User login
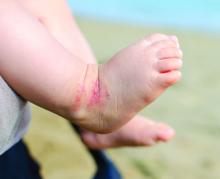
Database offers snapshot of common causes of pediatric allergic contact dermatitis
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
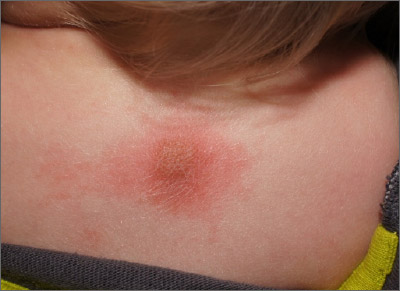
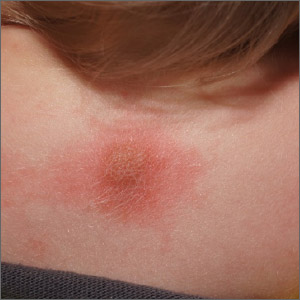
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
, according to an analysis of data from the Pediatric Allergic Contact Dermatitis Registry.
The registry is the first multicenter prospective database in the United States with a focus on pediatric allergic contact dermatitis. JiaDe (Jeff) Yu, MD, a dermatologist at Massachusetts General Hospital, Boston, was awarded a Dermatology Foundation Career Development Grant and formed the registry in 2018 “in an effort to gain a better understanding of allergic contact dermatitis in children,” Idy Tam, MS, said during the virtual annual meeting of the Society for Pediatric Dermatology. “There is currently limited data regarding the pediatric allergic contact dermatitis in the U.S., despite as many as 20% of children having allergic contact dermatitis.”
To date, the Pediatric Allergic Contact Dermatitis Registry consists of 10 academic medical centers with high volume pediatric patch testing across the United States: Massachusetts General Hospital, Boston; Brigham and Women’s Hospital, Boston; the University of Missouri–Columbia; Stanford (Calif.) University; the Medical University of South Carolina, Charleston; Texas Children’s Hospital, Houston; Northwestern University, Chicago; Emory University, Atlanta; Washington University, St. Louis; and the University of California, San Diego.
For the current analysis, Ms. Tam, a research fellow in the department of dermatology at Massachusetts General Hospital, and colleagues collected data on 218 patients under age 18 who were referred for an evaluation of allergic contact dermatitis at one of the 10 participating sites between January 2016 and June 2020.
The mean age of children at the time of their patch testing was 10 years, 62% were girls, and 66% had a history of atopic dermatitis (AD). Most (75%) were White, 14% were Black, 6% were Asian, the rest were from other racial backgrounds. The distribution of dermatitis varied; the top five most commonly affected sites were the face (62%), arms (35%), legs (29%), hands (27%), and neck (20%).
Ms. Tam reported that the mean number of allergens patch tested per child was 78. In all, 81% of children had one or more positive patch test reactions, with a similar rate among those with and without a history of AD (80% vs. 82%, respectively; P = .21). The five most common allergens were hydroperoxides of linalool (22%), nickel sulfate (19%), methylisothiazolinone (17%), cobalt chloride (13%), and fragrance mix I (12%).
The top two treatments at the time of patch testing were a topical corticosteroid (78%) and a topical calcineurin inhibitor (26%).
“This study has allowed for the increased collaboration among dermatologists with expertise in pediatric dermatology and allergic contact dermatitis,” concluded Ms. Tam, a fourth-year medical student at Tufts University, Boston. “We continue to actively seek further collaboration with a goal of creating the most comprehensive pediatric allergic contact dermatitis registry, which can improve our understanding of this condition in children and hopefully guide future research in this field.”
The work was recognized as one of the top poster abstracts at the meeting. The researchers reported having no relevant disclosures.
FROM SPD 2020
Americans getting more sunburns
, for reasons that are unclear, Nicole L. Bolick, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
On the plus side, utilization of indoor tanning plunged in the United States during the same period, a statistic worth celebrating as a public health and legislative success, noted Dr. Bolick, who was at the Harvard T.H. Chan School of Public Health, Boston, when she conducted her study and is now at East Carolina University, Greenville, N.C.
