User login
Racial Disparities in the Diagnosis of Psoriasis
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.
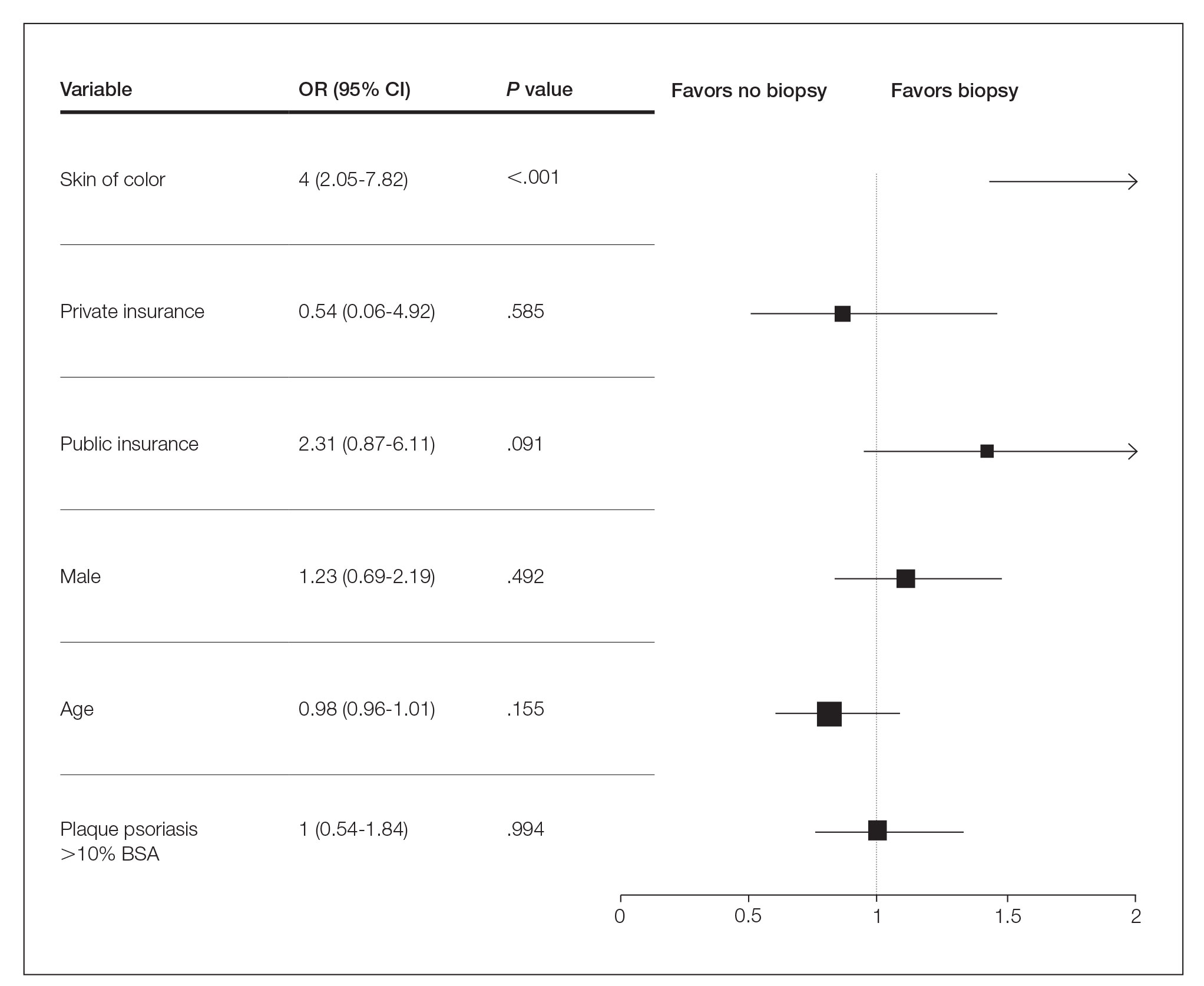
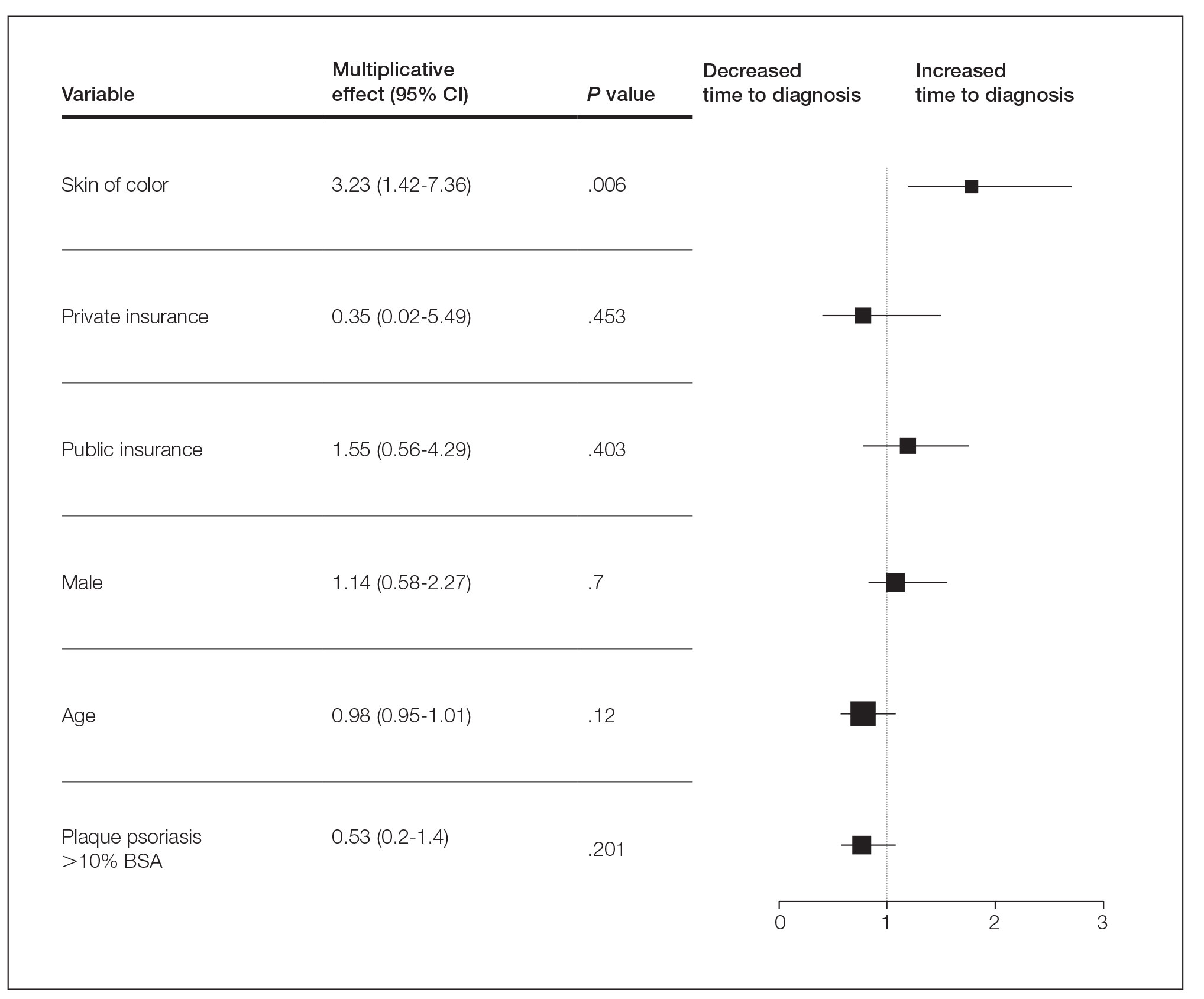
Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).
Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
Practice Points
- Skin of color (SOC) patients can wait 3 times longer to receive a diagnosis of psoriasis than non-SOC patients.
- Patients with SOC more often present with severe forms of psoriasis and are more likely to have palmoplantar psoriasis.
- Skin of color patients can be 4 times as likely to require a biopsy to confirm psoriasis diagnosis compared to non-SOC patients.
Funding of cosmetic clinical trials linked to racial/ethnic disparity
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.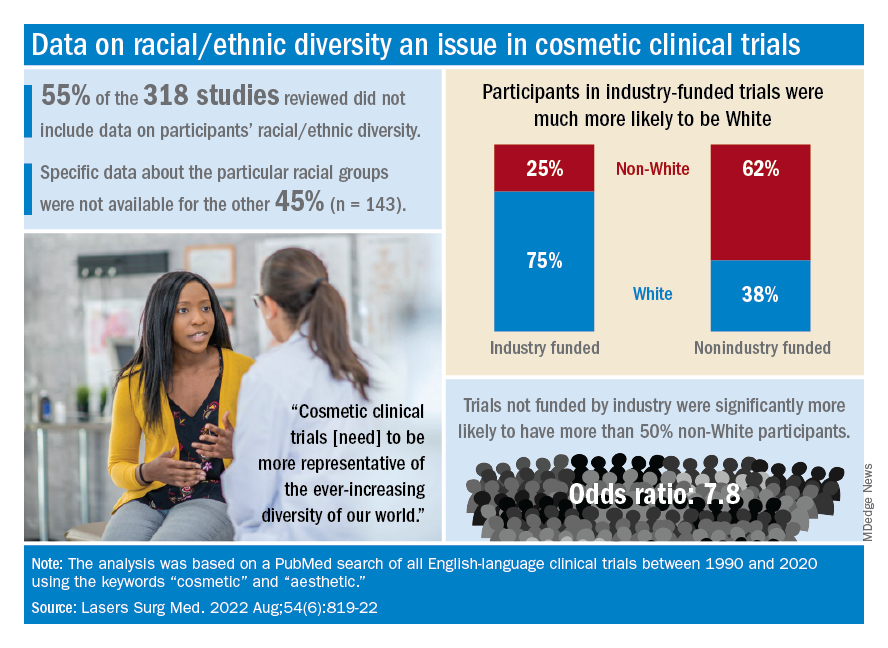
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
FROM LASERS IN SURGERY AND MEDICINE
Differences in Underrepresented in Medicine Applicant Backgrounds and Outcomes in the 2020-2021 Dermatology Residency Match
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.
