User login
FDA approves omadacycline for pneumonia and skin infections
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
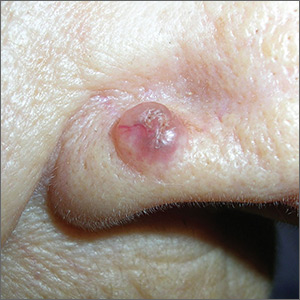
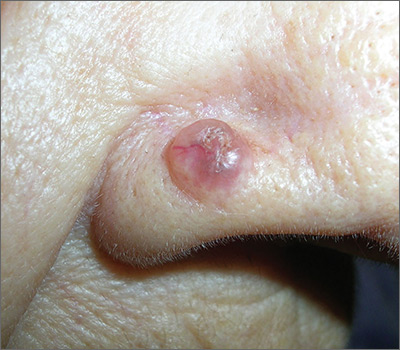
Growth on nose
The FP made the presumptive diagnosis of a nodular basal cell carcinoma.
He explained the importance of performing a biopsy and obtained informed consent. On the same day of the patient’s visit, he injected 1% lidocaine with epinephrine under the lesion with a single stick of a 30 gauge needle. He knew that it was safe to use epinephrine on the nose, and that it would prevent excessive bleeding during the biopsy. Contrary to the myth frequently taught in medical school, the nose is a very vascular area. The physician performed a shave biopsy that removed the top of the lesion flush with the skin around it. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular basal cell carcinoma. On the following visit, the physician recommended Mohs surgery as a way to preserve the vital anatomy of the nasal ala and achieve the highest cure rate.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP made the presumptive diagnosis of a nodular basal cell carcinoma.
He explained the importance of performing a biopsy and obtained informed consent. On the same day of the patient’s visit, he injected 1% lidocaine with epinephrine under the lesion with a single stick of a 30 gauge needle. He knew that it was safe to use epinephrine on the nose, and that it would prevent excessive bleeding during the biopsy. Contrary to the myth frequently taught in medical school, the nose is a very vascular area. The physician performed a shave biopsy that removed the top of the lesion flush with the skin around it. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular basal cell carcinoma. On the following visit, the physician recommended Mohs surgery as a way to preserve the vital anatomy of the nasal ala and achieve the highest cure rate.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
The FP made the presumptive diagnosis of a nodular basal cell carcinoma.
He explained the importance of performing a biopsy and obtained informed consent. On the same day of the patient’s visit, he injected 1% lidocaine with epinephrine under the lesion with a single stick of a 30 gauge needle. He knew that it was safe to use epinephrine on the nose, and that it would prevent excessive bleeding during the biopsy. Contrary to the myth frequently taught in medical school, the nose is a very vascular area. The physician performed a shave biopsy that removed the top of the lesion flush with the skin around it. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular basal cell carcinoma. On the following visit, the physician recommended Mohs surgery as a way to preserve the vital anatomy of the nasal ala and achieve the highest cure rate.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Basal cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:989-998.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Adalimumab safety update finds no new signals
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
not included in the previous 2009 analysis; their evaluation of data from these 18 trials found no new safety signals, they reported in the British Journal of Dermatology.
Adverse event incidence rates were expressed as events per 100 patient-years of exposure to adalimumab and, among the 3,727 patients who were aged 18 years or older and had moderate to severe plaque psoriasis for at least 6 months, there were 5,430 patient-years of cumulative exposure at the December 2015 cutoff date.
There were 3,798 treatment-related events altogether (70 events/100 patient-years); 269 events (5 events/100 patient-years ) led to discontinuation of treatment. The rates for serious adverse events and serious infections were 8.4 and 1.8 events per 100 patient-years, respectively; the most common types of serious infections were pneumonia and cellulitis.
The rates of the most frequently reported adverse events were comparable with those in the 2009 data set, with the most common being nasopharyngitis, upper respiratory tract infection, and headache. Furthermore, the rates of serious adverse events, serious infections, and malignancies were also stable, even with the increasing adalimumab exposure, and these were mostly consistent with what has been seen in large real-world registries.