More good news: Her analysis of data from 67,471 nationally representative participants in the Centers for Disease Control and Prevention’s National Health Information Survey for the years 2005, 2010, and 2015 also demonstrated that the public’s adoption of several key skin cancer prevention behaviors is on the rise, although she added that rates clearly remain suboptimal.
For example, the proportion of Americans who practice sun avoidance climbed from 31.7% in 2005 to 35.5% in 2010, and 36.8% in 2015 in a multivariate logistic regression analysis adjusted for demographics, alcohol use, location, smoking status, education level, health insurance, and family and personal history of skin cancer.
Similarly, the use of sunscreen always or most of the time when outdoors for more than 1 hour on a warm, sunny day rose from an adjusted 31.5% in 2005 to 33.1% in 2010 and to 34.3% in 2015.
Also, sun protective clothing – long pants, hats, and/or long-sleeved shirts – was utilized always or most of the time by 35.9% of respondents in 2005, 38.4% in 2010, and 37.2% in 2015.
In 2005, 19% of Americans reported having a lifetime history of a physician-performed full body skin examination. The prevalence of this secondary skin cancer prevention measure rose to 22.4% in 2010 and remained the same in 2015.
In the 2005 national survey, 14.1% of respondents reported engaging in indoor tanning within the past year. This figure dropped to 6.2% in 2010 and fell further to 4.1% in 2015.
A history of two or more sunburns within the past year was reported by 18.2% of subjects in 2005, by 21.1% in 2010, and by 19.9% in 2015. It’s unclear whether this unwelcome phenomenon is due to inadequate use of sun protection or increased awareness of the link between sun exposure and skin cancer, with a resultant increase in reporting of sunburns. The influence of climate change is another possible explanation worthy of further study, according to Dr. Bolick.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
, for reasons that are unclear, Nicole L. Bolick, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
On the plus side, utilization of indoor tanning plunged in the United States during the same period, a statistic worth celebrating as a public health and legislative success, noted Dr. Bolick, who was at the Harvard T.H. Chan School of Public Health, Boston, when she conducted her study and is now at East Carolina University, Greenville, N.C.
More good news: Her analysis of data from 67,471 nationally representative participants in the Centers for Disease Control and Prevention’s National Health Information Survey for the years 2005, 2010, and 2015 also demonstrated that the public’s adoption of several key skin cancer prevention behaviors is on the rise, although she added that rates clearly remain suboptimal.
For example, the proportion of Americans who practice sun avoidance climbed from 31.7% in 2005 to 35.5% in 2010, and 36.8% in 2015 in a multivariate logistic regression analysis adjusted for demographics, alcohol use, location, smoking status, education level, health insurance, and family and personal history of skin cancer.
Similarly, the use of sunscreen always or most of the time when outdoors for more than 1 hour on a warm, sunny day rose from an adjusted 31.5% in 2005 to 33.1% in 2010 and to 34.3% in 2015.
Also, sun protective clothing – long pants, hats, and/or long-sleeved shirts – was utilized always or most of the time by 35.9% of respondents in 2005, 38.4% in 2010, and 37.2% in 2015.
In 2005, 19% of Americans reported having a lifetime history of a physician-performed full body skin examination. The prevalence of this secondary skin cancer prevention measure rose to 22.4% in 2010 and remained the same in 2015.
In the 2005 national survey, 14.1% of respondents reported engaging in indoor tanning within the past year. This figure dropped to 6.2% in 2010 and fell further to 4.1% in 2015.
A history of two or more sunburns within the past year was reported by 18.2% of subjects in 2005, by 21.1% in 2010, and by 19.9% in 2015. It’s unclear whether this unwelcome phenomenon is due to inadequate use of sun protection or increased awareness of the link between sun exposure and skin cancer, with a resultant increase in reporting of sunburns. The influence of climate change is another possible explanation worthy of further study, according to Dr. Bolick.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
, for reasons that are unclear, Nicole L. Bolick, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
On the plus side, utilization of indoor tanning plunged in the United States during the same period, a statistic worth celebrating as a public health and legislative success, noted Dr. Bolick, who was at the Harvard T.H. Chan School of Public Health, Boston, when she conducted her study and is now at East Carolina University, Greenville, N.C.
