User login
Should antibiotic treatment be used toward the end of life?
Diagnosing an infection is complex because of the presence of symptoms that are often nonspecific and that are common in patients in decline toward the end of life. Use of antibiotic therapy in this patient population is still controversial, because the clinical benefits are not clear and the risk of pointless overmedicalization is very high.
Etiology
For patients who are receiving palliative care, the following factors predispose to an infection:
- Increasing fragility.
- Bedbound status and anorexia/cachexia syndrome.
- Weakened immune defenses owing to disease or treatments.
- Changes to skin integrity, related to venous access sites and/or bladder catheterization.
Four-week cutoff
For patients who are expected to live for fewer than 4 weeks, evidence from the literature shows that antimicrobial therapy does not resolve a potential infection or improve the prognosis. Antibiotics should therefore be used only for improving symptom management.
In practice, the most common infections in patients receiving end-of-life care are in the urinary and respiratory tracts. Antibiotics are beneficial in the short term in managing symptoms associated with urinary tract infections (effective in 60%-92% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death.
Antibiotics are also beneficial in managing symptoms associated with respiratory tract infections (effective in up to 53% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death. However, the risk of futility is high. As an alternative, opioids and antitussives could provide greater benefit for patients with dyspnea and cough.
No benefit has been observed with the use of antibiotics to treat symptoms associated with sepsis, abscesses, and deep and complicated infections. Antibiotics are therefore deemed futile in these cases.
In unclear cases, the “2-day rule” is useful. This involves waiting for 2 days, and if the patient remains clinically stable, prescribing antibiotics. If the patient’s condition deteriorates rapidly and progressively, antibiotics should not be prescribed.
Alternatively, one can prescribe antibiotics immediately. If no clinical improvement is observed after 2 days, the antibiotics should be stopped, especially if deterioration of the patient’s condition is rapid and progressive.
Increased body temperature is somewhat common in the last days and hours of life and is not generally associated with symptoms. Fever in these cases is not an indication for the use of antimicrobial therapy.
The most common laboratory markers of infection (C-reactive protein level, erythrocyte sedimentation rate, leukocyte level) are not particularly useful in this patient population, because they are affected by the baseline condition as well as by any treatments given and the state of systemic inflammation, which is associated with the decline in overall health in the last few weeks of life.
The choice should be individualized and shared with patients and family members so that the clinical appropriateness of the therapeutic strategy is evident and that decisions regarding antibiotic treatment are not regarded as a failure to treat the patient.
The longer term
In deciding to start antibiotic therapy, consideration must be given to the patient’s overall health, the treatment objectives, the possibility that the antibiotic will resolve the infection or improve the patient’s symptoms, and the estimated prognosis, which must be sufficiently long to allow the antibiotic time to take effect.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Diagnosing an infection is complex because of the presence of symptoms that are often nonspecific and that are common in patients in decline toward the end of life. Use of antibiotic therapy in this patient population is still controversial, because the clinical benefits are not clear and the risk of pointless overmedicalization is very high.
Etiology
For patients who are receiving palliative care, the following factors predispose to an infection:
- Increasing fragility.
- Bedbound status and anorexia/cachexia syndrome.
- Weakened immune defenses owing to disease or treatments.
- Changes to skin integrity, related to venous access sites and/or bladder catheterization.
Four-week cutoff
For patients who are expected to live for fewer than 4 weeks, evidence from the literature shows that antimicrobial therapy does not resolve a potential infection or improve the prognosis. Antibiotics should therefore be used only for improving symptom management.
In practice, the most common infections in patients receiving end-of-life care are in the urinary and respiratory tracts. Antibiotics are beneficial in the short term in managing symptoms associated with urinary tract infections (effective in 60%-92% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death.
Antibiotics are also beneficial in managing symptoms associated with respiratory tract infections (effective in up to 53% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death. However, the risk of futility is high. As an alternative, opioids and antitussives could provide greater benefit for patients with dyspnea and cough.
No benefit has been observed with the use of antibiotics to treat symptoms associated with sepsis, abscesses, and deep and complicated infections. Antibiotics are therefore deemed futile in these cases.
In unclear cases, the “2-day rule” is useful. This involves waiting for 2 days, and if the patient remains clinically stable, prescribing antibiotics. If the patient’s condition deteriorates rapidly and progressively, antibiotics should not be prescribed.
Alternatively, one can prescribe antibiotics immediately. If no clinical improvement is observed after 2 days, the antibiotics should be stopped, especially if deterioration of the patient’s condition is rapid and progressive.
Increased body temperature is somewhat common in the last days and hours of life and is not generally associated with symptoms. Fever in these cases is not an indication for the use of antimicrobial therapy.
The most common laboratory markers of infection (C-reactive protein level, erythrocyte sedimentation rate, leukocyte level) are not particularly useful in this patient population, because they are affected by the baseline condition as well as by any treatments given and the state of systemic inflammation, which is associated with the decline in overall health in the last few weeks of life.
The choice should be individualized and shared with patients and family members so that the clinical appropriateness of the therapeutic strategy is evident and that decisions regarding antibiotic treatment are not regarded as a failure to treat the patient.
The longer term
In deciding to start antibiotic therapy, consideration must be given to the patient’s overall health, the treatment objectives, the possibility that the antibiotic will resolve the infection or improve the patient’s symptoms, and the estimated prognosis, which must be sufficiently long to allow the antibiotic time to take effect.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Diagnosing an infection is complex because of the presence of symptoms that are often nonspecific and that are common in patients in decline toward the end of life. Use of antibiotic therapy in this patient population is still controversial, because the clinical benefits are not clear and the risk of pointless overmedicalization is very high.
Etiology
For patients who are receiving palliative care, the following factors predispose to an infection:
- Increasing fragility.
- Bedbound status and anorexia/cachexia syndrome.
- Weakened immune defenses owing to disease or treatments.
- Changes to skin integrity, related to venous access sites and/or bladder catheterization.
Four-week cutoff
For patients who are expected to live for fewer than 4 weeks, evidence from the literature shows that antimicrobial therapy does not resolve a potential infection or improve the prognosis. Antibiotics should therefore be used only for improving symptom management.
In practice, the most common infections in patients receiving end-of-life care are in the urinary and respiratory tracts. Antibiotics are beneficial in the short term in managing symptoms associated with urinary tract infections (effective in 60%-92% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death.
Antibiotics are also beneficial in managing symptoms associated with respiratory tract infections (effective in up to 53% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death. However, the risk of futility is high. As an alternative, opioids and antitussives could provide greater benefit for patients with dyspnea and cough.
No benefit has been observed with the use of antibiotics to treat symptoms associated with sepsis, abscesses, and deep and complicated infections. Antibiotics are therefore deemed futile in these cases.
In unclear cases, the “2-day rule” is useful. This involves waiting for 2 days, and if the patient remains clinically stable, prescribing antibiotics. If the patient’s condition deteriorates rapidly and progressively, antibiotics should not be prescribed.
Alternatively, one can prescribe antibiotics immediately. If no clinical improvement is observed after 2 days, the antibiotics should be stopped, especially if deterioration of the patient’s condition is rapid and progressive.
Increased body temperature is somewhat common in the last days and hours of life and is not generally associated with symptoms. Fever in these cases is not an indication for the use of antimicrobial therapy.
The most common laboratory markers of infection (C-reactive protein level, erythrocyte sedimentation rate, leukocyte level) are not particularly useful in this patient population, because they are affected by the baseline condition as well as by any treatments given and the state of systemic inflammation, which is associated with the decline in overall health in the last few weeks of life.
The choice should be individualized and shared with patients and family members so that the clinical appropriateness of the therapeutic strategy is evident and that decisions regarding antibiotic treatment are not regarded as a failure to treat the patient.
