secondary infection with BAL, which is significantly higher than the rate in standard patients with acute respiratory distress syndrome,” Dr. Chang said. “Additionally, the high rate of bronchoscopy (33% in intubated patients) is also significantly higher than that of standard viral ARDS patients. This increased rate of superimposed infection and need for bronchoscopy may be due to the abnormally thick secretions seen in patients with COVID-19.”
Of the 10 cardiothoracic surgery team members, 1 resident was COVID-19 positive by reverse transcriptase polymerase chain reaction (rtPCR) prior to performing any bronchoscopies. The remaining nine team members tested negative for COVID-19 via nasal pharyngeal swab for rtPCR assay, with at least one negative test performed 2 weeks after the last bronchoscopy performed during the study period.
“The use of apnea was well tolerated by the patients and likely contributed to the lack of transmission of COVID-19 to the health care providers,” Dr. Chang said. “Additionally, this work demonstrates a higher rate of superinfection with bacterial or fungal pneumonia, compared to other reports. It is also the only one that describes the false negative rate for negative tracheal aspirates, which is the current recommended diagnostic test for secondary pneumonia in patients with COVID-19.” She acknowledged certain limitation of the study, including its retrospective design. “Thus, the clinical impact of bronchoscopy on patient outcomes cannot be accurately assessed.”
The authors reported having no financial disclosures.
SOURCE: Chang S et al. CHEST. 2020 Oct 8. doi: 10.1016/j.chest.2020.09.263.
FROM CHEST
COVID-19 experience forced residents to quickly improve patient communication skills
While the spring peak of COVID-19 was tough and traumatic for many residents and interns in a New York City health system, the experience may have accelerated their patient communication skills regarding difficult goals-of-care discussions, results of a recent survey suggest.
Breaking bad news was an everyday or every-other-day occurrence at the peak of the pandemic for nearly all of 50 of the trainees surveyed, who had worked at hospitals affiliated with the internal medicine residency program at the at the Icahn School of Medicine at Mount Sinai from March to June 2020.
However, trainees became significantly more comfortable and fluent in goals-of-care discussions during the pandemic, according to Patrick Tobin-Schnittger, MBBS, a third-year internal medicine resident in the Mount Sinai program.
“COVID-19 has obviously made a huge impact on the world, but I think it’s also made a huge impact on a whole generation of junior doctors,” said Dr. Tobin-Schnittger, who presented the findings in a late-breaking abstract session at the CHEST Annual Meeting, held virtually this year.
“It’ll be interesting to see what happens in the future as that generation matures, and I think one of the things is that we’re a lot more comfortable with end-of-life care,” he said in an interview conducted during the conference.
Nevertheless, coping with death may still be a challenge for many residents, according to Dr. Tobin-Schnittger. In the survey, internal medicine residents who had rarely encountered patient deaths suddenly found themselves experiencing deaths weekly, with more than one in five saying they were encountering it every day.
When asked to self-rate themselves according to Bugen’s Coping With Death scale, most participants had scores that suggested their ability to cope was suboptimal, the researcher said.
To help trainees cope with local COVID-19 surges, internal medicine residency programs should be implementing “breaking bad news” workshops and educating house staff on resilience in times of crisis, especially if it can be done virtually, according to Dr. Tobin-Schnittger.
“That could be done pretty quickly, and it could be done remotely so people could practice this from home,” he explained. “They wouldn’t even need to congregate in a big room.”
As a “mini-surge” of COVID-19 cases hits the United States, teaching self-care and coping techniques may also be important, said Mangala Narasimhan, DO, FCCP, director of critical care services at Northwell Health in New York City.
“We’ve had several sessions in our health system of letting people vent, talk about what happened, and tell stories about patients that they are still thinking about and haunted by – there was so much death,” Dr. Narasimhan said in an interview.
“People will be suffering for a long time thinking about what happened in March and April and May, so I think our focus now needs to be how to fix that in any way we can and to support people, as we’re dealing with these increases in numbers,” she said. “I think everyone’s panicking over the increase in numbers, but they’re panicking because of the fear of going through what they went through before.”
Dr. Tobin-Schnittger and colleagues sent their survey to 94 residents and interns in the Mount Sinai program who had worked through the peak of the pandemic. They received 50 responses. Of those individuals, the mean age was 29.5 years, and about 46% had worked for more than 3 years.
Before the pandemic, only 3 of the 50 respondents reported having goals-of-care conversations every day or every other day, while during the pandemic, those conversations were happening at least every other day for 38 of the respondents, survey data show.
Self-reported fluency and comfort with those discussions increased significantly, from a mean of about 50 on a scale of 100 before the pandemic to more than 75 during the pandemic, according to Dr. Tobin-Schnittger.
When asked how they remembered coping with patient death, one respondent described holding up a phone so a dying patient could hear his daughter’s voice. Another reported not being able to sleep at night.
“I constantly would have dreams that my patients were dying and there was nothing I could do about it,” the respondent said in a survey response.
A third respondent described the experience as ”humbling” but said there were rewarding aspects in patient care during the peak of the pandemic, which helped in being able to focus during difficult days.
Three participants (7.7%) said they changed their career plans as a result of the pandemic experience, the researchers reported.
Negative consequences of the peak pandemic experience included anger, anxiety, professional strain, trauma, and emotional distancing, some respondents reported.
However, others called attention to positive outcomes, such as more professional pride, resilience, confidence, and camaraderie.
“While we did encounter a lot of traumatic experiences, overall, there’s a huge sense that there is a lot more camaraderie within our department, but also within other departments,” said Dr. Tobin-Schnittger. “So I think there are some positives that come from this, and I think there’s been a bit of a culture change.”
Dr. Tobin-Schnittger said that he and his coauthors had no conflicts of interest or relationships with commercial interests to report.
SOURCE: Tobin-Schnittger P. CHEST 2020. Late-breaking abstract. doi: 10.1016/j.chest.2020.09.040.
While the spring peak of COVID-19 was tough and traumatic for many residents and interns in a New York City health system, the experience may have accelerated their patient communication skills regarding difficult goals-of-care discussions, results of a recent survey suggest.
Breaking bad news was an everyday or every-other-day occurrence at the peak of the pandemic for nearly all of 50 of the trainees surveyed, who had worked at hospitals affiliated with the internal medicine residency program at the at the Icahn School of Medicine at Mount Sinai from March to June 2020.
