User login
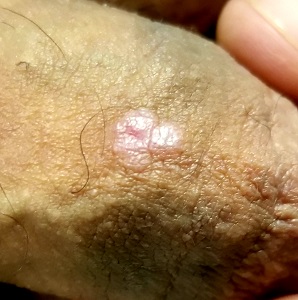
He Doesn’t Love It Warts and All
ANSWER
The correct answer is lichen planus (choice “c”).
DISCUSSION
Condyloma accuminata can demonstrate amazing lability, sometimes appearing decades after exposure. And spouses may not always be truthful when questioned about such exposure. To further confuse the issue, it's entirely possible that a patient may be unaware he or she has condyloma. So, this might well have been condyloma. But the differential for penile lesions would include this condition—and more.
Psoriasis (choice “a”) commonly affects the penis, manifesting as pinkish plaques and papules. But there is a good chance that examination would have revealed corroborative signs of this disease. Furthermore, the histologic results would have been entirely different.
While syphilis (choice “b”), especially in its primary stage, can present with nonhealing sores, in no way do they resemble the patient’s lesions. There is also no source for such an infection. And biopsy would have shown a predominately plasma cell infiltrate in an entirely different pattern.
Lichen sclerosus et atrophicus (choice “d”) is quite uncommon, especially on the penis, where it is usually known as balanitis xerotica obliterans (BXO). As its name suggests, BXO is usually atrophic—therefore macular—and whitish. Exclusive to uncircumcised men, it bears no resemblance to condyloma.
Though idiopathic, lichen planus is not contagious. Unless neglected, this condition seldom causes any suffering aside from mental anguish over its appearance. To make a more accurate diagnosis, it is always helpful for providers to consider a mnemonic device for “7 Ps” associated with lichen planus:
- Penile
- Pruritic
- Plaque-like
- Purple
- Papular
- Planar
- Puzzling.
TREATMENT
Fortunately, lichen planus affecting the penis responds readily to treatment with mid-strength topical steroid cream and the "tincture of time," which improves its appearance until it eventually disappears. For this patient, the PCP treated the affected area with triamcinolone 0.1% cream bid for 2 weeks. This was then applied once a day every other day for a month, which cleared the patient’s lesions.
ANSWER
The correct answer is lichen planus (choice “c”).
DISCUSSION
Condyloma accuminata can demonstrate amazing lability, sometimes appearing decades after exposure. And spouses may not always be truthful when questioned about such exposure. To further confuse the issue, it's entirely possible that a patient may be unaware he or she has condyloma. So, this might well have been condyloma. But the differential for penile lesions would include this condition—and more.
Psoriasis (choice “a”) commonly affects the penis, manifesting as pinkish plaques and papules. But there is a good chance that examination would have revealed corroborative signs of this disease. Furthermore, the histologic results would have been entirely different.
While syphilis (choice “b”), especially in its primary stage, can present with nonhealing sores, in no way do they resemble the patient’s lesions. There is also no source for such an infection. And biopsy would have shown a predominately plasma cell infiltrate in an entirely different pattern.
Lichen sclerosus et atrophicus (choice “d”) is quite uncommon, especially on the penis, where it is usually known as balanitis xerotica obliterans (BXO). As its name suggests, BXO is usually atrophic—therefore macular—and whitish. Exclusive to uncircumcised men, it bears no resemblance to condyloma.
Though idiopathic, lichen planus is not contagious. Unless neglected, this condition seldom causes any suffering aside from mental anguish over its appearance. To make a more accurate diagnosis, it is always helpful for providers to consider a mnemonic device for “7 Ps” associated with lichen planus:
- Penile
- Pruritic
- Plaque-like
- Purple
- Papular
- Planar
- Puzzling.
TREATMENT
Fortunately, lichen planus affecting the penis responds readily to treatment with mid-strength topical steroid cream and the "tincture of time," which improves its appearance until it eventually disappears. For this patient, the PCP treated the affected area with triamcinolone 0.1% cream bid for 2 weeks. This was then applied once a day every other day for a month, which cleared the patient’s lesions.
ANSWER
The correct answer is lichen planus (choice “c”).
DISCUSSION
Condyloma accuminata can demonstrate amazing lability, sometimes appearing decades after exposure. And spouses may not always be truthful when questioned about such exposure. To further confuse the issue, it's entirely possible that a patient may be unaware he or she has condyloma. So, this might well have been condyloma. But the differential for penile lesions would include this condition—and more.
Psoriasis (choice “a”) commonly affects the penis, manifesting as pinkish plaques and papules. But there is a good chance that examination would have revealed corroborative signs of this disease. Furthermore, the histologic results would have been entirely different.
While syphilis (choice “b”), especially in its primary stage, can present with nonhealing sores, in no way do they resemble the patient’s lesions. There is also no source for such an infection. And biopsy would have shown a predominately plasma cell infiltrate in an entirely different pattern.
Lichen sclerosus et atrophicus (choice “d”) is quite uncommon, especially on the penis, where it is usually known as balanitis xerotica obliterans (BXO). As its name suggests, BXO is usually atrophic—therefore macular—and whitish. Exclusive to uncircumcised men, it bears no resemblance to condyloma.
Though idiopathic, lichen planus is not contagious. Unless neglected, this condition seldom causes any suffering aside from mental anguish over its appearance. To make a more accurate diagnosis, it is always helpful for providers to consider a mnemonic device for “7 Ps” associated with lichen planus:
- Penile
- Pruritic
- Plaque-like
- Purple
- Papular
- Planar
- Puzzling.
TREATMENT
Fortunately, lichen planus affecting the penis responds readily to treatment with mid-strength topical steroid cream and the "tincture of time," which improves its appearance until it eventually disappears. For this patient, the PCP treated the affected area with triamcinolone 0.1% cream bid for 2 weeks. This was then applied once a day every other day for a month, which cleared the patient’s lesions.