More good news: Her analysis of data from 67,471 nationally representative participants in the Centers for Disease Control and Prevention’s National Health Information Survey for the years 2005, 2010, and 2015 also demonstrated that the public’s adoption of several key skin cancer prevention behaviors is on the rise, although she added that rates clearly remain suboptimal.
For example, the proportion of Americans who practice sun avoidance climbed from 31.7% in 2005 to 35.5% in 2010, and 36.8% in 2015 in a multivariate logistic regression analysis adjusted for demographics, alcohol use, location, smoking status, education level, health insurance, and family and personal history of skin cancer.
Similarly, the use of sunscreen always or most of the time when outdoors for more than 1 hour on a warm, sunny day rose from an adjusted 31.5% in 2005 to 33.1% in 2010 and to 34.3% in 2015.
Also, sun protective clothing – long pants, hats, and/or long-sleeved shirts – was utilized always or most of the time by 35.9% of respondents in 2005, 38.4% in 2010, and 37.2% in 2015.
In 2005, 19% of Americans reported having a lifetime history of a physician-performed full body skin examination. The prevalence of this secondary skin cancer prevention measure rose to 22.4% in 2010 and remained the same in 2015.
In the 2005 national survey, 14.1% of respondents reported engaging in indoor tanning within the past year. This figure dropped to 6.2% in 2010 and fell further to 4.1% in 2015.
A history of two or more sunburns within the past year was reported by 18.2% of subjects in 2005, by 21.1% in 2010, and by 19.9% in 2015. It’s unclear whether this unwelcome phenomenon is due to inadequate use of sun protection or increased awareness of the link between sun exposure and skin cancer, with a resultant increase in reporting of sunburns. The influence of climate change is another possible explanation worthy of further study, according to Dr. Bolick.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
FROM AAD 20
Lenalidomide may be an answer for refractory cutaneous lupus
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
Cutaneous lupus erythematosus (CLE) is present in 25% of patients with systemic lupus at the time of diagnosis, but it can also occur in up to 85% of cases at some point in their disease course, Eveline Y. Wu, MD, said during the virtual annual meeting of the Society for Pediatric Dermatology.
“CLE can also occur without any systemic disease,” said Dr. Wu, associate professor of pediatrics at the University of North Carolina at Chapel Hill. “It’s been shown that the risk of developing systemic lupus differs according to the type of skin involvement, meaning that cutaneous lupus can be classified into acute, subacute, chronic, and intermittent forms.”
Malar rash is the prototypical acute cutaneous lesion and is associated with active systemic lupus erythematosus (SLE) and anti–double stranded DNA antibody positivity, while discoid lupus erythematosus is the most common chronic lesion. “A small percentage of patients with discoid lupus can develop systemic lupus, particularly when the lesions are more disseminated,” said Dr. Wu, who specializes in pediatric rheumatology as well as allergy and immunology.
In the American College of Rheumatology’s 1997 classification system, mucocutaneous manifestations constitute 4 out of the 11 criteria that clinicians use to make a diagnosis of SLE: malar rash, discoid-lupus rash, photosensitivity, and oral or nasal mucocutaneous ulcerations. Dr. Wu recommends performing an oral exam on suspect cases, “because the oral ulcers that we see in systemic lupus tend to be painless, so oftentimes patients don’t realize they have them.”
Five other organ-specific manifestations of SLE include nonerosive arthritis, nephritis, encephalopathy, pleuritis or pericarditis, and cytopenia. The two other criteria are positive immunoserology and a positive antinuclear antibody test. “If you have any individuals present with one of these [mucocutaneous manifestations criteria], you want to think about getting a CBC to look for cytopenia or a urinalysis to look for evidence of nephritis, and potentially some additional blood studies, depending on your level of suspicion for systemic lupus,” Dr. Wu said.
Other rarer CLE manifestations include lupus pernio or chilblains, lupus panniculitis, livedo reticularis, bullous LE, urticarial vasculitis, neutrophilic dermatoses, and alopecia.