However, trainees became significantly more comfortable and fluent in goals-of-care discussions during the pandemic, according to Patrick Tobin-Schnittger, MBBS, a third-year internal medicine resident in the Mount Sinai program.
“COVID-19 has obviously made a huge impact on the world, but I think it’s also made a huge impact on a whole generation of junior doctors,” said Dr. Tobin-Schnittger, who presented the findings in a late-breaking abstract session at the CHEST Annual Meeting, held virtually this year.
“It’ll be interesting to see what happens in the future as that generation matures, and I think one of the things is that we’re a lot more comfortable with end-of-life care,” he said in an interview conducted during the conference.
Nevertheless, coping with death may still be a challenge for many residents, according to Dr. Tobin-Schnittger. In the survey, internal medicine residents who had rarely encountered patient deaths suddenly found themselves experiencing deaths weekly, with more than one in five saying they were encountering it every day.
When asked to self-rate themselves according to Bugen’s Coping With Death scale, most participants had scores that suggested their ability to cope was suboptimal, the researcher said.
To help trainees cope with local COVID-19 surges, internal medicine residency programs should be implementing “breaking bad news” workshops and educating house staff on resilience in times of crisis, especially if it can be done virtually, according to Dr. Tobin-Schnittger.
“That could be done pretty quickly, and it could be done remotely so people could practice this from home,” he explained. “They wouldn’t even need to congregate in a big room.”
As a “mini-surge” of COVID-19 cases hits the United States, teaching self-care and coping techniques may also be important, said Mangala Narasimhan, DO, FCCP, director of critical care services at Northwell Health in New York City.
“We’ve had several sessions in our health system of letting people vent, talk about what happened, and tell stories about patients that they are still thinking about and haunted by – there was so much death,” Dr. Narasimhan said in an interview.
“People will be suffering for a long time thinking about what happened in March and April and May, so I think our focus now needs to be how to fix that in any way we can and to support people, as we’re dealing with these increases in numbers,” she said. “I think everyone’s panicking over the increase in numbers, but they’re panicking because of the fear of going through what they went through before.”
Dr. Tobin-Schnittger and colleagues sent their survey to 94 residents and interns in the Mount Sinai program who had worked through the peak of the pandemic. They received 50 responses. Of those individuals, the mean age was 29.5 years, and about 46% had worked for more than 3 years.
Before the pandemic, only 3 of the 50 respondents reported having goals-of-care conversations every day or every other day, while during the pandemic, those conversations were happening at least every other day for 38 of the respondents, survey data show.
Self-reported fluency and comfort with those discussions increased significantly, from a mean of about 50 on a scale of 100 before the pandemic to more than 75 during the pandemic, according to Dr. Tobin-Schnittger.
When asked how they remembered coping with patient death, one respondent described holding up a phone so a dying patient could hear his daughter’s voice. Another reported not being able to sleep at night.
“I constantly would have dreams that my patients were dying and there was nothing I could do about it,” the respondent said in a survey response.
A third respondent described the experience as ”humbling” but said there were rewarding aspects in patient care during the peak of the pandemic, which helped in being able to focus during difficult days.
Three participants (7.7%) said they changed their career plans as a result of the pandemic experience, the researchers reported.
Negative consequences of the peak pandemic experience included anger, anxiety, professional strain, trauma, and emotional distancing, some respondents reported.
However, others called attention to positive outcomes, such as more professional pride, resilience, confidence, and camaraderie.
“While we did encounter a lot of traumatic experiences, overall, there’s a huge sense that there is a lot more camaraderie within our department, but also within other departments,” said Dr. Tobin-Schnittger. “So I think there are some positives that come from this, and I think there’s been a bit of a culture change.”
Dr. Tobin-Schnittger said that he and his coauthors had no conflicts of interest or relationships with commercial interests to report.
SOURCE: Tobin-Schnittger P. CHEST 2020. Late-breaking abstract. doi: 10.1016/j.chest.2020.09.040.
While the spring peak of COVID-19 was tough and traumatic for many residents and interns in a New York City health system, the experience may have accelerated their patient communication skills regarding difficult goals-of-care discussions, results of a recent survey suggest.
Breaking bad news was an everyday or every-other-day occurrence at the peak of the pandemic for nearly all of 50 of the trainees surveyed, who had worked at hospitals affiliated with the internal medicine residency program at the at the Icahn School of Medicine at Mount Sinai from March to June 2020.
However, trainees became significantly more comfortable and fluent in goals-of-care discussions during the pandemic, according to Patrick Tobin-Schnittger, MBBS, a third-year internal medicine resident in the Mount Sinai program.
“COVID-19 has obviously made a huge impact on the world, but I think it’s also made a huge impact on a whole generation of junior doctors,” said Dr. Tobin-Schnittger, who presented the findings in a late-breaking abstract session at the CHEST Annual Meeting, held virtually this year.
“It’ll be interesting to see what happens in the future as that generation matures, and I think one of the things is that we’re a lot more comfortable with end-of-life care,” he said in an interview conducted during the conference.
Nevertheless, coping with death may still be a challenge for many residents, according to Dr. Tobin-Schnittger. In the survey, internal medicine residents who had rarely encountered patient deaths suddenly found themselves experiencing deaths weekly, with more than one in five saying they were encountering it every day.
When asked to self-rate themselves according to Bugen’s Coping With Death scale, most participants had scores that suggested their ability to cope was suboptimal, the researcher said.
To help trainees cope with local COVID-19 surges, internal medicine residency programs should be implementing “breaking bad news” workshops and educating house staff on resilience in times of crisis, especially if it can be done virtually, according to Dr. Tobin-Schnittger.
“That could be done pretty quickly, and it could be done remotely so people could practice this from home,” he explained. “They wouldn’t even need to congregate in a big room.”
As a “mini-surge” of COVID-19 cases hits the United States, teaching self-care and coping techniques may also be important, said Mangala Narasimhan, DO, FCCP, director of critical care services at Northwell Health in New York City.
“We’ve had several sessions in our health system of letting people vent, talk about what happened, and tell stories about patients that they are still thinking about and haunted by – there was so much death,” Dr. Narasimhan said in an interview.
“People will be suffering for a long time thinking about what happened in March and April and May, so I think our focus now needs to be how to fix that in any way we can and to support people, as we’re dealing with these increases in numbers,” she said. “I think everyone’s panicking over the increase in numbers, but they’re panicking because of the fear of going through what they went through before.”