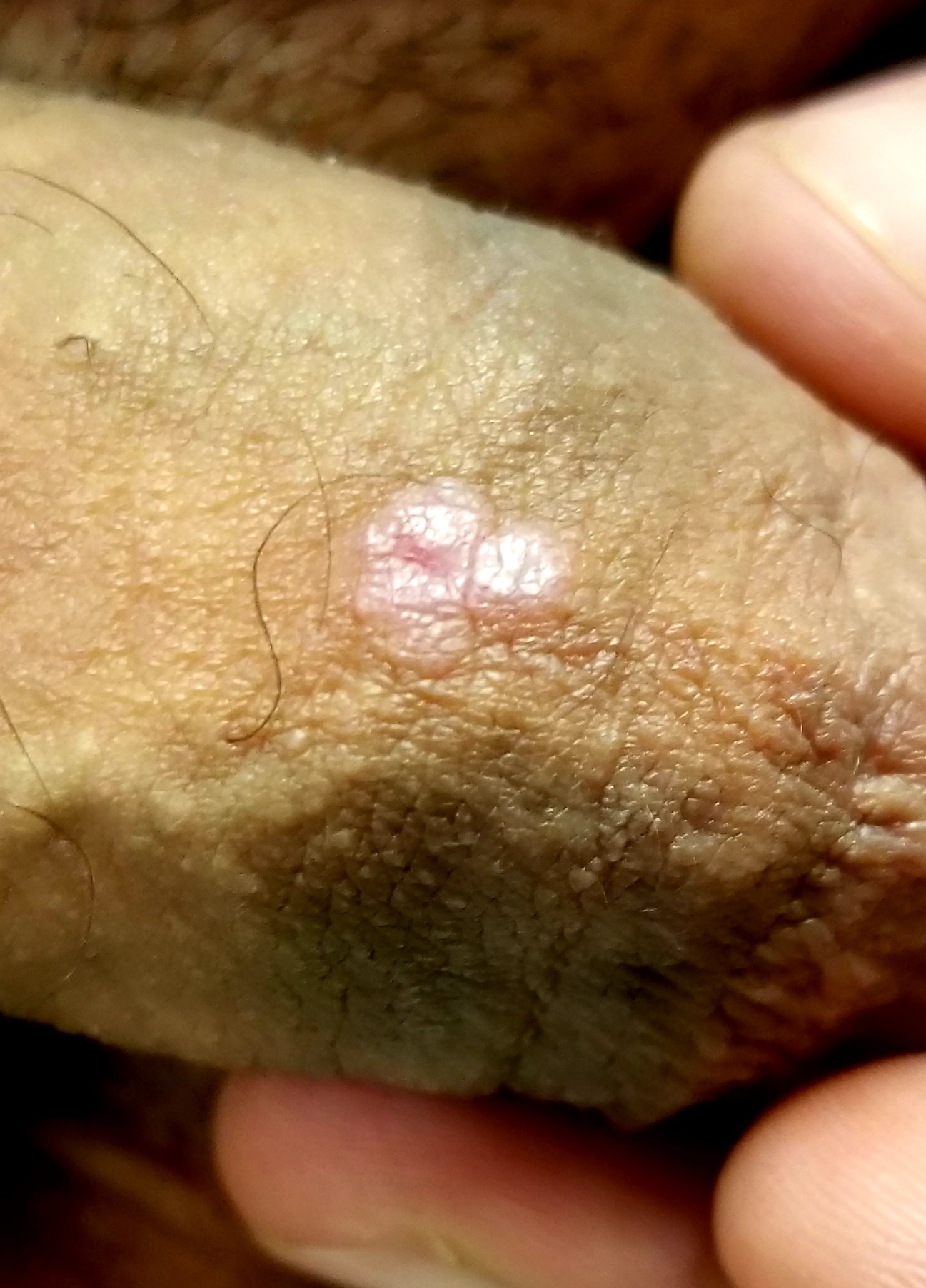
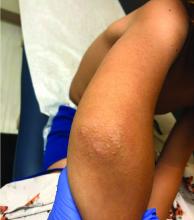
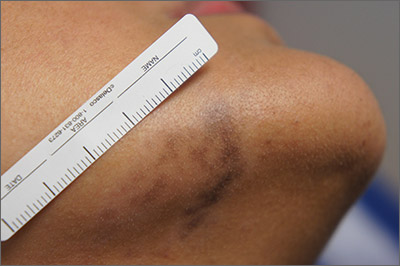
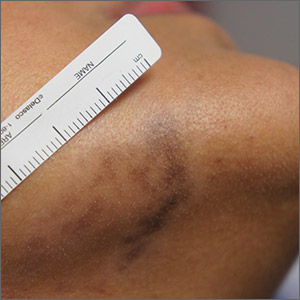
A 41-year-old man is understandably upset when his primary care provider (PCP) diagnoses him with penile warts. Still, he is more than willing to allow his PCP to treat the area with liquid nitrogen, which clears the affected area. However, after about a month, the warts reappear in the same area, with the same appearance, and the patient decides to consult his PCP about additional treatment.
To his distress, his PCP suggests that the warts may continue to return despite treatment. This prompts the patient to ask a more upsetting question: How had he even acquired the warts? Neither he nor his wife of 20 years has had any other sexual contact. Prior to marriage, he had no sexual encounters by which he might have acquired human papillomavirus (HPV).
The patient is otherwise quite healthy, though anxious to have his warts treated again despite the possibility of recurrence. At no point have the warts been symptomatic. His wife's Pap smears have been completely normal.
Examination reveals 4 tiny, pink, planar (flat-topped), 2-to-4-mm papules in 2 locations on the penile shaft. Each has a soft shiny surface. There is also a soft, smooth, pink, annular, 2-cm plaque on the distal shaft that spills over onto the corona focally.
Shave biopsy of 1 lesion shows a brisk lymphocytic infiltrate, which obliterated the dermo-epidermal junction, imparting a jagged sawtooth pattern to its usually smooth wave-like pattern. There are no signs of HPV. The patient has no other remarkable lesions or changes on his elbows, knees, trunk, legs, nails, or scalp.
Clinicians address psoriatic disease risk in the era of COVID-19
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
FROM THE GRAPPA 2020 VIRTUAL ANNUAL MEETING
Racial differences in rates of atopic dermatitis observed early in life
, results from a single-center retrospective study found.
“Atopic dermatitis is a very common pediatric skin condition with significant morbidity for patients and their families,” lead study author Reesa L. Monir, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “Existing studies show increased disease prevalence in Black and Asian children relative to White children, with conflicting data for Hispanic children. The methodology behind many of these existing studies, however, is somewhat questionable. Many were survey-based studies asking parents to remember a diagnosis of eczema or even asking parents to just report an itchy rash and using that as a diagnosis.”
