User login
Lifestyle coaching for obesity associated with improved cardiometabolic numbers in study
Patients who received intensive lifestyle training by coaches in the primary care setting experienced improvement in several indicators of cardiometabolic health in a 2-year trial.
The 803 trial participants comprised a racially diverse, low-income population with obesity. In this study, primary care clinics were randomly assigned to provide weight-loss coaching or usual care. Patients at the intensive training clinics lost significantly more weight than the other patients, as reported in a paper published in September in the New England Journal of Medicine on the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial. The patients who received weight loss coaching also had significantly more improvement in HDL cholesterol levels, total to HDL cholesterol ratios, and metabolic syndrome severity score, said researchers in the new paper on the PROPEL trial, which was published in Circulation on February 8 .
“We believe that one reason for success of the program was the use of a health coach [who] was embedded in the primary care office,” said lead author Peter Katzmarzyk, PhD, associate executive director for population and public health sciences at the Pennington Biomedical Research Center, Baton Rouge, La. “This way, the patients could get their counseling in a familiar environment and did not have to go to a different setting. The coaches developed close relationships with the patients over the 2 years, and this helped develop a sense of responsibility in the patients as the coaches were helping the patients to set goals and kept them accountable.”
In the PROPEL study, 67% of patients were Black and had low health literacy scores that corresponded with less than a ninth-grade education level. The intensive lifestyle intervention program included weekly sessions with the trained health coaches over the first 6 months — 16 face-to-face and 6 over the phone — and then at least monthly for the last 18 months. The coaches had higher education degrees in nutrition, physical activity, or behavioral medicine. Before the program started, the coaches also received training in the management of obesity and related health issues, health literacy, and patient communication and education. The goal of the program was 10% weight loss, using personalized action plans on eating, dieting, and physical activity.
Those in the usual-care clinics continued receiving normal care and received newsletters on health topics, such as the importance of sleep and tips for limiting time spent sitting. The primary care physicians at those clinics also were given a presentation with Centers for Medicare & Medicaid Services (CMS) information on intensive lifestyle interventions for obesity.
Cholesterol changes in intervention vs. control group
HDL cholesterol improved significantly among the coached patients, compared with the other patients, with a mean difference of 4.1 mg/dL at 1 year and 4.6 mg/dL at 2 years (P less than .01 for both). The total cholesterol to HDL cholesterol ratio showed a similarly significant difference in decline, with a between-group difference of –0.29 at 1 year and –0.31 at 2 years (P less than .01 for both). Also, the difference in the change in metabolic severity scores were –0.40 at 1 year and –0.21 at 2 years (P less than .01 for both).
Fasting blood glucose had declined after the 1st year by a significantly greater degree in the clinics with coaching, compared with the others, but not after the second year, researchers found.
There were no significant differences seen in total cholesterol, LDL cholesterol, non-HDL cholesterol, or blood pressure. Dr. Katzmarzyk said the likely reason for no change in blood pressure was that it was already relatively well-controlled at baseline for all the patients.
Funding barriers to obesity treatment
The CMS currently cover intensive training for obesity if delivered directly by a primary care physician, according to the authors of the new paper. Dr. Katzmarzyk said he hopes that will change.
“We are hoping that the evidence provided in this study may change the way that CMS funds obesity treatment in the future by allowing an expansion of the care team,” he said.
John Flack, MD, chair of internal medicine at Southern Illinois University, Springfield, said that the main achievement of the study was that it showed that intensive weight-loss training in the primary-care setting could be accomplished in a racially diverse population with low health literacy.
“You can’t just automatically assume just because you’ve seen it in some other populations that you can replicate this in every population, so they’ve done a really good job,” he said.
That programs are eligible for reimbursement only if they’re run by primary-care physicians is an ongoing problem, he said.
“You don’t necessarily need to be a physician to do this,” Dr. Flack said.
For best results, payment for coaching should not be tied to office visits, Dr. Flack noted.
“If they’re de-tethered from the office visits and you’re paid for quality ... you’re going to build out your infrastructure differently to care for people,” he said.
Andrew Freeman, MD, associate professor of medicine at the University of Colorado, Denver, and cochair of the American College of Cardiology’s nutrition and lifestyle work group, said the findings dovetail with his experience.
“I’m a huge believer that when people need to make lifestyle changes, having someone hold their hand and guide them through the effort is incredibly rewarding and incredibly powerful,” said Dr. Freeman, who also oversees the intensive cardiac rehab program at National Jewish Health in Denver.
A program like this needs proper funding in order to work, Dr, Freeman noted. He added that, even with coaches being paid well, “if you are able to prevent just one readmission for, say, heart failure a month . . . you could be saving millions of dollars over just a couple of years.”
Dr. Katzmarzyk, Dr. Flack, and Dr. Freeman reported no relevant disclosures. Louisiana State University, Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding a weight graph used in the study. The other researchers reported grants and/or fees from Bayer, Boehringer Ingelheim, Gilead, Takeda, Novo Nordisk, and other companies.
Patients who received intensive lifestyle training by coaches in the primary care setting experienced improvement in several indicators of cardiometabolic health in a 2-year trial.
The 803 trial participants comprised a racially diverse, low-income population with obesity. In this study, primary care clinics were randomly assigned to provide weight-loss coaching or usual care. Patients at the intensive training clinics lost significantly more weight than the other patients, as reported in a paper published in September in the New England Journal of Medicine on the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial. The patients who received weight loss coaching also had significantly more improvement in HDL cholesterol levels, total to HDL cholesterol ratios, and metabolic syndrome severity score, said researchers in the new paper on the PROPEL trial, which was published in Circulation on February 8 .
“We believe that one reason for success of the program was the use of a health coach [who] was embedded in the primary care office,” said lead author Peter Katzmarzyk, PhD, associate executive director for population and public health sciences at the Pennington Biomedical Research Center, Baton Rouge, La. “This way, the patients could get their counseling in a familiar environment and did not have to go to a different setting. The coaches developed close relationships with the patients over the 2 years, and this helped develop a sense of responsibility in the patients as the coaches were helping the patients to set goals and kept them accountable.”
In the PROPEL study, 67% of patients were Black and had low health literacy scores that corresponded with less than a ninth-grade education level. The intensive lifestyle intervention program included weekly sessions with the trained health coaches over the first 6 months — 16 face-to-face and 6 over the phone — and then at least monthly for the last 18 months. The coaches had higher education degrees in nutrition, physical activity, or behavioral medicine. Before the program started, the coaches also received training in the management of obesity and related health issues, health literacy, and patient communication and education. The goal of the program was 10% weight loss, using personalized action plans on eating, dieting, and physical activity.
Those in the usual-care clinics continued receiving normal care and received newsletters on health topics, such as the importance of sleep and tips for limiting time spent sitting. The primary care physicians at those clinics also were given a presentation with Centers for Medicare & Medicaid Services (CMS) information on intensive lifestyle interventions for obesity.
Cholesterol changes in intervention vs. control group
HDL cholesterol improved significantly among the coached patients, compared with the other patients, with a mean difference of 4.1 mg/dL at 1 year and 4.6 mg/dL at 2 years (P less than .01 for both). The total cholesterol to HDL cholesterol ratio showed a similarly significant difference in decline, with a between-group difference of –0.29 at 1 year and –0.31 at 2 years (P less than .01 for both). Also, the difference in the change in metabolic severity scores were –0.40 at 1 year and –0.21 at 2 years (P less than .01 for both).
Fasting blood glucose had declined after the 1st year by a significantly greater degree in the clinics with coaching, compared with the others, but not after the second year, researchers found.
There were no significant differences seen in total cholesterol, LDL cholesterol, non-HDL cholesterol, or blood pressure. Dr. Katzmarzyk said the likely reason for no change in blood pressure was that it was already relatively well-controlled at baseline for all the patients.
Funding barriers to obesity treatment
The CMS currently cover intensive training for obesity if delivered directly by a primary care physician, according to the authors of the new paper. Dr. Katzmarzyk said he hopes that will change.
“We are hoping that the evidence provided in this study may change the way that CMS funds obesity treatment in the future by allowing an expansion of the care team,” he said.
John Flack, MD, chair of internal medicine at Southern Illinois University, Springfield, said that the main achievement of the study was that it showed that intensive weight-loss training in the primary-care setting could be accomplished in a racially diverse population with low health literacy.
“You can’t just automatically assume just because you’ve seen it in some other populations that you can replicate this in every population, so they’ve done a really good job,” he said.
That programs are eligible for reimbursement only if they’re run by primary-care physicians is an ongoing problem, he said.
“You don’t necessarily need to be a physician to do this,” Dr. Flack said.
For best results, payment for coaching should not be tied to office visits, Dr. Flack noted.
“If they’re de-tethered from the office visits and you’re paid for quality ... you’re going to build out your infrastructure differently to care for people,” he said.
Andrew Freeman, MD, associate professor of medicine at the University of Colorado, Denver, and cochair of the American College of Cardiology’s nutrition and lifestyle work group, said the findings dovetail with his experience.
“I’m a huge believer that when people need to make lifestyle changes, having someone hold their hand and guide them through the effort is incredibly rewarding and incredibly powerful,” said Dr. Freeman, who also oversees the intensive cardiac rehab program at National Jewish Health in Denver.
A program like this needs proper funding in order to work, Dr, Freeman noted. He added that, even with coaches being paid well, “if you are able to prevent just one readmission for, say, heart failure a month . . . you could be saving millions of dollars over just a couple of years.”
Dr. Katzmarzyk, Dr. Flack, and Dr. Freeman reported no relevant disclosures. Louisiana State University, Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding a weight graph used in the study. The other researchers reported grants and/or fees from Bayer, Boehringer Ingelheim, Gilead, Takeda, Novo Nordisk, and other companies.
Patients who received intensive lifestyle training by coaches in the primary care setting experienced improvement in several indicators of cardiometabolic health in a 2-year trial.
The 803 trial participants comprised a racially diverse, low-income population with obesity. In this study, primary care clinics were randomly assigned to provide weight-loss coaching or usual care. Patients at the intensive training clinics lost significantly more weight than the other patients, as reported in a paper published in September in the New England Journal of Medicine on the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial. The patients who received weight loss coaching also had significantly more improvement in HDL cholesterol levels, total to HDL cholesterol ratios, and metabolic syndrome severity score, said researchers in the new paper on the PROPEL trial, which was published in Circulation on February 8 .
“We believe that one reason for success of the program was the use of a health coach [who] was embedded in the primary care office,” said lead author Peter Katzmarzyk, PhD, associate executive director for population and public health sciences at the Pennington Biomedical Research Center, Baton Rouge, La. “This way, the patients could get their counseling in a familiar environment and did not have to go to a different setting. The coaches developed close relationships with the patients over the 2 years, and this helped develop a sense of responsibility in the patients as the coaches were helping the patients to set goals and kept them accountable.”
In the PROPEL study, 67% of patients were Black and had low health literacy scores that corresponded with less than a ninth-grade education level. The intensive lifestyle intervention program included weekly sessions with the trained health coaches over the first 6 months — 16 face-to-face and 6 over the phone — and then at least monthly for the last 18 months. The coaches had higher education degrees in nutrition, physical activity, or behavioral medicine. Before the program started, the coaches also received training in the management of obesity and related health issues, health literacy, and patient communication and education. The goal of the program was 10% weight loss, using personalized action plans on eating, dieting, and physical activity.
Those in the usual-care clinics continued receiving normal care and received newsletters on health topics, such as the importance of sleep and tips for limiting time spent sitting. The primary care physicians at those clinics also were given a presentation with Centers for Medicare & Medicaid Services (CMS) information on intensive lifestyle interventions for obesity.
Cholesterol changes in intervention vs. control group
HDL cholesterol improved significantly among the coached patients, compared with the other patients, with a mean difference of 4.1 mg/dL at 1 year and 4.6 mg/dL at 2 years (P less than .01 for both). The total cholesterol to HDL cholesterol ratio showed a similarly significant difference in decline, with a between-group difference of –0.29 at 1 year and –0.31 at 2 years (P less than .01 for both). Also, the difference in the change in metabolic severity scores were –0.40 at 1 year and –0.21 at 2 years (P less than .01 for both).
Fasting blood glucose had declined after the 1st year by a significantly greater degree in the clinics with coaching, compared with the others, but not after the second year, researchers found.
There were no significant differences seen in total cholesterol, LDL cholesterol, non-HDL cholesterol, or blood pressure. Dr. Katzmarzyk said the likely reason for no change in blood pressure was that it was already relatively well-controlled at baseline for all the patients.