Dr. Tobin-Schnittger and colleagues sent their survey to 94 residents and interns in the Mount Sinai program who had worked through the peak of the pandemic. They received 50 responses. Of those individuals, the mean age was 29.5 years, and about 46% had worked for more than 3 years.
Before the pandemic, only 3 of the 50 respondents reported having goals-of-care conversations every day or every other day, while during the pandemic, those conversations were happening at least every other day for 38 of the respondents, survey data show.
Self-reported fluency and comfort with those discussions increased significantly, from a mean of about 50 on a scale of 100 before the pandemic to more than 75 during the pandemic, according to Dr. Tobin-Schnittger.
When asked how they remembered coping with patient death, one respondent described holding up a phone so a dying patient could hear his daughter’s voice. Another reported not being able to sleep at night.
“I constantly would have dreams that my patients were dying and there was nothing I could do about it,” the respondent said in a survey response.
A third respondent described the experience as ”humbling” but said there were rewarding aspects in patient care during the peak of the pandemic, which helped in being able to focus during difficult days.
Three participants (7.7%) said they changed their career plans as a result of the pandemic experience, the researchers reported.
Negative consequences of the peak pandemic experience included anger, anxiety, professional strain, trauma, and emotional distancing, some respondents reported.
However, others called attention to positive outcomes, such as more professional pride, resilience, confidence, and camaraderie.
“While we did encounter a lot of traumatic experiences, overall, there’s a huge sense that there is a lot more camaraderie within our department, but also within other departments,” said Dr. Tobin-Schnittger. “So I think there are some positives that come from this, and I think there’s been a bit of a culture change.”
Dr. Tobin-Schnittger said that he and his coauthors had no conflicts of interest or relationships with commercial interests to report.
SOURCE: Tobin-Schnittger P. CHEST 2020. Late-breaking abstract. doi: 10.1016/j.chest.2020.09.040.
FROM CHEST 2020
Link between vitamin D and ICU outcomes unclear
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CHEST 2020
Remdesivir effective, well-tolerated in final trial report
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
Drug beats placebo across multiple endpoints in COVID-19 patients
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
Optimal sedation strategies for COVID-19 ICU patients: A work in progress
According to the best available evidence, analagosedation remains the focus for managing COVID-19 ICU patients, according to Steven B. Greenberg, MD, FCCP, FCCM.
“The choice of sedation and analgesia is important,” Dr. Greenberg, vice chair of education in the department of anesthesiology at Evanston Hospital, part of NorthShore University Health System, Chicago, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. “We know that the right choice of these two components may increase liberation from ventilators, earlier ICU discharge, and return to normal brain function and independent functional status.”
Analgesia first
Prior to the current pandemic, the approach to sedation of patients in the ICU was based on the PADIS Guidelines of 2018, which call for an assessment-driven, protocol-based stepwise approach to pain and sedation management in critically ill adults (Crit Care Med. 2018;46:e825-73). “” Dr. Greenberg said. “We know that pain management should be a priority of sedation, because pain may increase the risk of delirium, anxiety, and endocrine suppression, and may increase the risk of release of endogenous catecholamines, ischemia, and hypermetabolic states.”
Fentanyl appears to be the most common opioid analgesic used for patients in the ICU, “but fentanyl is a very lipophilic drug and has a long context-sensitive half-life,” he said. “There are components to fentanyl that allow it to become a very long-acting drug upon days and days of infusion. Another opioid used is remifentanil, which is typically short-acting because it is broken down in the blood by esterases, but may cause rigidity at higher doses. Dilaudid seems to be the least affected by organ dysfunction. In our very critically ill, prolonged mechanically ventilated COVID-19 patients, we’ve been using methadone for its NMDA [N-methyl-D-aspartate] antagonistic effect and its opioid-sparing effects.”
As for nonopioid analgesics, Dr. Greenberg said that clinicians have shied away from using NSAIDs because of their side effects. “Tramadol indirectly inhibits reuptake of norepinephrine and serotonin, and ketamine is being used a lot more because of its NMDA antagonist effect,” he said. “Lidocaine and gabapentin have also been used.”
In a recent systematic review and meta-analysis, researchers assessed 34 trials that examined adjuvant analgesic use with an opioid in critically ill patients versus an opioid alone (Crit Care Expl. 2020;2:e0157). They found that when using an adjuvant such as acetaminophen, clonidine, dexmedetomidine, gabapentin, ketamine, magnesium, nefopam, NSAIDs, pregabalin, and tramadol, there was a reduction in pain scores as well as a reduction in opioid consumption. “So, clinicians should consider using adjuvant agents to limit opioid exposure and improve pain scores in the critically ill,” Dr. Greenberg said.
ICU delirium: Risk factors, prevention
Delirium in COVID-19 patients treated in the ICU of particular concern. According to a systematic review of 33 studies, 11 risk factors for delirium in the ICU were supported by strong or moderate levels of evidence (Crit Care Med. 2015;43:40-7). These include age, dementia, hypertension, emergency surgery, trauma, APACHE score of II, need for mechanical ventilation, metabolic acidosis, delirium on prior day, coma, and dexmedetomidine use. Risk factors for ICU delirium among COVID-19 patients, however, “are far different,” Dr. Greenberg said. “Why? First and foremost, we are restricting visitation of family,” he said. “That family connection largely can be lost. Second, there are limitations of nonpharmacologic interventions. There is less mobility and physical therapy employed because of the risk of health care workers’ exposure to the virus. There’s also uncertainty about the global pandemic. Anxiety and depression come with that, as well as disruptions to spiritual and religious services.”
Strategies for preventing delirium remain the same as before the pandemic and in accord with recent clinical practice guidelines: Reduce the use of certain drugs such as benzodiazepines and narcotics, reorient the patients, treat dehydration, use hearing aids and eyeglasses in patients who have them, use ear plugs to cancel noise, mobilize patients, maintain sleep/awake cycles, and encourage sedation holidays (Crit Care Med. 2018;46[9]:e825-73).
A recent study from France found that among 58 patients with COVID-19, 65% had positive Confusion Assessment Method (CAM)–ICU findings and 69% had agitation (N Engl J Med 2020;382:2268-70). Most of the patients (86%) received midazolam, 47% received propofol, and all received sufentanil. “In the pre-COVID days, we would use midazolam as a second-line agent for many of these patients,” Dr. Greenberg said. “So, times really have changed.”