Common treatments for cutaneous manifestations associated pediatric SLE include hydroxychloroquine, low dose corticosteroids, topical steroids, methotrexate, and leflunomide. Other options for increasing severity of systemic disease include lenalidomide/thalidomide, azathioprine, calcineurin inhibitors, belimumab (Benlysta), high-dose corticosteroids, mycophenolate mofetil (CellCept), rituximab (Rituxan), and cyclophosphamide. Cutaneous manifestations of pediatric SLE can often be refractory to treatments.
In 2017, Dr. Wu and associates published a retrospective chart review of 10 adolescents who received lenalidomide for refractory CLE. One of the subjects was a 21-year-old male with a significant malar rash despite being on hydroxychloroquine, azathioprine, and prednisone 40 mg daily. “One month after being on lenalidomide he had a pretty impressive response,” Dr. Wu said. “It’s not quite clear how lenalidomide works in cutaneous lupus. Currently it’s only approved for use in myelodysplastic syndromes, multiple myeloma, as well as certain lymphomas. It’s thought to modulate different parts of the immune system, which collectively result in the cytotoxicity against tumor cells.”
Lenalidomide is supplied in capsule sizes ranging from 2.5 mg to 25 mg and is given once daily. “For a smaller child, I would think about starting 5 mg once a day,” Dr. Wu said. “For an adult-sized adolescent, you could start at 10 mg once a day and then titrate up based on response. Side effects that you need to worry about are cytopenia and GI symptoms. The venous and arterial thromboembolism risk has been seen in patients with multiple myeloma, and it is unclear if this risk is applicable to all indications.” Use of the medication requires enrollment into a safety monitoring program.
She reported having no financial disclosures.
FROM SPD 2020
Is the presence of enanthem a clue for COVID-19?
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
Larger studies should explore and confirm this association, the study’s authors and other experts suggested.
Dermatologists are already aware of the connection between enanthem and viral etiology. “As seen with other viral infections, we wondered if COVID-19 could produce enanthem in addition to skin rash exanthem,” one of the study author’s, Juan Jiménez-Cauhe, MD, a dermatologist with Hospital Universitario Ramon y Cajal, Madrid, said in an interview. He and his colleagues summarized their findings in a research letter in JAMA Dermatology.
They examined the oral cavity of 21 COVID-19 patients at a tertiary care hospital who also had a skin rash from March 30 to April 8. They classified enanthems into four categories: petechial, macular, macular with petechiae, or erythematovesicular. Six of the patients presented with oral lesions, all of them located in the palate; in one patient, the enanthem was macular, it was petechial in two patients and was macular with petechiae in three patients. The six patients ranged between the ages of 40 and 69 years; four were women.
Petechial or vesicular patterns are often associated with viral infections. In this particular study, the investigators did not observe vesicular lesions.
On average, mucocutaneous lesions appeared about 12 days after the onset of COVID-19 symptoms. “Interestingly, this latency was shorter in patients with petechial enanthem, compared with those with a macular lesion with petechiae appearance,” the authors wrote.
This shorter time might suggest an association for SARS-CoV-2, said Dr. Jiménez-Cauhe. Strong cough may have also caused petechial lesions on the palate, but it’s unlikely, as they appeared close in time to COVID-19 symptoms. It’s also unlikely that any drugs caused the lesions, as drug rashes can take 2-3 weeks to appear.
This fits in line with other evidence of broader skin manifestations appearing at the same time or after COVID-19, Esther Freeman, MD, said in an interview. Dr. Freeman, director of global health dermatology at Massachusetts General Hospital, Boston, is the principal investigator of the COVID-19 Dermatology Registry, a collaboration of the American Academy of Dermatology and International League of Dermatological Societies.
The study’s small cohort made it difficult to establish a solid association between the oral lesions and SARS-CoV-2. “However, the presence of enanthem in a patient with a skin rash is a useful finding that suggests a viral etiology rather than a drug reaction. This is particularly useful in COVID-19 patients, who were receiving many drugs as part of the treatment,” Dr. Jimenez-Cauhe said. Future studies should assess whether the presence of enanthem and exanthem lead physicians to consider SARS-CoV-2 as possible agents, ruling out infection with a blood or nasopharyngeal test.