The longer term
In deciding to start antibiotic therapy, consideration must be given to the patient’s overall health, the treatment objectives, the possibility that the antibiotic will resolve the infection or improve the patient’s symptoms, and the estimated prognosis, which must be sufficiently long to allow the antibiotic time to take effect.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Antibiotics for acute exacerbation of COPD: It’s still controversial
In late 2021, the Rome Proposal for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and grading their severity was published. The 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) Report has adopted the Rome Proposal criteria. Given that an endorsement by GOLD is tantamount to acceptance by clinicians, researchers, and policymakers alike, I guess we’re all using them now.
Anyone who’s ever cared for patients with COPD knows that treatment and reduction of exacerbations is how we improve outcomes. AECOPD are associated with considerable morbidity, greater health care utilization and costs, and a long-term decline in lung function. While we hope our pharmacotherapies improve symptoms, we know they reduce AECOPD. If our pharmacotherapies have any impact on mortality, it’s probably via AECOPD prevention.
Since antibiotic indications are tied to severity, using the Rome Proposal criteria may affect management in unpredictable ways. As such, it’s worth reviewing the data on antibiotics for AECOPD.
What do the data reveal?
To start, it’s important to note that GOLD doesn’t equate having an AECOPD with needing an antibiotic. I myself have conflated the diagnosis with the indication and thereby overprescribed. The bar for diagnosis is quite low. In previous GOLD summaries, any “change in respiratory symptoms” would warrant the AECOPD label. Although the Rome Proposal definition is more specific, it leaves room for liberal interpretation. It’s likely to have a greater effect on research than on clinical practice. My guess is that AECOPD prevalence doesn’t change.
The antibiotic hurdle is slightly higher than that for diagnosis but is equally open to interpretation. In part, that’s related to the inherent subjectivity of judging symptoms, sputum production, and changes in color, but it’s also because the data are so poor. The meta-analyses that have been used to establish the indications include fewer than 1000 patients spread across 10 to 11 trials. Thus, the individual trials are small, and the sample size remains nominal even after adding them together. The addition of antibiotics – and it doesn’t seem to matter which class, type, or duration – will decrease mortality and hospital length of stay. One study says these effects are limited to inpatients while the other does not. After reading GOLD 2013, GOLD 2023, and both the meta-analyses they used to support their recommendations, I’m still not sure who benefits. Do you have to be hospitalized? Is some sort of ventilatory support required? Does C-reactive protein help or not?
In accordance with the classic Anthonisen criteria, GOLD relies on sputum volume and color as evidence of a bacterial infection. Soon after GOLD 2023 was published, a meta-analysis found that sputum color isn’t particularly accurate for detecting bacterial infection. Because it doesn’t seem to matter which antibiotic class is used, I always thought we were using antibiotics for their magical, pleiotropic anti-inflammatory effects anyway. I didn’t think the presence of an actual bacterial infection was important. If I saw an infiltrate on chest x-ray, I’d change my diagnosis from AECOPD to community-acquired pneumonia (CAP) and switch to CAP coverage. I’ve been doing this so long that I swear it’s in a guideline somewhere, though admittedly I couldn’t find said guideline while reading for this piece.
Key takeaways
In summary, I believe that the guidance reflects the data, which is muddy. The Rome Proposal should be seen as just that – a framework for moving forward with AECOPD classification and antibiotic indications that will need to be refined over time as better data become available. In fact, they allow for a more objective, point-of-care assessment of severity that can be validated and tied to antibiotic benefits. The Rome criteria aren’t evidence-based; they’re a necessary first step toward creating the evidence.
In the meantime, if your AECOPD patients are hospitalized, they probably warrant an antibiotic. If they’re not, sputum changes may be a reasonable surrogate for a bacterial infection. Considerable uncertainty remains.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
In late 2021, the Rome Proposal for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and grading their severity was published. The 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) Report has adopted the Rome Proposal criteria. Given that an endorsement by GOLD is tantamount to acceptance by clinicians, researchers, and policymakers alike, I guess we’re all using them now.
Anyone who’s ever cared for patients with COPD knows that treatment and reduction of exacerbations is how we improve outcomes. AECOPD are associated with considerable morbidity, greater health care utilization and costs, and a long-term decline in lung function. While we hope our pharmacotherapies improve symptoms, we know they reduce AECOPD. If our pharmacotherapies have any impact on mortality, it’s probably via AECOPD prevention.
Since antibiotic indications are tied to severity, using the Rome Proposal criteria may affect management in unpredictable ways. As such, it’s worth reviewing the data on antibiotics for AECOPD.
What do the data reveal?
To start, it’s important to note that GOLD doesn’t equate having an AECOPD with needing an antibiotic. I myself have conflated the diagnosis with the indication and thereby overprescribed. The bar for diagnosis is quite low. In previous GOLD summaries, any “change in respiratory symptoms” would warrant the AECOPD label. Although the Rome Proposal definition is more specific, it leaves room for liberal interpretation. It’s likely to have a greater effect on research than on clinical practice. My guess is that AECOPD prevalence doesn’t change.
The antibiotic hurdle is slightly higher than that for diagnosis but is equally open to interpretation. In part, that’s related to the inherent subjectivity of judging symptoms, sputum production, and changes in color, but it’s also because the data are so poor. The meta-analyses that have been used to establish the indications include fewer than 1000 patients spread across 10 to 11 trials. Thus, the individual trials are small, and the sample size remains nominal even after adding them together. The addition of antibiotics – and it doesn’t seem to matter which class, type, or duration – will decrease mortality and hospital length of stay. One study says these effects are limited to inpatients while the other does not. After reading GOLD 2013, GOLD 2023, and both the meta-analyses they used to support their recommendations, I’m still not sure who benefits. Do you have to be hospitalized? Is some sort of ventilatory support required? Does C-reactive protein help or not?
In accordance with the classic Anthonisen criteria, GOLD relies on sputum volume and color as evidence of a bacterial infection. Soon after GOLD 2023 was published, a meta-analysis found that sputum color isn’t particularly accurate for detecting bacterial infection. Because it doesn’t seem to matter which antibiotic class is used, I always thought we were using antibiotics for their magical, pleiotropic anti-inflammatory effects anyway. I didn’t think the presence of an actual bacterial infection was important. If I saw an infiltrate on chest x-ray, I’d change my diagnosis from AECOPD to community-acquired pneumonia (CAP) and switch to CAP coverage. I’ve been doing this so long that I swear it’s in a guideline somewhere, though admittedly I couldn’t find said guideline while reading for this piece.
Key takeaways
In summary, I believe that the guidance reflects the data, which is muddy. The Rome Proposal should be seen as just that – a framework for moving forward with AECOPD classification and antibiotic indications that will need to be refined over time as better data become available. In fact, they allow for a more objective, point-of-care assessment of severity that can be validated and tied to antibiotic benefits. The Rome criteria aren’t evidence-based; they’re a necessary first step toward creating the evidence.
In the meantime, if your AECOPD patients are hospitalized, they probably warrant an antibiotic. If they’re not, sputum changes may be a reasonable surrogate for a bacterial infection. Considerable uncertainty remains.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
In late 2021, the Rome Proposal for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and grading their severity was published. The 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) Report has adopted the Rome Proposal criteria. Given that an endorsement by GOLD is tantamount to acceptance by clinicians, researchers, and policymakers alike, I guess we’re all using them now.
Anyone who’s ever cared for patients with COPD knows that treatment and reduction of exacerbations is how we improve outcomes. AECOPD are associated with considerable morbidity, greater health care utilization and costs, and a long-term decline in lung function. While we hope our pharmacotherapies improve symptoms, we know they reduce AECOPD. If our pharmacotherapies have any impact on mortality, it’s probably via AECOPD prevention.
Since antibiotic indications are tied to severity, using the Rome Proposal criteria may affect management in unpredictable ways. As such, it’s worth reviewing the data on antibiotics for AECOPD.