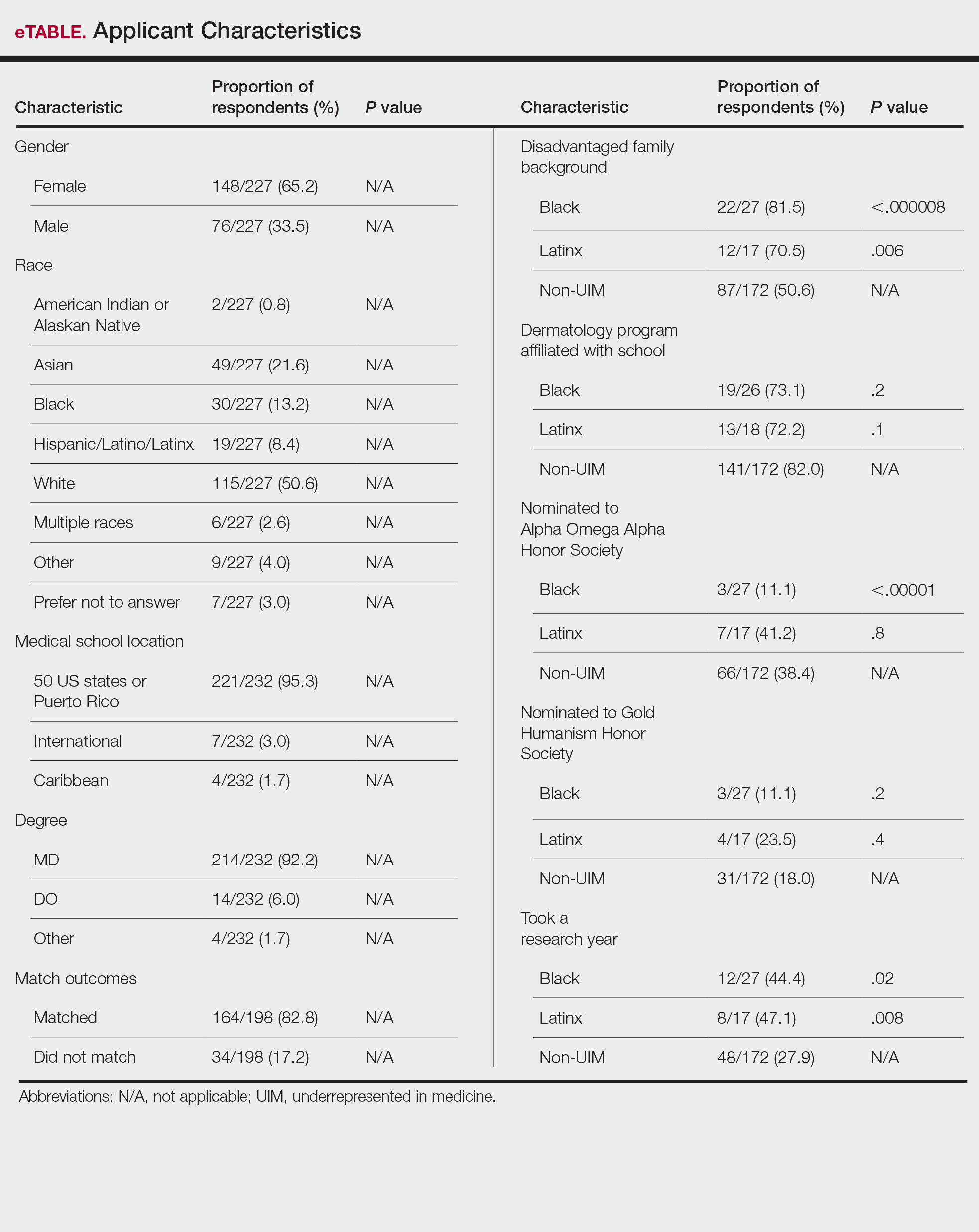
Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.
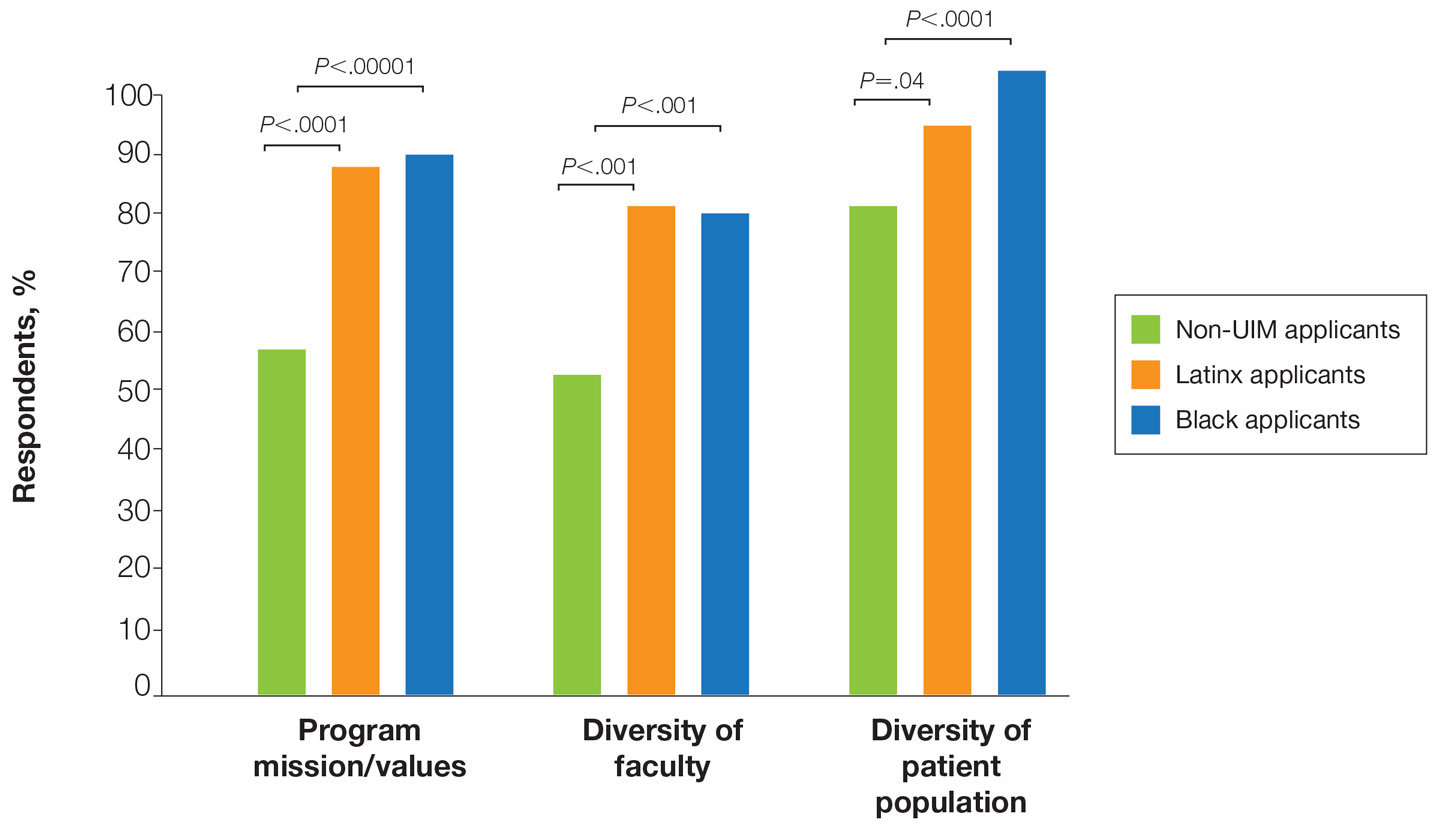
There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).
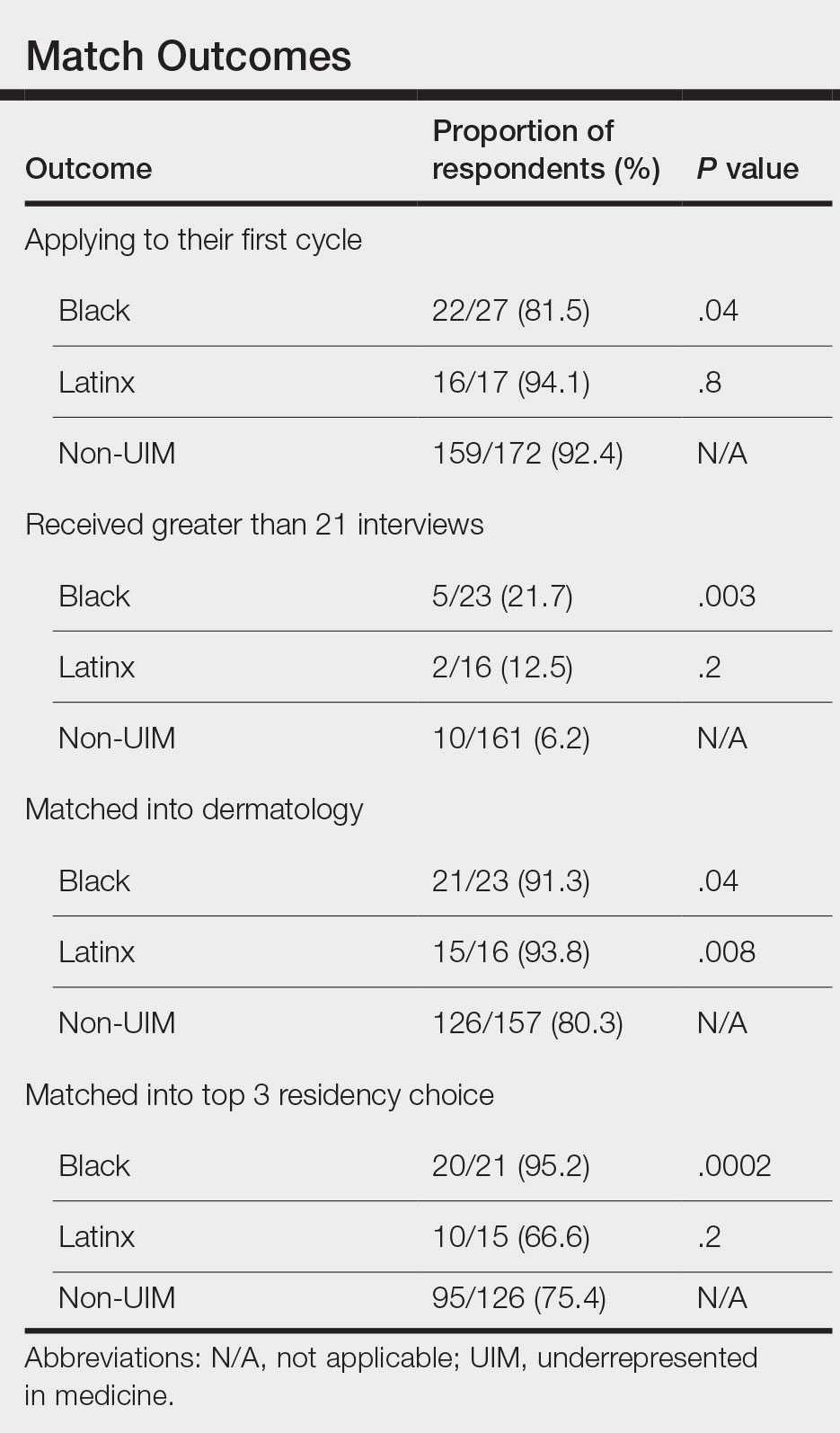
In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.

Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.

There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).

In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.

Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.

There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).

In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
Practice Points
- Underrepresented in medicine (UIM) dermatology residency applicants (Black and Latinx) are more likely to come from disadvantaged backgrounds and to have financial concerns about the residency application process.
- When choosing a dermatology residency program, diversity of patients and faculty are more important to UIM dermatology residency applicants than to their non-UIM counterparts.
- Increased awareness of and focus on a holistic review process by dermatology residency programs may contribute to higher rates of matching among Black applicants in our study.
Pigmented Papules on the Face, Neck, and Chest
The Diagnosis: Syringoma
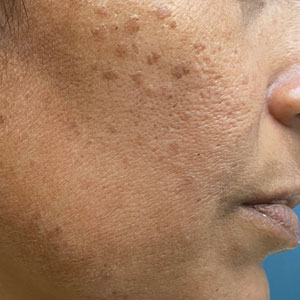
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.
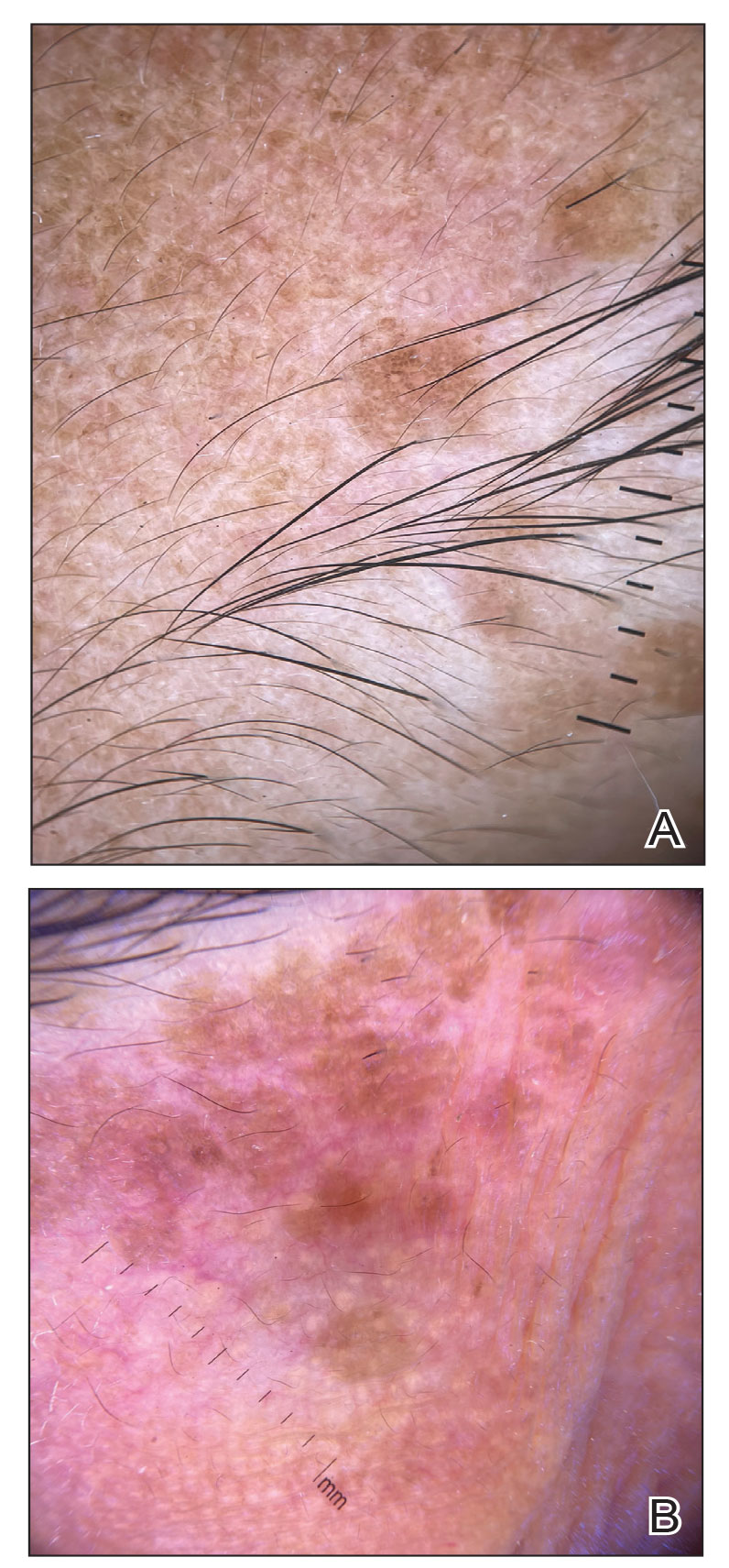
In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).
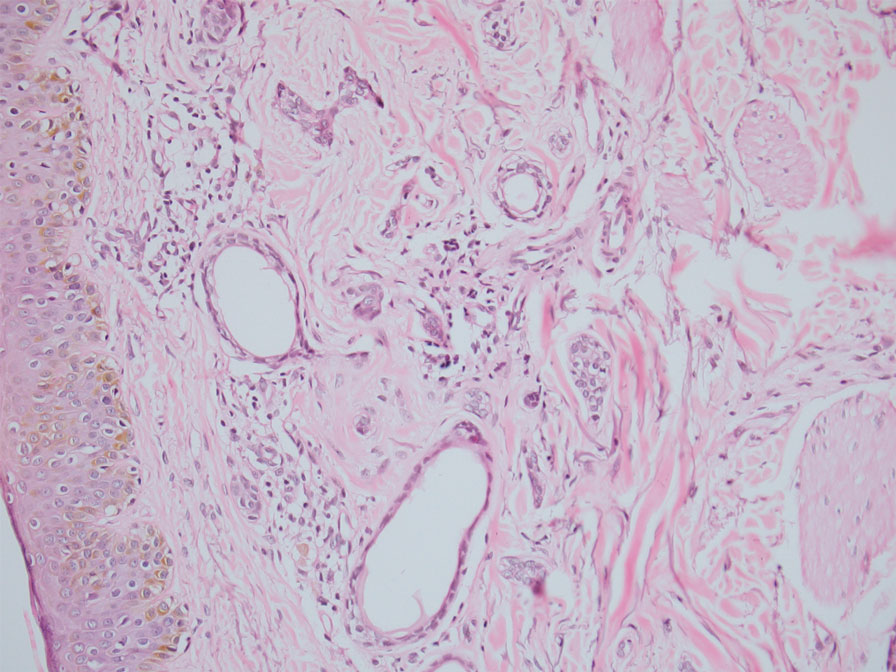
Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
A 46-year-old woman presented with multiple asymptomatic, flesh-colored, hyperpigmented papules on the face of 5 to 6 months’ duration that were progressively increasing in number. The lesions first appeared near the eyebrows and cheeks (top) and subsequently spread to involve the neck. She had no notable medical history. Cutaneous examination revealed multiple tan to brown papules over the periorbital, malar, and neck regions ranging in size from 1 to 5 mm. The lesions over the periorbital region were arranged in a linear pattern (bottom). Similar lesions also were present on the chest and arms. No other sites were involved, and systemic examination was normal.
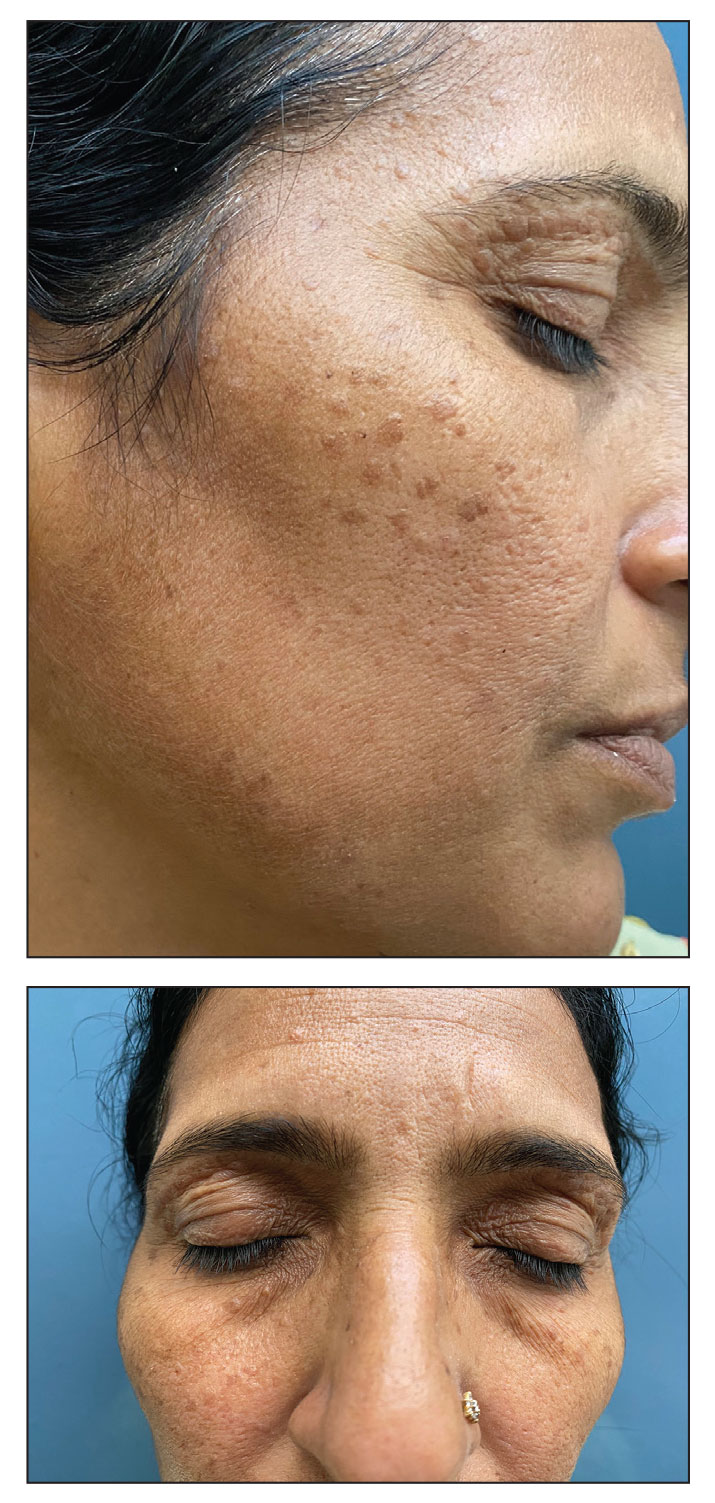
Social isolation, loneliness tied to death, MI, stroke: AHA
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Onset and awareness of hypertension varies by race, ethnicity
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.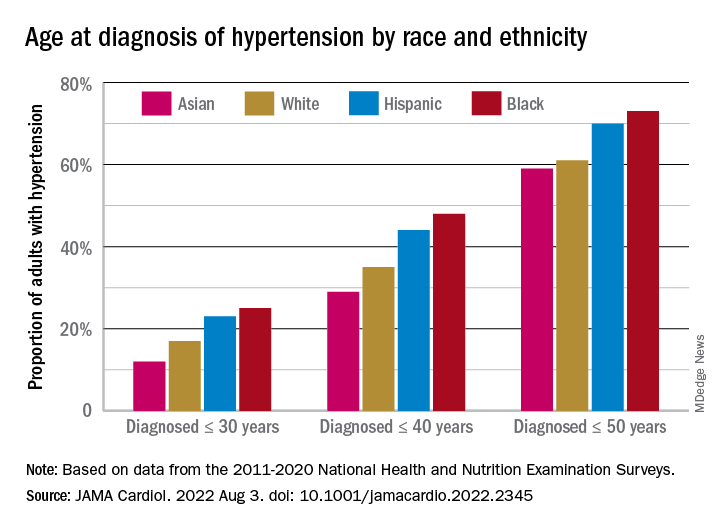
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
FROM JAMA CARDIOLOGY
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
Addressing racial bias in pulse oximetry
Pulse oximetry is a vital monitoring tool in the ICU and in pulmonary medicine. Regrettably, re-emerging data show that pulse oximeters do not accurately measure blood oxygen levels in Black patients, presumably due to their skin tone. (i.e., low arterial oxygen saturation despite a seemingly normal pulse oximetry reading). While inaccuracy of pulse oximeter measurements in patients with darker skin has been recognized for decades, recent studies have highlighted this as an ongoing problem with potentially severe consequences for Black patients and other patients of color.