For the current study, Dr. Monir and colleagues reviewed the records of 4,016 infants born between June 1, 2011, and April 30, 2017, who were followed in the University of Florida’s health care system. The researchers defined this as having two or more well-child visits after birth and at least one visit at 300 days of life or later, and the used documentation of specific ICD-9 or ICD-10 codes to capture an objective diagnosis of atopic dermatitis (AD). Of the 4,016 patients, 39.2% were Black, 38.5% were White, 7.1% were Hispanic, 5.3% were Asian, 6.5% were from other racial backgrounds, and 3.4% were multiracial.
Dr. Monir, who is a resident in the department of dermatology at the University of Florida, Gainesville, reported that Black infants had the highest prevalence of AD at 37%, followed by Asian infants (25.8%), Hispanic infants (24.1%), multiracial infants (23%), infants from other racial backgrounds (19.1%), and non-Hispanic White infants (17.9%). Compared with White infants, the odds ratio estimates for AD was highest for Black infants (OR, 2.62), followed by Asian infants (OR, 1.55), multiracial infants (OR, 1.42), Hispanic infants (OR, 1.41), and infants from other racial backgrounds (OR, .97).
On unadjusted analysis, the following factors were significantly associated with race: delivery mode (P = .006), insurance type (P less than .001), NICU stay (P less than .001), and gestational age (P less than .0001). However, on multivariate logistic regression, only two factors were significantly associated with the diagnosis of AD: race (P less than .0001) and NICU stay (P = .0385).
“When we looked at the early childhood period specifically, we found striking racial differences in the rates of AD arising early in life,” Dr. Monir concluded. “The diagnosis was independently associated with race and NICU stay. We suggest that further investigation into these disparities and ways we can mitigate them should focus on this early childhood period.”
The researchers reported having no relevant financial disclosures.
, results from a single-center retrospective study found.
“Atopic dermatitis is a very common pediatric skin condition with significant morbidity for patients and their families,” lead study author Reesa L. Monir, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “Existing studies show increased disease prevalence in Black and Asian children relative to White children, with conflicting data for Hispanic children. The methodology behind many of these existing studies, however, is somewhat questionable. Many were survey-based studies asking parents to remember a diagnosis of eczema or even asking parents to just report an itchy rash and using that as a diagnosis.”
For the current study, Dr. Monir and colleagues reviewed the records of 4,016 infants born between June 1, 2011, and April 30, 2017, who were followed in the University of Florida’s health care system. The researchers defined this as having two or more well-child visits after birth and at least one visit at 300 days of life or later, and the used documentation of specific ICD-9 or ICD-10 codes to capture an objective diagnosis of atopic dermatitis (AD). Of the 4,016 patients, 39.2% were Black, 38.5% were White, 7.1% were Hispanic, 5.3% were Asian, 6.5% were from other racial backgrounds, and 3.4% were multiracial.
Dr. Monir, who is a resident in the department of dermatology at the University of Florida, Gainesville, reported that Black infants had the highest prevalence of AD at 37%, followed by Asian infants (25.8%), Hispanic infants (24.1%), multiracial infants (23%), infants from other racial backgrounds (19.1%), and non-Hispanic White infants (17.9%). Compared with White infants, the odds ratio estimates for AD was highest for Black infants (OR, 2.62), followed by Asian infants (OR, 1.55), multiracial infants (OR, 1.42), Hispanic infants (OR, 1.41), and infants from other racial backgrounds (OR, .97).
On unadjusted analysis, the following factors were significantly associated with race: delivery mode (P = .006), insurance type (P less than .001), NICU stay (P less than .001), and gestational age (P less than .0001). However, on multivariate logistic regression, only two factors were significantly associated with the diagnosis of AD: race (P less than .0001) and NICU stay (P = .0385).
“When we looked at the early childhood period specifically, we found striking racial differences in the rates of AD arising early in life,” Dr. Monir concluded. “The diagnosis was independently associated with race and NICU stay. We suggest that further investigation into these disparities and ways we can mitigate them should focus on this early childhood period.”
The researchers reported having no relevant financial disclosures.
, results from a single-center retrospective study found.
“Atopic dermatitis is a very common pediatric skin condition with significant morbidity for patients and their families,” lead study author Reesa L. Monir, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology. “Existing studies show increased disease prevalence in Black and Asian children relative to White children, with conflicting data for Hispanic children. The methodology behind many of these existing studies, however, is somewhat questionable. Many were survey-based studies asking parents to remember a diagnosis of eczema or even asking parents to just report an itchy rash and using that as a diagnosis.”
For the current study, Dr. Monir and colleagues reviewed the records of 4,016 infants born between June 1, 2011, and April 30, 2017, who were followed in the University of Florida’s health care system. The researchers defined this as having two or more well-child visits after birth and at least one visit at 300 days of life or later, and the used documentation of specific ICD-9 or ICD-10 codes to capture an objective diagnosis of atopic dermatitis (AD). Of the 4,016 patients, 39.2% were Black, 38.5% were White, 7.1% were Hispanic, 5.3% were Asian, 6.5% were from other racial backgrounds, and 3.4% were multiracial.
Dr. Monir, who is a resident in the department of dermatology at the University of Florida, Gainesville, reported that Black infants had the highest prevalence of AD at 37%, followed by Asian infants (25.8%), Hispanic infants (24.1%), multiracial infants (23%), infants from other racial backgrounds (19.1%), and non-Hispanic White infants (17.9%). Compared with White infants, the odds ratio estimates for AD was highest for Black infants (OR, 2.62), followed by Asian infants (OR, 1.55), multiracial infants (OR, 1.42), Hispanic infants (OR, 1.41), and infants from other racial backgrounds (OR, .97).
On unadjusted analysis, the following factors were significantly associated with race: delivery mode (P = .006), insurance type (P less than .001), NICU stay (P less than .001), and gestational age (P less than .0001). However, on multivariate logistic regression, only two factors were significantly associated with the diagnosis of AD: race (P less than .0001) and NICU stay (P = .0385).
“When we looked at the early childhood period specifically, we found striking racial differences in the rates of AD arising early in life,” Dr. Monir concluded. “The diagnosis was independently associated with race and NICU stay. We suggest that further investigation into these disparities and ways we can mitigate them should focus on this early childhood period.”
The researchers reported having no relevant financial disclosures.