What do the data reveal?
To start, it’s important to note that GOLD doesn’t equate having an AECOPD with needing an antibiotic. I myself have conflated the diagnosis with the indication and thereby overprescribed. The bar for diagnosis is quite low. In previous GOLD summaries, any “change in respiratory symptoms” would warrant the AECOPD label. Although the Rome Proposal definition is more specific, it leaves room for liberal interpretation. It’s likely to have a greater effect on research than on clinical practice. My guess is that AECOPD prevalence doesn’t change.
The antibiotic hurdle is slightly higher than that for diagnosis but is equally open to interpretation. In part, that’s related to the inherent subjectivity of judging symptoms, sputum production, and changes in color, but it’s also because the data are so poor. The meta-analyses that have been used to establish the indications include fewer than 1000 patients spread across 10 to 11 trials. Thus, the individual trials are small, and the sample size remains nominal even after adding them together. The addition of antibiotics – and it doesn’t seem to matter which class, type, or duration – will decrease mortality and hospital length of stay. One study says these effects are limited to inpatients while the other does not. After reading GOLD 2013, GOLD 2023, and both the meta-analyses they used to support their recommendations, I’m still not sure who benefits. Do you have to be hospitalized? Is some sort of ventilatory support required? Does C-reactive protein help or not?
In accordance with the classic Anthonisen criteria, GOLD relies on sputum volume and color as evidence of a bacterial infection. Soon after GOLD 2023 was published, a meta-analysis found that sputum color isn’t particularly accurate for detecting bacterial infection. Because it doesn’t seem to matter which antibiotic class is used, I always thought we were using antibiotics for their magical, pleiotropic anti-inflammatory effects anyway. I didn’t think the presence of an actual bacterial infection was important. If I saw an infiltrate on chest x-ray, I’d change my diagnosis from AECOPD to community-acquired pneumonia (CAP) and switch to CAP coverage. I’ve been doing this so long that I swear it’s in a guideline somewhere, though admittedly I couldn’t find said guideline while reading for this piece.
Key takeaways
In summary, I believe that the guidance reflects the data, which is muddy. The Rome Proposal should be seen as just that – a framework for moving forward with AECOPD classification and antibiotic indications that will need to be refined over time as better data become available. In fact, they allow for a more objective, point-of-care assessment of severity that can be validated and tied to antibiotic benefits. The Rome criteria aren’t evidence-based; they’re a necessary first step toward creating the evidence.
In the meantime, if your AECOPD patients are hospitalized, they probably warrant an antibiotic. If they’re not, sputum changes may be a reasonable surrogate for a bacterial infection. Considerable uncertainty remains.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: The Hospitalist Triage Role for Reducing Admission Delays
The Hospitalist Triage Role for Reducing Admission Delays: Impacts on Throughput, Quality, Interprofessional Practice, and Clinician Experience of Care
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).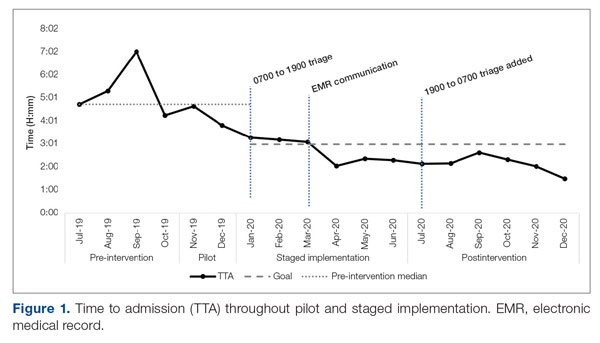
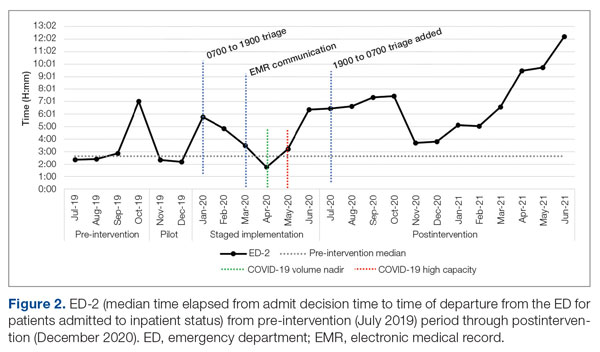
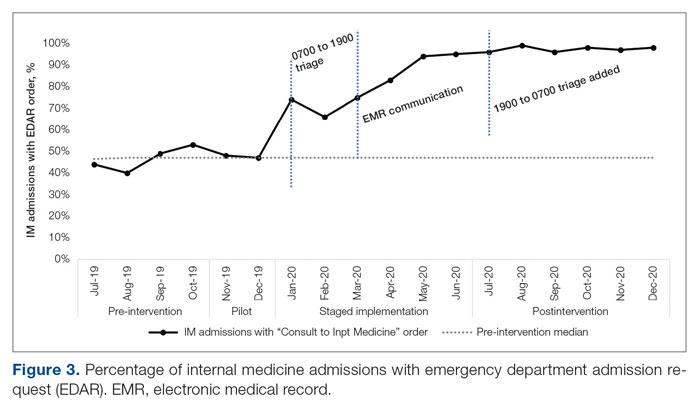
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).
There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.
Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
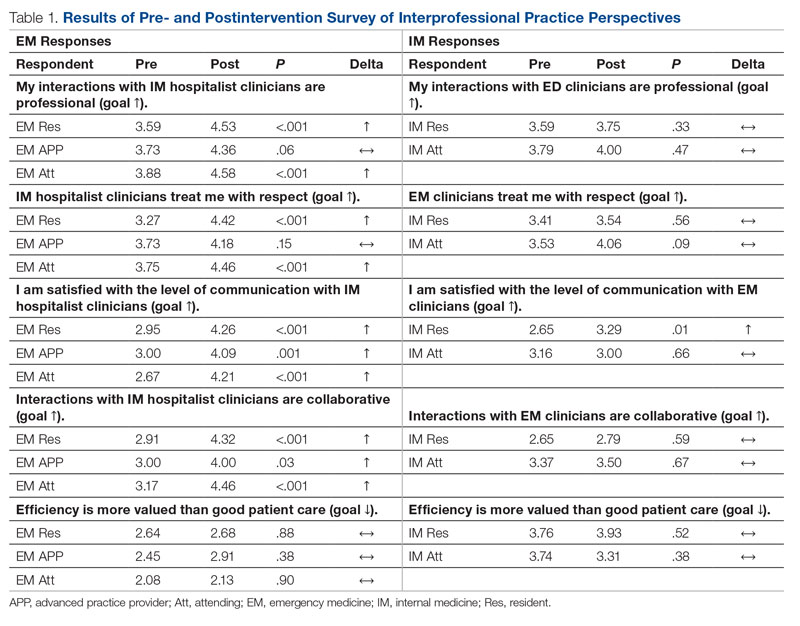
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions
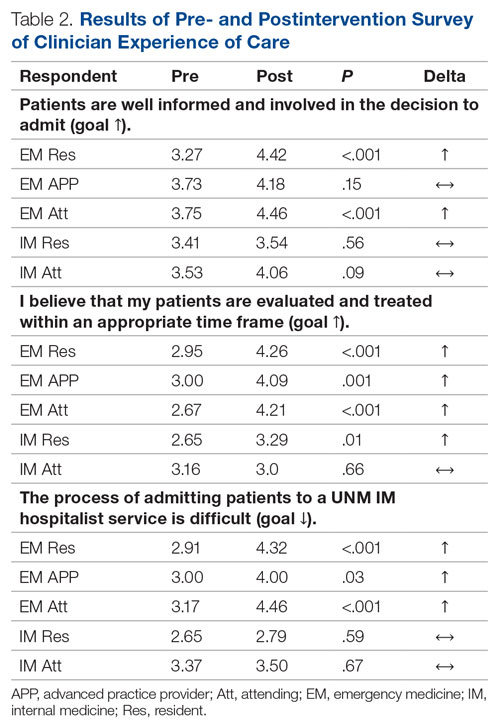
For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.
Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; CSBartlett@salud.unm.edu
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; CSBartlett@salud.unm.edu
Disclosures: None reported.
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; CSBartlett@salud.unm.edu
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
Helmet interface for ventilation likely superior in acute hypoxemic respiratory failure
For adults with acute hypoxemic respiratory failure (AHRF), treatment using a helmet interface is likely superior to a face mask interface, according to a systematic review of recent randomized controlled trials examining different noninvasive oxygenation strategies for AHRF treatment.
The COVID-19 pandemic has underscored the benefits of optimizing noninvasive strategies to avoid unnecessary intubation. Intubation may be avoided in patients with AHRF through noninvasive oxygenation strategies, including high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) and noninvasive bilevel ventilation, noted Tyler Pitre, MD, department of medicine, McMaster University, Hamilton, Ont., and colleagues. CPAP and bilevel ventilation can be delivered through different interfaces, most commonly face mask or helmet. While research has shown noninvasive strategies to be associated with reductions in risk for invasive mechanical ventilation, mortality assessments and analyses comparing specific modalities (i.e., CPAP vs. bilevel ventilation) have been limited. The incremental reduction in diaphragmatic effort and improved gas exchange demonstrated for bilevel ventilation compared with CPAP in COPD patients suggests that responses in AHRF may differ for CPAP and bilevel ventilation, state Dr. Pitre and colleagues. On the other hand, the increased drive pressure of bilevel ventilation may compound patient self-induced lung injury with concomitant lung inflammation and need for prolonged respiratory support. New evidence from several large, high quality randomized controlled trials (RCTs) in COVID-19-related AHRF offered an opportunity to reassess comparative efficacies, the researchers noted.
The retrospective study encompassed RCTs with all types of AHRF, including COVID-19 related, with a total of 7,046 patients whose median age was 59.4 years (61.4% were males). Thirty of the 36 RCTs reported on mortality (6,114 patients and 1,539 deaths). The study’s analysis showed with moderate certainty that helmet CPAP reduces mortality (231 fewer deaths per 1,000 [95% confidence interval (CI), 126-273]) while the 63 fewer deaths per 1,000 (95% CI, 15-102) indicated with low certainty that HFNC may reduce mortality compared with standard oxygen therapy (SOT). The analysis showed also that face mask bilevel (36 fewer deaths per 1,000 [84.0 fewer to 24.0 more]) and helmet bilevel ventilation (129.0 fewer deaths per 1,000 [195.0 to 24.0 fewer]) may reduce death compared with SOT (all low certainty). The mortality benefit for face mask CPAP compared with SOT was uncertain (very low certainty) (9 fewer deaths per 1,000 [81 fewer deaths to 84 more]). For helmet CPAP vs. HFNC ventilation, the mortality benefit had moderate certainty (198.1 fewer events per 1,000 [95% CI, 69.75-248.31].
Mechanical ventilation and ICU duration
The authors found that HFNC probably reduces the need for invasive mechanical ventilation (103.5 fewer events per 1,000 [40.5-157.5 fewer]; moderate certainty). Helmet bilevel ventilation and helmet CPAP may reduce the duration of ICU stay compared with SOT (both low certainty) at (4.84 days fewer [95% CI 2.33 to 16 7.36 days fewer]) and (1.74 days fewer [95% CI 4.49 fewer to 1.01 more]), respectively. Also, SOT may be more comfortable than face mask noninvasive ventilation (NIV) and no different in comfort compared with HFNC (both low certainty).
“Helmet noninvasive ventilation interfaces is probably effective in acute hypoxic respiratory failure and is superior to face mask interfaces. All modalities including HFNC probably reduce the risk of need for invasive mechanical ventilation,” the researchers wrote.
“This meta-analysis shows that helmet noninvasive ventilation is effective in reducing death, and need for invasive mechanical ventilation based on a moderate certainty of evidence,” Shyamsunder Subramanian, MD, chief, division of pulmonary critical care and sleep medicine, Sutter Health, Tracy, Calif., said in an interview. “It is premature based on the results of this meta-analysis to conclude that guideline changes are needed. Use of helmet based ventilation remains limited in scope. We need appropriately designed prospective trials across multiple centers to get sufficient rigor of scientific evidence before any change in guidelines or practice recommendations can be formulated about the appropriate use of helmet NIV in acute respiratory failure.”
The researchers cited the relative heterogeneity of the population included in this analysis as a study limitation.
Dr. Pitre and Dr. Subramanian disclosed that they have no relevant conflicts of interest.
For adults with acute hypoxemic respiratory failure (AHRF), treatment using a helmet interface is likely superior to a face mask interface, according to a systematic review of recent randomized controlled trials examining different noninvasive oxygenation strategies for AHRF treatment.
The COVID-19 pandemic has underscored the benefits of optimizing noninvasive strategies to avoid unnecessary intubation. Intubation may be avoided in patients with AHRF through noninvasive oxygenation strategies, including high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) and noninvasive bilevel ventilation, noted Tyler Pitre, MD, department of medicine, McMaster University, Hamilton, Ont., and colleagues. CPAP and bilevel ventilation can be delivered through different interfaces, most commonly face mask or helmet. While research has shown noninvasive strategies to be associated with reductions in risk for invasive mechanical ventilation, mortality assessments and analyses comparing specific modalities (i.e., CPAP vs. bilevel ventilation) have been limited. The incremental reduction in diaphragmatic effort and improved gas exchange demonstrated for bilevel ventilation compared with CPAP in COPD patients suggests that responses in AHRF may differ for CPAP and bilevel ventilation, state Dr. Pitre and colleagues. On the other hand, the increased drive pressure of bilevel ventilation may compound patient self-induced lung injury with concomitant lung inflammation and need for prolonged respiratory support. New evidence from several large, high quality randomized controlled trials (RCTs) in COVID-19-related AHRF offered an opportunity to reassess comparative efficacies, the researchers noted.
The retrospective study encompassed RCTs with all types of AHRF, including COVID-19 related, with a total of 7,046 patients whose median age was 59.4 years (61.4% were males). Thirty of the 36 RCTs reported on mortality (6,114 patients and 1,539 deaths). The study’s analysis showed with moderate certainty that helmet CPAP reduces mortality (231 fewer deaths per 1,000 [95% confidence interval (CI), 126-273]) while the 63 fewer deaths per 1,000 (95% CI, 15-102) indicated with low certainty that HFNC may reduce mortality compared with standard oxygen therapy (SOT). The analysis showed also that face mask bilevel (36 fewer deaths per 1,000 [84.0 fewer to 24.0 more]) and helmet bilevel ventilation (129.0 fewer deaths per 1,000 [195.0 to 24.0 fewer]) may reduce death compared with SOT (all low certainty). The mortality benefit for face mask CPAP compared with SOT was uncertain (very low certainty) (9 fewer deaths per 1,000 [81 fewer deaths to 84 more]). For helmet CPAP vs. HFNC ventilation, the mortality benefit had moderate certainty (198.1 fewer events per 1,000 [95% CI, 69.75-248.31].
Mechanical ventilation and ICU duration
The authors found that HFNC probably reduces the need for invasive mechanical ventilation (103.5 fewer events per 1,000 [40.5-157.5 fewer]; moderate certainty). Helmet bilevel ventilation and helmet CPAP may reduce the duration of ICU stay compared with SOT (both low certainty) at (4.84 days fewer [95% CI 2.33 to 16 7.36 days fewer]) and (1.74 days fewer [95% CI 4.49 fewer to 1.01 more]), respectively. Also, SOT may be more comfortable than face mask noninvasive ventilation (NIV) and no different in comfort compared with HFNC (both low certainty).