Funding barriers to obesity treatment
The CMS currently cover intensive training for obesity if delivered directly by a primary care physician, according to the authors of the new paper. Dr. Katzmarzyk said he hopes that will change.
“We are hoping that the evidence provided in this study may change the way that CMS funds obesity treatment in the future by allowing an expansion of the care team,” he said.
John Flack, MD, chair of internal medicine at Southern Illinois University, Springfield, said that the main achievement of the study was that it showed that intensive weight-loss training in the primary-care setting could be accomplished in a racially diverse population with low health literacy.
“You can’t just automatically assume just because you’ve seen it in some other populations that you can replicate this in every population, so they’ve done a really good job,” he said.
That programs are eligible for reimbursement only if they’re run by primary-care physicians is an ongoing problem, he said.
“You don’t necessarily need to be a physician to do this,” Dr. Flack said.
For best results, payment for coaching should not be tied to office visits, Dr. Flack noted.
“If they’re de-tethered from the office visits and you’re paid for quality ... you’re going to build out your infrastructure differently to care for people,” he said.
Andrew Freeman, MD, associate professor of medicine at the University of Colorado, Denver, and cochair of the American College of Cardiology’s nutrition and lifestyle work group, said the findings dovetail with his experience.
“I’m a huge believer that when people need to make lifestyle changes, having someone hold their hand and guide them through the effort is incredibly rewarding and incredibly powerful,” said Dr. Freeman, who also oversees the intensive cardiac rehab program at National Jewish Health in Denver.
A program like this needs proper funding in order to work, Dr, Freeman noted. He added that, even with coaches being paid well, “if you are able to prevent just one readmission for, say, heart failure a month . . . you could be saving millions of dollars over just a couple of years.”
Dr. Katzmarzyk, Dr. Flack, and Dr. Freeman reported no relevant disclosures. Louisiana State University, Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding a weight graph used in the study. The other researchers reported grants and/or fees from Bayer, Boehringer Ingelheim, Gilead, Takeda, Novo Nordisk, and other companies.
Glucosuria Is Not Always Due to Diabetes
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
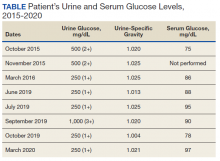
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
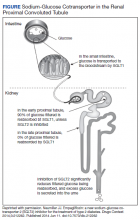
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Long-term metformin use linked to fewer ER+ breast cancers
.
Conversely, the results also showed higher rates of ER-negative and triple-negative breast cancer among women with type 2 diabetes who received metformin, although case numbers were small.
“Our conclusion that having type 2 diabetes increases the risk of developing breast cancer but taking metformin may protect against developing ER-positive breast cancer – but not other types of breast cancer – is biologically plausible and supported by our results, even though some [endpoints] are not statistically significant,” senior author Dale P. Sandler, PhD, chief of the epidemiology branch, National Institute of Environmental Health Sciences, Research Triangle Park, N.C., said in an interview.
“Among our findings that are not statistically significant are several that helped us get a better picture of the relationships between type 2 diabetes, metformin treatment, and breast cancer risk,” Dr. Sandler added.
The results were published online Jan. 28 in Annals of Oncology by Yong-Moon Mark Park, MD, PhD, now an epidemiologist at the University of Arkansas for Medical Sciences in Little Rock, and colleagues.
Sara P. Cate, MD, a breast cancer surgeon at Mount Sinai Medical Center in New York, who was not involved with the study, said: “Certainly, metformin helps with weight loss, which is linked with estrogen-driven breast cancers, so this may explain why fewer patients on metformin got this type of breast cancer.”
A tangled web ... with no clear conclusions yet
But in an accompanying editorial, Ana E. Lohmann, MD, PhD, and Pamela J. Goodwin, MD, say that, while this is “a large, well-designed prospective cohort study,” it tells a complicated story.
“The report by Park adds to the growing evidence linking type 2 diabetes and its treatment to breast cancer risk, but definitive conclusions regarding these associations are not yet possible,” they observe.
The “largely negative” results of the new study perhaps in part occurred because the cohort included only 277 women with type 2 diabetes diagnosed with incident breast cancer, note Dr. Lohmann, of London Health Sciences Centre, University of Western Ontario, and Dr. Goodwin, of Mount Sinai Hospital, Toronto.
“Clearly, this is an important area, and additional research is needed to untangle the web of inter-related associations of type 2 diabetes, its treatment, and breast cancer risk,” they write.
Examination of the effects of metformin in studies such as the Canadian Cancer Trial Group MA.32, a phase 3 trial of over 3,500 women with hormone receptor–positive early-stage breast cancer who are being randomized to metformin or placebo for up to 5 years in addition to standard adjuvant therapy, will provide further insights, they observe. The trial is slated to be completed in February 2022.
Study followed women whose sisters had breast cancer
The new data come from the Sister Study, which followed more than 50,000 women without a history of breast cancer who had sisters or half-sisters with a breast cancer diagnosis. The study, run by the NIEHS, enrolled women 35-74 years old from all 50 U.S. states and Puerto Rico in 2003-2009.
The current analysis excluded women with a history of any other type of cancer, missing data about diabetes, or an uncertain breast cancer diagnosis during the study, which left 44,541 available for study. At entry, 7% of the women had type 2 diabetes, and another 5% developed new-onset type 2 diabetes during follow-up.
Among those with diabetes, 61% received treatment with metformin either alone or with other antidiabetic drugs.
During a median follow-up of 8.6 years, 2,678 women received a diagnosis of primary breast cancer, either invasive or ductal carcinoma in situ.
In a series of multivariate analyses that adjusted for numerous potential confounders, the authors found that, overall, no association existed between diabetes and breast cancer incidence, with a hazard ratio of 0.99, compared with women without diabetes.
But, said Dr. Sandler, “there is a strong biological rationale to hypothesize that type 2 diabetes increases the risk for breast cancer, and results from earlier studies support this.”
Association of metformin and breast cancer
Women with type 2 diabetes who received metformin had a 14% lower rate of ER-positive breast cancer, compared with women with diabetes not taking metformin, a nonsignificant association.
Among women taking metformin for at least 10 years, the associated reduction in ER-positive breast cancer, compared with those who did not take it, was 38%, a difference that just missed significance, with a 95% confidence interval of 0.38-1.01.
In contrast, cases of ER-negative and triple-negative breast cancers increased in the women with diabetes taking metformin. The hazard ratio for ER-negative tumors showed a nonsignificant 25% relative increase in women taking metformin and a significant 74% increase in triple-negative cancers.
The editorialists note, however, that “the number of patients who were found to have triple-negative breast cancer was small [so] we cannot draw any practice-changing conclusions from it.”
In conclusion, Dr. Park and colleagues reiterate: “Our analysis is consistent with a potential protective effect of metformin and suggests that long-term use of metformin may reduce breast cancer risk associated with type 2 diabetes.”
The study received no commercial funding. Dr. Sandler, Dr. Park, Dr. Lohmann, Dr. Goodwin, and Dr. Cate have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Conversely, the results also showed higher rates of ER-negative and triple-negative breast cancer among women with type 2 diabetes who received metformin, although case numbers were small.
“Our conclusion that having type 2 diabetes increases the risk of developing breast cancer but taking metformin may protect against developing ER-positive breast cancer – but not other types of breast cancer – is biologically plausible and supported by our results, even though some [endpoints] are not statistically significant,” senior author Dale P. Sandler, PhD, chief of the epidemiology branch, National Institute of Environmental Health Sciences, Research Triangle Park, N.C., said in an interview.
“Among our findings that are not statistically significant are several that helped us get a better picture of the relationships between type 2 diabetes, metformin treatment, and breast cancer risk,” Dr. Sandler added.
The results were published online Jan. 28 in Annals of Oncology by Yong-Moon Mark Park, MD, PhD, now an epidemiologist at the University of Arkansas for Medical Sciences in Little Rock, and colleagues.
Sara P. Cate, MD, a breast cancer surgeon at Mount Sinai Medical Center in New York, who was not involved with the study, said: “Certainly, metformin helps with weight loss, which is linked with estrogen-driven breast cancers, so this may explain why fewer patients on metformin got this type of breast cancer.”
A tangled web ... with no clear conclusions yet
But in an accompanying editorial, Ana E. Lohmann, MD, PhD, and Pamela J. Goodwin, MD, say that, while this is “a large, well-designed prospective cohort study,” it tells a complicated story.
“The report by Park adds to the growing evidence linking type 2 diabetes and its treatment to breast cancer risk, but definitive conclusions regarding these associations are not yet possible,” they observe.
The “largely negative” results of the new study perhaps in part occurred because the cohort included only 277 women with type 2 diabetes diagnosed with incident breast cancer, note Dr. Lohmann, of London Health Sciences Centre, University of Western Ontario, and Dr. Goodwin, of Mount Sinai Hospital, Toronto.
“Clearly, this is an important area, and additional research is needed to untangle the web of inter-related associations of type 2 diabetes, its treatment, and breast cancer risk,” they write.
Examination of the effects of metformin in studies such as the Canadian Cancer Trial Group MA.32, a phase 3 trial of over 3,500 women with hormone receptor–positive early-stage breast cancer who are being randomized to metformin or placebo for up to 5 years in addition to standard adjuvant therapy, will provide further insights, they observe. The trial is slated to be completed in February 2022.
Study followed women whose sisters had breast cancer
The new data come from the Sister Study, which followed more than 50,000 women without a history of breast cancer who had sisters or half-sisters with a breast cancer diagnosis. The study, run by the NIEHS, enrolled women 35-74 years old from all 50 U.S. states and Puerto Rico in 2003-2009.
The current analysis excluded women with a history of any other type of cancer, missing data about diabetes, or an uncertain breast cancer diagnosis during the study, which left 44,541 available for study. At entry, 7% of the women had type 2 diabetes, and another 5% developed new-onset type 2 diabetes during follow-up.
Among those with diabetes, 61% received treatment with metformin either alone or with other antidiabetic drugs.
During a median follow-up of 8.6 years, 2,678 women received a diagnosis of primary breast cancer, either invasive or ductal carcinoma in situ.
In a series of multivariate analyses that adjusted for numerous potential confounders, the authors found that, overall, no association existed between diabetes and breast cancer incidence, with a hazard ratio of 0.99, compared with women without diabetes.
But, said Dr. Sandler, “there is a strong biological rationale to hypothesize that type 2 diabetes increases the risk for breast cancer, and results from earlier studies support this.”
Association of metformin and breast cancer
Women with type 2 diabetes who received metformin had a 14% lower rate of ER-positive breast cancer, compared with women with diabetes not taking metformin, a nonsignificant association.
Among women taking metformin for at least 10 years, the associated reduction in ER-positive breast cancer, compared with those who did not take it, was 38%, a difference that just missed significance, with a 95% confidence interval of 0.38-1.01.
In contrast, cases of ER-negative and triple-negative breast cancers increased in the women with diabetes taking metformin. The hazard ratio for ER-negative tumors showed a nonsignificant 25% relative increase in women taking metformin and a significant 74% increase in triple-negative cancers.
The editorialists note, however, that “the number of patients who were found to have triple-negative breast cancer was small [so] we cannot draw any practice-changing conclusions from it.”
In conclusion, Dr. Park and colleagues reiterate: “Our analysis is consistent with a potential protective effect of metformin and suggests that long-term use of metformin may reduce breast cancer risk associated with type 2 diabetes.”
The study received no commercial funding. Dr. Sandler, Dr. Park, Dr. Lohmann, Dr. Goodwin, and Dr. Cate have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
Conversely, the results also showed higher rates of ER-negative and triple-negative breast cancer among women with type 2 diabetes who received metformin, although case numbers were small.
“Our conclusion that having type 2 diabetes increases the risk of developing breast cancer but taking metformin may protect against developing ER-positive breast cancer – but not other types of breast cancer – is biologically plausible and supported by our results, even though some [endpoints] are not statistically significant,” senior author Dale P. Sandler, PhD, chief of the epidemiology branch, National Institute of Environmental Health Sciences, Research Triangle Park, N.C., said in an interview.
“Among our findings that are not statistically significant are several that helped us get a better picture of the relationships between type 2 diabetes, metformin treatment, and breast cancer risk,” Dr. Sandler added.
The results were published online Jan. 28 in Annals of Oncology by Yong-Moon Mark Park, MD, PhD, now an epidemiologist at the University of Arkansas for Medical Sciences in Little Rock, and colleagues.