FROM SPD 2020
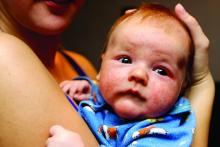
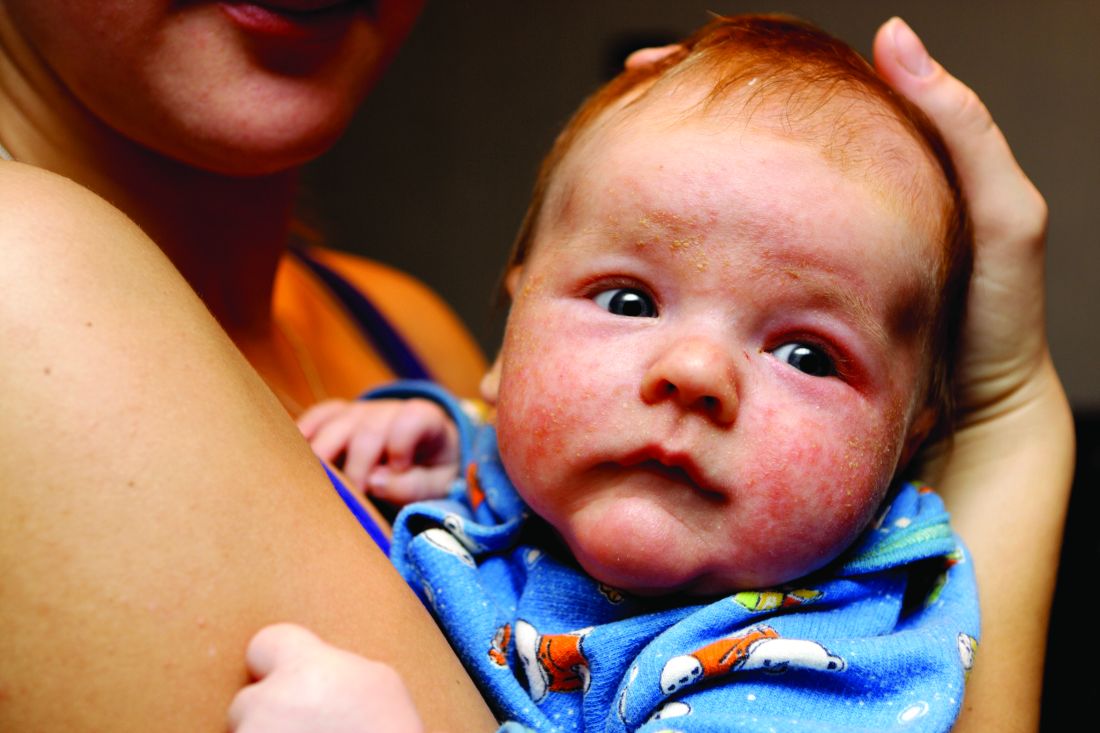
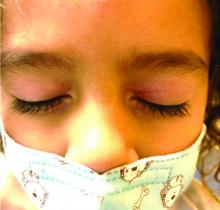
Four-year-old boy presents with itchy rash on face, extremities
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
Contact dermatitis is an eczematous, pruritic eruption caused by direct contact with a substance and an irritant or allergic reaction. While it may not be contagious or life-threatening, contact dermatitis may be tremendously uncomfortable and impactful. Contact dermatitis may occur from exposure to chemicals in soaps, shampoos, cosmetics, metals, plants and topical products, and medications. The hallmark of contact dermatitis is localized eczematous reactions on the portion of the body that has been directly exposed to the reaction-causing substance. – often with oozing and crusting.
Irritant contact dermatitis is the most common type, which occurs when a substance damages the skin’s outer protective layer and does not require prior exposure or sensitization. Allergic contact dermatitis (ACD) can develop after exposure and sensitization, with an external allergen triggering an acute inflammatory response.1 Common causes of ACD include nickel, cobalt, gold, chromium, poison ivy/oak/sumac, cosmetics/personal care products that contain formaldehyde, fragrances, topical medications (anesthetics, antibiotics, corticosteroids), baby wipes, sunscreens, latex materials, protective equipment, soap/cleansers, resins, and acrylics. Among children, nickel sulfate, ammonium persulfate, gold sodium thiosulfate, thimerosal, and toluene-2,5-diamine are the most common sensitizers. Rarely, ACD can be triggered by something that enters the body through foods, flavorings, medicine, or medical or dental procedures (systemic contact dermatitis).
An Id reaction, or autoeczematization, is a generalized acute cutaneous reaction to a variety of stimuli, including infectious and inflammatory skin conditions such as contact dermatitis, stasis dermatitis, or other eczematous dermatitis.3 Id reactions usually are preceded by a preexisting dermatitis. Lesions are, by definition, at a site distant from the primary infection or dermatitis. They often are distributed symmetrically. Papular or papular-vesicular lesions of the extremities and or trunk are common in children.
Our patient had evidence of a localized periocular contact dermatitis reaction that preceded the symmetric papular, eczematous eruption consistent with an id reaction. Our patient was prescribed hydrocortisone 2.5 % ointment for the eyes and triamcinolone 0.1% ointment for the rash on the body, which resulted in significant improvement.
Rosacea is a chronic and relapsing inflammatory skin disorder that primarily involves the central face. Common clinical features include facial erythema, telangiectasias, and inflammatory papules or pustules. Ocular involvement may occur in the presence or absence of cutaneous manifestations. Patients may report the presence of ocular foreign body sensation, burning, photophobia, blurred vision, redness, and tearing. Ocular disease is usually bilateral and is not proportional to the severity of the skin disease.4 Common skin findings are blepharitis, lid margin telangiectasia, tear abnormalities, meibomian gland inflammation, frequent chalazion, bilateral hordeolum, conjunctivitis, and, rarely, corneal ulcers and vascularization. Our patient initially did have bilateral hordeolum in what may seem to be ocular rosacea. However, given the use of a recent topical antibiotic with subsequent eczematous rash of the eyelids and then resulting distant rash on the body 1week later made the rash likely allergic contact dermatitis with id reaction.
Seborrheic dermatitis is a chronic, relapsing, and usually mild form of dermatitis that occurs in infants and in adults. The severity may vary from minimal, asymptomatic scaliness of the scalp (dandruff) to more widespread involvement. It is usually characterized by well-demarcated, erythematous plaques with greasy-looking, yellowish scales distributed on areas rich in sebaceous glands, such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas.