One recent study found that Black patients had almost three times the likelihood of occult hypoxemia compared with White patients (Sjoding, MW, et al. N Engl J Med. 2020;383[25]:2477-8).
Subsequent studies have confirmed this to be a widespread problem across various clinical settings in hundreds of hospitals (Wong AI, et al. JAMA Netw Open. 2021;4[11]:e2131674; Valbuena VS, et al. Chest. 2022;161[4]:971-8). A recent retrospective cohort study of patients with COVID-19 found that occult hypoxemia in Black and Hispanic patients was associated with delayed eligibility for potentially lifesaving COVID-19 therapies (Fawzy AF, et al. JAMA Intern Med. 2022; published online May 31, 2022).
Now that numerous studies have demonstrated the inaccuracy of pulse oximetry with the potential to cause harm to historically marginalized racial and ethnic groups, must we abandon the use of pulse oximetry? We would argue that pulse oximeters remain valuable tools, but for now, we must adapt our practice until better devices are widely adopted.
First, it is crucial that health professionals are aware that pulse oximeters may underestimate the true extent of hypoxemia for all patients, but particularly for patients with darker skin. Acknowledging this device flaw is essential to avoid harm to our patients.
Second, clinicians must have heightened skepticism for seemingly normal pulse oximetry values when caring for symptomatic patients at risk of occult hypoxemia.
Until better pulse oximeters are widely available, clinicians must consider workarounds aimed at ensuring timely identification of hypoxemia in Black patients and other patients of color.
These patients may need invasive monitoring of arterial oxygenation, including arterial blood gas checks or an arterial catheter. However, invasive monitoring comes at the cost of discomfort to patients and potential complications, such as vessel or nerve damage.
Invasive monitoring of patients at risk for occult hypoxemia is not an equitable or acceptable long-term solution for this problem. As advocates for patients, clinicians and professional organizations should lobby regulatory bodies to ensure pulse oximeters are accurate for all patients.
We must also call on government leaders to move this process forward. For example, in response to efforts by the United Kingdom’s Intensive Care Society, the Health Secretary of the UK, Sajid Javid, has called for a review of pulse oximeters as part of a larger review assessing structural issues in health care that lead to worse outcomes in racial and ethnic minorities (BBC News. https://www.bbc.com/news/uk-59363544. Published online Nov. 21, 2021).
Device companies are largely for-profit corporations with obligations to their shareholders. It seems that existing incentives are insufficient to motivate investment in less biased technology and real-world evaluations of their devices.
We previously called for buyers of pulse oximeters to change the incentives of device companies – that is, for “hospitals to commit to only purchasing pulse oximeters that have been shown to work equally well in patients of colour.” (Hidalgo DC, et al. Lancet Respir Med. 2021;9[4]:E37). And, indeed, we worry that hospitals are putting themselves at medicolegal risk by not raising their purchasing standards. Since it is now widely known that pulse oximeters are inaccurate in certain patients, could there be liability for hospitals that continue to use devices we know to be disproportionately inaccurate by race?
Device companies must commit to fixing racial bias in pulse oximeters. Change is feasible, and pulse oximeters can be redesigned to be accurate and reliable among all patients using existing technology that is decades-old.
In the 1960s and 1970s, Hewlett Packard worked with NASA to noninvasively measure oxygen saturation in astronauts (Moran-Thomas, M. Wired. Published online June 4, 2021. https://www.wired.com/story/pulse-oximeters-equity). The device was designed to work for all skin tones and could be calibrated based on an individual’s skin tone. However, Hewlett Packard moved away from medical devices in the 1980s, shelving their design while other companies took over the oximeter market.
Lastly, as new devices are designed, they must be proven to work for all patients. Testing should be conducted in real-world clinical settings using metrics aligned with clinical care, since we know testing in artificial environments may not generalize to critically ill patients. Testing standards historically used by the FDA, such as only requiring device testing in a small number of non-White individuals, may miss clinically relevant hypoxemia. Non-inferiority studies are particularly susceptible to poor design or under-powering, and rigorous standards are needed from unbiased sources.
While potential solutions are currently being evaluated, the fact remains that the inaccuracy of pulse oximeters has been known for decades without any meaningful action taken to correct the problem.
As Valeria Valbuena, author of a study demonstrating inaccuracy of pulse oximetry in patients about to undergo ECMO, points out, “Using White patients as the standard in biomedical design has led to both differential care and innovation inertia for optimizing the way devices and algorithms work for patients of racial and ethnic minoritized groups” (Valbuena VS. JAMA Intern Med. 2022. doi: 10.1001/jamainternmed.2022.1903).
We know that hypoxemia is dangerous for our patients and that this is only one example of the long-standing systemic racism leading to harm in historically marginalized racial and ethnic groups. It is unacceptable that the devices we rely on to care for our patients are disproportionately inaccurate in non-White patients.
We hope that with increased awareness of this problem, meaningful action will be taken by device companies to ensure pulse oximeters work accurately for all patients.
From the Division of Pulmonary and Critical Care, Department of Medicine and the Center for Bioethics and Social Sciences in Medicine, University of Michigan Medical School (Drs. Harlan and Valley), and the Institute for Healthcare Policy and Innovation (Dr. Valley), University of Michigan, Ann Arbor, MI; and the Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado School of Medicine, Aurora, CO (Dr. Colon Hidalgo).
Pulse oximetry is a vital monitoring tool in the ICU and in pulmonary medicine. Regrettably, re-emerging data show that pulse oximeters do not accurately measure blood oxygen levels in Black patients, presumably due to their skin tone. (i.e., low arterial oxygen saturation despite a seemingly normal pulse oximetry reading). While inaccuracy of pulse oximeter measurements in patients with darker skin has been recognized for decades, recent studies have highlighted this as an ongoing problem with potentially severe consequences for Black patients and other patients of color.
One recent study found that Black patients had almost three times the likelihood of occult hypoxemia compared with White patients (Sjoding, MW, et al. N Engl J Med. 2020;383[25]:2477-8).
Subsequent studies have confirmed this to be a widespread problem across various clinical settings in hundreds of hospitals (Wong AI, et al. JAMA Netw Open. 2021;4[11]:e2131674; Valbuena VS, et al. Chest. 2022;161[4]:971-8). A recent retrospective cohort study of patients with COVID-19 found that occult hypoxemia in Black and Hispanic patients was associated with delayed eligibility for potentially lifesaving COVID-19 therapies (Fawzy AF, et al. JAMA Intern Med. 2022; published online May 31, 2022).
Now that numerous studies have demonstrated the inaccuracy of pulse oximetry with the potential to cause harm to historically marginalized racial and ethnic groups, must we abandon the use of pulse oximetry? We would argue that pulse oximeters remain valuable tools, but for now, we must adapt our practice until better devices are widely adopted.
First, it is crucial that health professionals are aware that pulse oximeters may underestimate the true extent of hypoxemia for all patients, but particularly for patients with darker skin. Acknowledging this device flaw is essential to avoid harm to our patients.
Second, clinicians must have heightened skepticism for seemingly normal pulse oximetry values when caring for symptomatic patients at risk of occult hypoxemia.
Until better pulse oximeters are widely available, clinicians must consider workarounds aimed at ensuring timely identification of hypoxemia in Black patients and other patients of color.
These patients may need invasive monitoring of arterial oxygenation, including arterial blood gas checks or an arterial catheter. However, invasive monitoring comes at the cost of discomfort to patients and potential complications, such as vessel or nerve damage.
Invasive monitoring of patients at risk for occult hypoxemia is not an equitable or acceptable long-term solution for this problem. As advocates for patients, clinicians and professional organizations should lobby regulatory bodies to ensure pulse oximeters are accurate for all patients.
We must also call on government leaders to move this process forward. For example, in response to efforts by the United Kingdom’s Intensive Care Society, the Health Secretary of the UK, Sajid Javid, has called for a review of pulse oximeters as part of a larger review assessing structural issues in health care that lead to worse outcomes in racial and ethnic minorities (BBC News. https://www.bbc.com/news/uk-59363544. Published online Nov. 21, 2021).
Device companies are largely for-profit corporations with obligations to their shareholders. It seems that existing incentives are insufficient to motivate investment in less biased technology and real-world evaluations of their devices.
We previously called for buyers of pulse oximeters to change the incentives of device companies – that is, for “hospitals to commit to only purchasing pulse oximeters that have been shown to work equally well in patients of colour.” (Hidalgo DC, et al. Lancet Respir Med. 2021;9[4]:E37). And, indeed, we worry that hospitals are putting themselves at medicolegal risk by not raising their purchasing standards. Since it is now widely known that pulse oximeters are inaccurate in certain patients, could there be liability for hospitals that continue to use devices we know to be disproportionately inaccurate by race?