Sara P. Cate, MD, a breast cancer surgeon at Mount Sinai Medical Center in New York, who was not involved with the study, said: “Certainly, metformin helps with weight loss, which is linked with estrogen-driven breast cancers, so this may explain why fewer patients on metformin got this type of breast cancer.”
A tangled web ... with no clear conclusions yet
But in an accompanying editorial, Ana E. Lohmann, MD, PhD, and Pamela J. Goodwin, MD, say that, while this is “a large, well-designed prospective cohort study,” it tells a complicated story.
“The report by Park adds to the growing evidence linking type 2 diabetes and its treatment to breast cancer risk, but definitive conclusions regarding these associations are not yet possible,” they observe.
The “largely negative” results of the new study perhaps in part occurred because the cohort included only 277 women with type 2 diabetes diagnosed with incident breast cancer, note Dr. Lohmann, of London Health Sciences Centre, University of Western Ontario, and Dr. Goodwin, of Mount Sinai Hospital, Toronto.
“Clearly, this is an important area, and additional research is needed to untangle the web of inter-related associations of type 2 diabetes, its treatment, and breast cancer risk,” they write.
Examination of the effects of metformin in studies such as the Canadian Cancer Trial Group MA.32, a phase 3 trial of over 3,500 women with hormone receptor–positive early-stage breast cancer who are being randomized to metformin or placebo for up to 5 years in addition to standard adjuvant therapy, will provide further insights, they observe. The trial is slated to be completed in February 2022.
Study followed women whose sisters had breast cancer
The new data come from the Sister Study, which followed more than 50,000 women without a history of breast cancer who had sisters or half-sisters with a breast cancer diagnosis. The study, run by the NIEHS, enrolled women 35-74 years old from all 50 U.S. states and Puerto Rico in 2003-2009.
The current analysis excluded women with a history of any other type of cancer, missing data about diabetes, or an uncertain breast cancer diagnosis during the study, which left 44,541 available for study. At entry, 7% of the women had type 2 diabetes, and another 5% developed new-onset type 2 diabetes during follow-up.
Among those with diabetes, 61% received treatment with metformin either alone or with other antidiabetic drugs.
During a median follow-up of 8.6 years, 2,678 women received a diagnosis of primary breast cancer, either invasive or ductal carcinoma in situ.
In a series of multivariate analyses that adjusted for numerous potential confounders, the authors found that, overall, no association existed between diabetes and breast cancer incidence, with a hazard ratio of 0.99, compared with women without diabetes.
But, said Dr. Sandler, “there is a strong biological rationale to hypothesize that type 2 diabetes increases the risk for breast cancer, and results from earlier studies support this.”
Association of metformin and breast cancer
Women with type 2 diabetes who received metformin had a 14% lower rate of ER-positive breast cancer, compared with women with diabetes not taking metformin, a nonsignificant association.
Among women taking metformin for at least 10 years, the associated reduction in ER-positive breast cancer, compared with those who did not take it, was 38%, a difference that just missed significance, with a 95% confidence interval of 0.38-1.01.
In contrast, cases of ER-negative and triple-negative breast cancers increased in the women with diabetes taking metformin. The hazard ratio for ER-negative tumors showed a nonsignificant 25% relative increase in women taking metformin and a significant 74% increase in triple-negative cancers.
The editorialists note, however, that “the number of patients who were found to have triple-negative breast cancer was small [so] we cannot draw any practice-changing conclusions from it.”
In conclusion, Dr. Park and colleagues reiterate: “Our analysis is consistent with a potential protective effect of metformin and suggests that long-term use of metformin may reduce breast cancer risk associated with type 2 diabetes.”
The study received no commercial funding. Dr. Sandler, Dr. Park, Dr. Lohmann, Dr. Goodwin, and Dr. Cate have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oily fish linked to lower risk of diabetes in largest study to date
People who report regularly eating oily fish had a significantly reduced risk for developing type 2 diabetes in a prospective, observational study of nearly 400,000 UK residents.
The results also show a significant, but weaker, positive link between regular use of fish oil supplements and a drop in the incidence of type 2 diabetes, Qibin Qi, PhD, and colleagues wrote in a report published in Diabetes Care. Their analysis failed to show a significant link between consumption of non-oily fish and type 2 diabetes onset.
The study is notable for being “the largest so far” to examine the link between fish consumption and type 2 diabetes incidence, and the first to establish a clear, significant association between regularly eating oily fish and a drop in the incidence of diabetes, said Dr. Qi, an epidemiologist at Albert Einstein College of Medicine in New York.
“At present, it is prudent to recommend fresh oily fish as a part of a healthy dietary pattern instead of fish oil supplements for diabetes prevention,” said Dr. Qi and coauthors.
The study included just over 392,000 adults without type 2 diabetes or cardiovascular disease at baseline enrolled in the UK Biobank. Median follow-up was just over 10 years, during which 7,262 participants developed diabetes.
Participants who ate either one, or two or more, servings of oily fish weekly each had a significant 22% lower rate of incident type 2 diabetes than that of those who ate no oily fish, after adjustment for multiple confounders. Those who reported regularly taking a fish oil supplement had a significant 9% lower incidence of type 2 diabetes than that of those who didn’t.
Evidence growing to add oily fish to diet to prevent type 2 diabetes
“Many current dietary guidelines recommend consumption of two servings of fish, preferably oily, per week, primarily based on cardiovascular benefits,” Dr. Qi said in an interview.
“No prior statements recommended oily fish for prevention of type 2 diabetes,” he explained, adding: “Our findings support future recommendations, but the evidence is not strong enough to make a [formal] recommendation now. We need evidence from clinical trials.”
Jason Wu, PhD, an epidemiologist at the University of New South Wales in Sydney, Australia, who specializes in this field but was not involved with the current study, said it “is a very well-conducted study, and certainly generates important new evidence supporting the potential benefits of regular consumption of oily fish.”
But he agrees that the evidence remains too preliminary for any official recommendations on eating oily fish for preventing the development of type 2 diabetes, including targeting advice to high-risk subgroups such as those with prediabetes or people who are obese.
Before any groups make recommendations, “we need to thoroughly review all the literature in this space to appraise the overall body of evidence,” Dr. Wu noted in an interview.
Oily fish: Solid evidence for prevention of CVD events
In contrast, the case for including oily fish in the diet to prevent CVD events seems settled. In 2018, a panel assembled by the American Heart Association to address the issue released a statement that concluded: “Current scientific evidence strongly supports the recommendation that seafood be an integral component of a heart-healthy dietary pattern.” It added that “a large body of evidence supports the recommendation to consume nonfried seafood, especially species higher in long-chain n-3 fatty acids, one to two times per week for cardiovascular benefits, including reduced risk of cardiac death, coronary heart disease, and ischemic stroke.”
The statement highlighted that “cold-water oily fish such as salmon, anchovies, herring, mackerel (Atlantic and Pacific), tuna (bluefin and albacore), and sardines have the highest levels” of long-chain n-3 fatty acids, notably eicosapentaenoic acid and docosahexaenoic acid, also collectively known as omega-3 fatty acids.
These fish types were among the oily fishes tallied in the UK Biobank data used by Dr. Qi and colleagues.
The case for fish oil supplements for preventing CVD events is much rockier, as summarized in a 2019 editorial, with some studies reporting no discernible effect while others indicate efficacy.
A second commentary from December 2020 highlighted how results from the REDUCE-IT trial showed clear benefit for preventing CVD using a highly purified form of fish oil, icosapent ethyl (Vascepa, Amarin). However, findings from two other recent reports, the STRENGTH and OMENI studies, failed to show CVD benefits from more conventional fish oil formulations.
Composite CVD and diabetes prevention effects?
The new findings by Dr. Qi and colleagues “highlight the need to specifically test the effect of fish oil supplements on glucose metabolism in people who cannot or choose not to regularly eat oily fish,” said Dr. Wu, a researcher at the George Institute for Global Health in Newtown, Australia.
“If eventually there is really strong evidence that fish, fish oil, or both have independent effects on both CVD and type 2 diabetes” it would be reasonable to integrate both outcomes into a single, composite, efficacy endpoint for the purpose of future studies, he added.
Dr. Qi agreed on both points. “A randomized, controlled trial of fish oil on type 2 diabetes as a primary outcome is needed. Most existing data are based on secondary analyses in the randomized trials for CVD,” he explained.
But, he added, “our results suggest a potential beneficial effect from fish oil supplements,” which implies that these may be “better than nothing” for people who can’t add oily fish to their regular diet.
The means by which fish and fish oil might slow or stop progression to type 2 diabetes remains uncertain.
The mechanisms for preventing both diabetes and CVD events may overlap, Dr. Qi noted, such as anti-inflammatory effects and improved insulin sensitivity, both of which have been observed in animal studies.
Evidence is “still lacking from human studies,” he explained, but if such mechanisms were at play, Dr. Wu said that would “add biologic plausibility” to a possible causal link between oily fish consumption and diabetes prevention.
“But we can’t assume that omega-3 fatty acids alone will have the same effect as oily fish, which obviously contains many other components.”
The study received no commercial funding. Dr. Qi and Dr. Wu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who report regularly eating oily fish had a significantly reduced risk for developing type 2 diabetes in a prospective, observational study of nearly 400,000 UK residents.
The results also show a significant, but weaker, positive link between regular use of fish oil supplements and a drop in the incidence of type 2 diabetes, Qibin Qi, PhD, and colleagues wrote in a report published in Diabetes Care. Their analysis failed to show a significant link between consumption of non-oily fish and type 2 diabetes onset.
The study is notable for being “the largest so far” to examine the link between fish consumption and type 2 diabetes incidence, and the first to establish a clear, significant association between regularly eating oily fish and a drop in the incidence of diabetes, said Dr. Qi, an epidemiologist at Albert Einstein College of Medicine in New York.
“At present, it is prudent to recommend fresh oily fish as a part of a healthy dietary pattern instead of fish oil supplements for diabetes prevention,” said Dr. Qi and coauthors.
The study included just over 392,000 adults without type 2 diabetes or cardiovascular disease at baseline enrolled in the UK Biobank. Median follow-up was just over 10 years, during which 7,262 participants developed diabetes.
Participants who ate either one, or two or more, servings of oily fish weekly each had a significant 22% lower rate of incident type 2 diabetes than that of those who ate no oily fish, after adjustment for multiple confounders. Those who reported regularly taking a fish oil supplement had a significant 9% lower incidence of type 2 diabetes than that of those who didn’t.
Evidence growing to add oily fish to diet to prevent type 2 diabetes
“Many current dietary guidelines recommend consumption of two servings of fish, preferably oily, per week, primarily based on cardiovascular benefits,” Dr. Qi said in an interview.
“No prior statements recommended oily fish for prevention of type 2 diabetes,” he explained, adding: “Our findings support future recommendations, but the evidence is not strong enough to make a [formal] recommendation now. We need evidence from clinical trials.”
Jason Wu, PhD, an epidemiologist at the University of New South Wales in Sydney, Australia, who specializes in this field but was not involved with the current study, said it “is a very well-conducted study, and certainly generates important new evidence supporting the potential benefits of regular consumption of oily fish.”
But he agrees that the evidence remains too preliminary for any official recommendations on eating oily fish for preventing the development of type 2 diabetes, including targeting advice to high-risk subgroups such as those with prediabetes or people who are obese.
Before any groups make recommendations, “we need to thoroughly review all the literature in this space to appraise the overall body of evidence,” Dr. Wu noted in an interview.
Oily fish: Solid evidence for prevention of CVD events
In contrast, the case for including oily fish in the diet to prevent CVD events seems settled. In 2018, a panel assembled by the American Heart Association to address the issue released a statement that concluded: “Current scientific evidence strongly supports the recommendation that seafood be an integral component of a heart-healthy dietary pattern.” It added that “a large body of evidence supports the recommendation to consume nonfried seafood, especially species higher in long-chain n-3 fatty acids, one to two times per week for cardiovascular benefits, including reduced risk of cardiac death, coronary heart disease, and ischemic stroke.”
The statement highlighted that “cold-water oily fish such as salmon, anchovies, herring, mackerel (Atlantic and Pacific), tuna (bluefin and albacore), and sardines have the highest levels” of long-chain n-3 fatty acids, notably eicosapentaenoic acid and docosahexaenoic acid, also collectively known as omega-3 fatty acids.
These fish types were among the oily fishes tallied in the UK Biobank data used by Dr. Qi and colleagues.