The researchers did note that the rates of melanoma and nonmelanoma skin cancer were higher than would be expected in the general population, but they suspected this was at least partly because these psoriasis patients were receiving more frequent skin examinations and more skin cancers were being detected. (Incidence rates for these two cancers were stable during 2009-2015).
The analysis had certain limitations, such as a lack of a long-term comparator group. Also, while some patients continue to receive adalimumab for more than 10 years, the maximum duration of treatment in this analysis was only 5.5 years. Finally, the population in these clinical trials may differ from that seen in general practice settings because of the inclusion/exclusion criteria.
Six authors of the study reported multiple disclosures with pharmaceutical companies, including serving as a consultant, speaker, and/or adviser for, receiving honoraria from, and/or receiving grant/research support from AbbVie, which developed adalimumab and funded/advised this study; two authors are AbbVie employees, one is a former employee.
SOURCE: Leonardi C et al. Br J Dermatol. 2018 Aug 31. doi: 10.1111/bjd.17084.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Anti-TNF agents preferred for severe psoriasis in pregnancy
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
EXPERT ANALYSIS FROM SUMMER AAD 2018
Adalimumab safety profile similar in children and adults
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: The most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY).
Study details: An analysis of data for 577 pediatric patients from seven clinical trials between September 2002 and December 2015.
Disclosures: AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
Source: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
Dual-frequency ultrasound promising for refractory rosacea
, according to a new study in Dermatologic Surgery.
In the study, a retrospective medical record analysis of 42 rosacea patients, DFU improved symptoms, including erythema index (EI) and transepithelial water loss (TEWL), and also improved outcomes on the patient self-assessment (PSA), reported Jun Yeong Park, MD, and coauthors, from the department of dermatology, Hallym University Sacred Heart Hospital in Anyang, South Korea.
Of the 42 patients, 26 had erythematotelangiectatic rosacea, 14 had papulopustular rosacea, and 2 had mixed disease; their mean age was 48 years, and they had had rosacea for a mean of 2 years. Patients had started DFU treatment between September 2016 and December 2016, and were not taking oral medication (besides antihistamines), topical ointments, or other laser treatments at the time. Most had been treated with various systemic therapies, topical therapies, or lasers, but had not had adequate improvement of flushing and erythema.
Patients received DFU treatment of the entire face twice per week for the first week, followed by one-week intervals, for a total of 12 weeks. Each treatment session lasted 10 minutes, and included DFU frequencies of 3/4.5 MHz at an ultrasound intensity of 2.0W/cm2 for 5 minutes, followed by 4.5 MHz at an intensity of 2.0W/cm2. Responses to treatment were based on EI, TEWL values (measured on three different sites on each cheek according to guidelines established by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products), and PSA. PSA was completed on a scale of 0 (absent) to 4 (severe) for erythema, itching sensation, and burning sensation.
At 12 weeks follow-up, the mean EI dropped from 16.3 at baseline to 12.7 at 12 weeks (P less than .01). Mean TEWL values dropped from a baseline of 35.8 g m–1 h–1 to 22.8 g m–1 h–1 at 12 weeks (P less than .01).
When evaluated by rosacea subtype, a slightly higher reduction in the group with papulopustular rosacea was seen for EI and TEWL, compared with those with the erythematotelangiectatic subtype, but the differences were not statistically significant for either, the authors reported.
Between baseline and 12 weeks, the PSA values for erythema decreased from 2.6 to 1.1. Itching and burning grades also decreased, from 2.4 to 0.4 and from 2.4 to 0.3, respectively.
The findings verify that there were “improvements in the barrier function of patients with refractory rosacea, based on the TEWL level before and after treatment,” the authors noted. “Therefore, DFU may be an additional treatment option for rosacea that is resistant to other treatments.”
This study is the first to evaluate DFU in patients with refractory rosacea “who did not show signs of recovery after undergoing previous therapies,” they noted.
No disclosures were reported.
SOURCE: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
, according to a new study in Dermatologic Surgery.
In the study, a retrospective medical record analysis of 42 rosacea patients, DFU improved symptoms, including erythema index (EI) and transepithelial water loss (TEWL), and also improved outcomes on the patient self-assessment (PSA), reported Jun Yeong Park, MD, and coauthors, from the department of dermatology, Hallym University Sacred Heart Hospital in Anyang, South Korea.