Psoriasis typically affects the outside of the elbows, knees, or scalp, although it can appear on any location. It tends to go through cycles, flaring for a few weeks or months, then subsiding for a while or going into remission. Ocular involvement is a well known manifestation of psoriasis.5 Psoriatic lesions of the eyelid are rare, even in the erythrodermic variant of the disease. Occasionally, pustular psoriasis may involve the eyelids, with typical psoriatic lesions visible on the skin and lid margin. The reason for the relative sparing of the eyelid skin in patients with psoriasis is unknown. Other manifestations include meibomian gland dysfunction, decreased tear film break-up time, a nonspecific conjunctivitis, and corneal disease secondary to lid disease such as trichiasis.
Gianotti-Crosti syndrome (GCS), also known as papular acrodermatitis, papular acrodermatitis of childhood, and infantile papular acrodermatitis, is a self-limited skin disorder that most often occurs in young children. Viral infections are common GCS precipitating factors . GCS typically manifests as a symmetric, papular eruption, often with larger (3- to 10-mm) flat topped papulovesicles. Classic sites of involvement include the cheeks, buttocks, and extensor surfaces of the forearms and legs. GCS may be pruritic or asymptomatic, and papules typically resolve spontaneously within 2 months. Occasionally, GCS persists for longer periods. The eyelid lesions and localized pattern, with the absence of larger symmetric papules of the buttocks and legs, was not consistent with papular acrodermatitis of childhood.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. They had no conflicts of interest to disclose. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol 2016 Jun; 74(6):1043-54.
2. Pediatr Dermatol 2016 Jul; 33(4):399-404.
3. Evans M & Bronson D. (2019) Id Reaction (Autoeczematization). Retrieved from emedicine.medscape.com/article/1049760-overview.
4. Curr Opin Ophthalmol. 2004 Dec;15(6):499-502.
5. Clin Dermatol. Mar-Apr 2016;34(2):146-50.
A 4-year-old healthy male with no significant prior medical history presents for evaluation of "itchy bumps" on the face and extremities of 2 weeks' duration.
The child was well until around 2 and a half weeks ago when he presented for evaluation of two lesions on the lower eyelids, diagnosed as hordeolum (a stye). He was prescribed ofloxacin ophthalmic solution.
One week later he developed bilateral itchy red eyes with red, thickened areas on the upper lids, followed several days later by pruritic papules on the ears, wrists, elbows, knees, and ankles. His mother used Vaseline for the eyelids for 1 week with no improvement. Physical exam at the dermatologist's office showed mild erythema, induration, and lichenification of the upper eyelids, and bilateral periocular eczematous patches with overlying scale. Subtle papules were evident on the elbows and feet.
Jaw pigmentation
Exam and biopsy led to a diagnosis of erythema dyschromicum perstans. Notably, the punch biopsy was performed as superficially as possible to avoid injuring the marginal mandibular branch of the facial nerve. Histology showed focal vacuolar interface changes, pigmentary incontinence, and prominent dermal melanophages—consistent with erythema dyschromicum perstans.
Erythema dyschromicum perstans is an uncommon dermal macular hyperpigmentation that can affect patients of all ages. It is more often seen in patients with Fitzpatrick skin types III and IV. Some patients have a preceding inflammatory phase with associated erythema.
It is believed that erythema dyschromicum perstans may be a form of pigmented lichen planus with similar histologic changes. Genetic predisposition and medications, such as penicillamine and omeprazole, have been suggested as predisposing risk factors, although these risk factors are not always present. Similarly, cases of erythema dyschromicum perstans associated with human immunodeficiency virus and hepatitis C virus have been reported, but a causal link has not been established. Commonly affected sites include the face and neck, although patches on the trunk and extremities occur, as well.
The differential diagnosis includes lichen planus, discoid lupus, drug-induced hyperpigmentation, Hansen disease, and fixed drug eruption.
Erythema dyschromicum perstans is resistant to most therapies, yet it may clear spontaneously over the years. Therapies that have been successful in case reports include Q-switched ruby laser, topical steroids, UV therapy, isotretinoin, and clofazimine. This patient used desonide cream 0.05% bid for 3 weeks without improvement. She then used camouflage concealer for a year, at which point the area had faded almost completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Gutierrez D, Krueger LD, Tan A, et al. Proton pump inhibitor-induced erythema dyschromicum perstans-like pigmentation. JAAD Case Rep. 2019;5:701-703.
Exam and biopsy led to a diagnosis of erythema dyschromicum perstans. Notably, the punch biopsy was performed as superficially as possible to avoid injuring the marginal mandibular branch of the facial nerve. Histology showed focal vacuolar interface changes, pigmentary incontinence, and prominent dermal melanophages—consistent with erythema dyschromicum perstans.
Erythema dyschromicum perstans is an uncommon dermal macular hyperpigmentation that can affect patients of all ages. It is more often seen in patients with Fitzpatrick skin types III and IV. Some patients have a preceding inflammatory phase with associated erythema.
It is believed that erythema dyschromicum perstans may be a form of pigmented lichen planus with similar histologic changes. Genetic predisposition and medications, such as penicillamine and omeprazole, have been suggested as predisposing risk factors, although these risk factors are not always present. Similarly, cases of erythema dyschromicum perstans associated with human immunodeficiency virus and hepatitis C virus have been reported, but a causal link has not been established. Commonly affected sites include the face and neck, although patches on the trunk and extremities occur, as well.
The differential diagnosis includes lichen planus, discoid lupus, drug-induced hyperpigmentation, Hansen disease, and fixed drug eruption.
Erythema dyschromicum perstans is resistant to most therapies, yet it may clear spontaneously over the years. Therapies that have been successful in case reports include Q-switched ruby laser, topical steroids, UV therapy, isotretinoin, and clofazimine. This patient used desonide cream 0.05% bid for 3 weeks without improvement. She then used camouflage concealer for a year, at which point the area had faded almost completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Exam and biopsy led to a diagnosis of erythema dyschromicum perstans. Notably, the punch biopsy was performed as superficially as possible to avoid injuring the marginal mandibular branch of the facial nerve. Histology showed focal vacuolar interface changes, pigmentary incontinence, and prominent dermal melanophages—consistent with erythema dyschromicum perstans.
Erythema dyschromicum perstans is an uncommon dermal macular hyperpigmentation that can affect patients of all ages. It is more often seen in patients with Fitzpatrick skin types III and IV. Some patients have a preceding inflammatory phase with associated erythema.