The case for fish oil supplements for preventing CVD events is much rockier, as summarized in a 2019 editorial, with some studies reporting no discernible effect while others indicate efficacy.
A second commentary from December 2020 highlighted how results from the REDUCE-IT trial showed clear benefit for preventing CVD using a highly purified form of fish oil, icosapent ethyl (Vascepa, Amarin). However, findings from two other recent reports, the STRENGTH and OMENI studies, failed to show CVD benefits from more conventional fish oil formulations.
Composite CVD and diabetes prevention effects?
The new findings by Dr. Qi and colleagues “highlight the need to specifically test the effect of fish oil supplements on glucose metabolism in people who cannot or choose not to regularly eat oily fish,” said Dr. Wu, a researcher at the George Institute for Global Health in Newtown, Australia.
“If eventually there is really strong evidence that fish, fish oil, or both have independent effects on both CVD and type 2 diabetes” it would be reasonable to integrate both outcomes into a single, composite, efficacy endpoint for the purpose of future studies, he added.
Dr. Qi agreed on both points. “A randomized, controlled trial of fish oil on type 2 diabetes as a primary outcome is needed. Most existing data are based on secondary analyses in the randomized trials for CVD,” he explained.
But, he added, “our results suggest a potential beneficial effect from fish oil supplements,” which implies that these may be “better than nothing” for people who can’t add oily fish to their regular diet.
The means by which fish and fish oil might slow or stop progression to type 2 diabetes remains uncertain.
The mechanisms for preventing both diabetes and CVD events may overlap, Dr. Qi noted, such as anti-inflammatory effects and improved insulin sensitivity, both of which have been observed in animal studies.
Evidence is “still lacking from human studies,” he explained, but if such mechanisms were at play, Dr. Wu said that would “add biologic plausibility” to a possible causal link between oily fish consumption and diabetes prevention.
“But we can’t assume that omega-3 fatty acids alone will have the same effect as oily fish, which obviously contains many other components.”
The study received no commercial funding. Dr. Qi and Dr. Wu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People who report regularly eating oily fish had a significantly reduced risk for developing type 2 diabetes in a prospective, observational study of nearly 400,000 UK residents.
The results also show a significant, but weaker, positive link between regular use of fish oil supplements and a drop in the incidence of type 2 diabetes, Qibin Qi, PhD, and colleagues wrote in a report published in Diabetes Care. Their analysis failed to show a significant link between consumption of non-oily fish and type 2 diabetes onset.
The study is notable for being “the largest so far” to examine the link between fish consumption and type 2 diabetes incidence, and the first to establish a clear, significant association between regularly eating oily fish and a drop in the incidence of diabetes, said Dr. Qi, an epidemiologist at Albert Einstein College of Medicine in New York.
“At present, it is prudent to recommend fresh oily fish as a part of a healthy dietary pattern instead of fish oil supplements for diabetes prevention,” said Dr. Qi and coauthors.
The study included just over 392,000 adults without type 2 diabetes or cardiovascular disease at baseline enrolled in the UK Biobank. Median follow-up was just over 10 years, during which 7,262 participants developed diabetes.
Participants who ate either one, or two or more, servings of oily fish weekly each had a significant 22% lower rate of incident type 2 diabetes than that of those who ate no oily fish, after adjustment for multiple confounders. Those who reported regularly taking a fish oil supplement had a significant 9% lower incidence of type 2 diabetes than that of those who didn’t.
Evidence growing to add oily fish to diet to prevent type 2 diabetes
“Many current dietary guidelines recommend consumption of two servings of fish, preferably oily, per week, primarily based on cardiovascular benefits,” Dr. Qi said in an interview.
“No prior statements recommended oily fish for prevention of type 2 diabetes,” he explained, adding: “Our findings support future recommendations, but the evidence is not strong enough to make a [formal] recommendation now. We need evidence from clinical trials.”
Jason Wu, PhD, an epidemiologist at the University of New South Wales in Sydney, Australia, who specializes in this field but was not involved with the current study, said it “is a very well-conducted study, and certainly generates important new evidence supporting the potential benefits of regular consumption of oily fish.”
But he agrees that the evidence remains too preliminary for any official recommendations on eating oily fish for preventing the development of type 2 diabetes, including targeting advice to high-risk subgroups such as those with prediabetes or people who are obese.
Before any groups make recommendations, “we need to thoroughly review all the literature in this space to appraise the overall body of evidence,” Dr. Wu noted in an interview.
Oily fish: Solid evidence for prevention of CVD events
In contrast, the case for including oily fish in the diet to prevent CVD events seems settled. In 2018, a panel assembled by the American Heart Association to address the issue released a statement that concluded: “Current scientific evidence strongly supports the recommendation that seafood be an integral component of a heart-healthy dietary pattern.” It added that “a large body of evidence supports the recommendation to consume nonfried seafood, especially species higher in long-chain n-3 fatty acids, one to two times per week for cardiovascular benefits, including reduced risk of cardiac death, coronary heart disease, and ischemic stroke.”
The statement highlighted that “cold-water oily fish such as salmon, anchovies, herring, mackerel (Atlantic and Pacific), tuna (bluefin and albacore), and sardines have the highest levels” of long-chain n-3 fatty acids, notably eicosapentaenoic acid and docosahexaenoic acid, also collectively known as omega-3 fatty acids.
These fish types were among the oily fishes tallied in the UK Biobank data used by Dr. Qi and colleagues.
The case for fish oil supplements for preventing CVD events is much rockier, as summarized in a 2019 editorial, with some studies reporting no discernible effect while others indicate efficacy.
A second commentary from December 2020 highlighted how results from the REDUCE-IT trial showed clear benefit for preventing CVD using a highly purified form of fish oil, icosapent ethyl (Vascepa, Amarin). However, findings from two other recent reports, the STRENGTH and OMENI studies, failed to show CVD benefits from more conventional fish oil formulations.
Composite CVD and diabetes prevention effects?
The new findings by Dr. Qi and colleagues “highlight the need to specifically test the effect of fish oil supplements on glucose metabolism in people who cannot or choose not to regularly eat oily fish,” said Dr. Wu, a researcher at the George Institute for Global Health in Newtown, Australia.
“If eventually there is really strong evidence that fish, fish oil, or both have independent effects on both CVD and type 2 diabetes” it would be reasonable to integrate both outcomes into a single, composite, efficacy endpoint for the purpose of future studies, he added.
Dr. Qi agreed on both points. “A randomized, controlled trial of fish oil on type 2 diabetes as a primary outcome is needed. Most existing data are based on secondary analyses in the randomized trials for CVD,” he explained.
But, he added, “our results suggest a potential beneficial effect from fish oil supplements,” which implies that these may be “better than nothing” for people who can’t add oily fish to their regular diet.
The means by which fish and fish oil might slow or stop progression to type 2 diabetes remains uncertain.
The mechanisms for preventing both diabetes and CVD events may overlap, Dr. Qi noted, such as anti-inflammatory effects and improved insulin sensitivity, both of which have been observed in animal studies.
Evidence is “still lacking from human studies,” he explained, but if such mechanisms were at play, Dr. Wu said that would “add biologic plausibility” to a possible causal link between oily fish consumption and diabetes prevention.
“But we can’t assume that omega-3 fatty acids alone will have the same effect as oily fish, which obviously contains many other components.”
The study received no commercial funding. Dr. Qi and Dr. Wu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PURE: High refined-grain intake boosts death, CVD events
That’s one finding from an assessment of a more than 137,000 people in 21 countries that documented a clear link between a high level of consumption of refined grains and a significantly increased risk for death from any cause or major cardiovascular disease (CVD) event during a median follow-up of 9.5 years.
The results showed that people who reported eating at least 350 g (seven servings) of refined grain daily had a significant 29% increased risk of either death or a major CVD event (MI, stroke, or heart failure), compared with those who consumed less than one serving per day (fewer than 50 g) of refined grain after adjustment for multiple potential confounders, according to a report from the Prospective Urban Rural Epidemiology (PURE) study published in the BMJ on Feb. 3, 2021.
The analysis also showed no significant association between levels of whole grains or white rice in the diet and CVD events. Rice was considered a separate grain in the analysis because nearly two-thirds of the PURE study population reside in Asia, where rice is a staple food.
The findings show that “reduction in the quantity of refined grains and sugar, and improvement in the quality of carbohydrates is essential for better health outcomes, although we do not suggest complete elimination of refined grains,” said Mahshid Dehghan, PhD, lead investigator for this report and a researcher in nutrition epidemiology at the Population Health Research Institute of McMaster University, Hamilton, Ont.
‘Widely applicable’ results from large, diverse study
Although prior evidence had already shown the CVD risk from eating larger amounts of refined grains, “our findings are robust and more widely applicable because our large study recorded over 9,000 deaths and 3,500 major CVD events across a broad range of refined grain intake, and in a variety of different settings and cultures with varying dietary patterns,” Dr. Dehghan said in an interview.
“This is an important paper, with the strength of data from diverse countries. The associations are robust,” commented Dariush Mozaffarian, MD, DrPH, professor and dean of the Friedman School of Nutrition Science and Policy at Tufts University, Boston, who was not involved in the new report.
“The public and the public health community think about added sugar in food as harmful, but starch has gotten a free pass,” he said in an interview. Recently revised U.S. dietary guidelines recommend that refined grains constitute less than half of a person’s carbohydrate consumption, but that limitation remains set too high, Dr. Mozaffarian cautioned. A much safer daily consumption limit would cap refined grains to no more than one serving a day.
The data for the current PURE analysis came from more than 148,000 people aged 35-70 years at entry in 21 geographically and economically diverse countries. Excluding patients with known CVD at baseline left a cohort of 137,130 people.
The results showed no significant association between the quantity of whole grains consumed and the main outcome, nor a link between higher amounts of white rice consumption and the main outcome.
“Our findings suggest that intake of up to 350 g of cooked rice daily may not pose a significant health risk,” said Dr. Dehghan.
Refined grains produce a glucose surge
Dr. Dehghan and associates speculated that possible explanations for their findings are that “varieties of rice such as long-grain rice and especially parboiled white rice may have both a definite glycemic advantage and an overall nutritional advantage over refined wheat products. Also, depending on the culture and the nature of the rice eaten, rice may be displacing less desirable foods.”
In contrast, refined grains undergo “rapid action by digestive enzymes and quick absorption from the small intestines [that] could lead to an increase in postprandial blood glucose concentrations. The rise in glucose concentrations increases the insulin concentrations, which leads to hypoglycemia, lipolysis, and the stimulation of hunger and food intake,” the authors wrote.
“It’s similar to eating sugar, or candy,” noted Dr. Mozaffarian, as refined grain “is 100% glucose.” Whole grains differ by entering the gut packaged in cell structures that slow digestion and avoid delivering sugar in an unnaturally rapid way.
“We are providing new evidence, and we hope that dietary guidelines in North America encourage individuals to lower their refined grain and sugar intake,” Dr. Dehghan said.
PURE has received partial funding with unrestricted grants from several drug companies. Dr. Dehghan had no disclosures. Dr. Mozaffarian has been an adviser to or has received personal fees from several food companies, but had no relevant disclosures.
That’s one finding from an assessment of a more than 137,000 people in 21 countries that documented a clear link between a high level of consumption of refined grains and a significantly increased risk for death from any cause or major cardiovascular disease (CVD) event during a median follow-up of 9.5 years.
The results showed that people who reported eating at least 350 g (seven servings) of refined grain daily had a significant 29% increased risk of either death or a major CVD event (MI, stroke, or heart failure), compared with those who consumed less than one serving per day (fewer than 50 g) of refined grain after adjustment for multiple potential confounders, according to a report from the Prospective Urban Rural Epidemiology (PURE) study published in the BMJ on Feb. 3, 2021.
The analysis also showed no significant association between levels of whole grains or white rice in the diet and CVD events. Rice was considered a separate grain in the analysis because nearly two-thirds of the PURE study population reside in Asia, where rice is a staple food.
The findings show that “reduction in the quantity of refined grains and sugar, and improvement in the quality of carbohydrates is essential for better health outcomes, although we do not suggest complete elimination of refined grains,” said Mahshid Dehghan, PhD, lead investigator for this report and a researcher in nutrition epidemiology at the Population Health Research Institute of McMaster University, Hamilton, Ont.
‘Widely applicable’ results from large, diverse study
Although prior evidence had already shown the CVD risk from eating larger amounts of refined grains, “our findings are robust and more widely applicable because our large study recorded over 9,000 deaths and 3,500 major CVD events across a broad range of refined grain intake, and in a variety of different settings and cultures with varying dietary patterns,” Dr. Dehghan said in an interview.