Of the 42 patients, 26 had erythematotelangiectatic rosacea, 14 had papulopustular rosacea, and 2 had mixed disease; their mean age was 48 years, and they had had rosacea for a mean of 2 years. Patients had started DFU treatment between September 2016 and December 2016, and were not taking oral medication (besides antihistamines), topical ointments, or other laser treatments at the time. Most had been treated with various systemic therapies, topical therapies, or lasers, but had not had adequate improvement of flushing and erythema.
Patients received DFU treatment of the entire face twice per week for the first week, followed by one-week intervals, for a total of 12 weeks. Each treatment session lasted 10 minutes, and included DFU frequencies of 3/4.5 MHz at an ultrasound intensity of 2.0W/cm2 for 5 minutes, followed by 4.5 MHz at an intensity of 2.0W/cm2. Responses to treatment were based on EI, TEWL values (measured on three different sites on each cheek according to guidelines established by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products), and PSA. PSA was completed on a scale of 0 (absent) to 4 (severe) for erythema, itching sensation, and burning sensation.
At 12 weeks follow-up, the mean EI dropped from 16.3 at baseline to 12.7 at 12 weeks (P less than .01). Mean TEWL values dropped from a baseline of 35.8 g m–1 h–1 to 22.8 g m–1 h–1 at 12 weeks (P less than .01).
When evaluated by rosacea subtype, a slightly higher reduction in the group with papulopustular rosacea was seen for EI and TEWL, compared with those with the erythematotelangiectatic subtype, but the differences were not statistically significant for either, the authors reported.
Between baseline and 12 weeks, the PSA values for erythema decreased from 2.6 to 1.1. Itching and burning grades also decreased, from 2.4 to 0.4 and from 2.4 to 0.3, respectively.
The findings verify that there were “improvements in the barrier function of patients with refractory rosacea, based on the TEWL level before and after treatment,” the authors noted. “Therefore, DFU may be an additional treatment option for rosacea that is resistant to other treatments.”
This study is the first to evaluate DFU in patients with refractory rosacea “who did not show signs of recovery after undergoing previous therapies,” they noted.
No disclosures were reported.
SOURCE: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
, according to a new study in Dermatologic Surgery.
In the study, a retrospective medical record analysis of 42 rosacea patients, DFU improved symptoms, including erythema index (EI) and transepithelial water loss (TEWL), and also improved outcomes on the patient self-assessment (PSA), reported Jun Yeong Park, MD, and coauthors, from the department of dermatology, Hallym University Sacred Heart Hospital in Anyang, South Korea.
Of the 42 patients, 26 had erythematotelangiectatic rosacea, 14 had papulopustular rosacea, and 2 had mixed disease; their mean age was 48 years, and they had had rosacea for a mean of 2 years. Patients had started DFU treatment between September 2016 and December 2016, and were not taking oral medication (besides antihistamines), topical ointments, or other laser treatments at the time. Most had been treated with various systemic therapies, topical therapies, or lasers, but had not had adequate improvement of flushing and erythema.
Patients received DFU treatment of the entire face twice per week for the first week, followed by one-week intervals, for a total of 12 weeks. Each treatment session lasted 10 minutes, and included DFU frequencies of 3/4.5 MHz at an ultrasound intensity of 2.0W/cm2 for 5 minutes, followed by 4.5 MHz at an intensity of 2.0W/cm2. Responses to treatment were based on EI, TEWL values (measured on three different sites on each cheek according to guidelines established by the European Group for Efficacy Measurements on Cosmetics and Other Topical Products), and PSA. PSA was completed on a scale of 0 (absent) to 4 (severe) for erythema, itching sensation, and burning sensation.
At 12 weeks follow-up, the mean EI dropped from 16.3 at baseline to 12.7 at 12 weeks (P less than .01). Mean TEWL values dropped from a baseline of 35.8 g m–1 h–1 to 22.8 g m–1 h–1 at 12 weeks (P less than .01).