It is believed that erythema dyschromicum perstans may be a form of pigmented lichen planus with similar histologic changes. Genetic predisposition and medications, such as penicillamine and omeprazole, have been suggested as predisposing risk factors, although these risk factors are not always present. Similarly, cases of erythema dyschromicum perstans associated with human immunodeficiency virus and hepatitis C virus have been reported, but a causal link has not been established. Commonly affected sites include the face and neck, although patches on the trunk and extremities occur, as well.
The differential diagnosis includes lichen planus, discoid lupus, drug-induced hyperpigmentation, Hansen disease, and fixed drug eruption.
Erythema dyschromicum perstans is resistant to most therapies, yet it may clear spontaneously over the years. Therapies that have been successful in case reports include Q-switched ruby laser, topical steroids, UV therapy, isotretinoin, and clofazimine. This patient used desonide cream 0.05% bid for 3 weeks without improvement. She then used camouflage concealer for a year, at which point the area had faded almost completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Gutierrez D, Krueger LD, Tan A, et al. Proton pump inhibitor-induced erythema dyschromicum perstans-like pigmentation. JAAD Case Rep. 2019;5:701-703.
Gutierrez D, Krueger LD, Tan A, et al. Proton pump inhibitor-induced erythema dyschromicum perstans-like pigmentation. JAAD Case Rep. 2019;5:701-703.
Be wary of ‘for eczema’ claims on labels of popular moisturizers
Be wary of “for eczema” advertising claims contained on the labels of popular skin moisturizers.
Results from a study presented during the virtual annual meeting of the Society for Pediatric Dermatology found that .
“Prescription medications are important for managing eczema flares, but a lot of the work in treating eczema is preventative, done by consistently moisturizing the skin at home with drug store products,” co-first study author Catherine L. Ludwig, said in an interview. “Allergic contact dermatitis occurs more commonly in people with eczema. A previous study was done in characterizing the allergenic potential of drug store moisturizers and found that 88% of moisturizers contain at least one common allergen. Many moisturizers are marketed specifically to eczema, but the allergen content of these products are unknown.”
For the current study, Ms. Ludwig, a medical student at the University of Illinois at Chicago and co-first author Alyssa M. Thompson, a medical student at the University of Arizona, Tucson, and their colleagues compiled a list of the top 30 moisturizers “for eczema” sold by Amazon, Target, and Walmart. For each moisturizer they recorded common ingredients and marketing claims related to benefits for atopic dermatitis, including eczema relief, sensitive/gentle skin, hypoallergenic, anti-itch, anti-inflammatory, clinically proven, oatmeal, dermatologist recommended/approved, organic, fragrance-free, for baby, or National Eczema Association approved. To establish allergenic potential, the researchers used MATLAB to compare ingredient lists to compounds listed as common allergens in the American Contact Dermatitis Society’s Contact Allergen Management Program database (ACDS CAMP). Next, they used the Mann-Whitney U test to evaluate differences in allergen count between products with and without specific marketing claims.
Ms. Ludwig and her associates found that 28 of 30 products analyzed (93%) contained at least one allergen, with an overall average allergen count of 3.60. The three most prevalent allergens were cetyl alcohol (70%), phenoxyethanol (50%), and aloe (33%). “Anti-inflammatory” moisturizers had the greatest average number of allergens (4.00), followed by “anti-itch” (3.71) and “oatmeal” (3.71). Only products claiming to be “hypoallergenic” had significantly lower allergenic ingredient count (an average of 2.45) than those without the claim (P = .011).
“It was validating to see that eczema moisturizer products marketed as ‘hypoallergenic’ truly do have fewer allergenic ingredients than moisturizers without the claim,” Ms. Ludwig said. “However, it was surprising to see that even products marketed to eczema patients, who have a higher prevalence of allergic contact dermatitis, contain an average of 3.6 common allergens. As dermatology providers, we can relay to patients and parents that relying solely on ‘for eczema’ claims is not advisable. Clinicians should acquaint themselves with the top allergens (cetyl alcohol, phenoxyethanol, and aloe) and keep these ingredients, as well as affordability and patient preferences, in mind when making product recommendations.”
The study’s senior author, Vivian Y. Shi, MD, is a stock shareholder of Learn Health and has served as an advisory board member and/or investigator, and/or received research funding from AbbVie, Burt’s Bees, GpSkin, LEO Pharma, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Skin Actives Scientific, and SUN Pharma, and the Foundation for Atopic Dermatitis, Global Parents for Eczema Research, and the National Eczema Association. The other study authors reported having no financial disclosures.
Be wary of “for eczema” advertising claims contained on the labels of popular skin moisturizers.
Results from a study presented during the virtual annual meeting of the Society for Pediatric Dermatology found that .
“Prescription medications are important for managing eczema flares, but a lot of the work in treating eczema is preventative, done by consistently moisturizing the skin at home with drug store products,” co-first study author Catherine L. Ludwig, said in an interview. “Allergic contact dermatitis occurs more commonly in people with eczema. A previous study was done in characterizing the allergenic potential of drug store moisturizers and found that 88% of moisturizers contain at least one common allergen. Many moisturizers are marketed specifically to eczema, but the allergen content of these products are unknown.”
For the current study, Ms. Ludwig, a medical student at the University of Illinois at Chicago and co-first author Alyssa M. Thompson, a medical student at the University of Arizona, Tucson, and their colleagues compiled a list of the top 30 moisturizers “for eczema” sold by Amazon, Target, and Walmart. For each moisturizer they recorded common ingredients and marketing claims related to benefits for atopic dermatitis, including eczema relief, sensitive/gentle skin, hypoallergenic, anti-itch, anti-inflammatory, clinically proven, oatmeal, dermatologist recommended/approved, organic, fragrance-free, for baby, or National Eczema Association approved. To establish allergenic potential, the researchers used MATLAB to compare ingredient lists to compounds listed as common allergens in the American Contact Dermatitis Society’s Contact Allergen Management Program database (ACDS CAMP). Next, they used the Mann-Whitney U test to evaluate differences in allergen count between products with and without specific marketing claims.
Ms. Ludwig and her associates found that 28 of 30 products analyzed (93%) contained at least one allergen, with an overall average allergen count of 3.60. The three most prevalent allergens were cetyl alcohol (70%), phenoxyethanol (50%), and aloe (33%). “Anti-inflammatory” moisturizers had the greatest average number of allergens (4.00), followed by “anti-itch” (3.71) and “oatmeal” (3.71). Only products claiming to be “hypoallergenic” had significantly lower allergenic ingredient count (an average of 2.45) than those without the claim (P = .011).