Device companies must commit to fixing racial bias in pulse oximeters. Change is feasible, and pulse oximeters can be redesigned to be accurate and reliable among all patients using existing technology that is decades-old.
In the 1960s and 1970s, Hewlett Packard worked with NASA to noninvasively measure oxygen saturation in astronauts (Moran-Thomas, M. Wired. Published online June 4, 2021. https://www.wired.com/story/pulse-oximeters-equity). The device was designed to work for all skin tones and could be calibrated based on an individual’s skin tone. However, Hewlett Packard moved away from medical devices in the 1980s, shelving their design while other companies took over the oximeter market.
Lastly, as new devices are designed, they must be proven to work for all patients. Testing should be conducted in real-world clinical settings using metrics aligned with clinical care, since we know testing in artificial environments may not generalize to critically ill patients. Testing standards historically used by the FDA, such as only requiring device testing in a small number of non-White individuals, may miss clinically relevant hypoxemia. Non-inferiority studies are particularly susceptible to poor design or under-powering, and rigorous standards are needed from unbiased sources.
While potential solutions are currently being evaluated, the fact remains that the inaccuracy of pulse oximeters has been known for decades without any meaningful action taken to correct the problem.
As Valeria Valbuena, author of a study demonstrating inaccuracy of pulse oximetry in patients about to undergo ECMO, points out, “Using White patients as the standard in biomedical design has led to both differential care and innovation inertia for optimizing the way devices and algorithms work for patients of racial and ethnic minoritized groups” (Valbuena VS. JAMA Intern Med. 2022. doi: 10.1001/jamainternmed.2022.1903).
We know that hypoxemia is dangerous for our patients and that this is only one example of the long-standing systemic racism leading to harm in historically marginalized racial and ethnic groups. It is unacceptable that the devices we rely on to care for our patients are disproportionately inaccurate in non-White patients.
We hope that with increased awareness of this problem, meaningful action will be taken by device companies to ensure pulse oximeters work accurately for all patients.
From the Division of Pulmonary and Critical Care, Department of Medicine and the Center for Bioethics and Social Sciences in Medicine, University of Michigan Medical School (Drs. Harlan and Valley), and the Institute for Healthcare Policy and Innovation (Dr. Valley), University of Michigan, Ann Arbor, MI; and the Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado School of Medicine, Aurora, CO (Dr. Colon Hidalgo).
Pulse oximetry is a vital monitoring tool in the ICU and in pulmonary medicine. Regrettably, re-emerging data show that pulse oximeters do not accurately measure blood oxygen levels in Black patients, presumably due to their skin tone. (i.e., low arterial oxygen saturation despite a seemingly normal pulse oximetry reading). While inaccuracy of pulse oximeter measurements in patients with darker skin has been recognized for decades, recent studies have highlighted this as an ongoing problem with potentially severe consequences for Black patients and other patients of color.
One recent study found that Black patients had almost three times the likelihood of occult hypoxemia compared with White patients (Sjoding, MW, et al. N Engl J Med. 2020;383[25]:2477-8).
Subsequent studies have confirmed this to be a widespread problem across various clinical settings in hundreds of hospitals (Wong AI, et al. JAMA Netw Open. 2021;4[11]:e2131674; Valbuena VS, et al. Chest. 2022;161[4]:971-8). A recent retrospective cohort study of patients with COVID-19 found that occult hypoxemia in Black and Hispanic patients was associated with delayed eligibility for potentially lifesaving COVID-19 therapies (Fawzy AF, et al. JAMA Intern Med. 2022; published online May 31, 2022).
Now that numerous studies have demonstrated the inaccuracy of pulse oximetry with the potential to cause harm to historically marginalized racial and ethnic groups, must we abandon the use of pulse oximetry? We would argue that pulse oximeters remain valuable tools, but for now, we must adapt our practice until better devices are widely adopted.
First, it is crucial that health professionals are aware that pulse oximeters may underestimate the true extent of hypoxemia for all patients, but particularly for patients with darker skin. Acknowledging this device flaw is essential to avoid harm to our patients.
Second, clinicians must have heightened skepticism for seemingly normal pulse oximetry values when caring for symptomatic patients at risk of occult hypoxemia.
Until better pulse oximeters are widely available, clinicians must consider workarounds aimed at ensuring timely identification of hypoxemia in Black patients and other patients of color.
These patients may need invasive monitoring of arterial oxygenation, including arterial blood gas checks or an arterial catheter. However, invasive monitoring comes at the cost of discomfort to patients and potential complications, such as vessel or nerve damage.
Invasive monitoring of patients at risk for occult hypoxemia is not an equitable or acceptable long-term solution for this problem. As advocates for patients, clinicians and professional organizations should lobby regulatory bodies to ensure pulse oximeters are accurate for all patients.
We must also call on government leaders to move this process forward. For example, in response to efforts by the United Kingdom’s Intensive Care Society, the Health Secretary of the UK, Sajid Javid, has called for a review of pulse oximeters as part of a larger review assessing structural issues in health care that lead to worse outcomes in racial and ethnic minorities (BBC News. https://www.bbc.com/news/uk-59363544. Published online Nov. 21, 2021).
Device companies are largely for-profit corporations with obligations to their shareholders. It seems that existing incentives are insufficient to motivate investment in less biased technology and real-world evaluations of their devices.
We previously called for buyers of pulse oximeters to change the incentives of device companies – that is, for “hospitals to commit to only purchasing pulse oximeters that have been shown to work equally well in patients of colour.” (Hidalgo DC, et al. Lancet Respir Med. 2021;9[4]:E37). And, indeed, we worry that hospitals are putting themselves at medicolegal risk by not raising their purchasing standards. Since it is now widely known that pulse oximeters are inaccurate in certain patients, could there be liability for hospitals that continue to use devices we know to be disproportionately inaccurate by race?
Device companies must commit to fixing racial bias in pulse oximeters. Change is feasible, and pulse oximeters can be redesigned to be accurate and reliable among all patients using existing technology that is decades-old.
In the 1960s and 1970s, Hewlett Packard worked with NASA to noninvasively measure oxygen saturation in astronauts (Moran-Thomas, M. Wired. Published online June 4, 2021. https://www.wired.com/story/pulse-oximeters-equity). The device was designed to work for all skin tones and could be calibrated based on an individual’s skin tone. However, Hewlett Packard moved away from medical devices in the 1980s, shelving their design while other companies took over the oximeter market.
Lastly, as new devices are designed, they must be proven to work for all patients. Testing should be conducted in real-world clinical settings using metrics aligned with clinical care, since we know testing in artificial environments may not generalize to critically ill patients. Testing standards historically used by the FDA, such as only requiring device testing in a small number of non-White individuals, may miss clinically relevant hypoxemia. Non-inferiority studies are particularly susceptible to poor design or under-powering, and rigorous standards are needed from unbiased sources.
While potential solutions are currently being evaluated, the fact remains that the inaccuracy of pulse oximeters has been known for decades without any meaningful action taken to correct the problem.
As Valeria Valbuena, author of a study demonstrating inaccuracy of pulse oximetry in patients about to undergo ECMO, points out, “Using White patients as the standard in biomedical design has led to both differential care and innovation inertia for optimizing the way devices and algorithms work for patients of racial and ethnic minoritized groups” (Valbuena VS. JAMA Intern Med. 2022. doi: 10.1001/jamainternmed.2022.1903).
We know that hypoxemia is dangerous for our patients and that this is only one example of the long-standing systemic racism leading to harm in historically marginalized racial and ethnic groups. It is unacceptable that the devices we rely on to care for our patients are disproportionately inaccurate in non-White patients.
We hope that with increased awareness of this problem, meaningful action will be taken by device companies to ensure pulse oximeters work accurately for all patients.
From the Division of Pulmonary and Critical Care, Department of Medicine and the Center for Bioethics and Social Sciences in Medicine, University of Michigan Medical School (Drs. Harlan and Valley), and the Institute for Healthcare Policy and Innovation (Dr. Valley), University of Michigan, Ann Arbor, MI; and the Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado School of Medicine, Aurora, CO (Dr. Colon Hidalgo).
Perceptions of Community Service in Dermatology Residency Training Programs: A Survey-Based Study of Program Directors, Residents, and Recent Dermatology Residency Graduates
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.
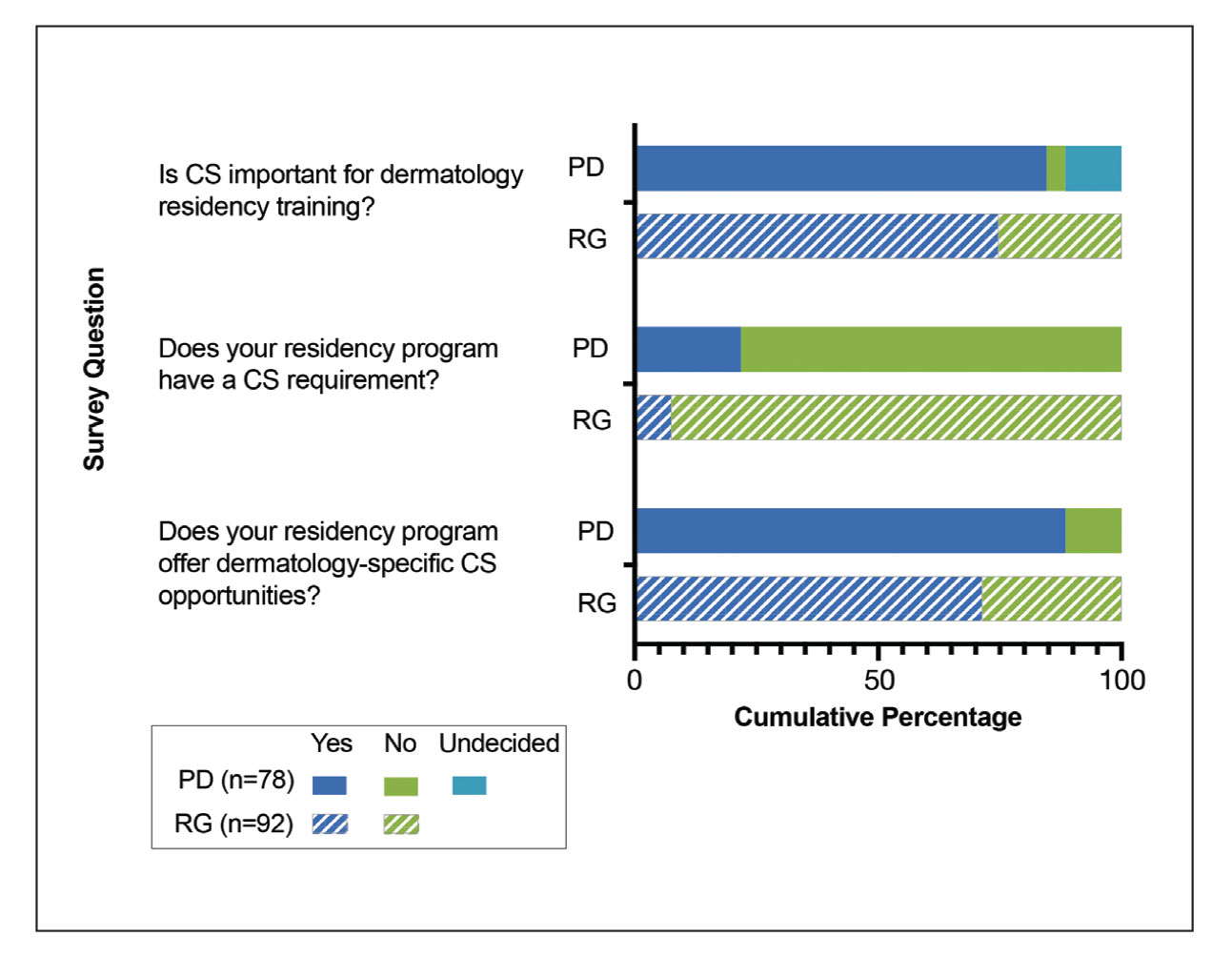
Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).