The fate of COVID-19 patients following discharge from the ICU remains a concern, continued Dr. Greenberg, clinical professor of anesthesiology at the University of Chicago. A recent journal article by Michelle Biehl, MD, and Denise Sese, MD, noted that post–intensive care syndrome (PICS) or new or worsening impairment in any physical, cognitive, or mental domain is of significant concern among COVID-19 patients following their ICU stay (Cleveland Clin J Med 2020 Aug doi: 10.3949/ccjm.87a.ccc055). The authors stated that COVID-19 patients may face a higher risk of PICS because of restricted family visitation, prolonged mechanical ventilation, exposure to higher amounts of sedatives, and limited physical therapy during hospital stay.
No ideal sedative agent
The 2018 PADIS Guidelines on the use of ICU sedation suggested strong evidence for modifiable risk factors producing delirium in the context of benzodiazepines and blood transfusion. They recommend a light level of sedation and the use of propofol or dexmedetomidine over benzodiazepines. They also recommend routine delirium testing such as using the CAM-ICU or Intensive Care Delirium Screening Checklist (ICDSC) and nonpharmacologic therapies such as reorientation, cognitive stimulation, sleep improvement, and mobilization.
Several sedation-related factors may be related to an increased risk of delirium. “The type, dose, duration, and mode of delivery are very important,” Dr. Greenberg said. “The ideal sedative agent has a rapid, predictable onset; is short-acting; has anxiolytic, amnestic, and analgesic properties; is soluble; has a high therapeutic index; and no toxicity. The ideal sedative is also easy to administrate, contains no active metabolites, has minimal actions with other drugs, is reversible, and is cost effective. The problem is, there really is no ideal sedative agent. There is inadequate knowledge about the drugs [used to treat COVID-19 in the ICU] available to us, the dosage, and importantly, the pharmacokinetics and dynamics of these medications.”
The classic types of sedation being used in the ICU, he said, include the benzodiazepines midazolam, lorazepam, and diazepam, as well as propofol. Alternatives include dexmedetomidine, clonidine, ketamine, and the neuroleptics – haloperidol, quetiapine, olanzapine, ziprasidone, and risperidone. “The advantages of benzos are that they are anxiolytics, amnestics, and they are good sedatives with minimal hemodynamic effects,” Dr. Greenberg said.
Advantages of propofol include its sedative, hypnotic, and anxiolytic properties, he said. It reduces the cerebral metabolic rate and can relieve bronchospasm. “However, small studies have found that its use may be associated with an increased risk of delirium,” he said. “It is a respiratory depressant, and it can cause hypotension and decreased contractility. It has no analgesic properties, and two of the big concerns of its use in COVID-19 are the potential for hypertriglyceridemia and propofol infusion syndrome, particularly at doses of greater than 5 mg/kg per hour for greater than 48 hours. It is being given in high doses because patients are requiring higher doses to maintain ventilator synchrony.”
Choosing the right drug
The keys to success for sedation of ICU patients are choosing the right drug at the right dose for the right duration and the right mode of delivery, and applying them to the right population. However, as noted in a recent study, the pandemic poses unique challenges to clinicians in how they care for critically ill COVID-19 patients who require sedation (Anesth Analg. 2020 Apr 22. doi: 10.1213/ANE.0000000000004887). The use of provisional work areas “has escalated because of the amount of patients we’ve had to care for over the past nine months,” Dr. Greenberg said. “We’ve used alternate providers who are not necessarily familiar with the sedation and analgesic protocols and how to use these specific medications. Drug shortages have been on the rise, so there’s a need to understand alternative agents that can be used.”
COVID-19 patients face the potential risk for an increase in drug-drug interactions and side effects due to the polypharmacy that is often required to provide adequate sedation during mechanical ventilation. He noted that these patients may have “unusually high” analgesia and sedation requirements, particularly when they’re mechanically ventilated. A hypothesis as to why patients with COVID-19 require so much sedation and analgesia is that they often have a high respiratory drive and ventilator dyssynchrony, which requires increased neuromuscular blockade. “They also have an intense inflammatory response, which may be linked to tolerance of specific opioids and other medications,” Dr. Greenberg said. “Many ventilated COVID-19 patients are of younger age and previously in good health, and therefore, have an excellent metabolism. Health care providers are concerned about self-extubation. This prompts bedside providers to administer more sedatives to prevent this unwanted complication. There may also be a reduction of drip modifications by health care workers because of the potential risk of contracting COVID-19 when going into the room multiple times and for long periods of time” (Anesth Analg. 2020;131[1]:e34-e35).
According to a sedation resource on the SCCM website, about 5% of COVID-19 patients require mechanical ventilation. “There has been a massive shortage of the usual drugs that we use,” Dr. Greenberg said. “The demand for sedatives has increased by approximately 91%, while the demand for analgesics has increased by 79%, and neuromuscular blocker demand has increased by 105%.”
A retrospective study of 24 COVID-19 patients who required ventilation in the ICU found that the median daily dose of benzodiazepines was significantly higher, compared with the median daily dose used in the OSCILLATE trial (a median of 270 mg vs. 199 mg, respectively; Anesth Analg. 2020;131[4]e198-e200. doi: 10.1213/ane.0000000000005131). In addition, their median daily dose of opioid was approximately three times higher, compared with patients in the OSCILLATE trial (a median of 775 mg vs. 289 mg). Other agents used included propofol (84%), dexmedetomidine (53%), and ketamine (11%).
“A potential strategy for COVID-19 ICU patient sedation should be analgesia first, as indicated in the 2018 PADIS guidelines,” Dr. Greenberg advised. “We should also apply nonpharmacologic measures to reduce delirium. In nonintubated patients, we should use light to moderate sedation, targeting a RASS of –2 to +1, using hydromorphone or fentanyl boluses for analgesia and midazolam boluses or dexmedetomidine for sedation,.”
For intubated patients, he continued, target a RASS of –3 to –4, or –4 to –5 in those who require neuromuscular blockade. “Use propofol first then intermittent boluses of benzodiazepines,” said Dr. Greenberg, editor-in-chief of the Anesthesia Patient Safety Foundation newsletter. “For heavy sedation, use midazolam and supplement with ketamine and other analgesics and sedatives such as barbiturates, methadone, and even inhalation anesthetics in some cases.”