“Helmet noninvasive ventilation interfaces is probably effective in acute hypoxic respiratory failure and is superior to face mask interfaces. All modalities including HFNC probably reduce the risk of need for invasive mechanical ventilation,” the researchers wrote.
“This meta-analysis shows that helmet noninvasive ventilation is effective in reducing death, and need for invasive mechanical ventilation based on a moderate certainty of evidence,” Shyamsunder Subramanian, MD, chief, division of pulmonary critical care and sleep medicine, Sutter Health, Tracy, Calif., said in an interview. “It is premature based on the results of this meta-analysis to conclude that guideline changes are needed. Use of helmet based ventilation remains limited in scope. We need appropriately designed prospective trials across multiple centers to get sufficient rigor of scientific evidence before any change in guidelines or practice recommendations can be formulated about the appropriate use of helmet NIV in acute respiratory failure.”
The researchers cited the relative heterogeneity of the population included in this analysis as a study limitation.
Dr. Pitre and Dr. Subramanian disclosed that they have no relevant conflicts of interest.
For adults with acute hypoxemic respiratory failure (AHRF), treatment using a helmet interface is likely superior to a face mask interface, according to a systematic review of recent randomized controlled trials examining different noninvasive oxygenation strategies for AHRF treatment.
The COVID-19 pandemic has underscored the benefits of optimizing noninvasive strategies to avoid unnecessary intubation. Intubation may be avoided in patients with AHRF through noninvasive oxygenation strategies, including high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) and noninvasive bilevel ventilation, noted Tyler Pitre, MD, department of medicine, McMaster University, Hamilton, Ont., and colleagues. CPAP and bilevel ventilation can be delivered through different interfaces, most commonly face mask or helmet. While research has shown noninvasive strategies to be associated with reductions in risk for invasive mechanical ventilation, mortality assessments and analyses comparing specific modalities (i.e., CPAP vs. bilevel ventilation) have been limited. The incremental reduction in diaphragmatic effort and improved gas exchange demonstrated for bilevel ventilation compared with CPAP in COPD patients suggests that responses in AHRF may differ for CPAP and bilevel ventilation, state Dr. Pitre and colleagues. On the other hand, the increased drive pressure of bilevel ventilation may compound patient self-induced lung injury with concomitant lung inflammation and need for prolonged respiratory support. New evidence from several large, high quality randomized controlled trials (RCTs) in COVID-19-related AHRF offered an opportunity to reassess comparative efficacies, the researchers noted.
The retrospective study encompassed RCTs with all types of AHRF, including COVID-19 related, with a total of 7,046 patients whose median age was 59.4 years (61.4% were males). Thirty of the 36 RCTs reported on mortality (6,114 patients and 1,539 deaths). The study’s analysis showed with moderate certainty that helmet CPAP reduces mortality (231 fewer deaths per 1,000 [95% confidence interval (CI), 126-273]) while the 63 fewer deaths per 1,000 (95% CI, 15-102) indicated with low certainty that HFNC may reduce mortality compared with standard oxygen therapy (SOT). The analysis showed also that face mask bilevel (36 fewer deaths per 1,000 [84.0 fewer to 24.0 more]) and helmet bilevel ventilation (129.0 fewer deaths per 1,000 [195.0 to 24.0 fewer]) may reduce death compared with SOT (all low certainty). The mortality benefit for face mask CPAP compared with SOT was uncertain (very low certainty) (9 fewer deaths per 1,000 [81 fewer deaths to 84 more]). For helmet CPAP vs. HFNC ventilation, the mortality benefit had moderate certainty (198.1 fewer events per 1,000 [95% CI, 69.75-248.31].
Mechanical ventilation and ICU duration
The authors found that HFNC probably reduces the need for invasive mechanical ventilation (103.5 fewer events per 1,000 [40.5-157.5 fewer]; moderate certainty). Helmet bilevel ventilation and helmet CPAP may reduce the duration of ICU stay compared with SOT (both low certainty) at (4.84 days fewer [95% CI 2.33 to 16 7.36 days fewer]) and (1.74 days fewer [95% CI 4.49 fewer to 1.01 more]), respectively. Also, SOT may be more comfortable than face mask noninvasive ventilation (NIV) and no different in comfort compared with HFNC (both low certainty).
“Helmet noninvasive ventilation interfaces is probably effective in acute hypoxic respiratory failure and is superior to face mask interfaces. All modalities including HFNC probably reduce the risk of need for invasive mechanical ventilation,” the researchers wrote.
“This meta-analysis shows that helmet noninvasive ventilation is effective in reducing death, and need for invasive mechanical ventilation based on a moderate certainty of evidence,” Shyamsunder Subramanian, MD, chief, division of pulmonary critical care and sleep medicine, Sutter Health, Tracy, Calif., said in an interview. “It is premature based on the results of this meta-analysis to conclude that guideline changes are needed. Use of helmet based ventilation remains limited in scope. We need appropriately designed prospective trials across multiple centers to get sufficient rigor of scientific evidence before any change in guidelines or practice recommendations can be formulated about the appropriate use of helmet NIV in acute respiratory failure.”
The researchers cited the relative heterogeneity of the population included in this analysis as a study limitation.
Dr. Pitre and Dr. Subramanian disclosed that they have no relevant conflicts of interest.
FROM CHEST
Meet the JCOM Author with Dr. Barkoudah: A Multidisciplinary Team–Based Clinical Care Pathway for Infective Endocarditis
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; hwc2pd@health.missouri.edu
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; hwc2pd@health.missouri.edu
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; hwc2pd@health.missouri.edu
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
The Shifting Landscape of Thrombolytic Therapy for Acute Ischemic Stroke
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
From PERT to AI, high-risk PE care evolves
In 2012, a small group of specialists, consisting of a critical care pulmonologist, cardiologist, cardiac surgeon, and vascular specialist, at Massachusetts General Hospital, Boston, met to Monday morning quarterback an acute pulmonary embolism case that didn’t go as well as they’d hoped. They came up with a concept known as the pulmonary embolism response team – PERT for short – an idea that soon took hold in other centers and served as the vanguard to other innovative approaches to managing critical care patients with PE, which is the third-leading cause of cardiovascular death in the United States (Intern Emerg Med. 2023. doi: 10.1007/s11739-022-03180-w).
Three years later the PERT Consortium came together, which today has 102 members, according to the organization’s website (www.pertconsortium.org), and members in South America, Europe, Asia, and Australia. Since then, and apps to expedite diagnosis and treatment. The PERT Consortium, meanwhile, is in the process of creating the PE Centers of Excellence program to certify centers that meet certain requirements.
“Part of the reason we recognized that a discussion across specialties was important was because there weren’t the large clinical trials that could tell us exactly what to do for any given case,” said Christopher Kabrhel, MD, MPH, director of the Center for Vascular Emergencies at Mass General and a professor at Harvard Medical School in Boston, who assembled that formative meeting. “Without a clear basis in data, it was really important to have all the different specialists weigh in and give their perspective and talk about what was the best approach for the patient’s care.”
Filling data gaps
Some of those data gaps persist today, Dr. Kabrhel said. “It’s precisely that lack of head-to-head data that existed in 2012, and to a great extent still exists today, that led us to create this system.” The American Heart Association just this January issued a scientific statement on surgical management and mechanical circulatory support in high-risk PE (Circulation. 2023;147:e628-47).
But the intervening research has been uneven. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 evaluated systemic thrombolysis and anticoagulation alone (N Engl J Med. 2014;370:1402-11), but head-to-head studies of catheter-directed thrombolysis (CDT), which was just emerging in 2012, and either systemic thrombolysis or anticoagulation have been lacking, Dr. Kabrhel said. The Hi-PEITHO trial in high-risk PE patients is evaluating ultrasound-guided CDT plus anticoagulation vs. anticoagulation alone (Am Heart J. 2022:251:43-54), but it isn’t complete.