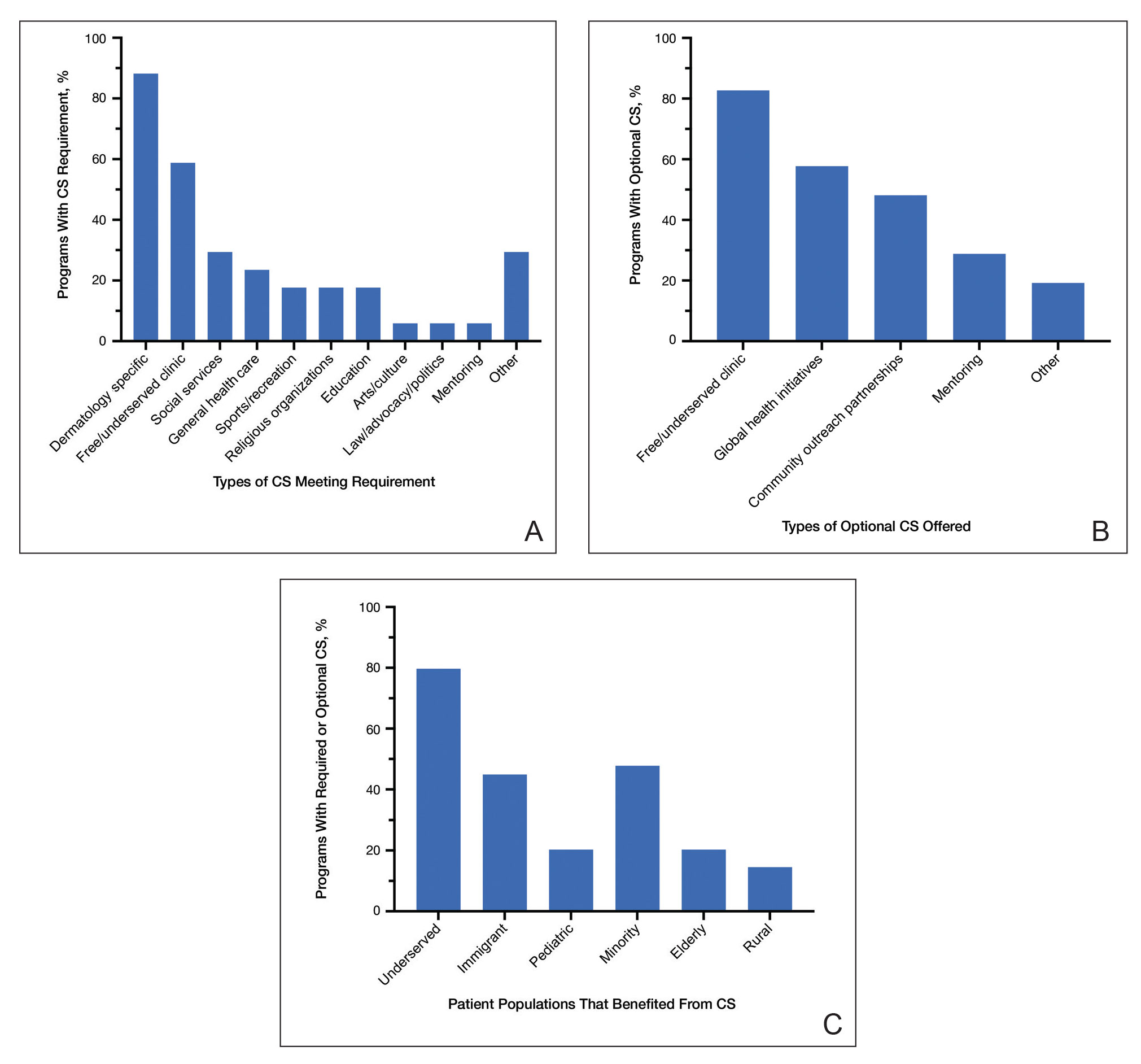
Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.
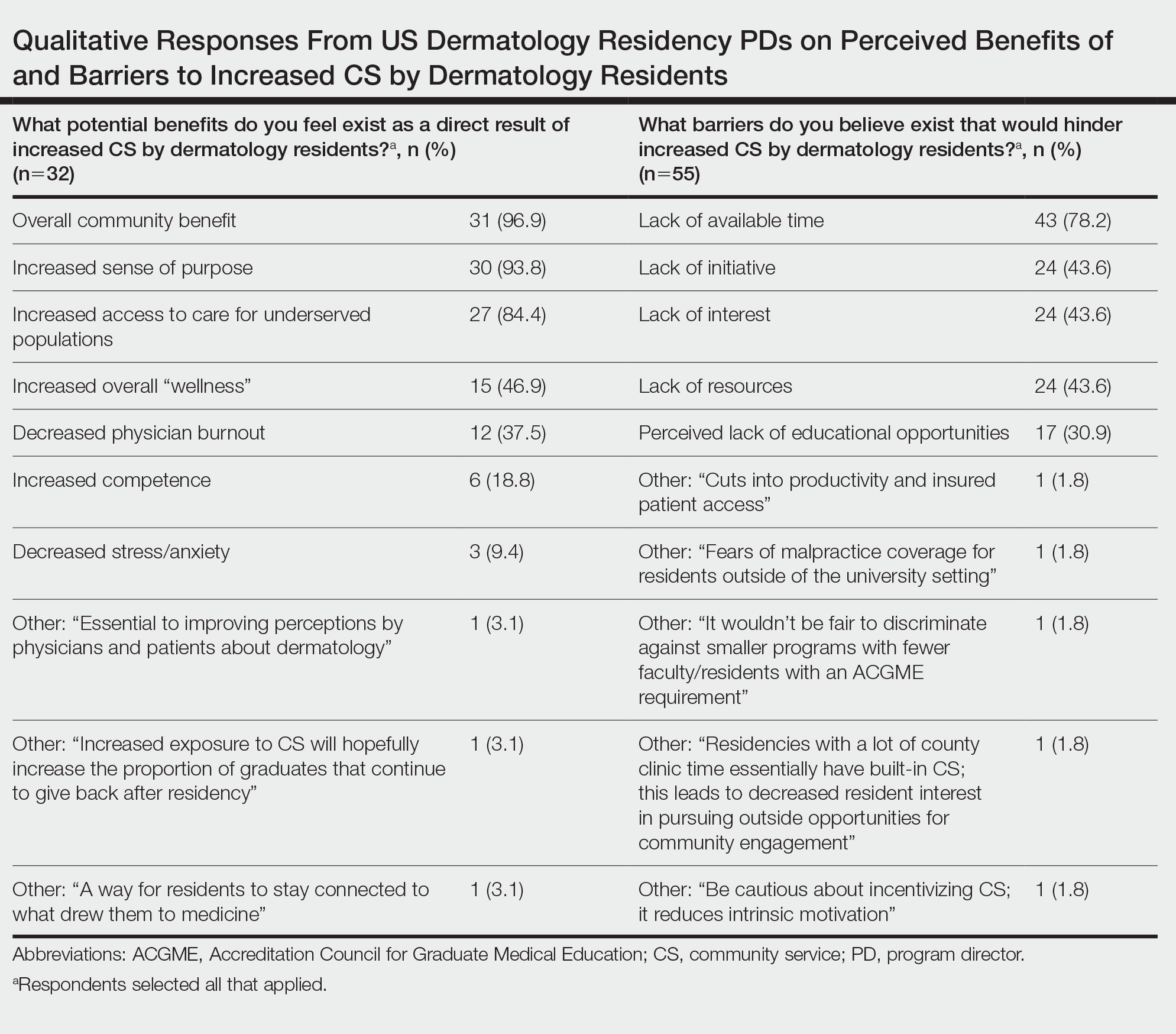
Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
Community service (CS) or service learning in dermatology (eg, free skin cancer screenings, providing care through free clinics, free teledermatology consultations) is instrumental in mitigating disparities and improving access to equitable dermatologic care. With the rate of underinsured and uninsured patients on the rise, free and federally qualified clinics frequently are the sole means by which patients access specialty care such as dermatology.1 Contributing to the economic gap in access, the geographic disparity of dermatologists in the United States continues to climb, and many marginalized communities remain without dermatologists.2 Nearly 30% of the total US population resides in geographic areas that are underserved by dermatologists, while there appears to be an oversupply of dermatologists in urban areas.3 Dermatologists practicing in rural areas make up only 10% of the dermatology workforce,4 whereas 40% of all dermatologists practice in the most densely populated US cities.5 Consequently, patients in these underserved communities face longer wait times6 and are less likely to utilize dermatology services than patients in dermatologist-dense geographic areas.7
Service opportunities have become increasingly integrated into graduate medical education.8 These service activities help bridge the health care access gap while fulfilling Accreditation Council of Graduate Medical Education (ACGME) requirements. Our study assessed the importance of CS to dermatology residency program directors (PDs), dermatology residents, and recent dermatology residency graduates. Herein, we describe the perceptions of CS within dermatology residency training among PDs and residents.
Methods
In this study, CS is defined as participation in activities to increase dermatologic access, education, and resources to underserved communities. Using the approved Association of Professors of Dermatology listserve and direct email communication, we surveyed 142 PDs of ACGME-accredited dermatology residency training programs. The deidentified respondents voluntarily completed a 17-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
We also surveyed current dermatology residents and recent graduates of ACGME-accredited dermatology residency programs via PDs nationwide. The deidentified respondents voluntarily completed a 19-question Qualtrics survey with a 5-point Likert scale (extremely, very, moderately, slightly, or not at all), yes/no/undecided, and qualitative responses.