For analgesia in intubated patients, use fentanyl boluses then infusion. “Patients can easily become tachyphylactic to fentanyl, and it has a long context-sensitive half time,” he said. “Hydromorphone may be least affected by organ dysfunction.”
Dr. Greenberg concluded his presentation by stating that more studies are required “to delineate the best analgesia/sedation strategies and monitoring modalities for COVID-19 ICU patients.”
In commenting on the presentation, Mangala Narasimhan, DO, FCCP, senior vice president and director of critical care services at Northwell Health, said that the recommendations regarding sedation highlight a struggle that ICU providers have been dealing with during the COVID-19 epidemic.
“There have been unique challenges with COVID-19 and intubated patients. We have seen severe ventilator dyssynchrony and prolonged duration of mechanical ventilation. I think we can all agree that these patients have extremely high metabolic rates, have required high levels of sedation, have an increased need for neuromuscular blockade, and have high levels of delirium for extended periods of time. The recommendations provided here are reasonable. Strategies to prevent delirium should be employed, pain management should be prioritized, analgesics can help reduce the need for opioids. Alternatives to sedation are useful in this patient population and are well tolerated. Drug shortages have provided additional challenges to these strategies and have required us to think about the use of alternative agents. The recommendations echo the experience we have had with large numbers of intubated COVID-19 patients.”
Dr. Greenberg disclosed that he receives a stipend from the Anesthesia Patient Safety Foundation for serving as editor-in-chief of the foundation’s newsletter.
According to the best available evidence, analagosedation remains the focus for managing COVID-19 ICU patients, according to Steven B. Greenberg, MD, FCCP, FCCM.
“The choice of sedation and analgesia is important,” Dr. Greenberg, vice chair of education in the department of anesthesiology at Evanston Hospital, part of NorthShore University Health System, Chicago, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. “We know that the right choice of these two components may increase liberation from ventilators, earlier ICU discharge, and return to normal brain function and independent functional status.”
Analgesia first
Prior to the current pandemic, the approach to sedation of patients in the ICU was based on the PADIS Guidelines of 2018, which call for an assessment-driven, protocol-based stepwise approach to pain and sedation management in critically ill adults (Crit Care Med. 2018;46:e825-73). “” Dr. Greenberg said. “We know that pain management should be a priority of sedation, because pain may increase the risk of delirium, anxiety, and endocrine suppression, and may increase the risk of release of endogenous catecholamines, ischemia, and hypermetabolic states.”
Fentanyl appears to be the most common opioid analgesic used for patients in the ICU, “but fentanyl is a very lipophilic drug and has a long context-sensitive half-life,” he said. “There are components to fentanyl that allow it to become a very long-acting drug upon days and days of infusion. Another opioid used is remifentanil, which is typically short-acting because it is broken down in the blood by esterases, but may cause rigidity at higher doses. Dilaudid seems to be the least affected by organ dysfunction. In our very critically ill, prolonged mechanically ventilated COVID-19 patients, we’ve been using methadone for its NMDA [N-methyl-D-aspartate] antagonistic effect and its opioid-sparing effects.”
As for nonopioid analgesics, Dr. Greenberg said that clinicians have shied away from using NSAIDs because of their side effects. “Tramadol indirectly inhibits reuptake of norepinephrine and serotonin, and ketamine is being used a lot more because of its NMDA antagonist effect,” he said. “Lidocaine and gabapentin have also been used.”
In a recent systematic review and meta-analysis, researchers assessed 34 trials that examined adjuvant analgesic use with an opioid in critically ill patients versus an opioid alone (Crit Care Expl. 2020;2:e0157). They found that when using an adjuvant such as acetaminophen, clonidine, dexmedetomidine, gabapentin, ketamine, magnesium, nefopam, NSAIDs, pregabalin, and tramadol, there was a reduction in pain scores as well as a reduction in opioid consumption. “So, clinicians should consider using adjuvant agents to limit opioid exposure and improve pain scores in the critically ill,” Dr. Greenberg said.
ICU delirium: Risk factors, prevention
Delirium in COVID-19 patients treated in the ICU of particular concern. According to a systematic review of 33 studies, 11 risk factors for delirium in the ICU were supported by strong or moderate levels of evidence (Crit Care Med. 2015;43:40-7). These include age, dementia, hypertension, emergency surgery, trauma, APACHE score of II, need for mechanical ventilation, metabolic acidosis, delirium on prior day, coma, and dexmedetomidine use. Risk factors for ICU delirium among COVID-19 patients, however, “are far different,” Dr. Greenberg said. “Why? First and foremost, we are restricting visitation of family,” he said. “That family connection largely can be lost. Second, there are limitations of nonpharmacologic interventions. There is less mobility and physical therapy employed because of the risk of health care workers’ exposure to the virus. There’s also uncertainty about the global pandemic. Anxiety and depression come with that, as well as disruptions to spiritual and religious services.”
Strategies for preventing delirium remain the same as before the pandemic and in accord with recent clinical practice guidelines: Reduce the use of certain drugs such as benzodiazepines and narcotics, reorient the patients, treat dehydration, use hearing aids and eyeglasses in patients who have them, use ear plugs to cancel noise, mobilize patients, maintain sleep/awake cycles, and encourage sedation holidays (Crit Care Med. 2018;46[9]:e825-73).
A recent study from France found that among 58 patients with COVID-19, 65% had positive Confusion Assessment Method (CAM)–ICU findings and 69% had agitation (N Engl J Med 2020;382:2268-70). Most of the patients (86%) received midazolam, 47% received propofol, and all received sufentanil. “In the pre-COVID days, we would use midazolam as a second-line agent for many of these patients,” Dr. Greenberg said. “So, times really have changed.”
The fate of COVID-19 patients following discharge from the ICU remains a concern, continued Dr. Greenberg, clinical professor of anesthesiology at the University of Chicago. A recent journal article by Michelle Biehl, MD, and Denise Sese, MD, noted that post–intensive care syndrome (PICS) or new or worsening impairment in any physical, cognitive, or mental domain is of significant concern among COVID-19 patients following their ICU stay (Cleveland Clin J Med 2020 Aug doi: 10.3949/ccjm.87a.ccc055). The authors stated that COVID-19 patients may face a higher risk of PICS because of restricted family visitation, prolonged mechanical ventilation, exposure to higher amounts of sedatives, and limited physical therapy during hospital stay.