“This is an important paper, with the strength of data from diverse countries. The associations are robust,” commented Dariush Mozaffarian, MD, DrPH, professor and dean of the Friedman School of Nutrition Science and Policy at Tufts University, Boston, who was not involved in the new report.
“The public and the public health community think about added sugar in food as harmful, but starch has gotten a free pass,” he said in an interview. Recently revised U.S. dietary guidelines recommend that refined grains constitute less than half of a person’s carbohydrate consumption, but that limitation remains set too high, Dr. Mozaffarian cautioned. A much safer daily consumption limit would cap refined grains to no more than one serving a day.
The data for the current PURE analysis came from more than 148,000 people aged 35-70 years at entry in 21 geographically and economically diverse countries. Excluding patients with known CVD at baseline left a cohort of 137,130 people.
The results showed no significant association between the quantity of whole grains consumed and the main outcome, nor a link between higher amounts of white rice consumption and the main outcome.
“Our findings suggest that intake of up to 350 g of cooked rice daily may not pose a significant health risk,” said Dr. Dehghan.
Refined grains produce a glucose surge
Dr. Dehghan and associates speculated that possible explanations for their findings are that “varieties of rice such as long-grain rice and especially parboiled white rice may have both a definite glycemic advantage and an overall nutritional advantage over refined wheat products. Also, depending on the culture and the nature of the rice eaten, rice may be displacing less desirable foods.”
In contrast, refined grains undergo “rapid action by digestive enzymes and quick absorption from the small intestines [that] could lead to an increase in postprandial blood glucose concentrations. The rise in glucose concentrations increases the insulin concentrations, which leads to hypoglycemia, lipolysis, and the stimulation of hunger and food intake,” the authors wrote.
“It’s similar to eating sugar, or candy,” noted Dr. Mozaffarian, as refined grain “is 100% glucose.” Whole grains differ by entering the gut packaged in cell structures that slow digestion and avoid delivering sugar in an unnaturally rapid way.
“We are providing new evidence, and we hope that dietary guidelines in North America encourage individuals to lower their refined grain and sugar intake,” Dr. Dehghan said.
PURE has received partial funding with unrestricted grants from several drug companies. Dr. Dehghan had no disclosures. Dr. Mozaffarian has been an adviser to or has received personal fees from several food companies, but had no relevant disclosures.
That’s one finding from an assessment of a more than 137,000 people in 21 countries that documented a clear link between a high level of consumption of refined grains and a significantly increased risk for death from any cause or major cardiovascular disease (CVD) event during a median follow-up of 9.5 years.
The results showed that people who reported eating at least 350 g (seven servings) of refined grain daily had a significant 29% increased risk of either death or a major CVD event (MI, stroke, or heart failure), compared with those who consumed less than one serving per day (fewer than 50 g) of refined grain after adjustment for multiple potential confounders, according to a report from the Prospective Urban Rural Epidemiology (PURE) study published in the BMJ on Feb. 3, 2021.
The analysis also showed no significant association between levels of whole grains or white rice in the diet and CVD events. Rice was considered a separate grain in the analysis because nearly two-thirds of the PURE study population reside in Asia, where rice is a staple food.
The findings show that “reduction in the quantity of refined grains and sugar, and improvement in the quality of carbohydrates is essential for better health outcomes, although we do not suggest complete elimination of refined grains,” said Mahshid Dehghan, PhD, lead investigator for this report and a researcher in nutrition epidemiology at the Population Health Research Institute of McMaster University, Hamilton, Ont.
‘Widely applicable’ results from large, diverse study
Although prior evidence had already shown the CVD risk from eating larger amounts of refined grains, “our findings are robust and more widely applicable because our large study recorded over 9,000 deaths and 3,500 major CVD events across a broad range of refined grain intake, and in a variety of different settings and cultures with varying dietary patterns,” Dr. Dehghan said in an interview.
“This is an important paper, with the strength of data from diverse countries. The associations are robust,” commented Dariush Mozaffarian, MD, DrPH, professor and dean of the Friedman School of Nutrition Science and Policy at Tufts University, Boston, who was not involved in the new report.
“The public and the public health community think about added sugar in food as harmful, but starch has gotten a free pass,” he said in an interview. Recently revised U.S. dietary guidelines recommend that refined grains constitute less than half of a person’s carbohydrate consumption, but that limitation remains set too high, Dr. Mozaffarian cautioned. A much safer daily consumption limit would cap refined grains to no more than one serving a day.
The data for the current PURE analysis came from more than 148,000 people aged 35-70 years at entry in 21 geographically and economically diverse countries. Excluding patients with known CVD at baseline left a cohort of 137,130 people.
The results showed no significant association between the quantity of whole grains consumed and the main outcome, nor a link between higher amounts of white rice consumption and the main outcome.
“Our findings suggest that intake of up to 350 g of cooked rice daily may not pose a significant health risk,” said Dr. Dehghan.
Refined grains produce a glucose surge
Dr. Dehghan and associates speculated that possible explanations for their findings are that “varieties of rice such as long-grain rice and especially parboiled white rice may have both a definite glycemic advantage and an overall nutritional advantage over refined wheat products. Also, depending on the culture and the nature of the rice eaten, rice may be displacing less desirable foods.”
In contrast, refined grains undergo “rapid action by digestive enzymes and quick absorption from the small intestines [that] could lead to an increase in postprandial blood glucose concentrations. The rise in glucose concentrations increases the insulin concentrations, which leads to hypoglycemia, lipolysis, and the stimulation of hunger and food intake,” the authors wrote.
“It’s similar to eating sugar, or candy,” noted Dr. Mozaffarian, as refined grain “is 100% glucose.” Whole grains differ by entering the gut packaged in cell structures that slow digestion and avoid delivering sugar in an unnaturally rapid way.
“We are providing new evidence, and we hope that dietary guidelines in North America encourage individuals to lower their refined grain and sugar intake,” Dr. Dehghan said.
PURE has received partial funding with unrestricted grants from several drug companies. Dr. Dehghan had no disclosures. Dr. Mozaffarian has been an adviser to or has received personal fees from several food companies, but had no relevant disclosures.
Bariatric surgery gives 10-year cure for some advanced diabetes
A small, single-center randomized trial of patients with obesity and advanced type 2 diabetes, defined as diabetes for ≥ 5 years and A1c ≥ 7%, found that a quarter to a half of patients who had metabolic surgery had diabetes remission (cure) that lasted 5-9 years.
That is, of the 60 randomized patients, 50% who had biliopancreatic diversion and 25% who had Roux-en-Y gastric bypass – but none who had received current medical therapy – still had diabetes remission a decade later.
Until now, there had only been 5-year follow-up data from this and similar trials, Geltrude Mingrone, MD, PhD, and colleagues noted in the study published online Jan. 23 in The Lancet.
These results provide “the most robust scientific evidence yet that full-blown type 2 diabetes is a curable disease, not inevitably progressive, and irreversible,” senior author Francesco Rubino, MD, chair of bariatric and metabolic surgery at King’s College London, said in a statement from his institution.
“The results of this trial will make a noticeable difference in the field and convince even the most skeptical of clinicians about the role of metabolic surgery as part of optimal care for their patients with difficult to control type 2 diabetes,” predicted two editorialists.
Alexander D. Miras, PhD, section of metabolism, digestion, and reproduction, Imperial College London, and Carel le Roux, MBChB, PhD, of the Diabetes Complications Research Centre, University College Dublin, penned the accompanying commentary.
Patients who had metabolic surgery also had greater weight loss, reduced medication use, lower cardiovascular risk, better quality of life, and a lower incidence of diabetes-related complications compared with those who received medical therapy.
“Clinicians and policymakers should ensure that metabolic surgery is appropriately considered in the management of patients with obesity and type 2 diabetes,” advised Dr. Mingrone of King’s College London and the Catholic University of Rome, and colleagues.
“Reassuring results, will make a difference in the field”
“It is reassuring that we now have 10-year data showing greater efficacy of metabolic surgery than conventional medical therapy,” Dr. Miras and Dr. le Roux wrote in their commentary.
There were no unexpected risks associated with surgery, they noted, and the findings are consistent with those of 12 other randomized controlled trials in the past 12 years.
“New generations of diabetologists are now more open to the use of metabolic surgery for patients with suboptimal responses to medical treatments,” they wrote, rather than endlessly intensifying insulin and blaming poor response on poor compliance.
And Dr. Miras and Dr. le Roux “eagerly await” 10-year data from the 150-patient STAMPEDE trial – which is examining sleeve gastrectomy, currently the most widely performed bariatric procedure, as well as Roux-en-Y gastric bypass and medical therapy – following the 5-year results published in 2017.
Diabetes for at least 5 years, mid 40s, half on insulin
Dr. Mingrone and colleagues previously reported 5-year findings from the 60 patients with obesity and advanced diabetes who were seen in a single center in Rome and randomized to three treatments (20 in each group) in 2009-2011.
Biliopancreatic diversion “remains infrequently performed but is still considered the best operation for glycemic control,” the researchers noted.
The primary endpoint was diabetes remission at 2 years (fasting plasma glucose < 100 mg/dL [5.6 mmol/L] and A1c < 6.5%) without the need for ongoing pharmacological treatment for at least 1 year.
Patients were a mean age of 44 years and had a mean body mass index of 44 kg/m2. About half were men. They had diabetes for a mean of 5.8 years and an average A1c of 8.6%. About half were taking insulin.
Patient retention rate was high (95%) and trial outcomes were assessed by nonsurgeons.
At 10 years, patients’ mean A1c had dropped to 6.4%, 6.7%, and 7.6%, in the biliopancreatic diversion, Roux-en-Y gastric bypass, and medical therapy groups, respectively; only 2.5% of patients in the surgery groups, versus 53% in the medical therapy group, required insulin.
At study end, patients in the surgery groups had lost about 29% of their initial weight versus a weight loss of 4.2% in the medical therapy group.
First 2 years after surgery is key
“We also learnt that patients who do not go into remission after 2 years are very unlikely to ever do so,” Dr. Miras and Dr. le Roux observed, which “might help us to intensify modern and potent glucose-lowering therapies like SGLT2 inhibitors and GLP-1 receptor agonists earlier after metabolic surgery.”
Ten of 19 patients (53%) in the biliopancreatic diversion group and 10 of 15 patients (67%) in the Roux-en-Y gastric bypass group who had diabetes remission at 2 years had a diabetes relapse, but at 10 years, they all had adequate glycemic control (mean A1c 6.7%), despite drastically reduced use of diabetes medications.
The two patients who crossed over to surgery from the medical therapy group had postoperative diabetes remission, which was maintained at 10 years in one patient.
Better risk-to-benefit ratio with Roux-en-y gastric bypass
No patient in the medical therapy group had a serious adverse event, but one patient in each surgery group had deep vein thrombosis or pulmonary embolism, and one patient in the biliopancreatic diversion group had an episode of atrial fibrillation. There were no late surgical complications.
Iron deficiency and mild osteopenia occurred in both surgical groups, but were more common in the biliopancreatic diversion group. And osteoporosis, transient nyctalopia (night blindness) due to vitamin A deficiency, and kidney stones were observed only with biliopancreatic diversion.
This suggests that despite the greater antidiabetic potential of biliopancreatic diversion, Roux-en-Y gastric bypass might have a more favorable risk-to-benefit profile as a standard surgical option for the treatment of type 2 diabetes, Dr. Mingrone and colleagues concluded.
The authors and Dr. Miras have reported no relevant financial relationships. Dr. le Roux has reported receiving funding from the Science Foundation Ireland, the Health Research Board, and the Irish Research Council for type 2 diabetes research, and serves on several advisory boards outside of the scope of the current study.
A version of this article first appeared on Medscape.com.
A small, single-center randomized trial of patients with obesity and advanced type 2 diabetes, defined as diabetes for ≥ 5 years and A1c ≥ 7%, found that a quarter to a half of patients who had metabolic surgery had diabetes remission (cure) that lasted 5-9 years.
That is, of the 60 randomized patients, 50% who had biliopancreatic diversion and 25% who had Roux-en-Y gastric bypass – but none who had received current medical therapy – still had diabetes remission a decade later.
Until now, there had only been 5-year follow-up data from this and similar trials, Geltrude Mingrone, MD, PhD, and colleagues noted in the study published online Jan. 23 in The Lancet.