Descriptive statistics were used for data analysis for both Qualtrics surveys. The University of Pittsburgh institutional review board deemed this study exempt.

Results
Feedback From PDs—Of the 142 PDs, we received 78 responses (54.9%). For selection of dermatology residents, CS was moderately to extremely important to 64 (82.1%) PDs, and 63 (80.8%) PDs stated CS was moderately to extremely important to their dermatology residency program at large. For dermatology residency training, 66 (84.6%) PDs believed CS is important, whereas 3 (3.8%) believed it is not important, and 9 (11.5%) remained undecided (Figure 1). Notably, 17 (21.8%) programs required CS as part of the dermatology educational curriculum, with most of these programs requiring 10 hours or less during the 3 years of residency training. Of the programs with required CS, 15 (88.2%) had dermatology-specific CS requirements, with 10 (58.8%) programs involved in CS at free and/or underserved clinics and some programs participating in other CS activities, such as advocacy, mentorship, educational outreach, or sports (Figure 2A).

Community service opportunities were offered to dermatology residents by 69 (88.5%) programs, including the 17 programs that required CS as part of the dermatology educational curriculum. Among these programs with optional CS, 43 (82.7%) PDs reported CS opportunities at free and/or underserved clinics, and 30 (57.7%) reported CS opportunities through global health initiatives (Figure 2B). Other CS opportunities offered included partnerships with community outreach organizations and mentoring underprivileged students. Patient populations that benefit from CS offered by these dermatology residency programs included 55 (79.7%) underserved, 33 (47.8%) minority, 31 (44.9%) immigrant, 14 (20.3%) pediatric, 14 (20.3%) elderly, and 10 (14.5%) rural populations (Figure 2C). At dermatology residency programs with optional CS opportunities, 22 (42.3%) PDs endorsed at least 50% of their residents participating in these activities.
Qualitative responses revealed that some PDs view CS as “a way for residents to stay connected to what drew them to medicine” and “essential to improving perceptions by physicians and patients about dermatology.” Program directors perceived lack of available time, initiative, and resources as well as minimal resident interest, malpractice coverage, and lack of educational opportunities as potential barriers to CS involvement by residents (Table). Forty-six (59.0%) PDs believed that CS should not be an ACGME requirement for dermatology training, 23 (29.5%) believed it should be required, and 9 (11.5%) were undecided.

Feedback From Residents—We received responses from 92 current dermatology residents and recent dermatology residency graduates; 86 (93.5%) respondents were trainees or recent graduates from academic dermatology residency training programs, and 6 (6.5%) were from community-based training programs. Community service was perceived to be an important part of dermatology training by 68 (73.9%) respondents, and dermatology-specific CS opportunities were available to 65 (70.7%) respondents (Figure 1). Although CS was required of only 7 (7.6%) respondents, 36 (39.1%) respondents volunteered at a free dermatology clinic during residency training. Among respondents who were not provided CS opportunities through their residency program, 23 (85.2%) stated they would have participated if given the opportunity.
Dermatology residents listed increased access to care for marginalized populations, increased sense of purpose, increased competence, and decreased burnout as perceived benefits of participation in CS. Of the dermatology residents who volunteered at a free dermatology clinic during training, 27 (75.0%) regarded the experience as a “high-yield learning opportunity.” Additionally, 29 (80.6%) residents stated their participation in a free dermatology clinic increased their awareness of health disparities and societal factors affecting dermatologic care in underserved patient populations. These respondents affirmed that their participation motivated them to become more involved in outreach targeting underserved populations throughout the duration of their careers.
Comment
The results of this nationwide survey have several important implications for dermatology residency programs, with a focus on programs in well-resourced and high socioeconomic status areas. Although most PDs believe that CS is important for dermatology resident training, few programs have CS requirements, and the majority are opposed to ACGME-mandated CS. Dermatology residents and recent graduates overwhelmingly conveyed that participation in a free dermatology clinic during residency training increased their knowledge base surrounding socioeconomic determinants of health and practicing in resource-limited settings. Furthermore, most trainees expressed that CS participation as a resident motivated them to continue to partake in CS for the underserved as an attending physician. The discordance between perceived value of CS by residents and the lack of CS requirements and opportunities by residency programs represents a realistic opportunity for residency training programs to integrate CS into the curriculum.
Residency programs that integrate service for the underserved into their program goals are 3 times more successful in graduating dermatology residents who practice in underserved communities.9 Patients in marginalized communities and those from lower socioeconomic backgrounds face many barriers to accessing dermatologic care including longer wait times and higher practice rejection rates than patients with private insurance.6 Through increased CS opportunities, dermatology residency programs can strengthen the local health care infrastructure and bridge the gap in access to dermatologic care.
By establishing a formal CS rotation in dermatology residency programs, residents will experience invaluable first-hand educational opportunities, provide comprehensive care for patients in resource-limited settings, and hopefully continue to serve in marginalized communities. Incorporating service for the underserved into the dermatology residency curriculum not only enhances the cultural competency of trainees but also mandates that skin health equity be made a priority. By exposing dermatology residents to the diverse patient populations often served by free clinics, residents will increase their knowledge of skin disease presentation in patients with darker skin tones, which has historically been deficient in medical education.10,11
The limitations of this survey study included recall bias, the response rate of PDs (54.9%), and the inability to determine response rate of residents, as we were unable to establish the total number of residents who received our survey. Based on geographic location, some dermatology residency programs may treat a high percentage of medically underserved patients, which already improves access to dermatology. For this reason, follow-up studies correlating PD and resident responses with region, program size, and university/community affiliation will increase our understanding of CS participation and perceptions.
Conclusion
Dermatology residency program participation in CS helps reduce barriers to access for patients in marginalized communities. Incorporating CS into the dermatology residency program curriculum creates a rewarding training environment that increases skin health equity, fosters an interest in health disparities, and enhances the cultural competency of its trainees.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59.
- Vaidya T, Zubritsky L, Alikhan A, et al. Socioeconomic and geographic barriers to dermatology care in urban and rural US populations. J Am Acad Dermatol. 2018;78:406-408.
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307.
- Resneck J, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Resneck J, Pletcher MJ, Lozano N. Medicare, Medicaid, and access to dermatologists: the effect of patient insurance on appointment access and wait times. J Am Acad Dermatol. 2004;50:85-92.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Vance MC, Kennedy KG. Developing an advocacy curriculum: lessons learned from a national survey of psychiatric residency programs. Acad Psychiatry. 2020;44:283-288.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Ebede T, Papier A. Disparities in dermatology educational resources.J Am Acad Dermatol. 2006;55:687-690.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Participation of dermatology residents in service-learning experiences increases awareness of health disparities and social factors impacting dermatologic care and promotes a lifelong commitment to serving vulnerable populations.
- Integrating service learning into the dermatology residency program curriculum enhances trainees’ cultural sensitivity and encourages the prioritization of skin health equity.
- Service learning will help bridge the gap in access to dermatologic care for patients in medically marginalized communities.
Racism tied to cognition in middle-aged, elderly
It is generally understood that racism, whether structural or personal, harms the well-being of the individual who experiences it. It has harmful health effects, and it contributes to ethnic inequality.
That was the fundamental message behind two studies presented at a press conference at the Alzheimer’s Association International Conference.
“We know that there are communities like black African Americans and Hispanic Latinos who are at greater risk for developing Alzheimer’s or another dementia,” said Carl Hill, PhD, who served as a moderator during the press conference. He pointed out that the genetic and lifestyle factors linked to dementia tell only part of the story. “It’s important that the science also examines the unique experiences of those at greater risk for dementia in our society,” said Dr. Hill, who is Alzheimer’s Association Chief Diversity Equity and Inclusion Officer.
Racism, memory, and cognition in middle-aged patients
Jennifer J. Manly, PhD, professor of neuropsychology at Columbia University, New York, presented a study of experience of racism and memory scores among a highly diverse, middle-aged cohort.
“There’s little understanding of how the multiple levels of racism – including intrapersonal, institutional, and structural racism – influence cognitive aging and dementia risk,” Dr. Manly said during the press conference.
Among 1,095 participants, 19.5% were non-Latinx White (61% female, mean age 57), 26.0% were non-Latinx Black (63% female, mean age 56), 32.3% were English-speaking Latinx (66% female, mean age 50), and 21.2% were Spanish-speaking Latinx (68% female, mean age 58).
The researchers used the Everyday Discrimination (ED) scale to measure experience of individual racism, the Major Discrimination (MD) scale to measure experience of institutional racism, and residential segregation of the census block group for an individual’s parents to measure residential segregation. Outcome measures included the Digit Span to assess attention and working memory, and the Selective Reminding Test to assess episodic memory.
The study found a clear association between racism and cognition. “The association of interpersonal racism to memory corresponds to 3 years of chronological age, and was driven by non-Hispanic black participants. Next, there was a reliable relationship between institutional racism and memory scores among non-Hispanic black participants, such that each reported civil rights violation corresponded to the effect of about 4.5 years of age on memory,” said Dr. Manly.
“The bottom line is that our results suggest that exposure to racism is a substantial driver of later life memory function, even in middle age, and especially for Black people,” Dr. Manly added.
The results should alert physicians to the complexities of racism and its impact. “Health providers need to be aware that many accumulated risks are historical and structural, and not controlled by the individual. Maybe more importantly, the medical system itself may perpetuate discriminatory experiences that contribute to worse health,” said Dr. Manly.
Latinx concerns
Also at the press conference, Adriana Perez, PhD, emphasized the challenges that Spanish-speaking Latinxs have with health care. Just 5%-7% of nurses are Latinx. “The same could be said for physicians, for clinical psychologists ... as you look at the really critical positions to address brain health equity, we are not represented there,” said Dr. Perez, an assistant professor and senior fellow at the University of Pennsylvania School of Nursing in Philadelphia.
She also pointed out that Latinx representation in clinical trials is very low, even though surveys performed by the Alzheimer’s Association show that this population values medical science and is willing to participate. In fact, 85% said they would participate if invited. The trouble is that many clinical trial announcements state that participants must speak English. Even the many Latinos who are bilingual may be put off by that wording: “That is a message that you’re not invited. That’s how it’s perceived,” said Dr. Perez.
Racism and cognition in the elderly
At the press conference, Kristen George, PhD, presented results from a study of individuals over age 90. “Racial disparities in dementia have been well characterized, particularly among those people who are aged 65 and older, but we don’t know very much about the oldest old individuals who are aged 90 and older. This group is one of the fastest growing segments of the population, and it’s becoming increasingly diverse,” said Dr. George, assistant professor of epidemiology at the University of California, Davis.