No ideal sedative agent
The 2018 PADIS Guidelines on the use of ICU sedation suggested strong evidence for modifiable risk factors producing delirium in the context of benzodiazepines and blood transfusion. They recommend a light level of sedation and the use of propofol or dexmedetomidine over benzodiazepines. They also recommend routine delirium testing such as using the CAM-ICU or Intensive Care Delirium Screening Checklist (ICDSC) and nonpharmacologic therapies such as reorientation, cognitive stimulation, sleep improvement, and mobilization.
Several sedation-related factors may be related to an increased risk of delirium. “The type, dose, duration, and mode of delivery are very important,” Dr. Greenberg said. “The ideal sedative agent has a rapid, predictable onset; is short-acting; has anxiolytic, amnestic, and analgesic properties; is soluble; has a high therapeutic index; and no toxicity. The ideal sedative is also easy to administrate, contains no active metabolites, has minimal actions with other drugs, is reversible, and is cost effective. The problem is, there really is no ideal sedative agent. There is inadequate knowledge about the drugs [used to treat COVID-19 in the ICU] available to us, the dosage, and importantly, the pharmacokinetics and dynamics of these medications.”
The classic types of sedation being used in the ICU, he said, include the benzodiazepines midazolam, lorazepam, and diazepam, as well as propofol. Alternatives include dexmedetomidine, clonidine, ketamine, and the neuroleptics – haloperidol, quetiapine, olanzapine, ziprasidone, and risperidone. “The advantages of benzos are that they are anxiolytics, amnestics, and they are good sedatives with minimal hemodynamic effects,” Dr. Greenberg said.
Advantages of propofol include its sedative, hypnotic, and anxiolytic properties, he said. It reduces the cerebral metabolic rate and can relieve bronchospasm. “However, small studies have found that its use may be associated with an increased risk of delirium,” he said. “It is a respiratory depressant, and it can cause hypotension and decreased contractility. It has no analgesic properties, and two of the big concerns of its use in COVID-19 are the potential for hypertriglyceridemia and propofol infusion syndrome, particularly at doses of greater than 5 mg/kg per hour for greater than 48 hours. It is being given in high doses because patients are requiring higher doses to maintain ventilator synchrony.”
Choosing the right drug
The keys to success for sedation of ICU patients are choosing the right drug at the right dose for the right duration and the right mode of delivery, and applying them to the right population. However, as noted in a recent study, the pandemic poses unique challenges to clinicians in how they care for critically ill COVID-19 patients who require sedation (Anesth Analg. 2020 Apr 22. doi: 10.1213/ANE.0000000000004887). The use of provisional work areas “has escalated because of the amount of patients we’ve had to care for over the past nine months,” Dr. Greenberg said. “We’ve used alternate providers who are not necessarily familiar with the sedation and analgesic protocols and how to use these specific medications. Drug shortages have been on the rise, so there’s a need to understand alternative agents that can be used.”
COVID-19 patients face the potential risk for an increase in drug-drug interactions and side effects due to the polypharmacy that is often required to provide adequate sedation during mechanical ventilation. He noted that these patients may have “unusually high” analgesia and sedation requirements, particularly when they’re mechanically ventilated. A hypothesis as to why patients with COVID-19 require so much sedation and analgesia is that they often have a high respiratory drive and ventilator dyssynchrony, which requires increased neuromuscular blockade. “They also have an intense inflammatory response, which may be linked to tolerance of specific opioids and other medications,” Dr. Greenberg said. “Many ventilated COVID-19 patients are of younger age and previously in good health, and therefore, have an excellent metabolism. Health care providers are concerned about self-extubation. This prompts bedside providers to administer more sedatives to prevent this unwanted complication. There may also be a reduction of drip modifications by health care workers because of the potential risk of contracting COVID-19 when going into the room multiple times and for long periods of time” (Anesth Analg. 2020;131[1]:e34-e35).
According to a sedation resource on the SCCM website, about 5% of COVID-19 patients require mechanical ventilation. “There has been a massive shortage of the usual drugs that we use,” Dr. Greenberg said. “The demand for sedatives has increased by approximately 91%, while the demand for analgesics has increased by 79%, and neuromuscular blocker demand has increased by 105%.”
A retrospective study of 24 COVID-19 patients who required ventilation in the ICU found that the median daily dose of benzodiazepines was significantly higher, compared with the median daily dose used in the OSCILLATE trial (a median of 270 mg vs. 199 mg, respectively; Anesth Analg. 2020;131[4]e198-e200. doi: 10.1213/ane.0000000000005131). In addition, their median daily dose of opioid was approximately three times higher, compared with patients in the OSCILLATE trial (a median of 775 mg vs. 289 mg). Other agents used included propofol (84%), dexmedetomidine (53%), and ketamine (11%).
“A potential strategy for COVID-19 ICU patient sedation should be analgesia first, as indicated in the 2018 PADIS guidelines,” Dr. Greenberg advised. “We should also apply nonpharmacologic measures to reduce delirium. In nonintubated patients, we should use light to moderate sedation, targeting a RASS of –2 to +1, using hydromorphone or fentanyl boluses for analgesia and midazolam boluses or dexmedetomidine for sedation,.”
For intubated patients, he continued, target a RASS of –3 to –4, or –4 to –5 in those who require neuromuscular blockade. “Use propofol first then intermittent boluses of benzodiazepines,” said Dr. Greenberg, editor-in-chief of the Anesthesia Patient Safety Foundation newsletter. “For heavy sedation, use midazolam and supplement with ketamine and other analgesics and sedatives such as barbiturates, methadone, and even inhalation anesthetics in some cases.”
For analgesia in intubated patients, use fentanyl boluses then infusion. “Patients can easily become tachyphylactic to fentanyl, and it has a long context-sensitive half time,” he said. “Hydromorphone may be least affected by organ dysfunction.”
Dr. Greenberg concluded his presentation by stating that more studies are required “to delineate the best analgesia/sedation strategies and monitoring modalities for COVID-19 ICU patients.”
In commenting on the presentation, Mangala Narasimhan, DO, FCCP, senior vice president and director of critical care services at Northwell Health, said that the recommendations regarding sedation highlight a struggle that ICU providers have been dealing with during the COVID-19 epidemic.