When evaluated by rosacea subtype, a slightly higher reduction in the group with papulopustular rosacea was seen for EI and TEWL, compared with those with the erythematotelangiectatic subtype, but the differences were not statistically significant for either, the authors reported.
Between baseline and 12 weeks, the PSA values for erythema decreased from 2.6 to 1.1. Itching and burning grades also decreased, from 2.4 to 0.4 and from 2.4 to 0.3, respectively.
The findings verify that there were “improvements in the barrier function of patients with refractory rosacea, based on the TEWL level before and after treatment,” the authors noted. “Therefore, DFU may be an additional treatment option for rosacea that is resistant to other treatments.”
This study is the first to evaluate DFU in patients with refractory rosacea “who did not show signs of recovery after undergoing previous therapies,” they noted.
No disclosures were reported.
SOURCE: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
Key clinical point: Dual-frequency ultrasound may be an effective option for treatment-resistant rosacea.
Major finding: In 12 weeks, the erythema index dropped from 16.3 to 12.7 (P less than .01), along with drops in patient self assessment measures for erythema, itching, and burning.
Study details: A retrospective electronic medical records analysis of 42 rosacea patients.
Disclosures: No disclosures were reported.
Source: Park J et al. Dermatol Surg 2018 Sep;44(9):1209-15. doi: 10.1097/DSS.0000000000001552.
FDA approves Seysara for treatment of moderate to severe acne
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
Small fibers, large impact
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
Pancreatitis: The great masquerader?
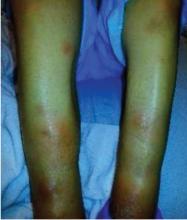
A 55-year-old man presented to the emergency department with 1 week of bilateral lower-extremity joint pain associated with painful skin nodules. He had a history of chronic recurrent alcoholic pancreatitis. He denied abdominal pain, nausea, or vomiting.
Results of initial laboratory testing:
- Alkaline phosphatase 300 IU/L (reference range 36–108)
- Erythrocyte sedimentation rate 81 mm/h (0–15)
- Lipase 20,000 U/L (16–61).
AN ATYPICAL PRESENTATION OF A COMMON DISEASE
Epidemiology and pathophysiology
Pancreatitis, panniculitis, and polyarthritis syndrome is a rare systemic complication of pancreatic disease occurring most often in middle-aged men with an acute exacerbation of chronic pancreatitis and a history of alcohol use disorder.1,2 It is also associated with pancreatic pseudocyst, pancreas divisum, and pancreatic adenocarcinoma.3–6 It is characterized by systemic fat necrosis secondary to severe and persistent elevation of pancreatic enzymes. The mortality rate is high; in a case series of 25 patients, 24% died within days to weeks after admission.1
Clinical presentation and treatment
The diagnosis of pancreatitis, panniculitis, and polyarthritis syndrome is often missed. Abdominal pain is mild or absent in over 60% of patients.1 Therefore, a high index of suspicion is required for early diagnosis.
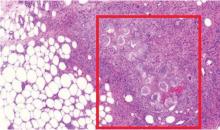
The differential diagnosis includes sarcoidosis (including Löfgren syndrome), subcutaneous infection, and vasculitis. “Ghost adipocytes” on skin biopsy are pathognomonic for pancreatic panniculitis and are the result of saponification; they appear to be anuclear, with basophilic material throughout the cytoplasm.7 Arthrocentesis of affected joints may reveal thick, creamy material, rich in triglycerides, which is diagnostic of pancreatic arthritis.1,8
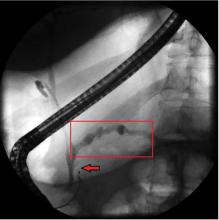
Treatment relies on correction of the underlying pancreatic pathology. Pancreatitis, panniculitis, and polyarthritis syndrome has been successfully treated by cyst gastrostomy, pancreatic duct stenting, and pancreaticoduodenectomy.7,9–11
TAKE-HOME POINTS
- Pancreatitis, panniculitis, and polyarthritis syndrome mimics rheumatologic disease and often presents without abdominal pain.
- The diagnosis is confirmed by the presence of elevated serum lipase or amylase, pancreatic imaging showing pancreatitis, and ghost adipocytes on skin biopsy.