“The therapeutic landscape for PE is evolving incredibly rapidly,” he said. “When we first started PERT we were just starting to see CDT. Since then, we’ve seen several new thrombolytic catheters come onto the market, but there’s also been a proliferation of suction embolectomy catheters and we’ve seen a potentially larger role for surgery and the use of ECMO [extracorporeal membrane oxygenation] or cardiac bypass to bridge patients to definitive therapy. With the rapid evolution and the seemingly daily addition of new therapeutic options, I think the need for PERT is only increasing.”
A recent study out of the University of Michigan reported that the PERT there led to a decrease in the use of advanced therapies given to acute PE patients without reducing mortality or extending hospital stays (Thromb Res. 2023;221:73-8). A study in Spain reported that patients with high-risk and intermediate high-risk PE who had PERT-coordinated care had half the 12-month mortality rate of non-PERT counterparts, 9% vs. 22.2% (P = .02) (Med Clin [Barc]. 2023;S0025-7753(23)00017-9). And a 2021 study at University Hospitals in Cleveland reported that PERT-managed PE patients had a 60% lower rate of adverse outcomes at 90 days than non–PERT-managed patients (J Invasive Cardiol. 2021;33:E173-E180).
Nelish Ardeshna, MD, MA, the lead author of the Michigan study, said the PERT there was formed in 2017. Besides the multispecialty team that can be summoned to a teleconference on short notice, the protocol includes having at least one noninvasive specialist, such as a cardiologist or hospitalist, and one interventionalist, such as a radiologist, always on call. The PERT gets activated through the paging system after a hospital or emergency department physician identifies a suspected or established high-risk PE.
“High-risk PE patients can present in all settings, including the emergency department, ICU, surgical floor, or medical floor,” said Dr. Ardeshna, an internal medicine resident. “Management for these patients is equally varied from anticoagulation to systemic thrombolytics. Not all providers may be familiar with current guidelines to select the optimal therapy for high-risk pulmonary embolism patients. PERT aims to bridge that gap by providing a multidisciplinary discussion with PE specialists that can help identify the correct therapeutic options for optimal outcomes.”
At Cleveland Clinic, where the PERT has been in place since 2012, the PERT can consist of six to eight different specialties and involve up to 15 providers on a conference call, said Leben Tefera, MD, a vascular specialist and head of the PERT team there.
“Each patient will come in and have certain comorbidities,” Dr. Tefera said. “The unfortunate thing about a majority of the PEs that we see, in particular ones [in patients] that are very sick and require inpatient treatment, is that they don’t really fit into a box; you can’t come up with one kind of generic care routine or care path that treats the majority of patients with PE.”
Evolving to follow-up care
As the PERT protocol led to better inpatient outcomes, the teams became more aware that discharged PE patients were struggling with mental health and other quality-of-life issues – symptoms that have been understudied, according to a protocol Dr. Tefera coauthored for a prospective observational study of psychological distress symptoms in PE survivors. By contrast, the protocol noted, these symptoms have been studied extensively in myocardial infarction and stroke patients (Res Pract Thromb Hemost. 2023. doi: 10.1016/j.rpth.2023.10045). Other studies have found that 35%-50% of patients reported mental health symptoms 3 months after PE (Chest. 2021;159:2428-38; Qual Life Res. 2019;28:2111-24).
“A lot of physicians have known it for quite some time, but it wasn’t really until the last couple of years that physicians started saying psychological stress is something that we need to quantify and that we need to actually treat, that we actually need to address,” Dr. Tefera said. That led Dr. Tefera and his Cleveland Clinic PERT colleagues to set up a follow-up clinic for PE patients.
At their follow-up visits, patients complete validated questionnaires about anxiety, depression, fear of recurrence, PE-specific quality of life, and posttraumatic stress disorder. “If they flag as positive, we give them a referral to an in-house psychologist,” he said. “One thing I can report is that patients absolutely, positively love this, because it’s something that they are all experiencing that a lot of physicians just aren’t addressing.”
Artificial intelligence emerges
At the University of Pittsburgh Medical Center, the PERT has started evaluating artificial intelligence to aid in PE diagnosis. Belinda Rivera-Lebron, MD, director of the acute and chronic embolism program at Pitt, explained that the AI protocol hasn’t been adopted yet, but the concept is to have a platform that’s compatible with the hospital system’s electronic medical record.
She described how AI would work once the PERT is activated. “Once the patient goes through the CT scanner, within 60 seconds of that scan being completed, the scan gets uploaded into the cloud and the app or the platform is able to tell you whether there is PE present or absent, and whether there is right ventricle dilation on that scan. This is even before you probably even think about opening up the computer to look at the scan, and even before radiology opens up the scan to read,” she said. “It’s so fast.”
The idea is to send the scans rapidly to the PERT. “It will send you a text, a notification on your phone that will tell you Mr. Smith is PE positive,” Dr. Rivera-Lebron said. “Then you open it and you are able to scroll through the CT scan in your phone. So, it’s really remarkable.”
Clinical trials worth watching
Meanwhile, a number of clinical trials have started to enroll patients, or will soon, that Dr. Rivera-Lebron said are worth paying attention to.
PEITHO-3 is a randomized, placebo-controlled trial with long-term follow-up comparing the efficacy of a reduced-dose alteplase regimen or standard heparin anticoagulation in patients with intermediate to high-risk PE (Thromb Haemost. 2022;122:867-66).
PEERLESS is a prospective randomized trial comparing mechanical thrombectomy and CDT (ClinicalTrials.gov identifier NCT05111613).
PE-Thrombus Removal with Catheter-directed Therapy (PE-TRACT) is an open-label Phase 3 trial comparing anticoagulation and CDT that’s not yet recruiting (ClinicalTrials.gov identifier NCT05591118).
FlowTriever for Acute Massive Pulmonary Embolism (FLAME) is a prospective cohort study evaluating a clot-retrieving device in high-risk PE patients (ClinicalTrials.gov identifier NCT04795167).
When completed and published, these trials could provide PERTs more evidence for their decision-making.
Dr. Ardeshna and Dr. Tefera have no relevant relationships to disclose. Dr. Rivera-Lebron disclosed relationships with INARI Catheter and Johnson & Johnson. Dr. Kabrhel disclosed relationships with Bristol Myers Squibb and Pfizer.
In 2012, a small group of specialists, consisting of a critical care pulmonologist, cardiologist, cardiac surgeon, and vascular specialist, at Massachusetts General Hospital, Boston, met to Monday morning quarterback an acute pulmonary embolism case that didn’t go as well as they’d hoped. They came up with a concept known as the pulmonary embolism response team – PERT for short – an idea that soon took hold in other centers and served as the vanguard to other innovative approaches to managing critical care patients with PE, which is the third-leading cause of cardiovascular death in the United States (Intern Emerg Med. 2023. doi: 10.1007/s11739-022-03180-w).
Three years later the PERT Consortium came together, which today has 102 members, according to the organization’s website (www.pertconsortium.org), and members in South America, Europe, Asia, and Australia. Since then, and apps to expedite diagnosis and treatment. The PERT Consortium, meanwhile, is in the process of creating the PE Centers of Excellence program to certify centers that meet certain requirements.
“Part of the reason we recognized that a discussion across specialties was important was because there weren’t the large clinical trials that could tell us exactly what to do for any given case,” said Christopher Kabrhel, MD, MPH, director of the Center for Vascular Emergencies at Mass General and a professor at Harvard Medical School in Boston, who assembled that formative meeting. “Without a clear basis in data, it was really important to have all the different specialists weigh in and give their perspective and talk about what was the best approach for the patient’s care.”