“There have been unique challenges with COVID-19 and intubated patients. We have seen severe ventilator dyssynchrony and prolonged duration of mechanical ventilation. I think we can all agree that these patients have extremely high metabolic rates, have required high levels of sedation, have an increased need for neuromuscular blockade, and have high levels of delirium for extended periods of time. The recommendations provided here are reasonable. Strategies to prevent delirium should be employed, pain management should be prioritized, analgesics can help reduce the need for opioids. Alternatives to sedation are useful in this patient population and are well tolerated. Drug shortages have provided additional challenges to these strategies and have required us to think about the use of alternative agents. The recommendations echo the experience we have had with large numbers of intubated COVID-19 patients.”
Dr. Greenberg disclosed that he receives a stipend from the Anesthesia Patient Safety Foundation for serving as editor-in-chief of the foundation’s newsletter.
According to the best available evidence, analagosedation remains the focus for managing COVID-19 ICU patients, according to Steven B. Greenberg, MD, FCCP, FCCM.
“The choice of sedation and analgesia is important,” Dr. Greenberg, vice chair of education in the department of anesthesiology at Evanston Hospital, part of NorthShore University Health System, Chicago, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. “We know that the right choice of these two components may increase liberation from ventilators, earlier ICU discharge, and return to normal brain function and independent functional status.”
Analgesia first
Prior to the current pandemic, the approach to sedation of patients in the ICU was based on the PADIS Guidelines of 2018, which call for an assessment-driven, protocol-based stepwise approach to pain and sedation management in critically ill adults (Crit Care Med. 2018;46:e825-73). “” Dr. Greenberg said. “We know that pain management should be a priority of sedation, because pain may increase the risk of delirium, anxiety, and endocrine suppression, and may increase the risk of release of endogenous catecholamines, ischemia, and hypermetabolic states.”
Fentanyl appears to be the most common opioid analgesic used for patients in the ICU, “but fentanyl is a very lipophilic drug and has a long context-sensitive half-life,” he said. “There are components to fentanyl that allow it to become a very long-acting drug upon days and days of infusion. Another opioid used is remifentanil, which is typically short-acting because it is broken down in the blood by esterases, but may cause rigidity at higher doses. Dilaudid seems to be the least affected by organ dysfunction. In our very critically ill, prolonged mechanically ventilated COVID-19 patients, we’ve been using methadone for its NMDA [N-methyl-D-aspartate] antagonistic effect and its opioid-sparing effects.”
As for nonopioid analgesics, Dr. Greenberg said that clinicians have shied away from using NSAIDs because of their side effects. “Tramadol indirectly inhibits reuptake of norepinephrine and serotonin, and ketamine is being used a lot more because of its NMDA antagonist effect,” he said. “Lidocaine and gabapentin have also been used.”
In a recent systematic review and meta-analysis, researchers assessed 34 trials that examined adjuvant analgesic use with an opioid in critically ill patients versus an opioid alone (Crit Care Expl. 2020;2:e0157). They found that when using an adjuvant such as acetaminophen, clonidine, dexmedetomidine, gabapentin, ketamine, magnesium, nefopam, NSAIDs, pregabalin, and tramadol, there was a reduction in pain scores as well as a reduction in opioid consumption. “So, clinicians should consider using adjuvant agents to limit opioid exposure and improve pain scores in the critically ill,” Dr. Greenberg said.
ICU delirium: Risk factors, prevention
Delirium in COVID-19 patients treated in the ICU of particular concern. According to a systematic review of 33 studies, 11 risk factors for delirium in the ICU were supported by strong or moderate levels of evidence (Crit Care Med. 2015;43:40-7). These include age, dementia, hypertension, emergency surgery, trauma, APACHE score of II, need for mechanical ventilation, metabolic acidosis, delirium on prior day, coma, and dexmedetomidine use. Risk factors for ICU delirium among COVID-19 patients, however, “are far different,” Dr. Greenberg said. “Why? First and foremost, we are restricting visitation of family,” he said. “That family connection largely can be lost. Second, there are limitations of nonpharmacologic interventions. There is less mobility and physical therapy employed because of the risk of health care workers’ exposure to the virus. There’s also uncertainty about the global pandemic. Anxiety and depression come with that, as well as disruptions to spiritual and religious services.”
Strategies for preventing delirium remain the same as before the pandemic and in accord with recent clinical practice guidelines: Reduce the use of certain drugs such as benzodiazepines and narcotics, reorient the patients, treat dehydration, use hearing aids and eyeglasses in patients who have them, use ear plugs to cancel noise, mobilize patients, maintain sleep/awake cycles, and encourage sedation holidays (Crit Care Med. 2018;46[9]:e825-73).
A recent study from France found that among 58 patients with COVID-19, 65% had positive Confusion Assessment Method (CAM)–ICU findings and 69% had agitation (N Engl J Med 2020;382:2268-70). Most of the patients (86%) received midazolam, 47% received propofol, and all received sufentanil. “In the pre-COVID days, we would use midazolam as a second-line agent for many of these patients,” Dr. Greenberg said. “So, times really have changed.”
The fate of COVID-19 patients following discharge from the ICU remains a concern, continued Dr. Greenberg, clinical professor of anesthesiology at the University of Chicago. A recent journal article by Michelle Biehl, MD, and Denise Sese, MD, noted that post–intensive care syndrome (PICS) or new or worsening impairment in any physical, cognitive, or mental domain is of significant concern among COVID-19 patients following their ICU stay (Cleveland Clin J Med 2020 Aug doi: 10.3949/ccjm.87a.ccc055). The authors stated that COVID-19 patients may face a higher risk of PICS because of restricted family visitation, prolonged mechanical ventilation, exposure to higher amounts of sedatives, and limited physical therapy during hospital stay.
No ideal sedative agent
The 2018 PADIS Guidelines on the use of ICU sedation suggested strong evidence for modifiable risk factors producing delirium in the context of benzodiazepines and blood transfusion. They recommend a light level of sedation and the use of propofol or dexmedetomidine over benzodiazepines. They also recommend routine delirium testing such as using the CAM-ICU or Intensive Care Delirium Screening Checklist (ICDSC) and nonpharmacologic therapies such as reorientation, cognitive stimulation, sleep improvement, and mobilization.
Several sedation-related factors may be related to an increased risk of delirium. “The type, dose, duration, and mode of delivery are very important,” Dr. Greenberg said. “The ideal sedative agent has a rapid, predictable onset; is short-acting; has anxiolytic, amnestic, and analgesic properties; is soluble; has a high therapeutic index; and no toxicity. The ideal sedative is also easy to administrate, contains no active metabolites, has minimal actions with other drugs, is reversible, and is cost effective. The problem is, there really is no ideal sedative agent. There is inadequate knowledge about the drugs [used to treat COVID-19 in the ICU] available to us, the dosage, and importantly, the pharmacokinetics and dynamics of these medications.”