These results provide “the most robust scientific evidence yet that full-blown type 2 diabetes is a curable disease, not inevitably progressive, and irreversible,” senior author Francesco Rubino, MD, chair of bariatric and metabolic surgery at King’s College London, said in a statement from his institution.
“The results of this trial will make a noticeable difference in the field and convince even the most skeptical of clinicians about the role of metabolic surgery as part of optimal care for their patients with difficult to control type 2 diabetes,” predicted two editorialists.
Alexander D. Miras, PhD, section of metabolism, digestion, and reproduction, Imperial College London, and Carel le Roux, MBChB, PhD, of the Diabetes Complications Research Centre, University College Dublin, penned the accompanying commentary.
Patients who had metabolic surgery also had greater weight loss, reduced medication use, lower cardiovascular risk, better quality of life, and a lower incidence of diabetes-related complications compared with those who received medical therapy.
“Clinicians and policymakers should ensure that metabolic surgery is appropriately considered in the management of patients with obesity and type 2 diabetes,” advised Dr. Mingrone of King’s College London and the Catholic University of Rome, and colleagues.
“Reassuring results, will make a difference in the field”
“It is reassuring that we now have 10-year data showing greater efficacy of metabolic surgery than conventional medical therapy,” Dr. Miras and Dr. le Roux wrote in their commentary.
There were no unexpected risks associated with surgery, they noted, and the findings are consistent with those of 12 other randomized controlled trials in the past 12 years.
“New generations of diabetologists are now more open to the use of metabolic surgery for patients with suboptimal responses to medical treatments,” they wrote, rather than endlessly intensifying insulin and blaming poor response on poor compliance.
And Dr. Miras and Dr. le Roux “eagerly await” 10-year data from the 150-patient STAMPEDE trial – which is examining sleeve gastrectomy, currently the most widely performed bariatric procedure, as well as Roux-en-Y gastric bypass and medical therapy – following the 5-year results published in 2017.
Diabetes for at least 5 years, mid 40s, half on insulin
Dr. Mingrone and colleagues previously reported 5-year findings from the 60 patients with obesity and advanced diabetes who were seen in a single center in Rome and randomized to three treatments (20 in each group) in 2009-2011.
Biliopancreatic diversion “remains infrequently performed but is still considered the best operation for glycemic control,” the researchers noted.
The primary endpoint was diabetes remission at 2 years (fasting plasma glucose < 100 mg/dL [5.6 mmol/L] and A1c < 6.5%) without the need for ongoing pharmacological treatment for at least 1 year.
Patients were a mean age of 44 years and had a mean body mass index of 44 kg/m2. About half were men. They had diabetes for a mean of 5.8 years and an average A1c of 8.6%. About half were taking insulin.
Patient retention rate was high (95%) and trial outcomes were assessed by nonsurgeons.
At 10 years, patients’ mean A1c had dropped to 6.4%, 6.7%, and 7.6%, in the biliopancreatic diversion, Roux-en-Y gastric bypass, and medical therapy groups, respectively; only 2.5% of patients in the surgery groups, versus 53% in the medical therapy group, required insulin.
At study end, patients in the surgery groups had lost about 29% of their initial weight versus a weight loss of 4.2% in the medical therapy group.
First 2 years after surgery is key
“We also learnt that patients who do not go into remission after 2 years are very unlikely to ever do so,” Dr. Miras and Dr. le Roux observed, which “might help us to intensify modern and potent glucose-lowering therapies like SGLT2 inhibitors and GLP-1 receptor agonists earlier after metabolic surgery.”
Ten of 19 patients (53%) in the biliopancreatic diversion group and 10 of 15 patients (67%) in the Roux-en-Y gastric bypass group who had diabetes remission at 2 years had a diabetes relapse, but at 10 years, they all had adequate glycemic control (mean A1c 6.7%), despite drastically reduced use of diabetes medications.
The two patients who crossed over to surgery from the medical therapy group had postoperative diabetes remission, which was maintained at 10 years in one patient.
Better risk-to-benefit ratio with Roux-en-y gastric bypass
No patient in the medical therapy group had a serious adverse event, but one patient in each surgery group had deep vein thrombosis or pulmonary embolism, and one patient in the biliopancreatic diversion group had an episode of atrial fibrillation. There were no late surgical complications.
Iron deficiency and mild osteopenia occurred in both surgical groups, but were more common in the biliopancreatic diversion group. And osteoporosis, transient nyctalopia (night blindness) due to vitamin A deficiency, and kidney stones were observed only with biliopancreatic diversion.
This suggests that despite the greater antidiabetic potential of biliopancreatic diversion, Roux-en-Y gastric bypass might have a more favorable risk-to-benefit profile as a standard surgical option for the treatment of type 2 diabetes, Dr. Mingrone and colleagues concluded.
The authors and Dr. Miras have reported no relevant financial relationships. Dr. le Roux has reported receiving funding from the Science Foundation Ireland, the Health Research Board, and the Irish Research Council for type 2 diabetes research, and serves on several advisory boards outside of the scope of the current study.
A version of this article first appeared on Medscape.com.
A small, single-center randomized trial of patients with obesity and advanced type 2 diabetes, defined as diabetes for ≥ 5 years and A1c ≥ 7%, found that a quarter to a half of patients who had metabolic surgery had diabetes remission (cure) that lasted 5-9 years.
That is, of the 60 randomized patients, 50% who had biliopancreatic diversion and 25% who had Roux-en-Y gastric bypass – but none who had received current medical therapy – still had diabetes remission a decade later.
Until now, there had only been 5-year follow-up data from this and similar trials, Geltrude Mingrone, MD, PhD, and colleagues noted in the study published online Jan. 23 in The Lancet.
These results provide “the most robust scientific evidence yet that full-blown type 2 diabetes is a curable disease, not inevitably progressive, and irreversible,” senior author Francesco Rubino, MD, chair of bariatric and metabolic surgery at King’s College London, said in a statement from his institution.
“The results of this trial will make a noticeable difference in the field and convince even the most skeptical of clinicians about the role of metabolic surgery as part of optimal care for their patients with difficult to control type 2 diabetes,” predicted two editorialists.
Alexander D. Miras, PhD, section of metabolism, digestion, and reproduction, Imperial College London, and Carel le Roux, MBChB, PhD, of the Diabetes Complications Research Centre, University College Dublin, penned the accompanying commentary.
Patients who had metabolic surgery also had greater weight loss, reduced medication use, lower cardiovascular risk, better quality of life, and a lower incidence of diabetes-related complications compared with those who received medical therapy.
“Clinicians and policymakers should ensure that metabolic surgery is appropriately considered in the management of patients with obesity and type 2 diabetes,” advised Dr. Mingrone of King’s College London and the Catholic University of Rome, and colleagues.
“Reassuring results, will make a difference in the field”
“It is reassuring that we now have 10-year data showing greater efficacy of metabolic surgery than conventional medical therapy,” Dr. Miras and Dr. le Roux wrote in their commentary.
There were no unexpected risks associated with surgery, they noted, and the findings are consistent with those of 12 other randomized controlled trials in the past 12 years.
“New generations of diabetologists are now more open to the use of metabolic surgery for patients with suboptimal responses to medical treatments,” they wrote, rather than endlessly intensifying insulin and blaming poor response on poor compliance.
And Dr. Miras and Dr. le Roux “eagerly await” 10-year data from the 150-patient STAMPEDE trial – which is examining sleeve gastrectomy, currently the most widely performed bariatric procedure, as well as Roux-en-Y gastric bypass and medical therapy – following the 5-year results published in 2017.
Diabetes for at least 5 years, mid 40s, half on insulin
Dr. Mingrone and colleagues previously reported 5-year findings from the 60 patients with obesity and advanced diabetes who were seen in a single center in Rome and randomized to three treatments (20 in each group) in 2009-2011.
Biliopancreatic diversion “remains infrequently performed but is still considered the best operation for glycemic control,” the researchers noted.
The primary endpoint was diabetes remission at 2 years (fasting plasma glucose < 100 mg/dL [5.6 mmol/L] and A1c < 6.5%) without the need for ongoing pharmacological treatment for at least 1 year.
Patients were a mean age of 44 years and had a mean body mass index of 44 kg/m2. About half were men. They had diabetes for a mean of 5.8 years and an average A1c of 8.6%. About half were taking insulin.
Patient retention rate was high (95%) and trial outcomes were assessed by nonsurgeons.
At 10 years, patients’ mean A1c had dropped to 6.4%, 6.7%, and 7.6%, in the biliopancreatic diversion, Roux-en-Y gastric bypass, and medical therapy groups, respectively; only 2.5% of patients in the surgery groups, versus 53% in the medical therapy group, required insulin.
At study end, patients in the surgery groups had lost about 29% of their initial weight versus a weight loss of 4.2% in the medical therapy group.
First 2 years after surgery is key
“We also learnt that patients who do not go into remission after 2 years are very unlikely to ever do so,” Dr. Miras and Dr. le Roux observed, which “might help us to intensify modern and potent glucose-lowering therapies like SGLT2 inhibitors and GLP-1 receptor agonists earlier after metabolic surgery.”
Ten of 19 patients (53%) in the biliopancreatic diversion group and 10 of 15 patients (67%) in the Roux-en-Y gastric bypass group who had diabetes remission at 2 years had a diabetes relapse, but at 10 years, they all had adequate glycemic control (mean A1c 6.7%), despite drastically reduced use of diabetes medications.
The two patients who crossed over to surgery from the medical therapy group had postoperative diabetes remission, which was maintained at 10 years in one patient.
Better risk-to-benefit ratio with Roux-en-y gastric bypass
No patient in the medical therapy group had a serious adverse event, but one patient in each surgery group had deep vein thrombosis or pulmonary embolism, and one patient in the biliopancreatic diversion group had an episode of atrial fibrillation. There were no late surgical complications.
Iron deficiency and mild osteopenia occurred in both surgical groups, but were more common in the biliopancreatic diversion group. And osteoporosis, transient nyctalopia (night blindness) due to vitamin A deficiency, and kidney stones were observed only with biliopancreatic diversion.
This suggests that despite the greater antidiabetic potential of biliopancreatic diversion, Roux-en-Y gastric bypass might have a more favorable risk-to-benefit profile as a standard surgical option for the treatment of type 2 diabetes, Dr. Mingrone and colleagues concluded.
The authors and Dr. Miras have reported no relevant financial relationships. Dr. le Roux has reported receiving funding from the Science Foundation Ireland, the Health Research Board, and the Irish Research Council for type 2 diabetes research, and serves on several advisory boards outside of the scope of the current study.
A version of this article first appeared on Medscape.com.
Plant-based or keto diet? Novel study yields surprising results
For appetite control, a low-fat, plant-based diet has advantages over a low-carbohydrate, animal-based ketogenic diet, although the keto diet wins when it comes to keeping post-meal glucose and insulin levels in check, new research suggests.
In a highly controlled crossover study conducted at the National Institutes of Health, people consumed fewer daily calories when on a low-fat, plant-based diet, but their insulin and blood glucose levels were higher than when they followed a low-carbohydrate, animal-based diet.
“There is this somewhat-outdated idea now that higher-fat diets, because they have more calories per gram, tend to make people overeat – something called the passive overconsumption model,” senior investigator Kevin Hall, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, said in an interview.
The other more popular model these days, he explained, is the carbohydrate-insulin model, which holds that following a diet high in carbohydrates and sugar that causes insulin levels to spike will increase hunger and cause a person to overeat.
In this study, Dr. Hall and colleagues tested these two hypotheses head to head.
“The short answer is that we got exactly the opposite predictions from the carbohydrate-insulin model of obesity. In other words, instead of making people eat more and gaining weight and body fat, they actually ended up eating less on that diet and losing body fat compared to the higher-fat diet,” Dr. Hall said.
“Yet, the passive overconsumption model also failed, because despite them eating a very energy-dense diet and high fat, they didn’t gain weight and gain body fat. And so both of these models of why people overeat and gain weight seem to be inadequate in our study,” he said. “This suggests that things are a little bit more complicated.”
The study was published online Jan. 21, 2021 in Nature Medicine.
Pros and cons to both diets
For the study, the researchers housed 20 healthy adults who did not have diabetes for 4 continuous weeks at the NIH Clinical Center. The mean age of the participants was 29.9 years, and the mean body mass index was 27.8 kg/m2.
The participants were randomly allocated to consume ad libitum either a plant-based, low-fat diet (10.3% fat, 75.2% carbohydrate) with low-energy density (about 1 kcal/g−1), or an animal-based, ketogenic, low-carbohydrate diet (75.8% fat, 10.0% carbohydrate) with high energy density (about 2 kcal/g−1) for 2 weeks. They then crossed over to the alternate diet for 2 weeks.