Filling data gaps
Some of those data gaps persist today, Dr. Kabrhel said. “It’s precisely that lack of head-to-head data that existed in 2012, and to a great extent still exists today, that led us to create this system.” The American Heart Association just this January issued a scientific statement on surgical management and mechanical circulatory support in high-risk PE (Circulation. 2023;147:e628-47).
But the intervening research has been uneven. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 evaluated systemic thrombolysis and anticoagulation alone (N Engl J Med. 2014;370:1402-11), but head-to-head studies of catheter-directed thrombolysis (CDT), which was just emerging in 2012, and either systemic thrombolysis or anticoagulation have been lacking, Dr. Kabrhel said. The Hi-PEITHO trial in high-risk PE patients is evaluating ultrasound-guided CDT plus anticoagulation vs. anticoagulation alone (Am Heart J. 2022:251:43-54), but it isn’t complete.
“The therapeutic landscape for PE is evolving incredibly rapidly,” he said. “When we first started PERT we were just starting to see CDT. Since then, we’ve seen several new thrombolytic catheters come onto the market, but there’s also been a proliferation of suction embolectomy catheters and we’ve seen a potentially larger role for surgery and the use of ECMO [extracorporeal membrane oxygenation] or cardiac bypass to bridge patients to definitive therapy. With the rapid evolution and the seemingly daily addition of new therapeutic options, I think the need for PERT is only increasing.”
A recent study out of the University of Michigan reported that the PERT there led to a decrease in the use of advanced therapies given to acute PE patients without reducing mortality or extending hospital stays (Thromb Res. 2023;221:73-8). A study in Spain reported that patients with high-risk and intermediate high-risk PE who had PERT-coordinated care had half the 12-month mortality rate of non-PERT counterparts, 9% vs. 22.2% (P = .02) (Med Clin [Barc]. 2023;S0025-7753(23)00017-9). And a 2021 study at University Hospitals in Cleveland reported that PERT-managed PE patients had a 60% lower rate of adverse outcomes at 90 days than non–PERT-managed patients (J Invasive Cardiol. 2021;33:E173-E180).
Nelish Ardeshna, MD, MA, the lead author of the Michigan study, said the PERT there was formed in 2017. Besides the multispecialty team that can be summoned to a teleconference on short notice, the protocol includes having at least one noninvasive specialist, such as a cardiologist or hospitalist, and one interventionalist, such as a radiologist, always on call. The PERT gets activated through the paging system after a hospital or emergency department physician identifies a suspected or established high-risk PE.
“High-risk PE patients can present in all settings, including the emergency department, ICU, surgical floor, or medical floor,” said Dr. Ardeshna, an internal medicine resident. “Management for these patients is equally varied from anticoagulation to systemic thrombolytics. Not all providers may be familiar with current guidelines to select the optimal therapy for high-risk pulmonary embolism patients. PERT aims to bridge that gap by providing a multidisciplinary discussion with PE specialists that can help identify the correct therapeutic options for optimal outcomes.”
At Cleveland Clinic, where the PERT has been in place since 2012, the PERT can consist of six to eight different specialties and involve up to 15 providers on a conference call, said Leben Tefera, MD, a vascular specialist and head of the PERT team there.
“Each patient will come in and have certain comorbidities,” Dr. Tefera said. “The unfortunate thing about a majority of the PEs that we see, in particular ones [in patients] that are very sick and require inpatient treatment, is that they don’t really fit into a box; you can’t come up with one kind of generic care routine or care path that treats the majority of patients with PE.”
Evolving to follow-up care
As the PERT protocol led to better inpatient outcomes, the teams became more aware that discharged PE patients were struggling with mental health and other quality-of-life issues – symptoms that have been understudied, according to a protocol Dr. Tefera coauthored for a prospective observational study of psychological distress symptoms in PE survivors. By contrast, the protocol noted, these symptoms have been studied extensively in myocardial infarction and stroke patients (Res Pract Thromb Hemost. 2023. doi: 10.1016/j.rpth.2023.10045). Other studies have found that 35%-50% of patients reported mental health symptoms 3 months after PE (Chest. 2021;159:2428-38; Qual Life Res. 2019;28:2111-24).
“A lot of physicians have known it for quite some time, but it wasn’t really until the last couple of years that physicians started saying psychological stress is something that we need to quantify and that we need to actually treat, that we actually need to address,” Dr. Tefera said. That led Dr. Tefera and his Cleveland Clinic PERT colleagues to set up a follow-up clinic for PE patients.
At their follow-up visits, patients complete validated questionnaires about anxiety, depression, fear of recurrence, PE-specific quality of life, and posttraumatic stress disorder. “If they flag as positive, we give them a referral to an in-house psychologist,” he said. “One thing I can report is that patients absolutely, positively love this, because it’s something that they are all experiencing that a lot of physicians just aren’t addressing.”
Artificial intelligence emerges
At the University of Pittsburgh Medical Center, the PERT has started evaluating artificial intelligence to aid in PE diagnosis. Belinda Rivera-Lebron, MD, director of the acute and chronic embolism program at Pitt, explained that the AI protocol hasn’t been adopted yet, but the concept is to have a platform that’s compatible with the hospital system’s electronic medical record.
She described how AI would work once the PERT is activated. “Once the patient goes through the CT scanner, within 60 seconds of that scan being completed, the scan gets uploaded into the cloud and the app or the platform is able to tell you whether there is PE present or absent, and whether there is right ventricle dilation on that scan. This is even before you probably even think about opening up the computer to look at the scan, and even before radiology opens up the scan to read,” she said. “It’s so fast.”
The idea is to send the scans rapidly to the PERT. “It will send you a text, a notification on your phone that will tell you Mr. Smith is PE positive,” Dr. Rivera-Lebron said. “Then you open it and you are able to scroll through the CT scan in your phone. So, it’s really remarkable.”
Clinical trials worth watching
Meanwhile, a number of clinical trials have started to enroll patients, or will soon, that Dr. Rivera-Lebron said are worth paying attention to.
PEITHO-3 is a randomized, placebo-controlled trial with long-term follow-up comparing the efficacy of a reduced-dose alteplase regimen or standard heparin anticoagulation in patients with intermediate to high-risk PE (Thromb Haemost. 2022;122:867-66).
PEERLESS is a prospective randomized trial comparing mechanical thrombectomy and CDT (ClinicalTrials.gov identifier NCT05111613).
PE-Thrombus Removal with Catheter-directed Therapy (PE-TRACT) is an open-label Phase 3 trial comparing anticoagulation and CDT that’s not yet recruiting (ClinicalTrials.gov identifier NCT05591118).
FlowTriever for Acute Massive Pulmonary Embolism (FLAME) is a prospective cohort study evaluating a clot-retrieving device in high-risk PE patients (ClinicalTrials.gov identifier NCT04795167).
When completed and published, these trials could provide PERTs more evidence for their decision-making.
Dr. Ardeshna and Dr. Tefera have no relevant relationships to disclose. Dr. Rivera-Lebron disclosed relationships with INARI Catheter and Johnson & Johnson. Dr. Kabrhel disclosed relationships with Bristol Myers Squibb and Pfizer.
In 2012, a small group of specialists, consisting of a critical care pulmonologist, cardiologist, cardiac surgeon, and vascular specialist, at Massachusetts General Hospital, Boston, met to Monday morning quarterback an acute pulmonary embolism case that didn’t go as well as they’d hoped. They came up with a concept known as the pulmonary embolism response team – PERT for short – an idea that soon took hold in other centers and served as the vanguard to other innovative approaches to managing critical care patients with PE, which is the third-leading cause of cardiovascular death in the United States (Intern Emerg Med. 2023. doi: 10.1007/s11739-022-03180-w).