The classic types of sedation being used in the ICU, he said, include the benzodiazepines midazolam, lorazepam, and diazepam, as well as propofol. Alternatives include dexmedetomidine, clonidine, ketamine, and the neuroleptics – haloperidol, quetiapine, olanzapine, ziprasidone, and risperidone. “The advantages of benzos are that they are anxiolytics, amnestics, and they are good sedatives with minimal hemodynamic effects,” Dr. Greenberg said.
Advantages of propofol include its sedative, hypnotic, and anxiolytic properties, he said. It reduces the cerebral metabolic rate and can relieve bronchospasm. “However, small studies have found that its use may be associated with an increased risk of delirium,” he said. “It is a respiratory depressant, and it can cause hypotension and decreased contractility. It has no analgesic properties, and two of the big concerns of its use in COVID-19 are the potential for hypertriglyceridemia and propofol infusion syndrome, particularly at doses of greater than 5 mg/kg per hour for greater than 48 hours. It is being given in high doses because patients are requiring higher doses to maintain ventilator synchrony.”
Choosing the right drug
The keys to success for sedation of ICU patients are choosing the right drug at the right dose for the right duration and the right mode of delivery, and applying them to the right population. However, as noted in a recent study, the pandemic poses unique challenges to clinicians in how they care for critically ill COVID-19 patients who require sedation (Anesth Analg. 2020 Apr 22. doi: 10.1213/ANE.0000000000004887). The use of provisional work areas “has escalated because of the amount of patients we’ve had to care for over the past nine months,” Dr. Greenberg said. “We’ve used alternate providers who are not necessarily familiar with the sedation and analgesic protocols and how to use these specific medications. Drug shortages have been on the rise, so there’s a need to understand alternative agents that can be used.”
COVID-19 patients face the potential risk for an increase in drug-drug interactions and side effects due to the polypharmacy that is often required to provide adequate sedation during mechanical ventilation. He noted that these patients may have “unusually high” analgesia and sedation requirements, particularly when they’re mechanically ventilated. A hypothesis as to why patients with COVID-19 require so much sedation and analgesia is that they often have a high respiratory drive and ventilator dyssynchrony, which requires increased neuromuscular blockade. “They also have an intense inflammatory response, which may be linked to tolerance of specific opioids and other medications,” Dr. Greenberg said. “Many ventilated COVID-19 patients are of younger age and previously in good health, and therefore, have an excellent metabolism. Health care providers are concerned about self-extubation. This prompts bedside providers to administer more sedatives to prevent this unwanted complication. There may also be a reduction of drip modifications by health care workers because of the potential risk of contracting COVID-19 when going into the room multiple times and for long periods of time” (Anesth Analg. 2020;131[1]:e34-e35).
According to a sedation resource on the SCCM website, about 5% of COVID-19 patients require mechanical ventilation. “There has been a massive shortage of the usual drugs that we use,” Dr. Greenberg said. “The demand for sedatives has increased by approximately 91%, while the demand for analgesics has increased by 79%, and neuromuscular blocker demand has increased by 105%.”
A retrospective study of 24 COVID-19 patients who required ventilation in the ICU found that the median daily dose of benzodiazepines was significantly higher, compared with the median daily dose used in the OSCILLATE trial (a median of 270 mg vs. 199 mg, respectively; Anesth Analg. 2020;131[4]e198-e200. doi: 10.1213/ane.0000000000005131). In addition, their median daily dose of opioid was approximately three times higher, compared with patients in the OSCILLATE trial (a median of 775 mg vs. 289 mg). Other agents used included propofol (84%), dexmedetomidine (53%), and ketamine (11%).
“A potential strategy for COVID-19 ICU patient sedation should be analgesia first, as indicated in the 2018 PADIS guidelines,” Dr. Greenberg advised. “We should also apply nonpharmacologic measures to reduce delirium. In nonintubated patients, we should use light to moderate sedation, targeting a RASS of –2 to +1, using hydromorphone or fentanyl boluses for analgesia and midazolam boluses or dexmedetomidine for sedation,.”
For intubated patients, he continued, target a RASS of –3 to –4, or –4 to –5 in those who require neuromuscular blockade. “Use propofol first then intermittent boluses of benzodiazepines,” said Dr. Greenberg, editor-in-chief of the Anesthesia Patient Safety Foundation newsletter. “For heavy sedation, use midazolam and supplement with ketamine and other analgesics and sedatives such as barbiturates, methadone, and even inhalation anesthetics in some cases.”
For analgesia in intubated patients, use fentanyl boluses then infusion. “Patients can easily become tachyphylactic to fentanyl, and it has a long context-sensitive half time,” he said. “Hydromorphone may be least affected by organ dysfunction.”
Dr. Greenberg concluded his presentation by stating that more studies are required “to delineate the best analgesia/sedation strategies and monitoring modalities for COVID-19 ICU patients.”
In commenting on the presentation, Mangala Narasimhan, DO, FCCP, senior vice president and director of critical care services at Northwell Health, said that the recommendations regarding sedation highlight a struggle that ICU providers have been dealing with during the COVID-19 epidemic.
“There have been unique challenges with COVID-19 and intubated patients. We have seen severe ventilator dyssynchrony and prolonged duration of mechanical ventilation. I think we can all agree that these patients have extremely high metabolic rates, have required high levels of sedation, have an increased need for neuromuscular blockade, and have high levels of delirium for extended periods of time. The recommendations provided here are reasonable. Strategies to prevent delirium should be employed, pain management should be prioritized, analgesics can help reduce the need for opioids. Alternatives to sedation are useful in this patient population and are well tolerated. Drug shortages have provided additional challenges to these strategies and have required us to think about the use of alternative agents. The recommendations echo the experience we have had with large numbers of intubated COVID-19 patients.”
Dr. Greenberg disclosed that he receives a stipend from the Anesthesia Patient Safety Foundation for serving as editor-in-chief of the foundation’s newsletter.
FROM AN SCCM VIRTUAL MEETING
COVID-19 airway management: Expert tips on infection control
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
FROM AN SCCM VIRTUAL MEETING