“It was validating to see that eczema moisturizer products marketed as ‘hypoallergenic’ truly do have fewer allergenic ingredients than moisturizers without the claim,” Ms. Ludwig said. “However, it was surprising to see that even products marketed to eczema patients, who have a higher prevalence of allergic contact dermatitis, contain an average of 3.6 common allergens. As dermatology providers, we can relay to patients and parents that relying solely on ‘for eczema’ claims is not advisable. Clinicians should acquaint themselves with the top allergens (cetyl alcohol, phenoxyethanol, and aloe) and keep these ingredients, as well as affordability and patient preferences, in mind when making product recommendations.”
The study’s senior author, Vivian Y. Shi, MD, is a stock shareholder of Learn Health and has served as an advisory board member and/or investigator, and/or received research funding from AbbVie, Burt’s Bees, GpSkin, LEO Pharma, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Skin Actives Scientific, and SUN Pharma, and the Foundation for Atopic Dermatitis, Global Parents for Eczema Research, and the National Eczema Association. The other study authors reported having no financial disclosures.
Be wary of “for eczema” advertising claims contained on the labels of popular skin moisturizers.
Results from a study presented during the virtual annual meeting of the Society for Pediatric Dermatology found that .
“Prescription medications are important for managing eczema flares, but a lot of the work in treating eczema is preventative, done by consistently moisturizing the skin at home with drug store products,” co-first study author Catherine L. Ludwig, said in an interview. “Allergic contact dermatitis occurs more commonly in people with eczema. A previous study was done in characterizing the allergenic potential of drug store moisturizers and found that 88% of moisturizers contain at least one common allergen. Many moisturizers are marketed specifically to eczema, but the allergen content of these products are unknown.”
For the current study, Ms. Ludwig, a medical student at the University of Illinois at Chicago and co-first author Alyssa M. Thompson, a medical student at the University of Arizona, Tucson, and their colleagues compiled a list of the top 30 moisturizers “for eczema” sold by Amazon, Target, and Walmart. For each moisturizer they recorded common ingredients and marketing claims related to benefits for atopic dermatitis, including eczema relief, sensitive/gentle skin, hypoallergenic, anti-itch, anti-inflammatory, clinically proven, oatmeal, dermatologist recommended/approved, organic, fragrance-free, for baby, or National Eczema Association approved. To establish allergenic potential, the researchers used MATLAB to compare ingredient lists to compounds listed as common allergens in the American Contact Dermatitis Society’s Contact Allergen Management Program database (ACDS CAMP). Next, they used the Mann-Whitney U test to evaluate differences in allergen count between products with and without specific marketing claims.
Ms. Ludwig and her associates found that 28 of 30 products analyzed (93%) contained at least one allergen, with an overall average allergen count of 3.60. The three most prevalent allergens were cetyl alcohol (70%), phenoxyethanol (50%), and aloe (33%). “Anti-inflammatory” moisturizers had the greatest average number of allergens (4.00), followed by “anti-itch” (3.71) and “oatmeal” (3.71). Only products claiming to be “hypoallergenic” had significantly lower allergenic ingredient count (an average of 2.45) than those without the claim (P = .011).
“It was validating to see that eczema moisturizer products marketed as ‘hypoallergenic’ truly do have fewer allergenic ingredients than moisturizers without the claim,” Ms. Ludwig said. “However, it was surprising to see that even products marketed to eczema patients, who have a higher prevalence of allergic contact dermatitis, contain an average of 3.6 common allergens. As dermatology providers, we can relay to patients and parents that relying solely on ‘for eczema’ claims is not advisable. Clinicians should acquaint themselves with the top allergens (cetyl alcohol, phenoxyethanol, and aloe) and keep these ingredients, as well as affordability and patient preferences, in mind when making product recommendations.”
The study’s senior author, Vivian Y. Shi, MD, is a stock shareholder of Learn Health and has served as an advisory board member and/or investigator, and/or received research funding from AbbVie, Burt’s Bees, GpSkin, LEO Pharma, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Skin Actives Scientific, and SUN Pharma, and the Foundation for Atopic Dermatitis, Global Parents for Eczema Research, and the National Eczema Association. The other study authors reported having no financial disclosures.
FROM SPD 2020
Topical PDE-4 inhibitor for psoriasis effective in phase 2b trial
Once-daily – including challenging lesions in tough-to-treat intertriginous areas – in a phase 2b, randomized, double-blind, vehicle-controlled clinical trial, Mark G. Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The clinical improvement occurred rapidly. And topical roflumilast’s side effect profile was essentially the same as in vehicle-treated controls, which suggests a potential major advantage for the novel drug in future clinical practice. After all, topical treatment is the mainstay of psoriasis therapy, but the current topical agents – high-potency corticosteroids, vitamin D derivatives, and retinoids – have long-term tolerability, efficacy, or side effect issues, especially in treating sensitive skin areas, including the face and intertriginous areas.
“Roflumilast cream could really be a game changer,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Phosphodiesterase-4 (PDE-4) activity is elevated in psoriatic skin. Indeed, inhibition of PDE-4 via oral apremilast (Otezla) is an established strategy for improving psoriasis through down-regulation of inflammatory cytokines including tumor necrosis factor–alpha, interleukins-17 and -23, and interferon-gamma. Notably, however, roflumilast is orders of magnitude more potent than any other PDE-4 inhibitor. An oral version marketed as Daliresp has been available for treatment of chronic obstructive pulmonary disease for nearly a decade.
The 12-week, multicenter, phase 2b study included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had a baseline Investigator Global Assessment (IGA) score of 3, indicative of moderate disease.
The primary endpoint was achievement of an IGA score of 0 or 1 (clear or almost clear) at week 6. The observed improvement was dose related, although both doses of roflumilast were significantly more effective than vehicle. However, peak improvement occurred at week 8, not week 6, with subsequent plateauing of response through week 12. A week 8 IGA of 0 or 1 plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls.
“The effect in improvement was very rapid, with a statistically significant improvement compared to vehicle for both concentrations as early as week 2,” Dr. Lebwohl said.