Three years later the PERT Consortium came together, which today has 102 members, according to the organization’s website (www.pertconsortium.org), and members in South America, Europe, Asia, and Australia. Since then, and apps to expedite diagnosis and treatment. The PERT Consortium, meanwhile, is in the process of creating the PE Centers of Excellence program to certify centers that meet certain requirements.
“Part of the reason we recognized that a discussion across specialties was important was because there weren’t the large clinical trials that could tell us exactly what to do for any given case,” said Christopher Kabrhel, MD, MPH, director of the Center for Vascular Emergencies at Mass General and a professor at Harvard Medical School in Boston, who assembled that formative meeting. “Without a clear basis in data, it was really important to have all the different specialists weigh in and give their perspective and talk about what was the best approach for the patient’s care.”
Filling data gaps
Some of those data gaps persist today, Dr. Kabrhel said. “It’s precisely that lack of head-to-head data that existed in 2012, and to a great extent still exists today, that led us to create this system.” The American Heart Association just this January issued a scientific statement on surgical management and mechanical circulatory support in high-risk PE (Circulation. 2023;147:e628-47).
But the intervening research has been uneven. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 evaluated systemic thrombolysis and anticoagulation alone (N Engl J Med. 2014;370:1402-11), but head-to-head studies of catheter-directed thrombolysis (CDT), which was just emerging in 2012, and either systemic thrombolysis or anticoagulation have been lacking, Dr. Kabrhel said. The Hi-PEITHO trial in high-risk PE patients is evaluating ultrasound-guided CDT plus anticoagulation vs. anticoagulation alone (Am Heart J. 2022:251:43-54), but it isn’t complete.
“The therapeutic landscape for PE is evolving incredibly rapidly,” he said. “When we first started PERT we were just starting to see CDT. Since then, we’ve seen several new thrombolytic catheters come onto the market, but there’s also been a proliferation of suction embolectomy catheters and we’ve seen a potentially larger role for surgery and the use of ECMO [extracorporeal membrane oxygenation] or cardiac bypass to bridge patients to definitive therapy. With the rapid evolution and the seemingly daily addition of new therapeutic options, I think the need for PERT is only increasing.”
A recent study out of the University of Michigan reported that the PERT there led to a decrease in the use of advanced therapies given to acute PE patients without reducing mortality or extending hospital stays (Thromb Res. 2023;221:73-8). A study in Spain reported that patients with high-risk and intermediate high-risk PE who had PERT-coordinated care had half the 12-month mortality rate of non-PERT counterparts, 9% vs. 22.2% (P = .02) (Med Clin [Barc]. 2023;S0025-7753(23)00017-9). And a 2021 study at University Hospitals in Cleveland reported that PERT-managed PE patients had a 60% lower rate of adverse outcomes at 90 days than non–PERT-managed patients (J Invasive Cardiol. 2021;33:E173-E180).
Nelish Ardeshna, MD, MA, the lead author of the Michigan study, said the PERT there was formed in 2017. Besides the multispecialty team that can be summoned to a teleconference on short notice, the protocol includes having at least one noninvasive specialist, such as a cardiologist or hospitalist, and one interventionalist, such as a radiologist, always on call. The PERT gets activated through the paging system after a hospital or emergency department physician identifies a suspected or established high-risk PE.
“High-risk PE patients can present in all settings, including the emergency department, ICU, surgical floor, or medical floor,” said Dr. Ardeshna, an internal medicine resident. “Management for these patients is equally varied from anticoagulation to systemic thrombolytics. Not all providers may be familiar with current guidelines to select the optimal therapy for high-risk pulmonary embolism patients. PERT aims to bridge that gap by providing a multidisciplinary discussion with PE specialists that can help identify the correct therapeutic options for optimal outcomes.”
At Cleveland Clinic, where the PERT has been in place since 2012, the PERT can consist of six to eight different specialties and involve up to 15 providers on a conference call, said Leben Tefera, MD, a vascular specialist and head of the PERT team there.
“Each patient will come in and have certain comorbidities,” Dr. Tefera said. “The unfortunate thing about a majority of the PEs that we see, in particular ones [in patients] that are very sick and require inpatient treatment, is that they don’t really fit into a box; you can’t come up with one kind of generic care routine or care path that treats the majority of patients with PE.”
Evolving to follow-up care
As the PERT protocol led to better inpatient outcomes, the teams became more aware that discharged PE patients were struggling with mental health and other quality-of-life issues – symptoms that have been understudied, according to a protocol Dr. Tefera coauthored for a prospective observational study of psychological distress symptoms in PE survivors. By contrast, the protocol noted, these symptoms have been studied extensively in myocardial infarction and stroke patients (Res Pract Thromb Hemost. 2023. doi: 10.1016/j.rpth.2023.10045). Other studies have found that 35%-50% of patients reported mental health symptoms 3 months after PE (Chest. 2021;159:2428-38; Qual Life Res. 2019;28:2111-24).
“A lot of physicians have known it for quite some time, but it wasn’t really until the last couple of years that physicians started saying psychological stress is something that we need to quantify and that we need to actually treat, that we actually need to address,” Dr. Tefera said. That led Dr. Tefera and his Cleveland Clinic PERT colleagues to set up a follow-up clinic for PE patients.
At their follow-up visits, patients complete validated questionnaires about anxiety, depression, fear of recurrence, PE-specific quality of life, and posttraumatic stress disorder. “If they flag as positive, we give them a referral to an in-house psychologist,” he said. “One thing I can report is that patients absolutely, positively love this, because it’s something that they are all experiencing that a lot of physicians just aren’t addressing.”
Artificial intelligence emerges
At the University of Pittsburgh Medical Center, the PERT has started evaluating artificial intelligence to aid in PE diagnosis. Belinda Rivera-Lebron, MD, director of the acute and chronic embolism program at Pitt, explained that the AI protocol hasn’t been adopted yet, but the concept is to have a platform that’s compatible with the hospital system’s electronic medical record.
She described how AI would work once the PERT is activated. “Once the patient goes through the CT scanner, within 60 seconds of that scan being completed, the scan gets uploaded into the cloud and the app or the platform is able to tell you whether there is PE present or absent, and whether there is right ventricle dilation on that scan. This is even before you probably even think about opening up the computer to look at the scan, and even before radiology opens up the scan to read,” she said. “It’s so fast.”
The idea is to send the scans rapidly to the PERT. “It will send you a text, a notification on your phone that will tell you Mr. Smith is PE positive,” Dr. Rivera-Lebron said. “Then you open it and you are able to scroll through the CT scan in your phone. So, it’s really remarkable.”
Clinical trials worth watching
Meanwhile, a number of clinical trials have started to enroll patients, or will soon, that Dr. Rivera-Lebron said are worth paying attention to.
PEITHO-3 is a randomized, placebo-controlled trial with long-term follow-up comparing the efficacy of a reduced-dose alteplase regimen or standard heparin anticoagulation in patients with intermediate to high-risk PE (Thromb Haemost. 2022;122:867-66).
PEERLESS is a prospective randomized trial comparing mechanical thrombectomy and CDT (ClinicalTrials.gov identifier NCT05111613).
PE-Thrombus Removal with Catheter-directed Therapy (PE-TRACT) is an open-label Phase 3 trial comparing anticoagulation and CDT that’s not yet recruiting (ClinicalTrials.gov identifier NCT05591118).
FlowTriever for Acute Massive Pulmonary Embolism (FLAME) is a prospective cohort study evaluating a clot-retrieving device in high-risk PE patients (ClinicalTrials.gov identifier NCT04795167).
When completed and published, these trials could provide PERTs more evidence for their decision-making.
Dr. Ardeshna and Dr. Tefera have no relevant relationships to disclose. Dr. Rivera-Lebron disclosed relationships with INARI Catheter and Johnson & Johnson. Dr. Kabrhel disclosed relationships with Bristol Myers Squibb and Pfizer.