Both diets contained about 14% protein and were matched for total calories, although the low-carb diet had twice as many calories per gram of food than the low-fat diet. Participants could eat what and however much they chose of the meals they were given.
One participant withdrew, owing to hypoglycemia during the low-carbohydrate diet phase. For the primary outcome, the researchers compared mean daily ad libitum energy intake between each 2-week diet period.
They found that energy intake from the low-fat diet was reduced by approximately 550-700 kcal/d−1, compared with the low-carbohydrate keto diet. Yet, despite the large differences in calorie intake, participants reported no differences in hunger, enjoyment of meals, or fullness between the two diets.
Participants lost weight on both diets (about 1-2 kg on average), but only the low-fat diet led to a significant loss of body fat.
“Interestingly, our findings suggest benefits to both diets, at least in the short term,” Dr. Hall said in a news release.
“While the low-fat, plant-based diet helps curb appetite, the animal-based, low-carb diet resulted in lower and more steady insulin and glucose levels. We don’t yet know if these differences would be sustained over the long term,” he said.
Dr. Hall added that it’s important to note that the study was not designed to make diet recommendations for weight loss, and the results might have been different had the participants been actively trying to lose weight.
“In fact, they didn’t even know what the study was about; we just said we want you to eat the two diets, and we’re going to see what happens in your body either as you eat as much or as little as you want,” he said.
“It’s a bit of a mixed bag in terms of which diet might be better for an individual. I think you can interpret this study as that there are positives and negatives for both diets,” Dr. Hall said.
Diet ‘tribes’
In a comment, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said it’s important to note that “a ‘low-carb diet’ has yet to be defined, and many definitions exist.
“We really need a standard definition of what constitutes ‘low-carb’ so that studies can be designed and evaluated in a consistent manner. It’s problematic because, without a standard definition, the ‘diet tribe’ researchers (keto versus plant-based) always seem to find the answer that is in their own favor,” Dr. Wallace said. “This study does seem to use less than 20 grams of carbs per day, which in my mind is pretty low carb.”
Perhaps the most important caveat, he added, is that, in the real world, “most people don’t adhere to these very strict diets – not even for 2 weeks.”
The study was supported by the NIDDK Intramural Research Program, with additional NIH support from a National Institute of Nursing Research grant. One author has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. Dr. Hall and the other authors disclosed no relevant financial relationships. Dr. Wallace is principal and CEO of the Think Healthy Group, editor of the Journal of Dietary Supplements, and deputy editor of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
For appetite control, a low-fat, plant-based diet has advantages over a low-carbohydrate, animal-based ketogenic diet, although the keto diet wins when it comes to keeping post-meal glucose and insulin levels in check, new research suggests.
In a highly controlled crossover study conducted at the National Institutes of Health, people consumed fewer daily calories when on a low-fat, plant-based diet, but their insulin and blood glucose levels were higher than when they followed a low-carbohydrate, animal-based diet.
“There is this somewhat-outdated idea now that higher-fat diets, because they have more calories per gram, tend to make people overeat – something called the passive overconsumption model,” senior investigator Kevin Hall, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, said in an interview.
The other more popular model these days, he explained, is the carbohydrate-insulin model, which holds that following a diet high in carbohydrates and sugar that causes insulin levels to spike will increase hunger and cause a person to overeat.
In this study, Dr. Hall and colleagues tested these two hypotheses head to head.
“The short answer is that we got exactly the opposite predictions from the carbohydrate-insulin model of obesity. In other words, instead of making people eat more and gaining weight and body fat, they actually ended up eating less on that diet and losing body fat compared to the higher-fat diet,” Dr. Hall said.
“Yet, the passive overconsumption model also failed, because despite them eating a very energy-dense diet and high fat, they didn’t gain weight and gain body fat. And so both of these models of why people overeat and gain weight seem to be inadequate in our study,” he said. “This suggests that things are a little bit more complicated.”
The study was published online Jan. 21, 2021 in Nature Medicine.
Pros and cons to both diets
For the study, the researchers housed 20 healthy adults who did not have diabetes for 4 continuous weeks at the NIH Clinical Center. The mean age of the participants was 29.9 years, and the mean body mass index was 27.8 kg/m2.
The participants were randomly allocated to consume ad libitum either a plant-based, low-fat diet (10.3% fat, 75.2% carbohydrate) with low-energy density (about 1 kcal/g−1), or an animal-based, ketogenic, low-carbohydrate diet (75.8% fat, 10.0% carbohydrate) with high energy density (about 2 kcal/g−1) for 2 weeks. They then crossed over to the alternate diet for 2 weeks.
Both diets contained about 14% protein and were matched for total calories, although the low-carb diet had twice as many calories per gram of food than the low-fat diet. Participants could eat what and however much they chose of the meals they were given.
One participant withdrew, owing to hypoglycemia during the low-carbohydrate diet phase. For the primary outcome, the researchers compared mean daily ad libitum energy intake between each 2-week diet period.
They found that energy intake from the low-fat diet was reduced by approximately 550-700 kcal/d−1, compared with the low-carbohydrate keto diet. Yet, despite the large differences in calorie intake, participants reported no differences in hunger, enjoyment of meals, or fullness between the two diets.
Participants lost weight on both diets (about 1-2 kg on average), but only the low-fat diet led to a significant loss of body fat.
“Interestingly, our findings suggest benefits to both diets, at least in the short term,” Dr. Hall said in a news release.
“While the low-fat, plant-based diet helps curb appetite, the animal-based, low-carb diet resulted in lower and more steady insulin and glucose levels. We don’t yet know if these differences would be sustained over the long term,” he said.
Dr. Hall added that it’s important to note that the study was not designed to make diet recommendations for weight loss, and the results might have been different had the participants been actively trying to lose weight.
“In fact, they didn’t even know what the study was about; we just said we want you to eat the two diets, and we’re going to see what happens in your body either as you eat as much or as little as you want,” he said.
“It’s a bit of a mixed bag in terms of which diet might be better for an individual. I think you can interpret this study as that there are positives and negatives for both diets,” Dr. Hall said.
Diet ‘tribes’
In a comment, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said it’s important to note that “a ‘low-carb diet’ has yet to be defined, and many definitions exist.
“We really need a standard definition of what constitutes ‘low-carb’ so that studies can be designed and evaluated in a consistent manner. It’s problematic because, without a standard definition, the ‘diet tribe’ researchers (keto versus plant-based) always seem to find the answer that is in their own favor,” Dr. Wallace said. “This study does seem to use less than 20 grams of carbs per day, which in my mind is pretty low carb.”
Perhaps the most important caveat, he added, is that, in the real world, “most people don’t adhere to these very strict diets – not even for 2 weeks.”
The study was supported by the NIDDK Intramural Research Program, with additional NIH support from a National Institute of Nursing Research grant. One author has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. Dr. Hall and the other authors disclosed no relevant financial relationships. Dr. Wallace is principal and CEO of the Think Healthy Group, editor of the Journal of Dietary Supplements, and deputy editor of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
For appetite control, a low-fat, plant-based diet has advantages over a low-carbohydrate, animal-based ketogenic diet, although the keto diet wins when it comes to keeping post-meal glucose and insulin levels in check, new research suggests.
In a highly controlled crossover study conducted at the National Institutes of Health, people consumed fewer daily calories when on a low-fat, plant-based diet, but their insulin and blood glucose levels were higher than when they followed a low-carbohydrate, animal-based diet.
“There is this somewhat-outdated idea now that higher-fat diets, because they have more calories per gram, tend to make people overeat – something called the passive overconsumption model,” senior investigator Kevin Hall, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, said in an interview.
The other more popular model these days, he explained, is the carbohydrate-insulin model, which holds that following a diet high in carbohydrates and sugar that causes insulin levels to spike will increase hunger and cause a person to overeat.
In this study, Dr. Hall and colleagues tested these two hypotheses head to head.
“The short answer is that we got exactly the opposite predictions from the carbohydrate-insulin model of obesity. In other words, instead of making people eat more and gaining weight and body fat, they actually ended up eating less on that diet and losing body fat compared to the higher-fat diet,” Dr. Hall said.
“Yet, the passive overconsumption model also failed, because despite them eating a very energy-dense diet and high fat, they didn’t gain weight and gain body fat. And so both of these models of why people overeat and gain weight seem to be inadequate in our study,” he said. “This suggests that things are a little bit more complicated.”
The study was published online Jan. 21, 2021 in Nature Medicine.
Pros and cons to both diets
For the study, the researchers housed 20 healthy adults who did not have diabetes for 4 continuous weeks at the NIH Clinical Center. The mean age of the participants was 29.9 years, and the mean body mass index was 27.8 kg/m2.
The participants were randomly allocated to consume ad libitum either a plant-based, low-fat diet (10.3% fat, 75.2% carbohydrate) with low-energy density (about 1 kcal/g−1), or an animal-based, ketogenic, low-carbohydrate diet (75.8% fat, 10.0% carbohydrate) with high energy density (about 2 kcal/g−1) for 2 weeks. They then crossed over to the alternate diet for 2 weeks.
Both diets contained about 14% protein and were matched for total calories, although the low-carb diet had twice as many calories per gram of food than the low-fat diet. Participants could eat what and however much they chose of the meals they were given.
One participant withdrew, owing to hypoglycemia during the low-carbohydrate diet phase. For the primary outcome, the researchers compared mean daily ad libitum energy intake between each 2-week diet period.
They found that energy intake from the low-fat diet was reduced by approximately 550-700 kcal/d−1, compared with the low-carbohydrate keto diet. Yet, despite the large differences in calorie intake, participants reported no differences in hunger, enjoyment of meals, or fullness between the two diets.
Participants lost weight on both diets (about 1-2 kg on average), but only the low-fat diet led to a significant loss of body fat.
“Interestingly, our findings suggest benefits to both diets, at least in the short term,” Dr. Hall said in a news release.
“While the low-fat, plant-based diet helps curb appetite, the animal-based, low-carb diet resulted in lower and more steady insulin and glucose levels. We don’t yet know if these differences would be sustained over the long term,” he said.
Dr. Hall added that it’s important to note that the study was not designed to make diet recommendations for weight loss, and the results might have been different had the participants been actively trying to lose weight.
“In fact, they didn’t even know what the study was about; we just said we want you to eat the two diets, and we’re going to see what happens in your body either as you eat as much or as little as you want,” he said.
“It’s a bit of a mixed bag in terms of which diet might be better for an individual. I think you can interpret this study as that there are positives and negatives for both diets,” Dr. Hall said.
Diet ‘tribes’
In a comment, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said it’s important to note that “a ‘low-carb diet’ has yet to be defined, and many definitions exist.
“We really need a standard definition of what constitutes ‘low-carb’ so that studies can be designed and evaluated in a consistent manner. It’s problematic because, without a standard definition, the ‘diet tribe’ researchers (keto versus plant-based) always seem to find the answer that is in their own favor,” Dr. Wallace said. “This study does seem to use less than 20 grams of carbs per day, which in my mind is pretty low carb.”
Perhaps the most important caveat, he added, is that, in the real world, “most people don’t adhere to these very strict diets – not even for 2 weeks.”
The study was supported by the NIDDK Intramural Research Program, with additional NIH support from a National Institute of Nursing Research grant. One author has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. Dr. Hall and the other authors disclosed no relevant financial relationships. Dr. Wallace is principal and CEO of the Think Healthy Group, editor of the Journal of Dietary Supplements, and deputy editor of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
Gestational diabetes carries CVD risk years later
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
FROM CIRCULATION
Incidence of autoimmune hepatitis may be rising
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Protecting patients with diabetes from impact of COVID-19
Experts discuss how to best protect people with diabetes from serious COVID-19 outcomes in a newly published article that summarizes in-depth discussions on the topic from a conference held online last year.
Lead author and Diabetes Technology Society founder and director David C. Klonoff, MD, said in an interview: “To my knowledge this is the largest article or learning that has been written anywhere ever about the co-occurrence of COVID-19 and diabetes and how COVID-19 affects diabetes ... There are a lot of different dimensions.”
The 37-page report covers all sessions from the Virtual International COVID-19 and Diabetes Summit, held Aug. 26-27, 2020, which had 800 attendees from six continents, on topics including pathophysiology and COVID-19 risk factors, the impact of social determinants of health on diabetes and COVID-19, and psychological aspects of the COVID-19 pandemic for people with diabetes.