The group enrolled 445 Asian, Black, Latinx, White, and multiracial individuals who were members of Kaiser Permanente Northern California, with a mean age of 92.7 years. They used the Major Experiences of Discrimination Scale to assess discrimination.
The researchers divided them into three groups based on gender, race, and responses to the 10-item scale. Class 1 included largely White men who had reported workplace discrimination, with an average of two major discrimination experiences. Class 2 was made up of White women and non-Whites who reported little or no discrimination, with an average of 0 experiences. Class 3 included all non-White participants, and they reported a mean of four discrimination experiences.
Using class 2 as a reference, executive function was better among class 1 individuals (beta = 0.28; 95% CI, 0.03-0.52) but there was no significant difference between class 3 and class 2. Class 1 had better baseline semantic memory than class 2 (beta = 0.33; 95% CI, 0.07-0.58), and those in class 3 performed significantly worse than class 2 (beta = –0.24; 95% CI, –0.48 to –0.00). There were no between-group differences in baseline verbal or episodic memory.
Dr. Perez, Dr. Manly, Dr. George, and Dr. Hill have no relevant financial disclosures.
It is generally understood that racism, whether structural or personal, harms the well-being of the individual who experiences it. It has harmful health effects, and it contributes to ethnic inequality.
That was the fundamental message behind two studies presented at a press conference at the Alzheimer’s Association International Conference.
“We know that there are communities like black African Americans and Hispanic Latinos who are at greater risk for developing Alzheimer’s or another dementia,” said Carl Hill, PhD, who served as a moderator during the press conference. He pointed out that the genetic and lifestyle factors linked to dementia tell only part of the story. “It’s important that the science also examines the unique experiences of those at greater risk for dementia in our society,” said Dr. Hill, who is Alzheimer’s Association Chief Diversity Equity and Inclusion Officer.
Racism, memory, and cognition in middle-aged patients
Jennifer J. Manly, PhD, professor of neuropsychology at Columbia University, New York, presented a study of experience of racism and memory scores among a highly diverse, middle-aged cohort.
“There’s little understanding of how the multiple levels of racism – including intrapersonal, institutional, and structural racism – influence cognitive aging and dementia risk,” Dr. Manly said during the press conference.
Among 1,095 participants, 19.5% were non-Latinx White (61% female, mean age 57), 26.0% were non-Latinx Black (63% female, mean age 56), 32.3% were English-speaking Latinx (66% female, mean age 50), and 21.2% were Spanish-speaking Latinx (68% female, mean age 58).
The researchers used the Everyday Discrimination (ED) scale to measure experience of individual racism, the Major Discrimination (MD) scale to measure experience of institutional racism, and residential segregation of the census block group for an individual’s parents to measure residential segregation. Outcome measures included the Digit Span to assess attention and working memory, and the Selective Reminding Test to assess episodic memory.
The study found a clear association between racism and cognition. “The association of interpersonal racism to memory corresponds to 3 years of chronological age, and was driven by non-Hispanic black participants. Next, there was a reliable relationship between institutional racism and memory scores among non-Hispanic black participants, such that each reported civil rights violation corresponded to the effect of about 4.5 years of age on memory,” said Dr. Manly.
“The bottom line is that our results suggest that exposure to racism is a substantial driver of later life memory function, even in middle age, and especially for Black people,” Dr. Manly added.
The results should alert physicians to the complexities of racism and its impact. “Health providers need to be aware that many accumulated risks are historical and structural, and not controlled by the individual. Maybe more importantly, the medical system itself may perpetuate discriminatory experiences that contribute to worse health,” said Dr. Manly.
Latinx concerns
Also at the press conference, Adriana Perez, PhD, emphasized the challenges that Spanish-speaking Latinxs have with health care. Just 5%-7% of nurses are Latinx. “The same could be said for physicians, for clinical psychologists ... as you look at the really critical positions to address brain health equity, we are not represented there,” said Dr. Perez, an assistant professor and senior fellow at the University of Pennsylvania School of Nursing in Philadelphia.
She also pointed out that Latinx representation in clinical trials is very low, even though surveys performed by the Alzheimer’s Association show that this population values medical science and is willing to participate. In fact, 85% said they would participate if invited. The trouble is that many clinical trial announcements state that participants must speak English. Even the many Latinos who are bilingual may be put off by that wording: “That is a message that you’re not invited. That’s how it’s perceived,” said Dr. Perez.
Racism and cognition in the elderly
At the press conference, Kristen George, PhD, presented results from a study of individuals over age 90. “Racial disparities in dementia have been well characterized, particularly among those people who are aged 65 and older, but we don’t know very much about the oldest old individuals who are aged 90 and older. This group is one of the fastest growing segments of the population, and it’s becoming increasingly diverse,” said Dr. George, assistant professor of epidemiology at the University of California, Davis.
The group enrolled 445 Asian, Black, Latinx, White, and multiracial individuals who were members of Kaiser Permanente Northern California, with a mean age of 92.7 years. They used the Major Experiences of Discrimination Scale to assess discrimination.
The researchers divided them into three groups based on gender, race, and responses to the 10-item scale. Class 1 included largely White men who had reported workplace discrimination, with an average of two major discrimination experiences. Class 2 was made up of White women and non-Whites who reported little or no discrimination, with an average of 0 experiences. Class 3 included all non-White participants, and they reported a mean of four discrimination experiences.
Using class 2 as a reference, executive function was better among class 1 individuals (beta = 0.28; 95% CI, 0.03-0.52) but there was no significant difference between class 3 and class 2. Class 1 had better baseline semantic memory than class 2 (beta = 0.33; 95% CI, 0.07-0.58), and those in class 3 performed significantly worse than class 2 (beta = –0.24; 95% CI, –0.48 to –0.00). There were no between-group differences in baseline verbal or episodic memory.
Dr. Perez, Dr. Manly, Dr. George, and Dr. Hill have no relevant financial disclosures.
It is generally understood that racism, whether structural or personal, harms the well-being of the individual who experiences it. It has harmful health effects, and it contributes to ethnic inequality.
That was the fundamental message behind two studies presented at a press conference at the Alzheimer’s Association International Conference.
“We know that there are communities like black African Americans and Hispanic Latinos who are at greater risk for developing Alzheimer’s or another dementia,” said Carl Hill, PhD, who served as a moderator during the press conference. He pointed out that the genetic and lifestyle factors linked to dementia tell only part of the story. “It’s important that the science also examines the unique experiences of those at greater risk for dementia in our society,” said Dr. Hill, who is Alzheimer’s Association Chief Diversity Equity and Inclusion Officer.
Racism, memory, and cognition in middle-aged patients
Jennifer J. Manly, PhD, professor of neuropsychology at Columbia University, New York, presented a study of experience of racism and memory scores among a highly diverse, middle-aged cohort.
“There’s little understanding of how the multiple levels of racism – including intrapersonal, institutional, and structural racism – influence cognitive aging and dementia risk,” Dr. Manly said during the press conference.
Among 1,095 participants, 19.5% were non-Latinx White (61% female, mean age 57), 26.0% were non-Latinx Black (63% female, mean age 56), 32.3% were English-speaking Latinx (66% female, mean age 50), and 21.2% were Spanish-speaking Latinx (68% female, mean age 58).
The researchers used the Everyday Discrimination (ED) scale to measure experience of individual racism, the Major Discrimination (MD) scale to measure experience of institutional racism, and residential segregation of the census block group for an individual’s parents to measure residential segregation. Outcome measures included the Digit Span to assess attention and working memory, and the Selective Reminding Test to assess episodic memory.
The study found a clear association between racism and cognition. “The association of interpersonal racism to memory corresponds to 3 years of chronological age, and was driven by non-Hispanic black participants. Next, there was a reliable relationship between institutional racism and memory scores among non-Hispanic black participants, such that each reported civil rights violation corresponded to the effect of about 4.5 years of age on memory,” said Dr. Manly.
“The bottom line is that our results suggest that exposure to racism is a substantial driver of later life memory function, even in middle age, and especially for Black people,” Dr. Manly added.
The results should alert physicians to the complexities of racism and its impact. “Health providers need to be aware that many accumulated risks are historical and structural, and not controlled by the individual. Maybe more importantly, the medical system itself may perpetuate discriminatory experiences that contribute to worse health,” said Dr. Manly.
Latinx concerns
Also at the press conference, Adriana Perez, PhD, emphasized the challenges that Spanish-speaking Latinxs have with health care. Just 5%-7% of nurses are Latinx. “The same could be said for physicians, for clinical psychologists ... as you look at the really critical positions to address brain health equity, we are not represented there,” said Dr. Perez, an assistant professor and senior fellow at the University of Pennsylvania School of Nursing in Philadelphia.
She also pointed out that Latinx representation in clinical trials is very low, even though surveys performed by the Alzheimer’s Association show that this population values medical science and is willing to participate. In fact, 85% said they would participate if invited. The trouble is that many clinical trial announcements state that participants must speak English. Even the many Latinos who are bilingual may be put off by that wording: “That is a message that you’re not invited. That’s how it’s perceived,” said Dr. Perez.
Racism and cognition in the elderly
At the press conference, Kristen George, PhD, presented results from a study of individuals over age 90. “Racial disparities in dementia have been well characterized, particularly among those people who are aged 65 and older, but we don’t know very much about the oldest old individuals who are aged 90 and older. This group is one of the fastest growing segments of the population, and it’s becoming increasingly diverse,” said Dr. George, assistant professor of epidemiology at the University of California, Davis.
The group enrolled 445 Asian, Black, Latinx, White, and multiracial individuals who were members of Kaiser Permanente Northern California, with a mean age of 92.7 years. They used the Major Experiences of Discrimination Scale to assess discrimination.
The researchers divided them into three groups based on gender, race, and responses to the 10-item scale. Class 1 included largely White men who had reported workplace discrimination, with an average of two major discrimination experiences. Class 2 was made up of White women and non-Whites who reported little or no discrimination, with an average of 0 experiences. Class 3 included all non-White participants, and they reported a mean of four discrimination experiences.
Using class 2 as a reference, executive function was better among class 1 individuals (beta = 0.28; 95% CI, 0.03-0.52) but there was no significant difference between class 3 and class 2. Class 1 had better baseline semantic memory than class 2 (beta = 0.33; 95% CI, 0.07-0.58), and those in class 3 performed significantly worse than class 2 (beta = –0.24; 95% CI, –0.48 to –0.00). There were no between-group differences in baseline verbal or episodic memory.
Dr. Perez, Dr. Manly, Dr. George, and Dr. Hill have no relevant financial disclosures.
FROM AAIC 2022