- Treatment is aimed at correcting the underlying pancreatic abnormality.
- Narváez J, Bianchi MM, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum 2010; 39(5):417–423. doi:10.1016/j.semarthrit.2008.10.001
- Mourad FH, Hannoush HM, Bahlawan M, Uthman I, Uthman S. Panniculitis and arthritis as the presenting manifestation of chronic pancreatitis. J Clin Gastroenterol 2001; 32(3):259–261. pmid:11246359
- Borowicz J, Morrison M, Hogan D, Miller R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: a rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J Drugs Dermatol 2010; 9(9):1145–1150. pmid:20865849
- Hudson-Peacock MJ, Regnard CF, Farr PM. Liquefying panniculitis associated with acinous carcinoma of the pancreas responding to octreotide. J R Soc Med 1994; 87(6):361–362. pmid:8046712
- Vasdev V, Bhakuni D, Narayanan K, Jain R. Intramedullary fat necrosis, polyarthritis and panniculitis with pancreatic tumor: a case report. Int J Rheum Dis 2010; 13(4):e74–e78. doi:10.1111/j.1756-185X.2010.01548.x
- Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol 1986; 14(2 pt 2):331–334. pmid:3950133
- Francombe J, Kingsnorth AN, Tunn E. Panniculitis, arthritis and pancreatitis. Br J Rheumatol 1995; 34(7):680–683. pmid:7670790
- Price-Forbes AN, Filer A, Udeshi UL, Rai A. Progression of imaging in pancreatitis panniculitis polyarthritis (PPP) syndrome. Scand J Rheumatol 2006; 35(1):72–74. doi:10.1080/03009740500228073
- Harris MD, Bucobo JC, Buscaglia JM. Pancreatitis, panniculitis, polyarthritis syndrome successfully treated with EUS-guided cyst-gastrostomy. Gastrointest Endosc 2010; 72(2):456–458. doi:10.1016/j.gie.2009.11.040
- Lambiase P, Seery JP, Taylor-Robinson SD, Thompson JN, Hughes JM, Walters JR. Resolution of panniculitis after placement of pancreatic duct stent in chronic pancreatitis. Am J Gastroenterol 1996; 91(9):1835–1837. pmid:8792709
- Potts JR. Pancreatic-portal vein fistula with disseminated fat necrosis treated by pancreaticoduodenectomy. South Med J 1991; 84(5):632–635. pmid:2035087
A 55-year-old man presented to the emergency department with 1 week of bilateral lower-extremity joint pain associated with painful skin nodules. He had a history of chronic recurrent alcoholic pancreatitis. He denied abdominal pain, nausea, or vomiting.
Results of initial laboratory testing:
- Alkaline phosphatase 300 IU/L (reference range 36–108)
- Erythrocyte sedimentation rate 81 mm/h (0–15)
- Lipase 20,000 U/L (16–61).
AN ATYPICAL PRESENTATION OF A COMMON DISEASE
Epidemiology and pathophysiology
Pancreatitis, panniculitis, and polyarthritis syndrome is a rare systemic complication of pancreatic disease occurring most often in middle-aged men with an acute exacerbation of chronic pancreatitis and a history of alcohol use disorder.1,2 It is also associated with pancreatic pseudocyst, pancreas divisum, and pancreatic adenocarcinoma.3–6 It is characterized by systemic fat necrosis secondary to severe and persistent elevation of pancreatic enzymes. The mortality rate is high; in a case series of 25 patients, 24% died within days to weeks after admission.1
Clinical presentation and treatment
The diagnosis of pancreatitis, panniculitis, and polyarthritis syndrome is often missed. Abdominal pain is mild or absent in over 60% of patients.1 Therefore, a high index of suspicion is required for early diagnosis.