The freely available report was published online Jan. 21 in the Journal of Diabetes Science and Technology by Jennifer Y. Zhang of the Diabetes Technology Society, Burlingame, Calif., and colleagues.
Other topics include medications and vaccines, outpatient diabetes management during the COVID-19 pandemic and the growth of telehealth, inpatient management of diabetes in patients with or without COVID-19, ethical considerations, children, pregnancy, economics of care for COVID-19, government policy, regulation of tests and treatments, patient surveillance/privacy, and research gaps and opportunities.
“A comprehensive report like this is so important because it covers such a wide range of topics that are all relevant when it comes to protecting patients with diabetes during a pandemic. Our report aims to bring together all these different aspects of policy during the pandemic, patient physiology, and patient psychology, so I hope it will be widely read and widely appreciated,” Ms. Zhang said in an interview.
Two important clinical trends arising as a result of the pandemic – the advent of telehealth in diabetes management and the use of continuous glucose monitoring (CGM) in hospital – are expected to continue even after COVID-19 abates, said Dr. Klonoff, medical director of the Diabetes Research Institute at Mills-Peninsula Medical Center, San Mateo, Calif.
Telehealth in diabetes here to stay, in U.S. at least
Dr. Klonoff noted that with diabetes telehealth, or “telediabetes” as it’s been dubbed, by using downloaded device data patients don’t have to travel, pay for parking, or take as much time off work. “There are advantages ... patients really like it,” he said.
And for health care providers, an advantage of remote visits is that the clinician can look at the patient while reviewing the patient’s data. “With telehealth for diabetes, the patient’s face and the software data are right next to each other on the same screen. Even as I’m typing I’m looking at the patient ... I consider that a huge advantage,” Dr. Klonoff said.
Rule changes early in the pandemic made the shift to telehealth in the United States possible, he said.
“Fortunately, Medicare and other payers are covering telehealth. It used to be there was no coverage, so that was a damper. Now that it’s covered I don’t think that’s going to go back. Everybody likes it,” he said.
CGM in hospitals helps detect hypoglycemia on wards
Regarding the increase of inpatient CGM (continuous glucose monitoring) prompted by the need to minimize patient exposure of nursing staff during the pandemic and the relaxing of Food and Drug Administration rules about its use, Dr. Klonoff said this phenomenon has led to two other positive developments.
“For FDA, it’s actually an opportunity to see some data collected. To do a clinical trial [prior to] March 2020 you had to go through a lot of processes to do a study. Once it becomes part of clinical care, then you can collect a lot of data,” he noted.
Moreover, Dr. Klonoff said there’s an important new area where hospital use of CGM is emerging: detection of hypoglycemia on wards.
“When a patient is in the ICU, if they become hypoglycemic or hyperglycemic it will likely be detected. But on the wards, they simply don’t get the same attention. Just about every doctor has had a case where somebody drifted into hypoglycemia that wasn’t recognized and maybe even died,” he explained.
If, however, “patients treated with insulin could all have CGMs that would be so useful. It would send out an alarm. A lot of times people don’t eat when you think they will. Suddenly the insulin dose is inappropriate and the nurse didn’t realize. Or, if IV nutrition stops and the insulin is given [it can be harmful].”
Another example, he said, is a common scenario when insulin is used in patients who are treated with steroids. “They need insulin, but then the steroid is decreased and the insulin dose isn’t decreased fast enough. All those situations can be helped with CGM.”
Overall, he concluded, COVID-19 has provided many lessons, which are “expanding our horizons.”
Ms. Zhang has reported no relevant financial relationships. Dr. Klonoff has reported being a consultant for Dexcom, EOFlow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, Samsung, and Thirdwayv.
A version of this article first appeared on Medscape.com.
Experts discuss how to best protect people with diabetes from serious COVID-19 outcomes in a newly published article that summarizes in-depth discussions on the topic from a conference held online last year.
Lead author and Diabetes Technology Society founder and director David C. Klonoff, MD, said in an interview: “To my knowledge this is the largest article or learning that has been written anywhere ever about the co-occurrence of COVID-19 and diabetes and how COVID-19 affects diabetes ... There are a lot of different dimensions.”
The 37-page report covers all sessions from the Virtual International COVID-19 and Diabetes Summit, held Aug. 26-27, 2020, which had 800 attendees from six continents, on topics including pathophysiology and COVID-19 risk factors, the impact of social determinants of health on diabetes and COVID-19, and psychological aspects of the COVID-19 pandemic for people with diabetes.
The freely available report was published online Jan. 21 in the Journal of Diabetes Science and Technology by Jennifer Y. Zhang of the Diabetes Technology Society, Burlingame, Calif., and colleagues.
Other topics include medications and vaccines, outpatient diabetes management during the COVID-19 pandemic and the growth of telehealth, inpatient management of diabetes in patients with or without COVID-19, ethical considerations, children, pregnancy, economics of care for COVID-19, government policy, regulation of tests and treatments, patient surveillance/privacy, and research gaps and opportunities.
“A comprehensive report like this is so important because it covers such a wide range of topics that are all relevant when it comes to protecting patients with diabetes during a pandemic. Our report aims to bring together all these different aspects of policy during the pandemic, patient physiology, and patient psychology, so I hope it will be widely read and widely appreciated,” Ms. Zhang said in an interview.
Two important clinical trends arising as a result of the pandemic – the advent of telehealth in diabetes management and the use of continuous glucose monitoring (CGM) in hospital – are expected to continue even after COVID-19 abates, said Dr. Klonoff, medical director of the Diabetes Research Institute at Mills-Peninsula Medical Center, San Mateo, Calif.
Telehealth in diabetes here to stay, in U.S. at least
Dr. Klonoff noted that with diabetes telehealth, or “telediabetes” as it’s been dubbed, by using downloaded device data patients don’t have to travel, pay for parking, or take as much time off work. “There are advantages ... patients really like it,” he said.
And for health care providers, an advantage of remote visits is that the clinician can look at the patient while reviewing the patient’s data. “With telehealth for diabetes, the patient’s face and the software data are right next to each other on the same screen. Even as I’m typing I’m looking at the patient ... I consider that a huge advantage,” Dr. Klonoff said.
Rule changes early in the pandemic made the shift to telehealth in the United States possible, he said.
“Fortunately, Medicare and other payers are covering telehealth. It used to be there was no coverage, so that was a damper. Now that it’s covered I don’t think that’s going to go back. Everybody likes it,” he said.
CGM in hospitals helps detect hypoglycemia on wards
Regarding the increase of inpatient CGM (continuous glucose monitoring) prompted by the need to minimize patient exposure of nursing staff during the pandemic and the relaxing of Food and Drug Administration rules about its use, Dr. Klonoff said this phenomenon has led to two other positive developments.
“For FDA, it’s actually an opportunity to see some data collected. To do a clinical trial [prior to] March 2020 you had to go through a lot of processes to do a study. Once it becomes part of clinical care, then you can collect a lot of data,” he noted.
Moreover, Dr. Klonoff said there’s an important new area where hospital use of CGM is emerging: detection of hypoglycemia on wards.
“When a patient is in the ICU, if they become hypoglycemic or hyperglycemic it will likely be detected. But on the wards, they simply don’t get the same attention. Just about every doctor has had a case where somebody drifted into hypoglycemia that wasn’t recognized and maybe even died,” he explained.
If, however, “patients treated with insulin could all have CGMs that would be so useful. It would send out an alarm. A lot of times people don’t eat when you think they will. Suddenly the insulin dose is inappropriate and the nurse didn’t realize. Or, if IV nutrition stops and the insulin is given [it can be harmful].”
Another example, he said, is a common scenario when insulin is used in patients who are treated with steroids. “They need insulin, but then the steroid is decreased and the insulin dose isn’t decreased fast enough. All those situations can be helped with CGM.”
Overall, he concluded, COVID-19 has provided many lessons, which are “expanding our horizons.”
Ms. Zhang has reported no relevant financial relationships. Dr. Klonoff has reported being a consultant for Dexcom, EOFlow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, Samsung, and Thirdwayv.
A version of this article first appeared on Medscape.com.
Experts discuss how to best protect people with diabetes from serious COVID-19 outcomes in a newly published article that summarizes in-depth discussions on the topic from a conference held online last year.
Lead author and Diabetes Technology Society founder and director David C. Klonoff, MD, said in an interview: “To my knowledge this is the largest article or learning that has been written anywhere ever about the co-occurrence of COVID-19 and diabetes and how COVID-19 affects diabetes ... There are a lot of different dimensions.”
The 37-page report covers all sessions from the Virtual International COVID-19 and Diabetes Summit, held Aug. 26-27, 2020, which had 800 attendees from six continents, on topics including pathophysiology and COVID-19 risk factors, the impact of social determinants of health on diabetes and COVID-19, and psychological aspects of the COVID-19 pandemic for people with diabetes.
The freely available report was published online Jan. 21 in the Journal of Diabetes Science and Technology by Jennifer Y. Zhang of the Diabetes Technology Society, Burlingame, Calif., and colleagues.
Other topics include medications and vaccines, outpatient diabetes management during the COVID-19 pandemic and the growth of telehealth, inpatient management of diabetes in patients with or without COVID-19, ethical considerations, children, pregnancy, economics of care for COVID-19, government policy, regulation of tests and treatments, patient surveillance/privacy, and research gaps and opportunities.
“A comprehensive report like this is so important because it covers such a wide range of topics that are all relevant when it comes to protecting patients with diabetes during a pandemic. Our report aims to bring together all these different aspects of policy during the pandemic, patient physiology, and patient psychology, so I hope it will be widely read and widely appreciated,” Ms. Zhang said in an interview.
Two important clinical trends arising as a result of the pandemic – the advent of telehealth in diabetes management and the use of continuous glucose monitoring (CGM) in hospital – are expected to continue even after COVID-19 abates, said Dr. Klonoff, medical director of the Diabetes Research Institute at Mills-Peninsula Medical Center, San Mateo, Calif.
Telehealth in diabetes here to stay, in U.S. at least
Dr. Klonoff noted that with diabetes telehealth, or “telediabetes” as it’s been dubbed, by using downloaded device data patients don’t have to travel, pay for parking, or take as much time off work. “There are advantages ... patients really like it,” he said.
And for health care providers, an advantage of remote visits is that the clinician can look at the patient while reviewing the patient’s data. “With telehealth for diabetes, the patient’s face and the software data are right next to each other on the same screen. Even as I’m typing I’m looking at the patient ... I consider that a huge advantage,” Dr. Klonoff said.
Rule changes early in the pandemic made the shift to telehealth in the United States possible, he said.
“Fortunately, Medicare and other payers are covering telehealth. It used to be there was no coverage, so that was a damper. Now that it’s covered I don’t think that’s going to go back. Everybody likes it,” he said.
CGM in hospitals helps detect hypoglycemia on wards
Regarding the increase of inpatient CGM (continuous glucose monitoring) prompted by the need to minimize patient exposure of nursing staff during the pandemic and the relaxing of Food and Drug Administration rules about its use, Dr. Klonoff said this phenomenon has led to two other positive developments.
“For FDA, it’s actually an opportunity to see some data collected. To do a clinical trial [prior to] March 2020 you had to go through a lot of processes to do a study. Once it becomes part of clinical care, then you can collect a lot of data,” he noted.
Moreover, Dr. Klonoff said there’s an important new area where hospital use of CGM is emerging: detection of hypoglycemia on wards.
“When a patient is in the ICU, if they become hypoglycemic or hyperglycemic it will likely be detected. But on the wards, they simply don’t get the same attention. Just about every doctor has had a case where somebody drifted into hypoglycemia that wasn’t recognized and maybe even died,” he explained.
If, however, “patients treated with insulin could all have CGMs that would be so useful. It would send out an alarm. A lot of times people don’t eat when you think they will. Suddenly the insulin dose is inappropriate and the nurse didn’t realize. Or, if IV nutrition stops and the insulin is given [it can be harmful].”
Another example, he said, is a common scenario when insulin is used in patients who are treated with steroids. “They need insulin, but then the steroid is decreased and the insulin dose isn’t decreased fast enough. All those situations can be helped with CGM.”
Overall, he concluded, COVID-19 has provided many lessons, which are “expanding our horizons.”
Ms. Zhang has reported no relevant financial relationships. Dr. Klonoff has reported being a consultant for Dexcom, EOFlow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, Samsung, and Thirdwayv.
A version of this article first appeared on Medscape.com.