This study adds to the growing body of knowledge on cutaneous and mucocutaneous findings associated with SARS-CoV-2 infection, Jules Lipoff, MD, of the department of dermatology, University of Pennsylvania, Philadelphia, said in an interview. “One challenge in evaluating these findings is that these findings are nonspecific, and medication reactions can often cause similar rashes, such as morbilliform eruptions that can be associated with both viruses and medications.”
Enanthems, as the study authors noted, are more specific to viral infections and are less commonly associated with medication reactions. “So, even though this is a small case series with significant limitations, it does add more evidence that COVID-19 is directly responsible for findings in the skin and mucous membranes,” said Dr. Lipoff.
Dr. Freeman noted that the study may also encourage clinicians to look in a patient’s mouth when assessing for SARS-CoV-2. Additional research should examine these data in a larger population.
Several studies by Dr. Freeman, Dr. Lipoff, and others strongly suggest that SARS-CoV-2 has a spectrum of associated dermatologic manifestations. One evaluated perniolike skin lesions (J Am Acad Dermatol. 2020 Aug; 83[2]:486-92). The other was a case series from the COVID-19 registry that examined 716 cases of new-onset dermatologic symptoms in patients from 31 countries with confirmed/suspected SARS-CoV-2 (J Am Acad Dermatol. 2020 Jul 2;S0190-9622[20]32126-5.).
The authors of the report had no disclosures.
SOURCE: Jimenez-Cauhe J et al. JAMA Dermatol. 2020 Jul 15. doi: 10.1001/jamadermatol.2020.2550.
FROM JAMA DERMATOLOGY
New developments in pustular psoriasis
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
FROM THE GRAPPA 2020 VIRTUAL ANNUAL MEETING
Patch testing in children: An evolving science
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
“Time needs to be allocated for a patch test consultation, placement, removal, and reading,” she said at the virtual annual meeting of the Society for Pediatric Dermatology. “You will need more time in the day that you’re reading the patch test for patient education. However, your staff will need more time on the front end of the patch test process for application. Also, if they are customizing patch tests, they’ll need time to make the patch tests along with access to a refrigerator and plenty of counter space.”
Other factors to consider are the site of service, your payer mix, and if you need to complete prior authorizations for patch testing.
Dr. Martin, associate professor of dermatology and child health at the University of Missouri–Columbia, said that the diagnosis of allergic contact dermatitis (ACD) crosses her mind when she sees a patient with new dermatitis, especially in an older child; if the dermatitis is patterned or regional; if there’s exacerbation of an underlying, previously stable skin disease; or if it’s a pattern known to be associated with systemic contact dermatitis. “In fact, 13%-25% of healthy, asymptomatic kids have allergen sensitization,” she said. “If you take that a step further and look at kids who are suspected of having allergic contact dermatitis, 25%-96% have allergen sensitization. Still, that doesn’t mean that those tests are relevant to the dermatitis that’s going on. If you take kids who are referred to tertiary centers for patch testing, about half will have relevant patch test results.”
Pediatric ACD differs from adult ACD in three ways, Dr. Martin said. First, children have a different clinical morphology and distribution on presentation, compared with adults. “In adults, the most common clinical presentation is hand dermatitis, while kids more often present with a scattered generalized morphology of dermatitis,” she said. “This occurs in about one-third of children with ACD. Their patterns of allergen exposure are also different. For the most part, adults are in control of their own environments and what is placed on their skin, whereas kids are not. When thinking about what you might need to patch test a child to if you’re considering ACD, it’s important to think about not only what the parent or caregiver puts directly on the child’s skin but also any connubial or consort allergen exposure – the most common ones coming from the caregivers themselves, such as fragrance or hair dyes that are transferred to a young child.”