The differential diagnosis includes sarcoidosis (including Löfgren syndrome), subcutaneous infection, and vasculitis. “Ghost adipocytes” on skin biopsy are pathognomonic for pancreatic panniculitis and are the result of saponification; they appear to be anuclear, with basophilic material throughout the cytoplasm.7 Arthrocentesis of affected joints may reveal thick, creamy material, rich in triglycerides, which is diagnostic of pancreatic arthritis.1,8
Treatment relies on correction of the underlying pancreatic pathology. Pancreatitis, panniculitis, and polyarthritis syndrome has been successfully treated by cyst gastrostomy, pancreatic duct stenting, and pancreaticoduodenectomy.7,9–11
TAKE-HOME POINTS
- Pancreatitis, panniculitis, and polyarthritis syndrome mimics rheumatologic disease and often presents without abdominal pain.
- The diagnosis is confirmed by the presence of elevated serum lipase or amylase, pancreatic imaging showing pancreatitis, and ghost adipocytes on skin biopsy.
- Treatment is aimed at correcting the underlying pancreatic abnormality.
A 55-year-old man presented to the emergency department with 1 week of bilateral lower-extremity joint pain associated with painful skin nodules. He had a history of chronic recurrent alcoholic pancreatitis. He denied abdominal pain, nausea, or vomiting.
Results of initial laboratory testing:
- Alkaline phosphatase 300 IU/L (reference range 36–108)
- Erythrocyte sedimentation rate 81 mm/h (0–15)
- Lipase 20,000 U/L (16–61).
AN ATYPICAL PRESENTATION OF A COMMON DISEASE
Epidemiology and pathophysiology
Pancreatitis, panniculitis, and polyarthritis syndrome is a rare systemic complication of pancreatic disease occurring most often in middle-aged men with an acute exacerbation of chronic pancreatitis and a history of alcohol use disorder.1,2 It is also associated with pancreatic pseudocyst, pancreas divisum, and pancreatic adenocarcinoma.3–6 It is characterized by systemic fat necrosis secondary to severe and persistent elevation of pancreatic enzymes. The mortality rate is high; in a case series of 25 patients, 24% died within days to weeks after admission.1
Clinical presentation and treatment
The diagnosis of pancreatitis, panniculitis, and polyarthritis syndrome is often missed. Abdominal pain is mild or absent in over 60% of patients.1 Therefore, a high index of suspicion is required for early diagnosis.
The differential diagnosis includes sarcoidosis (including Löfgren syndrome), subcutaneous infection, and vasculitis. “Ghost adipocytes” on skin biopsy are pathognomonic for pancreatic panniculitis and are the result of saponification; they appear to be anuclear, with basophilic material throughout the cytoplasm.7 Arthrocentesis of affected joints may reveal thick, creamy material, rich in triglycerides, which is diagnostic of pancreatic arthritis.1,8
Treatment relies on correction of the underlying pancreatic pathology. Pancreatitis, panniculitis, and polyarthritis syndrome has been successfully treated by cyst gastrostomy, pancreatic duct stenting, and pancreaticoduodenectomy.7,9–11
TAKE-HOME POINTS
- Pancreatitis, panniculitis, and polyarthritis syndrome mimics rheumatologic disease and often presents without abdominal pain.
- The diagnosis is confirmed by the presence of elevated serum lipase or amylase, pancreatic imaging showing pancreatitis, and ghost adipocytes on skin biopsy.
- Treatment is aimed at correcting the underlying pancreatic abnormality.