A key secondary endpoint focused on treatment response in intertriginous areas, since “those are the areas where we really don’t want to use steroids because of major irritation problems,” the dermatologist explained. At week 12, treatment success as defined by an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls.
About 65% of subjects on high-dose roflumilast cream reported at least a 4-point reduction in the Worst Itch–Numerical Rating Scale by week 8, as did 58% of those on the low-dose version and 42% of controls. Another secondary endpoint – patient-reported burden of disease as captured in a Psoriasis Symptoms Diary – showed a significant divergence between both doses of roflumilast and vehicle as early as week 2.
“Adverse events were negligible,” Dr. Lebwohl said. “In fact, there was only one discontinuation in the 0.3% arm, compared to none with 0.15% and two with vehicle.”
The phase 3 program is now recruiting participants.
The phase 2b study was funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving research funding from and serving as a consultant to that company and numerous others.
Once-daily – including challenging lesions in tough-to-treat intertriginous areas – in a phase 2b, randomized, double-blind, vehicle-controlled clinical trial, Mark G. Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The clinical improvement occurred rapidly. And topical roflumilast’s side effect profile was essentially the same as in vehicle-treated controls, which suggests a potential major advantage for the novel drug in future clinical practice. After all, topical treatment is the mainstay of psoriasis therapy, but the current topical agents – high-potency corticosteroids, vitamin D derivatives, and retinoids – have long-term tolerability, efficacy, or side effect issues, especially in treating sensitive skin areas, including the face and intertriginous areas.
“Roflumilast cream could really be a game changer,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Phosphodiesterase-4 (PDE-4) activity is elevated in psoriatic skin. Indeed, inhibition of PDE-4 via oral apremilast (Otezla) is an established strategy for improving psoriasis through down-regulation of inflammatory cytokines including tumor necrosis factor–alpha, interleukins-17 and -23, and interferon-gamma. Notably, however, roflumilast is orders of magnitude more potent than any other PDE-4 inhibitor. An oral version marketed as Daliresp has been available for treatment of chronic obstructive pulmonary disease for nearly a decade.
The 12-week, multicenter, phase 2b study included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had a baseline Investigator Global Assessment (IGA) score of 3, indicative of moderate disease.
The primary endpoint was achievement of an IGA score of 0 or 1 (clear or almost clear) at week 6. The observed improvement was dose related, although both doses of roflumilast were significantly more effective than vehicle. However, peak improvement occurred at week 8, not week 6, with subsequent plateauing of response through week 12. A week 8 IGA of 0 or 1 plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls.
“The effect in improvement was very rapid, with a statistically significant improvement compared to vehicle for both concentrations as early as week 2,” Dr. Lebwohl said.
A key secondary endpoint focused on treatment response in intertriginous areas, since “those are the areas where we really don’t want to use steroids because of major irritation problems,” the dermatologist explained. At week 12, treatment success as defined by an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls.
About 65% of subjects on high-dose roflumilast cream reported at least a 4-point reduction in the Worst Itch–Numerical Rating Scale by week 8, as did 58% of those on the low-dose version and 42% of controls. Another secondary endpoint – patient-reported burden of disease as captured in a Psoriasis Symptoms Diary – showed a significant divergence between both doses of roflumilast and vehicle as early as week 2.
“Adverse events were negligible,” Dr. Lebwohl said. “In fact, there was only one discontinuation in the 0.3% arm, compared to none with 0.15% and two with vehicle.”
The phase 3 program is now recruiting participants.
The phase 2b study was funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving research funding from and serving as a consultant to that company and numerous others.
Once-daily – including challenging lesions in tough-to-treat intertriginous areas – in a phase 2b, randomized, double-blind, vehicle-controlled clinical trial, Mark G. Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The clinical improvement occurred rapidly. And topical roflumilast’s side effect profile was essentially the same as in vehicle-treated controls, which suggests a potential major advantage for the novel drug in future clinical practice. After all, topical treatment is the mainstay of psoriasis therapy, but the current topical agents – high-potency corticosteroids, vitamin D derivatives, and retinoids – have long-term tolerability, efficacy, or side effect issues, especially in treating sensitive skin areas, including the face and intertriginous areas.
“Roflumilast cream could really be a game changer,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Phosphodiesterase-4 (PDE-4) activity is elevated in psoriatic skin. Indeed, inhibition of PDE-4 via oral apremilast (Otezla) is an established strategy for improving psoriasis through down-regulation of inflammatory cytokines including tumor necrosis factor–alpha, interleukins-17 and -23, and interferon-gamma. Notably, however, roflumilast is orders of magnitude more potent than any other PDE-4 inhibitor. An oral version marketed as Daliresp has been available for treatment of chronic obstructive pulmonary disease for nearly a decade.
The 12-week, multicenter, phase 2b study included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had a baseline Investigator Global Assessment (IGA) score of 3, indicative of moderate disease.
The primary endpoint was achievement of an IGA score of 0 or 1 (clear or almost clear) at week 6. The observed improvement was dose related, although both doses of roflumilast were significantly more effective than vehicle. However, peak improvement occurred at week 8, not week 6, with subsequent plateauing of response through week 12. A week 8 IGA of 0 or 1 plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls.
“The effect in improvement was very rapid, with a statistically significant improvement compared to vehicle for both concentrations as early as week 2,” Dr. Lebwohl said.
A key secondary endpoint focused on treatment response in intertriginous areas, since “those are the areas where we really don’t want to use steroids because of major irritation problems,” the dermatologist explained. At week 12, treatment success as defined by an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls.
About 65% of subjects on high-dose roflumilast cream reported at least a 4-point reduction in the Worst Itch–Numerical Rating Scale by week 8, as did 58% of those on the low-dose version and 42% of controls. Another secondary endpoint – patient-reported burden of disease as captured in a Psoriasis Symptoms Diary – showed a significant divergence between both doses of roflumilast and vehicle as early as week 2.
“Adverse events were negligible,” Dr. Lebwohl said. “In fact, there was only one discontinuation in the 0.3% arm, compared to none with 0.15% and two with vehicle.”
The phase 3 program is now recruiting participants.
The phase 2b study was funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving research funding from and serving as a consultant to that company and numerous others.
FROM AAD 20
Subcutaneous nemolizumab eases itching for atopic dermatitis
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Diagnosing molluscum contagiosum can be tricky
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
Oral difelikefalin quells severe chronic kidney disease–associated itch
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
FROM AAD 2020