The third factor that differs between pediatric and adult ACD is the allergen source. Dr. Martin noted that children and adults use different personal care products, wear different types of clothing, and spend different amounts of time in play versus work. “Children have many more hobbies in general that are unfortunately lost as many of us age,” she said. That means “thinking through the child’s entire day and how the seasons differ for them, such as what sports they’re in and what protective equipment may be involved with where their dermatitis is, or what musical instruments they play.”
Applying the T.R.U.E. patch test panel or a customized patch test panel to young children poses certain challenges, considering their limited body surface area and propensity to squirm. Dr. Martin often employs distraction techniques when placing patches on young patients, including the use of bubbles, music, movies, and games. “The goal is always to get as much of the patches on the back or the flanks as possible,” she said. “If you need additional space you can use the upper outer arms, the abdomen, or the anterior lateral thighs. Another thing to consider is how to set up your week for pediatric patch testing. There’s a standardized process for adults where we place the patches on day 0, read them on day 2, with removal of the patches at that time, and then perform a delayed read between day 4-7.”
The process is similar for postpubescent children, despite the lack of clear guidelines in the medical literature. “There is much controversy and different practices between different pediatric patch test centers,” Dr. Martin said. “There is more consensus between the older kids and the prepubescent group ages 6-12. Most clinicians will still do a similar placement on day 0 with removal and initial read on day 2, with a delayed read on day 4-7. However, some groups will remove patches at 24 hours, especially in those with atopic dermatitis (AD) or a generalized dermatitis, to reduce irritant reactions. Others will also use half-strength concentrations of allergens.”
The most controversy lies with children younger than 6 years, she said. For those aged 3-6 years, who do not have AD, most practices use a standardized pediatric tray with a 24- to 48-hour contact time. However, patch testing can be “very challenging” for children who are under 3 years of age, and children with AD who are under 6 years, “so there needs to be a very high degree of suspicion for ACD and very careful selection of the allergens and contact time that is used in those particular cases,” she noted.
The most common allergens in children are nickel, fragrance mix I, cobalt, balsam of Peru, neomycin, and bacitracin, which largely match the common allergens seen in adults. However, allergens more common in children, compared with adults, include gold, propylene glycol, 2-Bromo-2-nitropropane-1,3-diol, and cocamidopropyl betaine. “If the child presents with a regional dermatitis or a patterned dermatitis, sometimes you can hone in on your suspected allergens and only test for a few,” Dr. Martin said. “In a child with eyelid dermatitis, you’re going to worry more about cocamidopropyl betaine in their shampoos and cleansers. Also, a metal allergen could be transferred from their hands from toys or coins, specifically nickel and cobalt. They also may have different sports gear such as goggles that may be affecting their eyelid dermatitis, which you would not necessarily see in an adult.”
Periorificial contact dermatitis can also differ in presentation between children and adults. “In kids, think about musical instruments, flavored lip balms, gum, and pacifiers,” she said. “For ACD on the buttocks and posterior thighs, think about toilet seat allergens, especially those in the potty training ages, and the nickel bolts on school chairs.”
In 2018, Dr. Martin and her colleagues on the Pediatric Contact Dermatitis Workgroup published a pediatric baseline patch test series as a way to expand on the T.R.U.E. test (Dermatitis. 2018;29[4]:206-12). “It’s nice to have this panel available as a baseline screening tool when you’re unsure of possible triggers of the dermatitis but you still have high suspicion of allergic dermatitis,” Dr. Martin said. “This also is helpful for patients who present with generalized dermatitis. It’s still not perfect. We are collecting prospective data to fine-tune this baseline series.”
She reported having no financial disclosures.
FROM SPD 2020
Fixed scaly lesion
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
Hyperpigmentation of the legs
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
No link between topical steroids and fracture risk found in children with atopic dermatitis
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
FROM SPD 2020
Doctors hesitated to embrace biosimilar infliximab in first 2 years
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A total of 17% of patients new to infliximab received a biosimilar in 2018, compared with 11% of returning patients.
Major finding: Biosimilar infliximab accounted for 10% of the market share 2 years after the product was introduced.
Study details: The data come from a review of infliximab claims across 49,771 patients and 4,289 physicians who prescribed infliximab in 2018.
Disclosures: The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
Source: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.