- Narváez J, Bianchi MM, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum 2010; 39(5):417–423. doi:10.1016/j.semarthrit.2008.10.001
- Mourad FH, Hannoush HM, Bahlawan M, Uthman I, Uthman S. Panniculitis and arthritis as the presenting manifestation of chronic pancreatitis. J Clin Gastroenterol 2001; 32(3):259–261. pmid:11246359
- Borowicz J, Morrison M, Hogan D, Miller R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: a rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J Drugs Dermatol 2010; 9(9):1145–1150. pmid:20865849
- Hudson-Peacock MJ, Regnard CF, Farr PM. Liquefying panniculitis associated with acinous carcinoma of the pancreas responding to octreotide. J R Soc Med 1994; 87(6):361–362. pmid:8046712
- Vasdev V, Bhakuni D, Narayanan K, Jain R. Intramedullary fat necrosis, polyarthritis and panniculitis with pancreatic tumor: a case report. Int J Rheum Dis 2010; 13(4):e74–e78. doi:10.1111/j.1756-185X.2010.01548.x
- Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol 1986; 14(2 pt 2):331–334. pmid:3950133
- Francombe J, Kingsnorth AN, Tunn E. Panniculitis, arthritis and pancreatitis. Br J Rheumatol 1995; 34(7):680–683. pmid:7670790
- Price-Forbes AN, Filer A, Udeshi UL, Rai A. Progression of imaging in pancreatitis panniculitis polyarthritis (PPP) syndrome. Scand J Rheumatol 2006; 35(1):72–74. doi:10.1080/03009740500228073
- Harris MD, Bucobo JC, Buscaglia JM. Pancreatitis, panniculitis, polyarthritis syndrome successfully treated with EUS-guided cyst-gastrostomy. Gastrointest Endosc 2010; 72(2):456–458. doi:10.1016/j.gie.2009.11.040
- Lambiase P, Seery JP, Taylor-Robinson SD, Thompson JN, Hughes JM, Walters JR. Resolution of panniculitis after placement of pancreatic duct stent in chronic pancreatitis. Am J Gastroenterol 1996; 91(9):1835–1837. pmid:8792709
- Potts JR. Pancreatic-portal vein fistula with disseminated fat necrosis treated by pancreaticoduodenectomy. South Med J 1991; 84(5):632–635. pmid:2035087
- Narváez J, Bianchi MM, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum 2010; 39(5):417–423. doi:10.1016/j.semarthrit.2008.10.001
- Mourad FH, Hannoush HM, Bahlawan M, Uthman I, Uthman S. Panniculitis and arthritis as the presenting manifestation of chronic pancreatitis. J Clin Gastroenterol 2001; 32(3):259–261. pmid:11246359
- Borowicz J, Morrison M, Hogan D, Miller R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: a rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J Drugs Dermatol 2010; 9(9):1145–1150. pmid:20865849
- Hudson-Peacock MJ, Regnard CF, Farr PM. Liquefying panniculitis associated with acinous carcinoma of the pancreas responding to octreotide. J R Soc Med 1994; 87(6):361–362. pmid:8046712
- Vasdev V, Bhakuni D, Narayanan K, Jain R. Intramedullary fat necrosis, polyarthritis and panniculitis with pancreatic tumor: a case report. Int J Rheum Dis 2010; 13(4):e74–e78. doi:10.1111/j.1756-185X.2010.01548.x
- Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol 1986; 14(2 pt 2):331–334. pmid:3950133
- Francombe J, Kingsnorth AN, Tunn E. Panniculitis, arthritis and pancreatitis. Br J Rheumatol 1995; 34(7):680–683. pmid:7670790
- Price-Forbes AN, Filer A, Udeshi UL, Rai A. Progression of imaging in pancreatitis panniculitis polyarthritis (PPP) syndrome. Scand J Rheumatol 2006; 35(1):72–74. doi:10.1080/03009740500228073
- Harris MD, Bucobo JC, Buscaglia JM. Pancreatitis, panniculitis, polyarthritis syndrome successfully treated with EUS-guided cyst-gastrostomy. Gastrointest Endosc 2010; 72(2):456–458. doi:10.1016/j.gie.2009.11.040
- Lambiase P, Seery JP, Taylor-Robinson SD, Thompson JN, Hughes JM, Walters JR. Resolution of panniculitis after placement of pancreatic duct stent in chronic pancreatitis. Am J Gastroenterol 1996; 91(9):1835–1837. pmid:8792709
- Potts JR. Pancreatic-portal vein fistula with disseminated fat necrosis treated by pancreaticoduodenectomy. South Med J 1991; 84(5):632–635. pmid:2035087
FDA approves cemiplimab for advanced cutaneous squamous cell carcinoma
The U.S. Food and Drug Administration has approved cemiplimab-rwlc (Libtayo) for the treatment of metastatic or locally advanced cutaneous squamous cell carcinoma, the agency announced in a press release.
The approval was granted based on data from two open-label clinical trials involving a total of 108 patients: the phase 2 EMPOWER-CSCC-1 trial (NCT02760498) and two expansion cohorts from an open-label, nonrandomized phase 1 trial.
These trials, which included 75 patients with metastatic disease and 33 with locally advanced disease, found an overall response rate of 47.2%, and most of those patients still showed ongoing responses at the time of data analysis. Among patients with metastatic disease, 5% had a complete response, according to a press release from the manufacturer, Sanofi.
This is the sixth FDA approval for a checkpoint inhibitor targeting the PD-1/PD-L1 pathway. The drug was evaluated under the FDA’s Priority Review program for drugs that represent significant improvements in the safety or effectiveness of treatments for serious conditions. Manufacturer Sanofi was granted Breakthrough Therapy designation for cemiplimab in 2017 for advanced cutaneous squamous cell carcinoma, and the drug is also being reviewed by the European Medicines Agency.
Cemiplimab is administered as a 350-mg intravenous therapy every 3 weeks – costing $9,100 per treatment – until the disease progresses or patients experience unacceptable toxicity, according to the manufacturer. The most common side effects include fatigue, rash and diarrhea, but more serious adverse events can include immune-mediated reactions such as pneumonitis, colitis, hepatitis, endocrinopathies, and skin and kidney problems.
The U.S. Food and Drug Administration has approved cemiplimab-rwlc (Libtayo) for the treatment of metastatic or locally advanced cutaneous squamous cell carcinoma, the agency announced in a press release.
The approval was granted based on data from two open-label clinical trials involving a total of 108 patients: the phase 2 EMPOWER-CSCC-1 trial (NCT02760498) and two expansion cohorts from an open-label, nonrandomized phase 1 trial.
These trials, which included 75 patients with metastatic disease and 33 with locally advanced disease, found an overall response rate of 47.2%, and most of those patients still showed ongoing responses at the time of data analysis. Among patients with metastatic disease, 5% had a complete response, according to a press release from the manufacturer, Sanofi.
This is the sixth FDA approval for a checkpoint inhibitor targeting the PD-1/PD-L1 pathway. The drug was evaluated under the FDA’s Priority Review program for drugs that represent significant improvements in the safety or effectiveness of treatments for serious conditions. Manufacturer Sanofi was granted Breakthrough Therapy designation for cemiplimab in 2017 for advanced cutaneous squamous cell carcinoma, and the drug is also being reviewed by the European Medicines Agency.
Cemiplimab is administered as a 350-mg intravenous therapy every 3 weeks – costing $9,100 per treatment – until the disease progresses or patients experience unacceptable toxicity, according to the manufacturer. The most common side effects include fatigue, rash and diarrhea, but more serious adverse events can include immune-mediated reactions such as pneumonitis, colitis, hepatitis, endocrinopathies, and skin and kidney problems.
The U.S. Food and Drug Administration has approved cemiplimab-rwlc (Libtayo) for the treatment of metastatic or locally advanced cutaneous squamous cell carcinoma, the agency announced in a press release.
The approval was granted based on data from two open-label clinical trials involving a total of 108 patients: the phase 2 EMPOWER-CSCC-1 trial (NCT02760498) and two expansion cohorts from an open-label, nonrandomized phase 1 trial.
These trials, which included 75 patients with metastatic disease and 33 with locally advanced disease, found an overall response rate of 47.2%, and most of those patients still showed ongoing responses at the time of data analysis. Among patients with metastatic disease, 5% had a complete response, according to a press release from the manufacturer, Sanofi.
This is the sixth FDA approval for a checkpoint inhibitor targeting the PD-1/PD-L1 pathway. The drug was evaluated under the FDA’s Priority Review program for drugs that represent significant improvements in the safety or effectiveness of treatments for serious conditions. Manufacturer Sanofi was granted Breakthrough Therapy designation for cemiplimab in 2017 for advanced cutaneous squamous cell carcinoma, and the drug is also being reviewed by the European Medicines Agency.
Cemiplimab is administered as a 350-mg intravenous therapy every 3 weeks – costing $9,100 per treatment – until the disease progresses or patients experience unacceptable toxicity, according to the manufacturer. The most common side effects include fatigue, rash and diarrhea, but more serious adverse events can include immune-mediated reactions such as pneumonitis, colitis, hepatitis, endocrinopathies, and skin and kidney problems.